- 1Henan Key Laboratory of Cancer Epigenetics, Cancer Hospital, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 2Department of Pathology, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
Esophageal squamous cell carcinoma (ESCC) remains a global health challenge, with immune checkpoint inhibitors (ICIs) reshaping therapeutic strategies. However, heterogeneous responses underscore the urgent need for robust predictive biomarkers. While PD-L1 expression remains the most widely used marker, its limitations, including spatial heterogeneity and inducible expression, have prompted exploration of alternative and composite indicators. Recent advances highlight the predictive potential of tumor immune microenvironment (TME) features such as tumor-infiltrating lymphocytes (TILs), tertiary lymphoid structures (TLSs), stromal maturity, and T cell–inflamed gene expression profiles. Concurrently, tumor-intrinsic biomarkers, including microsatellite instability, tumor mutational burden, neoantigen load, and chromosomal alterations—have shown promise in stratifying immunotherapy responders. Multi-omics approaches, liquid biopsies, and integration of host factors such as gut microbiota are emerging to refine patient selection. This review comprehensively examines evolving biomarkers and therapeutic trials, emphasizing the need for integrative precision strategies to optimize immunotherapy efficacy in ESCC.
1 Introduction
Esophageal squamous cell carcinoma (ESCC), ranking seventh in global incidence and sixth in cancer-related mortality (1), is typically diagnosed at advanced stages with limited treatment efficacy. Immune checkpoint inhibitors (ICIs) have shown promising efficacy and manageable safety in advanced ESCC, as evidenced by trials such as KEYNOTE-181 (2), ESCORT (3), and KEYNOTE-590 (4), leading to their integration from later-line to first-line and neoadjuvant settings. However, the clinical benefit of ICIs is heterogeneous and influenced by multiple factors, including tumor immunogenicity, the tumor immune microenvironment (TIME), and host-related characteristics. Not all patients derive satisfied responses, underscoring the urgent need for robust predictive biomarkers to identify likely responders (5).
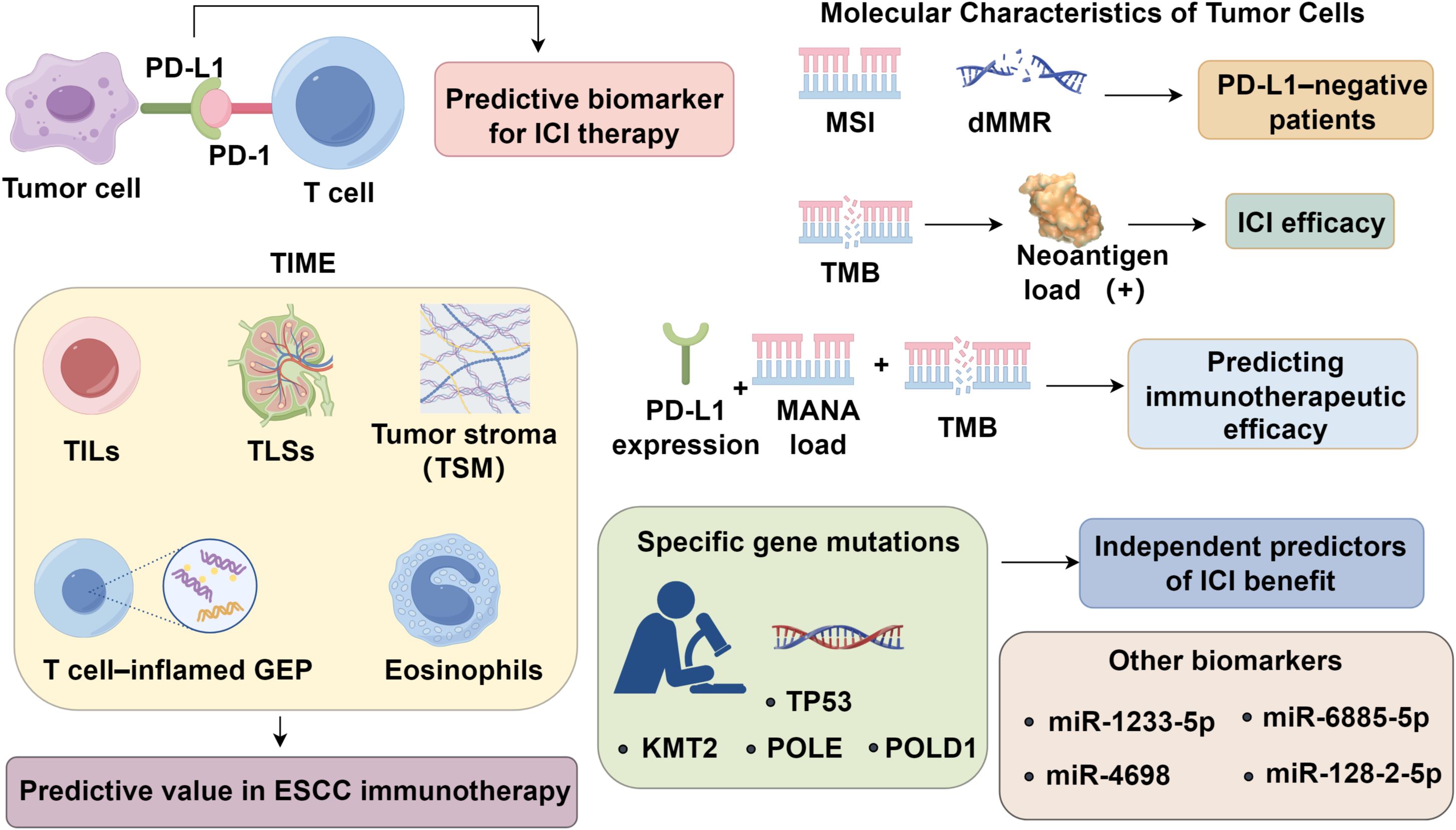
Programmed death-ligand 1 (PD-L1) expression remains the most widely recognized biomarker for predicting response to ICIs. Nevertheless, its predictive accuracy is limited, and a substantial proportion of patients with high PD-L1 expression still fail to respond to treatment (5). Ongoing research efforts have identified two broad categories of emerging biomarkers with potential predictive value in immunotherapy. The first category encompasses features of the TIME, such as tumor-infiltrating lymphocytes (TILs), the presence of tertiary lymphoid structures (TLS), and T cell–inflamed gene expression profiles. These factors reflect the immune contexture within the tumor and its potential responsiveness to immune modulation (6). The second category involves molecular characteristics intrinsic to tumor cells, including high microsatellite instability (MSI-H)/deficient mismatch repair (dMMR), tumor mutational burden (TMB), and neoantigen load, all of which are associated with enhanced immunogenicity and increased likelihood of immune recognition (7). Additional exploratory biomarkers include serum non-coding RNAs, DNA methylation signatures, and components of the gut microbiome, though their predictive relevance in ESCC remains to be validated through larger, high-quality datasets (8, 9). This review aims to provide a comprehensive synthesis of the current understanding of immunotherapeutic biomarkers in ESCC, with particular emphasis on PD-L1, the TIME, tumor cell–related molecular features, and their clinical implications in guiding immunotherapy strategies.
2 Mechanistic and spatial heterogeneity of PD-L1 expression in ESCC
PD-L1, a pivotal immunoregulatory molecule, is broadly expressed across solid tumors and infiltrating immune cells, and remains the most extensively investigated biomarker for predicting clinical responses to ICI therapy. In the KEYNOTE-181 trial, pembrolizumab improved median overall survival (OS) to 12.5 months in ESCC patients with PD-L1 combined positive score (CPS) ≥10, compared to 10.0 months in the overall cohort (2). Consistent outcomes were observed in the KEYNOTE-590 subgroup with CPS ≥10 (4). Nevertheless, PD-L1 fails to serve as a definitive predictor of therapeutic efficacy. In the ESCORT trial, demonstrated no significant correlation between PD-L1 expression levels and clinical response parameters (3), and responses have been reported even in PD-L1 negative patients (10). Despite its clinical utility, PD-L1 as a predictive biomarker is limited by spatial heterogeneity and TME complexity (11, 12). Expression may differ across tumor regions and metastatic sites, leading to sampling bias. Moreover, PD-L1 upregulation is not exclusively IFN-γ–driven; alternative pathways, such as PTEN loss or EGFR mutations, can induce expression independent of antitumor immunity (13). Its inducible nature also results in dynamic changes under therapeutic or immune pressure, undermining its stability as a predictive marker. As immunotherapy shifts toward combination regimens, reliance on PD-L1 alone has diminished. These limitations underscore the need for integrative biomarker strategies, incorporating tumor-infiltrating lymphocytes, T cell–inflamed gene signatures, or composite immune scores, to improve stratification and optimize treatment efficacy in ESCC and beyond (14).
3 The tumor immune microenvironment
3.1 Tertiary lymphoid structures
TILs, particularly CD8+ cytotoxic T cells, are central mediators of anti-tumor immunity and have emerged as prognostic and predictive biomarkers across malignancies (15–17). Immunophenotyping based on CD3+ and CD8+ T cell density and localization delineates tumors into immune-desert, inflamed, immune-excluded, and immunosuppressed categories, with ICIs demonstrating greater efficacy in inflamed phenotypes (18). Integrating PD-L1 expression with TIL density improves predictive accuracy for PD-1/PD-L1 blockade, with PD-L1+/TIL+ tumors showing superior responses (19). In ESCC, however, the prognostic and predictive roles of TILs remain to be fully elucidated, necessitating refined stratification strategies based on TIL subtypes and additional immune markers. Eosinophils, beyond their role in eosinophilic esophagitis, are found to inversely correlate with lymph node metastasis in ESCC and may serve as dynamic markers of immunotherapy response (20, 21). Tumor-associated macrophages (TAMs), especially M2-like subsets, drive immune evasion by secreting IL-10 and TGF-β, fostering Treg recruitment and CTL inhibition (22), with high TAM density correlating with poor prognosis in ESCCs (23). Functional exhaustion of NK cells in ESCC is characterized by diminished granzyme B and activating receptors (NKp30, NKG2D), driven by IL-6/IL-8–mediated STAT3 activation, and correlates with disease progression (24). Myeloid-derived suppressor cells (MDSCs) further contribute to immunosuppression via ROS, arginase-1, and nitric oxide production (23, 25, 26). Collectively, these immune components establish a profoundly immunosuppressive tumor microenvironment, attenuating cytotoxic responses and limiting immunotherapeutic efficacy. TLSs are ectopic lymphoid aggregates formed in non-lymphoid tissues, including tumor sites and regions of chronic inflammation, and are composed of diverse immune cell populations (27). While studies directly examining the role of TLSs in ESCC remain limited, accumulating evidence indicates that TLSs are robust predictors of ICI efficacy in several tumor types, independent of PD-L1 expression status (28). Notably, a recent study in melanoma demonstrated that patients exhibiting high TLS-related gene signature scores (TLS-H) experienced significantly improved survival following CTLA-4 blockade, highlighting the critical contribution of TLSs to the maintenance of effective anti-tumor immune responses (29). The potential predictive value of TLSs in ESCC immunotherapy warrants further investigation.
3.2 Tumor stromal maturity
TSM is assessed based on the organization and morphology of collagen fibers and the presence of myxoid changes within the tumor stroma, and is generally categorized into mature, intermediate, and immature subtypes. TSM has been strongly associated with tumor metastasis and prognosis in malignancies such as colorectal cancer (30) and gastric cancer (31). Immature stromal subtype in ESCC is correlated with more aggressive biological behavior and poorer clinical outcomes. Furthermore, TSM was found to be associated with PD-L1 expression, suggesting its potential utility as a predictive biomarker for immunotherapeutic efficacy in ESCC (32). In head and neck squamous cell carcinoma, specific subtypes of cancer-associated fibroblasts (CAFs) have also been implicated in modulating immunotherapy responses, further highlighting the role of stromal components as determinants of treatment efficacy (33). Importantly, recognizing TSM may help guide therapeutic stratification, wherein patients with immature stroma—characterized by a dense, disorganized matrix and immunosuppressive fibroblast phenotypes—might benefit from combined stromal-targeting and immunotherapeutic approaches (32, 34). Thus, integrating TSM assessment into routine pathological evaluation could enhance precision in tailoring immunotherapy regimens.
3.3 T cell–inflamed gene expression profile
The T cell–inflamed gene expression profile (GEP) captures the immunogenic characteristics of the TME (35). An RNA-based transcriptomic analysis of baseline tumor samples from patients treated with pembrolizumab revealed that the T cell–inflamed GEP comprises interferon-γ-responsive genes associated with antigen presentation, chemokine expression, cytotoxic activity, and adaptive immune resistance (36). Cristescu et al. performed whole-genome and RNA expression profiling of patients with advanced solid tumors and melanoma across four KEYNOTE clinical trials. Based on combined stratification of tumor mutational burden and GEP levels, they identified four distinct clinical response groups, with the TMBhigh/GEPhigh cohort exhibiting the strongest therapeutic responses, thus positioning T cell–inflamed GEP as a potential predictive biomarker for immunotherapy efficacy (37). Therefore, the integration of TMB and GEP holds promise as a robust strategy to guide precision immunotherapy, particularly in anti–PD-1 contexts.
4 Molecular characteristics of tumor cells as predictive biomarkers
4.1 Microsatellite instability and mismatch repair deficiency
Microsatellite instability (MSI) refers to alterations in the length of microsatellites—short tandem repeats scattered throughout the genome—resulting in the appearance of novel alleles. The DNA mismatch repair (MMR) system, composed of a set of highly conserved genes and their encoded enzymes—including MLH1, MSH2, MSH6, and PMS2—functions to correct base-pair mismatches during DNA replication. Deficiency in MMR (dMMR) impairs this repair capacity, thereby promoting the accumulation of replication errors and contributing to MSI development (38). High-level microsatellite instability (MSI-H) is associated with aberrations in cancer-related genes, facilitating tumorigenesis. Moreover, MSI-H tumors often display increased neoantigen expression and TIL density, both of which enhance responsiveness to ICIs (39). Despite its immunologic relevance, the prevalence of MSI-H in ESCC remains low, ranging from 0% to 27% across studies (40), with discrepancies likely stemming from variations in MSI-H definitions, assay loci, and detection thresholds. Based on findings from multiple clinical trials involving various solid tumors (38, 41), pembrolizumab, a PD-1 inhibitor, was approved for the treatment of MSI-H/dMMR-positive solid tumors irrespective of histology (42), encompassing MSI-H/dMMR esophageal cancer. Although only a small subset of ESCC patients may qualify under this indication, it offers a potential immunotherapy avenue for PD-L1–negative patients.
4.2 Tumor mutational burden and neoantigen burden
TMB quantifies the number of somatic nonsynonymous mutations within a defined genomic region. A higher TMB correlates with increased neoantigen load, enhancing immunogenicity and the likelihood of ICI efficacy. In the multicenter Phase II KEYNOTE-158 trial involving diverse advanced solid tumors (43), a threshold of 10 mutations per megabase (Mut/Mb) was established to define TMB-high (TMB-H) status. Patients with TMB-H demonstrated superior clinical responses to pembrolizumab monotherapy, achieving an objective response rate (ORR) of 30.3%, compared to 6.7% in TMB-low (TMB-L) counterparts. These findings made pembrolizumab approved for TMB-H tumors patients with progressive, unresectable, or metastatic disease regardless of tumor origin. A pan-cancer analysis further confirmed that high TMB was associated with improved response rates and prolonged survival following ICI therapy (44). Nonetheless, TMB varies widely across cancer types, and the top 20% threshold for defining TMB-H differs significantly between malignancies (45), precluding the use of a uniform cutoff. Notably, a consensus threshold for TMB-H specific to esophageal cancer remains undefined. Somatic mutations may give rise to tumor neoantigens, which represent biomarkers of highly immunogenic tumors Neoantigen burden has been correlated with patient survival and ICI responsiveness across various cancer types (46, 47). In ESCC patients treated with the anti–PD-1 antibody camrelizumab (SHR-1210), both TMB and mutation-associated neoantigens (MANAs) were positively associated with therapeutic response (48). A composite biomarker approach combining PD-L1 expression, MANA load, and TMB may offer a promising strategy for predicting immunotherapeutic efficacy (Figure 1).
4.3 Chromosomal amplification and specific gene mutations
Chromosomal amplification events have emerged as potential predictive markers of ICI resistance. Notably, ESCC patients exhibit amplification of chromosome 11q13, which is associated with advanced tumor stage. Mechanistically, 11q13 amplification often includes genes such as CCND1, FGF3/4/19, and ORAOV1. Overexpression of CCND1 can activate the CDK4/6-Rb axis, leading to cell cycle progression and immune evasion through decreased tumor immunogenicity. Additionally, CDK4/6 activation has been shown to downregulate MHC class I expression, thereby impairing antigen presentation and cytotoxic T cell recognition. These effects collectively blunt antitumor immunity and may underlie the reduced responsiveness to ICIs observed in 11q13-amplified ESCC. Evidence from a clinical trial of toripalimab in esophageal cancer suggests that 11q13 amplification may serve as a negative predictor of response to PD-1 blockade in metastatic ESCC (49). Mutations in TP53 are implicated in the pathogenesis of numerous cancers, including esophageal carcinoma. However, the predictive value of TP53 mutations for immunotherapy response remains ambiguous and may be context-dependent. While TP53 mutations have been associated with positive ICI response in breast and lung adenocarcinomas, they correlate with poor response in gastric, colorectal, and head and neck squamous cell carcinomas (50). In addition, mutations in genes such as KMT2 (51), POLE, and POLD1 (52) have been identified as independent predictors of ICI benefit across multiple tumor types, yet their mutation frequency in ESCC is exceedingly low. The relevance of other ESCC-associated gene alterations in shaping immunotherapeutic outcomes remains to be elucidated.
4.4 Other biomarkers
Several noncoding RNA and serum-based biomarkers have shown potential in predicting immunotherapy efficacy. For example, patients with advanced ESCC who responded to second-line nivolumab therapy exhibited reduced baseline levels of miR-1233-5p, as well as decreased expression of miR-6885-5p, miR-4698, and miR-128-2-5p during treatment, suggesting that specific circulating microRNAs may serve as predictive indicators (53). In other cancers, circulating long non-coding RNAs (54) and circular RNAs (55) have also been linked to ICI responsiveness. Elevated levels of peripheral biomarkers—such as serum albumin, neutrophils, inflammatory cytokines, and C-reactive protein—during nivolumab treatment have been associated with disease progression (56). Additionally, methylation profiling of CpG sites has been used to develop risk-scoring models with prognostic and predictive value for PD-1 inhibitor therapy (57). Emerging evidence also implicates host microbiota and their metabolic products as key modulators of ICI efficacy (58). Furthermore, host factors such as age and obesity may also influence immunotherapeutic outcomes (59). The identification and validation of novel predictive biomarkers and composite models remain active areas of investigation.
5 Immunotherapy
5.1 Pembrolizumab
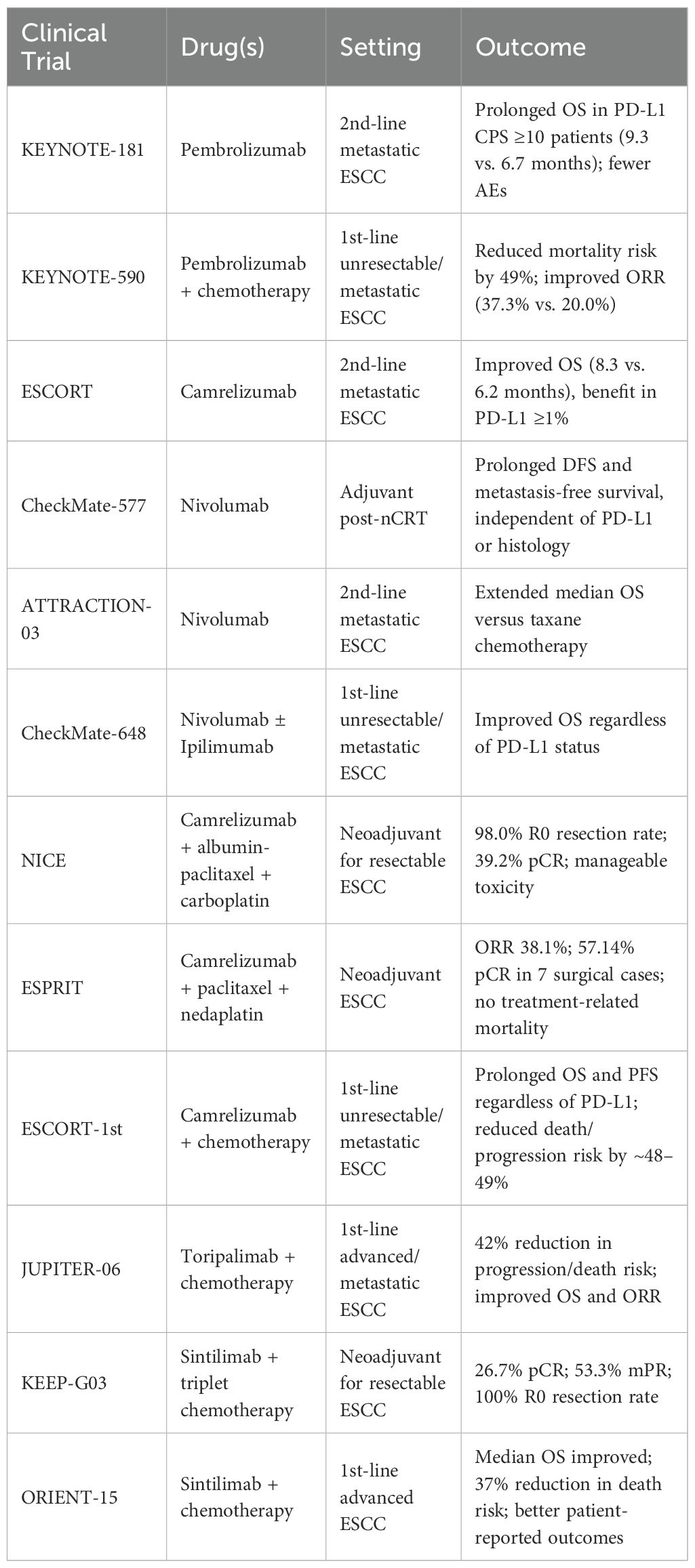
PD-1, expressed on T, B, and NK cells, maintains immune tolerance by regulating T cell differentiation (60). Its ligand PD-L1 is overexpressed in various cancers and suppresses T cell activity via PD-1 binding. PD-1/PD-L1 inhibitors are the main immunotherapy in ESCC. The PALACE-1 trial evaluated neoadjuvant chemoradiotherapy (nCRT) combined with pembrolizumab in 20 patients with resectable ESCC, all of whom experienced treatment-related adverse events (AEs) (61). Eighteen underwent surgery, with a pathologic complete response (pCR) achieved in 56%. While nCRT improves pCR rates, survival benefits remain uncertain, prompting an ongoing phase II multicenter trial (NCT04807673). For advanced esophageal cancer post-first-line chemotherapy, the KEYNOTE-181 trial demonstrated that pembrolizumab significantly prolonged median OS versus chemotherapy in patients with PD-L1 CPS ≥10, with fewer AEs (62), leading to its approval by the NMPA in June 2020. Furthermore, the KEYNOTE-590 trial established the superiority of first-line chemoimmunotherapy over chemotherapy alone. Pembrolizumab plus chemotherapy was first-line treatment and reduced mortality risk by 49% and improved ORR in ESCC (4, 63) (Table 1).
5.2 Nivolumab
nCRT followed by surgery has demonstrated the most substantial survival benefit for resectable, locally advanced esophageal cancer (64, 65). However, recurrence remains problematic. The CheckMate-577 trial (66, 67) addressed this by evaluating adjuvant nivolumab in patients with residual disease post-nCRT and R0 resection, revealing prolonged disease-free survival and metastasis-free survival, independent of PD-L1 expression or histology. In advanced settings, the ATTRACTION-03 trial (68, 69) established nivolumab as a second-line standard for ESCC, extending median overall survival versus taxane chemotherapy. Furthermore, the CheckMate-648 study (70)showed that both nivolumab plus chemotherapy and nivolumab plus ipilimumab significantly improved overall survival compared to chemotherapy alone in unresectable/metastatic ESCC, with consistent benefits regardless of PD-L1 status. Collectively, these trials support nivolumab’s role across multiple disease stages and treatment lines in ESCC.
5.3 Camrelizumab
Camrelizumab-based regimens have demonstrated promising efficacy and safety in both neoadjuvant and palliative settings for ESCC. The NICE study (71) enrolled 60 patients with resectable, locally advanced thoracic ESCC and multi-station lymph node metastases. Neoadjuvant camrelizumab combined with albumin-bound paclitaxel and carboplatin yielded a 98.0% R0 resection rate and a 39.2% pCR, with manageable toxicity. Similarly, the ESPRIT study (72) reported an objective response rate of 38.1% and 57.14% pCR in 7 surgical cases following camrelizumab plus paclitaxel and nedaplatin, with no treatment-related mortality. In the palliative setting, the ESCORT study (3) showed improved median OS with second-line camrelizumab, particularly in PD-L1 ≥1% patients. Furthermore, the ESCORT-1st trial (73) established camrelizumab combined with chemotherapy as an effective first-line treatment in unresectable or metastatic ESCC, prolonging OS and progression-free survival (PFS) regardless of PD-L1 expression. Notably, in patients with tumor proportion scores ≥10, camrelizumab reduced death and progression risks, while even PD-L1–negative patients derived clinical benefit.
5.4 Toripalimab and sintilimab
Perioperative toripalimab combined with neoadjuvant chemoradiotherapy (nCRT) was evaluated in 20 patients with locally advanced ESCC (74). Among them, 13 underwent surgery, yielding a pathological complete response (pCR) rate of 54%. Lymphopenia and leukopenia were the most common adverse events (AEs), with no postoperative recurrences observed at a 6-month median follow-up. These findings support the feasibility and tolerability of toripalimab–nCRT regimens in resectable ESCC, though long-term efficacy warrants further validation. In metastatic ESCC, toripalimab monotherapy showed preliminary activity, and the phase III JUPITER-06 trial (75) demonstrated that toripalimab plus chemotherapy significantly improved median OS and ORR by 17.2%, reducing the risk of progression or death by 42% versus chemotherapy alone. Similarly, the KEEP-G03 study (76) assessed neoadjuvant sintilimab with triplet chemotherapy (liposomal paclitaxel, cisplatin, and S-1) in resectable ESCC. Among 15 surgical patients, all achieved R0 resection; pCR and major pathological response (mPR) rates were 26.7% and 53.3%, respectively. Grade 3–4 AEs included leukopenia, neutropenia, and anemia, with no grade 5 events or surgery delays. In the ORIENT-15 phase III trial (77), sintilimab plus chemotherapy significantly prolonged median OS and PFS in advanced ESCC, reducing death and progression risks by 37% and 44%, respectively, with a manageable safety profile. Patient-reported quality-of-life outcomes also favored the sintilimab combination arm, underscoring its clinical benefit.
6 Conclusion
The integration of immune checkpoint inhibitors has significantly expanded the therapeutic arsenal against esophageal squamous cell carcinoma, yet substantial heterogeneity in response underscores the limitations of current biomarkers—particularly PD-L1—as standalone predictive tools. The immune landscape of ESCC is shaped by dynamic and complex interactions among tumor-intrinsic genetic alterations, immune-infiltrating cell populations, stromal architecture, and systemic host factors, all of which modulate immunotherapeutic responsiveness. Accumulating evidence supports the incorporation of tumor mutational burden, microsatellite instability, neoantigen load, and gene expression signatures as adjunctive or composite biomarkers that may refine patient stratification beyond PD-L1 status. Similarly, immune-rich microenvironments defined by TILs, TLSs, and mature stroma portend more favorable outcomes and offer additional layers of predictive value.
Moving forward, precision immunotherapy in ESCC will require the standardization and clinical validation of multi-parametric biomarker platforms—spanning genomics, transcriptomics, epigenetics, proteomics, and microbiome profiling. Therapeutically, rational combinations involving ICIs and agents targeting the tumor stroma, immunosuppressive myeloid cells, or oncogenic signaling pathways hold promise for overcoming resistance. Finally, future clinical trials must prioritize biomarker-driven design and incorporate patient-reported outcomes to ensure personalized, effective, and tolerable treatment strategies. By decoding the complex interplay between tumor biology and immune contexture, the field is poised to transform ESCC management through next-generation immunotherapy.
Author contributions
JM: Writing – original draft. DW: Writing – original draft. YL: Writing – original draft. GZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Key R&D and Promotion Projects of Henan Province (Grant No: 232102310130).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Cao Y, Qin S, Luo S, Li Z, Cheng Y, Fan Y, et al. Pembrolizumab versus chemotherapy for patients with esophageal squamous cell carcinoma enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open. (2022) 7:100341. doi: 10.1016/j.esmoop.2021.100341
3. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8
4. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
5. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. JNrCo. (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
6. Zhang D, Jiang D, Jiang L, Ma J, Wang X, Xu X, et al. HLA-A(+) tertiary lymphoid structures with reactivated tumor infiltrating lymphocytes are associated with a positive immunotherapy response in esophageal squamous cell carcinoma. Br J Cancer. (2024) 131:184–95. doi: 10.1038/s41416-024-02712-9
7. Tong X, Jin M, Wang L, Zhang D, Yin Y, and Shen Q. Prognostic biomarkers for immunotherapy in esophageal cancer. Front Immunol. (2024) 15:1420399. doi: 10.3389/fimmu.2024.1420399
8. Xu J, Pan HW, Wang XQ, and Chen KP. Status of diagnosis and treatment of esophageal cancer and non-coding RNA correlation research: a narrative review. Transl Cancer Res. (2021) 10:4532–52. doi: 10.21037/tcr-21-687
9. Xu Y, Wang Z, Pei B, Wang J, Xue Y, and Zhao G. DNA methylation markers in esophageal cancer. Front Genet. (2024) 15:1354195. doi: 10.3389/fgene.2024.1354195
10. Yang H, Wang K, Wang T, Li M, Li B, Li S, et al. The combination options and predictive biomarkers of PD-1/PD-L1 inhibitors in esophageal cancer. Front Oncol. (2020) 10:300. doi: 10.3389/fonc.2020.00300
11. Qin Y, Huo M, Liu X, and Li SC. Biomarkers and computational models for predicting efficacy to tumor ICI immunotherapy. Front Immunol. (2024) 15:1368749. doi: 10.3389/fimmu.2024.1368749
12. Yu B, Qi C, Liu Z, Ma N, Tian C, Wang Y, et al. Spatial heterogeneity of PD-L1 expression influence its assessment in esophageal squamous cell carcinoma. Transl Oncol. (2025) 59:102442. doi: 10.1016/j.tranon.2025.102442
13. Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, and Di Rosa F. Regulation of PD-L1 expression by NF-κB in cancer. Front Immunol. (2020) 11:584626. doi: 10.3389/fimmu.2020.584626
14. Butterfield LH and Najjar YG. Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nat Rev Immunol. (2024) 24:399–416. doi: 10.1038/s41577-023-00973-8
15. Brummel K, Eerkens AL, de Bruyn M, and Nijman HW. Tumour-infiltrating lymphocytes: from prognosis to treatment selection. JBJoC. (2023) 128:451–8. doi: 10.1038/s41416-022-02119-4
16. Xie H, Xi X, Lei T, Liu H, and Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
17. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
18. Galon J and Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
19. Teng MW, Ngiow SF, Ribas A, and Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. (2015) 75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255
20. Fukuchi M, Sakurai S, Suzuki M, Naitoh H, Tabe Y, Fukasawa T, et al. Esophageal squamous cell carcinoma with marked eosinophil infiltration. Case Rep Gastroenterol. (2011) 5:648–53. doi: 10.1159/000332441
21. Grisaru-Tal S, Itan M, Klion AD, and Munitz A. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. (2020) 20:594–607. doi: 10.1038/s41568-020-0283-9
22. Sezginer O and Unver N. Dissection of pro-tumoral macrophage subtypes and immunosuppressive cells participating in M2 polarization. Inflammation Res. (2024) 73:1411–23. doi: 10.1007/s00011-024-01907-3
23. Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. (2020) 111:3132–41. doi: 10.1111/cas.14541
24. Wang L, Chen Z, Liu G, and Pan Y. Functional crosstalk and regulation of natural killer cells in tumor microenvironment: Significance and potential therapeutic strategies. Genes Dis. (2023) 10:990–1004. doi: 10.1016/j.gendis.2022.07.009
25. Ali A, Molska GR, Yeo H, Esfandiari N, Jeong W, Huang M, et al. Immune microenvironment in oral potentially Malignant disorders and oral cancer: A narrative review. Int J Mol Sci. (2025) 26:6650. doi: 10.3390/ijms26146650
26. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, and Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
27. Guillaume SM, Beccaria CG, Iannacone M, and Linterman MA. Tertiary lymphoid structures across organs: context, composition, and clinical levers. JIR. (2025) 335:e70063. doi: 10.1111/imr.70063
28. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
29. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
30. Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, et al. A multicenter study of the prognostic value of desmoplastic reaction categorization in stage II colorectal cancer. Am J Surg Pathol. (2019) 43:1015–22. doi: 10.1097/PAS.0000000000001272
31. Kemi NA, Eskuri M, Pohjanen VM, Karttunen TJ, and Kauppila JH. Histological assessment of stromal maturity as a prognostic factor in surgically treated gastric adenocarcinoma. Histopathology. (2019) 75:882–9. doi: 10.1111/his.13934
32. Cheng N, Wang B, Xu J, Xue L, and Ying J. Tumor stroma ratio, tumor stroma maturity, tumor-infiltrating immune cells in relation to prognosis, and neoadjuvant therapy response in esophagogastric junction adenocarcinoma. JVA. (2025) 486:257–66. doi: 10.1007/s00428-024-03755-2
33. Obradovic A, Graves D, Korrer M, Wang Y, Roy S, Naveed A, et al. Immunostimulatory cancer-Associated fibroblast subpopulations can predict immunotherapy response in head and neck cancer. Clin Cancer Res. (2022) 28:2094–109. doi: 10.1158/1078-0432.CCR-21-3570
34. Valkenburg KC, de Groot AE, and Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. (2018) 15:366–81. doi: 10.1038/s41571-018-0007-1
35. Jiang H, Zhou L, Zhang Q, Yu T, and Yu Z. Radiomics-based prediction of T cell-inflamed gene expression profile and prognosis in head and neck squamous cell carcinoma. Med Phys. (2025) 52:e18028. doi: 10.1002/mp.18028
36. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. (2017) 127:2930–40. doi: 10.1172/JCI91190
37. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362:3593. doi: 10.1126/science.aar3593
38. Asaoka Y, Ijichi H, and Koike K. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 373:1979. doi: 10.1056/NEJMc1510353
39. Chang L, Chang M, Chang HM, and Chang F. Microsatellite instability: A predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. (2018) 26:e15–21. doi: 10.1097/PAI.0000000000000575
40. Hewitt LC, Inam IZ, Saito Y, Yoshikawa T, Quaas A, Hoelscher A, et al. Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: A large multi-centre study. Eur J Cancer. (2018) 94:104–14. doi: 10.1016/j.ejca.2018.02.014
41. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
42. Lemery S, Keegan P, and Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. (2017) 377:1409–12. doi: 10.1056/NEJMp1709968
43. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
44. Lee M, Samstein RM, Valero C, Chan TA, and Morris LGT. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccin Immunother. (2020) 16:112–5. doi: 10.1080/21645515.2019.1631136
45. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
46. Sun S, Liu L, Zhang J, Sun L, Shu W, Yang Z, et al. Oncology: The role of neoantigens and tumor mutational burden in cancer immunotherapy: advances, mechanisms, and perspectives. JoH. (2025) 18:84. doi: 10.1186/s13045-025-01732-z
47. Richman LP, Vonderheide RH, and Rech AJ. Neoantigen dissimilarity to the self-proteome predicts immunogenicity and response to immune checkpoint blockade. Cell Syst. (2019) 9:375–382.e374. doi: 10.1016/j.cels.2019.08.009
48. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. (2018) 24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439
49. Wang F, Ren C, Zhao Q, Xu N, Shen L, Dai G, et al. Association of frequent amplification of chromosome 11q13 in esophageal squamous cell cancer with clinical benefit to immune check point blockade. Am Soc Clin Oncol. (2019) 37:4036. doi: 10.1200/JCO.2019.37.15_suppl.4036
50. Li L, Li M, and Wang X. Cancer type-dependent correlations between TP53 mutations and antitumor immunity. DNA Repair (Amst). (2020) 88:102785. doi: 10.1016/j.dnarep.2020.102785
51. Zhang P and Huang Y. Genomic alterations in KMT2 family predict outcome of immune checkpoint therapy in multiple cancers. J Hematol Oncol. (2021) 14:39. doi: 10.1186/s13045-021-01050-0
52. Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. (2019) 5:1504–6. doi: 10.1001/jamaoncol.2019.2963
53. Sudo K, Kato K, Matsuzaki J, Takizawa S, Aoki Y, Shoji H, et al. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol. (2020) 50:114–21. doi: 10.1093/jjco/hyz146
54. Huang S, Zhang J, Lai X, Zhuang L, and Wu J. Identification of novel tumor microenvironment-related long noncoding RNAs to determine the prognosis and response to immunotherapy of hepatocellular carcinoma patients. Front Mol Biosci. (2021) 8:781307. doi: 10.3389/fmolb.2021.781307
55. Luo YH, Yang YP, Chien CS, Yarmishyn AA, Ishola AA, Chien Y, et al. Plasma level of circular RNA hsa_circ_0000190 correlates with tumor progression and poor treatment response in advanced lung cancers. Cancers (Basel). (2020) 12:1740. doi: 10.3390/cancers12071740
56. Kato R, Yamasaki M, Urakawa S, Nishida K, Makino T, Morimoto-Okazawa A, et al. Increased Tim-3(+) T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother. (2018) 67:1673–83. doi: 10.1007/s00262-018-2225-x
57. Kim JY, Choi JK, and Jung H. Genome-wide methylation patterns predict clinical benefit of immunotherapy in lung cancer. Clin Epigenet. (2020) 12:119. doi: 10.1186/s13148-020-00907-4
58. Malczewski AB, Navarro S, Coward JI, and Ketheesan N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J Immunother Cancer. (2020) 8:e001383. doi: 10.1136/jitc-2020-001383
59. Baiden-Amissah REM and Tuyaerts S. Contribution of aging, obesity, and microbiota on tumor immunotherapy efficacy and toxicity. Int J Mol Sci. (2019) 20:3586. doi: 10.3390/ijms20143586
60. Lampis A, Ratti M, Ghidini M, Mirchev MB, Okuducu AF, Valeri N, et al. Challenges and perspectives for immunotherapy in oesophageal cancer: A look to the future (Review). Int J Mol Med. (2021) 47:97. doi: 10.3892/ijmm.2021.4930
61. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
62. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
63. Li Z, Sun Y, Ye F, Ma D, Yin X, Zhuang W, et al. First-line pembrolizumab plus chemotherapy versus chemotherapy in patients with advanced esophageal cancer: Chinese subgroup analysis of KEYNOTE-590. Wolters Kluwer Health. (2021) 39:4049. doi: 10.1200/JCO.2021.39.15_suppl.4049
64. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
65. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-Label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
66. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
67. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab (NIVO) in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiotherapy (CRT): Expanded efficacy and safety analyses from CheckMate 577. Wolters Kluwer Health. (2021) 384:1191–203. doi: 10.1200/JCO.2021.39.15_suppl.4003
68. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
69. Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, et al. Three-Year follow-Up and response-Survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin Cancer Res. (2022) 28:3277–86. doi: 10.1158/1078-0432.CCR-21-0985
70. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-Cell carcinoma. N Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
71. Liu J, Li Z, Fu X, Yang Y, Li H, and Chen Y. 127P A prospective phase II clinical trial exploring neoadjuvant immunotherapy combined with chemotherapy in resectable thoracic esophageal squamous cell cancer (TESCC) with multi-station lymph node metastases (NICE study): Preliminary results. Ann Oncol. (2020) 31:S1292. doi: 10.1016/j.annonc.2020.10.148
72. Ma J, Zhang J, Yang Y, Zheng D, Wang X, Liang H, et al. 65P Camrelizumab combined with paclitaxel and nedaplatin as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESPRIT): A phase II, single-arm, exploratory research. Ann Oncol. (2021) 32:S1400. doi: 10.1016/j.annonc.2021.10.083
73. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-Free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. Jama. (2021) 326:916–25. doi: 10.1001/jama.2021.12836
74. Xu X, Sun Z, Liu Q, Zhang Y, Shen L, Zhang C, et al. Neoadjuvant chemoradiotherapy combined with sequential perioperative toripalimab in locally advanced esophageal squamous cell cancer. J Immunother Cancer. (2024) 12:e008631. doi: 10.1136/jitc-2023-008631
75. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. (2022) 40:277–288.e273. doi: 10.1016/j.ccell.2022.02.007
76. Gu Y, Chen X, Wang D, Ding M, Xue L, Zhen F, et al. 175P A study of neoadjuvant sintilimab combined with triplet chemotherapy of lipo-paclitaxel, cisplatin, and S-1 for resectable esophageal squamous cell carcinoma (ESCC). Ann Oncol. (2020) 31:S1307–8. doi: 10.1016/j.annonc.2020.10.196
77. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. Bmj. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
Keywords: esophageal cancer, programmed death-ligand 1, tumor immune microenvironment, predictive biomarkers, immunotherapy, chemoimmunotherapy
Citation: Ma J, Wu D, Liu Y and Zhang G (2025) Advancing immunotherapy for esophageal cancer: decoding the roles of PD-L1, TME, and tumor-intrinsic biomarkers. Front. Immunol. 16:1679365. doi: 10.3389/fimmu.2025.1679365
Received: 04 August 2025; Accepted: 29 October 2025;
Published: 18 November 2025.
Edited by:
Shangke Huang, Southwest Medical University, ChinaReviewed by:
Xin Zhang, Affiliated Foshan Hospital of Southern Medical University, ChinaCopyright © 2025 Ma, Wu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangping Zhang, eWZ5emdwQDE2My5jb20=
 Jiakang Ma
Jiakang Ma Dangrou Wu2
Dangrou Wu2