- Department of Hematology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
Although pediatric Burkitt lymphoma is considered curable, some patients still have a poor prognosis. Lack of early or complete response is an adverse prognostic factor. Therefore, improving patients’ sensitivity to initial treatment and achieving complete remission early are crucial for enhancing efficacy. Herein, we report two cases of children with newly diagnosed clinical stage IV Burkitt lymphoma who achieved early complete metabolic remission after only two cycles of treatment with polatuzumab vedotin plus rituximab combined with chemotherapy. Notably, this represents the first reported application of polatuzumab vedotin in pediatric patients with newly diagnosed Burkitt lymphoma.
Introduction
Burkitt lymphoma (BL) is the most common pediatric B-cell non-Hodgkin lymphoma (B-NHL), characterized by high aggressiveness and a tendency to invade the central nervous system (CNS), with early bone marrow metastasis (1–3). Over the past 20 years, the application of high-dose, short-course chemotherapy regimens has significantly improved the survival rate of children with BL, but some patients still have a poor prognosis. Risk factors leading to unfavorable prognosis include: higher staging, elevated lactate dehydrogenase (LDH) levels, leukemia bone marrow and CNS involvement, as well as treatment-related factors such as lack of early or complete response (4–6). Once disease progression or recurrence occurs, the survival rate is less than 30% (7). Therefore, optimizing the treatment regimen to achieve complete remission as early as possible is essential to enhance efficacy. Herein, we report two cases of children with clinical stage IV Burkitt lymphoma who achieved complete metabolic remission (CMR) after only two cycles of immunochemotherapy incorporating polatuzumab vedotin plus rituximab. This marks the first documented use of polatuzumab vedotin in the treatment of children with newly diagnosed Stage IV Burkitt lymphoma.
Case report
Case 1
A 13-year-old boy was presented to our hospital on July 1, 2024, with a fifteen-day history of toothache and pain in the lower back and buttocks. The patient had no significant medical history. No personal or family history of malignancies was documented. Physical examination showed positive tenderness in the lumbosacral vertebrae. Magnetic resonance imaging (MRI) of the vertebral body showed abnormal signals in the thoracolumbar and sacral vertebrae and corresponding accessory areas, accompanied by spinal cord occupancy at the T3-T5 and T11-L1 levels. Laboratory data showed that LDH was elevated (2061 U/L). Epstein-Barr virus-DNA test was negative. Positron emission tomography-computed tomography (PET-CT) showed high uptake of 18F-fluoro-2-deoxy-D-glucose (18-FDG) in multiple areas of the body distributed in the abdominal and pelvic cavity, retroperitoneal space, thorax, bilateral neck regions I and II, multiple bones, thoracolumbar spinal cord, penis, and spleen. Then, a biopsy of the tumor in the right lower abdomen’s appendix area was performed. Histopathological examination confirmed Burkitt lymphoma. The results of the immunohistochemical method (IHC) were as follows: CK (-), CD20 (+), CD79a (+), PAX5 (+), CD21 (-), CD23 (-), Bcl2 (-), Bcl6 (70%+), CD10 (+), MUM-1 (-), Ki67 (90%+), CD30 (-), CD19 (+), p53 (-), ALK (-), TDT (-), CD99 (-), c-Myc (90%+); CD3 (+), CD5 (+) in background T cells. In situ hybridization: EBER negative. FISH: C-MYC/IGH (+), C-MYC break-apart (+). Bone marrow (BM) examination revealed 57.80% infiltration of abnormal mature B lymphocytes. Cerebrospinal fluid (CSF) examination, including cytospin morphology and flow cytometric immunophenotyping, revealed no abnormal lymphocytes. Diagnosed with BL, leukemic phase (stage IV, R4), the patient developed rapid disease progression during diagnostic workup. This was accompanied by new-onset sensory loss below T11 vertebra, first documented on July 3, 2024.
The treatment was initiated after obtaining approval from the Institutional Ethics Committee and written informed consent from the guardian. Following cytoreductive pretreatment with regimen V consisted of low-dose cyclophosphamide and prednisone, a modified BFM95 immunochemotherapy regimen was initiated. This 6-cycle protocol comprised: (1) Polatuzumab vedotin plus Rituximab (Pola-R). (2) Sequentially alternating treatment blocks (AA-BB-CC-AA-BB-CC) (Table 1). All cycles administered at 21-day intervals and required treatment delay not exceeding 7 days for hematologic toxicity, maintaining relative dose intensity (RDI) ≥85%. During cycle 1 immunochemotherapy, the patient developed grade 4 myelosuppression complicated by febrile neutropenia (FN) and grade 2 mucositis, both resolving with supportive measures. After two cycles of immunochemotherapy, PET-CT confirmed CMR, with BM evaluation showing morphological remission. The patient completed six cycles of immunochemotherapy on November 10, 2024. Following cessation of anticancer therapy, neurological rehabilitation was initiated at the Department of Neurorehabilitation. Showing remarkable symptomatic improvement, the patient had significant recovery of sensory function below the T11 level and complete resolution of his initial back pain and toothache. Bimonthly follow-up visits showed no evidence of disease recurrence (Figure 1).
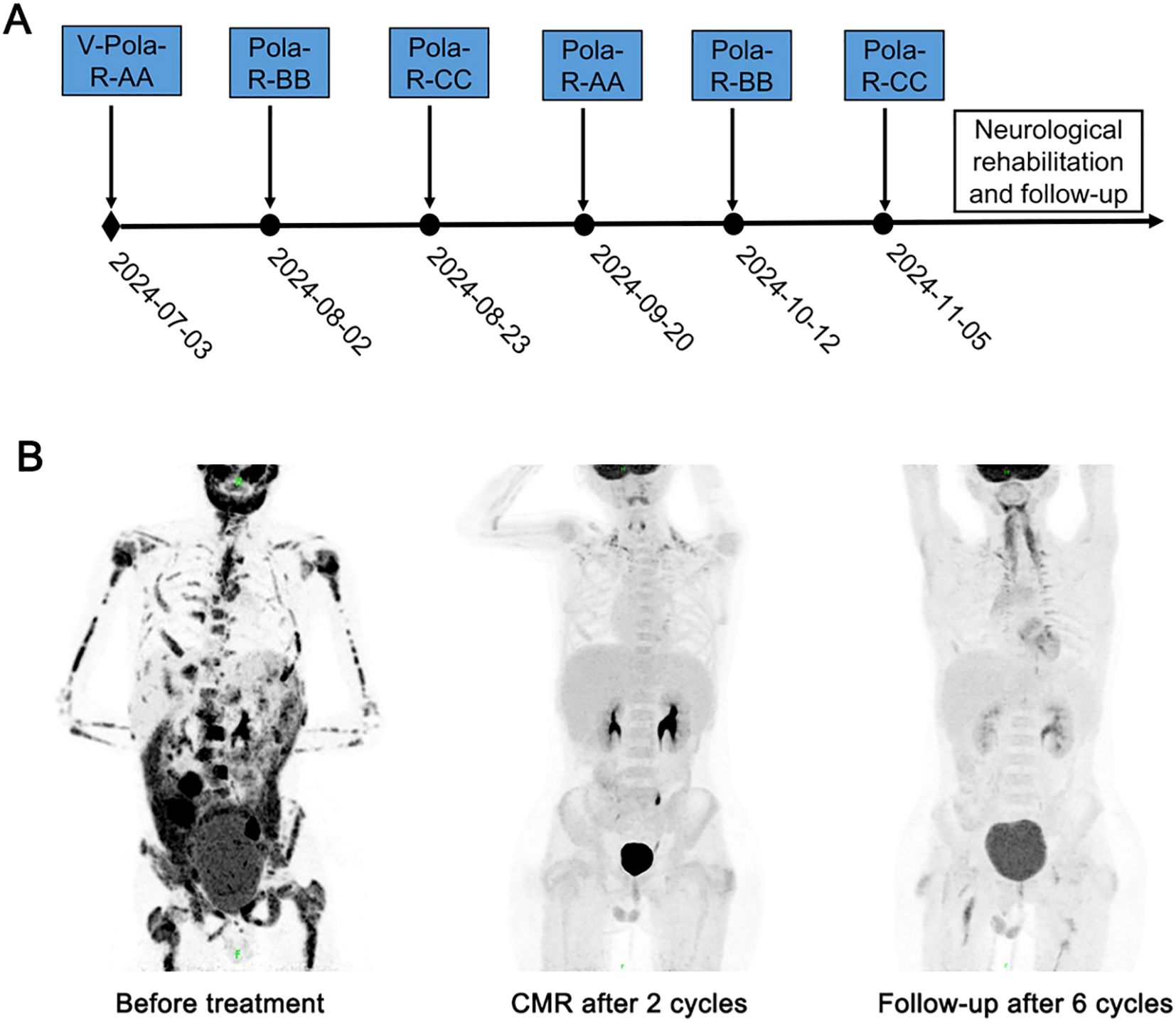
Figure 1. Treatment timeline and dynamic evaluation using PET-CT of case 1. (A) The timeline of treatment process in this case. (B) A dynamic evaluation of tumors with PET-CT.
Case 2
A 6-year-old boy was presented to our hospital on March 14, 2025, with a 20-day history of a right neck mass. The patient’s past medical history was unremarkable. There was no personal or family history of malignancy. Physical examination revealed a palpable 6.0 × 3.0 cm mass in the right cervical region. Cervical ultrasonography revealed multiple abnormal enlarged lymph nodes in bilateral cervical regions. Laboratory data showed that LDH was elevated (2193 U/L). Epstein-Barr virus-DNA test was negative. Then, the right neck lymph node biopsy was performed. Histopathological examination confirmed Burkitt lymphoma. The results of the IHC were as follows: CK (-), CD20 (+), CD79a (+), CD3 (T+), CD5 (T+), Bcl2 (-), Bcl6 (70%+), CD10 (80%+), MUM-1 (60%+), Ki67 (90%+), CD30 (-), ALK (<1%), TDT (-), c-Myc (90%+), CyclinD1 (-). In situ hybridization: EBER negative. FISH: C-MYC/IGH (+), C-MYC break-apart (+). PET-CT showed high uptake of 18-FDG in multiple areas of the body distributed in bilateral cervical level II regions, right cervical level III-IV regions, left supra-adrenal soft tissue, peripancreatic area, nasopharynx, multiple bones and spleen. BM examination revealed 15.2% infiltration of abnormal mature B lymphocytes. CSF examination was negative. The patient was diagnosed with BL (stage IV, R4).
After obtaining approval from the Institutional Ethics Committee and written consents from the guardian, treatment was initiated. Similar to the treatment of patient 1, this patient received immunochemotherapy based on BFM95 following cytoreductive pretreatment (Table 1). After two cycles of immunochemotherapy, PET-CT confirmed CMR, with bone marrow evaluation showing morphological remission. Clinically, the patient experienced rapid and complete resolution of the neck mass following the initial cycle of therapy. After the first cycle of immunochemotherapy, the patient developed grade 4 BM suppression with FN, and broad-spectrum antibiotics were administered for empirical treatment. The patient completed six cycles of immunochemotherapy on July 29, 2025 and was scheduled for follow-up similar to patient 1 (Figure 2).
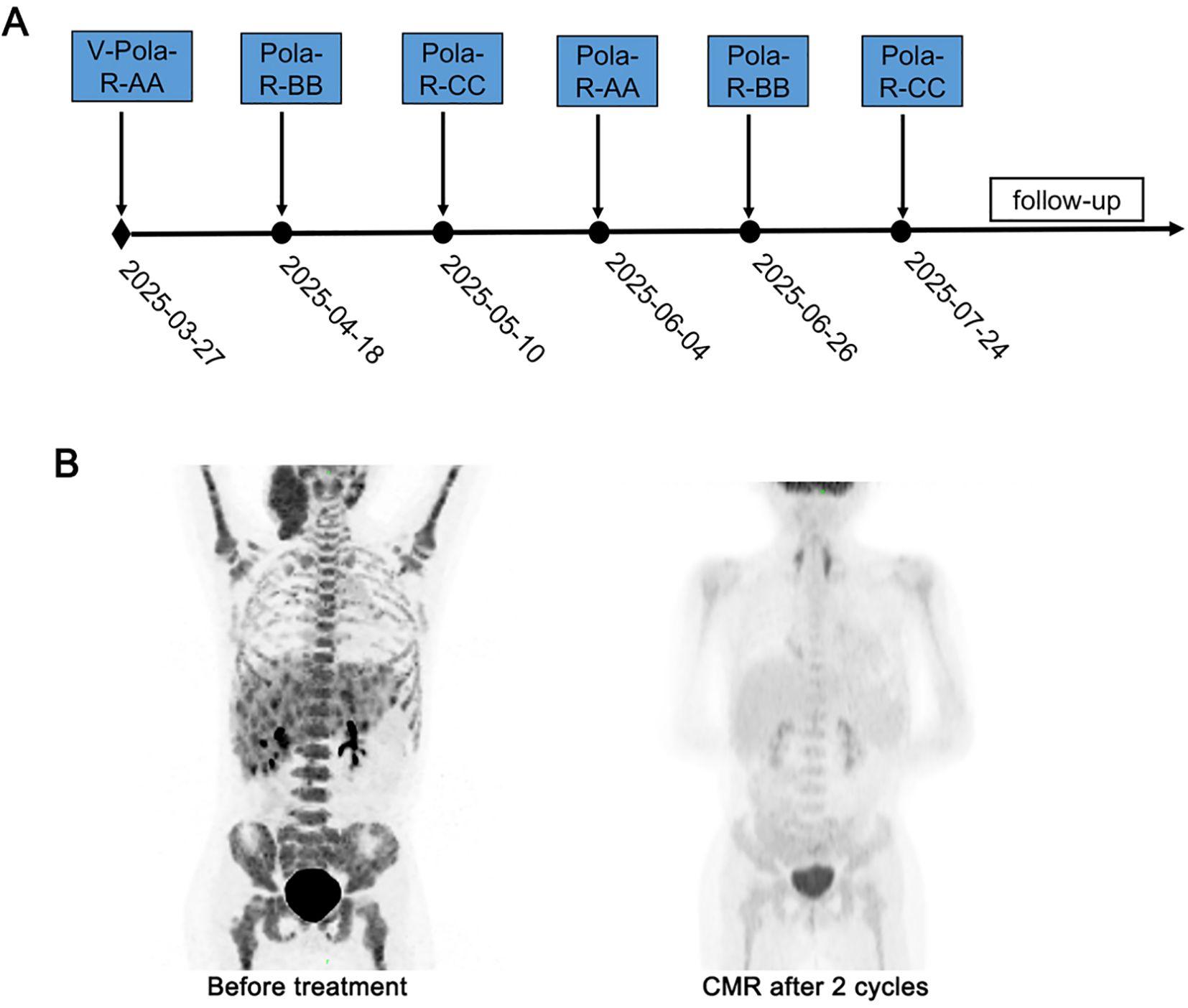
Figure 2. Treatment timeline and dynamic evaluation using PET-CT of case 2. (A) The timeline of treatment process in this case. (B) A dynamic evaluation of tumors with PET-CT.
Discussion
In our two cases of children with clinical stage IV, high-risk Burkitt lymphoma (R4), both achieved CMR rapidly (after only two cycles of treatment) with chemotherapy combined with polatuzumab vedotin plus rituximab. The main complications were myelosuppression and mucositis, manageable with supportive care without significant treatment delays or dose reductions.
Administering risk-adapted, short-course combination chemotherapy is fundamental for BL treatment. Protocols like LMB 89/96 (French-American-British group) and BFM90/95 (Berlin-Frankfurt-Münster group) are widely recognized as standard regimens (1, 3, 8). While adding rituximab to chemotherapy has improved outcomes for pediatric high-grade B-NHL, a significant subset of high-risk BL patients fail to achieve an early complete response (CR) (9–11). Accumulating data suggest that failure to achieve early CR is associated with poorer survival outcomes and represents an adverse prognostic factor in pediatric Burkitt lymphoma (12, 13). Novel strategies to improve early CR rates are therefore crucial for this population.
Polatuzumab vedotin, an antibody-drug conjugate targeting CD79b, delivers cytotoxic drugs directly to tumor cells (14). It has shown efficacy in adult diffuse large B-cell lymphoma (DLBCL) (15, 16). Given that most BL cells highly express CD79b, polatuzumab vedotin is a rational therapeutic target. Encouragingly, polatuzumab vedotin-based regimens have achieved 100% CMR in small series of adult patients with refractory BL (17, 18). The similar CD79b expression pattern in pediatric BL suggests potential benefit.
In these two pediatric patients, we employed a regimen combining polatuzumab vedotin, rituximab, and a modified BFM95 backbone (excluding vincristine to mitigate potential neurotoxicity). Notably, both patients achieved a CMR very early in the course of therapy (after only two treatment cycles). The primary toxicities were grade 4 myelosuppression with febrile neutropenia and mucositis, which resolved with supportive care.
This report describes the first application of polatuzumab vedotin plus rituximab combined with chemotherapy in pediatric patients with newly diagnosed, stage IV, high-risk Burkitt lymphoma. Given the established poor prognosis associated with lack of early CR in high-risk pediatric BL, the rapid achievement of CMR in both cases is particularly encouraging. These promising results strongly support the initiation of prospective clinical trials investigating polatuzumab vedotin in children and adolescents with Burkitt lymphoma.
However, the study has several limitations. The findings, derived from only two patients, severely limit definitive conclusions regarding the regimen’s efficacy and generalizability. Additionally, the short follow-up duration hinders assessment of long-term outcomes—including relapse-free survival, overall survival, and potential late toxicities—associated with polatuzumab vedotin in pediatric patients. Therefore, robust evaluation of it’s efficacy, long-term safety, and survival benefit when added to standard immunochemotherapy for high-risk pediatric Burkitt lymphoma requires prospective, multi-center trials with larger cohorts and extended follow-up.
Conclusion
Polatuzumab vedotin-rituximab-chemotherapy induced rapid complete metabolic response in two children with newly diagnosed, high-risk stage IV Burkitt lymphoma, supporting further clinical evaluation for improving early complete response rates in this population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University, Henan, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Data curation, Formal Analysis, Investigation, Writing – original draft. MD: Investigation, Methodology, Writing – original draft. CZ: Formal Analysis, Writing – original draft, Resources. YC: Data curation, Formal Analysis, Writing – original draft. YZ: Data curation, Formal Analysis, Investigation, Writing – original draft. WZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by Science and Technology Project of Henan Province (242102310149).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1684856/full#supplementary-material
References
1. Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, et al. The societe francaise d’oncologie pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with b-cell lymphomas and L3 leukemia. Blood. (2001) 97:3370–9. doi: 10.1182/blood.v97.11.3370
2. Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with b-cell neoplasms: a report of the BFM group study NHL-BFM95. Blood. (2005) 105:948–58. doi: 10.1182/blood-2004-03-0973
3. Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk b-cell non-hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. (2007) 109:2773–80. doi: 10.1182/blood-2006-07-036673
4. Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature b-cell non-hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. (2012) 30:387–93. doi: 10.1200/JCO.2010.33.3369
5. Miles RR, Arnold S, and Cairo MS. Risk factors and treatment of childhood and adolescent burkitt lymphoma/leukaemia. Br J Haematol. (2012) 156:730–43. doi: 10.1111/j.1365-2141.2011.09024.x
6. Guo J, Zhu Y, Gao J, Li Q, Jia C, Zhou C, et al. Clinical and prognostic analysis of 43 children with mature b-cell non-hodgkin’s lymphoma/acute lymphoblastic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2016) 24:72–9. doi: 10.7534/j.issn.1009-2137.2016.01.014
7. Cairo M, Auperin A, Perkins SL, Pinkerton R, Harrison L, Goldman S, et al. Overall survival of children and adolescents with mature b cell non-hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: a report from the FAB/LMB 96 study group. Br J Haematol. (2018) 182:859–69. doi: 10.1111/bjh.15491
8. Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, et al. Results of a randomized international study of high-risk central nervous system b non-hodgkin lymphoma and b acute lymphoblastic leukemia in children and adolescents. Blood. (2007) 109:2736–43. doi: 10.1182/blood-2006-07-036665
9. Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, et al. Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV b-cell non-hodgkin lymphoma: a children’s oncology group report. Leukemia. (2013) 27:1174–7. doi: 10.1038/leu.2012.255
10. Goldman S, Smith L, Galardy P, Perkins SL, Frazer JK, Sanger W, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive burkitt lymphoma/leukaemia: a children’s oncology group report. Br J Haematol. (2014) 167:394–401. doi: 10.1111/bjh.13040
11. Minard-Colin V, Auperin A, Pillon M, Burke GAA, Barkauskas DA, Wheatley K, et al. Rituximab for high-risk, mature b-cell non-hodgkin’s lymphoma in children. N Engl J Med. (2020) 382:2207–19. doi: 10.1056/NEJMoa1915315
12. Zhang M, Jin L, Yang J, Duan YL, Huang S, Zhou CJ, et al. Clinical and prognostic analysis of 186 children with burkitt’s lymphoma. Zhonghua Er Ke Za Zhi. (2018) 56:605–10. doi: 10.3760/cma.j.issn.0578-1310.2018.08.010
13. Zhang M, Wu P, Duan YL, Jin L, Yang J, Huang S, et al. Mid-term efficacy of China net childhood lymphoma-mature b-cell lymphoma 2017 regimen in the treatment of pediatric burkitt lymphoma. Zhonghua Er Ke Za Zhi. (2022) 60:1011–8. doi: 10.3760/cma.j.cn112140-20220429-00390
14. Li D, Lee D, Dere RC, Zheng B, Yu S, Fuh FK, et al. Evaluation and use of an anti-cynomolgus monkey CD79b surrogate antibody-drug conjugate to enable clinical development of polatuzumab vedotin. Br J Pharmacol. (2019) 176:3805–18. doi: 10.1111/bph.14784
15. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large b-cell lymphoma. J Clin Oncol. (2020) 38:155–65. doi: 10.1200/JCO.19.00172
16. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trneny M, Sharman JP, et al. Polatuzumab vedotin in previously untreated diffuse large b-cell lymphoma. N Engl J Med. (2022) 386:351–63. doi: 10.1056/NEJMoa2115304
17. Alanzi M, Abu-Tineh M, Szabados L, Sharaf Eldean MZ, Alatasi S, Taha RY, et al. Polatuzumab vedotin in a patient with refractory burkitt lymphoma, a case report. Onco Targets Ther. (2023) 16:133–9. doi: 10.2147/OTT.S394193
Keywords: Burkitt lymphoma, pediatric, polatuzumab vedotin, early complete remission, case report
Citation: Sun Y, Deng M, Zhang C, Chen Y, Zhang Y and Zuo W (2025) Polatuzumab vedotin plus rituximab with chemotherapy in newly diagnosed pediatric stage IV Burkitt lymphoma: case report highlighting early complete remission. Front. Immunol. 16:1684856. doi: 10.3389/fimmu.2025.1684856
Received: 13 August 2025; Accepted: 27 October 2025;
Published: 10 November 2025.
Edited by:
Claudio Cerchione, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Liqiong Liu, Affiliated Nanshan Hospital of Shenzhen University, ChinaJason Butler, Royal Brisbane and Women’s Hospital, Australia
Hutton Chapman, Duke University, United States
Copyright © 2025 Sun, Deng, Zhang, Chen, Zhang and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenli Zuo, d2VubGl6dW9xcUBhbGl5dW4uY29t
 Yanyan Sun
Yanyan Sun Mei Deng
Mei Deng Yanli Chen
Yanli Chen Yanli Zhang
Yanli Zhang