- Department of Infectious Diseases and Public Health, Faculty of Veterinary Medicine, University of Agriculture in Cracow, Krakow, Poland
1 Introduction
One of the most unexpected consequences of the COVID-19 pandemic was the resurgence of numerous bacterial and viral infections in its aftermath. Notably, the incidence of several infectious diseases in 2022–2023 returned to—or even surpassed—pre-pandemic levels (1–5). A number of high-income countries with well-developed healthcare systems, including the United Kingdom, Ireland, the Netherlands, France, Denmark, Sweden, the United States, Australia, and New Zealand, reported a marked increase in Streptococcus pyogenes (GAS) infections, particularly scarlet fever and invasive GAS hospitalizations, compared to pre-COVID-19 levels (6–8).
During the pandemic lockdown, widespread implementation of long-term non-pharmaceutical interventions (NPIs) led to a dramatic reduction in the incidence of various infectious diseases (9, 10). This was largely attributed to decreased circulation of environmental microbiota and pathogens, resulting in reduced natural exposure and a subsequent decline in population-level immunity (11). This concept—often referred to as “immunity debt”—is especially relevant to the post-pandemic resurgence of respiratory viral infections (e.g., RSV and influenza) and GAS infections (12–14). It underscores the relationship between reduced pathogen exposure and increased population susceptibility (15, 16). While several mechanisms have been proposed to explain this resurgence, a comprehensive explanation remains elusive.
A recent comprehensive review by Nygaard et al. in The Lancet explored possible contributors to the post-pandemic infection surge. Their analysis considered multiple factors, including antimicrobial resistance, reduced vaccine coverage, emergence of more virulent pathogen strains, virus–bacteria interactions, and altered immune function due to limited pathogen exposure. They concluded that the primary driver of the resurgence was the accumulation of immunity debt, coinciding with the re-emergence of common viral and bacterial pathogens (5, 10). Importantly, the role of COVID-19 in modulating trained immunity has been extensively investigated. However, to date, no association with upsurge of GAS infections has been reported (17–23).
It is challenging to formulate a universal explanation for this phenomenon, given the diversity of the pathogens involved—ranging from bacterial to viral, vaccine-preventable to non-vaccine-preventable, and those primarily governed by innate versus adaptive immunity. Nevertheless, the global lockdown created an unprecedented natural experiment in which an estimated four billion individuals experienced prolonged reduction in pathogen exposure (18). This scale of immunological isolation may never be replicated again, offering a rare opportunity to study population-level infectious immunity under such conditions (24, 25) In this study, we narrowed our focus to the post-pandemic surge in Group A Streptococcus pyogenes (GAS) infections. GAS lacks an available vaccine and is primarily controlled by innate immune mechanisms (26). Furthermore, this infection provides a unique lens through which it is possible to examine impaired trained (innate) immunity at the herd level—a phenomenon we previously described shortly after the COVID-19 pandemic (27).
1.1 S. pyogenes and the immune system: a model for post-pandemic immunity debt
Group A Streptococcus pyogenes (GAS) is a Gram-positive, beta-hemolytic bacterium that exclusively infects humans. It causes a range of diseases, most of which are self-limiting, but some can be severe or even fatal. GAS typically colonizes the upper respiratory tract and can evade host defense mechanisms, leading to conditions such as pharyngitis, pyoderma, scarlet fever, and invasive infections like necrotizing fasciitis and streptococcal toxic shock syndrome (STSS) (28–30).
GAS produces several virulence factors, including superantigens and the M1 protein. Superantigens, encoded by 13 distinct genes, induce massive T-cell proliferation and cytokine release, potentially triggering a cytokine storm and leading to sepsis (31). Such fatal outcomes are often linked to defects in the host’s innate immune response (15, 32). The M1 protein, another major virulence factor, prevents immunoglobulins from effectively attacking the pathogen by inhibiting immunoglobulin-mediated phagocytosis and enhancing resistance to neutrophil bactericidal activity (33). The M1 protein is part of the emm family, with over 250 distinct emm types identified based on variability in the N-terminal region. The emm1 genotype, particularly the M1UK sub-lineage, first identified in the United Kingdom in 2019—has been associated with an increased incidence of scarlet fever and invasive GAS infections in the 21st century. Importantly, M1UK is strongly linked to an increase risk of STSS due to elevated superantigens production and enhanced ability to evade the immune system (14, 34). In addition, the M1UK lineage may acquire integrative conjugative elements (ICEs), thereby gaining new characteristics such as antibiotic resistance (26, 27, 35).
Notably, during the post-COVID-19 era, there has been a shift in the distribution of emm types in high-income countries, with emm1 (especially M1UK) and emm12 emerging as the most prevalent and virulent strains, capable of evading the immune system more effectively (36, 37).
1.2 Post-pandemic immunity debt and loss of trained immunity
Immunity debt, refers to the reduction in pathogen exposure and subsequent weakening of population immunity during the COVID-19 pandemic due to widespread non-pharmaceutical interventions (NPIs) (10). As a result, individuals experienced diminished levels of immunity typically acquired through regular exposure to pathogens. Proposed consequences of immunity debt include reduced antibody levels, a diminished pool of memory B and T cells, impaired innate defenses, and heightened susceptibility to infections. Recent reports have shown a decrease in circulating antibodies to common pathogens like respiratory syncytial virus (RSV) and GAS (13, 16). However, the impact of immunity debt on innate immunity, particularly on trained immunity, remains less well understood (38).
Trained immunity is a relatively new concept that redefines the role of the innate immune system in pathogen defense (39). Unlike the adaptive immune system, which develops antigen-specific memory, the innate immune system can “learn” from previous encounters with pathogens and enhance its response upon re-exposure. This process is triggered by interactions between microbiota and the host’s innate immune system (40). Notably, certain pathogens, vaccines (e.g., the BCG vaccine), and immune-modulating substances like β-glucans (derived from yeast species such as Candida and Saccharomyces) are potent inducers of trained immunity (41–43).
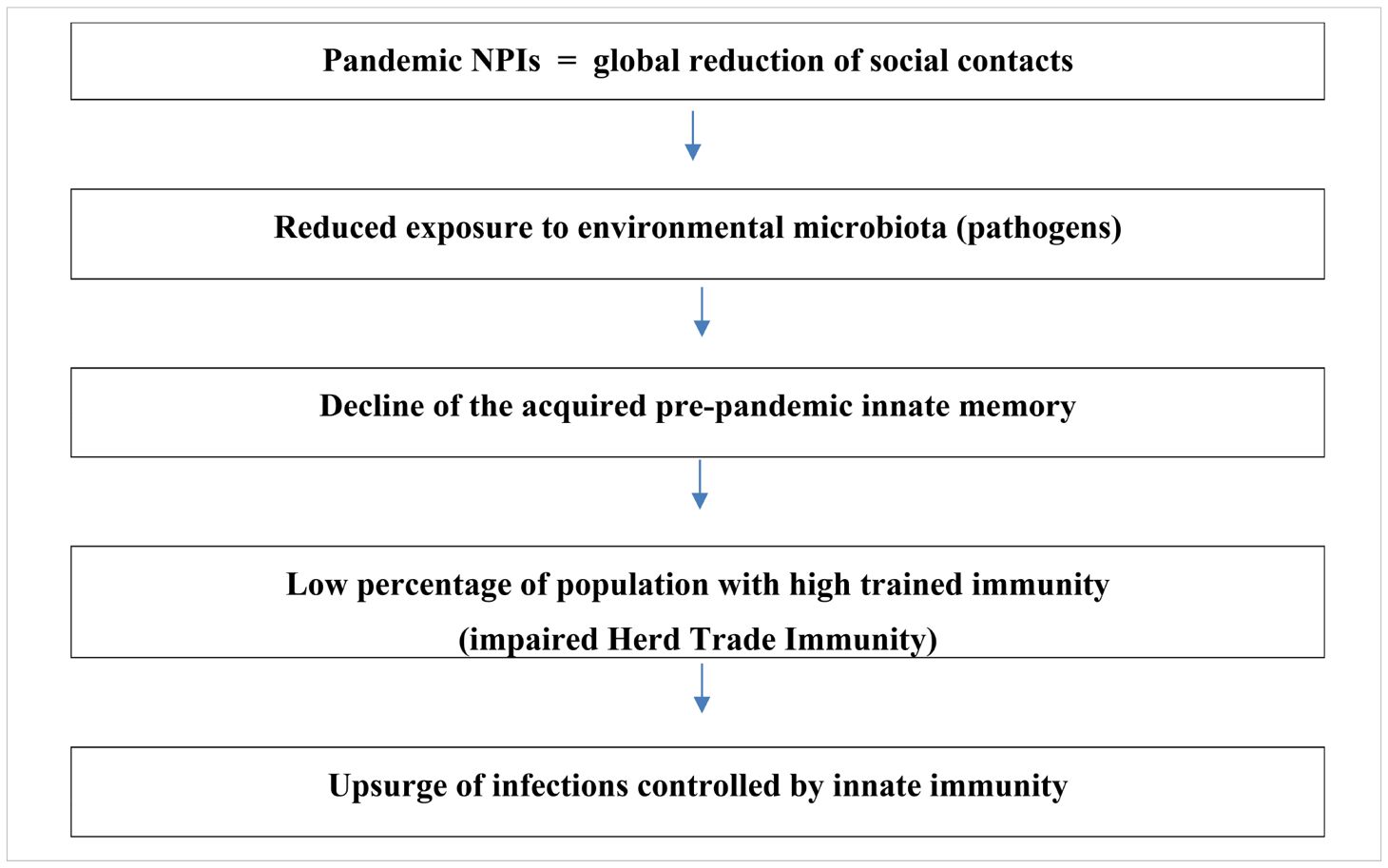
Trained immunity has revolutionized our understanding of innate immunity’s role in infectious disease defense. It enables a faster, more robust response to pathogens, preventing their multiplication and the onset of disease (44). The global reduction in microbial exposure during the COVID-19 lockdown has led to widespread depletion of trained immunity, weakening population-level defenses. As a result, herd trained immunity (HTI) has been impaired (27).
1.3 Innate immunity and GAS infections
The immune response to GAS infections is predominantly innate rather than adaptive. Recent studies have highlighted the role of macrophages and IFN-γ in protecting against GAS infection, with adaptive immune responses playing a lesser role in immediate defense (45). While adaptive immune responses can generate GAS-specific antibodies, these are often insufficient for long-term protection. Recurrent GAS infections do not confer effective, antigen-specific immunity, suggesting a critical role for the innate immune system in preventing infection. GAS exposure, however, does lead to the development of trained immunity (TI), wherein innate immune cells develop enhanced, non-specific memory and stronger responses to subsequent infections (32, 46, 47). Importantly, TI requires ongoing interaction with the microbiota, as the lifespan of trained immune cells, such as monocytes, is relatively short—typically no longer than three months (39, 44). In conclusion, the transmission and control of GAS infections may be governed more by population-level trained innate immunity rather than the generation of antigen-specific memory B and T cells (38–40). (41–44).
1.4 Herd immunity vs. herd trained immunity
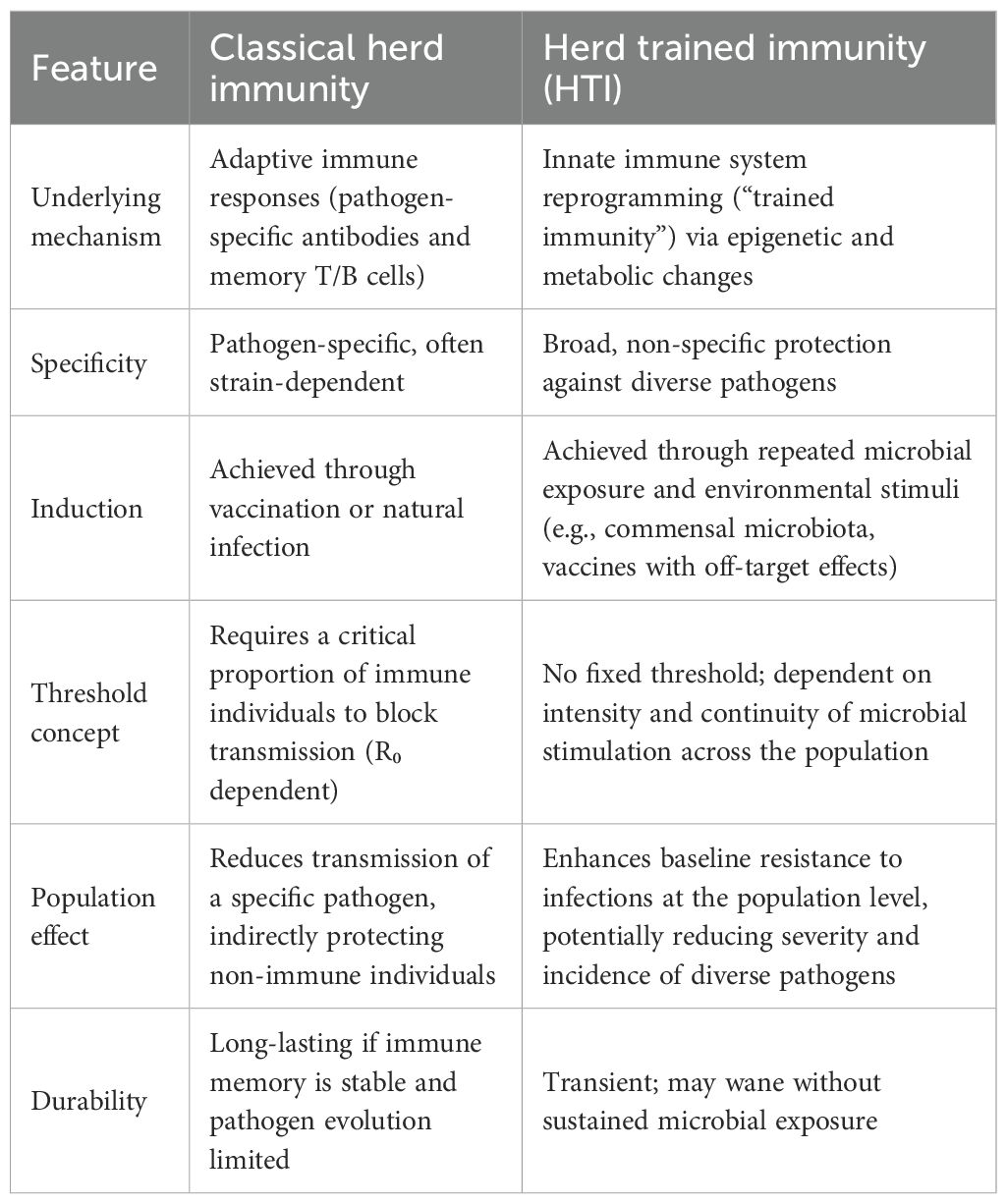
The concept of herd immunity traditionally refers to the protection of a population from infectious disease through widespread acquisition of antigen-specific adaptive immunity, particularly by memory B and T cells (48). In contrast, herd trained immunity (HTI) refers to the defense mechanisms conferred by the innate immune system, which operates through nonspecific memory and enhanced responsiveness to pathogens. Unlike adaptive immune memory, trained immunity involves cells such as monocytes and macrophages that have a much shorter lifespan—typically weeks to months (40). I have created with the assistance of AI, the comparison table between classical herd immunity and my concept of HTI to clarify their impact on infectious immunity at the populational level (Table 1).
2 Discussion
The COVID-19 pandemic lockdown created a unique and unexpected opportunity to investigate infectious immunity following a prolonged period of reduced exposure to common pathogens, including Streptococcus pyogenes (GAS). Of particular note is the sharp post-pandemic surge in GAS infections, with reported rates exceeding pre-pandemic prevalence by 2–4-fold (1, 2). This atypical rise is believed to be multifactorial, involving both host and pathogen-related factors, as postulated by previous studies.
Key contributing elements include: i) emergence or resurgence of virulent GAS strains, notably the emm1 lineage (M1UK) (36). In Europe, early evidence indicated a predominance of the highly virulent M1UK variant; however, no corresponding increase in antibiotic resistance was observed (12, 49); ii) an expanded reservoir of asymptomatic GAS carriers, potentially increasing community transmission (8); iii) a rise in respiratory viral infections (e.g., RSV, influenza), which may predispose individuals to secondary bacterial infections, though these alone cannot fully explain the observed GAS increase (3); iv) immunity debt, a phenomenon characterized by reduced innate and adaptive immune preparedness due to decreased pathogen exposure during prolonged lockdown periods (38).
While each of these factors likely contributed to the unique epidemiological pattern observed, immunity debt—particularly its impact on trained innate immunity—appears to be the dominant driver. Trained immunity refers to a form of innate immune memory that enhances the host’s nonspecific defense mechanisms after repeated microbial encounters (31). At the population level, this concept extends to what has been termed Herd Trained Immunity (HTI), which complements classical antigen-specific herd immunity mediated by B and T cells. HTI may play a crucial role in preventing the spread of infections such as invasive GAS (iGAS) (27).
Importantly, the global pandemic restrictions have underscored the importance of HTI, a phenomenon that had not been widely recognized until now. While adaptive immune memory can persist for years, trained immunity offers a rapid, short-term defense that is crucial in controlling infections, especially in the absence of vaccine-induced immunity (45). The lockdown-induced reduction in pathogen exposure has thus exposed a critical vulnerability in our immune system—one that was previously underestimated. Impact of the long-term pandemic lockdown on overthrow of Herd Trained Immunity (HTI) is explained in Table 2.
Moreover, the post-pandemic resurgence of infections highlights the critical role of continual immune stimulation by environmental microbes in maintaining immune readiness. Social distancing and other NPIs, while essential in controlling SARS-CoV-2 transmission, inadvertently disrupted this natural immune training. Notably, trained immunity has a limited duration—estimated at around three months —suggesting that extended lockdowns led to the population-wide waning of innate immune protection acquired pre-pandemically (14, 23). The timeline of GAS infections in England (2019-2024), aligned with the implementation and relaxation of NPIs, further supports the hypothesis of impaired HTI during this period (Figure 1).
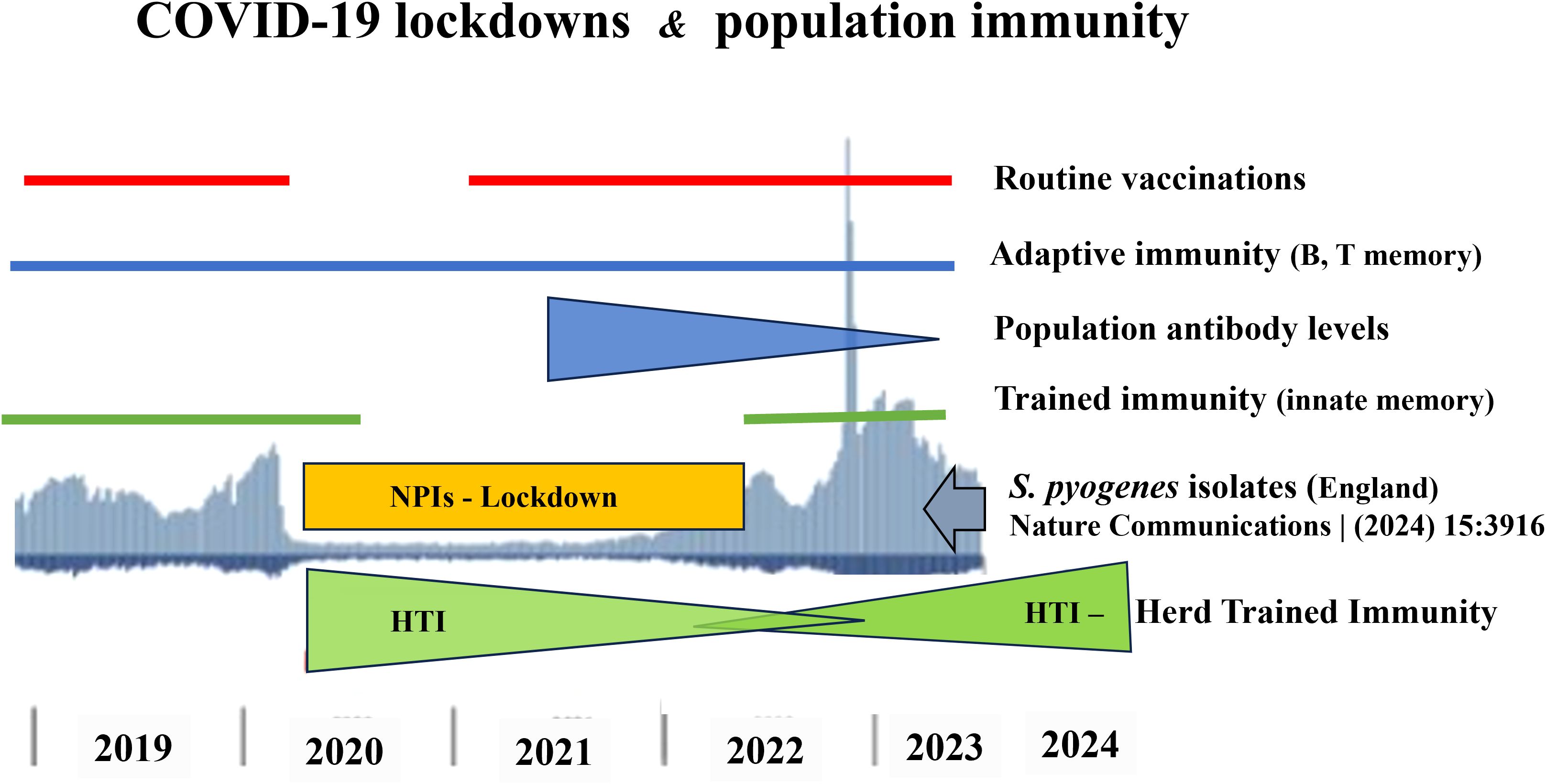
Figure 1. Timeline of S. pyogenes infections (2019–2024) and their relationship with the COVID-19 NPIs (reduced exposure to environmental microbiota 2020-2022), temporary suspension of routine vaccinations (2020), presence of pre-pandemic B and T memory cells (2019-2024), decline of population antibody levels (2021-2023), gap of trained immunity (2020-2022), disappearance (2020-2022) and re-creating of HTI (2022-2024).
Crucially, the decline in GAS incidence observed in some countries during 2024–2025 provides compelling evidence for this hypothesis (49). This decline likely reflects the re-establishment of innate immune memory across populations through renewed microbial exposure, thereby restoring HTI (50). However, the pace of recovery of innate immunity varies between countries and depends on both the duration of lockdowns and the degree of compliance during the epidemic. In my opinion, the global incidence of GAS infections may return to pre-pandemic levels within two years. Nevertheless, strong evidence indicates that the incidence of extremely severe iGAS infections, such as STSS, has returned to pre-pandemic levels. For example, in Poland the reported number of STSS cases was 19–20 in the pre-pandemic years (2018-2019), decreased to 2 cases during the lockdown year (2020), surged to 100 cases in the post-pandemic period (2023), and declined to 15 cases in the current year (01.01-31.08.2025) (51, 52).
3 Conclusion
The COVID-19 pandemic lockdown has underscored the essential role of regular microbial exposure in sustaining immune competence. The post-pandemic surge in infections such as iGAS illustrates the impact of immunity debt—particularly innate immunity debt—resulting from prolonged NPIs. The emerging concept of HTI provides novel insights into the interplay between innate and adaptive immune responses and offers a new framework for understanding and managing infectious disease risks in the context of public health interventions. By acknowledging HTI, we can better inform future strategies for epidemic preparedness and immune system resilience.
Finally, this hypothesis, which associates impaired Herd Trained Immunity (HTI) with the post-pandemic resurgence of GAS infections, does not exclude the contribution of additional factors or triggers. Notably, it indicates for the first time, that population-level of trained innate immunity exists and may be compromised by prolonged reduced contact with microbiota. However, it should be confirmed in the future by retrospective epidemiological studies covering pre- and post-pandemic few years. Nevertheless, the observed association between COVID-19 lockdown and impaired HTI suggests that future pandemic response strategies should, in addition to widespread vaccination, carefully balance the extent of lockdown measures with the preservation of HTI.
Author contributions
JM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
I would like to thank Dr. Grzegorz Majka (Department of Immunology, Faculty of Medicine, Jagiellonian University Medical College) for his assistance in editing the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abo Y-N, Oliver J, McMinn A, Osowicki J, Baker C, Clark JE, et al. Increase in invasive group a streptococcal disease among Australian children coinciding with Northern hemisphere surges. Lancet Regional Health – Western Pacific. (2023) 41:1–11. doi: 10.1016/j.lanwpc.2023.100873
2. Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus Pneumoniae, Haemophilus Influenzae, and Neisseria Meningitidisduring the COVID-19 Pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet DigitHealth. (2021) 3:e360–e70. doi: 10.1016/S2589-7500(21)00077-7
3. Musumeci S, MacPhail A, Weisser Rohacek M, Erba A, Goldenberger D, Lang C, et al. Role of viral coinfection in post-pandemic invasive group a streptococcal infections in adults, a nation-wide cohort study (Igaswiss). Eur J Clin Microbiol Infect Dis. (2025). doi: 10.1007/s10096-025-05229-y
4. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. Covid-19 in children and adolescents in europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
5. Nygaard U, Holm M, Rabie H, and Rytter M. The pattern of childhood infections during and after the covid-19 pandemic. Lancet Child Adolesc Health. (2024) 8:910–20. doi: 10.1016/S2352-4642(24)00236-0
6. Nygaard U, Hartling UB, Munkstrup C, Nielsen AB, Dungu KHS, Schmidt LS, et al. Invasive group a streptococcal infections in children and adolescents in Denmark during 2022-23 compared with 2016-17 to 2021-22: A nationwide, multicentre, population-based cohort study. Lancet Child Adolesc Health. (2024) 8:112–21. doi: 10.1016/S2352-4642(23)00295-X
7. Ammar S, Anglemyer A, Bennett J, Lees J, Addidle M, Morgan J, et al. Post-pandemic increase in invasive group a strep infections in New Zealand. J Infection Public Health. (2024) 17:102545. doi: 10.1016/j.jiph.2024.102545
8. Tomidis Chatzimanouil MK, Rößler S, Nurjadi D, Iakovidis I, Berner R, Toepfner N, et al. Post-Covid-19-pandemic changes and clinical characteristics of invasive group a streptococcal infections from 2015 to 2023. Infection. (2025) 53:991–1000. doi: 10.1007/s15010-024-02413-8
9. Dinleyici EC, Borrow R, Safadi MAP, van Damme P, and Munoz FM. Vaccines and routine immunization strategies during the Covid-19 pandemic. Hum Vaccines Immunotherapeutics. (2021) 17:400–7. doi: 10.1080/21645515.2020.1804776
10. Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, and Grenfell BT. The impact of Covid-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci. (2020) 117:30547–53. doi: 10.1073/pnas.2013182117
11. Graham DB and Xavier RJ. Conditioning of the immune system by the microbiome. Trends Immunol. (2023) 44:499–511. doi: 10.1016/j.it.2023.05.002
12. Karapati E, Tsantes AG, Iliodromiti Z, Boutsikou T, Paliatsiou S, Domouchtsidou A, et al. Group a Streptococcus Infections in Children: Epidemiological Insights before and after the Covid-19 Pandemic. Pathogens. (2024) 13(11):1007–1028. doi: 10.3390/pathogens13111007
13. den Hartog G, van Kasteren PB, Schepp RM, Teirlinck AC, van der Klis FRM, and van Binnendijk RS. Decline of rsv-specific antibodies during the Covid-19 pandemic. Lancet Infect Dis. (2023) 23:23–5. doi: 10.1016/s1473-3099(22)00763-0
14. Munro AP and House T. Cycles of susceptibility: immunity debt explains altered infectious disease dynamics post -pandemic. Clin Infect Dis. (2024). doi: 10.1093/cid/ciae493
15. Needle RF and Russell RS. Immunity debt, a gap in learning, or immune dysfunction? Viral Immunol. (2023) 36:1–2. doi: 10.1089/vim.2022.0204
16. Lorenz N, James A, Van Rooyen T, Paterson A, Ramiah C, Carlton LH, et al. Decline of antibodies to major viral and bacterial respiratory pathogens during the Covid-19 pandemic. J Infect Dis. (2025) 231:e77–81. doi: 10.1093/infdis/jiae611
17. Netea MG, Ziogas A, Benn CS, Giamarellos-Bourboulis EJ, Joosten LAB, Arditi M, et al. The role of trained immunity in Covid-19: lessons for the next pandemic. Cell Host Microbe. (2023) 31:890–901. doi: 10.1016/j.chom.2023.05.004
18. Brueggeman JM, Zhao J, Schank M, Yao ZQ, and Moorman JP. Trained Immunity: An Overview and the Impact on Covid-19, Vol. 13. Front Immunol. (2022). 13:837524. doi: 10.3389/fimmu.2022.837524.
19. Gu J, Liu Q, Zhang J, and Xu S. Covid-19 and Trained Immunity: The Inflammatory Burden of Long Covid, Vol. 14. Front Immunol. (2023). 14:1294959. doi: 10.3389/fimmu.2023.1294959.
20. Murphy DM, Cox DJ, Connolly SA, Breen EP, Brugman AAI, Phelan JJ, et al. Trained immunity is induced in humans after immunization with an adenoviral vector Covid-19 vaccine. J Clin Invest. (2023) 133(2):e162581. doi: 10.1172/JCI162581
21. Ziogas A, Bruno M, van der Meel R, Mulder WJM, and Netea MG. Trained immunity: target for prophylaxis and therapy. Cell Host Microbe. (2023) 31:1776–91. doi: 10.1016/j.chom.2023.10.015
22. Angulo M and Angulo C. Trained immunity-based vaccines: A vision from the one health initiative. Vaccine. (2025) 43:126505. doi: 10.1016/j.vaccine.2024.126505
23. Jentho E, Ruiz-Moreno C, Novakovic B, Kourtzelis I, Megchelenbrink WL, Martins R, et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc Natl Acad Sci USA (2021) 118:. doi: 10.1073/pnas.2102698118
24. Tay MZ, Poh CM, Rénia L, MacAry PA, and Ng LFP. The trinity of covid-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
25. Merad M, Blish CA, Sallusto F, and Iwasaki A. The immunology and immunopathology of Covid-19. Science. (2022) 375:1122–7. doi: 10.1126/science.abm8108
26. Kang A, Ye G, Singh R, Afkhami S, Bavananthasivam J, Luo X, et al. Subcutaneous bcg vaccination protects against streptococcal pneumonia via regulating innate immune responses in the lung. EMBO Mol Med. (2023) 15:e17084. doi: 10.15252/emmm.202217084
27. Marcinkiewicz J. Increase in the incidence of invasive bacterial infections following the Covid-19 pandemic: potential links with decreased herd trained immunity - a novel concept in medicine. Polish Arch Internal Med. (2024) 134(9):16794. doi: 10.20452/pamw.16794
28. Walker Mark J, Barnett Timothy C, McArthur Jason D, Cole Jason N, Gillen Christine M, Henningham A, et al. Disease manifestations and pathogenic mechanisms of group a streptococcus. Clin Microbiol Rev. (2014) 27:264–301. doi: 10.1128/cmr.00101-13
29. Tse H, Bao JYJ, Davies MR, Maamary P, Tsoi H-W, Tong AHY, et al. Molecular characterization of the 2011 hong kong scarlet fever outbreak. J Infect Dis. (2012) 206:341–51. doi: 10.1093/infdis/jis362
30. Lappin E and Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. (2009) 9:281–90. doi: 10.1016/S1473-3099(09)70066-0
31. Commons RJ, Smeesters PR, Proft T, Fraser JD, Robins-Browne R, and Curtis N. Streptococcal superantigens: categorization and clinical associations. Trends Mol Med. (2014) 20:48–62. doi: 10.1016/j.molmed.2013.10.004
32. Fieber C and Kovarik P. Responses of Innate Immune Cells to Group a Streptococcus, Vol. 4. Front Cell Infect Microbiol. (2014). 4:140. doi: 10.3389/fcimb.2014.00140.
33. Cunningham Madeleine W. Pathogenesis of group a streptococcal infections. Clin Microbiol Rev. (2000) 13:470–511. doi: 10.1128/cmr.13.3.470
34. Rodriguez-Ruiz JP, Lin Q, Lammens C, Smeesters PR, van Kleef-van Koeveringe S, Matheeussen V, et al. Increase in bloodstream infections caused by emm1 group a streptococcus correlates with emergence of toxigenic M1(Uk), Belgium, May 2022 to August 2023. Euro surveillance: Bull Europeen sur les maladies transmissibles = Eur communicable Dis Bull. (2023) 28(36):2300422. doi: 10.2807/1560-7917.Es.2023.28.36.2300422
35. Cai J, Zhou X, Zhang C, Jiang Y, Lv P, Zhou Y, et al. Ongoing epidemic of scarlet fever in Shanghai and the emergence of M1UK lineage Group a Streptococcus: a 14-year Surveillance Study across the COVID-19 Pandemic Period. Lancet Reg Health – West Pac. (2025) 58:101576. doi: 10.1016/j.lanwpc.2025.101576
36. Vieira A, Wan Y, Ryan Y, Li HK, Guy RL, Papangeli M, et al. Rapid expansion and international spread of M1uk in the post-pandemic uk upsurge of streptococcus pyogenes. Nat Commun. (2024) 15:3916. doi: 10.1038/s41467-024-47929-7
37. You Y, Davies MR, Protani M, McIntyre L, Walker MJ, and Zhang J. Scarlet fever epidemic in China caused by streptococcus pyogenesSerotype M12: epidemiologic and molecular analysis. eBioMedicine. (2018) 28:128–35. doi: 10.1016/j.ebiom.2018.01.010
38. Boraschi D and Italiani P. Innate Immune Memory: Time for Adopting a Correct Terminology, Vol. 9. Front Immunol. (2018). 9:799. doi: 10.3389/fimmu.2018.00799.
39. Netea Mihai G, Quintin J, and van der Meer Jos WM. Trained immunity: A memory for innate host defense. Cell Host Microbe. (2011) 9:355–61. doi: 10.1016/j.chom.2011.04.006
40. Vuscan P, Kischkel B, Joosten LAB, and Netea MG. Trained immunity: general and emerging concepts. Immunological Reviews (2024) 323:164–85. doi: 10.1111/imr.13326
41. Vuscan P, Kischkel B, Hatzioannou A, Markaki E, Sarlea A, Tintoré M, et al. Potent Induction of Trained Immunity by Saccharomyces Cerevisiae Β-Glucans, Vol. 15. Front Immunol. (2024). 55:1323333. doi: 10.3389/fimmu.2024.1323333.
42. Ciszek-Lenda M, Nowak B, Majka G, Suski M, Walczewska M, Fedor A, et al. Saccharomyces cerevisiae Β-glucan improves the response of trained macrophages to severe P. Aeruginosa Infections. Inflammation Res. (2024) 73:1283–97. doi: 10.1007/s00011-024-01898-1
43. Chen J, Gao L, Wu X, Fan Y, Liu M, Peng L, et al. Bcg-induced trained immunity: history, mechanisms and potential applications. J Trans Med. (2023) 21:106. doi: 10.1186/s12967-023-03944-8
44. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
45. Emami S, Westerlund E, Rojas Converso T, Johansson-Lindbom B, and Persson JJ. Protection acquired upon intraperitoneal group a Streptococcus immunization is independent of concurrent adaptive immune responses but relies on macrophages and IFN-γ. Virulence. (2025) 16:2457957. doi: 10.1080/21505594.2025.2457957
46. Matsumura T and Takahashi Y. The role of myeloid cells in prevention and control of group a streptococcal infections. Biosafety Health. (2020) 2:130–4. doi: 10.1016/j.bsheal.2020.05.006
47. Su Marcia S-W, Cheng Y-L, Lin Y-S, and Wu J-J. Interplay between group a streptococcus and host innate immune responses. Microbiol Mol Biol Rev. (2024) 88:e00052–22. doi: 10.1128/mmbr.00052-22
48. Randolph HE and Barreiro LB. Herd immunity: understanding covid-19. Immunity. (2020) 52:737–41. doi: 10.1016/j.immuni.2020.04.012
49. Goldberg-Bockhorn E, Hagemann B, Furitsch M, and Hoffmann TK. Invasive group a streptococcal infections in europe after the Covid-19 pandemic. Deutsches Arzteblatt Int. (2024) 121:673–80. doi: 10.3238/arztebl.m2024.0127
50. Uk Health Security Agency. (2025). Available online at: https://www.gov.uk/government/organisations/uk-health-security-agency (Accessed April 15, 2025).
51. Infectious Diseases and Poisonings in Poland (Annual Reports for 2019–2024 and Forthnight Reports for 2025) (2025). Available online at: https://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html (Accessed August 7, 2025).
Keywords: group A streptococcus, innate memory, herd trained immunity, pandemic lockdown, resurge of infections
Citation: Marcinkiewicz J (2025) Post-pandemic upsurge of group A streptococcus infections: potential link to impaired herd trained immunity following COVID-19 lockdowns. Front. Immunol. 16:1684332. doi: 10.3389/fimmu.2025.1684332
Received: 12 August 2025; Accepted: 06 October 2025;
Published: 15 October 2025.
Edited by:
Melese Abate Reta, University of Pretoria, South AfricaReviewed by:
Chuan Chiang-Ni, Chang Gung University, TaiwanCopyright © 2025 Marcinkiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janusz Marcinkiewicz, amFudXN6Lm1hcmNpbmtpZXdjekB1cmsuZWR1LnBs
 Janusz Marcinkiewicz
Janusz Marcinkiewicz