- 1Division of Molecular Endocrinology, Department of Molecular Oncology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2Department of Medicine, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Graves’ disease (GD) is an autoimmune disorder that results in hyperthyroidism, in which the immune system mistakenly targets the thyroid gland, causing it to produce excessive amounts of thyroid hormones. Genetic predisposition, environmental factors such as infections and stress, disruptions in the gut microbiome, excessive iodine intake, and epigenetic changes have all been implicated in the development of GD. The recent pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) posed a serious global health crisis. The emergence of COVID-19 vaccines has been pivotal in combating the viral infection and its spread. However, reports of rare adverse events, including the development of autoimmune disorders such as GD following vaccination, have raised concerns. Autoimmune factors play a critical role in the pathogenesis of GD, particularly through the production of autoantibodies targeting the thyroid gland. In this review, reported cases are critically analyzed to elucidate commonalities and potential triggers for the development of this autoimmune disorder, highlighting the vital role of autoimmune mechanisms in inducing GD. We also discuss the molecular mechanisms underlying vaccine-induced autoimmunity, including antigen presentation, bystander activation, molecular mimicry, and the induction of inflammatory factors following vaccination. Understanding these mechanisms in COVID-19 vaccine-induced GD could enhance patient care and guide vaccination policies.
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first identified in Wuhan, China, in December 2019. It rapidly escalated into a global outbreak, leading the World Health Organization (WHO) to declare it a pandemic in March 2020. The virus primarily transmits through respiratory droplets released when an infected individual coughs, sneezes, or speaks. Symptoms can range from mild, such as fever, fatigue, and cough, to more severe complications, including pneumonia and difficulty breathing (1). The SARS-CoV-2 “spike” (S) glycoprotein uses human angiotensin-converting enzyme 2 (ACE2) as a receptor for cellular entry, facilitating infection. ACE2 is highly expressed in various vital organs, including the heart, lungs, kidneys, blood vessels, small intestine, thyroid, and parathyroid glands (2, 3). Endocrine tissues, particularly the thyroid and parathyroid, are therefore likely to be affected by SARS-CoV-2. Viruses like SARS-CoV-2 preferentially infect cells with high ACE2 expression, such as thyroid and parathyroid cells, enabling rapid viral replication in these tissues. The immune system, in attempting to eliminate the virus, may inadvertently attack ACE2-expressing cells. This misdirected response can potentially lead to subsequent autoimmune complications. Consequently, the SARS-CoV-2 infection has been shown to induce various thyroid pathogeneses, including subacute thyroiditis, Graves’ disease (GD), thyroid sick syndrome, and Hashimoto’s thyroiditis (4, 5). Recently, a similar effect was also observed in the parathyroid gland (3). Overall, older adults, individuals with obesity, and those with preexisting chronic health conditions are at higher risk of severe illness.
As of 9 February 2025, a total of 777,385,370 COVID-19 cases had been reported globally, resulting in 7,088,757 deaths (1).
Multiple strategies, including vaccines, public health measures, and treatments, have been developed to combat the spread of SARS-CoV-2 and mitigate its impact. The rapid development and deployment of COVID-19 vaccines have been pivotal in reducing the pandemic’s severity (6). Moreover, these vaccines have significantly decreased illness severity and mortality caused by SARS-CoV-2 (7). Vaccinated individuals also have a lower risk of developing postacute sequelae of COVID-19 (PASC), commonly known as long COVID, highlighting another key benefit of vaccination (8). Since the introduction of COVID-19 vaccines, a total of 13.64 billion doses have been administered worldwide. By 31 December 2023, 67% of the global population had completed the full primary series of a COVID-19 vaccination. Additionally, 32% of the world’s population had received at least one booster dose (9). The rapid development and deployment of vaccines targeting SARS-CoV-2, the virus responsible for COVID-19, have played a crucial role in curbing the spread of the pandemic. However, COVID-19 vaccines have been associated with rare acute adverse events, including myocarditis, pericarditis, thrombosis, thrombocytopenia, Guillain–Barré syndrome, transverse myelitis, and Bell’s palsy (10). One such potential complication is the development of autoimmune thyroid disorders, including GD. According to the WHO, GD is an autoimmune disorder characterized by the overproduction of thyroid hormones (thyrotoxicosis) due to circulating autoantibodies that stimulate the thyroid-stimulating hormone receptor or thyrotropin receptor (TSHR). Symptoms can include weight loss, rapid heartbeat, anxiety, tremors, heat intolerance, diffuse goiter, bulging eyes (Graves’ orbitopathy), and, less commonly, dermopathy. Suppressed thyroid-stimulating hormone (TSH) levels, along with elevated free T4 and/or T3 and positivity for TSHR autoantibodies (TRAb), are indicative of GD. Imaging findings typically show diffusely increased radioactive iodine uptake (RAIU) and enhanced blood flow on thyroid ultrasonography with color Doppler, often described as a “thyroid inferno”. Treatment options include antithyroid medications, radioactive iodine therapy, or surgical intervention (9, 11).
This review aims to summarize the current evidence on the emergence of GD following SARS-CoV-2 vaccination, with a focus on the underlying autoimmune and inflammatory processes. By elucidating the pathophysiology and clinical implications of this phenomenon, it may help improve vaccination strategies and patient management during the ongoing COVID-19 pandemic.
Postvaccine syndrome
Some individuals have reported experiencing postvaccination symptoms similar to long-COVID shortly after receiving the COVID-19 vaccine. This condition, known as postvaccination syndrome (PVS) or postacute COVID-19 vaccination syndrome (PACVS), is characterized by symptoms such as extreme fatigue, reduced exercise tolerance, numbness, brain fog, nerve pain, insomnia, palpitations, muscle pain, tinnitus, headaches, burning sensations, and dizziness (12, 13). Unlike long-COVID, PVS is not officially recognized by health authorities, which limits access to appropriate patient care and support. The underlying molecular mechanisms of PVS remain largely unclear.
However, there is a significant overlap in self-reported symptoms between long-COVID and PVS, both of which involve exposure to the SARS-CoV-2 “S” protein in the context of immune responses triggered by infection or vaccination (13, 14). In individuals with underlying susceptibility, vaccines might trigger persistent symptoms through various biological mechanisms. First, vaccine components such as mRNA, lipid nanoparticles, and adenoviral vectors activate pattern recognition receptors, potentially leading to excessive immune activation and chronic inflammation (15, 16). Additionally, studies have shown that the “S” protein produced after BNT162b2 or mRNA-1273 vaccination can circulate in plasma within a day of vaccination (17). Interactions between the full-length “S” protein, its subunits (S1, S2), and peptide fragments with host molecules may contribute to prolonged symptoms in certain individuals (17). Recent research has also identified a subset of nonclassical monocytes containing the “S” protein in patients with PVS (18). Furthermore, animal studies on mRNA–lipid nanoparticle (LNP) platforms suggest that these components can cross the blood–brain barrier, leading to localized “S” protein expression that may contribute to neurocognitive symptoms (19). Lastly, vaccine-induced immune responses may stimulate autoreactive lymphocytes, potentially triggering autoimmune reactions (20). Recent studies and case reports have raised concerns about a possible link between COVID-19 vaccination and the development of GD (11, 21). While the exact mechanisms remain unclear, it is hypothesized that the immune response triggered by the vaccine may lead to the production of autoantibodies targeting the thyroid gland, resulting in hyperthyroidism and the clinical manifestations of GD. Moreover, many other factors are implicated in the development of classical GD. Understanding the potential association between COVID-19 vaccination and autoimmune thyroid disorders is essential for managing vaccinated individuals who develop GD.
Factors implicated in inducing Graves’ disease
Graves’ disease is a complex autoimmune disorder influenced by multiple factors. Although its exact cause remains unknown, several factors have been implicated in triggering the development of GD.
Genetic factors
Genetic predisposition plays a significant role in the development of GD. Certain genetic variations are associated with an increased risk of the condition, with genetic factors contributing to ~ 70%–80% of autoimmune thyroid disease (AITD). Variants in certain genes involved in immune regulation have been implicated in GD. For instance, human leukocyte antigen (HLA) genes encode proteins that present antigens to T cells, which are crucial for immune recognition. Specific HLA class II alleles (e.g., HLA-DR3) are strongly associated with GD, as they may present self-antigens, like TSHR peptides, more effectively to immune cells, thereby promoting an autoimmune response. Cytotoxic T-lymphocyte-associated protein 4 (CTLA4) is a checkpoint inhibitor that downregulates T-cell activity and helps maintain self-tolerance. Variants in the CTLA4 gene may impair this inhibitory function, allowing autoreactive T cells to proliferate and attack the thyroid. Protein tyrosine phosphatase nonreceptor type 22 (PTPN22) genes regulate T-cell activation by negatively modulating signaling pathways. The R620W polymorphism in PTPN22 is associated with several autoimmune diseases and likely disrupts T-cell regulation, enhancing autoimmunity and contributing to the development of GD (22). In addition, non-HLA genes have also been associated with GD. For example, the B-cell surface antigen CD40 (CD40) is involved in B-cell activation and antibody production under normal physiological conditions. Variants in CD40 have been shown to enhance B-cell activation and promote the production of TRAb, which stimulates the thyroid. The TSHR regulates normal thyroid hormone synthesis, and polymorphisms in the TSHR gene may lead to abnormal expression or structural changes, rendering it more immunogenic and triggering the production of autoantibodies against it. Moreover, thyroglobulin (TG) is a key protein in thyroid hormone synthesis and can serve as a potential autoantigen. Certain TG gene variants may increase the likelihood of it being recognized as foreign, triggering an autoimmune attack (23). These genetic variants could disrupt immune tolerance and promote the development of autoantibodies, particularly against TSHR, which is central to GD pathogenesis. The combination of immune dysregulation and enhanced presentation of thyroid autoantigens underlies the autoimmune nature of GD.
Epigenetic factors: methylation, miRNAs, and lncRNAs
Epigenetic mechanisms, which involve changes in gene expression without alterations to the underlying DNA sequence, have been implicated in the development of GD. These changes can be influenced by environmental factors and may contribute to the dysregulation of immune responses observed in GD (24). Various epigenetic mechanisms, including DNA methylation—such as rs2228612 in DNA-methyltransferase 1 (DNMT1)—are associated with DNA hypomethylation and GD intractability (25). Regarding histone modifications, several genes, including CD247, CD3D, CD3E, CD3G, CD8A, LCK, ZAP70, and CTLA4, show decreased H3K4me3 levels in their promoters, leading to downregulation of their expression in both CD4+ and CD8+ T cells of GD cases (26). Many noncoding RNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), have also been implicated in the development of GD. For instance, altered expression of let-7b and miR-146a-5p has been associated with GD onset (27). Additionally, the long noncoding RNA Heg has been found to reduce CD14 mRNA levels in mononuclear cells of individuals with GD (28). However, the same group reported that antithyroid treatment did not affect lncRNA Heg levels in GD cases, suggesting that further studies are needed to clarify this relationship (29). Furthermore, exosomes have also been implicated in the development of GD. For instance, exosomes derived from thyrocytes, which target dendritic cells and contain TPO, heat shock protein 60, major histocompatibility complex (MHC) class II, and activated dendritic cells, can significantly enhance CD4+ T lymphocyte responses, contributing to the onset and progression of AITD (30).
Stress and psychological factors
Stress and psychological factors have been implicated in the development of autoimmune diseases, including GD. Stress can impact immune function and may contribute to the development or exacerbation of autoimmune responses. Studies have shown that people with posttraumatic stress disorder (PTSD) are more likely to develop GD, highlighting the need for further research into the relationship between PTSD and thyroid disorders (31). Stress has also been associated with various other autoimmune conditions, including GD (32). The proposed mechanisms include activation of the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system, leading to altered cytokine profiles and immune dysregulation. Chronic stress may also impair regulatory T-cell function, thereby increasing the risk of autoantibody production against thyroid antigens.
Gut microbiome
Disruption of the gut microbiome, known as dysbiosis, may contribute to immune dysregulation and autoimmune diseases such as GD (33, 34). A relationship between GD and Yersinia enterocolitica has been reported; mice exclusively fed with Y. enterocolitica did not develop GD (35). Although some studies have examined gut microbiota in hyperthyroid patients, research specifically linking GD to gut microbiota remains limited (36). Thyroid hormone levels have also been shown to influence gut microbiota composition in GD patients (37). Bacteroidetes and Firmicutes dominate the gut microbiome. In GD, the proportion of Bacteroidetes increases while Firmicutes decrease, resulting in reduced alpha diversity (38–41). Other microbial shifts associated with GD include increases in Lactobacillales, Bacilli, Megamonas, Prevotella, and Veillonella, and decreases in Rikenellaceae, Ruminococcus, and Alistipes (42). Moreover, patients with GD show lower overall microbial richness (42). Bacteroides, Prevotella, and Alistipes have been shown to distinguish GD patients from healthy controls with 85% accuracy (43). TRAb, a key marker of GD, exhibits over 95% sensitivity and specificity (44). TRAb levels positively correlate with Succinivibrionaceae and Subdoligranulum and negatively with Parabacteroides distasonis (45). TRAb-positive patients may also have a higher risk of Graves’ orbitopathy (GO) (45). Further studies are needed to confirm interactions between the thyroid and gut microbiome.
Iodine intake
Excessive iodine intake has long been associated with the development of autoimmune thyroid disorders, including GD. While high iodine intake usually leads to mild and short-term thyroid issues, it can trigger severe and potentially life-threatening hyperthyroidism in some individuals (46). Iodine is essential for thyroid hormone production, and excessive iodine intake can trigger or exacerbate thyroid autoimmunity in susceptible individuals (47). The proposed mechanism may involve, firstly, increased iodination of thyroglobulin, which could enhance its immunogenicity and trigger an autoimmune response. Secondly, excess iodine can induce oxidative stress and apoptosis in thyroid cells, resulting in the release of autoantigens that activate autoreactive T and B cells.
Dietary factor: selenium
Certain dietary factors, particularly selenium deficiency or excess, have been suggested to influence the development of GD. Selenium is a vital trace element essential for maintaining proper thyroid function and supporting immune system regulation. Several studies have shown that selenium deficiency is a risk factor for the development of GD (48). Mechanistically, selenium deficiency impairs the activity of glutathione peroxidase and other selenoproteins, resulting in increased oxidative stress in thyroid tissue. This oxidative damage can promote the release of autoantigens and disrupt immune tolerance, contributing to the initiation of autoimmune responses against the thyroid. Conversely, excess dietary selenium can result in selenosis, characterized by symptoms such as hair loss, brittle nails, nausea, and fatigue. In severe cases, it may cause neurological damage and gastrointestinal disturbances (33).
Vitamin D deficiency
Vitamin D deficiency has been associated with an increased risk of autoimmune diseases, including GD. Vitamin D plays an important role in immune function and may help regulate the immune response in AITD (49, 50). A high prevalence of GD has been observed in vitamin D-deficient women (50), and low serum vitamin D levels have been reported in GD cases with no remission (51), collectively indicating that vitamin D has a considerable impact on GD. While these factors have been implicated in the development of GD, it is important to note that the underlying mechanisms of the disease are complex and multifactorial. Vitamin D deficiency can impair regulatory T-cell function and promote the activation of proinflammatory T helper (Th)1 and Th17 cells, resulting in an enhanced autoimmune response. Additionally, low vitamin D levels may increase the expression of MHC class II molecules and costimulatory signals on antigen-presenting cells, thereby enhancing autoantigen presentation and thyroid-specific immune activation (50).
Viruses
Several viruses have been linked to an increased risk of autoimmune thyroid disorders, including GD. For instance, Epstein–Barr virus (EBV) infects B cells and triggers autoimmunity. Hepatitis C virus (HCV) has been associated with thyroid dysfunction in infected individuals (3, 4). Coxsackievirus has been implicated in autoimmune thyroiditis and possibly GD, while human herpesvirus-6 (HHV-6) has also been reported in some autoimmune disorders. Recent evidence suggests that SARS-CoV-2 infections may trigger thyroid autoimmunity, including GD (4).
Vaccines
Several vaccines have been reported to potentially induce or exacerbate GD. Particularly, influenza vaccines, for example, activate the immune system and may trigger autoimmune thyroid disorders, particularly in individuals with a genetic or immunological predisposition. The hepatitis B vaccine, through immune responses to its recombinant proteins or adjuvants, may stimulate the production of thyroid autoantibodies (anti-TPO, anti-TG, or TRAb) (52). Similarly, the human papillomavirus (HPV) vaccine can activate the immune system via adjuvants (e.g., aluminum). Immune activation following yellow fever vaccination may also lead to the production of thyroid-stimulating immunoglobulins (TSI) (4).
Chemical constituents and characteristics of various SARS-CoV-2 vaccines
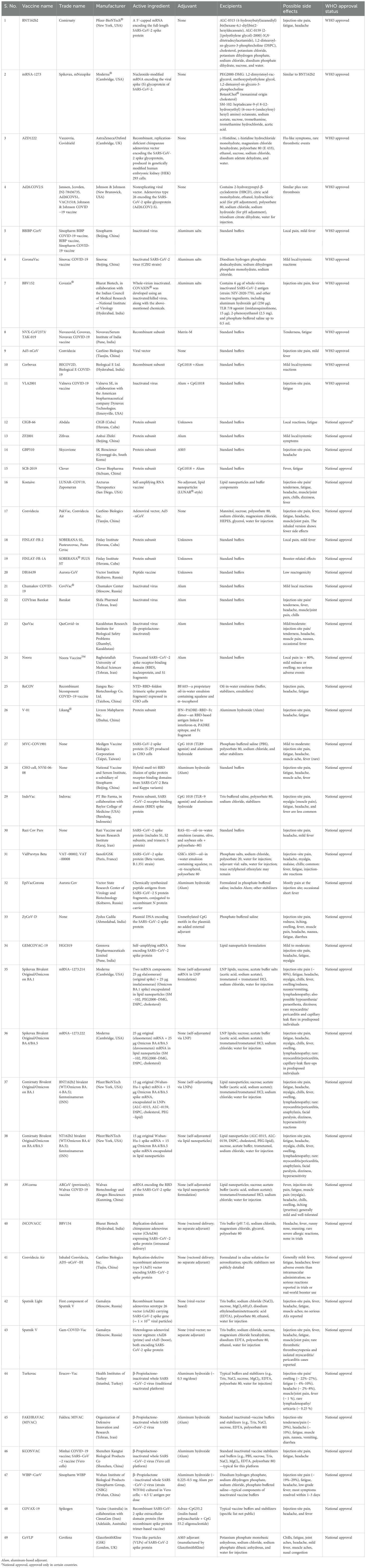
As listed in Table 1, a total of 242 COVID-19 vaccines have been developed to date. Among these, 66 underwent phase I trials, 72 proceeded to phase II, and 92 reached phase III trials, while 12 vaccines were discontinued during the process. Of the 242 vaccines, 49 are currently in widespread use for COVID-19 immunization (11 approved by the WHO and 38 approved only in a few countries), and one was withdrawn after initial approval. Each vaccine contains unique active ingredients, excipients, and, in some cases, adjuvants, which may influence their effects in immunized individuals. There are three major types of vaccines administered against COVID-19: mRNA-based vaccines, inactivated virus vaccines, and viral vector-based vaccines. The mRNA-based vaccines include Pfizer-BioNTech® BNT162b and Moderna® mRNA-1273. These vaccines use nucleoside-modified mRNA encoding the full-length SARS-CoV-2 “S” protein, encapsulated in lipid nanoparticles. Notably, no adjuvants are included, as the mRNA itself elicits a strong immune response. Excipients consist of polyethylene glycol (PEG)-based lipids, sucrose for stabilization, and buffers to maintain pH. As illustrated in Figure 1, these vaccines may trigger allergic or anaphylactic reactions, primarily due to PEG components, which are known to cause hypersensitivity in susceptible individuals. Inactivated-virus vaccines include the Sinovac COVID-19 vaccine (Vero cell) and Covaxin. These vaccines contain a chemically inactivated SARS-CoV-2 virus as the active component, inducing immunity without viral replication. Aluminum hydroxide (Alum) is used as an adjuvant to enhance immunogenicity, while excipients such as phosphate buffers and sodium chloride help maintain isotonicity. The presence of adjuvants in vaccines is primarily associated with local injection-site reactions and systemic responses such as fever and fatigue. Viral vector-based vaccines include ChAdOx1 nCoV-19 (AstraZeneca) and Jcovden™ (Janssen). These vaccines use genetically modified adenoviruses—chimpanzee adenovirus for AstraZeneca and human adenovirus type 26 for Janssen—to deliver the SARS-CoV-2 “S” protein. They do not contain adjuvants, as viral vectors inherently stimulate innate immune pathways. Excipients include polysorbate 80, histidine, and various salts to stabilize the formulation. These vaccines can produce various effects, including injection-site irritation, systemic immune responses, and, in rare cases, hypersensitivity reactions. mRNA vaccines use lipid nanoparticle delivery, which triggers immune activation with minimal excipients but carries a risk of PEG-induced allergies. Inactivated-virus vaccines require adjuvants, such as aluminum hydroxide, to enhance the immune response, often causing localized inflammation. Viral vector vaccines utilize adenoviral delivery systems to induce immunogenicity, with excipients ensuring stability, though they pose a risk of systemic inflammatory reactions (53). Table 1 summarizes differences in vaccine design, immunogenic components, and potential adverse effects, providing a clearer understanding of their safety profiles. To identify literature on COVID-19 vaccine-induced GD, we used the following search strategies.
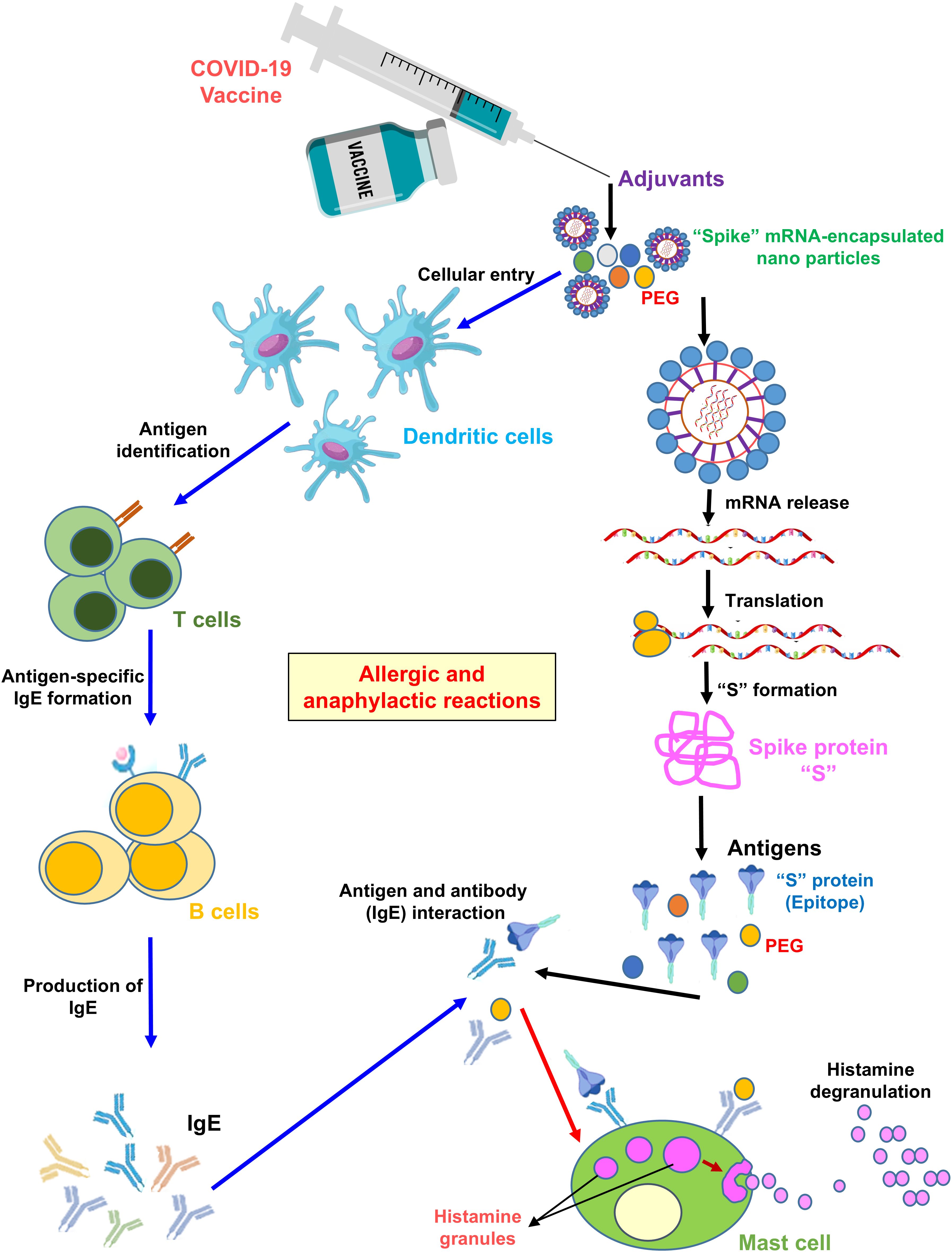
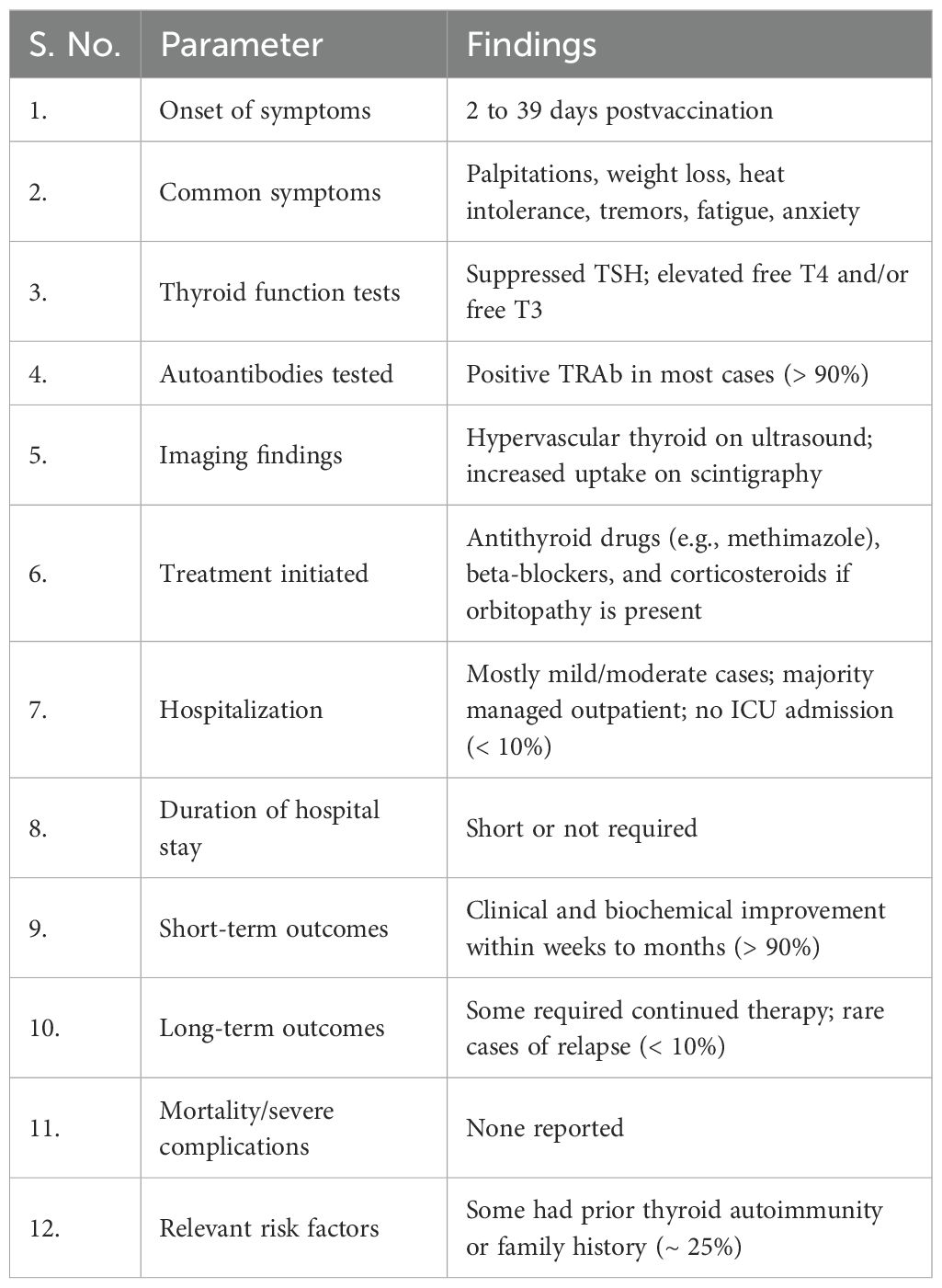
Figure 1. Mechanism of allergic and anaphylactic reactions following mRNA vaccination. As shown in the illustration, PEG nanoparticle packaging of the “S” protein mRNA enters an APC cell, such as dendritic cells, via endocytosis. The mRNA is then translated into the S protein by ribosomes. The APC presents free-floating PEG or S protein epitopes as antigens to T helper cells. The latter secrete cytokines, leading to B-cell activation. B cells produce IgE antibodies against PEG or S protein epitopes. Antigen-specific (PEG and S protein specific) IgE antibodies bind to the FcϵRI receptor. Engagement of this receptor triggers histamine release from basophils and/or mast cells, resulting in allergic/anaphylactic reactions.
Search strategy and selection criteria
We comprehensively searched the literature in PubMed, Web of Science, SCOPUS, and Google Scholar databases. The search terms used were “SARS-CoV-2 vaccine, Graves’ disease”, “COVID-19 vaccine, Graves’ disease”, “hyperthyroidism, COVID-19 vaccine”, and “hyperthyroidism, SARS-CoV-2 vaccine”. Only articles published in English were included. All articles published on/or before 31 December 2024 were considered, regardless of type, including full-length articles, short reports, case reports, and letters to the editor. We excluded articles reporting nonthyroid disease, non-Graves’ disease, SARS-CoV-2-induced Graves’ disease, and thyroid eye disease (TED). In total, 203 articles were retrieved through the database search. Of these, 100 duplicates, six nonthyroid disease articles, 10 non-Graves’ disease articles, five SARS-CoV-2-induced Graves’ disease articles, and seven TED articles were removed. All details are summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart (http://www.prisma-statement.org) in Figure 2.
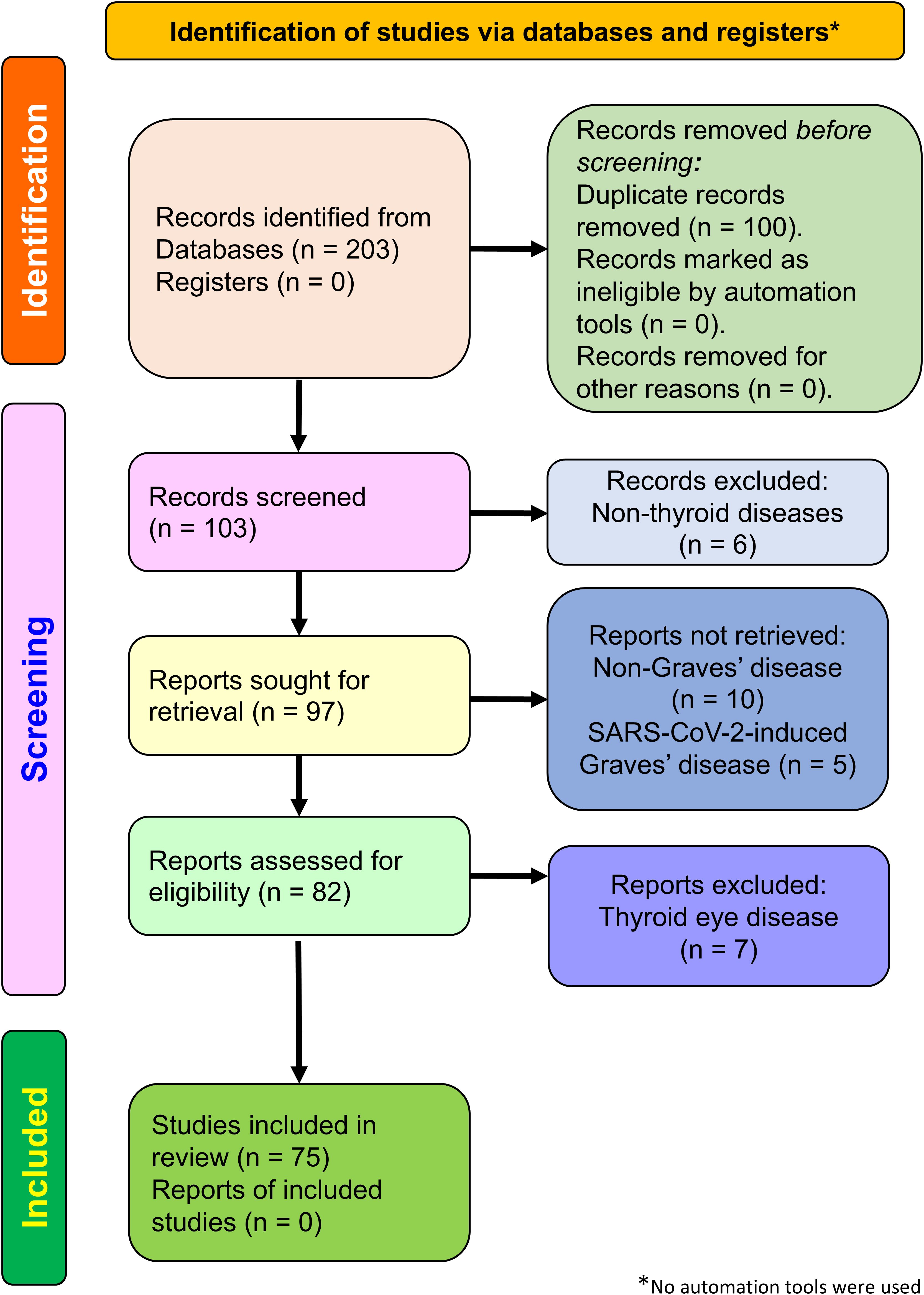
Figure 2. Flowchart depicting the PRISMA 2020 study selection process. The illustration shows the detailed study selection process for COVID-19 vaccine-induced hyperthyroidism of Graves’ disease.
COVID-19 vaccine induces Graves’ disease: an autoimmune hyperthyroidism
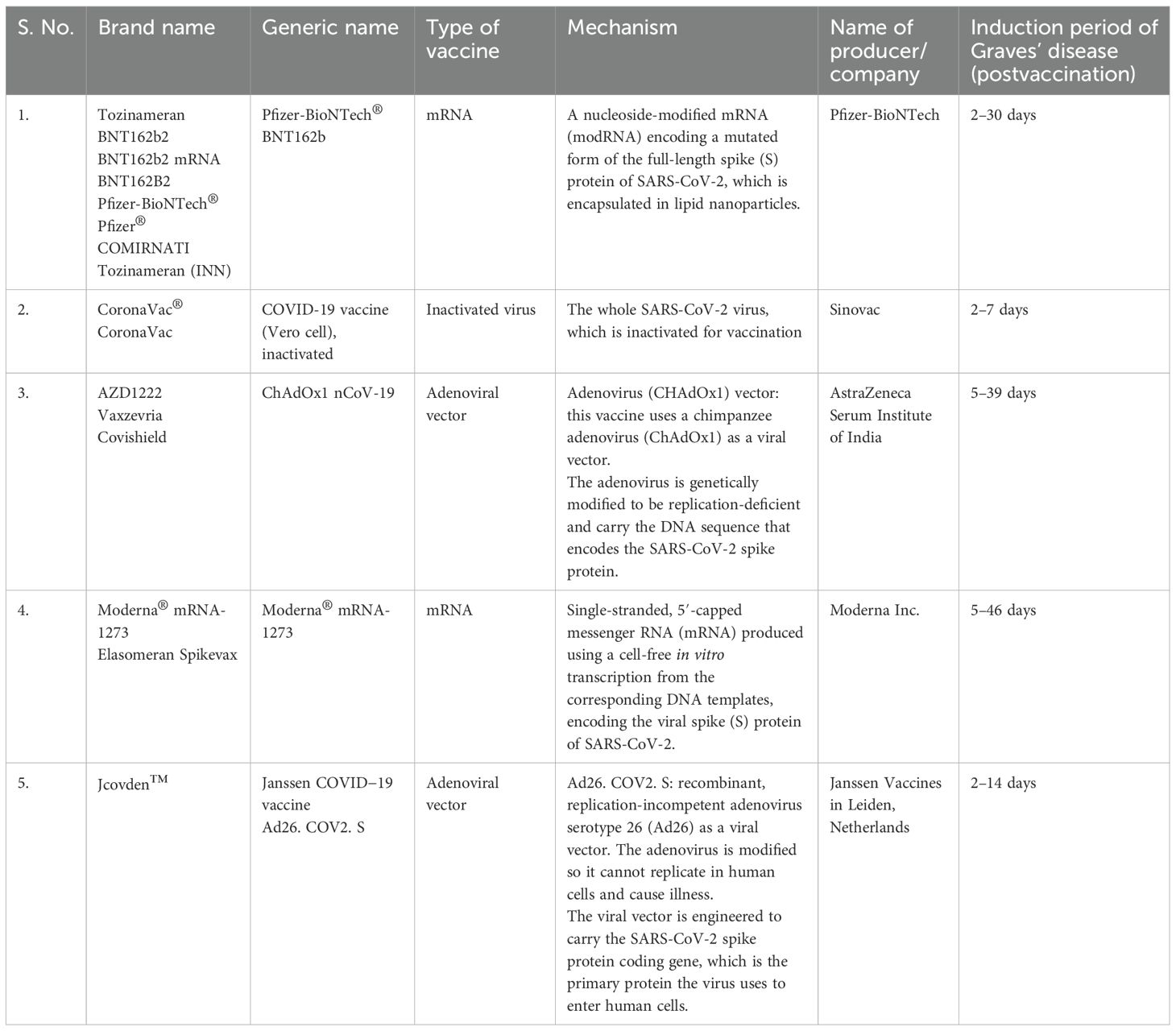
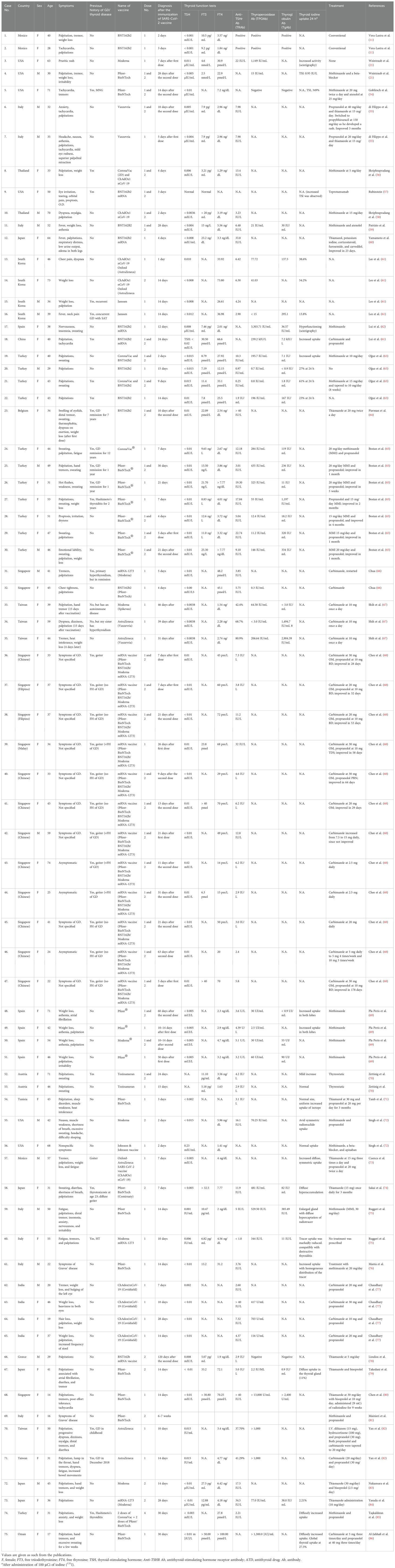
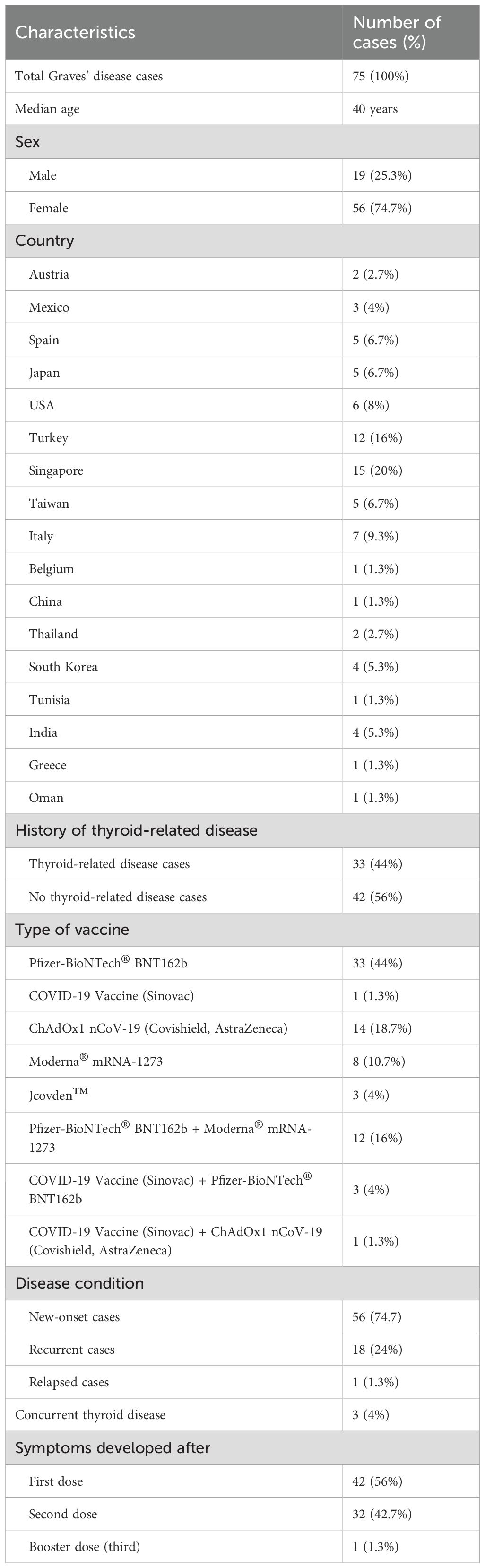
There have been many reports and studies suggesting a potential link between COVID-19 vaccination and the development of autoimmune conditions such as GD. As shown in Table 2, the induction period of GD in postvaccination varies depending on the vaccine type. Specifically, Pfizer-BioNTech® ranges from 2 to 30 days, CoronaVac® from 2 to 7 days, ChAdOx1 nCoV-19 from 5 to 46 days, and Jcovden™ from 2 to 14 days. To date, and as listed in Table 3, a total of 75 cases of vaccine-induced GD have been reported, and the detailed characteristics of each case are documented as described in the respective original articles (11, 21, 54–86). The characteristics of SARS-CoV-2 vaccine-induced GD are summarized in Table 4. The median age of affected individuals is 40 years, indicating a predilection for middle-aged adults. Out of 75 cases, 56 were women (74.7%) and 19 were men (25.3%), supporting the known female predominance in autoimmune diseases. Cases were reported globally, with the highest numbers from Singapore (15 cases), followed by Turkey (12 cases), Italy (seven cases), and the USA (six cases). These numbers may reflect the intensity of vaccination campaigns or genetic/environmental predispositions in specific populations. In total, 33 cases (44%) had a preexisting thyroid condition, such as autoimmune thyroiditis or GD in remission, whereas 42 cases (56%) had no prior thyroid-related diseases. Vaccine-associated GD occurred both with individual vaccines and through combinations of different vaccines (heterologous vaccination). The Pfizer-BioNTech® BNT162b vaccine was the most frequently associated, inducing GD in 33 cases (44%), followed by ChAdOx1 nCoV-19 (Covishield, AstraZeneca) in 14 cases (18.7%), and Moderna® mRNA-1273 in eight cases (10.7%). The remaining cases were linked to combinations such as Pfizer + Moderna or Sinovac with other vaccines, highlighting the effects of heterologous vaccination. Among them, 56 cases (74.7%) were new-onset, emphasizing that vaccine-induced GD is not restricted to individuals with prior thyroid disorders. This finding demonstrates the immune-activating potential of vaccines in triggering autoimmunity in some previously healthy individuals. Recurrent GD cases accounted for 18 cases (24%), representing a recurrence in those with resolved or remitted GD. Relapsed GD was identified in only one case (1.3%) involving individuals with ongoing thyroid dysfunction. Only three cases had documented concurrent thyroid diseases, such as Hashimoto’s thyroiditis, indicating that thyroid dysfunction postvaccination may primarily arise as a new autoimmune phenomenon. Onset and symptom timing were reported after the first dose in 42 cases (56%), after the second dose in 32 cases (42.7%), and after a booster dose in only one case (1.3%). This suggests a higher likelihood of onset during initial vaccine exposure, possibly due to immune priming. Major limitations of this observation include potential underreporting of cases due to mild symptoms or lack of awareness. Variations in case definitions, diagnostic criteria, and follow-up durations across studies may also influence the reported prevalence. Table 3 highlights the predominance of new-onset GD cases, female susceptibility, and a strong association with mRNA vaccines (Pfizer-BioNTech). It also emphasizes the importance of postvaccine surveillance and patient education for timely diagnosis and management, and suggests future research directions, such as investigating genetic predispositions and mechanisms of vaccine-induced thyroid autoimmunity. Tables 3, 4 systematically synthesize and critically appraise the currently dispersed case reports, providing a comprehensive overview of this emerging phenomenon and highlighting potential future research directions, such as investigating genetic predispositions and mechanisms of vaccine-induced thyroid autoimmunity. Table 5 details the hospital course and outcomes of COVID-19 vaccine-induced Graves’ disease. Symptom onset ranged from 2 to 39 days after vaccination, with common clinical features including palpitations, weight loss, heat intolerance, tremors, fatigue, and anxiety—typical manifestations of hyperthyroidism. Thyroid function tests typically revealed suppressed TSH levels with elevated free T4 and/or free T3, and over 90% of patients tested positive for TRAb, confirming the autoimmune nature of the condition. Imaging studies often showed a hypervascular thyroid on ultrasound and increased uptake on scintigraphy, consistent with active Graves’ disease. Treatment primarily involved antithyroid medications such as methimazole and beta-blockers, with corticosteroids reserved for cases with eye involvement. Most patients experienced mild to moderate illness, managed on an outpatient basis, with minimal hospitalization and no ICU admissions. The short-term outlook was favorable, with over 90% of patients demonstrating clinical and biochemical improvement within weeks to months. Long-term follow-up indicated that a minority required ongoing therapy or experienced relapse, although such instances were uncommon. Importantly, no cases of mortality or severe complications were reported. Approximately 25% of individuals had a personal or family history of thyroid autoimmunity, suggesting a potential predisposition.
However, it is important to note that these cases are rare, and further research is needed to establish a clear causal relationship. Autoimmune reactions may theoretically be triggered by vaccination, as the immune response stimulated by the vaccine could inadvertently target the body’s own tissues. Nonetheless, vaccines undergo rigorous testing for safety and efficacy prior to approval, and adverse effects are closely monitored. Evaluating patients’ medical histories and individual risk factors can provide critical guidance for addressing concerns regarding the COVID-19 vaccine and autoimmune conditions such as GD.
Molecular mechanism of COVID-19 vaccine-induced Graves’ disease
Graves’ disease is an autoimmune disorder characterized by the production of TSI, which activates the TSHR and leads to hyperthyroidism. While vaccines, including those against COVID-19, play a crucial role in preventing infectious diseases; however, emerging reports suggest that, in rare cases, they may trigger autoimmune responses, including GD. The molecular mechanisms underlying COVID-19 vaccine-induced GD remain under investigation, but several hypotheses are plausible. One of the key mechanisms likely to be involved is molecular mimicry, in which vaccine components, such as the “S” protein, share structural similarities with thyroid antigens, potentially leading to cross-reactivity and the development of autoimmunity. Additionally, bystander activation can result from vaccine-induced immune stimulation, leading to the activation of autoreactive T and B cells. Adjuvant components in the vaccine formulation may further contribute to immune dysregulation, increasing the risk of autoimmunity. Molecules involved in inflammatory pathways may also play a significant role in vaccine-induced GD. Understanding these molecular pathways is essential for identifying individuals at risk and developing strategies to mitigate such adverse effects while preserving the overall benefits of COVID-19 vaccination. Further research is needed to elucidate the precise immunological triggers and improve the management of postvaccine autoimmune conditions.
Molecular mimicry
Molecular mimicry, also known as the cross-reactivity theory, is a phenomenon in which antigens from infectious agents share structural similarities with self-antigens. When the immune system mounts a response against the infectious agent, it may inadvertently target self-antigens due to these similarities. As illustrated in Figure 3, some researchers hypothesize that molecular mimicry between antigens of the SARS-CoV-2 virus and self-antigens in the thyroid gland could contribute to the development of autoimmune thyroid disorders, including GD, following COVID-19 vaccination. In this context, the amino acid sequences of thyroid peroxidase (TPO) and the SARS-CoV-2 “S” protein share similarities (87, 88). As a result, the immune system may mistakenly produce antibodies against thyroid tissue due to its resemblance to the “S” protein generated by the mRNA vaccine. In addition, it has been observed that Japanese healthcare workers who received repeated mRNA vaccinations showed a gradual increase in mean TRAb levels (89). This rise may be attributed to cross-reactivity between thyroid tissue and the SARS-CoV-2 “S” protein, suggesting that repeated vaccinations could have induced higher TRAb production. However, the present study did not measure TPO levels in these individuals; assessing TPO alongside TRAb could provide a more comprehensive evaluation of postvaccine GD. It is important to note that, although these mechanisms are plausible, they have not been conclusively proven to contribute to COVID-19 vaccine-induced GD. On the other hand, it remains unclear whether total antibody levels are elevated in these cases. An increase in total antibodies (including anti-SARS-CoV-2 antibodies) could indicate that the rise in TRAb is part of a generalized immune activation following vaccination. In this study, no association was found between SARS-CoV-2 IgG antibodies and TRAb levels. While IgG responses are known to correlate with protection against COVID-19, assessments of cellular immunity or neutralizing antibody titers may provide a more precise connection to thyroid autoimmunity. Moreover, it is important to note that TRAb is a specific autoantibody, not typically produced during standard immune responses. A generalized increase in antibodies could theoretically unmask or trigger latent autoimmunity in genetically susceptible individuals. Therefore, while elevated total antibodies might reflect a broad immune response, the presence of TRAb indicates a more targeted autoimmune phenomenon rather than a nonspecific antibody surge. The rise in TRAb, therefore, likely reflects specific autoimmune activation. Further research is needed to elucidate the molecular mechanisms underlying this potential association.
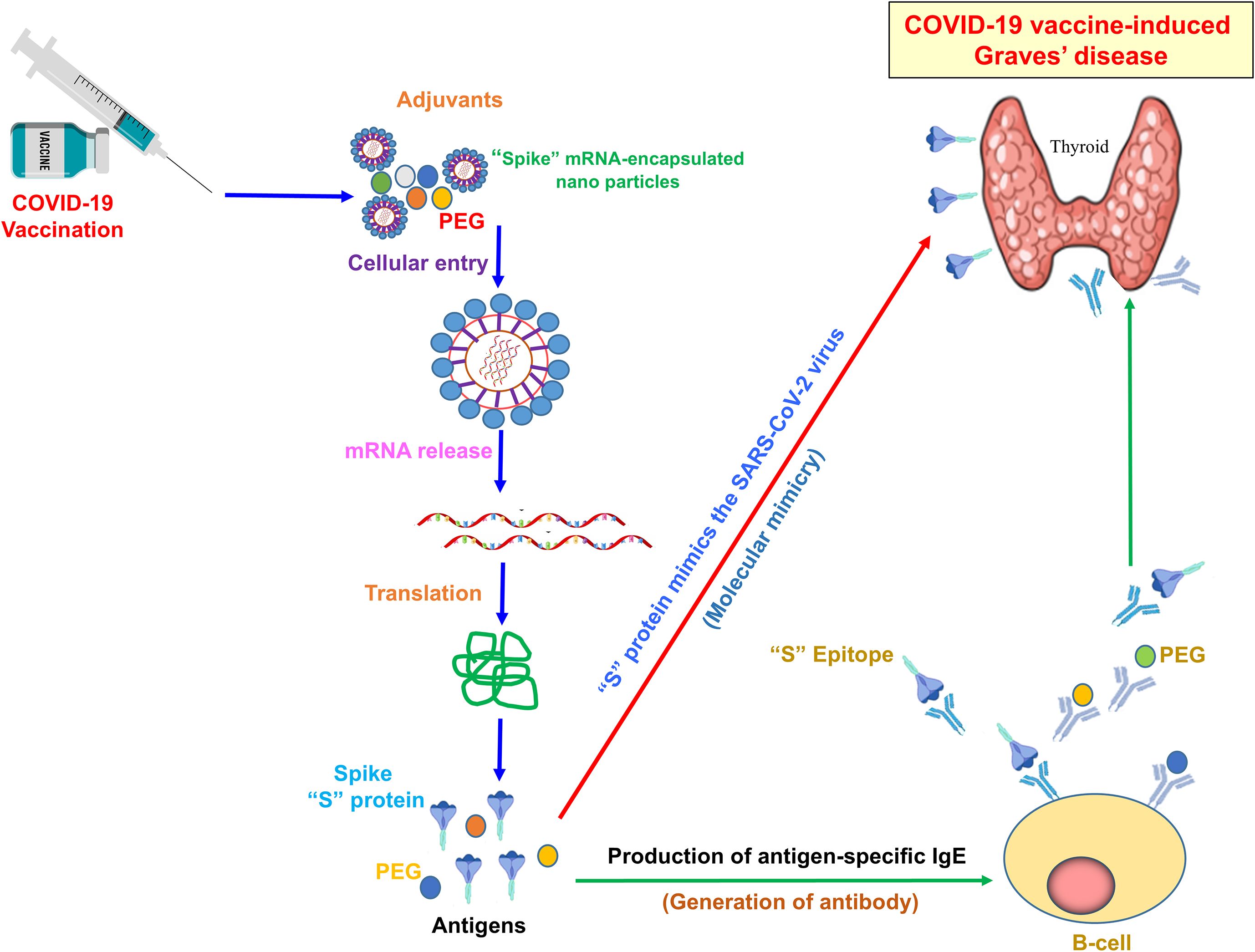
Figure 3. Mechanism of COVID-19 vaccine-induced Graves’ disease. The illustration typically shows how adjuvants and antigen-specific antibodies induce GD. The SARS-CoV-2 “S” protein mimics the viral antigen, triggering an immune response post-COVID-19 vaccination. Adjuvants, including PEG-encapsulated spike mRNA nanoparticles, enhance antigen delivery and immune activation. Upon mRNA release and translation, the “S” protein is expressed, leading to B-cell activation and the production of antigen-specific IgE antibodies. Molecular mimicry between the spike protein epitope and thyroid antigens may contribute to the development of autoimmune thyroid dysfunction, including GD.
Antigen presentation
Vaccines, including those for COVID-19, introduce antigens—typically spike proteins or their genetic blueprints—to stimulate the immune system. After administration, antigen-presenting cells (APCs), such as dendritic cells, take up these antigens. Within the APCs, the antigens are processed into peptide fragments and loaded onto MHC class II molecules, which are then displayed on the cell surface. These MHC-antigen complexes are recognized by CD4+ T helper cells, which become activated upon binding. In most individuals, this response results in protective immunity against the virus. However, in genetically predisposed individuals, particularly those carrying autoimmune susceptibility genes such as the HLA-DRB1 haplotype, this process may inadvertently activate autoreactive T cells. These T cells, which normally remain dormant or suppressed, may then target self-antigens that resemble viral peptides due to molecular mimicry (Figure 3).
In Graves’ disease, the autoreactive immune response targets the TSHR, leading to the production of TRAb. These autoantibodies mimic TSH, overstimulating the thyroid and causing hyperthyroidism. Adjuvants, such as aluminum salts, used to enhance immune activation, may further exacerbate this process by promoting a stronger and longer-lasting immune response, contributing to autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Graves’ disease following COVID-19 vaccination has been primarily associated with autoimmunity (62, 90, 91). Thus, COVID-19 vaccine-related GD may arise not merely from immune stimulation but from a complex interaction of antigen presentation, genetic predisposition, autoreactive T-cell activation, and heightened adjuvant-induced immune signaling. ASIA has previously been associated with autoimmune diseases following vaccinations for papillomavirus, influenza, and hepatitis B. While adjuvants are included in vaccines to enhance immune responses, they can also contribute to ASIA. A commonly used adjuvant is aluminum salt, present in vaccines such as those for papillomavirus, hepatitis B, and pneumococcal infections (89, 91, 92). In addition, genetic predisposition to ASIA has been reported, particularly among individuals carrying the HLA DRB1 haplotype (93).
Bystander activation
In addition to specific antigen recognition, vaccines can activate immune responses through a phenomenon known as bystander activation (94). This occurs when the immune system responds to a nonspecific stimulus, such as inflammation induced by the vaccine (94). Bystander activation can trigger autoreactive T cells that may target self-antigens, including those present in the thyroid gland. T-cell antigen specificity arises from T-cell receptors (TCRs), which recognize antigen-derived peptides presented by MHC molecules on APCs (95, 96). Upon activation, T cells undergo clonal expansion and differentiate into memory cells, establishing long-term immunity. However, some T cells that are not specific to the antigen can also participate in immune responses, as they can be activated independently of antigen recognition (94, 95). This phenomenon is known as “bystander activation”. Bystander activation mainly occurs through two mechanisms. Firstly, during pathogen infections and inflammatory responses, cytokines, toll-like receptor (TLR) ligands, and other immune mediators can stimulate self-reactive T cells, potentially triggering autoimmune responses (95). Secondly, an initial inflammatory response driven by self-antigens, including activation of self-antigen-specific T cells, can lead to the bystander activation of other memory T cells with different antigen specificities. These activated T cells then release pathogenic inflammatory cytokines, contributing to the development of autoimmune diseases (96).
Vital role of inflammatory syndrome/autoimmune factors in COVID-19 vaccine-induced Graves’ disease
This refers to the involvement of inflammatory molecules in activating the immune system during the development of GD following COVID-19 vaccination. In typical GD, the immune system targets the thyroid gland, causing overactivity and excessive production of thyroid hormones. When a person receives a COVID-19 vaccine, their immune system is stimulated to generate a response against the virus. In some individuals, however, vaccination can also trigger inflammation—partly due to vaccine adjuvants—leading to the production of autoantibodies that target the thyroid gland (75, 89). These autoantibodies can stimulate the thyroid gland to produce excessive thyroid hormones, leading to hyperthyroidism and the clinical manifestations of GD (89).
Notably, thyroid tissues have been shown to highly express ACE2, regardless of pathological condition, including cancerous stages (90). COVID-19 vaccines contain either mRNA alone (e.g., Moderna, Pfizer) or whole attenuated virus (e.g., Sinovac, Covaxin). The “S” protein produced by these vaccines binds to ACE2, leading to its downregulation, which has been found to induce the release the IL-1β and IL-6 (97). Additionally, the “S” protein may directly stimulate the production of these cytokines (98). Furthermore, mRNA vaccines contain adjuvants such as LNPs, which can trigger inflammatory cytokines, including interleukin (IL)-1β and IL-6, potentially contributing to ASIA (99, 100). The LNPs significantly increase IL-1β production by more than 22-fold and IL-6 synthesis by at least 12-fold, while generating higher antibody levels than AddaVax, an MF59-like adjuvant (98). Elevation of IL-1β and IL-6 has been shown to contribute to autoimmune disease by activating Th17 cells and suppressing regulatory T cells (88, 101) (Figure 4). Notably, increased IL−6 expression has been observed in patients with Graves’ disease (102). Furthermore, another study demonstrated that IL−1β induces elevated IL-6 expression in human orbital fibroblasts (103). Taken together, these findings highlight a strong mechanistic link between IL−1 and IL−6 in the pathogenesis of Graves’ disease. The development of autoimmune reactions following vaccination may be influenced by several factors, including genetic predisposition, environmental triggers, and specific characteristics of the vaccine. Understanding the role of autoimmune factors in COVID-19 vaccine-induced GD is essential for identifying individuals at risk and for developing strategies to mitigate these risks, while ensuring the continued effectiveness of COVID-19 vaccination programs.
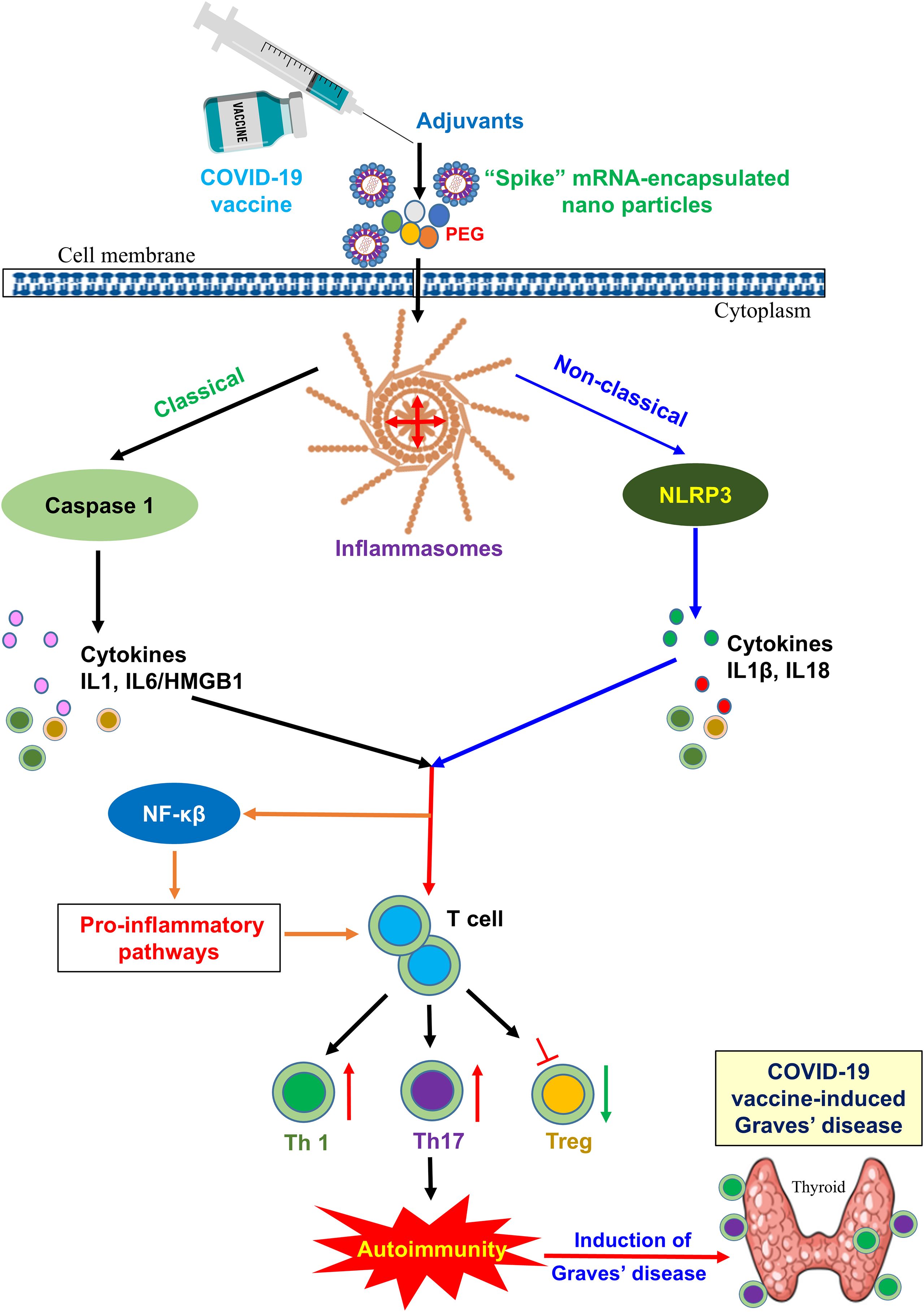
Figure 4. Potential mechanisms inducing autoimmunity through vaccine adjuvants: a vital role of inflammatory pathways. Vaccine adjuvants can activate inflammasomes. Canonical inflammasomes recruit caspase-1 via the adaptor molecule ASC, leading to activation of caspase-1 and the release of proinflammatory DAMPs such as IL-1α or HMGB1. Noncanonical inflammasomes activate the NLRP3 inflammasome, which indirectly induces the maturation and secretion of IL-1β and IL-18 via the noncanonical route, which binds to the receptors and activates the NF-κB signaling pathway, ultimately leading to the upregulation of NLRP3, pro-IL-1β, and pro-IL-18. In addition, preexisting antibody recognition of PEGs and direct mast cell activation, coupled with potential genetic or environmental predispositions to hypersensitivity, account for anaphylaxis to COVID-19 mRNA vaccines.
Benefit–risk context and rarity of post-COVID-19 vaccination Graves’ disease
It is important to acknowledge that the association between COVID-19 vaccination and the onset of Graves’ disease remains anecdotal, supported mainly by descriptive clinical reports rather than robust analytical or epidemiological data. The currently available evidence is limited to isolated case observations, which may reflect coincidental temporal relationships rather than true causality. In the absence of large-scale, controlled studies, any inference of a causal link remains speculative. Therefore, the findings summarized in this review should be interpreted with caution, considering the potential influence of publication bias and reporting variability. Our goal is not to establish causation but to consolidate existing clinical observations and propose potential immunological hypotheses that warrant further investigation. Future prospective, multicenter, and mechanistic studies are essential to confirm or refute any possible association between COVID-19 vaccination and autoimmune thyroid dysfunction.
While it is important to acknowledge the occurrence of rare autoimmune events such as Graves’ disease following COVID-19 vaccination, these cases should be interpreted within the broader context of vaccine safety and global benefit. To date, only a limited number of GD cases have been reported despite the administration of billions of vaccine doses worldwide, highlighting the exceptional rarity of this outcome. It is also plausible that some cases of vaccine-related Graves’ disease remain unrecognized, as they may be treated symptomatically without linking the onset to recent vaccination. Additionally, underreporting of mild or transient cases could lead to an underestimation of the true incidence in postvaccination surveillance data. However, the overall benefit–risk profile of COVID-19 vaccines remains highly favorable, as vaccination has played a crucial role in preventing severe disease, hospitalization, and mortality worldwide. The reporting of these rare adverse events primarily serves to enhance clinical vigilance and support timely diagnosis and management in predisposed individuals, rather than to question vaccine safety. Continued postvaccination surveillance and systematic data collection will further clarify the true incidence and immunological mechanisms underlying such uncommon autoimmune manifestations.
Conclusions and future prospects
In conclusion, emerging reports of COVID-19 vaccine-induced GD highlight the important role of autoimmune factors in its development. The molecular mechanisms underlying vaccine-induced autoimmunity, including GD, are complex and multifactorial. Genetic predisposition, environmental triggers, and immune system dysregulation likely contribute to autoimmune reactions following vaccination. Furthermore, other recently implicated factors—such as disruptions in the gut microbiome, excessive iodine intake, stress, and epigenetic changes—further underscore the multifactorial nature of this disorder. Understanding these factors is essential for identifying individuals at risk and for developing strategies to minimize these risks while maintaining the effectiveness of COVID-19 vaccination programs. Further research is needed to elucidate the specific molecular mechanisms linking COVID-19 vaccination to the development of GD. A better understanding of these mechanisms could improve our ability to predict, prevent, and manage vaccine-induced autoimmune reactions, ultimately enhancing the safety and efficacy of vaccination efforts worldwide.
Author contributions
AM: Investigation, Supervision, Software, Writing – original draft, Project administration, Validation, Resources, Formal Analysis, Conceptualization, Visualization, Methodology, Data curation. AA: Supervision, Writing – review & editing, Software, Formal Analysis, Methodology, Visualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; ACE2, angiotensin-converting enzyme 2; ILs, interleukins; ATDs, antithyroid drugs; TNF, tumor necrosis factor; TNF-α, tumor necrosis factor-α; Th1, T helper cell 1, Th2, T helper cell 2; AITD, autoimmune thyroid disease; TCR, T-cell receptor; RT-PCR, real-time PCR; ASC, apoptosis-associated speck-like protein containing a CARD; HMGB1, high-mobility group box 1; IL-1α, interleukin-1α; IL-1β, interleukin-1β; IL-18, interleukin-18; NF-κB, nuclear factor kappa B; PEG, polyethylene glycol.
References
1. World Health Organization (WHO). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed September 1, 2025).
2. Li MY, Li L, Zhang Y, and Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. (2020) 9:45. doi: 10.1186/s40249-020-00662-x
3. Murugan AK and Alzahrani AS. Potential impacts of SARS-CoV-2 on parathyroid: current advances and trends. Endocrine. (2023) 81:391–408. doi: 10.1007/s12020-023-03415-6
4. Murugan AK and Alzahrani AS. SARS-CoV-2 plays a pivotal role in inducing hyperthyroidism of Graves’ disease. Endocrine. (2021) 73:243–54. doi: 10.1007/s12020-021-02770-6
5. Murugan AK and Alzahrani AS. SARS-coV-2: emerging role in the pathogenesis of various thyroid diseases. J Inflammation Res. (2021) 14:6191–221. doi: 10.2147/JIR.S332705
6. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, and Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. (2022) 22:1293–302. doi: 10.1016/S1473-3099(22)00320-6
7. Zheng C, Shao W, Chen X, Zhang B, Wang G, and Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. (2022) 114:252–60. doi: 10.1016/j.ijid.2021.11.009
8. De Domenico M. Prevalence of long COVID decreases for increasing COVID-19 vaccine uptake. PloS Glob Public Health. (2023) 3:e0001917. doi: 10.1371/journal.pgph.0001917
9. COVID-19 vaccine data . Available online at: https://data.who.int/dashboards/covid19/vaccines?n=o (Accessed September 1, 2025).
10. Faksova K, Walsh D, Jiang Y, Griffin J, Phillips A, Gentile A, et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. (2024) 42:2200–11. doi: 10.1016/j.vaccine.2024.01.100
11. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, and Jara LJ. Two cases of graves’ Disease following SARS-coV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. (2021) 31:1436–9. doi: 10.1089/thy.2021.0142
12. Semmler A, Mundorf AK, Kuechler AS, Schulze-Bosse K, Heidecke H, Schulze-Forster K, et al. Chronic fatigue and dysautonomia following COVID-19 vaccination is distinguished from normal vaccination response by altered blood markers. Vaccines (Basel). (2023) 11:1642. doi: 10.3390/vaccines11111642
13. Krumholz HM, Wu Y, Sawano M, Shah R, Zhou T, Arun AS, et al. Post-vaccination syndrome: A descriptive analysis of reported symptoms and patient experiences after covid-19 immunization. medRxiv. (2023) 10:2023. doi: 10.1101/2023.11.09.23298266
14. Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, et al. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. (2022) 28:542–54. doi: 10.1016/j.molmed.2022.04.007
15. Verbeke R, Hogan MJ, Loré K, and Pardi N. Innate immune mechanisms of mRNA vaccines. Immunity. (2022) 55:1993–2005. doi: 10.1016/j.immuni.2022.10.014
16. Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. (2008) 181:2134–44. doi: 10.4049/jimmunol.181.3.2134
17. Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. (2022) 74:715–8. doi: 10.1093/cid/ciab465
18. Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, et al. Persistence of SARS coV-2 S1 protein in CD16+ Monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front Immunol. (2022) 12:746021. doi: 10.3389/fimmu.2021.746021
19. Theoharides TC. Could SARS-coV-2 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. (2022) 59:1850–61. doi: 10.1007/s12035-021-02696-0
20. Segal Y and Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. (2018) 15:586–94. doi: 10.1038/cmi.2017.151
21. Weintraub MA, Ameer B, and Sinha Gregory N. Graves disease following the SARS-coV-2 vaccine: case series. J Investig Med High Impact Case Rep. (2021) 9:23247096211063356. doi: 10.1177/23247096211063356
22. Grixti L, Lane LC, and Pearce SH. The genetics of Graves’ disease. Rev Endocr Metab Disord. (2024) 25:203–14. doi: 10.1007/s11154-023-09848-8
23. Płoski R, Szymański K, and Bednarczuk T. The genetic basis of Graves’ disease. Curr Genomics. (2011) 12:542–63. doi: 10.2174/138920211798120772
24. Razmara E, Salehi M, Aslani S, Bitaraf A, Yousefi H, Colón JR, et al. Graves’ disease: introducing new genetic and epigenetic contributors. J Mol Endocrinol. (2021) 66:R33–55. doi: 10.1530/JME-20-0078
25. Arakawa Y, Watanabe M, Inoue N, Sarumaru M, Hidaka Y, and Iwatani Y. Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clin Exp Immunol. (2012) 170:194–201. doi: 10.1111/j.1365-2249.2012.04646.x
26. Limbach M, Saare M, Tserel L, Kisand K, Eglit T, Sauer S, et al. Epigenetic profiling in CD4+ and CD8+ T cells from Graves’ disease patients reveals changes in genes associated with T cell receptor signaling. J Autoimmun. (2016) 67:46–56. doi: 10.1016/j.jaut.2015.09.006
27. Al-Heety RA, Al-Hadithi HS, and Turki KM. Correlation of circulating miRNA-146a-5p and let-7b expression with thyroid-stimulating hormone receptor antibody in patients with graves’ disease. Gene Rep. (2020) 19:100608. doi: 10.1016/j.genrep.2020.100608
28. Christensen NJ, Habekost G, and Bratholm P. A RNA transcript (Heg) in mononuclear cells is negatively correlated with CD14 mRNA and TSH receptor autoantibodies. Clin Exp Immunol. (2008) 154:209–15. doi: 10.1111/j.1365-2249.2008.03744.x
29. Christensen NJ, Habekost G, and Bratholm P. Decrease in TSH Receptor Autoantibodies during Antithyroid Treatment: Relationship with a Long Noncoding Heg RNA and Cdk1 mRNA in Mononuclear Cells. ISRN Endocrinol. (2011) 2011:287052. doi: 10.5402/2011/287052
30. Cui X, Wang S, Zhao N, Wang S, Wang Z, Huang M, et al. Thyrocyte-derived exosome-targeted dendritic cells stimulate strong CD4+ T lymphocyte responses. Mol Cell Endocrinol. (2020) 506:110756. doi: 10.1016/j.mce.2020.110756
31. Chen Z, Yu Y, Yao J, Guo Z, Cui Y, Li F, et al. Causal effects of post-traumatic stress disorder on autoimmune thyroid disease: insights from mendelian randomization. Front Psychiatry. (2024) 15:1417302. doi: 10.3389/fpsyt.2024.1417302
32. Sharif K, Watad A, Coplan L, Lichtbroun B, Krosser A, Lichtbroun M, et al. The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmun Rev. (2018) 17:967–83. doi: 10.1016/j.autrev.2018.04.005
33. Wiersinga WM. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol Metab (Seoul). (2016) 31:213–22. doi: 10.3803/EnM.2016.31.2.213
34. Köhling HL, Plummer SF, Marchesi JR, Davidge KS, and Ludgate M. The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin Immunol. (2017) 183:63–74. doi: 10.1016/j.clim.2017.07.001
35. Wang Z, Zhang Q, Lu J, Jiang F, Zhang H, Gao L, et al. Identification of outer membrane porin f protein of Yersinia enterocolitica recognized by antithyrotopin receptor antibodies in Graves’ disease and determination of its epitope using mass spectrometry and bioinformatics tools. J Clin Endocrinol Metab. (2010) 95:4012–20. doi: 10.1210/jc.2009-2184
36. Zhou L, Li X, Ahmed A, Wu D, Liu L, Qiu J, et al. Gut microbe analysis between hyperthyroid and healthy individuals. Curr Microbiol. (2014) 69:675–80. doi: 10.1007/s00284-014-0640-6
37. Ejtahed HS, Angoorani P, Soroush AR, Siadat SD, Shirzad N, Hasani-Ranjbar S, et al. Our little friends with big roles: alterations of the gut microbiota in thyroid disorders. Endocr Metab Immune Disord Drug Targets. (2020) 20:344–50. doi: 10.2174/1871530319666190930110605
38. Chen S, Cheng H, Wyckoff KN, and He Q. Linkages of Firmicutes and Bacteroidetes populations to methanogenic process performance. J Ind Microbiol Biotechnol. (2016) 43:771–81. doi: 10.1007/s10295-016-1760-8
39. Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, and Parisotto TM. Childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: A systematic review. Child Obes. (2018) 14:501–9. doi: 10.1089/chi.2018.0040
40. Jiang W, Yu X, Kosik RO, Song Y, Qiao T, Tong J, et al. Gut microbiota may play a significant role in the pathogenesis of graves’ Disease. Thyroid. (2021) 31:810–20. doi: 10.1089/thy.2020.0193
41. Ishaq HM, Mohammad IS, Shahzad M, Ma C, Raza MA, Wu X, et al. Molecular alteration analysis of human gut microbial composition in graves’ disease patients. Int J Biol Sci. (2018) 14:1558–70. doi: 10.7150/ijbs.24151
42. Yan HX, An WC, Chen F, An B, Pan Y, Jin J, et al. Intestinal microbiota changes in Graves’ disease: a prospective clinical study. Biosci Rep. (2020) 40:BSR20191242. doi: 10.1042/BSR20191242
43. Su X, Yin X, Liu Y, Yan X, Zhang S, Wang X, et al. Gut dysbiosis contributes to the imbalance of treg and th17 cells in graves’ Disease patients by propionic acid. J Clin Endocrinol Metab. (2020) 105:dgaa511. doi: 10.1210/clinem/dgaa511
44. Cooper GS and Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. (2003) 2:119–25. doi: 10.1016/s1568-9972(03)00006-5
45. Shi TT, Xin Z, Hua L, Zhao RX, Yang YL, Wang H, et al. Alterations in the intestinal microbiota of patients with severe and active Graves’ orbitopathy: a cross-sectional study. J Endocrinol Invest. (2019) 42:967–78. doi: 10.1007/s40618-019-1010-9
46. Farebrother J, Zimmermann MB, and Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. (2019) 1446:44–65. doi: 10.1111/nyas.14041
47. Sun X, Shan Z, and Teng W. Effects of increased iodine intake on thyroid disorders. Endocrinol Metab (Seoul). (2014) 29:240–7. doi: 10.3803/EnM.2014.29.3.240
48. Wang Y, Zhao F, Rijntjes E, Wu L, Wu Q, Sui J, et al. Role of selenium intake for risk and development of hyperthyroidism. J Clin Endocrinol Metab. (2019) 104:568–80. doi: 10.1210/jc.2018-01713
49. Czarnywojtek A, Florek E, Pietrończyk K, Sawicka-Gutaj N, Ruchała M, Ronen O, et al. The role of vitamin D in autoimmune thyroid diseases: A narrative review. J Clin Med. (2023) 12:1452. doi: 10.3390/jcm12041452
50. Yamashita H, Noguchi S, Takatsu K, Koike E, Murakami T, Watanabe S, et al. High prevalence of vitamin D deficiency in Japanese female patients with Graves’ disease. Endocr J. (2001) 48:63–9. doi: 10.1507/endocrj.48.63
51. Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine. (2013) 43:230–2. doi: 10.1007/s12020-012-9789-6
52. Olivieri B, Betterle C, and Zanoni G. Vaccinations and autoimmune diseases. Vaccines (Basel). (2021) 9:815. doi: 10.3390/vaccines9080815
53. World Health Organization. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (Accessed September 1, 2025).
54. Goblirsch TJ, Paulson AE, Tashko G, and Mekonnen AJ. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. (2021) 14:e246432. doi: 10.1136/bcr-2021-246432
55. Di Filippo L, Castellino L, and Giustina A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine. (2022) 75:19–21. doi: 10.1007/s12020-021-02919-3
56. Sriphrapradang C and Shantavasinkul PC. Graves’ disease following SARS-CoV-2 vaccination. Endocrine. (2021) 74:473–4. doi: 10.1007/s12020-021-02902-y
57. Rubinstein TJ. Thyroid eye disease following COVID-19 vaccine in a patient with a history graves’ Disease: A case report. Ophthalmic Plast Reconstr Surg. (2021) 37:e221–3. doi: 10.1097/IOP.0000000000002059
58. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine. (2021) 74:226–7. doi: 10.1007/s12020-021-02879-8
59. Patrizio A, Ferrari SM, Antonelli A, and Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. (2021) 125:102738. doi: 10.1016/j.jaut.2021.102738
60. Yamamoto K, Mashiba T, Takano K, Suzuki T, Kami M, Takita M, et al. A Case of Exacerbation of Subclinical Hyperthyroidism after First Administration of BNT162b2 mRNA COVID-19 Vaccine. Vaccines (Basel). (2021) 9:1108. doi: 10.3390/vaccines9101108
61. Lee KA, Kim YJ, and Jin HY. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. (2021) 74:470–2. doi: 10.1007/s12020-021-02898-5
62. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, and Tan KCB. Development of graves’ Disease after SARS-coV-2 mRNA vaccination: A case report and literature review. Front Public Health. (2021) 9:778964. doi: 10.3389/fpubh.2021.778964
63. Oğuz SH, Şendur SN, İremli BG, Gürlek A, Erbas T, and Ünlütürk U. SARS-CoV-2 vaccine- induced thyroiditis: Safety of Re-vaccinations and Clinical Follow-up. J Clin Endocrinol Metab. (2022), dgac049. doi: 10.1210/clinem/dgac049
64. Pierman G, Delgrange E, and Jonas C. Recurrence of graves’ Disease (a th1-type cytokine disease) following SARS-coV-2 mRNA vaccine administration: A simple coincidence? Eur J Case Rep Intern Med. (2021) 8:2807. doi: 10.12890/2021_002807
65. Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, et al. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun. (2022) 128:102809. doi: 10.1016/j.jaut.2022.102809
66. Chua MWJ. Graves’ disease after COVID-19 vaccination. Ann Acad Med Singap. (2022) 51:127–8. doi: 10.47102/annals-acadmedsg.2021398
67. Shih SR and Wang CY. SARS-CoV-2 vaccination related hyperthyroidism of graves’ disease. J Formos Med Assoc. (2022) 121:1881–2. doi: 10.1016/j.jfma.2022.02.010
68. Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. SARS-CoV-2 mRNA Vaccination and Graves’ Disease: a report of 12 cases and review of the literature. J Clin Endocrinol Metab. (2022), 107:e2324–30. doi: 10.1210/clinem/dgac119
69. Pla Peris B, Merchante Alfaro AÁ, Maravall Royo FJ, Abellán Galiana P, Pérez Naranjo S, and González Boillos M. Thyrotoxicosis following SARS-COV-2 vaccination: a case series and discussion. J Endocrinol Invest. (2022) 11:1–7. doi: 10.1007/s40618-022-01739-0
70. Zettinig G and Krebs M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J Endocrinol Invest. (2022) 45:227–8. doi: 10.1007/s40618-021-01650-0
71. Taieb A, Sawsen N, Asma BA, Ghada S, Hamza E, Yosra H, et al. A rare case of grave’s disease after SARS-CoV-2 vaccine: is it an adjuvant effect? Eur Rev Med Pharmacol Sci. (2022) 26:2627–30. doi: 10.26355/eurrev_202204_28500
72. Singh G and Howland T. Graves’ Disease following COVID-19 vaccination. Cureus. (2022) 14:e24418. doi: 10.7759/cureus.24418
73. Cuenca D, Aguilar-Soto M, and Mercado M. A case of graves’ Disease following vaccination with the oxford-astraZeneca SARS-coV-2 vaccine: case report and review of the literature. Eur J Case Rep Intern Med. (2022) 9:3275. doi: 10.12890/2022_003275
74. Sakai M, Takao K, Kato T, Ito K, Kubota S, Hirose T, et al. Graves’ Disease after administration of severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) vaccine in a type 1 diabetes patient. Intern Med. (2022) 61:1561–5. doi: 10.2169/internalmedicine.9231-21
75. Ruggeri RM, Giovanellla L, and Campennì A. SARS-CoV-2 vacine may trigger thyroid autoimmunity: real-life experience and review of the literature. J Endocrinol Invest. (2022) 45:2283–9. doi: 10.1007/s40618-022-01863-x
76. Manta R, Martin C, Muls V, and Poppe KG. New-onset Graves’ disease following SARS- CoV-2 vaccination: a case report. Eur Thyroid J. (2022) 11:e220049. doi: 10.1530/ETJ-22-0049
77. Chaudhary S, Dogra V, and Walia R. Four cases of Graves’ disease following viral vector severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) vaccine. Endocr J. (2022) 69:1431–5. doi: 10.1507/endocrj.EJ22-0208
78. Lioulios G, Tsouchnikas I, Dimitriadis C, Giamalis P, Pella E, Christodoulou M, et al. Two cases of autoimmune thyroid disorders after COVID vaccination in dialysis patients. Int J Mol Sci. (2022) 23:11492. doi: 10.3390/ijms231911492
79. Takedani K, Notsu M, Ishiai N, Asami Y, Uchida K, and Kanasaki K. Graves’ disease after exposure to the SARS-CoV-2 vaccine: a case report and review of the literature. BMC Endocr Disord. (2023) 23:132. doi: 10.1186/s12902-023-01387-2
80. Chen RY. Severe Graves’ disease in a child following a single dose of Pfizer-BioNTech SARS-CoV-2 vaccine. Singapore Med J. (2023) 10:4103. doi: 10.4103/Singaporemedj.SMJ-2021-357
81. Mainieri F, Chiarelli F, Betterle C, and Bernasconi S. Graves’ disease after COVID mRNA vaccination for the first time diagnosed in adolescence-case report. Cause and effect relationship or simple coincidence? J Pediatr Endocrinol Metab. (2023) 36:993–7. doi: 10.1515/jpem-2023-0181
82. Yan BC and Luo RR. Thyrotoxicosis in patients with a history of Graves’ disease after SARS-CoV-2 vaccination (adenovirus vector vaccine): Two case reports. World J Clin cases. (2023) 11:1122–8. doi: 10.12998/wjcc.v11.i5.1122
83. Nakamura F, Awaya T, Ohira M, Enomoto Y, Moroi M, and Nakamura M. Graves’ Disease after mRNA COVID-19 Vaccination, with the Presence of Autoimmune Antibodies Even One Year Later. Vaccines (Basel). (2023) 11:934. doi: 10.3390/vaccines11050934
84. Yasuda S, Suzuki S, Yanagisawa S, Morita H, Haisa A, Satomura A, et al. HLA typing of patients who developed subacute thyroiditis and Graves’ disease after SARS-CoV-2 vaccination: a case report. BMC Endocr Disord. (2023) 23:54. doi: 10.1186/s12902-023-01287-5
85. Taşkaldıran I, Altay FP, Bozkuş Y, İyidir ÖT, Nar A, and Tütüncü NB. A case report of concurrent graves’ Disease and subacute thyroiditis following SARS-coV-2 vaccination: an autoimmune/inflammatory syndrome (ASIA). Endocr Metab Immune Disord Drug Targets. (2023) 23:242–6. doi: 10.2174/1871530322666220621101209
86. Al-Jahhafi AS, Al-Sawaai AA, Al-Bimani ZK, and Al-Bulushi NK. Graves’ disease post-COVID-19 m-RNA vaccine in pediatric age group. Asia Ocean J Nucl Med Biol. (2024) 12:65–8. doi: 10.22038/AOJNMB.2023.73051
87. Vojdani A, Vojdani E, and Kharrazian D. Reaction of human monoclonal antibodies to SARS-coV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. (2021) 11:617089. doi: 10.3389/fimmu.2020.617089
88. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. (2022) 165:386–401. doi: 10.1111/imm.13443
89. Morita S, Takagi T, Inaba H, Furukawa Y, Kishimoto S, Uraki S, et al. Effect of SARS-CoV-2 BNT162b2 mRNA vaccine on thyroid autoimmunity: A twelve-month follow-up study. Front Endocrinol (Lausanne). (2023) 14:1058007. doi: 10.3389/fendo.2023.1058007
90. Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves’ disease to silent thyroiditis. J Endocrinol Invest. (2022) 45:875–82. doi: 10.1007/s40618-021-01707-0
91. Cohen Tervaert JW, Martinez-Lavin M, Jara LJ, Halpert G, Watad A, Amital H, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun Rev. (2023) 22:103287. doi: 10.1016/j.autrev.2023.103287
92. Şendur SN, Oğuz SH, and Ünlütürk U. COVID-19 vaccination and thyroiditis. Best Pract Res Clin Endocrinol Metab. (2023) 37:101759. doi: 10.1016/j.beem.2023.101759
93. Soriano A, Nesher G, and Shoenfeld Y. Predicting post-vaccination autoimmunity: who might be at risk? Pharmacol Res. (2015) 92:18–22. doi: 10.1016/j.phrs.2014.08.002
94. Tough DF, Borrow P, and Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. (1996) 272:1947–50. doi: 10.1126/science.272.5270.1947
95. Tough DF, Sun S, and Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med. (1997) 185:2089–94. doi: 10.1084/jem.185.12.2089
96. Gangappa S, Deshpande SP, and Rouse BT. Bystander activation of CD4(+) T cells can represent an exclusive means of immunopathology in a virus infection. Eur J Immunol. (1999) 29:3674–82. doi: 10.1002/(SICI)1521-4141(199911)29
97. Ramos SG, Rattis BADC, Ottaviani G, Celes MRN, and Dias EP. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am J Pathol. (2021) 191:1154–64. doi: 10.1016/j.ajpath.2021.04.010
98. Robles JP, Zamora M, Adan-Castro E, Siqueiros-Marquez L, Martinez De La Escalera G, and Clapp C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J Biol Chem. (2022) 298:101695. doi: 10.1016/j.jbc.2022.101695
99. Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. (2021) 54:2877–2892.e7. doi: 10.1016/j.immuni.2021.11.001
100. Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, and Igyártó BZ. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. (2021) 24:103479. doi: 10.1016/j.isci.2021.103479
101. Kimura A and Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. (2010) 40:1830–5. doi: 10.1002/eji.201040391
102. Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang K, et al. Increased expression of IL-37 in patients with Graves’ disease and its contribution to suppression of proinflammatory cytokines production in peripheral blood mononuclear cells. PloS One. (2014) 9:e107183. doi: 10.1371/journal.pone.0107183
Keywords: thyroid, COVID-19 vaccine, hyperthyroidism, autoimmune, SARS-CoV-2, Graves’ disease, inflammatory syndrome, ACE2
Citation: Murugan AK and Alzahrani AS (2025) COVID-19 vaccine-induced autoimmune hyperthyroidism: Graves’ disease. Front. Immunol. 16:1699210. doi: 10.3389/fimmu.2025.1699210
Received: 05 September 2025; Accepted: 07 November 2025; Revised: 05 November 2025;
Published: 03 December 2025.
Edited by:
Stefan Tukaj, University of Gdansk, PolandReviewed by:
Immadi Sudhakar Vamshidhar, All India Institute of Medical Sciences, IndiaAlireza Arefzadeh, Islamic Azad University Central Tehran Branch, Iran
Copyright © 2025 Murugan and Alzahrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avaniyapuram Kannan Murugan, YWttdXJ1Z2FuQGdtYWlsLmNvbQ==
 Avaniyapuram Kannan Murugan
Avaniyapuram Kannan Murugan Ali S. Alzahrani1,2
Ali S. Alzahrani1,2