- 1School of Physical Education, Southwest University, Chongqing, China
- 2Key Laboratory of Cognition and Personality, Faculty of Psychology, Ministry of Education, Southwest University, Chongqing, China
- 3School of Physical Education, Shanghai University of Sport, Shanghai, China
- 4School of Physical Education, Yunnan Normal University, Yunnan, China
- 5Department of Physical Education, Tianjin University, Tianjin, China
Objective: Depression is one of the most common mental disorders and is the leading cause of disability worldwide. The objective of this systematic review was to synthesize the latest evidence from randomized controlled trials (RCTs) regarding the effectiveness of mind-body therapies (MBTs) on pro-inflammatory cytokines in patients with depression.
Methods: A literature search was conducted in five electronic databases—PubMed, Embase, Web of Science, EBSCOhost, and Scopus. The quality of the included studies was evaluated using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). A narrative synthesis of the included studies was conducted.
Results: The 12 RCTs provided 21 pieces of evidence involving a total of 1,058 patients with depression. The risk of bias among the included studies ranged from low to high, with 4 studies assessed as low risk, 4 as some concerns, and 4 as high risk. Among the 21 pieces of evidence evaluated, 14 supported the positive impact of MBTs on pro-inflammatory cytokine levels in patients with depression.
Conclusion: MBTs have been widely recognized in nursing for their low risk and substantial benefits, and they hold promise as a complementary therapy to improve physiological health outcomes in patients with depression. However, the studies included commonly exhibit potential limitations in terms of intervention materials, adherence, and outcome measures. It is suggested that future research should further examine the existing evidence to strengthen the empirical foundation for incorporating MBTs into nursing care for depression.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251113095.
1 Background
Depression is one of the most common mental disorders and is the leading cause of disability worldwide (1–3). The clinical characteristics of depression primarily include a depressed mood, diminished interest or pleasure in activities, reduced ability to think or concentrate, and feelings of worthlessness or guilt (4). In recent years, the global prevalence of depression has been steadily increasing, placing a substantial disease burden on society (5, 6). Additionally, depression has been identified as an important risk factor for various physical illnesses, including coronary artery disease (7), sleep disorders (8), and cognitive impairments (9).
Pharmacological treatment is a common approach for alleviating depression; however, its therapeutic effects typically take at least four weeks to manifest, and during this period, patients may experience side effects such as headaches, anxiety, and agitation (10). Several complementary therapies have been developed to effectively address this challenge. Among them, mind-body therapies (MBTs) have become an important approach for alleviating depression due to their lower treatment risks and higher potential for efficacy (11). MBTs are rooted in ancient Eastern traditions and aim to enhance overall well-being by harnessing the interplay between the mind, body, and spirit (12, 13). The efficacy of MBTs in alleviating depression in both clinical and non-clinical populations has been supported by numerous studies (14–19).
Recent research suggests that the potential mechanism through which MBTs exert their antidepressant effects is related to the body’s inflammatory response (20, 21), primarily mediated by cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (22–24). Compared to healthy individuals, patients with depression may exhibit higher levels of pro-inflammatory cytokines. MBTs can reduce the levels of inflammatory markers by modulating the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) (20). For example, meditation can alter the brain’s neural response to stress or threats, and these changes may be associated with a reduction in the levels of inflammatory markers such as IL-6 (25). Yoga has been found to effectively reduce stress and improve cognition, benefits that are particularly important for reducing pro-inflammatory responses (26, 27). However, the above evidence primarily focuses on other clinical populations, and it remains uncertain whether it is applicable to patients with depression.
To the best of our knowledge, no study has yet comprehensively evaluated the overall effectiveness of MBTs in improving pro-inflammatory cytokine levels in patients with depression. Applying a complementary therapy that offers both low risk and substantial benefits to improve physiological health outcomes in patients with depression lays the foundation for subsequent treatment and promotes the comprehensive recovery of physical and mental health. Given the aforementioned evidence gap, this systematic review aimed to synthesize the latest evidence from randomized controlled trials (RCTs) regarding the effectiveness of MBTs on pro-inflammatory cytokines in patients with depression.
2 Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (28) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD420251113095.
2.1 Search methods
We conducted a literature search in five electronic databases—PubMed, Embase, Web of Science, EBSCOhost, and Scopus—using the Boolean algorithm established for this systematic review. In addition, we manually searched Google Scholar and reference lists of studies with similar designs to ensure the comprehensiveness of the literature search. The literature search covered the period from the inception of each database to June 2025. The search strategy is presented in Table 1, per the PubMed database.
2.2 Inclusion and exclusion criteria
The inclusion and exclusion criteria for this systematic review strictly followed the Population, Intervention, Comparator, Outcome, and Study design (PICOS) framework. Regarding the inclusion criteria, the population was restricted to patients with depression (age ≥ 18 years); the intervention was limited to MBTs; the comparator included both non-active controls (e.g., no exercise, wait-list) and active controls (e.g., treatment as usual, standard care, placebo); the outcome focused on pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α; and the study design was restricted to RCTs. Studies targeting non-depressed patients, non-MBTs, and non-RCTs were excluded. Data from the same group of subjects were only included in a single study that provided more comprehensive information.
2.3 Study selection and quality appraisal
According to the inclusion and exclusion criteria, two independent researchers conducted literature screening using EndNote 20.6 reference management software. After removing duplicates, the remaining records were screened sequentially based on their titles, abstracts, and full texts, with the reasons for exclusion systematically documented for each record. Two independent researchers evaluated the quality of the included studies using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (29). Any disagreements arising during this process were resolved through consultation with a third author.
2.4 Data extraction and synthesis
Upon identifying studies that met the inclusion criteria, two independent researchers extracted the following information from each included study: sociodemographic characteristics, intervention and comparator, implementation parameters, and outcome. A narrative synthesis of the included studies was conducted. We utilized the Template for Intervention Description and Replication (TIDieR) checklist to evaluate the adequacy of intervention reporting (30).
3 Results
3.1 Search outcomes
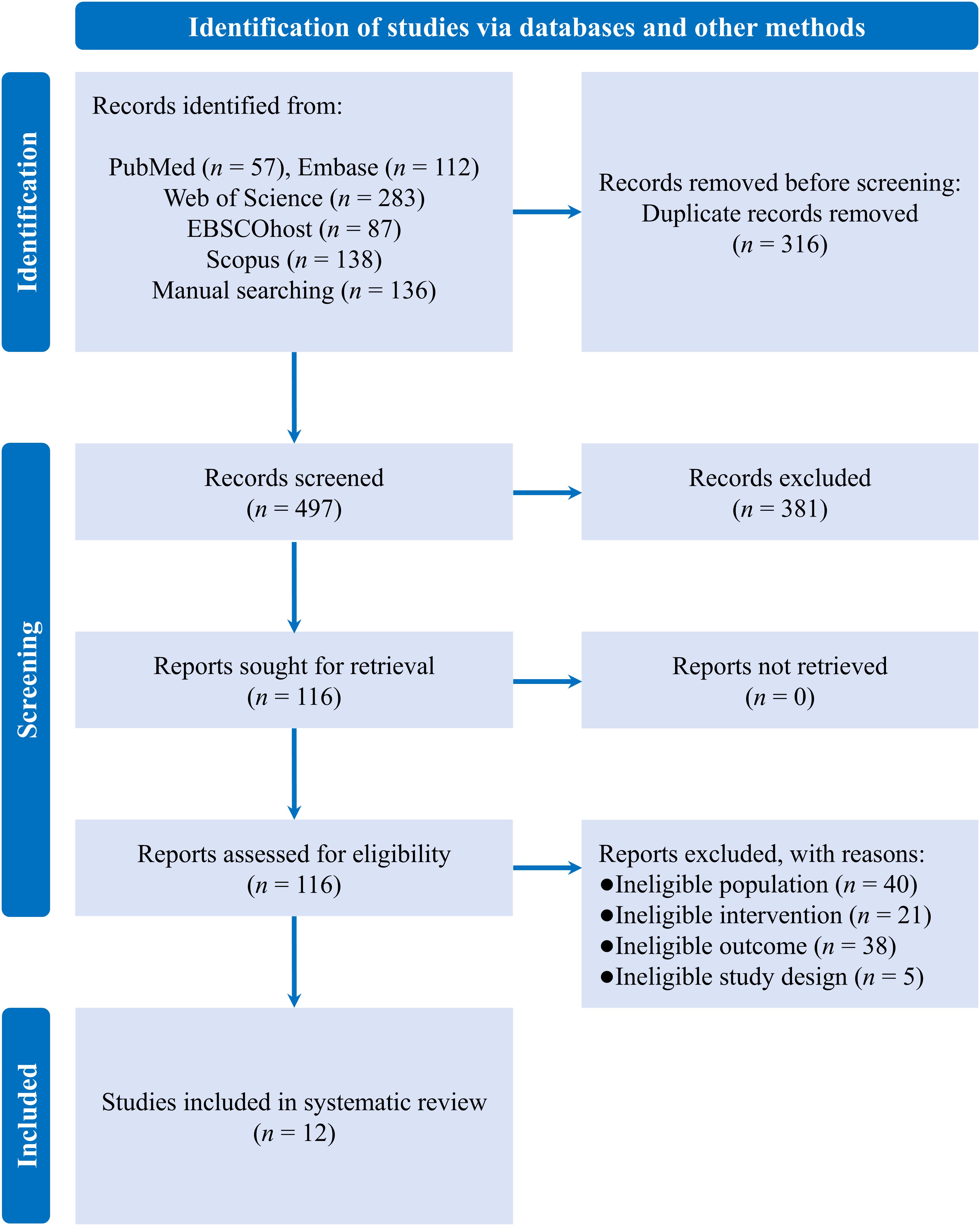
A literature search of various databases yielded a total of 813 records, of which 316 were duplicates. After screening the remaining records, 12 eligible RCTs were included in the systematic review (see Figure 1) (31–42).
3.2 Study characteristics and quality appraisal
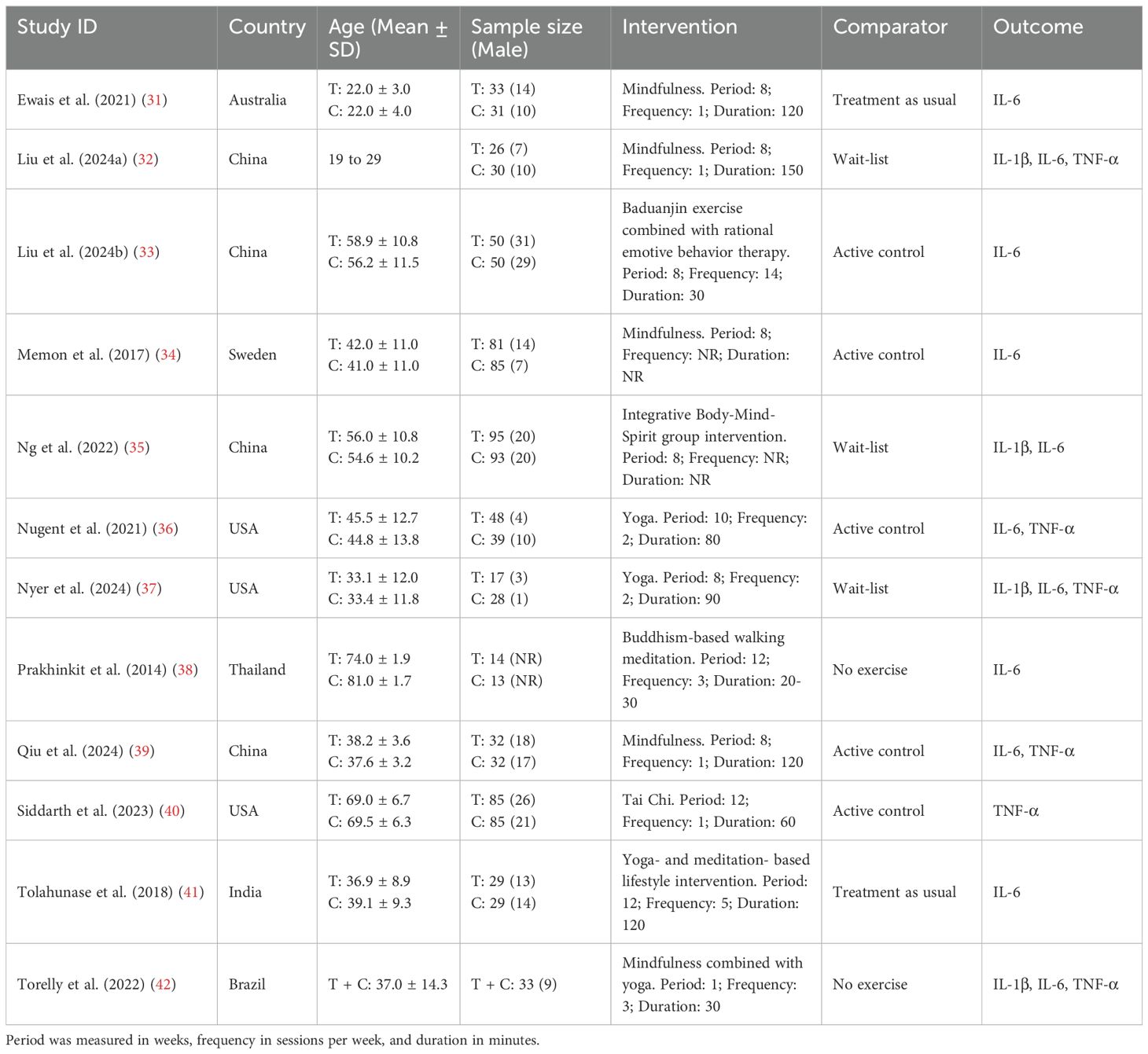
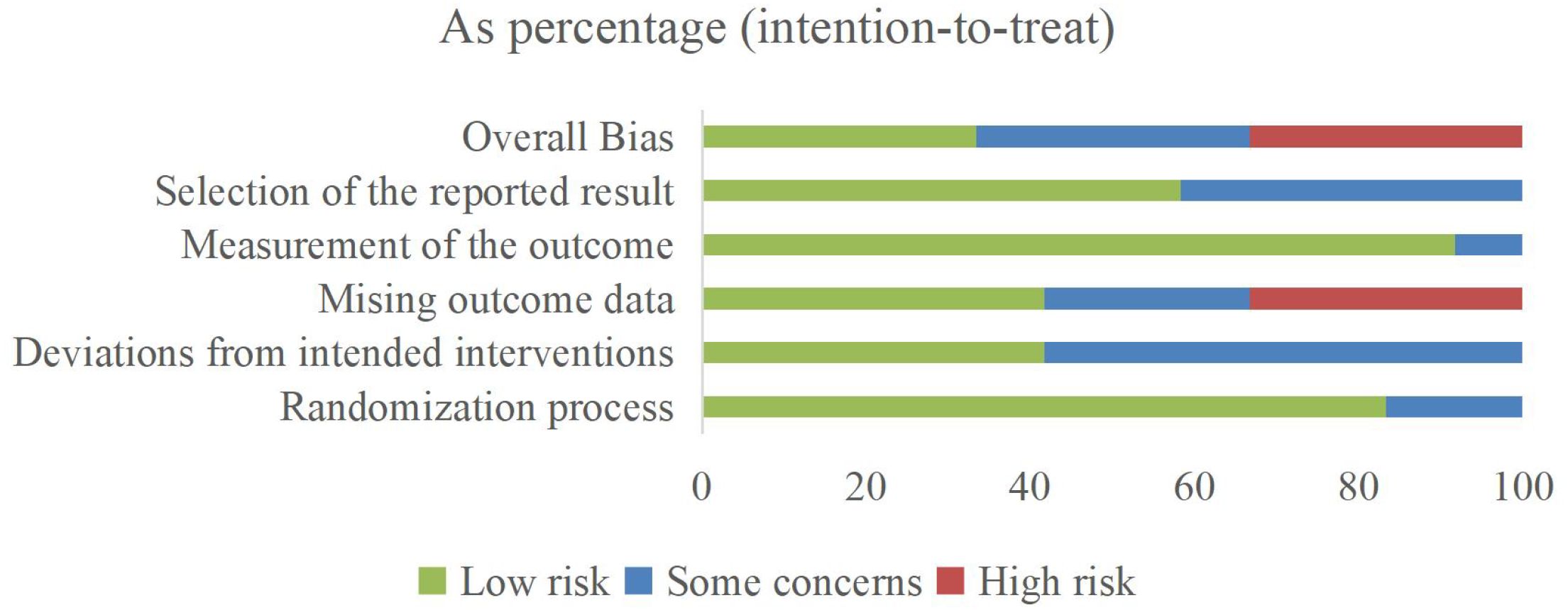
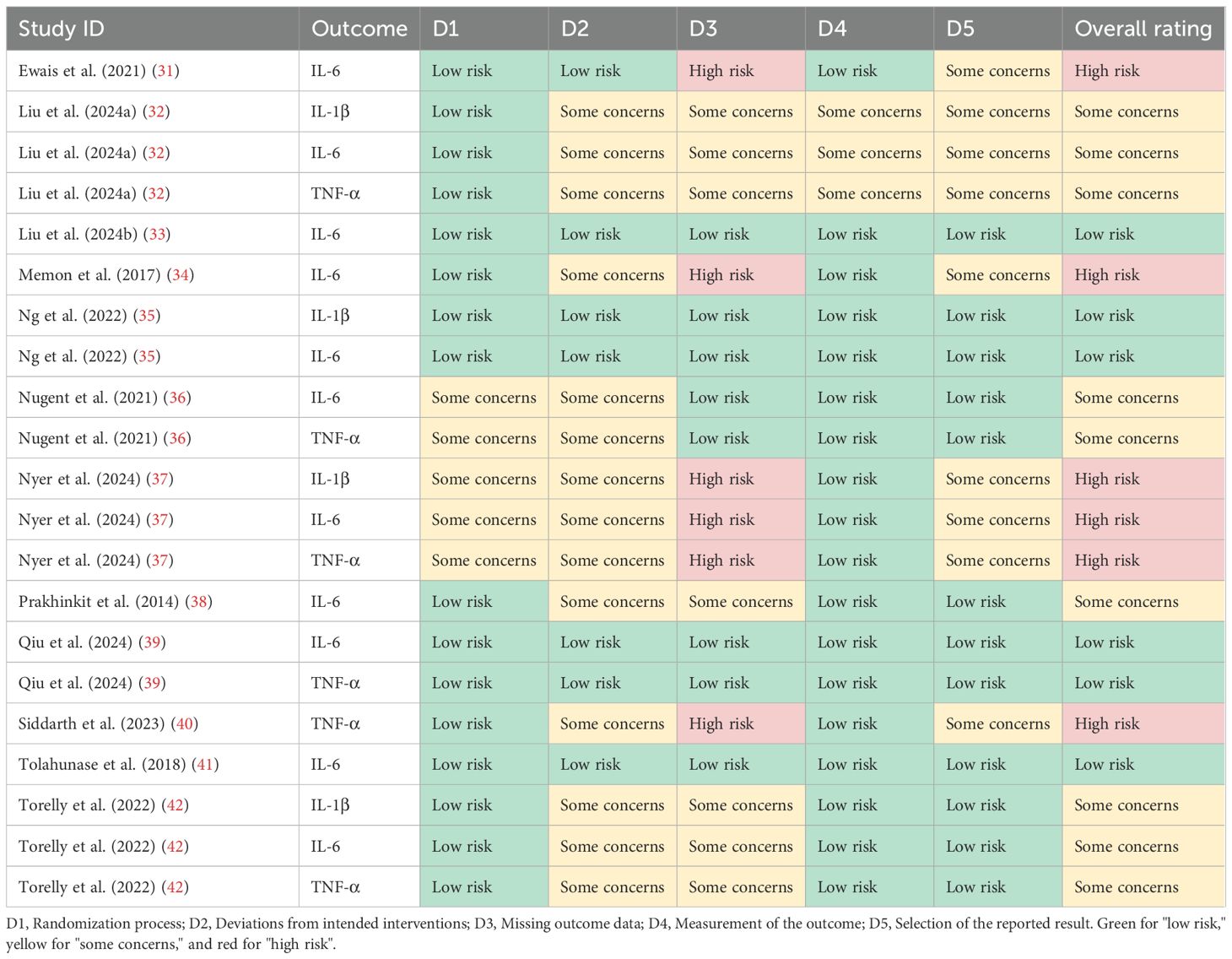
Evidence regarding the effectiveness of MBTs on pro-inflammatory cytokine levels in patients with depression was distributed across multiple countries, including Australia (one trial), Brazil (one trial), China (four trials), India (one trial), Sweden (one trial), Thailand (one trial), and the United States (three trials). The evidence involved 1,058 patients with depression, ranging in age from 18 to 82 years. The interventions included Baduanjin, mindfulness, meditation, Tai Chi, yoga, and combined forms of MBTs. Regarding implementation parameters, the included studies had an average intervention period of 8.6 weeks, an average frequency of 3.3 sessions per week, and an average duration of 82.5 minutes. The controls included active controls, no exercise, treatment as usual, and wait-list. In terms of outcome measures, evidence for IL-1β was reported in 4 studies, IL-6 in 11 studies, and TNF-α in 6 studies. The main characteristics of the included RCTs are presented in Table 2. The risk of bias among the included studies ranged from low to high, with 4 studies assessed as low risk, 4 as some concerns, and 4 as high risk (see Figure 2; Table 3).
3.3 Interventions and controls evaluated in the studies
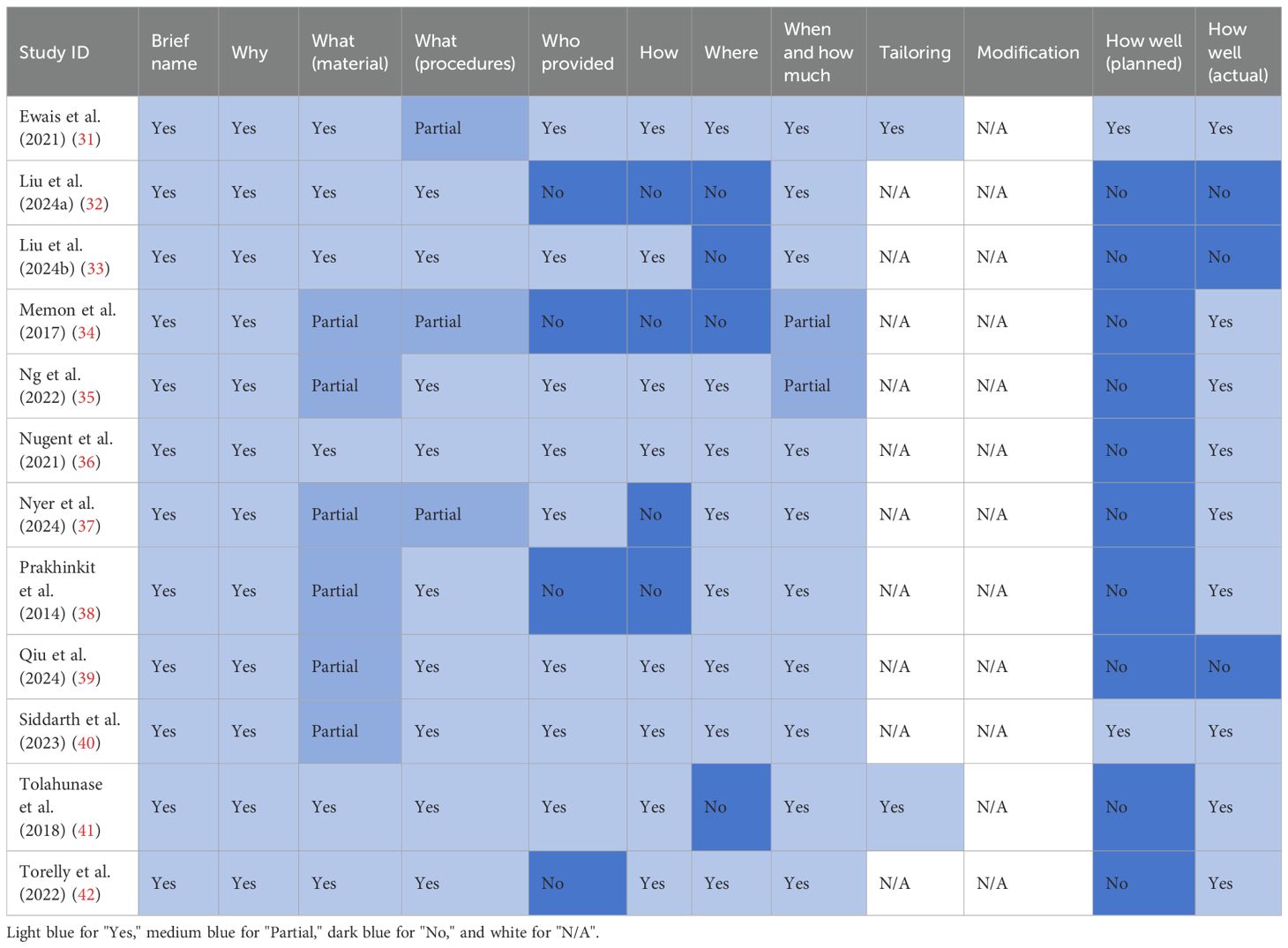
Among the 10 primary domains of the Template for Intervention Description and Replication checklist (with most studies not applicable to the other two domains), all studies had 1–7 domains that were under-reported or not reported (see Table 4). Adequate reporting of the domains was as follows: intervention name and rationale (12 studies, 100.0%), materials used (6 studies, 50.0%), procedure (9 studies, 75.0%), intervention provider (8 studies, 66.7%), mode of delivery (8 studies, 66.7%), location (8 studies, 66.7%), duration and intensity (10 studies, 83.3%), expected effects (2 studies, 16.7%), and actual effects (9 studies, 75.0%).
3.4 Research findings included in the studies
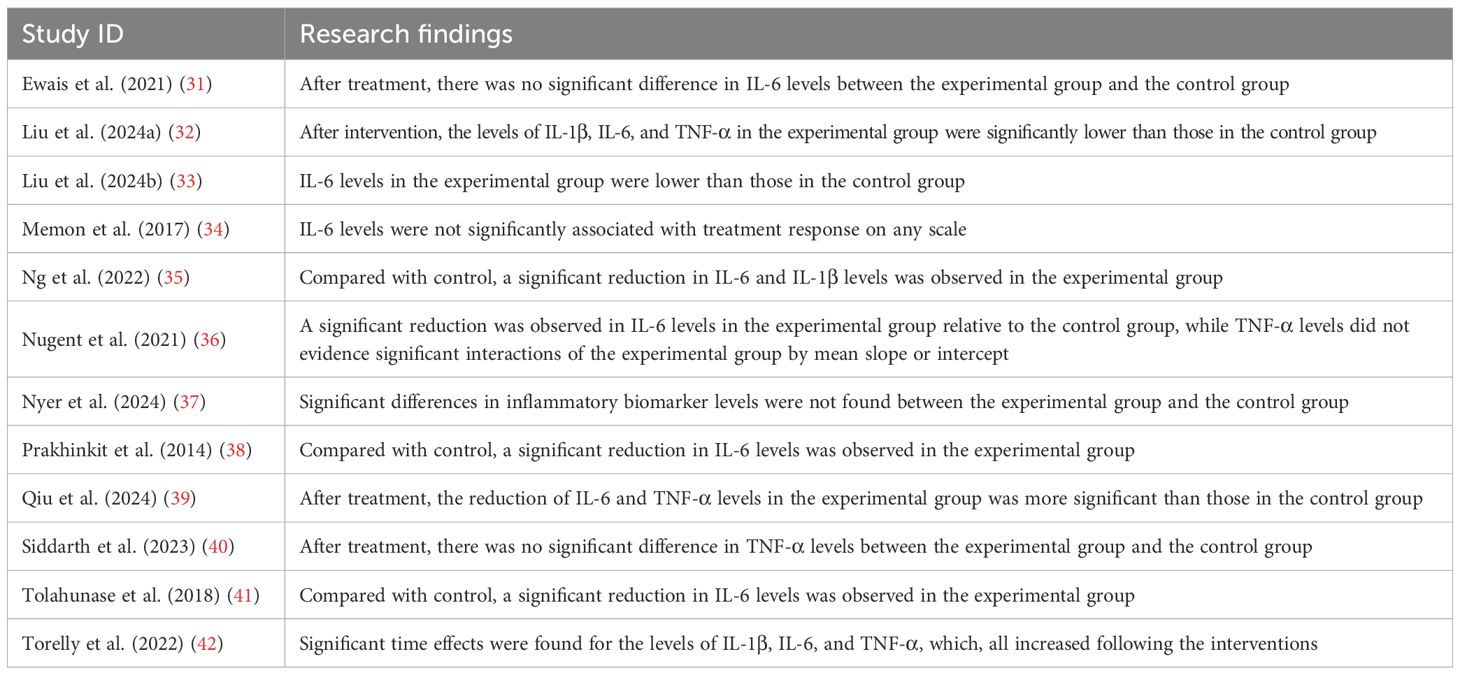
The included studies provided 21 pieces of evidence evaluating the effectiveness of MBTs on pro-inflammatory cytokine levels in patients with depression. Specifically, among the 4 pieces of evidence assessing IL-1β levels, 3 reported significant improvements following the intervention. Of the 11 pieces of evidence examining IL-6 levels, 8 reported significant improvements following the intervention. Among the 6 pieces of evidence evaluating TNF-α levels, 3 reported significant improvements following the intervention. Research findings included in the studies are presented in Table 5.
4 Discussion
Given the potential risks associated with pharmacological treatments, the application of a promising complementary therapy to improve physiological health outcomes in patients with depression is particularly critical for their disease management and physical and mental recovery. The objective of this systematic review was to synthesize the latest evidence from RCTs regarding the effectiveness of MBTs on pro-inflammatory cytokines in patients with depression. The 12 RCTs provided 21 pieces of evidence involving a total of 1,058 patients with depression. The risk of bias among the included studies ranged from low to high, and their overall quality was relatively low. Among the 10 primary domains of the Template for Intervention Description and Replication checklist, all studies had 1–7 domains that were under-reported or not reported. Out of four pieces of evidence, three reported significant improvements in IL-1β levels following the intervention. Eight pieces of evidence reported significant improvements in IL-6 levels out of eleven, and three pieces of evidence reported significant improvements in TNF-α levels out of six.
Among the 21 pieces of evidence evaluated, 14 supported the positive impact of MBTs on pro-inflammatory cytokine levels in patients with depression. The mechanism underlying the antidepressant effects of MBTs can be explained from a neurobiological perspective. Specifically, MBTs such as mindfulness and meditation contribute to decreased activity of the sympathetic nervous system (SNS) and increased activity of the parasympathetic nervous system (PNS), reflecting a greater sympathetic-vagal balance. This balance is thought to reduce the body’s inflammatory response by diminishing adrenergic signaling (20, 43–45). Notably, increased activity of the SNS has been found to promote the expression of pro-inflammatory genes while inhibiting the expression of anti-viral genes (46). MBTs can reverse the impact of acute and chronic stress and reduce the activation of the SNS, which in turn helps regulate immune-related transcription (45, 47). These transcriptional regulations are primarily characterized by a reduction in NF-κB-related transcription of pro-inflammatory cytokines and an enhancement in IRF1-related transcription of innate anti-viral response (47). Accordingly, parasympathetic activation induced by the vagus nerve has been shown to increase levels of brain-derived neurotrophic factor (BDNF) (48, 49), which can inhibit glial cell activation in the central nervous system through its signaling pathways, thereby alleviating inflammatory responses (50). The increased activity of the PNS may also reduce inflammation through the cholinergic anti-inflammatory pathway (51). Additionally, alterations in cortisol production or in glucocorticoid receptor sensitivity may also modulate inflammatory processes (21). Although evidence regarding the impact of MBTs on cortisol levels remains inconsistent, several studies have found that interventions such as yoga, mindfulness, and Tai Chi can enhance glucocorticoid receptor-mediated anti-inflammatory signaling pathways, accompanied by a decrease in NF-κB activity (52–54). In terms of neural mechanisms, the activity of the ANS and the HPA axis is primarily regulated by the brain regions associated with stress or threat, including the amygdala, dorsal anterior cingulate cortex, anterior insula, and periaqueductal gray (21). Studies have shown that MBTs, such as meditation, can lead to increased thickness of the prefrontal cortex and reduce the size and activity of the amygdala (20, 43). These changes help individuals better regulate emotional responses and respond to stress or threat in a more balanced manner.
Although MBTs have shown potential in improving pro-inflammatory cytokines in patients with depression, the directionality between inflammation reduction and symptom improvement remains unclear. Clarifying this limitation is crucial for understanding the clinical implications of cytokine changes. Given that the most studies only involved short-term interventions and failed to dynamically monitor the temporal relationship between changes in inflammatory markers and clinical symptoms, we suggest conducting longitudinal studies to better infer how MBTs may alleviate depression through anti-inflammatory mechanisms. Additionally, since the included studies vary in terms of implementation parameters, including intervention period, frequency, and duration, this may lead to various dose-response relationships. Therefore, future research should establish standardized intervention protocols and conduct long-term follow-ups to examine the relationship between the efficacy of the intervention and symptom improvement. This approach will help clarify the practical value of MBTs as anti-inflammatory adjunctive strategies in clinical practice. Notably, patients’ initial condition plays a crucial role in determining the efficacy of the established intervention. Significant differences may exist among patients at baseline in terms of inflammation levels, depression severity, and treatment adherence. These differences may moderate intervention efficacy and thus influence the relationship between inflammation reduction and symptom improvement. We advocate that future research should emphasize the moderating role of patient characteristics in this context and develop personalized intervention protocols based on these features. This approach will facilitate a more comprehensive evaluation of the anti-inflammatory potential and clinical applicability of MBTs across different types and severities of depression.
In terms of the outcome, this study primarily selected IL-1β, IL-6, and TNF-α as inflammatory biomarkers associated with depression. Notably, IL-1 exists in two isoforms, IL-1α and IL-1β, both of which may possess comparable potency in activating the body’s inflammatory response (55). Although existing studies have primarily focused on the effectiveness of established interventions on IL-1β, IL-1α—being the inducible form released in an inflammatory disease state—may be more closely associated with depression than IL-1β (55, 56). Future research is recommended to further investigate the effectiveness of MBTs on additional inflammatory biomarkers in patients with depression, if sufficient evidence becomes available. This would contribute to a more comprehensive understanding of the mechanism underlying the antidepressant effects of MBTs. In terms of intervention, most of the included studies lacked adequate attention to intervention materials and adherence. On the one hand, the lack of detailed reporting on intervention materials may hinder future research from accurately replicating the original intervention process. On the other hand, heterogeneity in intervention efficacy is related to patients’ adherence levels. A lack of strategies to engage patients and maintain their participation during the intervention phase may lead to underestimation or overestimation of the intervention efficacy. Therefore, the feasibility and adherence of the intervention are important factors influencing clinical implementation. Future research should enhance transparency in the design and reporting of the intervention. We recommend that researchers follow established intervention reporting guidelines, such as the TIDieR checklist, to systematically present key information including intervention materials, implementation procedures, and parameters. This will help improve the replicability and scalability of the intervention. In addition, researchers should place greater emphasis on the assessment of adherence and strategies to promote it in their study designs. Regarding assessment, adherence indicators (e.g., actual participation frequency, completion rate) should be clearly reported, and their potential impact on intervention efficacy should be explored. Regarding strategies, it is recommended to incorporate measures designed to enhance participant motivation during the intervention implementation, such as personalized feedback and intelligent reminder systems. These approaches can help improve the feasibility and sustainability of the intervention.
The findings of this study should be interpreted in light of its limitations. First, due to the limited number of studies and available quantitative data, a meta-analysis could not be conducted. This may make it difficult to quantify the effect size of MBTs on pro-inflammatory cytokine levels in patients with depression. It is suggested that future research should examine the pooled efficacy of MBTs in this field based on a more comprehensive body of evidence. Second, this study focused exclusively on IL-1β, IL-6, and TNF-α as inflammatory biomarkers associated with depression and did not examine the effectiveness of MBTs on other inflammatory markers. Future research should expand the scope of outcome measures to more comprehensively evaluate the antidepressant effects of MBTs. Finally, the overall low quality of the included studies may affect the robustness and external validity of the findings. Future research is recommended to enhance the quality of study design, particularly in intervention materials, adherence, and outcome measures. By adhering to established intervention reporting guidelines, adopting standardized adherence monitoring, and applying uniform cytokine assays, researchers can further improve the reliability and generalizability of intervention efficacy.
5 Conclusion
Overall, MBTs have been widely recognized in nursing for their low risk and substantial benefits, and they hold promise as a complementary therapy to improve physiological health outcomes in patients with depression. Among the 21 pieces of evidence evaluated, 14 supported the positive impact of MBTs on pro-inflammatory cytokine levels in patients with depression. However, the included studies commonly exhibit potential limitations in terms of intervention materials, adherence, and outcome measures, which may affect the reliability and generalizability of the above findings. Therefore, future research should further examine the existing evidence to strengthen the empirical foundation for incorporating MBTs into nursing care for depression.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
ZM: Methodology, Data curation, Writing – review & editing, Software, Writing – original draft, Investigation, Conceptualization. SL: Writing – original draft, Resources, Data curation, Conceptualization, Project administration. CC: Methodology, Writing – original draft, Visualization, Data curation. CL: Software, Writing – original draft, Methodology, Investigation. TW: Investigation, Writing – review & editing, Visualization. HJ: Supervision, Validation, Methodology, Writing – review & editing. LC: Software, Writing – review & editing, Validation, Supervision. RH: Project administration, Writing – review & editing, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the National Social Science Fund of China (project 24BTY098).
Acknowledgments
Research team would like to thank Southwest University for the support of this research, and also thank Shi Luo and Ranran He for the help in developing the research programme.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Chisholm D, Sweeny K, Sheehan P, Rasmussen B, Smit F, Cuijpers P, et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry. (2016) 3:415–24. doi: 10.1016/S2215-0366(16)30024-4
3. Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev. (2011) 31:1117–25. doi: 10.1016/j.cpr.2011.07.004
4. Maj M, Stein DJ, Parker G, Zimmerman M, Fava GA, De Hert M, et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry. (2020) 19:269–93. doi: 10.1002/wps.20771
5. Moreno-Agostino D, Wu Y-T, Daskalopoulou C, Hasan MT, Huisman M, and Prina M. Global trends in the prevalence and incidence of depression: a systematic review and meta-analysis. J Affect Disord. (2021) 281:235–43. doi: 10.1016/j.jad.2020.12.035
6. Herrman H, Kieling C, McGorry P, Horton R, Sargent J, and Patel V. Reducing the global burden of depression: a Lancet–World Psychiatric Association Commission. Lancet. (2019) 93:e42–e3. doi: 10.1016/S0140-6736(18)32408-5
7. Carney RM and Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. (2017) 14:145–55. doi: 10.1186/1741-7015-11-131
8. Fang H, Tu S, Sheng J, and Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
9. Semkovska M, Quinlivan L, O'Grady T, Johnson R, Collins A, O'Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6:851–61. doi: 10.1016/s2215-0366(19)30291-3
10. Marwaha S, Palmer E, Suppes T, Cons E, Young AH, and Upthegrove R. Novel and emerging treatments for major depression. Lancet. (2023) 401:141–53. doi: 10.1016/s0140-6736(22)02080-3
11. McClafferty H. Complementary, holistic, and integrative medicine: mind-body medicine. Pediatr Rev. (2011) 32:201–3. doi: 10.1542/pir.32-5-201
12. Laird KT, Paholpak P, Roman M, Rahi B, and Lavretsky H. Mind-body therapies for late-life mental and cognitive health. Curr Psychiatry Rep. (2018) 20:2. doi: 10.1007/s11920-018-0864-4
13. Mei Z, Jiang W, Zhang Y, Luo S, and Luo S. Mind-body therapies for resilience in adolescents: A systematic review of randomized controlled trials. Gen Hosp Psychiatry. (2024) 91:43–51. doi: 10.1016/j.genhosppsych.2024.08.014
14. Luo S, Mei Z, Fang G, Mu G, Zhang X, and Luo S. Effects of mind–body therapies on depression among adolescents: a systematic review and network meta-analysis. Front Public Health. (2024) 12:1431062. doi: 10.3389/fpubh.2024.1431062
15. Dong Y, Zhang X, Zhao R, Cao L, Kuang X, and Yao J. The effects of mind-body exercise on anxiety and depression in older adults: a systematic review and network meta-analysis. Front Psychiatry. (2024) 15:1305295. doi: 10.3389/fpsyt.2024.1305295
16. Li Z, Liu S, Wang L, and Smith L. Mind–body exercise for anxiety and depression in copd patients: A systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:22. doi: 10.3390/ijerph17010022
17. Jin X, Wang L, Liu S, Zhu L, Loprinzi PD, and Fan X. The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:31. doi: 10.3390/ijerph17010031
18. D'Silva S, Poscablo C, Habousha R, Kogan M, and Kligler B. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. (2012) 53:407–23. doi: 10.1016/j.psym.2012.04.006
19. Little SA, Kligler B, Homel P, Belisle SS, and Merrell W. Multimodal mind/body group therapy for chronic depression: a pilot study. Explore. (2009) 5:330–7. doi: 10.1016/j.explore.2009.08.004
20. Moreno JJ. Modulation of inflammatory response and pain by mind-body therapies as meditation. Brain Behav Immun Integr. (2024) 5:100036. doi: 10.1016/j.bbii.2023.100036
21. Bower JE and Irwin MR. Mind–body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun. (2016) 51:1–11. doi: 10.1016/j.bbi.2015.06.012
22. Abdelrahman SA, Samak MA, and Shalaby SM. Fluoxetine pretreatment enhances neurogenic, angiogenic and immunomodulatory effects of MSCs on experimentally induced diabetic neuropathy. Cell Tissue Res. (2018) 374:83–97. doi: 10.1007/s00441-018-2838-6
23. Leighton S, Nerurkar L, Krishnadas R, Johnman C, Graham G, and Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. (2018) 23:48–58. doi: 10.1038/mp.2017.205
24. Miller AH and Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. (2016) 16:22–34. doi: 10.1038/nri.2015.5
25. Dutcher JM, Boyle CC, Eisenberger NI, Cole SW, and Bower JE. Neural responses to threat and reward and changes in inflammation following a mindfulness intervention. Psychoneuroendocrinology. (2021) 125:105114. doi: 10.1016/j.psyneuen.2020.105114
26. Rajbhoj PH, Shete SU, Verma A, and Bhogal RS. Effect of yoga module on pro-inflammatory and anti-inflammatory cytokines in industrial workers of lonavla: a randomized controlled trial. J Clin Diagn Res. (2015) 9:CC01–CC5. doi: 10.7860/JCDR/2015/11426.5551
27. Twal WO, Wahlquist AE, and Balasubramanian S. Yogic breathing when compared to attention control reduces the levels of pro-inflammatory biomarkers in saliva: a pilot randomized controlled trial. BMC Complement Altern Med. (2016) 16:294. doi: 10.1186/s12906-016-1286-7
28. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
29. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:1–8. doi: 10.1136/bmj.l4898
30. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
31. Ewais T, Begun J, Kenny M, Hay K, Houldin E, Chuang KH, et al. Mindfulness based cognitive therapy for youth with inflammatory bowel disease and depression - Findings from a pilot randomised controlled trial. J Psychosom Res. (2021) 149:110549. doi: 10.1016/j.jpsychores.2021.110594
32. Liu W, Yuan J, Wu Y, Xu L, Wang X, Meng J, et al. A randomized controlled trial of mindfulness-based cognitive therapy for major depressive disorder in undergraduate students: Dose- response effect, inflammatory markers and BDNF. Psychiatry Res. (2024) 331:115671. doi: 10.1016/j.psychres.2023.115671
33. Liu Y, Chen C, Du H, Xue M, and Zhu N. Impact of Baduanjin exercise combined with rational emotive behavior therapy on sleep and mood in patients with poststroke depression: A randomized controlled trial. Med (Baltimore). (2024) 103:E38180. doi: 10.1097/MD.0000000000038180
34. Memon AA, Sundquist K, Ahmad A, Wang X, Hedelius A, and Sundquist J. Role of IL-8, CRP and epidermal growth factor in depression and anxiety patients treated with mindfulness-based therapy or cognitive behavioral therapy in primary health care. Psychiatry Res. (2017) 254:311–6. doi: 10.1016/j.psychres.2017.05.012
35. Ng SM, Yin MXC, Chan JSM, Chan CHY, Fong TCT, Li A, et al. Impact of mind–body intervention on proinflammatory cytokines interleukin 6 and 1β: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav Immun. (2022) 99:166–76. doi: 10.1016/j.bbi.2021.09.022
36. Nugent NR, Brick L, Armey MF, Tyrka AR, Ridout KK, and Uebelacker LA. Benefits of yoga on IL-6: findings from a randomized controlled trial of yoga for depression. Behav Med. (2021) 47:21–30. doi: 10.1080/08964289.2019.1604489
37. Nyer MB, Foster SL, Petrie SR, Mac Giollabhui N, Gould DA, Flux M, et al. Inflammatory biomarker findings from a randomized controlled trial of heated yoga for depression. Brain Behav Immun Integr. (2024) 8:100089. doi: 10.1016/j.bbii.2024.100089
38. Prakhinkit S, Suppapitiporn S, Tanaka H, and Suksom D. Effects of buddhism walking meditation on depression, functional fitness, and endothelium-dependent vasodilation in depressed elderly. J Altern Complement Med. (2014) 20:411–6. doi: 10.1089/acm.2013.0205
39. Qiu J, Gong Y, Zhang X, and Mao W. Effectiveness of mindfulness-based cognitive therapy on depressive symptoms, brain potential, and neuroimmunoinflammatory factors in depressed patients. Clin Neuropharmacol. (2024) 47:128–33. doi: 10.1097/wnf.0000000000000601
40. Siddarth P, Abikenari M, Grzenda A, Cappelletti M, Oughli H, Liu C, et al. Inflammatory markers of geriatric depression response to Tai Chi or health education adjunct interventions. Am J Geriatr Psychiatry. (2023) 31:22–32. doi: 10.1016/j.jagp.2022.08.004
41. Tolahunase MR, Sagar R, Faiq M, and Dada R. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor Neurol Neurosci. (2018) 36:423–42. doi: 10.3233/RNN-170810
42. Torelly GA, Novak PDS, Bristot G, Schuch FB, and Pio de Almeida Fleck M. Acute effects of mind-body practices and exercise in depressed inpatients: A randomized clinical trial. Ment Health Phys Act. (2022) 23:100479. doi: 10.1016/j.mhpa.2022.100479
43. Creswell JD and Lindsay EK. How does mindfulness training affect health? A mindfulness stress buffering account. Curr Dir Psychol Sci. (2014) 23:401–7. doi: 10.1177/0963721414547415
44. Audette JF, Jin YS, Newcomer R, Stein L, Duncan G, and Frontera WR. Tai Chi versus brisk walking in elderly women. Age Ageing. (2006) 35:388–93. doi: 10.1093/ageing/afl006
45. Motivala SJ, Sollers J, Thayer J, and Irwin MR. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. (2006) 61:1177–80. doi: 10.1093/gerona/61.11.1177
46. Morgan N, Irwin MR, Chung M, and Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PloS One. (2014) 9:e100903. doi: 10.1371/journal.pone.0100903
47. Black DS, Cole SW, Irwin MR, Breen E, Cyr NMS, Nazarian N, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. (2013) 38:348–55. doi: 10.1016/j.psyneuen.2012.06.011
48. Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. (2007) 1179:28–34. doi: 10.1016/j.brainres.2007.08.045
49. Furmaga H, Carreno FR, and Frazer A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PloS One. (2012) 7:e34844. doi: 10.1371/journal.pone.0034844
50. Cahn BR, Goodman MS, Peterson CT, Maturi R, and Mills PJ. Yoga, meditation and mind-body health: increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front Hum Neurosci. (2017) 11:315. doi: 10.3389/fnhum.2017.00315
52. Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. (2014) 43:20–9. doi: 10.1016/j.psyneuen.2014.01.019
53. Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. (2015) 121:1231–40. doi: 10.1002/cncr.29194
54. Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Institute Monographs. (2014) 2014:295–301. doi: 10.1093/jncimonographs/lgu028
55. Voronov E, Dotan S, Krelin Y, Song X, Elkabets M, Carmi Y, et al. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front Immunol. (2013) 4:177. doi: 10.3389/fimmu.2013.00177
Keywords: mind-body therapies, inflammatory markers, inflammation, depression, systematic review
Citation: Mei Z, Luo S, Cai C, Lam C, Wang T, Jia H, Chen L and He R (2025) Mind-body therapies for pro-inflammatory cytokines in patients with depression: findings from a systematic review of randomized controlled trials. Front. Immunol. 16:1677872. doi: 10.3389/fimmu.2025.1677872
Received: 01 August 2025; Accepted: 30 September 2025;
Published: 21 October 2025.
Edited by:
Iris Paola Guzmán-Guzmán, Autonomous University of Guerrero, MexicoReviewed by:
Marcos Edgar Herkenhoff, Santa Catarina State University, BrazilEnrique Becerril-Villanueva, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), Mexico
Copyright © 2025 Mei, Luo, Cai, Lam, Wang, Jia, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranran He, MTM0NDcwMzYzNkBxcS5jb20=
 Zhengyang Mei
Zhengyang Mei Shi Luo
Shi Luo Chenyi Cai
Chenyi Cai Chifong Lam
Chifong Lam Tingfeng Wang
Tingfeng Wang Haichang Jia
Haichang Jia Longjiang Chen
Longjiang Chen Ranran He5*
Ranran He5*