- 1Regulation of Host Defense Team, Division of Microbiology and Immunology, Center for Infectious Disease Education and Research, The University of Osaka, Osaka, Japan
- 2Department of Molecular Systems Immunology, University of Tokyo Pandemic Preparedness, Infection, and Advanced Research Center (UTOPIA), Tokyo, Japan
- 3Center for Advanced Modalities and Drug Delivery System (DDS), The University of Osaka, Osaka, Japan
Long-lived plasma cells (LLPCs), which continuously secrete antibodies, play a central role in humoral immunity and form the foundation of effective vaccine strategies. The anatomical segregation between the tissues where plasma cells are generated and where they are maintained suggests that both cell-intrinsic and extrinsic factors contribute to their longevity; however, the underlying cellular and molecular mechanisms remain largely unclear. In this review, we summarize recent advances in elucidating the regulation of plasma cell survival at both induction and effector sites. We particularly highlight potential LLPC precursors among newly generated plasma cells in secondary lymphoid tissues, and their subsequent maturation and differentiation into bona fide LLPCs within the bone marrow.
1 Introduction
Plasma cells are terminally differentiated effector cells responsible for the production of circulating antibodies, which confer protection against invading pathogens (1). Because of the half-life of antibodies is typically only a few days to weeks, long-term humoral immunity depends on the sustained presence of antigen-specific plasma cells.
While most plasma cells generated during infection or vaccination are generally short-lived, a subset acquires longevity and can persist for months, years, or even decades in human (2, 3). These cells, referred to as long-lived plasma cells (LLPCs), continuously secrete protective antibodies into the circulation (4–6). Durable antibody responses to certain viral infection or vaccines are thought to reflect the successful establishment of LLPCs within the bone marrow (5). Understanding the ontogeny and survival mechanisms of LLPCs may therefore help explain why the duration of antibody production varies widely across different vaccine (7) and, in turn, inform rational vaccine design.
It is well established that there is anatomical segregation between the sites where plasma cells are generated (induction sites) and the tissues where they home, secret antibodies, and persist long term (effector sites). Induction occurs almost exclusively in secondary lymphoid organs (SLOs), whereas LLPCs are maintained in a variety of anatomical locations (8). Two conceptual models have been proposed to explain how plasma cells acquire longevity (9) (Figure 1). The first, known as the effector site model, posits that newly formed short-lived plasma cells (SLPCs) migrate in response to the chemoattractants, such as CXCL12 (10), and those that fortuitously settle in survival-supportive niches, rich in pro-survival factors, mature into LLPCs and acquire extended longevity. In contrast, the second model proposes that heterogeneity in lifespan is programmed intrinsically at the induction site, with some plasma cells being fated to become long-lived immediately upon generation.
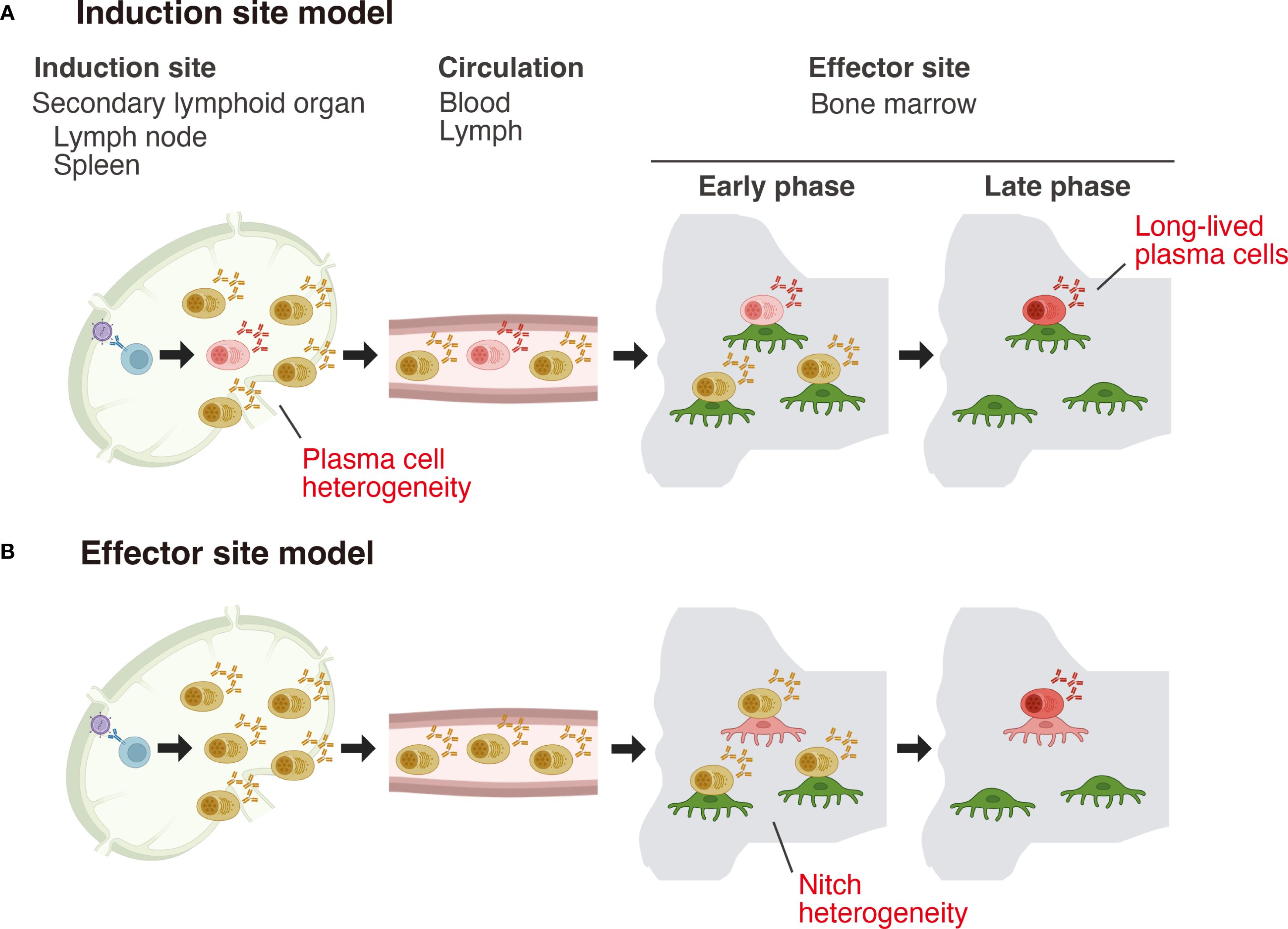
Figure 1. Two models for the acquisition of long-term survival capacity by plasma cells. (A) Induction site model: In this model, differences in plasma cell lifespan are programmed at the induction site, with the fate of becoming long-lived already imprinted at this stage. (B) Effector site model: In this model, plasma cells generated at the induction site are initially functionally uniform and stochastically migrate to the bone marrow. Within the bone marrow, a heterogeneous population of niche cells exists, and only plasma cells that interact with niche cells possessing high “nursing” capacity mature and acquire long-term survival potential.
These extrinsic (niche-dependent) and intrinsic (cell-autonomous) determinants of plasma cell longevity are not mutually exclusive and likely act in concert. However, until recently, these models had not been formally tested due to the lack of suitable experimental tools. Advances in genetic models and single-cell sequencing technologies over the past several years are beginning to unravel the developmental trajectories of LLPCs, both at their induction sites and within their effector sites (11–13). In this review, we summarize recent progress in understanding the regulation of plasma cell longevity, with a particular focus on the characteristics of potential LLPC precursors found in the spleen and lymph nodes (as induction sites) and their subsequent turnover and survival within the bone marrow (as a representative effector site).
2 Plasma cell fate determination at the induction site
The prevailing view has long held that LLPCs or their precursors arise predominantly from germinal centers (GCs) in response to T cell-dependent antigens (14–16). This concept is supported by evidence that most bone marrow plasma cells generated in response to such antigens produce high-affinity, class-switched antibodies (17–19).
However, several findings have challenged this dogma. LLPCs are detectable in the bone marrow after T-independent immunization (20–22). A recent timestamping study, discussed later, revealed that while IgG and IgA LLPCs predominantly consist of somatically hypermutated clones generated after immunization or infection, IgM LLPCs are highly enriched in public clones that arise through T cell-independent differentiation, and display affinity for self-antigens or microbial-derived antigens (11). Direct evidence was further provided by Koike et al. who demonstrated that GC-independent plasma cells can persist in the bone marrow with similar decay kinetics to GC-derived plasma cells of the same antigen specificity (23). Together, these findings indicate that developmental trajectories leading to LLPCs are more diverse than previously assumed, and that GC experience is not a strict prerequisite for LLPC generation.
Two recent studies have identified subsets of plasma cells in secondary lymphoid organs (SLOs) that exhibit bone marrow tropism and likely serve as precursors to LLPCs. Manakkat Vijay et al. employed an NP-KLH immunization model and found that TIGIT+ plasma cells in the spleen give rise to bone marrow plasma cells (12). Adoptive transfer experiments using splenic CD138+ plasma cells isolated at various time points after immunization revealed that cells generated at a later stage (day 35) gave rise to more sustained serum NP-specific antibody responses and more effectively seeded the bone marrow compared to those generated earlier (day 21 or day 28). Combined single-cell RNA sequencing and B cell receptor (BCR) sequencing identified TIGIT+ splenic plasma cells as precursors of bone marrow plasma cells. The frequency of TIGIT-expressing plasma cells increased during the later stages of the immune response. Notably, only TIGIT+ (and not TIGIT-) plasma cells produced NP-specific antibodies in the serum and gave rise to NP-specific LLPCs in the bone marrow of recipient mice following adoptive transfer. Furthermore, TIGIT deficiency impaired the generation of both splenic and bone marrow plasma cells upon immunization, underscoring the functional importance of TIGIT in plasma cell biology. Mechanistically, TIGIT was shown to regulate plasma cell proliferation: TIGIT+ plasma cells exhibited enhanced proliferative capacity than their TIGIT- counterparts. Consistently, TIGIT-deficient plasma cells showed reduced cell cycling, suggesting that TIGIT-dependent clonal expansion is critical for the migration of splenic plasma cells to the bone marrow survival niches.
In parallel, our group identified a subset of plasma cells in SLOs marked by high expression of integrin β7, which includes precursors of bone marrow plasma cells (13). Newly generated, antigen-specific IgG+ plasma cells in SLOs comprise both integrin β7lo and β7hi populations, whereas plasma cells that have recently arrived in the bone marrow are predominantly β7hi. Transcriptomic profiling and clonal tracing analyses revealed that β7hi cells preferentially egress from SLOs to the bone marrow. Notably, bone marrow-tropic plasma cells were found in both GC-derived and GC-independent populations. This egress-prone subset expressed elevated levels of the transcription factor KLF2, a regulator of immune cell differentiation and migration (24). Conditional Klf2 deletion in plasma cells impaired their exit from SLOs and subsequent bone marrow migration. Although integrin β7 itself was unexpectedly dispensable, S1PR1 was identified as a key downstream effector of KLF2 in regulating plasma cell egress. This finding is consistent with previous studies showing that mice lacking S1pr1 in B cells, or treated with FTY720 (a functional S1PR1 antagonist), exhibit reduced numbers of IgG plasma cells in both the blood and bone marrow (25). Integrin β7hi plasma cells also expressed elevated CD11b, which further contributed to their egress from SLOs.
Although the TIGIT+ (12) and integrin β7hi subsets show slightly different induction kinetics, they likely overlap. Integrin β7hi cells were Ki67hi, indicating that they are actively cycling, similar to TIGIT+ plasma cells. TIGIT is highly expressed in integrin β7hi cells, and is downregulated in KLF2-deficient plasma cells (unpublished data), suggesting that TIGIT may be a downstream target of KLF2 in plasma cells. Functionally, Klf2 deficiency impaired the migration of plasma cells to the bone marrow following influenza vaccination, resulting in reduced durability of anti-HA IgG antibody responses and compromised protection against influenza infection (13). This aligns with findings in IgA+ plasma cells, where KLF2 controls migration from mesenteric lymph nodes (mLNs) to effector sites (26). In B cell-specific Klf2-deficient mice, IgA plasma cells accumulated in mLNs but were reduced in bone marrow, blood, spleen, and intestinal lamina propria. Transcriptomic analysis revealed KLF2-dependent regulation of migration-related genes, including integrins (Itgb7, Itgb1, ItgbM), selectins (L-selectin), chemokine receptors (Ccr9), and S1P receptors (S1pr1, S1pr3, S1pr4) in IgA+ plasma cells in the mLNs. The upstream signals that induce or sustain KLF2 expression in nascent plasma cells remain unclear. In mature B cells, the transcription factor Foxo1 has been implicated in the regulation of Klf2 gene expression: Foxo1 binds to the Klf2 promoter (27), and Klf2 mRNA levels are reduced in Foxo1-deficient B cells (28). Defining the molecular control of KLF2 in plasma cells will be critical for vaccine optimization.
Our working model, illustrated in Figure 2, proposes that plasma cell fate is determined early by KLF2 expression. Upon vaccination or infection, a KLF2-dependent, egress-prone subset characterized by high integrin β7, CD11b, S1PR1, and TIGIT, is induced in SLOs. Cells lacking sufficient KLF2 fail to exit and die in situ. S1PR1 is essential for plasma cell egress into the bloodstream via the S1P gradient, but subsequent S1PR1 internalization is required for entry into the bone marrow (29). Once in circulation, plasma cells home to the bone marrow in response to the chemoattractant CXCL12 (10). Upon lodging in available survival niches, they gradually mature into LLPCs, as discussed below, by receiving critical survival cues, such as APRIL or BAFF, from the local microenvironment (30).
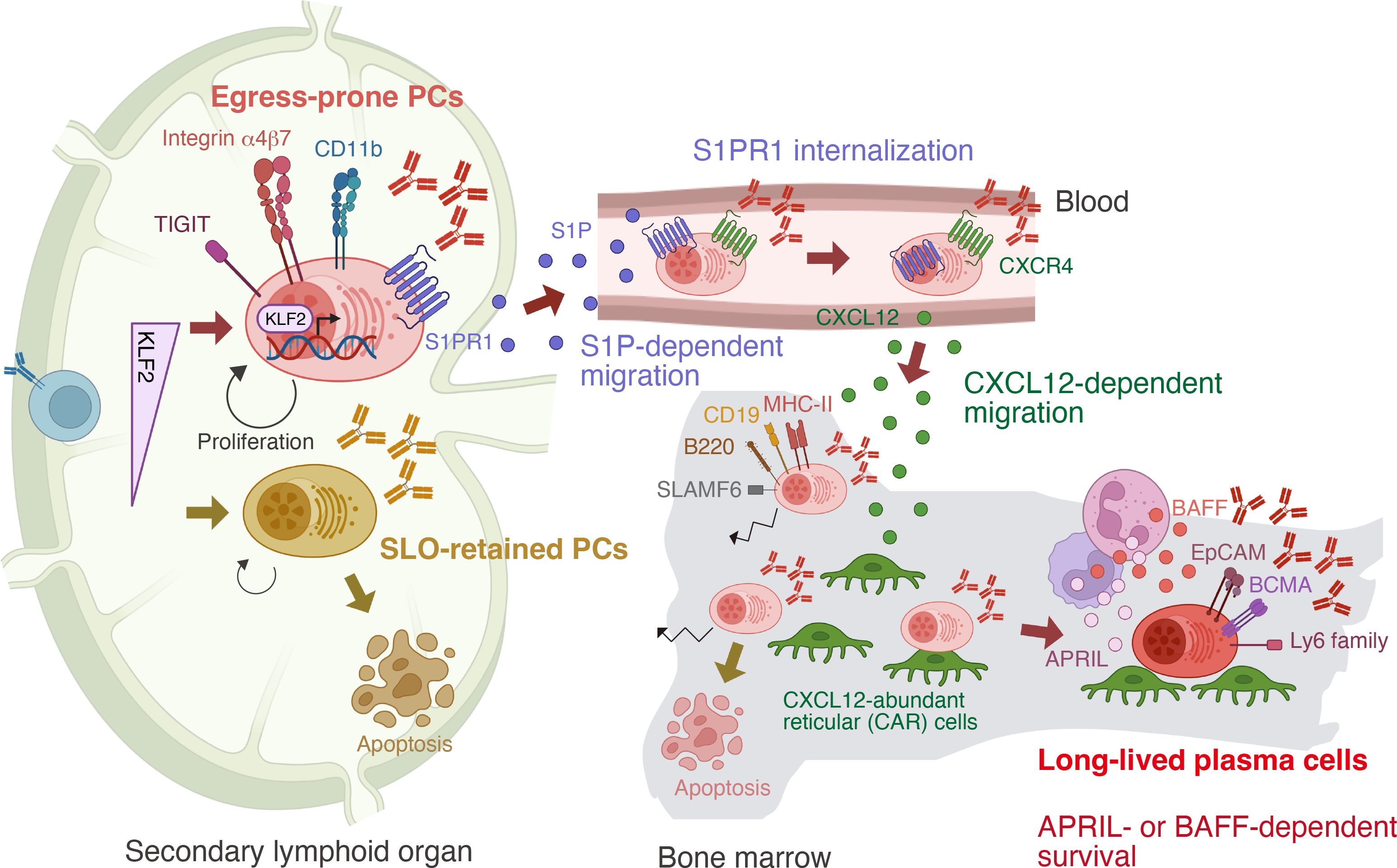
Figure 2. Stepwise differentiation toward long-lived plasma cells (LLPCs). In secondary lymphoid organs—the sites of plasma cell (PC) generation—PCs can be divided into two subsets based on expression of the transcription factor KLF2. Integrin β7lo TIGIT– PCs remain in the secondary lymphoid organs (SLOs), whereas Integrin β7hi TIGIT+ PCs acquire the capacity to migrate to the bone marrow. This migration is driven by KLF2-mediated upregulation of S1PR1, which senses the gradient of sphingosine-1-phosphate (S1P). Upon entering the bloodstream, where S1P levels are high, ligand engagement leads to internalization of S1PR1. These PCs then respond to the chemokine CXCL12, which is produced in the bone marrow, and migrate into the bone marrow. Only a fraction establish long-term residency and mature into LLPCs, which can be distinguished from recently arrived PCs by distinct surface marker expression. Within bone marrow niches, LLPCs are sessile and receive survival support from factors such as APRIL and BAFF produced by niche cells.
3 Timestamping analysis of plasma cell
Addressing the question of LLPC longevity has been a challenging task. LLPCs are not only extremely rare, but they also lack definitive phenotypic markers that distinguish them from SLPCs. Previous studies have primarily relied on the detection of antigen-specific plasma cells or the measurement of antigen-specific antibodies in the serum to evaluate the persistence of plasma cells following immunization (5, 31). However, because plasma cells are continuously generated as long as the immune response persists, the resulting plasma cell pool comprises a mixture of newly generated and pre-existing cells (32). Another strategy has involved labeling newly formed plasma cells with thymidine analogs such as BrdU and tracking them overtime (4, 20). While this method allows for temporal discrimination of plasma cell populations, it is incompatible with downstream molecular analyses, such as transcriptomic profiling, due to technical limitations associated with BrdU detection.
A major methodological breakthrough came with the development of a genetic plasma cell fate-mapping system, commonly referred to as plasma cell “timestamping”. Five research groups independently generated transgenic mouse lines expressing tamoxifen-inducible Cre recombinase (CreERT2) under the control of plasma cell-specific genes, such as Ighj or Prdm1 (11, 23, 33–35). When combined with a Cre-dependent reporter allele (e.g., Rosa26-LSL-tdTomato), administration of tamoxifen induces permanent expression of a reporter specifically in plasma cells present at the time of treatment. As a result, these timestamped cells can be tracked over time, while plasma cells generated after tamoxifen administration remain reporter-negative. This approach enables researchers to follow the fate of plasma cells present at a defined time point. Reporter-positive cells that persist for several months after tamoxifen treatment can be considered bona fide LLPCs, while the reporter-negative population contains a heterogeneous mixture of newly generated plasma cells of varying ages.
A series of timestamping studies have indeed yielded several novel insights into plasma cell biology, the details of which have been comprehensively reviewed elsewhere (36). The key points can be summarized as follows. First, homeostatic plasma cells are continuously replenished by newly generated populations, some of which progressively differentiated into LLPCs (23, 37). Second, following immunization with a model antigen, LLPCs accumulate in the bone marrow at a relatively constant rate from the early stages of the immune response, with no apparent bias toward the later phases (23, 33). Third, bone marrow plasma cells are highly heterogeneous in terms of maturation status and immunoglobulin isotype, with LLPCs comprising transcriptionally distinct subsets expressing IgA, IgM, or IgG (11, 34, 37). Fourth, LLPCs are enriched for a unique gene expression signature and display a distinct pattern of surface markers, enabling their distinction from bulk plasma cells or SLPCs (11, 34, 37). Fifth, plasma cells gradually lose motility within the bone marrow microenvironment, with LLPCs exhibiting a sessile, immobilized state within their survival niches (23, 34).
4 Regulation of plasma cell survival in the bone marrow
Timestamping approaches have provided new insight into one of the long-standing questions in the field: how newly generated plasma cells persist in the bone marrow despite the presence of pre-existing, established plasma cells. Two mutually exclusive models have been proposed to explain this phenomenon. The saturable niche model posits that the bone marrow contains a finite number of survival niches; newly formed plasma cells must compete for these limited sites, displacing resident cells to achieve long-term persistence (38). In contrast, the unlimited niche model suggests that new plasma cells occupy unfilled or newly created niches without directly competing with existing cells.
Recent timestamping studies appear to favor the latter model. Koike et al. reported no significant difference in bone marrow persistence between early (pre-GC) and late (post-GC) plasma cells, indicating that the timing of arrival does not dictate survival (23). Moreover, the absolute number of homeostatic plasma cells in the bone marrow gradually increases with age, indicating that niche capacity is not saturated over time (23). Similarly, Robinson et al., found that altering the influx of newly generated plasma cells, either increasing or decreasing their numbers, did not affect the turnover rate of pre-existing, timestamped plasma cells (37). Collectively, these findings suggest that plasma cell turnover in the bone marrow is governed primarily by intrinsic factors rather than by direct competition for limited niche resources, at least under homeostatic conditions or following immunization with model antigens and adjuvants. Whether different types of antigens and adjuvants can alter the turnover of bone marrow plasma cells remains an open question.
The possibility that bone marrow survival niches are heterogeneous in their capacity to support plasma cell longevity (Figure 1) also remains to be fully addressed. Jing et al. found that LLPCs are more frequently found in clusters than bulk plasma cells (34). This clustering depends on hematopoietic-derived APRIL (39), as acute APRIL inhibition disrupts these clusters and mobilizes plasma cells (30). Megakaryocytes are a known source of APRIL in the bone marrow (40), and their activation by thrombopoietin (TPO) administration has been shown to enhance the longevity of vaccine-induced antibody titers (41), indicating that megakaryocytes form a key component of LLPC survival niches. Furthermore, recent transinteractome analyses suggest that distinct subsets of plasma cells may depend on different cellular interaction partners, implying that their retention within the bone marrow is mediated by more diverse molecular mechanisms than previously appreciated (42). These observations point to the existence of functionally specialized microenvironments within the bone marrow that differentially regulate plasma cell survival. Fully elucidating the nature and organization of LLPC-supportive niches will require more refined approaches, such as spatial transcriptomics.
5 Conclusion and outstanding questions
Accumulating evidence has begun to reveal the developmental trajectories to LLPCs. A key emerging concept is that plasma cell longevity is “imprinted” early during their generation at the induction site. Depending on BCR signal and T cell help, activated B cells give rise to a small fraction of pre-plasma cells committed to the plasma cell lineage (43, 44). Their progeny, however, remain heterogenous, and only a fraction possesses the potential to become BM-resident LLPCs. These LLPC precursors are further shaped by turnover within extrinsic survival niches, gradually acquiring the features required for longevity. Collectively, both early imprinting at the induction site and subsequent refinement within effector sites cooperate to establish bona fide LLPCs.
Several key questions remain to be answered. First, the mechanisms governing the generation of LLPC precursors, such as TIGIT+ cells (12) or Integrin β7+ cells (13) at the induction site require further clarification. A central issue is which signals induce these precursors, and whether they are already specified at the pre-plasma cell stage or instead emerge only after plasma cell identity has been established. Moreover, it remains unresolved whether analogous precursors exist across other antibody isotypes or arise within distinct anatomical induction sites. Second, it remains unclear whether different forms of antigen and/or adjuvants influence the generation of LLPC precursors or their cell-intrinsic longevity. This may explain why durability of antibody response varies among vaccines (7) and addressing it could guide the design of next-generation vaccines capable of providing lifelong protection. Third, the precise characteristics and potential heterogeneity of bone marrow survival niches need to be defined. High-resolution single cell spatial omics will be indispensable for this task. Lastly, the signals and molecular pathways that drive LLPC maturation within these niches remain to be identified. The contribution of candidate regulators, including APRIL, BAFF (45), metabolites (46, 47), or adhesion molecules (39, 48), to LLPC maturation and survival needs to be determined. Clarifying these outstanding questions will be critical not only for the rational design of vaccines capable of inducing long-lasting humoral immunity, but also for the development of therapeutic strategies targeting autoimmune diseases in which chronically produced antibodies drive pathology.
Author contributions
TK: Writing – review & editing. WI: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Nippon Foundation (to WI) and the Japan Agency for Medical Research and Development (JP223fa627002 to WI and JP223fa627001 to TK).
Acknowledgments
Figures were created with BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nutt SL, Hodgkin PD, Tarlinton DM, and Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. (2015) 15:160–71. doi: 10.1038/nri3795
2. Landsverk OJB, Snir O, Casado RB, Richter L, Mold JE, Réu P, et al. Antibody-secreting plasma cells persist for decades in human intestine. J Exp Med. (2017) 214:309–17. doi: 10.1084/jem.20161590
3. Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity. (2015) 43:132–45. doi: 10.1016/j.immuni.2015.06.016
4. Manz RA, Thiel A, and Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. (1997) 388:133–4. doi: 10.1038/40540
5. Slifka MK, Matloubian M, and Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. (1995) 69:1895–902. doi: 10.1128/jvi.69.3.1895-1902.1995
6. Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, et al. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. (2004) 200:967–77. doi: 10.1084/jem.20040973
7. Amanna IJ, Carlson NE, and Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. (2007) 357:1903–15. doi: 10.1056/NEJMoa066092
8. Tellier J, Tarasova I, Nie J, Smillie CS, Fedele PL, Cao WHL, et al. Unraveling the diversity and functions of tissue-resident plasma cells. Nat Immunol. (2023) 25:330–42. doi: 10.1038/s41590-023-01712-w
9. Tarlinton DM, Ding Z, Tellier J, and Nutt SL. Making sense of plasma cell heterogeneity. Curr Opin Immunol. (2023) 81:102297. doi: 10.1016/j.coi.2023.102297
10. Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. (2001) 194:45–56. doi: 10.1084/jem.194.1.45
11. Liu X, Yao J, Zhao Y, Wang J, and Qi H. Heterogeneous plasma cells and long-lived subsets in response to immunization, autoantigen and microbiota. Nat Immunol. (2022) 23:1564–76. doi: 10.1038/s41590-022-01345-5
12. Manakkat Vijay GK, Zhou M, Thakkar K, Rothrauff A, Chawla AS, Chen D, et al. Temporal dynamics and genomic programming of plasma cell fates. Nat Immunol. (2024) 25:1097–109. doi: 10.1038/s41590-024-01831-y
13. Ise W, Koike T, Shimada N, Yamamoto H, Tai Y, Shirai T, et al. KLF2 expression in IgG plasma cells at their induction site regulates the migration program. J Exp Med. (2025) 222:e20241019. doi: 10.1084/jem.20241019
14. Elsner RA and Shlomchik MJ. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity. (2020) 53:1136–50. doi: 10.1016/j.immuni.2020.11.006
15. Good-Jacobson KL and Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. (2010) 185:3117–25. doi: 10.4049/jimmunol.1001155
16. Chan TD and Brink R. Affinity-based selection and the germinal center response. Immunol Rev. (2012) 247:11–23. doi: 10.1111/j.1600-065X.2012.01118.x
17. Smith KG, Light A, Nossal GJ, and Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. (1997) 16:2996–3006. doi: 10.1093/emboj/16.11.2996
18. Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. (2006) 203:2419–24. doi: 10.1084/jem.20061254
19. Weisel FJ, Zuccarino-Catania GV, Chikina M, and Shlomchik MJ. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. (2016) 44:116–30. doi: 10.1016/j.immuni.2015.12.004
20. Bortnick A, Chernova I, Quinn WJ 3rd, Mugnier M, Cancro MP, and Allman D. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol. (2012) 188:5389–96. doi: 10.4049/jimmunol.1102808
21. Taillardet M, Haffar G, Mondière P, Asensio M-J, Gheit H, Burdin N, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. (2009) 114:4432–40. doi: 10.1182/blood-2009-01-200014
22. Foote JB, Mahmoud TI, Vale AM, and Kearney JF. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol. (2012) 188:57–67. doi: 10.4049/jimmunol.1100783
23. Koike T, Fujii K, Kometani K, Butler NS, Funakoshi K, Yari S, et al. Progressive differentiation toward the long-lived plasma cell compartment in the bone marrow. J Exp Med. (2023) 220:e20221717. doi: 10.1084/jem.20221717
24. Wittner J and Schuh W. Krüppel-like factor 2 (KLF2) in immune cell migration. Vaccines (Basel). (2021) 9:1171. doi: 10.3390/vaccines9101171
25. Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. (2006) 203:2683–90. doi: 10.1084/jem.20061289
26. Wittner J, Schulz SR, Steinmetz TD, Berges J, Hauke M, Channell WM, et al. Krüppel-like factor 2 controls IgA plasma cell compartmentalization and IgA responses. Mucosal Immunol. (2022) 15:668–82. doi: 10.1038/s41385-022-00503-0
27. Yusuf I, Kharas MG, Chen J, Peralta RQ, Maruniak A, Sareen P, et al. KLF4 is a FOXO target gene that suppresses B cell proliferation. Int Immunol. (2008) 20:671–81. doi: 10.1093/intimm/dxn024
28. Chen J, Limon JJ, Blanc C, Peng SL, and Fruman DA. Foxo1 regulates marginal zone B-cell development. Eur J Immunol. (2010) 40:1890–6. doi: 10.1002/eji.200939817
29. Lo CG, Xu Y, Proia RL, and Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. (2005) 201:291–301. doi: 10.1084/jem.20041509
30. Park R, Benet Z, Jing Z, Enright J, and Fooksman DR. CD138 and APRIL regulate plasma cell survival, competition, and retention in the bone marrow niche. Cell Rep. (2025) 44:116123. doi: 10.1016/j.celrep.2025.116123
31. Slifka MK, Antia R, Whitmire JK, and Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. (1998) 8:363–72. doi: 10.1016/S1074-7613(00)80541-5
32. Chernova I, Jones DD, Wilmore JR, Bortnick A, Yucel M, Hershberg U, et al. Lasting antibody responses are mediated by a combination of newly formed and established bone marrow plasma cells drawn from clonally distinct precursors. J Immunol. (2014) 193:4971–9. doi: 10.4049/jimmunol.1401264
33. Robinson MJ, Dowling MR, Pitt C, O’Donnell K, Webster RH, Hill DL, et al. Long-lived plasma cells accumulate in the bone marrow at a constant rate from early in an immune response. Sci Immunol. (2022) 7:eabm8389. doi: 10.1126/sciimmunol.abm8389
34. Jing Z, Galbo P, Ovando L, Demouth M, Welte S, Park R, et al. Fine-tuning spatial-temporal dynamics and surface receptor expression support plasma cell-intrinsic longevity. Elife. (2024) 12:RP89712. doi: 10.7554/eLife.89712
35. Xu AQ, Barbosa RR, and Calado DP. Genetic timestamping of plasma cells in vivo reveals tissue-specific homeostatic population turnover. Elife. (2020) 9:e59850. doi: 10.7554/eLife.59850
36. Fooksman DR, Jing Z, and Park R. New insights into the ontogeny, diversity, maturation and survival of long-lived plasma cells. Nat Rev Immunol. (2024) 24:461–70. doi: 10.1038/s41577-024-00991-0
37. Robinson MJ, Ding Z, Dowling MR, Hill DL, Webster RH, McKenzie C, et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity. (2023) 56:1596–612. doi: 10.1016/j.immuni.2023.04.015
38. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dörner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. (2006) 6:741–50. doi: 10.1038/nri1886
39. Benet Z, Jing Z, and Fooksman DR. Plasma cell dynamics in the bone marrow niche. Cell Rep. (2021) 34:108733. doi: 10.1016/j.celrep.2021.108733
40. Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. (2010) 116:1867–75. doi: 10.1182/blood-2009-12-259457
41. Cortese M, Hagan T, Rouphael N, Wu S-Y, Xie X, Kazmin D, et al. System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans. Nat Immunol. (2025) 26:116–30. doi: 10.1038/s41590-024-02036-z
42. Bonaud A, Larraufie P, Khamyath M, Szachnowski U, Flint SM, Brunel-Meunier N, et al. Transinteractome analysis reveals distinct niche requirements for isotype-based plasma cell subsets in the bone marrow. Eur J Immunol. (2023) 53:e2250334. doi: 10.1002/eji.202250334
43. Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, et al. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. (2018) 48:702–715.e4. doi: 10.1016/j.immuni.2018.03.027
44. Luo W, Conter L, Elsner RA, Smita S, Weisel F, Callahan D, et al. IL-21R signal reprogramming cooperates with CD40 and BCR signals to select and differentiate germinal center B cells. Sci Immunol. (2023) 8:eadd1823. doi: 10.1126/sciimmunol.add1823
45. Cornelis R, Chang H-D, and Radbruch A. Keeping up with the stress of antibody production: BAFF and APRIL maintain memory plasma cells. Curr Opin Immunol. (2021) 71:97–102. doi: 10.1016/j.coi.2021.06.012
46. Lam WY and Bhattacharya D. Metabolic links between plasma cell survival, secretion, and stress. Trends Immunol. (2018) 39:19–27. doi: 10.1016/j.it.2017.08.007
47. Ishikawa M, Hasanali ZS, Zhao Y, Das A, Lavaert M, Roman CJ, et al. Bone marrow plasma cells require P2RX4 to sense extracellular ATP. Nature. (2024) 626:1102–7. doi: 10.1038/s41586-024-07047-2
Keywords: LLPC, bone marrow, antibody, niche, vaccine
Citation: Koike T and Ise W (2025) Developmental trajectory of long-lived plasma cells. Front. Immunol. 16:1684210. doi: 10.3389/fimmu.2025.1684210
Received: 12 August 2025; Accepted: 09 September 2025;
Published: 29 September 2025.
Edited by:
David R. Fooksman, Albert Einstein College of Medicine, United StatesReviewed by:
Godhev Kumar Manakkat Vijay, University of Pittsburgh, United StatesCopyright © 2025 Koike and Ise. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wataru Ise, d2lzZUBjaWRlci5vc2FrYS11LmFjLmpw
 Takuya Koike1,2
Takuya Koike1,2 Wataru Ise
Wataru Ise