- 1Department of Oncology, Weill Cornell Medicine, New York, NY, United States
- 2Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 4Department of Oncology, Stanford University School of Medicine, Stanford, CA, United States
Immune checkpoint inhibitors (ICI) have revolutionized the treatment of advanced cancers, but overall response rates remain modest and adjunct therapies to enhance efficacy of ICI are of great interest. This retrospective study examines the association between exercise and clinical outcomes in 258 patients with advanced solid tumors receiving ICI. The results suggest an association between exercise and better clinical outcomes, particularly in patients with high tumor mutation burden, though improvements in clinical benefit rate (58% vs. 51% for exercisers and non-exercisers, respectively) and one-year overall survival (67% vs. 58% for exercisers and non-exercisers, respectively) are not statistically significant. Our discovery-based findings in conjunction with preclinical evidence create a strong rationale for translational studies to formally investigate the effects of structured exercise therapy in combination with ICI in patients with solid tumors.
Introduction
Use of immune checkpoint inhibitors (ICI) has revolutionized cancer care and formed a new pillar of cancer treatment with potential for durable control of advanced cancer. Despite tremendous successes with ICI, overall response rates across solid tumors remain modest, creating an unmet need for predictive factors of response. Identifying adjunct, low toxicity, combination strategies to augment response to ICI is an area of intense investigation. Host factors such as genetic predisposition, diet, and body mass index (BMI) contribute to and/or modify the antitumor efficacy of ICI (1). BMI predicts response to immune checkpoint inhibitor therapy in multiple tumor types (2). As another modifiable host factor, the role of exercise has been less well-delineated. Preclinical studies demonstrate exercise, a potent regulator of host physiology, promotes anti-tumor immunity in solid tumors that, when combined with ICI, enhances tumor suppressive activity (3, 4). In patients with cancer, exercise decreases circulating myeloid cells and increases circulating NK cell number and cytotoxic function (5). Preliminary data suggest that exercise may augments response to ICI in patients with cancer but further research is needed (6). Clinical translation of whether exercise improves response to ICI in patients with solid tumors has received minimal attention. Accordingly, we examined the impact of exercise on clinical outcomes in patients receiving ICI for solid tumors.
Methods
We performed a retrospective analysis of 258 patients with solid tumor malignancies receiving ICI regimens for advanced, unresectable disease with annotation of exercise within one year prior to ICI initiation at Memorial Sloan Kettering Cancer Center (Table 1). Patients receiving adjuvant ICI were excluded. Best overall response (BOR) of complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) to ICI-containing regimen were assessed based on clinician determination of response and review of imaging. Clinical benefit (CR, PR, SD) by exercise status was evaluated using logistic regression, adjusting for sex and BMI. Overall survival (OS), defined as the time from first dose of ICI to death, was analyzed using Kaplan-Meier methods and modeled using Cox proportional hazards regression as a function of exercise status, sex and BMI.
Exercise assessment
Exercise was assessed using a validated survey and defined as any moderate or strenuous exercise per week; non-exercisers were defined as no moderate or strenuous exercise. Exercise history was prospectively evaluated using the Godin Leisure Time Exercise Questionnaire (GLTEQ) (7). The GLTEQ contains three questions that assess the average frequency of mild, moderate, and strenuous-intensity exercise sessions of at least 15 mins/session in a typical 7-day period during leisure-time. Participants also reported the average duration of exercise within each intensity category. The frequency of sessions per week within each intensity category was multiplied by the average duration to calculate exercise minutes per week in each intensity category, which was then summed for calculation of total minutes of exercise per week. Non-exercise was defined as 0 minutes of moderate or vigorous intensity exercise per week and exercise >0 minutes of moderate or vigorous intensity exercise per week.
TMB assessment
Tumor genomic sequencing data (8) was available on 226 patients (88%), enabling analysis of whether tumor mutational burden (TMB), an established predictor of ICI response, influenced clinical response to ICI and exercise, assessed via interaction terms in the models. Patients entered the risk set at the time of genomic sequencing in OS analyses by TMB to avoid introducing a delayed entry bias. Low TMB defined as <10 mt/Mb, high TMB defined as ≥10 mt/Mb.
Results
The clinical benefit rate was 58% for exercisers and 51% for non-exercisers (odds ratio (OR), 1.37, 95% CI, 0.82, 2.29). Median follow-up was 1.8 years (interquartile range 1.1, 2.7) among patients alive at the end of the study. During follow-up, a total of 181 deaths were observed. One year OS was 67% (95% CI, 60, 75) for exercisers and 58% (95% CI, 48, 69) for non-exercisers; two year OS was 38% (95% CI, 31, 47) for exercisers and 33% (95% CI, 24, 45) for non-exercisers (hazard ratio [HR], 0.87, 95% CI, 0.64, 1.19; Figure 1A).
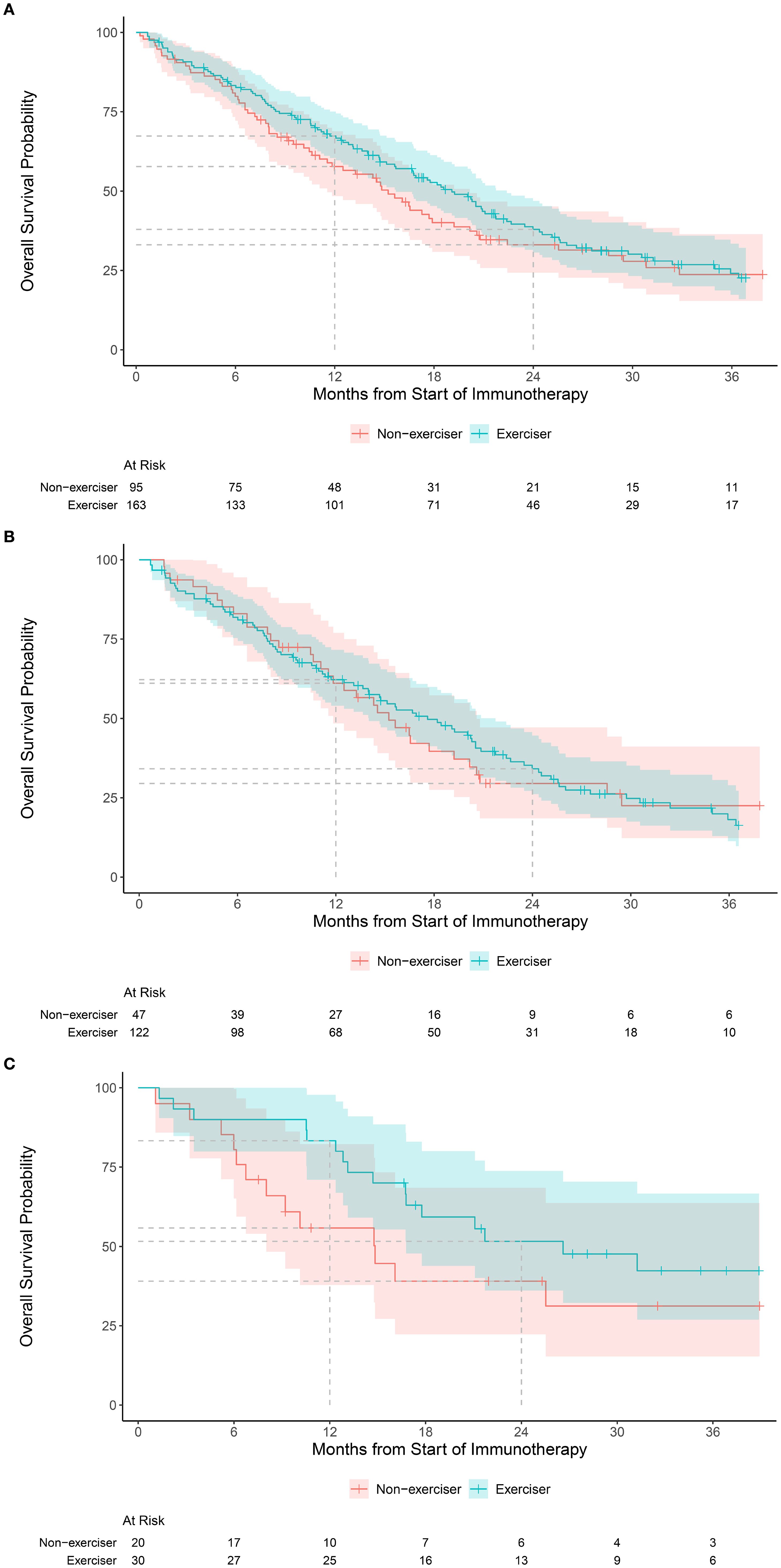
Figure 1. Kaplan-Meier estimates of overall survival in the: (A) overall cohort according to exercise status, categorized as exercisers (any moderate or vigorous exercise per week) versus non-exercise (no moderate or vigorous exercise per week), (B) Kaplan-Meier estimates of overall survival according to exercise status for tumors with low (<10 mt/mb) mutational burden, and (C) Kaplan-Meier estimates of overall survival according to exercise status for tumors with high (≥10 mt/mb) mutational burden.
The rate of clinical benefit for low (<10 mt/mb) TMB (n=174) was 55% for exercisers and non-exercisers; for high ( 10 mt/mb) TMB (n=52) was 73% for exercisers and 55% for non-exercisers. One year OS for patients with low TMB was 62% (95% CI, 54, 72) for exercisers and 61% (95% CI, 48, 77) for non-exercisers (Figure 1B); for high TMB, one year OS was 83% (95% CI, 71, 98) for exercisers and 56% (95% CI, 38, 82) for non-exercisers (Figure 1C). Interaction terms between TMB and exercise were not statistically significant for clinical benefit or OS.
Discussion
Identifying modifiable host factors that can influence the efficacy of ICI-based regimen is of great interest. Preclinical models indicate that exercise retards tumor growth and enhances the efficacy of ICIs (9–15). Multiple mechanisms have been proposed including augmentation of inflammation and immune infiltration tumor microenvironment (e.g. making tumors “hot”) (11, 13, 16), enhanced cytokine signaling mediating CD8+ T-cell and NK cell dependent cytotoxicity (10, 14), and alteration of the gut microbiome promoting accumulation of metabolites that augment the efficacy of ICI via CD8+ T cells (15). Translating these findings to clinical benefit for patients receiving ICIs is of the utmost urgency.
This single-center cohort study suggests that exercise may be associated with better clinical outcomes in patients receiving ICI for advanced solid tumors, although results were not statistically significant. Our results indicate that for patients with high tumor mutation burdens may be more likely to elicit an effect exercise-immune response and improved overall survival. This is consistent with prior work demonstrating that moderate and high levels of physical activity were associated with prolonged survival following ICI treatment in ICI responsive tumors (melanoma, Non small cell lung cancer (NSCLC), Renal Cell Carcinoma (RCC)), including those treated in the adjuvant setting (4).
Study limitations include small sample size, observational retrospective design, reliance on self-reported exercise, and restriction to survivors who were alive and willing to complete an exercise survey after initial cancer diagnosis. Moreover, objective measures of exercise capacity were not available at baseline or follow-up, which is a known prognostic factor across cancer entities (17). The lack of information on exercise type (endurance, resistance, or balance training), degree of supervision, and intensity further limits interpretation, underscoring the need for prospective studies with structured exercise phenotyping. In addition, the inclusion of different cancer entities may obscure disease-specific effects of exercise, as exercise interventions may need to be tailored by tumor type. Lung cancer patients, which represents the majority of our cohort, typically carry higher cardiovascular risk and ventilatory limitations, which may influence exercise, participation, and subsequent outcomes. Prior evidence has demonstrated that even small amounts of exercise may reduce mortality in cancer patients and improve the quality of life, fatigue, and exercise capacity (18, 19). Future studies examining exercise by unique tumor types with associated baseline cardiovascular risk factors and impact on exercise fitness is needed. Finally, cardiovascular comorbidities and acute-ICI related events (e.g. myocarditis, pneumonitis) were not fully captured, yet are important determinants of survival in this population. Larger retrospective or prospective studies are needed to increase statistical power, clarify these associations, and validate our exploratory findings.
Notwithstanding these limitations, our discovery-based findings in conjunction with existing preclinical evidence create a strong rationale for translational studies to formally investigate the effects of structured exercise therapy in combination with ICI in patients with solid tumors. Our work extends on these findings by including TMB analysis, demonstrating that another prognostic factor to ICI in conjunction with exercise associates with improved OS to ICI treatment among patients with unresectable or metastatic solid tumors, and highlighting a hypothesis-generating association that warrants confirmation in prospective studies. Prospective trials examining exercise physiology of patients undergoing ICI treatment are ongoing (NCT06026111, NCT06983899, NCT06672120). Taken together, our study provides a strong rationale for ongoing studies to investigate the effects of integrating structured exercise therapy with immunotherapy strategies in patients with advanced solid tumors to improve clinical outcomes. Our intriguing molecular findings support a novel hypothesis that tumors with a high number of clonal neoantigens propagated by high TMB may be more likely to elicit effective exercise-induced immune response.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Memorial Sloan Kettering Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This was a Retrospective Study.
Author contributions
KL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Formal analysis, Methodology, Writing – review & editing. JP: Data curation, Investigation, Writing – review & editing. WG: Data curation, Investigation, Writing – review & editing. WU: Formal analysis, Methodology, Writing – review & editing. CM: Formal analysis, Writing – review & editing. LWJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported, in part, by a research grant from AKTIV Against Cancer to LWJ. LWJ is supported by the City of Hope Cancer Center Support Grant (P30CA033572) and the Cherng Family Center for Integrative Oncology. JAL, WPU, CSM, and LWJ were supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). AB is supported by the Stanford Cancer Institute Cancer Center Support Grant (P30 CA124435). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
LJ owns stock in Pacylex, Inc and Illumisonics, Inc. JL discloses salary support from the American Association of Cancer Research Project Genomics Evidence Neoplasia Information Exchange Biopharma Collaborative GENIE BPC. AB has consulting and advisory roles with Adaptimmune, Bristol Myers Squibb, BluePath Solutions, cTRL Therapeutics, Genmab, Immatics, IO Biotech, Iovance Biotherapeutics, Novartis, Merck, Pfizer, and Replimune; and that she receives research funding institutional from Bristol Myers Squibb, Iovance Biotherapeutics, Lyell Immunopharma, Obsidian Therapeutics, and Replimune.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Warner AB and McQuade JL. Modifiable host factors in melanoma: emerging evidence for obesity, diet, exercise, and the microbiome. Curr Oncol Rep. (2019) 21:72. doi: 10.1007/s11912-019-0814-2
2. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ, et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. (2020) 6:512-518.
3. Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP, et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. (2021) 9:765–78. doi: 10.1158/2326-6066.CIR-20-0499
4. Kurz E, Hirsch CA, Dalton T, Shadaloey SA, Khodadadi-Jamayran A, Miller G, et al. Exercise-induced engagement of the IL-15/IL-15Ralpha axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. (2022) 40:720–737 e725.
5. Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, and Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol (1985). (2005) 98:1534–40. doi: 10.1152/japplphysiol.00566.2004
6. Verheijden RJ, Cabane Ballester A, Smit KC, van Eijs MJM, Bruijnen CP, van Lindert ASR, et al. Physical activity and checkpoint inhibition: association with toxicity and survival. J Natl Cancer Inst. (2024) 116:573–9. doi: 10.1093/jnci/djad245
7. Godin G and Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. (1985) 10:141–6.
8. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. (2015) 17:251–64. doi: 10.1016/j.jmoldx.2014.12.006
9. Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, and Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: A critical systematic review of in vivo preclinical data. Cancer Res. (2016) 76:4032–50. doi: 10.1158/0008-5472.CAN-16-0887
10. Kurz E, Hirsch CA, Dalton T, Shadaloey SA, Khodadadi-Jamayran A, Miller G, et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. (2022) 40:720–737.e5. doi: 10.1016/j.ccell.2022.05.006
11. Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP, et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. (2021) 9:765–78. doi: 10.1158/2326-6066.CIR-20-0499
12. Rundqvist H, Veliça P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M, et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife. 9:e59996. doi: 10.7554/eLife.59996
13. Savage H, Pareek S, Lee J, Ballarò R, Minussi DC, Hayek K, et al. Aerobic exercise alters the melanoma microenvironment and modulates ERK5 S496 phosphorylation. Cancer Immunol Res. (2023) 11:1168–83. doi: 10.1158/2326-6066.CIR-22-0465
14. Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. (2016) 23:554–62. doi: 10.1016/j.cmet.2016.01.011
15. Phelps CM, Willis NB, Duan T, Lee AH, Zhang Y, Rodriguez JM, et al. Exercise-induced microbiota metabolite enhances CD8 T cell antitumor immunity promoting immunotherapy efficacy. Cell. (2025). doi: 10.1016/j.cell.2025.06.018
16. Koelwyn GJ, Zhuang X, Tammela T, Schietinger A, and Jones LW. Exercise and immunometabolic regulation in cancer. Nat Metab. (2020) 2:849–57. doi: 10.1038/s42255-020-00277-4
17. Wernhart S and Rassaf T. Exercise, cancer, and the cardiovascular system: clinical effects and mechanistic insights. Basic Res Cardiol. (2025) 120:35–55. doi: 10.1007/s00395-024-01034-4
18. Anker MS, Mahabadi AA, Totzeck M, Tewes M, Khan MS, Mincu RI, et al. Heart failure therapy in patients with advanced cancer receiving specialized palliative care (EMPATICC trial). Eur Heart J. (2025), ehaf705. doi: 10.1093/eurheartj/ehaf705
Keywords: exercise oncology, immunotherapy response, immune check inhibitor (ICI), solid tumor, tumor mutation burden
Citation: Loo K, Lavery JA, Palmer J, Guo W, Underwood WP, Moskowitz CS, Jones LW and Betof AS (2025) Association between exercise and clinical outcomes in patients treated with immunotherapy for solid tumors. Front. Immunol. 16:1694045. doi: 10.3389/fimmu.2025.1694045
Received: 27 August 2025; Accepted: 13 October 2025;
Published: 31 October 2025.
Edited by:
Sebastian Theurich, LMU Munich University Hospital, GermanyReviewed by:
Mauro Vaccarezza, Curtin University, AustraliaSimon Wernhart, Technical University of Munich, Germany
Copyright © 2025 Loo, Lavery, Palmer, Guo, Underwood, Moskowitz, Jones and Betof. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allison S. Betof, YWxsaXNvbi5iZXRvZkBzdGFuZm9yZC5lZHU=; Lee W. Jones, bGVlam9uZXNAY29oLm9yZw==
†These authors have contributed equally to this work
 Kimberly Loo
Kimberly Loo Jessica A. Lavery
Jessica A. Lavery Jessica Palmer1
Jessica Palmer1 Whitney P. Underwood
Whitney P. Underwood Lee W. Jones
Lee W. Jones