- 1Department of Nephrology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 2College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou, China
- 3National Health Commission (NHC) Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, China
Introduction: M-type phospholipase A2 receptor (PLA2R) is the predominant autoantigen in primary membranous nephropathy (PMN), accounting for approximately 70%–80% of cases. Circulating anti-PLA2R IgG is a widely used biomarker to monitor disease activity and treatment. In recent years, antibodies targeting specific PLA2R domains and epitope spreading of PLA2R have been identified and suggested to be correlated with disease severity and resistance to treatment. However, its clinical relevance remains controversial. This study aimed to evaluate whether epitope spreading offers superior prognostic value compared to total anti-PLA2R IgG levels in patients with PMN.
Methods: This retrospective study enrolled 74 patients with biopsy-proven PMN who underwent at least 6 months of follow-up. Clinical data and serum samples were collected at baseline (M0), 6 months (M6), and 12 months (M12). PLA2R-IgG, domain-specific antibodies (CysR-, CTLD1-, and CTLD7/8-IgG/IgG4), and anti-rituximab antibodies (ARAs) were measured using time-resolved fluorescence immunoassay. Logistic regression and receiver operating characteristic curve analyses were used to assess prognostic factors and model performance. The patients were divided into cyclophosphamide (CTX) and rituximab (RTX) treatment groups.
Results: There were no significant differences in remission rates between the groups at M6 (CTX: 37.9% vs. RTX: 60.0%, P = 0.875) or M12 (61.5% vs. 75.6%, P = 0.220). However, the RTX group showed faster antibody clearance at M6 and a significantly higher immunological remission rate at M12 (96.2% vs. 65.6%, P = 0.017). In the RTX group, epitope spreading significantly decreased at M6 (P = 0.004), and four patients (22.2%) with no clinical remission were ARA-positive. Multivariate logistic regression analysis identified epitope spreading as an independent risk factor for non-remission at M6 (P = 0.031; AUC = 0.932). All four ARA-positive patients achieved partial or complete remission within 3–9 months after switching to obinutuzumab.
Discussion: Compared with CTX, RTX induced a higher rate of immunologic remission at M12. Epitope spreading of PLA2R was identified as an independent risk factor for clinical remission after 6 months of treatment with RTX.
1 Introduction
Membranous nephropathy (MN) is classified as either primary membranous nephropathy (PMN) or secondary membranous nephropathy according to the etiological classification and generally manifests as significant proteinuria (>3.5 g/24 h), hypoalbuminemia (<30 g/L), edema, hyperlipidemia, and other metabolic disorders (1, 2). PMN is an autoimmune renal glomerular disease mediated by the autoantigen antibody complex, among which phospholipase A2 receptor (PLA2R)-associated PMN accounts for 70%–80% (3).
According to the KDIGO guidelines, serum PLA2R-IgG is recommended as an efficient biomarker for risk stratification and efficacy determination in PMN. However, there are still some open questions, such as understanding the mechanisms of epitope spreading and immunodominance and determining whether the analysis of epitope reactivity has a greater predictive value than that of the PLA2R-antibody level (4). To date, a range of domain-specific epitopes of the PLA2R antigen have been identified through the discovery of specific antibodies for the PLA2R-CysR, PLA2R-CTLD1, and PLA2R-CTLD7/-CTLD8 domains. In 2016, Polski et al. demonstrated that both anti-PLA2R antibodies and epitope spreading could independently predict poor renal prognosis and serve as prognostic tools to monitor disease severity (5). However, different perspectives exist regarding the clinical significance of epitope spreading. In 2020, Reinhard et al. observed that clinical outcomes were only related to total anti-PLA2R antibody levels at the time of diagnosis but not to specific domain antibodies (6). Our previous study revealed that PLA2R-CTLD1-IgG4 might be a more effective biomarker to predict proteinuria remission at the 6th month of treatment in PMN compared with total PLA2R-IgG (7). Therefore, more studies are needed to confirm the association of epitope spreading and domain-specific antibodies with clinical remission and prognosis.
In addition, rituximab (RTX), a CD20 monoclonal antibody that eliminates autoantibodies by depleting B cells, has been recommended for patients with moderate- and high-risk PMN (4). Various studies on RTX used in PMN have also confirmed its efficacy, achieving clinical remission in 60%–80% of PMN patients (8). However, 20%–40% of PMN patients still develop resistance during RTX treatment, which may be related to the production of anti-rituximab antibodies (ARAs) (8). In 2020, Boyer-Suavet et al. enrolled 44 MN patients who were treated with RTX. Anti-rituximab antibodies were detected in 10 patients. Moreover, the rate of relapse was significantly higher in patients with ARAs (9). Changes in treatment regimens may be required for these patients. Studies on obinutuzumab, a humanized monoclonal antibody, in RTX treatment failure patients are ongoing progressively (10).
In this study, we aimed to elucidate whether epitope spreading offers superior clinical predictive value compared to PLA2R antibody levels, which will help facilitate the development of more accurate clinical prediction models in PMN.
2 Materials and methods
2.1 Patients and samples
In this retrospective study, the PMN cohort of 74 patients was followed up for at least 6 months. All patients were confirmed by renal biopsy during hospitalization in The Affiliated Wuxi People’s Hospital of Nanjing Medical University from January 2020 to December 2023 and positive for PLA2R antibody. Serum specimens were obtained at baseline (M0) and 6 (M6) and 12 months (M12) of treatment, which were processed through standardized centrifugation (3,000 rpm for 5 min), cryopreserved at -80 °C, and subsequently subjected to batch analysis in the central laboratory.
All patients provided signed informed consent. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (ethical approval no. kyl2016001).
2.2 Interventions and follow-up
Risk stratification and therapeutic regimen options were based on the KDIGO guideline. Patients in the RTX group received intravenous RTX (1,000 mg) on days 1 and 15. If complete remission (CR) was not achieved or CD19+ B cell counts exceeded 5 cells/mL after 6 months, an additional treatment with RTX (375 mg/m2) was administered. Patients who remained no remission at M6 and tested positive for ARA were switched to obinutuzumab treatment (1,000 mg on days 1 and 15, respectively). If complete remission (CR) was not achieved or the CD19+ B cell counts exceeded 5 cells/mL after 6 months, an additional treatment with obinutuzumab (375 mg/m2) was administered. The cyclophosphamide (CTX) group was administered with intravenous CTX (0.8–1.0 g/month) combined with oral prednisone (0.6–0.8 mg/kg/day adjusted for clinical response). All patients were treated with the maximum tolerated dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and followed up regularly. At M6 and M12, serum specimens were obtained from the patients before the next treatment to detect the PLA2R epitope-specific antibody (PLA2R-IgG, PLA2R-IgG4, PLA2R-CysR-IgG/IgG4, PLA2R-CTLD1-IgG/IgG4, and PLA2R-CTLD678-IgG/IgG4) anti-rituximab antibody and understand the epitope spreading.
2.3 Detection of PLA2R and its domain antibodies
Purified PLA2R and domain-specific proteins (CysR, CTLD1, and CTLD678) were coated onto 96-well plates (2 μg/mL, 100 μL/well) and incubated overnight at 4 °C. The next day, the plates were washed once with wash buffer, then 150 μL blocking buffer was added to each well, and the plates were blocked at room temperature for 2 h. After blocking, the plates were dried and stored at -20 °C.
Serum samples were diluted to 1:100 for -IgG antibodies and 1:20 for -IgG4 antibodies, respectively, in an assay buffer and then added to the antigen-coated plates in quadruplicate and incubated on a shaker at 25 °C for 1 h. After three washes with wash buffer, Eu3+-labeled goat anti-human IgG antibodies (diluted 1:100 in assay buffer) and mouse anti-human IgG4 antibodies (1:100 dilution) were added to the plates (100 μL/well) and administered according to previous protocols. After six washes, 200 μL of enhancement solution was added to the plates and agitated on a shaker at 25 °C for 5 min.
Fluorescence was measured using a DR6608 time-resolved fluorescence immunoassay (TRFIA) analyzer. The cutoff values for serum PLA2R-IgG and PLA2R-IgG4 levels were 14 and 200 ng/mL, respectively. For serum PLA2R-CysR/-CTLD1/-CTLD678-IgG, the cutoff values were 10.02, 16.01, and 14.38 RU/mL, respectively. Similarly, the cutoff values for serum PLA2R-CysR/-CTLD1/-CTLD678-IgG4 were 100, 50, and 100 RU/mL, respectively (11–14).
2.4 Detection of RTX antibody
TRFIA for RTX antibodies was performed using the bridging capture method (15). RTX was diluted to a concentration of 0.125 μg/mL in coating buffer, added to 96-well plates (100 μL/well), and incubated overnight at 4 °C. The next day, the plates were washed once with wash buffer, blocking buffer was added to each well, and the plates were blocked at room temperature for 2 h. After blocking, the plates were dried and stored at -20 °C. RTX was labeled with Eu3+ using a Eu3+ labeling kit according to the manufacturer’s instructions and diluted to the concentration of 1:400. Anti-rituximab idiotypic antibodies were diluted to standards in an assay buffer. Standards or serum samples (100 μL/well) were added to RTX-coated plates and incubated with agitation at 37 °C for 60 min. After two washes, 100 μL of diluted Eu3+-labeled RTX solution was added to each well. Subsequently, the plates were incubated with agitation at 37 °C for 60 min again and washed six times. Furthermore, 100 μL of enhancement solution was added per well, and the plates were shaken at 25 °C for 5 min. Finally, fluorescence was measured using an HG-1000 TRFIA analyzer. The cutoff value for serum ARAs was 2 ng/mL (15).
2.5 Definition
Remission criteria: (1) partial remission (PR) characterized by proteinuria reduction ≥50% from baseline with 24-h urinary protein <3.5 g, accompanied by stable renal function (serum creatinine fluctuation <20%); (2) CR required achievement of 24-h urinary protein <0.3 g with a normal glomerular filtration rate (GFR ≥90 mL/min/1.73 m²) or stabilization of pre-existing GFR (variation <10%); (3) non-response (NR) was defined as failure to achieve ≥50% proteinuria reduction from baseline or deterioration in renal function (serum creatinine increase >20% from baseline); and (4) immune remission was defined as PLA2R-IgG <2 RU/mL (ELISA).
Epitope spreading was defined as positive for PLA2R-CysR-IgG4 and positive for either PLA2R-CTLD1-IgG4 or PLA2R-CTLD678-IgG4 (5).
2.6 Statistical analyses
Statistical analyses were performed using SPSS version 27.0. To minimize data variability, PLA2R and domain-specific antibodies were natural-log-transformed. In descriptive statistics, the variables were assessed for normality using Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation (SD), non-normally distributed variables as median (interquartile range), and categorical variables as frequencies with percentages (n [%]). Qualitative variables were compared using chi-square test or Fisher’s exact test, whereas quantitative variables were compared using t-test or Wilcoxon–Mann–Whitney test. Chi-squared test or one-way analysis of variance was used to assess inter-sample differences. Pearson or Spearman correlation coefficient was used to evaluate correlations. Logistic regression analysis was performed to identify potential risk or protective factors for treatment response. The validity of the model was evaluated using receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) as the primary discriminative metric. All probabilities were two-sided, and statistical significance was set at P < 0.05. These P-values were corrected for multiple comparisons by Benjamini–Hochberg to control the false discovery rate (FDR) at the 10% level.
3 Results
3.1 Characteristics of patients at baseline (M0)
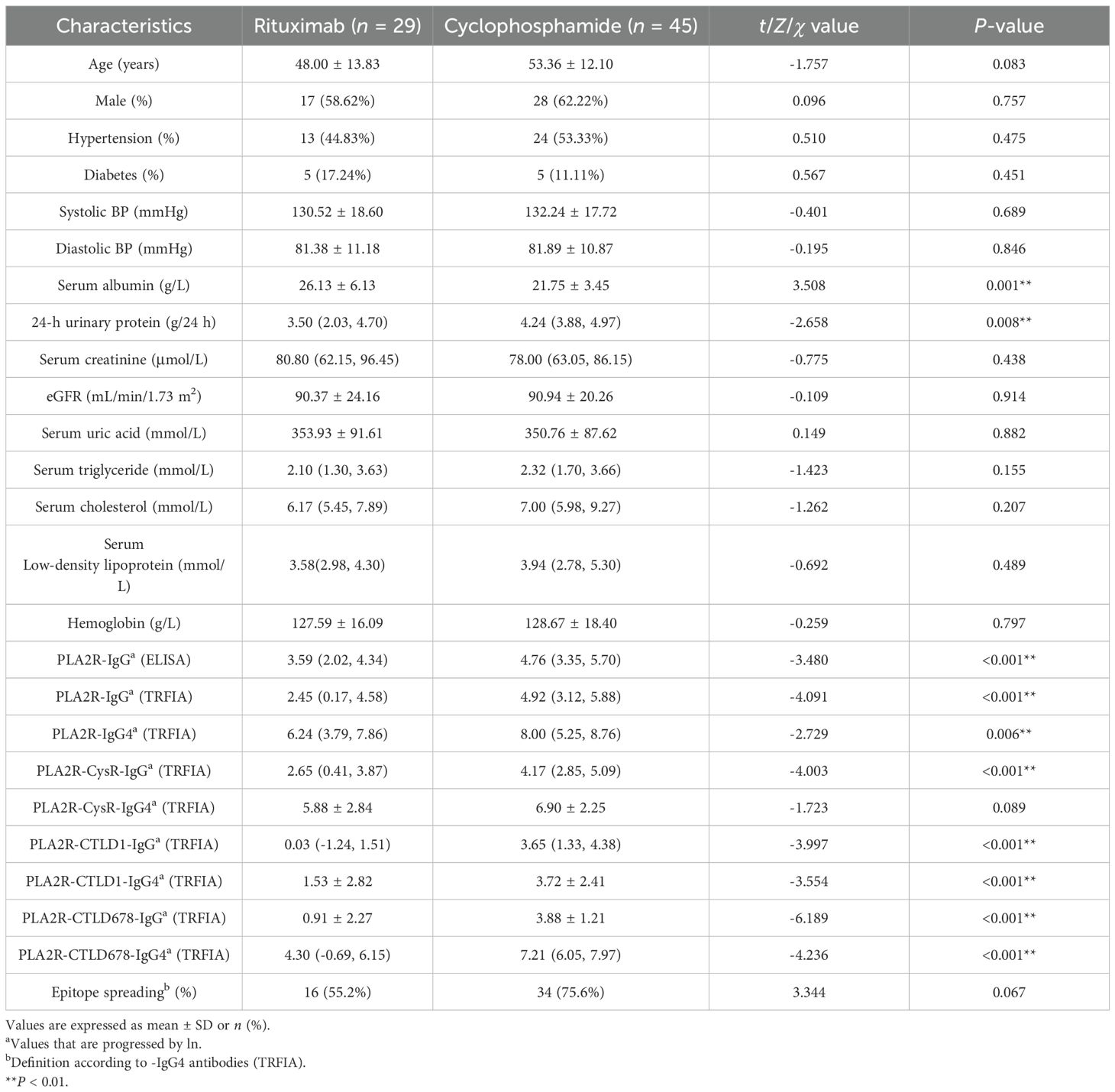
A total of 74 patients with PMN diagnosed by renal biopsy were enrolled in this study. Compared with the RTX group, the CTX group appeared to have lower serum albumin (26.13 ± 6.13 vs. 21.75 ± 3.45, P = 0.001) and higher 24-h urinary protein levels [3.50 (2.03, 4.70) vs. 4.24 (3.88, 4.97), P = 0.008)]. Furthermore, PLA2R-IgG/-IgG4 and some PLA2R domain-specific antibodies showed statistically significant differences between the two groups (Table 1).
3.2 Characteristics of patients at M6 and M12 of treatment
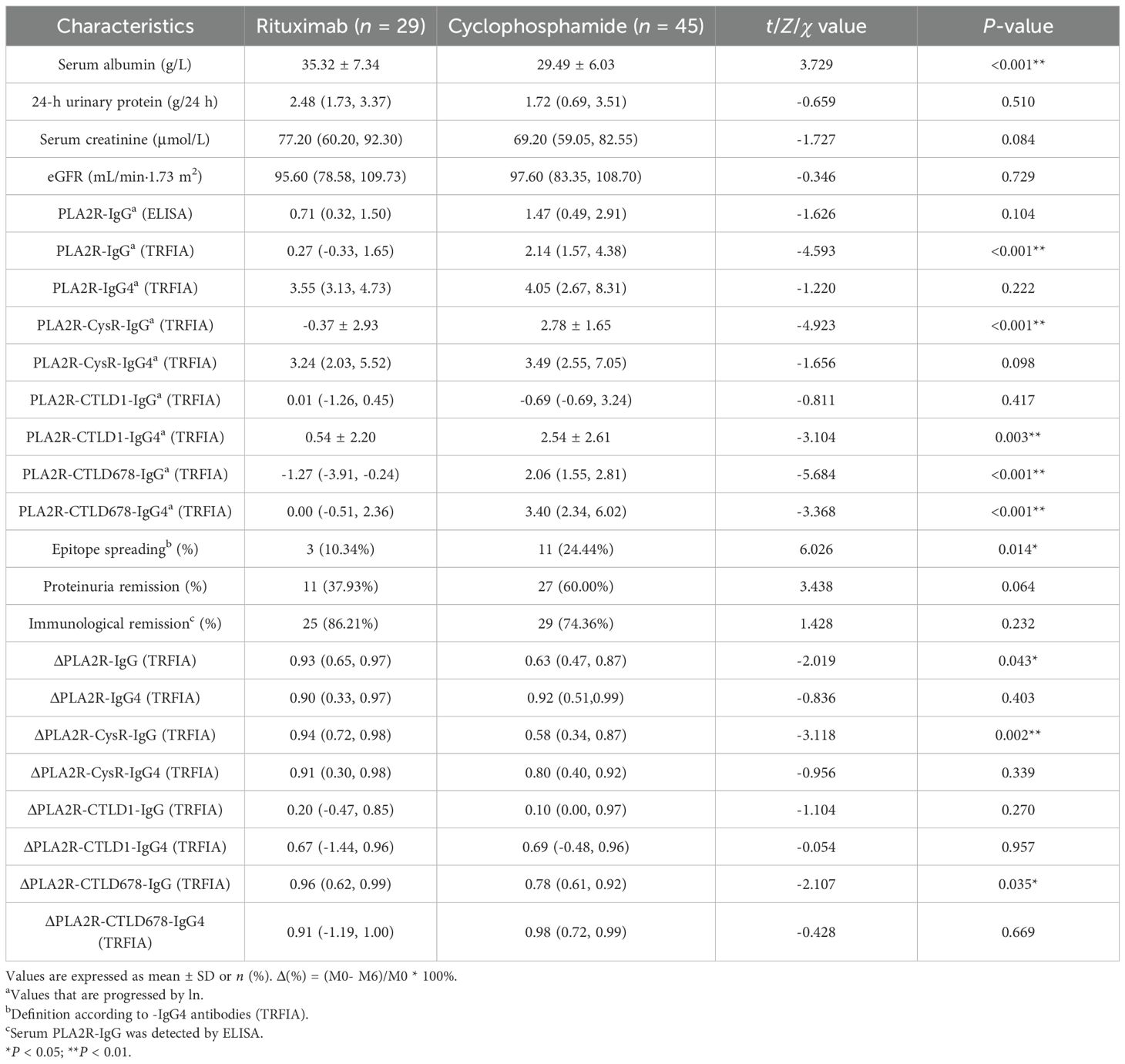
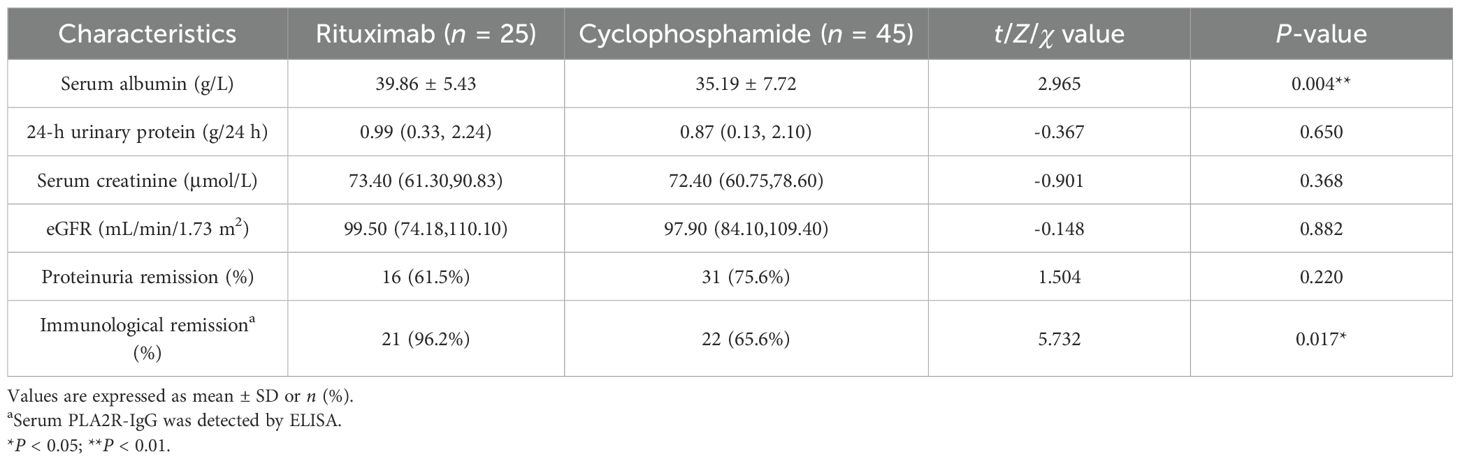
Comparing the RTX and CTX groups, although a discrepancy in serum albumin level still existed (35.06 ± 7.33 vs. 29.49 ± 6.03, P < 0.001) at M6, the 24-h urinary protein levels showed no significant statistical difference at M6 (P = 0.379) and M12 (P = 0.714) (Tables 2, 3) Both the CTX and RTX groups showed a decreasing trend in proteinuria over the 12-month treatment period, while serum albumin levels exhibited an increasing trend (Supplementary Figure S1).
Moreover, PLA2R-IgG (P < 0.001), PLA2R-CysR-IgG (P < 0.001), PLA2R-CTLD678-IgG (P < 0.001), and PLA2R-CTLD678-IgG4 (P < 0.001) showed lower levels in the RTX group, which had fewer patients with epitope spreading (20.7% vs. 26.7%, P < 0.001) at M6. Although no statistical differences in proteinuria remission rates were found at M6 (37.9% vs. 60.0%, P = 0.875) and M12 (61.5% vs. 75.6%, P = 0.220) in the two groups, the immunological remission rates (96.2% vs. 65.6%, P = 0.017) defined by PLA2R-IgG (ELISA) at M12 was higher in the RTX group. Simultaneously, as shown in the scatter plot and the table, the clearance rates from M0 to M6 of antibodies, including PLA2R-IgG (P = 0.043), PLA2R-CysR-IgG (P = 0.002), and PLA2R-CTLD678-IgG (P = 0.035) were also higher in the RTX group (Tables 2, 3; Supplementary Figures S2, S3).
3.3 Analysis of remission in the RTX group
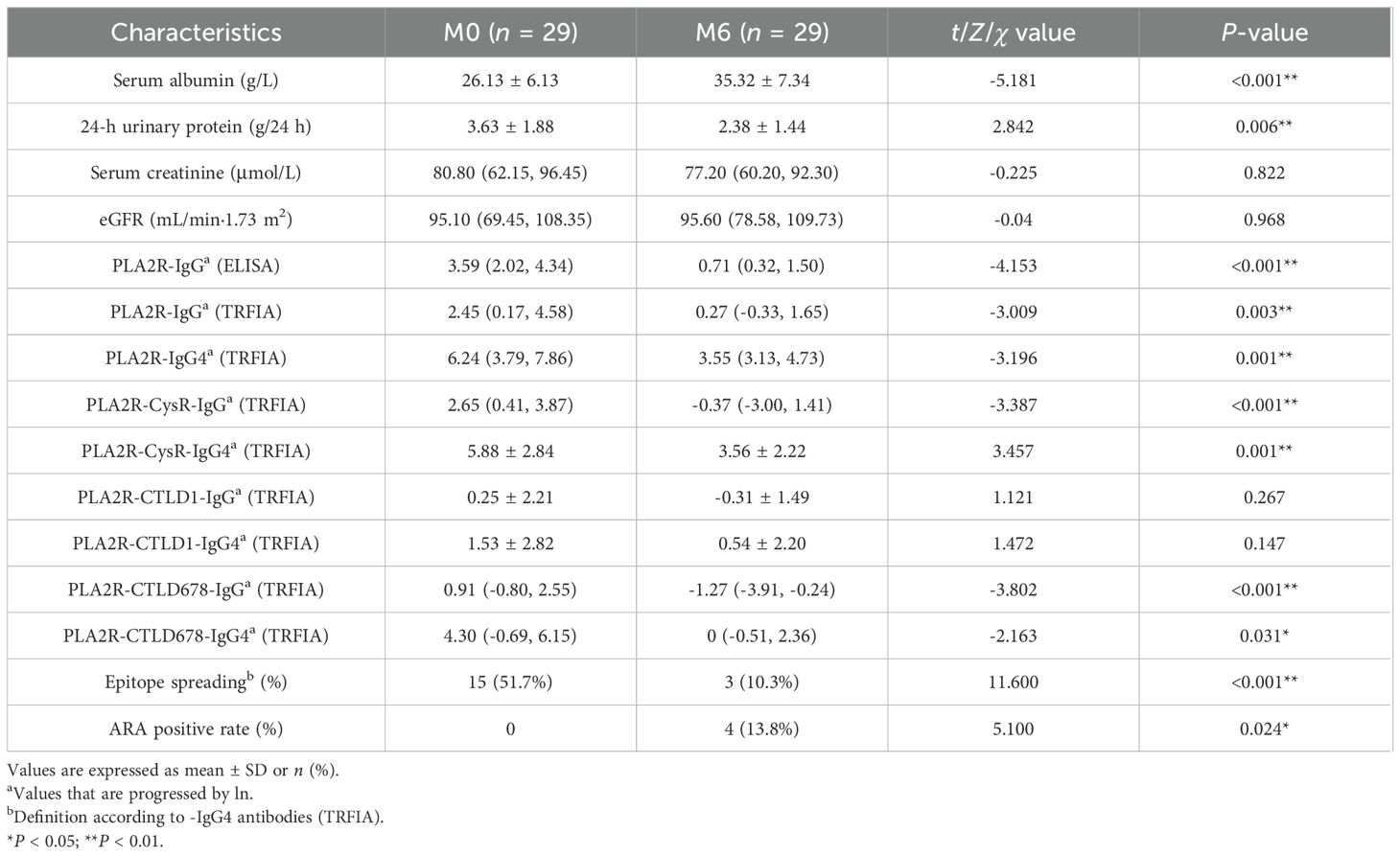
In the RTX group, the serum albumin levels (P < 0.001) decreased significantly at M6, whereas the level of proteinuria showed no statistical difference. Compared with the data at baseline, PLA2R-IgG (ELISA) (P <0.001), PLA2R-IgG (TRFIA) (P = 0.003), PLA2R-IgG4 (P = 0.001), PLA2R-CysR-IgG (P<0.001), PLA2R-CysR-IgG4 (P = 0.001), PLA2R-CTLD678-IgG (P < 0.001), and PLA2R-CTLD678-IgG4 (P = 0.031) significantly declined at 6 months, and epitope spreading also appeared to be significantly reduced (P = 0.027). After 6 months of RTX therapy, ARAs were detected in four patients (Table 4).
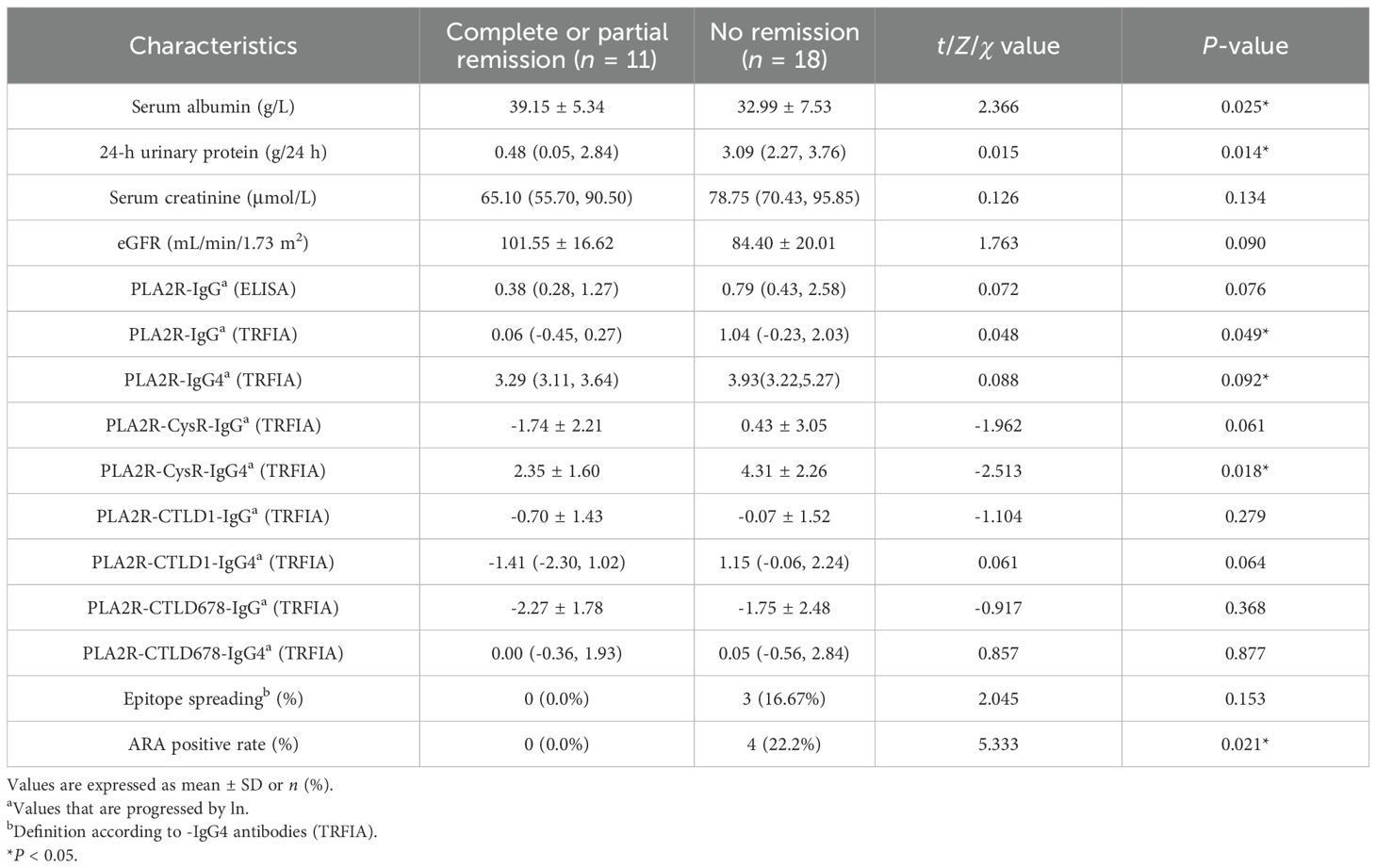
A further comparison between the proteinuria remission and non-remission groups after 6 months of RTX treatment revealed that PLA2R-IgG (TRFIA) (P = 0.049) and PLA2R-CysR-IgG4 (P = 0.018) were lower in the CR/PR group. Notably, epitope spreading was eliminated in the CR/PR group while remaining in the NR group (0% vs. 14.3%). Four ARA-positive PMN patients (0% vs. 22.2%, P = 0.009) were identified in the NR group by detecting ARAs at M6 (Table 5; Supplementary Figure S4).
3.4 Correlation analysis of the total PLA2R-IgG/IgG4 and the domain-specific antibodies of PLA2R
The correlation analysis revealed that the total PLA2R-IgG/IgG4 was significantly associated with PLA2R-CysR-IgG/IgG4 and PLA2R-CTLD678-IgG. Spearman’s correlations of PLA2R-IgG with PLA2R-CysR-IgG/IgG4 and PLA2R-CTLD-IgG were 0.703, 0.730, and 0.522, respectively, and the correlations of the PLA2R-IgG4 with the same antibodies mentioned above were 0.723, 0.792, and 0.545, respectively (Supplementary Figure S5).
3.5 Analysis of factors related to proteinuria remission
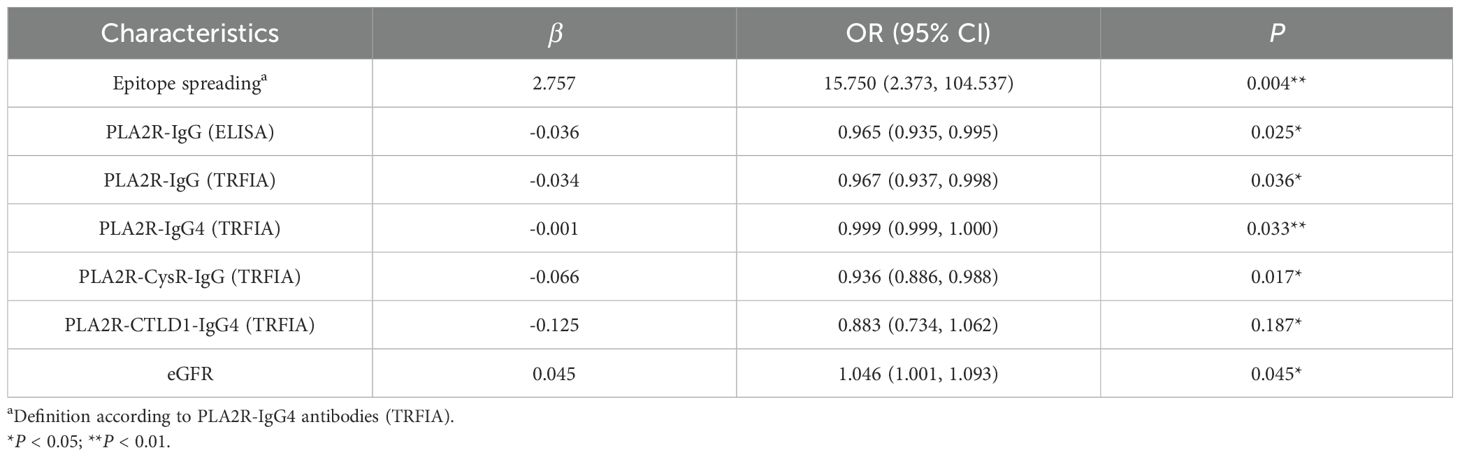
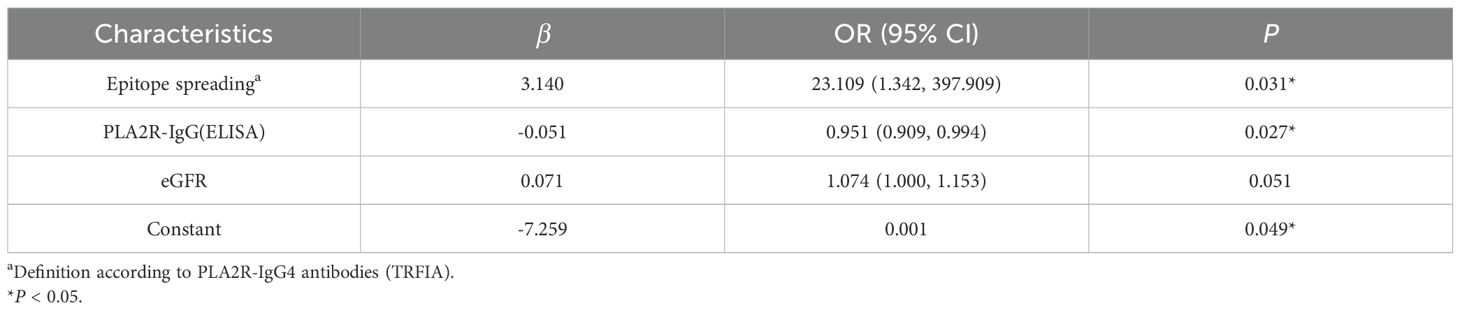
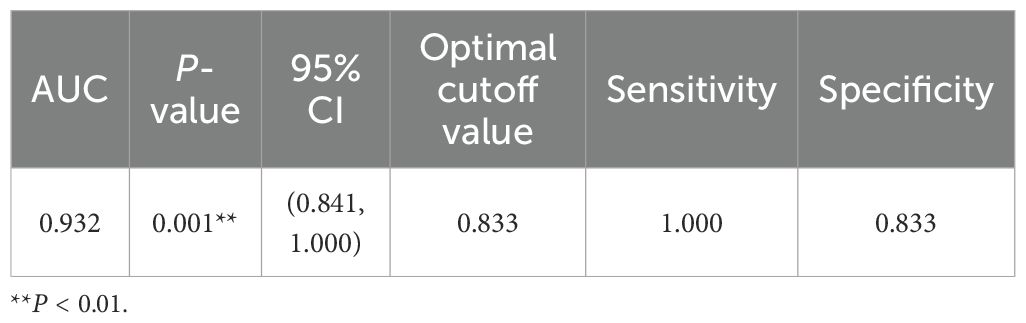
We explored 25 clinical factors associated with proteinuria remission, including serum albumin, PLA2R-IgG/-IgG4, its domain-specific antibodies, and epitope spreading. In the univariate logistic regression analyses, it was found that eGFR (P = 0.045), PLA2R-IgG (ELISA) (P = 0.025), PLA2R-IgG (TRFIA) (P = 0.036), PLA2R-IgG4 (TRFIA) (P = 0.033), PLA2R-CysR-IgG (TRFIA) (P = 0.017), and epitope spreading (P = 0.004) were correlated with proteinuria remission statistically, and after Benjamini–Hochberg correction, epitope spreading remained. Multivariable logistic regression analysis of the abovementioned relevant variables revealed that epitope spreading (P = 0.031) was an independent risk factor for proteinuria remission at M6 after adjusting for baseline eGFR and PLA2R concentrations (Tables 6, 7). The ROC curve of the regression equation demonstrated an AUC of 0.932 (Supplementary Figures S5, S6; Table 8).
3.6 Outcome of the PMN patients who were ARA-positive
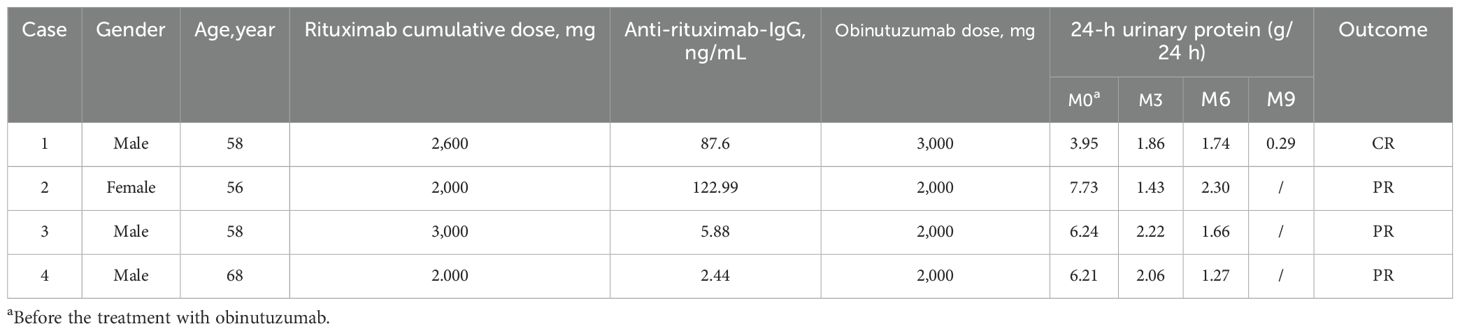
In this study, ARAs were detected in four patients after 6 months of RTX therapy, and none of them had achieved CR/PR at M6. Based on the clinical condition and wishes of the patients, replacement of treatment with obinutuzumab was put into effect. All patients achieved CR or PR within 3–9 months. Although the follow-up period was short, one of these patients (case 1) achieved CR at M9 after receiving 2,000 mg of obinutuzumab (Table 9).
4 Discussion
PMN is induced by antigen–antibody binding and deposition in the basilar membrane. Targeted treatment regimens have emerged with the discovery of pathogenic mechanisms. The 2021 KIDGO guidelines recommend that patients assessed as moderate and high risk according to risk stratification use RTX as the first-line treatment, whereas CTX is used in very-high-risk patients. The efficacy of RTX has also been confirmed (16–19). However, some patients using RTX still do not achieve CR or PR. Therefore, this study aimed to identify the best prognostic predictor in patients with PMN treated with RTX, which will contribute to the establishment of a clinical prediction model.
In the study, there was no statistical difference in the clinical remission between the CTX and RTX groups either at M6 (37.9% vs. 60.0%, P = 0.875) or M12 (75.6% vs. 61.5%, P = 0.220), while for immunological remission, the RTX group demonstrated a higher immunological remission rate at M12 (96.2% vs. 65.6%, P = 0.017) and more efficient PLA2R antibody clearance rate at M6, which may be due to the direct effect of RTX targeting CD20+ B cells. Proteinuria remission occurs later than immunological remission in PMN patients (6, 20, 21), suggesting that further validation of the efficacy of RTX treatment by extending the follow-up observation period is of great concern.
In a previous study, the clinical remission rates at M12 reported in the cohort of GMERITUX (17), MENTOR (19), and RI-CYCLO (18) were 64.9%, 60%, and 62%, respectively. In a prospective study (22), 43 of 70 patients (61.43%) in the RTX group and 54 of 71 patients (76.06%) in the CTX group achieved CR/PR (P = 0.06) at M12, with similar remission rates at M24 (75.41% vs. 68.97%, P = 0.54). In another study (23), compared with those in the NR group, PLA2R-IgG and PLA2R-CysR-IgG titers declined more in the PR or CR groups at M6 and M12, respectively. These results are consistent with the conclusions of the present study.
Despite differences in baseline characteristics between the RTX and CTX groups, given the aforementioned inconsistency between clinical and immunological remission, we decided to conduct a further analysis of the RTX group. In the subsequent analysis, compared with the RTX group at M0, it showed lower titers of PLA2R-specific antibodies and a lower positive rate of epitope spreading at M6, which may be related to the efficacy of RTX. Next, the RTX group was divided into CR/PR and NR groups based on the proteinuria remission of M6, which displayed PLA2R-IgG (P = 0.049) and PLA2R-CysR-IgG4 (P = 0.018) titers; the positive rate of epitope spreading (16.67%) was significantly higher in the NR group.
To further explore the relationship of PLA2R-specific antibodies and epitope spreading with proteinuria remission, we performed univariate logistic analysis and found that six indicators, including PLA2R-IgG (ELISA), PLA2R-IgG/-IgG4 (TRFIA), PLA2R-Cys-IgG, PLA2R-CTLD1-IgG4, and epitope spreading, were associated with clinical remission, and epitope spreading was still retained after Benjamini–Hochberg correction. Further multivariate logistic regression analysis (AUC-ROC = 0.932) showed that epitope spreading was an independent risk factor for proteinuria remission at M6. Experimental evidence that epitope spreading may occur and influence the severity of MN was documented in a Heymann nephritis model of MN in rats, which coincided with worsening proteinuria (24). Simultaneously, as an autoimmune disease, a proportion of PLA2R-MN cases show spreading of the immunodominant epitope from -CysR to -CTLD1 and/or -CTLD678, which is related to a worse prognosis (25).
In the NICE cohort (16), lower epitope spreading at diagnosis was associated with proteinuria remission at M6 (50% vs. 76%, P = 0.05), whereas epitope spreading at M0 (100% vs. 48%, P = 0.01) and incomplete clearance of PLA2R-IgG at M6 (0% vs. 9%, P = 0.05) were associated with recurrence. Another study conducted by Seitz-Polski et al. (25) reported that epitope spreading beyond the CysR epitope was an independent risk factor for poor renal prognosis. In a recent prospective study, the multi-domain recognition exhibited a reduced remission rate compared with the single-domain (44.44% vs. 82.61%, P = 0.011). Subsequent to the Kaplan–Meier analysis, it was demonstrated that the multi-domain group exhibited lower remission rates in comparison to the single-domain group at a number of designated time points (26). In contrast, Reinhard considered that the levels of domain-specific PLA2R antibodies against -CysR, -CTLD1, and -CTLD7 were strongly correlated with the levels of total anti-PLA2R1 antibodies but could not predict prognosis independtly of the total anti-PLA2R1 antibody levels (6). Similarly, in an Italian study, Ruggenenti et al. found that lower PLA2R-IgG (P = 0.002) and PLA2R-CysR-IgG (P = 0.001) titers at M0 were associated with a higher probability of clinical remission, whereas PLA2R-CTLD-IgG was not associated with remission (23). Our study confirmed that epitope spreading was an independent risk factor for proteinuria remission.
Although RTX could induce seasonable immunological remission, some patients did not maintain remission. In this study, four ARA antibody-positive cases were detected in the NR group after 6 months of treatment with RTX. Based on the wishes of the four patients, we changed the treatment regimen to obinutuzumab, and all four patients achieved PR or CR in 3 to 9 months of follow-up. As a fully human anti-CD20 monoclonal antibody, obinutuzumab is more likely to avoid the effects of anti-rituximab antibodies, depending on the immune response profiles of the different organisms.
In a clinical combined with in vitro experimental study, ARA was detected in 10 of 44 RTX-treated patients with PMN by ELISA, and in an in vitro test against ARA, anti-CD20 monoclonal antibody efficacy including obinutuzumab, ocrelizumab, and ofatumumab was not impaired in eight patients, while three ARA-positive patients were successfully treated with ofatumumab (9). To explore the factors influencing ARA production, Maxime Teisseyre et al. detected ARA in 21 (58%) out of 55 patients included and found that the patients in the ARA-positive group had a higher epitope spreading rate (82% vs. 44%, P < 0.05) (27). Epitope spreading indicates that the organism of that patient is more immunologically active, which means that it is more likely to produce ARA, and ARA-positive patients are more likely to experience failure of RTX treatment, consistent with the findings of the present study as previously described.
Xiaofan Hu et al. conducted a study that compared the efficacy of RTX and obinutuzumab. In a subgroup analysis of PLA2R-associated PMN, patients in the obinutuzumab group were more likely to reach immunological remission at M6 (92% vs. 64%, P = 0.06) (28). In a retrospective study in China (29), the response rates were significantly higher in 51 patients treated with obinutuzumab than in those treated with RTX (90.0% vs. 38.7%, P < 0.001) over a 24-month follow-up period. Cox proportional hazards survival regression analysis also showed superior efficacy of obinutuzumab (P < 0.001). All of these studies have shown that the efficacy of obinutuzumab is superior to that of RTX, but the choice of obinutuzumab treatment was not based on a positive ARA test but rather due to failure or resistance to treatments such as glucocorticoids, which is known as refractory MN. In contrast, all four patients in the present study selected obinutuzumab treatment because of positive ARA test results. Therefore, biomarkers or clinical indicators, such as ARA and epitope spreading, that can be used to determine the timing of selection of obinutuzumab or other fully human anti-CD20 monoclonal antibodies need to be explored further.
The limitations of this study include its retrospective design and a small number of patients in the RTX group, and some patients had a short follow-up observation period, which means that the conclusions of this study need to be confirmed by a larger sample size and long-term clinical studies.
5 Conclusion
In conclusion, compared with CTX, RTX induced a higher rate of immunological remission at M12. Epitope spreading of PLA2R was identified as an independent risk factor for clinical remission after 6 months of treatment with RTX.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (ethical approval no.kyl2016001). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XC: Formal Analysis, Data curation, Writing – review & editing, Writing – original draft. MZ: Writing – original draft, Formal Analysis. CC: Writing – review & editing, Data curation. JX: Writing – review & editing, Data curation. BL: Project administration, Writing – review & editing. ZZ: Writing – review & editing, Supervision. XZ: Writing – review & editing. LZ: Writing – review & editing. TC: Writing – review & editing. BH: Writing – review & editing, Funding acquisition. YZ: Writing – review & editing, Funding acquisition. LW: Validation, Writing – review & editing, Supervision. XL: Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Wuxi Medical Innovation Team Project (CXTD2021010), the Wuxi Science and Technology Development Fund project(2024217), the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (HB2023009), and the Cohort and Clinical Research Program of Wuxi Medical Center, Nanjing Medical University(WMCC202503). The funders was no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1685738/full#supplementary-material
References
1. Zabala Ramirez MJ, Stein EJ, and Jain K. Nephrotic syndrome for the internist. Med Clin North Am. (2023) 107:727–37. doi: 10.1016/j.mcna.2023.03.006
2. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. (2017) 12:983–97. doi: 10.2215/CJN.11761116
3. Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
4. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100(4S):S1–S276. doi: 10.1016/j.kint.2021.05.021
5. Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payré C, et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol. (2018) 29:401–8. doi: 10.1681/ASN.2017070734
6. Reinhard L, Zahner G, Menzel S, Koch-Nolte F, Stahl R a K, and Hoxha E. Clinical relevance of domain-specific phospholipase A(2) receptor 1 antibody levels in patients with membranous nephropathy. J Am Soc Nephrol. (2020) 31:197–207. doi: 10.1681/ASN.2019030273
7. Zhou K, Zhou J, Zhou L, Xue J, Liu B, Zhang Z, et al. Predictive value of the domain specific PLA2R antibodies for clinical remission in patients with primary membranous nephropathy: A retrospective study. PloS One. (2024) 19:e0302100. doi: 10.1371/journal.pone.0302100
8. Teisseyre M, Cremoni M, Boyer-Suavet S, Ruetsch C, Graça D, Esnault VLM, et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol. (2022) 13:859419. doi: 10.3389/fimmu.2022.859419
9. Boyer-Suavet S, Andreani M, Lateb M, Savenkoff B, Brglez V, Benzaken S, et al. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. (2019) 10:3069. doi: 10.3389/fimmu.2019.03069
10. Deng L and Xu G. Update on the application of monoclonal antibody therapy in primary membranous nephropathy. Drugs. (2023) 83:507–30. doi: 10.1007/s40265-023-01855-y
11. Qin Y, Wu Q, Sheng H, Li T, Liu X, Yang X, et al. Quantitative detection of anti-PLA2R antibodies targeting different epitopes and its clinical application in primary membranous nephropathy. Clin Chem Lab Med. (2023) 61:251–9. doi: 10.1515/cclm-2022-0720
12. Chen X, Zhou K, Xiang Z, Zhou X, Wang Y, Hong J, et al. Establishment and clinical application of time-resolved immunofluorescence assay of lipoprotein-associated phospholipase A2. Anal Biochem. (2022) 648:114674. doi: 10.1016/j.ab.2022.114674
13. Li T, Wu Q, Yang X, Zhang Y, Zhou X, Sheng H, et al. Establishment and application of an immunoassay for the simultaneous detection of IgG and its subtype IgG4 autoantibodies against M-type phospholipase A2 receptor. Clin Biochem. (2021) 96:49–55. doi: 10.1016/j.clinbiochem.2021.07.006
14. Li T, Wu Q, Yang X, Liu Y, Lin B, Zhou X, et al. A novel time-resolved fluoroimmunoassay based on magnetic microspheres method for detecting antibodies against the phospholipase A2 receptor. Anal Methods. (2021) 13:3017–23. doi: 10.1039/D1AY00369K
15. Kao S, Liu X, Jin J, Zhang L, Shen T, Wu J, et al. Development of a time-resolved fluoroimmunoassay for rituximab and its application to therapeutic drug monitoring. Clin Chim Acta. (2025) 568:120142. doi: 10.1016/j.cca.2025.120142
16. Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. (2019) 14:1173–82. doi: 10.2215/CJN.11791018
17. Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al. Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol. (2017) 28:348–58. doi: 10.1681/ASN.2016040449
18. Scolari F, Delbarba E, Santoro D, Gesualdo L, Pani A, Dallera N, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. (2021) 32:972–82. doi: 10.1681/ASN.2020071091
19. Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. (2019) 381:36–46. doi: 10.1056/NEJMoa1814427
20. Ronco P, Beck L, Debiec H, Fervenza FC, Hou F, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. (2021) 7:69. doi: 10.1038/s41572-021-00303-z
21. Stefan G, Stancu S, Zugravu A, Popa O, Zubidat D, Petre N, et al. Negative anti-phospholipase A2 receptor antibody status at three months predicts remission in primary membranous nephropathy. Ren Fail. (2022) 44:258–68. doi: 10.1080/0886022X.2022.2033265
22. Hu X, Ren H, Xu J, Gao C, Wu Y, Ouyang Y, et al. Treatment of membranous nephropathy in chinese patients: comparison of rituximab and intravenous cyclophosphamide with steroids. Kidney Dis (Basel). (2024) 10:359–68. doi: 10.1159/000540548
23. Ruggenenti P, Reinhard L, Ruggiero B, Perna A, Perico L, Peracchi T, et al. Anti-phospholipase A2 receptor 1 and anti-cysteine rich antibodies, domain recognition and rituximab efficacy in membranous nephropathy: A prospective cohort study. Am J Kidney Dis. (2024) 83:588–600.e1. doi: 10.1053/j.ajkd.2023.10.013
24. Shah P, Tramontano A, and Makker SP. Intramolecular epitope spreading in Heymann nephritis. J Am Soc Nephrol. (2007) 18:3060–6. doi: 10.1681/ASN.2007030342
25. Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol. (2016) 27:1517–33. doi: 10.1681/ASN.2014111061
26. Wu L, Su Z, Tang B, Chen Y, Hu H, Cheng Y, et al. Adverse prognosis in membranous nephropathy with phospholipase A2 receptor 1 epitope spreading: A prospective study. Am J Nephrol. (2025) 56(5):543–54. doi: 10.1159/000545133
27. Teisseyre M, Brglez V, Cremoni M, Fernandez C, Graça D, Boyer-Suavet S, et al. Risk factors associated with the occurrence of anti-rituximab antibodies in membranous nephropathy. Clin J Am Soc Nephrol. (2023) 18:785–7. doi: 10.2215/CJN.0000000000000152
28. Hu X, Zhang M, Xu J, Gao C, Yu X, Li X, et al. Comparison of obinutuzumab and rituximab for treating primary membranous nephropathy. Clin J Am Soc Nephrol. (2024) 19:1594–602. doi: 10.2215/CJN.0000000000000555
Keywords: primary membranous nephropathy, PLA2R, epitope spreading, rituximab, anti-rituximab antibody
Citation: Cheng X, Zhou M, Chen C, Xue J, Liu B, Zhang Z, Zhang X, Zhou L, Cai T, Huang B, Zhang Y, Wang L and Liu X (2025) Clinical study of PLA2R epitope spreading for predicting proteinuria remission in primary membranous nephropathy. Front. Immunol. 16:1685738. doi: 10.3389/fimmu.2025.1685738
Received: 14 August 2025; Accepted: 06 October 2025;
Published: 22 October 2025.
Edited by:
Emanuele Bizzi, Vita-Salute San Raffaele University, ItalyCopyright © 2025 Cheng, Zhou, Chen, Xue, Liu, Zhang, Zhang, Zhou, Cai, Huang, Zhang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wang, d2x3eHNua0AxNjMuY29t; Xiaobin Liu, bHhid3hzbmtAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xueyang Cheng
Xueyang Cheng Meiyi Zhou
Meiyi Zhou Caimei Chen1
Caimei Chen1 Biao Huang
Biao Huang Yi Zhang
Yi Zhang Liang Wang
Liang Wang