- 1Department of Pharmacy, Personalized Drug Research and Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Pharmacy, Chongqing Red Cross Hospital (People’s Hospital of Jiangbei District), Chongqing, China
- 3Department of Pharmacy, Bishan Hospital of Chongqing Medical University (Bishan Hospital of Chongqing), Chongqing, China
- 4School of Pharmacy, Southwest Medical University, Luzhou, China
Pregnant women and infants are more vulnerable to infectious diseases than the general population. Vaccination during pregnancy can protect not only mothers and fetuses from diseases but also safeguard infants through maternal antibodies transferred via the placenta. It stands as one of the most effective strategies to reduce the incidence and mortality of infectious diseases among pregnant women and infants. Globally, pregnancy vaccination strategies are increasingly diversified. This review discusses the effectiveness and safety of various vaccines for preventing infectious diseases during pregnancy, as well as vaccination recommendations for pregnant women across different countries.
Introduction
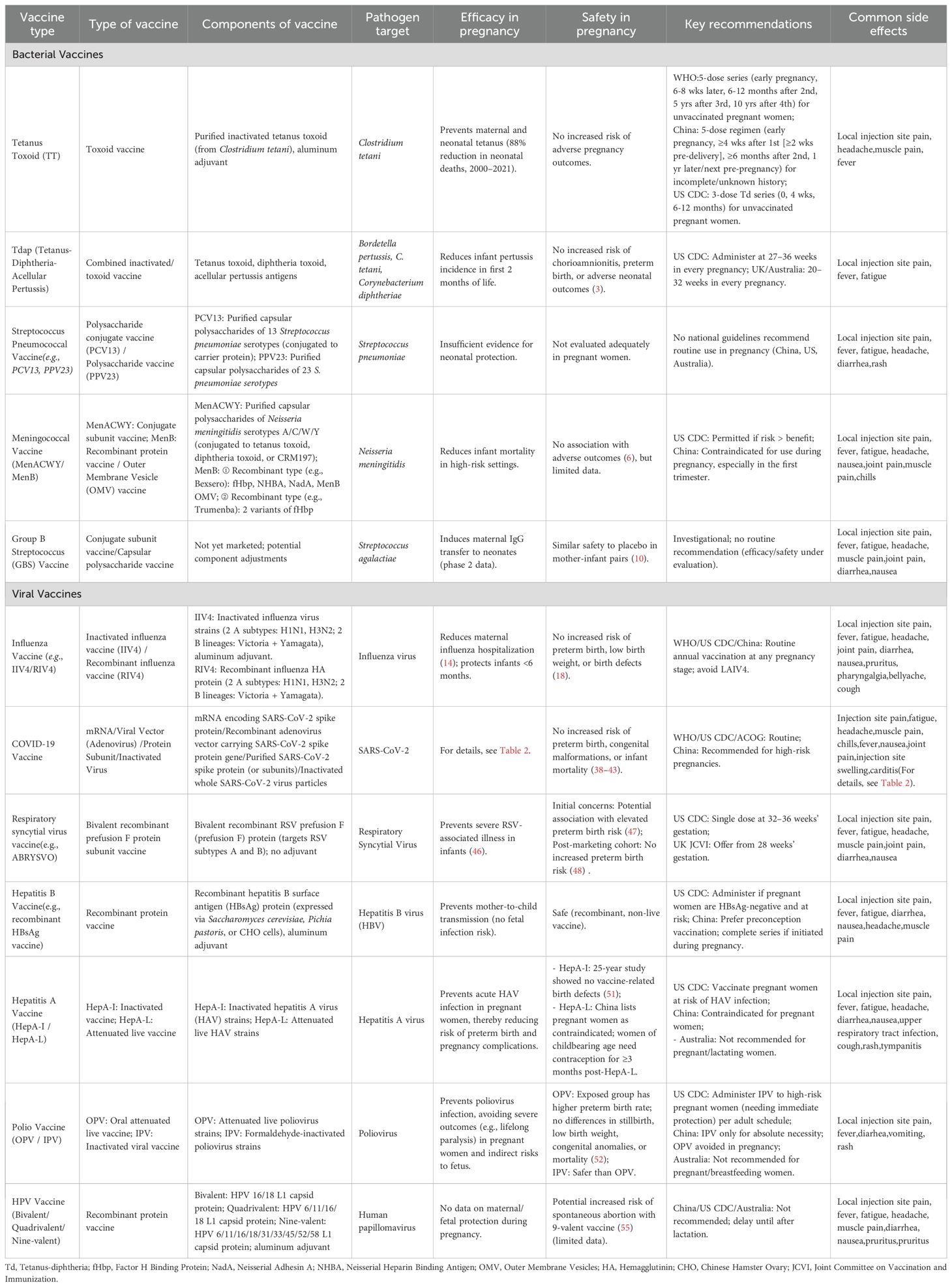
The immature immune system of fetuses and neonates renders them highly susceptible to infectious pathogens. Key factors contributing to this vulnerability include underdeveloped adaptive immunity, reduced antigen-presenting capacity, lack of immunological memory, and incomplete formation of mucosal barriers. These deficiencies result in a diminished capacity to combat microbial infections, often leading to more severe clinical manifestations and prolonged disease courses compared to adults. Maternal immunization represents a critical public health intervention to protect both mothers and their offspring. By eliciting pathogen-specific antibodies, vaccination during pregnancy facilitates transplacental transfer of immunoglobulin G (IgG), conferring passive immunity to fetuses and neonates during their period of highest susceptibility (Figure 1). Some vaccines have been proven safe and effective and are widely used in pregnant women, such as the tetanus vaccine, pertussis vaccine, and influenza vaccine. Other vaccines have varying recommendations for pregnant women across different countries or regions, while the effectiveness and safety of some vaccines in pregnant women remain controversial or under research. To provide a clear overview of the vaccines discussed in this review, Table 1 summarizes types, components, and key information for each vaccine—including their target pathogens, efficacy, safety profiles, and major recommendations for use during pregnancy.
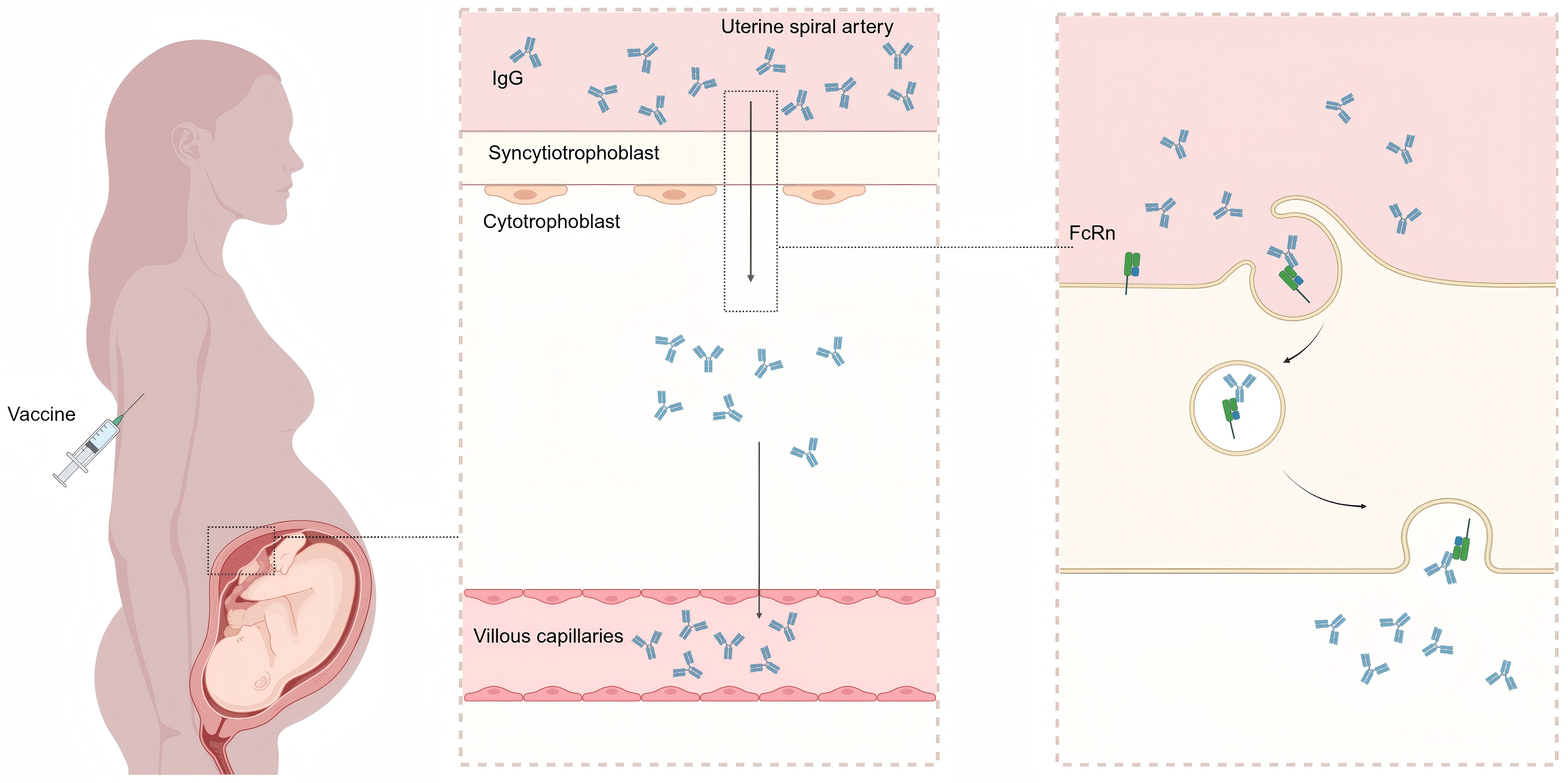
Figure 1. Mechanism of transplacental IgG transfer following maternal vaccination. Maternal IgG antibodies (produced postvaccination) bind to the fragment crystallizable neonatal receptor (FcRn) on placental trophoblast cells, undergo transcytosis across the syncytiotrophoblast layer, and are subsequently released into the fetal circulation. This figure was created with BioRender (https://biorender.com/).
Vaccines for preventing bacterial infections
Tetanus vaccine
Tetanus, an acute and specific infectious disease, is caused by the infiltration of Clostridium tetani into the human body through wound contamination. This condition predominantly affects individuals with traumatic injuries or burns, neonates born under unhygienic delivery conditions, and patients exposed to inadequately sterilized surgical instruments. Although tetanus can affect individuals of any age or demographic, its incidence and clinical severity are notably higher among unvaccinated neonates and pregnant women.
Maternal immunization with tetanus toxoid (TT) vaccine, an inactivated toxoid vaccine, has been demonstrated as an effective intervention for preventing both maternal and neonatal tetanus (MNT). The main component of the TT vaccine is detoxified tetanus toxin (toxoid), derived from the culture supernatant of Clostridium tetani. This toxoid retains immunogenicity, inducing the production of specific antibodies in the body, while losing toxic activity to avoid causing tetanus symptoms. In 1989, the 42nd World Health Assembly (WHA) issued a global call to eliminate MNT, defined as an incidence of fewer than one case per 1,000 live births in all regions. The integration of TT vaccination during pregnancy with improved obstetric hygiene practices has significantly reduced MNT-related mortality. As of 2024, only 10 countries remain noncompliant with the MNT elimination (MNTE) target.
According to World Health Organization (WHO) estimates, neonatal tetanus deaths declined by 88% between 2000 (200,000 deaths) and 2021 (24,000 deaths). To mitigate the risk of neonatal tetanus resulting from unsterile deliveries or other exposures, the WHO recommends that women of reproductive age receive TT immunization. This strategy confers passive immunity to infants during the vulnerable period from birth to 3 months of age.
The WHO immunization protocol specifies that unvaccinated pregnant women or women of childbearing age should receive a five-dose TT vaccination series, initiated as early as possible during pregnancy. In alignment with these guidelines, China’s public health policy mandates that pregnant women with incomplete or undocumented immunization records complete the five-dose regimen, prioritizing early antenatal administration. Conversely, the US Centers for Disease Control and Prevention (CDC) advises a three-dose series of tetanus-diphtheria (Td) vaccine for unvaccinated pregnant women.
Pertussis vaccine
Pertussis, caused by Bordetella pertussis, is a highly contagious acute respiratory infection associated with substantial morbidity and mortality in infants. Globally, the WHO estimates approximately 24 million cases and 160,700 deaths annually among children under 5 years of age. The CDC reports that the majority of pertussis deaths occur in infants under 3 months of age. In the USA, pertussis causes approximately 20 pediatric deaths annually, whereas in the Netherlands, 150–180 children under 2 years of age require hospitalization each year, with one infant death reported annually (1). Pertussis is a vaccine-preventable disease, and maternal immunization has been established as an effective strategy to protect infants who are too young to receive primary vaccination. The tetanus, diphtheria, and acellular pertussis (Tdap) vaccine—a combined inactivated/toxoid vaccine integrating three antigen components—when administered during pregnancy, provides passive immunity to neonates, significantly reducing pertussis incidence in the first 2 months of life (2). Its main components include the following: (1) Tetanus component; (2) Diphtheria component: detoxified diphtheria toxin (toxoid) from Corynebacterium diphtheriae; (3) Acellular pertussis component: purified acellular antigens of Bordetella pertussis. Research shows that Tdap vaccination during pregnancy can significantly reduce the incidence of pertussis in newborns (2). The Tdap vaccine demonstrates a favorable safety profile in pregnant women. A large-scale cohort study involving 118,211 pregnant women, of whom 103,258 (87%) received Tdap during gestation, found no increased risk of chorioamnionitis, preterm birth, or adverse neonatal outcomes (3). The WHO recommends maternal pertussis vaccination as a supplementary strategy in regions with high infant pertussis incidence or mortality. Similarly, the CDC advises Tdap administration during every pregnancy, regardless of prior vaccination history, with the optimal timing at 27–36 weeks of gestation to maximize transplacental antibody transfer. Nonetheless, vaccination is considered beneficial at any stage of pregnancy. Several high-income countries have successfully integrated maternal pertussis immunization into national programs: The USA introduced Tdap vaccination for pregnant women in 2011 to provide before infant immunization. The UK incorporated pertussis vaccination for pregnant women into its national program in 2012. Australia includes maternal pertussis vaccination in its National Immunization Program (NIP), recommending administration between 20 and 32 weeks of each pregnancy, although vaccination can still be provided until delivery. These policies have contributed to a measurable decline in neonatal pertussis morbidity and mortality, highlighting the critical role of maternal immunization as a public health intervention.
Streptococcus pneumoniae vaccine
Streptococcus pneumoniae (pneumococcus) is a leading invasive bacterial pathogen, contributing significantly to global morbidity and mortality, particularly among immunocompromised populations. Globally, pneumococcal diseases account for an estimated one million pediatric deaths annually. In the USA, pneumococcal pneumonia results in approximately 150,000 hospitalizations each year, with invasive pneumococcal disease (IPD)—including meningitis and bacteremia—causing 3,250 fatalities in 2019. No national guidelines currently endorse maternal pneumococcal vaccination due to insufficient evidence of neonatal protection. China has not included pregnant women in the target population for pneumococcal vaccines. The CDC recommends pneumococcal vaccination for all children under 5 years of age, adults over 65 years of age, and individuals aged 5–65 years with identified risk factors (4). Currently, the clinically most commonly used pneumococcal vaccines mainly include two types of subunit vaccines: (1) Pneumococcal polysaccharide vaccine (PPV, e.g., PPV23)—a polysaccharide-based subunit vaccine whose main component is purified capsular polysaccharides extracted from the cell wall of Streptococcus pneumoniae (typically covering 23 common pathogenic serotypes of Streptococcus pneumoniae); (2) Pneumococcal conjugate vaccine (PCV, e.g., PCV13, PCV15)—a conjugate subunit vaccine whose main component is Streptococcus pneumoniae capsular polysaccharides covalently bound to carrier proteins. However, there is currently no recommendation for pregnant women to receive the pneumococcal vaccine. The Australian Department of Health and Elderly Care (DOHAC) does not recommend that pregnant women receive pneumococcal vaccines.
Meningococcal vaccine
The mortality rate of invasive meningococcal disease is very high, with a mortality rate of up to one-sixth according to WHO data. One-fifth of survivors may experience long-term sequelae such as hearing loss, seizures, limb weakness, and visual impairment. In 2020, the WHO proposed the vision of “towards a world free of meningitis” by 2030, which includes three goals: elimination of bacterial meningitis epidemics, reduction of cases of vaccine-preventable bacterial meningitis by 50% and deaths by 70%, reduction of disability, and improvement of quality of life after meningitis due to any cause. Meningococcus, Streptococcus pneumoniae, Haemophilus influenzae, and Group B Streptococcus are common pathogenic bacteria that cause bacterial meningitis. The main serotypes of meningococcal bacteria that cause meningitis are A, B, C, Y, and W135 groups. Research shows that receiving the meningococcal vaccine during pregnancy can reduce infant mortality rates (5). Although current research shows that use of meningococcal vaccines during pregnancy is not associated with adverse pregnancy outcomes (6). However, due to the lack of reproductive toxicity testing or clinical studies in experimental animals and pregnant women, China has classified pregnant women as a prohibited population for the ACYW135 meningococcal polysaccharide vaccine (a polysaccharide subunit vaccine, whose main component is purified capsular polysaccharides extracted from A, C, Y, and W135 serogroups of Neisseria meningitidis), especially during the first three months of pregnancy. There are currently quadrivalent (serotypes A, C, W, and Y) meningococcal conjugate vaccines (MenACWY) and serotype B meningococcal vaccines (MenB) available in the United States. MenACWY is a conjugate subunit vaccine, with its main component being capsular polysaccharides from A, C, W, and Y serogroups of Neisseria meningitidis covalently bound to carrier proteins; MenB includes two types: recombinant protein vaccine or outer membrane vesicle (OMV) vaccine. Due to limited data on MenB vaccination during pregnancy, CDC recommends delaying MenB vaccination be deferred in pregnant and lactating women unless the woman is at increased risk, and, after consultation with her healthcare provider, the benefits of vaccination are considered to outweigh the potential risks. If indicated, pregnancy should not preclude vaccination with MenACWY. The DOHAC in Australia does not recommend pregnant or lactating women receive the meningococcal vaccine.
Group B Streptococcus vaccine
Group B Streptococcus (GBS) is an opportunistic pathogen that can transform from a colonized state to a pathogenic bacterium under certain conditions, leading to invasive GBS disease in pregnant women or newborns. For GBS colonization in pregnant women, without intervention, 50% will vertically transmit the bacterium to the fetus or newborn. Without antibiotic prophylaxis during childbirth, 1%–2% of these newborns will develop early-onset GBS disease (GBS-EOD), which can cause neonatal bacteremia, pneumonia, and meningitis (7). Prophylactic use of antibiotics during the perinatal period can effectively reduce the incidence of invasive GBS disease in neonates (8). The use of antibiotics during delivery in pregnant women carrying GBS can prevent early-onset invasive infections in newborns (within the first 7 days of birth) but does not protect against late-onset GBS disease (GBS-LOD) occurring between 7 days and 3 months after birth. Effective GBS vaccination has the potential to reduce the incidence of illness in mothers, fetuses, and infants. Currently, no GBS vaccines are widely marketed, and most candidates remain in clinical trials. The primary vaccine types entering clinical development are GBS capsular conjugate vaccines and capsular polysaccharide vaccines. Maternal vaccination with the Group B Streptococcus capsular conjugate vaccine can increase the serum IgG concentration against capsular polysaccharides in newborns at birth, thereby reducing the risk of Group B Streptococcus disease (9). Results of a phase 2 study in pregnant women indicate that the candidate vaccine GBS6 generally has good tolerability, produces strong maternal antibody responses, and can be effectively delivered to infants. The safety of the vaccine and placebo groups is similar in both mothers and infants (10). Further research is needed to determine the safety and efficacy of using the Group B Streptococcus capsular conjugate vaccine in pregnant women.
Vaccines for preventing viral infections
Influenza vaccine
Due to physiological changes in the immune, circulatory, and respiratory systems during pregnancy—including increased heart rate, higher oxygen consumption, and decreased lung capacity—pregnant women face a significantly higher risk of influenza infection (11). The probability of pregnant women becoming severely ill and requiring hospitalization after influenza infection is relatively high (12). In addition, influenza infection during pregnancy is associated with a decrease in the average birth weight of infants (13). Vaccination against influenza during pregnancy can reduce the risk of hospitalization for influenza by an average of 40% (14). At the same time, antibodies generated by maternal influenza vaccination can be transmitted to developing infants (15), providing protection against influenza and influenza-related hospitalization during the first few months of life (16), when infants are too young to receive vaccinations. Additionally, receiving the influenza vaccine during pregnancy can reduce the incidence of low birth weight and significantly lower the rate of premature birth (17). Influenza vaccines have a favorable safety profile, with multiple studies reporting no adverse effects on fetuses—including preterm birth, low birth weight, small-for-gestational-age infants, or major birth defects—following maternal vaccination (18). The WHO and Public Health England (PHE) recommend annual influenza vaccination for all pregnant women. The Advisory Committee on Immunization Practices (ACIP) recommends that all individuals over 6 months of age, including pregnant women, receive the influenza vaccine. The CDC recommends that inactivated influenza vaccine (IIV4) or recombinant influenza vaccine (RIV4) can be administered at any time during pregnancy (before or during the flu pandemic), and recommends that September and October are usually good months to receive the flu vaccine. This vaccination can provide immunity to infants born during the upcoming flu season. However, it is not recommended for pregnant women to receive the live attenuated influenza vaccine (LAIV4). The Chinese National Immunization Program Technical Working Group recommends that pregnant women may receive influenza vaccines at any stage of pregnancy. Similarly, the DOHAC in Australia recommends influenza vaccination for pregnant women. Furthermore, Australia has included influenza vaccination during pregnancy in its NIP.
COVID-19 vaccine
Current research has found that pregnant women infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have higher rates of ICU admission, need for invasive ventilation, need for extracorporeal membrane oxygenation (ECMO), and mortality compared to nonpregnant women (19, 20). The main pregnancy-related complications reported in pregnant women infected with SARS-CoV-2 include premature birth, stillbirth, preeclampsia, intrauterine growth restriction, and a higher risk of neonatal developmental defects (21). Pregnant women can obtain IgG antibodies by receiving the COVID-19 vaccine during pregnancy; these antibodies can be detected in the umbilical cord blood at delivery (22), providing protection to infants against COVID-19 infection (23). Currently, four main types of COVID-19 vaccines are marketed globally (23–32), with detailed information presented in Table 2. A cohort study confirmed that pregnant and lactating women exhibit robust vaccine-induced immune responses, with SARS-CoV-2 neutralizing antibody titers comparable to those in nonpregnant adults. These antibodies are efficiently transferred to fetuses via the placenta and to infants through breast milk (33). Regarding protective efficacy for infants, one study found that completing two doses of the messenger RNA (mRNA) COVID-19 vaccine during pregnancy may reduce the risk of COVID-19-related hospitalization in infants under 6 months, with an effectiveness rate of 61% (95% confidence interval [CI] = 31%–78%) (34). Another study found that administering two doses of the COVID-19 vaccine to pregnant women during pregnancy was 95% effective against SARS-CoV-2 Delta variant infection, and 97% effective in preventing related hospitalization in infants under 6 months (35). For maternal infection prevention, vaccination with the BNT162b2 mRNA vaccine was associated with a 78% reduced risk of SARS-CoV-2 infection in a 1:1 matched retrospective cohort (7,530 vaccinated vs. 7,530 unvaccinated pregnant women), confirming the efficacy of maternal vaccination against SARS-CoV-2 (36). Overall, receiving the COVID-19 vaccine during pregnancy can reduce the incidence of SARS-CoV-2 infections, including severe cases, lower the risk of COVID-19-related hospitalizations maternal mortality, and decrease the risk of SARS-CoV-2 infection in infants under 6 months (37). In terms of safety, evidence indicates that COVID-19 vaccination during pregnancy is safe and does not increase the risk of premature birth, low body weight, congenital malformations, or infant mortality (38–40). Multiple high-quality studies provide detailed supporting data. For example, a surveillance study included 35,691 pregnant participants, of whom 3,958 were enrolled in a pregnancy registry and received mRNA COVID-19 vaccines. Among these 3,958 participants, 827 had completed pregnancies, with a pregnancy loss rate of 13.9% and preterm birth rate of 9.4%—both comparable to prepandemic rates—and no vaccine-related severe adverse events were reported (41). An observational study of pregnant women who received the mRNA COVID-19 vaccine found that among 57 women who delivered during follow-up, there were no cases of preterm birth < 37 weeks or fetal/neonatal death (42). A study analyzing delivery outcomes of 2,002 pregnant women (140 vaccinated, 1,862 unvaccinated) found that the composite adverse outcome rate (5.0% vs. 4.9%) and preterm birth rate were not significantly different between the vaccinated and unvaccinated groups (43).
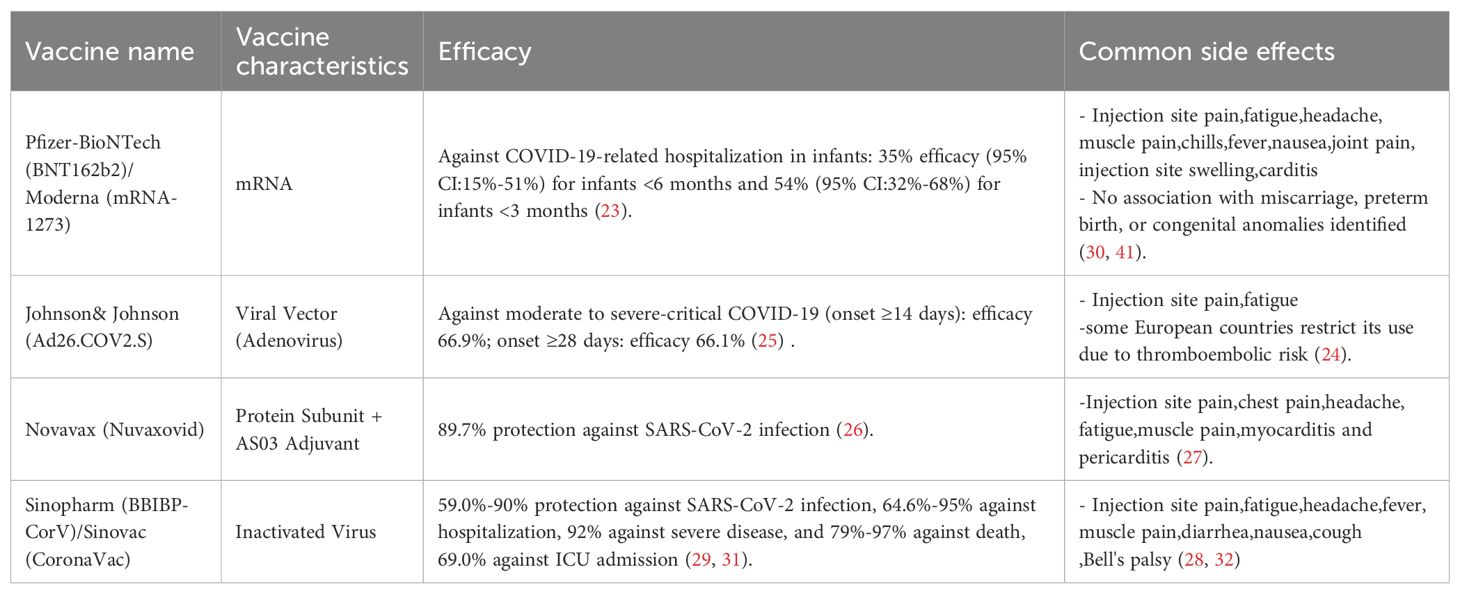
Table 2. Characteristics, efficacy and side effects of major currently used globally available covid-19 vaccines.
The WHO believes that the benefits of COVID-19 vaccination during pregnancy outweigh the potential risks and recommends that pregnant women receive the COVID-19 vaccine. The American College of Obstetricians and Gynecologists (ACOG) considers the COVID-19 vaccine safe for pregnant women and the fetus during any trimester. The COVID-19 vaccine generates antibodies that are passed to the fetus, which may protect against COVID-19 until a baby is eligible for vaccination at 6 months. The CDC recommends that people (including those who are pregnant, might become pregnant, were recently pregnant, or are breastfeeding) and infants 6 months of age or older be vaccinated with the latest version of the COVID-19 vaccine. The Society of Perinatal Medicine of the Chinese Medical Association suggests that, considering the inherent adverse effects of vaccination during pregnancy, COVID-19 vaccination may be postponed if there is no obvious risk of infection. However, if a pregnant woman faces a risk of infection—particularly during epidemics in daily life and/or workplace—it is recommended to receive an inactivated COVID-19 vaccine according to the standard procedure at any stage of pregnancy. Similarly, the DOHAC in Australia and the UK Medicines and Healthcare Products Regulatory Agency both recommend COVID-19 vaccination for pregnant women to help protect them and their infants from serious illness. The International Society of Infectious Diseases in Obstetrics and Gynecology (ISIDOG) recommends that pregnant women receive the COVID-19 vaccine and advises the use of mRNA-based vaccines (44).
Respiratory syncytial virus vaccine
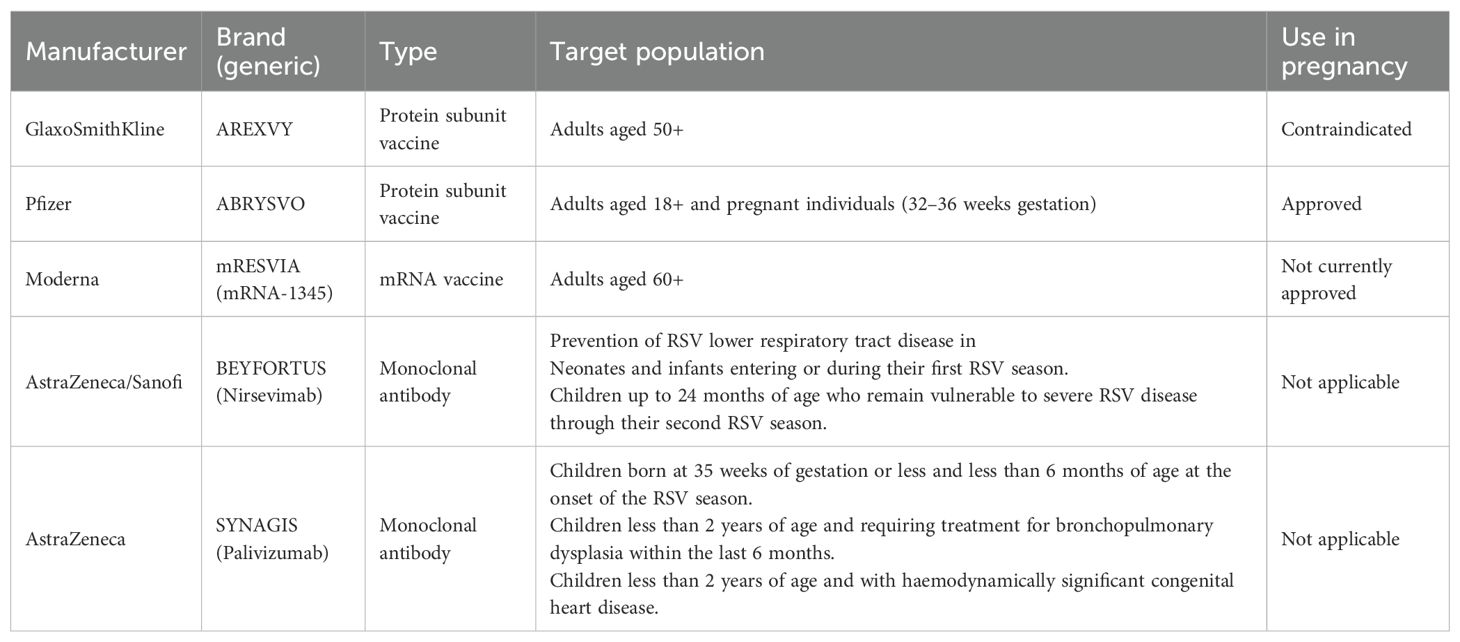
Respiratory syncytial virus (RSV) is a common respiratory virus and an important cause of morbidity and mortality in infants and young children worldwide. It is estimated that 2.0% of deaths in children aged 0 to 5 years and 3.6% of deaths in children aged 28 days to 6 months can be attributed to RSV (45). In 2019 alone, an estimated 33 million RSV-associated ALRTI cases occurred in children under five, resulting in 101,400 deaths worldwide (45). Given the persistent absence of efficacious antiviral therapeutics for RSV, prophylactic interventions constitute the cornerstone of disease management. Maternal RSV vaccination has emerged as a promising strategy to reduce severe RSV-related disease in infants. Clinical evidence demonstrates high protection against severe RSV-associated illness following vaccination during pregnancy, with efficacy rates of 81.8% within 90 days postpartum and 69.4% within 180 days (46). However, some studies suggest a potential association between maternal RSV vaccination and an elevated risk of preterm birth (47). The evolving landscape of RSV prophylaxis witnessed a paradigm shift in 2023 when the US Food and Drug Administration (FDA) granted approval to Pfizer’s bivalent RSV prefusion F protein subunit vaccine (ABRYSVO) for maternal immunization. Notably, while GSK’s adjuvanted recombinant vaccine (AREXVY)—an adjuvanted recombinant RSV protein subunit vaccine with main components of recombinant RSV prefusion F protein and AS01E adjuvant—and Moderna’s mRNA-1345 (mRESVIA)—an RSV mRNA vaccine with main components of mRNA encoding RSV prefusion F protein, lipid nanoparticles (LNP), and trace buffers—have received regulatory authorization for older adults (≥ 60 years), their safety profiles preclude antenatal application, establishing ABRYSVO as the sole maternal RSV vaccine globally. A cohort study involving 2,973 pregnant individuals post-marketing showed that among 1,026 participants (34.5%) who received prenatal RSVpreF vaccination, there was no association between prenatal RSVpreF vaccination and an increased risk of preterm birth (OR = 0.88; 95% CI: 0.64–1.20) (48). As of February 2025, the RSV immunoprophylaxis armamentarium encompasses five approved biologics categorized by target populations (Table 3). Accordingly, the CDC recommends a single dose of ABRYSVO at 32–36 weeks’ gestation. Similarly, the UK’s Joint Committee on Vaccination and Immunization (JCVI) advises offering RSV vaccination to pregnant individuals from 28 weeks’ gestation, with implementation scheduled to begin in September 2024.
Hepatitis B vaccine
Mother-to-child transmission is an important source of hepatitis B cases. The timing of transmission usually occurs during childbirth or the postpartum period. Hepatitis B virus (HBV) does not directly cause disease, and even if the fetus is exposed to the virus, fetal infection rarely occurs (49). However, during labor (including cesarean section), if the fetus or newborn is exposed to the mother’s blood and other bodily fluids, the virus can enter the newborn’s body. Close contact between newborns and their mothers after birth can also lead to transmission. Globally, the mainstream hepatitis B vaccine is a recombinant hepatitis B surface antigen (HBsAg) protein vaccine, with main components varying by expression system: (1) those produced via Saccharomyces cerevisiae—recombinant HBsAg protein and aluminum hydroxide adjuvant; (2) those produced via Pichia pastoris—recombinant HBsAg protein and aluminum adjuvant; and (3) those produced via Chinese hamster ovary (CHO) cells—recombinant HBsAg protein and aluminum phosphate adjuvant. As a recombinant protein vaccine, it contains no live hepatitis B virus and has no adverse effects on the fetus; therefore, pregnancy is not a contraindication for hepatitis B vaccine inoculation. However, the latest two hepatitis B vaccines—Heplisav-B and PreHevbrio—are not recommended for pregnant women due to insufficient data in this population. The CDC recommends that all pregnant women be screened for hepatitis B, and that adults aged 19–59 (including pregnant women) be vaccinated against hepatitis B. Newborns of HBsAg-positive pregnant women are at high risk of HBV infection. China’s Clinical Guidelines for Prevention of Maternal to Infant Transmission of Hepatitis B Virus (2020) recommend screening for hepatitis B serological indicators before pregnancy, and it is best to receive the hepatitis B vaccine prior to conception. However, if a woman becomes pregnant during the vaccination period, no special treatment is required, and the full vaccination course can be completed. According to the recommendations of the DOHAC in Australia, it is generally not recommended for pregnant or lactating women to receive the hepatitis B vaccine.
Hepatitis A vaccine
Hepatitis A virus is an acute intestinal infectious disease caused by the hepatitis A virus (HAV), mainly characterized by damage to liver parenchymal cells. Acute HAV infection in pregnant women may increase the risk of preterm birth and pregnancy complications (50). Currently, inactivated hepatitis A vaccine (HepA-I) and attenuated live hepatitis A vaccine (HepA-L; main components: attenuated live HAV strains with reduced pathogenicity that can induce an immune response without causing severe disease) are available globally. A study summarizing safety data from pregnant women vaccinated with HepA-I showed that among 378 pregnant women who received HepA-I over a 25-year period, no birth defects related to vaccination were observed in pregnancy outcomes (51). The CDC recommends that pregnant women be vaccinated with the HepA vaccine if they are identified as being at risk for HAV infection during pregnancy. However, China has listed pregnant women as a contraindicated group for the hepatitis A vaccine and recommends that women of childbearing age use contraception for at least 3 months after receiving HepA-L injection. The DOHAC in Australia also does not recommend that pregnant or lactating women receive the hepatitis A vaccine.
Polio vaccine
Poliovirus can cause poliomyelitis and lifelong paralysis. There are two types of polio vaccines: oral polio attenuated live vaccine (OPV) and inactivated polio vaccine (IPV), an inactivated viral vaccine with main components of formaldehyde-inactivated poliovirus strains (trivalent IPV [tIPV] containing serotypes 1, 2, 3 or monovalent IPV [mIPV] for single serotype) and an aluminum adjuvant in most formulations. Current evidence shows that exposure to OPV during pregnancy does not result in differences in stillbirth rates, low birth weight, congenital anomalies, neonatal mortality, or maternal mortality compared to nonexposed groups; however, the preterm birth rate is higher in the exposed group (18.4% vs. 11.0%, p = 0.011) (52). The CDC recommends that if a pregnant woman is at increased risk for infection and requires immediate protection against polio, IPV can be administered in accordance with the recommended schedules for adults. The instructions for the IPV launched in China state that pregnant women can only be vaccinated when it is absolutely necessary. The DOHAC in Australia does not recommend that pregnant or breastfeeding women receive the polio vaccine.
HPV vaccine
Human papillomavirus (HPV) infection is typically transmitted through direct contact with infected organs (via skin or mucous membranes) and is the most common sexually transmitted infection. Multiple research reports indicate that HPV-positive women have an increased risk of adverse pregnancy outcomes, including premature birth, miscarriage, gestational hypertension, intrauterine growth restriction, low birth weight, premature rupture of membranes, and fetal death (53). Currently, there is no evidence to suggest that administering bivalent HPV vaccines (a recombinant HPV protein subunit vaccine; main components: recombinant L1 capsid proteins of HPV types 16 and 18, plus aluminum hydroxide adjuvant) during pregnancy increases the risk of teratogenicity (54). However, a systematic review and meta-analysis showed that receiving the bivalent HPV vaccine within 45 days before the last menstrual period appears to increase the risk of spontaneous abortion (1.59 [1.04–2.45]) (55). Another systematic review showed that unintentional vaccination with bivalent HPV during pregnancy did not significantly increase the risk of miscarriage, stillbirth, small for gestational age, premature birth, or birth defects (56). Exposure to the quadrivalent HPV vaccine (a recombinant HPV protein subunit vaccine; main components: recombinant L1 capsid proteins of HPV types 6, 11, 16, and 18, plus aluminum adjuvant) during pregnancy or the peri-pregnancy is not associated with adverse pregnancy or delivery outcomes (57). A retrospective study in China found no stillbirths among 50 pregnant women who received the nine-valent HPV vaccine (a recombinant HPV protein subunit vaccine, main components: recombinant L1 capsid proteins of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, plus aluminum adjuvant), but one case of microtia was observed (58). A systematic review and meta-analysis showed that pregnancy exposure to the nine-valent HPV within 30 days of conception appeared to increase the risk of spontaneous abortion (2.04 [1.28–3.24]) (55). Due to limited data on HPV vaccination during pregnancy, China does not recommend prophylactic HPV vaccination for pregnant women. Women planning pregnancy in the near future are advised to postpone vaccination until after lactation. If an unexpected pregnancy occurs after initiating vaccination, incomplete vaccine doses should be discontinued. If the vaccination series has been completed, no intervention is required. Similarly, the CDC does not recommend HPV vaccination during pregnancy and advises that if a woman is found to be pregnant at the start of the vaccination schedule, the remaining doses should be postponed until after pregnancy. No pregnancy test is required before vaccination. If a vaccine dose is inadvertently administered during pregnancy, no intervention is needed. The DOHAC in Australia does not recommend HPV vaccination for pregnant women; however, lactating women may receive the vaccine.
Discussion
Pregnancy represents a unique immunological state characterized by maternal immune modulation, which increases susceptibility to viral and bacterial pathogens, thereby elevating risks of adverse outcomes, including severe infections, preterm birth, miscarriage, and stillbirth. Neonates remain vulnerable during the first months of life due to immature humoral immunity and incomplete vaccine-induced protection. Current evidence indicates that administration of inactivated viral/bacterial vaccines and toxoids during pregnancy does not pose fetal risks (59), with most nationally recommended maternal vaccines falling into this category. For instance, the US CDC, ACOG, and UK Health Security Agency endorse prenatal vaccination against influenza, pertussis, COVID-19, RSV, hepatitis B, and tetanus.
Live-attenuated vaccines, including measles, mumps, and rubella (MMR), varicella, and oral poliovirus vaccines, are generally contraindicated during pregnancy due to theoretical fetal risks, with postvaccination contraception advised for 28 days post-MMR/varicella administration. While unintended exposures require fetal risk counseling, termination is not indicated. Vaccines such as pneumococcal, meningococcal, and HPV require further safety validation, though meningococcal and hepatitis A vaccines may be administered under risk–benefit analysis.
Despite established safety profiles for pertussis and influenza vaccines, global coverage remains suboptimal (60, 61), attributed to sociopsychological barriers such as vaccine hesitancy, perceived low infection risk, and misinformation (62). Disparities in maternal immunization rates underscore the need for robust clinical evidence to address knowledge gaps and mitigate misconceptions. Multifaceted interventions targeting healthcare provider education, public health messaging, and equitable access are critical to optimizing prenatal vaccine uptake and safeguarding maternal–neonatal health outcomes.
Author contributions
JZ: Writing – original draft, Supervision. ZL: Writing – review & editing. SL: Investigation, Writing – review & editing. PL: Formal Analysis, Writing – original draft. JX: Supervision, Writing – review & editing. YY: Funding acquisition, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by: 1) Scientific Research and Development Centre for Higher Education, Ministry of Education (Grant No.: 2023HT070); 2) 2025 Annual High-Quality Development Research Project in Hospital Pharmacy, National Institute of Hospital Administration, NHC, China (Grant No.: NIHAYSZX2520).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van der Maas NAT, Hoes J, Sanders EAM, and de Melker HE. Severe underestimation of pertussis related hospitalizations and deaths in the Netherlands: A capture-recapture analysis. Vaccine. (2017) 35:4162–6. doi: 10.1016/j.vaccine.2017.06.037
2. Skoff TH, Deng L, Bozio CH, and Hariri S. US infant pertussis incidence trends before and after implementation of the maternal tetanus, diphtheria, and pertussis vaccine. JAMA Pediatr. (2023) 177:395–400. doi: 10.1001/jamapediatrics.2022.5689
3. Greenberg V, Vazquez-Benitez G, Kharbanda EO, Daley MF, Tseng HF, Klein NP, et al. Tdap vaccination during pregnancy and risk of chorioamnionitis and related infant outcomes. Vaccine. (2023) 41:3429–35. doi: 10.1016/j.vaccine.2023.04.043
4. Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, and Tolosa JE. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database systematic Rev. (2015) 1:CD004903. doi: 10.1002/14651858.CD004903.pub4
5. Clark DR, Omer SB, Tapia MD, Nunes MC, Cutland LC, Tielsch JM, et al. Influenza or meningococcal immunization during pregnancy and mortality in women and infants: A pooled analysis of randomized controlled trials. Pediatr Infect Dis J. (2020) 39:641–4. doi: 10.1097/INF.0000000000002629
6. Myers TR, McNeil MM, Ng CS, Li R, Lewis PW, and Cano MV. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010-2015. Vaccine. (2017) 35:1758–63. doi: 10.1016/j.vaccine.2017.02.030
7. Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion, number 797. Obstet Gynecol. (2020) 135:e51–72. doi: 10.1097/AOG.0000000000003668
8. Cho C-Y, Tang Y-H, Chen Y-H, Wang S-Y, Yang Y-H, Wang T-H, et al. Group B Streptococcal infection in neonates and colonization in pregnant women: An epidemiological retrospective analysis. J Microbiol Immunol Infect. (2019) 52:265–72. doi: 10.1016/j.jmii.2017.08.004
9. Saukkoriipi A, Silmon de Monerri NC, Toropainen M, Lindholm L, Veijola R, Toppari J, et al. Association between anti-capsular IgG levels at birth and risk of invasive group B streptococcus disease in Finnish newborns: a retrospective case-control study. Lancet Microbe. (2024) 5:689–96. doi: 10.1016/S2666-5247(24)00038-7
10. Madhi SA, Anderson AS, Absalon J, Radley D, Simon R, Jongihlati B, et al. Potential for maternally administered vaccine for infant group B streptococcus. N Engl J Med. (2023) 389:215–27. doi: 10.1056/NEJMoa2116045
11. Otieno NA, Nyawanda BO, McMorrow M, Oneko M, Omollo D, Lidechi S, et al. The burden of influenza among Kenyan pregnant and postpartum women and their infants, 2015-2020. Influenza Other Respir Viruses. (2022) 16:452–61. doi: 10.1111/irv.12950
12. Mertz D, Lo CKF, Lytvyn L, Ortiz JR, and Loeb M. FLURISK-INVESTIGATORS. Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis. (2019) 19:683. doi: 10.1186/s12879-019-4318-3
13. Dawood FS, Kittikraisak W, Patel A, Hunt DR, Suntarattiwong P, Wesley MG, et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis. (2021) 21:97–106. doi: 10.1016/S1473-3099(20)30592-2
14. Thompson MG, Kwong JC, Regan AK, Katz MA, Drews SJ, Azziz-Baumgartner EA, et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: A multi-country retrospective test negative design study, 2010-2016. Clin Infect Dis. (2019) 68:1444–53. doi: 10.1093/cid/ciy737
15. Munoz FM, Patel SM, Jackson LA, Swamy GK, Edwards KM, Frey SE, et al. Safety and immunogenicity of three seasonal inactivated influenza vaccines among pregnant women and antibody persistence in their infants. Vaccine. (2020) 38:5355–63. doi: 10.1016/j.vaccine.2020.05.059
16. Maltezou HC, Stavros S, Asimakopoulos G, Pergialiotis V, Raftopoulos V, Talias MA, et al. Effectiveness of maternal vaccination with quadrivalent inactivated influenza vaccine in pregnant women and their infants in 2019-2020. Expert Rev Vaccines. (2021) 21:983–92. doi: 10.1080/14760584.2022.2013820
17. Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. (2017) 17:981–9. doi: 10.1016/S1473-3099(17)30252-9
18. Olsen SJ, Mirza SA, Vonglokham P, Khanthamaly V, Chitry B, Pholsena V, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in lao PDR, 2014-2015. Clin Infect Dis. (2016) 63:487–94. doi: 10.1093/cid/ciw290
20. Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. (2022) 28:504–12. doi: 10.1038/s41591-021-01666-2
21. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, and Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J obstetrics gynecology. (2020) 222:415–26. doi: 10.1016/j.ajog.2020.02.017
22. Kugelman N, Nahshon C, Shaked-Mishan P, Cohen N, Sher ML, Gruber M, et al. Maternal and neonatal SARS-coV-2 immunoglobulin G antibody levels at delivery after receipt of the BNT162b2 messenger RNA COVID-19 vaccine during the second trimester of pregnancy. JAMA Pediatr. (2021) 176:290–5. doi: 10.1001/jamapediatrics.2021.5683
23. Simeone RM, Zambrano LD, Halasa NB, Fleming-Dutra KE, Newhams MM, Wu MJ, et al. Effectiveness of maternal mRNA COVID-19 vaccination during pregnancy against COVID-19-associated hospitalizations in infants aged <6 months during SARS-coV-2 omicron predominance - 20 states, march 9, 2022-may 31, 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:1057–64. doi: 10.15585/mmwr.mm7239a3
24. Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, et al. Safety monitoring of the janssen (Johnson & Johnson) COVID-19 vaccine - United States, march-april 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:680–4. doi: 10.15585/mmwr.mm7018e2
25. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose ad26.COV2.S vaccine against covid-19. N Engl J Med. (2021) 384:2187–201. doi: 10.1056/NEJMoa2101544
26. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-coV2373 covid-19 vaccine. N Engl J Med. (2021) 385:1172–83. doi: 10.1056/NEJMoa2107659
27. Clothier HJ, Parker C, Mallard JH, Effler P, Bloomfield L, Carcione D, et al. Nuvaxovid NVX-CoV2373 vaccine safety profile: real-world data evidence after 100,000 doses, Australia, 2022 to 2023. Euro Surveill. (2024) 29. doi: 10.2807/1560-7917.ES.2024.29.50.2400164
28. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
29. Wang C, Chen LY, Lu QB, and Cui F. Vaccination with the inactivated vaccine (Sinopharm BBIBP-corV) ensures protection against SARS-coV-2 related disease. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10060920
30. Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: A case-control study. Ann Intern Med. (2022) 175:362–70. doi: 10.7326/M21-3700
31. Jara A, Undurraga EA, Zubizarreta JR, González C, Acevedo J, Pizarro A, et al. Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. (2022) 28:1377–80. doi: 10.1038/s41591-022-01874-4
32. Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. (2022) 22:64–72. doi: 10.1016/S1473-3099(21)00451-5
33. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. (2021) 225:303.e1–.e17. doi: 10.1016/j.ajog.2021.03.023
34. Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Boom JA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, july 2021-january 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:264–70. doi: 10.15585/mmwr.mm7107e3
35. Harris E. COVID-19 vaccination during pregnancy protected infants. JAMA. (2023) 329:789. doi: 10.1001/jama.2023.2098
36. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-coV-2 infection in pregnant women. Jama. (2021) 326:728–35. doi: 10.1001/jama.2021.11035
37. Juliá-Burchés C. Martínez-VAREA A. An update on COVID-19 vaccination and pregnancy. J Pers Med. (2023) 13. doi: 10.3390/jpm13050797
38. Ha L, Levian C, Greene N, Goldfarb I, Hirsch A, and Naqvi M. Association between acceptance of routine pregnancy vaccinations and COVID-19 vaccine uptake in pregnant patients. J Infect. (2023) 87:551–5. doi: 10.1016/j.jinf.2023.10.010
39. Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. (2022) 327:1478–87. doi: 10.1001/jama.2022.4255
40. Kadali RAK, Janagama R, Peruru SR, Racherla S, Tirumala R, Madathala RR, et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am J Obstet Gynecol. (2021) 225:458–60. doi: 10.1016/j.ajog.2021.06.007
41. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med. (2021) 384:2273–82. doi: 10.1056/NEJMoa2104983
42. Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. (2021) 58:450–6. doi: 10.1002/uog.23729
43. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, and Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. (2021) 3:100467. doi: 10.1016/j.ajogmf.2021.100467
44. Donders GGG, Grinceviciene S, Haldre K, Lonnee-Hoffmann R, Donders F, Tsiakalos A, et al. ISIDOG Consensus Guidelines on COVID-19 Vaccination for Women before, during and after Pregnancy. J Clin Med. (2021) 10. doi: 10.3390/jcm10132902
45. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
46. Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. (2023) 388:1451–64. doi: 10.1056/NEJMoa2216480
47. Proaño A and Flannery DD. EBNEO Commentary: Respiratory syncytial virus vaccine in pregnancy for infant protection. Acta Paediatr. (2023) 112:2449–50. doi: 10.1111/apa.16950
48. Son M, Riley LE, Staniczenko AP, Cron J, Yen S, Thomas C, et al. Nonadjuvanted bivalent respiratory syncytial virus vaccination and perinatal outcomes. JAMA Netw Open. (2024) 7:e2419268. doi: 10.1001/jamanetworkopen.2024.19268
49. Liu J, Xu B, Chen T, Chen J, Feng J, Xu C, et al. Presence of hepatitis B virus markers in umbilical cord blood: Exposure to or infection with the virus? Digestive liver disease: Off J Ital Soc Gastroenterol Ital Assoc Study Liver. (2019) 51:864–9. doi: 10.1016/j.dld.2018.11.003
50. Seto MT, Cheung KW, and Hung IFN. Management of viral hepatitis A, C, D and E in pregnancy. Best Pract Res Clin Obstet Gynaecol. (2020) 68:44–53. doi: 10.1016/j.bpobgyn.2020.03.009
51. Celzo F, Buyse H, Welby S, and Ibrahimi A. Safety evaluation of adverse events following vaccination with Havrix, Engerix-B or Twinrix during pregnancy. Vaccine. (2020) 38:6215–23. doi: 10.1016/j.vaccine.2020.07.041
52. de Deus N, Chissaque A, Bauhofer A, Barata A, Jani IV, Lopez Cavestany R, et al. Safety of incidental exposure to the novel oral poliovirus vaccine type 2 in pregnancy: A longitudinal observational study in Mozambique, 2022-2023. Vaccine. (2024) 42:1326–31. doi: 10.1016/j.vaccine.2024.01.071
53. Condrat CE, Filip L, Gherghe M, Cretoiu D, and Suciu N. Maternal HPV infection: effects on pregnancy outcome. Viruses. (2021) 13. doi: 10.3390/v13122455
54. López-Fauqued M, Zima J, Maria-Genalin A, and Stegmann J-U. Results on exposure during pregnancy from a pregnancy registry for AS04-HPV-16/18 vaccine. Vaccine. (2017) 35:5325–30. doi: 10.1016/j.vaccine.2017.08.042
55. Tan J, Xiong Y-Q, He Q, Liu Y-M, Wang W, Chen M, et al. Peri-conceptional or pregnancy exposure of HPV vaccination and the risk of spontaneous abortion: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2019) 19:302. doi: 10.1186/s12884-019-2425-1
56. Wang A, Liu C, Wang Y, Yin A, Wu J, Zhang C, et al. Pregnancy outcomes after human papillomavirus vaccination in periconceptional period or during pregnancy: A systematic review and meta-analysis. Hum Vaccin Immunother. (2020) 16:581–9. doi: 10.1080/21645515.2019.1662363
57. Bukowinski AT, Hall C, Chang RN, Gumbs GR, and Conlin AMS. Maternal and infant outcomes following exposure to quadrivalent human papillomavirus vaccine during pregnancy. Vaccine. (2020) 38:5933–9. doi: 10.1016/j.vaccine.2020.06.073
58. Meng R, Ma R, Wang J, Liu P, Liu Z, He B, et al. Post-marketing surveillance for the safety of the 9-valent human papillomavirus vaccine: a retrospective real-world study in China. Expert Rev Vaccines. (2023) 22:696–703. doi: 10.1080/14760584.2023.2239911
59. ACOG committee opinion no. 741 summary: maternal immunization. Obstet Gynecol. (2018) 131:1188–91. doi: 10.1097/AOG.0000000000002665
60. Kristinsdottir I, Haraldsson A, and Thors V. Influenza vaccination in pregnant women in Iceland 2010-2020 and the burden of influenza in pregnant women and their infants. Vaccine. (2024) 42:2051–8. doi: 10.1016/j.vaccine.2024.02.046
61. Razzaghi H, Kahn KE, Calhoun K, Garacci E, Skoff TH, Ellington SR, et al. Influenza, tdap, and COVID-19 vaccination coverage and hesitancy among pregnant women - United States, april 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:1065–71. doi: 10.15585/mmwr.mm7239a4
Keywords: pregnancy, vaccination, maternal immunization, vaccine safety, vaccine efficacy
Citation: Zhang J, Li Z, Li S, Li P, Xie J and Yang Y (2025) Research progress in vaccination during pregnancy. Front. Immunol. 16:1687362. doi: 10.3389/fimmu.2025.1687362
Received: 17 August 2025; Accepted: 13 October 2025;
Published: 29 October 2025.
Edited by:
Daniela Hozbor, Institute of Biotechnology and Molecular Biology (IBBM), ArgentinaReviewed by:
Hare Krishna, All India Institute of Medical Sciences Jodhpur, IndiaGregory Todd Pharr, Mississippi State University, United States
Copyright © 2025 Zhang, Li, Li, Li, Xie and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Yang, eXl4cG93ZXJAMTI2LmNvbQ==
 Jiao Zhang
Jiao Zhang Zhimin Li
Zhimin Li Shiran Li
Shiran Li Pengfei Li
Pengfei Li Jingxian Xie
Jingxian Xie Yong Yang
Yong Yang