- 1Department of Emergency, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
- 3Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
Sepsis-induced multiple organ dysfunction syndrome is the leading cause of mortality among patients with sepsis. Its pathophysiological mechanisms encompass various factors, including dysregulated inflammatory responses, endothelial injury and microcirculatory disturbances, abnormal activation of cell death pathways, as well as metabolic reprogramming and immune interactions. The central nervous system (CNS) is one of the earliest and most susceptible organs affected during the septic process. This involvement not only results in brain dysfunction due to neuronal damage, excessive activation of microglia, and neuroinflammatory responses but also contributes to systemic organ damage through diverse neural regulatory mechanisms. Specifically, the CNS influences the function of distant organs via the autonomic nervous system—comprising inhibition of the vagus nerve cholinergic anti-inflammatory pathway and excessive activation of sympathetic nerve pathways—the neuroimmune regulatory network, central trained immunity regulation, extravasation of brain-derived inflammatory factors, and exosome transport. This paper provides a systematic review of key pathogenic mechanisms underlying sepsis-related organ damage while emphasizing the pivotal regulatory role played by the central nervous system in this pathological process along with its potential therapeutic implications.
1 Introduction
Sepsis is a life-threatening syndrome characterized by organ dysfunction resulting from a dysregulated host response to infection (1). This condition can lead to multi-organ system damage, primarily affecting vital organs such as the brain, heart, lungs, liver, and kidneys (2, 3). However, current clinical practice for treating sepsis-related organ damage predominantly relies on antibiotics and supportive care, with a notable absence of specific targeted therapeutic agents. Importantly, the central nervous system (CNS), recognized as one of the earliest and most vulnerable target organs (4), plays a critical role in the pathophysiology of sepsis. Clinical studies indicate that up to 70% of patients with sepsis develop sepsis-associated encephalopathy (SAE), which is characterized by diffuse brain dysfunction without direct evidence of central nervous system infection (5). his acute brain injury not only elevates the acute mortality rate among sepsis patients (ranging from 9% to 76%) (6) but also contributes to long-term cognitive impairment that severely impacts patients’ quality of life (7). Moreover, recent research has revealed that the CNS serves as a key driver in both the onset and progression of peripheral organ dysfunction and its long-term sequelae by modulating autonomic neural pathways and neuroinflammatory responses (8, 9). An experimental study have demonstrated that protective interventions targeting the nervous system—such as cholinergic pathway-targeted therapies—can effectively reverse multi-organ damage (10). These significant findings underscore the CNS’s central regulatory role in sepsis-related multi-organ dysfunction; however, elucidation of its regulatory mechanisms remains elusive. Therefore, an in-depth investigation into how the CNS influences sepsis-related organ damage will provide essential theoretical foundations and therapeutic targets for developing novel intervention strategies aimed at mitigating multi-organ damage associated with sepsis.
2 The core mechanism of sepsis-induced organ damage
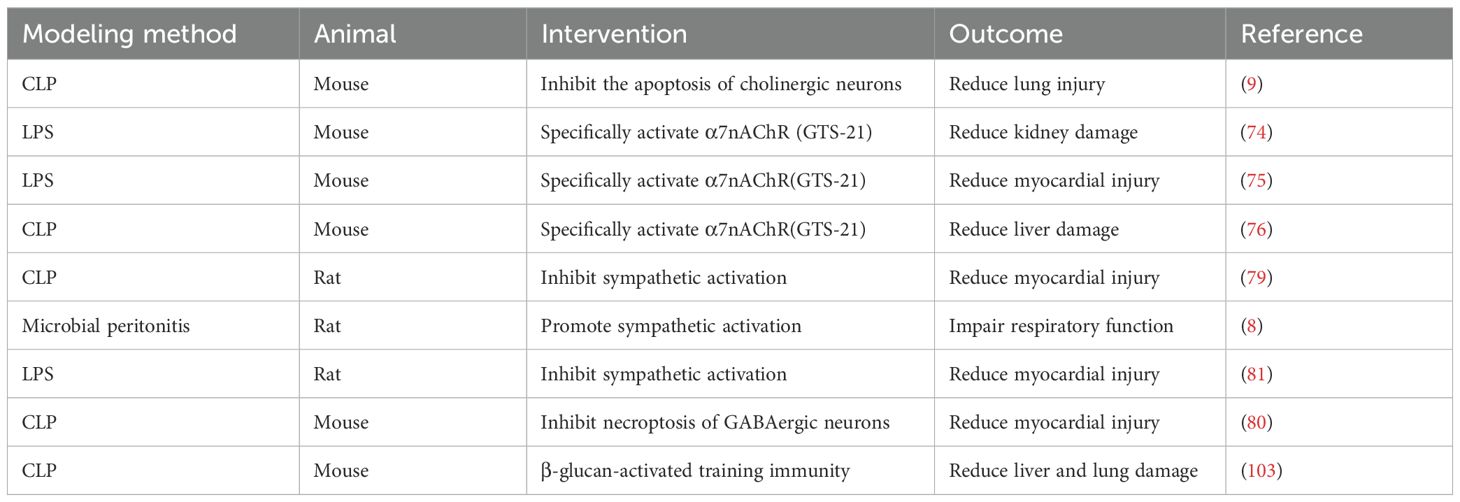
The fundamental mechanism underlying sepsis-induced multiple organ damage arises from the dysregulated inflammatory response of the host to infection, which initiates a cascade of interrelated pathophysiological processes (Figure 1). The resultant excessive cytokine storm leads to endothelial cell injury and aberrant activation, subsequently promoting an overactivation of the coagulation system (11, 12). This series of events culminates in microcirculatory thrombosis, tissue ischemia and hypoxia, as well as cellular apoptosis, thereby resulting in multiple organ dysfunction (13). This core process is frequently accompanied by an immunosuppressive state and metabolic reprogramming (14–16), both of which further intensify disease progression. Such alterations increase the risk of immune paralysis and secondary infections, ultimately exacerbating patient prognosis.
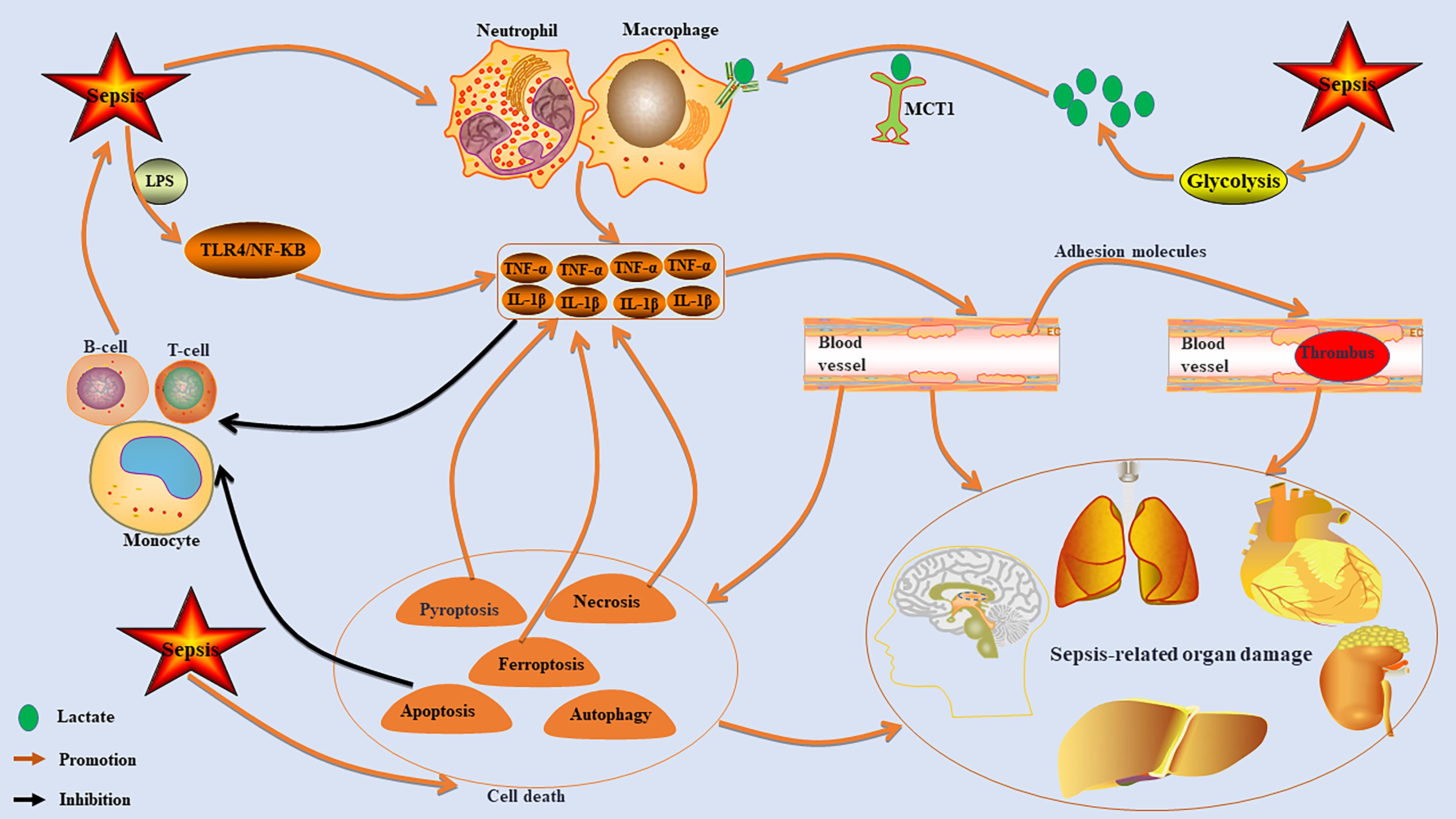
Figure 1. The core mechanism of sepsis-induced organ damage. Sepsis can lead to excessive activation of immune cells, such as macrophages and neutrophils, resulting in the release of high levels of pro-inflammatory factors like TNF-α and IL-1β. This cytokine storm damages endothelial cells, increases adhesion molecules, promotes microcirculation thrombosis, and causes ischemia and hypoxia, which can trigger multiple organ dysfunction. The inflammatory response also inhibits monocyte, T cell, and B cell functions, leading to immunosuppression that exacerbates sepsis. Additionally, sepsis activates cell death pathways that further increase inflammatory cytokine production. It also stimulates the glycolytic pathway to enhance lactate production, promoting macrophage activation via MCT1 transport.
2.1 Uncontrolled inflammatory response and immune suppression
Sepsis is characterized by an exaggerated inflammatory response in its early stages (17). The infection by pathogens activates immune cells, such as macrophages and neutrophils, prompting them to release substantial quantities of pro-inflammatory mediators, including tumor necrosis factor-α (TNF-α) and lnterleukin-1β (IL-1β), which in turn initiates a systemic inflammatory response (17). Among these immune cells, the abnormal activation of macrophages—particularly through M1 polarization—plays a pivotal role in driving the inflammatory cascade. The inflammatory mediators released can directly inflict damage on endothelial cells and parenchymal organs (18, 19). Furthermore, pathogen-associated molecular patterns, such as lipopolysaccharides (LPS), further intensify this uncontrolled systemic inflammatory response by continuously activating signaling pathways associated with inflammation, notably TLR4/NF-κB (20, 21). Significantly, the body may concurrently enter or swiftly transition into an immune-suppressed state marked by lymphocyte depletion and monocyte dysfunction (22). This condition elevates the risk for secondary infections. In summary, heightened inflammation and immune suppression frequently coexist within sepsis patients, creating an “immune paradox” that contributes to multiple organ dysfunction.
2.2 Endothelial injury and microcirculation disorder
Under septic conditions, the heparan sulfate glycocalyx structure on the surface of endothelial cells is compromised, leading to impaired vascular barrier function and an abnormal increase in permeability (23, 24). Consequently, this results in tissue edema and insufficient microcirculatory perfusion, ultimately causing ischemic injury to various organs (23, 24). Simultaneously, endothelial cell apoptosis intensifies, accompanied by a marked upregulation of adhesion molecules such as ICAM-1 and VCAM-1 (25, 26). This promotes aberrant leukocyte-endothelial cell adhesion and microthrombosis (25, 26). These pathological alterations further exacerbate microcirculatory disorders, creating a positive feedback loop of tissue hypoxia-inflammation-coagulation that ultimately leads to ischemic hypoxic injury across multiple organs.
2.3 Activation of cell death pathways
Apoptosis in sepsis exhibits a cell type-specific activation pattern. The excessive apoptosis of immune cells, such as dendritic cells, results in a reduction of their numbers and functional impairments, thereby promoting an immunosuppressive state that heightens the risk of secondary infections (16). Conversely, the inhibition of neutrophil apoptosis may lead to alternative death pathways, including necroptosis or pyroptosis, which can further aggravate organ damage (27). Necroptosis plays a pivotal role during both the early and progressive stages of sepsis by mediating the release of substantial quantities of damage-associated molecular patterns (28). This process continuously amplifies the inflammatory cascade and is closely associated with sepsis-related organ dysfunction (28). Furthermore, excessive activation of pyroptosis—driven by Gasdermin D-induced membrane perforation and subsequent release of inflammatory mediators such as IL-1β—exacerbates oxidative stress, endothelial dysfunction, and thrombosis, particularly in cases involving septic cardiomyopathy (29, 30). For instance, the activation of the NLRP3 inflammasome and subsequent caspase-1 activation lead to the release of IL-1β and pyroptosis, which can directly result in myocardial injury and lesions in other organs (31, 32). Ferroptosis, characterized as a form of iron-dependent lipid peroxidation-driven cell death, is significantly implicated in the pathological processes associated with sepsis-induced brain injury, cardiomyopathy, acute kidney injury, lung damage, and liver dysfunction (1, 33). This type of cell death can aggravate the inflammatory cascade during sepsis by promoting the release of inflammatory mediators and contributing to multi-organ damage (34). Notably, ferroptosis inhibitors exhibit organ-protective effects (35). Furthermore, dysregulation of autophagy within endothelial cells and parenchymal cells—such as obstruction of autophagic flux—can intensify cellular damage and is closely linked to both the onset and progression of sepsis-related organ dysfunction (31). In all, sepsis has the capacity to activate multiple pathways leading to programmed cell death; this not only results in direct cellular demise but also exacerbates multi-organ dysfunction through mechanisms such as amplification of inflammatory cascades and disruption of immune homeostasis. However, further exploration is required to elucidate the inter-regulatory network relationships among these pathways.
2.4 Metabolic-immune interactions
Metabolic disorders serve as a critical molecular foundation for the onset of sepsis and subsequent organ dysfunction (36). Throughout the progression of sepsis, host cells undergo metabolic reprogramming, which is primarily characterized by a shift in energy metabolism from oxidative phosphorylation to glycolysis (37). This metabolic transition is essential for the activation and pro-inflammatory function of immune cells, particularly macrophages (38). Enhanced glycolysis during the acute phase not only supports the pro-inflammatory response necessary to combat infection (39), but also partially mitigates the “energy crisis” induced by sepsis (37). Persistent metabolic reprogramming fosters a transformation in immune status from pro-inflammatory to immunosuppressive, thus increasing susceptibility to secondary infections (40). The metabolites derived from glycolysis exhibit dual roles: they serve as energy substrates while also acting as crucial regulatory signaling molecules within the immune system (41). For instance, lactate—a product of metabolism—can influence immune cell functionality through specific transporters (such as MCT1 and MCT4) and receptors like GPR81 (42). Distinct patterns of immune dysfunction are associated with specific metabolic disorder profiles (43, 44). The immunosuppressive state observed in sepsis is accompanied by significant metabolic disturbances (45, 46). These manifest as enrichments across multiple pathways along with distinct variations in metabolites (notably involving amino acid and lipid metabolism disorders) (45, 46). This indicates that metabolic reprogramming drives the immune response from an initial pro-inflammatory phase to later immunosuppression, while being intricately regulated by underlying immune dysfunctions.
3 Key pathophysiological mechanisms of sepsis-associated CNS injury
SAE represents the primary clinical manifestation of CNS injury in patients suffering from sepsis (47–49). The pathophysiological underpinnings of SAE encompass four critical components (Figure 2): neuronal injury (50), aberrant activation of microglia (51), neuroinflammation and dysfunction of the blood-brain barrier (BBB) (52). Activated microglia initiate neuroinflammatory cascades and oxidative stress, leading to neuronal damage (48, 53). Importantly, this neuroimmune activation extends beyond the confines of brain tissue; it can also have systemic effects by releasing pro-inflammatory factors (such as TNF-α and IL-1β), thereby contributing to the onset and progression of dysfunction in peripheral organs (2, 48, 51, 52).
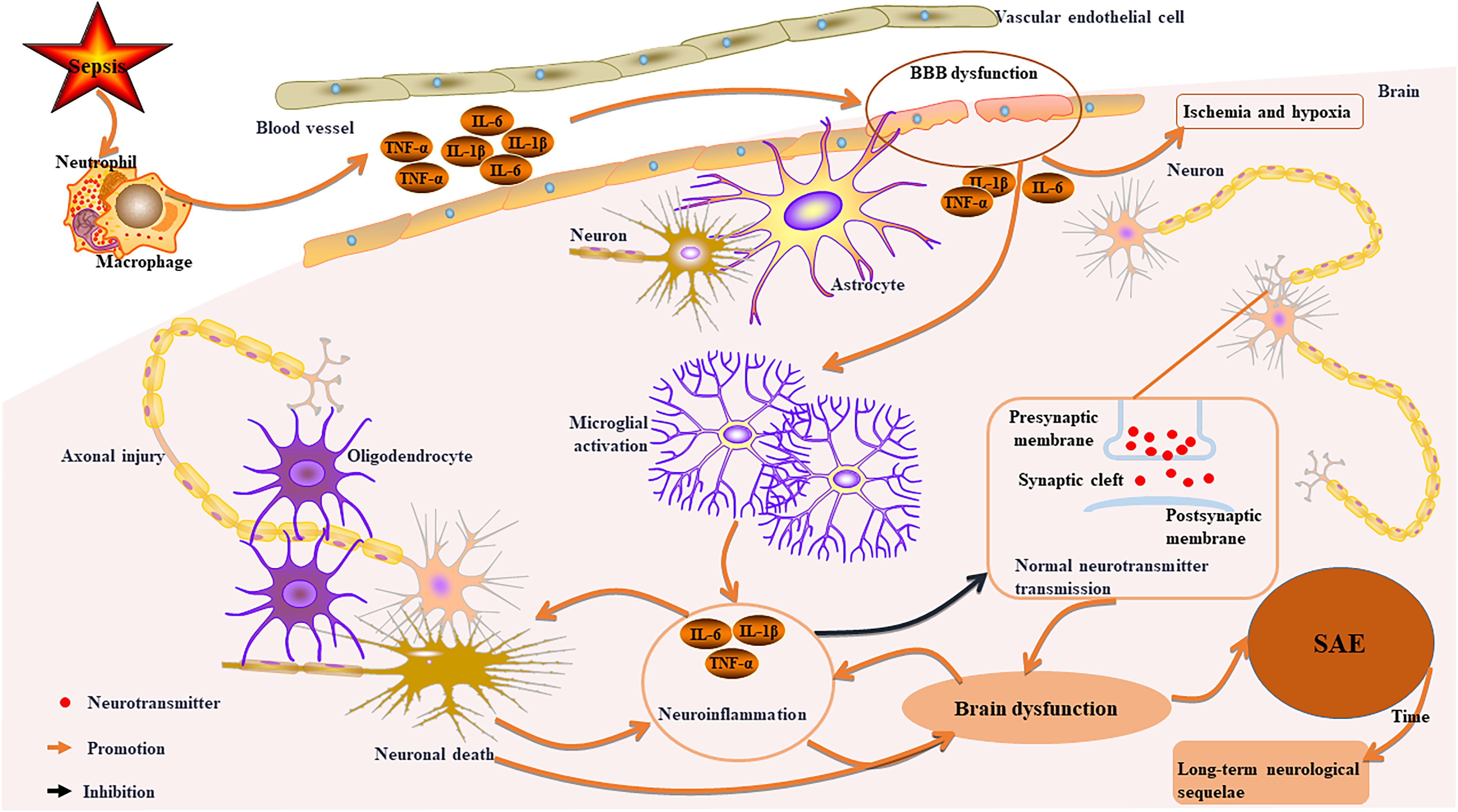
Figure 2. Key pathophysiological mechanisms of sepsis-related CNS injury. Sepsis activates immune cells, leading to systemic inflammation and damage to the BBB. This disruption allows inflammation into the brain, causing cerebral ischemia and hypoxia. Activated microglia release pro-inflammatory factors, resulting in neuroinflammation, axonal damage, neuronal death, and impaired neurotransmitter transmission, which contributes to brain dysfunction and SAE. Over time, survivors may experience long-term neurological sequelae. Furthermore, neuronal death can worsen neuroinflammation and brain dysfunction in a reciprocal manner.
3.1 BBB dysfunction and neuroinflammation
The systemic inflammatory response initiated by sepsis serves as the primary catalyst for SAE (54). This pathological process results in significant damage to both the structure and function of the BBB, characterized by a down-regulation of tight junction proteins and an abnormal increase in permeability (55, 56). Such alterations facilitate the translocation of peripheral inflammatory mediators across the BBB into the central nervous system, ultimately disrupting neural microenvironment homeostasis (55, 56). Once the integrity of the BBB is compromised, microglia become markedly activated and adopt a pro-inflammatory phenotype (48).These activated microglia may cause damage to the structural integrity of neurons, impair synaptic plasticity, and disrupt neurotransmitter transmission by releasing pro-inflammatory factors (49, 57).
3.2 Abnormal cerebral perfusion and ischemic injury
Sepsis-induced BBB dysfunction and impaired cerebral autoregulation lead to cerebral hypoperfusion (58). The inflammatory response from sepsis facilitates the abnormal transfer of peripheral inflammatory molecules to the CNS, causing pathological accumulation of amyloid-β (Aβ) and tau proteins, which are linked to neurodegenerative diseases (59, 60). Clinical studies indicate that this accumulation in SAE patients not only worsens neurodegenerative conditions but is also associated with cerebrovascular diseases (59). Pathological evidence shows characteristic edema changes, ischemic injury, and infarction foci in the brain tissue of SAE patients, all closely related to microcirculation disorders (61).
3.3 Axonal injury and neuronal death
Sepsis can lead to axonal injury and neuronal death (62). In sepsis mouse models, axonal damage has been noted particularly in the cerebral cortex, thalamus, and hippocampus (57). This occurs due to a significant release of pro-inflammatory cytokines from the systemic inflammatory response triggered by sepsis (48). These cytokines activate microglia, which then release toxic mediators that harm neurons and axons directly (48). Furthermore, activated microglia may promote synaptic pruning, resulting in synaptic loss and neuronal death, which further exacerbates inflammation (63). Such changes may be causally linked to long-term cognitive dysfunction in patients with SAE (57).
3.4 Mechanism of long-term neurological sequelae
Survivors of SAE frequently experience what is known as “post-sepsis syndrome,” a condition characterized by persistent cognitive deficits, abnormal emotional regulation, and functional disabilities, among other symptoms (64, 65). These phenomena are associated with disrupted neural circuits and sustained neuroinflammation, which propagate systemic effects through the neuroimmune axis, ultimately impacting the functionality of various organ systems and perpetuating a detrimental cycle (65, 66).
4 CNS as a core driver of organ damage in sepsis
In sepsis, the CNS regulates peripheral immune responses and organ functions through complex interactions within the neuro-immune-endocrine network (8, 67). The mechanisms can be summarized into the below core pathways (Figure 3, Table 1).
Figure 3. The mechanism of CNS as a core driver of organ damage in sepsis. Sepsis can worsen central nervous system (CNS) injury, which in turn exacerbates peripheral inflammation and organ damage. CNS injury may overactivated the sympathetic nervous system by disrupting autonomic nerve function, leading to excessive catecholamine release and impairing the vagal anti-cholinergic pathway, thus worsening peripheral inflammation. It also disrupts the neuroimmune regulatory network, hyperactivates the hypothalamic-pituitary-adrenal (HPA) axis, causes neurotransmitter transmission disorders, and promotes immune cell migration, further impacting peripheral immunity. Additionally, CNS injury can enhance peripheral inflammation by regulating central trained immunity and causing immune homeostasis imbalance. It may aggravate inflammation through the extravasation of brain-derived inflammatory factors and their transport via exosomes. Long-term CNS injury can lead to neurological sequelae and further intensify peripheral organ damage.
4.1 Autonomic nerve dysfunction
4.1.1 Damage to the vagus nerve cholinergic anti-inflammatory pathway
In animal models of sepsis, stimulation of the vagus nerve has been demonstrated to inhibit the synthesis of inflammatory cytokines, the recruitment of white blood cells, and the activation of endothelial cells (68, 69). The efferent fibers of the vagus nerve release acetylcholine, which interacts with nicotinic receptors on macrophages’ surface to suppress the production of pro-inflammatory cytokines (70, 71). Sepsis can induce inflammation in the brainstem, leading to damage in cholinergic anti-inflammatory pathway (CAP) function. This results in diminished efferent signals from the vagus nerve and a compromised ability to effectively mitigate peripheral inflammatory responses (9). Dysfunction within CAP contributes to an excessive release of pro-inflammatory factors, thereby exacerbating inflammatory damage to vital organs including the heart, lungs, and liver (72). Furthermore, downregulation of central cholinergic signaling mediated by M1 muscarinic acetylcholine receptors (M1AChR) may intensify peripheral inflammation (73–76). Experimental evidence also indicates that septic mice exhibit impaired functionality in vagus nerve preganglionic neurons located in the brainstem; this impairment weakens their capacity to regulate immune responses in peripheral organs such as the spleen (9).
4.1.2 Excessive sympathetic nerve activation
Peripheral infection activates the brainstem’s sympathetic nerve via humoral or neural pathways, resulting in increased sympathetic output (8). In an Escherichia coli sepsis model, brainstem inflammation leads to sustained sympathetic hyperactivity and a massive release of catecholamines (8). This hyperactivity directly harms the myocardium (e.g., causing arrhythmias) (77) and worsens renal ischemic injury through vasoconstriction (8). Inhibition of the sympathetic nerve and the suppression of catecholamine release can mitigate myocardial dysfunction associated with sepsis (78–81). Catecholamines also promote neutrophil infiltration and oxidative stress, further aggravating lung (82) and liver injuries (83).
4.2 The neuro-immune regulatory network
The CNS not only responds to peripheral inflammation in sepsis but also regulates immune cell migration and inflammatory factor production, creating a “brain-peripheral organ” feedback loop (6). Sepsis can induce intestinal immune cells (e.g., IL-7R CD8 γδ T17 cells) to migrate to the CNS, altering local immunity and amplifying systemic inflammation, which exacerbates oxidative stress and tissue damage in organs like the liver, lungs, and heart (84). In addition, sepsis-induced neurotransmitter disorders can contribute to peripheral organ damage by influencing immune metabolism. For example, dopamine imbalance can affect immune metabolism via the dopamine-tyrosine decarboxylase 1 axis, resulting in inflammation-related immunosuppression during sepsis (85). Central neurotransmitters (like acetylcholine and γ-aminobutyric acid (GABA)) and cytokines (such as IL-1β) regulate peripheral immunity bidirectionally (86). Acetylcholine released from the vagal nucleus inhibits the inflammatory response of splenic macrophages; reduced acetylcholine transmission in the hippocampus during sepsis weakens this immunoinhibition (86). GABA influences macrophage maturation and inflammatory responses through GABA transporters (GAT2), with GAT2 deficiency leading to decreased IL-1β production in pro-inflammatory macrophages, thus alleviating inflammation (87). The activation of α2A adrenergic receptors in spinal astrocytes mitigates sepsis-induced cardiac injury by inhibiting the necroptosis of GABAergic neurons (80).Notably, uncontrolled peripheral immunity due to neurotransmitter disorders may worsen damage chains like the brain-liver and brain-gut axes (88, 89). The hypothalamic-pituitary-adrenal (HPA) axis is a key part of the neuroendocrine system that regulates peripheral immune responses through hormones such as glucocorticoids (e.g., cortisol), primarily exerting anti-inflammatory effects (90). HPA axis activation commonly occurs in acute sepsis phases, characterized by significantly elevated circulating cortisol levels (91, 92). Persistent hyperactivity of the HPA axis can disrupt negative feedback mechanisms (e.g., α1-adrenergic receptor desensitization), leading to excessive glucocorticoid exposure and worsening multi-organ damage (93, 94). This imbalance in communication may lead to persistent organ dysfunction (5, 95, 96). The aforementioned evidence suggests that CNS damage in sepsis plays a crucial role in the associated peripheral organ dysfunction by influencing immune cell migration, neurotransmitter transmission, and the HPA axis. However, the potential interactions among these factors require further investigation.
4.3 The regulatory role of central training immunity
Central training immunity refers to the immune memory established by progenitor cells in the bone marrow through metabolic and epigenetic reprogramming (e.g., histone modification) in response to specific stimuli (e.g., microorganisms), which enhances reactions to subsequent stimuli (97). Research indicates that sepsis can create persistent innate immune memory in the mouse brain, termed “trained innate immunity,” independent of peripheral immune systems (98). Specifically, microglia in the hippocampus of sepsis-surviving mice undergo epigenetic changes, entering a long-term high-responsiveness state (like pro-inflammatory M1 polarization) that increases vulnerability to neurotoxins and causes cognitive dysfunction and neural damage (98). Central trained immunity enhances the body’s defense against sepsis by boosting bone marrow progenitor cells’ response to pathogens (99). However, abnormal immune responses can lead to excessive inflammation and autoimmune damage (99). For example, immune memory in the brain may increase vulnerability to neurodegenerative diseases, contributing to cognitive dysfunction in sepsis survivors (98). Notably, specific stimuli like β-glucan can induce “trained immunity” in central immune cells, allowing for a stronger anti-inflammatory response upon secondary stimulation (100–102). Research indicates that central trained immunity may reduce organ damage from sepsis—such as in the liver and lung (103)—by regulating peripheral monocyte/macrophage phenotypic transformation (e.g., promoting conversion from pro-inflammatory M1 to anti-inflammatory M2) (100, 101, 104). The above explanation indicates that central training immunity has a dual role in sepsis: it enhances immune memory for protection while potentially increasing long-term vulnerability of the nervous and immune systems post-sepsis.
4.4 Brain-derived inflammatory factor extravasation
Sepsis can disrupt the BBB, resulting in an elevation of inflammatory factors within the brain, such as interleukin-6 (IL-6) and high mobility group box 1 (HMGB1) (48, 54). Radioactive labeling experiments have demonstrated that HMGB1 is capable of bidirectional translocation across the BBB—moving from blood to brain tissue and vice versa. Moreover, inflammation induced by lipopolysaccharides can accelerate its transport rate into peripheral circulation (105). Additionally, research indicates a significant increase in IL-6 expression within the brains of septic mice, with BBB dysfunction facilitating cytokine infiltration from brain tissue into peripheral regions (106). Furthermore, factors derived from the brain may indirectly influence vascular endothelial cell injury and contribute to damage in organs such as the lungs and kidneys through mechanisms related to neuroinflammation (47, 54, 107). It has also been established that exosomes containing inflammatory factors are capable of traversing the BBB and entering cerebral tissues to promote neuronal damage (108, 109). However, whether inflammatory factors produced in the brain can be transmitted via exosomes to affect peripheral organ integrity remains an area requiring further investigation. In summary, these findings suggest that sepsis compromises the integrity of the BBB through various mechanisms including HMGB1 nuclear translocation, receptor activation, and immune cell infiltration; this leads to leakage of inflammatory mediators like HMGB1. This process may involve structural damage that establishes a positive feedback loop for “brain-peripheral” inflammatory dissemination; however, additional studies are necessary for a comprehensive understanding of these underlying mechanisms.
4.5 Others
In addition, the SAE resulting from nervous system injury not only causes acute cognitive impairment but also has long-term effects on autonomic nerve regulation of organs. This is closely linked to “post-sepsis syndrome,” characterized by abnormalities in cardiovascular and renal functions observed in survivors (110, 111).
5 Intervention strategies targeting the core role of the CNS
Based on the core role of the CNS, current treatment strategies mainly focus on regulating CNS inflammation and immune imbalance (112, 113). First, direct neuroinflammation inhibition: 1) Selective elimination of reactive oxygen species, such as hydroxyl radicals, significantly reduces oxidative stress and neuroinflammation in the CNS, improving cognitive dysfunction related to sepsis (114); 2)Ferroptosis inhibitors like Liproxstatin-1 reduce neuronal lipid peroxidation while enhancing SAE outcomes and multi-organ function (1, 115); 3)Regulation of TLR4 signaling involves targeted inhibition of the microglial TLR4/NF-κB pathway to prevent neuroinflammation from spreading peripherally (116). Second, regulation of the neuro-immune axis: 1) Adenosine-lidocaine-magnesium therapy balances autonomic nerve activity, alleviating hyperactivity in sympathetic nerves and reducing organ inflammation (113); 2) Enhancing cholinergic pathways with α7nAChR agonists restores vagus nerve anti-inflammatory functions (9, 72). Third, BBB protection and repair: Glycoprotein modulation and APOH protein application improve BBB integrity while decreasing neuroinflammation and systemic inflammation (23, 117).; Vitamin C therapy offers antioxidant benefits that protect BBB structure and limit neuroinflammatory overflow (118). However, these intervention strategies remain in research stages requiring further clinical translation (119). Further optimization is essential. Current research indicates a discrepancy between the outcomes of animal experiments and human clinical trials regarding sepsis treatment drugs (120). Considering the pivotal role of the central nervous system in the pathophysiological processes associated with sepsis, future drug development should explore the integration of neuroprotective strategies alongside systemic anti-inflammatory treatments. This approach holds promise for disrupting the detrimental cycle characteristic of sepsis.
5.1 Limitations and challenges of current research
Although existing evidence suggests that a comprehensive treatment strategy centered on the CNS may pave the way for new clinical intervention pathways for patients with sepsis, several key scientific issues remain unresolved. First, the pathogenesis of sepsis is complex, and patients exhibit highly heterogeneous clinical manifestations. Current animal models still fail to fully replicate the disease process observed in humans. While some studies have compared cellular changes in brain tissue samples from patients with SAE and murine models, revealing certain similarities in immune microenvironment signaling (121), there is also evidence indicating that humans, mice, and other species display fundamental differences in blood immune characteristics due to varying sensitivities to inflammation (122). Furthermore, during bacteremia, significant disparities exist between humans and mouse models regarding bacterial clearance ability, phagocytic function, and cytokine induction levels in the bloodstream (123). These differences limit the reliability of current animal models in simulating human neuroimmune responses and consequently constrain the clinical translational value of related research and drug development. Second, current research predominantly relies on single-organ studies conducted within animal models; systematic multi-organ comparative analyses are lacking. Moreover, data derived from human tissue samples remains scarce. Third, the pathogenesis and intervention strategies for systemic inflammatory response syndrome (SIRS) differ between the acute and chronic phases. Currently, the specific mechanisms and dynamic changes regarding neuroimmune regulation in peripheral organ damage at various stages remain poorly understood. Fourth, the regulatory interactions among multiple organs present a highly complex network. In sepsis, the central nervous system modulates peripheral organ function through several mechanisms; concurrently, intestinal microbiota can influence brain inflammatory responses via the gut-brain axis. The extent to which other organs—such as the lungs, liver, and kidneys—may exacerbate central nervous system injury through analogous feedback loops warrants further investigation. Fifth, conclusive evidence demonstrating that brain-derived inflammatory factors directly induce peripheral organ damage is still lacking. Additionally, whether these brain inflammatory factors contribute to further harm in other organs through carriers such as exosomes needs to be thoroughly examined. Sixth, the dynamic regulatory mechanisms of the neuro-immune-organ axis are not yet fully elucidated. For instance, it remains unclear how specific interactions between neuroendocrine factors (including HPA axis hormones and catecholamines) and the immune system mediate peripheral organ injury.
Given the aforementioned research limitations and challenges, future investigations should prioritize the development of sepsis models that more closely mimic the characteristics of human disease, such as organoids or organ-on-a-chip systems, to effectively simulate the neuro-immune-organ regulatory network. Furthermore, it is essential to integrate multi-modal data—including clinical parameters, imaging information, and biomarkers—to construct predictive models for sepsis neuro-immune regulation, thereby facilitating individualized treatment approaches. Moreover, adopting a longitudinal research strategy that combines single-cell sequencing with spatial transcriptomics techniques is recommended to systematically illustrate the dynamic evolution of the neuro-immune-organ network throughout the disease course and analyze its regulatory patterns. The utilization of multi-organ chip or organoid co-culture systems to emulate neuro-organ interactions under septic conditions can also provide valuable insights into molecular mechanisms at play.
5.2 Prospects
Overall, the CNS plays a crucial role in the pathogenesis and prognosis of sepsis, acting as both an early target for damage and a key regulator driving multi-organ dysfunction through neuroinflammation, neuroimmune dysregulation, and autonomic dysfunction. A comprehensive understanding of the CNS’s fundamental role in sepsis is essential for elucidating its underlying pathophysiology and provides a theoretical foundation for developing an integrative treatment strategy focused on neuroimmune regulation. Ultimately, this approach aims to enhance clinical outcomes for patients suffering from sepsis.
Author contributions
FT: Writing – original draft, Writing – review & editing. SW: Conceptualization, Software, Writing – review & editing. ZZ: Investigation, Methodology, Writing – review & editing. XL: Funding acquisition, Supervision, Visualization, Writing – review & editing. LQ: Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present study was supported by the National Science Foundation of China (grant nos. 82071353).
Acknowledgments
We thank EditSprings (https://www.editsprings.com) for assisting with English language editing of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CNS: central nervous system; SAE: sepsis-associated encephalopathy; TNF-α: tumor necrosis factor-α; IL-1β: lnterleukin-1β; LPS: lipopolysaccharides; BBB: blood-brain barrier; Aβ: amyloid-β; CAP: cholinergic anti-inflammatory pathway; M1AChR: M1 muscarinic acetylcholine receptors; GABA: γ-aminobutyric acid; GAT2: GABA transporters; HPA: hypothalamic-pituitary-adrenal; IL-6: interleukin-6; HMGB1: high mobility group protein B1.
References
1. Liang H, Chunfeng L, Yujun Y, Xueyan L, and Qingjun C. Pharmacological inhibition of ferroptosis as a therapeutic target for sepsis-associated organ damage. Eur J Med Chem. (2023) 257:115438. doi: 10.1016/j.ejmech.2023.115438
2. Atsushi M, Max B, Monowar A, and Ping W. Exosomes in Sepsis. Front Immunol. (2020) 11:2140. doi: 10.3389/fimmu.2020.02140
3. Xuan L, Yun-Mei Y, and Yuan-Qiang L. Immunosenescence: A critical factor associated with organ injury after sepsis. Front Immunol. (2022) 13:917293. doi: 10.3389/fimmu.2022.917293
4. Teneille EG and Bryan Y G. Sepsis-associated encephalopathy. Nat Rev Neurol. (2012) 8:557–66. doi: 10.1038/nrneurol.2012.183
5. Mariusz S, Karolina S-R, Jacek S, and Klaudia K. Potential neuroprotective role of melatonin in sepsis-associated encephalopathy due to its scavenging and anti-oxidative properties. Antioxidants (Basel). (2023) 12:1786. doi: 10.3390/antiox12091786
6. John Sieh D, Siqi L, Lili L, and Qiyi Z. Paediatric sepsis-associated encephalopathy (SAE): a comprehensive review. Mol Med. (2023) 29:27. doi: 10.1186/s10020-023-00621-w
7. Ryuta N, Tetsuro S, Takashi T, Yasuo M, Eigo K, Geoffrey W, et al. Rapidly progressive brain atrophy in septic ICU patients: a retrospective descriptive study using semiautomatic CT volumetry. Crit Care. (2021) 25:411. doi: 10.1186/s13054-021-03828-7
8. Gjinovefa K, Caitlyn WC, Cara KC, Rishi RD, Mathias D, Frank JJ, et al. Peritoneal sepsis caused by Escherichia coli triggers brainstem inflammation and alters the function of sympatho-respiratory control circuits. J Neuroinflammation. (2024) 21:45. doi: 10.1186/s12974-024-03025-7
9. Junli J, Shiqing A, Chengdong Y, Qianjin W, Bo X, Liang Z, et al. Dysfunction of cholinergic neuron in nucleus ambiguous aggravates sepsis-induced lung injury via a GluA1-dependment mechanism. Brain Behav Immun. (2025) 128:689–702. doi: 10.1016/j.bbi.2025.05.006
10. Yuki S, Maki M, Sena Y, Norihiro M, Tsukasa S, Arisa K, et al. Intrathecal injection of mesenchymal stromal cell cultured on 3D fiber ameliorates multiple organ damage in murine lupus. Stem Cells Transl Med. (2022) 11:644–58. doi: 10.1093/stcltm/szac021
11. Chiara V, Maria Kerstin P, Jamila W, Enrica M, Gustavo FA, Massimo C, et al. Spleen tyrosine kinase: a novel pharmacological target for sepsis-induced cardiac dysfunction and multi-organ failure. Front Immunol. (2024) 15:1447901. doi: 10.3389/fimmu.2024.1447901
12. Yiqi W, Lu W, Yun L, Yuan C, Min W, Zihui D, et al. Immunotherapy in the context of sepsis-induced immunological dysregulation. Front Immunol. (2024) 15:1391395. doi: 10.1016/j.jtha.2024.02.006
13. Toshiaki I, Julie H, Cheryl LM, Marcel L, Ecaterina S, and Jerrold HL. The role of thromboinflammation in acute kidney injury among patients with septic coagulopathy. J Thromb Haemost. (2024) 22:1530–40. doi: 10.1016/j.jtha.2024.02.006
14. Yuhao C, Lihong Y, and Xihong L. Advances in Mesenchymal stem cells regulating macrophage polarization and treatment of sepsis-induced liver injury. Front Immunol. (2023) 14:1238972. doi: 10.3389/fimmu.2023.1238972
15. Lexin F, Yu S, Jiangtao C, and Yueping D. The dual role of neutrophils in sepsis-associated liver injury. Front Immunol. (2025) 16:1538282. doi: 10.3389/fimmu.2025.1538282
16. Li-Yu Z, Yu D, Peng-Yi H, Meng-Yao W, Shu-Ting W, Xiao-Hui D, et al. Dysregulated dendritic cells in sepsis: functional impairment and regulated cell death. Cell Mol Biol Lett. (2024) 29:81. doi: 10.1186/s11658-024-00602-9
17. Joost W W. Tom vdP: Biological drivers of the host response in sepsis. Thorax. (2025) 7:222012. doi: 10.1136/thorax-2024-222012
18. Wenlin F, Chunli W, Kang X, Huaping L, and Qingjia C. Ccl5(+) Macrophages drive pro-inflammatory responses and neutrophil recruitment in sepsis-associated acute kidney injury. Int Immunopharmacol. (2024) 143:113339. doi: 10.1016/j.intimp.2024.113339
19. Grazia Maria V, Chiara B, Chiara P, Silvia P, Alessandra B, Massimo DC, et al. Direct effect of septic plasma in human cell lines viability. Blood Purif. (2018) 47:270–6. doi: 10.1159/000494597
20. Miao W, Jia L, Wan L, Lucen J, Kangli P, Xingyu S, et al. YL-109 attenuates sepsis-associated multiple organ injury through inhibiting the ERK/AP-1 axis and pyroptosis by upregulating CHIP. BioMed Pharmacother. (2024) 175:116633. doi: 10.1016/j.biopha.2024.116633
21. Zhenzhen S, Quan L, Shuang W, and Zhixia C. Protective effects of PNU 282987 on sepsis induced acute lung injury in mice. Mol Med Rep. (2019) 19:3791–8. doi: 10.3892/mmr.2019.10016
22. Min C, Guozheng W, and Jianfeng X. Immune dysregulation in sepsis: experiences, lessons and perspectives. Cell Death Discovery. (2023) 9:465. doi: 10.1038/s41420-023-01766-7
23. Risa I, Hideshi O, Genzou T, Kodai S, Chihiro T, Hirohisa Y, et al. Ultrastructural alteration of pulmonary capillary endothelial glycocalyx during endotoxemia. Chest. (2018) 154:317–25. doi: 10.1016/j.chest.2018.03.003
24. Yimu Y, Sarah MH, Matthew AS, Fuming Z, Kyrie LD, James FC, et al. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol. (2017) 56:727–37. doi: 10.1165/rcmb.2016-0338OC
25. Forough J, Sareh P, Ronan MNN, Ferrante SG, Rachel GK, Kimberly FM, et al. Characterization of systemic and regional hemodynamics and vascular dysfunction in mice with fecal induced peritonitis. Biomedicines. (2022) 10:470. doi: 10.3390/biomedicines10020470
26. Yuexian L, Liangyuan S, Zhiling F, Guoqing L, and Jin Z. Pivotal role of endothelial cell autophagy in sepsis. Life Sci. (2021) 276:119413. doi: 10.1016/j.lfs.2021.119413
27. Cheng-Long Z, Yi W, Qiang L, Hui-Ru L, Chang-Meng Y, Peng L, et al. Dysregulation of neutrophil death in sepsis. Front Immunol. (2022) 13:963955. doi: 10.3389/fimmu.2022.963955
28. Lu W, Yuan C, Yiqi W, Yun L, Yang B, Qiyuan Z, et al. Necroptosis in sepsis: mechanisms and therapeutic potential. Cytokine Growth Factor Rev. (2025) 84:74–86. doi: 10.1016/j.cytogfr.2025.06.008
29. Zilong Y, Xiaoyan P, Xiaoxia W, Qiuyun L, Yongxia C, Shuting C, et al. TREM-1 induces pyroptosis in cardiomyocytes by activating NLRP3 inflammasome through the SMC4/NEMO pathway. FEBS J. (2022) 290:1549–62. doi: 10.1111/febs.16644
30. Yixuan F, Baoyi G, Jianxing X, He Z, Liang Y, and Zhixu Y. Role of toll-like receptor-mediated pyroptosis in sepsis-induced cardiomyopathy. BioMed Pharmacother. (2023) 167:115493. doi: 10.1016/j.biopha.2023.115493
31. Song C, Renyu D, Ziwei H, Xiaohan Y, Feng X, Wei Z, et al. MicroRNA-34a inhibition alleviates lung injury in cecal ligation and puncture induced septic mice. Front Immunol. (2020) 11:1829. doi: 10.3389/fimmu.2020.01829
32. Juanjuan Q, Xiaoguang X, Xue G, and Yongli Z. Ulinastatin protects against sepsis induced myocardial injury by inhibiting NLRP3 inflammasome activation. Mol Med Rep. (2021) 24:730. doi: 10.3892/mmr.2021.12369
33. Xiaoye W, Qi T, Miaomiao L, and Yi L. METTL5 triggers the ferroptosis of cardiomyocytes in sepsis-induced myocardial injury. Free Radic Biol Med. (2025) 237:1–10. doi: 10.1016/j.freeradbiomed.2025.05.392
34. Xinye L, Ning M, Juping X, Yanchi Z, Pan Y, Xin S, et al. Targeting ferroptosis: pathological mechanism and treatment of ischemia-reperfusion injury. Oxid Med Cell Longev. (2021) 2021:1587922. doi: 10.1155/2021/1587922
35. Man Z, Yuying N, Jiahao H, and Liehua D. Advances in neutrophil extracellular traps and ferroptosis in sepsis-induced cardiomyopathy. Front Immunol. (2025) 16:1590313. doi: 10.3389/fimmu.2025.1590313
36. André B and Luís B. Organ crosstalk and dysfunction in sepsis. Ann Intensive Care. (2024) 14:147. doi: 10.1186/s13613-024-01377-0
37. Jingjing L, Gaosheng Z, Xiaoting W, and Dawei L. Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell Mol Life Sci. (2022) 79:456. doi: 10.1007/s00018-022-04490-0
38. Chao F, Pan R, Ganlan B, Jian W, Jiaxin B, Jiaxing H, et al. Enhancing Spns2/S1P in macrophages alleviates hyperinflammation and prevents immunosuppression in sepsis. EMBO Rep. (2023) 24:e56635. doi: 10.15252/embr.202256635
39. Shilei C, Yufei L, Xiaoliang S, Zhirui L, Liang G, Jueheng W, et al. The impact of glucose metabolism on inflammatory processes in sepsis-induced acute lung injury. Front Immunol. (2024) 15:1508985. doi: 10.3389/fimmu.2024.1508985
40. Katharina R and Michael A. Aquaporins in sepsis—an update. Front Immunol. (2024) 15:1495206. doi: 10.3389/fimmu.2024.1495206
41. Louise N, Jolien V, and Claude L. Sepsis-induced changes in pyruvate metabolism: insights and potential therapeutic approaches. EMBO Mol Med. (2024) 16:2678–98. doi: 10.1038/s44321-024-00155-6
42. Louise N, Marah H, Geike H, Elise M, Maxime R, Céline VD, et al. Unraveling mitochondrial pyruvate dysfunction to mitigate hyperlactatemia and lethality in sepsis. Cell Rep. (2025) 44:116032. doi: 10.1016/j.celrep.2025.116032
43. Ling X, Dustin B, Na L, Tina L, Sophie N, Sascha S, et al. Targeting protein kinase C-α prolongs survival and restores liver function in sepsis: evidence from preclinical models. Pharmacol Res. (2025) 212:107581. doi: 10.1016/j.phrs.2025.107581
44. Josh GC, Joost B, Deborah AS, Pavol G, Elizabeth C, Paul WB, et al. Sepsis endotypes identified by host gene expression across global cohorts. Commun Med (Lond). (2024) 4:120. doi: 10.1038/s43856-024-00542-7
45. Bianca LF, Mônica BS, Giuseppe GF, Milena KC, Erika SN, Alexandre KT, et al. Glucose metabolism is upregulated in the mononuclear cell proteome during sepsis and supports endotoxin-tolerant cell function. Front Immunol. (2022) 13:1051514. doi: 10.3389/fimmu.2022.1051514
46. Eleftheria K, Isis RP, Nikolaos A, Evdoxia K, Antigone K, Iraklis T, et al. Modulation of metabolomic profile in sepsis according to the state of immune activation. Crit Care Med. (2024) 52:e536–44. doi: 10.1097/ccm.0000000000006391
47. Shangwen P, Zheng L, Rui W, Huaqing S, Shiying Y, Yuan Y, et al. Sepsis-induced brain dysfunction: pathogenesis, diagnosis, and treatment. Oxid Med Cell Longev. (2022) 2022:1328729. doi: 10.1155/2022/1328729
48. Xiaoqian Y, Kaiying Y, Qi X, Rongyao H, Xudong P, and Xiaoyan Z. Central role of microglia in sepsis-associated encephalopathy: from mechanism to therapy. Front Immunol. (2022) 13:929316. doi: 10.3389/fimmu.2022.929316
49. Lijiao C, Shiyuan L, Ting L, Zhewei S, Yifan S, Qianzi Y, et al. Growth differentiation factor 15 aggravates sepsis-induced cognitive and memory impairments by promoting microglial inflammatory responses and phagocytosis. J Neuroinflammation. (2025) 22:44. doi: 10.1186/s12974-025-03369-8
50. Cuiping G, Wensheng L, Yi L, Abdoul Razak MY, Jianzhi W, Rong L, et al. LCN2 induces neuronal loss and facilitates sepsis-associated cognitive impairments. Cell Death Dis. (2025) 16:146. doi: 10.1038/s41419-025-07469-4
51. Dandan Z, Peng W, Xiyue C, Kaituo W, Yunsong W, Min Z, et al. Astrocyte-derived interleukin 11 modulates astrocyte-microglia crosstalk via nuclear factor-κB signaling pathway in sepsis-associated encephalopathy. Res (Wash D C). (2025) 8:598. doi: 10.34133/research.0598
52. Nils M, vS N, Jonathan W, Christian G, and Ha-Yeun C. Low-dose PLX5622 treatment prevents neuroinflammatory and neurocognitive sequelae after sepsis. J Neuroinflammation. (2023) 20:289. doi: 10.1186/s12974-023-02975-8
53. Jianhao X, Canyu M, Menglu H, Jiarui L, Ze X, and Jian W. CNS and CNS diseases in relation to their immune system. Front Immunol. (2022) 13:1063928. doi: 10.3389/fimmu.2022.1063928
54. Ramona DA, Mario T, Daniela I, Marika C, Rosalba S, Livia I, et al. Mechanism of action of natural compounds in peripheral multiorgan dysfunction and hippocampal neuroinflammation induced by sepsis. Antioxidants (Basel). (2023) 12:635. doi: 10.3390/antiox12030635
55. Xiaowen H, Pengju W, Cheng F, Min Y, Shilun Y, Linhui Q, et al. Compromised endothelial Wnt/β-catenin signaling mediates the blood-brain barrier disruption and leads to neuroinflammation in endotoxemia. J Neuroinflammation. (2024) 21:265. doi: 10.1186/s12974-024-03261-x
56. Yuwen S, Wanwan Z, Tong S, Lian H, Mubing Q, Qingyu W, et al. Endothelial TREM-1 mediates sepsis-induced blood‒brain barrier disruption and cognitive impairment via the PI3K/Akt pathway. J Neuroinflammation. (2025) 22:142. doi: 10.1186/s12974-025-03469-5
57. Jessica C, Yijen LW C, Edward D, Jeremy H, Marc S J, Ashok P, et al. Abcc8 (sulfonylurea receptor-1) knockout mice exhibit reduced axonal injury, cytotoxic edema and cognitive dysfunction vs. wild-type in a cecal ligation and puncture model of sepsis. J Neuroinflammation. (2023) 20:12. doi: 10.1186/s12974-023-02692-2
58. Ilaria AC, Carles S, Jean-Louis V, Rafael FF, Silvia CH, Federica ZC, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care. (2018) 22:327. doi: 10.1186/s13054-018-2258-8
59. Nobufumi S, Magdy S, and Amjad S. Sepsis-associated brain injury: underlying mechanisms and potential therapeutic strategies for acute and long-term cognitive impairments. J Neuroinflammation. (2022) 19:101. doi: 10.1186/s12974-022-02464-4
60. Molly CM, Danial A S, Louis PW, Pelin K, Benjamin DG, Eric VS, et al. Pericytes enrich the basement membrane and reduce neutrophil transmigration in an in vitro model of peripheral inflammation at the blood-brain barrier. Biomater Res. (2024) 28:81. doi: 10.34133/bmr.0081
61. Tatiana B, Jaqueline SG, Diogo D, Emily C, Vijayasree VG, Taha AS, et al. Postmortem evidence of brain inflammatory markers and injury in septic patients: a systematic review. Crit Care Med. (2022) 50:e241–52. doi: 10.1097/CCM.0000000000005307
62. Benedikt G, Jonathan W, Nina H, Vahid R, Hanna R, Ha-Yeun C, et al. Targeted rescue of synaptic plasticity improves cognitive decline in sepsis-associated encephalopathy. Mol Ther. (2024) 32:2113–29. doi: 10.1016/j.ymthe.2024.05.001
63. Ha-Yeun C, Jonathan W, Nina H, Nils M, Meike S, Philipp K, et al. Microglia mediate neurocognitive deficits by eliminating C1q-tagged synapses in sepsis-associated encephalopathy. Sci Adv. (2023) 9:eabq7806. doi: 10.1126/sciadv.abq7806
64. Felipe D-P, Gabriela FD-M, Monique M, Aurélien M, Fernando AB, Cristiane R, et al. What animal models can tell us about long-term psychiatric symptoms in sepsis survivors: a systematic review. Neurotherapeutics. (2021) 18:1393–413. doi: 10.1007/s13311-020-00981-9
65. Zachary M, Abraham P, Matthew M, Syed FM, Barbara L, Sagar B, et al. Post-sepsis syndrome – an evolving entity that afflicts survivors of sepsis. Mol Med. (2020) 26:6. doi: 10.1186/s10020-019-0132-z
66. Patricio TH, Sergio R, Tomás SH, Anchal S, Rose AB, Maya F, et al. Preclinical models of overwhelming sepsis implicate the neural system that encodes contextual fear memory. Mol Med. (2016) 22:789–99. doi: 10.2119/molmed.2015.00201
67. Mélanie GG. The microbiota-gut-brain axis in sepsis-associated encephalopathy. mSystems. (2022) 7:e0053322. doi: 10.1128/msystems.00533-22
69. Boeckxstaens G. The clinical importance of the anti-inflammatory vagovagal reflex. Handb Clin Neurol. (2013) 117:119–34. doi: 10.1016/b978-0-444-53491-0.00011-0
71. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. (2000) 405:458–62. doi: 10.1038/35013070
72. Lena B, Aurélien M, Luis HAC, Jarod L, Damien R, Estéban H, et al. Silencing of amygdala circuits during sepsis prevents the development of anxiety-related behaviours. Brain. (2022) 145:1391–409. doi: 10.1093/brain/awab475
73. Mabel NA, Ana N-K, Tiago DF, Omar Y, Mariana RB, Daniel EL, et al. M1 cholinergic signaling in the brain modulates cytokine levels and splenic cell sub-phenotypes following cecal ligation and puncture. Mol Med. (2024) 30:22. doi: 10.1186/s10020-024-00787-x
74. Aobing Y, Chia-Hsien W, Sayumi M, Ryusuke U, Yasuna N, Tsuyoshi I, et al. Activation of the α7nAChR by GTS-21 mitigates septic tubular cell injury and modulates macrophage infiltration. Int Immunopharmacol. (2024) 138:112555. doi: 10.1016/j.intimp.2024.112555
75. Weilan K, Kai K, Yang G, Haitao L, Xianglin M, Yanhui C, et al. GTS-21 protected against LPS-induced sepsis myocardial injury in mice through α7nAChR. Inflammation. (2018) 41:1073–83. doi: 10.1007/s10753-018-0759-x
76. Napat P, Qinghe M, Raymond G, Han X, Yu L, Chunli L, et al. α7 nicotinic acetylcholine receptor agonists regulate inflammation and growth hormone resistance in sepsis. Shock. (2021) 56:1057–65. doi: 10.1097/shk.0000000000001792
77. Wenwen Y, Yanting C, Jiayan L, Xin Z, Xiaoyi L, Ye T, et al. Pathogenesis and treatment strategies of sepsis-induced myocardial injury: modern and traditional medical perspectives. Int J Biol Sci. (2025) 21:3478–504. doi: 10.7150/ijbs.111288
78. Suzuki T, Suzuki Y, Okuda J, Kurazumi T, Suhara T, Ueda T, et al. Sepsis-induced cardiac dysfunction and β-adrenergic blockade therapy for sepsis. J Intensive Care. (2017) 5:22. doi: 10.1186/s40560-017-0215-2
79. Antoine K, Huguette L, Narimane AK, Julie D, Nicolas D, Chaojie W, et al. β1-adrenergic inhibition improves cardiac and vascular function in experimental septic shock. Crit Care Med. (2015) 43:e332–40. doi: 10.1097/ccm.0000000000001078
80. Ruilin H, Bin W, Liuhu H, Mingde L, Wenli G, Jiajing F, et al. Activation of spinal astrocyte α2A adrenoceptors protects against sepsis-induced heart injury through inhibition of GABAergic neuronal necroptosis. Adv Sci (Weinh). (2025) 12:e04406. doi: 10.1002/advs.202504406
81. Yuan M, Hao Y, Ping W, Yuxin M, Xu T, Sheng J, et al. Tyrosine hydroxylase-positive neurons in the rostral ventrolateral medulla mediate sympathetic activation in sepsis. Life Sci. (2024) 358:123118. doi: 10.1016/j.lfs.2024.123118
82. Zhaoyuan C, Jie Z, Shenjia G, Yi J, Mengdi Q, Jiahui G, et al. Suppression of Skp2 contributes to sepsis-induced acute lung injury by enhancing ferroptosis through the ubiquitination of SLC3A2. Cell Mol Life Sci. (2024) 81:325. doi: 10.1007/s00018-024-05348-3
83. Na G, Jingjing C, Yunchao L, Ying D, Zixinying H, Haiwei X, et al. The CYP2E1 inhibitor Q11 ameliorates LPS-induced sepsis in mice by suppressing oxidative stress and NLRP3 activation. Biochem Pharmacol. (2023) 214:115638. doi: 10.1016/j.bcp.2023.115638
84. Yuming W, Yujing Z, Yuan Y, Xin W, Zifan Z, Yin Y, et al. Small intestinal γδ T17 cells promote SAE through STING/C1q-induced microglial synaptic pruning in male mice. Nat Commun. (2025) 16:6779. doi: 10.1038/s41467-025-62181-3
85. Nian W, Jiao L, Runliu W, Feng C, Ruoxi Z, Chunhua Y, et al. A neuroimmune pathway drives bacterial infection. Sci Adv. (2025) 11:eadr2226. doi: 10.1126/sciadv.adr2226
86. Lu Y, Jinming Z, Hongwei M, Xinxin Z, Zhongmin F, Yongheng Y, et al. Selective activation of cholinergic neurotransmission from the medial septal nucleus to hippocampal pyramidal neurones improves sepsis-induced cognitive deficits in mice. Br J Anaesth. (2023) 130:573–84. doi: 10.1016/j.bja.2023.01.019
87. Jian F, Ziyi H, Zebiao W, Yaoyao X, Guan Y, Yulong Y, et al. GABA regulates IL-1β production in macrophages. Cell Rep. (2022) 41:111770. doi: 10.1016/j.celrep.2022.111770
88. Jianya P, Chandler BS, John JP, Alexander DL, Christina MH, Juan MI-R, et al. Monocytes maintain central nervous system homeostasis following helminth-induced inflammation. Proc Natl Acad Sci U S A. (2022) 119:e2201645119. doi: 10.1073/pnas.2201645119
89. Michael C, Valerie TR, Kristina S, Emmy T, Kaitlin M, Ingrid B-M, et al. Substance P receptor signaling contributes to host maladaptive responses during enteric bacterial infection. Proc Natl Acad Sci U S A. (2025) 122:e2415287122. doi: 10.1073/pnas.2415287122
90. Noorisah K, Supreet K, Carly MK, Marc G, et al. CNS-Spleen axis – a close interplay in mediating inflammatory responses in burn patients and a key to novel burn therapeutics. Front Immunol. (2021) 12:720221. doi: 10.3389/fimmu.2021.720221
91. Lies L, Arno T, Jan G, VdB G, et al. The hypothalamus-pituitary-adrenocortical response to critical illness: a concept in need of revision. Endocr Rev. (2023) 44:1096–106. doi: 10.1210/endrev/bnad021
92. Greet V, Arno T, Lies L, Jan G, et al. The hypothalamus-pituitary-adrenal axis in sepsis- and hyperinflammation-induced critical illness: gaps in current knowledge and future translational research directions. EBioMedicine. (2022) 84:104284. doi: 10.1016/j.ebiom.2022.104284
93. Zhiying J, Chun C, Grant LW, Xin F, Claire ES, Brook LWS, et al. Stress-induced glucocorticoid desensitizes adrenoreceptors to gate the neuroendocrine response to somatic stress in male mice. Cell Rep. (2022) 41:111509. doi: 10.1016/j.celrep.2022.111509
94. Delcio EJr P, Fabiana CV, Alexandre G-P, et al. Ghrelin receptor antagonist attenuated sickness behavior and activation of HPA-axis induced by immunological challenge in male rats. Life Sci. (2021) 288:120173. doi: 10.1016/j.lfs.2021.120173
95. Hong Z, Li C, De-Zhi L, Shuai F, Ke-Xin W, Li-Li X, et al. Unbiased multitissue transcriptomic analysis reveals complex neuroendocrine regulatory networks mediated by spinal cord injury-induced immunodeficiency. J Neuroinflammation. (2023) 20:219. doi: 10.1186/s12974-023-02906-7
96. Shuyi D, Anqi G, Zhengwei H, Kaiyu G, Ya Z, Cheekai C, et al. The exploration of neuroinflammatory mechanism by which CRHR2 deficiency induced anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 128:110844. doi: 10.1016/j.pnpbp.2023.110844
97. Jordi O, Willem JMM, Joren CM, Mihai GN, Raphaël D, et al. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol. (2022) 19:23–7. doi: 10.1038/s41581-022-00633-5
98. Virginia LDS, Suzana BA, Leticia MA, Mariana S-Q, Lilian CC, Carolina S, et al. Innate immune memory mediates increased susceptibility to Alzheimer's disease-like pathology in sepsis surviving mice. Brain Behav Immun. (2021) 95:287–98. doi: 10.1016/j.bbi.2021.04.001
99. Janire C, Sarai A-A, Diego B, Samuel TP, Iratxe S, Ainize P-C, et al. The microbiota metabolite, phloroglucinol, confers long-term protection against inflammation. Gut Microbes. (2024) 16:2438829. doi: 10.1080/19490976.2024.2438829
100. Zhuang W, Yuanyuan L, Jing H, Xinwei Y, Jin Y, Yuanxing Z, et al. Tissue-resident trained immunity in hepatocytes protects against septic liver injury in zebrafish. Cell Rep. (2024) 43:114324. doi: 10.1016/j.celrep.2024.114324
101. Yoon-Young K, Dong-Young K, Sang-Yong L, Hee-Joong K, Taehawn K, Jeong AC, et al. Innate immune training initiates efferocytosis to protect against lung injury. Adv Sci (Weinh). (2024) 11:e2308978. doi: 10.1002/advs.202308978
102. Wenjuan W, Lisi M, Bin L, Liangliang O, et al. The role of trained immunity in sepsis. Front Immunol. (2024) 15:1449986. doi: 10.3389/fimmu.2024.1449986
103. Yuchen P, Jingman L, Xiaoyu X, Jiali W, Qi J, Jingjing Y, et al. β-glucan-coupled superparamagnetic iron oxide nanoparticles induce trained immunity to protect mice against sepsis. Theranostics. (2022) 12:675–88. doi: 10.7150/thno.64874
104. Qiang L, Linmao K, Dandan Y, Han X, Zixuan Z, Rilei Y, et al. Discovery of D25, a potent and selective MNK inhibitor for sepsis-associated acute spleen injury. J Med Chem. (2024) 67:3167–89. doi: 10.1021/acs.jmedchem.3c02441
105. William AB, Kim MH, Michelle AE, Fulton TC, et al. High-mobility group box 1 (HMGB1) crosses the BBB bidirectionally. Brain Behav Immun. (2023) 111:386–94. doi: 10.1016/j.bbi.2023.04.018
106. Masafumi S, Yoshihisa F, Yuko O, Shohei O, Kazumichi F, Kimihiro Y, et al. Infiltrated regulatory T cells and Th2 cells in the brain contribute to attenuation of sepsis-associated encephalopathy and alleviation of mental impairments in mice with polymicrobial sepsis. Brain Behav Immun. (2020) 92:25–38. doi: 10.1016/j.bbi.2020.11.010
107. Jean-Marc C. During sepsis and COVID-19, the pro-inflammatory and anti-inflammatory responses are concomitant. Clin Rev Allergy Immunol. (2023) 65:183–7. doi: 10.1007/s12016-023-08965-1
108. Chanhee P, Zhuofan L, Yun L, Boyang R, Junyun H, Huang H, et al. Extracellular vesicles in sepsis plasma mediate neuronal inflammation in the brain through miRNAs and innate immune signaling. J Neuroinflammation. (2024) 21:252. doi: 10.1186/s12974-024-03250-0
109. Minshu L, Xiuping L, Dan W, Xiaolin G, Shiyao L, Xiaojing C, et al. Inhibition of exosome release augments neuroinflammation following intracerebral hemorrhage. FASEB J. (2021) 35:e21617. doi: 10.1096/fj.202002766R
110. Elisabeth CVDS, Andy YA, Robert EWH, Hjalmar RB, et al. Exploring the pathophysiology of post-sepsis syndrome to identify therapeutic opportunities. EBioMedicine. (2020) 61:103044. doi: 10.1016/j.ebiom.2020.103044
111. Ying L, Muhuo J, and Jianjun Y. Current understanding of long-term cognitive impairment after sepsis. Front Immunol. (2022) 13:855006. doi: 10.3389/fimmu.2022.855006
112. Yulei G, Xin T, Xiang Z, Grace Divine MN, Fang C, Yancun L, et al. The roles of tissue-resident macrophages in sepsis-associated organ dysfunction. Heliyon. (2023) 9:e21391. doi: 10.1016/j.heliyon.2023.e21391
113. Geoffrey PD, Hayley LL, and Jodie LM. Revolution in sepsis: a symptoms-based to a systems-based approach? J BioMed Sci. (2024) 31:57. doi: 10.1186/s12929-024-01043-4
114. Aline AJ, Patrícia P, Bruna MS, Isabelle R-S, Rafael AF, Marcelo EB, et al. Chronic molecular hydrogen inhalation mitigates short and long-term memory loss in polymicrobial sepsis. Brain Res. (2020) 1739:146857. doi: 10.1016/j.brainres.2020.146857
115. Yi L, Lu Y, Zhongmin F, Binxiao S, Yu C, Yan M, et al. Microglia: a potential therapeutic target for sepsis-associated encephalopathy and sepsis-associated chronic pain. Front Pharmacol. (2020) 11:600421. doi: 10.3389/fphar.2020.600421
116. Ying L and Guo F. TLR4 inhibitor alleviates sepsis-induced organ failure by inhibiting platelet mtROS production, autophagy, and GPIIb/IIIa expression. J Bioenerg Biomembr. (2022) 54:155–62. doi: 10.1007/s10863-022-09940-9
117. Zhicai Y, Changxue X, Rong L, Dandan P, Bian J, Zhen Z, et al. The protective effect of apolipoprotein H in paediatric sepsis. Crit Care. (2024) 28:36. doi: 10.1186/s13054-024-04809-2
118. Paul EM. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol Ther. (2018) 189:63–70. doi: 10.1016/j.pharmthera.2018.04.007
119. Simone CT and Roland N. Treatment of septic encephalopathy and encephalitis – a critical appraisal. Expert Rev Neurother. (2023) 23:1069–80. doi: 10.1080/14737175.2023.2288652
120. Cavaillon JM, Singer M, and Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. (2020) 12:e10128. doi: 10.15252/emmm.201810128
121. Wang J, Zhong Z, Luo H, Han Q, Wu K, Jiang A, et al. Modulation of brain immune microenvironment and cellular dynamics in systemic inflammation. Theranostics. (2025) 15:5153–71. doi: 10.7150/thno.107061
122. Gregory DJ, Han F, Li P, Gritsenko M, Kyle J, Riley FE, et al. Multi-Omic blood analysis reveals differences in innate inflammatory sensitivity between species. medRxiv. (2023) 2023:11. doi: 10.1101/2023.11.30.23299243
Keywords: sepsis, neuroimmune regulation, autonomic nerve function, inflammation, sepsis-associated encephalopathy
Citation: Tang F, Wu S, Zou Z, Li X and Qiao L (2025) The core role of central nervous system in sepsis-related organ damage. Front. Immunol. 16:1694003. doi: 10.3389/fimmu.2025.1694003
Received: 27 August 2025; Accepted: 06 November 2025; Revised: 08 October 2025;
Published: 21 November 2025.
Edited by:
Yihui Wang, Shanghai Jiao Tong University, ChinaReviewed by:
Hao Wang, Shandong University, ChinaJunHao Wang, Baylor College of Medicine, United States
Copyright © 2025 Tang, Wu, Zou, Li and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihong Li, bGl4aWhvbmdoeGV5QDE2My5jb20=; Lina Qiao, cWlhb2xpbmFAc2N1LmVkdS5jbg==
 Fajuan Tang
Fajuan Tang ShanShan Wu1,2
ShanShan Wu1,2 Zhuan Zou
Zhuan Zou Xihong Li
Xihong Li Lina Qiao
Lina Qiao