- 1Guizhou Aerospace Hospital, Dermatolog, Zunyi, China
- 2Key Laboratory of Basic Pharmacology of Guizhou Province and School of Pharmacy, Zunyi Medical University, Zunyi, China
- 3Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China
Vesicle trafficking mechanisms play indispensable roles throughout the viral replication cycle, though their stage-specific regulatory mechanisms during infection require further elucidation. Notably, the latest research reveals that diverse viruses strategically exploit host vesicle trafficking proteins to orchestrate critical infection phases, including receptor-mediated endocytosis initiation, viral attachment/membrane fusion, intracellular component transport, genome replication complex reorganization, and viral assembly/budding. By commandeering these trafficking pathways, viruses not only optimize cellular entry efficiency and immune evasion capabilities but also establish dynamic organelle microenvironments conducive to genome replication. Consequently, therapeutic strategies targeting vesicular transport nodes—through functional inhibition of trafficking proteins or disruption of vesicle homeostasis—have emerged as promising antiviral approaches with clinical translation potential. This review systematically examines viral phase-dependent mechanisms of host vesicular networks, elucidates infection optimization through transport pathway subversion, and evaluates current efforts in developing vesicle-targeted antivirals, thereby providing conceptual frameworks for novel therapeutic design.
Highlights
● Viruses hijack vesicle trafficking proteins to enhance replication;
● Viruses exploit vesicle trafficking to immune evasion;
● Targeting vesicle system enables multi-pronged antiviral strategies.
1 Introduction
Vesicular trafficking facilitates biomolecule transport through intracellular movement, membrane fusion and budding (1), thereby supporting cellular cargo distribution and secretion. This dynamic process is mediated by interconnected compartments—including the endoplasmic reticulum (ER), Golgi apparatus, trans-Golgi network (TGN), endosomes, and lysosomes—which collectively form an integrated cargo-processing network. These compartments operate through five evolutionarily conserved pathways that govern this system: (1) ER-to-Golgi transport (2), (2) Golgi-to-ER recycling (3), (3) TGN sorting (4), (4) endocytic trafficking (5), and (5) autophagic flux (6). These pathways are tightly regulated by key molecular components such as clathrin (7), adaptor protein complexes (8), dynein (9) and Rab proteins (10). Furthermore, emerging evidence highlights non-canonical vesicle trafficking pathways. For example, mitochondria-derived vesicles (MDVs) mediate UFMylation-dependent sorting of MAVS for lysosomal elimination (11), while Golgi-derived secretory granules (SGs) coordinate amphisome-mediated fusion through phosphoinositide conversion cascades (PI(3,4,5)P3→PI(4,5)P2) and CD63/PTPN9-regulated membrane fission (12). Building on these findings, intriguingly, core machinery like SNAREs and Rabs—originally characterized in canonical pathways—also orchestrate non-canonical trafficking through competitive SNARE complex assembly (e.g., Ykt6-Syx17-Snap29 vs Vamp7-Syx17-Snap29) and structure-specific regulatory mechanisms (RQ mutation tolerance in Ykt6’s zero ionic layer) (13), whereas Rab effectors coordinate vesicle tethering via HOPS complex interactions (14). Crucially, the conservation of both canonical and non-canonical trafficking modules not only underpins their essential role in cellular homeostasis but also creates vulnerabilities exploited by viruses, thereby amplifying the complexity of host-pathogen interdependence.
Virus particles are minimally composed of a nucleic acid genome (RNA or DNA) enclosed within a protein capsid, often surrounded by a host-derived lipid envelope studded with viral glycoproteins. Their replication cycle progresses through four host-dependent stages: entry/uncoating (15), genome replication (16), protein synthesis/processing (17), and virion assembly/egress (18). These processes critically exploit the dynamic membrane systems of eukaryotic cells, which perpetually remodel membrane-bound organelles—such as the endoplasmic reticulum, Golgi apparatus, and endosomes—to regulate interorganellar connectivity in response to intracellular and extracellular signals (19). Following cellular entry, viruses strategically hijack vesicular trafficking pathways to establish replication hubs. For example, (+) ssRNA viruses like SARS-CoV-2 co-opt endoplasmic reticulum-derived vesicles for replication organelle formation (20), while enveloped viruses such as HIV-1 redirect Rab GTPase-regulated endosomal networks for virion budding (21). Such viral interference restructures organelle networks to optimize resource allocation for replication (22) or repurposes transport vesicles for immune evasion and dissemination (23). These adaptations highlight how pathogens exploit conserved membrane remodeling mechanisms to balance replication efficiency with stealth, often at the cost of host cell homeostasis (Figure 1).
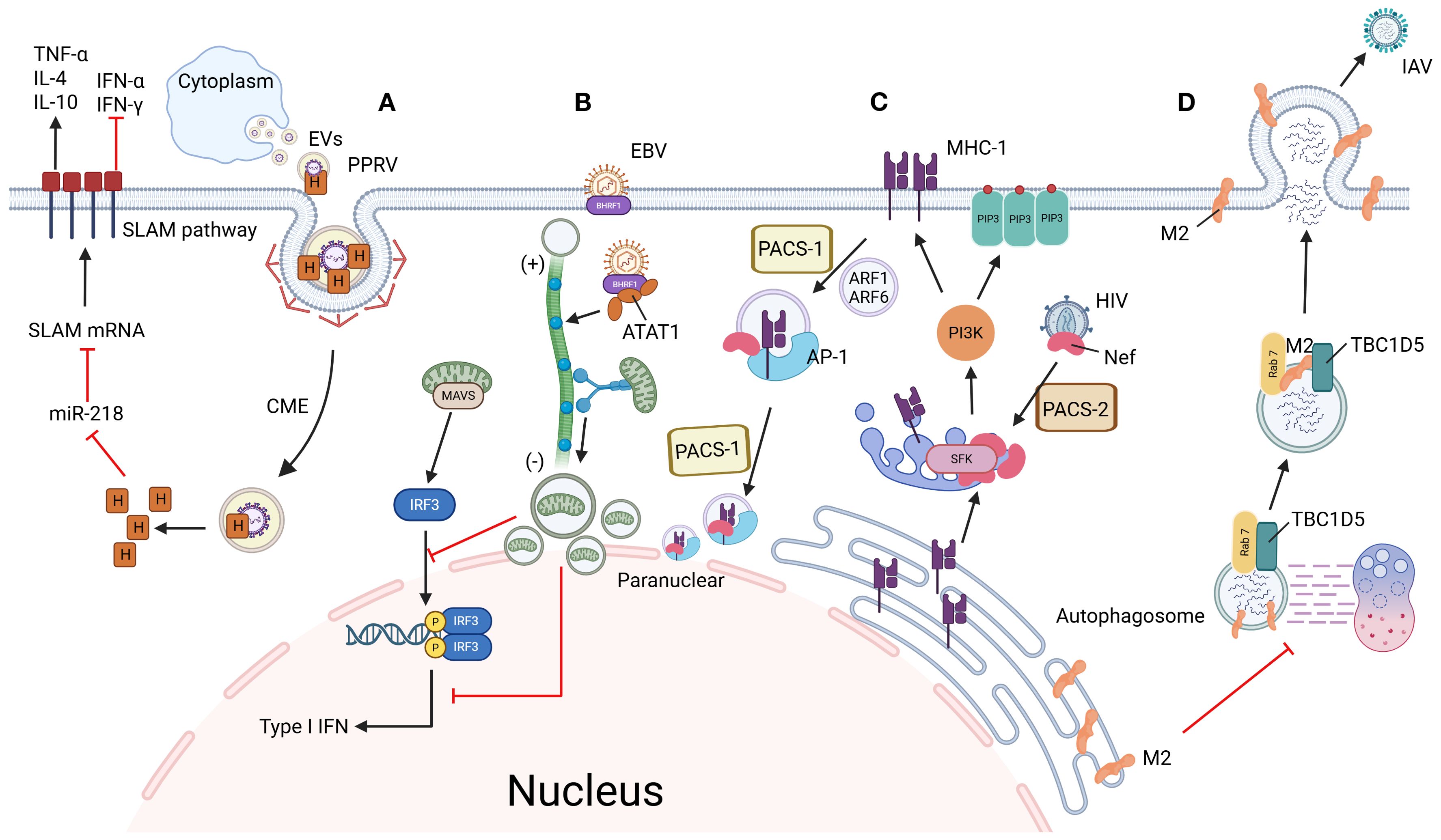
Figure 1. Mechanisms of viral immune evasion through hijacking vesicle trafficking systems. (A) PPRV enhances infectivity via SLAM receptor modulation: PPRV enters host cells through CME using extracellular vesicles. Its H protein suppresses miR-218, thereby upregulating SLAM receptor expression to facilitate viral entry. Concurrently, PPRV activates the SLAM signaling pathway, altering cytokine expression to create an immunosuppressive microenvironment and promote persistent infection. (B) EBV disrupts mitochondrial dynamics to evade innate immunity: EBV-encoded BHRF1 induces microtubule hyperacetylation, which hijacks dynein-mediated retrograde transport. This drives mitochondrial clustering near the nucleus, disrupting their dynamic network required for MAVS assembly. Consequently, interferon production is blocked, allowing EBV to evade innate immune surveillance. (C) HIV hijacks MHC-I recycling via AP-1/PACS-1 axis: HIV Nef is recruited to the TGN by PACS-2, where it activates SFK kinases and triggers PI3K-dependent PIP3 accumulation on the plasma membrane. Activated PI3K recruits ARF1/6 to drive MHC-I internalization into coated vesicles. Internalized MHC-I forms a ternary complex with Nef and AP-1, which is redirected to the perinuclear region via PACS-1-mediated sorting, ultimately blocking MHC-I recycling to the cell surface and suppressing antigen presentation. (D) IAV blocks autophagic degradation via Rab7 inactivation: IAV M2 protein disrupts the interaction between Rab7 GTPase and its GAP protein TBC1D5, preventing GTP hydrolysis and locking Rab7 in an inactive state. This failure of Rab7 activation impairs autophagosome-lysosome fusion, allowing viral particles to escape lysosomal degradation. By maintaining intracellular viral survival and facilitating plasma membrane budding, M2 ensures efficient IAV propagation. Figure created with BioRender.
Notwithstanding the pivotal role of vesicular trafficking across viral infection stages, its specific contribution to viral replication remains to be determined. Based on the close relationship between vesicle trafficking related proteins and virus replication, this review systematically summarizes the functional characterization of key trafficking proteins hijacked during infection, comparative analysis of molecular mechanisms through which diverse viruses subvert vesicular pathways, and deeply analyzes how these mechanisms cooperate to promote the dynamic process of virus replication and transmission.
2 Virus infection and vesicular trafficking-related host proteins
Vesicular trafficking mediates viral replication through host factor cooperativity, with mechanistic studies revealing lineage-specific adaptations. Strikingly, COPII vesicles drive rotavirus particle maturation through direct interaction with the enterotoxin NSP4 (24), while COPI components scaffold replication complex assembly during Drosophila C virus infection (25). Parallel analyses of divergent systems reveal distinct host machinery dependencies: HCV cellular entry requires Septin-6 isoforms for membrane anchoring (26), whereas DENV replication in mosquito cells hinges on Septin-2-mediated modulation of ER rearrangement (27). To systematically dissect these interactions, the following subsections focus on key vesicle trafficking components—clathrin-coated vesicles, dynein motor complexes, adaptor protein assemblies, and Rab GTPase networks—that enable viral exploitation of host transport systems. These evidences suggest that the virus has established a conserved evolutionary strategy to promote its own replication by hijacking the vesicle associated protein induced transport system (28) (Table 1).
2.1 Clathrin
The molecular mechanism of virus entry into host cells is the key to regulate infectivity, and clathrin mediated endocytosis (CME) has been established as the main entry pathway after long-term research. During CME, clathrin trimers composed of heavy and light chains self-assemble to form a polyhedral lattice, driving the generation of clathrin coated pits (CCP) (29, 30). This mechanism confers dual virological advantages: hijacking CME not only enhances invasion efficiency but also promotes rapid entry into endosomal compartments via clathrin-encapsulated vesicles. Strategically, this pathway evades cell membrane receptor surveillance while simultaneously delaying activation of host innate immune responses, thereby extending the temporal window for successful infection establishment (31).
As the central regulatory framework of mammalian vesicular transport, CME orchestrates spatiotemporal coordination of diverse cargoes—including signaling molecules, synaptic vesicles, and surface receptors—through clathrin-coated vesicle formation (32, 33). This hub-like regulatory capacity establishes CME as the preferred entry portal for multiple enveloped viruses, with canonical examples encompassing HIV-1 (34), PPRSV (35) and DENV (36). Notably, the evolutionary co-option of CME by viruses underscores the intricate equilibrium in host-pathogen dynamics: while utilizing this highly conserved pathway maximizes viral entry efficiency, it concurrently preserves host countermeasures targeting critical CME nodes. In HIV-1 pathogenesis, post-CME entry triggers a dual immunosuppressive cascade—accelerated endosomal acidification facilitates capsid disassembly while synchronized viral protease activation degrades restriction factors (37). This dual strategy of physical compartmentalization and enzymatic elimination substantially diminishes host antiviral capacity. Crucially, CME’s systemic influence spans the entire viral life cycle, governing initial receptor engagement (38), cellular invasion (39, 40), intracellular trafficking (41) and progeny virion egress (42, 43), thereby demonstrating its critical pathological significance as a fundamental cellular process co-opted by viral pathogens.
CME serves as a fundamental entry strategy for diverse enveloped viruses, though viral molecular mechanisms exhibit marked heterogeneity in CCP dynamics and immune modulation. While SARS-CoV-2 (44) (Figures 2A, 3B), IAV, and PEDV all exploit CME for invasion, they employ distinct strategies: SARS-CoV-2 and IAV activate canonical CME by recruiting the AP-2 complex for Rab5-dependent trafficking to early endosomes (EE), where pH-dependent fusion occurs. Notably, SARS-CoV-2 selectively retains clathrin adaptors to suppress STING-dependent immune signaling during endosomal transport, enabling early immune evasion (45). In contrast, PEDV employs an evolved RNASEK-EPS15 hijacking mechanism—its S2 glycoprotein binds RNASEK (a CME initiation factor) to drive EPS15/AP-2/clathrin assembly at CCPs. This facilitates transport to late endosomes (LE) for genome release while boosting virion-endosome affinity and accelerating clathrin polymerization (46–48). Concurrently, this pathway redirects virions to immune-silent compartments and attenuates adaptive immunity by blocking MHC-I antigen presentation (49). Analogously, Peste des petits ruminants virus (PPRV) utilizes CME through extracellular vesicles but enhances infectivity via SLAM receptor modulation: its H protein suppresses miR-218 to upregulate SLAM expression, while activating SLAM signaling to reshape cytokine expression and establish an immunosuppressive microenvironment for persistent infection (50) (Figure 1A). Collectively, these divergent mechanisms underscore the dual role of the clathrin system as both a conserved entry gateway and a pliable interface for viral adaptation through tailored CCP regulation and immune evasion tactics.
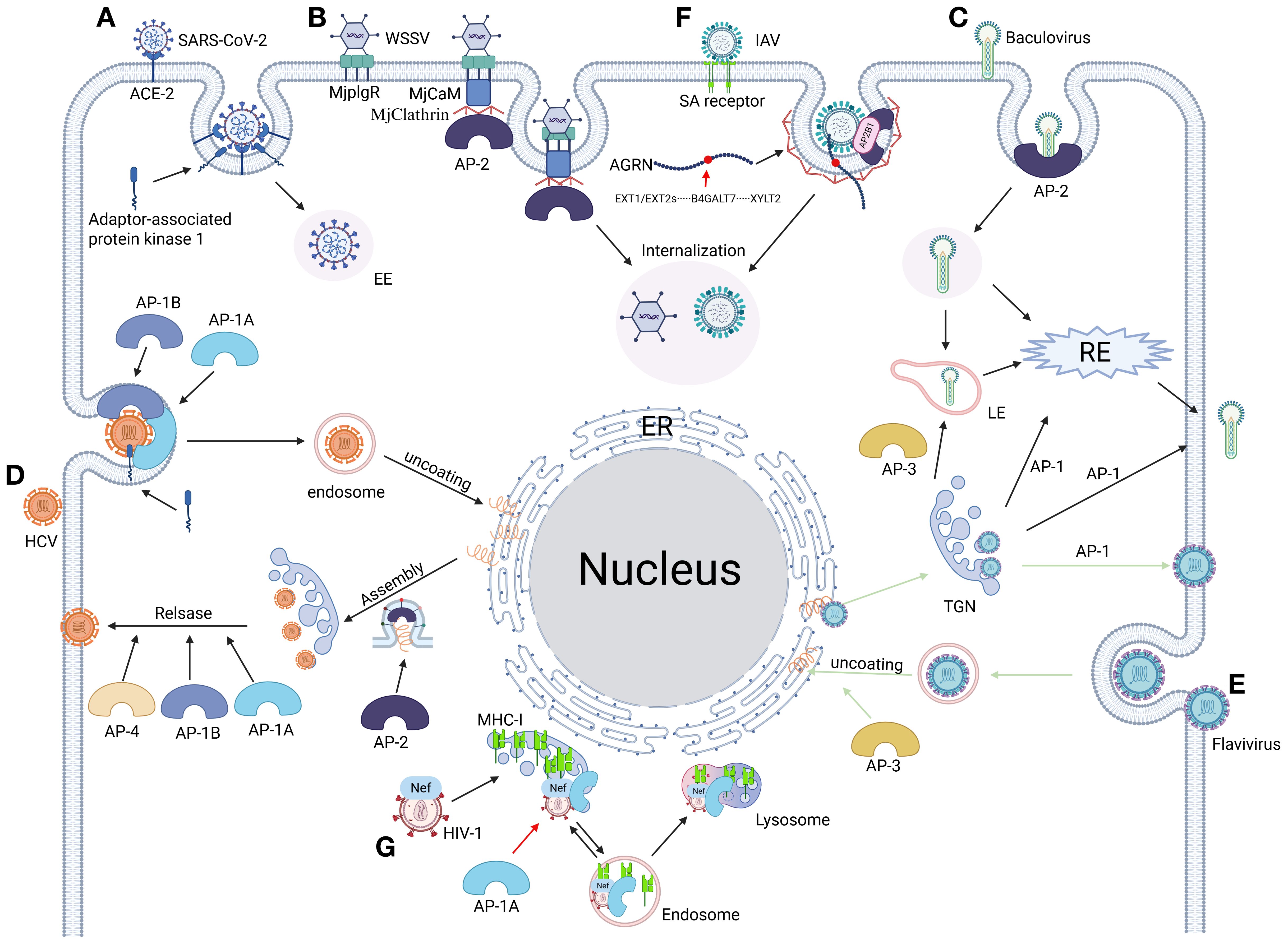
Figure 2. From invasion to release: the full cycle of viral replication mediated by adaptor proteins. (A) SARS-CoV-2 hijacks clathrin regulator AAK1 (AP-2-associated kinase 1) to drive AP-2-dependent endocytosis, mediating efficient viral entry (155, 275). (B) WSSV engages host receptor MjPlgR through luminal domain binding, activating a stepwise assembly: the receptor’s cytosolic domain recruits calmodulin (MjCaM), propagates a CaM-clathrin-AP2 cascade, and executes clathrin-mediated viral uptake (329). (C) Baculovirus coopts AP complexes for compartment-specific trafficking: AP-1 orchestrates virion assembly at the TGN and routes progeny to RE or plasma membranes. AP-2 steers clathrin-mediated viral internalization into EE and subsequent RE trafficking. AP-3 mediates the formation and transport of virions from the Golgi membrane to LE (410). (D) HCV commandeers adaptor protein complexes AP-1a, AP-1b, AP-2, and AP-4 to orchestrate stage-specific vesicular trafficking: AP-1a/1b-AP-2 axis directs entry, assembly, and cell-cell spread via host/viral protein interfaces. AP-1a/1b-AP-4 nexus coordinates late egress and cell-free virion dissemination (111). (E) Flaviviruses commandeer AP complexes for compartmentalized infection: AP-3 licenses endosomal maturation, enabling capsid uncoating in LE for cytoplasmic RNA release. AP-1 orchestrates TGN egress by packaging immature virions into secretory vesicles after trans-Golgi trafficking (151). (F) IAV undergoes heparan sulfate (HS)-mediated transport to the plasma membrane via Agrn protein binding, interacts with its HA surface protein, and recruits the AP2B1 subunit of the endocytic adaptor complex AP-2. This step initiates AP-2-dependent endocytosis, thereby achieving viral internalization (411). (G) The HIV-1 Nef protein subverts host immunity by hijacking the AP-1 complex’s μ1 subunit, redirecting newly synthesized MHC-I molecules from the Golgi apparatus to lysosomal degradation. This immune evasion strategy effectively blocks antigen presentation through Nef-mediated bridging of AP-1 to a non-canonical binding motif on the cytoplasmic tail of MHC-I (412). Figure created with BioRender.
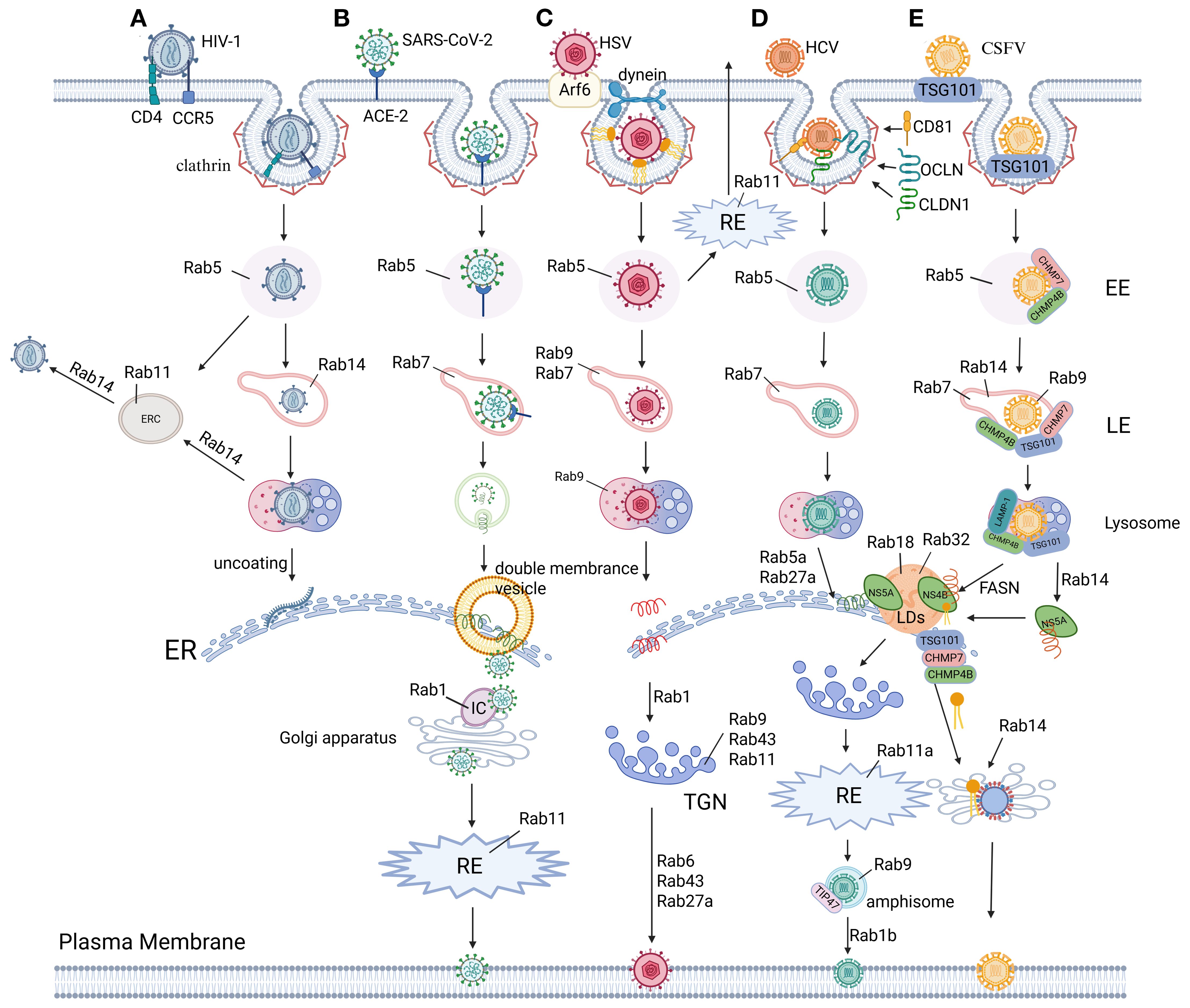
Figure 3. The role of Rab proteins in virus replication. (A) HIV-1 Env hijacks Rab GTPases for compartmentalized trafficking: Rab5 licenses EE sorting post-internalization. RAB14-dependent triage partitions Env into Rab11-ERC or LE/lysosomal degradation via endocytic intermediates. In addition, RAB14 steers retrograde trafficking of intact Env to the ER for viral replication. RAB14 restricts Env’s secretory axis, limiting PM delivery of viral glycoproteins (342, 344, 345). (B) SARS-CoV-2 entry initiates via ACE2 receptor binding and EMC. Post-internalization, the nucleocapsid uncoats in the cytoplasm to release the viral RNA genome. Viral envelopment occurs during ER-Golgi intermediate compartment (ERGIC) budding, followed by Rab1- and Rab11-dependent vesicular trafficking to the plasma membrane for virion release (10, 270–272). (C) HSV hijacks Rab GTPase networks for compartmentalized trafficking: CME via Rab5 delivers virions to EE, followed by Rab7/Rab9-mediated sorting into LE/lysosomes enroute to the ER for replication. Rab1 coordinates ER-to-Golgi transport of envelope proteins, while Rab43/Rab9/Rab11 mediate endosome-to-TGN trafficking for secondary envelopment. Export via trans-Golgi secretory vesicles requires Rab6, Rab43, and Rab27A, with Rab11 alternatively recycling plasma membrane-bound virions through RE (185–188, 314). (D) HCV hijacks Rab GTPases for compartmentalized trafficking: Post-attachment to CD81/CLDN1/OCLN, clathrin-mediated entry delivers virions to Rab5/Rab7-regulated endolysosomal transport for uncoating. Replication at the ER is coordinated by RAB5A and Rab27A, while Rab18/Rab32 direct core/NS5A trafficking to assembly membranes. Secretion involves Rab1b-mediated Golgi targeting, Rab11a-RE routing, and Rab9/TIP47-dependent exocytic sorting for non-lytic egress (180, 182, 184, 304). (E) CSFV hijacks ESCRT machinery and Rab GTPases for compartmentalized replication: Clathrin/TSG101-mediated entry delivers virions to Rab5-positive EE via ESCRT-I/III (TSG101/VPS25/CHMP4b/7). Rab5/Rab7/9/14 coordinate endolysosomal trafficking, with lysosomal LAMP-1/TSG101/CHMP4b enabling uncoating and ER-directed RNA release. NS4B recruits FASN to ER-lipid droplet interfaces, amplifying fatty acid synthesis via Rab18-FASN feedback to boost replication. Concurrently, NS5A redirects RAB14 to ER-Golgi ceramide flux, fueling sphingomyelin-enriched membranes for replication. Immature virions assemble in the ER lumen, maturing in the Golgi via Rab-dependent secretory trafficking (51, 53, 54, 97, 283–286). Figure created with BioRender.
In addition to the aforementioned viruses, the infection cycles of CSFV and ZIKV are also intricately linked to CME mechanisms. CSFV utilizes clathrin-coated vesicles to traffic into endolysosomal compartments (51) (Figure 3E), where its NS3 protease specifically cleaves mitochondrial antiviral-signaling protein (MAVS) to block type I interferon (IFN) signaling (52), thereby facilitating subsequent ER membrane remodeling and replication complex assembly (6, 53, 54). Similarly, ZIKV exploits HAVCR1-mediated clathrin endocytosis for endosomal entry (55), with its uncoating and replication processes being coordinately regulated by ubiquitination modifications (56, 57) and endoplasmic reticulum membrane reorganization (58–60). Strikingly, recent studies demonstrate that ZIKV’s NS4A protein hijacks the CME transport pathway to translocate to mitochondria, where it binds MAVS to inhibit RIG-I signaling complex formation (61). This vesicle-coupled immune evasion strategy markedly enhances viral replication efficiency. Collectively, these examples underscore CME’s conserved biological role across phylogenetically distinct viruses: by precisely modulating endosomal trafficking and immune signaling checkpoints, it establishes a molecular foundation for viruses to gain early infection advantages.
While CME persists as the canonical entry pathway for numerous viruses, emerging evidence delineates alternative mechanisms that expand our understanding of viral entry biology. Recent studies have characterized non-canonical strategies: HSV-1 hijacks PAK1-dependent macropinocytosis through Na+/H+ exchanger activation (62, 63), while LASV exploits cholesterol-rich membrane microdomains via glycoprotein-mediated docking before leveraging Rab7-coupled endosomal maturation for fusion (38, 64). These discoveries not only reaffirm CME’s central role in viral pathogenesis but also reveal the increasing complexity of entry route diversification, demanding refined models of tropism to inform therapeutic targeting. Crucially, such alternative pathways exhibit intrinsic immunomodulatory properties—HSV-1’s macropinocytic entry shortens cytoplasmic exposure of viral nucleic acids to limit pattern recognition receptor activation (63), whereas LASV’s preferential use of LE as fusion sites circumvents TLR3-mediated surveillance mechanisms predominant in early compartments (65). Collectively, these findings illustrate the dual evolutionary logic of viral entry strategies: they balance mechanistic conservation (leveraging established pathways like CME) with context-driven innovation (adapting alternative routes), ultimately optimizing host-cell adaptation while evading immune detection. This dynamic equilibrium positions viral entry as a prime target for therapies exploiting pathway vulnerabilities at the host-pathogen interface.
2.2 Dynein
Dynein, a microtubule-associated motor protein, coordinates intracellular trafficking of cellular cargo and viral particles across critical stages of pathogenesis. This molecular motor directly facilitates viral entry (66), genome replication (14), virion assembly (67), and progeny release (68), with its transport machinery hijacked by viruses to propel capsids through the cytoplasmic milieu (69). Notably, dynein-mediated microtubule trafficking exhibits a precisely balanced dual role in immune regulation during infection. In host defense, dynein collaborates with TAOK1 kinase to translocate TAOK1 along microtubules to the perinuclear region, where phosphorylation-dependent binding to IRF3 stabilizes the TBK1-IRF3 signaling complex. This spatial reorganization activates the type I interferon pathway, inducing antiviral effector protein expression to establish a cell-wide antiviral state (70). Conversely, viruses exploit dynein-mediated pathways to subvert immunity through distinct mechanisms. For instance, IAV interacts with dynein via the LC3-PCNT autophagy adaptor complex, which simultaneously promotes nucleocapsid uncoating and disrupts antiviral signal translocation to perinuclear reaction centers. Mechanistically, after endosomal fusion releases vRNPs into the cytoplasm, the LC3-PCNT complex acts as a dynein adaptor through two distinct interactions: (1) LC3 binds to vRNP-associated M1 matrix proteins, while (2) PCNT recruits dynein heavy chains. This assembly enables dynein-mediated transport of vRNPs along microtubules, facilitating their uncoating and nuclear import (71). Concurrently, viral nucleoprotein (NP) hijacks mitophagy to degrade mitochondrial antiviral-signaling protein (MAVS), thereby blocking the assembly of perinuclear antiviral complexes dependent on MAVS-mediated RIG-I signaling (72). By spatiotemporally decoupling host immune responses from viral replication, this strategy enables immune escape and infection spread. Sizmilarly, EBV employs an alternative strategy through its BHRF1 protein, which induces microtubule hyperacetylation to hijack dynein-mediated retrograde transport. This drives mitochondrial clustering near the nucleus, disrupting the dynamic mitochondrial network required for MAVS assembly. Consequently, interferon production is blocked, allowing EBV to evade innate immune surveillance (73) (Figure 1B). These contrasting mechanisms underscore dynein’s dual nature: while essential for antiviral signaling, its trafficking pathways are co-opted by viruses to either spatially coordinate immune suppression or dismantle organelle-based antiviral platforms, highlighting its pivotal yet conflicted role in infection outcomes.
Structurally, dynein motor family comprises three evolutionarily conserved subfamilies with distinct roles: cytoplasmic dynein-1 (DYN1), responsible for organelle transport (endosomes (74), vesicles (75), lysosomes (76)) and mitotic spindle organization (77); cytoplasmic dynein-2 (DYN2), specialized in retrograde intraflagellar transport (IFT) essential for ciliogenesis (78, 79); and axonemal dynein, which powers motile cilia function (80). Despite sharing structural components—heavy, intermediate, light intermediate, and light chains (81) —DYN1 and DYN2 exhibit functional divergence (82, 83), largely governed by IC-LC subunit interactions that regulate ciliary assembly and retrograde trafficking (84–87). Virions exploit this machinery by hijacking dynein heavy chains to traffic replication complexes to perinuclear regions thereby suppressing type I interferon production (88) (Figure 4). Pathologically, dynein-2 subunit mutations correlate with ciliopathies, including thoracic dystrophy (89), ciliary dysfunction (90), and neural tube defects (91). A striking example of dynein’s duality is the light chain Tctex-1, which enhances poliovirus replication by stabilizing host-cell receptor interactions (92) yet is indispensable for DENV assembly, evidenced by defective subviral particle formation in Tctex-1-depleted systems (93). Together, these mechanisms underscore dynein’s central role as a spatiotemporal conductor of viral pathogenesis, coordinating infection dynamics from cellular entry to immune evasion.
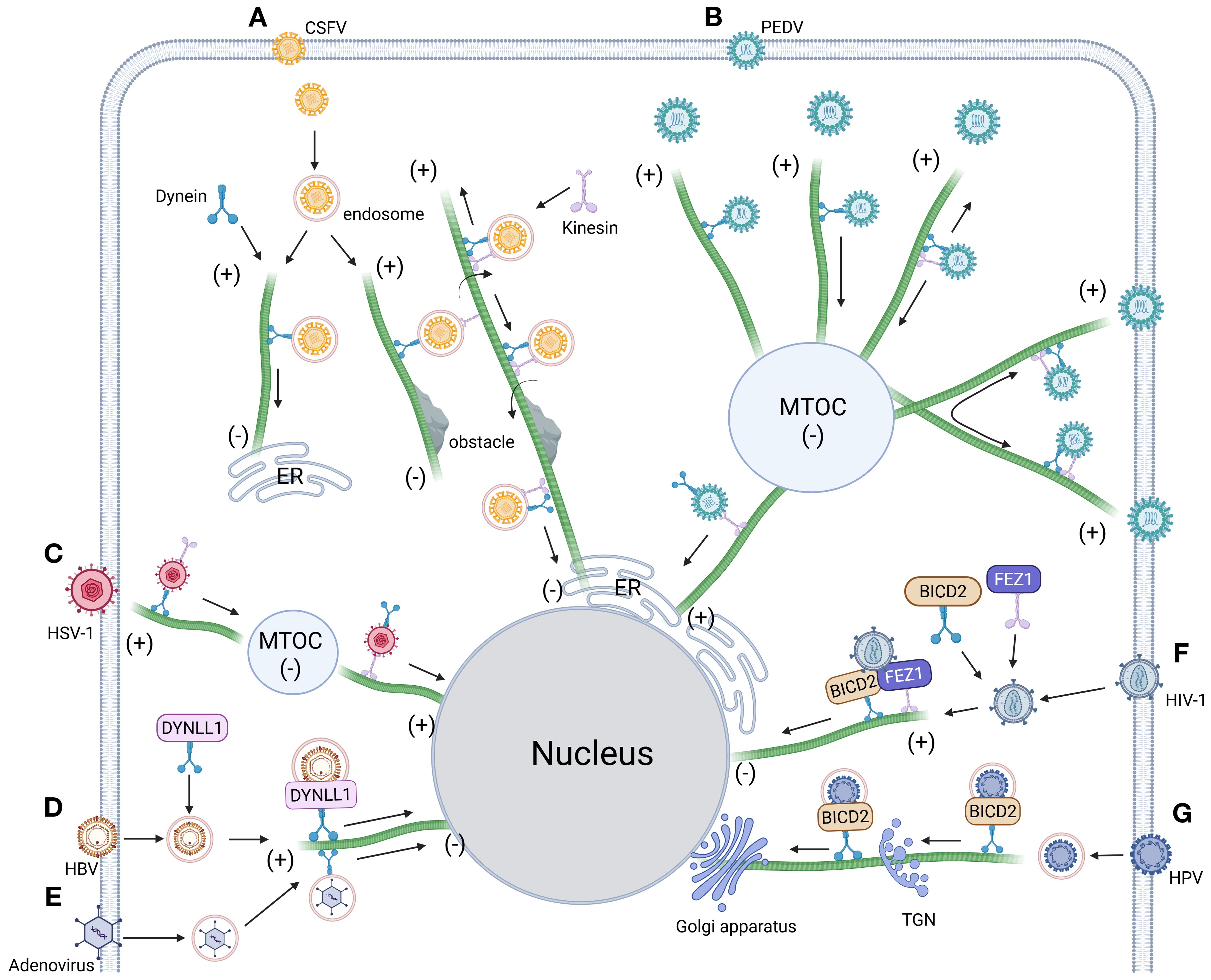
Figure 4. Intracellular trafficking model of virus dependent dynein. (A) Dynein orchestrates CSFV-endosome transport along microtubules toward polarized regions. Obstacle encounters trigger dynein-kinesin-1 coordinated navigation for barrier circumvention, followed by resumed microtubule transport culminating in ER docking (97). (B) The intercellular transport of PEDV along microtubules driven by dynein and kinesin-1 includes quiescent state, unidirectional movement toward the negative end of microtubules, bidirectional movement along the same microtubule and bidirectional movement along different microtubules (103). (C) HSV-1 hijacks the microtubule network to drive retrograde transport of viral cargoes, navigating first to the microtubule organizing center (MTOC) before ultimately localizing to the nuclear compartment (315). (D) HBV co-opts dynein light chain DYNLL1 to form capsid-associated complexes, orchestrating microtubule-dependent trafficking toward the nuclear compartment (303). (E) Post-entry adenovirus hijacks dynamin-mediated transport to navigate microtubule minus-end-directed trafficking toward the nuclear periphery (408). (F) Post-entry HIV-1 co-opts dynein adaptor BICD2 and kinesin-1 adaptor FEZ1 to coordinate microtubule-dependent retrograde transport, ensuring targeted nuclear entry (100). (G) HPV commandeers dynein adaptor BICD2 via capsid binding, coupling virions to retrograde motors for microtubule-driven trafficking along the endosome-TGN/Golgi axis (409). Figure created with BioRender.
Microtubules, polarized cytoskeletal highways composed of α/β-tubulin heterodimers, serve as directional tracks for dynein-driven retrograde transport—a system systematically exploited by viruses to navigate the cytoplasm toward replication sites. These dynamic filaments, operating alongside actin and intermediate filaments in eukaryotic cells (94–96), are co-opted by CSFV following clathrin-mediated entry (51). Live-cell imaging reveals that dynein and kinesin-1 coordinately mediate CSFV trafficking along acetylated microtubules, with their opposing motor activities fine-tuning viral replication efficiency through bidirectional transport regulation (97). After TSG101/Rab7-dependent sorting into LE, CSFV undergoes dynein-powered retrograde transport to lysosomes for capsid disassembly before ER docking (Figures 4A, 3E). Intriguingly, pharmacological inhibition of retrograde motility triggers an adaptive response: kinesin-1-dependent anterograde movement bypasses endolysosomal processing bottlenecks, rerouting virions directly to ER replication hubs. This compensatory mechanism underscores the evolutionary sophistication of viral trafficking strategies in overcoming host transport barriers.
Viral exploitation of host microtubule transport systems reflects an evolutionary balancing act, where pathogens optimize transport efficiency while evading host defense pathways. HIV-1 and HSV-1 exemplify this dichotomy: while both hijack dynein-dependent microtubule transport and utilize dynactin subunit 1 (DCTN1) as a trafficking adaptor (98, 99) (Figures 4C, F), their mechanisms diverge significantly. HSV-1 maintains infectivity across varying DCTN1 expression levels without perturbing mitochondrial function, whereas DCTN1 competitively disrupts HIV-1’s early infection cycle by sequestering cytoplasmic linker protein 170 (CLIP-170). CLIP-170 promotes HIV-1 core uncoating and microtubule-dependent nuclear trafficking via its zinc finger 2 (ZnF2) domain, either through direct core binding or recruitment of proviral cofactors (100–102). DCTN1 antagonizes this process by displacing CLIP-170 from viral complexes through competitive ZnF2 domain binding, thereby impairing nuclear transport. These interactions underscore the precise regulatory interplay between microtubule motors (dynein/kinesin) and adaptor proteins in establishing bidirectional transport highways critical for viral pathogenesis. Expanding this paradigm, Hou et al. (103) (Figure 4B) identified five microtubule trafficking modes during PEDV infection: anterograde, retrograde, bidirectional oscillation, microtubule-switching, and static anchoring—all mediated by dynein-kinesin-1 coordination. Notably, during static anchoring, PEDV induces microtubule deacetylation to suppress HDAC6-dependent innate immune signaling, creating an immune-evasive niche favorable for replication (104). Collectively, these studies illuminate conserved viral strategies for co-opting microtubule dynamics—orchestrating transport efficiency while neutralizing host defenses through spatiotemporal regulation of motor complexes and post-translational modifications.
2.3 Adaptor protein complex
The adaptor protein (AP) complex, a heterotetrameric assembly regulating CME and retroviral morphogenesis (105, 106), comprises five evolutionarily conserved subfamilies (AP-1 to AP-5) that coordinate compartment-specific cargo sorting. These molecular orchestrators exhibit distinct subcellular localizations across membranous organelles (Figure 2), governing processes from synaptic vesicle recycling to lysosomal maturation. Mechanistically, AP-2 enhances IAV and coronavirus entry by stabilizing CCP formation (107, 108), while AP-1 facilitates HIV-1 replication by mediating Gag polyprotein trafficking between the TGN and endosomes (109). In contrast, AP-3 supports herpesvirus assembly through lysosome-related organelle (LRO) membrane remodeling (110), whereas AP-4 directs HCV secretion via non-canonical endosomal trafficking pathways that bypass Golgi processing (111). Notably, AP-5 exacerbates ZIKV neurotropism by disrupting autophagosome-lysosome fusion (112), with pathogenic mutations in its associated spastic paraplegia genes (SPG11/SPG15) potentially sensitizing neurons to viral triggers of Parkinsonian neurodegeneration (113). Collectively, these findings position AP complexes as central players in viral pathogenesis, mediating tropism-specific interactions that bridge host trafficking machinery to viral replication cycles.
AP-1, a Golgi basal body/endosome-associated adaptor protein complex, serves as a central hub for intracellular membrane trafficking, with its modular subunit architecture and dynamic functionality making it a strategic focal point in the virus-host interplay (114–116) (Figure 2D). This complex exhibits a dual role during viral pathogenesis: its γ subunit-mediated transport machinery supports viral assembly, such as HIV-1 leveraging γ1/γ2 isoforms to antagonize CD317/tetherin and facilitate viral budding (109, 117, 118), while structural vulnerabilities like the μ subunit’s tyrosine-binding domain are hijacked for immune evasion—exemplified by HIV Nef degrading MHC-I via the μ1 subunit (119) and CMV-m154 disrupting AP-1 function to eliminate T cell costimulatory molecules (120). This pan-viral dependency extends across diverse pathogens: ASFV remodels viral factories through CD2v-AP-1 interactions (121), alphaherpesviruses exploit the μ1B subunit to redirect basolateral glycoproteins for transmission tunnel formation (122), DENV and VZV rely on AP-1 for viral factory reorganization and maturation (123, 124), while HEV and HCV employ AP-1A/B isoforms to mediate capsid generation and dual-release pathways, respectively (105). Notably, the functional specialization of AP-1 subtypes—AP-1A orchestrating lysosomal trafficking and AP-1B regulating basolateral recycling (125)—originally evolved as a host defense strategy, yet is subverted by viruses for spatiotemporally targeted attacks. For instance, HIV hijacks the AP-1/PACS-1 axis to manipulate MHC-I recycling: Nef is recruited to the Golgi via PACS-2, activating the SFK-PI3K signaling axis to induce PIP3 accumulation on the plasma membrane. This drives ARF1/6-dependent internalization of MHC-I into AP-1-coated vesicles, which are then redirected by the Nef-AP-1-PACS-1 ternary complex to perinuclear compartments, systematically blocking antigen presentation (126, 127) (Figure 1C). These mechanisms underscore AP-1’s structural vulnerabilities—its tyrosine-binding domains and basic clusters—as dual-purpose hubs, essential for physiological trafficking yet exploited as viral targets (128, 129). Consequently, therapeutic strategies targeting AP-1-virus interaction nodes, such as blocking μ1-mediated immune evasion, modulating AAK1/GAK kinase activity, or developing subtype-specific inhibitors, offer precision interventions to disrupt viral lifecycles while preserving cellular homeostasis, thereby opening new avenues for antiviral therapeutics.
The AP-2 complex—a heterotetramer of α, β2, μ2 (AP2M1), and σ2 subunits—acts as a central nexus for viral exploitation of host trafficking machinery, leveraging its structural plasticity and cargo-sorting motifs to mediate infection stages from entry to egress (130–132) (Figure 2B). Viral pathogens strategically target AP-2 subunits through precise motif recognition: HIV-2 Env binds the AP-2 complex via its cytoplasmic GYxxΦ motif to orchestrate viral particle release (133), while MLV glycoGag enhances HIV-1 infectivity by engaging the μ2 subunit’s YXXL sorting signal (134). Immune evasion mechanisms are similarly dependent on AP-2, exemplified by HIV-1 Nef hijacking the α/σ2 subunits—via its central loop and critical residues like Arg-134—to drive CD4 receptor endocytosis and immune evasion (135–137). During viral entry, AP-2-dependent CME is exploited by diverse pathogens including HCV (138), HBV (139), PRV (140), and human coronavirus HCoV-229E (107). HBV further underscores this dependency, with its pre-S1 domain directly binding AP-2 and clathrin heavy chain, and pharmacological inhibitors like chlorpromazine blocking infection by disrupting AP-2 function (139). Beyond entry, AP-2 coordinates viral dissemination through subunit-specific mechanisms: DENV requires BIKE kinase-mediated phosphorylation of AP-2 μ2 at T156 to drive both post-internalization trafficking and assembly/egress, with BIKE inhibitors (e.g., 5Z-7-oxozeaenol) potently blocking infection in vitro and ex vivo. The antiviral effect is mechanistically linked to BIKE activity, as its overexpression rescues viral replication. Notably, 5Z-7-oxozeaenol exhibits broad-spectrum activity against diverse RNA viruses with a high resistance barrier, highlighting host-targeted antiviral advantages (141, 142). Vaccinia virus recruits AP-2 and clathrin via the NPF motif of its A36 protein to activate Cdc42/N-WASP-driven actin polymerization, enabling cell-to-cell spread (143); and lentiviruses co-opt AP-2 to degrade the host restriction factor tetherin, illustrating dual-pathway hijacking for viral release and immune suppression (144). These findings position BIKE-AP-2 interplay as a nexus linking post-translational modification (T156 phosphorylation) and antiviral gene regulation—e.g., interferon-induced AP2M1 downregulation counteracts viral egress, while DENV subverts this through translational reprogramming (142). These collective interactions position AP-2 as a master regulator of viral pathogenesis, with therapeutic targeting of its subunit interfaces (e.g., μ2 cargo-binding domains), regulatory kinases (AAK1/BIKE), or motif-driven trafficking networks offering a promising strategy for broad-spectrum antivirals that disrupt viral commandeering of host transport systems.
The AP-3 complex, composed of β3a, δ, μ3, and σ3 subunits, orchestrates lysosomal trafficking and organelle biogenesis through δ/μ3 HEAT domain-mediated sorting signal recognition (145, 146), a process co-opted by diverse viruses for pathogenesis. HIV-1 exemplifies multi-layered exploitation: its Gag protein hijacks the δ subunit to redirect virions to late endosomal compartments (147, 148), while μ3b subunit interactions facilitate endosomal membrane penetration during assembly—mechanisms pharmacologically disruptible via targeted inhibition (149, 150). Flaviviruses paradoxically rely on AP-3 for replication organelle formation, though AP-3 deficiency selectively impairs JEV and DENV RNA synthesis while inducing HPS-2 (151, 152) (Figure 2E). HHV-7 further exploits AP-3μ3 via its U21 protein to divert MHC-I molecules to lysosomes for immune evasion, countered by host μ3 regulatory mechanisms (110). Evolutionary divergence in viral hijacking is evident in HRSV, which engages μ3A via YXXL motifs in its matrix (M) protein to drive assembly (153), versus Nipah virus, which manipulates β3A hinge dynamics to subvert budding (154). Conversely, AP-3 also exerts antiviral functions, as SARS-CoV-2’s envelope (E) protein is sequestered by β3A binding, suppressing virion release (155). The complex’s structural plasticity, including δ/σ3 recognition of [DE]XXXL[LI] motifs (156), bridges lysosomal disorder mechanisms and therapeutic opportunities, exemplified by strategies targeting HIV-1 Gag-δ binding (147), blocking HRSV M-μ3A interactions (153), or disrupting paramyxovirus budding via β3A interference (154). These multifaceted interactions position AP-3’s modular architecture as both a critical vulnerability in viral replication cycles and a template for structure-guided antiviral design, balancing its roles as a viral accomplice and host defender.
2.4 Rab protein
Rab GTPases—a conserved family of small GTP-binding proteins—serve as master regulators of eukaryotic membrane trafficking, governing spatiotemporal precision in vesicular transport through GTPase-driven molecular switching (157). These proteins coordinate sequential vesicle dynamics (budding (158), directional transport (159), docking (160), and fusion (161)) via GTP/GDP cycling while integrating broader cellular processes such as secretory pathway modulation (162), clathrin-independent endocytic sorting (163), lysosomal cargo processing (164), and chemotactic signal transduction through membrane compartmentalization (165). By functioning as molecular integrators, Rab GTPases establish a regulatory nexus that links core trafficking fidelity to pleiotropic host cell functions, creating diverse mechanistic interfaces exploitable by viral pathogens (Table 2).
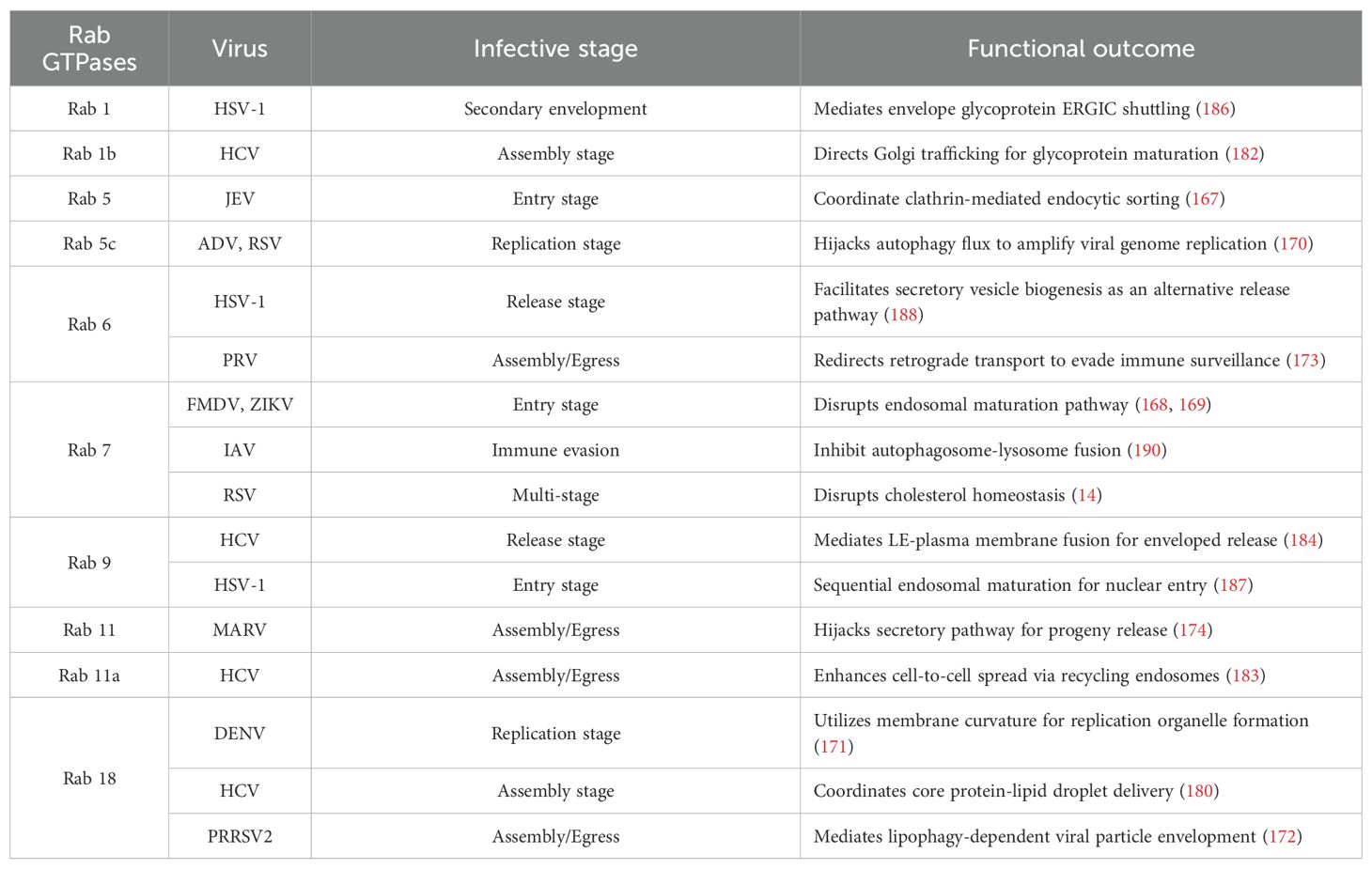
Rab GTPases serve as indispensable host factors enabling viral proliferation through spatiotemporal regulation of vesicular trafficking across infection stages (166). During viral entry, JEV exploits Rab5/Rab11-coordinated endocytic sorting (167), while FMDV and ZIKV subvert Rab5/Rab7-mediated endosomal maturation (168, 169). Replication strategies diverge: ADV and RSV hijack Rab5c-driven autophagic flux to amplify genome synthesis (170), whereas DENV utilizes Rab18-dependent membrane curvature for replication organelle formation (171). In assembly/egress phases, PRRSV2 co-opts Rab18-governed lipophagy for virion envelopment (172), PRV redirects Rab6-controlled retrograde transport to evade immunity (173), and MARV hijacks Rab11-modulated secretory pathways for progeny release (174). Strikingly, RSV exemplifies multifunctional exploitation—disrupting Rab7–RILP (Rab-interacting lysosomal protein) complexes to impair cholesterol homeostasis while commandeering Rab11a-enriched recycling endosomes to enhance cell-to-cell spread (14, 175)—highlighting Rab networks as versatile targets for viral hijacking.
Rab GTPases emerge as universal conductors for viral exploitation of membrane trafficking systems, operating with precise spatial and temporal control throughout infection cycles (Figure 3). During early infection, Rab5 orchestrates clathrin-mediated endocytic sorting to facilitate viral entry (176), while Rab7 governs endosomal maturation pathways that bifurcate towards either lysosomal degradation or replication-optimized compartments (177). Post-uncoating, Rab5A stabilizes ER-associated replication complexes to amplify viral RNA synthesis (178), concurrently partnering with Rab27A to shield viral genomes within transport vesicles from cytoplasmic immune sensors (179). The viral subversion of Rab networks extends through particle assembly to egress: HCV demonstrates a multi-Rab assembly cascade where Rab18/32 coordinate core protein-lipid droplet delivery (180, 181), Rab1b directs Golgi transit for glycoprotein maturation, and Rab11a activates RE machinery for pre-assembly intermediate processing (182, 183), culminating in Rab9-TIP47-mediated LE-plasma membrane fusion for enveloped release (184) (Figure 3D). HSV-1 expands this manipulation by sequentially engaging Rab5/Rab7/Rab9 for nuclear entry through endosomal maturation (185), followed by Rab1-mediated ERGIC shuttling of envelope glycoproteins and Rab43/Rab9/Rab11-driven TGN sorting during secondary envelopment (186, 187). The virus further subverts Rab11 recycling pathways for non-canonical plasma membrane egress while exploiting Rab6-mediated secretory vesicle biogenesis and Rab43/Rab27A-dependent vesicle docking for alternative release routes (188, 189) (Figure 3C). Notably, certain viruses inversely exploit Rab inactivation for immune evasion, exemplified by IAV hijacking M2 protein to block Rab7-mediated autophagic degradation: by disrupting Rab7-TBC1D5 interaction, M2 prevents GTP hydrolysis, trapping Rab7 in an inactive state. This selective impairment of Rab7 activity aborts autophagosome-lysosome fusion, preserving intracellular virions from degradation while enabling their plasma membrane budding (190) (Figure 1D). These coordinated strategies – ranging from Rab activation for trafficking optimization to targeted Rab inactivation for immune escape – reveal Rab GTPases as dual-purpose nodes whose conserved yet adaptable regulatory mechanisms are systematically repurposed across viral taxa. The evolutionary convergence on Rab-centric membrane trafficking subversion underscores their position as prime therapeutic targets for intercepting viral transport logistics across infection paradigms.
3 Vesicular trafficking mechanisms are effective antiviral targets
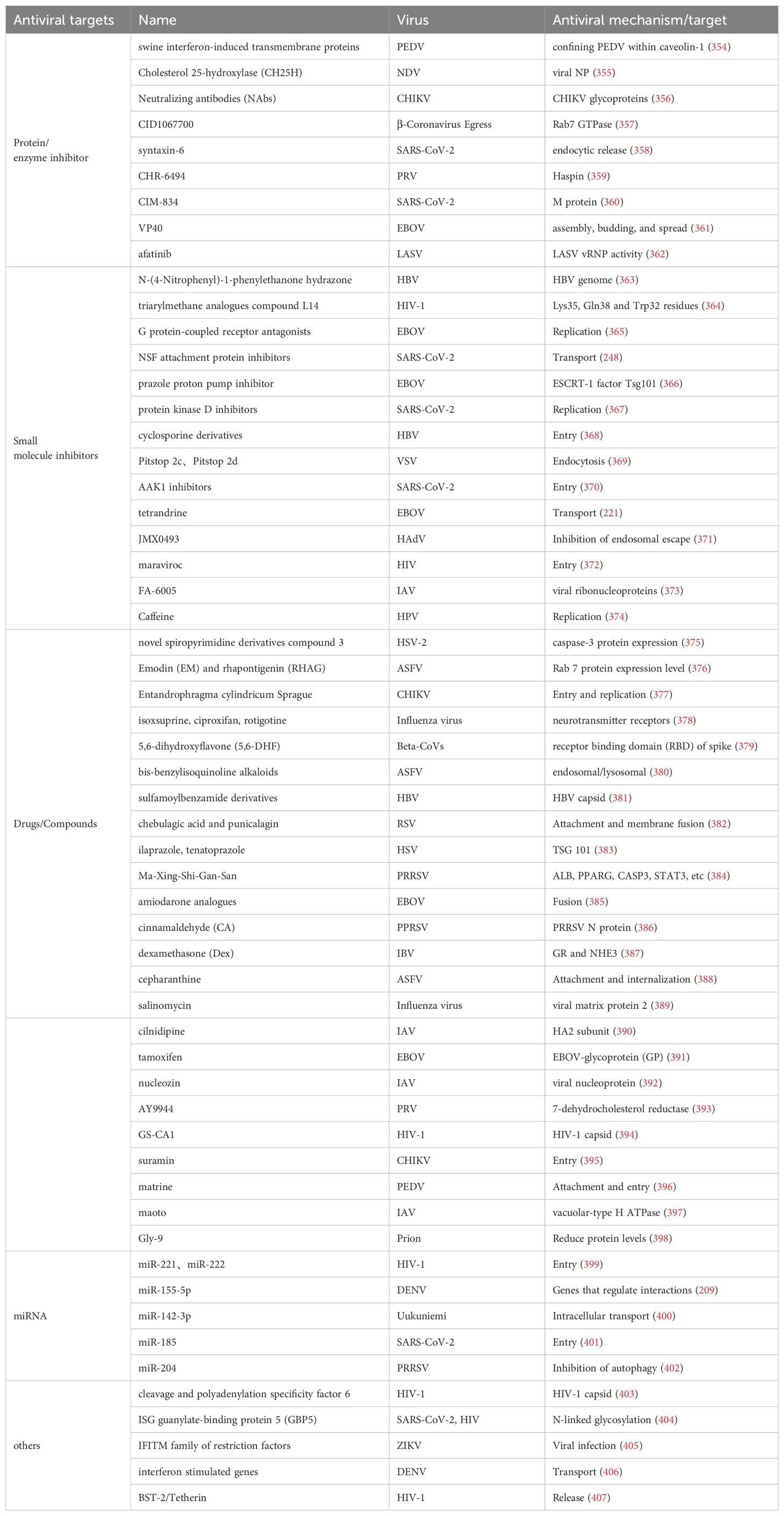
Viral infections persistently threaten global public health, driving an urgent need for innovative pharmacological strategies to counteract rapidly evolving pathogens. Small-molecule inhibitors and host-directed antivirals have emerged as key pillars of antiviral therapy, offering distinct advantages over traditional approaches. These agents disrupt viral proliferation through a dual mechanism: targeting either evolutionarily conserved host pathways—such as those regulating viral replication complexes or entry receptors [e.g., 1,3-diphenylurea derivatives inhibit viral entry (191), Tubeimosides blocking endosomal fusion (192)]—or hijacking pathogen-specific vulnerabilities (Figure 5) (Table 3). For example, the small-molecule inhibitor EDP-235 achieves broad-spectrum antiviral activity by irreversibly binding to the highly conserved 3CL protease (3CLpro), which is an essential viral enzyme for the replication of coronaviruses such as SARS-CoV-2 (193). In contrast, CD-MUS—an entry inhibitor based on β-cyclodextrin—targets the host heparan sulfate proteoglycan (HSPG), a universal entry receptor utilized by various respiratory viruses including RSV and DENV. This inhibitor blocks viruses’ attachment through multivalent sulfonate interactions and irreversibly neutralizes particles across multiple virus families (194). The dual approach leverages host-pathogen interdependence to achieve broad-spectrum efficacy against diverse RNA viruses while minimizing drug resistance development.
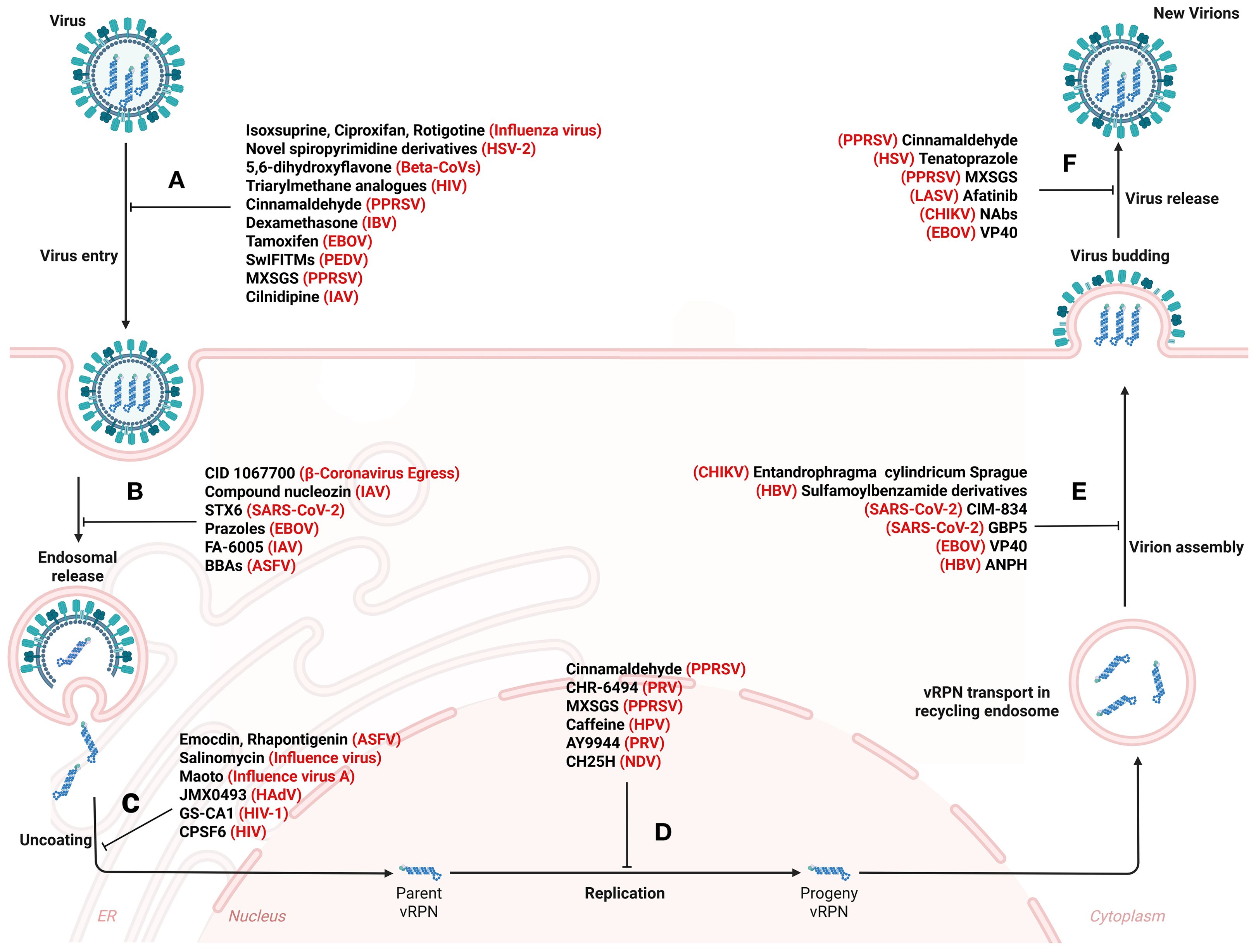
Figure 5. Systemic disruption of viral propagation: therapeutic roadblocks from invasion to particle release. Small molecule drugs act on vesicle transport pathways, thereby affecting virus attachment and entry (A), endosomal vesicle transport (B), uncoating (C), replication (D), assembly (E), budding and release (F). The detailed antiviral mechanism is shown in Table 3. Figure created with BioRender.
3.1 Orchestrating vesicular hijacking: multimodal antiviral strategies targeting the host pathogen interface
Antiviral therapies achieve multimodal disruption of viral life cycles through two complementary mechanisms—vesicle/organelle manipulation and host pathway engineering—that synergistically intercept vesicular trafficking networks. Specifically, vesicle rerouting strategies exploit transport pathway vulnerabilities to generate non-infectious virions, as exemplified by Rab5b-mediated rerouting diverting HBV surface proteins from ER trafficking to multivesicular body (MVB) sequestration (195), This spatial interference simultaneously activates MAVS-dependent mitochondrial antiviral signaling while exposing viral particles to innate immune “double hits” through enhanced type I interferon secretion prior to degradation (196). Similarly, Copb2 depletion traps SARS-CoV-2 progeny within ERGIC via COPII vesicle blockade (197), a mechanism that paradoxically improves MHC-I antigen presentation efficiency as proteasomes more effectively process viral antigens retained in ERGIC compartments (198). By exploiting viral evolutionary dependence on host trafficking systems, these tactics limit viral adaptation to therapeutic pressure while preserving cellular homeostasis. Complementing spatial diversion approaches, membrane fusion sabotage mechanisms are typified by interferon-induced transmembrane protein 3 (IFITM3), which neutralizes PRRSV fusion capacity through cholesterol-driven membrane curvature generation, creating steric hindrance for envelope fusion and reducing progeny infectivity (199). Notably, IFITM3 stabilizers under clinical evaluation demonstrate synergistic effects when combined with endocytosis inhibitors, though their lipid-modifying activity demands rigorous lipidomic monitoring to mitigate off-target effects on cholesterol metabolism in non-infected cells (200).
The latest research demonstrates that the dengue virus nonstructural protein achieves immune escape through a multistep mechanism involving PRDM1-mediated downregulation of host Nod-like receptor (NLRP12), hijacking of heat shock protein 90 (HSP90), and disruption of the NLRP12-HSP90 interaction essential for type I interferon signaling. This coordinated suppression not only inhibits antiviral interferon-stimulated genes such as IFITM3 but also cripples host defenses by dismantling the interferon pathway. These findings support therapeutic strategies combining IFITM3 stabilizers with endocytosis inhibitors to counteract viral immune evasion (201). Emerging clinical paradigms further integrate these approaches with PD-1 blocking antibodies, leveraging the dual benefit of viral replication suppression (via trafficking disruption) and T-cell reinvigoration (through checkpoint inhibition)—a strategy currently validated in preclinical models of chronic HBV coinfection (202). Concurrent interventions leverage interferon lambda 1 (IFN-λ1) to enhance autophagic flux through ATG10 activation, synchronizing accelerated lysosomal degradation (42) with reprogramming of intracellular trafficking networks via Rab1-regulated ER-Golgi transport, Rab11-dependent recycling endosome dynamics, and Rab6-mediated secretory vesicle exocytosis. The strategy further incorporates STING agonists to amplify the cGAS pathway activation, enabling autophagy-encapsulated viral DNA to trigger secondary interferon waves and establish a self-reinforcing innate immunity loop. This multilayer approach mirrors natural host defenses by converging modular viral lifecycle interventions with systemic cellular rewiring to undermine pathogen adaptability. Through simultaneous disruption of host-pathogen coadaptation and amplification of innate antiviral mechanisms, the dual-axis framework provides a blueprint for broad-spectrum therapies that target viral plasticity while maintaining cellular homeostasis.
Enzyme-targeted antiviral strategies achieve multimodal disruption of viral trafficking through three spatially coordinated mechanisms. The first axis targets ADP-ribosylation factor (ARF) GTPases, master regulators of post-Golgi transport. Their inhibition traps ZIKV, IAV, and SARS-CoV-2 virions in compromised Golgi compartments, forcing lysosomal degradation through impaired export competence (203). This leverages viruses’ evolutionary dependence on conserved host trafficking machinery, which constrains their mutational escape from such interventions. The second mechanism involves PIKfyve kinase blockade, which induces rapid phosphoinositide depletion to collapse ESCRT-dependent endosomal maturation. This dual-action strategy dismantles replication niches in VSV and ZIKV infections while suppressing NF-κB-driven inflammation (204). Concurrently, the resulting mitochondrial DNA stress activates the cGAS-STING pathway, establishing an interferon-independent antiviral state that broadens protection against diverse RNA viruses (205). The third frontier focuses on sphingosine kinases SK1/SK2. Their inhibition executes biphasic containment: chemically, it inverts ceramide-to-S1P ratios to disrupt LE-lysosome fusion in coronaviruses and EBOV; mechanically, it induces cortical actin polymerization to stiffen endocytic membranes, physically blocking enveloped virion penetration (206). This therapeutic triad—intercepting vesicle maturation checkpoints (ARF GTPases), destabilizing replication organelle integrity (PIKfyve), and reinforcing membrane rigidity (SK1/SK2)—establishes complementary metabolic barriers that counteract evolutionarily conserved viral entry/egress mechanisms through spatial compartmentalization. By strategically mirroring the layered defense logic of innate immunity, this tripartite strategy effectively converts the host’s subcellular compartmentalization into an insurmountable fortress against viral invasion through coordinated structural and functional interventions. Notably, current preclinical research has begun exploring the combination of this enzyme-targeting strategy with PD-1 blocking antibodies, which concurrently achieves viral replication suppression and cellular exhaustion alleviation (207). This dual therapeutic effect thereby demonstrates significant potential for transforming conventional antiviral approaches into an integrated paradigm of immune reconstitution therapy, as evidenced by recent experimental findings.
3.2 Vesicle-mediated antiviral drug delivery and miRNA modulation
The intricate interplay between viral pathogenesis and host vesicular transport systems has catalyzed therapeutic innovation through mechanistic insights, thereby driving the development of vesicle-centric strategies to intercept viral life cycles. Notably, vesicle-mediated drug delivery systems are emerging as precision tools targeting intracellular trafficking nodes, exemplified by the nitazoxanide derivative tizoxanide, which suppresses DENV-2 replication through dual disruption of vesicular transport dynamics and host post-translational modification pathways (208). These interventions exploit the evolutionary persistence of core cellular logistics networks, imposing mutational ceilings on viral escape by anchoring therapeutic pressure to immutable host constraints. Concurrently, microRNA-155-5p (miR-155-5p) orchestrates a biphasic antiviral response that simultaneously downregulates interferon signaling, TP53 tumor suppressor pathways, and vesicle trafficking machinery while potentiating cellular defense mechanisms, revealing novel therapeutic targets in dengue pathogenesis (209). This layered regulation exemplifies a systems-level therapeutic paradigm where host network modulation at critical inflection points supersedes direct viral targeting. Intriguingly, the ER-anchored STING protein exhibits paradoxical functionality: it inhibits coronavirus replication by blocking ER-derived double-membrane vesicle biogenesis (210), yet acts as a proviral scaffold for RNA viruses such as enterovirus D68 and SARS-CoV-2 during replication complex assembly (211). Such duality underscores the inherent biological trade-offs in manipulating host machinery—balancing antiviral potency against the risk of inadvertently supporting viral adaptation. Collectively, these discoveries delineate vesicle-centric therapeutic landscapes while emphasizing their translational potential and context-dependent biological constraints.
Extracellular vesicle (EV)-transported miRNAs coordinate antiviral defense by dually regulating viral replication dynamics and host immunomodulatory networks. Mechanistically, miR-431-5p establishes antiviral states by silencing CD95-mediated apoptotic signaling (212), while miR-136-5p suppresses M1 macrophage polarization through simultaneous inhibition of GNAS/PI3K/ERK/STAT3 signaling pathways, thereby attenuating viral-induced hepatic inflammation (213). This regulatory network reveals a new dimension in HBV infection: IFNα-activated macrophages deliver interferon-stimulated gene (ISG) products to HBV-infected hepatocytes via exosome secretion, significantly inhibiting HBV replication. Notably, the aldehyde glyoxylate system (AGS) enhances HBV replication while suppressing ISG activation by hijacking the host vesicle trafficking pathway. The exosome release inhibitor GW4869 completely abrogates IFNα’s antiviral effects, confirming exosomes as the central mediators of macrophage-hepatocyte antiviral crosstalk (214). This discovery uncovers a new paradigm of vesicle-mediated immune regulation, whereby activated immune cells restore ISG functionality in virus-infected cells through exosomal signaling, providing a host-directed precision strategy for chronic hepatitis therapy. This immune homeostasis modulation reflects an evolutionary balance between pathogen eradication and tissue protection, encoded within EV-mediated miRNA circuits through pathogen-host coadaptation. Additionally, EV-encapsulated miRNA clusters (miR-15b-5p, miR-24-3p, miR-223-3p) enhance mRNA vaccine efficacy by boosting antigen-presenting cell activation and germinal center B-cell responses (215), contrasting with milk-derived miR-let-7e and miR-27b that directly inhibit PEDV RNA-dependent RNA polymerase (RdRp) to block replication (216). Notably, mesenchymal stem cell-derived EVs enriched with the miR-92a-3p/26a-5p/23a-3p triad exert dual antiviral effects—suppressing SARS-CoV-2 genomic RNA synthesis via NSP12 helicase interference while mitigating NF-κB/IL-6-mediated cytokine storms in infected alveolar epithelium (217). This multidimensional strategy exploits the evolutionary conservation of viral replication checkpoints, restricting viral adaptability and co-opting host stress-response pathways to establish antiviral barriers. Together, these interconnected regulatory mechanisms highlight the therapeutic potential of EV-miRNA engineering for precision targeting of pathogen-host interactions across divergent viral families.
3.3 Small molecule inhibitors targeting host vesicular checkpoints against viral escape
Host-directed antiviral therapeutics overcome viral drug resistance by targeting evolutionarily conserved cellular machinery that governs pH homeostasis and vesicular trafficking, thereby establishing complementary defense layers against viral adaptation through three interdependent mechanisms: neutralizing endosomal acidification to block viral fusion, disrupting vesicular transport dynamics to prevent capsid uncoating, and competitively saturating host receptors to inhibit viral attachment. This strategy capitalizes on the host pathways’ evolutionary constraints, which viruses cannot readily mutate to bypass, thus creating durable antiviral barriers shaped by co-evolutionary pressures. Pharmacologically, pH regulation inhibitors like obatoclax exemplify this approach by inducing endosomal alkalinization, which locks coronavirus spike glycoproteins in prefusion conformations and renders virions fusion-incompetent (218). Notably, obatoclax demonstrates potent antiviral activity against SARS-CoV-2 in TMPRSS2-negative cells (EC50 = 0.06 μM) through pH-dependent entry blockade, with achievable therapeutic plasma concentrations (~0.4 μM) via intravenous administration. While phase I/II oncology trials established its safety profile at 20–40 mg/m² doses, transient neurological symptoms (grade 1-2) and reversible hepatotoxicity remain dose-limiting considerations for antiviral repurposing (219). Similarly, chloroquine and bafilomycin A1 disrupt acidification-dependent penetration routes, as confirmed by cryo-electron tomography, achieving comparable entry blockade through spatial interference (220). Beyond pH modulation, vesicular trafficking inhibitors such as tetrandrine suppress SARS-CoV-2 via dual mechanisms: impairing endolysosomal maturation through calcium flux disruption mediated by two-pore channel 2 (TPC2) while paradoxically stabilizing viral ribonucleoprotein complexes (221). Recent studies highlight tetrandrine demonstrates broad experimental efficacy, with reversible hepatosteatosis as a key toxicity linked to oral route-induced portal venous accumulation. Pulmonary delivery or structural analogs may mitigate hepatic risks while preserving antiviral activity, particularly in short-term SARS-CoV-2 regimens (222). These interventions are further amplified through synergistic therapeutic architectures, where calpeptin enhances remdesivir’s antiviral activity by simultaneously inhibiting papain-like protease-mediated polyprotein cleavage and host caspase-8-dependent viral egress (223). Such combinatorial strategies exploit viral dependency networks that bridge host-pathogen interfaces, effectively turning cellular defense mechanisms against viral replication checkpoints. For receptor competition, spatial hindrance is achieved through MDM2 antagonists like nutlin-3a, which mobilize ACE2-enriched EVs to saturate SARS-CoV-2 binding interfaces (224). Intriguingly, even replication fidelity mechanisms prove targetable, as camptothecin derivatives terminate flavivirus RNA synthesis through topoisomerase 1-DNA cleavage complex stabilization, effectively stalling replication (225). By synchronously intercepting viral lifecycle checkpoints while preserving host proteostatic resilience, this systems-level approach constructs an evolutionary firewall against pathogen adaptability, thereby providing a blueprint for preemptive countermeasures against viral mutational escape.
4 Vesicle small molecules serve as detection molecules for viral infection status
The co-option of host-derived EVs by viruses exemplifies an evolutionary refinement strategy that facilitates both stealth dissemination and systemic manipulation of cellular communication networks. Specifically, through pathogen-encoded molecular machinery, viruses subvert host proteostasis systems and commandeer vesicular trafficking pathways to reconfigure EV biology. This hijacking is epitomized by SARS-CoV-2, HIV-1, and HTLV-1, which remodel EV biogenesis through three-dimensional modulation of miRNA cargo profiles, immune-evasive surface marker expression, and vesicular dimensional plasticity to establish systemic infection (226–230). Hubert et al. (231) combined nanoparticle tracking analysis and enzymatic activity mapping to reveal an inverse correlation between circulating EV abundance and host miRNA bioavailability. Their study further demonstrated that reduced EV diameters quantitatively correlated with lymphocyte activation thresholds. These findings establish EV-mediated miRNA trafficking as a critical regulator of viral latency states (232). Such systemic rewiring underscores the dual-edged nature of EVs: their ancient role in cellular crosstalk becomes both a weapon and a vulnerability in host defense architectures. These findings not only position quantitative EV subpopulation profiling as a transformative liquid biopsy modality for monitoring infection progression and therapeutic efficacy through vesicle-encoded molecular fingerprints but also highlight EVs’ dual role as multimodal signaling platforms. Functioning as composite molecular carriers, EVs transport non-coding RNAs, post-translationally modified proteins, and bioactive lipids while executing precision regulation of intercellular crosstalk via spatiotemporally controlled signal transduction. Such dynamic orchestration coordinates pan-tissue homeostasis and immune microenvironment remodeling, ultimately rendering EVs pivotal yet vulnerable nodes in the host-pathogen interface.
As a key molecule of gene regulation, miRNAs carried by vesicles have shown important value in disease dynamic monitoring and precise diagnosis and treatment by targeting and regulating mRNA expression. First, exosomal miRNAs in HCV-infected patients function as dynamic biomarkers for monitoring host-virus interactions and antiviral immune response kinetics (233), whereas integrative profiling of SARS-CoV-2 Spike S1-bearing EVs against innate and adaptive immunity-associated EV signatures enables tissue-resolved tracking of viral reservoir persistence and disease severity stratification (234). These vesicular biomarkers exploit the evolutionary logic of intercellular communication systems, where viruses inadvertently co-opt host-derived signaling architectures to disseminate but in doing so create diagnostic vulnerabilities through molecular footprint leakage. Based on this, these technological advancements establish vesicular biomarkers as multidimensional diagnostic platforms capable of three-dimensional mapping: viral pathogenesis trajectories through longitudinal cargo analysis, therapeutic target engagement via pharmacodynamic marker quantification, and clinical outcome prediction using machine learning-driven signature deconvolution. Further, the molecular characteristics based on EV have demonstrated diagnostic precision across heterogeneous pathological contexts, including infection dynamics (Chagas disease parasite load monitoring (235), baculovirus replication kinetics (236), HIV-1 disease progression staging (237)), oncolytic virotherapy response assessment (immune activation states in adenovirus-treated tumors (238)), and neoplastic management (nasopharyngeal carcinoma immunotherapy efficacy (239, 240), glioblastoma tumor microenvironment profiling (241), metastatic colorectal cancer liquid biopsy (242)). The universality of EV-mediated diagnostics arises from their capacity to integrate multimodal cellular stress signatures—capturing both the immediate perturbations of pathogens and the adaptive reprogramming of host survival networks—thus positioning vesicles as sentinels of system-wide pathophysiological equilibrium shifts. Notably, vesicular biomarkers demonstrate cross-species applicability in simian immunodeficiency virus pathogenesis modeling (243) and veterinary diagnostics, particularly for porcine hemagglutinating encephalomyelitis virus surveillance (244). Their clinical utility further extends to specialized scenarios, including therapeutic resistance prediction in castration-resistant prostate cancer (245), severity indexing for pediatric viral pneumonia (246), and treatment response monitoring in tuberculosis (247). In addition, the emerging applications in hematologic malignancies (acute myeloid leukemia minimal residual disease detection (248)) and developmental disorders (nephroblastoma differential diagnosis (249)) further validate their pan-pathological diagnostic versatility.
5 Conclusion
Viruses, as obligate intracellular parasites, critically depend on host vesicular trafficking systems to execute their life cycles. Specifically, these pathogens systematically exploit membrane transport machinery to coordinate successive infection stages, including viral entry via receptor-mediated endocytosis (250), envelope fusion with host membranes (251), replication complex formation for genome amplification (252), virion assembly at specialized organelle interfaces (253), budding through membrane scission mechanisms (254), and extracellular release via exocytic pathways (255). While these trafficking networks are essential for cellular homeostasis, their functional complexity paradoxically constitutes an exploitable vulnerability for viral pathogenesis. Evolutionarily, viruses optimize strategies to hijack transport pathways for establishing replication niches, thereby underscoring the necessity to decode virus-host interplay at membrane trafficking checkpoints. For instance, elucidating molecular blueprints through which divergent viral families co-opt cellular conduits—from repurposing ER-Golgi intermediate compartments to retroviruses hijacking multivesicular bodies—not only provides dual benefits (advancing mechanistic virology and illuminating druggable targets) but also mandates the development of precision therapeutics. Such therapeutics must selectively inhibit viral glycoprotein interactions with vesicle-associated adaptors while sparing physiological transport functions—a strategy requiring combinatorial approaches integrating structural virology and membrane proteomics.
Author contributions
G-XC: Writing – original draft. F-XL: Writing – original draft. C-CM: Writing – review & editing. CD: Writing – review & editing. JD: Writing – review & editing. X-SQ: Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32272978), Natural Science Foundation of Shanghai (Grant No.23ZR1477100), Guizhou Provincial Natural Science Basic Research Program (No. QianKeHe Basic MS- (2025) 383), Guizhou Provincial Natural Science Basic Research Program (No. QianKeHe Basic - (2024) Youth 316), Zunyi Science and Technology Project (NO. (2024) 322) and Qian Ke Ping Tai Project (NO. (2021)1350-057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reiter CR and Bongarzone ER. The role of vesicle trafficking and release in oligodendrocyte biology. Neurochem Res. (2020) 45:620–9. doi: 10.1007/s11064-019-02913-2
2. Hay JC, Hirling H, and Scheller RH. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J Biol Chem. (1996) 271:5671–9. doi: 10.1074/jbc.271.10.5671
3. Kawabata M, Matsuo H, Koito T, Murata M, Kubori T, Nagai H, et al. Legionella hijacks the host Golgi-to-ER retrograde pathway for the association of Legionella-containing vacuole with the ER. PloS Pathog. (2021) 17:e1009437. doi: 10.1371/journal.ppat.1009437
4. Hoya M, Yanguas F, Moro S, Prescianotto-Baschong C, Doncel C, de León N, et al. Traffic through the trans-golgi network and the endosomal system requires collaboration between exomer and clathrin adaptors in fission yeast. Genetics. (2017) 205:673–90. doi: 10.1534/genetics.116.193458
5. Machesky LM. Rab11FIP proteins link endocytic recycling vesicles for cytoskeletal transport and tethering. Biosci Rep. (2019) 39:BSR20182219. doi: 10.1042/bsr20182219
6. Hao M, Yeo SK, and Guan JL. Autophagy inhibition perturbs ERBB2 trafficking and abolishes tumorigenesis in ERBB2-driven breast cancer. Autophagy. (2021) 17:1059–60. doi: 10.1080/15548627.2021.1907168
7. Mund M, Tschanz A, Wu YL, Frey F, Mehl JL, Kaksonen M, et al. Clathrin coats partially preassemble and subsequently bend during endocytosis. J Cell Biol. (2023) 222:e202206038. doi: 10.1083/jcb.202206038
8. Kwon SH, Oh S, Nacke M, Mostov KE, and Lipschutz JH. Adaptor protein CD2AP and L-type lectin LMAN2 regulate exosome cargo protein trafficking through the golgi complex. J Biol Chem. (2016) 291:25462–75. doi: 10.1074/jbc.M116.729202
9. Sanghavi P, Kumar P, Roy A, Madhusudhan MS, and Mallik R. On and off controls within dynein-dynactin on native cargoes. Proc Natl Acad Sci U.S.A. (2021) 118:e2103383118. doi: 10.1073/pnas.2103383118
10. Veeck C, Biedenkopf N, Rohde C, Becker S, and Halwe S. Inhibition of rab1B impairs trafficking and maturation of SARS-coV-2 spike protein. Viruses. (2023) 15:824. doi: 10.3390/v15040824
11. Yiu SPT, Zerbe C, Vanderwall D, Huttlin EL, Weekes MP, and Gewurz BE. An Epstein-Barr virus protein interaction map reveals NLRP3 inflammasome evasion via MAVS UFMylation. Mol Cell. (2023) 83:2367–2386.e2315. doi: 10.1016/j.molcel.2023.05.018
12. Omari S, Roded A, Eisenberg M, Ali H, Fukuda M, Galli SJ, et al. Mast cell secretory granule fusion with amphisomes coordinates their homotypic fusion and release of exosomes. Cell Rep. (2024) 43:114482. doi: 10.1016/j.celrep.2024.114482
13. Takáts S, Glatz G, Szenci G, Boda A, Horváth GV, Hegedűs K, et al. Non-canonical role of the SNARE protein Ykt6 in autophagosome-lysosome fusion. PloS Genet. (2018) 14:e1007359. doi: 10.1371/journal.pgen.1007359
14. Chen L, Zhang J, Xu W, Chen J, Tang Y, Xiong S, et al. Cholesterol-rich lysosomes induced by respiratory syncytial virus promote viral replication by blocking autophagy flux. Nat Commun. (2024) 15:6311. doi: 10.1038/s41467-024-50711-4
15. Fan B, Zhu L, Chang X, Zhou J, Guo R, Zhao Y, et al. Mortalin restricts porcine epidemic diarrhea virus entry by downregulating clathrin-mediated endocytosis. Vet Microbiol. (2019) 239:108455. doi: 10.1016/j.vetmic.2019.108455
16. Dixon LK, Chapman DA, Netherton CL, and Upton C. African swine fever virus replication and genomics. Virus Res. (2013) 173:3–14. doi: 10.1016/j.virusres.2012.10.020
17. Boersma S, Rabouw HH, Bruurs LJM, Pavlovič T, van Vliet ALW, Beumer J, et al. Translation and replication dynamics of single RNA viruses. Cell. (2020) 183:1930–1945.e1923. doi: 10.1016/j.cell.2020.10.019
18. Twarock R and Stockley PG. RNA-mediated virus assembly: mechanisms and consequences for viral evolution and therapy. Annu Rev Biophys. (2019) 48:495–514. doi: 10.1146/annurev-biophys-052118-115611
19. Uddin MK, Watanabe T, Arata M, Sato Y, Kimura H, and Murata T. Epstein-barr virus BBLF1 mediates secretory vesicle transport to facilitate mature virion release. J Virol. (2023) 97:e0043723. doi: 10.1128/jvi.00437-23
20. Ricciardi S, Guarino AM, Giaquinto L, Polishchuk EV, Santoro M, Di Tullio G, et al. The role of NSP6 in the biogenesis of the SARS-CoV-2 replication organelle. Nature. (2022) 606:761–8. doi: 10.1038/s41586-022-04835-6
21. Serrano T, Casartelli N, Ghasemi F, Wioland H, Cuvelier F, Salles A, et al. HIV-1 budding requires cortical actin disassembly by the oxidoreductase MICAL1. Proc Natl Acad Sci U.S.A. (2024) 121:e2407835121. doi: 10.1073/pnas.2407835121
22. Yadav V, Panganiban AT, Honer Zu Bentrup K, and Voss TG. Influenza infection modulates vesicular trafficking and induces Golgi complex disruption. Virusdisease. (2016) 27:357–68. doi: 10.1007/s13337-016-0347-3
23. Kumari S, Dash PK, Kumari T, Guo ML, Ghosh JK, Buch SJ, et al. HIV-1 Nef hijacks both exocytic and endocytic pathways of host intracellular trafficking through differential regulation of Rab GTPases. Biol Cell. (2022) 114:276–92. doi: 10.1111/boc.202100027
24. Crawford SE, Criglar JM, Liu Z, Broughman JR, and Estes MK. COPII vesicle transport is required for rotavirus NSP4 interaction with the autophagy protein LC3 II and trafficking to viroplasms. J Virol. (2019) 94:e01341–19. doi: 10.1128/jvi.01341-19
25. Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, and Perrimon N. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PloS Pathog. (2006) 2:e102. doi: 10.1371/journal.ppat.0020102
26. Kim CS, Seol SK, Song OK, Park JH, and Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J Virol. (2007) 81:3852–65. doi: 10.1128/jvi.01311-06
27. Rubio-Miranda J, Cázares-Raga FE, Coy-Arechavaleta AS, Viettri M, Cortes-Martínez L, Lagunes-Guillén A, et al. Septin 2 interacts with dengue virus replication complex proteins and participates in virus replication in mosquito cells. Virology. (2022) 570:67–80. doi: 10.1016/j.virol.2022.03.007
28. Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, and Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. (1992) 119:749–61. doi: 10.1083/jcb.119.4.749
29. Kirchhausen T and Toyoda T. Immunoelectron microscopic evidence for the extended conformation of light chains in clathrin trimers. J Biol Chem. (1993) 268:10268–73. doi: 10.1016/S0021-9258(18)82199-8
30. Kirchhausen T, Owen D, and Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol. (2014) 6:a016725. doi: 10.1101/cshperspect.a016725
31. Huang T, Lehmann MJ, Said A, Ma G, and Osterrieder N. Major histocompatibility complex class I downregulation induced by equine herpesvirus type 1 pUL56 is through dynamin-dependent endocytosis. J Virol. (2014) 88:12802–15. doi: 10.1128/jvi.02079-14
32. Shafaq-Zadah M, Dransart E, and Johannes L. Clathrin-independent endocytosis, retrograde trafficking, and cell polarity. Curr Opin Cell Biol. (2020) 65:112–21. doi: 10.1016/j.ceb.2020.05.009
33. Mettlen M, Chen PH, Srinivasan S, Danuser G, and Schmid SL. Regulation of clathrin-mediated endocytosis. Annu Rev Biochem. (2018) 87:871–96. doi: 10.1146/annurev-biochem-062917-012644
34. Shen QT, Ren X, Zhang R, Lee IH, and Hurley JH. HIV-1 Nef hijacks clathrin coats by stabilizing AP-1:Arf1 polygons. Science. (2015) 350:aac5137. doi: 10.1126/science.aac5137
35. Kreutz LC and Ackermann MR. Porcine reproductive and respiratory syndrome virus enters cells through a low pH-dependent endocytic pathway. Virus Res. (1996) 42:137–47. doi: 10.1016/0168-1702(96)01313-5
36. Panyasrivanit M, Khakpoor A, Wikan N, and Smith DR. Linking dengue virus entry and translation/replication through amphisomes. Autophagy. (2009) 5:434–5. doi: 10.4161/auto.5.3.7925
37. Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, et al. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PloS Pathog. (2009) 5:e1000450. doi: 10.1371/journal.ppat.1000450
38. Garry RF. Lassa virus structural biology and replication. Curr Top Microbiol Immunol. (2023) 440:147–64. doi: 10.1007/82_2023_262
39. Galindo I, Cuesta-Geijo MA, Hlavova K, Muñoz-Moreno R, Barrado-Gil L, Dominguez J, et al. African swine fever virus infects macrophages, the natural host cells, via clathrin- and cholesterol-dependent endocytosis. Virus Res. (2015) 200:45–55. doi: 10.1016/j.virusres.2015.01.022
40. Bauherr S, Larsberg F, Petrich A, Sperber HS, Klose-Grzelka V, Luckner M, et al. Macropinocytosis and clathrin-dependent endocytosis play pivotal roles for the infectious entry of puumala virus. J Virol. (2020) 94:e00184–20. doi: 10.1128/jvi.00184-20
41. Owczarek K, Chykunova Y, Jassoy C, Maksym B, Rajfur Z, and Pyrc K. Zika virus: mapping and reprogramming the entry. Cell Commun Signal. (2019) 17:41. doi: 10.1186/s12964-019-0349-z
42. Zhang MQ, Li JR, Yang L, Peng ZG, Wu S, and Zhang JP. ATG10S promotes IFNL1 expression and autophagic degradation of multiple viral proteins mediated by IFNL1. Autophagy. (2024) 20:2238–54. doi: 10.1080/15548627.2024.2361580
43. Liu CC, Liu YY, Zhou JF, Chen X, Chen H, Hu JH, et al. Cellular ESCRT components are recruited to regulate the endocytic trafficking and RNA replication compartment assembly during classical swine fever virus infection. PloS Pathog. (2022) 18:e1010294. doi: 10.1371/journal.ppat.1010294
44. Tiwari R, Mishra AR, Gupta A, and Nayak D. Structural similarity-based prediction of host factors associated with SARS-CoV-2 infection and pathogenesis. J Biomol Struct Dyn. (2022) 40:5868–79. doi: 10.1080/07391102.2021.1874532
45. Zhou Z, Zhang X, Lei X, Xiao X, Jiao T, Ma R, et al. Sensing of cytoplasmic chromatin by cGAS activates innate immune response in SARS-CoV-2 infection. Signal Transduct Target Ther. (2021) 6:382. doi: 10.1038/s41392-021-00800-3
46. Qin W, Kong N, Xie S, Liu H, Yang X, Wang Y, et al. RNASEK interacting with PEDV structural proteins facilitates virus entry via clathrin-mediated endocytosis. J Virol. (2025) 99:e0176024. doi: 10.1128/jvi.01760-24
47. Li Y, Wang J, Hou W, Shan Y, Wang S, and Liu F. Dynamic dissection of the endocytosis of porcine epidemic diarrhea coronavirus cooperatively mediated by clathrin and caveolae as visualized by single-virus tracking. mBio. (2021) 12:e00256–21. doi: 10.1128/mBio.00256-21
48. Wei X, She G, Wu T, Xue C, and Cao Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Vet Res. (2020) 51:10. doi: 10.1186/s13567-020-0739-7
49. Yin L, Liu X, Hu D, Luo Y, Zhang G, and Liu P. Swine enteric coronaviruses (PEDV, TGEV, and PDCoV) induce divergent interferon-stimulated gene responses and antigen presentation in porcine intestinal enteroids. Front Immunol. (2021) 12:826882. doi: 10.3389/fimmu.2021.826882
50. Chen Y, Wang T, Yang Y, Fang Y, Zhao B, Zeng W, et al. Extracellular vesicles derived from PPRV-infected cells enhance signaling lymphocyte activation molecular (SLAM) receptor expression and facilitate virus infection. PloS Pathog. (2022) 18:e1010759. doi: 10.1371/journal.ppat.1010759
51. Shi BJ, Liu CC, Zhou J, Wang SQ, Gao ZC, Zhang XM, et al. Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7. J Virol. (2016) 90:9194–208. doi: 10.1128/jvi.00688-16
52. Xie B, Zhao M, Song D, Wu K, Yi L, Li W, et al. Induction of autophagy and suppression of type I IFN secretion by CSFV. Autophagy. (2021) 17:925–47. doi: 10.1080/15548627.2020.1739445
53. Zhang YN, Liu YY, Xiao FC, Liu CC, Liang XD, Chen J, et al. Rab5, rab7, and rab11 are required for caveola-dependent endocytosis of classical swine fever virus in porcine alveolar macrophages. J Virol. (2018) 92:e00797–18. doi: 10.1128/jvi.00797-18
54. Liu CC, Liu YY, Cheng Y, Zhang YN, Zhang J, Liang XD, et al. The ESCRT-I subunit tsg101 plays novel dual roles in entry and replication of classical swine fever virus. J Virol. (2021) 95:e01928–20. doi: 10.1128/jvi.01928-20
55. Yu W, Tao J, Cao H, Zheng W, Zhang B, Zhang Y, et al. The HAVCR1-centric host factor network drives Zika virus vertical transmission. Cell Rep. (2025) 44:115464. doi: 10.1016/j.celrep.2025.115464
56. Rodrigo I, Albentosa-González L, Romero de Ávila MJ, Bassi MR, Sempere RN, Clemente-Casares P, et al. Ubiquitin-like modifier-activating enzyme 1 interacts with Zika virus NS5 and promotes viral replication in the infected cell. J Gen Virol. (2025) 106:002063. doi: 10.1099/jgv.0.002063
57. Giraldo MI, Xia H, Aguilera-Aguirre L, Hage A, van Tol S, Shan C, et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature. (2020) 585:414–9. doi: 10.1038/s41586-020-2457-8
58. Anton A, Mazeaud C, Freppel W, Gilbert C, Tremblay N, Sow AA, et al. Valosin-containing protein ATPase activity regulates the morphogenesis of Zika virus replication organelles and virus-induced cell death. Cell Microbiol. (2021) 23:e13302. doi: 10.1111/cmi.13302
59. Rodrigo I, Ballesta C, Nunes EB, Pérez P, García-Arriaza J, and Arias A. Eeyarestatin I, an inhibitor of the valosin-containing protein, exhibits potent virucidal activity against the flaviviruses. Antiviral Res. (2022) 207:105416. doi: 10.1016/j.antiviral.2022.105416
60. Gestuveo RJ, Royle J, Donald CL, Lamont DJ, Hutchinson EC, Merits A, et al. Analysis of Zika virus capsid-Aedes aEgypti mosquito interactome reveals pro-viral host factors critical for establishing infection. Nat Commun. (2021) 12:2766. doi: 10.1038/s41467-021-22966-8
61. Hu Y, Dong X, He Z, Wu Y, Zhang S, Lin J, et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell Biosci. (2019) 9:46. doi: 10.1186/s13578-019-0308-9
62. Praena B, Bello-Morales R, and López-Guerrero JA. Hsv-1 Endocytic Entry into a Human Oligodendrocytic Cell Line is Mediated by Clathrin and Dynamin but Not Caveolin. Viruses. (2020) 12:734. doi: 10.3390/v12070734
63. Devadas D, Koithan T, Diestel R, Prank U, Sodeik B, and Döhner K. Herpes simplex virus internalization into epithelial cells requires Na+/H+ exchangers and p21-activated kinases but neither clathrin- nor caveolin-mediated endocytosis. J Virol. (2014) 88:13378–95. doi: 10.1128/jvi.03631-13
64. Pasquato A, Fernandez AH, and Kunz S. Studies of lassa virus cell entry. Methods Mol Biol. (2018) 1604:135–55. doi: 10.1007/978-1-4939-6981-4_9
65. Azim KF, Lasker T, Akter R, Hia MM, Bhuiyan OF, Hasan M, et al. Combination of highly antigenic nucleoproteins to inaugurate a cross-reactive next generation vaccine candidate against Arenaviridae family. Heliyon. (2021) 7:e07022. doi: 10.1016/j.heliyon.2021.e07022
66. Keiffer TR, DiGiuseppe S, Guion L, Bienkowska-Haba M, Zwolinska K, Siddiqa A, et al. HPV16 entry requires dynein for minus-end transport and utilizes kinesin Kif11 for plus-end transport along microtubules during mitosis. J Virol. (2025) 99:e0093724. doi: 10.1128/jvi.00937-24
67. White S and Roller R. Herpes simplex virus type-1 cVAC formation in neuronal cells is mediated by dynein motor function and glycoprotein retrieval from the plasma membrane. J Virol. (2024) 98:e0071324. doi: 10.1128/jvi.00713-24
68. Pasdeloup D, McElwee M, Beilstein F, Labetoulle M, and Rixon FJ. Herpesvirus tegument protein pUL37 interacts with dystonin/BPAG1 to promote capsid transport on microtubules during egress. J Virol. (2013) 87:2857–67. doi: 10.1128/jvi.02676-12
69. Musarrat F, Chouljenko V, and Kousoulas KG. Cellular and viral determinants of HSV-1 entry and intracellular transport towards nucleus of infected cells. J Virol. (2021) 95:e02434–20. doi: 10.1128/jvi.02434-20
70. Luo X, Ji R, Liu Q, Xiao X, Song W, An H, et al. Ste20-like kinase TAOK1 positively regulates antiviral responses by controlling the TBK1-IRF3 signaling axis. J Innate Immun. (2023) 15:380–96. doi: 10.1159/000526324
71. Cong Y, Verlhac P, Green BB, de Vries-Idema J, Strauss LM, Río-Bergé C, et al. Influenza A virus subverts the LC3-pericentrin dynein adaptor complex for host cytoplasm entry. Sci Adv. (2025) 11:eadu7602. doi: 10.1126/sciadv.adu7602
72. Zhang B, Xu S, Liu M, Wei Y, Wang Q, Shen W, et al. The nucleoprotein of influenza A virus inhibits the innate immune response by inducing mitophagy. Autophagy. (2023) 19:1916–33. doi: 10.1080/15548627.2022.2162798
73. Glon D, Vilmen G, Perdiz D, Hernandez E, Beauclair G, Quignon F, et al. Essential role of hyperacetylated microtubules in innate immunity escape orchestrated by the EBV-encoded BHRF1 protein. PloS Pathog. (2022) 18:e1010371. doi: 10.1371/journal.ppat.1010371
74. Christensen JR, Kendrick AA, Truong JB, Aguilar-Maldonado A, Adani V, Dzieciatkowska M, et al. Cytoplasmic dynein-1 cargo diversity is mediated by the combinatorial assembly of FTS-Hook-FHIP complexes. Elife. (2021) 10:e74538. doi: 10.7554/eLife.74538
75. Insinna C, Baye LM, Amsterdam A, Besharse JC, and Link BA. Analysis of a zebrafish dync1h1 mutant reveals multiple functions for cytoplasmic dynein 1 during retinal photoreceptor development. Neural Dev. (2010) 5:12. doi: 10.1186/1749-8104-5-12
76. Saji T, Endo M, Okada Y, Minami Y, and Nishita M. KIF1C facilitates retrograde transport of lysosomes through Hook3 and dynein. Commun Biol. (2024) 7:1305. doi: 10.1038/s42003-024-07023-6
77. Horgan CP, Hanscom SR, and McCaffrey MW. Dynein LIC1 localizes to the mitotic spindle and midbody and LIC2 localizes to spindle poles during cell division. Cell Biol Int. (2011) 35:171–8. doi: 10.1042/cbi20100284
78. Toropova K, Zalyte R, Mukhopadhyay AG, Mladenov M, Carter AP, and Roberts AJ. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat Struct Mol Biol. (2019) 26:823–9. doi: 10.1038/s41594-019-0286-y
79. Asai DJ, Rajagopalan V, and Wilkes DE. Dynein-2 and ciliogenesis in tetrahymena. Cell Motil Cytoskeleton. (2009) 66:673–7. doi: 10.1002/cm.20397
81. Wickstead B and Gull K. Dyneins across eukaryotes: a comparative genomic analysis. Traffic. (2007) 8:1708–21. doi: 10.1111/j.1600-0854.2007.00646.x
82. Schmidt H, Zalyte R, Urnavicius L, and Carter AP. Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature. (2015) 518:435–8. doi: 10.1038/nature14023
83. Asante D, Stevenson NL, and Stephens DJ. Subunit composition of the human cytoplasmic dynein-2 complex. J Cell Sci. (2014) 127:4774–87. doi: 10.1242/jcs.159038
84. Wu C, Li J, Peterson A, Tao K, and Wang B. Loss of dynein-2 intermediate chain Wdr34 results in defects in retrograde ciliary protein trafficking and Hedgehog signaling in the mouse. Hum Mol Genet. (2017) 26:2386–97. doi: 10.1093/hmg/ddx127
85. Tsurumi Y, Hamada Y, Katoh Y, and Nakayama K. Interactions of the dynein-2 intermediate chain WDR34 with the light chains are required for ciliary retrograde protein trafficking. Mol Biol Cell. (2019) 30:658–70. doi: 10.1091/mbc.E18-10-0678
86. Vuolo L, Stevenson NL, Heesom KJ, and Stephens DJ. Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. Elife. (2018) 7:e39655. doi: 10.7554/eLife.39655
87. Hiyamizu S, Qiu H, Tsurumi Y, Hamada Y, Katoh Y, and Nakayama K. Dynein-2-driven intraciliary retrograde trafficking indirectly requires multiple interactions of IFT54 in the IFT-B complex with the dynein-2 complex. Biol Open. (2023) 12:bio059976. doi: 10.1242/bio.059976
88. Liao KC, Xie X, Sundstrom AKB, Lim XN, Tan KK, Zhang Y, et al. Dengue and Zika RNA-RNA interactomes reveal pro- and anti-viral RNA in human cells. Genome Biol. (2023) 24:279. doi: 10.1186/s13059-023-03110-9
89. Schmidts M, Vodopiutz J, Christou-Savina S, Cortés CR, McInerney-Leo AM, Emes RD, et al. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am J Hum Genet. (2013) 93:932–44. doi: 10.1016/j.ajhg.2013.10.003
90. Qiu H, Tsurumi Y, Katoh Y, and Nakayama K. Combinations of deletion and missense variations of the dynein-2 DYNC2LI1 subunit found in skeletal ciliopathies cause ciliary defects. Sci Rep. (2022) 12:31. doi: 10.1038/s41598-021-03950-0
91. Yan L, Yin H, Mi Y, Wu Y, and Zheng Y. Deficiency of Wdr60 and Wdr34 cause distinct neural tube malformation phenotypes in early embryos. Front Cell Dev Biol. (2023) 11:1084245. doi: 10.3389/fcell.2023.1084245
92. Mueller S, Cao X, Welker R, and Wimmer E. Interaction of the poliovirus receptor CD155 with the dynein light chain Tctex-1 and its implication for poliovirus pathogenesis. J Biol Chem. (2002) 277:7897–904. doi: 10.1074/jbc.M111937200
93. Brault JB, Kudelko M, Vidalain PO, Tangy F, Desprès P, and Pardigon N. The interaction of flavivirus M protein with light chain Tctex-1 of human dynein plays a role in late stages of virus replication. Virology. (2011) 417:369–78. doi: 10.1016/j.virol.2011.06.022
94. Binarová P and Tuszynski J. Tubulin: structure, functions and roles in disease. Cells. (2019) 8:1294. doi: 10.3390/cells8101294
95. Ma M, Stoyanova M, Rademacher G, Dutcher SK, Brown A, and Zhang R. Structure of the decorated ciliary doublet microtubule. Cell. (2019) 179:909–922.e912. doi: 10.1016/j.cell.2019.09.030
96. Wethekam LC and Moore JK. Microtubule cytoskeleton: Revealing new readers of the tubulin code. Curr Biol. (2022) 32:R960–r962. doi: 10.1016/j.cub.2022.08.023
97. Lou JX, Liu YY, Bai JS, Cheng Y, Zhang J, Liu CC, et al. Kinesin-1 regulates endocytic trafficking of classical swine fever virus along acetylated microtubules. J Virol. (2023) 97:e0192922. doi: 10.1128/jvi.01929-22
98. Lukic Z, Dharan A, Fricke T, Diaz-Griffero F, and Campbell EM. HIV-1 uncoating is facilitated by dynein and kinesin 1. J Virol. (2014) 88:13613–25. doi: 10.1128/jvi.02219-14
99. Carnes SK, Zhou J, and Aiken C. HIV-1 engages a dynein-dynactin-BICD2 complex for infection and transport to the nucleus. J Virol. (2018) 92:e00358–18. doi: 10.1128/jvi.00358-18
100. Shanmugapriya S, Santos da Silva E, Campbell JA, Boisjoli MP, and Naghavi MH. Dynactin 1 negatively regulates HIV-1 infection by sequestering the host cofactor CLIP170. Proc Natl Acad Sci U.S.A. (2021) 118:e2102884118. doi: 10.1073/pnas.2102884118
101. Badieyan S, Lichon D, Andreas MP, Gillies JP, Peng W, Shi J, et al. HIV-1 binds dynein directly to hijack microtubule transport machinery. bioRxiv. (2023) 11:eadn6796. doi: 10.1101/2023.08.29.555335
102. Santos da Silva E, Shanmugapriya S, Malikov V, Gu F, Delaney MK, and Naghavi MH. HIV-1 capsids mimic a microtubule regulator to coordinate early stages of infection. EMBO J. (2020) 39:e104870. doi: 10.15252/embj.2020104870
103. Hou W, Kang W, Li Y, Shan Y, Wang S, and Liu F. Dynamic dissection of dynein and kinesin-1 cooperatively mediated intercellular transport of porcine epidemic diarrhea coronavirus along microtubule using single virus tracking. Virulence. (2021) 12:615–29. doi: 10.1080/21505594.2021.1878748
104. Banerjee I, Miyake Y, Nobs SP, Schneider C, Horvath P, Kopf M, et al. Influenza A virus uses the aggresome processing machinery for host cell entry. Science. (2014) 346:473–7. doi: 10.1126/science.1257037
105. Ferrié M, Alexandre V, Montpellier C, Bouquet P, Tubiana T, Mézière L, et al. The AP-1 adaptor complex is essential for intracellular trafficking of the ORF2 capsid protein and assembly of Hepatitis E virus. Cell Mol Life Sci. (2024) 81:335. doi: 10.1007/s00018-024-05367-0
106. Maurer ME and Cooper JA. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. (2006) 119:4235–46. doi: 10.1242/jcs.03217
107. Andreu S, Ripa I, López-Guerrero JA, and Bello-Morales R. Human coronavirus 229E uses clathrin-mediated endocytosis as a route of entry in huh-7 cells. Biomolecules. (2024) 14:1232. doi: 10.3390/biom14101232
108. Wang G, Jiang L, Wang J, Zhang J, Kong F, Li Q, et al. The G protein-coupled receptor FFAR2 promotes internalization during influenza A virus entry. J Virol. (2020) 94:e01707–19. doi: 10.1128/jvi.01707-19
109. Camus G, Segura-Morales C, Molle D, Lopez-Vergès S, Begon-Pescia C, Cazevieille C, et al. The clathrin adaptor complex AP-1 binds HIV-1 and MLV Gag and facilitates their budding. Mol Biol Cell. (2007) 18:3193–203. doi: 10.1091/mbc.e06-12-1147
110. Kimpler LA, Glosson NL, Downs D, Gonyo P, May NA, and Hudson AW. Adaptor protein complexes AP-1 and AP-3 are required by the HHV-7 Immunoevasin U21 for rerouting of class I MHC molecules to the lysosomal compartment. PloS One. (2014) 9:e99139. doi: 10.1371/journal.pone.0099139
111. Xiao F, Wang S, Barouch-Bentov R, Neveu G, Pu S, Beer M, et al. Interactions between the hepatitis C virus nonstructural 2 protein and host adaptor proteins 1 and 4 orchestrate virus release. mBio. (2018) 9:e02233–17. doi: 10.1128/mBio.02233-17
112. Kaminska K, Cancellieri F, Quinodoz M, Moye AR, Bauwens M, Lin S, et al. Bi-allelic variants in three genes encoding distinct subunits of the vesicular AP-5 complex cause hereditary macular dystrophy. Am J Hum Genet. (2025) 112:808–28. doi: 10.1016/j.ajhg.2025.02.015
113. Hirst J, Edgar JR, Esteves T, Darios F, Madeo M, Chang J, et al. Loss of AP-5 results in accumulation of aberrant endolysosomes: defining a new type of lysosomal storage disease. Hum Mol Genet. (2015) 24:4984–96. doi: 10.1093/hmg/ddv220
114. Robinson MS, Antrobus R, Sanger A, Davies AK, and Gershlick DC. The role of the AP-1 adaptor complex in outgoing and incoming membrane traffic. J Cell Biol. (2024) 223:e202310071. doi: 10.1083/jcb.202310071
115. Deng H, Jia G, Li P, Tang Y, Zhao L, Yang Q, et al. The WDR11 complex is a receptor for acidic-cluster-containing cargo proteins. Cell. (2024) 187:4272–4288.e4220. doi: 10.1016/j.cell.2024.06.024
116. Wieffer M, Cibrián Uhalte E, Posor Y, Otten C, Branz K, Schütz I, et al. PI4K2β/AP-1-based TGN-endosomal sorting regulates Wnt signaling. Curr Biol. (2013) 23:2185–90. doi: 10.1016/j.cub.2013.09.017
117. Pujol FM, Laketa V, Schmidt F, Mukenhirn M, Müller B, Boulant S, et al. HIV-1 vpu antagonizes CD317/tetherin by adaptor protein-1-mediated exclusion from virus assembly sites. J Virol. (2016) 90:6709–23. doi: 10.1128/jvi.00504-16
118. Tavares LA, de Carvalho JV, Costa CS, Silveira RM, de Carvalho AN, Donadi EA, et al. Two functional variants of AP-1 complexes composed of either γ2 or γ1 subunits are independently required for major histocompatibility complex class I downregulation by HIV-1 nef. J Virol. (2020) 94:e02039–19. doi: 10.1128/jvi.02039-19
119. Wonderlich ER, Williams M, and Collins KL. The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J Biol Chem. (2008) 283:3011–22. doi: 10.1074/jbc.M707760200
120. Strazic Geljic I, Kucan Brlic P, Angulo G, Brizic I, Lisnic B, Jenus T, et al. Cytomegalovirus protein m154 perturbs the adaptor protein-1 compartment mediating broad-spectrum immune evasion. Elife. (2020) 9:e50803. doi: 10.7554/eLife.50803
121. Pérez-Núñez D, García-Urdiales E, Martínez-Bonet M, Nogal ML, Barroso S, Revilla Y, et al. CD2v interacts with adaptor protein AP-1 during african swine fever infection. PloS One. (2015) 10:e0123714. doi: 10.1371/journal.pone.0123714
122. Johnson DC, Webb M, Wisner TW, and Brunetti C. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J Virol. (2001) 75:821–33. doi: 10.1128/jvi.75.2.821-833.2001
123. Yasamut U, Tongmuang N, Yenchitsomanus PT, Junking M, Noisakran S, Puttikhunt C, et al. Adaptor protein 1A facilitates dengue virus replication. PloS One. (2015) 10:e0130065. doi: 10.1371/journal.pone.0130065
124. Lebrun M, Lambert J, Riva L, Thelen N, Rambout X, Blondeau C, et al. Varicella-zoster virus ORF9p binding to cellular adaptor protein complex 1 is important for viral infectivity. J Virol. (2018) 92:e00295–18. doi: 10.1128/jvi.00295-18
125. Fölsch H, Pypaert M, Maday S, Pelletier L, and Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. (2003) 163:351–62. doi: 10.1083/jcb.200309020
126. Kulpa DA, Del Cid N, Peterson KA, and Collins KL. Adaptor protein 1 promotes cross-presentation through the same tyrosine signal in major histocompatibility complex class I as that targeted by HIV-1. J Virol. (2013) 87:8085–98. doi: 10.1128/jvi.00701-13
127. Hooy RM, Iwamoto Y, Tudorica DA, Ren X, and Hurley JH. Self-assembly and structure of a clathrin-independent AP-1:Arf1 tubular membrane coat. Sci Adv. (2022) 8:eadd3914. doi: 10.1126/sciadv.add3914
128. Navarro Negredo P, Edgar JR, Wrobel AG, Zaccai NR, Antrobus R, Owen DJ, et al. Contribution of the clathrin adaptor AP-1 subunit µ1 to acidic cluster protein sorting. J Cell Biol. (2017) 216:2927–43. doi: 10.1083/jcb.201602058
129. Fujisawa R and Masuda M. Ecotropic murine leukemia virus envelope protein affects interaction of cationic amino acid transporter 1 with clathrin adaptor protein complexes, leading to receptor downregulation. Virology. (2007) 368:342–50. doi: 10.1016/j.virol.2007.06.036
130. Jatuyosporn T, Laohawutthichai P, Supungul P, Sotelo-Mundo RR, Ochoa-Leyva A, Tassanakajon A, et al. Role of Clathrin Assembly Protein-2 Beta Subunit during White Spot Syndrome Virus Infection in Black Tiger Shrimp Penaeus monodon. Sci Rep. (2019) 9:13489. doi: 10.1038/s41598-019-49852-0
131. Wang XF, Liu QH, Wu Y, and Huang J. Litopenaeus vannamei clathrin coat AP17 involved in white spot syndrome virus infection. Fish Shellfish Immunol. (2016) 52:309–16. doi: 10.1016/j.fsi.2016.03.007
132. Cho SH, Pak K, Jeong DC, Han ME, Oh SO, and Kim YH. The AP2M1 gene expression is a promising biomarker for predicting survival of patients with hepatocellular carcinoma. J Cell Biochem. (2019) 120:4140–6. doi: 10.1002/jcb.27699
133. Noble B, Abada P, Nunez-Iglesias J, and Cannon PM. Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release. J Virol. (2006) 80:2924–32. doi: 10.1128/jvi.80.6.2924-2932.2006
134. Usami Y, Popov S, and Göttlinger HG. The Nef-like effect of murine leukemia virus glycosylated gag on HIV-1 infectivity is mediated by its cytoplasmic domain and depends on the AP-2 adaptor complex. J Virol. (2014) 88:3443–54. doi: 10.1128/jvi.01933-13
135. Ren X, Park SY, Bonifacino JS, and Hurley JH. How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. Elife. (2014) 3:e01754. doi: 10.7554/eLife.01754
136. Staudt RP and Smithgall TE. Nef homodimers down-regulate SERINC5 by AP-2-mediated endocytosis to promote HIV-1 infectivity. J Biol Chem. (2020) 295:15540–52. doi: 10.1074/jbc.RA120.014668
137. Shu ST, Emert-Sedlak LA, and Smithgall TE. Cell-based fluorescence complementation reveals a role for HIV-1 nef protein dimerization in AP-2 adaptor recruitment and CD4 co-receptor down-regulation. J Biol Chem. (2017) 292:2670–8. doi: 10.1074/jbc.M116.770016
138. Neveu G, Ziv-Av A, Barouch-Bentov R, Berkerman E, Mulholland J, and Einav S. AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J Virol. (2015) 89:4387–404. doi: 10.1128/jvi.02705-14
139. Huang HC, Chen CC, Chang WC, Tao MH, and Huang C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J Virol. (2012) 86:9443–53. doi: 10.1128/jvi.00873-12
140. Andreu S, Agúndez C, Ripa I, López-Guerrero JA, and Bello-Morales R. Pseudorabies virus uses clathrin mediated endocytosis to enter PK15 swine cell line. Front Microbiol. (2024) 15:1332175. doi: 10.3389/fmicb.2024.1332175
141. Tongmuang N, Yasamut U, Noisakran S, Sreekanth GP, Yenchitsomanus PT, and Limjindaporn T. Suppression of µ1 subunit of the adaptor protein complex 2 reduces dengue virus release. Virus Genes. (2020) 56:27–36. doi: 10.1007/s11262-019-01710-x
142. Pu S, Schor S, Karim M, Saul S, Robinson M, Kumar S, et al. BIKE regulates dengue virus infection and is a cellular target for broad-spectrum antivirals. Antiviral Res. (2020) 184:104966. doi: 10.1016/j.antiviral.2020.104966
143. Snetkov X, Weisswange I, Pfanzelter J, Humphries AC, and Way M. NPF motifs in the vaccinia virus protein A36 recruit intersectin-1 to promote Cdc42:N-WASP-mediated viral release from infected cells. Nat Microbiol. (2016) 1:16141. doi: 10.1038/nmicrobiol.2016.141
144. Zhang F, Landford WN, Ng M, McNatt MW, Bieniasz PD, and Hatziioannou T. SIV Nef proteins recruit the AP-2 complex to antagonize Tetherin and facilitate virion release. PloS Pathog. (2011) 7:e1002039. doi: 10.1371/journal.ppat.1002039
145. Ma Z, Islam MN, Xu T, and Song E. AP-3 adaptor complex-mediated vesicle trafficking. Biophys Rep. (2021) 7:91–100. doi: 10.52601/bpr.2021.200051
146. Le Borgne R, Alconada A, Bauer U, and Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J Biol Chem. (1998) 273:29451–61. doi: 10.1074/jbc.273.45.29451
147. Dong X, Li H, Derdowski A, Ding L, Burnett A, Chen X, et al. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell. (2005) 120:663–74. doi: 10.1016/j.cell.2004.12.023
148. Garcia E, Nikolic DS, and Piguet V. HIV-1 replication in dendritic cells occurs through a tetraspanin-containing compartment enriched in AP-3. Traffic. (2008) 9:200–14. doi: 10.1111/j.1600-0854.2007.00678.x
149. Liu L, Sutton J, Woodruff E, Villalta F, Spearman P, and Dong X. Defective HIV-1 particle assembly in AP-3-deficient cells derived from patients with Hermansky-Pudlak syndrome type 2. J Virol. (2012) 86:11242–53. doi: 10.1128/jvi.00544-12
150. Alford JE, Marongiu M, Watkins GL, and Anderson EC. Human immunodeficiency virus type 2 (HIV-2) gag is trafficked in an AP-3 and AP-5 dependent manner. PloS One. (2016) 11:e0158941. doi: 10.1371/journal.pone.0158941
151. Agrawal T, Schu P, and Medigeshi GR. Adaptor protein complexes-1 and 3 are involved at distinct stages of flavivirus life-cycle. Sci Rep. (2013) 3:1813. doi: 10.1038/srep01813
152. Prandini A, Salvi V, Colombo F, Moratto D, Lorenzi L, Vermi W, et al. Impairment of dendritic cell functions in patients with adaptor protein-3 complex deficiency. Blood. (2016) 127:3382–6. doi: 10.1182/blood-2015-06-650689
153. Ward C, Maselko M, Lupfer C, Prescott M, and Pastey MK. Interaction of the Human Respiratory Syncytial Virus matrix protein with cellular adaptor protein complex 3 plays a critical role in trafficking. PloS One. (2017) 12:e0184629. doi: 10.1371/journal.pone.0184629
154. Sun W, McCrory TS, Khaw WY, Petzing S, Myers T, and Schmitt AP. Matrix proteins of Nipah and Hendra viruses interact with beta subunits of AP-3 complexes. J Virol. (2014) 88:13099–110. doi: 10.1128/jvi.02103-14
155. Subramanian G, Hage A, Feldmann F, Chiramel AI, McNally KL, Sturdevant GL, et al. AP3B1 has type I interferon-independent antiviral function against SARS-coV-2. Viruses. (2024) 16:1377. doi: 10.3390/v16091377
156. Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, et al. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. (2003) 163:1281–90. doi: 10.1083/jcb.200307157
157. Mima J. Reconstitution of membrane tethering mediated by Rab-family small GTPases. Biophys Rev. (2018) 10:543–9. doi: 10.1007/s12551-017-0358-3
158. McLauchlan H, Newell J, Morrice N, Osborne A, West M, and Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. (1998) 8:34–45. doi: 10.1016/s0960-9822(98)70018-1
159. Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, and Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. (2010) 12:645–54. doi: 10.1038/ncb2067
160. Patwardhan A, Bardin S, Miserey-Lenkei S, Larue L, Goud B, Raposo G, et al. Routing of the RAB6 secretory pathway towards the lysosome related organelle of melanocytes. Nat Commun. (2017) 8:15835. doi: 10.1038/ncomms15835
161. Norden PR, Sun Z, and Davis GE. Control of endothelial tubulogenesis by Rab and Ral GTPases, and apical targeting of caveolin-1-labeled vacuoles. PloS One. (2020) 15:e0235116. doi: 10.1371/journal.pone.0235116
162. Li X, Liu B, Wen Y, Wang J, Guo YR, Shi A, et al. Coordination of RAB-8 and RAB-11 during unconventional protein secretion. J Cell Biol. (2024) 223:e202306107. doi: 10.1083/jcb.202306107
163. Agola JO, Jim PA, Ward HH, Basuray S, and Wandinger-Ness A. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin Genet. (2011) 80:305–18. doi: 10.1111/j.1399-0004.2011.01724.x
164. Kumar R, Khan M, Francis V, Aguila A, Kulasekaran G, Banks E, et al. DENND6A links Arl8b to a Rab34/RILP/dynein complex, regulating lysosomal positioning and autophagy. Nat Commun. (2024) 15:919. doi: 10.1038/s41467-024-44957-1
165. Margiotta A, Progida C, Bakke O, and Bucci C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim Biophys Acta Mol Cell Res. (2017) 1864:367–81. doi: 10.1016/j.bbamcr.2016.11.020
166. Kushch AA and Ivanov AV. Exosomes in the life cycle of viruses and the pathogenesis of viral infections. Vopr Virusol. (2023) 68:181–97. doi: 10.36233/0507-4088-173
167. Liu CC, Zhang YN, Li ZY, Hou JX, Zhou J, Kan L, et al. Rab5 and rab11 are required for clathrin-dependent endocytosis of Japanese encephalitis virus in BHK-21 cells. J Virol. (2017) 91:e01113–17. doi: 10.1128/jvi.01113-17
168. Chen S, Yang F, Zhu Z, Cao W, Lian K, Zhang W, et al. The endocytosis of foot-and mouth disease virus requires clathrin and caveolin and is dependent on the existence of Rab5 and Rab7 in CHO-677 cells. Vet Microbiol. (2022) 274:109550. doi: 10.1016/j.vetmic.2022.109550
169. Hou L, Tong X, Pan Y, Shi R, Liu C, Guo J, et al. Seneca valley virus enters PK-15 cells via caveolae-mediated endocytosis and macropinocytosis dependent on low-pH, dynamin, rab5, and rab7. J Virol. (2022) 96:e0144622. doi: 10.1128/jvi.01446-22
170. Wang X, Cheng J, Shen L, Chen M, Sun K, Li J, et al. Rab5c promotes RSV and ADV replication by autophagy in respiratory epithelial cells. Virus Res. (2024) 341:199324. doi: 10.1016/j.virusres.2024.199324
171. Tang WC, Lin RJ, Liao CL, and Lin YL. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol. (2014) 88:6793–804. doi: 10.1128/jvi.00045-14
172. Li GL, Han YQ, Su BQ, Yu HS, Zhang S, Yang GY, et al. Porcine reproductive and respiratory syndrome virus 2 hijacks CMA-mediated lipolysis through upregulation of small GTPase RAB18. PloS Pathog. (2024) 20:e1012123. doi: 10.1371/journal.ppat.1012123
173. Liang DG, Guo YK, Zhao SB, Yang GY, Han YQ, Chu BB, et al. Pseudorabies virus hijacks the Rab6 protein to promote viral assembly and egress. Vet Res. (2024) 55:68. doi: 10.1186/s13567-024-01328-4
174. Furuyama W, Yamada K, Sakaguchi M, Marzi A, and Nanbo A. Marburg virus exploits the Rab11-mediated endocytic pathway in viral-particle production. Microbiol Spectr. (2024) 12:e0026924. doi: 10.1128/spectrum.00269-24
175. Cosentino G, Marougka K, Desquesnes A, Welti N, Sitterlin D, Gault E, et al. Respiratory syncytial virus ribonucleoproteins hijack microtubule Rab11 dependent transport for intracellular trafficking. PloS Pathog. (2022) 18:e1010619. doi: 10.1371/journal.ppat.1010619
176. Stone M, Jia S, Heo WD, Meyer T, and Konan KV. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J Virol. (2007) 81:4551–63. doi: 10.1128/jvi.01366-06
177. Wozniak AL, Long A, Jones-Jamtgaard KN, and Weinman SA. Hepatitis C virus promotes virion secretion through cleavage of the Rab7 adaptor protein RILP. Proc Natl Acad Sci U.S.A. (2016) 113:12484–9. doi: 10.1073/pnas.1607277113
178. Eyre NS, Fiches GN, Aloia AL, Helbig KJ, McCartney EM, McErlean CS, et al. Dynamic imaging of the hepatitis C virus NS5A protein during a productive infection. J Virol. (2014) 88:3636–52. doi: 10.1128/jvi.02490-13
179. Chen TC, Hsieh CH, and Sarnow P. Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect. PloS Pathog. (2015) 11:e1005116. doi: 10.1371/journal.ppat.1005116
180. Dansako H, Hiramoto H, Ikeda M, Wakita T, and Kato N. Rab18 is required for viral assembly of hepatitis C virus through trafficking of the core protein to lipid droplets. Virology. (2014) 462-463:166–74. doi: 10.1016/j.virol.2014.05.017
181. Pham TM, Tran SC, Lim YS, and Hwang SB. Hepatitis C virus-induced rab32 aggregation and its implications for virion assembly. J Virol. (2017) 91:e01662–16. doi: 10.1128/jvi.01662-16
182. Takacs CN, Andreo U, Dao Thi VL, Wu X, Gleason CE, Itano MS, et al. Differential regulation of lipoprotein and hepatitis C virus secretion by rab1b. Cell Rep. (2017) 21:431–41. doi: 10.1016/j.celrep.2017.09.053
183. Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, and Randall G. Molecular determinants and dynamics of hepatitis C virus secretion. PloS Pathog. (2012) 8:e1002466. doi: 10.1371/journal.ppat.1002466
184. Ploen D, Hafirassou ML, Himmelsbach K, Schille SA, Biniossek ML, Baumert TF, et al. TIP47 is associated with the hepatitis C virus and its interaction with Rab9 is required for release of viral particles. Eur J Cell Biol. (2013) 92:374–82. doi: 10.1016/j.ejcb.2013.12.003
185. Hollinshead M, Johns HL, Sayers CL, Gonzalez-Lopez C, Smith GL, and Elliott G. Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. (2012) 31:4204–20. doi: 10.1038/emboj.2012.262
186. Zenner HL, Yoshimura S, Barr FA, and Crump CM. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol. (2011) 85:8012–21. doi: 10.1128/jvi.00500-11
187. Tebaldi G, Pritchard SM, and Nicola AV. Herpes simplex virus entry by a nonconventional endocytic pathway. J Virol. (2020) 94:e01910–20. doi: 10.1128/jvi.01910-20
188. Bergeman MH, Velarde K, Hargis HL, Glenn HL, and Hogue IB. The Rab6 post-Golgi secretory pathway contributes to herpes simplex virus 1 (HSV-1) egress. J Virol. (2024) 98:e0059924. doi: 10.1128/jvi.00599-24
189. Bello-Morales R, Crespillo AJ, Fraile-Ramos A, Tabarés E, Alcina A, and López-Guerrero JA. Role of the small GTPase Rab27a during herpes simplex virus infection of oligodendrocytic cells. BMC Microbiol. (2012) 12:265. doi: 10.1186/1471-2180-12-265
190. Martin-Sancho L, Tripathi S, Rodriguez-Frandsen A, Pache L, Sanchez-Aparicio M, McGregor MJ, et al. Restriction factor compendium for influenza A virus reveals a mechanism for evasion of autophagy. Nat Microbiol. (2021) 6:1319–33. doi: 10.1038/s41564-021-00964-2
191. Kumar N, Taily IM, Singh C, Kumar S, Rajmani RS, Chakraborty D, et al. Identification of diphenylurea derivatives as novel endocytosis inhibitors that demonstrate broad-spectrum activity against SARS-CoV-2 and influenza A virus both in vitro and in vivo. PloS Pathog. (2023) 19:e1011358. doi: 10.1371/journal.ppat.1011358
192. Khan I, Li S, Tao L, Wang C, Ye B, Li H, et al. Tubeimosides are pan-coronavirus and filovirus inhibitors that can block their fusion protein binding to Niemann-Pick C1. Nat Commun. (2024) 15:162. doi: 10.1038/s41467-023-44504-4
193. Rhodin MHJ, Reyes AC, Balakrishnan A, Bisht N, Kelly NM, Gibbons JS, et al. The small molecule inhibitor of SARS-CoV-2 3CLpro EDP-235 prevents viral replication and transmission in vivo. Nat Commun. (2024) 15:6503. doi: 10.1038/s41467-024-50931-8
194. Heida R, Akkerman R, Jacob Silva PH, Lakerveld AJ, Ortiz D, Bigogno C, et al. Development of an inhalable antiviral powder formulation against respiratory syncytial virus. J Control Release. (2023) 357:264–73. doi: 10.1016/j.jconrel.2023.03.059
195. Inoue J, Ninomiya M, Umetsu T, Nakamura T, Kogure T, Kakazu E, et al. Small interfering RNA screening for the small GTPase rab proteins identifies rab5B as a major regulator of hepatitis B virus production. J Virol. (2019) 93:e00621–19. doi: 10.1128/jvi.00621-19
196. Suárez-Amarán L, Usai C, Di Scala M, Godoy C, Ni Y, Hommel M, et al. A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction. J Hepatol. (2017) 67:669–79. doi: 10.1016/j.jhep.2017.05.010
197. Hirabayashi A, Muramoto Y, Takenaga T, Tsunoda Y, Wakazaki M, Sato M, et al. Coatomer complex I is required for the transport of SARS-CoV-2 progeny virions from the endoplasmic reticulum-Golgi intermediate compartment. mBio. (2025) 16:e0333124. doi: 10.1128/mbio.03331-24
198. Chen T, Kim KY, Oh Y, Jeung HC, Chung KY, Roh MR, et al. Implication of COPB2 expression on cutaneous squamous cell carcinoma pathogenesis. Cancers (Basel). (2022) 14:2038. doi: 10.3390/cancers14082038
199. Zhang A, Duan H, Zhao H, Liao H, Du Y, Li L, et al. Interferon-induced transmembrane protein 3 is a virus-associated protein which suppresses porcine reproductive and respiratory syndrome virus replication by blocking viral membrane fusion. J Virol. (2020) 94:e01350–20. doi: 10.1128/jvi.01350-20
200. Klein S, Golani G, Lolicato F, Lahr C, Beyer D, Herrmann A, et al. IFITM3 blocks influenza virus entry by sorting lipids and stabilizing hemifusion. Cell Host Microbe. (2023) 31:616–633.e620. doi: 10.1016/j.chom.2023.03.005
201. Li X, Dong Z, Liu Y, Song W, Pu J, Jiang G, et al. A novel role for the regulatory nod-like receptor NLRP12 in anti-dengue virus response. Front Immunol. (2021) 12:744880. doi: 10.3389/fimmu.2021.744880
202. Ferrando-Martinez S, Snell Bennett A, Lino E, Gehring AJ, Feld J, Janssen HLA, et al. Functional exhaustion of HBV-specific CD8 T cells impedes PD-L1 blockade efficacy in chronic HBV infection. Front Immunol. (2021) 12:648420. doi: 10.3389/fimmu.2021.648420
203. Li MY, Deng K, Cheng XH, Siu LY, Gao ZR, Naik TS, et al. ARF4-mediated intracellular transport as a broad-spectrum antiviral target. Nat Microbiol. (2025) 10:710–23. doi: 10.1038/s41564-025-01940-w
204. Luo Z, Liang Y, Tian M, Ruan Z, Su R, Shereen MA, et al. Inhibition of PIKFYVE kinase interferes ESCRT pathway to suppress RNA virus replication. J Med Virol. (2023) 95:e28527. doi: 10.1002/jmv.28527
205. Zheng W, Zhou Z, Rui Y, Ye R, Xia F, Guo F, et al. TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion. Signal Transduct Target Ther. (2023) 8:79. doi: 10.1038/s41392-022-01287-2
206. Stewart CM, Bo Y, Fu K, Chan M, Kozak R, Apperley KY, et al. Sphingosine kinases promote ebola virus infection and can be targeted to inhibit filoviruses, coronaviruses, and arenaviruses using late endocytic trafficking to enter cells. ACS Infect Dis. (2023) 9:1064–77. doi: 10.1021/acsinfecdis.2c00416
207. Liu P, Shi M, Liu Y, Liu Y, Lin J, Zhai S, et al. Identification of a novel small molecule STING agonist reshaping the immunomicroenvironment of pancreatic ductal adenocarcinoma. Int J Biol Sci. (2025) 21:3555–72. doi: 10.7150/ijbs.107837
208. Yamamoto KA, Blackburn K, Goshe MB, Brown DT, Migoswski E, Campanhon IB, et al. Tizoxanide antiviral activity on dengue virus replication. Viruses. (2023) 15:696. doi: 10.3390/v15030696
209. Casadémont I, Ayala-Suárez R, Modhiran N, Tawfik A, Prot M, Paul R, et al. miRNome analysis reveals mir-155-5p as a protective factor to dengue infection in a resistant Thai cohort. Med Microbiol Immunol. (2025) 214:13. doi: 10.1007/s00430-025-00821-7
210. Song K, Hasan A, Hao W, Wu Y, Sun Y, Li W, et al. Stimulator of interferon genes (STING) inhibits coronavirus infection by disrupting viral replication organelles. J Med Virol. (2024) 96:e70020. doi: 10.1002/jmv.70020
211. Triantafilou K, Szomolay B, Shepherd MW, Ramanjulu J, and Triantafilou M. STING orchestrates EV-D68 replication and immunometabolism within viral-induced replication organelles. Viruses. (2024) 16:1541. doi: 10.3390/v16101541
212. Yuan M, Tian X, Ma W, Zhang R, Zou X, Jin Y, et al. miRNA-431-5p enriched in EVs derived from IFN-β stimulated MSCs potently inhibited ZIKV through CD95 downregulation. Stem Cell Res Ther. (2024) 15:435. doi: 10.1186/s13287-024-04040-4
213. Jiang X, Liu Z, You H, Tang Z, Ma Y, Nie R, et al. Quercetin-primed BMSC-derived extracellular vesicles ameliorate chronic liver damage through miR-136-5p and GNAS/STAT3 signaling pathways. Int Immunopharmacol. (2024) 142:113162. doi: 10.1016/j.intimp.2024.113162
214. Ganesan M, Pathania AS, Bybee G, Kharbanda KK, Poluektova LY, and Osna NA. Ethanol disrupts the protective crosstalk between macrophages and HBV-infected hepatocytes. Biomolecules. (2025) 15:57. doi: 10.3390/biom15010057
215. Almeida B, Dias TR, Cruz P, Sousa-Pimenta M, Teixeira AL, Pereira CE, et al. Plasma EV-miRNAs as potential biomarkers of COVID-19 vaccine immune response in cancer patients. Vaccines (Basel). (2024) 12:848. doi: 10.3390/vaccines12080848
216. Liang JQ, Xie MY, Hou LJ, Wang HL, Luo JY, Sun JJ, et al. miRNAs derived from milk small extracellular vesicles inhibit porcine epidemic diarrhea virus infection. Antiviral Res. (2023) 212:105579. doi: 10.1016/j.antiviral.2023.105579
217. Park JH, Choi Y, Lim CW, Park JM, Yu SH, Kim Y, et al. Potential therapeutic effect of micrornas in extracellular vesicles from mesenchymal stem cells against SARS-coV-2. Cells. (2021) 10:2393. doi: 10.3390/cells10092393
218. Varghese FS, Rausalu K, Hakanen M, Saul S, Kümmerer BM, Susi P, et al. Obatoclax inhibits alphavirus membrane fusion by neutralizing the acidic environment of endocytic compartments. Antimicrob Agents Chemother. (2017) 61:e02227–16. doi: 10.1128/aac.02227-16
219. Varghese FS, van Woudenbergh E, Overheul GJ, Eleveld MJ, Kurver L, van Heerbeek N, et al. Berberine and obatoclax inhibit SARS-cov-2 replication in primary human nasal epithelial cells in vitro. Viruses. (2021) 13:282. doi: 10.3390/v13020282
220. Yan C, Zhou W, Zhang Y, Zhou X, Qiao Q, Miao L, et al. Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-coV-2 infection. Chembiochem. (2024) 25:e202400404. doi: 10.1002/cbic.202400404
221. Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, et al. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. (2015) 347:995–8. doi: 10.1126/science.1258758
222. Chan WC, Zhao Q, Wong KH, Tang HH, Mok DK, Pardeshi L, et al. Tetrandrine regulates NAADP-mediated calcium signaling through a LIMP-2-dependent and sphingosine-mediated mechanism. Nat Commun. (2025) 16:6308. doi: 10.1038/s41467-025-61565-9
223. Kongsomros S, Suksatu A, Kanjanasirirat P, Manopwisedjaroen S, Prasongtanakij S, Jearawuttanakul K, et al. Anti-SARS-coV-2 activity of extracellular vesicle inhibitors: screening, validation, and combination with remdesivir. Biomedicines. (2021) 9:1230. doi: 10.3390/biomedicines9091230
224. Bortot B, Romani A, Ricci G, and Biffi S. Exploiting extracellular vesicles strategies to modulate cell death and inflammation in COVID-19. Front Pharmacol. (2022) 13:877422. doi: 10.3389/fphar.2022.877422
225. Wu KX and Chu JJ. Antiviral screen identifies EV71 inhibitors and reveals camptothecin-target, DNA topoisomerase 1 as a novel EV71 host factor. Antiviral Res. (2017) 143:122–33. doi: 10.1016/j.antiviral.2017.04.008
226. Fossat N, Lundsgaard EA, Costa R, Rivera-Rangel LR, Nielsen L, Mikkelsen LS, et al. Identification of the viral and cellular microRNA interactomes during SARS-CoV-2 infection. Cell Rep. (2023) 42:112282. doi: 10.1016/j.celrep.2023.112282
227. Liao Y, Liu Y, Li D, Luo S, Huang Y, Wu J, et al. COVID-19 patient serum-derived extracellular vesicles deliver miR-20b-5p induces neutrophil extracellular traps. Cell Commun Signal. (2025) 23:93. doi: 10.1186/s12964-025-02095-1
228. DeMarino C, Denniss J, Cowen M, Norato G, Dietrich DK, Henderson L, et al. HIV-1 RNA in extracellular vesicles is associated with neurocognitive outcomes. Nat Commun. (2024) 15:4391. doi: 10.1038/s41467-024-48644-z
229. Khabir M, Blanchet M, Angelo L, Loucif H, van Grevenynghe J, Bukong TN, et al. Exosomes as conduits: facilitating hepatitis B virus-independent hepatitis D virus transmission and propagation in hepatocytes. Viruses. (2024) 16:825. doi: 10.3390/v16060825
230. Polakowski N, Sarker MAK, Hoang K, Boateng G, Rushing AW, Kendle W, et al. HBZ upregulates myoferlin expression to facilitate HTLV-1 infection. PloS Pathog. (2023) 19:e1011202. doi: 10.1371/journal.ppat.1011202
231. Hubert A, Subra C, Jenabian MA, Tremblay Labrecque PF, Tremblay C, Laffont B, et al. Elevated abundance, size, and microRNA content of plasma extracellular vesicles in viremic HIV-1+ Patients: correlations with known markers of disease progression. J Acquir Immune Defic Syndr. (2015) 70:219–27. doi: 10.1097/qai.0000000000000756
232. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, and Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci. (2013) 14:5338–66. doi: 10.3390/ijms14035338
233. Santangelo L, Bordoni V, Montaldo C, Cimini E, Zingoni A, Battistelli C, et al. Hepatitis C virus direct-acting antivirals therapy impacts on extracellular vesicles microRNAs content and on their immunomodulating properties. Liver Int. (2018) 38:1741–50. doi: 10.1111/liv.13700
234. Yim KHW, Borgoni S, and Chahwan R. Serum extracellular vesicles profiling is associated with COVID-19 progression and immune responses. J Extracell Biol. (2022) 1:e37. doi: 10.1002/jex2.37
235. Madeira RP, Meneghetti P, Lozano N, Namiyama GM, Pereira-Chioccola VL, and Torrecilhas AC. Exploring peripheral blood-derived extracellular vesicles as biomarkers: implications for chronic chagas disease with viral infection or transplantation. Microorganisms. (2024) 12:116. doi: 10.3390/microorganisms12010116
236. Hausjell CS, Ernst W, Grünwald-Gruber C, Arcalis E, and Grabherr R. Quantitative proteomic analysis of extracellular vesicles in response to baculovirus infection of a Trichoplusia ni cell line. PloS One. (2023) 18:e0281060. doi: 10.1371/journal.pone.0281060
237. Bazié WW, Boucher J, Goyer B, Traoré IT, Kania D, Somé DY, et al. Plasma vesicular miR-155 as a biomarker of immune activation in antiretroviral treated people living with HIV. Front Immunol. (2022) 13:916599. doi: 10.3389/fimmu.2022.916599
238. Brachtlova T, Li J, van der Meulen-Muileman IH, Sluiter F, von Meijenfeldt W, Witte I, et al. Quantitative virus-associated RNA detection to monitor oncolytic adenovirus replication. Int J Mol Sci. (2024) 25:6551. doi: 10.3390/ijms25126551
239. He F, Gong Y, Tao G, Zhang J, Wu Q, Tan Y, et al. Targeting the LMP1-ALIX axis in EBV(+) nasopharyngeal carcinoma inhibits immunosuppressive small extracellular vesicle secretion and boosts anti-tumor immunity. Cancer Commun (Lond). (2024) 44:1391–413. doi: 10.1002/cac2.12619
240. Peng M, Zhou Y, Zhang Y, Cong Y, Zhao M, Wang F, et al. Small extracellular vesicle CA1 as a promising diagnostic biomarker for nasopharyngeal carcinoma. Int J Biol Macromol. (2024) 275:133403. doi: 10.1016/j.ijbiomac.2024.133403
241. Ricklefs FL, Wollmann K, Salviano-Silva A, Drexler R, Maire CL, Kaul MG, et al. Circulating extracellular vesicles as biomarker for diagnosis, prognosis, and monitoring in glioblastoma patients. Neuro Oncol. (2024) 26:1280–91. doi: 10.1093/neuonc/noae068
242. Yang CK, Hsu HC, Liu YH, Tsai WS, Ma CP, Chen YT, et al. EV-miRome-wide profiling uncovers miR-320c for detecting metastatic colorectal cancer and monitoring the therapeutic response. Cell Oncol (Dordr). (2022) 45:621–38. doi: 10.1007/s13402-022-00688-3
243. Huang Y, Liao Z, Dang P, Queen S, Abreu CM, Gololobova O, et al. Longitudinal characterization of circulating extracellular vesicles and small RNA during simian immunodeficiency virus infection and antiretroviral therapy. Aids. (2023) 37:733–44. doi: 10.1097/qad.0000000000003487
244. Li Z, Mu S, Tian Y, Shi J, Lan Y, Guan J, et al. Porcine hemagglutinating encephalomyelitis virus co-opts multivesicular-derived exosomes for transmission. mBio. (2023) 14:e0305422. doi: 10.1128/mbio.03054-22
245. Tavares I, Morais M, Dias F, Ferreira M, Martins G, Fernandes R, et al. Extracellular vesicles derived-microRNAs predicting enzalutamide-resistance in 3D spheroid prostate Cancer model. Int J Biol Macromol. (2025) 284:137993. doi: 10.1016/j.ijbiomac.2024.137993
246. Cheng J, Ji D, Yin Y, Wang S, Pan Q, Zhang Q, et al. Proteomic profiling of urinary small extracellular vesicles in children with pneumonia: a pilot study. Pediatr Res. (2023) 94:161–71. doi: 10.1038/s41390-022-02431-y
247. Carranza C, Herrera MT, Guzmán-Beltrán S, Salgado-Cantú MG, Salido-Guadarrama I, Santiago E, et al. A Dual Marker for Monitoring MDR-TB Treatment: Host-Derived miRNAs and M. tuberculosis-Derived RNA Sequences in Serum. Front Immunol. (2021) 12:760468. doi: 10.3389/fimmu.2021.760468
248. Wang J, Luo J, Wen Z, Wang X, Shuai L, Zhong G, et al. Alpha-soluble NSF attachment protein prevents the cleavage of the SARS-coV-2 spike protein by functioning as an interferon-upregulated furin inhibitor. mBio. (2022) 13:e0244321. doi: 10.1128/mbio.02443-21
249. Sharma D, Singh A, Wilson C, Swaroop P, Kumar S, Yadav DK, et al. Exosomal long non-coding RNA MALAT1: a candidate of liquid biopsy in monitoring of Wilms’ tumor. Pediatr Surg Int. (2024) 40:57. doi: 10.1007/s00383-023-05626-4
250. Cureton DK, Burdeinick-Kerr R, and Whelan SP. Genetic inactivation of COPI coatomer separately inhibits vesicular stomatitis virus entry and gene expression. J Virol. (2012) 86:655–66. doi: 10.1128/jvi.05810-11
251. Rahman K, Wilt I, Jolley AA, Chowdhury B, Datta SAK, and Compton AA. SNARE mimicry by the CD225 domain of IFITM3 enables regulation of homotypic late endosome fusion. EMBO J. (2025) 44:534–62. doi: 10.1038/s44318-024-00334-8
252. Sawaged S, Mota T, Piplani H, Thakur R, Lall D, McCabe E, et al. TBK1 and GABARAP family members suppress Coxsackievirus B infection by limiting viral production and promoting autophagic degradation of viral extracellular vesicles. PloS Pathog. (2022) 18:e1010350. doi: 10.1371/journal.ppat.1010350
253. Miao G, Zhao H, Li Y, Ji M, Chen Y, Shi Y, et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev Cell. (2021) 56:427–442.e425. doi: 10.1016/j.devcel.2020.12.010
254. Rahkila P, Alakangas A, Väänänen K, and Metsikkö K. Transport pathway, maturation, and targetting of the vesicular stomatitis virus glycoprotein in skeletal muscle fibers. J Cell Sci. (1996) 109:1585–96. doi: 10.1242/jcs.109.6.1585
255. Bär S, Rommelaere J, and Nüesch JP. Vesicular transport of progeny parvovirus particles through ER and Golgi regulates maturation and cytolysis. PloS Pathog. (2013) 9:e1003605. doi: 10.1371/journal.ppat.1003605
256. Huang J, Tan D, Wang Y, Liu C, Xu J, and Wang J. Egg drop syndrome virus enters duck embryonic fibroblast cells via clathrin-mediated endocytosis. Virus Res. (2015) 210:69–76. doi: 10.1016/j.virusres.2015.07.014
257. Shaw AB, Tse HN, Byford O, Plahe G, Moon-Walker A, Hover SE, et al. Cellular endosomal potassium ion flux regulates arenavirus uncoating during virus entry. mBio. (2024) 15:e0168423. doi: 10.1128/mbio.01684-23
258. Rojek JM, Perez M, and Kunz S. Cellular entry of lymphocytic choriomeningitis virus. J Virol. (2008) 82:1505–17. doi: 10.1128/jvi.01331-07
259. Byford O, Shaw AB, Tse HN, Todd E, Álvarez-Rodríguez B, Hewson R, et al. Lymphocytic choriomeningitis arenavirus requires cellular COPI and AP-4 complexes for efficient virion production. J Virol. (2024) 98:e0200623. doi: 10.1128/jvi.02006-23
260. Van Gorp H, Van Breedam W, Delputte PL, and Nauwynck HJ. The porcine reproductive and respiratory syndrome virus requires trafficking through CD163-positive early endosomes, but not late endosomes, for productive infection. Arch Virol. (2009) 154:1939–43. doi: 10.1007/s00705-009-0527-1
261. Wang K, Li S, Worku T, Hao X, Yang L, and Zhang S. Rab11a is required for porcine reproductive and respiratory syndrome virus induced autophagy to promote viral replication. Biochem Biophys Res Commun. (2017) 492:236–42. doi: 10.1016/j.bbrc.2017.08.057
262. Yang W, Li L, Zhang J, Wu J, Kang W, Wang Y, et al. SNX32 is a host restriction factor that degrades African swine fever virus CP204L via the RAB1B-dependent autophagy pathway. J Virol. (2024) 98:e0159923. doi: 10.1128/jvi.01599-23
263. Sánchez EG, Pérez-Núñez D, and Revilla Y. Mechanisms of entry and endosomal pathway of african swine fever virus. Vaccines (Basel). (2017) 5:42. doi: 10.3390/vaccines5040042
264. Chen X, Zheng J, Liu C, Li T, Wang X, Li X, et al. CD1d facilitates African swine fever virus entry into the host cells via clathrin-mediated endocytosis. Emerg Microbes Infect. (2023) 12:2220575. doi: 10.1080/22221751.2023.2220575
265. Alonso C, Miskin J, Hernáez B, Fernandez-Zapatero P, Soto L, Cantó C, et al. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J Virol. (2001) 75:9819–27. doi: 10.1128/jvi.75.20.9819-9827.2001
266. Chen X, Liang Y, Weng Z, Hu C, Peng Y, Sun Y, et al. ALIX and TSG101 are essential for cellular entry and replication of two porcine alphacoronaviruses. PloS Pathog. (2024) 20:e1012103. doi: 10.1371/journal.ppat.1012103
267. Song C, Li H, Han Y, Luo J, Zhao Y, Zhou C, et al. Host restriction factor Rab11a limits porcine epidemic diarrhea virus invasion of cells via fusion peptide-mediated membrane fusion. Int J Biol Macromol. (2024) 279:135299. doi: 10.1016/j.ijbiomac.2024.135299
268. Li J, Yuan C, Liu P, Li Y, Zhang P, and Yang Q. Red blood cells serve as a vehicle for PEDV transmission. Vet Microbiol. (2021) 257:109081. doi: 10.1016/j.vetmic.2021.109081
269. Li Z, Zhao K, Lan Y, Lv X, Hu S, Guan J, et al. Porcine Hemagglutinating Encephalomyelitis Virus Enters Neuro-2a Cells via Clathrin-Mediated Endocytosis in a Rab5-, Cholesterol-, and pH-Dependent Manner. J Virol. (2017) 91:e01083–17. doi: 10.1128/jvi.01083-17
270. Chen Y, Klute S, Sparrer KMJ, and Serra-Moreno R. RAB5 is a host dependency factor for the generation of SARS-CoV-2 replication organelles. mBio. (2025) 16:e0331424. doi: 10.1128/mbio.03314-24
271. Walia K, Sharma A, Paul S, Chouhan P, Kumar G, Ringe R, et al. SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle. Nat Commun. (2024) 15:2053. doi: 10.1038/s41467-024-46417-2
272. Saraste J and Prydz K. Assembly and cellular exit of coronaviruses: hijacking an unconventional secretory pathway from the pre-golgi intermediate compartment via the golgi ribbon to the extracellular space. Cells. (2021) 10:503. doi: 10.3390/cells10030503
273. Calderon-Dominguez M, Trejo-Gutierrez E, González-Rovira A, Beltrán-Camacho L, Rojas-Torres M, Eslava-Alcón S, et al. Serum microRNAs targeting ACE2 and RAB14 genes distinguish asymptomatic from critical COVID-19 patients. Mol Ther Nucleic Acids. (2022) 29:76–87. doi: 10.1016/j.omtn.2022.06.006
274. Eun SH, Noh SH, and Lee MG. Specific kinesin and dynein molecules participate in the unconventional protein secretion of transmembrane proteins. Korean J Physiol Pharmacol. (2024) 28:435–47. doi: 10.4196/kjpp.2024.28.5.435
275. Mao ND, Xu Y, Yao X, Gao Y, Hui Z, Che H, et al. Design, synthesis, and biological evaluation of novel AAK1/HDACs dual inhibitors against SARS-CoV-2 entry. Bioorg Chem. (2024) 153:107973. doi: 10.1016/j.bioorg.2024.107973
276. Posiri P, Thongsuksangcharoen S, Chaysri N, Panyim S, and Ongvarrasopone C. PmEEA1, the early endosomal protein is employed by YHV for successful infection in Penaeus monodon. Fish Shellfish Immunol. (2019) 95:449–55. doi: 10.1016/j.fsi.2019.10.054
277. Ongvarrasopone C, Chanasakulniyom M, Sritunyalucksana K, and Panyim S. Suppression of PmRab7 by dsRNA inhibits WSSV or YHV infection in shrimp. Mar Biotechnol (NY). (2008) 10:374–81. doi: 10.1007/s10126-007-9073-6
278. Prapavorarat A, Pongsomboon S, and Tassanakajon A. Identification of genes expressed in response to yellow head virus infection in the black tiger shrimp, Penaeus monodon, by suppression subtractive hybridization. Dev Comp Immunol. (2010) 34:611–7. doi: 10.1016/j.dci.2010.01.002
279. Jatuyosporn T, Supungul P, Tassanakajon A, and Krusong K. The essential role of clathrin-mediated endocytosis in yellow head virus propagation in the black tiger shrimp Penaeus monodon. Dev Comp Immunol. (2014) 44:100–10. doi: 10.1016/j.dci.2013.11.017
280. Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, et al. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PloS Pathog. (2010) 6:e1001121. doi: 10.1371/journal.ppat.1001121
281. Aleksandrowicz P, Marzi A, Biedenkopf N, Beimforde N, Becker S, Hoenen T, et al. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J Infect Dis. (2011) 204 Suppl 3:S957–967. doi: 10.1093/infdis/jir326
282. Kubota T, Matsuoka M, Chang TH, Bray M, Jones S, Tashiro M, et al. Ebolavirus VP35 interacts with the cytoplasmic dynein light chain 8. J Virol. (2009) 83:6952–6. doi: 10.1128/jvi.00480-09
283. Lin J, Wang C, Liang W, Zhang J, Zhang L, Lv H, et al. Rab1A is required for assembly of classical swine fever virus particle. Virology. (2018) 514:18–29. doi: 10.1016/j.virol.2017.11.002
284. Chen J, Yang H, Wan M, Cheng Y, Bai J, Li Y, et al. Classical swine fever virus recruits ALIX and ESCRT-III to facilitate viral budding. mBio. (2025) 16:e0261824. doi: 10.1128/mbio.02618-24
285. Liu YY, Liang XD, Liu CC, Cheng Y, Chen H, Baloch AS, et al. Fatty acid synthase is involved in classical swine fever virus replication by interaction with NS4B. J Virol. (2021) 95:e0078121. doi: 10.1128/jvi.00781-21
286. Liu YY, Bai JS, Liu CC, Zhou JF, Chen J, Cheng Y, et al. The small GTPase rab14 regulates the trafficking of ceramide from endoplasmic reticulum to golgi apparatus and facilitates classical swine fever virus assembly. J Virol. (2023) 97:e0036423. doi: 10.1128/jvi.00364-23
287. Acosta EG, Castilla V, and Damonte EB. Differential requirements in endocytic trafficking for penetration of dengue virus. PloS One. (2012) 7:e44835. doi: 10.1371/journal.pone.0044835
288. Shrivastava N, Sripada S, Kaur J, Shah PS, and Cecilia D. Insights into the internalization and retrograde trafficking of Dengue 2 virus in BHK-21 cells. PloS One. (2011) 6:e25229. doi: 10.1371/journal.pone.0025229
289. Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. (2015) 90:2767–82. doi: 10.1128/jvi.02949-15
290. Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, et al. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. (2007) 81:4881–5. doi: 10.1128/jvi.02210-06
291. Feeley EM, Sims JS, John SP, Chin CR, Pertel T, Chen LM, et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PloS Pathog. (2011) 7:e1002337. doi: 10.1371/journal.ppat.1002337
292. Kobayashi S, Suzuki T, Kawaguchi A, Phongphaew W, Yoshii K, Iwano T, et al. Rab8b regulates transport of west nile virus particles from recycling endosomes. J Biol Chem. (2016) 291:6559–68. doi: 10.1074/jbc.M115.712760
293. Chu JJ and Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. (2004) 78:10543–55. doi: 10.1128/jvi.78.19.10543-10555.2004
294. Beachboard DC, Park M, Vijayan M, Snider DL, Fernando DJ, Williams GD, et al. The small GTPase RAB1B promotes antiviral innate immunity by interacting with TNF receptor-associated factor 3 (TRAF3). J Biol Chem. (2019) 294:14231–40. doi: 10.1074/jbc.RA119.007917
295. Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. (2017) 18:324–33. doi: 10.1016/j.celrep.2016.12.045
296. Li M, Zhang D, Li C, Zheng Z, Fu M, Ni F, et al. Characterization of zika virus endocytic pathways in human glioblastoma cells. Front Microbiol. (2020) 11:242. doi: 10.3389/fmicb.2020.00242
297. Esteves E, Rosa N, Correia MJ, Arrais JP, and Barros M. New targets for zika virus determined by human-viral interactomic: A bioinformatics approach. BioMed Res Int. (2017) 2017:1734151. doi: 10.1155/2017/1734151
298. Zavala-Vargas DI, Visoso-Carbajal G, Cedillo-Barrón L, Filisola-Villaseñor JG, Rosales-Ramirez R, Ludert JE, et al. Interaction of the Zika virus with the cytoplasmic dynein-1. Virol J. (2023) 20:43. doi: 10.1186/s12985-023-01992-6
299. Macovei A, Petrareanu C, Lazar C, Florian P, and Branza-Nichita N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J Virol. (2013) 87:6415–27. doi: 10.1128/jvi.00393-13
300. Cui S, Xia T, Zhao J, Ren X, Wu T, Kameni M, et al. NDP52 mediates an antiviral response to hepatitis B virus infection through Rab9-dependent lysosomal degradation pathway. Nat Commun. (2023) 14:8440. doi: 10.1038/s41467-023-44201-2
301. Chu JYK, Chuang YC, Tsai KN, Pantuso J, Ishida Y, Saito T, et al. Autophagic membranes participate in hepatitis B virus nucleocapsid assembly, precore and core protein trafficking, and viral release. Proc Natl Acad Sci U.S.A. (2022) 119:e2201927119. doi: 10.1073/pnas.2201927119
302. Yousaf T, Sun Y, Naz W, Liu Y, Xu J, Yuan S, et al. Multiomics Analysis of Endocytosis upon HBV Infection and Identification of SCAMP1 as a Novel Host Restriction Factor against HBV Replication. Int J Mol Sci. (2022) 23:2211. doi: 10.3390/ijms23042211
303. Osseman Q, Gallucci L, Au S, Cazenave C, Berdance E, Blondot ML, et al. The chaperone dynein LL1 mediates cytoplasmic transport of empty and mature hepatitis B virus capsids. J Hepatol. (2018) 68:441–8. doi: 10.1016/j.jhep.2017.10.032
304. Manna D, Aligo J, Xu C, Park WS, Koc H, Heo WD, et al. Endocytic Rab proteins are required for hepatitis C virus replication complex formation. Virology. (2010) 398:21–37. doi: 10.1016/j.virol.2009.11.034
305. Benedicto I, Gondar V, Molina-Jiménez F, García-Buey L, López-Cabrera M, Gastaminza P, et al. Clathrin mediates infectious hepatitis C virus particle egress. J Virol. (2015) 89:4180–90. doi: 10.1128/jvi.03620-14
306. Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, and McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. (2008) 9:1268–82. doi: 10.1111/j.1600-0854.2008.00767.x
307. Chiou WC, Lu HF, Chen JC, Lai YH, Chang MF, Huang YL, et al. Identification of a novel interaction site between the large hepatitis delta antigen and clathrin that regulates the assembly of genotype III hepatitis delta virus. Virol J. (2022) 19:163. doi: 10.1186/s12985-022-01866-3
308. Yin X, Ambardekar C, Lu Y, and Feng Z. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J Virol. (2016) 90:4232–42. doi: 10.1128/jvi.02804-15
309. Bentaleb C, Hervouet K, Montpellier C, Camuzet C, Ferrié M, Burlaud-Gaillard J, et al. The endocytic recycling compartment serves as a viral factory for hepatitis E virus. Cell Mol Life Sci. (2022) 79:615. doi: 10.1007/s00018-022-04646-y
310. Kapur N, Thakral D, Durgapal H, and Panda SK. Hepatitis E virus enters liver cells through receptor-dependent clathrin-mediated endocytosis. J Viral Hepat. (2012) 19:436–48. doi: 10.1111/j.1365-2893.2011.01559.x
311. Kannan H, Fan S, Patel D, Bossis I, and Zhang YJ. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J Virol. (2009) 83:6375–82. doi: 10.1128/jvi.02571-08
312. Frampton AR Jr., Stolz DB, Uchida H, Goins WF, Cohen JB, and Glorioso JC. Equine herpesvirus 1 enters cells by two different pathways, and infection requires the activation of the cellular kinase ROCK1. J Virol. (2007) 81:10879–89. doi: 10.1128/jvi.00504-07
313. Frampton AR Jr., Uchida H, von Einem J, Goins WF, Grandi P, Cohen JB, et al. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet Microbiol. (2010) 141:12–21. doi: 10.1016/j.vetmic.2009.07.035
314. Xia Z, Ren Y, Li S, Xu J, Wu Y, and Cao Z. ML-SA1 and SN-2 inhibit endocytosed viruses through regulating TRPML channel expression and activity. Antiviral Res. (2021) 195:105193. doi: 10.1016/j.antiviral.2021.105193
315. Jambunathan N, Charles AS, Subramanian R, Saied AA, Naderi M, Rider P, et al. Deletion of a Predicted β-Sheet Domain within the Amino Terminus of Herpes Simplex Virus Glycoprotein K Conserved among Alphaherpesviruses Prevents Virus Entry into Neuronal Axons. J Virol. (2015) 90:2230–9. doi: 10.1128/jvi.02468-15
316. Zaichick SV, Bohannon KP, Hughes A, Sollars PJ, Pickard GE, and Smith GA. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe. (2013) 13:193–203. doi: 10.1016/j.chom.2013.01.009
317. Van Minnebruggen G, Favoreel HW, and Nauwynck HJ. Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex. J Virol. (2004) 78:8852–9. doi: 10.1128/jvi.78.16.8852-8859.2004
318. Shtanko O, Nikitina RA, Altuntas CZ, Chepurnov AA, and Davey RA. Crimean-Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PloS Pathog. (2014) 10:e1004390. doi: 10.1371/journal.ppat.1004390
319. Simon M, Johansson C, and Mirazimi A. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH- and cholesterol-dependent. J Gen Virol. (2009) 90:210–5. doi: 10.1099/vir.0.006387-0
320. Garrison AR, Radoshitzky SR, Kota KP, Pegoraro G, Ruthel G, Kuhn JH, et al. Crimean-Congo hemorrhagic fever virus utilizes a clathrin- and early endosome-dependent entry pathway. Virology. (2013) 444:45–54. doi: 10.1016/j.virol.2013.05.030
321. Yingvilasprasert W, Supungul P, and Tassanakajon A. PmTBC1D20, a Rab GTPase-activating protein from the black tiger shrimp, Penaeus monodon, is involved in white spot syndrome virus infection. Dev Comp Immunol. (2014) 42:302–10. doi: 10.1016/j.dci.2013.09.008
322. Lv LX, Gao J, Wang H, Zhao XF, and Wang JX. Infection and intracellular transport of white spot syndrome virus require the ESCRT machinery in shrimp. J Virol. (2024) 98:e0043324. doi: 10.1128/jvi.00433-24
323. Ye T, Tang W, and Zhang X. Involvement of Rab6 in the regulation of phagocytosis against virus infection in invertebrates. J Proteome Res. (2012) 11:4834–46. doi: 10.1021/pr300274k
324. Xiao B, Fu Q, Niu S, Zhu P, He J, and Li C. Penaeidins restrict white spot syndrome virus infection by antagonizing the envelope proteins to block viral entry. Emerg Microbes Infect. (2020) 9:390–412. doi: 10.1080/22221751.2020.1729068
325. Xu Y, Hu Y, Zhou Y, Jiang C, and Ye T. Rab9 defense against white spot syndrome virus by participation in autophagy in Marsupenaeus japonicas. Fish Shellfish Immunol. (2020) 104:245–51. doi: 10.1016/j.fsi.2020.06.012
326. Wang X, Zhu L, Zhao T, Li H, Hou L, Li C, et al. The molecular characterization of Rab11 and its immune roles in red swamp crayfish (Procambarus clarkii). Int J Biol Macromol. (2024) 274:133299. doi: 10.1016/j.ijbiomac.2024.133299
327. Li DL, Yang MH, Liu LK, Meng C, Li MQ, and Liu HP. Invasion and propagation of white spot syndrome virus: hijacking of the cytoskeleton, intracellular transport machinery, and nuclear import transporters. J Virol. (2022) 96:e0220521. doi: 10.1128/jvi.02205-21
328. Feng J, Tang X, and Zhan W. Cloning and characterization of cytoplasmic dynein intermediate chain in Fenneropenaeus chinensis and its essential role in white spot syndrome virus infection. Fish Shellfish Immunol. (2014) 39:407–14. doi: 10.1016/j.fsi.2014.05.034
329. Niu GJ, Wang S, Xu JD, Yang MC, Sun JJ, He ZH, et al. The polymeric immunoglobulin receptor-like protein from Marsupenaeus japonicus is a receptor for white spot syndrome virus infection. PloS Pathog. (2019) 15:e1007558. doi: 10.1371/journal.ppat.1007558
330. Tan L, Zhang Y, Zhan Y, Yuan Y, Sun Y, Qiu X, et al. Newcastle disease virus employs macropinocytosis and Rab5a-dependent intracellular trafficking to infect DF-1 cells. Oncotarget. (2016) 7:86117–33. doi: 10.18632/oncotarget.13345
331. Tan L, Zhang Y, Qiao C, Yuan Y, Sun Y, Qiu X, et al. NDV entry into dendritic cells through macropinocytosis and suppression of T lymphocyte proliferation. Virology. (2018) 518:126–35. doi: 10.1016/j.virol.2018.02.011
332. Krzyzaniak MA, Zumstein MT, Gerez JA, Picotti P, and Helenius A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PloS Pathog. (2013) 9:e1003309. doi: 10.1371/journal.ppat.1003309
333. Dai P, Tang Z, Qi M, Liu D, Bajinka O, and Tan Y. Dispersion and utilization of lipid droplets mediates respiratory syncytial virus-induced airway hyperresponsiveness. Pediatr Allergy Immunol. (2022) 33:e13651. doi: 10.1111/pai.13651
334. Leemans A, De Schryver M, van der Gucht W, Heykers A, Pintelon I, Hotard AL, et al. Antibody-induced internalization of the human respiratory syncytial virus fusion protein. J Virol. (2017) 91:e00184–17. doi: 10.1128/jvi.00184-17
335. Hu M, Schulze KE, Ghildyal R, Henstridge DC, Kolanowski JL, New EJ, et al. Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. Elife. (2019) 8:e42448. doi: 10.7554/eLife.42448
336. Harbison CE, Lyi SM, Weichert WS, and Parrish CR. Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking. J Virol. (2009) 83:10504–14. doi: 10.1128/jvi.00295-09
337. Parker JS and Parrish CR. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J Virol. (2000) 74:1919–30. doi: 10.1128/jvi.74.4.1919-1930.2000
338. Suikkanen S, Sääjärvi K, Hirsimäki J, Välilehto O, Reunanen H, Vihinen-Ranta M, et al. Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus. J Virol. (2002) 76:4401–11. doi: 10.1128/jvi.76.9.4401-4411.2002
339. Rivera-Serrano EE, González-López O, Das A, and Lemon SM. Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses. Elife. (2019) 8:e43983. doi: 10.7554/eLife.43983
340. Joki-Korpela P, Marjomäki V, Krogerus C, Heino J, and Hyypiä T. Entry of human parechovirus 1. J Virol. (2001) 75:1958–67. doi: 10.1128/jvi.75.4.1958-1967.2001
341. Nachmias D, Sklan EH, Ehrlich M, and Bacharach E. Human immunodeficiency virus type 1 envelope proteins traffic toward virion assembly sites via a TBC1D20/Rab1-regulated pathway. Retrovirology. (2012) 9:7. doi: 10.1186/1742-4690-9-7
342. Vidricaire G and Tremblay MJ. Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J Immunol. (2005) 175:6517–30. doi: 10.4049/jimmunol.175.10.6517
343. Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. (2008) 319:921–6. doi: 10.1126/science.1152725
344. Chaudhry A, Das SR, Jameel S, George A, Bal V, Mayor S, et al. HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic. (2008) 9:1925–35. doi: 10.1111/j.1600-0854.2008.00802.x
345. Hoffman HK, Aguilar RS, Clark AR, Groves NS, Pezeshkian N, Bruns MM, et al. Endocytosed HIV-1 envelope glycoprotein traffics to rab14(+) late endosomes and lysosomes to regulate surface levels in T-cell lines. J Virol. (2022) 96:e0076722. doi: 10.1128/jvi.00767-22
346. Imai K and Okamoto T. Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J Biol Chem. (2006) 281:12495–505. doi: 10.1074/jbc.M511773200
347. Harrison IP and McKnight A. Cellular entry via an actin and clathrin-dependent route is required for Lv2 restriction of HIV-2. Virology. (2011) 415:47–55. doi: 10.1016/j.virol.2011.04.001
348. Ahmad W, Li Y, Guo Y, Wang X, Duan M, Guan Z, et al. Rabies virus co-localizes with early (Rab5) and late (Rab7) endosomal proteins in neuronal and SH-SY5Y cells. Virol Sin. (2017) 32:207–15. doi: 10.1007/s12250-017-3968-9
349. Wang X, Wen Z, Cao H, Luo J, Shuai L, Wang C, et al. Transferrin receptor protein 1 is an entry factor for rabies virus. J Virol. (2023) 97:e0161222. doi: 10.1128/jvi.01612-22
350. Xu J, Gao J, Zhang M, Zhang D, Duan M, Guan Z, et al. Dynein- and kinesin- mediated intracellular transport on microtubules facilitates RABV infection. Vet Microbiol. (2021) 262:109241. doi: 10.1016/j.vetmic.2021.109241
351. Luo J, Zhang Y, Wang Y, Liu Q, Chen L, Zhang B, et al. Rhabdovirus infection is dependent on serine/threonine kinase AP2-associated kinase 1. Life (Basel). (2020) 10:170. doi: 10.3390/life10090170
352. Bernard E, Solignat M, Gay B, Chazal N, Higgs S, Devaux C, et al. Endocytosis of chikungunya virus into mammalian cells: role of clathrin and early endosomal compartments. PloS One. (2010) 5:e11479. doi: 10.1371/journal.pone.0011479
353. Hoornweg TE, van Duijl-Richter MKS, Ayala Nuñez NV, Albulescu IC, van Hemert MJ, and Smit JM. Dynamics of chikungunya virus cell entry unraveled by single-virus tracking in living cells. J Virol. (2016) 90:4745–56. doi: 10.1128/jvi.03184-15
354. Zhang Q, Wei Q, Guan T, Guo W, Jiang L, Cai S, et al. Swine interferon-induced transmembrane proteins inhibit porcine epidemic diarrhea virus replication. Vet Microbiol. (2025) 306:110495. doi: 10.1016/j.vetmic.2025.110495
355. Zhu G, Zong X, Xiao M, Wang D, Xu Z, Hu J, et al. Cholesterol 25-hydroxylase inhibits Newcastle disease virus replication by enzyme activity-dependent and direct interaction with nucleocapsid protein. J Virol. (2025) 99:e0042825. doi: 10.1128/jvi.00428-25
356. Jin J, Galaz-Montoya JG, Sherman MB, Sun SY, Goldsmith CS, O’Toole ET, et al. Neutralizing antibodies inhibit chikungunya virus budding at the plasma membrane. Cell Host Microbe. (2018) 24:417–428.e415. doi: 10.1016/j.chom.2018.07.018
357. Ghosh S, Dellibovi-Ragheb TA, Kerviel A, Pak E, Qiu Q, Fisher M, et al. β-coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. (2020) 183:1520–1535.e1514. doi: 10.1016/j.cell.2020.10.039
358. Sun H, Yang Q, Zhang Y, Cui S, Zhou Z, Zhang P, et al. Syntaxin-6 restricts SARS-CoV-2 infection by facilitating virus trafficking to autophagosomes. J Virol. (2025) 99:e0000225. doi: 10.1128/jvi.00002-25
359. Tan L, Yang Y, Huang X, Yuan Y, Wang K, Peng X, et al. Haspin kinase inhibition dampens pseudorabies virus infection in vitro. Front Vet Sci. (2025) 12:1572729. doi: 10.3389/fvets.2025.1572729
360. Laporte M, Jochmans D, Bardiot D, Desmarets L, Debski-Antoniak OJ, Mizzon G, et al. A coronavirus assembly inhibitor that targets the viral membrane protein. Nature. (2025) 640:514–23. doi: 10.1038/s41586-025-08773-x
361. Teimoori S, Seesuay W, Jittavisutthikul S, Chaisri U, Sookrung N, Densumite J, et al. Human transbodies to VP40 inhibit cellular egress of Ebola virus-like particles. Biochem Biophys Res Commun. (2016) 479:245–52. doi: 10.1016/j.bbrc.2016.09.052
362. Mizuma K, Takashima A, Cubitt B, de la Torre JC, and Iwasaki M. The Pan-ErbB tyrosine kinase inhibitor afatinib inhibits multiple steps of the mammarenavirus life cycle. Virology. (2022) 576:83–95. doi: 10.1016/j.virol.2022.09.005
363. Yamasaki M, Matsuda N, Matoba K, Kondo S, Kanegae Y, Saito I, et al. Acetophenone 4-nitrophenylhydrazone inhibits Hepatitis B virus replication by modulating capsid assembly. Virus Res. (2021) 306:198565. doi: 10.1016/j.virusres.2021.198565
364. Liang R, Guo J, Gu S, Wu Y, Huo S, Wang J, et al. Design, synthesis and biological evaluation of novel triarylmethane analogues as HIV-1 entry inhibitors. Eur J Med Chem. (2025) 291:117574. doi: 10.1016/j.ejmech.2025.117574
365. Cheng H, Lear-Rooney CM, Johansen L, Varhegyi E, Chen ZW, Olinger GG, et al. Inhibition of ebola and marburg virus entry by G protein-coupled receptor antagonists. J Virol. (2015) 89:9932–8. doi: 10.1128/jvi.01337-15
366. Mannemuddhu SS, Xu H, Bleck CKE, Tjandra N, Carter C, and Bhaduri-McIntosh S. Prazoles targeting tsg101 inhibit release of epstein-barr virus following reactivation from latency. J Virol. (2021) 95:e0246620. doi: 10.1128/jvi.02466-20
367. Han H, Liu H, Steiner R, Zhao Z, and Jin ZG. Inhibition of protein kinase D and its substrate phosphatidylinositol-4 kinase III beta blocks common human coronavirus replication. Microbiol Spectr. (2024) 12:e0150124. doi: 10.1128/spectrum.01501-24
368. Shimura S, Watashi K, Fukano K, Peel M, Sluder A, Kawai F, et al. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J Hepatol. (2017) 66:685–92. doi: 10.1016/j.jhep.2016.11.009
369. Horatscheck A, Krauß M, Bulut H, Chambon V, Zadah MS, Dransart E, et al. Next-generation small molecule inhibitors of clathrin function acutely inhibit endocytosis. Structure. (2025) 33:878–890.e877. doi: 10.1016/j.str.2025.02.011
370. Hui Z, Deng H, Xu Y, Gao Y, Zhai C, Mao ND, et al. Discovery and optimization of AAK1 inhibitors based on 1H-indazole scaffold for the potential treatment of SARS-CoV-2 infection. Mol Divers. (2025). doi: 10.1007/s11030-025-11135-4
371. Xu J, Berastegui-Cabrera J, Carretero-Ledesma M, Chen H, Xue Y, Wold EA, et al. Discovery of a small molecule inhibitor of human adenovirus capable of preventing escape from the endosome. Int J Mol Sci. (2021) 22:1617. doi: 10.3390/ijms22041617
372. Gu S, Maurya S, Lona A, Borrega Roman L, Salanga C, Gonzalez DJ, et al. Traffic control: Mechanisms of ligand-specific internalization and intracellular distribution of CCR5. Mol Pharmacol. (2025) 107:100020. doi: 10.1016/j.molpha.2025.100020
373. Yang F, Pang B, Lai KK, Cheung NN, Dai J, Zhang W, et al. Discovery of a novel specific inhibitor targeting influenza A virus nucleoprotein with pleiotropic inhibitory effects on various steps of the viral life cycle. J Virol. (2021) 95:e01432–20. doi: 10.1128/jvi.01432-20
374. Kanginakudru S, Gilson T, Jose L, and Androphy EJ. Effects of caffeine, a DNA damage response inhibitor, on papillomavirus genome replication. Pathogens. (2022) 11:1298. doi: 10.3390/pathogens11111298
375. Manie EA, Elzayat EM, Ragab SS, Sweed AM, Ibrahim H, Nasser S, et al. A novel approach utilizing spirocyclic thiopyrimidinone compounds against herpes simplex virus with underlying antiviral mechanisms of action. Virol J. (2025) 22:97. doi: 10.1186/s12985-025-02707-9
376. Guo Y, Chen Y, Wang Q, Wang Z, Gong L, Sun Y, et al. Emodin and rhapontigenin inhibit the replication of African swine fever virus by interfering with virus entry. Vet Microbiol. (2023) 284:109794. doi: 10.1016/j.vetmic.2023.109794
377. Simo Nemg FB, De S, Keshry SS, Mamidi P, Njayou FN, Demanou M, et al. Plants extracts from Cameroon pharmacopeia strongly inhibit the Chikungunya virus infection by targeting entry and replication steps. J Ethnopharmacol. (2022) 296:115458. doi: 10.1016/j.jep.2022.115458
378. Gao Y, Liu G, Ma Y, Su Y, Lian X, Jiang L, et al. Screening of neurotransmitter receptor modulators reveals novel inhibitors of influenza virus replication. Front Cell Infect Microbiol. (2025) 15:1562650. doi: 10.3389/fcimb.2025.1562650
379. Cao Y, Lai KM, Zheng H, Tan YJ, and Huang D. 5,6-dihydroxyflavone exerts anti-betacoronavirus activity by blocking viral entry to host cells. Virus Res. (2025) 356:199578. doi: 10.1016/j.virusres.2025.199578
380. Zhu J, Chen H, Gao F, Jian W, Huang G, Sunkang Y, et al. Bis-benzylisoquinoline alkaloids inhibit African swine fever virus internalization and replication by impairing late endosomal/lysosomal function. J Virol. (2024) 98:e0032724. doi: 10.1128/jvi.00327-24
381. Campagna MR, Liu F, Mao R, Mills C, Cai D, Guo F, et al. Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. J Virol. (2013) 87:6931–42. doi: 10.1128/jvi.00582-13
382. Xiong Y, Tao K, Li T, Zhou Y, Zhang Z, Ou W, et al. Both chebulagic acid and punicalagin inhibit respiratory syncytial virus entry via multi-targeting glycoprotein and fusion protein. J Virol. (2024) 98:e0153624. doi: 10.1128/jvi.01536-24
383. Leis J, Luan CH, Audia JE, Dunne SF, and Heath CM. Ilaprazole and other novel prazole-based compounds that bind Tsg101 inhibit viral budding of HSV-1/2 and HIV from cells. J Virol. (2021) 95:e00190–21. doi: 10.1128/jvi.00190-21
384. Zhang M, Huang J, Chi Q, Ran X, and Wen X. Antiviral effects and mechanism of Ma-Xing-Shi-Gan-San on porcine reproductive and respiratory syndrome virus. Front Microbiol. (2025) 16:1539094. doi: 10.3389/fmicb.2025.1539094
385. Salata C, Baritussio A, Munegato D, Calistri A, Ha HR, Bigler L, et al. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog Dis. (2015) 73:ftv032. doi: 10.1093/femspd/ftv032
386. Song J, Zhang J, Chen J, Chen S, Yu Z, He L, et al. Cinnamaldehyde inhibits the replication of porcine reproductive and respiratory syndrome virus type 2 in vitro. Viruses. (2025) 17:506. doi: 10.3390/v17040506
387. Dai J, Feng Y, Long H, Liao Y, Tan L, Sun Y, et al. Dexamethasone disrupts intracellular pH homeostasis to delay coronavirus infectious bronchitis virus cell entry via sodium hydrogen exchanger 3 activation. J Virol. (2025) 99:e0189424. doi: 10.1128/jvi.01894-24
388. Qi C, Lee J, Zhang Y, Chen H, Lv J, Wang Z, et al. Identification of cepharanthine as an effective inhibitor of African swine fever virus replication. Emerg Microbes Infect. (2024) 13:2429624. doi: 10.1080/22221751.2024.2429624
389. Jang Y, Shin JS, Yoon YS, Go YY, Lee HW, Kwon OS, et al. Salinomycin inhibits influenza virus infection by disrupting endosomal acidification and viral matrix protein 2 function. J Virol. (2018) 92:e01441–18. doi: 10.1128/jvi.01441-18
390. Li Y, Yang S, Jiang F, Luo S, Liang J, Jiang L, et al. Cilnidipine exerts antiviral effects in vitro and in vivo by inhibiting the internalization and fusion of influenza A virus. BMC Med. (2025) 23:200. doi: 10.1186/s12916-025-04022-0
391. Dada L, Nagai E, Agrawal S, Wirchnianski AS, Wilson IA, Chandran K, et al. SuFEx-enabled high-throughput medicinal chemistry for developing potent tamoxifen analogs as Ebola virus entry inhibitors. Front Immunol. (2025) 16:1533037. doi: 10.3389/fimmu.2025.1533037
392. Amorim MJ, Kao RY, and Digard P. Nucleozin targets cytoplasmic trafficking of viral ribonucleoprotein-Rab11 complexes in influenza A virus infection. J Virol. (2013) 87:4694–703. doi: 10.1128/jvi.03123-12
393. Ma Z, Guo L, Pan M, Jiang C, Liu D, Gao Y, et al. Inhibition of pseudorabies virus replication via upregulated interferon response by targeting 7-dehydrocholesterol reductase. Vet Microbiol. (2024) 290:110000. doi: 10.1016/j.vetmic.2024.110000
394. Selyutina A, Hu P, Miller S, Simons LM, Yu HJ, Hultquist JF, et al. GS-CA1 and lenacapavir stabilize the HIV-1 core and modulate the core interaction with cellular factors. iScience. (2022) 25:103593. doi: 10.1016/j.isci.2021.103593
395. Ho YJ, Wang YM, Lu JW, Wu TY, Lin LI, Kuo SC, et al. Suramin inhibits chikungunya virus entry and transmission. PloS One. (2015) 10:e0133511. doi: 10.1371/journal.pone.0133511
396. Qiao WT, Yao X, Lu WH, Zhang YQ, Malhi KK, Li HX, et al. Matrine exhibits antiviral activities against PEDV by directly targeting Spike protein of the virus and inducing apoptosis via the MAPK signaling pathway. Int J Biol Macromol. (2024) 270:132408. doi: 10.1016/j.ijbiomac.2024.132408
397. Masui S, Nabeshima S, Ajisaka K, Yamauchi K, Itoh R, Ishii K, et al. Maoto, a traditional Japanese herbal medicine, inhibits uncoating of influenza virus. Evid Based Complement Alternat Med. (2017) 2017:1062065. doi: 10.1155/2017/1062065
398. Nishizawa K, Oguma A, Kawata M, Sakasegawa Y, Teruya K, and Doh-ura K. Efficacy and mechanism of a glycoside compound inhibiting abnormal prion protein formation in prion-infected cells: implications of interferon and phosphodiesterase 4D-interacting protein. J Virol. (2014) 88:4083–99. doi: 10.1128/jvi.03775-13
399. Lodge R, Ferreira Barbosa JA, Lombard-Vadnais F, Gilmore JC, Deshiere A, Gosselin A, et al. Host microRNAs-221 and -222 inhibit HIV-1 entry in macrophages by targeting the CD4 viral receptor. Cell Rep. (2017) 21:141–53. doi: 10.1016/j.celrep.2017.09.030
400. Meier R, Franceschini A, Horvath P, Tetard M, Mancini R, von Mering C, et al. Genome-wide small interfering RNA screens reveal VAMP3 as a novel host factor required for Uukuniemi virus late penetration. J Virol. (2014) 88:8565–78. doi: 10.1128/jvi.00388-14
401. Ahmed N, Francis ME, Ahmed N, Kelvin AA, and Pezacki JP. microRNA-185 inhibits SARS-coV-2 infection through the modulation of the host’s lipid microenvironment. Viruses. (2023) 15:1921. doi: 10.3390/v15091921
402. Yao Y, Li S, Zhu Y, Xu Y, Hao S, Guo S, et al. miR-204 suppresses porcine reproductive and respiratory syndrome virus (PRRSV) replication via inhibiting LC3B-mediated autophagy. Virol Sin. (2023) 38:690–8. doi: 10.1016/j.virs.2023.07.004
403. Zhong Z, Ning J, Boggs EA, Jang S, Wallace C, Telmer C, et al. Cytoplasmic CPSF6 regulates HIV-1 capsid trafficking and infection in a cyclophilin A-dependent manner. mBio. (2021) 12:e03142–20. doi: 10.1128/mBio.03142-20
404. Wang S, Li W, Wang L, Tiwari SK, Bray W, Wu L, et al. Interferon-inducible guanylate-binding protein 5 inhibits replication of multiple viruses by binding to the oligosaccharyltransferase complex and inhibiting glycoprotein maturation. bioRxiv. (2024). doi: 10.1101/2024.05.01.591800
405. Savidis G, Perreira JM, Portmann JM, Meraner P, Guo Z, Green S, et al. The IFITMs inhibit zika virus replication. Cell Rep. (2016) 15:2323–30. doi: 10.1016/j.celrep.2016.05.074
406. Shu Q, Lennemann NJ, Sarkar SN, Sadovsky Y, and Coyne CB. ADAP2 is an interferon stimulated gene that restricts RNA virus entry. PloS Pathog. (2015) 11:e1005150. doi: 10.1371/journal.ppat.1005150
407. Han Z, Lv M, Shi Y, Yu J, Niu J, Yu XF, et al. Mutation of Glycosylation Sites in BST-2 Leads to Its Accumulation at Intracellular CD63-Positive Vesicles without Affecting Its Antiviral Activity against Multivesicular Body-Targeted HIV-1 and Hepatitis B Virus. Viruses. (2016) 8:62. doi: 10.3390/v8030062
408. Scherer J, Yi J, and Vallee RB. Role of cytoplasmic dynein and kinesins in adenovirus transport. FEBS Lett. (2020) 594:1838–47. doi: 10.1002/1873-3468.13777
409. Speckhart K, Choi J, DiMaio D, and Tsai B. The BICD2 dynein cargo adaptor binds to the HPV16 L2 capsid protein and promotes HPV infection. PloS Pathog. (2024) 20:e1012289. doi: 10.1371/journal.ppat.1012289
410. Hodgson JJ, Buchon N, and Blissard GW. Identification of cellular genes involved in baculovirus GP64 trafficking to the plasma membrane. J Virol. (2022) 96:e0021522. doi: 10.1128/jvi.00215-22
411. Wang G, Jiang L, Yan Y, Kong F, Li Q, Zhang J, et al. Cellular SLC35B4 promotes internalization during influenza A virus entry. mBio. (2025) 16:e0019425. doi: 10.1128/mbio.00194-25
Keywords: vesicle trafficking, virus, hijack, immune escape, antiviral
Citation: Cao G-X, Liu F-X, Meng C-C, Ding C, Dai J and Qiu X-S (2025) Virus infection and vesicle trafficking. Front. Immunol. 16:1682139. doi: 10.3389/fimmu.2025.1682139
Received: 08 August 2025; Accepted: 17 September 2025;
Published: 09 October 2025.
Edited by:
Guido Poli, Vita-Salute San Raffaele University, ItalyReviewed by:
Selvin Noé Palacios Rápalo, Institut Pasteur, FranceAnismrita Lahon, Institute of Advanced Virology (IAV), India
Copyright © 2025 Cao, Liu, Meng, Ding, Dai and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Dai, MTM0MTQ4MTI1NUBxcS5jb20=; Xu-Sheng Qiu, eHNxaXUxOTgxQHNodnJpLmFjLmNu
†These authors have contributed equally to this work
 Guo-Xiu Cao
Guo-Xiu Cao Fan-Xin Liu
Fan-Xin Liu Chun-Chun Meng
Chun-Chun Meng Chan Ding
Chan Ding Jun Dai
Jun Dai Xu-Sheng Qiu
Xu-Sheng Qiu