- 1Department of Pulmonary and Critical Care Medicine, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People′s Hospital, Quzhou, Zhejiang, China
- 2Department of Gastroenterology, Jiaxing Second Hospital, Jiaxing, Zhejiang, China
- 3Department of Radiation Oncology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People′s Hospital, Quzhou, Zhejiang, China
- 4Department of International Ward, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People′s Hospital, Quzhou, Zhejiang, China
Background: Extensive-stage small cell lung cancer (ES-SCLC) has a poor prognosis, with historical median overall survival (OS) of 8–13 months under platinum-etoposide chemotherapy. While phase III trials established adebrelimab (anti-PD-L1) plus chemotherapy as a new standard, real-world evidence remains scarce. This study evaluated real-world efficacy, safety, and prognostic factors of first-line adebrelimab-based therapy.
Methods: In this retrospective study, thirty-five patients with ES-SCLC receiving adebrelimab as first-line treatment were analyzed. Endpoints included objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), OS, and adverse events (AEs). Prognostic factors were assessed via Cox regression.
Results: Median age was 72 years; 88.6% were male, 85.7% had Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–1, and 51.4% had ≥2 metastatic sites. ORR was 62.8%, DCR 77.1%. Median PFS was 7.1 months (95% CI: 5.47–8.53), and median OS was 15.0 months (95% CI: 10.47–19.53). Multivariate analysis identified ECOG PS ≥2 as an independent predictor of inferior PFS (HR = 9.446, p=0.013), while ≥2 metastatic organs (HR = 3.594, p=0.046) and C-reactive Protein (CRP) ≥5 mg/L (HR = 3.337, p=0.044) predicted worse OS. Grade 3–4 AEs occurred in 74.3% of patients, primarily hematologic toxicities (neutropenia: 51.4%); two cases (5.7%) of myocarditis were observed.
Conclusions: Adebrelimab suggests potentially promising efficacy in ES-SCLC, aligning with pivotal trial data despite an older cohort. ECOG PS ≥2, high metastatic burden, and elevated CRP independently predict poorer outcomes. Vigilant monitoring for hematologic toxicity and rare cardiotoxicity is warranted.
1 Introduction
Small cell lung cancer (SCLC) represents an aggressive neuroendocrine malignancy characterized by rapid proliferation, early metastatic dissemination, and initially high sensitivity to chemotherapy and radiotherapy, followed by almost inevitable relapse and chemoresistance (1, 2). Approximately two-thirds of patients present with extensive-stage disease (ES-SCLC) at diagnosis, historically associated with a dismal median overall survival (OS) of 8–13 months with platinum-etoposide (PE) chemotherapy alone (3, 4). Despite decades of research, therapeutic advancements for ES-SCLC remained limited until the recent integration of immune checkpoint inhibitors (ICIs) targeting the programmed death-ligand 1 (PD-L1) pathway.
The addition of PD-L1 inhibitors to first-line platinum-based chemotherapy marked a paradigm shift, demonstrating significant survival benefits in phase III trials. Atezolizumab (IMpower133 trial) and durvalumab (CASPIAN trial), both targeting PD-L1, significantly improved OS compared to chemotherapy alone, establishing the current standard of care (5, 6). More recently, adebrelimab (SHR-1316), a novel, highly selective, humanized anti-PD-L1 monoclonal antibody developed in China, demonstrated compelling efficacy in the phase III CAPSTONE-1 trial (7). When combined with carboplatin and etoposide, adebrelimab significantly prolonged both progression-free survival (PFS) and OS compared to chemotherapy alone in Chinese patients with previously untreated ES-SCLC, with a manageable safety profile (7). Based on these results, adebrelimab gained regulatory approval in China for this indication.
While randomized controlled trials (RCTs) like CAPSTONE-1 provide high-level evidence of efficacy under controlled conditions, their stringent eligibility criteria often exclude patients commonly encountered in routine clinical practice, such as older individuals, those with poorer Eastern Cooperative Oncology Group Performance Status (ECOG PS ≥2), significant comorbidities, or specific metastatic burdens (8, 9). Consequently, the real-world effectiveness and safety profile of novel therapies can differ from trial results (10). Real-world evidence (RWE) derived from observational studies is therefore crucial to complement RCT findings, offering insights into treatment patterns, outcomes, and tolerability in broader, unselected patient populations reflective of everyday oncology practice (11, 12). Such studies are particularly valuable for understanding the performance of therapies like adebrelimab in diverse clinical scenarios and identifying real-world prognostic factors (13).
Despite the established role of PD-L1 inhibitors in ES-SCLC and the approval of adebrelimab, real-world data specifically focusing on adebrelimab in the first-line setting remain scarce. To address this gap and provide valuable insights into the effectiveness, prognostic determinants, and safety of adebrelimab-based therapy outside the controlled trial environment, we conducted this retrospective, single-center, real-world study at Quzhou People’s Hospital. This study aimed to evaluate the real-world clinical outcomes, including objective response rate (ORR), disease control rate (DCR), PFS, OS, and treatment-related adverse events (AEs), in an unselected cohort of patients with ES-SCLC receiving adebrelimab as part of first-line treatment. Furthermore, we sought to identify clinical and laboratory factors associated with survival outcomes in this real-world context.
2 Methods
2.1 Study design
We retrieved data from the electronic medical record system for patients diagnosed with extensive-stage small cell lung cancer (SCLC) and treated with Adebrelimab and chemotherapy [etoposide plus cisplatin (EP), etoposide plus carboplatin (EC), or irinotecan plus cisplatin (IP)] at Quzhou People’s Hospital between September 2021 and March 2025. Patients who met the following criteria were eligible for inclusion in this retrospective real-world study: (1) a histological or cytological diagnosis of extensive-stage SCLC; (2) adequate hepatic and renal function reserve; (3) receipt of a treatment regimen that included Adebrelimab as first-line treatment; and (4) at least one measurable lesion. Additionally, the following exclusion criteria were applied: (1) a history of autoimmune disease; (2) a poor ECOG performance status score greater than 2; (3) pregnant women; and (4) multiple primary malignant neoplasms. The follow-up deadline was set for June 30, 2025. This study was approved by the Ethics Committee of Quzhou People’s Hospital, and all investigations were conducted in accordance with the Declaration of Helsinki (revised in 2013).
2.2 Data source and outcomes evaluations
Clinical responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Radiologic assessments were performed every 2 cycles (6–8 weeks) during treatment. The objective response rate (ORR) was defined as the percentage of patients achieving a complete response (CR: complete remission of all target lesions) or a partial response (PR: at least a 30% reduction in the sum of the diameters of target lesions). Progressive disease (PD) was defined as a 20% increase in the sum of the diameters of target lesions. A disease that could not be classified as either PR or PD was evaluated as stable disease (SD). The percentage of patients with CR, PR, or SD was defined as the disease control rate (DCR). Progression-free survival (PFS) is calculated as the time from the start of adebrelimab-based treatment to the occurrence of PD or death. Overall survival (OS) is defined as the time from the start of treatment to death from any cause. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE v4.0).
2.3 Statistical analysis
Descriptive statistics (percentages, means, and medians) were used to describe the baseline characteristics and clinical features of the extensive-stage SCLC patients. Short-term efficacy was evaluated using ORR and DCR. Survival curves were calculated using the Kaplan-Meier method and were compared via the log-rank test based on ECOG PS. K-M curves were plotted using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). Cut-off values for continuous variables (e.g., CRP, LDH) were determined based on established normal range. These analyses were performed using SPSS software, version 23.0 (SPSS Inc., Chicago, USA). P ≤ 0.05 was considered to indicate statistical significance.
3 Results
3.1 Patient characteristics and outcomes
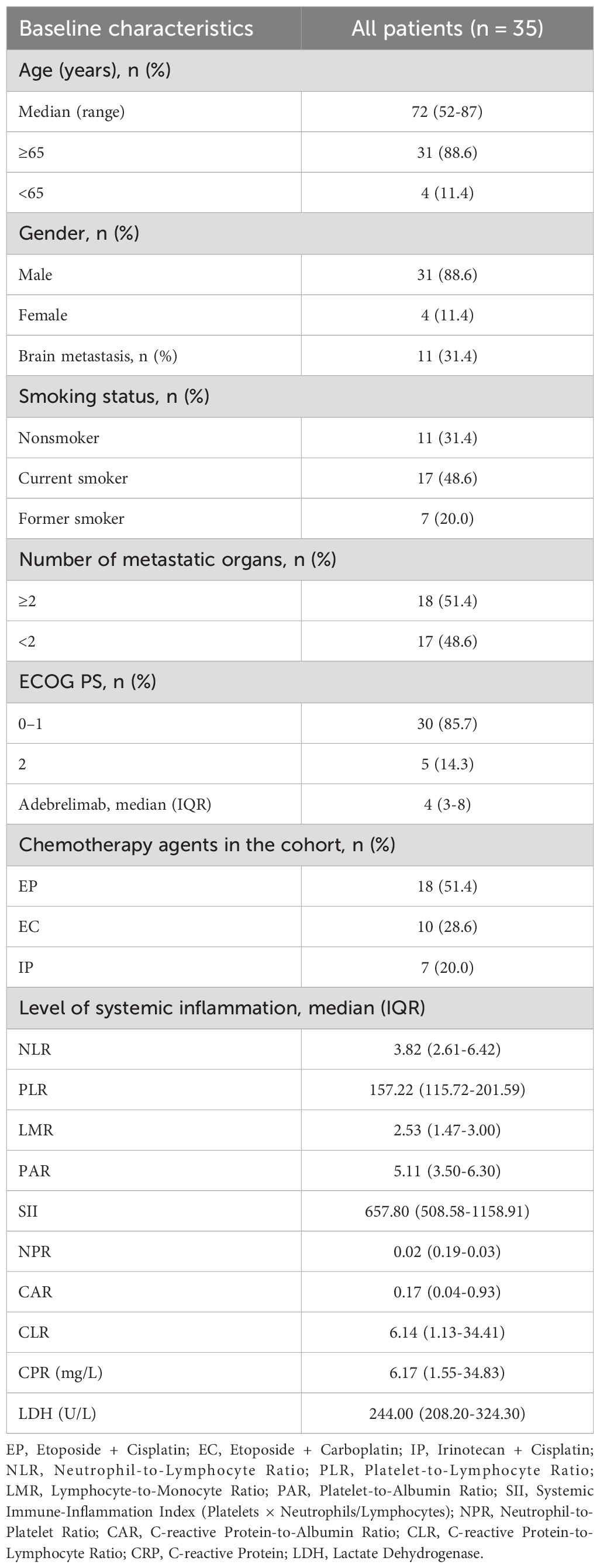
A total of 35 patients were enrolled in this study. Patient characteristics are summarized in Table 1. The median age was 72 years. The majority of patients were male (88.6%), had a history of smoking (68.6%), and had a performance status (PS) score of 0–1 in 85.7% of cases. The distribution of metastatic organs was as follows: fewer than 2 (48.6%) and 2 or more (51.4%), with brain metastases accounting for 31.4%. No patient received prior local therapy for metastatic lesions before beginning first-line treatment. Two patients (5.7%) received thoracic radiotherapy during treatment. In this cohort, 28.6%, 51.4%, and 20% of patients received EC, EP, and IP treatments, respectively. The median number of treatment cycles for adebrelimab was 4. Additionally, the systemic inflammatory markers for patients were as follows: NLR 4.97 ± 0.64, PLR 186.08 ± 20.63, LMR 2.51 ± 0.23, PAR 5.70 ± 0.77, SII 1217.37 ± 425.90, NPR 0.04 ± 0.01, CAR 0.95 ± 0.37, CLR 34.27 ± 11.46, C-reactive Protein (CRP) 30.61 ± 10.30 mg/L, and Lactate Dehydrogenase (LDH) 329.37 ± 46.29 U/L.
3.2 Clinical outcomes
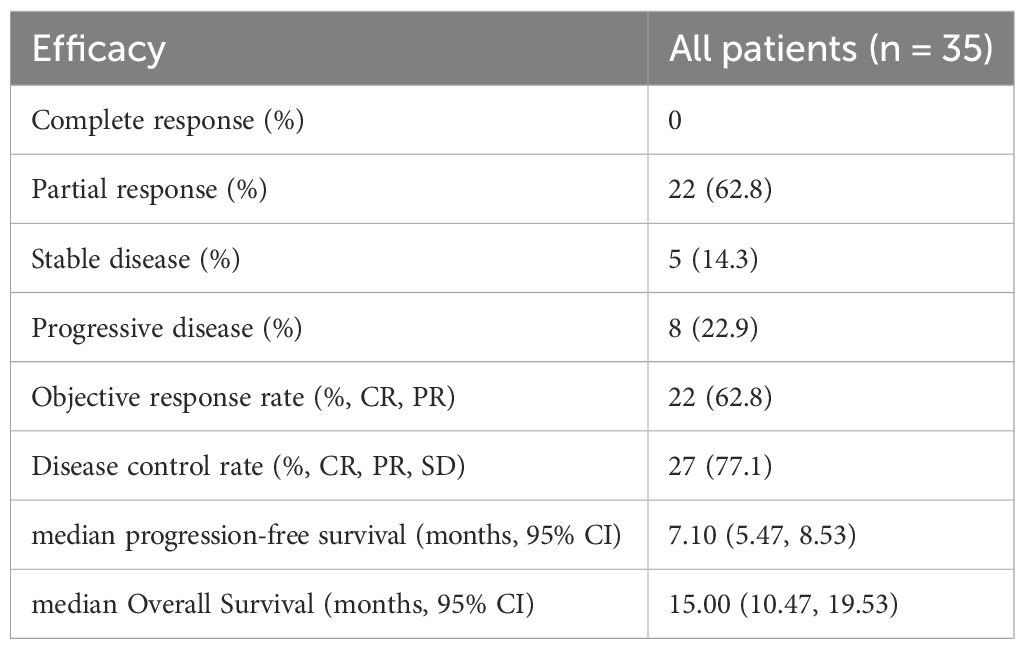
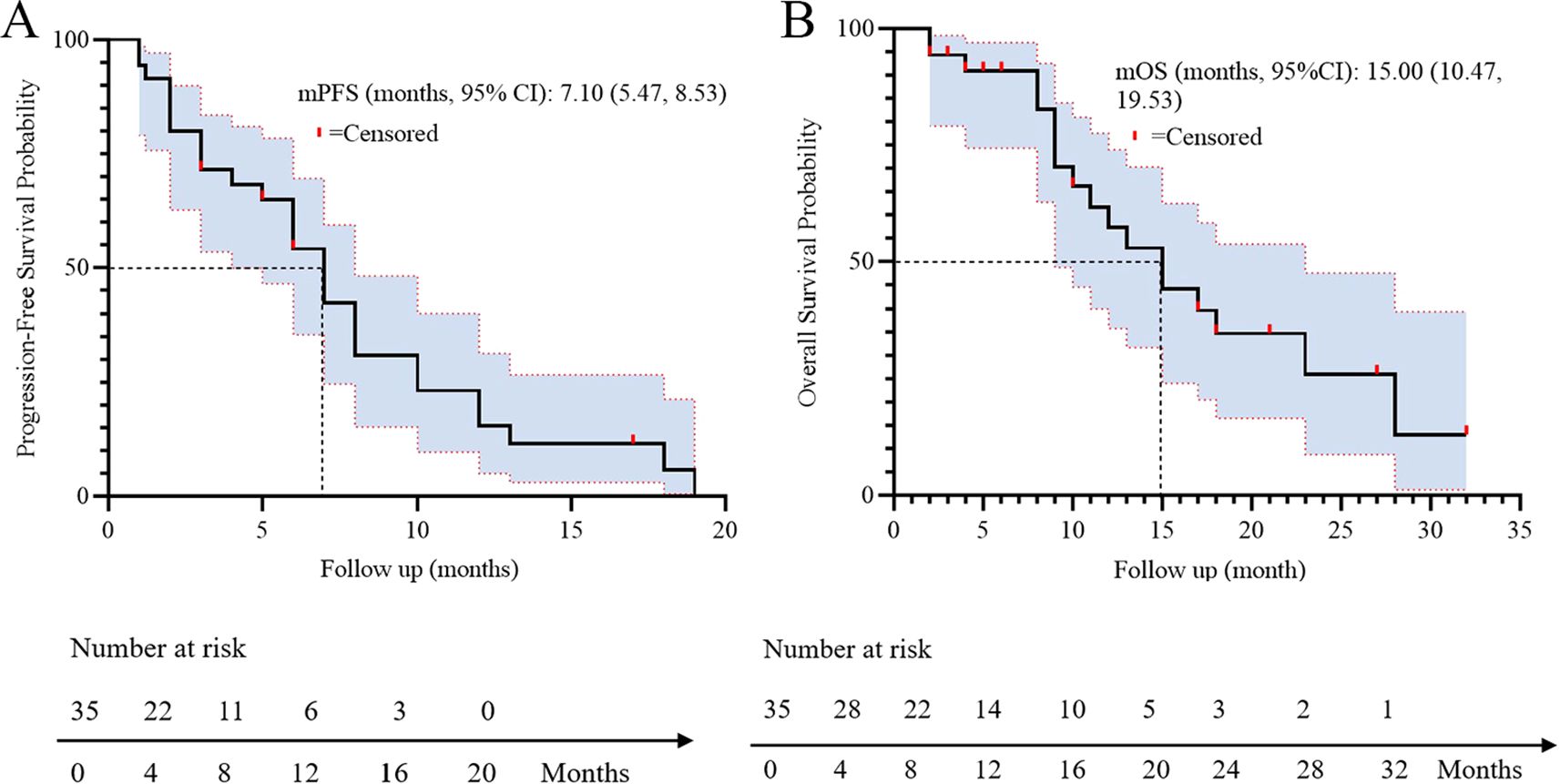
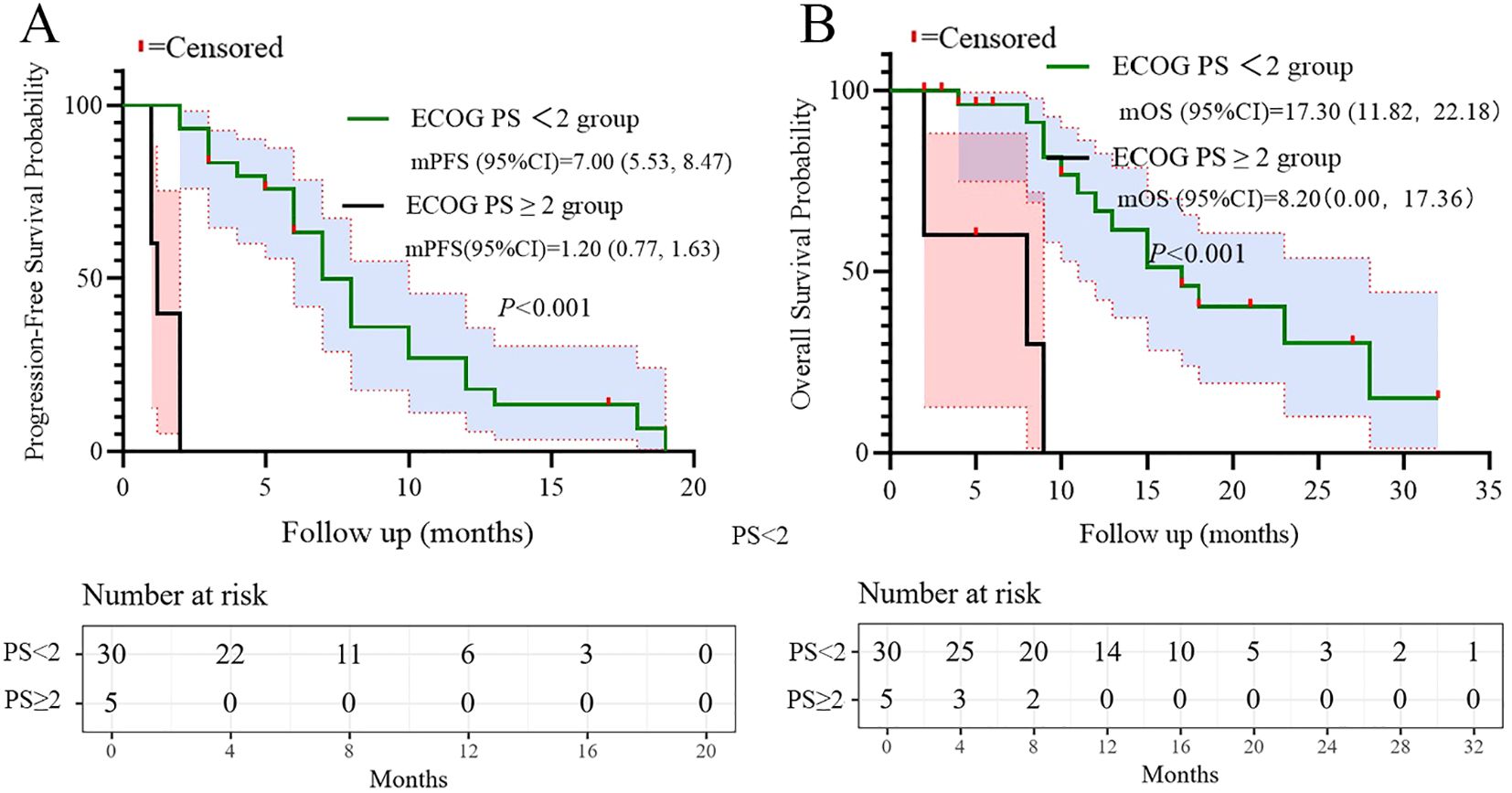
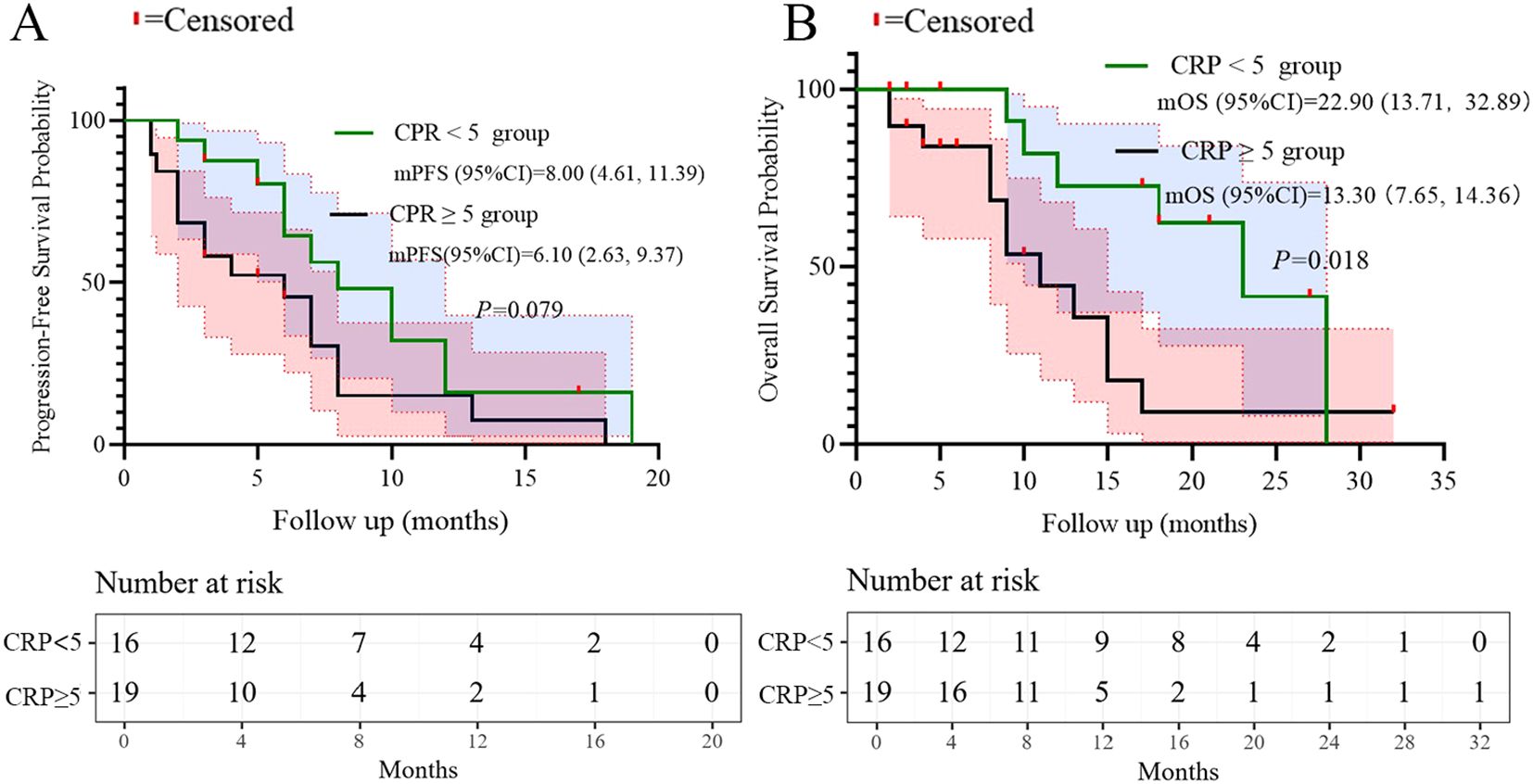
All patients underwent regular imaging reviews during treatment. The median follow-up time was 18.5 months (IQR: 12.3–24.1). As shown in Table 2, 22 of the 35 patients (62.8%) achieved a partial response (PR), 5 (14.3%) achieved stable disease (SD), and 8 (22.9%) experienced progressive disease. The disease control rate (DCR) and overall response rate (ORR) were 77.1% and 62.8%, respectively. The median progression-free survival (PFS) was 7.1 months, with a 95% confidence interval (CI) of 5.47 to 8.53 (Figure 1A). The median overall survival (OS) was 15.0 months, with a 95% confidence interval (CI) of 10.47 to 19.53 (Figure 1B).
3.3 Prognostic factors for PFS and OS
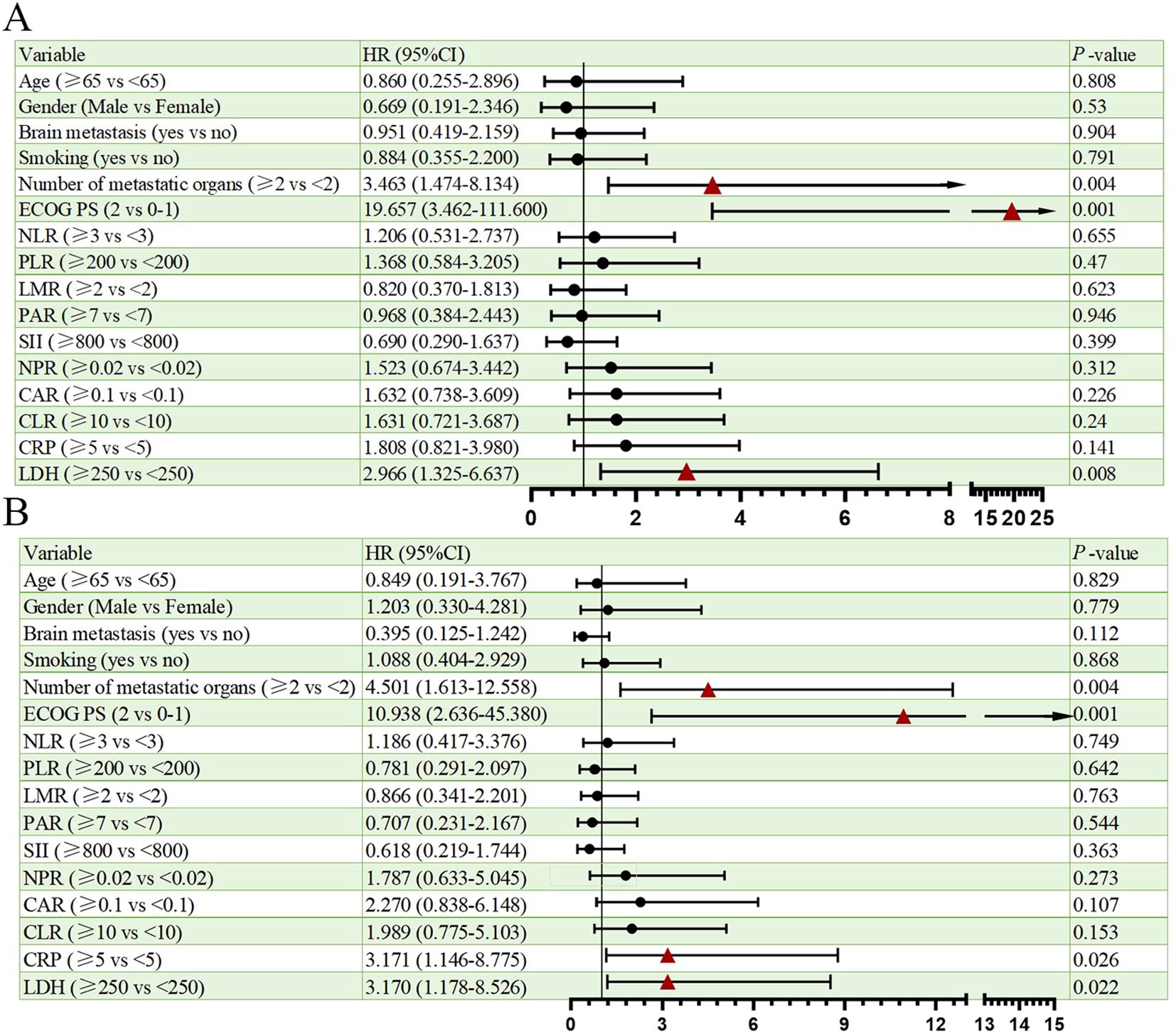
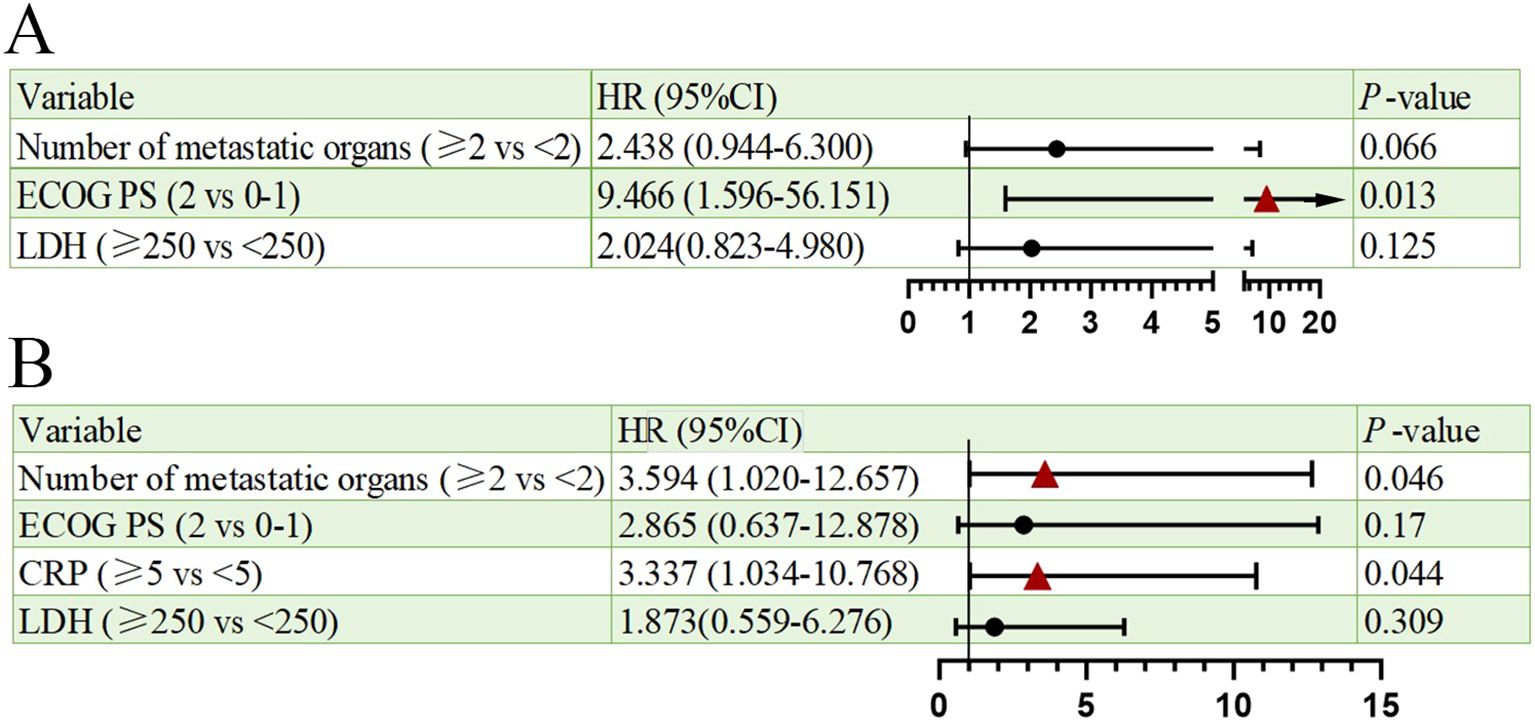
Univariate analysis of PFS and OS showed that the number of metastatic organs (≥2 vs <2; HR = 3.463, 95% CI: 1.474-8.134, p=0.004, Figure 2A), ECOG PS (2 vs 0-1; HR = 19.657, 95% CI: 3.462-111.600, p=0.001, Figure 2A), and LDH (≥250 vs <250; HR = 2.966, 95% CI: 1.325-6.637, p=0.008, Figure 2A) were potential risk factors for PFS. Similarly, the number of metastatic organs (≥2 vs <2; HR = 4.501, 95% CI: 1.613-12.558, p=0.004, Figure 2B), ECOG PS (2 vs 0-1; HR = 10.938, 95% CI: 2.636-45.380, p=0.001, Figure 2B), CRP (≥5 vs <5; HR = 3.171, 95% CI: 1.146-8.775, p=0.026, Figure 2B), and LDH (≥250 vs <250; HR = 3.170, 95% CI: 1.178-8.526, p=0.022, Figure 2B) were potential risk factors for OS. Furthermore, when these risk factors were incorporated into a multivariate analysis, the results demonstrated that a high ECOG PS (HR = 9.446, 95% CI: 1.596-56.151, p=0.013, Figure 3A) significantly influenced patients’ PFS, while CRP levels ≥ 5 (HR = 3.337, 95% CI: 1.034-10.768, p=0.044, Figure 3B) and the presence of ≥ 2 metastatic organs (HR = 3.594, 95% CI: 1.020-12.657, p=0.046, Figure 3B) were associated with a reduction in patients’ OS.
3.4 Subgroup analysis
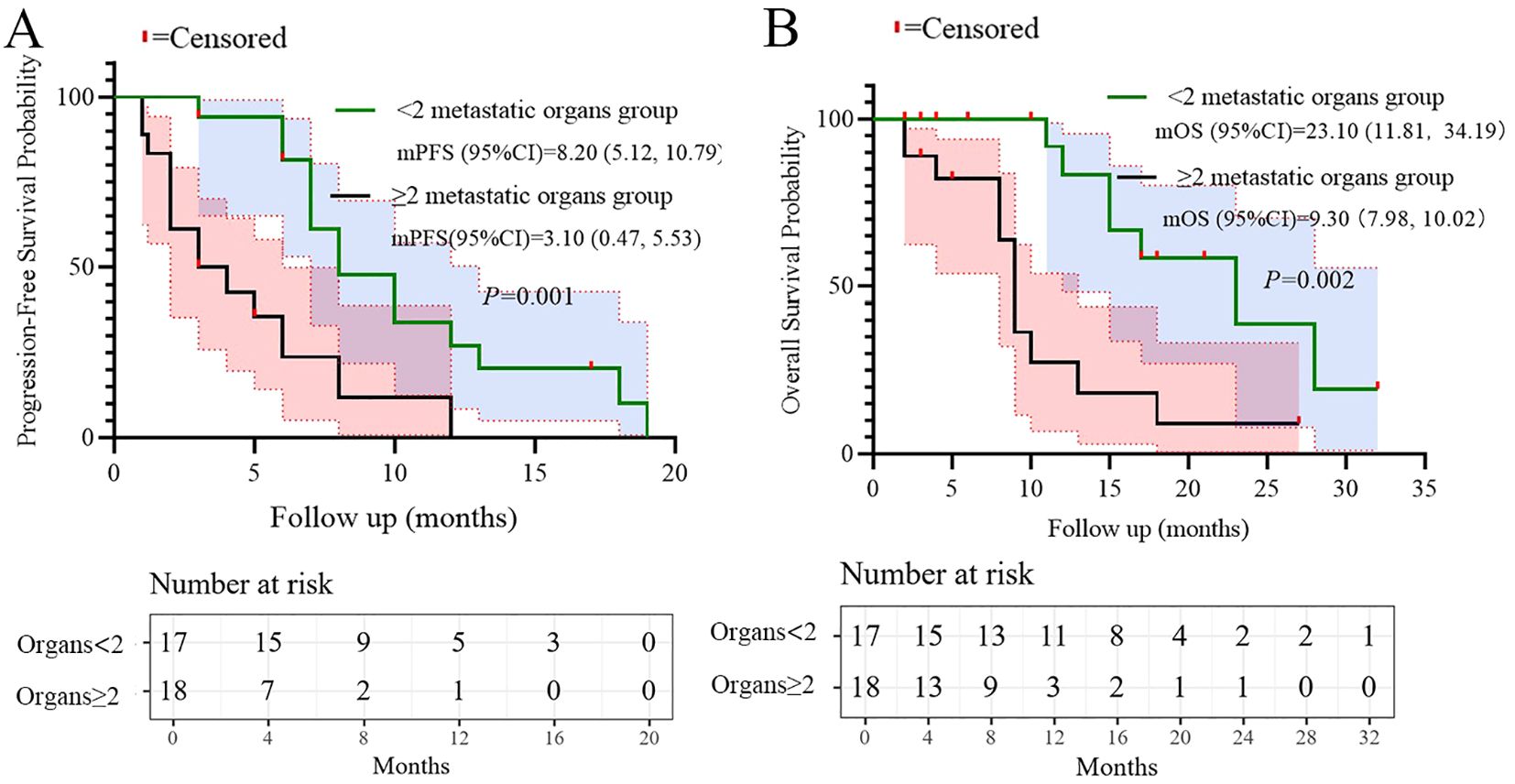
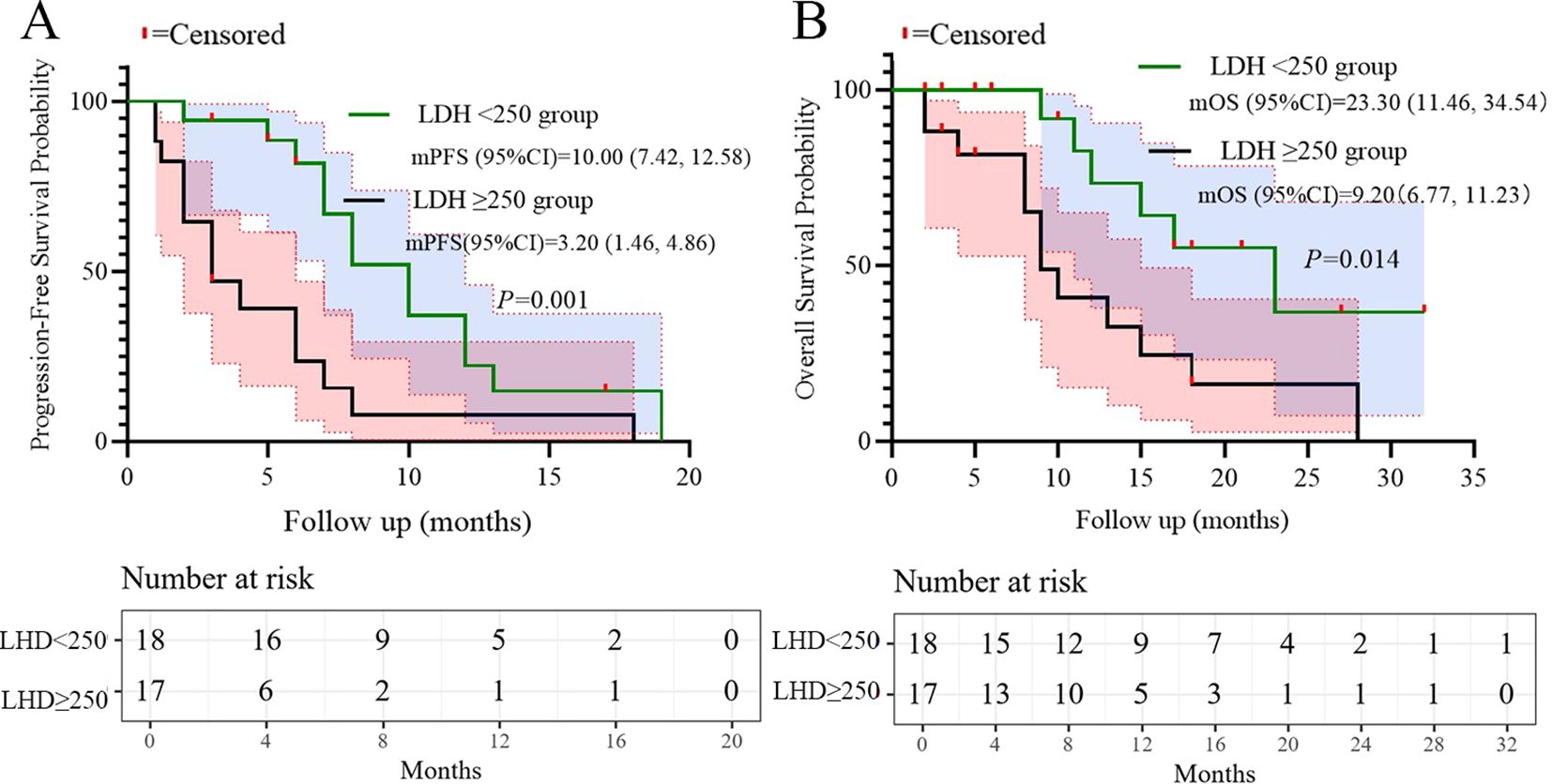
Based on the multivariate results, we performed subgroup analysis of PFS and OS based on CRP, LDH, PS, and the number of metastatic organs. The results showed that high PS (log-rank test p<0.001 for PFS and OS; Figure 4), heavier metastatic burden (log-rank test p=0.001 for PFS and p=0.002 for OS; Figure 5), and high LDH level (log-rank test p=0.001 for PFS and p=0.014 for OS; Figure 6) were independent risk factors for PFS and OS. In addition, CRP (log-rank p=0.018) was an independent risk factor for OS, but there was no statistical difference for PFS (Figure 7).
3.5 Safety
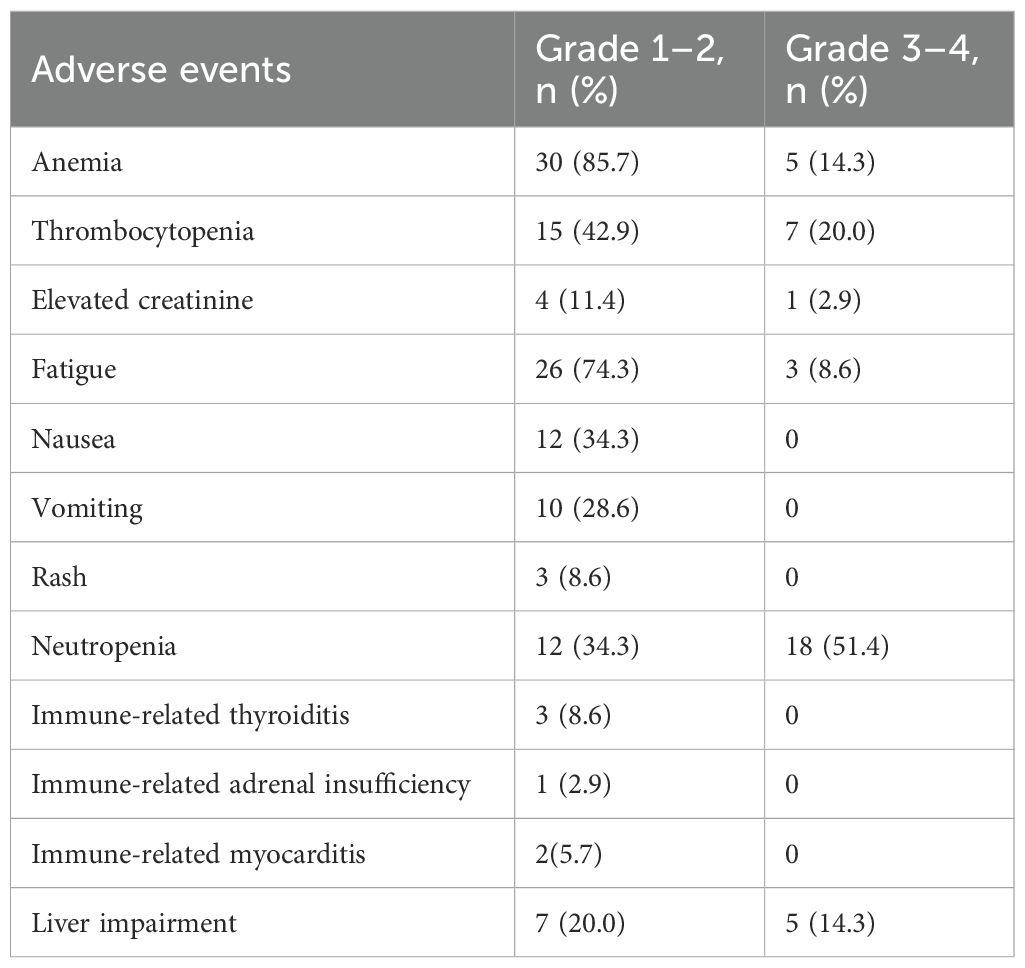
Treatment-related adverse events are summarized in Table 3. The most frequently reported grade 1–2 adverse events were anemia (85.7%), fatigue (74.3%), and thrombocytopenia (42.9%). Other grade 1–2 events included nausea (34.3%), neutropenia (34.3%), vomiting (28.6%), liver impairment (20.0%), elevated creatinine (11.4%), rash (8.6%), immune-related thyroiditis (8.6%), myocarditis (5.7%), and adrenal insufficiency (2.9%).
Grade 3–4 adverse events occurred most commonly as neutropenia (51.4%), followed by thrombocytopenia (20.0%), anemia (14.3%), liver impairment (14.3%), fatigue (8.6%), and elevated creatinine (2.9%). Notably, no grade 3–4 events were observed for nausea, vomiting, rash, or any of the immune-related adverse events. No grade 5 adverse events or treatment-related deaths were reported during the study period.
4 Discussion
This real-world study provides critical insights into the effectiveness, safety, and prognostic determinants of first-line adebrelimab plus chemotherapy in 35 unselected patients with ES-SCLC. Our results demonstrate a median OS of 15.0 months (95% CI: 10.47–19.53) and median PFS of 7.1 months (95% CI: 5.47–8.53), with an ORR of 62.8% and DCR of 77.1%. These outcomes align with the pivotal CAPSTONE-1 trial (OS: 15.3 months; PFS: 5.8 months) despite our cohort’s older median age (72 vs. 62 years) and inclusion of ECOG PS 2 patients (14.3%) (7). The slightly longer median PFS observed in our study compared to CAPSTONE-1 may be influenced by differences in assessment frequency or real-world imaging interpretation practices. The consistency underscores adebrelimab’s promising efficacy in routine practice, particularly relevant given the historically poor prognosis of ES-SCLC (median OS: 8–13 months with chemotherapy alone) (14, 15).
Multivariate analysis identified ECOG PS ≥2 as an independent predictor of inferior PFS (HR = 9.446, p=0.013), while metastatic burden ≥2 organs (HR = 3.594, p=0.046) and CRP ≥5 mg/L (HR = 3.337, p=0.044) independently predicted worse OS. These findings extend prior real-world studies of PD-L1 inhibitors (16, 17). However, our novel observation that baseline CRP elevation independently correlates with survival highlights systemic inflammation’s role in SCLC progression. Elevated CRP may reflect underlying IL-6-driven inflammation, as supported by prior studies (18–20), but our study did not directly assess this mechanism. This aligns with data linking CRP to poor outcomes in ICI-treated NSCLC (21, 22). Future prospective studies incorporating serial cytokine measurements (e.g., IL-6) and tissue-based analyses are warranted to elucidate the mechanistic role of inflammatory pathways in SCLC immunotherapy resistance.
Additionally, LDH ≥250 U/L predicted poorer PFS and OS in univariate analysis (p=0.008 and p=0.022), consistent with its established role as a surrogate for high tumor metabolic activity and aggressive biology in SCLC (23). Subgroup analyses further validated that patients with high metastatic burden, ECOG PS 2, or elevated LDH had significantly shorter survival (log-rank p<0.05, Figures 4–6). These factors may aid risk stratification for personalized therapy intensification.
Grade 3–4 adverse events occurred in 74.3% of patients, predominantly hematologic toxicities: neutropenia (51.4%), thrombocytopenia (20.0%), and anemia (14.3%). This exceeds CAPSTONE-1’s grade 3–4 neutropenia rate (33.2%) (7), likely due to our cohort’s advanced age (88.6% ≥65 years) and higher comorbidity burden typical in real-world settings. The higher incidence of hematologic toxicity observed in our older real-world population may necessitate more aggressive supportive care, such as primary prophylaxis with granulocyte-colony stimulating factor (G-CSF), particularly in patients receiving concurrent chemotherapy and immunotherapy. Non-hematologic irAEs were infrequent (thyroiditis: 8.6%; myocarditis: 5.6%), mirroring CAPSTONE-1’s manageable irAE profile. However, two cases of myocarditis warrant vigilance, as cardiovascular irAEs carry high mortality in SCLC patients with smoking-related comorbidities. Proactive monitoring via troponin/ECG is recommended, especially with rising cardiotoxicity reports from PD-L1 inhibitors.
The key limitations in the present study include: single-center retrospective design with limited sample size (n=35), reducing statistical power for subgroup analyses. exclusion of ECOG PS >2 patients, omitting those with poorest prognosis. lack of PD-L1/TMB data, preventing biomarker correlation with outcomes.
The primary limitation of this study is its small sample size (n=35), which restricts the statistical power for robust multivariate modeling and increases the risk of overfitting. Therefore, the results of the multivariate analysis, including the identified prognostic factors, should be interpreted as exploratory and require validation in larger cohorts.
In addition, heterogeneous chemotherapy backbones (EP/EC/IP), may introduce confounding effects, although the sample size precluded regimen-specific subgroup analysis. Future multi-center studies with larger cohorts should validate prognostic biomarkers (e.g., CRP, LDH) and explore molecular predictors (e.g., DLL3 expression, SLFN11 positivity).
5 Conclusions
This study suggests potential real-world effectiveness of first-line adebrelimab in ES-SCLC, achieving survival benchmarks set by RCTs despite older, comorbid patients. ECOG PS ≥2, metastatic burden ≥2 organs, and elevated CRP/LDH identify high-risk subgroups needing tailored approaches. The safety profile remains manageable, though hematologic toxicity and rare cardiotoxicity necessitate vigilant monitoring. These data support adebrelimab’s role in real-world ES-SCLC management while highlighting the critical need for biomarker-driven strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethical Committee of People′s Hospital of Quzhou. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KX: Data curation, Writing – original draft. JW: Methodology, Software, Writing – original draft. ZW: Writing – original draft, Software. JHW: Writing – original draft, Investigation, Visualization. JC: Writing – original draft, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by Instructional Project of Quzhou (2020057), Instructional Project of Quzhou (2021005), Science and Technology Key Project of Quzhou (2022K48), ‘New 115’ Talent Project of Quzhou, ‘551’ Health High-level Talents of Zhejiang Province, and ‘258’ Talent Project of Quzhou.
Acknowledgments
The authors thank the patients for their participation and agreement to publication of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1678020/full#supplementary-material
References
1. Byers LA and Rudin CM. Small cell lung cancer: where do we go from here? Cancer. (2015) 121:664–72. doi: 10.1002/cncr.29098
2. Gazdar AF, Bunn PA, and Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. (2017) 17:725–37.
3. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859
4. Kalemkerian GP and Schneider BJ. Advances in small cell lung cancer. Hematol Oncol Clin North Am. (2017) 31:143–56. doi: 10.1016/j.hoc.2016.08.005
5. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
6. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
7. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:739–47. doi: 10.1016/S1470-2045(22)00224-8
8. Unger JM, Vaidya R, Hershman DL, Minasian LM, and Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. (2019) 111:245–55. doi: 10.1093/jnci/djy221
9. Murthy VH, Krumholz HM, and Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. (2004) 291:2720–6. doi: 10.1001/jama.291.22.2720
10. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med. (2016) 375:2293–7.
11. Berger ML, Sox H, Willke RJ, Brixner DL, Eichler HG, Goettsch W, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. (2017) 26:1033–9.
12. Corrao G and Cantarutti A. Building reliable evidence from real-world data: Needs, methods, cautiousness and recommendations. Pulm Pharmacol Ther. (2018) 53:61–7. doi: 10.1016/j.pupt.2018.09.009
13. Booth CM, Karim S, and Mackillop WJ. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. (2019) 16:312–25. doi: 10.1038/s41571-019-0167-7
14. Farago AF and Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. (2018) 7:69–79. doi: 10.21037/tlcr.2018.01.16
15. Valette CA, Filleron T, Debieuvre D, Lena H, Perol M, Chouaid C, et al. Treatment patterns and clinical outcomes of extensive stage small cell lung cancer (SCLC) in the real-world evidence ESME cohort before the era of immunotherapy. Respir Med Res. (2023) 84:101012. doi: 10.1016/j.resmer.2023.101012
16. Vince M, Naqvi SMH, Pellini B, Verbosky M, and Melzer D. Real-world comparison of the efficacy and safety of atezolizumab versus durvalumab in extensive-stage small cell lung cancer. Lung Cancer. (2024) 198:107999. doi: 10.1016/j.lungcan.2024.107999
17. Misawa K, Watanabe K, Seike M, and Hosomi Y. Durvalumab plus platinum-etoposide chemotherapy for extensive-stage small cell lung cancer: a retrospective real-world study. Transl Lung Cancer Res. (2024) 13:1585–94. doi: 10.21037/tlcr-24-128
18. Ma H, Zhang S, Jiao P, Ding H, Wang F, Zhao Y, et al. Serum IL-6 predicts immunotherapy-related adverse and outcome in advanced gastric and esophageal cancer patients with Anti-PD-1 treatment. Front Immunol. (2025) 16:1553882. doi: 10.3389/fimmu.2025.1553882
19. Akdogan O, Turkmen S, Uyar GC, Yucel KB, Tufekci B, Gurler F, et al. Impact of serum GDF-15 and IL-6 on immunotherapy response in cancer: A prospective study. Cancers. (Basel). (2024) 16. doi: 10.3390/cancers16244146
20. Zhang L, Guo X, Sun X, Liao J, Liu Q, Ye Y, et al. Analysis of tumor-infiltrating exhausted T cells highlights IL-6 and PD1 blockade as a combined immunotherapy strategy for non-small cell lung cancer. Front Immunol. (2025) 16:1486329. doi: 10.3389/fimmu.2025.1486329
21. Matsuzawa R, Morise M, Kinoshita F, Tanaka I, Koyama J, Kimura T, et al. Non-invasive early prediction of immune checkpoint inhibitor efficacy in non-small-cell lung cancer patients using on-treatment serum CRP and NLR. J Cancer Res Clin Oncol. (2023) 149:3885–93. doi: 10.1007/s00432-022-04300-x
22. Riedl JM, Barth DA, Brueckl WM, Zeitler G, Foris V, Mollnar S, et al. C-reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: A bi-center study. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12082319
Keywords: adebrelimab, extensive-stage small cell lung cancer, real-world study, first-line therapy, prognostic factors
Citation: Xu K, Wang J, Weng Z, Wang J and Chen J (2025) Real-world study of adebrelimab as first-line therapy for extensive-stage small cell lung cancer: a retrospective study. Front. Immunol. 16:1678020. doi: 10.3389/fimmu.2025.1678020
Received: 01 August 2025; Accepted: 29 September 2025;
Published: 13 October 2025.
Edited by:
Thangavel Chellappagounder, Thomas Jefferson University, United StatesReviewed by:
Xin Tong, Ragon Institute, United StatesDaisuke Morinaga, Hokkaido University Hospital, Japan
Copyright © 2025 Xu, Wang, Weng, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhui Wang, d2FuZ2p1bmh1aTc1MjZAMTYzLmNvbQ==; Jianxin Chen, Y2p4ODEzN0AxNjMuY29t
†These authors have contributed equally to this work
 Kaili Xu1†
Kaili Xu1† Jian Wang
Jian Wang Junhui Wang
Junhui Wang Jianxin Chen
Jianxin Chen