- 1Laboratory of Intracellular Signaling in Health and Disease, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
- 2Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia
- 3Moscow Center for Advanced Studies, Moscow, Russia
For half a century, the quiet work of a specialized immunosuppressive B cell subset has been slowly unveiled, revealing its profound impact on immune balance. This review provides a comprehensive retrospective on the history of regulatory B cell (Breg) investigation, tracing their journey from initial elusive observations to their current recognition as crucial immunomodulators. We explore the paradigm shift from B cells solely as antibody producers to their multifaceted roles in immunosuppression. Key milestones include the earliest suggestions of suppressive B cell activity around 1970, the formal coining of the currently used term "regulatory B cells" in the early 2000s, and the subsequent elucidation of diverse Breg subsets and their suppressive mechanisms. Finally, we discuss contemporary advances, including the application of single-cell multi-omics, the identification of novel markers and metabolic regulators, and the promising yet challenging path toward Breg-based therapeutic strategies. This historical perspective underscores the remarkable progress in Breg biology and illuminates future directions for harnessing their clinical potential.
1 Introduction
B cells have long been recognized as central components of the adaptive immune system, primarily for their capacity to produce antibodies, facilitate opsonization, present antigens, and activate T cells (1). The foundational understanding of B cell biology was significantly advanced by landmark studies throughout history. For instance, the late 19th century saw Emil von Behring and Shibasaburo Kitasato identify circulating "antitoxins" (now known as antibodies) as crucial for immunity to diphtheria and tetanus (2). Paul Ehrlich later proposed that cells with pre-formed antibody receptors were the producers of these "antitoxins," laying theoretical groundwork (3). The cellular source of antibodies, B cells, was more definitively identified in the late 1940s, with plasma cell development correlating with antibody responses after immunization (4). A pivotal moment arrived in 1965 with Max Cooper and Robert Good's landmark study using chicken models, which established B cells as a distinct lineage responsible for antibody production, separate from T cells involved in delayed-type hypersensitivity (5).
The initial discovery and characterization of B cells focused predominantly on their role as antibody producers and promoters of adaptive immunity, establishing a long-held view of their function. However, this established immunological paradigm began to shift fundamentally with the emergence of observations suggesting a suppressive capacity for B cells. The first hints of B cells possessing immunosuppressive properties emerged in the late 1960s (6) However, given the lack of understanding regarding the molecular mechanisms underlying the function of these B cells, the concept of anti-inflammatory B lymphocytes faced significant challenges in gaining widespread acceptance within the scientific community. The currently used well-established term "regulatory B cells" (Bregs) itself emerged relatively recently after the publication of data on the immunosuppressive role of B lymphocytes in chronic intestinal inflammation (7). Later, it has been demonstrated that Bregs play a direct role in the pathogenesis of a wide range of pathologies, including cancer, autoimmune diseases, infectious diseases, and transplantation immunity (8, 9). In addition, these cells have been shown to contribute to the maintenance of homeostasis in a healthy organism (8). Recent progress in the field has led to a significant advancement in our understanding of Breg origin, differentiation pathways and molecular mechanisms of immunosuppression. While numerous reviews have discussed the mechanisms and functional roles of Bregs, few have synthesized their scientific journey – how the concept first emerged, how acceptance within the immunology community evolved, and how successive methodological advances reshaped our understanding, from early skepticism to recognition of Bregs as key immunoregulatory players. We also highlight unresolved controversies and translational frontiers. Major milestones in the Breg field are shown in Table 1 and represented in the timeline (Figure 1).
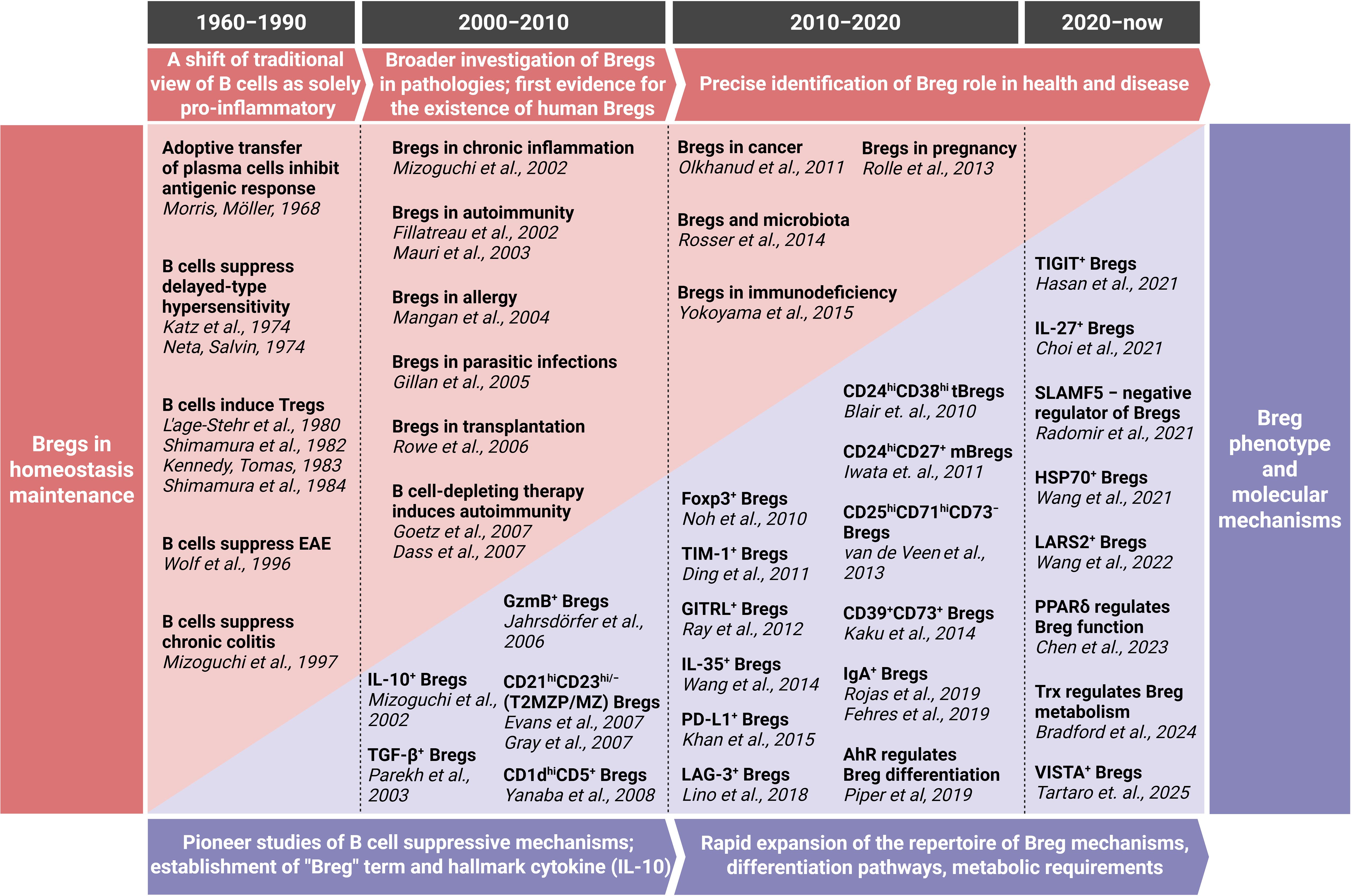
Figure 1. Timeline of Breg journey. For a more comprehensive overview, including additional key discoveries and detailed contextual descriptions, please refer to Table 1. EAE, experimental autoimmune encephalomyelitis; GzmB, granzyme B; LARS2, leucine-tRNA-synthetase-2; PPARδ, peroxisome proliferator-activated receptor delta; Trx, thioredoxin. Created with BioRender.com.
2 The genesis of immunosuppressive B cells (1960s – 1990s)
The journey into understanding the immunosuppressive capabilities of B cells began in the late 1960s, challenging the then-prevailing view of B lymphocytes solely as antibody-producing effector cells (10). This direct contradiction to the perceived "promoter" role of B cells necessitated a re-evaluation of their fundamental functions, introducing a new dimension to B cell functionality and indicating that B cells possess a dual potential: both pro-inflammatory and anti-inflammatory.
Morris and Möller were the first to discover that adoptively transferred plasma cells can inhibit response to antigenic stimulation (6). A significant turning point came with experiments demonstrating that B cell-depleted splenocytes exhibited a reduced capacity to suppress delayed-type hypersensitivity (DTH) reactions in guinea pigs. Specifically, studies by Katz, Parker, and Turk in 1974, and Neta and Salvin in the same year, showed that B cell depletion led to a more intense and prolonged DTH response compared to controls (11, 12). These findings directly implied that B cells could actively inhibit T cell activity, introducing the concept of a "suppressor B cell" (10). While the precise mechanisms remained unclear at that early stage, these observations were crucial in broadening the understanding of B cell functionality beyond antibody production (10). The notion that B cells could contribute to immune regulation, similar to the emerging understanding of suppressor T cells, laid the conceptual groundwork for future investigations into Breg subsets (13). Subsequent studies in the 1980s further supported this hypothesis, demonstrating that adoptive transfer of activated splenic B cells could induce tolerance and promote the differentiation of T cells into suppressor T cells (14–17). The 1990s marked a period of more definitive in vivo demonstrations of B cell-mediated immune suppression, largely facilitated by the development of genetically modified mouse models (10). A pivotal advancement was the establishment of a B cell-deficient mouse strain, µMT, achieved by disrupting the immunoglobulin µ chain gene (18). These mice, lacking mature B cells, became an invaluable tool for dissecting the roles of B cells in various immune contexts (10). A landmark study by Janeway and colleagues in 1996 utilized these µMT mice to investigate the role of B cells in experimental autoimmune encephalomyelitis (EAE), a widely used murine model for multiple sclerosis (19). While the incidence and initial severity of EAE were comparable between wild-type and µMT mice, the B cell-deficient mice exhibited a significantly longer disease duration and rarely achieved full recovery. This observation strongly suggested that B cells played a crucial role in the resolution or immune modulation of an acute autoimmune reaction in the central nervous system, rather than solely contributing to its pathogenesis (19). Further solidifying the concept of B cell-mediated immune suppression, similar findings were reported in a chronic colitis model in 1997 (20). Research demonstrated that mice lacking B cells developed colitis at an earlier age and experienced more severe disease compared to their B cell-sufficient counterparts (20). The adoptive transfer of purified B cells from healthy mice could prevent the development of colitis in recipient mice, indicating a protective, suppressive role for B cells in inflammatory bowel conditions.
These in vivo demonstrations in well-defined experimental murine models provided compelling evidence for the existence of B cells with immunoregulatory properties, setting the stage for their formal identification and characterization in the subsequent decade.
3 Coining the term and early characterization (2000s – 2010s)
The early 2000s marked a pivotal moment in B cell immunology with the formal coining of the term "regulatory B cells" (7). This distinct identity for the B cell subset responsible for immunosuppressive functions has moved beyond the earlier, less defined "suppressor B cell" hypothesis.
In 2006, Mizoguchi and colleagues were instrumental in introducing the term “Bregs” to the scientific community (21). Their research, conducted in mouse models of colitis and intestinal inflammation, identified a discrete population of B cells that exhibited suppressive functions (7, 20). These B cells were observed to expand in chronic inflammatory environments and were capable of dampening the progression of intestinal inflammation by down-regulating inflammatory signaling pathways. This work provided a clear conceptual framework and a specific name for this immunomodulatory B cell population.
The hypothesis of human Bregs was initially based on clinical observations. One of the first pieces of evidence came from observations related to the use of the B cell-depleting antibody rituximab. In some patients treated with rituximab, B cell depletion was associated with the development of psoriasis or a worsening of ulcerative colitis (22, 23). These paradoxical outcomes implied that B cells can exert a suppressive function in humans, which was lost upon their depletion. Further support for the human Breg hypothesis emerged when a clinical trial involving anti-CD20 monoclonal antibody therapy in transplant recipients was halted due to an increased rate of organ rejection (24). These early indications suggested that B cells also exert immunosuppressive functions in humans, similar to findings in murine models (25).
More definitive identification of human Bregs occurred in 2010 when Claudia Mauri's group identified them in the context of systemic lupus erythematosus (SLE) (26). They identified a specific immature B cell population in human peripheral blood, characterized by the CD24hiCD38hi phenotype. These cells were shown to produce high amounts of IL-10 upon in vitro CD40 engagement and were capable of suppressing Th1 differentiation and converting CD4+ T cells into regulatory T cells (Tregs).
Following the formal coining of the term, IL-10 rapidly emerged as the hallmark suppressive cytokine associated with Breg activity in the early 2000s. Its consistent involvement in Breg-mediated immune suppression across various models established it as the primary functional molecule for many years (1). For a long time, Bregs were identified solely by the expression of IL-10. Later, the existence of IL-10-independent Bregs has been convincingly demonstrated, and the term “B10 cells” was commonly used to refer to this population. However, it has become increasingly clear that IL-10 expression alone does not fully capture the phenotypic and functional heterogeneity of Bregs.
4 Advances in Breg subset characterization and differentiation pathways (2010s – 2020s)
The next era of Breg research marked the discovery of a large variety of human and murine Breg subsets, including common interspecies subpopulations such as CD5+CD1d+, as well as unique ones, such as CD24hiCD38hi (transitional Bregs), CD24hiCD27+ (memory Bregs, also termed “B10” in humans), CD25+CD71+CD73− (Br1) for humans, and CD21hiCD23hi (T2-MZP, transitional 2 marginal zone precursor B cells), CD21hiCD23− (MZ, marginal zone B cells), CD138+CD44hi (plasmablasts) for mice (1, 27). Breg subpopulations can also be identified based on their effector anti-inflammatory molecules, which broadly fall into three categories: cytokine-mediated mechanisms (TGF-β+, IL-35+, IL-10+, etc.), cell-cell contact mechanisms (PD-L1, TIM-1, FasL, TIGIT, etc.), and metabolic or unconventional mechanisms (CD39/CD73/adenosine, thioredoxin, GABA, HSP70) (28). It is noteworthy that despite this phenotypic heterogeneity some of these populations may overlap. With the discovery of a wide range of Breg subsets, scientists kept questioning the origin of Bregs. Hypotheses of pathways for Breg differentiation have long been debated. The basic concept suggested the presence of a universal Breg lineage marker (more possibly – transcription factor), and a lot of attempts have been made to find one. In 2019, Claudia Mauri’s group succeeded in finding a transcriptional factor aryl hydrocarbon receptor (AhR), which was shown to contribute to the CD21hiCD24hi Breg differentiation (29). Another transcription factor, hypoxia-inducible factor-1α, was also found to regulate CD1dhiCD5+ Bregs. The master-regulator of Tregs, FOXP3, was also identified in a subset of Bregs (30). However, to date, no study has succeeded in identifying a universal Breg lineage marker. Taking into account the high heterogeneity of Breg subsets and the ability of B cells to acquire regulatory functions in response to specific stimuli, the concept of an inducible Breg nature has arisen. This concept has been proven in a wide variety of studies showing that Bregs can be induced from different subsets of B cells (31–33). Breg-inducing stimuli include CD40L, IL-21, IL-35, CpG, BAFF, and APRIL in different combinations (33–36). This concept of "induced Bregs" highlights the plasticity of B cells and their ability to adapt their function based on the local immune milieu, also providing a basis for their therapeutic implication.
This era marked the burst of articles in the Breg field. The majority of these publications consist of observations regarding the involvement of Bregs in the pathogenesis of various diseases, mainly different types of cancer, autoimmune diseases, infections, and graft-versus-host disease (25). It was essential to transition towards a detailed investigation of their mechanisms of immunoregulation, intensifying the study of their molecular characteristics and methods for precise manipulation of Bregs.
5 Omics revolution in Breg immunology (2020s – onwards)
The current era is profoundly shaped by the advent and widespread adoption of single-cell and multi-omics technologies. These high-resolution analytical techniques are deepening the understanding of Breg heterogeneity and function, providing an unprecedented level of precision that was previously unattainable with traditional methods. Traditional bulk sequencing and flow cytometry often masked the true diversity within B cell populations by providing an average view of gene expression. Single-cell RNA sequencing (scRNA-seq), single-cell B cell receptor sequencing (scBCR-seq), and integrated multi-omics approaches overcome these limitations. This capability is critical for unraveling the true diversity of Bregs, which are known to exhibit remarkable phenotypic variability depending on their tissue location and the specific disease context.
Recent research leveraging these advanced technologies has already yielded significant discoveries, such as the identification of novel Breg subsets and Breg effector mechanisms (TIGIT+ Bregs, LARS2+ Bregs, VISTA+ Bregs, etc.) (37–40). Single-cell analysis has recently delineated seven organ-specific Breg subsets with variable immunosuppressive functions in mice, each with distinct gene expression profiles and immunosuppressive functionalities (41). These modern techniques have also helped reveal the functional diversity of Bregs. In particular, functionally specific Bregs that minimally express IL-10 (previously considered Breg hallmark) but show high levels of TGF-β and IL-35 were characterized, which proved that IL-10− Breg cells also possess specific immunosuppressive properties distinct from conventional Bregs (41). Recently, transcriptomic analysis revealed a distinct subpopulation of IL-27+ Bregs, which is also developmentally and functionally distinct from IL-10+ and IL-35+ Bregs (42). Notably, Bregs exhibit organ-specific heterogeneity with diverse phenotypes and functions depending on their organ of residence, such as the spleen, lymph nodes, or peritoneal cavity (41). This heterogeneity highlights the need for detailed characterization of Breg subsets across different tissues, and technologies like scRNA-seq offer a powerful approach to map their functional diversity in various contexts. Complementing omics-powered insights, recent research emphasizes that epigenetic and genetic regulation critically shape Breg functionality: histone modifications and DNA methylation, regulatory RNAs (lncRNA, miRNA, circRNA, etc.) and non-coding SNPs influence B cell subset differentiation and activation, thereby contributing to disease pathogenesis (43–48).
Another key milestone has been the refinement of our understanding of Breg differentiation and its requirements. A recent study by Mauri’s group performed trajectory analysis of scRNA-seq data of B cell culture activated with Breg-inducing CpG and revealed pathways of Breg differentiation. Within these pathways, they identified a specific metabolic regulator of Breg differentiation – a redox-regulating protein thioredoxin, highlighting that Breg differentiation, unlike non-Breg cells, highly depends on mitochondrial electron transport and controlled reactive oxygen species levels, while inhibition of other metabolic pathways made no difference (49). It is important to note that some other important regulators of Breg differentiation and function have also been recently identified, including PPARδ and SLAMF5, posing new potential therapeutic targets for Breg modulation (50, 51).
6 Emerging implications of Bregs
Bregs have attracted increasing attention as potential agents for immune modulation with human studies providing evidence across key contexts such as in autoimmune diseases (impaired transitional Bregs in SLE, paradoxical flares after rituximab in ulcerative colitis, etc.) (23, 26), transplantation (Breg-associated cytokines and the frequency of circulating Bregs as biomarkers of allograft rejection, implementation of Bregs to prevent graft-versus-host disease, etc.) (52–54), and cancer (VISTA+, PD-1+PD-L1+ and other tumor-associated Bregs as drivers of immune evasion, etc.) (40, 55). Recent advances have paved the way for several promising therapeutic approaches aimed at harnessing the immunosuppressive functions of Bregs.
While adoptive transfer of Tregs has advanced further clinically (56), with numerous trials and translational studies already underway, development of Breg-based cell therapy is still in its early stages (57). Nevertheless, Bregs offer unique advantages in some contexts at least due to their ability of antigen presentation and production of tolerogenic antibodies, providing a wider spectrum of immunoregulatory mechanisms. Adoptive Breg cell therapy represents a rapidly evolving strategy, involving the ex vivo expansion of Bregs and their subsequent reintroduction into the patient. Protocols have been developed to generate human Bregs from either autologous or allogeneic peripheral B cells using specific inducers. These expanded Bregs exhibit strong suppressive activity and the capacity to modulate effector cell responses, offering a foundation for personalized Breg-based immunotherapies (58). Engineering of antigen-specific Bregs also holds promise for the treatment of autoimmune pathologies characterized by involvement of well-defined autoantigens.
Given Breg ability to interact with and promote other immunoregulatory cell populations, including Tregs, myeloid-derived suppressor cells, and invariant natural killer T cells, they present an attractive option for combination immunotherapeutic strategies (1). Co-administration or co-induction of Bregs (for example, with in vivo inductors such as short-chain fatty acids, etc.) with other regulatory cells may enhance immune tolerance and provide longer-lasting immunosuppressive effects.
Advances in the identification of Breg-specific markers and regulatory pathways have opened new avenues for in vivo modulation. Even though Breg-specific lineage markers are yet to be identified, molecules such as TIM-1, TIGIT, and others have emerged as functional markers and potential therapeutic targets (38, 59). Pharmacologic agents or biologics designed to enhance or inhibit these pathways may allow for precise manipulation of Breg function in disease-specific contexts, enabling a shift toward immune tolerance without broad immunosuppression.
7 Conclusions and perspectives
The journey of Bregs has evolved from the initial recognition of a suppressive B cell population primarily defined by IL-10 production to a sophisticated understanding of a heterogeneous family of cells employing diverse suppressive mechanisms. The early 2000s established the fundamental concept of Bregs as critical regulators of immune homeostasis, particularly in autoimmune contexts. However, the subsequent decades, culminating in the current era, have revealed that Bregs are not a singular entity but rather a dynamic and adaptable population capable of acquiring regulatory functions through various pathways.
The current landscape, characterized by an enhanced ability to dissect cellular heterogeneity using single-cell and multi-omics technologies, has led to the identification of novel markers, subsets, and intricate regulatory networks beyond the B10 concept. The understanding that Bregs can arise from conventional B cell subsets in a context-dependent manner represents a major paradigm shift. Despite all the advances in the field, a significant challenge persists: Bregs still lack a unique lineage marker, which absence makes their identification and classification a persistent challenge; however, it remains uncertain whether such a marker exists. Beyond this, additional barriers remain, including the plasticity of Breg phenotypes, their strong context-dependency across diseases and tissues, and methodological limitations that complicate translation from experimental models to human biology. Deeper appreciation of Breg biology is not just academic: it directly informs the development of targeted therapeutic strategies. The creation of composite Breg-specific signatures with predictive potential could allow for the classification of risk groups in different Breg-associated pathologies, as well as for more precise therapy selection. Exploration of organ-specific Bregs is also critical, as distinct tissue-resident subsets may employ unique suppressive mechanisms that influence disease outcomes. Moreover, investigating combinatorial regulatory cell therapies could reveal synergistic approaches to enhance immunomodulation. Further challenges stem from pronounced heterogeneity of Bregs, as distinct subpopulations may use non-overlapping suppressive mechanisms, making it unclear which subset should be expanded or targeted therapeutically; their localization, since certain Breg subsets are scarce in peripheral blood and thus difficult to harvest for adoptive transfer; and the potential risks of Breg-based cellular products, which could cause excessive immunosuppression and predispose to malignancy. Moreover, it remains a major challenge to direct Bregs selectively to diseased tissues, in order to avoid systemic suppression and maximize their therapeutic benefit in a localized manner. A clear reflection of these barriers is the current translational gap: despite promising preclinical data, Breg-based therapies have not yet entered clinical trials. The new era of Breg immunology is poised to unlock the full therapeutic potential of these cells, offering novel avenues for treating a wide array of immune-mediated diseases and significantly advancing the field of immune tolerance.
Author contributions
EZ: Conceptualization, Visualization, Writing – original draft. NK: Writing – original draft. AU: Writing – original draft. ES: Writing – original draft. EB: Writing – original draft. MM: Writing – original draft. AU: Writing – review & editing. DD: Writing – review & editing. DK: Writing – review & editing. KK: Conceptualization, Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grant #24-14-00444 from the Russian Science Foundation (https://rscf.ru/en/project/24-14-00444/).
Acknowledgments
We apologize for not being able to cite all relevant publications. We thank Olga Karavashkova for her assistance and valuable comments on the manuscript. BioRender (https://biorender.com/) was used to make the timeline.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ahsan NF, Lourenço S, Psyllou D, Long A, Shankar S, and Bashford-Rogers R. The current understanding of the phenotypic and functional properties of human regulatory B cells (Bregs). Oxf Open Immunol. (2024) 5:iqae012. doi: 10.1093/oxfimm/iqae012
2. Behring E and Kitasato S. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch Med Wochenschr. (1890) 16:1113–4. doi: 10.1055/s-0029-1207589
4. Fagraeus A. Plasma cellular reaction and its relation to the formation of antibodies in vitro. Nature. (1947) 159:499. doi: 10.1038/159499a0
5. Cooper MD, Peterson RD, and Good RA. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature. (1965) 205:143–6. doi: 10.1038/205143a0
6. Morris A and Möller G. Regulation of cellular antibody synthesis. J Immunol. (1968) 101:439–45. doi: 10.4049/jimmunol.101.3.439
7. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, and Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. (2002) 16:219–30. doi: 10.1016/s1074-7613(02)00274-1
8. Mauri C and Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest. (2017) 127:772–9. doi: 10.1172/JCI85113
9. Zheremyan EA, Ustiugova AS, Karamushka NM, Uvarova AN, Stasevich EM, Bogolyubova AV, et al. Breg-mediated immunoregulation in the skin. Int J Mol Sci. (2024) 25:583. doi: 10.3390/ijms25010583
10. Matsumura Y, Watanabe R, and Fujimoto M. Suppressive mechanisms of regulatory B cells in mice and humans. Int Immunol. (2023) 35:55–65. doi: 10.1093/intimm/dxac048
11. Katz SI, Parker D, and Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. (1974) 251:550–1. doi: 10.1038/251550a0
12. Neta R and Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. (1974) 113:1716–25. doi: 10.4049/jimmunol.113.6.1716
13. Mauri C and Ehrenstein MR. The “short” history of regulatory B cells. Trends Immunol. (2008) 29:34–40. doi: 10.1016/j.it.2007.10.004
14. L’age-Stehr J, Teichmann H, Gershon RK, and Cantor H. Stimulation of regulatory T cell circuits by immunoglobulin-dependent structures on activated B cells. Eur J Immunol. (1980) 10:21–6. doi: 10.1002/eji.1830100105
15. Shimamura T, Hashimoto K, and Sasaki S. Feedback suppression of the immune response in vivo. I. Immune B cells induce antigen-specific suppressor T cells. Cell Immunol. (1982) 68:104–13. doi: 10.1016/0008-8749(82)90093-4
16. Shimamura T, Habu S, Hashimoto K, and Sasaki S. Feedback suppression of the immune response in vivo. III. Lyt-1+ B cells are suppressor-inducer cells. Cell Immunol. (1984) 83:221–4. doi: 10.1016/0008-8749(84)90242-9
17. Kennedy MW and Thomas DB. A regulatory role for the memory B cell as suppressor-inducer of feedback control. J Exp Med. (1983) 157:547–58. doi: 10.1084/jem.157.2.547
18. Kitamura D, Roes J, Kühn R, and Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. (1991) 350:423–6. doi: 10.1038/350423a0
19. Wolf SD, Dittel BN, Hardardottir F, and Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. (1996) 184:2271–8. doi: 10.1084/jem.184.6.2271
20. Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, and Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. (1997) 186:1749–56. doi: 10.1084/jem.186.10.1749
21. Mizoguchi A and Bhan AK. A case for regulatory B cells. J Immunol. (2006) 176:705–10. doi: 10.4049/jimmunol.176.2.705
22. Dass S, Vital EM, and Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. (2007) 56:2715–8. doi: 10.1002/art.22811
23. Goetz M, Atreya R, Ghalibafian M, Galle PR, and Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflammation Bowel Dis. (2007) 13:1365–8. doi: 10.1002/ibd.20215
24. Clatworthy MR, Watson CJE, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. (2009) 360:2683–5. doi: 10.1056/NEJMc0808481
25. Veh J, Ludwig C, Schrezenmeier H, and Jahrsdörfer B. Regulatory B cells-immunopathological and prognostic potential in humans. Cells. (2024) 13:357. doi: 10.3390/cells13040357
26. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. (2010) 32:129–40. doi: 10.1016/j.immuni.2009.11.009
27. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, and van de Veen W. Regulatory B cells, A to Z. Allergy. (2021) 76:2699–715. doi: 10.1111/all.14763
28. Goldmann O, Nwofor OV, Chen Q, and Medina E. Mechanisms underlying immunosuppression by regulatory cells. Front Immunol. (2024) 15:1328193. doi: 10.3389/fimmu.2024.1328193
29. Piper CJM, Rosser EC, Oleinika K, Nistala K, Krausgruber T, Rendeiro AF, et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. (2019) 29:1878–1892.e7. doi: 10.1016/j.celrep.2019.10.018
30. Meng X, Grötsch B, Luo Y, Knaup KX, Wiesener MS, Chen X-X, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat Commun. (2018) 9:251. doi: 10.1038/s41467-017-02683-x
31. Lighaam LC, Unger P-PA, Vredevoogd DW, Verhoeven D, Vermeulen E, Turksma AW, et al. In vitro-induced human IL-10+ B cells do not show a subset-defining marker signature and plastically co-express IL-10 with pro-inflammatory cytokines. Front Immunol. (2018) 9:1913. doi: 10.3389/fimmu.2018.01913
32. Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. (2019) 177:492–3. doi: 10.1016/j.cell.2019.03.037
33. Fehres CM, van Uden NO, Yeremenko NG, Fernandez L, Franco Salinas G, van Duivenvoorde LM, et al. APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front Immunol. (2019) 10:1368. doi: 10.3389/fimmu.2019.01368
34. Correale J, Marrodan M, and Carnero Contentti E. Interleukin-35 is a critical regulator of immunity during helminth infections associated with multiple sclerosis. Immunology. (2021) 164:569–86. doi: 10.1111/imm.13389
35. Zheremyan EA, Ustiugova AS, Uvarova AN, Karamushka NM, Stasevich EM, Gogoleva VS, et al. Differentially activated B cells develop regulatory phenotype and show varying immunosuppressive features: a comparative study. Front Immunol. (2023) 14:1178445. doi: 10.3389/fimmu.2023.1178445
36. Zhang Y, Li J, Zhou N, Zhang Y, Wu M, Xu J, et al. The unknown aspect of BAFF: Inducing IL-35 production by a CD5+CD1dhiFcγRIIbhi regulatory B-cell subset in lupus. J Invest Dermatol. (2017) 137:2532–43. doi: 10.1016/j.jid.2017.07.843
37. Wang Z, Lu Z, Lin S, Xia J, Zhong Z, Xie Z, et al. Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity. (2022) 55:1067–1081.e8. doi: 10.1016/j.immuni.2022.04.017
38. Hasan MM, Nair SS, O’Leary JG, Thompson-Snipes L, Nyarige V, Wang J, et al. Implication of TIGIT+ human memory B cells in immune regulation. Nat Commun. (2021) 12:1534. doi: 10.1038/s41467-021-21413-y
39. Zheremyan EA, Ustiugova AS, Radko AI, Stasevich EM, Uvarova AN, Mitkin NA, et al. Novel potential mechanisms of regulatory B cell-mediated immunosuppression. Biochem (Mosc). (2023) 88:13–21. doi: 10.1134/S0006297923010029
40. Lo Tartaro D, Aramini B, Masciale V, Paschalidis N, Lofaro FD, Neroni A, et al. Metabolically activated and highly polyfunctional intratumoral VISTA+ regulatory B cells are associated with tumor recurrence in early-stage NSCLC. Mol Cancer. (2025) 24:16. doi: 10.1186/s12943-024-02209-2
41. Yang S-Y, Long J, Huang M-X, Luo P-Y, Bian Z-H, Xu Y-F, et al. Characterization of organ-specific regulatory B cells using single-cell RNA sequencing. Front Immunol. (2021) 12:711980. doi: 10.3389/fimmu.2021.711980
42. Choi JK, Yu C-R, Bing SJ, Jittayasothorn Y, Mattapallil MJ, Kang M, et al. IL-27-producing B-1a cells suppress neuroinflammation and CNS autoimmune diseases. Proc. Natl Acad Sci U.S.A. (2021) 118:e2109548118. doi: 10.1073/pnas.2109548118
43. Xiao F, Rui K, Shi X, Wu H, Cai X, Lui KO, et al. Epigenetic regulation of B cells and its role in autoimmune pathogenesis. Cell Mol Immunol. (2022) 19:1215–34. doi: 10.1038/s41423-022-00933-7
44. Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, and Jamali E. The emerging role non-coding RNAs in B cell-related disorders. Cancer Cell Int. (2022) 22:91. doi: 10.1186/s12935-022-02521-1
45. Uvarova A, Zheremyan EA, Ustiugova A, Murashko MM, Bogomolova EA, Demin D, et al. Autoimmunity-associated SNP rs3024505 disrupts STAT3 binding in B cells, leading to IL10 dysregulation. Int J Mol Sci. (2024) 25:10196. doi: 10.3390/ijms251810196
46. Min KY, Lee MB, Hong SH, Lee D, Jo MG, Lee JE, et al. Entinostat, a histone deacetylase inhibitor, increases the population of IL-10+ regulatory B cells to suppress contact hypersensitivity. BMB Rep. (2021) 54:534–9. doi: 10.5483/bmbrep.2021.54.10.092
47. Uvarova AN, Ustiugova AS, Zheremyan EA, Stasevich EM, Korneev KV, and Kuprash DV. Functional characteristics of the gene promoters of anti-inflammatory cytokines TGF-b and IL-10 in B lymphocyte cell models. Med Immunol. (2024) 26:701–6. doi: 10.15789/1563-0625-fco-16940
48. Huang B, Guo F, Chen J, Lu L, Gao S, Yang C, et al. Regulation of B-cell function by miRNAs impacting Systemic lupus erythematosus progression. Gene. (2025) 933:149011. doi: 10.1016/j.gene.2024.149011
49. Bradford HF, McDonnell TCR, Stewart A, Skelton A, Ng J, Baig Z, et al. Thioredoxin is a metabolic rheostat controlling regulatory B cells. Nat Immunol. (2024) 25:873–85. doi: 10.1038/s41590-024-01798-w
50. Radomir L, Kramer MP, Perpinial M, Schottlender N, Rabani S, David K, et al. The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5. Nat Commun. (2021) 12:1893. doi: 10.1038/s41467-021-22230-z
51. Chen C, Ma J, Pi C, Huang W, Zhang T, Fu C, et al. PPARδ inhibition blocks the induction and function of tumor-induced IL-10+ regulatory B cells and enhances cancer immunotherapy. Cell Discov. (2023) 9:54. doi: 10.1038/s41421-023-00568-6
52. Cherukuri A, Salama AD, Mehta R, Mohib K, Zheng L, Magee C, et al. Transitional B cell cytokines predict renal allograft outcomes. Sci Transl Med. (2021) 13:eabe4929. doi: 10.1126/scitranslmed.abe4929
53. Zhou H, Zhan F, Zhang H, Gu J, Mu X, Gao J, et al. The proportion of CD19+CD24hiCD27+ regulatory B cells predicts the occurrence of acute allograft rejection in liver transplantation. Ann Transl Med. (2019) 7:465. doi: 10.21037/atm.2019.08.05
54. Sarvaria A, Basar R, Mehta RS, Shaim H, Muftuoglu M, Khoder A, et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood. (2016) 128:1346–61. doi: 10.1182/blood-2016-01-695122
55. Wang X, Wang G, Wang Z, Liu B, Han N, Li J, et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol Immunol. (2019) 109:20–6. doi: 10.1016/j.molimm.2019.02.009
56. Gooderham M, Lynde C, Alam MS, Sadick N, Rohan CA, Werschler WP, et al. 729 - A phase 2b, randomized, double-blinded, parallel-group, placebo-controlled, international, multicenter, study to evaluate the efficacy and safety of rezpegaldesleukin in adults with moderate-to-severe atopic dermatitis. Br J Dermatol. (2024) 191:ljae266.102. doi: 10.1093/bjd/ljae266.102
57. Bluestone JA, McKenzie BS, Beilke J, and Ramsdell F. Opportunities for Treg cell therapy for the treatment of human disease. Front Immunol. (2023) 14:1166135. doi: 10.3389/fimmu.2023.1166135
58. McNee A, Kannan A, Jull P, and Shankar S. Expanding human Breg for cellular therapy in transplantation: Time for translation. Transplantation. (2025) 109:926–37. doi: 10.1097/TP.0000000000005243
59. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. (2011) 121:3645–56. doi: 10.1172/JCI46274
60. Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornard T, et al. Activated B cells express functional Fas ligand. Eur J Immunol. (1996) 26:721–4. doi: 10.1002/eji.1830260332
61. D’Orazio TJ, Mayhew E, and Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen. II. Evidence presentation by Qa-1. J Immunol. (2001) 166:26–32. doi: 10.4049/jimmunol.166.1.26
62. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, and Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. (2002) 3:944–50. doi: 10.1038/ni833
63. Mauri C, Gray D, Mushtaq N, and Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. (2003) 197:489–501. doi: 10.1084/jem.20021293
64. Parekh VV, Prasad DVR, Banerjee PP, Joshi BN, Kumar A, and Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. (2003) 170:5897–911. doi: 10.4049/jimmunol.170.12.5897
65. Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, and Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. (2004) 173:6346–56. doi: 10.4049/jimmunol.173.10.6346
66. Gillan V, Lawrence RA, and Devaney E. B cells play a regulatory role in mice infected with the L3 of Brugia pahangi. Int Immunol. (2005) 17:373–82. doi: 10.1093/intimm/dxh217
67. Jahrsdörfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. (2006) 108:2712–9. doi: 10.1182/blood-2006-03-014001
68. Rowe V, Banovic T, MacDonald KP, Kuns R, Don AL, Morris ES, et al. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. (2006) 108:2485–92. doi: 10.1182/blood-2006-04-016063
69. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. (2007) 178:7868–78. doi: 10.4049/jimmunol.178.12.7868
70. Gray M, Miles K, Salter D, Gray D, and Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U.S.A. (2007) 104:14080–5. doi: 10.1073/pnas.0700326104
71. Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, and Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. (2008) 28:639–50. doi: 10.1016/j.immuni.2008.03.017
72. Yanaba K, Bouaziz J-D, Matsushita T, Tsubata T, and Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. (2009) 182:7459–72. doi: 10.4049/jimmunol.0900270
73. Noh J, Choi WS, Noh G, and Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells: Human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg). Immune Netw. (2010) 10:247–9. doi: 10.4110/in.2010.10.6.247
74. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. (2011) 71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316
75. Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. (2012) 11:670–7. doi: 10.1016/j.autrev.2011.11.018
76. Ray A, Basu S, Williams CB, Salzman NH, and Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. (2012) 188:3188–98. doi: 10.4049/jimmunol.1103354
77. Rolle L, Memarzadeh Tehran M, Morell-García A, Raeva Y, Schumacher A, Hartig R, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. (2013) 70:448–53. doi: 10.1111/aji.12157
78. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. (2013) 131:1204–12. doi: 10.1016/j.jaci.2013.01.014
79. Liu ZQ, Wu Y, Song JP, Liu X, Liu Z, Zheng PY, et al. Tolerogenic CX3CR1+ B cells suppress food allergy-induced intestinal inflammation in mice. Allergy. (2013) 68:1241–8. doi: 10.1111/all.12218
80. Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. (2014) 41:1040–51. doi: 10.1016/j.immuni.2014.10.016
81. Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. (2014) 507:366–70. doi: 10.1038/nature12979
82. Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med. (2014) 20:1334–9. doi: 10.1038/nm.3680
83. Kaku H, Cheng KF, Al-Abed Y, and Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol. (2014) 193:5904–13. doi: 10.4049/jimmunol.1400336
84. Nouël A, Pochard P, Simon Q, Ségalen I, Le Meur Y, Pers JO, et al. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J Autoimmun. (2015) 59:53–60. doi: 10.1016/j.jaut.2015.02.004
85. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, and Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. (2015) 6:5997. doi: 10.1038/ncomms6997
86. Yokoyama T, Yoshizaki A, Simon KL, Kirby MR, Anderson SM, and Candotti F. Age-dependent defects of regulatory B cells in Wiskott-Aldrich syndrome gene knockout mice. PloS One. (2015) 10:e0139729. doi: 10.1371/journal.pone.0139729
87. Lino AC, Dang VD, Lampropoulou V, Welle A, Joedicke J, Pohar J, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. (2018) 49:120–133.e9. doi: 10.1016/j.immuni.2018.06.007
88. Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature. (2021) 599:471–6. doi: 10.1038/s41586-021-04082-1
89. Wang L, Fu Y, Yu B, Jiang X, Liu H, Liu J, et al. HSP70, a novel regulatory molecule in B cell-mediated suppression of autoimmune diseases. J Mol Biol. (2021) 433:166634. doi: 10.1016/j.jmb.2020.08.019
Keywords: regulatory B cells, Bregs, immunotherapy, immunosuppression, immunoregulation
Citation: Zheremyan EA, Kon NR, Ustiugova AS, Stasevich EM, Bogomolova EA, Murashko MM, Uvarova AN, Demin DE, Kuprash DV and Korneev KV (2025) The half-century odyssey of regulatory B cells: from Breg discovery to emerging frontiers. Front. Immunol. 16:1681082. doi: 10.3389/fimmu.2025.1681082
Received: 06 August 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Paolo Casali, Md, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Yutaka Matsumura, Osaka University, JapanXiujia Yang, Southern Medical University, China
Copyright © 2025 Zheremyan, Kon, Ustiugova, Stasevich, Bogomolova, Murashko, Uvarova, Demin, Kuprash and Korneev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elina A. Zheremyan, ZWx5YXpoZXJlbXlhbkBtYWlsLnJ1; Kirill V. Korneev, a2lya29ybmVldkBnbWFpbC5jb20=
 Elina A. Zheremyan
Elina A. Zheremyan Nikolai R. Kon1,2
Nikolai R. Kon1,2 Aksinya N. Uvarova
Aksinya N. Uvarova Dmitry V. Kuprash
Dmitry V. Kuprash Kirill V. Korneev
Kirill V. Korneev