Abstract
Background:
Inflammatory Bowel Diseases (IBDs) are characterized by intestinal dysbiosis and immune dysregulation. Annexin A1 (AnxA1) promotes epithelial repair and inhibits immune responses during IBD. However, AnxA1’s impact on gut microbiota during IBD remains unclear. Here, we experimentally investigated the microbiota profile during colitis in wild-type (WT) and AnxA1-deficient mice (AnxA1-/-), and evaluated an observational cohort in IBD patients with high or low AnxA1 expression.
Methods:
Colitis was induced in C57BL/6 WT and AnxA1-/- mice via oral administration of 2% DSS for six days. Fecal samples were collected at baseline, peak inflammation (day 6), and during the recovery phase (day 10) for 16S rRNA sequencing. Human microbiota data from the Lifelines Dutch Microbiome Project cohort, including IBD and healthy subjects, were analyzed for AnxA1 expression using R software.
Results:
Healthy AnxA1-/- mice exhibited reduced microbial richness and a distinct gut microbiota composition, marked by increased Proteobacteria and Parasutterella, and reduced Deferribacterota, Campylobacterota, and Verrucomicrobiota. During DSS-induced colitis, AnxA1-/- mice showed greater weight loss and heightened inflammation, displaying earlier and more pronounced microbial shifts, including increased Proteobacteria, Cyanobacteria, Parabacteroides, Bacteroides, and Escherichia-Shigella. In contrast, WT mice exhibited delayed changes, with expansion of Alloprevotella, Akkermansia, and Faecalibaculum after day 6. In human IBD samples, Crohn’s disease (CD) patients with low AnxA1 expression and active inflammation presented an altered microbiota enriched in Lachnoclostridium and Parabacteroides, while ulcerative colitis (UC) patients showed phylum-level shifts modulated by AnxA1 levels. Notably, non-inflamed CD and UC patients with low AnxA1 differed significantly in microbiota composition. Moreover, inflamed CD patients with high AnxA1 expression showed microbial profiles resembling those of healthy controls, while low AnxA1 expression was associated with a more pronounced dysbiotic state.
Conclusion:
AnxA1 is implicated in microbiota control under healthy and IBD conditions. Accordingly, the microbiota of healthy AnxA1-/- mice, colitic AnxA1-/- mice, and IBD patients with low AnxA1 expression exhibit dysbiosis compared to their respective controls. Together, these unprecedented findings reveal AnxA1 as a potential regulatory protein in the immune–microbiota axis involved in IBD pathogenesis.
1 Introduction
Inflammatory Bowel Diseases (IBDs), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), are complex disorders influenced by genetic, environmental, and microbial factors. Clinically, IBD symptoms often include diarrhea, abdominal pain, rectal bleeding, and weight loss, with an increased risk of colorectal cancer (1–6). Diagnosis and monitoring rely on invasive procedures such as endoscopy and biopsy, underscoring the need for non-invasive biomarkers to enhance clinical management (7, 8). Treatments range from lifestyle changes and conventional anti-inflammatory drugs to advanced TNF-α, IL-12/IL-23, JAK and integrin inhibitors, as well as surgical procedures (9). However, many patients still experience non-responsiveness or relapse, reinforcing the need for continued research into the multifaceted mechanisms underlying IBDs (10, 11).
Alterations in mucosal integrity and gut microbial composition play a critical role in the progression of IBDs. Patients with IBD exhibit a state of dysbiosis, primarily characterized by reduced microbial diversity and depletion of beneficial phyla such as Firmicutes and Bacteroidetes, along with an expansion of potentially pathogenic phyla like Proteobacteria and Actinobacteriota (12, 13). Notably, commensal butyrate-producing bacteria that support mucosal homeostasis and exert anti-inflammatory effects, such as Faecalibacterium prausnitzii and Roseburia spp. are significantly diminished. In contrast, the abundance of pathobionts, including Escherichia coli (adherent-invasive strains), was markedly increased (14–17). This microbial imbalance contributes to a reduction in the production of short-chain fatty acids (SCFAs), particularly butyrate (18), which are essential for maintaining microbial diversity, epithelial integrity, and host–microbiota metabolic balance (19).
Annexin A1 (AnxA1) is a 37 kDa endogenous anti inflammatory protein expressed by neutrophils, monocytes, macrophages, epithelial and cancer cells (20–22). AnxA1 synthesis and release are induced by glucocorticoids and inflammatory mediators. It is found in the cytosol, associated with the plasma membrane or endosomal vesicles, and released via exocytosis or through microvesicles and exosomes (23, 24). Under stress or disease conditions, intracellular AnxA1 can translocate to the nucleus, potentially influencing gene regulation, and also interact with the cytoskeleton, modulating actin and microtubule reorganization (25–28). Secreted AnxA1 undergoes calcium-dependent conformational changes and phosphorylation, exposing its N-terminal domain responsible for its biological activities. Once phosphorylated, AnxA1 binds to membrane phospholipids and to formyl peptide receptors (FPRs), members of the G protein-coupled receptors, and controls inflammation and wound healing (24, 29, 30). Its functions include inhibition of phospholipase A2, neutrophil surveillance, inhibition of leukocyte trafficking into tissues, secretion of chemical mediators, induction of macrophage polarization and efferocytosis and epithelial cell proliferation, adhesion and migration during tissue repair (21, 31, 32). The AnxA1/FPRs’ roles in the development and resolution of inflammation and tissue repair have been extensively studied in IBD experimental and clinical research. In experimental models, AnxA1-deficient mice exhibit exacerbated and non-resolving colitis. In human studies, AnxA1 is highly expressed in inflamed intestinal tissue of IBD patients (21, 33–35).
Understanding AnxA1’s multifunctionality has driven therapeutic strategies, with recent studies indicating that targeting the AnxA1/FPRs pathways may reduce inflammatory disease severity and aid tissue regeneration. Indeed, genetic deficiency of AnxA1 impaired the therapeutic effects of the monoclonal antibody anti-TNF-α or pioglitazone in mice. In contrast, clinical observations in Crohn’s disease patients revealed that those treated with anti-TNF-α who experienced relapse showed lower expression of AnxA1 and FPRs in the inflamed epithelium (33, 36, 37). Emerging nanoparticle-based therapies using AnxA1-mimetic peptides are also being explored to deliver drugs into the inflamed zone, stabilize the intestinal barrier, and promote tissue repair, offering new perspectives in personalized IBD treatment (38–41).
Although the role of AnxA1 in IBD is well recognized, no studies to date have characterized the microbiota profile in the absence of AnxA1, nor the microbial dynamics during IBD development under AnxA1-deficient conditions. To address this, we analyzed the microbiota of wild-type (WT) or AnxA1-/- mice in a DSS-induced colitis model and evaluated a cohort of human IBD patients to identify key differences in microbiota profiles associated with the absence or impaired function of AnxA1.
2 Materials and methods
2.1 Animals
Male C57BL/6NCrl wild-type (WT) and Annexin A1-deficient (AnxA1-/-) mice aged 7–8 weeks and weighing 20–25 g were obtained from the Animal Facility of the School of Medicine at UNIFESP. Mice had conventional sanitary standards and were kept in barrier conditions on a 12-hour light/dark cycle at temperatures between 20 and 25°C with free access to water and food. All procedures were performed following the Ethical Principles for Animal Experimentation by the National Council for Animal Experimentation Control (CONCEA). The protocol of the study was approved by the Ethics Committee of Animal Use of the Faculty of Pharmaceutical Sciences of the University of São Paulo (CEUA/FCF/USP, protocol n° 617) and the Internal Biosafety Commission (CIBio).
2.2 Experimental colitis induced by dextran sulfate sodium
To induce experimental colitis, 2% dextran sulfate sodium (DSS; MW 40,000, Dextran Products Limited, Ontario, Canada) was administered in the drinking water for six consecutive days (days 0–6), while control animals received only filtered water. Wild-type (WT) and AnxA1-/- mice were divided into two groups: DSS-treated and control, and the experimental protocol lasted 10 days in total (Supplementary Figure 1A).
2.3 Assessment of colitis clinical score and FACS
Colitis development was monitored daily by body weight measures, in which the progression was expressed as the difference between initial and final weight, as percentage. On day 10 of the experiment, animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) via intraperitoneal injection. A surgical incision was made in the mice’s abdominal region and the colon was removed from the ileocecal junction to the anus, washed with an isotonic solution and the organ was fragmented for FACS analysis. Intestinal tissue was subjected to enzymatic digestion with collagenase, filtered through a 40 μm strainer, and washed with PBS. After centrifugation, cells were resuspended in FACS buffer (PBS, 5% FBS, 0.02% NaN2) and incubated on ice for 15 min to block nonspecific binding (42). Cells were stained with anti-Ly6G (1:50, BD Biosciences), anti-F4/80 (1:100, eBioscience), anti-CD3e (1:50, eBioscience) and anti-CD4 (1:100, Biolegend) antibodies. Analyses were performed on a BD Accuri C6 Plus cytometer and analyzed using BD Accuri C6 software.
2.4 Experimental statistical analyses
All results were subjected to normality tests, including D’Agostino-Pearson and Kolmogorov-Smirnov. Clinical analyses were evaluated using Two-way ANOVA, followed by Tukey’s post-test, with results expressed as mean ± standard deviation. Tissue analyses were performed using One-way ANOVA with Tukey’s post-test and expressed as mean ± standard deviation. Values of p<0.05 were considered significant. Statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA).
2.5 DNA sequencing of fecal mice samples
Feces were harvested on days 0, 6 and 10 of the experiment for 16S sequencing of the intestinal microbiota. DNA was extracted from the feces using the DNeasy Power Soil Kit (Qiagen) and Power Soil Pro Kit (Qiagen). The V4 region of the 16S ribosomal gene was amplified using the AccuPrime™ Taq DNA Polymerase System (Invitrogen), using the primers 515F–806R, as described by Caporaso et al. (43) and protocolized in the Earth Microbiome Project. Genetic material was purified using beads via the Beckman Coulter Agencourt AMPure XP Kit (Beckman Coulter), and quality and size were checked by gel electrophoresis. DNA quantification was performed using the Quant-iT PicoGreen dsDNA Kit (Invitrogen). Sequencing was performed on the Illumina MiSeq® System (Illumina INC, San Diego, CA, USA) at the Research Support Facilities Center (CEFAP) - ICB-USP, following the manufacturer’s protocol, using the Illumina V2 500-cycle kit (Illumina).
2.6 Experimental bioinformatics analyses
Bioinformatics analyses were performed using the QIIME2 software version 2022.2. Sequences were imported into the program and demultiplexed to associate indexes with their respective samples. A total of 5,011,692 sequences were obtained, with an average of 27,998 sequences per sample. The sequences were processed through the Dada2 pipeline to filter low-quality sequences, singletons, chimeric sequences, and to dereplicate sequences to reduce repetition. The first 10 bases of the sequence were trimmed, and sequences were truncated at base 248 based on data quality scores. Results were tables of Amplicon Sequence Variants (ASVs), containing information about the frequency and sequence of ASVs in the samples. After quality control with Dada2, 3,888,180 sequences were obtained across all samples, resulting in 1,574 ASVs. Diversity and taxonomy analyses were then performed, using a sampling depth of 10,367. The ASVs were aligned using the mafft pipeline and used to build phylogeny with fast tree. Alpha diversity was assessed using Faith and Evenness metrics with statistical analysis by Kruskal-Wallis, while beta diversity was analyzed using UniFrac weighted and unweighted, with PERMANOVA and principal coordinate analysis (PCoA). Taxonomy analysis was performed using the q2‐feature‐classifier pipeline with Silva database (silva-138-99-nb-weighted-classifier). All analyses were performed using rarefied tables subsampled to 10,367 sequences per sample.
2.7 Patient cohort and ethics committee
The data used for bioinformatics analyses in humans originates from the Lifelines Dutch Microbiome Project (DMP) cohort, a multidisciplinary prospective study designed to monitor health and health-related behaviors in 167,729 individuals living in the northern Netherlands. For this study, data from a total of 882 individuals were analyzed, including 458 individuals with Crohn’s disease, 363 with ulcerative colitis, and 61 controls. The IBD patients were further subdivided based on the presence or absence of biopsy tissue inflammation and the expression level of the AnxA1 protein (high vs. normal-low). The Lifelines study was approved by the ethics committee of the University of Groningen under the protocol number METc: 2017/152. The data can be accessed and/or requested through the following website: https://ega-archive.org/studies/EGAS00001005027.
2.8 Human cohort bioinformatics analyses
The bioinformatics analyses for the translational component were performed using the R software, version 4.3.1. Pre-normalized text files containing sample counts were provided for use in the study. For alpha diversity analysis, a minimum filter of 50 counts per genus was applied. The Vegan package was used to calculate Shannon, Richness, Evenness, and Chao1 metrics. Normality was tested using the Shapiro-Wilk test, and statistical differences were analyzed using the Kruskal-Wallis test followed by Dunn’s post hoc test. For beta diversity analysis, a filter of 50 counts per genus was applied, and the relative abundance of each genus within each sample was calculated. Using the Vegan package, Bray-Curtis distance was computed, and PERMANOVA was used for statistical analyses, with pairwise comparisons conducted using the Pairwise package. For the taxonomic analysis, the data were transformed into relative abundance and visualized using the ggplot2 package.
3 Results
3.1 The deficiency of AnxA1 influences the gut microbiota of healthy mice
Healthy AnxA1-/- mice exhibited a distinct gut microbiota profile compared to the WT mice. Beta diversity analysis at day 0 revealed a significant difference between groups (Figure 1A). Regarding alpha diversity, AnxA1-/- mice showed reduced ASV richness, while evenness did not differ between groups (Figure 1B).
Figure 1
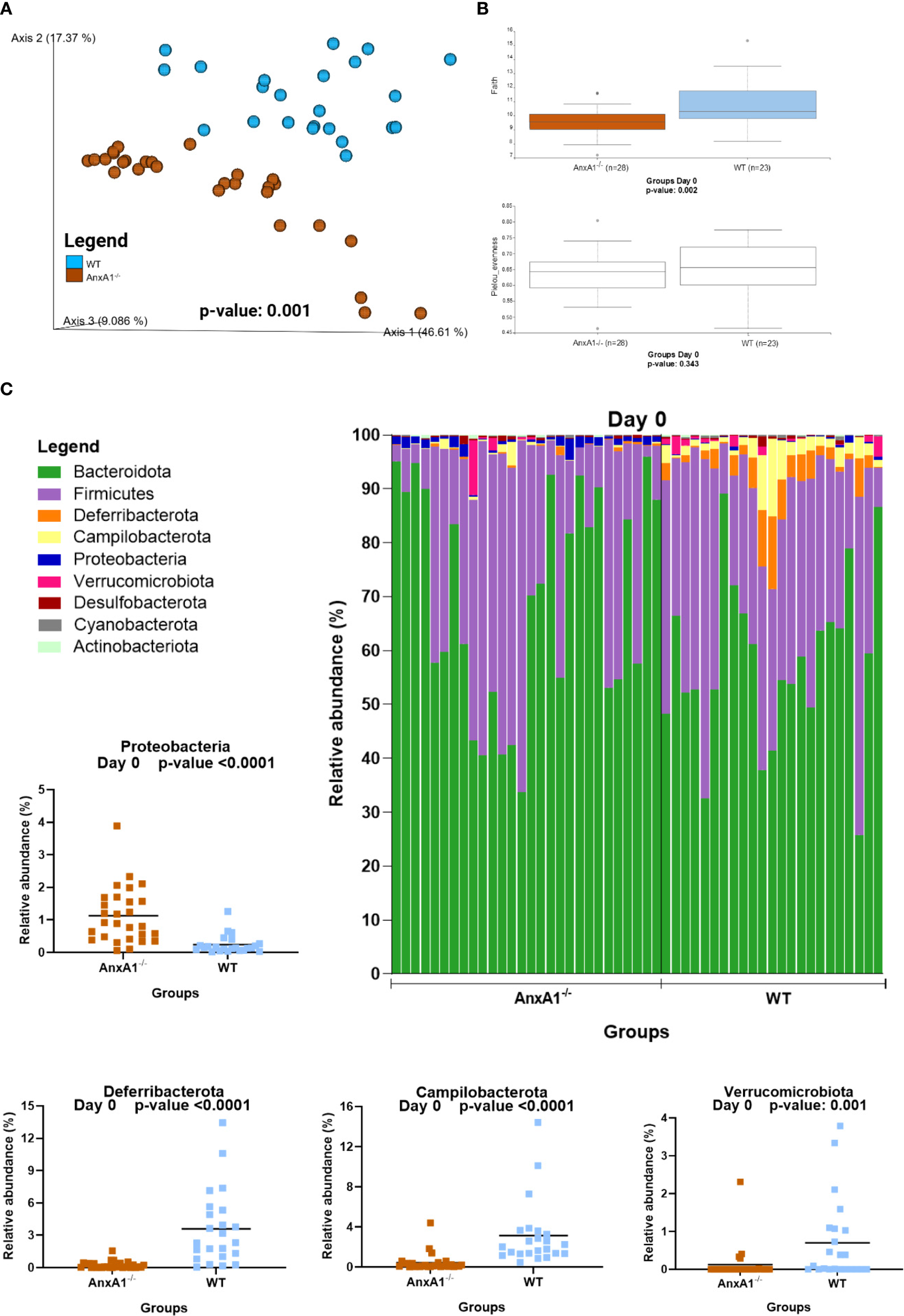
Microbiota shifts in the absence of AnxA1 in basal conditions (Day 0). (A) UniFrac weighted metric for beta diversity comparing WT and AnxA1-/- mice. P-value: 0.001 (B) Alpha diversity comparing WT and AnxA1-/- mice performing Richness (upper graph; p-value: 0.002) and Evenness (bottom graph; p-value: 0.343) metrics. (C) Relative abundance of phyla between WT and AnxA1-/- mice. ASVs tested with Mann-Whitney. Proteobacteria, Deferribacterota and Campilobacterota p-values <0.001, while Verrucomicrobiota: 0.001. n= WT: 23 and AnxA1-/-: 28. For all analyses, p-values <0.05 were considered statistically significant.
In AnxA1-/- mice, a higher prevalence of the phylum Proteobacteria was observed, while Deferribacterota, Campylobacterota, and Verrucomicrobiota were reduced compared to the WT group (Figure 1C). Within these phyla, AnxA1-/- mice exhibited higher abundance of Parasutterella (a member of Proteobacteria), along with decreased abundance in Helicobacter (a member of Campylobacterota) and Mucispirillum (a member of Deferribacterota) compared to WT mice (Figure 2B).
Figure 2
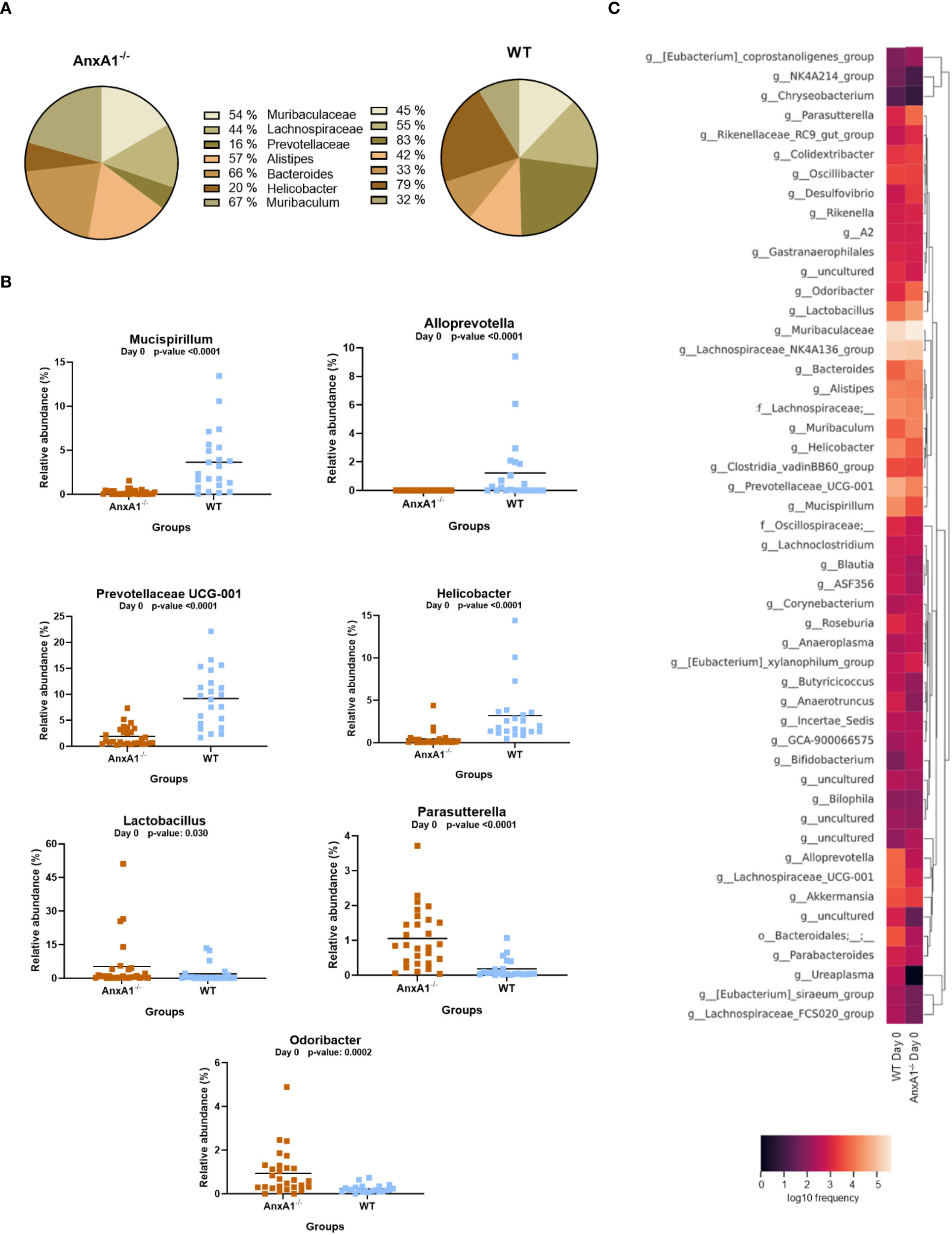
Genera shifts in the absence of AnxA1 in basal conditions (Day 0). (A) Percentage graph of the main genera commonly found in WT and AnxA1-/- mice. (B) Relative abundance of genera between WT and AnxA1-/- mice. ASVs tested with Mann-Whitney. Mucispirillum, Alloprevotella, Prevotellaceae UCG-001, Helicobacter and Parasutterella p-values<0.001. Lactobacillus p-value: 0.030 and Odoribacter: 0.0002. (C) Heatmap with the main shifts in genus level between WT and AnxA1-/- mice. n= WT: 23 and AnxA1-/-: 28. For all analyses, p-values <0.05 were considered statistically significant.
The main genera found in both mouse strains are presented in Figure 2A. In the absence of AnxA1, significant differences were also observed in less abundant genera. For instance, Odoribacter and Lactobacillus were more abundant in AnxA1-/- mice, whereas Prevotellaceae UCG-001 was less abundant compared to WT. Notably, Alloprevotella was detected exclusively in WT mice (Figure 2B). The primary differences in genus level in healthy animals are visualized in the heatmap in Figure 2C.
FACS analysis of the colon revealed that in healthy conditions, WT and AnxA1-/- mice showed similar percentages of colonic Ly6G+ neutrophils, F4/80+ macrophages, and CD3+ T lymphocytes (Supplementary Figure 1B).
3.2 Microbiota exhibits pronounced inflammatory profile in AnxA1-/- mice during colitis development
Between days 6 and 10 of DSS-induced colitis, all treated groups showed significant weight loss compared to controls, with AnxA1-/- DSS mice exhibiting greater loss on days 6 to 8 (Supplementary Figure 1C), suggesting a more severe inflammatory response in the absence of AnxA1, consistent with previous studies (33, 36, 37). During colitis, AnxA1-/- mice exhibited a marked increase in Ly6G+ neutrophils and reduced influx of F4/80+ macrophages compared to WT. The percentage of CD3+ T cells declined in AnxA1-/- mice during inflammation, while remaining stable in WT. Interestingly, CD4+ T cells were more abundant in healthy AnxA1-/- mice, but this population significantly decreased during colitis, whereas it remained unchanged in WT animals (Supplementary Figure 1B).
Day 6 of the model corresponds to the peak of clinical manifestations, while day 10 marks the recovery phase of the disease. As observed under healthy conditions, the bacteria composing the gut microbiota during experimental colitis maintain an individual pattern in the presence or absence of AnxA1 (Figure 3A). No differences in richness or evenness of the ASVs were detected between the groups through the colitis (Figure 3B).
Figure 3
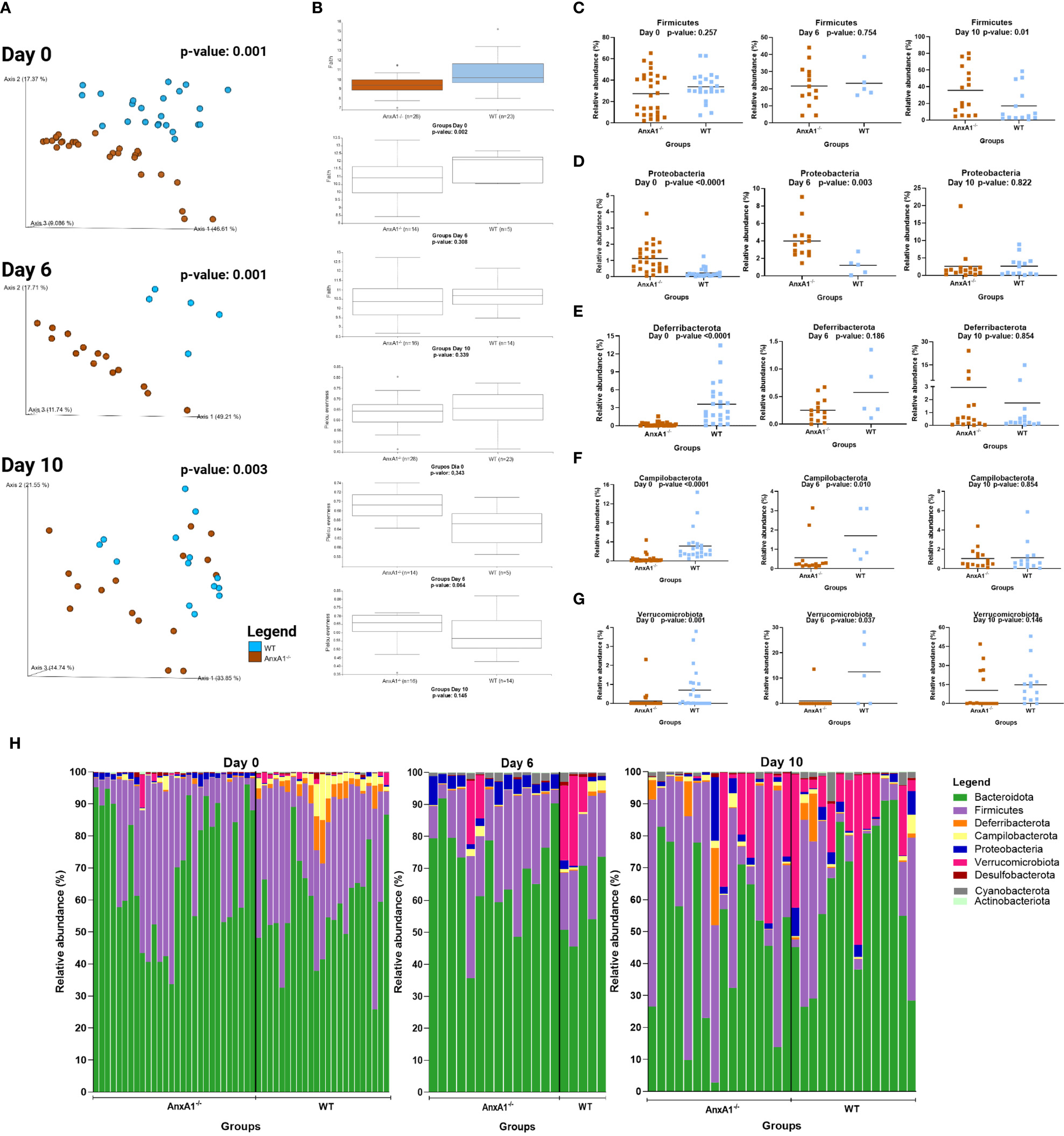
Microbiota shifts during DSS-induced colitis between WT and AnxA1-/- mice. (A) UniFrac weighted metric for beta diversity comparing WT and AnxA1-/- mice through the colitis model. P-value day 0 and 6 = 0.001, day 10 = 0.003. (B) Alpha diversity comparing WT and AnxA1-/- mice performing Richness (upper graph; p-values day 0 = 0.002, day 6 = 0.308 and day 10 = 0.339) and Evenness (bottom graph; p-values day 0 = 0.343, day 6 = 0.064 and day 10 = 0.145) metrics. (C-G) Relative abundance of phyla between WT and AnxA1-/- mice. ASVs were tested with Mann-Whitney. (H) Relative abundance shifts for all phyla through the colitis model. n day 0 WT = 23 and AnxA1-/-= 28; n day 6 WT = 5 and AnxA1-/-= 14; n day 10 WT = 14 and AnxA1-/-=16. For all analyses, p-values <0.05 were considered statistically significant.
The most abundant phyla in both groups remained Bacteroidota and Firmicutes before and after inducing colitis. However, the abundance of Firmicutes was increased in AnxA1-/- compared to WT on the 10th day (Figure 3C). Although AnxA1-/- maintained higher abundance of Proteobacteria in the peak of inflammation, this difference between groups lost significance on day 10 though (Figure 3D). Deferribacterota and Campilobacterota phyla were less abundant in AnxA1-/- mice on day 0, with slight variation on day 6 and 10. In contrast, WT mice lost abundance of this phylum through the development of the disease, so no more differences were observed between the two groups over the days (Figures 3E, F). Verrucomicrobiota bacteria were increased in both groups with colitis, although lower abundance of these bacteria was observed in AnxA1-/- mice in the beginning, and on the 10th, these differences disappeared (Figure 3G). The changes in the microbiota phyla profile through colitis can be observed in Figure 3H.
The genera Lachnospiraceae NK4A136 and Bacteroides increased on day 10, overlapping the prevalence of Muribaculaceae in the AnxA1-/- animals (Figure 4A), while Alloprevotella continued to be identified only in WT mice (Figure 4B). The genus Parasuterella, more abundant at the beginning of the disease, increased in abundance on the peak of inflammation and decreased on the 10th day; no differences were observed in WT DSS mice (Figure 4C). The shift in abundance at the genus level across the days can be observed in the heatmap in Figure 4D.
Figure 4
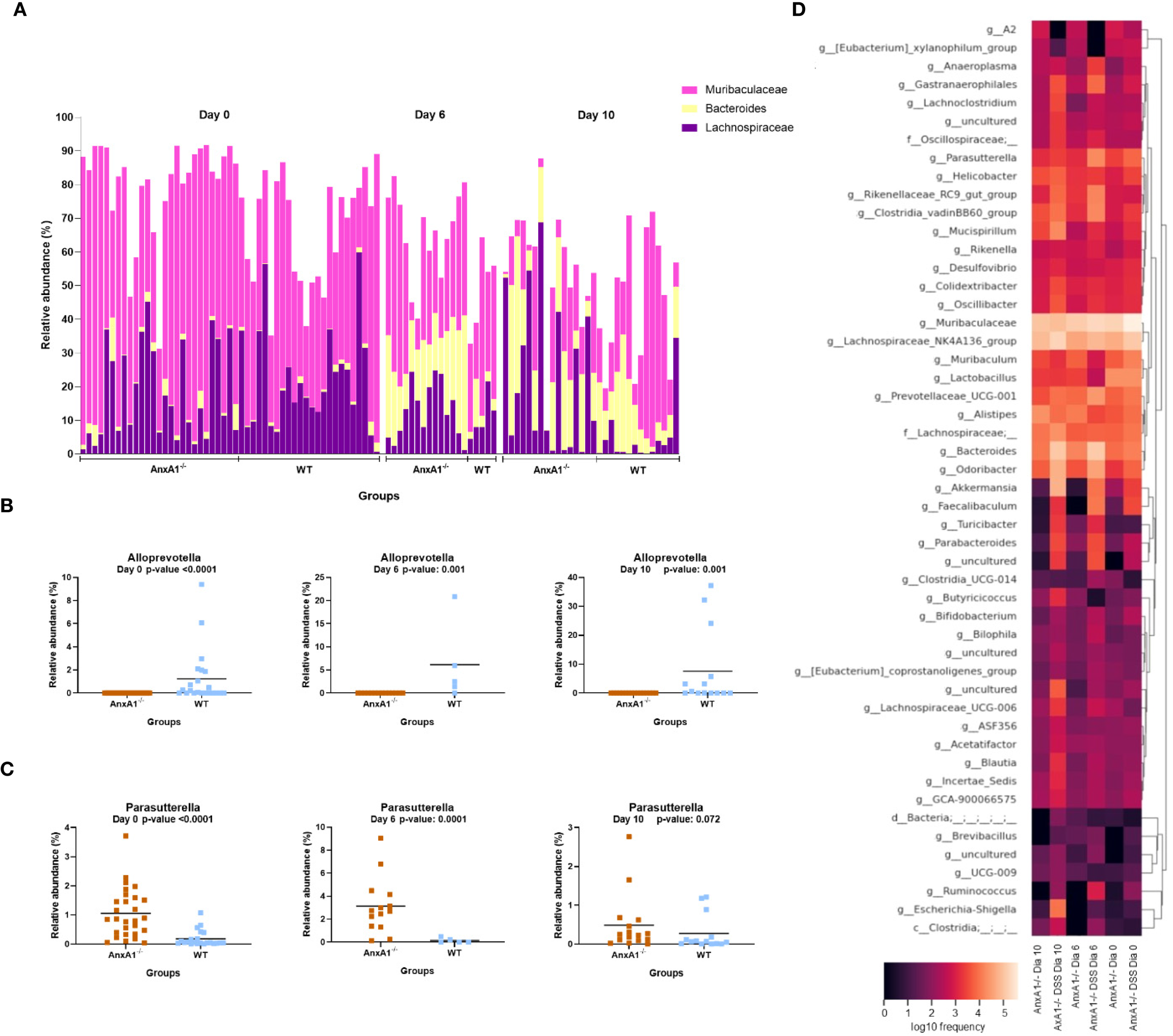
Genera shifts during DSS-induced colitis between WT and AnxA1-/- mice. (A) Relative abundance shifts for the genera belonging to Lachnospiraceae NK4A136, Bacteroides and Muribaculaceae through the colitis model. (B, C) Relative abundance shifts for Alloprevotella and Parasuterella genera through the colitis model. ASVs were tested with Mann-Whitney. (D) Heatmap with the main shifts in genus level between WT and AnxA1-/- mice through the colitis model. n day 0 WT = 23 and AnxA1-/-= 28; n day 6 WT = 5 and AnxA1-/-= 14; n day 10 WT = 14 and AnxA1-/-=16. For all analyses, p-values <0.05 were considered statistically significant.
3.3 AnxA1-/- mice are more susceptible to changes in microbiota during experimental colitis
The microbiota of WT mice started to meaningfully change from a healthy condition after the 6th day of the disease, as shown in Figure 5A. In contrast, in the absence of AnxA1, differences in microbial diversity were already observed on day 6 (Figure 5B).
Figure 5
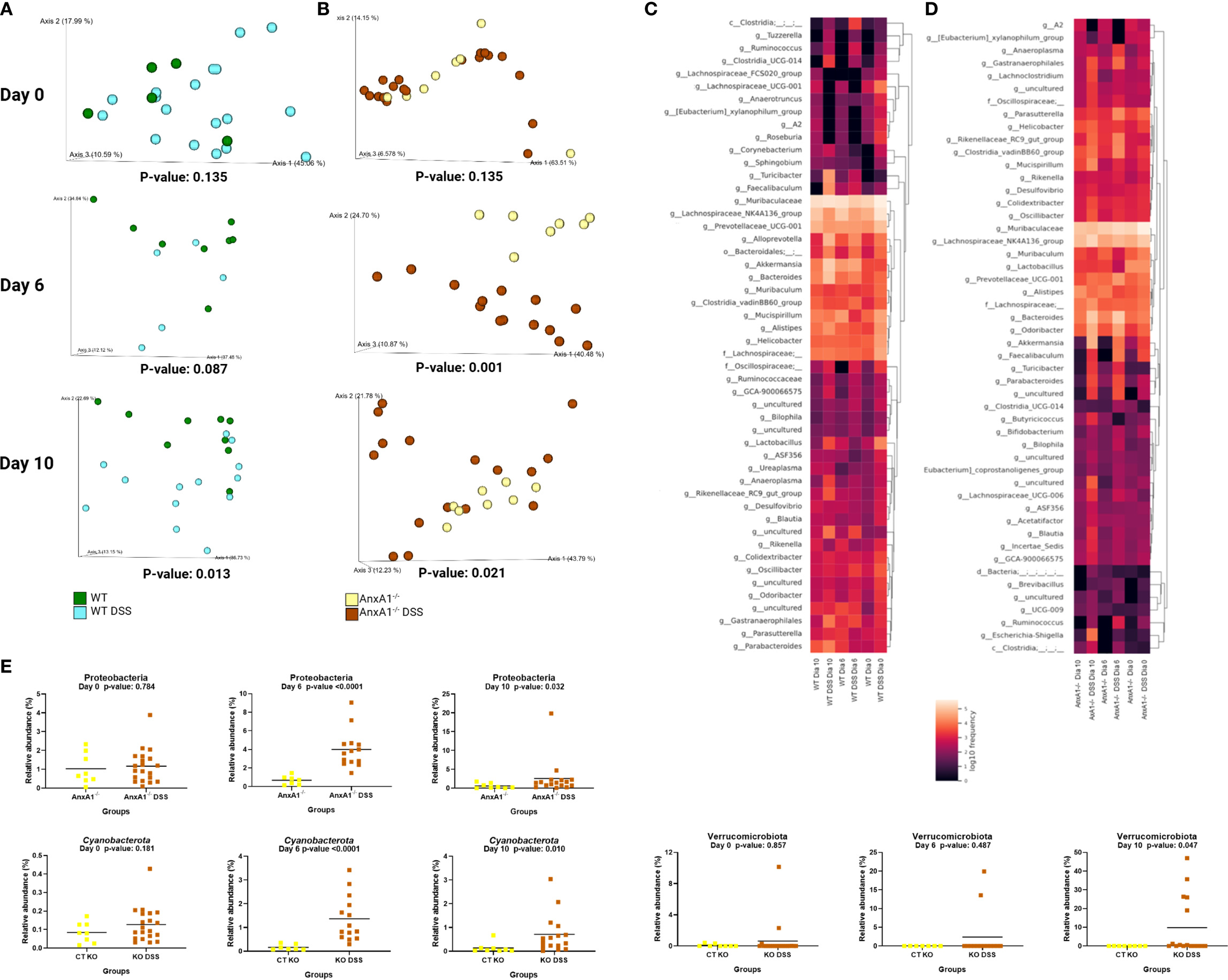
Early alterations in microbiota profile in the absence of AnxA1. (A) UniFrac weighted metric for beta diversity comparing WT control and WT DSS mice through the colitis model. P-values day 0 = 0.135, day 6 = 0.087 and day 10 = 0.013. (B) UniFrac weighted metric for beta diversity comparing AnxA1-/- control and AnxA1-/- DSS mice through the colitis model. P-values day 0 = 0.135, day 6 = 0.001 and day 10 = 0.021. (C) Heatmap with the main shifts in genus level between WT control and WT DSS mice through the colitis model. (D) Heatmap with the main shifts in genus level between AnxA1-/- control and AnxA1-/- DSS mice through the colitis model. (E) Relative abundance of phyla between AnxA1-/- and AnxA1-/- DSS mice. ASVs were tested with Mann-Whitney. n day 0 WT = 5 and WT DSS = 18, AnxA1-/-= 8 and AnxA1-/- DSS = 20; n day 6 WT = 8 and WT DSS = 5, AnxA1-/-= 7 and AnxA1-/- DSS = 14; n day 10 WT = 9 and WT DSS = 13, AnxA1-/-= 8 and AnxA1-/- DSS = 16. For all analyses, p-values <0.05 were considered statistically significant.
In WT mice, the most notable shifts were increments in Turicibacter, Alloprevotella, Akkermansia, Bacteroides, and Faecalibaculum genera. Modifications detected in lesser pronounced genus levels are presented in Figure 5C.
In contrast, AnxA1-/- mice exhibited earlier shifts at the phylum level, with increased abundance of Proteobacteria and Cyanobacteria already detectable on day 6 (Figure 5E). On day 10, a notable enrichment of the Verrucomicrobiota phylum was observed. However, the most prominent changes at the genus level were associated with members of the Firmicutes and Bacteroidota phyla. These included increased levels of Parabacteroides, Bacteroides and Turicibacter. Gastranaerophilales, a member of the Cyanobacteriota phyla and Escherichia-Shigella genera from the Proteobacteriota phyla were also enriched by the end of the disease in AnxA1-/- mice (Figure 5D).
3.4 Different expression in Annexin A1 levels affects the microbiota in IBD patients
The identified phyla in the human cohort subjects were Acidobacteriota, Actinobacteriota, Bacteroidota, Cyanobacteria, Campylobacterota, Firmicutes, Fusobacteriota, Patescibacteria, Proteobacteria, Spirochaetota, and Verrucomicrobiota. The most abundant bacterial genera found in this study were Bacteroides, Faecalibacterium, Parabacteroides, Sutterella, and Alistipes (Figure 6).
Figure 6
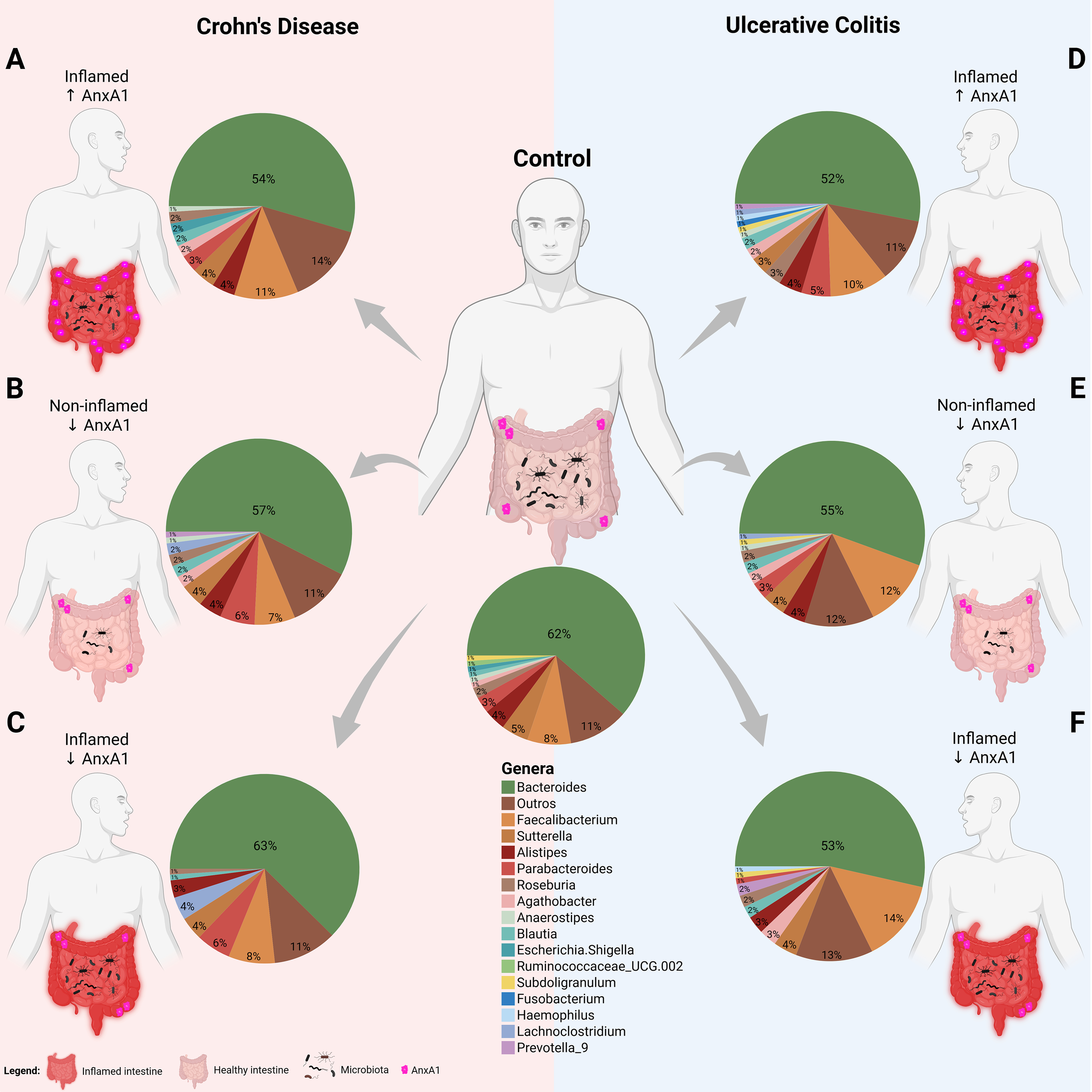
Microbiota shifts in IBD with AnxA1 differential expression. Relative abundance of main bacterial genera shifts in the colon of CD and UC subjects compared to control (centered). (A) CD patient with inflamed colon and high AnxA1 expression. (B) CD patient non-inflamed with basal AnxA1 expression. (C) CD patient with inflamed colon and low levels of AnxA1 expression. (D) UC patient with inflamed colon and high AnxA1 expression. (E) UC patient non-inflamed with basal AnxA1 expression. (F) UC patient with inflamed colon and low levels of AnxA1 expression.
CD patients with active inflammation and low AnxA1 expression exhibited distinct microbiota composition compared to healthy controls, mainly presenting an increase in Lachnoclostridium and Parabacteroides genera (Figure 6C). This pattern was not observed in inflamed patients with high AnxA1 levels though (Figure 6A), suggesting that elevated AnxA1 may partially preserve the microbial profile, maintaining it at an intermediate level, still dysbiotic, but less disrupted than in low levels of AnxA1. Moreover, CD patients in the relapse phase with basal or low AnxA1 expression (Figure 6B) showed no significant differences in microbiota composition when compared to healthy controls, but reduced bacterial richness.
UC patients presented the same microbial genera composition regardless of AnxA1 expression level (Figures 6D-F). However, in active disease, the magnitude of AnxA1 expression influenced the phylum, class and order levels of bacteria compared to relapsing patients. In this scenario, high levels of AnxA1 reflected in an increase at the Actinobacteriota and Bacteroidota phyla, as an increase in Parabacteroides, although the latter has not reached statistical significance (Figure 6D). Likewise, active disease in UC patients with low AnxA1 levels was marked by an increase in Faecalibacterium and Prevotellaceae, along with a decrease in Parabacteroides, although not significantly (Figure 6F). This shift reflects a more dysbiotic and pro-inflammatory microbial profile in the absence of AnxA1. Altogether, these findings suggest impaired anti-inflammatory regulation in AnxA1 deficiency.
If the microbiota of both IBD diseases are compared, it is possible to infer that the disease type may influence bacterial composition in the AnxA1-reduced condition. Non-inflamed CD and UC patients with reduced AnxA1 expression displayed different microbiota profiles (p-value <0.01), with higher abundance of Lachnoclostridium and Parabacteroides in CD patients, and lower abundance of Faecalibacterium (Figures 6B, E). Bacterial genus richness and evenness were also lower in CD patients. Together, the data obtained here point out that levels of AnxA1, even in the latent or relapsing inflammation, affect the microbiota profile in humans.
4 Discussion
The gut microbiota and the immune system are tightly interconnected, and intestinal homeostasis relies on a delicate balance between microbial surveillance, inflammatory responses and their resolution (44). Disruption of this balance, particularly due to deficiencies in pro-resolving mediators, such as AnxA1, impairs the regulation of inflammation and contributes to the pathogenesis of IBDs (33, 37, 41, 45). Although the immunomodulatory roles of AnxA1 on IBD have been described, its influence on microbial dynamics remains poorly understood. In this study, unprecedented data strengthen the evidence for AnxA1’s involvement in microbiota modulation, either dependent or independent of the inflammatory state.
Healthy AnxA1-deficient mice presented reduced richness in comparison to WT, which, along with compositional differences in the bacterial community, suggests resilient or functionally limited microbiota with predisposition to exaggerated inflammation upon stimulation. Among the taxonomic shifts, AnxA1-/- mice presented the overgrowth of Proteobacteria, which is considered a marker of microbiota instability, leading to immune activation, associated with Toll-like receptor (TLR) expression and inflammatory cytokines release, such as TNF-α, IL-6, and IL-1β (46), rendering the host more susceptible to inflammatory stimuli (18, 47–49). Notwithstanding, a reduction in microbial richness, as observed in AnxA1-/- mice, has been correlated with a relative expansion of Proteobacteria in IBDs (50). Consistent with this pattern, an increase in Parasutterella, which is linked to metabolic and inflammatory imbalances (51), indicates underlying alterations in epithelial barrier integrity or immune signaling pathways in the absence of AnxA1. In addition, the lower abundance of Deferribacterota and Campylobacterota in healthy AnxA1-/- mice, including their representative genera Mucispirillum and Helicobacter, which typically colonize mucus-adjacent niches, suggests that AnxA1 influences mucosal microbial colonization under physiological conditions. Notably, Helicobacter hepaticus, a species within Campylobacterota, has been implicated in shaping Th17 responses, being considered a pathobiont strain by disrupting Treg/Th17 axis in immunodeficient conditions (52).
Helicobacter was more abundant in WT and AnxA1-/- mice under basal conditions, but its levels markedly increased in the absence of AnxA1 during colitis, erasing the initial differences. This expansion under inflammatory stress may reflect the inability of AnxA1-/- mice to effectively restrain immune activation, allowing overgrowth of potentially pro-inflammatory taxa. While Helicobacter-driven IL-17A responses (52) may be tightly regulated in WT mice, maintaining mucosal balance, its dysregulation in AnxA1-/- animals could amplify inflammation and worsen disease outcomes.
Another genus decreased in AnxA1-/- mice was Prevotellaceae UCG-001, a bacterium associated with fiber fermentation and SCFA production (53). Its reduction may reflect impaired microbial metabolic function and a shift toward a more pro-inflammatory gut environment (54). The absence of Alloprevotella in AnxA1-/- mice is also notable, given that this genus is typically associated with the production of SCFAs. Moreover, its presence is often linked to greater microbial diversity, which is generally considered favorable for gut health (55, 56). In contrast, the higher abundance of Lactobacillus and Odoribacter in AnxA1-/- mice may reflect a microbial response associated with the maintenance of intestinal homeostasis. Odoribacter, for instance, can modulate macrophage function and epithelial barrier integrity through its metabolites. Loss of Odoribacter in colitis is linked to reduced SCFA availability, leading to intestinal inflammation (57, 58). Meanwhile, in dysbiosis, Lactobacillus may promote Th1 responses, enhancing the secretion of inflammatory cytokines like IFN-γ and TNF-α, contributing to IBD development (59). However, the functional impact of this shift remains unclear, as quantitative changes do not always translate into equivalent functional outcomes. The associated data clearly show a basal difference in microbiota constitution of AnxA1-/-, clearly showing that alteration in genotype affected bacteria in the gut. Although the leukocyte population in the gut of healthy AnxA1 or WT are similar, it remains to be evaluated how the absence of AnxA1 affects the microbiota.
As observed in the healthy state, the microbiota of AnxA1-/- and WT mice maintained distinct profiles throughout the colitis timeline, suggesting that the lack of AnxA1 actively drives microbiota divergence even under inflammatory conditions. The persistent enrichment of Proteobacteria in AnxA1-/- mice during the peak of inflammation (day 6) is consistent with prior reports linking this phylum to intestinal dysbiosis, potentially contributing to a more severe inflammatory profile compared to the WT group (60). Furthermore, dysbiosis with a predominance of Proteobacteria may lead to persistent innate immune activation, intensifying neutrophil and macrophage infiltration into the intestinal mucosa and worsening chronic inflammation (61). Interestingly, by day 10, this difference was no longer significant between both genotypes, which may reflect a delayed or altered resolution of the inflammatory response in the absence of AnxA1.
Firmicutes, one of the dominant phyla in the healthy gut, were more abundant in AnxA1-/- DSS mice at day 10, potentially indicating a compensatory bloom of taxa involved in butyrate production during the recovery phase (62). However, the functional relevance of this expansion remains to be elucidated. The progressive increase in Verrucomicrobiota across colitis is aligned with reports of Akkermansia muciniphila expansion during epithelial barrier damage or remodeling (63). Also, it was observed that the enrichment of Akkermansia through the days in both groups of animals. The disappearance of initial differences by day 10 between the groups may indicate a common mucosal healing process in both genotypes. Importantly, the persistence of Alloprevotella exclusively in WT mice across time points reinforces its sensitivity to the AnxA1-dependent mucosal environment. Given the association of this genus with SCFA production and immunoregulatory effects, its absence in AnxA1-/- mice may contribute to their distinct inflammatory profile.
Additionally, the increase of Lachnospiraceae NK4A136 group and Bacteroides in AnxA1-/- mice by day 10, overtaking Muribaculaceae, may reflect a shift in fermentative capacity or epithelial nutrient availability during recovery (64, 65). Bacteroides enrichment during recovery may not signal resolution, but rather a dysbiotic rebound shaped by host-microbiota disequilibrium (66–68). Parasutterella, initially enriched in AnxA1-/- mice, showed a transient bloom at peak inflammation before returning to WT levels, which may indicate a context-dependent interaction with host immunity or metabolic state. These bacteria produce succinate, which aids microbiota recovery but also promotes ulceration in DSS-colitis (69). In IBDs, excessive succinate drives inflammation by activating immune cells, HIF-1α, and IL-1β, while also fueling pathogens like C. difficile, disrupting microbial balance (70–72).
The dynamics of gut microbiota during experimental colitis reveal a distinct temporal pattern between WT and AnxA1-/- mice, suggesting that AnxA1 plays a critical role in modulating the microbial response to inflammation. In WT mice, meaningful microbiota changes only emerged later during disease progression in comparison to AnxA1-/-, whereas AnxA1-/- mice exhibited earlier shifts. These findings suggest that the absence of AnxA1 predisposes the gut environment to dysbiosis more rapidly during inflammatory stress, in line with its known regulatory functions in mucosal immunity and tissue homeostasis (73).
Although both WT and AnxA1-/- mice developed colitis, the heightened severity of the model may have obscured subtle differences between strains. Nevertheless, AnxA1-/- mice showed greater weight loss and increased circulating neutrophils, suggesting a more intense and unresolved inflammatory response. This is consistent with the known role of AnxA1 in regulating neutrophil physiology, including their release from the bone marrow, migration to inflamed tissues, apoptosis, and clearance by M2 macrophages in homing tissues (24, 74–76). In its absence, these processes become dysregulated, leading to persistent neutrophilia and impaired resolution. Additionally, the observed lymphopenia likely reflects lymphocyte recruitment to the inflamed colon, a process also influenced by AnxA1 through its modulation of leukocyte trafficking (34, 76, 77).
While AnxA1 also governs monocyte infiltration and promotes anti-inflammatory M2 macrophage polarization, no increase in macrophage numbers was detected by day 10 of colitis. This may be due to a predominance of pro-inflammatory M1 macrophages in AnxA1-/- mice or limitations in detection, as F4/80 alone may not adequately distinguish between macrophage subtypes, highlighting the need for more specific markers in future analyses (26, 78–80).
Together, these data indicate that AnxA1 plays a key role in modulating gut microbiota dynamics during experimental colitis. While overall alpha diversity remained stable, AnxA1-/- mice exhibited earlier and more pronounced taxonomic shifts compared to WT, suggesting increased susceptibility to inflammation-associated dysbiosis. These changes, particularly involving reductions in SCFA-producing bacteria and expansions of Proteobacteria, likely reflect impaired regulation of immune–microbiota interactions in the absence of AnxA1. The findings indicate that AnxA1 contributes to gut homeostasis by fine-tuning microbial composition over time and orchestrating a more balanced response and recovery to inflammatory stress, highlighting its relevance in mucosal immunity and the resolution of inflammation.
To determine whether these findings translate to human disease, we further investigated the relationship between AnxA1 expression and microbiota composition in patients with IBD. Studies have shown that alpha-diversity in IBDs, particularly in CD, is typically reduced compared to healthy individuals, with lower species richness and uneven microbial distribution, often marked by a loss of beneficial bacteria. This decrease is associated with more severe symptoms and chronic inflammation (81). In this study, non-inflamed CD patients with low AnxA1 expression had reduced species richness compared to controls. Such differences were not seen in patients with higher AnxA1, regardless of inflammation. These findings suggest that AnxA1 may be associated with maintaining the balance of alpha-diversity in IBD, sustaining homeostasis and preventing the worsening of inflammation.
Although UC patients showed no difference in richness compared to controls, the evenness of bacteria in UC-inflamed subjects with high AnxA1 was decreased compared to controls. Thus, while bacterial distribution varied in this group, the genera and total number of bacteria still resembled those of a healthy control, suggesting that AnxA1, particularly its high expression, may contribute to maintaining eubiosis. Non-inflamed CD subjects with low expression of AnxA1 also presented less richness and evenness when compared to UC ones. This change may highlight how disease basal dynamics and location are also influenced by overall microbial composition and its interactions with the immune resolutive system.
In the present study, differences in the microbiota of inflamed patients with low AnxA1 expression in CD were found compared to controls, but not in individuals with high AnxA1 expression. No differences were found between inflamed individuals with high AnxA1 expression and controls. This shows that while healthy individuals and those inflamed with low AnxA1 expression have distinct microbiotas, individuals with high levels of AnxA1 display an intermediate profile between the two of them.
Moreover, UC patients with active inflammation but with low AnxA1 expression exhibited different microbial composition than their counterparts with higher AnxA1 levels. These differences were observed primarily at the phylum, class, and order levels, suggesting subtle but specific shifts in community structure even in the absence of overt inflammation. However, no significant differences were found between these UC subgroups and healthy controls, indicating that changes associated with reduced AnxA1 in UC may not yet reach the level of dysbiosis detectable against a healthy baseline. In contrast, CD patients without inflammation but with low AnxA1 expression showed a markedly different microbiota composition compared to UC subjects with similarly low AnxA1 expression. The most notable alterations also included an increase in Lachnoclostridium and Parabacteroides in CD, alongside a reduction in Faecalibacterium. These findings suggest that the impact of AnxA1 deficiency on microbiota composition may be disease-specific and more pronounced in the context of CD. Altogether, these results highlight a potential role for AnxA1 in shaping gut microbial ecology even in the absence of inflammation and raise the possibility that low AnxA1 expression may prime the intestinal environment for disease progression in a disease-dependent manner.
Faecalibacterium is a key butyrate-producing genus involved in maintaining intestinal homeostasis by promoting anti-inflammatory macrophage polarization (M2), inducing Treg differentiation, and stimulating IL-10 production (82). Its reduction in IBD has been associated with increased inflammation, impaired epithelial barrier function, and heightened innate immune activation (83, 84). Despite its known relevance, this study did not reveal a clear difference in Faecalibacterium abundance across stratified patient groups.
Parabacteroides is considered a context-dependent genus within the gut microbiota. Its role in the gut is not universally good or bad, but shaped by host–microbe interactions, microbial context, and immune status. In murine models, Parabacteroides distasonis has been shown to stimulate IL-10 production and attenuate experimental colitis (85, 86). Furthermore, a synergistic effect between P. distasonis and Akkermansia muciniphila has been reported to protect against both acute and chronic colitis by enhancing epithelial barrier integrity and promoting the accumulation of group 3 innate lymphoid cells (ILC3s) in the colonic mucosa (87). In CD, however, its colonization has been associated with depressive-like behaviors, possibly due to gut–brain axis interactions (88). The abundance of Parabacteroides is influenced by various factors, with some species displaying notable resilience to antimicrobial treatments (89, 90). In this study, inflamed CD patients with low AnxA1 expression exhibited approximately twice the abundance of Parabacteroides compared to controls and those with high AnxA1 levels, a pattern not observed in UC. In AnxA1-/- mice, Parabacteroides levels increased throughout experimental colitis, with Parabacteroides goldsteinii being the only species identified. By day 10 of colitis, P. goldsteinii abundance was higher in AnxA1-/- mice compared to WT. Overall, the genus remained more abundant in WT animals, suggesting complex and context-dependent regulatory dynamics mediated by AnxA1.
A key limitation of our study is that, although AnxA1 deficiency was associated with distinct microbial shifts and worsened colitis outcomes, our data do not allow us to determine whether these alterations in the microbiota act as causal drivers of pathology or merely represent secondary consequences of inflammation. Establishing this directionality would require dedicated mechanistic approaches, such as fecal microbiota transfer (FMT), to directly test whether the observed microbial profiles can modulate disease severity in the context of AnxA1 signaling. In this regard, our discussion of potential metabolic consequences was grounded in previously published functional studies of the bacterial taxa identified, providing a conceptual framework and rationale for future investigations to address this cause–consequence interplay.
Other resolvins, especially the ones derived from eicosapentaenoic acid-EPA RvE1 and RvE2, and from the docosahexaenoic acid-DHA RvD1, AT-RvD1 and RvD2, have emerged as pivotal regulators of intestinal inflammation (91). In experimental colitis models, they mitigate classical features of disease activity, including weight loss, colon shortening, neutrophil infiltration, and cytokine production, while simultaneously enhancing macrophage phagocytosis and epithelial barrier protection (92–94). Mechanistically, these resolvins signal through specific receptors, such as Leukotriene B4 receptor 1 (BLT1), Lipoxin A4 receptor (ALX)/FPR2, and Chemokine-like receptor 1 (CMKLR1), thereby dampening nuclear factor kappa B (NF-κB) activation, reducing adhesion molecule expression, and facilitating the non-inflammatory clearance of apoptotic cells (93, 95, 96).
In the clinical context, differential expression of resolvins has been linked to disease activity in both CD and UC. Notably, RvE1 levels were consistently lower in CD than in UC, and in the active phase, this lipid mediator emerged as a potential discriminator between the two entities. In UC patients, remission was also more strongly associated with RvE1 and correlated with lower clinical and endoscopic activity, suggesting its potential utility as a biomarker of mucosal healing (97, 98). Importantly, RvD2 also attenuates DSS-induced colitis, demonstrating efficacy comparable to anti-TNF therapy in preclinical settings, underscoring its translational potential (99).
Campbell et al. (100) demonstrated that RvE1 protects against DSS-induced colitis, likely through the activation of intestinal alkaline phosphatase, which helps detoxify bacterial lipopolysaccharides. Similarly, Zeng et al. (101) showed that RvD1 ameliorates hepatic steatosis in DSS-induced chronic colitis by reshaping the gut microbiota. RvD1 treatment restored a healthier microbial profile resembling that of control mice, normalized the Firmicutes-to-Bacteroidetes ratio, increased beneficial bacteria such as Akkermansia, and reduced genera like Bacteroides and Desulfovibrio. These microbiota changes were associated with improved intestinal barrier integrity and reduced systemic inflammation, suggesting that RvD1 exerts its protective effects through modulation of the gut-liver axis. Taken together, these observations highlight resolvins as not only candidate therapeutic targets but also as biomarkers capable of distinguishing disease phases and entities. Functionally, they appear to mirror several actions attributed to Annexin A1, supporting the notion that convergent pro-resolving pathways act in parallel to restrain intestinal inflammation and promote mucosal homeostasis.
In this context, studies investigating AnxA1 in the setting of IBD treatment have been increasingly reported. AnxA1-mimetic peptides, such as Ac2-26, reproduce many of the anti-inflammatory and pro-resolving actions of the native protein, including inhibition of neutrophil infiltration, promotion of macrophage-mediated clearance, and restoration of epithelial barrier integrity (38). Polymeric nanoparticles loaded with Ac2–26 have been tested in murine models of colitis, where they accelerated epithelial wound healing and promoted intestinal tissue regeneration (40). Li et al. (102) further demonstrated that Ac2-26-containing nanoparticles reshaped the microbiota of DSS-induced mice, reducing the expansion of Escherichia–Shigella and increasing levels of Prevotellaceae. Alpha and beta diversity did not differ from control groups after nanotherapy treatment. Taken together with our findings, and supported by previous evidence showing that AnxA1 deficiency alters gut microbial composition and exacerbates colitis, these peptides may represent a dual-action therapeutic strategy: directly resolving inflammation while indirectly modulating the gut microbiota. Future studies are warranted to determine whether AnxA1-mimetic peptides can favorably reshape microbial communities and translate these mechanistic insights into therapeutic benefit for patients with IBD.
Altogether, the results presented here suggest that AnxA1 plays a key role in shaping the intestinal microbiota through its immunomodulatory effects, both in experimental colitis and in IBD patients. In murine models, the absence of AnxA1 led to early and more pronounced dysbiotic shifts during colitis. Even under healthy conditions, AnxA1-/- mice exhibited reduced microbial richness and distinct community structures, indicating that AnxA1 contributes to maintaining microbiota resilience and mucosal homeostasis. In human cohorts, patients with higher AnxA1 expression, regardless of inflammatory status, had microbial profiles more similar to healthy controls, with greater uniformity and richness, suggesting that AnxA1 may protect against microbiota disruption and excessive inflammation. Differences in microbial composition among IBD subtypes further suggest that the influence of AnxA1 on the gut ecosystem may be disease-specific and dependent on the inflammatory context. Hence, these findings underscore the critical role of AnxA1 in orchestrating host–microbiota interactions and maintaining intestinal equilibrium. They also highlight the therapeutic potential of targeting AnxA1 pathways to restore microbiota balance and reduce inflammation in IBD.
Statements
Data availability statement
The datasets analyzed for this study can be found in the European Genome-Phenome Archive https://ega-archive.org/studies/EGAS00001005027.
Ethics statement
The studies involving humans were approved by Ethics committee of the University of Groningen. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. The animal study was approved by Ethics Committee of Animal Use of the Faculty of Pharmaceutical Sciences of the University of São Paulo-CEUA/FCF/USP. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. RG: Conceptualization, Investigation, Methodology, Writing – review & editing. Gd: Investigation, Methodology, Writing – review & editing. MB: Investigation, Methodology, Writing – review & editing. PS: Investigation, Methodology, Writing – review & editing. FL: Investigation, Methodology, Writing – review & editing. KF: Methodology, Project administration, Resources, Supervision, Writing – review & editing. HH: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. CH: Methodology, Project administration, Resources, Supervision, Writing – review & editing. SF: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), PrInt-CAPES (Programa Institucional de Internacionalização da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), grant number 2022/11602-0/2024/15297-3. PS is a FAPESP fellowship (grant number 2020/14368-3).
Acknowledgments
We thank Dr. Sandra Farsky, Dr. Christian Hoffmann, Dr. Hermie Harmsen, and Dr. Klaas Nico Faber for their financial support and valuable scientific input in the conception of this work. We also acknowledge the collaborative efforts between the University of São Paulo and the University of Groningen, which were essential for the development of this study. Finally, we thank our colleagues for their contributions and constructive discussions throughout the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1679071/full#supplementary-material
References
1
Dolinger M Torres J Vermeire S . Crohn’s disease. Lancet. (2024) 403:1177–91. doi: 10.1016/S0140-6736(23)02586-2
2
Gordon H Biancone L Fiorino G Katsanos KH Kopylov U Al Sulais E et al . ECCO guidelines on inflammatory bowel disease and Malignancies. J Crohns Colitis. (2023) 17:827–54. doi: 10.1093/ecco-jcc/jjac187
3
Gros B Kaplan GG . Ulcerative colitis in adults: A review. JAMA. (2023) 330:951–65. doi: 10.1001/jama.2023.15389
4
Nakase H Uchino M Shinzaki S Matsuura M Matsuoka K Kobayashi T et al . Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. (2021) 56:489–526. doi: 10.1007/s00535-021-01784-1
5
Shah SC Itzkowitz SH . Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. (2022) 162:715–30.e3. doi: 10.1053/j.gastro.2021.10.035
6
Zhernakova DV Wang D Liu L Andreu-Sánchez S Zhang Y Ruiz-Moreno AJ et al . Host genetic regulation of human gut microbial structural variation. Nature. (2024) 625:813–21. doi: 10.1038/s41586-023-06893-w
7
Guo X Huang C Xu J Xu H Liu L Zhao H et al . Gut microbiota is a potential biomarker in inflammatory bowel disease. Front Nutr. (2022) 8:818902. doi: 10.3389/fnut.2021.818902
8
Mestrovic A Perkovic N Bozic D Kumric M Vilovic M Bozic J . Precision medicine in inflammatory bowel disease: A spotlight on emerging molecular biomarkers. Biomedicines. (2024) 12:1520. doi: 10.3390/biomedicines12071520
9
Vieujean S Jairath V Peyrin-Biroulet L Dubinsky M Iacucci M Magro F et al . Understanding the therapeutic toolkit for inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2025) 22:371–94. doi: 10.1038/s41575-024-01035-7
10
Al Radi ZMA Prins FM Collij V Vich Vila A Festen EAM Dijkstra G et al . Exploring the predictive value of gut microbiome signatures for therapy intensification in patients with inflammatory bowel disease: A 10-year follow-up study. Inflammation Bowel Dis. (2024) 30:1642–53. doi: 10.1093/ibd/izae064
11
Chen L Zhang C Niu R Xiong S He J Wang Y et al . Multi-omics biomarkers for predicting efficacy of biologic and small-molecule therapies in adults with inflammatory bowel disease: A systematic review. United Eur Gastroenterol J. (2025) 13:517–30. doi: 10.1002/ueg2.12720
12
Hu S Bourgonje AR Gacesa R Jansen BH Björk JR Bangma A et al . Mucosal host-microbe interactions associate with clinical phenotypes in inflammatory bowel disease. Nat Commun. (2024) 15:1470. doi: 10.1038/s41467-024-45855-2
13
Quaglio AEV Grillo TG De Oliveira ECS Di Stasi LC Sassaki LY . Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. (2022) 28:4053–60. doi: 10.3748/wjg.v28.i30.4053
14
Haneishi Y Furuya Y Hasegawa M Picarelli A Rossi M Miyamoto J . Inflammatory bowel diseases and gut microbiota. Int J Mol Sci. (2023) 24:3817. doi: 10.3390/ijms24043817
15
Iliev ID Ananthakrishnan AN Guo C-J . Microbiota in inflammatory bowel disease: mechanisms of disease and therapeutic opportunities. Nat Rev Microbiol. (2025) 23:509–24. doi: 10.1038/s41579-025-01163-0
16
Pandey H Jain D Tang DWT Wong SH Lal D . Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest Res. (2024) 22:15–43. doi: 10.5217/ir.2023.00080
17
Qiu P Ishimoto T Fu L Zhang J Zhang Z Liu Y . The gut microbiota in inflammatory bowel disease. Front Cell Infect Microbiol. (2022) 12:733992. doi: 10.3389/fcimb.2022.733992
18
Shin Y Han S Kwon J Ju S Choi TG Kang I et al . Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients. (2023) 15:4466. doi: 10.3390/nu15204466
19
Yi C Huang S Zhang W Guo L Xia T Huang F et al . Synergistic interactions between gut microbiota and short chain fatty acids: Pioneering therapeutic frontiers in chronic disease management. Microb Pathog. (2025) 199:107231. doi: 10.1016/j.micpath.2024.107231
20
Araújo TG Mota STS Ferreira HSV Ribeiro MA Goulart LR Vecchi L . Annexin A1 as a regulator of immune response in cancer. Cells. (2021) 10:2245. doi: 10.3390/cells10092245
21
Leoni G Nusrat A . Annexin A1: shifting the balance towards resolution and repair. Biol Chem. (2016) 397:971–9. doi: 10.1515/hsz-2016-0180
22
Sandri S Hebeda CB Broering MF de Paula Silva M Moredo LF de Barros e Silva MJ et al . Role of annexin A1 secreted by neutrophils in melanoma metastasis. Cells. (2023) 12:425. doi: 10.3390/cells12030425
23
Perez GI Bernard MP Vocelle D Zarea AA Saleh NA Gagea MA et al . Phosphatidylserine-exposing Annexin A1-positive extracellular vesicles: potential cancer biomarkers. Vaccines (Basel). (2023) 11:639. doi: 10.3390/vaccines11030639
24
Perretti M D’Acquisto F . Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. (2009) 9:62–70. doi: 10.1038/nri2470
25
Moreli JB Santos MRD Calderon I de MP Hebeda CB Farsky SHP et al . The role of Annexin A1 in DNA damage response in placental cells: impact on gestational diabetes mellitus. Int J Mol Sci. (2023) 24:10155. doi: 10.3390/ijms241210155
26
Sheikh MH Solito E . Annexin A1: uncovering the many talents of an old protein. Int J Mol Sci. (2018) 19:1045. doi: 10.3390/ijms19041045
27
Sousa SO Santos MRD Teixeira SC Ferro EAV Oliani SM . ANNEXIN A1: roles in placenta, cell survival, and nucleus. Cells. (2022) 11:2057. doi: 10.3390/cells11132057
28
Xia Q Li X Zhou H Zheng L Shi J . S100A11 protects against neuronal cell apoptosis induced by cerebral ischemia via inhibiting the nuclear translocation of annexin A1. Cell Death Dis. (2018) 9:657. doi: 10.1038/s41419-018-0686-7
29
D’Acquisto F Perretti M Flower RJ . Annexin-A1: a pivotal regulator of the innate and adaptive immune systems. Br J Pharmacol. (2008) 155:152–69. doi: 10.1038/bjp.2008.252
30
Qin CX Norling LV Vecchio EA Brennan EP May LT Wootten D et al . Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br J Pharmacol. (2022) 179:4617–39. doi: 10.1111/bph.15919
31
Grewal T Wason SJ Enrich C Rentero C . Annexins - insights from knockout mice. Biol Chem. (2016) 397:1031–53. doi: 10.1515/hsz-2016-0168
32
Zharkova O Salamah MF Babak MV Rajan E Lim LHK Andrade F et al . Deletion of annexin A1 in mice upregulates the expression of its receptor, fpr2/3, and reactivity to the anxA1 mimetic peptide in platelets. Int J Mol Sci. (2023) 24:3424. doi: 10.3390/ijms24043424
33
de Paula-Silva M Barrios BE Macció-Maretto L Sena AA Farsky SHP Correa SG et al . Role of the protein annexin A1 on the efficacy of anti-TNF treatment in a murine model of acute colitis. Biochem Pharmacol. (2016) 115:104–13. doi: 10.1016/j.bcp.2016.06.012
34
Leoni G Alam A Neumann P-A Lambeth JD Cheng G McCoy J et al . Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. (2013) 123:443–54. doi: 10.1172/JCI65831
35
Vergnolle N Pagès P Guimbaud R Chaussade S Buéno L Escourrou J et al . Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflammation Bowel Dis. (2004) 10:584–92. doi: 10.1097/00054725-200409000-00013
36
da Rocha GHO de Paula-Silva M Broering MF Scharf PRDS Matsuyama LSAS Maria-Engler SS et al . Pioglitazone-mediated attenuation of experimental colitis relies on cleaving of Annexin A1 released by macrophages. Front Pharmacol. (2020) 11:591561. doi: 10.3389/fphar.2020.591561
37
de Paula-Silva M da Rocha GHO Broering MF Queiroz ML Sandri S Loiola RA et al . Formyl peptide receptors and Annexin A1: complementary mechanisms to infliximab in murine experimental colitis and Crohn’s disease. Front Immunol. (2021) 12:714138. doi: 10.3389/fimmu.2021.714138
38
Broering MF Oseliero Filho PL Borges PP da Silva LCC Knirsch MC Xavier LF et al . Development of Ac2–26 mesoporous microparticle system as a potential therapeutic agent for inflammatory bowel diseases. Int J Nanomedicine. (2024) 19:3537–54. doi: 10.2147/IJN.S451589
39
Broering MF Leão M de C da Rocha GHO Scharf P Xavier LF et al . Development of Annexin A1-surface-functionalized metal-complex multi-wall lipid core nanocapsules and effectiveness on experimental colitis. Eur J Pharm Biopharm. (2022) 181:49–59. doi: 10.1016/j.ejpb.2022.10.022
40
Leoni G Neumann P-A Kamaly N Quiros M Nishio H Jones HR et al . Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. (2015) 125:1215–27. doi: 10.1172/JCI76693
41
Xu R Weber M-C Hu X Neumann P-A Kamaly N . Annexin A1 based inflammation resolving mediators and nanomedicines for inflammatory bowel disease therapy. Semin Immunol. (2022) 61–64:101664. doi: 10.1016/j.smim.2022.101664
42
Valle-Noguera A Gómez-Sánchez MJ Girard-Madoux MJH Cruz-Adalia A . Optimized protocol for characterization of mouse gut innate lymphoid cells. Front Immunol. (2020) 11:563414. doi: 10.3389/fimmu.2020.563414
43
Caporaso JG Lauber CL Walters WA Lozupone CA Turnbaugh PJ Fierer N et al . Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Nat Acad Sci. (2011) 108:4516–22. doi: 10.1073/pnas.1000080107
44
Wang J He M Yang M Ai X . Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. (2024) 345:122612. doi: 10.1016/j.lfs.2024.122612
45
Sena A Grishina I Thai A Goulart L Macal M Fenton A et al . Dysregulation of anti-inflammatory annexin A1 expression in progressive Crohns Disease. PLoS One. (2013) 8:e76969. doi: 10.1371/journal.pone.0076969
46
Peña-Durán E García-Galindo JJ López-Murillo LD Huerta-Huerta A Balleza-Alejandri LR Beltrán-Ramírez A et al . Microbiota and inflammatory markers: A review of their interplay, clinical implications, and metabolic disorders. Int J Mol Sci. (2025) 26:1773. doi: 10.3390/ijms26041773
47
Mukhopadhya I Hansen R El-Omar EM Hold GL . IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. (2012) 9:219–30. doi: 10.1038/nrgastro.2012.14
48
Ooi JH Waddell A Lin Y-D Albert I Rust LT Holden V et al . Dominant effects of the diet on the microbiome and the local and systemic immune response in mice. PloS One. (2014) 9:e86366. doi: 10.1371/journal.pone.0086366
49
Yee S-M Choi H Seon J-E Ban Y-J Kim M-J Seo J-E et al . Axl alleviates DSS-induced colitis by preventing dysbiosis of gut microbiota. Sci Rep. (2023) 13:5371. doi: 10.1038/s41598-023-32527-2
50
Zheng J Sun Q Zhang M Liu C Su Q Zhang L et al . Noninvasive, microbiome-based diagnosis of inflammatory bowel disease. Nat Med. (2024) 30:3555–67. doi: 10.1038/s41591-024-03280-4
51
Ju T Kong JY Stothard P Willing BP . Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. (2019) 13:1520–34. doi: 10.1038/s41396-019-0364-5
52
Morrison PJ Bending D Fouser LA Wright JF Stockinger B Cooke A et al . Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal Immunol. (2013) 6:1143–56. doi: 10.1038/mi.2013.11
53
Song X Zhong L Lyu N Liu F Li B Hao Y et al . Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genomics Proteomics Bioinf. (2019) 17:64–75. doi: 10.1016/j.gpb.2019.03.001
54
Hong C-T Chan L Chen K-Y Lee H-H Huang L-K Yang Y.-C.S.H. et al . Rifaximin modifies gut microbiota and attenuates inflammation in Parkinson’s disease: preclinical and clinical studies. Cells. (2022) 11:3468. doi: 10.3390/cells11213468
55
Han B Shi L Bao M-Y Yu F-L Zhang Y Lu X-Y et al . Dietary ellagic acid therapy for CNS autoimmunity: Targeting on Alloprevotella rava and propionate metabolism. Microbiome. (2024) 12:114. doi: 10.1186/s40168-024-01819-8
56
Pietrucci D Teofani A Milanesi M Fosso B Putignani L Messina F et al . Machine learning data analysis highlights the role of Parasutterella and Alloprevotella in autism spectrum disorders. Biomedicines. (2022) 10:2028. doi: 10.3390/biomedicines10082028
57
Dai J Jiang M Wang X Lang T Wan L Wang J . Human-derived bacterial strains mitigate colitis via modulating gut microbiota and repairing intestinal barrier function in mice. BMC Microbiol. (2024) 24:96. doi: 10.1186/s12866-024-03216-5
58
Zhuang J Zhuang Z Chen B Yang Y Chen H Guan G . Odoribacter splanchnicus-derived extracellular vesicles alleviate inflammatory bowel disease by modulating gastrointestinal inflammation and intestinal barrier function via the NLRP3 inflammasome suppression. Mol Med. (2025) 31:56. doi: 10.1186/s10020-025-01063-2
59
Guo N Lv L-L . Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun Inflammation Dis. (2023) 11:e1045. doi: 10.1002/iid3.1045
60
Ma J Wang K Wang J Zeng Q Liu K Zheng S et al . Microbial disruptions in inflammatory bowel disease: A comparative analysis. Int J Gen Med. (2024) 17:1355–67. doi: 10.2147/IJGM.S448359
61
Molloy MJ Grainger JR Bouladoux N Hand TW Koo LY Naik S et al . Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. (2013) 14:318–28. doi: 10.1016/j.chom.2013.08.003
62
Esquerre N Basso L Defaye M Vicentini FA Cluny N Bihan D et al . Colitis-induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell Mol Gastroenterol Hepatol. (2020) 10:225–44. doi: 10.1016/j.jcmgh.2020.04.003
63
Qu S Zheng Y Huang Y Feng Y Xu K Zhang W et al . Excessive consumption of mucin by over-colonized Akkermansia muciniphila promotes intestinal barrier damage during Malignant intestinal environment. Front Microbiol. (2023) 14:1111911. doi: 10.3389/fmicb.2023.1111911
64
Dou X Gao N Yan D Shan A . Sodium butyrate alleviates mouse colitis by regulating gut microbiota dysbiosis. Anim (Basel). (2020) 10:1154. doi: 10.3390/ani10071154
65
Schwab C Berry D Rauch I Rennisch I Ramesmayer J Hainzl E et al . Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. (2014) 8:1101–14. doi: 10.1038/ismej.2013.223
66
Bloom SM Bijanki VN Nava GM Sun L Malvin NP Donermeyer DL et al . Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. (2011) 9:390–403. doi: 10.1016/j.chom.2011.04.009
67
Bourgonje AR Roo-Brand G Lisotto P Sadaghian Sadabad M Reitsema RD de Goffau MC et al . Patients with inflammatory bowel disease show IgG immune responses towards specific intestinal bacterial genera. Front Immunol. (2022) 13:842911. doi: 10.3389/fimmu.2022.842911
68
Li F Yu C Zhao Q Wang Z Wang Z Chang Y et al . Exploring the intestinal ecosystem: from gut microbiota to associations with subtypes of inflammatory bowel disease. Front Cell Infect Microbiol. (2023) 13:1304858. doi: 10.3389/fcimb.2023.1304858
69
Osaka T Moriyama E Arai S Date Y Yagi J Kikuchi J et al . Meta-analysis of fecal microbiota and metabolites in experimental colitic mice during the inflammatory and healing phases. Nutrients. (2017) 9:1329. doi: 10.3390/nu9121329
70
Auria E Deschamps J Briandet R Dupuy B . Extracellular succinate induces spatially organized biofilm formation in Clostridioides difficile. Biofilm. (2023) 5:100125. doi: 10.1016/j.bioflm.2023.100125
71
Fernández-Veledo S Grau-Bové C Notararigo S Huber-Ruano I . The role of microbial succinate in the pathophysiology of inflammatory bowel disease: mechanisms and therapeutic potential. Curr Opin Microbiol. (2025) 85:102599. doi: 10.1016/j.mib.2025.102599
72
Huang H Li G He Y Chen J Yan J Zhang Q et al . Cellular succinate metabolism and signaling in inflammation: implications for therapeutic intervention. Front Immunol. (2024) 15:1404441. doi: 10.3389/fimmu.2024.1404441
73
Gobbetti T Cooray SN . Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature. Biol Chem. (2016) 397:981–93. doi: 10.1515/hsz-2016-0200
74
Dalli J Jones CP Cavalcanti DM Farsky SH Perretti M Rankin SM . Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow. FASEB J. (2012) 26:387–96. doi: 10.1096/fj.11-182089
75
MaChado ID Spatti M Hastreiter A Santin JR Fock RA Gil CD et al . Annexin A1 is a physiological modulator of neutrophil maturation and recirculation acting on the CXCR4/CXCL12 pathway. J Cell Physiol. (2016) 231:2418–27. doi: 10.1002/jcp.25346
76
Sugimoto MA Vago JP Teixeira MM Sousa LP . Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res. (2016) 2016:8239258. doi: 10.1155/2016/8239258
77
Gerke V Creutz CE Moss SE . Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. (2005) 6:449–61. doi: 10.1038/nrm1661
78
Italiani P Boraschi D . From monocytes to M1/M2 macrophages: phenotypical vs. Funct Differentiation. Front Immunol. (2014) 5:514. doi: 10.3389/fimmu.2014.00514
79
Lima KM Vago JP Caux TR Negreiros-Lima GL Sugimoto MA Tavares LP et al . The resolution of acute inflammation induced by cyclic AMP is dependent on annexin A1. J Biol Chem. (2017) 292:13758–73. doi: 10.1074/jbc.M117.800391
80
Yona S Heinsbroek SEM Peiser L Gordon S Perretti M Flower RJ . Impaired phagocytic mechanism in annexin 1 null macrophages. Br J Pharmacol. (2006) 148:469–77. doi: 10.1038/sj.bjp.0706730
81
Buffet-Bataillon S Durão G Le Huërou-Luron I Rué O Le Cunff Y Cattoir V et al . Gut microbiota dysfunction in Crohn’s disease. Front Cell Infect Microbiol. (2025) 15:1540352. doi: 10.3389/fcimb.2025.1540352
82
Qiu X Zhang M Yang X Hong N Yu C . Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis. (2013) 7:e558–568. doi: 10.1016/j.crohns.2013.04.002
83
Cao Y Shen J Ran ZH . Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol Res Pract. (2014) 2014:872725. doi: 10.1155/2014/872725
84
Fagundes RR Bravo-Ruiseco G Hu S Kierans SJ Weersma RK Taylor CT et al . Faecalibacterium prausnitzii promotes intestinal epithelial IL-18 production through activation of the HIF1α pathway. Front Microbiol. (2023) 14:1298304. doi: 10.3389/fmicb.2023.1298304
85
Cekanaviciute E Yoo BB Runia TF Debelius JW Singh S Nelson CA et al . Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. (2017) 114:10713–8. doi: 10.1073/pnas.1711235114
86
Cui Y Zhang L Wang X Yi Y Shan Y Liu B et al . Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol Lett. (2022) 369:fnac072. doi: 10.1093/femsle/fnac072
87
Gaifem J Mendes-Frias A Wolter M Steimle A Garzón MJ Ubeda C et al . Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut. mBio. (2024) 15:e0007824. doi: 10.1128/mbio.00078-24
88
Gomez-Nguyen A Basson AR Dark-Fleury L Hsu K Osme A Menghini P et al . Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav Immun. (2021) 98:245–50. doi: 10.1016/j.bbi.2021.08.218
89
Karlowsky JA Walkty AJ Adam HJ Baxter MR Hoban DJ Zhanel GG . Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. (2012) 56:1247–52. doi: 10.1128/AAC.05823-11
90
Snydman DR Jacobus NV McDermott LA Goldstein EJC Harrell L Jenkins SG et al . Trends in antimicrobial resistance among Bacteroides species and Parabacteroides species in the United States from 2010–2012 with comparison to 2008-2009. Anaerobe. (2017) 43:21–6. doi: 10.1016/j.anaerobe.2016.11.003
91
Schwanke RC Marcon R Bento AF Calixto JB . EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. Eur J Pharmacol. (2016) 785:156–64. doi: 10.1016/j.ejphar.2015.08.050
92
Arita M Yoshida M Hong S Tjonahen E Glickman JN Petasis NA et al . Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. (2005) 102:7671–6. doi: 10.1073/pnas.0409271102
93
Bento AF Claudino RF Dutra RC Marcon R Calixto JB . Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. (2011) 187:1957–69. doi: 10.4049/jimmunol.1101305
94
Ishida T Yoshida M Arita M Nishitani Y Nishiumi S Masuda A et al . Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium–induced colitis. Inflammation Bowel Dis. (2010) 16:87–95. doi: 10.1002/ibd.21029
95
Arita M Bianchini F Aliberti J Sher A Chiang N Hong S et al . Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. (2005) 201:713–22. doi: 10.1084/jem.20042031
96
Schwab JM Chiang N Arita M Serhan CN . Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. (2007) 447:869–74. doi: 10.1038/nature05877
97
Kikut J Drozd A Mokrzycka M Grzybowska-Chlebowczyk U Ziętek M Szczuko M . Are EPA and DHA derivatives involved in IBD remission? J Clin Med. (2022) 11:2388. doi: 10.3390/jcm11092388
98
Küçük İ. Tural E Güney B.Ç. Salmanoğlu M Kaplan M . Evaluation of serum resolvin d1 and e1 levels in patients with inflammatory bowel diseases. Eskisehir Med J. (2023) 4:212–9. doi: 10.48176/esmj.2023.135
99
Chaim FHM Pascoal LB de Castro MM Palma BB Rodrigues BL Fagundes JJ et al . The resolvin D2 and omega-3 polyunsaturated fatty acid as a new possible therapeutic approach for inflammatory bowel diseases. Sci Rep. (2024) 14:28698. doi: 10.1038/s41598-024-80051-8
100
Campbell EL MacManus CF Kominsky DJ Keely S Glover LE Bowers BE et al . Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. (2010) 107:14298–303. doi: 10.1073/pnas.0914730107
101
Zeng C Liu X Zhu S Xiong D Zhu L Hou X et al . Resolvin D1 ameliorates hepatic steatosis by remodeling the gut microbiota and restoring the intestinal barrier integrity in DSS-induced chronic colitis. Int Immunopharmacol. (2022) 103:108500. doi: 10.1016/j.intimp.2021.108500
102
Li C Zhao Y Cheng J Guo J Zhang Q Zhang X et al . A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv Sci (Weinh). (2019) 6:1900610. doi: 10.1002/advs.201900610
Summary
Keywords
ANXA1, microbiota, DSS-induced colitis, Crohn’s disease, ulcerative colitis, 16S, metagenomics
Citation
Filippi Xavier L, Gacesa R, da Rocha GHO, Broering MF, Scharf P, Lima FdS, Faber KN, Harmsen H, Hoffmann C and Farsky SHP (2025) Annexin A1 levels affect microbiota in health and DSS-induced colitis/inflammatory bowel disease development. Front. Immunol. 16:1679071. doi: 10.3389/fimmu.2025.1679071
Received
04 August 2025
Accepted
17 September 2025
Published
03 October 2025
Volume
16 - 2025
Edited by
Kathrin S. Michelsen, Cedars Sinai Medical Center, United States
Reviewed by
Ata Ur Rehman, Duke University, United States
Yu-Jen Wang, China Medical University, Taiwan
Updates
Copyright
© 2025 Filippi Xavier, Gacesa, da Rocha, Broering, Scharf, Lima, Faber, Harmsen, Hoffmann and Farsky.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandra Helena Poliselli Farsky, sfarsky@usp.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.