- 1Center of Thoracic Cancer, Chongqing University Cancer Hospital, Chongqing, China
- 2Chongqing Key Laboratory for the Mechanism and Intervention of Cancer Metastasis, Chongqing, China
- 3State Key Laboratory of Power Transmission Equipment Technology, School of Electrical Engineering, Chongqing University, Chongqing, China
Cancer remains a significant threat to human health, and conventional treatments such as surgery, chemotherapy, and radiotherapy have their limitations. In recent years, pulsed electric fields (PEFs) has garnered attention as an emerging method for cancer treatment. It primarily utilizes high-intensity pulse electric fields applied to tumor cells, inducing effects such as electroporation or internal electrical processing, which lead to cell death. This review will introduce the principles of PEFs, its application fields, and its prospects in cancer treatment, aiming to provide readers with a comprehensive understanding of the research areas related to PEFs and cancer therapy.
1 Introduction
Cancer treatment remains a complex and highly challenging field despite significant progress over the past few decades. Key obstacles include tumor heterogeneity, where diverse cell subpopulations and genetic variations lead to different treatment responses, complicating strategy development (1). Drug resistance arises as tumor cells evade therapy through mechanisms like gene mutations, altered drug transport, and activated survival pathways, reducing treatment efficacy (2). Toxic side effects from traditional therapies like chemotherapy and radiotherapy damage healthy cells, causing severe burdens such as nausea, vomiting, hair loss, and fatigue (3). The intricate tumor microenvironment (TME), comprising tumor cells, blood vessels, immune cells, and extracellular matrix, forms an ecosystem that significantly impacts treatment effectiveness and complicates design (4). Early diagnosis and treatment, crucial for success, are hindered by the frequent absence of symptoms in early-stage tumors and the highly invasive behavior of some cancers (5). Finally, individual differences in tumor biology and patient characteristics render standardized approaches inadequate, necessitating personalized strategies focused on genomics and molecular profiles for customized care (6).
In the face of these challenges, scientists and physicians are actively exploring new treatment strategies and technologies. First of all, physical therapy offers significant advantages. Pulsed electric fields (PEFs), as an emerging method in physical therapy for cancer treatment, offers unique advantages and promising applications, potentially providing new solutions to overcome the challenges in cancer therapy (7–9). PEFs, as an emerging method for cancer treatment, originated from the electroporation technology of the 1980s. Initially, electroporation was used as a research tool to enhance cellular membrane permeability. By applying high-intensity PEFs, transient pores are created in the cell membrane, allowing large molecules such as drugs or DNA to enter the cell (10). As research into electroporation deepened, it was realized that the effect of PEFs on cells was not only to increase membrane permeability but could also directly destroy the integrity of the cell membrane, leading to cell death-a phenomenon known as irreversible electroporation (IRE) (11). IRE have become hot topics in the field of cancer treatment among scholars (11–15). The earliest experiments with PEFs therapy were conducted in mouse tumor models and showed that PEFs could significantly inhibit tumor growth (16). Subsequent studies indicated that PEFs could induce tumor cell death through various mechanisms, including membrane electrical breakdown (17), apoptosis (18), cell cycle arrest (19), and activation of immune responses (20). With further technological development, researchers began to explore the application of PEFs in clinical cancer treatment. The first clinical trials applying PEFs to human cancer treatment were conducted in the 1990s, primarily targeting localized lesions such as skin tumors (21, 22). These trials demonstrated that PEFs could effectively control and reduce tumor volume. As the technology has continued to improve and clinical research has deepened, the application scope of PEFs has gradually expanded to include various types of solid tumors and hematological malignancies (23, 24). Additionally, PEFs can be combined with other treatments such as chemotherapy (25), radiotherapy (26), and immunotherapy (27) to enhance therapeutic efficacy.
In summary, the development background of PEFs can be traced back to the research on electroporation, which has gradually evolved into a unique method for cancer treatment. With further research and clinical practice, PEFs is expected to become one of the important means of cancer treatment, offering patients more effective and personalized treatment options (Figure 1).
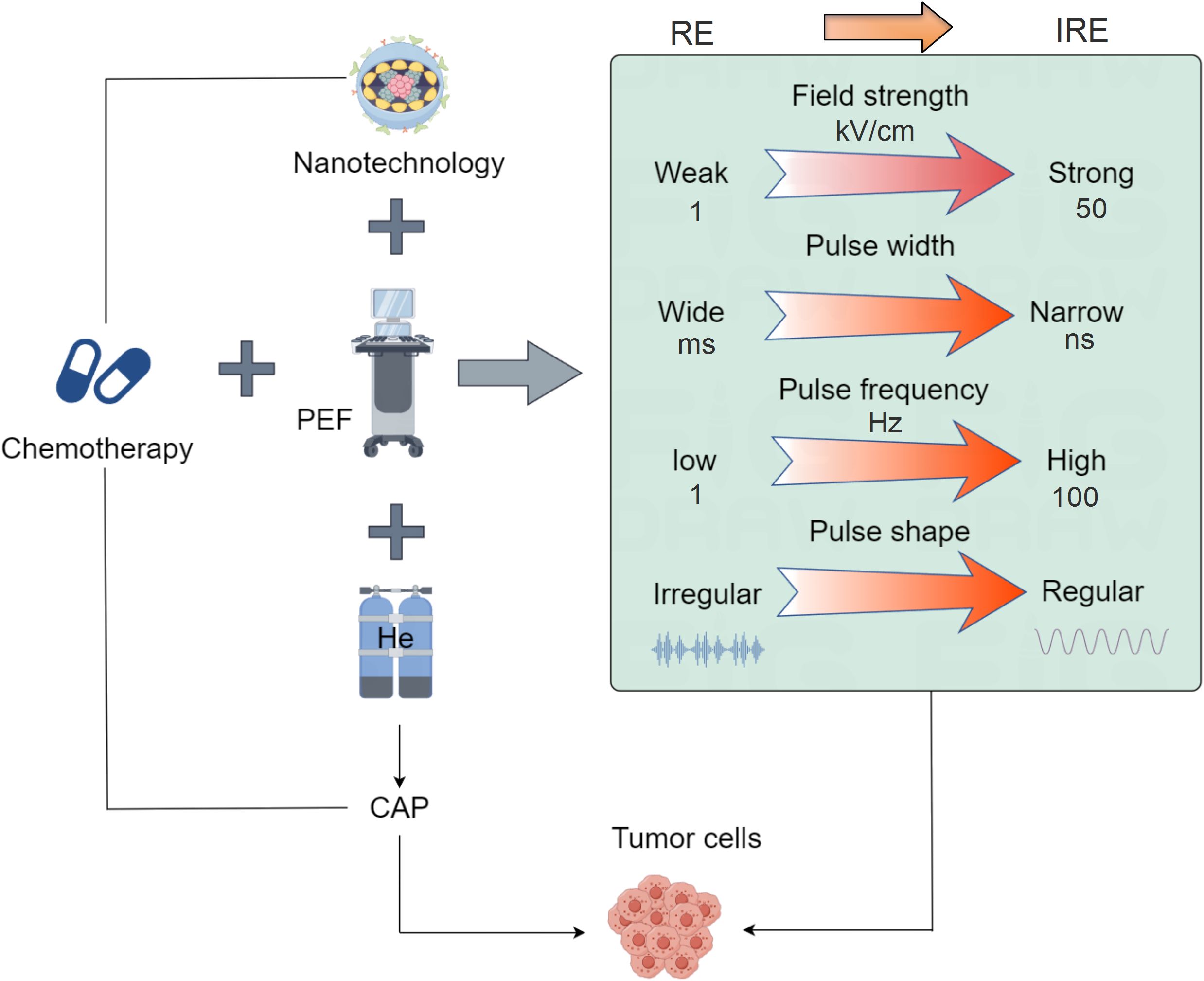
Figure 1. Schematic diagram of PEFs technology development. The application of PEFs technology in oncology has evolved from reversible electroporation to irreversible electroporation. The key parameters involved in PEFs have also progressed: the electric field intensity has increased from low (1 kV/cm) to high (over 50 kV/cm), the pulse width has shortened from long (ms) to short (ns), the frequency has risen from low (1 Hz) to high (over 100 Hz), and the pulse shape has become more regular from irregular. The development of PEFs combined with other modalities includes integration with radiotherapy and chemotherapy, combination with nanomaterials, pairing with cold atmospheric plasma (CAP), and coupling with immunotherapy, ultimately achieving targeted minimally/non-invasive tumor treatment.
2 Working principles and treatment modalities of PEFs in tumor therapy
2.1 The working principles of PEFs
PEFs refers to a high-voltage pulse applied over an extremely short period of time. It is a special type of electrical signal characterized by high electric field strength and a brief duration of action. In cancer treatment, PEFs are used to directly target tumor tissue to achieve therapeutic objectives.
The core principles governing PEFs encompass several critical parameters. Electric field strength–measured in kilovolts per centimeter (kV/cm)–serves as a pivotal determinant, where elevated intensities induce electrical breakdown and irreversible membrane disruption to eliminate tumor cells. Pulse width, quantified in nanoseconds (ns) or microseconds (μs), directly influences thermal and electrolytic side effects; shorter durations enhance selectivity for neoplastic cells while minimizing collateral damage. Shorter pulse widths and wavelengths deliver higher energy, which penetrates the outer cell membrane to directly target internal structures. Consequently, adjusting these parameters allows for selective targeting of internal cellular components. Pulse frequency, expressed in hertz (Hz), must be optimized according to therapeutic objectives and tumor-specific characteristics. Additionally, pulse waveform variations–including square, exponential decay, and triangular configurations–modulate cellular interactions and biological outcomes, enabling tailored bioelectrical effects. Collectively, these parameters define PEFs’s mechanism of action through controlled electroporation.
In cancer treatment, PEFs acts on tumor tissue by applying high-intensity pulsed electric fields, producing electrical breakdown and destructive effects on the cell membrane, known as irreversible electroporation (IRE). As opposed to reversible electroporation (RE), which is characterized by lower field strength, wider pulses, and lower frequency. Its action is confined to the outer cell membrane, enabling membrane recovery and thus preventing cell death (Figure 1). This disrupts the ionic balance inside and outside the cell, increases membrane permeability, impairs the exchange of substances across the membrane, and ultimately leads to the death of tumor cells. Additionally, PEFs can activate the immune system to promote an immune response against the tumor (28, 29). It is important to note that the specific application methods and parameter choices of PEFs in cancer treatment may vary depending on the type of tumor, treatment goals, and individual patient differences (9). Therefore, doctors will determine the appropriate PEFs parameters and treatment plans based on the patient’s condition and treatment needs for specific cases.
2.2 The strategies of PEFs in tumor treatment
PEFs employs electric field energy to target tumors through diverse implementation approaches tailored to specific therapeutic objectives (Table 1). In its standard form, PEFs therapy applies intra- or extracorporeal electric fields using micro-to-submicrosecond pulses at kV/cm strengths, inducing cell membrane electroporation to trigger apoptosis, necrosis, or cell cycle arrest for tumor suppression. Electrochemotherapy (ECT) synergistically combines PEFs with chemotherapeutic agents: drugs are administered intratumorally before PEFs application, where transient membrane permeabilization enhances intracellular drug uptake, significantly potentiating cytotoxicity against treatment-resistant malignancies. Electrohyperthermia (EHT) utilizes high-frequency currents to generate localized thermal effects, either directly ablating tumor cells to augment radiotherapy/chemotherapy efficacy. Alternatively, Electroporation-Mediated Gene Therapy (EMGT) leverages PEFs to reversibly permeabilize membranes, enabling targeted delivery of genetic payloads (therapeutic genes/DNA constructs) for precision genome editing, cellular reprogramming, or molecular interventions (Figure 1).
Collectively, these modalities demonstrate PEFs’ adaptability across mechanistic paradigms—from physical ablation to molecular-scale interventions.
3 The application of PEFs technology in cancer treatment
3.1 Pancreatic cancer
Pancreatic cancer stands as one of the malignancies most frequently targeted by PEFs therapy, which not only significantly suppresses tumor progression but also remodels the immunosuppressive TME, thereby preventing recurrence and metastasis. For example, IRE delivers high-voltage electric pulses to disrupt cellular membrane integrity, inducing immunogenic cell death (ICD) through damage-associated molecular pattern (DAMP) release and loss of intracellular homeostasis (30). In preclinical models, IRE monotherapy suppresses pancreatic tumor growth in a dose-dependent manner while enhancing CD8+ T-cell infiltration (30, 31). Its immunomodulatory capacity—evidenced by HMGB1-mediated MAPK-p38 activation and M1-macrophage polarization (32)—synergizes with immunotherapies: combined IRE and CD40 antibody therapy stimulates systemic immunity and inhibits metastasis in orthotopic models (31); IRE/OX40 agonist regimens enhance CD8+ T-cell quality/quantity and extend survival (33); and IRE-induced interferon-γ expression supports checkpoint inhibitor combinations to prevent recurrence (34). IRE further overcomes immunosuppression in the TME, improving dendritic cell vaccine efficacy (35) and promoting CD4+ T-cell conversion to antitumor Th1/Th17 subsets in MHC-I-low PDAC, where subsequent PD-L1 blockade activates compensatory cDC2-CD4+ T-cell axes (36). Advanced delivery strategies amplify these effects—nanoparticles loaded with TGF-β inhibitors (SB525334) or gemcitabine (NE/Lip-GEM) enhance neutrophil-mediated drug delivery (27, 37, 38), while M-TK-OA nanotherapeutics combined with IRE activate cGAS-STING pathways to induce durable immune memory via PARP/ATM inhibition (39). Clinically, adding PD-1/PD-L1 blockers to IRE-chemotherapy regimens improves survival in locally advanced pancreatic cancer (LAPC) (40). Complementary electrical modalities like nsPEF inhibit growth/metastasis while remodeling myeloid compartments (41–43), and electro-antibacterial therapy (EAT) enhances intracellular pathogen clearance (44). Collectively, IRE-based combinatorial approaches reshape the TME toward proinflammatory states (19), establishing a promising paradigm for immunologically resistant pancreatic malignancies.
3.2 Hepatocellular carcinoma
Clinical studies have established IRE as a safe and effective hepatocellular carcinoma (HCC) ablation modality, with ongoing optimization of efficacy assessment methods and device engineering complementing robust evidence of its antitumor effects. Preclinical validation includes rabbit HCC models demonstrating significant histological advantages of IRE over RE and untreated controls: IRE zones exhibited 51-60% fewer tumor cells, 66-67% reduced microvasculature, and 185-228% increased cell death (45). Technical refinements further define therapeutic parameters—thresholds for near-complete HepG2 cell eradication require IRE pulses at 4kV/cm, while RE permeabilization occurs at 1kV/cm (8 pulses) with maximum temperatures ≤30.1 °C, confirming non-thermal mechanisms (46). nsPEF demonstrates compelling preclinical efficacy: it inhibits Hep3B cell growth in vitro with distinct ultrastructural changes observed via TEM, achieves complete ablation in rabbit VX2 liver tumors under contrast-enhanced ultrasound guidance (47), and alters osteopontin-mediated glycogen metabolism in HCC (48). Crucially, nsPEF induces immunogenic cell death that stimulates systemic immunity against residual/metastatic disease (49), though it simultaneously elevates membrane PD-L1 levels and promotes PD-L1+ extracellular vesicle release, causing CD8+ T-cell dysfunction—an effect reversed by PD-L1 blockade to significantly suppress tumor growth and improve survival (50, 51). IRE monotherapy in H22 murine models similarly enhances CD8+ T-cell and dendritic cell infiltration in peri-ablational zones (52), while IRE-immunotherapy combinations in orthotopic models remodel tumor immunity by enhancing infiltrating CD8+ T cell necroptosis yet attenuating pro-tumor inflammatory populations (15). Clinical translation progresses rapidly: nsPEF safely alters sphingolipid metabolism to drive Ly6c2+ mononuclear phagocyte differentiation and memory CD8+ T-cell formation in human trials (53), while ultrasound-guided IRE fulfills clinical guidelines by enabling tumor control, symptom alleviation, and survival extension (54). Emerging combinatorial approaches include IRE with Chlorella vulgaris/polydopamine-encapsulated PD-1 inhibitors to boost local drug concentration and immune activation (55), and high-frequency repetitive nsPEF (rnsPEF) which achieves effective ablation at 4536.4 ± 618.2 V/cm in rabbit livers with preserved vasculature. rnsPEF synergizes with doxorubicin to enhance cell death and long-term tumor control (56), reinforcing nsPEF’s preclinical safety profile for high-risk HCC locations (57). Microbiome and serum metabolome analyses further suggest novel prognostic markers post-ablation (58). Collectively, these advances establish PEFs—particularly through IRE and nsPEF platforms—as transformative tools that overcome limitations of thermal ablation, with clinical integration accelerated by standardized protocols, immune modulation strategies, and precision energy delivery systems.
3.3 Prostate cancer
Emerging clinical evidence establishes IRE as a safe and effective modality for prostate cancer treatment, with recent studies highlighting its capacity to potentiate checkpoint immunotherapy. Specifically, IRE enhances systemic antitumor T-cell activation while downregulating immunosuppressive mechanisms in localized disease, promoting tumor antigen-specific expansion of tissue-resident memory CD8+ T cells (TRM) for durable immune surveillance (20, 59). Complementary nsPEF technology exerts distinct cytotoxic effects: high-frequency nsPEF induces profound cytoskeletal alterations that disrupt cellular mobility and enhance membrane permeability, ultimately triggering prostate cancer cell death (60). Parametric optimization studies in murine models demonstrate voltage-dependent efficacy, with histological, immunohistochemical, and immunoblot analyses confirming that 900 V represents the minimal threshold voltage for significant tumor growth reduction via IRE-induced cell death (61). In vitro and magnetic resonance imaging (MRI) assessments further validate IRE’s ability to achieve sustained tumor regression (62). When combined with microwave ablation, PEFs synergistically reduce PC3 cell viability, induce apoptosis, and inhibit migratory capacity—as quantitatively demonstrated through scratch assays (63). Current reviews comprehensively summarize these advances, analyzing IRE’s mechanisms, clinical outcomes, advantages, and limitations while highlighting critical research gaps and future directions for optimizing PEFs-based prostate cancer management (14). Collectively, these findings position IRE and nsPEF as transformative tools that bridge focal ablation with systemic immunomodulation in prostate oncology.
3.4 Melanoma
PEFs represent a highly suitable therapeutic approach for melanoma, primarily because these tumors are typically located in superficial layers, allowing for direct percutaneous minimally invasive intervention that yields clinically significant outcomes. For instance, nsPEF exert tumor-ablation effects primarily through subcellular membrane electroporation, offering cell-type specificity with minimal thermal damage while synergizing with chemotherapeutics—positioning it as a promising modality for melanoma treatment (64). Preclinically, nsPEF-mediated local ablation in murine melanoma models restores (though does not enhance) dormant antitumor immunity in tumor-bearing hosts (65), while nsPEF treatment induces non-cytotoxic membrane permeabilization and morphological changes characterized by vesicle externalization, cell contraction, and lipid migration. Critically, this elevates PD-1 checkpoint expression, suggesting therapeutic synergy with immune checkpoint inhibitors (66). Nanosecond pulse stimulation (NPS) demonstrates superior efficacy over cryoablation, permanently eliminating up to 91% of B16-F10 melanoma lesions (vs. 66% with cryoablation) with minimal fibrosis, muscle atrophy, or permanent skin damage—establishing it as a less destructive yet more effective alternative (67). Complementary approaches include high-frequency nsPEF combined with magneto-poration of iron oxide nanoparticles to suppress A375 cell viability in vitro (68), and integrin-targeted nsPEF IRE (INSPIRE) achieving voltage-dependent tumor reduction in equine spontaneous melanoma (84-88% volume reduction at 2kV) (69). Clinically, PEFs-mediated mRNA delivery of cyclin B1 knockdown induces tumor regression (70), while nsPEF upregulates melanoma-specific MAGE antigen expression to sensitize tumors to targeted therapies (71). Early human trials report rapid resolution of immunotherapy-resistant uveal melanoma metastases following PEFs treatment (72), underscoring its translational potential. Collectively, these modalities leverage unique bioelectrical mechanisms—from subcellular electroporation to immune checkpoint modulation—to overcome therapeutic resistance in melanoma.
3.5 Gliomas
For gliomas, PEFs (specifically IRE and nsPEF) effectively address the limitation of traditional chemotherapeutic agents being unable to penetrate the blood-brain barrier (BBB), enabling targeted treatment of tumor lesions while minimizing collateral damage to healthy brain cells (73). Studies utilizing high-intensity ultrashort PEFs on U87 GBM cells and U87-derived neurospheres revealed, through the analysis of diverse in vitro biological endpoints and transcriptomic and bioinformatic analyses, that PEFs affects cell proliferation, differentially regulates hypoxia, inflammation, and p53/cell cycle checkpoints, significantly reduces the capacity to form new neurospheres, inhibits invasive potential, and alters the expression of stemness/differentiation genes (74). High-frequency irreversible electroporation (H-FIRE) was applied to suspensions of F98 glioma and LL/2 Lewis lung carcinoma cells to model primary and metastatic brain cancer. Data indicate that H-FIRE induces both reversible and irreversible cell damage in a dose-dependent manner, and the existence of dose-dependent recovery mechanisms allows tumor cell proliferation (75). Furthermore, H-FIRE has been shown to improve survival and immune cell infiltration in rodent models of malignant glioma (76). Through cell ablation and survival experiments, this study investigated the bipolar cancellation (BPC) effect in U87-MG cells exposed to nsPEF with varying pulse numbers and electric field amplitudes. Results demonstrated the highest BPC efficiency (163.9%) occurred at 15 kV/cm and 15 pulses, while unipolar nsPEF at 20 kV/cm and 15 pulses achieved 90% lethality in cell suspensions; this latter field was subsequently used as the reference for ablation experiments. Ablation studies revealed that the electric field threshold for ablation was lower in 3D (3D-like tissue) models (5.805 ± 1.455 kV/cm) compared to monolayer walled cells (8.95 ± 0.75 kV/cm), resulting in larger ablation areas under identical pulse conditions. Additionally, the BPC effect was more pronounced for 3D cells, although ablation area and BPC efficiency followed similar trends when pulse number was modulated (77). Collectively, this work highlights the potential of ultrashort pulsed electric fields to disrupt the dense structure of glioma (78).
3.6 Breast cancer
PEFs effectively overcome chemoresistance in breast cancer while offering a minimally invasive, safe, and efficacious therapeutic approach. For example, this study aimed to evaluate the treatment of MCB-7 human breast cancer cells using nsPEF and low electric fields (LEFs) unipolar electrical pulses. At repetitive frequencies starting from 0.01 Hz, cell viability was significantly reduced by approximately 35%, reaching complete cell loss with microsecond pulses at 1 Hz. Uptake of non-permeant drugs occurred not via classical electroporation-mediated membrane permeabilization, but through endocytosis. Microsecond electric pulses were able to disrupt the membranes of endocytotic vesicles, releasing the cytotoxic drug bleomycin (79). PEFs treatment combined with azithromycin significantly inhibited human breast cancer cell proliferation (80). nsPEF effectively ablated tumors, elicited an immune response, and suppressed residual breast cancer growth in mice via a CXCL9 axis-dependent mechanism (81). Treating orthotopic breast cancer-bearing mice with PEF revealed significant immunomodulatory effects compared to radiofrequency ablation (RFA). Distinct serum and tumor cytokine profiles were observed, including intratumoral downregulation of vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1 alpha (HIF-1α), c-MET, interleukin-10 (IL-10), Ki67, and tumor necrosis factor alpha (TNF-α). PEFs increased innate immune activation, enhancing the recruitment of dendritic cells, M1 macrophages, and natural killer cells, while decreasing M2 macrophages and myeloid-derived suppressor cells. Concurrently, PEFs enhanced adaptive immunity compared to RFA, characterized by increased antigen-specific T cells and reduced regulatory T cells. By the study endpoint, PEFs suppressed tumor growth and increased survival. Finally, PEFs promoted an abscopal effect clearing lung metastases, with the effect being stronger when combined with anti-PD-1 therapy than PEFs alone (29). Research applying nsPEF to electrically stimulate breast cancer MCF-7 cells demonstrated that nsPEF distinctly impacted intracellular functions and dynamics in both MCF-7 and MCF-10A cells. This study proved the selective killing of breast cancer cells using microelectrodes, paving the way for developing nsPEF-based breast cancer therapies (82). This work investigated how sub-ablative H-FIRE affects lymphatic and blood microvascular remodeling in the 4T1 murine breast cancer model. Histological examination revealed a transient increase in blood vessel density on day 1 post-treatment, followed by a peak in lymphatic vessel density within surviving tumor regions on day 3, alongside increased lymphatic vessel density in the surrounding fat pad; minimal remodeling occurred in tumor-draining lymph nodes within 3 days. Gene expression analysis indicated elevated CCL21 and CXCL2 levels on day 1, while VEGFA and VEGFC did not appear to drive vascular remodeling. Similarly, CCL21 protein content in tumor-draining axillary lymph nodes correlated with gene expression data from surviving tumor regions. These findings demonstrate dynamic changes in lymphatic and blood microvascular architecture post-SA-HFIRE, potentially enhancing adaptive immune responses through CCL21-mediated lymphatic homing and subsequent lymph node microvascular remodeling (83). Finally, we demonstrate that μsPEF induces MCB-7 cell death through a mechanism dependent on Ca2+ electropermeation (CaEP) and calpain activity (84).
3.7 Other cancers
Beyond the malignancies mentioned above, PEFs demonstrate robust therapeutic efficacy in lung carcinoma, colon carcinoma, head and neck carcinoma, gastric carcinoma, and ovarian carcinoma, among others. Firstly, high-frequency irreversible electroporation (H-FIRE) treatment of primary lung tumors in dogs revealed tumor ablation shrinkage, and immunohistochemical staining results suggested H-FIRE may alter the tumor immune microenvironment (13). Combining high-frequency unipolar nsPEF with Ca2+ inhibited lung cancer cells growth (85). Clinical trials indicate that PEFs therapy is feasible and safe for early-stage non-small cell lung cancer (NSCLC), showing potential signals of immune system activation (86). Furthermore, PEFs treatment improved progression-free survival and overall survival (OS) in patients with progressive stage IV NSCLC (87). Calcium ions and optimized pulse parameters can enhance PEFs efficacy and induce oxidative changes in lung cancer cells. Therefore, the anticancer efficacy of PEFs combined with standard cytotoxic drugs or calcium ions should be considered for lung cancer treatment (88). Secondly, combining PEFs with daunorubicin in two gastric adenocarcinoma cell lines (ERG85-257P and ERG85–257 RDB) inhibited proteasome activity, leading to increased protein degradation, elevated cell death percentage, and heightened reactive oxygen species (89). Thirdly, nsPEF induced endoplasmic reticulum stress accompanied by immunogenic cell death in murine lymphoma and colorectal cancer models (90). nsPEF treatment reduced viability, proliferation, and mucin production in mucinous colorectal cancer (MCRC) cells while promoting cell death, indicating its potential clinical application for MCRC (91). Fourthly, the combination of IRE and L-BEZ effectively eradicated tumors and prevented recurrence in nude mice bearing head and neck tumors (24). Electrochemotherapy involves treating solid tumors by combining non-permeant cytotoxic drugs (e.g., bleomycin) with locally applied PEFs. Effective use of this method crucially depends on utilizing optimal PEFs protocols and concentrations of drugs that lack inherent cytotoxicity (25). Fifthly, results demonstrate that nsPEF can exert preferential ablation effects on highly invasive and malignant ovarian cancer cells compared to benign cells. This study provides an experimental foundation for research into killing malignant cells via electrotherapy and may hold clinical significance for tumor treatment and preventing post-treatment recurrence (92). Research shows that pulse asymmetry influences the bipolar cancellation (BPC) effect. Following Ca2+ electrochemotherapy, cell membrane poration decreased while cell death increased. The BPC phenomenon can be controlled using pulse asymmetry or a delay between the positive and negative phases of the pulse (93).
4 The mechanisms of PEFs in promoting cancer cells death
PEFs, including nsPEF, IRE, and H-FIRE, have been demonstrated to effectively induce cancer cell death through multiple mechanisms. Among these, IRE primarily promotes tumor cell death through its characteristic effects. However, due to its lower energy and shorter pulse width, RE often results in transient, repairable membrane permeabilization that is insufficient to cause tumor cell death. The cancer cells that survive IRE treatment, or those targeted by nsPEF, can subsequently undergo death via pathways such as apoptosis (94–98), autophagy (99, 100), and ferroptosis (101, 102).
Fundamental studies indicate that high-intensity electric pulses can significantly increase cell membrane permeability. Molecular dynamics simulations and free energy calculations reveal that this membrane permeabilization lesion undergoes secondary oxidation, inducing substantial lipid peroxidation that triggers ferroptosis (101). In various cell models, PEFs induce programmed cell death. For example, nsPEF treatment of human ovarian cancer cells (SKOv3) may induce apoptosis by activating the release of intracellular calcium stores (95); nsPEF significantly reduces viability, inhibits growth, and induces apoptosis in prostate cancer cells (PPC-1) (94); and IRE treatment of rat tissues activates caspase-3 and promotes apoptosis, as observed via immunohistochemistry and TUNEL assay (97). Furthermore, research on glioma cells (U251) shows that PEFs not only mediate apoptosis via the AP-1/Bim pathway but also involve other forms of regulated cell death (RCD), such as autophagy, necroptosis, and immunogenic cell death (ICD) (96).
Autophagy plays a complex role in the effects of PEFs. For instance: Sub-toxic doses of nsPEF activate autophagy as a compensatory mechanism to repair membrane damage, but prolonged exposure increases cell death while concomitantly reducing autophagy markers (99). Conversely, in a pancreatic cancer model using KPC-A548 or Panc02 murine cell line xenografts, IRE increased the expression of autophagy markers LC3 and p62. Inhibiting autophagy (e.g., with hydroxychloroquine) significantly enhanced the efficacy of IRE. Subsequent combination of IRE with inhibitors targeting both HMGB1 receptors (RAGE and TLR4) further suppressed tumor growth, indicating that IRE promotes cell death, in part, by modulating autophagy (100).
Notably, nsPEF can also exert cell cycle-specific effects on tumor cells, such as inhibiting the proliferation of S-phase cells without significantly affecting their viability (103). Importantly, PEFs can produce synergistic effects with traditional chemotherapy. For example, the chemotherapeutic agent 5-fluorouracil (5-FU) demonstrated effectiveness when combined with nsECT (nanosecond electrochemotherapy) and Fe(III). This combined approach significantly reduced the expression of the mitochondrial protein frataxin under a microsecond electroporation protocol, inducing ferroptosis (102). Additionally, H-FIRE was shown to inhibit the invasion and metastasis of highly aggressive tumor cells by suppressing SIRT1/2 expression and inducing mitochondrial cell death (98).
Collectively, these studies reveal the potential of PEFs to exert multi-faceted anti-tumor effects by inducing apoptosis, modulating autophagy, triggering ferroptosis, activating other RCD pathways, and potentially synergizing with chemotherapeutic drugs (Figure 2). Furthermore, different PEFs modalities trigger distinct cell death pathways. For instance, nsPEF primarily induces cell death through apoptosis, autophagy, and ferroptosis. In contrast, IRE can not only initiate these same pathways but also disrupt the ionic balance across both the inner and outer cell membranes to cause cell death.
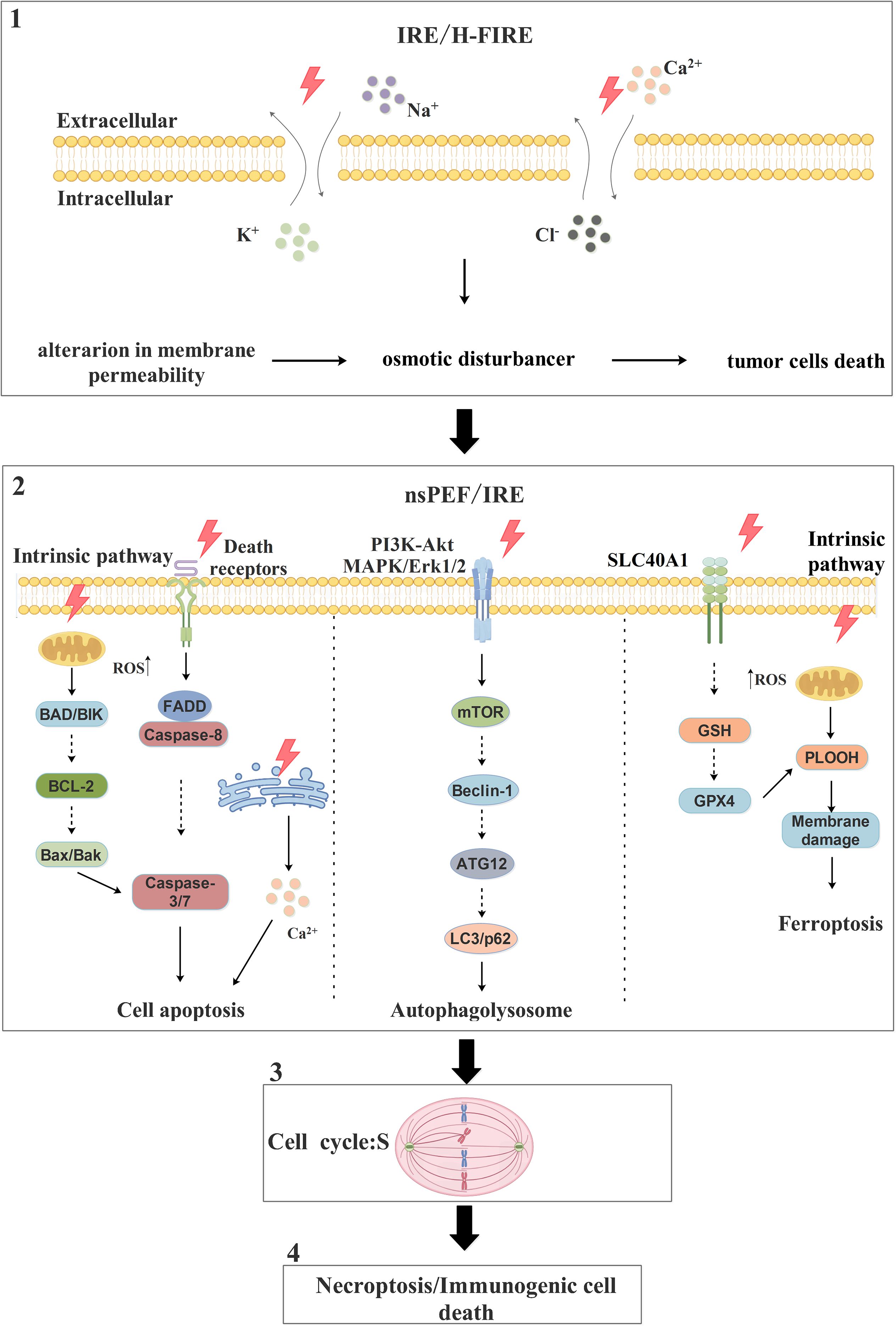
Figure 2. The pathways and molecular mechanisms of PEFs in promoting tumor cells death. PEFs primarily promote tumor cells death through three main pathways: apoptosis, autophagy, and ferroptosis. In apoptosis, one mechanism involves PEFs inducing an increase in endogenous ROS levels, which activates downstream pro-apoptotic proteins BAD and BIK; this activation inhibits the anti-apoptotic protein BCL-2, leading to the release of Bax and Bak, which subsequently activate caspase-3/7 and trigger apoptosis. Alternatively, PEFs promote apoptosis by triggering death receptors on the cell surface, leading to the recruitment of the adaptor protein FADD and the activation of caspase-8; this in turn activates caspase-3/7, resulting in substrate protein hydrolysis and cell death. Autophagy is induced by PEFs influencing the PI3K-Akt and MAPK/Erk1/2 signaling pathways to activate the downstream mTOR pathway; this activation promotes the formation of the Beclin-1/ATG12/LC3 complex, leading to autophagosome generation, fusion with lysosomes, and ultimately cell death. For ferroptosis, one mechanism involves PEFs affecting the metal transporter SLC40A1 to increase Fe²+ uptake; this increased iron influx inhibits the GSH/GPX4 antioxidant system, resulting in the accumulation of large amounts of phospholipid hydroperoxides (PLOOH), which disrupt membrane integrity and induce ferroptosis. Note: Solid lines indicate reported cases, while dashed lines indicate unreported cases.
5 Immune regulatory mechanisms of PEFs in the TME
PEFs (IRE and nsPEF) drive anti-tumor responses by systemically reshaping the myeloid immune landscape: They significantly reduce MDSCs proportions (19, 20) and inhibit CCR2+TAM-mediated immunosuppression (104), while driving the sphingolipid metabolism-mediated differentiation of Ly6c2+ monocytes into dendritic cells (53). Dendritic cells (DCs) act as central hubs—IRE combined with VMT/CaO2 NSs captures tumor antigens to form in situ vaccines that promote DC migration (105); a substantial intraprocedural resistance drop (large ΔR) upregulates the CD80 costimulatory molecule on cDC1s (106); PD-L1 blockade specifically activates cDC2s to enhance antigen presentation (36); and nsPEF activates the NLRP3 inflammasome to trigger IL-1β release (107)—collectively strengthening myeloid immune initiation. Therefore, in myeloid cells, PEFs primarily modulate MDSCs through the NLRP3/IL-1β signaling pathway and regulate tumor-associated macrophages via the CCLs/CCR2 signaling axis (Figure 3).
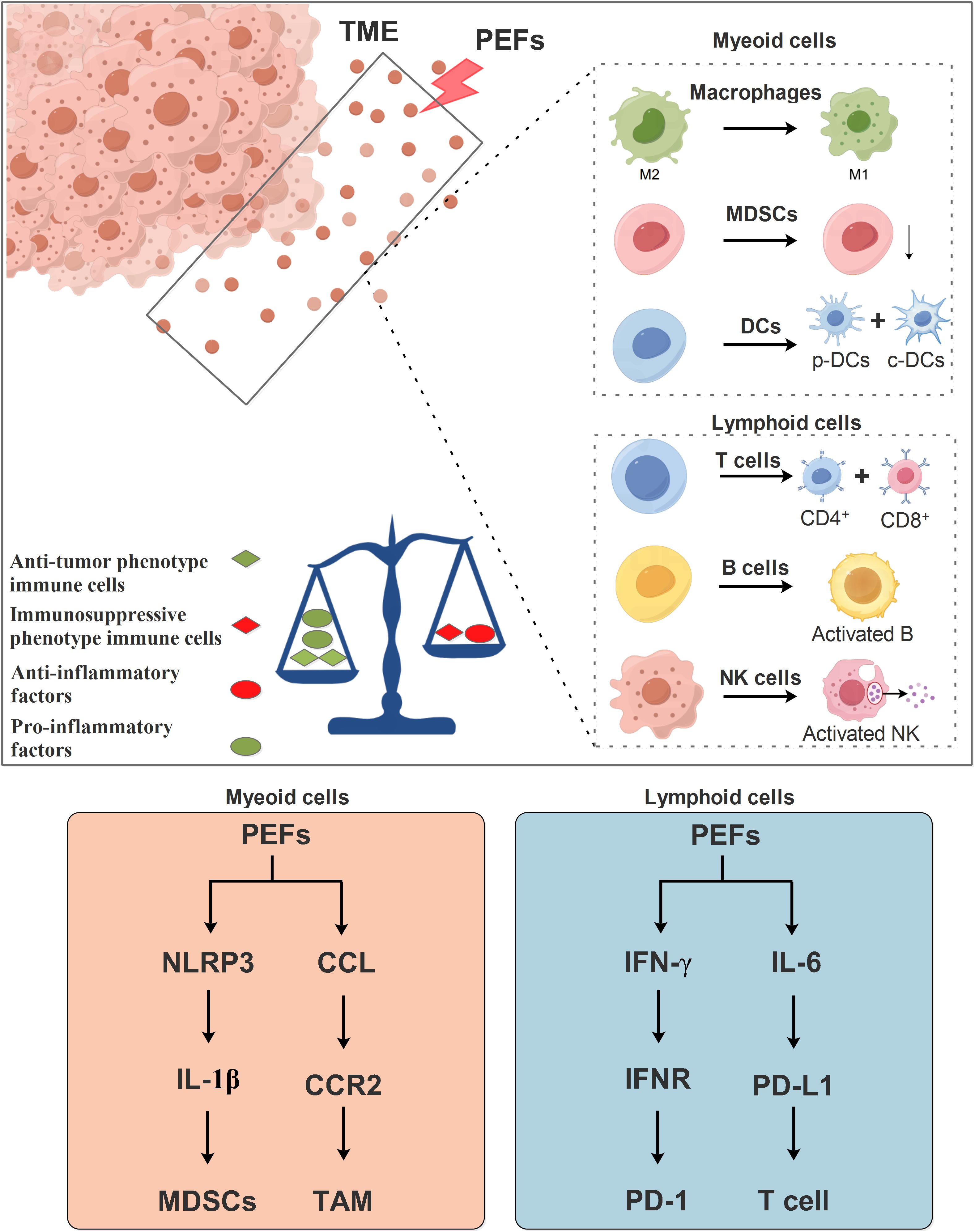
Figure 3. Mechanism of PEFs in regulating immune responses within the TME. PEFs influence immune cells in the TME, including both myeloid and lymphoid lineage cells. PEFs promote the repolarization of macrophages from the M2 phenotype toward the M1 phenotype, suppress myeloid-derived suppressor cell accumulation (MDSCs), and facilitate the differentiation of dendritic cells (DCs) into p-DCs and c-DCs. Furthermore, PEFs enhance the differentiation of T lymphocytes into CD4+ and CD8+ subsets, and activate B cells and NK cells. Collectively, PEFs drive a shift from an immunosuppressive TME toward an immunostimulatory state. This is characterized by increased pro-inflammatory cytokine production and decreased anti-inflammatory factors, thereby regulating antitumor immunity.
In the lymphoid compartment, PEFs induce profound responses: Enhanced CD8+ T-cell infiltration with dual Ki-67+/PD-1+ activation (106, 108); sustained central memory T cells (Tcm) strongly correlating with recurrence-free survival (109); CD4+ T-cell polarization toward IFN-γ+ Th1/Th17 phenotypes (36), expandable via PD-L1/IL-6 dual blockade to amplify the Th1-NK cell axis (36); alongside early NK-cell expansion (109) and increased B-cell infiltration (51, 76) synergistically counteracting Treg-mediated immunosuppression (20, 108). Synergistic strategies further augment efficacy: PD-1/CTLA-4 blockade remodels cytokine profiles and T-cell function (20, 51); oncolytic viruses (OVs) recruit CTLs to intensify local attack (110); and pH-responsive hydrogel microspheres promote cDC1 migration to lymph nodes (111). Thus, it is plausible that PEFs regulate T cells of the lymphoid lineage via the IFN-γ/IFNR and IL-6/PD-L1 signaling axes (Figure 3).
In summary, PEFs transform “cold” tumors into immune-permissive states (31, 36, 111) through myeloid reprogramming (enhanced antigen presentation/suppressive cell clearance) and lymphoid activation (effector-memory cell expansion). Their efficacy is modulated by intraprocedural resistance dynamics (ΔR), sphingolipid metabolism, and inflammasome pathways, establishing a novel “local ablation-systemic immunity” paradigm for solid tumors.
6 Prospects
PEFs offers significant advantages in tumor therapy, positioning it as a promising tool for both treatment and research (Table 2). A key strength is its minimally invasive nature, requiring no major surgery or incisions; instead, electrodes are inserted directly into the tumor area, reducing procedural risks and patient discomfort. The high selectivity of PEFs enables precise targeting of specific cells or tissues through parameter adjustment, sparing surrounding healthy structures (112). Its versatility further enhances utility–PEFs can directly kill or inhibit cancer cells, modulate immune responses, and enhance drug delivery efficacy, supporting applications across diverse therapeutic contexts. Rapid effectiveness is another critical advantage, with tumors often showing swift regression post-treatment, a feature particularly valuable for acute conditions or urgent symptom management. Finally, the adjustability of PEFs parameters allows customization to tumor-specific requirements, facilitating personalized treatment optimization.
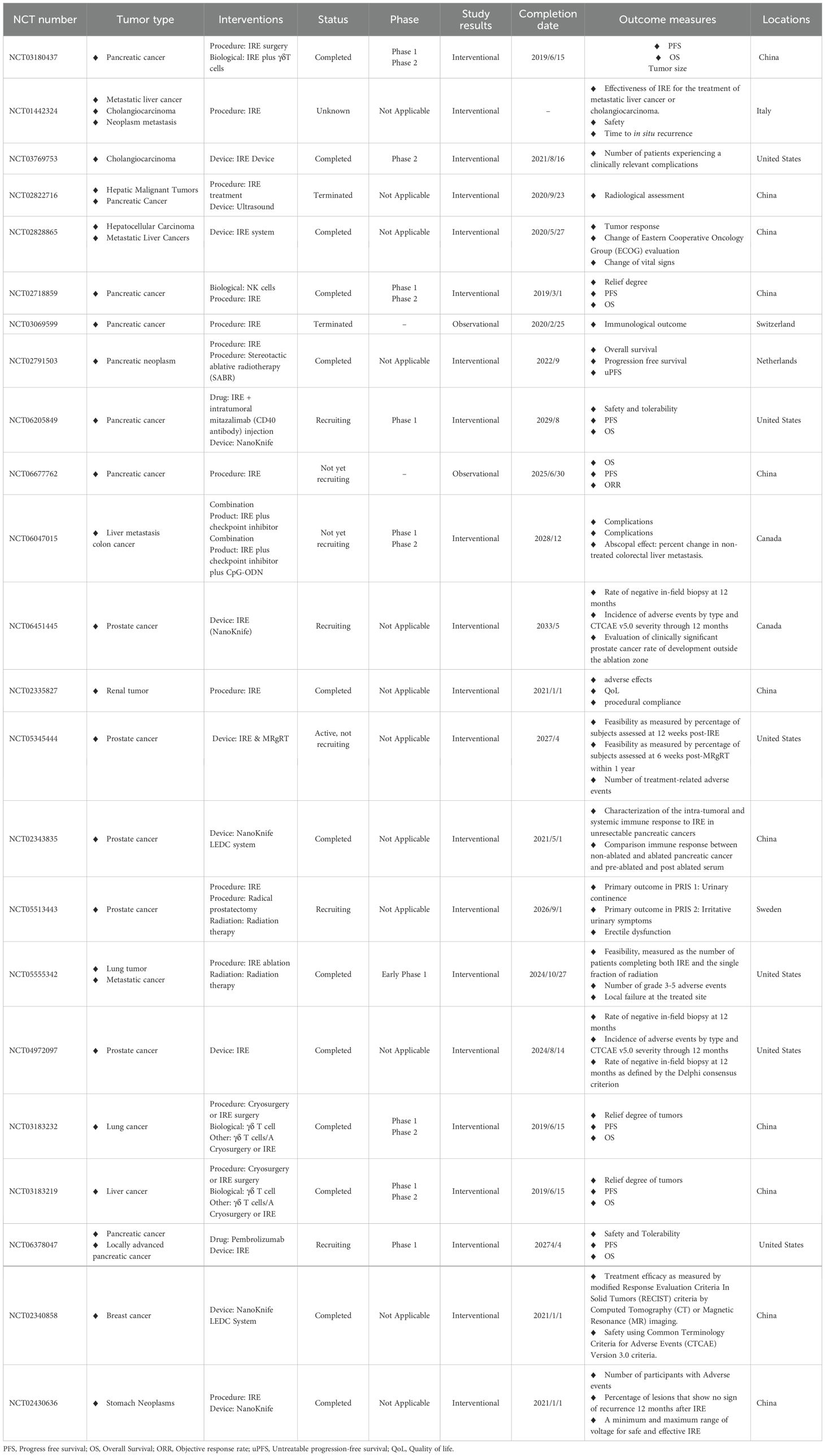
Table 2. The application of PEFs for cancers in clinical trials (https://clinicaltrials.gov/search?cond=Tumor&intr=IRE&viewType=Table).
Despite its significant advantages, PEFs face several challenges and limitations in the field of tumor therapy. A primary challenge lies in parameter optimization, where therapeutic efficacy depends on variables such as voltage, frequency, pulse width, and pulse number–identifying the optimal combination for diverse tumor types demands extensive research. Tumor specificity presents another obstacle, as heterogeneous tissue responses to PEFs can compromise treatment consistency and reproducibility, necessitating deeper mechanistic studies (113). Safety concerns also arise, with potential adverse effects including muscle contractions, tissue damage, pain, and inflammation during therapy, underscoring the need for enhanced safety protocols. Limited applicability further constrains PEFs, as certain tumor types exhibit suboptimal responses or unsuitability for this modality, requiring clearer definition of its scope. Finally, the translation to clinical practice remains challenging, demanding large-scale trials to validate efficacy and safety, alongside standardized protocols, equipment, and operational frameworks to ensure reliability (114). Additionally, a more critical analysis of the current limitations in clinical translation is needed, such as the standardization of treatment protocols, the management of off-target effects in complex anatomical locations, and the long-term efficacy and safety data from clinical trials.
In summary, while PEFs hold transformative potential, addressing these practical hurdles through sustained research and technological refinement is essential for advancing their clinical adoption.
7 Conclusion
PEFs, as an emerging method for cancer treatment, holds vast potential applications. By disrupting the integrity of tumor cell membranes, PEFs can effectively induce the death of tumor cells and can be combined with other treatment methods to enhance treatment outcomes. Despite some successes in practice, further research is still needed to address the challenges faced by PEFs and to expand its application in clinical practice.
Author contributions
LZ: Conceptualization, Project administration, Funding acquisition, Writing – review & editing, Methodology, Writing – original draft. SD: Conceptualization, Writing – review & editing, Project administration. FT: Project administration, Writing – review & editing. YW: Writing – review & editing, Methodology. WX: Supervision, Writing – review & editing. YC: Investigation, Writing – review & editing. LY: Writing – review & editing, Conceptualization. CY: Writing – review & editing, Conceptualization. ZW: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This Research Project Supported by Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJQN202300127), the Natural Science Foundation of Chongqing (CSTB2024NSCQ-KJFZZDX0011 & CSTB2025NSCQ-GPX0003 & CSTB2025NSCQ-GPX0004), the National Natural Science Foundation of China (82303363), and the Research Promotion Fund of Chongqing University Cancer Hospital (2023nlts008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roerden M and Spranger S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat Rev Immunol. (2025) 25:353–69. doi: 10.1038/s41577-024-01111-8
2. Vasan N, Baselga J, and Hyman DM. A view on drug resistance in cancer. Nature. (2019) 575:299–309. doi: 10.1038/s41586-019-1730-1
3. Dumontet C, Reichert JM, Senter PD, Lambert JM, and Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. (2023) 22:641–61. doi: 10.1038/s41573-023-00709-2
4. Elhanani O, Ben-Uri R, and Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. (2023) 41:404–20. doi: 10.1016/j.ccell.2023.01.010
5. Chen ZH, Lin L, Wu CF, Li CF, Xu RH, and Sun Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun (Lond). (2021) 41:1100–15. doi: 10.1002/cac2.12215
6. Sajti E, Kavelaars A, van Meeteren N, Teunis M, Gispen WH, and Heijnen C. Tumor angiogenesis and metastasis formation are associated with individual differences in behavior of inbred Lewis rats. Brain Behav Immun. (2004) 18:497–504. doi: 10.1016/j.bbi.2003.11.009
7. Petrov AA, Moraleva AA, Antipova NV, Amirov RK, Samoylov IS, and Savinov SY. The action of the pulsed electric field of the subnanosecond range on human tumor cells. Bioelectromagnetics. (2022) 43:327–35. doi: 10.1002/bem.22408
8. Tasu JP, Tougeron D, and Rols MP. Irreversible electroporation and electrochemotherapy in oncology: State of the art. Diagn Interv Imaging. (2022) 103:499–509. doi: 10.1016/j.diii.2022.09.009
9. Arciga BM, Walters DM, Kimchi ET, Staveley-O'Carroll KF, Li G, Teixeiro E, et al. Pulsed electric field ablation as a candidate to enhance the anti-tumor immune response to immune checkpoint inhibitors. Cancer Lett. (2025) 609:217361. doi: 10.1016/j.canlet.2024.217361
10. Batista Napotnik T, Polajžer T, and Miklavčič D. Cell death due to electroporation - A review. Bioelectrochemistry. (2021) 141:107871. doi: 10.1016/j.bioelechem.2021.107871
11. Zhang N, Li Z, Han X, Zhu Z, Li Z, Zhao Y, et al. Irreversible electroporation: an emerging immunomodulatory therapy on solid tumors. Front Immunol. (2021) 12:811726. doi: 10.3389/fimmu.2021.811726
12. Kwon W, Thomas A, and Kluger MD. Irreversible electroporation of locally advanced pancreatic cancer. Semin Oncol. (2021) 48:84–94. doi: 10.1053/j.seminoncol.2021.02.004
13. Hay AN, Aycock KN, Lorenzo MF, David K, Coutermarsh-Ott S, Salameh Z, et al. Investigation of high frequency irreversible electroporation for canine spontaneous primary lung tumor ablation. Biomedicines. (2024) 12(9):2038. doi: 10.3390/biomedicines12092038
14. Liu X, Wang H, Zhao Z, Zhong Q, Wang X, Liu X, et al. Advances in irreversible electroporation for prostate cancer. Discov Oncol. (2024) 15:713. doi: 10.1007/s12672-024-01570-4
15. Shi X, O'Neill C, Wang X, Chen Y, Yu Y, Tan M, et al. Irreversible electroporation enhances immunotherapeutic effect in the off-target tumor in a murine model of orthotopic HCC. Am J Cancer Res. (2021) 11:3304–19.
16. Taibi A, Perrin ML, Albouys J, Jacques J, Yardin C, Durand-Fontanier S, et al. 10 ns PEFs induce a histological response linked to cell death and cytotoxic T-lymphocytes in an immunocompetent mouse model of peritoneal metastasis. Clin Transl Oncol. (2021) 23:1220–37. doi: 10.1007/s12094-020-02525-1
17. Yao C, Ning J, Liu H, Lv Y, Zhao Y, and Dong S. Nanosecond pulses targeting intracellular ablation increase destruction of tumor cells with irregular morphology. Bioelectrochemistry. (2020) 132:107432. doi: 10.1016/j.bioelechem.2019.107432
18. Michel O, Błasiak P, Saczko J, Kulbacka J, Drąg-Zalesińska M, and Rzechonek A. Electropermeabilization of metastatic chondrosarcoma cells from primary cell culture. Biotechnol Appl Biochem. (2019) 66:945–54. doi: 10.1002/bab.1809
19. Imran KM, Brock RM, Beitel-White N, Powar M, Orr K, Aycock KN, et al. Irreversible electroporation promotes a pro-inflammatory tumor microenvironment and anti-tumor immunity in a mouse pancreatic cancer model. Front Immunol. (2024) 15:1352821. doi: 10.3389/fimmu.2024.1352821
20. Geboers B, Scheltema MJ, Jung J, Bakker J, Timmer FEF, Cerutti X, et al. Irreversible electroporation of localised prostate cancer downregulates immune suppression and induces systemic anti-tumour T-cell activation - IRE-IMMUNO study. BJU Int. (2025) 135:319–28. doi: 10.1111/bju.16496
21. Heller R, Jaroszeski M, Perrott R, Messina J, and Gilbert R. Effective treatment of B16 melanoma by direct delivery of bleomycin using electrochemotherapy. Melanoma Res. (1997) 7:10–8. doi: 10.1097/00008390-199702000-00003
22. Rols MP, Bachaud JM, Giraud P, Chevreau C, Roché H, and Teissié J. Electrochemotherapy of cutaneous metastases in Malignant melanoma. Melanoma Res. (2000) 10:468–74. doi: 10.1097/00008390-200010000-00009
23. Costa FP, Tuszynski J, Iemma AF, Trevizan WA, Wiedenmann B, and Schöll E. External low energy electromagnetic fields affect heart dynamics: surrogate for system synchronization, chaos control and cancer patient's health. Front Netw Physiol. (2024) 4:1525135. doi: 10.3389/fnetp.2024.1525135
24. Tian L, Wang L, Qiao Y, Lu L, Lee P, Chang A, et al. Antitumor efficacy of liposome-encapsulated NVP-BEZ235 combined with irreversible electroporation for head and neck cancer. Molecules. (2019) 24(19):3560. doi: 10.3390/molecules24193560
25. Morozas A, Malyško-Ptašinskė V, Kulbacka J, Ivaška J, Ivaškienė T, and Novickij V. Electrochemotherapy for head and neck cancers: possibilities and limitations. Front Oncol. (2024) 14:1353800. doi: 10.3389/fonc.2024.1353800
26. Yilmaz M, Karaaslan M, Şirin ME, Aybal H, Polat ME, Tonyali S, et al. Salvage irreversible electroporation for locally recurrent prostate cancer after definitive radiotherapy: a systematic review. Prostate Cancer Prostatic Dis. (2024) 28(3):707–17. doi: 10.1038/s41391-024-00926-9
27. Peng H, Shen J, Long X, Zhou X, Zhang J, Xu X, et al. Local release of TGF-β Inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci (Weinh). (2022) 9:e2105240. doi: 10.1002/advs.202105240
28. Zhao J, Chen S, Zhu L, Zhang L, Liu J, Xu D, et al. Antitumor effect and immune response of nanosecond pulsed electric fields in pancreatic cancer. Front Oncol. (2020) 10:621092. doi: 10.3389/fonc.2020.621092
29. Pastori C, Nafie EHO, Wagh MS, Mammarappallil JG, and Neal RE. 2nd, pulsed electric field ablation versus radiofrequency thermal ablation in murine breast cancer models: anticancer immune stimulation, tumor response, and abscopal effects. J Vasc Interv Radiol. (2024) 35:442–451.e7. doi: 10.1016/j.jvir.2023.11.021
30. He C, Huang X, Zhang Y, Lin X, and Li S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin Transl Med. (2020) 10:e39. doi: 10.1002/ctm2.39
31. Shankara Narayanan JS, Hayashi T, Erdem S, McArdle S, Tiriac H, Ray P, et al. Treatment of pancreatic cancer with irreversible electroporation and intratumoral CD40 antibody stimulates systemic immune responses that inhibit liver metastasis in an orthotopic model. J Immunother Cancer. (2023) 11(1):e006133. doi: 10.1136/jitc-2022-006133
32. He C, Sun S, Zhang Y, Xie F, and Li S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology. (2021) 10:1897295. doi: 10.1080/2162402X.2021.1897295
33. Zhang QW, Guo XX, Zhou Y, Wang QB, Liu Q, Wu ZY, et al. OX40 agonist combined with irreversible electroporation synergistically eradicates established tumors and drives systemic antitumor immune response in a syngeneic pancreatic cancer model. Am J Cancer Res. (2021) 11:2782–801.
34. Imran KM, Nagai-Singer MA, Brock RM, Alinezhadbalalami N, Davalos RV, and Allen IC. Exploration of novel pathways underlying irreversible electroporation induced anti-tumor immunity in pancreatic cancer. Front Oncol. (2022) 12:853779. doi: 10.3389/fonc.2022.853779
35. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, and Zhang Z. Irreversible electroporation ablation overcomes tumor-associated immunosuppression to improve the efficacy of DC vaccination in a mice model of pancreatic cancer. Oncoimmunology. (2021) 10:1875638. doi: 10.1080/2162402X.2021.1875638
36. Wu Z, Shan Q, Jiang Y, Huang W, Wang Z, Zhuang Y, et al. Irreversible electroporation combined with PD-L1/IL-6 dual blockade promotes anti-tumor immunity via cDC2/CD4(+)T cell axis in MHC-I deficient pancreatic cancer. Cancer Lett. (2025) 617:217620. doi: 10.1016/j.canlet.2025.217620
37. Huang T, Wen X, Liang Y, Liu X, Zhao J, and Long X. Irreversible electroporation-induced inflammation facilitates neutrophil-mediated drug delivery to enhance pancreatic cancer therapy. Mol Pharm. (2024) 21:1998–2011. doi: 10.1021/acs.molpharmaceut.4c00006
38. Zhao J, Lu H, Xu D, Sun R, Fang C, Zhao Q, et al. Neutrophil membrane-coated nanoparticles for enhanced nanosecond pulsed electric field treatment of pancreatic cancer. Int J Hyperthermia. (2022) 39:1026–35. doi: 10.1080/02656736.2022.2093994
39. Long X, Dai A, Huang T, Niu W, Liu L, Xu H, et al. Simultaneous delivery of dual inhibitors of DNA damage repair sensitizes pancreatic cancer response to irreversible electroporation. ACS Nano. (2023) 17:12915–32. doi: 10.1021/acsnano.3c05009
40. Ma Y, Xing Y, Li H, Yuan T, Liang B, Li R, et al. Irreversible electroporation combined with chemotherapy and PD-1/PD-L1 blockade enhanced antitumor immunity for locally advanced pancreatic cancer. Front Immunol. (2023) 14:1193040. doi: 10.3389/fimmu.2023.1193040
41. Liang YY, Lu Z, Liu HW, Huang Q, Zheng XT, Li XA, et al. Anti-tumor effects of nanosecond pulsed electric fields in a murine model of pancreatic cancer. Bioelectrochemistry. (2025) 161:108803. doi: 10.1016/j.bioelechem.2024.108803
42. Szlasa W, Michel O, Sauer N, Novickij V, Lewandowski D, Kasperkiewicz P, et al. Nanosecond pulsed electric field suppresses growth and reduces multi-drug resistance effect in pancreatic cancer. Sci Rep. (2023) 13:351. doi: 10.1038/s41598-023-27605-4
43. Zhao J, Xu M, Sun R, Zhao J, Zhao Q, Wang Y, et al. Single-cell analysis reveals nanosecond pulsed electric field ablation induced myeloid cells remodeling in pancreatic cancer. Bioelectrochemistry. (2022) 148:108266. doi: 10.1016/j.bioelechem.2022.108266
44. Duncan JL, Ahmad RN, Danesi H, Slade DJ, Davalos RV, and Verbridge SS. Electro-antibacterial therapy (EAT) to enhance intracellular bacteria clearance in pancreatic cancer cells. Bioelectrochemistry. (2024) 157:108669. doi: 10.1016/j.bioelechem.2024.108669
45. Figini M, Zhou K, Pan L, Sun C, Wang B, Hu S, et al. Transcatheter intra-arterial perfusion (TRIP)-MRI biomarkers help detect immediate response to irreversible electroporation of rabbit VX2 liver tumor. Magn Reson Med. (2020) 84:365–74. doi: 10.1002/mrm.28104
46. Lindelauf KHK, Baragona M, Baumann M, Maessen RTH, and Ritter A. Pulse parameters and thresholds for (ir)Reversible electroporation on hepatocellular carcinoma cells in vitro. Technol Cancer Res Treat. (2023) 22:15330338221136694. doi: 10.1177/15330338221136694
47. Li QG, Liu ZG, Dong G, Sun Y, Zou YW, Chen XL, et al. Nanosecond pulsed electric field ablates rabbit VX2 liver tumors in a non-thermal manner. PloS One. (2023) 18:e0273754. doi: 10.1371/journal.pone.0273754
48. Hu S, Zhu Y, Chen Y, Cheng P, Wang GR, Wang LQ, et al. Nanosecond pulsed electric field interrupts the glycogen metabolism in hepatocellular carcinoma by modifying the osteopontin pathway. Hepatobiliary Pancreat Dis Int. (2022) 21:199–201. doi: 10.1016/j.hbpd.2021.12.001
49. Liu J, Chen X, and Zheng S. Immune response triggered by the ablation of hepatocellular carcinoma with nanosecond pulsed electric field. Front Med. (2021) 15:170–7. doi: 10.1007/s11684-020-0747-z
50. Qian J, Chen T, Wu Q, Zhou L, Zhou W, Wu L, et al. Blocking exposed PD-L1 elicited by nanosecond pulsed electric field reverses dysfunction of CD8(+) T cells in liver cancer. Cancer Lett. (2020) 495:1–11. doi: 10.1016/j.canlet.2020.09.015
51. Yimingjiang M, Tuergan T, Chen X, Wen H, Shao Y, Zhang R, et al. Comparative analysis of immunoactivation by nanosecond pulsed electric fields and PD-1 blockade in murine hepatocellular carcinoma. Anal Cell Pathol (Amst) 2020. (2020) p:9582731. doi: 10.1155/2020/9582731
52. Dai Z, Wang Z, Lei K, Liao J, Peng Z, Lin M, et al. Irreversible electroporation induces CD8(+) T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. (2021) 503:1–10. doi: 10.1016/j.canlet.2021.01.001
53. Liu J, Fang C, Jin X, Tian G, Sun Z, Hong L, et al. Nanosecond pulsed electric field ablation-induced modulation of sphingolipid metabolism is associated with Ly6c2(+) mononuclear phagocyte differentiation in liver cancer. Mol Oncol. (2023) 17:1093–111. doi: 10.1002/1878-0261.13372
54. Xu M, Xie LT, Xiao YY, Liang P, Zhao QY, Wang ZM, et al. Chinese clinical practice guidelines for ultrasound-guided irreversible electroporation of liver cancer (version 2022). Hepatobiliary Pancreat Dis Int. (2022) 21:462–71. doi: 10.1016/j.hbpd.2022.08.006
55. Zeng C, Hua S, Zhou J, Zeng T, Chen J, Su L, et al. Oral microalgae-based biosystem to enhance irreversible electroporation immunotherapy in hepatocellular carcinoma. Adv Sci (Weinh). (2025) 12:e2409381. doi: 10.1002/advs.202409381
56. Wang Y, Ma R, Huang Z, Zhou Y, Wang K, Xiao Z, et al. Investigation of lethal thresholds of nanosecond pulsed electric field in rabbit VX2 hepatic tumors through finite element analysis and verification with a single-needle bipolar electrode: A prospective strategy employing three-dimensional comparisons. Comput Biol Med. (2024) 168:107824. doi: 10.1016/j.compbiomed.2023.107824
57. Xu M, Zhang W, Xu D, Dong G, Ren Z, Aji T, et al. Nanosecond pulsed electric field ablation as first-line curative therapy for hepatocellular carcinoma in high-risk locations a prospective multicenter. Int J Surg. (2025) 111:3289–98. doi: 10.1097/JS9.0000000000002361
58. Zou Y, Sun Y, Chen X, Hong L, Dong G, Bai X, et al. Nanosecond pulse effectively ablated hepatocellular carcinoma with alterations in the gut microbiome and serum metabolites. Front Pharmacol. (2023) 14:1163628. doi: 10.3389/fphar.2023.1163628
59. Burbach BJ, O'Flanagan SD, Shao Q, Young KM, Slaughter JR, Rollins MR, et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat Commun. (2021) 12:3862. doi: 10.1038/s41467-021-24132-6
60. Kiełbik A, Szlasa W, Novickij V, Szewczyk A, Maciejewska M, Saczko J, et al. Effects of high-frequency nanosecond pulses on prostate cancer cells. Sci Rep. (2021) 11:15835. doi: 10.1038/s41598-021-95180-7
61. Kim HB, Zeng CH, Kim Y, Jeong S, Kim SH, Kang JM, et al. Effects of different applied voltages of irreversible electroporation on prostate cancer in a mouse model. Sci Rep. (2022) 12:22336. doi: 10.1038/s41598-022-25258-3
62. Kim SH, Kang JM, Park Y, Kim Y, Lim B, and Park JH. Effects of bipolar irreversible electroporation with different pulse durations in a prostate cancer mouse model. Sci Rep. (2024) 14:9902. doi: 10.1038/s41598-024-60413-y
63. Murat C, Kaya A, Kaya D, and Erdoğan MA. Experimental study for in vitro prostate cancer treatment with microwave ablation and pulsed electromagnetic field. Electromagn Biol Med. (2024) 43:135–44. doi: 10.1080/15368378.2024.2345606
64. Zhou H, Wang Z, Dong Y, Alhaskawi A, Tu T, Hasan Abdullah Ezzi S, et al. New advances in treatment of skin Malignant tumors with nanosecond pulsed electric field: A literature review. Bioelectrochemistry. (2023) 150:108366. doi: 10.1016/j.bioelechem.2023.108366
65. Rossi A, Pakhomova ON, Pakhomov AG, Weygandt S, Bulysheva AA, Murray LE, et al. Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Sci Rep. (2019) 9:431. doi: 10.1038/s41598-018-36527-5
66. Sauer N, Szlasa W, Szewczyk A, Novickij V, Saczko J, Baczyńska D, et al. Effects of nanosecond pulsed electric field on immune checkpoint receptors in melanoma cells. Pharm (Basel). (2023) 16(10):1362. doi: 10.3390/ph16101362
67. McDaniel A, Freimark B, Navarro C, Von Rothstein K, Gonzalez D, Linder K, et al. Nano-pulse stimulation™ therapy (NPS™) is superior to cryoablation in clearing murine melanoma tumors. Front Oncol. (2022) 12:948472. doi: 10.3389/fonc.2022.948472
68. Mi Y, Dai L, Xu N, Zheng W, Ma C, Chen W, et al. Viability inhibition of A375 melanoma cellsin vitroby a high-frequency nanosecond-pulsed magnetic field combined with targeted iron oxide nanoparticles via membrane magnetoporation. Nanotechnology. (2021) 32(38). doi: 10.1088/1361-6528/ac0caf
69. Fesmire CC, Peal B, Ruff J, Moyer E, McParland TJ, Derks K, et al. Investigation of integrated time nanosecond pulse irreversible electroporation against spontaneous equine melanoma. Front Vet Sci. (2024) 11:1232650. doi: 10.3389/fvets.2024.1232650
70. Paganin-Gioanni A, Rols MP, Teissié J, and Golzio M. Cyclin B1 knockdown mediated by clinically approved pulsed electric fields siRNA delivery induces tumor regression in murine melanoma. Int J Pharm. (2020) 573:118732. doi: 10.1016/j.ijpharm.2019.118732
71. Szlasa W, Sauer N, Baczyńska D, Ziętek M, Haczkiewicz-Leśniak K, Karpiński P, et al. Pulsed electric field induces exocytosis and overexpression of MAGE antigens in melanoma. Sci Rep. (2024) 14:12546. doi: 10.1038/s41598-024-63181-x
72. Knight DB, Hillard H, Handran C, and Kuhlman P. Pulsed electric field (PEF) treatment of refractory vulvar melanoma concurrently treated with immune checkpoint blockade demonstrating an abscopal response: A case report. Gynecol Oncol Rep. (2025) 57:101671. doi: 10.1016/j.gore.2024.101671
73. Jenkins EPW, Finch A, Gerigk M, Triantis IF, Watts C, and Malliaras GG. Electrotherapies for glioblastoma. Adv Sci (Weinh). (2021) 8:e2100978. doi: 10.1002/advs.202100978
74. Casciati A, Tanori M, Gianlorenzi I, Rampazzo E, Persano L, Viola G, et al. Effects of ultra-short pulsed electric field exposure on glioblastoma cells. Int J Mol Sci. (2022) 23(6):3001. doi: 10.3390/ijms23063001
75. Murphy KR, Aycock KN, Hay AN, Rossmeisl JH, Davalos RV, and Dervisis NG. High-frequency irreversible electroporation brain tumor ablation: exploring the dynamics of cell death and recovery. Bioelectrochemistry. (2022) 144:108001. doi: 10.1016/j.bioelechem.2021.108001
76. Campelo SN, Lorenzo MF, Partridge B, Alinezhadbalalami N, Kani Y, Garcia J, et al. High-frequency irreversible electroporation improves survival and immune cell infiltration in rodents with Malignant gliomas. Front Oncol. (2023) 13:1171278. doi: 10.3389/fonc.2023.1171278
77. Luo Z, Guo F, Xiang S, Dong S, Yao C, and Liu H. Nanosecond pulsed bipolar cancellation of the killing effect on glioblastoma. IEEE Trans BioMed Eng. (2025) 72:2138–46. doi: 10.1109/TBME.2025.3536477
78. Qian K, Yao C, Wang Y, Yang Q, Xiang S, Pei Q, et al. Potential of ultrashort pulsed electric fields to disrupt dense structure in glioma tumors. IEEE Trans BioMed Eng. (2025) 72(11):3233–43. doi: 10.1109/TBME.2025.3565520
79. Abd-Elghany AA. Incorporation of electroendocytosis and nanosecond pulsed electric field in electrochemotherapy of breast cancer cells. Electromagn Biol Med. (2022) 41:25–34. doi: 10.1080/15368378.2021.1978479
80. Rembiałkowska N, Novickij V, Baczyńska D, Dubińska-Magiera M, Saczko J, Rudno-Rudzińska J, et al. Micro- and nanosecond pulses used in doxorubicin electrochemotherapy in human breast and colon cancer cells with drug resistance. Molecules. (2022) 27(7):2052. doi: 10.3390/molecules27072052
81. Xu Z, Pan C, Chen L, Qian J, Chen X, Zhou L, et al. Nanosecond pulsed electric field induces an antitumor effect in triple-negative breast cancer via CXCL9 axis dependence in mice. Cancers (Basel). (2023) 15(7):2076. doi: 10.3390/cancers15072076
82. Awasthi K, Huang WC, Wei CY, Hsu HY, and Ohta N. Unveiling the susceptibility of nanosecond pulsed electric field on intracellular function in breast cancerous and normal cells using fluorescence imaging. Biosens Bioelectron. (2025) 272:117129. doi: 10.1016/j.bios.2025.117129
83. Esparza S, Jacobs E, Hammel JH, Michelhaugh SK, Alinezhadbalalami N, Nagai-Singer M, et al. Transient lymphatic remodeling follows sub-ablative high-frequency irreversible electroporation therapy in a 4T1 murine model. Ann BioMed Eng. (2025) 53:1148–64. doi: 10.1007/s10439-024-03674-y
84. Safaei Z and Thompson GL. Caspase-dependent cell death and HDAC4 translocation following microsecond pulsed electric field (μsPEF) exposure in MCF-7 breast cancer cells. Bioelectromagnetics. (2025) 46:e70009. doi: 10.1002/bem.70009
85. Rembiałkowska N, Kucharczyk J, Radzevičiūtė-Valčiukė E, Novickij V, Tonci M, Dündar A, et al. Enhancing lung cancer growth inhibition with calcium ions: Role of mid- and high-frequency electric field pulses. BioMed Pharmacother. (2024) 181:117691. doi: 10.1016/j.biopha.2024.117691
86. Jimenez M, Flandes J, van der Heijden E, Ng CSH, Iding JS, Garcia-Hierro JF, et al. Safety and feasibility of pulsed electric field ablation for early-stage non-small cell lung cancer prior to surgical resection. J Surg Oncol. (2025) (8):1529–42. doi: 10.1002/jso.28110
87. Moore WH, Silk M, Bhattacharji P, Pua BB, Mammarappallil J, Sterman DH, et al. Early experience with PEF in the setting of recalcitrant stage IV lung cancer. Lung Cancer. (2025) 204:108575. doi: 10.1016/j.lungcan.2025.108575
88. Novickij V, Rembiałkowska N, Kasperkiewicz-Wasilewska P, Baczyńska D, Rzechonek A, Błasiak P, et al. Pulsed electric fields with calcium ions stimulate oxidative alternations and lipid peroxidation in human non-small cell lung cancer. Biochim Biophys Acta Biomembr. (2022) 1864:184055. doi: 10.1016/j.bbamem.2022.184055
89. Kulbacka J, Rembiałkowska N, Szewczyk A, Rossowska J, Drąg-Zalesińska M, Kulbacki M, et al. Nanosecond PEF induces oxidative stress and apoptosis via proteasomal activity inhibition in gastric adenocarcinoma cells with drug resistance. Int J Mol Sci. (2022) 23(21):12943. doi: 10.3390/ijms232112943
90. Rossi A, Pakhomova ON, Mollica PA, Casciola M, Mangalanathan U, Pakhomov AG, et al. Nanosecond pulsed electric fields induce endoplasmic reticulum stress accompanied by immunogenic cell death in murine models of lymphoma and colorectal cancer. Cancers (Basel). (2019) 11(12):2034. doi: 10.3390/cancers11122034
91. Gu Y, Zhang L, Yang H, Zhuang J, Sun Z, Guo J, et al. Nanosecond pulsed electric fields impair viability and mucin expression in mucinous colorectal carcinoma cell. Bioelectrochemistry. (2021) 141:107844. doi: 10.1016/j.bioelechem.2021.107844
92. Liu H, Zhao Y, Yao C, Schmelz EM, and Davalos RV. Differential effects of nanosecond pulsed electric fields on cells representing progressive ovarian cancer. Bioelectrochemistry. (2021) 142:107942. doi: 10.1016/j.bioelechem.2021.107942
93. Łapińska Z, Novickij V, Rembiałkowska N, Szewczyk A, Dubińska-Magiera M, Kulbacka J, et al. The influence of asymmetrical bipolar pulses and interphase intervals on the bipolar cancellation phenomenon in the ovarian cancer cell line. Bioelectrochemistry. (2023) 153:108483. doi: 10.1016/j.bioelechem.2023.108483
94. Liu Z, Zou Y, Sun Y, Chen X, Chen X, and Ren Z. Effects of nanosecond pulsed electric fields in cell vitality, apoptosis, and proliferation of TPC-1 cells. Anal Cell Pathol (Amst) 2021. (2021) p:9913716. doi: 10.1155/2021/9913716
95. Yao C, Mi Y, Hu X, Li C, Sun C, Tang J, et al. Experiment and mechanism research of SKOV3 cancer cell apoptosis induced by nanosecond pulsed electric field. Annu Int Conf IEEE Eng Med Biol Soc. (2008) 2008:1044–7. doi: 10.1109/IEMBS.2008.4649338
96. Yu S, Chen L, Song K, Shu T, Fang Z, Ding L, et al. Irreversible electroporation mediates glioma apoptosis via upregulation of AP-1 and bim: transcriptome evidence. Brain Sci. (2022) 12(11):1465. doi: 10.3390/brainsci12111465
97. Jeon HJ, Chun HJ, Choi HS, Keum B, Kim HB, and Kim JH. Biphasic regulation of apoptosis following gastric irreversible electroporation using tissue immunohistochemistry of activated caspase-3 with TUNEL method. Cancers (Basel). (2024) 16(7):1389. doi: 10.3390/cancers16071389
98. Wang X, Hong T, Liu G, Rao J, Shi F, Wang H, et al. High-frequency irreversible electroporation suppresses invasion and metastasis by targeting SIRT1/2 in highly invasive tumor cells: an in vitro study. Bioelectrochemistry. (2025) 166:109036. doi: 10.1016/j.bioelechem.2025.109036
99. Ullery JC, Tarango M, Roth CC, and Ibey BL. Activation of autophagy in response to nanosecond pulsed electric field exposure. Biochem Biophys Res Commun. (2015) 458:411–7. doi: 10.1016/j.bbrc.2015.01.131
100. Ye CF, Wu JD, Li LR, Sun SG, Wang YG, Jiang TA, et al. Co-inhibition of RAGE and TLR4 sensitizes pancreatic cancer to irreversible electroporation in mice by disrupting autophagy. Acta Pharmacol Sin. (2025) 46:1757–71. doi: 10.1038/s41401-025-01487-w
101. Wiczew D, Szulc N, and Tarek M. Molecular dynamics simulations of the effects of lipid oxidation on the permeability of cell membranes. Bioelectrochemistry. (2021) 141:107869. doi: 10.1016/j.bioelechem.2021.107869
102. Szlasa W, Mazurek W, Szewczyk A, Rembiałkowska N, Tunikowska J, and Kulbacka J. The antagonistic and synergistic role of fe(3+) compounds in chemo- and electrochemotherapy in human colon cancer in vitro. Pharm (Basel). (2024) 17(5):651. doi: 10.3390/ph17050651
103. Hall EH, Schoenbach KH, and Beebe SJ. Nanosecond pulsed electric fields have differential effects on cells in the S-phase. DNA Cell Biol. (2007) 26:160–71. doi: 10.1089/dna.2006.0514
104. Xu W, Li S, Shan X, Wang Q, Chen X, Wu S, et al. Targeting tumor-associated CCR2(+) macrophages to inhibit pancreatic cancer recurrence following irreversible electroporation. Sci Adv. (2025) 11:eadw2937. doi: 10.1126/sciadv.adw2937
105. Li R, Niu G, She Y, Li R, Yuan M, Pei Z, et al. Enhanced tumor ablation and immune activation via irreversible electroporation and functionalized vermiculite nanosheets. Small. (2025) 21:e2411879. doi: 10.1002/smll.202411879
106. Timmer FEF, Geboers B, Scheffer HJ, Bakker J, Ruarus AH, Dijkstra M, et al. Tissue resistance decrease during irreversible electroporation of pancreatic cancer as a biomarker for the adaptive immune response and survival. J Vasc Interv Radiol. (2023) 34:1777–1784.e4. doi: 10.1016/j.jvir.2023.06.027
107. Mazzarda F, Chittams-Miles AE, Pittaluga J, Sözer EB, Vernier PT, and Muratori C. Inflammasome activation and IL-1β Release triggered by nanosecond pulsed electric fields in murine innate immune cells and skin. J Immunol. (2024) 212:335–45. doi: 10.4049/jimmunol.2200881
108. Guo X, Du F, Liu Q, Guo Y, Wang Q, Huang W, et al. Immunological effect of irreversible electroporation on hepatocellular carcinoma. BMC Cancer. (2021) 21:443. doi: 10.1186/s12885-021-08176-x
109. O'Neill CH, Tan M, Yan J, Li Y, and Martin RCG. Perioperative systemic immunophenotype following irreversible electroporation (IRE) predicts recurrence. Am J Cancer Res. (2022) 12:165–75.
110. Xia ZY, Xiang JC, Xu JZ, Sun JX, Wang S, and Xia QD. Irreversible electroporation synergizes with oncolytic virus enhances the infiltration of cytotoxic T lymphocytes in the tumor immune microenvironment: a leap from focal therapy to immunotherapy for prostate cancer. J Immunother Cancer. (2025) 13(4):e009794. doi: 10.1136/jitc-2024-009794
111. Liu X, Zhuang Y, Huang W, Wu Z, Chen Y, Shan Q, et al. Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy. Nat Commun. (2023) 14:4106. doi: 10.1038/s41467-023-39759-w
112. Yun JH, Fang A, Khorshidi F, Habibollahi P, Kutsenko O, Etezadi V, et al. New developments in image-guided percutaneous irreversible electroporation of solid tumors. Curr Oncol Rep. (2023) 25:1213–26. doi: 10.1007/s11912-023-01452-y
113. Fang Z, Chen L, Moser MAJ, Zhang W, Qin Z, and Zhang B. Electroporation-based therapy for brain tumors: A review. J Biomech Eng. (2021) 143(10):100802. doi: 10.1115/1.4051184
Keywords: pulsed electric fields, cancer, irreversible electroporation, immunotherapy, mechanism of action, clinical prospects
Citation: Zhang L, Dong S, Teng F, Wang Y, Xu W, Chen Y, Yu L, Yao C and Wang Z (2025) Pulsed electric fields: a sharp sword in the battle against cancers. Front. Immunol. 16:1682539. doi: 10.3389/fimmu.2025.1682539
Received: 09 August 2025; Accepted: 03 November 2025;
Published: 20 November 2025.
Edited by:
Xing Li, Nanjing University of Aeronautics and Astronautics, ChinaReviewed by:
Minmin Wang, Westlake University, ChinaXinsheng Yang, Hebei University of Technology, China
Copyright © 2025 Zhang, Dong, Teng, Wang, Xu, Chen, Yu, Yao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Zhang, bHV6aGFuZ181MjRAYWxpeXVuLmNvbQ==; Liang Yu, eXVfbGlhbmdAY3F1LmVkdS5jbg==; Chenguo Yao, eWFvY2hlbmd1b0BjcXUuZWR1LmNu; Zhiqiang Wang, d2FuZ3poaXFpYW5nQGNxdS5lZHUuY24=
†These authors have contributed equally to this work
 Lu Zhang
Lu Zhang Shoulong Dong
Shoulong Dong Fei Teng1,2†
Fei Teng1,2† Chenguo Yao
Chenguo Yao