- 1College of Basic Medical Sciences, Shanxi University of Chinese Medicine, Jinzhong, China
- 2Basic Laboratory of Integrated Traditional Chinese and Western Medicine, Shanxi University of Chinese Medicine, Jinzhong, China
- 3Engineering Research Center of Cross Innovation for Chinese Traditional Medicine of Shanxi Province, Jinzhong, China
- 4Department of Acute General Medicine, John Radcliffe Hospital, Oxford, United Kingdom
As a key lymphocyte population in shaping and controlling adaptive immune response, T cells play an important immunomodulatory role in the early stages of autoimmune diseases. Although CD3+CD4−CD8− T (DNT) cells constitute only a small proportion of peripheral T lymphocytes, they may be closely linked to the occurrence and development of autoimmune diseases. However, the role of DNT cells in autoimmune disease pathogenesis still needs to be elucidated. In this review, we first present the origin, functions, and heterogeneity of DNT cells. We then summarize the role of DNT cells in the pathogenesis of various autoimmune diseases. Subsequently, we clarify the recent advances in the applications of DNT cell-based therapy for autoimmune diseases and outline potential drugs (including active ingredients extracted from Chinese medicinal treatments) and approaches that can target the proliferation and expansion of DNT cells. Lastly, the limitations and challenges of applying DNT-cell-based therapy are analyzed. In conclusion, we present an overview to further the understanding of the role of DNT cells in autoimmune disease pathogenesis and of DNT cells as a potential therapeutic tool for immune disorders.
1 Introduction
It is widely accepted that CD4+ helper and CD8+ cytotoxic T cells form the majority of adaptive immune cells, which shape and control the immune response, and play crucial roles in the development of immune diseases and tumors (1). They are characterized by the positive expression of T-cell receptor (TCR) (alpha and beta chains, αβ) and either CD4 or CD8 (2). The aberrant activation and accumulation of the CD4+ and CD8+ T cells are the foremost hallmarks of autoimmune diseases (3). However, another small population of T cells, termed CD3+CD4−CD8− T cells (Double-Negative T cells, DNT), has gained attention for its contribution to the pathophysiology of several autoimmune diseases (4, 5).
DNT cells are T cells which express CD3 but lack both CD4 and CD8 coreceptors (6). They also do not express NK cell markers such as CD56 (7). DNT cells do express TCR αβ or TCR gamma and delta (γδ), which enables them to recognize and respond to pathogens, mounting a functional adaptive immune response (7, 8). In humans, DNT cells make up approximately 1-5% of the total lymphocyte population in the peripheral blood and lymphoid organs (9). DNT cells, like CD4+ and CD8+ T cells, possess innate and adaptive immune functions, and hence play an important role in the pathogenesis of autoimmune diseases, including in autoimmune lymphoproliferative syndrome (ALPS), systemic lupus erythematosus (SLE), systemic sclerosis, and Sjogren’s syndrome (10–13). Nevertheless, our understanding of the functions and cellular origins of DNT cells remains limited.
Here, DNT cells play diverse roles in autoimmune diseases and immunotherapy, with potential molecular mechanisms. We will also discuss the potential combination of Chinese medicinal materials and DNT Cells in treating autoimmune diseases. This review aims to broaden current understanding of the complex roles of DNT cells.
2 Ontogeny and classification of DNT cells
2.1 Ontogeny of DNT cells
Progenitor lymphocytes originate in the bone marrow and generally migrate to the thymus to undergo differentiation into mature T cells. Developing T cells progress through multiple stages of differentiation, defined by the expression or lack of the CD4 and CD8 coreceptor molecules, including the Double Negative stage (DN), Double Positive stage (DP), and Single Positive stage (SP) (14). According to the expression of CD25 and CD44, murine DN thymocytes are further divided into four differentiation stages, which are DN1 (CD44+CD25-), DN2 (CD44+CD25+), DN3 (CD44-CD25+), and DN4 (CD44-CD25-). Human DN thymocytes undergo three distinct DN stages (DN1–DN3), defined by the expression or lack of CD34, CD38 and CD1a (15).
There is evidence that early T cells may develop into mature DNT cells in both the thymic and peripheral environments, however, the origin of DNT cells is still poorly understood (16). There are multiple theories regarding this, as shown in Figure 1. Peripheral DNT cells may originate from immature DN thymocytes which do not undergo negative selection in the thymus, and hence they escape further thymic development and migrate to peripheral blood. One theory hypothesizes that T cells which express CD4 or CD8 become DNT cells in the periphery by downregulating the expression of the co-receptor (possibly by demethylation of the CD8 or CD4 genes) (17). A second theory is that the DNT cells do not complete differentiation from DN4 to DP T cells, and instead exit the thymus and enter the periphery (18). There is a third theory that hematopoietic stem cells can leave the bone marrow, bypassing the thymus and mature into DNT cells in the periphery without needing to first develop from CD4+ or CD8+ T cell precursors (19). A fourth theory suggests that common lymphoid progenitor (early T) cells underwent an alternative developmental pathway in the thymus, hence not developing further into CD4+ or CD8+ T cells (9). A major limitation in understanding DNT cell ontogeny is that most studies use murine models, many of which are transgenic. Therefore, the application of this data to human immunology should be undertaken with caution.

Figure 1. Proposed mechanisms of DNT cell development. Four proposed theories exist on the origin of DNT cells. Theory 1 suggests that CD4+ or CD8+ T cells in the periphery may downregulate their co-receptors, possibly through demethylation, leading to the formation of DNT cells. Theory 2 proposes that an incomplete transition from DN4 to C stages in the thymus results in DNT cells migrating to the periphery. Theory 3 posits that HSCs directly mature into DNT cells in the periphery. Theory 4 indicates that CLPs migrate to the periphery and develop into DNT cells utilizing an alternate thymic pathway. DN, double negative stage; DP, double positive; HSCs, hematopoietic stem cells; CLPs, common lymphoid progenitors.
2.2 Classification and function of DNT cells
DNT cells are difficult to characterize into different phenotypes because there is a lack of well-defined cell markers, and thus, exclusion criteria are often used instead. As shown in Figure 2, DNT cells can be divided into three subpopulations that include DN natural killer T cells (NKT), TCR αβ+ DNT cells, and TCR γδ+ DNT cells (20). DN NKT cells have a close relationship with autoimmunity and infection. TCR αβ+ DNT cells can have both inflammatory and anti-inflammatory functions. TCR γδ+ DNT cells have potential roles in cytotoxicity against tumor cells, as well as bacterial and viral infections.
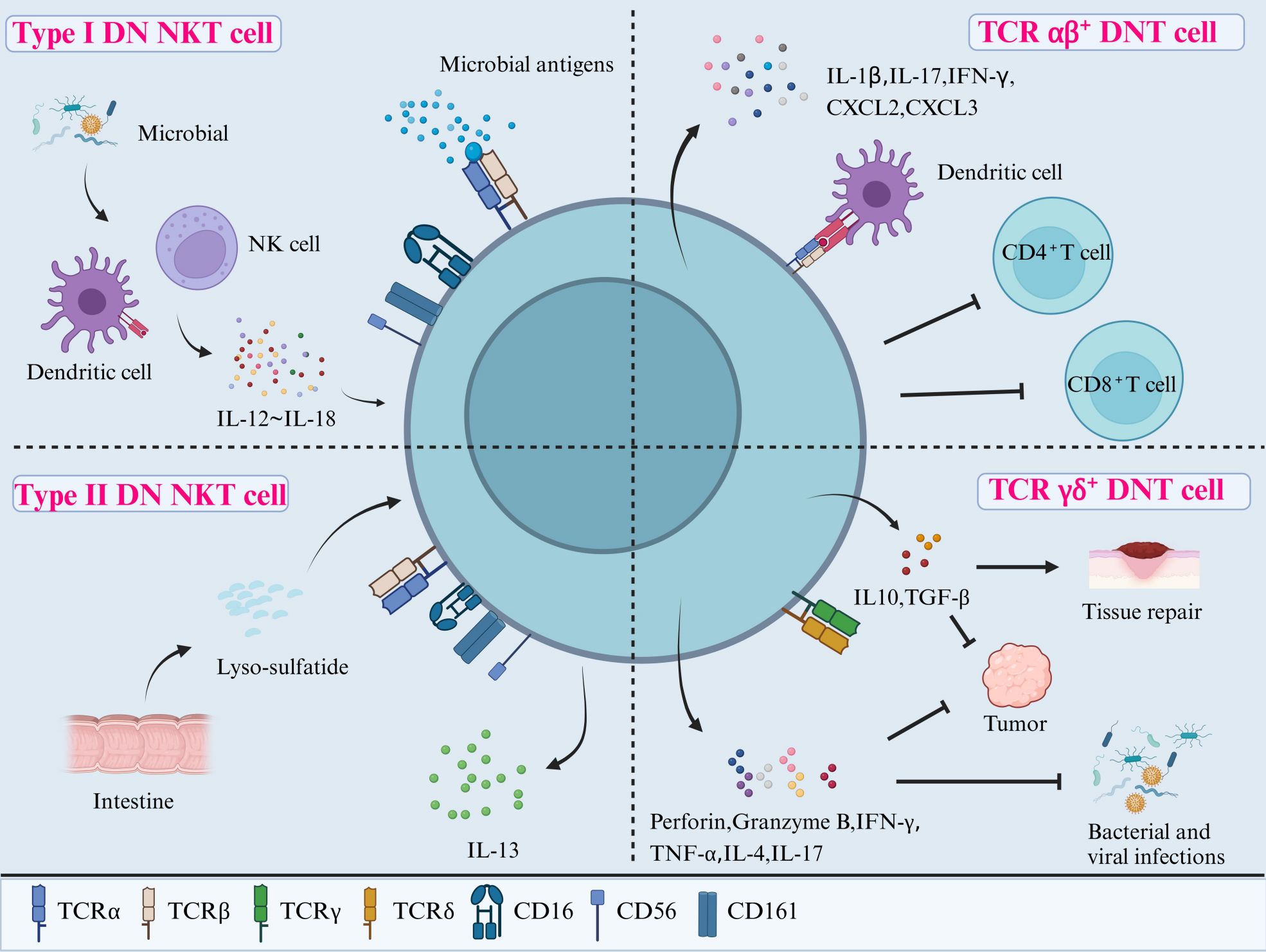
Figure 2. Classification and functions of DNT cells. DNT cells can be divided into three subpopulations according to their cell surface receptors: DN NKT cells (including Type I and II), TCR αβ+ DNT cells, and TCR γδ+ DNT cells. Type I DN NKT cells may protect the host from infections after being activated through the direct recognition of microbial antigens via TCR or by cytokines (ranging from IL-12 to IL-18) secreted from DCs and NK cells in response to microbial antigens. Type II DN NKT cells interact with tissue-specific antigens, such as lyso-sulfatide, to regulate the immune reactions in the gut and other tissues. TCR αβ+ DNT cells have pro-inflammatory roles through producing cytokines such as IL-1β, IL-17, and IFN-γ. They can also exert potent immunosuppressive activity on CD4+ and CD8+ T cells after activation by APCs. Lastly, TCR γδ+ DNT cells play a role in combatting infections and tumors by releasing perforin, granzyme B, IFN-γ, TNF-α, IL-4, and IL-17, as well as promoting tissue repair via IL-10 and TGF-β. NKT, natural killer T cells; IL, interleukin; DCs, dendritic cells; TCR, T cell receptor; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β.
2.2.1 DN NKT cells
DN NKT cells express NK-related surface markers, such as CD16, CD56, and CD161, as well as the TCR (21). They can be further divided into DN NKT type I and II cells. Type I DN NKT cells (also known as invariant natural killer T cells (iNKT) have a highly conserved TCR α chain and therefore are more restricted in their TCR repertoire (22). Type I DN NKT cells are activated by lipid antigens presented by CD1d (a non-classical major histocompatibility complex (MHC) member), for example, human and murine type DN NKT cells recognize marine sponge glycolipid and α-galactosylceramide (α-GalCer) (22). Once activated, they have a cytotoxic action within minutes (23). In contrast, the type II DN NKT cells are not activated by α-GalCer, but do react with a wider range of lipid antigens, including self-lipids. For example, they are activated by sulfatide, a glycolipid in myelin found in the central nervous system, when presented by CD1d, which could play a role in autoimmune demyelinating diseases (24).
Type I DN NKT cells can be activated by antigens through two different mechanisms: a. direct binding of TCR to microbial antigens presented by CD1d, known as direct recognition; and b. expansion mediated by cytokines (IL-12 and IL-18) released by other cells (antigen-presenting cells like dendritic cells, other NK cells, other T cells), known as indirect recognition. The cytokine response in indirect recognition can lower the threshold for TCR-mediated activation of NKT cells, however, there is also evidence to show that IL-12 and IL-18 alone can activate NKT cells without the need for CD1d-mediated antigen binding (25, 26). The type II DN NKT cells, also named sulfatide-reacting NKT cells, also recognize antigens through CD1d-mediated TCR activation. They may display protective or pro-inflammatory functions according to the surrounding types of tissue-specific ligands. For example, activation of type II DN NKT cells in a murine CNS model prevented induction of autoimmune encephalomyelitis, potentially through inducing the anergy of type I DN NKT cell and dendritic cells (27). Conversely, type II DN NKT cells in the gut responded to stimulation by lyso-sulfatide by secreting IL-13 and inducing epithelial cell cytotoxicity, thought to play a role in the pathogenesis of ulcerative colitis (28).
2.2.2 TCR αβ+ DNT
TCR αβ+ DNT cells are characterized by the expression of polyclonal TCR αβ molecules without NK cell-related markers (such as CD16 and CD56). They also do not express regulatory T (Treg) cell-related markers (such as CTLA-4 and CD25) (7). Furthermore, some inhibitory molecules, including programmed cell death protein (PD-1) and Helios, are highly expressed by TCR αβ+ DNT cells. Studies in murine models have shown that PD-1 and Helios are expressed by DNT cells derived from self-reactive CD8+ T cells, through loss of CD8 expression after self-antigen recognition (29).
TCR αβ+ DNT cells, especially a phenotype derived from self-reactive CD8+ T cells, can produce an array of pro-inflammatory mediators including IL-1β, IL-17 and interferon (IFN)-γ (30). On the other hand, TCR αβ+ DNT cells are a type of inducible Treg cell and can also exert potent immunosuppressive activity. Activated human TCR αβ+ DNT cells can reduce the proliferation and cytokine production of CD4+ and CD8+ T cells, thought to be through a cell contact-dependent mechanism and TCR binding (31). Murine TCR αβ+ DNT cells have also been found to contribute to peripheral tolerance to alloantigens in vitro and in vivo by killing activated syngeneic CD8+ T cells through the Fas-FasL pathway (32). Another function of TCR-αβ+ DNT cells is that they make up a subset of the mucosal-associated invariant T-cell family (MAIT), where they can respond to mucosal microbial antigens. They have important functions in mucosal immune responses in the oral mucosa (33), the lung (34), and against several infectious pathogens, such as E. Coli, H. Pylori, and H. influenzae (35). MAIT cells also play a role in several types of cancers, such as cervical cancer, where DN MAIT cells have been linked to survival benefit (36), as well as in the development of several autoimmune diseases (37, 38).
2.2.3 TCR γδ+ DNT cells
TCR γδ+ DNT cells, classified by the expression of TCR γδ, play an important regulatory role (39). They account for over 70% of all γδ T cells (40).
Single cell RNA sequencing has improved our understanding and definition of DNT cells into subgroups, based on differential gene expression. Yang et al. used RNA sequencing on murine immune cells to identify five distinct groups of naïve DNT (nDNT) cells. These included nDNT at rest, nDNT helper, nDNT intermediate, nDNT cytotoxic and innate nDNT. There five groups of nDNT cells have different transcription signatures and roles within the immune system, hence this progresses our understanding of how DNT cells are linked to autoimmune diseases (41).
TCR γδ+ DNT cells are activated by antigen presentation by MHC I, MHC II and CD1 molecules (26). They function as potent cytotoxic effector cells through the release of perforin, granzyme B, IFN-γ, tumor necrosis factor (TNF)-α, IL-4, and IL-17, hence help to combat intracellular and extracellular pathogens and malignant cells (42). In addition to their cytotoxic functions, these cells can downregulate the innate and adaptive immune response by secreting IL-10 and transforming growth factor (TGF)-β. They also contribute to tissue repair and wound healing, through the secretion of epithelial cell growth factors. However, this regulatory activity may also suppress anti-tumor activity, hence highlighting the dual role of TCR γδ+ DNT cells in immune regulation (43).
2.3 Heterogeneity and plasticity of DNT cells
DNT cells tend to exhibit effector cell phenotypes with a terminal differentiation status and poor proliferation upon anti-CD3 and TCR complex activation (5). Expanded DNT cells show increased Ki67 expression, narrowed TCR Vβ repertoire, and diluted TREC content during clonal proliferation in SLE and acute kidney injury (44, 45). It may be that there is heterogeneity in the DNT cell pool in differentiation states and potential for plasticity, therefore, some cells may be fully differentiated, whilst others may be partially differentiated (46).
DNT cells display diverse cytokine profiles in different disease models. IL-17 is the main pro-inflammatory cytokine secreted by DNT cells in SLE and chronic infection settings. IL-17 production can be promoted by IL-23 and reduced by IL-2 (47, 48). In contrast, DNT cells produce immunosuppressive IL-10 to regulate immune response in other models, such as in non-obese diabetes and allograft rejection (7, 9, 49). Current evidence suggests naïve DNT cells primarily exhibit regulatory functions to maintain self-tolerance, but chronic inflammation disrupts this balance, leading to predominant pro-inflammatory DNT cells (50).
3 The role of DNT cells in the development of autoimmune diseases
A summary of the autoimmune conditions discussed in this section and the evidence for the role of DNT cells is presented in Table 1.
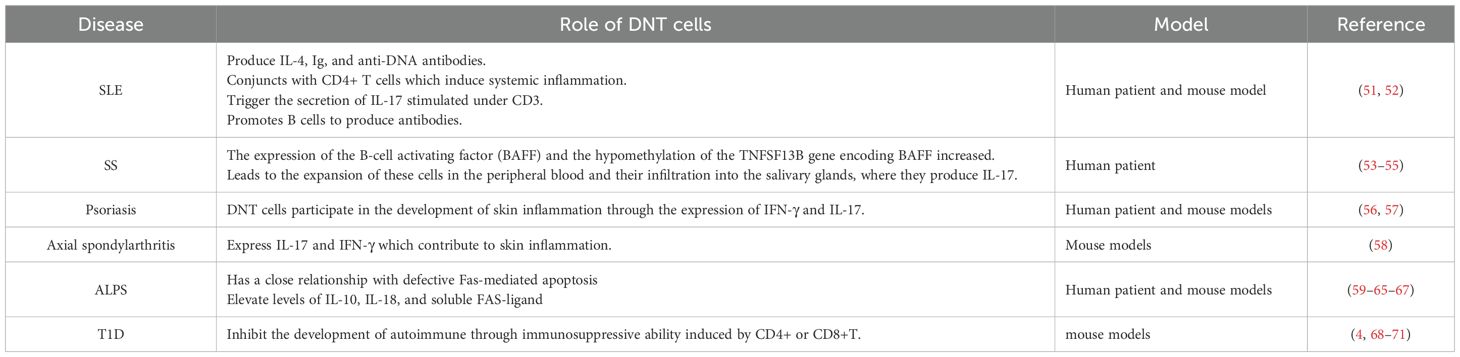
Table 1. The role of DNT cells in the pathogenesis of different autoimmune diseases described in this article.
3.1 Rheumatological conditions
3.1.1 Systemic lupus erythematosus
SLE is a systemic autoimmune disease arising from inappropriate immune responses to self-antigens. This leads to the deposition of autoantibodies and immune complexes, as well as lymphocyte infiltration, which affects several organs including the skin, kidneys, and joints (72). The infiltrating lymphocytes in SLE are composed of activated T cells expressing high levels of adhesion molecules (51).
The population of DNT cells in the peripheral blood of SLE patients are significantly expanded compared to healthy controls (52). They are known to induce systemic inflammation synergistically with CD4+ T cells, by producing pro-inflammatory cytokines such as IL-4 and IL-17, and anti-DNA IgG autoantibodies (17, 52, 73). DNT cells have been shown in a murine model to produce IL-17 and proliferate rapidly following anti-CD3 stimulation and exposure to self-antigens, and as noted earlier, the PD1+ but not PD1- phenotypes of DNT cells are the main source of IL-17 (25). In a murine model, activation of the mTORC1 pathway in DNT cells is associated with higher IL-17A levels, and phosphatidic acid, a lipid metabolite in T cells, can increase mTORC1 downstream signaling and lead to the expansion of IL-17A-producing DNT cells (11). Moreover, an in vitro study showed that DNT cells can also promote B cells to produce autoantibodies, contributing to the development of SLE (52).
It has been reported that a large proportion of the expanded DNT cells is likely derived from self-reactive CD8+ T cells (17). The conversion from CD8+ T cells into DNT cells is due to the downregulation of CD8 expression induced by the inflammatory cytokine environment. This contributes to the pathogenesis of SLE through the loss of CD8-dependent immunosuppressive mechanisms (17).
3.1.2 Sjögren’s syndrome
Sjögren’s syndrome (SS) is a chronic autoimmune disease which leads to chronic inflammation, tissue damage, and impaired secretory function of exocrine glands, hence the hallmark of the disease is dryness of the eyes and mouth (74, 75).
DNT cell expansion has been reported in the peripheral blood of SS patients, as well as in the lymphocytes which infiltrate into the salivary glands of SS patients (53). These DNT cells from patients with SS produce IL-17, which plays an important role in the destruction of glandular tissues (54), and have been found to show no response to corticosteroids in vitro (53). Furthermore, DNT cells from patients with SS promote the pathological activation of B cells by increasing the expression of B cell activating factor (BAFF), via epigenetic modulation of the BAFF-encoding gene, TNFSF13B (55). As a member of the tumor necrosis factor (TNF) family, BAFF promotes the maturation and activation of B cells, whereas, an excess of BAFF leading to B cell hyperactivity and autoantibody production are known features of SS (76).
3.1.3 Psoriasis and axial spondylarthritis
Psoriasis is a common systemic autoimmune, characterized by dryness, scaling and redness of the skin (77). Its underlying pathogenesis involves immune cell infiltration into the epidermis, inducing skin tissue inflammation and excessive proliferation of keratinocytes (56). In a murine model of psoriasis, DNT cells were found to infiltrate lesional skin and to produce IL-17, promoting skin inflammation (57). DNT cells isolated from the peripheral blood of psoriasis patients show reduced DNA methylation of the IFNG gene (which permits greater production of IFN-γ). These DNT cells also express higher levels of PD-1 (56). Together, these findings indicate that DNT cells are involved in the pathogenesis of psoriasis and contribute to skin inflammation via the expression of IFN-γ and IL-17.
Axial spondylarthritis is another chronic autoimmune disease that affects primarily the spine, the sacroiliac joints and tendon-bone attachments (entheses) but shares many genetic features with psoriasis (78). Interestingly, in a murine model of spondyloarthropathy, DNT cells expressing IL-23 receptor were shown to be present in inflamed entheses, and responded to IL-23 by secreting further inflammatory interleukins and chemokines (58). This supports the role of DNT cells in the pathogenesis of axial spondylarthritis.
3.2 Autoimmune lymphoproliferative syndrome
Autoimmune lymphoproliferative syndrome (ALPS) is a rare disorder leading to splenomegaly, non-malignant lymphadenopathy cytopenia and autoimmune complications such as glomerulonephritis, hepatitis and vasculitis (59). ALPS is caused by genetic mutations which lead to defective lymphocyte apoptosis, resulting in the abnormal accumulation of lymphocytes. Notably, one of the diagnostic criteria for ALPS is the expansion of DNT cells in peripheral blood and secondary lymphoid organs, which contributes to the disease’s pathogenesis (60, 61).
The classical genetic mutations causing ALPS are found in FAS, FASL and CASP10, causing defective Fas-mediated apoptosis and hence accumulation of lymphocytes (62). Fas and Fas ligand (FasL) interact, leading to the formation of the death-inducing signaling complex, which triggers a cascade of caspase enzymes which facilitate apoptosis (63). Other biochemical changes associated with ALPS are higher peripheral blood levels of IL-10, IL-18, and soluble FAS-ligand (62).
DNT cells from ALPS patients may also be derived from CD8+ T cells because the two T cell types share a significant proportion of CDR3 sequences across several TCRVβ families (64). They express abnormally high levels of the B cell antigen B220 (an isoform of the CD45 antigen), which is also expressed on activated T cells that undergo apoptosis (65). DNT cells from ALPS patients co-express CD27 and CD28, which is a characteristic of naïve and central memory T-cell subsets and is usually absent in effector T cells (66, 67). Despite this, DNT cells are mature antigen-experienced effector T lymphocytes, and DNT cells from ALPS patients do proliferate, in contrast to DNT cells from healthy individuals (32, 61).
The role of the accumulation of DNT cells in contributing to the pathogenesis of ALPS is well evidenced, based on several studies. The presence of autoantibodies in most ALPS patients correlates with the abundance of DNT cells in peripheral blood, which may be due to the B cell-promoting effects of IL-10, which is highly expressed by DNT cells from ALPS patients (63, 79). Furthermore, effective treatment of ALPS in murine models (using rapamycin) improved the pathological features of ALPS (such as splenomegaly, lymphadenopathy), in association with decreasing DNT cell levels and autoantibody levels (80, 81). Whilst this supports the key role of DNT cells in the pathogenesis of ALPS, further evidence is still needed to elucidate the pathophysiological mechanisms.
3.3 Type 1 diabetes
Type 1 diabetes (T1D) is an autoimmune disease in which there is destruction of insulin-producing pancreatic β cells, caused by self-reactive CD4+ and CD8+ T cells targeting β -cell antigens in genetically susceptible individuals (82, 83).
DNT cells have been found to prevent the development of autoimmune diabetes through their immunosuppressive effects (9), which is supported by several studies. Firstly, in the non-obese diabetic (NOD) murine model of T1D, diabetes develops around 12–14 weeks of age, which is correlated with a decrease in DNT cell numbers at the same time (49). Next, several studies using different diabetic mouse models show that transfer of DNT cells can slow the progression or even reverse autoimmune diabetes (68–70). Furthermore, transfer of NOD CD8+ T cells resulted in diabetes but co-transfer of NOD CD8+ T cells with DNT cells did not. It suggests that DNT cells act directly on the pathogenic effector T cells to carry out their immunoregulatory function (49). This may be through Fas/FasL-mediated apoptosis (71), perforin-mediated killing (84, 85) or the modulation of antigen-presenting cells through secretion of IL-10 and IFN γ (68, 86).
4 DNT cells in autoimmune disease immunotherapy
4.1 Roles and applications of DNT cells in treating autoimmune diseases
As discussed, DNT cells display diverse functions (including both inflammatory and anti-inflammatory activity) in autoimmune diseases. Therefore, there are several potential therapeutic approaches to autoimmune diseases which use our understanding of the role of DNT cells, such as selective inhibition to render DNT cells less pathogenic, specific modulation to promote their proliferation and inhibiting their apoptosis. Increasingly, approaches directly or indirectly targeting DNT cells have been trialed. Multiple potential approaches are depicted in Figure 3.
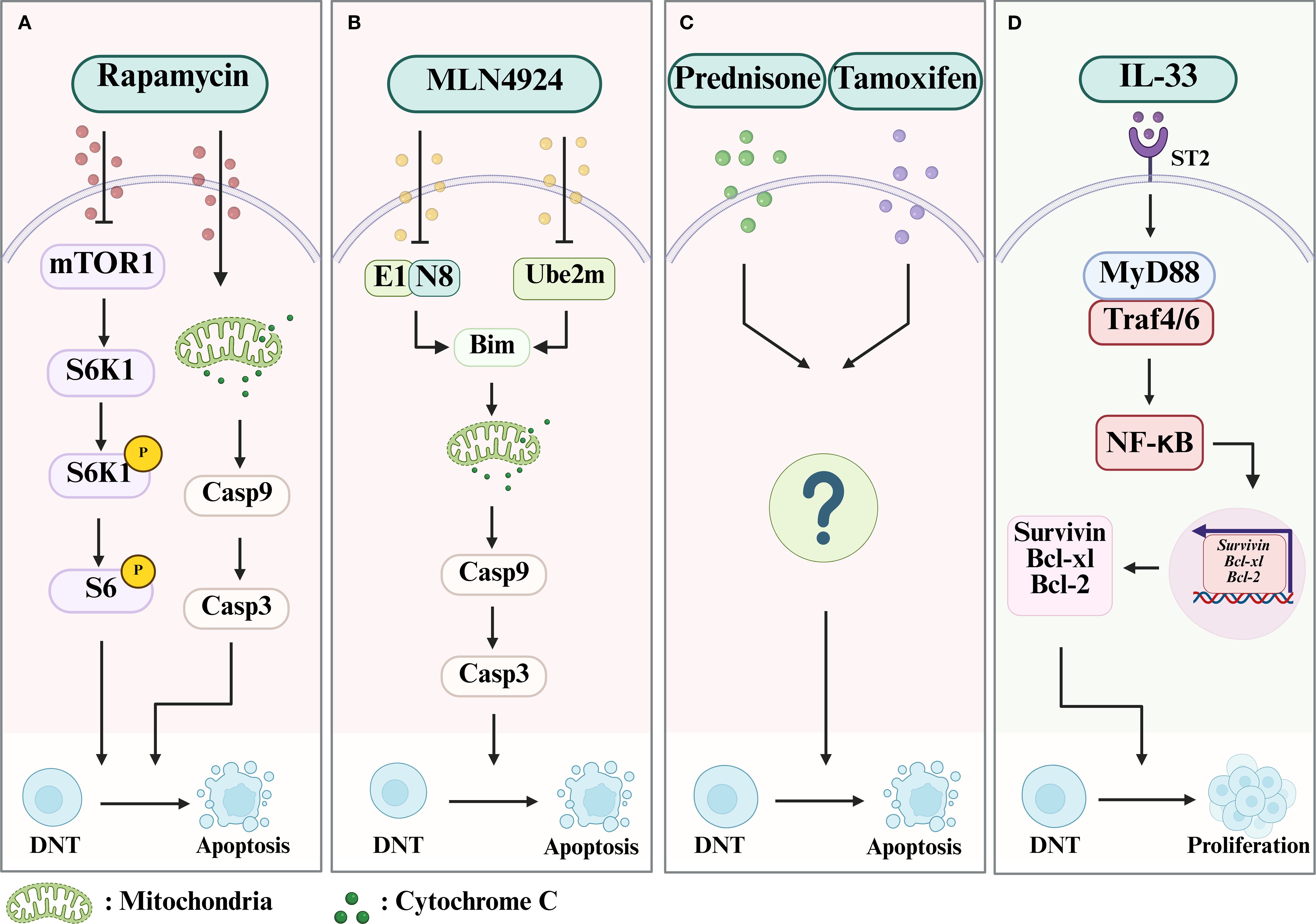
Figure 3. The mechanism of drugs which limit or promote the expansion of DNT cells. (A) Rapamycin can lead to the apoptosis of DNT cells by both inhibiting the mTOR/S6K1 pathway and Cytochrome C/Casp 9/Casp 3 pathway. (B) MLN4924 can inhibit NAE1 or Ube2m to block degradation of Bim, which will increase the mitochondrial membrane permeability and lead to the activation of the Cytochrome C/Casp 9/Casp 3 pathway, resulting in the apoptosis of DNT cells. (C) Prednisone and Tamoxifen can promote the apoptosis of DNT cells via an undefined mechanism. (D) IL-33 can contribute to the survival and proliferation of DNT cells by activating the MyD88/NF-κB pathway, which will subsequently induce the expression of Bcl-xl, Bcl-2, and Survivin, through the ST2 receptor. Case, Caspase; NEDD8, neuronal precursor cell-expressed developmentally downregulated protein 8; NAE1, NEDD8-activating enzyme E1; Ube2m, genetic knockout of NEDD8-conjugating enzyme E2.
4.1.1 Drug treatments limiting the expansion of pathogenic DNT cells
Rapamycin (also called sirolimus) is a drug which inhibits the mammalian target of rapamycin (mTOR) and is an immunosuppressive and antitumor agent with a tolerable side-effect profile (87). which may induce apoptosis in T and B lymphocytes (88, 89). In a preclinical study with murine models of ALPS, rapamycin induced a dramatic decrease in DNT cells (both in peripheral blood and lymphoid tissue), as well as clinical signs of the condition and the levels of anti-dsDNA IgG autoantibodies) (80). The authors also found that rapamycin could downregulate phosphor-S6 by inhibiting the activation of S6K1 (p70S6 kinase) and phospho-Bad (a proapoptotic molecule that is regulated by phosphorylation), and hence trigger caspase-dependent apoptosis. Subsequently, the function of rapamycin in suppressing the proliferation of DNT cells was verified again in a clinical trial including six ALPS patients who had failed therapy with steroids, where all patients showed significant response to treatment. It was suggested that rapamycin could be an effective second-line treatment for ALPS (81). A similar result was reported in a patient with a de novo FAS mutation with a severe phenotype of ALPS (90). Peripheral blood samples from the case report patient showed T-cell lymphocytosis with a higher proportion of DNT cells and a lower proportion of Treg cells compared to healthy controls. The patient displayed rapid clinical improvement and decrease in DNT cell numbers after being started on rapamycin.
Resembled to ubiquitination, neddylation-a form of protein post-translational modification involving binding of neuronal precursor cell-expressed developmentally downregulated protein 8 (NEDD8) to other proteins-is known to regulate T cell immune responses. It plays an important role in regulating CD4+ T cell activation, proliferation, and differentiation into T helper subsets such as Th1, Th2, T follicular helper cells and Treg cells (91–93). In a murine lupus model using MRL/lpr mice, it has been shown that inactivation of neddylation with MLN4924, a specific inhibitor of NEDD8-activating enzyme E1 (NAE1), or genetic knockout of NEDD8-conjugating enzyme E2 (Ube2m) in T cells, decreased DNT-cell accumulation and slowed the development of lupus (94). Further investigations revealed that inactivation of neddylation reduced the degradation of Bim (Bcl-2 interacting mediator of cell death, a pro-apoptotic protein) by ubiquitination and maintained Bim at higher levels in DNT cells, hence contributing to the apoptosis of the expanded DNT cells in lupus mice. In double knockout lupus mice (Ube2m-/-Bim-/-lpr), the inactivation of Bim prevented increase in apoptosis of DNT cells and the anti-lupus effect seen when expression of Ube2m alone was inhibited, suggesting that the apoptosis triggered by neddylation is dependent on Bim (94). Compared to healthy controls, SLE patients are known to have lower levels of Bim and higher levels of Cullin1 neddylation, and the inhibition of neddylation can cause apoptosis of SLE patient DNT cells (94). Altogether, these new findings suggest a promising therapeutic approach to SLE through targeting DNT cell neddylation.
Glucocorticoids are effective in treating inflammation in many autoimmune conditions, including in the induction and maintenance of remission in patients with SLE (95). CD138 (syndecan-1) is a member of the transmembrane heparan sulfate proteoglycan family and is important in mediating cell-cell adhesion and cell-matrix adhesion (96). It is also a marker of differentiated plasma cells and is associated with the production of anti-dsDNA antibodies in the pathogenesis of SLE (97). A large proportion of DNT cells in MRL/lpr mice (a model of spontaneous SLE) are CD138 positive (CD3+CD4-CD8-CD138+ phenotype) (98). Similar to the pathogenesis of ALPS, it has been reported that Fas deficiency leads to DNT cell accumulation in the spleens of MRL/lpr mice (a model of spontaneous SLE) and results in splenomegaly and lymphadenopathy (99). The CD138+ DNT cells which accumulate in the spleens of MRL/lpr mice overexpress FasL, and therefore can attack tissues in MRL/lpr mice that express low levels of the Fas receptor (100). Prednisone, a commonly used GC, when used to treat SLE in MRL/lpr mice, cause reduced accumulation of CD138+ T cells in the spleens of MRL/Lpr mice. This also leads to prednisolone causing reduced levels of anti-dsDNA autoantibodies (101). This finding provides new insights into the therapeutic effects and mechanisms of GCs as treatment for SLE, although the signaling pathways for reducing the frequency of CD138+ DNT cells in MRL/lpr mice are yet to be elucidated.
As a selective estrogen receptor modulator, tamoxifen (TAM) is used to treat estrogen receptor-positive breast cancer. Its therapeutic effect is mediated by endoxifen, the principal metabolite of tamoxifen (102). It is a synthetic non-steroidal triphenylethylene compound (103). Studies using MRL-lpr/lpr murine SLE models have shown that estrogen treatment can exacerbate the pathologies in SLE, whilst TAM induces positive response to treatment, such as reducing splenomegaly and lymphadenopathy (104–107). In addition, TAM-treated MRL-lpr/lpr mice had a significantly lower proportion of DN T cells (108). In vitro, TAM also led to dose-dependent inhibition of the IL-2-activated proliferation of DNT cells from lymph nodes (108). These findings offer a novel approach to exploring the feasibility of using selective estrogen receptor modulators as treatment for autoimmune diseases (including SLE).
4.1.2 Specific drugs to promote the proliferation and activation of DNT cells
Autoimmune hepatitis (AIH) is an inflammatory disease of the liver mediated by abnormal autoimmune responses, which can occur in different age groups and ethnic groups, and is more common in women (109). In 2021, the global incidence of AIH was 0.4-2.39/100,000 people, and the global prevalence was 4.8-42.9/100,000 people, and the incidence and prevalence continue to increase yearly (110). It is widely understood that liver injury is induced by the aberrant activation of T cells (mainly Th1 and Th 17), B cells, and macrophages directed against liver autoantigens, due to a loss of tolerance (111).
A recent study demonstrated that transfer of DN T cells converted from CD4+ T cells could suppress inflammatory responses in hepatitis, using a typical Concanavalin (Con A)-induced murine liver injury model (112). IL-33 is a cytokine released during tissue injury, with important roles in regulating inflammation, tissue homeostasis and repair and type 2 immune responses, as well as affecting the development of autoimmune diseases, viral infections, and cancer (113). It activates many immune cell subsets including NK cells, CD8+ T cells, neutrophils, regulatory macrophages, and Treg cells, via the IL-33 (ST2) receptor (114). Recent studies have found that DN T cells also express ST2 and that stimulation by IL-33 promotes DN T cell proliferation and suppresses their apoptosis, both in the Con A-induced murine liver injury model and in vitro during the conversion of DNT cells from CD4+ T cells isolated from human peripheral blood (112). In conclusion, these studies reveal a role for IL-33 in regulating DN T cells, and introduce new potential pathways to induce the expansion of DNT cells in the immune environment.
4.2 Combination of Chinese medicinal materials and DNT cells in treating autoimmune diseases
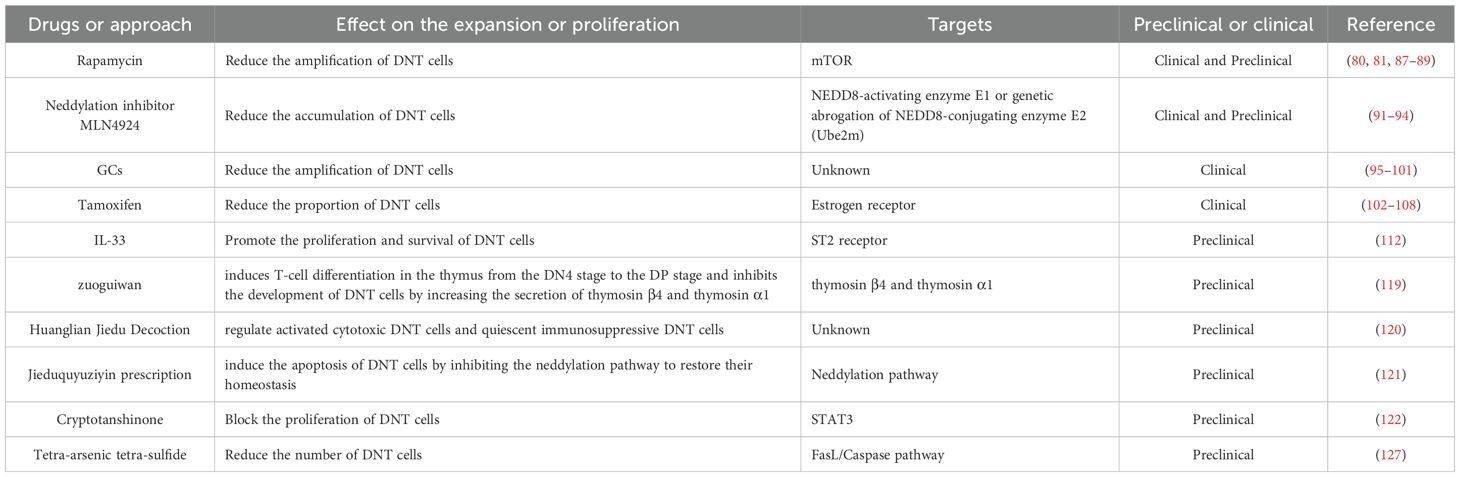
It has been shown that various traditional Chinese medicines (TCMs) can ameliorate autoimmune diseases, including in rheumatoid arthritis, osteoarthritis SLE and AIH, which has been demonstrated by both clinical trials and preliminary research in vivo (115–117). These TCMs include monomers isolated from Chinese herbs, extracts of Chinese herbs, and Chinese medicinal formulae which have been applied for centuries. TCM treatment focuses on regulating the body’s immune microenvironment and has unique advantages in the treatment of autoimmune diseases. These advantages are mainly reflected in the following aspects: it can adapt to the functional complexity of DNT cells, reduce disease recurrence, is suitable for elderly and frail patients, improve the universality of treatment, and alleviate the side effect of immune hypofunction caused by modern medicines (118). There is potential to integrate TCM and Western medicine in the approach to treating autoimmune diseases.
There are thus far few studies investigating the use of TCM treatments to regulate DNT cells in autoimmune disease. Of note, the effect of zuoguiwan (a typical TCM documented in the Complete Compendium of Zhang Jingyue) on thymic differentiation of DNT cells has been studied (119). In the embryonic rat thymus, zuoguiwan induces T-cell differentiation in the thymus from the DN4 stage to the DP stage and inhibits the development of DNT cells by increasing the secretion of thymosin β4 and thymosin α1 (119). Huanglian Jiedu Decoction (HLJD) can influence the changes of DNT cells in the treatment of ischemic stroke. Specifically, it can regulate activated cytotoxic DNT cells and quiescent immunosuppressive DNT cells (120). In the treatment of SLE, the Jieduquyuziyin prescription (JP) therapy can induce the apoptosis of DNT cells by inhibiting the neddylation pathway to restore their homeostasis, while Ube2m serves as a crucial therapeutic target for the JP therapy in regulating the homeostasis of DNT cells (121). Several other active ingredients extracted from TCM herbs have been shown to treat autoimmune diseases by effectively modulating DNT cell differentiation (122, 123). They are summarized in Figure 4.
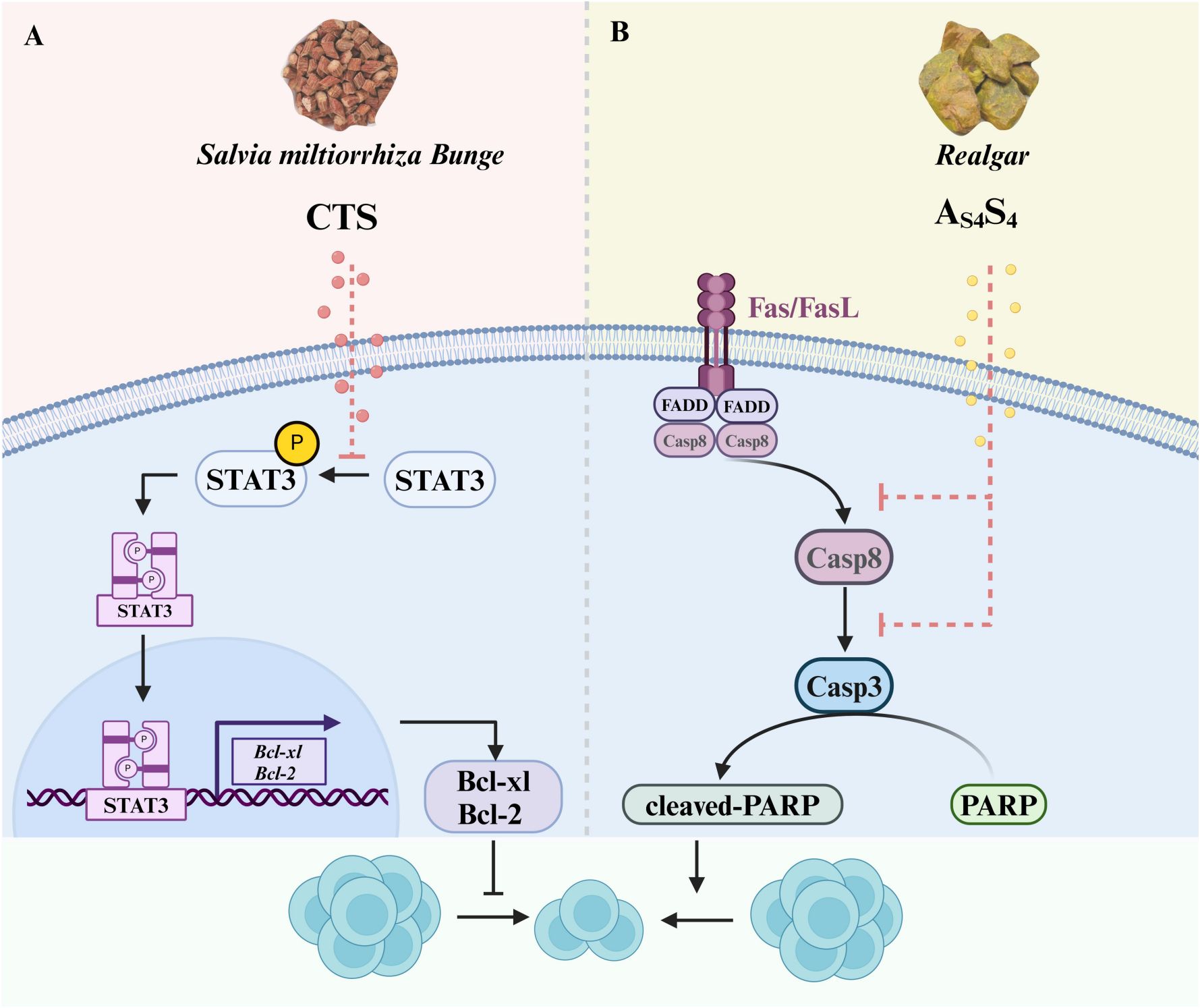
Figure 4. Effects of CTS and As4S4 on the differentiation of DNT cells. (A) CTS, which is isolated from Salvia miltiorrhiza Bunge (Danshen), can inhibit the proliferation of DNT cells by blocking the phosphorylation of STAT3 to reduce the expression of Bcl-xL and Bcl-2. (B) As4S4, derived from Realgar, can trigger Fas-mediated apoptosis of DNT cells by activating the Caspase8/Caspase 3 pathway. CTS, Cryptotanshinone; As4S4, Tetra-arsenic Tetra-sulfide.
Cryptotanshinone (CTS), a key bioactive quinoid diterpene derived from the dried roots of Salvia miltiorrhiza Bunge (Danshen, a traditional Chinese herbal medicine), has been utilized in the management of cardiovascular, cerebrovascular, and autoimmune disorders (122, 123). It is an effective inhibitor of STAT3 (124), which is overactive in the development of SLE (125).
STAT3 is activated by kinase-mediated phosphorylation at Tyr705, and two phosphorylated STAT3 dimerize to form a transcription factor complex that translocates to the nucleus to induce target gene expression (126). In a preclinical study using CTS to treat SLE in MRL/lpr mice, CTS decreased the total number of T cells and especially DNT cells. CTS treatment also led to a decrease in levels of autoantibodies and pro-inflammatory cytokines. Clinically, CTS treatment improved the signs and symptoms of SLE, such as reducing the size of skin lesions, reversing splenomegaly and improving kidney function (122). This study also showed that CTS reversed the excessive STAT3 signaling in the spleens of lupus-prone MRL/lpr mice, and that inhibition of T cell proliferation was through the inhibition of STAT3 activation (122). This suggests that CTS, as a potential therapeutic drug for SLE patients, can block DNT cell proliferation through reducing STAT3 phosphorylation and hence activation, to attenuate the development and progression of SLE.
Tetra-arsenic tetra-sulfide (As4S4), the major active ingredient of the traditional Chinese medicine realgar, is known to have little toxicity and has long been used for treating various diseases, including SLE and leukemia (127, 128). As4S4 treatment of lupus-prone BXSB mice reversed monocytosis in the spleen and decreased serum IL-6 levels (127). Furthermore, As4S4 in lupus-prone MRL/lpr mice significantly decreases the number of DNT cells, reduces their production of IL-17 and the production of antinuclear antibodies (129). The As4S4-induced decrease in DNT-cell number could be due to increased apoptosis, as suggested by the findings of decreased FasL expression and activation of several caspases after treatment (129). This study strongly supports the therapeutic potential of As4S4 in SLE through the suppression of DNT cells.
Table 2 provides a summary of the discussed therapeutic approaches using DNT immunotherapy to treat autoimmune diseases.
5 Potential limitations and challenges
Despite the relatively small numbers of DNT cells in peripheral blood and lymphoid organs, their immunoregulatory and pathogenic activities in the development and progression of autoimmune diseases have been widely acknowledged. Targeting the differentiation and expansion of DNT cells has potential as a valuable new approach for treating autoimmune diseases. The discovery of potential targets, including the IL-33 receptor, ST2 (112), mTOR (81), and STAT3 (122), for regulating DNT cells lays the foundation for the development of targeted drugs. Nonetheless, there are still some limitations and challenges in the application of DNT cells in treating autoimmune diseases.
Firstly, DNT cells perform diverse functions in different autoimmune diseases, and hence this may mean that different strategies to regulate DNT cells are required depending on the disease. The complexity of studying the role of DNT cells in different autoimmune disease settings is due to their phenotypic and functional diversity in different immune environments and tissues. DNT cells can inhibit the progression of autoimmune diabetes through their immunosuppressive functions, such as IL-10 secretion (4, 9, 68). On the other hand, DNT cells proliferate and produce IL-17 in SLE, leading to increased inflammation and tissue damage (17, 25). Therefore, the choice of whether to promote or inhibit DNT-cell activity requires consideration on a case-by-case basis for different autoimmune conditions. It will be crucial to elucidate the gaps in knowledge about the roles of DNT cells and their interactions with other immune cells in different pathologies and tissue types.
Secondly, most of the molecular targets that regulate the differentiation and expansion of DNT cells have multiple biological functions, and direct intervention may lead to unexpected side effects. For example, although mTOR could be a potential target to regulate the differentiation of DNT cells, mTOR activation also inhibits the functions of CD8+ effector memory T cells and CD4+ Treg cells, which may lead to disruption of immune homeostasis (130).
Additionally, the controversy on the origin of DNT cells continues. There are several hypotheses summarized in Figure 1. The lack of clear understanding of the ontogeny of DNT cells makes it difficult to regulate DNT cells from their origin, hence current approaches can only target the DNT cells which are already differentiated.
Another consideration is that several studies have suggested that adoptive DNT cell therapy may serve as a strategy to treat autoimmune diseases such as T1D (47, 71). However, the survival time of DNT cells in vivo limits its application. The human DNT cells can persist in vivo for several weeks (131), then, reinfusion of cryopreserved DNT cells may be required once DNT cell levels decrease below an established threshold. This means that the interval and number of cycles for DNT cell infusion need to be investigated before a treatment protocol using adoptive DNT cell therapy can be established. We can use gene editing technologies to enhance the functional activity of DNT cells, or developing strategies for long-term cell-based therapeutic regimens.
Finally, most studies investigating DNT cells are based on models using immunodeficient mice, hence, further clinical trials with larger study populations would need to be carried out to assess the efficacy of potential treatments in humans.
6 Conclusion
As a rare but important subset of mature T lymphocytes, DNT cells play a vital role in immune homeostasis. They can function as Treg cells, cytotoxic T cells, or Th cells and regulate the innate and adaptive immune systems. They are also closely linked with the development of several autoimmune diseases. A growing understanding of DNT cell origin and functional features has prompted the consideration of DNT-cell-based therapeutic approaches, including precise modulation of DNT cell activation and survival and inhibition of proinflammatory metabolic pathways. In brief, by summarizing the current understanding of the role of DNT cells in autoimmune disease, we have highlighted the heterogeneity and diverse functions of DNT cells. Hence, there is still a need for the use of DNT cells as potential therapeutic tools to be further explored, in the era of precision and personalized medicine.
Author contributions
XL: Writing – original draft, Visualization. DG: Writing – original draft, Visualization. IZ: Writing – review & editing, Validation. LZ: Conceptualization, Writing – original draft. NY: Formal Analysis, Writing – review & editing. YL: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Construction project of high-level Traditional Chinese Medicine key discipline of National Administration of Traditional Chinese Medicine (Grant Number: zyyzdxk-2023022); Discipline construction project of basic research of integrated Chinese and Western medicine on rheumatic immune diseases (Grant Number: 2024XKJS-03); Science and Technology Special project of scientific and technological cooperation and exchange in Shanxi Province (Grant Number: 202104041101013); Prevention and treatment of rheumatism and immune disease by combination of traditional Chinese and Western medicine Shanxi scientific and technological innovation talent team project (Grant Number: 202204051002033); Innovation Project of Shanxi University of Chinese Medicine (Grant Number: 2022TD2003); Shanxi Province Science Foundation (Grant Number: 202203021222272); Key laboratory of rheumatological and immunological diseases treated by integrated Chinese and Western medicine (Grant Number: zyyyjs2024021); Scientific Research Project of Shanxi Administration of Traditional Chinese Medicine in Shanxi Province (Grant Number: 2024ZYYAD008); Excellent graduate tutor team project of Shanxi Province (Grant Number: 2024TD33).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miceli MC and Parnes JR. The roles of CD4 and CD8 in T cell activation. Semin Immunol. (1991) 3:133–41.
2. Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. (1998) 16:523–44. doi: 10.1146/annurev.immunol.16.1.523
3. Sun L, Su Y, Jiao A, Wang X, and Zhang B. T cells in health and disease. Signal Transduct Target Ther. (2023) 8:235. doi: 10.1038/s41392-023-01471-y
4. D’Acquisto F and Crompton T. CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. (2011) 82:333–40. doi: 10.1016/j.bcp.2011.05.019
5. Brandt D and Hedrich CM. TCRαβ+CD3+CD4-CD8- (double negative) T cells in autoimmunity. Autoimmun Rev. (2018) 17:422–30. doi: 10.1016/j.autrev.2018.02.001
6. Bafor EE, Valencia JC, and Young HA. Double negative T regulatory cells: an emerging paradigm shift in reproductive immune tolerance? Front Immunol. (2022) 13:886645. doi: 10.3389/fimmu.2022.886645
7. Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR αβ+ CD4-CD8- Double-negative regulatory T Cells. Blood. (2005) 105:2828–35. doi: 10.1182/blood-2004-07-2583
8. Paul S, Shilpi, and Lal G. Role of Gamma-Delta (γδ) T cells in autoimmunity. J Leukoc Biol. (2015) 97:259–71. doi: 10.1189/jlb.3RU0914-443R
9. Juvet SC and Zhang L. Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. J Mol Cell Biol. (2012) 4:48–58. doi: 10.1093/jmcb/mjr043
10. Oliveira Mendonça L, Matucci-Cerinic C, Terranova P, Casabona F, Bovis F, Caorsi R, et al. The challenge of early diagnosis of autoimmune lymphoproliferative syndrome in children with suspected autoinflammatory/autoimmune disorders. Rheumatol (Oxford). (2022) 61:696–704. doi: 10.1093/rheumatology/keab361
11. Li W, Tang X, Zheng Y, Xu X, Zhao N, Tsao BP, et al. Phosphatidic acid promoting the generation of interleukin-17A producing double-negative T Cells by enhancing mTORC1 signaling in lupus. Arthritis Rheumatol. (2024) 76:1096–108. doi: 10.1002/art.42840
12. Zhang Q and Huang Y. Regarding the double-negative T cells in patients with systemic sclerosis. Clin Rheumatol. (2024) 43:2361. doi: 10.1007/s10067-024-06989-2
13. Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Bigerna B, et al. CD4(-)CD8(-) T-cells in primary Sjögren’s syndrome: association with the extent of glandular involvement. J Autoimmun. (2014) 51:38–43. doi: 10.1016/j.jaut.2014.01.030
14. Wang F, Qi Z, Yao Y, Yu G, Feng T, Zhao T, et al. Exploring the stage-specific roles of Tcf-1 in T cell development and Malignancy at single-cell resolution. Cell Mol Immunol. (2021) 18:644–59. doi: 10.1038/s41423-020-00527-1
15. Joachims ML, Chain JL, Hooker SW, Knott-Craig CJ, and Thompson LF. Human alpha beta and gamma delta thymocyte development: TCR gene rearrangements, intracellular TCR beta expression, and gamma delta developmental potential--differences between men and mice. J Immunol. (2006) 176(3):1543–1552. doi: 10.4049/jimmunol.176.3.1543
16. Newman-Rivera AM, Kurzhagen JT, and Rabb H. TCRαβ+CD4-/CD8- “double negative” T cells in health and disease-implications for the kidney. Kidney Int. (2022) 102:25–37. doi: 10.1016/j.kint.2022.02.035
17. Rodríguez-Rodríguez N, Flores-Mendoza G, Apostolidis SA, Rosetti F, Tsokos GC, and Crispín JC. TCR-α/β CD4- CD8- double negative T cells arise from CD8+ T cells. J Leukoc Biol. (2020) 108:851–7. doi: 10.1002/JLB.1AB0120-548R
18. Takahama Y, Kosugi A, and Singer A. Phenotype, ontogeny, and repertoire of CD4-CD8- T cell receptor alpha beta+ thymocytes. Variable influence of self-antigens on T cell receptor V beta usage. J Immunol. (1991) 146:1134–41. doi: 10.4049/jimmunol.146.4.1134
19. Ford MS, Zhang ZX, Chen W, and Zhang L. Double-negative T regulatory cells can develop outside the thymus and do not mature from CD8+ T cell precursors. J Immunol. (2006) 177:2803–9. doi: 10.4049/jimmunol.177.5.2803
20. Velikkakam T, Gollob KJ, and Dutra WO. Double-negative T cells: Setting the stage for disease control or progression. Immunology. (2022) 165:371–85. doi: 10.1111/imm.13441
21. Torina A, Guggino G, La Manna MP, and Sireci G. The janus face of NKT cell function in autoimmunity and infectious diseases. Int J Mol Sci. (2018) 19:440. doi: 10.3390/ijms19020440
22. Krovi SH and Gapin L. Invariant natural killer T cell subsets-more than just developmental intermediates. Front Immunol. (2018) 9:1393. doi: 10.3389/fimmu.2018.01393
23. Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. (1997) 278:1623–6. doi: 10.1126/science.278.5343.1623
24. Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, and Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. (2004) 199:947–57. doi: 10.1084/jem.20031389
25. Rodríguez-Rodríguez N, Apostolidis SA, Penaloza-MacMaster P, Martín Villa JM, Barouch DH, Tsokos GC, et al. Programmed cell death 1 and Helios distinguish TCR-αβ+ double-negative (CD4-CD8-) T cells that derive from self-reactive CD8 T cells. J Immunol. (2015) 194:4207–14. doi: 10.4049/jimmunol.1402775
26. Paul S, Singh AK, Shilpi, and Lal G. Phenotypic and functional plasticity of gamma-delta (γδ) T cells in inflammation and tolerance. Int Rev Immunol. (2014) 33:537–58. doi: 10.3109/08830185.2013.863306
27. Kalyan S and Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. (2013) 10:21–9. doi: 10.1038/cmi.2012.44
28. Yang L, Zhu Y, Tian D, Wang S, Guo J, Sun G, et al. Transcriptome landscape of double negative T cells by single-cell RNA sequencing. J Autoimmun. (2021) 121:102653. doi: 10.1016/j.jaut.2021.102653
29. Zajonc DM and Girardi E. Recognition of microbial glycolipids by natural killer T cells. Front Immunol. (2015) 6:400. doi: 10.3389/fimmu.2015.00400
30. Nagarajan NA and Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. (2007) 178:2706–13. doi: 10.4049/jimmunol.178.5.2706
31. Maricic I, Halder R, Bischof F, and Kumar V. Dendritic cells and anergic type I NKT cells play a crucial role in sulfatide-mediated immune regulation in experimental autoimmune encephalomyelitis. J Immunol. (2014) 193:1035–46. doi: 10.4049/jimmunol.1302898
32. Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, et al. IL-13Rα2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. (2014) 63:1728–36. doi: 10.1136/gutjnl-2013-305671
33. Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-) CD8- double-negative regulatory T cells. Blood. (2005) 105:2828–35. doi: 10.1182/blood-2004-07-2583
34. Voelkl S, Gary R, and Mackensen A. Characterization of the immunoregulatory function of human TCR-αβ+ CD4- CD8- double-negative T cells. Eur J Immunol. (2011) 41:739–48. doi: 10.1002/eji.201040982
35. Zhang ZX, Yang L, Young KJ, DuTemple B, and Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. (2000) 6:782–9. doi: 10.1038/77513
36. Sobkowiak MJ, Davanian H, Heymann R, Gibbs A, Emgård J, Dias J, et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur J Immunol. (2019) 49:133–43. doi: 10.1002/eji.201847759
37. Meierovics A, Yankelevich WJ, and Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U.S.A. (2013) 110:E3119–3128. doi: 10.1073/pnas.1302799110
38. Rudak PT, Choi J, and Haeryfar SMM. MAIT cell-mediated cytotoxicity: Roles in host defense and therapeutic potentials in infectious diseases and cancer. J Leukoc Biol. (2018) 104:473–86. doi: 10.1002/JLB.4RI0118-023R
39. Lu Z, Zhu M, Marley JL, Bi K, Wang K, Zhai M, et al. The combined action of monocytic myeloid-derived suppressor cells and mucosal-associated invariant T cells promotes the progression of cervical cancer. Int J Cancer. (2021) 148:1499–507. doi: 10.1002/ijc.33411
40. Böttcher K, Rombouts K, Saffioti F, Roccarina D, Rosselli M, Hall A, et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. (2018) 68:172–86. doi: 10.1002/hep.29782
41. Nel I, Beaudoin L, Gouda Z, Rousseau C, Soulard P, Rouland M, et al. MAIT cell alterations in adults with recent-onset and long-term type 1 diabetes. Diabetologia. (2021) 64:2306–21. doi: 10.1007/s00125-021-05527-y
42. Muller CKS, Spagnuolo J, Audigé A, Chancellor A, Russenberger D, Scherrer AU, et al. Immunophenotypic characterization of TCR γδ T cells and MAIT cells in HIV-infected individuals developing Hodgkin’s lymphoma. Infect Agent Cancer. (2021) 16:24. doi: 10.1186/s13027-021-00365-4
43. Bonneville M, O’Brien RL, and Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. (2010) 10:467–78. doi: 10.1038/nri2781
44. Li H, Adamopoulos IE, Moulton VR, Stillman IE, Herbert Z, Moon JJ, et al. Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun. (2020) 11:2859. doi: 10.1038/s41467-020-16636-4
45. Sadasivam M, Noel S, Lee SA, Gong J, Allaf ME, Pierorazio P, et al. Activation and proliferation of PD-1+ kidney double-negative T Cells is dependent on nonclassical MHC proteins and IL-2. J Am Soc Nephrol. (2019) 30:277–92. doi: 10.1681/ASN.2018080815
46. Dörner T and Lipsky PE. B cells: depletion or functional modulation in rheumatic diseases. Curr Opin Rheumatol. (2014) 26:228–36. doi: 10.1097/BOR.0000000000000000
47. Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. (2015) 21:719–29. doi: 10.1038/nm.3895
48. Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8- IL-17-producing T cells. J Immunol. (2014) 193:2168–77. doi: 10.4049/jimmunol.1400977
49. Duncan B, Nazarov-Stoica C, Surls J, Kehl M, Bona C, Casares S, et al. Double negative (CD3 + 4- 8-) TCR alpha beta splenic cells from young NOD mice provide long-lasting protection against type 1 diabetes. PloS One. (2010) 5:e11427. doi: 10.1371/journal.pone.0011427
50. Zhu J and Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. (2010) 20:4–12. doi: 10.1038/cr.2009.138
51. Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. (2007) 178:1938–47. doi: 10.4049/jimmunol.178.3.1938
52. Shivakumar S, Tsokos GC, and Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. (1989) 143:103–12. doi: 10.4049/jimmunol.143.1.103
53. Alunno A, Bistoni O, Bartoloni E, Caterbi S, Bigerna B, Tabarrini A, et al. IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann Rheum Dis. (2013) 72:286–92. doi: 10.1136/annrheumdis-2012-201511
54. Gan Y, Zhao X, He J, Liu X, Li Y, Sun X, et al. Increased interleukin-17F is associated with elevated autoantibody levels and more clinically relevant than interleukin-17A in primary sjögren’s syndrome. J Immunol Res. (2017) 2017:4768408. doi: 10.1155/2017/4768408
55. Renauer PA, Coit P, and Sawalha AH. The DNA methylation signature of human TCRαβ+CD4-CD8- double negative T cells reveals CG demethylation and a unique epigenetic architecture permissive to a broad stimulatory immune response. Clin Immunol. (2015) 156:19–27. doi: 10.1016/j.clim.2014.10.007
56. Brandt D, Sergon M, Abraham S, Mäbert K, and Hedrich CM. TCR+CD3+CD4-CD8- effector T cells in psoriasis. Clin Immunol. (2017) 181:51–9. doi: 10.1016/j.clim.2017.06.002
57. Ueyama A, Imura C, Fusamae Y, Tsujii K, Furue Y, Aoki M, et al. Potential role of IL-17-producing CD4/CD8 double negative αβ T cells in psoriatic skin inflammation in a TPA-induced STAT3C transgenic mouse model. J Dermatol Sci. (2017) 85:27–35. doi: 10.1016/j.jdermsci.2016.10.007
58. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. (2012) 18:1069–76. doi: 10.1038/nm.2817
59. Sneller MC, Dale JK, and Straus SE. Autoimmune lymphoproliferative syndrome. Curr Opin Rheumatol. (2003) 15:417–21. doi: 10.1097/00002281-200307000-00008
60. Teachey DT. New advances in the diagnosis and treatment of autoimmune lymphoproliferative syndrome. Curr Opin Pediatr. (2012) 24:1–8. doi: 10.1097/MOP.0b013e32834ea739
61. Bleesing JJ, Brown MR, Dale JK, Straus SE, Lenardo MJ, Puck JM, et al. TcR-alpha/beta (+) CD4(-) CD8(-) T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clin Immunol. (2001) 100:314–24. doi: 10.1006/clim.2001.5069
62. Palmisani E, Miano M, Grossi A, Lanciotti M, Lupia M, Terranova P, et al. Autoimmune lymphoproliferative syndrome (ALPS) disease and ALPS phenotype: are they two distinct entities? Hemasphere. (2023) 7:e845. doi: 10.1097/HS9.0000000000000845
63. Li P, Huang P, Yang Y, Hao M, Peng H, and Li F. Updated understanding of autoimmune lymphoproliferative syndrome (ALPS). Clin Rev Allergy Immunol. (2016) 50:55–63. doi: 10.1007/s12016-015-8466-y
64. Bristeau-Leprince A, Mateo Véronique, Lim A, Magerus-Chatinet A, Solary E, Fischer A, et al. Human TCR α/β+ CD4–CD8– double-negative T cells in patients with autoimmune lymphoproliferative syndrome express restricted Vβ TCR diversity and are clonally related to CD8+ T cells. J Immunol. (2008) 181:440–8. doi: 10.4049/jimmunol.181.1.440
65. Bleesing JJ, Morrow MR, Uzel G, and Fleisher TA. Human T cell activation induces the expression of a novel CD45 isoform that is analogous to murine B220 and is associated with altered O-glycan synthesis and onset of apoptosis. Cell Immunol. (2001) 213:72–81. doi: 10.1006/cimm.2001.1865
66. Mahnke YD, Brodie TM, Sallusto F, Roederer M, and Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. (2013) 43:2797–809. doi: 10.1002/eji.201343751
67. Bleesing JJ, Brown MR, Novicio C, Guarraia D, Dale JK, Straus SE, et al. A composite picture of TcR alpha/beta(+) CD4(-)CD8(-) T Cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clin Immunol. (2002) 104:21–30. doi: 10.1006/clim.2002.5225
68. Hillhouse EE, Beauchamp C, Chabot-Roy G, Dugas V, and Lesage S. Interleukin-10 limits the expansion of immunoregulatory CD4-CD8- T cells in autoimmune-prone non-obese diabetic mice. Immunol Cell Biol. (2010) 88:771–880. doi: 10.1038/icb.2010.84
69. Liu T, Cong M, Sun G, Wang P, Tian Y, Shi W, et al. Combination of double negative T cells and anti-thymocyte serum reverses type 1 diabetes in NOD mice. J Transl Med. (2016) 14:57. doi: 10.1186/s12967-016-0815-y
70. Dugas V, Beauchamp C, Chabot-Roy G, Hillhouse EE, and Lesage S. Implication of the CD47 pathway in autoimmune diabetes. J Autoimmun. (2010) 35:23–32. doi: 10.1016/j.jaut.2010.01.002
71. Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. (1994) 263:1759–62. doi: 10.1126/science.7510905
72. Tsokos GC. The immunology of systemic lupus erythematosus. Nat Immunol. (2024) 25:1332–43. doi: 10.1038/s41590-024-01898-7
73. Dean GS, Anand A, Blofeld A, Isenberg DA, and Lydyard PM. Characterization of CD3+ CD4- CD8- (double negative) T cells in patients with systemic lupus erythematosus: production of IL-4. Lupus. (2002) 11:501–7. doi: 10.1191/0961203302lu234oa
74. Mariette X and Criswell LA. Primary sjögren’s syndrome. New Engl J Med. (2018) 378:931–9. doi: 10.1056/NEJMcp1702514
75. Voulgarelis M and Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol. (2010) 6:529–37. doi: 10.1038/nrrheum.2010.118
76. Mackay F and Schneider P. Cracking the BAFF code. Nat Rev Immunol. (2009) 9:491–502. doi: 10.1038/nri2572
77. Liu H, Wang G, Liu X, Ren Y, Wang Y, Li J, et al. Novel insights into immune checkpoints in psoriasis and atopic dermatitis: From expression and function to treatments. Int Immunopharmacol. (2024) 139:112663. doi: 10.1016/j.intimp.2024.112663
78. Sieper J and Poddubnyy D. Axial spondyloarthritis. Lancet. (2017) 390:73–84. doi: 10.1016/S0140-6736(16)31591-4
79. Lisco A, Wong CS, Price S, Ye P, Niemela J, Anderson M, et al. Paradoxical CD4 lymphopenia in autoimmune lymphoproliferative syndrome (ALPS). Front Immunol. (2019) 10:1193. doi: 10.3389/fimmu.2019.01193
80. Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, et al. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS). Blood. (2006) 108:1965–71. doi: 10.1182/blood-2006-01-010124
81. Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. (2009) 145:101–6. doi: 10.1111/j.1365-2141.2009.07595.x
82. Huang Q and Zhu J. Regulatory T cell-based therapy in type 1 diabetes: Latest breakthroughs and evidence. Int Immunopharmacol. (2024) 140:112724. doi: 10.1016/j.intimp.2024.112724
83. Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. (2017) 127:2881–91. doi: 10.1172/JCI94549
84. Zhang ZX, Ma Y, Wang H, Arp J, Jiang J, Huang X, et al. Double-negative T cells, activated by xenoantigen, lyse autologous B and T cells using a perforin/granzyme-dependent, Fas-Fas ligand-independent pathway. J Immunol. (2006) 177:6920–9. doi: 10.4049/jimmunol.177.10.6920
85. Zhang D, Yang W, Degauque N, Tian Y, Mikita A, and Zheng XX. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. (2007) 109:4071–9. doi: 10.1182/blood-2006-10-050625
86. Zhang L and Thomson AW. New partners for tolerogenic dendritic cells. Am J Transplant. (2011) 11:2003–4. doi: 10.1111/j.1600-6143.2011.03654.x
87. Douros J and Suffness M. New antitumor substances of natural origin. Cancer Treat Rev. (1981) 8:63–87. doi: 10.1016/S0305-7372(81)80006-0
88. Strauss G, Osen W, and Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. (2002) 128:255–66. doi: 10.1046/j.1365-2249.2002.01777.x
89. Majchrzak A, Witkowska M, and Smolewski P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B-cell lymphoma: current knowledge and clinical significance. Molecules. (2014) 19:14304–15. doi: 10.3390/molecules190914304
90. Gu H, Chen Z, Ma J, Ma J, Fu L, Zhang R, et al. Case report: Effectiveness of sirolimus in a de novo FAS mutation leading to autoimmune lymphoproliferative syndrome-FAS and elevated DNT/Treg ratio. Front Pediatr. (2022) 10:868193. doi: 10.3389/fped.2022.868193
91. Wu D, Li H, Liu M, Qin J, and Sun Y. The Ube2m-Rbx1 neddylation-Cullin-RING-Ligase proteins are essential for the maintenance of Regulatory T cell fitness. Nat Commun. (2022) 13:3021. doi: 10.1038/s41467-022-30707-8
92. Cheng Q, Liu J, Pei Y, Zhang Y, Zhou D, Pan W, et al. Neddylation contributes to CD4+ T cell-mediated protective immunity against blood-stage Plasmodium infection. PloS Pathog. (2018) 14:e1007440. doi: 10.1371/journal.ppat.1007440
93. Jin HS, Liao L, Park Y, and Liu YC. Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling. Proc Natl Acad Sci U.S.A. (2013) 110:624–9. doi: 10.1073/pnas.1213819110
94. Zhang Y, Du L, Wang C, Jiang Z, Duan Q, Li Y, et al. Neddylation is a novel therapeutic target for lupus by regulating double negative T cell homeostasis. Signal Transduct Target Ther. (2024) 9:18. doi: 10.1038/s41392-023-01709-9
95. Lourdudoss C, Hafström I, Frostegård J, and van Vollenhoven R. The association between diet and glucocorticoid treatment in patients with SLE. Lupus Sci Med. (2016) 3:e000135. doi: 10.1136/lupus-2015-000135
96. O’Connell FP, Pinkus JL, and Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. (2004) 121:254–63. doi: 10.1309/617DWB5GNFWXHW4L
97. Lu LD, Stump KL, Wallace NH, Dobrzanski P, Serdikoff C, Gingrich DE, et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol. (2011) 187:3840–53. doi: 10.4049/jimmunol.1101228
98. Liu L, Takeda K, and Akkoyunlu M. Disease stage-specific pathogenicity of CD138 (Syndecan 1)-expressing T cells in systemic lupus erythematosus. Front Immunol. (2020) 11:1569. doi: 10.3389/fimmu.2020.01569
99. Martina MN, Noel S, Saxena A, Rabb H, and Hamad AR. Double negative (DN) αβ T cells: misperception and overdue recognition. Immunol Cell Biol. (2015) 93:305–10. doi: 10.1038/icb.2014.99
100. Benihoud K, Bonardelle D, Bobé P, and Kiger N. MRL/lpr CD4- CD8- and CD8+ T cells, respectively, mediate Fas-dependent and perforin cytotoxic pathways. Eur J Immunol. (1997) 27:415–20. doi: 10.1002/eji.1830270211
101. Xie T, Liu H, and Li P. Glucocorticoid prevents CD138 expression in T cells of autoimmune MRL/lpr mice. Mol Med Rep. (2022) 25:211. doi: 10.3892/mmr.2022.12727
102. Kruger B, Shamley D, Soko ND, and Dandara C. Pharmacogenetics of tamoxifen in breast cancer patients of African descent: Lack of data. Clin Transl Sci. (2024) 17:e13761. doi: 10.1111/cts.13761
103. Butta A, MacLennan K, Flanders KC, Sacks NP, Smith I, McKinna A, et al. Induction of transforming growth factor beta 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. (1992) 52:4261–4.
104. Steinberg AD, Roths JB, Murphy ED, Steinberg RT, and Raveche ES. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. (1980) 125:871–3. doi: 10.4049/jimmunol.125.2.871
105. Roubinian JR, Talal N, Greenspan JS, Goodman JR, and Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. (1978) 147:1568–83. doi: 10.1084/jem.147.6.1568
106. Sthoeger ZM, Bentwich Z, Zinger H, and Mozes E. The beneficial effect of the estrogen antagonist, tamoxifen, on experimental systemic lupus erythematosus. J Rheumatol. (1994) 21:2231–8.
107. Dayan M, Zinger H, Kalush F, Mor G, Amir-Zaltzman Y, Kohen F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. (1997) 90:101–8. doi: 10.1046/j.1365-2567.1997.00122.x
108. Wu WM, Suen JL, Lin BF, and Chiang BL. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. (2000) 100:110–8. doi: 10.1046/j.1365-2567.2000.00998.x
109. Muratori L, Lohse AW, and Lenzi M. Diagnosis and management of autoimmune hepatitis. BMJ. (2023) 380:e070201. doi: 10.1136/bmj-2022-070201
110. Tanaka A, Mori M, Matsumoto K, Ohira H, Tazuma S, and Takikawa H. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol Res. (2019) 49:881–9. doi: 10.1111/hepr.13342
111. Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, et al. Autoimmune hepatitis. Nat Rev Dis Primers. (2018) 4:18017. doi: 10.1038/nrdp.2018.17
112. Sun X, Zhang C, Sun F, Li S, Wang Y, Wang T, et al. IL-33 promotes double negative T cell survival via the NF-κB pathway. Cell Death Dis. (2023) 14:242. doi: 10.1038/s41419-023-05766-4
113. Cayrol C and Girard JP. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. (2022) 156:155891. doi: 10.1016/j.cyto.2022.155891
114. Dwyer GK, D’Cruz LM, and Turnquist HR. Emerging functions of IL-33 in homeostasis and immunity. Annu Rev Immunol. (2022) 40:15–43. doi: 10.1146/annurev-immunol-101320-124243
115. Zhang R, Han L, Lin W, Ba X, Yan J, Li T, et al. Mechanisms of NLRP3 inflammasome in rheumatoid arthritis and osteoarthritis and the effects of traditional Chinese medicine. J Ethnopharmacol. (2024) 321:117432. doi: 10.1016/j.jep.2023.117432
116. Xu F, Zhang H, Chen J, Zhan J, Liu P, Liu W, et al. Recent progress on the application of compound formulas of traditional Chinese medicine in clinical trials and basic research in vivo for chronic liver disease. J Ethnopharmacol. (2024) 321:117514. doi: 10.1016/j.jep.2023.117514
117. Zhou L, Luo JL, Sun A, Yang HY, Lin YQ, and Han L. Clinical efficacy and molecular mechanism of Chinese medicine in the treatment of autoimmune thyroiditis. J Ethnopharmacol. (2024) 323:117689. doi: 10.1016/j.jep.2023.117689
118. Li DN, Guo H, Niu L, Yin QS, Zhang YJ, and Zhuang PW. Clinical value-oriented research paradigm about inheritance and innovation development of TCM dominant diseases. Chin Herbal Medicines. (2023) 15:476–84. doi: 10.1016/j.chmed.2023.09.002
119. Min ZHAO, Mei-rong REN, Yan-yan ZHOU, Long-yun CHEN, and Ji-gang CAO. Effect of zuoguiwan on differentiation of CD4-CD8-T cells in thymus of kidney-deficient offspring rats. Chin J Exp Traditional Med Formulae. (2020) 26:49–55. doi: 10.13422/j.cnki.syfjx.20200802
120. Wang K, Sun Z, Shao Q, Wang Z, Zhang H, Li Y, et al. Modulation of double-negative T cells by Huang-Lian-Jie-Du Decoction attenuates neuroinflammation in ischemic stroke: insights from single-cell transcriptomics. Front Immunol. (2025) 16:1537277. doi: 10.3389/fimmu.2025.1537277
121. Li Y, Duan Q, Wang C, Du L, Jiang Z, Li S, et al. Jieduquyuziyin prescription alleviates lupus development via inhibiting neddylation pathway to promote Bim-induced apoptosis of double negative T cells. J Ethnopharmacology. (2025) 337:118884. doi: 10.1016/j.jep.2024.118884
122. Du Y, Du L, He Z, Zhou J, Wen C, and Zhang Y. Cryptotanshinone ameliorates the pathogenesis of systemic lupus erythematosus by blocking T cell proliferation. Int Immunopharmacol. (2019) 74:105677. doi: 10.1016/j.intimp.2019.105677
123. Tang J and Zhao X. Research progress on regulation of immune response by tanshinones and salvianolic acids of danshen (Salvia miltiorrhiza bunge). Molecules. (2024) 29:1201. doi: 10.3390/molecules29061201
124. Wang J, Zhang G, Dai C, Gao X, Wu J, Shen L, et al. Cryptotanshinone potentiates the antitumor effects of doxorubicin on gastric cancer cells via inhibition of STAT3 activity. J Int Med Res. (2017) 45:220–30. doi: 10.1177/0300060516685513
125. Edwards LJ, Mizui M, and Kyttaris V. Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice. Clin Immunol. (2015) 158:221–30. doi: 10.1016/j.clim.2015.04.004
126. Leung KH, Liu LJ, Lin S, Lu L, Zhong HJ, Susanti D, et al. Discovery of a small-molecule inhibitor of STAT3 by ligand-based pharmacophore screening. Methods. (2015) 71:38–43. doi: 10.1016/j.ymeth.2014.07.010
127. Zhao Y, Wen G, Qiao Z, Xu H, Sun Q, Huang H, et al. Effects of tetra-arsenic tetra-sulfide on BXSB lupus-prone mice: a pilot study. Lupus. (2013) 22:469–76. doi: 10.1177/0961203313478302
128. Liu Y, Jia Y, Liu Y, Chen X, and Zhang M. Tetra-arsenic tetra-sulfide enhances NK-92MI mediated cellular immunotherapy in all-trans retinoic acid-resistant acute promyelocytic leukemia. Invest New Drugs. (2022) 40:1231–43. doi: 10.1007/s10637-022-01313-8
129. Zhao Y, Mu Z, Cai L, Liu X, Jia J, and Zhang J. Tetra-arsenic tetra-sulfide ameliorates lupus syndromes by inhibiting IL-17 producing double negative T cells. Dermatol Ther. (2019) 32:e12849. doi: 10.1111/dth.12849
130. Caza T, Wijewardena C, Al-Rabadi L, and Perl A. Cell type-specific mechanistic target of rapamycin-dependent distortion of autophagy pathways in lupus nephritis. Transl Res. (2022) 245:55–81. doi: 10.1016/j.trsl.2022.03.004
Keywords: autoimmune diseases, ontogeny, heterogeneity, traditional Chinese medicine, immunotherapy, CD3+CD4−CD8− T cells
Citation: Li X, Guo D, Zou IX, Zhao L, Yang N and Liu Y (2025) CD3+CD4−CD8− T cells: a new potential therapeutic target in treating autoimmune diseases. Front. Immunol. 16:1683418. doi: 10.3389/fimmu.2025.1683418
Received: 12 August 2025; Accepted: 09 September 2025;
Published: 26 September 2025.
Edited by:
Chunjing Wang, University College London, United KingdomReviewed by:
Yuan Peng, Shanghai University of Traditional Chinese Medicine, ChinaKunze Du, Tianjin University of Traditional Chinese Medicine, China
Jinfang Gao, Shanxi Bethune Hospital, China
Copyright © 2025 Li, Guo, Zou, Zhao, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, bGl1eWFuZzE5ODBAc3h0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xin Li
Xin Li Di Guo
Di Guo Isabelle Xinyue Zou
Isabelle Xinyue Zou Lingyan Zhao1,2,3
Lingyan Zhao1,2,3 Na Yang
Na Yang Yang Liu
Yang Liu