- 1Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Genova, Italy
- 2UOC Reumatologia e Malattie Autoinfiammatorie, IRCCS Istituto Giannina Gaslini, Genova, Italy
- 3Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 4Division of Nephrology, Dialysis and Transplantation, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 5Pediatric Gastroenterology and Endoscopy Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy
Background: Patients affected by very early onset inflammatory bowel disease (VEO-IBD) are frequently refractory to standard treatments. Despite the lack of randomized clinical trials, vedolizumab emerged as an effective and safe alternative treatment in VEO-IBD resistant to TNF antagonists. Here, we present a case of VEO-IBD with ulcerative colitis (UC) phenotype developing renal injury after vedolizumab administration.
Case presentation: An 11-year-old female patient with VEO-UC was referred to our clinic for fever, nausea, and fatigue. She was treated with vedolizumab for 1 year due to steroid-dependent disease and failure of multiple therapies, including anti-TNF agents. At admission, she was in steroid-free clinical and endoscopic remission, with leukocytosis, increased inflammatory markers, and a rise in serum creatinine. Urine samples revealed persistent leukocyturia over the past 8–10 months with the absence of lower urinary tract symptoms and negative serial urine culture. MRI showed swollen-looking kidneys with bilateral irregular Diffusion-Weighted Imaging (DWI) signal restriction. Kidney biopsy revealed the presence of acute tubular damage with a mixed interstitial inflammatory infiltrate consistent with drug-induced acute tubulointerstitial nephritis (TIN). After prompt start of systemic glucocorticoid therapy and temporary discontinuation of vedolizumab, normalization of renal function and urinalysis was observed. Vedolizumab was restarted after 2 months, pre-medicated with steroids. The follow-up renal biopsy performed after 6 months showed a regression of the histological pattern, with chronic signs characterized by mild tubular atrophy and interstitial fibrosis.
Conclusion: Vedolizumab-related acute TIN is a potentially severe complication, rarely described in adult patients. We report the first case of VEO-IBD with a probable vedolizumab-related acute TIN treated with corticosteroids, with a good response and maintenance of vedolizumab. Persisting sterile leukocyturia could represent an early sign.
Introduction
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders that affect the digestive tract, including Crohn’s disease (CD), ulcerative colitis (UC), and inflammatory bowel disease-unclassified (IBD-U). Albeit it is traditionally considered a disease of adulthood, there was an increased rate of very early onset IBD (VEO-IBD), defined by the onset of symptoms before the age of 6 years (1). VEO-IBD is sustained by monogenic pathological variants in 8%–32% of cases (2, 3), characterized by inborn errors of immunity (IEI) in more than 70% of genetic cases (4).
Therapeutic failure in VEO-IBD is more frequent than in older pediatric subjects with IBD. In approximately 70% of cases, VEO-IBD is poorly responsive to the standard treatment of IBD, including 5-Aminosalicylic Acid, steroids, and immunomodulators (mercaptopurine, azathioprine, and methotrexate), therefore needing an alternative approach (5). Anti-tumor necrosis factor (anti-TNF) agents have recently shown relative efficacy in patients with VEO-IBD, especially in patients with CD phenotype, although they are not effective in maintaining long-term remission in more than 20% of treated patients (6). Vedolizumab, a gut-selective anti-α4β7 integrin humanized monoclonal antibody, has shown promising results in the treatment of pediatric IBD with primary failure or loss of response to other treatments, especially in UC (7–11). Although it represents a promising rescue therapy, data on safety in the pediatric population should be expanded (10–12).
Here, we present a pediatric case of renal injury due to drug-induced acute tubulointerstitial nephritis (TIN) in an 11-year-old female patient affected by VEO-IBD.
Case presentation
We present a case involving an 11-year-old female patient diagnosed with VEO-IBD exhibiting UC symptoms, which first manifested at the age of 4. Next-generation sequencing (NGS) genetic panel for genes associated with VEO-IBD was negative (Supplementary Table 1). Due to the limited response to first-line therapy (5-ASA, topical steroid, and azathioprine) and steroid-dependent disease, infliximab (IFX) was started at 8 years old without achieving either clinical improvement or mucosal healing despite the maximized dose (8.3 mg/kg every 4 weeks). For this reason, an approach with thalidomide was attempted with subsequent discontinuation after less than 6 months due to the appearance of a thalidomide-related pseudotumor cerebri. When the patient was 9, due to persistent steroid dependence, adalimumab, a different anti-TNF agent, was administered with endoscopic and histological evidence of active disease Ulcerative Colitis Endoscopic Index of Severity after 3 months of therapy. After failure of multiple lines of therapy, vedolizumab was begun with a good clinical and endoscopic response at the optimized dosage of 10 mg/kg every 8 weeks in order to keep levels between 10 and 15 μg/mL during maintenance. Mesalamine was maintained from the diagnosis and associated with every major treatment change.
She was referred to the emergency department for a persisting fever poorly responsive to oral antibiotic therapy, vomiting, fatigue, and loss of weight. Symptoms persisted for 2 weeks before and started a few days after the last infusion of vedolizumab. At admission, laboratory tests showed leukocytosis (White Blood Cells, 16,540 cells/mmc), thrombocytosis (Platelets, 569,000 cells/mmc), increased C-reactive protein (CRP; 10.74 mg/dL), and acute kidney injury (AKI), as well as risk stage according to the RIFLE criteria (serum creatinine increased by 0.4 to 0.7 mg/dL, eGFR 9 mL/min/1.73 m2 with revised Schwartz equation). Urine samples revealed leukocyturia (519 cells/μL) with a negative urine culture and without symptoms consistent with lower urinary tract infection. Seriate blood and stool cultures, chest X-ray, and abdominal ultrasound were negative.
She was admitted to the pediatric gastroenterology department for further investigations. Regarding the VEO-IBD, she was in steroid-free clinical remission (Pediatric Ulcerative Colitis Activity Index 0). Pancolonoscopy confirmed the mucosal healing. CT abdomen revealed kidneys with slightly bumpy margins, characterized by a non-homogeneous contrast enhancement with a minimal amount of pelvic fluid. Supposing a culture-negative urosepsis, a urinary tract infection, we administered the antibiotic piperacillin-tazobactam, with no effect in terms of clinical symptoms and serum inflammatory markers, which remained elevated.
Reviewing urinalysis and laboratory data, we noted an increase in urinary leukocytes and a really limited rise in serum creatinine also after the first vedolizumab infusion (Figure 1). Therefore, due to persistent fever and worsening of renal function (creatinine 0.89 mg/dL, eGFR 67 mL/min/1.73 m2), vedolizumab and mesalamine were discontinued due to suspicion of drug-induced nephropathy, and an ultrasound-guided kidney biopsy was performed.

Figure 1. Trends in serum creatinine and leukocyturia over the past 2 years. Black arrows and lines indicate I vedolizumab (VEDO) infusion and restart. Red arrows and lines indicate the start of prednisone (PDN) following tubulointerstitial nephritis (TIN) diagnosis. Orange arrows and lines indicate the treatment with mesalamine (MESA). The dashed horizontal red line indicates the cut-off of normal leukocyturia (<20 cells/μL)..
Hematoxylin and eosin stain showed diffuse interstitial inflammatory infiltrate and focal tubular atrophy localized mostly in the medullary compartment (Figure 2A). The inflammatory infiltrate was composed mostly of T (CD3+) and B (CD20+) lymphocytes, with intermixed histiocytes (CD68+), neutrophils, and rare eosinophils (Figure 2A).
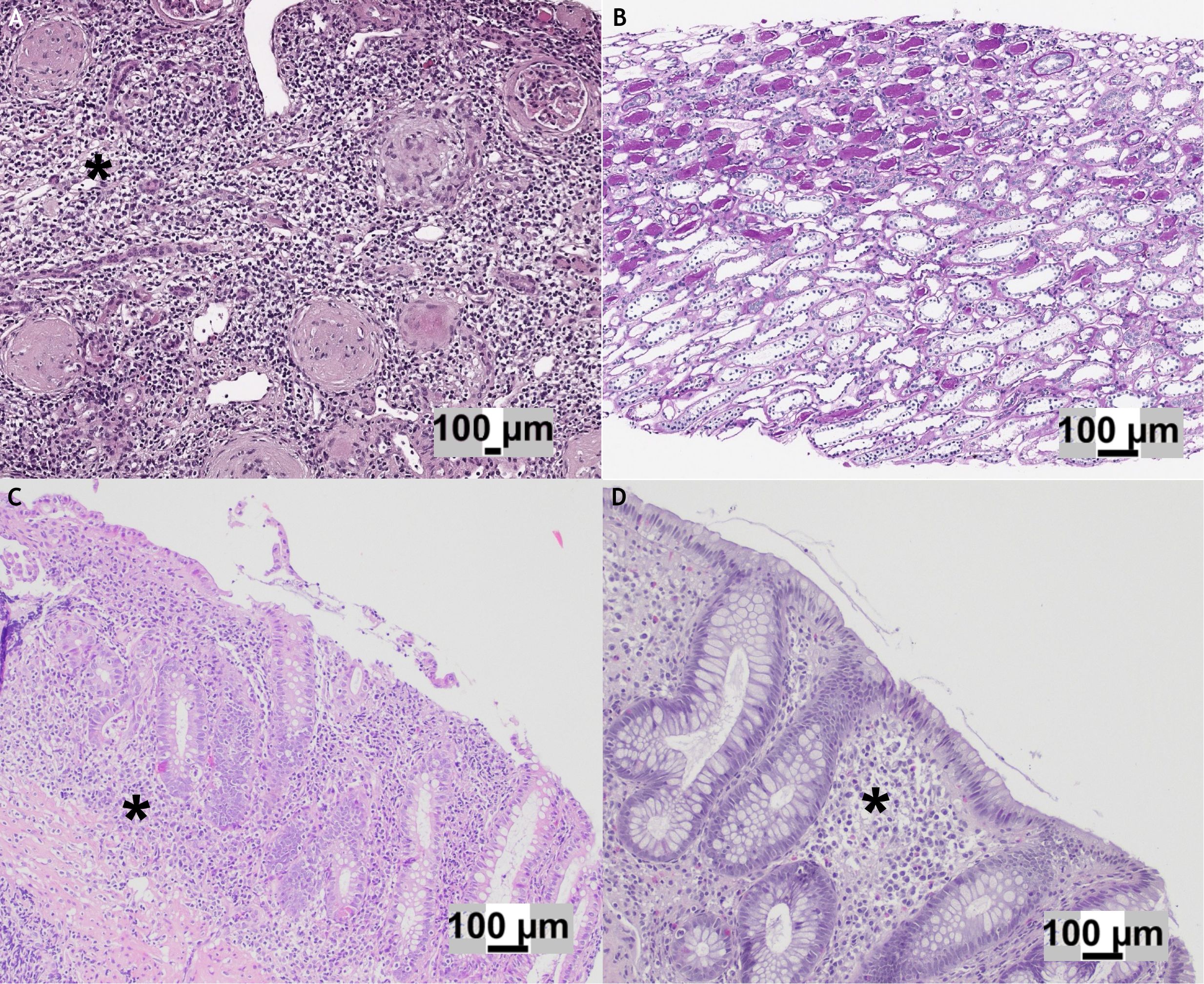
Figure 2. (A) Hematoxylin and eosin (H&E) stain of the initial kidney biopsy showing global glomerulosclerosis associated with an interstitial inflammatory infiltrate (*) predominantly composed of lymphocytes. (B) H&E stain of the follow-up kidney biopsy demonstrating mild tubular atrophy in the medullary compartment, with no evidence of active inflammation. (C) Gastrointestinal biopsy displaying an ulcerative colitis-like pattern, characterized by continuous, marked inflammatory infiltrate (*) with cryptitis and ulcerations. (D) Colon biopsy obtained at the time of kidney injury: in comparison with panel C, no active inflammation, cryptitis, or ulcerations were observed, although crypt architectural distortion was present..
More than half of the glomeruli (10/16) were characterized by a fibrous reaction affecting Bowman’s capsule and capillary collapse, and three of them were globally sclerotic. Immunofluorescence was negative (IgA, IgG, IgM, C3, C4, and C1q). The final diagnosis was consistent with acute TIN.
Based on histological findings, anti-inflammatory treatment with prednisone (1 mg/kg) was administered for 1 month and a withdrawal of 2 weeks, with a prompt decrease of serum creatinine and normalization of leukocyturia (Figure 1). After 2 months, due to the lack of an effective therapeutic alternative for IBD, vedolizumab therapy was administered combined with glucocorticoids (prednisone 0.5 mg/kg/die from the day before to the day after the infusion). At 1 year of follow-up, the patient received a total of nine vedolizumab infusions combined with the same steroid scheme. Blood and urine tests showed no further worsening of renal function. Following the reintroduction of low-dose mesalamine (35 mg/kg), mild leukocyturia reappeared on two occasions (Figure 1). A new kidney biopsy performed at 6 months of follow-up after the previous one demonstrated a regression of the interstitial inflammatory infiltrate and the absence of obsolescent glomeruli (Figure 2B).
Discussion
To the best of our knowledge, we report here the first pediatric case of AKI likely related to vedolizumab-induced TIN in a child with VEO-UC. Following the failure of multiple previous therapies, including two anti-TNF agents, vedolizumab was started with a good clinical response and mucosal healing. After 2 years, she presented with AKI due to drug-related TIN, clinically and histologically responsive to glucocorticoid treatment. The previously applied “long premedication” scheme with the steroid (11) allowed vedolizumab infusions to be restarted without relapses.
VEO-IBD treatment remains highly challenging for the pediatric gastroenterologist, and it needs to be tailored, given the clinical and genetic heterogeneity of this condition. This leads to the requirement for multidisciplinary management and prompt genetic counselling. Although vedolizumab has carved out a role in the treatment of VEO-UC in recent years, data on possible adverse effects in this challenging cohort are certainly needed.
According to the latest evidence (12–14) in pediatric subjects, adverse events mostly correspond to infusion-related reactions (fever, arthralgia/myalgia, headache, and nausea/vomiting) and viral upper airway infections, ranging from 4% to 9%. Severe adverse events leading to drug discontinuation are rare: anaphylaxis, septic arthritis, deep vein thrombosis, and leukocytoclastic vasculitis (14).
Kidney involvement due to vedolizumab is rare, and to the best of our knowledge, AKI related to vedolizumab infusion was not previously described in childhood. A recent systematic review by Forss et al. reported nine adult cases of TIN in patients with IBD receiving vedolizumab (Table 1) (15). Clinical presentation was heterogeneous: fever and systemic symptoms occurred in 4/9 cases, while 3/9 were asymptomatic (15). Concomitant aminosalicylates were used in 3/9 (15). Corticosteroids were administered in 8/9, with complete recovery within 4 weeks in 2/9 and persistent renal impairment in 5/9 (Table 1). Taken together with our case, these findings underscore the need for baseline and periodic renal function testing (serum creatinine and urinalysis) before and throughout vedolizumab therapy. Kidney biopsies typically showed T lymphocyte-predominant TIN with variable eosinophils and early tubular injury/fibrosis. The tubulointerstitial cellular infiltrate was reported in all cases and was mainly characterized by CD4+ T cells (11, 15–22). As with other drug-induced TIN, the monoclonal antibody may act as a hapten or planted antigen in the tubulointerstitium, triggering a T cell-mediated inflammatory cascade that injures renal tubules (23). Notably, the α4β7 integrin target of vedolizumab has no well-defined role in kidney tissue, suggesting the nephritis arises from off-target immune dysregulation rather than direct integrin blockade (24, 25). Blocking gut-homing α4β7 may alter lymphocyte trafficking or immune homeostasis, potentially triggering misdirected T-cell responses against renal tissue.

Table 1. Main clinical reports describing vedolizumab-induced TIN in adults (adapted from Forss et al. (15)).
Acute interstitial nephritis is an important cause of kidney injury, causing 3%–7% of AKI in children (26). Urinalysis monitoring allows the early detection of chronic sterile leukocyturia, a known sign of drug-induced TIN (27). Tubulointerstitial involvement in IBD is frequently related to chronic exposure to aminosalicylate or, rarely, may be a possible extraintestinal manifestation of active bowel disease (28, 29). Considering the absence of recent exacerbation of the colitis (Figures 2C, D) and the lack of granuloma evidence on renal biopsy (Figures 2A, B), we reasonably exclude that the TIN manifested itself as an extraintestinal manifestation of IBD.
As a limitation of our case, we report the chronic exposure of the patient to mesalamine, which was discontinued at the AKI. The reintroduction of mesalamine therapy was correlated with a slight rise in leukocyturia, with subsequent discontinuation, even considering the clinical remission. Nevertheless, the extended duration of 5-ASA treatment without any adverse effects and the significant temporal association between the start of vedolizumab and the onset of leukocyturia (Figure 1) led us to lean toward attributing the tubulointerstitial damage to the biological therapy rather than the exposure to aminosalicylates (29), or at least to a cumulative effect of the two drugs.
In conclusion, we report the first case of VEO-IBD with a probable vedolizumab-related acute TIN. Despite severe kidney damage, steroid therapy was effective, with functional recovery, normalization of the urinalysis, and resumption of vedolizumab infusions without relapses. Persistence of sterile leukocyturia may represent an early sign of drug-related TIN.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ED: Conceptualization, Data curation, Formal analysis, Writing – original draft. RC: Data curation, Writing – review & editing. BC: Investigation, Methodology, Resources, Writing – review & editing. GG: Writing – review & editing. LP: Writing – review & editing. EL: Supervision, Writing – review & editing. SS: Writing – review & editing. PG: Writing – review & editing. VV: Writing – review & editing. AA: Supervision, Writing – review & editing. SA: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. This work was supported by the Italian Minitry of Health 5x1000 project 2019 (5M-2019-23680421).
Acknowledgments
The authors would like to thank the patients and family members for supporting their research projects. The authors are grateful to all those Italian citizens who allocated the 5x1000 share of their income tax payment to health research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1684476/full#supplementary-material
References
1. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, and Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflammation Bowel Dis. (2011) 17:423–39. doi: 10.1002/ibd.21349
2. Charbit-Henrion F, Parlato M, Hanein S, Duclaux-Loras R, Nowak J, Begue B, et al. Diagnostic Yield of Next-generation Sequencing in Very Early-onset Inflammatory Bowel Diseases: A Multicentre Study [published correction appears in J Crohns Colitis. 2021 Mar 5;15(3):517-518. J Crohns Colitis. (2018) 12:1104–12. doi: 10.1093/ecco-jcc/jjy068
3. Collen LV, Kim DY, Field M, Okoroafor I, Saccocia G, Whitcomb SD, et al. Clinical phenotypes and outcomes in monogenic versus non-monogenic very early onset inflammatory bowel disease. J Crohns Colitis. (2022) 16:1380–96. doi: 10.1093/ecco-jcc/jjac045
4. Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, et al. Very early onset inflammatory bowel disease: A clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflammation Bowel Dis. (2020) 26:820–42. doi: 10.1093/ibd/izz259
5. Kelsen JR, Conrad MA, Dawany N, Patel T, Shraim R, Merz A, et al. The unique disease course of children with very early onset-inflammatory bowel disease. Inflammation Bowel Dis. (2020) 26:909–18. doi: 10.1093/ibd/izz214
6. Kerur B, Fiedler K, Stahl M, Hyams J, Stephens M, Lu Y, et al. Utilization of antitumor necrosis factor biologics in very early onset inflammatory bowel disease: A multicenter retrospective cohort study from North America. J Pediatr Gastroenterol Nutr. (2022) 75:64–9. doi: 10.1097/MPG.0000000000003464
7. Tyler CJ, Guzman M, Lundborg LR, Yeasmin S, Zgajnar N, Jedlicka P, et al. Antibody secreting cells are critically dependent on integrin α4β7/MAdCAM-1 for intestinal recruitment and control of the microbiota during chronic colitis. Mucosal Immunol. (2022) 15:109–19. doi: 10.1038/s41385-021-00445-z
8. Rogler G. Mechanism of action of vedolizumab: do we really understand it? Gut. (2019) 68:4–5. doi: 10.1136/gutjnl-2018-316777
9. Shah P and McDonald D. Vedolizumab: an emerging treatment option for pediatric inflammatory bowel disease. J Pediatr Pharmacol Ther. (2021) 26:795–801. doi: 10.5863/1551-6776-26.8.795
10. Garcia-Romero R, Martinez de Zabarte Fernandez JM, Pujol-Muncunill G, Donat-Aliaga E, Segarra-Cantón O, Irastorza-Terradillos I, et al. Safety and effectiveness of vedolizumab in paediatric patients with inflammatory bowel disease: an observational multicentre Spanish study [published correction appears in Eur J Pediatr. 2021 May 23. Eur J Pediatr. (2021) 180:3029–38. doi: 10.1007/s00431-021-04063-6
11. Bailly E, Von Tokarski F, Beau-Salinas F, Picon L, Miquelestorena-Standley E, Rousseau G, et al. Interstitial nephritis secondary to vedolizumab treatment in crohn disease and safe rechallenge using steroids: A case report. Am J Kidney Dis. (2018) 71:142–5. doi: 10.1053/j.ajkd.2017.08.008
12. Atia O, Shavit-Brunschwig Z, Mould DR, Stein R, Matar M, Aloi M, et al. Outcomes, dosing, and predictors of vedolizumab treatment in children with inflammatory bowel disease (VEDOKIDS): a prospective, multicentre cohort study. Lancet Gastroenterol Hepatol. (2023) 8:31–42. doi: 10.1016/S2468-1253(22)00307-7
13. Fabiszewska S, Derda E, Szymanska E, Osiecki M, and Kierkus J. Safety and effectiveness of vedolizumab for the treatment of pediatric patients with very early onset inflammatory bowel diseases. J Clin Med. (2021) 10:2997. doi: 10.3390/jcm10132997
14. Fang S, Song Y, Zhang C, and Wang L. Efficacy and safety of vedolizumab for pediatrics with inflammatory bowel disease: a systematic review. BMC Pediatr. (2022) 22:175. doi: 10.1186/s12887-022-03229-x
15. Forss A, Flis P, Sotoodeh A, Kapraali M, and Rosenborg S. Acute interstitial nephritis in patients with inflammatory bowel disease treated with vedolizumab: a systematic review. Scand J Gastroenterol. (2024) 59:821–9. doi: 10.1080/00365521.2024.2345383
16. Subhaharan D, Ramaswamy PK, Francisco S, and Ishaq N. Vedolizumab-induced acute interstitial nephritis in ulcerative colitis. ACG Case Rep J. (2022) 9:e00788. doi: 10.14309/crj.0000000000000788
17. Zhang PL, Pancioli T, Li W, and Kanaan HD. Electron microscopic findings can support multiple etiologies of nephrotoxicity in renal tubules. Ultrastruct Pathol. (2020) 44:481–8. doi: 10.1080/01913123.2020.1839152
18. Simpson N, Seenan JP, Patel R, and Kipgen D. Acute interstitial nephritis secondary to vedolizumab. BMJ Case Rep. (2021) 14:e243568. doi: 10.1136/bcr-2021-243568
19. Jahan S, Xu A, Danner R, Sellars I, and Coates TP. Acute interstitial nephritis with inflammatory bowel disease. Nephrology. (2022) 27:69–70.
20. Kepley A, Marino D, and DeCross A. P053 Interstitial nephritis from IBD: complicated conclusions. Am J Gastroenterol. (2021) 116:S14. doi: 10.14309/01.ajg.0000798812.13521.04
21. Muzib A, Parikh R, Bijol V, Uppal NN, and Sachdeva M Vedolizumab-induced acute interstitial nephritis and acute tubular necrosis: PO2260. J Am Soc Nephrol. (2020) 31:688. doi: 10.1681/ASN.20203110S1688c
22. O’Leary C, Wong D, and Wilson G. Vedolizumab-induced acute interstitial nephritis with failure of steroid prophylaxis on vedolizumab rechallenge. BMJ Case Rep. (2023) 16:e254715. doi: 10.1136/bcr-2023-254715
23. Krishnan N and Perazella MA. Drug-induced acute interstitial nephritis: pathology, pathogenesis, and treatment. Iran J Kidney Dis. (2015) 9:3–13.
24. McLean LP and Cross RK. Pharmacodynamic assessment of vedolizumab for the treatment of ulcerative colitis. Expert Opin Drug Metab Toxicol. (2016) 12:833–42. doi: 10.1080/17425255.2016.1181171
25. Pozzi A and Zent R. Integrins in kidney disease. J Am Soc Nephrol. (2013) 24:1034–9. doi: 10.1681/ASN.2013010012
26. Greising J, Trachtman H, Gauthier B, and Valderrama E. Acute interstitial nephritis in adolescents and young adults. Child Nephrol Urol. (1990) 10:189–95.
27. Perazella MA and Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. (2010) 6:461–70. doi: 10.1038/nrneph.2010.71
28. Angeletti A, Arrigo S, Madeo A, Molteni M, Vietti E, Arcuri L, et al. Different renal manifestations associated with very early onset pediatric inflammatory bowel disease: case report and review of literature. BMC Nephrol. (2021) 22:146. doi: 10.1186/s12882-021-02358-2
Keywords: VEO-IBD, vedolizumab, ulcerative colitis, tubulointerstitial nephritis (TIN), drug-induced acute interstitial nephritis
Citation: Drago E, Carfora R, Cafferata B, Gaggero G, Puzone L, La Porta E, Signa S, Gandullia P, Vellone VG, Angeletti A and Arrigo S (2025) Case Report: Severe kidney involvement in a case of very early onset inflammatory bowel disease. Front. Immunol. 16:1684476. doi: 10.3389/fimmu.2025.1684476
Received: 12 August 2025; Accepted: 31 October 2025;
Published: 19 November 2025.
Edited by:
Jan De Laffolie, University of Giessen, GermanyReviewed by:
Anders Forss, Karolinska Institutet, SwedenTejas S. Desai, London Health Sciences Centre, Canada
Copyright © 2025 Drago, Carfora, Cafferata, Gaggero, Puzone, La Porta, Signa, Gandullia, Vellone, Angeletti and Arrigo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enrico Drago, ZW5yaWNvLmRyYWdvQGNoaWxkcmVucy5oYXJ2YXJkLmVkdQ==, ZW5yaXB1LmRyYWdvQGdtYWlsLmNvbQ==
 Enrico Drago
Enrico Drago Roberta Carfora
Roberta Carfora Barbara Cafferata
Barbara Cafferata Gabriele Gaggero3
Gabriele Gaggero3 Edoardo La Porta
Edoardo La Porta Sara Signa
Sara Signa Valerio Gaetano Vellone
Valerio Gaetano Vellone Andrea Angeletti
Andrea Angeletti Serena Arrigo
Serena Arrigo