- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Ovarian cancer(OC) remains a major threat to women’s health, ranking among the top gynecologic malignancies in both incidence and mortality. Current clinical management continues to center on cytoreductive surgery combined with a multidisciplinary approach incorporating chemotherapy, targeted therapy, and immunotherapy. Notably, while single-agent immunotherapy has demonstrated limited efficacy in recurrent OC, recent breakthrough advances in dual-target immunotherapy have brought new hope for advanced-stage and recurrent patients. Clinical evidence indicates that programmed death-1/cytotoxic T-lymphocyte-associated protein 4 (PD-1/CTLA-4) dual immune checkpoint blockade strategies (e.g., durvalumab plus tremelimumab, nivolumab plus ipilimumab) exhibit differential therapeutic effects: durable treatment responses have been observed in recurrent/platinum-resistant advanced OC, while neoadjuvant applications have significantly improved complete resection rates. However, these therapeutic benefits demonstrate marked heterogeneity across different histological subtypes. The review of current research reveals several critical issues: first, the safety profile of dual immunotherapy requires further characterization; second, data on first-line treatment for advanced OC remain scarce; and third, optimal treatment strategies have yet to be established. Nevertheless, multiple ongoing clinical trials are paving the way for future research directions, including optimization of combination regimens and exploration of predictive biomarkers. In conclusion, despite existing challenges, dual-target immunotherapy has demonstrated clinically meaningful benefits, offering new therapeutic options for advanced and recurrent OC patients and heralding a new era of combination immunotherapy in OC treatment. Future large-scale clinical studies are warranted to further validate efficacy and establish individualized precision treatment strategies.
1 Introduction
Ovarian cancer (OC) ranks among the most common and lethal gynecologic malignancies worldwide (1). Owing to the absence of reliable early screening methods and its insidious clinical presentation, approximately 70% of patients are diagnosed at an advanced stage (2). Epidemiological studies report an overall 5-year survival rate of around 40%, with the 10-year survival plummeting to a mere 13% in advanced cases (3). The current therapeutic paradigm centers on cytoreductive surgery, supplemented by a multimodal approach integrating chemotherapy, targeted therapy, and immunotherapy (4). Immune checkpoint inhibitors (ICIs), now established as a cornerstone in treating various solid tumors (including cervical and endometrial cancers), have shown promise in OC. Preclinical evidence demonstrates that T-cell infiltration within ovarian tumor tissues correlates with anti-tumor immune activation at the molecular level, and this immune signature is positively associated with improved survival—lending a strong rationale for ICI-based interventions (5, 6). Contemporary immunotherapeutic strategies focus on enhancing cytotoxic immune responses to mitigate tumor burden. Nevertheless, response heterogeneity to ICIs persists across histological subtypes and individual patients, driven by factors such as tumor microenvironment composition, tumor mutational burden (TMB), immune checkpoint expression patterns, and genetic/epigenetic regulatory mechanisms. The intricate interplay of these elements ultimately dictates therapeutic outcomes (7–10). Amidst the rapid development of novel agents and combination regimens, the management of advanced and metastatic OC is witnessing a transformative shift, offering renewed hope for this historically recalcitrant disease.
In gynecologic oncology, ICIs targeting programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have advanced from second-line to first-line treatment for advanced/recurrent cervical and endometrial carcinomas. However, OC remains refractory to ICI therapy due to its immunologically “cold” tumor phenotype (11). This immunotherapeutic resistance is characterized by TMB, inadequate immune cell infiltration, and a profoundly immunosuppressive tumor microenvironment (TME) dominated by regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), coupled with limited tumor antigen expression that impedes immune recognition (7, 8).
The biological significance of PD-1 was elucidated following the discovery of its ligands PD-L1/PD-L2 (12, 13). CTLA-4, an immune checkpoint molecule highly expressed on activated T cells and Tregs, was first functionally characterized by Dr. James Allison’s team. It suppresses T cell proliferation and IL-2 production through competitive binding with B7 ligands (14). Although both anti-CTLA-4 and anti-PD-1/PD-L1 therapies restore T cell function by blocking inhibitory signals, their mechanisms differ fundamentally: CTLA-4 primarily modulates CD4+ T cell activation and migration by regulating antigen-presenting cell (APC)-mediated CD28-B7 interactions (15–17), whereas PD-1 acts downstream of T cell receptor (TCR) signaling to reverse CD8+ T cell exhaustion without affecting clonal expansion (15, 18). This mechanistic complementarity provides a strong rationale for combination immunotherapy strategies. The history of therapies targeting PD-1/PD-L1 and CTLA-4 is summarized in Figure 1.
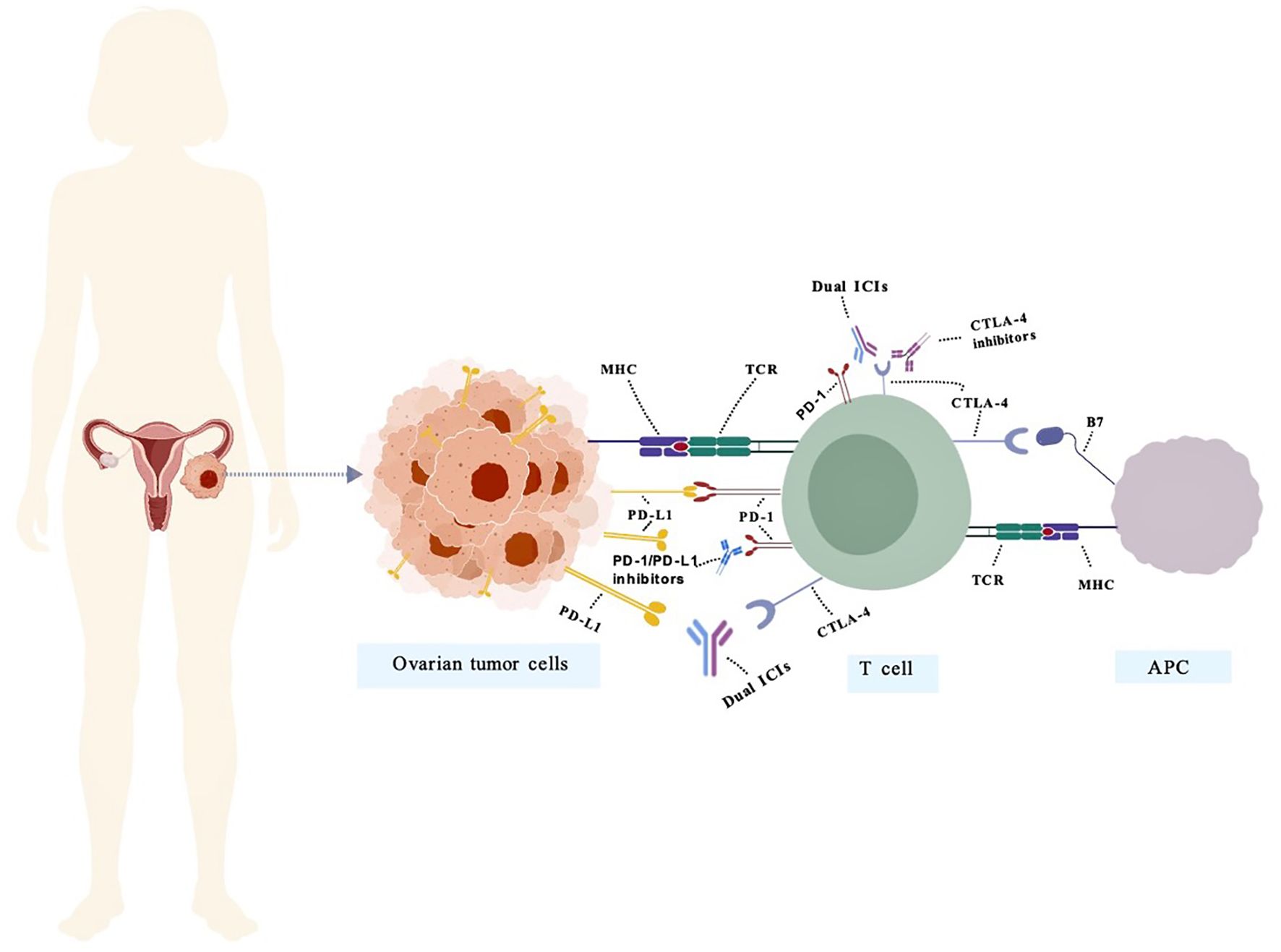
Figure 1. The mechanism of action of bispecific antibodies targeting PD-1/PD-L1 and CTLA-4 in OC. PD-1 negatively regulates effector T-cell activity by binding to its ligand PD-L1. Inhibitors targeting PD-1 or PD-L1 disrupt this interaction, thereby restoring T-cell function and promoting anti-tumor immunity. Representative PD-1/PD-L1 inhibitors include pembrolizumab, nivolumab, atezolizumab, and durvalumab. CTLA-4 binds to B7 molecules on antigen-presenting cells (APCs), transmitting inhibitory signals to T cells. CTLA-4 inhibitors restore immune activity by blocking this interaction. Representative CTLA-4 inhibitors include Ipilimumab and Tremelimumab. Dual ICIs mitigate immunosuppression by concurrently targeting multiple inhibitory pathways, thereby overcoming immune tolerance within the tumor microenvironment. (PD-1/PD-L1&CTLA-4) Representative Dual ICIs include Cadonilimab.
2 The evolving role of immunotherapy in OC
The efficacy of ICI monotherapy in recurrent OC remains suboptimal. ICI responses are typically associated with high tumor mutational burden (TMB-H, generally >10–20 mut/Mb), mismatch repair deficiency (dMMR), or microsatellite instability-high (MSI-H) status—features that enhance tumor antigen presentation and lymphocyte infiltration (19). However, OC is characterized by a low mutational burden (1–3.5 mut/Mb), with only 5–15% of cases exhibiting dMMR/MSI-H. Although dostarlimab and pembrolizumab have been approved in the US for dMMR/MSI-H/TMB-H tumors, this subgroup represents a small fraction of OC patients, significantly limiting the applicability of immunotherapy (20, 21).
The KEYNOTE-100 trial demonstrated that while the anti-PD-1 antibody pembrolizumab yielded an objective response rate (ORR) of <10% in advanced recurrent OC, a subset of responders exhibited durable clinical benefit lasting beyond 6 months (22). Similarly, the JAVELIN Ovarian 200 trial evaluated the anti-PD-L1 antibody avelumab in combination with pegylated liposomal doxorubicin, yet the combination achieved only a 13% response rate without significant survival improvement, with long-term benefit observed in only a minority of patients (23). Notably, certain OC histologic subtypes (e.g., clear cell carcinoma) display heightened sensitivity to immunotherapy (24), whereas the predominant high-grade serous and mucinous subtypes remain largely refractory (25).
The immunosuppressive TME of OC further complicates therapeutic efficacy. OC TME is characterized by low-to-moderate lymphocytic infiltration and abundant immunosuppressive myeloid cells (e.g., MDSCs, tumor-associated macrophages (TAMs)), which impair residual T-cell antitumor activity (26–28). Additionally, the “cold” tumor phenotype of OC—marked by low antigenicity and immune evasion mechanisms—limits ICI effectiveness (26). To overcome resistance, combinatorial strategies involving ICIs with chemotherapy, antiangiogenic agents, or PARP inhibitors have been explored, but no significant synergistic effects have been observed to date (25). Recently, dual immune checkpoint blockade (e.g., CTLA-4 plus PD-1/PD-L1 inhibitors) has gained attention due to its mechanistic complementarity, yet whether this approach can effectively overcome OC’s immunosuppressive barriers requires further validation. Future research should focus on novel combination strategies targeting TME reprogramming, enhanced antigen presentation, or myeloid cell modulation to expand the population benefiting from immunotherapy. To better illustrate the current progress in dual-ICI therapy, we summarize key clinical trial data in Table 1.
3 Advances in dual immune checkpoint inhibitor therapy for recurrent or platinum-resistant OC
Recent years have witnessed significant advancements in dual ICIs therapy for recurrent or platinum-resistant ovarian cancer (PROC), with emerging clinical evidence highlighting both its therapeutic potential and associated challenges. The Korean NCT03699449 trial (33) systematically evaluated efficacy differences among various immunotherapeutic regimens in 70 platinum-resistant patients. Notably, the combination of durvalumab (anti-PD-L1, 1500mg) with tremelimumab (anti-CTLA-4, 75mg) plus chemotherapy (n=18) demonstrated a superior ORR of 33.3% (95% CI: 13.3%-59.0%) compared to single-agent immunotherapy combined with chemotherapy (n=5). However, this regimen raised safety concerns, with grade 3/4 treatment-related adverse events (TRAEs) occurring in 66.7% of patients - substantially higher than other treatment groups (20%-47.1%). The investigators proposed that weekly low-dose paclitaxel administration might improve the safety profile. The NRG GY003 study (29, 30), involving 100 patients with recurrent OC (1–3 prior lines of chemotherapy and platinum-free interval <12 months), demonstrated that nivolumab (anti-PD-1) plus ipilimumab (anti-CTLA-4) significantly improved ORR (31.4% vs 12.2%, P = 0.034) and median progression-free survival (3.9 vs 2.0 months; HR = 0.53, P = 0.004) compared to nivolumab monotherapy. However, no statistically significant difference was observed in median overall survival (28.1 vs 21.8 months, P = 0.43). Importantly, biomarker analysis revealed no significant correlation between PD-L1 expression and treatment efficacy. The combination therapy maintained a manageable safety profile, with grade ≥3 TRAEs occurring in 49% of patients, consistent with previous reports.
The recently published phase Ib expansion study (39) demonstrated promising clinical outcomes with the novel dual immunotherapy combination of botensilimab (a next-generation multifunctional CTLA-4 inhibitor) plus balstilimab (PD-1 inhibitor) in 17 evaluable patients with recurrent platinum-resistant/refractory OC. Key efficacy data showed a median treatment duration of 3.2 months (range 0.9-19.6) and median follow-up of 8.8 months (range 2.0-29.2), with an ORR of 29% (5/17; 95% CI:13-53), including 4 confirmed partial responses and 1 unconfirmed complete response. The safety profile was favorable, with no reported cases of hypophysitis, myocarditis, or pneumonitis of any grade. TRAEs occurred at grade 1/2 in 94%, grade 3 in 24%, and grade 4 in 6% of patients, with no grade 5 events observed. Diarrhea/colitis was the most common grade 3/4 TRAE (18%). These findings suggest this dual checkpoint blockade combination achieves meaningful clinical activity with manageable toxicity in this heavily pretreated population, warranting further investigation in larger clinical trials to confirm its long-term efficacy and safety. A clinical study conducted by Hinchcliff et al. (37) investigated different administration strategies of CTLA-4 and PD-L1 inhibitors to evaluate their impact on progression-free survival (PFS) in platinum-resistant/refractory high-grade OC. The trial enrolled 61 platinum-resistant patients randomized to either sequential therapy (n=38; tremelimumab followed by durvalumab) or combination therapy (n=23; concurrent administration). Results demonstrated no statistically significant difference in median PFS between groups (P = 0.402). The sequential arm showed no objective responses, with 31.6% (12/38) achieving stable disease (SD), while the combination arm yielded partial responses in 8.7% (2/23) plus one SD case. Current data reflect outcomes in the high-grade serous ovarian carcinoma (HGSOC) subgroup, with enrollment ongoing for clear cell carcinoma patients.
In novel immunotherapy development, the bispecific antibody ubamatamab (targeting CD3 and MUC16) has shown therapeutic potential (40, 41). The ongoing first-in-human R16-ONC-3 study (REGN4018) evaluates ubamatamab monotherapy or combined with cemiplimab in recurrent advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer patients (≥18 years, ≥1 prior platinum-based regimen, CA-125≥2×ULN) using a dose-escalation design. As an innovative bispecific antibody (anti-MUC16×CD3), ubamatamab represents a promising immunotherapeutic strategy, though efficacy data await further analysis as the trial continues recruitment. These studies not only validate the clinical potential of dual immune checkpoint inhibition in recurrent/resistant OCbut also provide critical insights for treatment optimization, safety management, and biomarker exploration. Larger phase III trials are warranted to confirm these findings and identify optimal beneficiary populations.
4 Current advances in neoadjuvant dual immune checkpoint inhibition for OC
4.1 Combination therapy strategies with single-target ICIs
Recent years have witnessed significant breakthroughs in clinical research investigating neoadjuvant immunotherapy combinations for advanced OC. The Ib phase randomized clinical trial presented at the 2024 ASCO Annual Meeting substantiated this progress (36). This study enrolled patients with unresectable stage IIIC/IV OC, demonstrating that neoadjuvant chemotherapy (NACT) combined with immunotherapy significantly improved complete cytoreduction rate (CC0), pathological complete response (pCR) rate, and PFS. Notably, while the dual immune checkpoint blockade (Group B, durvalumab + tremelimumab) showed no superior tumor immune microenvironment infiltration or survival outcomes compared to durvalumab monotherapy (Group A), the study identified pCR, CC0, and BRCA mutation status as independent predictors of long-term efficacy, rather than conventional immune-related biomarkers. These findings provide novel insights for precision medicine in OC, though the underlying mechanisms and clinical implications require further investigation.
The results suggest that NACT combined with dual ICIs (durvalumab + tremelimumab) may enhance antitumor effects through immune synergy, offering a potential therapeutic strategy for advanced epithelial ovarian cancer (EOC). The innovative KGOG 3046/TRUD study (NCT03899610) (34, 35) explored this dual immunotherapy-chemotherapy approach in the neoadjuvant setting. Initial 2023 results showed patients receiving durvalumab (1500mg) + tremelimumab (75mg) + NACT achieved 73.9% R0 resection and 17.4% pCR rates, with 12- and 24-month PFS rates of 63.6% and 45.0%, respectively. The 2025 final analysis further stratified 45 patients into multiple low-dose (n=23) and single high-dose (n=22) cohorts, revealing superior outcomes in the former for ORR (95.7% vs 77.3%, P = 0.048), chemotherapy response score (CRS) grade 3 response (39.1% vs 22.7%), and pCR rates (17.4% vs 4.6%). The entire cohort maintained 65.9% 12-month and 36.4% 30-month PFS rates. Safety analysis showed universal TRAEs occurrence, with rash being most common and neutropenia predominating grade 3–4 events. Beyond clinical validation, comprehensive biomarker analysis identified predictive potential for PD-L1 expression, mutational signature 3, and extracellular matrix components, providing critical evidence for precision immunotherapy approaches.
4.2 Dual-specificity immune checkpoint blockade as monotherapy
Cadonilimab (AK104), a novel tetravalent bispecific antibody targeting both PD-1 and CTLA-4, has demonstrated enhanced tumor-targeting capability through its unique structural design and is currently approved in China for metastatic/recurrent cervical cancer (42). The ongoing phase II NCT05430906 trial (38) is evaluating its efficacy and safety in combination with NACT for advanced OC, with R0 resection rate as the primary endpoint. Among 17 enrolled patients (94.1% high-grade serous carcinoma, 82.4% stage IVb), the regimen achieved an R0 resection rate of 66.7%, the ORR of 94.1% (including 1 complete and 15 partial responses), a pCR rate of 11.8%, and a CRS of 3 in 17.6% of patients. TRAEs occurred in 76.5% of patients, primarily thyroid dysfunction (23.5%) and myelosuppression (17.6%), with grade ≥3 events observed in 17.6% of cases including one grade 3 immune-related colitis (5.9%), all of which were manageable. These promising preliminary results support the potential clinical value of cadonilimab plus NACT in advanced OC though longer-term outcomes require further validation. In a separate case report by Zhang et al. (43), a PD-L1-positive (CPS = 10) OC patient who showed limited response to NACT plus bevacizumab subsequently achieved complete response with normalized CA-125 levels following cadonilimab maintenance therapy, suggesting that dual immune checkpoint inhibition may offer significant clinical benefit for HR-proficient/PD-L1-positive OC patients and providing important insights for optimizing treatment strategies in this population.
5 Clinical evaluation of dual immune checkpoint inhibition in OC subtypes
A phase II clinical trial (NCT03158064) (44) evaluated the efficacy of durvalumab plus tremelimumab in 29 patients with recurrent/refractory germ cell tumors, with only one ovarian primary case included according to the conference abstract. Although the overall treatment efficacy was limited (16-week PFS rate: 13.8%; median PFS: 1.4 months), one patient achieved sustained partial response (59% tumor reduction with PFS reaching 33 months), and two additional patients demonstrated tumor shrinkage exceeding 20%. Safety data showed grade 3–4 adverse events occurring in 21% of patients, suggesting potential benefit for specific patient subsets that warrants further exploration of biomarker-guided treatment strategies. These findings indicate that dual immune checkpoint inhibition may provide clinical benefit for certain germ cell tumor patients. Despite the modest overall efficacy, this study provides preliminary evidence supporting further investigation of immunotherapy in germ cell malignancies, including those of ovarian origin.
For clear cell carcinoma, updated results from the phase II trial (NCT03355976) (32) presented at ASCO 2024 demonstrated superior efficacy of nivolumab plus ipilimumab in recurrent extrarenal clear cell carcinomas (n=44). The combination arm showed significantly higher ORR (33.3% vs 14.3%) and median PFS (5.6 vs 2.2 months) compared to monotherapy, with a manageable safety profile (47% grade 3–4 AEs; no new safety signals). These data support the clinical utility of dual ICIs in ovarian clear cell carcinoma and other extrarenal variants. Collectively, these studies provide foundational evidence for immunotherapy in rare OC subtypes while underscoring the imperative for precision patient selection. Larger prospective trials are needed to validate these observations and optimize therapeutic strategies.
Clinical research on dual immune checkpoint blockade as first-line therapy for advanced OC remains limited. A pivotal phase III trial (NCT07002346) (45) registered on ClinicalTrials.gov is evaluating the efficacy of iparomlimab and tuvonralimab (QL1706) plus bevacizumab versus standard chemotherapy (paclitaxel plus carboplatin) in advanced ovarian clear cell carcinoma. Iparomlimab and tuvonralimab is an innovative bifunctional combination antibody comprising a recombinant humanized IgG4 monoclonal antibody targeting PD-1 and a recombinant humanized IgG1 monoclonal antibody targeting CTLA-4. This randomized, open-label, parallel-assignment phase III study plans to enroll 226 patients who will be randomized to receive either QL1706 plus bevacizumab or standard paclitaxel/carboplatin chemotherapy. The primary endpoint is PFS, with investigator-assessed overall survival (OS) as a key secondary endpoint. This trial will provide crucial evidence for immunotherapy combinations in advanced ovarian clear cell carcinoma, though data remain pending. Overall, investigation of dual immune checkpoint inhibition in first-line OC treatment is still in its early stages. Future research should focus on optimizing combination strategies, assessing safety profiles, and identifying predictive biomarkers to better select potential beneficiaries.
6 Conclusions and future perspectives
6.1 Future perspectives
Despite the demonstrated potential of ICIs in oncology, their clinical efficacy in OC remains suboptimal, characterized by low to modest objective response rates in most unselected patient cohorts (46, 47). This limitation stems from a confluence of factors intrinsic to OC biology, including a profoundly immunosuppressive TME, a relatively low TMB, the absence of validated predictive biomarkers, and both primary and acquired resistance mechanisms (38, 39). Given the remarkable inter- and intra-tumoral heterogeneity of OC, a paradigm shift is urgently required. Future therapeutic advancements hinge on a dual-pronged strategy: the development of mechanistically rational combination therapies and the implementation of sophisticated, biomarker-driven patient selection.
The future of ICIs in OC lies in strategic combinations designed to reverse immunosuppression and trigger robust anti-tumor immunity. The synergy between ICIs and PARPi extends beyond simple cytotoxicity. Evidence suggests that PARPi can upregulate PD-L1 expression on tumor cells, creating a biological rationale for combined blockade (48). In homologous recombination (HR)-deficient models, PARPi-induced DNA damage leads to the cytoplasmic accumulation of double-stranded DNA, which activates the cGAS-STING pathway (49, 50). This activation promotes the production of type I interferons and other inflammatory cytokines, thereby enhancing the recruitment and activation of CD8+ T-cells and converting immunologically “cold” tumors into “hot” ones (51–53). Clinical trials such as MEDIOLA (NCT02734004) have explored this combination, showing promising efficacy in BRCA-mutated OC (54). The combination of ICIs with antiangiogenic drugs like bevacizumab represents another rational approach. Beyond its role in inhibiting neovascularization, bevacizumab promotes vascular normalization, which alleviates tumor hypoxia and improves the perfusion and intratumoral infiltration of cytotoxic T lymphocytes (55). Concurrently, it counteracts VEGF-mediated immunosuppression by reducing the recruitment of MDSCs and Tregs within the TME (56). The phase III IMagyn050 trial, while not meeting its primary endpoint in the intent-to-treat population, provided insights into the potential of this combination and underscored the need for biomarker identification (57).
The profound heterogeneity of OC demands a paradigm shift toward precision medicine, underscored by the failure of single-agent ICIs and inconsistent combination trial outcomes that highlight the insufficiency of PD-L1 as a standalone biomarker. Therefore, developing and validating integrated, multi-parametric biomarker panels is a critical future direction. Key components should encompass: (i) immunohistochemical profiling, including quantitative and spatial analysis of tumor-infiltrating lymphocytes (TILs), particularly CD8+ T cells in the tumor core and invasive margin, which has been consistently associated with improved clinical outcomes (58); (ii) genomic and transcriptomic signatures, such as TMB, BRCA1/2 mutational status, and genomic scarring patterns (e.g., loss of heterozygosity), to identify tumors with elevated neoantigen burden and inherent genomic instability (59, 60), complemented by bulk and single-cell RNA sequencing to delineate the functional state of the TME and classify it into distinct phenotypes such as “immune-inflamed,” “immune-excluded,” or “immune-desert” (61, 62); and (iii) advanced integrative analytics that leverage machine learning algorithms to synthesize these disparate data streams—genomics, transcriptomics, proteomics, and digital pathology—to deconvolute OC complexity and generate predictive signatures for identifying patients most likely to achieve long-term benefit from immunotherapy (63).
Therefore, the next generation of clinical trials must be prospectively designed with the dual objective of evaluating the efficacy of rational drug combinations and rigorously validating sophisticated biomarker panels. This integrated strategy, which couples mechanistically grounded regimens with precision patient selection, is indispensable for unlocking the full therapeutic potential of ICIs in OC.
6.2 Conclusions
In summary, bispecific antibody-based immunotherapy represents a promising strategy for OC, particularly in platinum-resistant or recurrent cases. Early-phase clinical trials have demonstrated preliminary efficacy of agents simultaneously targeting dual immune checkpoints (e.g., PD-1/CTLA-4). However, challenges such as treatment-related toxicity, tumor heterogeneity, and the immunosuppressive microenvironment remain. Future success will depend on improved toxicity management, innovative combination therapies, validated biomarker development, and continued engineering of more effective and safer bispecific antibody constructs. Through collaborative and multidisciplinary efforts, the full therapeutic potential of ICP inhibition in OC may ultimately be realized.
Author contributions
D-ML: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. K-GP: Conceptualization, Investigation, Methodology, Writing – review & editing. X-ZY: Conceptualization, Investigation, Methodology, Writing – review & editing. MX: Investigation, Methodology, Writing – review & editing. LZ: Conceptualization, Methodology, Writing – review & editing. LS: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. China Foundation for Youth Entrepreneurship and Employment (25H0209), China Health & Medical Development Foundation (24H1124), Sichuan Medical Science and Technology Innovation Research Society (24H0355), Sichuan Cancer Society (25H0764).
Acknowledgments
The authors acknowledge all researchers whose work contributed to the field discussed in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang L, Wang X, Zhu X, Zhong L, Jiang Q, Wang Y, et al. Drug resistance in ovarian cancer: from mechanism to clinical trial. Mol Cancer. (2024) 23:66. doi: 10.1186/s12943-024-01967-3
2. Konstantinopoulos PA and Matulonis UA. Clinical and translational advances in ovarian cancer therapy. Nat Cancer. (2023) 4:1239–57. doi: 10.1038/s43018-023-00617-9
3. Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, and Kruitwagen R. No improvement in long-term survival for epithelial ovarian cancer patients: A population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. (2018) 88:31–7. doi: 10.1016/j.ejca.2017.10.030
4. Richardson DL, Eskander RN, and O’Malley DM. Advances in ovarian cancer care and unmet treatment needs for patients with platinum resistance: A narrative review. JAMA Oncol. (2023) 9:851–9. doi: 10.1001/jamaoncol.2023.0197
5. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. (2003) 348:203–13. doi: 10.1056/NEJMoa020177
6. Hwang WT, Adams SF, Tahirovic E, Hagemann IS, and Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol Oncol. (2012) 124:192–8. doi: 10.1016/j.ygyno.2011.09.039
7. Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. First-line pembrolizumab + Chemotherapy versus placebo + Chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of keynote-826. J Clin Oncol. (2023) 41:5505–11. doi: 10.1200/JCO.23.00914
8. Bogani G, Monk BJ, Powell MA, Westin SN, Slomovitz B, Moore KN, et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer. Ann Oncol. (2024) 35:414–28. doi: 10.1016/j.annonc.2024.02.006
9. Lakhani N, Cosman R, Banerji U, Rasco D, Tomaszewska-Kiecana M, Garralda E, et al. A first-in-human phase I study of the pd-1 inhibitor, retifanlimab (Incmga00012), in patients with advanced solid tumors (Pod1um-101). ESMO Open. (2024) 9:102254. doi: 10.1016/j.esmoop.2024.102254
10. Santoro A, Angelico G, Inzani F, Arciuolo D, d’Amati A, Addante F, et al. The emerging and challenging role of pd-L1 in patients with gynecological cancers: an updating review with clinico-pathological considerations. Gynecol Oncol. (2024) 184:57–66. doi: 10.1016/j.ygyno.2024.01.032
11. Ghisoni E, Imbimbo M, Zimmermann S, and Valabrega G. Ovarian cancer immunotherapy: turning up the heat. Int J Mol Sci. (2019) 20:2927. doi: 10.3390/ijms20122927
12. Krummel MF and Allison JP. Cd28 and ctla-4 have opposing effects on the response of T cells to stimulation. J Exp Med. (1995) 182:459–65. doi: 10.1084/jem.182.2.459
13. Dong H, Zhu G, Tamada K, and Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. (1999) 5:1365–9. doi: 10.1038/70932
14. Krummel MF and Allison JP. Cd28 and ctla-4 have opposing effects on the response of T-cells to stimulation. J Exp Med. (1995) 182:459–65. doi: 10.1084/jem.182.2.459
15. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-ctla-4 and anti-pd-1 checkpoint blockade. Cell. (2017) 170:1120–33 e17. doi: 10.1016/j.cell.2017.07.024
16. Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative ctla-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. (2010) 16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569
17. Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. Ctla-4 blockade increases ifngamma-producing cd4+Icoshi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U.S.A. (2008) 105:14987–92. doi: 10.1073/pnas.0806075105
18. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discov. (2021) 11:838–57. doi: 10.1158/2159-8290.CD-20-1680
19. Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, and Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. (2020) 10:1808–25. doi: 10.1158/2159-8290.CD-20-0522
20. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. (2017) 9:34. doi: 10.1186/s13073-017-0424-2
21. Pal T, Permuth-Wey J, Kumar A, and Sellers TA. Systematic review and meta-analysis of ovarian cancers: estimation of microsatellite-high frequency and characterization of mismatch repair deficient tumor histology. Clin Cancer Res. (2008) 14:6847–54. doi: 10.1158/1078-0432.CCR-08-1387
22. Matulonis UA, Shapira R, Santin A, Lisyanskaya AS, Pignata S, Vergote I, et al. Final results from the keynote-100 trial of pembrolizumab in patients with advanced recurrent ovarian cancer. J Clin Oncol. (2020) 38:6005. doi: 10.1200/JCO.2020.38.15_suppl.6005
23. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (Javelin ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. (2021) 22:1034–46. doi: 10.1016/S1470-2045(21)00216-3
24. Peng Z, Li H, Gao Y, Sun L, Jiang J, Xia B, et al. Sintilimab combined with bevacizumab in relapsed or persistent ovarian clear cell carcinoma (Inova): A multicentre, single-arm, phase 2 trial. Lancet Oncol. (2024) 25:1288–97. doi: 10.1016/S1470-2045(24)00437-6
25. Colombo I, Karakasis K, Suku S, and Oza AM. Chasing immune checkpoint inhibitors in ovarian cancer: novel combinations and biomarker discovery. Cancers (Basel). (2023) 15:3220. doi: 10.3390/cancers15123220
26. Pawlowska A, Rekowska A, Kurylo W, Panczyszyn A, Kotarski J, and Wertel I. Current understanding on why ovarian cancer is resistant to immune checkpoint inhibitors. Int J Mol Sci. (2023) 24:10859. doi: 10.3390/ijms241310859
27. Santoiemma PP and Powell DJ Jr.Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. (2015) 16:807–20. doi: 10.1080/15384047.2015.1040960
28. Webb PM and Jordan SJ. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. (2024) 21:389–400. doi: 10.1038/s41571-024-00881-3
29. Zamarin D. Randomized phase ii trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an nrg oncology study (Vol 56, pg 770, 2020). J Clin Oncol. (2020) 38:2702–. doi: 10.1200/Jco.20.01986
30. Zamarin D, Burger RA, Sill MW, Powell DJ Jr., Lankes HA, Feldman MD, et al. Randomized phase ii trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an nrg oncology study. J Clin Oncol. (2020) 38:1814–23. doi: 10.1200/JCO.19.02059
31. Chae YK, Othus M, Patel SP, Wilkinson KJ, Whitman-Purves EM, Lea J, et al. Swog/nci phase ii dual anti-ctla-4/pd-1 blockade in rare tumors: nonepithelial ovarian cancer. Clin Cancer Res. (2024) 30:5593–600. doi: 10.1158/1078-0432.CCR-24-0606
32. Dizon DS, Mathews CA, David SM, Machan JT, Hadfield MJ, Marks E, et al. Final results of bruog 354: A randomized phase ii trial of nivolumab alone or in combination with ipilimumab for people with ovarian and other extra-renal clear cell carcinomas. J Clin Oncol. (2024) 42:Lba5500–Lba. doi: 10.1200/JCO.2024.42.17_suppl.LBA5500
33. Lee JY, Kim BG, Kim JW, Lee JB, Park E, Joung JG, et al. Biomarker-guided targeted therapy in platinum-resistant ovarian cancer (Ambition; kgog 3045): A multicentre, open-label, five-arm, uncontrolled, umbrella trial. J Gynecol Oncol. (2022) 33:e45. doi: 10.3802/jgo.2022.33.e45
34. Park J, Lee JB, Lim MC, Kim BG, Kim JW, Kim S, et al. Phase ii study of durvalumab and tremelimumab with front-line neoadjuvant chemotherapy in patients with advanced-stage ovarian cancer: primary analysis in the original cohort of kgog3046/tru-D. J Immunother Cancer. (2023) 11:e007444. doi: 10.1136/jitc-2023-007444
35. Park J, Joung JG, Lim MC, Lee J, Kim BG, Kim JW, et al. Neoadjuvant chemotherapy with dual immune checkpoint inhibitors for advanced-stage ovarian cancer: final analysis of tru-D phase ii nonrandomized clinical trial. Clin Cancer Res. (2025) 31:1865–76. doi: 10.1158/1078-0432.CCR-24-3753
36. Leary A, Chardin L, Lortholary A, Asselain B, Alexandre J, Lebreton C, et al. Phase ib ineov neoadjuvant trial of durvalumab plus/- tremelimumab with platinum chemotherapy for patients (Pts) with unresectab le ovarian cancer (Oc): survival outcomes and immune correlates. J Clin Oncol. (2024) 42:5569. doi: 10.1200/jco.2024.42.16_suppl.5569
37. Hinchcliff E, Patel A, Fellman B, Westin S, Sood A, Soliman P, et al. Randomized phase ii trial of durvalumab (Anti-pdl1) and tremelimumab (Anti-ctla4) administered in combination versus sequentially for the treatment of recurrent platinum-resistant non-clear cell ovarian cancer (Nct03026062). Gynecologic Oncol. (2021) 162:S39–S. doi: 10.1016/S0090-8258(21)00718-6
38. Tang J, Tian WF, Huang S, Yang J, Yang HY, and Zhang JQ. An open, prospective, single arm, phase ii study of cadonilimab (Pd-1/ctla-4 bispecific antibody) with neoadjuvant chemotherapy in patients with advanced ovarian cancer: interim analysis from the ak104-iit-003 study. J Clin Oncol. (2024) 42:e17552. doi: 10.1200/JCO.2024.42.16_suppl.e17552
39. Bockorny B, Matulonis U, O’Day S, Margolin K, El-Khoueiry A, Wilky B, et al. Botensilimab, a novel innate/adaptive immune activator, plus balstilimab (Anti-pd-1) in patients with recurrent platinum refractory/resistant ovarian cancer. Gynecologic Oncol. (2023) 176:S35–S6. doi: 10.1016/j.ygyno.2023.06.514
40. Moore K, Bouberhan S, Hamilton E, Liu JY, O’Cearbhaill R, O’Malley D, et al. First-in-human phase 1/2 study of ubamatamab, a muc16xcd3 bispecific antibody, administered alone or in combination with cemiplimab in patients with recurrent ovarian cancer. Int J Gynecological Cancer. (2023) 33:A254–A5. doi: 10.1136/ijgc-2023-IGCS.480
41. Liu J, O’Malley D, Van Nieuwenhuysen E, O’Cearbhaill R, Bouberhan S, Moore K, et al. A phase I/ii study of ubamatamab (Regn4018), a muc16xcd3 bispecific antibody, administered alone or in combination with cemiplimab (Anti-pd-1) in patients with recurrent ovarian cancer or muc16+Endometrial cancer: trial in progress update. Gynecologic Oncol. (2024) 190:S284–S. doi: 10.1016/j.ygyno.2024.07.422
42. Romero D. Cadonilimab is effective and safe in recurrent cervical cancer. Nat Rev Clin Oncol. (2025) 22:2. doi: 10.1038/s41571-024-00962-3
43. Zhang C, Dai M, Yang J, Tian W, Huang S, Bi F, et al. Patient with hr-proficient advanced ovarian cancer achieved a complete response with cadonilimab combined chemotherapy (Pd-1/ctla-4 bispecific): A case report and literature review. Gynecol Oncol Rep. (2025) 60:101785. doi: 10.1016/j.gore.2025.101785
44. Funt SA, Knezevic A, Freeman BA, Bolos M, Martorana V, Donoghue M, et al. A single-arm, phase ii study of durvalumab (D) and tremelimumab (T) for relapsed/refractory germ cell tumors (Gct). J Clin Oncol. (2023) 41:5040. doi: 10.1200/JCO.2023.41.16_suppl.5040
45. Randomized active-controlled trial evaluating ql1706 plus bevacizumab versus platinum-based chemotherapy for advanced first-line ovarian clear cell carcinoma (2025). Available online at: https://clinicaltrials.gov/study/NCT07002346. (Accessed June 20, 2025).
46. Kim HS, Kim JY, Lee YJ, Kim SH, Lee JY, Nam EJ, et al. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol Oncol. (2018) 151:414–21. doi: 10.1016/j.ygyno.2018.08.023
47. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-pd-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
48. Martinez A, Delord JP, Ayyoub M, and Devaud C. Preclinical and clinical immunotherapeutic strategies in epithelial ovarian cancer. Cancers (Basel). (2020) 12:1761. doi: 10.3390/cancers12071761
49. Yang B, Li X, Fu Y, Guo E, Ye Y, Li F, et al. Mek inhibition remodels the immune landscape of mutant kras tumors to overcome resistance to parp and immune checkpoint inhibitors. Cancer Res. (2021) 81:2714–29. doi: 10.1158/0008-5472.CAN-20-2370
50. Harter P, Trillsch F, Okamoto A, Reuss A, Kim JW, Rubio-Pérez MJ, et al. Durvalumab with paclitaxel/carboplatin (Pc) and bevacizumab (Bev), followed by maintenance durvalumab, bev, and olaparib in patients (Pts) with newly diagnosed advanced ovarian cancer (Aoc) without a tumor brca1/2 mutation (Non-tbrcam): results from the randomized, placebo (Pbo)-controlled phase iii duo-O trial. J Clin Oncol. (2023) 41:16. doi: 10.1200/JCO.2023.41.17_suppl.LBA5506
51. Budczies J, Kluck K, Beck S, Ourailidis I, Allgauer M, Menzel M, et al. Homologous recombination deficiency is inversely correlated with microsatellite instability and identifies immunologically cold tumors in most cancer types. J Pathol Clin Res. (2022) 8:371–82. doi: 10.1002/cjp2.271
52. Huang M, Cha Z, Liu R, Lin M, Gafoor NA, Kong T, et al. Enhancing immunotherapy outcomes by targeted remodeling of the tumor microenvironment via combined cgas-sting pathway strategies. Front Immunol. (2024) 15:1399926. doi: 10.3389/fimmu.2024.1399926
53. Liu YT and Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. (2021) 11:5365–86. doi: 10.7150/thno.58390
54. Drew Y, Kim JW, Penson RT, O’Malley DM, Parkinson C, Roxburgh P, et al. Olaparib plus durvalumab, with or without bevacizumab, as treatment in parp inhibitor-naive platinum-sensitive relapsed ovarian cancer: A phase ii multi-cohort study. Clin Cancer Res. (2024) 30:50–62. doi: 10.1158/1078-0432.CCR-23-2249
55. Yang F, Lee G, and Fan Y. Navigating tumor angiogenesis: therapeutic perspectives and myeloid cell regulation mechanism. Angiogenesis. (2024) 27:333–49. doi: 10.1007/s10456-024-09913-z
56. Guo Z, Jing X, Sun X, Sun S, Yang Y, and Cao Y. Tumor angiogenesis and anti-angiogenic therapy. Chin Med J (Engl). (2024) 137:2043–51. doi: 10.1097/CM9.0000000000003231
57. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage iii or iv ovarian cancer: placebo-controlled randomized phase iii trial (Imagyn050/gog 3015/engot-ov39). J Clin Oncol. (2021) 39:1842–55. doi: 10.1200/JCO.21.00306
58. Gao P, Peng T, Cao C, Lin S, Wu P, Huang X, et al. Association of cldn6 and cldn10 with immune microenvironment in ovarian cancer: A study of the claudin family. Front Genet. (2021) 12:595436. doi: 10.3389/fgene.2021.595436
59. Landen CN, Molinero L, Hamidi H, Sehouli J, Miller A, Moore KN, et al. Influence of genomic landscape on cancer immunotherapy for newly diagnosed ovarian cancer: biomarker analyses from the imagyn050 randomized clinical trial. Clin Cancer Res. (2023) 29:1698–707. doi: 10.1158/1078-0432.CCR-22-2032
60. Cerina D, Matkovic V, Katic K, Belac Lovasic I, Separovic R, Canjko I, et al. Comprehensive genomic profiling in the management of ovarian cancer-national results from Croatia. J Pers Med. (2022) 12:1176. doi: 10.3390/jpm12071176
61. Pomponio R, Tang Q, Mei A, Caron A, Coulibaly B, Theilhaber J, et al. An integrative approach of digital image analysis and transcriptome profiling to explore potential predictive biomarkers for tgfbeta blockade therapy. Acta Pharm Sin B. (2022) 12:3594–601. doi: 10.1016/j.apsb.2022.03.013
62. Gronauer R, Madersbacher L, Monfort-Lanzas P, Floriani G, Sprung S, Zeimet AG, et al. Integrated immunogenomic analyses of high-grade serous ovarian cancer reveal vulnerability to combination immunotherapy. Front Immunol. (2024) 15:1489235. doi: 10.3389/fimmu.2024.1489235
Keywords: ovarian cancer, dual immune checkpoint inhibitors, PD-1, CTLA-4, neoadjuvant therapy
Citation: Li D-M, Pei K-G, Yu X-Z, Qie M-R, Zhong L and Song L (2025) Advances in PD-1 and CTLA-4 dual-target immunotherapy for ovarian cancer. Front. Immunol. 16:1686532. doi: 10.3389/fimmu.2025.1686532
Received: 15 August 2025; Accepted: 21 October 2025;
Published: 03 November 2025.
Edited by:
Xinhua Li, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
He Li, Central South University, ChinaAnna Pawłowska-Łachut, Medical University of Lublin, Poland
Copyright © 2025 Li, Pei, Yu, Qie, Zhong and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Song, ZHJzb25nbGlhbmdAMTYzLmNvbQ==
 Dong-Mei Li
Dong-Mei Li Kai-Ge Pei1,2
Kai-Ge Pei1,2 Ming-Rong Qie
Ming-Rong Qie