- 1Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai Immune Therapy Institute, Shanghai, China
Follicular helper T cells (Tfh) are a Th cell subset that directly assists B cells in functioning, and their development is regulated by various factors. Among them, the initial regulation leads to phenotypic heterogeneity, while the regulation of their migration process results in spatial heterogeneity. The phenotypic heterogeneity is manifested by the presence of Tfh subsets with characteristics helper T cells (Th) of other lineages, namely Tfh1, Tfh2, and Tfh17, with different transcriptional programs and secrete distinct cytokines, potentially possessing different functions. The spatial heterogeneity is mainly manifested by the positional relationship between Tfh and germinal centers (GC), which are mainly divided into GC-Tfh, follicular mantle Tfh, and circulating Tfh, possibly reflecting the process of Tfh occurrence. This review summarizes the spatial and phenotypic heterogeneity of Tfh cells, and suggests a Tfh cell type framework with nodes of previous studied cell types and the edges of switching between specific celltypes, which is affected by the summation of imprinted plasticity part and de novo plasticity part in Tfh development, connecting the hypothesis Crotty et al. proposed in 2018. Discrete cell type is still eligible in qualifying the diseases state and quantifying the activity and severity of diseases, but it could also be beneficial to look Tfh from the view of cell states and expression programs, which, in the future studies, might better model the through process of Tfh development and unifying the contradiction caused by separate Tfh cell type view.
1 Introduction
Follicular helper T (Tfh) cells are a specialized CD4+ T helper subset of adaptive humoral immunity. Orchestrated by the lineage-defining transcription factor BCL6(B-cell lymphoma 6), Tfh cells are primed in the T cell zone and undergo full differentiation within GCs (germinal centers) of secondary lymphoid organs, where they provide critical signals for B cell selection, antibody affinity maturation, and class-switch recombination through direct cell-contact and cytokine production (e.g., IL-21) (1).
A defining hallmark of Tfh cells is their profound heterogeneity, which can be categorized along two primary dimensions: spatial distribution and functional phenotype. Based on their anatomical positioning, Tfh cells are compartmentalized into distinct subsets: GC-Tfh residing within GCs, FM-Tfh (follicular mantle Tfh) in FM (follicular mantle zone), cTfh (circulating Tfh) patrolling the periphery blood, and peripheral helper T cells (Tph) (2), as well as mucosal tissue Tfh. They share B cells helper function, although they possess differences in phenotype. Tfh cells exhibit functional plasticity, giving rise to subsets that co-express the typical transcription factors and cytokines of other T helper cell lineages (e.g., T-bet(T-box Transcription Factor, TBX21)/IFN-γ in Tfh1, GATA3/IL-4 in Tfh2, and RORγT(RAR-related orphan receptor C)/IL-17 in Tfh17), thereby tailoring antibody responses to specific immunological challenges.
Pathological conditions, including infections, autoimmune disorders, and cancers, often feature significant perturbations in the abundance, subset composition, and function of Tfh cells and their relatives, underscoring their pivotal role in disease pathogenesis. However, studying these relationships is complicated by the dynamic and plastic nature of these cells. This review aims to synthesize current understanding of Tfh cell heterogeneity, integrating spatial, phenotypic, and functional perspectives. We will explore the developmental pathways, migration dynamics, and diverse roles of canonical Tfh subsets, while also dedicating focused discussion to the emerging roles of Tph cells and Tfh/Tfh-like populations in mucosal immunity.
1.1 A multi-stage journey: Tfh cell differentiation, migration, and maturation
The differentiation of Tfh cells is a tightly regulated, multi-stage process that can be broadly delineated into initiation, migration, and effector phases, culminating in their functional specialization within GCs (3). Tfh cell fate is primed at the T-B border of secondary lymphoid organs. Here, naive CD4+ T cells receive critical signals from antigen-presenting cells (APCs) (primarily dendritic cells (DCs)), including MHC-II complexes, costimulation molecules, and cytokine signaling (4–6). This synergistic signaling cascade activates BCL6, which defines the Tfh lineage. BCL6 orchestrates Tfh commitment by driving the expression of canonical Tfh markers like CXCR5 and repressing genes that promote alternative T helper cell fates (e.g., T-bet/Th1, GATA3/Th2, RORγT/Th17) and the Tfh antagonist BLIMP1 (7, 8), making naive CD4 polarize towards Tfh rather than non-Tfh T helper cells (4, 9). Additional transcription factors, including BATF (Basic-Leucine Zipper Transcription Factors), ASCL2 (Achaete-scute family bHLH transcription factor 2), c-Maf (Musculoaponeurotic Fibrosarcoma Protein), and act upstream or in concert with BCL6 to reinforce this developmental program and promote IL-21 production (10–13).
Upon upregulating CXCR5 and downregulating the T-zone homing receptor CCR7, newly committed pre-Tfh cells migrate towards the CXCL13 chemokine gradient into the follicle and mature into GC-Tfh (14–16). The initial positioning of pre-Tfh within GC is regulated by a balance of CXCR5, CCR7, and EBI2 signaling (17). Within GCs, sustained interactions with APC (particularly through ICOSL (Inducible T-cell costimulator Ligand), CD40 by B cells, and CD115/CD112 by DCs) are indispensable for the final maturation of GC-Tfh cells (18–20). Mature GC-Tfh cells are characterized by high expression of BCL6, CXCR5, PD-1, and ICOS, and are proficient secretors of IL-21, which is essential for supporting B cell differentiation and antibody production (3). By downregulating CCR7 (17) and responding to CCL19/CCL21 signal (16), pre-Tfh could also migrate into FM to mature into FM-Tfh.
GC-Tfh could form a transitional CXCR5+CCR7loPD-1hi migrating subset which traverses the subcapsular sinus into efferent lymphatics, migrating toward the thoracic duct with a phenotype converging with circulating Tfh (cTfh) (21) by CD69, CD90, Ki-67 downregulation, and KLF2(Kruppel-like factor), S1PR1(Sphingosine-1-Phosphate Receptor 1), S1PR2(Sphingosine-1-Phosphate Receptor 2) upregulation (22, 23). Moreover, thoracic duct Tfh (TDL-Tfh) retain the transcriptional and epigenetic imprints of GC-Tfh, their TCR clonotypes are enriched in peripheral cTfh, confirming the migration route and the phenotype variation from GC-Tfh to TDL-Tfh and then to cTfh (23), which can be further proven by the clonal overlap between cTfh and GC-Tfh and their shared feature of expressing BCL6 and IL-21 (24, 25) (Figure 1).
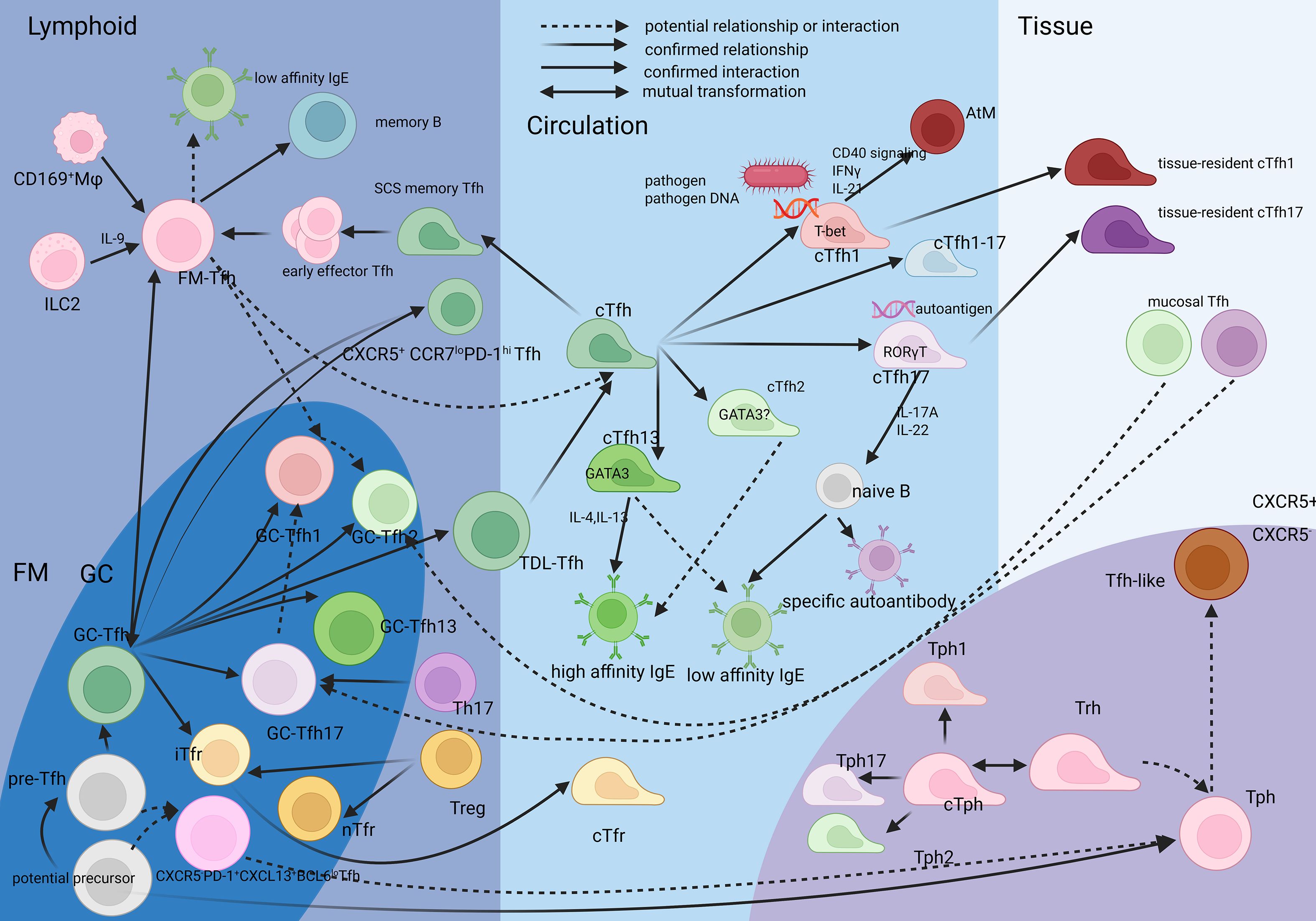
Figure 1. The relationship between the Tfh, Tph and their spatio and lineage subsets FM-Tfh is a stable subset different from pre-Tfh and GC-Tfh, while it is generated from GC-Tfh and pre-Tfh. cTfh is considered originated from GC-Tfh and also FM-Tfh. The initiation milleus is deduced to influence their phenotype. cTfh1 and cTfh17 can migrate into pathogenic tissue. Most mucosal Tfh has a Tfh17 skewing. Tph and Tfh have a common precursor closer than Th22, Tph has itsr circulating counterpart like cTfh, also relevant to memort phenotype.Tfh-like cells are considered relevent to Tph. Created in BioRender. SHEN, C. (2025) https://BioRender.com/jah121c, with permission.
1.2 Tfr: hybrid regulators of germinal center dynamics
Follicular regulatory T cells (Tfr) serve as a pivotal immunoregulatory subset within GCs, co-expressing BCL6 (shared with Tfh cells) and FOXP3 (characteristic of Tregs), alongside a hybrid phenotype combining Tfh-like surface markers CXCR5+PD-1+ICOS+ and Treg-like surface markers CTLA-4+GITR+. The Tfr derived from thymic Tregs (CD25hiCD38+) with closer resemblance to Treg TCR repertoires are called natural Tfrs (nTfrs), they localize to GC mantle/dark zones, and their limited TCR diversity support their role in suppressing autoreactive GC B cells clones via their preference in autoantigen, assisted by suppressive molecules (e.g., CTLA4), neuritin (preventing GC B expansion), and decoy receptor IL-1R2/IL-1R antagonist(preventing GC B activation) (26, 27).
Beyond direct B cell targeting, Tfr also modulates the GC microenvironment to prevent excessive immune responses. The timing of naive T cells’ entry into the GC influences their differentiation due to the microenvironment of GCs: early-arriving precursors exposed to strong TCR and costimulatory signals tend to adopt a Tfh phenotype, whereas late-arriving cells—encountering declining antigen availability, reduced IL-6/IL-21, and enhanced inhibitory cues (e.g., CTLA-4, IL-10)—preferentially differentiate into Tfr (28), which is referred to as induced Tfrs (iTfrs) and considered to originate from CD25hiTfh precursors under IL-2 signaling and GC microenvironment cues. They operate predominantly in germinal center light zones, where they modulate GC contraction by suppressing excessive Tfh responses while providing help to affinity-matured GC-B (e.g., via IL-21 and IL-10 secretion) (29, 30) to maintain their survival in dark zone microenvironments while inhibiting their activation (26, 28). Moreover, “late Tfr”, which enters GCs late, is a subset of iTfr, which induces GC B apoptosis via prolonged interactions (likely by FasL signal) (31). It mediates GC termination and could also be considered as immune suppression. Therefore, it can be hypothesized that the functional polarization of Tfr cells is influenced by their developmental proximity to either Tfh or Treg lineages, implying that the transition from Treg or Tfh to Tfr may represent a functional and phenotypic continuum shaped by both cellular origin and microenvironmental cues (Figure 1).
Systemic immune surveillance involves circulating Tfrs (cTfrs, BCL6+CXCR5+CD4+FOXP3+CD25+), which are hypothesized to patrol between blood and lymphoid tissues via S1P and CXCR5-CXCL13 axis and home to nascent GCs to prime regulatory networks. This subset is considered as “immature Tfr”: it exhibits reduced immune suppression and may accelerate secondary antibody responses (32); it has a lower expression of BCL6 and Tfh-like surface markers than Tfh, while maintaining a similar expression of FOXP3 with Treg and lower suppressive function than GC-Tfr (33). Anti-PD-1 therapy could increase cTfr, consequently increasing the quantity of plasmablasts and the titer of immunoglobulin, both of which exhibit positive linear correlation with CD38+ cTfr (34) (might be considered as “i-cTfr” subset), suggesting a deviation from traditional Tfr function.
To conclude, Tfh biology is governed by multilayered control: Transcriptional antagonism (BCL6 vs. BLIMP1), cellular positioning guided by spatiotemporal molecular concentration gradients, and functional cross-talk with Tfr. Defining these subsets necessitates integrating phenotypic markers, functional outputs, and anatomic context to resolve their roles in immunity and autoimmunity.
2 Heterogeneity of canonical Tfh cells: GC-Tfh, FM-Tfh, cTfh
As outlined in the introduction, Tfh heterogeneity is defined by spatial and phenotypic criteria. This section will now detail the characteristics of the canonical spatial subsets: GC-Tfh, FM-Tfh, and cTfh.
2.1 Germinal center Tfh
GC-Tfh cells, characterized by high BCL6, PD-1, CXCR5 expression and a signature of CD44hiCD62LloCD90loCCR7loPSGL1loCXCR5hiICOShiPD-1hi (16), are not a monolithic population but exhibit remarkable functional plasticity, driven by disparate cytokine milieus and transcriptional programs that confer T helper lineage-like characteristics (Tfh1, Tfh2, Tfh17). This heterogeneity is empirically supported by in situ multiplex immunofluorescence and CyTOF analyses (35).
2.1.1 Distribution-specificity and lineage subset characteristics of GC-Tfh
The abundance and activation state of GC-Tfh cells vary significantly across secondary lymphoid organs, reflecting their distinct immunological roles. Flow cytometry reveals a quantitative gradient in Tfh frequency of tonsil>mesenteric lymph nodes (mLNs)>spleen and activation markers (e.g., Ki-67, CXCR5, PD-1, ICOS, HLA-DR, CD38) of tonsil > spleen > mLN.Tfr populations mirrored this activation gradient (tonsil > spleen > mLN) for marker expression but demonstrated an inverse frequency distribution pattern of being enriched in mLNs compared to tonsils or spleen (36). This suggests tonsils are active reaction centers, the spleen is a storage site, and mLNs may function as regulated transit hubs, consistent with their roles in antigen trafficking. Despite these quantitative differences, the qualitative properties and defining features of Tfh lineage subsets skewing remain comparable across these sites, allowing for generalized subset classification.
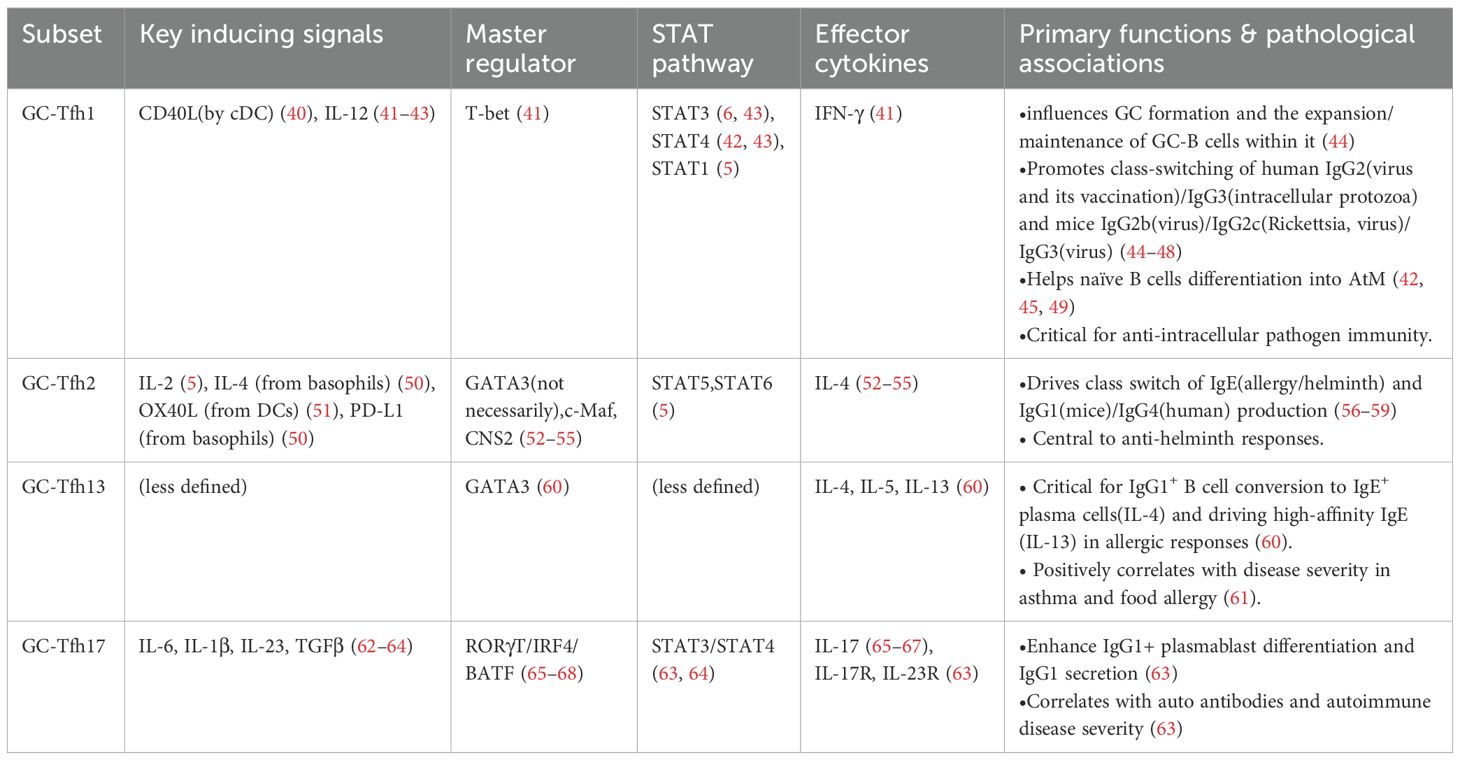
GC-Tfh skewing is highly context-dependent (Figure 2). Under homeostasis, it exhibits limited heterogeneity and similar transcriptional profiles. However, during disease disturbance, the APCs (mainly DCs) and cytokine milieu potently reshape GC-Tfh polarization, prompting the expression of transcription factors associated with other Th lineages and secretion of related cytokines (37–39), thereby tailoring antibody responses to specific immunological challenges. Their differentiation is initiated by specific cues from antigen-presenting cells (APCs), predominantly dendritic cells (DCs), which shape the ensuing polarization. The characteristics of the major subsets are summarized in Table 1.
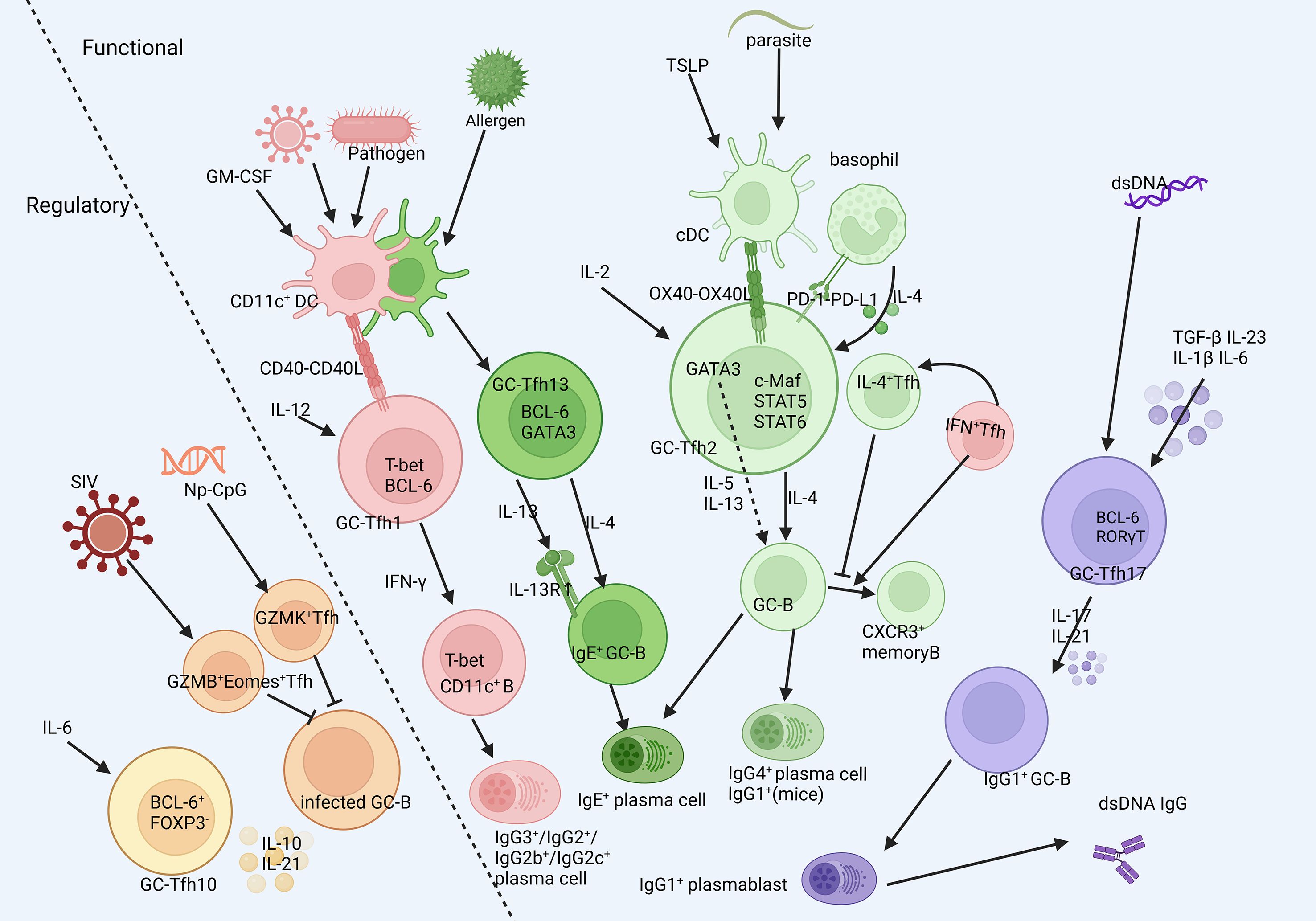
Figure 2. The heterogenity of GC-Tfh cells. GC-Tfh could be classified into several subsets different in functions according to their surface markers and classical transcription factors and cytokines. They are initiated in different milleus, experience deviate developmental process and respond to different stimulation. Created in BioRender. SHEN, C. (2025) https://BioRender.com/cej3yjb, with permission.
Beyond these main subsets, regulatory subsets also exist in GC-Tfh, including Tfh10 and cytotoxic Tfh. Tfh10 shares features with conventional Tfh but produces high levels of IL-10 while lacking Foxp3 and CD25, distinguishing them from Tfr. They sense inflammation (e.g., via IL-6) and feedback with IL-10 to establish an anti-inflammatory balance (69). Cytotoxic Tfh cells highly and specifically express GZMK (granzyme K) and GZMA (granzyme A), and also GZMB (granzyme B). They can kill GC B cells to suppress high-affinity antibody production or eliminate infected cells, but may also disrupt GC homeostasis if overactivated. They appear more closely related to the Tfh1 subset and play a role in chronic inflammatory and fibrotic diseases (70–72).
2.1.2 Issues in GC-Tfh subtyping
Traditionally, Tfh subsets were classified by cytokine profiles or transcription factors, but both methods present limitations. There are misalignments of cytokines and transcription factor. It has been proved that GC-Tfh2 relies on c-Maf and CNS2 rather than GATA3(which is low in Tfh2); the generation of IL-5/IL-13 strictly depends on GATA3, and IL-4+ Tfh itself does not co-express IL-5 or IL-13 (52–55). Also, some argue that IL-4 expression does not necessarily confer a Th2-biased heterogeneity to GC-Tfh (73), yet in parasite models, these IL-4+ Tfh do promote host IgE and IgG1 responses against helminths (74). Additionally, the more Th2-like Tfh13 subset (producing IL-4/5/13 and GATA3-dependent) better qualifies as “bona fide” GC-Tfh2 than IL-4-only producers. Therefore, it seems as if the commonly referred to “IL-4+ GC-Tfh2” is not an exact classification, and its heterogeneity warrants specific investigation. Similarly, IL-17 production in GC-Tfh17 is not strictly RORγT-dependent but is ICOS-dependent. In the absence of RORγT, IL-6/IL-23 signaling could activate STAT3, which cooperates with IRF4/BATF to bind the IL-17A promoter and thus sustain IL-17A production (68), indicating that GC-Tfh17 generation is a process coordinated by multiple transcription factors and cytokines. Thus, cytokine and transcription factor signatures often misalign, underscoring the need for a more precise and dynamic classification system.
Surface marker-based classification used for cTfh (e.g., CXCR3/CCR6) also fails to fully align with cytokine/transcription factor subset, suggesting the subpopulations defined in GC-Tfh and cTfh may not be entirely analogous. Simultaneous analysis using CXCR3/CCR6 markers and cytokines (IFN-γ, IL-17) found some of the GC-Tfh subset(e.g., Tfh1 and Tfh17) identified by surface markers(CXCR3/CCR6) without coexpression of IL-21 and their corresponding cytokines, but this misaligned fraction of cells in marker-identified GC-Tfh1/GC-Tfh17 are decreased as the increase of corresponding environmental stimuli, suggesting the “aligned” GC-Tfh might be considered as an activated state. Interestingly, there actually exist Tfh1/17 cells (CXCR3+CCR6+) producing IL-17, IL-21, and IFN-γ simultaneously, although they are rare in the CXCR3+CCR6+CXCR5+ subset, where IL-17/IFN-γ single-expressing subsets dominate (37). Single-cell RNA sequencing also revealed cross-polarization in a particular environment: ~8% of Tfh under Tfh1-enriched conditions exhibited Tfh2 traits, and ~15% under Tfh2 conditions showed Tfh1 features (72). These discoveries suggest the misalignment of surface markers and cytokines might also largely derive from the impact of environmental stimuli and their corresponding state, other than the inaccuracy of certain markers.
2.2 Tfh in follicular mantle zone is distinct from pre-Tfh and GC-Tfh
Follicular mantle zone(FM), the area surrounding the GC, is localized between the GC and the capsule of follicles. It is enriched by IgD+ naive B cells, and thus could be distinguished from GC by CD157 or CD21(both positive in GC) and IgD(positive in FM) markers. And by technique based on IgD+naive B cell enrichment and Tfh migration velocity gradients (lower migration velocity in FM), the span of the FM zone is measured to be about 50 μm (21, 75). FM-Tfh denotes a spatially defined functional subset that stably occupies the FM and scans antigens via subcapsular sinus (SCS) macrophages (16, 21), while having shared but a little lower expression of surface molecule features CXCR5, PD-1, and ICOS than their GC counterpart (GC-Tfh) (76) (Figure 1).
Approximately 30% of FM-Tfh cells differentiate directly from pre-Tfh without traversing GCs, indicating a GC-independent developmental pathway (77). This process is orchestrated through BCL6-mediated regulation of PSGL1, EBI2, and S1PR1 genes, which disrupts the barrier of exchange Tfh between GC and FM (78), and correspondingly, transformation could happen between FM-Tfh and GC-Tfh (21), thus FM-Tfh serves as one of the sources of GC-Tfh.
However, despite this exchange, FM-Tfh presents different molecular profile, shaping their function deviated from GC-Tfh under resting state: While expressing Bcl6 transcript levels comparable to GC-Tfh, they exhibit significantly reduced BCL6 protein expression (~60% decrease) (21), which stems from IL-9 receptor (IL-9R) signaling sustaining baseline BCL6 expression, as evidenced by further BCL6 downregulation upon IL-9 deprivation (79), suggesting their low polarization state. Cytokine secretion profiles demonstrate elevated IL-10 production but diminished IL-4 output compared to GC-Tfh (21). The IL-10 secretion inhibits memory B cell apoptosis, complementing GC-Tfh-driven proliferation, both of which are vital in memory pool maintenance. Under disturbance such as LCMV infection, FM-Tfh can express T-bet and IFN-γ, acquiring Th1-like effector functions (80), in which STAT3 is also vital (6). This plasticity is facilitated by persistent IL-9R expression, enabling responses to IL-9 secreted by ILC2s activated via B cell-derived leukotriene LTC4 (79). Critically, an intermediate KLF2 expression gradients dictate spatial specificity of FM-Tfh and renders them susceptible to perforin-dependent NK cell clearance, which is also counteracted by IL-9 signaling (79, 81), suggesting IL-9 might be an indispensable factor in FM-Tfh development and regulation.
The transcriptional reprogramming of FM-Tfh resembles GC-Tfh profiles during rechallenge, accelerating antibody production rates five-fold versus primary responses (21). FM-Tfh gain access to antigens from CD169+ macrophages, which enables efficient presentation to memory B cells, reactivating secondary GC reactions (25). Thus, FM-Tfh may function as a multifunctional subset that orchestrates the interplay between innate immunity and GC/EF(extrafollicular) reactions, maintains immune stability at steady state, and mounts rapid responses upon antigen re-encounter.
Pathologically, FM-Tfh causes allergy while avoiding autoimmune responses and presents age-association. Constrained by the absence of somatic hypermutation(SHM) in follicular mantle B cells, FM-Tfh could only induce IgE+B cells, without generating autoreactive IgE antibodies (60), and eventually induce low affinity IgE, which play a role in allergy, due to their rapid antibody generation and reaction, for example, IL-9R+FM-Tfh drive five-fold increases in house dust mite (HDM)-specific IgE, with IL-9R blockade significantly ameliorating allergic responses (79). On the contrary, since GC-Tfh experiences SHM, high-affinity antibodies could be induced, for example, GC-Tfh13 are capable of inducing the differentiation of B cells into plasma cells producing high-affinity IgE (60). Accumulation of FM-Tfh (increasing by 40%) and the gradual decrease of GC-Tfh both correlate with age and impaired GC reactions, explaining reduced vaccine efficacy in elderly populations (21, 76). Therefore, it could be deduced that recalibrating FM-Tfh/GC-Tfh balance by targeting KLF2-S1PR1 or IL-9R signaling might potentially offer a novel intervention for allergic disorders and age-related immune decline.
FM-Tfh is rarely studied due to the difficulties in dissecting (owing to the localization and the overlap phenotype with GC-Tfh), and the pathological role is less significant than GC-Tfh and cTfh, while studying it might give an insight of the potential relationship between GC-Tfh and cTfh. Real-time visualization of pre-Tfh, FM-Tfh, and GC-Tfh trafficking is important in directly observing their relationship; and studying the crosstalk of KLF2 and IL-9R(including whether KLF2 directly suppresses Il9r transcription to regulate FM-Tfh expansion) and the consistency of KLF2int IL-9R+ co-expression in human lymph nodes versus tonsils could further decode the development of FM-Tfh and deepen the insight into their roles in Tfh plasticity.
2.3 Circulating follicular helper T cell
Circulating follicular helper T cells (cTfh) are characterized by the surface markers CXCR5+CD45RA−CD45RO+. Although considered to originate from GC-Tfh, cTfh exhibit reduced BCL6 expression alongside elevated CCR7 levels and moderately decreased CXCR5 expression (77, 82–84). Their function alterations are associated with phenotypic regulation and spatial localization. Besides acting as a long-lived memory pool, cTfh is activated upon re-stimulation and undergoes clonal expansion (32, 77, 85) to stimulate B cell differentiation for antibody production. They play vital roles in anti-viral neutralizing antibody production (39, 86), while the abnormal increase of cTfh correlates with autoimmunity (87). They facilitate the differentiation of naïve and memory B cells into plasmablasts secreting IgG, IgE, and IgA via T–B interactions (84), mainly depending on their secretion of IL-21 (88, 89). This process is antigen-specific, with variations in quantity and effector function. For instance, following influenza, tetanus, or Candida albicans vaccination, MHC-II peptide tetramer technology allows isolation of cTfh specific for each antigen (90). Intriguingly, cTfh cells are more potent than effector Tfh cells in lymph nodes: they can be reactivated by DCs, home to GCs, and produce more cytokines (23), aligning with their memory function and re-activation under stimuli.
2.3.1 Circulating Tfh with memory phenotype
cTfh has four developmental states: naïve (Tn), stem-like memory (Tscm), central memory (Tcm), and effector memory (Tem). Finally, cTfh can mainly display either Tem or Tcm phenotypes (91). Upon initial generation, cTfh exhibit a Tem phenotype (low CCR7), consistent with GC-Tfh and later gradually transition to a Tcm state (high CCR7) following antigen stimulation (83, 92, 93), to be a predominate relatively stable memory subset in homeostasis conditions, it adopts a more “stem-like” phenotype, including intrinsically expression of CCR7 and CD62L mediating migration (93) and a lower expression of transcription factors and cytokines of Tfh lineage subset than in cTfh-Tem, whereas Tfh-Tem are less frequent (94), and expand abnormally under specific diseases (95), probably due to a dominant effect in the corresponding milieu (91). The extent of cTfh memory states activity is determined by CCR7(stem-like markers), ICOS, and PD-1(activation markers). PD-1++CCR7lo cells rapidly differentiate into mature Tfh upon antigen re-encountering (77). Among these, ICOS+PD-1++CCR7lo cells (<1% of cTfh) express Ki-67, representing activated memory cTfh, whereas ICOS−PD-1+CCR7int and ICOS−PD-1−CCR7hi subsets correspond to resting memory cTfh (96). Shifts toward activated Tem phenotypes are clinically significant; for example, RA patients exhibit increased frequencies of Tfh-Tem (PD-1hiCCR7lo/ICOS+PD-1hiCCR7lo), which correlate with disease activity (95, 97), indicating antigen-driven activation. Thus, the acquisition of an activated Tem phenotype (characterized by PD-1hiCCR7lo) brings cTfh closer both phenotypically and functionally to GC-Tfh, suggesting that cTfh become active because of the transformation of their memory state.
2.3.2 Heterogeneity of cTfh
Blood cTfh is categorized into three functional subsets based on surface chemokine receptors analogous to Th lineages: cTfh1 (CXCR3+CCR6-), cTfh17 (CXCR3-CCR6+), and cTfh2 (CXCR3-CCR6-) (96). A rare cTfh1–17 subset (CXCR5+CXCR3+CCR6+) has also been identified and implicated in B-cell responses (28, 98), though its scarcity has limited mechanistic insight. cTfh2 secrete IL-4, IL-5, and IL-13 but not IL-6 or IL-10, distinguishing them from Th2 cells by their lack of anti-inflammatory function. cTfh1 secrete IFN-γ and express T-bet, while cTfh17 secrete IL-17A and IL-22 and express RORγT (84, 99), resembling Th1 and Th17 profiles, respectively (Figure 1).
During viral infections,cTfh1 expand and promote virus-specific IgG production by assisting memory—but not naïve—B cells, underscoring a specific helper phenotype (99–101). Pathogen-associated DNA-induced cTfh stimuli further induce IFN-γ+ cTfh (102), reinforcing their role in antiviral immunity. cTfh1 also exhibits pathogenic potential: they provide CD40L, IFN-γ, and IL-21 to promote CD21loT-bethi with CD40 activation outweighing TLR signaling (103). CXCR3 mediates cTfh1 migration to inflammatory sites such as the CSF in multiple sclerosis, where they display a cytotoxic gene signature (104). Tfh1 are found in lupus nephritis kidneys (105)and IgA vasculitis intestine (106). and their cTfh1 reduction in peripheral blood suggests tissue homing.
cTfh17 can produce IL-21 and help naive B cells (84). They are strongly implicated in autoimmunity: about 60% of autoreactive cTfh are IL-17 positive and exclusively express RORγT and drive specific autoantibody induction by memory B cells in a RORγT-dependent manner, thus qualitatively and quantitatively associated with autoimmune disease (102, 107). Furthermore, beyond their helper function, cTfh17 cells also present expansion in serum and target tissue infiltration, such as the kidneys of IgAV nephritis and the aorta of Takayasu arteritis (108, 109). However, CCR6 is not Tfh17-specific (73), and BCL6 itself can suppress CCR6 expression (78),complicating subset identification.
cTfh2 also produces IL-21, but its specific functions remain unclear. Their defining markers (CXCR3-CCR6-) are negative, and the previously assumed key cytokine IL-4 is not specific to cTfh2. Classification by CXCR3 and CCR6 double negativity includes a significant amount of non-GATA-3/non-IL-4 expressing cells (56), suggesting CXCR3/CCR6 double negative does not fully characterize cTfh2. Some studies have begun using CXCR3-CCR4+ markers for cTfh2 (37, 110, 111). Functionally, cTfh2(referred to as GATA-3+IL-4+BCL6+CXCR3-CCR6-) promotes B cell differentiation into antigen-secreting cells and positively correlates with total and antigen-specific IgE and allergic reactions (112). It also participates in autoimmune, considering its positive correlation with SLEDAI and anti-dsDNA antibodies in SLE (56). Furthermore, an IL-13-producing cTfh13 subset is elevated in peanut allergy and asthma, correlated with IgE, and airway hyperreactivity (60), suggesting the crucial role of cTfh13 in high-affinity IgE in allergy, consistent with GC-Tfh13 (113), calling for studies on the relationship of cTfh2 and cTfh13.
Notably, distinct cTfh subsets (e.g., cTfh1/cTfh17) may adopt different memory stages according to the immune milieu; in healthy individuals, cTfh1 primarily exibits Tem and Tcm is enriched in cTfh17, and in viral infection cTfh1 signatures effector state while cTfh17 still in memory state (91), suggesting the role of cTfh1 in normal secondary re-challenging, by sustaining a more active state within memory state. It is different in autoimmune diseases, such as active SLE, in which cTfh1 primarily exhibits Tcm, while cTfh17 shows a Tem phenotype (114), consistent with the effect of Tfh17 skewing. Thus, cTfh subsets enter Tem state to exert their function of different lineages in various conditions.
2.4 Plasticity of GC-Tfh and cTfh
Heterogeneous Tfh phenotypes are stimulus-dependent. Crotty proposed three models—’imprinted partial plasticity,’ ‘de novo partial plasticity,’ and ‘FM-Tfh origin’—to explain the mechanisms underlying cTfh heterogeneity (73), which, as we noticed in recent researches, could be borrowed to model the heterogeneity and plasticity of all Tfh subsets. These plasticity models operate within the established framework of the BCL6-BLIMP1 balancing axis (section 1.1), often starting from a low-polarity state (84). In vitro, under IL-12/IL-4/TGF-β conditions, memory-like induced-Tfh1/2/17(iTfh) can be generated, respectively. These cytokine-induced Tfh1/2/17 express Tfh core markers and “canonical markers” for each Tfh lineage, with BCL6 lower than in GC-Tfh but comparable to pre-Tfh and cTfh (91), supporting the idea that decreased BCL6 inhibition contributes to Tfh subset generation (13, 64). and their effect in antibody class switching is consistent with the corresponding lineage, suggesting the existence of a relatively stable state in Tfh skewing (91), supporting the concept of low-polarity Tfh and environment-driven polarization (“de novo partial plasticity”). By downregulating RORγT and IL-17 while upregulating IL-21 and BCL6, Th17 cells in Peyer’s patches(PP) can transform into Tfh-like cells (115). Gut segmented filamentous bacteria(SFB) could also generate Tfh17 by inducing c-Maf rather than BCL-6; these Tfh17 share origin and TCR profile with Th17 rather than Tfh. S1PR1+ Tfh17 enter circulation and migrate to the spleen, potentially contributing to cTfh17. Aligned with cTfh17 effects we described above, these splenic Tfh17 promote RA manifestation, enhance GC-B responses, and elevate anti-GPI antibody titer (116), supporting the “imprinted” plasticity models, and its adjustment of c-Maf suggests that BCL-6 is not the only factor of tuning Tfh and Th lineage phenotype, and considering c-Maf being the key of GC-Tfh2 generation, supporting the imprinted plasticity model applied to GC-Tfh, where c-Maf is an important participant, as a supplement to its de novo plasticity model (5) supported by evidences in 2.1.2 and 2.1.3.
These models help explain plasticity from bona fide Tfh to lineage subsets, but direct evidence for plasticity between established Tfh subsets is still scarce, with most data limited to subset ratio change (117), rather than the direct evidence of transition between the “individual” Tfh lineage subset cells. But it is certain that there might be a shared period between Tfh1 and Tfh2 in their early stage. GC-Tfh1 transiently express T-bet during early maturation and undergo epigenetic modifications at the Ifng locus, enabling sustained IFN-γ production (41), and surprisingly, so-called “GC-Tfh2” cells can transiently produce IFN-γ before switching to IL-4, which could be referred to as an IL-4-free window, which is a transition regulated by T-bet (51, 58). IFN-γ production promotes the differentiation of germinal center B cells (GC-B) into CXCR3+ memory B cell precursors. When secretion switches to IL-4, this program gradually downregulates to modulate GC dynamics (118), suggesting that the early-stage-GC-Tfh1 actually has the same function as “mature” Tfh1. These could be interpreted to assume the potential transformation from (transient) Tfh1 to Tfh2; the possibility of plasticity might lie in the misalignment of Tfh subset markers. It has been indicated that Tfh2 only secretes IL-4 in helminth infection to help low-affinity IgE generation, and Tfh13 in allergen stimulation helps high-affinity IgE generation (60). As Tfh13 co-expresses IL-4 and IL-13, it might be hypothesized that Tfh2 could transfer to Tfh13. Another potential evidence of potential plasticity between Tfh lineage subsets is that under autoimmune uveitis(AE), the expression of decreasing or deficient STING (Stimulator of interferon genes) could drive Tfh17 polarization, and in the infiltrated tissue, the proportion of Th1 is decreased; however, in the DLN of AE, the Th1 is high (119). Given that STING is the driver of IRF3, IFN-I and IFN-γ receptor expression and Th1 differentiation (120), despite the necessity of direct “Tfh1 to Tfh17” evidence, it could be deduced that the polarization of Tfh17 could also be a consequence of Tfh17 skewing overcoming Tfh1 skewing. Additionally, the presence of Tfh1–17 cells (37) might also hint at a potential connection between Tfh1 and Tfh17 subsets, which could be worth exploring. There is no hint of the transformation between Tfh lineage subsets after their skewing is mature, suggesting that upon maturation, Tfh subsets enter a relatively stable state, and their relatively pliable state exists prior to this stable state.
3 Non-canonical Tfh-like populations
The conventional definition of Tfh cells—based on the co-expression of BCL6, CXCR5, and PD-1—fails to capture the full spectrum of CD4+ T cells capable of providing B cell help. Emerging evidence has revealed populations of non-canonical Tfh-like cells that, while lacking one or more of these classic markers (most notably CXCR5), nonetheless exhibit core Tfh functional attributes and a potent capacity to drive B cell differentiation and antibody production in peripheral and mucosal tissues. The recognition and classification of these non-canonical populations are essential for a complete understanding of humoral immunity, particularly in pathological contexts where they often play prominent roles.
3.1 Peripheral helper T cells
Peripheral helper T cells (Tph) are a distinct CD4+ T cell subset with a CXCR5-PD-1+CXCR3+CXCL13+IL-21+IL-10+BLIMP1hiBCL6lo signature (121). They are originally identified in rheumatoid arthritis (RA) synovium, and have a unique capacity to drive memory B differentiation into plasma cells in extralymphoid tissues (2). A hallmark of Tph differentiation is the TGFβ and ICOS-driven transcriptional program via Sox4, which promotes CXCL13/IL-21 secretion, PD-1 upregulation, and downregulates CXCR5, simultaneously enabling their homing to inflammatory sites via alternative chemokine receptors (e.g., CCR2/CCR5/CX3CR1) while precluding their entry into B cell follicles (122–125). While sharing a dependency on c-Maf, BATF, TIGIT, etc. with Tfh cells, their low BCL-6 and inability to access B cell follicles due to CXCR5 deficiency represent key distinctions (2, 62, 126, 127).
Tph cells exhibit significant plasticity and contextual function, while originally upregulating IFN-γ secretion (124) and acquiring Tfh1-like features when driven by combined ICOS and TGFβ signaling (2, 127). Upon acute viral infection, Tph upregulates CXCR3, TBX21, and STAT1, produces IFN-γ, to induce CXCR3 expression and neutralizing antibodies of plasmablasts (121). In chronic inflammation and autoimmunity, they are pivotal drivers of pathogenic antibody production by stimulating memory B cells to differentiate into plasmablasts and pathogenic antibody, especially IgG production in infiltrated tissues (125, 128), including synovium(RA) (128) and kidneys(SLE) (127). Similar to GC-Tfh1 and cTfh1, Tph can also promote CD21lowCD11c+ B cell differentiation, also by offering IFN-γ, CD40 and IL-21 (129). All these evidences suggest that Tph has a Th1 skewing like Tfh1, thus it might also be recognized as “Tph1”. But it could also be possible that those Tph studied in autoimmune disease are originally generated under Interferon milieus, for it is observed that by blocking IFNAR, Tph and CXCL13 generation can be halted (130). Besides, a spectrum of Tph subsets (Tph1, Tph2, Tph17, Tph1-17) exists in the circulation pool of Tph(referred to as circulating Tph, cTph), with the dominant subset influencing disease manifestation. They are also classified using CXCR3/CCR6. Among them, Tph2 and Tph1–17 subsets expressed only low level of IL21 and offer less help to B cells; Tph2 presents a cytotoxicity related transcripts correlated with their alignment of severity and activity of autoimmune disease (131); SLE patients exhibit increased Tph1/Tph2 frequencies positively linked to SLEDAI scores, with Tph1 enrichment in cutaneous/musculoskeletal involvement and Tph2 in lupus nephritis (132), suggesting the different roles Tph subsets play across the system of SLE. Interestingly, these Tph2 upregulate T-bet, Eomes, etc, indicating the participation of Tfh1-skewing programs. However, there are also evidence that Tph2 expand in allergic disease, secreting IL-5, IL-13, IL-21, likely to induce IgE-producing B cells (45, 112), different from that in autoimmunity (131), thus Tph2 might be heterogeneous, although it is necessary to clarify whether this heterogeneous is “imprinted” or from the stimulation of milieu in “de novo” development.
The relationship between Tph and Tfh is complex and may represent a continuum rather than a strict dichotomy. Beyond shared differentiation mechanisms and some common signatures mentioned previously, Tph and Tfh might also share the same developing pathway: IL-2–STAT5 signaling negatively regulates both lineages, while aryl hydrocarbon receptor (AHR) synergizes with AP-1(Activator Protein-1) family member JUN(Jun proto-oncogene) to inhibit CXCL13+ Tph/Tfh differentiation and promote Th22 development (130), so it could be deduced that at least, at the time point T helper cell develops Th22, Tph and Tfh are still sharing the same trajectory, and Tph/Tfh could be considered as subsets with shared mechanism but possess separate fate at last, probably under the effect of different milieus, the IFN-γ fate overcome the AHR/JUN signal. Besides, a distinct population of CXCR5-PD-1+CXCL13+BCL6lo Tph-like Tfh cells within breast cancer TLS promotes robust B cell recruitment and TLS formation, and might present a phenotypically “in-between” subset between Tfh and Tph (133). Moreover, although with a low expression, BCL6 is vital in Tfh and most Tph generation(although exceptions exist in lung resident Tph) and correlates with auto-antibody generation (134), again indicating the closer relationship of Tph with Tfh than other T helper cells.
cTph are homologous to pathological tissue resident Tph with shared features, functions, and, most importantly, TCR repertoire with cTfh cells (134), again suggesting deep correlation of Tfh and Tph. There is also evidence that cTph comes from and could in turn become tissue resident T helper cells(Trh) by adjusting their CD69 and PSGL1 levels, and due to their shared phenotype and highly overlapped TCR clonal type, the Trh could be considered as Tph reside in affected tissue. Trh can be distinguished from cTph by a CD69+PSGL1lo pattern (135). The Tph in synovium also highly expresses CD69, CXCR6 as Trm(tissue resident memory T helper cells) markers (136), suggesting that Trh might be a Tph compartment partially overlapped with Trm, BLIMP-1, and GPR56 participate in tissue residency (136, 137) (Figure 1). More importantly, although the IL-21R expression is lower in Trh and cTph, the IL-21-IL-21R-BCL6 signaling axis is still vital in their development (135), suggesting their strong relationship with Tfh as a tissue resident compartment.
In total, it is previously considered that Tph is a stable subset apart from Tfh as they have different differentiation track, but the transcriptome and experimental researches argues that Tph might also be regarded as a cell state of “generalized” Tfh, so Tph and Tfh in blood and pathogenic tissues might have redundant functions, but due to the difficulty of recruiting Tfh in chronic inflammatory tissues like synovium, their amount overwhelms Tfh (2) Furthermore, given that Tph strongly produce CXCL13, and CXCL13 can recruit Tfh and B cells by CXCR5-CXCL13 axis we discussed previously, Tph might be the initiator of autoimmune. As a result, quantitative comparison of Tfh and Tph B cell helping efficiency will further reveal the significance of recruiting Tfh, either as an enhancer of building ectopic GC or just recruited from the circulating system unspecifically. However the findings of tumor-infiltrated Tph that do not secrete IL-21 but instead recruit Breg cells by providing TGF-β and PD-L1 challenges this unified view (138), these cells may not have the same origin with Tph in inflammary tissues, but it is also rational to reason that it is within the tumor microenvironment that Tph changes its state or become a new subset, and it could be valuable to compare this Tph with “bona-fide” Tph on the a larger panel of Tph signatures, tracking how Tph enter these pathogenic tissues may be significant in further illustrating Tfh and Tph relationship.
3.2 Canonical and non-canonical Tfh in mucosal tissues
Tfh cells across various mucosal sites typically retain the canonical CXCR5+BCL6+ phenotype but exhibit tissue-specific functional biases. Significant conceptual and phenotypic challenge arises from the discovery of CXCR5- cells that closely mimic key features of Tfh cells, termed Tfh-like cells. They occur in mucosal tissues and might play a role in pathological mucosal immunity. In this part, we will focus on these mucosal Tfh populations.
3.2.1 Tfh and Tfh-like cells in GALT
As referred to at 2.1.1, the Tfh in tonsil, spleen and mLN are comparable despite quantitative differences, but Tfh in Peyer’s patches(PP) presents a Th17 bias apart from integrin, whereas the core program is the same; these could be considered as another evidence for the effect of imprinted plasticity (73). The Tfr in PP express BATF and c-Maf and secrete IL-4 and IL-21, a small group of Tfr also express IFN-γ under the regulation of T-bet (139), presenting a Th1 skewing. These evidences suggest an intrinsic skewing milieu in PP.
It was previously thought that GC-Tfh17 in GALT induces IgA production (115). However, recent findings argue that intestinal IgA does not depend on Th17 or IL-17. TGF-β produced by Peyer’s patch Tregs is the primary driver of IgA production, and IL-21 produced by Tfh is essential for terminal differentiation of IgA plasma cells; both factors are indispensable. These IgA-inducing Tfh may originate directly from naïve T cells and do not appear to exhibit a polarized phenotype (140, 141).
IgE production is also regulated in GALT. Food antigen could induce IL-4 producing Tfh(referred to as Tfh2) in mesenteric lymph node and Peyer’s patches, CD40 provided by DCs and ICOSL provided by B cells together with their presenting antigen induce and maintain this Tfh in germ-free mice, which is associated with IgE producing plasma cells generating and IgE increasing (142), suggesting the actual IgE-promoting effect of Tfh2 and the importance of gut microbiota. Moreover, peanut-specific cTfh13 is sensitively decreased in peanut oral immunotherapy and might help decrease IgE, although it does not necessarily predict the success of this therapy (143). Together, it is suggested that IgA rather than IgE is the most important factor in oral immunization.
There exist Tfh-like cells characterized by their CCR9+CXCR5- phenotype in GALT. They share a strikingly similar transcriptional profile with bona fide Tfh cells, including elevated expression of BCL6, c-Maf, PD-1 and ICOS, and retain the capacity to produce IL-21. These cells primarily localize to mucosal sites, especially the gut-associated lymphoid tissue (GALT), reflecting the role of CCR9 in gut homing. Thus, according to its residency and markers, it would not be too courageous to infer that CCR9+Tfh-like cells are the Tph counterpart in GALT, especially in the large picture of inflammatory and autoimmune states. They could also display remarkable functional plasticity, producing high levels of diverse cytokines such as IFN-γ, IL-17, IL-4, and IL-10 (144), in response to complex microenvironmental cues. Consequently, mounting evidence implicates these CCR9+ Tfh-like cells in the pathogenesis of autoimmune conditions. They contribute to mucosal inflammation in inflammatory bowel disease (IBD) (likely via IL-17/IFN-γ driven pathways) (145) and participate in the exocrine gland damage seen in primary Sjögren’s syndrome (pSS) (potentially through ectopic IL-21 production supporting autoreactive B cells) (146), all of which indicates that Tfh-like cells might have a close relationship, or at least emerge in very similar state with Tph.
3.2.2 Tfh-like cells in BALT
It has recently been revealed that ectopic inducible bronchus-associated tissues (iBALT) in the lung formed in inflammation and infection have similar ectopic GC-like structures, which could be distinguished from typical GC or other ectopic GC by a vague T-B aggregation structure. In viral and Mycobacterium tuberculosis infection, iBALT-Tfh has the canonical GC-Tfh phenotype (147), particularly, in tuberculosis background this Tfh represent a Tfh1 skewing with a CXCR3+T-bet+IFN-γ+ phenotype (148), while in G- bactorial infection background, a non-canonical “Tfh-like” cell without CXCR5, but with a normal PD-1, could offer help to GC-B-like cells with CD40L, IL-21, and IL-4, interestingly this non-canonical subset shows a valid Th2 skewing, with GATA3 expression and IL-10, IL-13 secretion (149).
3.2.3 Tfh-like cells in NALT
Although sharing a canonical GC-Tfh phenotype, the frequency of NALT(Nasopharyngeal-associated lymphoreticular tissue) Tfh is declined in adults, compared to those in children, which might be associated with the degradation of the adenoid. Inactivated antigen vaccine and LAIV(Live-attenuated influenza vaccines) could induce the proliferation of NALT Tfh, the generation of GC-B cells and the enhancement of the antibody production in NALT. In addition, the adjuvant CpG-DNA could enhance Tfh and antibody responses, which might have potential linkages with Tfh17 biased phenotype (150, 151).
In conclusion, Tfh have their counterpart in mucosal tissues, according to the milieus, these Tfh presents lineage-skewing, and the Tfh cells (particularly in GALT/NALT) is age-associated, suggesting that they might be temporal subsets that mainly functions in the environment of immature immunology; while the CXCR5- Tfh-like cells might be considered as a unique Tfh subset owing to their B cell helping function, it could be proposed that these “Tfh-like” cells might be Tph counterpart in mucosal, they loss CXCR5 possibly due to the lack of typical GC or ectopic GC-structures in mucosal, and they are not very prominent in producing CXCL13, which might be owed to the way B cells enter the corresponding mucosal structure is quite different from which in normal GC and normal ectopic GCs, these reason might also explain the lack of CXCR5 in Tph, suggesting that Tph and Tfh are “counterpart” in different milieus. Ultimately, it might be the environmental context that endow Tph with different lineage phenotypes or gene expression programs and signatures.
4 Discussion
The differentiation of Tfh cells is governed by a complex and precise regulatory network. Their spatial positioning is largely related to chemokine-mediated migration processes, which contribute to the remarkable heterogeneity of Tfh cells. Based on spatial localization, Tfh cells can be classified into GC-Tfh, FM-Tfh, and cTfh. Tph and mucosal Tfh-like cells may also be incorporated into this model due to their close relationship with conventional Tfh cells. Based on their close relationship with conventional Tfh cells, we propose an integrated system in which tissue-resident Tph and mucosal Tph constitute a tissue-resident pool, cTph and cTfh represent a circulating pool, and GC/FM-Tfh form a central pool. cTfh have been shown to be memory-pool, whereas cTph/Tph exhibit a memory phenotype. This suggests that cTfh may comprise the Tcm, Tem, and Tscm compartments of the Tfh lineage, while Tph/cTph correspond to its Trm pool. Thus, the memory pool can be considered an additional dimension—distinct from spatial localization and lineage commitment—consisting of memory states, with lineage subsets skewed toward particular memory phenotypes according to their different activating states.
We propose that the control of Tfh lineage commitment can be divided into two components: an imprinted part, wherein Tfh subsets may originate from other Th lineages (e.g., Tfh17 from Th17 and nTfr from Treg), and a de novo part orchestrated by BCL6-BLIMP1 balance (3). The generation of a Tfh subset with specific lineage features can be viewed as the summation of both imprinted and de novo contributions. That is, imprinted features initiate the expression of specific transcription factors, which shape initial differences in cytokine receptor profiles that ultimately manifest as major distinctions among Tfh subsets. These differences imply that variation in the microenvironment plays a critical role, reciprocally influencing the expression patterns of transcription factors, cytokines, and their receptors. Therefore, defining subsets based on surface markers, transcription factors, cytokines, and function remains a necessary foundation for clinical translation. High-dimensional technologies such as single-cell RNA sequencing and Cytometry by Time of Flight (CyTOF)—utilizing unsupervised clustering—could provide a greater panel of signatures utilized for machine learning to distinguish Tfh subsets more accurately (72).
However, to better capture the dynamics and plasticity of these states, which arise from variation in the imprinted and de novo components, non-clustering methods such as consensus non-negative matrix factorization (cNMF) are essential for modeling gene expression programs independent of limited marker sets. Pseudotime and trajectory analysis are valuable for inferring cellular dynamics, and single-cell TCR sequencing can elucidate clonal relationships among Tfh subsets.
The classification of Tfh cells retains clinical utility—for example, in assessing disease activity, severity, and therapeutic response—particularly in biopsy samples, where microenvironmental variation is limited. Caution is advised when interpreting results from blood samples, however, as circulating subset dynamics reflect the integrated immune state across multiple target organs. Defining Tfh subsets helps identify skewed states and recognize potentially pathological cell types, such as cTfh1 and Th1-skewed Tph, offering deeper immunological insight into diseases and therapies (such as selective cytokine targeting and JAK targeting therapies) and enabling more precise therapies. Current checkpoint blockade strategies targeting Tfh primarily focus on PD-1 and IL-21, and the insight into Tfh subsets might provide new targets. It may be beneficial to develop therapies that target skewing mechanisms, such as T-bet in Th1-oriented Tfh/Tph and atypical memory B cells in SLE. Elucidating Tfh memory states and their skewing could also improve our understanding of disease persistence, as in long COVID and other refractory or recurrent diseases, in which CAR-T therapies targeting the pathological memory cell subset might be beneficial.
In conclusion, defining Tfh heterogeneity extends beyond frequency-based cohort studies. A systemic understanding of relatively stable Tfh state and the dynamics of their development and plasticity will be crucial for unraveling disease mechanisms and ultimately guiding the development of more precise therapeutic interventions.
Author contributions
CS: Writing – original draft, Writing – review & editing. QF: Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Dr. Fu’s work is supported by National Natural Science Foundation of China “Immunity Deciphering Project” grant 92474112, Grant 82371805, Shanghai Municipal Science and Technology Fund (21ZR1438800,22Y11902400), Shanghai Immune Therapy Institute and the Shanghai Hospital Development Center (SHDC) (No. SHDC2023CRD012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviation
BCL6, B-cell Lymphoma 6; BLIMP1, B lymphocyte-induced maturation protein 1; CXCR3, C-X-C Motif Chemokine Receptor 3; CXCR5, C-X-C Motif Chemokine Receptor 5; CCR6, CC Motif Chemokine Receptor 6; CCR7, CC Motif Chemokine Receptor 7; C-Maf, the transcript of musculoaponeurotic fibrosarcoma(MAF) gene; FM, Follicular mantle; FM-Tfh, Follicular mantle follicular T helper cells; FOXP3, Forkhead box protein P3; GATA3, GATA-Binding Factor 3; GC, Germinal center; GC-Tfh, Germinal center follicular T helper cells; ICOS, inducible synergistic co-stimulation molecules; ICOSL, inducible synergistic co-stimulation molecules ligand; JAK-STAT pathway, Janus kinase-Signal Transducer and Activator of Transcription pathway; KLF2, Kruppel-like factor 2; OX40, Tumor necrosis factor receptor superfamily, member 4; OX40L, OX40 ligand; RORγT, Retinoic acid-related orphan receptor gamma t; SLE, Systemic Lupus Erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; S1PR1, Sphingosine-1-Phosphate Receptor 1; S1PR2, Sphingosine-1-Phosphate Receptor 2; T-Bet, T-box expressed in T cell; TIGIT, T cell immune receptor with Ig and ITIM domains; Tfh, follicular T helper cell; cTfh, Circulating follicular T helper cell; Tph, peripheral T helper cell.
References
1. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, and Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. (2000) 192:1553–62. doi: 10.1084/jem.192.11.1553
2. Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. (2017) 542:110–4. doi: 10.1038/nature20810
3. Crotty S. T follicular helper cell biology: A decade of discovery and diseases. Immunity. (2019) 50:1132–48. doi: 10.1016/j.immuni.2019.04.011
4. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. (2011) 34:932–46. doi: 10.1016/j.immuni.2011.03.023
5. Ma X, Nakayamada S, and Wang J. Multi-source pathways of T follicular helper cell differentiation. Front Immunol. (2021) 12:621105. doi: 10.3389/fimmu.2021.621105
6. Sulczewski FB, Martino LA, Salles D, Yamamoto MM, Rosa DS, and Boscardin SB. STAT3 signaling modulates the immune response induced after antigen targeting to conventional type 1 dendritic cells through the DEC205 receptor. Front Immunol. (2022) 13:1006996. doi: 10.3389/fimmu.2022.1006996
7. Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, et al. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity. (2015) 42:1159–70. doi: 10.1016/j.immuni.2015.05.012
8. Tahiliani V, Hutchinson TE, Abboud G, Croft M, and Salek-Ardakani S. OX40 cooperates with ICOS to amplify follicular th cell development and germinal center reactions during infection. J Immunol. (2017) 198:218–28. doi: 10.4049/jimmunol.1601356
9. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. (2009) 325:1006–10. doi: 10.1126/science.1175870
10. Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. (2014) 507:513–8. doi: 10.1038/nature12910
11. Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. (2012) 188:3734–44. doi: 10.4049/jimmunol.1103246
12. Wu H, Deng Y, Long D, Yang M, Li Q, Feng Y, et al. The IL-21-TET2-AIM2-c-MAF pathway drives the T follicular helper cell response in lupus-like disease. Clin Transl Med. (2022) 12:e781. doi: 10.1002/ctm2.781
13. Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. (2011) 12:536–43. doi: 10.1038/ni.2037
14. Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, et al. Migratory CD11b+ conventional dendritic cells induce T follicular helper cell–dependent antibody responses. Sci Immunol. (2017) 2:eaam9169. doi: 10.1126/sciimmunol.aam9169
15. Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci. (2016) 113:2702–7. doi: 10.1073/pnas.1520112113
16. Qi H. T follicular helper cells in space-time. Nat Rev Immunol. (2016) 16:612–25. doi: 10.1038/nri.2016.94
17. Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, and Cyster JG. Role of CXCR5 and CCR7 in follicular th cell positioning and appearance of a programmed cell death gene-1High germinal center-associated subpopulation1. J Immunol. (2007) 179:5099–108. doi: 10.4049/jimmunol.179.8.5099
18. Yasutomi M, Christiaansen AF, Imai N, Martin-Orozco N, Forst CV, Chen G, et al. CD226 and TIGIT cooperate in the differentiation and maturation of human tfh cells. Front Immunol. (2022) 13:840457. doi: 10.3389/fimmu.2022.840457
19. Zhu F, McMonigle RJ, Schroeder AR, Xia X, Figge D, Greer BD, et al. Spatiotemporal resolution of germinal center Tfh cell differentiation and divergence from central memory CD4+ T cell fate. Nat Commun. (2023) 14:3611. doi: 10.1038/s41467-023-39299-3
20. Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. (2015) 517:214–8. doi: 10.1038/nature13803
21. Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. (2015) 42:704–18. doi: 10.1016/j.immuni.2015.03.002
22. Yeh C-H, Finney J, Okada T, Kurosaki T, and Kelsoe G. Primary germinal center-resident T follicular helper cells are a physiologically distinct subset of CXCR5hiPD-1hi T follicular helper cells. Immunity. (2022) 55:272–289.e7. doi: 10.1016/j.immuni.2021.12.015
23. Vella LA, Buggert M, Manne S, Herati RS, Sayin I, Kuri-Cervantes L, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest. (2019) 129:3185–200. doi: 10.1172/JCI125628
24. Schattgen SA, Turner JS, Ghonim MA, Crawford JC, Schmitz AJ, Kim H, et al. Influenza vaccination stimulates maturation of the human T follicular helper cell response. Nat Immunol. (2024) 25:1742–53. doi: 10.1038/s41590-024-01926-6
25. Ise W, Inoue T, McLachlan JB, Kometani K, Kubo M, Okada T, et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc Natl Acad Sci U.S.A. (2014) 111:11792–7. doi: 10.1073/pnas.1404671111
26. Maceiras AR, Almeida SCP, Mariotti-Ferrandiz E, Chaara W, Jebbawi F, Six A, et al. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun. (2017) 8:15067. doi: 10.1038/ncomms15067
27. Ritvo P-GG, Churlaud G, Quiniou V, Florez L, Brimaud F, Fourcade G, et al. Tfr cells lack IL-2Rα but express decoy IL-1R2 and IL-1Ra and suppress the IL-1–dependent activation of Tfh cells. Sci Immunol. (2017) 2(15):eaan0368. doi: 10.1126/sciimmunol.aan0368
28. Merkenschlager J, Berz R-M, Ramos V, Uhlig M, MacLean AJ, Nowosad CR, et al. Continually recruited naïve T cells contribute to the follicular helper and regulatory T cell pools in germinal centers. Nat Commun. (2023) 14:6944. doi: 10.1038/s41467-023-41880-9
29. Le Coz C, Oldridge DA, Herati RS, De Luna N, Garifallou J, Cruz Cabrera E, et al. Human T follicular helper clones seed the germinal center–resident regulatory pool. Sci Immunol. (2023) 8:eade8162. doi: 10.1126/sciimmunol.ade8162
30. Graca L, Jacobsen J, and Kumar S. The expanding family of T follicular regulatory cells. Sci Immunol. (2023) 8(82):eadg7526. doi: 10.1126/sciimmunol.adg7526
31. Jacobsen JT, Hu WR. Castro TB, Solem S, Galante A, Lin Z, Allon SJ, et al. Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science. (2021) 373:eabe5146. doi: 10.1126/science.abe5146
32. Sage PT, Alvarez D, Godec J, Andrian UHV, and Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. (2014) 124:5191–204. doi: 10.1172/JCI76861
33. Fonseca VR, Agua-Doce A, Maceiras AR, Pierson W, Ribeiro F, Romão VC, et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol. (2017) 2(14):eaan1487. doi: 10.1126/sciimmunol.aan1487
34. Zhang H, Zheng H, Wang Y, Chen C, Tong Y, Xie S, et al. PD-1 suppresses human CD38+ circulating Tfr cells and regulates humoral immunity. J Immunother Cancer. (2025) 13(1):e010026. doi: 10.1136/jitc-2024-010026
35. Ioannidou K, Ndiaye D-R, Noto A, Fenwick C, Fortis SP, Pantaleo G, et al. In situ characterization of follicular helper CD4 T cells using multiplexed imaging. Front Immunol. (2021) 11:607626. doi: 10.3389/fimmu.2020.607626
36. Vaineau R, Jeger-Madiot R, Ali-Moussa S, Prudhomme L, Debarnot H, Coatnoan N, et al. IL-1β signaling modulates T follicular helper and regulatory cells in human lymphoid tissues. JCI Insight. (2025) 10(12):e188724. doi: 10.1172/jci.insight.188724
37. Verma A, Hawes CE, Elizaldi SR, Smith JC, Rajasundaram D, Pedersen GK, et al. Tailoring Tfh profiles enhances antibody persistence to a clade C HIV-1 vaccine in rhesus macaques. eLife. (2024) 12:RP89395. doi: 10.7554/eLife.89395
38. Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3–CXCR5+ Memory tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. (2013) 39:758–69. doi: 10.1016/j.immuni.2013.08.031
39. Ponnan SM, Vidyavijayan KK, Thiruvengadam K, Hilda JN, Mathayan M, Murugavel KG, et al. Role of circulating T follicular helper cells and stem-like memory CD4+ T cells in the pathogenesis of HIV-2 infection and disease progression. Front Immunol. (2021) 12:666388. doi: 10.3389/fimmu.2021.666388
40. Korniotis S, Saichi M, Trichot C, Hoffmann C, Amblard E, Viguier A, et al. GM-CSF-activated human dendritic cells promote type 1 T follicular helper cell polarization in a CD40-dependent manner. J Cell Sci. (2022) 135:jcs260298. doi: 10.1242/jcs.260298
41. Fang D, Cui K, Mao K, Hu G, Li R, Zheng M, et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. J Exp Med. (2018) 215:2705–14. doi: 10.1084/jem.20180927
42. Alterauge D, Bagnoli JW, Dahlström F, Bradford BM, Mabbott NA, Buch T, et al. Continued Bcl6 Expression Prevents the Transdifferentiation of Established Tfh Cells into Th1 Cells during Acute Viral Infection. Cell Rep. (2020) 33(1):108232. doi: 10.1016/j.celrep.2020.108232
43. Powell MD, Read KA, Sreekumar BK, Jones DM, and Oestreich KJ. IL-12 signaling drives the differentiation and function of a TH1-derived TFH1-like cell population. Sci Rep. (2019) 9:13991. doi: 10.1038/s41598-019-50614-1
44. Mendoza A, Yewdell WT, Hoyos B, Schizas M, Bou-Puerto R, Michaels AJ, et al. Assembly of a spatial circuit of T-bet-expressing T and B lymphocytes is required for antiviral humoral immunity. Sci Immunol. (2021) 6:eabi4710. doi: 10.1126/sciimmunol.abi4710
45. Levack RC, Newell KL, Popescu M, Cabrera-Martinez B, and Winslow GM. CD11c+ T-bet+ B cells require IL-21 and IFN-γ from type 1 T follicular helper cells and intrinsic bcl-6 expression but develop normally in the absence of T-bet. J Immunol. (2020) 205:1050–8. doi: 10.4049/jimmunol.2000206
46. Obeng-Adjei N, Portugal S, Holla P, Li S, Sohn H, Ambegaonkar A, et al. Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PloS Pathog. (2017) 13:e1006576. doi: 10.1371/journal.ppat.1006576
47. Liang H, Tang J, Liu Z, Liu Y, Huang Y, Xu Y, et al. ZIKV infection induces robust Th1-like Tfh cell and long-term protective antibody responses in immunocompetent mice. Nat Commun. (2019) 10:3859. doi: 10.1038/s41467-019-11754-0
48. Miyauchi K, Sugimoto-Ishige A, Harada Y, Adachi Y, Usami Y, Kaji T, et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat Immunol. (2016) 17:1447–58. doi: 10.1038/ni.3563
49. Weinstein JS, Laidlaw BJ, Lu Y, Wang JK, Schulz VP, Li N, et al. STAT4 and T-bet control follicular helper T cell development in viral infections. J Exp Med. (2018) 215:337–55. doi: 10.1084/jem.20170457
50. Tchen J, Simon Q, Chapart L, Thaminy MK, Vibhushan S, Saveanu L, et al. PD-L1- and IL-4-expressing basophils promote pathogenic accumulation of T follicular helper cells in lupus. Nat Commun. (2024) 15:3389. doi: 10.1038/s41467-024-47691-w
51. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. (2017) 214:1529–46. doi: 10.1084/jem.20150402
52. Bao K, Isik Can U, Miller MM, Brown IK, Dell’Aringa M, Dooms H, et al. A bifurcated role for c-Maf in Th2 and Tfh2 cells during helminth infection. Mucosal Immunol. (2023) 16:357–72. doi: 10.1016/j.mucimm.2023.04.002
53. Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, et al. Interleukin-4 production by follicular helper T cells requires the conserved il4 enhancer hypersensitivity site V. Immunity. (2012) 36:175–87. doi: 10.1016/j.immuni.2011.12.014
54. Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, et al. The 3′ Enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. (2012) 36:188–200. doi: 10.1016/j.immuni.2012.02.002
55. Ruterbusch M, Pruner KB, Shehata L, and Pepper M. In vivo CD4 + T cell differentiation and function: revisiting the th1/th2 paradigm. Annu Rev Immunol. (2020) 38:705–25. doi: 10.1146/annurev-immunol-103019-085803
56. Kim CJ, Lee C-G, Jung J-Y, Ghosh A, Hasan SN, Hwang S-M, et al. The transcription factor ets1 suppresses T follicular helper type 2 cell differentiation to halt the onset of systemic lupus erythematosus. Immunity. (2018) 49:1034–1048.e8. doi: 10.1016/j.immuni.2018.10.012
57. Kobayashi T, Iijima K, Dent AL, and Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. (2017) 139:300–313.e7. doi: 10.1016/j.jaci.2016.04.021
58. Dell’Aringa M, Reinhardt RL, Friedman RS, and Jacobelli J. Live imaging of IL-4-expressing T follicular helper cells in explanted lymph nodes. Methods Mol Biol. (2018) 1799:225–35. doi: 10.1007/978-1-4939-7896-0_17
59. Seth A, Yokokura Y, Choi J-Y, Shyer JA, Vidyarthi A, and Craft J. AP-1-independent NFAT signaling maintains follicular T cell function in infection and autoimmunity. J Exp Med. (2023) 220:e20211110. doi: 10.1084/jem.20211110
60. Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. (2019) 365:eaaw6433. doi: 10.1126/science.aaw6433
61. Yu B, Pei C, Peng W, Zheng Y, Fu Y, Wang X, et al. Microbiota-derived butyrate alleviates asthma via inhibiting Tfh13-mediated IgE production. Sig Transduct Target Ther. (2025) 10:181. doi: 10.1038/s41392-025-02263-2
62. Chang Y, Bach L, Hasiuk M, Wen L, Elmzzahi T, Tsui C, et al. TGF-β specifies TFH versus TH 17 cell fates in murine CD4+ T cells through c-Maf. Sci Immunol. (2024) 9:eadd4818. doi: 10.1126/sciimmunol.add4818
63. Kim V, Lee K, Tian H, Jang SH, Diamond B, and Kim SJ. IL-17–producing follicular Th cells enhance plasma cell differentiation in lupus-prone mice. JCI Insight. (2022) 7(11):e157332. doi: 10.1172/jci.insight.157332
64. Hong H, Gao M, Wu Q, Yang P, Liu S, Li H, et al. IL-23 promotes a coordinated B cell germinal center program for class-switch recombination to igG2b in BXD2 mice. J Immunol. (2020) 205:346–58. doi: 10.4049/jimmunol.2000280
65. Schmitt N, Liu Y, Bentebibel S-E, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. (2014) 15:856–65. doi: 10.1038/ni.2947
66. Zhang S. The role of transforming growth factor β in T helper 17 differentiation. Immunology. (2018) 155:24–35. doi: 10.1111/imm.12938
67. Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, et al. IL-23 and IL-1β Drive human th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. (2018) 22:2642–53. doi: 10.1016/j.celrep.2018.02.044
68. Wichner K, Stauss D, Kampfrath B, Krüger K, Müller G, Rehm A, et al. Dysregulated development of IL-17- and IL-21-expressing follicular helper T cells and increased germinal center formation in the absence of RORγt. FASEB J (2016) 30(2):761–74. doi: 10.1096/fj.15-274001
69. Almanan M, Raynor J, Ogunsulire I, Malyshkina A, Mukherjee S, Hummel SA, et al. IL-10–producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci Adv. (2020) 6:eabb0806. doi: 10.1126/sciadv.abb0806
70. Xie MM, Fang S, Chen Q, Liu H, Wan J, and Dent AL. Follicular regulatory T cells inhibit the development of granzyme B–expressing follicular helper T cells. JCI Insight. (2019) 4(16):e128076. doi: 10.1172/jci.insight.128076
71. Aoyagi R, Maehara T, Koga R, Munemura R, Tomonaga T, Murakami Y, et al. Single-cell transcriptomics reveals granzyme K–expressing cytotoxic Tfh cells in tertiary lymphoid structures in IgG4-RD. J Allergy Clin Immunol. (2024) 153:513–520.e10. doi: 10.1016/j.jaci.2023.08.019
72. Kumar S, Basto AP, Ribeiro F, Almeida SCP, Campos P, Peres C, et al. Specialized Tfh cell subsets driving type-1 and type-2 humoral responses in lymphoid tissue. Cell Discov. (2024) 10:1–21. doi: 10.1038/s41421-024-00681-0
73. Crotty S. Do Memory CD4 T Cells Keep Their Cell-Type Programming: Plasticity versus Fate Commitment? Complexities of Interpretation due to the Heterogeneity of Memory CD4 T Cells, Including T Follicular Helper Cells. Cold Spring Harb Perspect Biol. (2018) 10:a032102. doi: 10.1101/cshperspect.a032102
74. Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. (2016) 17:1197–205. doi: 10.1038/ni.3554
75. Kasashima S, Kawashima A, Kurose N, Ozaki S, Ikeda H, and Harada K. The disturbance of the distribution of T helper cell subsets in the mantle area surrounding germinal centers in immunoglobulin G4–related sclerosing sialadenitis. Virchows Arch. (2022) 481:767–77. doi: 10.1007/s00428-022-03384-7
76. Pastor R, Puyssegur J, de la Guardia MP, Varón LS, Beccaglia G, Spada N, et al. Role of germinal center and CD39highCD73+ B cells in the age-related tonsillar involution. Immun Ageing. (2024) 21:24. doi: 10.1186/s12979-024-00425-4
77. He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. (2013) 39:770–81. doi: 10.1016/j.immuni.2013.09.007
78. Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. (2015) 212:539–53. doi: 10.1084/jem.20141380
79. Sato T, Ikegami I, Yanagi M, Ohyu T, Sugaya T, Shirato S, et al. Interleukin 9 mediates T follicular helper cell activation to promote antibody responses. Front Immunol. (2024) 15:1441407. doi: 10.3389/fimmu.2024.1441407
80. Ray JP, Staron MM, Shyer JA, Ho P-C, Marshall HD, Gray SM, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity. (2015) 43:690–702. doi: 10.1016/j.immuni.2015.08.017
81. Waggoner S, Cox A, Canaday L, Katko A, Feldman H, Warrick K, et al. KLF2 determines the susceptibility of T cells to immunoregulatory NK cells. bioRxiv (2024). doi: 10.21203/rs.3.rs-4921081/v1
82. Brenna E, Davydov AN, Ladell K, McLaren JE, Bonaiuti P, Metsger M, et al. CD4+ T follicular helper cells in human tonsils and blood are clonally convergent but divergent from non-tfh CD4+ Cells. Cell Rep. (2020) 30:137–152.e5. doi: 10.1016/j.celrep.2019.12.016
83. Deng J, Wei Y, Fonseca VR, Graca L, and Yu D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat Rev Rheumatol. (2019) 15:475–90. doi: 10.1038/s41584-019-0254-2
84. Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. (2011) 34:108–21. doi: 10.1016/j.immuni.2010.12.012
85. Herati RS, Muselman A, Vella L, Bengsch B, Parkhouse K, Del Alcazar D, et al. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci Immunol. (2017) 2:eaag2152. doi: 10.1126/sciimmunol.aag2152
86. Cubas R and Perreau M. The dysfunction of T follicular helper cells. Curr Opin HIV AIDS. (2014) 9:485. doi: 10.1097/COH.0000000000000095
87. Deng J, Fan C, Gao X, Zeng Q, Guo R, Wei Y, et al. Signal transducer and activator of transcription 3 hyperactivation associates with follicular helper T cell differentiation and disease activity in rheumatoid arthritis. Front Immunol. (2018) 9:1226. doi: 10.3389/fimmu.2018.01226
88. Ricard L, Jachiet V, Malard F, Ye Y, Stocker N, Rivière S, et al. Circulating follicular helper T cells are increased in systemic sclerosis and promote plasmablast differentiation through the IL-21 pathway which can be inhibited by ruxolitinib. Ann Rheum Dis. (2019) 78:539–50. doi: 10.1136/annrheumdis-2018-214382
89. De Leur K, Dor FJMF, Dieterich M, van der Laan LJW, Hendriks RW, and Baan CC. IL-21 Receptor Antagonist Inhibits Differentiation of B Cells toward Plasmablasts upon Alloantigen Stimulation. Front Immunol. (2017) 8:306. doi: 10.3389/fimmu.2017.00306
90. Hu M, Notarbartolo S, Foglierini M, Jovic S, Mele F, Jarrossay D, et al. Clonal composition and persistence of antigen-specific circulating T follicular helper cells. Eur J Immunol. (2023) 53:2250190. doi: 10.1002/eji.202250190
91. Gao X, Luo K, Wang D, Wei Y, Yao Y, Deng J, et al. T follicular helper 17 (Tfh17) cells are superior for immunological memory maintenance. eLife. (2023) 12:e82217. doi: 10.7554/eLife.82217
92. Sallusto F, Lenig D, and Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature (1999) 401:708–12. doi: 10.1038/44385
93. Sallusto F, Geginat J, and Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. (2004) 22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702
94. Takeshita M, Suzuki K, Kassai Y, Takiguchi M, Nakayama Y, Otomo Y, et al. Polarization diversity of human CD4+ stem cell memory T cells. Clin Immunol. (2015) 159:107–17. doi: 10.1016/j.clim.2015.04.010
95. Takeshita M, Suzuki K, Kondo Y, Morita R, Okuzono Y, Koga K, et al. Multi-dimensional analysis identified rheumatoid arthritis-driving pathway in human T cell. Ann Rheumatic Dis. (2019) 78:1346–56. doi: 10.1136/annrheumdis-2018-214885
96. Schmitt N, Bentebibel S-E, and Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. (2014) 35:436–42. doi: 10.1016/j.it.2014.06.002
97. Ferrero PV, Onofrio LI, Acosta CDV, Zacca ER, Ponce NE, Mussano E, et al. Dynamics of circulating follicular helper T cell subsets and follicular regulatory T cells in rheumatoid arthritis patients according to HLA-DRB1 locus. Front Immunol. (2022) 13:1000982. doi: 10.3389/fimmu.2022.1000982
98. Su R, Wang Y, Hu F, Li B, Guo Q, Zheng X, et al. Altered distribution of circulating T follicular helper-like cell subsets in rheumatoid arthritis patients. Front Med. (2021) 8:690100. doi: 10.3389/fmed.2021.690100
99. Ueno H, Banchereau J, and Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. (2015) 16:142–52. doi: 10.1038/ni.3054
100. Bentebibel S-E, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+ CXCR3+ CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. (2013) 5(176):176ra32. doi: 10.1126/scitranslmed.3005191
101. Juno JA, Tan H-X, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. (2020) 26:1428–34. doi: 10.1038/s41591-020-0995-0
102. Wen Z, Xu L, Xu W, and Xiong S. Retinoic acid receptor–related orphan nuclear receptor γt licenses the differentiation and function of a unique subset of follicular helper T cells in response to immunogenic self-DNA in systemic lupus erythematosus. Arthritis Rheumatol. (2021) 73:1489–500. doi: 10.1002/art.41687
103. Keller B, Strohmeier V, Harder I, Unger S, Payne KJ, Andrieux G, et al. The expansion of human T-bethighCD21low B cells is T cell dependent. Sci Immunol. (2021) 6:eabh0891. doi: 10.1126/sciimmunol.abh0891
104. Morille J, Mandon M, Rodriguez S, Roulois D, Leonard S, Garcia A, et al. Multiple sclerosis CSF is enriched with follicular T cells displaying a th1/eomes signature. Neurol-Neuroimmunol Neuroinflamm. (2022) 9:e200033. doi: 10.1212/NXI.0000000000200033
105. Enghard P, Humrich JY, Rudolph B, Rosenberger S, Biesen R, Kuhn A, et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheumatism. (2009) 60:199–206. doi: 10.1002/art.24136
106. Liu D, Liu J, Wang J, Guo L, Liu C, Jiang Y, et al. Distribution of circulating T follicular helper cell subsets is altered in immunoglobulin A vasculitis in children. PloS One. (2017) 12:e0189133. doi: 10.1371/journal.pone.0189133
107. Holstein J, Solimani F, Baum C, Meier K, Pollmann R, Didona D, et al. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J Allergy Clin Immunol. (2021) 147:2358–69. doi: 10.1016/j.jaci.2020.11.008
108. Jiang Q, Chi X, Wei T, Nakayamada S, Shan Y, Sun Y, et al. Amelioration of immunoglobulin A vasculitis by suppression of the pathological expansion of T follicular helper 17 cells. J Autoimmun. (2024) 149:103304. doi: 10.1016/j.jaut.2024.103304
109. Desbois AC, Régnier P, Quiniou V, Lejoncour A, Maciejewski-Duval A, Comarmond C, et al. Specific follicular helper T cell signature in takayasu arteritis. Arthritis Rheumatol. (2021) 73:1233–43. doi: 10.1002/art.41672
110. Starshinova A, Malkova A, Zinchenko Y, Kudryavtsev I, Serebriakova M, Akisheva T, et al. Identification of autoimmune markers in pulmonary tuberculosis. Front Immunol. (2023) 13:1059714. doi: 10.3389/fimmu.2022.1059714
111. Huang Y, Wu X, Gui L, Jiang Y, Tu L, Li X, et al. Age-specific imbalance of circulating tfh cell subsets and its association with gout-targeted kidney impairment. Front Immunol. (2021) 11:625458. doi: 10.3389/fimmu.2020.625458
112. Zhu Y, Jiang Q, Lei C, Yu Q, and Qiu L. The response of CD27+CD38+ plasmablasts, CD24hiCD38hi transitional B cells, CXCR5–ICOS+PD-1+ Tph, Tph2 and Tfh2 subtypes to allergens in children with allergic asthma. BMC Pediatr. (2024) 24:154. doi: 10.1186/s12887-024-04622-4
113. Ollé L, García-García L, Ruano-Zaragoza M, Bartra J, González-Navarro E, Pérez M, et al. Mast cell activation profile and TFH13 detection discriminate between food anaphylaxis and sensitization. J Investig Allergol Clin Immunol. (2025) 35:203–15. doi: 10.18176/jiaci.0995
114. Khunsri T, Thawornpan P, Tianpothong P, Suangtamai T, Ngamjanyaporn P, Leepiyasakulchai C, et al. Activation of circulating TFH17 cells associated with activated naive and double negative 2 B cell expansion, and disease activity in systemic lupus erythematosus patients. Arthritis Res Ther. (2024) 26:159. doi: 10.1186/s13075-024-03394-7
115. Hirota K, Turner J-E, Villa M, Duarte JH, Demengeot J, Steinmetz OM, et al. Plasticity of TH17 cells in Peyer’s patches is responsible for the induction of T cell–dependent IgA responses. Nat Immunol. (2013) 14:372–9. doi: 10.1038/ni.2552
116. Fan T, Tai C, Sleiman KC, Cutcliffe MP, Kim H, Liu Y, et al. Aberrant T follicular helper cells generated by TH17 cell plasticity in the gut promote extraintestinal autoimmunity. Nat Immunol. (2025) 26:790–804. doi: 10.1038/s41590-025-02125-7
117. Yang Z, Hai L, Chen X, Wu S, Lv Y, Cui D, et al. OX40 ligand promotes follicular helper T cell differentiation and development in mice with immune thrombocytopenia. J Zhejiang Univ Sci B. (2025) 26:240–53. doi: 10.1631/jzus.B2300947
118. Arroyo-Díaz NM, Bachus H, Papillion A, Randall TD, Akther J, Rosenberg AF, et al. Interferon-γ production by Tfh cells is required for CXCR3+ pre-memory B cell differentiation and subsequent lung-resident memory B cell responses. Immunity. (2023) 56:2358–2372.e5. doi: 10.1016/j.immuni.2023.08.015
119. Li Z, Liu X, Li Z, Xiao Z, Chen G, Li Y, et al. STING deficiency promotes th17-like tfh to aggravate the experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. (2025) 66:8. doi: 10.1167/iovs.66.3.8
120. Benoit-Lizon I, Jacquin E, Rivera Vargas T, Richard C, Roussey A, Dal Zuffo L, et al. CD4 T cell-intrinsic STING signaling controls the differentiation and effector functions of TH 1 and TH 9 cells. J Immunother Cancer. (2022) 10:e003459. doi: 10.1136/jitc-2021-003459
121. Asashima H, Mohanty S, Comi M, Ruff WE, Hoehn KB, Wong P, et al. PD-1highCXCR5-CD4+ peripheral helper T cells promote CXCR3+ plasmablasts in human acute viral infection. Cell Rep. (2023) 42:111895. doi: 10.1016/j.celrep.2022.111895
122. Kim T, Martínez-Bonet M, Wang Q, Hackert N, Sparks JA, Baglaenko Y, et al. Non-coding autoimmune risk variant defines role for ICOS in T peripheral helper cell development. Nat Commun. (2024) 15:2150. doi: 10.1038/s41467-024-46457-8
123. Shan Y, Nakayamada S, Nawata A, Yamagata K, Sonomoto K, Tanaka H, et al. TGF-β3 in differentiation and function of Tph-like cells and its relevance to disease activity in patients with systemic lupus erythematosus. Rheumatology. (2023) 62:2464–74. doi: 10.1093/rheumatology/keac646
124. Yoshitomi H, Kobayashi S, Miyagawa-Hayashino A, Okahata A, Doi K, Nishitani K, et al. Human Sox4 facilitates the development of CXCL13-producing helper T cells in inflammatory environments. Nat Commun. (2018) 9:3762. doi: 10.1038/s41467-018-06187-0
125. Yoshitomi H and Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol. (2021) 18:523–7. doi: 10.1038/s41423-020-00529-z
126. Kojima M, Suzuki K, Takeshita M, Ohyagi M, Iizuka M, Yamane H, et al. Anti-human-TIGIT agonistic antibody ameliorates autoimmune diseases by inhibiting Tfh and Tph cells and enhancing Treg cells. Commun Biol. (2023) 6:500. doi: 10.1038/s42003-023-04874-3
127. Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G, et al. PD-1hiCXCR5– T peripheral helper cells promote B cell responses in lupus via MAF and IL-21. JCI Insight. (2019) 4(20). doi: 10.1172/jci.insight.130062
128. Murray-Brown W, Guo Y, Small A, Lowe K, Weedon H, Smith MD, et al. Differential expansion of T peripheral helper cells in early rheumatoid arthritis and osteoarthritis synovium. RMD Open. (2022) 8:e002563. doi: 10.1136/rmdopen-2022-002563
129. Fischer J, Dirks J, Klaussner J, Haase G, Holl-Wieden A, Hofmann C, et al. Effect of clonally expanded PD-1high CXCR5 – CD4 + Peripheral T helper cells on B cell differentiation in the joints of patients with antinuclear antibody–positive juvenile idiopathic arthritis. Arthritis Rheumatol. (2022) 74:150–62. doi: 10.1002/art.41913
130. Law C, Wacleche VS, Cao Y, Pillai A, Sowerby J, Hancock B, et al. Interferon subverts an AHR–JUN axis to promote CXCL13+ T cells in lupus. Nature. (2024) 631:857–66. doi: 10.1038/s41586-024-07627-2
131. Seki N, Tsujimoto H, Tanemura S, Kojima S, Miyoshi F, Kikuchi J, et al. Cytotoxic Tph subset with low B-cell helper functions and its involvement in systemic lupus erythematosus. Commun Biol. (2024) 7:277. doi: 10.1038/s42003-024-05989-x
132. Lin J, Yu Y, Ma J, Ren C, and Chen W. PD-1+CXCR5–CD4+T cells are correlated with the severity of systemic lupus erythematosus. Rheumatology. (2019) 58:2188–92. doi: 10.1093/rheumatology/kez228
133. Gu-Trantien C, Migliori E, Buisseret L, De Wind A, Brohée S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. (2017) 2:e91487. doi: 10.1172/jci.insight.91487. C.V. LD.
134. Sasaki T, Sowerby J, Xiao Y, Wang R, Marks KE, Horisberger A, et al. Clonal relationships between Tph and Tfh cells in patients with SLE and in murine lupus. bioRxiv. (2025). doi: 10.1101/2025.01.27.635189
135. Kong X, Wu X, Wang B, Zeng D, Cassady K, Nasri U, et al. Trafficking between clonally related peripheral T-helper cells and tissue-resident T-helper cells in chronic GVHD. Blood. (2022) 140:2740–53. doi: 10.1182/blood.2022016581
136. Argyriou A, Wadsworth MH, Lendvai A, Christensen SM, Hensvold AH, Gerstner C, et al. Single cell sequencing identifies clonally expanded synovial CD4+ TPH cells expressing GPR56 in rheumatoid arthritis. Nat Commun. (2022) 13:4046. doi: 10.1038/s41467-022-31519-6
137. Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. (2016) 352:459–63. doi: 10.1126/science.aad2035
138. Yu H, Shi M, Li X, Liang Z, Li K, Hu Y, et al. Tissue-resident peripheral helper T cells foster hepatocellular carcinoma immune evasion by promoting regulatory B-cell expansion. Chin Med J. (2025) 138:2148–58. doi: 10.1097/CM9.0000000000003544
139. Georgiev H, Ravens I, Papadogianni G, Halle S, Malissen B, Loots GG, et al. Shared and unique features distinguishing follicular T helper and regulatory cells of peripheral lymph node and peyer’s patches. Front Immunol. (2018) 9:714. doi: 10.3389/fimmu.2018.00714
140. Gribonika I, Strömberg A, Lebrero-Fernandez C, Schön K, Moon J, Bemark M, et al. Peyer’s patch TH 17 cells are dispensable for gut IgA responses to oral immunization. Sci Immunol. (2022) 7:eabc5500. doi: 10.1126/sciimmunol.abc5500
141. Gribonika I, Eliasson DG, Chandode RK, Schön K, Strömberg A, Bemark M, et al. Class-switch recombination to IgA in the Peyer’s patches requires natural thymus-derived Tregs and appears to be antigen independent. Mucosal Immunol. (2019) 12:1268–79. doi: 10.1038/s41385-019-0202-0
142. Hong S-W, Lee JY, Lee M, Han D, Ko H-J, Sprent J, et al. Food antigens drive spontaneous IgE elevation in the absence of commensal microbiota. Sci Adv. (2019) 5:eaaw1507. doi: 10.1126/sciadv.aaw1507. O E.
143. Wang W, Lyu S-C, Ji X, Gupta S, Manohar M, Dhondalay GKR, et al. Transcriptional changes in peanut-specific CD4+ T cells over the course of oral immunotherapy. Clin Immunol. (2020) 219:108568. doi: 10.1016/j.clim.2020.108568
144. McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, et al. A subset of interleukin-21+ Chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. (2011) 34:602–15. doi: 10.1016/j.immuni.2011.01.021
145. Cosorich I, McGuire HM, Warren J, Danta M, and King C. CCR9 expressing T helper and T follicular helper cells exhibit site-specific identities during inflammatory disease. Front Immunol. (2019) 9:2899. doi: 10.3389/fimmu.2018.02899
146. Hinrichs AC, Kruize AA, Lafeber FPJG, Leavis HL, and van Roon JAG. CCR9/CXCR5 co-expressing CD4 T cells are increased in primary sjögren’s syndrome and are enriched in PD-1/ICOS-expressing effector T cells. Int J Mol Sci. (2023) 24:11952. doi: 10.3390/ijms241511952
147. Tan H-X, Esterbauer R, Vanderven HA, Juno JA, Kent SJ, and Wheatley AK. Inducible bronchus-associated lymphoid tissues (iBALT) serve as sites of B cell selection and maturation following influenza infection in mice. Front Immunol. (2019) 10:611. doi: 10.3389/fimmu.2019.00611
148. Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. (2013) 123(2):JCI65728. doi: 10.1172/JCI65728
149. Vu Van D, Beier KC, Pietzke L-J, Al Baz MS, Feist RK, Gurka S, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun. (2016) 7:10875. doi: 10.1038/ncomms10875
150. Aljurayyan AN, Sharma R, Upile N, Beer H, Vaughan C, Xie C, et al. A critical role of T follicular helper cells in human mucosal anti-influenza response that can be enhanced by immunological adjuvant CpG-DNA. Antiviral Res. (2016) 132:122–30. doi: 10.1016/j.antiviral.2016.05.021
151. Aljurayyan A, Puksuriwong S, Ahmed M, Sharma R, Krishnan M, Sood S, et al. Activation and induction of antigen-specific T follicular helper cells play a critical role in live-attenuated influenza vaccine-induced human mucosal anti-influenza antibody response. J Virol. (2018) 92:e00114–18. doi: 10.1128/JVI.00114-18
Keywords: Tfh, germinal center, follicular mantle, circulating Tfh, Tfh plasticity, Tfh1, Tfh2, Tfh17
Citation: Shen C and Fu Q (2025) Follicular helper T cells (Tfh): heterogeneity in spatial distribution and phenotypic characteristics. Front. Immunol. 16:1686687. doi: 10.3389/fimmu.2025.1686687
Received: 15 August 2025; Accepted: 29 September 2025;
Published: 16 October 2025.
Edited by:
Shingo Ichimiya, Sapporo Medical University, JapanReviewed by:
Aquib Ehtram, La Jolla Institute for Immunology (LJI), United StatesJingjing Qi, Dalian Medical University, China
Copyright © 2025 Shen and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Fu, ZnVxaW9uZzVAMTYzLmNvbQ==
 Caifeng Shen
Caifeng Shen Qiong Fu
Qiong Fu