- 1Department of Spine Surgery, Ganzhou Hospital-Nanfang Hospital, Southern Medical University (Ganzhou People’s Hospital), Ganzhou, Jiangxi, China
- 2Department of Spine Surgery, The Affiliated Ganzhou Hospital, Jiangxi Medical College, Nanchang University, Ganzhou, Jiangxi, China
Traumatic spinal cord injury (tSCI) is a severe disabling central nervous system injury caused by external forces directly acting on the spinal cord. It can rapidly trigger the release of a large number of pro-inflammatory mediators after the injury, leading to significant neurological dysfunction and, in severe cases, paralysis. Currently, symptoms are mainly alleviated, and endogenous repair mechanisms are improved through drug intervention, surgery, stem cell transplantation, behavioral interventions, physical stimulation, and supportive therapies. However, these methods do not directly promote nerve regeneration and functional recovery. The inflammatory response after injury is an important pathological process leading to secondary damage and plays a crucial role in regulating the pathological progression of acute and chronic tSCI. However, prolonged inflammatory stimulation can further worsen the microenvironment at the injury site, leading to neurological function decline. Therefore, regulating the inflammatory microenvironment and restoring cytokine balance are expected to promote the recovery of neurological function after injury. This review summarizes the formation of the inflammatory microenvironment after tSCI, focusing on the recruitment and activation characteristics of major inflammation-related cells, and elaborates on the expression regulation, pathological effects, and impacts of key cytokines—including the interleukin family, TNF-α, and various chemokines—on neuronal survival and axonal regeneration. Additionally, we summarize multiple inflammatory signaling pathways closely related to secondary injury, such as NF-κB, JAK/STAT, and MAPK, emphasizing that these pathways are interconnected. For example, TNF-α and IL-1β can jointly activate NF-κB and MAPK to amplify the pro-inflammatory response and disrupt the blood-spinal cord barrier. Meanwhile, JAK-STAT3 amplifies inflammation while driving reactive proliferation of astrocytes and glial scar formation, thereby limiting later axonal regeneration. Based on this mutually amplifying inflammatory network, we also briefly summarize the exploratory applications of chemical antagonists, biologic agents, neuroprotective molecules, plant-derived active compounds, and hormonal interventions in regulating this microenvironment. This article aims to provide a reference for the analysis of inflammation-mediated pathological mechanisms after tSCI and the development of targeted anti-inflammatory treatment strategies.
1 Introduction
Traumatic spinal cord injury (tSCI) is a severe neurological injury caused by external forces (such as impact, falls, or compression) directly acting on the spinal cord. It often results in long-term or even permanent loss of motor, sensory, and autonomic function. The pathological processes of tSCI can be divided into distinct stages, including primary mechanical injury and subsequent secondary injury, with the primary mechanical injury initiating the secondary injury. The latter can trigger a series of secondary pathological changes, such as neuroinflammation, cell death, and limited nerve regeneration (1, 2). Because of the complex pathophysiological processes of tSCI and the very weak neural repair capacity, the damaged spinal cord is difficult to repair and to reconstruct functionally (3, 4), posing significant challenges for healthcare workers and medical research (5, 6). In this context, current clinical treatment strategies mainly focus on the early stage of injury, aiming to preserve residual neurological function as much as possible and to limit further deterioration. Common methods include surgical decompression and spinal stabilization during the acute phase, high-dose corticosteroid therapy, pharmacological interventions, and later rehabilitation training (7). Although some progress has been made in the treatment of tSCI, these conventional treatments can only improve symptoms in the short term and are difficult to prevent long-term neurological deficits and disabilities, leading survivors to still face severe motor, sensory, and autonomic dysfunction for a long time (8). Therefore, in recent years, treatment concepts have gradually shifted from purely mechanical decompression and supportive therapy to interventions targeting the secondary damage process itself, especially focusing on inhibiting the persistent tissue destruction caused by secondary inflammation. Current clinical consensus also suggests that, in addition to implementing spinal decompression as early as possible after injury, efforts should be made to prevent and limit the progression of secondary damage (9). Meanwhile, relevant in vivo and in vitro studies indicate that neutralizing the local inflammatory microenvironment and inhibiting excessive inflammatory responses may create more favorable regenerative conditions for axonal preservation, myelin protection, and subsequent tissue engineering repair (10). Therefore, a deeper understanding of the inflammatory mechanisms during the secondary damage phase is becoming a key entry point for optimizing tSCI treatment strategies and improving functional recovery.
The inflammatory microenvironment shaped by pro-inflammatory enzymes, inflammatory cells, inflammatory mediators, and other inflammation-related molecules is an important driving force for the occurrence and progression of various central nervous system diseases, including tSCI (11). In tSCI, this inflammatory microenvironment is considered one of the core driving factors of secondary injury, which can directly or indirectly determine the degree of tissue preservation and functional outcomes after injury (12, 13). Following the initial mechanical injury, immune cells such as neutrophils, infiltrating monocytes and macrophages, and T lymphocytes accumulate and become activated at the injury site. At the same time, microglia and astrocytes transform into reactive phenotypes (e.g., M1-like or A1-like states), initiating and amplifying the inflammatory response. This response affects the survival and function of surrounding neurons and creates a microenvironment that inhibits nerve regeneration (14, 15). Multiple in vivo and in vitro studies have shown that, in the inflammatory microenvironment after tSCI, specific cellular factors such as TNF-α, IL-1β, and IL-6, along with chemokines like CCL2 and CXCL10, drive the inflammatory cascade, disrupt the blood-spinal cord barrier, and promote neuronal apoptosis by regulating signaling between microglia, infiltrating macrophages, and T lymphocytes. These processes thereby influence the balance between nerve injury and repair (16, 17).
It is worth noting that the inflammatory microenvironment after tSCI has a typical ‘double-edged sword’ effect. In the early stages, a moderate inflammatory response clears necrotic tissue and myelin debris, creating conditions for local tissue stabilization and initial repair. When the inflammatory response is continuously amplified through multiple key signaling pathways, it shifts to a pathological and destructive secondary injury process. This amplification is driven by several signaling pathways. The NF-κB pathway, when activated in microglia and infiltrating macrophages, continuously induces the expression of pro-inflammatory factors such as TNF-α, IL-1β, and IL-6. The NLRP3 inflammasome promotes the maturation and release of IL-1β and triggers pyroptosis. The JAK/STAT pathway regulates macrophages and microglia to maintain a pro-inflammatory phenotype during the acute phase or shift to a neuroprotective or repair phenotype in the chronic phase. The MAPK pathway (p38 MAPK, ERK, JNK) is associated with oxidative stress and apoptosis, driving neuronal loss, axonal breakage, and oligodendrocyte death. The sustained activation of these signals not only induces pathological cell death such as apoptosis, pyroptosis, and ferroptosis but also drives reactive astrogliosis, leading to the formation of dense glial scars. These glial scars inhibit axonal regeneration and neural circuit reconstruction during the chronic phase(beyond 28 days after injury), thereby limiting functional recovery (18–20).
Inflammatory signals related to secondary injury can spread along the white matter tracts to adjacent segments, leading to more widespread neuronal death, synaptic connection loss, and long-term impairment of conduction pathways. In the chronic phase, residual activated microglia continuously release pro-inflammatory mediators, sustaining the activation of pathways such as NF-κB, JAK/STAT, and MAPK at a low but persistent level, thereby maintaining a chronic, low-level inflammatory environment. This chronic inflammation not only continues to inhibit axonal regeneration and remyelination but is also closely associated with neuropathic pain, spasticity, and limited recovery of sensory and motor functions (21, 22). Therefore, the inflammatory microenvironment after tSCI should be regarded as a dynamic network shaped by various immune cells (neutrophils, macrophages/microglia, T cells, reactive astrocytes) and key signaling pathways (NF-κB, JAK/STAT, MAPK). The sustained activation of this network is the core driving force behind the expansion of secondary injury, neuronal death, and glial scar formation, as well as the key molecular basis for the limitation of axonal regeneration and functional recovery. Therefore, in-depth analysis of how these signaling pathways coordinate and maintain chronic inflammation over time is crucial for elucidating the pathological mechanisms of tSCI and designing more precise, staged anti-inflammatory treatment strategies.
This narrative review primarily conducts a comprehensive analysis based on representative literature from recent studies on animal models, cellular experiments, and early-stage clinical observational studies in the field of tSCI and related neuroinflammation. This article focuses on and attempts to answer the following core questions: (1) How is the inflammatory microenvironment formed after tSCI, specifically which cell types (such as neutrophils, macrophages and microglia, astrocytes, and T/B lymphocytes) are recruited and activated. (2) How do the cytokines, chemokines, and other mediators released by these cells drive secondary injury through key signaling pathways (such as NF-κB, JAK/STAT, MAPK, etc.)? Specifically, how do these pathways lead to neuronal death, demyelination, and glial scar formation. (3) What intervention strategies are currently available to modulate this inflammatory microenvironment (such as cytokine/receptor antagonists, immunomodulatory drugs, hormones, biological agents, natural products, etc.). What are their action targets and potential translational value. By organizing around these questions, our goal is to systematically elucidate the composition, molecular regulatory mechanisms, and pathological consequences of the inflammatory microenvironment after tSCI. Additionally, we aim to summarize existing and candidate anti-inflammatory/immunomodulatory therapeutic approaches, thereby providing a theoretical basis and direction for future basic research design and clinical intervention strategies.
2 Composition and characteristics of inflammatory microenvironment
The inflammatory response after tSCI follows a characteristic temporal sequence, which can be summarized into three stages: acute phase (minutes to hours after injury), early amplification phase (approximately 1–3 days), and sustained amplification and expansion phase (subsequent days to the subacute stage (from approximately 3 days to 14 or 28 days after injury)). Immediately after injury, the blood-spinal cord barrier (BSCB) is disrupted, exposing spinal cord tissue to the circulatory system, and peripheral immune cells (neutrophils, monocytes/macrophages, activated T lymphocytes) begin entering the injury area (23, 24). At the same time, local cells (microglia, astrocytes, endothelial cells, etc.) rapidly release pro-inflammatory cytokines such as TNF-α and IL-1β, along with chemokines including CCL2, CXCL1, and CXCL10, establishing a chemotactic gradient that drives cell recruitment (23, 24). Within 1–3 days, neutrophils and monocyte-derived macrophages infiltrating the spinal cord parenchyma are further activated, becoming the main source of inflammatory mediators such as TNF-α, IL-1β, IL-6, CCL2, and CXCL10 (25). Meanwhile, microglia and astrocytes transform into pro-inflammatory phenotypes and produce the same pro-inflammatory cytokines and chemokines, further amplifying the local inflammatory response (26). In the following days, activated microglia, macrophages, neutrophils, astrocytes, as well as recruited B cells and T lymphocytes continuously release inflammatory mediators, forming a positive feedback loop (27). These signals spread to areas adjacent to the injury, leading to sustained disruption of the BSCB, neuronal death, demyelination, and axonal rupture, thereby promoting the expansion of secondary injury (28, 29)(Figure 1).

Figure 1. Spatiotemporal cellular changes in the injured spinal cord at 7 and 14 days post-injury. The schematic illustrates the evolution of the lesion microenvironment in the spinal cord following injury, comparing the situation at 7 days post-injury (top panel) and 14 days post-injury (bottom panel). The central lesion core is composed of the primary injury site (orange) surrounded by a region of secondary injury (purple), characterized by progressive inflammatory cell infiltration and glial activation. By 7 days post-injury, the lesion core contains abundant infiltrating peripherally derived macrophages (red), neutrophils (purple), lymphocytes (yellow), and T cells (green), together with pericytes (blue) and reactive astrocytes (violet, stellate with hypertrophic processes) forming a dense inflammatory milieu. Activated microglia (dark red) accumulate around the lesion border, while ramified/resting microglia (brown) and neurons (light pink) remain in the relatively preserved parenchyma distal to the lesion. Astrocytes (light purple) at the periphery of the lesion show marked reactive changes and begin to contribute to glial scar formation. By 14 days post-injury, the lesion core becomes more compact and organized. Peripherally derived macrophages remain prominent, but the relative composition shifts toward a more macrophage-dominated environment with reduced neutrophil presence, indicating a transition from acute to subacute/chronic inflammation. Reactive astrocytes more clearly delineate the lesion border, suggesting maturation of the astroglial scar. Microglia in adjacent tissue persist in an activated state near the injury core, while more distal regions continue to display ramified microglia and relatively preserved neural architecture. (This figure was adapted from Hellenbrand et al., Journal of Neuroinflammation, 2021, (https://doi.org/10.1186/s12974-021-02337-2)).
2.1 Infiltration and activation of inflammatory cells
After tSCI, the recruitment and activation of inflammatory cells are core features in the formation of the inflammatory microenvironment and exhibit clear temporal characteristics. Neutrophils are among the first inflammatory cells to arrive at the site of tSCI. Within hours after the injury, neutrophils rapidly migrate from the peripheral blood into the damaged spinal cord tissue, driven by chemokine signals (30, 31). Early neutrophils can clear necrotic cell debris and potential pathogens, providing an acute defense response (32). However, neutrophils also release cytotoxic molecules such as myeloperoxidase (MPO), elastase, and reactive oxygen species (ROS) and form neutrophil extracellular traps (NETs). These molecules and structures can directly damage surrounding neurons, disrupt oligodendrocytes, and compromise the stability of the myelin sheath, thereby exacerbating tissue edema, demyelination, and deterioration of motor function (33, 34).
Microglia, as the innate immune cells in the CNS, maintain the homeostasis of the spinal cord environment under physiological conditions (35). After tSCI, microglia are rapidly activated in the early stage of injury (within the first few hours to 1–3 days after injury), with their morphology and phenotype shifting from a resting state to a pro-inflammatory state. Activated microglia can exhibit functionally polarized phenotypes that resemble the ‘M1 type’ and ‘M2 type’ microglia. M1-type microglia display pro-inflammatory and neurotoxic characteristics, producing a large amount of neurotoxic mediators such as NO and ROS, as well as pro-inflammatory mediators such as TNF-α and IL-1β. These molecules can amplify the local inflammatory cascade response by activating inflammatory signaling pathways such as NF-κB, inducing neuronal apoptosis and exacerbating axonal/neurotrophic damage (36). In contrast, M2-type microglia tend toward an anti-inflammatory/repair phenotype. They participate in limiting excessive inflammation. Additionally, they support tissue remodeling and axonal survival by releasing IL-10 and transforming growth factor-β (TGF-β) and clearing cellular debris (37, 38). Moreover, in a mouse contusion model, it was found that damage-associated molecular patterns (DAMPs) and inflammatory mediators released at the injury site can activate microglia, altering their morphology and function (39, 40). Subsequently, activated microglia can also release cytokines such as IL-1α and TNF-α, which can activate neurotoxic reactive astrocytes, further mediating neuronal death (41). If microglia remain excessively activated and maintain an M1-type pro-inflammatory state, it can lead to chronic low-grade inflammation, resulting in persistent axonal degenerative damage and limited functional recovery (42).
Macrophages mainly derive from peripheral blood monocytes that are recruited and subsequently differentiate after reaching the injury site. In mouse thoracic contusion models and rat compression models, it was found that in the early stages of injury (within hours up to 3 days), chemokines such as CCL2 and CXCL12 in the injury area were significantly upregulated. These chemokines recruit peripheral monocytes into the spinal cord through the CCR2/CXCR4 axis, where they differentiate locally into tissue-infiltrating macrophages (43, 44). These macrophages exhibit high plasticity and can polarize into M1 or M2 phenotypes depending on their microenvironment (30). M1 macrophages, also known as classically activated macrophages, are produced in response to stimuli such as interferon-γ (IFN-γ) and lipopolysaccharides (LPS) (45, 46). In mouse compression models, M1 macrophages were found to have a strong pro-inflammatory effect, capable of secreting large amounts of inflammatory mediators such as TNF-α, IL-1β, IL-6, and nitric oxide (NO). These inflammatory mediators can directly damage neurons and glial cells, disrupt the blood-spinal cord barrier, and exacerbate inflammatory responses and tissue edema (47). Additionally, M1 macrophages can induce neuronal apoptosis or necrosis directly by releasing toxic substances such as ROS and reactive nitrogen species (RNS). They also damage the blood-spinal cord barrier, aggravate local edema, and harm oligodendrocytes, thereby promoting secondary demyelination (48, 49). In contrast, M2 macrophages, also known as alternatively activated macrophages, are produced in response to stimuli such as IL-4 and IL-13. M2 macrophages have anti-inflammatory and pro-repair effects and secrete anti-inflammatory factors such as IL-10 and TGF-β. These factors inhibit inflammatory responses, promote tissue repair, and facilitate nerve regeneration (49, 50). Moreover, M2 macrophages phagocytose and clear cellular debris and pathogens at the injury site, thereby creating a microenvironment conducive to nerve regeneration (50, 51). Therefore, the dynamic balance between M1 and M2 macrophages after injury is considered a key factor in determining the extent of secondary injury and the quality of neurological recovery.
T cells and B cells also participate in the persistent inflammation and immune regulation after tSCI. T lymphocytes are activated after tSCI and enter the injured spinal cord. They play an important role in secondary neuroinflammation, neurodegenerative changes, and repair responses (52). After injury, the balance between pro-inflammatory T cell subsets, such as Th1 and Th17, and regulatory T cells (Tregs) is disrupted. This disruption leads to a Th1/Th17-dominated pro-inflammatory state, which is associated with elevated levels of cytokines such as IFN-γ, TNF-α, and IL-17. These cytokines further amplify focal inflammatory cascades and exacerbate tissue damage (26, 53). These T cell responses subsequently drive the activation of B cells. Both mouse compressive tSCI models and human injured spinal cord specimens show that B cells and plasma cells deposit immunoglobulins in the injury area along with complement deposition. This deposition is associated with local demyelination and axonal rupture (54, 55). In mouse contusion tSCI models, reducing B cells levels in the injured area can improve the subsequent motor function recovery, suggesting that B cell-mediated autoantibody responses contribute to persistent tissue damage (56).
In addition, astrocytes play a dual role in the pathological process after tSCI. On one hand, astrocytes are rapidly activated in response to pro-inflammatory signals (such as TNF-α, IL-1α, and IL-1β). They can acquire a neurotoxic phenotype and secrete additional mediators such as TNF-α, IL-6, and nitric oxide. This secretion further amplifies the local inflammatory response and induces the death of adjacent neurons (57, 58). On the other hand, during the subacute to chronic phase after injury, activated astrocytes form glial scar boundaries and upregulate extracellular matrix components derived from glial cells, such as chondroitin sulfate proteoglycans. These components physically isolate necrotic and high-inflammatory areas, limiting the spread of inflammation to undamaged tissues. Additionally, astrocytes participate in reconstructing the blood-spinal cord barrier, stabilizing the local ionic environment, and supporting the survival of axonal stumps (59). This “neurotoxic/neuroprotective” biphasic characteristic indicates that astrocytes are neither purely harmful nor purely beneficial. Instead, they can exacerbate secondary injury during the acute phase or lay the structural foundation for subsequent tissue stabilization and nerve regeneration during the subacute and chronic phases under varying microenvironmental conditions (60, 61), as summarized in Table 1.
2.2 The role of inflammatory factors and chemokines
Inflammatory cytokines and chemokines play a crucial role in the inflammatory microenvironment after tSCI, interacting through a complex network to participate in the recruitment and activation of inflammatory cells as well as the occurrence and development of nerve damage. TNF-α is a pro-inflammatory cytokine with broad biological activity; it plays a central role in the inflammatory response after tSCI (62). During the acute phase after tSCI (Within several hours to approximately 72 hours after injury), infiltrating macrophages and activated microglia in the CNS rapidly secrete large amounts of TNF-α (63). TNF-α can induce apoptosis in neurons and glial cells by activating the caspase family in a primary neuron and astrocyte co-culture system. This indicates its direct neurotoxic effect (64). Furthermore, in a rat contusion tSCI model, TNF-α was found to increase the permeability of the BSCB, leading to plasma protein extravasation and local edema. At the same time, TNF-α upregulates the expression of IL-1β and IL-6, amplifying the NF-κB-dependent pro-inflammatory cascade (65). Additionally, during the early post-injury phase, the expression level of TNF-α rapidly rises, and the extent of this increase is closely related to the severity of tSCI (66). Higher levels of TNF-α are associated with decreased motor function scores (such as reduced scores on the hind limb motor function scale), enlarged injury volume, increased number of TUNEL-positive cells, and increased extent of axonal rupture (67). Moreover, in mice, blocking TNF-α signaling through gene knockout or administration of TNF-α neutralizing antibodies can reduce secondary tissue damage and improve motor function recovery (68, 69). Therefore, TNF-α is not only an inflammatory marker but also a core mediator and intervention target driving acute secondary damage, including cell apoptosis, barrier disruption, and tissue edema.
Multiple members of the interleukin (IL) family play important roles in the inflammatory microenvironment after tSCI. Specifically, members of the IL family have distinct roles at different stages of tSCI. IL-1β is a potent pro-inflammatory cytokine primarily secreted by activated macrophages and microglia, and its production is closely related to the activation of the NLRP3 inflammasome, which, upon activation, leads to caspase-1 activation that promotes the maturation of pro-IL-1β (70, 71). In a rat compression model, IL-1β activated inflammatory cells, promoted the production of prostaglandin E2 (PGE2) and nitric oxide (NO), and upregulated the expression of cell adhesion molecules, thereby enhancing inflammatory cell infiltration (72). Additionally, IL-1β can directly damage neurons and inhibit the growth and regeneration of nerve axons (73–75). Therefore, IL-1β is considered a key factor linking acute inflammatory amplification with inhibition of axonal regeneration; it plays a dual role in promoting inflammation and suppressing neural regeneration.
IL-6 is a key cytokine that is highly upregulated after tSCI, primarily produced by activated microglia, infiltrating monocyte-derived macrophages, and reactive astrocytes, and is one of the main secretory products of these cells in the injury area (76, 77). In the tSCI mouse contusion model, IL-6 exhibits a phase-dependent dual role: during the acute phase, IL-6 synergizes with TNF-α, and IL-1β to promote immune cell activation and amplify pro-inflammatory signals (78). This process partially relies on the JAK/STAT3 signaling axis, suggesting that IL-6 is not merely a passive bystander but one of the active driving factors maintaining a pro-inflammatory environment. In the subacute and recovery phases, IL-6 regulates immune cells (macrophages and microglia) to transition towards a more reparative phenotype through the JAK/STAT3 pathway, promoting axonal regeneration, glial scar stabilization, and tissue repair and remodeling (79). Therefore, IL-6 is one of the few cytokines that participate in both the amplification of acute injury and the stabilization and repair and remodeling of tissues in later stages, indicating that the JAK/STAT3 axis may be a phase-specific rather than unidirectional therapeutic target. In contrast, IL-10 represents a classic anti-inflammatory pathway regulatory cytokine. It is primarily secreted by M2 macrophages and regulatory T cells (80) and can inhibit the activation of inflammatory cells while reducing the release of pro-inflammatory mediators (TNF-α and IL-1β), thereby exerting anti-inflammatory and neuroprotective effects. Analysis of human tSCI serum samples shows elevated levels of IL-10, which in turn alleviates inflammatory responses and promotes the recovery of neurological function (81). IL-10 is therefore regarded as a key immune regulatory cytokine in the chronic phase that inhibits persistent inflammation, limits further tissue damage, and enhances the preservation of pathway function.
Chemokines are a class of small signaling proteins that direct the migration of inflammatory cells and play a key role in their recruitment (82). In the inflammatory microenvironment after tSCI, the expression of various chemokines undergoes dynamic changes. CCL2 is an important chemokine primarily secreted by activated macrophages, microglia, and endothelial cells (83). CCL2 specifically attracts monocytes, macrophages, and other inflammatory cells to the injury site, promoting their infiltration (84). In an experimental mouse thoracic spinal cord contusion model, during the acute phase after injury, CCL2 expression in the damaged spinal cord tissue is significantly upregulated at the injury site and surrounding areas; this increase is closely related to the degree of infiltration of CCR2+ peripheral monocytes/macrophages into the spinal cord parenchyma (85). In the same model, blocking the CCL2-CCR2 axis significantly reduces the recruitment of these peripheral monocyte-derived inflammatory cells to the injury site, thereby inhibiting local inflammatory responses and alleviating secondary tissue damage (86). CXCL12 is another important chemokine that plays a crucial role in nerve regeneration and repair after tSCI (87). After binding to its receptor CXCR4, CXCL12 promotes the migration, proliferation, and differentiation of neural stem cells and progenitor cells, which benefits nerve regeneration (88). However, in the inflammatory microenvironment, CXCL12 can also attract inflammatory cells, such as neutrophils and monocytes, into the injury site, contributing to the amplification of inflammation (89). Therefore, CXCL12 is involved both in the localization of reparative cells and in enhancing inflammation; this dual role reflects its bidirectional nature in tissue reconstruction and inflammation maintenance. CCL20 is also associated with the amplification of neuroinflammation after spinal cord injury. In a rat compressive tSCI model, CCL20 is significantly upregulated in the affected spinal cord tissue after injury and is related to the recruitment of local immune cells, including infiltrating CCR6+ lymphocytes and monocyte-derived macrophages, leading to persistent inflammation (90, 91). In the same model, researchers blocked the interaction between CCL20 and its receptor CCR6 by administering neutralizing antibodies against CCL20, resulting in a significant reduction in tissue edema and inflammatory cell infiltration at the injury site, followed by improved recovery of hind limb motor function (92). These results suggest that CCL20 is not merely a passive inflammatory marker, but part of a targetable pro-inflammatory chemokine-receptor signaling axis (Table 2).
3 The impact of inflammatory microenvironment on tSCI
3.1 Neurotoxicity
Inflammatory factors are released in large quantities in the inflammatory microenvironment after tSCI, producing significant neurotoxic effects on neurons, and their damage mechanisms involve multiple aspects. TNF-α, a key pro-inflammatory cytokine, is significantly upregulated within hours after injury in the rat compression injury model. TNF-α can lead to neuronal apoptosis by activating intracellular apoptosis signaling pathways (93). Specifically, after TNF-α binds to TNF receptor 1 (TNFR1) on the neuronal membrane surface, it first recruits the adaptor protein TRADD containing a death domain, and then further recruits Fas-associated death domain protein (FADD) and caspase-8, which assemble to form the death-inducing signaling complex (DISC) (94, 95). This complex mediates the activation of caspase-8. The activated caspase-8 amplifies neuronal damage signals through two pathways: one is the extrinsic (receptor-mediated) apoptotic pathway, where caspase-8 directly cleaves and activates downstream effector caspases (such as caspase-3 and caspase-7), leading to DNA fragmentation, chromatin condensation, and neuronal apoptosis (96). The other pathway is the mitochondrial-dependent intrinsic apoptotic pathway, where caspase-8 cleaves Bid to generate truncated Bid (tBid), which increases mitochondrial outer membrane permeability, leading to the release of cytochrome c, further activating the caspase cascade reaction, thereby amplifying the apoptotic signal (97). In addition to directly inducing apoptosis, TNF-α can also amplify the secondary injury network: it promotes the abnormal accumulation of glutamate at the injury site (exacerbating excitotoxicity) and activates astrocytes, causing them to release pro-inflammatory cytokines such as IL-6 and IFN-γ, thereby driving the inflammatory cascade, ROS/RNS-mediated neuronal damage, and degenerative changes in axons and synapses (98). Overall, this TNF-α/TNFR1–TRADD/FADD–caspase-8 axis not only induces apoptosis in individual neurons but also promotes secondary tissue loss and deterioration of neurological function. Consistent with these findings, in the rat spinal cord contusion model, pharmacological inhibition of TNF-α expression or blockade of the TNF-α signaling pathway can significantly reduce the level of caspase-dependent apoptosis in neurons within the injury area, alleviate secondary neural tissue damage, and improve subsequent motor function recovery (99). In addition to TNF-α, IL-1β has also been proven to be an important neurotoxic factor. Evidence from the rat spinal cord contusion model shows that local elevation of IL-1β disrupts the integrity of the BSCB, increases microvascular permeability, leading to the extravasation of plasma proteins and peripheral inflammatory cells into the injury area, inducing local edema and secondary tissue compression, thereby exacerbating mechanical stress, and functional impairment on remaining neurons (100, 101). Additionally, in an in vitro co-culture system of primary spinal cord neurons and glial cells, exogenous IL-1β can reshape neurotransmitter homeostasis. On one hand, it promotes the release of the excitatory neurotransmitter glutamate, while on the other hand, it inhibits the synthesis and release of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Excess glutamate can trigger excitotoxicity. Meanwhile, the decrease in GABA weakens the inhibitory regulation of neuronal excitability, and this excitatory–inhibitory imbalance is considered an important molecular basis for secondary neuronal damage (102). In addition to the in vitro findings, significant elevation of IL-1β has also been detected in biopsy samples from human injured tissues surrounding the lesion, where IL-1β can activate microglia and astrocytes, prompting them to release more inflammatory mediators such as TNF-α and IL-6, forming an inflammatory cascade that exacerbates neuronal damage (103). This suggests that the aforementioned inflammation–excitotoxicity axis is not limited to rodent models. In addition to TNF-α and IL-1β, inflammatory cytokines such as IL-6 and IFN-γ also contribute to neuronal damage mediated by the inflammatory microenvironment. IL-6 is significantly upregulated during the acute phase in spinal cord contusion models in rats (104). In these animal models and neuronal in vitro culture systems, IL-6 binds to the IL-6 receptor complex on the surface of neurons and activates the JAK/STAT3 pathway. This activation induces transcriptional programs associated with apoptosis, inflammatory stress, and axonal dysfunction, which manifest as decreased neuronal survival and impaired axonal conduction (105, 106). In contrast, IFN-γ mainly originates from activated T lymphocytes; a large number of IFN-γ–positive T cells have been observed in mouse spinal cord compression injury models and among infiltrating lymphocytes around human tSCI lesions (107, 108). IFN-γ enhances the pro-inflammatory activity of infiltrating macrophages and resident microglia, increasing their production of highly reactive molecules such as NO. At the same time, IFN-γ can also directly act on neurons, inducing them to produce neurotoxic molecules, including NO, and exacerbating mitochondrial stress, thereby amplifying secondary neuronal damage (109, 110) (Figure 2).

Figure 2. Schematic diagram of the influence of the inflammatory microenvironment on the pathological process of tSCI. The inflammatory microenvironment mainly affects tSCI through neurotoxicity, excitotoxicity and calcium overload, glial scar formation, inhibition of axon growth and spinal cord demyelination.
3.2 Excitatory toxicity and calcium overload
Excitotoxicity refers to the excessive extracellular excitatory neurotransmitter accumulation (mainly glutamate) and the overactivation of neuronal excitatory receptors, which induces pathological ionic influx and cell death in neurons (111, 112). Calcium overload, on the other hand, refers to the abnormal and sustained increase of free Ca2+ concentration within neurons to toxic levels, thereby inducing intracellular protein degradation, membrane damage, mitochondrial failure, and apoptosis (113). In the inflammatory microenvironment following tSCI, excitotoxicity and calcium overload are core mechanisms that lead to progressive neuronal death and functional loss. These two processes amplify each other through interactions with inflammatory responses and glial scar formation, ultimately affecting axonal regeneration and functional recovery. They represent sequential upstream and downstream events: glutamate-driven excitotoxicity induces and maintains calcium overload, and calcium overload causes irreversible neuronal structural damage by converting the initial excitotoxic stimulus (114). Under physiological conditions, excitatory and inhibitory neurotransmitters in the spinal cord are in dynamic balance to maintain neuronal firing, synaptic plasticity, and ionic homeostasis (115). However, in the rat tSCI model, the local microenvironment rapidly exhibits pathological accumulation of extracellular glutamate after injury, disrupting this balance. The reasons mainly include two aspects: first, mechanical injury leads to the rupture of neuronal cell membranes, causing passive leakage of high concentrations of intracellular glutamate; at the same time, the inflammatory response further promotes excessive release of glutamate. Activated microglia, neutrophils, and monocyte-derived macrophages infiltrating the injury site can actively release glutamate under the stimulation of pro-inflammatory signals such as TNF-α and IL-1β, thereby continuously amplifying excitotoxicity in the core injury area (116, 117). Second, the ability of astrocytes to clear glutamate significantly decreases after spinal cord injury. Under normal conditions, astrocytes rely on high-affinity glutamate transporters (such as GLT-1/EAAT2) to uptake synaptic glutamate into cells and convert it into glutamine for metabolic’detoxification’via glutamine synthetase (118, 119). However, in the injury area, astrocytes undergo reactive changes and swelling, and the expression and transport activity of GLT-1/EAAT2 are downregulated (120). Meanwhile, local energy crisis (ATP depletion, Na+/K+ gradient collapse, membrane potential destabilization) weakens the driving force of Na+-dependent glutamate transporters, leading to impaired reuptake and disrupted metabolic cycling of glutamate (121). Consequently, glutamate is continuously released and cannot be effectively cleared, causing extracellular glutamate to sharply and persistently increase around the injury site. Glutamate is the main excitatory neurotransmitter in the central nervous system. An appropriate amount of glutamate is crucial for the transmission of neural signals and the normal function of neurons (122). However, when the local glutamate concentration in the spinal cord is excessively elevated, it strongly and persistently activates the ionotropic glutamate receptors on the surface of spinal cord neurons and glial cells. These receptors include the N-methyl-D-aspartate (NMDA) and AMPA receptors (123). Damage-Associated Molecular Patterns (DAMPs) and pro-inflammatory signals can drive astrocytes into a highly reactive state, continuously activating pathways such as JAK/STAT3. This leads to their proliferation, hypertrophy, and increased expression of GFAP at the injury site, ultimately forming a dense glial scar barrier. Furthermore, this excessive activation induces the typical excitotoxic response, where receptor-mediated cation channels remain open for an extended period. This results in a massive influx of Ca2+ and Na+ into neurons, with the pathological influx of Ca2+ considered a key early event in secondary neuronal death (124). It is important to emphasize that calcium overload after tSCI does not solely arise from the opening of NMDA/AMPA receptors themselves. Continuous activation of glutamate receptors leads to significant membrane depolarization and excessive Na+ influx, which can trigger further opening of voltage-dependent Ca2+ channels and reverse the Na+/Ca2+ exchanger from a physiological mode of “pumping out Ca2+” to a pathological mode of “pumping in Ca2+”, thereby continuously pushing Ca2+ into the cells (125, 126). In addition, oxidative stress can also promote the release of stored Ca2+ from intracellular calcium stores such as the endoplasmic reticulum, further increasing the concentration of free Ca2+ in the cytoplasm (127). In summary, calcium overload after tSCI is a complex result driven by excessive activation of glutamate receptors, membrane electrophysiological destabilization, reverse operation of ion transporters, and abnormal leakage of intracellular calcium stores.
Calcium overload has a severe damaging effect on neurons. Under normal circumstances, intracellular Ca2+ levels are tightly regulated to remain low and act as controlled signaling molecules involved in intracellular regulation. When Ca2+ is abnormally elevated, it activates a series of calcium-dependent enzymes, including proteases, phospholipases, and nucleases (128). The activation of proteases leads to the degradation of cytoskeletal proteins, disrupting the structural integrity of neurons (129). The activation of phospholipases hydrolyzes phospholipids on cell membranes, resulting in damage and dysfunction of the cell membrane (130). The activation of nucleases degrades DNA and RNA, affecting the transmission of genetic information and protein synthesis in cells (131). Meanwhile, excessive Ca2+ entering mitochondria interferes with mitochondrial membrane potential and inhibits ATP synthesis. It also induces mitochondria to release cytochrome c and other pro-apoptotic factors, thereby triggering the mitochondrial-dependent apoptosis pathway (132, 133). In addition, excessive Ca2+ can induce neurons and activated microglia to upregulate NOS, leading to increased production of NO. In tissue samples around the injury site of the spinal cord compression injury model in mice, the increase of NO is often accompanied by the accumulation of superoxide anion (O2·−) (113). NO and O2·− can rapidly react to form peroxynitrite (ONOO−), which is a strong oxidizing and nitrating agent capable of attacking key macromolecules such as proteins, membrane lipids, and nucleic acids, causing irreversible oxidative damage and further amplifying neuronal death signals (134) (Figure 2).
3.3 Glial scar formation and axonal growth inhibition
Astrocytes are the most abundant glial cells in the central nervous system. After tSCI, various stimuli in the inflammatory microenvironment (including DAMPs and pro-inflammatory cytokines such as TNF-α and IL-1β), rapidly activate astrocytes, inducing their transition from a resting state to a reactive state. Reactive astrocytes show typical morphological and functional remodeling: their cell bodies enlarge, and the number and thickness of astrocytic processes significantly increase. They also begin to express various cytokines, chemokines, and neurotrophic factors at high levels (135, 136). In the injury area, inflammatory cells (such as infiltrating monocyte-macrophage-like cells and T cells) migrate in and release TNF-α, IL-1β, and chemokines CCL2 and CXCL10. These molecules not only continuously amplify the local inflammatory response but also directly promote the proliferation and reactive changes of astrocytes.
During the acute phase of the inflammatory response after tSCI, reactive astrocytes mainly present a pro-inflammatory phenotype. They secrete high levels of TNF-α, IL-1β, and IL-6, which can further activate local microglia and monocyte-derived macrophages infiltrating the injury area, maintaining the sustained activation of pro-inflammatory signaling pathways such as TNF-α and IL-1β, thereby exacerbating the inflammatory response (137). In addition, TNF-α and IL-1β can upregulate the expression of inflammation-related genes by activating the nuclear factor-κB (NF-κB) signaling pathway, promoting the massive release of inflammatory mediators (138). Furthermore, activated astrocytes can also produce chemokines (such as CCL2 and CXCL10), which have been shown in rat tSCI models to recruit peripheral immune cells (including monocyte-macrophage-like cells and T cells) to further infiltrate the injury site, forming a sustained supply of immune cells and enhancing the local inflammatory amplification loop (139). This high-level exposure to pro-inflammatory cytokines and chemokines can have direct cytotoxic effects on surrounding surviving neurons, oligodendrocytes, and non-dead glial cells, inducing apoptosis and necrosis, and are closely related to the limited recovery of motor and sensory functions (140). Therefore, astrocytes at this stage are not only responders driven by inflammation but also amplifiers of the inflammatory cascade.
Under stimulation by continuous inflammatory signals (such as TNF-α, IL-1β, CCL2, CXCL10), astrocytes undergo excessive activation and proliferation, gradually forming a dense glial scar structure at the boundary of the injury site. The glial scar, which is mainly composed of astrocytes and the extracellular matrix they secrete, exhibits significant dual functions (141). On one hand, from the perspective of spatial structure, the cell bodies and thickened processes of activated astrocytes intertwine to form a dense network that wraps and encloses the core area of injury, creating a physical barrier; this barrier can limit the spread of inflammatory cells and cytotoxic molecules to the surrounding, less severely damaged spinal cord tissue, playing an acute protective role of “encapsulation” and “isolation,” thereby reducing further secondary injury (142). On the other hand, from the perspective of regeneration, this glial boundary also hinders axon regeneration. In the rat tSCI model, axon regeneration is significantly restricted within the glial scar formed at the injury site; the disordered fiber orientation further impedes the establishment of orderly and long-range axonal connections necessary for neural circuit reconstruction (143, 144). In addition to the physical barrier, the glial scar also forms a chemical barrier. Reactive astrocytes secrete and enrich various inhibitory extracellular matrix components (such as chondroitin sulfate proteoglycans) during this stage; these molecules can inhibit axonal extension, anchor, and physically trap regenerating nerve fibers, limiting their ability to traverse the injury area for axonal reconstruction (145, 146). Furthermore, astrocytes and accompanying myelin/oligodendrocyte debris can release a series of growth-inhibitory molecules, including Nogo-A (reticulon-4A), myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp). These inhibitory molecules can bind to corresponding receptors on the surface of neurons, initiating axonal growth inhibition signaling pathways, thereby directly limiting axon regeneration and extension (147, 148). Therefore, the glial scar serves as a protective structure that limits the spread of inflammation and stabilizes tissue boundaries during the acute phase after tSCI, while also acting as a barrier that hinders axon regeneration and obstructs the reconstruction of long-range neural circuits during the chronic phase (Figure 2).
3.4 Spinal cord demyelination
The main function of oligodendrocytes is to form myelin sheaths that wrap around the axons of neurons, facilitating the rapid conduction of nerve impulses. In the inflammatory microenvironment after tSCI, oligodendrocytes are highly susceptible to damage, leading to apoptosis and functional impairment, which results in myelin loss and severe disruption of nerve conduction (149, 150). This process is not caused by a single pathway but results from the synergistic effects of direct toxic actions mediated by inflammatory factors, oxidative stress-mediated structural damage, and sustained immune attacks mediated by adaptive immunity. Firstly, inflammatory factors can directly induce the death of oligodendrocytes. Moreover, TNF-α is significantly upregulated in the inflammatory microenvironment after tSCI and can directly act on oligodendrocytes. TNF-α binding to TNFR1 on the surface of oligodendrocytes activates intracellular apoptotic signaling pathways, triggering the cascade activation of caspase family proteases, ultimately inducing apoptosis (151, 152). Similarly, pro-inflammatory cytokines such as IL-18 are also believed to promote oligodendrocyte apoptosis and inhibit the synthesis of myelin proteins, driving the occurrence of secondary demyelination. During this process, not only does the number of oligodendrocytes decrease, but the structural proteins necessary for synthesizing and maintaining myelin are also suppressed. IL-1β can damage oligodendrocytes through multiple pathways by inhibiting the proliferation and maturation of oligodendrocytes and reducing the expression of key myelin-related proteins, such as myelin basic protein, MBP, proteolipid protein, PLP, etc., thereby weakening their ability to maintain myelin homeostasis (153). In addition, IL-1β promotes the production of ROS and RNS, leading to oxidative stress damage that disrupts the structure and function of oligodendrocytes (154).
Secondly, the inflammatory microenvironment can trigger significant oxidative/nitrosative stress, causing further damage to oligodendrocytes and myelin structures. In the context of inflammatory cell activation and metabolic abnormalities, a large number of ROS and RNS (such as O2−, hydrogen peroxide, H2O2, hydroxyl radical, ·OH, nitric oxide, NO, and peroxynitrite, ONOO−) are continuously released. These highly reactive molecules can attack key target structures such as the plasma membrane, mitochondria, and nucleic acids of oligodendrocytes (155, 156). After lipid peroxidation of the membrane lipids, the permeability of the cell membrane increases abnormally, disrupting ionic homeostasis and significantly impairing cell function. Mitochondrial damage leads to restricted energy metabolism and reduced ATP synthesis, which makes it more difficult for cells to maintain myelin synthesis and repair. Additionally, nucleic acid damage can cause genomic instability and induce apoptotic pathways (157). Furthermore, oxidative stress can further activate apoptotic signals such as the mitochondrial pathway and death receptor-mediated pathway, inducing apoptosis in oligodendrocytes (158). Therefore, ROS and RNS are not merely byproducts but are core mediators that promote oligodendrocyte death, myelin damage, and axonal exposure.
Furthermore, the adaptive immune response can form a sustained and directed attack on oligodendrocytes and myelin components. After tSCI, B cells/plasma cells can produce autoantibodies against myelin components and oligodendrocyte antigens (159). After deposition of these autoantibodies in the damaged area, they can activate the complement system, triggering complement-dependent cytotoxic reactions that directly damage oligodendrocytes and myelin sheaths (160). At the same time, autoantibodies can also recruit immune cells such as natural killer (NK) cells and macrophages through antibody-dependent cell-mediated cytotoxicity to mediate cytotoxic attack on oligodendrocytes (161). Additionally, activated T lymphocytes can directly attack oligodendrocytes, exacerbating their apoptosis and shedding. Following oligodendrocyte damage, myelin integrity is compromised, resulting in demyelination. Demyelination can slow down or even interrupt the conduction speed of nerve impulses, severely affecting neurological function (162). In tSCI patients, demyelination is one of the important causes of neurological dysfunction such as limb paralysis and sensory disturbances (163). Axons that lose myelin are also more susceptible to continuous inflammation and oxidative stress, further undergoing degeneration and disruption, forming a vicious cycle of “oligodendrocyte loss – demyelination – secondary axonal injury,” ultimately exacerbating long-term neurological deficits after tSCI (164) (Figure 2).
4 Inflammatory signaling pathways and molecular regulatory mechanisms
The inflammatory microenvironment after tSCI is a highly dynamic and coordinated network, where multiple signaling pathways jointly regulate immune activation, oxidative stress response, and neural tissue remodeling. The previous section has generally introduced the inflammation and immune imbalance mechanism after tSCI, and this section will further focus on the four core signaling pathways that play a key role in the regulation of inflammation in tSCI—namely, the TLR4/NF-κB pathway, MAPK pathway, JAK/STAT pathway, and PI3K/Akt pathway. These pathways are currently the most commonly reported important molecular mechanisms mediating the inflammatory response and neuroimmune regulation after tSCI in the literature.
4.1 NF-κB signaling pathway
NF-κB is a member of the Rel protein family and is an evolutionarily conserved transcription factor (165), involved in the adaptation and response of cells to various environmental changes (166, 167). NF-κB usually exists in an inactive form in the cytoplasm. Upon stimulation by DAMPs, bacterial endotoxins, or pro-inflammatory cytokines (such as TNF-α and IL-1β), IκBα is phosphorylated and degraded. This process allows NF-κB to translocate into the nucleus and initiate transcription programs, inducing the expression of various inflammation-related genes (such as TNF-α, IL-1β, IL-6) as well as chemokines and adhesion molecules (168, 169). After tSCI, the NF-κB signaling pathway is regarded as a key driving factor of secondary inflammatory responses. Studies from rat acute spinal cord injury models show that mechanical injury rapidly leads to the release of a large amount of cellular debris, DAMPs, and pro-inflammatory cytokines, thereby activating infiltrating macrophages, resident microglia, and reactive astrocytes in the injury site and surrounding areas. Among these cells, upstream signals such as cytokines like TNF-α can trigger NF-κB nuclear translocation, thereby amplifying the inflammatory transcription response (170, 171). Activated microglia can secrete large amounts of mediators such as TNF-α, IL-1β, and IL-6 within hours after injury. These mediators, in turn, continuously stimulate the NF-κB pathway, forming a self-amplifying inflammatory loop (172, 173). Meanwhile, NF-κB-dependent transcription also promotes the release of factors such as CXCL10, CCL2, TGF-β2, and adhesion molecules by reactive astrocytes. These molecules can recruit more peripheral immune cells into the injury area, enhance adhesion and infiltration, and promote BSCB disruption, cytotoxic accumulation, and secondary tissue damage, thereby exacerbating neurological deficits after tSCI (174–177). Since NF-κB is located at the upstream hub of “initial injury signals → inflammation amplification → tissue damage,” inhibiting its activation can reduce the expression of mediators such as TNF-α, IL-1β, and IL-6, decrease immune cell infiltration and BSCB disruption, and alleviate secondary neuronal damage. Therefore, NF-κB is considered a potential therapeutic target for controlling excessive inflammation and limiting secondary damage after tSCI.
4.2 JAK/STAT signaling pathway
The JAK/STAT signaling pathway plays an important role in the inflammatory response of astrocytes, especially the activation of JAK2 and STAT3 (178). In a rat model of acute spinal cord injury, rapid and sustained phosphorylation of STAT3 in astrocytes can be detected in the injury area and its surrounding region, closely matching the early focal neuroinflammation after injury (179, 180). Similarly, in vitro experiments with primary astrocytes exposed to inflammatory stimuli such as bacterial endotoxin LPS or cytokines like TNF-α, IL-1β, and IL-6 rapidly activate JAK2, leading to STAT3 phosphorylation. Phosphorylated STAT3 then translocates to the nucleus, initiating a transcriptional program activating multiple inflammation-related genes, including typical pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β (181, 182). These mediators, in turn, can exacerbate the activation of local microglia and macrophages as well as neurotoxic stress, thereby amplifying the secondary neuroinflammatory response associated with tSCI (183). Notably, the activation of STAT3 is observed not only in rodent models but also in GFAP-positive reactive astrocytes at the injury margin of severe tSCI patients. In these cells, enhanced nuclear localization of STAT3 and upregulation of its downstream inflammatory factors can also be detected (184).
In addition to mediating the expression of pro-inflammatory cytokines, the JAK2/STAT3 pathway is closely related to the reactive proliferation, morphological remodeling, and scar formation of astrocytes (185). During the subacute phase of the spinal cord contusion/compression injury model in rodents, persistent activation of STAT3 signaling coincides with the dense arrangement of astrocytes at the injury boundary, thickened processes, and high levels of GFAP expression, which together form the typical astrocyte-dominated glial scar (184). This glial scar structurally forms a covering and an isolating barrier around the injury core, while simultaneously creating both a mechanical barrier and a molecularly inhibitory environment for regenerating axons. This environment is believed to limit axonal regeneration and subsequently neural circuit reconstruction, thereby posing a long-term obstacle to functional recovery (186). A similar dense GFAP+/STAT3+ reactive astrocyte barrier has also been described at the margins of severe human tSCI lesions, correlating with limited functional recovery in the chronic phase (187, 188). Therefore, the JAK2/STAT3 pathway serves as a core signaling module that drives astrocytes to produce pro-inflammatory cytokines. Moreover, it amplifies secondary neuroinflammation and acts as a key regulator, inducing reactive proliferation of astrocytes and glial scar formation, thereby affecting axonal regeneration and functional recovery. Consequently, targeting JAK2/STAT3 signaling may provide a potential therapeutic strategy to limit the harmful inflammatory activation and excessive scar formation of astrocytes after tSCI.
4.3 TLR4/NF-κ B pathway
Toll-like receptor 4 (TLR4) is a pattern recognition receptor that plays an important role in the body’s response to infection and injury by mediating pro-inflammatory responses through activation of the NF-κB signaling pathway. In rat spinal cord injury, TLR4 is significantly upregulated in glial cells and infiltrating immune cells (macrophages and neutrophils) in the injury area within hours to days after injury. This upregulation is accompanied by enhanced phosphorylation and nuclear translocation of NF-κB. The activated NF-κB then induces excessive release of pro-inflammatory factors such as TNF-α, IL-1β, and IL-6. These factors not only exacerbate local inflammatory responses, but may also lead to further neuronal damage and apoptosis (189, 190). Furthermore, abnormal activation of TLR4 is closely related to the progression of various neurological diseases. For example, high expression of TLR4 has been observed in human spinal cord injury tissue samples, chronic activation occurs in neurodegenerative disease models, and sustained inflammatory activation is present in chronic pain states (191, 192). In the rat tSCI model, the use of TLR4 inhibitors or NF-κB signaling blockers can significantly reduce inflammatory infiltration and edema in the injury area, reduce levels of pro-inflammatory factors, and improve motor function recovery (193, 194). This indicates that the TLR4/NF-κB pathway plays a key amplifying role in the secondary inflammatory response following tSCI. Additionally, in vitro cell culture studies have found that activation of TLR4 can drive microglia to adopt M1 polarization, further exacerbating neuroinflammation. In contrast, inhibiting TLR4 or inhibiting NF-κB activation can enhance the proportion of microglia exhibiting M2 polarization and increase the secretion of anti-inflammatory factors such as IL-10 and TGF-β (195). Therefore, regulating the balance of the TLR4/NF-κB signaling axis and microglial polarization state may become a potential therapeutic strategy to alleviate neuroinflammation and promote neurological recovery after tSCI.
4.4 Mitogen activated protein kinase
MAPK is a group of evolutionarily highly conserved serine/threonine kinases and is one of the core regulatory pathways in the eukaryotic signal transduction network (196). This pathway can respond to different stimuli, link extracellular signals with fundamental cellular processes, and coordinate cellular responses and intercellular signaling (197, 198), thereby regulating cell growth, cell proliferation, cell differentiation, apoptosis, and stress responses (199, 200). After tSCI, the activation of MAPK is primarily triggered by acute tissue damage. Mechanical injury leads to the accumulation of a large number of DAMPs, pro-inflammatory cytokines (such as TNF-α, IL-1β, IL-6), and cell debris in the core injury area. These molecules can bind to and activate pattern recognition receptors (such as Toll-like receptors, TLRs) on the surface of immune cells and glial cells, subsequently triggering the phosphorylation cascade of p38, ERK, and JNK (201, 202). Once MAPK is phosphorylated and activated, it can initiate downstream transcriptional programs in microglia, infiltrating macrophages, and astrocytes. This activation induces pro-inflammatory transcription factors to enter the nucleus, which then significantly upregulate pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, as well as chemokines like CCL2 and CXCL1. These mediators recruit more peripheral monocytes/macrophages and T cells into the injury area and maintain the activated state of glial cells. Consequently, local inflammation is amplified, the BSCB is disrupted, neuronal death is promoted, and the range of secondary tissue damage expands (203–206). In vitro cell experiments have shown that inhibiting the phosphorylation of the MAPK pathway reduces the activation and translocation of inflammation-related transcription factors and decreases the expression of pro-inflammatory cytokines and chemokines both in vitro and in vivo, thereby alleviating inflammatory damage (207–209). Furthermore, not only does MAPK signaling drive inflammation during the acute phase, but it is also critical in the maintenance of neuropathic pain during the subacute to chronic stages (210, 211). tSCI leads to the sustained activation of microglia and astrocytes in the dorsal horn of the spinal cord. These glial cells, through sustained activation of MAPK (especially p38 and ERK), release pro-inflammatory cytokines and chemokines, which enhance the excitability of local synaptic transmission and upregulate pain signaling pathways, thereby inducing and maintaining central sensitization, hyperalgesia, and chronic neuropathic pain (212, 213). The activation of the MAPK signaling pathway has been confirmed to trigger neuropathic pain in a rat model of chronic pain after tSCI. Inhibiting the activity of this pathway can effectively suppress the expression of inflammatory factors in lipopolysaccharide-induced microglia, thereby alleviating symptoms associated with neuropathic pain (214, 215) (Figure 3).

Figure 3. Signaling pathways related to the regulation of the inflammatory microenvironment after participating in tSCI.
5 Methods for controlling or eliminating the inflammatory microenvironment in tSCI
The highly complex local microenvironment following tSCI, particularly the persistent inflammatory milieu, amplifies secondary tissue damage, inhibits axon regeneration, and disrupts neural circuit reconstruction. These effects collectively hinder neuronal regeneration and functional recovery. Various anti-inflammatory strategies aim to modulate this inflammatory milieu by reducing inflammation at the injury site, regulating the balance between pro-inflammatory and anti-inflammatory responses, subsequently preventing further tissue damage, and promoting neuronal tissue repair and axonal regeneration. This section outlines several therapeutic approaches designed to modulate the inflammatory environment after tSCI (Figure 4).
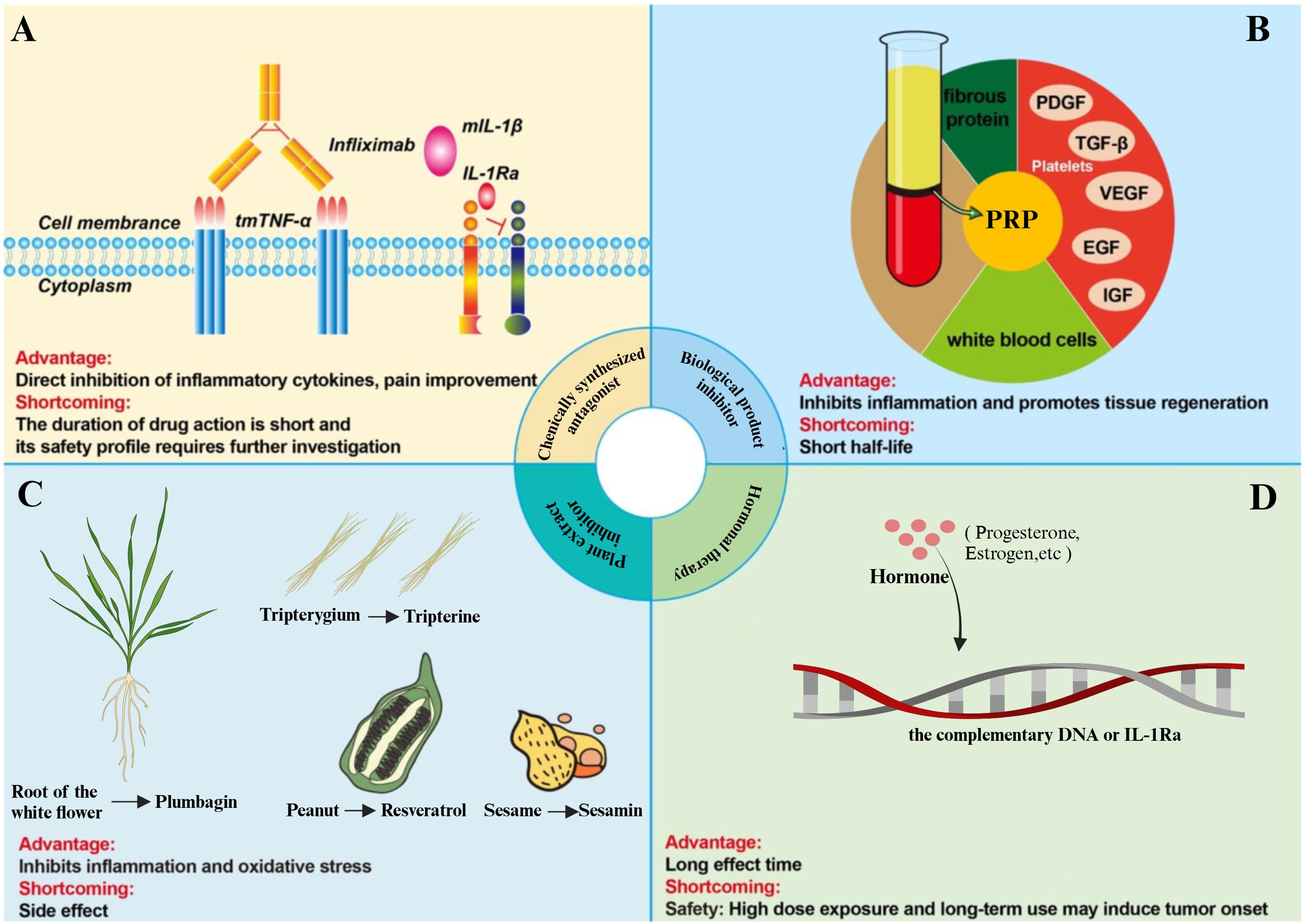
Figure 4. Methods to control or eliminate the inflammatory microenvironment of tSCI. (A) The TNF-α inhibitor, Infliximab, inhibits the binding of TNF-α to its receptor, thereby rendering TNF-α biologically inactive in triggering inflammatory pathways. The IL-1 inhibitor, IL-1Ra, binds to IL-1R, blocking downstream inflammatory signaling. (B) The biological product platelet-rich plasma (PRP) inhibits inflammation and promotes tissue regeneration. (C) Various plant extracts can attenuate the progression of tSCI by inhibiting inflammation and oxidative stress. (D) Hormonal therapy, such as corticosteroids, modulates the inflammatory response in tSCI by inhibiting key inflammatory pathways.
5.1 Chemical synthesis antagonist
TNF-α inhibitors and IL-1 inhibitors are widely used for other inflammation-related diseases (such as rheumatoid arthritis) and have been proposed as potential therapeutic strategies for post-inflammatory regulation after tSCI. However, they are currently still mainly in the experimental and exploratory stages, with no clear evidence supporting their routine clinical applications in tSCI patients (216–218). Focusing on TNF-α inhibitors, Infliximab and Adalimumab are both monoclonal antibodies capable of neutralizing TNF-α activity. Infliximab can block the TNF-α/TNFR signaling pathway by specifically binding to TNF-α, thereby reducing the intensity of pro-inflammatory responses (219). In rat and rabbit models of tSCI, the application of Infliximab (a TNF-α neutralizing antibody) can inhibit TNF-α-mediated inflammatory vascular responses; reduce vascular permeability in the injured area and secondary edema; and significantly decrease neuronal loss (220, 221). In addition, Infliximab can reduce neuronal apoptosis by downregulating the expression of caspase-3, promoting the survival and regeneration of nerve cells, thus exerting neuroprotective effects (222).
Adalimumab (ADM) is a humanized monoclonal antibody whose unique structure can reduce immunogenicity and improve the safety of treatment. ADM can effectively inhibit TNF-α when used alone and can also be combined with other drugs (such as erythropoietin) to synergistically inhibit the M1 polarization of microglia, thereby alleviating inflammatory responses (223). In the tSCI rat model, the use of ADM combined with erythropoietin treatment can inhibit neuroinflammation and apoptosis mediated by microglial M1 polarization, thereby improving recovery from tSCI (224). Furthermore, Adalimumab reduces the release of pro-inflammatory factors by regulating the NF-κB signaling pathway, thereby enhancing the anti-inflammatory environment and promoting functional recovery of the spinal cord (225).
Interleukin-1 receptor antagonist (IL-1RA) is a molecule that antagonizes IL-1α and IL-1β by competitively binding to the IL-1 receptor (IL-1R), thereby preventing IL-1-mediated downstream inflammatory signal transduction and exerting anti-inflammatory effects (226). In the tSCI animal model, peripheral systemic administration of IL-1RA can weaken the excessive inflammatory reaction during the acute phase—including the elevation of pro-inflammatory factors, such as TNF-α and IL-1β—and reduce the infiltration of neutrophils, monocyte-derived macrophages, and T cells into the injury site, thus improving motor function recovery (227). Moreover, the abnormally high expression of IL-1 after tSCI is closely related to the amplification of local tissue damage. IL-1RA treatment can significantly reduce the level of IL-1β in the injured area, which alleviates secondary damage to neurons and glial cells (228). IL-1RA can also inhibit the activation of the NLRP3 inflammasome, thereby reducing caspase-1-mediated pyroptosis. This effect alleviates cell death and functional impairment caused by spinal cord injury (229). Additionally, the anti-inflammatory mechanism of IL-1RA includes regulating the phenotypic conversion of macrophages and microglia, promoting the proliferation of M2 macrophages, and thereby forming an anti-inflammatory microenvironment that is conducive to nerve regeneration (230).
COX-2 is another target for modulating the inflammatory microenvironment, as it regulates the synthesis of PGE2 in this environment (231). In the tSCI rat model, intraperitoneal injection of COX-2 inhibitors can alleviate the inflammatory response by inhibiting neutrophil infiltration and reducing the synthesis of prostaglandins and free radicals (232). Furthermore, in tSCI animal models, the selective COX-2 inhibitor meloxicam has been found to inhibit COX-2-mediated PGE2 production, significantly reducing neutrophil infiltration into the injury site, alleviating local tissue edema and secondary mechanical compression, and promoting motor function recovery (233). Therefore, COX-2 inhibitors, as an adjunctive therapeutic approach to standard treatments, have significant potential in the modulation of inflammation after tSCI and may become a new strategy for future clinical treatment.
5.2 Biological product inhibitors
The main components of platelet-rich plasma (PRP) are platelets, leukocytes, and plasma. Platelets are the core of PRP because they contain high concentrations of various growth factors that play an important role in promoting tissue repair and regeneration (234). PRP contains growth factors such as platelet-derived growth factor, TGF-β, epidermal growth factor, insulin-like growth factor 1, and VEGF. It also plays a significant role in regulating the inflammatory microenvironment after spinal cord injury. In a rat tSCI model, PRP treatment can downregulate the transcription levels of TNF-α and IL-6, primarily by blocking the TLR4/MyD88 pathway, thereby inhibiting neuroinflammatory responses (235, 236). Furthermore, in an experimental spinal cord contusion model, local injection of PRP can enhance the expression of tight junction proteins, such as ZO-1, alleviate the inflammatory environment in the damaged area, and repair the blood-spinal cord barrier. These effects improve the microenvironment after spinal cord injury, contributing to the recovery of neurological function and nerve regeneration (237).
Mesenchymal stem cells (MSCs) are multipotent stem cells derived from the mesoderm, possessing self-renewal and multi-lineage differentiation potential. MSCs are regarded as multipotent progenitor “seed cells” that can inhibit local inflammation and apoptosis. They also stimulate the regeneration and differentiation of resident tissue progenitor cells by secreting soluble growth factors. In the context of tSCI, MSCs can be induced to differentiate into neuron-like cells, providing a potential cellular source for nerve regeneration (238–240). In rat spinal cord contusion and compressive tSCI models, transplanted MSCs reduce the inflammatory response at the injury site by secreting anti-inflammatory cytokines and inhibiting pro-inflammatory cytokines, such as TNF, IFN-γ, and IL-6, thereby playing a crucial role in promoting tissue repair (241–243). Additionally, in a mouse contusion tSCI model, transplanting neural stem cells (NSCs) to the injury site can reduce neutrophil infiltration and regulate macrophage activation by inhibiting the pro-inflammatory M1 macrophage phenotype. NSCs lower the mRNA levels of inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-12. They also inhibit the activation of bone marrow-derived macrophages, reduce the release of cytokines such as TNF-α and IL-1β, and improve functional recovery after tSCI (244).
5.3 Plant extract inhibitors
In recent years, a large number of rodent model studies have utilized plant-derived components to alleviate the inflammatory microenvironment of tSCI (245, 246). For example, compounds derived from plants such as curcumin, geniposide, sesamin, baicalin, rutin, and resveratrol have been shown to promote the recovery of neurological function after injury in tSCI models by inhibiting the inflammatory response induced by IL-1β or TNF-α (247).
Geniposide is an active ingredient of gardenia, a traditional Chinese medicinal herb, which exhibits various pharmacological effects, such as anti-inflammatory, antioxidant, and anti-apoptotic properties (248, 249). In tSCI cell models, geniposide reduces the expression and release of IL-1β, IL-6, IL-8, and TNF-α induced by LPS (250). In tSCI rat models, geniposide treatment significantly inhibited the IKKs/NF-κB signaling pathway, reduced neutrophil infiltration at the injury site, and significantly downregulated TNF-α, IL-1β, and IL-6, thereby alleviating spinal cord damage caused by the inflammatory response (251, 252). Moreover, geniposide reduces inflammatory factor expression by inhibiting the activation of the NF-κB signaling pathway, thereby exerting its anti-inflammatory effects (253). Similarly, sesamin is a component of sesame oil with anti-inflammatory and neuroprotective effects (254). In rat tSCI models, sesamin exerts its anti-inflammatory effects and promotes the recovery of motor function by inhibiting NF-κB activation and upregulating AMPK signaling (255). Furthermore, sesamin promotes cellular autophagy by activating the AMPK signaling pathway, which plays a key role in neuroprotection. The activation of AMPK helps regulate cell metabolism and inflammatory response, allowing nerve cells to better recover function when injured (256, 257). Baicalin, extracted from the roots of Scutellaria baicalensis, exhibits multiple biological activities, such as anti-inflammatory and analgesic effects (258). In tSCI rat models, baicalin can improve neurological function after spinal cord injury by activating Nrf-2, inhibiting the activity of NF-κB, and alleviating cellular inflammatory response and oxidative stress (259). In addition to the aforementioned plant-derived compounds, rutin (260), mangiferin (261), tripterine (262), and resveratrol glycoside (245) can also reduce NLRP3 expression in tSCI animal models, thereby decreasing the release of pro-inflammatory factors such as IL-1β and IL-18, effectively inhibiting the inflammatory response and promoting the recovery of neurological function.
5.4 Hormone therapy
Estrogens, as an important sex hormone, mainly exert their biological effects by activating estrogen receptors (ERα and ERβ). In the regulation of inflammation after tSCI, estrogens can inhibit the activation of inflammatory signaling pathways such as NF-κB. This inhibition downregulates the gene expression of TNF-α and iNOS and reduces local inflammation levels. In a rat spinal cord injury contusion model, treatment with 17-β estradiol (E2) significantly reduced the levels of IL-1β and IL-18 in spinal cord tissue, while improving neuronal survival and enhancing the recovery of hind limb motor function (263). Furthermore, estrogens have also been found to alleviate the neuroinflammatory response after tSCI by inhibiting the activity of the NLRP3 inflammasome, which may provide a new explanation for the neuroprotective effects of estrogens in tSCI (264, 265). Progestogens exert their biological effects by binding to progesterone receptors (PR); in tSCI, exogenous progestogen administration can inhibit the activation of the NLRP3 inflammasome, reducing the release of IL-1β and MCP-1 in the injured area, accompanied by a reduction in local inflammatory infiltration (266). In a mouse tSCI model, systemic administration of progestogens significantly alleviated spinal cord edema and oxidative stress levels and improved motor function scores by reducing neuronal apoptosis, suggesting that progestogens have potential therapeutic value in promoting neuronal survival and functional recovery (267). Additionally, progestogens demonstrate significant effects in regulating the immune response after tSCI, further promoting the recovery of neurological function by modulating mechanisms that protect neurons (268). Besides estrogens and progestogens, research on corticosteroids and androgens in the regulation of inflammation in tSCI has also attracted considerable research interest. Corticosteroids exhibit typical anti-inflammatory effects in acute tSCI animal models, inhibiting the release of pro-inflammatory cytokines and alleviating local tissue inflammation and secondary injury; however, their long-term use or high-dose application may lead to side effects such as immunosuppression and osteoporosis (269). Androgens are believed to have potential neuroprotective effects in promoting nerve regeneration and repair, with early experimental administration in rodent tSCI models showing that androgens may promote axonal preservation/regeneration and subsequent improvement in motor function (270). Nevertheless, existing evidence remains insufficient regarding the specific mechanisms of hormonal intervention, optimal administration windows, dosage ranges, and individualized medication strategies, especially given the limited research foundation for translating findings from rodent models to clinical patients (271).
6 Future prospects and conclusions
Traumatic spinal cord injury is a common neurotrauma disease that can lead to disability and negatively impact quality of life. Inflammation is a relevant process in the pathophysiology of tSCI, and the inflammatory microenvironment is considered an important factor in the occurrence and development of tSCI, where various inflammatory cells and mediators play a crucial role. These cell types and inflammatory cytokines trigger inflammatory cascade reactions through multiple mechanisms, accelerating the deterioration of the inflammatory microenvironment, which in turn leads to further damage to the spinal cord. Regulating the inflammatory microenvironment can effectively inhibit the pathological progression of spinal cord injury, thereby alleviating tSCI symptoms and improving prognosis. Therefore, regulating the inflammatory microenvironment may be an effective approach to treating tSCI.
Despite the progress made in this research area, several limitations remain. In terms of mechanism research, although key mechanisms such as the activation and infiltration of immune cells—such as microglia, macrophages, and neutrophils—the release of inflammatory factors, and inflammatory cascade reactions have been thoroughly explored, the complex interactions between various cells and molecules in the inflammatory microenvironment have not been fully clarified. The functions and mechanisms of newly discovered cell subpopulations and molecules, such as recently identified immune cell subsets and novel cytokines, need further exploration. Additionally, the interaction mechanisms between the inflammatory microenvironment and neuroregenerative cells, including neural stem cells and neural progenitor cells, require in-depth study. Regarding intervention strategies, most existing treatment methods remain in the basic research or clinical trial stages and have not yet resulted in mature and effective clinical treatment plans. The safety, efficacy, and long-term effects of various treatment methods still need further validation. Moreover, combination therapy strategies—such as the concurrent or sequential use of different treatments—also need to be studied in depth to achieve optimal therapeutic effects. In clinical case analyses, the sample sizes are relatively small, which may not fully reflect the situation of tSCI patients. Additionally, the research durations are short, necessitating further observation and study on the long-term effects of intervention strategies.
In the future, mechanistic studies of the inflammatory microenvironment in spinal cord injury and the development of therapeutic strategies have broad prospects. With the continuous advancement of biotechnology, such as single-cell sequencing and multi-omics analysis, it will be possible to reveal the functions and interaction mechanisms of various cells and molecules in the inflammatory microenvironment more deeply. Combined with gene editing technologies, such as CRISPR/Cas9, research will further explore the role of specific genes in the inflammatory microenvironment and nerve repair, providing a theoretical basis for developing new therapeutic targets. Regarding therapeutic strategies, it is necessary to further optimize existing treatment methods to improve efficacy and safety. Research on the combined application of different treatment methods should be strengthened to explore the best combination therapy plans for synergistic effects. Additionally, there is a need to develop new treatment methods and technologies, such as personalized therapy strategies based on artificial intelligence and the application of nanotechnology in both drug delivery and cell therapy. In clinical research, the sample size should be expanded, and multicenter, large-sample clinical trials should be conducted to further verify the effectiveness and safety of these interventional strategies. Strengthening long-term follow-up studies to assess the sustained efficacy and potential adverse reactions of therapeutic strategies is also crucial. Through continuous research and exploration, researchers are expected to reveal the mechanisms of the inflammatory microenvironment in spinal cord injury in depth, develop more effective therapeutic strategies, improve nerve function recovery in spinal cord injury patients, and enhance their quality of life.
Author contributions
HG: Writing – review & editing, Writing – original draft. FL: Writing – review & editing, Software. NL: Software, Writing – review & editing. CX: Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by “The "Science and Technology + Healthcare" Joint Plan Project of the Science and Technology Plan of Ganzhou City, Jiangxi Province (GZ2024YLJ074)”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu C, Liu Y, Ma B, Zhou M, Zhao X, Fu X, et al. Mitochondrial regulatory mechanisms in spinal cord injury: A narrative review. Medicine. (2022) 101:e31930. doi: 10.1097/MD.0000000000031930
2. Liu F, Wang X, Ren M, He P, Li Y, Cui J, et al. A shielded cascade of targeted nanocarriers spanning multiple microenvironmental barriers for inflammatory disease therapy. J nanobiotechnology. (2024) 22:789. doi: 10.1186/s12951-024-03075-2
3. Wang H, Liu X, Zhao Y, Ou L, Zhou Y, Li C, et al. Incidence and pattern of traumatic spinal fractures and associated spinal cord injury resulting from motor vehicle collisions in China over 11 years: An observational study. Medicine. (2016) 95:e5220. doi: 10.1097/MD.0000000000005220
4. Chen J, Chen Z, Zhang K, Song D, Wang C, and Xuan T. Epidemiological features of traumatic spinal cord injury in Guangdong Province, China. J spinal cord Med. (2021) 44:276–81. doi: 10.1080/10790268.2019.1654190
5. Zhou K, Nan W, Feng D, Yi Z, Zhu Y, Long Z, et al. Spatiotemporal expression of Ski after rat spinal cord injury. Neuroreport. (2017) 28:149–57. doi: 10.1097/WNR.0000000000000729
6. Burns AS and O’Connell C. The challenge of spinal cord injury care in the developing world. J spinal cord Med. (2012) 35:3–8. doi: 10.1179/2045772311Y.0000000043
7. Karsy M and Hawryluk G. Modern medical management of spinal cord injury. Curr Neurol neurotSCIence Rep. (2019) 19:65. doi: 10.1007/s11910-019-0984-1
8. Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. (2019) 30:683–99. doi: 10.3171/2018.10.SPINE18802
9. Hilton BJ, Moulson AJ, and Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. (2017) 652:3–10. doi: 10.1016/j.neulet.2016.12.004
10. Zhao J, Zhang X, Wang Y, Zeng Y, Sun H, and Cui X. The role of m6A modification in regulating MSC differentiation and immunomodulation: implications for regenerative medicine and therapeutic applications. Front Biosci (Landmark Ed). (2025) 30:36475. doi: 10.31083/FBL36475
11. Zhang G, Ma L, Bai L, Li M, Guo T, Tian B, et al. Inflammatory microenvironment-targeted nanotherapies. J Control Release. (2021) 334:114–26. doi: 10.1016/j.jconrel.2021.04.018
12. Yao XQ, Liu ZY, Chen JY, Huang ZC, Liu JH, Sun BH, et al. Proteomics and bioinformatics reveal insights into neuroinflammation in the acute to subacute phases in rat models of spinal cord contusion injury. FASEB J. (2021) 35:e21735. doi: 10.1096/fj.202100081RR
13. Xu S, Zhu W, Shao M, Zhang F, Guo J, Xu H, et al. Ecto-5’-nucleotidase (CD73) attenuates inflammation after spinal cord injury by promoting macrophages/microglia M2 polarization in mice. J Neuroinflamm. (2018) 15:155. doi: 10.1186/s12974-018-1183-8
14. Li J, Zhai X, and Yu C. Spatial distribution-based progression of spinal cord injury pathology: a key role for neuroimmune cells. Front Immunol. (2024) 15:1505755. doi: 10.3389/fimmu.2024.1505755
15. Liu H, Pan M, Liu M, Zeng L, Li Y, Huang Z, et al. Lactate: a rising star in tumors and inflammation. Front Immunol. (2024) 15:1496390. doi: 10.3389/fimmu.2024.1496390
16. Li J, Cui S, Li Y, Zhang C, Chang C, and Jian F. Sirtuin1 in spinal cord injury: regulatory mechanisms, microenvironment remodeling and therapeutic potential. CNS Neurosci Ther. (2025) 31:e70244. doi: 10.1111/cns.70244
17. Jiu J, Liu H, Li D, Li X, Zhang J, Yan L, et al. 3D mechanical response stem cell complex repairs spinal cord injury by promoting neurogenesis and regulating tissue homeostasis. Advanced healthcare materials. (2025) 14:e2404925. doi: 10.1002/adhm.202404925
18. Nakamura M, Okada S, Toyama Y, and Okano H. Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allergy Immunol. (2005) 28:197–204. doi: 10.1385/CRIAI:28:3:197
19. Takeuchi O and Akira S. Pattern recognition receptors and inflammation. Cell. (2010) 140:805–20. doi: 10.1016/j.cell.2010.01.022
20. Macleod C and Bryant CE. Visualising pattern recognition receptor signalling. Biochem Soc Trans. (2017) 45:1077–85. doi: 10.1042/BST20160459
21. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. (2018) 9:7204–18. doi: 10.18632/oncotarget.23208
22. Okada S. The pathophysiological role of acute inflammation after spinal cord injury. Inflammation regeneration. (2016) 36:20. doi: 10.1186/s41232-016-0026-1
23. Park CS, Lee JY, Choi HY, Ju BG, Youn I, and Yune TY. Protocatechuic acid improves functional recovery after spinal cord injury by attenuating blood-spinal cord barrier disruption and hemorrhage in rats. (2019) 124:181–92. doi: 10.1016/j.neuint.2019.01.013
24. Li X-Q, Lv H-W, Wang Z-L, Tan W-F, Fang B, and Ma H. MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR 4 signaling pathway. (2015) 12:1–13. doi: 10.1186/s12974-015-0246-3
25. Paramos-De-Carvalho D, Martins I, Cristovao AM, Dias AF, Neves-Silva D, Pereira T, et al. Targeting senescent cells improves functional recovery after spinal cord injury. (2021) 36:. doi: 10.1016/j.celrep.2021.109334
26. Garcia E, Aguilar-Cevallos J, Silva-Garcia R, and Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflammation. (2016) 2016:9476020. doi: 10.1155/2016/9476020
27. Cekanaviciute E and Buckwalter MS. Astrocytes: integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics. (2016) 13:685–701. doi: 10.1007/s13311-016-0477-8
28. García E, Mondragón-Caso J, and Ibarra A. Spinal cord injury: potential neuroprotective therapy based on neural-derived peptides. Neural regeneration Res. (2016) 11:1762–3. doi: 10.4103/1673-5374.194718
29. Mortazavi MM, Verma K, Harmon OA, Griessenauer CJ, Adeeb N, Theodore N, et al. The microanatomy of spinal cord injury: a review. Clin Anat (New York NY). (2015) 28:27–36. doi: 10.1002/ca.22432
30. Gensel JC and Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. (2015) 1619:1–11. doi: 10.1016/j.brainres.2014.12.045
31. Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. (1997) 79:1177–82. doi: 10.1016/S0306-4522(97)00011-0
32. Pineau I and Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. (2007) 500:267–85. doi: 10.1002/cne.21149
33. Kubota K, Saiwai H, Kumamaru H, Maeda T, Ohkawa Y, Aratani Y, et al. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. (2012) 37:1363–9. doi: 10.1097/BRS.0b013e31824b9e77
34. Kang J, Jiang MH, Min HJ, Jo EK, Lee S, Karin M, et al. IKK-β-mediated myeloid cell activation exacerbates inflammation and inhibits recovery after spinal cord injury. (2011) 41:1266–77. doi: 10.1002/eji.201040582
35. Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, and Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. (2010) 133:433–47. doi: 10.1093/brain/awp322
36. Chen J, Wang Z, Zheng Z, Chen Y, Khor S, Shi K, et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. (2017) 8:e3090–e. doi: 10.1038/cddis.2017.490
37. Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, and Koo BN. Regulation of Microglia and Macrophage Polarization via Apoptosis Signal-Regulating Kinase 1 Silencing after Ischemic/Hypoxic Injury. Front Mol Neurosci. (2017) 10:261. doi: 10.3389/fnmol.2017.00261
38. Fu SP, Chen SY, Pang QM, Zhang M, Wu XC, Wan X, et al. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. (2022) 13:1014013. doi: 10.3389/fimmu.2022.1014013
39. Sroga JM, Jones TB, Kigerl KA, McGaughy VM, and Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. (2003) 462:223–40. doi: 10.1002/cne.10736
40. Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. (2006) 129:3249–69. doi: 10.1093/brain/awl296
41. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. (2017) 541:481–7. doi: 10.1038/nature21029
42. David S and Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. (2011) 12:388–99. doi: 10.1038/nrn3053
43. Greenhalgh AD and David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. (2014) 34:6316–22. doi: 10.1523/JNEUROSCI.4912-13.2014
44. Herz J, Filiano AJ, Wiltbank AT, Yogev N, and Kipnis J. Myeloid cells in the central nervous system. Immunity. (2017) 46:943–56. doi: 10.1016/j.immuni.2017.06.007
45. Guo Q, Zhu X, Wei R, Zhao L, Zhang Z, Yin X, et al. miR-130b-3p regulates M1 macrophage polarization via targeting IRF1. J Cell Physiol. (2021) 236:2008–22. doi: 10.1002/jcp.29987
46. Roy S, Guler R, Parihar SP, Schmeier S, Kaczkowski B, Nishimura H, et al. Batf2/Irf1 induces inflammatory responses in classically activated macrophages, lipopolysaccharides, and mycobacterial infection. J Immunol. (2015) 194:6035–44. doi: 10.4049/jimmunol.1402521
47. Dooley D, Lemmens E, Vangansewinkel T, Le Blon D, Hoornaert C, Ponsaerts P, et al. Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. (2016) 7:1099–115. doi: 10.1016/j.stemcr.2016.11.005
48. Sica A and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. (2012) 122:787–95. doi: 10.1172/JCI59643
49. Kobashi S, Terashima T, Katagi M, Nakae Y, Okano J, Suzuki Y, et al. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. (2020) 28:254–65. doi: 10.1016/j.ymthe.2019.09.004
50. Han GH, Kim SJ, Ko WK, Lee D, Han IB, Sheen SH, et al. Transplantation of tauroursodeoxycholic acid–inducing M2-phenotype macrophages promotes an anti-neuroinflammatory effect and functional recovery after spinal cord injury in rats. (2021) 54:e13050. doi: 10.1111/cpr.13050
51. Zhou X, He X, and Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. (2014) 9:1787–95. doi: 10.4103/1673-5374.143423
52. Ankeny DP and Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. (2009) 158:1112–21. doi: 10.1016/j.neuroscience.2008.07.001
53. Xu Y, Li Y, Zhang Z, Sun X, Gong A, Ding H, et al. Expressions and implications of Th1/Th2 cytokines in injured rat brain and spinal cord. (2013) 29:919–22, 26.
54. Liu X, Zhang Y, Wang Y, and Qian T. Inflammatory response to spinal cord injury and its treatment. World Neurosurg. (2021) 155:19–31. doi: 10.1016/j.wneu.2021.07.148
55. Anjum A, Yazid MDI, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. (2020) 21:7533. doi: 10.3390/ijms21207533
56. Ankeny DP, Guan Z, and Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. (2009) 119:2990–9. doi: 10.1172/JCI39780
57. Lin ST, Wang Y, Xue Y, Feng DC, Xu Y, Xu LY, et al. Tetrandrine suppresses LPS-induced astrocyte activation via modulating IKKs-IkappaBalpha-NF-kappaB signaling pathway. Mol Cell Biochem. (2008) 315:41–9. doi: 10.1007/s11010-008-9787-4
58. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. (2017) 541:481–7. doi: 10.1038/nature21029
59. Clifford T, Finkel Z, Rodriguez B, Joseph A, and Cai L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. (2023) 12. doi: 10.3390/cells12060853
60. Nakagawa S, Izumi Y, Takada-Takatori Y, Akaike A, and Kume T. Increased CCL6 expression in astrocytes and neuronal protection from neuron-astrocyte interactions. Biochem Biophys Res Commun. (2019) 519:777–82. doi: 10.1016/j.bbrc.2019.09.030
61. Cameron EG, Nahmou M, Toth AB, Heo L, Tanasa B, Dalal R, et al. A molecular switch for neuroprotective astrocyte reactivity. Nature. (2024) 626:574–82. doi: 10.1038/s41586-023-06935-3
62. Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, and Boos N. Analysis of tissue distribution of TNF-α, TNF-α-receptors, and the activating TNF-α–converting enzyme suggests activation of the TNF-α system in the aging intervertebral disc. (2007) 1096:44–54. doi: 10.1196/annals.1397.069
63. Yan P, Li Q, Kim G-M, Xu J, Hsu CY, and Xu XM. Cellular localization of tumor necrosis factor-α following acute spinal cord injury in adult rats. (2001) 18:563–8. doi: 10.1089/089771501300227369
64. Martin DA and Elkon KB. Mechanisms of apoptosis. Rheumatic Dis Clinics North America. (2004) 30:441–54. doi: 10.1016/j.rdc.2004.04.008
65. Garcia E, Aguilar-Cevallos J, Silva-Garcia R, and Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflammation. (2016) 2016:9476020. doi: 10.1155/2016/9476020
66. Brewer KL and Nolan TA. Spinal and supraspinal changes in tumor necrosis factor-alpha expression following excitotoxic spinal cord injury. J Mol Neurosci. (2007) 31:13–21. doi: 10.1007/BF02686114
67. Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, and Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. (2000) 164:280–91. doi: 10.1006/exnr.2000.7399
68. Wang L, Wei F-X, Cen J-S, Ping S-n, Li Z-q, Chen N-n, et al. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. (2014) 1575:87–100. doi: 10.1016/j.brainres.2014.05.038
69. Tolosa L, Caraballo-Miralles V, Olmos G, Lladó J, and Wilms K. TNF-α potentiates glutamate-induced spinal cord motoneuron death via NF-κB. (2011) 46:176–86. doi: 10.1016/j.mcn.2010.09.001
70. Navone SE, Marfia G, Giannoni A, Beretta M, Guarnaccia L, Gualtierotti R, et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. (2017) 32:523–42. doi: 10.14670/HH-11-846
71. Pineau I and Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. (2007) 500:267–85. doi: 10.1002/cne.21149
72. Jorge A, Taylor T, Agarwal N, Hamilton DK, Rizzo P, Fillmore W, et al. Current agents and related therapeutic targets for inflammation after acute traumatic spinal cord injury. World Neurosurg. (2019) 132:138–47. doi: 10.1016/j.wneu.2019.08.108
73. Burke SJ, Lu D, Sparer TE, Karlstad MD, and Collier JJ. Transcription of the gene encoding TNF-α is increased by IL-1β in rat and human islets and β-cell lines. (2014) 62:54–62. doi: 10.1016/j.molimm.2014.05.019
74. Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S, et al. Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. (2009) 7:1–12. doi: 10.1186/1477-3155-7-8
75. Wang X-J, Kong K-M, Qi W-L, Ye W-l, and Song P-s. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. (2005) 26:934–42. doi: 10.1111/j.1745-7254.2005.00152.x
76. Lee JY, Park CS, Seo KJ, Kim IY, Han S, Youn I, et al. IL-6/JAK2/STAT3 axis mediates neuropathic pain by regulating astrocyte and microglia activation after spinal cord injury. Exp Neurol. (2023) 370:114576. doi: 10.1016/j.expneurol.2023.114576
77. Guptarak J, Wanchoo S, Durham-Lee J, Wu Y, Zivadinovic D, Paulucci-Holthauzen A, et al. Inhibition of IL-6 signaling: A novel therapeutic approach to treating spinal cord injury pain. Pain. (2013) 154:1115–28. doi: 10.1016/j.pain.2013.03.026
78. Anwar MA, Al Shehabi TS, and Eid AH. Inflammogenesis of secondary spinal cord injury. (2016) 10:98. doi: 10.3389/fncel.2016.00098
79. Nakamura M, Okada S, Toyama Y, and Okano H. Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allergy Immunol. (2005) 28:197–204. doi: 10.1385/CRIAI:28:3:197
80. Saraiva M and O’garra A. The regulation of IL-10 production by immune cells. (2010) 10:170–81. doi: 10.1038/nri2711
81. Patilas C, Varsamos I, Galanis A, Vavourakis M, Zachariou D, Marougklianis V, et al. The role of interleukin-10 in the pathogenesis and treatment of a spinal cord injury. Diagnostics (Basel Switzerland). (2024) 14. doi: 10.3390/diagnostics14020151
82. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Cox M, Hanley EN, et al. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. (2015) 98:102–5. doi: 10.1016/j.yexmp.2014.12.002
83. Nayak D, Roth TL, and Mcgavern DB. Microglia development and function. Annu Rev Immunol. (2014) 32:367–402. doi: 10.1146/annurev-immunol-032713-120240
84. Biber K and Boddeke E. Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front Cell Neurosci. (2014) 8:210. doi: 10.3389/fncel.2014.00210
85. Ciechanowska A and Mika J. CC chemokine family members’ Modulation as a novel approach for treating central nervous system and peripheral nervous system injury-A review of clinical and experimental findings. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25073788
86. Xu P, Zhang F, Chang MM, Zhong C, Sun CH, Zhu HR, et al. Recruitment of γδ T cells to the lesion via the CCL2/CCR2 signaling after spinal cord injury. J Neuroinflamm. (2021) 18:64. doi: 10.1186/s12974-021-02115-0
87. Jaerve A, Bosse F, and Müller HW. SDF-1/CXCL12: its role in spinal cord injury. Int J Biochem Cell Biol. (2012) 44:452–6. doi: 10.1016/j.biocel.2011.11.023
88. Li J, Wu Y, Chen P, Huang X, Liu Y, Peng M, et al. CXCL12 promotes spinal nerve regeneration and functional recovery after spinal cord injury. Neuroreport. (2021) 32:450–7. doi: 10.1097/WNR.0000000000001613
89. Mckeon RJ, Schreiber RC, Rudge JS, and Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. (1991) 11:3398–411. doi: 10.1523/JNEUROSCI.11-11-03398.1991
90. Wang YH, Chen J, Zhou J, Nong F, Lv JH, Liu J, et al. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. (2017) 13:203–7. doi: 10.3892/etm.2016.3941
91. Welsh-Bacic D, Lindenmeyer M, Cohen CD, Draganovici D, Mandelbaum J, Edenhofer I, et al. Expression of the chemokine receptor CCR6 in human renal inflammation. Nephrol Dial Transplant. (2011) 26:1211–20. doi: 10.1093/ndt/gfq560
92. Smith-Thomas LC, Fok-Seang J, Stevens J, Du JS, Muir E, Faissner A, et al. An inhibitor of neurite outgrowth produced by astrocytes. J Cell Sci. (1994) 107:1687–95. doi: 10.1242/jcs.107.6.1687
93. Zhao J, Shen S, Dai Y, Chen F, and Wang K. Methamphetamine induces intestinal inflammatory injury via nod-like receptor 3 protein (NLRP3) inflammasome overexpression in vitro and in vivo. Med Sci monitor. (2019) 25:8515–26. doi: 10.12659/MSM.920190
94. Casha S, Yu WR, and Fehlings MG. FAS deficiency reduces apoptosis, spares axons and improves function after spinal cord injury. Exp Neurol. (2005) 196:390–400. doi: 10.1016/j.expneurol.2005.08.020
95. Yu WR and Fehlings MG. Fas/FasL-mediated apoptosis and inflammation are key features of acute human spinal cord injury: implications for translational, clinical application. Acta neuropathologica. (2011) 122:747–61. doi: 10.1007/s00401-011-0882-3
96. Werida RH, Elshafiey RA, Ghoneim A, Elzawawy S, and Mostafa TM. Role of alpha-lipoic acid in counteracting paclitaxel- and doxorubicin-induced toxicities: a randomized controlled trial in breast cancer patients. Supportive Care Cancer. (2022) 30:7281–92. doi: 10.1007/s00520-022-07124-0
97. Xiromerisiou G, Marogianni C, Lampropoulos IC, Dardiotis E, Speletas M, Ntavaroukas P, et al. Peripheral inflammatory markers TNF-α and CCL2 revisited: association with Parkinson’s disease severity. Int J Mol Sci. (2022) 24. doi: 10.3390/ijms24010264
98. Surina NM, Fedotova IB, Nikolaev GM, Grechenko VV, Gankovskaya LV, Ogurtsova AD, et al. Neuroinflammation in pathogenesis of audiogenic epilepsy: altered proinflammatory cytokine levels in the rats of krushinsky-molodkina seizure-prone strain. Biochem (Mosc). (2023) 88:481–90. doi: 10.1134/S0006297923040041
99. Inyang HO, Ezemagu UK, Okori SO, Ijomone OK, and Ijomone OM. D-ribose-L-cysteine attenuates manganese-induced oxidative stress, neuromorphological deficits, Bax/Bcl-2 response and TNF-α/ERK signalling in rats. Neurochemical Res. (2025) 50:223. doi: 10.1007/s11064-025-04466-z
100. Fan Y, Yang W, Wu W, Wang X, Lin Y, Wu L, et al. Serum neurotransmitter analysis of motor and non-motor symptoms in Parkinson’s patients. Front Aging Neurosci. (2024) 16:1423120. doi: 10.3389/fnagi.2024.1423120
101. Xiong M, Feng Y, Luo C, Guo J, Zeng J, Deng L, et al. Teriparatide: an innovative and promising strategy for protecting the blood-spinal cord barrier following spinal cord injury. Front Pharmacol. (2024) 15:1386565. doi: 10.3389/fphar.2024.1386565
102. Fergany A, Zong C, Ekuban FA, Suzuki A, Kimura Y, Ichihara S, et al. Deletion of IL-1β exacerbates acrylamide-induced neurotoxicity in mice. Toxicological Sci. (2023) 195:246–56. doi: 10.1093/toxsci/kfad077
103. Huang H, Li S, Zhang Y, He C, and Hua Z. Microglial priming in bilirubin-induced neurotoxicity. Neurotoxicity Res. (2023) 41:338–48. doi: 10.1007/s12640-023-00643-6
104. Xia Y, Yang R, Wang H, Hou Y, Li Y, Zhu J, et al. Biomaterials delivery strategies to repair spinal cord injury by modulating macrophage phenotypes. J Tissue Eng. (2022) 13:20417314221143059. doi: 10.1177/20417314221143059
105. Guan H, Yan T, Wu D, and Tripathi AS. Epifriedelinol ameliorates the neuropathic pain and recovers the function in spinal cord injury by downregulation of neuronal apoptosis and NMDA receptor. Tohoku J Exp Med. (2022) 258:143–8. doi: 10.1620/tjem.2022.J065
106. Liu Z, Qiu YH, Li B, Ma SH, and Peng YP. Neuroprotection of interleukin-6 against NMDA-induced apoptosis and its signal-transduction mechanisms. Neurotox Res. (2011) 19:484–95. doi: 10.1007/s12640-010-9215-x
107. Zhang M, Han X, Yan L, Fu Y, Kou H, Shang C, et al. Inflammatory response in traumatic brain and spinal cord injury: The role of XCL1-XCR1 axis and T cells. CNS Neurosci Ther. (2024) 30:e14781. doi: 10.1111/cns.14781
108. Gattlen C, Clarke CB, Piller N, Kirschmann G, Pertin M, Decosterd I, et al. Spinal cord T-cell infiltration in the rat spared nerve injury model: A time course study. Int J Mol Sci. (2016) 17:352. doi: 10.3390/ijms17030352
109. Chen W, Gao X, Yang W, Xiao X, Pan X, and Li H. Htr2b Promotes M1 Microglia Polarization and Neuroinflammation after Spinal Cord Injury via Inhibition of Neuregulin-1/ErbB Signaling. Mol Neurobiol. (2024) 61:1643–54. doi: 10.1007/s12035-023-03656-6
110. Hamrangsekachaee M, Baumann HJ, Pukale DD, Shriver LP, and Leipzig ND. Investigating mechanisms of subcutaneous preconditioning incubation for neural stem cell embedded hydrogels. ACS Appl Bio materials. (2022) 5:2176–84. doi: 10.1021/acsabm.2c00017
111. Hu YY, Li L, Xian XH, Zhang M, Sun XC, Li SQ, et al. GLT-1 upregulation as a potential therapeutic target for ischemic brain injury. Curr Pharm Des. (2017) 23:5045–55. doi: 10.2174/1381612823666170622103852
112. Wang J, Wang F, Mai D, and Qu S. Molecular mechanisms of glutamate toxicity in parkinson’s disease. Front Neurosci. (2020) 14:585584. doi: 10.3389/fnins.2020.585584
113. Gong QH, Wang Q, Shi JS, Huang XN, Liu Q, and Ma H. Inhibition of caspases and intracellular free Ca2+ concentrations are involved in resveratrol protection against apoptosis in rat primary neuron cultures. Acta Pharmacol Sin. (2007) 28:1724–30. doi: 10.1111/j.1745-7254.2007.00666.x
114. Angelova PR, Vinogradova D, Neganova ME, Serkova TP, Sokolov VV, Bachurin SO, et al. Pharmacological sequestration of mitochondrial calcium uptake protects neurons against glutamate excitotoxicity. Mol Neurobiol. (2019) 56:2244–55. doi: 10.1007/s12035-018-1204-8
115. Patel JC, Shukla M, and Shukla M. Cellular and molecular interactions in CNS injury: the role of immune cells and inflammatory responses in damage and repair. Cells. (2025) 14. doi: 10.3390/cells14120918
116. Park E, Velumian AA, and Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. (2004) 21:754–74. doi: 10.1089/0897715041269641
117. Cao J, Yu X, Liu J, Fu J, Wang B, Wu C, et al. Ruxolitinib improves the inflammatory microenvironment, restores glutamate homeostasis, and promotes functional recovery after spinal cord injury. Neural Regener Res. (2024) 19:2499–512. doi: 10.4103/NRR.NRR-D-23-01863
118. Sandhu JK, Sikorska M, and Walker PR. Characterization of astrocytes derived from human NTera-2/D1 embryonal carcinoma cell. J Neurosci Res. (2002) 68:604–14. doi: 10.1002/jnr.10236
119. Rose CF, Verkhratsky A, and Parpura V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans. (2013) 41:1518–24. doi: 10.1042/BST20130237
120. van Landeghem FK, Weiss T, Oehmichen M, and von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma. (2006) 23:1518–28. doi: 10.1089/neu.2006.23.1518
121. Moriarty C, Gupta N, and Bhattacharya D. Role of glutamate excitotoxicity in glioblastoma growth and its implications in treatment. Cell Biol Int. (2025) 49:421–34. doi: 10.1002/cbin.70005
122. Liao R, Wood TR, and Nance E. Superoxide dismutase reduces monosodium glutamate-induced injury in an organotypic whole hemisphere brain slice model of excitotoxicity. J Biol Eng. (2020) 14:3. doi: 10.1186/s13036-020-0226-8
123. Neves D, Salazar IL, Almeida RD, and Silva RM. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. (2023) 328:121814. doi: 10.1016/j.lfs.2023.121814
124. Nicotera P and Bano D. The enemy at the gates. Ca2+ entry through TRPM7 channels and anoxic neuronal death. Cell. (2003) 115:768–70. doi: 10.1016/S0092-8674(03)01019-5
125. Vale C, Alfonso A, Suñol C, Vieytes MR, and Botana LM. Modulation of calcium entry and glutamate release in cultured cerebellar granule cells by palytoxin. J Neurosci Res. (2006) 83:1393–406. doi: 10.1002/jnr.20841
126. Wang BW, Liao WN, Chang CT, and Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. (2006) 59:491–501. doi: 10.1002/syn.20267
127. Hoga K, Wakuzawa M, Nakamura T, Kato Y, and Fukui K. Effect of disruption of mitochondrial and endoplasmic reticulum calcium homeostasis on neurites in hydrogen peroxide- and ionomycin-treated cells. J Clin Biochem Nutr. (2025) 76:253–63. doi: 10.3164/jcbn.24-122
128. Park J, Kim SH, Kim YJ, Kim H, Oh Y, Choi KY, et al. Elevation of phospholipase C-β1 expression by amyloid-β facilitates calcium overload in neuronal cells. Brain Res. (2022) 1788:147924. doi: 10.1016/j.brainres.2022.147924
129. Batista-Silva H, Rodrigues K, Sousa de Moura KR, Van Der Kraak G, Delalande-Lecapitaine C, and Mena Barreto Silva FR. Role of bisphenol A on calcium influx and its potential toxicity on the testis of Danio rerio. Ecotoxicology Environ Saf. (2020) 202:110876. doi: 10.1016/j.ecoenv.2020.110876
130. Fu T, Ma Y, Li Y, Wang Y, Wang Q, and Tong Y. Mitophagy as a mitochondrial quality control mechanism in myocardial ischemic stress: from bench to bedside. Cell Stress chaperones. (2023) 28:239–51. doi: 10.1007/s12192-023-01346-9
131. Spencer SA, Suárez-Pozos E, Escalante M, Myo YP, and Fuss B. Sodium-calcium exchangers of the SLC8 family in oligodendrocytes: functional properties in health and disease. Neurochemical Res. (2020) 45:1287–97. doi: 10.1007/s11064-019-02949-4
132. Cheng Q, He K, Zhu J, Li X, Wu X, Zeng C, et al. Memantine attenuates the development of osteoarthritis by blocking NMDA receptor mediated calcium overload and chondrocyte senescence. J orthopaedic translation. (2024) 48:204–16. doi: 10.1016/j.jot.2024.08.007
133. Chen T, Xie Q, Tan B, Yi Q, Xiang H, Wang R, et al. Inhibition of pyruvate dehydrogenase kinase 4 protects cardiomyocytes from lipopolysaccharide-induced mitochondrial damage by reducing lactate accumulation. Inflammation. (2024) 47:1356–70. doi: 10.1007/s10753-024-01981-z
134. Moazamian A, Gharagozloo P, Aitken RJ, and Drevet JR. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: Sperm telomeres, oxidative stress, and infertility. Reprod (Cambridge England). (2022) 164:F125–f33. doi: 10.1530/REP-22-0189
135. Zhou ZL, Xie H, Tian XB, Xu HL, Li W, Yao S, et al. Microglial depletion impairs glial scar formation and aggravates inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural regeneration Res. (2023) 18:1325–31. doi: 10.4103/1673-5374.357912
136. Feng S, Liu L, Cheng Y, Zhou M, Zhu H, Zhao X, et al. Icariin promotes functional recovery in rats after spinal cord injury by inhibiting YAP and regulating PPM1B ubiquitination to inhibiting the activation of reactive astrocytes. Front Pharmacol. (2024) 15:1434652. doi: 10.3389/fphar.2024.1434652
137. Lawrence JM, Schardien K, Wigdahl B, and Nonnemacher MR. Roles of neuropathology-associated reactive astrocytes: a systematic review. Acta neuropathologica Commun. (2023) 11:42. doi: 10.1186/s40478-023-01526-9
138. Zheng JH, Yuan N, Zhang P, Liu DF, Lin W, and Miao J. Acupuncture combined with moxibustion mitigates spinal cord injury-induced motor dysfunction in mice by NLRP3-IL-18 signaling pathway inhibition. J orthopaedic Surg Res. (2023) 18:419. doi: 10.1186/s13018-023-03902-6
139. Mordillo-Mateos L, Sánchez-Ramos A, Coperchini F, Bustos-Guadamillas I, Alonso-Bonilla C, Vargas-Baquero E, et al. Development of chronic pain in males with traumatic spinal cord injury: role of circulating levels of the chemokines CCL2 and CXCL10 in subacute stage. Spinal cord. (2019) 57:953–9. doi: 10.1038/s41393-019-0311-3
140. Sun M, Song Y, Hu X, Zhang Z, Tan R, Cai Z, et al. Leptin reduces LPS-induced A1 reactive astrocyte activation and inflammation via inhibiting p38-MAPK signaling pathway. Glia. (2025) 73:25–37. doi: 10.1002/glia.24611
141. Chen J, Zeng X, Wang L, Zhang W, Li G, Cheng X, et al. Mutual regulation of microglia and astrocytes after Gas6 inhibits spinal cord injury. Neural regeneration Res. (2025) 20:557–73. doi: 10.4103/NRR.NRR-D-23-01130
142. Khankan RR, Griffis KG, Haggerty-Skeans JR, Zhong H, Roy RR, Edgerton VR, et al. Olfactory ensheathing cell transplantation after a complete spinal cord transection mediates neuroprotective and immunomodulatory mechanisms to facilitate regeneration. J Neurosci. (2016) 36:6269–86. doi: 10.1523/JNEUROSCI.0085-16.2016
143. Luo D, Zhang Y, Yuan X, Pan Y, Yang L, Zhao Y, et al. Oleoylethanolamide inhibits glial activation via moudulating PPARα and promotes motor function recovery after brain ischemia. Pharmacol Res. (2019) 141:530–40. doi: 10.1016/j.phrs.2019.01.027
144. Gao X, Zeb S, He YY, Guo Y, Zhu YM, Zhou XY, et al. Valproic acid inhibits glial scar formation after ischemic stroke. Pharmacology. (2022) 107:263–80. doi: 10.1159/000514951
145. Alizadeh J, Kochan MM, Stewart VD, Drewnik DA, Hannila SS, Ghavami S, et al. Inhibition of autophagy flux promotes secretion of chondroitin sulfate proteoglycans in primary rat astrocytes. Mol Neurobiol. (2021) 58:6077–91. doi: 10.1007/s12035-021-02533-4
146. Zhang H, Uchimura K, and Kadomatsu K. Brain keratan sulfate and glial scar formatio. Ann N Y Acad Sci. (2006) 1086:81–90. doi: 10.1196/annals.1377.014
147. Pang QM, Chen SY, Xu QJ, Fu SP, Yang YC, Zou WH, et al. Neuroinflammation and scarring after spinal cord injury: therapeutic roles of MSCs on inflammation and glial scar. Front Immunol. (2021) 12:751021. doi: 10.3389/fimmu.2021.751021
148. Xu L, Liu X, Guo C, Wang C, Zhao J, Zhang X, et al. Inhibition of ROCK2 kinase activity improved behavioral deficits and reduced neuron damage in a DEACMP rat model. Brain Res Bull. (2022) 180:24–30. doi: 10.1016/j.brainresbull.2021.12.018
149. Li J, Luo W, Xiao C, Zhao J, Xiang C, Liu W, et al. Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair. Theranostics. (2023) 13:3966–87. doi: 10.7150/thno.84133
150. Yao L, Cai X, Yang S, Song Y, Xing L, Li G, et al. A single-cell landscape of the regenerating spinal cord of zebrafish. Neural regeneration Res. (2026) 21:780–9. doi: 10.4103/NRR.NRR-D-24-01163
151. Yue Y, Stanojlovic M, Lin Y, Karsenty G, and Lin W. Oligodendrocyte-specific ATF4 inactivation does not influence the development of EAE. J Neuroinflamm. (2019) 16:23. doi: 10.1186/s12974-019-1415-6
152. Kamali S, Rajendran R, Stadelmann C, Karnati S, Rajendran V, Giraldo-Velasquez M, et al. Oligodendrocyte-specific deletion of FGFR2 ameliorates MOG(35-55) -induced EAE through ERK and Akt signalling. Brain Pathol (Zurich Switzerland). (2021) 31:297–311. doi: 10.1111/bpa.12916
153. Jahanbazi Jahan-Abad A, Karima S, Sahab Negah S, Noorbakhsh F, Borhani-Haghighi M, Gorji A, et al. Therapeutic potential of conditioned medium derived from oligodendrocytes cultured in a self-assembling peptide nanoscaffold in experimental autoimmune encephalomyelitis. Brain Res. (2019) 1711:226–35. doi: 10.1016/j.brainres.2019.01.035
154. Xiao Y, Guan T, Yang X, Xu J, Zhang J, Qi Q, et al. Baicalin facilitates remyelination and suppresses neuroinflammation in rats with chronic cerebral hypoperfusion by activating Wnt/β-catenin and inhibiting NF-κB signaling. Behav Brain Res. (2023) 442:114301. doi: 10.1016/j.bbr.2023.114301
155. Spaas J, Van Veggel L, Schepers M, Tiane A, van Horssen J, Wilson DM, et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol Life sciences: CMLS. (2021) 78:4615–37. doi: 10.1007/s00018-021-03802-0
156. Zhou J, Terluk MR, Basso L, Mishra UR, Orchard PJ, Cloyd JC, et al. N-acetylcysteine provides cytoprotection in murine oligodendrocytes through heme oxygenase-1 activity. Biomedicines. (2020) 8. doi: 10.3390/biomedicines8080240
157. Kim JY, Kim CH, Lee EY, and Seo JH. Alpha B-crystallin overexpression protects oligodendrocyte precursor cells against oxidative stress-induced apoptosis through the akt pathway. J Mol neuroscience: MN. (2020) 70:751–8. doi: 10.1007/s12031-020-01485-z
158. Zhang H, Yang Y, Zhang J, Huang L, Niu Y, Chen H, et al. Oligodendrocytes play a critical role in white matter damage of vascular dementia. Neuroscience. (2024) 538:1–10. doi: 10.1016/j.neuroscience.2023.10.018
159. López-Muguruza E, Peiró-Moreno C, Ruiz A, and Matute C. Oligodendrocyte and myelin pathophysiology in multiple sclerosis. Adv Neurobiol. (2025) 43:317–61. doi: 10.1007/978-3-031-87919-7_12
160. Elkhodiry AA, Zamzam DA, and El Tayebi HM. MicroRNA−155 modulation of CD8(+) T−cell activity personalizes response to disease−modifying therapies of patients with relapsing−remitting multiple sclerosis. Med Int. (2023) 3:20. doi: 10.3892/mi.2023.80
161. Yandamuri SS, Filipek B, Obaid AH, Lele N, Thurman JM, Makhani N, et al. MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.165373
162. Pukoli D and Vécsei L. Kynurenines and mitochondrial disturbances in multiple sclerosis. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26115098
163. Wu Z, Li G, Wang S, Zhang N, Li X, Zhang F, et al. Single-cell analysis of spinal cord injury reveals functional heterogeneity of oligodendrocyte lineage cells. Gene. (2023) 886:147713. doi: 10.1016/j.gene.2023.147713
164. Adams AA, Wood TL, and Kim HA. Mature and myelinating oligodendrocytes are specifically vulnerable to mild fluid percussion injury in mice. Neurotrauma Rep. (2023) 4:433–46. doi: 10.1089/neur.2023.0037
165. Jimi E, Fei H, and Nakatomi C. NF-κB signaling regulates physiological and pathological chondrogenesis. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20246275
166. Vrábel D, Pour L, and Ševčíková S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. (2019) 34:56–66. doi: 10.1016/j.blre.2018.11.003
167. Jimi E and Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. (2005) 208:80–7. doi: 10.1111/j.0105-2896.2005.00329.x
168. Tang R, Botchway BOA, Meng Y, Zhang Y, Zhou C, Jiang J, et al. The inhibition of inflammatory signaling pathway by secretory leukocyte protease inhibitor can improve spinal cord injury. Cell Mol Neurobiol. (2020) 40:1067–73. doi: 10.1007/s10571-020-00799-1
169. Gilmore TD. NF-κB and human cancer: what have we learned over the past 35 years? Biomedicines. (2021) 9. doi: 10.3390/biomedicines9080889
170. Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta neurobiologiae experimentalis. (2011) 71:281–99. doi: 10.55782/ane-2011-1848
171. Norden DM, Trojanowski PJ, Villanueva E, Navarro E, and Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. (2016) 64:300–16. doi: 10.1002/glia.22930
172. Jiang J, Luo Y, Qin W, Ma H, Li Q, Zhan J, et al. Electroacupuncture suppresses the NF-κB signaling pathway by upregulating cylindromatosis to alleviate inflammatory injury in cerebral ischemia/reperfusion rats. Front Mol Neurosci. (2017) 10:363. doi: 10.3389/fnmol.2017.00363
173. Liu G, Fan G, Guo G, Kang W, Wang D, Xu B, et al. FK506 attenuates the inflammation in rat spinal cord injury by inhibiting the activation of NF-κB in microglia cells. Cell Mol Neurobiol. (2017) 37:843–55. doi: 10.1007/s10571-016-0422-8
174. Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. (2012) 32:6391–410. doi: 10.1523/jneurosci.6221-11.2012
175. Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. (2011) 12:844–52. doi: 10.1038/ni.2080
176. Awane M, Andres PG, Li DJ, and Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol (Baltimore Md: 1950). (1999) 162:5337–44. doi: 10.4049/jimmunol.162.9.5337
177. Lawrence T. The nuclear factor NF-κB pathway in inflammation. (2009) 1:a001651. doi: 10.1101/cshperspect.a001651
178. Acarin L, González B, and Castellano B. STAT3 and NFkappaB activation precedes glial reactivity in the excitotoxically injured young cortex but not in the corresponding distal thalamic nuclei. J Neuropathol Exp Neurol. (2000) 59:151–63. doi: 10.1093/jnen/59.2.151
179. Zheng XQ, Huang JF, Lin JL, Zhu YX, Wang MQ, Guo ML, et al. Controlled release of baricitinib from a thermos-responsive hydrogel system inhibits inflammation by suppressing JAK2/STAT3 pathway in acute spinal cord injury. Colloids Surf B Biointerfaces. (2021) 199:111532. doi: 10.1016/j.colsurfb.2020.111532
180. Li J, Guo Y, Zeng X, Tian H, Han Y, Cai S, et al. Electroacupuncture stimulation inhibited astrogliosis and microglia polarisation to alleviate spinal cord injury via Janus kinase 2/signal transducer and activator of transcription 3 signalling pathway. Folia Histochem Cytobiol. (2025) 63:28–40. doi: 10.5603/fhc.104273
181. Zhang Z, He Y, Liu H, Liu Y, Wu T, Li R, et al. NLRP3 regulates ferroptosis via the JAK2/STAT3 pathway in asthma inflammation: Insights from in vivo and in vitro studies. Int Immunopharmacol. (2024) 143:113416. doi: 10.1016/j.intimp.2024.113416
182. Kim YK, Lee JY, and Suh HN. Cytokine-induced JAK2-STAT3 activates tissue regeneration under systemic or local inflammation. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23042262
183. Birck C, Ginolhac A, Pavlou MAS, Michelucci A, Heuschling P, and Grandbarbe L. NF-κB and TNF affect the astrocytic differentiation from neural stem cells. Cells. (2021) 10. doi: 10.3390/cells10040840
184. Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. (2008) 28:7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008
185. Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, et al. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol Med. (2019) 11. doi: 10.15252/emmm.201809665
186. Ageeva T, Rizvanov A, and Mukhamedshina Y. NF-κB and JAK/STAT signaling pathways as crucial regulators of neuroinflammation and astrocyte modulation in spinal cord injury. Cells. (2024) 13. doi: 10.3390/cells13070581
187. Zhao C, Zhou T, Li M, Liu J, Zhao X, Pang Y, et al. Argatroban promotes recovery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway. Neural Regener Res. (2024) 19:434–9. doi: 10.4103/1673-5374.375345
188. Agarwal G, Roy A, Singh AA, Kumar H, Mandoli A, Srivastava A, et al. BM-MSC-loaded graphene-collagen cryogels ameliorate neuroinflammation in a rat spinal cord injury model. ACS Appl Bio Mater. (2024) 7:1478–89. doi: 10.1021/acsabm.3c00876
189. Wang D, Zhao S, Pan J, Wang Z, Li Y, Xu X, et al. Ginsenoside Rb1 attenuates microglia activation to improve spinal cord injury via microRNA-130b-5p/TLR4/NF-κB axis. J Cell Physiol. (2021) 236:2144–55. doi: 10.1002/jcp.30001
190. Chen W, Zhang L, Zhong G, Liu S, Sun Y, Zhang J, et al. Regulation of microglia inflammation and oligodendrocyte demyelination by Engeletin via the TLR4/RRP9/NF-κB pathway after spinal cord injury. Pharmacol Res. (2024) 209:107448. doi: 10.1016/j.phrs.2024.107448
191. Han X, Shao J, Ren X, Li Y, Yu W, Lin C, et al. The different mechanisms of peripheral and central TLR4 on chronic postsurgical pain in rats. J Anat. (2021) 239:111–24. doi: 10.1111/joa.13406
192. Zhao Y, Lv W, Wen L, Liu W, Zhao Y, Li Y, et al. Relationship between GTP binding protein RAB10, toll-like receptor 4, and nuclear factor kappa-B and prognosis in patients with breast cancer. Sci Rep. (2024) 14:23287. doi: 10.1038/s41598-024-74501-6
193. Souza LKM, Nogueira KM, Araújo TSL, Sousa NA, Sousa FBM, Oliveira AP, et al. Anti-diarrheal therapeutic potential of diminazene aceturate stimulation of the ACE II/Ang-(1-7)/Mas receptor axis in mice: A trial study. Biochem Pharmacol. (2021) 186:114500. doi: 10.1016/j.bcp.2021.114500
194. Liu Y, Xiao Y, Gao J, Gao J, Li R, Qi Z, et al. MSR405: inhibiting neuroinflammation after spinal cord injury in rats. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12030614
195. Deng M, Xie P, Liu J, Zhou Y, Chen Z, Ma Y, et al. α-cyperone improves rat spinal cord tissue damage via akt/nrf2 and NF-κB pathways. J Surg Res. (2022) 276:331–9. doi: 10.1016/j.jss.2022.02.006
196. Miao L, Qing SW, and Tao L. Huntingtin-associated protein 1 ameliorates neurological function rehabilitation by facilitating neurite elongation through TrKA-MAPK pathway in mice spinal cord injury. Front Mol Neurosci. (2023) 16:1214150. doi: 10.3389/fnmol.2023.1214150
197. Ronkina N and Gaestel M. MAPK-activated protein kinases: servant or partner? Annu Rev Biochem. (2022) 91:505–40. doi: 10.1146/annurev-biochem-081720-114505
198. Dong C, Davis RJ, and Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. (2002) 20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133
199. Morrison DK and Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. (2003) 19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942
200. Lavoie H, Gagnon J, and Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. (2020) 21:607–32. doi: 10.1038/s41580-020-0255-7
201. Kigerl KA, De Rivero Vaccari JP, Dietrich WD, Popovich PG, and Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. (2014) 258:5–16. doi: 10.1016/j.expneurol.2014.01.001
202. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. (2010) 58:253–63. doi: 10.1002/glia.20928
203. Beattie MS, Farooqui AA, and Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J neurotrauma. (2000) 17:915–25. doi: 10.1089/neu.2000.17.915
204. Stirling DP, Liu S, Kubes P, and Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J neuroscience: Off J Soc Neurosci. (2009) 29:753–64. doi: 10.1523/JNEUROSCI.4918-08.2009
205. Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, and Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. (2009) 29:3956–68. doi: 10.1523/JNEUROSCI.3992-08.2009
206. Jung HW, Son HY, Minh CV, Kim YH, and Park YK. Methanol extract of Ficus leaf inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated microglia via the MAPK pathway. Phytotherapy research: PTR. (2008) 22:1064–9. doi: 10.1002/ptr.2442
207. Pang T, Wang J, Benicky J, and Saavedra JM. Minocycline ameliorates LPS-induced inflammation in human monocytes by novel mechanisms including LOX-1, Nur77 and LITAF inhibition. Biochim Biophys Acta. (2012) 1820:503–10. doi: 10.1016/j.bbagen.2012.01.011
208. Corsaro A, Thellung S, Chiovitti K, Villa V, Simi A, Raggi F, et al. Dual modulation of ERK1/2 and p38 MAP kinase activities induced by minocycline reverses the neurotoxic effects of the prion protein fragment 90–231. Neurotoxicity Res. (2009) 15:138–54. doi: 10.1007/s12640-009-9015-3
209. Song ZP, Xiong BR, Guan XH, Cao F, Manyande A, Zhou YQ, et al. Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta pharmacologica Sin. (2016) 37:753–62. doi: 10.1038/aps.2016.1
210. Jin SX, Zhuang ZY, Woolf CJ, and Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. (2003) 23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003
211. Ji RR and Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. (2007) 3:33. doi: 10.1186/1744-8069-3-33
212. Kawasaki Y, Zhang L, Cheng JK, and Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. (2008) 28:5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008
213. Moalem G and Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. (2006) 51:240–64. doi: 10.1016/j.brainresrev.2005.11.004
214. Clark AK, D’aquisto F, Gentry C, Marchand F, McMahon SB, and Malcangio M. Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J neurochemistry. (2006) 99:868–80. doi: 10.1111/j.1471-4159.2006.04126.x
215. Ji RR, Gereau RWT, Malcangio M, and Strichartz GR. MAP kinase and pain. Brain Res Rev. (2009) 60:135–48. doi: 10.1016/j.brainresrev.2008.12.011
216. Zou Y, Zhang J, Xu J, Fu L, Xu Y, Wang X, et al. SIRT6 inhibition delays peripheral nerve recovery by suppressing migration, phagocytosis and M2-polarization of macrophages. Cell bioscience. (2021) 11:210. doi: 10.1186/s13578-021-00725-y
217. Schizas N, Perry S, Andersson B, Wählby C, Kullander K, and Hailer NP. Differential neuroprotective effects of interleukin-1 receptor antagonist on spinal cord neurons after excitotoxic injury. Neuroimmunomodulation. (2017) 24:220–30. doi: 10.1159/000484607
218. Börcek A, Çivi S, Öcal Ö, and Gülbahar Ö. Effects of tumor necrosis factor alpha blocker adalimumab in experimental spinal cord injury. J Korean Neurosurgical Soc. (2015) 57:73–6. doi: 10.3340/jkns.2015.57.2.73
219. Gordon M, Sinopoulou V, Akobeng AK, Radford SJ, Eldragini M, Darie AM, et al. Infliximab for medical induction of remission in Crohn’s disease. Cochrane Database systematic Rev. (2023) 11:Cd012623. doi: 10.1002/14651858.CD012623.pub2
220. Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, et al. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. (2010) 17:1563–7. doi: 10.1016/j.jocn.2010.04.027
221. Kurt G, Ergün E, Cemil B, Börcek AO, Börcek P, Gülbahar O, et al. Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol. (2009) 71:332–6. doi: 10.1016/j.surneu.2008.01.038
222. Zhang X, Xu L, Chen X, Zhou X, and Cao L. Acacetin alleviates neuroinflammation and oxidative stress injury via the Nrf2/HO-1 pathway in a mouse model of spinal cord injury. Trans Neurosci. (2022) 13:483–94. doi: 10.1515/tnsci-2022-0266
223. Wang M, Gao X, Zhao K, Chen H, Xu M, and Wang K. Effect of TREM2 on release of inflammatory factor from LPS-stimulated microglia and its possible mechanism. Ann Clin Lab Sci. (2019) 49:249–56.
224. Liu Y, Zhang F, Sun Q, and Liang L. Adalimumab combined with erythropoietin improves recovery from spinal cord injury by suppressing microglial M1 polarization-mediated neural inflammation and apoptosis. Inflammopharmacology. (2023) 31:887–97. doi: 10.1007/s10787-022-01090-z
225. Shimizu N, Wada N, Shimizu T, Suzuki T, Kurobe M, Kanai AJ, et al. Role of p38 MAP kinase signaling pathways in storage and voiding dysfunction in mice with spinal cord injury. Neurourology urodynamics. (2020) 39:108–15. doi: 10.1002/nau.24170
226. Hoyland JA, Le Maitre C, and Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatol (Oxford England). (2008) 47:809–14. doi: 10.1093/rheumatology/ken056
227. Yates AG, Jogia T, Gillespie ER, Couch Y, Ruitenberg MJ, and Anthony DC. Acute IL-1RA treatment suppresses the peripheral and central inflammatory response to spinal cord injury. J Neuroinflamm. (2021) 18:15. doi: 10.1186/s12974-020-02050-6
228. Guan YZ, Sun C, Wang HL, Xia XL, Lu FZ, Song J, et al. MiR-223-5p inhibitor suppresses microglia inflammation and promotes Nrg-1 in rats of spinal cord injury. Eur Rev Med Pharmacol Sci. (2019) 23:9746–53. doi: 10.26355/eurrev_201911_19537
229. Amo-Aparicio J, Garcia-Garcia J, Puigdomenech M, Francos-Quijorna I, Skouras DB, Dinarello CA, et al. Inhibition of the NLRP3 inflammasome by OLT1177 induces functional protection and myelin preservation after spinal cord injury. Exp Neurol. (2022) 347:113889. doi: 10.1016/j.expneurol.2021.113889
230. Wang P, Li ZW, Zhu Z, Zhang ZY, and Liu J. Inhibition of miR-214-5p attenuates inflammatory chemotaxis and nerve regeneration obstruction after spinal cord injury in rats. Eur Rev Med Pharmacol Sci. (2019) 23:2332–9. doi: 10.26355/eurrev_201903_17376
231. Bjarnason I. The impact of recent data on our understanding of the roles of COX-1 and COX-2 in gastrointestinal pathophysiology. Clin Drug Invest. (2007) 27 Suppl 1:7–13. doi: 10.2165/00044011-200727001-00003
232. Yuksel U, Bakar B, Dincel GC, Budak Yildiran FA, Ogden M, and Kisa U. The investigation of the Cox-2 selective inhibitor parecoxib effects in spinal cord injury in rat. J Invest Surg. (2019) 32:402–13. doi: 10.1080/08941939.2017.1423423
233. Balachandran SK, Iyer K, Senthil SA, Ramalingam AP, and Venkatachalam S. Evaluating the anti-inflammatory efficacy of steroids, COX-2 selective, and nonselective NSAIDs in contusion spinal cord injury: an experimental analysis. J Pharm bioallied Sci. (2025) 17:S1877–s81. doi: 10.4103/jpbs.jpbs_604_25
234. Melnikov DV, Kirillova KA, Zakharenko AS, Sinelnikov MY, Ragimov AA, Istranov AL, et al. Effect of cryo-processing on platelet-rich autoplasma preparations. Sovremennye tekhnologii v meditsine. (2021) 12:54–60. doi: 10.17691/stm2020.12.6.07
235. Karagianni K, Pettas S, Kanata E, Lioulia E, Thune K, Schmitz M, et al. Carnosic acid and carnosol display antioxidant and anti-prion properties in in vitro and cell-free models of prion diseases. Antioxidants (Basel Switzerland). (2022) 11. doi: 10.3390/antiox11040726
236. Wu C-C, Chen W-H, Zao B, Lai P-L, Lin T-C, Lo H-Y, et al. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. (2011) 32:5847–54. doi: 10.1016/j.biomaterials.2011.05.002
237. Nie X, Liu Y, Yuan T, Yu T, Yun Z, Xue W, et al. Platelet-rich plasma-derived exosomes promote blood-spinal cord barrier repair and attenuate neuroinflammation after spinal cord injury. J nanobiotechnology. (2024) 22:456. doi: 10.1186/s12951-024-02737-5
238. Antonios JP, Farah GJ, Cleary DR, Martin JR, Ciacci JD, Pham MH, et al. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. (2019) 46:E9. doi: 10.3171/2018.12.FOCUS18589
239. Tsai M-J, Liou D-Y, Lin Y-R, Weng C-F, Huang M-C, Huang W-C, et al. Attenuating spinal cord injury by conditioned medium from bone marrow mesenchymal stem cells. (2018) 8:23. doi: 10.3390/jcm8010023
240. Chandanala S, Mohan G, Manukonda DL, Kumar A, and Prasanna J. A novel role of CD73-IFNγ signalling axis in human mesenchymal stromal cell mediated inflammatory macrophage suppression. Regenerative Ther. (2024) 26:89–101. doi: 10.1016/j.reth.2024.05.011
242. Kim Y, Jo S-H, Kim WH, and Kweon O-K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. (2015) 6:1–10. doi: 10.1186/s13287-015-0236-5
243. Yang Z, Zheng C, Zhang F, Lin B, Cao M, Tian X, et al. Magnetic resonance imaging of enhanced nerve repair with mesenchymal stem cells combined with microenvironment immunomodulation in neurotmesis. Muscle Nerve. (2020) 61:815–25. doi: 10.1002/mus.26862
244. Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, et al. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. (2016) 17:1380. doi: 10.3390/ijms17091380
245. Lv R, Du L, Liu X, Zhou F, Zhang Z, and Zhang L. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. (2019) 70:28–36. doi: 10.1016/j.intimp.2019.02.006
246. Jiang W, Huang Y, Han N, He F, Li M, Bian Z, et al. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. (2016) 54:592–6. doi: 10.1038/sc.2015.227
247. Coyoy-Salgado A, Segura-Uribe JJ, Guerra-Araiza C, Orozco-Suárez S, Salgado-Ceballos H, Feria-Romero IA, et al. The importance of natural antioxidants in the treatment of spinal cord injury in animal models: an overview. (2019) 2019:3642491. doi: 10.1155/2019/3642491
248. Cheng S, Zhou F, Xu Y, Liu X, Zhang Y, Gu M, et al. Geniposide regulates the miR-101/MKP-1/p38 pathway and alleviates atherosclerosis inflammatory injury in ApoE-/-mice. (2019) 224:296–306. doi: 10.1016/j.imbio.2018.12.005
249. Lu W, Zhao Y, Kong Y, Zhang W, Ma W, Li W, et al. Geniposide prevents H2O2-induced oxidative damage in melanocytes by activating the PI3K–Akt signalling pathway. (2018) 43:667–74. doi: 10.1111/ced.13409
250. Ma S, Zhang C, Zhang Z, Dai Y, Gu R, and Jiang R. Geniposide protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via up-regulation of miR-145-5p. Artif cells nanomedicine Biotechnol. (2019) 47:2875–81. doi: 10.1080/21691401.2019.1626406
251. Li Y, Qiu H, Yao S, Li Q, Ding Y, Cao Y, et al. Geniposide exerts protective effects on spinal cord injury in rats by inhibiting the IKKs/NF-κB signaling pathway. (2021) 100:108158. doi: 10.1016/j.intimp.2021.108158
252. Choi PG, Park SH, Jeong HY, Kim HS, Hahm JH, Seo HD, et al. Geniposide attenuates muscle atrophy via the inhibition of FoxO1 in senescence-accelerated mouse prone-8. Phytomedicine: Int J phytotherapy phytopharmacology. (2024) 123:155281. doi: 10.1016/j.phymed.2023.155281
253. Li M, Chen S, Luo K, Li X, Wang R, Yang J, et al. Geniposide from Gardeniae Fructus exerts antipyretic effect in febrile rats through modulating the TLR4/NF-κB signaling pathway. J ethnopharmacology. (2024) 326:117934. doi: 10.1016/j.jep.2024.117934
254. Ren B, Yuan T, Diao Z, Zhang C, Liu Z, Liu X, et al. Protective effects of sesamol on systemic oxidative stress-induced cognitive impairments via regulation of Nrf2/Keap1 pathway. (2018) 9:5912–24. doi: 10.1039/C8FO01436A
255. Romero N and Favoreel HW. Pseudorabies Virus Infection Triggers NF-κB Activation via the DNA Damage Response but Actively Inhibits NF-κB-Dependent Gene Expression. J Virol. (2021) 95:e0166621. doi: 10.1128/JVI.01666-21
256. Gina NNT, Kuo JL, Wu ML, and Chuang SM. Sesamin and sesamolin potentially inhibit adipogenesis through downregulating the peroxisome proliferator-activated receptor γ protein expression and activity in 3T3-L1 cells. Nutr Res (New York NY). (2024) 123:4–17. doi: 10.1016/j.nutres.2023.12.011
257. Lu Y, Duan Y, Dai Y, Ni X, Li J, Zeng X, et al. Decoding the pharmacodynamics of Fufang Tongye Shaoshang You: A promising ethnomedicine for diabetic ulcer healing. J ethnopharmacology. (2025) 347:119727. doi: 10.1016/j.jep.2025.119727
258. Zhan S, Lu L, Pan SS, Wei XQ, Miao RR, Liu XH, et al. Targeting NQO1/GPX4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br J Cancer. (2022) 127:364–76. doi: 10.1038/s41416-022-01800-y
259. Zhao X, Chen X, and Yue C. Rutin ameliorates inflammation and oxidative stress in ulcerative colitis by inhibiting NLRP3 inflammasome signaling pathway. Cell Biochem biophysics. (2024) 82:3715–26. doi: 10.1007/s12013-024-01459-7
260. Zhang P and Ma X. Effect of rutin on spinal cord injury through inhibition of the expression of MIP-2 and activation of MMP-9, and downregulation of Akt phosphorylation. (2015) 12:7554–60. doi: 10.3892/mmr.2015.4357
261. Zhou Z and Zhang P. Formononetin ameliorates the LPS-induced inflammatory response and apoptosis of neuronal cells via NF-κB/NLRP3 signaling pathway. Funct Integr Genomics. (2023) 23:321. doi: 10.1007/s10142-023-01247-1
262. Dai W, Wang X, Teng H, Li C, Wang B, and Wang J. Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int Immunopharmacol. (2019) 66:215–23. doi: 10.1016/j.intimp.2018.11.029
263. Majidpoor J, Mortezaee K, Khezri Z, Fathi F, Zali A, Derakhshan HB, et al. The effect of the “segment” of spinal cord injury on the activity of the nucleotide-binding domain-like receptor protein 3 inflammasome and response to hormonal therapy. Cell Biochem Funct. (2021) 39:267–76. doi: 10.1002/cbf.3574
264. Majidpoor J, Khezri Z, Rostamzadeh P, Mortezaee K, Rezaie MJ, Fathi F, et al. The expressions of NLRP1, NLRP3, and AIM2 inflammasome complexes in the contusive spinal cord injury rat model and their responses to hormonal therapy. Cell Tissue Res. (2020) 381:397–410. doi: 10.1007/s00441-020-03250-5
265. Coyoy-Salgado A, Segura-Uribe J, Salgado-Ceballos H, Castillo-Mendieta T, Sánchez-Torres S, Freyermuth-Trujillo X, et al. Evaluating sex steroid hormone neuroprotection in spinal cord injury in animal models: is it promising in the clinic? Biomedicines. (2024) 12. doi: 10.3390/biomedicines12071478
266. Xie L, Wu H, Huang X, and Yu T. Melatonin, a natural antioxidant therapy in spinal cord injury. Front Cell Dev Biol. (2023) 11:1218553. doi: 10.3389/fcell.2023.1218553
267. Rahimi B, Aliaghaei A, Ramezani F, Behroozi Z, and Nasirinezhad F. Sertoli cell transplantation attenuates microglial activation and inhibits TRPC6 expression in neuropathic pain induced by spinal cord injury. Physiol Behav. (2022) 251:113807. doi: 10.1016/j.physbeh.2022.113807
268. Claus D, Etter D, and Ryder SC. Nuances of gender affirming therapy for transgender women with spinal cord injury. J spinal cord Med. (2025) 48:542–4. doi: 10.1080/10790268.2024.2396644
269. Ying Y, Cai X, Dai P, Zhang Y, Lv J, Huang Z, et al. Neurological emergency treatment strategy: A neuron-targeted regulation system for reactive oxygen species metabolism through ferroptosis modulation. ACS nano. (2025) 19:8753–72. doi: 10.1021/acsnano.4c15705
270. Zhong G, Yang Y, Feng D, Wei K, Chen J, Chen J, et al. Melatonin protects injured spinal cord neurons from apoptosis by inhibiting mitochondrial damage via the SIRT1/Drp1 signaling pathway. Neuroscience. (2023) 534:54–65. doi: 10.1016/j.neuroscience.2023.10.010
271. Martin-Rojas T, Sastre-Oliva T, Esclarín-Ruz A, Gil-Dones F, Mourino-Alvarez L, Corbacho-Alonso N, et al. Effects of growth hormone treatment and rehabilitation in incomplete chronic traumatic spinal cord injury: insight from proteome analysis. J personalized Med. (2020) 10. doi: 10.3390/jpm10040183
Keywords: traumatic spinal cord injury, inflammatory microenvironment, cytokines, chemokines, signal transduction
Citation: Gu H-y, Lin F-x, Liu N and Xu C-h (2025) Mechanism analysis and intervention strategies of the inflammatory microenvironment in traumatic spinal cord injury. Front. Immunol. 16:1692346. doi: 10.3389/fimmu.2025.1692346
Received: 25 August 2025; Accepted: 19 November 2025; Revised: 07 November 2025;
Published: 18 December 2025.
Edited by:
Guillem Paniagua Soriano, Principe Felipe Research Center (CIPF), SpainReviewed by:
Lien Beckers, University of Hasselt, BelgiumGhazaleh Moshkdanian, Kashan University of Medical Sciences, Iran
Copyright © 2025 Gu, Lin, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Can-hua Xu, eHVjYW5odWEyMDI0QDE2My5jb20=
 Hou-yun Gu
Hou-yun Gu Fei-xiang Lin
Fei-xiang Lin Ning Liu1,2
Ning Liu1,2 Can-hua Xu
Can-hua Xu
