- Department of Medical Oncology, Zhejiang University School of Medicine Sir Run Run Shaw Hospital, Hangzhou, China
Anaplastic lymphoma kinase (ALK) rearrangements are exceedingly rare in gastric cancer, and uncommon fusion types add to the difficulties of proper, precise treatment strategies. Although detected in non-small cell lung cancer (NSCLC), inflammatory myofibroblastic tumors (IMTs), and Spitz tumors, the DCTN1–ALK fusion has not previously been reported in gastric cancer. This report describes the first case of gastric adenocarcinoma harboring a DCTN1–ALK fusion that was successfully treated with the ALK-targeted agent alectinib after first- and second-line chemotherapy-based regimens had failed. Progression-free survival on alectinib was 11.5 months until KRAS amplification emerged on serial circulating tumor DNA analysis, leading to rapid systemic relapse. The other documented cases with DCTN1–ALK fusion treated with the first or second generation of ALK inhibitors indicated this rare fusion as an actionable driver gene mutation. This successful personalized anti-tumor strategy highlights the clinical utility of comprehensive genomic profiling and liquid biopsy in detecting and monitoring actionable ALK fusions in solid tumors.
Introduction
Gastric cancer still poses a significant challenge in oncology due to its heterogeneity and unsatisfactory response to traditional chemotherapy. Recent advancements in cancer research have identified gastric cancer as subgroups based on epidemiologic (1), genomic (2), or molecular (3) classifications that may benefit from potential therapeutic targets. Among the various subtypes of gastrointestinal (GI) cancers, although very rare, anaplastic lymphoma kinase (ALK) rearrangements represent a particularly intriguing group, suggesting a potential for targeted therapy with ALK inhibitors (ALKi), offering a ray of hope for personalized treatment approaches.
The ALK gene encodes a receptor tyrosine kinase that is involved in cell growth, proliferation, and survival. Mutations in the ALK gene can lead to the aberrant activation of the ALK protein, resulting in uncontrolled cell growth and tumor formation. ALK rearrangements are extremely rare in GI cancers, with a frequency of <1% in colorectal cancer (4), 0.2% in pancreatic cancer (5), and 0.9% to 2.3% in gastric cancer (1, 6), but may offer personalized treatment strategies in selected patients. A Korean gastric cancer cohort (n = 455) consisted of 38 ALK Immunohistochemistry (IHC)-positive (34 of 1+, and 4 of 2+/3+) patients who were younger and more likely to have signet ring cells (1), associated with worse disease-free survival (DFS) and overall survival (OS) (1). Fortunately, ALKi, such as alectinib and lorlatinib (7), have demonstrated efficacy in inhibiting tumor growth and improving patient outcomes in ALK-mutant gastric cancer, offering a more effective and less toxic alternative to traditional chemotherapy.
The appearance of the DCTN1–ALK fusion gene in cancer is a rare genetic rearrangement event primarily associated with the occurrence and progression of tumors. Dynactin subunit 1 (DCTN1) is a protein related to the cytoskeleton, involved in the transport of materials within the cell. When DCTN1 undergoes a gene fusion with ALK, DCTN1 is suspected to promote the dimerization of ALK and lead to the abnormal activation of the ALK kinase by transphosphorylation, thereby promoting the proliferation and survival of tumor cells (8). To date, published case reports of DCTN1–ALK fusion solid tumors included non-small cell lung cancer (NSCLC) (9–11), inflammatory myofibroblastic tumors (IMTs) (12, 13), Spitz tumors (14), and some rare types of neoplasms.
Given the rarity of both ALK mutation and the fusion type, here, we report a case of a gastric cancer patient with a driver mutation of DCTN1–ALK fusion. Although the first and second lines of standard chemotherapy ended in progression of disease, subsequent targeted therapy improved the general health of the patient and prolonged the survival.
Case presentation
The patient, a 58-year-old woman, was admitted to the local hospital due to symptoms of cough, decline in appetite, and mild weight loss in August 2022. Laboratory tests revealed a significant increase in carcinoembryonic antigen (CEA) to 323.26 ng/mL and CA724 to 266.83 U/mL. The patient was then transferred to the Department of General Practice. Physical examinations did not show positive findings. A chest computed tomography (CT) scan showed enlarged mediastinal lymph nodes. An enhanced abdominal CT scan revealed thickening with enhancement of the lateral wall of the gastric cardia, fundus, and lesser curvature of the gastric body, indicating a T4a malignant tumor (Figure 1a). In addition, L3 vertebral metastasis was suspected. Multiple lymph node enlargements were observed in the perigastric, hilar, mesenteric, and retroperitoneal regions, consistent with metastasis (cT4aN3M1, stage IV). On September 8, 2022, the patient received a gastroduodenoscopy, and biopsies of the gastric angle, fundus, and lower esophagus were pathologically diagnosed as poorly differentiated carcinoma (Figure 2). Immunohistochemical analysis showed positive staining for cytokeratin (CK7), cytokeratin pan-cocktail (AE1/AE3), chromogranin A (focal positive), Ki-67 (40%), and ALK (D5F3) (Figure 2), and negative staining for S-100, thyroid transcription factor 1 (TTF1), hepatocyte, estrogen receptor, synaptophysin (Syn), CD56, CD3, CD20, GATA-3, NapsinA, SOX10, and human epidermal growth factor receptor 2 (HER2) (Supplementary Figure 1), as well as intact expression of mismatch repair proteins.
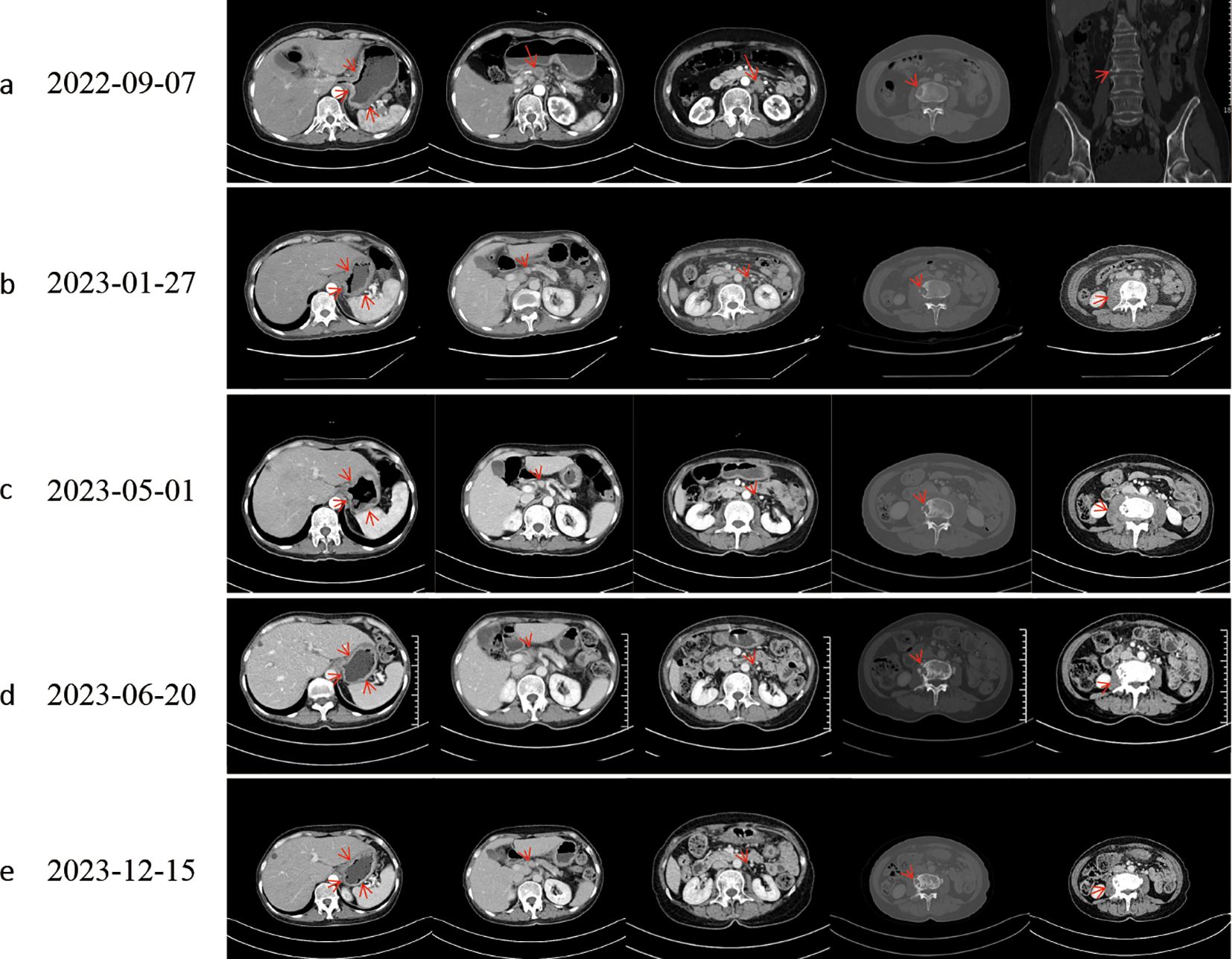
Figure 1. Computed tomography evaluation. Following effective therapy, the patient exhibited decreased gastric wall thickness, reduced osteolytic destruction of bone metastases, increased osteoblastic changes, and diminished perilesional soft-tissue components around osseous metastases. (a) Baseline. (b) Evaluation after progression of first-line chemotherapy. (c) Evaluation after progression of second-line chemotherapy; L3 vertebral metastasis showed progressive osteolytic destruction with an expanding perilesional soft-tissue mass. (d, e) The best response to targeted therapy was a partial response (PR).
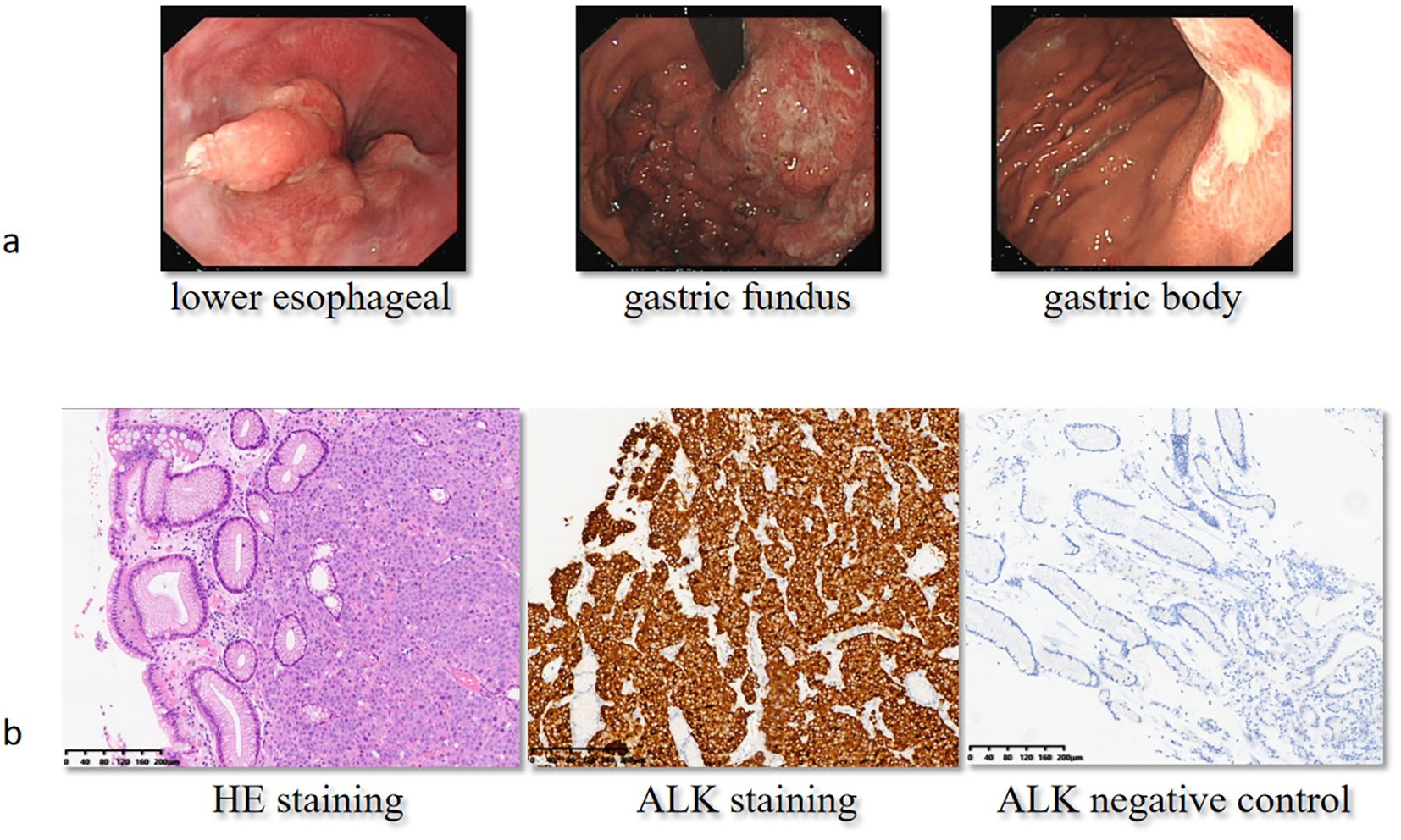
Figure 2. Gastroduodenoscopy and immunohistochemical analysis at baseline. On September 8, 2022, the patient received a gastroduodenoscopy (a), and the pathologist reported poorly differentiated carcinoma with positive ALK staining (b). H&E, hematoxylin and eosin; ALK, anaplastic lymphoma kinase.
Standard chemotherapy phase
From September 20, 2022, to December 14, 2022, the patient received chemotherapy combined with immunotherapy for five cycles, consisting of capecitabine and oxaliplatin (XELOX) plus sintilimab. Meanwhile, a whole-body bone CT scan on November 22, 2022, showed multiple abnormal increases in bone metabolism at the seventh cervical vertebra, 10th and 11th thoracic vertebrae, third lumbar vertebra, right third anterior rib, sacrum, bilateral iliac bones, right ischium, and upper segment of the left femur, primarily indicating tumor bone metastasis. The patient received bisphosphonate therapy to retard osteolysis.
On January 27, 2023, due to elevated tumor markers (Figure 3) and increased soft tissue at the L3 metastatic lesion (Figure 1b), the disease was considered to be progressing. From January 31, 2023, to April 17, 2023, the patient received single-agent chemotherapy with albumin-bound paclitaxel as second-line treatment.
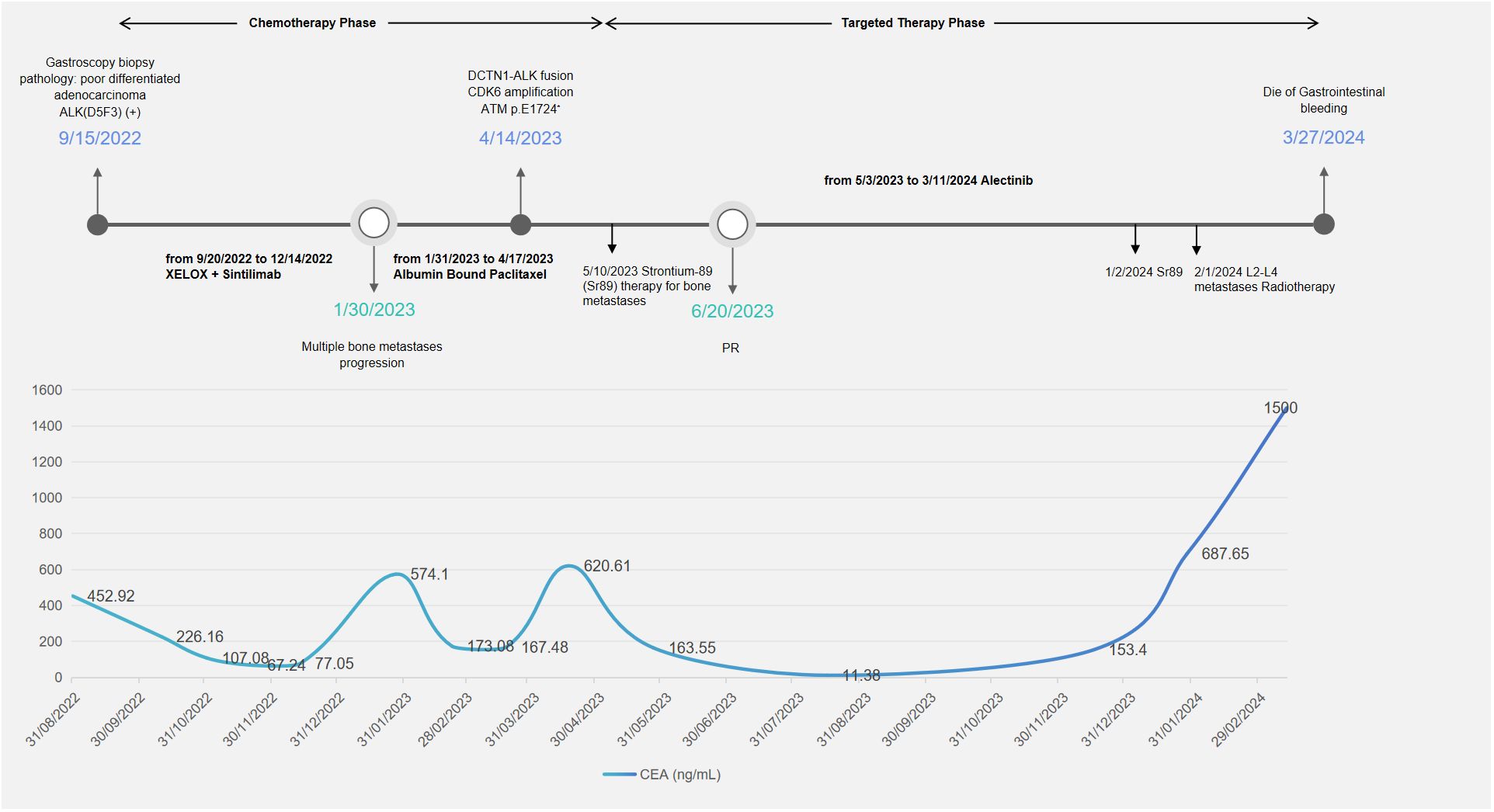
Figure 3. Timeline of treatment and CEA changes. During the chemotherapy phase, the patient received two lines of treatment: XELOX plus sintilimab and albumin-bound paclitaxel. The patient received strontium-89 therapy and localized radiotherapy for bone metastases. XELOX, capecitabine and oxaliplatin; PR, partial response; CEA, carcinoembryonic antigen.
Targeted therapy phase
During second-line chemotherapy, the patient’s CEA level decline remained suboptimal (Figure 3), indicating a limited therapeutic response (Figure 1c); consequently, the patient opted for next-generation sequencing (NGS)-based genomic profiling. On April 14, 2023, NGS gene testing was completed, revealing a DCTN1–ALK gene fusion, ATM p.E1724*, and amplification of the CDK6 gene by hybrid-capture NGS performed on DNA extracted from the gastric-biopsy formalin-fixed paraffin-embedded (FFPE) tissue on September 8, 2022. On May 3, 2023, the patient received targeted therapy with alectinib. On May 10, 2023, the patient underwent strontium-89 (Sr-89) therapy for metastatic bone tumor control and severe pain relief. During follow-up, the patient demonstrated excellent disease control: CT revealed gastric wall thinning, and the previously lytic L3 metastasis exhibited osteogenic changes (Figures 1d, e).
Post-resistance phase
In January 2024, surveillance revealed a marked surge in CEA, suggesting incipient, gradual resistance to targeted therapy. Subsequent MRI of the L3 vertebra revealed epidural spinal cord compression from osseous metastasis, prompting palliative radiotherapy to the L2–L4 metastatic lesion in February 2024. The whole blood circulating tumor DNA (ctDNA) gene test revealed the disappearance of the original DCTN1–ALK fusion and the concomitant emergence of a de novo KRAS amplification mutation. The patient subsequently experienced fulminant systemic progression and ultimately died of uncontrollable gastrointestinal hemorrhage.
Discussion
ALK rearrangements occur at low prevalence in a spectrum of non-pulmonary solid tumors, yet they have emerged as actionable, pan-cancer driver events among solid neoplasms. Activating ALK alterations—comprising gain-of-function point mutations, high-level gene amplifications, and oncogenic fusions/rearrangements—have been documented in a spectrum of malignancies that includes NSCLC, anaplastic large-cell lymphoma (ALCL; accounting for approximately 0.5% of adult lymphomas and roughly 10% of pediatric non-Hodgkin lymphomas), IMT, neuroblastoma, cutaneous spitzoid neoplasms, and inflammatory breast carcinoma. Population-level genomic profiling identifies ALK alterations in ~3.3% of all cancers, with fusions/rearrangements representing a minority subset (~0.5%–0.8%) (15). Within NSCLC, the prevalence of ALK rearrangements exceeds 3%, whereas non-NSCLC malignancies exhibit a markedly lower incidence (~0.2%). Beyond NSCLC, IMT (~50%) and ALCL (50%–80%) are the entities most frequently driven by ALK fusions. While the overwhelming majority of ALK-positive NSCLCs harbor an EML4–ALK fusion (83.5%), this chimeric transcript is encountered in only ~31% of ALK-rearranged non-NSCLC tumors, which instead display marked heterogeneity in 5′ fusion partners (16). In gastric cancer, ALK fusions reported involve RAB10 (17) or HMBOX (7); here, we report the first case of DCTN1–ALK fusion.
The appearance of the DCTN1–ALK fusion gene in cancer is a rare genetic rearrangement event, primarily associated with the occurrence and progression of tumors. DCTN1 is a protein related to the cytoskeleton, involved in the transport of materials within the cell (18). When DCTN1 undergoes a gene fusion with ALK, it may lead to the abnormal activation of the ALK kinase, thereby promoting the proliferation and survival of tumor cells. To date, the DCTN1–ALK fusion gene has been reported in three cases of NSCLC, one case of IMT (12), six cases of Spitz tumors (14), one case of epithelioid fibrous histiocytoma (19), and one case of pancreatic tumors (20).
With the advancement of NGS technology, rare ALK fusion subtypes, such as DCTN1–ALK, are being increasingly identified, which has facilitated the development of more precise, targeted treatment strategies for patients with these rare fusions. All published cases have confirmed a clinical response to targeted therapy in tumors harboring the DCTN1–ALK fusion protein. Among the three lung cases, the lung cancer patient (9) with a single DCTN1–ALK mutation received crizotinib orally at a dose of 250 mg twice daily, which resulted in a significant symptomatic improvement and radiographic response after 3 months of therapy. The female lung adenocarcinoma patient (10) (concurrent EGFR mutations and DCTN1–ALK fusion) with brain metastases developed resistance to chemotherapy or targeted therapy. CtDNA was dynamically monitored and confirmed the coexistence of a primary EGFR T790M mutation/EGFR exon 19 deletion and DCTN1–ALK translocation. She responded to osimertinib and alectinib treatment, and after acquiring osimertinib resistance, the patient still responded to alectinib and achieved a partial response (PR) for lung and brain lesions. The lung adenocarcinoma patient (11) harboring dual DCTN1–ALK and ALK-CLIP4 rearrangements showed PR to crizotinib with a 12-month progression-free survival (PFS) and received alectinib as second-line treatment. In the IMT case (12), the patient, who had confirmed a diagnosis of a DCTN1–ALK fusion through genetic profiling, was enrolled in a phase I clinical trial (ClinicalTrials.gov Identifier: NCT01548144) of crizotinib in combination with pazopanib. The patient was treated with crizotinib 250 mg orally on alternating days and pazopanib 200 mg orally daily and had confirmed PR for over 6 months. In our gastric adenocarcinoma case, the chemotherapy phase lasted for approximately 8 months. During chemotherapy, the primary gastric lesion demonstrated a limited response to chemotherapy +/− immunotherapy. However, the bone metastases exhibited primary resistance, resulting in severe pain and impairment of activities of daily living. On the contrary, alectinib targeted therapy reached PR, and the PFS reached 11.5 months.
In the other two cases of ALK fusion gastric cancer, one with RAB10–ALK mutation refused off-label use of agents such as crizotinib and ceritinib, and the other patient with ALK–HMBOX fusion received alectinib as first-line targeted therapy and achieved complete response (CR) in thoracic and cervical metastatic lymph nodes and PR in brain metastases, which reached a 6-month PFS. The second-line targeted therapy of lorlatinib also showed a 6-month PFS, with the best overall response of stable disease (SD). Likewise, other rare ALK-altered digestive system neoplasms confront therapeutic decision challenges. In a case series report of 13 GI cancer patients (7), regardless of the prior standard systemic treatment, ALKi chosen as the first-line target agent were alectinib for seven patients, crizotinib for five patients, and entrectinib for one patient. Further lines of ALKi included alectinib, lorlatinib, and ceritinib. Ten of 12 evaluable patients (83%) achieved a PR or SD response, and the median PFS was 5.0 months; the median OS was 9.3 months. Overall, patients with non-pulmonary malignancies harboring ALK alterations exhibited shorter PFS on targeted therapy than those with ALK-positive lung cancer. The breakpoint identified in our case with baseline tumor specimen (DCTN1-E19:ALK-E20) mirrors that described in NSCLC, implying that the shorter progression-free survival observed in GI malignancies does not merely depend on structural differences in the fusion itself but may be comprehensively affected by patient-related tumor microenvironment or co-occurring genomic events, such as CDK6 amplification and ATM mutation.
In summary, DCTN1–ALK fusion behaves biologically like other gastric ALK fusions [same kinase domain, equal in vitro Tyrosine kinase Inhibitor (TKI) sensitivity], but three features appear unique to the DCTN1 partner. First, DCTN1–ALK retains the same ALK kinase domain and in vitro drug sensitivity as other gastric ALK fusions, indicating equal intrinsic signaling potency. Second, it reproducibly associates with poorly differentiated histology and bone metastases, suggesting a phenotypic footprint linked to the DCTN1 cytoskeletal role. Third, our case and the published lung tumors frequently harbor co-alterations such as ATM loss or CDK6 amplification that shorten TKI durability. Taken together, the fusion is not inherently “stronger”, but its biological distinctiveness lies in histology, tropism, and genomic context, supporting comprehensive gene profiling in specific situations, especially for those with refractory gastric cancer. Therefore, based on an actionable fusion frequency ≥0.5%, a clinically relevant response rate to ALK TKIs, and marginal additional cost when incorporated into existing comprehensive NGS workflows, we advocate routine ALK fusion assessment in all cases of metastatic or refractory gastric cancer.
Dynamic genomic profiling to monitor resistance mechanisms can guide precise regimen adjustments. Given that rare variants lack evidence-based therapeutic guidance and the challenges of repeat tissue biopsies, liquid biopsy options, such as peripheral blood ctDNA testing, offer a practical alternative. Several cases have reported the use of dynamic ctDNA sequencing to identify resistance mechanisms after first-line targeted therapy, thereby informing subsequent targeted treatment selection. In the case of carcinoma of unknown primary with an EML4–ALK fusion (21), ctDNA detection revealed two resistance mutations (L1196M and G1269A) to crizotinib. Therefore, the targeted therapy was switched to brigatinib 180 mg once a day p.o., and CT examination confirmed PR. The gastric cancer case with ALK–HMBOX fusion failed to reveal the resistance mechanism by a second FoundationOne CDx test on the new cervical lymph node tissue specimen; however, the p.Val1180Leu ALKi resistance mutation was identified in ctDNA analyzed on a Illumina NextSeq 550 NGS instrument. Then, the patient started lorlatinib 50 mg twice a day as second-line treatment. After progression, an STK11 intronic loss-of-function mutation (c.734 + 1G.T) was detected as a resistance mechanism through liquid biopsy using pleural effusion and plasma. In our case, the DCTN1–ALK fusion was first detected in the primary gastric tissue specimen; cfDNA analysis was employed later for longitudinal surveillance and revealed the emergence of KRAS amplification at progression. It is supposed that the KRAS amplification mutation replaced DCTN1–ALK fusion to emerge as the driver gene, precipitating rapid and widespread tumor progression. The patient ultimately lost all further anti-tumor therapeutic opportunities. Although the index detection of DCTN1–ALK was tissue-based, subsequent resistance profiling relied on cfDNA; therefore, we cannot determine whether the KRAS-amplified sub-clone originated from the primary tumor, bone metastases, or both.
To date, a limited number of basket trials have been initiated to investigate better targeted therapy strategies and resistance mechanisms in patients with ALK-altered non-pulmonary malignancies. There are several ongoing basket trials listed on the ClinicalTrials.gov website, with identifiers NCT04644315, NCT03868423, NCT01284192, and NCT04439266 (7). With continuing advances and innovations in gene sequencing technologies, we anticipate an expansion of regional and global basket trials that will establish a robust evidence base for precision-targeted therapies against rare ALK alterations.
Conclusion
Despite the progress made in understanding and targeting ALK fusions in non-lung cancers, challenges remain in optimizing treatment strategies and overcoming resistance to ALK inhibitors. GI cancer patients harboring ALK mutations are at risk of being excluded from personalized treatment, which may dramatically improve survival due to the conventional test and treatment routine. To our knowledge, this is the first case report of gastric cancer harboring DCTN1–ALK fusion with clinical response to targeted therapy. Liquid biopsy plays a pivotal role in the dynamic genomic surveillance of patients in real-world clinical practice. Further research is needed to identify novel therapeutic combinations that can improve patient outcomes and elucidate the mechanisms of resistance to ALK-driven solid tumors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW: Writing – original draft, Writing – review & editing. LY: Conceptualization, Supervision, Writing – review & editing. HP: Data curation, Writing – review & editing. XQ: Data curation, Writing – review & editing. JS: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1686666/full#supplementary-material
References
1. Chon HJ, Kim HR, Shin E, Kim C, Heo SJ, Lee C, et al. The clinicopathologic features and prognostic impact of ALK positivity in patients with resected gastric cancer. Ann Surg Oncol. (2015) 22:3938–45. doi: 10.1245/s10434-015-4376-8
2. Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. (2012) 61:673–84. doi: 10.1136/gutjnl-2011-301839
3. Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: A new paradigm. Clin Cancer Res. (2011) 17:2693–701. doi: 10.1158/1078-0432.CCR-10-2203
4. Aisner DL, Nguyen TT, Paskulin DD, Le AT, Haney J, Schulte N, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. (2014) 12:111–8. doi: 10.1158/1541-7786.MCR-13-0479-T
5. Singhi AD, Ali SM, Lacy J, Hendifar A, Nguyen K, Koo J, et al. Identification of targetable ALK rearrangements in pancreatic ductal adenocarcinoma. J Natl Compr Canc Netw. (2017) 15:555–62. doi: 10.6004/jnccn.2017.0058
6. Alese OB, El-Rayes BF, Sica G, Zhang G, Alexis D, La Rosa FG, et al. Anaplastic lymphoma kinase (ALK) gene alteration in signet ring cell carcinoma of the gastrointestinal tract. Ther Adv Med Oncol. (2015) 7:56–62. doi: 10.1177/1758834014567117
7. Ambrosini M, Del Re M, Manca P, Hendifar A, Drilon A, Harada G, et al. ALK inhibitors in patients with ALK fusion-positive GI cancers: an international data set and a molecular case series. JCO Precis Oncol. (2022) 6:e2200015. doi: 10.1200/PO.22.00015
8. Chiarle R, Voena C, Ambrogio C, Piva R, and Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. (2008) 8:11–23. doi: 10.1038/nrc2291
9. Vendrell JA, Taviaux S, Béganton B, Godreuil S, Audran P, Grand D, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep. (2017) 7:12510. doi: 10.1038/s41598-017-12679-8
10. Yin Q, Guo T, Zhou Y, Sun L, Meng M, Ma L, et al. Effectiveness of alectinib and osimertinib in a brain metastasized lung adenocarcinoma patient with concurrent EGFR mutations and DCTN1-ALK fusion. Thorac Cancer. (2022) 13:637–42. doi: 10.1111/1759-7714.14291
11. Gao F, Gao F, Wu H, Lu J, Xu Y, and Zhao Y. Response to ALK-TKIs in a lung adenocarcinoma patient harboring dual DCTN1-ALKandALK-CLIP4 rearrangements. Thorac Cancer. (2022) 13:1088–90. doi: 10.1111/1759-7714.14345
12. Subbiah V, McMahon C, Patel S, Zinner R, Silva EG, Elvin JA, et al. STUMP un”stumped”: anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol. (2015) 8:66. doi: 10.1186/s13045-015-0160-2
13. Denis D, Elayoubi K, Weil A, Berthelet F, and Bojanowski M. Inflammatory myofibroblastic tumors of the central nervous system that express anaplastic lymphoma kinase have a high recurrence rate. Surg Neurol Int. (2013) 4:70. doi: 10.4103/2152-7806.112614
14. Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. (2014) 5:3116. doi: 10.1038/ncomms4116
15. The AACR Project GENIE Consortium, The AACR Project GENIE Consortium, André F, Arnedos M, Baras AS, Baselga J, Bedard PL, et al. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. (2017) 7:818–31. doi: 10.1158/2159-8290.CD-17-0151
16. Ross JS, Ali SM, Fasan O, Block J, Pal S, Elvin JA, et al. ALK fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncol. (2017) 22:1444–50. doi: 10.1634/theoncologist.2016-0488
17. Wen Z, Xiong D, Zhang S, Liu J, Li B, Li R, et al. Case report: RAB10-ALK: A novel ALK fusion in a patient with gastric cancer. Front Oncol. (2021) 11:645370. doi: 10.3389/fonc.2021.645370
18. Lee YD, Kim B, Jung S, Kim H, Kim MK, Kwon J-O, et al. The dynactin subunit DCTN1 controls osteoclastogenesis via the Cdc42/PAK2 pathway. Exp Mol Med. (2020) 52:514–28. doi: 10.1038/s12276-020-0406-0
19. Dickson BC, Swanson D, Charames GS, Fletcher CD, and Hornick JL. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Modern Pathol. (2018) 31:753–62. doi: 10.1038/modpathol.2017.191
20. Shimada Y, Kohno T, Ueno H, Ino Y, Hayashi H, Nakaoku T, et al. An oncogenic ALK fusion and an RRAS mutation in KRAS mutation-negative pancreatic ductal adenocarcinoma. Oncol. (2017) 22:158–64. doi: 10.1634/theoncologist.2016-0194
Keywords: anaplastic lymphoma kinase (ALK), circulating tumor DNA (ctDNA), next generation sequencing (NGS), case report, review
Citation: Wang H, You L, Pan H, Qiu X and Sheng J (2025) Response to anaplastic lymphoma kinase inhibitor in gastric cancer harboring DCTN1–ALK fusion: a case report and review. Front. Immunol. 16:1686666. doi: 10.3389/fimmu.2025.1686666
Received: 18 August 2025; Accepted: 20 October 2025;
Published: 30 October 2025.
Edited by:
Khyati Maulik Kariya, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Sharanya Nag, Memorial Sloan Kettering Cancer Center, United StatesAyantika Sen Gupta, Stowers Institute for Medical Research, United States
Copyright © 2025 Wang, You, Pan, Qiu and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Sheng, c2hlbmdqaW5Aemp1LmVkdS5jbg==
 Huadi Wang
Huadi Wang Liangkun You
Liangkun You Hong Pan
Hong Pan Jin Sheng
Jin Sheng