- 1Department of Hematology, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 2Harbin Medical University, Harbin, Heilongjiang, China
Innate immune cells and pathways are central to shaping the tumor microenvironment (TME), where they influence tumor growth, metastasis, and responsiveness to immunotherapy. Although research on innate immunity in cancer has expanded considerably, the mechanisms driving immune dysfunction remain incompletely understood. This review summarizes current knowledge on the functional states of innate immune cells within the TME and highlights how metabolic reprogramming contributes to immune suppression and tumor progression. We further discuss recent advances in therapeutic strategies targeting innate immune pathways, emphasizing their translational potential. Importantly, we also examine unresolved controversies and knowledge gaps across innate immune cells, metabolic networks, and innate immune factors such as complement and cytokines, outlining key challenges for clinical translation. By linking mechanistic insights with emerging interventions and identifying future directions, this review provides a framework for integrating innate immunity into next-generation cancer treatment.
1 Introduction
Innate immunity represents the body’s intrinsic, non-specific defense mechanism and plays an equally critical role in tumor immune responses (Figure 1). Under ideal circumstances, abnormal cells would be promptly recognized and eliminated by the host immune system. However, as tumors progress, cancer cells acquire the ability to secrete diverse cytokines and chemokines that progressively impair immune cell function and foster immune evasion (1).
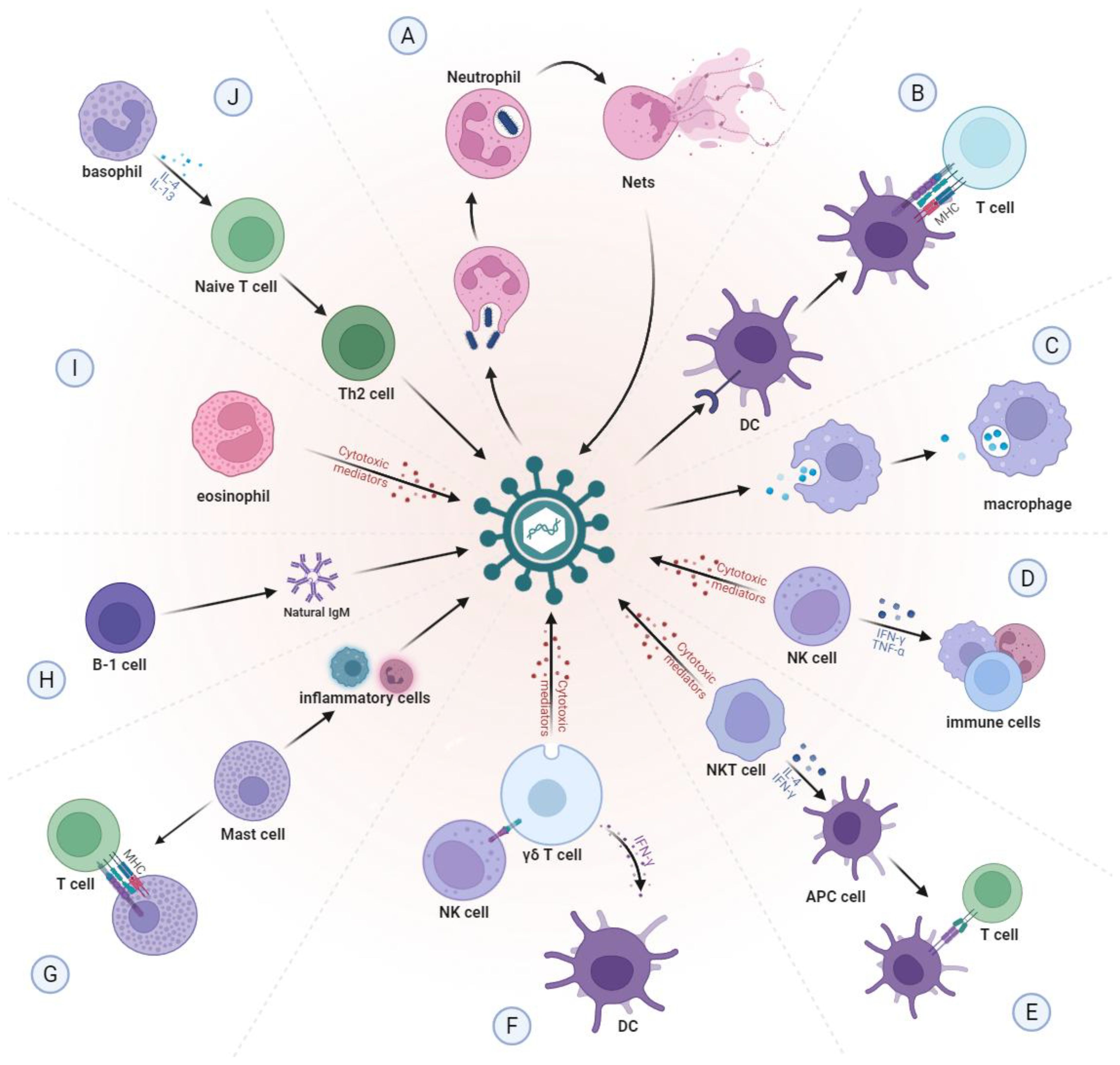
Figure 1. Normal function of innate immune cells. (A) Neutrophils can kill pathogens by directly engulfing pathogens or by self-sacrifice to create neutrophil extracellular traps (NETs). (B) DCs capture, process and present antigens to T cells through MHC molecules. (C) Macrophages phagocytose and remove foreign bodies and senescent cells from the body. (D) NK cells can quickly recognize and clear pathogens, while activating other immune cells by secreting cytokines. (E) NKT cells can directly kill pathogens and can also produce IFN-γ and IL-4 to activate APC cells. (F) γδ T cells can recognize and respond to a variety of antigens, act as APCs to present antigens to T cells, and activate other immune cells. (G) When pathogens invade, mast cells are stimulated and release various inflammatory mediators, which are able to attract more leukocytes to the site of infection. Mast cells also function as APCs. (H) B-1 cells can produce natural IgM antibodies, which can bind to a variety of pathogen associated carbohydrate antigens and play an early defense role. (I) Eosinophils mainly regulate type I hypersensitivity and kill foreign pathogens through cytotoxic effects. (J) Basophils can promote the activation of Th2 cells and enhance the humoral immune response by secreting cytokines. NETs, neutrophil extracellular traps; DCs, dendritic cells; MHC, major histocompatibility complex; NK, natural killer; NKT, natural killer T; IFN-γ, interferon-γ; IL, interleukin; APC, antigen-presenting cell; IgM, immunoglobulin M; Th2, T helper type 2.
Immunotherapy has transformed the landscape of oncology, yet its clinical benefit remains restricted to a subset of patients. Current approaches predominantly focus on adaptive immunity—particularly T cell checkpoint blockade—while the crucial roles of innate immune cells and pathways have not been fully appreciated. Growing evidence now indicates that innate immunity not only initiates antitumor responses but also shapes the tumor microenvironment (TME) in ways that ultimately determine the effectiveness of adaptive immunity (2).
In this review, we provide a comprehensive overview of the innate immune system in cancer and analyze its therapeutic implications. We place particular emphasis on the intersection between innate immune metabolism and immunotherapy, and we highlight feasible strategies to harness innate immune pathways in the design of next-generation therapies. Importantly, we also discuss ongoing controversies, unresolved knowledge gaps, and future research directions, aiming to provide a balanced perspective that can inform the rational integration of innate immunity into personalized cancer treatment.
2 Innate immune cells in tumors
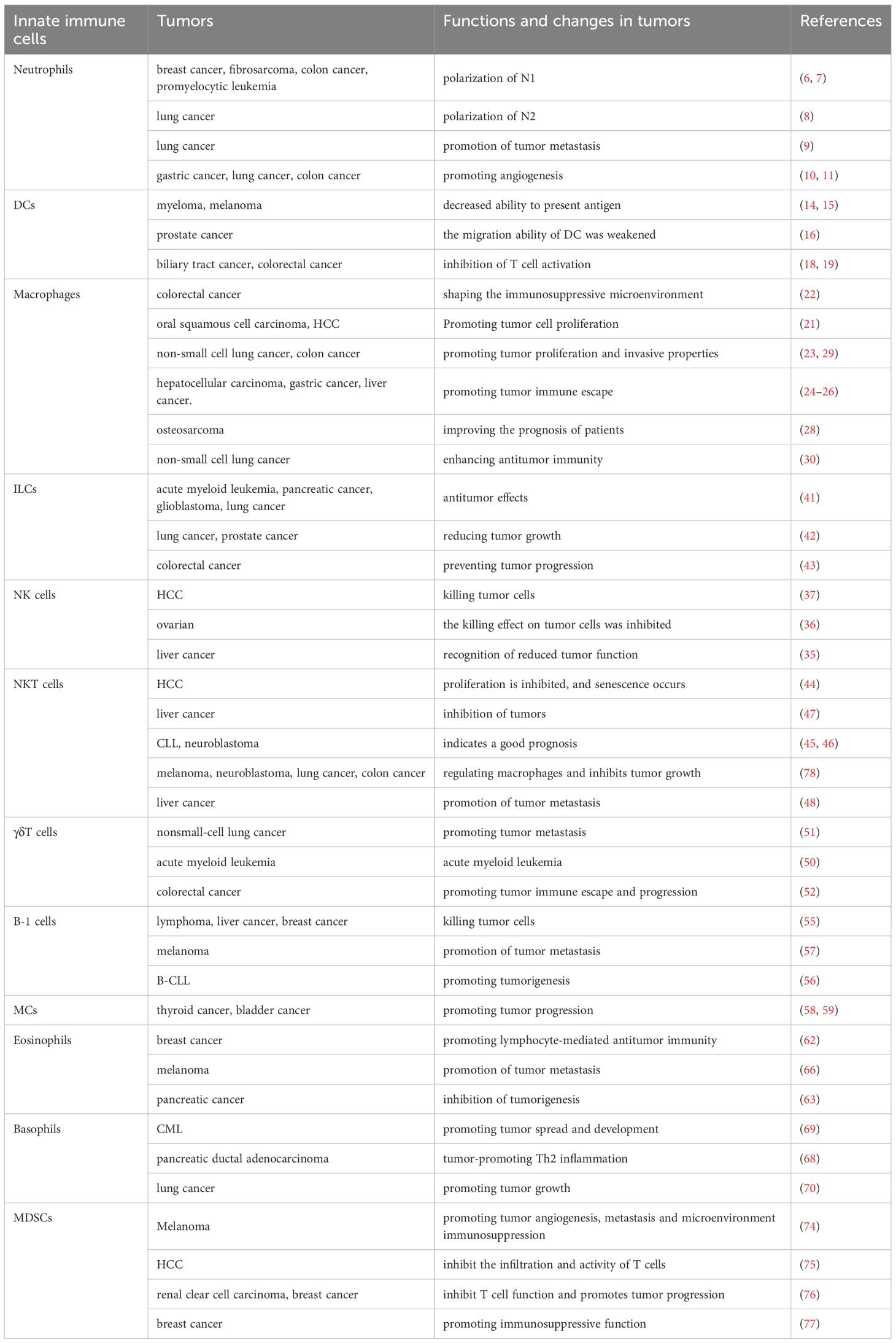
Innate immunity involves various bone marrow-derived cells, each contributing significantly to the innate immune response (3). As tumors develop, a corresponding TME is established, which is the internal milieu that nurtures tumor cells. Within the TME, tumor cells have the capacity to boost or suppress the innate immune response through the secretion of cytokines and chemokines (4). The interplay among various components of the TME significantly influences the functionality of innate immune cells. The following sections will further explore the functional alterations of innate immune cells within tumors and the underlying mechanisms that drive these changes (Table 1).
2.1 Neutrophils
Neutrophils are the predominant cell type within the innate immune system and the first line of defense against infections. Within the TME, there is a significantly increase in neutrophil infiltration, accompanied by alterations in the function (5).
Although traditionally viewed as immunosuppressive, recent studies have highlighted their complex roles in tumors. In the early stages of tumor development, signaling molecules such as CXCL9 and IFN-β drive neutrophils toward an anti-tumor N1 phenotype. These cells are highly cytotoxic and enhance local immune responses by secreting pro-inflammatory chemokines (6, 7).
However, as tumors progress, cytokines such as IL-6, IL-10, TGF-β1, and G-CSF, neutrophils are polarized to the pro-tumor N2 phenotype, which exhibits immunosuppressive properties (8, 9). N2 neutrophils facilitate tumor angiogenesis by releasing factors such as vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9), and fibroblast growth factor-2 (FGF-2), ultimately promoting tumor proliferation and metastasis (10, 11). Additionally, neutrophils can form neutrophil extracellular traps (NETs) in response to specific stimuli. These web-like structures, while capable of trapping tumor cells and limiting their dissemination, also contribute to tumor progression by fostering an immunosuppressive microenvironment (12).
2.2 Dendritic cells
Antigen-presenting cells (APCs), primarily consisting of dendritic cells (DCs), play a crucial role in the innate immune system’s ability to recognize tumors. Widely distributed in nearly all tissues, DCs serve as a vital link between the innate and adaptive immune systems. They are responsible for capturing, processing, and presenting tumor antigens to naive T cells via major histocompatibility complex (MHC) molecules. MHC class I (MHC-I) presents endogenous antigens, while MHC class II (MCH-II) is responsible for handling exogenous antigens, thereby triggering adaptive immune responses characterized by CD8+ or CD4+ T cell activation (13).
The number of DCs is decreased in various tumors, correlating with tumor size and stage. These cells also show notable alterations in the phenotypic profiles. Specifically, reduced expression of HLA-A, B, C, DR, CCR5, CCR7 and DEC-205 in DCs compared to healthy controls (14). Among these, HLA-DR serves as a critical marker of DC maturation, while the other MHC molecules are essential for effective antigen presentation. CCR5 and CCR7, as chemokine receptors, are important for DC migration, and their downregulation further impairs DC trafficking and T cell priming within the tumor microenvironment (15–18). Moreover, abnormal upregulation of immunosuppressive signals critically contributes to DC dysfunction. Evidence indicates that lowering PD-L1 expression enhances CD8+ T cell anti-tumor activity and suppresses tumor growth (19).
2.3 Macrophages
Macrophages are essential components of the innate immune system, involved in various physiological processes such as pathogen clearance, tissue repair, and inflammation regulation. In tumor development, macrophage infiltration is a hallmark of solid tumors. Within the TME, macrophages are exposed to complex stimuli and exhibit high plasticity with diverse activation states. Traditionally, macrophages are categorized into two primary phenotypes: M1 and M2 (20). M1 macrophages are activated by pro-inflammatory factors such as IFN, CSF, and TNF. In contrast, M2 macrophages are activated by cytokines like TGF-β, IL-4, IL-13, and others.
Tumor-associated macrophages (TAMs) are highly heterogeneous and can either promote tumor progression or support anti-tumor immunity, depending on their functional states and the microenvironmental context. Beyond the classical M1/M2 dichotomy, multiple specialized TAM subsets have been identified. SPP1+ macrophages contribute to tumor metastasis, angiogenesis, and activation of cancer stem cells, and interact with FAP+ fibroblasts to form immuno-repulsive tissue structures that limit T cell infiltration (21–23). TREM2+ TAMs accumulate lipids via scavenger receptors, suppress CD8+ T cell activity, and enhance Treg-mediated immunosuppression (24). Additionally, inhibition of MARCO+ macrophages promotes the activation of CD8+ T cells and NK cells, thereby reprogramming the TME (25). In contrast, FOLR2+ macrophages positively correlate with CD8+ T cells and activate their cytotoxicity through antigen cross-presentation (26). Notably, C1q+ TAMs display a dual role. These cells can promote tumor progression by driving T cell exhaustion and correlating with poor prognosis, while also mediating anti-tumor immunity through proinflammatory and phagocytic functions that enhance therapeutic responses (27–29). Mechanistically, C1q+ TAMs interact with cancer stem cells via C1q–C1q receptor signaling, modulate cytokine secretion (e.g., IL-6, MCP-1) to either promote tumor progression or, through the AMPK/JAK/STAT pathway, enhance anti-tumor immunity (29, 30). Therapeutically, targeting TAMs can be achieved by inhibiting immunosuppressive subsets (e.g., C1Q+TPP1+), activating antitumor subsets (e.g., FABP4+C1q+), or combining with immune checkpoint blockade, highlighting macrophage-centered strategies as promising avenues for cancer immunotherapy (29–31). Collectively, these findings underscore the importance of recognizing TAM heterogeneity and leveraging specific subsets to optimize anti-tumor efficacy.
2.4 NK cells
Natural killer (NK) cells are a critical component of the innate immune system, capable of eliminating viral infections and certain tumor cells. In addition to their cytotoxic function, NK cells play essential roles in immune surveillance and regulation.
However, within the TME, NK cells often become functionally impaired, resulting in reduced anti-tumor activity (32). Tumor and stromal cells in the TME secrete various immunosuppressive factors, which directly or indirectly inhibit NK cell activation (33). These factors also suppress the activation of key NK cell receptors such as NKG2D and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (34). Additionally, NK cells in the TME frequently exhibit reduced membrane protrusions, a morphological alteration that may compromise their ability to recognize and eliminate tumor cells (35). Their proliferation, cytotoxicity, and granzyme B secretion are also significantly diminished under such conditions (36). Despite the immunosuppressive nature of the TME, NK cells in hepatocellular carcinoma (HCC) have been shown to retain strong cytotoxic activity, highlighting their potential as a target for cancer immunotherapy (37).
2.5 ILCs
Innate lymphoid cells (ILCs) are key components of the innate immune system and include three major subsets—ILC1, ILC2, and ILC3—as well as NK cells. Although NK cells are functionally considered cytotoxic ILC1s, they are usually classified separately because they represent “mature” killer cells, whereas other ILC1s primarily regulate immunity through cytokine secretion (38).
Under pathological conditions, such as follicular lymphoma, ILC populations are markedly perturbed compared with non-malignant tissues (39). ILC1s generally exert anti-tumor activity through IFN-γ production, but sustained activation can drive functional exhaustion (40). ILC2s secrete granzyme B and directly lyse tumor cells via pyroptosis and/or apoptosis, regulated by DNAM-1–CD112/CD155 interactions that inactivate the negative regulator FOXO1 (41). In tumor-bearing mice, pulmonary ILC2s, as well as tumor-infiltrating ILC2s adoptively transferred at a ratio of 1:60 relative to tumor cells, significantly enhance the infiltration of CD4+ and CD8+ T cells and eosinophils into the tumor microenvironment, thereby suppressing tumor growth. Human ILC2s similarly demonstrate potent anti-tumor activity in vivo (42). ILC3s, on the other hand, interact with T cells through MHC-II, supporting microbial colonization and promoting type 1 immune responses within both the intestine and the tumor (43).
2.6 NKT cells
Natural killer T (NKT) cells, a unique subset of T cells, bridge the gap between innate and adaptive immune responses. They are characterized by the expression of invariant T cell receptors (TCRs) and possess the ability to recognize lipid antigens presented by the non-polymorphic MHC-I molecules, specifically CD1d.
Within the TME, however, the expansion of NKT cells is often impaired, leading to a reduced population and an increased rate of cellular senescence (44). Moreover, dysregulated lipid metabolism in the TME alters the composition of lipid antigens presented by CD1d, thereby compromising NKT cell activation and function (45). Despite these inhibitory conditions, NKT cells retain significant anti-tumor potential. Studies have demonstrated that higher infiltration levels of NKT cells are associated with favorable prognoses in several malignancies, including chronic lymphocytic leukemia (CLL) and neuroblastoma (45, 46). In HCC, NKT cells play a crucial role in tumor suppression by secreting IFN-γ (47). Their activation shifts macrophage polarization toward the M1 phenotype while inhibiting M2 macrophages, thereby promoting the clearance of TAMs and suppressing tumor proliferation. In certain tumors, their protective effects are diminished, NKT cells may even facilitate tumor metastasis and progression through the secretion of IL-22 (48).
2.7 γδ T cells
T lymphocytes can be classified into αβ T cells and γδ T cells, distinguished by the expression of the αβ TCR and γδ TCR, respectively. γδ T cells are a unique subset of T cells with innate immune characteristics. As rapid responders in the innate immune system, they quickly recognize and eliminate infected or abnormal cells. Unlike conventional T cells, which rely on MHC molecules to present antigens, γδ T cells can directly recognize lipid antigens or stress-induced molecules on the surface of tumor cells, greatly expanding their range of antigen recognition (49).
Based on the TCR chain types (e.g., Vδ1, Vδ2, Vγ4, Vγ6) and their tissue-specific distribution, γδ T cells can be further categorized into subsets with distinct immune functions. For instance, Vδ1+ γδ T cells recognize and kill tumor cells via NKG2D ligands; γδT17 cells secrete IL-17, which is correlated with tumor metastasis. Conversely, γδ regulatory T cells (γδTreg) can recruit myeloid-derived suppressor cells (MDSCs), thereby promoting the formation of an immunosuppressive microenvironment (50–52).
2.8 B-1 cells
Conventional B cells are a crucial component of the adaptive immune system, primarily generated in the bone marrow, where they play essential roles in antigen recognition, antibody production, and antigen presentation. In contrast, B-1 cells are a subset of B lymphocytes that are mainly involved in innate immunity and are primarily found in the pleural and peritoneal cavities (53). B-1 cells synthesize natural immunoglobulin M (IgM) antibodies and bind to various pathogen-associated carbohydrate antigens, making them pivotal in the initial defense against bacterial infections. In addition to their strong, non-specific response to bacteria and carbohydrate antigens, B-1 cells are also capable of phagocytosing and clearing apoptotic cells (54).
In abdominal tumors, B-1 cells can rapidly produce and secrete natural IgM antibodies that target tumor-associated carbohydrate antigens, thereby promoting tumor cell killing (55). However, the overactivation of B-1 cells is linked to certain diseases, particularly CLL (56). Within the TME, interactions between B-1 cells and melanoma cells enhance the survival of B-1 cells and promote tumor cell metastasis by upregulating the expression of metastasis-associated genes, such as MMP-9 and CXCR-4 (57).
2.9 Mast cells
Mast cells (MCs) play a crucial role in immune regulation, performing functions such as secreting various cytokines, expressing MHC molecules, and presenting antigens. They are often significantly increased in both tumor tissues and adjacent areas, and are among the first immune cells recruited to the tumor site, where they participate in tumor initiation and progression (58).
In bladder cancer, mast cells activate interferon and NF-κB signaling pathways, leading to increased secretion of CCL2 and IL-13. This attracts THP-1 monocytes and further promotes tumor progression (59). In contrast, in prostate cancer, mast cells exhibit a negative regulatory role, inhibiting tumor angiogenesis and growth. However, those located in the surrounding tumor microenvironment contribute to tumor cell proliferation (60). In summary, mast cells play a complex and context-dependent role in tumor biology, with their functions varying significantly across different tumor types and microenvironments.
2.10 Eosinophils
Eosinophils are a key subpopulation of white blood cells that play diverse roles in immune defense, particularly in parasitic infections, allergic reactions, and immune regulation. In certain solid tumors, elevated eosinophil levels are often associated with favorable prognoses (61, 62). However, in pancreatic cancer, tumor cells can inhibit eosinophil accumulation by secreting chemokines through an autocrine feedback mechanism, thereby facilitating tumor progression (63). Beyond their regulatory roles, eosinophils also contribute to immune activation. For instance, they secrete IL-4, which inhibits CD8+ T cell apoptosis and supports the formation of memory CD8+ T cells (64). Within the TME, eosinophils activated by TNF-α and IFN-γ release chemokines such as CXCL9 and CXCL10, promoting CD4+ and CD8+ T cell infiltration and enhancing anti-tumor immune responses (65). Conversely, eosinophils can also facilitate tumor cell migration and metastasis by secreting CCL6 (66). Therefore, eosinophils play a dual and context-dependent role in tumor immunity, capable of both enhancing immune responses and contributing to tumor progression depending on the tumor type and microenvironment.
2.11 Basophils
Basophils are a subset of white blood cells found in both blood and tissues, traditionally recognized for their role in allergic responses. However, emerging research highlights their equally important involvement in innate immunity (67). Within the TME, basophils display complex and context-dependent functions, capable of both suppressing and promoting tumor progression. In pancreatic ductal carcinoma, activated basophils have been shown to exert significant tumor-suppressive effects, helping to inhibit further tumor development. Conversely, in other contexts, basophils may support tumor growth by secreting cytokines such as IL-13, which reduce the proportion of Th1-like immune cells and dampen anti-tumor immunity (68). The pro-tumorigenic role of basophils is particularly evident in hematologic malignancies. For instance, in patients with chronic myeloid leukemia (CML), basophils express hepatocyte growth factor (HGF), which promotes the proliferation of CML cells (69). In solid tumors, basophils can influence the immune microenvironment to support tumor development. In lung cancer, for instance, they drive the generation of immunosuppressive myeloid cells through the IL-4 signaling axis, thereby facilitating tumor growth (70).
2.12 MDSCs
MDSCs are derived from myeloid cells, which are key components of the innate immune system, their classification as innate immune cells remains controversial. The primary role of innate immune cells is to rapidly identify and eliminate pathogens, whereas MDSCs primarily function to suppress immune responses (71). In healthy individuals, MDSCs are virtually absent and typically expand only under pathological conditions. Thus, despite their myeloid origin and close ties to the innate immune system, MDSCs primarily act as immunosuppressive cells rather than typical innate immune effectors. However, some researchers argue that MDSCs can be considered a “regulatory branch” of the innate immune system due to their role in maintaining immune balance in the inflammatory microenvironment (72).
The recruitment of MDSCs is a critical step in the formation of an immunosuppressive TME, primarily mediated by chemokine receptors CXCR2, CCR2, and CCR5 (73, 74). In the TME, MDSCs not only foster immune suppression through interactions with diverse lymphoid and myeloid cells but also secrete inhibitory cytokines that limit the infiltration and activity of cytotoxic CD8+ T cells (75). Additionally, MDSCs produce large amounts of ROS and nitric oxide, both of which inhibit T cell function and further promote tumor progression (76). Moreover, studies have shown that MDSCs and TAMs engage in bidirectional interactions, significantly amplifying the immunosuppressive effect (77).
3 Innate immune factors
In the previous section, we discussed the critical roles of innate immune cells in tumor immune surveillance and immune escape. As regulators of these immune cells, innate immune factors play an essential role in quickly identifying and eliminating pathogens, while also serving as a bridge for immune responses. Not only do they provide the foundation for the body’s early defense, but they also facilitate adaptive immunity by delivering crucial signals, forming a comprehensive immune defense network. In this section, we will explore the role of innate immune factors in greater detail.
3.1 Complement system
The complement system, composed of over 60 proteins and regulatory factors, represents a complex branch of innate immunity. Its activation can promote tumor clearance through opsonization, phagocytosis, and complement-dependent cytotoxicity (CDC) (79–81). However, aberrant or sustained complement activity within the tumor microenvironment often drives pro-tumorigenic processes, including TAM polarization, angiogenesis, recruitment of immunosuppressive MDSCs, and MMP-9–mediated metastasis, highlighting its dual role in regulating tumor metabolism (82).
Mechanistic studies provide illustrative examples of complement-mediated modulation of TAMs. In ovarian cancer, aberrant C5aR expression on TAMs induces an immunosuppressive phenotype, whereas genetic or pharmacological C5aR blockade reprograms TAMs, restores CXCL9 production, enhances CD8+ T cell infiltration, and improves the efficacy of immune checkpoint inhibition (83). In glioma, the NFAT1–C3a–C3aR feedback loop maintains M2-like TAM polarization and promotes mesenchymal transition of tumor stem cells, while C3aR inhibition reverses TAM-mediated immunosuppression and limits tumor growth (84). Conversely, complement-activating immunotherapeutic complexes (CoMiX) targeting HER2-positive tumor cells via the alternative pathway (FHR4-mediated) or the classical pathway (tri-Fc dimer–mediated) significantly enhance C3b/C5b-9 deposition and CDC, effectively suppressing tumor growth even in resistant models (85).
Collectively, these findings position the complement system at the interface of innate and adaptive immunity, emphasizing its context-dependent and multifaceted roles in cancer. They highlight the potential of complement-targeted strategies—ranging from pathway-specific inhibitors to innovative complement-activating immunotherapies—to modulate the tumor microenvironment and enhance antitumor immunity (86, 87). Integrating such approaches with existing immunotherapies, guided by context-specific complement profiling, offers a promising path toward more precise, effective, and personalized cancer treatments.
3.2 Cytokines
Cytokines are crucial signaling molecules in innate immunity. They rapidly establish a defense network by regulating immune cell recruitment, activation, and function, while also providing a bridge for the activation of adaptive immunity. As key players in the innate immune response, inflammatory cytokines are mainly produced by immune cells in response to pathogens. These cytokines increase vascular permeability and promote the recruitment of immune cells such as neutrophils and monocytes. Chronic inflammation is strongly associated with tumor initiation and progression. Many inflammatory cytokines, such as IL-17, and IL-23, play significant roles in tumor cell proliferation, immunosuppression, and metastasis within the tumor microenvironment (88, 89). These findings underscore the role of chronic inflammation in the tumor microenvironment as a driving force for tumor immune escape.
Chemokines, a subset of cytokines, regulate the migration of immune cells. They are categorized into four main types: CC, CXC, CX3C, and C. By interacting with various G protein-coupled receptors, chemokines form a complex receptor-ligand network that controls immune cell recruitment, activation, and function (90). Within the tumor microenvironment, both tumor cells and immune cells secrete different chemokines that regulate the recruitment of T cells and regulatory T cells (Tregs), influencing the overall tumor immune response (91). For example, the chemokine CXCL10 promotes the infiltration of T cells into the tumor microenvironment, enhancing anti-tumor immunity, while CCL20 recruits Tregs via the FOXO1/CEBPB/NF-κB signaling pathway, thereby promoting chemotherapy resistance in colorectal cancer (91, 92). In addition to immune cell migration, chemokines also contribute to angiogenesis. Tumors maintain their growth and metastasis by secreting pro-angiogenic factors, which help establish an abnormal vascular network (93, 94).
In conclusion, the complement system and cytokines play complex and multifaceted roles in tumor immunity. While they enhance immune defense, they can also create conditions that promote tumor immune escape.
4 Mechanisms of innate immune disorders
Tumors significantly impact the immune system, leading to the progressive attenuation of both innate and adaptive immune responses. The role of innate immune cells and immune factors in tumors has been discussed earlier. In this section, we will delve into the mechanisms underlying innate immune disorders in TME, focusing particularly on the interactions between immune signaling pathways, metabolic disorders, and immune cell senescence.
4.1 Innate immune signaling is impaired
Innate immune signaling pathways within the TME are crucial for tumor immune surveillance and the immune response. Alterations in these pathways affect the immunogenicity of tumor cells, the activity and functionality of immune cells, as well as the tumor’s response to immunotherapeutic interventions. Recent studies have identified the involvement of various signaling pathways in tumor innate immunity, notably the cGAS-STING pathway, Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and C-type lectin receptors (CLRs).
The cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS), a cytoplasmic receptor for double-stranded DNA (dsDNA), promotes the synthesis of the second messenger cGAMP upon activation. cGAMP then interacts with the STING protein to activate downstream signaling pathways, triggering immune responses (95). Notably, type I interferon (I-IFN) plays a key role in detecting tumor cell immunogenicity and activating tumor-specific CD8+ T cells, a process that depends on STING-mediated signaling (96). Moreover, STING has been shown to enhance tumor cell survival through the activation of the IFN/STAT1 signaling pathway, underscoring its complex and dual role in tumor immunity (97).
TLRs, as pattern recognition receptors (PRRs), are essential for innate immune responses. Aberrant TLR activation can promote tumorigenesis and immune evasion (98). TLRs recognize specific components of pathogens and initiate signaling cascades, such as those mediated by myeloid differentiation factor 88 (MyD88). Most TLRs activate NF-κB via MyD88-dependent signaling, triggering inflammatory responses and influencing DC maturation (99). In lung cancer, silencing TLR2, TLR4, and TLR9, along with epithelial-specific MyD88/NF-κB signaling, significantly reduces the expression of pro-tumor immunosuppressive factors like RETNLB, while enhancing the expression of anti-tumor cytokines like IFN-γ (100). In breast cancer cells, activation of TLR2 and TLR4 stimulates NF-κB, regulating the secretion of cytokines such as IL-6, TGF-β, VEGF, and MMP9, promoting tumor invasion, migration, and progression (101, 102).
RLRs are key immune receptors that detect viral RNA in the cytoplasm. Upon binding to RNA ligands, RLRs trigger downstream signaling cascades that activate transcription factors like IRF3 and NF-κB, leading to the production of I-IFN and inflammatory cytokines, which are critical for antiviral responses (103). In bladder cancer, the upregulation of the m6A reading factor YTHDF2 inhibits RIG-I, reducing CD8+ T cell recruitment to the TME (104). In bladder cancer, RIG-I–mediated type I interferon signaling is suppressed, contributing to immune evasion (105).
NLRs play an important role in the innate immune response. NLRs recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), and participate in immune defense by activating NF-κB and MAPK signaling pathways (106). Studies show that activation of the NOD1 pathway accelerates tumor progression, while NOD2 loss is associated with a protective effect in colitis-related tumors (107, 108). However, another study found an increased tumor incidence in NOD2 knockout mice, suggesting that the role of NOD2 in tumorigenesis may not be related to intestinal microbial imbalance (109).
CLRs are a key class of pattern recognition receptors predominantly expressed by myeloid cells. CLRs have significant roles in both innate and adaptive immune responses by recognizing pathogens and DAMPs released from necrotic cells (110). In gastric cancer, CLR Dectin-1 is associated with poor prognosis and promotes immune evasion by modulating the immunosuppressive activity of TAMs (111). Furthermore, CLR Dectin-2-mediated activation of the Nlrp3 inflammasome enhances NK cell function and inhibits liver metastasis (112). These observations illustrate that CLR signaling intersects with inflammasome pathways to produce divergent effects. CLRs also play a role in tumor glycan recognition and dendritic cell dysfunction, driving immunosuppression and tumor immune escape (113). Such seemingly contradictory outcomes likely reflect differences in the cellular compartments involved (tumor, myeloid, or stromal cells), the temporal dynamics of inflammasome activation, and the influence of organ-specific microenvironments.
4.2 Metabolic disorders
In the TME, the metabolic profile of innate immune cells plays a pivotal role in regulating their function. Glycolysis and lipid metabolism are two key pathways involved in immune cell activation and response. Disruptions in these metabolic processes not only impair immune cell efficacy but also contribute to tumor growth, metastasis, and immune evasion.
Glycolysis provides a rapid source of energy for innate immune cells following activation. Key glycolytic enzymes such as hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) are upregulated in this process (Figure 2) (114). Meanwhile, the rapid proliferation of tumor cells and angiogenesis create a hypoxic TME, which activates HIF-1α. HIF-1α further enhances the expression of glycolytic enzymes and glucose transporters (e.g., GLUT1), increasing glycolytic flux to meet the energy demands of both tumor and immune cells (115). Innate immune cells in the TME also display distinct glucose metabolism patterns. For instance, TAMs show enhanced glycolysis, which supports their immunosuppressive phenotype. In breast cancer, the transcription factor ZEB1 promotes macrophage glycolysis via the PI3K/Akt/HIF-1α pathway, facilitating their shift to a pro-tumor state and reinforcing immunosuppression within the TME (116). Moreover, upregulation of GLUT1 further supports tumor progression. In contrast, NK cells in the TME exhibit downregulation of glycolysis-related genes. Elevated expression of fructose-1,6-bisphosphatase (FBP1), which inhibits glycolysis, weakens NK cell activity and reduces IFN-γ secretion (117, 118). Lactate—the end product of glycolysis—accumulates in the TME and acts as a key metabolic signal, disrupting CD8+ T cell activation and antitumor immunity by interfering with GLUT10-mediated glucose transport (119).
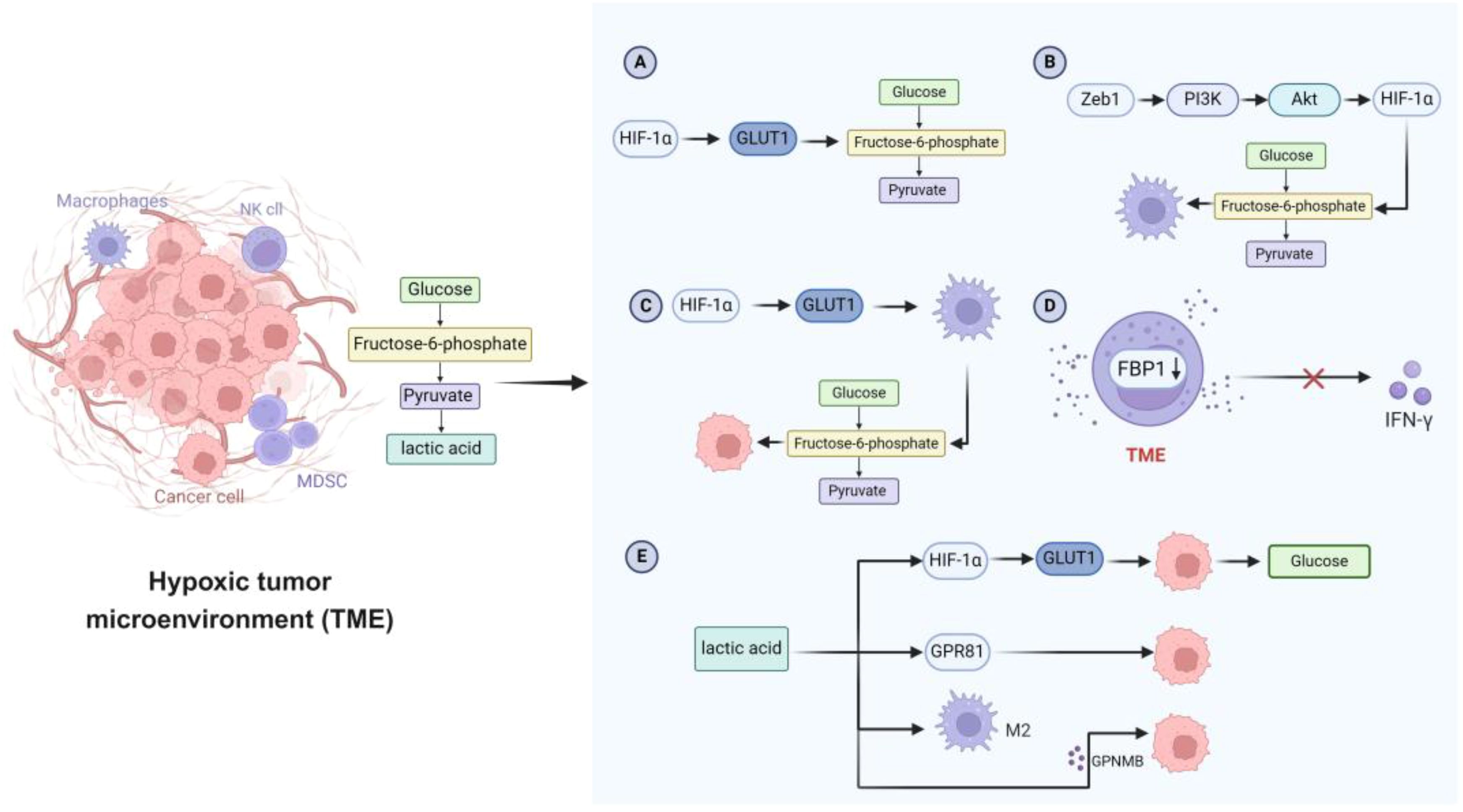
Figure 2. Characteristics of glucose metabolism in hypoxic TME. (A) Tumor cells activate HIF-1α, thereby increasing the expression of GLUT and promoting the glycolytic flux of tumor cells. (B) The transcription factor Zeb1 enhances glycolytic activity through the PI3K/Akt/HIF-1α signaling pathway and promotes the transformation of macrophages to a preneoplastic phenotype. (C) GLUT1 is involved in enhancing macrophage glycolysis and supporting tumor cell growth. (D) Abnormal expression of FBP1 in NK cells in TME inhibits glycolysis, impairs NK cell viability, and limits IFN-γ secretion. (E) Lactate enhances glucose uptake in tumor cells by up-regulating the expression of GLUT1. Lactate also affects tumor cell growth and metastasis through GPR81 receptor. In addition, lactate induces the polarization of macrophages to an M2-like phenotype and regulates the secretion of GPNMB, which further promotes the migration and invasion of tumor cells. TME, tumor microenvironment; HIF-1α, hypoxia-inducible factor 1α; GLUT, glucose transporter; Zeb1, zinc finger E-box binding homeobox 1; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; FBP1, fructose-1,6-bisphosphatase 1; NK, natural killer; IFN-γ, interferon-γ; GPR81, G-protein coupled receptor 81; GPNMB, glycoprotein non-metastatic melanoma protein B.
Beyond glycolysis, lipid metabolism is equally critical in innate immune regulation. Fatty acid oxidation (FAO), a key lipid metabolic pathway, supplies significant energy to tumor cells. Enzymes involved in FAO, such as fatty acid synthase (FASN), sterol regulatory element-binding protein 1 (SREBP1), and carnitine palmitoyltransferase 1 (CPT1), are commonly upregulated in various tumors, supporting their rapid growth (120, 121). Under hypoxic conditions, HIF-1α inhibits FAO by suppressing acyl-CoA dehydrogenase, thereby promoting tumor survival (122). Pyruvate, a glycolytic intermediate, may either be converted into lactate or enter the mitochondria to fuel fatty acid synthesis (123). Lipid accumulation within the TME also influences immune cell function. Oxidized lipids taken up by DCs form covalent complexes with heat shock protein 70 (HSP70), impairing MHC-I translocation to the cell membrane and disrupting antigen presentation (124). Similarly, CD8+ T cells lose their cytotoxic function after excessive lipid uptake via CD36, due to lipid peroxidation and activation of the p38 signaling pathway (125).
Cholesterol homeostasis within immune cells is regulated mainly by SREBP2, which promotes cholesterol synthesis and uptake, and liver X receptor (LXR), which drives cholesterol efflux (126). Tumor cells exploit these pathways to accumulate cholesterol, supporting their continuous proliferation (127, 128). Interestingly, cholesterol levels vary across cell types in the TME: tumor-infiltrating lymphocytes (TILs) and TAMs often exhibit cholesterol deficiency, whereas tumor cells and bone marrow-derived immunosuppressive cells maintain elevated cholesterol levels (126, 129). Cholesterol depletion, particularly in CD8+ cytotoxic T cells, has a notable impact on their anti-tumor capacity. Several tumor-derived factors also influence cholesterol metabolism in the TME. For example, APOA1 from glioblastoma, and CSF1 from prostate cancer can promote cholesterol efflux from TAMs (129, 130). The released cholesterol is then reabsorbed by prostate cancer cells and used for dihydrotestosterone synthesis, which activates androgen receptor signaling and downstream gene expression—ultimately accelerating tumor progression (131, 132). Nevertheless, the broader regulatory effects of cholesterol efflux from innate immune cells on other TME components remain to be fully understood.
4.3 Cellular senescence
In the TME, cellular senescence is increasingly recognized not only as a stress response of tumor cells but also as a key regulator of immune function. Tumor cells are subjected to a variety of chronic stressors—such as oncogenic signals, replication stress, hypoxia, ROS, nutrient deprivation, and inflammatory factors—which can drive them into a state of senescence. Although senescent cells lose their proliferative capacity, they remain metabolically active and influence the surrounding microenvironment through the senescence-associated secretory phenotype (SASP). This secretory profile enables senescent cells to exert strong paracrine effects, particularly on immune cell behavior. For instance, DCs co-cultured with senescent tumor cells show enhanced antigen-presenting capabilities, suggesting that SASP factors can promote antigen presentation under specific conditions (133). The TME can also facilitate the senescence of immune cells. Tumor stem cells release the IL-6, a pro-aging factor that induces macrophage senescenc (134). Furthermore, tumor cells and Tregs can influence lipid metabolism and induce T cells senescence by upregulating the expression of phospholipase A2, contributing to tumor immune evasion (135, 136).
5 Targeted innate immunotherapy
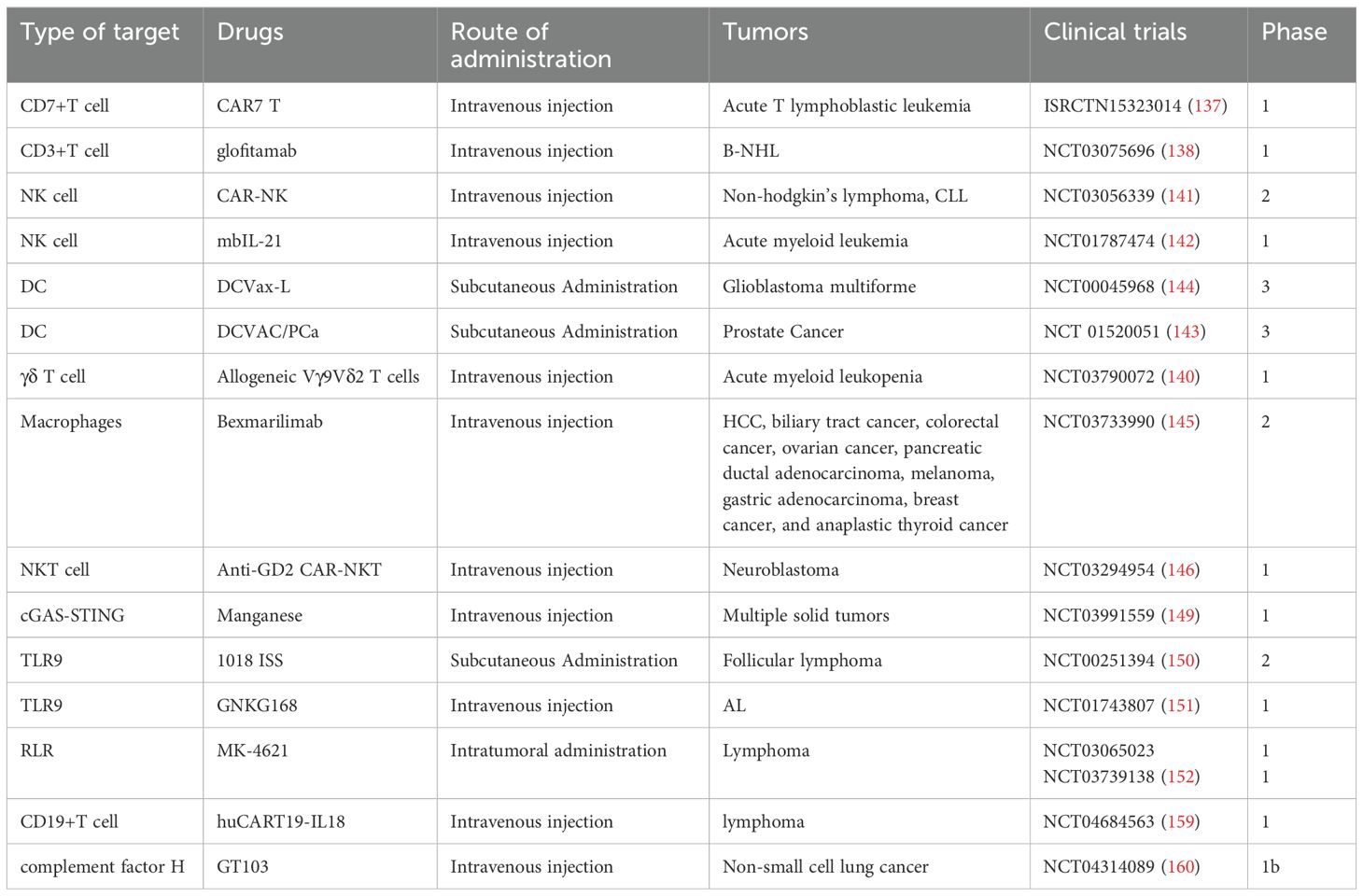
Innate immunity plays a critical role in tumor initiation and progression, and accumulating evidence from both basic research and clinical practice indicates that its therapeutic modulation can substantially enhance anti-tumor efficacy. To provide a comprehensive overview of this rapidly evolving field, we summarize the main therapeutic targets—including innate immune cells, innate signaling pathways, innate checkpoints, and innate immune factors such as cytokines and complement—along with their impact on the TME and key opportunities and challenges for clinical translation (Figure 3). Complementing this conceptual summary, representative clinical trials are highlighted to illustrate the progress and limitations of current strategies (Table 2). The following subsections expand on these approaches in detail.
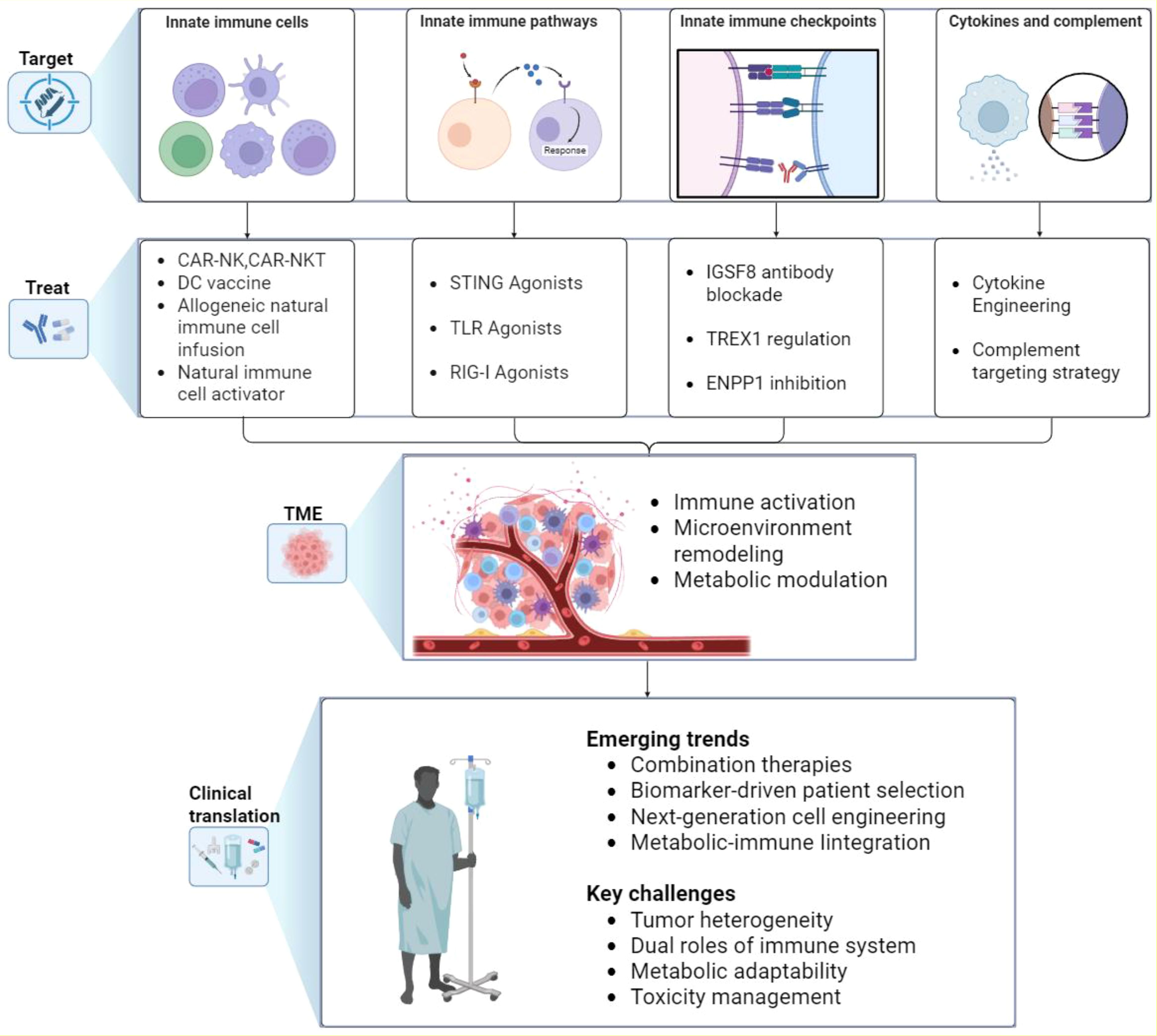
Figure 3. Therapeutic strategies targeting innate immunity in cancer. The figure summarizes current and emerging approaches to harness innate immunity in cancer therapy. Four major target areas are illustrated: innate immune cells, innate immune pathways, innate immune checkpoints, and innate immune factors such as cytokines and complement. Corresponding therapeutic strategies aim to reprogram innate immune responses, which in turn modulate the TME by enhancing immune activation, remodeling tissue context, and reshaping metabolic activity. These effects are being translated into clinical applications, with key emerging trends including rational combination therapies, biomarker-driven patient selection, next-generation cell engineering, and metabolic–immune integration. Major challenges that remain include tumor heterogeneity, the dual roles of innate immune mediators, metabolic adaptability, and toxicity management. TME, tumor microenvironment; TAM, tumor-associated macrophage; NK, natural killer; DC, dendritic cell; ILC, innate lymphoid cell; CAR, chimeric antigen receptor; TLR, Toll-like receptor; STING, stimulator of interferon genes.
5.1 Targeting innate immune cells
Targeting innate immune cells represents a novel immunotherapeutic approach aimed at enhancing or reactivating their ability to identify and eliminate tumor cells. Among these, chimeric antigen receptor T cell (CAR-T) therapy remains one of the most extensively studied modalities. Several CD19-targeting CAR-T therapies have already been approved for clinical use, demonstrating promising therapeutic outcomes. Notably, Robert Chiesa’s team is developing a CD7-targeted CAR-T therapy, which has shown preliminary efficacy in specific tumor types (137). Additionally, Glofitamab, a bispecific antibody targeting CD3 on T cells, has achieved sustained complete remission in patients with refractory and aggressive B-cell non-Hodgkin’s lymphoma (B-NHL), further expanding the landscape of immunotherapy (138). Merging strategies targeting γδ T cells have also shown broad therapeutic potential across various tumor types (139). γδ T cells derived from haploidentical donors have demonstrated promising safety and efficacy in a phase I clinical trial involving patients with refractory or relapsed acute myeloid leukemia (140).
Beyond CAR-T cells, chimeric antigen receptor-modified natural killer cells (CAR-NK) have attracted growing interest. By introducing CARs into NK cells, their tumor recognition and cytotoxic capabilities are significantly enhanced. Clinical trials have reported that CAR-NK therapy improves response rates in CD19+ malignancies and prolongs both overall survival (OS) and progression-free survival (PFS) (141). Moreover, NK cells derived from patients or healthy donors can be expanded ex vivo and reinfused, offering a feasible and scalable therapeutic option (142).
DC vaccines also hold considerable promise in cancer immunotherapy. For instance. Early-phase studies demonstrated immunogenicity and potential survival benefit, but outcomes in larger randomized trials have been inconsistent—for example, DCVAC/PCa in mCRPC showed no OS advantage, whereas DCVax-L in glioblastoma reported encouraging signals but raised concerns about trial design and patient selection (143, 144).
Macrophage-targeted therapy has also emerged as a promising avenue. Bexmarilimab, an inhibitor of CLEVER-1-mediated macrophage activation, has demonstrated early signs of improving survival in patients with various solid tumors (145). Furthermore, chimeric antigen receptor natural killer T cell (CAR-NKT) therapy, an innovative approach, showed good safety and preliminary efficacy in a first-in-human clinical trial, achieving an objective response rate of 25% (3 out of 12 patients) (146).
5.2 Targeting innate immune pathways
Activation of innate immune pathways plays a crucial role in initiating antitumor immune responses. In recent years, agonists TLRs and the STING pathway have shown promising potential in clinical trials. Although clinical application of the cGAS-STING pathway is still in its early stages, several studies have reported encouraging safety profiles and preliminary efficacy in immune activation (147, 148). For instance, research by Lv Mengze’s team demonstrated that the synergistic use of manganese ions and PD-1 antibodies activates the cGAS-STING signaling pathway, thereby promoting the infiltration and maturation of CD8+ T cells, DCs, and macrophages, ultimately enhancing antitumor immunity (149).
TLR agonists have also gained attention for their potent immunomodulatory properties. The TLR-9 agonist 1018 ISS, when combined with rituximab, significantly increased CD8+ T cell and macrophage infiltration in tumor tissues (150). Another TLR-9 agonist, GNKG168, was shown to independently reduce NK cell immunosuppression in patients with acute leukemia (151). Additionally, the RIG-I agonist MK-4621 exhibited notable antitumor activity both as a monotherapy and in combination with PD-1 blockade (152). Currently, multiple innate immune pathway agonists are undergoing clinical evaluation, and the results are eagerly anticipated.
5.3 Targeting innate immune checkpoint
Immune checkpoint inhibitors (ICIs) have profoundly transformed the landscape of tumor immunotherapy by unleashing adaptive immune responses, particularly through blockade of classical targets such as PD-1 and CTLA-4. However, accumulating evidence suggests that the innate immune system also harbors functionally significant checkpoint molecules, thereby representing promising candidates for next-generation immunotherapeutic strategies (153). Among these, IGSF8 has emerged as a novel inhibitory checkpoint, capable of suppressing NK cell activity via its interaction with human KIR3DL2 or murine Klra9 receptors. Preclinical studies have demonstrated that antibody-mediated blockade of this pathway can restore NK cell cytotoxicity against malignant cells in vitro, highlighting its potential therapeutic value (154). In addition to surface checkpoint receptors, intracellular regulators such as the DNA exonuclease TREX1 have been implicated in shaping antitumor immunity. The loss of TREX1 in tumor cells can initiate robust activation of both CD8+ T cells and NK cells, mitigate T cell exhaustion, and reprogram the immunosuppressive myeloid microenvironment, collectively enhancing the efficacy of immunotherapy (155). Another promising target is ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), frequently overexpressed in a variety of malignancies and closely associated with the formation of an immunosuppressive tumor microenvironment (156).
5.4 Targeting cytokines and complement
In addition to innate immune cells, signaling pathways, and checkpoint molecules, innate immune factors such as cytokines and the complement system are increasingly recognized as actionable targets in cancer immunotherapy.
Cytokines have long been studied for their potent immunomodulatory activity. Early agents such as IFNα and high-dose IL-2 were approved for selected malignancies but were limited by modest efficacy and significant toxicities (157). Building on these milestones, next-generation cytokine therapeutics are being developed, including engineered “superkines,” fusion proteins with extended half-lives and tumor-targeted activity, and antagonists of immunosuppressive cytokines (158). An innovative approach is armored CAR-T cells engineered to secrete IL-18 (huCART19-IL18), which demonstrated robust expansion, acceptable safety, and durable clinical responses in relapsed/refractory lymphoma (159). These advances underscore how cytokine engineering can enhance both the efficacy and persistence of cell-based therapies.
The complement system, another ancient arm of innate immunity, exerts dual roles in cancer progression: while capable of mediating opsonization and cytotoxicity, persistent activation via the C5a/C5aR1 axis fosters TAM recruitment, immune suppression, and metastasis (86). Complement regulators such as CD46, CD55, CD59, and factor H are frequently upregulated in tumors, contributing to immune evasion (87). Clinical translation is now underway, exemplified by the first-in-class anti–factor H antibody GT103, which showed safety and disease stabilization in a phase 1b trial of refractory non-small cell lung cancer (160). Complement-targeted strategies are also being tested in combination with checkpoint blockade to reprogram the tumor microenvironment and promote effector T cell infiltration.
Together, cytokine- and complement-based therapies expand the therapeutic armamentarium of innate immunotherapy. By integrating these innate immune factors–targeting approaches with established cell- and checkpoint-directed strategies, next-generation immunotherapies may achieve more durable and personalized clinical benefit.
6 Challenges, controversies, and future directions
Despite major progress in dissecting the role of innate immunity in tumor development and therapy, many unresolved questions and conflicting findings remain. These challenges span innate immune cells, innate immune factors, and metabolic regulation, each presenting both opportunities and uncertainties for clinical translation.
6.1 Innate immune cells
Innate immune cells often display paradoxical, context-dependent roles. For example, subsets of γδ T cells exert potent cytotoxicity, whereas γδT17 or γδTreg cells can drive angiogenesis and immunosuppression. Dendritic cell vaccines have demonstrated immunogenicity in early studies, yet randomized clinical trials have produced inconsistent benefits. TAMs also exhibit duality, with some subsets enhancing antigen presentation while others facilitate immune evasion. Similarly, NK cells show strong efficacy in hematologic malignancies but limited persistence and activity in solid tumors, partly due to inhibitory checkpoint interactions. These examples highlight the high heterogeneity of innate immune cells and underscore the need for standardized definitions, patient stratification based on immune subsets, and reprogramming strategies rather than indiscriminate expansion or depletion.
6.2 Innate immune factors
Innate immune mediators, including complement and cytokines, likewise play dual roles. Complement can drive cytotoxicity and opsonization, yet sustained C3a/C5a signaling recruits immunosuppressive myeloid cells and supports angiogenesis. Cytokine therapies illustrate a similar paradox: while early agents such as IFNα and IL-2 validated the concept, they were limited by toxicity and variable efficacy. Next-generation cytokines, such as engineered superkines and fusion proteins, offer improved activity but remain highly dependent on tumor microenvironmental context. Progress will therefore require predictive biomarkers of efficacy and toxicity, along with rational combinations with checkpoint inhibitors or cell-based therapies.
6.3 Metabolic pathways
Metabolic reprogramming and innate immune signaling are closely intertwined, together shaping either pro- or anti-tumor responses. The accumulation of metabolites such as lactate, oxidized lipids, and altered cholesterol flux reshapes bone marrow and lymphoid function, promoting immunosuppressive TAM and MDSC phenotypes while impairing T and NK cell activity. Dysregulation of innate pathways, exemplified by contradictory reports on NOD2, further illustrates this complexity: depending on context, such signaling may enhance immune surveillance or drive tumor progression through chronic inflammation and microbiota-related dysbiosis.
These intertwined mechanisms provide therapeutic opportunities but also significant barriers. Although metabolic interventions—such as glycolysis inhibitors, LDH inhibitors, FAO/FASN modulators, and agents targeting cholesterol metabolism—can restore immune activity in preclinical models, their translation is hindered by systemic toxicity, compensatory metabolic rewiring, and tumor-type–specific effects. Overcoming these obstacles will require tumor-targeted delivery systems (e.g., nanoparticles, tumor-activated prodrugs) and biomarker-guided patient selection.
In summary, whether at the level of innate immune cells, immune factors, or metabolism, cancer immunity is characterized by profound context dependence and frequent contradictions. Addressing these challenges will require standardized models, integration of single-cell and spatial multi-omics, and metabolic flux tracing to establish causal links. Ultimately, biomarker-driven clinical trials and rational combinations of metabolic modulators with immunotherapies—such as checkpoint blockade, adoptive cell transfer, or engineered cytokines—will be essential to safely and effectively harness innate immunity as a foundation for durable and personalized cancer treatment.
7 Conclusion
This review explores the complex interplay between innate immunity and cancer. Innate immunity plays a critical role in anti-tumor responses by recognizing and eliminating tumor cells and contributing to immune surveillance. However, tumor cells employ various immune evasion mechanisms that weaken immune recognition, thereby promoting tumor growth and metastasis. Within the TME, metabolic disturbances further suppress innate immune function, enhance immunosuppression, and facilitate immune escape, accelerating cancer progression. A comprehensive understanding of the mechanisms underlying the interaction between innate immunity and tumor cells is therefore essential for developing more effective immunotherapeutic strategies.
Despite progress in this field, significant challenges remain. For instance, no universal tumor-associated patterns have been identified that are consistently recognized by the innate immune system. While innate immunity can detect certain tumor-related molecular or cellular changes, these changes are not exclusive to tumors and may also occur in non-cancerous conditions such as tissue injury or metabolic stress, limiting specificity and selectivity. Enhancing the tumor recognition and cytotoxic capabilities of the innate immune system has thus become a key objective in improving cancer control. Additionally, the safety and long-term efficacy of therapies targeting innate immunity require further investigation.
Future research should aim to better characterize the immune and metabolic landscape of the TME and elucidate the precise role of innate immunity in anti-tumor defense. Exploring combination therapies that harness the full potential of innate immunity alongside other treatment modalities may offer promising new avenues for cancer treatment.
Author contributions
SL: Investigation, Methodology, Writing – original draft. YR: Investigation, Validation, Writing – original draft. YT: Validation, Writing – review & editing. WZ: Investigation, Writing – review & editing. YM: Investigation, Writing – review & editing. XC: Writing – review & editing. HZ: Writing – review & editing. WW: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of Heilongjiang Province (Grant No. LH2022H021) and by a transverse research project funded by the Beijing Huikang Charity Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li C, Yu X, Han X, Lian C, Wang Z, Shao S, et al. Innate immune cells in tumor microenvironment: A new frontier in cancer immunotherapy. iScience. (2024) 27:110750. doi: 10.1016/j.isci.2024.110750
2. Carpenter S and O’Neill LAJ. From periphery to center stage: 50 years of advancements in innate immunity. Cell. (2024) 187:2030–51. doi: 10.1016/j.cell.2024.03.036
3. Tarannum M, Ding X, Barisa M, Hu S, Anderson J, Romee R, et al. Engineering innate immune cells for cancer immunotherapy. Nat Biotechnol. (2025) 43:516–33. doi: 10.1038/s41587-025-02629-5
4. Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP, et al. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond). (2023) 43:525–61. doi: 10.1002/cac2.12416
5. Zhang J, Gu J, Wang X, Ji C, Yu D, Wang M, et al. Engineering and targeting neutrophils for cancer therapy. Adv Mater. (2024) 36:e2310318. doi: 10.1002/adma.202310318
6. Wang P, Xu M-H, Xu W-X, Dong Z-Y, Shen Y-H, and Qin W-Z. CXCL9 overexpression predicts better HCC response to anti-PD-1 therapy and promotes N1 polarization of neutrophils. J Hepatocellular Carcinoma. (2024) 11:787–800. doi: 10.2147/JHC.S450468
7. Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Köckritz-Blickwede M, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. (2015) 138:1982–93. doi: 10.1002/ijc.29945
8. Chung JY-F, Tang PC-T, Chan MK-K, Xue VW, Huang X-R, Ng CS-H, et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat Commun. (2023) 14:1794. doi: 10.1038/s41467-023-37515-8
9. Zhang S, Sun L, Zuo J, and Feng D. Tumor associated neutrophils governs tumor progression through an IL-10/STAT3/PD-L1 feedback signaling loop in lung cancer. Trans Oncol. (2024) 40:101866. doi: 10.1016/j.tranon.2023.101866
10. Han L, Chen Y, Huang N, Zhou X, Lv Y, Li H, et al. Cancer-educated neutrophils promote lung cancer progression via PARP-1-ALOX5-mediated MMP-9 expression. Cancer Biol Med. (2024) 21:175–92. doi: 10.20892/j.issn.2095-3941.2023.0248
11. Yang S, Sun B, Li J, Li N, Zhang A, Zhang X, et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell Commun Signal. (2023) 21:176. doi: 10.1186/s12964-023-01196-z
12. Adrover JM, McDowell SAC, He XY, Quail DF, and Egeblad M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. (2023) 41:505–26. doi: 10.1016/j.ccell.2023.02.001
13. Liu H, Lu Y, Zong J, Zhang B, Li X, Qi H, et al. Engineering dendritic cell biomimetic membrane as a delivery system for tumor targeted therapy. J Nanobiotechnology. (2024) 22:663. doi: 10.1186/s12951-024-02913-7
14. Cencini E, Sicuranza A, Ciofini S, Fabbri A, Bocchia M, and Gozzetti A. Tumor-associated macrophages in multiple myeloma: key role in disease biology and potential therapeutic implications. Curr Oncol. (2023) 30:6111–33. doi: 10.3390/curroncol30070455
15. Chen C, He L, Wang X, Xiao R, Chen S, Ye Z, et al. Leonurine promotes the maturation of healthy donors and multiple myeloma patients derived-dendritic cells via the regulation on arachidonic acid metabolism. Front Pharmacol. (2023) 14:1104403. doi: 10.3389/fphar.2023.1104403
16. Feriz AM, Khosrojerdi A, Lotfollahi M, Shamsaki N, GhasemiGol M, HosseiniGol E, et al. Single-cell RNA sequencing uncovers heterogeneous transcriptional signatures in tumor-infiltrated dendritic cells in prostate cancer. Heliyon. (2023) 9:e15694. doi: 10.1016/j.heliyon.2023.e15694
17. Zheng J, Wang M, Pang L, Wang S, Kong Y, Zhu X, et al. Identification of a novel DEC-205 binding peptide to develop dendritic cell-targeting nanovaccine for cancer immunotherapy. J Control Release. (2024) 373:568–82. doi: 10.1016/j.jconrel.2024.07.056
18. Hu Y, Wang K, Chen Y, Jin Y, Guo Q, and Tang H. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front Immunol. (2024) 15:1430551. doi: 10.3389/fimmu.2024.1430551
19. Zhou J, Li Y, Jiang X, Xin Z, Liu W, Zhang X, et al. PD-L1 siRNA incorporation into a cationic liposomal tumor mRNA vaccine enhances cytotoxic T cell activation and prevents immune evasion. Mater Today Bio. (2025) 31:101603. doi: 10.1016/j.mtbio.2025.101603
20. Li M, Yang Y, Xiong L, Jiang P, Wang J, and Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. (2023) 16:80. doi: 10.1186/s13045-023-01478-6
21. Dong L, Hu S, Li X, Pei S, Jin L, Zhang L, et al. SPP1(+) TAM regulates the metastatic colonization of CXCR4(+) metastasis-associated tumor cells by remodeling the lymph node microenvironment. Adv Sci (Weinh). (2024) 11:e2400524. doi: 10.1002/advs.202400524
22. Hong SM, Lee AY, Kim BJ, Lee JE, Seon SY, Ha YJ, et al. NAMPT-driven M2 polarization of tumor-associated macrophages leads to an immunosuppressive microenvironment in colorectal cancer. Advanced Sci. (2024) 11:e2303177. doi: 10.1002/advs.202303177
23. Xiao M, Deng Y, Guo H, Ren Z, He Y, Ren X, et al. Single-cell and spatial transcriptomics profile the interaction of SPP1(+) macrophages and FAP(+) fibroblasts in non-small cell lung cancer. Transl Lung Cancer Res. (2025) 14:2646–69. doi: 10.21037/tlcr-2025-244
24. Tan J, Fan W, Liu T, Zhu B, Liu Y, Wang S, et al. TREM2+ macrophages suppress CD8+ T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatology. (2023) 79:126–40. doi: 10.1016/j.jhep.2023.02.032
25. Ding L, Qian J, Yu X, Wu Q, Mao J, Liu X, et al. Blocking MARCO+ tumor-associated macrophages improves anti-PD-L1 therapy of hepatocellular carcinoma by promoting the activation of STING-IFN type I pathway. Cancer Lett. (2024) 582:216568. doi: 10.1016/j.canlet.2023.216568
26. He Y, Wang J, Deng Z, Feng H, Du M, Zhang D, et al. FOLR2(+) macrophage depletion from intestinal metaplasia to early gastric cancer: single-cell sequencing insight into gastric cancer progression. J Exp Clin Cancer Res. (2024) 43:326. doi: 10.1186/s13046-024-03245-y
27. Revel M, Sautes-Fridman C, Fridman WH, and Roumenina LT. C1q+ macrophages: passengers or drivers of cancer progression. Trends Cancer. (2022) 8:517–26. doi: 10.1016/j.trecan.2022.02.006
28. Tu J, Wang D, Zheng X, and Liu B. Single-cell RNA datasets and bulk RNA datasets analysis demonstrated C1Q+ tumor-associated macrophage as a major and antitumor immune cell population in osteosarcoma. Front Immunol. (2023) 14:911368. doi: 10.3389/fimmu.2023.911368
29. Veschi V, Verona F, Di Bella S, Turdo A, Gaggianesi M, Di Franco S, et al. C1Q(+) TPP1(+) macrophages promote colon cancer progression through SETD8-driven p53 methylation. Mol Cancer. (2025) 24:102. doi: 10.1186/s12943-025-02293-y
30. Zhang D, Wang M, Liu G, Li X, Yu W, Hui Z, et al. Novel FABP4(+)C1q(+) macrophages enhance antitumor immunity and associated with response to neoadjuvant pembrolizumab and chemotherapy in NSCLC via AMPK/JAK/STAT axis. Cell Death Dis. (2024) 15:717. doi: 10.1038/s41419-024-07074-x
31. Mantovani A, Allavena P, Marchesi F, and Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. (2022) 21:799–820. doi: 10.1038/s41573-022-00520-5
32. Tang F, Li J, Qi L, Liu D, Bo Y, Qin S, et al. A pan-cancer single-cell panorama of human natural killer cells. Cell. (2023) 186:4235–51.e20. doi: 10.1016/j.cell.2023.07.034
33. Jia H, Yang H, Xiong H, and Luo KQ. NK cell exhaustion in the tumor microenvironment. Front Immunol. (2023) 14:1303605. doi: 10.3389/fimmu.2023.1303605
34. Liu T, Fan L, Huang W, Chen P, Liu Y, Wang S, et al. Harnessing NKG2D CAR-T cells with radiotherapy: a novel approach for esophageal squamous cell carcinoma treatment. Front Immunol. (2025) 16:1589379. doi: 10.3389/fimmu.2025.1589379
35. Zheng X, Hou Z, Qian Y, Zhang Y, Cui Q, Wang X, et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat Immunol. (2023) 24:802–13. doi: 10.1038/s41590-023-01462-9
36. Vidal-Manrique M, Nieuwenstein T, Hooijmaijers L, de Jonge P, Djojoatmo M, Jansen J, et al. IL-15 transpresentation by ovarian cancer cells improves CD34(+) progenitor-derived NK cell’s anti-tumor functionality. Oncoimmunology. (2025) 14:2465010. doi: 10.1080/2162402X.2025.2465010
37. Li J, Liu Z, Zhang G, Yin X, Yuan X, Xie W, et al. Uncovering the heterogeneity of NK cells on the prognosis of HCC by integrating bulk and single-cell RNA-seq data. Front Oncol. (2025) 15:1570647. doi: 10.3389/fonc.2025.1570647
38. Nouari W and Aribi M. Innate lymphoid cells, immune functional dynamics, epithelial parallels, and therapeutic frontiers in infections. Int Rev Immunol. (2025) 44:245–72. doi: 10.1080/08830185.2025.2490233
39. Van Acker N, Frenois FX, Gravelle P, Tosolini M, Syrykh C, Laurent C, et al. Spatial mapping of innate lymphoid cells in human lymphoid tissues and lymphoma at single-cell resolution. Nat Commun. (2025) 16:4545. doi: 10.1038/s41467-025-59811-1
40. Lopes N, Vivier E, and Narni-Mancinelli E. Natural killer cells and type 1 innate lymphoid cells in cancer. Semin Immunol. (2023) 66:101709. doi: 10.1016/j.smim.2022.101709
41. Li Z, Ma R, Tang H, Guo J, Shah Z, Zhang J, et al. Therapeutic application of human type 2 innate lymphoid cells via induction of granzyme B-mediated tumor cell death. Cell. (2024) 187:624–41 e23. doi: 10.1016/j.cell.2023.12.015
42. Saranchova I, Xia CW, Besoiu S, Finkel PL, Ellis SLS, Kari S, et al. A novel type-2 innate lymphoid cell-based immunotherapy for cancer. Front Immunol. (2024) 15:1317522. doi: 10.3389/fimmu.2024.1317522
43. Goc J, Lv M, Bessman NJ, Flamar AL, Sahota S, Suzuki H, et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell. (2021) 184:5015–30 e16. doi: 10.1016/j.cell.2021.07.029
44. Cheng X, Tan X, Wang W, Zhang Z, Zhu R, Wu M, et al. Long-chain acylcarnitines induce senescence of invariant natural killer T cells in hepatocellular carcinoma. Cancer Res. (2023) 83:582–94. doi: 10.1158/0008-5472.CAN-22-2273
45. Lee MS and Webb TJ. Novel lipid antigens for NKT cells in cancer. Front Immunol. (2023) 14:1173375. doi: 10.3389/fimmu.2023.1173375
46. Nishimura K, Aoki T, Kobayashi M, Takami M, Ozaki K, Ogawa K, et al. Antibody-Dependent Cellular Cytotoxicity of iPS Cell-Derived Natural Killer T Cells by Anti-GD2 mAb for Neuroblastoma. Cancer Sci. (2025) 116:884–96. doi: 10.1111/cas.70008
47. Morgan RC, Frank C, Greger M, Attaway M, Sigvardsson M, Bartom ET, et al. TGF-beta promotes the postselection thymic development and peripheral function of IFN-gamma-producing invariant NKT cells. J Immunol. (2023) 211:1376–84. doi: 10.4049/jimmunol.2200809
48. Giannou AD, Kempski J, Shiri AM, Lücke J, Zhang T, Zhao L, et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity. (2023) 56:125–42.e12. doi: 10.1016/j.immuni.2022.12.014
49. Wiesheu R and Coffelt SB. From backstage to the spotlight: gammadeltaT cells in cancer. Cancer Cell. (2024) 42:1637–42. doi: 10.1016/j.ccell.2024.08.017
50. Mensurado S, Condeco C, Sanchez-Martinez D, Shirley S, Coelho RML, Tirado N, et al. CD155/PVR determines acute myeloid leukemia targeting by Delta One T cells. Blood. (2024) 143:1488–95. doi: 10.1182/blood.2023022992
51. Xu R, Ke X, Shang W, Liu S, Fu X, Wang T, et al. Distribution and clinical significance of IL-17A in tumor-infiltrating lymphocytes of non-small cell lung cancer patients. Pathol Oncol Res. (2022) 28:1610384. doi: 10.3389/pore.2022.1610384
52. Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu X, et al. Tumor-infiltrating CD39(+)gammadeltaTregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. (2017) 6:e1277305. doi: 10.1080/2162402X.2016.1277305
53. Rodriguez-Zhurbenko N and Hernandez AM. The role of B-1 cells in cancer progression and anti-tumor immunity. Front Immunol. (2024) 15:1363176. doi: 10.3389/fimmu.2024.1363176
54. Mattos MS, Vandendriessche S, Waisman A, and Marques PE. The immunology of B-1 cells: from development to aging. Immun Ageing. (2024) 21:54. doi: 10.1186/s12979-024-00455-y
55. Haro MA, Dyevoich AM, Phipps JP, and Haas KM. Activation of B-1 cells promotes tumor cell killing in the peritoneal cavity. Cancer Res. (2019) 79:159–70. doi: 10.1158/0008-5472.CAN-18-0981
56. de Oliveira VC, de Lacerda MP, Moraes BBM, Gomes CP, Maricato JT, Souza OF, et al. Deregulation of Ikaros expression in B-1 cells: New insights in the Malignant transformation to chronic lymphocytic leukemia. J Leukocyte Biol. (2019) 106:581–94. doi: 10.1002/JLB.MA1118-454R
57. Pérez EC, MaChado J, Aliperti F, Freymüller E, Mariano M, and Lopes JD. B-1 lymphocytes increase metastatic behavior of melanoma cells through the extracellular signal-regulated kinase pathway. Cancer Science. (2008) 99:920–8. doi: 10.1111/j.1349-7006.2008.00776.x
58. Hou Y, Wang Q, Su L, Zhu Y, Xiao Y, and Feng F. Increased tumor-associated mast cells facilitate thyroid cancer progression by inhibiting CD8+ T cell function through galectin-9. Braz J Med Biol Res. (2023) 56:e12370. doi: 10.1590/1414-431x2023e12370
59. Liu Z, Huang C, Mao X, Mi J, Zhang Q, Xie Y, et al. Single cell RNA-sequencing reveals mast cells enhance mononuclear phagocytes infiltration in bladder cancer microenvironment. J Cancer. (2024) 15:5672–90. doi: 10.7150/jca.99554
60. Shin J, Kim MJ, Quan X, Kim JW, Lee S, Park S, et al. Thrombopoietin receptor agonist antibody for treating chemotherapy-induced thrombocytopenia. BMC Cancer. (2023) 23:490. doi: 10.1186/s12885-023-10975-3
61. Kaczmarek F, Marcinkowska-Gapinska A, Bartkowiak-Wieczorek J, Nowak M, Kmiecik M, Brzezinska K, et al. Blood-based biomarkers as predictive and prognostic factors in immunotherapy-treated patients with solid tumors-currents and perspectives. Cancers (Basel). (2025) 17:2001. doi: 10.3390/cancers17122001
62. Hu G, Wang S, Zhong K, Xu F, Huang L, Chen W, et al. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis. BMC Cancer. (2020) 20:454. doi: 10.1186/s12885-020-06966-3
63. Bhattacharyya S, Oon C, Diaz L, Sandborg H, Stempinski ES, Saoi M, et al. Autotaxin–lysolipid signaling suppresses a CCL11–eosinophil axis to promote pancreatic cancer progression. Nat Cancer. (2024) 5:283–98. doi: 10.1038/s43018-023-00703-y
64. Zhou J, Liu J, Wang B, Li N, Liu J, Han Y, et al. Eosinophils promote CD8+ T cell memory generation to potentiate anti-bacterial immunity. Signal Transduction Targeted Ther. (2024) 9:43. doi: 10.1038/s41392-024-01752-0
65. Grisaru-Tal S, Dulberg S, Beck L, Zhang C, Itan M, Hediyeh-zadeh S, et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. (2021) 81:5555–71. doi: 10.1158/0008-5472.CAN-21-0839
66. Li F, Du X, Lan F, Li N, Zhang C, Zhu C, et al. Eosinophilic inflammation promotes CCL6-dependent metastatic tumor growth. Sci Adv. (2021) 7:eabb5943. doi: 10.1126/sciadv.abb5943
67. Charles N and Blank U. IgE-mediated activation of mast cells and basophils in health and disease. Immunol Rev. (2025) 331:e70024. doi: 10.1111/imr.70024
68. Wei J, Mayberry CL, Lv X, Hu F, Khan T, Logan NA, et al. IL3-driven T cell-basophil crosstalk enhances antitumor immunity. Cancer Immunol Res. (2024) 12:822–39. doi: 10.1158/2326-6066.CIR-23-0851
69. Cerny-Reiterer S, Ghanim V, Hoermann G, Aichberger KJ, Herrmann H, Muellauer L, et al. Identification of basophils as a major source of hepatocyte growth factor in chronic myeloid leukemia: A novel mechanism of BCR-ABL1-independent disease progression. Neoplasia. (2012) 14:572–IN10. doi: 10.1593/neo.12724
70. LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. (2024) 625:166–74. doi: 10.1038/s41586-023-06797-9
71. He S, Zheng L, and Qi C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol Cancer. (2025) 24:5. doi: 10.1186/s12943-024-02208-3
72. Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, and Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. (2024) 21:147–64. doi: 10.1038/s41571-023-00846-y
73. Lim HX, Kim TS, and Poh CL. Understanding the differentiation, expansion, recruitment and suppressive activities of myeloid-derived suppressor cells in cancers. Int J Mol Sci. (2020) 21:3599. doi: 10.3390/ijms21103599
74. Groth C, Arpinati L, Shaul ME, Winkler N, Diester K, Gengenbacher N, et al. Blocking migration of polymorphonuclear myeloid-derived suppressor cells inhibits mouse melanoma progression. Cancers (Basel). (2021) 13:726. doi: 10.3390/cancers13040726
75. Zhang Z, Huang W, Hu D, Jiang J, Zhang J, Wu Z, et al. E-twenty-six-specific sequence variant 5 (ETV5) facilitates hepatocellular carcinoma progression and metastasis through enhancing polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC)-mediated immunosuppression. Gut. (2025) 74:1137–49. doi: 10.1136/gutjnl-2024-333944
76. Mo W, Liu S, Zhao X, Wei F, Li Y, Sheng X, et al. ROS scavenging nanozyme modulates immunosuppression for sensitized cancer immunotherapy. Adv Healthc Mater. (2023) 12:e2300191. doi: 10.1002/adhm.202300191
77. Zheng Y, Wang N, Wang S, Zhang J, Yang B, and Wang Z. Chronic psychological stress promotes breast cancer pre-metastatic niche formation by mobilizing splenic MDSCs via TAM/CXCL1 signaling. J Exp Clin Cancer Res. (2023) 42:129. doi: 10.1186/s13046-023-02696-z
78. Lin W, Luo Y, Wu J, Zhang H, Jin G, Guo C, et al. Loss of ADAR1 in macrophages in combination with interferon gamma suppresses tumor growth by remodeling the tumor microenvironment. J ImmunoTherapy Cancer. (2023) 11:726. doi: 10.1136/jitc-2023-007402
79. Balduit A, Agostinis C, and Bulla R. Beyond the Norm: The emerging interplay of complement system and extracellular matrix in the tumor microenvironment. Semin Immunol. (2025) 77:101929. doi: 10.1016/j.smim.2025.101929
80. Revel M and Merle NS. Local and Cell-intrinsic complement: The new player in cancer progression. Semin Immunol. (2025) 79:101976. doi: 10.1016/j.smim.2025.101976
81. Artero MR, Minery A, Nedelcev L, Radanova M, and Roumenina LT. Complement and the hallmarks of cancer. Semin Immunol. (2025) 78:101950. doi: 10.1016/j.smim.2025.101950
82. Garlanda C, Dambra M, and Magrini E. Interplay between the complement system and other immune pathways in the tumor microenvironment. Semin Immunol. (2025) 78:101951. doi: 10.1016/j.smim.2025.101951
83. Luan X, Lei T, Fang J, Liu X, Fu H, Li Y, et al. Blockade of C5a receptor unleashes tumor-associated macrophage antitumor response and enhances CXCL9-dependent CD8(+) T cell activity. Mol Ther. (2024) 32:469–89. doi: 10.1016/j.ymthe.2023.12.010
84. Zhang Y, Song Y, Wang X, Shi M, Lin Y, Tao D, et al. An NFAT1-C3a-C3aR positive feedback loop in tumor-associated macrophages promotes a glioma stem cell Malignant phenotype. Cancer Immunol Res. (2024) 12:363–76. doi: 10.1158/2326-6066.CIR-23-0418
85. Seguin-Devaux C, Brandus B, Plesseria JM, Iserentant G, Servais JY, Pitiot A, et al. Directed-complement activation as a novel immunotherapeutic approach for HER2-breast cancer. Immunotargets Ther. (2025) 14:979–95. doi: 10.2147/ITT.S517584
86. Merle NS and Roumenina LT. The complement system as a target in cancer immunotherapy. Eur J Immunol. (2024) 54:e2350820. doi: 10.1002/eji.202350820
87. Saxena R, Gottlin EB, Campa MJ, He YW, and Patz EF Jr. Complement regulators as novel targets for anti-cancer therapy: A comprehensive review. Semin Immunol. (2025) 77:101931. doi: 10.1016/j.smim.2025.101931
88. Dang C, Liu M, Liu P, Liu J, Yu X, Dong Y, et al. Causal relationship between inflammatory factors and gynecological cancer: a Bayesian Mendelian randomization study. Sci Rep. (2024) 14:29868. doi: 10.1038/s41598-024-80747-x
89. Vangilbergen M, Stockman A, Van De Velde A, Garmyn M, Punie K, and Hillary T. The role of interleukin-17 and interleukin-23 inhibitors in the development, progression, and recurrence of cancer: A systematic review. JAAD Int. (2024) 17:71–9. doi: 10.1016/j.jdin.2024.06.006
90. Kureshi CT and Dougan SK. Cytokines in cancer. Cancer Cell. (2025) 43:15–35. doi: 10.1016/j.ccell.2024.11.011
91. Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8+ T cell antitumor immunity. Immunity. (2023) 56:1613–30.e5. doi: 10.1016/j.immuni.2023.06.003
92. Mao J, Li J, Chen J, Wen Q, Cao M, Zhang F, et al. CXCL10 and Nrf2-upregulated mesenchymal stem cells reinvigorate T lymphocytes for combating glioblastoma. J Immunother Cancer. (2023) 11:e007481. doi: 10.1136/jitc-2023-007481
93. Liu Z-L, Chen H-H, Zheng L-L, Sun L-P, and Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduction Targeted Ther. (2023) 8:198. doi: 10.1038/s41392-023-01460-1
94. Ghalehbandi S, Yuzugulen J, Pranjol MZI, and Pourgholami MH. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur J Pharmacol. (2023) 949:175586. doi: 10.1016/j.ejphar.2023.175586
95. Zhang B, Xu P, and Ablasser A. Regulation of the cGAS-STING pathway. Annu Rev Immunol. (2025) 43:667–92. doi: 10.1146/annurev-immunol-101721-032910
96. Huang B, Li H, Jiang Q, Li Y, Jiang Z, Cao H, et al. Elevated type I IFN signalling directly affects CD8(+) T-cell distribution and autoantigen recognition of the skeletal muscles in active JDM patients. J Autoimmun. (2024) 146:103232. doi: 10.1016/j.jaut.2024.103232
97. Li JX, Zhang J, Li CH, Li YF, Chen HM, Li T, et al. Human papillomavirus E1 proteins inhibit RIG-I/MDA5-MAVS, TLR3-TRIF, cGAS-STING, and JAK-STAT signaling pathways to evade innate antiviral immunity. Front Immunol. (2025) 16:1549766. doi: 10.3389/fimmu.2025.1549766
98. Berouti M, Wagner M, Greulich W, Piseddu I, Gartig J, Hansbauer L, et al. Pseudouridine RNA avoids immune detection through impaired endolysosomal processing and TLR engagement. Cell. (2025) 188:4880–95 e15. doi: 10.1016/j.cell.2025.05.032
99. Chen R, Li F, Zhou K, Xing M, Zhang X, Zhao X, et al. Component identification of modified sanmiao pills by UPLC-Xevo G2-XS QTOF and its anti-gouty arthritis mechanism based on network pharmacology and experimental verification. J Ethnopharmacol. (2023) 311:116394. doi: 10.1016/j.jep.2023.116394
100. Velasco WV, Khosravi N, Castro-Pando S, Torres-Garza N, Grimaldo MT, Krishna A, et al. Toll-like receptors 2, 4, and 9 modulate promoting effect of COPD-like airway inflammation on K-ras-driven lung cancer through activation of the MyD88/NF-ĸB pathway in the airway epithelium. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1118721
101. Cossu C, Di Lorenzo A, Fiorilla I, Todesco AM, Audrito V, and Conti L. The role of the toll-like receptor 2 and the cGAS-STING pathways in breast cancer: friends or foes? Int J Mol Sci. (2023) 25:456. doi: 10.3390/ijms25010456
102. Thompson AL, Grenald SA, Ciccone HA, Mohty D, Smith AF, Coleman DL, et al. Morphine-induced osteolysis and hypersensitivity is mediated through toll-like receptor-4 in a murine model of metastatic breast cancer. Pain. (2023) 164:2463–76. doi: 10.1097/j.pain.0000000000002953
103. Stewart H, Lu Y, O’Keefe S, Valpadashi A, Cruz-Zaragoza LD, Michel HA, et al. The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity. iScience. (2023) 26:108080. doi: 10.1016/j.isci.2023.108080
104. Zhang L, Li Y, Zhou L, Zhou H, Ye L, Ou T, et al. The m6A reader YTHDF2 promotes bladder cancer progression by suppressing RIG-I-mediated immune response. Cancer Res. (2023) 83:1834–50. doi: 10.1158/0008-5472.CAN-22-2485
105. Wei W, Li H, Tian S, Zhang C, Liu J, Tao W, et al. Asparagine drives immune evasion in bladder cancer via RIG-I stability and type I IFN signaling. J Clin Invest. (2025) 135:e186648. doi: 10.1172/JCI186648
106. Chen R, Zou J, Chen J, Zhong X, Kang R, and Tang D. Pattern recognition receptors: function, regulation and therapeutic potential. Signal Transduct Target Ther. (2025) 10:216. doi: 10.1038/s41392-025-02264-1
107. Chauvin C, Radulovic K, Boulard O, Delacre M, Waldschmitt N, Regnier P, et al. Loss of NOD2 in macrophages improves colitis and tumorigenesis in a lysozyme-dependent manner. Front Immunol. (2023) 14:1252979. doi: 10.3389/fimmu.2023.1252979
108. Ma W, Zhang L, Chen W, Chang Z, Tu J, Qin Y, et al. Microbiota enterotoxigenic Bacteroides fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance through its functional receptor NOD1. Protein Cell. (2024) 15:419–40. doi: 10.1093/procel/pwae005
109. Udden SMN, Peng L, Gan J-L, Shelton JM, Malter JS, Hooper LV, et al. NOD2 suppresses colorectal tumorigenesis via downregulation of the TLR pathways. Cell Rep. (2017) 19:2756–70. doi: 10.1016/j.celrep.2017.05.084
110. Reis e Sousa C, Yamasaki S, and Brown GD. Myeloid C-type lectin receptors in innate immune recognition. Immunity. (2024) 57:700–17. doi: 10.1016/j.immuni.2024.03.005
111. Liu X, Lv K, Wang J, Lin C, Liu H, Zhang H, et al. C-type lectin receptor Dectin-1 blockade on tumour-associated macrophages improves anti-PD-1 efficacy in gastric cancer. Br J Cancer. (2023) 129:721–32. doi: 10.1038/s41416-023-02336-5
112. Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, et al. The nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity. (2015) 43:751–63. doi: 10.1016/j.immuni.2015.08.013
113. Niveau C, Sosa Cuevas E, Roubinet B, Pezet M, Thepaut M, Mouret S, et al. Melanoma tumour-derived glycans hijack dendritic cell subsets through C-type lectin receptor binding. Immunology. (2024) 171:286–311. doi: 10.1111/imm.13717
114. Kooshan Z, Cardenas-Piedra L, Clements J, and Batra J. Glycolysis, the sweet appetite of the tumor microenvironment. Cancer Lett. (2024) 600:217156. doi: 10.1016/j.canlet.2024.217156
115. Chen Z, Han F, Du Y, Shi H, and Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduction Targeted Ther. (2023) 8:70. doi: 10.1038/s41392-023-01332-8
116. Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. (2021) 593:282–8. doi: 10.1038/s41586-021-03442-1
117. Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. (2018) 28:243–55.e5. doi: 10.1016/j.cmet.2018.06.021
118. Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. (2021) 33:1205–20.e5. doi: 10.1016/j.cmet.2021.03.023
119. Liu Y WF, Peng D, Zhang D, Liu L, Wei J, Yuan J, et al. Activation and antitumor immunity of CD8+ T cells are supported by the glucose transporter GLUT10 and disrupted by lactic acid. Sci Transl Med. (2024) 16:eadk7399. doi: 10.1126/scitranslmed.adk7399
120. Zheng Y, Jiang Z, Yuan L, Cheng X, He W, and Chen X. Targeting fatty acid oxidation: A potential strategy for treating gastrointestinal tumors. Int J Cancer. (2025) 157:7–17. doi: 10.1002/ijc.35380
121. Ma J, Wang S, Zhang P, Zheng S, Li X, Li J, et al. Emerging roles for fatty acid oxidation in cancer. Genes Dis. (2025) 12:101491. doi: 10.1016/j.gendis.2024.101491
122. Matsufuji S, Kitajima Y, Higure K, Kimura N, Maeda S, Yamada K, et al. A HIF-1alpha inhibitor combined with palmitic acid and L-carnitine treatment can prevent the fat metabolic reprogramming under hypoxia and induce apoptosis in hepatocellular carcinoma cells. Cancer Metab. (2023) 11:25. doi: 10.1186/s40170-023-00328-w
123. Sun W, Jia M, Feng Y, and Cheng X. Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy. (2023) 19:3240–1. doi: 10.1080/15548627.2023.2246356
124. Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. (2017) 8:2122. doi: 10.1038/s41467-017-02186-9
125. Qin Y HF, Feng Z, Hou J, Ding Y, Wang Q, Gui Y, et al. CD36 promotes iron accumulation and dysfunction in CD8+ T cells via the p38-CEBPB-TfR1 axis in earlystage hepatocellular carcinoma. Clin Mol Hepatol. (2025) 31:960–80. doi: 10.3350/cmh.2024.0948
126. Yan C, Zheng L, Jiang S, Yang H, Guo J, Jiang L-Y, et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell. (2023) 41:1276–93.e11. doi: 10.1016/j.ccell.2023.04.016
127. Lagunas-Rangel FA. Cholesterol effects on the tumor immune microenvironment: from fundamental concepts to mechanisms and implications. Front Oncol. (2025) 15:1579054. doi: 10.3389/fonc.2025.1579054
128. Geng F and Guo D. SREBF1/SREBP-1 concurrently regulates lipid synthesis and lipophagy to maintain lipid homeostasis and tumor growth. Autophagy. (2024) 20:1183–5. doi: 10.1080/15548627.2023.2275501
129. Feng X, Zhang J, Yu B, An W, and Jin X. Lipid metabolic reprogramming in tumor-associated macrophages: A key driver of functional polarization and tumor immunomodulation. Crit Rev Oncol Hematol. (2025) 215:104881. doi: 10.1016/j.critrevonc.2025.104881
130. Wang S, Yan W, Kong L, Zuo S, Wu J, Zhu C, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat Commun. (2023) 14:4367. doi: 10.1038/s41467-023-39683-z
131. El-Kenawi A, Dominguez-Viqueira W, Liu M, Awasthi S, Abraham-Miranda J, Keske A, et al. Macrophage-derived cholesterol contributes to therapeutic resistance in prostate cancer. Cancer Res. (2021) 81:5477–90. doi: 10.1158/0008-5472.CAN-20-4028
132. Zhang Z, Wang W, Kong P, Feng K, Liu C, Sun T, et al. New insights into lipid metabolism and prostate cancer (Review). Int J Oncol. (2023) 62:74. doi: 10.3892/ijo.2023.5522
133. Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. (2023) 13:410–31. doi: 10.1158/2159-8290.CD-22-0523
134. Wada H, Otsuka R, Germeraad WTV, Murata T, and Kondo T. Seino K-i. Tumor cell-induced macrophage senescence plays a pivotal role in tumor initiation followed by stable growth in immunocompetent condition. J ImmunoTherapy Cancer. (2023) 11:e006677. doi: 10.1136/jitc-2023-006677
135. Liu X, Hartman CL, Li L, Albert CJ, Si F, Gao A, et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci Transl Med. (2021) 13:eaaz6314. doi: 10.1126/scitranslmed.aaz6314
136. Xu Y, Ding L, Wu M, Wang X, Wang L, Xu Z, et al. Lipid metabolic remodeling delays senescence of T cells to potentiate their immunity against solid tumors. J Immunother Cancer. (2025) 13:e010403. doi: 10.1136/jitc-2024-010403
137. Chiesa R, Georgiadis C, Syed F, Zhan H, Etuk A, Gkazi SA, et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. New Engl J Med. (2023) 389:899–910. doi: 10.1056/NEJMoa2300709
138. Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: A phase I trial. J Clin Oncol. (2021) 39:1959–70. doi: 10.1200/JCO.20.03175
139. Wang Y, Han J, Wang D, Cai M, Xu Y, Hu Y, et al. Anti-PD-1 antibody armored gammadelta T cells enhance anti-tumor efficacy in ovarian cancer. Signal Transduct Target Ther. (2023) 8:399. doi: 10.1038/s41392-023-01646-7
140. Vydra J CE, Lesný P, Wanless RS, Anderson J, Clark AG, Scott A, et al. A phase I trial of allogeneic γδ T lymphocytes from haploidentical donors in patients with refractory or relapsed acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2023) 23:e232–e9. doi: 10.1016/j.clml.2023.02.003
141. Marin D, Li Y, Basar R, Rafei H, Daher M, Dou J, et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat Med. (2024) 30:772–84. doi: 10.1038/s41591-023-02785-8
142. Ciurea SO, Kongtim P, Srour S, Chen J, Soebbing D, Shpall E, et al. Results of a phase I trial with Haploidentical mbIL-21 ex vivo expanded NK cells for patients with multiply relapsed and refractory AML. Am J Hematology. (2024) 99:890–9. doi: 10.1002/ajh.27281
143. Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, et al. Efficacy and safety of autologous dendritic cell–based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. (2022) 8:546–52. doi: 10.1001/jamaoncol.2021.7298
144. Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma. JAMA Oncol. (2023) 9:112–21. doi: 10.1001/jamaoncol.2022.5370
145. Rannikko JH, Verlingue L, de Miguel M, Pasanen A, Robbrecht D, Skytta T, et al. Bexmarilimab-induced macrophage activation leads to treatment benefit in solid tumors: The phase I/II first-in-human MATINS trial. Cell Rep Med. (2023) 4:101307. doi: 10.1101/2023.04.17.23288693
146. Heczey A, Xu X, Courtney AN, Tian G, Barragan GA, Guo L, et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med. (2023) 29:1379–88. doi: 10.1038/s41591-023-02363-y
147. Jiang W, Li M, Peng S, Hu T, Long Y, Zhang J, et al. Ubiquitin ligase enzymes and de-ubiquitinating enzymes regulate innate immunity in the TLR, NLR, RLR, and cGAS-STING pathways. Immunol Res. (2023) 71:800–13. doi: 10.1007/s12026-023-09400-5
148. Wang Y and Shao W. Innate immune response to viral vectors in gene therapy. Viruses. (2023) 15:1801. doi: 10.3390/v15091801
149. Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. (2020) 30:966–79. doi: 10.1038/s41422-020-00395-4
150. Friedberg JW, Kelly JL, Neuberg D, Peterson DR, Kutok JL, Salloum R, et al. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. Br J Haematology. (2009) 146:282–91. doi: 10.1111/j.1365-2141.2009.07773.x
151. Ronsley R, Kariminia A, Ng B, Mostafavi S, Reid G, Subrt P, et al. The TLR9 agonist (GNKG168) induces a unique immune activation pattern in vivo in children with minimal residual disease positive acute leukemia: Results of the TACL T2009-008 phase I study. Pediatr Hematol Oncol. (2019) 36:468–81. doi: 10.1080/08880018.2019.1667461
152. Moreno V, Calvo E, Middleton MR, Barlesi F, Gaudy-Marqueste C, Italiano A, et al. Treatment with a retinoic acid-inducible gene I (RIG-I) agonist as monotherapy and in combination with pembrolizumab in patients with advanced solid tumors: results from two phase 1 studies. Cancer Immunology Immunother. (2022) 71:2985–98. doi: 10.1007/s00262-022-03191-8
153. Sensi B, Angelico R, Toti L, Conte L, Coppola A, Tisone G, et al. Mechanism, potential, and concerns of immunotherapy for hepatocellular carcinoma and liver transplantation. Curr Mol Pharmacol. (2024) 17:e18761429310703. doi: 10.2174/0118761429310703240823045808
154. Li Y, Wu X, Sheng C, Liu H, Liu H, Tang Y, et al. IGSF8 is an innate immune checkpoint and cancer immunotherapy target. Cell. (2024) 187:2703–16 e23. doi: 10.1016/j.cell.2024.03.039
155. Lim J RR, Williams K, Silva J, Gutierrez AG, Tyler P, Baharom F, et al. The exonuclease TREX1 constitutes an innate immune checkpoint limiting cGAS/STING-mediated antitumor immunity. Cancer Immunol Res. (2024) 12:663–72. doi: 10.1158/2326-6066.CIR-23-1078
156. Wang S, Bohnert V, Joseph AJ, Sudaryo V, Skariah G, Swinderman JT, et al. ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway in breast cancer. Proc Natl Acad Sci U S A. (2023) 120:e2313693120. doi: 10.1073/pnas.2313693120
157. Conlon KC, Miljkovic MD, and Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res. (2019) 39:6–21. doi: 10.1089/jir.2018.0019
158. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. (2019) 120:6–15. doi: 10.1038/s41416-018-0328-y
159. Svoboda J, Landsburg DJ, Gerson J, Nasta SD, Barta SK, Chong EA, et al. Enhanced CAR T-cell therapy for lymphoma after previous failure. N Engl J Med. (2025) 392:1824–35. doi: 10.1056/NEJMoa2408771
Keywords: innate immunity, tumor microenvironment, immunotherapy, immune cells, immune factors
Citation: Leng S, Ren Y, Tian Y, Zhao W, Mou Y, Chen X, Zhou H and Wang W (2025) Innate immunity in tumors: roles and therapeutic targets. Front. Immunol. 16:1689714. doi: 10.3389/fimmu.2025.1689714
Received: 20 August 2025; Accepted: 06 October 2025;
Published: 22 October 2025.
Edited by:
Jagadeesh Bayry, Indian Institute of Technology Palakkad, IndiaReviewed by:
Andrea Balduit, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyPramod Kumar Gupta, Bhabha Atomic Research Centre (BARC), India
Copyright © 2025 Leng, Ren, Tian, Zhao, Mou, Chen, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2Vpd2FuZzBfMEAxNjMuY29t
 Songze Leng1,2
Songze Leng1,2 Wei Wang
Wei Wang