- 1Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 2Department of Faculty of Medicine and Health Sciences, UCSI University, Port Dickson, Negeri Sembilan, Malaysia
- 3Department of Medical Oncology III, Yunnan Cancer Hospital, Kunming, Yunnan, China
- 4Department of Gastrointestinal Oncology, Yunnan Cancer Hospital, Yunnan Cancer Hospital, Kunming, Yunnan, China
- 5Department of Respiratory and Critical Care Medicine, Yanan Hospital of Kunming City, Kunming, Yunnan, China
- 6School of Information Science & Engineering, Yunnan University, Kunming, China
- 7Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Background: Immune checkpoint inhibitors (ICIs) have improved outcomes in advanced lung cancer. β-adrenergic signaling may promote tumor initiation and progression, and β-blockers (BBs) have emerged as anti-tumor sensitizing agents. This study evaluates the impact of BBs use during ICIs treatment in advanced lung cancer.
Methods: This multicenter retrospective real-world study included 462 patients treated with ICIs from June 2019 to December 2024. Patients were divided into BBs and No BBs groups. Primary endpoints were overall survival (OS) and progression-free survival (PFS); efficacy evaluation and objective response rate (ORR) were secondary. Propensity score matching (PSM) balances baseline characteristics. Kaplan–Meier method, Cox, and logistic regression models were used for survival and multivariate analyses. Subgroup analyses assessed clinical factors. A P value < 0.05 is considered statistically significant.
Results: After PSM, 318 patients were included (88 BBs, 230 No BBs). BBs use was associated with longer median PFS (mPFS) (15.8 vs. 11.8 months; HR = 0.67, 95% CI: 0.49–0.92, P = 0.038) and higher ORR (51.1% vs. 35.2%, P = 0.014), but not improved median OS (mOS) (29.0 vs. 31.5 months; HR = 1.38, 95% CI: 0.93–2.03, P = 0.108). BBs use independently predicted improved ORR (OR = 0.45, 95% CI: 0.26–0.78, P = 0.004) and longer PFS (HR = 0.67, 95% CI: 0.49–0.92, P = 0.014). In patients with cardiovascular comorbidities (CVD), BBs use was linked to longer mPFS (15.8 vs. 10.9 months, P = 0.0066) and higher ORR(51.1% vs 27.0%, P<0.001), with no mOS difference (P = 0.82). Among non-small cell lung cancer (NSCLC) patients, mPFS (17.5 vs. 12.3 months, P = 0.04) and ORR (56.0% vs 35.9%, P = 0.004) were also improved in the BBs group, whereas OS did not differ significantly (P = 0.3).
Conclusion: In stage-advanced lung cancer, BBs combined with ICIs were associated with improved ORR and prolonged PFS, but did not significantly improve OS. PFS and ORR benefits were also observed in patients with CVD or NSCLC. Further prospective studies are needed to validate these findings and clarify whether BBs directly contribute to ICIs’ efficacy.
1 Introduction
Lung cancer is one of the most common cancers worldwide, with consistently high incidence and mortality rates (1). Early-stage lung cancer often presents without obvious symptoms, and most patients are diagnosed at an advanced stage when clinical manifestations emerge. The overall 5-year survival rate for patients with advanced lung cancer is approximately 20% (2). In recent years, the emergence of immune checkpoint inhibitors (ICIs) has brought about a breakthrough of advanced lung cancer. ICIs function by blocking cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or the programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) pathways, thereby relieving the inhibition of T cell and natural killer cell activity, restoring their anti-tumor activity, and ultimately suppressing tumor growth and metastasis. ICIs have already been applied in first-line treatment for advanced lung cancer (3, 4).
CD8+ T cells are a key component of the anti-tumor immune response and are capable of suppressing cancer cells. When antigens cannot be effectively eliminated, T cells may enter a state of exhaustion (5). T cell exhaustion limits the efficacy of T cell-mediated responses in cancer, resulting in immunotherapy failure or resistance in a substantial proportion of lung cancer patients (6). Preclinical studies have shown that stress-induced β-adrenergic signaling may increase the number and activity of immunosuppressive cells, reduce the production of T cell growth-promoting factors, and inhibit T cell cytotoxicity, ultimately leading to T cell exhaustion (7). Globig et al. found that exhausted T cells expressed higher levels of the adrenergic receptor β1 (ADRB1) in mice with chronic viral infections or cancer. They reported that deletion of ADRB1 in T cells, or pharmacological blockade of ADRB1 using β-blockers (BBs), enhanced the secretion of effector molecules and cytokines and improved T cell function in chronic infection and tumor settings (8). These findings highlight the potential of combining BBs with existing immunotherapies to improve cancer treatment by enhancing cytotoxic T cell function.
Repurposing drugs already approved for other indications is an attractive strategy to optimize treatment outcomes in cancer patients (9). BBs are a class of safe, cost-effective drugs with mild side effects and are widely used for hypertension, cardiovascular diseases, hyperthyroidism, and anxiety (10, 11). Preclinical evidence supports the potential of BBs to enhance lung cancer immunotherapy (8, 12), and there is growing interest in the role of BBs as modulators of tumor initiation, progression, and metastasis (12, 13).
Several clinical studies have found that the use of BBs is associated with prolonged progression-free survival (PFS) in patients with melanoma, while no significant association has been observed with PFS in colorectal or breast cancer (13). In hepatocellular carcinoma, BBs use was also not found to be associated with overall survival (OS), PFS, or objective response rate (ORR) in patients receiving immunotherapy (14). However, a recent large population-based cohort study reported that BBs use was associated with improved cancer-specific survival and recurrence-free interval in triple-negative breast cancer, and reduced risk of distant recurrence (15). Regarding lung cancer, some studies have shown that BBs use is associated with prolonged OS and PFS in non-small cell lung cancer (NSCLC) patients receiving chemoradiotherapy, and may serve as an independent prognostic factor (16–18). In the context of ICIs treatment, the role of BBs in lung cancer remains limited and conflicting. Michael S. Oh et al. reported that BBs use may be associated with improved PFS in NSCLC patients receiving ICIs, but no association with OS (19). While other studies have suggested the use of BBs not associated with OS or PFS, albeit with trends toward improved ORR (11, 20). In contrast, Leshem et al. reported that the use of β1-selective blockers was associated with shorter OS and PFS in NSCLC (21). However, most existing studies are single-center retrospective analyses with limited sample sizes, and their conclusions remain to be further validated.
Given the widespread use of BBs in clinical oncology and their potential immunomodulatory effects, we hypothesized that inhibition of β-adrenergic signaling may enhance the efficacy of immunotherapy in lung cancer. Therefore, we conducted this multicenter real-world retrospective study to investigate the impact of BBs use on the prognosis of advanced lung cancer patients receiving immunotherapy.
2 Materials and methods
2.1 Study population
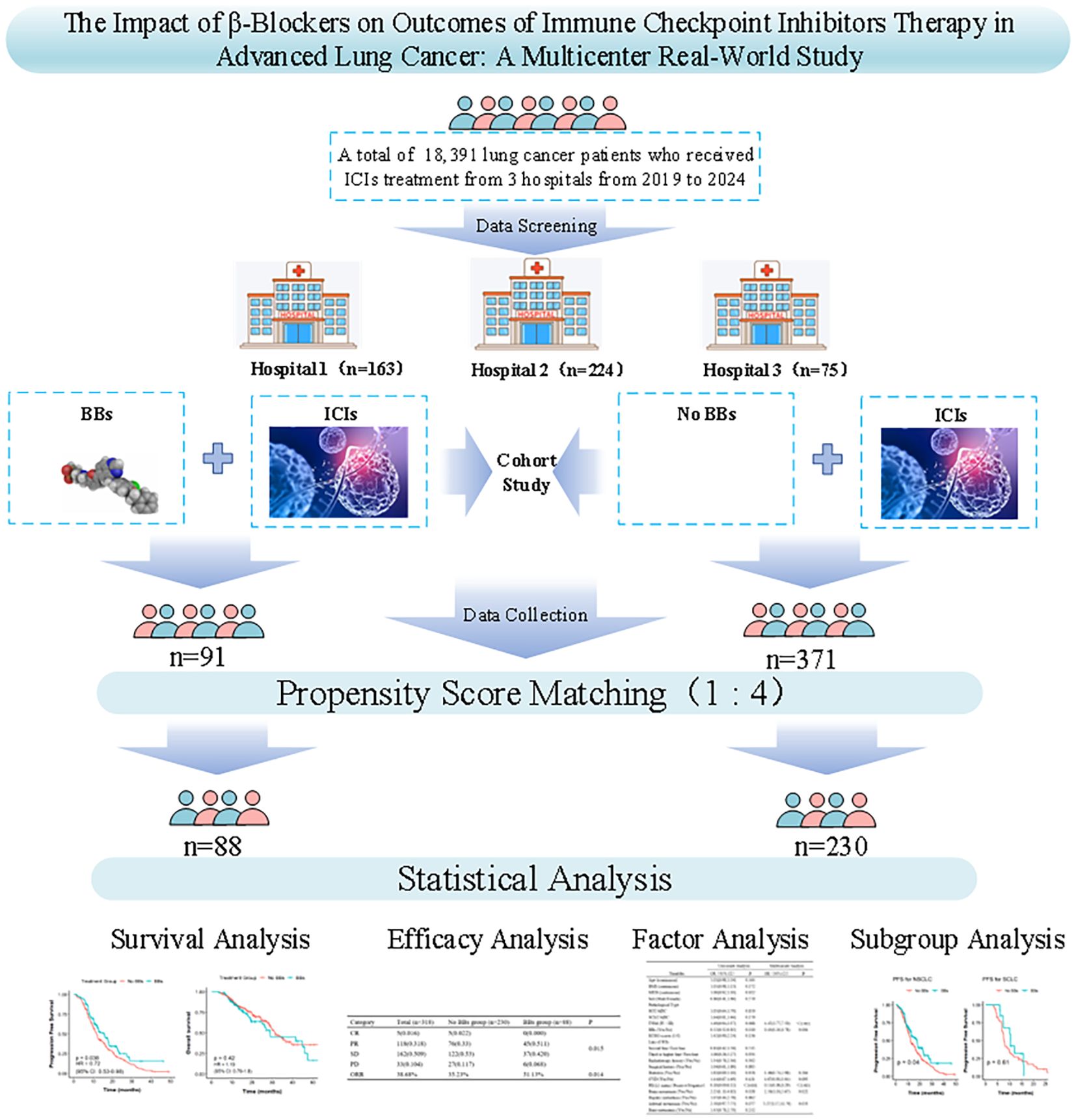
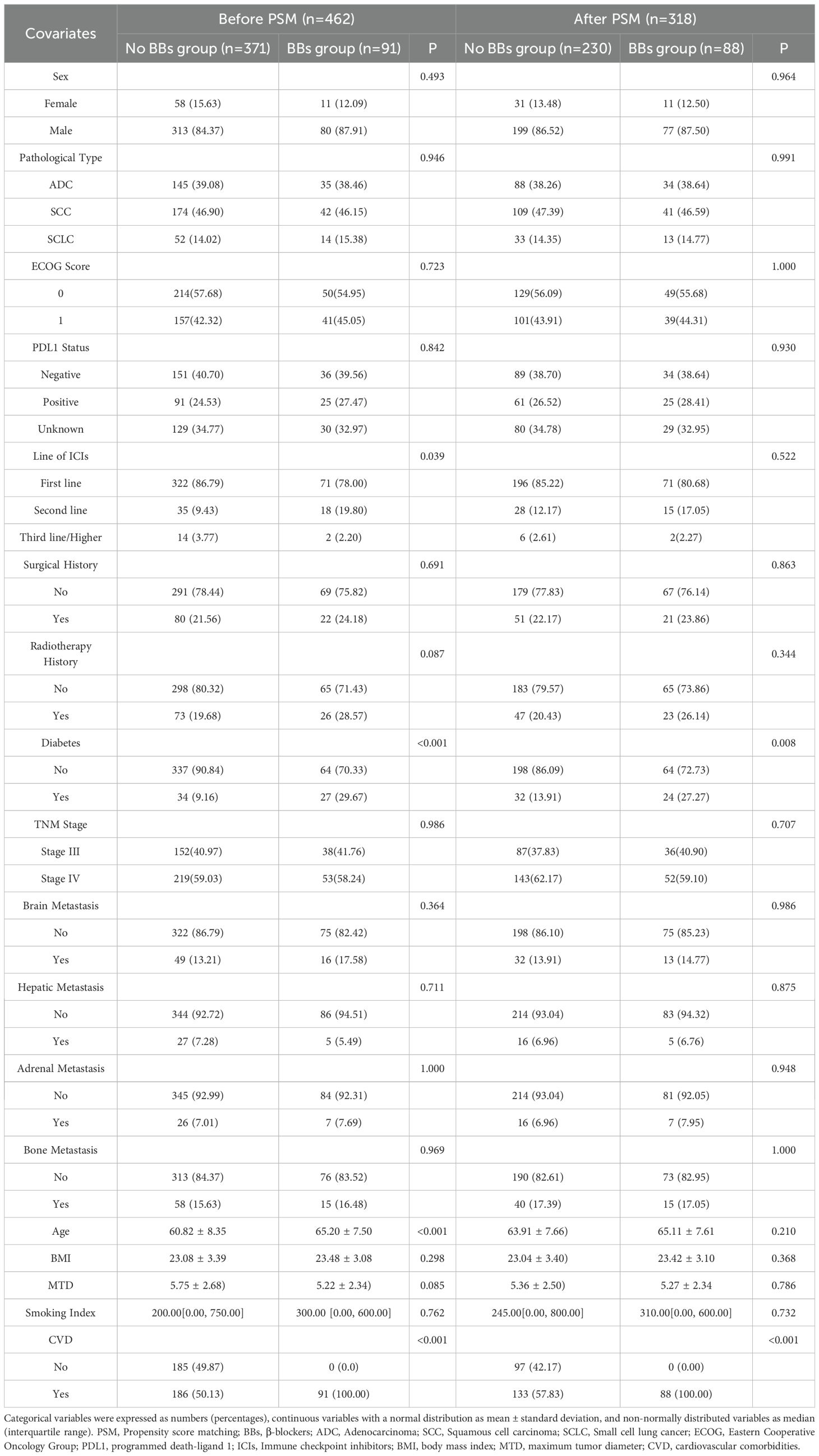
This retrospective study included 462 patients with stage III-IV lung cancer who received ICIs treatment at the Second Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, and Yan an Hospital of Kunming city from June 2019 to December 2024. The No BBs group consisted of lung cancer patients who did not use BBs but received at least 2 cycles of ICIs. The BBs group included those who received both BBs and ICIs for at least two cycles. Both groups of patients received other treatments in accordance with clinical guidelines. This study complies with the Helsinki Declaration (22) and has been reviewed by the ethics committees of the three hospitals. The ethics approval numbers are: Shen-PJ-Ke-2024-23, KYLX2024-217, and 2024-232-01, respectively.
2.2 Inclusion criteria
(1) Patients diagnosed with lung cancer through pathological and imaging examinations; (2) Patients classified as stage III-IV lung cancer according to the AJCC 8th edition of the TNM staging criteria (23); (3) Patients who received at least two cycles of single-agent or combination ICIs therapy after diagnosis; (4) Age ≥ 18 years, with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-1 (24) (5); According to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) version (25, 26), there is at least one measurable lesion; (7) No second primary tumors; (8) Complete clinical and follow-up data of the patients are available.
2.3 Exclusion criteria
(1) An expected survival of less than 3 months; (2) ECOG score ≥ 2; (3) Use of ICIs and BBs for less than 2 treatment cycles; (4) Presence of immunotherapy contraindications; (5) No measurable lesions; (6) Patients for whom treatment efficacy evaluation is impossible; (7) Patients with incomplete or unavailable data.
2.4 Data collection
Clinical data collected included: demographic characteristics (age and gender), body mass index (BMI), smoking index (pack-years), history of CVD (e.g., coronary artery disease and hypertension), and history of diabetes. Collect the tumor-related data, including surgical and radiotherapy history, pathological type, stage, size, metastatic status, baseline treatment situation, pathological and imaging examinations, immunohistochemical features, and ECOG score etc.
2.5 Study endpoints
2.5.1 Primary endpoints
OS and PFS. OS was defined as the time from treatment initiation to the date of death from any cause. PFS was defined as the time from treatment initiation to the first documented disease progression or death from any cause, whichever occurred first.
2.5.2 Secondary endpoints
Efficacy evaluation and ORR. According to RECIST 1.1, treatment response was defined as: 1) Complete Response (CR): Disappearance of all target lesions, with all pathological lymph nodes reduced to a short axis of <10 mm, sustained for at least 4 weeks. 2) Partial Response (PR): At least a 30% decrease in the sum of the diameters of target lesions, sustained for at least 4 weeks. 3) Progressive Disease (PD): At least a 20% increase in the sum of the diameters of target lesions, or the appearance of new lesions. 4) Stable Disease (SD): The target lesion neither shows sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. ORR: was defined as the proportion of patients achieving CR or PR. In this study, a favorable efficacy evaluation was defined as the CR or PR state. An unfavorable efficacy evaluation was defined as the SD or PD state.
2.6 Statistical analyses
Descriptive statistics were performed for categorical and continuous variables. Categorical variables were expressed as numbers (percentages), continuous variables with a normal distribution as mean ± standard deviation, and non-normally distributed variables as median (interquartile range, IQR). Between-group comparisons used the independent-samples t-test or Wilcoxon rank-sum test for continuous variables, and the chi-square or Fisher’s exact test for categorical variables. PSM was conducted with a caliper of 0.2 times the standard deviation of the propensity score, using nearest-neighbor matching at a 1:4 ratio to maximize baseline comparability between groups. PFS and OS were estimated by the Kaplan-Meier method and compared using the log-rank test. Binary logistic regression was used for univariate and multivariate analyses of CR + PR versus PD + SD, and Cox proportional hazards models for PFS and OS to identify independent prognostic factors. Variables with P < 0.1 in univariate analyses and important clinical factors were included in multivariate models. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 27.0 and R 4.5.1.
3 Results
3.1 Baseline characteristics of patients
A total of 462 lung cancer patients who received ICIs treatment were enrolled in this study, including 163 patients from the Second Affiliated Hospital of Kunming Medical University, 224 from Yunnan Cancer Hospital, and 75 from Yan an Hospital of Kunming city. Based on BBs use, 371 patients were in the No BBs group and 91 in the BBs group. Before PSM, the two groups were comparable in the gender, BMI, smoking index, ECOG score, PDL1 status, TNM stage, immunotherapy line, pathological stage of lung cancer, history of radiotherapy, surgical history, and metastasis sites (all P > 0.05). However, patients in the BBs group were older (65.20 vs 60.82 years, P < 0.001), had a higher proportion of diabetes (P < 0.001), and all had CVD. At the same time, the distribution of treatment lines differed significantly between groups (P = 0.018), with the proportion of first-line treatment higher in the No BBs group (86.79% vs. 78.02%) and the proportion of second-line treatment higher in the BBs group (32.97% vs. 9.43%). To adjust for the above confounding factors, we used 1:4 PSM (covariates included: age, gender, BMI, smoking index, ECOG score, PDL1 status, TNM stage, diabetes, radiotherapy history, surgical history, ICIs treatment lines, maximum diameter of target lesion, metastasis site, etc.) to obtain 230 patients in the No BBs group and 88 in the BBs group. After PSM, baseline characteristics were balanced between groups except for history of CVD (P < 0.001) and diabetes (P = 0.008). Figure 1 provides the flowchart of the study, and Table 1 lists the clinical characteristics of the two groups before and after PSM.
3.2 Efficacy evaluation
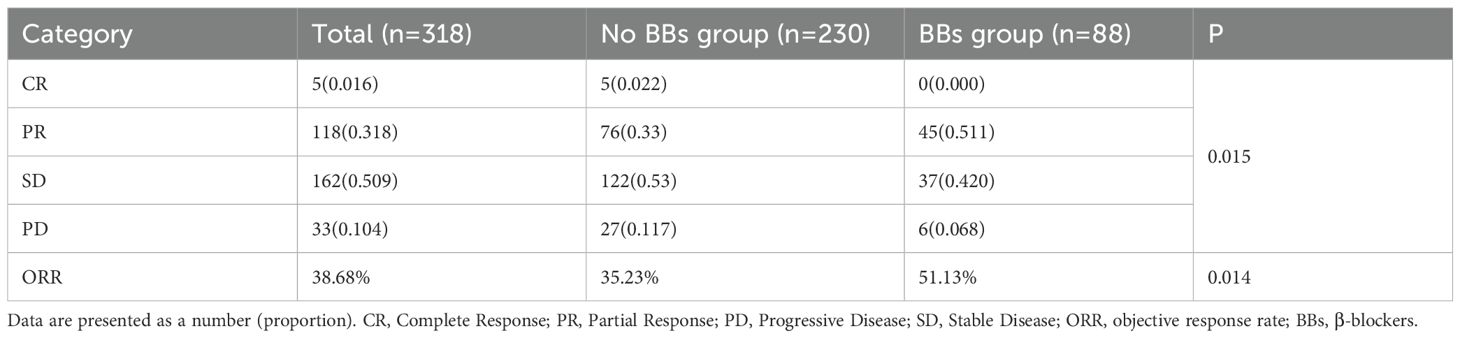
The best radiological response was evaluated according to RECIST 1.1. In the No BBs group, the proportions of CR, PD, PR, and SD were 2.2%, 11.7%, 33.0%, and 53.0%, respectively; in the BBs group, the corresponding proportions were 0%, 6.8%, 51.1%, and 42.0%. The distribution of response categories differed significantly between the two groups (P = 0.015). The ORR was 35.23% in the No BBs group and 51.13% in the BBs group, with a statistically significant difference (P = 0.014). These results suggest that BBs use may enhance the therapeutic response to ICIs in lung cancer patients and improve ORR (Table 2).
To control for confounding factors affecting ORR, univariate and multivariate analyses were performed using a binary logistic regression model comparing patients with CR + PR versus PD + SD (Supplementary Table S1). Univariate analysis showed that brain metastasis was significantly associated with poor response (PD+SD) (OR = 2.25, 95% CI: 1.13–4.82, P = 0.028), suggesting it as a negative factor for ORR. Conversely, BBs use (OR = 0.52, 95% CI: 0.32–0.85, P = 0.010) and positive PD-L1 expression (OR = 0.18, 95% CI: 0.09–0.32, P < 0.001) were correlated with higher ORR, indicating protective effects. Multivariate analysis further identified brain metastasis (OR = 2.50, 95% CI: 1.18–5.60, P = 0.022), stage IV TNM (vs. stage III, OR = 4.47, 95% CI: 2.77–7.50, P < 0.001), and adrenal metastasis (OR = 3.27, 95% CI: 1.17–10.76, P = 0.033) as independent risk factors for poor response. Meanwhile, BBs use (OR = 0.45, 95% CI: 0.26–0.78, P = 0.004) and PD-L1 positivity (OR = 0.15, 95% CI: 0.08–0.29, P < 0.001) remained independent protective factors reducing the risk of poor response after adjusting for multiple covariates.
3.3 PFS
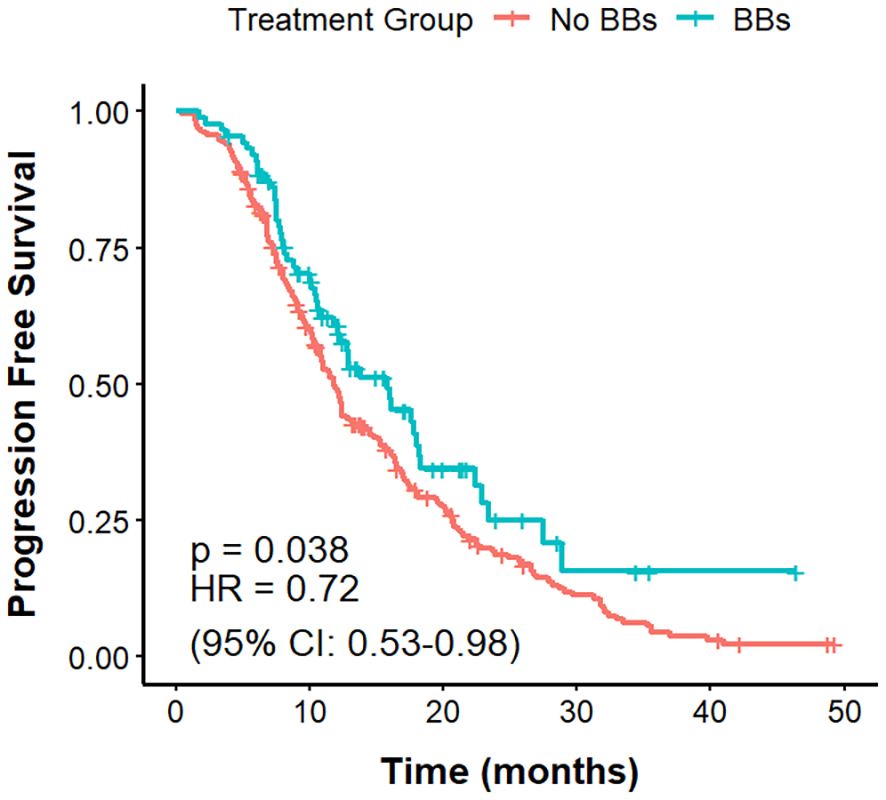
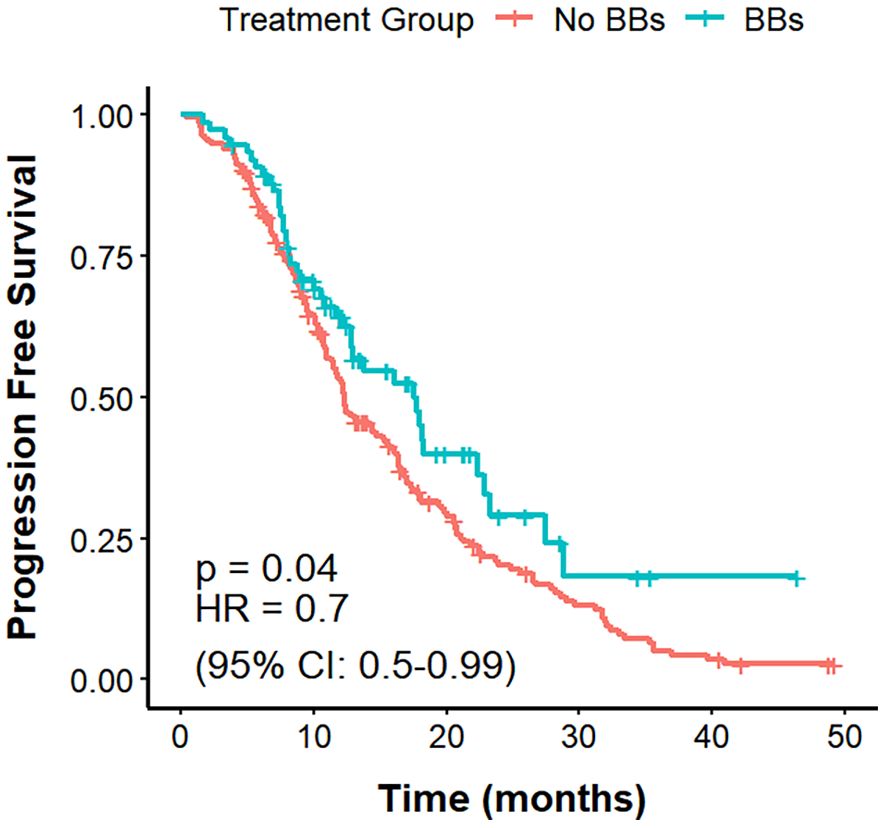
The mPFS was 11.8 months in the No BBs cohort and 15.8 months in the BBs cohort (Figure 2). The difference in PFS between the two groups was statistically significant (P = 0.038, HR = 0.72, 95% CI: 0.53–0.98).
Cox proportional hazards models were used to evaluate prognostic factors associated with PFS (Supplementary Table S2). In univariate analysis, small cell lung cancer (SCLC) histology (HR = 1.83, 95% CI: 1.26–2.66, P = 0.002), higher ECOG performance status (HR = 1.59, 95% CI: 1.24–2.05, P < 0.001), and brain metastases (HR = 1.61, 95% CI: 1.14–2.27, P = 0.007) were significantly associated with shorter PFS, whereas BBs use (HR = 0.72, 95% CI: 0.53–0.98, P = 0.039) and PD-L1 positivity (HR = 0.41, 95% CI: 0.29–0.56, P < 0.001) were linked to longer PFS. Multivariate analysis, adjusting for variables with P < 0.1 in univariate analysis and key clinical factors, confirmed SCLC histology (HR = 3.08, 95% CI: 2.05–4.62, P < 0.001), ECOG score (HR = 1.66, 95% CI: 1.28–2.15, P < 0.001), brain metastases (HR = 1.45, 95% CI: 1.02–2.08, P = 0.041), adrenal metastases (HR = 1.87, 95% CI: 1.15–3.04, P = 0.012), and bone metastases (HR = 1.51, 95% CI: 1.06–2.15, P = 0.022) as independent predictors of shorter PFS. BBs use (HR = 0.67, 95% CI: 0.49–0.92, P = 0.014) and PD-L1 positivity (HR = 0.32, 95% CI: 0.23–0.46, P < 0.001) remained significant protective factors, with stronger effect sizes after adjustment.
3.4 OS
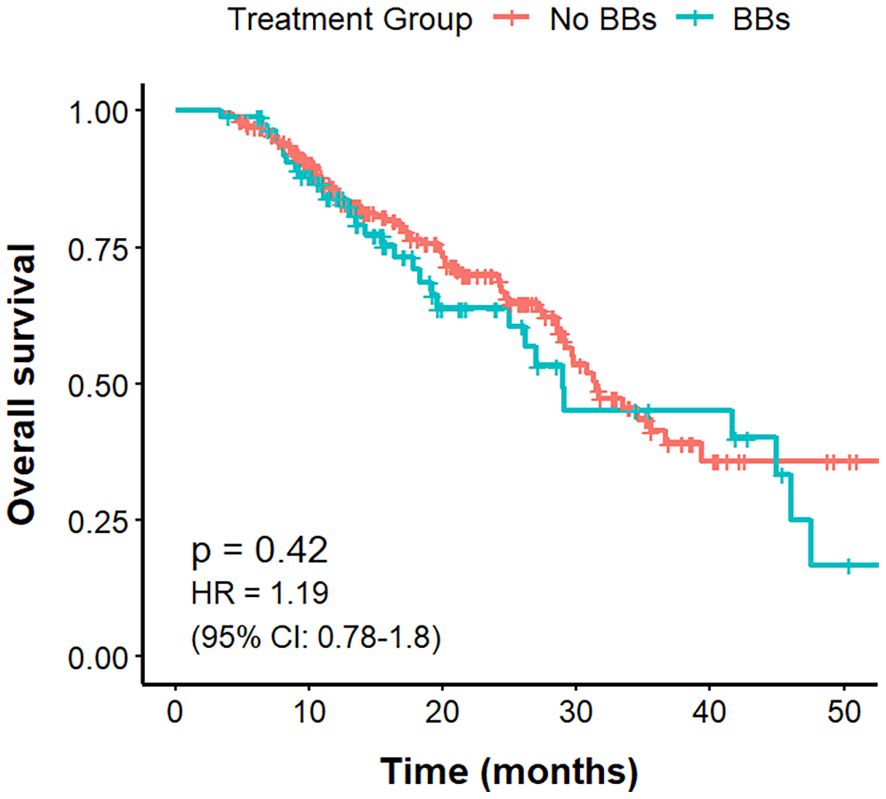
The mOS was 31.5 months in the No BBs cohort and 29.0 months in the BBs cohort, with no statistically significant difference between the groups (P = 0.43, HR = 1.19, 95% CI: 0.78–1.79; Figure 3).
Univariate and multivariate Cox regression analyses were performed to identify prognostic factors for OS (Supplementary Table S3). In the univariate analysis, brain metastases (HR = 1.70, 95% CI: 1.02–2.83, P = 0.040) and bone metastases (HR = 1.63, 95% CI: 1.02–2.60, P = 0.043) were significantly associated with shorter OS, whereas PD-L1 positive expression (HR = 0.53, 95% CI: 0.33–0.86, P = 0.011) was associated with longer OS, suggesting a protective effect. The multivariate model incorporated variables with P < 0.1 from the univariate analysis, and other clinically relevant covariates, revealed SCLC histology (HR = 2.01, 95% CI: 1.08–3.77, P = 0.029) emerged as an independent adverse prognostic factor for OS, while PD-L1 positive expression (HR = 0.74, 95% CI: 0.57–0.95, P = 0.017) remained an independent protective factor. BBs use showed no significant impact on OS (HR = 0.88, 95% CI: 0.65–1.18, P = 0.38).
3.5 Subgroup analyses
3.5.1 PFS, OS, and ORR by CVD and BBs status
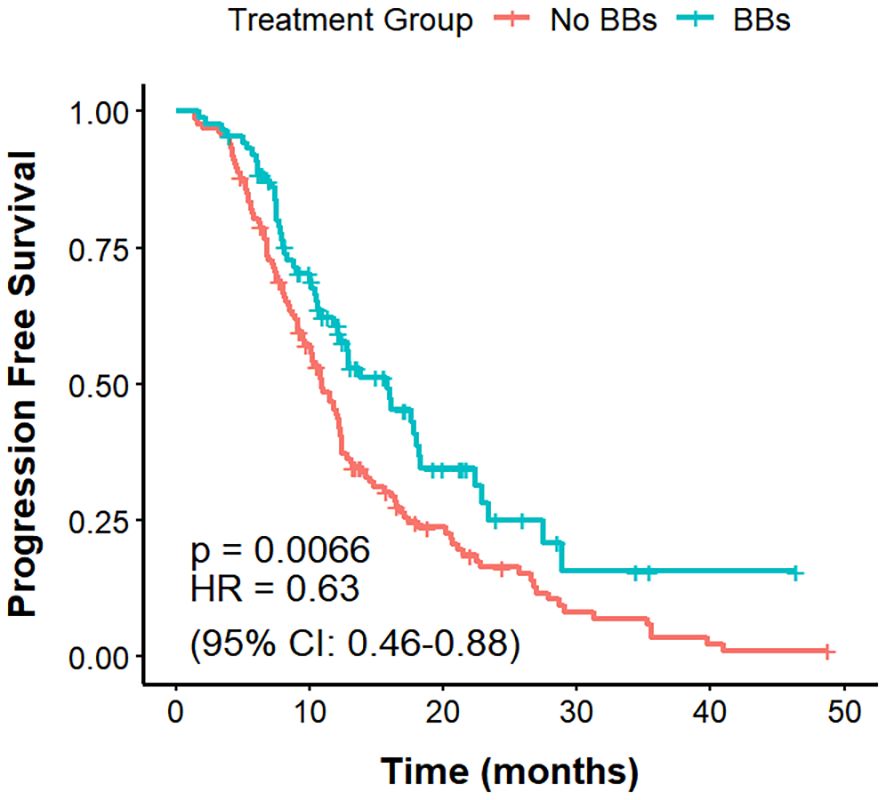
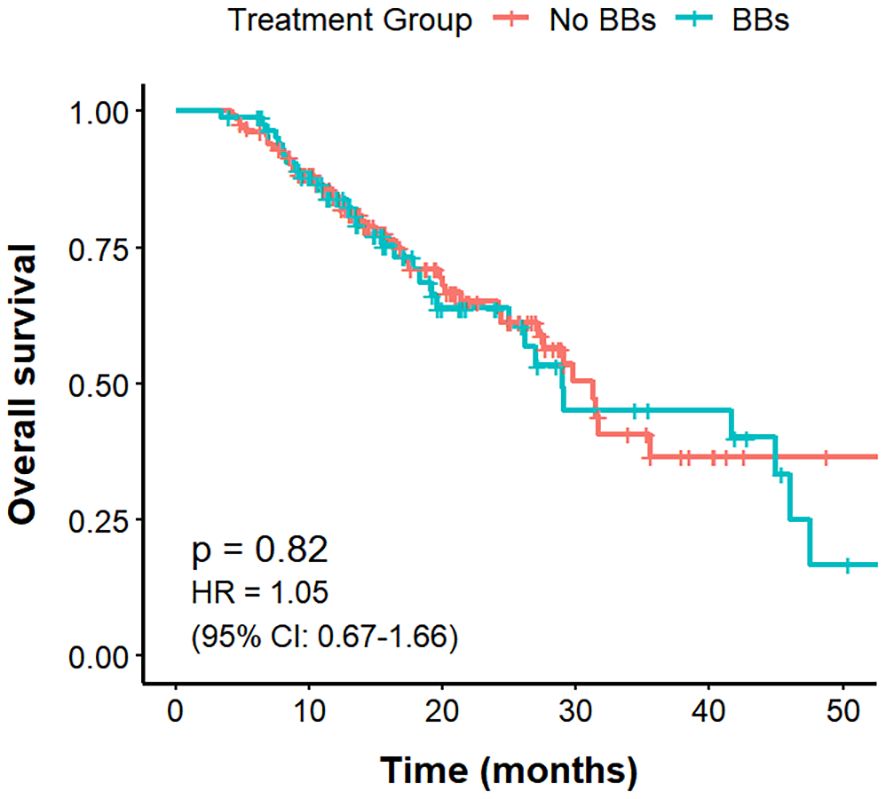
Among the 221 lung cancer patients with CVD, those in the No BBs group (n = 133) had a mPFS of 10.9 months, compared with 15.8 months in the BBs group (n = 88), with a clear difference (P = 0.0066; HR = 0.63, 95% CI: 0.46–0.88). The mOS was 31.2 months in the No BBs group and 29 months in the BBs group, showing no meaningful difference (P = 0.82). The ORR was significantly higher in the BBs group compared with the No BBs group (51.1% vs 27.0%, P<0.001). These findings indicate that, in lung cancer patients with coexisting CVD, treated with BBs exhibit a better short-term efficacy than those without BBs; however, no significant impact on OS was observed (Figures 4, 5, Table 3).
In the No BBs cohort (n=230), patients with CVD (No BBs + CVD, n=133) and those without CVD (No BBs + No CVD, n=97) had mPFS of 10.9 months and 15.3 months, respectively, with a borderline statistical significance (P = 0.051). The mOS was 31.2 months versus 34.6 months, respectively, with no significant difference (P = 0.234). The ORR was significantly higher in the No BBs + No CVD group compared with the No BBs + CVD group (46.4% vs. 27.1%, P = 0.004). These results suggest that some patients with CVD in the no BBs group may be receiving specific treatments that exclude beta-blockers, potentially reducing short-term efficacy (primarily ORR), but have no significant effect on long-term survival (Supplementary Tables S4, S5, Supplementary Figures S1, S2).
Further comparison among three groups—BBs + CVD, No BBs + CVD, and No BBs + No CVD — showed that PFS, OS, and ORR were comparable between the BBs + CVD group and the No BBs + No CVD group. These findings indicate that BBs was associated with improved short-term efficacy of ICIs in lung cancer patients with CVD, bringing their outcomes closer to those of patients without CVD (Supplementary TableS4, Supplementary Figures S3, S4).
3.5.2 PFS, OS, and ORR in different histologies
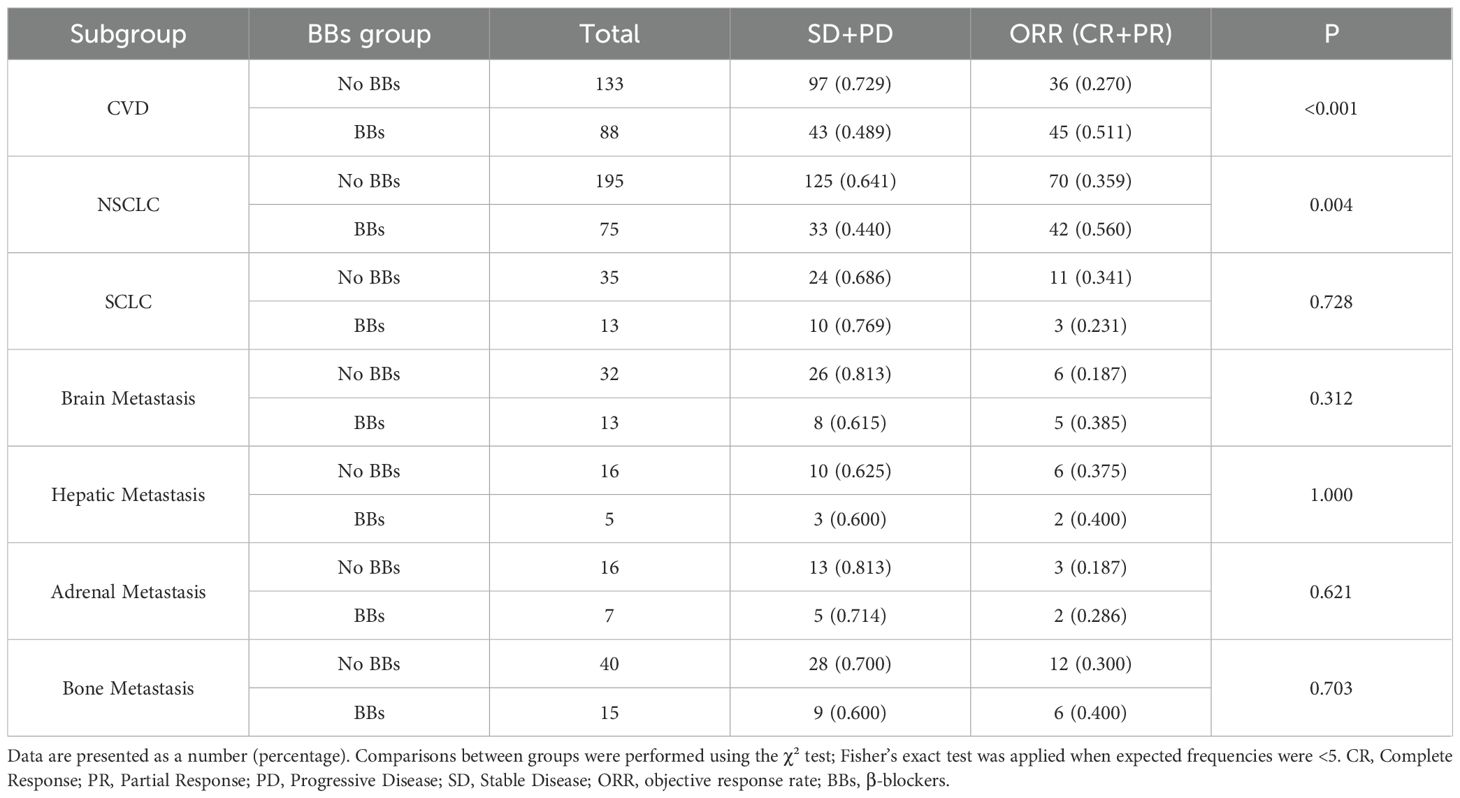
By pathological type, among 270 patients with NSCLC, the BBs group (n = 75) had a longer mPFS compared with the No BBs group (n = 195) (17.5 vs. 12.3 months; P = 0.04, HR = 0.70, 95% CI: 0.40–0.99). However, OS did not differ significantly between the two groups (29.0 vs. 33.5 months; P = 0.30). ORR in the BBs group was notably higher than that in the No BBs group (56.0% vs. 35.9%; P = 0.004). These findings indicate that BBs use in NSCLC is associated with improved PFS but has no evident impact on OS (Figures 6, 7). In 48 patients with SCLC, the mPFS was 7.7 months in the No BBs group (n = 35) and 10.4 months in the BBs group (n = 13), with no statistical difference observed (P = 0.61). The mOS was 30.8 months in the No BBs group and 41.7 months in the BBs group, also without a significant difference (P = 0.56). The ORR likewise showed no difference between the two groups (23.1% vs. 34.1%; P = 0.728). These results suggest a potential clinical benefit of BBs in SCLC, though confirmation in larger cohorts is warranted due to the limited sample size (Supplementary Figures S5, S6, Table 3).
3.5.3 PFS, OS, and ORR by distant metastasis sites
In subgroup analyses by metastatic site, BBs use did not demonstrate a clear survival and efficacy advantage. Among 45 patients with brain metastases, the BBs group (n = 13) had an mPFS of 8.8 months versus 9.4 months in the No BBs group (n = 32) (P = 0.43); mOS was 19.5 vs. 29.2 months, respectively (P = 0.43). In 21 patients with liver metastases, the BBs group (n = 5) was too small to reach mPFS or mOS, whereas the No BBs group (n = 16) had an mPFS of 9.1 months (P = 0.48) and an mOS of 29.8 months (P = 0.39). Among 23 patients with adrenal metastases, the mPFS was 10.4 months in the BBs group (n = 7) and 8.4 months in the No BBs group (n = 16) (P = 0.46). The No BBs group had an mOS of 39.4 months, while mOS was not reached in the BBs group due to insufficient events (P = 0.64). In 55 patients with bone metastases, the BBs group (n = 15) had an mPFS of 15.8 months compared with 8.8 months in the No BBs group (n = 40) (P = 0.35); mOS was 29 months in both groups (P = 0.99). The ORR did not differ significantly between the BBs and No BBs groups in any of these metastatic subgroups (all P > 0.05). Given the small sample sizes in these subgroups, these findings should be interpreted with caution (Supplementary Figure S7, Table 3).
4 Discussion
This study investigated the association between BBs and the clinical outcomes of ICIs in patients with advanced lung cancer. We found that in patients treated with ICIs, BBs use was linked to longer PFS and higher ORR, but showed no effect on OS. This finding is consistent with the results reported by Michael S. Oh et al., who suggested that BBs could extend PFS (19), and aligns with reports in other cancers, such as melanoma, renal cell carcinoma, urothelial carcinoma, and breast cancer, where BBs have been suggested to enhance ICIs efficacy (12, 13, 27), suggesting a potential immune-sensitizing effect of BBs. Unlike most previous single-center, small-sample retrospective studies, our study was based on multicenter real-world data with a larger sample size, and applied PSM and multivariable regression analyses to minimize confounding, providing more robust evidence. Duarte Mendes et al. also observed a favorable trend of BBs use in ICIs-treated lung cancer patients, but it was not statistically significant (11); our larger sample and rigorous methodology likely yield more reliable results.
β-adrenergic receptors (β-AR) are widely expressed in tumor cells, immune cells, and vascular endothelial cells. They promote an immunosuppressive microenvironment by activating multiple pathways, including cAMP/PKA, MAPK, and PI3K/AKT, and can induce exhaustion of CD8+ T cells (28–31). For example, β-AR activation increases intracellular cAMP levels, which activate protein kinase A (PKA). PKA phosphorylates key signaling molecules, disrupting T cell receptor-mediated signaling and inhibiting CD8+ T cell activation, proliferation, glucose uptake, and glycolysis (32). BBs can reverse stress-induced immunosuppression by blocking β-AR signaling, restoring CD8+ T cell antitumor activity (33). In animal models, combining propranolol with anti-PD-1 therapy enhances tumor control (34). Our findings in lung cancer patients provide strong support for an independent association between BBs use and improved ORR and PFS.
Our study is a multicenter retrospective analysis of 462 patients with advanced lung cancer from three hospitals. In the unmatched original cohort, the BBs group exhibited more unfavorable baseline characteristics, including older age, a higher prevalence of diabetes, and universal presence of underlying CVD, indicating a generally poorer health status and greater comorbidity burden. Furthermore, the BBs group had a lower proportion of patients receiving ICIs as first-line therapy, while previous studies have shown ICIs to be more effective in the first-line setting (35, 36). These factors may have introduced bias favoring the No BBs group. Therefore, to control for these potential confounding factors, PSM was performed, resulting in 230 patients in the No BBs group and 88 patients in the BBs group. After matching, baseline characteristics were comparable between the two groups except for differences in history of CVD (P < 0.001) and diabetes (P = 0.008). Notably, the imbalance in CVD stems from the clinical indications for BBs, which are primarily prescribed for hypertension, arrhythmia, heart failure, and other cardiovascular conditions (10). Thus, this imbalance reflects clinical practice realities rather than a methodological flaw. However, in subsequent multivariate Cox and logistic regression analyses, diabetes and CVD were not identified as independent prognostic factors for PFS, OS, or ORR, suggesting a limited impact of these differences on the primary outcomes.
Using logistic regression and Cox proportional hazards models, we identified independent risk and protective factors for ORR, PFS, and OS in lung cancer patients receiving ICIs therapy, which may provide valuable guidance for treatment and prognosis. Regarding efficacy, the BBs group showed a higher ORR than the No BBs group (51.13% vs. 35.23%, P = 0.014), indicating a reduced risk of disease stabilization or progression. Multivariate logistic regression analysis confirmed BBs use as an independent protective factor for ORR (OR = 0.45, 95% CI: 0.26–0.78, P = 0.004). Similarly, the Cox proportional hazards model indicated a protective effect of BBs on PFS (HR = 0.67, 95% CI: 0.49–0.92, P = 0.014), but the association with OS did not reach statistical significance (HR = 0.82, 95% CI: 0.56–1.21, P = 0.32). These results suggest that BBs may enhance early treatment response by modulating sympathetic nervous system activity and improving the immune microenvironment, while their long-term survival benefit may be attenuated by other confounding factors (37). PD-L1 positivity emerged an independent protective factor for ORR, PFS, and OS, consistent with the current consensus in immunotherapy research (38, 39), underscoring its reliability and clinical value as a biomarker and the robustness of our data. In addition, our study found that an elevated ECOG score was an independent risk factor for PFS, with higher scores associated with reduced benefit. ECOG is a recognized prognostic factor for PFS across cancers, patients with poor ECOG scores often receive less intensive treatment, which may affect cancer control and survival. Those in poorer overall health also tend to have more comorbidities and healthcare-related issues; thus, the observed close association between ECOG status and PFS is not surprising (40–42). SCLC histology was also confirmed as an independent risk factor for both PFS and OS, consistent with its aggressive biology and poor prognosis. Literature reports a 5-year survival rate below 7% in SCLC, and its rapid progression, early metastasis, and relatively low response rate to immunotherapy make it one of the lung cancer subtypes with the poorest prognosis (43, 44). Meanwhile, the presence of distant metastases further highlights the complexity of disease progression: brain metastasis was identified as an independent risk factor for both ORR and PFS, while adrenal metastasis and TNM stage IV were independent risk factors for ORR. These findings suggest that distant metastases not only indicate extensive disease spread and aggressive tumor biology but may also be linked to high tumor burden, a strongly immunosuppressive microenvironment, or limited drug penetration (45–47).
This study further explored the prognostic differences of BBs use among patients with varying clinical characteristics through subgroup analysis. Among lung cancer patients with comorbid CVD, BBs use was associated with prolonged mPFS (15.8 vs. 10.9 months; P = 0.0066) and an increased ORR (51.1% vs. 27.0%; P <0.001) relative to the No BBs group, while no difference was observed in OS. Within the No BBs group, patients with CVD had slightly lower ORR than those without CVD, but PFS and OS were comparable. Comparison between the BBs group and the No BBs + No CVD group revealed no significant differences in PFS, OS, or ORR. These findings suggest that some patients with CVD in the no BBs group may be receiving specific treatments that exclude beta-blockers, potentially reducing short-term efficacy. Meanwhile, BBs may influence tumor progression and mitigate CVD complications (17). Relevant preclinical studies indicate that chronic stress associated with CVD leads to sustained activation of adrenergic signaling, which reduces immune cell infiltration in the tumor microenvironment and suppresses antitumor immunity and tumor cell apoptosis (48, 49); and BBs can antagonize these processes. BBs may partly contribute to improved short-term efficacy in patients with CVD, but further studies are needed to confirm whether this reflects a true synergistic effect with ICIs or reduced efficacy in CVD patients not receiving BBs or other confounding variables.
Subgroup analysis by lung cancer histology showed that in NSCLC patients, those using BBs had a longer mPFS (17.5 vs. 12.3 months, P = 0.04) and a higher ORR (56.0% vs. 35.9%; P = 0.004) than the No BBs group, further supporting the potential role of BBs as ICIs sensitizers. In SCLC patients, BBs use showed trends toward prolonged PFS and OS (PFS: 10.4 vs. 7.7 months; OS: 41.7 vs. 30.8 months), but these differences were not statistically significant (P > 0.05). Given the small sample size in SCLC (n = 48) and limited statistical power, these findings warrant cautious interpretation. SCLC is a highly aggressive tumor characterized by high tumor mutational burden, but with low immune cell infiltration, reduced major histocompatibility complex expression, presence of immunosuppressive cells and cytokines, ischemic tumor regions, and rapid growth enabling immune evasion, all of which limit ICI efficacy (50–52). Real-world studies show that durable benefit from ICIs occurs only in a minority of SCLC patients (53). Our results provide preliminary evidence for further exploration of BBs in SCLC immunotherapy, warranting validation in larger cohorts. Additionally, subgroup analyses by sites of distant metastasis revealed no clinically meaningful differences, possibly due to limited sample sizes.
This study has several limitations. First, although PSM was applied, significant differences in the CVD and diabetes history remained between groups. More stringent matching would have led to substantial sample loss and reduced statistical power. To address residual confounding, multivariate Logistic and Cox regression analyses were performed for further adjustment. Second, as a retrospective study, unmeasured confounders such as BBs type, dosage, adherence, psychological stress, and inflammatory markers may still exist despite PSM and multivariable adjustments. Prospective cohort studies and preclinical experiments are needed to confirm these findings. Third, due to a lack of data on lung cancer patients using BBs for non-CVD reasons, subgroup analyses in patients without CVD were not conducted; future studies should include this population. Finally, the relatively small sample size in the BBs group, particularly in subgroup analyses, limits statistical power and calls for larger studies to validate these results. Despite its limitations, the study based on multicenter real-world, provides a foundation for future prospective studies and supports further exploration of BBs as immune-modulating agents in clinical practice.
5 Conclusion
This multicenter real-world retrospective study found that among patients with stage III–IV lung cancer treated with ICIs, the use of BBs exhibited a higher ORR and longer PFS, but no improvement in OS. Subgroup analysis showed that BBs also improved PFS and ORR in patients with CVD or NSCLC. Multivariate analysis confirmed BBs as an independent protective factor for ORR and PFS. However, it is still uncertain whether these benefits are directly attributable to BBs or may be influenced by other factors, including concomitant CVD treatments and other confounding factors. Further prospective studies and clinical trials are needed to clarify the causal relationship and validate these results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of The Second Affiliated Hospital of Kunming Medical University, Ethics Committee of Yunnan Cancer Hospital, Ethics Committee of Kunming Yanan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LC: Writing – review & editing, Supervision. HZ: Writing – original draft, Writing – review & editing. YxL: Investigation, Data curation, Conceptualization, Writing – review & editing. JY: Data curation, Writing – review & editing, Resources. YL: Writing – review & editing, Data curation, Methodology. GW: Writing – review & editing, Validation, Formal analysis, Methodology, Visualization. JZ: Writing – review & editing, Supervision, Funding acquisition, Resources. HS: Writing – review & editing, Writing – original draft, Conceptualization, Validation, Visualization. BH: Writing – review & editing, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article.The author(s) declare that financial support was received for the 2023 Yunnan Revitalization Talent Support Program (Jinsong Zhang, Grant No: XDYC-YLWS-2023-0021) and 2024 Yunnan Revitalization Talent Support Program (Bing Hai, Grant No: XDYC-YLWS-2024-0023)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1693249/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Ribas A and Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
4. Li S, Zhang C, Wang X, Wang J, and Li K. Correlation of inflammatory markers with progression-free survival in advanced lung cancer patients treated with immune checkpoint inhibitors in combination with chemotherapy: a real-world study. J Thorac Dis. (2025) 17:3456–63. doi: 10.21037/jtd-2024-2198
5. Amezquita RA and Kaech SM. Immunology: The chronicles of T-cell exhaustion. Nature. (2017) 543:190–1. doi: 10.1038/nature21508
6. Li Q, Xu S, Ren Y, Zhang C, Li K, and Liu Y. Single-cell RNA sequencing reveals adrb1 as a sympathetic nerve-regulated immune checkpoint driving T cell exhaustion and impacting immunotherapy in esophageal squamous cell carcinoma. Front Immunol. (2025) 16:1520766. doi: 10.3389/fimmu.2025.1520766
7. Zhu J, Naulaerts S, Boudhan L, Martin M, Gatto L, and Van den Eynde BJ. Tumour immune rejection triggered by activation of α2-adrenergic receptors. Nature. (2023) 618:607–15. doi: 10.1038/s41586-023-06110-8
8. Globig AM, Zhao S, Roginsky J, Maltez VI, Guiza J, Avina-Ochoa N, et al. The β(1)-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature. (2023) 622:383–92. doi: 10.1038/s41586-023-06568-6
9. Caparica R, Bruzzone M, Agostinetto E, De Angelis C, Fêde Â, Ceppi M, et al. Beta-blockers in early-stage breast cancer: a systematic review and meta-analysis. ESMO Open. (2021) 6:100066. doi: 10.1016/j.esmoop.2021.100066
10. DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D’Ascenzo F, Cerrato E, et al. β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. (2015) 2:e000230. doi: 10.1136/openhrt-2014-000230
11. Duarte Mendes A, Freitas AR, Vicente R, Ferreira R, Martins T, Ramos MJ, et al. Beta-adrenergic blockade in advanced non-small cell lung cancer patients receiving immunotherapy: A multicentric study. Cureus. (2024) 16:e52194. doi: 10.7759/cureus.52194
12. Mellgard G, Patel VG, Zhong X, Joshi H, Qin Q, Wang B, et al. Effect of concurrent beta-blocker use in patients receiving immune checkpoint inhibitors for advanced solid tumors. J Cancer Res Clin Oncol. (2023) 149:2833–41. doi: 10.1007/s00432-022-04159-y
13. Sharma AE, Chan S, Komorowski AS, Cao X, Gao Y, Kshatri K, et al. The impact of beta blockers on survival in cancer patients: A systematic review and meta-analysis. Cancers (Basel). (2025) 17(8):1357. doi: 10.3390/cancers17081357
14. Wu YL, van Hyfte G, Özbek U, Reincke M, Gampa A, Mohamed YI, et al. Outcomes of beta blocker use in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Front Oncol. (2023) 13:1128569. doi: 10.3389/fonc.2023.1128569
15. Scott OW, Tin Tin S, and Botteri E. Beta blocker use and breast cancer survival by subtypes: A population-based cohort study. N/A. (2025) 81:104474. doi: 10.1016/j.breast.2025.104474
16. Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. (2013) 24:1312–9. doi: 10.1093/annonc/mds616
17. Liang X, Li P, Qin Y, Mo Y, and Chen D. Beta-adrenergic receptor blockers improve survival in patients with advanced non-small cell lung cancer combined with hypertension undergoing radiotherapy. Sci Rep. (2025) 15:10702. doi: 10.1038/s41598-025-93205-z
18. Zaborowska-Szmit M, Szmit S, Olszyna-Serementa M, Badurak P, Zajda K, Janowicz-Żebrowska A, et al. Beta blockers with statins may decrease all-cause mortality in patients with cardiovascular diseases and locally advanced unresectable non-small-cell lung cancer after chemoradiotherapy. Cancers (Basel). (2023) 15(4):1277. doi: 10.3390/cancers15041277
19. Oh MS, Guzner A, Wainwright DA, Mohindra NA, Chae YK, Behdad A, et al. The impact of beta blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. (2021) 22:e57–62. doi: 10.1016/j.cllc.2020.07.016
20. Yan X, Liu P, Li D, Hu R, Tao M, Zhu S, et al. Novel evidence for the prognostic impact of β-blockers in solid cancer patients receiving immune checkpoint inhibitors. Int Immunopharmacol. (2022) 113:109383. doi: 10.1016/j.intimp.2022.109383
21. Leshem Y, Etan T, Dolev Y, Nikolaevski-Berlin A, Miodovnik M, Shamai S, et al. The prognostic value of beta-1 blockers in patients with non-small-cell lung carcinoma treated with pembrolizumab. Int J Cardiol. (2024) 397:131642. doi: 10.1016/j.ijcard.2023.131642
22. World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human participants. JAMA. (2025) 333:71–4. doi: 10.1001/jama.2024.21972
23. Lababede O and Meziane MA. The eighth edition of TNM staging of lung cancer: reference chart and diagrams. Oncologist. (2018) 23:844–8. doi: 10.1634/theoncologist.2017-0659
24. Datta SS, Ghosal N, Daruvala R, Chakraborty S, Shrimali RK, van Zanten C, et al. How do clinicians rate patient’s performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience. (2019) 13:913. doi: 10.3332/ecancer.2019.913
25. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. (2017) 18:e143–e52. doi: 10.1016/S1470-2045(17)30074-8
26. Persigehl T, Lennartz S, and Schwartz LH. iRECIST: how to do it. Cancer Imag. (2020) 20:2. doi: 10.1186/s40644-019-0281-x
27. Fiala O, Ostašov P, Rozsypalová A, Hora M, Šorejs O, Šustr J, et al. Impact of concomitant cardiovascular medication on survival of metastatic renal cell carcinoma patients treated with sunitinib or pazopanib in the first line. Target Oncol. (2021) 16:643–52. doi: 10.1007/s11523-021-00829-y
28. Baker FL, Bigley AB, Agha NH, Pedlar CR, O’Connor DP, Bond RA, et al. Systemic β-Adrenergic Receptor Activation Augments the ex vivo Expansion and Anti-Tumor Activity of Vγ9Vδ2 T-Cells. Front Immunol. (2019) 10:3082. doi: 10.3389/fimmu.2019.03082
29. Qiao G, Chen M, Mohammadpour H, MacDonald CR, Bucsek MJ, Hylander BL, et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol Res. (2021) 9:651–64. doi: 10.1158/2326-6066.CIR-20-0445
30. Li W, Wan J, Chen C, Zhou C, Liao P, Hu Q, et al. Dissecting the role of cell signaling versus CD8(+) T cell modulation in propranolol antitumor activity. J Mol Med (Berl). (2022) 100:1299–306. doi: 10.1007/s00109-022-02238-8
31. Liao P, Song K, Zhu Z, Liu Z, Zhang W, Li W, et al. Propranolol suppresses the growth of colorectal cancer through simultaneously activating autologous CD8(+) T cells and inhibiting tumor AKT/MAPK pathway. N/A. (2020) 108:606–15. doi: 10.1002/cpt.1894
32. Qiao G, Bucsek MJ, Winder NM, Chen M, Giridharan T, Olejniczak SH, et al. β-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. N/A. (2019) 68:11–22. doi: 10.1007/s00262-018-2243-8
33. Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, et al. Blockade of β-adrenergic receptors improves CD8(+) T-cell priming and cancer vaccine efficacy. Cancer Immunol Res. (2019) 7:1849–63. doi: 10.1158/2326-6066.CIR-18-0833
34. Gandhi S, Pandey MR, Attwood K, Ji W, Witkiewicz AK, Knudsen ES, et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. (2021) 27:87–95. doi: 10.1158/1078-0432.CCR-20-2381
35. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
36. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2019) 37:537–46. doi: 10.1200/JCO.18.00149
37. Massalee R and Cao X. Repurposing beta-blockers for combinatory cancer treatment: effects on conventional and immune therapies. Front Pharmacol. (2023) 14:1325050. doi: 10.3389/fphar.2023.1325050
38. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
39. Majem M, Cobo M, Isla D, Marquez-Medina D, Rodriguez-Abreu D, Casal-Rubio J, et al. PD-(L)1 inhibitors as monotherapy for the first-line treatment of non-small-cell lung cancer patients with high PD-L1 expression: A network meta-analysis. J Clin Med. (2021) 10(7):1365. doi: 10.3390/jcm10071365
40. Dall’Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, and Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. (2020) 145:95–104. doi: 10.1016/j.lungcan.2020.04.027
41. Krishnan M, Kasinath P, High R, Yu F, and Teply BA. Impact of performance status on response and survival among patients receiving checkpoint inhibitors for advanced solid tumors. JCO Oncol Pract. (2022) 18:e175–e82. doi: 10.1200/OP.20.01055
42. Yang TM, Chen IA, Yu CC, Yang CR, Chen WC, Lin PH, et al. Preoperative ECOG performance status as a predictor of outcomes in upper tract urothelial cancer surgery. Sci Rep. (2025) 15:10356. doi: 10.1038/s41598-025-95128-1
43. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. (2023) 73:620–52. doi: 10.3322/caac.21785
44. Zhou L, Li Y, Wang L, Chen K, Zhou S, Chen Y, et al. Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis. Ther Adv Med Oncol. (2025) 17:17588359251348310. doi: 10.1177/17588359251348310
45. Liu B, Chen J, and Luo M. Efficacy and safety of immune checkpoint inhibitors for brain metastases of non-small cell lung cancer: a systematic review and network meta-analysis. Front Oncol. (2025) 15:1513774. doi: 10.3389/fonc.2025.1513774
46. Zhang X, Gao H, Dang S, Dai L, and Zhang J. Extracranial metastasis sites correlate to the incidence risk of brain metastasis in stage IV non-small cell lung cancer: a population-based study. J Cancer Res Clin Oncol. (2023) 149:6293–301. doi: 10.1007/s00432-022-04548-3
47. Gutierrez M, Lam WS, Hellmann MD, Gubens MA, Aggarwal C, Tan DSW, et al. Biomarker-directed, pembrolizumab-based combination therapy in non-small cell lung cancer: phase 2 KEYNOTE-495/KeyImPaCT trial interim results. Nat Med. (2023) 29:1718–27. doi: 10.1038/s41591-023-02385-6
48. Zhang H, Yang Y, Cao Y, and Guan J. Effects of chronic stress on cancer development and the therapeutic prospects of adrenergic signaling regulation. BioMed Pharmacother. (2024) 175:116609. doi: 10.1016/j.biopha.2024.116609
49. Chen Y, Qian Y, Huang W, Zhang Y, Wu M, Cheng Y, et al. Chronic stress promotes tumor immune evasion via the suppression of MHC-I expression and the upregulation of PD-L1. Am J Cancer Res. (2022) 12:5286–99.
50. Nguyen EM, Taniguchi H, Chan JM, Zhan YA, Chen X, Qiu J, et al. Targeting lysine-specific demethylase 1 rescues major histocompatibility complex class I antigen presentation and overcomes programmed death-ligand 1 blockade resistance in SCLC. J Thorac Oncol. (2022) 17:1014–31. doi: 10.1016/j.jtho.2022.05.014
51. Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, et al. Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. (2021) 11:1952–69. doi: 10.1158/2159-8290.CD-20-0913
52. Ouyang W, Xu Z, Guan S, Hu Y, Gou X, Liu Z, et al. Advancement opportunities and endeavor of innovative targeted therapies for small cell lung cancer. Int J Biol Sci. (2025) 21:1322–41. doi: 10.7150/ijbs.105973
53. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. (2021) 39:619–30. doi: 10.1200/JCO.20.01055
Keywords: lung cancer, immunotherapy, immune checkpoint inhibitors, β-adrenergic signaling, β-blockers
Citation: Chang L, Zhang H, Li Y, Yang J, Li Y, Wang G, Zhang J, Shi H and Hai B (2025) The impact of β-blockers on outcomes of immune checkpoint inhibitors therapy in advanced lung cancer: a multicenter real-world study. Front. Immunol. 16:1693249. doi: 10.3389/fimmu.2025.1693249
Received: 26 August 2025; Accepted: 09 October 2025;
Published: 21 October 2025.
Edited by:
Italia Falcone, Regina Elena National Cancer Institute (IRCCS), ItalyReviewed by:
Alexa Meara, The Ohio State University, United StatesSalvatore Cortellino, Università degli Studi di Napoli Federico II, Italy
Copyright © 2025 Chang, Zhang, Li, Yang, Li, Wang, Zhang, Shi and Hai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Zhang, OTQ1OTMzMzkyempzQHNpbmEuY29t; Hongjin Shi, MjAxOTA3OTdAa21tdS5lZHUuY24=; Bing Hai, YmluZ2hhaTk5OUAxNjMuY29t
†These authors have contributed equally to this work
 Lingdan Chang1†
Lingdan Chang1† Jinsong Zhang
Jinsong Zhang Bing Hai
Bing Hai