- 1School of Medical and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Respiratory Medicine, Jianyang People’s Hospital, Jianyang, China
- 3School of Modern Chinese Medicine Industry/School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
COPD complicated by pulmonary aspergillosis (COPD-PA) encompasses invasive, chronic, and allergic phenotypes and is increasingly recognized as a high-burden comorbidity. Clinical recognition is often hindered by nonspecific manifestations, corticosteroid-suppressed inflammatory signs, and the suboptimal performance of serum galactomannan in non-neutropenic hosts. To define the translational utility of immune biomarkers in this population, evidence was synthesized from international guidelines and contemporary studies in COPD-enriched cohorts, and performance was appraised across diagnostic, monitoring, and prognostic domains. Convergent findings indicate that bronchoalveolar-lavage galactomannan facilitates early diagnosis; serum galactomannan indices stratify risk during exacerbations; Aspergillus-specific IgG supports rule-in for invasive and chronic disease; and pentraxin-3 adds prognostic information. Cytokines central to COPD-PA pathobiology, including interleukin-1β, interleukin-6, interleukin-8, and interleukin-17, provide adjunctive diagnostic value, whereas (1→3)-β-D-glucan shows limited specificity. On this basis, a three-tier framework was developed that classifies biomarkers as clinically validated, mechanistically promising, or exploratory, and this framework was translated into subtype-tailored panels and decision rules that favor either-positive criteria for screening and both-positive criteria for confirmation. It is concluded that immune biomarkers complement microbiology and imaging, expand access when bronchoscopy or biopsy is not feasible, and enable longitudinal risk stratification. Priorities include COPD-specific thresholds, assay standardization, and multicenter validation, with particular emphasis on chronic pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Biomarker-guided immunomodulation may benefit selected phenotypes but requires rigorous evaluation before clinical adoption.
1 Introduction
Chronic obstructive pulmonary disease with pulmonary aspergillosis (COPD–PA) refers to COPD complicated by infections across the Aspergillus spectrum—primarily invasive pulmonary aspergillosis (IPA) (1), chronic pulmonary aspergillosis (CPA) (2), and allergic bronchopulmonary aspergillosis (ABPA) (3). The clinical burden of COPD–PA is considerable. A 2020 global systematic review estimated that 1.3–3.9% of hospitalized COPD patients develop invasive aspergillosis annually, based on studies conducted across multiple regions from 2000 to 2019 (4). Numerous studies have highlighted that COPD–PA significantly exacerbates outcomes by accelerating the decline in lung function, diminishing quality of life, and increasing mortality (5–7). Diagnosing COPD–PA in clinical practice presents several challenges: COPD symptoms often obscure aspergillosis, and the clinical manifestations of aspergillosis are nonspecific (8–11); corticosteroid exposure can suppress fever and inflammatory responses (12); the sensitivity of galactomannan (GM) is lower in non-neutropenic COPD patients than in classic immunocompromised individuals (13).
In recent years, immune markers have attracted growing interest in COPD–PA, including GM, Aspergillus-specific IgG (Asp IgG), C-reactive protein (CRP), and pentraxin-3 (PTX3) (14–17). The immunopathogenesis of COPD–PA is complex; nevertheless, the pursuit of immune markers remains feasible. COPD-related epithelial injury (18), impaired mucociliary clearance (19), neutrophil/macrophage dysfunction (20, 21), and skewed T-cell responses create a permissive environment for Aspergillus persistence and overgrowth, thereby providing a biological rationale for early diagnosis, prognostic assessment, and therapeutic stratification (22).
Although interest in COPD–PA has been growing, dedicated investigations remain limited, with most studies treating COPD and PA as separate entities. To the best of our knowledge, no review has yet consolidated immune markers specific to COPD–PA across IPA, CPA, and ABPA. In this review, we analyze immune dysregulation in COPD–PA, synthesize the current evidence, and propose an evidence-tiered marker framework specifically tailored to COPD–PA, aimed at guiding research priorities and facilitating its translational applications.
2 Immune dysregulation in COPD–PA
Chronic inflammation in COPD damages airway and lung structures, thereby impairing the respiratory tract’s natural defenses and promoting Aspergillus colonization and invasion (23). Moreover, treatments commonly used for COPD, such as inhaled corticosteroids (ICS) and broad-spectrum antibiotics, can exacerbate immune dysregulation and impair the clearance of pulmonary Aspergillus infections (24). Following infection, fungal virulence factors directly damage airway epithelial cells (25). Concurrently, excessive secretion of pro-inflammatory cytokines and chemokines by immune cells further exacerbates immune dysregulation, ultimately worsening COPD. Importantly, this entire pathogenic cycle is inextricably associated with immune dysregulation.
2.1 Adhesion as the initial step in infection: epithelial cells as the first line of defense against Aspergillus
Aspergillus species are ubiquitous in the environment, and most Aspergillus conidia are likely cleared from the airway by the ciliary action of the airway epithelium, making the inhalation of conidia rarely cause disease in immunocompetent hosts (19, 26). In addition to ciliary action, specialized epithelial secretory cells prevent spore colonization by secreting mucus (27). Human bronchial epithelial cells, when exposed to Aspergillus fumigatus (A. fumigatus) conidia in vitro, inhibit conidial germination through FleA recognition, thereby reducing fungal invasiveness (28). However, in COPD patients, factors such as cigarette smoke and recurrent infections compromise epithelial function, impair conidial clearance, and increase susceptibility to Aspergillus infection (18). For instance, inhalation of cigarette smoke, the primary risk factor for COPD (29), induces epithelial injury and triggers the release of damage-associated molecular patterns (DAMPs) (30).
Adherence to the epithelium is the first step in infection development, mediated by multiple receptor-ligand interactions between Aspergillus and the epithelium, including pattern-recognition receptors (PRRs) recognizing GM and (1→3)-β-D-glucan (BDG), among others. Epithelial PRRs—including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and NOD-like receptors (NLRs)—can activate innate inflammatory cascades. These receptors detect DAMPs that initiate sterile inflammation, as well as persistent fungal pathogen-associated molecular patterns (PAMPs), especially GM and BDG, that sustain chronic inflammation (31, 32). The relationship between COPD and PA is bidirectional, persistent Aspergillus drives excessive immune responses that exacerbate airway inflammation and tissue damage in COPD (33). A. fumigatus-derived extracellular polysaccharides and gliotoxins directly disrupt epithelial integrity (34), impairing airway repair in COPD.
TLRs, including TLR2 and TLR4, are critical components of the innate immunity in COPD–PA. A study has shown that through airway brushings, the mRNA expression of TLR2 and TLR4 in the airway epithelial cells of patients with mild-to-moderate COPD was increased by approximately 2.4-fold and 8.7-fold, and their expression is also increased in severe COPD (35). This increase is likely associated with the chronic inflammatory processes underlying COPD. In lung parenchyma biopsy samples, mRNA expression of TLR2 and TLR4 was elevated in mild-to-moderate COPD, but reduced in severe cases, possibly related to bacterial infection and to protective responses that limit oxidative stress in lung tissue (35, 36). Ou et al. demonstrated that cigarette smoke activates the TLR4–MyD88–NF-κB signaling cascade (37), and cigarette smoke is the primary risk factor for COPD (29). In COPD, TLR expression is upregulated in most settings, thereby amplifying airway inflammation. TLR2 and TLR4 mediate multiple immune responses during Aspergillus infection (38, 39), with TLR2 inducing Dectin-1 expression and recognizing BDG to activate the SYK/CARD9–NF-κB and RAF-1 pathways, releasing pro-inflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8), as well as C-X-C motif chemokine ligand 1 (CXCL1) and C-X-C motif chemokine ligand 2 (CXCL2), and promoting neutrophil recruitment (40). In vitro stimulation of lung epithelial cells with A. fumigatus conidia demonstrated that loss of function of TLR2 or Dectin-1 results in reduced production of inflammatory cytokines (41).
TLR/MyD88 activation not only activates NF-κB but also activates the JNK pathway in the MAPK signaling cascade, leading to the activation of the AP-1 transcription factor (42, 43). These downstream transcription factors regulate inflammation, immune cell recruitment, and pathogen clearance (44, 45). JNK pathway activation correlates with epithelial damage and airway inflammation in COPD (46, 47). Cui et al. found that stimulation of human bronchial epithelial BEAS-2B cells with A. fumigatus conidia activates the MAPK-JNK pathway, leading to the upregulation of IL-27 and tumor necrosis factor-alpha (TNF-α); The JNK inhibitor SP600125 significantly attenuated this cytokine production (48). These findings indicate that TLRs, Dectin-1, and JNK are likely involved in the pathophysiological processes of COPD–PA. Their downstream products promote infection defense but may also aggravate airway inflammation in COPD.
2.2 The phagocytic system as the second line of host defense
Failure of mucociliary clearance enables Aspergillus conidia to adhere to and potentially colonize the epithelial surface (49). This event activates downstream immune effectors through multiple signaling pathways (outlined earlier), thereby initiating the host’s second line of defense. The second line of defense includes alveolar macrophages (AMs), which are crucial in destroying the conidia of Aspergillus, as well as neutrophils that kill fungal hyphae and germinating spores (50).
Macrophages perform multiple interrelated functions crucial to pulmonary host defense, including phagocytosis of inhaled particles and pathogens (e.g., cigarette smoke particulates and Aspergillus conidia), immune surveillance via PRRs, antigen processing and presentation to prime adaptive responses, and orchestration of inflammatory signaling (51). Excessive oxidative stress (e.g., glutathione-mediated) impairs phagocytosis, permitting cytotoxic release from necrotic cells, a dysregulation that exacerbates COPD inflammation (52). Consistently, elevated oxidative stress in COPD is associated with diminished bacterial phagocytosis, linked to mitochondrial dysfunction and the accumulation of intracellular oxidants (53). COPD macrophages significantly increase the expression of TNF-α, IL-1β, and IL-6 upon exposure to A. fumigatus conidia, contributing to COPD exacerbation during PA comorbidity (33). In addition, compromised immune surveillance increases susceptibility to fungal infections that trigger acute exacerbations (54). Macrophages derived from smokers and individuals with COPD exhibit impaired recognition and clearance of A. fumigatus, potentially due to altered receptor expression and blunted pathogen-responsive signaling, thereby increasing the risk of COPD–PA (55, 56). Collectively, macrophage dysfunction provides a mechanistic link between COPD pathobiology and susceptibility to PA.
AMs recognize fungal PAMPs primarily via CLRs and cooperate with humoral opsonins such as PTX3 and surfactant proteins (SP), thereby activating pro-inflammatory signaling, promoting phagocytosis, and favoring M1-skewed responses (51, 57). The diagnostic and prognostic value of PTX3 in patients with non-neutropenic IPA has garnered increasing attention (14). PTX3 is a soluble humoral pattern-recognition molecule that binds multiple ligands and thereby contributes to immune defense, inflammation, and diverse biological processes (58). Compared with CRP, PTX3 more faithfully reflects local inflammatory activity, and its covalently stabilized multimeric architecture is conducive to assay consistency and interpretability (59).Inflammatory dysregulation related to COPD promotes M2 anti-inflammatory polarization (60), reducing TNF-α, IL-1β, IL-6, and IL-8 secretion and impairing antifungal defense.
Neutrophils are key effector cells in COPD pathogenesis and anti-Aspergillus defense. Neutrophil accumulation directly depends on IL-6, IL-8, and CXC chemokine recruitment (61, 62). As noted, epithelial SYK/CARD9–NF-κB signaling drives neutrophil recruitment during Aspergillus pneumonia through inflammatory factor release, including IL-6 and IL-8 (38, 63). Furthermore, the neutrophil interleukin-1 receptor (IL-1R) regulates cell-autonomous survival during inflammation by modulating apoptosis and pro-survival pathways (64). Neutrophils execute intracellular and extracellular antifungal functions. Intracellular killing involves NADPH oxidase (NOX)-generated reactive oxygen species (ROS) (65). Extracellular killing is mediated by neutrophil extracellular traps (NETs) and antibody-dependent cellular cytotoxicity (ADCC) (65). ADCC requires antibody binding to Fc receptors (FcRs) on neutrophils, but the evidence regarding the mechanism of ADCC in the airways of COPD–PA patients remains limited, with research primarily focusing on NETs.
Aspergillus induces the formation of NETs, which entangle conidia and limit fungal dissemination (66). In COPD, neutrophil dysfunction can generate aberrant NETs that accumulate in the airway and form fibrin-rich networks, thereby amplifying chronic inflammation and tissue injury (67). Moreover, excessive release of neutrophil elastase in COPD degrades immunoglobulins and complement components, undermining opsonization and the clearance of A. fumigatus; this constitutes a mechanism of immune evasion by A. fumigatus (68).
Figure 1 depicts the initial host–pathogen interface, illustrating how innate immune cells convert fungal recognition into inflammatory and antifungal responses and thereby couple early containment to downstream adaptive immunity.
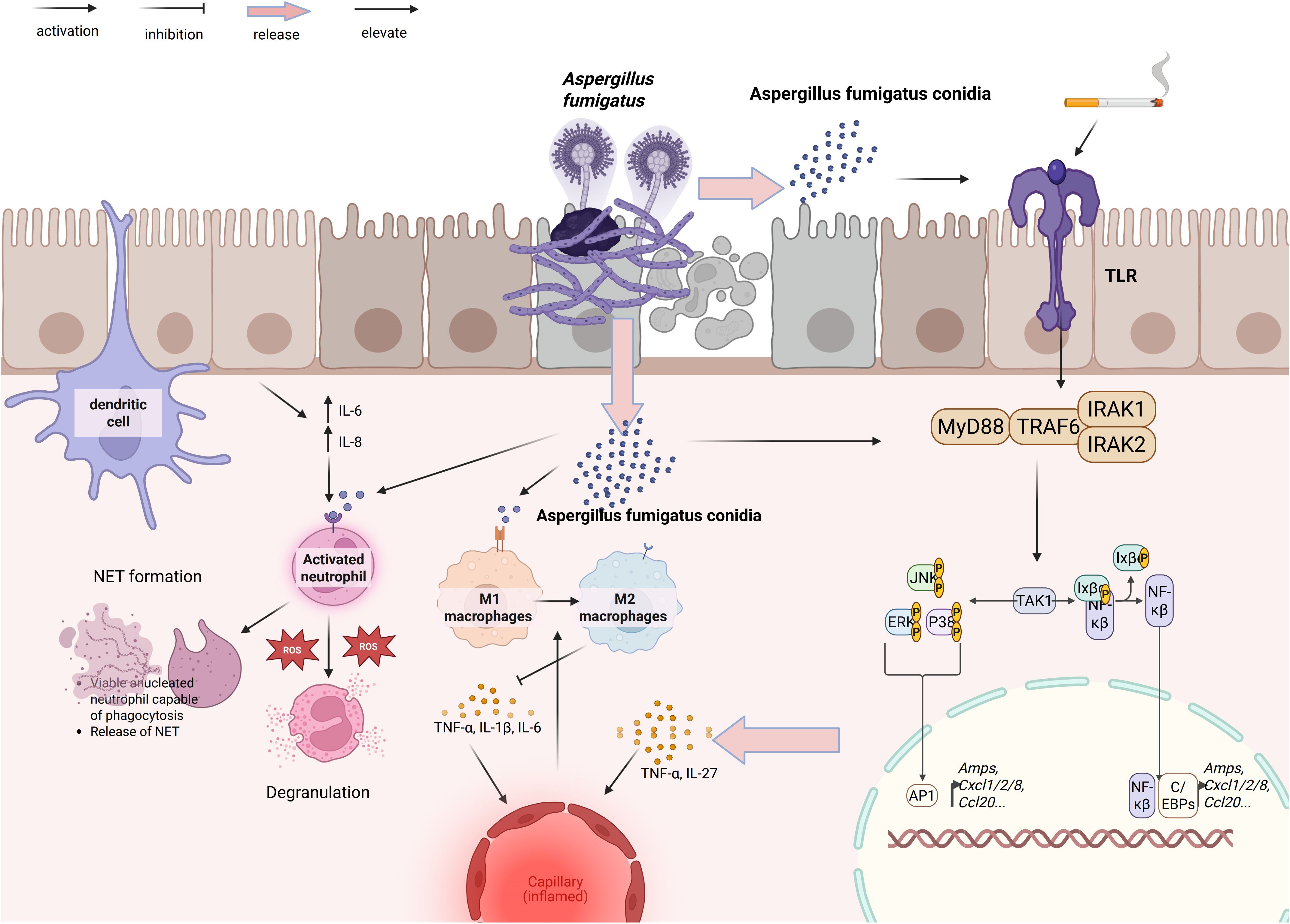
Figure 1. Epithelial–myeloid responses to A. fumigatus in COPD. Conidia activate epithelial TLR–MyD88–IRAK1/2–TRAF6–TAK1, triggering MAPK modules JNK/ERK/p38 and NF-κB/AP-1/C/EBPs to induce AMPs and chemokines CXCL1/2/8 and CCL20. DCs sample fungal material; recruited neutrophils degranulate, generate ROS, and form NETs. Macrophages polarize to M1 (TNF-α, IL-1β, IL-6) or M2 (TNF-α, IL-27). These circuits promote fungal control yet amplify airway and microvascular inflammation in COPD–PA. Arrowheads denote activation/flow; blunt bars denote inhibition; pale arrows indicate release/elevation.
2.3 Adaptive immunity: the final line of host defense
Dendritic cells (DCs) bridge innate and adaptive immunity and constitute a central arm of antifungal defense against A. fumigatus (65). DCs translate innate pathogen recognition into programmed T-cell responses (69). Adaptive immunity is centered on T cells: T cells comprise CD8+ cytotoxic T lymphocytes (CTLs) that mediate targeted cytolysis, whereas CD4+ T helper cells differentiate into T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17), and regulatory T (Treg) lineages. Through their cytokine networks, these subsets condition the airway microenvironment, calibrating the balance between pathogen clearance and immunopathology. This immunologic set-point, in turn, influences fungal burden, the risk of acute exacerbations, and the progression of structural lung injury.
CTLs, a CD8+ T-cell subset that recognizes and eliminates infected or transformed cells via apoptosis, are central to antiviral and antitumor immunity (70). Upon priming by dendritic cells via antigen presentation on MHC class I with costimulatory signals, and by Th1-derived cytokines such as interferon-γ (IFN-γ), naïve CD8+ T cells become activated and undergo clonal expansion (71). Activated CTLs induce target cell death predominantly through perforin–granzyme–dependent cytolysis (72).
DCs also orchestrate T-helper differentiation. Depending on the cytokine milieu, DCs activate naive CD4+ T cells and drive their differentiation into distinct effector lineages (e.g., Th1, Th2, Th17) or Treg (73). The cytokine milieu is partially determined by morphological forms of A. fumigatus and its cell-wall components recognized by DCs (74). CD4+ T lymphocytes amplify adaptive immunity by providing B-cell help and by licensing and enhancing CD8+ T-cell responses, whereas Treg restrain these processes, thereby modulating the magnitude and quality of inflammation (75).
Th1 and Th2 cells represent two principal subsets of CD4+ T cells: Th1 cells principally secrete IFN-γ and IL-2, coordinating cellular immunity, whereas Th2 cells produce IL-4, IL-5, and IL-13, thereby modulating humoral and type-2 inflammatory responses (76). Dysregulated CD4+ T-cell differentiation in COPD drives immune imbalance, with elevated Th1/Th2 ratios promoting persistent inflammation, airway remodeling, and emphysema (77). DCs stimulated by conidia of Aspergillus produce IL-12, which drives Th1 differentiation and induces IFN-γ production (78). Allergic manifestations induced by Aspergillus, such as ABPA, predominantly trigger Th2 responses, characterized by increased production of IL-4, IL-5, and IL-13 but reduced IFN-γ (79). IL-5 promotes eosinophil differentiation and chemotaxis, while IL-4 and IL-13 are involved in IgE production (79).
Accordingly, the Th1/Th2 axis serves as a central immunologic rheostat. Th1 responses driven by IFN-γ are associated with favorable anti-Aspergillus outcomes. However, when the Th1/Th2 balance shifts upward and IFN-γ signaling remains persistently elevated—particularly during acute exacerbations or in the presence of concurrent infection—COPD airway inflammation and tissue injury can worsen (80). Therefore, any use of exogenous or induced IFN-γ should be explored only as a context-specific adjunct to immunomodulatory therapy and evaluated cautiously in rigorously designed clinical trials (45). By contrast, Th2 polarization driven by IL-4, IL-5, and IL-13 is closely linked to ABPA, elevated IgE, eosinophilic airway inflammation, and mucus plugging, which exacerbate airflow obstruction and worsen prognosis. Across COPD cohorts, sensitization to A. fumigatus correlates with poorer lung function and more frequent exacerbations (prevalence≈13%; FEV1% predicted: 39% vs 51%, p=0.01), suggesting that heightened Th2 reactivity in COPD undermines effective antifungal immunity and amplifies allergic inflammation (81). The 2024 ERS/ISHAM guidelines identify COPD as a predisposing condition for ABPA (3).
Beyond the Th1/Th2 axis, the Th17/Treg axis constitutes a key arm of the immune processes underlying COPD–PA. Th17 cell differentiation depends critically on IL-6 and IL-23 (82). In fungal infection, CD4+ T cells binding DCs via Dectin-1 activate the Syk/CARD9 pathway, inducing IL-1β, IL-6, and IL-23 production that promotes Th17 differentiation and activation (83). Th17 cells secrete IL-17A and IL-17F, which enhance neutrophil recruitment and promote fungal clearance. While Th17 cells mediate crucial fungal clearance, dysregulated activation causes lung injury and chronic inflammation (84). COPD-derived pulmonary T cells demonstrate Th17 polarization (85); these cells exert pro-inflammatory effects via IL-17A, IL-17F, and IL-22 release (86).
In COPD–PA, whether Th17 cells exacerbate or mitigate disease progression remains debated. An analysis of a U.S. national inpatient database showed that, among admissions for acute exacerbations of COPD (AECOPD), in-hospital mortality was 14.5% in patients with IPA versus 3.6% in those without (5). Expert consensus recommends voriconazole as the preferred first-line agent for the initial treatment of COPD–IPA (87). Overall, the evidence indicates that invasive fungal infection is the principal driver in COPD–IPA, and timely antifungal therapy is critical (8). Accordingly, during the acute phase, the antifungal activity of the Th17 response appears to predominate, whereas COPD-related chronic inflammation is likely secondary. In a COPD–IPA mouse model, Geng et al. reported that Th17 cells failed to mount an effective response to pulmonary Aspergillus infection in the COPD setting, likely due to COPD-associated T-cell exhaustion (87). Lycan et al. reported that, despite the chronically inflamed milieu of COPD, T cells exhibit exhaustion-like dysfunction with upregulated immune checkpoints such as PD-1. This observation helps explain heightened susceptibility and mortality and argues against indiscriminate inhibition of the IL-17 pathway. Current work is concentrated on COPD–IPA; evidence regarding Th17 in CPA and ABPA is limited and requires more rigorous study.
Th17 cells and their signature cytokine IL-17 function as a double-edged sword. Their regulation is complex and is shaped by fungal immune evasion and activation strategies, as well as cigarette smoke exposure (74, 88, 89). Cell-wall components of A. fumigatus differentially regulate TLR2- and TLR4-dependent pathways; notably, α-glucan constrains IL-6 production via TLR2/TLR4 signaling, thereby dampening Th17 responses as an immune-evasion mechanism (74). A. fumigatus triggers Th1 and Th17 responses in mice via the TLR–MyD88 and Dectin-1 pathways, respectively; Dectin-1 recognition of BDG is a key driver of the Th17 response (88). Exposure to cigarette smoke extract increased IL-17 production by approximately 3–4-fold within hours (89).
Tregs exert anti-inflammatory effects through multiple pathways, notably the production of IL-10 and TGF-β, and CTLA-4–mediated, cell-contact–dependent inhibition of leukocyte function (90). Upon airway exposure to A. fumigatus, peripheral-blood A. fumigatus–specific Tregs undergo marked expansion, which is considered a mechanism that restrains Th2-dominant allergic responses (91). Tregs restrain excessive Th2 activity, thereby maintaining balance along the Th1/Th2 axis and sustaining Th1-mediated antifungal defense; they also curb dysregulated Th17 responses (92). This counter-regulatory architecture is advantageous in principle, preserving antifungal competence while limiting inflammation driven by Th2- or Th17-pathway hyperactivation. However, in COPD, Kalathil et al. reported that functionally suppressive Tregs and exhausted PD-1+ T cells contribute to effector T-cell dysfunction (93). Such T-cell dysfunction impairs fungal clearance, while inflammatory mediators such as IL-17 remain elevated (94), creating an immune milieu that favors the coexistence of persistent fungal burden and structural airway damage in COPD–PA.
B cell-mediated immune responses primarily generate antibodies crucial for pathogen neutralization and immunological memory (92). However, antigen-stimulated B lymphocytes promote pulmonary inflammation and damage through autoantibodies, cellular components, extracellular matrix proteins, and immune complex formation (92); they additionally enhance cellular immunity by functioning as antigen-presenting cells (APCs) (95). B lymphocytes producing IL-6 are classified as effector B cells (B-effs), while IL-10-producing cells are regulatory B cells (Bregs) that maintain immune homeostasis by preventing excessive inflammation and tissue damage (96). Peripheral blood lymphocytes in COPD show significantly reduced Breg counts and elevated B-eff/Breg ratios, causing adaptive immunity imbalance (97). DCs process A. fumigatus antigens and present them to naive T cells, activating Th1 cells and initiating targeted immune responses. Th1-derived cytokines—particularly IFN-γ—promote B-cell class switching to IgG production (98). The Th2-mediated IgE production mechanism was previously detailed. This provides the pathophysiological rationale for employing IgG and IgE as diagnostic markers.
Mucosal B cells produce secretory IgA (sIgA) that mediates immune exclusion at the airway surface. In COPD, epithelial barrier dysfunction together with focal sIgA deficiency facilitates adhesion and colonization of Aspergillus conidia. In parallel, reduced abundance or impaired function of regulatory Bregs diminishes regulatory control over Aspergillus-driven allergic inflammation. Asp IgG reflects sustained antigen exposure and is often markedly elevated in CPA (2, 99). Th2-driven class-switch recombination to IgE is the central mechanism of Aspergillus-related allergic disease, typified by ABPA. Allergen-specific IgE (and total IgE) is directly associated with eosinophilic inflammation, mucus impaction, and an increased risk of acute exacerbations (100). These pathophysiological links underpin the use of these antibodies as potential immunologic markers.
Figure 2 summarizes how DCs sensing of Aspergillus instructs CD4+ T-cell lineage commitment (Th1, Th2, Th17, and Treg). These subsets, in turn, activate macrophage, neutrophil, and eosinophil effector programs and drive B-cell class-switch recombination to IgG and IgE, whereas Treg-derived IL-10 and TGF-β restrain excessive inflammation.
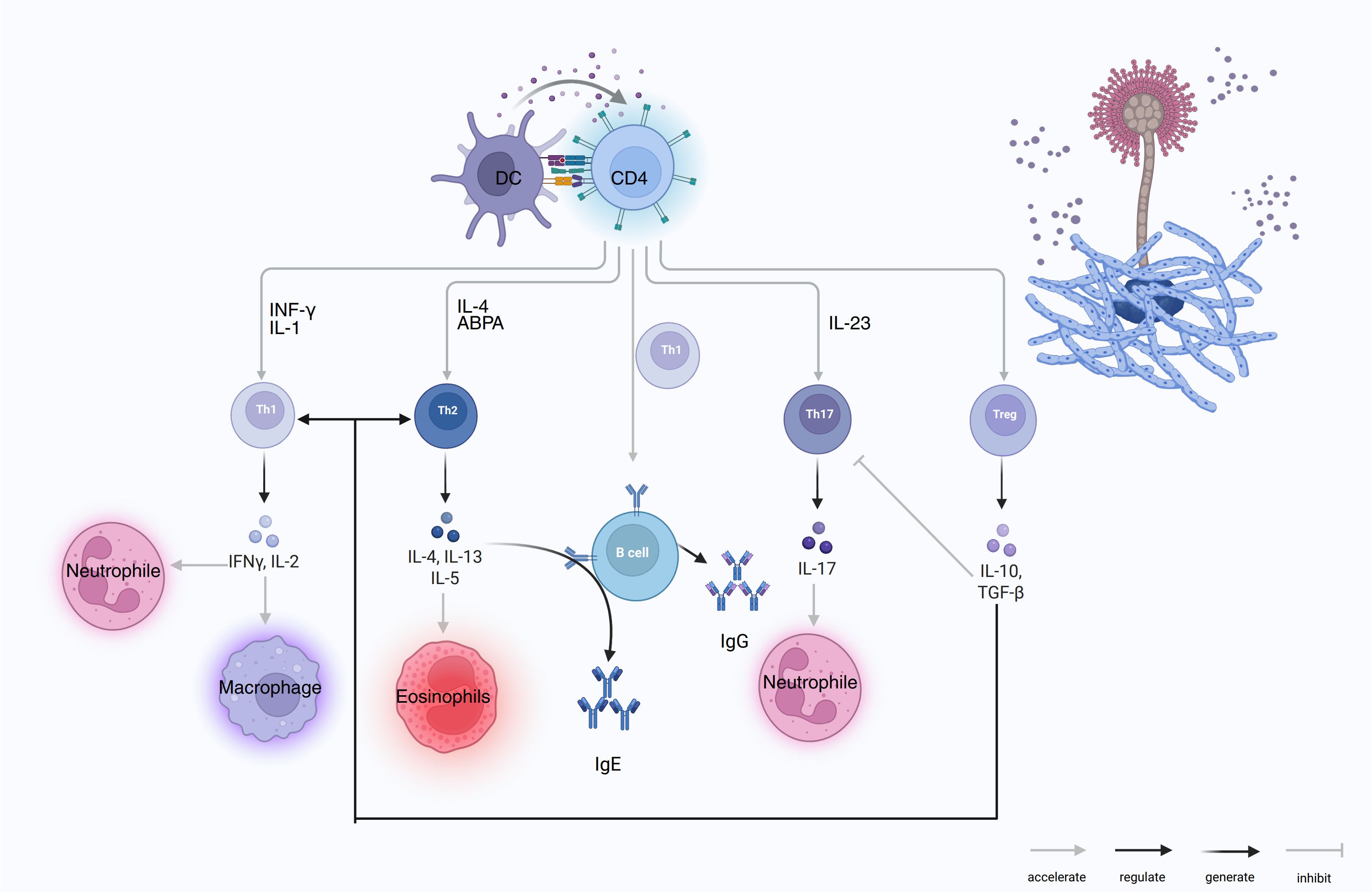
Figure 2. DC-CD4 polarization to Th1/Th2/Th17/Treg and effector outputs in COPD–PA. DCs present A. fumigatus antigens to CD4 T cells and drive lineage programs: Th1 (IFN-γ, IL-2) activates macrophages and supports neutrophils; Th2 (IL-4, IL-13, IL-5) induces eosinophilia and B-cell IgE class switching, the hallmark of ABPA; Th17 (IL-23→IL-17) recruits/activates neutrophils; Treg (IL-10, TGF-β) inhibits Th1/Th17 and restrains B-cell effector functions. With T-cell help, B cells generate IgG and IgE. Arrow keys: accelerate/regulate/generate/inhibit.
3 From disease trajectory and immune phenotypes to biomarker candidates
Building on the preceding overview of shared immune mechanisms in COPD–PA, it is clear that its clinical subtypes exhibit distinct immunologic profiles. In COPD–IPA, clinical presentations often mirror an acute exacerbation, with fever, cough, and dyspnea, thereby mimicking bacterial infection and complicating timely diagnosis (51). Among patients with AECOPD, concomitant IPA is associated with higher rates of cough, purulent sputum, hemoptysis, and fever compared with non-IPA cases (101). Early in the disease course, infection may remain confined to the tracheobronchial compartment, with vascular invasion emerging at later stages (102, 103). Accordingly, molecules engaged early in the airway immune response, such as GM, are best sampled from bronchoscopic specimens for early diagnosis, whereas serum measurements can assist in tracking disease progression. Consistent with this, bronchoalveolar lavage fluid (BALF) GM generally outperforms serum GM diagnostically (1, 104, 105), while serum GM may carry prognostic information (106). This perspective is necessarily incomplete: acute-phase reactants such as CRP may contribute to PA diagnosis, albeit with limited specificity (16). As the host response evolves, more specific antibodies and cytokines may accumulate and serve as adjunctive markers for diagnosis and longitudinal assessment.
COPD–CPA typically follows a chronic, progressive course, characterized by chronic cough, sputum production, fatigue, weight loss, and dyspnea; some patients remain asymptomatic for prolonged periods (51). According to ERS/ESCMID guidelines, CPA is categorized by chronicity, imaging, and mycologic evidence into simple aspergilloma, chronic cavitary CPA (CCPA), chronic fibrosing CPA (CFPA), Aspergillus nodule, and subacute invasive aspergillosis (SAIA) (2). Although CPA elicits immune responses, overall immunity, particularly cell-mediated immunity, is considered suboptimal (107), suggesting that cell-mediated effector products (e.g., IFN-γ) may be altered in CPA (2), with additional complexity introduced by underlying COPD. Sustained antigen exposure can drive persistent elevations of antibodies and cytokines beyond cell-mediated immunity, including IL-1, IL-6, and IL-8, which are amenable to serum-based investigation. ABPA features a type-2–skewed immune profile—elevated IL-4, IL-5, and IL-13, eosinophil activation, and high-titer allergen-specific IgE (79). These hallmarks naturally orient the search for COPD–ABPA immune markers.
Because COPD lacks pathogen-specific immune signatures, biomarker discovery from a COPD-only vantage is difficult (108, 109); however, mapping the distinct pathobiology and immune profiles of IPA, ABPA, and CPA reveals plausible biomarkers across these subtypes.
4 Immune markers
Based on extant evidence, we categorize immune biomarkers for COPD–PA into three tiers: (i) Experimentally Validated, (ii) Mechanistically Promising, and (iii) Requiring Further Investigation, reflecting current research maturity in this domain. Experimentally Validated biomarkers meet at least one of the following criteria: supported by international guidelines or expert consensus (such as ECCMID/ERS) and/or validated in COPD–PA–specific clinical cohorts. Mechanistically Promising biomarkers exhibit compelling evidence of involvement in COPD and aspergillosis pathways, demonstrate potential as standalone diagnostic indicators, but lack validation in dedicated COPD–PA cohorts. Requiring Further Investigation encompasses biomarkers with contradictory roles in COPD versus aspergillosis pathogenesis, necessitating mechanistic clarification prior to clinical application.
4.1 Experimentally validated
4.1.1 GM and BDG
GM and BDG are key fungal cell wall components involved in multiple immune processes. Both are included as diagnostic markers in aspergillosis clinical practice guidelines (104, 110). Serum and BALF GM plus serum BDG serve as IPA diagnostic indicators, with BALF GM showing superior reliability (104). A four-year multicenter study demonstrated 88.9% sensitivity for BALF GM (ODI ≥0.5) in COPD-associated IPA diagnosis versus 11.1% for serum GM (ODI ≥0.5) (111). An earlier study evaluating GM in COPD-associated IPA identified an optimal BALF GM cutoff of approximately 0.8 (ODI), with 88.9% sensitivity and 100% specificity (105). However, frequent BALF sampling is contraindicated in comorbid or respiratory-impaired COPD patients (112), making serum testing a more feasible clinical alternative. Expert consensus endorses BALF GM positivity as microbiological evidence for CPA diagnosis in COPD (15). Salzer et al. demonstrated superior GM test diagnostic performance in COPD patients with CPA versus isolated CPA cases (113). Evidence indicates that GM has poor diagnostic performance for ABPA (114), and clinical practice guidelines explicitly discourage its use as a diagnostic tool (3); therefore, its utility in COPD–ABPA is unlikely to be favorable.
Taken together, GM shows favorable diagnostic performance in COPD–IPA and COPD–CPA. In practice, “serum GM” refers to the galactomannan index (GMI) measured in serum. In COPD–IPA, GMI also functions as a prognostic marker, particularly among AECOPD patients for whom invasive sampling is not feasible. In 153 acute AECOPD patients with IPA, positive GMI during initial ICU admission week correlated with poorer outcomes including reduced 28-day survival (106). These findings position serum GM as a prognostic biomarker for IPA in AECOPD. Multiple studies define serum GM positivity as GMI ≥ 0.5, whereas Yoshimura et al. regarded GMI ≥ 0.7 as a high level significantly associated with severe AECOPD and respiratory mortality (106, 115). Collectively, these observations support a screening threshold of GMI 0.5–0.7, to be interpreted alongside imaging and complementary biomarkers.
The diagnostic performance of BDG in IPA patients is suboptimal (116), so its use for detecting IPA in COPD patients is not regarded favorably. In a study involving 11 COPD patients with concomitant IPA, BDG testing was performed on 5 patients, with 3 testing positive (117). Potential explanations for BDG’s suboptimal performance include reduced specificity compared with GM because BDG is a ubiquitous fungal cell-wall component across many species, environmental glucan contamination, and interference from intravenous immunoglobulin (118). Clinical guidelines recommend combining BDG with GM to improve diagnostic performance (104). Reports on combined GM and BDG testing for diagnosing COPD–PA are limited. In a prospective single-center study (October 2006–November 2008) of 261 hospitalized AECOPD patients, dual-positive serum GM and BDG achieved 98.8% specificity—useful when BALF is not feasible—although the number of events was small (119). Hsu AJ et al. evaluated combined GM and BDG testing for invasive aspergillosis in immunocompromised hosts. Relative to GM alone, a both-positive rule markedly reduced sensitivity but yielded very high specificity, whereas an either-positive rule increased sensitivity with minimal loss of specificity (118). By extrapolation to COPD–PA, an either-positive GM/BDG approach may maximize sensitivity for early screening—particularly when BALF is not feasible—whereas a both-positive rule is preferable for confirmation to minimize false positives.
Although ibrexafungerp—a novel BDG-targeting antifungal—shows broad anti-Aspergillus activity and has shown efficacy in preclinical and clinical studies (120, 121), evidence in COPD patients with IPA remains limited. Multicenter randomized trials are needed to validate its utility in this population. Therefore, both BALF and serum GM represent valuable immune biomarkers for the IPA in COPD. BALF GM is primarily diagnostic, whereas serum GM provides greater prognostic value. In clinical practice, a combined GM–BDG testing strategy may be considered.
4.1.2 IgG and IgM
Plasma Asp IgG is highly informative for diagnosing IPA in non-neutropenic patients (17, 122). Very high titers (>150 AU/mL) achieve ≥95% specificity. Using an optimal cutoff of 56.6 AU/mL, the sensitivity and specificity are 77.8% and 63.9%, respectively. In non-neutropenic IPA, its diagnostic performance exceeds that of serum GM but is slightly inferior to BALF GM (17). In the COPD subgroup, an Asp IgG threshold of approximately 72 AU/mL yields 73% sensitivity and 72% specificity, supporting Asp IgG as a blood-based alternative when bronchoscopy is impractical (17). Asp IgG can be measured by ELISA and, more recently, by a lateral flow assay (LFA). An “either positive” strategy that combines the IgG LFA with BALF GM or with sputum culture or serum GM markedly increases sensitivity (87.5 percent and 85.0 percent, respectively) while maintaining high specificity, indicating a useful noninvasive adjunct for patients who cannot undergo bronchoscopy (122). Comparative and combined use of GM, Asp IgG, and BDG may represent a promising direction for future research. Experts also recommend using Asp IgG as microbiological evidence for the diagnosis of CPA in COPD patients (15). Despite its well-established diagnostic performance, in a prospective multicenter cohort of non-neutropenic IPA (including a COPD subgroup), plasma Asp-IgG positivity was not associated with 90-day mortality; Asp-IgG levels did not differ between survivors and non-survivors, and Asp-IgG did not emerge as an independent prognostic factor in multivariable analysis (14). In a cohort of 59 patients with CPA (30 with COPD), regression analysis identified Asp IgG as a predictor of mortality (123).
Asp IgG and total serum IgE have been included in the diagnostic guidelines for ABPA (3). A retrospective study that analyzed clinical data from 251 bronchiectasis patients found significantly higher serum total IgE and Asp IgG levels in ABPA patients than in controls (124). Research has shown that IgE levels are elevated in AECOPD patients with Aspergillus colonization compared to those without (125). While the current utility of IgG and IgM is predominantly diagnostic, their prognostic value for disease progression in COPD-associated pulmonary aspergillosis warrants prospective validation.
4.1.3 IL-1β
IL-1β, a key pro-inflammatory factor in COPD and PA. Aspergillus β-glucan and other PAMPs activate NF-κB via Dectin-1/CARD9 and TLR/MyD88 pathways, inducing IL1B transcription; subsequently, the NLRP3 inflammasome activates caspase-1 to process pro-IL-1β into its mature form (126). In airway models of aspergillosis, IL-1 receptor signaling sustains neutrophil survival and preserves antifungal activity (127). In COPD airways, cigarette smoke drives NLRP3–GSDMD-dependent IL-1β release, thereby amplifying airway inflammation (128). Analogous to IL-17, IL-1β contributes to antifungal defense yet exacerbates airway injury. Although macrophages in COPD may skew toward an M2 phenotype and dampen inflammation by reducing IL-1β (60), recent murine and cellular COPD models show that A. fumigatus exposure markedly elevates TNF-α, IL-1β, IL-6, and IL-33 (33).
Research on IL-1β has focused mainly on CPA, and its diagnostic performance for COPD–CPA appears suboptimal. One study suggested that the sensitivity of IL-1β, IL-6, and IL-8 as diagnostic biomarkers for CPA is higher in patients without COPD than in those with COPD (113). Nonetheless, IL-1β holds promise for monitoring and prognostic assessment in COPD–CPA. Serum IL-1β levels >2 pg/mL are associated with poorer outcomes in patients with CPA (129). In a cohort of 88 newly diagnosed CPA patients, AECOPD and active tuberculosis were associated with heightened pro-inflammatory responses and elevated IL-1β levels; an IL-1β concentration >20.3 pg/mL was defined as “high” (130). In the AECOPD–CPA subgroup (n=44), the high-IL-1β group exhibited greater disease activity, including more cavities (27/44 vs 13/44) and aspergillomas (25/44 vs 11/44); notably, IL-1β levels declined after surgical resection of lesions. Additionally, the administration of recombinant IL-1 receptor antagonist (IL-1Ra) reduces the pro-inflammatory Th17-mediated responses caused by Aspergillus infections, thereby improving prognosis (87). In corticosteroid-treated immunosuppressed mice with Aspergillus infection, IL-1Ra reduces lung tissue damage, thereby attenuating hypoxia (131). IL-1Ra thus represents a potential adjunct therapy for COPD–PA. Evidence for IL-1β in COPD–PA derives primarily from studies of CPA. Going forward, multicenter prospective cohorts enrolling COPD–CPA cases alongside COPD controls are needed, with methodological harmonization to define robust thresholds and enable external validation. In parallel, COPD–PA animal models should be developed to assess the safety and efficacy of IL-1Ra-based immunotherapy.
4.1.4 IL-6 and IL-8
IL-6 and IL-8 are key cytokines in COPD and PA that mediate neutrophil recruitment. Their production and regulation in COPD–PA have been described earlier (33, 60, 63, 83, 113). In a prospective cohort of 106 patients with COPD, elevated IL-6 and IL-8 levels served as adjunctive diagnostic markers for IPA. For serum IL-6, the sensitivity and specificity were 74.32% and 81.25% at a cutoff of 92.82 pg/mL; for BALF, the corresponding values were 68.92% and 71.88% at 229.4 pg/mL. For serum IL-8, the sensitivity and specificity were 83.78% and 81.25% at 93.46 pg/mL; in BALF, they were 85.14% and 75.00% at 325.4 pg/mL, indicating marginally better diagnostic performance in serum than in BALF (132). Additionally, IL-6, IL-8, and TNF-α levels help differentiate patients with CPA-TB from those with TB alone (133). Huang et al. further showed that pro-inflammatory mediators—including TNF-α, IL-6, and IL-8—increase with worsening CPA in both univariate and multivariate analyses (129). Whether IL-6 and IL-8, akin to IL-1β, can serve as prognostic markers for disease progression in COPD–CPA remains to be determined. A case report of invasive aspergillosis occurring after tocilizumab (anti–IL-6R) therapy in a patient with COVID-19 cautions against indiscriminate extrapolation of anti-IL-6/IL-8 strategies to COPD–PA (134), and many questions remain unresolved.
4.1.5 IL-17
He et al. reported that in non-neutropenic IPA, including a subgroup with COPD, plasma and BALF IL-17 demonstrated useful diagnostic performance, with sensitivity exceeding that of GM but lower specificity (94). Optimal cutoffs were identified as 12.02 pg/mL for plasma IL-17 (sensitivity 72.6%, specificity 69.4%) and 21.32 pg/mL for BALF IL-17 (sensitivity 81.2%, specificity 72.6%) (94). Geng et al. established an IL-17A gene knockout COPD–IPA mouse model and found that its Aspergillus fungal load was nearly twice that of the regular COPD–IPA mouse model, suggesting that IL-17 contributes to antifungal defense (87). Given the predominantly antifungal activity of IL-17 observed in experimental models, IL-17–based adjunctive strategies for COPD–IPA merit cautious evaluation rather than direct supplementation.
4.1.6 Pentraxins
Pentraxins, defined by a conserved pentraxin domain, comprise short pentraxins (such as CRP) and long pentraxins (such as PTX3). CRP serves as a biomarker for disease severity and prognosis in COPD and PA (123, 135). In a cohort of hospitalized patients with COPD (GOLD III–IV), a day-1 CRP level >1.29 mg/L was identified as the optimal diagnostic cutoff for COPD-associated IPA, with sensitivity 91.2% and specificity 57.7%. Pairwise combinations further improved discrimination, such as CRP plus ESR and CRP plus LDH (16). CRP may have dual utility for monitoring and diagnosing IPA in patients with COPD. However, coexisting bacterial infections—common in COPD-associated IPA—can confound the interpretation of CRP (136, 137), necessitating further validation of its standalone or combined diagnostic utility.
PTX3, an acute-phase pattern recognition receptor (138), demonstrates diagnostic value in non-neutropenic IPA (139). In a multicenter prospective cohort of non-neutropenic IPA that included a COPD subgroup, Sun et al. identified 90-day mortality stratification thresholds of plasma PTX3 ≥7.11 ng/mL (AUC 0.82; sensitivity 82.8%; specificity 73.4%) and BALF PTX3 ≥4.29 ng/mL (AUC 0.76; sensitivity 81.4%; specificity 67.1%) (14). Recombinant PTX3 has shown therapeutic efficacy against aspergillosis in murine and rat IPA models; however, human studies are still lacking (140). Novel anti-Aspergillus agents continue to emerge in the pharmaceutical pipeline.
4.2 Mechanistically promising
4.2.1 IL-5
ABPA, a type 2 inflammatory disease, is characterized by elevated IL-5 (141). Biologics targeting IL-5 and its receptor α (IL-5Rα) may benefit patients with COPD; in selected eosinophilic COPD populations, these agents significantly reduce annual exacerbation rates and serious adverse events (142, 143). Based on this, attempts have been made to treat ABPA with biologics, including omalizumab (anti-IgE), mepolizumab (anti-IL-5), benralizumab (anti-IL-5R), dupilumab (anti-IL-4Rα), and tezepelumab (anti-TSLP) (144). According to the ISHAM-ABPA guidelines, biologics are not recommended as first-line therapy for acute ABPA. Initial treatment is oral prednisolone or itraconazole, and combination therapy is considered only for relapses (3). Direct evidence of efficacy of anti-IL-5/IL-5Rα in COPD–ABPA is lacking. In ABPA with comorbid asthma, real-world and retrospective studies report that anti-IL-5/anti-IL-5Rα monoclonal antibodies reduce exacerbation frequency, decrease oral corticosteroid requirements, and improve lung function (145). Therefore, large-scale randomized trials with extended follow-up are required to evaluate the efficacy of biologics in COPD–ABPA. Key endpoints should include annualized exacerbation rate, cumulative oral corticosteroid exposure, relief of mucus plugging, and radiologic improvement. Randomization should be stratified by blood eosinophil count and Aspergillus sensitization, with comparator arms including anti-IgE therapy and azole monotherapy.
4.2.2 TARC
Thymus and activation-regulated chemokine (TARC) is associated with eosinophilic inflammation and IgE activation, and mechanistically aligns with the type-2 inflammatory axis of ABPA (146). In ABPA, Kozlova et al. reported markedly elevated serum TARC (median, 734.0 pg/mL) that decreased significantly after 12 weeks of itraconazole, paralleling improvements in FEV1 and reductions in total IgE, thereby supporting its use for monitoring antifungal treatment response (147). In COPD higher TARC concentrations are associated with rapid decline in lung function: a serum TARC ≥211 pg/mL identified rapid FEV1 decline (sensitivity 100%, specificity 26.8%), and TARC ≥335 pg/mL identified rapid %FEV1 decline (sensitivity 67.5%, specificity 55.4%), suggesting that TARC may serve as an independent predictor of accelerated deterioration (148). However, sensitivity/specificity thresholds in COPD–PA/ABPA require prospective validation. At present, TARC is best considered an exploratory companion marker.
4.2.3 Th17/Treg
The potential immunologic roles of Th17 and Treg in COPD–PA have been outlined above. In a prospective cohort of acute respiratory distress syndrome (ARDS), a Th17/Treg ratio >0.79 predicted 28-day mortality (sensitivity 87.5%, specificity 68.1%) (149), which initially suggested potential prognostic utility for COPD–PA; however, closer appraisal tempered this expectation. In COPD, the Th17/Treg ratio is not uniformly elevated and varies by disease stage and sampling compartment (86). Even within the same PA subtype (ABPA), studies report opposing directions (79, 150), and differences across subtypes are likely—though direct evidence is limited. Given the added immunologic complexity of COPD with PA, other markers, such as IL-1β, may offer greater predictive value.
4.2.4 SP-D
Surfactant protein D (SP-D) is a critical component of the pulmonary innate immune system. It mediates functions of innate and adaptive immune cells and participates in clearing apoptotic cells, allergens, and other harmful particles. Cigarette smoking promotes SP-D translocation from alveolar spaces into the bloodstream, establishing serum SP-D as a validated biomarker of smoke-induced pulmonary damage (151). One study indicated that serum SP-D levels are not associated with the development of COPD (152). Notably, serum SP-D demonstrates limited diagnostic or monitoring utility for ABPA exacerbations (153). Although SP-D demonstrated protective efficacy in immunosuppressed murine models challenged with A. fumigatus conidia, enhancing fungal clearance, the available evidence does not support SP-D as a diagnostic or prognostic biomarker in COPD–PA (154).
4.3 Requiring further investigation
4.3.1 Immune receptors and signaling molecules
NF-κB, TLR2, TLR4, JNK, and Dectin-1 have been discussed in earlier sections as potential immunological biomarkers. Due to their established cross-reactivity within immune pathways, these molecules are frequently explored as therapeutic targets. However, the activation of these signaling molecules and immune receptors can serve a dual function: while they exert antifungal effects, they may also exacerbate airway inflammation. Taking the JNK pathway as an example, epithelial cells are stimulated by A. fumigatus conidia to activate the JNK signaling pathway, which facilitates fungal clearance (48) However, activation of the JNK pathway is also associated with epithelial damage and airway inflammation in COPD (46, 47). Moreover, evidence supporting their use as immunological biomarkers for COPD–PA remains limited, particularly in clinical cohorts. Most studies have primarily focused on their roles in immunomodulation and inflammatory cascades, with systematic evaluations of their biomarker potential still notably absent.
4.3.2 IFN-γ
In COPD, heightened Th1 and Th17 activity drives IFN-γ production, mediating pulmonary inflammation and tissue damage (21). IFN-γ is a central antifungal cytokine that activates macrophages, augments antimicrobial-peptide expression, and promotes effector T-cell recruitment to coordinate neutrophil-mediated fungal clearance (63). In parallel, IL-12 signaling downstream of the TLR–MyD88 and Dectin-1/CARD9 axes drives Th1 differentiation, thereby upregulating IFN-γ production (40, 78). Notably, many cytokines—including IL-1β, IL-6, IL-8, IL-17, and IFN-γ—support antifungal defense yet concurrently exacerbate airway inflammation and tissue injury in COPD. However, a study observed that CPA patients exhibit defective production of IFN-γ and IL-17A (155). IFN-γ replacement therapy has also been included in CPA clinical treatment guidelines (50–60 μg subcutaneously, three times weekly) (2). ABPA involves predominant Th2 responses with concomitant Th1 suppression, inducing IFN-γ deficiency. Additionally, IPA is commonly observed in immunocompromised patients (156). Russo et al. observed that IFN-γ knockout mice exhibited increased susceptibility to IPA, and also noted a marked decrease in IFN-γ expression in severe IPA patients (157). IFN-γ replacement therapy may hold substantial promise for the treatment of PA, yet excessive IFN-γ can exacerbate COPD. This paradox complicates IFN-γ replacement therapy in COPD–PA: in the COPD-dominant phenotype, elevated IFN-γ contraindicates supplementation, whereas in the PA-dominant phenotype, IFN-γ therapy may be feasible but likely within a narrow therapeutic window. Accordingly, IFN-γ replacement should first be evaluated in COPD–PA animal models using dose-escalation designs to define an exposure–response threshold, followed by rigorously controlled studies in COPD–PA patients. Table 1 summarizes the immunological biomarkers discussed in the preceding sections.
5 Translational clinical applications of immune biomarkers
5.1 Early diagnosis
In COPD–PA, the usefulness of conventional microbiology and imaging for early recognition is limited by several factors, particularly the relative rarity in COPD patients of classical IPA radiologic signs such as the air-crescent sign and the halo sign (12, 13, 158, 159). Immune markers: (i) may serve as complementary evidence alongside standard microbiologic and radiologic assessments; (ii) may improve diagnostic accessibility when bronchoscopy or tissue biopsy is not feasible or is contraindicated; and (iii) may be applied in combination, whereby different panels and decision rules, including the “either-positive” and “both-positive” rules, yield distinct sensitivities and specificities, thereby informing clinical decision-making.
5.2 Monitoring and prognostic assessment
In COPD–PA, disease activity fluctuates in response to airway inflammation, acute exacerbations, bacterial co-infection, and the intensity of antifungal or adjunctive therapies, which may blunt or amplify conventional clinical signals. IPA and ABPA typically present acutely and are associated with poorer prognoses, whereas CPA generally follows a more indolent course (3, 158, 160). Immune markers: (i) may aid early risk stratification during acute episodes and thereby inform interventions that improve outcomes and survival; (ii) in chronic disease, may help delineate disease stage and support a biomarker-based staging framework for COPD–CPA; and (iii) if staging can be anchored to immune-biomarker profiles, it may enable more systematic, stage-specific therapeutic strategies while acknowledging interindividual variability. Taken together, integrating immune-biomarker readouts with clinical, radiologic, and microbiologic assessments may strengthen longitudinal monitoring and prognostic assessment across the COPD–PA spectrum.
Based on current studies evaluating the diagnostic and prognostic utility of immune markers in COPD- and non-neutropenic patient–associated IPA, we synthesized and summarized the relevant data (Table 2). Given the paucity of studies on COPD-associated CPA and ABPA—we did not present corresponding tables.
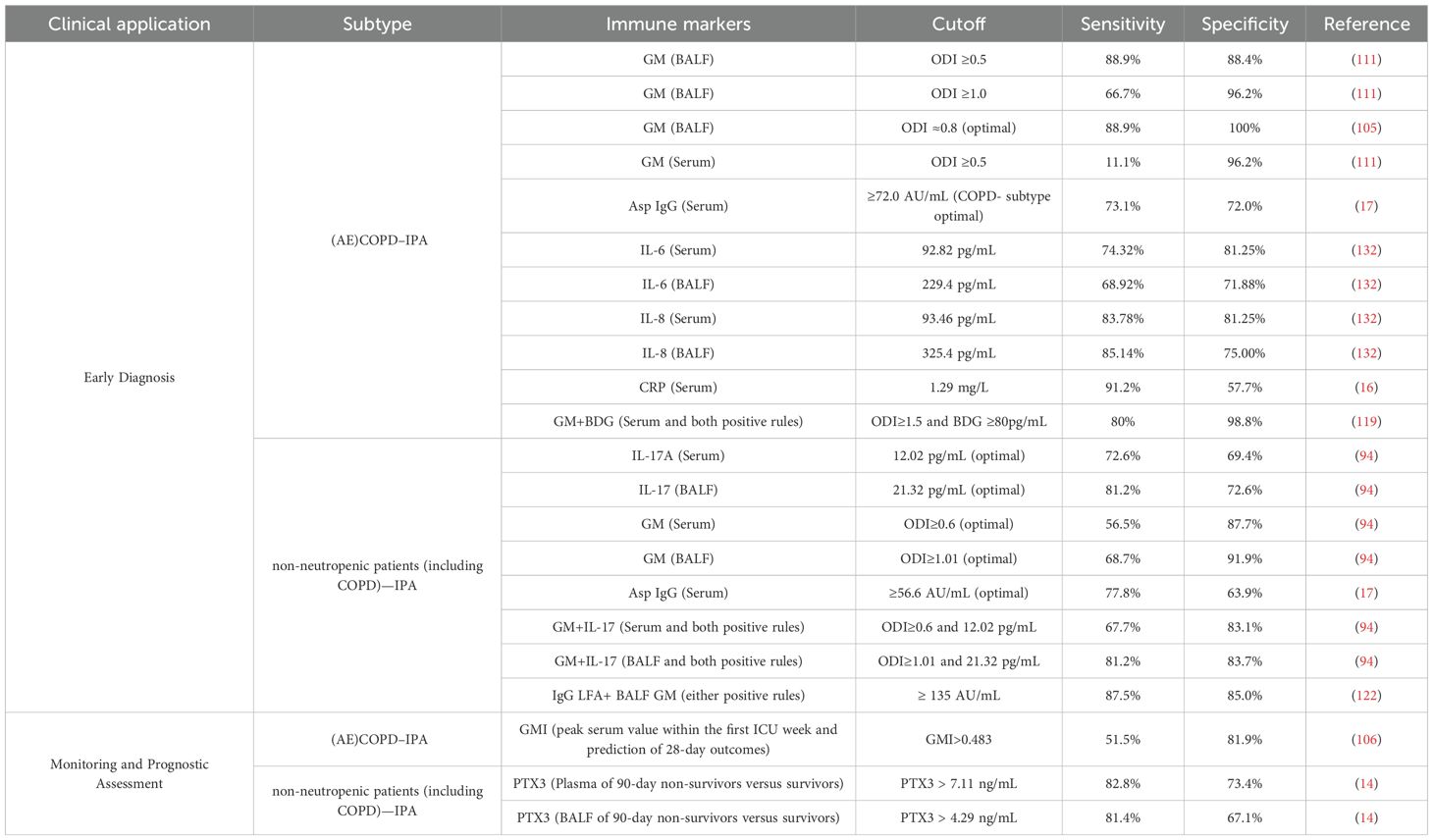
Table 2. Immune markers for early diagnosis and prognostic assessment of IPA in COPD/non-neutropenic patients: validated cutoffs, sensitivities and specificities.
5.3 Immunotherapy
Traditional antifungal therapy is limited by toxicity and pharmacologic constraints: for example, amphotericin B is nephrotoxic (161); triazoles have extensive drug–drug interactions that necessitate therapeutic drug monitoring (TDM) (1–3, 110); and azole resistance in Aspergillus is increasingly reported across multiple regions, with resistance in invasive aspergillosis associated with higher mortality and diminishing the effectiveness of first-line azoles (162). Immune markers: (i) may be combined with antifungal agents to enhance therapeutic efficacy; (ii) may aid the management of refractory disease; and (iii) may help mitigate antifungal-related adverse effects.
5.4 Biomarker-guided algorithms and cutoffs for COPD–PA subtypes
Anchored in the tiered framework (Table 1) and the standardized cutoffs and reporting conventions (Table 2), the following subtype-specific recommendations translate biomarker evidence into bedside algorithms. For suspected COPD–IPA, prioritize testing BALF GM (optimal ODI ≥ 1.01; sensitivity 68.7%, specificity 91.9%) when bronchoscopic sampling is feasible and safe; otherwise, employ a serum-centered panel comprising GM (optimal ODI ≥ 0.6; 56.5%, 87.7%) with or without PTX3, IL-6, and IL-8. An any-positive rule supports early screening, whereas a dual-positive rule can serve for confirmation—for example, serum GM + BDG (ODI ≥ 1.5 and BDG ≥ 80 pg/mL; 80.0%, 98.8%) or GM + IL-17 (serum: 0.6 and 12.02 pg/mL; 67.7%, 83.1%; BALF: 1.01 and 21.32 pg/mL; 81.2%, 83.7%)—before initiating antifungal therapy that entails toxicity and resistance. For suspected COPD–CPA, base the diagnosis on Asp IgG (optimal ≥ 56.6 AU/mL; 77.8%, 63.9%; very high titers > 150 AU/mL achieve ≥ 95% specificity; COPD subgroup approximately 72 AU/mL yields 73%/72%) and incorporate IL-1β for activity and prognostic assessment; employ sequential retesting when clinically indicated. For COPD–ABPA, follow the guideline pathway centered on total IgE and Asp IgG; when feasible, consider TARC and IL-5 as activity markers or companion readouts to therapy. For longitudinal monitoring and prognostication in non-neutropenic patients (including COPD) with IPA, consider GMI (peak value in the first ICU week > 0.483; 51.5%, 81.9%) and PTX3 (plasma > 7.11 ng/mL; 82.8%, 73.4%; BALF > 4.29 ng/mL; 81.4%, 67.1%), alongside CRP and selected airway cytokines (such as IL-8: serum 93.46 pg/mL; 83.78%, 81.25%; BALF 325.4 pg/mL; 85.14%, 75.00%).
6 Discussion
This review synthesizes evidence on immune biomarkers in COPD complicated by IPA, CPA, and ABPA and advances a tiered, clinically oriented framework (Table 1). Tier 1 comprises GM, BDG, Asp IgG, IgE, CRP, PTX3, IL-1β, IL-17, IL-6, and IL-8; taken together, these biomarkers support diagnosis, with BALF GM as the anchor, and inform risk stratification and prognostication, with particular relevance of PTX3, the serum GMI, and IL-1β. Tier 2/3 encompasses candidates that are mechanistically grounded or promising yet require further validation, including IL-5 and TARC, as well as pathway nodes such as NF-κB, TLR2/4, JNK, and Dectin-1. The role of IFN-γ appears context-dependent and bidirectional, underscoring the need for clarification specifically in COPD–PA.
Pathophysiologically, the evidence is consistent with a proximal-airway to systemic spillover trajectory: in early disease, airway fungal burden and mucosal inflammation predominate, which explains the higher sensitivity of BALF GM and selected airway cytokines; as the disease progresses, serum readouts become more informative, such as the GMI, PTX3, and systemic inflammatory mediators. To enhance comparability and clinical translation, Table 2 collates commonly used cutoffs and reporting conventions as practical reference points for future studies and pilot implementation.
A cross-study synthesis revealed recurring themes. Study-population heterogeneity was pervasive: many cohorts were not COPD–PA exclusive and enrolled non-neutropenic or non-immunosuppressed patients in whom COPD formed only a subgroup; small sample sizes, stringent exclusion criteria, and categorization bias (such as liberal assignment to “possible IPA”) hampered effect-size estimation and constrained the generalizability of thresholds (14, 17, 94). Assay platforms and workflows also differed, yielding non-uniform cutoffs; for example, serum GMI is commonly interpreted around 0.5–0.7 (106, 115). In the context of COPD–CPA, IL-1β thresholds of 2 pg/mL and 20.3 pg/mL have been reported for monitoring and prognostication; the former signals poorer outcomes and the latter defines a higher activity tier, underscoring that cutoff selection depends on study objectives (129, 130). Combination rules behaved predictably: dual-positive panels, such as GM plus BDG or GM plus IL-17, increased specificity at the cost of sensitivity and thus favored confirmation, whereas any-positive panels maximized sensitivity with only modest specificity loss, supporting early screening when BALF cannot be obtained promptly (94, 119). Evidence is skewed toward COPD–IPA, followed by COPD–CPA, with COPD–ABPA markedly underrepresented. Across the research pipeline, the field remains diagnosis-centric, then monitoring and prognostication, and only lastly treatment, reflecting an early emphasis on identifying high-sensitivity and high-specificity markers to enable timely antifungal therapy. Finally, cytokines act as a double-edged sword: they can aid fungal clearance yet aggravate COPD airway inflammation. We hypothesize that, during acute IPA, fungal infection is the primary driver whereas COPD airway inflammation is secondary—a provisional inference that aligns with portions of the literature. IFN-γ, immune receptors, and signaling molecules were highlighted in Tier 3 because IL-6, IL-8, IL-1β, and IL-17 already have clinical or preclinical support in COPD–PA, whereas these Tier-3 targets currently lack comparably robust evidence and remain exploratory.
Future research on COPD–PA should be organized around several themes. First, increase attention to COPD–CPA and COPD–ABPA, with parallel emphasis on immune biomarkers for risk stratification, prediction, and immunotherapeutic decision-making. Second, multicenter studies must be expanded to increase statistical power and external validity. Current COPD–PA studies typically enroll several dozen to two or three hundred participants; sample sizes tend to be larger in IPA—such as 153 and 261 cases (106, 119)—are commonly in the tens for CPA (123, 130), and dedicated reports on COPD–ABPA remain scarce. Future work should prioritize multicenter designs; Third, combinatorial application should become standard practice: single immune biomarkers have inherent limitations; antibiotic exposure can produce false-positive GM results (163), and corticosteroid use together with COPD-related immune dysregulation diminishes the diagnostic utility of antibody testing for IPA in this population (164). Immune markers panels should be interpreted together with clinical findings, mycological results, and imaging. When appropriate, they can also be incorporated into machine-learning diagnostic models that use multiple predictors (165).
Author contributions
YH: Writing – review & editing, Investigation, Writing – original draft, Conceptualization. XW: Visualization, Writing – review & editing, Conceptualization. HW: Investigation, Writing – original draft. HD: Writing – review & editing, Investigation. FG: Supervision, Writing – review & editing. YW: Supervision, Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grants from The Sichuan Provincial Medical Research Project Plan (project number: S23038).
Acknowledgments
The author(s) gratefully acknowledge the seminal work of the Department of Respiratory Medicine, Jianyang People’s Hospital, through their research project “COPD Lung Microbiome Study”. This project profoundly inspired the conceptual framework and scientific perspectives presented in this review. All figures were created using BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT (OpenAI; ChatGPT-5, accessed September 2025; and GPT-4o, accessed May 2025) strictly for language polishing. No analyses, data interpretation, or content generation were delegated to the tool, and no patient-level or confidential data were entered. All outputs were reviewed, verified, and edited by the authors, who take full responsibility for the final text.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABPA, Allergic Bronchopulmonary Aspergillosis; ADCC, Antibody-Dependent Cellular Cytotoxicity; AECOPD, Acute Exacerbation of Chronic Obstructive Pulmonary Disease; Asp IgG, Aspergillus-specific Immunoglobulin G; A. fumigatus, Aspergillus fumigatus; AUC, Area Under the Curve; BALF, Bronchoalveolar Lavage Fluid; BDG, (1→3)-β-D-Glucan; CCPA, Chronic Cavitary Pulmonary Aspergillosis; CFPA, Chronic Fibrosing Pulmonary Aspergillosis; COPD, Chronic Obstructive Pulmonary Disease; COPD–PA, COPD complicated by Pulmonary Aspergillosis; CPA, Chronic Pulmonary Aspergillosis; CRP, C-Reactive Protein; CTL, Cytotoxic T Lymphocyte; CXCL1/2, C-X-C Motif Chemokine Ligand 1/2; DCs, Dendritic Cells; DAMPs, Damage-Associated Molecular Patterns; ESR, Erythrocyte Sedimentation Rate; FEV1, Forced Expiratory Volume in One Second; FcR, Fc Receptor; GM, Galactomannan; GMI, Galactomannan Index; GSDMD, Gasdermin D; ICU, Intensive Care Unit; ICS, Inhaled Corticosteroid; IFN-γ, Interferon-gamma; IgE/IgG/IgM, Immunoglobulin E/G/M; IL-1β/2/5, Interleukin-1β/2/5; IPA, Invasive Pulmonary Aspergillosis; JNK, c-Jun N-terminal Kinase; LDH, Lactate Dehydrogenase; LFA, Lateral Flow Assay; NETs, Neutrophil Extracellular Traps; NLR, NOD-like Receptor; NLRP3, NLR Family Pyrin Domain Containing 3; NOX, NADPH Oxidase; ODI, Optical Density Index; PA, Pulmonary Aspergillosis; PAMP, Pathogen-Associated Molecular Pattern; PD-1, Programmed Cell Death Protein 1; PRR, Pattern-Recognition Receptor; PTX3, Pentraxin 3; ROS, Reactive Oxygen Species; sIgA, Secretory Immunoglobulin A; SP-D, Surfactant Protein D; TARC, Thymus and Activation-Regulated Chemokine (CCL17); TDM, Therapeutic Drug Monitoring; TGF-β, Transforming Growth Factor-beta; Th1/Th2/Th17, T helper 1/2/17; TLR2/4, Toll-like Receptor 2/4; TNF-α, Tumor Necrosis Factor-α; Treg, Regulatory T cell; TSLP, Thymic Stromal Lymphopoietin.
References
1. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect diseases: an Off Publ Infect Dis Soc America. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
2. Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. (2016) 47:45–68. doi: 10.1183/13993003.00583-2015
3. Agarwal R, Sehgal IS, Muthu V, Denning DW, Chakrabarti A, Soundappan K, et al. Revised ISHAM-ABPA working group clinical practice guidelines for diagnosing, classifying and treating allergic bronchopulmonary aspergillosis/mycoses. Eur Respir J. (2024) 63:2400061. doi: 10.1183/13993003.00061-2024
4. Hammond EE, McDonald CS, Vestbo J, and Denning DW. The global impact of aspergillus infection on COPD. BMC pulmonary Med. (2020) 20:241. doi: 10.1186/s12890-020-01259-8
5. Mir T, Uddin M, Khalil A, Lohia P, Porter L, Regmi N, et al. Mortality outcomes associated with invasive aspergillosis among acute exacerbation of chronic obstructive pulmonary disease patient population. Respir Med. (2022) 191:106720. doi: 10.1016/j.rmed.2021.106720
6. Tiew PY, Dicker AJ, Keir HR, Poh ME, Pang SL, Mac Aogáin M, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J. (2021) 57:2002050. doi: 10.1183/13993003.02050-2020
7. Tiew PY, Narayana JK, Quek MSL, Ang YY, Ko FWS, Poh ME, et al. Sensitisation to recombinant aspergillus fumigatus allergens and clinical outcomes in COPD. Eur Respir J. (2023) 61:2200507. doi: 10.1183/13993003.00507-2022
8. Gu Y, Ye X, Liu Y, Wang Y, Shen K, Zhong J, et al. A risk-predictive model for invasive pulmonary aspergillosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Res. (2021) 22:176. doi: 10.1186/s12931-021-01771-3
9. Guo L, Wu X, and Wu X. Aspergillus infection in chronic obstructive pulmonary diseases. Clin Respir J. (2023) 17:129–38. doi: 10.1111/crj.13585
10. Bulpa P, Dive A, and Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. (2007) 30:782–800. doi: 10.1183/09031936.00062206
11. Guinea J, Torres-Narbona M, Gijón P, Muñoz P, Pozo F, Peláez T, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol infection: Off Publ Eur Soc Clin Microbiol Infect Dis. (2010) 16:870–7. doi: 10.1111/j.1469-0691.2009.03015.x
12. Nates JL, Pène F, Darmon M, Mokart D, Castro P, David S, et al. Septic shock in the immunocompromised cancer patient: A narrative review. Crit Care (London England). (2024) 28:285. doi: 10.1186/s13054-024-05073-0
13. Cordonnier C, Botterel F, Ben Amor R, Pautas C, Maury S, Kuentz M, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol infection: Off Publ Eur Soc Clin Microbiol Infect Dis. (2009) 15:81–6. doi: 10.1111/j.1469-0691.2008.02122.x
14. Sun C, Cai X, Zhong H, Lu Y, Li Y, Cai Y, et al. Pentraxin-3 as a novel prognostic biomarker in non-neutropenic invasive pulmonary aspergillosis patients. Microbiol Spectr. (2025) 13:e0294524. doi: 10.1128/spectrum.02945-24
15. Chinese Thoracic Society of Chinese Medical A. Expert consensus on the diagnosis and treatment of pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. (2024) 47:604–22. doi: 10.3760/cma.j.cn112147-20231228-00399
16. Long Z, Li X, Li Z, Hu J, Qiu Y, Li S, et al. Improved diagnostic markers for invasive pulmonary aspergillosis in copd patients. Front Cell infection Microbiol. (2024) 14:1294971. doi: 10.3389/fcimb.2024.1294971
17. Lu Y, Liu L, Li H, Chen B, Gu Y, Wang L, et al. The clinical value of aspergillus-specific IgG antibody test in the diagnosis of nonneutropenic invasive pulmonary aspergillosis. Clin Microbiol infection. (2023) 29:797. doi: 10.1016/j.cmi.2023.02.002
18. Bertuzzi M, Howell GJ, Thomson DD, Fortune-Grant R, Möslinger A, Dancer P, et al. Epithelial uptake leads to fungal killing in vivo and is aberrant in copd-derived epithelial cells. iScience. (2024) 27:109939. doi: 10.1016/j.isci.2024.109939
19. Bigot J, Guillot L, Guitard J, Ruffin M, Corvol H, Balloy V, et al. Bronchial epithelial cells on the front line to fight lung infection-causing aspergillus fumigatus. Front Immunol. (2020) 11:1041. doi: 10.3389/fimmu.2020.01041
20. Chen J, Wang T, Li X, Gao L, Wang K, Cheng M, et al. DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease. Signal transduction targeted Ther. (2024) 9:163. doi: 10.1038/s41392-024-01881-6
21. Qi Y, Yan Y, Tang D, Han J, Zhu X, Cui M, et al. Inflammatory and immune mechanisms in COPD: current status and therapeutic prospects. J Inflammation Res. (2024) 17:6603–18. doi: 10.2147/jir.S478568
22. Xu J, Zeng Q, Li S, Su Q, and Fan H. Inflammation mechanism and research progress of COPD. Front Immunol. (2024) 15:1404615. doi: 10.3389/fimmu.2024.1404615
23. Raby KL, Michaeloudes C, Tonkin J, Chung KF, and Bhavsar PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol. (2023) 14:1201658. doi: 10.3389/fimmu.2023.1201658
24. Gu Y, Ye X, Wang Y, Shen K, Zhong J, Chen B, et al. Clinical features and prognostic analysis of patients with aspergillus isolation during acute exacerbation of chronic obstructive pulmonary disease. BMC pulmonary Med. (2021) 21:69. doi: 10.1186/s12890-021-01427-4
25. Sy KA, Valsecchi I, Briottet M, Vaudin M, John S, Faye M, et al. Impact of specialized pro-resolving lipid mediators on the colonization of CF respiratory epithelia by aspergillus fumigatus. Rev Des Maladies Respiratoires. (2025) 42:212. doi: 10.1016/j.rmr.2025.02.061
26. Liu F, Zeng M, Zhou X, Huang F, and Song Z. Aspergillus fumigatus escape mechanisms from its harsh survival environments. Appl Microbiol Biotechnol. (2024) 108:53. doi: 10.1007/s00253-023-12952-z
27. Feldman MB, Dutko RA, Wood MA, Ward RA, Leung HM, Snow RF, et al. Aspergillus fumigatus cell wall promotes apical airway epithelial recruitment of human neutrophils. Infection Immun. (2020) 88:e00813-19. doi: 10.1128/iai.00813-19
28. Richard N, Marti L, Varrot A, Guillot L, Guitard J, Hennequin C, et al. Human bronchial epithelial cells inhibit aspergillus fumigatus germination of extracellular conidia via flea recognition. Sci Rep. (2018) 8:15699. doi: 10.1038/s41598-018-33902-0
29. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, and Rudan I. Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (COPD) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir Med. (2022) 10:447–58. doi: 10.1016/s2213-2600(21)00511-7
30. Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, et al. Cigarette smoke-induced necroptosis and damp release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. (2016) 310:L377–86. doi: 10.1152/ajplung.00174.2015
31. Ma M, Jiang W, and Zhou R. Damps and damp-sensing receptors in inflammation and diseases. Immunity. (2024) 57:752–71. doi: 10.1016/j.immuni.2024.03.002
32. Gong T, Liu L, Jiang W, and Zhou R. Damp-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. (2020) 20:95–112. doi: 10.1038/s41577-019-0215-7
33. Bouyssi A, Trecourt A, Déméautis T, Persat F, Glehen O, Wallon M, et al. Aspergillus fumigatus is responsible for inflammation in a murine model of chronic obstructive pulmonary disease exacerbation. Respir Res. (2025) 26:25. doi: 10.1186/s12931-024-03092-7
34. Gayathri L, Akbarsha MA, and Ruckmani K. In vitro study on aspects of molecular mechanisms underlying invasive aspergillosis caused by gliotoxin and fumagillin, alone and in combination. Sci Rep. (2020) 10:14473. doi: 10.1038/s41598-020-71367-2
35. Haw TJ, Starkey MR, Pavlidis S, Fricker M, Arthurs AL, Nair PM, et al. Toll-like receptor 2 and 4 have opposing roles in the pathogenesis of cigarette smoke-induced chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L298–l317. doi: 10.1152/ajplung.00154.2017
36. Sidletskaya K, Vitkina T, and Denisenko Y. The role of toll-like receptors 2 and 4 in the pathogenesis of chronic obstructive pulmonary disease. Int J chronic obstructive pulmonary Dis. (2020) 15:1481–93. doi: 10.2147/copd.S249131
37. Ou G, Zhu M, Huang Y, Luo W, Zhao J, Zhang W, et al. HSP60 regulates the cigarette smoke-induced activation of TLR4-NF-κB-myD88 signalling pathway and NLRP3 inflammasome. Int Immunopharmacol. (2022) 103:108445. doi: 10.1016/j.intimp.2021.108445
38. Zhao GQ, Lin J, Hu LT, Yin XN, Wang Q, Xu Q, et al. The role of dectin-1/raf-1 signal cascade in innate immune of human corneal epithelial cells against aspergillus fumigatus infection. Int J Ophthalmol. (2016) 9:1371–5. doi: 10.18240/ijo.2016.10.01
39. Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, et al. TLRs govern neutrophil activity in aspergillosis. J Immunol (Baltimore Md: 1950). (2004) 173:7406–15. doi: 10.4049/jimmunol.173.12.7406
40. Wang Y, Zhang D, Hou Y, Shen S, and Wang T. The adaptor protein CARD9, from fungal immunity to tumorigenesis. Am J Cancer Res. (2020) 10:2203–25.
41. Khosravi AR, Alheidary S, Nikaein D, and Asghari N. Aspergillus fumigatus conidia stimulate lung epithelial cells (TC-1 JHU-1) to produce IL-12, IFNγ, IL-13 and IL-17 cytokines: modulatory effect of propolis extract. J mycologie medicale. (2018) 28:594–8. doi: 10.1016/j.mycmed.2018.09.006
42. Liu W, Ouyang X, Yang J, Liu J, Li Q, Gu Y, et al. AP-1 activated by toll-like receptors regulates expression of IL-23 P19. J Biol Chem. (2009) 284:24006–16. doi: 10.1074/jbc.M109.025528
43. Takeda K and Akira S. TLR signaling pathways. Semin Immunol. (2004) 16:3–9. doi: 10.1016/j.smim.2003.10.003
44. Thrikawala SU, Anderson MH, and Rosowski EE. Glucocorticoids suppress NF-κb-mediated neutrophil control of aspergillus fumigatus hyphal growth. J Immunol (Baltimore Md: 1950). (2024) 213:971–87. doi: 10.4049/jimmunol.2400021
45. Hohl TM. Immune responses to invasive aspergillosis: new understanding and therapeutic opportunities. Curr Opin Infect Dis. (2017) 30:364–71. doi: 10.1097/qco.0000000000000381
46. Yu Y, Yang A, He X, Wu B, Wu Y, Li Y, et al. Soluble epoxide hydrolase deficiency attenuates airway inflammation in COPD via IRE1α/JNK/AP-1 signaling pathway. J Inflammation (London England). (2023) 20:36. doi: 10.1186/s12950-023-00361-y
47. Wagner C, Uliczka K, Bossen J, Niu X, Fink C, Thiedmann M, et al. Constitutive immune activity promotes JNK- and foxO-dependent remodeling of drosophila airways. Cell Rep. (2021) 35:108956. doi: 10.1016/j.celrep.2021.108956
48. Cui X, Chen F, Zhao J, Li D, Hu M, Chen X, et al. Involvement of JNK signaling in aspergillus fumigatus-induced inflammatory factors release in bronchial epithelial cells. Sci Rep. (2023) 13:1293. doi: 10.1038/s41598-023-28567-3
49. Tiew PY, Thng KX, and Chotirmall SH. Clinical aspergillus signatures in COPD and bronchiectasis. J fungi (Basel Switzerland). (2022) 8:480. doi: 10.3390/jof8050480
50. Shoham S and Levitz SM. The immune response to fungal infections. Br J haematology. (2005) 129:569–82. doi: 10.1111/j.1365-2141.2005.05397.x
51. Otu A, Kosmidis C, Mathioudakis AG, Ibe C, and Denning DW. The clinical spectrum of aspergillosis in chronic obstructive pulmonary disease. Infection. (2023) 51:813–29. doi: 10.1007/s15010-022-01960-2
52. Rodríguez-Pérez J, Andreu-Martínez R, Daza R, Fernández-Arroyo L, Hernández-García A, Díaz-García E, et al. Oxidative stress and inflammation in hypoxemic respiratory diseases and their comorbidities: molecular insights and diagnostic advances in chronic obstructive pulmonary disease and sleep apnea. Antioxidants (Basel Switzerland). (2025) 14:839. doi: 10.3390/antiox14070839
53. Belchamber KBR, Singh R, Batista CM, Whyte MK, Dockrell DH, Kilty I, et al. Defective bacterial phagocytosis is associated with dysfunctional mitochondria in copd macrophages. Eur Respir J. (2019) 54:1802244. doi: 10.1183/13993003.02244-2018
54. Tu Y, Chen Y, Li X, Wang Y, Fang B, Ren Y, et al. Advances in acute COPD exacerbation: clarifying specific immune mechanisms of infectious and noninfectious factors. Ther Adv Respir Dis. (2025) 19:17534666241308408. doi: 10.1177/17534666241308408
55. Wrench C, Belchamber KBR, Bercusson A, Shah A, Barnes PJ, Armstrong-James D, et al. Reduced clearance of fungal spores by chronic obstructive pulmonary disease GM-CSF- and M-CSF-derived macrophages. Am J Respir Cell Mol Biol. (2018) 58:271–3. doi: 10.1165/rcmb.2017-0351LE
56. Belchamber K, Barnes P, and Donnelly L. Altered scavenger receptor expression and response to oxidative stress is linked to defective bacterial phagocytosis in COPD macrophages. Eur Respir J. (2016) 48:PA4657. doi: 10.1183/13993003.congress-2016.PA4657
57. Latgé JP and Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev. (2019) 33:e00140-18. doi: 10.1128/cmr.00140-18
58. Dellière S, Chauvin C, Wong SSW, Gressler M, Possetti V, Parente R, et al. Interplay between host humoral pattern recognition molecules controls undue immune responses against aspergillus fumigatus. Nat Commun. (2024) 15:6966. doi: 10.1038/s41467-024-51047-9
59. Ristagno G, Fumagalli F, Bottazzi B, Mantovani A, Olivari D, Novelli D, et al. Pentraxin 3 in cardiovascular disease. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00823
60. Kim GD, Lim EY, and Shin HS. Macrophage polarization and functions in pathogenesis of chronic obstructive pulmonary disease. Int J Mol Sci. (2024) 25:5631. doi: 10.3390/ijms25115631
61. Henrot P, Prevel R, Berger P, and Dupin I. Chemokines in COPD: from implication to therapeutic use. Int J Mol Sci. (2019) 20:2785. doi: 10.3390/ijms20112785
62. Lin WC and Fessler MB. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell Mol Life sciences: CMLS. (2021) 78:4095–124. doi: 10.1007/s00018-021-03768-z
63. Lionakis MS, Drummond RA, and Hohl TM. Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol. (2023) 23:433–52. doi: 10.1038/s41577-022-00826-w
64. Noseykina EM, Schepetkin IA, and Atochin DN. Molecular mechanisms for regulation of neutrophil apoptosis under normal and pathological conditions. J evolutionary Biochem Physiol. (2021) 57:429–50. doi: 10.1134/s0022093021030017
65. Earle K, Valero C, Conn DP, Vere G, Cook PC, Bromley MJ, et al. Pathogenicity and virulence of aspergillus fumigatus. Virulence. (2023) 14:2172264. doi: 10.1080/21505594.2023.2172264
66. Zhong H, Lu RY, and Wang Y. Neutrophil extracellular traps in fungal infections: A seesaw battle in hosts. Front Immunol. (2022) 13:977493. doi: 10.3389/fimmu.2022.977493
67. Keir HR and Chalmers JD. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir review: an Off J Eur Respir Soc. (2022) 31:210241. doi: 10.1183/16000617.0241-2021
68. Voynow JA and Shinbashi M. Neutrophil elastase and chronic lung disease. Biomolecules. (2021) 11:1065. doi: 10.3390/biom11081065
69. Warrick KA, Vallez CN, Meibers HE, and Pasare C. Bidirectional communication between the innate and adaptive immune systems. Annu Rev Immunol. (2025) 43:489–514. doi: 10.1146/annurev-immunol-083122-040624
70. Raskov H, Orhan A, Christensen JP, and Gögenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. (2021) 124:359–67. doi: 10.1038/s41416-020-01048-4
71. Lei X, de Groot DC, Welters MJP, de Wit T, Schrama E, van Eenennaam H, et al. CD4(+) T cells produce IFN-I to license cDC1s for induction of cytotoxic T-cell activity in human tumors. Cell Mol Immunol. (2024) 21:374–92. doi: 10.1038/s41423-024-01133-1
72. Sharma J, Mudalagiriyappa S, and Nanjappa SG. T cell responses to control fungal infection in an immunological memory lens. Front Immunol. (2022) 13:905867. doi: 10.3389/fimmu.2022.905867
73. Zhu J, Yamane H, and Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. (2010) 28:445–89. doi: 10.1146/annurev-immunol-030409-101212
74. Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, et al. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes infection. (2011) 13:151–9. doi: 10.1016/j.micinf.2010.10.005
75. Topchyan P, Lin S, and Cui W. The role of CD4 T cell help in CD8 T cell differentiation and function during chronic infection and cancer. Immune network. (2023) 23:e41. doi: 10.4110/in.2023.23.e41
76. Mosmann TR and Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. (1989) 7:145–73. doi: 10.1146/annurev.iy.07.040189.001045
77. Qin K, Xu B, Pang M, Wang H, and Yu B. The functions of CD4 T-helper lymphocytes in chronic obstructive pulmonary disease. Acta Biochim Biophys Sin. (2022) 54:173–8. doi: 10.3724/abbs.2021009
78. Thompson A and Orr SJ. Emerging IL-12 family cytokines in the fight against fungal infections. Cytokine. (2018) 111:398–407. doi: 10.1016/j.cyto.2018.05.019
79. Luo RG, Wu YF, Lu HW, Weng D, Xu JY, Wang LL, et al. Th2-skewed peripheral T-helper cells drive B-cells in allergic bronchopulmonary aspergillosis. Eur Respir J. (2024) 63:2400386. doi: 10.1183/13993003.00386-2024
80. Yun JH, Lee S, Srinivasa P, Morrow J, Chase R, Saferali A, et al. An interferon-inducible signature of airway disease from blood gene expression profiling. Eur Respir J. (2022) 59:2100569. doi: 10.1183/13993003.00569-2021
81. Bafadhel M, McKenna S, Agbetile J, Fairs A, Desai D, Mistry V, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J. (2014) 43:64–71. doi: 10.1183/09031936.00162912
82. García-Domínguez M. The role of IL-23 in the development of inflammatory diseases. Biology. (2025) 14:347. doi: 10.3390/biology14040347
83. Gringhuis SI, Kaptein TM, Remmerswaal EBM, Drewniak A, Wevers BA, Theelen B, et al. Fungal sensing by dectin-1 directs the non-pathogenic polarization of T(H)17 cells through balanced type I IFN responses in human DCs. Nat Immunol. (2022) 23:1735–48. doi: 10.1038/s41590-022-01348-2
84. Paroli M, Caccavale R, Fiorillo MT, Spadea L, Gumina S, Candela V, et al. The double game played by Th17 cells in infection: host defense and immunopathology. Pathog (Basel Switzerland). (2022) 11:1547. doi: 10.3390/pathogens11121547
85. Thomas R, Qiao S, and Yang X. Th17/Treg imbalance: implications in lung inflammatory diseases. Int J Mol Sci. (2023) 24:4865. doi: 10.3390/ijms24054865
86. Lourenço JD, Ito JT, Martins MA, Tibério I, and Lopes F. Th17/Treg imbalance in chronic obstructive pulmonary disease: clinical and experimental evidence. Front Immunol. (2021) 12:804919. doi: 10.3389/fimmu.2021.804919
87. Geng WR, He HY, Zhang Q, and Tong ZH. Th17 cells are involved in mouse chronic obstructive pulmonary disease complicated with invasive pulmonary aspergillosis. Chin Med J. (2020) 134:555–63. doi: 10.1097/cm9.0000000000001183
88. Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, et al. Dectin-1 diversifies aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. (2011) 208:369–81. doi: 10.1084/jem.20100906
89. Chang Y, Al-Alwan L, Alshakfa S, Audusseau S, Mogas AK, Chouiali F, et al. Upregulation of IL-17A/F from human lung tissue explants with cigarette smoke exposure: implications for COPD. Respir Res. (2014) 15:145. doi: 10.1186/s12931-014-0145-7
90. Vignali DA, Collison LW, and Workman CJ. How regulatory T cells work. Nat Rev Immunol. (2008) 8:523–32. doi: 10.1038/nri2343
91. Bacher P, Kniemeyer O, Schönbrunn A, Sawitzki B, Assenmacher M, Rietschel E, et al. Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol. (2014) 7:916–28. doi: 10.1038/mi.2013.107
92. Dewi IMW, van de Veerdonk FL, and Gresnigt MS. The multifaceted role of T-helper responses in host defense against aspergillus fumigatus. J fungi (Basel Switzerland). (2017) 3:55. doi: 10.3390/jof3040055
93. Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, et al. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2014) 190:40–50. doi: 10.1164/rccm.201312-2293OC
94. He Q, Cao J, Zhang M, and Feng C. IL-17 in plasma and bronchoalveolar lavage fluid in non-neutropenic patients with invasive pulmonary aspergillosis. Front Cell infection Microbiol. (2024) 14:1402888. doi: 10.3389/fcimb.2024.1402888
95. Hua Z and Hou B. The role of B cell antigen presentation in the initiation of cd4+ T cell response. Immunol Rev. (2020) 296:24–35. doi: 10.1111/imr.12859
96. Kitano T, Horii M, Kudo K, Nishio J, Fujii K, Fushida N, et al. Disrupted B-cell cytokine homeostasis in psoriasis: the impact of elevated IL-6 and impaired IL-10 production. J Dermatol. (2025) 52:1297–303. doi: 10.1111/1346-8138.17804
97. Jacobs M, Verschraegen S, Salhi B, Anckaert J, Mestdagh P, Brusselle GG, et al. IL-10 producing regulatory B cells are decreased in blood from smokers and copd patients. Respir Res. (2022) 23:287. doi: 10.1186/s12931-022-02208-1
98. Cramer RA, Rivera A, and Hohl TM. Immune responses against aspergillus fumigatus: what have we learned? Curr Opin Infect Dis. (2011) 24:315–22. doi: 10.1097/QCO.0b013e328348b159
99. Tashiro M, Takazono T, and Izumikawa K. Chronic pulmonary aspergillosis: comprehensive insights into epidemiology, treatment, and unresolved challenges. Ther Adv Infect Dis. (2024) 11:20499361241253751. doi: 10.1177/20499361241253751
100. Qian H, Xu JY, Fan R, Shi J, Lu HW, Ye L, et al. High level of initial aspergillus fumigatus-specific IgE links increased risk of exacerbation in allergic bronchopulmonary aspergillosis patients. Respir Res. (2025) 26:95. doi: 10.1186/s12931-025-03171-3
101. Zhang XH, Zhou C, Luo YM, Ge HQ, Liu HG, Wei HL, et al. Clinical features and related factors of invasive pulmonary aspergillosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Zhonghua yi xue za zhi. (2023) 103:1692–9. doi: 10.3760/cma.j.cn112137-20221106-02333
102. van de Veerdonk FL, Brüggemann RJM, Vos S, De Hertogh G, Wauters J, Reijers MHE, et al. Covid-19-associated aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med. (2021) 9:795–802. doi: 10.1016/s2213-2600(21)00138-7
103. Ledoux MP and Herbrecht R. Invasive pulmonary aspergillosis. J fungi (Basel Switzerland). (2023) 9:131. doi: 10.3390/jof9020131
104. Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol infection: Off Publ Eur Soc Clin Microbiol Infect Dis. (2018) 24 Suppl 1:e1–e38. doi: 10.1016/j.cmi.2018.01.002
105. He H, Ding L, Sun B, Li F, and Zhan Q. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: A prospective study. Crit Care (London England). (2012) 16:R138. doi: 10.1186/cc11443
106. He H, Li Q, Chang S, Ding L, Sun B, Li F, et al. Prognostic value of serum galactomannan index in critically ill patients with chronic obstructive pulmonary disease at risk of invasive pulmonary aspergillosis. Chin Med J. (2014) 127:23–8. doi: 10.3760/cma.j.issn.0366-6999.20131651
107. Bongomin F, Harris C, Foden P, Kosmidis C, and Denning DW. Innate and adaptive immune defects in chronic pulmonary aspergillosis. J fungi (Basel Switzerland). (2017) 3:26. doi: 10.3390/jof3020026
108. Mathioudakis AG, Janssens W, Sivapalan P, Singanayagam A, Dransfield MT, Jensen JS, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax. (2020) 75:520–7. doi: 10.1136/thoraxjnl-2019-214484
109. Wedzicha JAEC-C, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: A european respiratory society/american thoracic society guideline. Eur Respir J. (2017) 49:1600791. doi: 10.1183/13993003.00791-2016
110. Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect diseases: an Off Publ Infect Dis Soc America. (2016) 63:e1–e60. doi: 10.1093/cid/ciw326
111. Fortún J, Martín-Dávila P, Gomez Garcia de la Pedrosa E, Silva JT, Garcia-Rodríguez J, Benito D, et al. Galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis in non-hematological patients. J infection. (2016) 72:738–44. doi: 10.1016/j.jinf.2016.02.019
112. Ren J, Chen W, Zhong Z, Wang N, Chen X, Yang H, et al. Bronchoalveolar lavage fluid from chronic obstructive pulmonary disease patients increases neutrophil chemotaxis measured by a microfluidic platform. Micromachines. (2023) 14:1740. doi: 10.3390/mi14091740
113. Salzer HJF, Prattes J, Flick H, Reimann M, Heyckendorf J, Kalsdorf B, et al. Evaluation of galactomannan testing, the aspergillus-specific lateral-flow device test and levels of cytokines in bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. Front Microbiol. (2018) 9:2223. doi: 10.3389/fmicb.2018.02223
114. Agarwal R, Aggarwal AN, Sehgal IS, Dhooria S, Behera D, and Chakrabarti A. Performance of serum galactomannan in patients with allergic bronchopulmonary aspergillosis. Mycoses. (2015) 58:408–12. doi: 10.1111/myc.12334
115. Yoshimura K, Suzuki Y, Inoue Y, Nishimoto K, Mori K, Karayama M, et al. Utility of serum aspergillus-galactomannan antigen to evaluate the risk of severe acute exacerbation in chronic obstructive pulmonary disease. PloS One. (2018) 13:e0198479. doi: 10.1371/journal.pone.0198479
116. Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J, et al. Use and limits of (1-3)-β-D-glucan assay (Fungitell), compared to galactomannan determination (Platelia aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol. (2014) 52:2328–33. doi: 10.1128/jcm.03567-13
117. Tutar N, Metan G, Koç AN, Yilmaz I, Bozkurt I, Simsek ZO, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Multidiscip Respir Med. (2013) 8:59. doi: 10.1186/2049-6958-8-59
118. Hsu AJ, Tamma PD, and Zhang SX. Challenges with utilizing the 1,3-beta-D-glucan and galactomannan assays to diagnose invasive mold infections in immunocompromised children. J Clin Microbiol. (2021) 59:e0327620. doi: 10.1128/jcm.03276-20
119. Gao X, Chen L, Hu G, and Mei H. Invasive pulmonary aspergillosis in acute exacerbation of chronic obstructive pulmonary disease and the diagnostic value of combined serological tests. Ann Saudi Med. (2010) 30:193–7. doi: 10.4103/0256-4947.62828
120. Thompson GR, King T, Azie N, Angulo DA, and Prattes J. 871. Oral ibrexafungerp outcomes by fungal disease in patients from an interim analysis of a phase 3 open-label study (FURI). Open Forum Infect Dis. (2022) 9:Supplement_2. doi: 10.1093/ofid/ofac492.064
121. Petraitis V, Petraitiene R, Katragkou A, Maung BBW, Naing E, Kavaliauskas P, et al. Combination therapy with ibrexafungerp (Formerly SCY-078), a first-in-class triterpenoid inhibitor of (1→3)-β-d-Glucan Synthesis, and isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrobial Agents chemotherapy. (2020) 64:e02429-19. doi: 10.1128/aac.02429-19
122. Lu Y, Zhong H, Wang Y, Sun C, Li Y, Cai Y, et al. Evaluation of a novel aspergillus IgG lateral flow assay for the diagnosis of non-neutropenic patients with acute and subacute invasive aspergillosis. Front Cell infection Microbiol. (2025) 15:1599425. doi: 10.3389/fcimb.2025.1599425
123. Kosmidis C, Smith H, Mollett G, Harris C, Akili S, and Bazaz R. Predictive factors for treatment response and mortality in chronic pulmonary aspergillosis. Mycoses. (2023) 66:960–8. doi: 10.1111/myc.13641
124. He Q, Li M, Cao J, Zhang M, and Feng C. Clinical characteristics and risk factors analysis of allergic bronchopulmonary aspergillosis combined with bronchiectasis. Front Med. (2025) 12:1557241. doi: 10.3389/fmed.2025.1557241
125. Tong X, Cheng A, Xu H, Jin J, Yang Y, Zhu S, et al. Aspergillus fumigatus during COPD exacerbation: A pair-matched retrospective study. BMC pulmonary Med. (2018) 18:55. doi: 10.1186/s12890-018-0611-y
126. Obar JJ, Hohl TM, and Cramer RA. New advances in invasive aspergillosis immunobiology leading the way towards personalized therapeutic approaches. Cytokine. (2016) 84:63–73. doi: 10.1016/j.cyto.2016.05.015
127. Ralph BA, Lehoux M, Ostapska H, Snarr BD, Caffrey-Carr AK, Fraser R, et al. The IL-1 receptor is required to maintain neutrophil viability and function during aspergillus fumigatus airway infection. Front Immunol. (2021) 12:675294. doi: 10.3389/fimmu.2021.675294
128. Huot-Marchand S, Nascimento M, Culerier E, Bourenane M, Savigny F, Panek C, et al. Cigarette smoke-induced gasdermin D activation in bronchoalveolar macrophages and bronchial epithelial cells dependently on nlrp3. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.918507
129. Huang SF, Huang CC, Chou KT, Chan YJ, Yang YY, and Wang FD. Chronic pulmonary aspergillosis: disease severity using image analysis and correlation with systemic proinflammation and predictors of clinical outcome. J fungi (Basel Switzerland). (2021) 7:842. doi: 10.3390/jof7100842
130. Zhan M, Xu B, Zhao L, Li B, Xu L, Sun Q, et al. The serum level of IL-1B correlates with the activity of chronic pulmonary aspergillosis. Can Respir J. (2018) 2018:8740491. doi: 10.1155/2018/8740491
131. Gresnigt MS, Rekiki A, Rasid O, Savers A, Jouvion G, Dannaoui E, et al. Reducing hypoxia and inflammation during invasive pulmonary aspergillosis by targeting the interleukin-1 receptor. Sci Rep. (2016) 6:26490. doi: 10.1038/srep26490
132. Liu F, Zhang X, Du W, Du J, Chi Y, Sun B, et al. Diagnosis values of IL-6 and IL-8 levels in serum and bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. J Invest Med. (2021) 69:1344–9. doi: 10.1136/jim-2021-001857
133. Ren W, Li H, Guo C, Shang Y, Wang W, Zhang X, et al. Serum cytokine biomarkers for use in diagnosing pulmonary tuberculosis versus chronic pulmonary aspergillosis. Infection Drug resistance. (2023) 16:2217–26. doi: 10.2147/idr.S403401
134. Witting C, Quaggin-Smith J, Mylvaganam R, Peigh G, Angarone M, and Flaherty JD. Invasive pulmonary aspergillosis after treatment with tocilizumab in a patient with COVID-19 ARDS: A case report. Diagn Microbiol Infect Dis. (2021) 99:115272. doi: 10.1016/j.diagmicrobio.2020.115272
135. Fermont JM, Masconi KL, Jensen MT, Ferrari R, Di Lorenzo VAP, Marott JM, et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax. (2019) 74:439–46. doi: 10.1136/thoraxjnl-2018-211855
136. Mudrakola HV, Tandon YK, DeMartino E, Tosh PK, Yi ES, and Ryu JH. Autopsy study of fatal invasive pulmonary aspergillosis: often undiagnosed premortem. Respir Med. (2022) 199:106882. doi: 10.1016/j.rmed.2022.106882
137. Cherny SS, Brzezinski RY, Wasserman A, Adler A, Berliner S, Nevo D, et al. Characterizing CRP dynamics during acute infections. Infection. (2025) 53:1199–203. doi: 10.1007/s15010-024-02422-7
138. Massimino AM, Colella FE, Bottazzi B, and Inforzato A. Structural insights into the biological functions of the long pentraxin PTX3. Front Immunol. (2023) 14:1274634. doi: 10.3389/fimmu.2023.1274634
139. Ma L, Li D, Wen Y, and Shi D. Advances in understanding the role of pentraxin-3 in lung infections. Front Immunol. (2025) 16:1575968. doi: 10.3389/fimmu.2025.1575968
140. Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, et al. Anti-aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrobial Agents chemotherapy. (2004) 48:4414–21. doi: 10.1128/aac.48.11.4414-4421.2004
141. Buchheit KM, Shaw D, Chupp G, Lehtimaki L, Heffler E, Finney-Hayward T, et al. Interleukin-5 as a pleiotropic cytokine orchestrating airway type 2 inflammation: effects on and beyond eosinophils. Allergy. (2024) 79:2662–79. doi: 10.1111/all.16303
142. Zhang C, Wang Y, Zhang M, Su X, Lei T, Yu H, et al. Monoclonal antibodies targeting IL-5 or IL-5Rα in eosinophilic chronic obstructive pulmonary disease: A systematic review and meta-analysis. Front Pharmacol. (2021) 12:754268. doi: 10.3389/fphar.2021.754268
143. Day de Larrañaga F, Joubert A, Drouin I, Ouellet I, Li PZ, Ross BA, et al. Biological anti-IL-5/IL-5R therapeutics for chronic obstructive pulmonary disease (COPD) with specific treatable traits: A real-world retrospective analysis. Int J chronic obstructive pulmonary Dis. (2024) 19:217–23. doi: 10.2147/copd.S438153
144. Agarwal R, Muthu V, and Sehgal IS. New insights into the treatment of asthma complicated by allergic bronchopulmonary aspergillosis. Expert Rev Respir Med. (2025) 19:967–79. doi: 10.1080/17476348.2025.2517302
145. Tomomatsu K, Yasuba H, Ishiguro T, Imokawa S, Hara J, Soeda S, et al. Real-world efficacy of anti-IL-5 treatment in patients with allergic bronchopulmonary aspergillosis. Sci Rep. (2023) 13:5468. doi: 10.1038/s41598-023-32246-8
146. Catherine J and Roufosse F. What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin immunopathology. (2021) 43:439–58. doi: 10.1007/s00281-021-00857-w
147. Kozlova Y, Frolova E, Uchevatkina A, Filippova L, Aak O, Burygina E, et al. Diagnostic markers of allergic bronchopulmonary aspergillosis in patients with severe asthma. Mycoses. (2020) 63:596–603. doi: 10.1111/myc.13083
148. Machida H, Inoue S, Shibata Y, Kimura T, Sato K, Abe K, et al. Thymus and activation-regulated chemokine (TARC/CCL17) predicts decline of pulmonary function in patients with chronic obstructive pulmonary disease. Allergology Int. (2021) 70:81–8. doi: 10.1016/j.alit.2020.04.004
149. Yu ZX, Ji MS, Yan J, Cai Y, Liu J, Yang HF, et al. The ratio of Th17/treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care (London England). (2015) 19:82. doi: 10.1186/s13054-015-0811-2
150. Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against candida albicans. Cell. (2019) 176:1340–55.e15. doi: 10.1016/j.cell.2019.01.041
151. Dalgård C, Wang F, Titlestad IL, Kyvik KO, Vestbo J, and Sorensen GL. Increased serum SP-D in identification of high-risk smokers at high risk of COPD. Am J Physiol Lung Cell Mol Physiol. (2021) 320:L1005–l10. doi: 10.1152/ajplung.00604.2020
152. Liao JP, Wang X, Liu F, Cheng Y, Hu ZW, Zhang LN, et al. Lung function decline, and incident chronic obstructive pulmonary disease: A longitudinal study in beijing. J Thorac Dis. (2021) 13:92–100. doi: 10.21037/jtd-20-1675
153. Krane M and Griese M. Surfactant protein D in serum from patients with allergic bronchopulmonary aspergillosis. Eur Respir J. (2003) 22:592–5. doi: 10.1183/09031936.03.00060603
154. Madan T, Kishore U, Singh M, Strong P, Hussain EM, Reid KB, et al. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infection Immun. (2001) 69:2728–31. doi: 10.1128/iai.69.4.2728-2731.2001
155. Colombo SAP, Hashad R, Denning DW, Kumararatne DS, Ceron-Gutierrez L, Barcenas-Morales G, et al. Defective interferon-gamma production is common in chronic pulmonary aspergillosis. J Infect Dis. (2022) 225:1822–31. doi: 10.1093/infdis/jiab583
156. Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect diseases: an Off Publ Infect Dis Soc America. (2016) 63:433–42. doi: 10.1093/cid/ciw444
157. Russo A, Morrone HL, Rotundo S, Trecarichi EM, and Torti C. Cytokine profile of invasive pulmonary aspergillosis in severe covid-19 and possible therapeutic targets. Diagnostics (Basel). (2022) 12:1364. doi: 10.3390/diagnostics12061364
158. Dai Z, Zhao H, Cai S, Lv Y, and Tong W. Invasive pulmonary aspergillosis in non-neutropenic patients with and without underlying disease: A single-centre retrospective analysis of 52 subjects. Respirology (Carlton Vic). (2013) 18:323–31. doi: 10.1111/j.1440-1843.2012.02283.x
159. Woods JA, Wheeler JS, Finch CK, and Pinner NA. Corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Int J chronic obstructive pulmonary Dis. (2014) 9:421–30. doi: 10.2147/copd.S51012
160. Sengupta A, Ray A, Upadhyay AD, Izumikawa K, Tashiro M, Kimura Y, et al. Mortality in chronic pulmonary aspergillosis: A systematic review and individual patient data meta-analysis. Lancet Infect Dis. (2025) 25:312–24. doi: 10.1016/s1473-3099(24)00567-x
161. Lee JSF, Cohen RM, Khan RA, Burry J, Casas EC, Chung HY, et al. Paving the way for affordable and equitable liposomal amphotericin B access worldwide. Lancet Global Health. (2024) 12:e1552–e9. doi: 10.1016/s2214-109x(24)00225-0
162. Dladla M, Gyzenhout M, Marias G, and Ghosh S. Azole resistance in aspergillus fumigatus- comprehensive review. Arch Microbiol. (2024) 206:305. doi: 10.1007/s00203-024-04026-z
163. Sulahian A, Touratier S, and Ribaud P. False positive test for aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. New Engl J Med. (2003) 349:2366–7. doi: 10.1056/nejm200312113492424
164. Ader F, Bienvenu AL, Rammaert B, and Nseir S. Management of invasive aspergillosis in patients with copd: rational use of voriconazole. Int J chronic obstructive pulmonary Dis. (2009) 4:279–87. doi: 10.2147/copd.s4229
165. Wang X, Lu Y, Sun C, Zhong H, Cai Y, Cao M, et al. Development and validation of a machine learning-based diagnostic model for identifying nonneutropenic invasive pulmonary aspergillosis in suspected patients: A multicenter cohort study. Microbiol Spectr. (2025) 13:e0060725. doi: 10.1128/spectrum.00607-25
Keywords: chronic obstructive pulmonary disease, pulmonary aspergillosis, immune biomarkers, galactomannan, diagnostic algorithm
Citation: Hu Y, Wang X, Wu H, Deng H, Gao F and Wu Y (2025) Immune markers for pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: a narrative review. Front. Immunol. 16:1687790. doi: 10.3389/fimmu.2025.1687790
Received: 18 August 2025; Accepted: 08 October 2025;
Published: 23 October 2025.
Edited by:
Hector Mora Montes, University of Guanajuato, MexicoReviewed by:
Mona Ghazanfari, Mazandaran University of Medical Sciences, IranZhiwei Long, Guangzhou Medical University, China
Meiliyana Wijaya, Atma Jaya Catholic University of Indonesia, Indonesia
Copyright © 2025 Hu, Wang, Wu, Deng, Gao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Wu, MzE4NjcyMjIwQHFxLmNvbQ==; Fei Gao, ZmVpZ2FvMjA3QHllYWgubmV0
†These authors have contributed equally to this work
 Yuke Hu
Yuke Hu Xingbo Wang
Xingbo Wang Hao Wu
Hao Wu Haibo Deng2
Haibo Deng2 Fei Gao
Fei Gao Yonghong Wu
Yonghong Wu