- 1Hunan University of Chinese Medicine, Changsha, China
- 2Hunan Academy of Chinese Medicine, Changsha, China
- 3Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine, Changsha, China
As the body’s largest immunological interface, the intestine harbors a complex ecosystem of gut microbiota (GM) that orchestrates mucosal immune maturation while sustaining local immunological equilibrium. Emerging evidence reveals the gut’s influence on skeletal homeostasis via neuro-immune-endocrine pathways—termed the gut-bone axis—though its mechanistic intricacies remain incompletely defined. Since the concept of osteoimmunology was proposed in 2000 by Arron & Choi, immune-skeletal interactions have garnered significant research traction. Immune cells primarily contribute to the maintenance of bone homeostasis through the release of pro- and anti-inflammatory factors. Consequently, the immune system represents a crucial intermediary in understanding the relationship between GM and metabolic bone diseases. This review synthesizes the interrelationships among gut microbiota, immune cells, and osteoporosis, and elucidates how GM modulate bone metabolism in osteoporosis through this critical intermediary. Furthermore, building upon the microbiome–immune–bone axis, we highlight several emerging microbiota-targeted interventions—such as probiotics, prebiotics, dietary modifications, fecal microbiota transplantation, and engineered microbes—and evaluate their clinical translational potential, with the aim of advancing diagnostic and therapeutic strategies for metabolic bone disorders.
1 Introduction
Osteoporosis (OP), a common systemic metabolic bone disease, is characterized by low bone mass and structural deterioration of bone tissue leading to bone fragility and an increased risk for fractures (1). Globally, over 200 million people suffer from OP (2). And it is estimated that OP will be a significant healthcare burden in the future due to the increased incidence of the disease with age and female gender. Currently, the underlying molecular mechanisms of OP pathogenesis continue to be investigated. With the in-depth study of gut microbiome (GM), some studies have demonstrated decreased bacterial richness and diversity in patient with postmenopausal osteoporosis (PMOP) (3).
The GM is now viewed as a tissue that interacts bidirectionally with the gastrointestinal, immune, endocrine and nervous systems, affecting the cellular responses in numerous organs (4, 5). Accumulating evidence suggests that the GM plays an important role in the regulation of the bone homeostasis and serves as a contributing mechanism of OP (5). Current research reveals that bone homeostasis is linked to a healthy microbiome and that gut dysbiosis can exacerbate osteoclasts (OCs) activity and promote OP (6). The GM can affect bone metabolism through multiple pathways including the intestinal barrier, metabolic pathways, nutrient absorption such as calcium and phosphorus, the immune system, and hormonal environment (7). However, the mechanism by which intestinal flora regulate bone homeostasis remains to be elucidated.
Meanwhile, as the largest immune organ in the human body, the GM is where most lymphocytes interact with other immune factors. The GM could regulate the maturation of the mucosal immune system, whereas the pathogenic microbiome causes immune dysfunction leading to disease development. In recent years, a growing number of studies have reported a close relationship among immune cells, GM and OP. And some studies indicated that intestinal flora regulates immunity and affects bone metabolism. Here, we summarize the relationship between immune cells and GM and OP, and show how the GM regulates OP through immune cells.
2 Gut microbiota and osteoporosis
The human gastrointestinal tract contains more than 10 trillion bacteria. As a result, the GM is also known as the second largest human genome. Recently, accumulating research confirmed that the diverse community of microorganisms has been recognized as a profound role in maintaining the bone homeostasis (8). It was found that the bacterial composition and diversity are altered in patients with OP compared to normal people, supporting the view that the pathological process of OP is affected by the GM (9). Herein, we attempted to explore the intimate connection between the GM and OP from the following three aspects.
2.1 The gut-bone axis
The intestinal microbiota is actively involved in many necessary physiological reactions. The gut interacts with most of the organs through neural, immune and endocrine pathways, a connection known as the gut-organ axis (10). Among them, the gut-bone axis mainly refers to the bidirectional relationship between the GM and bone tissue (11). Recently, the correlation between OP and intestinal microflora has been studied. Sjögren et al. conducted a study on germ-free (GF) mice and found GF mice exhibited decreased bone mineral density (BMD) compared to conventionally raised mice. Conversely, when the GM from conventionally raised mice was transferred to the GF mice, reduced bone density was reversed (12).
Their interplay tends to emphasize that the GM is a key regulator of bone health, suggesting its potential therapeutic targets for multiple bone disorders, especially for OP. A cross-sectional study reported that increased abundance of Prevotellaceae was positively associated with systemic inflammation and rheumatoid arthritis (RA) onset, suggesting that GM dysbiosis may contribute to bone loss via inflammatory mediators (13). As we know that intestinal inflammation can be accompanied by OP, but their relationship remains unclear. A new study induced food-allergic bowel disease using a non-IgE-mediated food-allergic enteropathy model of ovalbumin (OVA) 23–3 mice, found that abnormally activated OVA-specific Th2 cells in the mesenteric lymph nodes that overproduce IL-4 migrated to the bone marrow, trigger an inflammatory cascade that promotes bone damage (14). The findings reveal the mechanism by which the gut-bone axis plays an important role in bone loss induced by food-allergic bowel disease. Experimental evidence demonstrates that Fusobacterium nucleatum modulates M1 macrophage polarization via the AKT2 signaling pathway, exacerbating colonic inflammation and revealing a mechanism through which microbes influence bone metabolism via immune regulation (15). Subsequently, another research found that gut inflammation promotes OCs differentiation generation and bone loss by promoting cytokine changes, and upregulation of OCs precursor surface receptor MDL-1 expression (16). This work shows a relationship between gut inflammation and bone health, providing new evidence for gut-bone axis interactions. Xie Hui’s research team found that children’s intestinal flora, or the probiotic Akkermansia muciniphila in it, can promote bone formation and inhibit bone absorption by releasing extracellular vesicles (EVs) into bone tissue (17). It can reduce bone loss in PMOP mice. This study reveals a novel “gut-bone axis” regulatory model of bone metabolism mediated by gut bacteria functional EVs.
Altogether, the gut-bone axis offers a rationale for a potential therapeutic option for treating OP.
2.2 Gut microbiota and bone formation
OP occurs when there is an imbalance between bone formation and bone resorption during bone remodeling. A study employing integrated 16S rRNA gene sequencing and liquid chromatography-mass spectrometry metabolomic analysis revealed a positive correlation between Bacteroides abundance and BMD. The abundance of Bacteroides was significantly higher in the control group compared with the OP group, suggesting a potential regulatory role through the tryptophan-indole metabolic pathway. This finding indicates that GM dysbiosis may represent a significant risk factor for OP (18). Dysbiosis of intestinal microflora can lead to malabsorption of essential elements for bone growth, such as calcium, carbohydrates, B vitamins, and vitamin K, and to the production of metabolites that affect cellular signaling (19). Changes in the intestinal flora may thereby affect the calcium regulation and bone density. Studies have demonstrated that Lactobacillus acidophilus-fermented Astragalus polysaccharides alter the GM by increasing the relative abundance of specific beneficial bacteria and stimulating the production of key metabolites, thereby enhancing calcium absorption and ameliorating OP (20). Separately, sheep bone protein hydrolysate was shown to increase Firmicutes abundance, reduce Proteobacteria and Verrucomicrobia, promote short-chain fatty acids (SCFAs) production, improve calcium uptake, and ultimately restore BMD (21).
He W investigated the effect of the GM on calcium absorption in ovariectomized (OVX) rats, and found that specific species (e.g. Acinetobacter and Propionibacterium) of gut bacteria were associated with higher calcium absorption efficiency (22). There is a decrease in beneficial intestinal bacteria for postmenopausal women with OP, always accompanied with reduced levels of bone formation markers (23). Furthermore, studies have revealed that OVX mice exhibit a significant increase in the Firmicutes/Bacteroidetes ratio and elevated serum Lipopolysaccharide (LPS) levels, suggesting that estrogen deficiency may represent a key mechanism through which GM dysbiosis contributes to bone loss (24).
One of these studies reported that Lactobacillus rhamnosus GG regulating the intestinal microbiome and intestinal barrier through probiotics, stimulating the Th17/Treg balance in the intestine and bones, and promoting bone formation can improve OP caused by estrogen deficiency (25). Moreover, the combination of Butyricicoccus pullicaecorum and 3-hydroxyanthranilic acid (3-HAA) was shown to prevent PMOP by modulating the Th17/Treg immune balance, highlighting the potential of targeting the microbiota-immune axis for OP treatment (26).
Parathyroid hormone (PTH) is a critical regulator of skeletal development that promotes bone formation. Jau-Yi Li.et showed that the microbiota was required for PTH to stimulate bone formation and increase bone mass, and found butyrate, a metabolite responsible for gut-bone communication, plays in triggering regulatory pathways, which suggested that supplementing butyric acid to increase the number of regulatory T cells may be a new method to treat OP or enhance the anabolic activity of PTH (27). Supplementation with Pseudobifidobacterium CECT 7765 has been shown to upregulate the Wnt/β-catenin signaling pathway, resulting in increased levels of PTH, serum C-terminal telopeptide (CTX), and osteocalcin (28). In addition, two independent studies demonstrated that Bacillus subtilis enhances bone formation either by modulating bacterial communities to elevate PTH levels or by reducing TNF-α expression, thereby attenuating inflammatory conditions (29, 30).
Therefore, these studies highlight the potential of targeting GM to regulate bone formation in OP.
2.3 Gut microbiota and bone resorption
GM can influence bone resorption by modulating the immune and endocrine system, affecting inflammation, and producing metabolites that impact bone cell activity, including OCs.
Ohlsson investigated the role of GM in PMOP in mice (31). They found that the GM promoted bone loss by increasing the differentiation and activity of OCs, the cells responsible for bone resorption. Provided evidence that probiotic supplementation partially reverses the suppression of bone marrow T-regulatory cells caused by ovariectomy. The probiotic intake resulted in reduced levels of bone TNF-α and other pro-inflammatory cytokines in OVX mice, consistent with the regulation of anti-inflammatory T-cells (32, 33). Additionally, the researchers observed an increase in TGF-β1 expression, which is associated with enhanced T-regulatory cells (32). Li investigated the impact of GM dysbiosis on bone resorption in a mouse model of PMOP (34). They found that dysbiosis led to an altered GM profile, increased inflammation, and enhanced OCs activity, resulting in accelerated bone loss. The study highlighted the potential of targeting GM to modulate bone resorption in OP.
Wallimann found that GM and the metabolites they produce, primarily SCFAs, have been shown to affect almost all organs in the human body, including bones (35). SCFAs have shown a wide range of activities in positively affecting bone healing outcomes by acting directly on cell types involved in fracture healing, such as osteoblasts (OBs), OCs, chondrocytes, and fibroblasts, or indirectly on appropriate anti-inflammatory and immunomodulatory responses (35, 36). It is well documented that the GM can increase bone mass and improve OP by inhibiting OCs proliferation and differentiation, inducing apoptosis, reducing bone resorption, or promoting OBs proliferation and maturation. Experimental studies have demonstrated that quercetin supplementation ameliorates bone loss in OVX rats by enhancing intestinal barrier function through upregulation of tight junction proteins (ZO-1 and occludin), increasing SCFAs levels, and reducing pro-inflammatory mediators such as LPS, IL-1β, and TNF-α (37). These findings underscore the role of microbial-derived metabolites (e.g., SCFAs) in inhibiting bone resorption, highlighting a microbiota-metabolite-immune mechanism in OP intervention.
Peripheral serotonin (5-HT), produced by enterochromaffin (EC) cells in the gastrointestinal tract, promotes OCs differentiation and suppresses OBs activity. GM dysbiosis resulting from chronic ethanol abuse has been shown to increase 5-HT levels and exacerbate bone resorption (38). In contrast, centrally-derived 5-HT in the brain promotes bone formation by binding to Htr2c receptors on ventromedial hypothalamic neurons (39). Experimental supplementation with Clostridium butyricum and 25-hydroxyvitamin D3 (25-OH-D3) in broilers was found to increase 5-HT levels, decrease bone Gla protein (BGP) and peptide YY (PYY) levels, and improve bone metabolism. Metagenomic analysis of the cecum further revealed an increased abundance of Alistipes and significant alterations in metabolic pathways such as PI3K-AKT signaling and bile acid biosynthesis (40). Additionally, soybean germ extract and Lactobacillus gasseri exerted positive effects on skeletal health in OVX rats, significantly improving alkaline phosphatase (ALP) and bone resorption markers, while also elevating levels of estrogen, 5-HT, and norepinephrine (41).
In summary, bone loss within the gut-bone axis is primarily driven by GM dysbiosis through multiple mechanisms, including inflammatory factor release, metabolite alterations, immune dysregulation, intestinal barrier impairment, and endocrine hormone disruption. The mechanisms through which GM dysbiosis influences bone metabolism are illustrated in Figure 1.
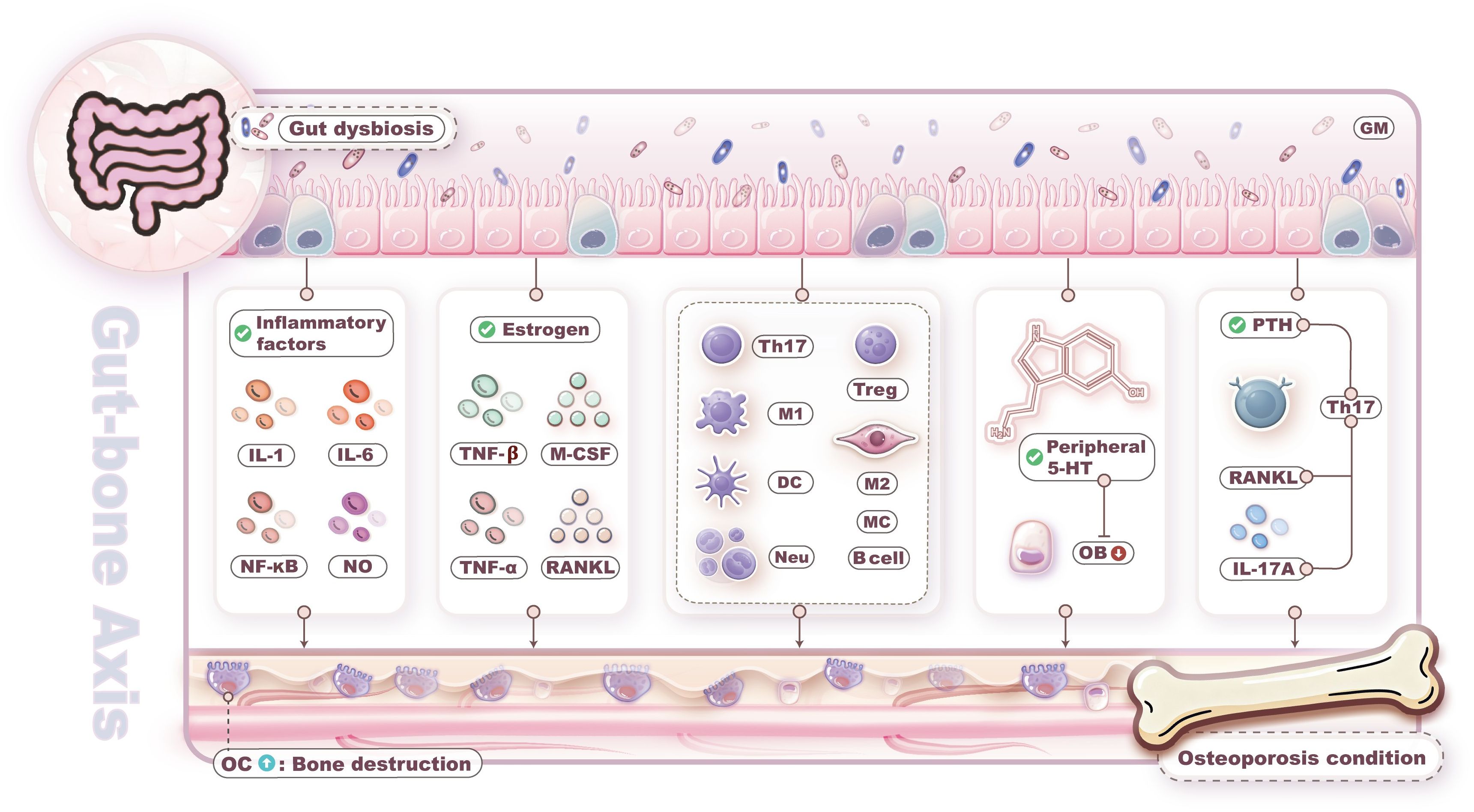
Figure 1. Gut microbiota (GM) dysbiosis leads to bone loss. Bone loss driven by GM dysbiosis through multiple mechanisms, including inflammatory factor release, metabolite alterations, immune dysregulation, intestinal barrier impairment, and endocrine hormone disruption.
3 Interaction between immune cells and osteoporosis
3.1 From osteoimmunology to immunoporosis: immune imbalance caused bone loss regulate bone remodeling plays an important role in regulating bone health and homeostasis
The relationship between the immune system and the bone system has long been studied. In 2000, Arron et al. proposed the concept of “osteoimmunology” and pointed out that there is an intricate interaction between the immune system and the bone system (42). Since then, immune-related factors have gradually become a hot research topic in metabolic bone diseases. In fact, the regulatory between the immune system and bone is bidirectional (43). On the one hand, as immune cells form in the bone marrow, the bone system exerts an important influence on the generation, function and regulation of the immune system through affecting the generation and differentiation of hematopoietic stem cells (HSCs) and the secretion of cytokines (44). On the other hand, the immune system regulates bone health and homeostasis by modulating the differentiation of OBs and OCs, as well as the inflammatory responses (45). It has been shown previously that the RANKL/RANK/OPG pathway was thought to act as a link between immune system and bone (46). Activated T cells release RANKL, a crucial cytokine in osteoclastogenesis and bone resorption, which binds to RANK and then regulates bone remodeling by activating a series of signal transduction pathways.
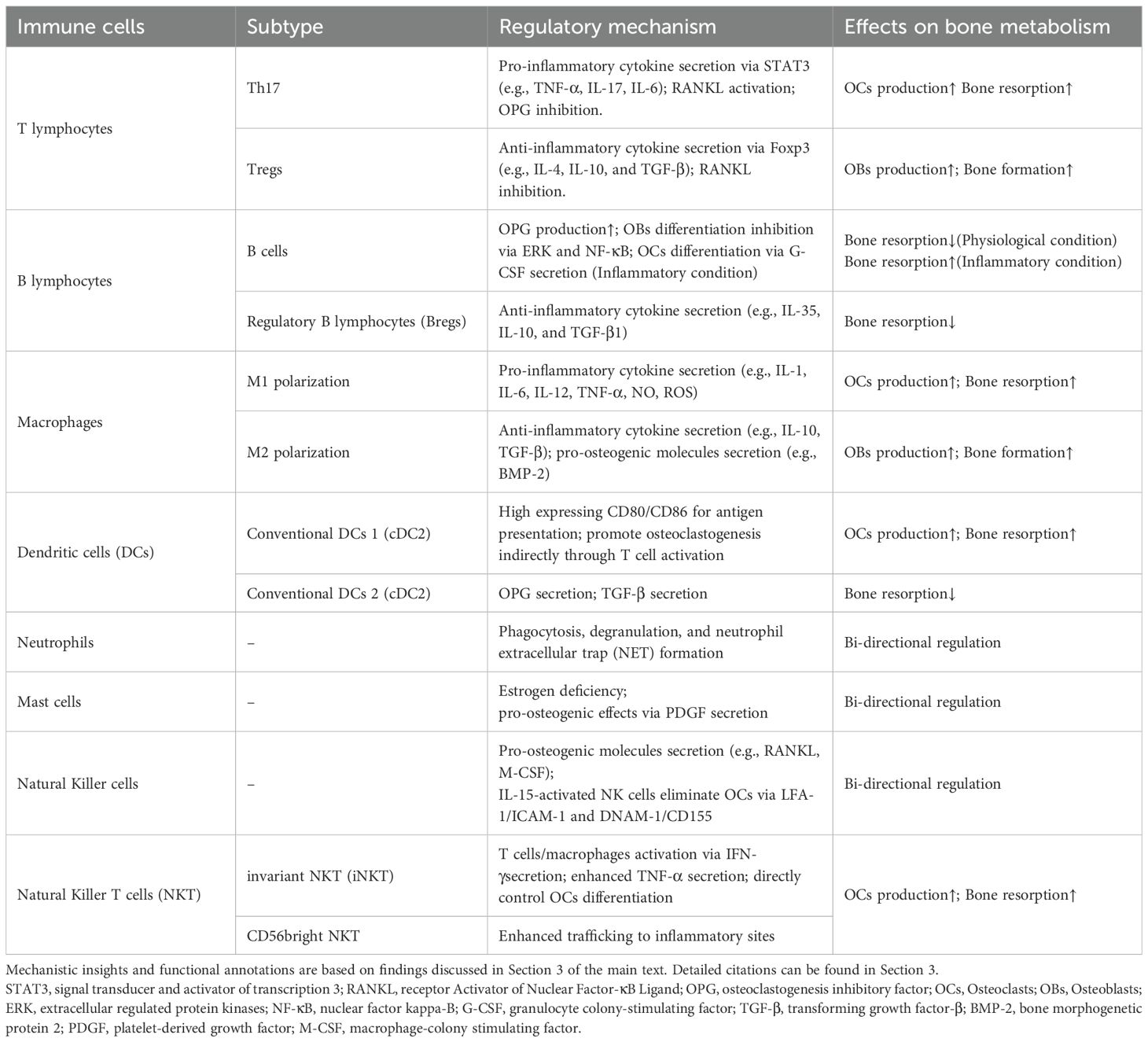
Recently, Srivastava et al. have coined the term “immunoporosis” to highlight the role of immune cells in the pathology of OP (47). Immune OP provides a good perspective for understanding the complex interactions between the immune system and the skeletal system. It enables us to use immune knowledge to explain the pathological mechanism of OP and provide important clues for the development of new treatments and drugs for OP in the future. A summary of the mechanisms by which immune cells regulate bone metabolism, as discussed in this article, is provided in Table 1.
3.2 T lymphocytes and osteoporosis
In particular, T lymphocytes (T cells) play pivotal roles in the regulation of bone health.
T cells are the core cells of immune regulation, and naïve T cells can differentiate into Treg cells, Th17 cells and other subsets after stimulation by exogenous antigens, and can produce different immune effects by secreting different characteristic cytokines (48). Th17 cells are essential core links in estrogen deficiency-induced bone loss (49). In addition, Treg cell deficiency and inactivation are associated with some chronic inflammatory diseases, and Treg cells can regulate OCs formation and prevent bone resorption by secreting IL-4, IL-10, and TGF-β (50).
The imbalance between Th17 cells and Treg cells constitutes a pivotal mechanism underlying OP pathogenesis (50). Th17 cells secrete IL-17 to activate RANKL expression in bone marrow stromal cells while suppressing osteoprotegerin (OPG) production, thereby disrupting the RANKL/OPG equilibrium (51). This ratio imbalance amplifies OCs differentiation through NF-κB signaling cascades (52). Experimental studies confirm that phosphorylation of STAT3, the Th17-specific transcription factor, upregulates Cathepsin K expression to enhance osteoclastic bone resorption activity (53).
Treg cells employ Foxp3-dependent pathways to secrete TGF-β and IL-10, directly inhibiting RANKL-induced osteoclastogenesis (54). Their surface molecule CTLA-4 interacts with CD80/CD86 on dendritic cells, blocking costimulatory signaling and suppressing pro-osteoclastogenic factors like TNF-α (55). Under physiological conditions, Treg cells maintain bone equilibrium by balancing Foxp3/RORγt transcriptional factor expression in CD4+ T cells, thereby counteracting Th17-mediated bone destruction (54). Postmenopausal estrogen deficiency reduces bone marrow Treg population and functional competence, concurrently promoting Th17 differentiation into IL-23 receptor-high subsets (56). This immunologic shift activates JAK2/STAT3 pathway to induce OBs apoptosis and enhances OCs precursor sensitivity to M-CSF, ultimately driving bone mass loss (57). Elucidation of this regulatory network provides theoretical foundation for immunologically targeted interventions in OP management.
3.3 B lymphocytes and osteoporosis
B lymphocytes, pivotal components of humoral immunity, develop from HSCs precursors in bone marrow and primarily function through antibody secretion to neutralize pathogens while enhancing effector functions of other immune cells (58). Emerging research increasingly implicates B lymphocytes in bone remodeling pathologies. Critically, these cells produce approximately 50% of bone marrow-derived OPG, which competitively inhibits RANKL binding to suppress osteoclastogenesis and prevent excessive bone resorption (59). Conversely, B lymphocytes secrete C-C motif chemokine ligand 3 (CCL3) and tumor necrosis factor (TNF) that impair OBs differentiation via extracellular signal-regulated kinase (ERK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways (60).
The osteoimmunological impact of B lymphocytes is context-dependent. Inflammatory conditions polarize B cells toward pro-osteolytic phenotypes: Activated B lymphocytes secrete granulocyte colony-stimulating factor (G-CSF) that expands OCs progenitor pools, while concurrent RANKL and G-CSF production drives OCs proliferation and differentiation, ultimately driving progressive osteopenia (61, 62). Clinically, postmenopausal osteoporotic women exhibit significantly reduced CD19+ B lymphocyte frequencies versus healthy controls, underscoring the pivotal contributions of B lymphocytes to OP pathogenesis through immunomodulatory and osteometabolic pathways (63).
Beyond conventional antibody-secreting subsets, regulatory B lymphocytes (Bregs) counterbalance OCs activity through anti-inflammatory mediators (64). IL-35 promotes Breg differentiation via STAT1/STAT3 signaling (65); IL-10 directly impedes osteoclastogenesis by disrupting Ca²+ mobilization and NFATc1 signaling in precursors while enhancing OBs differentiation through miR-7015-5p downregulation (66, 67); and TGF-β1 stimulates osteogenesis via SMAD/MAPK-mediated Runt-related transcription factor 2 (RUNX2) upregulation while suppressing osteolytic genes encoding tartrate-resistant acid phosphatase (TRAP) and cathepsin K (68). Thus, in TGF-β1-deficient osteoporotic microenvironments, sustained RANKL expression on OBs coupled with attenuated RUNX2-directed osteogenesis collectively drives pathologic bone remodeling toward resorption.
3.4 Macrophages and osteoporosis
As pivotal innate immune cells, macrophages orchestrate bone immunometabolic homeostasis through their remarkable phenotypic plasticity, dynamically transitioning between polarization states to regulate tissue balance (69). Proinflammatory cytokines including IL-1β, IL-6, IFN-γ, and TNF-α, along with bacterial components like LPS, drive M1 polarization of macrophages (70). These classically activated cells release inflammatory mediators such as IL-1β, IL-6, IL-12, TNF-α, nitric oxide (NO), and reactive oxygen species (ROS), which directly or indirectly stimulate osteoclastogenesis and enhance bone resorption (71). When sustained, this M1-dominant state propagates chronic inflammation, triggering a compensatory shift toward M2 polarization via anti-inflammatory cytokines (e.g., IL-4, IL-10, IL-13) to restore tissue equilibrium (72).
M2-polarized macrophages contribute to tissue repair through efferocytosis of apoptotic cells, secretion of anti-inflammatory cytokines like IL-10, and production of pro-osteogenic molecules such as bone morphogenetic protein 2 (BMP-2) and transforming growth factor β (TGF-β) (73). These factors induce bone marrow mesenchymal stem cells (BMSCs) differentiation into mature OBs, accelerating bone regeneration (74). Notably, elevated M1/M2 macrophage ratios in OP models underscore macrophage repolarization as a promising therapeutic target (75).
Recent mechanistic studies reveal novel regulatory pathways: Huang et al. demonstrated that M2-derived extracellular vesicles (M2-EVs) reprogram osteoclast precursors (OCPs) into M2-like macrophages, rebalancing the OC-macrophage axis to attenuate pathological bone loss (76). This strategy offers a bone-targeting approach for OP therapy. Complementary work by Qin et al. showed that bilobalide—a bioactive constituent of Ginkgo biloba—promotes dose-dependent M2 polarization while suppressing RANKL-induced osteoclastogenesis through SIRT3 upregulation and NF-κB pathway inhibition, revealing another promising intervention for OC-mediated bone loss (77).
Collectively, these advances position M2 macrophages as central orchestrators of osteogenic activity, with macrophage phenotype modulation representing a compelling therapeutic frontier for OP management.
3.5 Others immune cells and osteoporosis
3.5.1 Dendritic cells
Dendritic cells (DCs), specialized antigen-presenting cells (APCs), serve as pivotal initiators of adaptive immune responses. Distributed ubiquitously, they continuously monitor danger signals via pattern recognition receptors (PRRs) while constitutively expressing high levels of major histocompatibility complex class II (MHC-II) molecules and co-stimulatory receptors (CD80/CD86), essential for antigen presentation. Emerging research reveals DCs as critical regulators of bone homeostasis and pathological remodeling, demonstrating striking functional duality (78).
Under RANKL and M-CSF stimulation, DCs exhibit significantly greater efficiency in transdifferentiating into OCs than monocytes, with transcriptomic analyses confirming fewer regulatory steps required for this conversion, suggesting close developmental proximity between these lineages (79). Activated DCs promote osteoclastogenesis indirectly through T cell activation—particularly Th17 polarization—triggering IL-17 and TNF-α secretion that induces RANKL production in bone stromal cells (78, 80). Furthermore, DCs and T cells form pathological aggregates that exacerbate osteolytic conditions including synovitis and periodontitis (81, 82). Significantly, a self-amplifying loop emerges wherein newly formed OCs chemoattract additional DCs to bone resorption sites through chemokine signaling (83).
Conversely, DCs counterbalance bone destruction via cDC2-derived OPG—a decoy receptor that competitively inhibits RANKL binding to suppress OCs activation (59). Under specific conditions, DCs also secrete TGF-β and other anti-resorptive molecules that mitigate inflammatory bone loss (84). Crucially, DCs function as dynamic immunoregulatory buffers that fine-tune the osteolytic-osteogenic equilibrium during OP initiation (85).
3.5.2 Neutrophils
Neutrophils—polymorphonuclear granulocytes constituting 40%-60% of circulating leukocytes—serve as primary sentinels of innate immunity. Their production is regulated by G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF), with mature cells rapidly recruited to inflammatory sites where they orchestrate local microenvironments through phagocytosis, degranulation, and neutrophil extracellular trap (NET) formation (86). Beyond these canonical functions, neutrophils synthesize C-X-C and C-C chemokines facilitating crosstalk with bone cells and other cellular constituents (87).
Emerging research reveals neutrophils modulate skeletal metabolism through multiple pathways, where their senescence or dysfunction directly/indirectly impacts OCs, OBs, and MSCs (88, 89). Neutrophils exacerbate bone resorption by secreting RANKL, generating ROS, and releasing pro-inflammatory cytokines that potentiate osteoclastogenesis (90, 91). Simultaneously, they recruit pro-osteoporotic cells like Th17 lymphocytes via chemokines (IL-8, IL-17), further amplifying osteoclastic activity (92). Conversely, neutrophils enhance bone formation by inducing OBs expression of mineralization markers including ALP and osteocalcin, thereby promoting mineral deposition (93). They also regulate MSC osteogenic differentiation; though notably, in vitro studies demonstrate neutrophils inhibit MSC-mediated extracellular matrix production while G-CSF-induced neutrophil expansion triggers ROS-mediated apoptosis in MSCs and OBs, collectively attenuating osteogenesis (94, 95).
3.5.3 Mast cells
Mast cells (MCs) exhibit unique spatial positioning in osteoporotic bone, predominantly accumulating within endosteal marrow regions while closely adjacent to OCs surfaces (96). The resulting osteometabolic imbalance manifests initially as enhanced endosteal resorption within metaphyseal compartments, subsequently progressing to affect cortical endosteal surfaces in epiphyseal and diaphyseal regions. Estrogen deficiency constitutes a key permissive condition for MC-mediated osteopathology, as evidenced by significant MC expansion in OVX rat marrow compared to sham controls (97). Mechanistically, MC-deficient mice resist OVX-induced bone loss through suppressed OCs numbers and activity (98). Critically, mast cell accumulation in OVX mice directly correlates with elevated OCs numbers (99), exhibiting frequent spatiotemporal co-localization with OCs (96). Consequently, estrogen deficiency functions as a crucial molecular switch unlocking mast cell-mediated osteolytic activity.
Compared to OC interactions, MC influences on OBs remain less characterized. Lind et al. demonstrated elevated bone mass, formation markers, and mineralization rates in female mice lacking the MC protease chymase Mcpt4, suggesting chymase inhibits osteogenesis (100). Further supporting an anti-osteogenic role, MCs suppress OB differentiation via IL-1 secretion (96). However, conflicting reports indicate pro-osteogenic effects: Turner et al. identified MC-derived platelet-derived growth factor (PDGF) promoting stromal cell differentiation toward osteoprogenitors (101), and MC-deficient mice exhibit reduced OBs activity (102), revealing context-dependent regulatory duality.
3.5.4 Natural Killer cells
Natural killer (NK) cells, traditionally derived from bone marrow HSCs, now demonstrate maturation capacity within secondary lymphoid tissues (SLTs) where they acquire adaptive features including memory functions (103). Beyond canonical tumor-lytic activity, NK cells eliminate virus-infected and stress-altered cells (104, 105) through balanced signaling via activating/inhibitory receptors (106). These lymphocytes further sculpt local microenvironments by mediating antibody-dependent cellular cytotoxicity (ADCC) and secreting pro-inflammatory mediators (e.g., IFN-γ, chemokines) that regulate neighboring immune cells (107).
Notably, NK cells exhibit context-dependent functional duality in inflammatory osteopathies. In RA, synovial-infiltrating NK cells highly express osteoclastogenic factors RANKL and M-CSF—levels further augmented by IL-15 (108). Clinically, RA synovial fluid NK cells drive monocyte differentiation into functional OCs, with IL-15 conferring enhanced resorptive capacity (109). Correspondingly, NK cell depletion in collagen-induced arthritis (CIA) models significantly attenuates bone destruction (109). Conversely, IL-15-activated NK cells eliminate OCs via LFA-1/ICAM-1 and DNAM-1/CD155 contact-dependent cytolysis, an effect abrogated by receptor blockade (110). This phenotypic plasticity indicates NK cell OC-regulatory behavior depends critically on microenvironmental cues. Supporting clinical significance, bioinformatic analyses reveal expanded T and NK cell frequencies in PMOP bone (111).
3.5.5 Natural Killer T cells
Natural killer T (NKT) cells constitute critical regulators of skeletal metabolism, exhibiting a hybrid T-NK phenotype that enables both cytotoxic clearance of compromised cells (112) and cytokine-mediated modulation of T/B cell immunity (113, 114) and myeloid functions (115). Recent advances reveal invariant NKT (iNKT) subsets directly control OCs differentiation through specialized pathways (116). Within rheumatoid RA pathology, synovial NKT cells expand dramatically—comprising ≤ 20% of lymphocytes (117)—with CD56bright subsets exhibiting enhanced chemokine receptor-mediated trafficking to inflammatory sites (118). These infiltrating NKT cells activate monocytes to promote osteoclastogenesis (117), while concurrently secreting RANKL and M-CSF (potentiated by IL-15) (119). Significantly, NKT-derived IFN-γ stimulates T cells/macrophages to amplify TNF-α release (120), which subsequently drives osteoprogenitor differentiation through RANKL-dependent mechanisms while inducing OBs to secrete additional RANKL/M-CSF (121, 122). This multi-layered cooperativity establishes NKT cells as principal orchestrators of inflammatory bone loss.
Taken together, these findings indicate that both innate and adaptive immune cells persistently shape OCs and OBs functions. This interaction is crucial for deciphering physiological bone metabolic equilibrium and pathological remodeling processes in OP.
4 Gut microbiota influences immune system
The GM orchestrates maturation and functional development of the early human immune system from birth. Gnotobiotic models provide definitive evidence: studies comparing GF and specific pathogen-free mice reveal significant developmental impairments in gut-associated lymphoid tissue and defective formation of isolated lymphoid follicles—critical sites for IgA response induction (123). Critically, the GM further modulates systemic immunity and self-antigen responsiveness, with microbiota alterations reported across preclinical and clinical models of chronic diseases underscoring its influence on immune dysregulation during pathogenesis (124).
GM-immune crosstalk in extraintestinal organs operates through three interconnected mechanisms (125): firstly through direct cellular interactions where microbes engage immune cells via surface adherence or phagocytic uptake, and bind pattern-recognition receptors on mucosal epithelia and macrophages to trigger pro-inflammatory cascades that amplify immune activity; mechanistically through metabolite-mediated signaling where SCFAs and other immunomodulatory molecules directly govern immune cell proliferation, differentiation, and effector function; furthermore through barrier-dependent segregation wherein epithelial structures physically and chemically sequester luminal communities to maintain host-commensal homeostasis by preventing aberrant lymphocyte activation.
Thus, the immune-GM axis pivots on these molecular pathways, warranting detailed examination of their collective impact on immune regulation.
4.1 Gut microbiota and Immune cells
The GM primarily colonizes the gastrointestinal tract, with highest density in the colorectum, and is predominantly composed of Firmicutes and Bacteroidetes (collectively representing ~90% of total abundance), alongside minor constituents including Proteobacteria and Actinobacteria (126). Gut homeostasis is characterized by dominance of obligate anaerobes from Firmicutes and Bifidobacteriaceae, whereas expansions in facultative anaerobes of the Enterobacteriaceae family commonly signify dysbiosis (127). Functionally, Firmicutes abundance positively correlates with calcium absorption (128), while Bacteroidetes orchestrate immune equilibrium by restoring Th1/Th2 balance, redirecting lymphoid organogenesis in gnotobiotic animals, and directing cellular/physical maturation of the developing immune system (129).
The intestinal epithelium establishes sophisticated physical and chemical barriers that spatially segregate GM from immune cells, preventing excessive immune activation while concurrently functioning as a critical communication interface between these compartments (130). A subset of bacterial species adhere tightly to small intestinal epithelial cells (IECs), exploiting this unique niche colonization to induce specific gene expression programs in IECs that subsequently promote immune cell differentiation. This is exemplified by segmented filamentous bacteria (SFB), whose attachment to IECs triggers epithelial production of serum amyloid A (SAA), thereby driving Th17 response differentiation and enhancing antimicrobial defense against bacterial pathogens (131).
While most GM remain non-adherent to the intestinal epithelium, immune cells and IECs deploy PRRs for microbial surveillance, recognizing bacterial components through Toll-like receptors (TLRs) and NOD-like receptors (NLRs) (132, 133). Specific bacterial components—including lipopolysaccharide (LPS), flagellin, and Bacteroides fragilis capsular polysaccharide A (PSA)—are detected by these PRRs, orchestrating downstream innate immune signaling cascades.
4.1.1 Lipopolysaccharide
Gram-negative bacterial LPS exemplifies this signaling cascade: initially complexing with LPS-binding protein (LBP), it transfers to CD14 on myeloid cells before engaging the TLR4-MD2 complex. This binding induces TLR4 dimerization and conformational change, triggering dual signaling axes through myeloid differentiation factor 88 (MyD88) and IL-1R-associated kinase (IRAK) (134). Downstream activation of NF-κB and MAPK pathways drives production of pro-inflammatory mediators (IL-1β, IL-6, TNF-α, NO), promotes M1 macrophage polarization (70), and enhances DCs antigen presentation via upregulated MHC-II and co-stimulatory molecules (CD80/86) (135), while concurrently stimulating neutrophil recruitment through CXCL1/2 chemokine release (136). Concomitantly, the TLR4/TRIF axis phosphorylates TANK-binding kinase 1, which phosphorylates interferon regulatory factor 3 to drive type I interferon (IFN-β) production (137). This IFN-I signaling potentiates cytotoxic programs in NK cells and CD8+ T lymphocytes (138, 139).
4.1.2 Flagellin
Flagellin from Gram-negative bacteria (including invasive pathogens) is primarily recognized by lamina propria-resident DCs with high TLR5 expression, triggering MyD88-dependent signaling to generate abundant pro-inflammatory cytokines (140). Select pathogens like Salmonella further leverage type III secretion systems to translocate flagellin into host cytosol (141). During intracellular delivery, flagellin is initially sensed by NAIP5/6 proteins (NLR family apoptosis inhibitory proteins), which recruit NOD-like receptor NLRC4 to assemble inflammasomes. This cascade activates caspase-1, cleaving pro-interleukin-1β/18 into mature IL-1β/IL-18 to induce pyroptosis (142).
Intriguingly, commensal-derived flagellins exhibit distinct immunomodulatory properties, as demonstrated by Clasen et al. (143). While binding TLR5 with comparable affinity to pathogenic flagellins, these “silent flagellins” evade inflammatory activation—revealing novel recognition paradigms within innate immunity.
Beyond innate sensing, TLR5 coordination extends to humoral immunity: Intestinal epithelial TLR5 engagement activates NF-κB, stimulating B-cell activating factor and proliferation-inducing ligand secretion (144). These cytokines drive B-cell differentiation into IgA-producing plasma cells that neutralize pathogens (145).
4.1.3 Fragilis polysaccharide A
Bacteroides fragilis, a commensal bacterium inhabiting the colonic mucosa, PSA—an immune-tolerant symbiosis factor conferring therapeutic benefits against intestinal inflammation and systemic immune-mediated disorders like experimental autoimmune encephalomyelitis (EAE) (146, 147). PSA engages TLR2/TLR1 heterodimers on DCs to induce IL-10+/TGF-β+ Tregs while suppressing pro-inflammatory cytokines, establishing an immunosuppressive niche (129, 148).
Ertürk-Hasdemir et al. demonstrated that PSA-mediated EAE protection requires functional TLR2/TLR1 signaling (149). Concurrently, PSA activates Dectin-1 lectin receptors, triggering Syk kinase phosphorylation that converges with TLR2 signaling at PI3K (149). Notably, ablation of either receptor ablates PI3K-dependent Akt phosphorylation, disrupting anti-inflammatory gene programs.
Mechanistically, PSA-driven Akt phosphorylation inhibits GSK3β, preventing NF-κB/CBP complex formation while promoting CREB-dependent transcription of immunosuppressive mediators (IL-10, MHC-II, ICOS-L) (150). This epigenetic reprogramming initiates Foxp3+ IL-10+ Treg expansion, completing a multi-receptor immunosuppressive circuit (151).
4.2 Gut microbiota metabolites and Immune cells
Beyond the GM themselves, their metabolites significantly contribute to intestinal homeostasis and inflammation regulation by modulating immune responses.
4.2.1 Short-chain fatty acids
SCFAs—predominantly acetate, propionate, and butyrate—are abundantly produced in the colon through microbial fermentation of dietary fiber (152). SCFAs suppress inflammation and carcinogenesis by directly or indirectly inhibiting histone deacetylase (HDAC) activity, primarily via activation of G protein-coupled receptors (GPCRs), thereby influencing immune cell differentiation and function (152).
Specifically, butyrate and propionate enhance histone H3 acetylation, opening the promoters of the Foxp3 and IL-10 genes to drive Treg differentiation (153, 154). Concurrently, they activate the aryl hydrocarbon receptor (AhR), synergistically augmenting the anti-inflammatory effects of tryptophan metabolites (155). Functional evidence demonstrates that propionate mediates its protective effects through GPR43 signaling: GPR43-knockout mice with chronic inflammation exhibit reduced intestinal Tregs, elevated Th17 cell proportions, and aggravated experimentally induced colitis compared to wild-type mice following propionate administration (156). This establishes that propionate inhibits HDAC via GPR43 to enforce anti-inflammatory activity against T cell-driven colitis.
In innate immunity, butyrate-activated GPR109A signaling induces arginase-1 (Arg1) and IL-10 expression in macrophages and DCs, polarizing them toward an anti-inflammatory phenotype that promotes IL-10-producing CD4+ T cells and Treg differentiation in the colon (157). Furthermore, acetate binding to GPR43 on IECs stimulates potassium efflux and membrane hyperpolarization, triggering NLRP3 inflammasome activation. The consequent release of IL-18 contributes to intestinal homeostasis and colitis prevention (158).
4.2.2 Tryptophan
Tryptophan, an essential dietary amino acid, serves as a precursor for diverse metabolic transformations through direct microbial metabolism or conversion via the kynurenine pathway into indole derivatives, kynurenine (Kyn), and downstream metabolites (159). The AhR—a ligand-activated transcription factor expressed across immune cell populations—represents a central mechanism whereby tryptophan metabolites regulate immunity (160). Indole-3-propionic acid (IPA) activates AhR signaling in CD4+ T cells, driving differentiation of RORγt+ Tregs (161). Concurrently, AhR inhibits Th17 differentiation by suppressing STAT3 phosphorylation, consequently reducing IL-17A secretion (162). Substantiating AhR’s critical role in gut immunity, intestinal Tregs exhibit significantly higher AhR expression than Tregs at other anatomical sites (163).
Regarding innate immunity, in vitro studies demonstrate that tryptophan metabolites inhibit inflammatory responses by suppressing histamine production in macrophages (164). Kyn further induces M2 macrophage polarization via AhR activation, promoting expression of anti-inflammatory mediators IL-10 and arginase-1 (Arg1) (165). This mechanism confers protection against septic colonic injury through the PPARγ/NF-κB axis (166). The downstream Kyn metabolite 3-HAA suppresses dendritic cell maturation by downregulating CD40/CD80/CD86/I-A expression and reducing IL-6, IL-12, and TNF-α production in LPS-stimulated bone marrow-derived DCs (BMDCs). It concurrently inhibits phospho-JNK and phospho-p38 levels in both DC2.4 cells and BMDCs, collectively demonstrating that 3-HAA impairs CD4+ T cell activation and proliferation through DC suppression (167).
4.2.3 Secondary bile acids
Secondary bile acids (SBAs), including deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA), are primarily generated by GM (e.g., Clostridium spp.) via 7α-dehydroxylation of primary bile acids (168). These metabolites regulate immune cell function primarily through activation of the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (TGR5) (169).
SBAs interact with TGR5 in macrophages, inhibiting NLRP3 inflammasome assembly and suppressing NF-κB signaling through the cAMP-PKA pathway (170). This promotes IL-10 secretion and induces an M2-polarized phenotype (171, 172). The SBA 3β-hydroxydeoxycholic acid (isoDCA), produced via microbial epimerization of cholic acid, attenuates immunostimulatory properties in DCs through FXR signaling (173). This enhances Foxp3 induction, thereby promoting Treg generation and differentiation. Supporting this mechanistic insight, engineered minimal microbial consortia containing Bacteroides strains expanded intestinal RORγt+ Treg populations (173).
Notably, isoallolithocholic acid (isoalloLCA) amplifies Treg differentiation through mitochondrial ROS generation, elevating Foxp3 expression (174). Beyond Treg modulation, 3-oxolithocholic acid (3-oxoLCA) directly binds the transcription factor RORγt to inhibit Th17 cell differentiation (174). Thus, bile acid metabolites control host immunity by directly balancing Th17 and Treg responses. Crucially, RORγ+ Treg homeostasis depends on the collective intestinal bile acid pool rather than individual SBAs, underscoring the host-microbial biliary network’s role in gut immune homeostasis (175).
In the liver, SBAs reduce CXCL16 expression on sinusoidal endothelial cells, limiting hepatic recruitment of CXCR6-expressing NKT cells (176). Consistent with this immunomodulatory function, selective FXR agonists significantly suppress liver tumor growth in murine models (177, 178). Notably, an obeticholic acid (OCA, a clinically approved FXR agonist) nanoemulsion prepared via ultrasonication demonstrated superior efficacy to oral free OCA (178).
4.3 Gut microbiota and Intestinal epithelial cells
As the intermediary layer of the gut barrier, IECs physically separate the underlying lamina propria from luminal pathogenic threats and commensal microorganisms. This compartmentalization is essential for executing protective immune functions. Luminal antigens and microbiota-derived signals transmit information to IECs, mediating adaptation to intestinal environmental shifts through mucosal barrier modulation. Gut microbial signals engaging IECs are broadly categorized into three classes: bacteria themselves, bacterial components, and bacteria metabolites (125).
4.3.1 Bacteria themselves and bacterial components
As previously described, IECs express TLRs and NLRs for immune surveillance (179). These receptors rapidly detect bacterial components like LPS and flagellin, driving epithelial proliferation while inducing expression and secretion of cytokines, antimicrobial molecules, and mucus in IECs (125). The Regenerating Islet-derived 3 (Reg3) protein family—secreted by Paneth cells—constitutes a critical class of antimicrobial effectors that spatially segregate GM from the epithelial surface (180). In the small intestine, Paneth cells secrete antimicrobial molecules (including Reg3 proteins and α-defensins) via TLR/MyD88 signaling, thereby reducing pathogen colonization and maintaining microbial ecology (181). Within the colon, LPS and flagellin induce goblet cells to secrete mucin-2 (MUC2) through TLR ligand engagement (182). Critically, Myd88 deficiency in IECs reduces MUC2 expression and Reg3γ production, exacerbating susceptibility to colitis and impairing resistance to Salmonella Typhi or Citrobacter rodentium infections (180, 183).
Cytosolic NLR receptors further uphold mucosal barrier integrity. Lactobacillus reuteri (L. reuteri) activates NOD2 signaling in IECs to stimulate Paneth cell secretion of antimicrobial peptides (e.g., α-defensins), indirectly suppressing Th1/Th17 responses while enhancing Treg function (184). Moreover, NLRP6 promotes mucin exocytosis by goblet cells through autophagy-mediated vesicle trafficking, which crucially restricts colonization by pathobionts like Prevotellaceae and candidate phylum TM7 (185, 186).
4.3.2 Bacteria metabolites
SCFAs, particularly butyrate, serve not only as the preferred energy substrates for IECs but also as critical modulators of IEC and immune cell physiology. They play pivotal roles in maintaining epithelial integrity and repairing mucosal damage (187). Through activation of GPR41, GPR43, and GPR109A expressed on IECs, SCFAs induce expression of tight junction proteins (e.g., ZO-1, occludin, claudin-1), thereby reducing intestinal permeability (188). Notably, butyrate primarily signals via GPR109A on IECs or lamina propria dendritic cells, whereas acetate and propionate function through GPR43/GPR41 activation (189). Additionally, SCFAs enhance mucosal barrier function by promoting histone acetylation at the MUC2 locus to increase mucus layer thickness while simultaneously stimulating Paneth cell exocytosis of AMPs (190). This coordinated action—fortifying physical barrier integrity through enhanced intercellular junctions and reinforcing chemical defenses via AMP secretion—demonstrates how SCFAs regulate IEC-microbial crosstalk to prevent pathogen invasion.
AHR is constitutively expressed across IECs, enabling detection of indole and its derivatives derived from tryptophan metabolism. Animal studies demonstrate that AHR deficiency in IECs significantly reduces MUC2 and carbonic anhydrase 4 (Car4) expression, consequently compromising resistance to pathogenic infections (191). Additionally, AHR modulates the Wnt/β-catenin signaling pathway in IECs, thereby regulating the specification of epithelial and crypt stem cells (191). Crucially, AHR governs group 3 innate lymphoid cell (ILC3) development, where indole metabolites stimulate ILC3s to secrete IL-22—a cytokine essential for enhancing barrier function through induction of AMPs production and intestinal infection defense (192). Intriguingly, indole compounds concurrently activate the pregnane X receptor (PXR), upregulating junctional proteins including occludin and claudin-1 through ligand binding (193). These findings establish that indole-mediated barrier enhancement operates through coordinated AHR and PXR activation in IECs, playing indispensable roles in maintaining epithelial barrier integrity.
Bile acids exert dual effects on intestinal barrier integrity (194). Primary bile acids (e.g., cholic acid and chenodeoxycholic acid) exert cytotoxic effects on IECs (195), whereas secondary bile acids (e.g., DCA and LCA) enhance barrier function by modulating expression of tight junction proteins including claudin-1, claudin-4, and occludin (196). Specifically, DCA inhibits IEC proliferation and wound healing through FXR activation (197). Additionally, DCA stimulates TGR5 on enteroendocrine cells, triggering release of 5-hydroxytryptamine (5-HT) and calcitonin gene-related peptide (CGRP) to promote colonic motility (198).
Collectively, GM and their bioactive metabolites continuously shape IEC activity and proliferation. This dynamic regulation is indispensable for maintaining barrier competence—preventing bacterial translocation and mucosal damage while ensuring efficient pathogen exclusion.
5 Immune changes resulting from disruption of the microbiome composition can affect bones
The human GM encompasses approximately 1,000 species across 28 distinct phyla—exceeding human cell counts while expressing ~100-fold more genes than the human genome—illustrating remarkable complexity and diversity (199). Consequently, compositional shifts in this microbial community can exert either beneficial or detrimental effects on human health (200).
Animal studies reveal that GF mice—exhibiting GM dysbiosis—display reduced CD4+ T cells and OCs alongside diminished TNF-α and IL-1 expression compared to conventional counterparts. Remarkably, GF mice colonized with normal microbiota restored bone mass to physiological levels (12). Similarly, colonization with microbiota-derived SCFAs normalized bone mass in GF mice, potentially mediated via GM-induced insulin-like growth factor 1 (IGF-1) production that stimulates osteogenesis (201). Clinically, malnourished and growth-stunted children exhibit compromised microbiota relative to healthy controls (202). Critically, administering two bacterial strains (Ruminococcus and Clostridium symbiosum) rectified growth impairment in mice transplanted with malnourished children’s microbiota (202). A randomized double-blind trial demonstrated that six-month multi-species probiotic supplementation significantly decreased bone-specific ALP and CTX levels—key biomarkers of bone turnover—in PMOP patients, attenuating bone resorption (203). Collectively, these findings demonstrate that microbiota dysbiosis triggers immune alterations impacting skeletal integrity and development, whereas probiotic interventions can restore microbial ecology to promote bone health—though precise mechanistic details remain elusive.
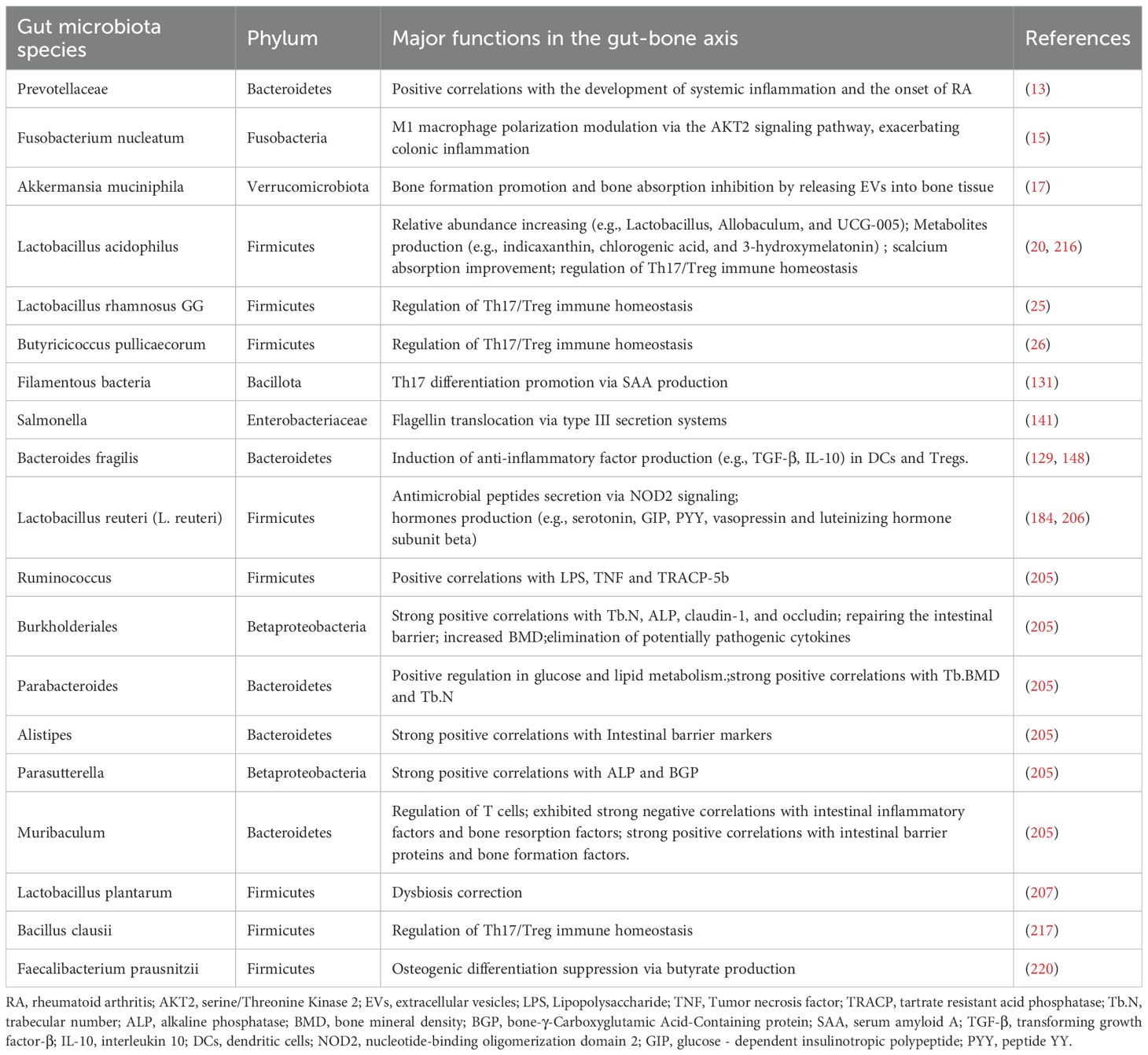
Beyond microbial complexity, limited culturability poses fundamental challenges to delineating GM regulatory mechanisms. Of approximately 1000 resident microbial species, fewer than 40% are currently culturable in vitro, leaving >60% of functional potentials unexplored. The discovery of “silent flagellins” —mutated bacterial proteins evading innate immune recognition—reveals novel microbial evasion strategies while underscoring critical knowledge gaps in host-microbe interactions (143). Advancements in molecular tools and technologies—such as metagenomics, metabolomics, lipidomics, and metatranscriptomics—have enabled the gradual deciphering of complex interactions between GM, their metabolites, and bone metabolism. These developments allow us to extend beyond classical immunology, thereby deepening our understanding of the gut-bone axis. All microbial species discussed herein and their mechanisms of bone metabolism regulation are summarized in Table 2.
A metagenomic study conducted in both Chinese and American populations revealed that Bacteroides vulgatus (B. vulgatus) was negatively correlated with BMD, while the metabolite serum valeric acid (VA) showed a positive correlation with BMD. Subsequent animal experiments confirmed that VA suppresses the production of the pro-inflammatory factor RELA and enhances the mRNA expression of the anti-inflammatory cytokine IL-10, thereby inhibiting bone resorption. In contrast, B. vulgatus was found to promote bone resorption by inhibiting VA production, highlighting the potential of the Bacteroides-valeric acid axis as a therapeutic target (204). A Mendelian randomization analysis further demonstrated that the abundance of Burkholderiales was strongly positively correlated with bone formation markers, gut barrier indicators (e.g., claudin-1, Claudin), and bone density parameters (e.g., Tb.N, ALP). Conversely, the genus Ruminococcus exhibited strong positive correlations with bone resorption markers and gut inflammatory factors (e.g., LPS, TNF, TRACP-5b). The study also identified Akkermansia, Parabacteroides, Alistipes, Parasutterella, and Muribaculum as potential mediators for the clinical diagnosis and treatment of OP (205). Additionally, metabolomic investigations revealed that L. reuteri promotes the production of hormones such as serotonin, GIP, and PYY, including vasopressin and luteinizing hormone subunit beta—newly identified hormones produced in gut epithelial cells—thereby broadening our understanding of microbial regulatory mechanisms in bone metabolism (206).
In addition to predicting potential therapeutic targets and biomarkers for OP, omics approaches have been utilized to validate the efficacy of pharmacological treatments, providing robust experimental evidence and offering new directions for elucidating microbial mechanisms. For instance, studies have demonstrated that Lactobacillus plantarum and curcumin increase beneficial GM while reducing harmful species, thereby ameliorating glucocorticoid-induced OP (207, 208). Another study revealed that alginate oligosaccharides alleviate estrogen-deficient osteosarcopenia by modulating bile acid metabolism, reducing Th17 cell prevalence and systemic inflammation (209). Additionally, a Chinese herbal extract, Isaria felina, was found to improve bone metabolism by correcting gut dysbiosis (particularly in Bacteroides and Ruminococcus), restoring Th17/Treg balance, and lowering levels of inflammatory cytokines such as IL-17 and TNF-α. Metabolomic analyses further indicated alterations in nucleotide and lipid metabolism (210).
The rational application of these advanced biotechnologies will facilitate the discovery of novel biomarkers and therapeutic targets, paving the way for the development of safer and more effective interventions.
6 How does gut flora affect bone metabolism through the immune system
Through comprehensive analysis of the GM-immune cell-OP relationship, we have established that the Th17/Treg equilibrium, Breg cells, and macrophage polarization profiles play key roles in maintaining bone homeostasis. As gut microbes continuously stimulate these immune cells, the GM thus indirectly influences bone metabolism via the immune system. The crosstalk mechanisms within the GM-immune cells-OP axis is described in Figure 2. Although mechanistic links between GM and skeletal health involve profound complexity, current hypotheses remain overwhelmingly focused on T-lymphocyte-mediated pathways. Research on non-T immune cells is critically underexplored. Beyond this T cell-centric paradigm, emerging evidence reveals that macrophages and DCs constitute a pivotal regulatory triumvirate with B lymphocytes. Neutrophils, NK, and NKT cells likewise function as putative modifiers of microbe-directed osteometabolic regulation.
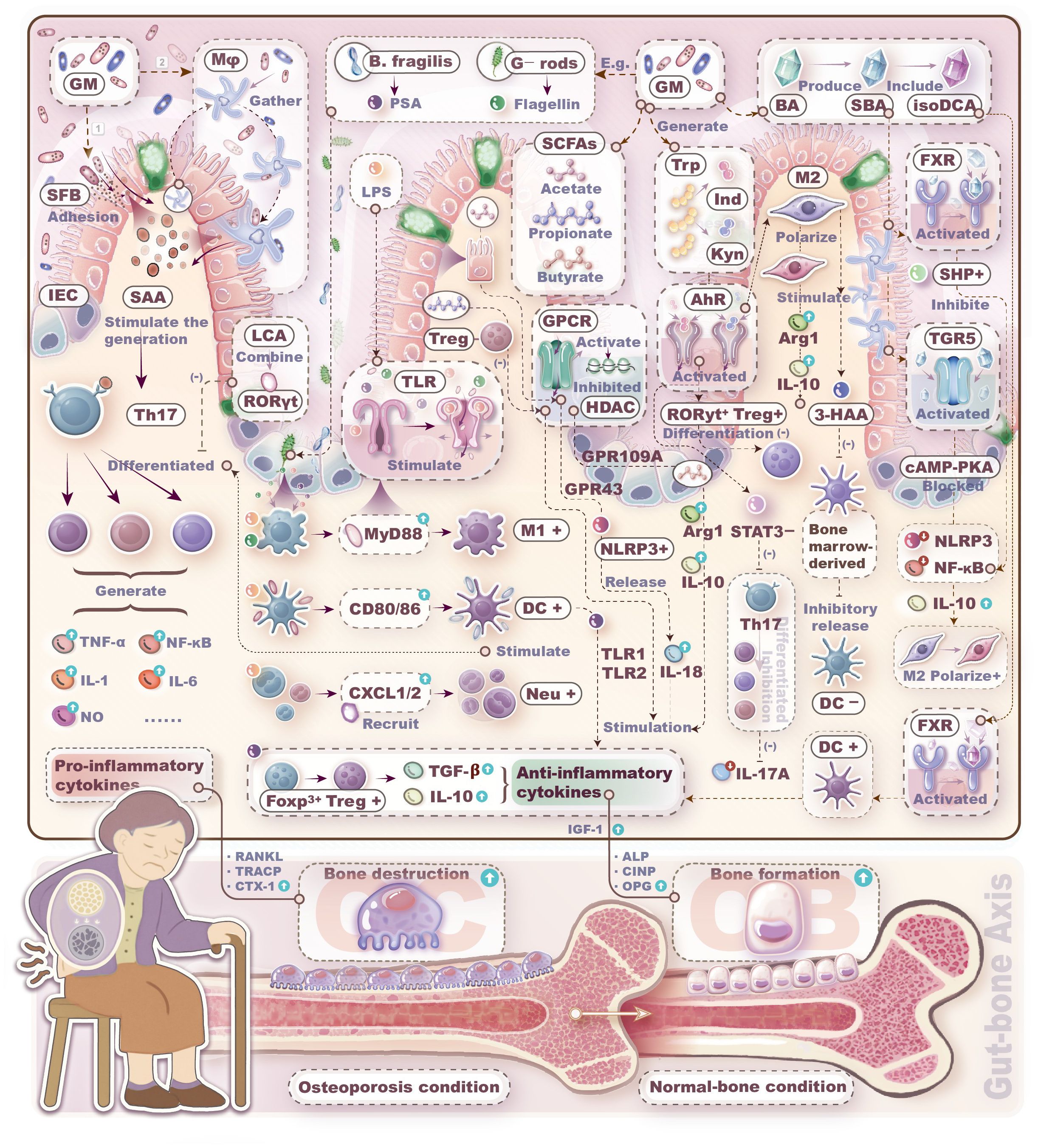
Figure 2. Mechanisms by which gut microbiota regulates bone metabolism through immune cells. Bacteria themselves, bacterial components, and bacteria metabolites can induce immune cells to produce pro-/anti-inflammatory factors, thereby promoting either bone resorption or bone formation.
While the mechanisms linking GM to skeletal homeostasis remain elusive, current hypotheses derived from recent literature have predominantly focused on T lymphocytes. Besides the mainstream proposed T-cell-mediated interactions, we have found that macrophages and dendritic cells may play pivotal roles, while B lymphocytes, neutrophils, NK cells, and NKT cells could also serve as potential targets in microbiota-mediated regulation of bone metabolism.
As delineated, bacterial flagellin activates B-lymphocyte proliferation/differentiation, triggering IgA-mediated pathogen neutralization. LPS polarizes macrophages toward pro-osteolytic M1 phenotypes that secrete bone-resorptive cytokines; conversely, microbial metabolites (e.g., SCFAs, tryptophan derivatives, secondary bile acids) induce anti-inflammatory M2 polarization while suppressing Th17 expansion. LPS and flagellin provoke DC inflammatory cascades via TLR/MyD88 pathways, whereas microbial metabolites (butyrate, kynurenine, isoDCA) attenuate DC immunogenicity by inducing IL-10 and Treg differentiation. Additionally, LPS releases CXCL1/2 to recruit neutrophils and primes NK cytolytic function. Thus, current limited evidence unequivocally underscores an urgent need for comprehensive investigations into multicellular crosstalk mechanisms orchestrating immune functions within the gut-bone axis. Elucidating gut-immunological pathways and decoding dysbiosis-induced pathophysiological cascades may unlock mechanistic insights into osteometabolic disorders.
Furthermore, microbial metabolites act directly on IECs, modulating gut barrier integrity and functionality—thereby indirectly governing microbiota-immune interplay. Compromised epithelial barrier integrity precipitates pathogen translocation into systemic circulation, triggering sterile inflammation and potentiating gastrointestinal pathologies (211). Critically, gut barrier dysfunction accelerates bone mass deterioration as demonstrated in preclinical models (212). A recent study analyzing fecal samples from postmenopausal women revealed a significant reduction in the abundance of Prevotella compared to healthy controls. Subsequent animal experiments demonstrated that treatment with Prevotella histicola partially restored bone mass in OVX mice, a effect potentially mediated through the modulation of intestinal permeability (213). Another study indicated that overexpression of neuropeptide Y exacerbates colonic inflammation and impairs gut barrier integrity in OVX rats, thereby increasing the systemic translocation of GM-derived metabolites such as LPS. These adverse effects were reversed following administration of a Y1 receptor antagonist (214). Furthermore, in a diabetic mouse model, treatment with angiotensin- (1-7) increased the abundance of Firmicutes, promoted the restoration of intestinal stem cells, and ameliorated diabetes-induced impairment of colonic barrier function (215). Collectively, these findings underscore the importance of intestinal permeability as a critical factor in elucidating the regulatory mechanisms of the gut-bone axis.
7 Intervention strategies based on the microbiome-immune-bone axis
Conventional anti-OP drugs are primarily classified into two categories: anti-resorptive and anabolic agents. However, their clinical utility is limited by adverse effects. In contrast, emerging microbiota-targeted therapies offer unique advantages, which are summarized in Figure 3.
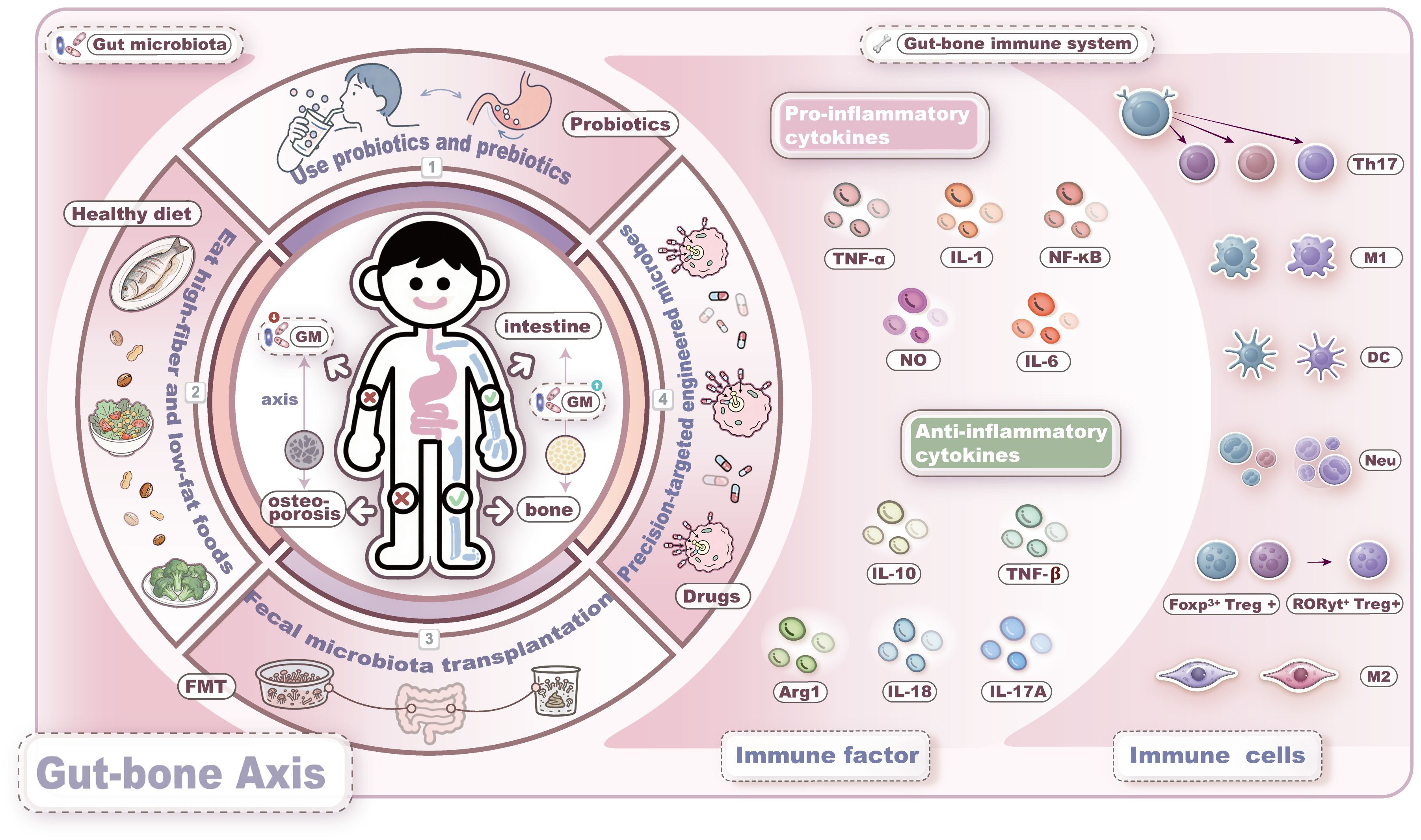
Figure 3. Intervention strategies based on the microbiome-immune-bone axis. Current strategies for gut microbiota-targeted therapy mainly include the following four approaches: 1. probiotics and prebiotics; 2. dietary interventions; 3. fecal microbiota transplantation; 4. engineered microbes. These strategies aim to improve bone metabolism by restoring gut microbiota dysbiosis, modulating immune responses, enhancing the gut barrier function, and regulating the endocrine system.
7.1 Probiotics, prebiotics and diet
In the second section, we summarize the mechanisms by which various probiotics and prebiotics ameliorate OP, including reduction of inflammatory factors, alteration of metabolites, regulation of immune and endocrine systems, and enhancement of the intestinal barrier. Animal studies have demonstrated that Lactobacillus acidophilus, Bacillus clausii, and Lactobacillus rhamnosus alleviate inflammatory bone loss in osteoporotic mouse models by modulating Treg/Th17 cell balance, highlighting the therapeutic potential of the microbiota-immune axis (216–218). Additionally, the indole-derived metabolite melatonin significantly increases SCFAs production and reduces trimethylamine N-oxide (TMAO)-related metabolites. By restoring M1/M2 macrophage equilibrium and lowering pro-inflammatory cytokine levels, MLT ultimately attenuates OP-related clinical symptoms and reverses gut dysbiosis, supporting the microbial-metabolite-immune network as a promising therapeutic target (219). Notably, butyrate produced by Faecalibacterium prausnitzii competitively inhibits lactylation at lysine 263 of GAPDH via induction of butyrylation at the same site, thereby suppressing the osteogenic differentiation of human aortic valve interstitial cells. This mechanism reveals the potential of butyrate in treating calcific aortic valve disease and offers novel insights into metabolite-mediated regulation of osteogenesis (220). Further evidence indicates that L. reuteri prevents bone loss by reversing antibiotic-induced gut dysbiosis and restoring intestinal barrier integrity, underscoring the importance of microbiota-modulated gut permeability in skeletal health (221). Moreover, synbiotics—formulations combining probiotics and prebiotics—modulate multiple gut microbial metabolites and improve bone metabolic markers, positioning them as promising next-generation interventions for OP (222).
Dietary patterns exert a decisive influence on the composition, diversity, and function of the GM (126). Experimental studies have demonstrated that a high-fiber diet restores Th17/Treg balance, enhances intestinal barrier integrity, and increases the abundance of SCFAs, thereby ameliorating autoimmune pathology in RA and significantly reducing bone loss (223, 224). A randomized controlled trial (RCT) revealed that blackcurrant supplementation increased the relative abundance of Ruminococcus 2 and effectively improved BMD in patients with PMOP (225). Another large three-year RCT involving 924 elderly participants demonstrated that a low-calorie Mediterranean diet intervention effectively mitigated age-related decline in BMD, confirming the efficacy of dietary therapy in ameliorating bone loss (226). Therefore, dietary strategies should be incorporated into clinical practice as foundational adjuvant treatments to enhance therapeutic outcomes in OP patients.
7.2 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is a therapeutic approach that involves extracting and transplanting beneficial microbial communities from healthy donor feces into the gastrointestinal tract of recipients to remodel gut microbial composition (227). The efficacy of FMT in treating OP primarily operates through improved intestinal barrier function, correction of GM dysbiosis, and modulation of immune responses to regulate bone metabolism (228). Animal studies have demonstrated that FMT upregulates tight junction proteins—such as zonula occludens-1 (ZO-1) and occludin—and inhibits the release of pro-osteoclastic cytokines, including TNF-α and IL-1β. It also optimizes the composition and abundance of GM, concomitantly elevating levels of acetate and propionate (229). Another study revealed that combining exercise with FMT enhances the enrichment of bile acid metabolites, including taurocholic acid and ursodeoxycholic acid, thereby mediating protective effects on bone mass (230).
Currently, the application of FMT in anti-OP therapy remains in the experimental stage, with limited clinical data available. The lack of standardized treatment protocols—including administration routes, donor selection, microbiota preparation, and storage methods—may influence treatment outcomes (227). Furthermore, FMT entails safety concerns such as the transmission of infectious diseases, adverse immune reactions, and long-term complications related to dysbiosis. Its therapeutic effects are often transient, necessitating repeated transplants or adjunct interventions to sustain microbial homeostasis, which presents ongoing challenges for clinical standardization. Future clinical studies should adhere to high safety standards, extend follow-up periods beyond one year to monitor potential long-term risks, and establish unified and standardized procedural guidelines.
7.3 Precision targeting therapies of engineered microbes
The engineering of probiotics using synthetic biology for precision-targeted therapy has emerged as a representative next-generation biotherapeutic strategy (231). A biomimetic self-adjuvating vaccine developed from programmable macrophage-derived vesicles significantly enhanced the activation rate of antigen-presenting cells by nearly fourfold at equivalent dosages against monkeypox virus, demonstrating substantial potential of engineered biological systems (232). Through a co-culture system, intestinal organoids (IOs) and their EVs were applied in combination, resulting in enhanced intestinal barrier integrity, reduced expression of inflammatory factors, and effective amelioration of inflammatory bowel disease-associated OP. This finding highlights the clinical translational potential of IOs and their EVs (233). Another research team improved microbial-based delivery methods by developing a colon-targeted drug delivery system using shellac resin-coated polyvinyl butyral nanoparticles, enabling sustained release of the postbiotic butyrate in the colorectum. This system effectively suppressed macrophage-mediated inflammation and modulated GM composition, highlighting its promise for targeted immuno-microbial therapy (234). In OVX mice, outer membrane vesicles (OMVs) derived from Proteus mirabilis inhibited osteoclastogenesis by elevating ROS and inducing mitochondrial dysfunction, thereby mitigating experimental bone loss (235). Researchers also constructed a recombinant probiotic Escherichia coli strain and isolated engineered BEV-BMP-2-CXCR4 vesicles (BEVs-BC), which markedly promoted osteogenic differentiation of BMSCs (236). Additionally, engineered small EVs modified with EXOmotif (CGGGAGC) and loaded with anti-miR-6359 exhibited precise targeting toward OCPs and ameliorated valproic acid-induced bone loss (237).
Collectively, these studies underscore the encouraging progress in employing engineered microbes to restore bone metabolic homeostasis, though clinical translation will require the establishment of stringent standardized protocols.
7.4 Clinical translation
Intervention strategies based on the microbiota-immune-bone axis have emerged as a promising approach for the clinical management of OP. A RCT demonstrated that supplementation with L. reuteri effectively prevented the deterioration of GM and inflammatory status in elderly women with low BMD (238). However, another double-blind, randomized, placebo-controlled clinical trial using L. reuteri did not yield positive outcomes, potentially due to insufficient dosage or viability of the probiotic strain (239). Combined supplementation with Bifidobacterium lactis subsp. Probio-M8, calcium, and calcitriol proved more effective than conventional drug therapy alone, providing a theoretical foundation for synergistic probiotic/prebiotic-mineral matrix combination strategies (240). Traditional Chinese medicine has also shown potential in modulating the gut-bone axis; clinical trials revealed that integrative therapy with conventional treatment and Chinese herbal medicine improved GM composition and modulated metabolite profiles—such as diclofenac, carbamazepine, D-pyroglutamic acid, and tamsulosin—facilitating recovery in OP patients (241).
Despite these promising findings, existing clinical trials are limited by small sample sizes, short follow-up durations, and considerable microbial heterogeneity. Safety concerns regarding GM interventions also warrant careful consideration, as complications from FMT may lead to unpredictable outcomes. Moreover, probiotics often influence bone metabolism through multiple pathways and targets, and systemic administration frequently results in low bioavailability at the intended sites and off-target effects. Therefore, achieving precise microbial intervention remains a significant challenge for clinical translation. Future advances in nanodelivery systems for engineered microbes may provide a viable solution.
8 Conclusions and future directions
The GM regulates bone metabolism through multiple mechanisms, including modulation of inflammatory factors, alterations in metabolites, and interactions with the immune and endocrine systems, as well as maintenance of intestinal barrier integrity. This review focuses on the interactions between GM and immune cells, and between immune cells and OP, aiming to elucidate and establish a comprehensive GM-immune cell-OP crosstalk network. Intervention strategies based on the microbiota-immune-bone axis show therapeutic potential in OP management, with promising applications of probiotics, prebiotics, dietary patterns, FMT, and engineered microbes. Nevertheless, numerous challenges remain unresolved, and future efforts should prioritize the following aspects.
Although preliminary insights into the GM-immune-bone axis have been established, the precise regulatory mechanisms remain to be fully elucidated. The considerable heterogeneity of GM and their metabolites, coupled with their multi-pathway, multi-receptor, and multi-target modes of action, make it exceptionally challenging to decipher their exact mechanisms. Future mechanistic studies should prioritize understanding how specific microbial species influence bone metabolism through distinct immune cells. Microbiome research may hold the key to accelerating progress in this area. Although multi-omics approaches—as discussed in Section 5—have helped correlate microbial and immune signatures with bone metabolic markers, the underlying mechanisms remain elusive. Future investigations should employ integrated biological animal models, combining advanced transcriptomic, proteomic, and multi-omics technologies to systematically unravel the mechanisms of microbial communities and their metabolites, thereby facilitating the development of robust biomarkers for precision medicine.
On the other hand, a substantial body of preclinical evidence supports the role of the GM in indirectly improving skeletal health through immune cell mediators, corroborating the mechanisms discussed herein. However, translating these foundational insights into effective and clinically viable applications remains a major challenge. In contrast, clinical studies in this area remain scarce and have produced inconsistent outcomes, particularly due to a lack of high-quality trials. Interindividual variability and microbial heterogeneity likely contribute to these discrepancies. For instance, to address compositional variability in the microbiome, future approaches could employ personalized bacterial profiling to select appropriate microbial consortia for precision therapeutics aimed at restoring skeletal health. Equally critical is the design of microbial interventions—such as FMT and engineered microbes—that can safely, precisely, and effectively target the immune system to maintain bone metabolic homeostasis. There is an urgent need for large-scale, multicenter RCTs with long-term follow-up, which must incorporate appropriate patient stratification based on complex microbial or metabolic signatures to rigorously evaluate the impact of microbiota-targeted therapies on key clinical outcomes and enhance the scientific rigor and translational credibility of the evidence.
Author contributions
TM: Writing – original draft. TZ: Writing – original draft, Visualization. CP: Writing – original draft. KL: Writing – original draft, Methodology. YX: Writing – original draft, Supervision. KC: Writing – original draft. NP: Writing – original draft. ZW: Writing – original draft. JK: Writing – review & editing, Resources. LO: Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work was supported by National Natural Science Foundation of China (82405447), Hunan Provincial Administration of Traditional Chinese Medicine (A20240125), Hunan Provincial Health Commission (20230479), Natural Science Foundation of Hunan Province (2023JJ60118), Natural Science Foundation of Changsha City (kq2403132), and Hunan University of Chinese Medicine (2024CX135).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used generative AI for the polishing work of the article.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Langdahl BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. (2021) 178:1891–906. doi: 10.1111/bph.15024
2. Guo M, Xu Y, Ren L, He S, and Pang AX. A systematic study on DNA barcoding of medicinally important genus epimedium L. (berberidaceae). Genes. (2018) 9:637. doi: 10.3390/genes9120637
3. He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Milano). (2020) 12:8583–604. doi: 10.18632/aging.103168
4. Heintz-Buschart A and Wilmes P. Human gut microbiome: function matters. Trends Microbiol. (2018) 26:563–74. doi: 10.1016/j.tim.2017.11.002
5. Lynch SV and Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
6. Guo H, Bi Y, Zhang G, Luo S, Jia X, Yang R, et al. Alcohol-induced bone loss driven by dysregulated spatial distribution of gut microbiota and PGD2-IL17 pathway-mediated osteoclast activation. Front Microbiol. (2025) 16:1551028. doi: 10.3389/fmicb.2025.1551028
7. Lu L, Chen X, Liu Y, and Yu X. Gut microbiota and bone metabolism. FASEB J. (2021) 35:e21740. doi: 10.1096/fj.202100451R
8. Pacifici R. Bone remodeling and the microbiome. Cold Spring Harb Perspect Med. (2018) 8:a031203. doi: 10.1101/cshperspect.a031203
9. Wang J, Wang Y, Gao W, Wang B, Zhao H, Zeng Y, et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. (2017) 5:e3450. doi: 10.7717/peerj.3450
10. Fukasawa N, Tsunoda J, Sunaga S, Kiyohara H, Nakamoto N, Teratani T, et al. The gut-organ axis: clinical aspects and immune mechanisms. Allergol Int: Off J Jpn Soc Allergol. (2025) 74:197–209. doi: 10.1016/j.alit.2025.01.004
11. Zaiss MM, Jones RM, Schett G, and Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. (2019) 129:3018–28. doi: 10.1172/JCI128521
12. Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. (2012) 27:1357–67. doi: 10.1002/jbmr.1588
13. Wells PM, Adebayo AS, Bowyer RCE, Freidin MB, Finckh A, Strowig T, et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. (2020) 2:e418–27. doi: 10.1016/S2665-9913(20)30064-3
14. Ono-Ohmachi A, Yamada S, Uno S, Tamai M, Soga K, Nakamura S, et al. Effector memory CD4+T cells in mesenteric lymph nodes mediate bone loss in food-allergic enteropathy model mice, creating IL-4 dominance. Mucosal Immunol. (2021) 14:1335–46. doi: 10.1038/s41385-021-00434-2
15. Liu L, Liang L, Liang H, Wang M, Lu B, Xue M, et al. Fusobacterium nucleatum aggravates the progression of colitis by regulating M1 macrophage polarization via AKT2 pathway. Front Immunol. (2019) 10:1324. doi: 10.3389/fimmu.2019.01324
16. Peek CT, Ford CA, Eichelberger KR, Jacobse J, Torres TP, Maseda D, et al. Intestinal inflammation promotes MDL-1+ osteoclast precursor expansion to trigger osteoclastogenesis and bone loss. Cell Mol Gastroenterol Hepatol. (2022) 14:731–50. doi: 10.1016/j.jcmgh.2022.07.002
17. Liu J-H, Chen C-Y, Liu Z-Z, Luo Z-W, Rao S-S, Jin L, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (weinh Baden-wurtt Ger). (2021) 8:2004831. doi: 10.1002/advs.202004831
18. Yan L, Wang X, Yu T, Qi Z, Li H, Nan H, et al. Characteristics of the gut microbiota and serum metabolites in postmenopausal women with reduced bone mineral density. Front Cell Infect Microbiol. (2024) 14:1367325. doi: 10.3389/fcimb.2024.1367325
19. Weiss GA and Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci: CMLS. (2017) 74:2959–77. doi: 10.1007/s00018-017-2509-x
20. Zhou J, Cheng J, Liu L, Luo J, and Peng X. Lactobacillus acidophilus (LA) fermenting astragalus polysaccharides (APS) improves calcium absorption and osteoporosis by altering gut microbiota. Foods (basel Switz). (2023) 12:275. doi: 10.3390/foods12020275
21. Hu G, Sun X, Hao S, Li X, Qian M, Dou L, et al. Effect of sheep bone protein hydrolysate on promoting calcium absorption and enhancing bone quality in low-calcium diet fed rats. Food Chem. (2024) 446:138763. doi: 10.1016/j.foodchem.2024.138763
22. He W, Xie Z, Thøgersen R, Rasmussen MK, Zachariassen LF, Jørgensen NR, et al. Effects of calcium source, inulin, and lactose on gut-bone associations in an ovarierectomized rat model. Mol Nutr Food Res. (2022) 66:e2100883. doi: 10.1002/mnfr.202100883
23. Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. (2017) 5:17046. doi: 10.1038/boneres.2017.46
24. Guan Z, Xuanqi Z, Zhu J, Yuan W, Jia J, Zhang C, et al. Estrogen deficiency induces bone loss through the gut microbiota. Pharmacol Res. (2023) 196:106930. doi: 10.1016/j.phrs.2023.106930
25. Guo M, Liu H, Yu Y, Zhu X, Xie H, Wei C, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/treg balance and gut microbiota structure. Gut Microbes. (2023) 15:2190304. doi: 10.1080/19490976.2023.2190304
26. Zhu F, Liu H, Cao Y, Dai B, Wu H, and Li W. The combination of butyricicoccus pullicaecorum and 3-hydroxyanthranilic acid prevents postmenopausal osteoporosis by modulating gut microbiota and Th17/treg. Eur J Nutr. (2024) 63:1945–59. doi: 10.1007/s00394-024-03400-3
27. Li J-Y, Yu M, Pal S, Tyagi AM, Dar H, Adams J, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. (2020) 130:1767–81. doi: 10.1172/JCI133473
28. Fernández-Murga ML, Olivares M, and Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reverses the adverse effects of diet-induced obesity through the gut-bone axis. Bone. (2020) 141:115580. doi: 10.1016/j.bone.2020.115580
29. Yang H-J, Zhang T, Yue Y, Jeong S-J, Ryu M-S, Wu X, et al. Protective effect of long-term fermented soybeans with abundant bacillus subtilis on glucose and bone metabolism and memory function in ovariectomized rats: modulation of the gut microbiota. Foods (basel Switz). (2023) 12:2958. doi: 10.3390/foods12152958
30. Yan F-F, Wang W-C, and Cheng H-W. Bacillus subtilis-based probiotic promotes bone growth by inhibition of inflammation in broilers subjected to cyclic heating episodes. Poult Sci. (2020) 99:5252–60. doi: 10.1016/j.psj.2020.08.051
31. Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. (2014) 9:e92368. doi: 10.1371/journal.pone.0092368
32. Tsai W-H, Lin W-C, Chou C-H, and Yang L-C. The probiotic lactiplantibacillus plantarum attenuates ovariectomy-induced osteoporosis through osteoimmunological signaling. Food Funct. (2023) 14:6929–40. doi: 10.1039/d3fo00681f
33. Zhang Y-W, Cao M-M, Li Y-J, Sheng R-W, Zhang R-L, Wu M-T, et al. The preventive effects of probiotic prevotella histicola on the bone loss of mice with ovariectomy-mediated osteoporosis. Microorganisms. (2023) 11:950. doi: 10.3390/microorganisms11040950
34. Li Y, Zhuang Q, Tao L, Zheng K, Chen S, Yang Y, et al. Urolithin B suppressed osteoclast activation and reduced bone loss of osteoporosis via inhibiting ERK/NF-κB pathway. Cell Proliferation. (2022) 55:e13291. doi: 10.1111/cpr.13291
35. Wallimann A, Magrath W, Thompson K, Moriarty T, Richards RG, Akdis CA, et al. Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing. Eur Cells Mater. (2021) 41:454–70. doi: 10.22203/eCM.v041a29
36. Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. (2018) 9:55. doi: 10.1038/s41467-017-02490-4
37. Feng R, Wang Q, Yu T, Hu H, Wu G, Duan X, et al. Quercetin ameliorates bone loss in OVX rats by modulating the intestinal flora-SCFAs-inflammatory signaling axis. Int Immunopharmacol. (2024) 136:112341. doi: 10.1016/j.intimp.2024.112341
38. Liu Z, Xu X, Shen Y, Hao Y, Cui W, Li W, et al. Altered gut microbiota and metabolites profile are associated with reduced bone metabolism in ethanol-induced osteoporosis. Cell Proliferation. (2022) 55:e13245. doi: 10.1111/cpr.13245
39. Yadav VK, Oury F, Suda N, Liu Z-W, Gao X-B, Confavreux C, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. (2009) 138:976–89. doi: 10.1016/j.cell.2009.06.051
40. Cao G, Yu Y, Wang H, Yang H, Tao F, Yang S, et al. Dietary clostridium butyricum and 25-hydroxyvitamin D3 modulate bone metabolism of broilers through the gut-brain axis. Poult Sci. (2024) 103:103966. doi: 10.1016/j.psj.2024.103966
41. Lee S-H, Lim T-J, Yun EJ, Kim KH, and Lim S. Anti-menopausal effect of soybean germ extract and lactobacillus gasseri in the ovariectomized rat model. Nutrients. (2023) 15:4485. doi: 10.3390/nu15204485
43. Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. (2006) 24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646
44. Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. (2005) 105:2631–9. doi: 10.1182/blood-2004-06-2480
45. Tsukasaki M and Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. (2019) 19:626–42. doi: 10.1038/s41577-019-0178-8
46. Boyce BF and Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. (2008) 473:139–46. doi: 10.1016/j.abb.2008.03.018
47. Srivastava RK, Dar HY, and Mishra PK. Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol. (2018) 9:657. doi: 10.3389/fimmu.2018.00657
48. Buck MD, O’Sullivan D, and Pearce EL. T cell metabolism drives immunity. J Exp Med. (2015) 212:1345–60. doi: 10.1084/jem.20151159
49. Yu M, Pal S, Paterson CW, Li J-Y, Tyagi AM, Adams J, et al. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. (2021) 131:e143137. doi: 10.1172/JCI143137
50. Zhu L, Hua F, Ding W, Ding K, Zhang Y, and Xu C. The correlation between the Th17/treg cell balance and bone health. Immun Ageing. (2020) 17:30. doi: 10.1186/s12979-020-00202-z
51. Cho K-A, Park M, Kim Y-H, Ryu K-H, and Woo S-Y. Mesenchymal stem cells inhibit RANK-RANKL interactions between osteoclasts and Th17 cells via osteoprotegerin activity. Oncotarget. (2017) 8:83419–31. doi: 10.18632/oncotarget.21379
52. Zeng X, He L, Wang S, Wang K, Zhang Y, Tao L, et al. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin. (2016) 37:255–63. doi: 10.1038/aps.2015.85
53. Ledesma-Colunga MG, Adán N, Ortiz G, Solís-Gutiérrez M, López-Barrera F, Martínez de la Escalera G, et al. Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res Ther. (2017) 19:93. doi: 10.1186/s13075-017-1290-4
54. Bozec A and Zaiss MM. T regulatory cells in bone remodelling. Curr Osteoporos Rep. (2017) 15:121–5. doi: 10.1007/s11914-017-0356-1
55. Tekguc M, Wing JB, Osaki M, Long J, and Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U.S.A. (2021) 118:e2023739118. doi: 10.1073/pnas.2023739118
56. Wang X, Sun B, Wang Y, Gao P, Song J, Chang W, et al. Research progress of targeted therapy regulating Th17/treg balance in bone immune diseases. Front Immunol. (2024) 15:1333993. doi: 10.3389/fimmu.2024.1333993
57. Chen B, Ning K, Sun M-L, and Zhang X-A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: a systematic review. Cell Commun Signal: CCS. (2023) 21:67. doi: 10.1186/s12964-023-01094-4
58. Cooper MD. Current concepts. B lymphocytes. Normal development and function. N Engl J Med. (1987) 317:1452–6. doi: 10.1056/NEJM198712033172306
59. Walsh MC and Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. (2014) 5:511. doi: 10.3389/fimmu.2014.00511
60. Sun W, Meednu N, Rosenberg A, Rangel-Moreno J, Wang V, Glanzman J, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun. (2018) 9:5127. doi: 10.1038/s41467-018-07626-8
61. Zhang Z, Yuan W, Deng J, Wang D, Zhang T, Peng L, et al. Granulocyte colony stimulating factor (G-CSF) regulates neutrophils infiltration and periodontal tissue destruction in an experimental periodontitis. Mol Immunol. (2020) 117:110–21. doi: 10.1016/j.molimm.2019.11.003
62. Yang M and Zhu L. Osteoimmunology: the crosstalk between T cells, B cells, and osteoclasts in rheumatoid arthritis. Int J Mol Sci. (2024) 25:2688. doi: 10.3390/ijms25052688
63. Breuil V, Ticchioni M, Testa J, Roux CH, Ferrari P, Breittmayer JP, et al. Immune changes in post-menopausal osteoporosis: the Immunos study. Osteoporos Int. (2010) 21:805–14. doi: 10.1007/s00198-009-1018-7
64. Horowitz MC, Bothwell ALM, Hesslein DGT, Pflugh DL, and Schatz DG. B cells and osteoblast and osteoclast development. Immunol Rev. (2005) 208:141–53. doi: 10.1111/j.0105-2896.2005.00328.x
65. Rosser EC and Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. (2015) 42:607–12. doi: 10.1016/j.immuni.2015.04.005
66. Evans KE and Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. (2007) 8:4. doi: 10.1186/1471-2121-8-4
67. Xiong Y, Yan C, Chen L, Endo Y, Sun Y, Zhou W, et al. IL-10 induces MC3T3-E1 cells differentiation towards osteoblastic fate in murine model. J Cell Mol Med. (2020) 24:1076–86. doi: 10.1111/jcmm.14832
68. Lee B, Oh Y, Jo S, Kim T-H, and Ji JD. A dual role of TGF-β in human osteoclast differentiation mediated by Smad1 versus Smad3 signaling. Immunol Lett. (2019) 206:33–40. doi: 10.1016/j.imlet.2018.12.003
69. Schlundt C, Fischer H, Bucher CH, Rendenbach C, Duda GN, and Schmidt-Bleek K. The multifaceted roles of macrophages in bone regeneration: a story of polarization, activation and time. Acta Biomater. (2021) 133:46–57. doi: 10.1016/j.actbio.2021.04.052
70. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
71. Tosun B, Wolff LI, Houben A, Nutt S, and Hartmann C. Osteoclasts and macrophages-their role in bone marrow cavity formation during mouse embryonic development. J Bone Miner Res. (2022) 37:1761–74. doi: 10.1002/jbmr.4629
72. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
73. Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. (2019) 196:80–9. doi: 10.1016/j.biomaterials.2017.12.025
74. Zhao S-J, Kong F-Q, Jie J, Li Q, Liu H, Xu A-D, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. (2020) 10:17–35. doi: 10.7150/thno.36930
75. Dou C, Ding N, Zhao C, Hou T, Kang F, Cao Z, et al. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J Bone Miner Res. (2018) 33:899–908. doi: 10.1002/jbmr.3364
76. Huang X, Lan Y, Shen J, Zhao X, Zhou Y, Wu W, et al. M2 macrophages secrete glutamate-containing extracellular vesicles to alleviate osteoporosis by reshaping osteoclast precursor fate. Mol Ther. (2024) 32:1158–77. doi: 10.1016/j.ymthe.2024.02.005
77. Qin Y, Hu C, Jin J, Chao Y, Wang D, Xia F, et al. Bilobalide ameliorates osteoporosis by influencing the SIRT3/NF-κB axis in osteoclasts and promoting M2 polarization in macrophages. Int J Biol Macromol. (2024) 281:136504. doi: 10.1016/j.ijbiomac.2024.136504
78. Wang B, Dong Y, Tian Z, Chen Y, and Dong S. The role of dendritic cells derived osteoclasts in bone destruction diseases. Genes Dis. (2021) 8:401–11. doi: 10.1016/j.gendis.2020.03.009
79. Gallois A, Lachuer J, Yvert G, Wierinckx A, Brunet F, Rabourdin-Combe C, et al. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J Bone Miner Res. (2010) 25:661–72. doi: 10.1359/jbmr.090829
80. Cline-Smith A, Axelbaum A, Shashkova E, Chakraborty M, Sanford J, Panesar P, et al. Ovariectomy activates chronic low-grade inflammation mediated by memory T cells, which promotes osteoporosis in mice. J Bone Miner Res. (2020) 35:1174–87. doi: 10.1002/jbmr.3966
81. Sarkar S and Fox DA. Dendritic cells in rheumatoid arthritis. Front Biosci. (2005) 10:656–65. doi: 10.2741/1560
82. Teng Y-TA. Protective and destructive immunity in the periodontium: part 2–T-cell-mediated immunity in the periodontium. J Dent Res. (2006) 85:209–19. doi: 10.1177/154405910608500302
83. Lapérine O, Blin-Wakkach C, Guicheux J, Beck-Cormier S, and Lesclous P. Dendritic-cell-derived osteoclasts: a new game changer in bone-resorption-associated diseases. Drug Discov Today. (2016) 21:1345–54. doi: 10.1016/j.drudis.2016.04.022
84. Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, and Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. (1996) 2:1132–6. doi: 10.1038/nm1096-1132
85. Elsayed R, Kurago Z, Cutler CW, Arce RM, Gerber J, Celis E, et al. Role of dendritic cell-mediated immune response in oral homeostasis: a new mechanism of osteonecrosis of the jaw. FASEB J. (2020) 34:2595–608. doi: 10.1096/fj.201901819RR
86. Liew PX and Kubes P. The neutrophil’s role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
87. Kolaczkowska E and Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
88. Ando Y, Tsukasaki M, Huynh NC-N, Zang S, Yan M, Muro R, et al. The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. Int J Oral Sci. (2024) 16:18. doi: 10.1038/s41368-023-00275-8
89. Cai B, Lin D, Li Y, Wang L, Xie J, Dai T, et al. N2-polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a missing piece of the bone regeneration puzzle. Adv Sci (weinh Baden-wurtt Ger). (2021) 8:e2100584. doi: 10.1002/advs.202100584
90. Chakravarti A, Raquil M-A, Tessier P, and Poubelle PE. Surface RANKL of toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. (2009) 114:1633–44. doi: 10.1182/blood-2008-09-178301
91. O’Neil LJ, Oliveira CB, Wang X, Navarrete M, Barrera-Vargas A, Merayo-Chalico J, et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann Rheum Dis. (2023) 82:630–8. doi: 10.1136/ard-2022-223568
92. Hajishengallis G, Moutsopoulos NM, Hajishengallis E, and Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. (2016) 28:146–58. doi: 10.1016/j.smim.2016.02.002
93. Herath TDK, Larbi A, Teoh SH, Kirkpatrick CJ, and Goh BT. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J Tissue Eng Regener Med. (2018) 12:e1221–36. doi: 10.1002/term.2521
94. Bastian OW, Croes M, Alblas J, Koenderman L, Leenen LPH, and Blokhuis TJ. Neutrophils inhibit synthesis of mineralized extracellular matrix by human bone marrow-derived stromal cells. In vitro. Front Immunol. (2018) 9:945. doi: 10.3389/fimmu.2018.00945
95. Singh P, Hu P, Hoggatt J, Moh A, and Pelus LM. Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia. (2012) 26:2375–83. doi: 10.1038/leu.2012.117
96. Ragipoglu D, Dudeck A, Haffner-Luntzer M, Voss M, Kroner J, Ignatius A, et al. The role of mast cells in bone metabolism and bone disorders. Front Immunol. (2020) 11:163. doi: 10.3389/fimmu.2020.00163
97. Lesclous P and Saffar JL. Mast cells accumulate in rat bone marrow after ovariectomy. Cells Tissues Organs. (1999) 164:23–9. doi: 10.1159/000016639
98. Kroner J, Kovtun A, Kemmler J, Messmann JJ, Strauss G, Seitz S, et al. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res. (2017) 32:2431–44. doi: 10.1002/jbmr.3234
99. Lesclous P, Guez D, Llorens A, and Saffar JL. Time-course of mast cell accumulation in rat bone marrow after ovariectomy. Calcif Tissue Int. (2001) 68:297–303. doi: 10.1007/BF02390837
100. Lind T, Gustafson A-M, Calounova G, Hu L, Rasmusson A, Jonsson KB, et al. Increased bone mass in female mice lacking mast cell chymase. PLoS One. (2016) 11:e0167964. doi: 10.1371/journal.pone.0167964
101. Turner RT, Iwaniec UT, Marley K, and Sibonga JD. The role of mast cells in parathyroid bone disease. J Bone Miner Res. (2010) 25:1637–49. doi: 10.1002/jbmr.49
102. Silberstein R, Melnick M, Greenberg G, and Minkin C. Bone remodeling in W/wv mast cell deficient mice. Bone. (1991) 12:227–36. doi: 10.1016/8756-3282(91)90068-t
103. Scoville SD, Freud AG, and Caligiuri MA. Modeling human natural killer cell development in the era of innate lymphoid cells. Front Immunol. (2017) 8:360. doi: 10.3389/fimmu.2017.00360
104. Brandstadter JD and Yang Y. Natural killer cell responses to viral infection. J Innate Immun. (2011) 3:274–9. doi: 10.1159/000324176
105. Trinchieri G. Biology of natural killer cells. Adv Immunol. (1989) 47:187–376. doi: 10.1016/s0065-2776(08)60664-1
106. Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. (2005) 5:201–14. doi: 10.1038/nri1570
107. Fauriat C, Long EO, Ljunggren H-G, and Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. (2010) 115:2167–76. doi: 10.1182/blood-2009-08-238469
108. McInnes IB, Leung BP, Sturrock RD, Field M, and Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. (1997) 3:189–95. doi: 10.1038/nm0297-189
109. Söderström K, Stein E, Colmenero P, Purath U, Müller-Ladner U, de Matos CT, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U.S.A. (2010) 107:13028–33. doi: 10.1073/pnas.1000546107
110. Feng S, Madsen SH, Viller NN, Neutzsky-Wulff AV, Geisler C, Karlsson L, et al. Interleukin-15-activated natural killer cells kill autologous osteoclasts via LFA-1, DNAM-1 and TRAIL, and inhibit osteoclast-mediated bone erosion. vitro. Immunol. (2015) 145:367–79. doi: 10.1111/imm.12449
111. Zhou X, Chen Y, Zhang Z, Miao J, Chen G, and Qian Z. Identification of differentially expressed genes, signaling pathways and immune infiltration in postmenopausal osteoporosis by integrated bioinformatics analysis. Heliyon. (2024) 10:e23794. doi: 10.1016/j.heliyon.2023.e23794
112. Lanier LL. NK cell recognition. Annu Rev Immunol. (2005) 23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526
113. Berzins SP, Uldrich AP, Pellicci DG, McNab F, Hayakawa Y, Smyth MJ, et al. Parallels and distinctions between T and NKT cell development in the thymus. Immunol Cell Biol. (2004) 82:269–75. doi: 10.1111/j.0818-9641.2004.01256.x
114. Nair S, Boddupalli CS, Verma R, Liu J, Yang R, Pastores GM, et al. Type II NKT-TFH cells against gaucher lipids regulate B-cell immunity and inflammation. Blood. (2015) 125:1256–71. doi: 10.1182/blood-2014-09-600270
115. Fujii S-I, Shimizu K, Smith C, Bonifaz L, and Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. (2003) 198:267–79. doi: 10.1084/jem.20030324
116. Hu M, Bassett JHD, Danks L, Howell PGT, Xu K, Spanoudakis E, et al. Activated invariant NKT cells regulate osteoclast development and function. J Immunol (baltim Md,: 1950). (2011) 186:2910–7. doi: 10.4049/jimmunol.1002353
117. de Matos CT, Berg L, Michaëlsson J, Felländer-Tsai L, Kärre K, and Söderström K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. (2007) 122:291–301. doi: 10.1111/j.1365-2567.2007.02638.x
118. Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol (baltim Md,: 1950). (2001) 166:6477–82. doi: 10.4049/jimmunol.166.11.6477
119. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. (2009) 31:184–96. doi: 10.1016/j.immuni.2009.08.006
120. Biburger M and Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of kupffer cells. J Immunol (baltim Md,: 1950). (2005) 175:1540–50. doi: 10.4049/jimmunol.175.3.1540
121. Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, and Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. (2000) 106:1481–8. doi: 10.1172/JCI11176
122. Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther. (2006) 8:201. doi: 10.1186/ar1857
123. Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Sci (n Y NY). (2016) 351:1296–302. doi: 10.1126/science.aad2571
124. Shi N, Li N, Duan X, and Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. (2017) 4:14. doi: 10.1186/s40779-017-0122-9
125. Kayama H, Okumura R, and Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu Rev Immunol. (2020) 38:23–48. doi: 10.1146/annurev-immunol-070119-115104
126. Zmora N, Suez J, and Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. (2019) 16:35–56. doi: 10.1038/s41575-018-0061-2
127. Arenas-Gómez CM, Garcia-Gutierrez E, Escobar JS, and Cotter PD. Human gut homeostasis and regeneration: the role of the gut microbiota and its metabolites. Crit Rev Microbiol. (2023) 49:764–85. doi: 10.1080/1040841X.2022.2142088
128. Whisner CM, Martin BR, Nakatsu CH, Story JA, MacDonald-Clarke CJ, McCabe LD, et al. Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: a randomized dose-response trial in free-living pubertal females. J Nutr. (2016) 146:1298–306. doi: 10.3945/jn.115.227256
129. Mazmanian SK, Liu CH, Tzianabos AO, and Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. (2005) 122:107–18. doi: 10.1016/j.cell.2005.05.007
130. Allaire JM, Crowley SM, Law HT, Chang S-Y, Ko H-J, and Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. (2018) 39:677–96. doi: 10.1016/j.it.2018.04.002
131. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. (2009) 139:485–98. doi: 10.1016/j.cell.2009.09.033
132. Carroll SL, Pasare C, and Barton GM. Control of adaptive immunity by pattern recognition receptors. Immunity. (2024) 57:632–48. doi: 10.1016/j.immuni.2024.03.014
133. Wan T, Wang Y, He K, and Zhu S. Microbial sensing in the intestine. Protein Cell. (2023) 14:824–60. doi: 10.1093/procel/pwad028
134. Lu Y-C, Yeh W-C, and Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. (2008) 42:145–51. doi: 10.1016/j.cyto.2008.01.006
135. Gröbner S, Lukowski R, Autenrieth IB, and Ruth P. Lipopolysaccharide induces cell volume increase and migration of dendritic cells. Microbiol Immunol. (2014) 58:61–7. doi: 10.1111/1348-0421.12116
136. Aries M, Cook M, and Hensley-McBain T. A pilot study to investigate peripheral low-level chronic LPS injection as a model of neutrophil activation in the periphery and brain in mice. Int J Mol Sci. (2024) 25:5357. doi: 10.3390/ijms25105357
137. Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. (2007) 37:306–9. doi: 10.1002/eji.200637009
138. Papaioannou S, See J-X, Jeong M, de la Torre C, Ast V, Reiners-Koch P-S, et al. Liver sinusoidal endothelial cells orchestrate NK cell recruitment and activation in acute inflammatory liver injury. Cell Rep. (2023) 42:112836. doi: 10.1016/j.celrep.2023.112836
139. Pallett LJ, Swadling L, Diniz M, Maini AA, Schwabenland M, Gasull AD, et al. Tissue CD14+CD8+ T cells reprogrammed by myeloid cells and modulated by LPS. Nature. (2023) 614:334–42. doi: 10.1038/s41586-022-05645-6
140. Scheithauer TPM, Herrema H, Yu H, Bakker GJ, Winkelmeijer M, Soukhatcheva G, et al. Gut-derived bacterial flagellin induces beta-cell inflammation and dysfunction. Gut Microbes. (2022) 14:2111951. doi: 10.1080/19490976.2022.2111951
141. Hajam IA, Dar PA, Shahnawaz I, Jaume JC, and Lee JH. Bacterial flagellin-a potent immunomodulatory agent. Exp Mol Med. (2017) 49:e373. doi: 10.1038/emm.2017.172
142. Guan C, Huang X, Yue J, Xiang H, Shaheen S, Jiang Z, et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics. (2021) 11:3981–95. doi: 10.7150/thno.55573
143. Clasen SJ, Bell MEW, Borbón A, Lee D-H, Henseler ZM, de la Cuesta-Zuluaga J, et al. Silent recognition of flagellins from human gut commensal bacteria by toll-like receptor 5. Sci Immunol. (2023) 8:eabq7001. doi: 10.1126/sciimmunol.abq7001
144. Markazi A, Bracci PM, McGrath M, and Gao S-J. Pseudomonas aeruginosa stimulates inflammation and enhances kaposi’s sarcoma herpesvirus-induced cell proliferation and cellular transformation through both lipopolysaccharide and flagellin. Mbio. (2020) 11:e02843–20. doi: 10.1128/mBio.02843-20
145. Cao AT, Yao S, Gong B, Elson CO, and Cong Y. Th17 cells upregulate polymeric ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol (baltim Md,: 1950). (2012) 189:4666–73. doi: 10.4049/jimmunol.1200955
146. Zhong Y, Chang X, Zhao Z, Zheng L, Kuang G, Li P, et al. Bacteroides fragilis capsular polysaccharide a ameliorates ulcerative colitis in rat by recovering intestinal barrier integrity and restoring gut microbiota. Front Pharmacol. (2024) 15:1402465. doi: 10.3389/fphar.2024.1402465
147. Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. (2010) 3:487–95. doi: 10.1038/mi.2010.29
148. Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, et al. A bacterial carbohydrate links innate and adaptive responses through toll-like receptor 2. J Exp Med. (2006) 203:2853–63. doi: 10.1084/jem.20062008
149. Erturk-Hasdemir D, Oh SF, Okan NA, Stefanetti G, Gazzaniga FS, Seeberger PH, et al. Symbionts exploit complex signaling to educate the immune system. Proc Natl Acad Sci U.S.A. (2019) 116:26157–66. doi: 10.1073/pnas.1915978116
150. Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Sci (n Y NY). (2016) 352:1116–20. doi: 10.1126/science.aad9948
151. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Sci (n Y NY). (2011) 332:974–7. doi: 10.1126/science.1206095
152. Koh A, De Vadder F, Kovatcheva-Datchary P, and Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
153. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
154. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
155. Zhang Y, Tu S, Ji X, Wu J, Meng J, Gao J, et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-kyn pathway. Nat Commun. (2024) 15:1333. doi: 10.1038/s41467-024-45636-x
156. Kim MH, Kang SG, Park JH, Yanagisawa M, and Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. (2013) 145:396–406.e1–10. doi: 10.1053/j.gastro.2013.04.056
157. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. (2014) 40:128–39. doi: 10.1016/j.immuni.2013.12.007
158. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. (2015) 6:6734. doi: 10.1038/ncomms7734
159. Su X, Gao Y, and Yang R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells. (2022) 11:2296. doi: 10.3390/cells11152296
160. Shinde R and McGaha TL. The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol. (2018) 39:1005–20. doi: 10.1016/j.it.2018.10.010
161. Ma N and Ma X. Dietary amino acids and the gut-microbiome-immune axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Saf. (2019) 18:221–42. doi: 10.1111/1541-4337.12401
162. Gao H, Sun M, Li A, Gu Q, Kang D, Feng Z, et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes. (2025) 17:2467235. doi: 10.1080/19490976.2025.2467235
163. Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, et al. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. (2017) 21:2277–90. doi: 10.1016/j.celrep.2017.10.114
164. Masuda K, Kimura A, Hanieh H, Nguyen NT, Nakahama T, Chinen I, et al. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. Int Immunol. (2011) 23:637–45. doi: 10.1093/intimm/dxr072
165. Dehhaghi M, Kazemi Shariat Panahi H, Heng B, and Guillemin GJ. The gut microbiota, kynurenine pathway, and immune system interaction in the development of brain cancer. Front Cell Dev Biol. (2020) 8:562812. doi: 10.3389/fcell.2020.562812
166. Wang F, Zhang M, Yin L, Zhou Z, Peng Z, Li W, et al. The tryptophan metabolite kynurenic acid ameliorates septic colonic injury through activation of the PPARγ signaling pathway. Int Immunopharmacol. (2025) 147:113651. doi: 10.1016/j.intimp.2024.113651
167. Lee W-S, Lee S-M, Kim M-K, Park S-G, Choi I-W, Choi I, et al. The tryptophan metabolite 3-hydroxyanthranilic acid suppresses T cell responses by inhibiting dendritic cell activation. Int Immunopharmacol. (2013) 17:721–6. doi: 10.1016/j.intimp.2013.08.018
168. Wells JE and Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. (2000) 66:1107–13. doi: 10.1128/AEM.66.3.1107-1113.2000
169. Fiorucci S, Marchianò S, Urbani G, Di Giorgio C, Distrutti E, Zampella A, et al. Immunology of bile acids regulated receptors. Prog Lipid Res. (2024) 95:101291. doi: 10.1016/j.plipres.2024.101291
170. Zhao C, Wu K, Hao H, Zhao Y, Bao L, Qiu M, et al. Gut microbiota-mediated secondary bile acid alleviates staphylococcus aureus-induced mastitis through the TGR5-cAMP-PKA-NF-κB/NLRP3 pathways in mice. NPJ Biofilms Microbiomes. (2023) 9:8. doi: 10.1038/s41522-023-00374-8
171. Pols TWH, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. (2011) 14:747–57. doi: 10.1016/j.cmet.2011.11.006
172. Biagioli M, Carino A, Cipriani S, Francisci D, Marchianò S, Scarpelli P, et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol (baltim Md,: 1950). (2017) 199:718–33. doi: 10.4049/jimmunol.1700183
173. Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. (2020) 581:475–9. doi: 10.1038/s41586-020-2193-0
174. Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and treg cell differentiation. Nature. (2019) 576:143–8. doi: 10.1038/s41586-019-1785-z
175. Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. (2020) 577:410–5. doi: 10.1038/s41586-019-1865-0
176. Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatol (baltim Md). (2007) 45:695–704. doi: 10.1002/hep.21458
177. Gou H, Liu S, Liu L, Luo M, Qin S, He K, et al. Obeticholic acid and 5β-cholanic acid 3 exhibit anti-tumor effects on liver cancer through CXCL16/CXCR6 pathway. Front Immunol. (2022) 13:1095915. doi: 10.3389/fimmu.2022.1095915
178. Ji G, Ma L, Yao H, Ma S, Si X, Wang Y, et al. Precise delivery of obeticholic acid via nanoapproach for triggering natural killer T cell-mediated liver cancer immunotherapy. Acta Pharm Sin B. (2020) 10:2171–82. doi: 10.1016/j.apsb.2020.09.004
179. Eshleman EM and Alenghat T. Epithelial sensing of microbiota-derived signals. Genes Immun. (2021) 22:237–46. doi: 10.1038/s41435-021-00124-w
180. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Sci (n Y NY). (2011) 334:255–8. doi: 10.1126/science.1209791
181. Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, et al. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal paneth cell α-defensins. J Innate Immun. (2013) 5:39–49. doi: 10.1159/000341630
182. Birchenough GMH, Nyström EEL, Johansson MEV, and Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Sci (n Y NY). (2016) 352:1535–42. doi: 10.1126/science.aaf7419
183. Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, et al. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. (2012) 5:501–12. doi: 10.1038/mi.2012.23
184. Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y, Rui-Long W, Bin-Qiang X, et al. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and paneth cells deficiency. Gut Microbes. (2022) 14:2112882. doi: 10.1080/19490976.2022.2112882
185. Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang J-P, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. (2014) 156:1045–59. doi: 10.1016/j.cell.2014.01.026
186. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. (2011) 145:745–57. doi: 10.1016/j.cell.2011.04.022
187. Marchix J, Goddard G, and Helmrath MA. Host-gut microbiota crosstalk in intestinal adaptation. Cell Mol Gastroenterol Hepatol. (2018) 6:149–62. doi: 10.1016/j.jcmgh.2018.01.024
188. Parada Venegas D, de la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. doi: 10.3389/fimmu.2019.00277
189. Yang S, Liu H, and Liu Y. Advances in intestinal epithelium and gut microbiota interaction. Front Microbiol. (2025) 16:1499202. doi: 10.3389/fmicb.2025.1499202
190. Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. (2018) 11:752–62. doi: 10.1038/mi.2017.118
191. Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. (2019) 50:1542. doi: 10.1016/j.immuni.2019.05.024
192. Ye Q, Huang S, Wang Y, Chen S, Yang H, Tan W, et al. Wogonin improves colitis by activating the AhR pathway to regulate the plasticity of ILC3/ILC1. Phytomed: Int J Phytother Phytopharm. (2024) 128:155425. doi: 10.1016/j.phymed.2024.155425
193. Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity. (2014) 41:296–310. doi: 10.1016/j.immuni.2014.06.014
194. Hegyi P, Maléth J, Walters JR, Hofmann AF, and Keely SJ. Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev. (2018) 98:1983–2023. doi: 10.1152/physrev.00054.2017
195. Camilleri M. Bile acid detergency: permeability, inflammation, and effects of sulfation. Am J Physiol Gastrointest Liver Physiol. (2022) 322:G480–8. doi: 10.1152/ajpgi.00011.2022
196. Di Vincenzo F, Puca P, Lopetuso LR, Petito V, Masi L, Bartocci B, et al. Bile acid-related regulation of mucosal inflammation and intestinal motility: from pathogenesis to therapeutic application in IBD and microscopic colitis. Nutrients. (2022) 14:2664. doi: 10.3390/nu14132664
197. Mroz MS, Lajczak NK, Goggins BJ, Keely S, and Keely SJ. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing. Am J Physiol Gastrointest Liver Physiol. (2018) 314:G378–87. doi: 10.1152/ajpgi.00435.2016
198. Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. (2013) 144:145–54. doi: 10.1053/j.gastro.2012.09.055
199. Fukuda S and Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. (2014) 36:103–14. doi: 10.1007/s00281-013-0399-z
200. Ley RE, Turnbaugh PJ, Klein S, and Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. (2006) 444:1022–3. doi: 10.1038/4441022a
201. Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U.S.A. (2016) 113:E7554–63. doi: 10.1073/pnas.1607235113
202. Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. (2016) 351(6275):aad3311. doi: 10.1126/science.aad3311
203. Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, and Keshavarz SA. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr. (2017) 36:497–506. doi: 10.1080/07315724.2017.1318724
204. Lin X, Xiao H-M, Liu H-M, Lv W-Q, Greenbaum J, Gong R, et al. Gut microbiota impacts bone via bacteroides vulgatus-valeric acid-related pathways. Nat Commun. (2023) 14:6853. doi: 10.1038/s41467-023-42005-y
205. Li N, Wang H, Pei H, Wu Y, Li L, Ren Y, et al. Genus_Ruminococcus and order_Burkholderiales affect osteoporosis by regulating the microbiota-gut-bone axis. Front Microbiol. (2024) 15:1373013. doi: 10.3389/fmicb.2024.1373013
206. Di Rienzi SC, Danhof HA, Forshee MD, Roberts A, and Britton RA. Limosilactobacillus reuteri promotes the expression and secretion of enteroendocrine- and enterocyte-derived hormones. FASEB J. (2025) 39:e70408. doi: 10.1096/fj.202401669R
207. Li S, Han X, Liu N, Chang J, Liu G, and Hu S. Lactobacillus plantarum attenuates glucocorticoid-induced osteoporosis by altering the composition of rat gut microbiota and serum metabolic profile. Front Immunol. (2023) 14:1285442. doi: 10.3389/fimmu.2023.1285442
208. Li S, Zhang Y, Ding S, Chang J, Liu G, and Hu S. Curcumin ameliorated glucocorticoid-induced osteoporosis while modulating the gut microbiota and serum metabolome. J Agric Food Chem. (2025) 73:8254–76. doi: 10.1021/acs.jafc.4c06689
209. Zhang M, Sun J, Zhao H, Liu Y, Tang Z, Wen Y, et al. Alginate oligosaccharides relieve estrogen-deprived osteosarcopenia by affecting intestinal Th17 differentiation and systemic inflammation through the manipulation of bile acid metabolism. Int J Biol Macromol. (2025) 295:139581. doi: 10.1016/j.ijbiomac.2025.139581
210. Li X, Xue C, Yang Y, Zhao L, Chen L, Wang J, et al. Therapeutic effects of isaria felina on postmenopausal osteoporosis: modulation of gut microbiota, metabolites, and immune responses. Front Immunol. (2025) 16:1508634. doi: 10.3389/fimmu.2025.1508634
211. France MM and Turner JR. The mucosal barrier at a glance. J Cell Sci. (2017) 130:307–14. doi: 10.1242/jcs.193482
212. Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, et al. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. (2017) 5:e13263. doi: 10.14814/phy2.13263
213. Wang Z, Chen K, Wu C, Chen J, Pan H, Liu Y, et al. An emerging role of prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutr. (2021) 114:1304–13. doi: 10.1093/ajcn/nqab194
214. Chen Z, Lv M, Liang J, Yang K, Li F, Zhou Z, et al. Neuropeptide Y-mediated gut microbiota alterations aggravate postmenopausal osteoporosis. Adv Sci (weinh Baden-wurtt Ger). (2023) 10:e2303015. doi: 10.1002/advs.202303015
215. Chittimalli K, Manandhar I, Adkins S, Rahman M, Toelle A, Tummala R, et al. Angiotensin-(1-7) modulates gut-bone marrow axis in diabetes. Biochem Pharmacol. (2025) 241:117168. doi: 10.1016/j.bcp.2025.117168
216. Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating treg-Th17 cell balance. Bone Rep. (2018) 8:46–56. doi: 10.1016/j.bonr.2018.02.001
217. Dar HY, Pal S, Shukla P, Mishra PK, Tomar GB, Chattopadhyay N, et al. Bacillus clausii inhibits bone loss by skewing treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutr (burbank Angeles Cty Calif). (2018) 54:118–28. doi: 10.1016/j.nut.2018.02.013
218. Sapra L, Dar HY, Bhardwaj A, Pandey A, Kumari S, Azam Z, et al. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing treg-Th17 cell balance in ovx mice. Sci Rep. (2021) 11:1807. doi: 10.1038/s41598-020-80536-2
219. Chen Y, Yang C, Deng Z, Xiang T, Ni Q, Xu J, et al. Gut microbially produced tryptophan metabolite melatonin ameliorates osteoporosis via modulating SCFA and TMAO metabolism. J Pineal Res. (2024) 76:e12954. doi: 10.1111/jpi.12954
220. Wang C, Liu Z, Zhou T, Wu J, Feng F, Wang S, et al. Gut microbiota-derived butyric acid regulates calcific aortic valve disease pathogenesis by modulating GAPDH lactylation and butyrylation. Imeta. (2025) 4:e70048. doi: 10.1002/imt2.70048
221. Schepper JD, Collins FL, Rios-Arce ND, Raehtz S, Schaefer L, Gardinier JD, et al. Probiotic lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J Bone Miner Res: Off J Am Soc Bone Miner Res. (2019) 34:681–98. doi: 10.1002/jbmr.3635
222. Song S, Guo Y, Yang Y, and Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. (2022) 237:108168. doi: 10.1016/j.pharmthera.2022.108168
223. Lou Y, Wen X, Song S, Zeng Y, Huang L, Xie Z, et al. Dietary pectin and inulin: a promising adjuvant supplement for collagen-induced arthritis through gut microbiome restoration and CD4+ T cell reconstitution. J Nutr Biochem. (2024) 133:109699. doi: 10.1016/j.jnutbio.2024.109699
224. Bai Y, Li Y, Marion T, Tong Y, Zaiss MM, Tang Z, et al. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J Autoimmun. (2021) 116:102564. doi: 10.1016/j.jaut.2020.102564
225. Nosal BM, Thornton SN, Darooghegi Mofrad M, Sakaki JR, Mahoney KJ, Macdonald Z, et al. Blackcurrants shape gut microbiota profile and reduce risk of postmenopausal osteoporosis via the gut-bone axis: evidence from a pilot randomized controlled trial. J Nutr Biochem. (2024) 133:109701. doi: 10.1016/j.jnutbio.2024.109701
226. Vázquez-Lorente H, García-Gavilán JF, Shyam S, Konieczna J, Martínez JA, Martín-Sánchez V, et al. Mediterranean diet, physical activity, and bone health in older adults: a secondary analysis of a randomized clinical trial. JAMA Netw Open. (2025) 8:e253710. doi: 10.1001/jamanetworkopen.2025.3710
227. Zhang Y-W, Cao M-M, Li Y-J, Zhang R-L, Wu M-T, Yu Q, et al. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab. (2022) 40:874–89. doi: 10.1007/s00774-022-01375-x
228. Li J, Fan R, Zhang Z, Zhao L, Han Y, Zhu Y, et al. Role of gut microbiota in rheumatoid arthritis: potential cellular mechanisms regulated by prebiotic, probiotic, and pharmacological interventions. Microbiol Res. (2025) 290:127973. doi: 10.1016/j.micres.2024.127973
229. Zhang Y-W, Cao M-M, Li Y-J, Lu P-P, Dai G-C, Zhang M, et al. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Transl. (2022) 37:46–60. doi: 10.1016/j.jot.2022.08.003
230. Yu C, Sun R, Yang W, Gu T, Ying X, Ye L, et al. Exercise ameliorates osteopenia in mice via intestinal microbial-mediated bile acid metabolism pathway. Theranostics. (2025) 15:1741–59. doi: 10.7150/thno.104186
231. Wei J, Ding W, Song K, Zhang Y, Luo Q, and Qi C. Next-generation probiotics and engineered BEVs for precision therapeutics in osteoporosis. Front Nutr. (2025) 12:1581971. doi: 10.3389/fnut.2025.1581971
232. Lin W, Shen C, Li M, Ma S, Liu C, Huang J, et al. Programmable macrophage vesicle based bionic self-adjuvanting vaccine for immunization against monkeypox virus. Adv Sci (weinh Baden-wurtt Ger). (2025) 12:e2408608. doi: 10.1002/advs.202408608
233. Wang M, Li R, Sheng S, Dong Z, Bai L, Wang X, et al. Combination therapy using intestinal organoids and their extracellular vesicles for inflammatory bowel disease complicated with osteoporosis. J Orthop Transl. (2025) 53:26–36. doi: 10.1016/j.jot.2025.05.008
234. Yu T, Bai R, Wang Z, Qin Y, Wang J, Wei Y, et al. Colon-targeted engineered postbiotics nanoparticles alleviate osteoporosis through the gut-bone axis. Nat Commun. (2024) 15:10893. doi: 10.1038/s41467-024-55263-1
235. Wang T, Mo L, Ou J, Fang Q, Wu H, Wu Y, et al. Proteus mirabilis vesicles induce mitochondrial apoptosis by regulating miR96-5p/Abca1 to inhibit osteoclastogenesis and bone loss. Front Immunol. (2022) 13:833040. doi: 10.3389/fimmu.2022.833040
236. Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. (2024) 13:e12429. doi: 10.1002/jev2.12429
237. Xie X, Cheng P, Hu L, Zhou W, Zhang D, Knoedler S, et al. Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss. Signal Transduction Targeted Ther. (2024) 9:24. doi: 10.1038/s41392-023-01726-8
238. Li P, Ji B, Luo H, Sundh D, Lorentzon M, and Nielsen J. One-year supplementation with lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes. (2022) 8:84. doi: 10.1038/s41522-022-00348-2
239. Gregori G, Pivodic A, Magnusson P, Johansson L, Hjertonsson U, Brättemark E, et al. Limosilactobacillus reuteri 6475 and prevention of early postmenopausal bone loss: a randomized clinical trial. JAMA Netw Open. (2024) 7:e2415455. doi: 10.1001/jamanetworkopen.2024.15455
240. Zhao F, Guo Z, Kwok L-Y, Zhao Z, Wang K, Li Y, et al. Bifidobacterium lactis probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr. (2023) 62:965–76. doi: 10.1007/s00394-022-03042-3
Keywords: gut microbiota, intestinal flora, immune cells, osteoporosis, osteolmmunology
Citation: Ma T, Zhang T, Peng C, Liu K, Xiong Y, Chen K, Peng N, Wei Z, Kuang J and Ou L (2025) Immune cells: the key mediator between the gut microbiota and osteoporosis. Front. Immunol. 16:1680021. doi: 10.3389/fimmu.2025.1680021
Received: 05 August 2025; Accepted: 19 September 2025;
Published: 02 October 2025.
Edited by:
Yueqi Chen, Army Medical University, ChinaReviewed by:
Bhupendra Gopalbhai Prajapati, Parul University, IndiaWenquan Liang, Southern Medical University, China
Copyright © 2025 Ma, Zhang, Peng, Liu, Xiong, Chen, Peng, Wei, Kuang and Ou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Kuang, MTM3ODYxNjU2NTZAMTYzLmNvbQ==; Liang Ou, OTkyMjU0MDQzQHFxLmNvbQ==
†These authors have contributed equally to this work and shared first authorship
 Tianyi Ma
Tianyi Ma Tiantian Zhang
Tiantian Zhang Chengqi Peng
Chengqi Peng Ke Liu1
Ke Liu1 Liang Ou
Liang Ou