- 1Translational Research Unit, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 2Clinical Epidemiology Unit, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 3Department of Neurosciences, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy
- 4Unità Operativa Semplice (UOS) Professioni Sanitarie Tecniche, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 5Cellular Immunology and Pharmacology Laboratory, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 6Laboratory of Virology, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 7U.O.C. Risk Management, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 8Center for Vaccine Innovation, La Jolla Institute for Immunology (LJI), La Jolla, CA, United States
- 9Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California San Diego (UCSD), La Jolla, CA, United States
- 10Clinical Division of Infectious Diseases, National Institute for Infectious Diseases “Lazzaro Spallanzani” Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
Background: The COVID-19 pandemic highlighted challenges in managing patients with multiple sclerosis (PwMS), as disease-modifying therapies (DMTs) can interfere with immune responses to infections and vaccines.
Objective: This study investigates the spike-specific T-cell response after the third dose of mRNA COVID-19 vaccines in PwMS undergoing DMTs, evaluating different cytokines, beyond IFN-γ, and exploring their potential association with SARS-CoV-2 breakthrough infections (BI).
Methods: We prospectively enrolled 31 PwMS and 27 healthcare workers (HCWs). The spike-specific T-cell response was evaluated by measuring Th1 cytokines (IFN-γ, IL-2, TNF-α) and IP-10 using an easy-to-use whole-blood assay.
Results: Most PwMS mounted a Wuhan spike-specific T-cell response by releasing Th1 cytokines (IFN-γ, IL-2, TNF-α) and IP-10, albeit with significantly reduced Th1 cytokine levels compared to HCWs. Fingolimod-treated patients showed the weakest response with significantly reduced IFN-γ and IL-2 levels compared to HCWs (both p<0.0001), as well as to ocrelizumab (p=0.0018 and p=0.0002, respectively) and cladribine/IFN-β-treated patients (p=0.041 and p<0.0001, respectively). Moreover, a cell-mediated response was observed against the Delta spike variant, and all cytokines correlated with each other. BI occurred in 38.7% of PwMS, with predominantly mild COVID-19 cases. Male sex (IRR: 4.05, p=0.017) and primary progressive MS (IRR: 3.65, p=0.052) were associated with a higher BI incidence rate. Spike-specific T-cell response did not associate with a higher protection against BI.
Conclusions: This study provides an in-depth immunological characterization of the spike-specific T-cell response in PwMS under DMTs, evaluating immunological biomarkers whose relevance may extend beyond COVID-19 for studying immune responses to other infections and vaccinations.
1 Introduction
Multiple sclerosis (MS) is an immune-mediated disease that affects the central nervous system, causing demyelination (1). A major breakthrough in the management of MS has been the advent of disease-modifying therapies (DMTs), such as ocrelizumab, fingolimod, cladribine, and interferon (IFN)-β. These therapies target the immune system at different levels, thus potentially compromising the immune response to both infections and vaccinations (2–4).
Consequently, during COronaVIrus Disease 2019 (COVID-19) pandemic, the management of patients with MS (PwMS) raised significant concerns (5). Key issues included the potential increased susceptibility to SARS-CoV-2 infection and the risk of severe COVID-19 outcomes, which vary depending on the DMT used (6, 7). In particular, a more severe outcome of COVID-19 has been reported in PwMS treated with anti-CD20 therapies (8, 9).
The vaccination campaign launched in early 2021 was an effective measure to mitigate the public health impact of COVID-19 by reducing the severity of the disease and hospitalization rates (10–12). Although vaccination against COVID-19 has proven effective, breakthrough infections (BI) have continued to occur due to the progressive weakening of vaccine-induced immunity (13–16) and the emergence of SARS-CoV-2 variants, likely favored by the viral replication in immunocompromised subjects, who are more susceptible to developing persistent infections (17).
However, vaccination continues to be the primary defense against COVID-19 in vulnerable individuals such as PwMS. Several studies, including ours, have demonstrated the immunogenicity of anti-SARS-CoV-2 vaccines in both healthy individuals (18–21) and PwMS (13, 22–27). The collective evidence indicates that most PwMS develop humoral and/or IFN-γ-specific T-cell responses to SARS-CoV-2 spike peptides. Nevertheless, the overall magnitude of these immune responses is reduced in PwMS compared with healthy individuals, and varies depending on the DMTs administered (13, 28–30). Specifically, fingolimod, a sphingosine-1 phosphate receptor modulator, predominantly impairs IFN-γ-specific T-cell response (31–33), while the B-cell-depleting anti-CD20 monoclonal antibody, ocrelizumab, is mostly associated with reduced anti-receptor binding domain (RBD) and neutralizing antibody production after COVID-19 vaccination (34–37). Moreover, the presence of both antibody and cell-mediated immune responses has been associated with a more rapid swab negativization in PwMS; indeed fingolimod-treated patients, who have a compromised IFN-γ-specific T-cell response, tend to require more time to achieve swab-negative status (38).
These results underscore the importance of studying the immune response to SARS-CoV-2 in PwMS, with a focus on the T-cell response (39, 40). In particular, T helper 1 (Th1) lymphocytes are known to play a fundamental role in the immune response against viral infections through the release of key cytokines such as IFN-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α, also known as Th1 cytokines (41, 42). IP-10/CXCL-10 (Interferon gamma-induced protein 10) is a chemokine induced by IFN-γ, which plays a pivotal role in the activation and chemoattraction of immune cells, especially T cells, to the sites of inflammation (43).
To date, most studies have primarily assessed the spike-specific T-cell response in terms of IFN-γ production (13, 18, 32, 36, 44), whereas some studies have evaluated the functional spike-specific CD4+ and CD8+ T cell responses by flow cytometry in PwMS after three doses of SARS-CoV-2 vaccines (22, 45–47). To the best of our knowledge, only one study conducted by Al Rahbani (48) evaluated, beyond IFN-γ, the SARS-CoV-2-specific immune cytokine profile in plasma supernatant of PwMS. However, this analysis did not compare the cytokine response with healthy controls nor correlate it with the risk of BI (48).
Our study aims to investigate additional biomarkers for a more complete evaluation of the cell-mediated immune response to COVID-19 vaccination. This study specifically examined the spike-specific immune response following a third dose of mRNA COVID-19 vaccines by assessing Th1 cytokines (IFN-γ, IL-2, and TNF-α) and IP-10 production using an easy-to-use whole-blood assay in PwMS undergoing various DMTs. Additionally, these responses were compared to those observed in healthcare workers (HCWs), alongside an evaluation of the risk of BI. Moreover, demographic and clinical variables were evaluated for their potential impact on cytokine production.
2 Materials and methods
2.1 Study cohort
This prospective, longitudinal, single-center study included a cohort of patients diagnosed with MS (PwMS) according to the 2017 revisions of McDonald criteria (49), along with an age- and sex-matched control group of healthcare workers (HCWs).
PwMS were recruited from patients regularly followed at the outpatient clinic at the MS Centre of the Department of Neurosciences of San Camillo Forlanini Hospital (Rome, Italy). Eligibility criteria included receiving three doses of COVID-19 mRNA vaccines (BNT162b2 or mRNA-1273) and ongoing treatment with ocrelizumab, fingolimod, cladribine, or IFN-β.
Healthy controls were recruited among HCWs without immune-suppressive conditions who had received three doses of COVID-19 mRNA vaccines at the National Institute for Infectious Diseases (INMI) – Lazzaro Spallanzani (Rome, Italy).
Enrolled PwMS were followed until either a confirmed SARS-CoV-2 infection or the administration of the fourth vaccine dose. Confirmed SARS-CoV-2 BI were classified by severity as mild, moderate, or severe (50). The enrolment began in March 2021 and was completed with the conclusion of the follow-up in December 2022.
Exclusion criteria for both cohorts included HIV infection, age below 18 years, and prior SARS-CoV-2 infection, defined by a positive antigenic or molecular test and/or detectable anti-nucleoprotein antibodies (anti-N IgG) at baseline.
The study was approved by the Ethical Committee of National Institute of Infectious Diseases “L. Spallanzani” (INMI)-IRCCS (approval numbers 319/2021, 443/2021, 297/2021) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants signed a written informed consent before their inclusion in the study.
2.2 Sample collection
Blood samples from PwMS were collected in BD Vacutainer tubes containing lithium heparin (Becton Dickinson, Florence, Italy, Cat. 367526) 4–6 weeks after the third dose of COVID-19 mRNA vaccines. Samples obtained at the MS Center of San Camillo Forlanini Hospital were transported to INMI and processed within 2 hours of collection. Blood samples from both PwMS and HCWs were handled according to a standardized protocol routinely used (13, 23, 51).
2.3 Antibody testing
The enrolled cohort was screened for prior SARS-CoV-2 infection by assessing anti-N-IgG as per the manufacturer’s instructions (Architect® i2000sr, Abbott Diagnostics, Chicago, IL, USA). Anti-N IgG were considered positive when index values, calculated as the ratio of sample (S) to cut-off (CO), were ≥1.4. The anti-SARS-CoV-2 antibody response to COVID-19 vaccination was assessed in terms of anti-receptor-binding domain (RBD) antibodies (anti-RBD Abs) and neutralizing ones. Anti-RBD IgG levels, expressed as binding antibody units (BAU)/mL, were measured according to the manufacturer’s instructions (Architect® i2000sr, Abbott Diagnostics, Chicago, IL, USA), and identify a positive response when ≥7.1 BAU/mL. Neutralising antibodies were assessed by the micro-neutralization assay previously reported (52), using the SARS-CoV-2/Human/ITA/PAVIA10734/2020 (provided by Fausto Baldanti, Pavia, Italy). A neutralizing titre ≥10, corresponding to the first dilution tested, was considered positive.
2.4 Spike-specific cell response
A whole blood assay was used to assess the spike-specific T-cell response. Specifically, 600 µL of blood was stimulated in a 48-well plate with peptide pools covering the spike protein sequence derived from SARS-CoV-2 Wuhan-Hu-1 (Wuhan spike) and the Delta variant (Delta spike). The Wuhan spike pool consisted of equal amounts of three peptide pools (PepTivator® Prot_S1, Prot_S, and Prot_S+, Miltenyi Biotec, Bergisch Gladbach, Germany, Cat. 130–127–048, Cat. 130–126–701, and Cat. 130–127–312) used at a final concentration of 0.1 µg/mL. The Delta spike pool, consisting of overlapping 15-mer peptides, was designed based on the GISAID ID: EPI_ISL_2020950, and used at 0.1 μg/mL. To verify the immunocompetence of the enrolled subjects, Staphylococcal enterotoxin B (SEB) (Merck Life Science, Milan, Italy, Cat. S4881) was used at 200 ng/mL. Following overnight incubation, the stimulated plasma was collected and stored at -80 °C until further analysis. Th1 cytokines (IFN-γ, TNF-α, IL-2) and IP-10 were quantified using the ELLA Simple Plex Human Assay (Bio-Techne, Minneapolis, MN, USA, Cat. SPCKC-PS-003978 customized kit). The detection limits for IP-10, IFN-γ, IL-2 and TNF-α were 0.60 pg/mL, 0.17 pg/mL, 0.54 pg/mL, and 0.3 pg/mL, respectively. Data were reported after subtracting the unstimulated value.
2.5 Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 8, Dotmatics, Boston, MA 02110) and Stata (StataCorp. 2021. Stata Statistical Software: Release 17. TX: StataCorp LLC, College Station, TX, USA). Categorical variables were reported as absolute values and relative percentages, whereas the continuous ones were expressed as medians and interquartile ranges (IQR). Mann-Whitney U and Kruskal-Wallis tests, followed by Dunn’s multiple comparisons test, were performed for pairwise and multiple comparisons, respectively. Wilcoxon signed-rank test was used to analyze paired data. For categorical variables, the Fisher’s Exact test was used. Correlations among immunological parameters were assessed by the non-parametric Spearman’s rank test (ρ coefficient).
To account for potential demographic and clinical confounders of cytokine production, a quantile regression analysis was performed. In the analysis, dependent variables (i.e., IFN-γ, IL-2, TNF-α and IP-10) and the following covariates were included: age, sex, body mass index (BMI), type of DMT, disease and treatment duration, lymphocyte count, Expanded Disability Status Scale (EDSS) score, presence of comorbidities, MS disease phenotype, and time from the third vaccine dose to sample collection. Covariates with p < 0.05 were entered in the stepwise regression model to identify the most influential factors.
To determine the incidence rate ratio (IRR) of SARS-CoV-2 infection based on demographic, clinical, and immunological parameters, an univariable Poisson regression model was applied. Two-tailed p values were considered statistically significant if <0.05.
3 Results
3.1 Characteristics of the enrolled cohort
From established cohorts of 167 HCWs and 134 PwMS previously evaluated for immune response to COVID-19 vaccination in related studies (13, 23, 38, 53), a subgroup of 27 HCWs and 31 PwMS, who completed blood sampling at all established time points, was selected for an in-depth analysis of the immunological response 4–6 weeks after the third vaccine dose (Figure 1).
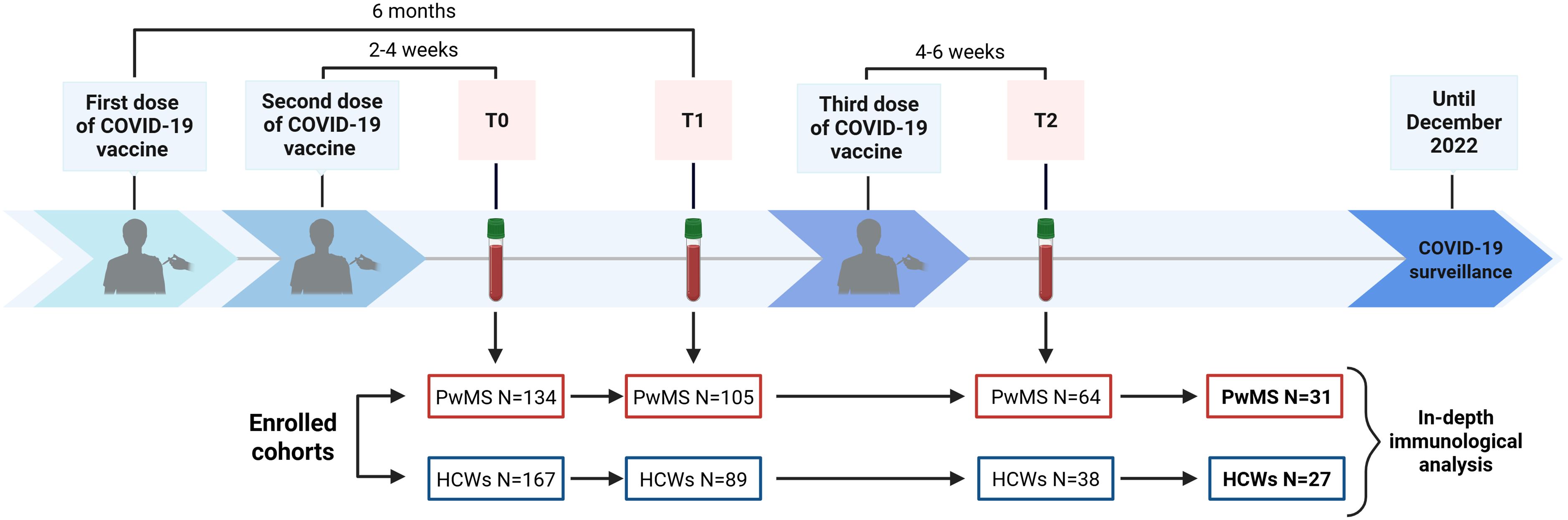
Figure 1. Timeline of COVID-19 vaccination and blood sample collection. The flow chart displays the enrolment and sample collection at T0 (2–4 weeks after the second dose), T1 (6 months after the first dose) and T2 (4–6 weeks after the third dose), excluding subjects lost to follow-up. Out of 64 PwMS and 38 HCWs who completed blood sampling at all designated time points, a convenience sample consisting of 31 PwMS and 27 HCWs was selected for an in-depth immunological characterization. COVID-19, COronaVIrus Disease 2019; HCWs, healthcare workers; PwMS, patients with multiple sclerosis. Created in https://BioRender.com.
The characteristics of the study cohort are described in Table 1. The two groups were matched for age and sex; both cohorts showed a female predominance exceeding 75%, reflecting the approximately 3:1 female-to-male ratio in MS, and the higher proportion of women among HCWs in Italy. Among the enrolled PwMS, 10 (32.2%) subjects were treated with ocrelizumab, 13 (41.9%) with fingolimod, 3 (9.7%) with cladribine, and 5 (16.1%) with IFN-β. Most PwMS (87.1%) had a relapsing-remitting MS, and the median duration of the disease was 16 years. The median MS treatment duration at the sample collection was 1.8 years (IQR: 1.0–2.3) for ocrelizumab, 7.4 years (IQR: 5.1–8.5) for fingolimod, 1.6 years (IQR: 0.8–1.9) for cladribine and 9.5 years (IQR: 7.6–18.7) for IFN-β. Only a few subjects reported comorbidities such as cardiovascular and metabolic diseases. Lymphocyte counts significantly differed among DMTs (p=0.003), with fingolimod-treated patients showing the lowest counts. Regarding the other blood cell subsets, no significant differences were found among the different DMTs as reported in Table 1. The median time from the third vaccine dose to the blood sample collection in PwMS was 48 days (IQR 43-51), with no differences among treatment subgroups.
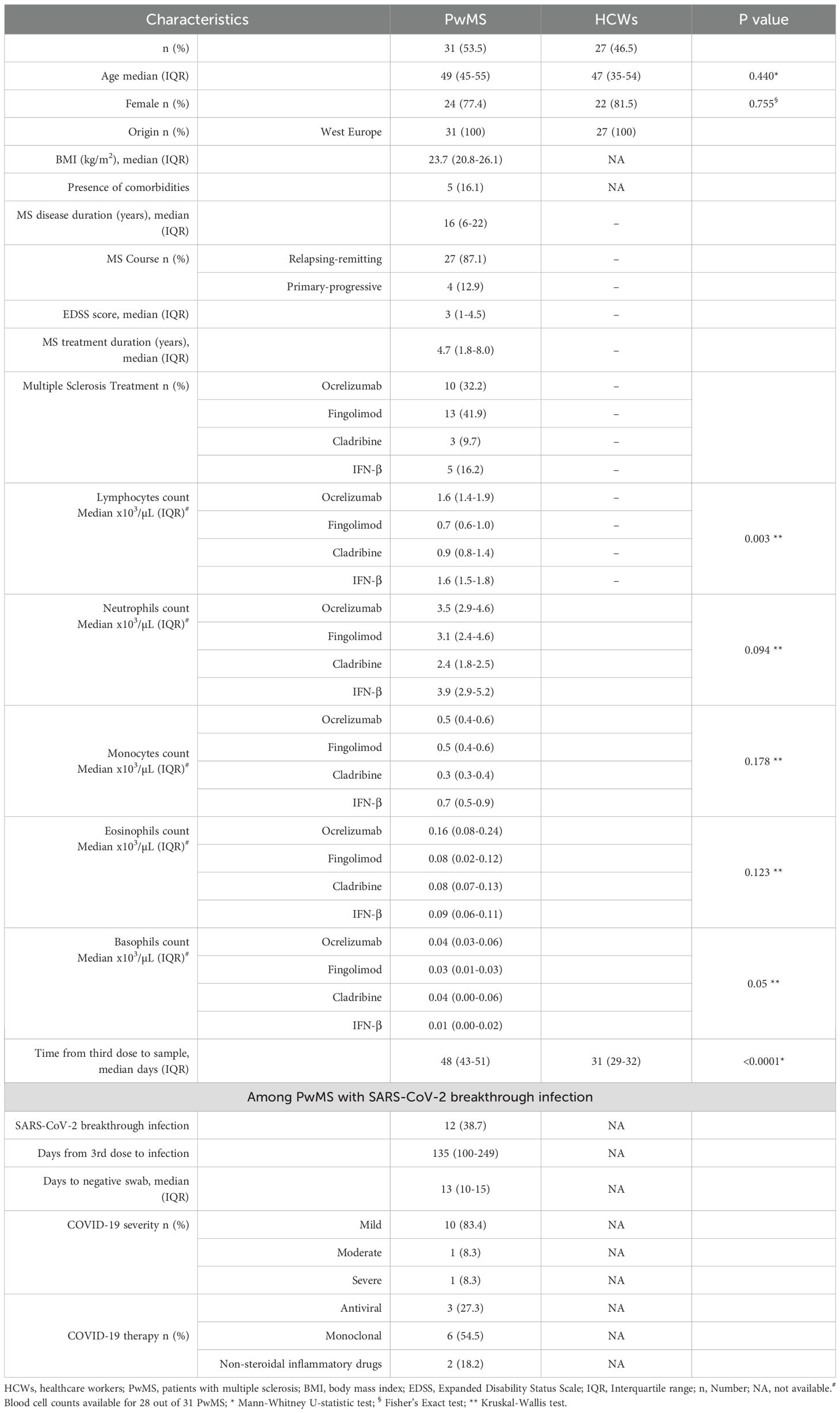
Table 1. Demographic and clinical characteristics of the 58 subjects enrolled after the third dose of COVID-19 vaccination.
3.2 Profile of cytokines induced by SARS-CoV-2 vaccination
The spike-specific cell response was assessed by measuring Th1 cytokines (IFN-γ, IL-2, and TNF-α) as well as IP-10, an IFN-γ-induced protein, in response to the Wuhan spike peptides. All HCWs responded to Wuhan spike stimulation by producing IFN-γ, IL-2 and TNF-α, while only 25/27 (92.6%) HCWs released IP-10 (Figure 2A). Compared with HCWs, PwMS showed a significantly reduced cytokine response to Wuhan spike peptides with a 6-fold decreased IFN-γ level (HCWs median: 525 pg/mL, IQR: 226–852 vs. PwMS median: 86 pg/mL, IQR: 1.5-476.5, p<0.0001) and TNF-α production (HCWs median: 98 pg/mL, IQR: 44.6–270 vs. PwMS median: 15.4 pg/mL, IQR: 3.7-38.7, p<0.0001), and an approximately 3-fold reduction in IL-2 production (HCWs median: 234 pg/mL, IQR: 96–447 vs. PwMS median: 81 pg/mL, IQR: 3.3-325, p=0.042). By contrast, no significant differences were observed for IP-10, between the two groups (Figure 2A).
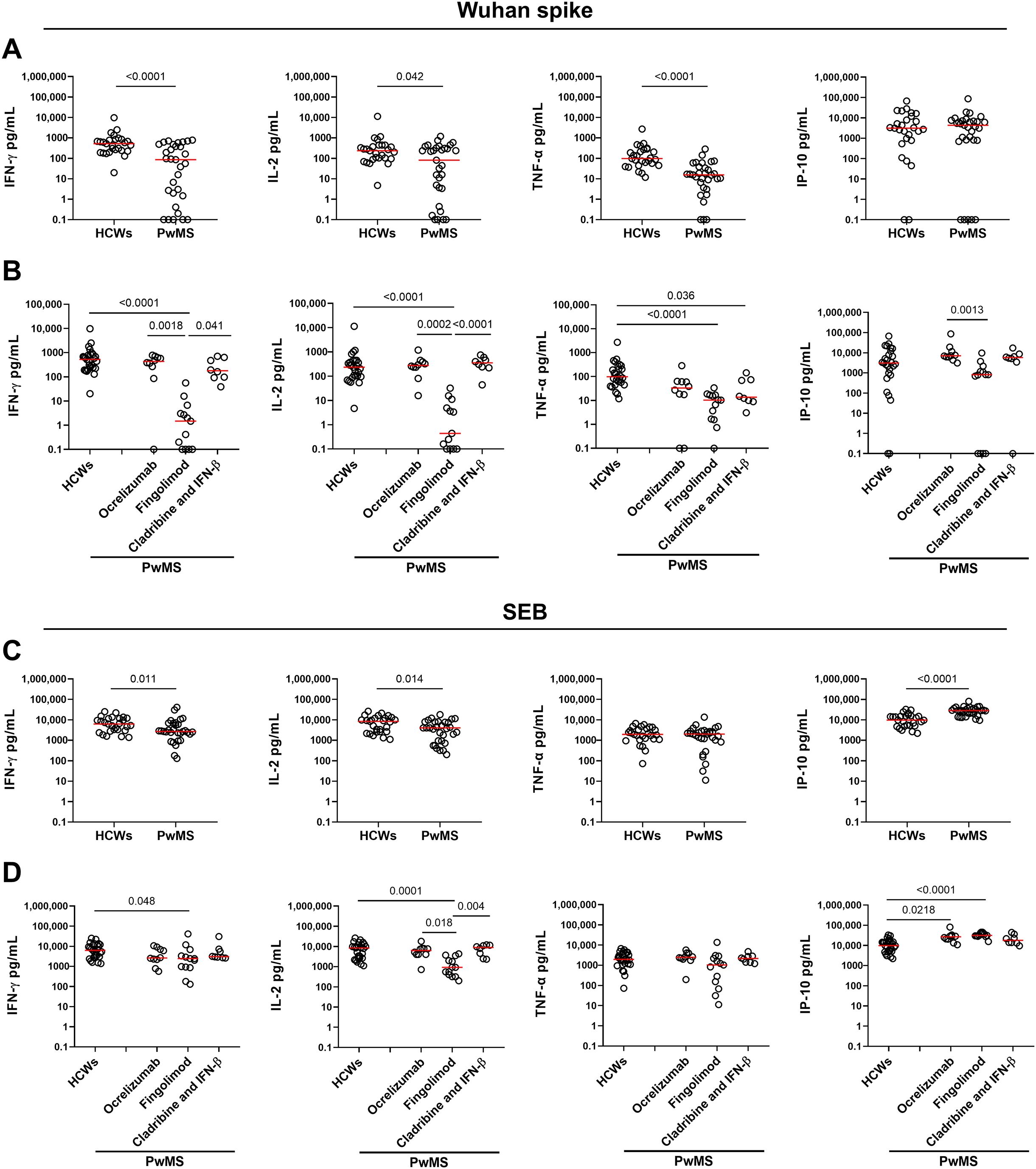
Figure 2. Cytokine/chemokine response to SARS-CoV-2 Wuhan spike peptides and SEB in HCWs and PwMS after 4–6 weeks from the third vaccine dose. Comparison of the cytokine response to Wuhan spike (A, B) and SEB (C, D) between HCWs and PwMS. (B, D) PwMS were stratified by the current therapy into three groups: ocrelizumab (n=10), fingolimod (n=13) and cladribine/IFN-β (n=8). IFN-γ, IL-2, TNF-α and IP-10 concentrations were expressed in pg/mL with median values indicated by red lines. For the statistical analysis, Mann-Whitney U test was performed to compare HCWs and PwMS (A, C), while for the comparison among groups the Kruskal-Wallis test followed by the Dunn’s multiple comparisons test was used (B, D). Differences with p values < 0.05 were considered significant. HCWs, healthcare workers; PwMS, patients with multiple sclerosis; SEB, Staphylococcal enterotoxin B; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; IP-10, interferon gamma-induced protein 10.
Although most PwMS showed a T-cell specific response, the quantitative response to Wuhan spike significantly varied among DMTs. In particular, patients receiving fingolimod showed an impaired immune response characterized by a significantly reduced production of both IFN-γ, IL-2, and TNF-α compared to HCWs (p<0.0001 for all Th1 cytokines) (Figure 2B). The significant differences in IFN-γ and IL-2 production observed between PwMS treated with fingolimod and HCWs persisted even after adjusting for the time interval between the third vaccine dose and sample collection (Table 2).
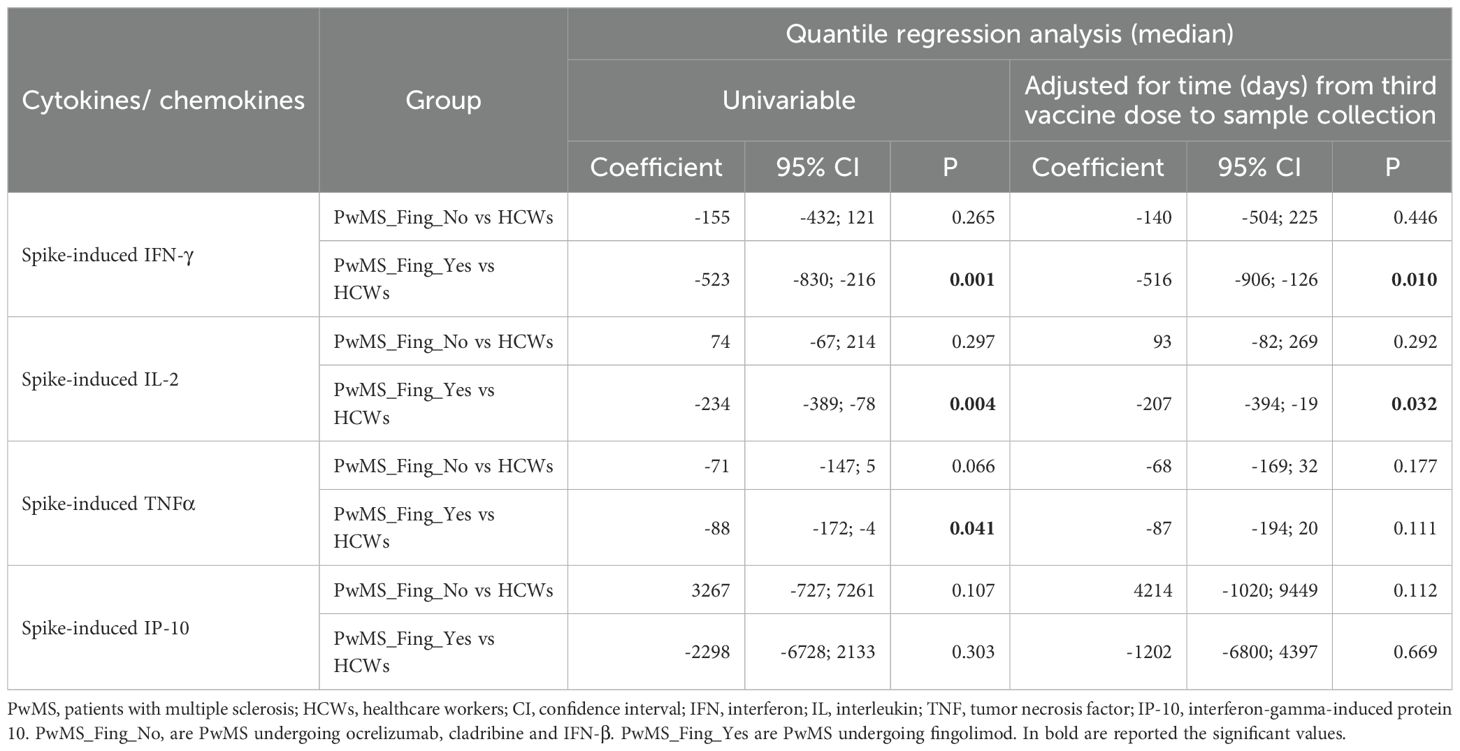
Table 2. Comparison between PwMS and HCWs for each cytokine/chemokine in response to the in vitro specific stimulation with Wuhan spike-peptides after the third dose of COVID-19 vaccination.
Within the PwMS cohort, fingolimod-treated patients exhibited significantly lower levels of IFN-γ and IL-2 compared to patients treated with ocrelizumab (p=0.0018 and p=0.0002, respectively) or cladribine/IFN-β (p=0.041 and p<0.0001, respectively). Moreover, a significant difference was observed in the IP-10 production between patients receiving ocrelizumab and fingolimod (p=0.0013). In contrast, no significant differences were reported for TNF-α production among PwMS stratified based on DMTs (Figure 2B).
To evaluate the impact of demographic and clinical variables on spike-specific responses in PwMS, a quantile regression analysis was conducted. While various covariates were initially identified as potential influencers depending on the cytokine or chemokine assessed, only some variables remained significant following stepwise regression (Table 3). The differences in Th1 cytokine and IP-10 levels among patients treated with fingolimod, as compared to those receiving alternative therapies, were validated through stepwise regression analysis (Table 3). The MS treatment emerged as the main variable that explained the differences observed in the MS cohort for IFN-γ, IL-2 and IP-10, (p=0.062, p<0.001, p<0.001, respectively). Moreover, the gender was identified as a variable affecting the specific-immune response, with male patients showing higher levels of IFN-γ and TNF-α (p=0.003 and p=0.010) (Table 3). Instead, the duration of MS treatment did not appear to influence the cytokine response.
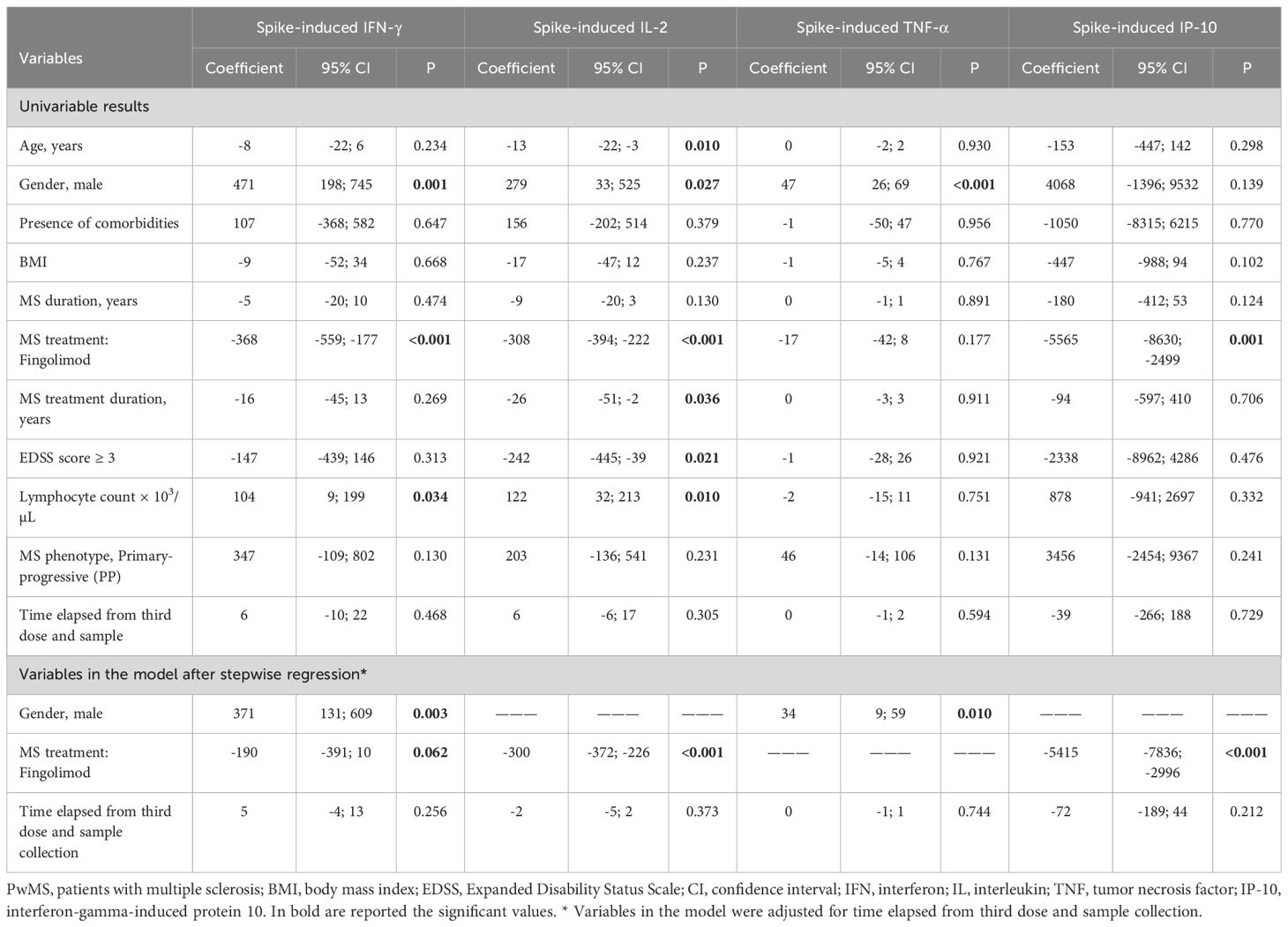
Table 3. Quantile and stepwise regression models for demographic and clinical factors affecting the Wuhan spike-specific immune response after the third dose of COVID-19 vaccination.
To assess the immune competence of the enrolled subjects, we analyzed the cytokine production in response to SEB, a non-specific stimulus used as a positive control (Figures 2C, D). All HCWs and PwMS demonstrated a robust response to SEB, thus confirming their preserved immune functionality (Figure 2C). However, significant differences were observed in the magnitude of IFN-γ, IL-2, and IP-10 production, thus indicating a dysregulated cytokine response in PwMS compared to HCWs. Particularly, PwMS showed a 2-fold reduction in IFN-γ (HCWs median: 6212 pg/mL, IQR: 2995–11663 vs. PwMS median: 2704 pg/mL, IQR: 2001-6830, p=0.011) and IL-2 production (HCWs median: 8433 pg/mL, IQR: 2648–12138 vs. PwMS median: 4050 pg/mL, IQR: 928-7904, p=0.014), while higher IP-10 levels were observed in PwMS than in HCWs (approximately 3-fold increase, p<0.0001). Consistent with the results of the spike-specific response, patients receiving fingolimod showed a more pronounced impairment of the immune response to SEB compared to HCWs, particularly regarding IFN-γ and IL-2 production (p=0.048 and p=0.0001). Nonetheless, the extent of impairment in fingolimod-treated patients is markedly lower than that observed in response to Wuhan spike peptides (IFN-γ SEB: 2.5-fold decrease vs. IFN-γ Wuhan spike: 300-fold decrease; IL-2 SEB: 9-fold decrease vs. IL-2 Wuhan spike: 500-fold decrease) (Figure 2D).
3.3 Profile of cytokines induced by Delta variant peptides of the spike protein
To investigate whether COVID-19 vaccination elicited an immune response against SARS-CoV-2 variants of concern, we evaluated cytokine production after stimulation with peptides derived from the SARS-CoV-2 Delta variant (B.1.617.2) in a subgroup of PwMS (n=14). As shown in Figure 3A, the T-cell response to Delta variant significantly differed among DMTs. Specifically, most PwMS treated with ocrelizumab, cladribine, or IFN-β mounted a T-cell response to Delta spike with production of both Th1 cytokines and IP-10. On the other hand, patients treated with fingolimod showed a markedly reduced response compared to other DMTs (IFN-γ: p=0.003; IL-2: p=0.0003; TNF-α, p=0.0016; IP-10, p=0.003). There is no response to Delta spike peptides in fingolimod-treated patients; only one to IFN-γ, three to TNF-α, and one to IP-10. Compared to the response induced by the Wuhan spike peptides, the magnitude of the T-cell response to the Delta variant peptides was significantly lower (IFN-γ: p=0.0002; IL-2: p=0.001; TNF-α, p=0.0015; IP-10, p=0.001) (Figure 3B).
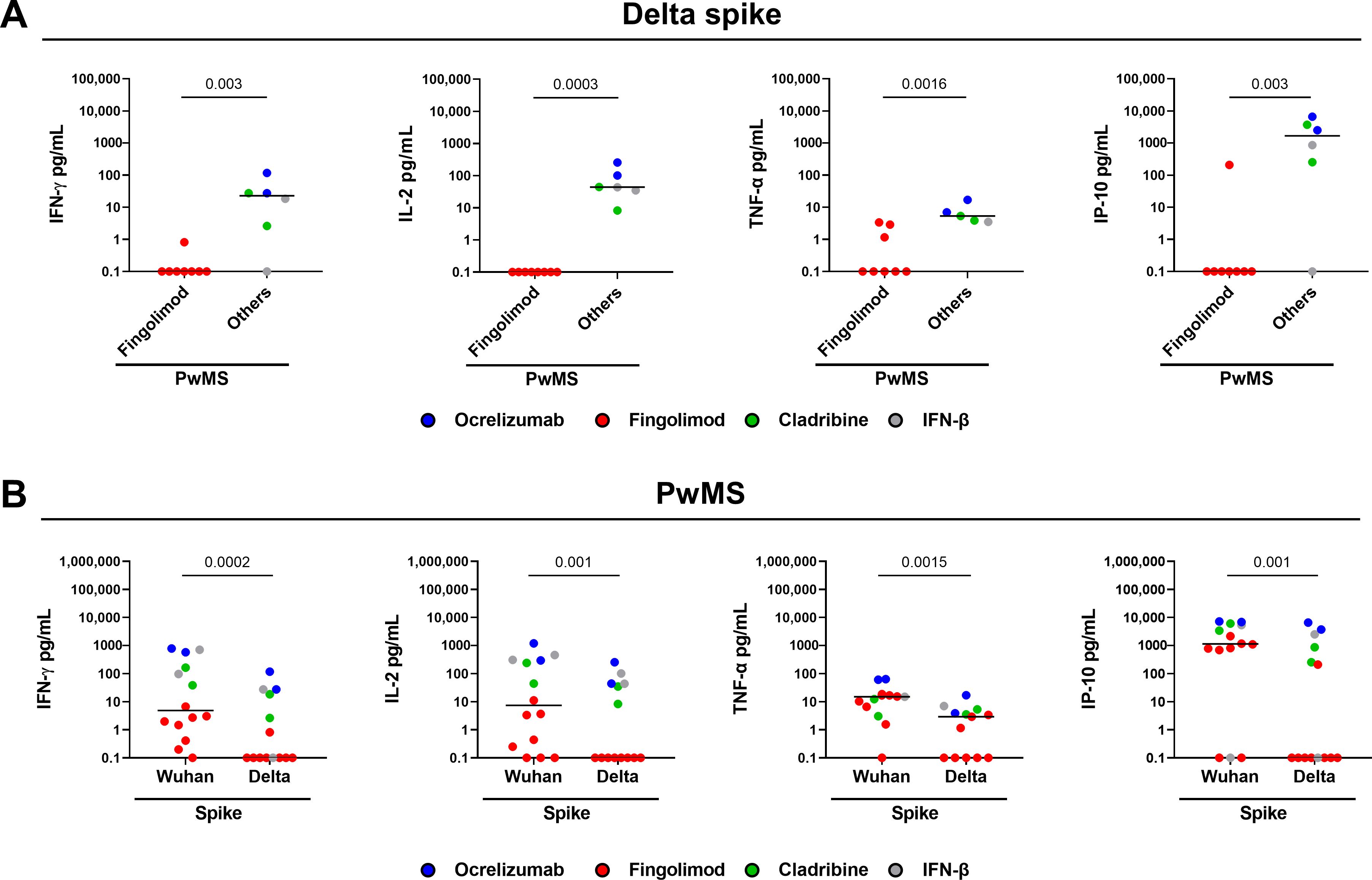
Figure 3. Cytokine/chemokine response to SARS-CoV-2 Delta variant of the spike peptides in PwMS. (A) PwMS (n=14) were stratified into two groups: fingolimod (n=8) and others, which includes PwMS treated with ocrelizumab (n=2), cladribine (n=2) and IFN-β (n=2). (B) Comparison between the T-cell response induced by the Wuhan spike peptides and that induced by the Delta variant. IFN-γ, IL-2, TNF-α and IP-10 concentrations were expressed in pg/mL with median values indicated by black lines. For the statistical analysis, the Mann-Whitney U test (A) and Wilcoxon signed-rank test (B) were used and p values < 0.05 were considered significant. PwMS, patients with multiple sclerosis; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; IP-10, interferon gamma-induced protein 10.
3.4 Correlations between immunological parameters
A coordinated cytokine response was observed in both cohorts. In PwMS, spike-specific IFN-γ production showed strong positive correlations with IP-10, IL-2, and TNF-α-specific responses (ρ=0.709, p<0.001; ρ=0.882, p<0.001; ρ=0.764, p<0.001, respectively). Moreover, IL-2-spike-specific response positively correlated with both IP-10 and TNF-α (ρ=0.708; p<0.001; ρ=0.650, p<0.001, respectively), and TNF-α-spike-specific production correlated with IP-10 levels (ρ=0.515, p<0.01) (Figure 4A). Spike-specific responses in PwMS also correlated with those elicited by SEB stimulation. Particularly, IL-2-spike-specific response was positively associated with SEB-induced IFN-γ, IL-2, and TNF-α levels (ρ=0.367, p<0.05; ρ=0.730, p<0.001; ρ=0.472, p<0.01, respectively), but negatively correlated with SEB-induced IP-10 levels (ρ=-0.370, p<0.05). Moreover, positive correlations were observed between spike-specific IFN-γ and IP-10 productions and SEB-induced IL-2 levels (ρ=0.598, p<0.001; ρ=0.389, p<0.05). These correlations likely emerged within the PwMS cohort due to the greater heterogeneity in lymphocyte counts, attributable to the administration of DMTs, which affect the magnitude of the specific T-cell response.
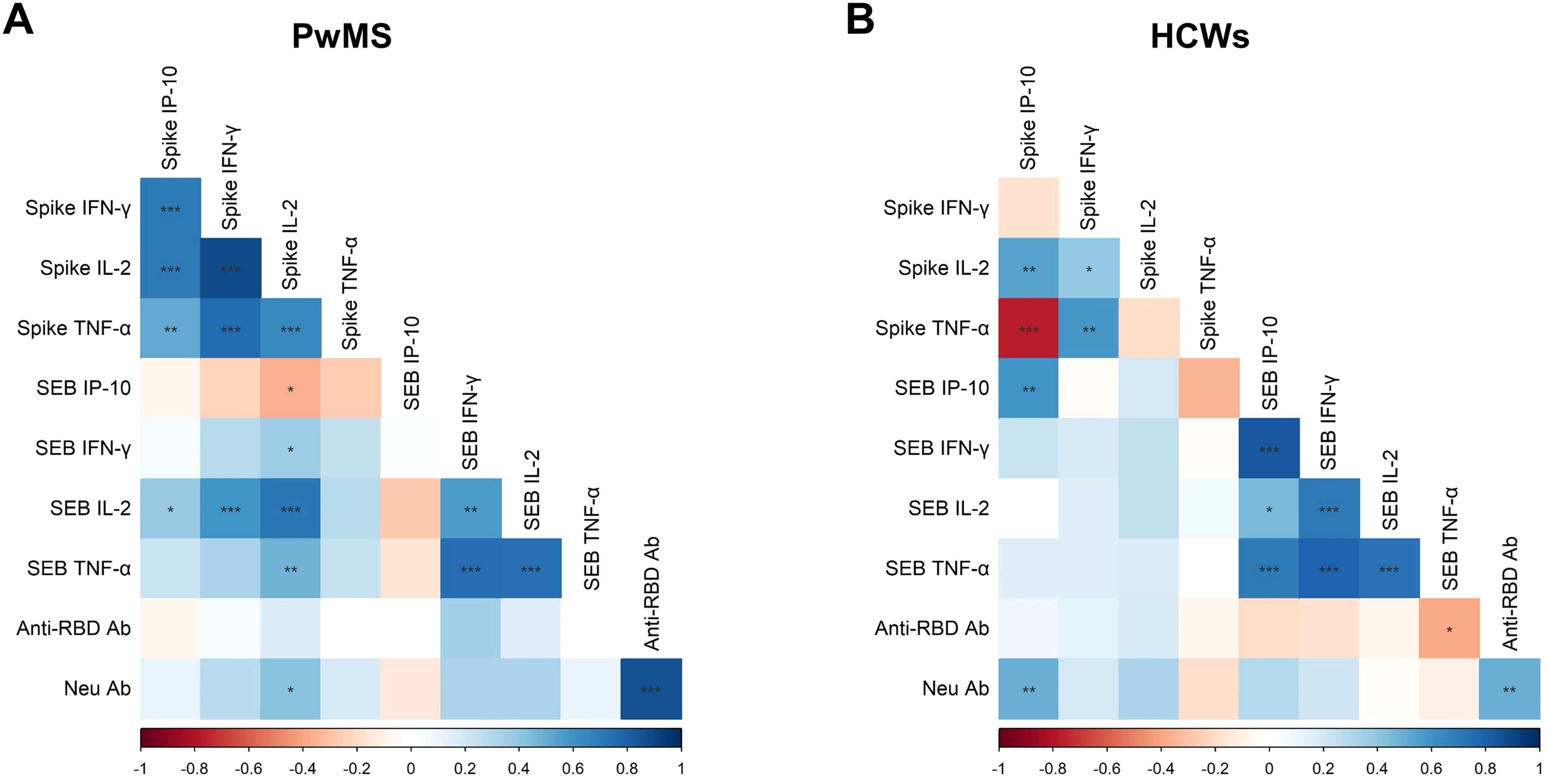
Figure 4. Correlations among the immunological parameters. Correlation matrices in PwMS (A) and HCWs (B) include the following variables: cytokine/chemokine (IFN-γ, IL-2, TNF-α and IP-10) response to SARS-CoV-2 Wuhan spike peptides and SEB, as well as antibody response measured as anti-RBD and neutralizing antibodies. For the analysis, the non-parametric Spearman’s rank test was performed. Positive (blue) and negative (red) correlations are indicated according to the colour-grade scale, and the colour intensity depends on the strength of the correlation coefficient (ρ). The significance level threshold used is the following: *p<0.05, **p<0.01, ***p<0.001.
Interestingly, neutralizing antibody titers, in addition to correlating with anti-RBD antibody levels (ρ=0.870, p<0.001), also showed a positive association with IL-2 production in response to Wuhan spike peptides (ρ=0.415, p<0.05).
In HCWs, cytokine responses elicited by SEB stimulation positively correlated with each other (Figure 4B). As for the Wuhan-spike specific response, positive correlations were observed between IFN-γ production and both IL-2 and TNF-α levels (ρ=0.390, p<0.05; ρ=0.590, p<0.01), and between spike-specific IP-10 response and IL-2 levels (ρ=0.549, p<0.01). In contrast, spike-specific IP-10 production negatively correlated with TNF-α levels (ρ=-0.771, p<0.001). Unlike PwMS, in HCWs no significant correlations were observed between spike-specific responses and those induced by SEB stimulation, except for IP-10 levels (ρ=0.594, p<0.01). Furthermore, there was a significant positive correlation between neutralizing antibody titers and spike-induced IP-10 levels (ρ = 0.491, p < 0.01).
3.5 SARS-CoV-2 breakthrough infection and immune response
Within the PwMS cohort, 12 patients (38.7%) experienced SARS-CoV-2 breakthrough infections (BIs) during the study period, with a median time of 135 days from administration of the third vaccine dose to BI onset (Table 1). Most PwMS (83.4%) developed mild COVID-19, with a median time to nasopharyngeal swab negativization of 13 days (IQR; 10-15), and were treated with antiviral agents or monoclonal antibodies. Among the BI, 50% (6/12) occurred in ocrelizumab-treated patients, 33.3% (4/12) in those receiving fingolimod, and the remaining 16.7% (2/12) in patients treated with IFN-β and cladribine (Table 4).
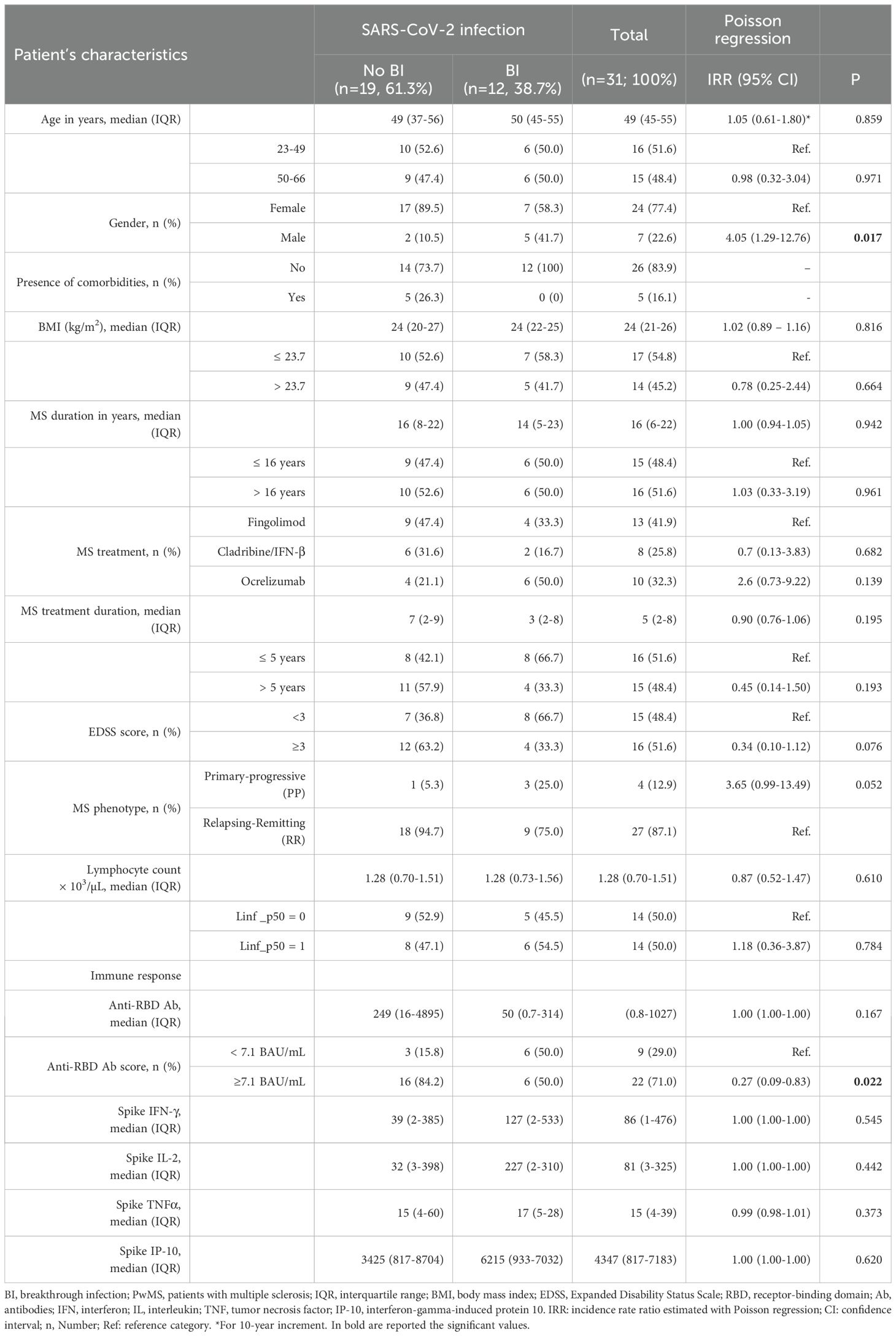
Table 4. Demographic, clinical, and immunological factors affecting the incidence of SARS-CoV-2 breakthrough infection in PwMS after the third dose of COVID-19 vaccination.
Interestingly, among the demographic and clinical factors, Poisson regression analysis identified sex as a significant factor associated with the risk of BI in PwMS. Male sex was significantly associated with a higher incidence rate of BI compared to female sex (IRR: 4.05, 95%CI:1.29-12.76, p=0.017). Moreover, PwMS with a primary progressive disease showed a higher incidence rate of BI (IRR: 3.65, 95%CI: 0.99-13.49, p=0.052) compared to those with relapsing-remitting MS.
Moreover, we evaluated whether the cell-mediated immunity induced after the third vaccine dose influenced the risk of subsequent SARS-CoV-2 infections in PwMS. As shown in Supplementary Figure 1 and supported by the Poisson regression analysis (Table 4), the spike-specific response was not associated with increased protection against BI. In contrast, a significant association was observed between antibody response and a reduced risk of BI (IRR: 0.27, IQR: 0.09–0.83, p = 0.022) (Table 4). This association explains the higher susceptibility of ocrelizumab-treated patients to SARS-CoV-2 infection, as ocrelizumab treatment significantly impairs the antibody response, as shown in previous studies (13, 23). By contrast, most fingolimod-treated patients induced an antibody response, although its magnitude was lower compared to that observed in healthy controls, while cladribine and IFN-β did not significantly affect the humoral response.
4 Discussion
This prospective study provides an in-depth immunological characterization of the spike-specific response following the third dose of COVID-19 vaccination in PwMS undergoing different DMTs by evaluating soluble biomarkers beyond IFN-γ, including IL-2, TNF-α and IP-10, as surrogate markers of the cell-mediated response. Moreover, the immune response was compared with that of a cohort of HCWs matched for age and sex, and the potential association between these immune factors and the risk of BI was investigated.
To date, most studies have focused on the evaluation of the spike-specific T-cell response, selectively assessing IFN-γ response (13, 18, 32, 36, 44). The identification of additional immunological biomarkers may be important to gain a more comprehensive view of the cell-mediated response induced by COVID-19 vaccination. Data on the vaccine-induced response, evaluated as production of both Th1 cytokines (IFN-γ, IL-2, TNF-α) as well as IP-10, are scarce (48, 54). Few studies have examined the functional spike-specific CD4+ and CD8+ T cell responses by evaluating IFN-γ-, IL-2, or TNF-α-producing T cells by flow cytometry in PwMS after the third dose of COVID-19 mRNA-vaccines (22, 45–47). In this study, we employed a user-friendly whole-blood assay to measure Th1 cytokines and IP-10, serving as surrogate markers for the cell-mediated immune response following COVID-19 vaccination.
We demonstrated that PwMS receiving DMTs have an immune system capable of responding to a nonspecific stimulus such as SEB. However, their cytokine response appears dysregulated compared to that observed in HCWs. This difference becomes even more significant when evaluating antigen-specific immune responses, such as those elicited in response to SARS-CoV-2 spike antigens after COVID-19 vaccination. In this case, most PwMS mounted a T-cell response by releasing Th1 cytokines (IFN-γ, IL-2, TNF-α) as well as IP-10. This data aligns with a recent study showing that the immune response to SARS-CoV-2 vaccines in PwMS is unbalanced towards a Th1 phenotype, predominantly characterized by IL-2 and IFN-γ (48).
Nonetheless, the magnitude of the Th1 response to the Wuhan spike peptides was significantly lower than that observed in HCWs, with IFN-γ and TNF-α levels reduced by six-fold, and IL-2 levels decreased by approximately three-fold. Notably, significant variations in the immune response were observed according to the ongoing DMTs, with fingolimod-treated patients presenting the most immunocompromised response, especially in terms of both IFN-γ and IL-2 production. These results confirm previous studies showing reduced IFN-γ levels (13, 48) or frequencies of IFN-γ and IL-2-producing T cells in fingolimod-treated patients (22, 46).
These findings are consistent with the fingolimod’s mechanism of action. Fingolimod acts as a sphingosine 1-phosphate (S1P) receptor modulator and hinders lymphocyte egress from lymph nodes, thereby leading to a reduced lymphocyte count in the peripheral blood (3) and a diminished capacity to mount an effective immune response (55). In addition to MS treatment, male sex was identified as an independent factor influencing the spike-specific immune response, particularly affecting TNF-α production.
Despite widespread vaccination efforts, the emergence of SARS-CoV-2 VOCs has reduced the protective efficacy of existing COVID-19 vaccines (56). However, whereas it has been shown that the antibody responses against VOCs are markedly reduced, because these variants have acquired the ability to evade the antibody recognition (57, 58), the T-cell response appears to be more heterogeneous (59, 60). Consistent with earlier findings (59, 61), we found that COVID-19 vaccines based on the original Wuhan spike protein induce T-cell responses that also cross-react with the Delta variant, as shown by various immunological biomarkers. In this context, PwMS generated a Delta spike-specific response by producing Th1 cytokines and IP-10, though certain DMTs, such as fingolimod, may impair this response, confirming results generated with the Wuhan spike antigen.
Although the cross-reactivity was maintained, the magnitude of the T-cell response to the Delta variant peptides in PwMS was significantly lower than that induced by the Wuhan spike peptides. This result is expected, considering that the antigen used in the first mRNA vaccines was based on the spike protein derived from the original Wuhan strain.
In the PwMS cohort, and to a lesser extent in HCWs, spike-specific cytokine levels showed positive correlations with one another, indicating that the immunological parameters analyzed reflect immune responsiveness. Interestingly, in addition to the already known correlation with anti-RBD antibodies (20, 23), we demonstrated a positive association between neutralizing antibody titers and the levels of IL-2 in PwMS. Altogether, this evidence supports the use of IL-2 as a valuable biomarker for assessing both the cell-mediated and antibody response to COVID-19 vaccination in PwMS.
IL-2, as well as IFN-γ and TNF-α, are cytokines mainly produced by Th1 lymphocytes. IFN-γ contributes to macrophage activation and controls the differentiation of naïve CD4+ T cells into Th1 effectors, which in turn mediate cellular immunity against viral and intracellular bacterial infections (41). TNF-α is involved in various processes, including cell survival, cell death, inflammation, and immune cell activation (42). IL-2 indirectly favors antibody response by promoting T-cell activation and proliferation as well as the differentiation of T follicular helper cells, which are important for B cell maturation (62). Moreover, IL-2 contributes to the generation of plasma cells responsible for antibody production (63, 64).
In HCWs, the neutralizing titer positively correlated with the levels of IP-10. The latter is a chemokine, induced by IFN-γ, that promotes the chemotaxis of activated T and B lymphocytes to the sites of inflammation (65, 66). Moreover, IP-10 drives activated B cells to differentiate into plasma cells (67).
Regarding the incidence of BI, 38.7% (12/31) of PwMS in our cohort experienced SARS-CoV-2 infection after three doses of COVID-19 vaccines. Most of them reported a mild COVID-19, likely explained also by the lower pathogenicity of the Omicron variant (68, 69), which was the predominant variant circulating in Italy during the follow-up period of this present study (70).
Among the demographic and clinical factors analyzed, sex emerged as a significant variable associated with the risk of BI in PwMS. Specifically, male patients exhibited a 4-fold increased risk of BI compared to females. This finding is consistent with previously reported sex-related differences in vaccine-induced response, wherein females mount a more robust immune compared to males (71, 72). Moreover, the relapsing-remitting phenotype of MS disease was associated with a greater protection compared to the primary progressive form, thus confirming previous data (38, 73).
Among DMTs, patients receiving ocrelizumab showed the highest incidence rate of BI, followed by those treated with fingolimod and, finally, patients with cladribine/IFN-β. These results are consistent with data from other studies, identifying patients on ocrelizumab and fingolimod as those at greatest risk of BI (74–77).
.Furthermore, this finding is consistent with the known mechanism of action of ocrelizumab, which acts as CD20-depleting B cell agent (3), underscoring the pivotal role of the antibody response (78). In our PwMS cohort, we confirmed that mounting an antibody response confers protection against infection with an estimated 70% reduction in BI risk, as previously demonstrated (38). To note, SARS-CoV-2 infection occurred approximately 5 months after the administration of the third dose, a time frame that corresponds to the decline of the vaccine-induced antibody response, as widely reported in vary longitudinal studies (13–16).
Unlike the antibody response (79), we did not find any association between the spike-specific cell-mediated response and the protection against SARS-CoV-2 infection for any of the soluble factors evaluated. However, although the lack of a proper T-cell response does not imply an increased BI risk, we have previously showed that a reduced IFN-γ T-cell response adversely affects the time to have a swab negativization by increasing the time required to achieve viral clearance (38). Indeed, fingolimod-treated patients require approximately 7 additional days to test negative. A prolonged persistence of the virus may promote its replication, increasing the likelihood of the emergence of new variants in the individual and spread the infection to the community (17).
Some limitations of the study are acknowledged. Firstly, the small sample size may have limited the ability to perform more in-depth analyses; however, the cohort was thoroughly characterized both immunologically and clinically. Secondly, among the SARS-CoV-2 VOC, only the immune response to Delta variant was evaluated, and a direct comparison with the corresponding response in HCWs is lacking.
On the other hand, a major strength of this study is the comprehensive characterization of the spike-specific immune response. In addition to assessing the IFN-γ production, this study also measured IL-2, TNF-α and IP-10 levels, thus providing a broader overview of the T-cell functionality and cytokine response. Moreover, as far as we know, this is the first study evaluating the association between these immunological biomarkers and the risk of BI. In addition, our immunological data were obtained using a friendly-to-use whole blood standardized method that has been thoroughly validated in previous studies in COVID-19 (51, 80) or in other diseases as tuberculosis (81). However, we cannot rule out that advanced techniques directly measuring the T-cell responses, such as intracellular cytokine staining, rather than using surrogate markers, may provide different results. To our knowledge, a recent flow cytometry study corroborated our findings by demonstrating lower percentages of responding and triple-positive (IFN-γ, IL-2, TNF-α) T cells in PwMS compared to healthy controls, particularly within the depleting/sequestering-out subgroup, such as patients treated with fingolimod (45). Unfortunately, the study did not examine the association between the T-cell response and the risk of SARS-CoV-2 breakthrough infections.
As a clinical implication of our findings, this study contributes to refining the stratification of SARS-CoV-2 infection risk in PwMS treated with DMTs, particularly highlighting patients treated with ocrelizumab and fingolimod as higher-risk groups. These results support the consideration of tailored vaccination approaches, including adjusted booster timing or additional prophylactic interventions. While our data focus on specific DMTs, this framework may be extended to other DMTs and warrants further investigation. Among the immunological biomarkers analyzed, IL-2 emerged as a promising complementary marker of vaccine-induced immunity, showing correlations with both humoral and cell-mediated responses. Nevertheless, additional studies are necessary to confirm its clinical utility and establish thresholds.
In conclusion, this study provides evidence of the spike-specific cytokine and chemokine response in PwMS undergoing DMTs following the third dose of COVID-19 vaccination. Our findings showed that PwMS mount a Th1-type immune response, broadly resembling that observed in HCWs, albeit with significantly reduced levels of IFN-γ and IL-2. Notably, this impaired Th1 response is not associated with a risk of SARS-CoV-2 infection. Importantly, our results identify immunological biomarkers beyond IFN-γ, particularly IL-2, as additional tools to assess the cell-mediated immune response to COVID-19 vaccination. The relevance of these immunological biomarkers may extend beyond COVID-19, for offering insight into host immune responses to other infectious agents and vaccinations.
Data availability statement
The raw data are available in our institutional repository (rawdata.inmi.it), subject to registration. The data can be found by selecting the article of interest from a list of articles ordered by the year of publication. No charge for granting access to data is required. In the event of a malfunction of the application, the request can be sent directly by e-mail to the library (YmlibGlvdGVjYUBpbm1pLml0).
Ethics statement
The studies involving humans were approved by Ethical Committee of National Institute of Infectious Diseases “L. Spallanzani” (INMI)-IRCCS (approval numbers 319/2021, 443/2021, 297/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AlA: Formal Analysis, Writing – original draft, Writing – review & editing. AN: Writing – review & editing, Formal Analysis. SH: Data curation, Writing – review & editing. SR: Writing – review & editing, Data curation. GC: Data curation, Writing – review & editing. VV: Methodology, Writing – review & editing. AnS: Writing – review & editing, Methodology. SN: Methodology, Writing – review & editing. AnA: Writing – review & editing, Methodology. SM: Methodology, Writing – review & editing. FC: Methodology, Writing – review & editing. EC: Writing – review & editing, Methodology. CT: Writing – review & editing, Data curation. LP: Data curation, Writing – review & editing. MQ: Data curation, Writing – review & editing. SG: Data curation, Writing – review & editing. VP: Data curation, Writing – review & editing. FM: Writing – review & editing, Methodology. AG: Writing – review & editing, Resources. AlS: Writing – review & editing, Resources. EN: Data curation, Writing – review & editing. CG: Writing – review & editing, Conceptualization, Data curation. DG: Writing – review & editing, Supervision, Writing – original draft, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by INMI “Lazzaro Spallanzani” Ricerca Corrente Linea 1 (Progetto n.1) on emerging infections funded by the Italian Ministry of Health and by generous liberal donation/funding for COVID-19 research from Camera di Commercio, Industria e Artigianato di Roma (resolution number 395 on May 25, 2021).
Acknowledgments
The authors gratefully acknowledge the nurses of the MS Centre of the San Camillo Forlanini Hospital and the patients who provided their consent to generate this study.
Conflict of interest
AlS is a consultant for Darwin Health, Desna Therapeutics, EmerVax, Gilead Sciences, Guggenheim Securities, Link Campus University and RiverVest Venture Partners. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materiall
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1682049/full#supplementary-material
References
1. McGinley MP, Goldschmidt CH, and Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: A review. JAMA. (2021) 325:765–79. doi: 10.1001/jama.2020.26858
2. Du Pasquier RA, Pinschewer DD, and Merkler D. Immunological mechanism of action and clinical profile of disease-modifying treatments in multiple sclerosis. CNS Drugs. (2014) 28:535–58. doi: 10.1007/s40263-014-0160-8
3. Yang JH, Rempe T, Whitmire N, Dunn-Pirio A, and Graves JS. Therapeutic advances in multiple sclerosis. Front Neurol. (2022) 13:824926. doi: 10.3389/fneur.2022.824926
4. Sormani MP, Schiavetti I, Landi D, Carmisciano L, De Rossi N, Cordioli C, et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: An international cohort study. Mult Scler. (2022) 28:1034–40. doi: 10.1177/13524585211035318
5. Prosperini L, Arrambide G, Celius EG, Goletti D, Killestein J, Kos D, et al. COVID-19 and multiple sclerosis: challenges and lessons for patient care. Lancet Reg Health Eur. (2024) 44:100979. doi: 10.1016/j.lanepe.2024.100979
6. Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. (2021) 89:780–9. doi: 10.1002/ana.26028
7. Prosperini L, Tortorella C, Haggiag S, Ruggieri S, Galgani S, and Gasperini C. Increased risk of death from COVID-19 in multiple sclerosis: a pooled analysis of observational studies. J Neurol. (2022) 269:1114–20. doi: 10.1007/s00415-021-10803-3
8. Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. (2021) 97:e1870–85. doi: 10.1212/WNL.0000000000012753
9. Iyer RB, S R, M JN, and R J. COVID-19 outcomes in persons with multiple sclerosis treated with rituximab. Mult Scler Relat Disord. (2022) 57:103371. doi: 10.1016/j.msard.2021.103371
10. Jakimovski D, Zakalik K, Awan S, Kavak KS, Pennington P, Hojnacki D, et al. COVID-19 vaccination in multiple sclerosis and inflammatory diseases: effects from disease-modifying therapy, long-term seroprevalence and breakthrough infections. Vaccines (Basel). (2022) 10:695. doi: 10.3390/vaccines10050695
11. Ioannou GN, Bohnert ASB, O’Hare AM, Boyko EJ, Maciejewski ML, Smith VA, et al. Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization, and death: A target trial emulation in the omicron (B.1.1.529) variant era. Ann Intern Med. (2022) 175:1693–706. doi: 10.7326/M22-1856
12. Capuano R, Prosperini L, Altieri M, Lorefice L, Fantozzi R, Cavalla P, et al. Symptomatic COVID-19 course and outcomes after three mRNA vaccine doses in multiple sclerosis patients treated with high-efficacy DMTs. Mult Scler. (2023) 29:856–65. doi: 10.1177/13524585231167515
13. Ruggieri S, Aiello A, Tortorella C, Navarra A, Vanini V, Meschi S, et al. Dynamic Evolution of Humoral and T-Cell Specific Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Followed until the Booster Dose. Int J Mol Sci. (2023) 24:8525. doi: 10.3390/ijms24108525
14. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. (2021) 385:e84. doi: 10.1056/NEJMoa2114583
15. Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. (2021) 398:385–7. doi: 10.1016/S0140-6736(21)01642-1
16. Menegale F, Manica M, Zardini A, Guzzetta G, Marziano V, d’Andrea V, et al. Evaluation of waning of SARS-coV-2 vaccine-induced immunity: A systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e2310650. doi: 10.1001/jamanetworkopen.2023.10650
17. Machkovech HM, Hahn AM, Garonzik Wang J, Grubaugh ND, Halfmann PJ, Johnson MC, et al. Persistent SARS-CoV-2 infection: significance and implications. Lancet Infect Dis. (2024) 24:e453–62. doi: 10.1016/S1473-3099(23)00815-0
18. Herzberg J, Fischer B, Becher H, Becker A-K, Honarpisheh H, Guraya SY, et al. Cellular and humoral immune response to a third dose of BNT162b2 COVID-19 vaccine - A prospective observational study. Front Immunol. (2022) 13:896151. doi: 10.3389/fimmu.2022.896151
19. Liu Y, Zeng Q, Deng C, Li M, Li L, Liu D, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. (2022) 8:10. doi: 10.1038/s41421-022-00373-7
20. Agrati C, Castilletti C, Goletti D, Meschi S, Sacchi A, Matusali G, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. (2021) 9:1315. doi: 10.3390/microorganisms9061315
21. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. (2021) 385:1393–400. doi: 10.1056/NEJMoa2114255
22. Aiello A, Coppola A, Ruggieri S, Farroni C, Altera AMG, Salmi A, et al. Longitudinal characterisation of B and T-cell immune responses after the booster dose of COVID-19 mRNA-vaccine in people with multiple sclerosis using different disease-modifying therapies. J Neurol Neurosurg Psychiatry. (2022) 94(4):290–99. doi: 10.1136/jnnp-2022-330175
23. Tortorella C, Aiello A, Gasperini C, Agrati C, Castilletti C, Ruggieri S, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. (2022) 98:e541–54. doi: 10.1212/WNL.0000000000013108
24. Bajwa HM, Novak F, Nilsson AC, Nielsen C, Holm DK, Østergaard K, et al. Persistently reduced humoral and sustained cellular immune response from first to third SARS-CoV-2 mRNA vaccination in anti-CD20-treated multiple sclerosis patients. Mult Scler Relat Disord. (2022) 60:103729. doi: 10.1016/j.msard.2022.103729
25. Dreyer-Alster S, Menascu S, Mandel M, Shirbint E, Magalashvili D, Dolev M, et al. COVID-19 vaccination in patients with multiple sclerosis: Safety and humoral efficacy of the third booster dose. J Neurol Sci. (2022) 434:120155. doi: 10.1016/j.jns.2022.120155
26. Milo R, Staun-Ram E, Karussis D, Karni A, Hellmann MA, Bar-Haim E, et al. Humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: an Israeli multi-center experience following 3 vaccine doses. Front Immunol. (2022) 13:868915. doi: 10.3389/fimmu.2022.868915
27. König M, Torgauten HM, Tran TT, Holmøy T, Vaage JT, Lund-Johansen F, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. (2022) 79:307–9. doi: 10.1001/jamaneurol.2021.5109
28. Achiron A, Mandel M, Dreyer-Alster S, Harari G, Magalashvili D, Sonis P, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. (2021) 14:17562864211012835. doi: 10.1177/17562864211012835
29. Sabatino JJ, Mittl K, Rowles WM, McPolin K, Rajan JV, Laurie MT, et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine–induced antibody and T cell immunity and function. JCI Insight. (2022) 7(4):e156978. doi: 10.1172/jci.insight.156978
30. Baba C, Ozcelik S, Kaya E, Samedzada U, Ozdogar AT, Cevik S, et al. Three doses of COVID-19 vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult Scler Relat Disord. (2022) 68:104119. doi: 10.1016/j.msard.2022.104119
31. Meyer-Arndt L, Braun J, Fauchere F, Vanshylla K, Loyal L, Henze L, et al. SARS-CoV-2 mRNA vaccinations fail to elicit humoral and cellular immune responses in patients with multiple sclerosis receiving fingolimod. J Neurol Neurosurg Psychiatry. (2022) 93(9):960–71. doi: 10.1136/jnnp-2022-329395
32. Palomares Cabeza V, Kummer LYL, Wieske L, Hagen RR, Duurland M, Konijn VAL, et al. Longitudinal T-cell responses after a third SARS-CoV-2 vaccination in patients with multiple sclerosis on ocrelizumab or fingolimod. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1178. doi: 10.1212/NXI.0000000000001178
33. Iannetta M, Landi D, Cola G, Campogiani L, Malagnino V, Teti E, et al. B- and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol. (2022) 12:796482. doi: 10.3389/fimmu.2021.796482
34. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. (2021) 27:1990–2001. doi: 10.1038/s41591-021-01507-2
35. Katz Sand I, Gnjatic S, Krammer F, Tuballes K, Carreño JM, Satyanarayan S, et al. Evaluation of immunological responses to third COVID-19 vaccine among people treated with sphingosine receptor-1 modulators and anti-CD20 therapy. Mult Scler Relat Disord. (2023) 70:104486. doi: 10.1016/j.msard.2022.104486
36. Drulovic J, Tamas O, Nikolovski N, Momcilovic N, Radisic V, Andabaka M, et al. Vaccine-induced humoral and cellular response to SARS-CoV-2 in multiple sclerosis patients on ocrelizumab. Vaccines (Basel). (2025) 13:488. doi: 10.3390/vaccines13050488
37. Habek M, Željko C, Savić Mlakar A, Bendelja K, Rogić D, Adamec I, et al. Humoral and cellular immunity in convalescent and vaccinated COVID-19 people with multiple sclerosis: Effects of disease modifying therapies. Mult Scler Relat Disord. (2022) 59:103682. doi: 10.1016/j.msard.2022.103682
38. Aiello A, Ruggieri S, Navarra A, Tortorella C, Vanini V, Haggiag S, et al. Anti-RBD antibody levels and IFN-γ-specific T cell response are associated with a more rapid swab reversion in patients with multiple sclerosis after the booster dose of COVID-19 vaccination. Vaccines (Basel). (2024) 12:926. doi: 10.3390/vaccines12080926
39. Petrone L, Sette A, de Vries RD, and Goletti D. The importance of measuring SARS-CoV-2-specific T-cell responses in an ongoing pandemic. Pathogens. (2023) 12:862. doi: 10.3390/pathogens12070862
40. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. (2022) 23:186–93. doi: 10.1038/s41590-021-01122-w
41. Schoenborn JR and Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. (2007) 96:41–101. doi: 10.1016/S0065-2776(07)96002-2
42. Wang R, Lan C, Benlagha K, Camara NOS, Miller H, Kubo M, et al. The interaction of innate immune and adaptive immune system. MedComm (2020). (2024) 5:e714. doi: 10.1002/mco2.714
43. Madhurantakam S, Lee ZJ, Naqvi A, and Prasad S. Importance of IP-10 as a biomarker of host immune response: Critical perspective as a target for biosensing. Curr Res Biotechnol. (2023) 5:100130. doi: 10.1016/j.crbiot.2023.100130
44. Torres P, Sancho-Saldaña A, Gil Sánchez A, Peralta S, Solana MJ, Bakkioui S, et al. A prospective study of cellular immune response to booster COVID-19 vaccination in multiple sclerosis patients treated with a broad spectrum of disease-modifying therapies. J Neurol. (2023) 270:2380–91. doi: 10.1007/s00415-023-11575-8
45. Dominelli F, Zingaropoli MA, Tartaglia M, Tortellini E, Guardiani M, Perri V, et al. Multiple sclerosis-disease modifying therapies affect humoral and T-cell response to mRNA COVID-19 vaccine. Front Immunol. (2022) 13:1050183. doi: 10.3389/fimmu.2022.1050183
46. Wolf A-S, Ravussin A, König M, Øverås MH, Solum G, Kjønstad IF, et al. T cell responses to SARS-CoV-2 vaccination differ by disease-modifying therapy for multiple sclerosis. JCI Insight. (2023) 8:e165111. doi: 10.1172/jci.insight.165111
47. Solchenberger H, Odendahl M, Schriefer D, Proschmann U, Rahbani GKA, Ziemssen T, et al. Extensive T-cell profiling following SARS-CoV-2 mRNA vaccination in multiple sclerosis patients treated with DMTs. Pathogens. (2025) 14:235. doi: 10.3390/pathogens14030235
48. Al Rahbani GK, Woopen C, Dunsche M, Proschmann U, Ziemssen T, and Akgün K. SARS-CoV-2-specific immune cytokine profiles to mRNA, viral vector and protein-based vaccines in patients with multiple sclerosis: beyond interferon gamma. Vaccines (Basel). (2024) 12:684. doi: 10.3390/vaccines12060684
49. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
50. CDC. Healthcare workers. In: Centers for Disease Control and Prevention (2020). National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Available online at: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/hcp/clinical-care/clinical-considerations-course.html.
51. Aiello A, Coppola A, Vanini V, Petrone L, Cuzzi G, Salmi A, et al. Accuracy of QuantiFERON SARS-CoV-2 research use only assay and characterization of the CD4+ and CD8+ T cell-SARS-CoV-2 response: comparison with a homemade interferon-γ release assay. Int J Infect Dis. (2022) 122:841–9. doi: 10.1016/j.ijid.2022.07.049
52. Matusali G, Colavita F, Lapa D, Meschi S, Bordi L, Piselli P, et al. SARS-CoV-2 serum neutralization assay: A traditional tool for a brand-new virus. Viruses. (2021) 13:655. doi: 10.3390/v13040655
53. Picchianti-Diamanti A, Aiello A, Laganà B, Agrati C, Castilletti C, Meschi S, et al. ImmunosuppressiveTherapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol. (2021) 12:740249. doi: 10.3389/fimmu.2021.740249
54. Corradini P, Agrati C, Apolone G, Mantovani A, Giannarelli D, Marasco V, et al. Humoral and T-cell immune response after 3 doses of messenger RNA severe acute respiratory syndrome coronavirus 2 vaccines in fragile patients: the italian VAX4FRAIL study. Clin Infect Dis. (2022) 76:e426–38. doi: 10.1093/cid/ciac404
55. Achiron A, Mandel M, Gurevich M, Dreyer-Alster S, Magalashvili D, Sonis P, et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol. (2022) 269:2286–92. doi: 10.1007/s00415-022-11030-0
56. Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. (2022) 387(11):1011–20. doi: 10.1056/NEJMra2206573
57. Jiang X-L, Song X-D, Shi C, Yang G-J, Wang X-J, Zhang Y-W, et al. Variant-specific antibody response following repeated SARS-CoV-2 vaccination and infection. Cell Rep. (2024) 43:114387. doi: 10.1016/j.celrep.2024.114387
58. He P, Liu B, Gao X, Yan Q, Pei R, Sun J, et al. SARS-CoV-2 Delta and Omicron variants evade population antibody response by mutations in a single spike epitope. Nat Microbiol. (2022) 7:1635–49. doi: 10.1038/s41564-022-01235-4
59. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. (2022) 185:847–859.e11. doi: 10.1016/j.cell.2022.01.015
60. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. (2021) 374:abm0829. doi: 10.1126/science.abm0829
61. Jordan SC, Shin B-H, Gadsden T-AM, Chu M, Petrosyan A, Le CN, et al. T cell immune responses to SARS-CoV-2 and variants of concern (Alpha and Delta) in infected and vaccinated individuals. Cell Mol Immunol. (2021) 18:2554–6. doi: 10.1038/s41423-021-00767-9
62. Faliti CE, Mesina M, Choi J, Bélanger S, Marshall MA, Tipton CM, et al. Interleukin-2-secreting T helper cells promote extra-follicular B cell maturation via intrinsic regulation of a B cell mTOR-AKT-Blimp-1 axis. Immunity. (2024) 57:2772–2789.e8. doi: 10.1016/j.immuni.2024.11.006
63. Le Gallou S, Caron G, Delaloy C, Rossille D, Tarte K, and Fest T. IL-2 requirement for human plasma cell generation: coupling differentiation and proliferation by enhancing MAPK-ERK signaling. J Immunol. (2012) 189:161–73. doi: 10.4049/jimmunol.1200301
64. Hipp N, Symington H, Pastoret C, Caron G, Monvoisin C, Tarte K, et al. IL-2 imprints human naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. Nat Commun. (2017) 8:1443. doi: 10.1038/s41467-017-01475-7
65. Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. (2011) 22:121–30. doi: 10.1016/j.cytogfr.2011.06.001
66. Griffith JW, Sokol CL, and Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. (2014) 32:659–702. doi: 10.1146/annurev-immunol-032713-120145
67. Xu W, Joo H, Clayton S, Dullaers M, Herve M-C, Blankenship D, et al. Macrophages induce differentiation of plasma cells through CXCL10/IP-10. J Exp Med. (2012) 209:1813–23. doi: 10.1084/jem.20112142
68. Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. (2022) 399:1303–12. doi: 10.1016/S0140-6736(22)00462-7
69. Bálint G, Vörös-Horváth B, and Széchenyi A. Omicron: increased transmissibility and decreased pathogenicity. Sig Transduct Target Ther. (2022) 7:151. doi: 10.1038/s41392-022-01009-8
70. Stefanelli P, Trentini F, Petrone D, Mammone A, Ambrosio L, Manica M, et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Euro Surveill. (2022) 27:2200125. doi: 10.2807/1560-7917.ES.2022.27.45.2200125
71. St Clair LA, Chaulagain S, Klein SL, Benn CS, and Flanagan KL. Sex-differential and non-specific effects of vaccines over the life course. Curr Top Microbiol Immunol. (2023) 441:225–51. doi: 10.1007/978-3-031-35139-6_9
72. Plebani M and Lippi G. Sex and gender differences in COVID-19: a narrative review. J Sex- Gender-Specific Med. (2022) 8:105–11. doi: 10.1723/0000.37953
73. Chaudhry F, Bulka H, Rathnam AS, Said OM, Lin J, Lorigan H, et al. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci. (2020) 418:117147. doi: 10.1016/j.jns.2020.117147
74. Novak F, Bajwa HM, Coia JE, Nilsson AC, Nielsen C, Holm DK, et al. Low protection from breakthrough SARS-CoV-2 infection and mild disease course in ocrelizumab-treated patients with multiple sclerosis after three mRNA vaccine doses. J Neurol Neurosurg Psychiatry. (2023) 94:934–7. doi: 10.1136/jnnp-2022-330757
75. Sormani MP, Schiavetti I, Inglese M, Carmisciano L, Laroni A, Lapucci C, et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. (2022) 80:104042. doi: 10.1016/j.ebiom.2022.104042
76. van Kempen ZLE, Stalman EW, Steenhuis M, Kummer LYL, van Dam KPJ, Wilbrink MF, et al. SARS-CoV-2 omicron breakthrough infections in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. (2023) 94:280–3. doi: 10.1136/jnnp-2022-330100
77. Schiavetti I, Cordioli C, Stromillo ML, Teresa Ferrò M, Laroni A, Cocco E, et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult Scler. (2022) 28:2106–11. doi: 10.1177/13524585221102918
78. Qi H, Liu B, Wang X, and Zhang L. The humoral response and antibodies against SARS-CoV-2 infection. Nat Immunol. (2022) 23:1008–20. doi: 10.1038/s41590-022-01248-5
79. Gillot C, Bayart J-L, Closset M, Cabo J, Maloteau V, Dogné J-M, et al. Peri-infection titers of neutralizing and binding antibodies as a predictor of COVID-19 breakthrough infections in vaccinated healthcare professionals: importance of the timing. Clin Chem Lab Med. (2023) 61(9):1670–75. doi: 10.1515/cclm-2023-0134
80. Petrone L, Petruccioli E, Vanini V, Cuzzi G, Najafi Fard S, Alonzi T, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. (2021) 27:286.e7–286.e13. doi: 10.1016/j.cmi.2020.09.051
81. Alonzi T, Petruccioli E, Aiello A, Repele F, and Goletti D. Diagnostic tests for tuberculosis infection and predictive indicators of disease progression: Utilizing host and pathogen biomarkers to enhance the tuberculosis elimination strategies. Int J Infect Dis. (2025) 155:107880. doi: 10.1016/j.ijid.2025.107880
Keywords: multiple sclerosis, Th1 cytokines, mRNA vaccines, SARS-CoV-2 infection, T-cell response, disease-modifying therapies
Citation: Aiello A, Navarra A, Haggiag S, Ruggieri S, Cuzzi G, Vanini V, Salmi A, Notari S, Altera AMG, Meschi S, Colavita F, Cimini E, Tortorella C, Prosperini L, Quartuccio ME, Galgani S, Puro V, Maggi F, Grifoni A, Sette A, Nicastri E, Gasperini C and Goletti D (2025) Minimal association between Th1-specific responses to COVID-19 vaccines and SARS-CoV-2 breakthrough infections in multiple sclerosis patients receiving disease-modifying therapies. Front. Immunol. 16:1682049. doi: 10.3389/fimmu.2025.1682049
Received: 08 August 2025; Accepted: 20 October 2025;
Published: 30 October 2025.
Edited by:
Alejandro Vallejo, Ramón y Cajal Institute for Health Research, SpainReviewed by:
Ezequiel Ruiz-Mateos, Spanish National Research Council (CSIC), SpainGüliz Tuba Barut, Institute of virology and immunology, Switzerland
Copyright © 2025 Aiello, Navarra, Haggiag, Ruggieri, Cuzzi, Vanini, Salmi, Notari, Altera, Meschi, Colavita, Cimini, Tortorella, Prosperini, Quartuccio, Galgani, Puro, Maggi, Grifoni, Sette, Nicastri, Gasperini and Goletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delia Goletti, ZGVsaWEuZ29sZXR0aUBpbm1pLml0
 Alessandra Aiello
Alessandra Aiello Assunta Navarra
Assunta Navarra Shalom Haggiag3
Shalom Haggiag3 Serena Ruggieri
Serena Ruggieri Silvia Meschi
Silvia Meschi Francesca Colavita
Francesca Colavita Eleonora Cimini
Eleonora Cimini Luca Prosperini
Luca Prosperini Alba Grifoni
Alba Grifoni Alessandro Sette
Alessandro Sette Emanuele Nicastri
Emanuele Nicastri Delia Goletti
Delia Goletti