- 1Department of Immunology, Tufts University School of Medicine, Boston, MA, United States
- 2Division of Infectious Diseases and Immunology, University of Massachusetts Chan Medical School, Worcester, MA, United States
Background: Neisseria gonorrhoeae, causative agent of the human sexually transmitted infection gonorrhea, is a significant global health concern because of increasing antimicrobial resistance and the lack of an effective vaccine. Recent ecological analyses have shown a reduced incidence of gonorrhea in recipients of detergent-extracted outer membrane vesicle (OMV)-containing meningococcal vaccine, which has contributed to identification of shared, protective antigens. Previously, our group has developed an immunobioinformatics-based pipeline (CASS, Candidate Antigen Selection Strategy) for identification of gonococcal hypothetical proteins expressed during human natural mucosal infections, as novel vaccine candidates.
Methods: In this study, we expanded the immunological characterization of three targets, NGO0690, NGO0948 and Csp (copper storage protein, previously called NGO1701) to include analysis of their efficacy in a mouse model of gonococcal vaginal infection when combined as a trivalent subunit vaccine and adjuvanted with Alum and MPLA.
Results: We reported induction of systemic and mucosal antibody responses, serum bactericidal activity against heterologous N. gonorrhoeae strains, and accelerated bacterial clearance in vivo. Immune profiling revealed a balanced Th1/Th2 response, based on IgG antibody subclasses and cytokines. Antigen dose de-escalation experiments in female and male mice showed sustained antibody production against the individual antigens and against whole bacteria. The latter were slightly lower than with the original dose vaccine particularly in male mice, who also exhibited a distinct cytokine pattern and weaker complement-mediated serum bactericidal activity (SBA) titers compared to female mice. These findings underscore the importance of considering sex-based differences in vaccine evaluation. A bivalent vaccine containing only NGO0690 and Csp was still protective in vivo, supporting the value of multivalent approaches to address gonococcal antigenic diversity.
Discussion: Overall, our results suggested that the rational design of our multi-antigen subunit vaccines holds translational potential for enhancing broadly protective immune responses and protection against N. gonorrhoeae.
Introduction
Neisseria gonorrhoeae is an obligate human pathogen and the causative agent of gonorrhea, a sexually transmitted infection (STI) with an estimated global burden exceeding 82 million cases annually, and in the United States alone, over 600,000 cases were reported in 2023 (1). In men, gonococcal urethral infections are generally symptomatic, but infection in women is frequently asymptomatic; this lack of symptoms can lead to a delayed diagnosis and treatment, with serious reproductive complications including pelvic inflammatory disease, ectopic pregnancy, and infertility (2). Rectal and pharyngeal infections in men who have sex with men (MSM) are also frequently asymptomatic. Unfortunately, natural gonococcal infection induces limited and strain-specific immunity, with scarce memory responses even after repeated exposure (3). In some instances, disseminated gonococcal infection (DGI) can also occur. Concurrent chlamydia infection increases the burden of gonococcal infection (4, 5) and gonorrhea is associated with enhanced transmission of human immunodeficiency virus 1 (HIV-1) infection (6, 7). Furthermore, widespread and increasing antimicrobial resistance (AMR) has been reported for gonorrhea in the last decade. Resistance has emerged even to extended-spectrum cephalosporins, the last FDA-recommended first-line treatment, raising fears of untreatable gonorrhea (8–10). While efforts to identify new treatment options are ongoing, developing gonococcal vaccines is essential for prevention of disease transmission (11).
Vaccine development for gonorrhea has been hampered by antigenic variation, immune evasion, and absence of natural immunity. Earlier approaches with killed whole–cell, pilin or porin subunit, and outer membrane (OM) protein vaccines failed due to genetic variability, and conferred only short-term or strain-specific protection [reviewed in (12)]. A significant breakthrough emerged from retrospective epidemiologic studies indicating that detergent-extracted meningococcal outer membrane vesicle (OMV)-based vaccines (MeNZB and 4CMenB (Bexsero®)) conferred partial protection against Neisseria gonorrhoeae (13). 4CMenB generated bactericidal antibodies and significantly accelerated clearance of N. gonorrhoeae in a mouse model of gonococcal vaginal colonization (14–17). While serum bactericidal activity (SBA) is an established correlate of protective immunity for N. meningitidis (18), the correlates of protection against gonorrhea have not been established, but SBA is accepted as in vitro surrogate assay in preclinical gonococcal vaccine studies (19). Building on these observations, multiple clinical trials are underway to evaluate the efficacy of 4CMenB against gonococcal infection, and to identify relevant correlates of protection (20–22). N. meningitidis and N. gonorrhoeae share multiple conserved outer membrane proteins (80–90% genome homology) (23–26), which is likely the cause of the observed cross-protective responses. Gonococcal native OMVs formulated with slow-release IL-12 microspheres and administered either intranasally or intravaginally elicited Th1 responses, suppressed non-protective Th17 pathways, and accelerated clearance of gonococci from mouse vaginas (27, 28). Importantly, protection was abrogated in B cell-deficient mice, underscoring the role of antibodies in vaccine-induced immunity.
Meanwhile, individual gonococcal components also have shown promise in the mouse model and provide an opportunity for developing a subunit vaccine. For example, lipooligosaccharide (LOS) and peptide mimics of conserved LOS epitopes (29, 30), and surface-exposed proteins such as PorB (25), NHBA, MetQ (31) or TbpA/TbpB (32) are a few examples of vaccine targets that have been explored in preclinical studies with various adjuvants (12). Subunit vaccine candidates have shown to be immunogenic in mice, to induce antibodies with bactericidal and/or opsonophagocytic activity, and, in some instances (e.g., MetQ, TbpB, and the LOS mimotope), protection against N. gonorrhoeae in a mouse model of gonococcal vaginal challenge. However, these antigens have been identified by growing N. gonorrhoeae in vitro in conditions that may not replicate protein expression levels during natural infection in humans. Our group has utilized a novel immunobioinformatics-based pipeline called CASS (Candidate Antigen Selection Strategy) and identified hypothetical proteins that are expressed during natural mucosal infection in men and women and could represent new vaccine antigens (33–35). Thus far, from a pool of 36 gonococcal targets, three antigens have undergone a detailed immunological characterization - NGO0690, NGO0948 (homolog of BamC) and Csp [previously referred to as NGO1701 (36)], either individually (33) or as a combination vaccine with different adjuvants (37). We reported robust immune responses in mice, with serum bactericidal activity against several N. gonorrhoeae strains, and recognition of these antigens by sera from human subjects with acute gonococcal infection as well as convalescent subjects (37), supporting the translational potential of these candidates for preclinical studies. Building on these results, and with the premise that a multi-antigen vaccine platform would elicit a broader epitope coverage and more durable response compared to a single antigen vaccine (38–43), we evaluated the efficacy of a NGO0690+NGO0948+Csp combination vaccine with Alum+MPLA as adjuvants (3-Ag vaccine) in vivo in a female mouse model of gonococcal vaginal colonization. A multi-antigen approach could also help to address the antigenic variability and overcome immune evasion strategies that have undermined previous gonococcal subunit vaccine attempts (44). In addition, the effect of antigen dose de-escalation was examined on the magnitude of immune responses in both female and male mice, as well as the ability of a simplified two-antigen combination that only included NGO0690 and Csp (2-Ag vaccine), on protection in vivo in the mouse model of gonococcal challenge. Our results showed that immunization with the 3-Ag vaccine led to protection against N. gonorrhoeae in vivo. Lowering the antigen concentration had a small effect on antibody production in female mice, accompanied by a slight decrease in the serum bactericidal activity titers, while these effects were more pronounced in male mice. Protection was also demonstrated using the 2-Ag vaccine, indicating that excluding the structurally more complex and low immunogenic antigen NGO0948, could still afford protective immune responses in vivo. Studies investigating other CASS antigen are currently ongoing to identify additional candidates that could potentiate a multi-antigen vaccine combination, as well as revisiting adjuvants, doses and/or routes of immunization.
Materials and methods
Antigens
Recombinant NGO0690, NGO0948 (BamC) and Csp were expressed and purified as previously described (33).
Immunization of mice
Female and male BALB/c mice (4–6 weeks old) (Jackson Labs, Bar Harbor, ME, USA) were housed, cared for and immunized according to NIH, Tufts University (Protocol number B2024-11) and University of Massachusetts Chan Medical School IACUC (Protocol number 202000074) approved protocols. Groups of mice (n=20) were immunized subcutaneously three times at 2-weeks apart with purified recombinant NGO0690+NGO0948+Csp (10 µg each) combined and adjuvanted with Alum (Imject) (Thermo Fisher Scientific; 1:1 v/v ratio with the antigen mixture) + Monophosphoryl Lipid A (MPLA) (Avanti Lipids; 10 µg/mouse/dose) (37) (referred to as 3-Ag vaccine in the text), or with NGO0690 and Csp (10 µg each) combined and Alum+MPLA as above (referred to as 2-Ag vaccine in the text). Control groups of mice (n = 20) were immunized with Alum+MPLA alone (referred to as Adjuvant in the text). Additional groups of female and male BALB/c mice (n = 5) were immunized with a low-dose 3-Ag vaccine containing 5 µg of each antigen and with Alum+MPLA as above. For all experiments, pre-immune (Pr) sera were collected before immunization, and immune sera two weeks after each immunization (referred to as 1st, 2nd, 3rd in the Figure legends). An additional aliquot of serum was collected four weeks after the last immunization and prior to infection (pre-challenge, p-c) from the mice undergoing gonococcal challenge described below. Vaginal lavages were collected two weeks after the last immunization. All sera and lavages were stored at -80°C until use.
Bacterial strains and growth conditions
N. gonorrhoeae strains F62 (Pil+/Opa+) and FA1090 were stored as frozen glycerol stocks. Bacteria were grown on GC base agar plates containing 1% (v/v) IsoVitaleX or on chocolate agar at 37°C in a 5% CO2 incubator, and in liquid GC broth (GCB) with 1% IsoVitaleX (33). Growth was monitored spectrophotometrically at O.D.600nm. For some experiments, aliquots of bacteria suspension at O.D.600nm > 1 were formalin-killed by incubation with 1% paraformaldehyde for 1h at 4°C, washed and resuspended in PBS.
Mouse model of gonococcal vaginal challenge
At the end of the immunization schedule, female mice were evaluated to select those in the diestrus phase of the estrous cycle. Groups of 10 mice each were treated with 0.5 mg Premarin (Pfizer) in 200 µL water subcutaneously for 3 days (day −2, 0, and +2 days relative to the challenge (Day 0) to allow for a longer estrus phase and to increase susceptibility to N. gonorrhoeae infection as previously described (45). Antibiotics (vancomycin, trimethoprim, and streptomycin, (VTS)) were used to control the mouse vaginal microflora without affecting N. gonorrhoeae survival. Mice were challenged intravaginally with N. gonorrhoeae FA1090 (2.7 x 107 – 3.1 x 107 colony forming units, CFUs) and infection burden was monitored daily by obtaining vaginal swabs, eluting the content of each swab in 100 µl of normal saline, and plating of serial dilutions of the eluted material onto chocolate agar containing VTS, colistin and neomycin, and counting CFUs after incubation of plates for 24 h at 37°C in 5% CO2.
Mouse antibody ELISA
ELISA plates were coated with purified recombinant NGO0690, NGO0948 or Csp proteins (2 μg/ml) or with formalin-fixed N. gonorrhoeae (1–1.5 x 108 bacteria/ml) as previously described (37). Serial dilutions of pooled mouse sera or vaginal lavages were added to measure total IgG, IgG1, IgG2a, IgM or IgA antibodies using AP-conjugated secondary anti-mouse antibodies (Southern Biotech) followed by 1-step PNPP (p-nitrophenyl phosphate) reagent (ThermoFisher Scientific) and spectrophotometric detection at O.D.405nm. IgG, IgG1, IgG2a and IgM were quantified in µg/ml ± SD using antibody reference standard curves (Southern Biotech) and a linear regression function (33). IgA (O.D.405nm values minus the blank) were normalized to the adjuvant alone and expressed as fold-change ± SD. The Th1:Th2 ratio was calculated as IgG2a/IgG1. Sera and vaginal lavages were evaluated in triplicate or quadruplicate.
Cytokine ELISA
Th1-type cytokines IL-12p70 and IFN-γ, Th2-type cytokines IL-4 and IL-10 and IL-6, TNF-α and IL-1β were measured in pooled mouse sera by ELISA using Opt-EIA kits (BD Biosciences, San Jose, CA, USA) or Invitrogen kit (ThermoFisher Scientific) per the manufacturers’ specifications. Cytokines were expressed in pg/ml ± SD.
Serum bactericidal activity
SBA was conducted as previously described (37). Briefly, N. gonorrhoeae F62 and FA1090 cultures (2–4 x 104 CFU/ml) were incubated in HBSS with 0.15 mM CaCl2 and 1 mM MgCl2 and 2% BSA for 20 minutes at room temperature with serial dilutions of heat-inactivated mouse sera. Commercially available IgG/IgM-depleted pooled normal human serum (Pel-Freez Biologicals) at a final concentration of 10% (v/v) was added as source of complement. Aliquots of the reaction mixture were plated immediately on GC agar plates in triplicate (Time 0) and after 30 minutes incubation at 37°C (Time 30). Plates were incubated at 37°C in 5% CO2 overnight, and survival was determined the CFUs at T30 relative to T0 and expressed as percentage ± SD. Bactericidal titers represent the lowest serum dilution that yielded ≤ 50% survival after 30 minutes. Controls reactions included bacteria alone and bacteria incubated with complement alone.
Statistical analysis
GraphPad Prism 10 (GraphPad Software, Inc., San Diego, CA) was used to determine statistical significance using unpaired t test and one-way analyses of variance (ANOVA) with Tukey’s multiple comparisons test or with Dunnett’s test. Statistically significant p values are indicated in the figure legends. For mouse challenge experiments, the median time to clearance was evaluated using Kaplan–Meier survival curves, the times to clearance were compared between groups using the Mantel-Cox log-rank test. The mean area under the curve (AUC) of the log10 CFU vs. time was computed for each mouse to estimate the bacterial burden over time (cumulative infection) and comparisons between groups were made using Mann-Whitney’s non-parametric test (29).
Results
Immunization with NGO0690+NGO0948+Csp and Alum+MPLA as adjuvants (3-Ag vaccine) protects mice from gonococcal vaginal colonization
We previously characterized immune responses elicited in mice by NGO0690, NGO0948 and Csp [NGO1701 (36)] as individual vaccine targets or in combination, with different adjuvants (33, 37). Here, we evaluated their efficacy as a combination vaccine with Alum+MPLA as adjuvants (3-Ag vaccine) in a mouse model of gonococcal vaginal challenge. Female BALB/c mice were immunized with the 3-Ag vaccine or with Alum+MPLA alone as a control, followed by intravaginal infection with N. gonorrhoeae strain FA1090 four weeks after the last immunization. Vaginal swabs were collected daily and plated to enumerate gonococcal CFUs. A significant reduction in the time to bacterial clearance was observed in the 3-Ag vaccine group compared to the adjuvant alone group, shown by the Kaplan–Meier curves (Figure 1A, open squares and black circles, respectively). A significant decrease in the number of bacteria recovered was also observed (Figure 1B), reflected in the area under the curve (AUC) analysis (Figure 1C). These results indicated that the 3-Ag vaccine was protective in a female mouse model of gonococcal colonization.
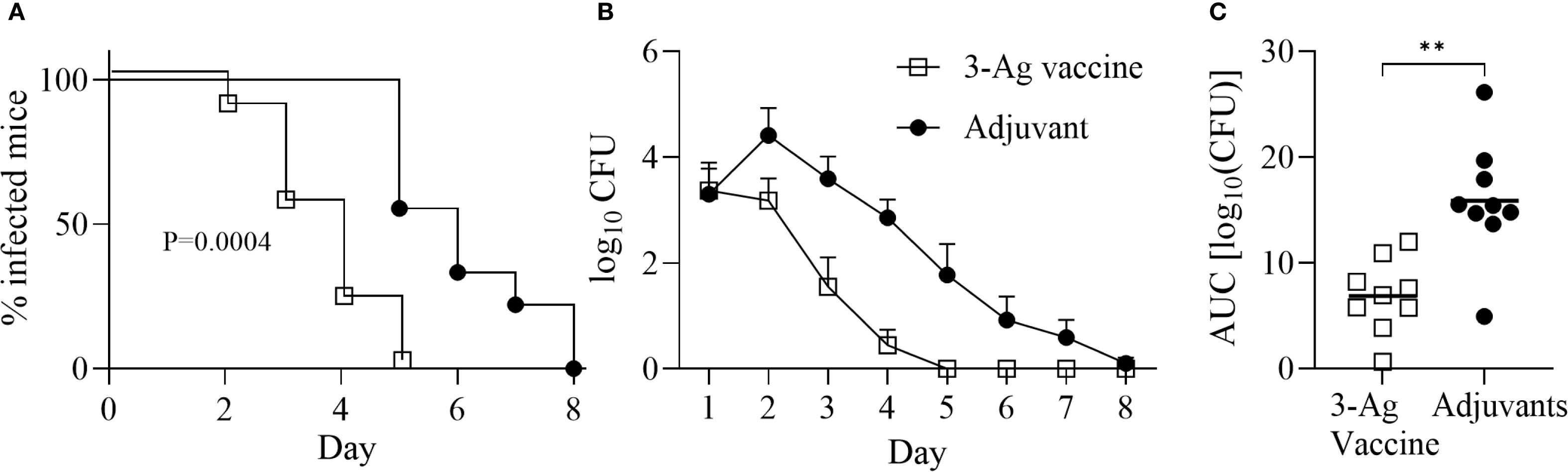
Figure 1. Immunization with NGO0690+NGO0948+Csp and Alum+MPLA (3-Ag vaccine) is protective in a mouse model of gonococcal vaginal colonization. Female BALB/c mice (n = 20 per group) were immunized with the 3-Ag vaccine (10 µg each antigen) (open squares) or with Alum+MPLA alone (black circles). Two weeks after the third immunization, mice in the diestrus phase of the estrous cycle (n = 10 per group) were challenged intravaginally with N. gonorrhoeae FA1090 (2.6 × 107 CFU). Vaginal swabs were collected daily and plated to enumerate gonococcal CFUs. (A) Time-to-clearance Kaplan–Meier curves (p = 0,0004, groups compared by Mantel-Cox analysis); (B) Bacterial burden (log10 CFU ± SEM); (C) Area under the curve (AUC) (means ± 95% confidence intervals compared across groups by Mann Whitney’s non-parametric test, **p = 0.0019).
Qualitative, quantitative, and functional antibody responses induced by the 3-Ag vaccine
In the current study, mice were immunized with the 3-Ag vaccine three times following a 2-weeks apart schedule, which is different from the prior 3-weeks apart schedule used to characterize the serum antibody responses (37); thus, it was important to evaluate antibody production. IgG levels induced by the 3-Ag vaccine were examined by ELISA against the individual purified antigens or whole N. gonorrhoeae using pooled sera aliquots collected throughout the immunization schedule and prior to the challenge. Higher total IgG antibody levels were detected against NGO0690 and Csp than against NGO0948 (Figures 2A-C, open circles) consistent with our previous results (37), and these remained elevated prior to the gonococcal challenge (4 weeks after the third immunization, p-c); a small increase in IgG antibodies was also observed in response to immunization with Alum+MPLA alone (Figures 2A-C, closed circles). Whole cell ELISA against N. gonorrhoeae FA1090 (the strain used in the challenge model) and of N. gonorrhoeae F62 (as an additional strain) showed a robust and similar serum IgG antibody response against both strains (Figures 2D, E). Analysis of the IgG subclasses confirmed a balanced Th1-Th2 response with a slight Th2 skew (Supplementary Table S1) [an IgG2a/IgG1 ratio > 2 indicates a Th1 response, a ratio < 0.5 a Th2-biased response, and between 0.5 and 2, a mixed response (46)]; IgM antibody levels against the antigens and both gonococcal strains (not shown) were also consistent with our previous results (37), also showing presence of non-specific, cross-reactive IgM antibodies in the preimmune sera and in the adjuvant-only group sera. Low serum IgA antibody levels were measured against N. gonorrhoeae, although these were about 2-fold higher than with the adjuvant alone (Supplementary Figure S1A, dashed bars). However, evaluation of mucosal antibodies showed a 2- to 6-fold increase in secreted IgA over the adjuvant alone group (Supplementary Figure S1A, dotted bars) in the vaginal lavages and confirmed induction of IgG antibodies against N. gonorrhoeae (Supplementary Figure S2A).
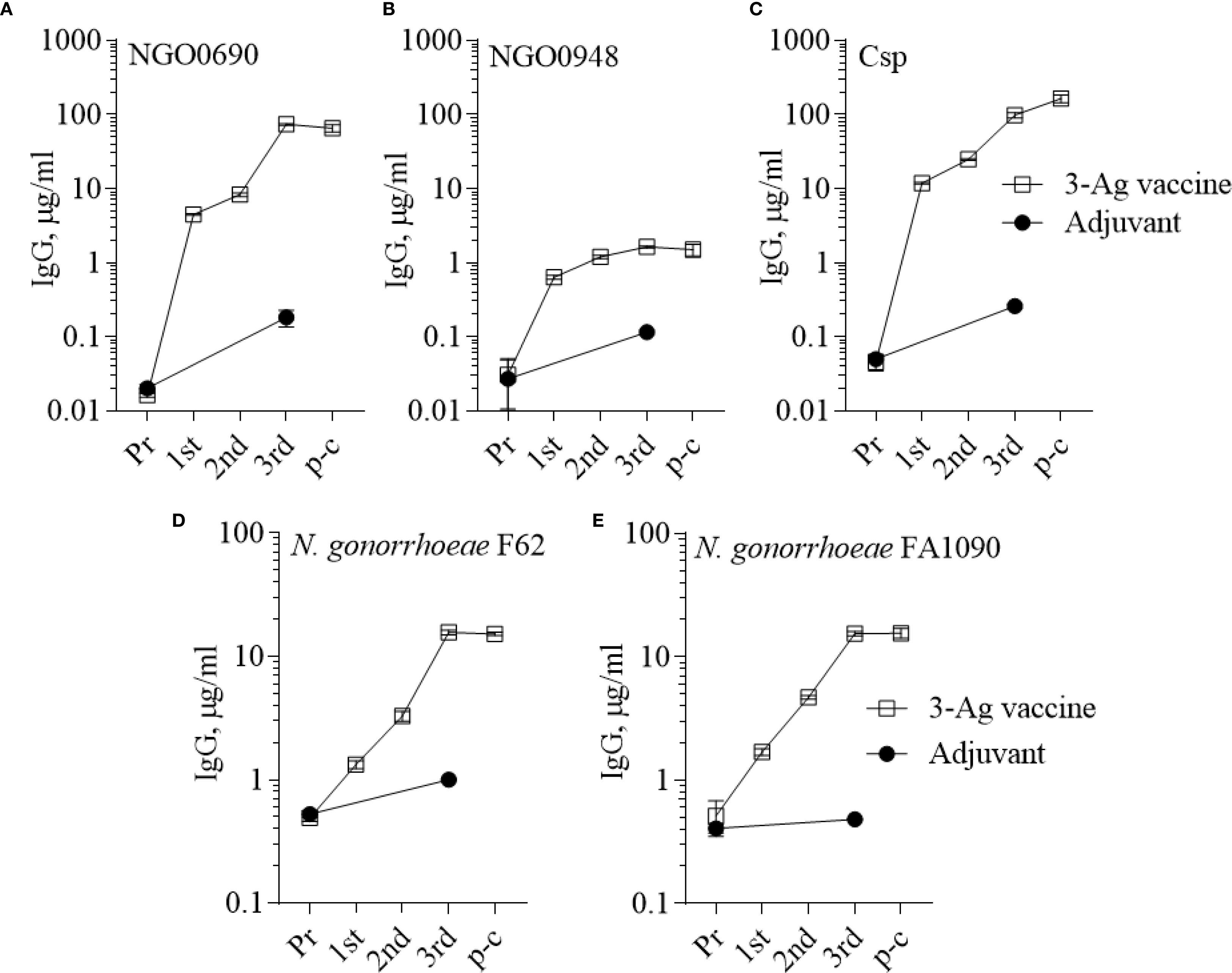
Figure 2. Serum IgG antibodies to the purified antigens and to N. gonorrhoeae. Total IgG antibodies measured by ELISA in sera from mice immunized with the 3-Ag vaccine (open squares) or Alum+MPLA alone (closed circles). Sera were tested against (A) purified recombinant NGO0690, (B) NGO0948 and (C) Csp, or (D) N. gonorrhoeae F62 and (E) N. gonorrhoeae FA1090 in the mouse preimmune sera (Pr), in sera collected 2 weeks after each immunization (1st, 2nd and 3rd) and 2 weeks prior to challenge (p-c). Pooled sera were evaluated in triplicate and antibodies were expressed as µg/ml ± SD.
As a measure of antibody function, the ability of the immune sera to kill N. gonorrhoeae was examined in a complement-dependent bactericidal assay. The 3-Ag vaccine produced sera with killing titers of about 1/160 against N. gonorrhoeae F62 (Figure 3A, black bars) and of about 1/40 against N. gonorrhoeae FA1090 (Figure 3B, black bars), also confirming our previous results (37). Differences in killing titers were attributable to the serum-sensitivity profile of these strains [FA1090 is intrinsically more serum-resistant than F62 (45)]. No significant killing was induced by the sera from mice vaccinated with Alum+MPLA alone (Figures 3A, B, gray bars). Collectively, these results confirmed that immunization of mice with the 3-Ag vaccine induced robust serum antibody responses and a more modest mucosal antibody response, and that the immune sera had complement-dependent serum bactericidal activity.
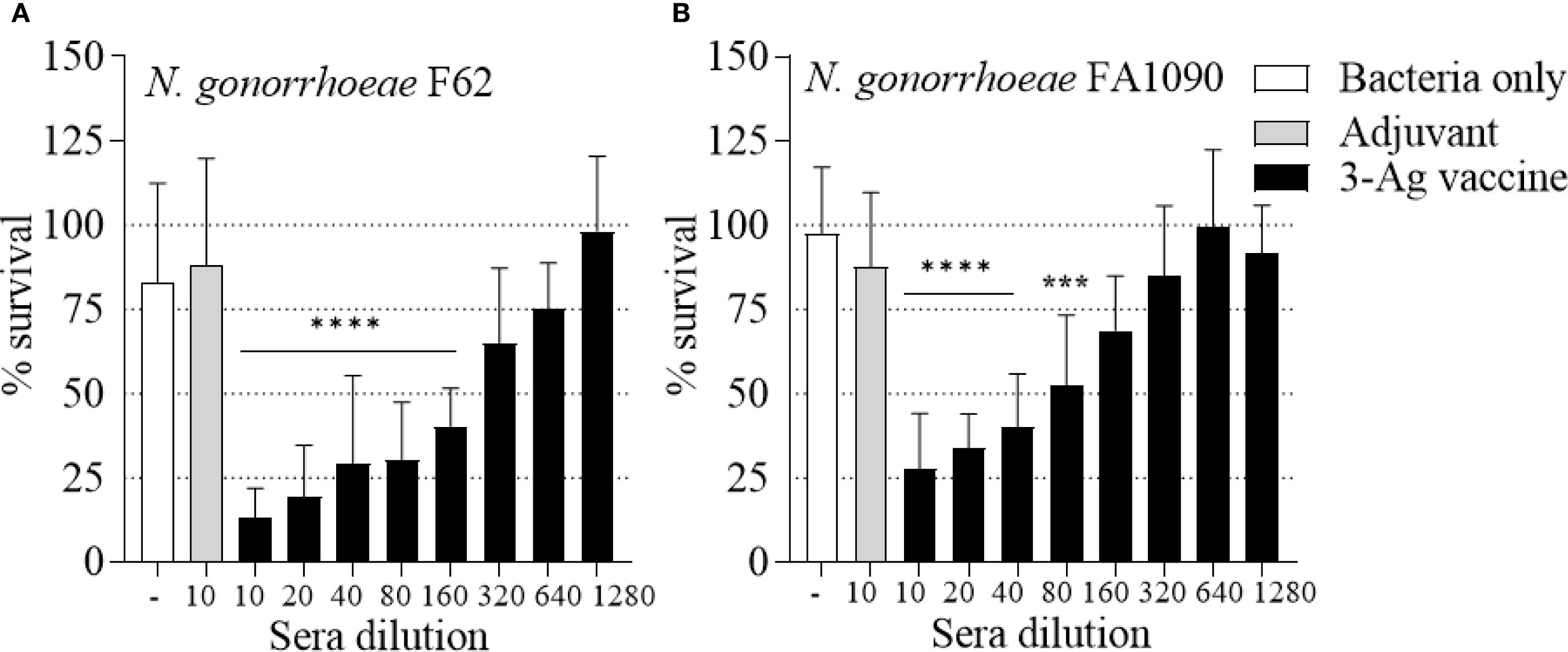
Figure 3. Serum Bactericidal Activity (SBA) of mouse sera elicited by immunization with the 3-Ag vaccine. N. gonorrhoeae survival (% CFU at T30/T0 ± SD) in pooled sera from mice immunized with the 3-Ag vaccine (black bars) or Alum+MPLA alone (gray bars). (A) N. gonorrhoeae F62 and (B) N. gonorrhoeae FA1090. Sera were evaluated in a minimum of seven repeats and dilutions are indicated on the X-axis. Bacteria alone, white bars. ***p = 0.0005 and ****p < 0.0001 by one-way ANOVA with Dunnett’s multiple comparisons test vs Adjuvant.
Serum cytokine responses
Serum cytokine levels induced by the 3-Ag vaccine were also measured by ELISA. Slightly higher IL-12p70 levels than IFN-γ (Figure 4A) and significantly higher IL-10 levels than IL-4 (Figure 4B) were detected, confirming the balanced Th1/Th2 immune response with a slight Th2 bias previously observed (37), along with the IgG subclasses profile. An increase of pro-inflammatory cytokines IL-6, TNF-α and IL-1β production was induced by the 3-Ag vaccine compared to Alum+MPLA (Figures 4C, D, black bars and gray bars, respectively); while these cytokines support immune activation, they can be also associated with inflammation and adverse effects (47).
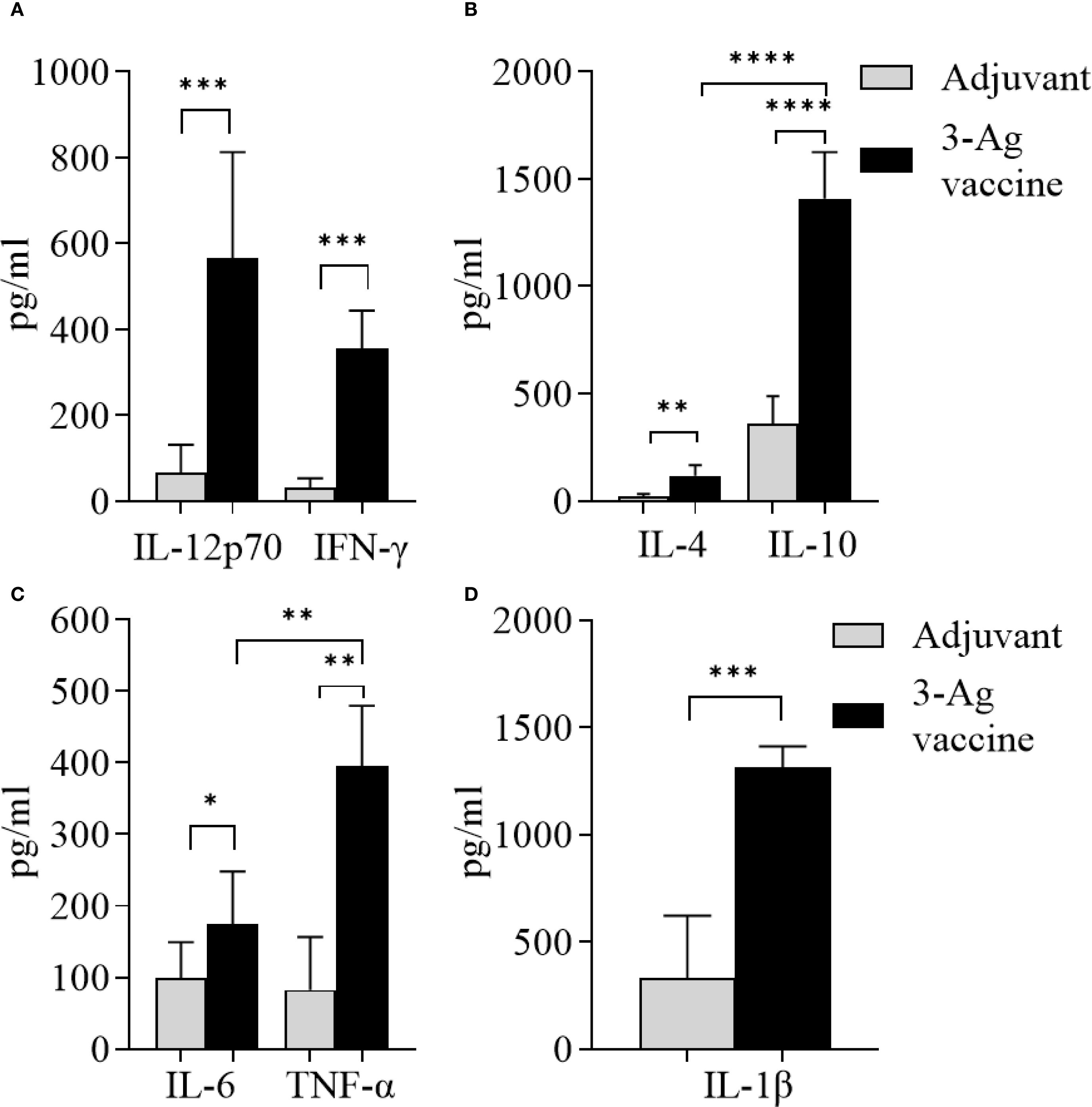
Figure 4. Analysis of cytokines production in sera from mice immunized with the 3-Ag vaccine. (A) Th1 cytokines IL-12p70 and IFN-γ, (B) Th2 cytokines IL-4 and IL-10, (C) Inflammatory cytokines IL-6 and TNF-α, and (D) IL-1β measured by ELISA in pooled sera from female BALB/c mice immunized with the 3-Ag vaccine (black bars) or Alum+MPLA alone (gray bars). Sera were evaluated in quadruplicate and cytokines were expressed in pg/ml ± SD. *p < 0.05; **p ≤ 0.008, ***p ≤ 0.0007, and ****p < 0.0001 by unpaired t test.
Immune responses to a low-dose 3-Ag vaccine in female and male mice
Since the 3-Ag vaccine induced high IgG antibody levels particularly against NGO0690 and Csp, as well as production of TNF-α and IL-1β, we investigated whether antigen dose de-escalation could decrease inflammatory responses without significantly affecting antibody responses and efficacy. A low-dose 3-Ag vaccine containing 50% less of each antigen (5 µg each) but the same amount of Alum and MPLA adjuvants, was used to immunize female BALB/c mice. In addition, male mice were also immunized to begin comparing sex-dependent immune responses. No significant change in IgG levels against the individual antigens was detected in sera from the female mice compared to the original dose vaccine (Figures 2A–C and 5A–C), but a decrease in IgG antibodies against N. gonorrhoeae F62 was observed (Figure 5D). In male mice, the amounts of anti-NGO0690 and anti-Csp IgG antibodies were similar to the female mice (Figures 5E, G), but anti-NGO0948 IgG antibodies (Figure 5F) and IgG antibodies against N. gonorrhoeae F62 (Figure 5H) appeared lower. In both sets of sera, slightly lower amounts of anti-gonococcal IgM antibodies were measured than with the original dose vaccine (not shown), as well as serum IgA antibodies reacting with N. gonorrhoeae (Supplementary Figure S1B, dashed and black bars, respectively). Mucosal IgA antibodies against N. gonorrhoeae F62 were similar to the original dose vaccine levels (Supplementary Figure S1B, dotted bar), while secreted IgG levels appeared lower (Supplementary Figure S2B). A decrease in serum IgG antibody subclasses was also determined against N. gonorrhoeae F62, with IgG1 = 0.77 ± 0.04 µg/ml and IgG2a = 0.52 ± 0.01 µg/ml in female mice, and IgG1 = 0.94 ± 0.07 µg/ml and IgG2a = 0.56 ± 0.005 µg/ml in male mice, but the IgG1/IgG2a ratio remained consistent with a Th1-Th2 balanced profile (Female mice, 0.68 and Male mice, 0.59). This was supported by the cytokine profile in female mice, with higher IL-12p70 than IFN-γ, and higher IL-10 than IL-4 (Figures 6A, B) and in male mice, although, interestingly, lower IL-12p70 and higher IL-10 were induced than in female mice (Figures 6A, B). The low-dose 3-Ag vaccine induced less TNF-α and IL-1β than the original dose vaccine in female mice, and no significant changes in IL-6 (Figures 6C, D); IL-6 and TNF-α levels were comparable to those in male mice (Figure 6C), but IL-1β production in male mice was higher (Figure 6D), even in the adjuvant-only group. These results were consistent with the reported variability in innate and adaptive immune responses to vaccination across sexes in mice and in humans (48–51).
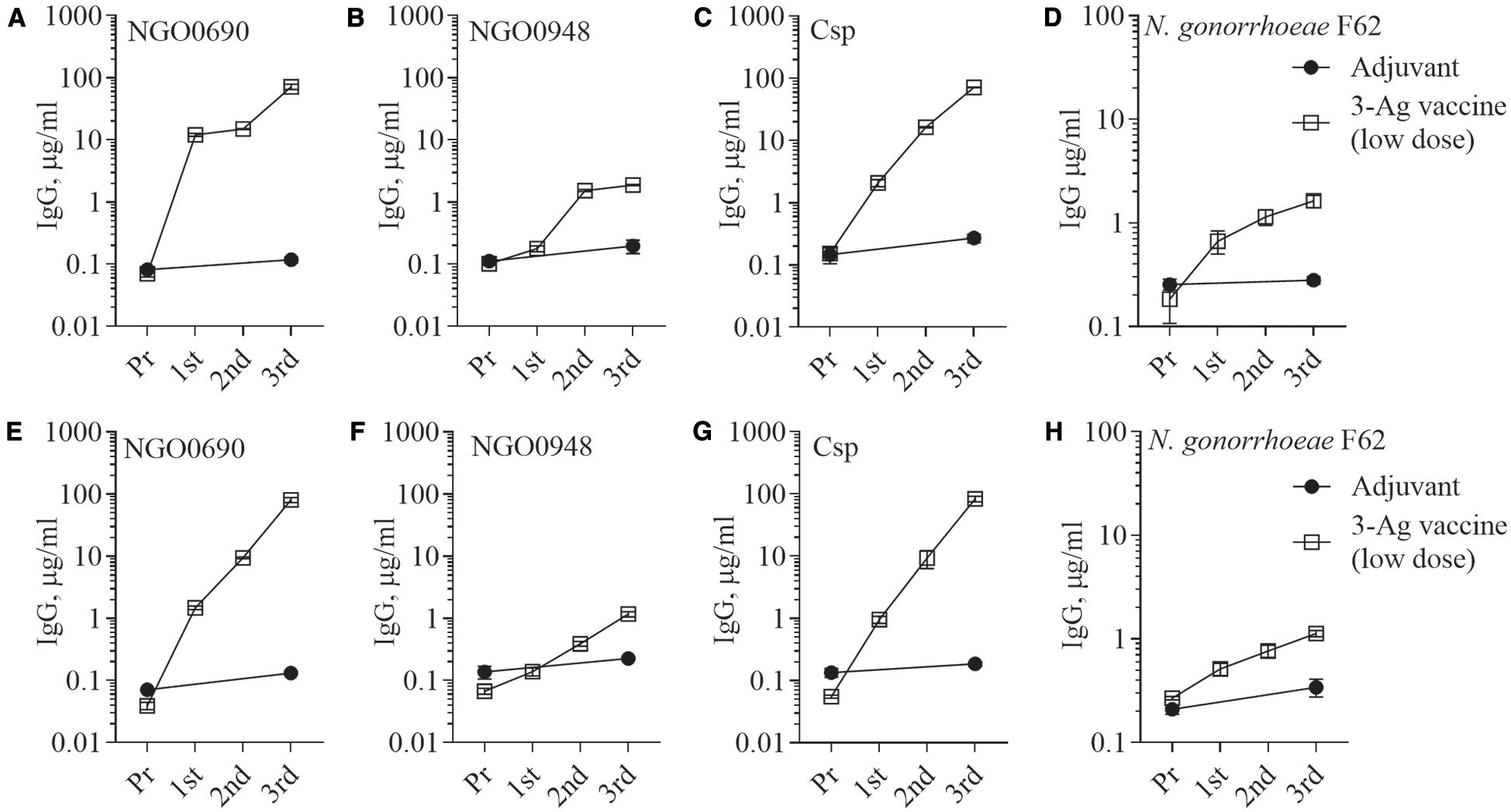
Figure 5. Serum IgG antibodies to the purified antigens and to N. gonorrhoeae. Total IgG antibodies measured by ELISA in sera from mice immunized with the low-dose 3-Ag vaccine (open squares) or Alum+MPLA alone (closed circles). Sera were evaluated against (A) purified recombinant NGO0690, (B) NGO0948 and (C) Csp, or (D) N. gonorrhoeae F62 in the mouse preimmune sera (Pr) and in sera collected 2 weeks after each immunization (1st, 2nd, and 3rd). (E-H) Pooled sera from male BALB/c mice as above. Pooled sera were evaluated in triplicate and antibodies were expressed as µg/ml ± SD.
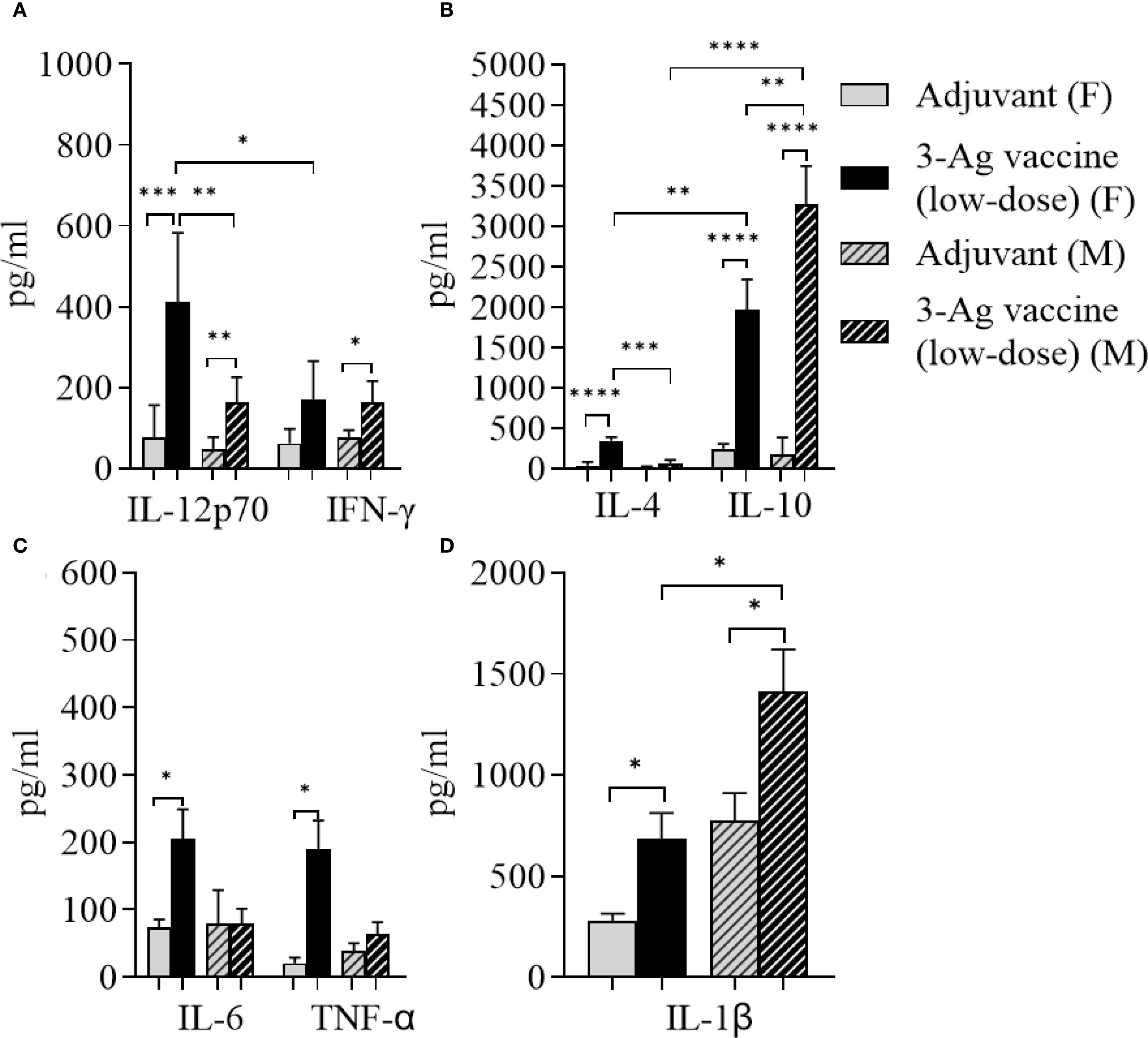
Figure 6. Analysis of cytokines production in sera from mice immunized with the low-dose 3-Ag vaccine in female and male mice. (A) Th1 cytokines IL-12p70 and IFN-γ, (B) Th2 cytokines IL-4 and IL-10, (C) Inflammatory cytokines IL-6 and TNF-α, and (D) IL-1β measured by ELISA in pooled sera from female BALB/c mice immunized with the low dose 3-Ag vaccine (black bars) or Alum+MPLA alone (gray bars), and pooled sera from male BALB/c mice immunized as above (gray dashed bars and black dashed bars). Sera were evaluated in quadruplicate and cytokines were expressed in pg/ml ± SD. *p < 0.05; **p ≤ 0.006, ***p ≤ 0.0005, and ****p < 0.0001 by unpaired t test.
Lastly, the SBA titers were evaluated. Sera from female and male mice immunized with the low-dose vaccine retained the ability to kill N. gonorrhoeae F62, but an approx. 50% decrease in the SBA titers was detected in female mouse sera (Figure 7A) than with the original dose 3-Ag vaccine (1/80-1/160). Notably, SBA titers of male mice sera only reached ~ 1/10 (Figure 7B), possibly mirroring the lower magnitude of the antibody responses to N. gonorrhoeae detected in these mice. These results indicated that a 50% decrease in antigen amount in the 3-Ag vaccine reduced inflammatory responses but also dampened antibody responses against N. gonorrhoeae.
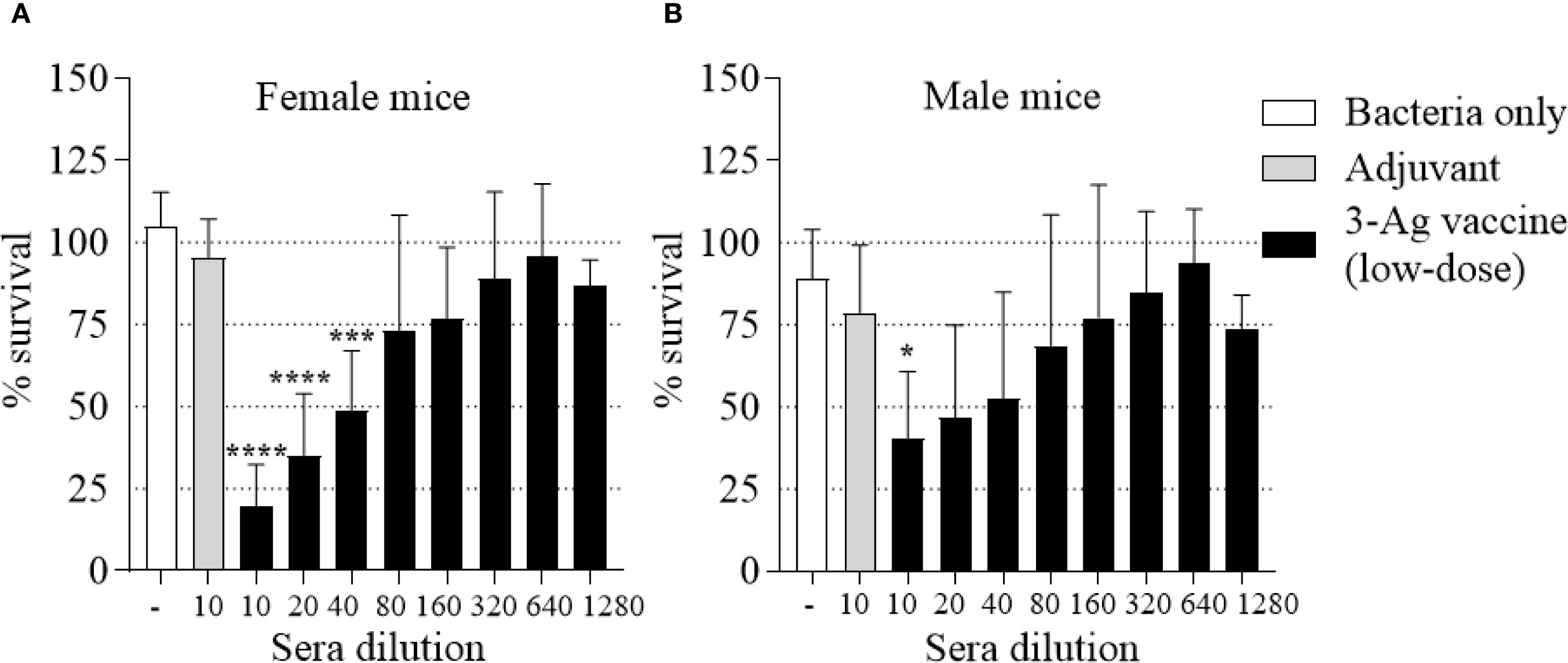
Figure 7. Serum Bactericidal Activity (SBA) of mouse sera elicited by immunization with the low-dose 3-Ag vaccine in female and male mice. N. gonorrhoeae F62 survival (% CFU at T30/T0 ± SD) in pooled sera from (A) Female BALB/c mice and (B) Male BALB/c mice immunized with the low-dose 3-Ag vaccine (black bars) or Alum+MPLA alone (gray bars). Sera were evaluated in a minimum of three repeats and dilutions are indicated on the X-axis. Bacteria alone, white bars. *p < 0.05, and ****p < 0.0001 by one-way ANOVA with Dunnett’s multiple comparisons test vs Adjuvant.
Immunization with NGO0690+Csp and Alum+MPLA (2-Ag vaccine) also protects mice from gonococcal vaginal colonization
Since NGO0690 and Csp were more immunodominant than NGO0948, and because the latter is a structurally more complex antigen that could be more difficult to scale up for manufacturing, we evaluated a bivalent vaccine only comprising NGO0690 and Csp. Female BALB/c mice were immunized with NGO0690+Csp (10 µg each) and Alum+MPLA as adjuvants (2-Ag vaccine) followed by challenge with N. gonorrhoeae FA1090 as previously described. The 2-Ag vaccine also protected mice when compared to the Alum+MPLA control group (Figures 8A–C), indicating induction of protective responses in vivo. Qualitative, quantitative, and functional antibody responses elicited by the 2-Ag vaccine were examined; as expected, anti-NGO0690 and anti-Csp IgG antibodies remained high (Figure 9A), and only a slight decrease in IgG levels against N. gonorrhoeae was observed (Figure 9B). Comparable IgM antibody responses were reported (not shown) and serum IgA antibodies (Supplementary Figure S1C, striped bars), as well as mucosal IgA against N. gonorrhoeae F62 (Supplementary Figure S2C, dotted bars) but an apparent decrease in mucosal IgA against N. gonorrhoeae FA1090. IgG levels in vaginal lavages remained high (Supplementary Figure S2C). The observed balanced Th1/Th2 response was also confirmed by evaluation of the IgG subclasses induced by the 2-Ag vaccine, with IgG1 = 1.19 ± 0.06 µg/ml and 1.67 ± 0.05 µg/ml for N. gonorrhoeae F62 and FA1090, respectively, and IgG2a: 0.7 ± 0.08 µg/ml for both strains. The Th1 and Th2 cytokines profile was quantitatively and qualitatively similar to that of the 3-Ag vaccine (not shown), including that of IL-6 and IL-1β. However, an approx. 50% reduction in TNF-α levels was observed (185 ± 31 pg/ml vs 395 ± 84 pg/ml for the 3-Ag vaccine) suggesting that NGO0948 contributed to inflammatory responses. The SBA titers of the 2-Ag vaccine sera also remained around 1/80 - 1/160 against N. gonorrhoeae F62 (Figure 10A) but were only about 1/10 - 1/20 against N. gonorrhoeae FA1090 (Figure 10B). These results indicated that NGO0690 and Csp were immunodominant antigens that induced majority of the antibody responses against N. gonorrhoeae and suggested that, while exclusion of NGO0948 only minimally affected antibody production in vivo, it decreased inflammatory responses.
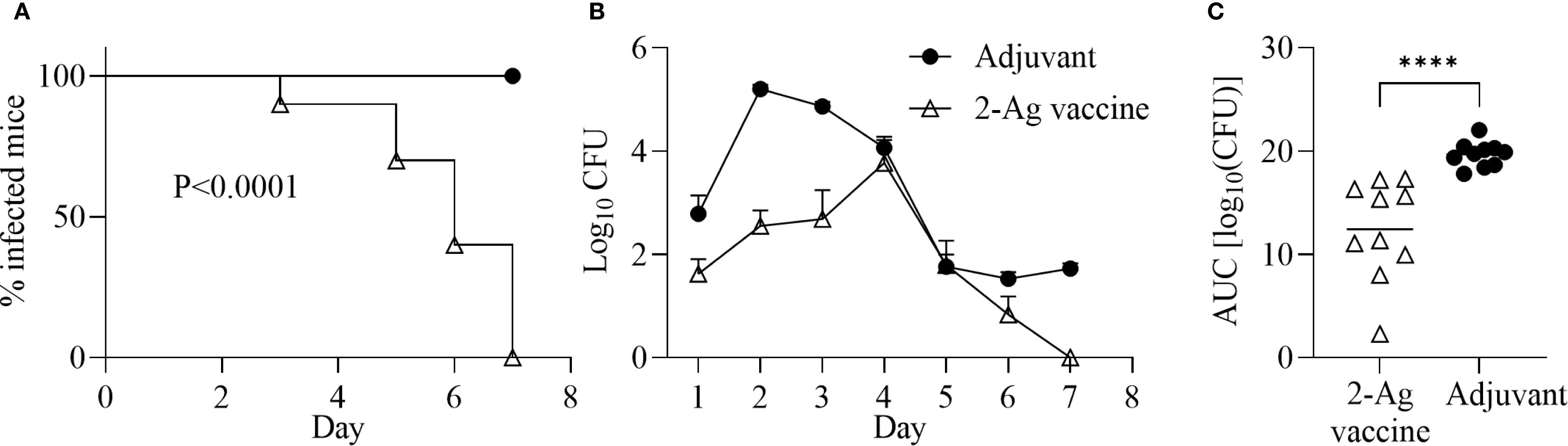
Figure 8. Immunization with NGO0690+Csp and Alum+MPLA (2-Ag vaccine) is protective in a mouse model of gonococcal vaginal colonization. Female BALB/c mice (n = 20) were immunized with the 2-Ag vaccine (10 µg each antigen) (open triangles) or with adjuvants alone (black circles). Two weeks after the third immunization, mice in the diestrus phase of the estrous cycle (n = 10 per group) were challenged intravaginally with N. gonorrhoeae FA1090 (3.1 x 107 CFU). Vaginal swabs were collected daily and plated to enumerate gonococcal CFUs. (A) Time-to-clearance Kaplan–Meier curves (p < 0.0001, groups compared by Mantel-Cox analysis); (B) Bacterial burden (log10 CFU ± SEM); (C) Area under the curve (AUC) (means ± 95% confidence intervals compared across groups by Mann Whitney’s non-parametric test). ****p < 0.0001).
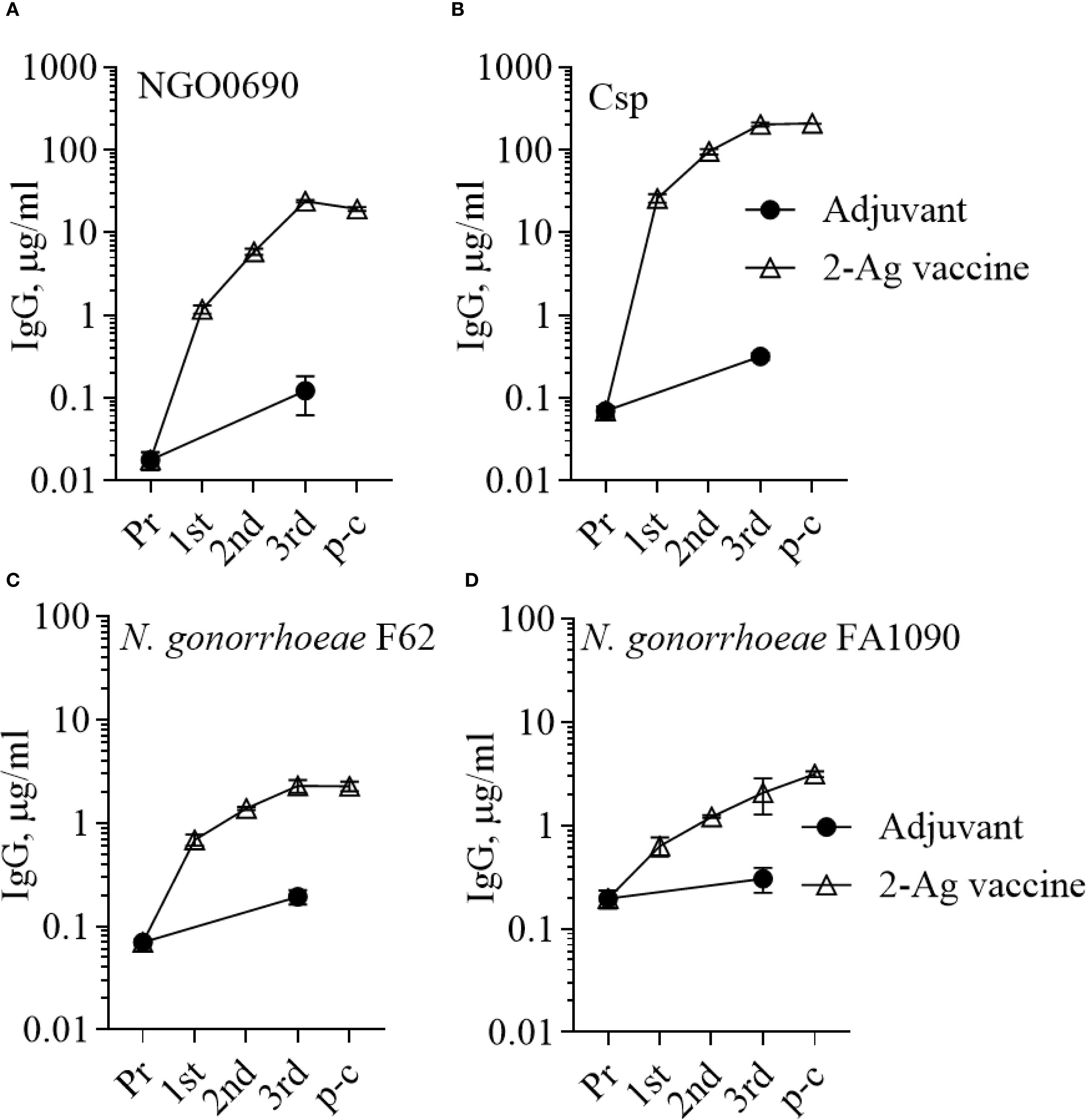
Figure 9. Serum IgG antibodies to the purified antigens and to N. gonorrhoeae. Total IgG antibodies measured by ELISA in pooled sera from mice immunized with the 2-Ag vaccine (open triangles) or with Alum+MPLA alone (closed circles) against (A) NGO0690, (B) Csp, (C) N. gonorrhoeae F62 and (D) N. gonorrhoeae FA1090. Pr, preimmune; p-c, pre-challenge. Sera were evaluated in triplicate and antibodies were expressed as µg/ml ± SD.
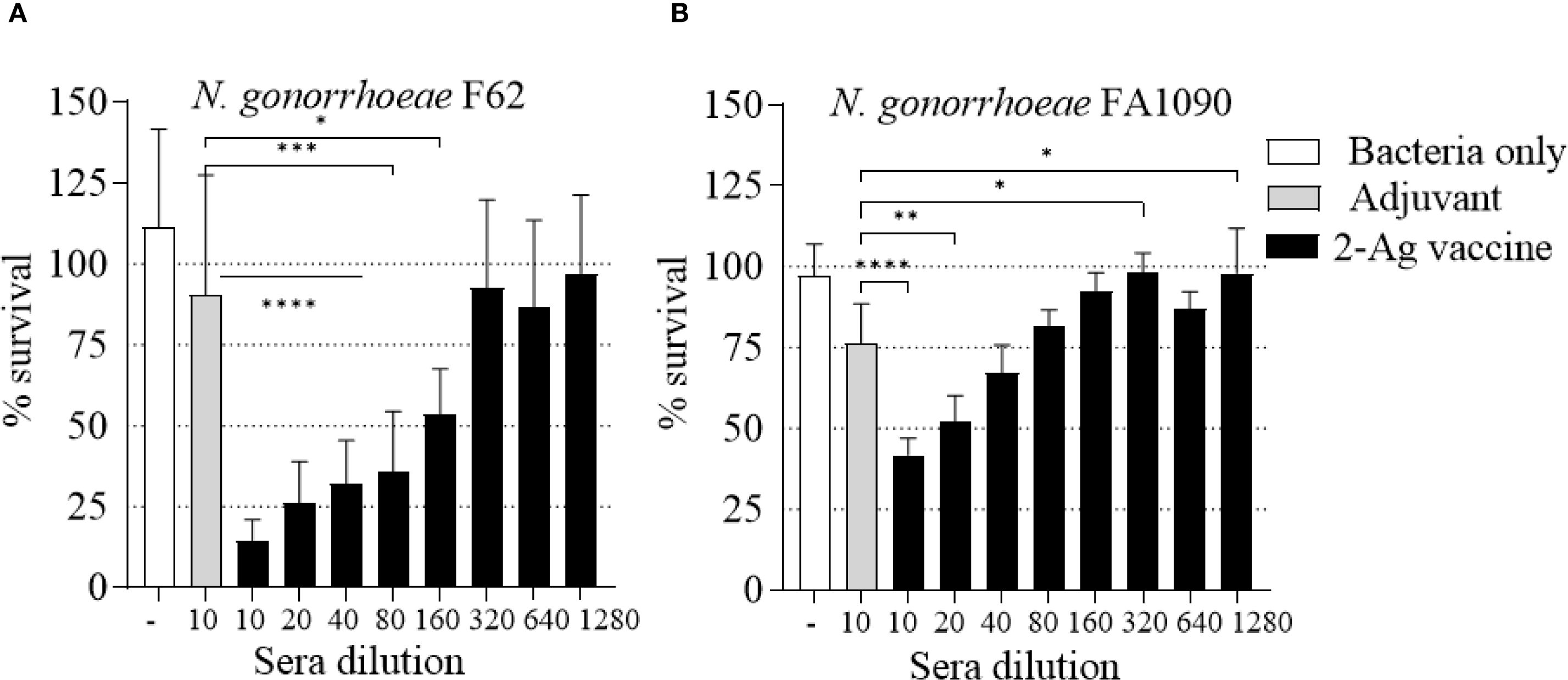
Figure 10. Serum Bactericidal Activity (SBA) of mouse sera elicited by immunization with the 2-Ag vaccine. N. gonorrhoeae survival (% CFU at T30/T0 ± SD). Pooled sera from female BALB/c mice immunized with the 2-Ag vaccine (black bars) or Alum+MPLA alone (gray bars). (A) N. gonorrhoeae F62 and (B) N. gonorrhoeae FA1090. Bacteria alone, white bars. Sera were evaluated in a minimum of three repeats and dilutions are indicated on the X-axis. *p ≤ 0.03, **p <= 0.005, ***p = 0.0001 and ****p < 0.0001 by one-way ANOVA with Dunnett’s multiple comparisons test vs Adjuvant.
Discussion
While N. gonorrhoeae whole-bacteria vaccines have failed to induce cross-strain protection because of antigen variability, they have served as a stepping-stone for identification of potential gonococcal targets for use in subunit vaccines (12). For some diseases, monovalent subunit vaccines may be suitable [for example, the H. influenzae type B (Hib) vaccine conjugate, the shingles vaccine, the Hepatitis B (HepB) vaccines or Covid-19 (52)], but a multivalent vaccine may offer significant advantages against a pathogen such as Neisseria gonorrhoeae, where antigenic variability and immune evasion strategies are a concern (12) (ideally, immune responses against multiple conserved antigens that target distinct bacterial functions will reduce the possibility of vaccine escape). From an immunological standpoint, multivalent vaccines may also enhance the magnitude or the quality of the immune response because of potential synergistic effects among the immunodominant and conserved antigens used (purified proteins, polysaccharides, or peptides) (38). Gonococcal proteins that have been tested in combination include LptD and LtgC (53), the passenger and translocator fragments of adhesion and penetration protein (App) (54), TbpA and TbpB (55), App, MetQ and NHBA (56), NGO0265 and NGO1549 (57), which have indeed shown more robust immune responses and sustained bactericidal titers than the individual antigens. Our previous studies of NGO0690, NGO0948 and Csp as a combination vaccine aligned with these observations (33, 37). Building on those results, we assessed the ability of the 3-Ag vaccine to induce protective responses in a mouse model of gonococcal vaginal colonization. Our results showed that the 3-Ag vaccine induced protection against N. gonorrhoeae in vivo, demonstrated by a reduced bacterial burden and an accelerated colonization clearance time compared to the adjuvant control group. We confirmed that the 3-Ag vaccine elicited strong systemic antibody responses against the individual antigens and against whole gonococci, along with mucosal IgG and low levels of mucosal IgA antibodies. Mucosal responses could be enhanced by exploring different adjuvants and/or routes of immunization in future studies (27, 37, 56, 58) including, for example, systemic priming followed by mucosal boost, as shown against Chlamydia (59, 60) and in other infection models (61). The bactericidal activity of sera from the vaccinated mice against N. gonorrhoeae strains showed titers aligning with those we previously reported, thus reinforcing the value of our vaccine targets. Based on the IgG subclasses and on the serum cytokine profile elicited, a balanced Th1/Th2 response was observed, marked primarily by IL-12p70 and IL-10. These results suggested a likely multifactorial immune mechanism involving both humoral and cellular components promoting a broad-based immune response that contributed to both bacterial clearance and antibody functionality. Detectable serum levels of proinflammatory cytokines TNF-α and IL-1β further supported immune system activation, but also suggested potential vaccine-induced inflammation (62).
For this reason, we investigated whether de-escalating the antigens dose could offer immunological advantages, such as minimizing potential inflammation without affecting functional antibody responses. In some cases, decreasing the antigen concentration may even improve T cell response and induction of memory B cells crucial for long-term immunity (63) although, if the antigen concentration is too low, the immune system might not be sufficiently stimulated to generate a robust and long-lasting protective response. Fine-tuning of the antigens dose in a multivalent vaccine is also important to avoid potential immune interference among the antigens (63). We reported no significant changes in the quantity and quality of the antibody responses to the purified proteins in sera from mice that were immunized with a 3-Ag vaccine that contained 50% less of each antigen, but antibody titers elicited by the low-dose vaccine against whole gonococci appeared lower. While it is possible that the immunoreactive epitopes are more accessible to the antibodies on the purified antigens than when these are expressed within the bacterial membrane, this finding realistically reflected the antigen dose-dependency of the immune responses induced.
Qualitatively, the antibody responses were similar in female and male mice, but a slightly dampened profile was observed in male mice, with lower antibody levels against N. gonorrhoeae and lower SBA titers. The serum cytokine profile was also different in male mice, with more elevated IL-10 and reduced IL-12p70, TNF-α and IL-6 than in female mice, and a higher IL-1β baseline. These results align with a more robust humoral and cell-mediated response often detected in females than in males, which is attributed to hormonal, genetic, or microbiota-driven factors increasingly recognized as key modulators of vaccine efficacy (48, 50, 51). These findings underscore the importance of including both sexes in preclinical vaccine evaluation, since differences in immune responses between sexes may affect translational outcomes in humans, especially for gonococcal-specific immune responses. Indeed, vaccine efficacy studies have suggested that, for certain vaccines, one sex may be better protected than the other, for example measles vaccination, the seasonal trivalent influenza vaccine or hepatitis B seem more effective in females, and BCG vaccination may be more effective in males, and thus requiring more fine-tuning (48–50).
Based on the strong antibody responses induced by NGO0690 and Csp, and on the higher SBA titers induced by the individual antigens previously reported (33), these candidates may be more immunodominant targets compared to NGO0948. We adopted an immunofocusing strategy by excluding NGO0948, an approach that could also be beneficial from a potential future manufacturing perspective, since NGO0948 is a large and structurally more complex protein. The NGO0690+Csp bivalent formulation was found to also protect mice in vivo, although the apparent longer time to infection clearance and the lower SBA titers measured than with the 3-Ag vaccine warrant additional investigation. However, the inherent variability across experiments in the mouse model makes quantitative comparisons between experiments problematic. It is possible that the breadth and magnitude of protection conferred by the 3-Ag combination was likely superior, despite an apparently more modest contribution of NGO0948 to the antibody responses. On the other hand, using exclusively immunodominant antigens in a vaccine may not always be beneficial, since it may lead to immune evasion or even induction of blocking antibodies, as with gonococcal Rmp protein (64). Thus, presence of sub-dominant antigens could be a strength for broadening the response to a multi-antigen vaccine (41, 65, 66); we cannot exclude the possibility that additional CASS antigens may be added to NGO0690 and Csp in a multivalent vaccine to achieve similar, or even better protection. The benefits of adding additional antigens to a vaccine preparation need to be weighed against increasing costs, an important consideration for this disease that disproportionately affects socioeconomically disadvantaged populations and persons in low- and middle-income countries (LMICs). Altogether, this work adds to a growing body of evidence supporting subunit-based vaccination strategies for gonorrhea because these may offer customizable approaches, particularly when optimized for conserved surface-exposed gonococcal antigens. In addition, different adjuvants should be explored to increase the effectiveness of our antigens, or different delivery platforms targeting mucosal tissues, for improving mucosal antibodies and memory responses critical for protection against gonorrhea. Alternatively, OMVs could be engineered in which CASS antigens are overexpressed, to benefit from the built-in adjuvanticity, ease of purification and flexibility of this platform. Besides antigen composition, future studies should also continue to focus on refining antigen doses and defining the immune correlates of protection across sexes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies were approved by Tufts University IACUC and University of Massachusetts Chan Medical School IACUC. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SKR: Formal Analysis, Investigation, Methodology, Visualization, Writing – review & editing. BZ: Investigation, Methodology, Writing – review & editing. SG: Formal Analysis, Investigation, Methodology, Writing – review & editing. CG: Resources, Writing – review & editing. SR: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. PM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by NIH/NIAID 1R01AI166537 grant and by Tufts Launchpad Accelerator Program to PM.
Acknowledgments
The authors thank Dr. Gary A. Jarvis, PhD, for critical reading of the manuscript.
Conflict of interest
SR is a cofounder of StiRx, Inc. and holds equity in the company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1688536/full#supplementary-material
Abbreviations
CASS, Candidate antigen selection strategy; Csp, copper storage protein; SBA, serum bactericidal activity; OM, outer membrane; OMV, outer membrane vesicle; LOS, lipooligosaccharide; MPLA, Monophosphoryl Lipid A; 3-Ag vaccine, NGO0690+NGO0948 (BamC)+Csp (NGO1701) with Alum+MPLA; 2-Ag Vaccine, NGO0690+Csp (NGO1701) with Alum+MPLA; Pr, Pre-immune sera; 1st, 2nd, 3rd, serum aliquots collected 2 weeks after each immunization; P-c, pre-challenge sera; CFUs, colony forming units.
References
1. CDC. Sexually transmitted disease surveillance(2023). Available online at: https://www.cdc.gov/sti-statistics/annual/index.html (Accessed August 01, 2025).
2. Unemo M, Seifert HS, Hook EW 3rd, Hawkes S, Ndowa F, and Dillon JR. Gonorrhoea. Nat Rev Dis Primers. (2019) 5:79. doi: 10.1038/s41572-019-0128-6
3. Lovett A and Duncan JA. Human immune responses and the natural history of Neisseria gonorrhoeae infection. Front Immunol. (2018) 9:3187. doi: 10.3389/fimmu.2018.03187
4. van Dessel HA, Dirks J, van Loo IHM, van der Veer B, Hoebe C, Dukers-Muijrers N, et al. Higher Neisseria gonorrhoeae bacterial load in coinfections with Chlamydia trachomatis compared with Neisseria gonorrhoeae single infections does not lead to more symptoms. Sex Transm Infect. (2024) 100:127–8. doi: 10.1136/sextrans-2023-055977
5. Su X, Le W, Zhu X, Li S, Wang B, Madico G, et al. Neisseria gonorrhoeae infection in women increases with rising gonococcal burdens in partners: chlamydia coinfection in women increases gonococcal burden. J Infect Dis. (2022) 226:2192–203. doi: 10.1093/infdis/jiac408
6. Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. (1993) 7:95–102. doi: 10.1097/00002030-199301000-00015
7. Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. (1997) 349:1868–73. doi: 10.1016/S0140-6736(97)02190-9
8. Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe. (2021) 2:e627–e36. doi: 10.1016/S2666-5247(21)00171-3
9. Hiruy H, Bala S, Byrne JM, Roche KG, Jang SH, Kim P, et al. FDA, CDC, and NIH co-sponsored public workshop summary-development considerations of antimicrobial drugs for the treatment of gonorrhea. Clin Infect Dis. (2024) 24:ciae386. doi: 10.1093/cid/ciae386
10. Marshall HS, Molina JM, Berlaimont V, Mulgirigama A, Sohn WY, Bercot B, et al. Management and prevention of Neisseria meningitidis and Neisseria gonorrhoeae infections in the context of evolving antimicrobial resistance trends. Eur J Clin Microbiol Infect Dis. (2025) 44:233–50. doi: 10.1007/s10096-024-04968-8
11. Gill DS, Ram S, and Rice PA. Biologic drug development for treatment and prevention of sexually transmitted infections. Clin Microbiol Rev. (2025) 29:e0010724. doi: 10.1128/cmr.00107-24
12. Williams E, Seib KL, Fairley CK, Pollock GL, Hocking JS, McCarthy JS, et al. Neisseria gonorrhoeae vaccines: a contemporary overview. Clin Microbiol Rev. (2024) 37:e0009423. doi: 10.1128/cmr.00094-23
13. Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. (2017) 390:1603–10. doi: 10.1016/S0140-6736(17)31449-6
14. Leduc I, Connolly KL, Begum A, Underwood K, Darnell S, Shafer WM, et al. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PloS Pathog. (2020) 16:e1008602. doi: 10.1371/journal.ppat.1008602
15. Semchenko EA, Tan A, Borrow R, and Seib KL. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis. (2018) 69(7):1101–1111. doi: 10.1093/cid/ciy1061
16. Tzeng YL, Sannigrahi S, and Stephens DS. NHBA antibodies elicited by 4CMenB vaccination are key for serum bactericidal activity against Neisseria gonorrhoeae. NPJ Vaccines. (2024) 9:223. doi: 10.1038/s41541-024-01018-4
17. Matthias KA, Connolly KL, Begum AA, Jerse AE, Macintyre AN, Sempowski GD, et al. Meningococcal detoxified outer membrane vesicle vaccines enhance gonococcal clearance in a murine infection model. J Infect Dis. (2022) 225:650–60. doi: 10.1093/infdis/jiab450
18. Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. (2006) 24:5093–107. doi: 10.1016/j.vaccine.2006.03.091
19. Matthias KA, Reveille A, Dhara K, Lyle CS, Natuk RJ, Bonk B, et al. Development and validation of a standardized human complement serum bactericidal activity assay to measure functional antibody responses to Neisseria gonorrhoeae. Vaccine. (2025) 43:126508. doi: 10.1016/j.vaccine.2024.126508
20. Thng C, Semchenko EA, Hughes I, O’Sullivan M, and Seib KL. An open-label randomised controlled trial evaluating the efficacy of a meningococcal serogroup B (4CMenB) vaccine on Neisseria gonorrhoeae infection in gay and bisexual men: the MenGO study protocol. BMC Public Health. (2023) 23:607. doi: 10.1186/s12889-023-15516-y
21. Abara WE, Kirkcaldy RD, Bernstein KT, Galloway E, and Learner ER. Effectiveness of MenB-4C vaccine against gonorrhea: A systematic review and meta-analysis. J Infect Dis. (2025) 231:61–70. doi: 10.1093/infdis/jiae383
22. Wang B, Giles L, Andraweera P, McMillan M, Beazley R, Sisnowski J, et al. Long-term 4CMenB vaccine effectiveness against gonococcal infection at four years post-program implementation: observational case-control study. Open Forum Infect Dis. (2025) 12:ofae726. doi: 10.1093/ofid/ofae726
23. Marjuki H, Topaz N, Joseph SJ, Gernert KM, Kersh EN, Antimicrobial-Resistant Neisseria gonorrhoeae Working G, et al. Genetic similarity of gonococcal homologs to meningococcal outer membrane proteins of serogroup B vaccine. mBio. (2019) 10(5):e01668-19. doi: 10.1128/mBio.01668-19
24. Tzeng YL, Sannigrahi S, Borrow R, and Stephens DS. Neisseria gonorrhoeae lipooligosaccharide glycan epitopes recognized by bactericidal IgG antibodies elicited by the meningococcal group B-directed vaccine, MenB-4C. Front Immunol. (2024) 15:1350344. doi: 10.3389/fimmu.2024.1350344
25. Vezzani G, Viviani V, Audagnotto M, Rossi A, Cinelli P, Pacchiani N, et al. Isolation of human monoclonal antibodies from 4CMenB vaccinees reveals PorB and LOS as the main OMV components inducing cross-strain protection. Front Immunol. (2025) 16:1565862. doi: 10.3389/fimmu.2025.1565862
26. Zhu W, Waltmann A, Little MB, Connolly KL, Matthias KA, Thomas KS, et al. Protection against N. gonorrhoeae induced by OMV-based meningococcal vaccines are associated with cross-species directed humoral and cellular immune responses. Front Immunol. (2025) 16:1539795. doi: 10.3389/fimmu.2025.1539795
27. Liu Y, Hammer LA, Daamen J, Stork M, Egilmez NK, and Russell MW. Microencapsulated IL-12 drives genital tract immune responses to intranasal gonococcal outer membrane vesicle vaccine and induces resistance to vaginal infection with diverse strains of Neisseria gonorrhoeae. mSphere. (2023) 8:e0038822. doi: 10.1128/msphere.00388-22
28. Liu Y, Hammer LA, Liu W, Hobbs MM, Zielke RA, Sikora AE, et al. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. (2017) 10:1594–608. doi: 10.1038/mi.2017.11
29. Gulati S, Pennington MW, Czerwinski A, Carter D, Zheng B, Nowak NA, et al. Preclinical efficacy of a lipooligosaccharide peptide mimic candidate gonococcal vaccine. mBio. (2019) 10(6):e02552-19. doi: 10.1128/mBio.02552-19
30. Gulati S, Shaughnessy J, Ram S, and Rice PA. Targeting lipooligosaccharide (LOS) for a gonococcal vaccine. Front Immunol. (2019) 10:321. doi: 10.3389/fimmu.2019.00321
31. Taha, Eskandari S, Slesarenko VA, Haselhorst T, Semchenko EA, and Seib KL. Refinement and optimisation of Neisseria gonorrhoeae NHBA and MetQ vaccine candidates. Vaccine. (2024) 42:126416. doi: 10.1016/j.vaccine.2024.126416
32. Fegan JE, Calmettes C, Islam EA, Ahn SK, Chaudhuri S, Yu RH, et al. Utility of hybrid transferrin binding protein antigens for protection against pathogenic neisseria species. Front Immunol. (2019) 10:247. doi: 10.3389/fimmu.2019.00247
33. Zhu T, McClure R, Harrison OB, Genco C, and Massari P. Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection. Vaccines (Basel). (2019) 7(4):153. doi: 10.3390/vaccines7040153
34. McClure R, Nudel K, Massari P, Tjaden B, Su X, Rice PA, et al. The gonococcal transcriptome during infection of the lower genital tract in women. PloS One. (2015) 10:e0133982. doi: 10.1371/journal.pone.0133982
35. Nudel K, McClure R, Moreau M, Briars E, Abrams AJ, Tjaden B, et al. Transcriptome Analysis of Neisseria gonorrhoeae during Natural Infection Reveals Differential Expression of Antibiotic Resistance Determinants between Men and Women. mSphere. (2018) 3(3):e00312-18. doi: 10.1128/mSphereDirect.00312-18
36. Roe SK, Mazgaj R, Zhu T, Esmaeeli M, Lewis LA, Genco C, et al. The gonococcal vaccine candidate antigen NGO1701 is a N. gonorrhoeae periplasmic copper storage protein. bioRxiv. (2025) 7:2025.05.01.651437. doi: 10.1101/2025.05.01.651437
37. Roe SK, Felter B, Zheng B, Ram S, Wetzler LM, Garges E, et al. In vitro pre-clinical evaluation of a gonococcal trivalent candidate vaccine identified by transcriptomics. Vaccines (Basel). (2023) 11(12):1846. doi: 10.3390/vaccines11121846
38. Watson PS, Novy PL, and Friedland LR. Potential benefits of using a multicomponent vaccine for prevention of serogroup B meningococcal disease. Int J Infect Dis. (2019) 85:22–7. doi: 10.1016/j.ijid.2019.05.019
39. Dorosti H, Eskandari S, Zarei M, Nezafat N, and Ghasemi Y. Design of a multi-epitope protein vaccine against herpes simplex virus, human papillomavirus and Chlamydia trachomatis as the main causes of sexually transmitted diseases. Infect Genet Evol. (2021) 96:105136. doi: 10.1016/j.meegid.2021.105136
40. Nisa A, Pinto R, Britton WJ, Triccas JA, and Counoupas C. Immunogenicity and protective efficacy of a multi-antigen mycobacterium tuberculosis subunit vaccine in mice. Vaccines (Basel). (2024) 12(9):997. doi: 10.3390/vaccines12090997
41. Alhashimi M, Sayedahmed EE, Elkashif A, Chothe SK, Wang WC, Murala MST, et al. A multi-antigen-based SARS-CoV-2 vaccine provides higher immune responses and protection against SARS-CoV-2 variants. NPJ Vaccines. (2025) 10:159. doi: 10.1038/s41541-025-01198-7
42. Vesikari T, Langley JM, Segall N, Ward BJ, Cooper C, Poliquin G, et al. Immunogenicity and safety of a tri-antigenic versus a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. Lancet Infect Dis. (2021) 21:1271–81. doi: 10.1016/S1473-3099(20)30780-5
43. Mellata M, Mitchell NM, Schodel F, Curtiss RR, and Pier GB. Novel vaccine antigen combinations elicit protective immune responses against Escherichia coli sepsis. Vaccine. (2016) 34:656–62. doi: 10.1016/j.vaccine.2015.12.014
44. Boslego JW, Tramont EC, Chung RC, McChesney DG, Ciak J, Sadoff JC, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine. (1991) 9:154–62. doi: 10.1016/0264-410X(91)90147-X
45. Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. (1999) 67:5699–708. doi: 10.1128/IAI.67.11.5699-5708.1999
46. Xiao J, Wang H, Callahan C, O’Donnell G, Rodriguez S, Staupe RP, et al. Immunogenicity of RSV fusion protein adsorbed to non-pathogenic bacillus subtilis spores: implications for mucosal vaccine delivery in nonclinical animal models. Biomedicines. (2025) 13(5):1112. doi: 10.3390/biomedicines13051112
47. Herve C, Laupeze B, Del Giudice G, Didierlaurent AM, and Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. (2019) 4:39. doi: 10.1038/s41541-019-0132-6
48. Fischinger S, Boudreau CM, Butler AL, Streeck H, and Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. (2019) 41:239–49. doi: 10.1007/s00281-018-0726-5
49. Harper A and Flanagan KL. Effect of sex on vaccination outcomes: important but frequently overlooked. Curr Opin Pharmacol. (2018) 41:122–7. doi: 10.1016/j.coph.2018.05.009
50. Ursin RL, Dhakal S, Liu H, Jayaraman S, Park HS, Powell HR, et al. Greater breadth of vaccine-induced immunity in females than males is mediated by increased antibody diversity in germinal center B cells. mBio. (2022) 13:e0183922. doi: 10.1128/mbio.01839-22
51. Binici B, Rattray Z, Schroeder A, and Perrie Y. The role of biological sex in pre-clinical (Mouse) mRNA vaccine studies. Vaccines (Basel). (2024) 12(3):282. doi: 10.3390/vaccines12030282
52. FDA. Available online at: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (Accessed August 01, 2025).
53. Noori Goodarzi N, Barzi SM, Ajdary S, Chiani M, Yekaninejad MS, Badmasti F, et al. Immunogenic evaluation of LptD + LtgC as a bivalent vaccine candidate against Neisseria gonorrhoeae. J Transl Med. (2025) 23:261. doi: 10.1186/s12967-025-06256-1
54. Xia L, Lu Q, Wang X, Jia C, Zhao Y, Wang G, et al. Characterization of protective immune responses against Neisseria gonorrhoeae induced by intranasal immunization with adhesion and penetration protein. Heliyon. (2024) 10:e25733. doi: 10.1016/j.heliyon.2024.e25733
55. Fegan JE, Islam EA, Curran DM, Ng D, Au N, Currie EG, et al. Rational selection of TbpB variants elucidates a bivalent vaccine formulation with broad spectrum coverage against Neisseria gonorrhoeae. bioRxiv. (2024) 10(1):10. doi: 10.1101/2024.09.07.611798
56. Lu Q, Yang H, Peng Y, Dong Z, Nie P, Wang G, et al. Intranasal trivalent candidate vaccine induces strong mucosal and systemic immune responses against Neisseria gonorrhoeae. Front Immunol. (2024) 15:1473193. doi: 10.3389/fimmu.2024.1473193
57. Gulati S, Mattsson AH, Schussek S, Zheng B, DeOliveira RB, Shaughnessy J, et al. Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence. mBio. (2023) 14(6):e0250023. doi: 10.1128/mbio.02500-23
58. Islam EA, Fegan JE, Zeppa JJ, Ahn SK, Ng D, Currie EG, et al. Adjuvant-dependent impacts on vaccine-induced humoral responses and protection in preclinical models of nasal and genital colonization by pathogenic Neisseria. Vaccine. (2025) 48:126709. doi: 10.1016/j.vaccine.2025.126709
59. Lorenzen E, Follmann F, Boje S, Erneholm K, Olsen AW, Agerholm JS, et al. Intramuscular priming and intranasal boosting induce strong genital immunity through secretory IgA in minipigs infected with chlamydia trachomatis. Front Immunol. (2015) 6:628. doi: 10.3389/fimmu.2015.00628
60. Garcia-Del Rio L, Diaz-Rodriguez P, Pedersen GK, Christensen D, and Landin M. Sublingual boosting with a novel mucoadhesive thermogelling hydrogel following parenteral CAF01 priming as a strategy against chlamydia trachomatis. Adv Healthc Mater. (2022) 11:e2102508. doi: 10.1002/adhm.202102508
61. Maltseva M, Galipeau Y, McCluskie P, Castonguay N, Cooper CL, and Langlois MA. Systemic and mucosal antibody responses to SARS-CoV-2 variant-specific prime-and-boost and prime-and-spike vaccination: A comparison of intramuscular and intranasal bivalent vaccine administration in a murine model. Vaccines (Basel). (2025) 13(4):351. doi: 10.3390/vaccines13040351
62. Schultheiss C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. (2022) 3:100663. doi: 10.1016/j.xcrm.2022.100663
63. Billeskov R, Beikzadeh B, and Berzofsky JA. The effect of antigen dose on T cell-targeting vaccine outcome. Hum Vaccin Immunother. (2019) 15:407–11. doi: 10.1080/21645515.2018.1527496
64. Gulati S, Mu X, Zheng B, Reed GW, Ram S, and Rice PA. Antibody to reduction modifiable protein increases the bacterial burden and the duration of gonococcal infection in a mouse model. J Infect Dis. (2015) 212:311–5. doi: 10.1093/infdis/jiv024
65. Holst PJ, Jensen BA, Ragonnaud E, Thomsen AR, and Christensen JP. Targeting of non-dominant antigens as a vaccine strategy to broaden T-cell responses during chronic viral infection. PloS One. (2015) 10:e0117242. doi: 10.1371/journal.pone.0117242
Keywords: gonorrhea, combination vaccine, bactericidal antibodies, protection, mouse model
Citation: Roe SK, Zheng B, Gulati S, Genco C, Ram S and Massari P (2025) Preclinical in vivo evaluation of a gonococcal multivalent vaccine containing antigens identified by CASS. Front. Immunol. 16:1688536. doi: 10.3389/fimmu.2025.1688536
Received: 19 August 2025; Accepted: 03 September 2025;
Published: 22 September 2025.
Edited by:
Catherine Mary O’Connell, University of North Carolina at Chapel Hill, United StatesReviewed by:
Viviane Maimoni Goncalves, Butantan Institute, BrazilTaylor Brooks Poston, University of North Carolina at Chapel Hill, United States
Copyright © 2025 Roe, Zheng, Gulati, Genco, Ram and Massari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Massari, cGFvbGEubWFzc2FyaUB0dWZ0cy5lZHU=
 Shea K. Roe
Shea K. Roe Bo Zheng
Bo Zheng Sunita Gulati
Sunita Gulati Caroline Genco
Caroline Genco Sanjay Ram
Sanjay Ram Paola Massari
Paola Massari