- National Key Laboratory of Veterinary Public Health and Safety, College of Veterinary Medicine, China Agricultural University, Beijing, China
Humoral Immunity plays an important role during Mycobacterium tuberculosis(Mtb) infection. In mouse models, polyvalent and monoclonal antibodies targeting Mtb provided some protection against tuberculosis (TB). The five human antibody isotypes (IgG, IgM, IgA, IgE, and IgD) mediate an array of functional activities against bacterial infections, including neutralization, antibody-dependent cellular cytotoxicity (ADCC), phagocytosis, and complement activation. Different antibody isotypes have functions through different protective mechanisms based on the biological structures and pathways involved. In this review, we summarize the research progress on the different isotypes of antibodies against TB, and discuss the antibody-based strategies against tuberculosis, the potentiality of antibodies in TB diagnosis, and suggest further research directions, including investigating the mechanisms of different isotypes of antibody-mediated protection against TB, identifying correlates of immunity, and novel vaccines development.
Introduction
Tuberculosis (TB) is still a leading cause of death from a single infectious disease. There were about 10.8 million cases of active TB globally, with 1.25 million deaths in 2023 (1). Currently, the only established TB preventive vaccine, Bacillus Calmettee-Guérin (BCG), because of its variable efficacy in adults, is not good enough to control TB despite its widespread use. Therefore, effective TB drugs and vaccines are the most urgent needs for TB control and treatment.
The mammalian immune system has innate and adaptive components including macrophages, dendritic cells, T cells, and B cells etc., which can cooperate to protect the host against bacterial infections (2, 3). In humans, the adaptive immune responses, including cellular and humoral immunity to Mtb, play an important role in the outcome of Mtb infection and disease progression (3–5). After Mtb enters the body, T cells and B cells can collaborate to control infection and direct the adaptive immune response. B cells have the functions to present Mtb antigens to activate T helper cells, which in turn stimulate B cells to produce antibodies and become memory B cells (6–8). Moreover, both cell types can produce cytokines, which can help regulate the immune responses to TB (6–8). Therefore, understanding the B-cell responses and protective roles of different antibody types in TB challenges the long-held dogma that cellular immunity alone is sufficient to fight Mtb infections.
In the late nineteenth century, it was thought that the animal’s serum therapy was effective in treating tuberculosis patients, but later the antibody effects were considered insignificant because of the inconsistent trial results (9, 10). There is now accumulated evidence that antibodies contribute to the prevention of M. tuberculosis infection and the progression of tuberculosis (11–19). However, evidence from passive monoclonal antibody (mAb) studies does not necessarily reflect the protective humoral immunity developed during a natural Mtb infection. Blocking of Th2 cytokines IL-4 can enhance host resistance and passive IgA protection against tuberculosis (20, 21). Understanding the antibodies’ roles, the targeting antigens, and the protective mechanisms will help us develop more effective diagnostics and novel TB vaccines in a more rational way (22). In this review, we summarize the research progress about antibody subclasses, various antibody effector functions, the antibodies in TB diagnosis, and raise important and potential scientific questions for TB antibody research in future studies, which can be a reference and help in the design and development of novel vaccines and therapeutics.
Antibody isotypes and subclasses
The antibody is a Y-shaped or T-shaped heterodimeric protein consisting of two heavy chains and two light chains, which are secreted by the plasma B cells (23). B cell receptor (BCR) -ligand interactions play a critical role in regulating B cell behavior, and primary B cell development and survival (24). The antibodies are further classified into five isotypes, named IgM, IgA, IgG, IgE, and IgD, depending on the unique constant region of the antibody (25) (Figures 1A, B). Major antibody isotypes display distinct serum abundances and half-lives: IgG (70–80% of total serum) has a ~21-day half-life; IgA (~15%) shows a short monomeric half-life (~1 day) but a longer secretory dimer half-life (~5–6 days); IgM (~10%) has a ~5-day half-life; IgD (<0.5%) and IgE (<0.01%) have short half-lives (~2–3 days and ~2 days, respectively) (26–28). Different quantities of antibodies and persistence can also affect immunological effects. For example, the extended half-life of IgG, mediated by FcRn recycling, is why this class is responsible for long-term immunity following vaccination or infection (26–28). The differences in antibodies’ constant regions could be reflected in the fact that each antibody isotype plays different roles throughout the infection process (29, 30). The antibodies have considerable diversity in the location and number of the conserved N-linked glycosylation sites that are located at the Fc as well as Fab regions. For IgG, it bears a single N-linked glycosylation site at asparagine 297 (N297) of each heavy chain and has shown importance in antibodies’ functions. The antibodies’ hinge region can contain N- and O-linked glycans (31, 32) (Figure 1A). IgG is the most important isotype in blood and extracellular fluids, while IgA is mainly secreted by plasma cells within mucosal membranes lining the intestines, airways, and reproductive tracts. IgG has a higher efficiency in regulating macrophage phagocytosis compared to IgA because of its functional location and easy access to T helper cells and molecules (33). IgA is the primary antibody protecting the mucosal surface, excelling at defending against invaders that can penetrate the mucosal barrier, with a unique tail structure that could resist acids and enzymes (34). IgE levels are very low in the blood and primarily bound to mast cell receptors located in the skin and submucosa. Antigen-binding IgE has induced mast cells to release chemicals to control pathogenic spread (35). Most antibodies are diffusely distributed throughout the body from the site of synthesis, while secretory IgA needs to be transported to the apical surface through the polymeric immunoglobulin receptor (pIgR) (36). Different isotypes of antibodies have been developed to work in various bodily regions. These antibody isotypes exhibit variable characteristics at different body locations to counteract pathogenic infections. Specifically, each antibody isotype has a specific structure that affects its function. For example, IgM, which exists as a pentamer, enhances antibody-antigen avidity in the form of multi-site binding and the ability to bind complement (37, 38). In the case of IgD, the heavy chain glycosylation leads to the formation of a T-shaped structure that increases the flexibility of the hinge region, which could well facilitate the epitope binding of the antigens and the synergistic action of IgM in the early stages of pathogenic infection (32, 39). IgD and IgG share the same basic structure but have a longer hinge region that is easily hydrolyzed by proteases (40). The other isotypes of antibodies, IgA and IgE, are smaller and can diffuse easily out of the blood into the tissues (40).
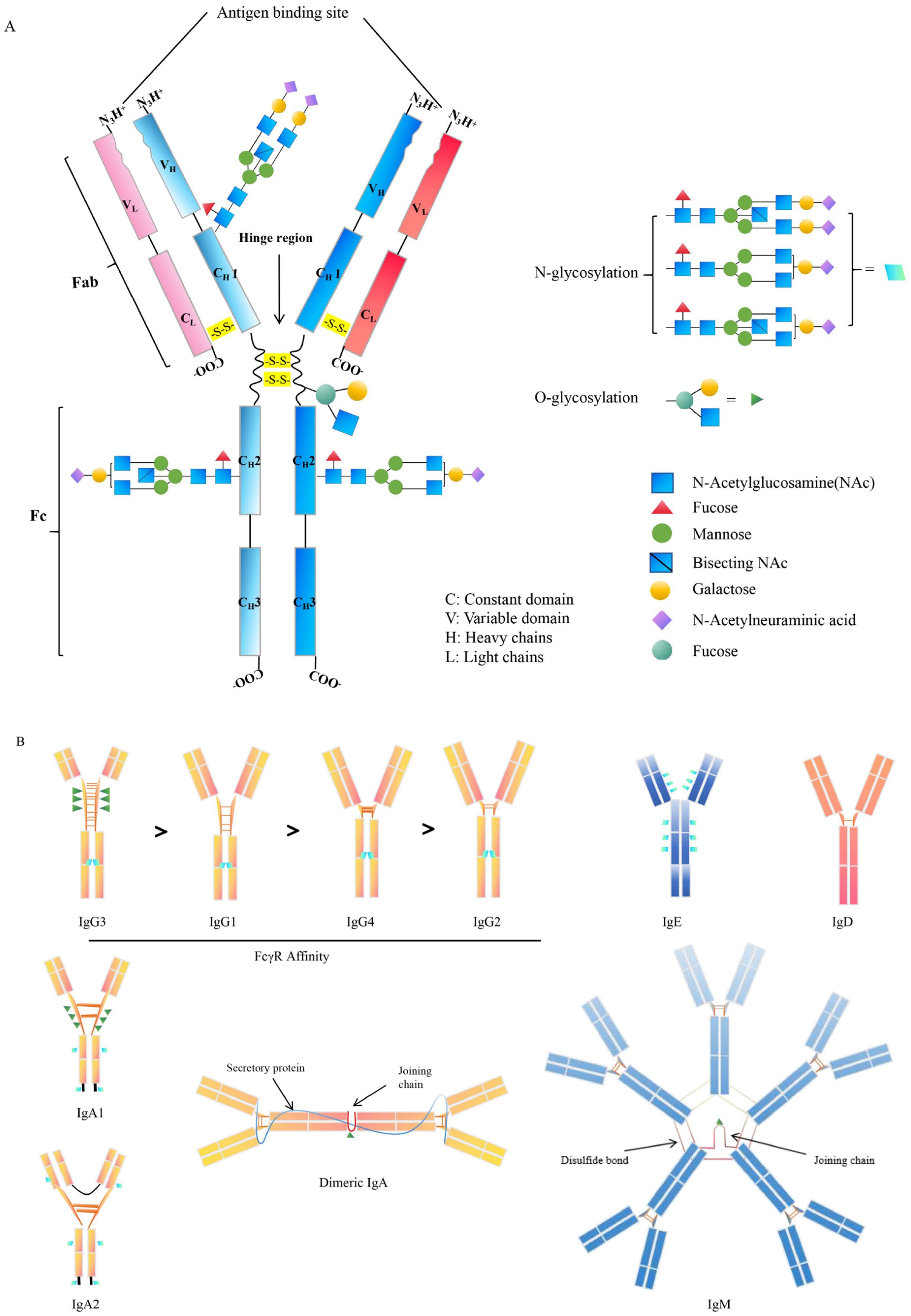
Figure 1. Structure of antibodies’ isotypes and subclasses. (A). The general structure of antibody. The variable glycosylated chains are distributed in different antibody fractions such as the Fab and Fc domain. (B). The structure and characteristics of antibody isotypes. The IgG is classed into 4 subclasses, including IgG1, IgG2, IgG3, and IgG4, according to the variations in heavy and light chain disulfide bonds. IgG subclass identified by FcγR affinity. In particular, IgG and IgE are only present as monomers, while the IgA exists either as monomers, such as IgA1 and IgA2 or as dimers. Additionally, IgM is solely presented as multimers, primarily as pentameric structures. N-glycosylation sites: ; O-glycosylation sites.
In addition to this, each antibody is further subdivided into several different subclasses based on the amino acid composition of the hinge region and the number and location of disulfide bonds (41). In mammals, there is only one type of IgM except for cattle (Bos taurus), which has two Igμ genes on the same chromosome and expresses two subclasses of IgM, IgM1 and IgM2 (42). Human IgG can be classified as IgG1, IgG2, IgG3, and IgG4, while mouse IgG is classified as IgG1, IgG2a/c, IgG2b, and IgG3. IgG1 is also the most promising subclass for tumor immunotherapy, and because human IgG1 is also able to bind effectively to the murine Fcγ receptor, significant effects can be observed in an in vivo mouse model (11, 43, 44) (Figure 1). IgG2 is mainly used to neutralize pathogen toxins or block the binding of receptor ligands, and its measured complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) effects have been shown to be very weak (45) (Table 1). IgG3 has the strongest binding capacity to FcγRs, triggers ADCC and antibody-dependent cell-mediated phagocytosis (ADCP), and has a stronger CDC effect than IgG1 (43–45) (Table 1). The hinge region of the IgG4 molecule is short, and its binding to FcγRs was weak (43, 55) (Figure 1). In humans, there are two subclasses of IgA, IgA1 and IgA2. Circulating monomeric immunoglobulin A (mIgA) and dimeric secretory IgA (sIgA) are two structures of IgA antibodies. Monomeric IgA is mostly IgA1 in the bloodstream and has functions as systemic immunity, while dimeric sIgA is the principal antibody of external secretions and the mucosal immune system (42). The differences between IgA1 and IgA2 are mainly in the structure of their hinge region and the number of glycosylation sites (57). In serum, the ratio of IgA1 and IgA2 is 9:1, whereas in mucosal tissues, IgA1 and IgA2 are evenly distributed. By contrast, mice have only one IgA isotype and lack a functional homolog to FcαRI, which is different from human beings (57) (Table 1). The studies indicate that IgA effector functions depend on subclass and glycosylation, and the balance of subclass distribution and IgA1/IgA2 ratio is associated with host immune responses and disease progression (58), and the mechanisms of these need to be further investigated.
Different cytokine stimulation in vitro can induce B cells to secrete different antibody subclasses: IFN-γ can induce the production of IgG2 (61), while IL-4 and IL-3 can induce the production of IgG4 (56), and IL-10 can act as a switch factor for IgG1 and IgG3 (51) (Table 1). Therefore, this area regarding the mechanisms of antibody subclass protection deserves to be explored in depth in the future.
The roles of antibody isotypes in TB prevention and therapies
The aerosol containing Mtb enters the human lungs and is phagocytosed by the resident alveolar macrophages (62–64). The tissue-resident alveolar macrophage can secrete IL-1 to activate interstitial macrophages for producing GM-CSF to activate monocyte-derived macrophages, which can lead to lung tissue damage (62–64) (Figure 2). Macrophages recognize the bacteria via different surface receptors, including complement receptors, Fc receptors, mannose receptors, and DC-SIGN receptor (65, 66). The phagosome and lysosome fusion is a critical pathway for inhibiting Mtb growth inside macrophages, and the granulysin and perforins secreted by CD8+ T cells can work together to decrease the viability of macrophage intracellular Mtb (67, 68). Humans with IFN-gamma receptor deficiency are susceptible to Mycobacterial infection (69, 70). CD4+ and CD8+ T cells are important mediators of protection against Mtb infection (71, 72) (Figure 2). Mtb is a kind of intracellular pathogen that can arouse the Th1 type immunity, comprising monocyte activation and T cell cytotoxicity. B cells and T cells can work together to prevent Mtb infection. T helper cells can stimulate B cells to become activated and produce high-affinity antibodies, become memory B cells, and help activate cytotoxic T cells to kill macrophages (8, 73, 74). In the host granulomas B cells and T follicular helper (TFH) -like cells are important for TB control, and Mtb antigen-specific B cells can direct TFH -like cells into lymphoid follicles, which can help in mediating Mtb control (75). On the other hand, B cells can modulate T cell immune response by different mechanisms such as antigen presentation, antibodies, and cytokines production (6, 7) (Figure 2). In primates, the subclasses of antibodies are related to the effectors’ response, such as cytotoxicity, phagocytosis, and secretion of immune cytokines (17, 76, 77). The amount and persistence of antibodies also have an important role in Mtb infection. The low quantity of antibodies may not be enough to confer protection, while the high amount of antibodies may not give protection, which is called a prozone effect (78–80). Studies indicate that the constant regions with the variable regions of antibodies can confer specific protective effects against Mtb infection (16, 17, 81, 82). Different murine isotypes of antibodies, such as IgM, IgG1, IgG3, and IgA, are passively protective against Mtb infection (Table 2).
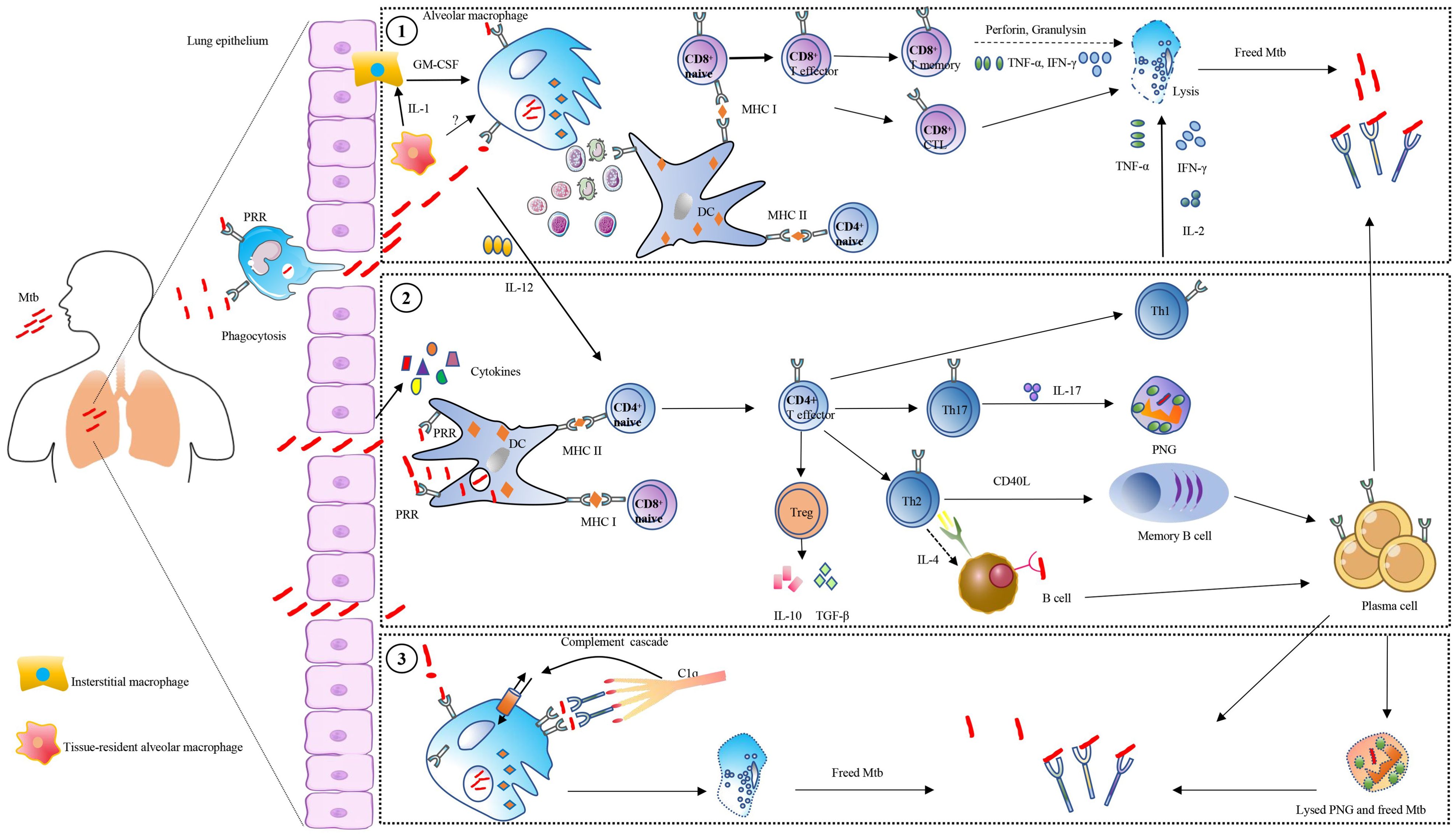
Figure 2. Overview of antibodies’ roles in tuberculosis. Control of Mycobacterium tuberculosis (Mtb) is the result of multiple immune cell interactions, such as T-cell, B-cell, macrophages, and dendritic cells (DC). Meanwhile, a variety of receptors and cytokines also mediated the process. The Mtb is internalized into macrophage and DC via pattern recognition receptors (PRR), and survives inside the phagosomal compartment, after which the apoptosis bodies carrying mycobacterial peptides are released. Several ways are employed by host cells to control Mtb: (1), These vesicles are acquired by DC, and the mycobacterial peptides are loaded on the MHC class I or MHC class II, which is presented to CD4+ T cells or CD8+ T cells. The CD8 T cells combined with MHC class I are activated, and act as cytolytic T lymphocytes (CTL) secreting perforin and granulysin to lyse host cells and kill Mtb. Moreover, the CD4+ T-helper (Th) cells combined with MHC class II are activated and polarized into different subsets including Th1, Th2, Th17, and Treg. Among them, Th1 cells can produce IL-2, TNF-α, and IFN-γ for interacting with macrophages. Th2 cells produce IL-4 to mediate B cells. Regulate T cells (Treg) produce IL-10 or transforming growth factor β (TGF-β). Th17 cells produce IL-17, which can activate polymorphonuclear granulocytes (PNG). (2), The Mtb is directly taken up by DC and is loaded on the MHC class I or MHC class II, with serial cells mediating as described above. (3) The affinity of antibody Fc domain with complement component 1 (C1q) causes complement-dependent cytotoxicity (CDC), which regulates host cells to eradicate Mtb. The lysed cells release Mtb which is affected by antibodies during cell-to-cell transfer.
IgM
IgM is expressed on the cell surface at the beginning of B-cell establishment in the bone marrow and accompanies the entire process of B-cell maturation. IgM is evolutionarily conserved and can specifically bind antigens in the absence of prior immunization (93, 94). IgM is the primary class of antibody produced early in host infection and provides a rapid antibody response. The protective roles of IgM against a wide range of pathogens, including intracellular bacteria such as Mtb and Ehrlichia muris (E. muris), have been demonstrated (95–100). IgM plays an indispensable role in the development of an optimal germinal center (GC) reaction, which is a prerequisite for the establishment of effective humoral immunity for chronic TB control. The variation of specific Ig classes may potentially affect TB disease progression (101). The immunodeficient mice, lacking IgM secretion, exhibit significant susceptibility to TB, indicating the protective role of IgM in TB progression (99, 102) Intravenous administration of the BCG vaccine can prevent Mtb infection in a rhesus monkey model (100), and the existence of Mtb-specific IgM in bronchoalveolar lavage fluids (BALF) of the BCG-vaccinated monkeys implies that IgM can have protection against TB in an early phase of Mtb-host interaction (98, 100). The above results initiate a new era in the study of IgM against TB and provide a new direction for the study of the mechanisms of humoral immunity in fighting against intracellular mycobacterial infection. However, early IgM antibodies do not undergo somatic hypermutation and therefore produce IgM with low affinity (33). Fortunately, IgM can form pentamers, which spontaneously bind multivalent antigenic molecules, such as bacterial capsule polysaccharides (103). Therefore, the deficiency of IgM monomer avidity is compensated by this multipoint binding ability (104). The rapid production and efficient activation of IgM can be very effective in controlling bacterial infections, which would have serious consequences if the pathogenic infection is not controlled as soon as possible. Specific long-lived IgM plasma cells can indeed demonstrate somatic mutations, produce IgM antibodies, and contribute to long-term protection (105). IgM can function as an antimicrobial activity by modulating multiple immune processes, including opsonization, dendritic cell functions, T cell immunity, and humoral responses (103, 106). During the active phase of TB, the IgM against different Mtb antigens is induced within one month of infection (107). In immunized rhesus monkeys, IgM titers are the strongest marker of reduced bacterial load, and intravenous BCG administration to rhesus monkeys elicited near complete immune protection against TB (98). The IgM exhibits very potent anti-bacterial and viral activity by fixing complement and mediating protection (108). Benefiting from its pentameric and hexameric structure, IgM has a high affinity to the complement component C1q and is therefore more likely than IgG to utilize the complement system to accomplish complement-dependent cytotoxic (CDC) processes (109) (Figure 3). BCG can bind to C1q in the presence of IgM in serum samples from BCG-vaccinated people (110). Mild tuberculosis meningitis (TBM) is associated with overall higher IgM titers to Mtb antigens in the CSF and is characterized by an enrichment of Mtb-specific antibodies that can activate complement and drive phagocytosis by monocytes and neutrophils (111). The mAb anti-LAM IgM A194 can inhibit Mtb growth in human whole blood but not in macrophages, which suggests that other factors, including complement, may be required for the restrictive effects (100). Mouse mAb anti-LAM IgM TMDU3 can bind C1q and iC3b, activate the classical complement pathway and enhance mycobacteria phagocytosis and promote the fusion of phagosome and lysosome in a CD11b-dependent manner (83). However, the detailed molecular mechanism by which IgM mediates the complement system against TB remains poorly characterized and warrants further investigation in future studies.
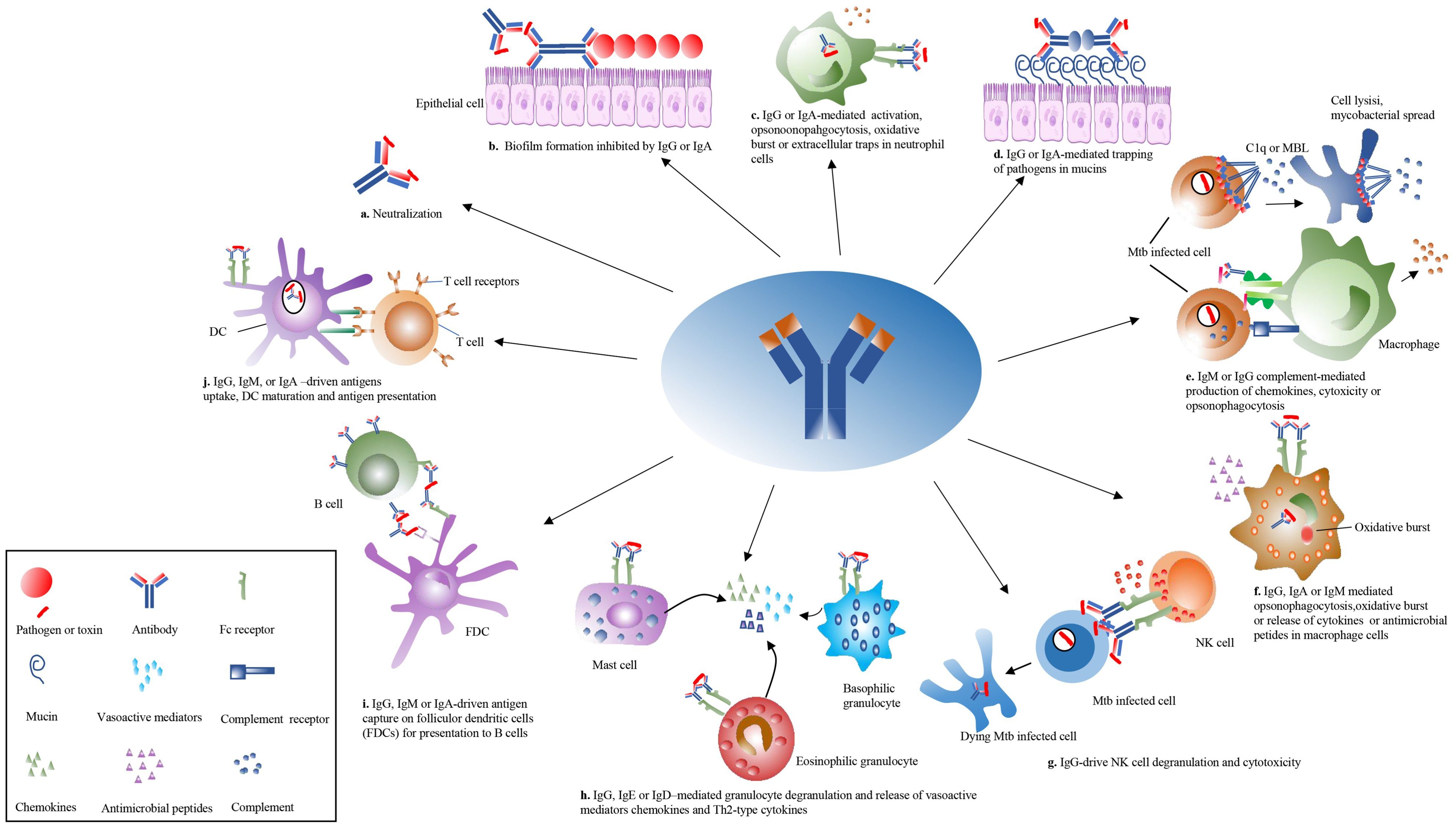
Figure 3. Overview of the antibodies’ effector functions. The antibody can play pleiotropic effectors functions during Mtb infection. (a) The antibody is directly neutralized with Mtb. (b) The antibody isotypes, IgG and IgA, inhibited biofilm formation. (c) The IgG and IgA activate neutrophil opsonophagocytosis, oxidative bursts, and extracellular traps. (d) The antibody isotypes, IgG and IgA, mediated the trapping of Mtb in mucins. (e) The IgM and IgG activate complement cascade to induce cell lysis through the membrane attack complex or drive Mtb clearance. (f) The IgG, IgM, and IgA mediate opsonophagocytosis, oxidative burst, or release of cytokines in macrophage cells. (g) The IgG isotypes drive natural killer (NK) cell degranulation to kill infected cells. (h) The IgG, IgE, and IgD mediate the degranulation of mast cells, eosinophilic granulocytes, and basophilic granulocytes to release vasoactive substances and cytokines in allergens or parasitic infections. (i) The follicular dendritic cells (FDCs) present mycobacterial antigen to B cells. (j) The IgG, IgM, and IgA mediate DC to enhance antigen uptake, processing, and presentation toward T cells. MBL, mannose-binding lectin.
IgD
In general, the IgM and IgD are co-expressed on the mature B cell surface before antigenic stimulation, and IgD may play a crucial role in regulating B cell maturation (25). IgD isotypes are not presented on the surface of primary B cells but on the mature B cells, and are mainly expressed in the upper respiratory tract, where they bind well to basophils and innate immune cells to promote bactericidal activity through unknown receptors (25). Structurally, IgD has the increasing flexibility of the hinge region, which greatly augments antigen-antibody binding. In addition, IgD also synergizes with IgM to have protection at the early stages of pathogenic infections to exert anti-infective effects (112). One study indicates that older TB patients have higher total IgD levels (113).
IgG
Monoclonal antibodies of the IgG isotype have been utilized for passive therapy, since IgG is the most abundant antibody isotype in serum with a long half-life. The increasing evidence suggests antibodies play a protective role in mouse infection models, including BALB/c and C57BL/6 (13, 17, 114). Intravenous administration of the high dose of total human immunoglobulin IgG (hdIVIg) to mice resulted in significantly reducing the organs’ bacterial loads in Mtb-infected BALB/c and C57BL/6 mice models (15, 115). The hdIVIg may have protective effects, maybe because it can modify the critical cells’ responses against TB, such as dendritic cells and T cells (115). In another research, the human IgG was administered into mice via the intranasal route, and the results showed a remarkable decrease in pulmonary bacterial load in mice (116), in this study, the gamma-globulin’s protection was abolished after incubation with Mtb, which suggests a potential role of Mtb-specific antibodies against TB (116). The di-glycosylated glycan structures found on the Fc region of IgG can differentiate tuberculosis infection (TBI) and active tuberculosis (ATB), as well as discriminate treated ATB from ATB (27). Moreover, the TB-specific IgG4 antibodies are evaluated in ATB but diminished after TB treatment (27). The total Ig isolated from highly exposed healthcare workers was injected into the mice 5 hours before aerosol infection and can offer protection in the lungs’ CFU reduction (13). The abundance of IgG3 from protective donors in this study was higher than that from a nonprotective donor when testing for decay kinetics in the mouse model in this study (13). The functions of antibody subclasses could be affected by hinge region length and disulfide bonding, and longer, more flexible structures implied an increased binding efficiency for antigens, complements, and Fc receptors (117, 118) (Figure 3). Different subclasses may play different regulatory functions at different stages of TB development (119). The IgG1 antibody subclass against TB could increase the TNF-α release, responsible for disease localization and granuloma formation at the early stages of infection (120, 121). The IgG1 monoclonal antibodies directed against LAM named SMITB14 can increase the survival rate and reduce bacterial load and weight loss in BALB/c mice (84). The human IgG1 P1AM25 targeting oligosaccharide (OS) motifs of AM can enhance Mtb phagocytosis by macrophages, reduce intracellular growth in an FcγR-dependent manner, and have protective effects in passive transfer with Mtb–infected FcγR-humanized mice (114). However, P1AM25 in murine IgG2a but not IgG1 can give protection against Mtb infection in C57BL/6 mice (114). Another study indicates that the IgG1 of monoclonal anti-LAM increased the mycobacterial load in A549 cells (85). The presence of only neonatal receptors in the A549 cells, lacking conventional FcγR and FcαR receptors expressed on the cell surface, may be the main reason for the differences (85). Antibodies targeting polysaccharide LAM and protein antigens in TB patients were predominantly of IgG regardless of the patient’s clinical status (122). In murine models of C. neoformans infection, the switch from IgG3 to IgG1 increases antibody protective efficacy against C. neoformans infection (123). The mouse IgG2b monoclonal antibodies targeting OmpA and MPB83 have shown a significant reduction in the bacterial burdens in mice infected with M. bovis (86, 87). In TB patients, different antibody isotypes are found to target lipoarabinomannan (LAM), which is a mycobacterial cell wall glycolipid component (54, 122). A significant switch from anti-LAM antibody subclass IgG1 to IgG2 was observed from tuberculoid toward lepromatous forms, despite a constant total amount of antibodies, suggesting that this conversion may be associated with changes in antibody protective effects (122).
IgA
In the early stages of Mtb infection, IgA antibodies secreted from the mucosal surfaces of the respiratory tracts play a key role in the anti-infection process (Figure 3). The anti-HBHA IgA monoclonal antibodies were shown to inhibit bacterial uptake in lung epithelial cells (85). The use of arabinomannan reactive monoclonal antibodies to opsonize Mtb demonstrates that IgG1 on THP1 monocytes has no significant difference in bacterial counts compared with the isotype control, whereas the IgA1 isotype does (85). A monoclonal antibody targeted Mtb surface lipoprotein LpqH from a highly exposed but uninfected healthcare worker, which was identified to be of IgA isotype in its natural form, was shown to have protection against tuberculosis (14).
Antigen specificity influences protection, since a mouse IgA monoclonal antibody binding to the Acr antigen named TBA61 was found to be far more protective than an antibody TBA84 against the PstS1 antigen (91). Mouse IgA monoclonal antibodies, regardless of their specificity, can inhibit the proliferation of mouse macrophage cell lines. The anti-proliferative activity is manifested by IgA binding to J774.1 cells, stimulating tumor necrosis factor (TNF)-alpha production and inducing apoptosis, but not by mouse monoclonal IgG and IgM (124). The TNF-α is essential for granuloma formation and macrophage recruitment at the early stages of Mtb infection; however, too much TNF-α can lead to the necrosis of the infected macrophage cells and help the mycobacteria release into the blood (125, 126). In addition, adding IFN-γ before macrophages infected with IgA-opsonized Mtb can increase nitric oxide and TNF-α production, and decrease the bacterial counts in macrophages (90). When Balb/c mice were inoculated with mouse IFNγ and an anti-Acr IgA TBA61 mAb via the intranasal route (i.n.), a synergistic protective effect on reducing bacterial burdens can be found for the lungs harvested 3 and 4 weeks after H37Rv aerosol infection, while neither component alone was protective (90, 127) (Table 2). Moreover, when co-inoculation of anti-Acr IgA TBA61 mAb and mouse IFNγ via i.n. route in Balb/c mice with IL-4 depletion by a neutralizing anti-IL4 mAb, a significant reduction of bacterial burdens can be observed compared with IgA and IFNγ treatment in wild-type mice after H37Rv infection (21) (Table 2). The studies indicate that the IFNγ and IL4-mediated macrophage functions are involved in the process which anti-Mtb IgA mAb inhibit Mtb growth (21, 90, 127).
IgE
Specific IgE has been reported in TB patients, suggesting that IgE may play an important role during TB disease progression (56, 128, 129). In TB patients, IgE levels are significantly elevated, and a decrease in IgE levels after treatment is observed. In addition, total IgE levels are significantly higher in TB patients with intestinal helminths and human immunodeficiency virus (HIV) co-infection than in those with helminths or without co-infection (p< 0.05) (130). Specific IgE levels are elevated in both tuberculosis and leprosy patients, and the differences can be observed between TB patients and healthy controls (131). Therefore, changes in the level of total IgE are often ignored in TB diagnosis (132, 133). Conventionally, IgE is often used as a characteristic marker of allergic diseases, and the relationship regarding specific IgE and TB is still not very clear (133).
The roles of antibody isotypes in TB diagnosis
The main component of immunologic diagnosis of TB is based on the detection of antibody responses to Mtb antigens. Mtb infection provokes a complex humoral immune response, with different antigens expressed at different stages of the infection (134, 135). Studies have shown that antigen or epitope-specific serology may help in diagnosis, assessment of prognosis, and monitoring of chemotherapy in TB patients (136–138). The antigens selected for antibody detection are important. A systems-level analysis of the antibody response to the entire Mtb proteome in TB patients indicates that Mtb immunoproteome is rich in membrane-associated and extracellular proteins, and the antibody responses to the same antigens varied among patients and are correlated with bacillary burden (139). Using serology for active TB patients finding could reduce the TB transmission, which can help treat the patients and limit the spread timely (140, 141). The previous studies have shown that the antigens such as 38-kDa antigen (PstS1), the 16-kDa antigen (Acr), and LAM are the immunodominant markers for ATB diagnosis (142–144). The smear-positive pulmonary tuberculosis patients have increased serum immunoglobulin titers against mycobacterial antigens; however, there are still 10% didn’t show any increase (145, 146). On the other hand, antibody-based assays have performed poorly when used to diagnose sputum smear-negative TB, and antibody responses can also be observed in past TB cases, which pose additional challenges for TB serodiagnosis (147).
As the most predominant antibody isotype in serum, IgG has been the focus of TB diagnosis. Serologically based detection of IgG levels of single or multiple antigens is by far the most common concept in TB diagnosis. 11 Mtb antigens are combined to detect IgG levels, and the results showed an astounding sensitivity of over 95% in sputum smear-positive samples (148). In 755 HIV-uninfected adults, the three-antigen model and the multi-antigen model have shown higher sensitivity compared with the single-antigen model (149). As such, IgG level against TB in the context of polyprotein fusion from TB antigens is used in diagnosis to discriminate TB patients. In terms of diagnostic sensitivity of up to 90%, it shows that six antigen fusion became an effective way to improve TB detection (150). The reason for using multiple antigens to detect TB was that each antigen is expressed at a different stage of infection, and the use of multiple antigens allowed for a more accurate diagnosis. Similarly, each antibody subtype and subclass significantly different at the stage of TB development, even when the total amount is constant. Therefore, the combined diagnosis of multiple isotypes becomes a possibility for efficient diagnosis. Moreover, the characteristics of the antibody are also a factor for an accurate diagnosis to distinguish the active TB and TBI. Some groups have revealed the presence of distinct glycosylation patterns in IgG Fc portion antibodies in active TB and TBI from South Africa, the USA, and Mexico, implying that the glycosylation could be a potential molecular target to differentiate between active TB and TBI (15, 27). Recent studies have shown that subclass IgG4 also serves as a new antibody signature for active TB, especially after significant changes in treated TB patients, indicating potential as a signature molecule for detection (27). FcγRI, an activating receptor of immune cells, can be significantly upregulated by IFN-γ and GM-CSF and binds to monomeric IgG1, IgG3, and IgG4 with a high affinity (151). Therefore, the FcγRI levels can be a marker that would help to improve the sensitivity of the detection to distinguish between the active and latent infection. Apart from that, the IgG1 and IgG3 are also major antibody subclasses for complement activation, with significantly higher levels of complement C1q in active TB patients compared to those with latent infection (52, 53). Therefore, the complement C1q expression related to active TB could be a potential marker to discriminate the TBI from active TB. The high-affinity antibody receptor FCγR1A, which principally binds the IgG1 and IgG3 subclasses, has been observed to be higher by analysis of whole blood transcription in active TB patients than in those with TBI, regardless of HIV status or ethnicity (152). Therefore, the FCγR1A expression level has the potential to be a biomarker for indicating acute tuberculosis in patients.
Mtb antigen-specific IgA antibodies could be used to develop accurate tests for TB diagnosis, the studies suggest that IgA targeting Acr could discriminate between clinical TB patients and healthy controls (153, 154). Moreover, the anti-16kDa IgA and anti-MPT64 IgA have been found suitable target molecules to discriminate the active TB and TBI, with >90% sensitivity in diagnosis (155). The role of IgM in TB diagnosis is not well understood. The previous studies indicate that the diagnosis of patients with TB by measuring the titer of IgM antibodies in serum alone showed low sensitivity (156, 157). However, the combination of IgG, IgM, and IgA antibody responses to protein antigens or polysaccharides like LAM can improve the sensitivity and specificity of active TB diagnosis (155, 158).The different mycobacterial species may have their unique characteristics of LAM structure. Rapid-growing nontuberculosis mycobacteria (NTM) such as M. smegmatis have uncapped ends or inositol phosphate caps (PILAMs), and slow-growing NTM such as M. avium are capped with mannopyranose residues, which leads to manLAM (159, 160). Therefore, it is important and possible to develop antibodies specifically targeting Mtb regions to enhance the specificity (161). Antigen variation in different lineages of Mtb should also be considered when the Mtb-specific antibodies are used for TB diagnosis. There is a 63bp deletion in Mtb lineage 4.2.2(L4.2.2) strains, which may affect the MPT64-based testing results in L4.2.2 isolates prevalent areas (162). Therefore, we should consider the changing levels of antibodies and also epitopes recognized by the diagnostic antibodies when conducting the TB diagnosis protocol design to effectively improve the accuracy and performance of the test.
Conclusions and perspectives
Tuberculosis is a contagious respiratory disease due to Mtb infection and is one of the top 10 single pathogens in the world in terms of mortality (163, 164). The problem of antibiotic therapy for tuberculosis has led to the emergence of Mtb drug resistance. In recent years, passive therapy with antibodies has become increasingly popular for research as an alternative to antimicrobial therapeutic agents. The effectiveness of various antibodies relies mostly on isotypes, which allows them to efficiently adapt to the most appropriate mode of transport across the epithelium to the site of function. Additionally, crucial research tools include broadly and powerfully antibodies, which may be used to find protective epitopes that can be developed into functional vaccines by structure-based reverse vaccinology. Currently, more than 70 monoclonal antibodies have been approved for the treatment of various pathogenic bacterial infections (165). However, many hurdles remain in the field of anti-infective mAbs: finding optimal targets for a pathogen, understanding how the isotypes, Fc receptors (FcRs), and other structural regions mediate protection, and developing better pre-clinical and clinical trials to investigate the therapeutic potential of these antibodies (165).
Traditionally, it is believed that, as intracellular bacteria, the protective immune response against TB is mainly exerted by T cell-mediated cellular immunity, including CD4+ and CD8+ T cells (166). However, in recent years, new findings suggest that B cells also play an important role in the anti-TB process, but the exact mechanisms are not well understood (167). Currently, BCG is the most commonly used TB vaccine. Its role is preventing the onset of meningitis and disseminating TB in infants and children. According to the World Health Organization in 2017, 120 of the 158 countries that allow BCG immunization have 90% BCG immunization coverage (168).
However, the failure of BCG to protect against pulmonary disease in adults has limited its use in a larger range of populations. In addition, significantly different immuno-protective effects were observed with different immunization routes of BCG, with significant immuno-protective effects observed with mucosal immunization with BCG compared to subcutaneous immunization (169, 170) and in intravenously BCG-injected experimental monkeys (98)Changes in both cellular immunity-related factors and antibody levels have been observed in these studies, but their roles in host protection and their modes of action remain to be answered.
Previous studies have mostly focused on eliciting cell-mediated immune responses against TB (171–173). More and more studies have shown that the host can produce protective antibodies against TB, and an increasing number of Mtb antigens have been reported to have the ability to induce protective antibodies (11, 14, 17, 174). Therefore, the integration of humoral immunity into tuberculosis vaccine development could be a potentially effective strategy. Recent studies have shown that the subunit vaccines incorporating the antigens arousing cellular immunity, such as Ag85A, and humoral immunity, such as PstS1 or LpqH, can significantly enhance the protective efficacy against TB (22, 175, 176), which gives us suggestions that it is rational to design the TB vaccines based on both cellular immunity and humoral immunity.
An ideal target is a prerequisite for an antibody to have its functions. It is worth investigating whether specific antigens, their relative concentrations, and post-translational lipid and carbohydrate moieties influence the class or subclass of antibody production in future studies. Mtb has secreted proteins playing important roles in its virulence and immune evasion. The secreted virulence proteins are secreted by secretion systems and can modulate the host immune responses (177). Therefore, some secreted proteins are possible therapeutic targets for TB treatment. The development of antibodies targeting secreted systems and proteins is a rational strategy for TB control and treatment. The cell walls of M.bovis BCG resemble those of Gram-negative bacteria and are presumed to have a four-layer membrane structure, from inner to outer: the cytoplasmic membrane, the peptidoglycan-arabinomannan complex, the extracellular membrane, and the outermost pod membrane (178). The presence of 144 outer membrane proteins in Mtb was deduced using signal peptide prediction, transmembrane protein prediction, and β-strands amphiphilicity, but only MctB and OmpATb of Mtb and MspA of M. smegmatis have been fully identified (179, 180). Antibodies that recognize bacterial outer membrane proteins generate immune protection (87), suggesting the potential value of screening efforts for antibodies against outer membrane proteins (87, 165). Given that most antibodies recognizing surface proteins are currently uncharacterized, their anti-infective value awaits further elucidation.
The antibody-dependent enhancement of infectious disease should also be considered. The antibodies’ receptor FcγRI inactivation can impact nitric oxide production by neutrophils, antigen presentation, and antibody-dependent killing of pathogens by macrophages (181, 182). Moreover, the improved Mtb control in the lungs of Fcgrt−/− mice compared to the control mice was observed and associated with reduced neutrophil recruitment (181, 182). Neutrophil accumulation is associated with increased disease severity in human TB and in mouse models of TB (183–185). Moreover, one study indicates that rabbit anti-H37Rv sera can facilitate the multiplication of BCG in the spleens of mice (186). Antibodies’ prozone-like effects should also be considered when developing antibody-based treatment methods for TB (78–80). The Mtb ‘decoy’ constituents also need to be considered. Antigens such as PstS1 may exacerbate disease by inducing a Th2 response, while anti-Acr antibodies show potential for protection, but the Th2 stimulant components need to be removed (21, 143, 187). Although obstacles remain in identifying effective targets and understanding how monoclonal antibodies protect against different infections, progress in these areas is a positive indication that monoclonal antibodies will be more widely accepted in the future as a treatment for bacterial infections.
Above all, the different roles of antibodies’ isotypes in tuberculosis need to be further investigated. The discovery of protective antibodies against TB can contribute to TB prevention and treatment. At the same time, the antigens identified following the isolation of protective antibodies will help in the design and development of novel tuberculosis vaccines.
Author contributions
HML: Writing – original draft, Writing – review & editing. HL: Project administration, Resources, Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China to Hao Li (No. 32070937), 2115 Talent Development Program of China Agricultural University to Hao Li (No. 00109029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Global tuberculosis report 2024. Geneva: World Health Organization. (2024). Licence: CC BY-NC-SA 3.0 IGO.
2. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. (2007) 449:819–26. doi: 10.1038/nature06246
3. Mayer-Barber KD and Barber DL. Innate and adaptive cellular immune responses to mycobacterium tuberculosis infection. Cold Spring Harb Perspect Med. (2015) 5. doi: 10.1101/cshperspect.a018424
4. Chandra P, Grigsby SJ, and Philips JA. Immune evasion and provocation by mycobacterium tuberculosis. Nat Rev Microbiol. (2022) 20:750–66. doi: 10.1038/s41579-022-00763-4
5. Jasenosky LD, Scriba TJ, Hanekom WA, and Goldfeld AE. T cells and adaptive immunity to mycobacterium tuberculosis in humans. Immunol Rev. (2015) 264:74–87. doi: 10.1111/imr.12274
6. Lund FE and Randall TD. Effector and regulatory B cells: modulators of cd4+ T cell immunity. Nat Rev Immunol. (2010) 10:236–47. doi: 10.1038/nri2729
7. Maglione PJ and Chan J. How B cells shape the immune response against mycobacterium tuberculosis. Eur J Immunol. (2009) 39:676–86. doi: 10.1002/eji.200839148
8. Vinuesa CG, Tangye SG, Moser B, and Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. (2005) 5:853–65. doi: 10.1038/nri1714
9. Kumararatne DS. Tuberculosis and immunodeficiency–of mice and men. Clin Exp Immunol (1997) 107(1):11–4. doi: 10.1046/j.1365-2249.1997.d01-910.x
10. Glatman-Freedman A and Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against mycobacterium tuberculosis. Clin Microbiol Rev. (1998) 11:514–32. doi: 10.1128/CMR.11.3.514
11. Watson A, Li H, Ma B, Weiss R, Bendayan D, Abramovitz L, et al. Human antibodies targeting a mycobacterium transporter protein mediate protection against tuberculosis. Nat Commun. (2021) 12:602. doi: 10.1038/s41467-021-20930-0
12. Casadevall A. Antibodies to mycobacterium tuberculosis. N Engl J Med. (2017) 376:283–5. doi: 10.1056/NEJMcibr1613268
13. Li H, Wang XX, Wang B, Fu L, Liu G, Lu Y, et al. Latently and uninfected healthcare workers exposed to tb make protective antibodies against mycobacterium tuberculosis. Proc Natl Acad Sci U.S.A. (2017) 114:5023–8. doi: 10.1073/pnas.1611776114
14. Krishnananthasivam S, Li H, Bouzeyen R, Shunmuganathan B, Purushotorman K, Liao X, et al. An anti-lpqh human monoclonal antibody from an asymptomatic individual mediates protection against mycobacterium tuberculosis. NPJ Vaccines. (2023) 8:127. doi: 10.1038/s41541-023-00710-1
15. Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. (2016) 167:433–43 e14. doi: 10.1016/j.cell.2016.08.072
16. Achkar JM and Prados-Rosales R. Updates on antibody functions in mycobacterium tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr Opin Immunol. (2018) 53:30–7. doi: 10.1016/j.coi.2018.04.004
17. Li H and Javid B. Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol. (2018) 18:591–6. doi: 10.1038/s41577-018-0028-0
18. Nimmerjahn F, Vidarsson G, and Cragg MS. Effect of posttranslational modifications and subclass on igg activity: from immunity to immunotherapy. Nat Immunol. (2023) 24:1244–55. doi: 10.1038/s41590-023-01544-8
19. Vordermeier HM, Venkataprasad N, Harris DP, and Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. (1996) 106:312–6. doi: 10.1046/j.1365-2249.1996.d01-845.x
20. Chan J, Mehta S, Bharrhan S, Chen Y, Achkar JM, Casadevall A, et al. The role of B cells and humoral immunity in mycobacterium tuberculosis infection. Semin Immunol. (2014) 26:588–600. doi: 10.1016/j.smim.2014.10.005
21. Buccheri S, Reljic R, Caccamo N, Ivanyi J, Singh M, Salerno A, et al. Il-4 depletion enhances host resistance and passive iga protection against tuberculosis infection in balb/C mice. Eur J Immunol. (2007) 37:729–37. doi: 10.1002/eji.200636764
22. Zeng L, Ma X, Qu M, Tang M, Li H, Lei C, et al. Immunogenicity and Protective Efficacy of Ag85a and Truncation of Psts1 Fusion Protein Vaccines against Tuberculosis. Heliyon. (2024) 10(5):e27034. doi: 10.1016/j.heliyon.2024.e27034
23. McLean MR, Lu LL, Kent SJ, and Chung AW. An inflammatory story: antibodies in tuberculosis comorbidities. Front Immunol. (2019) 10:2846. doi: 10.3389/fimmu.2019.02846
24. Heltemes-Harris L, Liu X, and Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J Immunol. (2004) 172:823–33. doi: 10.4049/jimmunol.172.2.823
25. Schroeder HW Jr. and Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. (2010) 125:S41–52. doi: 10.1016/j.jaci.2009.09.046
26. Saxena A and Wu D. Advances in therapeutic fc engineering - modulation of igg-associated effector functions and serum half-life. Front Immunol. (2016) 7:580. doi: 10.3389/fimmu.2016.00580
27. Grace PS, Dolatshahi S, Lu LL, Cain A, Palmieri F, Petrone L, et al. Antibody subclass and glycosylation shift following effective tb treatment. Front Immunol. (2021) 12:679973. doi: 10.3389/fimmu.2021.679973
28. Grace PS, Peters JM, Sixsmith J, Lu R, Irvine EB, Luedeman C, et al. Antibody-fab and -fc features promote mycobacterium tuberculosis restriction. Immunity (2025) 58(6):1586–97 e5. doi: 10.1016/j.immuni.2025.05.004
29. Nimmerjahn F and Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. (2008) 8:34–47. doi: 10.1038/nri2206
30. Nimmerjahn F and Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. (2007) 96:179–204. doi: 10.1016/S0065-2776(07)96005-8
31. Irvine EB and Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. (2020) 30:241–53. doi: 10.1093/glycob/cwaa018
32. Arnold JN, Wormald MR, Sim RB, Rudd PM, and Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. (2007) 25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702
33. Lu LL, Suscovich TJ, Fortune SM, and Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. (2018) 18:46–61. doi: 10.1038/nri.2017.106
34. Nicoara O and Twombley K. Immunoglobulin a nephropathy and immunoglobulin a vasculitis. Pediatr Clin North Am. (2019) 66:101–10. doi: 10.1016/j.pcl.2018.08.008
35. Phong BL, D’Souza SJ, Baudier RL, Wu E, Immethun VE, Bauer DL, et al. Ige-activated mast cells enhance tlr4-mediated antigen-specific cd4(+) T cell responses. Sci Rep. (2021) 11:9686. doi: 10.1038/s41598-021-88956-4
36. Ryman JT and Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. (2017) 6:576–88. doi: 10.1002/psp4.12224
37. Onoue K, Grossberg AL, Yagi Y, and Pressman D. Immunoglobulin M antibodies with ten combining sites. Science. (1968) 162:574–6. doi: 10.1126/science.162.3853.574
38. Czajkowsky DM and Shao Z. The human igm pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci U.S.A. (2009) 106:14960–5. doi: 10.1073/pnas.0903805106
39. Sun Z, Almogren A, Furtado PB, Chowdhury B, Kerr MA, and Perkins SJ. Semi-extended solution structure of human myeloma immunoglobulin D determined by constrained X-ray scattering. J Mol Biol. (2005) 353:155–73. doi: 10.1016/j.jmb.2005.07.072
40. Janeway CA Jr, Travers P, Walport M, and Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th Edition. (New York: Garland Science). (2001).
41. Burton DR and Woof JM. Human antibody effector function. Adv Immunol. (1992) 51:1–84. doi: 10.1016/s0065-2776(08)60486-1
42. de Sousa-Pereira P and Woof JM. Iga: structure, function, and developability. Antibodies (Basel). (2019) 8. doi: 10.3390/antib8040057
43. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human fcgamma receptors and their polymorphic variants for human igg subclasses. Blood. (2009) 113:3716–25. doi: 10.1182/blood-2008-09-179754
44. Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, et al. Affinity of human igg subclasses to mouse fc gamma receptors. MAbs. (2017) 9:767–73. doi: 10.1080/19420862.2017.1323159
45. de Taeye SW, Bentlage AEH, Mebius MM, Meesters JI, Lissenberg-Thunnissen S, Falck D, et al. Fcgammar binding and adcc activity of human igg allotypes. Front Immunol. (2020) 11:740. doi: 10.3389/fimmu.2020.00740
46. Ma L, Qin T, Chu D, Cheng X, Wang J, Wang X, et al. Internal duplications of dh, jh, and C region genes create an unusual igh gene locus in cattle. J Immunol. (2016) 196:4358–66. doi: 10.4049/jimmunol.1600158
47. Li Y, Wang G, Li N, Wang Y, Zhu Q, Chu H, et al. Structural insights into immunoglobulin M. Science. (2020) 367:1014–7. doi: 10.1126/science.aaz5425
48. Li Y, Shen H, Zhang R, Ji C, Wang Y, Su C, et al. Immunoglobulin M perception by fcmur. Nature. (2023) 615:907–12. doi: 10.1038/s41586-023-05835-w
49. Leslie GA and Swate TE. Structure and biologic functions of human igd. I. The presence of immunoglobulin D in human cord sera. J Immunol. (1972) 109:47–50. doi: 10.4049/jimmunol.109.1.47
50. Spiegelberg HL. The structure and biology of human igd. Immunol Rev. (1977) 37:3–24. doi: 10.1111/j.1600-065x.1977.tb00243.x
51. Briere F, Servet-Delprat C, Bridon JM, Saint-Remy JM, and Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (Sigd+) B cells to secrete igg1 and igg3. J Exp Med. (1994) 179:757–62. doi: 10.1084/jem.179.2.757
52. Cai Y, Yang Q, Tang Y, Zhang M, Liu H, Zhang G, et al. Increased complement C1q level marks active disease in human tuberculosis. PloS One. (2014) 9:e92340. doi: 10.1371/journal.pone.0092340
53. Michaelsen TE, Garred P, and Aase A. Human igg subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur J Immunol. (1991) 21:11–6. doi: 10.1002/eji.1830210103
54. Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, and Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by hiv status. Clin Vaccine Immunol. (2012) 19:198–208. doi: 10.1128/CVI.05550-11
56. Gauchat JF, Lebman DA, Coffman RL, Gascan H, and de Vries JE. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to ige production. J Exp Med. (1990) 172:463–73. doi: 10.1084/jem.172.2.463
57. van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, and van de Winkel JG. Iga and the iga fc receptor. Trends Immunol. (2001) 22:205–11. doi: 10.1016/s1471-4906(01)01873-7
58. Steffen U, Koeleman CA, Sokolova MV, Bang H, Kleyer A, Rech J, et al. Iga subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. (2020) 11:120. doi: 10.1038/s41467-019-13992-8
59. McDonnell JM, Dhaliwal B, Sutton BJ, and Gould HJ. Ige, ige receptors and anti-ige biologics: protein structures and mechanisms of action. Annu Rev Immunol. (2023) 41:255–75. doi: 10.1146/annurev-immunol-061020-053712
60. Sutton BJ and Davies AM. Structure and dynamics of ige-receptor interactions: fcepsilonri and cd23/fcepsilonrii. Immunol Rev. (2015) 268:222–35. doi: 10.1111/imr.12340
61. Kawano Y and Noma T. Role of interleukin-2 and interferon-gamma in inducing production of igg subclasses in lymphocytes of human newborns. Immunology. (1996) 88:40–8. doi: 10.1046/j.1365-2567.1996.d01-634.x
62. Pisu D, Huang L, Narang V, Theriault M, Le-Bury G, Lee B, et al. Single cell analysis of M. Tuberculosis phenotype and macrophage lineages in the infected lung. J Exp Med. (2021) 218. doi: 10.1084/jem.20210615
63. Hou F, Xiao K, Tang L, and Xie L. Diversity of macrophages in lung homeostasis and diseases. Front Immunol. (2021) 12:753940. doi: 10.3389/fimmu.2021.753940
64. Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. (2008) 3:399–407. doi: 10.1016/j.chom.2008.05.006
65. Ernst JD. Macrophage receptors for mycobacterium tuberculosis. Infect Immun. (1998) 66:1277–81. doi: 10.1128/IAI.66.4.1277-1281.1998
66. Cambi A, Koopman M, and Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. (2005) 7:481–8. doi: 10.1111/j.1462-5822.2005.00506.x
67. Flannagan RS, Jaumouille V, and Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. (2012) 7:61–98. doi: 10.1146/annurev-pathol-011811-132445
68. Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. (1998) 282:121–5. doi: 10.1126/science.282.5386.121
69. Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille calmette-guerin infection. N Engl J Med. (1996) 335:1956–61. doi: 10.1056/NEJM199612263352604
70. Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, et al. A human ifngr1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. (1999) 21:370–8. doi: 10.1038/7701
71. van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, and Andersen P. Control of latent mycobacterium tuberculosis infection is dependent on cd8 T cells. Eur J Immunol. (2000) 30:3689–98. doi: 10.1002/1521-4141(200012)30:12&60;3689::AID-IMMU3689&62;3.0.CO;2-4
72. Flynn JL, Goldstein MM, Triebold KJ, Koller B, and Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to mycobacterium tuberculosis infection. Proc Natl Acad Sci U.S.A. (1992) 89:12013–7. doi: 10.1073/pnas.89.24.12013
73. Engleman EG, Benike CJ, Grumet FC, and Evans RL. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. (1981) 127:2124–9. doi: 10.4049/jimmunol.127.5.2124
74. Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ, van der Voort EI, et al. Immature dendritic cells acquire cd8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. (2000) 192:145–50. doi: 10.1084/jem.192.1.145
75. Swanson RV, Gupta A, Foreman TW, Lu L, Choreno-Parra JA, Mbandi SK, et al. Antigen-specific B cells direct T follicular-like helper cells into lymphoid follicles to mediate mycobacterium tuberculosis control. Nat Immunol. (2023) 24:855–68. doi: 10.1038/s41590-023-01476-3
76. Ravetch JV and Bolland S. Igg fc receptors. Annu Rev Immunol. (2001) 19:275–90. doi: 10.1146/annurev.immunol.19.1.275
77. Samuelsson A, Towers TL, and Ravetch JV. Anti-inflammatory activity of ivig mediated through the inhibitory fc receptor. Science. (2001) 291:484–6. doi: 10.1126/science.291.5503.484
78. Taborda CP and Casadevall A. Immunoglobulin M efficacy against cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J Immunol. (2001) 166:2100–7. doi: 10.4049/jimmunol.166.3.2100
79. Taborda CP, Rivera J, Zaragoza O, and Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with igg. J Immunol. (2003) 170:3621–30. doi: 10.4049/jimmunol.170.7.3621
80. Casadevall A, Dadachova E, and Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. (2004) 2:695–703. doi: 10.1038/nrmicro974
81. Achkar JM, Chan J, and Casadevall A. B cells and antibodies in the defense against mycobacterium tuberculosis infection. Immunol Rev. (2015) 264:167–81. doi: 10.1111/imr.12276
82. Casadevall A. To be or not be a (Functional) antibody against tb. Cell. (2016) 167:306–7. doi: 10.1016/j.cell.2016.09.041
83. Nakayama H, Hanafusa K, Yamaji T, Oshima E, Hotta T, Takamori K, et al. Phylactic role of anti-lipoarabinomannan igm directed against mannan core during mycobacterial infection in macrophages. Tuberculosis (Edinb). (2023) 143:102391. doi: 10.1016/j.tube.2023.102391
84. Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, and Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(Ab’) fragment prolong survival of mice infected with mycobacterium tuberculosis. Clin Exp Immunol. (2004) 138:30–8. doi: 10.1111/j.1365-2249.2004.02593.x
85. Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, et al. Human isotype-dependent inhibitory antibody responses against mycobacterium tuberculosis. EMBO Mol Med. (2016) 8:1325–39. doi: 10.15252/emmm.201606330
86. Chambers MA, Gavier-Widen D, and Hewinson RG. Antibody bound to the surface antigen mpb83 of mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. (2004) 41:93–100. doi: 10.1016/j.femsim.2004.01.004
87. Li H, Ji J, Qu M, Ma X, Zuo Y, Tang M, et al. Isolation and characterization of a protective monoclonal antibody targeting outer membrane protein (Ompa) against tuberculosis. Microbiol Spectr. (2025) 13(4):e0294224. doi: 10.1128/spectrum.02942-24
88. Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mab recognizing a surface antigen of mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U.S.A. (1998) 95:15688–93. doi: 10.1073/pnas.95.26.15688
89. Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, et al. The heparin-binding haemagglutinin of M. Tuberculosis is required for extrapulmonary dissemination. Nature. (2001) 412:190–4. doi: 10.1038/35084083
90. Reljic R, Clark SO, Williams A, Falero-Diaz G, Singh M, Challacombe S, et al. Intranasal ifngamma extends passive iga antibody protection of mice against mycobacterium tuberculosis lung infection. Clin Exp Immunol. (2006) 143:467–73. doi: 10.1111/j.1365-2249.2006.03012.x
91. Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, et al. Passive protection with immunoglobulin a antibodies against tuberculous early infection of the lungs. Immunology. (2004) 111:328–33. doi: 10.1111/j.1365-2567.2004.01809.x
92. Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human iga monoclonal antibody protects against tuberculosis. J Immunol. (2011) 186:3113–9. doi: 10.4049/jimmunol.1003189
93. Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. (2002) 2:688–98. doi: 10.1038/nri889
94. Jayasekera JP, Moseman EA, and Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. (2007) 81:3487–94. doi: 10.1128/JVI.02128-06
95. Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, et al. Igm production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. (2011) 186:1011–21. doi: 10.4049/jimmunol.1002836
96. Mehrani T and Petri M. Igm anti-beta2 glycoprotein I is protective against lupus nephritis and renal damage in systemic lupus erythematosus. J Rheumatol. (2011) 38:450–3. doi: 10.3899/jrheum.100650
97. Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, and Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun. (2006) 74:4856–64. doi: 10.1128/IAI.00246-06
98. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous bcg immunization. Nature. (2020) 577:95–102. doi: 10.1038/s41586-019-1817-8
99. Hisert KB, Kirksey MA, Gomez JE, Sousa AO, Cox JS, Jacobs WR Jr., et al. Identification of mycobacterium tuberculosis counterimmune (Cim) mutants in immunodeficient mice by differential screening. Infect Immun. (2004) 72:5315–21. doi: 10.1128/IAI.72.9.5315-5321.2004
100. Irvine EB, O’Neil A, Darrah PA, Shin S, Choudhary A, Li W, et al. Robust igm responses following intravenous vaccination with bacille calmette-guerin associate with prevention of mycobacterium tuberculosis infection in macaques. Nat Immunol. (2021) 22:1515–23. doi: 10.1038/s41590-021-01066-1
101. Jain VK, Bishnoi HS, Beniwal OP, and Misra SN. Immunoglobulin profile in pulmonary tuberculosis. J Postgrad Med. (1984) 30(2):80–4.
102. Corthesy B. Role of secretory immunoglobulin a and secretory component in the protection of mucosal surfaces. Future Microbiol. (2010) 5:817–29. doi: 10.2217/fmb.10.39
103. Keyt BA, Baliga R, Sinclair AM, Carroll SF, and Peterson MS. Structure, function, and therapeutic use of igm antibodies. Antibodies (Basel). (2020) 9. doi: 10.3390/antib9040053
104. Seixas AMM, Sousa SA, and Leitao JH. Antibody-based immunotherapies as a tool for tackling multidrug-resistant bacterial infections. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10111789
105. Bohannon C, Powers R, Satyabhama L, Cui A, Tipton C, Michaeli M, et al. Long-lived antigen-induced igm plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat Commun. (2016) 7:11826. doi: 10.1038/ncomms11826
106. Liu J, Wang Y, Xiong E, Hong R, Lu Q, Ohno H, et al. Role of the igm fc receptor in immunity and tolerance. Front Immunol. (2019) 10:529. doi: 10.3389/fimmu.2019.00529
107. Chiang IH, Suo J, Bai KJ, Lin TP, Luh KT, Yu CJ, et al. Serodiagnosis of tuberculosis. A study comparing three specific mycobacterial antigens. Am J Respir Crit Care Med. (1997) 156:906–11. doi: 10.1164/ajrccm.156.3.9607122
108. Wibroe PP, Helvig SY, and Moein Moghimi S. The role of complement in antibody therapy for infectious diseases. Microbiol Spectr. (2014) 2. doi: 10.1128/microbiolspec.AID-0015-2014
109. Sharp TH, Boyle AL, Diebolder CA, Kros A, Koster AJ, and Gros P. Insights into igm-mediated complement activation based on in situ structures of igm-C1-C4b. Proc Natl Acad Sci U.S.A. (2019) 116:11900–5. doi: 10.1073/pnas.1901841116
110. Carroll MV, Lack N, Sim E, Krarup A, and Sim RB. Multiple routes of complement activation by mycobacterium bovis bcg. Mol Immunol. (2009) 46:3367–78. doi: 10.1016/j.molimm.2009.07.015
111. Spatola M, Nziza N, Irvine EB, Cizmeci D, Jung W, Van LH, et al. Distinctive antibody responses to mycobacterium tuberculosis in pulmonary and brain infection. Brain. (2024) 147:3247–60. doi: 10.1093/brain/awae066
112. Puissant-Lubrano B, Peres M, Apoil PA, Congy-Jolivet N, Roubinet F, and Blancher A. Immunoglobulin iga, igd, igg, igm and igg subclass reference values in adults. Clin Chem Lab Med. (2015) 53:e359–61. doi: 10.1515/cclm-2014-1186
113. Buckley CE and Trayer HR. Serum igd concentrations in sarcoidosis and tuberculosis. Clin Exp Immunol (1972) 10(2):257–65.
114. Liu Y, Chen T, Zhu Y, Furey A, Lowary TL, Chan J, et al. Features and protective efficacy of human mabs targeting mycobacterium tuberculosis arabinomannan. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.167960
115. Roy E, Stavropoulos E, Brennan J, Coade S, Grigorieva E, Walker B, et al. Therapeutic efficacy of high-dose intravenous immunoglobulin in mycobacterium tuberculosis infection in mice. Infect Immun. (2005) 73:6101–9. doi: 10.1128/IAI.73.9.6101-6109.2005
116. Olivares N, Puig A, Aguilar D, Moya A, Cadiz A, Otero O, et al. Prophylactic effect of administration of human gamma globulins in a mouse model of tuberculosis. Tuberculosis (Edinb). (2009) 89:218–20. doi: 10.1016/j.tube.2009.02.003
117. Gupta S, Shende N, Bhatia AS, Kumar S, and Harinath BC. Igg subclass antibody response to mycobacterial serine protease at different stages of pulmonary tuberculosis. Med Sci Monit (2005) 11(12):CR585–8.
118. Dall’Acqua WF, Cook KE, Damschroder MM, Woods RM, and Wu H. Modulation of the effector functions of a human igg1 through engineering of its hinge region. J Immunol. (2006) 177:1129–38. doi: 10.4049/jimmunol.177.2.1129
119. de Araujo LS, da Silva NBM, Leung JAM, Mello FCQ, and Saad MHF. Igg subclasses’ Response to a set of mycobacterial antigens in different stages of mycobacterium tuberculosis infection. Tuberculosis (Edinb). (2018) 108:70–6. doi: 10.1016/j.tube.2017.10.010
120. Olivares N, Marquina B, Mata-Espinoza D, Zatarain-Barron ZL, Pinzon CE, Estrada I, et al. The protective effect of immunoglobulin in murine tuberculosis is dependent on igg glycosylation. Pathog Dis. (2013) 69:176–83. doi: 10.1111/2049-632X.12069
121. Kindler V, Sappino AP, Grau GE, Piguet PF, and Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during bcg infection. Cell. (1989) 56:731–40. doi: 10.1016/0092-8674(89)90676-4
122. Sousa AO, Henry S, Maroja FM, Lee FK, Brum L, Singh M, et al. Igg subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Exp Immunol. (1998) 111:48–55. doi: 10.1046/j.1365-2249.1998.00452.x
123. Yuan RR, Spira G, Oh J, Paizi M, Casadevall A, and Scharff MD. Isotype switching increases efficacy of antibody protection against cryptococcus neoformans infection in mice. Infect Immun. (1998) 66:1057–62. doi: 10.1128/IAI.66.3.1057-1062.1998
124. Reljic R, Crawford C, Challacombe S, and Ivanyi J. Mouse iga inhibits cell growth by stimulating tumor necrosis factor-alpha production and apoptosis of macrophage cell lines. Int Immunol. (2004) 16:607–14. doi: 10.1093/intimm/dxh070
125. Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. (2012) 12:352–66. doi: 10.1038/nri3211
126. Yuk JM, Kim JK, Kim IS, and Jo EK. Tnf in human tuberculosis: A double-edged sword. Immune Netw. (2024) 24:e4. doi: 10.4110/in.2024.24.e4
127. Reljic R, Williams A, and Ivanyi J. Mucosal immunotherapy of tuberculosis: is there a value in passive iga? Tuberculosis (Edinb). (2006) 86:179–90. doi: 10.1016/j.tube.2006.01.011
128. Lynch NR, Hagel IA, Palenque ME, Di Prisco MC, Escudero JE, Corao LA, et al. Relationship between helminthic infection and ige response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. (1998) 101:217–21. doi: 10.1016/S0091-6749(98)70386-0
129. Adams JF, Scholvinck EH, Gie RP, Potter PC, Beyers N, and Beyers AD. Decline in total serum ige after treatment for tuberculosis. Lancet. (1999) 353:2030–3. doi: 10.1016/s0140-6736(98)08510-9
130. Kassu A, Mohammad A, Fujimaki Y, Moges F, Elias D, Mekonnen F, et al. Serum ige levels of tuberculosis patients in a tropical setup with high prevalence of hiv and intestinal parasitoses. Clin Exp Immunol. (2004) 138:122–7. doi: 10.1111/j.1365-2249.2004.02597.x
131. Yong AJ, Grange JM, Tee RD, Beck JS, Bothamley GH, Kemeny DM, et al. Total and anti-mycobacterial ige levels in serum from patients with tuberculosis and leprosy. Tubercle. (1989) 70:273–9. doi: 10.1016/0041-3879(89)90021-4
132. Ohrui T, Zayasu K, Sato E, Matsui T, Sekizawa K, and Sasaki H. Pulmonary tuberculosis and serum ige. Clin Exp Immunol. (2000) 122:13–5. doi: 10.1046/j.1365-2249.2000.01291.x
133. Ellertsen LK, Wiker HG, Egeberg NT, and Hetland G. Allergic sensitisation in tuberculosis and leprosy patients. Int Arch Allergy Immunol. (2005) 138:217–24. doi: 10.1159/000088722
134. Flynn JL and Chan J. Immune cell interactions in tuberculosis. Cell. (2022) 185:4682–702. doi: 10.1016/j.cell.2022.10.025
135. de Martino M, Lodi L, Galli L, and Chiappini E. Immune response to mycobacterium tuberculosis: A narrative review. Front Pediatr. (2019) 7:350. doi: 10.3389/fped.2019.00350
136. Bothamley GH, Rudd R, Festenstein F, and Ivanyi J. Clinical value of the measurement of mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax. (1992) 47:270–5. doi: 10.1136/thx.47.4.270
137. Bothamley GH, Schreuder GM, de Vries RR, and Ivanyi J. Association of antibody responses to the 19-kda antigen of mycobacterium tuberculosis and the hla-dq locus. J Infect Dis. (1993) 167:992–3. doi: 10.1093/infdis/167.4.992
138. Bothamley GH. Epitope-specific antibody levels demonstrate recognition of new epitopes and changes in titer but not affinity during treatment of tuberculosis. Clin Diagn Lab Immunol. (2004) 11:942–51. doi: 10.1128/CDLI.11.5.942-951.2004
139. Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, et al. Dynamic antibody responses to the mycobacterium tuberculosis proteome. Proc Natl Acad Sci U.S.A. (2010) 107:14703–8. doi: 10.1073/pnas.1009080107
140. Ivanyi J. Serodiagnosis of tuberculosis: due to shift track. Tuberculosis (Edinb). (2012) 92:31–7. doi: 10.1016/j.tube.2011.09.001
141. Ivanyi J. Could active case finding reduce the transmission of tuberculosis? Lancet. (2014) 383:1035–6. doi: 10.1016/S0140-6736(14)60510-9
142. Jackett PS, Bothamley GH, Batra HV, Mistry A, Young DB, and Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. (1988) 26:2313–8. doi: 10.1128/jcm.26.11.2313-2318.1988
143. Ivanyi J. Function and potentials of M. Tuberculosis epitopes. Front Immunol. (2014) 5:107. doi: 10.3389/fimmu.2014.00107
144. Ben-Selma W, Harizi H, and Boukadida J. Immunochromatographic igg/igm test for rapid diagnosis of active tuberculosis. Clin Vaccine Immunol. (2011) 18:2090–4. doi: 10.1128/CVI.05166-11
145. Jacobs AJ, Mongkolsapaya J, Screaton GR, McShane H, and Wilkinson RJ. Antibodies and tuberculosis. Tuberculosis (Edinb). (2016) 101:102–13. doi: 10.1016/j.tube.2016.08.001
146. Seibert FB. The significance of antigen-antibody reactions in tuberculosis. J Infect Dis. (1956) 99:76–83. doi: 10.1093/infdis/99.1.76
147. Kunnath-Velayudhan S and Gennaro ML. Immunodiagnosis of tuberculosis: A dynamic view of biomarker discovery. Clin Microbiol Rev. (2011) 24:792–805. doi: 10.1128/CMR.00014-11
148. Khaliq A, Ravindran R, Hussainy SF, Krishnan VV, Ambreen A, Yusuf NW, et al. Field evaluation of a blood based test for active tuberculosis in endemic settings. PloS One. (2017) 12:e0173359. doi: 10.1371/journal.pone.0173359
149. Broger T, Basu Roy R, Filomena A, Greef CH, Rimmele S, Havumaki J, et al. Diagnostic performance of tuberculosis-specific igg antibody profiles in patients with presumptive tuberculosis from two continents. Clin Infect Dis. (2017) 64:947–55. doi: 10.1093/cid/cix023
150. Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, and Reed SG. Identification of mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. (2010) 17:1539–47. doi: 10.1128/CVI.00198-10
151. Hogarth PM and Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov. (2012) 11:311–31. doi: 10.1038/nrd2909
152. Sutherland JS, Loxton AG, Haks MC, Kassa D, Ambrose L, Lee JS, et al. Differential gene expression of activating fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin Microbiol Infect. (2014) 20:O230–8. doi: 10.1111/1469-0691.12383
153. Abebe F, Belay M, Legesse M, LMCF K, and Ottenhoff THM. Iga and igg against mycobacterium tuberculosis rv2031 discriminate between pulmonary tuberculosis patients, mycobacterium tuberculosis-infected and non-infected individuals. PloS One. (2018) 13:e0190989. doi: 10.1371/journal.pone.0190989
154. Kaushik A, Singh UB, Porwal C, Venugopal SJ, Mohan A, Krishnan A, et al. Diagnostic potential of 16 kda (Hspx, alpha-crystalline) antigen for serodiagnosis of tuberculosis. Indian J Med Res. (2012) 135(5):771–7.
155. Melkie ST, Arias L, Farroni C, Jankovic Makek M, Goletti D, and Vilaplana C. The role of antibodies in tuberculosis diagnosis, prophylaxis and therapy: A review from the esgmyc study group. Eur Respir Rev. (2022) 31:210218. doi: 10.1183/16000617.0218-2021
156. Kaustova J. Serological igg, igm and iga diagnosis and prognosis of mycobacterial diseases in routine practice. Eur J Med Res. (1996) 1(8):393–403.
157. Querol JM, Oltra C, Granda D, Alonso MC, Climent JL, Labrador T, et al. Usefulness of igg and igm detection against antigen 60 in the diagnosis of thoracic tuberculosis. Med Interna. (1993) 10:271–4.
158. Awoniyi DO, Baumann R, Chegou NN, Kriel B, Jacobs R, Kidd M, et al. Detection of a combination of serum igg and iga antibodies against selected mycobacterial targets provides promising diagnostic signatures for active tb. Oncotarget. (2017) 8:37525–37. doi: 10.18632/oncotarget.16401
159. Khoo KH, Dell A, Morris HR, Brennan PJ, and Chatterjee D. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of mycobacterium. J Biol Chem. (1995) 270:12380–9. doi: 10.1074/jbc.270.21.12380
160. Angala SK, Palcekova Z, Belardinelli JM, and Jackson M. Covalent modifications of polysaccharides in mycobacteria. Nat Chem Biol. (2018) 14:193–8. doi: 10.1038/nchembio.2571
161. Chan CE, Gotze S, Seah GT, Seeberger PH, Tukvadze N, Wenk MR, et al. The diagnostic targeting of a carbohydrate virulence factor from M.Tuberculosis. Sci Rep. (2015) 5:10281. doi: 10.1038/srep10281
162. Song Z, He W, Pei S, Zhao B, Cao X, Wang Y, et al. Association of lineage 4.2.2 of mycobacterium tuberculosis with the 63-bp deletion variant of the mpt64 gene. Microbiol Spectr. (2023) 11:e0184223. doi: 10.1128/spectrum.01842-23
163. Wang X, Li H, Jiang G, Zhao L, Ma Y, Javid B, et al. Prevalence and drug resistance of nontuberculous mycobacteria, northern China, 2008-2011. Emerg Infect Dis. (2014) 20:1252–3. doi: 10.3201/eid2007.131801
164. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. (2016) 2:16076. doi: 10.1038/nrdp.2016.76
165. Motley MP, Banerjee K, and Fries BC. Monoclonal antibody-based therapies for bacterial infections. Curr Opin Infect Dis. (2019) 32:210–6. doi: 10.1097/QCO.0000000000000539
166. Delogu G, Sali M, and Fadda G. The biology of mycobacterium tuberculosis infection. Mediterr J Hematol Infect Dis. (2013) 5:e2013070. doi: 10.4084/MJHID.2013.070
167. Rijnink WF, Ottenhoff THM, and Joosten SA. B-cells and antibodies as contributors to effector immune responses in tuberculosis. Front Immunol. (2021) 12:640168. doi: 10.3389/fimmu.2021.640168
168. Global tuberculosis report 2017. Geneva: World Health Organization. (2017). Licence: CC BY-NC-SA 3.0 IGO.
169. Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, et al. Prevention of tuberculosis infection and disease by local bcg in repeatedly exposed rhesus macaques. Nat Med. (2019) 25:255–62. doi: 10.1038/s41591-018-0319-9
170. Scriba TJ and Nemes E. Protection against tuberculosis by mucosal bcg administration. Nat Med. (2019) 25:199–201. doi: 10.1038/s41591-019-0347-0
171. Izzo AA. Tuberculosis vaccines - perspectives from the nih/niaid mycobacteria vaccine testing program. Curr Opin Immunol. (2017) 47:78–84. doi: 10.1016/j.coi.2017.07.008
172. Scriba TJ, Netea MG, and Ginsberg AM. Key recent advances in tb vaccine development and understanding of protective immune responses against mycobacterium tuberculosis. Semin Immunol. (2020) 50:101431. doi: 10.1016/j.smim.2020.101431
173. Zhang Y, Xu JC, Hu ZD, and Fan XY. Advances in protein subunit vaccines against tuberculosis. Front Immunol. (2023) 14:1238586. doi: 10.3389/fimmu.2023.1238586
174. Dieli F and Ivanyi J. Role of antibodies in vaccine-mediated protection against tuberculosis. Cell Mol Immunol. (2022) 19:758–60. doi: 10.1038/s41423-022-00861-6
175. Zeng L and Li H. Protocol for rapid evaluation of antibody-mediated protection in macrophages using immunofluorescence techniques. STAR Protoc. (2024) 5:103324. doi: 10.1016/j.xpro.2024.103324
176. Zeng L, Zuo Y, Tang M, Lei C, Li H, Ma X, et al. A subunit vaccine ag85a-lpqh focusing on humoral immunity provides substantial protection against tuberculosis in mice. iScience. (2025) 28:111568. doi: 10.1016/j.isci.2024.111568
177. Pal R, Bisht MK, and Mukhopadhyay S. Secretory proteins of mycobacterium tuberculosis and their roles in modulation of host immune responses: focus on therapeutic targets. FEBS J. (2022) 289:4146–71. doi: 10.1111/febs.16369
178. Dulberger CL, Rubin EJ, and Boutte CC. The mycobacterial cell envelope - a moving target. Nat Rev Microbiol. (2020) 18:47–59. doi: 10.1038/s41579-019-0273-7
179. Song H, Sandie R, Wang Y, Andrade-Navarro MA, and Niederweis M. Identification of outer membrane proteins of mycobacterium tuberculosis. Tuberculosis (Edinb). (2008) 88:526–44. doi: 10.1016/j.tube.2008.02.004
180. Niederweis M, Danilchanka O, Huff J, Hoffmann C, and Engelhardt H. Mycobacterial outer membranes: in search of proteins. Trends Microbiol. (2010) 18:109–16. doi: 10.1016/j.tim.2009.12.005
181. Newbrough SA, Mocsai A, Clemens RA, Wu JN, Silverman MA, Singer AL, et al. Slp-76 regulates fcgamma receptor and integrin signaling in neutrophils. Immunity. (2003) 19:761–9. doi: 10.1016/s1074-7613(03)00305-4
182. Ahmed M, Thirunavukkarasu S, Rosa BA, Thomas KA, Das S, Rangel-Moreno J, et al. Immune correlates of tuberculosis disease and risk translate across species. Sci Transl Med. (2020) 12. doi: 10.1126/scitranslmed.aay0233
183. Dorhoi A and Kaufmann SH. Versatile myeloid cell subsets contribute to tuberculosis-associated inflammation. Eur J Immunol. (2015) 45:2191–202. doi: 10.1002/eji.201545493
184. Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, et al. S100a8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med. (2013) 188:1137–46. doi: 10.1164/rccm.201304-0803OC
185. Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. (2010) 466:973–7. doi: 10.1038/nature09247
186. Forget A, Benoit JC, Turcotte R, and Gusew-Chartrand N. Enhancement activity of anti-mycobacterial sera in experimental mycobacterium bovis (Bcg) infection in mice. Infect Immun. (1976) 13:1301–6. doi: 10.1128/iai.13.5.1301-1306.1976
Keywords: Mycobacterium tuberculosis, tuberculosis, humoral immunity, antibody isotype, BCG
Citation: Li H and Li H (2025) Different antibody isotypes against tuberculosis: what we know and what we need to know. Front. Immunol. 16:1682934. doi: 10.3389/fimmu.2025.1682934
Received: 10 August 2025; Accepted: 07 October 2025;
Published: 22 October 2025.
Edited by:
Bingdong Zhu, Lanzhou University, ChinaReviewed by:
Juraj Ivanyi, King’s College London, United KingdomKonstantin Lyashchenko, Chembio, United States
Fei Li, Lanzhou University, China
Copyright © 2025 Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Li, bGloYW9fdGh1QGhvdG1haWwuY29t
 Huoming Li
Huoming Li Hao Li
Hao Li