- Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou, China
Aging is a complex biological phenomenon, which involved in a large number of diseases such as cancer, neurodegeneration, and cardiovascular diseases. Understanding the mechanism of aging may facilitate the development of preventive strategies of age-related diseases. Immunoglobulin (Ig) includes proteins with antibody (Ab) activity or membrane-bound proteins that share a chemically analogous structure to Ab. Ig can recognize and neutralize numerous antigens, which constitutes the main characteristic of adaptive immunity. The quantity, glycosylation and function of Ig change with advancing age. Some Ig is found to be accumulated in aged tissues and appear to be regarded as a potential marker for aging, which indicates the critical role of Ig in aging. B cells are main producers of antibodies and undergo aging-related changes, leading to increased autoimmune responses and reduced vaccine responses. The immune dysregulation of B cells is also intensively involved in the alteration of Ig. In this review, we focus on the current research findings on Ig, discuss the relation between Ig and aging, highlight the complex interplay among B cell, gut microbiota, Ig, and aging, and explore potential therapeutic strategy. We hope this review may provide an insight for investigating the regulatory mechanism of Ig in aging, as well as for evaluating the therapeutic potential in treating age-related diseases.
1 Introduction
Aging is a complex process that involves pronounced decrease of biological functions. It is one of the primary causes for prevalent chronic diseases such as neurodegenerative disorders, cardiovascular system diseases, and cancer, which eventually, lead to death in the world (1). Gaining insight into the mechanism underlying aging can facilitate to enhance lifespan and quality.
Many factors contribute to aging, such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis (2). Of which, cellular senescence has been accepted as a critical trigger of aging and received intensive investigation. Senescent cells are accumulated in tissues and organs during aging. The clearance of senescent cells has successfully alleviated aging and age-related diseases. Interestingly, accumulated immunoglobulin (Ig) is detected adjacent to senescent cells. Ig-related genes such as Igκc, Igj, and Ighg2c are also significantly increased in aged tissues. These evidences suggest that Ig-associated senescence may be a hallmark of aging (3). In other situation such as dysbiosis or chronic inflammation, Ig can also be associated with aging through gut microbiota (4). All these findings not only highlight the mechanism of aging, but also require a fresh look at the complex role of Ig in aging.
Ig is a Y-shaped glycoprotein belongs to the immunoglobulin super-family which shares the same basic structure: two heavy and two light chains linked by disulfide bonds. The alteration of specific glycans on the chains is associated with aging (5). Most importantly, Ig comprises a heterogeneous group of proteins with antibody (Ab) function and serves as the main components of the body’s humoral immunity. In vertebrates, Ig mainly exists as B cell receptor (BCR) on the surface of B cells or as Ab secreted into extracellular fluid. B cells experience immunosenescence during the process of aging and induce the dysregulation of Ig. Apart from lymphoid cells, non-lymphoid lineage and cancer cells can also express Ig (6). With the ability to bind to a myriad of antigens, Ig can recognize antigens and activate cell, while soluble molecules can combine with external substances or pathogens and neutralize pathogens. As a result, Ig is significantly important for physiology and pathology of the human body. The dysregulation of Ig has been identified across different conditions. For example, the structure and composition of IgG or IgM are changed in neurodegenerative diseases such as Parkinson’s disease (PD) or Alzheimer’s disease (AD) (7), while increased IgE is associated with autoimmune diseases such as rheumatoid arthritis (RA) (8). Other type of Ig such as IgA is also involved in autoimmune diseases or centralnervous system diseases. These diseases are commonly thought to be age-related diseases and possess some similar pathological mechanisms with aging.
In this review, we summarize current knowledge on Ig alterations associated with aging and age-related diseases, discuss the underling mechanism of Ig in aging, which will help understanding the mechanism of aging and provide insights into the novel therapies in age-related diseases.
2 Immunoglobulins in brief
Ig is a class of proteins existed in gnathostomes such as mammals, birds, and amphibians et al. (9, 10). Although they had already emerged 500 million years ago, its existence was only described in the late 19th century. Ig is critical for protecting pathogen invasion and thought to be one of the hallmarks of adaptive immunity. They are composed of four polypeptide chains: two light chains and two heavy chains according to their molecular weight (about 23.000 and 50000 respectively). Disulfide bonds link the chains and form a Y-shaped structure. These chains contain looped structures and can be divided into different domains or regions. Light chains possess one constant region (CL) and one variable region (VL). The variable region contains three complementary decision regions (CDRs), which contribute to antigen binding. The constant domains specify effector functions including activation of complement or binding to Fc receptors (11). According to the difference in polypeptide sequence, the light chain is classified into kappa (κ) or lambda (λ), which possesses different antigenic properties. The light chains are usually short of carbohydrate components (12). Unlike light chains, heavy chains possess one variable region (VH) and 3-4 constant regions (CH). Based on the size and amino acid composition within constant region, the heavy chains can be classified into different classes. In mammals such as human and mice, five heavy chain isotypes have been identified: α, δ, ϵ, γ, and μ. Therefore, Ig is classified into five isotypes as IgA, IgD, IgE, IgG, and IgM. However, in other species such as bony fish, the heavy chains are μ, δ, and τ/ζ, which represent IgM, IgD and IgT/Z, respectively (13). And in amphibians, the heavy chain isotypes are μ, δ, χ, υ, and C, which represent IgM, IgD, IgX, IgY, and IgF (14).
A significant event called somatic recombination occurs in the V region, which crucially contributes to the diverse repertoire of Ig heavy and light chains. This process includes the recombination of variable (V), diversity (D) and joining (J) gene segments in an ostensibly random manner. Recombination‐activating gene1/2 initiate the V(D)J recombination by introducing double‐strand breaks at specific recombination signal sequences (15). Apart from V(D)J recombination, other factors contributing to Ig function include activation-induced cytidine deaminase-mediated somatic hypermutation (SHM) and class switch recombination (CSR). SHM can induce point mutations in some hotspots and alter the affinity of Ig (16), while CSR leads to the production of secondary isotypes including IgG, IgA and IgE (17). Following these processes, Ig acquires the ability to recognize and bind to a variety of antigens, and finally exert specific effector functions.
Ig is predominantly produced by B cells. Interestingly, macrophages and non-immune cells such as cancer cells and neurons can also produce Ig such as IgG, IgM, and IgA (18). However, their functions remain to be demonstrated.
3 Aging affects B cell development and function
In mammals, Ig is predominantly expressed by B cells which are originated from multipotent hematopoietic stem cells in the bone marrow (BM). Based on the surface marker and the rearrangement of Ig genes, the development of B cells can be categorized into distinct stages: progenitor B cells (pro-B cells), precursor B cells (pre-B cells), immature B cells, transitional B cells, mature B cells, germinal center (GC) B cells, memory B cells and plasma cells (19). Pro-B cells undergo V(D)J recombination and form the μ chains, and develop into pre-B cells. Pre-B cells then form the light chains and develop into IgM+ immature B cell (15). Immature B cells subsequently migrate to the spleen for terminal maturation. With the assistance of alternative splicing, immature B cells develop into mature IgM+IgD+ B cells. Upon antigen encounter, activated B cells differentiate into Ab-secreting plasma cells. The Ab (Ig) secreted by plasma cells shares structural homology with membrane-bound BCR (Figure 1).
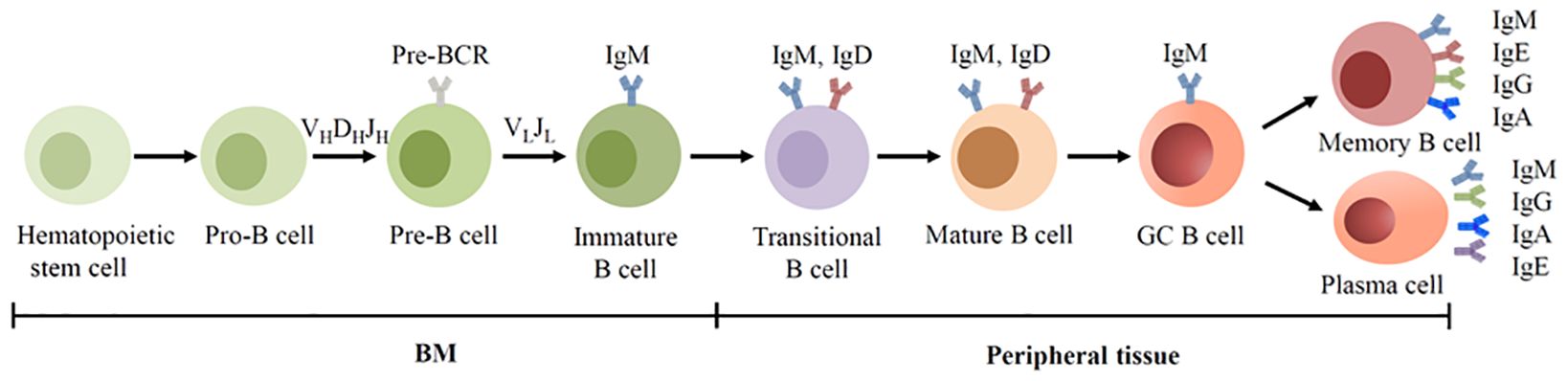
Figure 1. BCR expression during human B cell development. B cells are derived from hematopoietic stem cells in the BM, and acquire BCRs through V(D)J recombination of Ig genes in the early development. In the GC, B cells undergo SHM and CSR, allowing the BCR switched from IgM to other isotypes.
Two lineages can be divided in the early development of B cells, B1 and B2. B1 cells are originated from fetal liver progenitor cells, and notably, their BCR lacks nucleotides at the junctions between V and J segments (20). Located in the peritoneal cavity, most B1 cells can produce natural antibodies including IgM, IgA and IgG in humans and mice. Unlike conventional B2 cell-produced adaptive Ab, natural Ab is synthesized without exposure to foreign antigen. They are polyreactive and can recognize numerous autoantigens and new antigens (21, 22). Conventional B2 cells are mainly located in peripheral tissues and consist of two main populations: marginal zone (MZ) B cells that rapidly produce IgM upon antigen exposure and follicular (FO) B cells produce high-affinity Ab such as IgG, IgA, or IgE.
The emergence of autoantibody (autoAb) manifests a functional collapse of B cell tolerance. B cell acquires central and peripheral tolerance during development. Central tolerance is achieved by the rearrangement of Ig chains and subsequent BCR formation. This process can form autoreactive B cells. Upon reacting with autoantigens in the BM, these newly developed B cells undergo negative selection, a process results in the elimination of some autoreactive immature B cells. The surviving cells then immigrate to the periphery and undergo periphery tolerance, which further eliminate autoreactive cells. The breakdown of both central and peripheral tolerance contributes to the increase of autoreactive B cells, and thereby increased circulating autoantibodies.
Apart from B cells, other cells are involved in the production of autoAb in some conditions. T cells can regulate B cell function. The dysregulation of follicular helper T cells affects B cell maturation and promote the production of autoAb (23). Macrophages play important roles in antigen presentation, immune tolerance and inflammatory response. With increased antigen presentation, macrophage can help to activate B cells and boost the production of autoAb. Moreover, elevated apoptotic debris of macrophage is also involved in autoAb formation in autoimmune diseases (24, 25). Increasing studies have proved that autoAb exert both harmful and protective effect. On the one hand, autoAb is the major contributor in initiating autoimmune diseases. On the other hand, autoAb can suppress inflammation, infection, and kill tumor cells in various diseases (26).
Aging induces great changes in immune system, especially in humoral immune mediated by B cells. This phenomenon is called immunosenescence. Immunosenescence can increase both the frequency and severity of infectious diseases, and reduce the response to vaccination. In fact, aging can affect the development and subset of B cells, as well as Ab production (Figure 2).
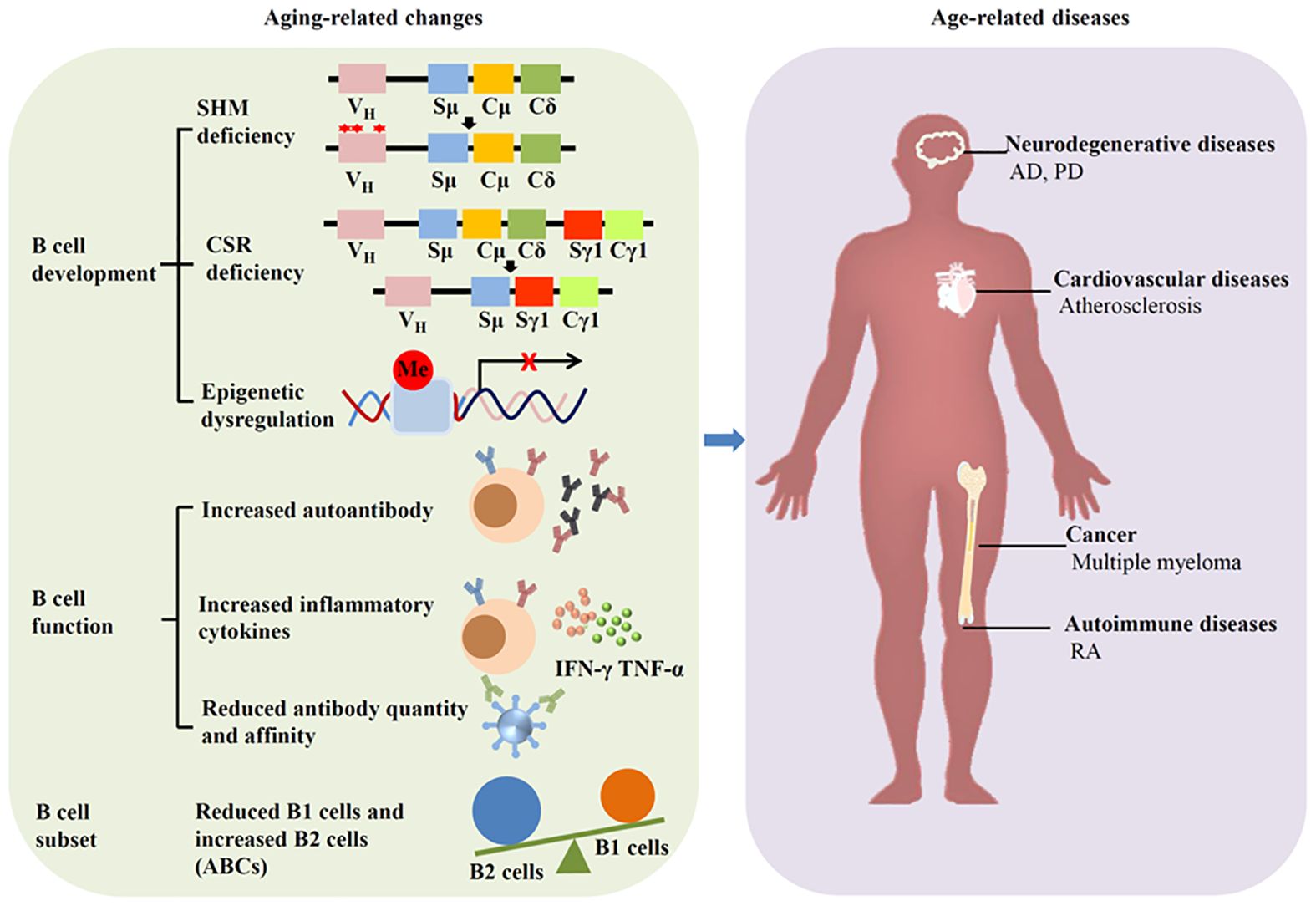
Figure 2. Aging affects B cells and induces diseases. Aging can alter the development, function and subset of B cells, leading to age-related diseases such as neurodegenerative diseases, cardiovascular diseases, autoimmune diseases, and cancer.
During aging, murine and human BM demonstrates diminished B cell precursors, likely attributable to age-associated microenvironmental changes. While murine B1 cell numbers remain stable with aging (27), human B 1 cells are reduced in the elderly (28). In accordance with reduce B1 cells, the amount and function of natural antibodies is reduced during aging, leading to age-related diseases such as atherosclerosis, cancer, and neurodegeneration (29–31). B2 cells exhibit aging-related functional impairments, as manifested by defective isotype switching in aged cells (32). Although peripheral B2 cell maintain unchanged in aging mice, the population of FO B cells decreases with age. The amount of MZ B cell is also reduced during aging, which accompanied by the augmentation of autoantibodies (33, 34).
Age-associated B cells (ABCs) are presumably derived from B2 cells and represent a memory B cells. They are accumulated in the spleen of aged mice. In humans, ABCs expand in elderly individuals and correlate with increased IgG1 levels. ABCs demonstrate autoreactive potential through autoAb secretion.
AutoAb is not only related to autoimmune disease, but also is associated with aging. In fact, there is a complex relationship between aging and autoAb. Increased autoAb is identified during aging. Among them, antinuclear Ab is related to diabetes and age-related diseases (35). Autoantibodies against AT1 receptor can promote endothelial cell senescence and vascular aging (36). Aging can alter the inflammation response as evidenced by increased proinflammatory cytokines such as TNF-α and IFN-γ. Chronic low-grade inflammation, as we all know, is a characteristic of aging.
4 Immunoglobulins and aging
4.1 Immunoglobulin level changed with advanced age
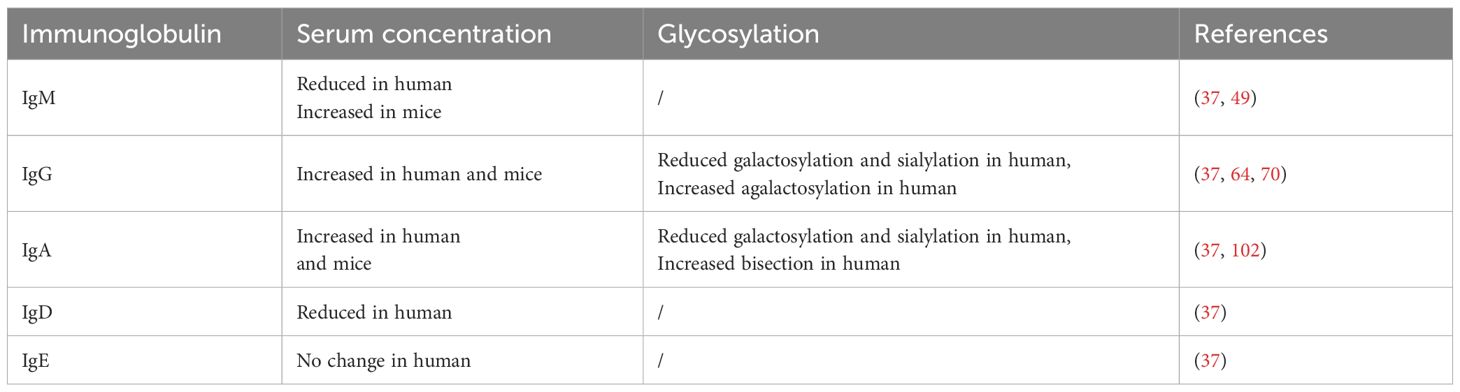
Aging significantly influences B cells, which can be reflected by the changes of Ig levels. Although serum IgE remains stable, the concentrations of switched Ig (IgA and IgG) exhibit increased expression in the human elderly, while IgM and IgD are reduced with age (37). This change implies a transition from naïve to memory B cells and impaired response to new antigens (38). Interestingly, the level of total serum Ig shows no difference or a mild elevation with advanced age in human (39, 40). In addition, the glycosylation of Ig can also be altered with age, which plays an important role in aging and age-related diseases (Table 1). The following sections will review age-related changes in different Ig classes, and go a step further to discuss the underlining mechanism, which will be helpful in understanding the complexity of Ig in aging.
4.2 IgM and aging
4.2.1 IgM structure and function
IgM is the predominant natural Ab and serves as the first responder to foreign invaders. It is the only Ab found in all vertebrate species. Monomeric IgM mainly exists as membrane-bound receptors on B cells, playing a vital role in B cell survival (41, 42). The monomeric IgM has two μ-heavy chains with a C-terminal extension that facilitates oligomerization. Under certain pathological condition such as autoimmune diseases, monomeric IgM can also be secreted (43, 44).
As the primordial class of Ab produced by activated B cells, IgM typically forms a pentameric structure upon secretion. Five IgM monomers are joined together by disulfide bonds and form this pentamer with the assistance of J-chain. The presence of the J-chain enables IgM to cross mucosal epithelia through interaction with the polymeric Ig receptor (45). The pentameric configuration enhances IgM’s ability to bind antigens with high avidity, allowing it to perform multiple functions in immune responses (46).
While human and mouse IgM primarily exist as pentamers, other forms, such as hexameric IgM lacking the J-chain, have been observed in frog. IgM plays a significant role in both humoral and mucosal immunity. It is highly effective in recruiting complement and inducing strong inflammatory responses. Additionally, pentameric autoreactive IgM has been implicated in various autoimmune diseases, including RA and autoimmune neuropathy (47).
4.2.2 IgM exerts a complex role in aging
Aging can increase the level of IgM in mice. Aged mice express more serum IgM than young mice. In response to S. aureus bacteremia infection, aged mice demonstrate higher IgM levels compared to their young counterparts (48–50). However, the situation is different in human. Human serum IgM was reduced with age, especially in women (37). A possible reason for the difference between humans and mice is related to the dynamic changes in B1 cells. B1 cells are mainly producers of IgM. Human B1 cells reduce with age, while mice B1 cells do not. Chinese centenarians with lower serum IgM levels had significantly shorter median survival time (51). These findings suggest that IgM may provide protective effects in human elders.
Time-restricted eating (TRE) is an intermittent fasting pattern that limits daily eating time to a window ranging from 4 to 12 hours. TRE possesses the anti-aging ability as evidenced by increased sphingosine-1-phosphate and L-serine expression. The percentage of IgM increased after 30 days of TRE. The activation of B cells is suppressed as demonstrated by reduced CSR from IgM to IgA (52). Despite the lack of direct evidence, this study indicates that IgM may possess potential anti-aging capability. The protein in cerebrospinal fluid (CSF) is closely associated with aging and neurodegenerative diseases. Using limited proteolysis-mass spectrometry, researchers find that the structure and expression level of IgM are changed in human CSF with aging (53). To be more specific, a complex composed of IgM and Cd5l is increased in mouse and human CSF with aging, which may provide protection against PD. Adaptive immunity is involved in the development of atherosclerosis. IgM can reduce atherosclerosis progression and cardiovascular events (54–56). Patients with coronary artery disease exhibit a significant decrease in circulating atheroprotective oxLDL-specific IgM compared to young healthy volunteers (57). Systemic lupus erythematosus (SLE) patients always show increased atherosclerosis. Serum IgM antibodies against phosphorylcholine (anti-PC), which can provide protection against atherosclerosis, are reduced with age in SLE patient. The protective role of IgM anti-PC antibodies may be associated with the senescence of T cells (58, 59). Based on these findings, it is suggested that IgM can be protective in aging and age-related diseases.
Notably, some investigations yield inconsistent results. Increased urine IgM is linked to the development of vascular aging and cardiovascular events (60). Vascular aging is evidenced by the changes of vascular structure and function, and plays a crucial role in brain and cognitive aging. Many factors such as arteriosclerosis or endothelial dysfunction emerged as the early stage of vascular aging (61). In young to middle-aged healthy people, the increased IgM expression in urine is not associated with hypertension but often means lower ankle brachial index and higher systolic blood pressure. Most importantly, elevated urinary IgM is closely related to increased urinary albumin excretion, an indicator of systemic inflammation and cardiovascular abnormalities. Based on these findings, urine IgM can be seen as an indicator of subclinical peripheral atherosclerosis (62).
4.3 IgG and aging
4.3.1 IgG structure and function
IgG is the predominant Ig class in healthy humans (about 80% of total serum Ig). Structurally, IgG is a 150 kDa glycoprotein composed of two identical heavy chains and two light chains. The heavy chain contains one VH and three CH (CH1, CH2, and CH3). The hinge region exists between the CH1 and CH2 region and contribute to conformational flexibility of the IgG molecule. The protease papain cleaves the hinge region at the N-terminal side of the disulfide bonds and split IgG into three pieces: two identical Fab fragments (fragment antigen binding) and one Fc fragment (fragment crystallizable). The Fab fragments are capable of recognizing and binding to a variety of antigens including bacteria, toxins or self-antigens. The Fc fragment interacts with specific receptors on the surface of immune cells and induce immune responses (49). Pepsin cleaves IgG molecule at the C-terminal of the hinge region and then produces an F(ab’)2 fragment and a smaller Fc fragments (pFc’) (63). Based on the heavy chain γ1-4, IgG can be divided into four subclasses: IgG1, IgG2, IgG3, and IgG4. Despite the sequence homology, these subclasses show subtle differences in conformational flexibility or binding affinity which affect their function.
IgG can cross the placenta and diffuse into extravascular areas. It is critical to humoral immune as protecting against pathogens. IgG exert their protective function through binding to Fcγ receptors (FcγR) and then activating FcγR-bearing cells. IgG can also activate complement which contribute to the recruitment of immune cells.
4.3.2 The reciprocal interaction between IgG and aging
IgG is the most extensively studied aging-related Ig to date. The latest research indicates that the accumulation of IgG is a hallmark of human aging. IgG is increased in human liver and lymph node with age. Moreover, accumulated IgG can induce cell senescence and contributes to tissue aging (3). IgG-producing cells are increased in aged mice, which are consistent with the levels of serum IgG (64, 65). However, in SAMP1 mice, serum IgG was reduced sharply, which contribute to aging-associated arterial stiffening. IgG treatment alleviates arterial stiffening and hypertension in old mice (66). Although the results may vary, these findings all demonstrate this idea: Aging can alter the expression and function of IgG, and IgG can in turn affect aging. Regulation of IgG can counteract aging (Figure 3).
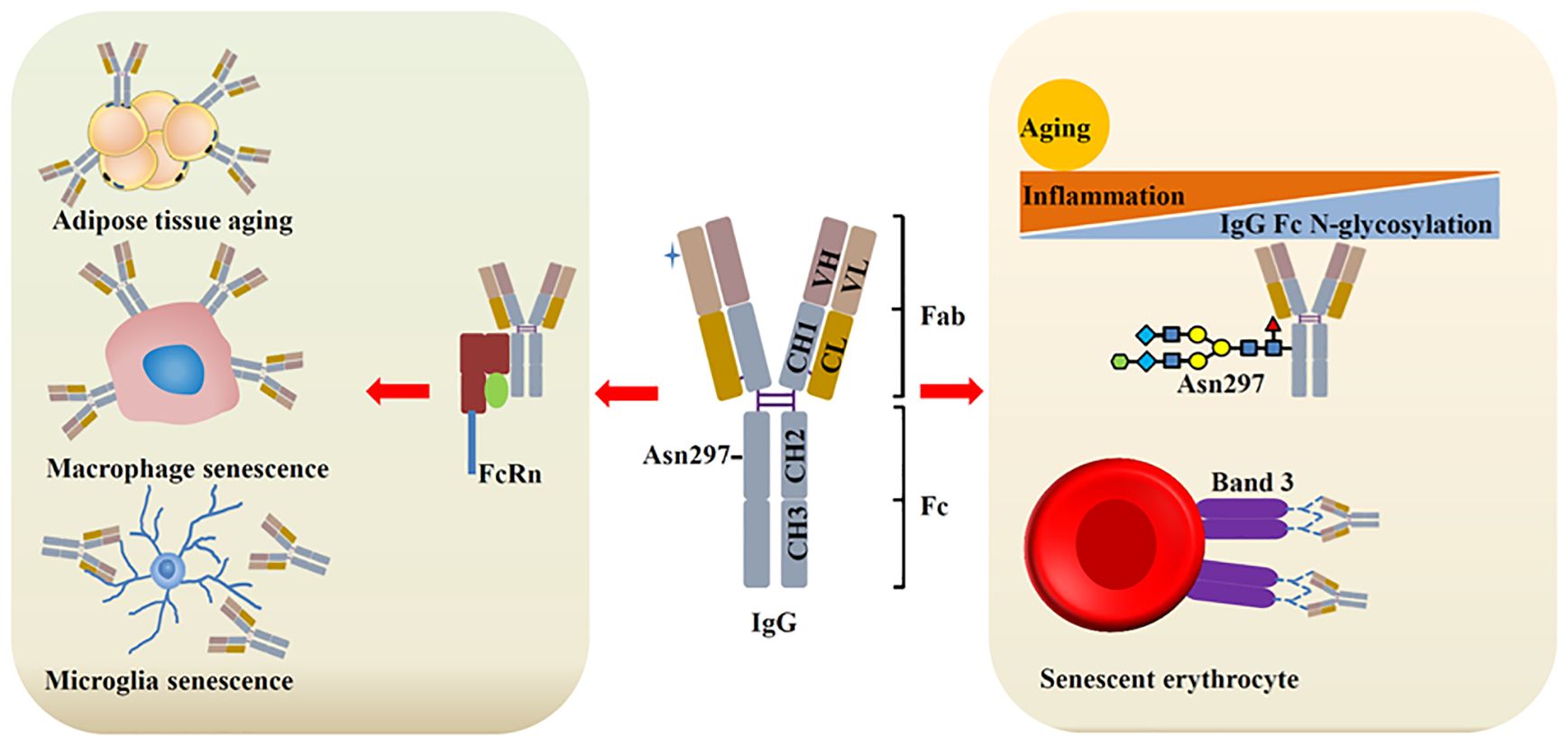
Figure 3. IgG and aging. During aging, IgG is increased, which contributes to cell senescence and tissue aging through binding with different ligands. Aging/inflammation can reduce the N-glycosylation (galactosylation and sialylation) of IgG, leading to increased inflammation, which further promote aging. Senescent erythrocytes show increased IgG binding with band 3 protein.
4.3.3 IgG N-glycosylation and aging
Glycosylation is the most frequent post-translational modification. It mediates cell adhesion, proliferation and differentiation. N-linked and O-linked glycosylation are the two main forms of glycosylation. The function of IgG is modulated through N-glycosylation, where glycans attach to Asn297 in the consensus sequence (Asn-X-Ser/Thr). Under homeostatic conditions, N-glycosylation of IgG remains stable. However, inflammation and aging can significantly alter IgG glycosylation. As a result, changes in IgG glycosylation are closely associated with aging and age-related diseases (67). N-glycosylation includes galactosylation, sialylation, core-fucosylation, and bisecting GlcNAcylation (68). Of which, core-fucosylation and bisecting GlcNAcylation exhibit slightly alteration with age. On the contrary, the level of galactosylated IgG glycans is increased from young and reduced with advancing age. IgG glycosylation can be a predictor of human aging (69, 70). In mice, serum IgG N-glycans also changed with aging. B-cell-specific ablation of β-1,4-galactosyltransferase1can maintain IgG glycans and attenuate aging in mice (71). Aberrant glycosylation of IgG is closely linked to age-related diseases, including dementia, hypertension, and diabetes. Chronic inflammation is crucial for aging and is a causal factor in age-related diseases (72, 73). IgG galactosylation can be reduced by chronic low-grade inflammation, and the attenuation of IgG galactosylation further promotes inflammation. A mendelian randomization study proved the potential causality between IgG N-glycosylation and aging, sialylation of IgG can reduce the inflammation and suppress the aging process (74). Although the mechanism by which glycosylated IgG regulation inflammation remains unclear, the latest study has identified a key transcription factor: repressor element-1 silencing transcription factor (REST). Sialylated IgG can activate the REST in macrophages, which can suppress nuclear factor κB-related signals, leading to reduced inflammation (75).
4.3.4 IgG and erythrocyte aging
Erythrocyte is originated from hematopoietic stem cell and shows a short lifespan of 115 days in the blood. Erythrocyte level is declined in the elderly. Band 3 protein is predominant expressed on erythrocyte membranes and plays an important role in erythrocytes homeostasis. Recent studies suggest that band 3 protein is important for erythrocyte senescence signaling (76). Band 3 anion exchanger can produce senescent cell antigen that binds to serum IgG. This process is important for the removal of erythrocytes (77, 78). Senescent erythrocyte binds with more IgG than young erythrocyte. In neurodegenerative diseases such as AD and PD, band 3 exhibits increased degradation and produce more senescent cell antigen, which may finally contribute to increased IgG on the surface of erythrocytes (79). Erythrocyte can be seen as a biomarker of AD, which indirectly indicates the involvement of IgG in neurodegenerative diseases. These findings are consistent with recent study that IgG is highly expressed near the senescent cell and promote senescence.
4.3.5 Tissue IgG and aging
IgG is accumulated in white adipose tissue and induces the degeneration of adipose tissue during aging (74, 80), which suggests that IgG not only exerts immune function in the plasma, but also plays an important role in metabolism in the tissue. In addition of adipose tissue, IgG has been found accumulated in different tissues and induce the senescence of nearby cells (53). The accumulated IgG is regulated by FcRn. FcRn can protect IgG from lysosomal degradation upon binding with it. Targeting FcRn can reduce IgG expression and aging. Bidirectional two-sample mendelian randomization analysis identifies the causal association with SASP (81). Another report finds that IgG is accumulated in the tissues of aged mice and elicit the senescence of macrophage and microglia, which further aggravates aging. Reducing IgG can attenuate aging.
4.4 IgA and aging
4.4.1 IgA structure and function
IgA serves as the predominant Ab class in humans, constituting approximately 3/4 of total Ig. Although IgA is mostly distributed in mucosal tissues, it ranks as the second most abundant plasma Ig following IgG. Based on the molecular forms, IgA exists in two distinct forms: monomeric IgA (mIgA) and polymeric IgA (pIgA). Plasma IgA exists mainly in a monomeric form. On the contrary, over 90% of mucosal IgA displays polymeric forms.
The human IgA comprises two subclasses (IgA1 and IgA2) distinguished by amino acid sequences of the hinge region. Both subclasses associate with either κ or λ light chain consisting of VL and CL domains. The heavy chains contain a VH followed by three constant domains (Cα1-Cα3). The ratio of IgA1 to IgA2 in serum is 9:1. IgA2 demonstrates two principal allotypes IgA2m (1) and IgA2m (2). A third variant, IgA2 (n) has been recently characterized. This subclass diversity contrasts with mouse or rat where a single IgA form predominates.
Functionally, IgA serves as the primary immunological barrier at mucosal surfaces. Its functional repertoire includes neutralization, complement activation, maintenance of host-commensal homeostasis, and receptor-mediated effector functions. Interestingly, the functions of IgA can be performed by other Ig such as IgM, IgD, or IgG. In fact, IgM is similar to IgA in many aspects such as evolution, structure, and function (42).
4.4.2 IgA interacts with microbiota during aging
IgA is responsive to age and show significant changes with aging. Human serum IgA is increased during aging, which is similar to mice (82). Older people have more IgA in the urine (83). Blood plasma therapy can decrease IgA expression in old rat (84). Moderate aerobic exercise can promote IgA production and improve homeostatic conditions during aging (85). CCL25 is a chemokine that recruits IgA-secreting cells into intestinal lamina propria. Aging can reduce the expression of CCL25. This reduction can in turn decrease IgA and IgA-secreting cells, ultimately affecting gut immunity (86, 87).
A notable phenomenon is that IgA, gut microbe and aging are interrelated and mutually influential. IgA is produced into the intestinal lumen in large numbers every day. These secreted IgA can interact with gut microbiome, and then maintain host-microbiota homeostasis (88, 89). IgA can change the bacterial composition and inflammatory response in the intestinal tract. Gut microbiota dysbiosis is detected in IgA deficient human (90). As a result, IgA is accepted as a controller of symbiotic microbiota (91). Studies have shown the composition of human gut microbiome changed during aging. Microbiota with beneficial functions (such as Oscillospira, Oxalobacter, Prevotellaceae) declined with age, while others (Parvimonas, Corynebacterium, and Corynebacterium) increased and are associated with aging-related inflammation and diseases (92–95).
Cellular senescence is a hallmark of aging. IgA seemed to serve as a bridge connecting the gut microbiota and cellular senescence during aging (96–98). In the ileal of aged mice, commensal bacteria may promote the senescence of GC B cells. The accumulated senescent cells lead to compromised diversity and production of IgA, which in turn changes the composition of gut microbiota and break the gut homeostasis.
It is noteworthy that gut microbiome not only influences IgA that is in the same location, but also exerts an influence on IgA secretion that is anatomically distant from gut. Endocrine dysfunction is associated with gut microbiome during aging. The pituitary gland is an important endocrine organ. During aging, pituitary hormone declined. Interestingly, IgA can be produced by hormone-secreting cells but not B cells in pituitary. The expression of IgA is increased significantly in aged pituitary, which is regulated by gut microbiota (99).
Gut microbiota can also regulate IgA in the central nervous system. IgA+ B cells are increased in the CSF of MS patients. These cells are related to the acute inflammation and neuroinflammatory conditions. Gut microbiota-specific IgA may transported to the CNS and induce neuroinflammatory diseases (100). Moreover, IgA can affect microbiota and further influence lymphocyte and glial cells in the central nervous system (101). Aging-related reduction of microbiota composition can reduce the maturation of microglia. Although microbio is important in CNS aging and IgA can regulate microbio, there is no direct evidence to support the idea that IgA can regulate CNS aging through microbe at present. The interplay among IgA, the microbiome and aging-related CNS disease require further investigation.
4.4.3 IgA glycosylation and aging
Like IgG, IgA contains abundant N- and O-glycosylation sites. These glycopeptide structures changed during aging. Notably, the glycomes of IgA and IgG are closely correlated and regulated by common genetic factors (102). While IgG glycosylation is important in aging, the role of IgA glycosylation in aging remains unknown. Given IgG’s established role in aging, the relationship between IgA glycosylation and aging is worthy of further exploration.
4.5 IgD and aging
4.5.1 IgD structure and function
IgD has two delta (δ) heavy chains which is different from IgG, IgA, and IgM. It exists in all vertebrate species and thought to be evolutionarily conserved Ig class. IgD exhibits pronounced structure plasticity in different species, probably through extensive modifications via both the duplication and deletion of exons. For example, mice and human IgD consisted of two and three Cδ domains, while catfish IgD has seven Cδ domains. Moreover, most jawed vertebrate species display significant alternative RNA splicing events compared to mammals, which also involved in the structural plasticity of IgD.
In mammals such as human and mice, IgD exists in two forms: membrane-anchored IgD (mIgD) and secreted IgD (sIgD). Expression of mIgD follows IgM during B cell development and is an important component of BCR. Most mature B cells coexpress surface IgD and IgM through alternative splicing of a pre-messenger RNA in the nucleus. Notably, a unique subset of mucosal B cells in nasopharyngeal lymphoid tissues exhibit exclusive IgD expression through an unconventional CSR mechanism. These IgM−IgD+ B cells then differentiate into IgD-secreting plasma cells (103, 104). sIgD remains minimally detected in the serum, which accounts for only 0.25% of total serum Ig (105). However, it demonstrates broad tissue distribution, detectable in the nasopharyngeal, oral and lachrymal secretions. Moreover, sIgD can traverse epithelial and placental barriers (106). The distribution of sIgD is correlated with the localization of IgD-producing B cells.
The functions of IgD are relatively enigmatic compared to other Ig. mIgD on B cells may be involved in the peripheral tolerance and B cell anergy, while sIgD may help to maintain mucosal homeostasis through regulating symbiotic host-microbiota interaction (107, 108). The relationship between IgD and aging is also unknown, with only indirect insights from the studies on B cells.
4.5.2 IgD−CD27− B cells and aging
IgD is an important marker of B cells. According to the expression of IgD and/or CD27, human B cells can be classified into four subsets: naïve B cells (CD27-IgD+), unswitched memory B cells (CD27+IgD+), switched memory B cells (CD27+IgD−) and double‐negative (DN) B cells (CD27−IgD−) (109). CD27−IgD− DN B cells are also thought as a subset of memory B cells (110, 111). IgD+IgM+CD27+ memory B cells are dramatically declined in the aged people (112). Although circulating IgD−CD27− B cells exhibit lower expression of SASP marker including TNF-α, IL-6, IL-8 and p16INK4 (113), they are now thought to be related to immunosenescence, aging, autoimmune and infectious diseases. It is increased in elder people and had been seen as senescent or exhausted B cells. In HIV patients, the number of IgD−CD27− cells is correlated with CD3+CD4+CD57+CD45RO−CD4+ T cells, a terminal effector cells that are prevail in aging (114). In addition, IgG+IgD−CD27− B cells are increased in RA patients. IL-6R blockade (tocilizumab) or TNF inhibitors can significantly reduce the expression of cells to normal levels (115, 116). Moreover, IgD−CD27− B cells are associated with severe atherosclerosis in human and can promote inflammation in male elders (117, 118). All these findings suggest that IgD−CD27− B cells may play a role in aging-related inflammation or diseases.
4.6 IgE and aging
4.6.1 IgE structure and function
Amphibians IgY underwent a gene duplication event and diverged into IgE, a unique Ab class which is exclusively found in mammals. IgE exists as a monomeric form composed of two heavy and two light chains, distinguished from other Ig isotypes by its characteristic epsilon constant region (Cϵ). The Cϵ region contains four constant domains (Cϵ1-Cϵ4), which is similar to the μ heavy chain of IgM. Despite sharing evolutionary origins with IgG through ancestral IgY molecules, IgE exhibits distinct structural features, particularly in its Cϵ2 domain positioning. These domains occupy spatial coordinates equivalent to the Fab-Fc hinge region found in IgG molecules, a key differentiating feature between these two Ab classes. Cϵ2 and Cϵ3 domains interact with the high‐affinity IgE‐receptor FcϵRI, while Cϵ3 and Cϵ4 domains interact with the low‐affinity IgE‐receptor CD23. CD23 on B cells is important for IgE synthesis and presentation.
IgE demonstrates the lowest serum concentration among Igs (approximately 10,000 fold less than other isotypes). However, it exhibits remarkable potency in host defense against parasites and certain toxins. This Ab class plays a dual biological role, mediating both protective immune responses and pathological hypersensitivity reactions. Membrane-bound IgE antibodies undergo crosslinking upon encounter antigens, and then initiate the release of bioactive molecules collectively induce allergic and inflammatory reactions.
4.6.2 IgE is involved in age-related diseases
Although previous studies showed that IgE production is reduced with age and is associated with reduced allergic symptom (119), recent findings require a fresh look at the role of IgE in aging. IgE is closely associated to allergy, a disease which is considered a pediatric disease rather than an adult one. Interestingly, some studies find that allergy, especially food allergy is increasing in elders, which is similar to other age-related diseases such as cardiovascular, neurodegenerative, and cancer (120, 121). Many factors seemed to be involved in this phenomenon. Immunosenescence play a crucial role in food allergy. Food allergy indicates the impairment of mucosal tolerance. Gut immune system experiences multiple changes during aging, such as increased local inflammation, impaired barrier function, and IgA deficiency. Aging also alters the composition and function of gut microbiota, leading to chronic inflammation. IgE is increased in the skin lesion of elders, which can induce inflammatory response and contribute to allergy in elders (122). In addition, B cells are previously thought to be cleared shortly after IgE production. However, long-lived B cells that constantly producing IgE have been identified, supporting the increased IgE with aging.
IgE also contributes to autoimmune diseases. Autoreactive IgE can activate basophils, and other FcϵRI-bearing cells, prompting Ab production and other pro-inflammatory signals. IgE autoantibodies have been identified in rheumatic diseases such as RA and SLE (8). Therapy targeting IgE can reduce autoimmune diseases. In addition, serum IgE is increased in atherosclerosis patients. This may attribute to IL-17, an important proinflammatory cytokine in the pathogenesis of atherosclerosis. IL-17 is increased in atherosclerosis, which can enhance B cell-produced IgE. IgE can promote macrophage polarization and cholesterol accumulation through binding with FcϵR1. IgE deficient mice demonstrate attenuated atherosclerosis (123, 124).
5 Therapeutic potential of Ig in aging
Ig therapy exerts anti-inflammation and immune modulatory function, and has been used in immune deficient diseases and other immune-mediated conditions such as dermatomyositis, autoimmune bullous diseases, and chronic inflammatory demyelinating polyradiculoneuropathy (125). However, the therapeutic effect in aging remains to be clarified. Recent studies find that antisense oligonucleotide against FcRn can inhibit the accumulation of IgG in adipose tissue, which helps reduce fibrosis and relieve aging (74, 80). In another study, suppressing IgG effectively reduces aging in mice. IgM transfer has been reported to induce B cell tolerance and inhibit autoimmunity (126). Considering the fact that Ig is deeply involved in the development of aging, there is a high possibility that regulation of Ig can be used to develop novel methods for counteracting aging. Chronic inflammation or inflammaging is the hallmark of immunosenescence and aging, and anti-inflammatory therapies can be seen as potential anti-aging treatment (127, 128). B cells are the main Ig-producing cells and are critical in immune response. Interventions targeting B cells may alleviate aging through regulating inflammation, senescence and Ig production. Rituximab, a monospecific Ab that targets CD20 on B cells, can alleviate RA, MS, and atherosclerosis. However, monospecific Ab only targets one specific molecule, leading to limited efficacy. Different approaches have been developed to improve therapeutic efficacy. Bispecific Ab (BsAb) can bind two different epitopes/antigens simultaneously, which aids in redirecting cytotoxic effector cells to target cells. Ab-drug conjugate (ADC) consists of a monoclonal Ab and a cytotoxic drug. This combination helps to achieve targeted and potent therapy. Besides bsAb and ADC, chimeric antigen receptor T (CAR-T) cell therapy also makes encouraging progress in B cell malignancies. It uses engineered T cells to target CD19 and B cell maturation antigen (BCMA). In summary, these new technologies offer considerable potential for therapeutic use and greatly enhance treatment efficacy in autoimmune diseases and cancer (129) (Figure 4).
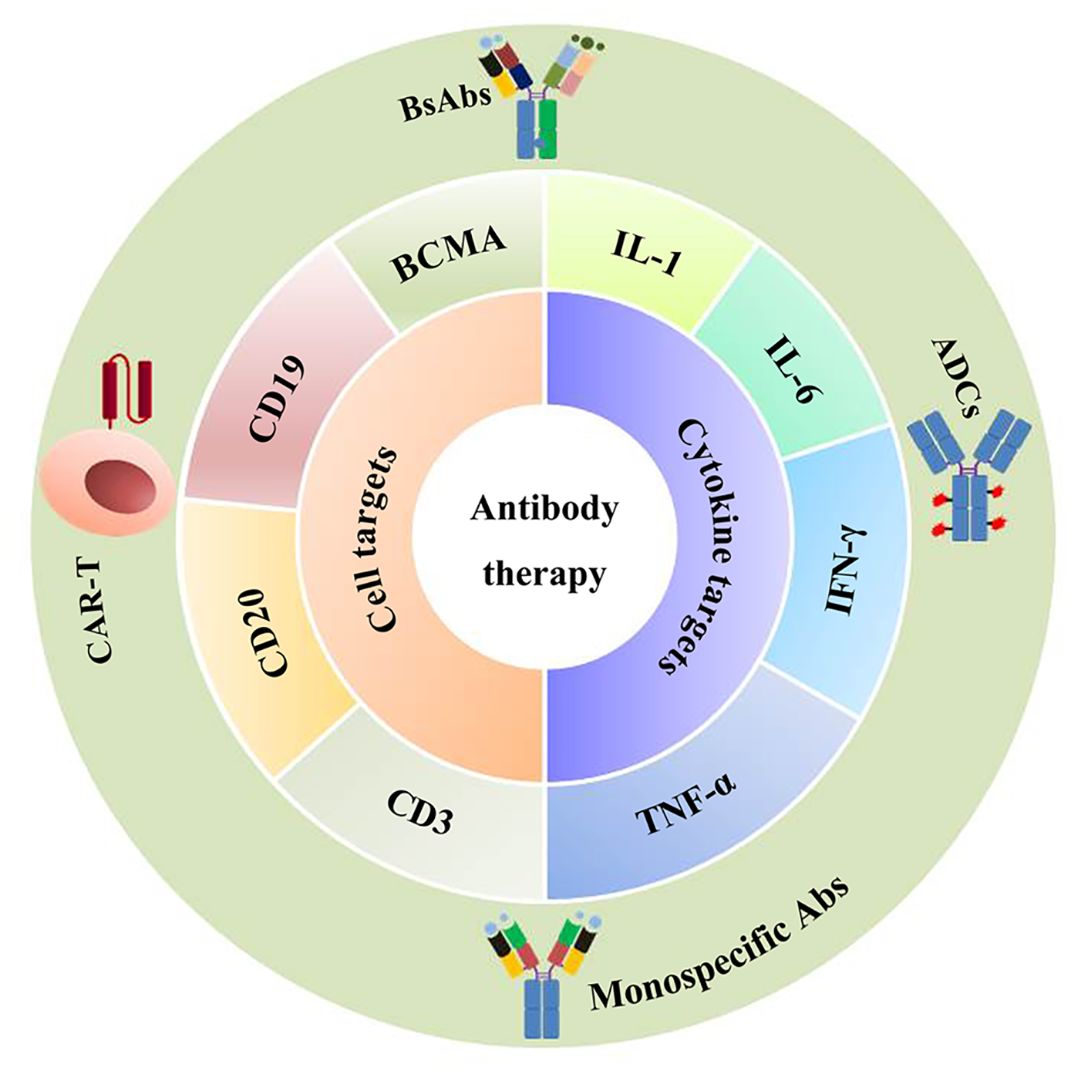
Figure 4. Antibody-based therapy. Antibody-based therapy is used to treat age-related diseases such as cancer or autoimmune diseases through regulating inflammation or senescence. The currently developed drugs include monospecific Abs, bsAbs (bispecific Abs), CAR-T (chimeric antigen receptor T), and ADCs (antibody-drug conjugates), which target CD19, CD20, BCMA (B cell maturation antigen), or target inflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ.
6 Conclusions
Aging is an interconnected process during which immune responses experience a gradual decline. Increasing studies suggest that aging can regulate the production and function of Ig through immunosenescence, chronic inflammation, epigenetic modification, and microbe disturbances. The immunosenescent B cells are deficient in Ig class switch and affinity maturation which affect the isotype and function of Ig. On the other hand, with increased inflammatory factor production and self-tolerance broken, aged B cells can produce autoAb which contributes to some age-related diseases. Glycosylation or Asp isomerization in Ig, especially in IgG, has changed during aging and is expected to be seen as a biomarker of aging. In addition, there is a complex relation between aging, microbes, and Ig. Ig and aging exhibit mutual influence and interaction, although the underling mechanisms require further clarification. Moreover, apart from B cells, Ig is produced by many other cells such macrophage. Macrophage is related to aging. However, the function of macrophage-derived Ig remains unknown. Overall, aging is related to almost all chronic diseases. Understanding the role of Ig in aging will facilitate the diagnosis of these diseases. Ig or Ig-producing cells-based therapy may be a hopeful strategy to intervene in aging and age-related diseases.
Author contributions
QG: Investigation, Visualization, Writing – original draft, Writing – review & editing. YW: Investigation, Visualization, Writing – review & editing, Writing – original draft. CZ: Resources, Writing – review & editing. XZ: Writing – review & editing. LN: Writing – review & editing. HZ: Writing – review & editing. HY: Supervision, Writing – review & editing. QS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82172485, 82372457), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Acknowledgments
We sincerely thank the reviewers for their valuable feedback on this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell. (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
2. López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. Hallmarks of aging: An expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
3. Ma S, Ji Z, Zhang B, Geng L, Cai Y, Nie C, et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell. (2024) 187:7025–44 e34. doi: 10.1016/j.cell.2024.10.019
4. Kawamoto S and Hara E. Crosstalk between gut microbiota and cellular senescence: a vicious cycle leading to aging gut. Trends Cell Biol. (2024) 34:626–35. doi: 10.1016/j.tcb.2023.12.004
5. Krištić J, Lauc G, and Pezer M. Immunoglobulin G glycans - Biomarkers and molecular effectors of aging. Clin Chim Acta. (2022) 535:30–45. doi: 10.1016/j.cca.2022.08.006
6. Chen Z, Qiu X, and Gu J. Immunoglobulin expression in non-lymphoid lineage and neoplastic cells. Am J Pathol. (2009) 174:1139–48. doi: 10.2353/ajpath.2009.080879
7. Paganelli R, Paganelli A, Pawelec G, and Di Iorio A. Natural IgG antibodies to β amyloid are decreased in patients with Parkinson's disease. Immun Ageing. (2023) 20:13. doi: 10.1186/s12979-023-00336-w
8. Charles N, Kortekaas-Krohn I, Kocaturk E, Scheffel J, Altrichter S, Steinert C, et al. Autoreactive IgE: Pathogenic role and therapeutic target in autoimmune diseases. Allergy. (2023) 78:3118–35. doi: 10.1111/all.15843
9. Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. (2002) 2:688–98. doi: 10.1038/nri889
10. Olivieri DN, Mirete-Bachiller S, and Gambón-Deza F. Insights into the evolution of IG genes in Amphibians and reptiles. Dev Comp Immunol. (2021) 114:103868. doi: 10.1016/j.dci.2020.103868
11. Sarkar A and Pitchumoni CS. The protean manifestations of IgG4-RD in gastrointestinal disorders. Dis Mon. (2015) 61:493–515. doi: 10.1016/j.disamonth.2015.09.008
12. Li H, Zhang Y, Zhu Y, Zhao Q, Xu J, Li X, et al. Functional insights into immunoglobulin superfamily proteins in invertebrate neurobiology and immunity. Front Immunol. (2025) 16:1552151. doi: 10.3389/fimmu.2025.1552151
13. Parra D, Takizawa F, and Sunyer JO. Evolution of B cell immunity. Annu Rev Anim Biosci. (2013) 1:65–97. doi: 10.1146/annurev-animal-031412-103651
14. Zhao Y, Pan-Hammarström Q, Yu S, Wertz N, Zhang X, Li N, et al. Identification of IgF, a hinge-region-containing Ig class, and IgD in Xenopus tropicalis. Proc Natl Acad Sci U S A. (2006) 103:12087–92. doi: 10.1073/pnas.0600291103
15. Chi X, Li Y, and Qiu X. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation. Immunology. (2020) 160:233–47. doi: 10.1111/imm.13176
16. Mikocziova I, Greiff V, and Sollid LM. Immunoglobulin germline gene variation and its impact on human disease. Genes Immun. (2021) 22:205–17. doi: 10.1038/s41435-021-00145-5
17. Chaudhuri J and Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. (2004) 4:541–52. doi: 10.1038/nri1395
18. Gong X, Yan H, Ma J, Zhu Z, Zhang S, Xu W, et al. Macrophage-derived immunoglobulin M inhibits inflammatory responses via modulating endoplasmic reticulum stress. Cells. (2021) 10:2812. doi: 10.3390/cells10112812
19. Martinis E, Tonon S, Colamatteo A, La Cava A, Matarese G, and Pucillo CEM. B cell immunometabolism in health and disease. Nat Immunol. (2025) 26:366–77. doi: 10.1038/s41590-025-02102-0
20. de Mol J, Kuiper J, Tsiantoulas D, and Foks AC. The dynamics of B cell aging in health and disease. Front Immunol. (2021) 12:733566. doi: 10.3389/fimmu.2021.733566
21. Rothstein TL. Natural antibodies as rheostats for susceptibility to chronic diseases in the aged. Front Immunol. (2016) 7:127. doi: 10.3389/fimmu.2016.00127
22. Mattos MS, Vandendriessche S, Waisman A, and Marques PE. The immunology of B-1 cells: from development to aging. Immun Ageing. (2024) 21:54. doi: 10.1186/s12979-024-00455-y
23. Walker LSK. The link between circulating follicular helper T cells and autoimmunity. Nat Rev Immunol. (2022) 22:567–75. doi: 10.1038/s41577-022-00693-5
24. Yang S, Zhao M, and Jia S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front Immunol. (2023) 14:1080310. doi: 10.3389/fimmu.2023.1080310
25. Li B, Yue Y, Dong C, Shi Y, and Xiong S. Blockade of macrophage autophagy ameliorates activated lymphocytes-derived DNA induced murine lupus possibly via inhibition of proinflammatory cytokine production. Clin Exp Rheumatol. (2014) 32:705–14.
26. Jaycox JR, Dai Y, and Ring AM. Decoding the autoantibody reactome. Sci (New York NY). (2024) 383:705–7. doi: 10.1126/science.abn1034
27. Alter-Wolf S, Blomberg BB, and Riley RL. Old mice retain bone marrow B1 progenitors, but lose B2 precursors, and exhibit altered immature B cell phenotype and light chain usage. Mech Ageing Dev. (2009) 130:401–8. doi: 10.1016/j.mad.2009.04.001
28. Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, and Hernandez AM. Human B-1 cells and B-1 cell antibodies change with advancing age. Front Immunol. (2019) 10:483. doi: 10.3389/fimmu.2019.00483
29. Binder CJ. Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol. (2010) 30 Suppl 1:S56–60. doi: 10.1007/s10875-010-9396-3
30. Szabo P, Relkin N, and Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev. (2008) 7:415–20. doi: 10.1016/j.autrev.2008.03.007
31. Haro MA, Dyevoich AM, Phipps JP, and Haas KM. Activation of B-1 cells promotes tumor cell killing in the peritoneal cavity. Cancer Res. (2019) 79:159–70. doi: 10.1158/0008-5472.can-18-0981
32. Frasca D, van der Put E, Riley RL, and Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol (Baltimore Md: 1950). (2004) 172:2155–62. doi: 10.4049/jimmunol.172.4.2155
33. Miller JP and Cancro MP. B cells and aging: balancing the homeostatic equation. Exp Gerontol. (2007) 42:396–9. doi: 10.1016/j.exger.2007.01.010
34. Dunn-Walters DK. The ageing human B cell repertoire: a failure of selection? Clin Exp Immunol. (2016) 183:50–6. doi: 10.1111/cei.12700
35. Meier HCS, Sandler DP, Simonsick EM, Weng NP, and Parks CG. Sex differences in the association between antinuclear antibody positivity with diabetes and multimorbidity in older adults: Results from the Baltimore Longitudinal Study of Aging. Exp Gerontol. (2020) 135:110906. doi: 10.1016/j.exger.2020.110906
36. Wang M, Yin X, Zhang S, Mao C, Cao N, Yang X, et al. Autoantibodies against AT1 receptor contribute to vascular aging and endothelial cell senescence. Aging Dis. (2019) 10:1012–25. doi: 10.14336/ad.2018.0919
37. Listì F, Candore G, Modica MA, Russo M, Di Lorenzo G, Esposito-Pellitteri M, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. (2006) 1089:487–95. doi: 10.1196/annals.1386.013
38. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
39. Bulati M, Caruso C, and Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by "inflamm-ageing. Ageing Res Rev. (2017) 36:125–36. doi: 10.1016/j.arr.2017.04.001
40. Martín S, Pérez A, and Aldecoa C. Sepsis and immunosenescence in the elderly patient: A review. Front Med (Lausanne). (2017) 4:20. doi: 10.3389/fmed.2017.00020
41. Pleass RJ, Moore SC, Stevenson L, and Hviid L. Immunoglobulin M: Restrainer of inflammation and mediator of immune evasion by plasmodium falciparum malaria. Trends Parasitol. (2016) 32:108–19. doi: 10.1016/j.pt.2015.09.007
42. Sun Y, Huang T, Hammarström L, and Zhao Y. The Immunoglobulins: New insights, implications, and applications. Annu Rev Anim Biosci. (2020) 8:145–69. doi: 10.1146/annurev-animal-021419-083720
43. Xu H, Geddes R, and Roberts-Thomson PJ. Low molecular weight IgM and CD5 B lymphocytes in rheumatoid arthritis. Ann Rheum Dis. (1994) 53:383–90. doi: 10.1136/ard.53.6.383
44. Roberts-Thomson PJ, Shepherd K, Bradley J, and Boey ML. Frequency and role of low molecular weight IgM in systemic lupus erythematosus. Study of patients from different ethnic origins. Rheumatol Int. (1990) 10:95–8. doi: 10.1007/bf02274821
45. Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. (1994) 12:63–84. doi: 10.1146/annurev.iy.12.040194.000431
46. Kridin K and Ahmed AR. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun Rev. (2020) 19:102466. doi: 10.1016/j.autrev.2020.102466
47. Amendt T and Yu P. TLR7 and IgM: Dangerous partners in autoimmunity. Antibodies (Basel). (2023) 12:4. doi: 10.3390/antib12010004
48. Webster SE, Tsuji NL, Clemente MJ, and Holodick NE. Age-related changes in antigen-specific natural antibodies are influenced by sex. Front Immunol. (2022) 13:1047297. doi: 10.3389/fimmu.2022.1047297
49. Gupta P, Hu Z, Kopparapu PK, Deshmukh M, Sághy T, Mohammad M, et al. The impact of TLR2 and aging on the humoral immune response to Staphylococcus aureus bacteremia in mice. Sci Rep. (2023) 13:8850. doi: 10.1038/s41598-023-35970-3
50. Holodick NE and Rothstein TL. B cells in the aging immune system: time to consider B-1 cells. Ann N Y Acad Sci. (2015) 1362:176–87. doi: 10.1111/nyas.12825
51. Zhang W, Duan Y, Li Z, Niu Y, Wang B, Feng Z, et al. Association between serum IgM and all-cause mortality risk in Chinese centenarians: a prospective cohort study. Immun Ageing. (2024) 21:70. doi: 10.1186/s12979-024-00475-8
52. Chen Y, Li X, Yang M, Jia C, He Z, Zhou S, et al. Time-restricted eating reveals a "younger" immune system and reshapes the intestinal microbiome in human. Redox Biol. (2024) 78:103422. doi: 10.1016/j.redox.2024.103422
53. Shuken SR, Rutledge J, Iram T, Losada PM, Wilson EN, Andreasson KI, et al. Limited proteolysis-mass spectrometry reveals aging-associated changes in cerebrospinal fluid protein abundances and structures. Nat Aging. (2022) 2:379–88. doi: 10.1038/s43587-022-00196-x
54. Chistiakov DA, Orekhov AN, and Bobryshev YV. Immune-inflammatory responses in atherosclerosis: Role of an adaptive immunity mainly driven by T and B cells. Immunobiology. (2016) 221:1014–33. doi: 10.1016/j.imbio.2016.05.010
55. Gigante B, Leander K, Vikström M, Baldassarre D, Veglia F, Strawbridge RJ, et al. Low levels of IgM antibodies against phosphorylcholine are associated with fast carotid intima media thickness progression and cardiovascular risk in men. Atherosclerosis. (2014) 236:394–9. doi: 10.1016/j.atherosclerosis.2014.07.030
56. Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, et al. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis. (2012) 220:59–65. doi: 10.1016/j.atherosclerosis.2011.10.002
57. Grievink HW, Smit V, Huisman BW, Gal P, Yavuz Y, Klerks C, et al. Cardiovascular risk factors: The effects of ageing and smoking on the immune system, an observational clinical study. Front Immunol. (2022) 13:968815. doi: 10.3389/fimmu.2022.968815
58. Rahman M, Sing S, Golabkesh Z, Fiskesund R, Gustafsson T, Jogestrand T, et al. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin Immunol. (2016) 166-167:27–37. doi: 10.1016/j.clim.2016.04.007
59. López P, Rodríguez-Carrio J, Martínez-Zapico A, Pérez-Álvarez ÁI, Benavente L, Caminal-Montero L, et al. IgM anti-phosphorylcholine antibodies associate with senescent and IL-17+ T cells in SLE patients with a pro-inflammatory lipid profile. Rheumatol (Oxford England). (2020) 59:407–17. doi: 10.1093/rheumatology/kez264
60. Tofik R, Torffvit O, Rippe B, and Bakoush O. Urine IgM-excretion as a prognostic marker for progression of type 2 diabetic nephropathy. Diabetes Res Clin Pract. (2012) 95:139–44. doi: 10.1016/j.diabres.2011.10.008
61. Nilsson PM. Early vascular ageing - A concept in development. Eur Endocrinol. (2015) 11:26–31. doi: 10.17925/ee.2015.11.01.26
62. Swärd P, Tofik R, Bakoush O, Torffvit O, Nilsson PM, and Christensson A. Patterns of urinary albumin and IgM associate with markers of vascular ageing in young to middle-aged individuals in the Malmö offspring study. BMC Cardiovasc Disord. (2020) 20:358. doi: 10.1186/s12872-020-01638-3
63. Liu J, Zhang Z, Bai A, Sha Y, Ma L, Qin S, et al. Prophylactic efficacy of equine immunoglobulin F(ab')2 fragments against feline parvovirus. Appl Biochem Biotechnol. (2021) 193:3151–62. doi: 10.1007/s12010-021-03591-z
64. Koga T, McGhee JR, Kato H, Kato R, Kiyono H, and Fujihashi K. Evidence for early aging in the mucosal immune system. J Immunol. (2000) 165:5352–9. doi: 10.4049/jimmunol.165.9.5352
65. Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, and Faria AM. Specific immune responses but not basal functions of B and T cells are impaired in aged mice. Cell Immunol. (2009) 256:1–5. doi: 10.1016/j.cellimm.2009.01.010
66. Fan J, Wang S, Chen K, and Sun Z. Aging impairs arterial compliance via Klotho-mediated downregulation of B-cell population and IgG levels. Cell Mol Life Sci. (2022) 79:494. doi: 10.1007/s00018-022-04512-x
67. Dall'Olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, and Franceschi C. N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing Res Rev. (2013) 12:685–98. doi: 10.1016/j.arr.2012.02.002
68. Wu Y, Zhang Z, Chen L, and Sun S. Immunoglobulin G glycosylation and its alterations in aging-related diseases. Acta Biochim Biophys Sin (Shanghai). (2024) 56:1221–33. doi: 10.3724/abbs.2024137
69. Ensinck A, Biondi CS, Marini A, García Borrás S, Racca LL, Cotorruelo CM, et al. Effect of membrane-bound IgG and desialysation in the interaction of monocytes with senescent erythrocytes. Clin Exp Med. (2006) 6:138–42. doi: 10.1007/s10238-006-0110-y
70. Giron LB, Liu Q, Adeniji OS, Yin X, Kannan T, Ding J, et al. Immunoglobulin G N-glycan markers of accelerated biological aging during chronic HIV infection. Nat Commun. (2024) 15:3035. doi: 10.1038/s41467-024-47279-4
71. Sha J, Fan J, Zhang R, Gu Y, Xu X, Ren S, et al. B-cell-specific ablation of β-1,4-galactosyltransferase 1 prevents aging-related IgG glycans changes and improves aging phenotype in mice. J Proteomics. (2022) 268:104717. doi: 10.1016/j.jprot.2022.104717
72. Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, et al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine. (2015) 2:1549–58. doi: 10.1016/j.ebiom.2015.07.029
73. Singh A, Schurman SH, Bektas A, Kaileh M, Roy R, Wilson DM 3rd, et al. Aging and inflammation. Cold Spring Harb Perspect Med. (2024) 14:a041197. doi: 10.1101/cshperspect.a041197
74. Yu L, Wan Q, Liu Q, Fan Y, Zhou Q, Skowronski AA, et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. (2024) 36:793–807 e5. doi: 10.1016/j.cmet.2024.01.015
75. Chakraborty S, Cheng BY, Edwards DL, Gonzalez JC, Chiu DK, Zheng H, et al. Sialylated IgG induces the transcription factor REST in alveolar macrophages to protect against lung inflammation and severe influenza disease. Immunity. (2025) 58:182–96 e10. doi: 10.1016/j.immuni.2024.10.002
76. Badior KE and Casey JR. Large conformational dynamics in Band 3 protein: Significance for erythrocyte senescence signalling. Biochim Biophys Acta Biomembr. (2021) 1863:183678. doi: 10.1016/j.bbamem.2021.183678
77. Loriamini M, Cserti-Gazdewich C, and Branch DR. Autoimmune hemolytic anemias: Classifications, pathophysiology, diagnoses and management. Int J Mol Sci. (2024) 25:4269. doi: 10.3390/ijms25084296
78. Buerck JP, Burke DK, Schmidtke DW, Snyder TA, Papavassiliou D, and O'Rear EA. A flow induced autoimmune response and accelerated senescence of red blood cells in cardiovascular devices. Sci Rep. (2019) 9:19443. doi: 10.1038/s41598-019-55924-y
79. Yazdani U, Zaman S, Hynan LS, Brown LS, Dewey RB Jr., Karp D, et al. Blood biomarker for Parkinson disease: peptoids. NPJ Parkinsons Dis. (2016) 2:16012–. doi: 10.1038/npjparkd.2016.12
80. Yu L, Yang YX, Gong Z, Wan Q, Du Y, Zhou Q, et al. FcRn-dependent IgG accumulation in adipose tissue unmasks obesity pathophysiology. Cell Metab. (2025) 37:656–72 e7. doi: 10.1016/j.cmet.2024.11.001
81. Wang H, Liu D, Meng X, Sun W, Li C, Lu H, et al. Bidirectional two-sample mendelian randomization study of immunoglobulin G N-glycosylation and senescence-associated secretory phenotype. Int J Mol Sci. (2024) 25:6337. doi: 10.3390/ijms25126337
82. Radl J, Sepers JM, Skvaril F, Morell A, and Hijmans W. Immunoglobulin patterns in humans over 95 years of age. Clin Exp Immunol. (1975) 22:84–90.
83. Bakun M, Senatorski G, Rubel T, Lukasik A, Zielenkiewicz P, Dadlez M, et al. Urine proteomes of healthy aging humans reveal extracellular matrix (ECM) alterations and immune system dysfunction. Age (Dordrecht Netherlands). (2014) 36:299–311. doi: 10.1007/s11357-013-9562-7
84. Asmaz ED, Teker HT, Sertkaya ZT, Ceylani T, and Genç A. Effect of middle-age plasma therapy on ileum morphology, immune defense (IgA) and cell proliferation (Ki-67) of female aged rats. Histochem Cell Biol. (2024) 163:17. doi: 10.1007/s00418-024-02344-3
85. Hernández-Urbán AJ, Drago-Serrano ME, Reséndiz-Albor AA, Sierra-Ramírez JA, Guzmán-Mejía F, Oros-Pantoja R, et al. Moderate aerobic exercise induces homeostatic IgA generation in senile mice. Int J Mol Sci. (2024) 25:8200. doi: 10.3390/ijms25158200
86. Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. (2004) 173:3668–75. doi: 10.4049/jimmunol.173.6.3668
87. Nagafusa H and Sayama K. Age-related chemokine alterations affect IgA secretion and gut immunity in female mice. Biogerontology. (2020) 21:609–18. doi: 10.1007/s10522-020-09877-9
88. Yang Y and Palm NW. Immunoglobulin A and the microbiome. Curr Opin Microbiol. (2020) 56:89–96. doi: 10.1016/j.mib.2020.08.003
89. van der Waaij LA, Mesander G, Limburg PC, and van der Waaij D. Direct flow cytometry of anaerobic bacteria in human feces. Cytometry. (1994) 16:270–9. doi: 10.1002/cyto.990160312
90. von Gunten S, Schneider C, Imamovic L, and Gorochov G. Antibody diversity in IVIG: Therapeutic opportunities for novel immunotherapeutic drugs. Front Immunol. (2023) 14:1166821. doi: 10.3389/fimmu.2023.1166821
91. Gleeson PJ, Camara NOS, Launay P, Lehuen A, and Monteiro RC. Immunoglobulin A antibodies: From protection to harmful roles. Immunol Rev. (2024) 328:171–91. doi: 10.1111/imr.13424
92. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) 488:178–84. doi: 10.1038/nature11319
93. Candela M, Biagi E, Brigidi P, O'Toole PW, and De Vos WM. Maintenance of a healthy trajectory of the intestinal microbiome during aging: a dietary approach. Mech Ageing Dev. (2014) 136-137:70–5. doi: 10.1016/j.mad.2013.12.004
94. Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. (2016) 16:90. doi: 10.1186/s12866-016-0708-5
95. Xu C, Zhu H, and Qiu P. Aging progression of human gut microbiota. BMC Microbiol. (2019) 19:236. doi: 10.1186/s12866-019-1616-2
96. Jang DH, Shin JW, Shim E, Ohtani N, and Jeon OH. The connection between aging, cellular senescence and gut microbiome alterations: A comprehensive review. Aging Cell. (2024) 23:e14315. doi: 10.1111/acel.14315
97. Kawamoto S, Uemura K, Hori N, Takayasu L, Konishi Y, Katoh K, et al. Bacterial induction of B cell senescence promotes age-related changes in the gut microbiota. Nat Cell Biol. (2023) 25:865–76. doi: 10.1038/s41556-023-01145-5
98. Mizuno H, Kawamoto S, Uemura K, Park JH, Hori N, Okumura Y, et al. B cell senescence promotes age-related changes in oral microbiota. Aging Cell. (2024) 23:e14304. doi: 10.1111/acel.14304
99. Li Y, Wang J, Wang R, Chang Y, and Wang X. Gut bacteria induce IgA expression in pituitary hormone-secreting cells during aging. iScience. (2023) 26:107747. doi: 10.1016/j.isci.2023.107747
100. Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. (2020) 5:eabc7191. doi: 10.1126/sciimmunol.abc7191
101. Pu A, Lee DSW, Isho B, Naouar I, and Gommerman JL. The Impact of IgA and the microbiota on CNS disease. Front Immunol. (2021) 12:742173. doi: 10.3389/fimmu.2021.742173
102. Visconti A, Rossi N, Bondt A, Ederveen AH, Thareja G, Koeleman CAM, et al. The genetics and epidemiology of N- and O-immunoglobulin A glycomics. Genome Med. (2024) 16:96. doi: 10.1186/s13073-024-01369-6
103. Chen K, Xu W, Wilson M, He B, Miller NW, Bengtén E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. (2009) 10:889–98. doi: 10.1038/ni.1748
104. Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, et al. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. (2007) 117:1558–65. doi: 10.1172/jci27628
105. Nguyen TG. The therapeutic implications of activated immune responses via the enigmatic immunoglobulin D. Int Rev Immunol. (2022) 41:107–22. doi: 10.1080/08830185.2020.1861265
106. Salonen EM, Hovi T, Meurman O, Vesikari T, and Vaheri A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J Med Virol. (1985) 16:1–9. doi: 10.1002/jmv.1890160102
107. Wan Z, Zhao Y, and Sun Y. Immunoglobulin D and its encoding genes: An updated review. Dev Comp Immunol. (2021) 124:104198. doi: 10.1016/j.dci.2021.104198
108. Gutzeit C, Chen K, and Cerutti A. The enigmatic function of IgD: some answers at last. Eur J Immunol. (2018) 48:1101–13. doi: 10.1002/eji.201646547
109. Klein U, Rajewsky K, and Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. (1998) 188:1679–89. doi: 10.1084/jem.188.9.1679
110. Velounias RL and Tull TJ. Human B-cell subset identification and changes in inflammatory diseases. Clin Exp Immunol. (2022) 210:201–16. doi: 10.1093/cei/uxac104
111. Beckers L, Somers V, and Fraussen J. IgD(-)CD27(-) double negative (DN) B cells: Origins and functions in health and disease. Immunol letters. (2023) 255:67–76. doi: 10.1016/j.imlet.2023.03.003
112. Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, and Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. Immunol Lett. (2005) 175:3262–7. doi: 10.4049/jimmunol.175.5.3262
113. Frasca D, Diaz A, Romero M, and Blomberg BB. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol. (2017) 87:113–20. doi: 10.1016/j.exger.2016.12.001
114. Cagigi A, Rinaldi S, Di Martino A, Manno EC, Zangari P, Aquilani A, et al. Premature immune senescence during HIV-1 vertical infection relates with response to influenza vaccination. J Allergy Clin Immunol. (2014) 133:592–4. doi: 10.1016/j.jaci.2013.10.003
115. Moura RA, Quaresma C, Vieira AR, Gonçalves MJ, Polido-Pereira J, Romão VC, et al. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PloS One. (2017) 12:e0182927. doi: 10.1371/journal.pone.0182927
116. Mahmood Z, Muhammad K, Schmalzing M, Roll P, Dörner T, and Tony HP. CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis. Arthritis Res Ther. (2015) 17:61. doi: 10.1186/s13075-015-0580-y
117. Nevalainen T, Autio A, Kummola L, Salomaa T, Junttila I, Jylhä M, et al. CD27- IgD- B cell memory subset associates with inflammation and frailty in elderly individuals but only in males. Immun Ageing. (2019) 16:19. doi: 10.1186/s12979-019-0159-6
118. Pattarabanjird T, Srikakulapu P, Ransegnola B, Marshall MA, Ghosheh Y, Gulati R, et al. Single-cell profiling of CD11c+ B cells in atherosclerosis. Front Immunol. (2023) 14:1296668. doi: 10.3389/fimmu.2023.1296668
119. al-Rayes H, Pachas W, Mirza N, Ahern DJ, Geha RS, and Vercelli D. IgE regulation and lymphokine patterns in aging humans. J Allergy Clin Immunol. (1992) 90:630–6. doi: 10.1016/0091-6749(92)90136-p
120. Möhrenschlager M and Ring J. Food allergy: an increasing problem for the elderly. Gerontology. (2011) 57:33–6. doi: 10.1159/000316576
121. De Martinis M, Sirufo MM, Viscido A, and Ginaldi L. Food allergies and ageing. Int J Mol Sci. (2019) 20:5580. doi: 10.3390/ijms20225580
122. Tanei R, Hasegawa Y, and Sawabe M. Abundant immunoglobulin E-positive cells in skin lesions support an allergic etiology of atopic dermatitis in the elderly. J Eur Acad Dermatol Venereol. (2013) 27:952–60. doi: 10.1111/j.1468-3083.2012.04612.x
123. Zhang X, Li J, Luo S, Wang M, Huang Q, Deng Z, et al. IgE contributes to atherosclerosis and obesity by affecting macrophage polarization, macrophage protein network, and foam cell formation. Arterioscler Thromb Vasc Biol. (2020) 40:597–610. doi: 10.1161/atvbaha.119.313744
124. Sage AP, Tsiantoulas D, Binder CJ, and Mallat Z. The role of B cells in atherosclerosis. Nat Rev Cardiol. (2019) 16:180–96. doi: 10.1038/s41569-018-0106-9
125. Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. (2017) 139:S1–S46. doi: 10.1016/j.jaci.2016.09.023
126. Nguyen TT, Elsner RA, and Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol. (2015) 194:1489–502. doi: 10.4049/jimmunol.1401880
127. Andonian BJ, Hippensteel JA, Abuabara K, Boyle EM, Colbert JF, Devinney MJ, et al. Inflammation and aging-related disease: A transdisciplinary inflammaging framework. GeroScience. (2025) 47:515–42. doi: 10.1007/s11357-024-01364-0
128. Accardi G and Caruso C. Immune-inflammatory responses in the elderly: an update. Immun Ageing. (2018) 15:11. doi: 10.1186/s12979-018-0117-8
Keywords: immunoglobulin, aging, senescence, B cell, immunosenescence, age-related diseases
Citation: Gu Q, Wang Y, Zhu C, Zhou X, Ni L, Zhao H, Yang H and Shi Q (2025) Immunoglobulin: unraveling its complex web in aging. Front. Immunol. 16:1690018. doi: 10.3389/fimmu.2025.1690018
Received: 21 August 2025; Accepted: 16 September 2025;
Published: 02 October 2025.
Edited by:
Amy L. Kenter, University of Illinois Chicago, United StatesReviewed by:
Michael William Washabaugh, The MITRE Corporation, United StatesPatricia Johanna Gearhart, National Institutes of Health (NIH), United States
Copyright © 2025 Gu, Wang, Zhu, Zhou, Ni, Zhao, Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Shi, c2hpcWluQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Qiaoli Gu
Qiaoli Gu Yi Wang†
Yi Wang† Can Zhu
Can Zhu Xichao Zhou
Xichao Zhou Huilin Yang
Huilin Yang Qin Shi
Qin Shi