- 1Department of Clinical Laboratory Medicine, Fifth Affiliated Hospital, Southern Medical University, Guangzhou, China
- 2Shenzhen Yilifang Biotech CO., LTD., Shenzhen, China
- 3Center for Medical Experiments (CME), Shenzhen Guangming District People’s Hospital, Shenzhen, China
Chikungunya fever (CHIKF), caused by the Chikungunya virus (CHIKV) and transmitted by Aedes mosquitoes, has rapidly evolved from localized outbreaks to a significant global health threat. While the initial high fever is often debilitating, it is the severe and frequently long-lasting pain, affecting joints (arthralgia), muscles (myalgia), and sometimes nerves (neuropathic pain), that truly characterizes the disease’s impact on sufferers. This review explores how CHIKV infection triggers both acute pain and persistent chronic pain. We examine the mechanisms by which the virus directly damages tissues, incites extensive inflammation, invades the nervous system, and potentially manipulates the immune response, leading to autoimmune-like attacks. Understanding these processes is essential, as current treatments mainly focus on symptom management, and there are no specific antiviral therapies available. Identifying the factors that contribute to the persistence of pain is critical for developing targeted and more effective therapeutic interventions, ultimately alleviating the long-term burden of this debilitating disease.
Introduction
Chikungunya fever (CHIKF) is an arboviral disease caused by the chikungunya virus (CHIKV), which belongs to the Togaviridae family and the Alphavirus genus (1). The virus is primarily transmitted by mosquitoes of the Aedes aegypti and Aedes albopictus species, which are widespread in many regions (2). CHIKV was first isolated in Tanzania in 1952. Initially, the disease was endemic to Africa, Asia, and the Indian subcontinent. However, since 2013, it has rapidly expanded into the Americas, infecting over 1.5 million individuals (3). The adaptation of CHIKV to Aedes albopictus, which is capable of transmitting the virus in temperate climates, has increased the risk of outbreaks in regions previously considered unaffected. The rapid spread of the virus is further driven by increased international travel and trade, raising concerns about its potential to establish new transmission cycles in Europe, the Americas, and other temperate zones (4, 5). The name “chikungunya” is derived from the Makonde language, meaning “that which bends up,” referencing the severe joint pain and postural changes characteristic of the disease.
CHIKF is characterized by acute, often debilitating symptoms, including high fever, severe polyarthralgia (joint pain), myalgia (muscle pain), headache, rash, and nausea (6). A hallmark feature is intense, acute joint pain, typically affecting small joints, and often accompanied by swelling and stiffness. Notably, a significant proportion of patients develop chronic arthralgia that can persist for months or even years, severely impairing daily function and quality of life (7). Factors influencing the severity and duration of symptoms include patient age, pre-existing health conditions, and the initial disease course (8, 9). Older adults and individuals with pre-existing rheumatological conditions are at higher risk of developing persistent symptoms, underscoring the importance of ongoing research to better understand and manage the disease (10). The virus invades human cells by binding to receptors such as MXRA8, rapidly replicates, and causes extensive tissue damage (11). This tropism for musculoskeletal tissues, including myocytes (muscle cells), directly contributes to the characteristic myalgia, while infection of joint tissues underlies the debilitating arthralgia. Currently, there is no specific antiviral treatment for CHIKF. Management remains symptomatic, primarily involving analgesics and anti-inflammatory agents. Recently, cases of CHIKV infection have been identified in the Guangdong province, China, with gradual spread, posing a certain threat to the health of local residents and socioeconomic stability (12, 13). Therefore, a better understanding of the underlying mechanisms of pain in CHIKF could help develop targeted therapies, improving pain management and overall patient outcomes. This review summarizes the occurrence, potential mechanisms, and emerging therapeutic approaches for the pain syndrome, a primary symptom of CHIKF, aiming to provide a reference for clinicians and patients alike.
Virology and pathogenesis of CHIKV
CHIKV is a positive-sense, single-stranded RNA virus with a genome approximately 12 kilobases in length, encoding four non-structural proteins (nsP1-4) and four structural proteins (C, E1, E2, and 6k) (14). The enveloped virus measures around 60–70 nm in diameter and is sensitive to desiccation and temperatures exceeding 58 °C (15).
The virus gains entry into human cells primarily via specific receptors such as MXRA8, which mediates attachment and internalization (11). Following receptor binding, the virus is endocytosed, and the viral envelope fuses with the endosomal membrane, releasing the genomic RNA into the cytoplasm. The viral RNA serves as mRNA for the translation of the non-structural polyprotein, which is processed into nsP1-4. These proteins form the replication complex, synthesizing a negative-strand RNA intermediate, which in turn templates the production of new genomic RNA and a subgenomic RNA that encodes the structural proteins. New virions assemble in the cytoplasm, are processed through the Golgi apparatus, and are released from the host cell by exocytosis (15).
It shows a strong tropism for musculoskeletal tissues, such as synoviocytes, fibroblasts, and myocytes, as well as neural tissues. Upon initial infection at the bite site, particularly of skin fibroblasts and macrophages, CHIKV begins replicating using the host’s cellular machinery (16). Subsequently, it disseminates through the bloodstream and lymphatic system to other tissues, including joints, muscles, and peripheral nerves. This tissue localization of virus results in cellular damage and triggers immune responses, both of which contribute to the hallmark symptoms of high fever, severe joint pain, and systemic inflammation observed in infected individuals (11, 15, 17).
Clinically, the disease manifests with an acute phase characterized by high fever, headache, and debilitating, bilateral, and symmetrical joint pain involving small joints such as wrists, ankles, and phalanges. The joint pain is often severe enough to limit mobility and may be accompanied by swelling in a significant proportion of cases. While neurological complications like encephalitis and meningitis are less common, they can occur, particularly in vulnerable populations. A distinctive and persistent feature of Chikungunya infection is the progression into a chronic phase, during which joint pain, fatigue, and musculoskeletal dysfunction can persist for months or even years, greatly affecting the patient’s quality of life. This chronicity tends to be associated with risk factors such as older age and pre-existing rheumatological conditions, with women showing a heightened susceptibility to severe or prolonged symptoms (7, 9, 18). Despite extensive research, the underlying mechanisms driving the persistent pain in the chronic phase remain incompletely understood, though immune-mediated processes, particularly the production of pro-inflammatory cytokines, are believed to play a significant role. The pathogenesis involves a complex interplay between the virus, the host immune system, and environmental factors, which together orchestrate both the acute inflammatory response and the sustained pain symptoms that characterize chronic CHIKF.
Mechanisms of pain syndrome in CHIKF
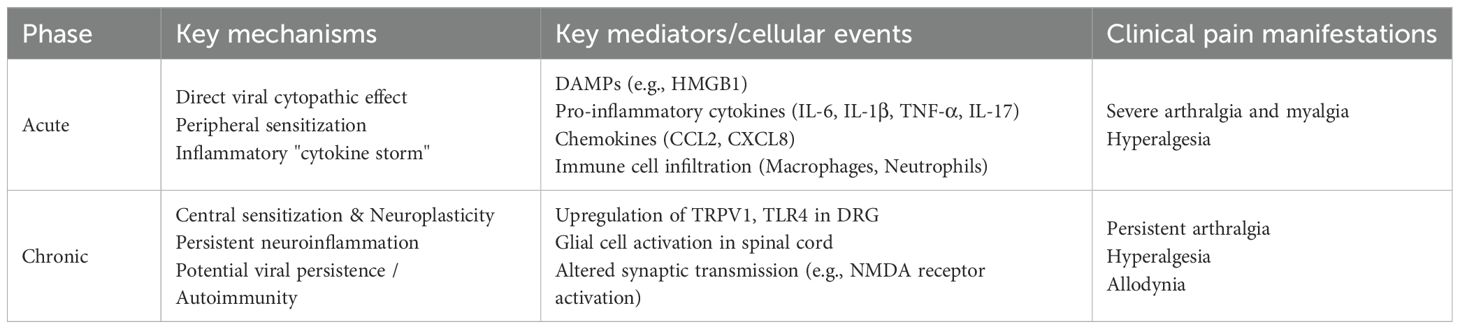
The mechanisms underlying pain in CHIKF involve a complex interplay of direct tissue damage, immune responses, and neural sensitization (19, 20). The virus induces cytopathic effects in synovial cells, muscle fibers, and endothelial cells, leading to cell injury and death (17, 21). This damage results in the release of damage-associated molecular patterns (DAMPs), which activate immune cells and nociceptive neurons, thereby amplifying pain signaling (22–24). In affected joints, the disruption of normal synovial homeostasis leads to synovitis and cartilage degradation, which are key contributors to joint pain (25). During the acute phase of infection, a robust immune response is triggered, characterized by the release of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β) (26). It has also been demonstrated that IL-17 plays as a putative hallmark of intense arthralgia and age-related serum immune mediator networks during acute CHIKF (27). These cytokines, along with DAMPs, provoke an “immune storm” that floods infected tissues with inflammatory mediators (28–30). These substances sensitize and activate nociceptors, the pain-sensing nerve endings, resulting in heightened pain perception (Table 1).
Moreover, inflammatory cytokines and chemokines such as CCL2 and CXCL8 recruit immune cells like macrophages, neutrophils, and lymphocytes into the tissues, where they release additional mediators that perpetuate inflammation and further sensitize nociceptors (31, 32). This creates an environment akin to adding fuel to a fire, intensifying pain sensations. Additionally, inflammatory mediators such as prostaglandins, which are targeted by non-steroidal anti-inflammatory drugs (NSAIDs), and bradykinin contribute to nociceptor sensitization, making even light touch painfully perceptible (33). This ongoing inflammatory response explains the intense pain characteristic of the acute phase of CHIKF (34). Evidence suggests that the virus can invade peripheral nerves, dorsal root ganglia (DRG) (20), and central nervous system structures (35, 36). Such neuroinvasion can disrupt neural function, alter signal transmission, and lead to neuropathic pain (19).
Persistent viral RNA in neural tissues may sustain chronic pain even after the initial infection has resolved (31). In the chronic phase of disease, pain persists and is often associated with upregulation of pain-related receptors on sensory neurons, such as TRPV1 and TLR4 (37–39). These changes induce neuroplasticity within the DRG and spinal cord, leading to remodeling of neural circuits and increased pain sensitivity, phenomena known as central sensitization (15).
Central sensitization represents a maladaptive state of the CNS
In the spinal cord, persistent nociceptive input from the periphery leads to increased excitability of dorsal horn neurons. This involves enhanced synaptic efficacy mediated by glutamate receptors (e.g., NMDA receptors), reduced inhibitory control by GABAergic and glycinergic interneurons, and increased activity of glial cells (astrocytes and microglia) which release pro-inflammatory cytokines that further amplify neuronal signaling (24, 38). Concurrently, within the DRG, the cell bodies of sensory neurons undergo transcriptional and translational changes, leading to the increased expression of pain-related channels and receptors (e.g., TRPV1, Nav1.7, TLR4). This neuroplastic remodeling lowers the activation threshold for pain (peripheral sensitization) and amplifies pain signals transmitted to the brain (central sensitization). As a result, the nervous system becomes hyperexcitable, and normal stimuli may evoke exaggerated pain responses (hyperalgesia), while normally non-painful stimuli (like light touch) can become painful (allodynia) (Table 1, Figure 1).
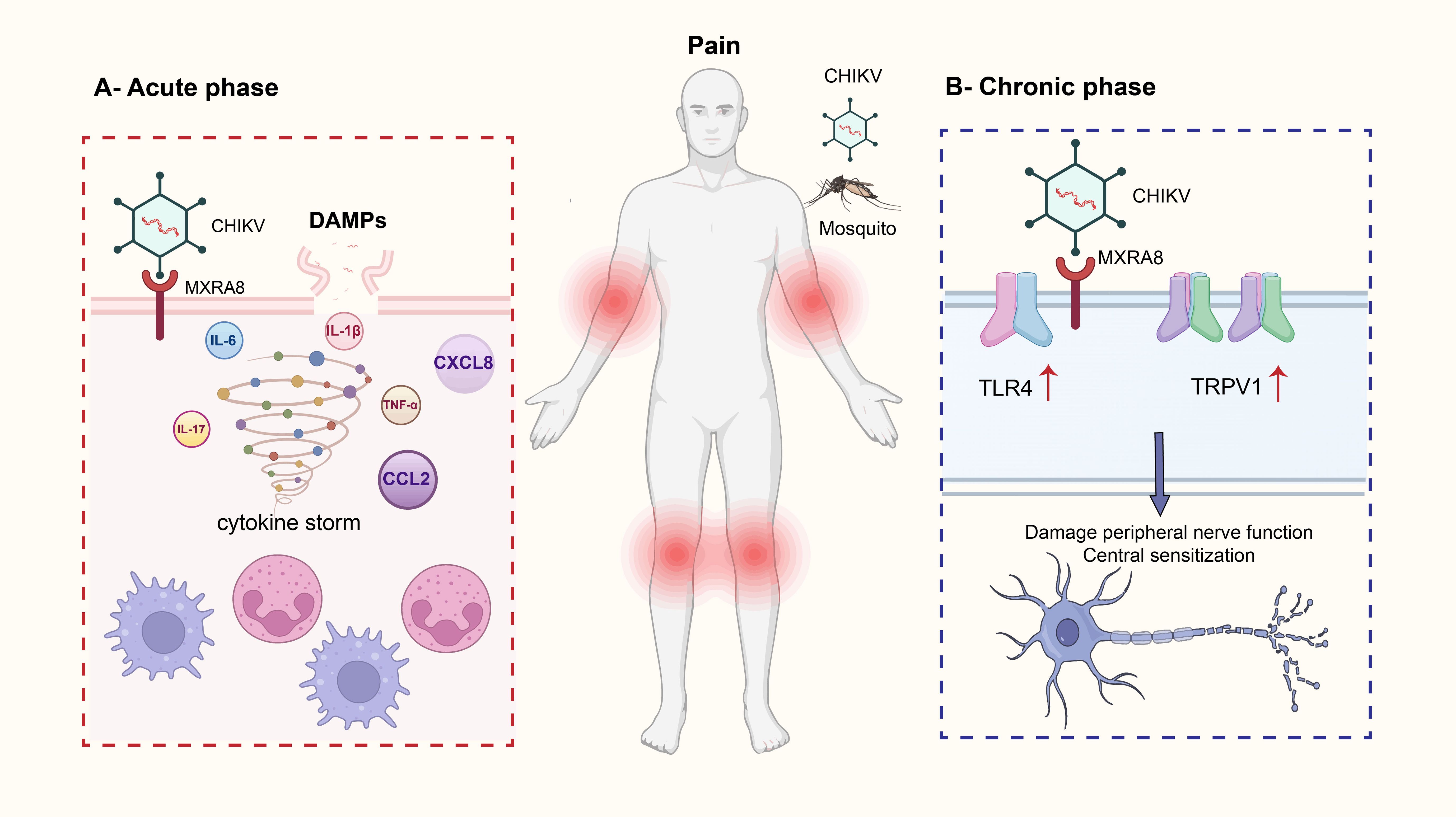
Figure 1. This image illustrates the mechanism by which Chikungunya virus (CHIKV) infection leads to pain. Chikungunya fever is primarily transmitted by the bites of Aedes aegypti and Aedes albopictus mosquitoes. Clinical symptoms typically include high fever, severe polyarthralgia, myalgia, headache, rash, and nausea. In acute phase, CHIKV enters host cells by binding to the MXRA8 receptor, which triggers the release of damage-associated molecular patterns (DAMPs). These DAMPs initiate a cytokine storm, characterized by the release of multiple pro-inflammatory cytokines and chemokines, including IL-6, IL-17, IL-1β, TNF-α, CXCL8, and CCL2. This immune response activates and recruits immune cells such as neutrophils and macrophages, leading to amplified inflammation and acute joint pain. In chronic phase, persistent viral invasion of neural structures results in abnormal nerve function. Chronic pain-related receptors such as TRPV1 and TLR4 become upregulated, promoting neuroplasticity in dorsal root ganglia (DRG) and spinal cord neurons. This contributes to central sensitization, which underlies prolonged and potentially worsening chronic pain.
Factors influencing pain
While the aforementioned mechanisms directly drive pain, several host-related factors can significantly influence an individual’s susceptibility to and the severity of CHIKF-associated pain. Polymorphisms in genes encoding cytokines or pain-related receptors may affect the body’s response to CHIKV infection. For example, certain gene polymorphisms may lead to excessive production of inflammatory cytokines, increasing the severity of pain and the risk of chronic pain. X-linked polymorphisms in TLR7 and TLR8 genes are associated with protection against Chikungunya fever (40, 41). Elderly patients and those with pre-existing joint disorders are more likely to develop chronic pain after CHIKV infection (7, 9). There is also gender-specific differences in pain perception and immune response. Generally, women may be more sensitive to pain, which may affect the course and severity of CHIKF (42). Taken together, the mechanisms underlying pain in CHIKF encompass direct viral damage, immune-mediated inflammation, neural plasticity, and genetic predispositions. Understanding these interconnected processes is crucial for developing effective therapeutics aimed at reducing both acute and chronic pain associated with the disease.
Clinical manifestations and challenges in pain management
Chikungunya is a mosquito-borne viral disease that causes acute and chronic pain symptoms, particularly polyarthralgia and myalgia. The pathogenesis begins with the infection of specific cells, which triggers an inflammatory response that underlies the clinical symptoms during the acute phase. In the acute phase, patients typically experience intense inflammatory arthralgia and myalgia, often accompanied by fever and joint swelling (43, 44). The pain develops rapidly and can be severe, significantly impairing mobility and daily functioning. As the disease progresses, some individuals develop persistent arthralgia and neuropathic pain, which are more difficult to treat and can substantially affect quality of life. These prolonged symptoms pose a major management challenge, partly because they often overlap with other arboviral infections such as dengue and Zika virus (45).
Diagnostic challenges exist because the symptoms of CHIKF overlap with those of other arboviral diseases, such as dengue fever and Zika virus infection. Persistent inflammatory joint pain, sometimes evolving into distinct neuropathic pain (burning, tingling). Telling CHIKF pain apart from dengue or Zika early on can be tricky, relying on symptom patterns and lab tests. During the acute phase, RT-PCR can detect viral RNA in blood, while serological assays are valuable for diagnosing past infections or ongoing immune responses during later stages (46–48). The incubation period ranges from 4 to 12 days, with most symptoms resolving within ten days, though joint pain may persist for weeks or months beyond the initial illness. Effective diagnosis in regions where multiple arboviruses are endemic relies on an integrated approach that combines clinical, laboratory, and epidemiological data. Although diagnostic advancements have improved accuracy, differentiating CHIKF remains complex, underscoring the importance of comprehensive testing to guide management.
Currently, the pain management of CHIKF are primarily supportive, aimed at relieving pain, inflammation, and swelling, which mainly relies on non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, analgesics (e.g., Acetamino-phen, Opioids) and neuropathic agents (e.g., Gabapentin, Amitriptyline). However, the effect of these drugs is limited, and long-term use may have adverse effects (49). Emerging therapies are exploring targeted approaches, such as cytokine inhibitors and TRPV1 antagonists, are being studied, which may provide new ideas for pain treatment. The emerging understanding of mechanisms points to promising future targets: drugs blocking specific cytokines (IL-6, TNF-α) (50), inhibiting overactive pain receptors (TRPV1 antagonists, TLR4 antagonists) (24, 38), or modulating the immune response driving chronicity, offering potential for more precise pain control. Despite ongoing research, there are no specific antiviral treatments for CHIKF. Supportive care remains the mainstay, emphasizing symptomatic relief. Although agents like ribavirin have been investigated, their efficacy is uncertain. Severe neurological or ocular complications may necessitate hospitalization. Patients are advised to rest, stay well-hydrated, and avoid medications like aspirin that increase bleeding risk. Prevention primarily relies on vector control and reducing mosquito exposure (51). Public health measures include the use of insect repellents, protective clothing, elimination of breeding sites, and insecticide-treated nets. Several vaccine candidates are currently in clinical trials, aiming to develop a safe and effective vaccine that offers long-term protection and reduces disease burden. Overall, while research continues, effective antiviral therapies and universally protective vaccines remain experimental. In the meantime, comprehensive supportive care, accurate diagnosis, and preventive measures are essential for managing CHIKF.
Future directions and research gaps
Although significant progress has been made in understanding the pain mechanisms of CHIKF, many questions remain unanswered. For instance, the molecular basis of CHIKV persistence within tissues, particularly in synovial and neural tissues, is not yet fully understood. Moreover, it is unclear what factors drive the transition from acute to chronic pain, whether it is related to viral RNA persistence, immune memory, or neural scarring. Unraveling these mechanisms is crucial for developing targeted interventions. Current animal models have limitations in accurately replicating human pain phenotypes, especially the complex, long-term aspects of CHIKF-associated pain. Developing more precise translational models is essential for advancing our understanding of the underlying mechanisms and for evaluating the efficacy of novel therapies. Creating models that closely mimic chronic arthralgia and neuropathic pain seen in humans would provide valuable insights.
The development of virus-specific antivirals represents an important future direction, as such treatments could prevent the source of pain altogether. In addition, targeted therapies for chronic pain, such as drugs that inhibit neuroinflammation or restore neural homeostasis, warrant further investigation. Improving our understanding of these pathways could offer new avenues for effective pain management in long-term patients. Future research should prioritize interdisciplinary approaches that integrate clinical, epidemiological, and basic biomedical investigations. Such collaboration can deepen our understanding of virus transmission dynamics and the long-term effect of CHIKF. The development of advanced diagnostic tools and innovative therapies, such as targeted antivirals and immunomodulators, has the potential to significantly improve patient outcomes. Overall, addressing these knowledge gaps will be key to advancing the prevention, diagnosis, and treatment of CHIKF and its associated pain.
Conclusion
CHIKF is a significant emerging infectious disease characterized by debilitating pain that can persist long after the initial infection. Its pathogenesis involves a complex interplay of direct viral tissue damage, extensive inflammatory responses, neural invasion, and host-specific factors. Understanding these mechanisms is crucial for developing targeted and effective therapies. Future research should focus on elucidating the molecular and cellular pathways involved, identifying novel therapeutic targets, and improving animal models to enhance the translation of research findings into clinical practice. Ultimately, effective management of chikungunya-associated pain, both during the acute and chronic phases, requires moving beyond symptomatic treatment. Strategies that target viral replication, modulate immune responses, and restore neural function hold promise for reducing long-term disease burden. Gaining deeper insights into these processes offers hope for lessening the long-term health impacts of this increasingly global threat.
Author contributions
WS: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. SL: Writing – review & editing, Methodology, Validation, Investigation. MZ: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 82171378, 82401438), Science, Technology and Innovation Commission of Shenzhen Municipality (No. JCYJ20240813114512016 and No. JCYJ20240813152049062), Shenzhen Nanshan District Healthcare System Science and Technology Key Projects (No. NSZD2023003), Medical-Engineering Interdisciplinary Research Foundation of Shenzhen University (2023YG031).
Conflict of interest
Author SL was employed by Shenzhen Yilifang Biotech CO., LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weaver SC and Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. (2015) 372:1231–9. doi: 10.1056/NEJMra1406035
2. Mourad O, Makhani L, and Chen LH. Chikungunya: an emerging public health concern. Curr Infect Dis Rep. (2022) 24:217–28. doi: 10.1007/s11908-022-00789-y
3. de Souza WM, Ribeiro GS, de Lima STS, de Jesus R, Moreira FRR, Whittaker C, et al. Chikungunya: a decade of burden in the Americas. Lancet Reg Health Am. (2024) 30:100673. doi: 10.1016/j.lana.2023.100673
4. Sharif N, Sarkar MK, Ferdous RN, Ahmed SN, Billah MB, Talukder AA, et al. Molecular epidemiology, evolution and reemergence of chikungunya virus in south asia. Front Microbiol. (2021) 12:689979. doi: 10.3389/fmicb.2021.689979
5. Eneh SC, Uwishema O, Nazir A, El Jurdi E, Olanrewaju OF, Abbass Z, et al. Chikungunya outbreak in Africa: a review of the literature. Ann Med Surg (Lond). (2023) 85:3545–52. doi: 10.1097/MS9.0000000000000979
6. Frezgi O, Berhane A, Ghebrewelde G, Tekie H, Kiflezgi T, Mohamedsied A, et al. Acute clinical features and persistence of joint pain in probable cases of chikungunya fever in Eritrea. Open Access Rheumatol. (2025) 17:13–24. doi: 10.2147/OARRR.S465082
7. Campos LO, Cunha Dos Santos ME, Esquirio AF, Gama GL, de Cassia Macedo M, Barbosa MA, et al. Post-Chikungunya chronic arthralgia effects on pain, balance, strength, and gait: do middle-aged differ from older adults? J Bodyw Mov Ther. (2025) 43:251–8. doi: 10.1016/j.jbmt.2025.05.019
8. Torres Dos Santos Lopes D, Cerutti Junior C, Areias Cabidelle A, Espinosa Miranda A, Drumond Louro I, Pamplona de Goes Cavalcanti L, et al. Factors associated with hospitalization in the acute phase of Chikungunya. PloS One. (2023) 18:e0296131. doi: 10.1371/journal.pone.0296131
9. Binti Adnan NAA, Kalam N, Lim Zi Jiunn G, Komarasamy TV, and Balasubramaniam V. Infectomics of chikungunya virus: roles played by host factors. Am J Trop Med Hyg. (2025) 112:481–90. doi: 10.4269/ajtmh.23-0819
10. Holanda JSCB, Mendes EF, Silva-Filho E, Queiroz de Medeiros AC, Di-Bonaventura S, Pegado R, et al. Clinical variables associated with functional deficits in patients affected by chronic chikungunya arthralgia: A cross-sectional study. J Back Musculoskelet Rehabil. (2025).
11. Zhang R, Kim AS, Fox JM, Nair S, Basore K, Klimstra WB, et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature. (2018) 557:570–4. doi: 10.1038/s41586-018-0121-3
12. Pedi VD, de Franca GVA, Rodrigues VB, Duailibe FT, Santos MTP, de Oliveira MRF, et al. Burden of chikungunya fever and its economic and social impacts worldwide: A systematic review. Trop Med Int Health. (2025). doi: 10.1111/tmi.70012
13. Ren J, Ling F, Liu Y, and Sun J. Chikungunya in zhejiang province, southeast China. Infect Med (Beijing). (2023) 2:315–23. doi: 10.1016/j.imj.2023.11.005
14. de Lima Cavalcanti TYV, Pereira MR, de Paula SO, and Franca RFO. A review on chikungunya virus epidemiology, pathogenesis and current vaccine development. Viruses. (2022) 14. doi: 10.3390/v14050969
15. Freppel W, Silva LA, Stapleford KA, and Herrero LJ. Pathogenicity and virulence of chikungunya virus. Virulence. (2024) 15:2396484. doi: 10.1080/21505594.2024.2396484
16. Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. (2010) 120:894–906. doi: 10.1172/JCI40104
17. Lidbury BA, Rulli NE, Suhrbier A, Smith PN, McColl SR, Cunningham AL, et al. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J Infect Dis. (2008) 197:1585–93. doi: 10.1086/587841
18. Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. (2011) 203:149–57. doi: 10.1093/infdis/jiq042
19. de Andrade DC, Jean S, Clavelou P, Dallel R, and Bouhassira D. Chronic pain associated with the Chikungunya Fever: long lasting burden of an acute illness. BMC Infect Dis. (2010) 10:31. doi: 10.1186/1471-2334-10-31
20. Huerta Albarran R, Weber A, Aviles Robles M, and Appendino JP. Chikungunya virus infection: A scoping review highlighting pediatric systemic and neurologic complications. Semin Pediatr Neurol. (2025) 54:101213. doi: 10.1016/j.spen.2025.101213
21. Zhang Y, Yan H, Li X, Zhou D, Zhong M, Yang J, et al. A high-dose inoculum size results in persistent viral infection and arthritis in mice infected with chikungunya virus. PloS Negl Trop Dis. (2022) 16:e0010149. doi: 10.1371/journal.pntd.0010149
22. Chang AY, Hernandez AS, Mejia JF, Tritsch SR, Mendoza-Torres E, Encinales L, et al. The natural history of post-chikungunya viral arthritis disease activity and T-cell immunology: A cohort study. J Cell Immunol. (2024) 6:64–75. doi: 10.33696/immunology.6.191
23. Brito RMM, de Melo MF, Fernandes JV, Valverde JG, Matta Guedes PM, de Araujo JMG, et al. Acute chikungunya virus infection triggers a diverse range of T helper lymphocyte profiles. Viruses. (2024) 16. doi: 10.3390/v16091387
24. Segato-Vendrameto CZ, Zanluca C, Zucoloto AZ, Zaninelli TH, Bertozzi MM, Saraiva-Santos T, et al. Chikungunya virus and its envelope protein E2 induce hyperalgesia in mice: inhibition by anti-E2 monoclonal antibodies and by targeting TRPV1. Cells. (2023) 12. doi: 10.3390/cells12040556
25. Amaral JK, Schoen RT, Bingham CO, Chang A, and Candido EL. Immunomodulatory therapy of chikungunya arthritis: systematic review and meta-analysis. J Travel Med. (2025). doi: 10.1093/jtm/taaf067
26. Venugopalan A, Ghorpade RP, and Chopra A. Cytokines in acute chikungunya. PloS One. (2014) 9:e111305. doi: 10.1371/journal.pone.0111305
27. Teixeira CW, Dias JP, Morgado-Santos L, da Costa-Rocha IA, Giarola-Silva S, Lopes-Ribeiro A, et al. IL-17 as a putative hallmark of intense arthralgia and age-related serum immune mediator networks during acute chikungunya fever. Inflammation Res. (2025) 74:16. doi: 10.1007/s00011-024-01977-3
28. Nunes JAL, Sousa JR, Smith VC, Quaresma JS, Vasconcelos P, Chiang JO, et al. Immunological impact of cytokines on the chikungunya virus pathophysiology: A literature narrative review. Rev Med Virol. (2023) 33:e2441. doi: 10.1002/rmv.2441
29. Morel Z, Martinez T, Galeano F, Coronel J, Quintero L, Jimenez R, et al. Cytokine storm in Chikungunya: Can we call it multisystem inflammatory syndrome associated with Chikungunya? Reumatol Clin (Engl Ed). (2024) 20:223–5.
30. Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM, et al. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. (2011) 204:115–23. doi: 10.1093/infdis/jiq006
31. Morrison T, Zarrella K, Sheridan R, Ware B, Davenport B, da Silva M, et al. Chikungunya virus persists in joint associated macrophages and promotes chronic disease. Res Sq. (2025). doi: 10.21203/rs.3.rs-6917990/v1
32. Jacob-Nascimento LC, Carvalho CX, Silva MMO, Kikuti M, Anjos RO, Fradico JRB, et al. Acute-phase levels of CXCL8 as risk factor for chronic arthralgia following chikungunya virus infection. Front Immunol. (2021) 12:744183. doi: 10.3389/fimmu.2021.744183
33. Bedoui Y, Septembre-Malaterre A, Giry C, Jaffar-Bandjee MC, Selambarom J, Guiraud P, et al. Robust COX-2-mediated prostaglandin response may drive arthralgia and bone destruction in patients with chronic inflammation post-chikungunya. PloS Negl Trop Dis. (2021) 15:e0009115. doi: 10.1371/journal.pntd.0009115
34. Chopra A, Saluja M, and Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. (2014) 66:319–26. doi: 10.1002/art.38221
35. Das T, Jaffar-Bandjee MC, Hoarau JJ, Krejbich Trotot P, Denizot M, Lee-Pat-Yuen G, et al. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog Neurobiol. (2010) 91:121–9. doi: 10.1016/j.pneurobio.2009.12.006
36. Porto Silva CN, Crispim JG, Pereira MC, Galdino da Rocha Pitta M, Barreto de Melo Rego MJ, Melgarejo da Rosa M, et al. The communication between chikungunya infection and the central nervous system. Microb Pathog. (2025) 206:107747. doi: 10.1016/j.micpath.2025.107747
37. Sanjai Kumar P, Nayak TK, Mahish C, Sahoo SS, Radhakrishnan A, De S, et al. Inhibition of transient receptor potential vanilloid 1 (TRPV1) channel regulates chikungunya virus infection in macrophages. Arch Virol. (2021) 166:139–55. doi: 10.1007/s00705-020-04852-8
38. da Silva LCM, Dos Santos Maia AC, de Sousa NCF, Pavi CP, Savi BP, Nagashima S, et al. Chikungunya particle and RNA induce mechanical and heat hypersensitivities in a TRPV1-dependent manner. Biomolecules. (2025) 15. doi: 10.3390/biom15020171
39. Mahish C, De S, Chatterjee S, Ghosh S, Keshry SS, Mukherjee T, et al. TLR4 is one of the receptors for Chikungunya virus envelope protein E2 and regulates virus induced pro-inflammatory responses in host macrophages. Front Immunol. (2023) 14:1139808. doi: 10.3389/fimmu.2023.1139808
40. Gotay WJP, Rodrigues RO, and Yaochite JNU. Influence of host genetic polymorphisms involved in immune response and their role in the development of Chikungunya disease: a review. Braz J Med Biol Res. (2023) 56:e12557. doi: 10.1590/1414-431x2023e12557
41. Gotay WJP, Maciel MSC, Rodrigues RO, Cardoso CC, Oliveira CN, Montenegro AFL, et al. X-linked polymorphisms in TLR7 and TLR8 genes are associated with protection against Chikungunya fever. Mem Inst Oswaldo Cruz. (2025) 120:e230224. doi: 10.1590/0074-02760230224
42. Bouquillard E, Fianu A, Bangil M, Charlette N, Ribera A, Michault A, et al. Rheumatic manifestations associated with Chikungunya virus infection: A study of 307 patients with 32-month follow-up (RHUMATOCHIK study). Joint Bone Spine. (2018) 85:207–10. doi: 10.1016/j.jbspin.2017.01.014
43. Santiago RA, Bavaresco SPP, Citrangulo SG, Medronho RA, Sampaio V, Costa AJL, et al. Clinical manifestations associated with the chronic phase of Chikungunya Fever: A systematic review of prevalence. PloS Negl Trop Dis. (2025) 19:e0012810. doi: 10.1371/journal.pntd.0012810
44. Goncalves WA, de Sousa CDF, Teixeira MM, and Souza DG. A brief overview of chikungunya-related pain. Eur J Pharmacol. (2025) 994:177322. doi: 10.1016/j.ejphar.2025.177322
45. Santos LLM, de Aquino EC, Fernandes SM, Ternes YMF, and Feres VCR. Dengue, chikungunya, and Zika virus infections in Latin America and the Caribbean: a systematic review. Rev Panam Salud Publica. (2023) 47:e34. doi: 10.26633/RPSP.2023.34
46. Sajith A, Iyengar V, Varamballi P, Mukhopadhyay C, and Nittur S. Diagnostic utility of real-time RT-PCR for chikungunya virus detection in the acute phase of infection: a retrospective study. Ann Med. (2025) 57:2523559. doi: 10.1080/07853890.2025.2523559
47. Simo FBN, Burt FJ, and Makoah NA. Chikungunya virus diagnosis: A review of current antigen detection methods. Trop Med Infect Dis. (2023) 8. doi: 10.3390/tropicalmed8070365
48. Li X, Wan X, Liu J, Wang H, Li A, Ke C, et al. Luciferase immunosorbent assay based on multiple E antigens for the detection of chikungunya virus-specific igG antibodies. Microbiol Spectr. (2022) 10:e0149621.
49. Cunha RVD and Trinta KS. Chikungunya virus: clinical aspects and treatment - A Review. Mem Inst Oswaldo Cruz. (2017) 112:523–31. doi: 10.1590/0074-02760170044
50. Moreira TP, de Sousa CDF, Melo Costa VR, Queiroz-Junior CM, Santos FM, Bonilha CS, et al. Tumour necrosis factor plays a deleterious role in the pathogenesis of chikungunya virus infection. Immunology. (2023) 168:444–58. doi: 10.1111/imm.13583
Keywords: chikungunya virus, arbovirus, pain, arthralgia, myalgia, inflammation, neuroinvasion, cytokines
Citation: Sun W, Shi S, Liao S and Zhai M (2025) Chikungunya fever: pathogenesis and mechanisms underlying pain symptoms. Front. Immunol. 16:1679385. doi: 10.3389/fimmu.2025.1679385
Received: 04 August 2025; Accepted: 28 October 2025;
Published: 11 November 2025.
Edited by:
Giada Amodeo, University of Milan, ItalyReviewed by:
Giulia Galimberti, University of Trento, ItalyCopyright © 2025 Sun, Shi, Liao and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wuping Sun, d3VwaW5nLnN1bkBmb3htYWlsLmNvbQ==; Mingzhu Zhai, bWluZ3podS56aGFpQGZveG1haWwuY29t
 Wuping Sun
Wuping Sun Shuqi Shi1
Shuqi Shi1