- 1Department of Rhinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2School of Acupuncture-Moxibustion and Tuina, Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Neurosurgery, Shanghai Tenth People’s Hospital of Tongji University, Shanghai, China
- 4Department of Geriatrics, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 5Department of Respiratory Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 6Department of Oncology, The Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
- 7Key Laboratory of Molecular Pathology in Tumors of Guangxi Higher Education Institutions, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
Laryngeal cancer remains a formidable clinical challenge, with growing evidence that vitamin D3 acts as a potential therapeutic modulator. However, its precise role is complex, largely due to poor understanding of the mechanisms underlying its variable efficacy. This review synthesizes current knowledge to establish a comprehensive framework for vitamin D3’s dichotomous role in laryngeal carcinogenesis. First, we clarify its two distinct mechanisms of action: (i) directly inhibiting laryngeal cancer cell proliferation and survival via the canonical vitamin D receptor (VDR) axis—triggering G0/G1 cell cycle arrest, inducing apoptosis, and reversing epithelial-mesenchymal transition (EMT); (ii) indirectly exerting anti-tumor effects by reprogramming the tumor immune microenvironment, including enhancing cytotoxicity of CD8+ T and natural killer (NK) cells, promoting dendritic cell maturation, and suppressing key inflammatory pathways such as the COX-2/PGE2 axis. Subsequently, we propose that the net effect of vitamin D3 signaling is context-dependent and double-edged, determined mainly by host-intrinsic and viral factors—most notably estrogen receptor α (ERα66) expression. Specifically, vitamin D3-related products promote cell growth in ERα66-positive laryngeal cancer cell lines, but suppress growth in ERα66-negative lines, thereby aiding cancer therapy. This integration provides a nuanced paradigm, highlighting the need for biomarker-driven patient stratification to harness vitamin D3’s therapeutic potential in laryngeal cancer.
1 Introduction
Head and neck malignancies rank seventh among the most prevalent cancers worldwide, with laryngeal carcinoma accounting for approximately one-fifth of these cases (1). Laryngeal cancer is a malignant neoplasm originating from laryngeal tissues and constitutes a major global health burden (2). According to 2022 data released by the World Health Organization, there were an estimated 188960 new cases of laryngeal cancer globally, resulting in approximately 103216 deaths. Both incidence and mortality display pronounced sex disparities. Epidemiological studies indicate a male-to-female incidence ratio of roughly 4:1. In certain regions, the incidence among men is markedly higher, with population-based surveys reporting ratios approaching 10:1 (3). A similar trend is observed in mortality, with more men than women succumbing to laryngeal cancer, likely attributable to differences in lifestyle factors and biological characteristics between sexes (3). Laryngeal cancer can be classified according to anatomical location and histological features. Anatomically, it is divided into three main types: supraglottic carcinoma, glottic carcinoma, and subglottic carcinoma (4). Histologically, most laryngeal cancers are identified as squamous cell carcinomas originating from the laryngeal squamous epithelium (5). Less common histological variants include adenocarcinomas (originating from glandular cells) and sarcomas (originating in connective tissues, including muscle and cartilage) (5). Vitamin D encompasses several fat-soluble compounds that are essential micronutrients required for maintaining human health. It comprises vitamin D2 and vitamin D3; the former is predominantly sourced from plants following ultraviolet activation, while the latter is chiefly acquired from animal products or produced endogenously in the skin in response to ultraviolet radiation. The present article centers on the function of vitamin D3 (6). Vitamin D3 itself is inactive and requires sequential hydroxylation to generate active metabolites. Specifically, upon entering the circulation, vitamin D3 associates with vitamin D-binding protein (DBP), facilitating its transport to the liver (7). Within the liver, vitamin D3 undergoes hydroxylation by 25-hydroxylase (encoded by CYP2R1), resulting in the formation of 25-hydroxyvitamin D3, the main storage form. Following hepatic conversion, the 25-(OH)D3-DBP complex is transported to renal tissue, where CYP27B1-encoded 1α-hydroxylase catalyzes the final hydroxylation step, producing the biologically active hormone 1,25-dihydroxyvitamin D3. While another enzyme in the kidney, 24-hydroxylase (encoded by the gene CYP24A1), can hydroxylate it into 24R,25-(OH)2D3(an active native conformer of 24,25-(OH)2D3). After exerting its biological effects in cells and tissues, 1,25-(OH)2D3 is further hydroxylated by 24-hydroxylase (encoded by CYP24A1) into inactive 1,24,25-trihydroxyvitamin D3 (1,24,25-(OH)3D3) in liver prior to excretion; this constitutes the classical pathway of vitamin D3 metabolism (8–10). However, accumulating evidence indicates that vitamin D3 can also be synthesized locally via paracrine pathways. Dendritic cells and macrophages secrete 1,25-(OH)2D3 to suppress excessive immunity or modulate cell differentiation (11). In the cutaneous microenvironment, keratinocytes together with skin-resident immune populations locally synthesize 1,25-dihydroxyvitamin D3, thereby orchestrating epidermal turnover, lineage-specific differentiation, inflammatory tone, and tissue repair after injury (12). Tissues including breast, prostate, pancreas, and larynx possess local vitamin D3-converting capacity, potentially participating in cell proliferation control and tissue homeostasis (13–17). Moreover, whereas 24R,25-(OH)2D3 was traditionally considered metabolically inert (18), recent studies reveal its biological activity in laryngeal cancer cells, modulating cell-cycle progression (19). Clinical evidence demonstrates that reduced vitamin D3 levels correlate significantly with poor survival in advanced laryngeal cancer patients undergoing total laryngectomy (20, 21), suggesting an important role of vitamin D3 in laryngeal cancer pathogenesis and therapy. “Vitamin D3 axis” in the title refers to the entire pathway, encompassing the dietary intake and endogenous synthesis of vitamin D3, its metabolic activation into 25-hydroxyvitamin D3 and the hormonal form 1,25-dihydroxyvitamin D3, VDR, and the subsequent downstream genomic and non-genomic signaling events. As an essential fat-soluble vitamin, vitamin D3 participates in laryngeal cancer pathophysiology via two primary mechanisms: First, direct regulation of tumor cell biology, and second, modulation of the host immune microenvironment (22). For the former, we focus on cell-cycle control and other cancer-cell-intrinsic mechanisms; for the latter, on immune-cell modulation and key immunoregulatory molecules. We also highlight estrogenic influences, as the hormone-sensitivity status of laryngeal cancer remains unresolved. Additionally, whether laryngeal cancer is hormone-sensitive remains debated. Although traditional views attribute sex disparities in incidence mainly to differential smoking rates, emerging evidence implicates estrogen signaling (23). Notably, when laryngeal cancer cells express estrogen receptors, estrogen may interfere with vitamin D3 bioactivity and modulate the paracrine processes of vitamin D3-active metabolites within the tumor microenvironment (24, 25). This underexplored crosstalk is clinically significant for understanding sex-based differences in laryngeal cancer and for developing sex-specific therapeutic strategies.
2 Vitamin D3 regulates laryngeal cancer cell growth and migration
2.1 Vitamin D3 influences the laryngeal cancer cell cycle
The biologically active form of D3(1,25-(OH)2D3)binds to the VDR and arrests cells in the G0/G1 phase, thereby inhibiting proliferation and inducing differentiation in various malignancies, such as cell lines of head and neck squamous cell carcinoma (SCCHN) (26). Specifically, it markedly induces expression of the cell-cycle inhibitors p21 and p27; p21 and p27 bind and inhibit CDK2–cyclin E and CDK2–cyclin A complexes, preventing phosphorylation of retinoblastoma protein (Rb). Hypophosphorylated Rb sequesters the transcription factor E2F, suppressing expression of S-phase genes (e.g., those required for DNA replication) and blocking G1-to-S transition, thus arresting cells in G0/G1 (27–30). 1,25-(OH)2D3 also promotes p38 phosphorylation to its active form; activated p38 indirectly controls the cell cycle by inducing p21 expression (31). Furthermore, the vitamin D3 analogue EB1089 up-regulates p57 expression and synergizes with p21 and other cell-cycle inhibitors to induce G1 arrest and inhibit cancer cell proliferation (32). Vitamin D3 arrests the laryngeal cancer cell cycle as a cornerstone of its antiproliferative effect, yet concurrently targets multiple signaling axes and phenotypic plasticity to orchestrate a multi-dimensional suppression of tumor cell behavior.
2.2 Additional regulatory mechanisms
Treatment with 1,25-(OH)2D3 exerts a significant inhibitory effect on the IL-6–JAK–STAT3 signaling pathway in cancer cells (33). Although direct studies in laryngeal cancer are lacking, existing evidence suggests that: VDR is expressed in laryngeal cancer cells and influences tumorigenesis and prognosis (21). VDR protein binds the Jak2 promoter, transcriptionally down-regulating Jak2 expression (34). When 1,25-(OH)2D3 binds, the VDR–RXR heterodimer can competitively bind the dimerization domain of STAT3, preventing formation of functional p-STAT3 dimers. The VDR–RXR dimer can also occupy NF-κB binding sites within the IL-6 promoter to inhibit NF-κB-mediated transcriptional activation of IL-6 (35). At appropriate concentrations, 1,25-(OH)2D3 time- and dose-dependently inhibits the PI3K/AKT/Bcl-2 pathway, inducing apoptosis in Hep-2 laryngeal carcinoma cells (36). Epithelial–mesenchymal transition (EMT) denotes a dynamic process whereby epithelial cells lose epithelial characteristics and acquire mesenchymal phenotypes, thereby enhancing migratory and invasive capacities. In vitro knockdown of Snail inhibits EMT in LSCC cells via the VDR signaling pathway (37, 38). Snail directly binds three E-boxes within the promoter of the epithelial marker E-cadherin, repressing its expression while up-regulating mesenchymal markers such as matrix metalloproteinases (MMP)-2 and MMP-9, disrupting epithelial cell–cell contacts and conferring increased motility, thereby facilitating invasion and metastasis (37, 39). As the tumor microenvironment concept matures, peritumoral cells gain prominence, and vitamin D3’s modulation of these bystanders in laryngeal cancer is equally pivotal.
3 Vitamin D3 modulates the laryngeal cancer immune microenvironment
3.1 Vitamin D3 enhances immune cell infiltration and differentiation
Vitamin D3 promotes infiltration of CD3+, CD8+, and NKR-P1C+ immune cells within the tumor microenvironment, reduces M2 macrophages and regulatory T cells (Tregs), and thus impedes tumor immune escape (33, 40). An elevated count of immunosuppressive CD34+ progenitor cells is detected in both peripheral blood and tumor tissues. Tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) induces expansion of these CD34+ cells, and tumor-secreted vascular endothelial growth factor (VEGF) chemoattracts them to the tumor site (41). These CD34+ cells suppress autologous T-cell function; removal of CD34+ cells markedly enhances IFN-γ production by T cells stimulated with anti-CD3 antibody and low-dose IL-2 (41, 42). Treatment with 1,25-(OH)2D3 decreases intratumoral CD34+ progenitor cells in HNSCC patients, promotes their differentiation into dendritic cells, and increases intratumoral T-cell infiltration (43, 44), supporting further investigation of 1,25-(OH)2D3-mediated immunomodulation within the tumor microenvironment (42, 44, 45). 25-(OH)D3 elevates HLA-DR expression and increases plasma IL-12 and IFN-γ levels while improving T-cell proliferative responses. 1,25-(OH)2D3 induces expression of the pattern-recognition receptor CD14 gene in epithelial cells (46) and drives the monocytic cell line HL60 toward monocyte or macrophage differentiation. The T1/ST2 protein (IL-1 receptor family member) gene is also strongly induced; murine knockout studies demonstrate that T1/ST2 signaling is essential for Th2 differentiation (46). 1,25-(OH)2D3 increases CD4+ and CD8+ T-cell levels and augments intratumoral populations expressing the early activation marker CD69. Additionally, 1,25-(OH)2D3 reduces tumor angiogenesis, thereby inhibiting tumor progression and metastasis (Figure 1). Next, we dissect at the molecular level how vitamin D3 precisely reprograms immune microenvironmental cells: (i) by modulating cyclo-oxygenase-2 activity and (ii) by reshaping the expression and secretion of key inflammatory mediators.
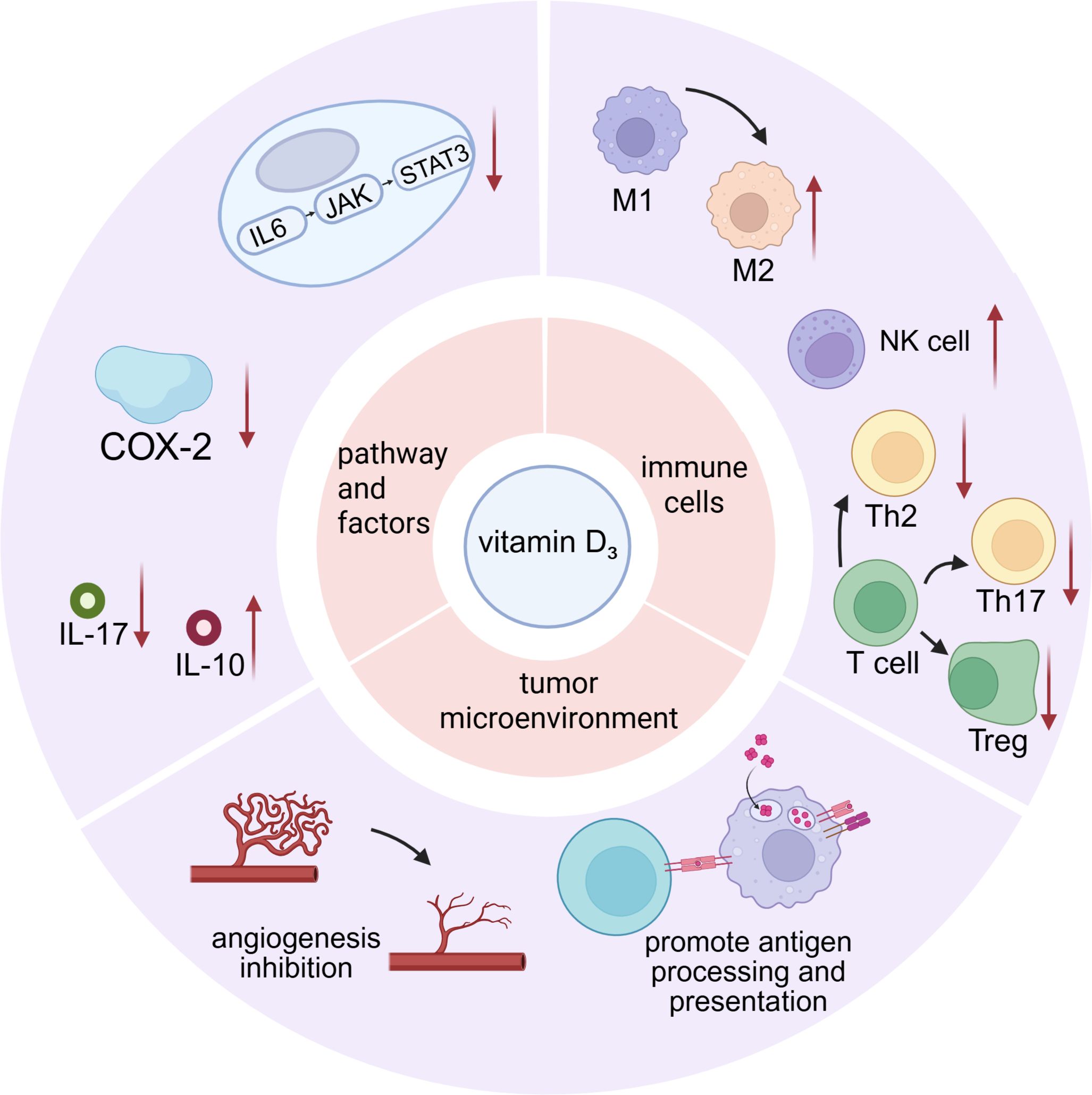
Figure 1. The multifaceted effects of vitamin D3 on the cells, molecules, and immune microenvironment.
3.2 COX-2 plays a pivotal role in the immune response modulated by vitamin D3
Cyclo-oxygenase-2 (COX-2) is an enzyme closely linked to immune responses. Its catalytic product PGE2 drives a shift of helper T cells from Th1 to Th2; imbalance of Th1/Th2 ratios causes immune dysregulation. PGE2 also polarizes macrophages from M1 to M2 phenotype; M2 macrophages possess immunosuppressive properties, secreting IL-10 and TGF-β to inhibit antitumor immunity. Furthermore, PGE2 induces development of Tregs, Th17 cells, and myeloid-derived suppressor cells (MDSCs) while suppressing dendritic and NK cell functions, thereby fostering a tumor-permissive immune milieu (40, 47, 48). 1,25-(OH)2D3 alone down-regulates overexpressed COX-2 in both tumor and immune cells, reducing production of inflammatory mediators such as prostaglandin E2 (PGE2) and thereby alleviating immunosuppression and inflammation (1). However, when combined with the commonly used chemotherapeutic agent cisplatin, vitamin D3 up-regulates COX-2 expression within the laryngeal mucosal epithelial stroma, potentially exacerbating mucosal injury and inflammation (49) (Figure 1).
3.3 Vitamin D3 modulates tumor microenvironment via pro-inflammatory cytokines
While delineating vitamin D3 mechanisms, we interrogate the determinants of its efficacy. Beyond smoking and HPV, we focus on sex steroids—an emerging but understudied modulator of laryngeal cancer outcome. As stated previously, IL-6 activates the IL-6–JAK–STAT3 pathway to promote tumor growth. 1,25-(OH)2D3 downregulates pro-inflammatory cytokines (IL-6, IL-17, TNF-α) and the immunosuppressive cytokine IL-1, thereby mitigating cancer-associated chronic inflammation. As an immunosuppressive cytokine, IL-10 limits T-cell activation via suppression of dendritic cell maturation and antigen presentation (50, 51). 1,25-(OH)2D3 increases HLA-DR expression, elevates plasma IL-12 and IFN-γ levels, and enhances T-cell proliferation (45). Within the tumor milieu, Th17 (CD4+) cells exhibit pro-inflammatory and pro-angiogenic properties and can differentiate into immunosuppressive Tregs (52). Th17 cells exert their effects primarily via IL-17 production, and 1,25-(OH)2D3 reduces IL-17 levels, thereby attenuating Th17-mediated disease progression (51). Additionally, studies reveal discordant cytokine responses to 1,25-(OH)2D3 between tumor tissue and peripheral blood; plasma cytokine profiles may not accurately reflect intratumoral immune status (53) (Figure 1).
4 Estrogen is a critical regulator of vitamin D3 actions on tumor and immune cells
The vitamin D3 derivative 24R,25-dihydroxyvitamin D3 exerts cell-type-specific effects on laryngeal carcinoma cells, which are modulated by the status of the estrogen receptor α66 (ERα66). 24R,25-dihydroxyvitamin D3, when acting on human head and neck squamous cell carcinoma cell line with estrogen receptor α66 negativity (UM-SCC-11A cells), suppresses proliferation, upregulates apoptosis-related markers (TUNEL positivity, p53 expression, and BAX/BCL2 ratio), and downregulates metastasis-associated markers, with these effects collectively reflecting its tumor-suppressive capacity (17). Conversely, in human head and neck squamous cell carcinoma cell line with estrogen receptor α66 positivity (UM-SCC-12 cells), 24R,25-(OH)2D3 promotes multiplication, reduces DNA fragmentation (TUNEL-negative), and increases total p53, reflecting tumor promotion (19). Previous research has established that laryngeal cancer cells exhibit individual differences in their responsiveness to vitamin D3 supplementation, with recent investigations identifying ERα66 expression as a key determinant of this variability. Laryngeal cancer cells possess the capacity for local vitamin D3 metabolism, a process that in turn modulates cellular fate and shapes the tumor microenvironment. Estrogen modulates expression of vitamin D3 hydroxylases (25), and laryngeal cancer cells possess the capacity to synthesize estrogen, exerting autocrine and paracrine effects (23). Hydroxylases CYP27B1 and CYP24A1 are critical enzymes in vitamin D3 metabolism: CYP27B1 converts 25-(OH)D3 to 1,25-(OH)2D3, whereas CYP24A1 hydroxylates 25-(OH)D3 to 24R,25-(OH)2D3 and further converts 1,25-(OH)2D3 to 1,24,25-(OH)3D3 (24). Both enzymes are expressed in laryngeal cancer cells; ERα66 exerts an inhibitory effect on these activities, resulting in decreased biosynthesis of active 1,25-(OH)2D3 and 24R,25-(OH)2D3 in UM-SCC-12 cells relative to UM-SCC-11A cells; this, in turn, impacts tumor progression and immune cell functionality within the microenvironment. Investigations have demonstrated that 24R,25-(OH)2D3 exerts its effects through the phospholipase D (PLD), caveolae, and palmitoylation pathways. For example, 24R,25-(OH)2D3 increases PLD activity in UM-SCC-12 cells but decreases it in UM-SCC-11A cells; inhibiting PLD activity or palmitoylation, or silencing caveolin-1 expression, alters p53 levels. p53 is a key cell-cycle checkpoint molecule, and these perturbations modulate tumor behavior—for instance, promoting p21 expression and G1/G2 arrest in UM-SCC-11A cells (54). In UM-SCC-12 cells, 24R,25-(OH)2D3 docks with a membrane complex composed of TLCD3B2, VDR and protein disulfide-isomerase A3 (PDIA3); this interaction is palmitoylation-dependent and requires coordinated PLD–PI3K–LPAR activity. In contrast, UM-SCC-11A cells utilize a VDR–PDIA3–ROR2 complex that triggers endosomal signaling cascades, the molecular details of which remain undefined (55). Additionally, estrogen via ERα66 modulates paracrine effects of vitamin D3 and its metabolites, influencing immune cell infiltration and differentiation within the tumor microenvironment. The paracrine effects of vitamin D3 and its active metabolites on cells modulate immune cell infiltration and differentiation within the tumor microenvironment. Specifically, active vitamin D3 secreted into the tumor milieu downregulates MHC class II molecules on dendritic cells (DCs), thereby attenuating their antigen-presenting capacity. Additionally, active vitamin D3 or its analogs suppress DC-derived cytokine production, particularly interleukin (IL)-12—which directs helper T-cell differentiation toward the Th1 phenotype—and IL-23, which promotes Th17 differentiation (56). In macrophages, active vitamin D3 primarily regulates polarization, shifting macrophages from the M2 to the M1 phenotype, as evidenced by upregulated M1 markers CD11c and concomitant suppression of M2 markers CD16 (40) (Figure 2).
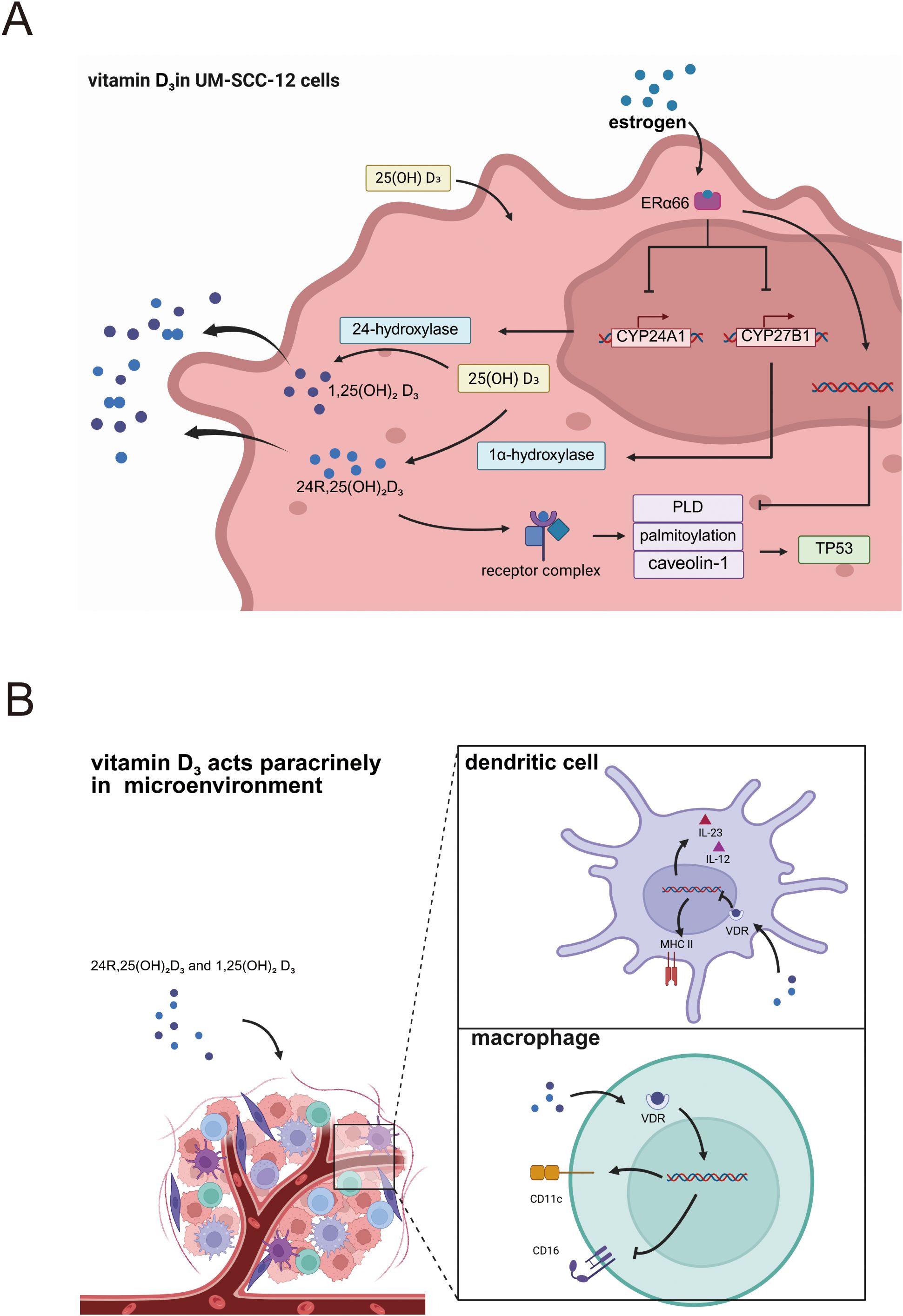
Figure 2. (A) Mechanisms of vitamin D3 action in laryngeal cancer cells expressing estrogen receptors. (B) Paracrine vitamin D3 shapes immune cells within the laryngeal-cancer immune microenvironment.
Research directly investigating the role of 24R,25-(OH)2D3 in laryngeal cancer is limited. Therefore, its potential molecular mechanisms in laryngeal cancer cells and their associated immune cells are largely extrapolated from studies in other cell types, such as osteoblasts. The proposed mechanism via the canonical VDR-dependent pathway is as follows: Upon cellular entry, 24R,25-(OH)2D3 first binds to the Vitamin D Receptor (VDR), forming a 24R,25-(OH)2D3-VDR complex (57). Subsequently, this complex must assemble with the Retinoid X Receptor (RXR) to form a heterodimer, a critical structural step for initiating downstream transcriptional regulation (57). The 24R,25-(OH)2D3-VDR-RXR heterodimer then targets and binds to Vitamin D Response Elements (VDREs) located in the promoter regions of target genes (58). However, due to its relatively weak binding affinity, this process often requires the assistance of Nuclear Auxiliary Factors (NAFs) to enhance binding efficiency. Ultimately, the heterodimer, once bound to the VDRE, modulates the transcriptional activity of target genes by recruiting co-activator or co-repressor complexes. This, in turn, influences the expression of downstream genes, thereby regulating biological functions such as cell proliferation, differentiation, and immune modulation (59). The mechanisms of action for 24R,25-(OH)2D3 also encompass a VDR-independent pathway. For instance, 24R,25-(OH)2D3 can bind to the cell membrane of chondrocytes, leading to the activation of Protein Kinase C (PKC). This subsequently influences the Mitogen-Activated Protein Kinase (MAPK) pathway, ultimately resulting in new gene expression through a process independent of VDR (60). Furthermore, the specific molecular interplay between VDR and the Estrogen Receptor (ER) is a key area of investigation. Insights can be drawn from breast cancer, another hormone-dependent malignancy analogous to laryngeal cancer. In breast cancer cells, Estrogen-Related Receptor alpha (ERRα) regulates gene expression and transcription through two primary mechanisms. First, ERRα can directly bind to the promoters of the CYP24A1, ERα, and aromatase (CYP19A1) genes, or recruit co-activators like p300 to alter chromatin conformation (61, 62). These actions respectively promote: the degradation of active vitamin D by CYP24A1, thereby interfering with calcitriol-VDR transcription, the enhancement of estrogen signaling by ERα, and the elevation of local estrogen levels by aromatase—all of which favor cancer cell growth. Second, the Ligand-Binding Domain (LBD) of ERRα binds to the LxxLL/LLxxL motifs of PGC-1α (63, 64). This complex then recruits the CBC and Mediator complexes via the CBM and RS domains of PGC-1α. Subsequently, it assembles with VDR to form a larger transcriptional complex. Upon binding to the target gene’s VDRE, this complex efficiently promotes target gene expression by recruiting RNA Polymerase II, facilitating transcriptional elongation, and preventing premature termination, thereby influencing cancer cell behavior (65). However, it is crucial to note that these molecular mechanisms cannot be directly extrapolated from breast cancer to laryngeal cancer, as the two malignancies exhibit distinct and sometimes contradictory experimental and clinical manifestations.
5 Discussion
Vitamin D3 orchestrates a complex regulatory network in laryngeal cancer initiation, progression, and immune microenvironmental remodeling. This study systematically delineates its dual-pathway impact: (i) via the canonical vitamin D receptor axis, directly modulating tumor cell biology, including p21/p27/p57-dependent G0/G1 arrest (27–30, 32), PI3K/AKT/Bcl-2 inhibition-mediated apoptosis (36), and Snail down-regulation-reversed EMT (37, 39); and (ii) by reshaping the immune microenvironment, it exerts anti-tumor effects through reducing CD34+ immunosuppressive progenitor infiltration and promoting their differentiation into dendritic cells (42–44), enhancing CD8+ T-cell and NK-cell activity (33, 45), and suppressing the COX-2/PGE2 pathway and pro-inflammatory cytokines IL-6/IL-17/TNF-α (33, 47, 51). Notably, these regulatory effects are constrained by a triad of factors, namely ERα66 status, HPV infection, and VDR/CYP24A1 polymorphisms (17, 19, 21, 33), constituting the molecular basis for heterogeneous therapeutic responsiveness. ERα66, a key mediator of sexual dimorphism in laryngeal cancer, plays a critical role in regulating vitamin D3 metabolism and its biological functions. Specifically, in ERα66-negative cells (UM-SCC-11A), 24R,25-(OH)2D3 inhibits cellular proliferation and triggers apoptosis through the activation of the p53/p21 pathway (17, 19), whereas in ERα66-positive cells (UM-SCC-12), the same metabolite promotes tumor progression (19). This paradoxical effect arises from ERα66-mediated suppression of local vitamin D3 hydroxylases: ERα66 downregulates CYP27B1 and CYP24A1 activities (23–25), diminishing the generation of anti-tumoral 1,25-(OH)2D3 and disrupting paracrine control of immune cells by vitamin D3 metabolites (54). These findings offer a mechanistic explanation for the higher incidence of laryngeal cancer in males and underscore the centrality of estrogen–VDR crosstalk in microenvironmental remodeling (3, 23). However, direct evidence demonstrating that estrogen modulates vitamin D3 signaling in laryngeal cancer remains scarce. Most inferences are extrapolated from breast-cancer models. More nuanced and context-specific investigations are therefore urgently required. Furthermore, smoking, body weight, gender, host genetic polymorphisms, and HPV status are also recognized as crucial factors influencing the progression and prognosis of laryngeal cancer (66). A significant proportion of patients with this malignancy have a history of smoking (67). Moreover, compared with current smokers, former smokers exhibit a substantially reduced laryngeal-cancer risk (68). Studies have indicated that men with lower abdominal adiposity are at a greater risk of developing laryngeal cancer than females with higher abdominal adiposity (69). HPV status also dictates therapeutic responsiveness: 1,25-(OH)2D3 suppresses the MYC oncogenic program in HPV-positive cells but may activate it in HPV-negative contexts 1 (33). Associations between VDR/CYP24A1 polymorphisms and the recurrence risk of glottic carcinoma further emphasize the need for genotype-guided therapy (21). Prospective studies further indicate that the therapeutic and prognostic impact of vitamin D3 in laryngeal cancer exhibits substantial inter-individual heterogeneity (70). These factors should serve as stratification criteria. Based on the foregoing evidence, the following clinically actionable strategies for laryngeal cancer can be advanced: patients should be pre-stratified based on ERα66 expression detected via immunohistochemistry: for ERα66-negative patients, 24R,25-(OH)2D3 should be administered as adjuvant therapy; for ERα66-positive patients, vitamin D3 analogs (e.g., EB1089) should be combined with ERα66 inhibitors. The core goal of these treatment strategies is to reverse the suppression of CYP27B1/CYP24A1 and thereby restore the biosynthesis of active metabolites (71). It is possible to combine vitamin D3 with immune checkpoint inhibitors (ICIs): in resectable cases, use high-dose 25-(OH)D3 for 2–4 weeks pre-ICI to enhance CD8+ T-cell infiltration and suppress COX-2/PGE2; for unresectable patients, further stratify by HPV (prioritizing HPV-positive cohorts, where 1,25-(OH)2D3 suppresses MYC) to optimize benefit (72, 73). In the clinical setting, CYP27B1/CYP24A1-targeted agents may also be considere. Test CYP24A1 inhibitors in patients with VDR/CYP24A1 polymorphisms, monitoring intratumoral 1,25-(OH)2D3 levels and Ki-67 to validate “metabolite-guided” dosing (74). Clinical translation of vitamin D3 faces multiple contradictions. Although low vitamin D3 levels correlate with poor prognosis (20, 21), several limitations exist. First, combined use with cisplatin may exacerbate mucositis via COX-2 upregulation (49), necessitating cautious combination strategies. Second, peripheral vitamin D3 levels and cytokine profiles poorly mirror the intratumoral immune landscape (53), limiting the utility of systemic biomarkers. Finally, head and neck cancer patients frequently exhibit vitamin D3 deficiency, yet supplementation strategies must be tailored to ERα66 strata. To date, multiple clinical studies have confirmed that vitamin D3 can improve the prognostic survival rate of patients with laryngeal cancer, while vitamin D3 deficiency is a risk factor for laryngeal cancer development (75, 76). More targeted clinical studies are needed to further enrich the evidence base in this field.
6 Conclusion
Vitamin D3 exerts a profound yet dichotomous influence on laryngeal cancer, acting as a master regulator at the nexus of direct tumor cell biology and immune microenvironment remodeling. Its function transcends a simple anti-proliferative role; instead, it operates as a context-dependent ‘rheostat,’ where its ultimate anti-tumor efficacy is contingent upon the tumor’s specific molecular landscape, notably the host’s ERα66 expression. This understanding necessitates a paradigm shift from a ‘one-size-fits-all’ supplementation strategy towards precision-guided interventions. Future research must prioritize clinical trials stratified by these biomarkers to validate therapeutic efficacy. Furthermore, exploring synergistic combinations of vitamin D3 with immune checkpoint inhibitors, and developing novel agents targeting key metabolic enzymes like CYP27B1/CYP24A1 to optimize local active metabolite concentrations, represent promising avenues. Integrating these multi-level insights will be pivotal for translating the complex biology of vitamin D3 into tangible, personalized therapeutic benefits for patients with laryngeal cancer.
Author contributions
QG: Writing – original draft, Writing – review & editing. YC: Writing – original draft, Writing – review & editing. QZ: Writing – original draft, Writing – review & editing. DD: Writing – original draft, Writing – review & editing. SH: Writing – original draft, Writing – review & editing. MS: Writing – original draft, Writing – review & editing. BZ: Writing – original draft, Writing – review & editing. LP: Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. This research was partially funded by the National Natural Science Foundation of China (82071023), the Key Project of Medical Science and Technology of Henan Province (SBGJ202102160), and the Scientific Research Project of Colleges and Universities in Henan Province (21A320053).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Starska-Kowarska K. Role of vitamin D in head and neck cancer-immune function, anti-tumour effect, and its impact on patient prognosis. Nutrients. (2023) 15:2592. doi: 10.3390/nu15112592
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
4. Steuer CE, El-Deiry M, Parks JR, Higgins KA, and Saba NF. An update on larynx cancer. CA Cancer J Clin. (2017) 67:31–50. doi: 10.3322/caac.21386
5. Thompson L. World health organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. (2006) 85:74. doi: 10.1177/014556130608500201
6. Saponaro F, Saba A, and Zucchi R. An update on vitamin D metabolism. Int J Mol Sci. (2020) 21:6573. doi: 10.3390/ijms21186573
7. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. (2004) 80:1678s–88s. doi: 10.1093/ajcn/80.6.1678S
8. Borel P, Caillaud D, and Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. (2015) 55:1193–205. doi: 10.1080/10408398.2012.688897
9. Ganmaa D, Bromage S, Khudyakov P, Erdenenbaatar S, Delgererekh B, and Martineau AR. Influence of vitamin D supplementation on growth, body composition, and pubertal development among school-aged children in an area with a high prevalence of vitamin D deficiency: A randomized clinical trial. JAMA Pediatr. (2023) 177:32–41. doi: 10.1001/jamapediatrics.2022.4581
10. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. (2008) 88:582s–6s. doi: 10.1093/ajcn/88.2.582S
11. Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells1. J Immunol. (2003) 170:5382–90. doi: 10.4049/jimmunol.170.11.5382
12. Segaert S and Simonart T. The epidermal vitamin D system and innate immunity: some more light shed on this unique photoendocrine system? Dermatology. (2008) 217:7–11. doi: 10.1159/000118506
13. Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. (2011) 347:55–60. doi: 10.1016/j.mce.2011.05.020
14. Voutsadakis IA. Vitamin D receptor (Vdr) and metabolizing enzymes cyp27b1 and cyp24a1 in breast cancer. Mol Biol Rep. (2020) 47:9821–30. doi: 10.1007/s11033-020-05780-1
15. Krishnan AV, Peehl DM, and Feldman D. The role of vitamin D in prostate cancer. Recent Results Cancer Res. (2003) 164:205–21. doi: 10.1007/978-3-642-55580-0_15
16. Morró M, Vilà L, Franckhauser S, Mallol C, Elias G, Ferré T, et al. Vitamin D receptor overexpression in β-cells ameliorates diabetes in mice. Diabetes. (2020) 69:927–39. doi: 10.2337/db19-0757
17. Dennis CD, Dillon JT, Patel PH, Cohen DJ, Halquist MS, Pearcy AC, et al. Laryngeal cancer cells metabolize 25-hydroxyvitamin D3 and respond to 24r,25-dihydroxyvitamin D3 via a mechanism dependent on estrogen receptor levels. Cancers. (2024) 16:1635. doi: 10.3390/cancers16091635
18. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. (2006) 92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016
19. Dennis CD, Cohen DJ, Boyan BD, and Schwartz Z. Abstract 2693: The vitamin D3 metabolite, 24R,25(OH)3D3 is differentially tumorigenic in ER+ and ER- laryngeal cancer cells in vitro. Cancer Res. (2022) 82:2693. doi: 10.1158/1538-7445.AM2022-2693
20. Radivojevic N, Grujicic SS, Suljagic V, Stojkovic S, Arsovic K, Jakovljevic S, et al. Prognostic value of serum 25-hydroxyvitamin D levels and malnutrition status on postoperative complications in patients following laryngectomy with neck dissection. Eur Arch Otorhinolaryngol. (2025) 282:341–9. doi: 10.1007/s00405-024-09046-5
21. Edizer DT, Leblebici A, Ergun U, Yilmaz F, Koc A, Basbinar Y, et al. Effects of vdr and cyp24a1 gene polymorphisms on the outcome of supraglottic larynx cancer. Eur Rev Med Pharmacol Sci. (2024) 28:2168–78. doi: 10.26355/eurrev_202403_35720
22. Mora JR, Iwata M, and von Andrian UH. Vitamin effects on the immune system: vitamins a and D take centre stage. Nat Rev Immunol. (2008) 8:685–98. doi: 10.1038/nri2378
23. Verma A, Schwartz N, Cohen DJ, Patel V, Nageris B, Bachar G, et al. Loss of estrogen receptors is associated with increased tumor aggression in laryngeal squamous cell carcinoma. Sci Rep. (2020) 10:4227. doi: 10.1038/s41598-020-60675-2
24. Jacobs ET, Van Pelt C, Forster RE, Zaidi W, Hibler EA, Galligan MA, et al. Cyp24a1 and cyp27b1 polymorphisms modulate vitamin D metabolism in colon cancer cells. Cancer Res. (2013) 73:2563–73. doi: 10.1158/0008-5472.Can-12-4134
25. Pike JW, Spanos E, Colston KW, MacIntyre I, and Haussler MR. Influence of estrogen on renal vitamin D hydroxylases and serum 1alpha,25-(Oh)2d3 in chicks. Am J Physiol. (1978) 235:E338–43. doi: 10.1152/ajpendo.1978.235.3.E338
26. Cai P, Wu Z, and Li J. in vitro inhibition of human laryngeal squamous cell carcinoma cell line by 1,25(Oh)2d3. Lin Chuang Er Bi Yan Hou Ke Za Zhi. (2006) 20:538–40.
27. Hager G, Kornfehl J, Knerer B, Weigel G, and Formanek M. Molecular analysis of P21 promoter activity isolated from squamous carcinoma cell lines of the head and neck under the influence of 1,25(Oh)2 vitamin D3 and its analogs. Acta Otolaryngol. (2004) 124:90–6. doi: 10.1080/00016480310015353
28. Yoon MK, Mitrea DM, Ou L, and Kriwacki RW. Cell cycle regulation by the intrinsically disordered proteins P21 and P27. Biochem Soc Trans. (2012) 40:981–8. doi: 10.1042/bst20120092
29. Gartel AL, Serfas MS, and Tyner AL. P21–negative regulator of the cell cycle. Proc Soc Exp Biol Med. (1996) 213:138–49. doi: 10.3181/00379727-213-44046
30. Hager G, Formanek M, Gedlicka C, Thurnher D, Knerer B, and Kornfehl J. 1,25(Oh)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Otolaryngol. (2001) 121:103–9. doi: 10.1080/000164801300006353
31. Lu LJ, Zha DJ, Xue T, and Qiu JH. Inhibitory effect of 1,25(Oh)2d3 on proliferation of human laryngeal carcinoma cells and potential mechanisms. Ai Zheng. (2009) 28:691–4. doi: 10.5732/cjc.008.10801
32. Lu L, Qiu J, Liu S, and Luo W. Vitamin D3 analogue eb1089 inhibits the proliferation of human laryngeal squamous carcinoma cells via P57. Mol Cancer Ther. (2008) 7:1268–74. doi: 10.1158/1535-7163.Mct-07-2222
33. Brust LA, Linxweiler M, Schnatmann J, Kühn JP, Knebel M, Braun FL, et al. Effects of vitamin D on tumor cell proliferation and migration, tumor initiation and anti-tumor immune response in head and neck squamous cell carcinomas. BioMed Pharmacother. (2024) 180:117497. doi: 10.1016/j.biopha.2024.117497
34. Zhang YG, Lu R, Wu S, Chatterjee I, Zhou D, Xia Y, et al. Vitamin D receptor protects against dysbiosis and tumorigenesis via the jak/Stat Pathway in Intestine. Cell Mol Gastroenterol Hepatol. (2020) 10:729–46. doi: 10.1016/j.jcmgh.2020.05.010
35. van Etten E and Mathieu C. Immunoregulation by 1,25-Dihydroxyvitamin D3: Basic Concepts. J Steroid Biochem Mol Biol. (2005) 97:93–101. doi: 10.1016/j.jsbmb.2005.06.002
36. Gui M, Li B, Qi S, and Zhou C. 1,25-Dihydroxyvitamin D_3 Induced hep-2 cells apoptosis through pi3k/akt/bcl-2 signaling pathway. Jounral Chongqing Med Univ. (2017) 42:1422–5.
37. Zhao X, Yu D, Yang J, Xue K, Liu Y, and Jin C. Knockdown of snail inhibits epithelial-mesenchymal transition of human laryngeal squamous cell carcinoma hep-2 cells through the vitamin D receptor signaling pathway. Biochem Cell Biol. (2017) 95:672–8. doi: 10.1139/bcb-2017-0039
38. Wang X, Li K, Wan Y, Chen F, Cheng M, Xiong G, et al. Methyltransferase like 13 mediates the translation of snail in head and neck squamous cell carcinoma. Int J Oral Sci. (2021) 13:26. doi: 10.1038/s41368-021-00130-8
39. Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by muc1 repression and zeb1 expression. J Biol Chem. (2002) 277:39209–16. doi: 10.1074/jbc.M206400200
40. Bochen F, Balensiefer B, Körner S, Bittenbring JT, Neumann F, Koch A, et al. Vitamin D deficiency in head and neck cancer patients - prevalence, prognostic value and impact on immune function. Oncoimmunology. (2018) 7:e1476817. doi: 10.1080/2162402x.2018.1476817
41. Young MR, Petruzzelli GJ, Kolesiak K, Achille N, Lathers DM, and Gabrilovich DI. Human squamous cell carcinomas of the head and neck chemoattract immune suppressive cd34(+) progenitor cells. Hum Immunol. (2001) 62:332–41. doi: 10.1016/s0198-8859(01)00222-1
42. Lathers DM, Clark JI, Achille NJ, and Young MR. Phase ib study of 25-hydroxyvitamin D(3) treatment to diminish suppressor cells in head and neck cancer patients. Hum Immunol. (2001) 62:1282–93. doi: 10.1016/s0198-8859(01)00317-2
43. Young MR and Lathers DM. Combination docetaxel plus vitamin D(3) as an immune therapy in animals bearing squamous cell carcinomas. Otolaryngol Head Neck Surg. (2005) 133:611–8. doi: 10.1016/j.otohns.2005.05.658
44. Kulbersh JS, Day TA, Gillespie MB, and Young MR. 1alpha,25-dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory cd34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. (2009) 140:235–40. doi: 10.1016/j.otohns.2008.11.011
45. Lathers DM, Clark JI, Achille NJ, and Young MR. Phase 1b study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. (2004) 53:422–30. doi: 10.1007/s00262-003-0459-7
46. Lin R, Nagai Y, Sladek R, Bastien Y, Ho J, Petrecca K, et al. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog eb1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol. (2002) 16:1243–56. doi: 10.1210/mend.16.6.0874
47. Jin K, Qian C, Lin J, and Liu B. Cyclooxygenase-2-prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front Oncol. (2023) 13:1099811. doi: 10.3389/fonc.2023.1099811
48. Chi H, Xie X, Yan Y, Peng G, Strohmer DF, Lai G, et al. Natural killer cell-related prognosis signature characterizes immune landscape and predicts prognosis of hnscc. Front Immunol. (2022) 13:1018685. doi: 10.3389/fimmu.2022.1018685
49. Basak K, Demir MG, Altintoprak N, and Aydin S. The effect of antioxidant agents on cisplatin-induced laryngeal histological alterations in rats. J Medicinal Food. (2021) 24:197–204. doi: 10.1089/jmf.2019.0235
50. Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, et al. 1,25-dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an il-10-dependent mechanism. Immunobiology. (2012) 217:1292–300. doi: 10.1016/j.imbio.2012.07.018
51. Young MR and Day TA. Immune regulatory activity of vitamin D3 in head and neck cancer. Cancers (Basel). (2013) 5:1072–85. doi: 10.3390/cancers5031072
52. Peng G, Chi H, Gao X, Zhang J, Song G, Xie X, et al. Identification and validation of neurotrophic factor-related genes signature in hnscc to predict survival and immune landscapes. Front Genet. (2022) 13:1010044. doi: 10.3389/fgene.2022.1010044
53. Walker DD, Reeves TD, de Costa AM, Schuyler C, and Young MR. Immunological modulation by 1α,25-dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine. (2012) 58:448–54. doi: 10.1016/j.cyto.2012.03.002
54. Dennis CD, Cohen DJ, Dillon JT, Boyan BD, and Schwartz Z. Autocrine regulation of laryngeal cancer tumorigenesis by 24r,25(Oh)2d3. Cancer Res. (2024) 84:2084. doi: 10.1158/1538-7445.Am2024-2084
55. Dennis CD, Cohen DJ, Debnath K, Schwartz N, Lodato BP, Dillon JT, et al. 24r,25(Oh)(2)D(3) regulates tumorigenesis in estrogen sensitive laryngeal cancer cells via membrane-associated receptor complexes in er+ and er- cells. Int J Cancer. (2025). doi: 10.1002/ijc.70141
56. Ao T, Kikuta J, and Ishii M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules. (2021) 11:1624. doi: 10.3390/biom11111624
57. Yasmin R, Williams RM, Xu M, and Noy N. Nuclear import of the retinoid X receptor, the vitamin D receptor, and their mutual heterodimer. J Biol Chem. (2005) 280:40152–60. doi: 10.1074/jbc.M507708200
58. Uchida M, Ozonco K, and Pike JW. In vitro binding of vitamin D receptor occupied by 24r,25-dihydroxyvitamin D3 to vitamin D responsive element of human osteocalcin gene. J Steroid Biochem Mol Biol. (1997) 60:181–7. doi: 10.1016/s0960-0760(96)00194-x
59. Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, et al. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. (1997) 154 Suppl:S57–73.
60. Boyan BD, Sylvia VL, Dean DD, and Schwartz Z. 24,25-(Oh)(2)D(3) regulates cartilage and bone via autocrine and endocrine mechanisms. Steroids. (2001) 66:363–74. doi: 10.1016/s0039-128x(00)00162-8
61. Liu D, Zhang Z, Gladwell W, and Teng CT. Estrogen stimulates estrogen-related receptor alpha gene expression through conserved hormone response elements. Endocrinology. (2003) 144:4894–904. doi: 10.1210/en.2003-0432
62. Rej RK, Thomas JE 2nd, Acharyya RK, Rae JM, and Wang S. Targeting the estrogen receptor for the treatment of breast cancer: recent advances and challenges. J Med Chem. (2023) 66:8339–81. doi: 10.1021/acs.jmedchem.3c00136
63. Carrier JC, Deblois G, Champigny C, Levy E, and Giguère V. Estrogen-related receptor alpha (Erralpha) is a transcriptional regulator of apolipoprotein a-iv and controls lipid handling in the intestine. J Biol Chem. (2004) 279:52052–8. doi: 10.1074/jbc.M410337200
64. Jia M, Andreassen T, Jensen L, Bathen TF, Sinha I, Gao H, et al. Estrogen receptor α Promotes breast cancer by reprogramming choline metabolism. Cancer Res. (2016) 76:5634–46. doi: 10.1158/0008-5472.Can-15-2910
65. Muduli K, Pradhan J, Prusty M, Samal AP, Reddy KS, and Elangovan S. Estrogen-related receptor alpha (Errα) promotes the migration, invasion and angiogenesis of breast cancer stem cell-like cells. Med Oncol. (2024) 41:78. doi: 10.1007/s12032-024-02329-1
66. Pei Y, Mou Z, Jiang L, Yang J, Gu Y, Min J, et al. Aging and head and neck cancer insights from single cell and spatial transcriptomic analyses. Discov Oncol. (2024) 15:801. doi: 10.1007/s12672-024-01672-z
67. Ramroth H, Dietz A, and Becher H. Environmental tobacco smoke and laryngeal cancer: results from a population-based case-control study. Eur Arch Otorhinolaryngol. (2008) 265:1367–71. doi: 10.1007/s00405-008-0651-7
68. Bosetti C, Garavello W, Gallus S, and La Vecchia C. Effects of smoking cessation on the risk of laryngeal cancer: an overview of published studies. Oral Oncol. (2006) 42:866–72. doi: 10.1016/j.oraloncology.2006.02.008
69. Garavello W, Randi G, Bosetti C, Dal Maso L, Negri E, Barzan L, et al. Body size and laryngeal cancer risk. Ann Oncol. (2006) 17:1459–63. doi: 10.1093/annonc/mdl166
70. Mäkitie A, Tuokkola I, Laurell G, Mäkitie O, Olsen K, Takes RP, et al. Vitamin D in head and neck cancer: A systematic review. Curr Oncol Rep. (2020) 23:5. doi: 10.1007/s11912-020-00996-7
71. Urba SG, Carey TE, Kudla-Hatch V, Wolf GT, and Forastiere AA. Tamoxifen therapy in patients with recurrent laryngeal squamous carcinoma. Laryngoscope. (1990) 100:76–8. doi: 10.1288/00005537-199001000-00015
72. Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol. (2018) 36:1143–69. doi: 10.1200/jco.2017.75.7385
73. Gong X, Xiong J, Gong Y, Zhang J, Zhang J, Yang G, et al. Deciphering the role of hpv-mediated metabolic regulation in shaping the tumor microenvironment and its implications for immunotherapy in hnscc. Front Immunol. (2023) 14:1275270. doi: 10.3389/fimmu.2023.1275270
74. Gioacchini FM, Alicandri-Ciufelli M, Magliulo G, Rubini C, Presutti L, and Re M. The clinical relevance of ki-67 expression in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. (2015) 272:1569–76. doi: 10.1007/s00405-014-3117-0
75. Fanidi A, Muller DC, Midttun Ø, Ueland PM, Vollset SE, Relton C, et al. Circulating vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the epic cohort. Sci Rep. (2016) 6:36017. doi: 10.1038/srep36017
Keywords: vitamin D3, laryngeal cancer, cell cycle, estrogen, immunity
Citation: Guo Q, Chen Y, Zhou Q, Dong D, Huang S, Shan M, Zhang B, Pan L and Zhao Y (2025) The vitamin D3 axis in laryngeal cancer: a double-edged sword modulated by estrogen signaling. Front. Immunol. 16:1688589. doi: 10.3389/fimmu.2025.1688589
Received: 19 August 2025; Accepted: 20 October 2025;
Published: 30 October 2025.
Edited by:
Zhe Pei, Virginia Tech, United StatesReviewed by:
Jiawen Xu, Johns Hopkins University, United StatesXushuai Dong, Charité Medical University of Berlin, Germany
Copyright © 2025 Guo, Chen, Zhou, Dong, Huang, Shan, Zhang, Pan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulin Zhao, emhhb3l1bGlubWFpbHZpcEAxNjMuY29t; Liuye Pan, Nzg1MDAyNTYwQHFxLmNvbQ==; Baiquan Zhang, YmFpcTIwMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Qian Guo
Qian Guo Yicheng Chen
Yicheng Chen Qiang Zhou
Qiang Zhou Dong Dong1
Dong Dong1 Shuman Huang
Shuman Huang Meirong Shan
Meirong Shan Baiquan Zhang
Baiquan Zhang Yulin Zhao
Yulin Zhao