- Department of Medical Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Objective: To evaluate the prognostic performance of six scoring systems in predicting outcomes of first-line chemo-immunotherapy in patients with extensive-stage small cell lung cancer (ES-SCLC), aiming to guide individualized treatment.
Methods: This single-center retrospective study included 197 ES-SCLC patients treated with first-line chemo-immunotherapy. Clinical and laboratory data were collected, including baseline characteristics, treatment responses, and survival outcomes. The prognostic impact of six scoring systems (RHM, MDACC, MDACC+NLR, MDA-ICI, LIPI, GRIm) was assessed using univariate and multivariate Cox regression analyses for progression-free survival (PFS) and overall survival (OS). Kaplan–Meier analysis was conducted for risk stratification.
Results: By the last follow-up (October 15, 2024), the median follow-up was 12 months, with 113 deaths (57.3%). The objective response rate was 75.6%. ECOG ≥1, lung metastasis, and liver metastasis were independent predictors of poorer PFS and OS. Among the scoring systems, only MDACC+NLR effectively stratified patients: low-risk patients had significantly longer PFS and OS (both p = 0.02). MDACC alone did not distinguish PFS among risk groups (p = 0.17) but showed significant OS differences (p = 0.02). Other systems (RHM, MDA-ICI, LIPI, GRIm) lacked significant discriminatory ability for both PFS and OS (all p > 0.05).
Conclusion: ECOG ≥1, lung metastasis, and liver metastasis are adverse prognostic factors for ES-SCLC patients receiving first-line chemo-immunotherapy. The MDACC+NLR scoring system provides superior predictive value for treatment outcomes and survival, supporting its potential utility for clinical risk stratification.
1 Introduction
According to the latest data released in 2025 by the International Agency for Research on Cancer (IARC), a specialized agency of the World Health Organization, lung cancer remains the leading cause of cancer incidence and mortality worldwide. It has ranked first in global cancer-related deaths for ten consecutive years. Among all lung cancer subtypes, small cell lung cancer (SCLC) accounts for a significant proportion of lung cancer–related deaths (1). In China, data from the National Cancer Center reported more than 1.06 million new lung cancer cases and over 730,000 deaths in 2022. Lung cancer continues to be the most common and deadliest malignancy in the country, posing a serious threat to public health (2).
Small cell lung cancer (SCLC) is a highly aggressive subtype of lung cancer (3), accounting for approximately 13% to 15% of all lung cancer cases (4). Its occurrence is strongly associated with tobacco smoking (5), SCLC is characterized by rapid growth, early dissemination, and a high propensity for recurrence and metastasis. Recent reviews have highlighted the molecular heterogeneity of SCLC and its implications for therapeutic response and drug development. Distinct transcriptional subtypes such as ASCL1, NEUROD1, POU2F3, and YAP1 not only exhibit unique biological behaviors but may also influence sensitivity to immunotherapy and targeted agents” (6). Although initially sensitive to chemotherapy and radiotherapy, the disease frequently relapses and has a poor overall prognosis. Clinically, SCLC is classified into limited-stage (LS) and extensive-stage (ES) disease. The 5-year survival rate for LS-SCLC is approximately 30%, while for ES-SCLC it is only around 3%. The median survival time is about 15–20 months for LS patients and 8–13 months for those with ES-SCLC (7). Despite a recent decline in the overall incidence of lung cancer, the mortality rate of SCLC remains alarmingly high. Due to the lack of effective screening methods, most patients are diagnosed at an advanced stage, presenting significant therapeutic challenges.
Over the past few decades, platinum-based doublet chemotherapy has been the standard first-line treatment for small cell lung cancer (SCLC). It usually consists of a platinum agent combined with etoposide or irinotecan. However, most patients experience disease recurrence within four months after completing initial therapy.
In recent years, immunotherapy—particularly immune checkpoint inhibitors (ICIs)—has become a major breakthrough in cancer treatment (8). In extensive-stage SCLC (ES-SCLC), combining immunotherapy with chemotherapy has significantly improved progression-free survival (PFS) compared with chemotherapy alone. As a result, chemoimmunotherapy (CIT) is now recommended as the standard first-line regimen for ES-SCLC (9).
Despite CIT being established as the first-line standard of care and demonstrating survival benefits in several clinical trials (10, 11), not all patients derive equal benefit from this approach. Most existing studies on first-line treatment for ES-SCLC are based on rigorously designed randomized controlled trials (RCTs). However, due to the strict inclusion criteria of RCTs, their findings may have limited generalizability to real-world patient populations. In contrast, real-world evidence provides a more comprehensive picture of the effectiveness and survival outcomes associated with CIT in clinical practice.
Currently, there are no reliable or validated biomarkers that can accurately predict the efficacy or prognosis of chemoimmunotherapy (CIT) in ES-SCLC. To address this gap, several large-scale clinical studies have developed prognostic scoring systems based on clinical risk factors. These include the Royal Marsden Hospital Index (RMH), the MD Anderson Clinical Center Score (MDACC), the MDACC score combined with the neutrophil-to-lymphocyte ratio (MDACC+NLR), the MD Anderson Immune Checkpoint Inhibitor Score (MDA-ICI), the Lung Immune Prognostic Index (LIPI), and the Gustave Roussy Immune Score (GRIm) (7, 12–14). These scoring systems were primarily designed to identify patients with malignant tumors who may derive greater benefit from novel therapies in clinical trials and have also demonstrated value in prognostic stratification. However, the predictive and prognostic utility of these scoring systems in the context of first-line CIT for ES-SCLC remains unclear and requires further investigation.
In this study, we retrospectively analyzed clinicopathological characteristics and survival data from 197 patients with ES-SCLC who received first-line chemoimmunotherapy (CIT) in a real-world setting. We evaluated the prognostic value and predictive performance of six established scoring systems—RHM, MDACC, MDACC+NLR, MDA-ICI, LIPI, and GRIm—in the context of first-line CIT. This study aimed to identify the most effective prognostic model for stratifying ES-SCLC patients and guiding treatment decisions. Our findings may provide important insights for selecting patients most likely to benefit from CIT and offer new evidence to support the development of personalized treatment strategies for ES-SCLC.
2 Materials and methods
2.1 Study population
2.1.1 Patient selection and treatment details
This retrospective study reviewed 275 patients diagnosed with extensive-stage small cell lung cancer (ES-SCLC) at our institution between December 2016 and June 2024.Eligible patients met the following inclusion criteria (1): histologically or cytologically confirmed ES-SCLC (2); receipt of first-line platinum-based chemotherapy combined with a PD-1 or PD-L1 inhibitor; and (3) availability of complete baseline clinical and laboratory data. Patients were excluded if they (1) had concurrent malignancies (2); lacked complete follow-up or treatment information; or (3) were lost to follow-up before the first efficacy assessment. After applying these criteria, 197 patients were included in the final analysis. A detailed summary of inclusion and exclusion criteria is presented below, and the patient selection process is depicted in Figure 1.
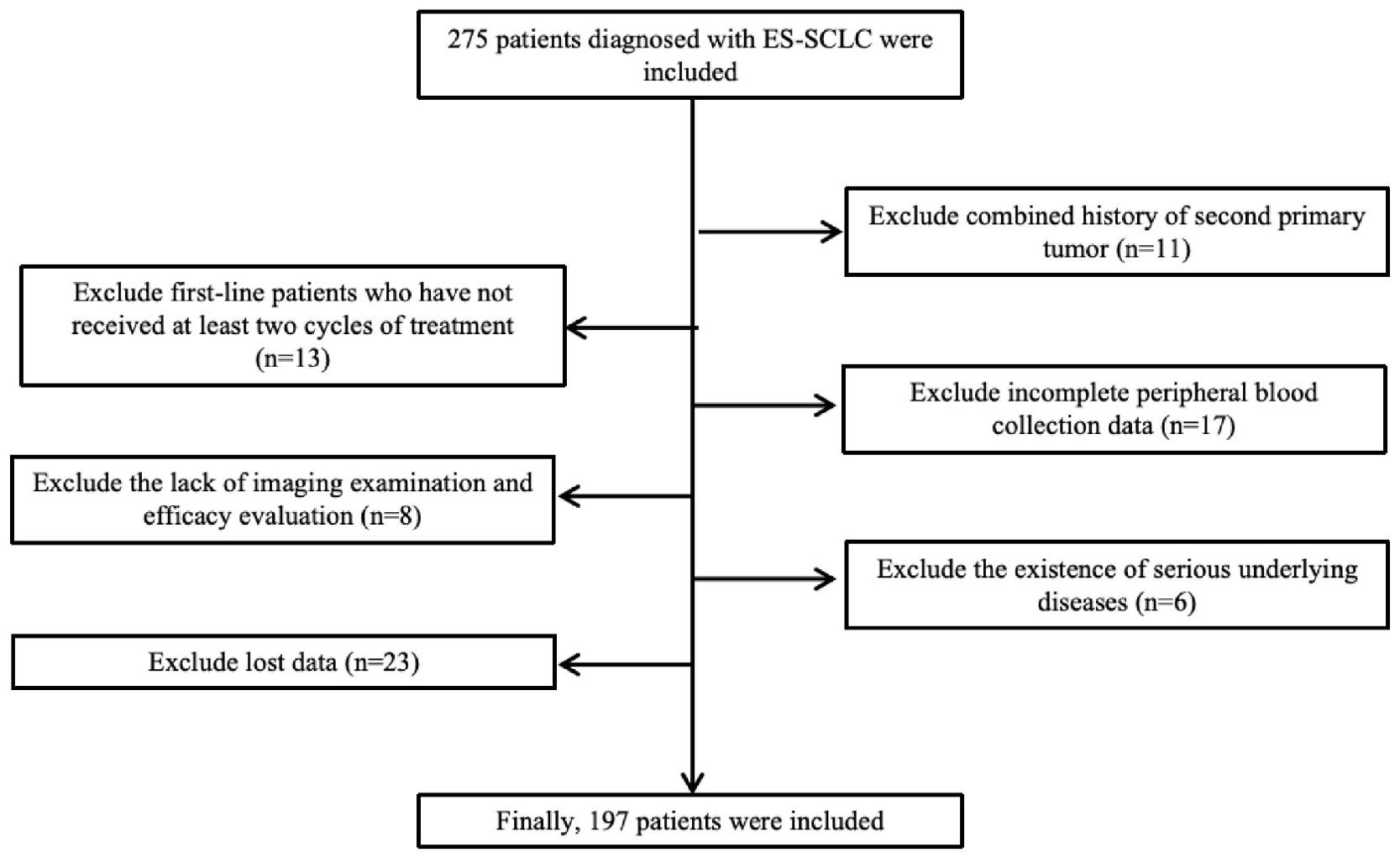
Figure 1. Flow diagram of patient enrollment. A total of 275 patients diagnosed with ES-SCLC were screened; 78 were excluded for predefined reasons, and 197 patients were finally included in the analysis.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
Patients were eligible for inclusion if they met the following criteria:
1. Pathologically confirmed SCLC according to WHO criteria;
2. Extensive-stage disease defined by VALSG classification;
3. Age ≥18 years;
4. No contraindications to systemic therapy based on baseline evaluations;
5. Received ≥2 cycles of first-line platinum-based chemoimmunotherapy with evaluable efficacy data;
6. Tumor response assessed by CT, MRI, bone scan, or PET-CT;
7. Availability of complete baseline clinical, laboratory, and follow-up data.
2.2.2 Exclusion criteria
Patients were excluded based on the following criteria:
1. Receipt of any prior antitumor therapy (e.g., radiotherapy or surgery) before first-line treatment;
2. History of another primary malignancy;
3. Lack of imaging data or unassessable tumor response;
4. Presence of active infection, severe autoimmune disease, or major organ dysfunction;
5. Incomplete or missing baseline clinical or follow-up information.
2.3 Study design
2.3.1 Treatment regimens
All enrolled patients with ES-SCLC were categorized into two groups: the chemoimmunotherapy (CIT) group and the chemotherapy (CT) group. Chemotherapy agents included etoposide, either alone or in combination with cisplatin, carboplatin, or lobaplatin. Immunotherapy agents included:
• Atezolizumab (Roche Diagnostics GmbH, 1200 mg every 3 weeks)
• Durvalumab (AstraZeneca UK Limited, 1500 mg every 3 weeks)
• Serplulimab (Shanghai Henlius Biotech Inc., 4.5 mg/kg every 3 weeks)
• Tislelizumab (BeiGene, Guangzhou, 200 mg every 3 weeks)
• Toripalimab (Zhonghe Biopharma, Suzhou, 3 mg/kg every 3 weeks)
• Adebrelimab (Hengrui Medicine Co., Ltd., Jiangsu, 20 mg/kg every 3 weeks)
Chemotherapy was administered for 2–6 cycles. Patients then either continued with maintenance therapy or discontinued treatment upon disease progression, unacceptable toxicity, or death.
2.3.2 Peripheral blood biomarkers
Baseline peripheral blood parameters within one week prior to initiation of CIT were extracted from the hospital electronic medical record system. These included white blood cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), platelet count (PLT), serum lactate dehydrogenase (LDH), and serum albumin (ALB).
2.4 Derived inflammatory indices
1. Neutrophil-to-lymphocyte ratio (NLR) was calculated as: NLR = ANC/ALC
2. Derived neutrophil-to-lymphocyte ratio (dNLR) was calculated as: dNLR = ANC/(WBC − ANC)
2.5 Response evaluation criteria
Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1:
Complete Response (CR): Disappearance of all target lesions, no new lesions, normalization of tumor markers, and sustained response for at least 4 weeks.
Partial Response (PR): At least a 30% reduction in the sum of the diameters of target lesions compared to baseline, with no new lesions, sustained for at least 4 weeks.
Stable Disease (SD): Disease status between PR and PD, with no new lesions, maintained for 6–8 weeks.
Progressive Disease (PD): A ≥20% increase in the sum of diameters of target lesions from the smallest value recorded or the appearance of one or more new lesions.
The Objective Response Rate (ORR) was calculated as: ORR = (CR + PR)/total number of patients × 100%.
2.6 Prognostic scoring systems
Detailed scoring criteria and grouping rules for the six prognostic systems (RMH Score (15), MDACC Score (16), MDACC+NLR Score (11), MDA-ICI Score (13), Definition of LIPI Score (7), GRIm Score (14)) are listed in Appendix Table 1.
2.7 Follow-up
Patient follow-up was conducted using the institutional follow-up center database, as well as the outpatient and inpatient medical record systems. For patients with incomplete follow-up information in the system, additional data were collected via telephone interviews. The final date of follow-up was October 15, 2024.
2.8 Study endpoints
The primary endpoints of this study were overall survival (OS) and progression-free survival (PFS).
OS was defined as the time interval from the initial diagnosis of ES-SCLC to death from any cause or last follow-up.
PFS was defined as the time from initial diagnosis to the date of disease progression or death from any cause.
Patients who had not experienced disease progression or death by the end of follow-up were censored at the date of their last follow-up.
2.9 Statistical analysis
All statistical analyses were conducted using SPSS software, version 25.0. Categorical variables were presented as frequencies and percentages. Group differences were assessed using the chi-square test. Survival comparisons between groups were performed using Kaplan–Meier survival curves, with statistical significance evaluated by the log-rank test. Prognostic factors were identified using Cox proportional hazards regression models, and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. All tests were two-sided, and p < 0.05 was considered statistically significant.
A Cox proportional hazards model using the MDACC+NLR composite score as the predictor was applied to evaluate its prognostic value for overall survival (OS). Model performance was assessed by Harrell’s concordance index (C-index, 1,000 bootstrap resamples) and bias-corrected calibration curves at 6 and 12 months. A nomogram was constructed to visualize individualized survival probabilities, and decision curve analysis (DCA) was used to estimate the clinical net benefit across threshold probabilities. All analyses were performed in R (version 4.x) using the rms, Hmisc, rmda, and timeROC packages, with a two-sided α = 0.05.
3 Results
3.1 Patient enrollment and data collection
A total of 275 patients diagnosed with extensive-stage small cell lung cancer (ES-SCLC) who received first-line chemoimmunotherapy (CIT) were initially screened. After excluding 78 patients due to receipt of fewer than two treatment cycles, history of another primary malignancy, incomplete peripheral blood data, severe comorbidities, or loss to follow-up, 197 patients with complete clinical and follow-up data were included in the final analysis. As of the last follow-up on October 15, 2024, the median follow-up time was 16 months (95% CI: 14.7–17.3), and 113 deaths had occurred.
3.2 Clinicopathological characteristics
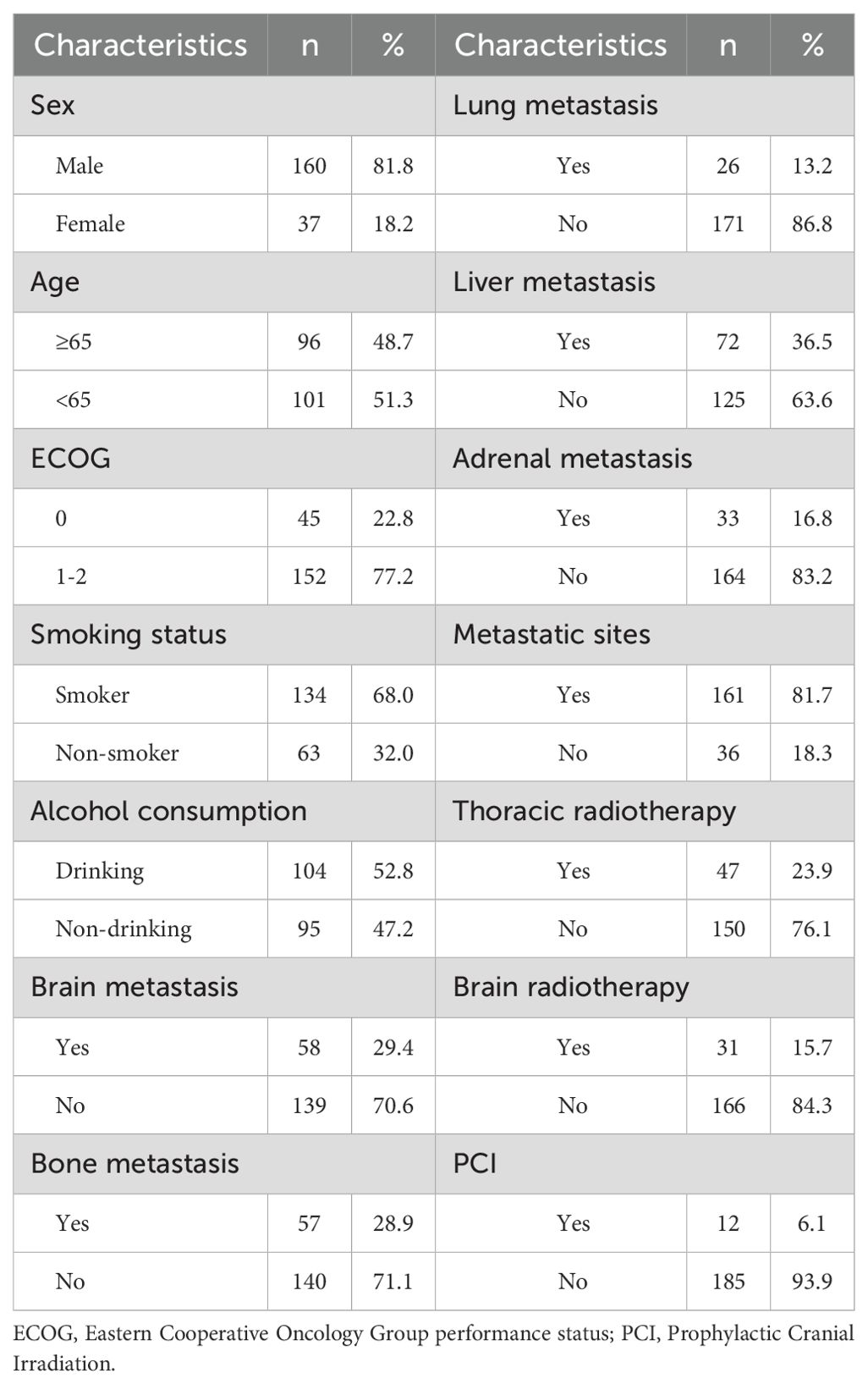
Among the 197 patients, 160 (81.8%) were male and 37 (18.2%) were female. The median age was 65 years; 101 patients (51.3%) were <65 years, and 96 (48.7%) were ≥65 years. Forty-five patients (22.8%) had an ECOG performance status <1, while 152 (77.2%) had ECOG ≥1.
At diagnosis, metastases were observed in the brain (58 patients, 29.4%), liver (72, 36.5%), lung (26, 13.2%), bone (140, 71.1%), and adrenal glands (33, 16.8%). Multi-organ metastases (≥2 organs) were present in 161 patients (81.7%). Forty-seven (23.9%) underwent brain radiotherapy, and 12 (6.1%) received prophylactic cranial irradiation (PCI). A smoking history was reported in 104 patients (52.8%), while 93 (47.2%) were non-smokers (Table 1).
3.3 Prognostic impact of clinicopathological characteristics
3.3.1 Progression-free survival
In univariate Cox regression, ECOG <1 was associated with longer PFS (HR: 0.60, 95% CI: 0.38–0.95; P = 0.02). Lung metastases (HR: 0.49, 95% CI: 0.31–0.76; P < 0.01) and liver metastases (HR: 0.65, 95% CI: 0.47–0.93; P = 0.02) were associated with shorter PFS. Other factors showed no significant association with PFS (P > 0.05). Multivariate analysis confirmed ECOG ≥1 (HR: 0.57, 95% CI: 0.36–0.91; P = 0.01), lung metastases (HR: 0.45, 95% CI: 0.28–0.73; P < 0.01), and liver metastases (HR: 0.67, 95% CI: 0.47–0.94; P = 0.02) as independent negative predictors of PFS (Table 2).
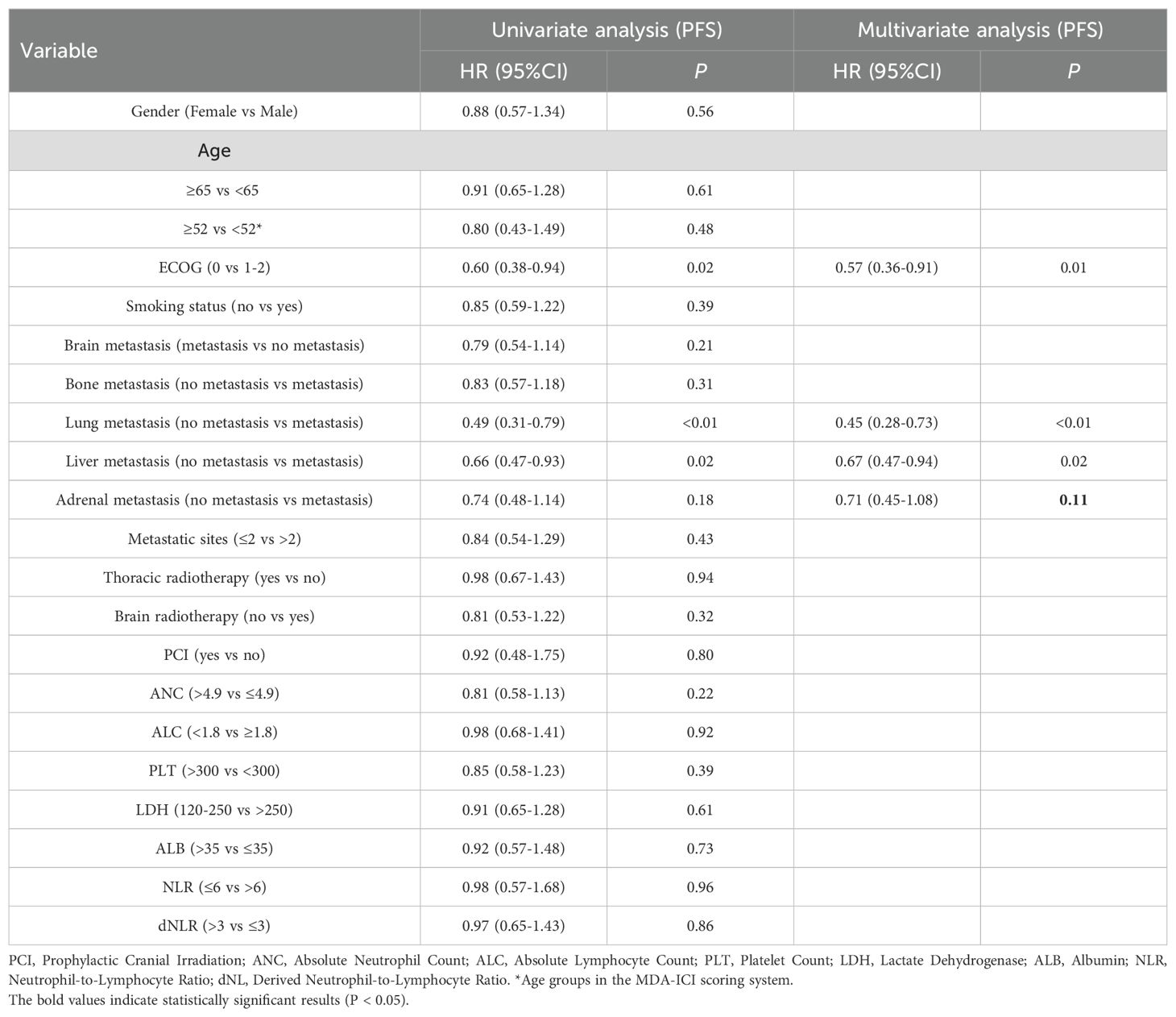
Table 2. Univariate and multivariate Cox regression analyses of clinicopathological characteristics associated with progression-free survival (PFS) in ES-SCLC patients receiving first-line chemoimmunotherapy.
3.3.2 Overall survival
Univariate analysis showed liver metastases (HR: 0.54, 95% CI: 0.37–0.79; P < 0.01) and lung metastases (HR: 0.54, 95% CI: 0.32–0.92; P = 0.02) were associated with worse OS. ECOG <1 trended toward longer OS (HR: 0.58, 95% CI: 0.34–1.01; P = 0.06), though not statistically significant. In multivariate analysis, ECOG <1 (HR: 0.56, 95% CI: 0.32–1.00; P = 0.05), lung metastases (HR: 0.56, 95% CI: 0.32–0.97; P = 0.04), and liver metastases (HR: 0.64, 95% CI: 0.42–0.98; P = 0.04) remained independent predictors of poorer OS (Table 3).
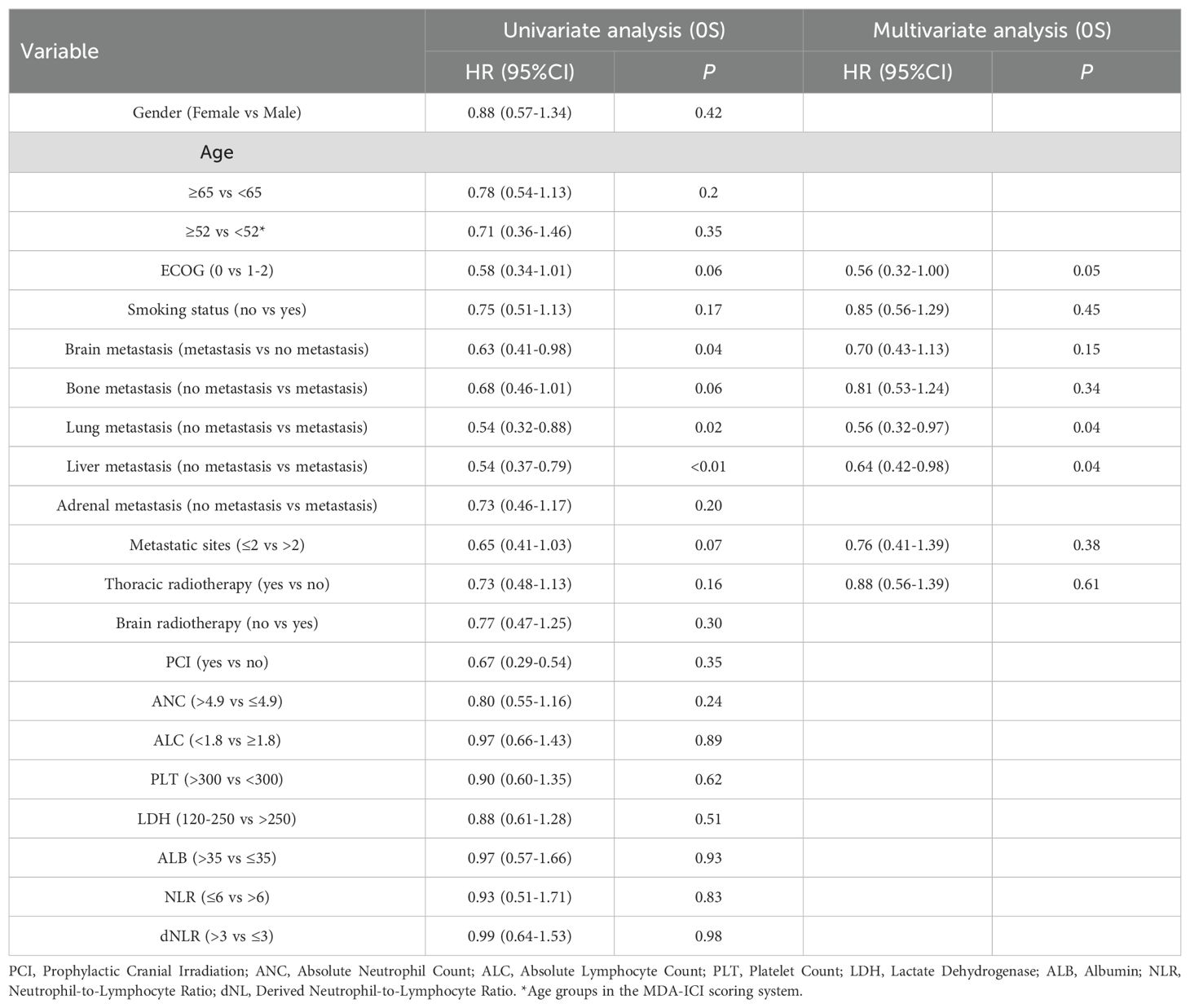
Table 3. Univariate and multivariate Cox regression analyses of clinicopathological characteristics associated with overall survival (OS) in ES-SCLC patients receiving first-line chemoimmunotherapy.
3.4 Prognostic value of scoring systems
3.4.1 1RMH score
Patients were stratified into low-risk (score 0–1) and high-risk (score 2–3) categories according to the RMH score.Median PFS was 8 months in both groups (P = 0.69; Figure 2A). Median OS was 15 months for the low-risk group and 18 months for the high-risk group (P = 0.69; Figure 2B).
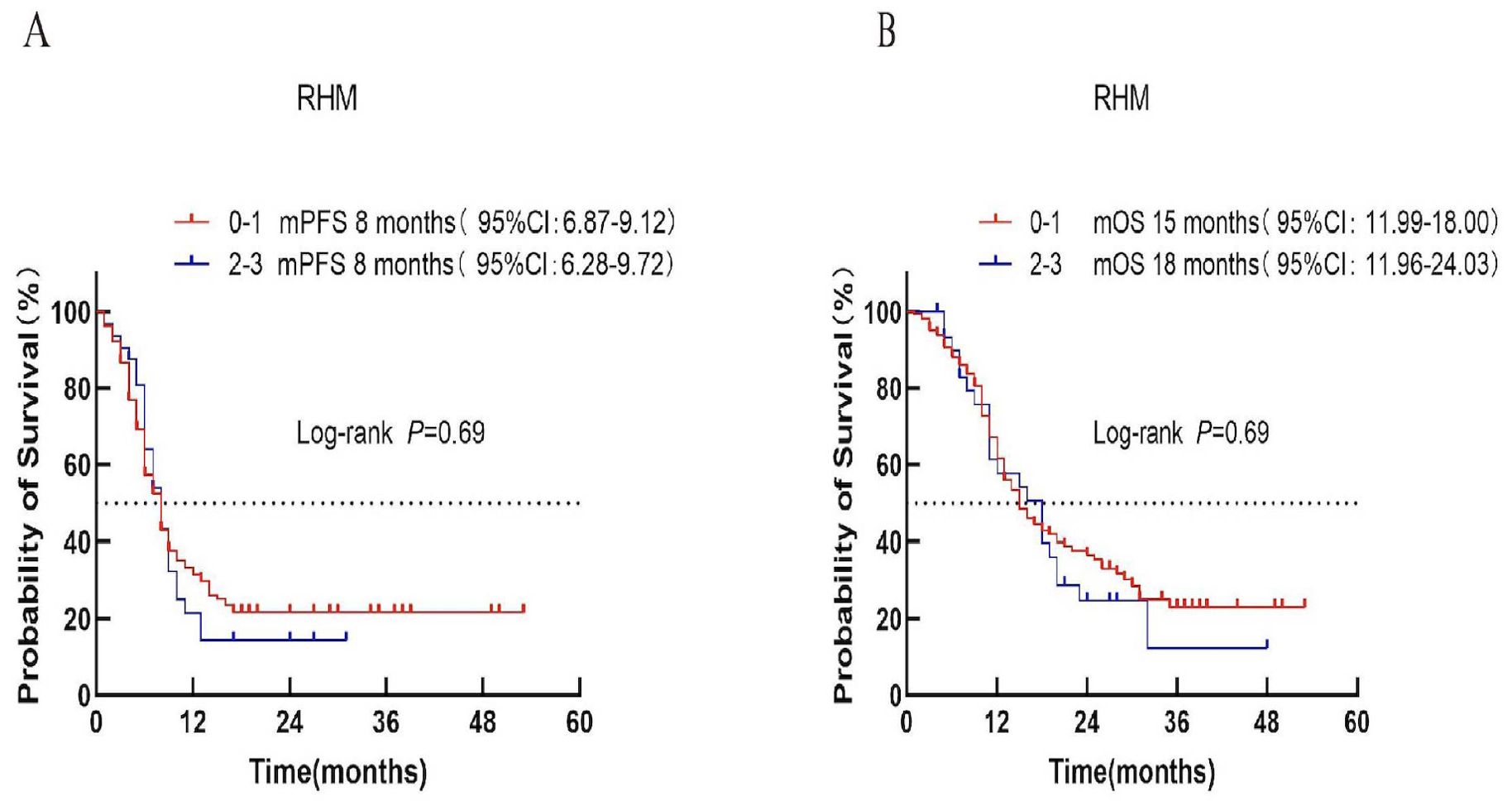
Figure 2. Survival analysis of the RMH scoring system in ES-SCLC patients treated with first-line chemoimmunotherapy. (A) Progression-free survival (PFS). (B) Overall survival (OS).
3.4.2 MDACC score
Based on the MDACC score, patients were divided into low-risk (score 0–1), intermediate-risk (score 2), and high-risk (score 3) categories. Median PFS was 8, 7, and 6 months for the respective groups (P = 0.69; Figure 3A). In contrast, median OS differed significantly among the three groups (17, 15, and 12 months; P = 0.02; Figure 3B).
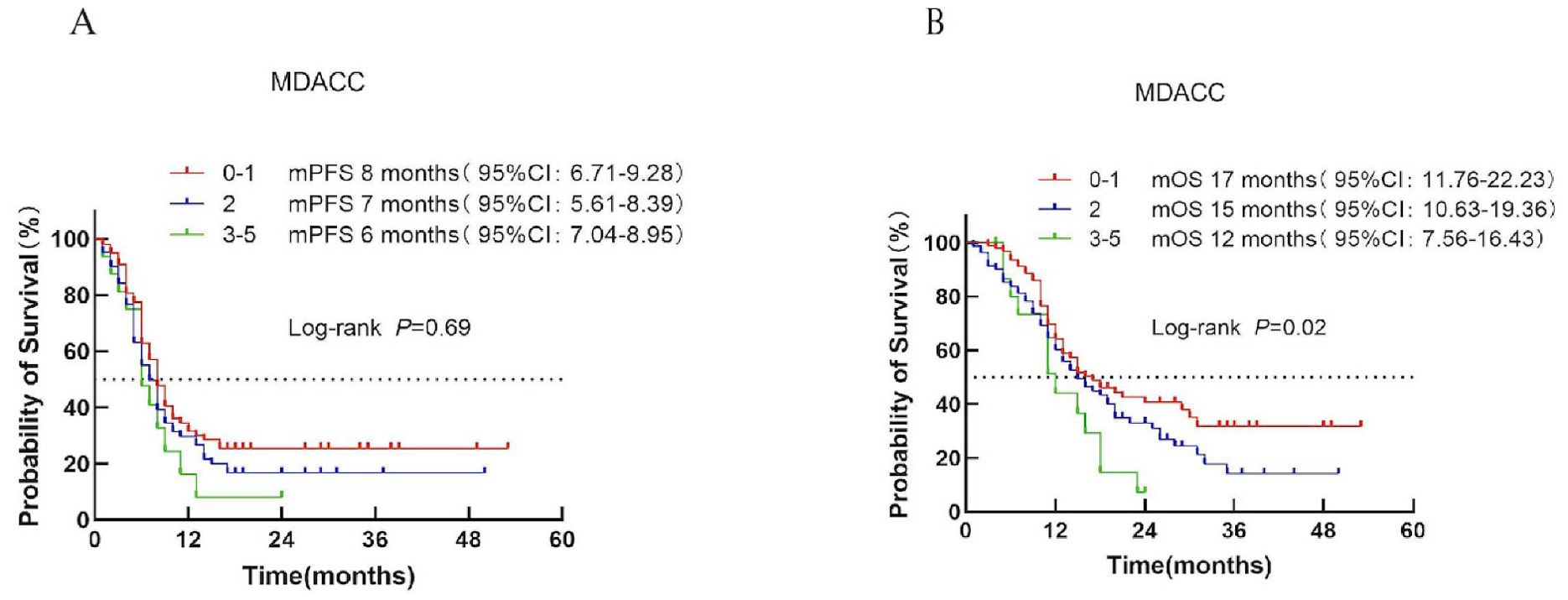
Figure 3. Survival analysis of the MDACC scoring system in ES-SCLC patients receiving first-line chemoimmunotherapy combined with immunotherapy. (A) PFS curves for each risk group. (B) OS curves with corresponding median survival times.
3.4.3 MDACC + NLR score
Patients were stratified into low-risk (score 0–1) and high-risk (score >1) categories according to the MDACC + NLR score. Median PFS was 9 months in the low-risk group and 7 months in the high-risk group (P = 0.02; Figure 4A). Median OS was 18 vs. 15 months, respectively (P = 0.02; Figure 4B), demonstrating a clear prognostic distinction between the two groups.
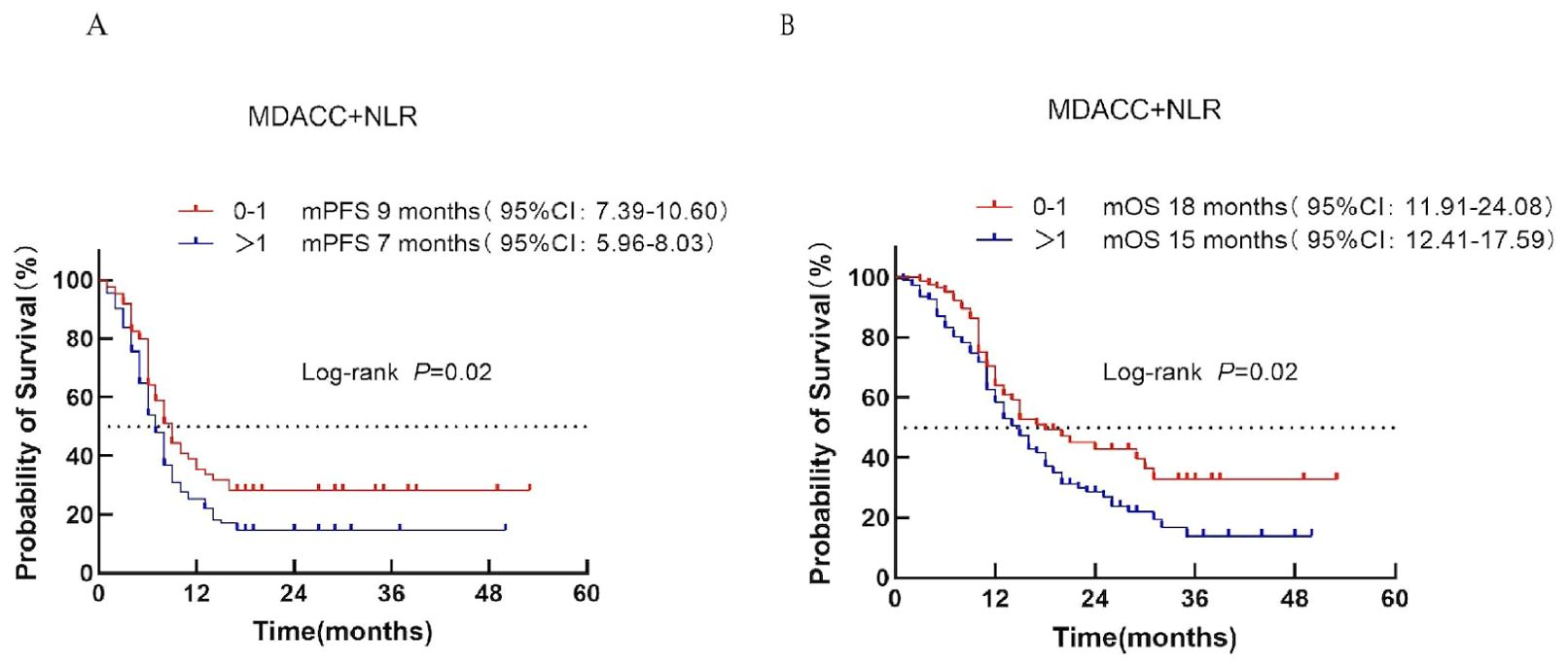
Figure 4. Survival analysis of the MDACC + NLR scoring system in ES-SCLC patients treated with first-line chemoimmunotherapy. (A) PFS. (B) OS.
3.4.4 MDA-ICI score
According to the MDA-ICI score, patients were classified into three risk groups: low risk (score 0–2), intermediate risk (score 3–4), and high risk (score 5–7). Median PFS was 10, 7, and 8 months for the low-, intermediate-, and high-risk groups, respectively (P = 0.15; Figure 5A). Median OS was 35, 15, and 16 months, respectively (P = 0.16; Figure 5B). No significant survival difference was observed across risk categories, indicating limited prognostic value for this model.
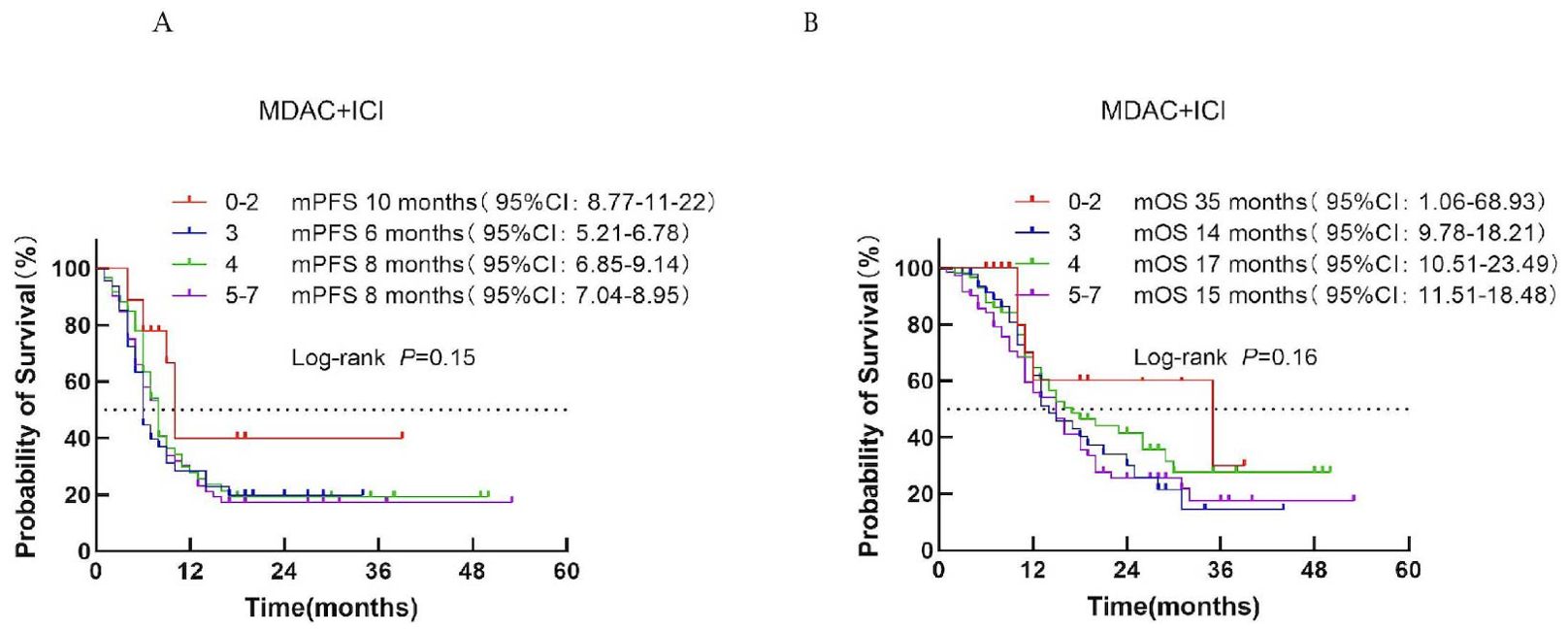
Figure 5. Survival analysis of the MDACC + ICI scoring system in ES-SCLC patients treated with first-line immunotherapy combination. (A) PFS. (B) OS.
3.4.5 LIPI score
Based on the Lung Immune Prognostic Index (LIPI), patients were classified into low-risk (score 0), intermediate-risk (score 1), and high-risk (score 2) groups corresponding to LIPI scores of 0, 1, and 2, respectively. Median PFS was 8, 7, and 8 months for the low-, intermediate-, and high-risk groups, respectively (P = 0.94; Figure 6A). Median OS was 15, 16, and 16 months, respectively (P = 0.85; Figure 6B).
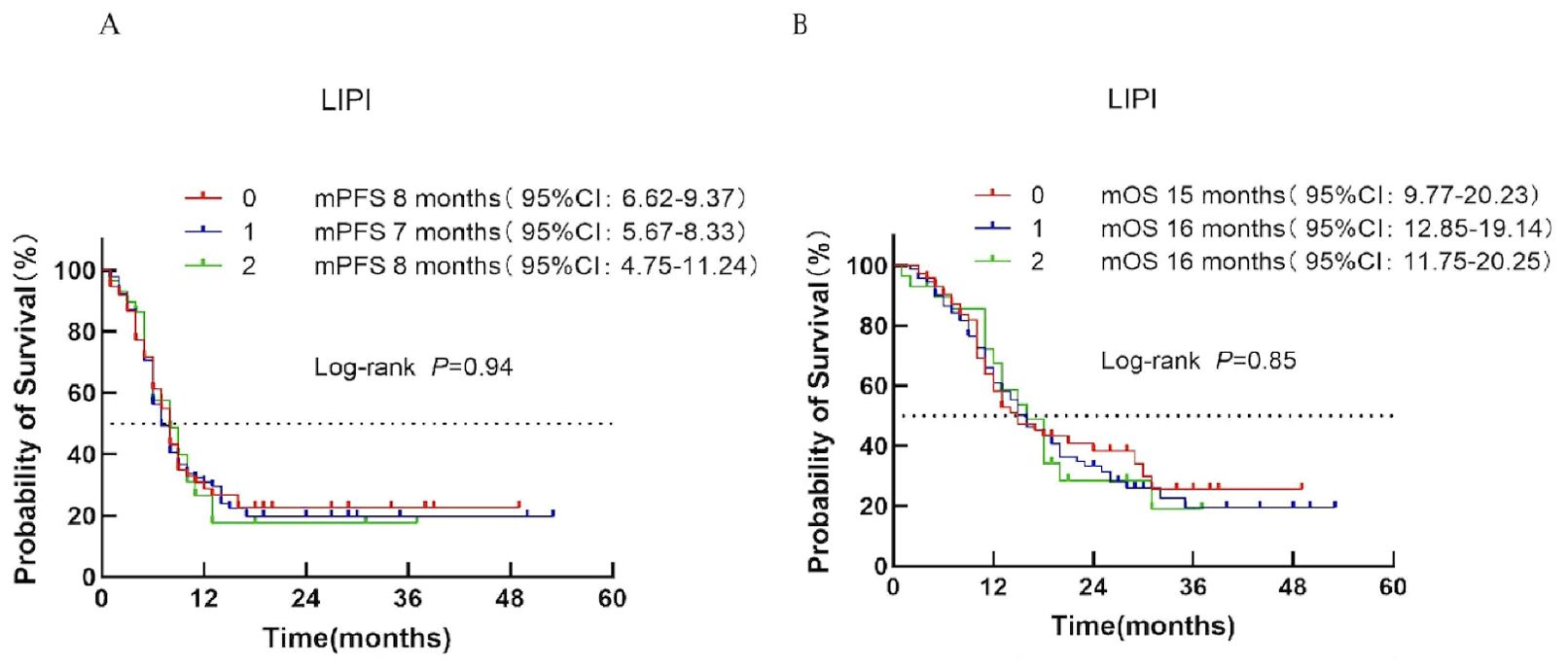
Figure 6. Survival analysis of the LIPI scoring system in ES-SCLC patients treated with first-line immunotherapy combination. (A) PFS. (B) OS.
3.4.6 GRIm score
Patients were divided into low-risk (score 0–1) and high-risk (score 2–3) categories according to the GRIm score. Median PFS was 8 months in both groups (P = 0.89; Figure 7A). Median OS was 15 months for the low-risk group and 18 months for the high-risk group (P = 0.87; Figure 7B), indicating no significant prognostic discrimination.
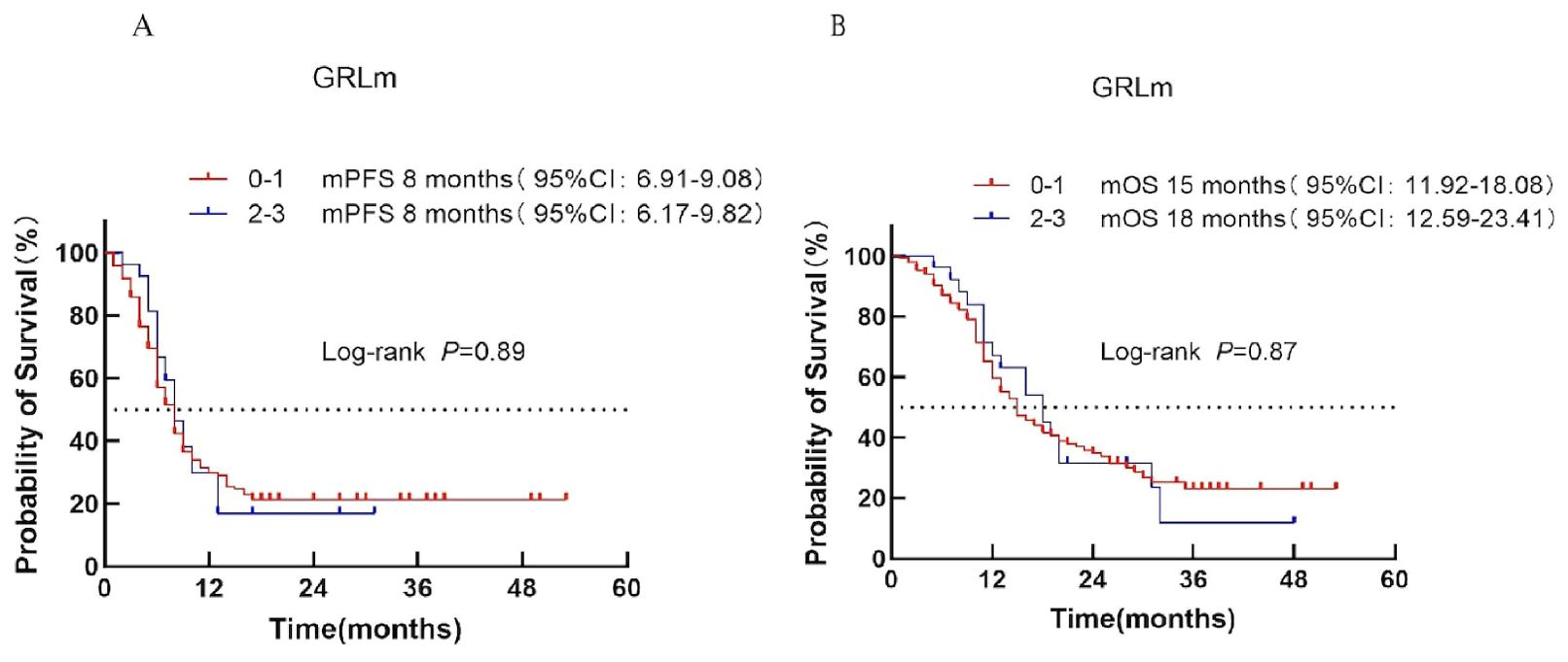
Figure 7. Survival analysis of the GRIm scoring system in ES-SCLC patients treated with first-line chemoimmunotherapy. (A) PFS. (B) OS.
As shown in Table 4, the comparative analysis of six prognostic scoring systems revealed that only the MDACC+NLR composite score provided significant prognostic separation for both PFS and OS. The MDACC score showed moderate prognostic relevance for OS, whereas the RMH, MDA-ICI, LIPI, and GRIm models failed to demonstrate significant survival discrimination. These findings indicate that the MDACC+NLR model may serve as a practical and reliable prognostic tool for risk stratification in real-world ES-SCLC patients receiving chemoimmunotherapy.
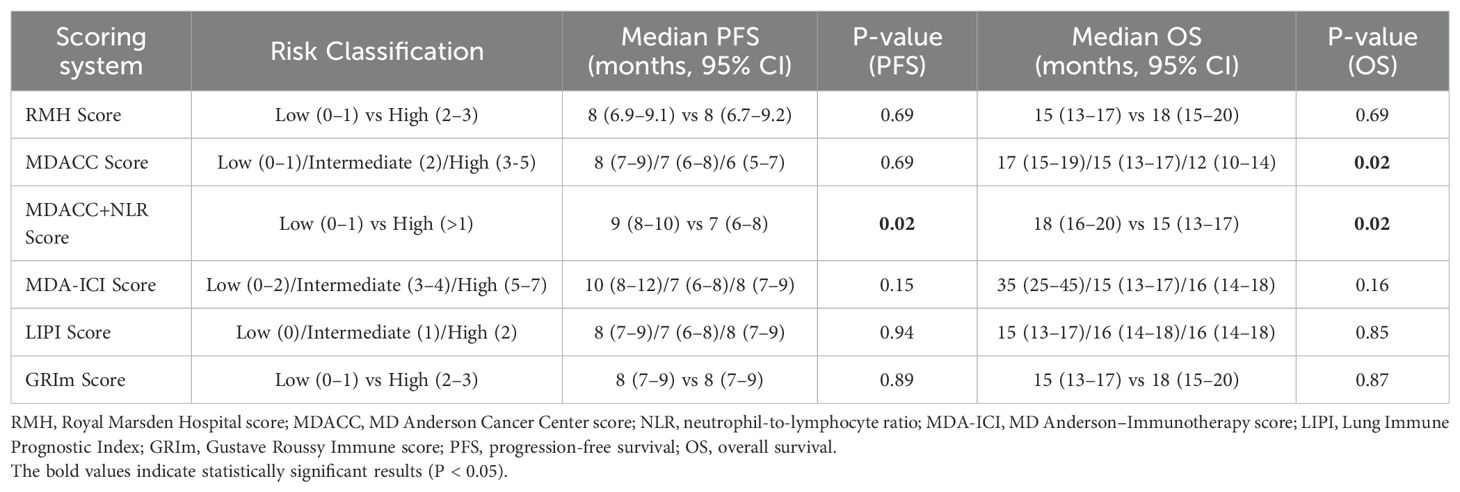
Table 4. Progression-free survival (PFS) and overall survival (OS) according to six prognostic scoring systems.
3.4.7 The predictive ability of MDACC+NLR scoring system
The MDACC + NLR–based Cox model demonstrated significant prognostic value for overall survival (OS) in patients with extensive-stage small-cell lung cancer (ES-SCLC) receiving first-line chemoimmunotherapy. The apparent Harrell’s concordance index (C-index) for OS was 0.62, and the bootstrap-corrected C-index was 0.61, indicating moderate discrimination. Calibration curves at 6 and 12 months showed good agreement between predicted and observed survival probabilities. The corresponding nomogram (Figure 8A) provides individualized estimates of 6- and 12-month OS probabilities according to the MDACC + NLR score. Decision curve analysis (DCA) (Figure 8B) revealed a positive net benefit across clinically relevant threshold probabilities of approximately 0.18–0.42, exceeding the “treat-all” and “treat-none” strategies. These findings confirm the satisfactory predictive accuracy and potential clinical utility of the MDACC + NLR model for prognostic assessment in ES-SCLC.
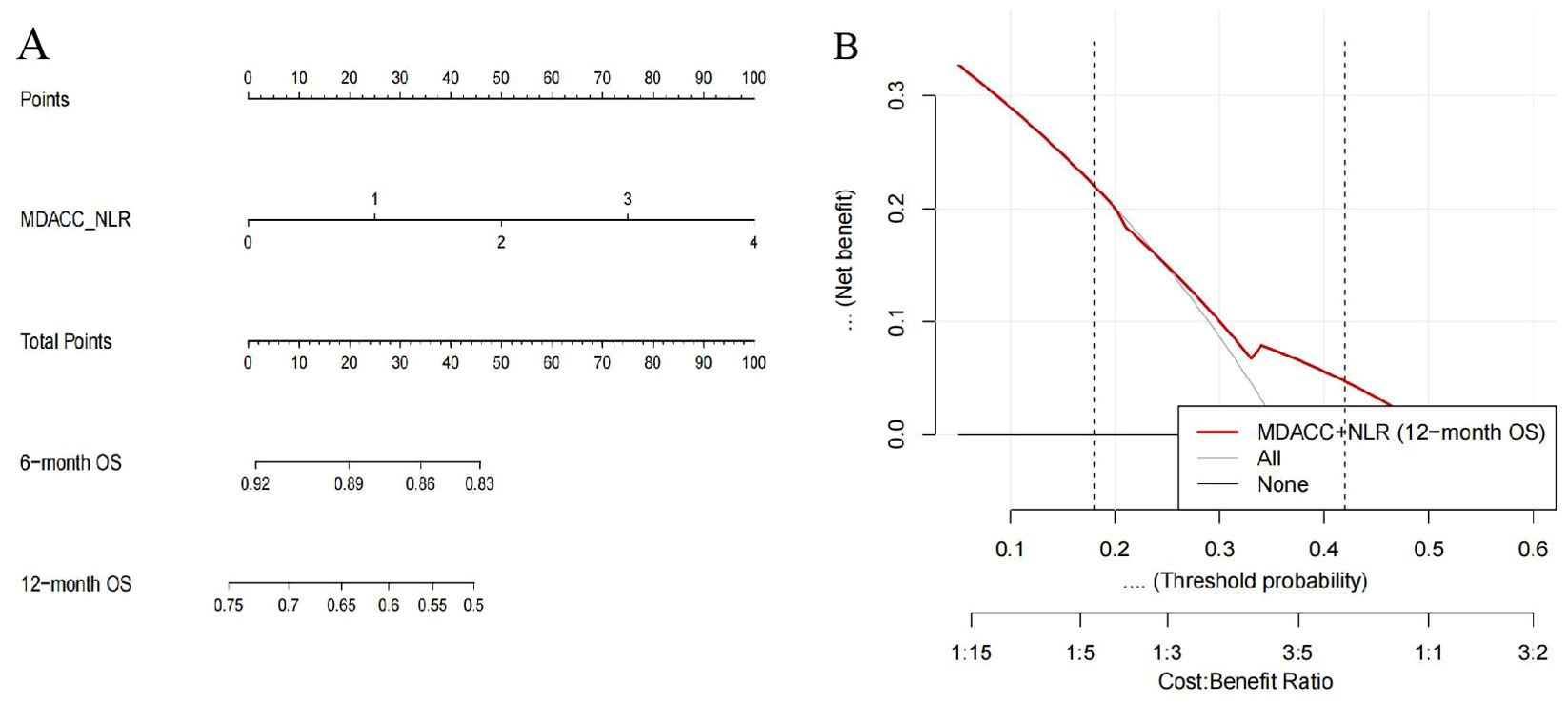
Figure 8. Nomogram and decision curve analysis (DCA) based on the MDACC+NLR scoring system for predicting overall survival (OS) in extensive-stage small-cell lung cancer (ES-SCLC) patients receiving first-line chemoimmunotherapy. (A) Nomogram developed from the MDACC+NLR score using a Cox proportional hazards model to predict 6- and 12-month OS. The upper “Points” axis assigns a score to each MDACC+NLR value; summing these yields the “Total Points,” which can be projected downward to estimate the predicted 6- and 12-month OS probabilities. The MDACC+NLR axis is displayed over a range of 0–4 for visualization, where 3–4 represent extrapolated ranges beyond the observed data (observed range = 0–2). (B) Decision curve analysis (DCA) evaluating the clinical net benefit of the MDACC+NLR-based model for 12-month OS. The red curve indicates the MDACC+NLR model, and the gray/black lines represent the reference “treat-all” and “treat-none” strategies. The model demonstrates a positive net benefit within threshold probabilities of approximately 0.18–0.42, supporting its potential clinical utility.
4 Discussion
4.1 Clinical implications
Small cell lung cancer (SCLC) is an aggressive malignancy classified as limited-stage (LS) or extensive-stage (ES), with ES-SCLC accounting for 70%–80% of cases and characterized by distant metastases and poor prognosis (17). Platinum-based chemotherapy has long been the first-line standard, achieving limited efficacy (median OS <12 months) (18). The advent of chemoimmunotherapy (CIT) has improved survival outcomes.
To date, few studies have systematically assessed immune–inflammation–based prognostic models such as NLR in SCLC. In this real-world cohort, the MDACC+NLR scoring system demonstrated the best predictive performance among all evaluated models. From a translational perspective, the MDACC+NLR model offers a pragmatic and easily applicable tool for risk stratification and individualized treatment planning in the immunotherapy era (19).
Although several prognostic models—such as LIPI, GRIm, RHM, and MDA-ICI—have shown prognostic value in NSCLC, none demonstrated significant discrimination in our ES-SCLC cohort (20–22). This discrepancy likely reflects the unique biological characteristics of SCLC, including rapid proliferation, genomic instability, and a neuroendocrine yet immunologically “cold” phenotype with low PD-L1 expression and limited T-cell infiltration. Consequently, inflammation-based indices originally derived from NSCLC fail to capture the distinct immune dysfunction of SCLC, and their risk cutoffs (e.g., for NLR or LDH) may not be directly applicable. These mechanistic and biological differences may underlie why only the MDACC+NLR model retained robust predictive performance in ES-SCLC (23).
Based on this framework, we further validated the prognostic performance of these models in our real-world ES-SCLC cohort (24). In our cohort, the MDACC+NLR model demonstrated the strongest predictive ability, with scores ≤1 associated with longer PFS and OS. The number of metastatic organs, also confirmed as an independent prognostic factor (25–27), may further enhance MDACC+NLR performance.
Landmark trials—IMpower133, CASPIAN, and KEYNOTE-604—have confirmed the survival benefit of CIT in ES-SCLC (10, 11, 18). IMpower133 demonstrated OS and PFS improvement with atezolizumab plus chemotherapy (11), CASPIAN verified similar results with durvalumab (10), and KEYNOTE-604 supported pembrolizumab in this setting (18). Consistent with these pivotal studies, our real-world analysis of 197 patients showed longer PFS (8 vs. 6 months; HR = 0.63; P < 0.01) and OS (15 vs. 13 months; HR = 0.71; P < 0.01) in the CIT group, confirming the effectiveness of CIT outside trial settings.
Multivariate Cox analysis identified ECOG ≥1, lung metastases, and liver metastases as independent adverse factors for both PFS and OS, consistent with prior evidence[23–25]. ECOG status reflects treatment tolerance and prognosis, while liver metastases are known to promote an immunosuppressive microenvironment impairing ICI efficacy. Although some HRs included 1 within their confidence intervals, these trends warrant validation in larger cohorts.
Effective prognostic tools should provide clear survival discrimination using accessible parameters. The MDACC+NLR system fulfills these criteria and represents a valuable, pragmatic model for prognosis and treatment planning. Previous studies have only explored MDACC+NLR in small or exploratory SCLC cohorts (19); our large-scale, real-world validation provides robust evidence to support its integration into future risk models and clinical practice.
4.2 Inflammation-based biomarkers
Peripheral blood biomarkers such as ANC, NLR, dNLR, PLT, and LDH are cost-effective indicators of systemic inflammation and immunotherapy response (28–31). Elevated levels correlate with poor outcomes, and recent studies confirm that high baseline inflammatory scores predict reduced response and shorter survival in SCLC patients receiving CIT (32). These easily accessible markers thus offer practical value for early risk stratification.
4.3 Biomarker perspective in SCLC
To date, the NCCN Clinical Practice Guidelines for small cell lung cancer (SCLC) have not recommended routine testing for PD-L1 expression or tumor mutational burden (TMB), due to insufficient evidence linking these biomarkers with clinical benefit from immunotherapy. Although PD-L1 and TMB are well-established predictive biomarkers in non-small cell lung cancer (NSCLC), their clinical utility in SCLC remains limited because of low PD-L1 expression, methodological heterogeneity, and restricted tissue availability.
In the CASPIAN trial, PD-L1 positivity was observed in approximately 5.7% of tumor cells and 25.8% of immune cells (28.3% in either), and durvalumab plus chemotherapy improved survival regardless of PD-L1 status (11). Similarly, the IMpower133 analysis showed that atezolizumab plus chemotherapy prolonged overall survival independently of PD-L1 and other biomarker subgroups (10). Consequently, the NCCN SCLC guideline does not currently recommend routine PD-L1 or TMB testing. These findings emphasize that, in contrast to molecularly driven strategies in NSCLC, SCLC still lacks validated biomarkers for immunotherapy selection—highlighting the rationale for exploring immune-inflammatory scoring model.
Similarly, tumor mutational burden (TMB) has been investigated as a potential biomarker for predicting the efficacy of immunotherapy in SCLC. The 2025 CSCO Clinical Practice Guidelines for Small Cell Lung Cancer indicate that TMB may serve as a predictive indicator of response to immune checkpoint inhibitors, and that NGS-based multigene panel testing represents a clinically feasible approach for estimating TMB (33). In the phase I/II CheckMate 032 trial, patients with high TMB who received nivolumab plus ipilimumab achieved an objective response rate (ORR) of 46.2% and a 1-year progression-free survival (PFS) rate of 30.0%, which were significantly higher than those in the low- and intermediate-TMB subgroups (34). Furthermore, when tumor tissue is insufficient, NGS-based circulating tumor DNA (ctDNA) testing is considered a promising alternative method for TMB evaluation (35, 36). Despite these encouraging results, TMB testing is currently classified as a Grade III recommendation, Level 2B evidence in the CSCO guidelines, and neither NCCN nor CSCO currently recommend routine TMB testing in SCLC. The predictive value of TMB in this disease still requires validation in larger prospective studies.
In our cohort, 197 patients were included, of whom only 11 had available PD-L1 testing data. Among these, 8 patients showed PD-L1 <1%, and 3 patients showed PD-L1 = 0%, consistent with previous studies reporting generally low PD-L1 expression in SCLC. In addition, TMB testing was not performed because comprehensive genomic profiling was not part of the standard diagnostic workflow during the study period. These findings align with current NCCN and CSCO recommendations, which do not support routine PD-L1 or TMB testing in SCLC.
Given these limitations, our study further highlights the clinical importance of developing immune–inflammation–based prognostic models, such as the MDACC+NLR scoring system, which utilize readily accessible clinical and hematological parameters. These models may provide a practical and cost-effective approach for prognostic stratification in real-world settings, complementing molecular biomarker research and bridging the gap between biological insight and clinical applicability.
4.4 Limitations and future directions
This study has several limitations. First, its retrospective, single-center design may introduce inherent selection bias. Second, the absence of an external validation cohort limits the generalizability of our findings. Third, molecular biomarkers such as PD-L1 expression and tumor mutational burden (TMB) were not routinely assessed, as these tests are not yet mandated by current clinical guidelines and remain relatively costly in real-world practice. Future prospective studies incorporating molecular and immune biomarkers are needed to confirm and expand these findings. Fourth, as this was a retrospective study, data on treatment-related adverse events (AEs) were incomplete, which precluded analysis of the potential association between AEs and prognostic scores. Future multicenter, prospective studies incorporating external validation, molecular biomarker analyses, and safety outcomes are warranted to confirm and extend our findings. Future multicenter prospective studies integrating molecular and immune biomarkers, external validation, and treatment toxicity analysis are warranted to refine and validate the MDACC+NLR model. In future work, we plan to further enlarge the dataset and integrate additional biological correlates to enhance the generalizability and mechanistic insight of our findings (37).
4.5 Conclusion
This study demonstrates that poor performance status (ECOG ≥1), lung metastasis, and liver metastasis are independent adverse prognostic factors for both progression-free survival (PFS) and overall survival (OS) in patients with extensive-stage small cell lung cancer (ES-SCLC) receiving first-line chemoimmunotherapy. Among the prognostic models evaluated, the MDACC+NLR scoring system exhibited superior predictive accuracy for treatment outcomes and survival, suggesting its potential utility as a valuable tool to guide personalized therapeutic strategies in ES-SCLC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Fourth Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin. This article is a retrospective study and has obtained ethical exemption.
Author contributions
DL: Writing – original draft, Writing – review & editing. XL: Writing – review & editing, Writing – original draft. NL: Writing – review & editing, Methodology, Writing – original draft. BW: Writing – original draft, Writing – review & editing. HJ: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing. XZ: Writing – original draft, Writing – review & editing. LW: Writing – original draft, Writing – review & editing. ZF: Writing – review & editing, Writing – original draft. LF: Writing – review & editing, Writing – original draft. JH: Writing – review & editing, Writing – original draft. JZ: Writing – review & editing, Writing – original draft. YW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Hebei Province (Grant No.H2025206698) and the Wu Jieping Medical Foundation (Approval No. 320.6750.2021-01-10).
Acknowledgments
The authors would like to thank all colleagues and patients who contributed to this study. We thank the editor and series editor for constructive criticisms of an earlier version of this chapter.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1681658/full#supplementary-material
References
1. Wu, et al. Prognostic factors in extensive-stage small cell lung cancer patients with organ-specific metastasis (2024). Available online at: https://link.springer.com/content/pdf/ (Accessed July 12, 2025).
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Solta A, Ernhofer B, Boettiger K, Megyesfalvi Z, Heeke S, Hoda MA, et al. Small cells - big issues: biological implications and preclinical advancements in small cell lung cancer. Mol Cancer. (2024) 23:41. doi: 10.1186/s12943-024-01953-9
4. Small cells - big issues biological implications and preclinical advancements in small cell lung (2024). Available online at: https://molecular-cancer.biomedcentral.com/counter/pdf/10.1186/s12943-024-01953–9 (Accessed October 17, 2025).
5. Leiter A, Veluswamy RR, and Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
6. Huang D, Wang J, Chen L, Jiang W, Inuzuka H, Simon DK, et al. Molecular subtypes and targeted therapeutic strategies in small cell lung cancer: advances, challenges, and future perspectives. Molecules. (2025) 30:1731. doi: 10.3390/molecules30081731
7. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. (2018) 4:351–7. doi: 10.1001/jamaoncol.2017.4771
8. Kim SY, Park HS, and Chiang AC. Small cell lung cancer: a review. JAMA. (2025) 333:1906–17. doi: 10.1001/jama.2025.0560
9. Xu T, Zhang H, Yang BB, Qadir J, Yuan H, and Ye T. Tumor-infiltrating immune cells state-implications for various breast cancer subtypes. Front Immunol. (2025) 16:1550003. doi: 10.3389/fimmu.2025.1550003
10. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
11. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
12. Maymani H, Hess K, Groisberg R, Hong DS, Naing A, Piha-Paul S, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer. (2018) 120:137–41. doi: 10.1016/j.lungcan.2018.03.020
13. Sen S, Hess K, Hong DS, Naing A, Piha-Paul S, Janku F, et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer. (2018) 118:763–9. doi: 10.1038/bjc.2017.480
14. Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the gustave roussy immune score (GRIm-score). Eur J Cancer (oxf Engl: 1990). (2017) 84:212–8. doi: 10.1016/j.ejca.2017.07.027
15. Tirelli U. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. (2008) 99:1364. doi: 10.1038/sj.bjc.6604648
16. Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, et al. Survival of 1,181 patients in a phase I clinic: the MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. (2012) 18:2922–9. doi: 10.1158/1078-0432.CCR-11-2217
17. Tao F, Zhu H, Xu J, Guo Y, Wang X, Shao L, et al. Prognostic value of PAX8 in small cell lung cancer. Heliyon. (2024) 10:e28251. doi: 10.1016/j.heliyon.2024.e28251
18. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. (2020) 38:2369–79. doi: 10.1200/JCO.20.00793
19. Mu Q, Jing Y, Ding Y, Wang J, Zhang H, Jiang Y, et al. Comprehensive prognostic model for immunotherapy in small cell lung cancer: a multi-center study integrating clinical and blood biomarkers. Front Oncol. (2025) 15:1680624. doi: 10.3389/fonc.2025.1680624
20. Blagden SP, Charman SC, Sharples LD, Magee LRA, and Gilligan D. Performance status score: do patients and their oncologists agree? Br J Cancer. (2003) 89:1022–7. doi: 10.1038/sj.bjc.6601231
21. Chow R, Bruera E, Temel JS, Krishnan M, Im J, and Lock M. Inter-rater reliability in performance status assessment among healthcare professionals: an updated systematic review and meta-analysis. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. (2020) 28:2071–8. doi: 10.1007/s00520-019-05261-7
22. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. (2017) 5:417–24. doi: 10.1158/2326-6066.CIR-16-0325
23. Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. (2019) 19:289–97. doi: 10.1038/s41568-019-0133-9
24. Gao Y, Zhang L, Yan M, Sun Z, Zhao H, and Zhao L. Development of a prognostic model for patients with extensive-stage small cell lung cancer undergoing immunotherapy and chemotherapy. Front Immunol. (2025) 16:1561333. doi: 10.3389/fimmu.2025.1561333
25. Uzel Şener M, Akın Kabalak P, Kavurgacı S, Yılmaz Demirci N, Kızılgöz D, Yanık F, et al. Different approach to M descriptor for future staging of oligometastatic disease in SCLC: a cross-sectional survival analysis. Clin Transl Oncol: Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. (2025) 27:2750–60. doi: 10.1007/s12094-024-03778-w
26. Wu Y, Zhang J, Zhou W, Yuan Z, and Wang H. Prognostic factors in extensive-stage small cell lung cancer patients with organ-specific metastasis: unveiling commonalities and disparities. J Cancer Res Clin Oncol. (2024) 150:74. doi: 10.1007/s00432-024-05621-9
27. Bauckneht M, Genova C, Rossi G, Rijavec E, Dal Bello MG, Ferrarazzo G, et al. The role of the immune metabolic prognostic index in patients with non-small cell lung cancer (NSCLC) in radiological progression during treatment with nivolumab. Cancers. (2021) 13:3117. doi: 10.3390/cancers13133117
28. Stares M, Swan A, Cumming K, Ding T-E, Leach J, Stratton C, et al. Hypoalbuminaemia as a prognostic biomarker of first-line treatment resistance in metastatic non-small cell lung cancer. Front Nutr. (2021) 8:734735. doi: 10.3389/fnut.2021.734735
29. Huai Q, Luo C, Song P, Bie F, Bai G, Li Y, et al. Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. (2023) 114:4484–98. doi: 10.1111/cas.15964
30. Kuang Z, Miao J, and Zhang X. Serum albumin and derived neutrophil-to-lymphocyte ratio are potential predictive biomarkers for immune checkpoint inhibitors in small cell lung cancer. Front Immunol. (2024) 15:1327449. doi: 10.3389/fimmu.2024.1327449
31. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother: CII. (2020) 69:1813–22. doi: 10.1007/s00262-020-02585-w
32. Chen T, Wang M, Chen Y, Cao Y, and Liu Y. Advances in predictive biomarkers associated with immunotherapy in extensive-stage small cell lung cancer. Cell Biosci. (2024) 14:117. doi: 10.1186/s13578-024-01283-9
33. Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
34. Pietanza MC, Kadota K, Huberman K, Sima CS, Fiore JJ, Sumner DK, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res: Off J Am Assoc Cancer Res. (2012) 18:1138–45. doi: 10.1158/1078-0432.CCR-11-2059
35. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. (2019) 5:696–702. doi: 10.1001/jamaoncol.2018.7098
36. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. (2018) 24:1441–8. doi: 10.1038/s41591-018-0134-3
Keywords: extensive-stage small cell lung cancer (ES-SCLC), chemoimmunotherapy, prognostic scoring model, MDACC-NLR, survival prediction
Citation: Li D, Li X, Liu N, Wang B, Jin H, Liu Y, Liu J, Zhang X, Wang L, Fan Z, Feng L, Han J, Zuo J and Wang Y (2025) Prognostic value of the MDACC–NLR score in extensive-stage small-cell lung cancer treated with first-line chemoimmunotherapy. Front. Immunol. 16:1681658. doi: 10.3389/fimmu.2025.1681658
Received: 07 August 2025; Accepted: 23 October 2025;
Published: 07 November 2025.
Edited by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilReviewed by:
Ting Ye, Southwest Medical University, ChinaAli Zarezadeh Mehrabadi, Tehran University of Medical Sciences, Iran
Wei Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2025 Li, Li, Liu, Wang, Jin, Liu, Liu, Zhang, Wang, Fan, Feng, Han, Zuo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yudong Wang, d3lkXzk5OUBoZWJtdS5lZHUuY24=
†These authors share first authorship
 Dan Li†
Dan Li† Yudong Wang
Yudong Wang