- 1Department of Respiratory and Critical Care Medicine, Central Laboratory, Translational Medicine Research Center, The Affiliated Jiangning Hospital of Nanjing Medical University, Nanjing, China
- 2School of Biomedical Engineering and Informatics, Nanjing Medical University, Nanjing, China
- 3Department of Thyroid and Breast Surgery, The Affiliated JiangNing Hospital of NanJing Medical University, Nanjing, China
The gut-brain-immune axis represents a paradigm shift in understanding systemic homeostasis and disease. While microbial dysbiosis is firmly linked to a spectrum of neurological and immunological disorders, a critical gap persists in our mechanistic understanding of how gut microbes precisely orchestrate the crosstalk between these two systems. This review moves beyond correlation to dissect the causative mechanisms by which microbial metabolites—including short-chain fatty acids, tryptophan derivatives, and neurotransmitters—directly modulate neuroimmune circuits. We synthesize emerging evidence delineating specific molecular circuits that govern microglial maturation, T cell differentiation, and blood–brain barrier integrity, and propose a novel framework for microbiota-mediated neuroimmune regulation. We evaluate cutting-edge microbiota-directed interventions, not merely as generic probiotics, but as precision tools to reestablish neuroimmune homeostasis, thereby outlining a roadmap for next-generation therapeutics in autoimmune, neurodegenerative, and psychiatric diseases.
1 Introduction
The gut microbiota exerts profound influence on both the nervous and immune systems, and growing evidence suggests that its most critical function lies in shaping their bidirectional communication (1). This tripartite interaction—the gut–brain–immune axis—has emerged as a key determinant of host homeostasis (2, 3). Yet a central question remains unresolved: through what molecular and cellular pathways does the microbiota calibrate this dynamic neuroimmune dialogue, and how does disruption of this regulation precipitate disease? Addressing this challenge requires disentangling the molecular signals that enable microbes to affect immune cell behavior and neuronal activity across distinct anatomical compartments. Emerging studies highlight metabolites, microbial antigens, and neuroactive compounds as candidate mediators of these effects (4–6), yet the precise mechanisms by which such signals modulate neurological function, educate peripheral immunity, and propagate through humoral, cellular, or neural routes remain incompletely defined (7, 8). Furthermore, whether deliberate modulation of the microbiota can rewire maladaptive neuroimmune circuits in established disease is an open and pressing question. In this review, we synthesize recent advances that illuminate the mechanistic basis of microbiota–neuroimmune communication. We highlight how microbial metabolites shape immune cell fate and neuronal integrity, and how perturbations in this dialogue contribute to conditions such as multiple sclerosis and autism spectrum disorders (ASD). By reframing the microbiota as an active conductor of neuroimmune crosstalk, we aim to establish a framework that integrates microbiology, immunology, and neuroscience, and to consider how microbial ecology might be leveraged for therapeutic innovation.
2 Gut microbiota
The human gut microbiota—a complex assemblage of bacteria, archaea, viruses, fungi, and other eukaryotes—acts as an endocrine and immunomodulatory organ central to host physiology (9–11). Beyond its established roles in nutrient metabolism and colonization resistance, it is now recognized as a key regulator of systemic homeostasis through the production of diverse bioactive metabolites. These metabolites constitute the primary medium of host–microbe communication, allowing the gut community to influence distal organs, including the brain (10). The functional capacity of the microbiota, encoded by its collective metagenome and exceeding that of the human genome, is often more consequential than its taxonomic composition (12). High-throughput sequencing has revealed that despite extensive inter-individual variation, core metabolic pathways are conserved (12). Among the most important products are short-chain fatty acids (SCFAs)—acetate, propionate, and butyrate—derived from dietary fiber fermentation. SCFAs not only sustain gut health locally but also act as histone deacetylase inhibitors and ligands for G-protein–coupled receptors (e.g., GPR41, GPR43, GPR109a), thereby modulating gene expression and function in immune cells and, potentially, neurons (13). Likewise, microbial metabolism of tryptophan yields aryl hydrocarbon receptor (AHR) ligands (e.g., indole derivatives) and serotonin (5-HT) precursors, which shape immune tolerance and neuroimmune interactions (14).
The spatial organization of microbial communities further contributes to functional diversity (15). Distinct niches along the gastrointestinal tract—from the small intestine to the colon—generate region-specific metabolic outputs that educate local immune populations and influence barrier integrity at both the gut–blood and blood–brain interfaces (15). These contributions depend on ecological stability. Perturbations from diet, antibiotics, or inflammation can induce dysbiosis, often marked by the loss of SCFA-producing taxa (e.g., Faecalibacterium prausnitzii) and the overgrowth of pathobionts (16). Such shifts alter the metabolic milieu, promote production of pro-inflammatory molecules such as lipopolysaccharide (LPS), and disrupt critical signaling pathways (17). Thus, the gut microbiota is best understood not as a static collection of species but as a dynamic, metabolically active interface that translates environmental inputs, particularly diet, into host biochemical signals (18, 19). The following sections examine how this microbial organ, through its metabolic repertoire and direct host interactions, calibrates the immune system, engages in bidirectional communication with the nervous system, and orchestrates integrated gut–brain–immune crosstalk.
3 Neuro-gut microbiota interactions: the gut–brain axis
Among the most intensively investigated topics in biomedical research is the bidirectional communication between the gut microbiota and the nervous system, commonly referred to as the gut–brain axis (GBA) (20, 21). This complex, multidimensional network facilitates continuous signaling between the gastrointestinal tract and the central nervous system (CNS), influencing not only gastrointestinal physiology but also mood, cognition, behavior, and overall systemic health. The GBA encompasses four interconnected components: the gut microbiota, the central and peripheral nervous systems, the neuroendocrine system, and the immune system (22). Within the CNS, regions critical to the GBA include the prefrontal cortex, limbic system, and hypothalamic–pituitary–adrenal (HPA) axis (23). The limbic system—particularly the hippocampus—serves as a central hub for emotional regulation, memory consolidation, and behavioral adaptation, integrating neural inputs from diverse brain regions (24). The peripheral arm of the GBA comprises the autonomic nervous system (ANS), including both sympathetic and parasympathetic branches, and the enteric nervous system (ENS) (25–27). The ENS, embedded within the gastrointestinal wall, functions semi-autonomously to regulate gut motility, secretion, and local reflexes. It maintains direct anatomical and functional connections to the brain via the vagus nerve, dorsal root ganglia, and nodose ganglia, thereby constituting the structural basis for gut–brain feedback loops (28). The ANS modulates gut activity in response to systemic cues: sympathetic activation during stress suppresses motility and modulates immune responses, whereas parasympathetic input, primarily through the vagus nerve, supports digestion, mucosal immunity, and microbial equilibrium under homeostatic conditions (29). Through these integrated pathways, the gut microbiota exerts influence on neural circuits by producing neuroactive metabolites, modulating neurotransmitter availability, and shaping immune signaling. Conversely, central nervous outputs regulate microbial composition and activity via neuroendocrine signaling and autonomic efferents. This intricate bidirectional crosstalk underlies the pathophysiology of numerous disorders, including depression, anxiety, irritable bowel syndrome (IBS), and neurodevelopmental conditions (30, 31).
3.1 Neurobiological pathways linking the CNS and gut microbiota
Bidirectional communication between the CNS and gut microbiota is mediated through four principal, interconnected pathways: neural circuits, intestinal barrier (IB) integrity and inflammation, neuroendocrine signaling, and microbial modulation of neurotransmitters (Figure 1). Neural signaling constitutes the most direct and evolutionarily conserved mode of interaction (32–34). The vagus nerve, a major afferent–efferent conduit of the GBA, senses microbial metabolites—including SCFAs, tryptophan derivatives, and secondary bile acids—via its peripheral terminals, transmitting these signals to brain regions implicated in emotion and homeostasis (35, 36). Vagal afferents serve as the primary gut-to-brain channel, while efferent vagal activity regulates intestinal immune tone through the cholinergic anti-inflammatory reflex, suppressing pro-inflammatory cytokines such as TNF-α and modulating macrophage responses. Enhanced vagal tone has been associated with changes in appetite regulation, mood, and systemic inflammation (37). Complementing vagal pathways, the ENS—comprising 200–600 million neurons and extensive enteric glia—functions semi-autonomously to control gut motility, secretion, and mucosal immunity, while maintaining reciprocal communication with the CNS via spinal and vagal afferents (38–40).
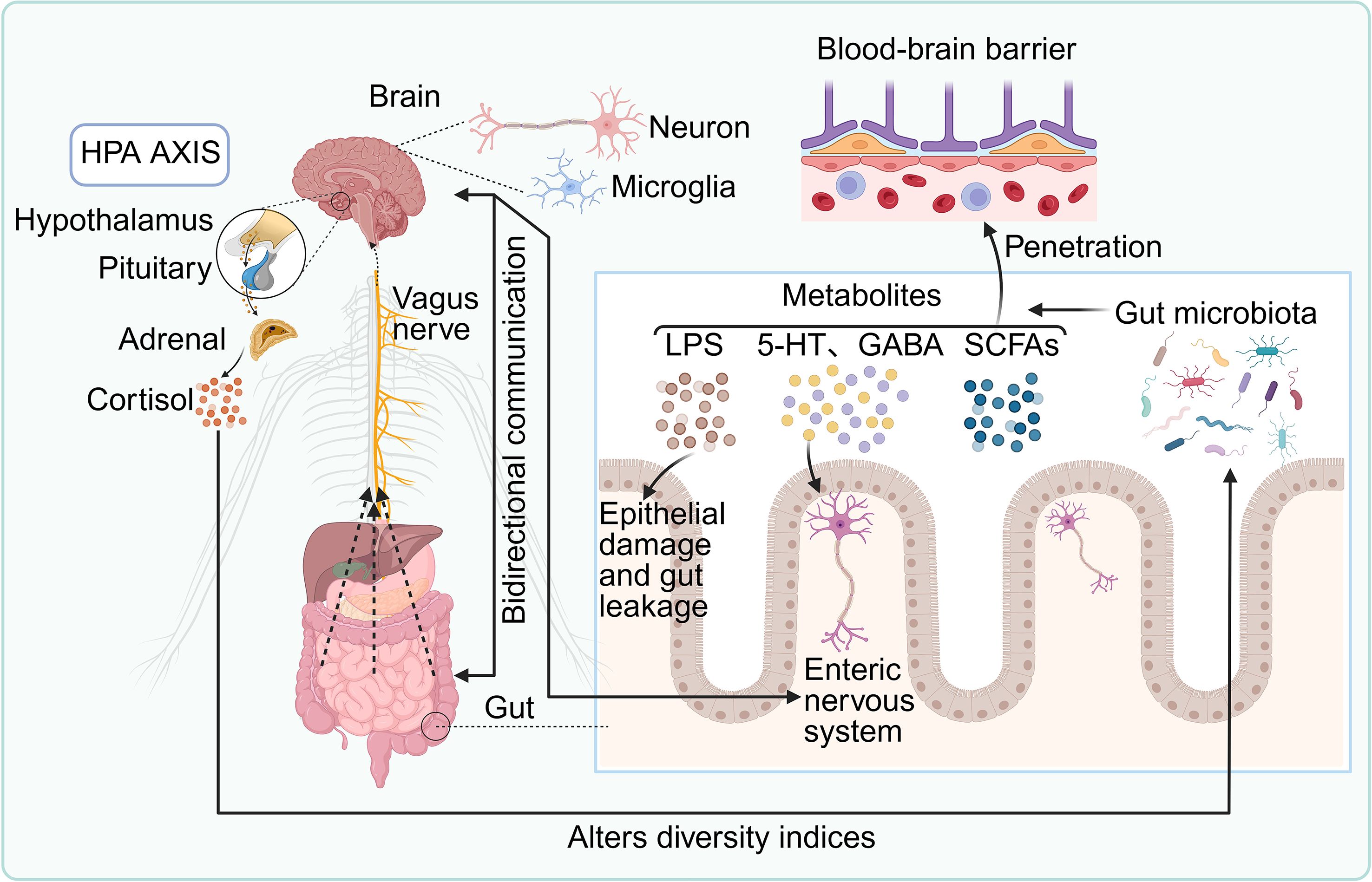
Figure 1. Bidirectional communication between the gut microbiota and the central nervous system (CNS). Microbial metabolites—including lipopolysaccharide (LPS), serotonin (5-HT), γ-aminobutyric acid (GABA), and short-chain fatty acids (SCFAs)—can cross the blood–brain barrier (BBB) to modulate neuronal and microglial function, thereby influencing brain physiology. The gut microbiota also interacts with the enteric nervous system (ENS), regulating epithelial barrier integrity and contributing to intestinal permeability. The hypothalamic–pituitary–adrenal (HPA) axis communicates with the gut directly via the vagus nerve and indirectly through cortisol secretion, which in turn modulates microbial composition. This reciprocal signaling network within the gut–brain axis (GBA) is critical for neuroimmune homeostasis and plays a central role in the pathogenesis and progression of neuroimmune disorders.
The IB–inflammation axis serves as a second key communication route. Dysbiosis or pathogen invasion can compromise tight junction integrity through downregulation of occludin, claudins, and zonula occludens-1, permitting translocation of microbial components into the lamina propria (41). Recognition of pathogen-associated molecular patterns, such as LPS, by pattern recognition receptors activates innate immune responses, triggering local and systemic inflammation (42). Immune signals then reach the CNS via humoral and neural pathways, contributing to neuroinflammation and altered brain function (43, 44).
The neuroendocrine axis, notably the HPA axis, represents the third mode of interaction. In response to stress, enteroendocrine cells and ENS neurons release hormones and neuropeptides—including corticotropin-releasing hormone (CRH), substance P, and various gut peptides—that activate the HPA axis through vagal and circulatory routes (45, 46). Cortisol, released by the adrenal cortex, modulates IB integrity, immune cell activity, and microbiota composition, forming a feedback loop that influences metabolism, immune tolerance, and neurobehavioral outcomes (47).
Finally, microbial modulation of neurotransmitters constitutes the fourth core pathway. The gut microbiota produces or modulates numerous neuroactive compounds, including catecholamines (dopamine, norepinephrine (NE), epinephrine), 5-HT, GABA, and histamine (4, 48, 49). Dopamine, primarily synthesized in the CNS, is influenced by gut microbes and local catecholamine production, impacting vascular tone, reward pathways, and stress responses in a dose-dependent manner (50, 51). Sympathetic neurons and the adrenal medulla synthesize NE and epinephrine, which, as key mediators of the ‘fight-or-flight’ response, potently suppress intestinal motility and reduce perfusion via vasoconstriction, while also altering blood flow distribution to modulate nutrient absorption and immune activity, effectively diverting energy away from digestion during stress (31, 49). Approximately 90% of 5-HT is produced in the gut, where microbial-derived SCFAs and tryptophan metabolites regulate its biosynthesis in enterochromaffin cells; 5-HT subsequently modulates CNS signaling via circulation and vagal pathways (52, 53). GABA, the principal inhibitory neurotransmitter in the CNS, is synthesized by Lactobacillus and Bifidobacterium through glutamate decarboxylase activity. Microbial GABA influences enteric neural function, visceral sensitivity, insulin secretion, and immune responses—including IL-6 suppression—and has been implicated in anxiety, depression, and IBS (54, 55). Histamine, generated by bacterial histidine decarboxylase (notably in Lactobacillus and Escherichia coli), modulates gut motility and immune tone via enteric H1–H4 receptors and may cross the BBB during inflammation, activating microglia and contributing to neuroimmune dysregulation (56, 57). While physiological histamine levels support arousal and memory, dysregulated microbial histamine production has been linked to migraines, IBS, and anxiety disorders (58).
Furthermore, indoleacetic acid (IAA), a tryptophan metabolite, plays regulatory role in neuroinflammation and neurodegeneration. Research indicates that Bacteroides fragilis, Bacteroides thetaiotaomicron, and Anaerostipes hadrus are tryptophan-metabolizing bacteria. The IAA they produce acts as a ligand for the AHR (59). By activating AHR, IAA inhibits glial cell activation and inflammatory mediator release, and suppresses the activation of the receptor for advanced glycation end products (RAGE) pathway via AHR-dependent signaling, thereby inhibiting the NF-κB signaling pathway. This reduces the expression of inflammatory cytokines (such as TNF-α, IL-1β, IL-6, and iNOS) and alleviates neuroinflammation (60). Similarly, research indicates that the synbiotic combination of Lactobacillus suilinensis and inulin increases indole-3-lactic acid synthesis, activates the AHR signaling pathway, suppresses the expression of pro-inflammatory factors like TNF-α, COX2, and IL-1β, reduces microglial activation, and alleviates systemic and CNS inflammatory responses (61). Additionally, studies have shown that gut microbiota alterations associated with mild cognitive impairment (MCI) in Down syndrome patients may induce systemic inflammation, thereby compromising BBB integrity. For example, increased Bacteroides levels correlate with elevated YKL-40 (a neuroinflammation marker), which may indirectly affect brain health by triggering immune responses and inflammatory processes (62). Collectively, these four pathways illustrate the complex, multimodal nature of CNS–microbiota communication, providing mechanistic insight into how the gut ecosystem shapes neural function and systemic health.
3.2 Gut microbiota regulation of neuroimmune function via BBB integrity
The BBB comprises brain microvascular endothelial cells (BECs), pericytes, basement membrane, and astrocytes. It maintains its structural and functional integrity through tight junctions and adherens junctions, effectively preventing harmful substances from entering the brain. Systemic inflammation can downregulate the expression of junctional proteins such as claudin-5, occludin, and VE-cadherin in BECs, thereby increasing BBB permeability (8). This process is precisely regulated by multiple signaling pathways, including Wnt/β-catenin, Sonic Hedgehog, and PDGF-β/PDGFRβ, and their dysregulation is closely associated with BBB disruption (63). Gut dysbiosis can induce intestinal inflammation, which in turn triggers systemic chronic inflammation. Inflammatory mediators such as IL-1β and TNF-α can enter the circulatory system through the compromised IB, disrupting BBB structure and facilitating the entry of inflammatory mediators or pathogens into brain tissue. This activates microglia, initiating neuroinflammatory responses (2).
Additionally, the gut microbiota influences the balance between Th17 cells and regulatory T cells (Tregs). For example, dysbiosis leads to excessive activation of Th17 cells, resulting in the production of pro-inflammatory factors such as IL-17A, IFN-γ, TNF-α, and IL-6 (64). These factors can upregulate inflammatory markers on BEC surfaces (e.g., VCAM-1 and ICAM-1), facilitating the transmigration of immune cells (such as Th17 and other CD4+ T cells) across the BBB into the brain, thereby exacerbating neuroinflammation and neurodegenerative diseases (65, 66).
In contrast to these pro-inflammatory mechanisms, certain gut microbiota metabolites—including SCFAs, bile acids, and H2S—exert beneficial effects on IB function and systemic inflammation. SCFAs possess anti-inflammatory properties, enhancing intestinal epithelial barrier integrity and suppressing systemic inflammatory responses (67). They can also cross the BBB to influence neurotransmitter levels and neuronal activity in the brain. For instance, SCFAs regulate GABAergic neurotransmission, thereby influencing mood and cognitive function (68).
3.3 Neuroendocrine regulation of host–microbiota interactions
In contrast to the well-studied bottom-up signaling from gut to brain, efferent pathways from the CNS back to the gut play an equally vital role in shaping the microbiota. The ANS and neuroendocrine signals allow the host to rapidly adapt to psychological and physiological stressors by altering the gut microenvironment (65, 69, 70). Activation of the sympathetic nervous system (SNS) under stress significantly influences gut function and microbial composition. NE released from sympathetic neurons can directly modulate bacterial growth, virulence, and gene expression (71). Certain pathogens, including Escherichia coli and Salmonella enterica, express adrenergic receptors that detect NE and enhance their growth and pathogenicity (72). Chronic stress-induced SNS activation promotes dysbiosis, reducing microbial diversity and depleting beneficial taxa such as Lactobacillus, likely through NE-mediated growth inhibition and changes in intestinal motility and mucus secretion (73, 74).
Conversely, the parasympathetic nervous system—primarily through the vagus nerve—supports a “rest-and-digest” state that favors commensal microbes. Vagal efferent activity enhances intestinal motility, stimulates epithelial secretion, and helps maintain mucosal barrier integrity (75, 76). Its cholinergic anti-inflammatory pathway further suppresses local immune activation, fostering a less inflammatory microbial environment (77). Vagotomy studies confirm that vagal signaling directly influences microbial community structure, underscoring its role in maintaining gut ecological balance (78, 79). Neuroendocrine hormones also serve as key mediators of brain-to-gut communication. CRH and glucocorticoids (cortisol in humans, corticosterone in rodents)—core components of the HPA axis—modulate gut function during stress. Glucocorticoids can inhibit the growth of certain commensals (80), compromise IB function (“leaky gut”) (81), and alter bile acid profiles, thereby applying selective pressure on the microbiota (82). Other stress hormones, including catecholamines, enter the gut lumen and may be used by bacteria as nutrients or signals, further influencing microbial ecology (83). Together, these top-down mechanisms ensure continuous adjustment of the gut microbiota by the host’s central state, forming a feedback loop in which cognitive and emotional states rapidly affect microbial composition and function, which in turn modulate brain activity.
3.4 Gut microbiota dysbiosis and neurological disorders
Accumulating evidence implicates gut microbiota dysbiosis in the pathogenesis of several major neurodegenerative and neuroinflammatory disorders, including Parkinson’s disease (PD), Alzheimer’s disease (AD), and MS (84). Alterations in microbial composition and metabolite profiles may affect neural health through immune activation, disruption of BBB integrity, altered neurotransmitter synthesis, and modulation of neuroinflammation and host metabolism.
3.4.1 Parkinson’s disease
PD is clinically characterized by progressive motor impairments—bradykinesia, resting tremor, and rigidity—as well as non-motor symptoms such as depression, cognitive decline, and autonomic dysfunction (85). Increasing studies reveal shifts in gut microbiota composition associated with PD. Compared to healthy controls, patients exhibit reduced abundance of anti-inflammatory and SCFA-producing taxa, including Lachnospiraceae, Prevotellaceae, Faecalibacterium, and Bacteroides, alongside increased levels of Akkermansia, Bifidobacterium, and Lactobacillus, taxa linked to LPS and toxin production (86, 87). The observed increase in typically beneficial genera (Akkermansia, Bifidobacterium, Lactobacillus) is not necessarily contradictory but reflects a broader ecological shift towards a pro-inflammatory state in PD. Their association with LPS and toxin-related pathology is primarily indirect and context-dependent (88, 89). For example, Akkermansia muciniphila is a specialized mucin-degrader. While beneficial in homeostasis, its overgrowth can degrade the protective mucus layer. This erosion facilitates the translocation of pro-inflammatory microbial products, including LPS from other Gram-negative bacteria, into the host system, potentially triggering systemic inflammation (88, 90). Bifidobacterium and Lactobacillus are Gram-positive and do not produce LPS. Their increase may occur alongside unmeasured expansions of LPS-producing pathobionts (e.g., Enterobacteriaceae) (91). Thus, they serve as a marker of dysbiosis rather than a direct source of LPS (17).
This microbial imbalance compromises IB integrity, increasing gut permeability and systemic inflammation, which may exacerbate CNS dysfunction (92, 93). A hallmark of PD pathology is the misfolding and aggregation of α-synuclein (α-syn) within enteric neurons, potentially originating in the submucosal plexus or olfactory bulb and propagating to the brainstem via retrograde transport along the vagus nerve (94). Transplantation of fecal microbiota from PD patients into mice worsens α-syn aggregation and motor deficits, supporting the gut-origin hypothesis (10). Recent studies indicate that PD patients exhibit an increased abundance of Streptococcus mutans (S. mutans) in their gut. This bacterium utilizes uridine reductase to produce the metabolite imidazole propionate (ImP), which can cross the BBB and enter the brain. Within the brain, ImP activates the mTORC1 signaling pathway, contributing to the specific loss of dopaminergic neurons—a hallmark of PD—and consequently triggering motor dysfunction (95). Furthermore, ImP exacerbates α-syn pathology by promoting its aggregation, which further intensifies neurotoxicity. Additionally, ImP activates microglia (as evidenced by enlarged cell bodies) and induces astrocyte activation, thereby driving neuroinflammation (95). Microbial-derived SCFAs can activate neuroprotective pathways by inducing glial cell-derived neurotrophic factor and brain-derived neurotrophic factor (BDNF), while dysbiosis also affects ghrelin and other neuropeptide signaling, further modulating disease progression (96).
3.4.2 Alzheimer’s disease
AD, the leading cause of dementia, is defined by extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein (97). Patients with AD show distinct gut microbial alterations characterized by depletion of key SCFA-producing taxa, particularly butyrate-producing members of the families Lachnospiraceae and Ruminococcaceae (within the phylum Bacillota) as well as the genus Bifidobacterium (98). This is accompanied by an increased abundance of pro-inflammatory bacteria, including those within the phylum Proteobacteria (e.g., Escherichia coli). This dysbiosis reduces neuroprotective metabolites like butyrate and increases endotoxin release (e.g., LPS), which activates Toll-like receptor 4 (TLR4) signaling, triggering peripheral immune responses and elevating pro-inflammatory cytokines IL-6 and TNF-α (99).
These cytokines cross the BBB or signal via neural pathways to activate microglia, sustaining neuroinflammation and accelerating Aβ deposition and tau hyperphosphorylation—key drivers of neurodegeneration (100). Compared to healthy individuals, patients with AD and those with MCI exhibit higher levels of bacterial extracellular vesicles (bEVs) in their blood. These bEVs, derived from the gut microbiota, contain elevated concentrations of LPS (101). It is proposed that these LPS-bearing bEVs can traverse the BBB. Upon entering the brain, the LPS cargo activates the Piezo1 mechanosensitive channel on microglia. This Piezo1 activation triggers the classical complement cascade, specifically the C1q-C3 pathway, which leads to excessive synaptic pruning (101). This aberrant microglial activity, driven by gut microbiota-derived bEVs, represents a novel mechanism contributing to synaptic loss and neuroinflammation in AD. Gut microbes also influence neurotransmitter bioavailability (dopamine, GABA, 5-HT, acetylcholine (ACh), melatonin) and affect autophagy, oxidative stress, and glial function, all implicated in AD pathology (21).
3.4.3 Multiple sclerosis
MS is an autoimmune demyelinating disorder of the CNS, shaped by genetic susceptibility, environmental factors, and immune dysregulation (102). MS patients exhibit altered gut microbial profiles compared to healthy controls, marked by reduced levels of Bacteroides, Prevotella, Faecalibacterium, Lactobacillus, and Clostridium, alongside increased abundance of Pseudomonas, Haemophilus, and Mycoplasma (103–105). Dysbiosis contributes to an imbalance between pro-inflammatory Th17 cells and anti-inflammatory Tregs. Segmented filamentous bacteria (SFB) promote Th17 differentiation and secretion of IL-17 and IL-22, exacerbating CNS inflammation, whereas SCFA-producing Clostridia are diminished, reducing Treg-mediated immunosuppression (66). Molecular mimicry between microbial antigens—such as from Klebsiella pneumoniae—and CNS myelin proteins (e.g., myelin oligodendrocyte glycoprotein) may activate autoreactive B cells (106). Microbial products like LPS increase BBB permeability and stimulate peripheral macrophages to release pro-inflammatory cytokines (5, 107). Additionally, tryptophan-derived microbial metabolites modulate microglial activation via vagal afferents, while increased BBB permeability facilitates CNS infiltration by CD4+ T cells (108–110). Collectively, these pathways underscore the multifactorial role of gut microbiota in MS pathogenesis, encompassing immune modulation, barrier disruption, and neuroinflammatory cascades.
3.5 Gut microbiota in neuropsychiatric disorders
Beyond classical neurodegenerative diseases, alterations of the gut microbiota are increasingly implicated in major neuropsychiatric disorders, including depression, anxiety, and ASD. In these contexts, microbial dysbiosis does not simply accompany disease but contributes to pathogenesis by reshaping systemic immunity, modulating neurotransmitter pathways, and altering neural signaling via microbial metabolites.
3.5.1 Depression and anxiety
Clinical and preclinical evidence supports a bidirectional relationship between dysbiosis and mood disorders. Patients with major depressive disorder (MDD) or anxiety frequently exhibit reduced microbial richness, characterized by depletion of butyrate-producing taxa such as Faecalibacterium and Coprococcus, alongside expansion of pro-inflammatory families like Enterobacteriaceae (111, 112). Causality has been demonstrated by fecal microbiota transplantation (FMT) from depressed individuals into germ-free rodents, which transfers depression-like phenotypes (113). Mechanistically, dysbiosis influences neuropsychiatric states through several converging pathways. Mechanistically, gut dysbiosis contributes to neuropsychiatric disorders through converging immune, metabolic, and neural pathways. Translocation of microbial products such as LPS elevates systemic pro-inflammatory cytokines (e.g., IL-6, TNF-α), creating an inflammatory milieu that impairs hippocampal neurogenesis and reduces BDNF, both essential for mood regulation (2). In parallel, disruption of microbial communities alters tryptophan metabolism, diverting it from 5-HT synthesis toward the kynurenine pathway and producing neurotoxic metabolites that exacerbate neuroinflammation (114). Additionally, specific probiotic strains, including Lactobacillus and Bifidobacterium, exert vagus-dependent effects on the HPA axis, reducing cortisol levels and mitigating anxiety-like behaviors (115). Together, these pathways reveal how dysbiosis can both initiate and amplify the neuroimmune and neuroendocrine disturbances characteristic of depression and anxiety.
3.5.2 Autism spectrum disorder
Gut microbiota disturbances are also prominent in ASD, where gastrointestinal comorbidities are frequent. Children with ASD often display increased abundance of Clostridium and decreased Bifidobacterium species (116, 117). Preclinical studies indicate a causal role: maternal immune activation induces offspring dysbiosis and ASD-like behavioral abnormalities, which can be ameliorated by probiotic administration of Bacteroides fragilis (116–118). Microbial metabolites emerge as critical mediators. Elevated levels of 4-ethylphenylsulfate, a microbial-derived aromatic compound, induce anxiety-like behaviors in mice (118). Similarly, propionic acid, an abundant SCFA, exerts dose-dependent effects—supporting gut physiology at low concentrations but provoking neuroinflammation and ASD-like behaviors when present in excess (119). Perturbations in bile acid metabolism and reductions in microbial-derived GABA and 5-HT precursors have also been described, linking dysbiosis to disrupted neurotransmitter homeostasis.
4 Gut microbiota–immune system crosstalk
Disruptions within the microbiota–immune axis have been linked to a broad array of immune-mediated disorders, including gastrointestinal infections, inflammatory bowel disease (IBD), metabolic conditions such as cardiovascular disease, diabetes, and hypertension, autoimmune diseases including rheumatoid arthritis, hypersensitivity reactions like asthma and food allergies, neuropsychiatric disorders such as anxiety, and malignancies including colorectal and hepatocellular carcinoma.
4.1 Microbiota-mediated immune development and homeostasis
Studies employing GF animal models have unequivocally demonstrated that the gut microbiota is indispensable for postnatal immune maturation (120). GF mice display profound immunodeficiencies, including impaired differentiation of Th17 cells, an imbalance in Tregs, underdeveloped Peyer’s patches, and reduced IgA production (121, 122). Colonization with defined microbial taxa rescues these defects, underscoring the causal role of microbiota in immune programming. For example, SFB promote Th17 differentiation via the IL-23/IL-1β axis, while Bacteroides fragilis produces polysaccharide A (PSA) that activates dendritic cells to expand Tregs and foster immune tolerance (123). Similarly, Clostridium species enhance Treg differentiation through SCFAs, particularly butyrate, via epigenetic mechanisms such as histone acetylation (124). Additionally, Bacteroides and probiotic strains like Escherichia coli Nissle 1917 facilitate B cell maturation and immunoglobulin class switching (IgA, IgG), supporting mucosal defense (124). These interactions occur both through direct microbe–immune cell contact and indirectly via microbial metabolites and pattern recognition receptor (PRR) signaling pathways, including Toll-like receptors and NOD-like receptors (Figure 2) (125). Collectively, these processes establish a finely tuned immune milieu balancing host defense and tolerance. The capacity of specific microbial strains to differentially modulate immune pathways highlights their potential as therapeutic agents. Rational probiotic design, metabolite supplementation (e.g., SCFAs), and precision microbial consortia represent promising immunomodulatory strategies for autoimmune, allergic, and inflammatory diseases.
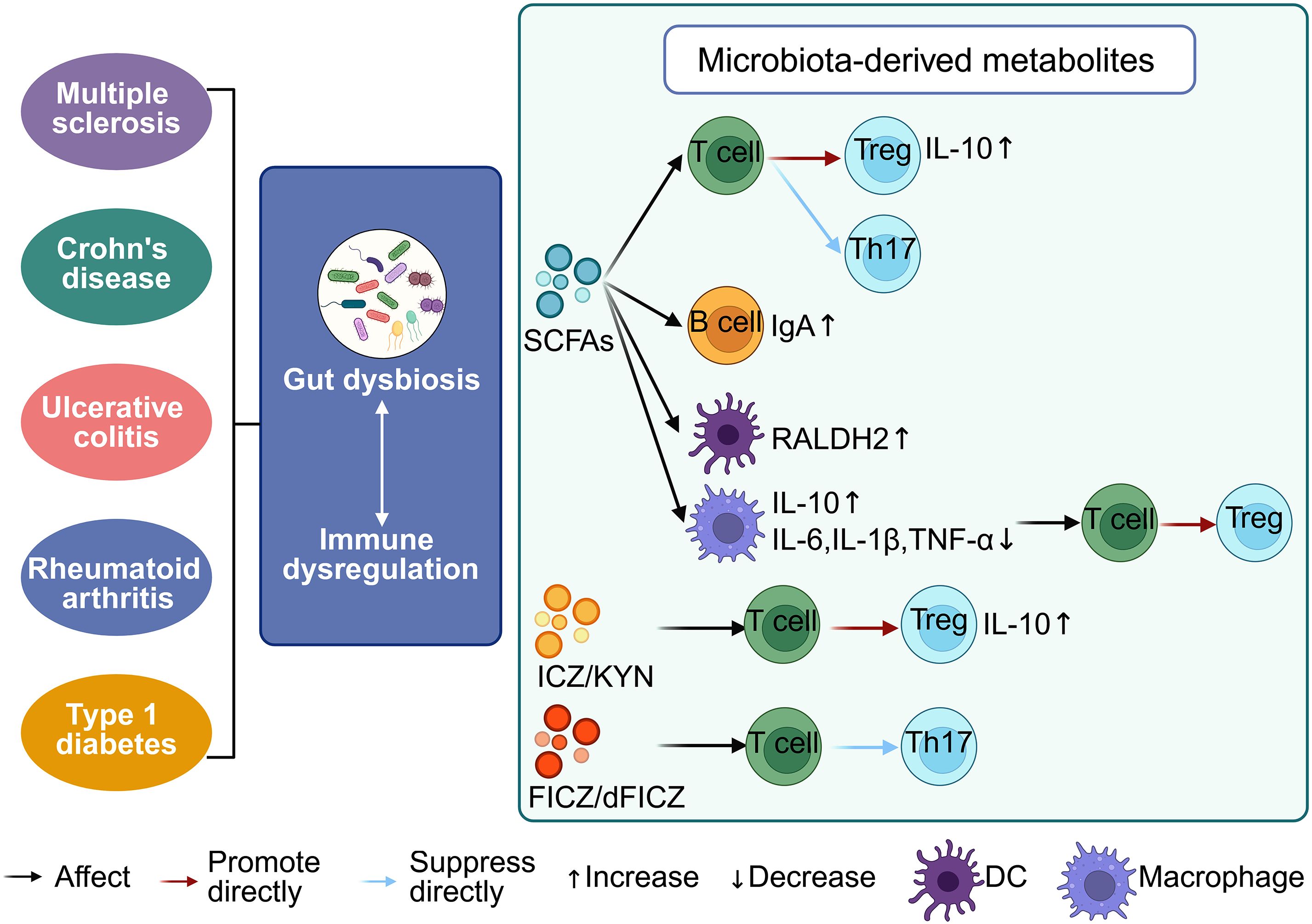
Figure 2. Interaction between the gut microbiota and the host immune system. Gut microbiota dysbiosis disrupts immune homeostasis, contributing to the pathogenesis of autoimmune diseases such as multiple sclerosis, Crohn’s disease, ulcerative colitis (UC), and rheumatoid arthritis. Microbial metabolites—including short-chain fatty acids (SCFAs), indole derivatives like indolo [3, 2-b] carbazole (ICZ), kynurenine (KYN), and 6-formylindolo [3, 2-b] carbazole (FICZ/dFICZ)—modulate immune cell function by influencing cytokine profiles and T cell differentiation. SCFAs suppress pro-inflammatory cytokines (IL-6, IL-1β, TNF-α), enhance anti-inflammatory IL-10 production, and promote IgA secretion by B cells. Both SCFAs and ICZ/KYN drive Treg differentiation, whereas FICZ/dFICZ inhibit Th17 cell development, underscoring the immunoregulatory potential of microbiota-derived metabolites.
4.2 Microbial metabolites as immunomodulators
Microbial-derived metabolites form a critical interface between the gut microbiota and host immunity, orchestrating immune tolerance, inflammatory responses, and cellular metabolism (126). The gut microbiota exerts profound influence on brain and neuroimmune function by modulating key neurotransmitter pathways (127). The GABAergic/Glutamatergic balance is a prime target, with microbes producing inhibitory GABA and consuming excitatory glutamate precursors, thereby regulating neuronal network activity (128). Simultaneously, microbial SCFAs enhance cholinergic function, which supports synaptic plasticity and provides potent anti-inflammatory signals via the cholinergic anti-inflammatory pathway (129). The serotonergic and dopaminergic systems are similarly modulated, as microbes supply essential precursors (tryptophan, tyrosine) and bioactive metabolites that influence the synthesis of serotonin and dopamine (53, 130). These monoamines subsequently regulate neuroimmune cells, such as microglia, and refine synaptic architecture, ultimately integrating gut-derived signals with central control of behavior, plasticity, and immunity (131). To provide a comprehensive overview of the roles of microbiota and their metabolites in neuroimmune function, we summarize key findings in Table 1.
4.2.1 GABA
As the principal inhibitory neurotransmitter in the CNS, GABA can also be synthesized by commensal bacteria such as Lactobacillus, Bifidobacterium, and Bacteroides. Beyond regulating neuronal excitability and anxiety-related behaviors, microbial GABA exerts direct immunoregulatory effects. Engagement of GABA-A receptors on T cells and macrophages dampens pro-inflammatory cytokine production (IL-6, IL-1β) and restrains T-cell proliferation, thereby attenuating systemic inflammation and autoimmune responses (152). In addition, GABA promotes Treg differentiation and biases microglia toward an anti-inflammatory phenotype, underscoring its dual role in maintaining immune tolerance and neural homeostasis.
4.2.2 5-HT
Over 90% of the body’s 5-HT is synthesized in the gut by enterochromaffin (EC) cells, which convert dietary L-tryptophan into 5-HT through the sequential actions of tryptophan hydroxylase 1 (TPH1) and aromatic L-amino acid decarboxylase (153). Certain intestinal bacteria, such as Bifidobacterium, Lactobacillus, and Clostridium species, possess tryptophan decarboxylase activity that enables them to directly convert dietary tryptophan into tryptamine or 5-hydroxytryptophan (154). These intermediates can then be absorbed by EC cells and further decarboxylated to complete 5-HT biosynthesis—essentially acting as a “pre-processing” step for serotonin precursors. In addition, microbial metabolites such as SCFAs can activate free fatty acid receptors 2 and 3 on EC cells, thereby upregulating TPH1 expression and enhancing tetrahydrobiopterin availability, which collectively increase 5-HT production (53). Other microbial products—including secondary bile acids (155), indole derivatives (153), and various cofactors—along with immuno-inflammatory modulation (156), can further activate TPH1 or L-amino acid decarboxylase activity, promoting serotonin synthesis within EC cells. SCFAs (53). Subsequently, this gut-derived 5-HT mediates key functions like propulsive motility by activating local receptors including the 5-HT4 receptor. Peripheral 5-HT influences immune function by acting on a broad spectrum of receptors (5-HT1A, 5-HT2A, 5-HT3, 5-HT4, 5-HT7) expressed on dendritic cells, T cells, and macrophages, thereby regulating cytokine secretion and T-cell fate decisions (157). In the CNS, 5-HT derived from microbial precursors directly influences microglial activity and synaptic remodeling, evidenced by suppressing pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and modulating the phagolysosomal compartment, while concurrently enhancing markers of synaptic plasticity such as BDNF and promoting dendritic spine complexity (2). Altered microbial control of serotonergic pathways has been linked to both neuroinflammation and psychiatric disease (114).
4.2.3 Dopamine
Gut microbiota, including genera such as Bacillus, Escherichia, and Enterococcus, can directly synthesize dopamine or convert dietary precursors like the amino acid tyrosine and L-dopa into dopamine, influencing local gut signalling and contributing to systemic dopaminergic pools (158). Immune cells express dopamine receptors (DRD1–DRD5), allowing dopamine to modulate both innate and adaptive responses. Low-dose signaling through these receptors generally promotes anti-inflammatory outcomes, including reduced T-cell activation and diminished release of cytokines such as TNF-α and IFN-γ (159). In the CNS, microbial-derived dopamine precursors contribute to dopaminergic tone, influencing motor control, reward processing, and motivation. Perturbations in this axis have been implicated in Parkinson’s disease, depression, and inflammatory disorders (137).
4.2.4 ACh
Certain commensal taxa, including Lactobacillus plantarum, produce Ach, a neurotransmitter central to both peripheral and central physiology (138). In the periphery, ACh is a critical mediator of the vagus nerve–driven “cholinergic anti-inflammatory pathway.” By engaging nicotinic and muscarinic receptors on macrophages, ACh suppresses the release of pro-inflammatory cytokines (TNF-α, IL-1β), thereby limiting systemic inflammation and protecting against septic shock. Within the CNS, cholinergic signaling is essential for learning, memory, and attention, and microbial modulation of this pathway may provide a mechanistic link between gut homeostasis, cognition, and neuroimmune balance (139).
4.2.5 SCFAs
SCFAs—primarily acetate, propionate, and butyrate—are generated by microbial fermentation of dietary fibers such as resistant starch and inulin. Butyrate exhibits potent anti-inflammatory effects by inhibiting NF-κB activation in intestinal epithelial cells, suppressing pro-inflammatory cytokines including IL-6, IL-1β, and TNF-α (160). Concurrently, butyrate functions as a histone deacetylase inhibitor, promoting transcription of anti-inflammatory genes (133). In vivo, butyrate enhances epithelial barrier integrity by stimulating mucin production, inducing goblet cell differentiation, and promoting epithelial turnover, thereby limiting translocation of inflammatory stimuli (133).
SCFAs modulate both innate and adaptive immunity via G-protein-coupled receptors (GPR41, GPR43, GPR109A) expressed on neutrophils, dendritic cells, and T cells, influencing chemotaxis, phagocytosis, and cytokine secretion (161). Activation of GPR43 on neutrophils enhances reactive oxygen species production and pathogen clearance, while GPR109A signaling in dendritic cells increases retinoic acid synthesis, promoting a tolerogenic phenotype (162). Notably, SCFAs facilitate differentiation of peripheral Tregs, reinforcing immune tolerance and preventing autoimmunity (163).
Metabolically, SCFAs serve as energy substrates for colonocytes and immune cells. Butyrate undergoes β-oxidation within intestinal epithelial mitochondria, supporting ATP production and epithelial homeostasis (164). In T cells, SCFAs influence metabolic reprogramming by elevating intracellular acetyl-CoA and activating AMP-activated protein kinase, which favors oxidative phosphorylation over glycolysis. This shift promotes differentiation of Tregs and memory T cells while restraining pro-inflammatory Th1 and Th17 subsets (164). Furthermore, SCFAs suppress mTOR signaling, reducing inflammatory T cell proliferation and enhancing anti-inflammatory responses (165).
4.2.6 Tryptophan metabolites
Tryptophan metabolism by gut microbes produces immunologically active compounds including indole derivatives (e.g., indole-3-acetic acid, indole [3, 2-b] carbazole), kynurenine (KYN), and downstream metabolites such as 3-hydroxykynurenine (166). Many act via the aryl AHR, a ligand-activated transcription factor broadly expressed in immune and epithelial cells (167). Upon ligand binding, AHR translocates to the nucleus, modulating gene expression linked to immune cell development and cytokine production. Commensal-derived indole derivatives like ICZ and FICZ activate AHR to suppress Th17 polarization and expand Tregs, thereby attenuating mucosal inflammation (136). Concurrently, AHR signaling regulates dendritic cell and macrophage maturation and function, fine-tuning antigen presentation and maintaining immune balance (136).
KYN pathway metabolites also influence adaptive immunity. KYN and 3-HK, endogenous AHR ligands, promote Foxp3 expression, facilitating Treg differentiation and creating an immunosuppressive environment (168). These tryptophan metabolites form a critical communication axis integrating microbial composition, metabolic output, and host inflammatory status. Thus, microbial metabolites such as SCFAs and tryptophan derivatives represent essential molecular mediators within the gut-immune axis, modulating immune cell programming and metabolic pathways with significant therapeutic implications.
Collectively, these microbiota-derived neurotransmitters exemplify the biochemical integration of microbial metabolism with host neuroimmune regulation. They not only facilitate direct communication between the gut and the brain but also calibrate immune responses, thereby influencing susceptibility to neuroinflammatory, autoimmune, and psychiatric disorders. Targeting the synthesis, reception, or degradation of these microbial neurotransmitters offers promising therapeutic avenues for diseases characterized by neuroimmune dysregulation.
5 Therapeutic implications and microbiota-targeted interventions
Elucidation of the gut–brain–immune axis provides a compelling rationale for microbiota-targeted therapies in neuroimmune disorders. Current strategies aim to reverse dysbiosis and restore homeostasis mainly through two primary approaches:
i. Microbiota-targeted supplementation. This includes the use of prebiotics, probiotics, engineered live biotherapeutic products (LBPs), and postbiotics. Prebiotic fibers (e.g., inulin) selectively promote beneficial commensals and enhance production of immunomodulatory metabolites such as SCFAs (169, 170). Defined probiotics or LBPs (e.g., Faecalibacterium prausnitzii, Clostridium clusters IV and XIVa, and specific Lactobacillus or Bifidobacterium strains) reintroduce keystone taxa to enhance SCFA production, promote Treg differentiation (171, 172), strengthen gut integrity (173), and modulate neuroactive signaling (e.g., GABAergic pathways) (115). Alternatively, direct administration of microbial metabolites (postbiotics) such as SCFAs (e.g., sodium butyrate) or tryptophan derivatives (e.g., indole-3-propionic acid) offers a non-living strategy to directly influence host immunity and barrier function (109, 174).
ii. Ecological Restoration via FMT. FMT seeks to holistically restore a healthy microbial community, thereby concurrently improving metabolic output, epithelial barrier function, and immune regulation. Although FMT is most established in gastrointestinal conditions, preclinical evidence supports its potential to ameliorate neuroinflammatory and behavioral phenotypes in models of MS and AD (65, 70).
Clinical implementation of these interventions faces challenges, including microbiota heterogeneity, limited engraftment efficiency of biotherapeutics, and interactions with conventional immunotherapies. Future efforts should emphasize personalized strategies based on deep phenotyping of host–microbiota interactions to enable precision modulation of neuroimmune activity.
6 Conclusion and future perspectives
The intricate interplay among the gut microbiota, immune system, and CNS—often framed as the “gut–brain–immune axis”—is increasingly recognized as a central regulatory network governing human health and disease. Gut microbes not only sustain intestinal homeostasis but also engage in dynamic, bidirectional communication with the brain through neuroactive metabolites, microbial signaling molecules, and immune modulation. This complex ecosystem profoundly influences the pathogenesis of neurodegenerative diseases, autoimmune disorders, and metabolic syndromes.
Recent advances have illuminated pivotal roles for microbial metabolites such as SCFAs and tryptophan derivatives in orchestrating host immunity and neuronal function. Yet, critical mechanistic questions remain unresolved. In particular, the molecular integration of microbiota-derived signals in immune cell programming, BBB integrity, and neuroinflammatory pathways requires deeper elucidation. Although microbial dysbiosis holds promise as a biomarker for disease diagnosis, its predictive accuracy for early detection or clinical progression mandates validation through comprehensive, longitudinal cohort studies.
Moving forward, research should emphasize high-resolution dissection of host–microbe interactions using single-cell and spatial transcriptomics, paired with functional validation in GF animal models and human organoid systems. Such integrative approaches have the potential to reveal novel microbial effectors and host pathways that drive disease onset and progression. Concurrently, development of precision microbiome-based diagnostics and therapeutics—including engineered probiotics, tailored microbial consortia, and bioactive postbiotics—will be essential to achieve individualized modulation of immune and neural functions based on personal microbiota and immune profiles.
Moreover, the gut microbiome exhibits remarkable plasticity, responding dynamically to environmental factors such as diet, lifestyle, antibiotics, and stress. Deciphering how these external influences reshape microbial communities and their functional outputs will be critical for designing sustainable, non-invasive interventions. Integrative multi-omics and systems biology frameworks will play an indispensable role in capturing the temporal and physiological complexity of host–microbiota crosstalk.
In summary, the gut microbiota represents a frontier of biomedicine with transformative potential to advance immune regulation and neuroprotection. Continued interdisciplinary efforts promise to uncover novel biomarkers, therapeutic targets, and personalized strategies that will redefine prevention, diagnosis, and treatment paradigms for immune-mediated and neurodegenerative diseases.
Author contributions
HO: Project administration, Writing – original draft. YY: Funding acquisition, Writing – review & editing. XZ: Funding acquisition, Writing – review & editing. YC: Conceptualization, Supervision, Writing – review & editing. YZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no. 81971726), and the Nanjing Healthcare Science and Technology Development Special Funded Project (YKK23219; YKK24224).
Acknowledgments
We created Figures 1, 2 using the online tool BioRender, and these images were authorized through a paid license from http://BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keane L, Clarke G, and Cryan JF. A Role for Microglia in Mediating the Microbiota-Gut-Brain Axis. Nat Rev Immunol. (2025) 25:847–61. doi: 10.1038/s41577-025-01188-9
2. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the Cns. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
3. Fung TC, Olson CA, and Hsiao EY. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat Neurosci. (2017) 20:145–55. doi: 10.1038/nn.4476
4. De Palma G, Shimbori C, Reed DE, Yu Y, Rabbia V, Lu J, et al. Histamine Production by the Gut Microbiota Induces Visceral Hyperalgesia through Histamine 4 Receptor Signaling in Mice. Sci Trans Med. (2022) 14:eabj1895. doi: 10.1126/scitranslmed.abj1895
5. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation Via the Aryl Hydrocarbon Receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
6. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Sci (New York NY). (2011) 332:974–7. doi: 10.1126/science.1206095
7. Bonaz B, Bazin T, and Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. (2018) 12:49. doi: 10.3389/fnins.2018.00049
8. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci Trans Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
9. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat Med. (2014) 20:159–66. doi: 10.1038/nm.3444
10. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. (2016) 167:1469–80.e12. doi: 10.1016/j.cell.2016.11.018
11. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, and Gordon JI. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
12. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
13. Du Y, He C, An Y, Huang Y, Zhang H, Fu W, et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int J Mol Sci. (2024) 25:7379. doi: 10.3390/ijms25137379
14. Gheorghe CE, Martin JA, Manriquez FV, Dinan TG, Cryan JF, and Clarke G. Focus on the Essentials: Tryptophan Metabolism and the Microbiome-Gut-Brain Axis. Curr Opin Pharmacol. (2019) 48:137–45. doi: 10.1016/j.coph.2019.08.004
15. Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated Cd8(+) T Cells. Immunity. (2019) 51:285–97.e5. doi: 10.1016/j.immuni.2019.06.002
16. Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-Resistant Enterococcus Domination of Intestinal Microbiota Is Enabled by Antibiotic Treatment in Mice and Precedes Bloodstream Invasion in Humans. J Clin Invest. (2010) 120:4332–41. doi: 10.1172/jci43918
17. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
18. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
19. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. (2018) 174:1388–405.e21. doi: 10.1016/j.cell.2018.08.041
20. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology. (2011) 141:599–609. doi: 10.1053/j.gastro.2011.04.052
21. Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, et al. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal transduction targeted Ther. (2024) 9:37. doi: 10.1038/s41392-024-01743-1
22. Carabotti M, Scirocco A, Maselli MA, and Severi C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann Gastroenterol. (2015) 28:203–9.
23. Truyens M, Lernout H, De Vos M, Laukens D, and Lobaton T. Unraveling the Fatigue Puzzle: Insights into the Pathogenesis and Management of Ibd-Related Fatigue Including the Role of the Gut-Brain Axis. Front IN Med. (2024) 11:1424926. doi: 10.3389/fmed.2024.1424926
24. Lehmann H, Lacanilao S, and Sutherland RJ. Complete or Partial Hippocampal Damage Produces Equivalent Retrograde Amnesia for Remote Contextual Fear Memories. Eur J Neurosci. (2007) 25:1278–86. doi: 10.1111/j.1460-9568.2007.05374.x
25. Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria Activate Sensory Neurons That Modulate Pain and Inflammation. Nature. (2013) 501:52–7. doi: 10.1038/nature12479
26. Bonaz BL and Bernstein CN. Brain-Gut Interactions in Inflammatory Bowel Disease. Gastroenterology. (2013) 144:36–49. doi: 10.1053/j.gastro.2012.10.003
27. Forsythe P and Kunze WA. Voices from Within: Gut Microbes and the Cns. Cell Mol Life sciences: CMLS. (2013) 70:55–69. doi: 10.1007/s00018-012-1028-z
28. Udit S and Gautron L. Molecular Anatomy of the Gut-Brain Axis Revealed with Transgenic Technologies: Implications in Metabolic Research. Front IN Neurosci. (2013) 7:134. doi: 10.3389/fnins.2013.00134
29. Jacobson A, Yang D, Vella M, and Chiu IM. The Intestinal Neuro-Immune Axis: Crosstalk between Neurons, Immune Cells, and Microbes. Mucosal Immunol. (2021) 14:555–65. doi: 10.1038/s41385-020-00368-1
30. Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, et al. The Microbiome Regulates Amygdala-Dependent Fear Recall. Mol Psychiatry. (2018) 23:1134–44. doi: 10.1038/mp.2017.100
31. Charitos IA, Inchingolo AM, Ferrante L, Inchingolo F, Inchingolo AD, Castellaneta F, et al. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients. (2024) 16:4404. doi: 10.3390/nu16244404
32. Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell. (2019) 177:1600–18.e17. doi: 10.1016/j.cell.2019.05.004
33. Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, et al. Gaba-Producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol Motil. (2017) 29:e12904. doi: 10.1111/nmo.12904
34. Ojeda J, Avila A, and Vidal PM. Gut Microbiota Interaction with the Central Nervous System Throughout Life. J OF Clin Med. (2021) 10:1299. doi: 10.3390/jcm10061299
35. Goswami C, Iwasaki Y, and Yada T. Short-Chain Fatty Acids Suppress Food Intake by Activating Vagal Afferent Neurons. J Nutr Biochem. (2018) 57:130–5. doi: 10.1016/j.jnutbio.2018.03.009
36. Matteoli G and Boeckxstaens GE. The Vagal Innervation of the Gut and Immune Homeostasis. Gut. (2013) 62:1214–22. doi: 10.1136/gutjnl-2012-302550
37. Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, et al. Modulation of Tnf Release by Choline Requires Alpha7 Subunit Nicotinic Acetylcholine Receptor-Mediated Signaling. Mol Med (Cambridge Mass). (2008) 14:567–74. doi: 10.2119/2008-00079.Parrish
38. Cipriani G, Terhaar ML, Eisenman ST, Ji S, Linden DR, Wright AM, et al. Muscularis Propria Macrophages Alter the Proportion of Nitrergic but Not Cholinergic Gastric Myenteric Neurons. Cell Mol Gastroenterol Hepatol. (2019) 7:689–91.e4. doi: 10.1016/j.jcmgh.2019.01.005
39. Baghdadi MB and Kim TH. The Multiple Roles of Enteric Glial Cells in Intestinal Homeostasis and Regeneration. Semin Cell Dev Biol. (2023) 150-151:43–9. doi: 10.1016/j.semcdb.2023.01.005
40. Fleming MA 2nd, Ehsan L, Moore SR, and Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract. (2020) 2020:8024171. doi: 10.1155/2020/8024171
41. Poritz LS, Harris LR, Kelly AA, and Koltun WA. Increase in the Tight Junction Protein Claudin-1 in Intestinal Inflammation. DIGESTIVE Dis AND Sci. (2011) 56:2802–9. doi: 10.1007/s10620-011-1688-9
42. Hattori N and Yamashiro Y. The Gut-Brain Axis. Ann OF Nutr AND Metab. (2021) 77:1–3. doi: 10.1159/000512226
43. Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, et al. Systemic Inflammation Induces Acute Working Memory Deficits in the Primed Brain: Relevance for Delirium. Neurobiol Aging. (2012) 33:603–16.e3. doi: 10.1016/j.neurobiolaging.2010.04.002
44. DiSabato DJ, Quan N, and Godbout JP. Neuroinflammation: The Devil Is in the Details. J OF NEUROCHEMISTRY. (2016) 139:136–53. doi: 10.1111/jnc.13607
45. Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, and Pittman QJ. Early Life Activation of Toll-Like Receptor 4 Reprograms Neural Anti-Inflammatory Pathways. J neuroscience: Off J Soc Neurosci. (2010) 30:7975–83. doi: 10.1523/jneurosci.6078-09.2010
46. Stengel A and Tache Y. Gut-Brain Neuroendocrine Signaling under Conditions of Stress-Focus on Food Intake-Regulatory Mediators. Front IN Endocrinol. (2018) 9:498. doi: 10.3389/fendo.2018.00498
47. Li M, Tong F, Wu B, and Dong X. Radiation-Induced Brain Injury: Mechanistic Insights and the Promise of Gut-Brain Axis Therapies. Brain Sci. (2024) 14:1295. doi: 10.3390/brainsci14121295
48. van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, et al. Gut Bacterial Tyrosine Decarboxylases Restrict Levels of Levodopa in the Treatment of Parkinson’s Disease. Nat Commun. (2019) 10:310. doi: 10.1038/s41467-019-08294-y
49. Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J Cell Physiol. (2017) 232:2359–72. doi: 10.1002/jcp.25518
50. Byrne CJ, Khurana S, Kumar A, and Tai TC. Inflammatory Signaling in Hypertension: Regulation of Adrenal Catecholamine Biosynthesis. Front Endocrinol (Lausanne). (2018) 9:343. doi: 10.3389/fendo.2018.00343
51. Ryan M and Ryznar R. The Molecular Basis of Resilience: A Narrative Review. Front Psychiatry. (2022) 13:856998. doi: 10.3389/fpsyt.2022.856998
52. Xu M, Zhou EY, and Shi H. Tryptophan and Its Metabolite Serotonin Impact Metabolic and Mental Disorders Via the Brain-Gut-Microbiome Axis: A Focus on Sex Differences. Cells. (2025) 14:384. doi: 10.3390/cells14050384
53. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
54. Braga JD, Thongngam M, and Kumrungsee T. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut-Brain Axis. NPJ Sci Food. (2024) 8:16. doi: 10.1038/s41538-024-00253-2
55. Barakat H and Aljutaily T. Role of Γ-Aminobutyric Acid (Gaba) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules. (2025) 15:399. doi: 10.3390/biom15030399
56. Yuan C, He Y, Xie K, Feng L, Gao S, and Cai L. Review of Microbiota Gut Brain Axis and Innate Immunity in Inflammatory and Infective Diseases. Front Cell infection Microbiol. (2023) 13:1282431. doi: 10.3389/fcimb.2023.1282431
57. Vanuytsel T, Bercik P, and Boeckxstaens G. Understanding Neuroimmune Interactions in Disorders of Gut-Brain Interaction: From Functional to Immune-Mediated Disorders. Gut. (2023) 72:787–98. doi: 10.1136/gutjnl-2020-320633
58. Fang Z, Chen J, Zheng Y, and Chen Z. Targeting Histamine and Histamine Receptors for Memory Regulation: An Emotional Perspective. Curr neuropharmacology. (2024) 22:1846–69. doi: 10.2174/1570159x22666240128003108
59. Krishnan S, Ding Y, Saeidi N, Choi M, Sridharan GV, Sherr DH, et al. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. (2019) 28:3285. doi: 10.1016/j.celrep.2019.08.080
60. Wang N, Sun C, Yang Y, Zhang D, Huang L, Xu C, et al. Gut Microbiota-Derived Indoleacetic Acid Attenuates Neuroinflammation and Neurodegeneration in Glaucoma through Ahr/Rage Pathway. J Neuroinflamm. (2025) 22:179. doi: 10.1186/s12974-025-03505-4
61. Yang C, Sun J, Li L, Zheng J, Wang C, Zhao Y, et al. Synbiotics of Lactobacillus Suilingensis and Inulin Alleviates Cognitive Impairment Via Regulating Gut Microbiota Indole-3-Lactic Acid Metabolism in Female Ad Mice. Alzheimer’s dementia: J Alzheimer’s Assoc. (2025) 21:e70406. doi: 10.1002/alz.70406
62. Rosas HD, Morgan XC, Tao Y, Lai F, and Mercaldo ND. Gut Dysbiosis in Down Syndrome: A Potentially Unexplored Culprit for Early Alzheimer’s Disease. Alzheimer’s dementia: J Alzheimer’s Assoc. (2025) 21:e70330. doi: 10.1002/alz.70330
63. Michinaga S, Inoue A, Sonoda K, Mizuguchi H, and Koyama Y. Down-Regulation of Astrocytic Sonic Hedgehog by Activation of Endothelin Et(B) Receptors: Involvement in Traumatic Brain Injury-Induced Disruption of Blood Brain Barrier in a Mouse Model. Neurochemistry Int. (2021) 146:105042. doi: 10.1016/j.neuint.2021.105042
64. Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal Microbiota and Myelin Autoantigen Cooperate to Trigger Autoimmune Demyelination. Nature. (2011) 479:538–41. doi: 10.1038/nature10554
65. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc Natl Acad Sci United States America. (2017) 114:10713–8. doi: 10.1073/pnas.1711235114
66. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia Xiva and Iv Clusters. PloS One. (2015) 10:e0137429. doi: 10.1371/journal.pone.0137429
67. Li M, van Esch B, Henricks PAJ, Folkerts G, and Garssen J. The Anti-Inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells Via Activation of Gpr41/43 and Inhibition of Hdacs. Front Pharmacol. (2018) 9:533. doi: 10.3389/fphar.2018.00533
68. Forte N, Marfella B, Nicois A, Palomba L, Paris D, Motta A, et al. The Short-Chain Fatty Acid Acetate Modulates Orexin/Hypocretin Neurons: A Novel Mechanism in Gut-Brain Axis Regulation of Energy Homeostasis and Feeding. Biochem Pharmacol. (2024) 226:116383. doi: 10.1016/j.bcp.2024.116383
69. Foster JA, Rinaman L, and Cryan JF. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
70. Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-Like Pathogenesis in App/Ps1 Transgenic Mice. Trans Psychiatry. (2019) 9:189. doi: 10.1038/s41398-019-0525-3
71. Freestone PP, Lyte M, Neal CP, Maggs AF, Haigh RD, and Williams PH. The Mammalian Neuroendocrine Hormone Norepinephrine Supplies Iron for Bacterial Growth in the Presence of Transferrin or Lactoferrin. J bacteriology. (2000) 182:6091–8. doi: 10.1128/jb.182.21.6091-6098.2000
72. Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, et al. Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio. (2016) 7:e00826–16. doi: 10.1128/mBio.00826-16
73. Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota Alteration Is Associated with the Development of Stress-Induced Despair Behavior. Sci Rep. (2017) 7:43859. doi: 10.1038/srep43859
74. Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, et al. Chronic Stress Promotes Colitis by Disturbing the Gut Microbiota and Triggering Immune System Response. Proc Natl Acad Sci United States America. (2018) 115:E2960–e9. doi: 10.1073/pnas.1720696115
75. Bonaz B, Sinniger V, and Pellissier S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front Immunol. (2017) 8:1452. doi: 10.3389/fimmu.2017.01452
76. Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, and Liberles SD. Sensory Neurons That Detect Stretch and Nutrients in the Digestive System. Cell. (2025) 188:3623–4. doi: 10.1016/j.cell.2025.05.043
77. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the Vagus Nerve Attenuates Macrophage Activation by Activating the Jak2-Stat3 Signaling Pathway. Nat Immunol. (2005) 6:844–51. doi: 10.1038/ni1229
78. Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, et al. Anti-Inflammatory Effect of Vagus Nerve Stimulation in a Rat Model of Inflammatory Bowel Disease. Autonomic neuroscience: basic Clin. (2011) 160:82–9. doi: 10.1016/j.autneu.2010.10.007
79. Perez-Burgos A, Wang L, McVey Neufeld KA, Mao YK, Ahmadzai M, Janssen LJ, et al. The Trpv1 Channel in Rodents Is a Major Target for Antinociceptive Effect of the Probiotic Lactobacillus Reuteri Dsm 17938. J Physiol. (2015) 593:3943–57. doi: 10.1113/jp270229
80. Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, and Bailey MT. The Prebiotics 3’sialyllactose and 6’sialyllactose Diminish Stressor-Induced Anxiety-Like Behavior and Colonic Microbiota Alterations: Evidence for Effects on the Gut-Brain Axis. Brain behavior Immun. (2015) 50:166–77. doi: 10.1016/j.bbi.2015.06.025
81. Zheng G, Victor Fon G, Meixner W, Creekmore A, Zong Y, KD M, et al. Chronic Stress and Intestinal Barrier Dysfunction: Glucocorticoid Receptor and Transcription Repressor Hes1 Regulate Tight Junction Protein Claudin-1 Promoter. Sci Rep. (2017) 7:4502. doi: 10.1038/s41598-017-04755-w
82. Winston JA and Theriot CM. Impact of Microbial Derived Secondary Bile Acids on Colonization Resistance against Clostridium Difficile in the Gastrointestinal Tract. Anaerobe. (2016) 41:44–50. doi: 10.1016/j.anaerobe.2016.05.003
83. Hughes DT and Sperandio V. Inter-Kingdom Signalling: Communication between Bacteria and Their Hosts. Nat Rev Microbiol. (2008) 6:111–20. doi: 10.1038/nrmicro1836
84. Ghaisas S, Maher J, and Kanthasamy A. Gut Microbiome in Health and Disease: Linking the Microbiome-Gut-Brain Axis and Environmental Factors in the Pathogenesis of Systemic and Neurodegenerative Diseases. Pharmacol Ther. (2016) 158:52–62. doi: 10.1016/j.pharmthera.2015.11.012
85. Parashar A and Udayabanu M. Gut Microbiota: Implications in Parkinson’s Disease. PARKINSONISM RELATED Disord. (2017) 38:1–7. doi: 10.1016/j.parkreldis.2017.02.002
86. Bicknell B, Liebert A, Borody T, Herkes G, McLachlan C, and Kiat H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int J Mol Sci. (2023) 24:9577. doi: 10.3390/ijms24119577
87. Zhang X, Tang B, and Guo J. Parkinson’s Disease and Gut Microbiota: From Clinical to Mechanistic and Therapeutic Studies. Trans neurodegeneration. (2023) 12:59. doi: 10.1186/s40035-023-00392-8
88. Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, et al. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in Nod Mice. Gut. (2018) 67:1445–53. doi: 10.1136/gutjnl-2017-314508
89. Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Movement disorders: Off J Movement Disord Soc. (2015) 30:350–8. doi: 10.1002/mds.26069
90. Cani PD and de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front Microbiol. (2017) 8:1765. doi: 10.3389/fmicb.2017.01765
91. Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, et al. Gut Inflammation Can Boost Horizontal Gene Transfer between Pathogenic and Commensal Enterobacteriaceae. Proc Natl Acad Sci United States America. (2012) 109:1269–74. doi: 10.1073/pnas.1113246109
92. Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut Microbiota from Multiple Sclerosis Patients Enables Spontaneous Autoimmune Encephalomyelitis in Mice. Proc Natl Acad Sci United States America. (2017) 114:10719–24. doi: 10.1073/pnas.1711233114
93. Kustrimovic N, Balkhi S, Bilato G, and Mortara L. Gut Microbiota and Immune System Dynamics in Parkinson’s and Alzheimer’s Diseases. Int J Mol Sci. (2024) 25:12164. doi: 10.3390/ijms252212164
94. Braak H, de Vos RA, Bohl J, and Del Tredici K. Gastric Alpha-Synuclein Immunoreactive Inclusions in Meissner’s and Auerbach’s Plexuses in Cases Staged for Parkinson’s Disease-Related Brain Pathology. Neurosci Lett. (2006) 396:67–72. doi: 10.1016/j.neulet.2005.11.012
95. Park H, Cheon J, Kim H, Kim J, Kim J, Shin JY, et al. Gut Microbial Production of Imidazole Propionate Drives Parkinson’s Pathologies. Nat Commun. (2025) 16:8216. doi: 10.1038/s41467-025-63473-4
96. Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, et al. Histone Deacetylase Inhibitors up-Regulate Astrocyte Gdnf and Bdnf Gene Transcription and Protect Dopaminergic Neurons. Int J Neuropsychopharmacol. (2008) 11:1123–34. doi: 10.1017/s1461145708009024
97. Chen Y and Yu Y. Tau and Neuroinflammation in Alzheimer’s Disease: Interplay Mechanisms and Clinical Translation. J Neuroinflamm. (2023) 20:165. doi: 10.1186/s12974-023-02853-3
98. Murray ER, Kemp M, and Nguyen TT. The Microbiota-Gut-Brain Axis in Alzheimer’s Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions. Arch OF Clin Neuropsychol. (2022) 37:595–607. doi: 10.1093/arclin/acac008
99. Kim H-J, Kim H, Lee J-H, and Hwangbo C. Toll-Like Receptor 4 (Tlr4): New Insight Immune and Aging. Immun Ageing. (2023) 20:67. doi: 10.1186/s12979-023-00383-3
100. Qin J, Ma Z, Chen X, and Shu S. Microglia Activation in Central Nervous System Disorders: A Review of Recent Mechanistic Investigations and Development Efforts. Front IN Neurol. (2023) 14:1103416. doi: 10.3389/fneur.2023.1103416
101. Zhao X, Yu J, Xu B, Xu Z, Lei X, Han S, et al. Gut-Derived Bacterial Vesicles Carrying Lipopolysaccharide Promote Microglia-Mediated Synaptic Pruning. Alzheimer’s dementia: J Alzheimer’s Assoc. (2025) 21:e70331. doi: 10.1002/alz.70331
102. Waubant E, Lucas R, Mowry E, Graves J, Olsson T, Alfredsson L, et al. Environmental and Genetic Risk Factors for Ms: An Integrated Review. Ann Clin Trans Neurol. (2019) 6:1905–22. doi: 10.1002/acn3.50862
103. Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, et al. Alterations of Host-Gut Microbiome Interactions in Multiple Sclerosis. EBioMedicine. (2022) 76:103798. doi: 10.1016/j.ebiom.2021.103798
104. Lehman PC, Ghimire S, Price JD, Ramer-Tait AE, and Mangalam AK. Diet-Microbiome-Immune Interplay in Multiple Sclerosis: Understanding the Impact of Phytoestrogen Metabolizing Gut Bacteria. Eur J Immunol. (2023) 53:e2250236. doi: 10.1002/eji.202250236
105. Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, et al. The Gut Microbiota in Multiple Sclerosis Varies with Disease Activity. Genome Med. (2023) 15:1. doi: 10.1186/s13073-022-01148-1
106. Bigdeli A, Ghaderi-Zefrehei M, Lesch BJ, Behmanesh M, and Arab SS. Bioinformatics Analysis of Myelin-Microbe Interactions Suggests Multiple Types of Molecular Mimicry in the Pathogenesis of Multiple Sclerosis. PloS One. (2024) 19:e0308817. doi: 10.1371/journal.pone.0308817
107. Montgomery TL, Peipert D, and Krementsov DN. Modulation of Multiple Sclerosis Risk and Pathogenesis by the Gut Microbiota: Complex Interactions between Host Genetics, Bacterial Metabolism, and Diet. Immunol Rev. (2024) 325:131–51. doi: 10.1111/imr.13343
108. Taylor CT and Colgan SP. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat Rev Immunol. (2017) 17:774–85. doi: 10.1038/nri.2017.103
109. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature. (2018) 557:724–8. doi: 10.1038/s41586-018-0119-x
110. Zhao YF, Wei DN, and Tang Y. Gut Microbiota Regulate Astrocytic Functions in the Brain: Possible Therapeutic Consequences. Curr neuropharmacology. (2021) 19:1354–66. doi: 10.2174/1570159x19666210215123239
111. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain behavior Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
112. Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat Microbiol. (2019) 4:623–32. doi: 10.1038/s41564-018-0337-x
113. Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
114. O’Mahony SM, Clarke G, Borre YE, Dinan TG, and Cryan JF. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
115. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central Gaba Receptor Expression in a Mouse Via the Vagus Nerve. Proc Natl Acad Sci United States America. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
116. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, and Conlon MA. Increased Abundance of Sutterella Spp. And Ruminococcus Torques in Feces of Children with Autism Spectrum Disorder. Mol Autism. (2013) 4:42. doi: 10.1186/2040-2392-4-42
117. Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PloS One. (2013) 8:e68322. doi: 10.1371/journal.pone.0068322
118. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
119. MacFabe DF, Cain NE, Boon F, Ossenkopp KP, and Cain DP. Effects of the Enteric Bacterial Metabolic Product Propionic Acid on Object-Directed Behavior, Social Behavior, Cognition, and Neuroinflammation in Adolescent Rats: Relevance to Autism Spectrum Disorder. Behav Brain Res. (2011) 217:47–54. doi: 10.1016/j.bbr.2010.10.005
120. Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, et al. Commensal Bacteria (Normal Microflora), Mucosal Immunity and Chronic Inflammatory and Autoimmune Diseases. Immunol Lett. (2004) 93:97–108. doi: 10.1016/j.imlet.2004.02.005
121. Ma Z, Zuo T, Frey N, and Rangrez AY. A Systematic Framework for Understanding the Microbiome in Human Health and Disease: From Basic Principles to Clinical Translation. Signal transduction targeted Ther. (2024) 9:237. doi: 10.1038/s41392-024-01946-6
122. Shim JA, Ryu JH, Jo Y, and Hong C. The Role of Gut Microbiota in T Cell Immunity and Immune Mediated Disorders. Int J Biol Sci. (2023) 19:1178–91. doi: 10.7150/ijbs.79430
123. Flannigan KL and Denning TL. Segmented Filamentous Bacteria-Induced Immune Responses: A Balancing Act between Host Protection and Autoimmunity. IMMUNOLOGY. (2018) 154:537–46. doi: 10.1111/imm.12950
124. Fessler J, Matson V, and Gajewski TF. Exploring the Emerging Role of the Microbiome in Cancer Immunotherapy. J FOR IMMUNOTHERAPY OF Cancer. (2019) 7:108. doi: 10.1186/s40425-019-0574-4
125. Lunjani N, Ahearn-Ford S, Dube FS, Hlela C, and O’Mahony L. Mechanisms of Microbe-Immune System Dialogue within the Skin. Genes AND Immun. (2021) 22:276–88. doi: 10.1038/s41435-021-00133-9
126. Dong F, Hao F, Murray IA, Smith PB, Koo I, Tindall AM, et al. Intestinal Microbiota-Derived Tryptophan Metabolites Are Predictive of Ah Receptor Activity. Gut Microbes. (2020) 12:1–24. doi: 10.1080/19490976.2020.1788899
127. Otaru N, Ye K, Mujezinovic D, Berchtold L, Constancias F, Cornejo FA, et al. Gaba Production by Human Intestinal Bacteroides Spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front Microbiol. (2021) 12:656895. doi: 10.3389/fmicb.2021.656895
128. Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, and Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. (2018) 173:1728–41.e13. doi: 10.1016/j.cell.2018.04.027
129. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation. (2019) 139:1407–21. doi: 10.1161/circulationaha.118.036652
130. Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, et al. A Gut-Derived Metabolite Alters Brain Activity and Anxiety Behaviour in Mice. Nature. (2022) 602:647–53. doi: 10.1038/s41586-022-04396-8
131. Chen HH, Chang PC, Chen C, and Chan MH. Protective and Therapeutic Activity of Honokiol in Reversing Motor Deficits and Neuronal Degeneration in the Mouse Model of Parkinson’s Disease. Pharmacol reports: PR. (2018) 70:668–76. doi: 10.1016/j.pharep.2018.01.003
132. Chang PV, Hao L, Offermanns S, and Medzhitov R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function Via Histone Deacetylase Inhibition. Proc Natl Acad Sci United States America. (2014) 111:2247–52. doi: 10.1073/pnas.1322269111
133. Mann ER, Lam YK, and Uhlig HH. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
134. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
135. Sharma A, Sharma G, and Im SH. Gut Microbiota in Regulatory T Cell Generation and Function: Mechanisms and Health Implications. Gut Microbes. (2025) 17:2516702. doi: 10.1080/19490976.2025.2516702
136. Hubbard TD, Murray IA, and Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab disposition: Biol fate chemicals. (2015) 43:1522–35. doi: 10.1124/dmd.115.064246
137. Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, et al. Dopaminergic Receptors on Cd4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease. Sci Rep. (2016) 6:33738. doi: 10.1038/srep33738
138. Forsythe P, Bienenstock J, and Kunze WA. Vagal Pathways for Microbiome-Brain-Gut Axis Communication. Adv Exp Med Biol. (2014) 817:115–33. doi: 10.1007/978-1-4939-0897-4_5
139. Breit S, Kupferberg A, Rogler G, and Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. (2018) 9:44. doi: 10.3389/fpsyt.2018.00044
140. Osadchiy V, Mayer EA, Gao K, Labus JS, Naliboff B, Tillisch K, et al. Analysis of Brain Networks and Fecal Metabolites Reveals Brain-Gut Alterations in Premenopausal Females with Irritable Bowel Syndrome. Trans Psychiatry. (2020) 10:367. doi: 10.1038/s41398-020-01071-2
141. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc Natl Acad Sci United States America. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
142. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc Natl Acad Sci United States America. (2008) 105:16731–6. doi: 10.1073/pnas.0804812105
143. Segui-Perez C, Huang LZX, Paganelli FL, Lievens E, and Strijbis K. Probiotic Bifidobacterium Bifidum Strains Desialylate Muc13 and Increase Intestinal Epithelial Barrier Function. Sci Rep. (2025) 15:8778. doi: 10.1038/s41598-025-92125-2
144. Al-Sadi R, Dharmaprakash V, Nighot P, Guo S, Nighot M, Do T, et al. Bifidobacterium Bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-Like Receptor-2 Pathway in an Nf-κb-Independent Manner. Int J Mol Sci. (2021) 22:8070. doi: 10.3390/ijms22158070
145. Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, and Iannitti T. Mechanisms and Therapeutic Effectiveness of Lactobacilli. J Clin Pathol. (2016) 69:187–203. doi: 10.1136/jclinpath-2015-202976
146. Zhang S, Yu Q, Sun Y, Zhang G, Zhang Y, and Xin H. Alleviating the Effect of Branched-Chain Fatty Acids on the Lipopolysaccharide-Induced Inflammatory Response in Calf Small Intestinal Epithelial Cells. Antioxidants (Basel Switzerland). (2025) 14:608. doi: 10.3390/antiox14050608
147. Facchin S, Bertin L, Bonazzi E, Lorenzon G, De Barba C, Barberio B, et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life (Basel Switzerland). (2024) 14:559. doi: 10.3390/life14050559
148. Huang Y, Zhang X, Yu C, Liu Y, Kang H, Liu Y, et al. Lactobacillus Acidophilus Promotes Cognitive Function Recovery Via Regulating Microglial Peroxisomal Function in Cerebral Ischemia. Cell Host Microbe. (2025) 33:1484–501.e12. doi: 10.1016/j.chom.2025.07.018
149. Zhong Y, Chang X, Zhao Z, Zheng L, Kuang G, Li P, et al. Bacteroides Fragilis Capsular Polysaccharide a Ameliorates Ulcerative Colitis in Rat by Recovering Intestinal Barrier Integrity and Restoring Gut Microbiota. Front Pharmacol. (2024) 15:1402465. doi: 10.3389/fphar.2024.1402465
150. Yang C, Lan R, Zhao L, Pu J, Hu D, Yang J, et al. Prevotella Copri Alleviates Hyperglycemia and Regulates Gut Microbiota and Metabolic Profiles in Mice. mSystems. (2024) 9:e0053224. doi: 10.1128/msystems.00532-24
151. Nakajima A, Sasaki T, Itoh K, Kitahara T, Takema Y, Hiramatsu K, et al. A Soluble Fiber Diet Increases Bacteroides Fragilis Group Abundance and Immunoglobulin a Production in the Gut. Appl Environ Microbiol. (2020) 86:e00405–20. doi: 10.1128/aem.00405-20
152. Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory Role for Gaba in Autoimmune Inflammation. Proc Natl Acad Sci United States America. (2010) 107:2580–5. doi: 10.1073/pnas.0915139107
153. Zhou M, Fan Y, Xu L, Yu Z, Wang S, Xu H, et al. Microbiome and Tryptophan Metabolomics Analysis in Adolescent Depression: Roles of the Gut Microbiota in the Regulation of Tryptophan-Derived Neurotransmitters and Behaviors in Human and Mice. Microbiome. (2023) 11:145. doi: 10.1186/s40168-023-01589-9
154. Sugisawa E, Takayama Y, Takemura N, Kondo T, Hatakeyama S, Kumagai Y, et al. Rna Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell. (2020) 182:609–24.e21. doi: 10.1016/j.cell.2020.06.022
155. Xu Y, Wang J, Wu X, Jing H, Zhang S, Hu Z, et al. Gut Microbiota Alteration after Cholecystectomy Contributes to Post-Cholecystectomy Diarrhea Via Bile Acids Stimulating Colonic Serotonin. Gut Microbes. (2023) 15:2168101. doi: 10.1080/19490976.2023.2168101
156. Zhai L, Huang C, Ning Z, Zhang Y, Zhuang M, Yang W, et al. Ruminococcus Gnavus Plays a Pathogenic Role in Diarrhea-Predominant Irritable Bowel Syndrome by Increasing Serotonin Biosynthesis. Cell Host Microbe. (2023) 31:33–44.e5. doi: 10.1016/j.chom.2022.11.006
157. Herr N, Bode C, and Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med. (2017) 4:48. doi: 10.3389/fcvm.2017.00048
158. Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical Role of Gut Microbiota in the Production of Biologically Active, Free Catecholamines in the Gut Lumen of Mice. Am J Physiol Gastrointestinal liver Physiol. (2012) 303:G1288–95. doi: 10.1152/ajpgi.00341.2012
159. Cosentino M and Marino F. Adrenergic and Dopaminergic Modulation of Immunity in Multiple Sclerosis: Teaching Old Drugs New Tricks? J neuroimmune pharmacology: Off J Soc NeuroImmune Pharmacol. (2013) 8:163–79. doi: 10.1007/s11481-012-9410-z
160. Mowat AM and Agace WW. Regional Specialization within the Intestinal Immune System. Nat Rev Immunol. (2014) 14:667–85. doi: 10.1038/nri3738
161. Sun M, Wu W, Liu Z, and Cong Y. Microbiota Metabolite Short Chain Fatty Acids, Gpcr, and Inflammatory Bowel Diseases. J OF Gastroenterol. (2017) 52:1–8. doi: 10.1007/s00535-016-1242-9
162. Kaisar MMM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, and Everts B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells Via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109a Signaling. Front Immunol. (2017) 8:1429. doi: 10.3389/fimmu.2017.01429
163. Goswami TK, Singh M, Dhawan M, Mitra S, Emran TB, Rabaan AA, et al. Regulatory T Cells (Tregs) and Their Therapeutic Potential against Autoimmune Disorders - Advances and Challenges. Hum Vaccines immunotherapeutics. (2022) 18:2035117. doi: 10.1080/21645515.2022.2035117
164. Roediger WE. Role of Anaerobic Bacteria in the Metabolic Welfare of the Colonic Mucosa in Man. Gut. (1980) 21:793–8. doi: 10.1136/gut.21.9.793
165. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the Mtor-S6k Pathway. Mucosal Immunol. (2015) 8:80–93. doi: 10.1038/mi.2014.44
166. Lu Y, Chong J, Shen S, Chammas J-B, Chalifour L, and Xia J. Trpnet: Understanding Tryptophan Metabolism across Gut Microbiome. METABOLITES. (2022) 12:10. doi: 10.3390/metabo12010010
167. Chen Y, Wang Y, Fu Y, Yin Y, and Xu K. Modulating Ahr Function Offers Exciting Therapeutic Potential in Gut Immunity and Inflammation. Cell bioscience. (2023) 13:85. doi: 10.1186/s13578-023-01046-y
168. Ma N, He T, Johnston LJ, and Ma X. Host-Microbiome Interactions: The Aryl Hydrocarbon Receptor as a Critical Node in Tryptophan Metabolites to Brain Signaling. Gut Microbes. (2020) 11:1203–19. doi: 10.1080/19490976.2020.1758008
169. Mitchell CM, Davy BM, Ponder MA, McMillan RP, Hughes MD, Hulver MW, et al. Prebiotic Inulin Supplementation and Peripheral Insulin Sensitivity in Adults at Elevated Risk for Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients. (2021) 13:3235. doi: 10.3390/nu13093235
170. Wenzel TJ, Gates EJ, Ranger AL, and Klegeris A. Short-Chain Fatty Acids (Scfas) Alone or in Combination Regulate Select Immune Functions of Microglia-Like Cells. Mol Cell Neurosci. (2020) 105:103493. doi: 10.1016/j.mcn.2020.103493
171. Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, et al. Butyrate Produced by Gut Commensal Bacteria Activates Tgf-Beta1 Expression through the Transcription Factor Sp1 in Human Intestinal Epithelial Cells. Sci Rep. (2018) 8:9742. doi: 10.1038/s41598-018-28048-y
172. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Sci (New York NY). (2013) 341:569–73. doi: 10.1126/science.1241165
173. Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp Mol Med. (2018) 50:e450. doi: 10.1038/emm.2017.282
Keywords: gut microbiota, neuro, immune, gut-brain-immune axis, neuroimmune homeostasis
Citation: Ouyang H, Yang Y, Zhang X, Cui Y and Zhang Y (2025) Microbial orchestration of neuroimmune crosstalk: from homeostasis to disease. Front. Immunol. 16:1679286. doi: 10.3389/fimmu.2025.1679286
Received: 04 August 2025; Accepted: 15 October 2025;
Published: 29 October 2025.
Edited by:
Amélia M. Sarmento, Fernando Pessoa University, PortugalReviewed by:
Zi-Hui Mao, First Affiliated Hospital of Zhengzhou University, ChinaSaurabh Kadyan, National Dairy Research Institute (ICAR), India
Copyright © 2025 Ouyang, Yang, Zhang, Cui and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlei Zhang, eXVubGVpemhhbmdAbmptdS5lZHUuY24=; Yiyao Cui, Y3l5dHQyMDAwQHNpbmEuY29t
 Huixia Ouyang
Huixia Ouyang Yang Yang1
Yang Yang1 Yunlei Zhang
Yunlei Zhang