- Department of Endocrinology and Metabolism, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
Diabetes mellitus is primarily categorized into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), which exhibit distinct pathogenic mechanisms. T1DM is characterized by an absolute deficiency of insulin secretion, predominantly resulting from the autoimmune-mediated destruction of pancreatic beta cells. In contrast, T2DM arises from a combination of insulin resistance in peripheral tissues and a compensatory insulin secretory response that ultimately becomes inadequate. The pathogenesis of diabetes mellitus is orchestrated through bidirectional crosstalk between autoimmune aggression and metabolic derangement. γδ T cells, innate-like lymphocytes bridging innate and adaptive immunity, play pivotal roles in tissue homeostasis, inflammation, and immunity through cytokine production and cytotoxicity. This review comprehensively examines the dual roles of γδ T cells across diabetes mellitus types. Furthermore, γδ T cells contribute to diabetic complications and are profoundly affected by the diabetic milieu, leading to defective anti-infection and anti-tumor immunity. We discuss emerging therapeutic strategies targeting γδ T cells or their effector pathways and highlight key knowledge gaps regarding subset-specific functions, dynamic changes during disease progression, and tissue-resident γδ T cell roles. Elucidating these mechanisms may provide a strong foundation for developing novel γδ T cell-based immunotherapies for diabetes mellitus and its complications.
1 Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent hyperglycemia. DM embodies a paradigm of autoimmune-metabolic crosstalk. Type 1 diabetes mellitus (T1DM) is defined by autoimmune destruction of pancreatic β-cells, while type 2 diabetes mellitus (T2DM) features metabolism-triggered autoinflammation. According to the 2021 Global Burden of Disease analysis, approximately 591 million individuals worldwide live with diabetes, a figure projected to reach 1.031 billion by 2050 (1). This escalating prevalence underscores DM’s status as a critical public health challenge. Suboptimal glycemic control predisposes patients to multi-system complications affecting ocular, renal, integumentary, and other organ systems (2, 3). Furthermore, DM significantly elevates mortality risks from infections and malignancies (4), with this systemic vulnerability linked to immune dysregulation. The innate immune system is involved in the pathogenesis of DM and its chronic complications (5). Within this context, γδ T cells emerge as unexplored arbiters of diabetic autoimmunity in T1DM and as drivers of obesity-related inflammation in T2DM. Serving as a bridge between innate and adaptive immunity (6), γδ T cells exert unique functions in tissue immunosurveillance and inflammatory modulation through major histocompatibility complex (MHC) -unrestricted activation and cytokine secretion, thereby regulating αβ T cells and other immune effectors (7). Notably, the diabetic milieu may reciprocally impair γδ T cell function.
As an endocrine-metabolic disease driven primarily by autoimmunity, γδ T cells may mediate either protective or destructive effects on pancreatic β-cells in T1DM. γδ T cells also critically interact with obesity-induced insulin resistance, which is the fundamental mechanism in T2DM development. Visceral adipose tissue (AT) in obese individuals shows marked γδ T cell expansion, accounting for over 95% of tissue-resident immune cells (8), highlighting their dominance in the adipose niche. IL-17, a key cytokine secreted by γδ T cells in AT (9, 10), suppresses glucose uptake in skeletal muscle and impairs insulin sensitivity in hepatocytes (11), positioning γδ T cells as drivers of obesity-related inflammation in T2DM. Additionally, γδ T cells participate in diabetic complications through epithelial repair mechanisms in lung and skin tissues (6), potentially influencing infection susceptibility and wound healing in DM. While γδ T cells exhibit context-dependent pro- or anti-inflammatory roles in DM, their subset-specific functions, temporal dynamics, and therapeutic targeting potential remain incompletely defined. Elucidating these mechanisms may provide a critical foundation for novel immunomodulatory strategies against DM.
2 Gamma delta T cell
2.1 Origin and development
γδ T cells are innate-like lymphocytes that fundamentally differ from conventional αβ T cells in developmental origin and activation mechanisms. T cell development broadly follows a sequential process. Initially, hematopoietic stem cells differentiate into lymphoid stem cells within the bone marrow hematopoietic inductive microenvironment. These lymphoid stem cells further develop into pro-T cells, which then migrate via the bloodstream to the thymus (12). Within the thymic microenvironment, pro-T cells differentiate sequentially through the double-negative (DN), double-positive (DP), and single-positive (SP) stages, ultimately maturing into functional T cells (13). The pro-T cell stage represents the branch point at which αβ T cells and γδ T cells begin to diverge into distinct lineages. During differentiation, bone marrow-derived progenitor T cells migrate to the thymus where they undergo TCR gene rearrangement at the pro-T cell stage. In the thymus, αβ T cells constitute over 95% of the total T cell population, whereas γδ T cells account for less than 5%. γδ T cells arise from DN thymocytes and undergo TCRγ and TCRδ rearrangement prior to TCRβ recombination, subsequently expressing the γδTCR/CD3 complex on their plasma membrane (14). Notably, rare thymocytes co-express both γδ and αβ TCRs (15). Their antigen recognition mechanisms differ significantly from those of αβ T cells. γδ T cells directly recognize diverse antigens in an MHC-independent manner. This non-classical recognition stems from unique TCR diversity generation mechanisms. Functionally, γδ T cells rapidly secrete cytokines or directly lyse target cells via the NKG2D–ligand pathway, playing pivotal roles in anti-pathogen defense, tumor immunosurveillance and tissue repair (16). The high conservation of TCR repertoires, tissue distribution, and functional subsets between human and murine γδ T cells establishes mice as essential model organisms for mechanistic studies.
2.2 Subsets
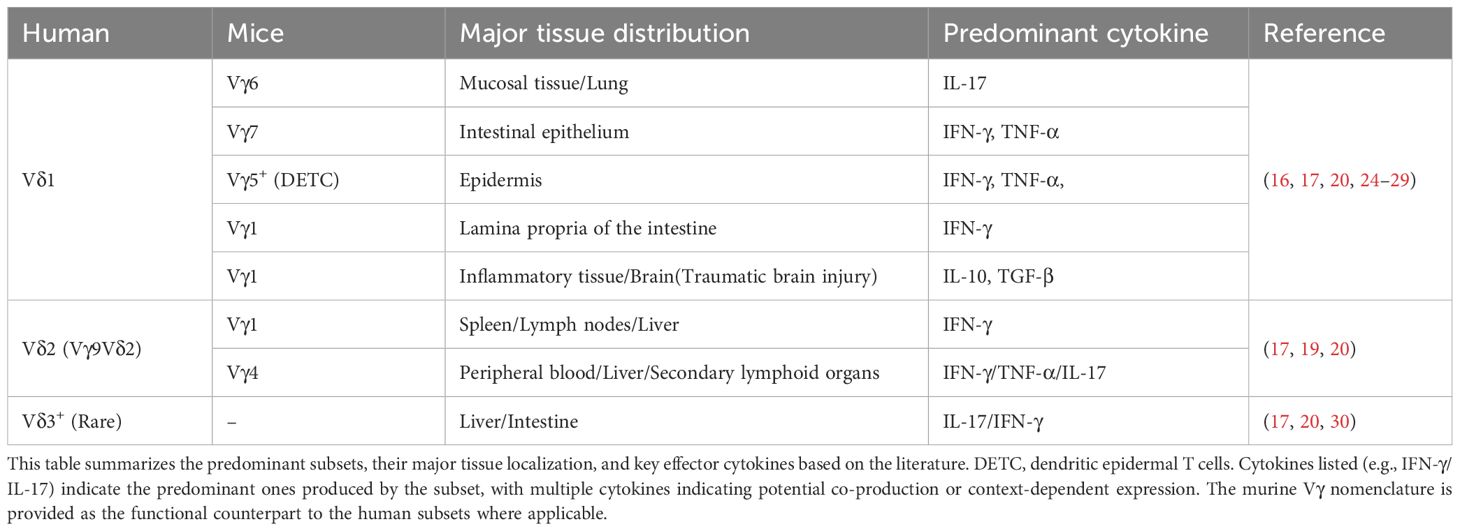
Human γδ T cells are classified into Vδ1, Vδ2, and Vδ3 subsets based on δ chain usage. From embryonic stages to childhood, the relative frequencies of the two primary human γδ T cell subsets (Vδ1 and Vδ2) undergo dynamic shifts (Figure 1A). The earliest rearrangements in the gamma/delta T cell lineage involve the Vγ9 and Vδ2 gene segments. Evidence of this process appears in the fetal liver during gestational 5–6 weeks and in the fetal thymus from the 8th week onward (17). Following this early development, Vγ9Vδ2+ T cells expand to constitute the majority of the gamma/delta repertoire by midgestation (20–30 weeks) (17). From birth to approximately 10 years of age, the peripheral γδ T cell compartment undergoes substantial maturation, marked not only by an increase in total numbers but also by a dramatic reconstitution of its subset composition, wherein the Vγ9Vδ2 population expands from a small fraction to a majority (>75% circulating γδ T cells) (18). In contrast to their minority status in adults, Vδ1 T cells represent the dominant γδ T cell subset in umbilical cord blood at birth (18). Vδ1 T cells, which account for only a minority in peripheral blood, are enriched in barrier tissues such as the skin and mucosa-associated lymphoid tissue (MALT), and recognize antigens presented by CD1 molecules; Vδ2 T cells, which dominate in peripheral blood and lymphoid organs, are primarily recognize phosphoantigens derived from microbial metabolism (e.g., HMBPP) (6, 19, 20). Zoledronate is a bisphosphonate. Vδ1 T cells are non-responsive to bisphosphonates or phosphoantigens. Culturing γδ T cells under high-glucose conditions requires an initial in vitro expansion step (Figure 1B). The established method of expanding Vδ2 T cells in vitro with zoledronate and IL-2 has made this subset a major subject of study in diabetic autoimmunity research (21). This subset-specific distribution and functional specialization underscore the need to avoid overgeneralization when studying γδ T cells in diabetic complications.
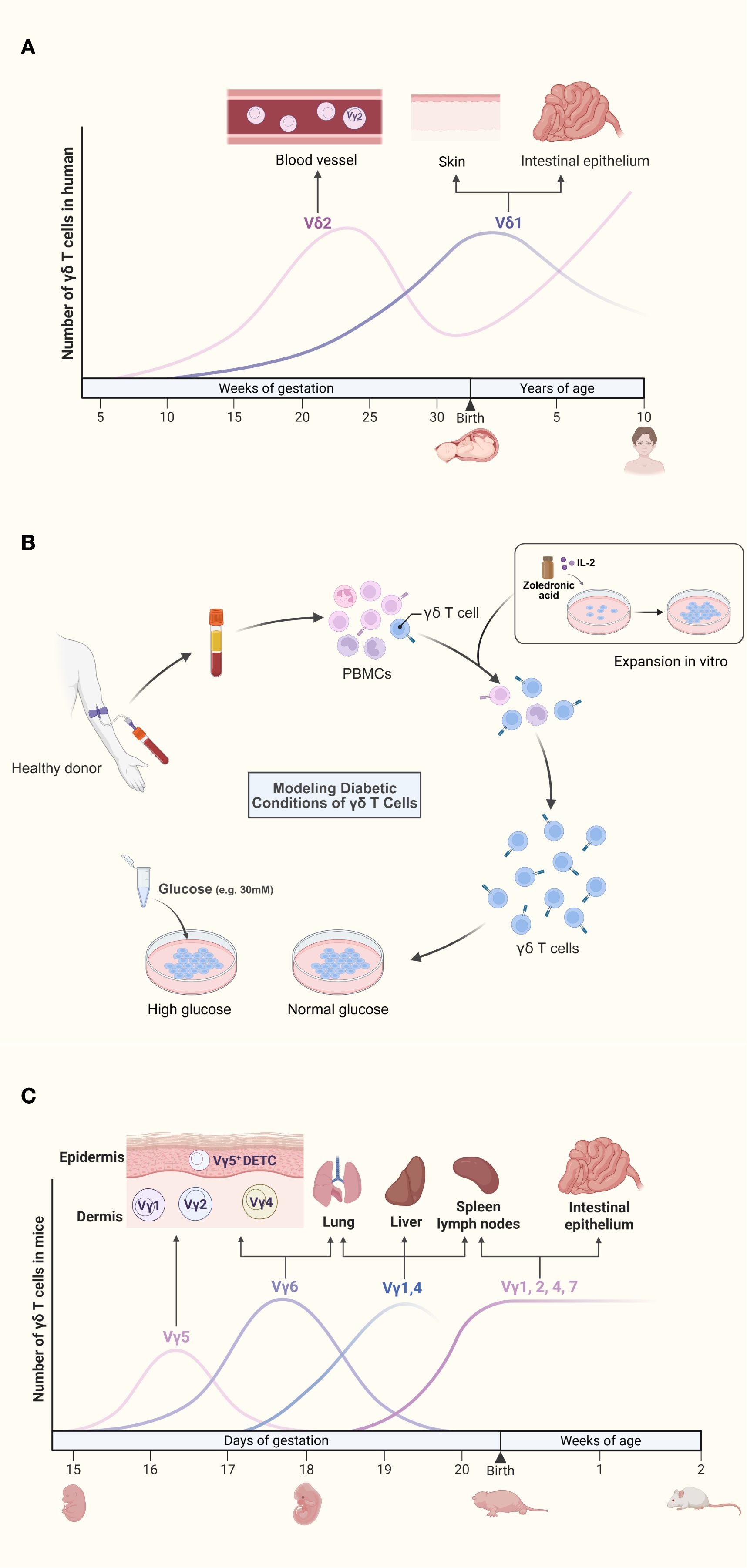
Figure 1. (A) Dynamic reconstitution of human γδ T cell subsets from fetal development to childhood. The earliest rearrangements involve the Vγ9 and Vδ2 gene segments. Although Vγ9Vδ2 T cells expand to form a major subset by mid-gestation, Vδ1 T cells are the dominant population in umbilical cord blood at birth. During postnatal development, the peripheral γδ T cell compartment undergoes a substantial reconstitution, characterized by a marked expansion of the Vγ9Vδ2 population, which becomes the predominant subset (>75% of circulating γδ T cells) by approximately 10 years of age. (B) Isolation, in vitro expansion, and high-glucose treatment of γδ T cells from diabetic models. Peripheral blood mononuclear cells (PBMCs) are isolated from peripheral venous blood via density-gradient centrifugation. Cultures are stimulated with zoledronate and recombinant human IL-2 to promote γδ T cell activation and proliferation. After 10–14 days of expansion, γδ T cells display robust proliferation, whereas other immune cell subsets progressively undergo apoptosis. Finally, the expanded γδ T cells are exposed to high-glucose conditions to mimic the diabetic microenvironment and assess their functional responses. (C). Spatiotemporal ontogeny and tissue distribution of murine γδ T cell subsets. During embryogenesis, the first wave of γδ T cells expresses monoclonal Vγ5Vδ1 TCRs, which migrate to and establish permanent residency within the epidermis. Subsequent Vγ6+ subsets traffic to the dermis, peritoneal cavity, and adipose tissue, while Vγ4+ subsets emerge concurrently and localize to the lung, skin dermis, and lymph nodes. In the perinatal period, Vγ7+ subsets colonize the intestinal tract, whereas polyclonal Vγ1+ and Vγ4+ subsets distribute broadly across peripheral lymphoid organs.
Murine γδ T cell subsets are primarily classified according to Vγ chain usage. Developmentally, γδ T cells represent the earliest T cell population emerging in the embryonic mouse thymus. During embryogenesis, the first wave expresses monoclonal Vγ5Vδ1 TCRs that home to and permanently reside in the skin epidermis (17). Subsequently, additional subsets develop and localize to specific niches (Figure 1C). The skin epidermis harbors a specialized subset termed dendritic epidermal T cells (DETC), originating from embryonic Vγ3Vδ1 precursors (22). Postnatally, polyclonal CD27+ Vγ1 and CD27+ Vγ4 subsets mature predominantly in the liver and lymph nodes (16). Functional specialization of murine γδ T cells is governed by Vγ chains, establishing mice as essential experimental models (17, 23). Despite conserved tissue distribution and phenotypic functions between human and murine γδ T cells, no strict subset equivalency exists. Researchers must judiciously select models and subsets based on target tissue microenvironments and specific biological questions (Table 1).
Beyond the aforementioned major subtype classification based on TCR chains, γδ T cells can also be categorized into distinct subsets according to their cluster of differentiation (CD) profiles. Fundamentally, the T-cell receptor (TCR) complex consists of receptor subunits (either TCRαβ or TCRγδ) and the associated CD3 subunits (CD3γ, δ, ϵ, and ζ) (31). Consequently, like all mature T cells, γδ T cells uniformly express the CD3 complex. γδ T cells predominantly exhibit a CD4-CD8- double-negative phenotype, with a minor subset expressing CD8+ (32). A single-cell RNA sequencing study in NOD mice revealed an abnormal expansion of double-negative T cells (33), although the specific role of γδ T cells within this population requires further investigation. Furthermore, Different functional γδ cell subsets can be classified by CD27. Mature TCRαβ+ thymocytes homogeneously express CD27, while γδ T cells represent only a small subset of the CD27+ thymocyte population. Human Vδ2 T cells can be functionally subdivided into naïve (CD45RA+CD27+), central memory (CD45RA-CD27+), effector memory (CD45RA-CD27-), and terminally differentiated effector (often CD45RA-CD27- or other combinations) phenotypes (17). CD27- thymocytes (approximately 10% of all γδ thymocytes) preferentially differentiate into IL-17A–secreting cells and CD27+ subsets primarily generate IFN-γ (34).
2.3 Effector functions
γδ T cells can be activated by specific cytokines to produce effector cytokines. While essential for tissue homeostasis at physiological levels, excessive concentrations of pro-inflammatory cytokines, such as TNF-α and IL-17, drive chronic inflammation (6). IFN-γ production requires synergistic IL-12 and IL-18 signaling, and IL-17A secretion is induced by IL-23 and IL-1β (19). Notably, single cytokine stimulation fails to elicit robust responses (24). Although Th17 cells are primary IL-17A producers, γδ T cells serve as significant contributors, particularly during early mucosal immune defense. Murine γδ T cell development critically depends on IL-7 and IL-15 (35). IL-15 and IL-2 drive IFN-γ+ subsets (Vγ5+ DETC, Vγ7+, Vγ1+), and IL-7 promotes IL-17A+ γδ T cells (predominantly CD27-Vγ6+) (17) (25).
γδ T cells have several activation pathways (Figure 2). The JAK2/STAT3/RORγt axis implicated in inflammatory and fibrotic diseases operates in immune cells (36–38). However, previous studies on this pathway have mostly focused on Th17 cells, with relatively few studies on γδ T cells that secrete IL-17. When pathogen-associated molecular patterns (PAMP) bind to pathogen recognition receptors(PRRs), DCs or macrophages release IL-23 and IL-1β directly triggers γδ T cell IL-17A secretion without TCR involvement (39). In addition, the TCR signaling pathway can also regulate the secretion of cytokines by γδ T cells. According to some research findings, γδ T cells may utilize molecular mechanisms during TCR signaling activation that differ from those of αβ T cells (40, 41). Although this pathway has not been fully elucidated, the signaling mechanisms of αβ TCR are considered largely similar to those of γδ TCR (14). Therefore, the γδ TCR signaling pathway can be understood within the framework established for αβ TCR signaling. Kinases act as critical drivers of TCR signaling. ZAP-70, a member of the Syk family kinases, is recruited to the TCR complex and the transmembrane adaptor protein LAT upon TCR activation, where it undergoes phosphorylation (42, 43). Phosphorylated LAT then provides docking sites for signaling enzymes such as PLCγ1. Subsequently, PLCγ1 hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG recruits Ras guanine nucleotide-releasing protein 1 (RasGRP1) to the plasma membrane, leading to activation of the Ras-ERK pathway (14). In γδ T cells, the main transcription factors regulating the expression of IL-17 or IFN-γ downstream of the TCR signaling pathway are RORγt or Tbx21(T-bet), which are partially transduced through the extracellular-signal related kinases/mitogen-activated protein kinases (ERK/MAPK) pathway (25).Functionally constrained by their developmental programming, γδ T cell effector fates remain largely unaltered in response to exogenous cytokines. Physiological cytokine secretion maintains tissue homeostasis, whereas pathological overproduction contributes to skin inflammation, atopic dermatitis, and autoimmune arthritis (44, 45). Therapeutic IL-17 blockade attenuates such inflammation (46), highlighting γδ T cell subset modulation as a promising, though still investigational, intervention strategy.
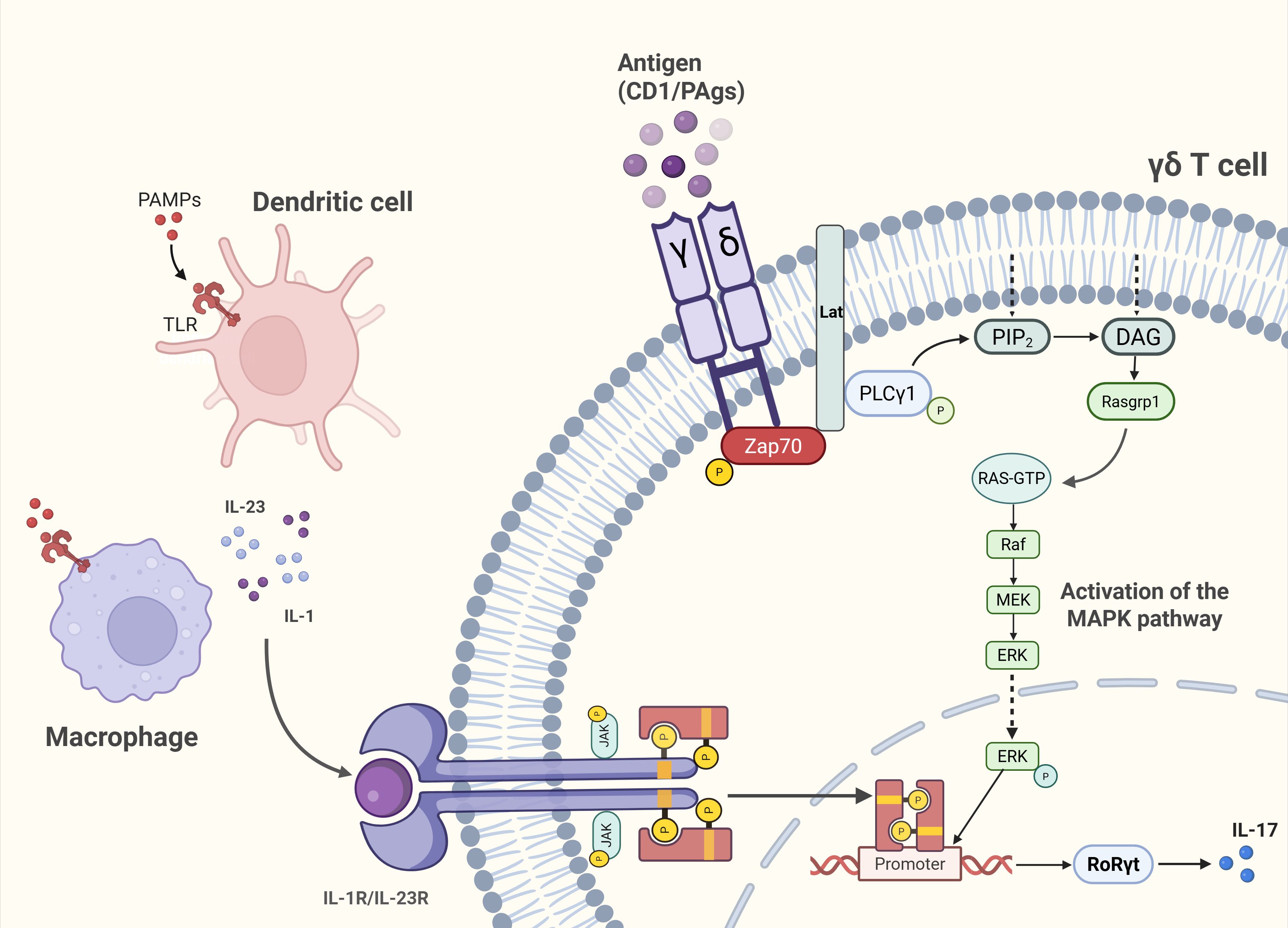
Figure 2. Unique activation pathways of γδ T cells. Pathogen-sensing route (TCR-independent): Engagement of PAMPs with PRRs on dendritic cells (DCs) and macrophages induces IL-23 and IL-1β release, directly stimulating γδ T cells to secrete IL-17A without TCR involvement. TCR-dependent route: Antigen recognition via the γδ TCR activates the ERK/MAPK signaling cascade, driving transcriptional polarization toward RORγt in IL-17–producing subsets.
3 Gamma delta T cells in diabetes mellitus pathogenesis
3.1 The dual role and therapeutic potential of γδ T cells in T1DM
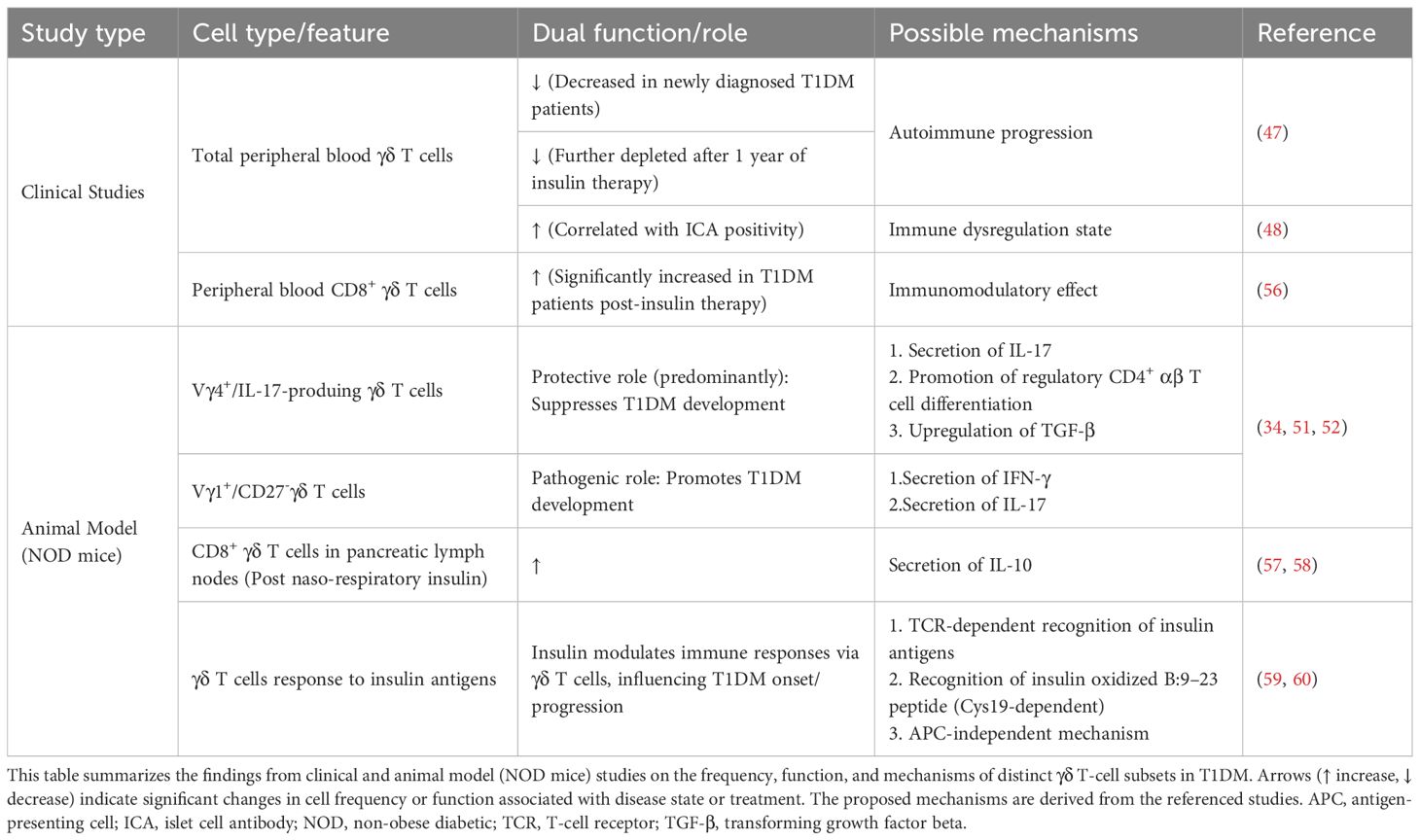
Several clinical studies suggest a potential association between γδ T cells and T1DM. Newly diagnosed T1DM patients exhibit a reduced mean proportion of peripheral blood γδ T cells, with further depletion observed after one year of insulin therapy (47). In islet cell antibody (ICA)-positive relatives of T1DM probands, who represent a high-risk group for future diabetes development, a high percentage of γδ T cells is associated with ICA positivity (48, 49), potentially reflecting stage-specific immune alterations. In T1DM animal models, distinct γδ T cell subsets have been specifically investigated. The non-obese diabetic (NOD) mouse serves as an ideal model for studying the immune basis and treatment of T1DM (50). Research indicates that Vγ4+ γδ T cells in NOD mice can suppress T1DM development by producing IL-17 and facilitating the differentiation of regulatory CD4+ αβ T cells in pancreatic lymph nodes; Vγ1+ cells, biased toward IFN-γ production, thereby promote a pro-inflammatory microenvironment conducive to T1DM pathogenesis (51). Within NOD mouse islets, the majority of infiltrating γδ T cells are IL-17-producing CD27- cells, while the IFN-γ-producing subset expresses CD27 (34, 52). Although the proportion of peripheral γδ T cells increases in NOD mice, predominantly IL-17-producing cells, they do not exacerbate diabetes; instead, they confer protection by upregulating TGF-β production (53). A gluten-free diet enriches splenic naïve CD27+ γδ T cells in mice, potentially reducing type 1 diabetes susceptibility by preventing their differentiation into pro-autoreactive effector cells (54). Collectively, these findings demonstrate a dual role for γδ T cells in T1DM pathogenesis, primarily governed by their effector cytokine profile. IL-17-producing γδ T cells appear primarily protective, suppressing islet inflammation. In contrast, the IFN-γ-producing subset promotes disease progression. Notably, γδ T cells coordinate with αβ T cells to drive T1DM development but are insufficient to independently cause disease (52). The role of IL-17 remains particularly challenging to define, as studies using IL-17 blockade have reported either protective effects or no significant impact, while in diabetic complications it demonstrates a dual nature (55). These discrepancies appear to depend on the experimental animal model employed, the timing of intervention, and the specific cytokine microenvironment, highlighting the need for further investigation in future studies.
Insulin therapy remains a cornerstone treatment for T1DM and has been shown to possess immunomodulatory properties. T1DM patients show a significant increase in peripheral blood CD8+γδ T cells after 3–6 months of insulin treatment (56), suggesting the expansion of a potential regulatory subset induced by exogenous insulin. This concept is strongly supported by animals studies in which mucosal insulin administration promotes immune tolerance. Aerosol insulin induces autoimmune tolerance mediated by regulatory CD8+ γδ T cells, preventing T1DM in mice (57). Similarly, naso-respiratory insulin administration in NOD mice increases IL-10-producing CD8+ γδ T cells in pancreatic lymph nodes (58). The underlying mechanism may involve the unique antigen-recognition capability of γδ T cells. The TCRs of NOD mouse γδ T cells exhibit specific reactivity to multiple insulin antigens, likely through an APC-independent mechanism (59), indicating TCR-dependent recognition. For instance, γδ TCRs can recognize the insulin oxidized B:9–23 peptide, naturally generated during insulin degradation in β cells, which contains the essential Cys19 residue for γδ T cell responses (60). Based on these findings, a mechanistic model can be proposed. Mucosal insulin administration may enhance the local presentation or availability of insulin-derived peptides (such as B:9–23) in respiratory mucosa. This setting likely promotes the engagement of insulin-reactive γδ T cell TCRs, leading to their activation and functional polarization. These activated CD8+ γδ T cells acquire a regulatory phenotype, characterized by secretion of the potent anti-inflammatory cytokine IL-10. These cells subsequently migrate to pancreatic lymph nodes, where local IL-10 production may suppress the activation and effector functions of autoreactive αβ T cells (61), thereby reestablishing immune tolerance and preventing β cell destruction. Collectively, these studies demonstrate that insulin, beyond its metabolic role, may exert immunomodulatory effects that counter T1DM pathogenesis. γδ T cells may modulate immune function through potential responses to insulin antigens, potentially influencing T1DM onset and progression.
The role of γδ T cells in T1DM is gradually being elucidated (Table 2). However, current human studies present limitations. Firstly, clinical assessments predominantly measure total γδ T cells without distinguishing functionally heterogeneous subsets (Vδ1/Vδ2), hindering the precise identification of protective versus pathogenic subsets and contributing to observed discrepancies. Defining the γδ T cell subset repertoire in human T1DM patients remains a future objective. Additionally, the lack of phenotypic and functional tracking of islet-resident γδ T cells in humans impedes understanding of their direct role in islet autoimmunity. The inherent difficulty in obtaining pancreatic tissue samples from T1DM patients, who rarely undergo surgical intervention, presents a major obstacle. Currently, there is experience in procuring pancreatic tissue from individuals with T1DM. Pancreatic tissue samples from donors with T1DM can be obtained through nPOD (https://npod.org). Studies utilizing laparoscopic pancreatic biopsy have revealed immunological changes in the islets of newly diagnosed T1DM patients, without reporting major complications (62). However, the Diabetes Virus Detection Study (DiViD) collected larger pancreatic tissue samples via caudal pancreatectomy from adults recently diagnosed with T1DM, which resulted in some patients experiencing postoperative bleeding and leakage of amylase-rich pancreatic juice (63). Consequently, it was deemed unethical to continue the study. Significant challenges remain in the acquisition of pancreatic tissue from patients with T1DM.
3.2 γδ T cells in T2DM: balancing systemic exhaustion and tissue inflammation
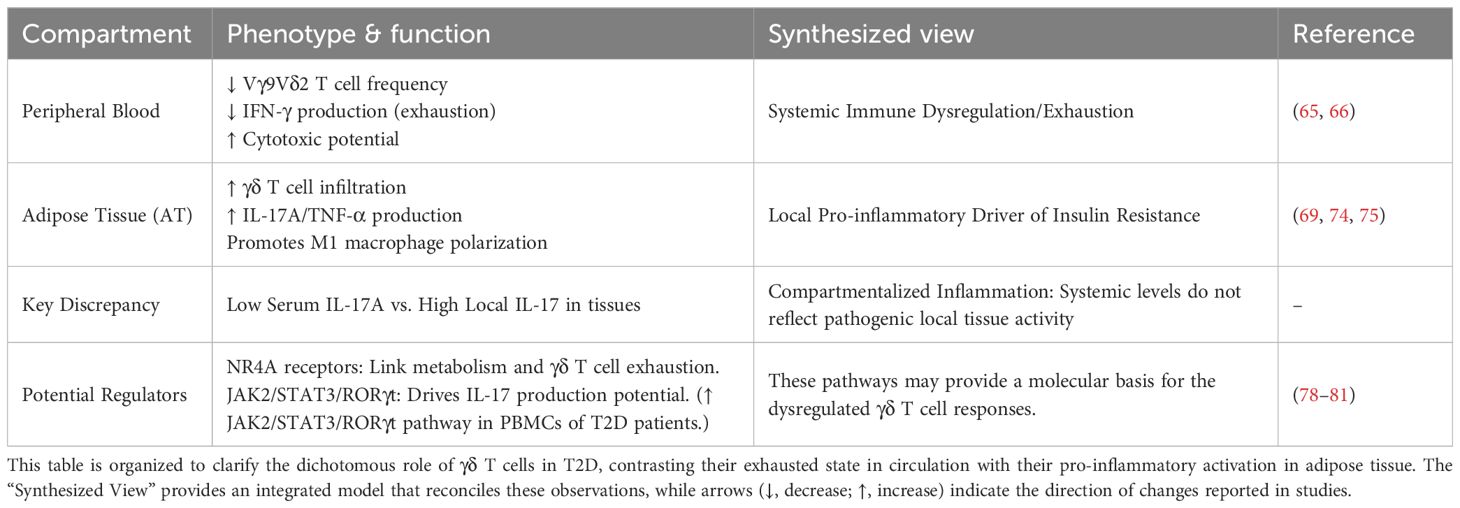
Studies have shown that patients with T2DM exhibit increased monocyte counts, reflecting exacerbated chronic inflammation and immune activation, which promotes insulin resistance through the secretion of pro-inflammatory cytokines (64). Insulin resistance (IR), a central feature of obesity-related metabolic dysregulation, manifests as reduced insulin responsiveness in adipose, hepatic, and muscle tissues, ultimately leading to β-cell failure and T2DM onset. Obesity-induced chronic low-grade inflammation is a key driver of IR. The chronic inflammatory process is embedded within an immune-mediated proinflammatory environment, wherein γδ T cells exhibit complex immunoregulatory roles. First, both obese individuals and obese T2DM patients display significant dysfunction in the peripheral Vγ9Vδ2 T cell subset, characterized by diminished IFN-γ secretion (65, 66). In obese individuals, the proportion of Vγ9Vδ2 T cells in late apoptosis (Annexin V+ PI+) is significantly higher compared to those in early apoptosis (Annexin V+ PI-) (65). This accelerated apoptotic process in Vγ9Vδ2 T cells under obese conditions represents a potential mechanism contributing to their depletion in obese individuals. In addition, IL-2 stimulation can reverse this IFN-γ secretory defect, suggesting reversible functional suppression (65). Furthermore, the study indicated that obesity does not impair the capacity of Vγ9Vδ2 T cells to produce IFN-γ upon strong HDMAPP stimulation. This observation may also partially explain the improved fasting glucose, HbA1c, and insulin sensitivity observed in postmenopausal women with prediabetes and osteopenia following alendronate treatment (67). Bisphosphonates may restore the capacity of Vγ9Vδ2 T cells to produce cytokines such as IFN-γ through their activation. Consequently, restoring peripheral γδ T cell function represents a promising therapeutic avenue for T2DM. Within peripheral blood mononuclear cells (PBMCs) of T2DM patients, γδ T cells demonstrate increased cytotoxicity and expansion (68). In contrast to their systemic exhaustion, γδ T cells within adipose tissue (AT) exhibit a pro-inflammatory, tissue-resident phenotype. In AT of mice, two major γδ T cell populations exist: a CD3ϵlowCD27+ subset secreting IFN-γ, and a CD3ϵhighCD27- subset producing IL-17A and TNF-α (69). Adipose-resident γδ T cells serve as the primary source of IL-17A in adipose tissue. These IL-17-producing γδ T cells exhibit robust diurnal rhythms in RORγt and IL-17A expression, playing a critical role in systemic metabolic homeostasis by sustaining de novo lipogenesis (DNL) (70). Dysregulation of DNL is associated with metabolic disorders such as obesity and type 2 diabetes (71).The proportion of γδ T cells increases in the livers of individuals with non-alcoholic steatohepatiti (72). Paradoxically, clinical studies report significantly lower serum IL-17A levels in T2DM patients compared to normoglycemic controls (73), indicating a potential dissociation between local tissue inflammation and systemic immune responses, reflecting compartmentalized inflammation. This may arise from systemic immune exhaustion in diabetes reducing serum IL-17, while persistent activation of γδ T cells within the local adipose microenvironment elevates IL-17. This dichotomy may be explained by chronic metabolic insults which globally dampen immune responsiveness, leading to reduced cytokine output in circulation. Conversely, within specific niches like inflamed adipose tissue, local pro-inflammatory cytokines provide potent, compartmentalized signals that drive IL-17 production from resident γδ T cells. Furthermore, in obese mice, the predominant γδ T cell subsets accumulating in epididymal AT (eAT) are Vγ4+ and Vγ6+ T cells, which promote eAT inflammation by inhibiting the accumulation of anti-inflammatory M2 macrophages (74). M1 macrophages, conversely, enhance adipocyte inflammation and reduce insulin sensitivity via TNF-α production (75). High-fat (HF) diet-fed TCRδ-/- mice exhibit reduced M1 macrophage accumulation and improved glucose clearance and insulin sensitivity post-insulin injection compared to TCRδ+/+ mice (74), suggesting adipose-resident γδ T cells promote insulin resistance.
NR4A nuclear receptors regulate hepatic gluconeogenesis and maintain inflammatory balance (76, 77). Dysregulated hepatic gluconeogenesis significantly impacts T2DM. NR4A1 and NR4A3 enhance insulin sensitivity in skeletal muscle and liver, yet are underexpressed in these tissues across various insulin-resistant animal models (78). In eAT, the abundance of γδ T cells decreased in mice fed with HF diet (79). In 3T3-L1 adipocytes, NR4A3 overexpression enhances insulin-stimulated glucose transport activity, potentially by increasing GLUT4 translocation to the plasma membrane or augmenting insulin-mediated IRS1 tyrosine phosphorylation and Akt phosphorylation (78). However, these studies primarily focus on NR4A in adipocytes or tissues. Research in cervical cancer cells indicates that transcription factors NR4A2/3 promote Vγ9Vδ2 T cell exhaustion (80), suggesting NR4A may regulate γδ T cells and influence immune-mediated inflammatory process in insulin resistance. Reduced adipocyte NR4A exacerbates insulin resistance, while diminished NR4A in adipose γδ T cells might delay their exhaustion, potentially contributing to sustained pro-inflammatory cytokine secretion and establishing a vicious cycle of metabolic and autoimmune inflammation. While NR4A receptors are implicated in metabolism and inflammation, their direct role in regulating γδ T cell function within the context of diabetic insulin resistance remains speculative and warrants dedicated investigation. If validated, NR4A could emerge as a key therapeutic target in T2DM. However, given divergent alterations in peripheral blood versus adipose tissue γδ T cells, NR4A expression may also exhibit opposing patterns. Specific investigations into NR4A family members within γδ T cells under diabetic conditions remain limited and warrant further study.
The JAK2/STAT3/RORγt pathway in γδ T cells may also contribute to T2DM pathogenesis. Enhanced expression of IL-23, JAK2, STAT3, and RORγt is observed in PBMCs of T2DM patients (81). RORγt is the key transcription factor for γδ T cell differentiation into IL-17–producing subsets, and this pathway is critically involved in Th17-mediated inflammation. Compared to non-diabetic or insulin-deficient islets, insulin-sufficient islets demonstrate elevated IL-17 expression in both β-cells and α-cells, though CD45+ cells are not the primary source of this IL-17 (82). In diabetic tissues, IL-17 contributes to impaired insulin signaling and β-cell dysfunction by activating the JNK pathway, promoting neutrophil infiltration into islets, and enhancing the expression of inflammatory cytokines and chemokines (83). Thus, investigating the specific molecular mechanisms underlying IL-17 production by CD45- γδ T cells in T2DM islets represents a promising area for future research. γδ T cells might amplify AT inflammation and accelerate IR progression via this pathway, although the precise mechanisms require elucidation.
In summary, the role of γδ T cells in T2D is marked by a critical functional compartmentalization: systemic exhaustion in the periphery—evidenced by reduced Vγ9Vδ2 T cell frequency and impaired IFN-γ production—coexists with pro-inflammatory activation within metabolic tissues. In AT, resident γδ T cells promote insulin resistance through secretion of IL-17 and TNF-α, as well as by inducing pro-inflammatory macrophage polarization. This dichotomy resolves the apparent paradox of low serum IL-17 levels alongside localized tissue inflammation. The NR4A receptor family and the JAK2/STAT3/RORγt pathway have emerged as key potential mechanisms linking metabolic dysregulation to γδ T cell–driven inflammation, thereby providing an integrated model of their dual role in T2D pathogenesis (Table 3).
3.3 Gestational state and gestational diabetes mellitus
Pregnancy represents a unique physiological state where γδ T cells contribute to localized immune responses. In healthy pregnant women, γδ T cells account for up to 50% of CD3+ T cells in the uterus. The majority of γδ T cells at the maternal-fetal interface (MFI) express Vδ1 and produce elevated levels of TGF-β and IL-10 (84). During early normal pregnancy, the Vδ1 subset at the MFI increases significantly (85), and exhibits fluctuations under progesterone regulation (86), highlighting its hormone-responsive functionality. Gestational diabetes mellitus (GDM), a common complication in pregnancy, prompts interest in the relationship between γδ T cells and GDM. The exploration of γδ T cells in GDM reveals alterations but lacks mechanistic clarity. Studies indicate alterations in lymphocyte subsets in GDM mothers and their newborns compared to health pregnancies. GDM mothers exhibit higher γδ T cell levels than healthy pregnant controls (87, 88). Specifically, GDM patients show increased peripheral blood total lymphocytes and CD8+ γδ T cells compared to normal glucose tolerance (NGT) controls, and GDM newborns have a higher proportion of CD8+ γδ T cell numbers than NGT newborns (89).
While current research has not yet directly elucidated the mechanistic pathways by which γδ T cells contribute to the pathogenesis of GDM, their known biological characteristics and the pathophysiology of GDM suggest several promising future research directions. First, given that progesterone regulates fluctuations in γδ T cells, the pronounced hormonal disturbances in GDM may disrupt the precise hormonal control of endometrial Vδ1 cells. This dysregulation could impair their production of cytokines such as IL-10 and TGF-β, thereby disturbing the immune-tolerant environment at the maternal-fetal interface and triggering local inflammation. Second, a shift of γδ T cells toward a pro-inflammatory profile may exacerbate insulin resistance. Approximately 80% of GDM cases arise from β-cell dysfunction against a background of chronic insulin resistance, a pathophysiology similar to that of T2DM (90). As significant producers of cytokines such as IFN-γ and IL-17, γδ T cells in GDM may undergo an abnormal shift toward such pro-inflammatory subsets, amplifying systemic and placental inflammation. This, in turn, can disrupt metabolic regulation via cytokine-mediated mechanisms and worsen insulin resistance in both maternal and fetal tissues. Finally, the observation that about 70% of prior GDM patients later develop T2DM (91) suggests the potential persistence of metabolic and immune dysregulation. The alterations in γδ T cells observed during GDM pregnancy may not be transient but rather represent a lasting immunological imprint. These long-lived, tissue-resident Vδ1 cells could sustain a low-grade inflammatory state, partially explaining the immunological link between GDM and subsequent T2DM. Future studies should directly analyze the functional status, subset distribution, and specific cytokine profiles of γδ T cells at the maternal-fetal interface and in the circulation of GDM patients to validate these hypotheses.
4 Diabetes-induced γδ T cells dysfunction
Clinical diabetes often involves pathological states like hyperglycemia or obesity. While γδ T cells contribute to diabetes pathogenesis, the diabetic milieu also impacts γδ T cells, inducing functional impairments that heighten susceptibility to infections and cancer in diabetic patients.
4.1 Anti-infection defects
γδ T cells constitute a crucial first line of defense against infections, acting early before primary αβ T cell responses develop. Vγ9Vδ2 T cells induce potent anti-infective effects by producing IFN-γ and lysing infected target cells (e.g., influenza, Mycobacterium tuberculosis) (65). T2DM patients are frequently overweight or obese. Obesity is associated with reduced peripheral Vγ9Vδ2 T cell numbers and weakened IFN-γ–dependent antiviral responses (65), potentially compromising anti-infective γδ T cell function in obese diabetics. Furthermore, hyperglycemia negatively impacts innate autoimmunity via oxidative stress induction and reduced cytokine production, increasing infection risk in DM (92–94). The lifetime risk of progressing from Mycobacterium tuberculosis infection to active tuberculosis (TB) significantly increases with immunosuppressive triggers like diabetes (95). Individuals with latent TB infection (LTBI) who have diabetes or prediabetes show reduced γδ T cells in PBMCs (96), potentially linked to diminished immune protection in LTBI. IL-17 is considered vital for anti-infective defense. γδ T cells secrete IL-17 early during mucosal surface infections, contributing to antibacterial immunity (39). Murine models of various pathogens (S. pneumoniae, S. aureus, Escherichia coli, influenza) demonstrate that γδ T cells can mobilize neutrophils via IL-17 secretion to combat infection (97–100). The IL-17-producing CD27- γδ T cell subset rapidly expands during acute infection (34). γδ T cells and αβ-γδ T cells are also significant sources of IL-17 in early S. aureus infection and experimental autoimmune encephalomyelitis (EAE) (101, 102). Hyperglycemia in DM may impair IL-17 secretion, thereby increasing infection risk.
4.2 Antitumor impairment
γδ T cells play indispensable roles in antitumor immunity. This section focuses on how hyperglycemia alters γδ T cell antitumor function. Vγ9Vδ2 T cell receptors recognize phosphorylated metabolites accumulating in cancer cells due to dysregulated mevalonate pathways or pharmacologic intervention (103). IL-17-secreting γδ T cells, relying on oxidative phosphorylation, often promote tumor progression, whereas IFN-γ–producing subsets, dependent on glycolysis, associate with tumor regression and favorable prognosis (104–106). Vγ9Vδ2 T cells from T2DM patients exhibit defects in synapse formation with target tumor cells and lytic granule polarization (21). The hyperglycemic diabetic environment induces pathological metabolic reprogramming, enhancing the Warburg effect (aerobic glycolysis) in Vγ9Vδ2 T cells, which suppresses AMPK activity and impedes lytic granule polarization and trafficking to the immunological synapse (21). The AMPK pathway also functions in tumor cells. Vγ9Vδ2 T cells recognize a cell surface complex containing Butyrophilin 2A1 (BTN2A1) and BTN3A1, overexpressed in malignancies (107). This complex can be activated by elevated levels of phosphoantigens in tumor cells. AMPK activation in tumor cells increases BTN2A1-BTN3A complex expression, enhancing Vγ9Vδ2 T cell-mediated tumor killing (107). Many antidiabetic drugs may modulate cancer risk (108). Metformin, as an AMPK agonist, may increase the expression of specific tumor cell surface proteins (BTN2A1 and BTN3A), potentially enhancing recognition by Vγ9Vδ2 T cells (21) (Figure 3). Various γδ T cell-based immunotherapeutic strategies, including ex vivo expanded allogeneic γδ T cells, γδ T cell infusion, and antibodies, are under clinical evaluation (103). This offers insight: deeper understanding of DM-γδ T cell interactions may enable analogous strategies to delay disease progression and improve quality of life in diabetic patients and those with complications.
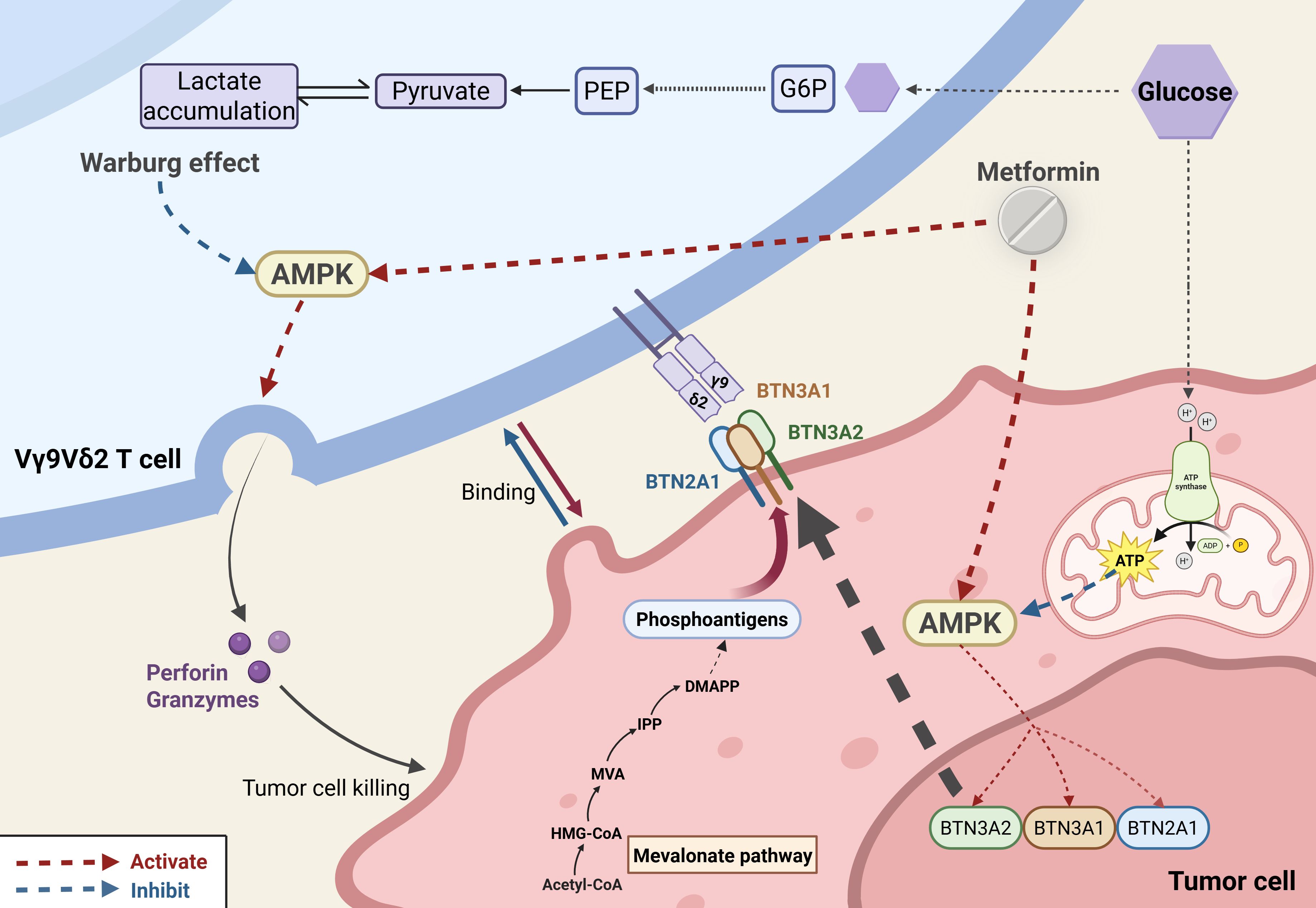
Figure 3. Metabolic–immunological interplay in hyperglycemia-driven tumor immunity dysfunction. Hyperglycemia impairs tumor immunity through two parallel mechanisms: (1) In γδ T cells, it induces a Warburg-like metabolic shift that suppresses AMPK activity, thereby disrupting lytic granule trafficking and reducing cytotoxicity. (2) In tumor cells, it downregulates AMPK-dependent expression of BTN2A1/BTN3A1 complexes, attenuating Vγ9Vδ2 TCR–mediated tumor recognition. Metformin reverses these defects via dual AMPK activation, restoring γδ T cell cytotoxicity and enhancing BTN2A1/BTN3A1 presentation on tumor cells.
4.3 Impaired wound healing
The skin harbors γδ T cells enriched in both the epidermis and dermis. These cells coordinate the complex interplay between keratinocytes and inflammatory cells by secreting growth factors and inflammatory mediators, thereby contributing to the regulation of epithelial homeostasis. Skin-resident γδ T cells exert protective functions and contribute critically to skin wound healing through multiple mechanisms, including costimulatory molecules, cytokine secretion, and chemokine production (109). This is highly relevant to understanding the mechanisms underlying chronic, non-healing wounds. Under diabetic conditions, hyperglycemia and obesity synergistically disrupt this repair network. Human chronic wound tissues contain more γδ T cells than normal tissues, but γδ T cell numbers are lower in chronic wounds of T2DM patients compared to non-diabetic chronic wound patients (26). Studies in diabetic mice reveal impaired IL-17 secretion by dermal Vγ4+ T cells due to reduced levels of IL-7, IL-23, and IL-1β; recruitment of Vγ4+ T cells is also diminished by attenuated CCL20/CCR6 chemokine signaling (109, 110). Notably, this research identified that impaired mTOR signaling in epidermal keratinocytes of diabetic mice leads to reduced IL-7 secretion, consequently impairing Vγ4+ T cell function. The mTOR inhibitor rapamycin can impair the wound-healing capacity of γδ T cells in mice (111). Similarly, in intact skin of streptozotocin (STZ)-induced diabetic rats, weakened mTOR pathway activation impairs the insulin like growth factor 1 (IGF-1)/IL-15 axis, disrupting keratinocyte-γδ T cell interactions and delaying wound closure (112, 113). Skin γδ T cells are activated via junctional adhesion molecule-like (JAML)-coxsackie and adenovirus receptor (CAR)costimulatory signals, inducing secretion of cytokines like IL-2 and TNF-α (114), thereby influencing wound healing processes involving outer-layer keratinocytes (26). IL-2 stimulation activates Jak1 and Jak3 in skin γδ T cells of mice (115), leading to phosphorylation of STAT5A and STAT5B, peaking at 30 minutes before rapidly declining (116). Under high-glucose conditions, IL-2 stimulation induces abnormally sustained STAT5A phosphorylation but fails to elicit STAT5B phosphorylation (116). Elevated TNF-α, associated with obesity and insulin resistance, suppresses the tissue-repair function of skin γδ T cells, whereas TNF-α blockade restores their epithelial responsiveness (116, 117). Collectively, hyperglycemia or obesity impairs skin γδ T cell proliferation and function via STAT5, aryl hydrocarbon receptor (AHR) signaling, mTOR, the IL-15–IGF-1 loop, and other pathways (22, 113, 116, 118) (Table 4). This renders γδ T cells unresponsive to epithelial damage and increases inflammation-associated gene expression.
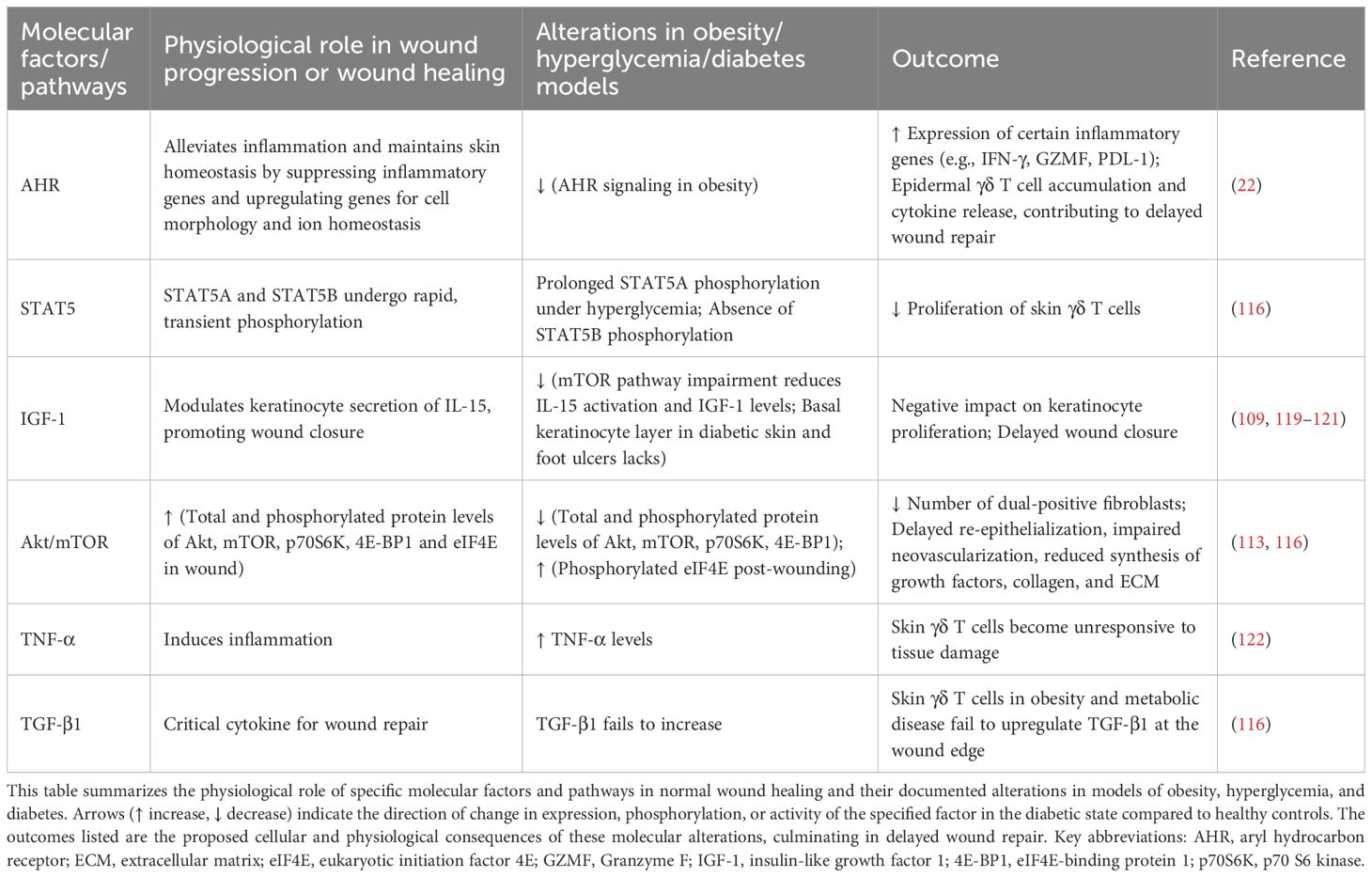
Table 4. Immunologic mechanisms of diabetic wound healing failure in γδ T cells: dysregulated pathways and compromised γδ T cell-epithelial communication.
Psoriasis, a chronic inflammatory skin disorder, is another skin-related comorbidity of DM. CCL20-mediated recruitment of γδ T cells via the CCR6 receptor, promoting increased IL-17 secretion, may be a key mechanism underlying delayed wound repair in diabetes. In psoriasis, enhanced CCR6 expression on skin γδ T cells of mice facilitates greater recruitment of dermal Vγ4+ T cells (123), which drive pathology through massive IL-17 secretion. IL-17, in turn, acts on keratinocytes to induce their proliferation (leading to epidermal hyperplasia) and the production of further pro-inflammatory cytokines and antimicrobial peptides, creating a self-amplifying inflammatory loop (124). This same pro-inflammatory pathway is hijacked in diabetic skin, contributing to impaired wound healing. CCL20 expression is elevated in the skin of T2DM patients (125), and dermal γδ T cells increase in the hind paws of 21-week-old db/db mice (126). The resulting IL-17-driven chronic inflammation disrupts the orderly process of wound repair by preventing the transition from the inflammatory to the proliferative phase. Chronic inflammation driven by pro-inflammatory cytokines from immune cells can stall diabetic wound repair (127). However, findings regarding γδ T cell numbers in wounds show discrepancies between studies. While Vγ4+ T cells play a significant role in chronic wound healing, both increasing and inhibiting IL-17A have been observed to accelerate wound healing in diabetic mice (109). This paradox may be explained by the concentration-dependent and temporal-specific role of IL-17; at early stages or low levels, it may aid in host defense and stem cell activation, whereas its persistent, high-level secretion is unequivocally pathogenic. Variations in wound pathology severity across non-healing models and limited sample sizes likely contribute to these conflicting results, highlighting the need for future research to explore whether skin γδ T cell loss or recruitment relates to other underlying factors. Furthermore, preventing chronic wounds may require a specific cytokine balance. These studies provide novel insights into the mechanisms of refractory wound formation in diabetic patients. As the autoimmunity mechanisms by which skin γδ T cells function in diabetic refractory wounds are further elucidated, fine-tuning cytokine levels holds promise for overcoming the therapeutic challenges of these wounds.
5 Immunomodulatory effects of antidiabetic drugs on γδ T cells
Current research on the mechanisms of antidiabetic drugs is shifting from solely metabolic regulation towards dual immunometabolic perspectives. As pivotal bridging cells, γδ T cells represent a functional target for multiple therapeutic approaches (Table 5). 1α,25(OH)2D3 is the active form of Vitamin D, which is also known as Calcitriol. It improves systemic insulin resistance by suppressing inflammatory responses in γδ T cells. Acting via the vitamin D receptor (VDR), 1α,25(OH)2D3 promotes FBP1 expression, inhibits glycolysis in human Vδ2 T cells, and consequently reduces pro-inflammatory cytokine production (105). Mucosal insulin administration, inducing regulatory CD8+ γδ T cells, represents another potential strategy for preventing human T1DM (57). PBMCs from T2DM patients treated with saxagliptin, a dipeptiyl peptidase 4 (DPP-4) inhibitor, exhibit reduced levels of IL-23, JAK2, STAT3, and RORγt (81). As RORγt is a key transcription factor for γδ T cells, these cells may be modulated by DPP-4 inhibitors, potentially contributing to improvements in glucose homeostasis and metabolic control in T2DM. Additionally, psoriasis exhibits a significant association with DM (131, 132). A prospective case study found that applying GLP-1 receptor agonists (GLP-1 RAs) in T2DM patients with comorbid psoriasis reduced dermal γδ T cell numbers and IL-17 production, correlating with improved clinical severity of psoriasis (128). Given that psoriasis involves elevated γδ T cells and IL-17 production, these findings suggest that GLP-1 RAs may offer benefits for psoriasis management alongside glucose-lowering and weight-loss effects. This finding provides a novel rationale for selecting glucose-lowering regimens in diabetic patients, specifically favoring GLP-1 RAs for T2DM patients with concurrent psoriasis (Figure 4). Further research reveals abundant GLP-1 receptor expression on murine small intestinal γδ T cells (129, 130), suggesting that GLP-1 RAs may directly modulate γδ T cells and influence autoimmune responses, potentially delaying diabetes onset and progression. However, specific mechanistic evidence for this remains lacking and warrants investigation.
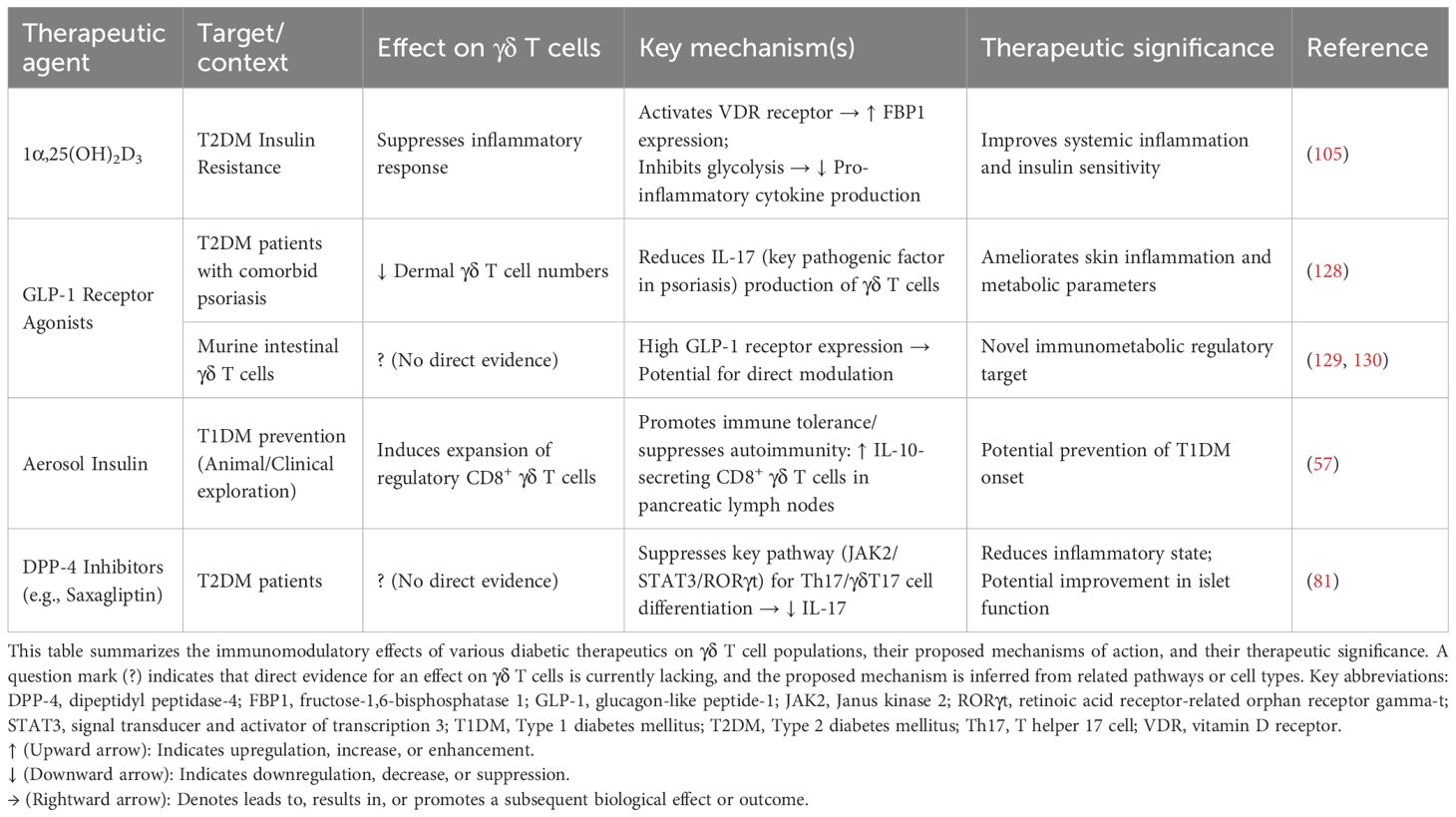
Table 5. Dual metabolic-immune targeting in DM and comorbidities: γδ T cell-directed therapeutic strategies.
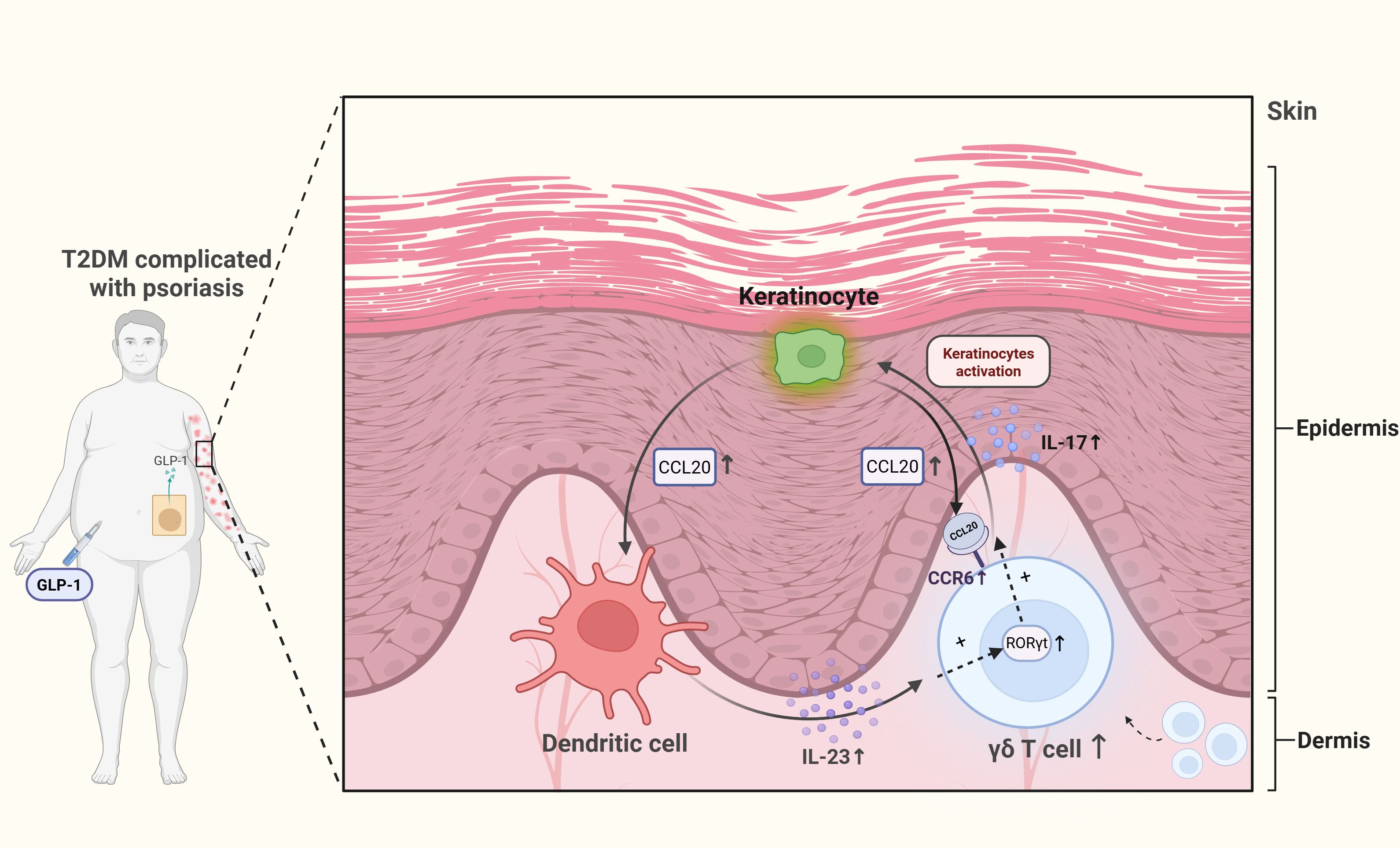
Figure 4. Dermal γδ T cell hyperactivation in T2DM-associated psoriasis and GLP-1RA intervention. Self-perpetuating inflammatory loop: Keratinocyte-derived CCL20 recruits dermal γδ T cells, whose IL-17 secretion further stimulates keratinocytes to upregulate CCL20, by binding to CCR6 receptors. Cytokine and metabolic amplifiers: DC-derived IL-23 polarizes γδ T cells toward a RORγt+IL-17+ phenotype, while T2DM-associated hyperglycemia synergistically enhances both CCL20 production and IL-17 output. GLP-1 receptor agonists (GLP-1RAs) interrupt this cycle by modulating metabolic and inflammatory pathways.
Therapeutically harnessing γδ T cells requires precision targeting of autoimmune-specific pathways. In T1DM, agents targeting IL-17 and related pathways, such as ustekinumab and ixekizumab, are currently in Phase II/III recruiting stage (39, 133, 134). Beyond monoclonal antibodies, safe and effective oral small molecule drugs (SMDs) targeting molecules like RORγt (39) hold future clinical promise, although such agents have not yet advanced to clinical studies. While IL-17 and RORγt are closely linked to γδ T cells, they are not γδ T cell-specific targets, as other immune cells also utilize these pathways. The potential of γδ T cells as early warning biomarkers or immunomodulatory targets remains underexplored, particularly for high-risk diabetes populations. Given that up to 50% of individuals with prediabetes progress to diabetes within 5 years (135), strategies to delay or reverse this progression are crucial. Significant potential also exists for novel γδ T cell-targeted drug development. Local insulin injection accelerates diabetic foot ulcer healing by stimulating AKT and ERK pathways (136). As γδ T cells recognize certain insulin antigens and their TCR activation involves MAPK pathways, investigating whether insulin promotes γδ T cell function to aid wound healing in diabetes presents a promising avenue for future research. Collectively, substantial gaps remain in developing clinically applicable γδ T cell-based strategies for preventing or treating diabetes, demanding further exploration.
6 Discussion
This review comprehensively summarizes the dual regulatory roles of γδ T cells in DM and its complications. On the one hand, γδ T cells contribute to diabetes pathogenesis through the secretion of effector molecules like IL-17, with functions exhibiting subset-specific characteristics. On the other hand, the diabetic pathological milieu reciprocally impairs γδ T cell functions in anti-infection, antitumor autoimmunity, and tissue repair.
γδ T cells may also modulate regulatory T cells (Tregs) or other immune cells, thereby potentially alleviating certain pathological processes. In AT of mice, both γδ T cells and Tregs increase with age, with γδ T cells and their secretion of IL-17 providing a conditioning signal for Treg accumulation (69). During influenza virus clearance or AT thermoregulation, IL-17-secreting γδ T cells may promote Treg accumulation via IL-33 upregulation (69, 137). Studies have identified impaired function or reduced numbers of Tregs in both T1DM and T2DM patients (138, 139). Tregs can also improve insulin resistance by suppressing the activity of Th1, Th2, and Th17 cells (138). Therefore, γδ T cell–mediated promotion of Treg accumulation and functional restoration via IL-17A secretion may represent a potential strategy for future diabetes immunotherapy. In addition to their direct antitumor functions, γδ T cells also exert indirect antitumor effects by modulating αβ T cell activity (140).
Research on the role of γδ T cells in DM still faces significant limitations that require resolution. Firstly, current investigations inadequately characterize the functions of human γδ T cell subsets; findings derived from mouse subsets cannot be directly extrapolated to humans. The specific role of the tissue-resident Vδ1 subset in diabetes and its complications remains unclear. This ambiguity stems partly from the difficulty in obtaining samples, as Vδ1 cells primarily reside in the skin and MALT. Utilizing organoid models to simulate tissue-resident subset function may offer a solution. Furthermore, the Vδ1 subset is scarce in peripheral blood and does not recognize phosphoantigens, presenting challenges for direct ex vivo expansion and culture in research settings. Secondly, the precise role of γδ T cells across different stages of diabetes progression remains poorly defined. The dynamic changes and underlying mechanisms of γδ T cells, from the stage of impaired glucose tolerance in prediabetes to the onset of acute or chronic diabetic complications, remain poorly understood.
While these unresolved complexities pose challenges, therapeutically targeting the metabolic susceptibility and TCR-dependent reprogramming of γδ T cells may help translate mechanistic insights into future clinical applications for DM and its comorbidities. Elucidating the context-dependent duality of γδ T cells in DM not only deciphers the immunometabolic crosstalk at the cellular frontier but also unlocks precision-targeted therapies.
Author contributions
BW: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. QL: Conceptualization, Investigation, Visualization, Writing – review & editing. QW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by LiaoNing Revitalization Talents Program (grant numbers, XLYC2412003).
Acknowledgments
All images were created in https://BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
DM: iabetes mellitus
T1DM: Type 1 diabetes mellitus
T2DM: Type 2 diabetes mellitus
AT: Adipose tissue
IR: Insulin resistance
MALT: Mucosa-associated lymphoid tissue
MHC: Major histocompatibility complex
HMBPP: (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
IL: Interleukin
IFN-γ: Interferon-gamma
TCR: T cell receptor
DETC: Dendritic epidermal T cell
DC: Dendritic cell
PAMP: Pathogen-associated molecular pattern
PRR: Pattern-recognition receptor
ERK/MAPK: Extracellular signal–regulated kinase/mitogen-activated protein kinase
NK: Natural killer
NR4A: Nuclear receptor subfamily 4 group A
GDM: Gestational diabetes mellitus
NOD: Non-obese diabetic (mouse)
ICA: Islet cell antibody
PBMCs: Peripheral blood mononuclear cells
DKD: Diabetic kidney disease
mTOR: Mechanistic target of rapamycin
AMPK: AMP-activated protein kinase
BTN: Butyrophilin
BTN2A1: Butyrophilin subfamily 2 member A1
BTN3A: Butyrophilin subfamily 3 member A
STAT: Signal transducer and activator of transcription
RORγt: Retinoic acid receptor-related orphan receptor gamma t
AHR: Aryl hydrocarbon receptor
IGF-1: Insulin-like growth factor 1
HF diet: High-fat diet
GLUT4: Glucose transporter type 4
IRS1: Insulin receptor substrate 1
References
1. GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet Lond Engl. (2023) 402:203–34. doi: 10.1016/S0140-6736(23)01301-6
2. Naaman SC and Bakris GL. Diabetic nephropathy: Update on pillars of therapy slowing progression. Diabetes Care. (2023) 46:1574–86. doi: 10.2337/dci23-0030
3. Tian Y, Qiu Z, Wang F, Deng S, Wang Y, Wang Z, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat Rev Cardiol. (2020) 17:1969–77. doi: 10.1038/s41569-020-0339-2
4. Tian Y, Qiu Z, Wang F, Deng S, Wang Y, Wang Z, et al. Associations of diabetes and prediabetes with mortality and life expectancy in China: A national study. Diabetes Care. (2024) 47:1969–77. doi: 10.2337/dca24-0012
5. Tang SCW and Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. (2020) 16:206–22. doi: 10.1038/s41581-019-0234-4
6. Fay NS, Larson EC, and Jameson JM. Chronic inflammation and γδ T cells. Front Immunol. (2016) 7:210. doi: 10.3389/fimmu.2016.00210
7. Kaufmann SH, Blum C, and Yamamoto S. Crosstalk between alpha/beta T cells and gamma/delta T cells in vivo: Activation of alpha/beta T-cell responses after gamma/delta T-cell modulation with the monoclonal antibody GL3. Proc Natl Acad Sci U S A. (1993) 90:9620–4. doi: 10.1073/pnas.90.20.9620
8. Goldberg EL, Shchukina I, Asher JL, Sidorov S, Artyomov MN, and Dixit VD. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat Metab. (2020) 2:50–61. doi: 10.1038/s42255-019-0160-6
9. Weinstock A, Moura Silva H, Moore KJ, Schmidt AM, and Fisher EA. Leukocyte heterogeneity in adipose tissue, including in obesity. Circ Res. (2020) 126:1590–612. doi: 10.1161/CIRCRESAHA.120.316203
10. Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. (2010) 185:6947–59. doi: 10.4049/jimmunol.1001269
11. Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. (2013) 145:366–374.e1-3. doi: 10.1053/j.gastro.2013.04.010
12. Golzari-Sorkheh M, Yoganathan K, Chen ELY, Singh J, and Zúñiga-Pflücker JC. T cell development: From T-lineage specification to intrathymic maturation. Adv Exp Med Biol. (2025) 1471:81–137. doi: 10.1007/978-3-031-77921-3_4
13. Nitta T and Takayanagi H. Non-epithelial thymic stromal cells: Unsung heroes in thymus organogenesis and T cell development. Front Immunol. (2021) 11:620894. doi: 10.3389/fimmu.2020.620894
14. Muro R, Takayanagi H, and Nitta T. T cell receptor signaling for γδT cell development. Inflammation Regen. (2019) 39:6. doi: 10.1186/s41232-019-0095-z
15. Itohara S, Nakanishi N, Kanagawa O, Kubo R, and Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: Analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. (1989) 86:5094–8. doi: 10.1073/pnas.86.13.5094
16. Ribot JC, Lopes N, and Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. (2021) 21:221–32. doi: 10.1038/s41577-020-00452-4
17. Qu G, Wang S, Zhou Z, Jiang D, Liao A, and Luo J. Comparing mouse and human tissue-resident γδ T cells. Front Immunol. (2022) 13:891687. doi: 10.3389/fimmu.2022.891687
18. Kalyan S and Kabelitz D. Defining the nature of human γδ T cells: A biographical sketch of the highly empathetic. Cell Mol Immunol. (2013) 10:21–9. doi: 10.1038/cmi.2012.44
19. Silva-Santos B, Mensurado S, and Coffelt SB. γδ T cells: Pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. (2019) 19:392–404. doi: 10.1038/s41568-019-0153-5
20. Cruz MS, Diamond A, Russell A, and Jameson JM. Human αβ and γδ T cells in skin immunity and disease. Front Immunol. (2018) 9:1304. doi: 10.3389/fimmu.2018.01304
21. Mu X, Xiang Z, Xu Y, He J, Lu J, Chen Y, et al. Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes. Cell Mol Immunol. (2022) 19:944–56. doi: 10.1038/s41423-022-00894-x
22. Merches K, Schiavi A, Weighardt H, Steinwachs S, Teichweyde N, Förster I, et al. AHR signaling dampens inflammatory signature in neonatal skin γδ T cells. Int J Mol Sci. (2020) 21:2249. doi: 10.3390/ijms21062249
23. Kazen AR and Adams EJ. Evolution of the V, D, and J gene segments used in the primate gammadelta T-cell receptor reveals a dichotomy of conservation and diversity. Proc Natl Acad Sci U S A. (2011) 108:E332–340. doi: 10.1073/pnas.1105105108
24. Paget C, Chow MT, Gherardin NA, Beavis PA, Uldrich AP, Duret H, et al. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol Cell Biol. (2015) 93:198–212. doi: 10.1038/icb.2014.94
25. Muñoz-Ruiz M, Sumaria N, Pennington DJ, and Silva-Santos B. Thymic determinants of γδ T cell differentiation. Trends Immunol. (2017) 38:336–44. doi: 10.1016/j.it.2017.01.007
26. Xu P, Fu X, Xiao N, Guo Y, Pei Q, Peng Y, et al. Involvements of γδT lymphocytes in acute and chronic skin wound repair. Inflammation. (2017) 40:1416–27. doi: 10.1007/s10753-017-0585-6
27. Dong P, Zhang S, Cai M, Kang N, Hu Y, Cui L, et al. Global characterization of differential gene expression profiles in mouse Vγ1+ and Vγ4+ γδ T cells. PloS One. (2014) 9:e112964. doi: 10.1371/journal.pone.0112964
28. Hahn YS, Ji XY, Woo SI, Choi YK, Song MS, Shin KS, et al. Vγ1+ γδ T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunol Lett. (2008) 121:87–92. doi: 10.1016/j.imlet.2008.09.001
29. Abou-El-Hassan H, Rezende RM, Izzy S, Gabriely G, Yahya T, Tatematsu BK, et al. Vγ1 and Vγ4 gamma-delta T cells play opposing roles in the immunopathology of traumatic brain injury in males. Nat Commun. (2023) 14:4286. doi: 10.1038/s41467-023-39857-9
30. Cieslak SG and Shahbazi R. Gamma delta T cells and their immunotherapeutic potential in cancer. biomark Res. (2025) 13:51. doi: 10.1186/s40364-025-00762-6
31. Alcover A, Alarcón B, and Di Bartolo V. Cell biology of T cell receptor expression and regulation. Annu Rev Immunol. (2018) 36:103–25. doi: 10.1146/annurev-immunol-042617-053429
32. Roy Chowdhury R, Valainis JR, Dubey M, von Boehmer L, Sola E, Wilhelmy J, et al. NK-like CD8+ γδ T cells are expanded in persistent mycobacterium tuberculosis infection. Sci Immunol. (2023) 8:eade3525. doi: 10.1126/sciimmunol.ade3525
33. Islam MZ, Zimmerman S, Lindahl A, Weidanz J, Ordovas-Montanes J, Kostic A, et al. Single-cell RNA-seq reveals disease-specific CD8+ T cell clonal expansion and a high frequency of transcriptionally distinct double-negative T cells in diabetic NOD mice. PloS One. (2025) 20:e0317987. doi: 10.1371/journal.pone.0317987
34. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. (2009) 10:427–36. doi: 10.1038/ni.1717
35. Giri S and Lal G. Differentiation and functional plasticity of gamma-delta (γδ) T cells under homeostatic and disease conditions. Mol Immunol. (2021) 136:138–49. doi: 10.1016/j.molimm.2021.06.006
36. Jie XL, Luo ZR, Yu J, Tong ZR, Li QQ, Wu JH, et al. Pi-pa-run-fei-tang alleviates lung injury by modulating IL-6/JAK2/STAT3/IL-17 and PI3K/AKT/NF-κB signaling pathway and balancing Th17 and treg in murine model of OVA-induced asthma. J Ethnopharmacol. (2023) 317:116719. doi: 10.1016/j.jep.2023.116719
37. Liang Z, Tang Z, Zhu C, Li F, Chen S, Han X, et al. Intestinal CXCR6+ ILC3s migrate to the kidney and exacerbate renal fibrosis via IL-23 receptor signaling enhanced by PD-1 expression. Immunity. (2024) 57:1306–1323.e8. doi: 10.1016/j.immuni.2024.05.004
38. Xia S, Chen L, Li Z, Li Y, Zhou Y, Sun S, et al. Qingchang wenzhong decoction reduce ulcerative colitis in mice by inhibiting Th17 lymphocyte differentiation. Phytomedicine Int J Phytother Phytopharm. (2022) 107:154460. doi: 10.1016/j.phymed.2022.154460
39. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23:38–54. doi: 10.1038/s41577-022-00746-9
40. Hayes SM and Love PE. Distinct structure and signaling potential of the gamma delta TCR complex. Immunity. (2002) 16:827–38. doi: 10.1016/s1074-7613(02)00320-5
41. van Oers NS, Lowin-Kropf B, Finlay D, Connolly K, and Weiss A. alpha beta T cell development is abolished in mice lacking both lck and fyn protein tyrosine kinases. Immunity. (1996) 5:429–36. doi: 10.1016/s1074-7613(00)80499-9
42. Gaud G, Lesourne R, and Love PE. Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol. (2018) 18:485–97. doi: 10.1038/s41577-018-0020-8
43. Lo WL, Shah NH, Ahsan N, Horkova V, Stepanek O, Salomon AR, et al. Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nat Immunol. (2018) 19:733–41. doi: 10.1038/s41590-018-0131-1
44. Alnahas S, Hagner S, Raifer H, Kilic A, Gasteiger G, Mutters R, et al. IL-17 and TNF-α are key mediators of moraxella catarrhalis triggered exacerbation of allergic airway inflammation. Front Immunol. (2017) 8:1562. doi: 10.3389/fimmu.2017.01562
45. Roark CL, French JD, Taylor MA, Bendele AM, Born WK, and O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol Baltim Md 1950. (2007) 179:5576–83. doi: 10.4049/jimmunol.179.8.5576
46. Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJJ, Joosten LAB, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheumatol. (2004) 50:650–9. doi: 10.1002/art.20001
47. Zubkiewicz-Kucharska A and Noczyńska A. Abnormal distribution of gamma-delta T lymphocytes and their subsets in type 1 diabetes. Adv Clin Exp Med Off Organ Wroclaw Med Univ. (2016) 25:665–71. doi: 10.17219/acem/60714
48. Lang FP, Schatz DA, Pollock BH, Riley WJ, Maclaren NK, Dumont-Driscoll M, et al. Increased T lymphocytes bearing the gamma-delta T cell receptor in subjects at high risk for insulin dependent diabetes. J Autoimmun. (1991) 4:925–33. doi: 10.1016/0896-8411(91)90055-h
49. Riley WJ, Maclaren NK, Krischer J, Spillar RP, Silverstein JH, Schatz DA, et al. A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med. (1990) 323:1167–72. doi: 10.1056/NEJM199010253231704
50. Kachapati K, Adams D, Bednar K, and Ridgway WM. The non-obese diabetic (NOD) mouse as a model of human type 1 diabetes. In: Joost HG, Al-Hasani H, and Schürmann A, editors. Animal models in diabetes research. Vol 933. Methods in molecular biology. 999 Riverview Dr. Suite 208, Totowa, NJ 07512 USA: Humana Press (2012). p. 3–16. doi: 10.1007/978-1-62703-068-7_1
51. O’Brien RL, Matsuda J, Aydintug MK, Jin N, Phalke S, and Born WK. A distinctive γδ T cell repertoire in NOD mice weakens immune regulation and favors diabetic disease. Biomolecules. (2022) 12:1406. doi: 10.3390/biom12101406
52. Markle JGM, Mortin-Toth S, Wong ASL, Geng L, Hayday A, and Danska JS. γδ T cells are essential effectors of type 1 diabetes in the nonobese diabetic mouse model. J Immunol Baltim Md 1950. (2013) 190:5392–401. doi: 10.4049/jimmunol.1203502
53. Han G, Wang R, Chen G, Wang J, Xu R, Wang L, et al. Interleukin-17-producing gammadelta+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-beta. Immunology. (2010) 129:197–206. doi: 10.1111/j.1365-2567.2009.03166.x
54. Niederlova V, Michalik J, Drabonova B, Cisarova R, Funda D, and Stepanek O. Gluten-free diet induces small-scale changes across multiple T-cell subsets in NOD mice. Eur J Immunol. (2025) 55:e202451559. doi: 10.1002/eji.202451559
55. Tan HB, Zheng YQ, and Zhuang YP. IL-17A in diabetic kidney disease: Protection or damage. Int Immunopharmacol. (2022) 108:108707. doi: 10.1016/j.intimp.2022.108707
56. Kretowski A, Myśliwiec J, and Kinalska I. Abnormal distribution of gammadelta T lymphocytes in graves’ disease and insulin-dependent diabetes type 1. Arch Immunol Ther Exp (Warsz). (2000) 48:39–42.
57. Harrison LC, Dempsey-Collier M, Kramer DR, and Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. (1996) 184:2167–74. doi: 10.1084/jem.184.6.2167
58. Harrison LC, Solly NR, and Martinez NR. (pro)insulin-specific regulatory T cells. Novartis Found Symp. (2003) 252:132–41.
59. Zhang L, Jin N, Nakayama M, O’Brien RL, Eisenbarth GS, and Born WK. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J Autoimmun. (2010) 34:478–84. doi: 10.1016/j.jaut.2009.12.008
60. Aydintug MK, Zhang L, Wang C, Liang D, Wands JM, Michels AW, et al. γδ T cells recognize the insulin B:9–23 peptide antigen when it is dimerized through thiol oxidation. Mol Immunol. (2014) 60:116–28. doi: 10.1016/j.molimm.2014.04.007
61. Tang A, Li C, Chen Z, and Li T. Anti-CD20 monoclonal antibody combined with adenovirus vector-mediated IL-10 regulates spleen CD4+/CD8+ T cells and T-bet/GATA-3 expression in NOD mice. Mol Med Rep. (2017) 16:3974–82. doi: 10.3892/mmr.2017.7111
62. Hanafusa T, Miyazaki A, Miyagawa J, Tamura S, Inada M, Yamada K, et al. Examination of islets in the pancreas biopsy specimens from newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia. (1990) 33:105–11. doi: 10.1007/BF00401048
63. Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyöty H, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: Experiences from the DiViD study. Diabetologia. (2014) 57:841–3. doi: 10.1007/s00125-013-3155-y
64. Li H, Zou L, Long Z, and Zhan J. Immunometabolic alterations in type 2 diabetes mellitus revealed by single-cell RNA sequencing: Insights into subtypes and therapeutic targets. Front Immunol. (2025) 15:1537909. doi: 10.3389/fimmu.2024.1537909
65. Costanzo AE, Taylor KR, Dutt S, Han PP, Fujioka K, Jameson JM, et al. Obesity impairs γδ T cell homeostasis and antiviral function in humans. PloS One. (2015) 10:e0120918. doi: 10.1371/journal.pone.0120918
66. Li Y, Woods K, Parry-Strong A, Anderson RJ, Capistrano C, Gestin A, et al. Distinct dysfunctional states of circulating innate-like T cells in metabolic disease. Front Immunol. (2020) 11:448. doi: 10.3389/fimmu.2020.00448
67. Karimi Fard M, Aminorroaya A, Kachuei A, Salamat MR, Hadi Alijanvand M, Aminorroaya Yamini S, et al. Alendronate improves fasting plasma glucose and insulin sensitivity, and decreases insulin resistance in prediabetic osteopenic postmenopausal women: A randomized triple-blind clinical trial. J Diabetes Investig. (2019) 10:731–7. doi: 10.1111/jdi.12944
68. Gu D, Lim J, Han KY, Seo IH, Jee JH, Cho SJ, et al. Single-cell analysis of human PBMCs in healthy and type 2 diabetes populations: Dysregulated immune networks in type 2 diabetes unveiled through single-cell profiling. Front Endocrinol. (2024) 15:1397661. doi: 10.3389/fendo.2024.1397661
69. Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. (2018) 19:464–74. doi: 10.1038/s41590-018-0094-2
70. Douglas A, Stevens B, Rendas M, Kane H, Lynch E, Kunkemoeller B, et al. Rhythmic IL-17 production by γδ T cells maintains adipose. novo lipogenesis. Nature. (8041) 2024:636. doi: 10.1038/s41586-024-08131-3
71. Song Z, Xiaoli AM, and Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. (2018) 10:1383. doi: 10.3390/nu10101383
72. Zhou Y, Zhang H, Yao Y, Zhang X, Guan Y, and Zheng F. CD4+ T cell activation and inflammation in NASH-related fibrosis. Front Immunol. (2022) 13:967410. doi: 10.3389/fimmu.2022.967410
73. Vasanthakumar R, Mohan V, Anand G, Deepa M, Babu S, and Aravindhan V. Serum IL-9, IL-17, and TGF-β levels in subjects with diabetic kidney disease (CURES-134). Cytokine. (2015) 72:109–12. doi: 10.1016/j.cyto.2014.10.009
74. Mehta P, Nuotio-Antar AM, and Smith CW. γδ T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J Leukoc Biol. (2015) 97:121–34. doi: 10.1189/jlb.3A0414-211RR
75. Suganami T, Nishida J, and Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. (2005) 25:2062–8. doi: 10.1161/01.ATV.0000183883.72263.13
76. Rodríguez-Calvo R, Tajes M, and Vázquez-Carrera M. The NR4A subfamily of nuclear receptors: Potential new therapeutic targets for the treatment of inflammatory diseases. Expert Opin Ther Targets. (2017) 21:291–304. doi: 10.1080/14728222.2017.1279146
77. Berriel Diaz M, Lemke U, and Herzig S. Discovering orphans’ sweet secret: NR4A receptors and hepatic glucose production. Cell Metab. (2006) 4:339–40. doi: 10.1016/j.cmet.2006.10.005
78. Fu Y, Luo L, Luo N, Zhu X, and Garvey WT. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: Potential role in insulin resistance. J Biol Chem. (2007) 282:31525–33. doi: 10.1074/jbc.M701132200
79. Tougaard P, Martinsen LO, Lützhøft DO, Jensen HE, Flethøj M, Vandenabeele P, et al. TL1A regulates adipose-resident innate lymphoid immune responses and enables diet-induced obesity in mice. Int J Obes 2005. (2020) 44:1062–74. doi: 10.1038/s41366-020-0539-1
80. Liu J, Wu M, Yang Y, Mei X, Wang L, Wang J, et al. BTN3A1 expressed in cervical cancer cells promotes Vγ9Vδ2 T cells exhaustion through upregulating transcription factors NR4A2/3 downstream of TCR signaling. Cell Commun Signal CCS. (2024) 22:459. doi: 10.1186/s12964-024-01834-0
81. Rezaeepoor M, Hoseini-Aghdam M, Sheikh V, Eftekharian MM, and Behzad M. Evaluation of interleukin-23 and JAKs/STATs/SOCSs/ROR-γt expression in type 2 diabetes mellitus patients treated with or without sitagliptin. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. (2020) 40:515–23. doi: 10.1089/jir.2020.0113
82. Rajendran S, Quesada-Masachs E, Zilberman S, Graef M, Kiosses WB, Chu T, et al. IL-17 is expressed on beta and alpha cells of donors with type 1 and type 2 diabetes. J Autoimmun. (2021) 123:102708. doi: 10.1016/j.jaut.2021.102708
83. Elahi R, Nazari M, Mohammadi V, Esmaeilzadeh K, and Esmaeilzadeh A. IL-17 in type II diabetes mellitus (T2DM) immunopathogenesis and complications; molecular approaches. Mol Immunol. (2024) 171:66–76. doi: 10.1016/j.molimm.2024.03.009
84. De Luccia TPB, Pendeloski KPT, Ono E, Mattar R, Pares DBS, Yazaki Sun S, et al. Unveiling the pathophysiology of gestational diabetes: Studies on local and peripheral immune cells. Scand J Immunol. (2020) 91:e12860. doi: 10.1111/sji.12860
85. Terzieva A, Dimitrova V, Djerov L, Dimitrova P, Zapryanova S, Hristova I, et al. Early pregnancy human decidua is enriched with activated, fully differentiated and pro-inflammatory gamma/delta T cells with diverse TCR repertoires. Int J Mol Sci. (2019) 20:687. doi: 10.3390/ijms20030687
86. Cai D, Tang Y, and Yao X. Changes of γδT cell subtypes during pregnancy and their influences in spontaneous abortion. J Reprod Immunol. (2019) 131:57–62. doi: 10.1016/j.jri.2019.01.003
87. Friebe-Hoffmann U, Antony L, Kruessel JS, Pawlowski B, and Hoffmann TK. Peripheral immunological cells in pregnant women and their change during diabetes. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. (2017) 125:677–83. doi: 10.1055/s-0043-104935
88. Lapolla A, Sanzari M, Betterle C, Dalfrà MG, Masin M, Zanchetta R, et al. Evaluation of T-cell receptor CD3+ gamma delta in gestational diabetes mellitus. Acta Diabetol. (2000) 37:207–11. doi: 10.1007/s005920070007
89. Lapolla A, Dalfrà MG, Sanzari M, Fedele D, Betterle C, Masin M, et al. Lymphocyte subsets and cytokines in women with gestational diabetes mellitus and their newborn. Cytokine. (2005) 31:280–7. doi: 10.1016/j.cyto.2005.05.004
90. McNestry C, Killeen SL, Crowley RK, and McAuliffe FM. Pregnancy complications and later life women’s health. Acta Obstet Gynecol Scand. (2023) 102:523–31. doi: 10.1111/aogs.14523
91. Plows JF, Stanley JL, Baker PN, Reynolds CM, and Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. (2018) 19:3342. doi: 10.3390/ijms19113342
92. Holt RIG, Cockram CS, Ma RCW, and Luk AOY. Diabetes and infection: Review of the epidemiology, mechanisms and principles of treatment. Diabetologia. (2024) 67:1168–80. doi: 10.1007/s00125-024-06102-x
93. Tong ZWM, Grant E, Gras S, Wu M, Smith C, Barrett HL, et al. The role of T-cell immunity in COVID-19 severity amongst people living with type II diabetes. FEBS J. (2021) 288:5042–54. doi: 10.1111/febs.16105
94. Kavazović I, Krapić M, Beumer-Chuwonpad A, Polić B, Turk Wensveen T, Lemmermann NA, et al. Hyperglycemia and not hyperinsulinemia mediates diabetes-induced memory CD8 T-cell dysfunction. Diabetes. (2022) 71:706–21. doi: 10.2337/db21-0209
95. Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn JDick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat Rev Microbiol. (2009) 7:845–55. doi: 10.1038/nrmicro2236
96. Kathamuthu GR, Kumar NP, Moideen K, Menon PA, and Babu S. Decreased frequencies of gamma/delta T cells expressing Th1/Th17 cytokine, cytotoxic, and immune markers in latent tuberculosis-diabetes/pre-diabetes comorbidity. Front Cell Infect Microbiol. (2021) 11:756854. doi: 10.3389/fcimb.2021.756854
97. Shibata K, Yamada H, Hara H, Kishihara K, and Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after escherichia coli infection via IL-17 production. J Immunol Baltim Md 1950. (2007) 178:4466–72. doi: 10.4049/jimmunol.178.7.4466
98. Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous staphylococcus aureus infection in mice. J Clin Invest. (2010) 120:1762–73. doi: 10.1172/JCI40891
99. Hassane M, Demon D, Soulard D, Fontaine J, Keller LE, Patin EC, et al. Neutrophilic NLRP3 inflammasome-dependent IL-1β secretion regulates the γδT17 cell response in respiratory bacterial infections. Mucosal Immunol. (2017) 10:1056–68. doi: 10.1038/mi.2016.113
100. Hamada H, Garcia-Hernandez M, de la L, Reome JB, Misra SK, Strutt TM, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol Baltim Md 1950. (2009) 182:3469–81. doi: 10.4049/jimmunol.0801814
101. Edwards SC, Sutton CE, Ladell K, Grant EJ, McLaren JE, Roche F, et al. A population of proinflammatory T cells coexpresses αβ and γδ T cell receptors in mice and humans. J Exp Med. (2020) 217:e20190834. doi: 10.1084/jem.20190834
102. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, and Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. (2009) 31:331–41. doi: 10.1016/j.immuni.2009.08.001
103. Saura-Esteller J, de Jong M, King LA, Ensing E, Winograd B, de Gruijl TD, et al. Gamma delta T-cell based cancer immunotherapy: Past-present-future. Front Immunol. (2022) 13:915837. doi: 10.3389/fimmu.2022.915837
104. Lopes N, McIntyre C, Martin S, Raverdeau M, Sumaria N, Kohlgruber AC, et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat Immunol. (2021) 22:179–92. doi: 10.1038/s41590-020-00848-3
105. Li P, Li K, Yuan W, Xu Y, Li P, Wu R, et al. 1α,25(OH)2D3 ameliorates insulin resistance by alleviating γδ T cell inflammation via enhancing fructose-1,6-bisphosphatase 1 expression. Theranostics. (2023) 13:5290–304. doi: 10.7150/thno.84645
106. Reis BS, Darcy PW, Khan IZ, Moon CS, Kornberg AE, Schneider VS, et al. TCR-Vγδ usage distinguishes protumor from antitumor intestinal γδ T cell subsets. Science. (2022) 377:276–84. doi: 10.1126/science.abj8695
107. Mamedov MR, Vedova S, Freimer JW, Sahu AD, Ramesh A, Arce MM, et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature. (2023) 621:188–95. doi: 10.1038/s41586-023-06482-x
108. Shlomai G, Neel B, LeRoith D, and Gallagher EJ. Type 2 diabetes mellitus and cancer: The role of pharmacotherapy. J Clin Oncol Off J Am Soc Clin Oncol. (2016) 34:4261–9. doi: 10.1200/JCO.2016.67.4044
109. Munoz LD, Sweeney MJ, and Jameson JM. Skin resident γδ T cell function and regulation in wound repair. Int J Mol Sci. (2020) 21:9286. doi: 10.3390/ijms21239286
110. Liu Z, Xu Y, Zhang X, Liang G, Chen L, Xie J, et al. Defects in dermal Vγ4 γ δ T cells result in delayed wound healing in diabetic mice. Am J Transl Res. (2016) 8:2667–80.
111. Mills RE, Taylor KR, Podshivalova K, McKay DB, and Jameson JM. Defects in skin gamma delta T cell function contribute to delayed wound repair in rapamycin-treated mice. J Immunol Baltim Md 1950. (2008) 181:3974–83. doi: 10.4049/jimmunol.181.6.3974
112. Liu Z, Liang G, Gui L, Li Y, Liu M, Bai Y, et al. Weakened IL-15 production and impaired mTOR activation alter dendritic epidermal T cell homeostasis in diabetic mice. Sci Rep. (2017) 7:6028. doi: 10.1038/s41598-017-05950-5
113. Huang H, Cui W, Qiu W, Zhu M, Zhao R, Zeng D, et al. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J Dermatol Sci. (2015) 79:241–51. doi: 10.1016/j.jdermsci.2015.06.002
114. Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. (2010) 329:1205–10. doi: 10.1126/science.1192698
115. Hennighausen L and Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. (2008) 22:711–21. doi: 10.1101/gad.1643908
116. Taylor KR, Mills RE, Costanzo AE, and Jameson JM. Gammadelta T cells are reduced and rendered unresponsive by hyperglycemia and chronic TNFalpha in mouse models of obesity and metabolic disease. PloS One. (2010) 5:e11422. doi: 10.1371/journal.pone.0011422
117. Wellen KE and Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. (2005) 115:1111–9. doi: 10.1172/JCI25102
118. Kerley-Hamilton JS, Trask HW, Ridley CJA, Dufour E, Ringelberg CS, Nurinova N, et al. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a western diet. Environ Health Perspect. (2012) 120:1252–9. doi: 10.1289/ehp.1205003
119. Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, et al. A role for human skin-resident T cells in wound healing. J Exp Med. (2009) 206. doi: 10.1084/jem.20081787
120. Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, and Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. (2000) 190:589–94. doi: 10.1002/(SICI)1096-9896(200004)190:5%3C589::AID-PATH553%3E3.0.CO;2-T
121. Brown DL, Kane CD, Chernausek SD, and Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am J Pathol. (1997) 151:715–24.
122. Bonneville M, O’Brien RL, and Born WK. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat Rev Immunol. (2010) 10:467–78. doi: 10.1038/nri2781
123. Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. (2015) 6:7687. doi: 10.1038/ncomms8687
124. Lawler W, Castellanos T, Engel E, Alvizo CR, Kasler A, Bshara-Corson S, et al. Impact of obesity on the CCR6-CCL20 axis in epidermal γδ T cells and IL-17A production in murine wound healing and psoriasis. BioRxiv Prepr Serv Biol. (2024) 2024:.04.09.588780. doi: 10.1101/2024.04.09.588780
125. Wu C, Chen X, Shu J, and Lee CT. Whole-genome expression analyses of type 2 diabetes in human skin reveal altered immune function and burden of infection. Oncotarget. (2017) 8:34601–9. doi: 10.18632/oncotarget.16118
126. Nichols JM, Pham HV, Lee EF, Mahalingam R, and Shepherd AJ. Single-cell analysis of age-related changes in leukocytes of diabetic mouse hindpaws. Cell Mol Life Sci CMLS. (2024) 81:146. doi: 10.1007/s00018-024-05128-z
127. Saltiel AR and Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. (2017) 127:1–4. doi: 10.1172/JCI92035
128. Buysschaert M, Baeck M, Preumont V, Marot L, Hendrickx E, Van Belle A, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal γδ T-cell number: A prospective case-series study. Br J Dermatol. (2014) 171:155–61. doi: 10.1111/bjd.12886
129. Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. (2015) 64:2537–49. doi: 10.2337/db14-1577
130. He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. (2019) 566:115–9. doi: 10.1038/s41586-018-0849-9
131. Brazzelli V, Maffioli P, Bolcato V, Ciolfi C, D’Angelo A, Tinelli C, et al. Psoriasis and diabetes, a dangerous association: evaluation of insulin resistance, lipid abnormalities, and cardiovascular risk biomarkers. Front Med. (2021) 8:605691. doi: 10.3389/fmed.2021.605691
132. Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, and Gelfand JM. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J Am Acad Dermatol. (2018) 78:315–322.e1. doi: 10.1016/j.jaad.2017.10.050
133. Trial | NCT04589325 . Available online at: https://cdek.pharmacy.purdue.edu/trial/NCT04589325/ (Accessed March 15, 2025).
134. Trial | NCT03941132 . Available online at: https://cdek.pharmacy.purdue.edu/trial/NCT03941132/ (Accessed March 15, 2025).
135. Richter B, Hemmingsen B, Metzendorf MI, and Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. (2018) 10:CD012661. doi: 10.1002/14651858.CD012661.pub2
136. Vatankhah N, Jahangiri Y, Landry GJ, Moneta GL, and Azarbal AF. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regener Off Publ Wound Heal Soc Eur Tissue Repair Soc. (2017) 25:288–91. doi: 10.1111/wrr.12514
137. Guo XZJ, Dash P, Crawford JC, Allen EK, Zamora AE, Boyd DF, et al. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity. (2018) 49:531–544.e6. doi: 10.1016/j.immuni.2018.07.011
138. Yuan N, Feng ZH, Wei Q, Wang P, and Ying GW. Expression of CD4+CD25+Foxp3+ regulatory T cells, interleukin 10 and transforming growth factor β in newly diagnosed type 2 diabetic patients. Exp Clin Endocrinol Diabetes. (2018) 126:96–101. doi: 10.1055/s-0043-113454
139. Qin W, Sun L, Dong M, An G, Zhang K, Zhang C, et al. Regulatory T cells and diabetes mellitus. Hum Gene Ther. (2021) 32:875–81. doi: 10.1089/hum.2021.024
Keywords: gamma delta T cells, cytokines, diabetes mellitus, autoimmunity, insulin resistance, inflammation, diabetic complications
Citation: Wang B, Li Q and Wang Q (2025) γδ T cells in diabetes mellitus: dual roles and therapeutic implications. Front. Immunol. 16:1693466. doi: 10.3389/fimmu.2025.1693466
Received: 27 August 2025; Accepted: 29 October 2025;
Published: 18 November 2025.
Edited by:
Dimitri Poddighe, VinUniversity, VietnamReviewed by:
Surya Prakash Pandey, University of Pittsburgh, United StatesKaren Cerosaletti, Benaroya Research Institute, United States
Sally C. Kent, University of Massachusetts Medical School, United States
Alice Cheung, Singapore General Hospital, Singapore
Veronika Niederlova, Institute of Molecular Genetics (ASCR), Czechia
Copyright © 2025 Wang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuyue Wang, d3F5Y211MTIzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Bing Wang
Bing Wang Qi Li
Qi Li Qiuyue Wang
Qiuyue Wang