- 1Department of Gastroenterology, Affiliated Hospital, Southwest Medical University, Luzhou, China
- 2Southwest Medical University, Luzhou, China
- 3First Teaching Hospital of Tianjin University of Traditional Chinese Medicine Tianjin, Tianjin, China
- 4Department of Specialty Medicine, Ohio University, Athens, OH, United States
T cells play a central role in the immune response to gastric cancer, and their dysfunction directly contributes to immune escape from the tumor and limits the efficacy of immunotherapy. The immune microenvironment of gastric cancer consists of a wide range of cells and molecules, and this complex and dynamic environment exerts profound inhibitory effects on T cell function. upregulation of PD-1, CTLA-4, and other inhibitory molecules is a key mechanism of T cell depletion, and metabolic reprogramming and chronic antigenic stimulation further weaken the anti-tumor activity of T cells. In recent years, PD-1/PD-L1 inhibitors have demonstrated some efficacy in gastric cancer, but the problem of drug resistance remains prominent. To address these challenges, combinatorial therapeutic strategies have gradually become the focus of research, especially combining immune checkpoint inhibitors with chemotherapy, radiotherapy, or targeted therapy to enhance the antitumor effect of immunotherapy. This review delves into the molecular mechanisms of T-cell depletion and its impact in gastric cancer immunotherapy, and analyzes the potential application of biomarkers in predicting treatment response. By comprehensively analyzing T-cell depletion and the immune microenvironment in gastric cancer, this paper provides a theoretical basis for the development of future personalized combinatorial therapeutic strategies, with the aim of improving patient prognosis and enhancing the overall therapeutic efficacy.
1 Introduction
Gastric cancer is the fifth most common malignant cancer worldwide and the fourth leading cause of cancer-related deaths (1). According to the World Health Organization (WHO), there will be approximately 1 million new cases of gastric cancer worldwide in 2020, accounting for 5.6% of all cancer cases, the incidence of gastric cancer in East Asia (China, Japan, Korea, etc.) is much higher than that in Europe and the United States, with significant geographical differences (2, 3). In addition to this, the incidence of gastric cancer also has obvious gender distribution differences, and the incidence of gastric cancer in men is approximately twice as high as that in women (1, 4). Adenocarcinoma is the most common histologic subtype of gastric cancer, in addition to mucinous adenocarcinoma, indolent cell carcinoma, and undifferentiated carcinoma (5). Gastric cancer has a rapid progression and is prone to lymph node metastasis and distant metastasis, resulting in a poor prognosis (6). Therefore, the study of the pathogenesis of gastric cancer and its therapeutic strategies is essential to improve clinical prognosis.
T cells are one of the core components of the adaptive immune system that recognize and kill tumors (7).CD8+ cytotoxic T lymphocytes (CTLs) directly kill tumor cells by recognizing tumor antigens and releasing perforin and granzyme (8, 9). And CD4+ helper T cells coordinate the immune response by secreting cytokines (10). The functional soundness of T cells is an important guarantee to maintain anti-tumor immune surveillance (11). However, in gastric cancer and other solid tumors, T cells often exhibit dysfunction (e.g., depletion or heterogeneity), and their antitumor efficacy is greatly diminished (12). A distinctive feature of T-cell dysfunction is the upregulation of immune checkpoint molecules, such as programmed death receptor-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4), the expression of which leads to T-cell suppression which in turn promotes tumor evasion of immune surveillance (13–15). In addition, chronic antigenic stimulation, metabolic abnormalities, and suppressive cytokines in the tumor microenvironment contribute to the gradual loss of effector function of T cells, further exacerbating immune escape from the tumor (16). Therefore, an in-depth understanding of the dysfunctional mechanisms of T cells in gastric cancer immunity can help develop novel immunotherapeutic strategies (17, 18).
This article delves into the molecular mechanisms underlying T cell dysfunction in gastric cancer and explores potential therapeutic strategies. By integrating basic and clinical research findings, we elucidate the intricate role of T cells in gastric cancer immunity. We focus on T cell depletion and heterogeneity, and highlight the potential application of biomarkers in predicting therapeutic response. Ultimately, we aim to provide novel insights for the development of future personalized immunotherapy strategies.T-cell dysfunction in gastric cancer.
1.1 Mechanisms of T-cell exhaustion
T-cell exhaustion is a state in which T cells gradually lose their effector function under continuous antigenic stimulation (19). Depleted T cells exhibit decreased proliferative capacity, decreased secretion of effector molecules (e.g., IFN-γ, TNF-α, IL-2), and high expression of multiple inhibitory receptors (PD-1, CTLA-4, TIM-3, etc.) (20). In gastric cancer, T cell depletion is one of the important mechanisms of tumor immune escape (21).
1.1.1 Immune checkpoint inhibitory pathways
The PD-1/PD-L1 pathway is one of the most well-studied mechanisms of T-cell exhaustion (22). Programmed Cell Death Protein 1 (PD-1), an inhibitory receptor on the surface of T-cells, is usually highly expressed in response to chronic antigenic exposure and inhibits T-cell activity by binding to its ligand, PD-L1, thereby preventing its killing of tumor cells (23). Gastric cancer cells often exploit this pathway to evade immune surveillance by overexpressing PD-L1 (24). This inhibitory mechanism not only plays a role in depleting T cells, but is also upregulated during acute T cell activation to prevent T cell overreaction. In acute infection models, inhibitory receptors including PD-1 and Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are up-regulated to counteract the activating effects of T-cell receptor (TCR) and co-stimulatory signals to maintain immune homeostasis (25).
During immune depletion, the presence of PD-1 was not essential. However, its existence notably diminished the extent of depletion and to some extent, prevented cells from becoming overstimulated. This implies that even in the context of depleted T cells, PD-1 may still be exerting a protective effect by inhibiting cell activation (26). In addition to the PD-1/PD-L1 pathway, CTLA-4 is an important immune checkpoint molecule that acts mainly during the initial activation of T cells (27). CTLA-4 inhibits the activity of T cells by preventing co-stimulatory signaling through binding to B7 molecules on the surface of antigen-presenting cells (28). In gastric cancer, tumor cells further enhance T-cell inhibition by upregulating PD-L1 and B7 expression. CTLA-4 acts mainly during initial T-cell activation, whereas PD-1 exerts a sustained inhibitory effect under chronic antigen exposure (29).
1.1.2 Metabolic reprogramming
Metabolic changes in the tumor microenvironment significantly affect T cell function (30). Gastric cancer cells and immunosuppressive cells (e.g. myeloid-derived suppressor cells, MDSCs) preferentially seize nutrients such as glucose and glutamine through metabolic reprogramming, weakening the metabolic activity of T cells and thus inhibiting their effector function (31). Tumor cells are predominantly aerobic glycolysis, which rapidly consumes glucose and generates large amounts of lactic acid, acidifying the microenvironment, thus further inhibiting T cell proliferation and effector function (32). In addition, lipid accumulation and fatty acid oxidation caused by metabolic reprogramming were enhanced, which promoted the impaired mitochondrial function and accelerated depletion of T cells. Notably, metabolic wastes such as lactate in the tumor microenvironment not only inhibit T cells, but also promote polarization of M2-type tumor-associated macrophages (TAMs) by inducing the expression of HIF-1α, which in turn drives tumor progression (33).
1.1.3 Chronic antigenic stimulation
In gastric cancer, tumor cells continuously express tumor-associated antigens, resulting in a long-term activation state of T cells (34). However, sustained antigenic stimulation not only causes T cells to gradually lose their effector function, but also further deepens the depletion state of T cells by upregulating inhibitory receptors such as PD-1, TIM-3, and LAG-3 (35). It has been pointed out that chronic antigen exposure accompanied by metabolic reprogramming promotes mitochondrial dysfunction, leading to excessive accumulation of reactive oxygen species (ROS) and exacerbating T cell depletion. Therefore, blocking these inhibitory signals or modulating metabolic pathways is expected to restore T cell activity and improve immunotherapy outcomes (36).
1.2 T-cell heterogeneity
In the microenvironment of gastric cancer, T cell heterogeneity is mainly reflected in the functional differentiation of subpopulations such as CD8+ effector T cells, memory T cells and regulatory T cells (Treg cells).CD8+ effector T cells (CTLs) are responsible for the direct killing of tumor cells, but they usually show depletion characteristics in gastric cancer patients, which manifests itself in the form of the high expression of inhibitory receptors, such as PD-1, LAG-3, etc., and in this state the CTLs lose their effector function and gradually reduce the secretion of perforin and granzyme, thus failing to effectively kill tumor cells (36). Memory T cells, such as central memory (Tcm) and effector memory (Tem) cells, can be rapidly activated upon re-encountering tumor antigens, but their function is often limited by metabolic defects and inhibitory factors (e.g., TGF-β, IL-10) in the gastric cancer microenvironment, resulting in reduced cell proliferation capacity and secretion of key effector molecules such as IFN-γ. In addition, the number of Treg cells was significantly elevated in gastric cancer tissues, and they suppressed effector T cells by secreting inhibitory factors such as TGF-β and IL-10, and competed with antigen-presenting cells for nutrients, thus weakening anti-tumor immunity (37). In conclusion, the heterogeneity among T cell subsets and their different depletion mechanisms in the microenvironment further reveal the complexity of immune escape in gastric cancer, providing multiple targets and optimized strategies for immunotherapy (38).
2 Biomarkers and clinical prediction
As the immune microenvironment of gastric cancer has been studied intensively, a variety of emerging biomarkers have shown significant potential in predicting immunotherapy response and assessing patient prognosis (39).
2.1 Emerging biomarkers of T cell dysfunction
CD39/CD73 are key enzymes in adenosine metabolism and are usually highly expressed in depleted T cells. Through the adenosine signaling pathway, CD39 and CD73 contribute to the accumulation of immunosuppressive adenosine in the tumor microenvironment, thereby suppressing the function of effector T cells (40). In gastric cancer patients, high levels of CD39/CD73 expression are closely associated with reduced T cell activity, allowing tumor cells to evade recognition by the immune system. Therapies that block the adenosine pathway, such as A2aR inhibitors, have been shown to restore T-cell function and enhance anti-tumor responses, and are one of the important current research directions in gastric cancer immunotherapy (41).
TIGIT, a newly discovered inhibitory receptor, significantly inhibits the glycolytic activity of CD8+ T cells by binding to its ligand CD155, which in turn reduces the secretion of anti-tumor factors such as IFN-γ (42). Preclinical studies have shown that blocking the TIGIT/CD155 pathway not only restores the metabolic activity of T cells, but also significantly enhances anti-tumor immune responses and improves patient survival. Therefore, the TIGIT/CD155 axis is considered a potential target in gastric cancer immunotherapy with important clinical translational prospects (43).
2.2 Biomarkers for predicting response to therapy
Along with the widespread use of immunotherapy in gastric cancer treatment, biomarkers that predict response to therapy are particularly important to help optimize treatment strategies and improve efficacy.
Tumor Mutational Burden (TMB) is considered an important marker of response to immunotherapy (44). A higher TMB usually means more tumor neoantigen generation, enhancing the chances of recognition and attack by the immune system (45). In gastric cancer patients, it has been found that those with higher TMB typically show more significant efficacy to PD-1/PD-L1 inhibitor therapies, as these neoantigens stimulate a stronger immune response (46).
Microsatellite instability (MSI) is another widely studied biomarker in immunotherapy (47). Due to defective DNA mismatch repair, MSI-H (high microsatellite instability) tumors typically have higher mutational loads and neoantigen generation rates, and thus show good response to immune checkpoint inhibitors in gastric cancer.MSI assays have become a commonly used method in the clinic to screen patients for suitability for immunotherapy (48).
In addition, factors such as the number of tumor-infiltrating lymphocytes (TILs) and specific metabolic markers (e.g., CXCL9, IDO, and LDH) have shown significant value in predicting the efficacy of immunotherapy (49). A high density of CD8+ TILs is usually associated with a better clinical prognosis, and high expression of markers such as CXCL9 and IDO predicts a stronger anti-tumor immune response (50). Personalized therapeutic regimens based on these markers can significantly improve the outcome of gastric cancer patients and promote the development of personalized immunotherapy.
3 Treatment opportunities and prospects
Immune checkpoint inhibitors (ICIs) and T-cell augmentation therapies have shown unprecedented promise in the treatment of gastric cancer (51). Through immune system modulation, the survival and treatment outcome of gastric cancer patients have been significantly improved (52).
3.1 Immune checkpoint inhibitors
In immunotherapy for gastric cancer, PD-1/PD-L1 inhibitors and CTLA-4 inhibitors have been widely used as the two main classes of ICIs (53). Pembrolizumab and Nivolumab are the most common PD-1 inhibitors, which restore the anti-tumor activity of T-cells by blocking the binding of the PD-1 receptor to the PD-L1 ligand (54). For example, the CheckMate 649 trial showed that the combination of Nivolumab and chemotherapy significantly prolonged the overall survival (OS) of gastric cancer patients compared to chemotherapy alone, with a particularly significant survival benefit in patients with high PD-L1 expression (55). In contrast, CTLA-4 inhibitors, such as Ipilimumab, boosted T-cell activity by competitively inhibiting the binding of CTLA-4 to B7 molecules (56, 57). However, due to the low response of gastric cancer to CTLA-4 inhibitors, clinical studies have mostly focused on their use in combination with other therapies to optimize treatment outcomes (58, 59).
In recent years, combination strategies of ICIs with other therapeutic agents have been explored and have shown initial success. For patients with locally advanced gastric cancer, a combination neoadjuvant therapy trial including Karelizumab, Apatinib and chemotherapy showed that patients achieved a complete pathological response rate of 15.8% after surgery, while demonstrating remarkable safety and tolerability. This combination therapy strategy not only acts directly on tumor cells, but also enhances the efficacy of ICIs by inhibiting tumor angiogenesis and elevating the degree of immune cell infiltration in the tumor microenvironment (60).
3.2 T-cell enhancement therapy
In addition to ICIs, T-cell augmentation therapies such as chimeric antigen receptor T-cell (CAR-T) therapy and T-cell receptor (TCR-T) therapy have shown great potential in gastric cancer immunotherapy (61).
3.2.1 CAR-T cell therapy
CAR-T therapy targets and kills tumor cells by genetically engineering patient T cells to express receptors for specific tumor antigens (62). For gastric cancer, CLDN18.2-specific CAR-T cells have demonstrated potential efficacy in a phase I clinical trial, with disease control in approximately 48.6% of patients (63). The therapy showed a high overall response rate, especially for gastric cancer patients expressing CLDN18.2, with a 6-month survival rate of 81.2% (63). In addition, ICAM-1-targeted CAR-T cells showed significant anti-tumor activity in gastric cancer models (64). The study showed that by combining with chemotherapy or IL-12, the therapy exhibited significant anti-tumor effects in abdominal metastatic gastric cancer.
3.2.2 TCR-T cell therapy
Unlike CAR-T therapies, TCR-T therapies target more diverse antigens (e.g., tumor-specific mutant antigens) by enhancing the recognition of tumor antigens by T cells (65). In gastric cancer treatment, TCR-T therapy research has focused on targeting antigens such as NY-ESO-1 and MAGE-A4 (66). Although TCR-T therapies have shown some anti-tumor activity in early trials, challenges remain in how to effectively respond to immunosuppression in the tumor microenvironment, especially in the management of off-target effects (67).
4 Combination therapy strategies
Combination therapy is becoming increasingly important in the treatment of gastric cancer, as monotherapies struggle to overcome the complexity of the tumor microenvironment and multiple drug resistance (68). By combining different therapies, studies have shown that the efficacy of ICIs can be significantly enhanced when combined with chemotherapy, radiotherapy or targeted therapy (69). Chemotherapy and radiotherapy not only kill tumor cells directly, but also enhance the immune system’s ability to recognize and attack tumors by inducing “immunogenic cell death” and releasing tumor antigens (70). These treatments can also alter the tumor microenvironment, reducing the number of immunosuppressive cells such as Treg cells while increasing the infiltration of effector T cells to further enhance the efficacy of ICIs (71). In gastric cancer clinical trials, PD-1 inhibitors combined with chemotherapy or radiotherapy showed significant synergistic effects, significantly prolonging patients’ overall survival (OS) and progression-free survival (PFS) (72). In addition, targeted therapies such as HER2 and VEGFR inhibitors reduce tumor resistance by blocking tumor-specific signaling pathways; studies have shown that the combination of HER2 inhibitors with PD-1/PD-L1 inhibitors significantly activates anti-tumor immune responses and improves prognosis (73). Looking forward, combination therapy will be more based on individual tumor characteristics and microenvironmental status, combined with TMB, MSI and other markers to design personalized programs. In addition, the combination of new immunotherapies such as bispecific antibodies and tumor vaccines with ICIs is also being explored, which is expected to bring more therapeutic options for gastric cancer patients (74) (Figure 1).
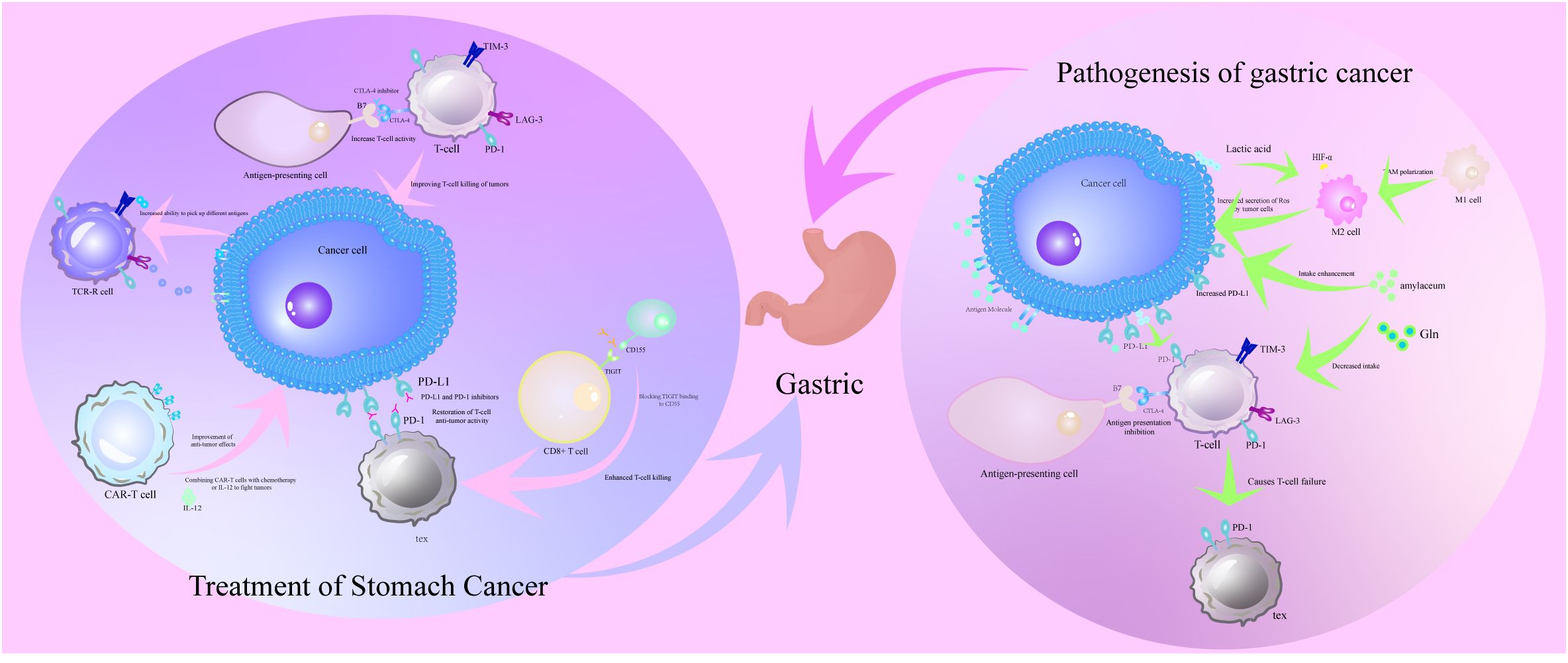
Figure 1. Pathogenesis of T cell dysfunction in the immune microenvironment of gastric cancer and its corresponding therapeutic strategies. The pathogenesis of T cell dysfunction in the immune microenvironment of gastric cancer is the evasion of immune surveillance by gastric cancer cells through metabolic reprogramming of lactate accumulation and HIF-1α activation, recruitment of immunosuppressive cells MDSCs and Tregs, and upregulation of immune checkpoint molecules PD-L1 and TIM-3. Therapeutic strategies for T-cell dysfunction in the immune microenvironment of gastric cancer are immune checkpoint inhibitors PD-1/PD-L1 and CTLA-4 inhibitors restoring T-cell antitumor activity by blocking inhibitory signals, emphasizing the potential of the combined therapeutic strategy of immune checkpoint inhibitors in conjunction with chemotherapy or radiotherapy in improving therapeutic efficacy.
5 Discussion
T cell dysfunction in the immune response to gastric cancer reveals a complex mechanism by which tumors evade immune surveillance (75). In this paper, we systematically review the multiple effects of the gastric cancer microenvironment on T-cell function, focusing on T-cell exhaustion and its resulting immunosuppression. The critical roles of inhibitory pathways such as PD-1 and CTLA-4 in T-cell exhaustion have been intensively investigated, while metabolic reprogramming and chronic antigenic stimulation further exacerbate the loss of T-cell effector function (76). A variety of emerging biomarkers, such as CD39, CD73, and TIGIT, have demonstrated important applications in predicting the response of gastric cancer patients to immunotherapy, providing a basis for individualized stratification and optimization of therapeutic strategies (77).
Although the use of immune checkpoint inhibitors in the treatment of gastric cancer has achieved some success, monotherapy still faces challenges in dealing with the complex tumor microenvironment (78). Combination strategies combining chemotherapy, radiotherapy and targeted therapies have demonstrated significant synergistic effects in the clinic, and future studies should focus on the optimization of these combination regimens and their efficacy differences in different patient populations (79). In addition, although T-cell enhancement therapies such as CAR-T and TCR-T have been successful in hematological tumors, their application in gastric cancer faces challenges such as the lack of tumor-specific antigens and microenvironmental inhibition (80). Personalized, multi-target combination therapy strategies for gastric cancer patients should be the future research direction (81).
In the future, immunotherapy for gastric cancer will further develop towards diversification and precision (82). Through the discovery of novel biomarkers and optimized combinations of treatment strategies, breakthroughs in overcoming drug resistance and improving efficacy are expected. Multidisciplinary collaboration will be key to advancing these studies, ultimately providing patients with a wider range of therapeutic options and longer-term survival benefits. These findings will not only dramatically improve gastric cancer treatment options, but also provide a valuable reference for immunotherapy of other solid tumors.
Author contributions
HL: Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. JW: Data curation, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. YY: Formal analysis, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. DX: Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. JZ: Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. XCZ: Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. GY: Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. XLZ: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Beijing Huaxia Cancer Prevention and Treatment Research Institute - 2023 Early Cancer Standardization Capacity Enhancement Fund Project, 2023.10-2025.09 (202401), Sichuan Medical Science and Technology Innovation Research Association (abbreviated as Sichuan Medical Innovation Association) launched the “Summit of Medical Innovation” Special Research Project. 2025.01-2027.03 (YCH-KY-YCZD2024-298) and Research Project on Educational and Teaching Reform at Southwest Medical University 2023. 12-2025. 12 (JG2023yb190)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lyons K, Le LC, Pham YT, Borron C, Park JY, Tran CTD, et al. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev. (2019) 28:397–412. doi: 10.1097/CEJ.0000000000000480
3. Chang C, Zheng A, Wang P, and Teng X. Circular RNA mitochondrial translation optimization 1 correlates with less lymph node metastasis, longer disease-free survival, and higher chemotherapy sensitivity in gastric cancer. J Clin Lab Anal. (2022) 36:e23918. doi: 10.1002/jcla.23918
4. Wu X-N, Su D, Mei YD, Xu MQ, Zhang H, Wang ZY, et al. Identified lung adenocarcinoma metabolic phenotypes and their association with tumor immune microenvironment. Cancer Immunol Immunother: CII. (2021) 70:2835–50. doi: 10.1007/s00262-021-02896-6
5. Wang Z, Liu Y, Mo Y, Zhang H, Dai Z, Zhang X, et al. The CXCL family contributes to immunosuppressive microenvironment in gliomas and assists in gliomas chemotherapy. Front Immunol. (2021) 12:731751. doi: 10.3389/fimmu.2021.731751
6. Zhu Q, Li L, Jiao X, Xiong J, Zhai S, Zhu G, et al. Rare metastasis of gastric cancer to the axillary lymph node: A case report. Front Oncol. (2022) 12:995738. doi: 10.3389/fonc.2022.995738
7. Zhou Y, Li M, Zhou K, Brown J, Tsao T, Cen X, et al. Engineering induced pluripotent stem cells for cancer immunotherapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14092266
8. Wang R, Zhuang J, Zhang Q, Wu W, Yu X, Zhang H, et al. Decoding the metabolic dialogue in the tumor microenvironment: from immune suppression to precision cancer therapies. Exp Hematol Oncol. (2025) 14:99. doi: 10.1186/s40164-025-00689-6
9. Song MK, Park BB, and Uhm JE. Resistance mechanisms to CAR T-cell therapy and overcoming strategy in B-cell hematologic Malignancies. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20205010
10. An SX, Yu ZJ, Fu C, Wei MJ, and Shen LH. Biological factors driving colorectal cancer metastasis. World J Gastrointest Oncol. (2024) 16:259–72. doi: 10.4251/wjgo.v16.i2.259
11. Zhang N, et al. Uncovering the predictive and immunomodulatory potential of transient receptor potential melastatin family-related CCNE1 in pan-cancer. Mol Cancer. (2024) 23:258. doi: 10.1186/s12943-024-02169-7
12. Posey AD Jr., Young RM, and June CH. Future perspectives on engineered T cells for cancer. Trends Cancer. (2024) 10:687–95. doi: 10.1016/j.trecan.2024.05.007
13. Cheng Y, Song Z, Chen J, Tang Z, and Wang B. Molecular basis, potential biomarkers, and future prospects of OSCC and PD-1/PD-L1 related immunotherapy methods. Heliyon. (2024) 10:e25895. doi: 10.1016/j.heliyon.2024.e25895
14. Jiang S, Geng S, Luo X, Zhang C, Yu Y, Cheng M, et al. Biomarkers of related driver genes predict anti-tumor efficacy of immune checkpoint inhibitors. Front Immunol. (2022) 13:995785. doi: 10.3389/fimmu.2022.995785
15. Xiao H, Pang Q, Wang Y, Lai S, and Chen H. The prediction of necroptosis-related lncRNAs in prognosis and anticancer therapy of colorectal cancer. Anal Cell Pathol (Amst). (2022) 2022:7158684. doi: 10.1155/2022/7158684
16. Schaible P, Bethge W, Lengerke C, and Haraszti RA. RNA therapeutics for improving CAR T-cell safety and efficacy. Cancer Res. (2023) 83:354–62. doi: 10.1158/0008-5472.CAN-22-2155
17. Wu Y, Zhuang J, Qu Z, Yang X, and Han S. Advances in immunotyping of colorectal cancer. Front Immunol. (2023) 14:1259461. doi: 10.3389/fimmu.2023.1259461
18. Yang R, Cheng S, Luo N, Gao R, Yu K, Kang B, et al. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. (2019) 21:2. doi: 10.1186/s13059-019-1921-y
19. Jansen JA, Omuro A, and Lucca LE. T cell dysfunction in glioblastoma: a barrier and an opportunity for the development of successful immunotherapies. Curr Opin Neurol. (2021) 34:827–33. doi: 10.1097/WCO.0000000000000988
20. Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. (2019) 19:665–74. doi: 10.1038/s41577-019-0221-9
21. Yang X, Gao M, Xu R, Tao Y, Luo W, Wang B, et al. Hyperthermia combined with immune checkpoint inhibitor therapy in the treatment of primary and metastatic tumors. Front Immunol. (2022) 13:969447. doi: 10.3389/fimmu.2022.969447
22. Li H, Cao GM, Gu GL, Li SY, Yan Y, Fu Z, et al. Expression characteristics of peripheral lymphocyte programmed death 1 and FoxP3(+) Tregs in gastric cancer during surgery and chemotherapy. World J Gastroenterol. (2023) 29:5582–92. doi: 10.3748/wjg.v29.i40.5582
23. Hou W, Kong L, Hou Z, and Ji H. CD44 is a prognostic biomarker and correlated with immune infiltrates in gastric cancer. BMC Med Genomics. (2022) 15:225. doi: 10.1186/s12920-022-01383-w
24. Chen X, Chen LJ, Peng XF, Deng L, Wang Y, Li JJ, et al. Anti-PD-1/PD-L1 therapy for colorectal cancer: Clinical implications and future considerations. Transl Oncol. (2024) 40:101851. doi: 10.1016/j.tranon.2023.101851
25. Zhao M, Guo W, Wu Y, Yang C, Zhong L, Deng G, et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. (2019) 9:304–15. doi: 10.1016/j.apsb.2018.08.009
26. Curnock AP, Bossi G, Kumaran J, Bawden LJ, Figueiredo R, Tawar R, et al. Cell-targeted PD-1 agonists that mimic PD-L1 are potent T cell inhibitors. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.152468
27. Díaz-Tejedor A, Lorenzo-Mohamed M, Puig N, García-Sanz R, Mateos MV, Garayoa M, et al. Immune system alterations in multiple myeloma: molecular mechanisms and therapeutic strategies to reverse immunosuppression. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13061353
28. Jiao Y, Li S, Wang X, Yi M, Wei H, Rong S, et al. A genomic instability-related lncRNA model for predicting prognosis and immune checkpoint inhibitor efficacy in breast cancer. Front Immunol. (2022) 13:929846. doi: 10.3389/fimmu.2022.929846
29. Deng D, Luo X, Zhang S, and Xu Z. Immune cell infiltration-associated signature in colon cancer and its prognostic implications. Aging (Albany NY). (2021) 13:19696–709. doi: 10.18632/aging.203380
30. Corsale AM, Di Simone M, Lo Presti E, Picone C, Dieli F, and Meraviglia S. Metabolic changes in tumor microenvironment: how could they affect γδ T cells functions? Cells. (2021) 10. doi: 10.3390/cells10112896
31. Ho PC and Kaech SM. Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. (2017) 46:38–44. doi: 10.1016/j.coi.2017.04.003
32. Cheng HR, van Vorstenbosch RWR, Pachen DM, Meulen LWT, Straathof JWA, Dallinga JW, et al. Detecting colorectal adenomas and cancer using volatile organic compounds in exhaled breath: A proof-of-principle study to improve screening. Clin Transl Gastroenterol. (2022) 13:e00518. doi: 10.14309/ctg.0000000000000518
33. She X, Wu Q, Rao Z, Song D, Huang C, Feng S, et al. SETDB1 methylates MCT1 promoting tumor progression by enhancing the lactate shuttle. Adv Sci (Weinh). (2023) 10:e2301871. doi: 10.1002/advs.202301871
34. Kono Y, Saito H, Miyauchi W, Shimizu S, Murakami Y, Shishido Y, et al. Increased PD-1-positive macrophages in the tissue of gastric cancer are closely associated with poor prognosis in gastric cancer patients. BMC Cancer. (2020) 20:175. doi: 10.1186/s12885-020-6629-6
35. Ferrer G, Álvarez-Errico D, and Esteller M. Biological and molecular factors predicting response to adoptive cell therapies in cancer. J Natl Cancer Inst. (2022) 114:930–9. doi: 10.1093/jnci/djac088
36. Lei ZN, Tian Q, Teng QX, Wurpel JND, Zeng L, Pan Y, et al. Understanding and targeting resistance mechanisms in cancer. MedComm. (2023) 4:e265. doi: 10.1002/mco2.265
37. Holtermann A, Gislon M, Angele M, Subklewe M, von Bergwelt-Baildon M, Lauber K, et al. Prospects of synergy: local interventions and CAR T cell therapy in solid tumors. BioDrugs. (2024) 38:611–37. doi: 10.1007/s40259-024-00669-y
38. Jin K, Cao Y, Gu Y, Fang H, Fei Y, Wang J, et al. Poor clinical outcomes and immunoevasive contexture in CXCL13+CD8+ T cells enriched gastric cancer patients. Oncoimmunology. (2021) 10:1915560. doi: 10.1080/2162402X.2021.1915560
39. Wang XX, Deng SZ, Wu LH, Liu QQ, Zheng G, Du K, et al. Cuproptosis-mediated patterns characterized by distinct tumor microenvironment and predicted the immunotherapy response for gastric cancer. ACS Omega. (2023) 8:10851–62. doi: 10.1021/acsomega.2c07052
40. Huang X, Chi H, Gou S, Guo X, Li L, Peng G, et al. An aggrephagy-related lncRNA signature for the prognosis of pancreatic adenocarcinoma. Genes. (2023) 14:124. doi: 10.3390/genes14010124
41. Ping Y, Shen C, Huang B, and Zhang Y. Reprogramming T-cell metabolism for better anti-tumor immunity. Cells. (2022) 11. doi: 10.3390/cells11193103
42. Gavali S, Liu J, Li X, and Paolino M. Ubiquitination in T-cell activation and checkpoint inhibition: new avenues for targeted cancer immunotherapy. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms221910800
43. He W, Zhang H, Han F, Chen X, Lin R, Wang W, et al. CD155T/TIGIT signaling regulates CD8(+) T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. (2017) 77:6375–88. doi: 10.1158/0008-5472.CAN-17-0381
44. Wei M, Shen D, Mulmi Shrestha S, Liu J, Zhang J, and Yin Y. The progress of T cell immunity related to prognosis in gastric cancer. BioMed Res Int. (2018) 2018:3201940. doi: 10.1155/2018/3201940
45. Zhao Y, Baldin AV, Isayev O, Werner J, Zamyatnin AA Jr., and Bazhin AV. Cancer vaccines: antigen selection strategy. Vaccines (Basel). (2021) 9. doi: 10.3390/vaccines9020085
46. Li W, Zhao Y, Zhang H, Zheng W, Wang R, and Gu X. Predictive value of tumor mutational burden for PD-1/PD-L1 inhibitors in NSCLC: A meta-analysis. Med (Baltimore). (2023) 102:e34990. doi: 10.1097/MD.0000000000034990
47. Shen Y, Chi H, Xu K, Li Y, Yin X, Chen S, et al. A novel classification model for lower-grade glioma patients based on pyroptosis-related genes. Brain Sci. (2022) 12:700. doi: 10.3390/brainsci12060700
48. Gong X and Karchin R. Pan-cancer HLA gene-mediated tumor immunogenicity and immune evasion. Mol Cancer Res. (2022) 20:1272–83. doi: 10.1158/1541-7786.MCR-21-0886
49. Yan A, Chen Y, Bian R, Wang C, Que H, Shen Y, et al. The landscape of enhancer RNA identify prognosis-related molecular subtypes in gastric cancer. Cancer Med. (2023) 12:2046–57. doi: 10.1002/cam4.4959
50. Gong J, Chehrazi-Raffle A, Reddi S, and Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. (2018) 6:8. doi: 10.1186/s40425-018-0316-z
51. Chi H, Huang J, Yan Y, Jiang C, Zhang S, Chen H, et al. Unraveling the role of disulfidptosis-related LncRNAs in colon cancer: a prognostic indicator for immunotherapy response, chemotherapy sensitivity, and insights into cell death mechanisms. Front Mol Biosci. (2023) 10:1254232. doi: 10.3389/fmolb.2023.1254232
52. Guo X, Bu X, Yuan L, and Ji L. Collagen type V alpha 2 promotes the development of gastric cancer via M2 macrophage polarization. Chin J Physiol. (2023) 66:93–102. doi: 10.4103/cjop.CJOP-D-22-00078
53. Dong X, Liao P, Liu X, Yang Z, Wang Y, Zhong W, et al. Construction and validation of a reliable disulfidptosis-related lncRNAs signature of the subtype, prognostic, and immune landscape in colon cancer. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241612915
54. Jiang L, Jiang Y, Zhou X, Wang L, Zhang S, Jiang C, et al. The key role of COA6 in pancreatic ductal adenocarcinoma: metabolic reprogramming and regulation of the immune microenvironment. J Cell Mol Med. (2025) 29:e70685. doi: 10.1111/jcmm.70685
55. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
56. Zhou X, Sang X, Jiang L, Zhang S, Jiang C, Gu Y, et al. Deciphering the role of acetylation-related gene NAT10 in colon cancer progression and immune evasion: implications for overcoming drug resistance. Discov Oncol. (2025) 16:774. doi: 10.1007/s12672-025-02617-w
57. Sekihara K, Himuro H, Toda S, Saito N, Hirayama R, Suganuma N, et al. Recent trends and potential of radiotherapy in the treatment of anaplastic thyroid cancer. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12061286
58. El Rami FE, Barsoumian HB, and Khneizer GW. Hereditary diffuse gastric cancer therapeutic roadmap: current and novel approaches in a nutshell. Ther Adv Med Oncol. (2020) 12:1758835920967238. doi: 10.1177/1758835920967238
59. Conway JR, Kofman E, Mo SS, Elmarakeby H, and Van Allen E. Genomics of response to immune checkpoint therapies for cancer: implications for precision medicine. Genome Med. (2018) 10:93. doi: 10.1186/s13073-018-0605-7
60. Laubach K, Turan T, Mathew R, Wilsbacher J, Engelhardt J, and Samayoa J. Tumor-intrinsic metabolic reprogramming and how it drives resistance to anti-PD-1/PD-L1 treatment. Cancer Drug Resist. (2023) 6:611–41. doi: 10.20517/cdr.2023.60
61. Zhu YJ, Li X, Chen TT, Wang JX, Zhou YX, Mu XL, et al. Personalised neoantigen-based therapy in colorectal cancer. Clin Transl Med. (2023) 13:e1461. doi: 10.1002/ctm2.1461
62. Szlasa W, Sztuder A, Kaczmar-Dybko A, Maciejczyk A, and Dybko J. Efficient combination of radiotherapy and CAR-T - A systematic review. BioMed Pharmacother. (2024) 174:116532. doi: 10.1016/j.biopha.2024.116532
63. Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. (2022) 28:1189–98. doi: 10.1038/s41591-022-01800-8
64. Jung M, Yang Y, McCloskey JE, Zaman M, Vedvyas Y, Zhang X, et al. Chimeric antigen receptor T cell therapy targeting ICAM-1 in gastric cancer. Mol Ther Oncol. (2020) 18:587–601. doi: 10.1016/j.omto.2020.08.009
65. Netsrithong R and Wattanapanitch M. Advances in adoptive cell therapy using induced pluripotent stem cell-derived T cells. Front Immunol. (2021) 12:759558. doi: 10.3389/fimmu.2021.759558
66. Johdi NA and Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. (2020) 11:1624. doi: 10.3389/fimmu.2020.01624
67. Xu R, Wang Q, Zhu J, Bei Y, Chu Y, Sun Z, et al. Membrane fusogenic nanoparticle-based HLA-peptide-addressing universal T cell receptor-engineered T (HAUL TCR-T) cell therapy in solid tumor. Bioeng Transl Med. (2023) 8:e10585. doi: 10.1002/btm2.10585
68. Yan XY, Yin PW, Wu XM, and Han JX. Prediction of the drug-drug interaction types with the unified embedding features from drug similarity networks. Front Pharmacol. (2021) 12:794205. doi: 10.3389/fphar.2021.794205
69. Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. (2022) 15:95. doi: 10.1186/s13045-022-01294-4
70. Hibino S, Eto S, Hangai S, Endo K, Ashitani S, Sugaya M, et al. Tumor cell-derived spermidine is an oncometabolite that suppresses TCR clustering for intratumoral CD8(+) T cell activation. Proc Natl Acad Sci U.S.A. (2023) 120:e2305245120. doi: 10.1073/pnas.2305245120
71. Wei M, Dong Q, Chen S, Wang J, Yang H, and Xiong Q. Identification of key biomarkers in systemic lupus erythematosus by a multi-cohort analysis. Front Immunol. (2022) 13:928623. doi: 10.3389/fimmu.2022.928623
72. Lee CK, Rha SY, Kim HS, Jung M, Kang B, Che J, et al. A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer. Nat Commun. (2022) 13:6002. doi: 10.1038/s41467-022-33267-z
73. Pan Y, Lu L, Liu H, Chen D, Han N, Yao R, et al. Case report: Long response to PD-1 blockade after failure of trastuzumab plus chemotherapy in advanced Epstein-Barr virus-associated gastric cancer. Front Immunol. (2022) 13:1003859. doi: 10.3389/fimmu.2022.1003859
74. Wang Y, Yang S, Wan L, Ling W, Chen H, and Wang J. New developments in the mechanism and application of immune checkpoint inhibitors in cancer therapy (Review). Int J Oncol. (2023) 63. doi: 10.3892/ijo.2023.5534
75. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, and Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometr Syst Pharmacol. (2017) 6:58–66. doi: 10.1002/psp4.12143
76. Kuehu DL, Fu Y, Nasu M, Yang H, Khadka VS, and Deng Y. Effects of heat-induced oxidative stress and astaxanthin on the NF-kB, NFE2L2 and PPARα Transcription factors and cytoprotective capacity in the thymus of broilers. Curr Issues Mol Biol. (2024) 46:9215–33. doi: 10.3390/cimb46080544
77. Sek K, Mølck C, Stewart GD, Kats L, Darcy PK, and Beavis PA. Targeting adenosine receptor signaling in cancer immunotherapy. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19123837
78. Liang J, Cheng K, Li Y, Xu J, Chen Y, Ma N, et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam Res. (2022) 2:23–36. doi: 10.1016/j.fmre.2021.11.032
79. Chen Y, Gao Z, Mohd-Ibrahim I, Yang H, Wu L, Fu Y, et al. Pan-cancer analyses of bromodomain containing 9 as a novel therapeutic target reveals its diagnostic, prognostic potential and biological mechanism in human tumours. Clin Trans Med. (2024) 14:e1543. doi: 10.1002/ctm2.1543
80. Wu Y, Huang Z, Harrison R, Liu L, Zhu L, Situ Y, et al. Engineering CAR T cells for enhanced efficacy and safety. APL Bioeng. (2022) 6:011502. doi: 10.1063/5.0073746
81. Levantini E. Novel therapeutic targets in cancers. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241914660
Keywords: T-cell exhaustion, immune checkpoint, biomarkers, cancer immune evasion, gastric cancer, immune microenvironment
Citation: Luo H, Wu J, Yan Y, Xu D, Zhang J, Zhou X, Yang G and Zhong X (2025) Mechanisms and therapeutic strategies to reveal and overcome T-cell dysfunction in gastric cancer: translation from basic research to clinical application. Front. Immunol. 16:1681539. doi: 10.3389/fimmu.2025.1681539
Received: 07 August 2025; Accepted: 11 September 2025;
Published: 30 September 2025.
Edited by:
Wantao Wu, Chongqing Medical University, ChinaReviewed by:
Zhe Pei, Virginia Tech, United StatesCopyright © 2025 Luo, Wu, Yan, Xu, Zhang, Zhou, Yang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuancheng Zhou, emhvdXh1YW5jaGVuZzA0QDE2My5jb20=; Guanhu Yang, Z3Vhbmh1eWFuZ0BnbWFpbC5jb20=; Xiaolin Zhong, eGlhb2xpbnpob25nQHN3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Huanyu Luo
Huanyu Luo Jianxi Wu
Jianxi Wu Yalan Yan
Yalan Yan Danqi Xu2
Danqi Xu2 Jieying Zhang
Jieying Zhang Xuancheng Zhou
Xuancheng Zhou Guanhu Yang
Guanhu Yang Xiaolin Zhong
Xiaolin Zhong