Abstract
Introduction:
Coronary involvement in immunoglobulin G4–related disease (IgG4-RD) has remained underexplored despite its risk posed in terms of major adverse cardiovascular events (MACEs). The study provides a comprehensive review, particularly focusing on multimodal imaging characteristics and clinical applicability.
Methods:
A systematic review was conducted on IgG4-related coronary involvement, supplemented by serial cases from our center included. We analyzed clinical features and multimodal imaging, focusing on the presence or absence of cardiovascular symptoms.
Results:
A total of 134 IgG4-RD patients with coronary involvement were included and analyzed, including 118 from the literature and 16 from our center. Seven (5%) patients died from secondary myocardial ischemia/infarction. Coronary anomalies commonly affected the left anterior descending artery (LAD) (79%) and presented as diffuse wall thickening or periarterial soft tissue encasement (85%). Stenosis was frequent (47%) and often secondary. Symptoms, primarily induced by myocardial ischemia or infarction (84%), were largely due to stenosis (68%). Chest computed tomography (CT) and coronary computed tomography angiography (CTA) were the primary imaging modalities (81%), particularly in symptomatic cases (88%). Positron emission tomography-computed tomography (PET-CT) was applied in 55 patients (41%) and often in asymptomatic cases (51%). CMR, though less adopted (23%), demonstrated potential in detecting coronary lesions (77%). Glucocorticoid therapy is the most common (76%), with the best response of periarterial encasement (66%). Surgery was less common (32%), primarily being applied to aneurysms (63%).
Conclusion:
Coronary involvement in IgG4-RD presents four phenotypes, sometimes with an insidious onset and as the sole affected site, poses a potential risk for MACEs. Multimodal imaging is essential for early diagnosis and effective monitoring, with coronary CMR showing promise for early detection without the risk of radiation-induced inflammation and fibrosis.
1 Introduction
Immunoglobulin G4–related disease (IgG4-RD) is a unique immune-mediated chronic fibro-inflammatory disorder characterized by elevated serum IgG4 levels and lymphoplasmacytic infiltration, predominantly consisting of IgG4-positive plasma cells, within the affected tissues (1). IgG4-RD shows a broad spectrum of organ involvement, with aortitis/peri-aortitis being the most commonly reported in the cardiovascular system (2, 3). However, vascular inflammation is common in IgG4-RD, including in the coronary arteries, which, as medium-sized vessels, have received comparatively limited attention. Notably, the coronary arteries are the second most frequently affected extra-aortic vessels in patients with periarteritis (4). Coronary involvement in IgG4-RD can result in life-threatening complications, including aneurysmal rupture and ischemic heart disease.
Identifying typical sites of involvement is essential for diagnosing IgG4-RD (5). Imaging serves as a crucial tool for the non-invasive detection of involved organs, particularly in areas where biopsy is challenging, such as coronary artery lesions. However, due to an incomplete understanding of this uncommon site and suboptimal imaging assessment, IgG4-related coronary involvement has been underestimated in clinical practice and remains poorly understood. Accordingly, the literature on coronary involvement in IgG4-RD was reviewed, spanning the past 24 years, and supplemented with magnetic resonance (MR) data from a center on IgG4-RD patients with typical coronary involvement. The research aims to elucidate the clinical features and further highlight the imaging characteristics and their clinical applicability.
2 Materials and methods
2.1 Literature review
2.1.1 Search strategy
The exploration of the literature involved a meticulous search across the PubMed/Medline, Scopus, and Web of Science databases, specifically targeting English-language publications. The MeSH term “Immunoglobulin G4–Related Diseases” along with its related Entry Terms was applied, combined with the MeSH term “Coronary artery” and its corresponding Entry Terms using the Boolean operator “AND”. Additionally, references cited in the papers initially selected to uncover any additional relevant literature for inclusion in the study were reviewed. The outcomes of these search strategies are listed in Supplementary Table S1.
2.1.2 Study selection and study screening
The design, data analysis, and interpretation of this study were conducted on the basis of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (6) (Figure 1). The study eligibility was defined employing the population, intervention, comparator, and outcome framework (7). Detailed inclusion and exclusion criteria are outlined in Supplementary Table S2. Publication Date: Articles published between 2000 and 2024.
Figure 1

PRISMA diagram for the selection of papers.
Two independent reviewers (Du YQ and Bai Y) evaluated the literature abiding by the inclusion criteria. Titles and abstracts of the retrieved articles were screened, and a quality assessment was carried out to identify potentially relevant studies. Each study was assessed for quality and risk of bias using the Joanna Briggs Institute checklist (8).
2.2 Retrospective cases review
A series of 77 patients identified as IgG4-RD from a center (database of The First Hospital of China Medical University, 2021-2024) were selected, conforming to the classification criteria for 2019 American College of Rheumatology/European League Against Rheumatism (5), with 21 of them suspected to show coronary involvement after systematic examination based on the patterns described in the previous literature (9). The charts of all patients were reviewed and the IgG4-RD patients with typical coronary involvement were extracted based on radiologic evidence as previously described (10). The case reports were reviewed and approved by the ethics committee of The First Affiliated Hospital of China Medical University. Written informed consent was obtained from all included patients.
2.3 Data extraction
The collected medical records include patient demographics (age and sex), clinical characteristics (medical history, treatment details, laboratory and imaging data), assessment of coronary artery lesions, clinical outcomes (response, relapse and remission), and documentation of adverse events.
2.4 Statistical analysis
Due to the high publication bias, a highly reliable statistical analysis could not be performed. For illustrative purposes, however, an analysis of a few relevant variables was performed. Normality of distributions was assessed using the Kolmogorov-Smirnov test. Categorical data are presented as percentages, and continuous variables are expressed as mean ± standard deviation or median (interquartile range), as per the data distribution. Comparisons between independent samples were made by virtue of one-way analysis of variance, Kruskal-Wallis test, or chi-squared test with Bonferroni post-hoc adjustments and Fisher’s exact tests, as appropriate for the type of data. All tests were two-tailed, with a p-value of less than.05 considered statistically significant.
3 Results
A total of 586 articles were retrieved, of which 111 met the inclusion criteria and were ultimately included (9, 11–120). These articles reported a total of 118 IgG4-RD patients. Combined with 16 IgG4-RD patients with typical coronary involvement from our center, the total number of cases analyzed reached 134. Detailed information on the patients from the center is provided below and summarized in Table 1.
Table 1
| Cases | Age, years | Sex | Comorbidities | Smoking history | Symptoms | Coronary abnormalities | Other cardiovascular involvement | Number of organ involvements | Serum IgG4, mg/dL (before and after treatment) | Serum IgG, mg/dL (before and after treatment) | ESR, mm/h (before and after treatment) | CRP, mg/L (before and after treatment) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 63 | Male | CAD | + | None | LAD: Type2 | Aorta, Pericardium, Myocardium | 10 | 2560 | 242 | 3071 | 1035 | 50 | 2 | 1.0 | 2.2 |
| Case 2 | 49 | Male | Asthma, Allergic rhinitis | – | Dyspnea, Chest distress | LAD: Type1 | None | 7 | 1549 | 74 | 1549 | 948 | 5 | 4 | 1.2 | 3.0 |
| Case 3 | 63 | Male | Hypertension | – | None | LAD, RCA: Type1 | Myocardium, mildly reduced RVEF% and LVEF% | 7 | 415 | 2079 | – | – | – | 1 | – | |
| Case 4 | 73 | Female | None | – | None | RCA: Type1 | Myocardium | 4 | 805 | – | – | – | 30 | – | 1.5 | – |
| Case 5 | 66 | Female | None | – | None | LAD: Type1 | None | 6 | 634 | – | 1220 | – | 28 | – | 7.6 | – |
| Case 6 | 68 | Female | None | + | None | RCA: Type1 | Pulmonary artery | 6 | 1410 | 354 | 1991 | 1298 | 20 | 9 | 0.69 | 1.48 |
| Case 7 | 56 | Male | None | – | None | RCA: Type1 | None | 2 | 1130 | 405 | 1419 | 1105 | 10 | 8 | 2.2 | 2.0 |
| Case 8 | 67 | Male | None | – | None | LAD: Type1, 4 | None | 7 | 3390 | – | 2464 | – | 36 | – | 3.7 | – |
| Case 9 | 68 | Male | None | – | Palpitation, Shortness of breath | LAD, LCX, RCA: Type1 | Myocardium, Reduced RVEF% and LVEF% | 5 | 1840 | 347 | 1977 | 1080 | 12 | 2 | 2.4 | 1.3 |
| Case 10 | 58 | Male | None | – | Palpitation, Shortness of breath, Chest distress | RCA: Type1,4; LAD: Type1 | None | 9 | 7030 | 799 | 5648 | 856 | >90 | 15 | 7.2 | 3.1 |
| Case 11 | 66 | Male | None | – | None | LAD: Type1 | None | 6 | 1360 | – | 2431 | – | 62 | – | 6.2 | – |
| Case 12 | 60 | Male | None | – | None | LAD: Type1 | None | 9 | 4030 | – | 3140 | – | 50 | – | 3.5 | – |
| Case 13 | 71 | Female | None | – | None | LAD: Type1 | None | 4 | 3020 | – | 2753 | – | 77 | – | 4.7 | – |
| Case 14 | 69 | Male | None | – | None | LAD, RCA: Type1 | Pericardium | 6 | 1940 | – | 1861 | – | 18 | – | 3.2 | – |
| Case 15 | 52 | Male | None | – | None | LCX: Type1 | None | 5 | 1300 | – | 1593 | – | 12 | – | 3.4 | – |
| Case 16 | 62 | Male | None | – | None | LAD: Type1 | Reduced RVEF% | 7 | 2030 | – | 2196 | – | 28 | – | 2.7 | – |
Characteristics of IgG4-RD patients with coronary artery involvement from the center.
Type 1: wall thickening, Type 2: periarterial soft tissue encasement, Type 3: aneurysm/ectasia and Type 4: stenosis.
LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery; CAD: coronary artery disease; LVEF: left ventricle ejection fraction; RVEF: right ventricle ejection fraction.
For further analysis, all included patients were classified into two groups based on their clinical presentation of coronary involvement: the symptomatic group (n = 75) and the asymptomatic group (n = 59) (Table 2).
Table 2
| Characteristics | Symptomatic (n = 75) | Asymptomatic (n = 59) | p-value |
|---|---|---|---|
| Age, years | 63 [54-70] | 63 [58-71] | .23 |
| Sex (male) | 63 [84] | 49 [83] | .88 |
| Disease duration, years | 5 [1-10] | 5 [2-8] | .45 |
| Hypertension | 12 [16] | 13 [22] | .37 |
| Diabetes | 6 [8] | 8 [14] | .30 |
| Atherosclerosis | 28[37] | 31[53] | .08 |
| Number of organs involvement | 3 [2-4] | 5 [3-6] | <.001 |
| Asthma | 6[8] | 8[14] | .30 |
| Myocardial ischemia/infarction | 63 [84] | 7 [12] | <.001 |
| Large vessel involvement | |||
| Aorta | 23 [31] | 23 [39] | .31 |
| Others | 15 [20] | 13 [22] | .77 |
| Coronary involvement | |||
| LAD | 58 [77] | 48 [81] | .57 |
| LCX | 37 [49] | 27 [46] | .68 |
| RCA | 60 [80] | 35 [59] | .009 |
| Coronary abnormalities | |||
| Type 1 | 30 [40] | 28 [48] | .39 |
| Type 2 | 36 [48] | 26 [44] | .65 |
| Type 3 | 43 [57] | 23 [39] | .04 |
| Type 4 | 45 [60] | 18 [31] | <.001 |
| Peak IgG4, mg/dL | 799 [278-1761] | 1410 [785-2600] | .006 |
| Peak IgG, mg/dL | 2080 [1618-4027] | 2464 [1991-3864] | .39 |
| Peak IgE, IU/mL | 1014 [338-1709] | 743 [361-2405] | .76 |
| ESR, mm/h | 58 [16-95] | 52 [22-80] | .71 |
| CRP, mg/L | 8.9 [4.1-24.8] | 3.6 [1.6-9.2] | .01 |
| Imaging examination | |||
| CT/CTA | 66 [88] | 42 [71] | .02 |
| ICA | 41 [55] | 16 [27] | .001 |
| CMR | 13 [17] | 18 [31] | .07 |
| PET-CT | 25 [33] | 30 [51] | .04 |
| IVUS | 12 [16] | 5 [9] | .19 |
| Echocardiography | 20 [27] | 24 [41] | .09 |
Characteristics of IgG4-RD with coronary involvement.
Values are n [%], or median [interquartile range].
One-way analysis of variance and Kruskal-Wallis with post-hoc tests for differences.
p-value for the one-way analysis of the whole cohort.
Type 1: wall thickening, Type 2: periarterial soft tissue encasement, Type 3: aneurysm/ectasia and Type 4: stenosis. Statistically significant values are shown in bold.
LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; CT: computed tomography; ICA: invasive coronary angiography; CMR: cardiac magnetic resonance; PET-CT: positron emission tomography-computed tomography; IVUS: intravascular ultrasound.
3.1 Characteristics of the patients included in the analysis
The patients were predominantly middle-aged males, consistent with the demographic profile of IgG4-RD patients reported in the literature (121). Notably, three younger patients (aged 13, 15, and 22 years) in the cohort exhibited significant coronary involvement (33, 80, 85), with two experiencing secondary myocardial infarction and reduced cardiac function (80, 85). There were no significant differences in age distribution across groups. The time from disease onset to the detection of coronary abnormalities varied widely, ranging from 12 weeks to 25 years.
Most patients (85%) presented multi-organ involvement in addition to coronary lesions, with lymph nodes being the most frequently affected site (27%). Within the cardiovascular system, the aorta was most commonly affected (34%). Detailed information on extra-cardiovascular involvement is provided in Supplementary Table S3. Interestingly, patients in the asymptomatic group had a significantly larger number of organs involved compared to those in the symptomatic group (p <.001).
3.2 Diagnosis of coronary artery involvement
Among the 134 cases studied, 20 patients (15%) presented with isolated coronary involvement, while the remaining patients had already been diagnosed with IgG4-RD through the identification of involvement in other organs before coronary lesions were detected. The diagnosis of IgG4-RD coronary artery involvement primarily relied on cardiovascular-related clinical symptoms, characteristic imaging findings, and elevated serum IgG4 levels, with biopsy occasionally performed for further confirmation.
In the cohort, 91 patients received a definitive IgG4-RD diagnosis confirmed by biopsy, with coronary involvement pathologically validated in 38 cases (42%), including 12 with isolated coronary lesions. Similar to other organs affected by IgG4-RD, the histologic hallmark of coronary involvement included diffuse lymphoplasmacytic infiltrates with abundant IgG4-positive plasma cells, a high IgG4-to-IgG plasma cell ratio, and storiform fibrosis.
While the 16 cases of IgG4-RD with coronary involvement from the center were not biopsy-confirmed, they exhibited no evidence of coronary artery diseases (CADs) and consistently displayed characteristic imaging features of IgG4-RD coronary involvement. Furthermore, the majority of these patients showed regression of coronary lesions following disease-modifying therapies, further supporting the diagnosis.
3.3 Imaging modalities for evaluating coronary artery involvement in IgG4-RD
Chest computed tomography (CT) and coronary computed tomography angiography (CTA) were the most commonly used imaging modalities, identifying coronary abnormalities in 108 patients (81%). Notably, 42 of these patients (39%) were incidentally diagnosed in the absence of symptoms (Table 2). Invasive coronary angiography (ICA) was the second most frequently utilized imaging technique, performed in 57 patients (43%), primarily as a complementary method to CT for confirming luminal stenosis or ectasia and assessing its severity. Positron emission tomography-computed tomography (PET-CT) was applied in 55 patients (41%), while intravascular ultrasound (IVUS) was performed in only 17 patients (13%). In the symptomatic group, CT/CTA and ICA were more frequently adopted compared to the asymptomatic group (CT/CTA: 88% v. 71%, p = .005; ICA: 55% v. 27%, p = .001). Conversely, PET-CT was more frequently employed in the asymptomatic group compared to the symptomatic group (51% v. 33%, p = .04).
Cardiovascular magnetic resonance (CMR) was not routinely used in clinical practice for evaluating coronary involvement, with only 31 patients (23%) assessed in this cohort. Among the 15 cases identified in the literature, coronary lesions were detected in eight cases through CMR. However, all 16 patients from the center underwent CMR, with coronary lesions consistently identified via contrast-enhanced coronary MR angiography (MRA).
Follow-up imaging was conducted on 57 post-treatment patients. Of these, 45 patients (79%) underwent chest CT/CTA to assess changes in coronary lesion size and luminal alterations. PET-CT was applied in 19 patients (33%) to evaluate inflammatory activity through changes in 18F-FDG uptake. At the center, six patients were followed up with CMR, allowing for the evaluation of coronary lesion size and the extent of coronary wall enhancement.
3.3.1 Characteristics of coronary involvement in IgG4-RD
Consistent with the descriptions of IgG4-related coronary involvement by Akiyama et al. and Katz et al. (10, 122), four types of coronary artery involvement were identified through multimodal imaging, often with multiple types coexisting (Figure 2):
Figure 2

Four types of IgG4-related coronary artery lesions by multimodal imaging evaluation. Type 1: wall thickening, Type 2: periarterial soft tissue encasement and Type 3: aneurysm/ectasia may coexist; Type 4: stenosis generally secondary to other types or atherosclerosis. (a) Thickening of the LAD coronary wall (red arrowheads on CMR and yellow arrowheads on CTA), with contrast enhancement on coronary CMR angiography even in segments without apparent wall thickening on CTA (red arrowheads), accompanied by secondary luminal stenosis. (b) Periarterial soft tissue encasement of LAD, resembling the “Pigs-in-a-Blanket Coronary Artery” on CTA (yellow arrowheads), coexists with wall thickening and contrast enhancement on coronary CMR angiography (red arrowheads), accompanied by secondary luminal stenosis. (c) Aneurysm formation of RCA with wall thickening and contrast enhancement on coronary CMR angiography (red arrowheads); localized ectasia of LAD (yellow arrow) with periarterial soft tissue formation (yellow arrowheads). LAD: left anterior descending artery; RCA: Right coronary artery; CMR: cardiac magnetic resonance.
Type 1: Wall thickening (43%) – Characterized by coronary wall thickening with contrast enhancement on CT/MRI (arteritis). Type 2: Periarterial soft tissue encasement (46%) – Appears as localized or diffuse peri-coronary soft tissue thickening (periarteritis), sometimes described as pseudo-tumors, which may cause luminal narrowing. Type 3: Aneurysm/ectasia (49%) – Characterized by localized or continuous luminal dilation, typically accompanied by mild coronary wall thickening or periarterial soft tissue encasement. Larger dilations are prone to mural thrombus formation (35%), potentially causing luminal occlusion due to thrombus detachment. Type 4: Stenosis (47%) – Generally considered a secondary phenomenon, often following Type 2 lesions.
These three types (1-3) frequently exhibited elevated uptake on 18F-FDG PET/CT and contrast enhancement on CT/CMR. Additionally, 44% of cases had concomitant atherosclerosis, with stenosis in some cases directly resulting from localized atherosclerosis (19%).
In comparing the two groups, the incidence of aneurysm/ectasia (Type 3) and stenosis (Type 4) was significantly higher in the symptomatic group than the asymptomatic group (symptomatic v. asymptomatic group: Type 3: 57% v. 39%, p = .04; Type 4: 60% v. 31%, p <.001). Additionally, the most frequently affected site of coronary involvement across the cohort was the left anterior descending artery (LAD) (79%), but the symptomatic group demonstrated a higher incidence of right coronary artery (RCA) involvement (symptomatic v. asymptomatic group: 80% v. 59%, p = .009). This may be attributed to the fact that involvement of multiple coronary arteries is more likely to induce cardiovascular symptoms, while multiple types of coronary lesions are more commonly overlapped in the LAD.
3.3.2 CMR manifestations of cardiovascular involvement and coronary MRA techniques
A total of 31 patients underwent CMR examination, with coronary involvement identified in 24 cases (77%), including eight cases from the literature review (Supplementary Table S4). CMR evaluation revealed three primary types of coronary involvement: wall thickening (75%), periarterial soft tissue encasement (29%), and aneurysm/ectasia (25%), with frequent overlap between these patterns; however, without the use of coronary MRA, a technique not routinely employed in IgG4-RD imaging evaluation, identifying coronary arterial luminal stenosis based solely on routine CMR remained challenging. Among the reviewed cases in the literature, only one case (Supplementary Table S4, case 7) underwent coronary MRA examination. All patients at the center received contrast-enhanced coronary wall MR imaging (123), which facilitated the detection of coronary wall abnormalities, including mild wall thickening. The most characteristic CMR findings in this cohort included enhancement of thickened coronary walls, peri-coronary masses, and aneurysm walls. Multi-parameter MR imaging also played a critical part in the identification of intracavitary thrombosis (Supplementary Table S4, cases 5 and 6).
Apart from coronary involvement, CMR identified both inflammatory non-ischemic myocardial abnormalities (19%) and secondary ischemic myocardial abnormalities (13%), even among asymptomatic patients. Non-ischemic myocardial abnormalities were typically characterized by subepicardial or mid-myocardial late gadolinium enhancements (LGEs) and/or elevated T2 values, which were often directly associated with the inflammatory infiltration of the disease rather than secondary to the coronary involvement. Ischemic myocardial abnormalities, on the other hand, shown as subendocardial or transmural LGEs, with coronary CTA/ICA confirming the culprit coronary arteries in three patients. Furthermore, CMR detected pericardial involvement in one patient from the center, who showed significant remission following treatment (Figures 3C, F).
Figure 3
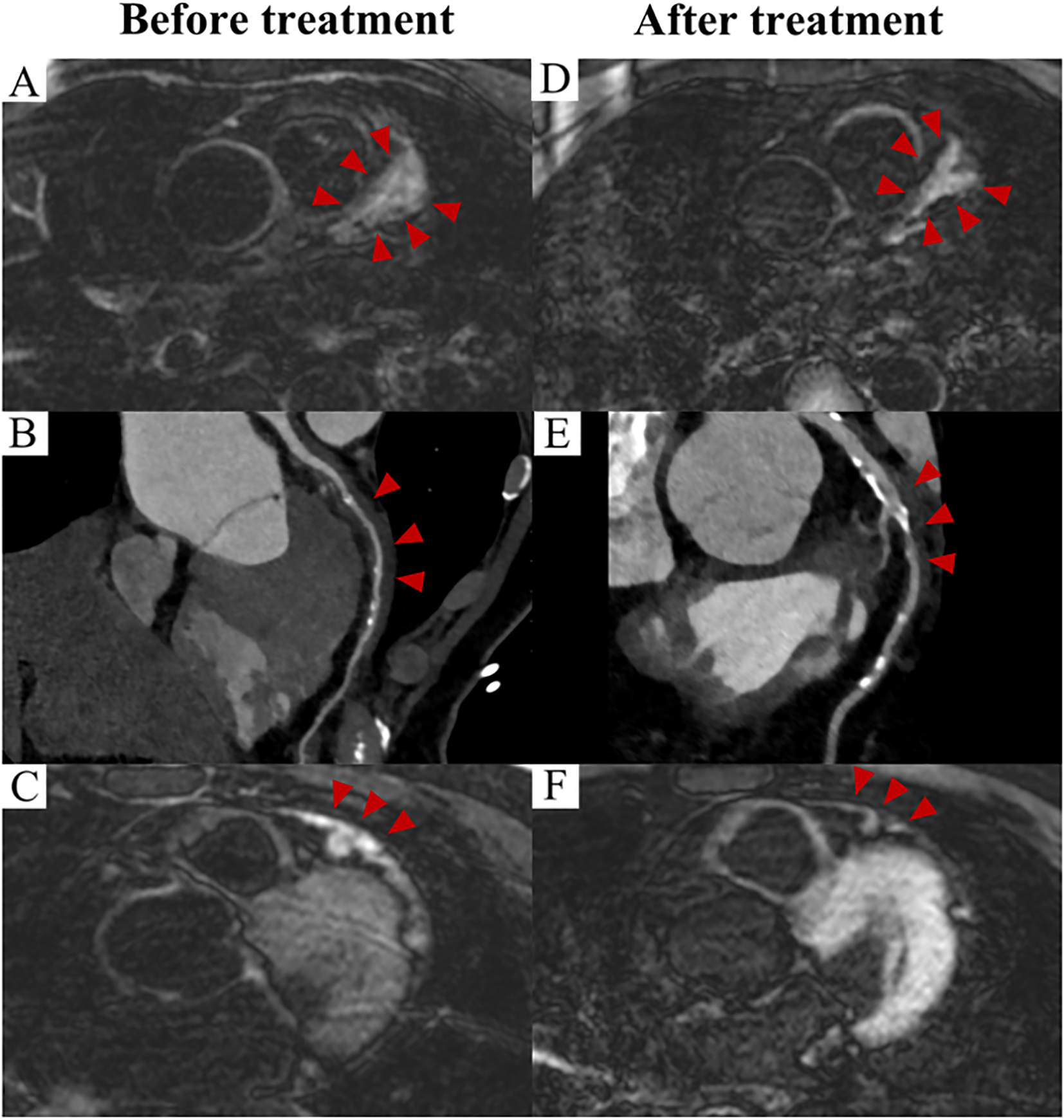
Prognosis of IgG4-related coronary involvement evaluated by imaging (Case 1). CMR image demonstrate wall thickening and enhancement of the LAD before treatment (A), and a reduction in both wall thickening and enhancement were observed after two years of treatment (D). (red arrowheads) CTA images demonstrate the corresponding findings (B, E). (red arrowheads) Pericardial thickening with enhancement was evident on CMR before treatment (C), with no abnormality observed after treatment (F). (red arrowheads). CMR: cardiac magnetic resonance; CTA: computed tomography angiography; LAD: left anterior descending artery.
3.4 Complications of coronary involvement
Chest tightness and chest pain/angina were the most common clinical symptoms. Patients with myocardial ischemia/infarction were significantly more common in the symptomatic group than the asymptomatic group (84% v. 12%, p <.001), with this condition most frequently observed in symptomatic patients with Type 4 involvement (68%). This indicates that myocardial ischemia/infarction caused by coronary stenosis may represent the primary cause of symptoms in these patients. In the asymptomatic group, four patients experienced sudden death due to myocardial ischemia/infarction (three with Type 4 involvement and one with Type 3), three of whom had a documented history of prior myocardial infarction.
3.5 Laboratory findings
In a cohort of 112 patients with known serum IgG4 levels, the majority showed markedly elevated serum IgG4 (95%), with a median level of 1160 mg/dL. Interestingly, it seems that the severity of clinical manifestations did not appear to correlate with the degree of elevation in serum IgG4 levels: the serum IgG4 level in the asymptomatic group was higher than that of the symptomatic group (1410 mg/dL v. 799 mg/dL, p = .006). Notably, six individuals (6%) with normal serum IgG4 levels still presented significant symptoms of coronary artery involvement. Serum C-reactive protein (CRP) levels were recorded in 48 cases across the cohort (median: 4.9 mg/L), with 25 patients (52%) exhibiting values above the normal range. The serum CRP level in the symptomatic group was higher than that of the asymptomatic group (8.9 mg/L v. 3.6 mg/L, p = .01). Other laboratory results were recorded in less than half of the cases, with over 70% within the normal range, revealing no significant differences between the two groups (Table 2).
3.6 Treatment and prognosis
The majority of IgG4-RD patients with coronary artery involvement were treated with glucocorticoids (76%), with more than 50% demonstrating remission of coronary lesions. The use of immunosuppressants alone (21%) or rituximab alone (7%) was uncommon, as these therapies were typically combined with glucocorticoids.
Surgical treatment is generally recommended for giant aneurysms (> 20 mm) or multiple aneurysms (124). Among patients with known lesion sizes, 28 (42%) had aneurysms larger than 20 mm, with 16 undergoing surgery, and most achieved remission or stabilization of their lesions (69%). Across the entire cohort, however, surgical treatment for coronary lesions was less frequent (32%), possibly because of operability limitations. Surgical cases included aneurysms (37%), periarterial soft tissue encasement (23%), a combination of both (26%), and wall thickening (14%). Among these, five patients (Type 3 combined with Type 1/2/4) received surgical treatment after failing to respond to glucocorticoid therapy.
The most commonly employed surgical approach was lesion excision combined with coronary artery bypass grafting (33%), with most patients receiving routine postoperative glucocorticoid therapy. The prognosis for surgical treatment was favorable, with 65% of patients achieving relief or stabilization of their condition. However, four cases (9%), including three with Type 3 and one with Type 2, demonstrated enlarged coronary lesions after coronary artery bypass grafting. Notably, two of these patients had not received glucocorticoid therapy (56, 58, 60, 92, 97).
Overall, most patients had a favorable prognosis after medical and/or surgical treatment, evidenced by reductions in peri-coronary mass size, aneurysm diameter, or alleviation of wall thickening on CT/CTA/CMR imaging (Figure 3), as well as reduced FDG uptake on 18F-FDG PET/CT scans. Prognosis varied across different types of coronary artery involvement, as displayed in Table 3. Type 2 involvement demonstrated the best prognosis, with the highest improvement rate (66%) and the lowest rate of progression (8%). Type 3 had a relatively higher rate of progression (15%).
Table 3
| Type of coronary involvement | Number of patients | improved | Progressive | Stable | Dead | Unknown |
|---|---|---|---|---|---|---|
| Type 1 | 58 | 19[33] | 5[9] | 11[19] | 7[12] | 16 |
| Type 2 | 62 | 41[66] | 5[8] | 7[11] | 5[8] | 4 |
| Type 3 | 66 | 33[50] | 9[14] | 11[17] | 3[5] | 10 |
| Type 4 | 63 | 34[54] | 5[8] | 9[8] | 8[13] | 7 |
Prognosis of IgG4-RD with different coronary abnormalities.
Type 1: wall thickening, Type 2: periarterial soft tissue encasement, Type 3: aneurysm/ectasia and Type 4: stenosis.
3.7 Characteristics of death cases
The total number of deceased patients in the cohort was 13 (10%), of whom 7 (54%) died from cardiac causes, all directly attributed to myocardial ischemia/infarction. The detailed characteristics are listed in Table 4. Among these, three deaths were due to wall thickening or periarterial soft tissue encasement, two were induced by thrombosis formation associated with atherosclerosis, and the remaining two resulted from thrombus formation within coronary artery aneurysms, leading to severe narrowing or occlusion of the coronary artery lumen. Notably, five patients exhibited no discernible signs of IgG4-RD involvement beyond the coronary arteries before death; postmortem examinations confirmed IgG4-RD influencing the coronary arteries. Additionally, four patients had not experienced any cardiovascular-related symptoms before their sudden death.
Table 4
| Study (reference) | Age at the discovery of CAI | Sex | Type of coronary artery involvement | Coronary involvement | Peak IgG4, mg/dL | Peak IgG, mg/dL | Peak IgE, IU/mL | ESR, mm/h | CRP, mg/L | Treatment | Cause of death | Other organs involvement | Imaging examination |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ramdin et al. (11) | 84 | Male | Type 1 | LAD, LCX, and RCA | 1160 mg/dL | 2890 mg/dL | Normal | Glucocorticoid and rituximab | Aspiration pneumonia | Kidney, ureter, biliary tract, pancreas, retroperitoneum, retroorbital tissue, lymph node | CT | ||
| Inokuchi et al. (12) | 38 | Male | Type 1 and type 4 | LCX and RCA | 109 mg/dL | 1563 mg/dL | 3.0 mg/L | NA | Coronary thrombosis led to ischemic heart disease | NA | CT | ||
| Patel et al. (13) | 53 | Male | Type 1, type 2 and type 4 | LAD, LCX, and RCA | NA | NA | Coronary thrombosis led to myocardial infarction | NA | NA | ||||
| Paratz et al. (18) | 48 | Male | Type 1 and type 4 | Multi-vessel | NA | NA | Coronary stenosis led to ischemic heart disease | Spleen enlargement, retroperitoneal soft tissue adjacent to the aorta | NA | ||||
| Paratz et al. (18) | 50 | Male | Type 2 and type 4 | LAD | NA | NA | Coronary stenosis led to myocardial ischemia | NA | NA | ||||
| Gutierrez et al. (19) | 54 | Male | Type 3 | LAD and RCA | NA | NA | Thrombosis in aneurysm led to myocardial infarction | NA | NA | ||||
| Bukiri et al. (20) | 55 | Male | Type 1 and type 4 | LAD and LM | NA | Glucocorticoid | Coronary occlusion led to myocardial ischemia | NA | NA | ||||
| Chan et al. (67) | 73 | Male | Type 3 | LAD, LM and RCA | NA | Glucocorticoid | Thrombosis in aneurysm led to myocardial ischemia | Surrounding soft tissue mass of right internal iliac artery | CT | ||||
| Yardimci et al. (9) | 61 | Male | Type 1 and type 4 | RCA | 148 mg/dL | 15.3 mg/L | Glucocorticoid | Heart failure, sepsis, systemic multi-organ failure | Orbital inflammation | CT and echocardiography | |||
| Urabe et al. (38) | 84 | Male | Type 2 and type 3 | LAD, LCX, and RCA | 2630 mg/dL | 8194 mg/dL | Glucocorticoid | Rupture of thoracic aortic aneurysm | NA | CT, DSA, IVUS and PET | |||
| Tajima et al. (52) | 68 | Male | Type 2 | LAD, LCX, and RCA | 2390 mg/dL | 4305 mg/dL | 9716 IU/mL | 97 mm/h | 9.7 mg/L | Glucocorticoid | Rupture of splenic aneurysm | Multiple systemic aneurysms of right internal carotid artery, bilateral subclavian arteries, left axillary artery, left brachial artery, common hepatic artery, and splenic artery; Salivary glands involvement | CT and PET-CT |
| Ibrahim et al. (100) | 70 | Male | Type 2 and type 4 | LAD, LCX, and RCA | 3030 mg/dL | Glucocorticoid and rituximab | Toxic megacolon | Orbital inflammatory pseudo-tumor, abdominal aortic aneurysm, and bilateral common iliac artery involvement | CT, PET-CT and echocardiography | ||||
| Fujii et al. (102) | 77 | Male | Type 1 and type 4 | LCX | 1750 mg/dL | 4813 mg/dl | Glucocorticoid | Shock and metabolic acidosis | Multi-organ involvement includes submandibular gland, sigmoid mesentery, retroperitoneal soft tissue, abdominal aorta, liver, extrahepatic bile duct, bilateral lungs, bilateral kidneys, bladder, prostate, epicardium, and lymph nodes | CT |
Characteristics of dead patients.
Type 1: wall thickening, Type 2: periarterial soft tissue encasement, Type 3: aneurysm/ectasia and Type 4: stenosis.
LAD: left anterior descending artery; LCX: left circumflex artery; RCA: Right coronary artery; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; CT: computed tomography; DSA: digital subtraction angiography; PET-CT: positron emission tomography-computed tomography; IVUS: intravascular ultrasound.
4 Discussion
In this review, a detailed analysis of the characteristics of IgG4-RD coronary involvement was provided on the basis of published reports and data from the center.
4.1 Epidemiology of IgG4-RD coronary involvement
Clinically, 60%-90% of IgG4-RD cases present with multi-organ involvement, with almost all organs potentially affected. Approximately 10%-30% of cases are complicated by vascular involvement, including arteritis and periarteritis (125), while coronary involvement accounts for only 7% of systemic organ involvement and 10% of vasculitis (126, 127). Despite increasing recognition, the understanding of IgG4-RD coronary involvement as a distinct clinical entity remains limited, and most coronary artery lesions are currently identified through CTA, which is primarily sensitive in detecting Type 2 and Type 3 lesions. However, the insensitivity of conventional imaging techniques to coronary wall inflammation probably results in a significant underestimation of the true extent of coronary involvement in IgG4-RD. In our previous single-center study, coronary MR imaging was utilized for visualizing and quantifying both luminal and wall changes in IgG4-RD coronary vasculitis. About 77% of patients were found to exhibit abnormal coronary wall enhancement, predominantly in a diffuse pattern, suggesting inflammatory involvement (128, 129). This finding indicates that coronary involvement may be more prevalent than currently appreciated and that its true burden remains underestimated.
4.2 Mechanisms of IgG4-RD vascular involvement
To date, the pathogenesis of IgG4-RD remains unclear. In recent years, most perspectives have suggested that its occurrence is related to interactions between innate and adaptive immune responses as well as the cooperation between B cells and T cells and the involvement of T follicular helper (Tfh) cells. These interactions can lead to IgG4 class switching, germinal center formation, and plasmablast differentiation, thereby initiating tissue damage and/or fibrosis (130). The typical pathological features of IgG4-RD involving the coronary arteries include coronary wall inflammation and lymphoplasmacytic infiltration (72), resulting in fibrosis and thickening of the coronary wall. Kasashima et al. conducted a retrospective study through the use of a novel histopathological method for whole-slide analysis of IgG4-related abdominal aortic aneurysms (IgG4-AAA). The results indicated that IgG4-AAA should be positioned as adventitial vasculitis with predominant T follicular regulatory and Tfh2 cells, accompanied by the abnormal appearance of arterial tertiary lymphoid organs (ATLOs) (131, 132). ATLOs typically formed in response to chronic inflammation due to infection or autoimmunity (133), and are rarely presented in the adventitia of normal arteries, making their presence a strong indicator of vascular disease (131). The development of ATLOs may sustain inflammatory processes within the arterial walls, driving vascular remodeling, damage, and fibrosis (134). Beyond aortic diseases, ATLOs have also been observed in the adventitia of abnormal medium-sized arteries, including coronary arteries affected by atherosclerosis (135). These findings may provide valuable insights into the pathological mechanisms underlying coronary involvement in IgG4-RD.
4.3 IgG4-related coronary involvement v. aortic involvement
Coronary involvement and aortic involvement refer to the vessel involvement in IgG4-RD, and have similar manifestations with wall thickening in arteritis, perivascular mass formation in periarteritis. However, they are not identical, especially in cases of periarteritis.
According to clinicopathological patterns, IgG4-RD can be divided into two subtypes: proliferative and fibrotic. These types differ significantly in clinical characteristics and response to glucocorticoid therapy. Lesions in the mediastinum, retroperitoneum and periaortic tissues are commonly associated with the fibrotic subtype (136), while coronary arteritis and periarteritis are more frequently observed in the proliferative subtype (10, 136).
Large vessel involvement in IgG4-RD is suggested as best being classified as primary manifestations of wall thickening (arteritis), and secondary manifestations of perivascular mass formation (periarteritis, mainly idiopathic retroperitoneal fibrosis) (137–139). Recent evidence indicates that idiopathic retroperitoneal fibrosis represents a manifestation of autoimmune or fibro-inflammatory diseases (139, 140). This helps explain why periarteritis/retroperitoneal fibrosis was classified as a secondary type. IgG4-related retroperitoneal fibrosis arises from an autoimmune response, resulting in the accumulation of inflammatory cells, such as lymphocytes and macrophages, in the retroperitoneal region, which release inflammatory and profibrotic mediators that drive fibrous tissue proliferation (136). In patients with periarteritis/retroperitoneal fibrosis, the vascular wall is rarely involved, however, in cases with peri-coronary soft tissue masses, distinct supplying vessel from the coronary artery was observed (58), suggesting that the mass is not entirely isolated from the coronary artery. Furthermore, biopsies have discovered positive cell infiltration in the adventitia of coronary arteries, accompanied by peri-coronary mass formation (38), as the adventitia is the initial site of various vascular diseases, including periarteritis and atherosclerosis (141). IgG4-related coronary arteritis/periarteritis may represent the same entity, both originating from the adventitia, coronary wall thickening may progress to peri-coronary masses as the disease advances. Definitive answers remain elusive due to a lack of longitudinal direct evidence, underlining the need for longitudinal studies integrating clinical and pathophysiological data.
CMR and 18F-FDG PET/CT have been suggested as tools to help distinguish arteritis from periarteritis to some extent (137). However, coronary periarteritis may occasionally present in a diffuse pattern, making it difficult to differentiate from arteritis characterized by diffuse and significant coronary wall thickening. Regardless of this distinction, both conditions increase the risk of coronary stenosis, potentially leading to myocardial infarction and other severe cardiovascular events. Early detection is therefore crucial.
4.4 Identification of IgG4-related coronary involvement
Symptomatic IgG4-related coronary involvement frequently manifests as myocardial ischemia or infarction, although the presence of symptoms does not necessarily correlate with number of organs involved. For some patients, coronary involvement may represent the initial and sole manifestation, carrying the risk of severe outcomes or even sudden death. The interval from symptom onset to detection of coronary artery lesions in IgG4-RD varies widely among individuals with different clinical presentations, underscoring the insidious and often overlooked nature of coronary involvement. Early recognition and timely intervention are therefore crucial for managing IgG4-RD-related coronary involvement.
The pathological diagnostic criteria for IgG4-related aortitis/periaortitis include: (1) Histology consistent with aortitis or periaortitis that cannot be readily explained by other processes such as atherosclerosis; (2) At least 50% of plasma cells staining positive for IgG4; and (3) A minimum of 50 IgG4+ plasma cells per 400× high-power field, assessed across at least three fields (142). In most clinical scenarios, pathologically confirming coronary artery involvement in IgG4-RD is particularly challenging due to the difficulty of obtaining biopsy samples from the affected coronary arteries.
4.4.1 Laboratory indicators
Although elevated serum IgG4 levels can support the diagnosis of IgG4-RD, Wallace et al. (143) reported that only 51% of biopsy-confirmed active IgG4-RD cases shad elevated serum IgG4. In the cohort, serum IgG4 levels did not correlate with the presence or absence of symptoms. This highlights the need for early screening to rule out coronary involvement, even in patients with modestly elevated or normal IgG4 levels.
CRP levels were normal in half of the patients, but CRP was associated with clinical symptoms. Most studies noted that patients with IgG4-related coronary arteritis were more likely to exhibit elevated serum CRP compared to those with non-vasculitis IgG4-RD or other autoimmune arteritis (122). However, previous studies did not demonstrate a consistent correlation among CRP levels CRP levels, disease activity, and coronary involvement, a discrepancy that maybe attributable to treatment received by some patients (128).
4.4.2 Diagnostic value of imaging examination
Due to the lack of specific clinical symptoms and laboratory markers, detecting coronary involvement in IgG4-RD is challenging and depends largely on imaging evidence. Multimodal imaging plays a critical role in diagnosing and assessing the prognosis of IgG4-RD coronary involvement. IgG4-RD predominantly affects middle-aged and elderly males, a population already at high risk for coronary atherosclerosis, highlighting the need to differentiate IgG4-RD coronary arteritis from coronary atherosclerosis (144).
4.4.2.1 Coronary CTA and ICA
Coronary CTA is effective in identifying coronary stenosis, tumor-like lesions, coronary ectasia, and aneurysmal formation, and can sometimes assist in differentiating arteritis from atherosclerosis (145). Atherosclerosis on coronary CTA typically manifests as focal plaques within the coronary arteries, leading to luminal narrowing, whereas IgG4-RD coronary arteritis often presents as diffuse, circumferential coronary wall thickening (80). However, the positive coronary CTA findings associated with IgG4-RD coronary arteritis often reflect advanced stages of inflammation, while false negatives in the early stages may cause an underestimation of the true extent and progression of the disease. Notably, coronary atherosclerosis and arteritis can coexist, with arteritis potentially accelerating the progression of atherosclerosis. While ICA can accurately depict coronary luminal anomalies, its ability to assess aneurysms with mural thrombosis is less precise compared to coronary CTA (87). Moreover, ICA is limited in visualizing changes in the coronary artery wall. Despite these constraints, coronary CTA and ICA remain the most commonly used imaging modalities for evaluating symptomatic patients in clinical practice.
4.4.2.2 PET/CT
As IgG4-RD often involves multiple organs, the application of (18)F-FDG PET/CT is not uncommon in clinical practice (18).F-FDG PET/CT could quantitatively evaluate disease activity in CADs, as elevated (18)F-FDG uptake indicates active inflammation. Even if coronary artery masses do not diminish in size after treatment, a reduction in (18)F-FDG uptake may still reflect the effectiveness of treatment (146). Additionally, the immunopathological characteristics of the arterial wall of IgG4-AAA patients suggest that the concentration of FDG uptake observed by PET/CT might reflect the amount or activity of ATLOs (131). Moreover, many asymptomatic patients with coronary involvement were incidentally identified through (18)F-FDG PET/CT, highlighting its value for screening multi-organ involvement. While (18)F-FDG PET/CT is effective in diagnosing large-vessel vasculitis, its diagnostic capacity remains limited to some extent for small and medium-sized vasculitis, including coronary arteritis, due to resolution constraints (147). Thus, combining (18)F-FDG PET/CT with CTA, particularly ECG-gating CTA, may provide a more comprehensive and complementary approach to assessing inflammatory IgG4-related cardiovascular diseases (137).
4.4.2.3 CMR
Although its use in clinical practice remains relatively uncommon, the sensitivity of CMR for early detection of coronary arteritis is increasingly recognized. As a non-invasive imaging modality free from radiation exposure, CMR provides a comprehensive, “one-stop” assessment of the cardiovascular system. This is particularly significant given that radiation exposure may contribute to the progression of IgG4-RD (148). Radiation-induced injury triggers inflammation, ultimately promoting the transdifferentiation of fibroblasts into myofibroblasts (149), a hallmark of fibrosis-related diseases and a key pathogenetic feature of IgG4-RD (150).
Recently, advancements in imaging technology have highlighted promising applications of coronary MRA in the evaluation of CADs. Coronary MRA techniques, such as bright arterial lumen imaging and black arterial wall imaging, allow for high-resolution visualization of inflammatory vasculitis changes, including luminal stenosis, occlusion, mural thickening, and contrast enhancement of the affected vessel wall (151). Contrast enhancement of the coronary artery wall may indicate abnormalities of the vascular wall, including fibrosis, neovascularization, or higher permeability associated with inflammation (152).
While the clinical application of CMR for diagnosing CADs remains limited—reflected by only eight reports in the current literature—our preliminary study suggests its potential for visualizing and quantifying both subclinical and clinical coronary involvement. Notably, arterial wall imaging with coronary MRA can detect coronary wall enhancement before significant wall thickening develops, rendering it a valuable tool for early lesion identification. Beyond the morphological insights offered by CTA, coronary MRA provides additional diagnostic capabilities, helping to distinguish vascular inflammation from atherosclerosis based on wall-enhancement patterns. It may also provide a more precise assessment of the severity of coronary involvement and disease activity compared to CTA (128). In IgG4-RD patients, CMR not only enables the integrated identification of coronary involvement but also facilitates the evaluation of myocardial inflammatory infiltration or ischemic changes. In addition, CMR’s unique advantages in noninvasiveness and lack of ionization make it an ideal modality for longitudinal monitoring of disease progression, as well as evaluation of the effects of therapies. However, the clinical utility of CMR is currently constrained by its long scan times and the requirement for patient cooperation, which remain significant limitations for widespread application.
4.5 Adverse cardiovascular events caused by secondary stenosis
The findings reveal that the most common manifestation of coronary involvement in IgG4-RD is wall thickening and/or periarterial soft tissue formation. Despite the primary thickening in the coronary adventitia (153), nearly half of the patients in the cohort developed coronary narrowing (49%), which often led to myocardial ischemia or infarction. This narrowing subsequently led to clinical symptoms and was identified as the leading cause of cardiac death in the cohort. Notably, luminal stenosis was never observed in isolation, suggesting that the stenosis type (Type 4) is more likely a secondary manifestation of IgG4-RD coronary involvement. The development of stenosis in IgG4-RD appears to be partially related to mechanical compression from peri-coronary soft tissue formation due to infiltration of IgG4-positive plasma cells. This mechanism differs from other rheumatic diseases, including rheumatoid arthritis and lupus, where systemic inflammation accelerates atherosclerosis and subsequent coronary stenosis (59). However, it has also been proposed that IgG4-RD coronary arteritis and periarteritis may contribute to or accelerate atherosclerosis in affected arteries (84). In the cohort, 44% of cases were accompanied by atherosclerosis, with 20% of these cases leading to stenosis. Notably, among the atherosclerosis-associated cases, six patients had a history of CADs before their IgG4-RD diagnosis. This raises uncertainty regarding the extent to which the development of atherosclerosis in these patients can be attributed directly to IgG4-RD.
The critical role of the adventitia in vascular remodeling under pathological conditions has been well-documented. During inflammation, the adventitial region becomes a focal point for the local immune response, producing cytokines and enzymes that stimulate immune cell mobilization and accumulation (154). A cross-talk between intimal lesions and adventitial ATLOs via medial vascular smooth muscle cells has been suggested in atherosclerotic vessels (155). It can be reasonably hypothesized that this interaction between IgG4-related adventitial inflammation and the intima may contribute to the development of coronary atherosclerosis in IgG4-RD. Nevertheless, the precise mechanisms underlying stenosis in IgG4-RD coronary involvement remain complex and warrant further investigation. Advanced imaging techniques, including specific contrast agents combined with dedicated MRI pulse sequences, have shown promise in visualization of macrophage-rich inflammation within atherosclerotic plaques (156, 157). Additionally, coronary MRA has proven effective in visualizing and quantitatively assessing coronary artery lesions in IgG4-RD (128). Future studies integrating these advanced imaging modalities may provide valuable insights into the pathophysiological mechanisms of IgG4-RD coronary lesions and the connection between intimal lesions and adventitial inflammation.
It is also worth noting that, while the concurrent development of coronary aneurysms was less common, it was always accompanied by mural thrombosis, which could sometimes lead to thromboembolism and myocardial infarction. Interestingly, in two patients who died suddenly from myocardial infarction caused by thromboembolism, no aneurysms were observed, and only mild atherosclerosis was present, without definite localized luminal narrowing. It is speculated that the inflammatory state itself promotes the release of procoagulant substances, representing a critical risk factor for cardiovascular events. Therefore, it is necessary to carefully evaluate the coronary artery wall using CTA or CMR in IgG4-RD patients, particularly when DSA indicates no significant luminal stenosis.
4.6 The effect of treatments on IgG4-related coronary involvement and prognosis
Glucocorticoids remain the cornerstone of IgG4-RD treatment, demonstrating effectiveness in improving most cases of diffuse wall thickening and/or periarterial soft tissue encasement (158). Rituximab, by depleting B cells and reducing myofibroblast activation, offers the potential of alleviating the fibrotic processes associated with the disease (159). Studies have shown that rituximab can induce remission in 67–83% of cases and may facilitate early tapering of glucocorticoid therapy (160). For refractory or complex IgG4-RD cases, multiple immunosuppressants are currently available as adjunctive therapies to glucocorticoids. These immunosuppressants have been verified to be both safe and effective, particularly in patients resistant to corticosteroid treatment (161).
The prognosis of the aneurysm phenotype is often unsatisfactory and difficult to predict, representing the primary pattern of disease progression, which may be attributed to irreversible damage including coronary artery wall remodeling (84). While high-dose corticosteroids can suppress inflammation and hinder further development of IgG4-related arterial aneurysms, they do not reverse pre-existing and stable aneurysms lacking signs of active inflammation (162). Previous studies have suggested that high-dose corticosteroid treatment might cause arterial wall thinning, potentially raising the risk of aneurysm rupture (163). Immunosuppressive therapy is generally not recommended for coronary aneurysms, surgical intervention is typically prioritized, particularly in patients at high risk for rupture. However, performing revascularization surgery alone without concomitant lesion resection provides only symptomatic relief and carries a risk of progression, particularly in patients not maintained on subsequent corticosteroid therapy.
5 Limitations
Given the limited literature on IgG4-related coronary involvement, conducting a truly comprehensive systematic review remains inherently difficult. The study aimed to provide a detailed overview of the topic, particularly highlighting the role of imaging diagnostics, including CMR. However, the scarcity of available references on this specific subject poses a significant limitation. It is acknowledged that the limited number of studies, along with incomplete data on laboratory findings and patient prognoses in the existing literature, may have introduced bias into the conclusions. Nonetheless, this work may serve as a valuable foundation to stimulate further research. Studies with larger, more diverse, and well-characterized cohorts are essential for advancing understanding of this complex condition.
6 Conclusions
Coronary involvement of IgG4-RD could be categorized into four phenotypes, sometimes with an insidious onset and as the sole affected site, posing a potential risk for major adverse cardiovascular events. Integrating multimodal imaging is crucial for early diagnosis and effective monitoring, with coronary CMR showing promise for early detection without the risk of radiation-induced inflammation and fibrosis. Glucocorticoid therapy is effective for most patients, sometimes in combination with immunosuppressive therapy, while surgical intervention is prioritized for established aneurysms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study involving human participants was approved by the Ethics of Committees of The First Hospital of China Medical University. Patients gave informed consent prior to participation. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
GW: Resources, Data curation, Visualization, Funding acquisition, Project administration, Conceptualization, Formal Analysis, Writing – review & editing, Validation, Investigation, Supervision, Methodology, Software. YD: Investigation, Writing – original draft, Formal Analysis, Conceptualization, Data curation, Methodology. YB: Methodology, Formal Analysis, Writing – original draft, Investigation, Data curation. YZ: Supervision, Methodology, Writing – review & editing, Investigation, Data curation, Validation, Visualization. SD: Formal Analysis, Data curation, Writing – review & editing. XW: Data curation, Writing – original draft. YX: Validation, Supervision, Writing – review & editing, Software, Resources, Visualization. H-jY: Supervision, Writing – review & editing. DL:Writing – review & editing, Supervision. ZF: Supervision, Writing – review & editing. GF: Writing – review & editing, Supervision, Resources, Project administration. ZL: Visualization, Validation, Resources, Supervision, Writing – review & editing. JW: Writing – review & editing, Supervision, Investigation, Validation, Project administration. YS: Writing – review & editing, Supervision, Resources, Investigation, Validation, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The National Natural Science Foundation of China (82471972), The Natural Science Foundation of Shenyang City (22-321-33-31), The Health Commission Foundation of Liaoning Province (2024-N013-03), LiaoNing Revitalization Talents Program (XLYC2412059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1685508/full#supplementary-material
Abbreviations
IgG4-RD, IgG4-related disease; MACEs, major adverse cardiovascular events; CMR, Cardiac magnetic resonance; CT, computed tomography; CTA, computed tomography angiography; PET-CT, Positron emission tomography-computed tomography; CADs, Coronary artery diseases; ICA, Invasive coronary angiography; IVUS, intravascular ultrasound; LGEs, late gadolinium enhancements.
References
1
StoneJHZenYDeshpandeV. IgG4-related disease. New Engl J Med. (2012) 366:539–51. doi: 10.1056/NEJMra1104650
2
InoueDZenYAboHGabataTDemachiHYoshikawaJet al. Immunoglobulin G4–related periaortitis and periarteritis: CT findings in 17 patients. Radiology. (2011) 261:625–33. doi: 10.1148/radiol.11102250
3
WallaceZSKhosroshahiACarruthersMDPeruginoCAChoiHCampochiaroCet al. An International multispecialty validation study of the IgG4-related disease responder index. Arthritis Care Res. (2018) 70:1671–8. doi: 10.1002/acr.23543
4
FragoulisGEEvangelatosGTektonidouMG. Vasculitis beyond aortitis in IgG4-related disease (IgG4-RD): case report and review of the literature. Clin Rheumatol. (2021) 40:1167–73. doi: 10.1007/s10067-020-05302-1
5
WallaceZSNadenRPChariSChoiHDella-TorreEDicaireJ-Fet al. The 2019 American College of Rheumatology/European league against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol. (2020) 72:7–19. doi: 10.1002/art.41120
6
MoherDLiberatiATetzlaffJAltmanDGPRISMA Group*, t. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
7
GuyattGHOxmanADKunzRAtkinsDBrozekJVistGet al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. (2011) 64:395–400. doi: 10.1016/j.jclinepi.2010.09.012
8
MunnZBarkerTHMoolaSTufanaruCSternCMcArthurAet al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. (2020) 18:2127–33. doi: 10.11124/JBISRIR-D-19-00099
9
YardimciGKArdali-DüzgünSBolekECKilicLCanpolatUHazirolanTet al. Coronary (peri)-arteritis in patients with IgG4-related disease: A case series from the Central Anatolia Region of Turkey. Int J Rheum Dis. (2021) 24:1004–13. doi: 10.1111/1756-185X.14153
10
KatzGHedgireSHStoneJRPerez-EspinaSFernandesAPeruginoCAet al. IgG4-related disease as a variable-vessel vasculitis: a case series of 13 patients with medium-sized coronary artery involvement. Semin Arthritis Rheum. (2023) 60:152184. doi: 10.1016/j.semarthrit.2023.152184
11
RamdinNOrdeMO'NeillSBLaiCPorsJDMultanMet al. Hidden igG4-related coronary disease: an autopsy study. Am J Clin Pathol. (2021) 156:471–7. doi: 10.1093/ajcp/aqaa258
12
InokuchiGHayakawaMKishimotoTMakinoYIwaseH. A suspected case of coronary periarteritis due to IgG4-related disease as a cause of ischemic heart disease. Forensic Sci Med Pathol. (2014) 10:103–8. doi: 10.1007/s12024-013-9516-5
13
PatelNRAnzaloneMLBujaLMElghetanyMT. Sudden cardiac death due to coronary artery involvement by IgG4-related disease: a rare, serious complication of a rare disease. Arch Pathol Lab Med. (2014) 138:833–6. doi: 10.5858/arpa.2012-0614-CR
14
LeeNJGlocknerJF. Multisystem IgG4-related disease involving the abdomen and coronary arteries and causing chronic abdominal pain. Acta Radiol Open. (2021) 10:20584601211044989. doi: 10.1177/20584601211044989
15
MaedaRNaitoDAdachiAShiraishiHSakamotoTMatobaSet al. IgG4-related disease involving the cardiovascular system: an intracardiac mass and a mass lesion surrounding a coronary artery. Internal Med. (2019) 58:2363–6. doi: 10.2169/internalmedicine.2509-18
16
KanzakiYMiuraTHashizumeNSaigusaTEbisawaSKuwaharaKet al. Unique angiographic findings in a patient with myocardial ischemia and immunoglobulin G4-related disease. SAGE Open Med Case Rep. (2017) 5:2050313X17728010. doi: 10.1177/2050313X17728010
17
IkutomiMMatsumuraTIwataHNishimuraGIshizakaNHirataYet al. Giant tumorous legions surrounding the right coronary artery associated with immunoglobulin-G4-related systemic disease. Cardiology. (2011) 120:22–6. doi: 10.1159/000332996
18
ParatzEDRossLZentnerDMorganNBouwerHLynchMet al. Intracoronary IgG4-related disease as an unusual cause of sudden cardiac arrest: a case series. Eur Heart Journal-Case Rep. (2022) 6:ytac050. doi: 10.1093/ehjcr/ytac050
19
GutierrezPSSchultzTSiqueiraSACde Figueiredo BorgesL. Sudden coronary death due to IgG4-related disease. Cardiovasc Pathol. (2013) 22:505–7. doi: 10.1016/j.carpath.2013.05.003
20
BukiriHRuhoySMBucknerJH. Sudden cardiac death due to coronary artery vasculitis in a patient with relapsing polychondritis. Case Rep Rheumatol. (2020) 2020:5620471. doi: 10.1155/2020/5620471
21
GoudotGYanamandalaMMitchellRTolisGGerhard HermanMD. Severe triple vessel disease secondary to IgG4-related coronary periarteritis. J Cardiac Surg. (2022) 37:5468–71. doi: 10.1111/jocs.17187
22
LanzafameLRMCarerjMLRizzoGMinutoliFBucoloGMIrreraNet al. Multimodality imaging evaluation of coronary IgG4-related disease: a “tumor-like” cardiac lesion. Diagnostics. (2022) 12:2814. doi: 10.3390/diagnostics12112814
23
KusumotoSKawanoHTakenoMKawaharaFAbeKHayashiHet al. Mass lesions surrounding coronary artery associated with immunoglobulin G4-related disease. J Cardiol cases. (2012) 5:e150–4. doi: 10.1016/j.jccase.2012.02.006
24
MatsumotoYKasashimaSKawashimaASasakiHEndoMKawakamiKet al. A case of multiple immunoglobulin G4–related periarteritis: a tumorous lesion of the coronary artery and abdominal aortic aneurysm. Hum Pathol. (2008) 39:975–80. doi: 10.1016/j.humpath.2007.10.023
25
YamamotoHItoYIsogaiJIshibashi-UedaHNakamuraY. Immunoglobulin G4-related multiple giant coronary artery aneurysms and a single left gastric artery aneurysm. Case Rep. (2020) 2:769–74. doi: 10.1016/j.jaccas.2020.03.015
26
ZhangYJChenYZhaoXChangXGuanSTianJet al. Coronary arteritis and periaortitis in IgG4-related disease. Can J Cardiol. (2020) 36:589.e585–589.e587. doi: 10.1016/j.cjca.2019.11.028
27
ShibasakiINakajimaTOtaniYNakanoSSatoNFukudaHet al. Immunoglobulin G4-related inflammatory pseudotumor of the right ventricle with right coronary artery occlusion. J Cardiol cases. (2022) 26:293–6. doi: 10.1016/j.jccase.2022.05.019
28
YamadaMUenoKKojimaYWatanabeMMoritaN. Long-term efficacy of drug-coated balloon only angioplasty for IgG4-related coronary artery disease: a case report. Eur Heart Journal-Case Rep. (2024) 8:ytae492. doi: 10.1093/ehjcr/ytae492
29
KadowakiHAmiyaEHoshinoYTamuraMUeharaMNakayamaAet al. Enormous aneurysm in coronary artery fistula with immunoglobulin G4–related disease. Can J Cardiol. (2019) 35:230.e231–230. doi: 10.1016/j.cjca.2018.11.030
30
KarmallySPancholyBLauRRapariaKPursnaniS. IgG4-related disease: Coronary arteritis masquerading as coronary “masses. J Cardiovasc Computed Tomography. (2022) 16:e29–30. doi: 10.1016/j.jcct.2021.12.009
31
TongAK-TTanS-YGoY-YLamWW-C. Cardiac structural abnormalities associated with IgG4-related coronary periarteritis and inflammation revealed by multimodality imaging. Can J Cardiol. (2014) 30:956.e915–956.e917. doi: 10.1016/j.cjca.2014.03.016
32
GuoYAnsdellDBrouhaSYenA. Coronary periarteritis in a patient with multi-organ IgG4-related disease. J Radiol Case Rep. (2015) 9:1. doi: 10.3941/jrcr.v9i1.1967
33
VasudevanAKKumarGARajeshSAhamedMZ. IgG4-related coronary aneurysm in a child. Indian J Pediatr. (2021) 88:593–3. doi: 10.1007/s12098-021-03743-3
34
HadaTAmanoMIrieYMoriuchiKOkadaAMatsumotoMet al. Left ventricular dysfunction caused by igG4-related small intramural coronary periarteritis. Internal Med. (2022) 61:59–63. doi: 10.2169/internalmedicine.7721-21
35
CochranRLBrideauHRWuMYStoneJHWallaceZSLittleBPet al. Pulmonary and coronary arterial abnormalities in patients with IgG4-related disease. Radiol Case Rep. (2022) 17:4924–7. doi: 10.1016/j.radcr.2022.09.070
36
KomiyaYSoejimaMTezukaDKohsakaH. Early detection and intervention of coronary artery involvement in immunoglobulin G4-related disease. Internal Med. (2018) 57:617–22. doi: 10.2169/internalmedicine.7816-16
37
KeraliyaARMurphyDJAghayevASteignerML. IgG4-related disease with coronary arteritis. Circulation: Cardiovasc Imaging. (2016) 9:e004583. doi: 10.1161/CIRCIMAGING.116.004583
38
UrabeYFujiiTKurushimaSTsujiyamaSKiharaY. Pigs-in-a-blanket coronary arteries: a case of immunoglobulin G4-related coronary periarteritis assessed by computed tomography coronary angiography, intravascular ultrasound, and positron emission tomography. Circulation: Cardiovasc Imaging. (2012) 5:685–7. doi: 10.1161/CIRCIMAGING.112.975946
39
RatwatteSDayMRidleyLJFungCNaoumCYiannikasJet al. Cardiac manifestations of IgG4-related disease: a case series. Eur Heart Journal-Case Rep. (2022) 6:ytac153. doi: 10.1093/ehjcr/ytac153
40
LiuXZhaoYWuNZhangW. Occult myocardial infarction due to an unusual cause: a case report of periarteritis involving the left coronary artery. Eur Heart Journal-Case Rep. (2022) 6:ytac182. doi: 10.1093/ehjcr/ytac182
41
Ansari-GilaniKGilkesonRC. Multimodality imaging of IgG4 related coronary artery aneurysm. Echocardiography. (2020) 37:979–81. doi: 10.1111/echo.14746
42
KosekiKYahagiKOkunoTKikushimaHSaitoANinomiyaKet al. Immunoglobulin G4-related coronary periarteritis with multiple intracoronary images. JACC: Cardiovasc Interventions. (2019) 12:e59–61. doi: 10.1016/j.jcin.2018.11.018
43
OkuyamaTTanakaTDNagoshiTYoshimuraM. Coronary artery disease concomitant with immunoglobulin G4-related disease: a case report and literature review. Eur Heart Journal-Case Rep. (2019) 3:ytz013. doi: 10.1093/ehjcr/ytz013
44
MatsudaJTakanoHShimizuW. IgG4-related periarteritis in the coronary artery and subclinical pericarditis assessed the presence and monitoring of therapy response by PET and CT scan. Case Rep. (2018) 2018:bcr–2018-225172. doi: 10.1136/bcr-2018-225172
45
RokutandaRNishihataYOkadaM. IgG4-related pericoronary arteritis. J Rheumatol. (2017) 44:1509–10. doi: 10.3899/jrheum.170262
46
SakamotoATanakaTHiranoKKoikeKKomuroI. Immunoglobulin G4-related coronary periarteritis and luminal stenosis in a patient with a history of autoimmune pancreatitis. Internal Med. (2017) 56:2445–50. doi: 10.2169/internalmedicine.8259-16
47
NishimuraSAmanoMIzumiCKurodaMYoshikawaYTakahashiYet al. Multiple coronary artery aneurysms and thoracic aortitis associated with IgG4-related disease. Internal Med. (2016) 55:1605–9. doi: 10.2169/internalmedicine.55.6314
48
ItoSHasuoTNimuraY. iMAP™ imaging of tumorous lesions surrounding the coronary arteries in a patient with an elevated serum level of immunoglobulin G4. Heart Vessels. (2016) 31:2061–7. doi: 10.1007/s00380-016-0852-8
49
BaruahDRubensteinJShahirK. ‘Coronary wrap’: IgG4 related disease of coronary artery presenting as a mass lesion. Int J Cardiovasc Imaging. (2014) 30:977–8. doi: 10.1007/s10554-014-0425-9
50
Kan-oMKadoYSadanagaATamiyaSToyoshimaSSakamotoMet al. Immunoglobulin G4-related multiple cardiovascular lesions successfully treated with a combination of open surgery and corticosteroid therapy. J Vasc Surg. (2015) 61:1599–603. doi: 10.1016/j.jvs.2013.10.106
51
NakamuraTGoryoYIsojimaTKawataH. Immunoglobulin G4-related masses surrounding coronary arteries: a case report. Eur Heart Journal-Case Rep. (2021) 5:ytab055. doi: 10.1093/ehjcr/ytab055
52
TajimaMHiroiYTakazawaYMuraokaHIwataHYamashitaHet al. Immunoglobulin G4–related multiple systemic aneurysms and splenic aneurysm rupture during steroid therapy. Hum Pathol. (2014) 45:175–9. doi: 10.1016/j.humpath.2013.07.035
53
BitoYSasakiYHiraiHHosonoMNakahiraASuehiroYet al. A surgical case of expanding bilateral coronary aneurysms regarded as immunoglobulin G4-related disease. Circulation. (2014) 129:e453–6. doi: 10.1161/CIRCULATIONAHA.114.008706
54
KubotaNOzakiKHoyanoMOkuboTKimuraSYanagawaTet al. Improvement of mass lesions around coronary arteries and fractional flow reserve after steroid therapy in immunoglobulin-G4-related coronary periarteritis. Internal Med. (2022) 61:351–6. doi: 10.2169/internalmedicine.7880-21
55
YadavAGodasuGBuxiTBSShethS. Multiple artery aneurysms: unusual presentation of IgG4 vasculopathy. J Clin Imaging Sci. (2021) 11:17. doi: 10.25259/JCIS_149_2020
56
IkawaAOkadaTYamashitaDFurukawaY. Progression of IgG4-related coronary aneurysm without corticosteroid treatment after surgical resection: A case report. J Cardiol cases. (2023) 27:275–8. doi: 10.1016/j.jccase.2023.02.012
57
BarbuMLindströmUNordborgCMartinssonADworeckCJeppssonAet al. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. (2017) 103(6):e487–9. doi: 10.1016/j.athoracsur.2016.12.048
58
NittaKHamamotoMFujiiTShokawaTZaitsuJ. Ten-year clinical observation of immunoglobulin G4-related coronary periarteritis with aneurysms. Int Heart J. (2023) 64:512–7. doi: 10.1536/ihj.23-035
59
WangYZhouHHuPZhaoJMaoYLiZet al. Case report: dual-energy computed tomography of cardiac changes in IgG4-related disease. Front Cardiovasc Med. (2022) 9:792531. doi: 10.3389/fcvm.2022.792531
60
PotaPSuwannasomPWoragidpoonpolSSrisuwanT. Coil embolization to giant left anterior descending artery and left circumflex artery coronary artery aneurysm after failed coronary aneurysmal repair in IgG4-related disease: a case report. Eur Heart Journal-Case Rep. (2021) 5:ytab452. doi: 10.1093/ehjcr/ytab452
61
TangMZhangZWangLQianHWuWLiuZet al. Coronary artery ectasia associated with IgG4-related disease: a case report and literature review. BMC Cardiovasc Disord. (2023) 23:347. doi: 10.1186/s12872-023-03369-7
62
SudoMFukamachiDYodaSOkumuraY. Coronary artery features of immunoglobulin-G4-related coronary periarteritis with multi-modality visualization. Circ J. (2019) 84:128. doi: 10.1253/circj.CJ-19-0477
63
TorresPPTESMagalhãesVOSpotonLRMarchioriE. Immunoglobulin G4-related systemic disease with vascular involvement. Vasc Med. (2018) 23:570–1. doi: 10.1177/1358863X18784663
64
YokoyamaTSuzukiRKurazumiHTanakaAKawanoHMikamoAet al. Open surgical treatment for aortic arch and coronary artery aneurysms associated with immunoglobulin G4-related disease: a case report. Gen Thorac Cardiovasc Surg cases. (2023) 2:47. doi: 10.1186/s44215-023-00063-0
65
YamauraHIshikawaHKasayukiNOtsukaK. Morphological and compositional alteration of pericoronary arteritis in a patient with immunoglobulin G4-related disease. Eur Heart Journal-Case Rep. (2022) 6:ytac027. doi: 10.1093/ehjcr/ytac027
66
TanigawaJDaimonMMuraiMKatsumataTTsujiMIshizakaNet al. Immunoglobulin G4–related coronary periarteritis in a patient presenting with myocardial ischemia. Hum Pathol. (2012) 43:1131–4. doi: 10.1016/j.humpath.2011.09.019
67
ChanS. A rare case of sudden death due to IgG4-related giant coronary artery aneurysms. J Forensic Sci. (2022) 67:363–9. doi: 10.1111/1556-4029.14824
68
HouraiRMiyamuraMTasakiRIwataATakedaYMoritaHet al. A case of IgG4-related lymphadenopathy, pericarditis, coronary artery periarteritis and luminal stenosis. Heart Vessels. (2016) 31:1709–13. doi: 10.1007/s00380-016-0794-1
69
de la FuenteJBirdJ. Coronary arteritis in IgG4-related disease. New Engl J Med. (2019) 380:2156–6. doi: 10.1056/NEJMicm1809588
70
TseY-HLoSS-WLauC-STseH-F. Regression of IgG4-related coronary arteritis and aortitis with immunosuppressive therapy. Eur Heart Journal-Case Rep. (2022) 6:ytac156. doi: 10.1093/ehjcr/ytac156
71
RoubertouYEuvrardR. Giant coronary aneurysm in IgG4–related disease. Eur Heart Journal-Case Rep. (2024) 8:ytae131. doi: 10.1093/ehjcr/ytae131
72
YoshinoYGodaAShimizuYMohriTTakeuchiSKoyamaKet al. Direct extension of igG4-related periarteritis of the coronary artery to the adjacent myocardium demonstrated using dual-energy cardiac computed tomography. Circ J. (2024) 88:434. doi: 10.1253/circj.CJ-23-0849
73
KiY-JChunEJYounT-J. Characteristic virtual histology-intravascular ultrasound imaging of immunoglobulin G4-related coronary artery disease patient. Korean Circ J. (2023) 53:189. doi: 10.4070/kcj.2022.0300
74
RathinasamyRGhatiNParakhNKumarSBisoiAKAravaSet al. South African flag sign to a giant coronary artery aneurysm. Eur Heart Journal-Case Rep. (2024) 8:ytae028. doi: 10.1093/ehjcr/ytae028
75
MendesGSMesquitaAERochaBAbecasisJRamosSTrabuloMet al. Relato de Caso de Doença Coronariana e Vascular Não Aterosclerótica: Em Busca de uma Entidade Clínica Rara. Arquivos Brasileiros Cardiol. (2022) 119:488–95. doi: 10.36660/abc.20210722
76
KamikawaYOhashiTTadakoshiMKojimaAYamauchiHHiokiKet al. Hybrid treatment of a giant coronary artery aneurysm in a patient with immunoglobulin G4-related disease. Gen Thorac Cardiovasc Surg. (2021) 69:1347–51. doi: 10.1007/s11748-021-01668-4
77
KaymakciMElfishawiMKosterMJHurstPDWarringtonKJ. Clinical Images: IgG4-related disease: a giant cell arteritis mimic. Arthritis Rheumatol. (2023) 75:1262. doi: 10.1002/art.42469
78
JinLFrahm-JensenG. A ruptured abdominal aortic aneurysm was just the tip of the iceberg–A rare case of IgG4-related disease and multiple giant aneurysms. Ann Vasc Surgery-Brief Rep Innov. (2023) 3:100227. doi: 10.1016/j.avsurg.2023.100227
79
IaconelliARovereGD’AmarioDGalliMSannaT. Aetiologic diagnosis of coronary artery aneurysm in a patient with pancreatitis, dacryoadenitis, and sialadenitis. Eur Heart J. (2023) 44:535–5. doi: 10.1093/eurheartj/ehac536
80
MohammadzadehAHoushmandGPouraliakbarHSoltaniZSalehabadiGAzimiAet al. Coronary artery involvement in a patient with IgG4-related disease. Radiol Case Rep. (2023) 18:3699–703. doi: 10.1016/j.radcr.2023.07.062
81
OdaNMatsuzoeHSatoSNishioRMatsumotoDAzamiTet al. Apical six chamber view: multiple giant coronary aneurysms associated with IgG4-related disease. J Echocardiography. (2024) 22:63–4. doi: 10.1007/s12574-023-00604-0
82
KoowattanatianchaiSTeawprasertNLimchareonSKaolawanichY. A rare case of immunoglobulin G4-related disease presenting with coronary artery pseudotumor and aneurysm. Eur J Case Rep Internal Med. (2023) 11:004215. doi: 10.12890/2023_004215
83
CaoJYangZ. IgG4-related disease involving coronary and pulmonary arteries: a case report and literature review. Cardiovasc Diagn Ther. (2023) 13:1128. doi: 10.21037/cdt-23-215
84
KawaharaHMizushimaIMatsumotoYSakataKTakamuraM. Solitary recurrence of IgG4-related giant coronary aneurysm: Case report and review of the literature focusing on treatment strategies and complications. Modern Rheumatol Case Rep. (2024) 8:182–94. doi: 10.1093/mrcr/rxad065
85
de Sainte MarieBBonnetGGonzalezSAppayRBarralPASchleinitzNet al. Coronary arteritis and aneurysm in a young adult revealing systemic igG4-related disease. Ann Internal Med: Clin cases. (2024) 3:e230846. doi: 10.7326/aimcc.2023.0846
86
TakeiYAbeNBohgakiMKasaharaH. Paradoxical enlargement of immunoglobulin G4 (IgG4)-related coronary aneurysms despite controlling serum IgG4 level. Eur Heart J. (2022) 43:2250–0. doi: 10.1093/eurheartj/ehac045
87
MiuraYKoyamaKKohnoTSoejimaKToriiSNakazawaGet al. Rapid progression of a coronary artery aneurysm caused by IgG4-related disease. Cardiovasc Pathol. (2024) 71:107647. doi: 10.1016/j.carpath.2024.107647
88
SuwaKOgawaNYoshizawaNFujihiroMSakamotoAOhtaniHet al. A case of coronary arteritis and myocardial involvement with associated IgG4-related disease. Case Rep. (2023) 14:101843. doi: 10.1016/j.jaccas.2023.101843
89
TsunetaSSunoKFujiedaYWatanabeMWatanabeSHirataKet al. Accumulation of technetium-99m tetrofosmin on myocardial perfusion scintigraphy in a patient with immunoglobulin G4-related coronary periarteritis. Can J Cardiol. (2024) 40:450–1. doi: 10.1016/j.cjca.2023.10.021
90
ByounJTLeeS-YChoJYYunKHOhSK. Spontaneous dissection in immunoglobulin G4-related coronary arteritis. Circulation: Cardiovasc Imaging. (2022) 15:e013958. doi: 10.1161/CIRCIMAGING.122.013958
91
NishijoDNakanishiKKonoMShimadaSKomuroI. A rare case of coronary involvement in igG4-related disease. Case Rep. (2024) 29:102254. doi: 10.1016/j.jaccas.2024.102254
92
YamauraHOtsukaKFukudaD. Rapidly progressed coronary aneurysm in immunoglobulin G4-related disease. Eur Heart Journal-Case Rep. (2023) 7:ytad278. doi: 10.1093/ehjcr/ytad278
93
OnoKNomuraTSakaueYWadaNKeiraNTatsumiTet al. Myocardial infarction due to immunoglobulin G4-related coronary artery aneurysm. Cardiovasc Intervent Ther. (2022) 37:405–6. doi: 10.1007/s12928-021-00799-y
94
MatsuyamaSKishigamiTSakamotoM. A case of giant right coronary artery aneurysm due to IgG4-related disease. Gen Thorac Cardiovasc Surg. (2020) 68:1453–6. doi: 10.1007/s11748-019-01272-7
95
FudimMThorpeMPChangLLSt ClairEWHurwitz KoweekLMWangAet al. Cardiovascular imaging with 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with fibroinflammatory disorders. JACC: Cardiovasc Imaging. (2018) 11:365–8. doi: 10.1016/j.jcmg.2017.10.020
96
XuXBaiWMaHDongPXieHShiYet al. Remission of “mistletoe sign” after treatment. J Cardiovasc Computed Tomography. (2020) 14:e118–9. doi: 10.1016/j.jcct.2019.08.002
97
KanzakiYMoritaHIshizakaN. Increased 18F-FDG uptake in IgG4-related coronary periarterial pseudotumor. Internal Med. (2017) 56:1603–4. doi: 10.2169/internalmedicine.56.8127
98
YongYRLathNCheahFKNgYL. Pictorial essay: uncommon causes of coronary artery encasement. J Cardiovasc Computed Tomography. (2016) 10:424–9. doi: 10.1016/j.jcct.2016.07.004
99
HouraiRKasashimaSFujitaSISohmiyaKDaimonMHiroseYet al. A case of aortic stenosis with serum IgG4 elevation, and IgG4-positive plasmacytic infiltration in the aortic valve, epicardium, and aortic adventitia. Int Heart J. (2018) 59:1149–54. doi: 10.1536/ihj.17-567
100
IbrahimTMuenzelDBabarykaGBarthelPThürmelK. Affection of the cardiovascular system by IgG4-related disease. Eur Heart Journal–Cardiovascular Imaging. (2017) 18:485–5. doi: 10.1093/ehjci/jew290
101
MatsudaKOshitaAKonoYMiyoshiTKawakamiHMatsuokaHet al. Intravascular ultrasound findings of immunoglobulin G4-related periarteritis. Cardiovasc Intervent Ther. (2019) 34:388–9. doi: 10.1007/s12928-019-00569-x
102
FujiiMSatoYOharaNHashimotoKKobashiHKoyamaYet al. Systemic IgG4-related disease with extensive peripheral nerve involvement that progressed from localized IgG4-related lymphadenopathy: an autopsy case. Diagn Pathol. (2014) 9:1–9. doi: 10.1186/1746-1596-9-41
103
KusunoseKHotchiJTakagawaYNishioSIseTTobiumeTet al. Serial imaging changes during treatment of immunoglobulin G4–related disease with multiple pseudotumors. Circulation. (2015) 131:1882–3. doi: 10.1161/CIRCULATIONAHA.115.015638
104
TranMNLangguthDHartGHeinerMRafterAFlemingSJet al. Doença sistêmica relacionada a IgG4 com arterite e aortite coronariana, causando isquemia coronariana crítica recorrente. Int J Cardiol. (2015) 201:33–4. doi: 10.1016/j.ijcard.2015.08.014
105
KoyanagawaKNayaMManabeOAnzaiT. What is this image? 2020: Image 6 result: A case of ventricular apical aneurysm with suspected IgG4-related arteritis on steroid therapy. J Nucl Cardiol. (2020) 27:719–22. doi: 10.1007/s12350-020-02155-8
106
RuggioAIaconelliAPanaioliEBernardiniFTinelliGSavinoGet al. Coronary artery aneurysms presenting as acute coronary syndrome: an unusual case of IgG4-related disease vascular involvement. Can J Cardiol. (2018) 34:1088.e1087–1088.e1010. doi: 10.1016/j.cjca.2018.04.026
107
HigashiHInabaSAzumaTSumimotoT. Effects of steroid therapy for IgG4-related coronary periarteritis. Internal Med. (2016) 55:1935–6. doi: 10.2169/internalmedicine.55.6484
108
WongPCFungATGerrieASMoloneyGMaberleyDRossmanDet al. I gG4-related disease with hypergammaglobulinemic hyperviscosity and retinopathy. Eur J Haematol. (2013) 90:250–6. doi: 10.1111/ejh.12059
109
BilginBYesilkayaYTurkerBVaranOAydinOIsikMet al. Periaortitis, coronary vasculitis and retro-orbital pseudotumor in a patient with immunoglobulin G4-related sclerosing vasculitis. Turkish J Rheumatol. (2012) 27. doi: 10.5606/tjr.2012.008
110
TakeiHNagasawaHSakaiRNishimuraKKurasawaTOkuyamaAet al. A case of multiple giant coronary aneurysms and abdominal aortic aneurysm coexisting with IgG4-related disease. Internal Med. (2012) 51:963–7. doi: 10.2169/internalmedicine.51.6944
111
TaguchiTNishiHKitaharaMShirasakiYYoshitatsuM. Surgical treatment for giant multiple coronary artery aneurysms caused by an igG4-related disease. Cureus. (2024) 16:e60115. doi: 10.7759/cureus.60115
112
ZhangLJZhouF. A patient with multiple coronary artery aneurysms. JAMA Cardiol. (2019) 4:388–9. doi: 10.1001/jamacardio.2018.4268
113
DebonnairePBammensBBlockmansDHerregodsMCDuboisCVoigtJUet al. Multimodality imaging of giant coronary artery aneurysms in immunoglobulin g4-related sclerosing disease. J Am Coll Cardiol. (2012) 59:e27–7. doi: 10.1016/j.jacc.2011.06.085
114
MingoiaGAstutiGOrlandoEComparatoFFarinaCD’AngeloFet al. COronary artery aneurysm iN IGG4–related disease. Eur Heart J Suppl. (2024) 26:ii185. doi: 10.1093/eurheartjsupp/suae036.448
115
DuYZhengYZhangHDuJZhangXXuLet al. Perivascular fat attenuation index mapping and tracking immunoglobulin G4-related coronary arteritis. Circulation: Cardiovasc Imaging. (2023) 16:e014912. doi: 10.1161/CIRCIMAGING.122.014912
116
ZhengYTanAWYongTHChaiSC. Giant coronary aneurysms in a patient with immunoglobulin G4-related disease. Circulation: Cardiovasc Imaging. (2020) 13:e010213. doi: 10.1161/CIRCIMAGING.119.010213
117
ChiengNDYapJLinM. Coronary IgG4: PET pigs in blankets. Circulation: Cardiovasc Imaging. (2022) 15:e014314. doi: 10.1161/CIRCIMAGING.122.014314
118
Delgado-GarcíaGSánchez-SalazarSRendón-RamírezECastro-MedinaMSáenz-IbarraBBarboza-QuintanaÁet al. Myocardial ischemia as presenting manifestation of IgG4-related disease: a case-based review. Clin Rheumatol. (2016) 35:2857–64. doi: 10.1007/s10067-016-3292-z
119
ZhangBLiX. Coronary arteritis in immunoglobulin G4–related disease. Radiology. (2024) 312:e240180. doi: 10.1148/radiol.240180
120
OtaniTMatsumotoYInoueKTanakaHHamadaM. Morphological characterization of coronary arteries in immunoglobulin G4-related disease estimated by using computed tomography and magnetic resonance imaging. Int J Cardiol. (2016) 215:111–3. doi: 10.1016/j.ijcard.2016.04.060
121
AkiyamaMTakeuchiT. IgG4-related disease: beyond glucocorticoids. Drugs Aging. (2018) 35:275–87. doi: 10.1007/s40266-018-0534-6
122
AkiyamaMKanekoYTakeuchiT. Characteristics and prognosis of IgG4-related periaortitis/periarteritis: a systematic literature review. Autoimmun Rev. (2019) 18:102354. doi: 10.1016/j.autrev.2019.102354
123
XieYKimYJPangJKimJSYangQWeiJet al. Coronary atherosclerosis T1-weighed characterization with integrated anatomical reference: comparison with high-risk plaque features detected by invasive coronary imaging. JACC: Cardiovasc Imaging. (2017) 10:637–48. doi: 10.1016/j.jcmg.2016.06.014
124
KawsaraANúñez GilIJAlqahtaniFMorelandJRihalCSAlkhouliMet al. Management of coronary artery aneurysms. JACC: Cardiovasc Interventions. (2018) 11:1211–23. doi: 10.1016/j.jcin.2018.02.041
125
MaritatiFPeyronelFVaglioA. IgG4-related disease: a clinical perspective. Rheumatology. (2020) 59:iii123–31. doi: 10.1093/rheumatology/kez667
126
KaradağÖErdenAAyhanEABölekEÇKalyoncuUArmağanBet al. The clinical features and outcomes of Turkish patients with IgG4-related disease: a single-center experience. Turkish J Med Sci. (2017) 47:1307–14. doi: 10.3906/sag-1704-150
127
QiLMaoDXiaoLJinXLiMHuaYet al. Immunoglobulin G4-related disease complicated with vascular lesions: CT findings in 21 patients. Diagn Interventional Radiol. (2019) 25:42. doi: 10.5152/dir.2018.18174
128
DuYDingSLiCBaiYWangXLiDet al. Coronary artery wall contrast enhancement imaging impact on disease activity assessment in IgG4-RD: a direct marker of coronary involvement. J Cardiovasc Magnetic Resonance. (2024) 26:101047. doi: 10.1016/j.jocmr.2024.101047
129
IsmailTF. Can cardiovascular magnetic resonance enhance our understanding of coronary involvement in immunoglobulin subclass 4-related disease? J Cardiovasc Magnetic Resonance. (2024) 26:101063. doi: 10.1016/j.jocmr.2024.101063
130
TouzaniFPozdzikA. New insights into immune cells cross-talk during IgG4-related disease. Clin Immunol. (2019) 198:1–10. doi: 10.1016/j.clim.2018.11.004
131
KasashimaSKawashimaAKuroseNOzakiSKasashimaFMatsumotoYet al. Disordered balance of T-cell subsets in arterial tertiary lymphoid organs in immunoglobulin G4–related vascular disease. J Am Heart Assoc. (2023) 12:e030356. doi: 10.1161/JAHA.123.030356
132
HuYXuQ. Adventitial biology: differentiation and function. Arteriosclerosis Thrombosis Vasc Biol. (2011) 31:1523–9. doi: 10.1161/ATVBAHA.110.221176
133
Van de PavertSAMebiusRE. New insights into the development of lymphoid tissues. Nat Rev Immunol. (2010) 10:664–74. doi: 10.1038/nri2832
134
WeihFGräbnerRHuDBeerMHabenichtAJ. Control of dichotomic innate and adaptive immune responses by artery tertiary lymphoid organs in atherosclerosis. Front Physiol. (2012) 3:226. doi: 10.3389/fphys.2012.00226
135
AkhavanpoorMGleissnerCAAkhavanpoorHLasitschkaFDoeschAOKatusHAet al. Adventitial tertiary lymphoid organ classification in human atherosclerosis. Cardiovasc Pathol. (2018) 32:8–14. doi: 10.1016/j.carpath.2017.08.002
136
ZhangWStoneJH. Management of igG4-related disease. Lancet Rheumatol. (2019) 1:e55–65. doi: 10.1016/S2665-9913(19)30017-7
137
Oyama-ManabeNYabusakiSManabeOKatoFKanno-OkadaHKudoKet al. IgG4-related cardiovascular disease from the aorta to the coronary arteries: multidetector CT and PET/CT. Radiographics. (2018) 38:1934–48. doi: 10.1148/rg.2018180049
138
PeruginoCAWallaceZSMeyersohnNOliveiraGStoneJRStoneJHet al. Large vessel involvement by IgG4-related disease. Medicine. (2016) 95:e3344. doi: 10.1097/MD.0000000000003344
139
VaglioASalvaraniCBuzioC. Retroperitoneal fibrosis. Lancet. (2006) 367:241–51. doi: 10.1016/S0140-6736(06)68035-5
140
VaglioAMaritatiF. Idiopathic retroperitoneal fibrosis. J Am Soc Nephrol. (2016) 27:1880–9. doi: 10.1681/ASN.2015101110
141
WangJWangYWangJGuoXChanECJiangFet al. Adventitial activation in the pathogenesis of injury-induced arterial remodeling: potential implications in transplant vasculopathy. Am J Pathol. (2018) 188:838–45. doi: 10.1016/j.ajpath.2017.12.002
142
StoneJR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol. (2011) 23:88–94. doi: 10.1097/BOR.0b013e3283412f7c
143
WallaceZSDeshpandeVMattooHMahajanVSKulikovaMPillaiSet al. IgG4-related disease: baseline clinical and laboratory features in 125 patients with biopsy-proven disease. Arthritis Rheumatol (Hoboken NJ). (2015) 67:2466. doi: 10.1002/art.39205
144
KikuchiSOkadaKHibiKMaejimaNYabuNUchidaKet al. Coronary arteritis: a case series. Eur Heart Journal-Case Rep. (2020) 4:1–6. doi: 10.1093/ehjcr/ytaa011
145
ShakirAWheelerYKrishnaswamyG. The enigmatic immunoglobulin G4-related disease and its varied cardiovascular manifestations. Heart. (2021) 107:790–8. doi: 10.1136/heartjnl-2020-318041
146
HuangHLFongWPehWMNirajKALamWW. The utility of FDG PET/CT in IgG4-related disease with a focus on coronary artery involvement. Nucl Med Mol Imaging. (2018) 52:53–61. doi: 10.1007/s13139-017-0494-5
147
ZerizerITanKKhanSBarwickTMarzolaMCRubelloDet al. Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol. (2010) 73:504–9. doi: 10.1016/j.ejrad.2010.01.021
148
AimiHMuraiTTakinoKNaitoWMiuraIHataMet al. A unique adverse event of radiotherapy in a patient with igG4-related disease: A case report. In Vivo. (2023) 37:2840–4. doi: 10.21873/invivo.13399
149
ZhuSWangYTangJCaoM. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front Immunol. (2022) 13:1074477. doi: 10.3389/fimmu.2022.1074477
150
CaiSHuZChenYZhongJDongL. Potential roles of non-lymphocytic cells in the pathogenesis of IgG4-related disease. Front Immunol. (2022) 13:940581. doi: 10.3389/fimmu.2022.940581
151
WeinrichJMLenzAAdamGFrançoisCJBannasP. Radiologic imaging in large and medium vessel vasculitis. Radiol Clinics. (2020) 58:765–79. doi: 10.1016/j.rcl.2020.02.001
152
YeonSBSabirAClouseMMartinezclarkPOPetersDCHauserTHet al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J Am Coll Cardiol. (2007) 50:441–7. doi: 10.1016/j.jacc.2007.03.052
153
KasashimaSZenYKawashimaAEndoMMatsumotoYKasashimaFet al. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg. (2009) 49:1264–71. doi: 10.1016/j.jvs.2008.11.072
154
PabstRTschernigT. Perivascular capillaries in the lung: an important but neglected vascular bed in immune reactions? J Allergy Clin Immunol. (2002) 110:209–14. doi: 10.1067/mai.2002.126836
155
GräbnerRLötzerKDöppingSHildnerMRadkeDBeerMet al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE–/– mice. J Exp Med. (2009) 206:233–48. doi: 10.1084/jem.20080752
156
KorosoglouGWeissRGKedziorekDAWalczakPGilsonWDSchärMet al. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J Am Coll Cardiol. (2008) 52:483–91. doi: 10.1016/j.jacc.2008.03.063
157
WuMLiXGuoQLiJXuGLiGet al. Magnetic mesoporous silica nanoparticles-aided dual MR/NIRF imaging to identify macrophage enrichment in atherosclerotic plaques. Nanomed: Nanotechnol Biol Med. (2021) 32:102330. doi: 10.1016/j.nano.2020.102330
158
MasakiYMatsuiSSaekiTTsuboiHHirataSIzumiYet al. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Modern Rheumatol. (2017) 27:849–54. doi: 10.1080/14397595.2016.1259602
159
Della-TorreEFeeneyEDeshpandeVMattooHMahajanVKulikovaMet al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. (2015) 74:2236–43. doi: 10.1136/annrheumdis-2014-205799
160
LanzillottaMFernàndez-CodinaACulverEEbboMMartinez-ValleFSchleinitzNet al. Emerging therapy options for IgG4-related disease. Expert Rev Clin Immunol. (2021) 17:471–83. doi: 10.1080/1744666X.2021.1902310
161
KhosroshahiAWallaceZSCroweJLAkamizuTAzumiACarruthersMNet al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. (2015) 67:1688–99. doi: 10.1002/art.39132
162
MizushimaIInoueDYamamotoMYamadaKSaekiTUbaraYet al. Clinical course after corticosteroid therapy in IgG4-related aortitis/periaortitis and periarteritis: a retrospective multicenter study. Arthritis Res Ther. (2014) 16:1–11. doi: 10.1186/ar4671
163
KhosroshahiAStoneJH. Treatment approaches to IgG4-related systemic disease. Curr Opin Rheumatol. (2011) 23:67–71. doi: 10.1097/BOR.0b013e328341a240
Summary
Keywords
IgG4-related disease, IgG4-related coronary arteritis, imaging, prognosis, cardiovascular
Citation
Wang G, Du Y, Bai Y, Zhou Y, Ding S, Wang X, Xie Y, Yang H-j, Li D, Fan Z, Fan G, Lou Z, Wei J and Sun Y (2025) Clinical characteristics and multimodal imaging insights of coronary involvement in immunoglobulin G4–related disease. Front. Immunol. 16:1685508. doi: 10.3389/fimmu.2025.1685508
Received
14 August 2025
Revised
13 November 2025
Accepted
19 November 2025
Published
04 December 2025
Volume
16 - 2025
Edited by
Kirill Peskov, Modeling & Simulation Decisions FZ-LLC, United Arab Emirates
Reviewed by
Gianluca Moroncini, Marche Polytechnic University, Italy
Nilesh C. Sharma, Western Kentucky University, United States
Updates
Copyright
© 2025 Wang, Du, Bai, Zhou, Ding, Wang, Xie, Yang, Li, Fan, Fan, Lou, Wei and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxian Sun, sunyingxian12@yahoo.com.cn; Jiayi Wei, weijycmu@163.com; Zhe Lou, blackpoppy@live.cn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.