- 1School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Tuina, Chengdu Pidu District Hospital of Traditional Chinese Medicine, Chengdu, China
- 3Department of Neurological Center, Traditional Chinese Medicine Hospital of Meishan, Meishan, China
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the loss of immune tolerance, leading to the production of autoantibodies and widespread organ damage. Th1, Th2, and Th17 cytokines play critical roles in driving inflammation and tissue injury in SLE. IL-17 and IL-23 have been identified as key mediators in disease progression, with ongoing clinical trials assessing the efficacy of their inhibitors. Additionally, cytokines like IL-10 and IL-22 exhibit dual roles, influencing both pathogenic and protective immune responses. While targeted therapies have shown clinical promise, challenges related to safety and long-term efficacy persist. Emerging targets such as MIF and IL-39 offer new insights into disease mechanisms. This review summarizes the immunoregulatory functions of these cytokines, focusing on their contributions to disease pathogenesis and potential therapeutic strategies, highlighting the importance of cytokine in SLE treatments.
1 Introduction
Systemic lupus erythematosus (SLE) is a persistent autoimmune condition that manifests with extensive organ involvement. Its pathological features are primarily defined by uncontrolled autoantibody synthesis, abnormal complement activation, and diffuse immune complex deposition across tissues (1, 2). The disease exhibits substantial heterogeneity, and its underlying mechanisms remain only partially understood. Current findings suggest that the interplay of genetic susceptibility, hormonal factors, environmental influences, and immune imbalances jointly fuels disease onset and progression (1, 3). Of particular importance, alterations in the functional states of CD4+ T helper (Th) cell subsets, along with disruption of their cytokine signaling networks, have been strongly linked to both disease pathogenesis and clinical expression (4).
On an immunological level, Th cells serve as key modulators of both innate and adaptive immunity through the secretion of lineage-defining cytokines. Studies have highlighted that proinflammatory mediators—including interleukin (IL)-12, IL-17, and IL-23—initiate feed-forward inflammatory circuits by acting on fibroblasts, epithelial cells, and various immune components. This interaction promotes the pathological release of chemokines, matrix metalloproteinases (MMPs), and other inflammatory molecules, thereby sustaining tissue injury and chronic inflammation seen in SLE (5). In this review, we focus on dissecting the immunoregulatory roles and clinical translational relevance of Th1-, Th2-, and Th17-associated cytokines under conditions of Th cell imbalance. Our goal is to elucidate the mechanistic underpinnings of cytokine-mediated immune dysregulation in SLE and to provide a foundation for more refined therapeutic strategies.
2 Th1-associated cytokines in SLE pathogenesis
2.1 Aberrant interferon signaling in SLE
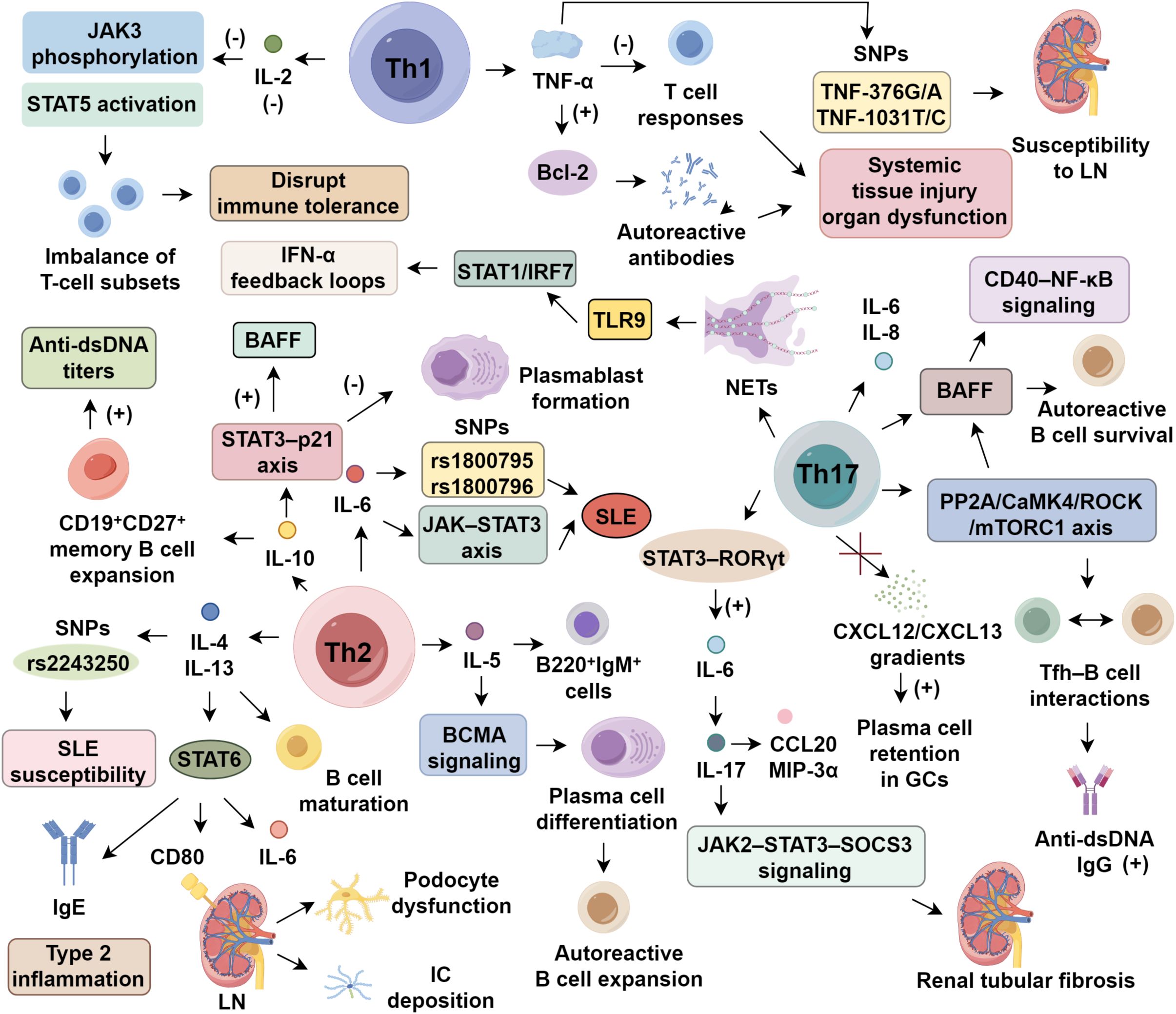
Dysregulated interferon-α (IFN-α) production via Toll-like receptor (TLR) signaling is a hallmark of SLE, with genetic polymorphisms in the IFN-α kinase cascade driving sustained type I interferon activation (6). Elevated serum IFN-α is linked to cutaneous lesions and lupus nephritis (LN) (7, 8), with levels paralleling disease activity indices like SLEDAI (9). Blocking IFN-α has shown clinical benefit: anifrolumab, an anti-IFN-α monoclonal antibody, achieved satisfied SRI-4 response in most patients during phase III trials, though long-term efficacy remains uncertain (10). In contrast, IFN-γ’s pathogenic relevance is less established. In murine lupus models, IFN-γ enhances anti-dsDNA production via FcγRIII-mediated complement activation, worsening renal pathology (11). Clinically, newly diagnosed SLE patients often exhibit elevated IFN-γ, correlating with anti-TPO antibody presence (12, 13). It is proposed that IFN-α drives early innate immune dysregulation, while IFN-γ contributes to adaptive immune activation during later stages (12, 14). However, the mechanisms—particularly involving Th1/Th17 interplay—underlying IFN-γ’s pathogenicity remain to be fully elucidated (Figure 1).
2.2 Bidirectional immunomodulation by IL-2 in SLE
IL-2 plays a dual role in SLE, where its deficiency disrupts immune tolerance while low-dose supplementation restores Treg homeostasis (15, 16). Impaired IL-2 signaling in SLE, characterized by diminished JAK3 phosphorylation and STAT5 activation, destabilizes T cell subset equilibrium (16). Therapeutic reconstitution with low-dose IL-2 selectively expands CD25+ regulatory T cells (Tregs) while limiting pathogenic helper and follicular helper T cells (Tfh), thereby reestablishing immune balance (16, 17). A randomized trial demonstrated that low-dose IL-2 significantly improved clinical outcomes within 6 months, with over 50% complete remission in LN and limited adverse events (18). To enhance efficacy and selectivity, IL-2/anti–IL-2 monoclonal antibody complexes have been developed, preferentially activating CD25+ Tregs while sparing effector T cells. In murine lupus models, these complexes boosted Treg proliferation and mitigated renal damage (19, 20). Next-generation IL-2 analogs—including muteins and pegylated variants such as bempegaldesleukin (NKTR-358)—demonstrate improved pharmacokinetics and sustained Treg expansion, with early clinical data suggesting favorable tolerability (21, 22). These strategies represent promising avenues for inducing durable immune tolerance in SLE.
2.3 TNF-α-driven inflammatory circuits
TNF-α functions as a multifunctional cytokine with paradoxical roles in SLE pathogenesis. Its deleterious effects are primarily mediated through the activation of macrophages by abnormal immune complexes, which induce TNF-α production and establish a proinflammatory milieu (23, 24). This, in turn, facilitates the generation of autoreactive antibodies by dampening T cell responses and enhancing the expression of anti-apoptotic regulators from the BCL-2 family, thereby contributing to systemic tissue injury and organ dysfunction (25). Clinical investigations underscore the aberrant regulation of TNF-α in SLE. Plasma concentrations of TNF-α were reported to be higher in SLE patients compared to healthy individuals, correlating inversely with C3 complement levels (26). Moreover, specific single nucleotide polymorphisms (SNPs) in the TNF-α gene, particularly TNF-376G/A, have been linked to heightened susceptibility to LN, whereas the TNF-308G/A variant appears unrelated to disease risk (27). Despite these findings, the epigenetic regulation bridging these SNPs to altered TNF-α signaling remains poorly understood. While existing studies reinforce TNF-α’s inflammatory contribution in lupus, its mechanistic involvement in Treg dysfunction and vascular endothelial activation warrants in-depth exploration using cell-specific gene deletion approaches (25, 28).
3 Th2-associated cytokines in SLE pathogenesis
3.1 The IL-4/IL-13 axis in lupus
IL-4 and IL-13 are canonical Th2 cytokines that share significant structural and functional overlap, particularly through co-activation of the STAT6 signaling cascade (29). Both cytokines are secreted by Th2 cells, mast cells, and basophils, and orchestrate humoral immune responses by promoting B cell maturation, IgE class switching, and type 2 inflammation (30, 31). In SLE, IL-4/IL-13 axis displays both immunoregulatory and pathogenic roles (31). In lupus-prone MRL/lpr mice, IL-4 signaling impairs apoptosis of autoreactive B cells. Deocharan et al. (32) demonstrated that IL-4 reduced B220+CD19+ B cell apoptosis by 53%, correlating with increased anti-dsDNA titers. While serum IL-4 levels are paradoxically lower in SLE patients than in healthy individuals (33), genetic polymorphisms—such as the rs2243250—have been linked to increased SLE susceptibility (34). Similarly, IL-13 contributes to disease progression via the STAT6 pathway, promoting IgE production, renal epithelial CD80 expression, and IL-6 secretion. Clinically, IL-13 levels are higher in active SLE and 3.4-fold higher in LN patients compared to non-LN individuals, correlating with SLEDAI and 24-hour proteinuria (35, 36). Brugos et al. (36) further demonstrated that elevated IL-13 exacerbates immune complex deposition and podocyte dysfunction in LN. Although IL-13 enhances Th2–B cell interactions and drives renal inflammation, its contribution to mesangial proliferation and complement dysregulation remains unclear. Future studies should employ conditional knockout models and spatial transcriptomic approaches to dissect the spatiotemporal contributions of each cytokine in SLE progression.
3.2 IL-5–induced B cell dysregulation
IL-5, a prototypical Th2 cytokine released by activated CD4+ T cells and mast cells, orchestrates eosinophil recruitment and B cell maturation during immune dysregulation (30). Functional studies have identified its dual immunomodulatory roles. In lupus-prone NZB/W F1 mice, IL-5 overproduction led to increase in proliferating splenic B220+IgM+ cells and accelerated plasma cell differentiation through BCMA signaling, linking IL-5 to autoreactive B cell expansion in SLE (37). Clinical findings, however, remain inconsistent. Some reports observed markedly elevated IL-5 levels in active SLE patients, correlating with anti-nuclear antibody titers (38, 39), while a larger multicenter cohort identified IL-5 upregulation in only 32% of patients, without a clear association with SLEDAI scores (40). This variability may reflect underlying genetic differences, as IL5RA polymorphisms appear to influence eosinophilic infiltration, though their downstream regulatory axes in SLE are not fully delineated (41). Although IL-5 may contribute to antibody isotype switching (IgA, IgE) and tissue pathology, its dynamic expression patterns and potential synergy with IL-4 and IL-13 warrant further exploration via single-cell transcriptomics and spatial profiling techniques.
3.3 IL-6 signaling and clinical heterogeneity
IL-6 is a pivotal mediator of SLE pathogenesis via the JAK–STAT3 axis. Promoter polymorphisms, notably rs1800795 and rs1800796, have been associated with increased SLE susceptibility (42). Clinically, serum IL-6 levels are elevated in SLE and correlate with SLEDAI scores (43). Targeting IL-6 signaling with tocilizumab, an anti-IL-6R monoclonal antibody, significantly reduces anti-dsDNA titers and ameliorates arthritis; however, neutropenia occurred in 30% of patients in early-phase trials (44). Beyond safety concerns, IL-6 inhibition may impair immunological memory: as IL-6 supports B cell maturation, germinal center dynamics, and plasma/memory B cell generation, its blockade can blunt vaccine responses, particularly relevant in SLE populations with elevated infection risk. IL-6 also promotes neutrophil differentiation and survival, and its sustained suppression may weaken host defense against pathogens (45). Diminished vaccine efficacy, notably against influenza and SARS-CoV-2, has been observed in patients on IL-6 inhibitors (46). This therapeutic paradox, attenuation of Th17-driven tissue damage versus disruption of humoral and innate immunity, underscores the need for precision in IL-6–targeted interventions. Future efforts must delineate context-specific signaling to balance efficacy and immunological safety.
3.4 IL-10 modulates immune balance in SLE
IL-10 exhibits dual immunomodulatory roles in SLE, primarily via STAT3 signaling downstream of monocytes, macrophages, regulatory B cells, and Th2 cells (47, 48). In vitro, IL-10 promotes CD19+CD27+ memory B cell expansion and elevates anti-dsDNA titers (49), while clinical data show serum IL-10 levels are higher in active SLE and correlate with renal pathology (50). Conversely, IL-10 deficiency aggravates disease: IL-10 knockout mice display a 65% reduction in CD19+CD24hiCD38hi Bregs and exacerbated proteinuria, underscoring its role in immune tolerance (51). This paradox arises from spatiotemporal and cell-type–specific IL-10Rα expression. In regulatory cells, IL-10 signals through the STAT3–p21 axis to inhibit plasmablast formation, while in autoreactive B cells it enhances BAFF production and survival (52, 53). Early IL-10 secretion may limit inflammation, yet chronic elevation sustains B cell hyperactivity (54, 55). This dynamic modulation is shaped by local cytokine milieus and differential downstream signaling strength. Despite its established relevance to SLE pathogenesis, the spatiotemporal regulation of IL-10, its interplay with type I interferons, and the cell-specific effects require further dissection using targeted gene deletion strategies.
4 Th17-associated cytokines in SLE pathogenesis
4.1 The IL-17 signaling axis in SLE pathogenesis
IL-17, primarily secreted by Th17 cells, is a central mediator of immunopathology in SLE, bridging innate and adaptive immunity via NF-κB, AP-1, and STAT3 activation (56). Elevated serum IL-17 correlates with SLEDAI scores, particularly in neuropsychiatric SLE, implicating it in neuroimmune dysfunction (57, 58). IL-17 drives pathology through multiple mechanisms: it stimulates keratinocyte-derived IL-6 and IL-8, enhances BAFF expression to sustain autoreactive B cell survival, and promotes NET formation, amplifying DNA antigen exposure and type I IFN production. In MRL/lpr mice, IL-17 knockout reduces germinal center (GC) B cells, anti-dsDNA antibodies, and renal immune complexes (59). Further, IL-17 reinforces Tfh–B cell interactions via the PP2A/CaMK4/ROCK/mTORC1 axis, increasing anti-dsDNA IgG by 3.2-fold, and synergizes with BAFF to activate B cell CD40–NF-κB signaling. By disrupting CXCL12/CXCL13 gradients, it elevates plasma cell retention in GCs by 58%, impairing affinity maturation (60, 61). NET formation, induced 4.7 times more frequently in SLE neutrophils, releases nuclear contents sensed by pDC TLR9, initiating STAT1/IRF7-driven IFN-α feedback loops (62, 63). At the tissue level, IL-17 establishes a reciprocal amplification loop with IL-6: STAT3–RORγt–driven Th17 polarization increases IL-6, which in turn enhances IL-17 secretion from renal tubular epithelial cells, promoting fibrosis via JAK2–STAT3–SOCS3 signaling (64). IL-17A/F, particularly in concert with IL-23, boosts RORγt activity and IL-22 production, driving keratinocyte secretion of CCL20 and MIP-3α (65). Despite its pathogenic significance, the tissue-specific roles of IL-17A versus IL-17F, receptor heterogeneity (IL-17RC/RE), and modulation by gut microbiota-derived metabolites remain poorly defined. IL-17 blockade (brodalumab) shows cutaneous benefit but variable efficacy in LN, likely reflecting microenvironmental variation (66). Future studies employing spatial transcriptomics and single-cell multi-omics are essential to unravel IL-17’s context-specific effects on NETosis, Th17/Treg balance, and metabolic programming within the SLE immune landscape.
4.2 Mechanisms of Th17 dysregulation in SLE pathogenesis
Clonal expansion of Th17 cells is central to SLE pathology, influenced by multiple regulatory disruptions (67). Environmental factors, such as gut microbiota imbalance and pathogen-derived signals, promote Th17 polarization by destabilizing T cell homeostasis (56, 68, 69). On the molecular level, persistent STAT3 activation cooperates with TGF-β, driving RORγt-mediated transcriptional reprogramming and boosting the conversion of naïve CD4+ T cells to Th17. This also diverts Tregs to Th17-like subsets (70). Pathologically, expanded Th17 cells release IL-17A/F, IL-22, and CCL20, activating endothelial ICAM-1/VEGF pathways, which promotes NET formation and CD138+ plasma cell differentiation, leading to a 4.7-fold increase in anti-dsDNA titers (71, 72). In SLE, Th17/Treg ratio was 5.6:1 in peripheral blood, with renal Th17 cells accounting for 35% of CD4+ T cells. This contributes to podocyte nephrin downregulation via NFATc1/CaMK4 signaling, worsening proteinuria (73, 74). Despite this understanding, the role of the gut-kidney axis, metabolic reprogramming, and tissue-resident memory Th17 (TRM) dynamics require further investigation using spatial transcriptomics.
4.3 Th17/IL-17 axis and its contribution to SLE pathogenesis
The Th17/IL-17 axis plays a central role in SLE immunopathogenesis. Following autoantigen exposure, TLR4 activation by DAMPs induces APC-derived IL-6, IL-23, and TGF-β, promoting STAT3-dependent Th17 differentiation and IL-17A/F production (75, 76). In LN, Th17 cells infiltrate renal tissue via CCL20–CCR6, exhibit enhanced chemotaxis, and drive epithelial–mesenchymal transition through NF-κB and CaMK4, markedly increasing fibrosis (77). Renal biopsies show Th17 enrichment in SLE, correlating with proteinuria (78). IL-17RA/RC expression on plasma cells augments pathogenic IgG and anti-dsDNA autoantibodies via PI3K/Akt/mTOR, with IL-17 stimulation increasing largely (79). Yet, the integration of Th17 activity, Treg dysregulation, and epigenetic remodeling in SLE remains incompletely defined and warrants high-resolution single-cell analyses. IL-23, essential for Th17 polarization, amplifies SLE inflammation via IL-23R/STAT3 signaling. It synergizes with TNF-α to enhance CXCL10 from monocytes and intensify Th1 and Th17 responses through RORγt activation, establishing a self-reinforcing inflammatory loop (80). Elevated IL-23 correlates with SLEDAI scores and is particularly prominent in LN (81). In phase IIb trials, risankizumab (anti–IL-23) achieved nearly 75% lesion reduction in 68% of cutaneous lupus patients, although infection risk increased (82). Moreover, IL-23’s influence on γδT17 trafficking, microbiota-immune crosstalk, and chromatin remodeling remains underexplored. Future investigations integrating single-cell and spatial transcriptomics may uncover novel regulatory axes and therapeutic windows in IL-23–driven SLE pathobiology.
5 Emerging cytokine targets in SLE pathogenesis
IL-22, produced by Th17 cells, γδ T cells, and ILC3s, contributes to SLE via STAT3 signaling, promoting SOCS3-mediated MMP-9 induction and podocyte nephrin loss, exacerbating LN progression (83). Serum IL-22 levels are markedly elevated in LN and correlate with proteinuria and fibrosis. In MRL/lpr mice, IL-22 blockade ameliorated renal injury, highlighting therapeutic promise (84). Additionally, microbial dysbiosis and intestinal barrier disruption drive IL-22 overproduction through TLR-IL-23–dependent activation of ILC3s and Th17 cells. Antibiotic-induced microbiota depletion reduces IL-22 and proteinuria, implicating a gut-kidney axis in LN pathogenesis (85). However, the interplay between IL-22, IL-17/IL-23 circuits, and Th17/Treg balance remains incompletely understood. IL-39, a novel IL-12 family heterodimer (p19/Ebi3), is secreted by activated B cells via TLR7–MyD88 pathways and induces BAFF production and plasma cell expansion through STAT1/STAT3 co-activation (86, 87). Murine models show that IL-39 deficiency reduces plasma cell differentiation and renal damage (88). Yet, IL-39 lacks conserved functionality in humans, as recombinant IL-39 fails to activate STAT3 or alter cytokine expression in human PBMCs (89). Its receptor complex (IL-23R/gp130) and biological relevance remain undefined, and no clinical trials have been initiated. Macrophage migration inhibitory factor (MIF), a pleiotropic cytokine, activates the NLRP3 inflammasome via CD74/CXCR4/mTOR signaling, promoting IL-1β release and pyroptosis in renal epithelium (90). Clinically, serum MIF levels correlate with proteinuria and creatinine and inversely with C3, especially in LN (91). IMTO-05, an anti-MIF monoclonal antibody, reduced proteinuria in early trials but was halted due to adverse events and limited enrollment (92). MIF also modulates T cell costimulation, Th17/Treg imbalance, and NET formation, though its mechanistic roles warrant validation through gene-editing studies.
6 Therapies targeting IL-17
Targeting IL-17 in SLE involves three primary strategies, including direct neutralization with secukinumab or ixekizumab, achieving 63% renal and 73% skin response but with increased infection risk (61, 77), indirect inhibition via CaMK4 and STAT3 blockade, which suppress IL-17A production and reduce proteinuria through H3K27ac modulation (62, 64, 93), and metabolic reprogramming, which decreases renal IL-17+ cells by 61% (94). Combined therapies restore Treg/Th17 balance and lower SLEDAI by 48% (77). However, CaMK4’s widespread expression in non-immune tissues raises off-target toxicity concerns, as current inhibitors lack immune cell specificity. To address this, immune-targeted strategies, such as CD4+ T cell–selective liposomes, antibody-drug conjugates, and conditional CRISPR/Cas9 systems, are under development. Novel regulators like miR-125a-3p reduce renal fibrosis by 61% (95), while secukinumab yields 77% reduction in anti-dsDNA titers in refractory LN (96). Challenges persist, including STAT3 inhibitor hepatotoxicity and compensatory IL-23/IL-1β upregulation (97). Future efforts should prioritize multi-omics biomarker integration and tissue-specific delivery systems to enhance precision and minimize systemic toxicity.
7 Conclusion
SLE is a multifaceted autoimmune disorder driven by a combination of genetic, environmental, and immune factors. Th1, Th2, and Th17-associated cytokines play central roles in the progression and clinical manifestation of the disease. Despite significant advances in understanding the immunopathology of SLE, including the roles of cytokines like IL-17, IL-23, and IL-10, therapeutic strategies remain limited by issues related to safety and efficacy durability. Targeting cytokines such as IL-17 has shown promise in clinical trials, but challenges such as compensatory upregulation of other inflammatory pathways and potential adverse effects require further exploration. Emerging targets like IL-22 and MIF offer new avenues for intervention, although their mechanisms and interactions within the immune microenvironment need more comprehensive investigation. To achieve precise and effective treatments, future research must focus on integrating multi-omics approaches, identifying reliable biomarkers, and exploring novel delivery systems to tailor therapies to individual patients, minimizing SLE heterogeneity and improving long-term outcomes.
Author contributions
ZC: Writing – original draft. SL: Writing – original draft. RX: Writing – original draft. PZ: Writing – original draft, Writing – review & editing. YF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Joint Innovation Fund Project of Chengdu University of Traditional Chinese Medicine (LH202402013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, et al. An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus. (2022) 14:e30330. doi: 10.7759/cureus.30330
2. Cocco C, Manca E, Corda G, Angioni MM, Noli B, Congia M, et al. Brain-reactive autoantibodies in neuropsychiatric systemic lupus erythematosus. Front Immunol. (2023) 14:1157149. doi: 10.3389/fimmu.2023.1157149
3. Woo JMP, Parks CG, Jacobsen S, Costenbader KH, and Bernatsky S. The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Intern Med. (2022) 291:755–78. doi: 10.1111/joim.13448
4. Park J, Jang W, Park HS, Park KH, Kwok SK, Park SH, et al. Cytokine clusters as potential diagnostic markers of disease activity and renal involvement in systemic lupus erythematosus. J Int Med Res. (2020) 48:300060520926882. doi: 10.1177/0300060520926882
5. Rafael-Vidal C, Pérez N, Altabás I, Garcia S, and Pego-Reigosa JM. Blocking IL-17: A promising strategy in the treatment of systemic rheumatic diseases. Int J Mol Sci. (2020) 21:7100. doi: 10.3390/ijms21197100
6. Infante B, Mercuri S, Dello Strologo A, Franzin R, Catalano V, Troise D, et al. Unraveling the link between interferon-α and systemic lupus erythematosus: from the molecular mechanisms to target therapies. Int J Mol Sci. (2022) 23:15998. doi: 10.3390/ijms232415998
7. Jinshan Z, Yong Q, Fangqi C, Juanmei C, Min L, and Changzheng H. The role of TNF-α as a potential marker for acute cutaneous lupus erythematosus in patients with systemic lupus erythematosus. J Dermatol. (2024) 51:1481–91. doi: 10.1111/1346-8138.17355
8. Nawata A, Nakayamada S, Hisano S, Miyazaki Y, Miyamoto T, Shiba E, et al. Differential expression of IFN-α, IL-12 and BAFF on renal immune cells and its relevance to disease activity and treatment responsiveness in patients with proliferative lupus nephritis. Lupus Sci Med. (2023) 10:e000962. doi: 10.1136/lupus-2023-000962
9. Abdel Galil SM, El-Shafey AM, Abdul-Maksoud RS, and El-Boshy M. Interferon alpha gene expression and serum level association with low vitamin D levels in Egyptian female patients with systemic lupus erythematosus. Lupus. (2018) 27:199–209. doi: 10.1177/0961203317716321
10. Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:1909–16. doi: 10.1136/annrheumdis-2015-208562
11. Baudino L, Azeredo da Silveira S, Nakata M, and Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol. (2006) 28:175–84. doi: 10.1007/s00281-006-0037-0
12. Dufour A, Bellac CL, Eckhard U, Solis N, Klein T, Kappelhoff R, et al. C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease. Nat Commun. (2018) 9:2416. doi: 10.1038/s41467-018-04717-4
13. Torell F, Eketjäll S, Idborg H, Jakobsson PJ, Gunnarsson I, Svenungsson E, et al. Cytokine profiles in autoantibody defined subgroups of systemic lupus erythematosus. J Proteome Res. (2019) 18:1208–17. doi: 10.1021/acs.jproteome.8b00811
14. Bolouri N, Akhtari M, Farhadi E, Mansouri R, Faezi ST, Jamshidi A, et al. Role of the innate and adaptive immune responses in the pathogenesis of systemic lupus erythematosus. Inflammation Res. (2022) 71:537–54. doi: 10.1007/s00011-022-01554-6
15. Wang B, Chen C, Liu X, Zhou S, Xu T, and Wu M. The effect of combining PD-1 agonist and low-dose Interleukin-2 on treating systemic lupus erythematosus. Front Immunol. (2023) 14:1111005. doi: 10.3389/fimmu.2023.1111005
16. La Cava A. Low-dose interleukin-2 therapy in systemic lupus erythematosus. Rheumatol Immunol Res. (2023) 4:150–6. doi: 10.2478/rir-2023-0021
17. Zhang R, Zhao Y, Chen X, Zhuang Z, Li X, and Shen E. Low-dose IL-2 therapy in autoimmune diseases: An update review. JIRoI. (2024) 43:113–37. doi: 10.1080/08830185.2023.2274574
18. He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2020) 79:141–9. doi: 10.1136/annrheumdis-2019-215396
19. Yan JJ, Lee JG, Jang JY, Koo TY, Ahn C, and Yang J. IL-2/anti-IL-2 complexes ameliorate lupus nephritis by expansion of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Kidney Int. (2017) 91:603–15. doi: 10.1016/j.kint.2016.09.022
20. Yokoyama Y, Iwasaki T, Kitano S, Satake A, Nomura S, Furukawa T, et al. IL-2-anti-IL-2 monoclonal antibody immune complexes inhibit collagen-induced arthritis by augmenting regulatory T cell functions. J Immunol. (2018) 201:1899–906. doi: 10.4049/jimmunol.1701502
21. Fanton C, Furie R, Chindalore V, Levin R, Diab I, Dixit N, et al. Selective expansion of regulatory T cells by NKTR-358 in healthy volunteers and patients with systemic lupus erythematosus. J Transl Autoimmun. (2022) 5:100152. doi: 10.1016/j.jtauto.2022.100152
22. Dixit N, Fanton C, Langowski JL, Kirksey Y, Kirk P, Chang T, et al. NKTR-358: A novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J Transl Autoimmun. (2021) 4:100103. doi: 10.1016/j.jtauto.2021.100103
23. Ghorbaninezhad F, Leone P, Alemohammad H, Najafzadeh B, Nourbakhsh NS, Prete M, et al. Tumor necrosis factor−α in systemic lupus erythematosus: Structure, function and therapeutic implications (Review). Int J Mol Med. (2022) 49:43. doi: 10.3892/ijmm.2022.5098
24. Richter P, Macovei LA, Mihai IR, Cardoneanu A, Burlui MA, and Rezus E. Cytokines in systemic lupus erythematosus-focus on TNF-α and IL-17. Int J Mol Sci. (2023) 24:14413. doi: 10.3390/ijms241914413
25. Postal M and Appenzeller S. The role of Tumor Necrosis Factor-alpha (TNF-α) in the pathogenesis of systemic lupus erythematosus. Cytokine. (2011) 56:537–43. doi: 10.1016/j.cyto.2011.08.026
26. Ma CY, Jiao YL, Zhang J, Yang QR, Zhang ZF, Shen YJ, et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-α and TNF-α in systemic lupus erythematosus. Rheumatol Int. (2012) 32:395–402. doi: 10.1007/s00296-010-1636-6
27. Ramírez-Bello J, Cadena-Sandoval D, Mendoza-Rincón JF, Barbosa-Cobos RE, Sánchez-Muñoz F, Amezcua-Guerra LM, et al. Tumor necrosis factor gene polymorphisms are associated with systemic lupus erythematosus susceptibility or lupus nephritis in Mexican patients. Immunol Res. (2018) 66:348–54. doi: 10.1007/s12026-018-8993-8
28. Chen L, Huang Z, Liao Y, Yang B, and Zhang J. Association between tumor necrosis factor polymorphisms and rheumatoid arthritis as well as systemic lupus erythematosus: a meta-analysis. Braz J Med Biol Res. (2019) 52:e7927. doi: 10.1590/1414-431x20187927
29. Wu W-J, Wang S-H, Wu C-C, Su Y-A, Chiang C-Y, Lai C-H, et al. IL-4 and IL-13 promote proliferation of mammary epithelial cells through STAT6 and IRS-1. JIJoMS. (2021) 22:12008. doi: 10.3390/ijms222112008
30. Ogulur I, Mitamura Y, Yazici D, Pat Y, Ardicli S, Li M, et al. Type 2 immunity in allergic diseases. Cell Mol Immunol. (2025) 22:211–42. doi: 10.1038/s41423-025-01261-2
31. Bernstein ZJ, Shenoy A, Chen A, Heller NM, and Spangler JB. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol Rev. (2023) 320:29–57. doi: 10.1111/imr.13230
32. Deocharan B, Marambio P, Edelman M, and Putterman C. Differential effects of interleukin-4 in peptide induced autoimmunity. Clin Immunol. (2003) 108:80–8. doi: 10.1016/S1521-6616(03)00096-2
33. Oon S, Monaghan K, Ng M, Hoi A, Morand E, Vairo G, et al. A potential association between IL-3 and type I and III interferons in systemic lupus erythematosus. Clin Transl Immunol. (2019) 8:e01097. doi: 10.1002/cti2.1097
34. Mohammadoo-Khorasani M, Salimi S, Tabatabai E, Sandoughi M, Zakeri Z, and Farajian-Mashhadi F. Interleukin-1β (IL-1β) & IL-4 gene polymorphisms in patients with systemic lupus erythematosus (SLE) & their association with susceptibility to SLE. Indian J Med Res. (2016) 143:591–6. doi: 10.4103/0971-5916.187107
35. Wang R, Lu YL, Huang HT, Qin HM, Lan Y, Wang JL, et al. Association of interleukin 13 gene polymorphisms and plasma IL 13 level with risk of systemic lupus erythematosus. Cytokine. (2018) 104:92–7. doi: 10.1016/j.cyto.2017.09.034
36. Brugos B, Vincze Z, Sipka S, Szegedi G, and Zeher M. Serum and urinary cytokine levels of SLE patients. Pharmazie. (2012) 67:411–3.
37. Wen X, Zhang D, Kikuchi Y, Jiang Y, Nakamura K, Xiu Y, et al. Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus. Eur J Immunol. (2004) 34:2740–9. doi: 10.1002/eji.200425267
38. Ko H, Kim CJ, and Im SH. T helper 2-associated immunity in the pathogenesis of systemic lupus erythematosus. Front Immunol. (2022) 13:866549. doi: 10.3389/fimmu.2022.866549
39. Zhu H, Mi W, Luo H, Chen T, Liu S, Raman I, et al. Whole-genome transcription and DNA methylation analysis of peripheral blood mononuclear cells identified aberrant gene regulation pathways in systemic lupus erythematosus. Arthritis Res Ther. (2016) 18:162. doi: 10.1186/s13075-016-1050-x
40. Timóteo RP, Micheli DC, Teodoro RB, Freire M, Bertoncello D, Murta EF, et al. Characterization of inflammatory markers associated with systemic lupus erythematosus patients undergoing treatment. Rev Bras Reumatol Engl Ed. (2016) 56:497–503. doi: 10.1016/j.rbre.2016.02.009
41. Iakovliev A, Castellini-Pérez O, Erabadda B, Consortium PC, Consortium PFC, Martín J, et al. : Discovery of core genes for systemic lupus erythematosus via genome-wide aggregated trans-effects analysis. JG Immun. (2025) 26:497–508. doi: 10.1038/s41435-025-00352-4
42. Qiu J, Qin X, Wen J, Wu L, Kong L, Ou Y, et al. Single-nucleotide polymorphisms influence the promoter activities of systemic lupus erythematosus-associated genes. Biotechnol Lett. (2020) 42:1887–96. doi: 10.1007/s10529-020-02916-y
43. Richter P, Rezus C, Burlui AM, Schreiner TG, and Rezus E. Serum interleukin-6 in systemic lupus erythematosus: insights into immune dysregulation, disease activity, and clinical manifestations. Cells. (2025) 14:1568. doi: 10.3390/cells14191568
44. Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. (2010) 62:542–52. doi: 10.1002/art.27221
45. Nepal D and Gazeley D. Role of IL-6 and IL-6 targeted therapy in systemic lupus erythematosus. Rheumatol (Oxford). (2023) 62:3804–10. doi: 10.1093/rheumatology/kead416
46. Della-Torre E, Criscuolo E, Lanzillotta M, Locatelli M, Clementi N, Mancini N, et al. IL-1 and IL-6 inhibition affects the neutralising activity of anti-SARS-CoV-2 antibodies in patients with COVID-19. JTLR. (2021) 3:e829–31. doi: 10.1016/S2665-9913(21)00321-0
47. Michée-Cospolite M, Boudigou M, Grasseau A, Simon Q, Mignen O, Pers J-O, et al. Molecular mechanisms driving IL-10-producing B cells functions: STAT3 and c-MAF as underestimated central key regulators? JFiI. (2022) 13:818814. doi: 10.3389/fimmu.2022.818814
48. Biswas S, Bieber K, and Manz RA. IL-10 revisited in systemic lupus erythematosus. JFiI. (2022) 13:970906. doi: 10.3389/fimmu.2022.970906
49. Ouyang W, Rutz S, Crellin NK, Valdez PA, and Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. (2011) 29:71–109. doi: 10.1146/annurev-immunol-031210-101312
50. Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, et al. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep. (2016) 6:34604. doi: 10.1038/srep34604
51. Heinemann K, Wilde B, Hoerning A, Tebbe B, Kribben A, Witzke O, et al. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol. (2016) 45:312–6. doi: 10.3109/03009742.2015.1126346
52. Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. (2003) 112:286–97. doi: 10.1172/JCI18025
53. Calame KL. Plasma cells: finding new light at the end of B cell development. Nat Immunol. (2001) 2:1103–8. doi: 10.1038/ni1201-1103
54. Facciotti F, Larghi P, Bosotti R, Vasco C, Gagliani N, Cordiglieri C, et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6(+)B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci U.S.A. (2020) 117:7305–16. doi: 10.1073/pnas.1917834117
55. Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. (2023) 14:1161067. doi: 10.3389/fimmu.2023.1161067
56. Yang Y, Yan C, Yu L, Zhang X, Shang J, Fan J, et al. The star target in SLE: IL-17. Inflammation Res. (2023) 72:313–28. doi: 10.1007/s00011-022-01674-z
57. Ye C, Chen L, Zhang L, Zheng Y, Liu X, Huang Z, et al. IL-17A, IL-23R, FCGR3A are associated with neuropsychiatric systemic lupus erythematosus susceptibility in pediatric patients with lupus nephritis. JC. (2025) 188:156874. doi: 10.1016/j.cyto.2025.156874
58. Vincent FB, Northcott M, Hoi A, Mackay F, and Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. (2013) 15:R97. doi: 10.1186/ar4277
59. Li Y, Wang R, Liu S, Liu J, Pan W, Li F, et al. Interleukin-25 is upregulated in patients with systemic lupus erythematosus and ameliorates murine lupus by inhibiting inflammatory cytokine production. Int Immunopharmacol. (2019) 74:105680. doi: 10.1016/j.intimp.2019.105680
60. Du LJ, Feng YX, He ZX, Huang L, Wang Q, Wen CP, et al. Norcantharidin ameliorates the development of murine lupus via inhibiting the generation of IL-17 producing cells. Acta Pharmacol Sin. (2022) 43:1521–33. doi: 10.1038/s41401-021-00773-7
61. Robert M and Miossec P. Interleukin-17 and lupus: enough to be a target? For which patients? Lupus. (2020) 29:6–14. doi: 10.1177/0961203319891243
62. Koga T, Ichinose K, Kawakami A, and Tsokos GC. Current insights and future prospects for targeting IL-17 to treat patients with systemic lupus erythematosus. Front Immunol. (2020) 11:624971. doi: 10.3389/fimmu.2020.624971
63. Frangou E, Chrysanthopoulou A, Mitsios A, Kambas K, Arelaki S, Angelidou I, et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis. (2019) 78:238–48. doi: 10.1136/annrheumdis-2018-213181
64. Du B, Fan X, Lei F, Zhang S, Li G, and Xi X. Comparative transcriptome analysis reveals a potential role for CaMK4 in γδT17 cells from systemic lupus erythematosus patients with lupus nephritis. Int Immunopharmacol. (2020) 80:106139. doi: 10.1016/j.intimp.2019.106139
65. Elkoumi MA, Allah MA, Mohamed FY, Boraey NF, Abdellatif SH, Shehab MM, et al. et al: Association of interleukin-17A gene polymorphisms and susceptibility to systemic lupus erythematosus in Egyptian children and adolescents: a multi-centre study. Lupus. (2020) 29:767–75. doi: 10.1177/0961203320922305
66. Chasov V, Zmievskaya E, Ganeeva I, Gilyazova E, Davletshin D, Khaliulin M, et al. Immunotherapy strategy for systemic autoimmune diseases: betting on CAR-T cells and antibodies. JA. (2024) 13:10. doi: 10.3390/antib13010010
67. Wu Y, Zhang W, Liao Y, Sun T, Liu Y, and Liu Y. Immune cell aberrations in Systemic Lupus Erythematosus: navigating the targeted therapies toward precision management. Cell Mol Biol Lett. (2025) 30:73. doi: 10.1186/s11658-025-00749-z
68. Chen PM and Tsokos GC. T cell abnormalities in the pathogenesis of systemic lupus erythematosus: an update. Curr Rheumatol Rep. (2021) 23:12. doi: 10.1007/s11926-020-00978-5
69. Yang J, Yang X, Yang J, and Li M. Hydroxychloroquine inhibits the differentiation of th17 cells in systemic lupus erythematosus. J Rheumatol. (2018) 45:818–26. doi: 10.3899/jrheum.170737
70. Jiang C, Wang H, Xue M, Lin L, Wang J, Cai G, et al. Reprograming of peripheral Foxp3(+) regulatory T cell towards Th17-like cell in patients with active systemic lupus erythematosus. Clin Immunol. (2019) 209:108267. doi: 10.1016/j.clim.2019.108267
71. Fu D, Ma J, Gong Q, Senouthai S, Wang J, You Y, et al. Fractalkine mediates lymphocyte inflammation and tubulointerstitial lesions by modifying the Treg/Th17 balance in lupus-prone MRL/lpr mice. Am J Transl Res. (2020) 12:6170–86.
72. Larosa M, Zen M, Gatto M, Jesus D, Zanatta E, Iaccarino L, et al. IL-12 and IL-23/Th17 axis in systemic lupus erythematosus. Exp Biol Med (Maywood). (2019) 244:42–51. doi: 10.1177/1535370218824547
73. Chen M, Chen X, and Wan Q. Altered frequency of Th17 and Treg cells in new-onset systemic lupus erythematosus patients. Eur J Clin Invest. (2018) 48:e13012. doi: 10.1111/eci.13012
74. Fu D, Senouthai S, Wang J, and You Y. Vasoactive intestinal peptide ameliorates renal injury in a pristane-induced lupus mouse model by modulating Th17/Treg balance. BMC Nephrol. (2019) 20:350. doi: 10.1186/s12882-019-1548-y
75. Jin X, Chen J, Wu J, Lu Y, Li B, Fu W, et al. Aberrant expansion of follicular helper T cell subsets in patients with systemic lupus erythematosus. Front Immunol. (2022) 13:928359. doi: 10.3389/fimmu.2022.928359
76. Yu Y, Fu S, Zhang X, Wang L, Zhao L, Wan W, et al. Leptin facilitates the differentiation of Th17 cells from MRL/Mp-Fas lpr lupus mice by activating NLRP3 inflammasome. Innate Immun. (2020) 26:294–300. doi: 10.1177/1753425919886643
77. Paquissi FC and Abensur H. The th17/IL-17 axis and kidney diseases, with focus on lupus nephritis. Front Med (Lausanne). (2021) 8:654912. doi: 10.3389/fmed.2021.654912
78. Zhao C, Chu Y, Liang Z, Zhang B, Wang X, Jing X, et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/Treg cells in refractory SLE patients. BMC Immunol. (2019) 20:32. doi: 10.1186/s12865-019-0305-0
79. Ma K, Du W, Xiao F, Han M, Huang E, Peng N, et al. IL-17 sustains the plasma cell response via p38-mediated Bcl-xL RNA stability in lupus pathogenesis. Cell Mol Immunol. (2021) 18:1739–50. doi: 10.1038/s41423-020-00540-4
80. Fischer K, Przepiera-Będzak H, Sawicki M, Walecka A, Brzosko I, and Brzosko M. Serum interleukin-23 in polish patients with systemic lupus erythematosus: association with lupus nephritis, obesity, and peripheral vascular disease. Mediators Inflammation. (2017) 2017:9401432. doi: 10.1155/2017/9401432
81. Shahin D, El-Farahaty RM, Houssen ME, Machaly SA, Sallam M, ElSaid TO, et al. Serum 25-OH vitamin D level in treatment-naïve systemic lupus erythematosus patients: Relation to disease activity, IL-23 and IL-17. Lupus. (2017) 26:917–26. doi: 10.1177/0961203316682095
82. van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. (2018) 392:1330–9. doi: 10.1016/S0140-6736(18)32167-6
83. Yang X, Weng Q, Hu L, Yang L, Wang X, Xiang X, et al. Increased interleukin-22 levels in lupus nephritis and its associated with disease severity: a study in both patients and lupus-like mice model. Clin Exp Rheumatol. (2019) 37:400–7.
84. Hu L, Hu J, Chen L, Zhang Y, Wang Q, and Yang X. Interleukin-22 from type 3 innate lymphoid cells aggravates lupus nephritis by promoting macrophage infiltration in lupus-prone mice. JFiI. (2021) 12:584414. doi: 10.3389/fimmu.2021.584414
85. Valeri M and Raffatellu M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis. (2016) 74:111. doi: 10.1093/femspd/ftw111
86. Lv K, Hu B, Xu M, Wan L, Jin Z, Xu M, et al. IL-39 promotes chronic graft-versus-host disease by increasing T and B Cell pathogenicity. Exp Hematol Oncol. (2022) 11:34. doi: 10.1186/s40164-022-00286-x
87. Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han G, et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol. (2016) 46:1343–50. doi: 10.1002/eji.201546095
88. Wang X, Liu X, Zhang Y, Wang Z, Zhu G, Han G, et al. Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol. (2016) 186:144–56. doi: 10.1111/cei.12840
89. Bridgewood C, Alase A, Watad A, Wittmann M, Cuthbert R, and McGonagle D. The IL-23p19/EBI3 heterodimeric cytokine termed IL-39 remains a theoretical cytokine in man. Inflammation Res. (2019) 68:423–6. doi: 10.1007/s00011-019-01235-x
90. Shin MS, Kang Y, Wahl ER, Park HJ, Lazova R, Leng L, et al. Macrophage migration inhibitory factor regulates U1 small nuclear RNP immune complex-mediated activation of the NLRP3 inflammasome. Arthritis Rheumatol. (2019) 71:109–20. doi: 10.1002/art.40672
91. Tu Y, Guo R, Li J, Wang S, Leng L, Deng J, et al. MiRNA regulation of MIF in SLE and attenuation of murine lupus nephritis with miR-654. Front Immunol. (2019) 10:2229. doi: 10.3389/fimmu.2019.02229
92. Bilsborrow JB, Doherty E, Tilstam PV, and Bucala R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther Targets. (2019) 23:733–44. doi: 10.1080/14728222.2019.1656718
93. Chen SY, Liu MF, Kuo PY, and Wang CR. Upregulated expression of STAT3/IL-17 in patients with systemic lupus erythematosus. Clin Rheumatol. (2019) 38:1361–6. doi: 10.1007/s10067-019-04467-8
94. Zhao Y, Mu Z, Cai L, Liu X, Jia J, and Zhang J. Tetra-arsenic tetra-sulfide ameliorates lupus syndromes by inhibiting IL-17 producing double negative T cells. Dermatol Ther. (2019) 32:e12849. doi: 10.1111/dth.12849
95. Costa R, Antunes P, Salvador P, Oliveira P, and Marinho A. Secukinumab on refractory lupus nephritis. Cureus. (2021) 13:e17198. doi: 10.7759/cureus.17198
96. Zhang Y, Chen X, and Deng Y. miR-125a-3p decreases levels of interlukin-17 and suppresses renal fibrosis via down-regulating TGF-β1 in systemic lupus erythematosus mediated Lupus nephritic mice. Am J Transl Res. (2019) 11:1843–53.
Keywords: systemic lupus erythematosus, cytokine, Th17 cells, precision medicine, immunopathology
Citation: Chen Z, Liu S, Xie R, Zhang P and Feng Y (2025) Cytokine networks and therapeutic advances in systemic lupus erythematosus. Front. Immunol. 16:1680418. doi: 10.3389/fimmu.2025.1680418
Received: 07 August 2025; Accepted: 27 October 2025;
Published: 07 November 2025.
Edited by:
Muna Saleh, Linköping University, SwedenReviewed by:
Yunfei Liu, Central South University, ChinaCopyright © 2025 Chen, Liu, Xie, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Zhang, enBfbHRjbUAxNjMuY29t; Yue Feng, ZmVuZ3l1ZTcxNEAxNjMuY29t
†These authors have contributed equally to this work
 Zeping Chen
Zeping Chen Shupei Liu1†
Shupei Liu1† Rui Xie
Rui Xie Pan Zhang
Pan Zhang Yue Feng
Yue Feng