- 1Department of Orthopedics, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 3Hebei Medical University, Shijiazhuang, Hebei, China
With global aging accelerating, cancer incidence among older adults is rapidly increasing. Individuals aged ≥65 years now represent 64% of new cancer cases and 71.3% of cancer-related deaths worldwide. This population exhibits a distinct immune imbalance—driven by tumor-induced immunosuppression, immunosenescence, and inflammaging—which contributes to poor tolerance of standard therapies and suboptimal outcomes with PD-1/PD-L1 inhibitors.
As an emerging immunotherapeutic strategy, oncolytic viruses (OVs) selectively infect tumor cells, induce immunogenic cell death (ICD), and activate the cGAS–STING pathway. Although clinical data in elderly patients with esophageal, lung, or pancreatic cancer are scarce, promising outcomes have been reported in melanoma/sarcoma subgroups, including objective response rates of 26.4–32.9% and a median duration of response of 33.7 months, highlighting the potent antitumor potential of OVs.
However, age-related immunological vulnerability—manifesting across different frailty stages as reflected by G8 scoring—may predispose elderly patients to immune overload, cytokine storm, and impaired tolerance, while this group remains underrepresented in OV trials. Systematic studies in this context are lacking. This review highlights the immunological characteristics of aging, emphasizes the importance of addressing immunological vulnerability across different age stages (G8 scoring), and outlines emerging challenges and future directions for OV-based therapies tailored to frail elderly populations.
1 Introduction
As global aging progresses, the incidence of newly diagnosed cancers is steadily rising. By 2050, it is estimated that approximately 35 million new cancer cases will occur annually worldwide (1). Presently, the elderly population (≥65 years) accounts for about 64% of new cancer cases and 71.3% of cancer-related deaths (2), with these proportions projected to increase further. Elderly cancer patients experience a distinct immune imbalance shaped by tumor-induced immunosuppression, age-associated immunosenescence, and inflammaging (3, 4). This unique immunological state contributes to the high toxicity of conventional therapies, with grade 3–5 adverse events occurring in 53–83% of cases and a treatment-related mortality rate of 2% (5), alongside overall poor tolerance to therapy (6). Moreover, immune checkpoint blockade with PD-1/PD-L1 inhibitors exhibits limited effectiveness in older patients (7) and a higher risk of immune-related adverse events affecting the skin, kidneys, and gastrointestinal tract (8, 9).
Oncolytic viruses (OVs), as a novel class of cancer immunotherapies, selectively infect tumor cells and induce immunogenic cell death (ICD), promoting the release of damage-associated molecular patterns (DAMPs) and tumor-associated antigens. Additionally, they activate the cGAS–STING innate immune pathway and stimulate type I interferon production, thereby converting immunologically “cold” tumors into “hot” ones (10). Notably, in the context of melanoma/sarcoma subgroups, OVs have achieved objective response rates of 26.4–32.9%, complete responses in 15.0%, durable response rates (DRR ≥ 6 months) in 16.3%, and extended the median duration of response to 33.7 months (11, 12). However, the overall incidence of adverse events (AEs) related to OV therapy is 26.6%, nearly 2.07 times higher than in control groups (13). Furthermore, elderly patients remain severely underrepresented in OV trials, especially with those aged ≥70 years comprising only 17.7% of early-phase clinical studies (14). This lack of age-specific data casts doubt on the generalizability of OV findings to older populations.
Due to their distinctive immunosuppressive profiles (15–17), elderly patients undergoing OV therapy may be vulnerable to multiple complications, including the dual burden of immune overload and exhaustion (18, 19), cytokine storm-induced inflammation (18, 20), and compromised immune tolerance (21–23). This complex and multifactorial immune state heightens both the risks and limitations of OV-based therapy in the elderly, posing significant safety and efficacy challenges (24–26). Despite this, there is still a dearth of systematic investigations into the mechanisms of OV therapy under the backdrop of immune frailty in aging populations (14). Most existing studies rely on young or adult models and cohorts, leaving gaps in our knowledge regarding elderly-specific immune microenvironmental changes, virus–host interaction patterns, and optimal treatment timing (27).
This review seeks to elucidate the immunological features unique to elderly individuals and their interactions with OV therapy, focusing on three key questions (Figure 1):
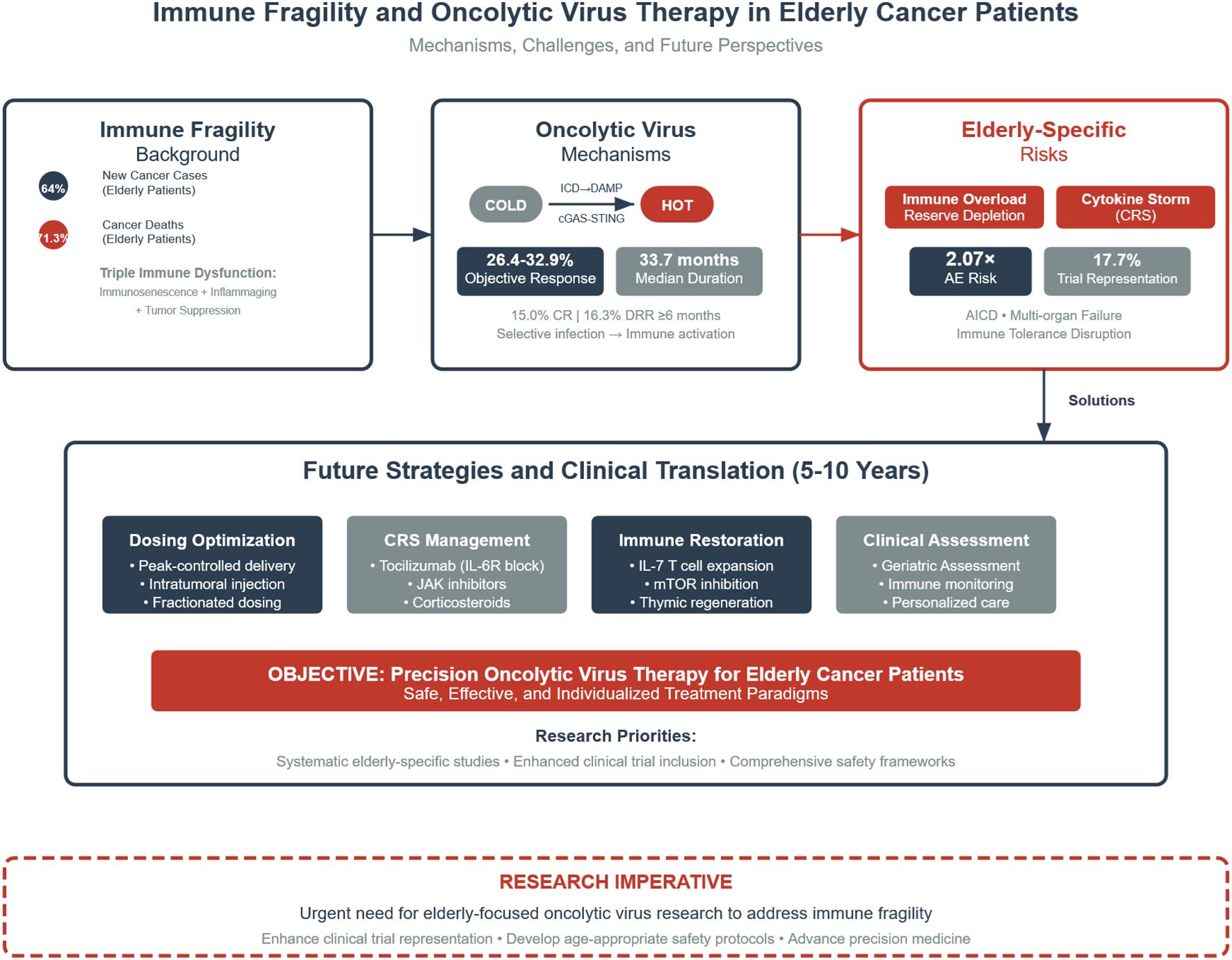
Figure 1. Integrated framework of immune fragility and oncolytic virus (OV) therapy in elderly cancer patients. ICD, immunogenic cell death; DAMP, damage-associated molecular patterns; cGAS–STING, cyclic GMP–AMP synthase–stimulator of interferon genes pathway; CRS, cytokine release syndrome. This figure illustrates the integrated framework of immunological frailty and oncolytic virus (OV) therapy in elderly cancer patients. Elderly individuals account for 64% of newly diagnosed cancers and 71.3% of cancer-related deaths, and exhibit a triple immune imbalance characterized by immunosenescence, inflammaging, and tumor-induced immunosuppression. OVs promote the conversion of “cold” tumors into “hot” tumors via immunogenic cell death (ICD), the release of damage-associated molecular patterns (DAMPs), and activation of the cGAS–STING pathway. Although clinical data remain limited for elderly patients with esophageal, lung, or pancreatic cancers, an objective response rate (ORR) of 26.4–32.9% and a median duration of response (DOR) of 33.7 months have been achieved in melanoma/sarcoma subgroups. However, elderly patients face unique risks, including immune overload and reserve exhaustion, cytokine storm (CRS), and disruption of immune tolerance, with a 2.07-fold increased risk of adverse events and underrepresentation in clinical trials (17.7%). Future strategies should focus on four key areas: optimized drug delivery, CRS management, immune reconstruction, and personalized frailty-based assessment. Dark blue elements indicate core mechanisms, grey indicates neutral or observational data, and red highlights clinical risk warnings. Arrows denote causal relationships and directional processes.
1. What are the pathophysiological characteristics of the immune microenvironment in elderly cancer patients? How do tumor-induced immunosuppression and age-related immunosenescence synergize at the molecular level to create a state of immune frailty? How does immunological vulnerability across different age stages affect immune function?
2. What unique immunotoxicities are associated with OV therapy in elderly patients? What are the mechanisms and clinical manifestations of the vicious cycle of immune overload and exhaustion, cytokine release syndrome, and loss of immune tolerance?
3. Why is it essential to prioritize the unique immune status of elderly cancer patients? Can precision interventions targeting immune frailty improve therapeutic windows? Can integrated strategies—such as immune reconstruction technologies, pharmacological optimization, and stratified management systems—maximize the benefits while minimizing the risks of OV therapy in older adults?How should this be achieved?
1.1 Unique immune frailty in elderly cancer patients
The immune microenvironment of elderly cancer patients (≥65 years) is distinctively complex (17), characterized by tumor-induced immunosuppression, age-related immunosenescence, and their synergistic disruption of immune homeostasis. Together, these factors constitute a unique state of immune frailty in elderly cancer patients.
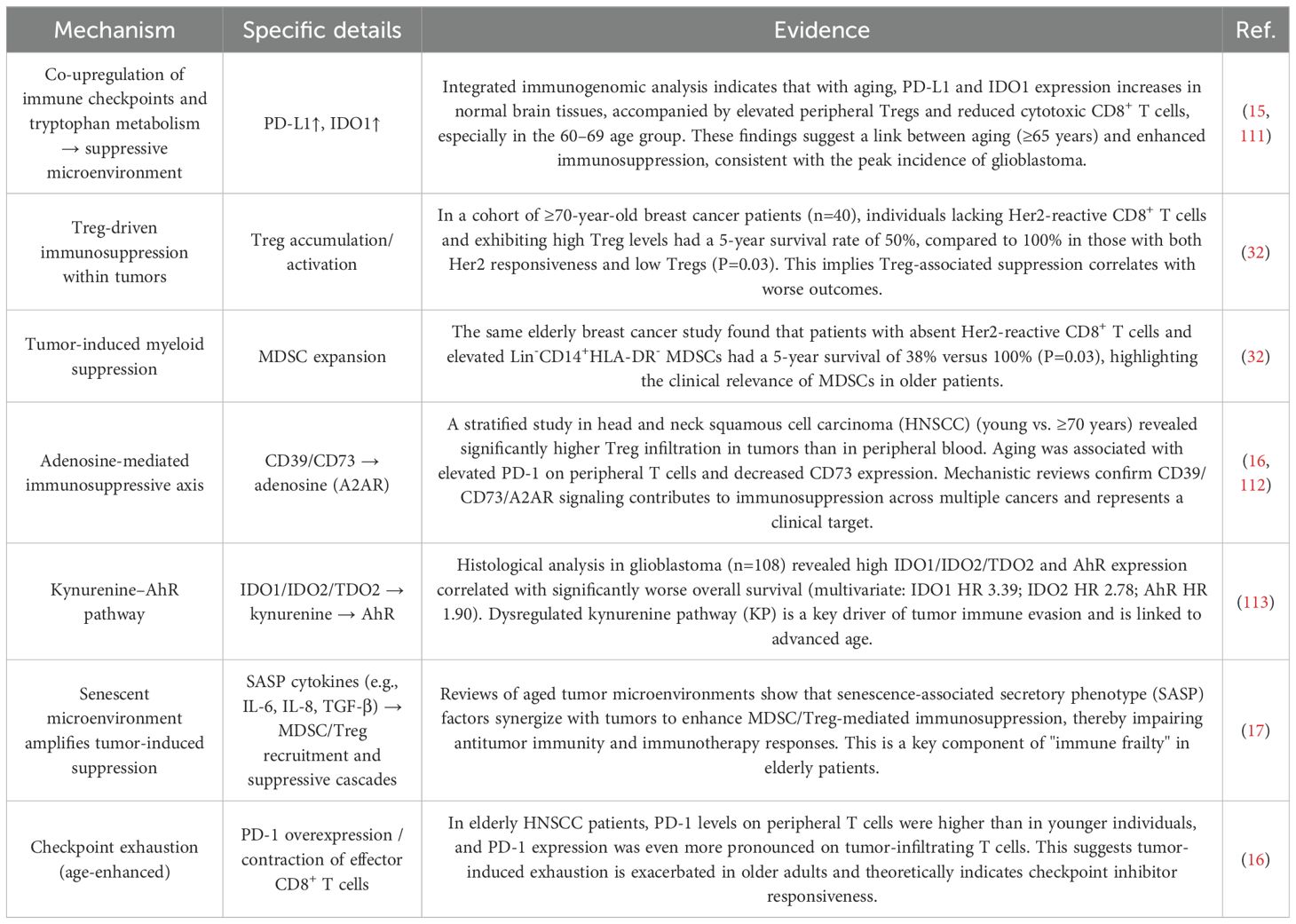
Tumor-induced immunosuppression is commonly observed in this population (Table 1). Specifically, cancer cells suppress effector T cell activity and function through the secretion of immunosuppressive cytokines such as transforming growth factor-β (TGF-β), interleukin-10 (IL-10), and vascular endothelial growth factor (VEGF) (28, 29). In addition, they activate suppressive metabolic pathways, including the denosine axis (CD39/CD73 → adenosine) and the tryptophan–kynurenine–AhR pathway (IDO1/IDO2/TDO2 → Kyn → AhR), further impairing T cell function (16). Tumor cells may also directly engage inhibitory immune checkpoints, such as programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), thereby inducing T cell apoptosis (30). Concurrently, the tumor immune microenvironment recruits and activates suppressive cell populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), both of which significantly inhibit antitumor immunity (31). The situation is further compounded by pro-inflammatory cytokines secreted via the senescence-associated secretory phenotype (SASP), including IL-6, IL-8, and TGF-β, which synergize with tumor-derived signals to exacerbate MDSC and Treg-mediated immunosuppression (17). This immunosuppressive milieu is frequently observed in elderly cancer patients and is associated with a reduced 5-year survival rate of just 38–50% among breast cancer patients aged over 70 years (32).
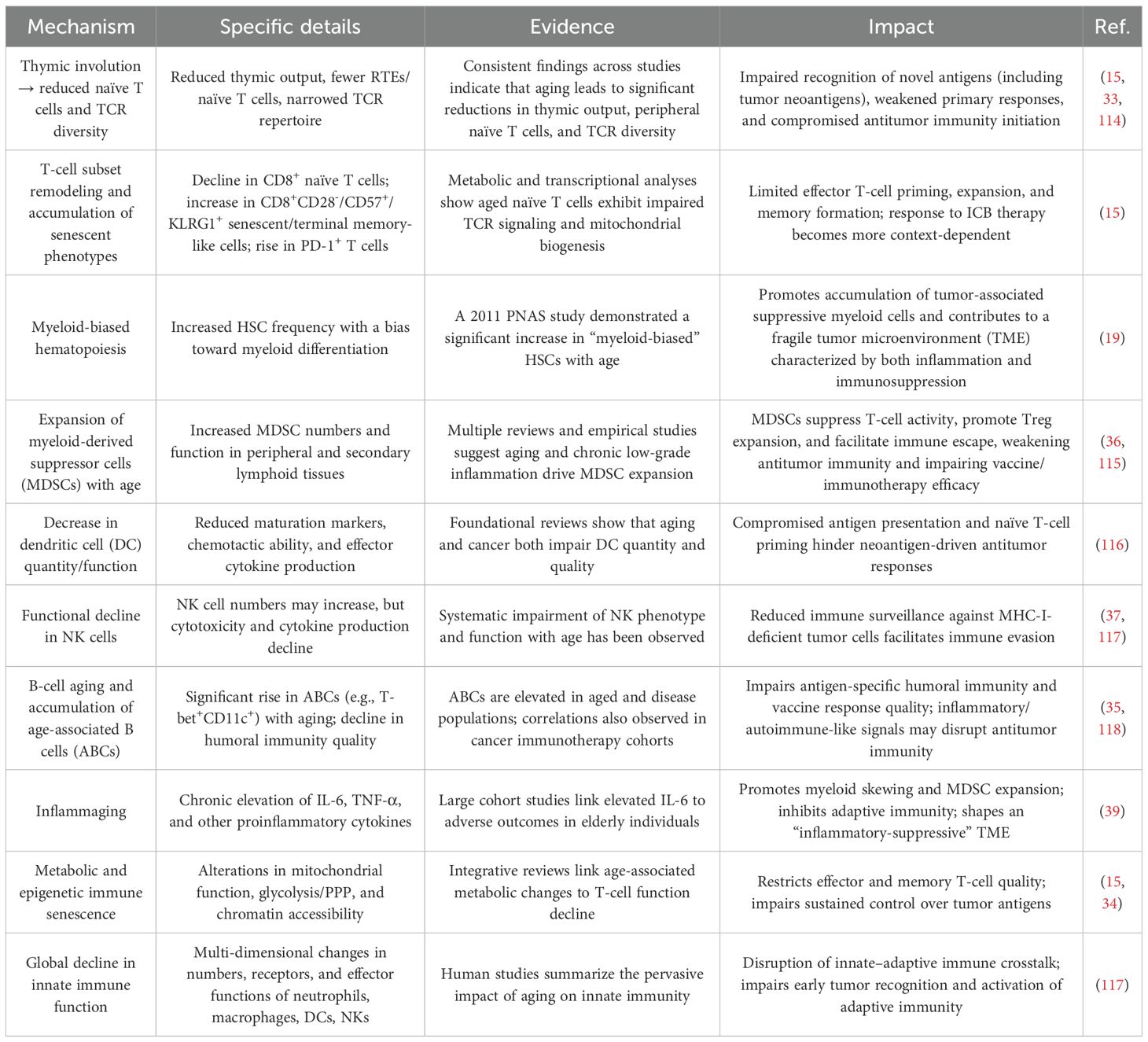
Age-related immunosenescence leads to thymic involution and a marked reduction in naïve T cells and T cell receptor (TCR) diversity and quantity, thereby compromising antigen recognition and immune responsiveness (15, 33). Metabolic reprogramming also occurs in aged T cells, affecting mitochondrial function, glycolytic pathways, and chromatin accessibility, which diminishes the quality of both effector and memory T cells (15, 34). In addition to T cell defects, age-related B cells (ABCs), particularly the T-bet+CD11c+ phenotype, become more prevalent, weakening humoral immune responses and impairing antigen-specific and antitumor immunity (35). Hematopoietic stem cells (HSCs) in the elderly exhibit a “myeloid bias” (19), resulting in increased MDSC production and activity, suppression of T cell function, and enhanced Treg expansion (36). Aging also impairs dendritic cell (DC) maturation, chemotactic ability, and cytokine secretion, leading to both quantitative and functional decline. Although natural killer (NK) cell numbers may increase with age, their cytotoxicity and cytokine production capacity are significantly diminished, compromising the first line of defense against tumors (37, 38). Moreover, elderly individuals often present with “inflammaging,” a pro-inflammatory state strongly associated with adverse outcomes such as frailty and mortality. This state is marked by chronically elevated levels of IL-6 and TNF-α (39), which further promote myeloid skewing and MDSC expansion, suppress adaptive immunity, and contribute to a tumor microenvironment characterized by both immunosuppression and chronic inflammation (40). These immunosenescence-related changes collectively result in profound immunosuppression (Table 2).
When tumor-induced immunosuppression coexists with age-related immunosenescence, their interplay gives rise to a vicious cycle of mutual reinforcement (Table 3). Chronically elevated IL-6 activates the JAK/STAT3 signaling pathway, upregulating PD-L1 expression on tumor cells. It also inhibits tumor antigen presentation via the NMD/SMG1 pathway, thereby facilitating immune escape and further weakening antitumor immunity (41, 42). SASP exacerbates immunosuppression by increasing the number of MDSCs and Tregs (17), upregulating Treg expression and FoxP3 levels (43). Tumor-derived extracellular vesicles (tEVs) carrying PD-L1 can induce T cell DNA damage and lipid metabolism reprogramming, thereby accelerating T cell senescence (44). Simultaneously, aged microenvironments release extracellular vesicles (aged-EVs) that remodel the tumor milieu to favor cancer progression. When combined with tEV-PD-L1, these factors further intensify immunosuppression (45). Tumor-secreted suppressive factors such as VEGF aggravate the existing decline in antigen presentation and IFN production capacity (46), leading to severely compromised tumor antigen recognition and presentation by antigen-presenting cells (APCs) like DCs (47).
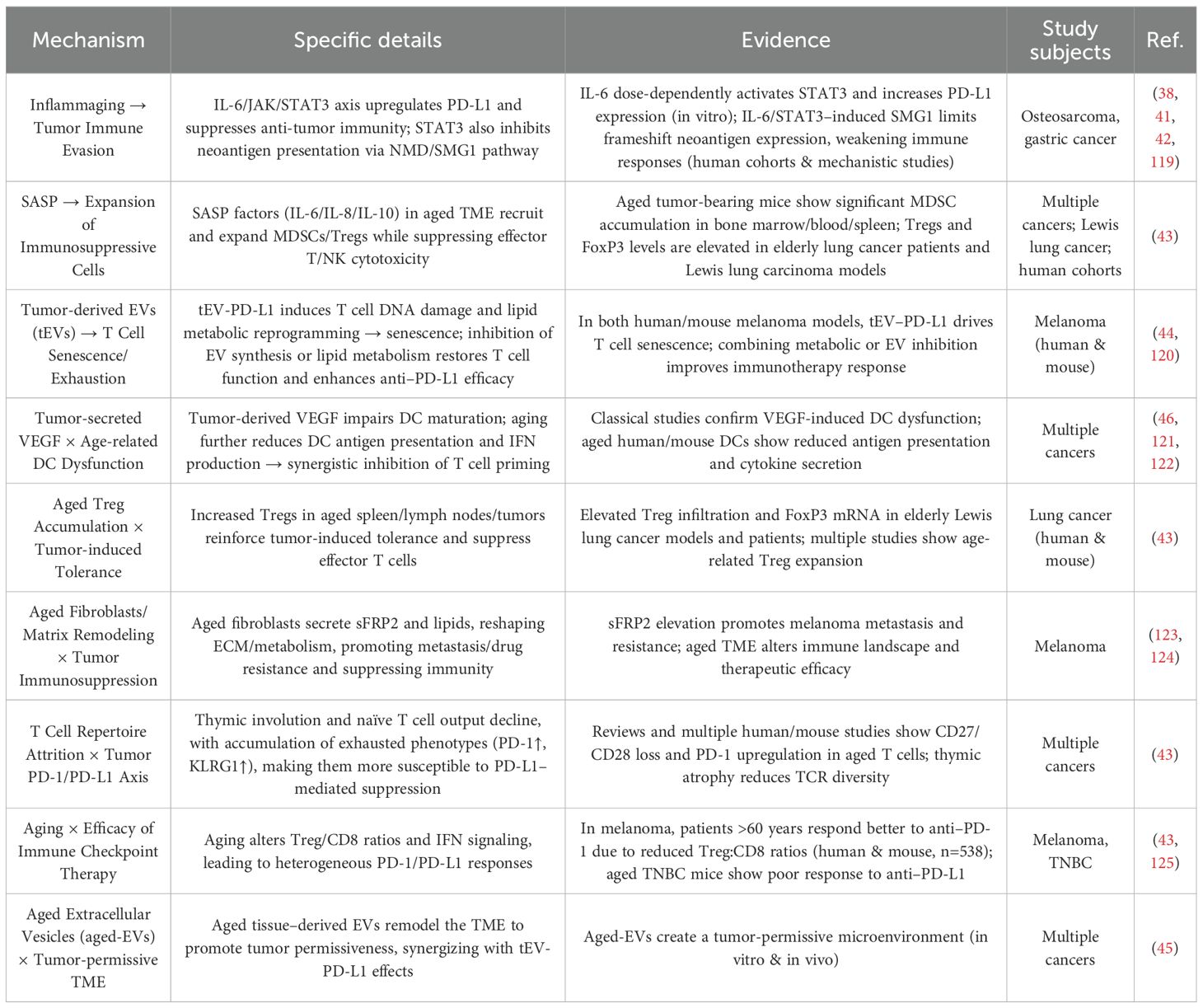
Table 3. Synergistic effects between tumor-induced immunosuppression and immunosenescence in elderly cancer patients.
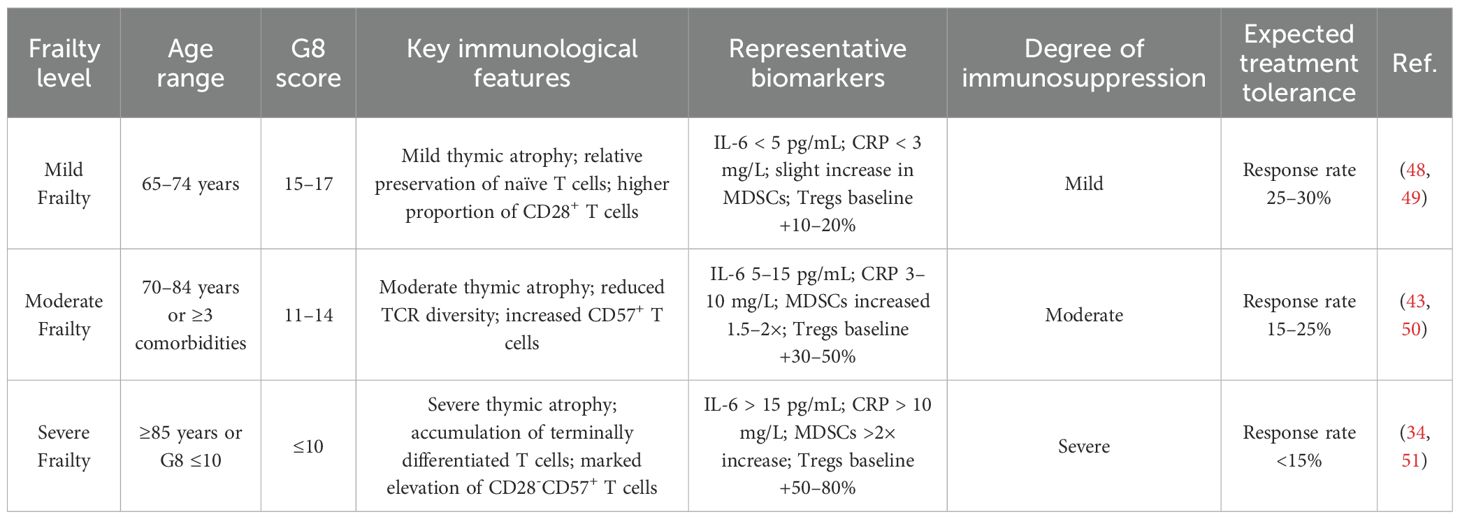
Moreover, the degree of frailty across different stages of aging significantly impacts the immune system of elderly cancer patients (Table 4). Patients with mild frailty (65–74 years, G8 score 15–17) typically exhibit only mild thymic involution. Naïve T cells are relatively preserved, baseline inflammatory markers such as IL-6 remain low (<5 pg/mL), MDSCs show only slight increases, and Tregs expand modestly (baseline +10–20%). As a result, these patients retain a 25–30% response rate to immunotherapy (48, 49). In contrast, moderately frail patients (70–84 years or ≥3 comorbidities, G8 score 11–14) exhibit more pronounced thymic atrophy, significant reductions in TCR diversity, elevated inflammatory load (IL-6 at 5–15 pg/mL), 1.5–2-fold expansion of MDSCs, and a 30–50% increase in Tregs, resulting in reduced immunotherapy response rates of 15–25% (43, 50). Severely frail patients (≥85 years or G8 score ≤10) show marked thymic atrophy, accumulation of terminally differentiated T cells (notably increased CD28–CD57+), and severe baseline inflammation (IL-6 >15 pg/mL). MDSCs are elevated by more than 2-fold, and Tregs expand by 50–80%, leading to immunotherapy response rates dropping below 15% (34, 51). These frailty-related immune differences across age groups directly affect the efficacy of OV therapy by modulating immune responsiveness, ultimately influencing therapeutic outcomes and associated risks.
1.2 Novel immunological challenges of oncolytic virus therapy in elderly cancer patients
Oncolytic virus (OV) therapy presents several immunological challenges in elderly cancer patients, including immune overload, exhaustion of immune reserves, cytokine storm-driven inflammatory cascades, and disruption of self-tolerance. These adverse effects can severely compromise the already fragile immune landscape in aged individuals (Figure 2).
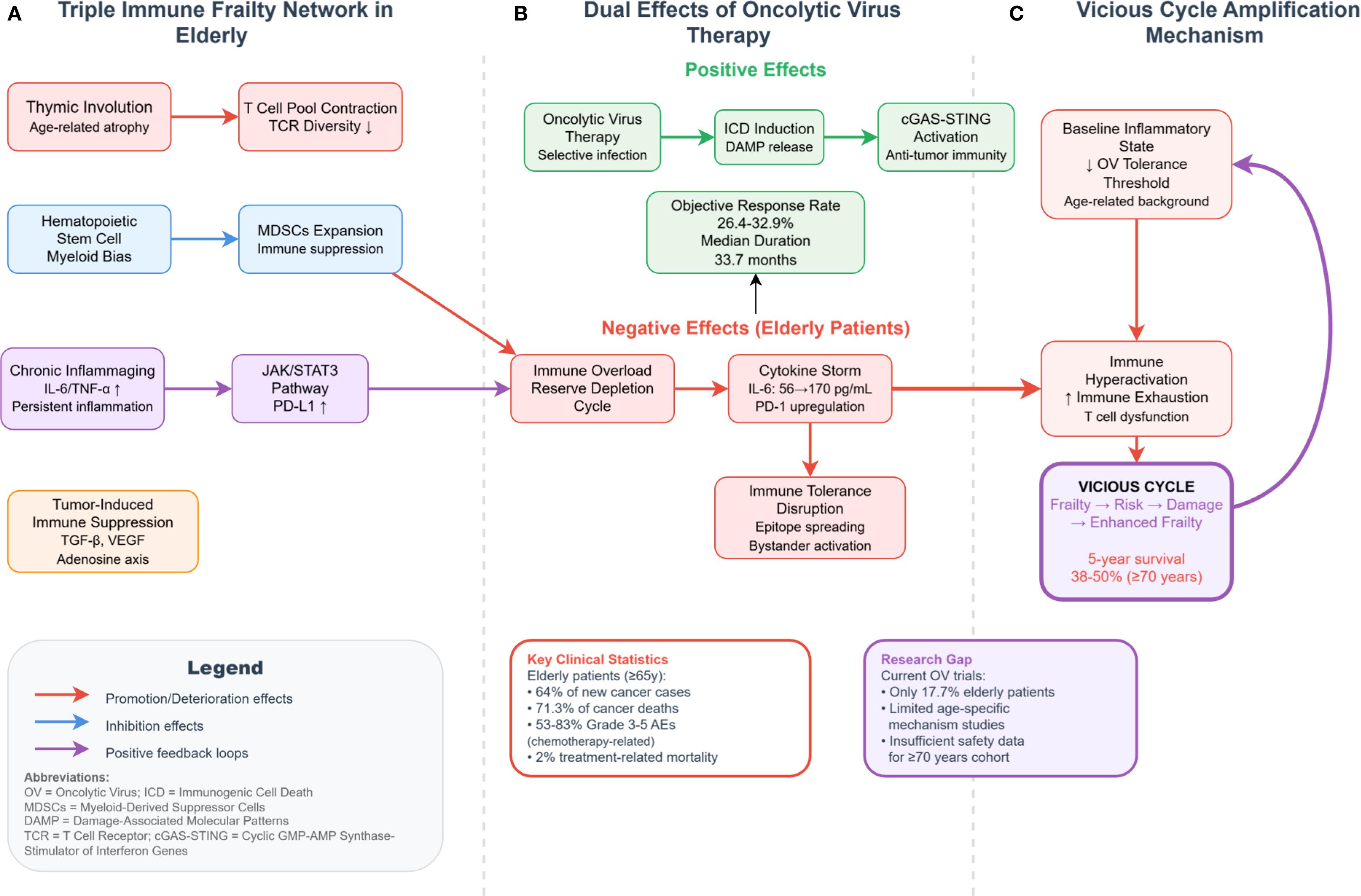
Figure 2. Molecular network of immune fragility and OV therapy interactions in elderly cancer patients. ICD, immunogenic cell death; DAMP, damage-associated molecular patterns; MDSCs, myeloid-derived suppressor cells; SASP, senescence-associated secretory phenotype; tEVs, tumor-derived extracellular vesicles; AICD, activation-induced cell death. This figure delineates the mechanistic interplay between immune fragility and OV therapy-associated risks in elderly cancer patients. (A) Triple-network of age-related immune dysfunction: Thymic involution reduces T-cell repertoire diversity; hematopoietic stem cell myeloid bias expands MDSCs; and chronic inflammaging (elevated IL-6/TNF-α) activates the JAK/STAT3–PD-L1 axis, which cooperates with tumor-derived immunosuppressive signals (e.g., TGF-β, VEGF, and adenosine pathway) to establish a coexisting immunosuppressive and inflammatory state. (B) Dual-edged effects of OV therapy: On the one hand, OVs trigger antitumor immunity via ICD–DAMP–cGAS–STING activation, facilitating immunologic conversion of cold tumors. On the other hand, their adverse effects are amplified in elderly patients, leading to: Immune overactivation and exhaustion cycles (e.g., surge in pro-inflammatory cytokines → PD-1 upregulation → T-cell dysfunction); Cytokine storms, with IL-6 levels rising from 56 to 170 pg/mL; Breakdown of immune tolerance via epitope spreading and bystander activation. (C) Feedback amplification loop: Preexisting low inflammatory thresholds in elderly patients reduce OV tolerability, while OV-induced immune hyperactivation further exacerbates immune exhaustion. This reinforces a vicious cycle of “fragility–risk–damage–increased fragility,” ultimately contributing to a 5-year survival rate of only 38–50% in patients aged 70 and above. Red arrows denote aggravating effects; blue arrows represent inhibitory effects; dashed arrows indicate positive feedback loops.
A major concern is the vicious cycle between immune overload and immune reserve exhaustion, which are tightly interconnected and dialectically unified (Table 5). Specifically, OV therapy triggers acute surges in pro-inflammatory cytokines such as IL-6, type I/II interferons, and TNF, which markedly upregulate Pdcd1 (PD-1) and Cd274 (PD-L1) transcription in T cells, leading to functional overload and subsequent depletion of immune reserves (18, 52). Moreover, combinatorial regimens involving T-VEC and chemotherapeutics (e.g., doxorubicin) can further escalate IL-6 levels from ~56 pg/mL to ~170 pg/mL, intensifying this immunological strain (53).
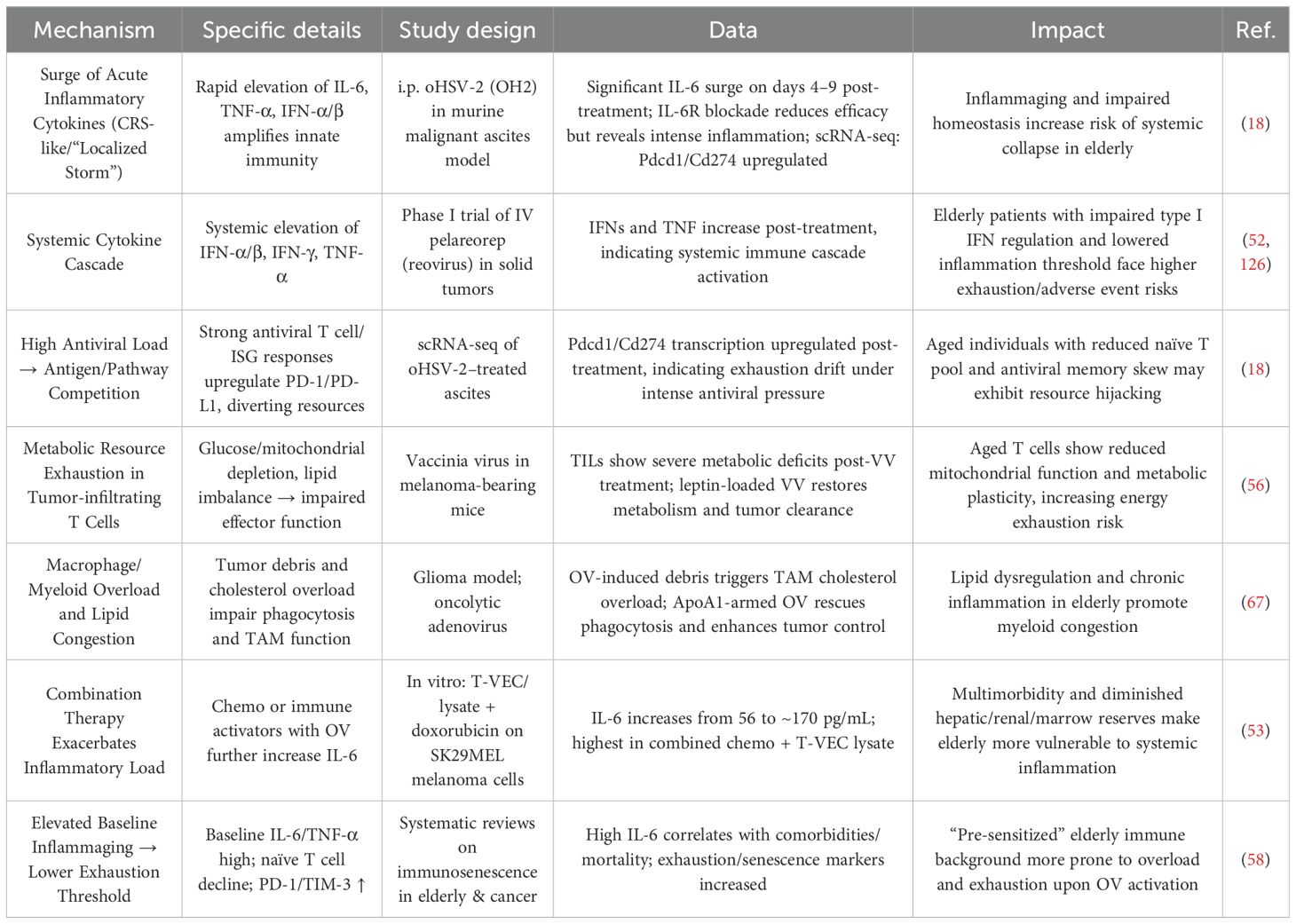
Table 5. The dual malignant cycle of immune overload and immunological resource exhaustion in elderly cancer patients under OV therapy.
Elderly individuals inherently exhibit elevated baseline levels of IL-6 and TNF-α, reduced naïve T-cell pools, and increased expression of exhaustion markers (e.g., PD-1, TIM-3), indicative of immunosenescence (54, 55). This aging-associated immune state exacerbates the risk of immune overload and exhaustion under OV therapy, contributing to lymphopenia and impaired T-cell metabolism (56–58). Additionally, OV-induced hematopoietic stem cell depletion worsens metabolic dysfunction and deepens immunosuppression (19).
Conversely, immune exhaustion can also precipitate further immune overload. OV treatment activates robust antiviral T-cell responses and upregulates interferon-stimulated genes (ISGs), fostering a suppressive immune milieu via checkpoint molecule induction. In elderly patients, prior viral exposures contribute to memory T-cell bias, reducing the naïve T-cell repertoire (59–61). OV-induced inflammation disproportionately burdens the remaining unskewed T cells, leading to their numerical expansion but diminished function (62–64). This paradoxical expansion is frequently followed by contraction via activation-induced cell death (AICD) (65, 66), culminating in accelerated immune reserve depletion. Moreover, tumor lysis by OVs generates debris accumulation, triggering cholesterol overload in tumor-associated macrophages and impairing their phagocytic capacity, further taxing immune resources (67).
OV therapy can also induce cytokine release syndrome (CRS), characterized by explosive surges of IL-6 and TNF-α within a short period (18). CRS represents one of the most life-threatening acute toxicities of OV therapy (68, 69). Despite prophylactic use of potent corticosteroids (e.g., dexamethasone) (70) or localized administration strategies (25), CRS manifestations such as fever, elevated AST, thrombocytopenia, and treatment interruptions remain common (20). The self-amplifying “inflammation–immunosuppression cycle” in the elderly, characterized by increased levels of immunosuppressive metabolites such as lactate, further amplifies the severity and long-term consequences of CRS (71–73). However, systematic age-specific incidence data remain lacking, limiting the ability to quantitatively assess the severity and exact frequency of adverse events in elderly patients (Table 6).
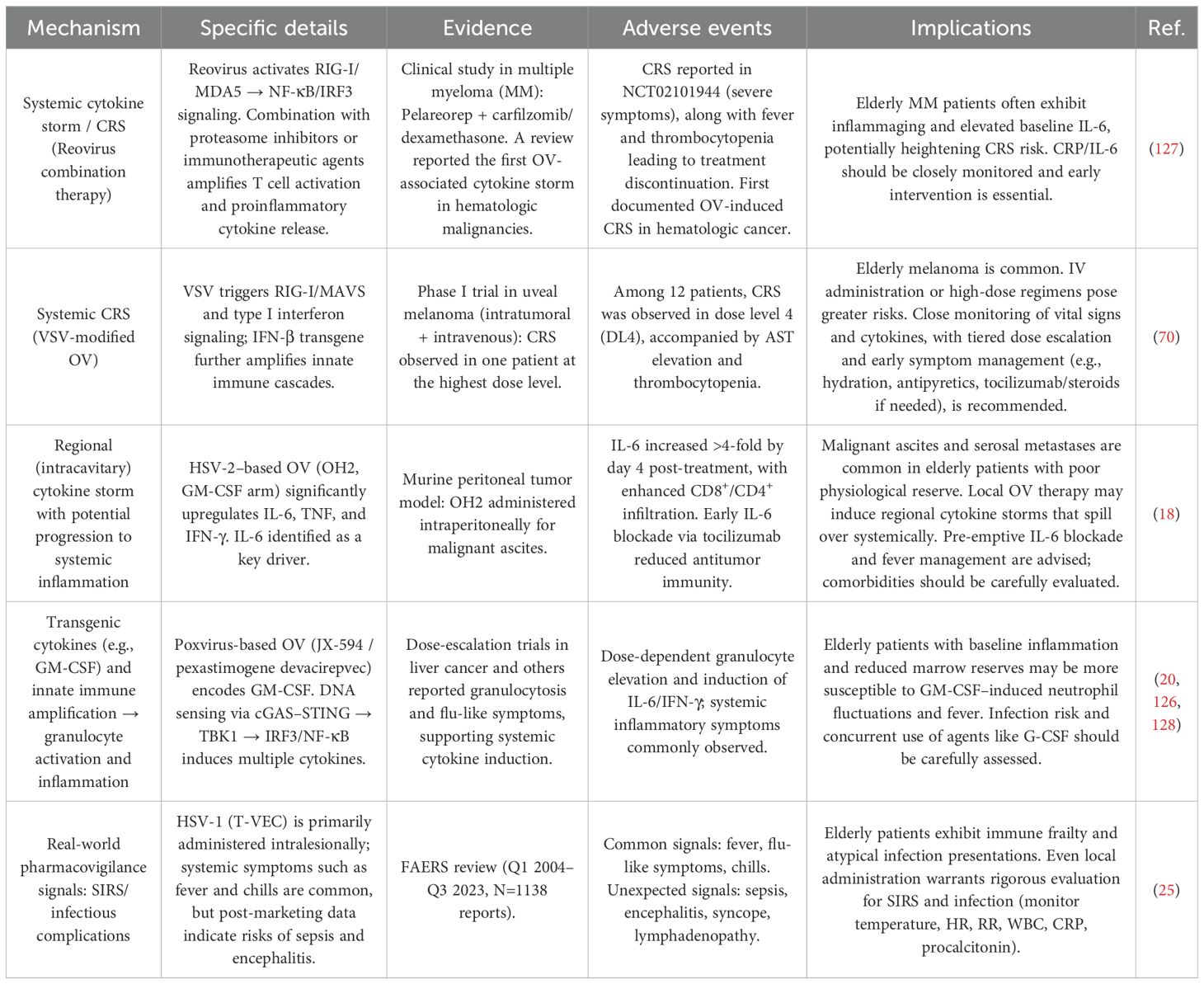
Table 6. Inflammatory cascade reactions (CRS/SIRS) induced by oncolytic viruses in elderly cancer patients.
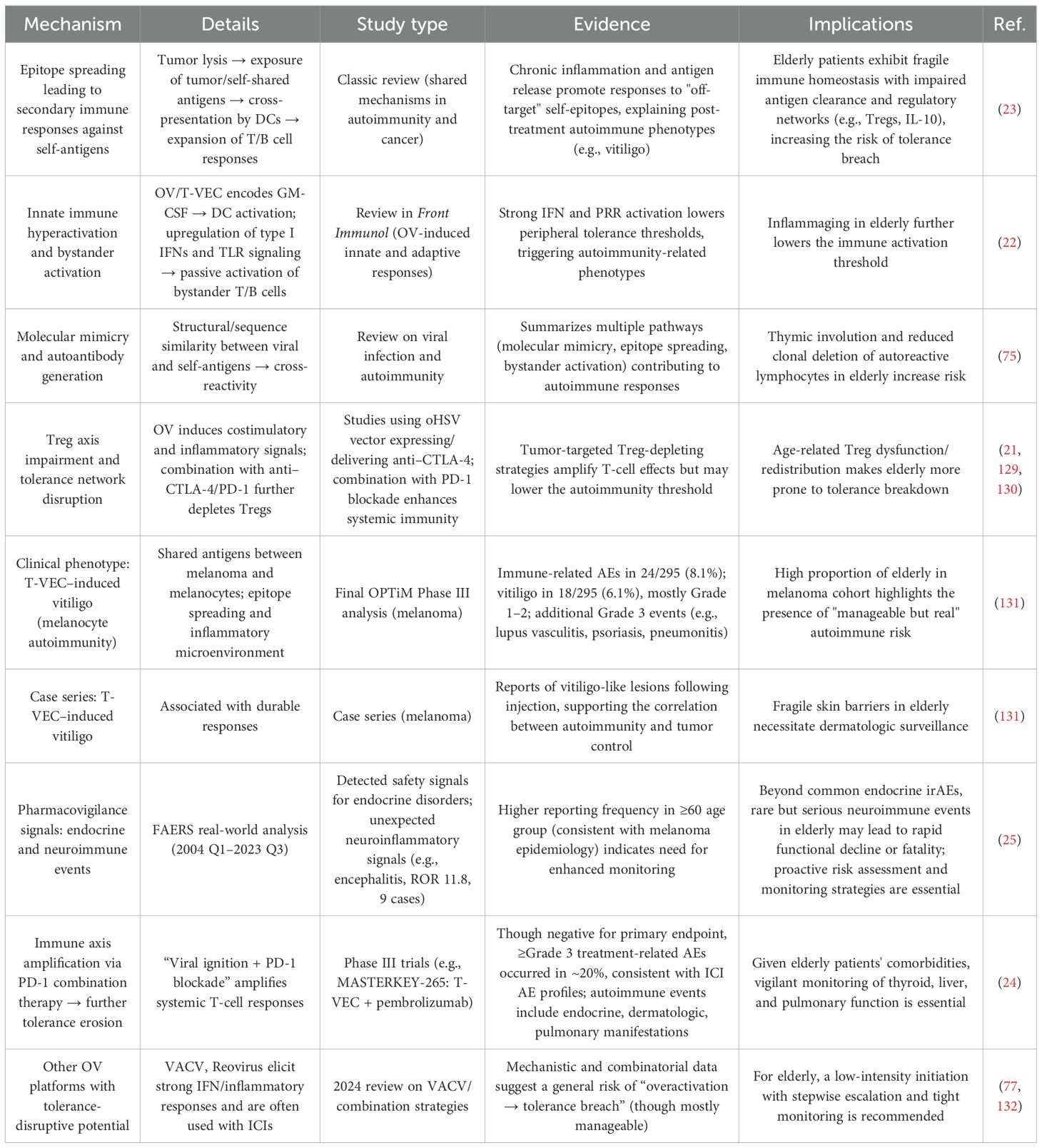
Furthermore, OV therapy disrupts immune tolerance in elderly individuals. Rapid lysis of tumor cells releases large quantities of shared self-tumor antigens, which are cross-presented by antigen-presenting cells (APCs), eliciting secondary T/B cell responses against non-target self-epitopes and triggering autoimmune reactions (23, 74). Potent activation of dendritic cells and the upregulation of type I IFN and TLR signaling lower the threshold for immune tolerance, promoting bystander activation of autoreactive lymphocytes (22). Additionally, molecular mimicry between OV components and self-antigens drives T cell–mediated autoimmune cross-reactivity (75).
Genetically engineered OVs expressing immune modulators such as anti–CTLA-4 further amplify T-cell responses, intensifying autoimmune pathology and impairing immune tolerance (21). As a result, OV therapy has been associated with various immune-related adverse events (irAEs), including vitiligo, lupus vasculitis, psoriasis, pneumonitis, and encephalitis (25). These risks are significantly heightened when OVs are combined with immune checkpoint inhibitors, leading to increased rates of grade ≥3 treatment-related adverse events (24), and underscoring the compounded vulnerability of immune tolerance in aged hosts (76, 77).
Table 7 provides a comprehensive overview of the mechanisms, evidence, and clinical data supporting OV-induced disruption of immune self-tolerance.
2 Discussion
Over the next 5–10 years, the immunological frailty of elderly cancer patients is expected to be progressively overcome (Table 8). Tumor-induced immunosuppression may be alleviated through a range of combination strategies, such as the use of the A2A receptor antagonist ciforadenant in conjunction with anti–PD-L1 therapy (78), CD73 inhibitors paired with immune checkpoint blockade (79, 80), and VEGF-Trap agents that correct dendritic cell differentiation defects (81). Furthermore, the development of novel drugs is anticipated based on evidence that the PDE5 inhibitor tadalafil effectively reduces MDSC and Treg levels (82).
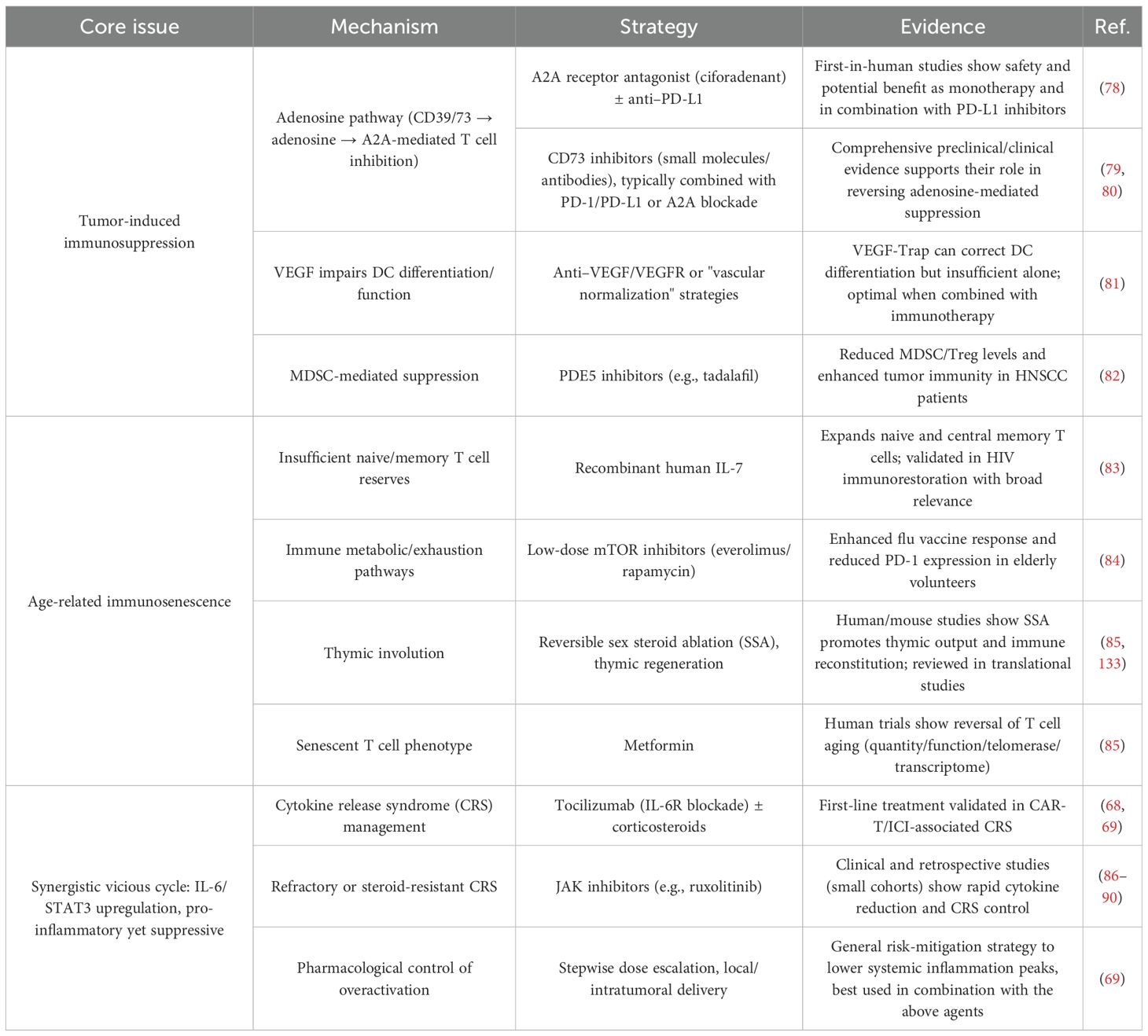
Table 8. Strategies to address the three core dimensions of immune fragility in elderly cancer patients.
Age-related immunosenescence is likely to be addressed by augmenting the quantity and quality of immune cells. Quantitative improvement may be achieved using cytokines such as recombinant human IL-7 to expand naïve and central memory T cells (83). Qualitative enhancement may involve low-dose mTOR inhibitors to downregulate PD-1 expression in T cells (84) and pharmacological agents such as metformin to attenuate T cell senescence through multidimensional mechanisms (85). In addition, reversible sex steroid ablation (SSA) and thymic regeneration approaches are under exploration to restore immune competency (85), providing a multifaceted strategy to counter immunosenescence.
To interrupt the vicious cycle between immunosuppression and immune exhaustion, standard treatment regimens such as tocilizumab combined with glucocorticoids—commonly used for CRS induced by CAR-T or checkpoint inhibitors—will be expanded (68, 69). JAK inhibitors like ruxolitinib, which can rapidly suppress cytokine surges and alleviate CRS, are also being investigated for broader application in immune-related toxicity control (86–90), paving the way for the development of next-generation interventions.
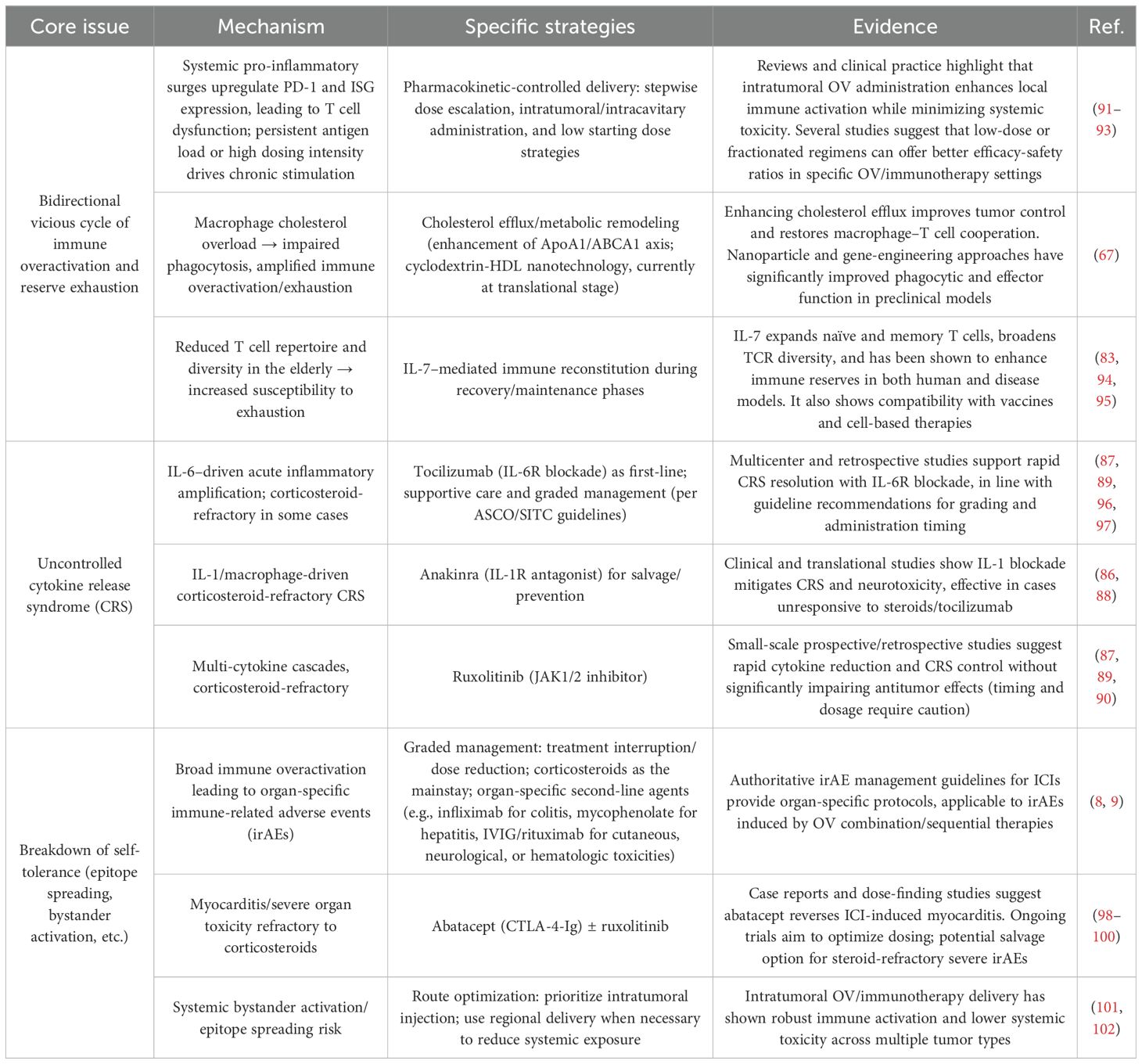
In parallel, the three core immune-related challenges induced by oncolytic virus (OV) therapy in elderly cancer patients will likely be tackled within the coming decade (Table 9). To break the bidirectional vicious cycle of immune overload and immune reserve exhaustion, emerging strategies include pharmacokinetic-based dosing (“controlled-peak” regimens), intratumoral administration to enhance local immune activation and reduce systemic toxicity, and low-dose fractionated schedules that optimize efficacy–safety ratios (91–93). Additional approaches involve enhancing cholesterol efflux and metabolic reprogramming to activate the ApoA1/ABCA1 pathway and improve macrophage phagocytosis and macrophage–T cell synergy (67), as well as IL-7–mediated expansion of T cell pools and diversification of the TCR repertoire to “replenish immune reserves” (83, 94, 95).
The challenge of cytokine storm (CRS) will be addressed through first-line use of IL-6R blockers such as tocilizumab for rapid symptom relief (87, 89, 96, 97). For steroid-refractory CRS, IL-1R blockers (e.g., anakinra) and JAK1/2 inhibitors (e.g., ruxolitinib) will be employed to mitigate cytokine levels while preserving antitumor activity (86–90). This comprehensive, multi-tiered control framework is essential for CRS prevention and management.
The disruption of immune tolerance associated with OV-based regimens will be managed using a structured irAE management protocol based on current ICI guidelines. First-line glucocorticoids will serve as foundational therapy, with second-line, organ-specific agents such as infliximab for colitis, mycophenolate mofetil for hepatitis, and IVIG/rituximab for dermatologic and hematologic toxicity (8, 9). For cardiotoxicity, the combination of abatacept (CTLA-4-Ig) and ruxolitinib has shown promise in reversing ICI-induced myocarditis (98–100). Drug delivery route optimization—via intratumoral or regional administration when possible—will further reduce systemic exposure and minimize the risk of bystander immune activation (101, 102), offering a holistic strategy to restore immune tolerance.
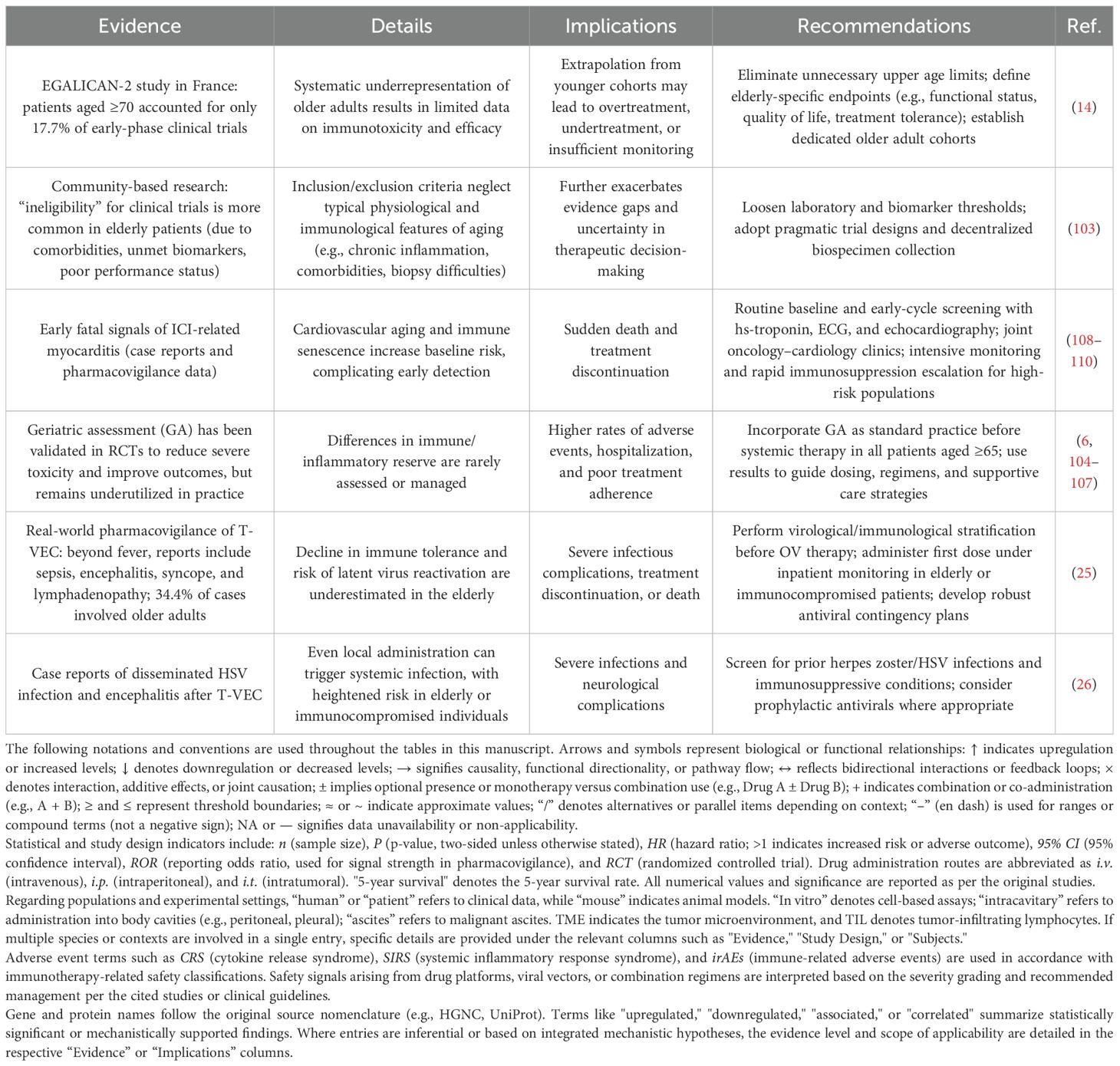
Despite these advancements, the unique immune landscape of elderly cancer patients remains marginalized (Table 10). Due to comorbidities, suboptimal biomarker profiles, and compromised performance status (103), elderly individuals comprise only 17.7% of early-phase clinical trial participants (14). Within the context of OV therapy, elderly patients account for 34.4% of immune-related adverse events (25), and are at heightened risk of severe complications—such as disseminated HSV infection or encephalitis—even with localized treatments like T-VEC (26).
However, geriatric assessment (GA), a tool proven to reduce severe toxicity and improve outcomes, remains underutilized in routine clinical practice. This oversight leads to insufficient immunological risk assessment, ultimately contributing to elevated rates of adverse events, hospitalizations, treatment non-compliance (6, 104–107), and even unexpected mortality or treatment discontinuation (108–110).
Therefore, at the clinical level, we recommend establishing a three-tiered frailty-based dosing strategy: standard-dose regimens for mildly frail patients, 25–50% dose reduction for moderately frail patients, and cautious risk–benefit evaluation for severely frail patients. In addition, a CRS early-warning system should be implemented, focusing on three critical markers: IL-6 levels, body temperature fluctuations, and platelet count. For high-risk individuals with a G8 score ≤14, enhanced monitoring protocols should be applied. Moreover, geriatric assessment (GA) should be standardized as a prerequisite for OV treatment in patients aged ≥65 years. In terms of clinical research, elderly patients should comprise ≥30% of enrolled participants, with at least three ongoing trials dedicated to dose optimization in this population. Furthermore, a dedicated OV safety database containing ≥200 elderly cases should be established to quantify age-specific risks and support precision medicine initiatives.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
J-WW: Conceptualization, Investigation, Validation, Visualization, Writing – original draft. J-HL: Project administration, Supervision, Writing – original draft. Y-LL: Supervision, Writing – review & editing. W-ZX: Supervision, Writing – review & editing. Z-BZ: Project administration, Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Li L, Shan T, Zhang D, and Ma F. Nowcasting and forecasting global aging and cancer burden: analysis of data from the GLOBOCAN and Global Burden of Disease Study. J Natl Cancer Cent. (2024) 4:223–32. doi: 10.1016/j.jncc.2024.05.002
3. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes. Front Immunol. (2017) 8:1960. doi: 10.3389/fimmu.2017.01960
4. Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. (2018) 19:10–9. doi: 10.1038/s41590-017-0006-x
5. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. (2011) 29:3457–65. doi: 10.1200/JCO.2011.34.7625
6. Li D, Sun CL, Kim H, Soto-Pérez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: A randomized clinical trial. JAMA Oncol. (2021) 7:e214158. doi: 10.1001/jamaoncol.2021.4158
7. Nie RC, Chen GM, Wang Y, Zhou J, Duan JL, Zhou ZW, et al. Efficacy of anti-PD-1/PD-L1 monotherapy or combinational therapy in patients aged 75 years or older: A study-level meta-analysis. Front Oncol. (2021) 11:538174. doi: 10.3389/fonc.2021.538174
8. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9(6):e002435. doi: 10.1136/jitc-2021-002435
9. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
10. Bommareddy PK, Zloza A, Rabkin SD, and Kaufman HL. Oncolytic virus immunotherapy induces immunogenic cell death and overcomes STING deficiency in melanoma. Oncoimmunology. (2019) 8:1591875. doi: 10.1080/2162402X.2019.1591875
11. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. (2015) 33:2780–8. doi: 10.1200/JCO.2014.58.3377
12. Wong MK, Milhem MM, Sacco JJ, Michels J, In GK, Couselo EM, et al. RP1 combined with nivolumab in advanced anti-PD-1-failed melanoma (IGNYTE). J Clin Oncol. (2025), 101200JCO2501346. doi: 10.1200/JCO-25-01346
13. Lau P, Liang L, Chen X, Zhang J, Liu H, et al. Comparative safety and efficacy of oncolytic virotherapy for the treatment of individuals with Malignancies: a systematic review, meta-analysis, and Bayesian network meta-analysis. EClinicalMedicine. (2025) 86:103362. doi: 10.1016/j.eclinm.2025.103362
14. Baldini C, Charton E, Schultz E, Auroy L, Italiano A, Robert M, et al. Access to early-phase clinical trials in older patients with cancer in France: the EGALICAN-2 study. ESMO Open. (2022) 7:100468. doi: 10.1016/j.esmoop.2022.100468
15. Han S, Georgiev P, Ringel AE, Sharpe AH, Haigis MC, et al. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. (2023) 35:36–55. doi: 10.1016/j.cmet.2022.11.005
16. Jeske SS, Schuler PJ, Doescher J, Theodoraki MN, Laban S, Brunner C, et al. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun Ageing. (2020) 17:3. doi: 10.1186/s12979-020-0174-7
17. Zhao B, Wu B, Feng N, Zhang X, Zhang X, Wei Y, et al. Aging microenvironment and antitumor immunity for geriatric oncology: the landscape and future implications. J Hematol Oncol. (2023) 16:28. doi: 10.1186/s13045-023-01426-4
18. Dong S, Liu B, Hu S, Guo F, Zhong Y, Cai Q, et al. A novel oncolytic virus induces a regional cytokine storm and safely eliminates Malignant ascites of colon cancer. Cancer Med. (2022) 11:4297–309. doi: 10.1002/cam4.4772
19. Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. (2011) 108:20012–7. doi: 10.1073/pnas.1116110108
20. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. (2013) 19:329–36. doi: 10.1038/nm.3089
21. Azar F, Deforges J, Demeusoit C, Kleinpeter P, Remy C, Silvestre N, et al. TG6050, an oncolytic vaccinia virus encoding interleukin-12 and anti-CTLA-4 antibody, favors tumor regression via profound immune remodeling of the tumor microenvironment. J Immunother Cancer. (2024) 12(7):e009302. doi: 10.1136/jitc-2024-009302
22. Marelli G, Howells A, Lemoine NR, and Wang Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front Immunol. (2018) 9:866. doi: 10.3389/fimmu.2018.00866
23. Vanderlugt CJ and Miller SD. Epitope spreading. Curr Opin Immunol. (1996) 8:831–6. doi: 10.1016/S0952-7915(96)80012-4
24. Chesney JA, Ribas A, Long GV, Kirkwood JM, Dummer R, Puzanov I, et al. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J Clin Oncol. (2023) 41:528–40. doi: 10.1200/JCO.22.00343
25. Hong Y, Cheng K, Qu H, Wang Y, Wang Y, Fan G, et al. Safety of talimogene laherparepvec: a real-world retrospective pharmacovigilance study based on FDA Adverse Event Reporting System (FAERS). J Pharm Health Care Sci. (2024) 10:79. doi: 10.1186/s40780-024-00388-0
26. Kimmis BD, Luu Y, and Dai H. Disseminated herpes infection following talimogene laherparepvec administration. JAMA Dermatol. (2022) 158:456–7. doi: 10.1001/jamadermatol.2022.0020
27. Pooladvand P, Yun C, Yoon A-, Kim PS, and Frascoli F. The role of viral infectivity in oncolytic virotherapy outcomes: A mathematical study. Math BIOSCIENCES. (2021) 334:108520. doi: 10.1016/j.mbs.2021.108520
28. Ni Y, Soliman A, Joehlin-Price A, Abdul-Karim F, Rose PG, and Mahdi H. Immune cells and signatures characterize tumor microenvironment and predict outcome in ovarian and endometrial cancers. Immunotherapy. (2021) 13:1179–92. doi: 10.2217/imt-2021-0080
29. Zhang Z, Yang A, Chaurasiya S, Park AK, Kim SI, Lu J, et al. Anti-tumor immunogenicity of the oncolytic virus CF33-hNIS-antiPDL1 against ex vivo peritoneal cells from gastric cancer patients. Int J Mol Sci. (2023) 24(18):14189. doi: 10.3390/ijms241814189
30. Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase ib study. Clin Cancer Res. (2020) 26:71–81. doi: 10.1158/1078-0432.CCR-19-2078
31. Qin H, Liu J, Qu Y, Li YY, Xu YL, and Yan YF. The intratumoral microbiota biomarkers for predicting survival and efficacy of immunotherapy in patients with ovarian serous cystadenocarcinoma. J Ovarian Res. (2024) 17:140. doi: 10.1186/s13048-024-01464-7
32. Bailur JK, Gueckel B, Derhovanessian E, and Pawelec G. Presence of circulating Her2-reactive CD8 + T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. (2015) 17:34. doi: 10.1186/s13058-015-0541-z
33. Palmer DB. The effect of age on thymic function. Front Immunol. (2013) 4:316. doi: 10.3389/fimmu.2013.00316
34. Liu Z, Liang Q, Ren Y, Zhu Y, Zhang H, Wang X, et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther. (2023) 8:200. doi: 10.1038/s41392-023-01451-2
35. Yam-Puc JC, Hosseini Z, Horner EC, Pereyra Gerber P, Beristain-Covarrubias N, Hughes R, et al. Age-associated B cells predict impaired humoral immunity after COVID-19 vaccination in patients receiving immune checkpoint blockade. Nat Commun. (2023) 14:3292. doi: 10.1038/s41467-023-38810-0
36. Pawelec G, Picard E, Bueno V, Verschoor CP, and Ostrand-Rosenberg S. MDSCs, ageing and inflammageing. Cell Immunol. (2021) 362:104297. doi: 10.1016/j.cellimm.2021.104297
37. Brauning A, Rae M, Zhu G, Chidambaram S, Saunders PTK, Nixon M, et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells. (2022) 11(6):1017. doi: 10.3390/cells11061017
38. Chan LC, Li CW, Xia W, Hsu JL, Lee HH, Cha JH, et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. (2019) 129:3324–38. doi: 10.1172/JCI126022
39. Ferrucci L and Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
40. Manyam M, Stephens AJ, Kennard JA, Donovan E, Thomas S, Galanis E, et al. A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer. Gynecol Oncol. (2021) 163:481–9. doi: 10.1016/j.ygyno.2021.10.069
41. Meraviglia-Crivelli D, Villanueva H, Zheleva A, Lambert J, Dietrich PY, Jandus C, et al. IL-6/STAT3 signaling in tumor cells restricts the expression of frameshift-derived neoantigens by SMG1 induction. Mol Cancer. (2022) 21:211. doi: 10.1186/s12943-022-01679-6
42. Rahmadiani N, Norahmawati E, Endharti AT, Hambalie AO, and Isma S. PD-L1, STAT3, IL6, and EGFR immunoexpressions in high-grade osteosarcoma. Adv Orthop. (2024) 2024:9036225. doi: 10.1155/2024/9036225
43. Lian J, Yue Y, Yu W, Zhang Y, et al. Immunosenescence: a key player in cancer development. J Hematol Oncol. (2020) 13:151. doi: 10.1186/s13045-020-00986-z
44. Ma F, Liu X, Zhang Y, Vayalil J, Lee G, Wang Y, et al. Tumor extracellular vesicle-derived PD-L1 promotes T cell senescence through lipid metabolism reprogramming. Sci Transl Med. (2025) 17:eadm7269. doi: 10.1126/scitranslmed.adm7269
45. Hüser L, Chhabra Y, Gololobova O, Wang Z, Liu A, Dixit R, et al. Aged fibroblast-derived extracellular vesicles promote angiogenesis in melanoma. Cell Rep. (2024) 43:114721. doi: 10.1016/j.celrep.2024.114721
46. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. (1996) 2:1096–103. doi: 10.1038/nm1096-1096
47. Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. (2018) 36:1658–67. doi: 10.1200/JCO.2017.73.7379
48. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. (2012) 23:2166–72. doi: 10.1093/annonc/mdr587
49. Kao C, Charmsaz S, Tsai HL, Aziz K, Shu DH, Munjal K, et al. Age-related divergence of circulating immune responses in patients with solid tumors treated with immune checkpoint inhibitors. Nat Commun. (2025) 16:3531. doi: 10.1038/s41467-025-58512-z
50. Thomas R, Wang W, and Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing. (2020) 17:2. doi: 10.1186/s12979-020-0173-8
51. Van Herck Y, Feyaerts A, Alibhai S, Migeotte I, Cools L, Deckx L, et al. Is cancer biology different in older patients? Lancet Healthy Longevity. (2021) 2:e663–77. doi: 10.1016/S2666-7568(21)00179-3
52. White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. (2008) 15:911–20. doi: 10.1038/gt.2008.21
53. Delic M, Boeswald V, Goepfert K, Pabst P, and Moehler M. In vitro characterization of enhanced human immune responses by GM-CSF encoding HSV-1-induced melanoma cells. Onco Targets Ther. (2022) 15:1291–307. doi: 10.2147/OTT.S350136
54. Jirovec E, Quixabeira D, Clubb J, Histola J, Cerullo V, Ranki T, et al. Single intravenous administration of oncolytic adenovirus TILT-123 results in systemic tumor transduction and immune response in patients with advanced solid tumors. J Exp Clin Cancer Res. (2024) 43:297. doi: 10.1186/s13046-024-03219-0
55. Pakola SA, Clubb J, Kudling TV, Mäkelä I, Ahtiainen L, Ranki T, et al. Transient lymphocyte count decrease correlates with oncolytic adenovirus efficacy in humans: mechanistic and biomarker findings from TUNIMO phase I trial. J Immunother Cancer. (2025) 13(1):e010493. doi: 10.1136/jitc-2024-010493
56. Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Cillo AR, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity. (2019) 51:548–560.e4. doi: 10.1016/j.immuni.2019.07.003
57. Graham AL, Schrom EC 2nd, and Metcalf C. The evolution of powerful yet perilous immune systems. Trends Immunol. (2022) 43:117–31. doi: 10.1016/j.it.2021.12.002
58. Møller SH, Hsueh PC, Yu YR, Zhang L, and Ho PC. Metabolic programs tailor T cell immunity in viral infection, cancer, and aging. Cell Metab. (2022) 34:378–95. doi: 10.1016/j.cmet.2022.02.003
59. Bajwa M, Vita S, Vescovini R, Larsen M, Sansoni P, Rinaldi A, et al. CMV-specific T-cell responses at older ages: broad responses with a large central memory component may be key to long-term survival. J Infect Dis. (2017) 215:1212–20. doi: 10.1093/infdis/jix080
60. Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. (2005) 175:8218–25. doi: 10.4049/jimmunol.175.12.8218
61. Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. (2014) 192:2143–55. doi: 10.4049/jimmunol.1301721
62. Kaufman HL, Kohlhapp FJ, and Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. (2015) 14:642–62. doi: 10.1038/nrd4663
63. Lin D, Shen Y, and Liang T. Oncolytic virotherapy: basic principles, recent advances and future directions. Signal Transduct Target Ther. (2023) 8:156. doi: 10.1038/s41392-023-01407-6
64. Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. (2006) 176:2645–53. doi: 10.4049/jimmunol.176.4.2645
65. Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene. (2015) 34:5960–70. doi: 10.1038/onc.2015.46
66. Green DR, Droin N, and Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. (2003) 193:70–81. doi: 10.1034/j.1600-065X.2003.00051.x
67. Wang S, Yan W, Kong L, Zheng W, Liu W, Gao Z, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat Commun. (2023) 14:4367. doi: 10.1038/s41467-023-39683-z
68. Morris EC, Neelapu SS, Giavridis T, and Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. (2022) 22:85–96. doi: 10.1038/s41577-021-00547-6
69. Shah D, Soper B, and Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. (2023) 14:1190379. doi: 10.3389/fimmu.2023.1190379
70. Smith K, Peng KW, Pulido JS, Dingli D, Russell SJ, Egan KM, et al. A phase I oncolytic virus trial with vesicular stomatitis virus expressing human interferon beta and tyrosinase related protein 1 administered intratumorally and intravenously in uveal melanoma: safety, efficacy, and T cell responses. Front Immunol. (2023) 14:1279387. doi: 10.3389/fimmu.2023.1279387
71. Lutzky J, Sullivan RJ, Cohen JV, Ren Y, Li A, and Haq R. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. J Cancer Res Clin Oncol. (2023) 149:6059–66. doi: 10.1007/s00432-022-04510-3
72. Sasso E, D’Alise AM, Zambrano N, Scarselli E, Folgori A, and Nicosia A. New viral vectors for infectious diseases and cancer. Semin Immunol. (2020) 50:101430. doi: 10.1016/j.smim.2020.101430
73. Zhang B, Huang J, Tang J, Hu S, Yuan Y, Song K, et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J immunotherapy Cancer. (2021) 9:e002224. doi: 10.1136/jitc-2020-002224
74. Hu C, Liu Y, Lin Y, Liang J, Xiao J, Wang G, et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. (2018) 9:274. doi: 10.1038/s41419-018-0325-3
75. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, and Yassine HM. Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses. (2019) 11:762. doi: 10.3390/v11080762
76. Yang Y, Xie H, Li D, Jia Y, Cui B, Zou J, et al. Real world pharmacovigilance study of antineoplastic drug related vitiligo risks. Sci Rep. (2025) 15:22733. doi: 10.1038/s41598-025-06314-0
77. Xu L, Sun H, Lemoine NR, Xuan Y, and Wang P. Oncolytic vaccinia virus and cancer immunotherapy. Front Immunol. (2023) 14:1324744. doi: 10.3389/fimmu.2023.1324744
78. Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. (2020) 10:40–53. doi: 10.1158/2159-8290.CD-19-0980
79. Bi C, Patel JS, and Liang SH. Development of CD73 inhibitors in tumor immunotherapy and opportunities in imaging and combination therapy. J Med Chem. (2025) 68:6860–9. doi: 10.1021/acs.jmedchem.4c02151
80. Kurago Z, Guo G, Shi H, Sgammeglia A, Ahmed MS, Nicosia A, et al. Inhibitors of the CD73-adenosinergic checkpoint as promising combinatory agents for conventional and advanced cancer immunotherapy. Front Immunol. (2023) 14:1212209. doi: 10.3389/fimmu.2023.1212209
81. Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Brown SW, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. (2007) 13:4840–8. doi: 10.1158/1078-0432.CCR-07-0409
82. Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. (2015) 21:39–48. doi: 10.1158/1078-0432.CCR-14-1711
83. Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelièvre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. (2009) 119:997–1007. doi: 10.1172/JCI38052
84. Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. (2014) 6:268ra179. doi: 10.1126/scitranslmed.3009892
85. Yang J, Liu HC, Zhang JQ, Li X, Wang L, Chen Y, et al. The effect of metformin on senescence of T lymphocytes. Immun Ageing. (2023) 20:73. doi: 10.1186/s12979-023-00394-0
86. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e325–31. doi: 10.1016/S2665-9913(20)30127-2
87. Pan J, Deng B, Ling Z, Hu S, Huang H, Gong F, et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J Cell Mol Med. (2021) 25:1089–99. doi: 10.1111/jcmm.16176
88. Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy–associated toxicity in large B-cell lymphoma. Blood Advances. (2020) 4:3123–7. doi: 10.1182/bloodadvances.2020002328
89. Wei S, Gu R, Xu Y, Chen S, Chen L, Wang X, et al. Adjuvant ruxolitinib therapy relieves steroid-refractory cytokine-release syndrome without impairing chimeric antigen receptor-modified T-cell function. Immunotherapy. (2020) 12:1047–52. doi: 10.2217/imt-2020-0116
90. Zi FM, Ye LL, Zheng JF, Cheng J, and Wang QM. Using JAK inhibitor to treat cytokine release syndrome developed after chimeric antigen receptor T cell therapy for patients with refractory acute lymphoblastic leukemia: A case report. Med (Baltimore). (2021) 100:e25786. doi: 10.1097/MD.0000000000025786
91. Cyrelle Ornella MS, Kim JJ, Cho E, Cho M, and Hwang TH. Dose considerations for vaccinia oncolytic virus based on retrospective reanalysis of early and late clinical trials. Vaccines (Basel). (2024) 12:1010. doi: 10.3390/vaccines12091010
92. Gu X, Zhang Y, Zhou W, Liu Y, Zhang X, Wang R, et al. Infusion and delivery strategies to maximize the efficacy of CAR-T cell immunotherapy for cancers. Exp Hematol Oncol. (2024) 13:70. doi: 10.1186/s40164-024-00542-2
93. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. (2020) 53:187–203.e8. doi: 10.1016/j.immuni.2020.06.016
94. Velardi E, Tsai JJ, and van den Brink M. T cell regeneration after immunological injury. Nat Rev Immunol. (2021) 21:277–91. doi: 10.1038/s41577-020-00457-z
95. Zhao Y, Wei K, Chi H, Xia Z, and Li X. IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines. Front Immunol. (2022) 13:1022808. doi: 10.3389/fimmu.2022.1022808
96. Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. (2020) 8:e001511. doi: 10.1136/jitc-2020-001511
97. Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. (2021) 39:3978–92. doi: 10.1200/JCO.21.01992
98. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. (2019) 380:2377–9. doi: 10.1056/NEJMc1901677
99. Salem JE, Ederhy S, Belin L, Cano NM, Grinda JM, Roquelaure B, et al. Abatacept dose-finding phase II triaL for immune checkpoint inhibitors myocarditis (ACHLYS) trial design. Arch Cardiovasc Dis. (2025) 118:106–15. doi: 10.1016/j.acvd.2024.12.005
100. Wadden E, Lai C, Grivas P, Peugh J, Ison J, Jaeger V, et al. Successful treatment of immune checkpoint inhibitor-associated fulminant myocarditis with abatacept and ruxolitinib: a case report. Eur Heart J Case Rep. (2025) 9:ytaf019. doi: 10.1093/ehjcr/ytaf019
101. De Lombaerde E, De Wever O, and De Geest BG. Delivery routes matter: Safety and efficacy of intratumoral immunotherapy. Biochim Biophys Acta Rev Cancer. (2021) 1875:188526. doi: 10.1016/j.bbcan.2021.188526
102. Tan Z, Wu Y, Fan Z, Tian W, Peng P, Zhu B, et al. Intratumoral oncolytic virus OH2 injection in patients with locally advanced or metastatic sarcoma: a phase 1/2 trial. J Immunother Cancer. (2025) 13:e010543. doi: 10.1136/jitc-2024-010543
103. Sedrak MS, Ji J, Tiwari A, Mohile SG, Dale W, and Le-Rademacher JG. Clinical trial enrollment, ineligibility, and reasons for decline in older vs younger patients with cancer in the national cancer institute community oncology research program. JAMA Netw Open. (2022) 5:e2235714. doi: 10.1001/jamanetworkopen.2022.35714
104. Dale W, Klepin HD, Williams GR, Wildes TM, Sanoff HK, Mohile SG, et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J Clin Oncol. (2023) 41:4293–312. doi: 10.1200/JCO.23.00933
105. Dale W, Williams GR R, MacKenzie A, Apostolidis T, Enright H, Guerard EJ, et al. How is geriatric assessment used in clinical practice for older adults with cancer? A survey of cancer providers by the american society of clinical oncology. JCO Oncol Pract. (2021) 17:336–44. doi: 10.1200/OP.20.00442
106. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. (2021) 398:1894–904. doi: 10.1016/S0140-6736(21)01789-X
107. Puts M, Alqurini N, Strohschein F, McWatters K, Phillips S, Tourangeau AE, et al. Impact of geriatric assessment and management on quality of life, unplanned hospitalizations, toxicity, and survival for older adults with cancer: the randomized 5C trial. J Clin Oncol. (2023) 41:847–58. doi: 10.1200/JCO.22.01007
108. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
109. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, and Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6
110. Sharma A, Alexander G, Chu JH, Bintoro BS, McGregor C, Delgado D, et al. Immune checkpoint inhibitors and cardiotoxicity: A comparative meta-analysis of observational studies and randomized controlled trials. J Am Heart Assoc. (2024) 13:e032620. doi: 10.1161/JAHA.123.032620
111. Kim M, Ladomersky E, Mozny A, Kocherginsky M, Chong HY, Wang J, et al. Glioblastoma as an age-related neurological disorder in adults. Neuro-Oncology Adv. (2021) 3:vdab125. doi: 10.1093/noajnl/vdab125
112. Xia C, Yin S, To K, and Fu L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer. (2023) 22:44. doi: 10.1186/s12943-023-01733-x
113. Jacquerie A, Hoeben A, Eekers D, Wesseling P, Venneti S, Vandertop WP, et al. Prognostic relevance of high expression of kynurenine pathway markers in glioblastoma. Sci Rep. (2024) 14:14975. doi: 10.1038/s41598-024-65907-3
114. Palmer S, Albergante L, Blackburn CC, and Newman TJ. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci U S A. (2018) 115:1883–8. doi: 10.1073/pnas.1714478115
115. Schroeter A, Roesel MJ, Matsunaga T, Xiao Y, Zhou H, and Tullius SG. Aging affects the role of myeloid-derived suppressor cells in alloimmunity. Front Immunol. (2022) 13:917972. doi: 10.3389/fimmu.2022.917972
116. Shurin MR, Shurin GV, and Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol. (2007) 64:90–105. doi: 10.1016/j.critrevonc.2007.03.002
117. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, and Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. (2012) 24:331–41. doi: 10.1016/j.smim.2012.04.008
118. Ma S, Wang C, Mao X, and Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. (2019) 10:318. doi: 10.3389/fimmu.2019.00318
119. Yi M, Niu M, Xu L, Luo S, and Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. (2021) 14:10. doi: 10.1186/s13045-020-01027-5
120. Ma F, Vayalil J, Lee G, Wang Y, and Peng G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J Immunother Cancer. (2021) 9:e003217. doi: 10.1136/jitc-2021-003217
121. Agrawal A, Agrawal S, and Gupta S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol. (2017) 8:896. doi: 10.3389/fimmu.2017.00896
122. Gabrilovich D, Ishida T, Oyama T, Ran S, Nadaf S, Kavanaugh D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. (1998) 92:4150–66. doi: 10.1182/blood.V92.11.4150
123. Fane M and Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. (2020) 20:89–106. doi: 10.1038/s41568-019-0222-9
124. Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH 3rd, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. (2016) 532:250–4. doi: 10.1038/nature17392
125. Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. (2018) 24:5347–56. doi: 10.1158/1078-0432.CCR-18-1116
126. Pol JG, Workenhe ST, Konda P, Gujar S, and Kroemer G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. (2020) 56:4–27. doi: 10.1016/j.cytogfr.2020.10.007
127. Raimondi V, Vescovini R, Dessena M, Donofrio G, Storti P, and Giuliani N. Oncolytic viruses: a potential breakthrough immunotherapy for multiple myeloma patients. Front Immunol. (2024) 15:1483806. doi: 10.3389/fimmu.2024.1483806
128. Lee J, Ghonime MG, Wang R, and Cassady KA. The antiviral apparatus: STING and oncolytic virus restriction. Mol Ther Oncolytics. (2019) 13:7–13. doi: 10.1016/j.omto.2019.02.002
129. Chen B, Zhao J, Zhang D, Zheng A, and Wu G. Immunotherapy of cancer by targeting regulatory T cells. Int Immunopharmacology. (2022) 104:108469. doi: 10.1016/j.intimp.2021.108469
130. Semmrich M, Marchand JB, Fend L, Merillon N, Jacob D, Villemin A, et al. Vectorized Treg-depleting αCTLA-4 elicits antigen cross-presentation and CD8(+) T cell immunity to reject ‘cold’ tumors. J Immunother Cancer. (2022) 10:e003488. doi: 10.1136/jitc-2021-003488
131. Iglesias P, Ribero S, Barreiro A, Quintas L, Patrao J, Carrera C, et al. Induced vitiligo due to talimogene laherparepvec injection for metastatic melanoma associated with long-term complete response. Acta Derm Venereol. (2019) 99:232–3. doi: 10.2340/00015555-3061
132. Mirbahari SN, Da Silva M, Zúñiga A, Rahman MM, McFadden G, et al. Recent progress in combination therapy of oncolytic vaccinia virus. Front Immunol. (2024) 15:1272351. doi: 10.3389/fimmu.2024.1272351
Keywords: immune frailty, elderly cancer patients, oncolytic virus therapy, immunosenescence, inflammaging, cytokine release syndrome
Citation: Wang J-W, Liu J-H, Liu Y-L, Xu W-Z and Zhang Z-B (2025) Oncolytic virus therapy in the elderly: immune frailty, challenges, and perspectives. Front. Immunol. 16:1686659. doi: 10.3389/fimmu.2025.1686659
Received: 15 August 2025; Accepted: 22 September 2025;
Published: 08 October 2025.
Edited by:
Laura Senovilla, Spanish National Research Council (CSIC), SpainReviewed by:
Yanna Zhang, University of Electronic Science and Technology of China, ChinaCopyright © 2025 Wang, Liu, Liu, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zi-Bo Zhang, NDkwMDE5MzlAaGVibXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jia-Wen Wang
Jia-Wen Wang Jia-Hui Liu1†
Jia-Hui Liu1† Zi-Bo Zhang
Zi-Bo Zhang