- 1The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
- 2School of Medical Technology and Information Engineering, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Department of Neurosurgery, The Second People’s Hospital of Yibin, Yibin, Sichuan, China
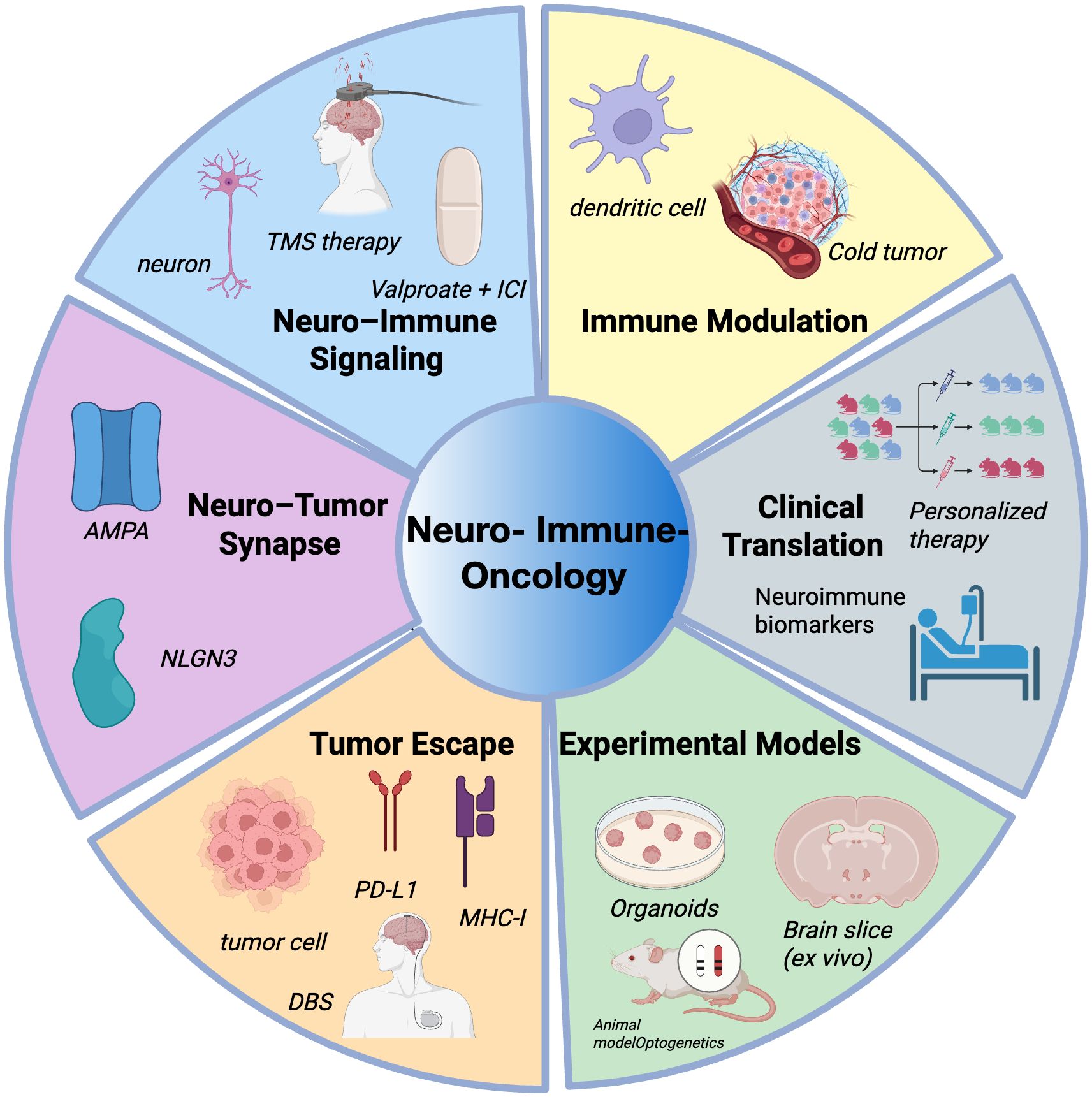
Neuroimmuno-oncology is an emerging interdisciplinary field that explores the complex interactions among the nervous system, the immune system, and tumor cells within the tumor microenvironment (TME). Recent studies have underscored the critical role of neurons in gliomas, where synaptic signaling and the release of neurotrophic factors contribute not only to tumor progression but also to mechanisms of immune evasion. Neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA), along with neuron-derived factors including brain-derived neurotrophic factor (BDNF) and neuroligin-3 (NLGN3), have been shown to modulate immune cell function and promote the formation of an immunosuppressive TME. In particular, neuronal electrical activity mediated through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) signaling facilitates immune escape in glioma cells, leading to the development of an “immune-excluded” phenotype that compromises the efficacy of immunotherapy. Therapeutic strategies that combine AMPAR antagonists with immune checkpoint inhibitors—alongside neuromodulatory techniques such as repetitive transcranial magnetic stimulation (rTMS) or deep brain stimulation (DBS)—hold potential to reprogram the neuro–immune–tumor axis, remodel the immune landscape, and improve immunotherapy responses in central nervous system malignancies. Advancing our understanding of how neuronal activity regulates the glioma immune microenvironment may open new avenues for precision-targeted therapeutic approaches in neuro-oncology.
1 Introduction
Neuroimmuno-oncology is an emerging interdisciplinary field that investigates the intricate interplay between the nervous system, the immune system, and cancer (1). As neuroscience, immunology, and oncology continue to converge, accumulating evidence suggests that the nervous system is not merely a conduit for information transmission but also an active regulator of tumor initiation, progression, and immune evasion (2–4). This field provides a novel framework to elucidate the mechanisms underlying immunologically “cold” tumors, supporting the development of combinatorial interventions aimed at enhancing immunotherapy responsiveness (5, 6). It is increasingly recognized as a promising direction for innovation in cancer treatment. The nervous system constitutes a critical component of the tumor microenvironment (TME) (7). Functionally active neurons can engage tumor cells through electrical and chemical synapses or modulate tumor behavior via the paracrine release of neuromodulatory factors (8, 9), including neuroligin-3 (NLGN3) (10), brain-derived neurotrophic factor (BDNF) (11, 12), glutamate (13), and norepinephrine (5). These signals reshape tumor cell metabolism, oncogenic signaling pathways, and immune phenotypes. Moreover, neuronal activity has been shown to promote the upregulation of immune checkpoints and the recruitment of immunosuppressive cell populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), thereby fostering an immunosuppressive TME (3).
Among central nervous system malignancies, gliomas represent a prototypical model of neuron-cancer crosstalk (10). Recent studies have demonstrated that glioma cells can form bona fide synaptic connections with active neurons, establishing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)–dependent synaptic networks (13). Neuronal activity synchronizes calcium signaling within the tumor, enhances intercellular coupling, and activates the phosphoinositide 3-kinase (PI3K) – mechanistic target of rapamycin (mTOR) axis through NLGN3, driving glioma proliferation via a feedforward loop (10, 14). These findings highlight a central role for neuronal input in glioma progression and suggest novel therapeutic targets within neuron–tumor signaling pathways. Beyond tumor-intrinsic effects, neurons also exert immunomodulatory influence. In various malignancies, neuron-derived neurotransmitters and neurotrophic factors modulate immune cell behavior through receptor-mediated signaling, orchestrating the transition between immune-inflamed (“hot”) and immune-excluded/deserted (“cold”) tumor states (15). This neuron–immune–tumor triad offers a mechanistic explanation for immune resistance in certain tumors and points toward rational strategies to combine neural modulation with immune interventions (3, 6). As research in neuroimmuno-oncology progresses, this field is poised to enhance the precision of immunotherapies, overcome resistance, and improve outcomes across multiple tumor types. In this review, we explore the molecular mechanisms through which neurons regulate anti-tumor immunity and discuss their potential implications for cancer immunotherapy (Figure 1).
2 Neuronal activity and glioma progression
The progression of high-grade gliomas is closely associated with neuronal activity. Clinical observations indicate that brain regions exhibiting higher neuronal firing rates frequently correlate with more aggressive tumor phenotypes and significantly reduced patient survival, highlighting the crucial role of neuronal excitability in glioma biology (4). Glioma cells preferentially migrate toward neuron-rich regions and actively establish synapse-like structural connections with neurons, enabling excitatory neuronal signals to directly stimulate tumor cells (16, 17). These “neuron–glioma synapses” predominantly rely on glutamate-mediated neurotransmission, activating calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (CP-AMPARs) on glioma cell membranes. This activation induces sustained membrane depolarization and downstream oncogenic signaling cascades, thereby enhancing glioma cell proliferation and invasiveness (13, 18). Furthermore, neurotransmitters such as glutamate and norepinephrine accumulate within the synaptic microenvironment, further elevating neuronal excitability. Concurrently, these neurotransmitters stimulate glioma cells through corresponding receptors, exacerbating metabolic reprogramming and malignant progression (13, 17, 19).
Intriguingly, glioma cells themselves may acquire neuron-like electrophysiological properties, actively participating within neuronal circuits and further integrating into existing neural networks. This neuronal phenotype potentially allows glioma cells to generate autonomous rhythmic activity, amplifying their invasiveness and resistance to therapy (9). Recent studies have also suggested that glioma-induced remodeling of neuronal circuits can reciprocally enhance neuronal hyperexcitability, establishing a self-reinforcing feedback loop between tumor growth and neuronal activity (4). Such a mechanism might contribute to both tumor progression and treatment resistance, underscoring the complexity and therapeutic challenge posed by neuron–glioma interactions. Collectively, these findings reveal the bidirectional and dynamic nature of neuron–glioma interactions. Insights into mechanisms such as synapse formation, neurotransmitter metabolism, and receptor-mediated signaling pathways, as well as the reciprocal modulation of neuronal circuits by gliomas, could provide novel therapeutic strategies aimed at disrupting pathological neuron–glioma crosstalk and effectively impeding tumor progression (11, 13, 14).
3 Mechanisms of neuromodulation of the TME
3.1 Effects of neurotransmitters on immune cells
Neurotransmitters, while classically mediating neural communication, also serve as critical regulators of immune responses by modulating the differentiation, activation, and function of immune cells (20). Key mediators such as glutamate, gamma-aminobutyric acid (GABA), and acetylcholine (ACh) shape the immune phenotype of T cells, macrophages, and dendritic cells through receptor-dependent signaling within the tissue microenvironment (19, 21, 22). Furthermore, neurotransmitters facilitate crosstalk and positive feedback loops between immune cells and the sympathetic or parasympathetic nervous systems, enabling dynamic regulation of immune and inflammatory states (5, 23).
In addition to glutamate, several other neurotransmitter systems have been implicated in shaping immune cell behavior. GABA, the primary inhibitory neurotransmitter in the CNS, can suppress T cell proliferation and cytokine production via GABA_A and GABA_B receptor signaling on lymphocytes and myeloid cells (24, 25). GABAergic signaling has been associated with increased Treg differentiation and impaired cytotoxic function, potentially contributing to immune escape in the tumor microenvironment (26, 27). Cholinergic signaling, mediated by ACh, modulates macrophage activation states and promotes anti-inflammatory responses through α7 nicotinic acetylcholine receptors (α7 nAChRs). Cholinergic macrophages have been shown to regulate peritoneal inflammation and may play roles in tumor immunity. Norepinephrine, a key neurotransmitter of the sympathetic nervous system, exhibits context-dependent effects: chronic adrenergic signaling may suppress CD8+ T cell activity and enhance immunosuppressive cell recruitment (5), whereas acute stimulation may facilitate dendritic cell mobilization and antigen presentation. These findings underscore the complexity and diversity of neurotransmitter-mediated immune regulation beyond glutamatergic pathways.
3.2 Neuronal regulation of the immunosuppressive microenvironment
The tumor immune microenvironment (TIME) plays a central role in shaping antitumor immunity, influencing therapeutic responses, disease progression, and clinical outcomes (28). Far beyond a simple aggregation of malignant cells, the TME comprises a complex network of infiltrating or resident immune cells—including T cells, MDSCs, Tregs, and tumor-associated macrophages (TAMs)—alongside stromal components such as cancer-associated fibroblasts (CAFs), astrocytes, and neurons (29). Accumulating evidence indicates that neurons actively contribute to the remodeling of the TME (3). Functionally active neurons not only engage in direct communication with tumor cells via electrical and chemical synapses but also influence local immune dynamics through the paracrine secretion of neuron-derived immunomodulatory molecules (30, 31). Among these, NLGN3 and BDNF have emerged as key neuronal mediators that drive both tumor progression and immunosuppressive remodeling (11, 32). In high-grade gliomas such as glioblastoma (GBM), neuronal activity promotes tumor growth through the secretion of soluble NLGN3, which activates the PI3K–mTOR signaling cascade and triggers feedforward expression of NLGN3 within glioma cells, thereby amplifying their proliferative potential (10, 14). Beyond promoting tumor proliferation, NLGN3 and BDNF have also been implicated in the reprogramming of the tumor immune microenvironment, potentially facilitating the recruitment and activation of immunosuppressive populations such as Tregs and MDSCs (3, 12). This neuron–immune axis forms a positive feedback loop that promotes immune evasion and sustains tumor growth (3, 33, 34).
Moreover, neuronal activity has been increasingly recognized as playing a pivotal role in the establishment of the “immune-cold” tumor phenotype (7). Compared to immune-inflamed tumors, immune-cold tumors are typically characterized by reduced immune infiltration, impaired antigen presentation, and T cell exhaustion (28, 35). Further investigations have demonstrated that heightened neuronal activity can upregulate the expression of immune-regulatory factors such as C–C motif chemokine ligand 2(CCL2), interleukin-1 beta (IL-1β), and prostaglandin E2 (PGE2), thereby promoting the accumulation of MDSCs, Tregs, and M2-polarized macrophages within the TME (5, 23). This process not only exacerbates immunosuppression but also impairs T cell activation, dampening the antitumor immune response. Moreover, single-cell transcriptomic analyses have revealed a strong association between neurogenic signaling pathways and immune exclusion signatures in immune-cold tumors, suggesting that the nervous system actively contributes to immune evasion through spatial and functional modulation of the TME (3, 36).
3.3 Immune escape mechanisms in glioma cells after neural influence
In high-grade gliomas, hyperactive neurons establish functional synaptic connections with tumor cells, delivering excitatory postsynaptic currents (EPSCs) mediated by CP-AMPARs (8, 13, 18). These EPSCs induce sustained membrane depolarization, thereby promoting tumor cell proliferation and invasiveness (10, 11, 18). Concurrently, neuronal secretion of BDNF activates the tropomyosin receptor kinase B (TrkB) and signals through calcium/calmodulin-dependent protein kinase II (CaMKII), promoting AMPAR trafficking to the glioma cell membrane and enhancing glutamate-evoked current amplitudes (11, 13). These processes induce synaptic strengthening in glioma cells, mimicking mechanisms of physiological synaptic plasticity in the healthy brain, and contribute to malignant progression. This neuron-driven “electro-metabolic axis” not only fuels glioma progression but also impairs immune surveillance by downregulating the expression of antigen-processing components such as transporter associated with antigen processing 1 (TAP1) and transporter associated with antigen processing 2 (TAP2) through AMPAR–mediated signaling (11), and by enhancing programmed death-ligand 1 (PD-L1) expression via mTOR-dependent pathways (37). These alterations collectively diminish antigen presentation and enhance PD-1 binding affinity, contributing to an immune-evasive tumor phenotype.
Emerging evidence further highlights that gliomas hijack additional signaling axes to consolidate immune evasion. Among these, the adenosine pathway—driven by ectonucleoside triphosphate diphosphohydrolase-1 (CD39)/ecto-5’-nucleotidase (CD73) ectoenzymes and signaling through adenosine A2A receptor (A2AR)—constitutes a dominant immunosuppressive mechanism that induces T cell exhaustion and promotes regulatory cell recruitment (38, 39). Recent findings also reveal that kynurenine–aryl hydrocarbon receptor (AHR) signaling in tumor-associated macrophages upregulates CD39, thereby reinforcing adenosine production and amplifying immunosuppression within the glioma microenvironment. Collectively, these interconnected pathways converge to establish a multilayered barrier against effective antitumor immunity. These immunosuppressive mechanisms lay the groundwork for further immune exclusion orchestrated by neuronal cues.
Importantly, such electrically driven gliomas are not only highly proliferative but also exhibit a distinctly immune-excluded tumor microenvironment (11). Neuronal activity reshapes the tumor microenvironment by promoting the recruitment of immunosuppressive cell populations, including Tregs and MDSCs, and by upregulating immunomodulatory molecules such as CD73 and PD-L1, thereby suppressing effector T cell infiltration and function (5, 40, 41). Unlike peripheral tumors such as breast cancer, which rarely receive direct synaptic input, gliomas uniquely convert neuronal hyperactivity into both oncogenic and immunosuppressive signals (4, 42). This neuron-driven immunological remodeling contributes to the establishment of an exclusionary immune niche, fostering immune escape and potentially compromising the efficacy of immune checkpoint blockade therapies (37, 43). These neuromodulatory effects on the immune environment pave the way for understanding direct neuronal influence on immune escape in gliomas.
4 A systematic model of neural-immune-tumor interactions
In recent years, accumulating evidence has demonstrated that the nervous system contributes to tumor initiation and progression not only by modulating tumor cell behavior but also by profoundly influencing the composition and functionality of the immune system (20, 44). This has led to the conceptualization of a multidimensional regulatory framework known as the neuron–immune–tumor axis (30). Neurons, through synaptic activity, neurotrophic factors (such as BDNF, neuroligin-3), and neurotransmitters (such as norepinephrine, glutamate), can directly enhance tumor cell proliferation and invasiveness, while also indirectly promoting immune evasion by recruiting immunosuppressive cells such as Tregs and MDSCs or depleting effector T cells (5, 45, 46). Further studies have shown that neuronal signals, including BDNF and norepinephrine, can modulate the phenotype and function of microglia or dendritic cells (12, 23). For example, BDNF signaling through the TrkB receptor has been reported to influence immune-related gene expression (47). Similarly, sympathetic neuron-derived norepinephrine has been shown to upregulate the secretion of chemokines such as CCL2 within the tumor microenvironment, thereby promoting the recruitment of immunosuppressive populations including Tregs and MDSCs (5, 33). Immune cells modulated by neural inputs can, in turn, promote tumor progression through diverse mechanisms, thus forming a functional coupling loop among neurons, immune cells, and tumor cells. For example, Tregs and MDSCs secrete immunosuppressive cytokines such as interleukin-10(IL-10) and transforming growth factor beta (TGF-β) to suppress effector T cell function (34), while activated microglia release tumor-promoting factors including epidermal growth factor (EGF) and matrix metalloproteinases (MMPs), enhancing glioma cell proliferation and invasiveness (48). Additionally, tumor cells under neural influence often upregulate immune checkpoint molecules such as PD-L1 and CD73, further impairing T cell–mediated immune clearance and reinforcing immune escape (41, 49).
While neurons actively regulate immune dynamics in the tumor microenvironment, immune cells can also reciprocally influence neural activity, forming a bidirectional signaling loop. For example, microglia and peripheral immune cells release cytokines such as IL-1β, tumor necrosis factor alpha (TNF-α), and Interferon-gamma (IFN-γ) that modulate synaptic plasticity, neuronal excitability, and neurotransmitter release. Chronic neuroinflammation may result in maladaptive remodeling of neural circuits, with implications for tumor-associated seizures and cognitive dysfunction (50). Tregs and MDSCs can influence neuronal and glial signaling via immunosuppressive mediators like IL-10 and TGF-β. In addition, activated microglia and macrophages may enhance neuronal excitability or alter synaptic pruning through the release of BDNF, reactive oxygen species (ROS), or complement proteins, further reinforcing the immunosuppressive microenvironment and potentially driving neuroplastic adaptations within the tumor niche (51–53).
The increasingly delineated “neuron → immune cell → tumor cell” signaling cascade forms a core mechanism underlying the neurogenic immunosuppressive phenotype observed in gliomas. In this multicellular signaling circuit, neurons first activate immune cells via synaptic activity and soluble factors, leading to the expression of immune checkpoints or secretion of immunosuppressive molecules, which in turn facilitate tumor progression and immune evasion (36, 54). Simultaneously, tumor-associated immune cells may feedback to regulate neural activity or sustain inflammatory signaling, forming a positive feedback loop that amplifies the immunosuppressive milieu (29, 55, 56). This neuron–immune–tumor axis not only enhances our understanding of immune escape mechanisms in central nervous system tumors such as high-grade gliomas but also provides a theoretical basis and strategic direction for developing combined neuro-modulatory and immunotherapeutic approaches.
To better investigate the dynamic interactions within the neuron–immune–tumor triad, organoids and brain organoid models are emerging as ideal experimental platforms. Compared to traditional 2D cultures or animal models, 3D organoid systems better preserve the structural architecture, gene expression profiles, and immune microenvironmental heterogeneity of primary tumors, allowing for more accurate modeling of complex interactions among tumor cells, stromal cells, and immune components (57, 58). Brain organoids further offer visualization and functional assessment of neuronal development, synaptic connectivity, and neurotransmitter release, thereby supporting the study of how neurons regulate immune cell behavior within a spatial and electrophysiological context (59–61). These models are also highly compatible with CRISPR-based gene editing, high-throughput drug screening, and single-cell omics, enabling the integration of mechanistic investigation with therapeutic target validation and holding promise for the development of individualized combination interventions (62–65). Recent technological advances have further enhanced the physiological relevance of organoid systems. For instance, 3D bioprinting allows spatially controlled deposition of induced pluripotent stem cells (iPSC)-derived neurons and immune cells using bioinks laden with functional sensory neuron populations, enabling the localized reconstruction of electrophysiologically active neural circuits and long-range axon guidance (66). The integration of microfluidic platforms permits dynamic perfusion and localized delivery of soluble factors, thereby supporting real-time tracking of neural–immune–tumor interactions under defined chemical and flow conditions (66, 67). These approaches facilitate modular and personalized modeling of synapse-driven immune modulation and offer scalable platforms for evaluating neuro-immune-targeted therapeutic strategies. Nonetheless, current organoid and brain organoid platforms still face limitations. Certain immune cell subpopulations may be lost during long-term culture, and the absence of vascular networks, neural innervation, and mechanical stress limits the faithful recapitulation of dynamic feedback processes within the neuron–immune–tumor axis (68–70). Future efforts integrating microfluidic chips, spatial transcriptomics, and multimodal imaging may enhance the physiological relevance and real-time monitoring capabilities of these models, thereby providing more accurate in vitro platforms for decoding neuro-immune regulation and optimizing therapeutic strategies (58) (Figure 2).
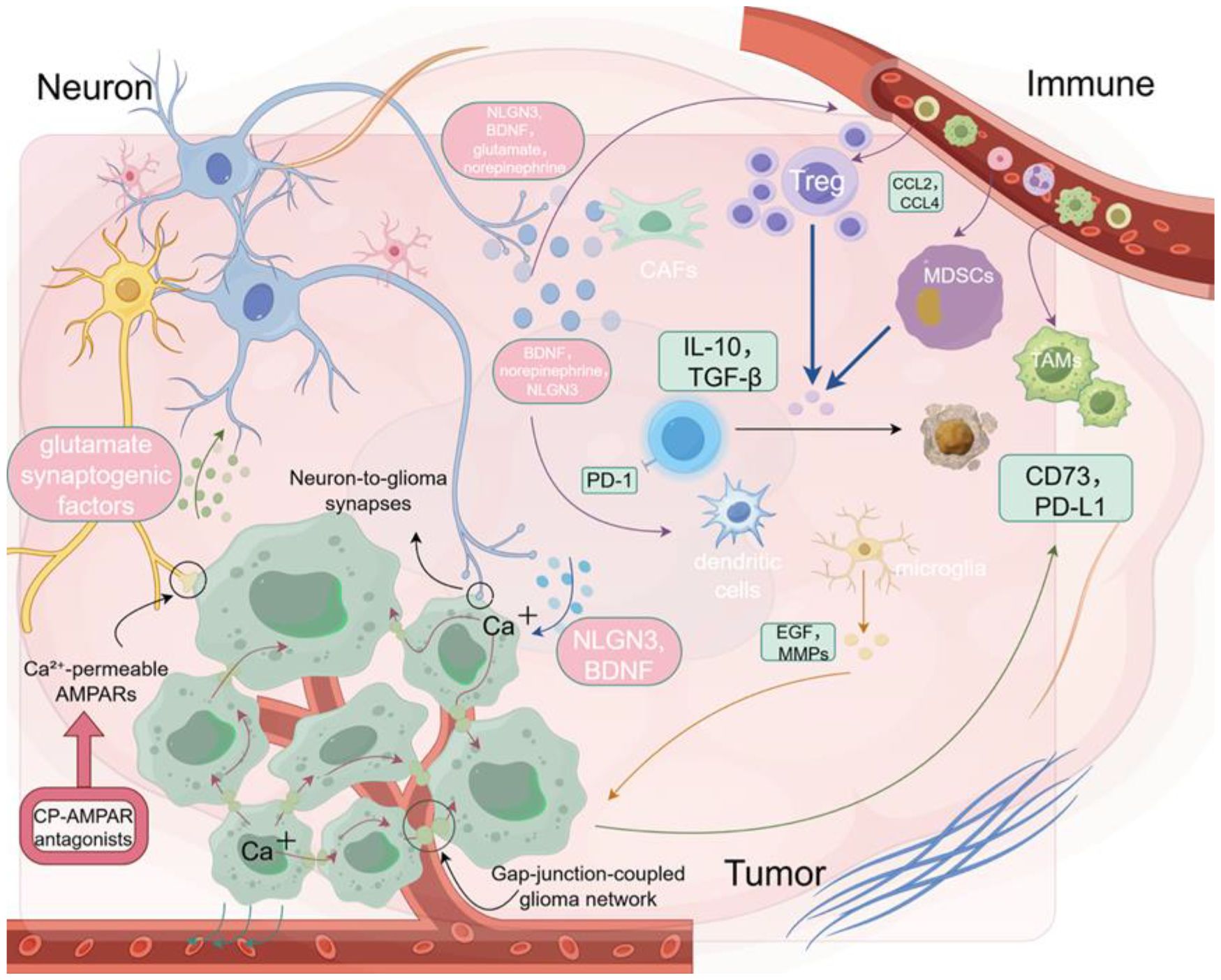
Figure 2. Neuron–tumor–immune interactions in the glioma microenvironment. Neurotransmitters such as glutamate and norepinephrine promote tumor growth and immune exclusion by modulating immune cell behavior.
5 Clinical translation of neuro-immune therapeutics
5.1 Interventional neuromodulation to enhance immunotherapy
In recent years, immune checkpoint inhibitors (ICIs) have achieved significant breakthroughs in the treatment of various solid tumors (71). However, their efficacy remains limited in neurogenic “cold tumors,” such as gliomas and small-cell lung cancer, largely due to the presence of a neuronally regulated immunosuppressive TME (7). Emerging evidence indicates that neurons release neurotransmitters such as glutamate and norepinephrine, which activate AMPAR—particularly CP-AMPARs—on tumor and glial cells (13). This process promotes the recruitment of immunosuppressive cells, including Tregs and MDSCs, induces T cell exhaustion, and shapes a profoundly immune-excluding TME (18). This neuro–tumor–immune axis not only undermines T cell-mediated immunity but also facilitates immune evasion, thereby limiting the therapeutic potential of ICIs (37, 72). Targeting this axis to reprogram the immune landscape has thus emerged as a promising strategy to enhance ICI efficacy. CP-AMPAR antagonists, such as 1-naphthylacetyl spermine trihydrochloride (NASPM), selectively inhibit glutamate-induced calcium influx and neuronal excitatory signaling, effectively disrupting tumor–neuron synaptic coupling and alleviating immunosuppression at its source (40). By mitigating T cell exhaustion and restoring antigen presentation capacity, this “de-excitation” strategy establishes a more favorable immune contexture for ICIs to exert their effects—enabling a synergistic transition from neural disinhibition to immune reactivation (40). Additionally, AMPAR blockade may modulate microglial and astrocyte-mediated synaptic remodeling and immunoregulation (40, 73), potentially improving the immunological tone of the tumor microenvironment and enhancing responsiveness to immune checkpoint inhibitors.
This combinatorial strategy holds substantial clinical potential, particularly in the context of tumors characterized by heightened neural activity and immune evasion (11). Inhibiting CP-AMPARs may function as a pre-conditioning approach to disrupt neuro–tumor crosstalk, alleviate immunosuppression, and enhance tumor immunogenicity—thereby sensitizing tumors to ICIs (13, 40, 44, 73). In this reprogrammed immune landscape, ICIs may subsequently amplify antitumor responses by promoting effector T cell expansion, fostering memory formation, and supporting durable immunity (74, 75). This synergy may be especially beneficial in malignancies with elevated PD-L1 expression yet suboptimal ICI responsiveness, or in those complicated by neuroinflammatory comorbidities (54, 76). To fully realize this potential, future studies should investigate the integration of this dual-targeted approach with radiotherapy, oncolytic virotherapy, or personalized cancer vaccines, while optimizing delivery systems and therapeutic sequencing to facilitate clinical translation.
In parallel with pharmacological blockade, non-invasive neuromodulation technologies are gaining traction as complementary strategies to modulate the immune microenvironment. Repetitive transcranial magnetic stimulation (rTMS), widely used in neuropsychiatric disorders, has been shown—particularly in its low-frequency (<1 Hz) form—to suppress oncogenic signaling cascades (such as PI3K/protein kinase B (AKT) and extracellular signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK)) and induce tumor cell apoptosis (77); its potential immunomodulatory effects, including on local T cell infiltration, remain to be further elucidated. Moreover, rTMS may influence sympathetic nervous system activity, potentially affecting norepinephrine-mediated modulation of the tumor microenvironment and thereby impacting the efficacy of immune checkpoint inhibitors (5, 78). Deep brain stimulation (DBS), as a precise and adjustable neurostimulation technique, also demonstrates potential for immune modulation by altering central excitability and inflammatory feedback (79, 80). In preclinical epilepsy models, anterior nucleus (AN)-targeted DBS significantly reduces hippocampal interleukin-6 (IL-6) levels and caspase-3 activity, indicating its anti-inflammatory and anti-apoptotic effects on the brain’s immune milieu (80). Clinical studies in Parkinson’s disease suggest that subthalamic nucleus (STN)-DBS can reduce dopaminergic medication burden, stabilize limbic system dynamics, and alleviate impulsivity-related behavioral disturbances (81). These effects may also contribute to the mitigation of neuroinflammation and psychological stress, both of which are common in cancer patients with central nervous system involvement (82). Collectively, both pharmacological AMPAR blockade and neuromodulatory interventions such as rTMS and DBS offer synergistic potential with ICIs by reconfiguring the neuro–immune axis, reversing immune suppression, and enhancing antitumor immunity. CP-AMPAR inhibition serves as a “de-excitation” strategy to suppress glutamatergic immune dampening, while electrical neuromodulation enables structural reprogramming of immune thresholds and inflammatory states (77, 79). Moving forward, the convergence of these approaches underscores the need to develop multimodal therapeutic models that integrate neural-targeted agents, neuromodulation, and immune checkpoint blockade—guided by biomarker-driven stratification, optimal treatment timing, and translational validation within the framework of precision oncology.
Beyond small molecules and electrical stimulation, biologically engineered vectors are emerging as the third pillar of neuroimmune modulation. A representative example is the oncolytic adenovirus with triple deletions expressing non-secreting interleukin-12 (Ad-TD-nsIL12), which is currently under investigation in two single-center phase I clinical trials (NCT05717712 for newly diagnosed and NCT05717699 for progressive isocitrate dehydrogenase (IDH)-wildtype gliomas) in pediatric patients. This agent not only promotes direct tumor cell lysis but also enhances local immune activation through the sustained expression of non-secreting interleukin-12 (IL-12) (83). It demonstrates the potential to reshape the immune microenvironment by increasing cytotoxic T lymphocyte infiltration and reducing immunosuppressive cell populations—effects that are particularly valuable in pediatric gliomas characterized by neural regulation and immune exclusion. Similarly, the novel agent DMAMCL, derived from the natural compound micheliolide, has shown potent antitumor activity in preclinical models by inducing apoptosis and impairing mitochondrial function in tumor cells, while simultaneously modulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)–mediated inflammatory signaling (84). These emerging technologies offer scalable, biologically integrated platforms to simulate and intervene in neuroimmune dynamics, and may serve as adjuncts to ICIs by transforming cold tumor microenvironments into immunologically responsive states (Table 1).
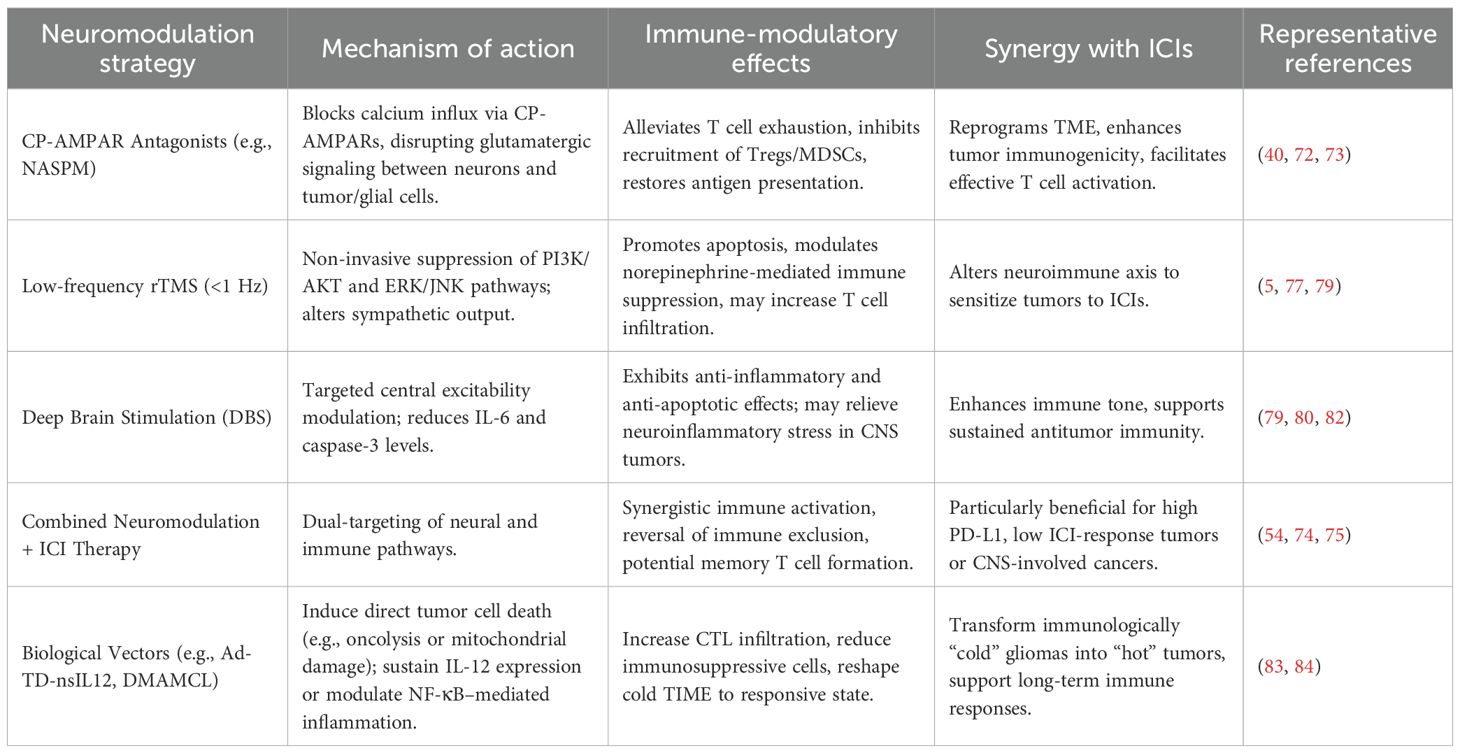
Table 1. Interventional neuromodulation strategies enhancing immune checkpoint inhibitor (ICI) efficacy in cold tumors.
5.2 Observations on the linkage between epilepsy control and immune response
Seizure activity is frequently accompanied by pronounced neuroinflammatory responses, characterized by microglial activation, peripheral T cell infiltration, and elevated levels of proinflammatory cytokines (85). Accumulating evidence has shown sustained increases in IL-1β, TNF-α, and IL-6 within brain parenchyma and cerebrospinal fluid of individuals with epilepsy, implicating these mediators not only in seizure initiation and propagation but also in the establishment of a self-reinforcing “seizure–immune” feedback loop via enhanced neuronal excitability (86, 87). This bidirectional interplay suggests that epilepsy should not be viewed solely as a disorder of aberrant neuronal discharges, but rather as a complex condition intricately linked to immune dysregulation. As such, therapeutic strategies targeting inflammatory and immune pathways—particularly those capable of modulating both neuronal and immune cell function—are gaining increasing attention. Valproic acid (VPA), a widely used broad-spectrum antiepileptic drug, has attracted interest for its immunomodulatory properties beyond seizure control. As a classical histone deacetylase (HDAC) inhibitor, VPA exerts multifaceted effects on immune signaling. It suppresses HDAC3 activity, thereby enhancing acetylation of transcription factors signal transducer and activator of transcription 1(STAT1) and NF-κB, promoting the phenotypic shift of microglia from proinflammatory M1 to anti-inflammatory M2 states (88). In parallel, VPA reduces the expression of key proinflammatory mediators such as TNF-α, IL-1β, and IL-6, alleviating neuroinflammation (88). Additionally, VPA upregulates immune metabolic genes including immune responsive gene 1(IRG1), increasing the production of its downstream metabolite itaconate and activating the nuclear factor erythroid 2–related factor 2(Nrf2) antioxidant pathway—an effect that contributes to maintaining redox and immune homeostasis at both central and systemic levels (89).
This dual function of VPA—as both an antiepileptic and immune-regulating agent—has been validated in a range of disease models. In hypertensive rats, long-term VPA administration attenuated cardiac oxidative stress and inflammation, independent of its effects on blood pressure (90). In cAMP response element modulator (CREM) transgenic mice, VPA delayed the onset of atrial fibrillation, reduced atrial remodeling, and reversed dysregulation of Ras homolog family member A (RhoA) and mitochondrial oxidative phosphorylation pathways (90). In experimental epilepsy, VPA has been shown to modulate CD4+/CD8+ T cell ratios, enhance Treg populations, and mitigate immune-mediated neuroinflammatory damage (91). These findings collectively support the therapeutic potential of VPA to not only stabilize neuronal excitability and suppress seizures, but also to reshape the immune microenvironment. Such a dual-action profile positions VPA as a promising candidate for treating inflammation-driven epilepsy and neuroimmune comorbidities.
While VPA exemplifies a systemically available dual-acting agent, other approaches such as CP-AMPAR inhibition offer localized neuromodulatory-immune benefits. However, the clinical application of CP-AMPAR antagonists must be approached with caution due to their involvement in normal synaptic transmission and cognitive processes. CP-AMPARs are enriched in hippocampal and cortical circuits where they contribute to excitatory synaptic plasticity and memory formation (92–95). Systemic blockade may therefore risk cognitive or behavioral side effects. To mitigate this, emerging strategies aim to achieve tumor-selective inhibition via nanoparticle delivery systems, or through local administration such as convection-enhanced delivery (CED), which minimizes off-target exposure. Additionally, low-dose or transient inhibition of CP-AMPARs has demonstrated immunomodulatory benefits in glioma models with limited neurological impairment, supporting a favorable therapeutic window (Table 2).
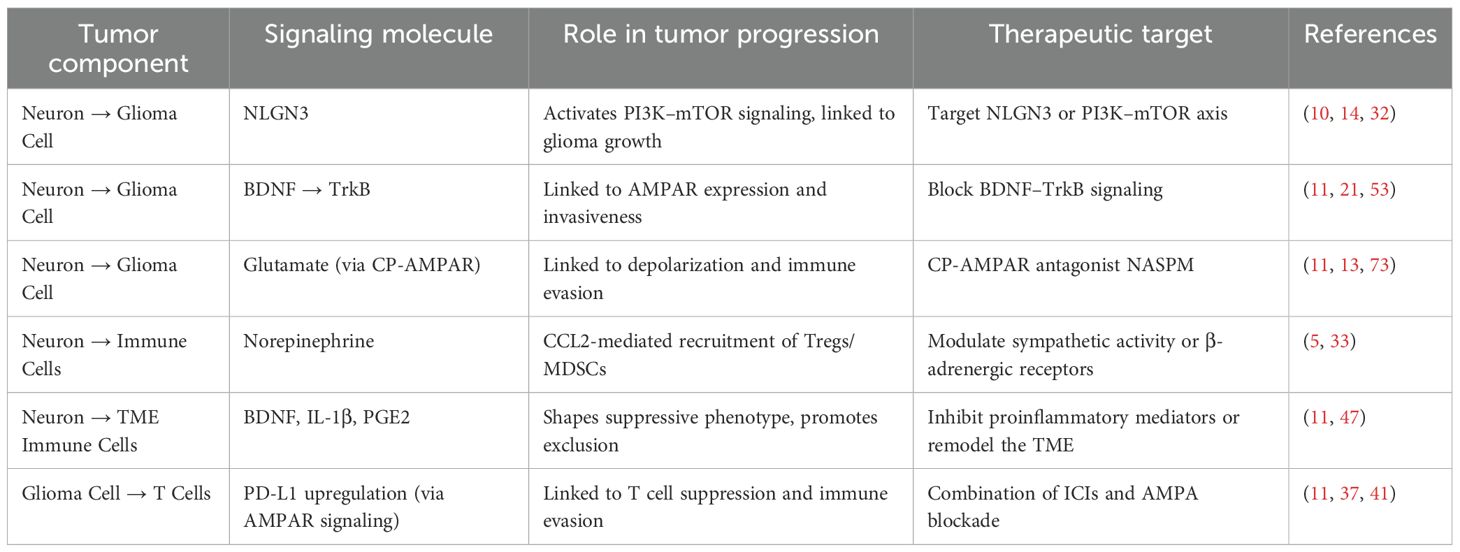
Table 2. Representative pathways of the neuron–immune–tumor axis in glioma and their potential therapeutic targets.
6 Challenges and prospects
While this review emphasizes the predominantly immunosuppressive influence of neuronal activity in gliomas, it is worth noting that neuronal signals may exert bidirectional immunological effects depending on context. For example, certain studies have shown that sympathetic neural activity can enhance antigen presentation and dendritic cell priming under specific conditions, potentially promoting anti-tumor responses. Similarly, BDNF has been linked to microglial modulation and might support immune surveillance in non-malignant contexts (96, 97). These findings underscore the context- and tissue-specific nature of neural influence on the immune system, and highlight the importance of precise spatial and temporal modeling in future investigations. In this regard, animal models—particularly murine systems—have played an indispensable role in elucidating the mechanisms of neuro–immune interaction. Their well-defined genetic backgrounds and experimental accessibility make them ideal platforms to explore how neural signals modulate immune cell differentiation, migration, and functional programming. Reproducible insights have emerged from models simulating sympathetic activation, Notch-mediated contact signaling, and neuron–immune co-culture systems. However, translating these findings to human applications remains a major challenge. Interspecies differences in neural architecture, immune cell ontogeny, and microbiota exposure often limit the predictive value of murine data (98). Compounding this issue, most neuromodulatory strategies rely on systemic inhibition—such as whole-brain electrical stimulation or broad receptor blockade—which risks off-target effects including cognitive impairment and metabolic disturbance. These limitations underscore the need for precise, context-specific neuromodulation. Promising directions include tumor-selective neuronal targeting, programmable stimulation platforms, and neuron-specific delivery vectors such as ligand–receptor engineering or localized biomaterial systems.
Future strategies should also integrate electrophysiological and immune profiling. Combining neurophysiological modalities—such as multi-channel cortical recordings or calcium imaging—with single-cell immune omics (including T cell receptor (TCR)/B cell receptor (BCR) repertoire sequencing and spatial proteomics) may help decode how specific neural signals shape immune cell states within the TME. Conversely, understanding how immune perturbations modulate neuronal excitability could uncover reciprocal control axes. Finally, circuit-level dissection remains limited. Despite evidence that neuron-derived cues like NLGN3 or BDNF regulate immune landscapes, the anatomical wiring of tumor-infiltrating neural circuits remains elusive. Techniques such as viral tracing, connectomics, and in vivo optogenetics may help reconstruct this architecture.
Altogether, overcoming these challenges will require human-relevant models, multimodal analysis frameworks, and cross-disciplinary innovation (Figure 3).
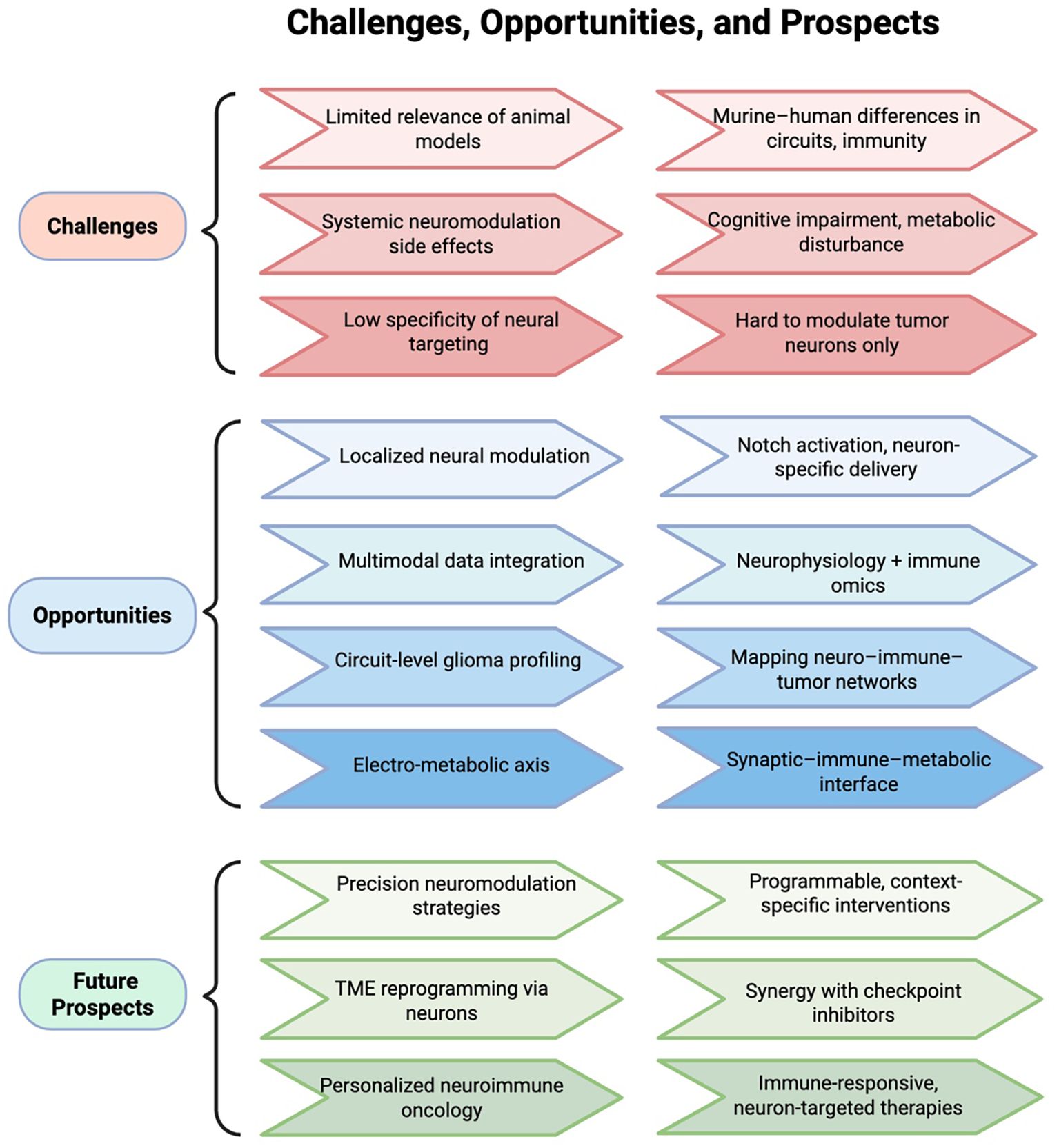
Figure 3. Challenges and prospects in neuro–immune–tumor modulation. The electro-metabolic axis refers to neuron-driven excitatory activity that reprograms metabolism and impairs immune surveillance in gliomas. The neuro–immune–tumor axis denotes the bidirectional regulatory loop linking neurons, immune cells, and tumor cells.
7 Conclusion
The neuron–immune–tumor axis is increasingly recognized as a critical regulatory pathway in glioma progression and immune evasion. Neuronal signals, including neurotransmitters and neurotrophic factors such as NLGN3, BDNF, glutamate, and norepinephrine, can alter immune cell phenotypes and contribute to the formation of an immunosuppressive tumor microenvironment. This review highlights the emerging role of neural activity in shaping antitumor immunity and summarizes current strategies targeting neuron-mediated pathways, including AMPA receptor inhibition, neuromodulation, biologically engineered immunotherapeutics, and the repurposing of antiepileptic agents. However, many aspects of spatiotemporal neural–immune interactions remain poorly understood. Future studies that integrate electrophysiological monitoring, organoid-based modeling and immune profiling may help elucidate context-dependent regulatory mechanisms and guide the development of precise, mechanism-based multimodal therapies to enhance immunotherapy efficacy in gliomas.
Author contributions
LX: Writing – original draft, Methodology, Visualization, Conceptualization. SC: Project administration, Visualization, Writing – review & editing. YF: Writing – review & editing, Validation, Data curation. TZ: Writing – review & editing, Visualization. JY: Formal Analysis, Writing – review & editing, Investigation. JL: Writing – review & editing, Supervision, Data curation. WC: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the 2024 Annual Construction Project of Key Disciplines in Traditional Chinese Medicine of Zhejiang Province (No. 2024-XK-26 by W.C).
Acknowledgments
We utilized the BioRender (https://www.biorender.com/) and Figdraw (https://www.figdraw.com/) online platforms to create the figures in this manuscript and extend our heartfelt thanks for the support and functionality they offer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZW declared a shared parent affiliation with the author YF to the handling editor at the time of review.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT4.0 in order to polishing the language. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
A2AR: adenosine A2A receptor
ACh: acetylcholine
α7 nAChRs: α7 nicotinic acetylcholine receptors
Ad-TD-nsIL12: oncolytic adenovirus with triple deletions expressing non-secreting interleukin-12
AHR: aryl hydrocarbon receptor
AKT: protein kinase B
AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
AN: anterior nucleus
BCR: B cell receptor
BDNF: brain-derived neurotrophic factor
CAFs: cancer-associated fibroblasts
CaMKII: calcium/calmodulin-dependent protein kinase II
CCL2: C–C motif chemokine ligand 2
CD39: ectonucleoside triphosphate diphosphohydrolase-1
CD73: ecto-5’-nucleotidase
CED: convection-enhanced delivery
CP-AMPARs: calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
CREM: cAMP response element modulator
DBS: deep brain stimulation
EGF: epidermal growth factor
EPSCs: excitatory postsynaptic currents
ERK: extracellular signal-regulated kinase
GABA: gamma-aminobutyric acid
GBM: glioblastoma
HDAC: histone deacetylase
ICIs: immune checkpoint inhibitors
IDH: isocitrate dehydrogenase
IFN-γ: Interferon-gamma
IL-1β: interleukin-1 beta
IL-6: interleukin-6
IL-10: interleukin-10
IL-12: interleukin-12
iPSC: induced pluripotent stem cells
IRG1: immune responsive gene 1
JNK: c-Jun N-terminal kinase
MDSCs: myeloid-derived suppressor cells
MMPs: matrix metalloproteinases
NASPM: 1-naphthylacetyl spermine trihydrochloride
NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells
NLGN3: neuroligin-3
Nrf2: nuclear factor erythroid 2–related factor 2
PD-L1: programmed death-ligand 1
PGE2: prostaglandin E2
PI3K: phosphoinositide 3-kinase
mTOR: mechanistic target of rapamycin
rTMS: repetitive transcranial magnetic stimulation
RhoA: Ras homolog family member A
ROS: reactive oxygen species
STAT1: signal transducer and activator of transcription 1
STN: subthalamic nucleus
TAMs: tumor-associated macrophages
TAP1: transporter associated with antigen processing 1
TAP2: transporter associated with antigen processing 2
TCR: T cell receptor
TGF-β: transforming growth factor beta
TIME: tumor immune microenvironment
TME: tumor microenvironment
TNF-α: tumor necrosis factor alpha
TrkB: tropomyosin receptor kinase B
Tregs: regulatory T cells
VPA: valproic acid.
References
1. Huang Y, Zhou X, Liu J, Cao Y, Fu W, and Yang J. Emerging neuroimmune mechanisms in cancer neuroscience. Cancer Lett. (2025) 612:217492. doi: 10.1016/j.canlet.2025.217492
2. Pan Y, Hysinger JD, Barron T, Schindler NF, Cobb O, Guo X, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature. (2021) 594:277–82. doi: 10.1038/s41586-021-03580-6
3. Tomaszewski WH, Waibl-Polania J, Chakraborty M, Perera J, Ratiu J, Miggelbrink A, et al. Neuronal CaMKK2 promotes immunosuppression and checkpoint blockade resistance in glioblastoma. Nat Commun. (2022) 13:6483. doi: 10.1038/s41467-022-34175-y
4. Krishna S, Choudhury A, Keough MB, Seo K, Ni L, Kakaizada S, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. (2023) 617:599–607. doi: 10.1038/s41586-023-06036-1
5. Geng Q, Li L, Shen Z, Zheng Y, Wang L, Xue R, et al. Norepinephrine inhibits CD8+ T-cell infiltration and function, inducing anti-PD-1 mAb resistance in lung adenocarcinoma. Br J Cancer. (2023) 128:1223–35. doi: 10.1038/s41416-022-02132-7
6. Guo X, Pan Y, Xiong M, Sanapala S, Anastasaki C, Cobb O, et al. Midkine activation of CD8+ T cells establishes a neuron–immune–cancer axis responsible for low-grade glioma growth. Nat Commun. (2020) 11:2177. doi: 10.1038/s41467-020-15770-3
7. Huang-Hobbs E, Cheng YT, Ko Y, Luna-Figueroa E, Lozzi B, Taylor KR, et al. Remote neuronal activity drives glioma progression via Sema4f. Nature. (2023) 619:844–50. doi: 10.1038/s41586-023-06267-2
8. Barron T, Yalçın B, Su M, Byun YG, Gavish A, Shamardani K, et al. GABAergic neuron-to-glioma synapses in diffuse midline gliomas. Nature. (2025) 639:1060–8. doi: 10.1038/s41586-024-08579-3
9. Tetzlaff SK, Reyhan E, Layer N, Bengtson CP, Heuer A, Schroers J, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell. (2025) 188:390–411.e36. doi: 10.1016/j.cell.2024.11.002
10. Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. (2015) 161:803–16. doi: 10.1016/j.cell.2015.04.012
11. Taylor KR, Barron T, Hui A, Spitzer A, Yalçin B, Ivec AE, et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature. (2023) 623:366–74. doi: 10.1038/s41586-023-06678-1
12. Tang R, Cao QQ, Hu SW, He LJ, Du PF, Chen G, et al. Sulforaphane activates anti-inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol Sin. (2022) 43:829–39. doi: 10.1038/s41401-021-00727-z
13. Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. (2019) 573:532–8. doi: 10.1038/s41586-019-1564-x
14. Venkatesh HS, Tam LT, Woo PJ, Lennon J, Nagaraja S, Gillespie SM, et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. (2017) 549:533–7. doi: 10.1038/nature24014
15. Galon J and Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
16. Zhang X, Duan S, Apostolou PE, Wu X, Watanabe J, Gallitto M, et al. CHD2 regulates neuron-glioma interactions in pediatric glioma. Cancer Discov. (2024) 14:1732–54. doi: 10.1158/2159-8290.CD-23-0012
17. Hsieh AL, Ganesh S, Kula T, Irshad M, Ferenczi EA, Wang W, et al. Widespread neuroanatomical integration and distinct electrophysiological properties of glioma-innervating neurons. Proc Natl Acad Sci U.S.A. (2024) 121:e2417420121. doi: 10.1073/pnas.2417420121
18. Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. (2019) 573:539–45. doi: 10.1038/s41586-019-1563-y
19. Huang D, Wang Y, Thompson JW, Yin T, Alexander PB, Qin D, et al. Cancer cell-derived GABA promotes β-catenin-mediated tumor growth and immunosuppression. Nat Cell Biol. (2022) 24:230–41. doi: 10.1038/s41556-021-00820-9
20. Khanmammadova N, Islam S, Sharma P, and Amit M. Neuro-immune interactions and immuno-oncology. Trends Cancer. (2023) 9:636–49. doi: 10.1016/j.trecan.2023.05.002
21. Luo S, Lin H, Wu C, Zhu L, Hua Q, Weng Y, et al. Cholinergic macrophages promote the resolution of peritoneal inflammation. Proc Natl Acad Sci U.S.A. (2024) 121:e2402143121. doi: 10.1073/pnas.2402143121
22. Best SA, Gubser PM, Sethumadhavan S, Kersbergen A, Negrón Abril YL, Goldford J, et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. (2022) 34:874–887.e6. doi: 10.1016/j.cmet.2022.04.003
23. Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. (2019) 22:1771–81. doi: 10.1038/s41593-019-0511-3
24. Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature. (2021) 599:471–6. doi: 10.1038/s41586-021-04082-1
25. Jin Z, Hammoud H, Bhandage AK, Korol SV, Trujeque-Ramos O, Koreli S, et al. GABA-mediated inhibition of human CD4+ T cell functions is enhanced by insulin but impaired by high glucose levels. EBioMedicine. (2024) 105:105217. doi: 10.1016/j.ebiom.2024.105217
26. Venkatesan T, Toumpourleka M, Niewiadomska M, Farhat K, Morris L, Elkholey K, et al. Vagal stimulation rescues HFpEF by altering cardiac resident macrophage function. Circ Res. (2025) 137:664–81. doi: 10.1161/CIRCRESAHA.125.326236
27. Zhou X, Wu Y, Zhang Y, Chu B, Yang K, Hong J, et al. Targeting α7 nicotinic acetylcholine receptor for modulating the neuroinflammation of dry eye disease via macrophages. Invest Ophthalmol Visual Sci. (2025) 66:13. doi: 10.1167/iovs.66.5.13
28. Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. (2021) 39:845–865.e7. doi: 10.1016/j.ccell.2021.04.014
29. Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. (2020) 10:1330–51. doi: 10.1158/2159-8290.CD-19-1384
30. Winkler F, Venkatesh HS, Amit M, Batchelor T, Demir IE, Deneen B, et al. Cancer neuroscience: state of the field, emerging directions. Cell. (2023) 186:1689–707. doi: 10.1016/j.cell.2023.02.002
31. Mancusi R and Monje M. The neuroscience of cancer. Nature. (2023) 618:467–79. doi: 10.1038/s41586-023-05968-y
32. Qin L, Liu Z, Guo S, Han Y, Wang X, Ren W, et al. Astrocytic Neuroligin-3 influences gene expression and social behavior, but is dispensable for synapse number. Mol Psychiatry. (2025) 30:84–96. doi: 10.1038/s41380-024-02659-6
33. Liu C, Yang Y, Chen C, Li L, Li J, Wang X, et al. Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat Commun. (2021) 12:5725. doi: 10.1038/s41467-021-25967-9
34. Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. (2016) 76:5671–82. doi: 10.1158/0008-5472.CAN-16-0144
35. Khosravi G, Mostafavi S, Bastan S, Ebrahimi N, Gharibvand RS, and Eskandari N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun (Lond). (2024) 44:521–53. doi: 10.1002/cac2.12539
36. Elguindy MM, Young JS, Ho WS, and Lu RO. Co-evolution of glioma and immune microenvironment. J Immunother Cancer. (2024) 12:e009175. doi: 10.1136/jitc-2024-009175
37. Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. (2018) 175:984–997.e24. doi: 10.1016/j.cell.2018.09.006
38. Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, et al. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human Malignant gliomas. Neuro-oncol. (2013) 15:1160–72. doi: 10.1093/neuonc/not067
39. Ott M, Tomaszowski KH, Marisetty A, Kong LY, Wei J, Duna M, et al. Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration. JCI Insight. (2020) 5:e134386, 134386. doi: 10.1172/jci.insight.134386
40. Wang M, Jia J, Cui Y, Peng Y, and Jiang Y. CD73-positive extracellular vesicles promote glioblastoma immunosuppression by inhibiting T-cell clonal expansion. Cell Death Dis. (2021) 12:1065. doi: 10.1038/s41419-021-04359-3
41. Ye J, Gavras NW, Keeley DC, Hughson AL, Hannon G, Vrooman TG, et al. CD73 and PD-L1 dual blockade amplifies antitumor efficacy of SBRT in murine PDAC models. J Immunother Cancer. (2023) 11:e006842. doi: 10.1136/jitc-2023-006842
42. de Ruiter Swain J, Michalopoulou E, Noch EK, Lukey MJ, and Van Aelst L. Metabolic partitioning in the brain and its hijacking by glioblastoma. Genes Dev. (2023) 37:681–702. doi: 10.1101/gad.350693.123
43. Jiacheng D, Jiayue C, Ying G, Shaohua W, Wenhui L, and Xinyu H. Research progress and challenges of the PD-1/PD-L1 axis in gliomas. Cell Biosci. (2024) :14:123. doi: 10.1186/s13578-024-01305-6
44. Lin H, Liu C, Hu A, Zhang D, Yang H, and Mao Y. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J Hematol Oncol. (2024) 17:31. doi: 10.1186/s13045-024-01544-7
45. Zhao H, Teng D, Yang L, Xu X, Chen J, Jiang T, et al. Myeloid-derived itaconate suppresses cytotoxic CD8+ T cells and promotes tumor growth. Nat Metab. (2022) 4:1660–73. doi: 10.1038/s42255-022-00676-9
46. Altas B, Tuffy LP, Patrizi A, Dimova K, Soykan T, Brandenburg C, et al. Region-specific phosphorylation determines neuroligin-3 localization to excitatory versus inhibitory synapses. Biol Psychiatry. (2024) 96:815–28. doi: 10.1016/j.biopsych.2023.12.020
47. Wu SY, Pan BS, Tsai SF, Chiang YT, Huang BM, Mo FE, et al. BDNF reverses aging-related microglial activation. J Neuroinflamm. (2020) 17:210. doi: 10.1186/s12974-020-01887-1
48. Nuñez RE, del Valle MM, Ortiz K, Almodovar L, and Kucheryavykh L. Microglial cytokines induce invasiveness and proliferation of human glioblastoma through pyk2 and FAK activation. Cancers. (2021) 13:6160. doi: 10.3390/cancers13246160
49. Zhang T, Liu H, Jiao L, Zhang Z, He J, Li L, et al. Genetic characteristics involving the PD-1/PD-L1/L2 and CD73/A2aR axes and the immunosuppressive microenvironment in DLBCL. J Immunother Cancer. (2022) 10:e004114. doi: 10.1136/jitc-2021-004114
50. Kim MW, Gao W, Lichti CF, Gu X, Dykstra T, Cao J, et al. Endogenous self-peptides guard immune privilege of the central nervous system. Nature. (2024) 637:176–83. doi: 10.1038/s41586-024-08279-y
51. Hu CF, Wu SP, Lin GJ, Shieh CC, Hsu CS, Chen JW, et al. Microglial nox2 plays a key role in the pathogenesis of experimental autoimmune encephalomyelitis. Front Immunol. (2021) 12:638381. doi: 10.3389/fimmu.2021.638381
52. Chagas L da S and Serfaty CA. The influence of microglia on neuroplasticity and long-term cognitive sequelae in long COVID: impacts on brain development and beyond. Int J Mol Sci. (2024) 25:3819. doi: 10.3390/ijms25073819
53. Vecchiarelli HA, Lopes LT, Paolicelli RC, Stevens B, Wake H, and Tremblay MÈ. Synapse regulation. Adv Neurobiol. (2024) 37:179–208. doi: 10.1007/978-3-031-55529-9_11
54. Wen J, Li Y, Deng W, and Li Z. Central nervous system and immune cells interactions in cancer: unveiling new therapeutic avenues. Front Immunol. (2025) 16:1528363. doi: 10.3389/fimmu.2025.1528363
55. Snell LM, McGaha TL, and Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. (2017) 38:542–57. doi: 10.1016/j.it.2017.05.005
56. Pang L, Guo S, Khan F, Dunterman M, Ali H, Liu Y, et al. Hypoxia-driven protease legumain promotes immunosuppression in glioblastoma. Cell Rep Med. (2023) 4:101238. doi: 10.1016/j.xcrm.2023.101238
57. Zhou Z, Pang Y, Ji J, He J, Liu T, Ouyang L, et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nat Rev Immunol. (2024) 24:18–32. doi: 10.1038/s41577-023-00896-4
58. Zhu J, Pang K, Hu B, He R, Wang N, Jiang Z, et al. Custom microfluidic chip design enables cost-effective three-dimensional spatiotemporal transcriptomics with a wide field of view. Nat Genet. (2024) 56:2259–70. doi: 10.1038/s41588-024-01906-4
59. Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, et al. Microglia innately develop within cerebral organoids. Nat Commun. (2018) 9:4167. doi: 10.1038/s41467-018-06684-2
60. Zhang W, Zhang M, Xu Z, Yan H, Wang H, Jiang J, et al. Human forebrain organoid-based multi-omics analyses of PCCB as a schizophrenia associated gene linked to GABAergic pathways. Nat Commun. (2023) 14:5176. doi: 10.1038/s41467-023-40861-2
61. Lancaster MA and Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. (2014) 9:2329–40. doi: 10.1038/nprot.2014.158
62. Sun F, Li H, Sun D, Fu S, Gu L, Shao X, et al. Single-cell omics: experimental workflow, data analyses and applications. Sci China Life Sci. (2025) 68:5–102. doi: 10.1007/s11427-023-2561-0
63. Villiger L, Joung J, Koblan L, Weissman J, Abudayyeh OO, and Gootenberg JS. CRISPR technologies for genome, epigenome and transcriptome editing. Nat Rev Mol Cell Biol. (2024) 25:464–87. doi: 10.1038/s41580-023-00697-6
64. Li C, Fleck JS, Martins-Costa C, Burkard TR, Themann J, Stuempflen M, et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature. (2023) 621:373–80. doi: 10.1038/s41586-023-06473-y
65. Boreström C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslöw A, et al. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell–derived kidney model for drug discovery. Kidney Int. (2018) 94:1099–110. doi: 10.1016/j.kint.2018.05.003
66. Hirano M, Huang Y, Vela Jarquin D, de la Garza Hernández RL, Jodat YA, Luna Cerón E, et al. 3D bioprinted human iPSC-derived somatosensory constructs with functional and highly purified sensory neuron networks. Biofabrication. (2021) 13:035046. doi: 10.1088/1758-5090/abff11
67. Michels KR, Sheih A, Hernandez SA, Brandes AH, Parrilla D, Irwin B, et al. Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering. J Immunother Cancer. (2023) 11:e006292. doi: 10.1136/jitc-2022-006292
68. Papaioannou MD, Sangster K, Sajid RS, Djuric U, and Diamandis P. Cerebral organoids: emerging ex vivo humanoid models of glioblastoma. Acta Neuropathol Commun. (2020) 8:209. doi: 10.1186/s40478-020-01077-3
69. Prior N, Inacio P, and Huch M. Liver organoids: from basic research to therapeutic applications. Gut. (2019) 68:2228–37. doi: 10.1136/gutjnl-2019-319256
70. Wang L, Owusu-Hammond C, Sievert D, and Gleeson JG. Stem cell based organoid models of neurodevelopmental disorders. Biol Psychiatry. (2023) 93:622–31. doi: 10.1016/j.biopsych.2023.01.012
71. Gao W, Wang X, Zhou Y, Wang X, and Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. (2022) 7:196. doi: 10.1038/s41392-022-01046-3
72. Bai X, Zhou Y, Yokota Y, Matsumoto Y, Zhai B, Maarouf N, et al. Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J Exp Clin Cancer Res. (2022) 41:132. doi: 10.1186/s13046-022-02307-3
73. Ishiuchi S, Yoshida Y, Sugawara K, Aihara M, Ohtani T, Watanabe T, et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J Neurosci. (2007) 27:7987–8001. doi: 10.1523/JNEUROSCI.2180-07.2007
74. Yin T, Wang G, Wang L, Mudgal P, Wang E, Pan CC, et al. Breaking NGF–TrkA immunosuppression in melanoma sensitizes immunotherapy for durable memory T cell protection. Nat Immunol. (2024) 25:268–81. doi: 10.1038/s41590-023-01723-7
75. Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. (2019) 50:195–211. doi: 10.1016/j.immuni.2018.12.021
76. Sharma P, Hu-Lieskovan S, Wargo JA, and Ribas A. Primary, adaptive and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
77. Jo S, Im SH, Kim SH, Baek D, Shim JK, Kang SG, et al. Tumor suppressive effect of low-frequency repetitive transcranial magnetic stimulation on glioblastoma progression. Neurotherapeutics. (2025) 22:e00569. doi: 10.1016/j.neurot.2025.e00569
78. Sattler A, Korzun T, Gupta K, Diba P, Kyprianou N, and Eksi SE. Sympathetic nerve signaling rewires the tumor microenvironment: a shift in “microenvironmental-ity. Cancer Metastasis Rev. (2025) 44:25. doi: 10.1007/s10555-025-10241-x
79. Puk O, Jabłońska M, and Sokal P. Immunomodulatory and endocrine effects of deep brain stimulation and spinal cord stimulation - A systematic review. BioMed Pharmacother. (2023) 168:115732. doi: 10.1016/j.biopha.2023.115732
80. Amorim BO, Covolan L, Ferreira E, Brito JG, Nunes DP, de Morais DG, et al. Deep brain stimulation induces antiapoptotic and anti-inflammatory effects in epileptic rats. J Neuroinflamm. (2015) :12:162. doi: 10.1186/s12974-015-0384-7
81. Pham U, Skogseid IM, Pripp AH, Bøen E, and Toft M. Impulsivity in Parkinson’s disease patients treated with subthalamic nucleus deep brain stimulation—An exploratory study. PloS One. (2021) 16:e0248568. doi: 10.1371/journal.pone.0248568
82. Kurtis MM, Rajah T, Delgado LF, and Dafsari HS. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. NPJ Parkinson’s Dis. (2017) 3:1–12. doi: 10.1038/npjparkd.2016.24
83. Qian X, Ning W, Yang J, Dunmall LC, Pandha HS, Shang G, et al. The oncolytic adenovirus ad-TD-nsIL12 in primary or progressive pediatric IDH wild-type diffuse intrinsic pontine glioma results of two phase I clinical trials. Nat Commun. (2025) 16:6934. doi: 10.1038/s41467-025-62260-5
84. Xu N, Hua Z, Ba G, Zhang S, Liu Z, Thiele CJ, et al. The anti-tumor growth effect of a novel agent DMAMCL in rhabdomyosarcoma in vitro and in vivo. J Exp Clin Cancer Res. (2019) 38:118. doi: 10.1186/s13046-019-1107-1
85. Sanz P, Rubio T, and Garcia-Gimeno MA. Neuroinflammation and epilepsy: from pathophysiology to therapies based on repurposing drugs. Int J Mol Sci. (2024) 25:4161. doi: 10.3390/ijms25084161
86. Soltani Khaboushan A, Yazdanpanah N, and Rezaei N. Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol. (2022) 59:1724–43. doi: 10.1007/s12035-022-02725-6
87. Vezzani A, Balosso S, and Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. (2019) 15:459–72. doi: 10.1038/s41582-019-0217-x
88. Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflamm. (2018) 15:150. doi: 10.1186/s12974-018-1193-6
89. Lei I, Huang W, Noly PE, Naik S, Ghali M, Liu L, et al. Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci Transl Med. (2023) 15:eade3782. doi: 10.1126/scitranslmed.ade3782
90. Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. (2010) 56:437–44. doi: 10.1161/HYPERTENSIONAHA.110.154567
91. Lin JR, Huang SH, Wu CH, Chen YW, Hong ZJ, Cheng CP, et al. Valproic acid suppresses autoimmune recurrence and allograft rejection in islet transplantation through induction of the differentiation of regulatory T cells and can be used in cell therapy for type 1 diabetes. Pharmaceuticals. (2021) 14:475. doi: 10.3390/ph14050475
92. Durakoglugil MS, Wasser CR, Wong CH, Pohlkamp T, Xian X, Lane-Donovan C, et al. Reelin regulates neuronal excitability through striatal-enriched protein tyrosine phosphatase (STEP61) and calcium permeable AMPARs in an NMDAR-dependent manner. J Neurosci. (2021) 41:JN–RM-0388-21. doi: 10.1523/JNEUROSCI.0388-21.2021
93. Cepeda-Prado EA, Khodaie B, Quiceno GD, Beythien S, Edelmann E, and Lessmann V. Calcium-permeable AMPA receptors mediate timing-dependent LTP elicited by low repeat coincident pre- and postsynaptic activity at schaffer collateral-CA1 synapses. Cereb Cortex. (2021) 32:1682–703. doi: 10.1093/cercor/bhab306
94. Yi JH, Moon S, Cho E, Kwon H, Lee S, Jeon J, et al. Hyperoside improves learning and memory deficits by amyloid β1–42 in mice through regulating synaptic calcium-permeable AMPA receptors. Eur J Pharmacol. (2022) :931:175188. doi: 10.1016/j.ejphar.2022.175188
95. Koek LA, Sanderson TM, Georgiou J, and Collingridge GL. The role of calcium stores in long-term potentiation and synaptic tagging and capture in mouse hippocampus. Philos T R Soc B. (2024) 379:20230241. doi: 10.1098/rstb.2023.0241
96. Maurya SK and Mishra R. Co-expression and interaction of pax6 with genes and proteins of immunological surveillance in the brain of mice. Neurotoxic Res. (2022) 40:2238–52. doi: 10.1007/s12640-022-00562-y
97. Boisserand LSB, Geraldo LH, Bouchart J, El Kamouh MR, Lee S, Sanganahalli BG, et al. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J Exp Med. (2024) 221:e20221983. doi: 10.1084/jem.20221983
Keywords: neuro–immune–tumor axis, glioma, tumor microenvironment, neuronal activity, immune suppression, AMPA receptor, immunotherapy
Citation: Xu L, Chen S, Fu Y, Zhou T, Yu J, Li J and Chen W (2025) Neuro–immune–tumor axis in gliomas: a review of mechanisms, models, and translational opportunities. Front. Immunol. 16:1682322. doi: 10.3389/fimmu.2025.1682322
Received: 08 August 2025; Accepted: 24 September 2025;
Published: 08 October 2025.
Edited by:
Qihang Yuan, Dalian Medical University, ChinaReviewed by:
Zhenpeng Wen, Sichuan University, ChinaHong-yi Zhangben, The University of Tokyo, Japan
Copyright © 2025 Xu, Chen, Fu, Zhou, Yu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Chen, Y2hlbndlaWJlbmJlbkAxNjMuY29t; Jiayang Li, amlheWFuZy5saUB6Y211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Lu Xu
Lu Xu Shuangyu Chen
Shuangyu Chen Yixin Fu
Yixin Fu Tingting Zhou
Tingting Zhou Jianghao Yu
Jianghao Yu Jiayang Li
Jiayang Li Wei Chen
Wei Chen