- 1Medical School, Jianghan University, Wuhan, Hubei, China
- 2Institute of Acupuncture, Jianghan University, Wuhan, Hubei, China
- 3Department of Integrated Traditional Chinese and Western Medicine, Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 4School of Acupuncture and Bone Injury, Hubei University of Chinese Medicine, Wuhan, Hubei, China
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with a complex etiology, in which gut microecological dysregulation plays a central role in disease onset and progression. Acupuncture, a non-pharmacological intervention rooted in traditional Chinese medicine, confers multi-target and systemic regulatory effects, and has been extensively applied in the prevention and management of T2DM. This review systematically examines the roles of gut microbiota dysbiosis, aberrant gut-derived metabolites, intestinal barrier dysfunction, and chronic low-grade inflammation in the pathophysiology of T2DM. It further delineates the potential mechanisms by which acupuncture indirectly mitigates T2DM through modulation of the “gut microbiota–metabolite–intestinal barrier–inflammation” axis, alongside its direct actions on insulin signaling pathways and pancreatic β-cell function. Drawing on recent advances in multi-omics technologies, this review discusses the prospects and challenges of applying acupuncture in individualized and precision treatment of T2DM, aiming to provide a conceptual framework and theoretical foundation for an integrated diabetes prevention and treatment paradigm with distinctive features of traditional Chinese medicine.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by insulin resistance and progressive β-cell dysfunction. With a steadily rising prevalence, it has emerged as a major global public health concern (1, 2). According to the 10th edition of the International Diabetes Federation Diabetes Atlas, an estimated 537 million people worldwide were living with diabetes in 2021, a figure projected to reach 783 million by 2045 (3). The onset and progression of T2DM impose a substantial burden on patient quality of life and socioeconomic systems (4). Although current hypoglycemic agents achieve variable glycemic control, their efficacy is often limited by pronounced inter-individual variability, transient effects, and a spectrum of adverse reactions coupled with significant economic costs. These factors severely undermine long-term patient adherence and constrain widespread clinical adoption (5). Consequently, the development of more effective, safe, and sustainable intervention strategies has emerged as a critical focus in T2DM research.
Compared to the non-specific side effects of pharmacological treatments, acupuncture is characterized by multi-pathway, multi-target regulation and a favorable safety profile. It holds distinct advantages in managing metabolic disorders, particularly through modulating gut microenvironment homeostasis, enhancing insulin sensitivity, and promoting insulin secretion (6–8). Recent preclinical and clinical studies have demonstrated that acupuncture exerts significant therapeutic effects in alleviating symptoms associated with T2DM, effects that are highly likely mediated through the modulation of gut microecological balance (9, 10). Gut microecological homeostasis primarily depends on the coordinated regulation of four core dimensions: the structure and composition of the gut microbiota, the production of gut-derived metabolites, the integrity of the intestinal barrier, and the dynamic balance of inflammation. Studies have shown that acupuncture intervention promotes the proliferation of beneficial bacteria while inhibiting the expansion of pathogenic species, thereby effectively ameliorating the dysbiotic state commonly observed in patients with T2DM (11). Gut microbiota dysregulation plays a critical role in the onset and progression of T2DM, characterized by imbalances in microbial community structure, aberrant gut-derived metabolites, and impaired intestinal barrier function (12, 13). These abnormalities collectively drive chronic low-grade inflammation, exacerbate insulin resistance, and impair pancreatic β-cell function, ultimately contributing to the development of T2DM. Focusing on the coordinated regulation of the functional dimensions of gut microecology represents a crucial research direction for elucidating the molecular mechanisms underlying acupuncture treatment of T2DM.
Current research has made preliminary progress in elucidating the role of acupuncture in modulating gut microecology to intervene in T2DM; however, its precise underlying mechanisms remain incompletely understood. The core components of the gut microbiota are depicted in Figure 1. This review aims to systematically delineate the critical roles of gut microbiota dysbiosis, aberrant gut-derived metabolites, intestinal barrier dysfunction, and chronic low-grade inflammation in the pathogenesis of T2DM. It focuses on summarizing the potential mechanisms by which acupuncture ameliorates T2DM through the modulation of gut microecological homeostasis, and further explores the prospects and challenges of its application in individualized, precision treatment of T2DM. Ultimately, this review seeks to provide a theoretical foundation and practical guidance to optimize basic research directions and facilitate clinical translation of acupuncture in the prevention and treatment of metabolic diseases.
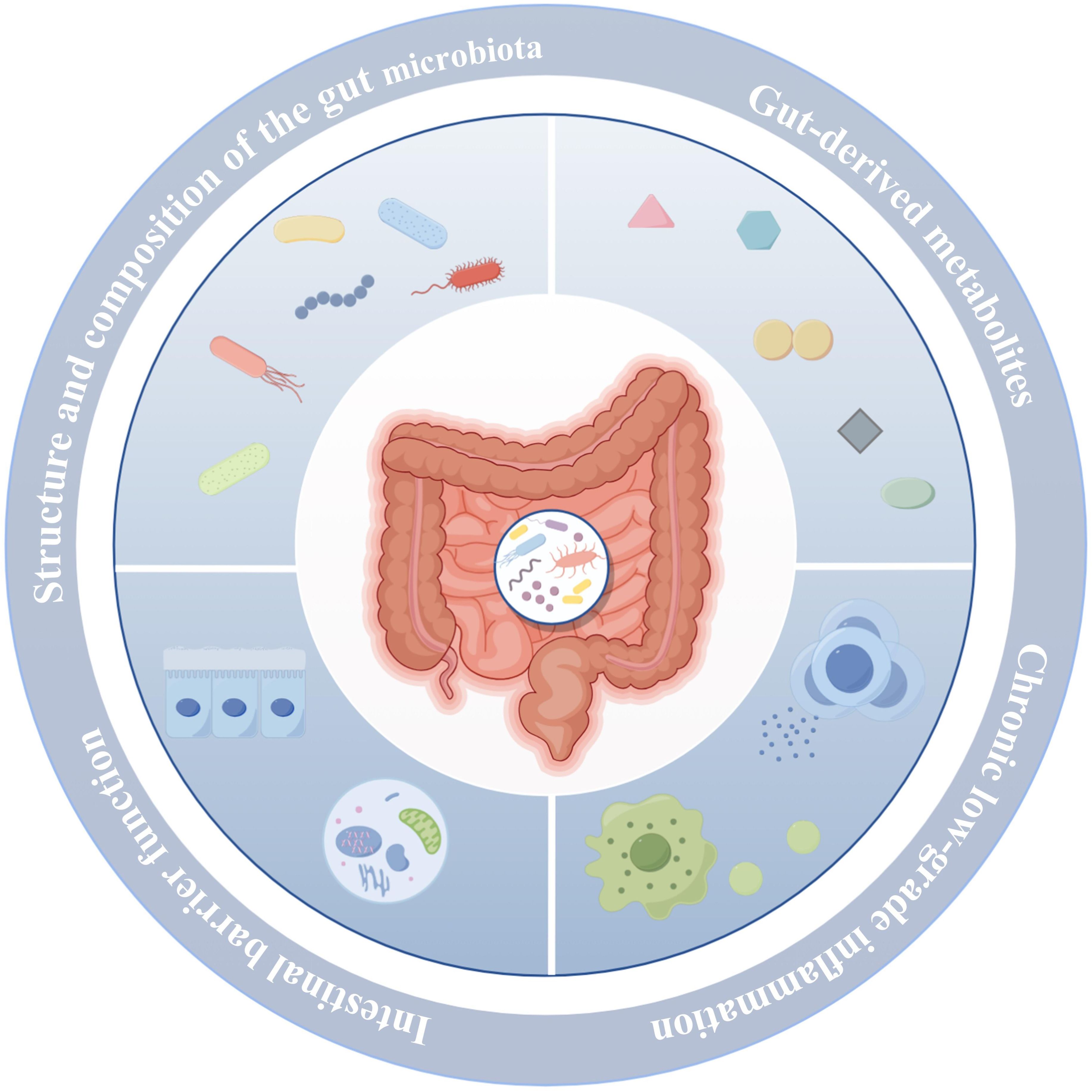
Figure 1. Four key functional dimensions of gut microbiota homeostasis. The figure was created using Figdraw 2.0.
2 Gut microecological dysregulation as a key contributor to the pathogenesis of T2DM
Gut microecological imbalance is widely recognized as a critical pathogenic factor in the onset of T2DM. It is characterized by a decline in beneficial bacteria, such as Bifidobacterium and Akkermansia, alongside an expansion of pathogenic species, including Escherichia coli and Clostridium spp., resulting in a disrupted microbial community structure and composition. This dysbiotic state leads to impaired synthesis and functional dysregulation of gut-derived metabolites, manifested by reduced production of short-chain fatty acids (SCFAs), elevated lipopolysaccharide (LPS) release, and aberrant secondary bile acid profiles. Microbial imbalance and aberrant gut-derived metabolites disrupt intestinal barrier function, initiating systemic chronic low-grade inflammation. This inflammatory state suppresses insulin secretion and impairs insulin sensitivity in target tissues, thereby exacerbating insulin deficiency and resistance—central pathological mechanisms driving the onset and progression of T2DM.
2.1 Imbalance of gut microbiota structure and composition
The gut microbiota constitutes a highly complex and dynamic micro-ecosystem composed of diverse phyla, including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia. It is involved in multiple physiological processes such as carbohydrate and lipid metabolism, maintenance of intestinal barrier integrity, immune modulation, and neural signaling. Collectively, the gut microbiota plays an indispensable role in preserving host health and metabolic homeostasis. Under healthy conditions, the gut microbiota maintains a relatively stable community structure characterized by diverse species and balanced abundance. Symbiotic interactions and metabolic cooperation among different microbial taxa collectively sustain this microecological equilibrium. However, in the context of T2DM, this balance is disrupted, leading to disturbances in the structure and composition of the microbiota.
Multiple independent studies have consistently reported a reduced relative abundance of genera such as Bifidobacterium, Bacteroides, Akkermansia, and Roseburia in the gut microbiota of T2DM patients, suggesting their potential protective roles in maintaining host metabolic homeostasis. Conversely, genera including Ruminococcus, Collinsella, and Blautia are frequently enriched in T2DM-associated microbiomes, implicating them as possible contributors to pro-inflammatory or pathogenic processes in the onset and progression of T2DM. Notably, the relative abundance of Lactobacillus exhibits inconsistent trends across T2DM studies, a variability that may reflect differences in strain diversity, host heterogeneity, intervention parameters, and sample origin. Moreover, although the Bacteroidetes-to-Firmicutes ratio has long been considered a potential microecological marker of metabolic diseases, current studies examining its association with T2DM yield inconsistent results, lacking robust and reproducible evidence. This variability suggests that reliance on broad taxonomic ratios alone may be insufficient to accurately capture the complexity of microecological dysbiosis associated with T2DM (Table 1). A visual representation of the content discussed in this subsection is provided in Figure 2.
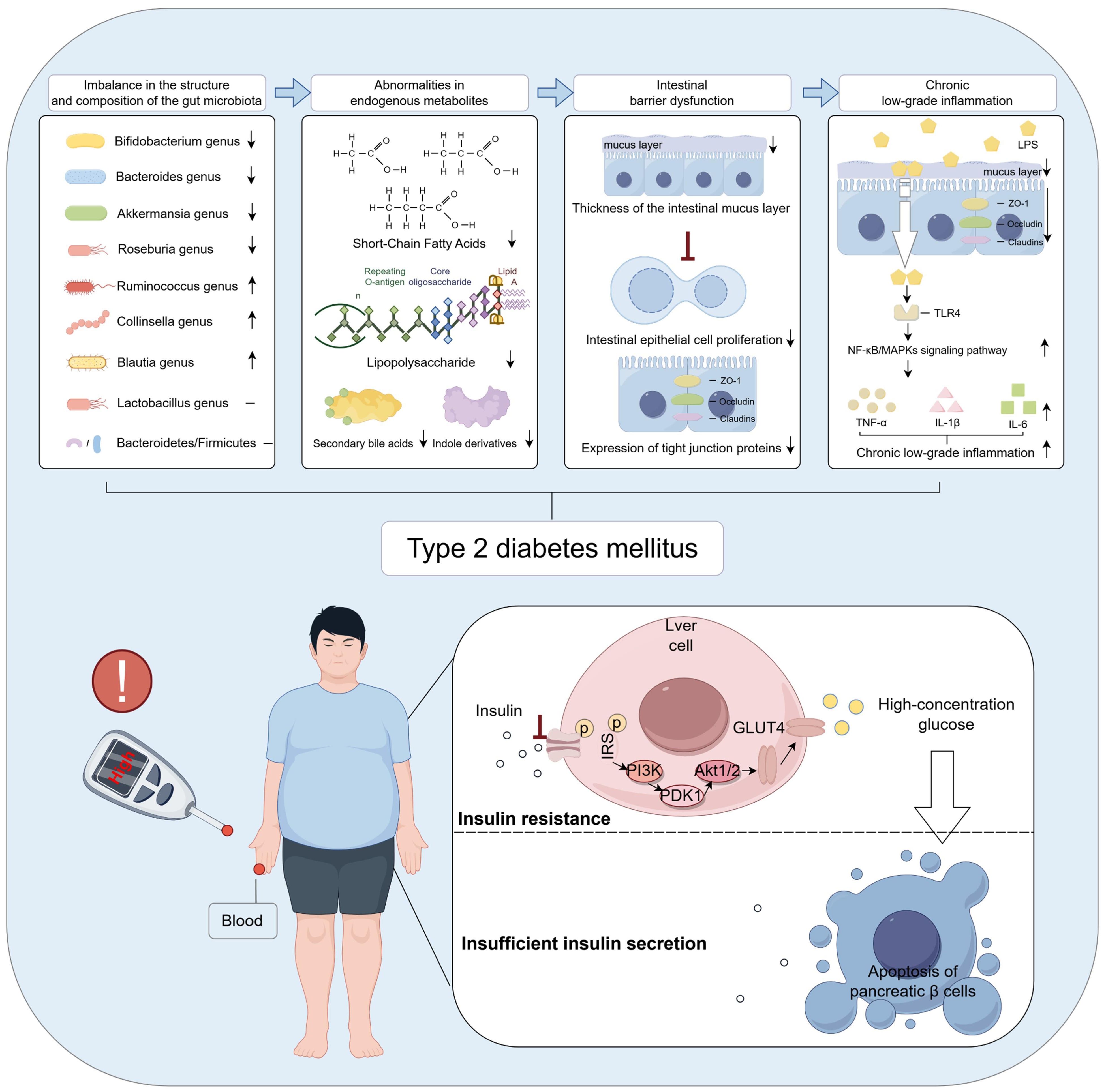
Figure 2. Gut microbiota dysbiosis is an important contributing factor in the pathogenesis of T2DM. The figure was created using Figdraw 2.0. TLR4: Toll-like receptor 4; TNF-α: Tumor necrosis factor-a; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IRS: Insulin receptor substrate-1; PI3K: Phosphoinositide 3-Kinase; PDK1: 3-Phosphoinositide-Dependent Protein Kinase-1; GLUT4: Glucose Transporter Type 4.
2.2 Aberrant gut-derived metabolites
Gut-derived metabolites refer to a class of bioactive low-molecular-weight compounds produced by the gut microbiota through the metabolism of dietary components, host endogenous substances such as bile acids and amino acids, or their own physiological activities. These metabolites are extensively involved in regulating diverse physiological processes, particularly carbohydrate and lipid metabolism. Gut-derived metabolites primarily include SCFAs, LPS, secondary bile acids, and indole derivatives. Gut microbiota dysbiosis can markedly alter the composition, abundance, and biological activity of these metabolites, manifested as follows (1): Reduced SCFA levels. Studies have shown that gut microbiota dysbiosis results in a significant decrease in SCFAs—namely acetate, propionate, and butyrate (22, 23)—primarily due to the diminished abundance of SCFA-producing bacteria such as Bifidobacterium, Akkermansia muciniphila, Bacteroides, and butyrate-producing bacteria in T2DM patients (24, 25) (2). Elevated LPS levels. Microbial dysbiosis leads to an increased relative abundance of Gram-negative bacteria in the gut, driving enhanced synthesis and release of LPS (26, 27). This exacerbates systemic chronic low-grade inflammation, thereby further promoting insulin resistance (3). Reduced levels of secondary bile acids. Gut microbiota dysbiosis leads to a decreased abundance of bacteria with dehydroxylation capabilities, such as Clostridium species (28), thereby impairing the biotransformation of primary to secondary bile acids. This disruption attenuates farnesoid X receptor (FXR) and TGR5-mediated signaling pathways, compromising glucose homeostasis and insulin sensitivity, and subsequently exacerbating dysregulated carbohydrate and lipid metabolism (4). Decreased levels of indole derivatives. Microbial dysbiosis also disrupts the metabolism of dietary tryptophan by gut microbes, resulting in reduced production of indolepropionic acid–a tryptophan metabolite known to enhance insulin secretion from pancreatic β-cells. Studies have demonstrated that circulating indolepropionic acid levels are significantly lower in individuals with T2DM compared to healthy controls (29). In summary, gut microbiota dysbiosis orchestrates the generation and regulation of multiple gut-derived metabolites, collectively driving dysregulated carbohydrate and lipid metabolism as well as chronic inflammation. This interplay provides a fundamental pathophysiological basis for the development of T2DM. A visual representation of the content discussed in this subsection is provided in Figure 2.
2.3 Intestinal barrier dysfunction
Gut microbiota dysbiosis and aberrant levels of gut-derived metabolites, particularly SCFAs, further exacerbate intestinal barrier dysfunction. The intestinal barrier serves as a critical physical and immunological interface between the internal and external environments. It is primarily composed of the gut microbiota, mucus layer, intestinal epithelial cells, and their tight junction proteins (30). This barrier performs multiple physiological functions, including preventing the translocation of harmful microbes and their metabolites across the intestinal wall (31), maintaining gut microecological balance, and regulating both local and systemic immune responses (32). Studies have shown that gut microbiota dysbiosis leads to a significant reduction in mucus layer thickness (33, 34). This alteration is primarily attributed to a decline in butyrate-producing bacteria, resulting in decreased synthesis of SCFAs–particularly butyrate–which suppresses goblet cell differentiation and the expression of the mucin MUC2 (35), thereby impairing mucus layer formation. Concurrently, overgrowth of mucus-degrading bacteria such as Bacteroides, along with inflammation-induced disruption of mucus secretion, further compromises the structural integrity and function of the mucus barrier, ultimately weakening the intestinal mucosa’s defense against pathogenic microbes and harmful metabolites. Furthermore, gut microbiota dysbiosis and aberrant gut-derived metabolites markedly inhibit the proliferation and renewal of intestinal epithelial cells (36, 37), while downregulating the expression of tight junction proteins such as ZO-1, occludin, and claudins (38, 39). This disruption compromises the epithelial barrier structure, increases intestinal permeability, and leads to barrier dysfunction. As a result, harmful bacterial metabolites like LPS and inflammatory mediators readily translocate into the lamina propria and systemic circulation, triggering immune activation and chronic low-grade inflammation, which further exacerbate metabolic dysregulation and insulin resistance. A visual representation of the content discussed in this subsection is provided in Figure 2.
2.4 Chronic low-grade inflammation
Chronic low-grade inflammation is widely recognized as a critical pathological feature in the onset and progression of T2DM. A central pathogenic mechanism involves gut microbiota dysbiosis, which elevates levels of LPS and impairs intestinal barrier function, thereby facilitating the translocation of LPS across the gut mucosal barrier into the systemic circulation. Circulating LPS binds to Toll-like receptor 4 (TLR4), activating canonical inflammatory signaling pathways including TLR4/MyD88/NF-κB and mitogen-activated protein kinases (MAPKs). This activation induces the excessive release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) (40, 41), thereby eliciting persistent low-grade inflammation across multiple target tissues. The resulting chronic inflammation activates serine kinases, including JNK and IKK, which phosphorylate the insulin receptor and impair its normal binding to insulin (42), culminating in insulin resistance. This mechanism profoundly compromises insulin-mediated glucose uptake, markedly attenuating the responsiveness of key target tissues such as skeletal muscle, liver, and adipose tissue. Furthermore, the sustained hyperglycemia consequent to insulin resistance intensifies metabolic stress within pancreatic β-cells, precipitating a cascade of deleterious events including oxidative stress, chronic inflammation, and endoplasmic reticulum stress. Collectively, these insults drive β-cell apoptosis (43–45), thereby accelerating the pathogenesis of T2DM. A visual representation of the content discussed in this subsection is provided in Figure 2.
3 Mechanisms underlying acupuncture therapy for T2DM
3.1 Mechanisms of acupuncture in improving T2DM via regulation of gut microecological balance
Acupuncture, as a longstanding, safe, and minimally invasive traditional intervention, has increasingly demonstrated unique advantages in the prevention and treatment of metabolic diseases. By modulating the gut microecology through multi-target and multi-pathway mechanisms, acupuncture effectively ameliorates T2DM. Its primary actions include regulating gut microbiota composition and diversity, restoring gut-derived metabolite profiles, enhancing intestinal barrier integrity, and suppressing chronic low-grade inflammation.
First, acupuncture markedly ameliorated gut microbiota dysbiosis associated with T2DM. Evidence from animal studies demonstrated that acupuncture intervention significantly enhanced gut microbial α-diversity and notably increased the relative abundance of key probiotics, including Lactobacillus and Bacteroides (10). These bacterial taxa can exert anti-inflammatory effects through the production of SCFAs, which reduce the generation of IKK and subsequently inhibit its interaction with the insulin receptor, thereby alleviating insulin resistance. In addition, SCFAs and their associated signaling pathways can promote the secretion of glucagon-like peptide-1 (GLP-1), which is closely linked to pancreatic β-cell proliferation and insulin secretion (46, 47). Corresponding clinical investigations revealed that acupuncture treatment induced substantial remodeling of the gut microbiota in T2DM patients, characterized by enrichment of beneficial microbes and reduction of potential pathogens. This ecological optimization suggested that acupuncture may indirectly modulate host glucose metabolism and insulin sensitivity by restoring microbial homeostasis (48). A study employing a T2DM mouse model revealed that electroacupuncture markedly reduced the relative abundance of diabetes-associated bacterial taxa, including Lachnoclostridium, Lachnospiraceae_UCG-006, Odoribacter, and Oscillibacter. Further fecal microbiota transplantation experiments demonstrated that the gut microbiota derived from electroacupuncture-treated mice significantly improved blood glucose levels and insulin resistance in antibiotic-treated diabetic mice (49). Moreover, when type 2 diabetic mice were co-treated with electroacupuncture and antibiotics, the hypoglycemic effect of electroacupuncture was abolished (50), suggesting that the gut microbiota plays a pivotal role in mediating the antidiabetic effects of electroacupuncture. Secondly, the role of acupuncture in modulating gut-derived metabolites has gained increasing attention. Multiple studies have demonstrated that stimulation of specific acupoints can influence microbial metabolic activity and promote the production of key metabolites. Acupuncture at “Zusanli” (ST36) and “Sanyinjiao” (SP6) has been shown to significantly elevate SCFA levels, particularly butyrate and propionate, thereby improving insulin sensitivity and enhancing glucose metabolic capacity (51). Similarly, acupuncture at “Pishu” (BL20) and “Weishu” (BL21) has also been reported to upregulate SCFA production, wherein butyrate activates the gut-expressed G protein-coupled receptor 43, thereby promoting insulin secretion and alleviating glucose metabolic disturbances (52). In addition to modulating SCFA production, acupuncture at “Tianshu” (ST25) and “Zusanli” (ST36) has been shown to enhance the synthesis of secondary bile acids, which, through activation of the FXR and TGR5 signaling pathways along the gut–liver axis, improve hepatic insulin sensitivity and facilitate glucose metabolism (53). Notably, FXR activation also suppresses hepatic cholesterol accumulation, thereby contributing to the amelioration of T2DM-associated lipid metabolic disturbances (54). Moreover, acupuncture has demonstrated pronounced biological effects in preserving intestinal barrier integrity. Multiple studies have shown that stimulation at “Tianshu” (ST25), “Zusanli” (ST36), “Zhongwan” (CV12), and “Pishu” (BL20) markedly upregulated the expression of key tight junction proteins in intestinal epithelial cells, including ZO-1, occludin, and claudin-1, thereby reinforcing mucosal barrier stability, reducing intestinal permeability, and restricting the translocation of luminal endotoxins such as LPS into the systemic circulation (52, 55). This barrier-protective effect helps attenuate systemic inflammation and interrupts the progression of insulin resistance driven by barrier dysfunction. Acupuncture also plays a pivotal role in suppressing chronic low-grade inflammation associated with T2DM. In chronic inflammation models driven by gut microbiota dysbiosis, acupuncture intervention effectively downregulated the expression of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β (56). Further investigations revealed that stimulation at “Guanyuan” (CV4) and “Shenshu” (BL23) mitigated systemic inflammatory responses by inhibiting the activation of inflammatory signaling pathways such as NF-κB, thereby ameliorating insulin resistance and slowing the pathological progression of T2DM (57). In summary, by orchestrating an integrated modulation of the critical “gut microbiota–metabolites–intestinal barrier–inflammation” regulatory axis, acupuncture effectively alleviates insulin resistance driven by gut microecological dysbiosis and holds promise as a valuable therapeutic strategy for targeting both T2DM and gut microecological dysbiosis. A visual representation of the content discussed in this subsection is provided in Figure 3.
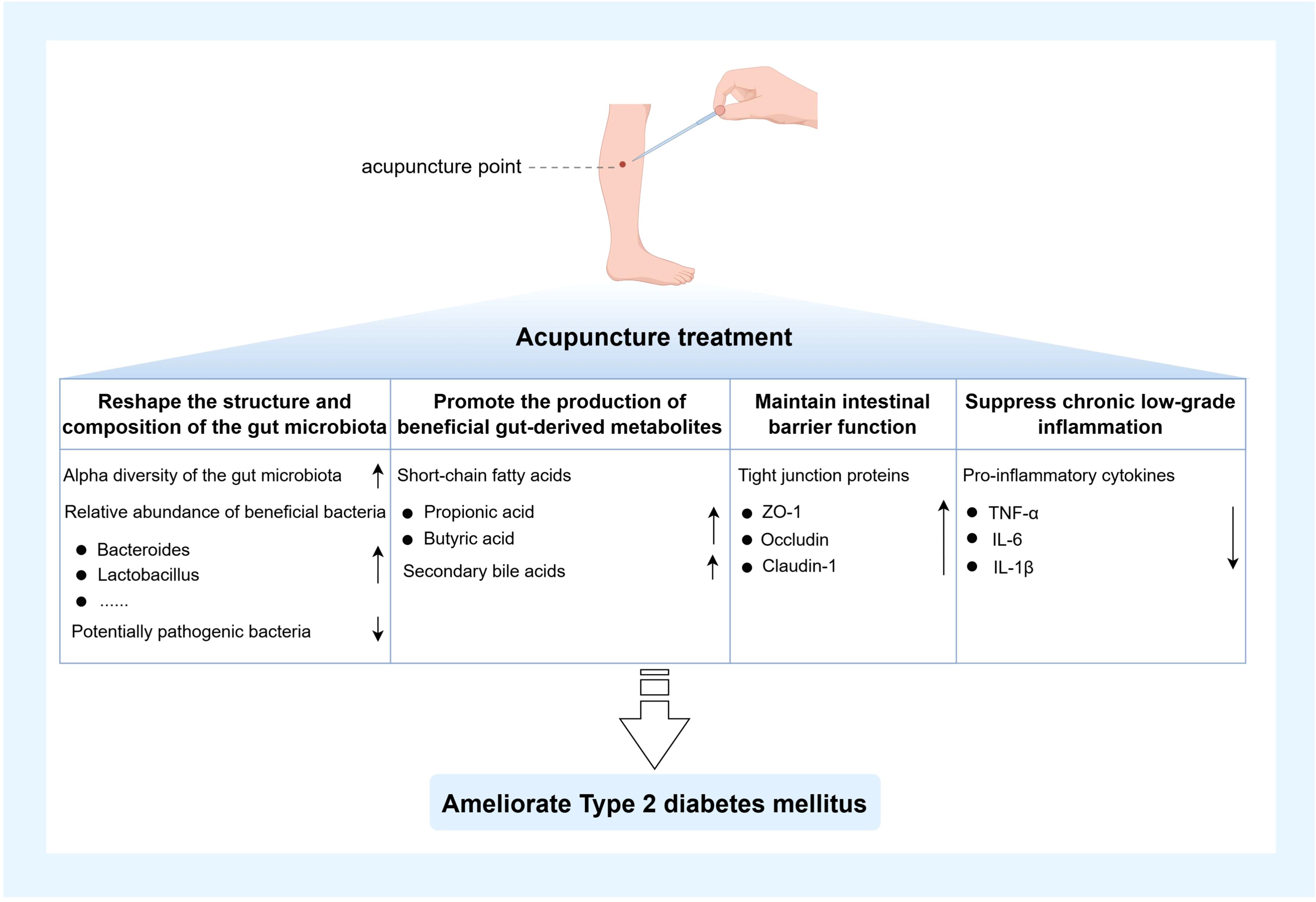
Figure 3. Mechanisms of acupuncture alleviating T2DM by modulating gut microbiota homeostasis. The figure was created using Figdraw 2.0. TNF-α: Tumor necrosis factor-α; IL-1β: Interleukin-1β; IL-6: Interleukin-6.
3.2 Direct mechanisms of acupuncture in ameliorating T2DM
Beyond indirectly improving glucose metabolism through modulation of the gut microecological balance, acupuncture also directly targets multiple pivotal pathways in the treatment of T2DM. It effectively modulates relevant signaling pathways involved in glucose metabolism. Studies have demonstrated that electroacupuncture at Zusanli (ST36) and Shenshu (BL23) reduces peripheral insulin levels while significantly upregulating hepatic mRNA expression of GLUT2 and glucokinase (GCK) (58), thereby enhancing hepatic glucose uptake and utilization, ultimately ameliorating hyperglycemia. Furthermore, acupuncture modulates the serine and tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) in skeletal muscle, upregulating the expression of IRS-1, IRS-2, and GLUT4. This enhances insulin signaling pathway activity and effectively reduces peripheral insulin resistance (59). Acupuncture also exhibits significant direct regulatory effects on pancreatic islet function. By activating the local pancreatic neural network, acupuncture promotes structural repair of the pancreas, enhances both the secretory capacity and mass of islet β-cells, and stimulates insulin secretion. Studies suggest that acupuncture may activate the PDX-1/GLUT2/GCK signaling pathway and upregulate gut-derived GLP-1 expression, thereby promoting β-cell proliferation and preserving their functional integrity (60, 61). Meanwhile, acupuncture also possesses anti-apoptotic properties toward pancreatic β-cells. Studies examining the effects of electroacupuncture on circRNA expression in plasma exosomes revealed that it inhibits β-cell apoptosis by modulating thyroid hormone and phosphatidylinositol signaling pathways (62). Additionally, acupuncture has been shown to markedly reduce aberrant mitochondrial cytochrome c release, suppress the activation of key pro-apoptotic factors such as caspase-9 and caspase-3, and thereby block the mitochondrial-mediated apoptotic cascade, preserving β-cell functional stability and viability (63, 64).
In summary, acupuncture not only indirectly modulates the progression of T2DM through regulation of the gut microecology but also directly targets glucose metabolic pathways, islet function, and cellular proliferation and apoptosis. This multi-level, multi-target systemic intervention provides a robust theoretical foundation for acupuncture as a non-pharmacological therapeutic strategy for T2DM.
4 Prospects and challenges of acupuncture modulation of gut microecology for T2DM treatment
4.1 Prospects
The application prospects of acupuncture in modulating gut microecology for the treatment of T2DM have garnered increasing attention (65). As a traditional medicine–derived, safe, and well-tolerated non-pharmacological intervention, acupuncture demonstrates multi-pathway and multi-target systemic regulatory capabilities. Acupuncture can play a positive role in regulating insulin resistance and improving abnormal glucose and lipid metabolism by reshaping gut microbiota composition and structure (66), promoting the synthesis of advantageous gut-derived metabolites (67), enhancing intestinal barrier integrity (68) and suppressing chronic low-grade inflammation (69). This not only provides a biological foundation for acupuncture’s therapeutic role in T2DM but also supports its development as a novel strategy for managing metabolic diseases. Secondly, driven by the principles of precision medicine, acupuncture interventions hold promise for integration with individualized gut microecological profiles, enabling a targeted therapeutic paradigm of “microbial typing–targeted acupoint selection–individual response prediction.” With advancing insights into the associations between gut microbial subtypes and metabolic phenotypes (70), the development of stratified intervention models based on microbiota characteristics is emerging as a key direction for future research. Furthermore, owing to its ease of administration and cost-effectiveness, acupuncture presents substantial potential for widespread implementation, particularly among T2DM patients with multiple metabolic comorbidities or those at risk of adverse drug reactions. Its adaptability across primary healthcare settings, rehabilitation centers, and community health management facilitates the establishment of a multi-tiered, sustainable integrated intervention framework for T2DM. With ongoing advances in mechanistic research, technological innovation, and the accumulation of robust clinical evidence, acupuncture-mediated modulation of gut microecology is poised to become a pivotal component of precision treatment strategies for T2DM, offering a model pathway for the modernization and global integration of traditional Chinese medicine.
4.2 Challenges
Despite demonstrating promising efficacy in clinical studies for the treatment of T2DM and other metabolic disorders, acupuncture research and application remain constrained by numerous limitations, which significantly hinder its broader adoption and deeper integration. The following provides an overview of the key challenges and issues currently confronting acupuncture interventions for T2DM.
4.2.1 Uncertainties in the mechanisms of acupuncture modulation of the autonomic nervous system
Acupuncture, as a therapeutic modality that modulates physiological functions through stimulation of specific acupoints, is generally believed to exert its effects via the autonomic nervous system (71). The autonomic nervous system, particularly the vagus and sympathetic nerves, plays a pivotal role in regulating metabolic diseases (72). Acupuncture influences visceral organ function by activating the vagus nerve, thereby ameliorating metabolic disorders such as diabetes. However, the precise mechanisms by which acupuncture modulates the autonomic nervous system remain incompletely understood. Specifically, how acupuncture activates the vagus nerve through stimulation of distinct acupoints and subsequently modulates endocrine and metabolic processes remains underexplored in a systematic manner. Current studies primarily focus on the activation effects of acupuncture on the vagus nerve, yet the differential outcomes elicited by various acupoints, the precise neural pathways involved, and the crosstalk between the nervous, endocrine, and immune systems warrant deeper investigation. Furthermore, individual variability presents a significant challenge, as differential neural responses among subjects may contribute to the heterogeneity of acupuncture’s therapeutic efficacy, necessitating rigorous quantification and optimization in future research.
4.2.2 Insufficiency of in-depth mechanistic validation
Despite emerging clinical evidence supporting acupuncture’s efficacy in treating metabolic diseases such as diabetes, the precise molecular mechanisms underlying its therapeutic effects remain insufficiently validated. Most existing studies have focused on acupuncture’s modulation of gut microecology, immune responses, and glucose-lipid metabolism to alleviate metabolic symptoms (11, 73). However, in-depth exploration of specific biomarkers, signaling pathways, and molecular mechanisms involved in acupuncture’s action is still markedly lacking. Although acupuncture is believed to improve diabetes metabolism by modulating gut microbial composition (74), the specific bacterial taxa or metabolic products mediating these effects remain unclear. Future investigations urgently require larger-scale animal studies, cellular experiments, and clinical trials to elucidate and validate the biological mechanisms of acupuncture, thereby providing a more robust theoretical foundation and clinical evidence.
4.3 Future research directions
Future research on acupuncture treatment for T2DM should leverage multi-omics integration to comprehensively investigate underlying mechanisms, establish standardized therapeutic protocols, and develop robust efficacy evaluation frameworks.
4.3.1 Multi-omics integrative research
Multi-omics integrative research, as a pivotal frontier in modern biomedical science, offers a systematic and comprehensive framework to elucidate the mechanisms underlying acupuncture treatment for T2DM and other metabolic disorders. By integrating multidimensional datasets encompassing genomics, transcriptomics, metabolomics, and microbiomics, this approach enables a holistic dissection of acupuncture’s biological effects across gene expression regulation, metabolic alterations, and gut microecological remodeling. A comprehensive study employing single-cell sequencing, transcriptomics, metabolomics, and metagenomics systematically evaluated the effects of acupuncture in patients undergoing methadone maintenance therapy. The findings demonstrated that acupuncture exerts regulatory effects through multiple biological layers, including the modulation of immune function, reshaping of gut microbial composition and metabolic activity, and regulation of cellular immune signaling pathways. These results provide systematic and molecular-level evidence supporting the potential of acupuncture as an adjunctive therapy for mitigating the adverse effects associated with long-term opioid use (75). Integrative analyses based on multi-omics data not only facilitate the identification of key molecular pathways and targets mediating acupuncture’s therapeutic effects but also enable the elucidation of individualized response profiles. Moreover, by combining genomic information with gut microbiome characteristics, these approaches can assist in predicting treatment outcomes and guide the development of personalized acupuncture regimens. With ongoing advancements in multi-omics technologies and continual refinement of data analytical methods, future studies are poised to further unravel the complex network mechanisms underlying acupuncture’s modulation of metabolic diseases and accelerate its translation and application within the realm of precision medicine.
4.3.2 Establishing standardized acupuncture protocols and evaluation frameworks
Standardization of acupuncture therapy remains a significant challenge in current research. Considerable individual variability in acupuncture treatment–including differences in acupoint selection, stimulation intensity, treatment frequency, and duration–has led to poor comparability and reproducibility across studies. Therefore, establishing unified, standardized treatment protocols and evaluation frameworks is essential to enhance the scientific rigor and clinical applicability of acupuncture. Firstly, standardization requires clear definition of key parameters such as acupoint selection, stimulation intensity, treatment frequency, and course duration. Existing studies have predominantly focused on traditional and commonly used acupoints, such as Zusanli (ST36) and Tianshu (ST25) (76–78), yet symptomatology, disease severity, and individual patient differences may necessitate more precise acupoint targeting. Future research could integrate artificial intelligence and big data technologies to tailor personalized acupuncture protocols based on patients’ clinical characteristics and biomarkers. In studies aimed at standardizing key acupuncture parameters, the inclusion of appropriate control groups (e.g., sham acupuncture) is essential to ensure the scientific rigor and reproducibility of the findings. Secondly, standardization of evaluation systems is equally crucial. Currently, efficacy assessments of acupuncture largely rely on changes in clinical symptoms and routine biochemical markers, which are relatively limited and fail to comprehensively capture therapeutic outcomes. Moving forward, a multidimensional evaluation framework incorporating clinical symptoms, metabolic biomarkers, immune responses, and gut microbiota alterations should be established to provide a more holistic and objective appraisal of acupuncture efficacy. Moreover, clinical trial data based on standardized evaluation criteria will offer robust support for the wider adoption of acupuncture and inform policy development.
5 Conclusion
T2DM, a global chronic metabolic disorder, is increasingly recognized to be closely associated with gut microecological imbalance. In recent years, the role of the gut microbiota in regulating glucose metabolism, maintaining insulin sensitivity, and mediating insulin resistance has emerged as a major focus of research. Acupuncture, as a traditional medicine intervention with systemic regulatory advantages and a non-pharmacological approach, has been demonstrated to act on multiple pathological pathways related to T2DM. These include remodeling gut microbial composition and diversity, enhancing the production of beneficial gut-derived metabolites such as SCFAs, strengthening intestinal epithelial barrier function, suppressing pro-inflammatory signaling pathways, and ameliorating systemic chronic inflammation. By modulating the critical axis of “gut microbiota–metabolites–intestinal barrier–inflammation”, acupuncture holds promise for upstream intervention in T2DM-associated glucose dysregulation, offering novel insights for comprehensive disease management.
However, the precise molecular mechanisms by which acupuncture modulates T2DM through the gut microecology remain incompletely understood, with a notable lack of systematic and reproducible mechanistic validation. Future research should fully integrate multi-omics technologies–including genomics, transcriptomics, metabolomics, and microbiomics–combined with bioinformatics approaches to elucidate the key signaling pathways and core targets of acupuncture intervention. Concurrently, there is an urgent need to establish standardized acupuncture treatment parameters encompassing acupoint selection, stimulation intensity, intervention duration, and clinical efficacy evaluation criteria. Such efforts will facilitate the standardization, mechanistic clarification, and clinical practicability of acupuncture in the precision management of T2DM, thereby broadening its translational potential in metabolic disease therapeutics.
Author contributions
HN: Writing – original draft, Funding acquisition, Conceptualization, Investigation. MZ: Supervision, Writing – review & editing, Methodology. LZ: Supervision, Writing – review & editing, Methodology. HW: Writing – review & editing, Methodology, Supervision, Data curation. FW: Supervision, Methodology, Writing – review & editing. SZ: Supervision, Methodology, Writing – review & editing. RC: Funding acquisition, Writing – review & editing, Supervision. FL: Writing – review & editing, Funding acquisition, Supervision, Methodology. HZ: Supervision, Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (82274663, 82274634, 82374585), Department of Science and Technology of Hubei Province (2025AFB117).
Acknowledgments
We sincerely thank all contributors to this review for their invaluable insights, rigorous efforts, and dedication throughout the research and writing process. Their expertise and collaboration were essential in shaping the comprehensive analysis presented here.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Valabhji J and Kar P. Rise in type 2 diabetes shows that prevention is more important than ever. BMJ. (2023) 381:910. doi: 10.1136/bmj.p910
2. Ladher N, Hinton R, and Veitch E. Challenges of obesity and type 2 diabetes require more attention to food environment. BMJ. (2023) 383:2269. doi: 10.1136/bmj.p2269
3. Magliano DJ and Boyko EJ. Idf diabetes atlas. In: Idf diabetes atlas, 10th ed. Brussels: IDF Diabetes Atlas 10th edition scientific committee (2021).
4. Taylor R, Ramachandran A, Yancy WS Jr., and Forouhi NG. Nutritional basis of type 2 diabetes remission. BMJ. (2021) 374:n1449. doi: 10.1136/bmj.n1449
5. Jin J. Screening for type 2 diabetes in children and adolescents. JAMA. (2022) 328:993. doi: 10.1001/jama.2022.15240
6. Xie Y, Huan MT, Sang JJ, Luo SS, Kong XT, Xie ZY, et al. Clinical effect of abdominal massage therapy on blood glucose and intestinal microbiota in patients with type 2 diabetes. Oxid Med Cell Longev. (2022) . 2022:2286598. doi: 10.1155/2022/2286598
7. Xie W, Ye Y, Ma C, Wei Y, and Yan X. Research progress on the “Neuro-endocrine-immune” Regulatory mechanism of acupuncture in the treatment of simple obesity (in chinese). Acupuncture Res. (2024) 50:1–13. doi: 10.13702/j.1000-0607.20231051
8. Wang W, Liang F, Song A, Huang Q, and Chen R. Current status and considerations of acupuncture in regulating gut microbiota (in chinese). Acupuncture Res. (2019) 44:71–4. doi: 10.13702/j.1000-0607.180118
9. Han Y. Mechanistic study on acupuncture combined with acupoint pressing to improve glucose and lipid metabolism disorders and intestinal microenvironment in type 2 diabetes mellitus based on intestinal mucosal barrier. [Doctoral Dissertation] (in chinese). Changchun University of Chinese Medicine. (2022).
10. Zhang S, Cui Y, Sun Z, Zhou X, Cao Y, Li X, et al. Research progress on the mechanisms of acupuncture in the treatment of type 2 diabetes mellitus. Acupuncture Res. (2024) 49:641–9. doi: 10.13702/j.1000-0607.20230372
11. Bao C, Wu L, Wang D, Chen L, Jin X, Shi Y, et al. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate crohn’s disease: A randomized controlled trial. EClinicalMedicine. (2022) 45:101300. doi: 10.1016/j.eclinm.2022.101300
12. Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2dm). Nutrients. (2020) 12:3719. doi: 10.3390/nu12123719
13. Li X, Watanabe K, and Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front Immunol. (2017) 8:1882. doi: 10.3389/fimmu.2017.01882
14. Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic ma-pi 2 diet. Br J Nutr. (2016) 116:80–93. doi: 10.1017/S0007114516001045
15. Sedighi M, Razavi S, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Mehrtash A, et al. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microbial pathogenesis. (2017) 111:362–9. doi: 10.1016/j.micpath.2017.08.038
16. Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, et al. Molecular characterisation of the faecal microbiota in patients with type ii diabetes. Curr Microbiol. (2010) 61:69–78. doi: 10.1007/s00284-010-9582-9
17. Larsen N, Vogensen FK, Van Den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. (2010) 5:e9085. doi: 10.1371/journal.pone.0009085
18. Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, et al. Decreased abundance of akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Advanced Science. (2021) 8:2100536. doi: 10.1002/advs.202100536
19. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in european women with normal, impaired and diabetic glucose control. Nature. (2013) 498:99–103. doi: 10.1038/nature12198
20. Salamon D, Sroka-Oleksiak A, Kapusta P, Szopa M, Mrozińska S, Ludwig-Słomczyńska AH, et al. Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next-generation sequencing of the 16s rrna gene fragment. Pol Arch Intern Med. (2018) 128:336–43. doi: 10.20452/pamw.4246
21. Egshatyan L, Kashtanova D, Popenko A, Tkacheva O, Tyakht A, Alexeev D, et al. Gut microbiota and diet in patients with different glucose tolerance. Endocrine Connections. (2016) 5:1–9. doi: 10.1530/EC-15-0094
22. Wang P, Deng X, Zhang C, and Yuan H. Gut microbiota and metabolic syndrome. Chin Med J. (2020) 133:808–16. doi: 10.1097/CM9.0000000000000696
23. Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. (2019) 148:104403. doi: 10.1016/j.phrs.2019.104403
24. Vitetta L, Gorgani NN, Vitetta G, and Henson JD. Prebiotics progress shifts in the intestinal microbiome that benefits patients with type 2 diabetes mellitus. Biomolecules. (2023) 13:1307. doi: 10.3390/biom13091307
25. Zhang L, Chu J, Hao W, Zhang J, Li H, Yang C, et al. Gut microbiota and type 2 diabetes mellitus: association, mechanism, and translational applications. Mediators Inflammation. (2021) 2021:5110276. doi: 10.1155/2021/5110276
26. Di Lorenzo F, De Castro C, Silipo A, and Molinaro A. Lipopolysaccharide structures of gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol Rev. (2019) 43:257–72. doi: 10.1093/femsre/fuz002
27. Salguero MV, Al-Obaide MA, Singh R, Siepmann T, and Vasylyeva TL. Dysbiosis of gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. (2019) 18:3461–9. doi: 10.3892/etm.2019.7943
28. Guo P, Zhang K, Ma X, and He P. Clostridium species as probiotics: potentials and challenges. J Anim science. (2020) 11:24. doi: 10.1186/s40104-019-0402-1
29. De Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the finnish diabetes prevention study. Sci Rep. (2017) 7:46337. doi: 10.1038/srep46337
30. Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. (2021) 8:718356. doi: 10.3389/fnut.2021.718356
31. Martens EC, Neumann M, and Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. (2018) 16:457–70. doi: 10.1038/s41579-018-0036-x
32. Chen Y, Cui W, Li X, and Yang H. Interaction between commensal bacteria, immune response and the intestinal barrier in inflammatory bowel disease. Front Immunol. (2021) 12:761981. doi: 10.3389/fimmu.2021.761981
33. Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep. (2019) 7:3–12. doi: 10.1093/gastro/goy052
34. Paone P and Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. (2020) 69:2232–43. doi: 10.1136/gutjnl-2020-322260
35. Augenlicht L, Shi L, Mariadason J, Laboisse C, and Velcich A. Repression of muc2 gene expression by butyrate, a physiological regulator of intestinal cell maturation. Oncogene. (2003) 22:4983–92.
36. Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M, et al. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. (2020) 11:997–1014. doi: 10.1080/19490976.2020.1734423
37. Salvi PS and Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. (2021) 10:1775. doi: 10.3390/cells10071775
38. Stolfi C, Maresca C, Monteleone G, and Laudisi F. Implication of intestinal barrier dysfunction in gut dysbiosis and diseases. Biomedicines. (2022) 10:289. doi: 10.1080/19490976.2020.1734423
39. Mucientes A, Lisbona-Montañez JM, Mena-Vázquez N, Ruiz-Limón P, Manrique-Arija S, García-Studer A, et al. Intestinal dysbiosis, tight junction proteins, and inflammation in rheumatoid arthritis patients: A cross-sectional study. Int J Mol Sci. (2024) 25:8649. doi: 10.3390/ijms25168649
40. Jin M, Fang J, J-j W, Shao X, Xu S-W, Liu P-Q, et al. Regulation of toll-like receptor (Tlr) signaling pathways in atherosclerosis: from mechanisms to targeted therapeutics. Pharmacologica Sinica. (2023) 44:2358–75. doi: 10.1038/s41401-023-01123-5
41. Yuan R, Huang L, Du L, Feng J, Li J, Luo Y, et al. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking tlr4 dimerization. Pharmacol Res. (2019) 142:102–14. doi: 10.1016/j.phrs.2019.02.017
42. Nandipati KC, Subramanian S, and Agrawal DK. Protein kinases: mechanisms and downstream targets in inflammation-mediated Obesity and insulin resistance. Mol Cell Biochem. (2017) 426:27–45. doi: 10.1007/s11010-016-2878-8
43. Yong J, Johnson JD, Arvan P, Han J, and Kaufman RJ. Therapeutic opportunities for pancreatic β-cell er stress in diabetes mellitus. Nat Rev Endocrinology. (2021) 17:455–67. doi: 10.1038/s41574-021-00510-4
44. Ježek P, Jabůrek M, and Plecitá-Hlavatá L. Contribution of oxidative stress and impaired biogenesis of pancreatic β-cells to type 2 diabetes. Antioxidants Redox Signaling. (2019) 31:722–51. doi: 10.1089/ars.2018.7656
45. Malin SK, Kirwan JP, Sia CL, and González F. Pancreatic β-cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-κb activation and systemic inflammation. Am J Physiology-Endocrinology Metab. (2015) 308:E770–E7. doi: 10.1152/ajpendo.00510.2014
46. Wang G, Li X, Zhao J, Zhang H, and Chen W. Lactobacillus casei ccfm419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. (2017) 8:3155–64. doi: 10.1039/C7FO00593H
47. Cheng J, Hu J, Geng F, and Nie S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci Hum Wellness. (2022) 11:1101–10. doi: 10.1016/j.fshw.2022.04.002
48. Sun L and Gao H. Research progress on traditional chinese medicine regulating gut microbiota in the treatment of type 2 diabetes mellitus (in chinese). J Difficult Complicated Cases. (2023) 22:1102–1105 + 10.
49. An J, Wang L, Song S, Tian L, Liu Q, Mei M, et al. Electroacupuncture reduces blood glucose by regulating intestinal flora in type 2 diabetic mice. J Diabetes. (2022) 14:695–710. doi: 10.1111/1753-0407.13323
50. Yue Z, Xiang W, Duping D, Yuanyuan G, Xuanyi C, Juan L, et al. Integrating 16s rdna and metabolomics to uncover the therapeutic mechanism of electroacupuncture in type 2 diabetic rats. Front Microbiol. (2025) 15:1436911. doi: 10.3389/fmicb.2024.1436911
51. Zhang B, Zhao L, Jin Z, and Tong X. Considerations on the relationship between the “Depression-heat” Pathogenesis of type 2 diabetes mellitus and gut microbiota (in chinese). Chin J Basic Med Traditional Chin Med. (2022) 28:1286–9. doi: 10.19945/j.cnki.issn.1006-3250.2022.08.015
52. Han Y, Lu Z, Chen S, Zhong C, Gang X, and Liu M. Study on the effects of acupuncture combined with acupressure on gut microbiota composition and homeostasis in type 2 diabetic rats based on the intestinal microenvironment (in chinese). China J Traditional Chin Med Pharmacy. (2022) 37:7337–41.
53. Pan T. Effects and mechanisms of electroacupuncture with “Regulating the viscera and unblocking the collaterals” Method in the treatment of type 2 diabetes mellitus based on gut microbiota–bile acid metabolism. [Doctoral Dissertation] (in chinese). Changchun University of Chinese Medicine. (2023).
54. Liu Z. Study on the effect of electroacupuncture on atherosclerosis based on gut microbiota regulation of bile acid metabolism. [Doctoral Dissertation] (in chinese). Liaoning University of Traditional Chinese Medicine. (2023).
55. Li X, Cai J, Lin Y, and Liu X. Effects of acupuncture on gut microbiota structure and promotion of intestinal tight junctions in rats with diarrhea-predominant irrita ble bowel syndrome (in chinese). J Guangzhou Univ Traditional Chin Med. (2019) 36:1022–8. doi: 10.13359/j.cnki.gzxbtcm.2019.07.019
56. Zheng Y, Zhai Q, Gao W, Gao H, and He X. Research progress on acupuncture regulating gut microbiota in the treatment of some related diseases (in chinese). J Changchun Univ Traditional Chin Med. (2024) 40:928–33. doi: 10.13463/j.cnki.cczyy.2024.08.024
57. Huang F and Yao Z. Effects of Acupuncture on Gut Microbiota in Patients with Diarrhea-Predominant Irritabl e Bowel Syndrome (Liver Qi Stagnation and Spleen Deficiency Syndrome) Analyzed by 16S rRNA Sequencing (in Chinese). China Med Herald. (2024) 26:407–11.
58. Jia N, Li R, Cao B, Tian HH, Hu XG, Ma YJ, et al. Effects of electroacupuncture at “Zusanli” and “Shenshu” on GLUT2 and GCK in rats with type 2 diabetes mellitus (in chinese). World Chin Med. (2017) 12:1114–9.
59. Song S, Li R, Cao B, Zhang J, Kim Y, Kim B, et al. Mechanism of electroacupuncture regulating irs-1 phosphorylation in skeletal muscle to improve insulin sensitivity. Evidence-Based Complementary Altern Med. (2021) 2021:8631475. doi: 10.1155/2021/8631475
60. Hu X. Mechanistic study on the effects of electroacupuncture at “Zusanli” on PDX-1 in rats with type 2 diabetes mellitus [Master’s thesis] (in chinese). Beijing Univ Chin Med. (2017).
61. Cao B, Li R, Tian H, Jia N, Hu X, Ma Y, et al. Effects of electroacupuncture at “Weiwanshuyu” on pancreatic morphology and glucagon-like peptide-1 receptor in the pancreas of rats with type 2 diabetes mellitus (in chinese). Acupuncture Res. (2017) 42:107–13. doi: 10.13702/j.1000-0607.2017.02.003
62. Shou Y, Hu L, Zhang W, Gao Y, Xu P, and Zhang B. Determination of electroacupuncture effects on circrnas in plasma exosomes in diabetic mice: an rna-sequencing approach. Evidence-Based Complementary Altern Med. (2019) 2019:7543049. doi: 10.1155/2019/7543049
63. Pang X. Study on the effect of acupuncture on apoptosis of pancreatic β Cells in type 2 diabetes mellitus via the mitochondrial pathway. [Master’s Thesis] (in chinese). Nanjing University of Chinese Medicine. (2014).
64. Tian H. Mechanistic study of electroacupuncture at “Yishu,” “Pishu,” and “Shenshu” in the intervention of type 2 diabetes mellitus based on MST1. [Doctoral Dissertation] (in Chinese). Beijing University of Chinese Medicine. (2017).
65. Chen C, Liu J, Sun M, Liu W, Han J, and Wang H. Acupuncture for type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Complementary therapies Clin practice. (2019) 36:100–12. doi: 10.1016/j.ctcp.2019.04.004
66. Yan X, Yao J, Li Y, Xiao X, Yang W, Chen S, et al. Effects of acupuncture on gut microbiota and short-chain fatty acids in patients with functional constipation: A randomized placebo-controlled trial. Front Pharmacol. (2023) 14:1223742. doi: 10.3389/fphar.2023.1223742
67. Chen L, Xu W, Pei L, Wu X, Geng H, Guo J, et al. Effect of tiaoshen jianpi acupuncture therapy on gut microbiota and fecal short-chain fatty acids in patients with diarrhea type irritab le bowel syndrome. Chin Acupuncture Moxibustion. (2021) 41:137–41. doi: 10.13703/j.0255-2930.20200205-k0002
68. Kang X, Zhang H, Li X, Zhang K, Huang Z, Li Y, et al. Electroacupuncture Improving Intestinal Barrier Function in Rats with Irrita ble Bowel Syndrome through Regulating Aquaporins. Digestive Dis Sci. (2024) 69:1143–55. doi: 10.1007/s10620-024-08288-x
69. Oh J-E and Kim S-N. Anti-inflammatory effects of acupuncture at st36 point: A literature review in animal studies. Front Immunol. (2022) 12:813748. doi: 10.3389/fimmu.2021.813748
70. Hu J, Ding J, Li X, Li J, Zheng T, Xie L, et al. Distinct signatures of gut microbiota and metabolites in different types of diabetes: A population-based cross-sectional study. EClinicalMedicine. (2023) 62:102132. doi: 10.1016/j.eclinm.2023.102132
71. Li Y, Li W, Wang S, Gong Y, Dou B, Lyu Z, et al. The autonomic nervous system: A potential link to the efficacy of acupuncture. Front Neurosci. (2022) 16:1038945. doi: 10.3389/fnins.2022.1038945
72. Carnagarin R, Matthews VB, Herat LY, Ho JK, and Schlaich MP. Autonomic regulation of glucose homeostasis: A specific role for sympathetic nervous system activation. Curr Diabetes Rep. (2018) 18:107. doi: 10.1007/s11892-018-1069-2
73. Han J, Guo X, Meng X-J, Zhang J, Yamaguchi R, Motoo Y, et al. Acupuncture improved lipid metabolism by regulating intestinal absorption in mice. World J Gastroenterology. (2020) 26:5118. doi: 10.3748/wjg.v26.i34.5118
74. Cao L, Zhou S, Li J, Chen K, Xue X, and Yi W. Effects of electroacupuncture on intestinal microflora and plasma metabolites in an insulin-resistant mouse model of type 2 diabetes mellitus. Acupuncture Med. (2024) 42:76–86. doi: 10.1177/09645284231207871
75. Chen Y, Fan B, Zeng J, Zou Y, Tao C, Chen C, et al. Single-cell rna transcriptomics and multi-omics analyses reveal the clinical effects of acupuncture on methadone reduction. Research. (2025) 8:0741. doi: 10.34133/research.07
76. Wang H, Chen X, Chen C, Pan T, Li M, Yao L, et al. Electroacupuncture at lower he-sea and front-mu acupoints ameliorates insulin resistance in type 2 diabetes mellitus by regulating the intestinal flora and gut barrier. Diabetes Metab Syndrome Obesity: Targets Ther. (2022) 15:2265–76. doi: 10.2147/DMSO.S374843
77. Srilestari A, Abdurrohim K, and Perkasa S. Effect of electroacupuncture combined with medical treatment on insulin resistance (Homa-ir) of patients with metabolic syndrome. J Physics: Conf Series. (2018) 1073:062030. doi: 10.1088/1742-6596/1073/6/062030
Keywords: acupuncture therapy, type 2 diabetes mellitus, gut microecology, gut microbiota, insulin signaling pathway
Citation: Niu H, Zhou M, Zhou L, Wu H, Wu F, Zhu S, Chen R, Liang F and Zhang H (2025) Mechanisms of acupuncture in the treatment of type 2 diabetes mellitus: insights from the regulation of the gut microecology. Front. Immunol. 16:1682521. doi: 10.3389/fimmu.2025.1682521
Received: 09 August 2025; Accepted: 30 October 2025;
Published: 20 November 2025.
Edited by:
Ding Shi, Zhejiang University, ChinaCopyright © 2025 Niu, Zhou, Zhou, Wu, Wu, Zhu, Chen, Liang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Chen, dW5pb25jckAxNjMuY29t; Fengxia Liang, ZnhsaWFuZzVAaG90bWFpbC5jb20=; Hongxing Zhang, emh4emhlbmppdUAxNjMuY29t
†These authors have contributed equally to this work
 Huifang Niu
Huifang Niu Minfeng Zhou
Minfeng Zhou Li Zhou
Li Zhou Huan Wu1,2
Huan Wu1,2 Rui Chen
Rui Chen Fengxia Liang
Fengxia Liang