- 1Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Shanghai Key Laboratory of Proton-therapy, Shanghai, China
Background: Neoadjuvant chemoradiotherapy (nCRT) is recommended as the standard of care for locally advanced esophageal squamous cell carcinoma (ESCC). Adding immunotherapy to nCRT (nICRT) has gained attention in clinical practice. We evaluated the differences in clinicopathologic outcomes and the patterns of lymph node metastasis in patients receiving nCRT and nICRT for locally advanced ESCC.
Methods: A total of 208 ESCC patients who completed transthoracic esophagectomy after neoadjuvant treatment were enrolled. Clinicopathologic parameters and the rates of lymph node metastasis in each station classified using both the eighth edition of the American Joint Committee on Cancer (AJCC) esophageal cancer staging system and the 11th edition of the Japanese Classification of Esophageal Cancer (JCEC) standard were recorded and evaluated.
Results: The rates of pathological complete response (pCR) and major pathological response (MPR) were 44.9% in nICRT vs. 37.0% in nCRT (p = 0.263) and 79.5% in nICRT vs. 65.4% in nCRT (p = 0.024), respectively. The common sites of lymph node metastasis after neoadjuvant treatment were station 112pulL (8.3%), followed by station 104L (4.9%), station 7 (4.5%), and station 3a (4.3%), according to the 11th JCEC standard. Compared with nCRT, nICRT can significantly reduce the rates of lymph node metastasis in station 2R (0.8% vs. 4.6%, p = 0.039) classified using the AJCC system, and those in station 106recR (0.8% vs. 4.6%, p = 0.042) and station 20 (0 vs. 12.5%, p = 0.030) classified using the JCEC standard.
Conclusion: nICRT followed by surgery may lead to a promising pathological response. For patients with lymph node metastasis in certain regions, nICRT should be considered as a better preoperative treatment option.
Introduction
Esophageal cancer is the sixth most common cause of cancer-related mortality and ranks seventh in incidence among all malignancies worldwide, with the highest incidence rates in eastern Asia (1). Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype of esophageal cancer globally, accounting for approximately 90% (2). Curative resection is still a major component of current treatment for ESCC. Based on the results of the ChemorRdiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) (3–5) and neoadjuvant chemoradiotheraoy for esophageal cancer 5010 (NEOCRTEC5010) (6) trials, neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy is applied as the standard of care for locally advanced ESCC (7). However, there are still 49% patients developing either locoregional or distant recurrence after this regimen, and the 5-year overall survival rate is approximately 47% (4). Novel and effective treatment strategies for locally advanced ESCC to further improve prognosis are desperately needed.
The application of immunotherapy in the neoadjuvant setting of locally advanced ESCC has gained attention in clinical practice (8). The addition of immune checkpoint inhibitors (ICIs) to neoadjuvant chemotherapy, namely, neoadjuvant immunochemotherapy (nICT), has achieved pathological complete response (pCR) rates between 16.7% and 35.3% for locally advanced ESCC patients in ESONICT-1 (9) and ESONICT-2 (10) trials. Other trials have also shown that this combination may have synergistic and greater effects compared with chemotherapy alone (11–14). Given that nCRT is recommended as the first-line strategy for locally advanced ESCC and the radiation-induced enhancement of antitumor efficacy of immunotherapy (15–17), researchers are moving forward with their work to combine ICIs with nCRT, namely, neoadjuvant immunochemoradiotherapy (nICRT), to attempt to further improve the efficacy of the existing neoadjuvant treatment and expand clinicians’ options. Zhu et al. found that the combination of pembrolizumab and nCRT as treatment for gastroesophageal junction adenocarcinoma achieved an improved pCR rate in patients with Programmed Cell Death Ligand 1 (PD-L1) Combined Positive Score (CPS) ≥ 10 compared with those with PD-L1 CPS < 10 (50% vs. 13.6%, p = 0.046) (18). The PERFECT trial conducted by van den Ende et al. showed that the addition of atezolizumab to nCRT revealed a pCR rate of 25% but without a survival benefit in patients with resectable esophageal adenocarcinoma (19). As for nICRT in locally advanced ESCC, the single-armed PALACE-1 trial conducted by our institute confirmed the safety and activity of preoperative pembrolizumab combined with nCRT with a preliminary 55.6% (10/18) pCR rate (20), and a subsequent multicenter single-arm PALACE-2 trial investigating the efficacy is ongoing to further confirm the safety of nICRT (21).
Postoperative pathological response and staging (ypTNM) are closely correlated with patients’ risk of recurrence. Previous studies have demonstrated that the depth of primary tumor residual (ypT) and the number of metastatic lymph nodes (ypN) were critical predictors for survival in patients with esophageal cancer receiving neoadjuvant treatment (22). Patients achieving pCR were more likely to have a better prognosis, while the 5-year survival rates of patients with ypT3+ or ypN+ were only 30% and 25.6%, respectively (23). Furthermore, ESCC has an earlier propensity for lymphatic spread since the esophageal wall has a rich lymphatic drainage network, and the lymphatic vessels of the thoracic esophagus can drain to the cervical, thoracic, and abdominal lymph node stations. This characteristic brings about difficulty in defining the proper sentinel lymph nodes (24). The nICRT strategy was given high expectations in disease control, but it has not been determined yet whether the pattern of primary tumor residual and lymph node spread in locally advanced ESCC patients treated with the nICRT regimen and whether there are differences from the traditional nCRT treatment. Moreover, there are currently two mainstream standards classifying lymph node stations in esophageal cancer applied by clinicians worldwide, namely, the eighth edition of the American Joint Committee on Cancer (AJCC) esophageal cancer staging system (25) and the 11th edition of Japanese Classification of Esophageal Cancer (JCEC) standards (26, 27). Considering that precise lymph node dissection is crucial to improve prognosis, it is necessary to evaluate the pattern of lymph node spread based on both these standards to cater to clinicians with different preferences.
In this study, we analyzed the differences in postoperative pathological characteristics and the pattern of lymph node spread in patients with thoracic ESCC receiving different neoadjuvant treatment strategies, including nCRT and nICRT, from a prospective database to provide a reference for determining the optimal lymph node removal scope and for the selection of treatment regimens.
Materials and methods
Patient enrollment
The study was designed retrospectively based on a prospective database (20, 28–31) and was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, while patients’ informed consent was exempted due to the retrospective nature of the study. Data were collected from patients with previously untreated, locally advanced, and surgically resectable ESCC at our center from April 2019 to December 2023. Pretreatment staging was undertaken for every patient. The key inclusion criteria were 1) pathologically confirmed locally advanced ESCC, 2) ages between 18 and 75 years, 3) Eastern Cooperative Oncology Group performance status score of 0–1, and 4) treated with standardized nCRT with or without pembrolizumab followed by curative surgery. Patients with a history of other malignancies, antitumor treatment, or salvage esophagectomy after definitive chemoradiotherapy were excluded.
Neoadjuvant strategy
Preoperative treatment was composed of chemotherapy, radiotherapy, and immunotherapy. nCRT was given according to the CROSS regimen (5). The concurrent chemotherapy regimen included carboplatin [area under the curve (AUC) of 2 mg·mL−1·min−1] and paclitaxel/nab-paclitaxel (50 mg/m2), which was administered intravenously once a week for 5 weeks (on days 1, 8, 15, 22, and 29 of the neoadjuvant treatment period). A total radiation dose of 41.4 Gy was given in 23 fractions, with five fractions each week and 1.8 Gy per fraction. Patients in the nICRT group received pembrolizumab concurrently on days 1 and 22 of the neoadjuvant treatment period at a dose of 200 mg in addition to the nCRT regimen. Involved-field irradiation (IFI) was used in the present study, and the detailed methods for countering gross tumor volume (GTV), clinical target volume (CTV), and planning treatment volume (PTV) were described in our previous study (30). The positive metastatic lymph nodes (GTV-nd) were determined according to one of the following criteria: 1) paraoesophageal, tracheoesophageal groove, at the angle of the diaphragm or abdominal lymph nodes with a short diameter ≥0.5 cm; or lymph nodes in other locations with a short diameter ≥1 cm; 2) multiple (≥5) small lymph nodes clustered together; and 3) PET–CT showed lymph nodes with high metabolism and standardized uptake value (SUV) ≥2.5.
Surgical treatment
After the completion of neoadjuvant treatment, radiology examination (such as contrast-enhanced CT scan and PET–CT), routine laboratory tests, pulmonary function, and electrocardiogram were conducted to reassess the disease and exclude cases with any surgical contraindications. Patients suitable for radical esophagectomy were evaluated by the multidisciplinary team, and the surgical approach was determined by thoracic surgeons. Surgery was arranged 4–6 weeks after the completion of neoadjuvant treatment. For patients with tumors located in the upper third of the esophagus or with cervical lymph node involvement, the McKeown esophagectomy with three-field lymphadenectomy was performed. For patients with tumors located in the middle and lower third of the esophagus, the Ivor Lewis esophagectomy with two-field lymphadenectomy was performed.
Pathological assessment
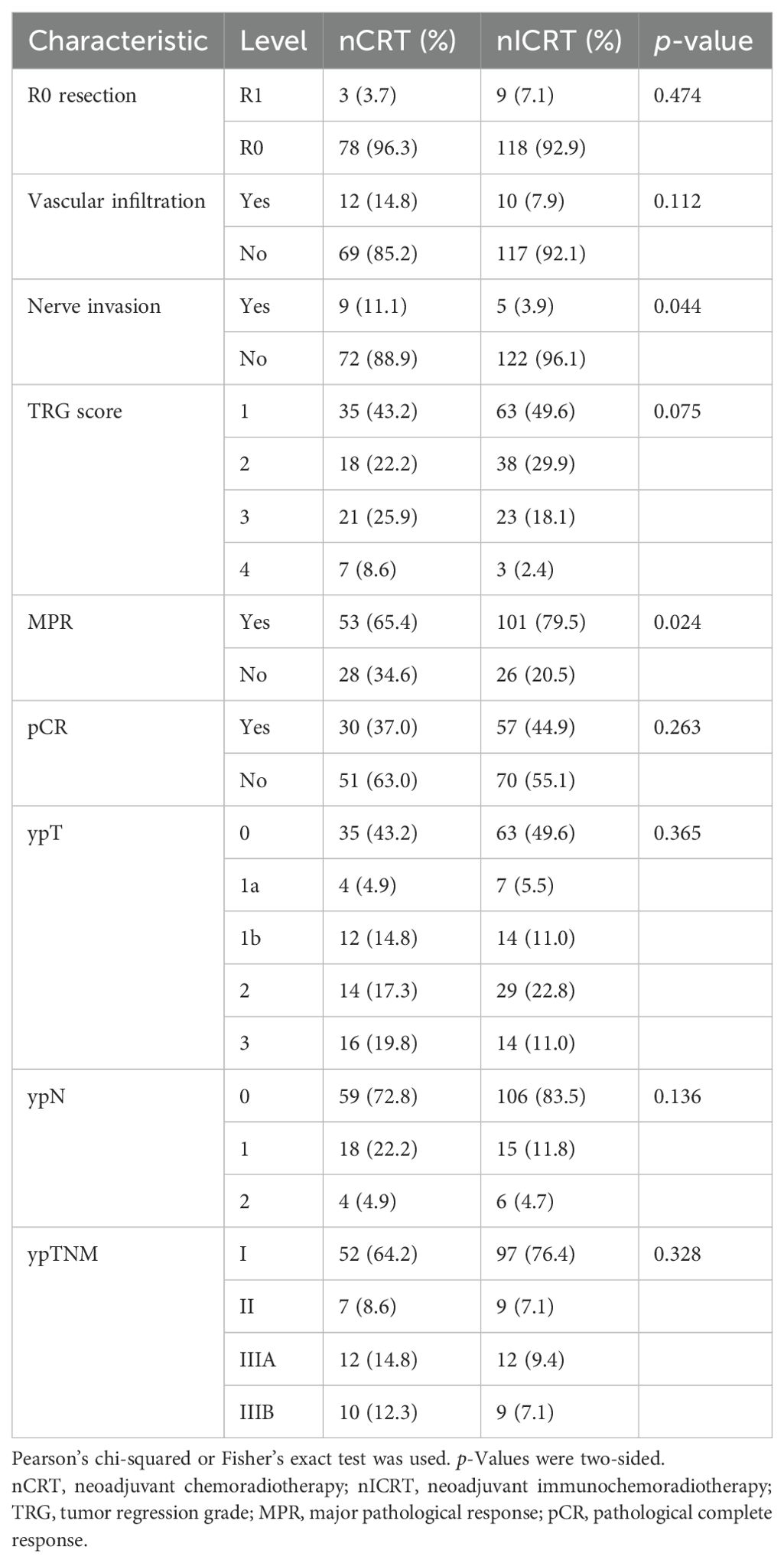
Each resected tumor specimen was independently reviewed by two experienced upper gastrointestinal pathologists. Pathological examination included pathological type, tumor length, depth and extent of tumor invasion, resection margin, grade of differentiation, grade of tumor regression, overall and positive lymph nodes dissected, and tumor stage determined by the eighth edition TNM classification of the AJCC for esophageal cancer. R0 resection was defined as a tumor-free resection margin, while R1 and R2 levels were referred to as a resection margin with microscopic and macroscopic residual tumors, respectively. The Chirieac modified tumor regression grade (TRG) system was applied to classify the pathological response of the primary lesion. The extent of residual tumor was divided into four categories: TRG1, no residual carcinoma; TRG2, 1%–10% vital residual carcinoma; TRG3, 11%–50% residual carcinoma; and TRG4, greater than 50% residual carcinoma (32). All dissected lymph nodes were reviewed for their status of metastases, their precise locations, or called stations and were recorded according to two currently mainstream standards, namely, the eighth edition of the AJCC esophageal cancer staging system (25) and the 11th edition of the JCEC standards (26, 27). The complete regression of primary lesion and lymph node metastases was considered pCR, while major pathological response (MPR) was defined as <10% residual viable tumor cells in the primary tumor.
Statistical analysis
The primary endpoint of our study was pCR, while the secondary endpoints were TRG, MPR, postoperative stage, and specific nodal response. For descriptive statistics, the continuous variable was expressed as a median with its range, while the categorical variable was expressed as frequency with percentage. To compare the differences in baseline characteristics, pathological parameters, and rates of lymph node metastasis in all stations between the nCRT and nICRT groups, a Wilcoxon rank-sum test for continuous variables and Pearson’s chi-squared or Fisher’s exact test for categorical variables were conducted. p-Values were two-sided, with a significance level of <0.05 for all analyses. All statistical analyses were performed using the SPSS software for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
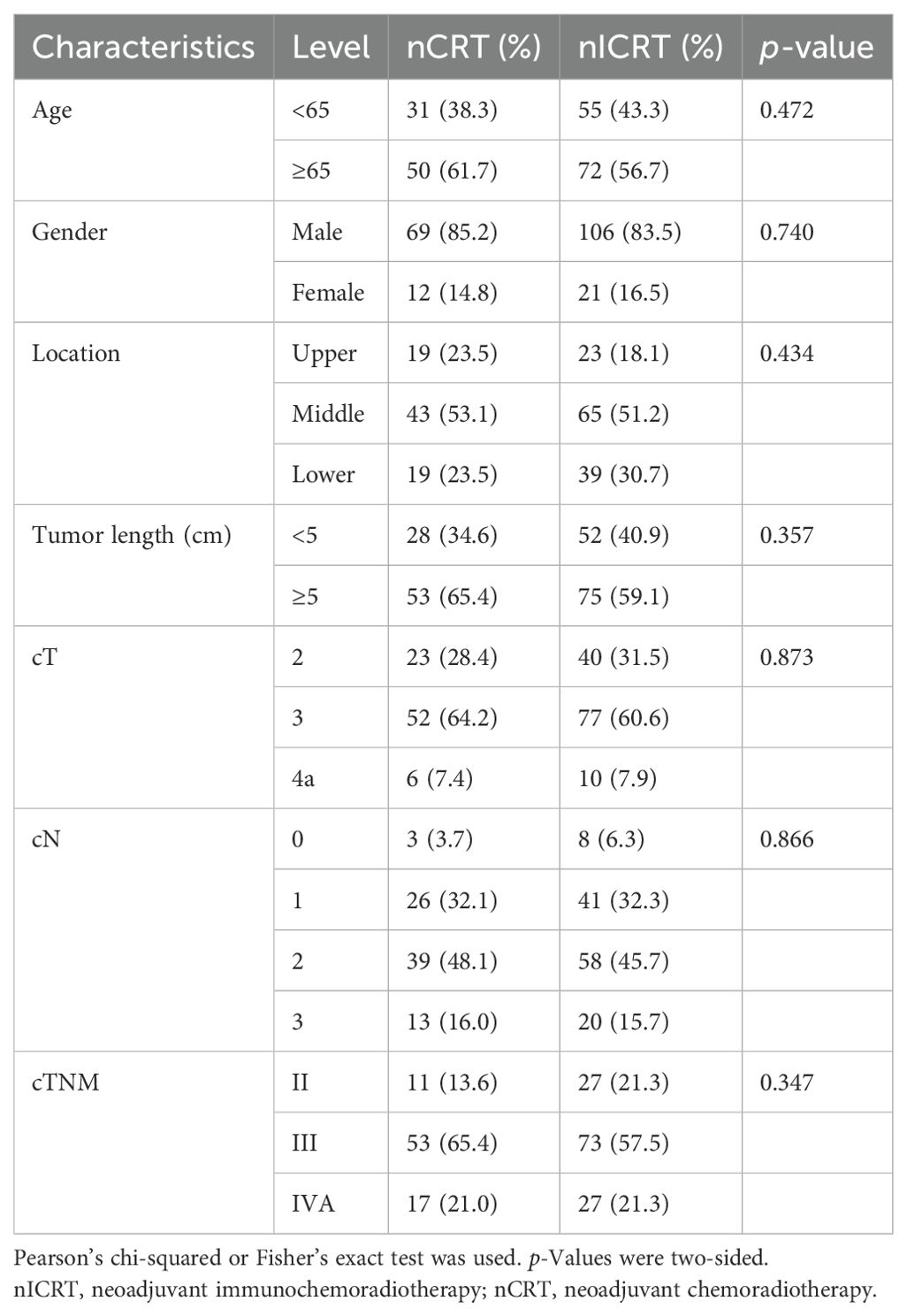
In our study, a total of 208 patients with locally advanced ESCC who completed transthoracic esophagectomy after neoadjuvant treatment were enrolled. Among them, 81 patients received conventional nCRT, whereas 127 patients received nICRT. The baseline characteristics were comparable between the two groups, including age, gender, primary tumor location, primary tumor length, and clinical staging, which are summarized in Table 1.
Pathological response to neoadjuvant treatment
We estimated the pathological response to neoadjuvant treatment in the 208 ESCC patients who underwent surgical resection. The results are shown in Table 2 and Figure 1. Patients receiving nICRT showed comparable rates of R0 resection and vascular infiltration to patients receiving conventional nCRT (R0 resection rate: 92.9% vs. 96.3%, p = 0.474; vascular infiltration rate: 7.9% vs. 14.8%, p = 0.112). However, the rate of nerve invasion in patients receiving nICRT was significantly lower than that of patients receiving nCRT (3.9% vs. 11.1%, p = 0.044). Patients in the nICRT group exhibited numerically lower TRG score (p = 0.075; Figure 1b) and higher pCR rate (44.9% vs. 37.0%, p = 0.263; Figure 1a), but significantly higher MPR rate (79.5% vs. 65.4%, p = 0.024; Figure 1a), compared to those in the nCRT group. Compared with nCRT, the nICRT regimen had an advantage in downgrading postoperative TNM stage despite a lack of statistical difference (Figures 1c, d).
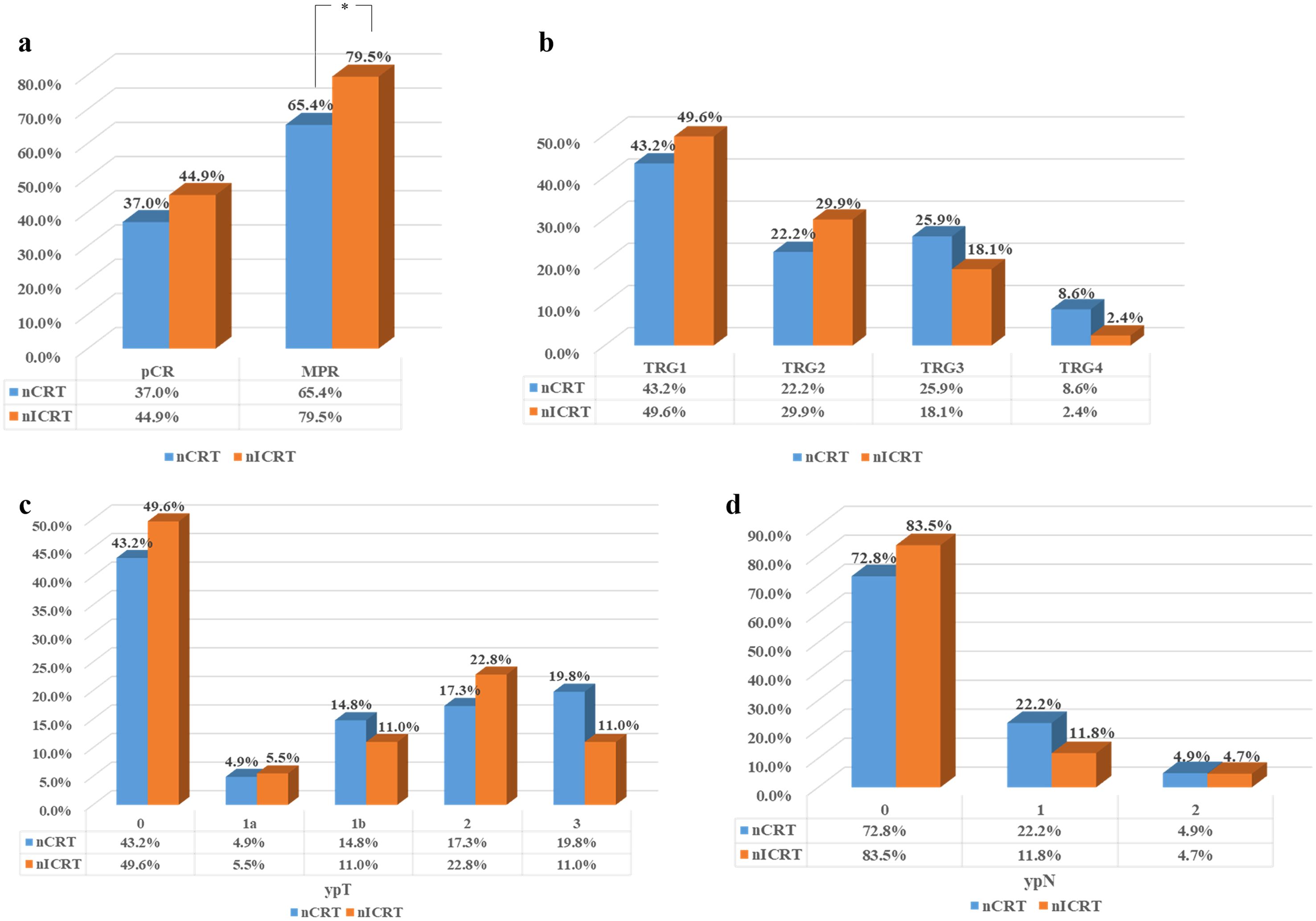
Figure 1. Residual tumor characteristics of all patients in both groups after neoadjuvant therapy. (a) Comparisons of the pathological complete response (pCR) and major pathological response (MPR) rates between two groups. (b) Tumor regression grade (TRG) score in two groups. Pathological response of primary tumor toward neoadjuvant therapy was evaluated using the Chirieac modified TRG system. (c) Comparison of the depth of tumor invasion (ypT) between two groups. (d) Comparison of the number of lymph node metastases (ypN) between two groups. *p < 0.05. Pearson’s chi-squared or Fisher’s exact test was used. p-Values were two-sided.
Patterns of lymphatic spread in overall and subgroup analyses
Among the 208 patients, 43 (20.7%) had pathologically positive lymph nodes postoperatively, with 22 and 21 patients in the nCRT and nICRT groups, respectively (27.2% vs. 16.5%, p = 0.19). We then estimated the metastasis rates of lymph nodes in different locations in the 208 ESCC patients who underwent surgical resection after neoadjuvant therapy. We identified a total of 1,176 and 2,230 lymph nodes, with 37 and 39 metastatic lymph nodes in the nCRT and nICRT groups, respectively (3.1% vs. 1.7%, p = 0.0069). The common sites of lymph node metastasis after neoadjuvant treatment were station 112pulL (8.3%, 3/36 nodes positive), followed by station 104L (4.9%, 2/41 nodes positive), station 7 (4.5%, 12/266 nodes positive), and station 3a (4.3%, 10/230 nodes positive) according to the 11th JCEC standard. Based on the eighth of the AJCC esophageal cancer staging system, the metastasis rate of lymph nodes in station 2R in patients receiving nICRT was significantly lower than that of patients receiving nCRT (0.8% vs. 4.6%, p = 0.039; Table 3). Based on the 11th JCEC standard, metastasis rates of lymph nodes in station 106recR (0.8% vs. 4.6%, p = 0.042; Table 3) and station 20 (0% vs. 12.5%, p = 0.030) had a significant difference between nICRT and nCRT. Among other stations, however, there was no statistical difference in lymph node metastasis rates whether patients received nICRT or nCRT.
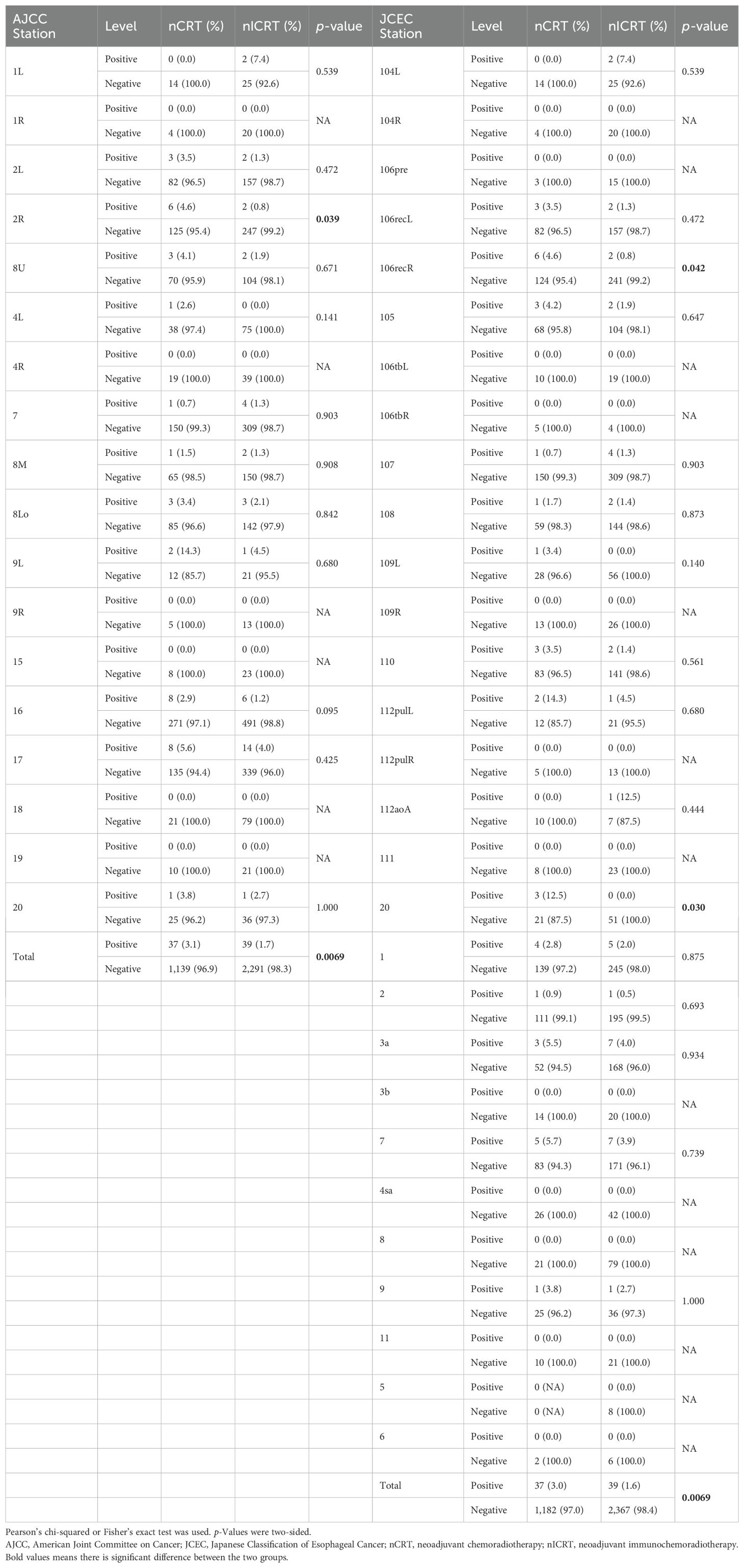
Table 3. Lymph node metastasis rates of different stations classified using AJCC and JCEC standards among two groups of patients.
Considering that the pattern of lymph node metastasis is related to the location of the tumor, we assessed the stations and frequencies of lymph node metastasis according to tumor location. The rates of lymph node metastasis in the upper mediastinum, middle mediastinum, lower mediastinum, and abdomen in different locations of thoracic ESCC are shown in Figure 2. The stations and frequencies of lymph node metastasis in different locations of thoracic ESCC are shown in Supplementary Tables 1–4 and Figure 3. In upper thoracic cases, the rate of upper mediastinal lymph node metastasis in the nICRT group was significantly lower than that in the nCRT group (0.8% vs. 6.5%, p = 0.044; Figure 2). In middle thoracic cases, the rate of lower mediastinal lymph node metastasis in the nICRT group was significantly lower than that in the nCRT group (1.0% vs. 8.9%, p = 0.038; Figure 2). The nCRT group had a higher lymph node metastasis rate in station 8Lo of the eighth edition of the AJCC esophageal cancer staging system and station 110 of the 11th JCEC standard compared with the nICRT group (8.8% vs. 0%, p = 0.033; Supplementary Table 2, Figure 3). In lower thoracic cases, the nICRT group had a lower lymph node metastasis rate in station 7 of the 11th JCEC standard compared with the nCRT group (0% vs. 12.5%, p = 0.022; Supplementary Table 3, Figure 3).
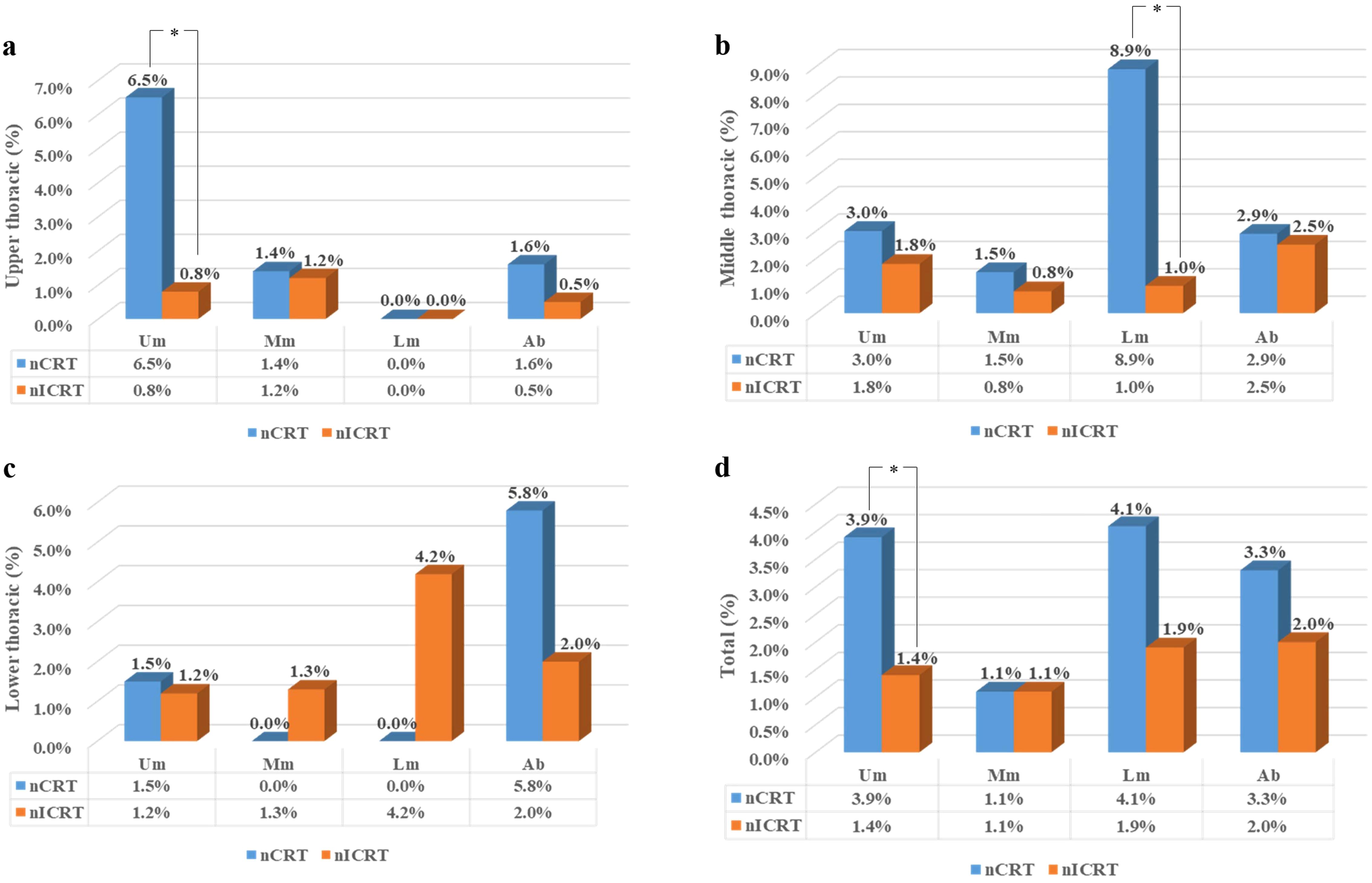
Figure 2. Rates of lymph node metastasis according to the location of the primary tumor. (a) Rates of lymph node metastasis in upper thoracic esophageal cancer (b) Rates of lymph node metastasis in middle thoracic thoracic esophageal cancer, (c) Rates of lymph node metastasis in lower thoracic thoracic esophageal cancer, and (d) Rates of lymph node metastasis in all esophageal cancer.nCRT, neoadjuvant chemoradiotherapy; nICRT, neoadjuvant immunochemoradiotherapy; Um, upper mediastinum; Mm, middlemediastinum; Lm, lower mediastinum; Ab, abdomen. *p < 0.05. Pearson’s chi-squared or Fisher’s exact test was used. p-Values were two-sided.
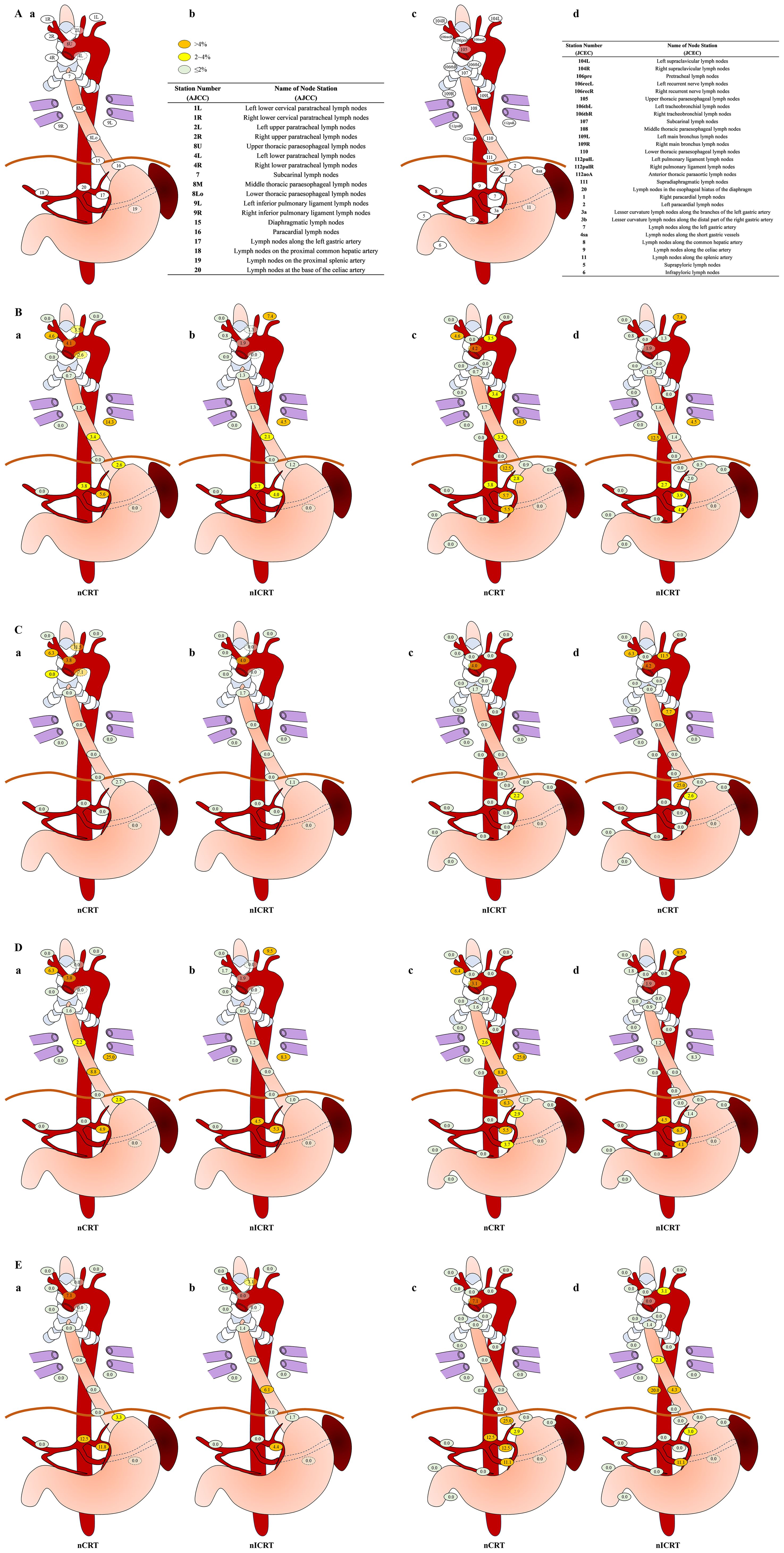
Figure 3. Rates of lymph node metastasis in subgroup analysis. (A) Sites and terminology of the regional lymph nodes in esophageal squamous cell carcinoma (ESCC) classified using both eighth edition of American Joint Committee on Cancer (AJCC) esophageal cancer staging system (Ia, Ib) and 11th edition of Japanese Classification of Esophageal Cancer (JCEC) standards (Ic, Id). (B) Sites and rates of lymph node metastasis in neoadjuvant chemoradiotherapy (nCRT) (Bb, Bc) and neoadjuvant immunochemoradiotherapy (nICRT) (Bb, Bd) according to both AJCC (Ba, Bb) system and JCEC (Bc, Bd) standards. (C) In upper thoracic cases, sites and rates of lymph node metastasis in nCRT (Ca, Cc) and nICRT (Cb, Cd) according to both AJCC (Ca, Cb) system and JCEC (Cc,Cd) standards. (D) In middle thoracic cases, sites and rates of lymph node metastasis in nCRT (Da, Dc) and nICRT (Db, Dd) according to both AJCC (Da, Db) system and JCEC (Dc, Dd) standards. (E) In lower thoracic cases, sites and rates of lymph node metastasis in nCRT (Ea, Ec) and nICRT (Eb, Ed) according to both AJCC (Ea, Eb) system and JCEC (Ec, Ed) standards. Note. According to the metastasis rate, three different colors are used to label lymph node metastasis, as follows: <2%, light green; 2% to 4%, yellow; >4%, orange. The numbers with decimal points on each labeled lymph node station represent the rates of lymph node metastasis involved in each location. nCRT, neoadjuvant chemoradiotherapy; nICRT, neoadjuvant immunochemoradiotherapy. Pearson’s chi-squared or Fisher’s exact test was used. p-Values were two-sided.
Discussion
Since the publication of two large Phase III trials, the CROSS trial (3) and the NEOCRTEC5010 trial (33), nCRT followed by esophagectomy has become the standardized treatment option for locally advanced ESCC. However, the long-term outcomes of ESCC patients after nCRT and surgery remain poor, with the 5-year cumulative incidence rates of local, distant, and overall recurrence being 15.3%, 24.3%, and 32.2%, respectively (33). Immunotherapy, especially for immune checkpoint inhibitors, has recently emerged as an effective antitumor treatment among various solid tumors, including ESCC. Currently, chemotherapy combined with ICIs has become the standardized treatment for metastatic ESCC patients (34–37). Recently, more and more trials have been performed to investigate the efficacy and toxicity of ICIs in neoadjuvant or definitive radiotherapy settings (38). In our center, we initiated the PALACE-1 trial and demonstrated that the combination of pembrolizumab and nCRT was safe and efficient with a pCR of 55.6% (10/18) (20). We then conducted a multicenter single-arm PALACE-2 trial to validate this new treatment strategy in a large sample size cohort (39). Although neoadjuvant therapy could effectively downstage locally advanced ESCC, standardized lymph node dissection remains important. Previous studies have demonstrated that lymph node status after neoadjuvant treatment and the extent of lymph node dissection were two independent predictors of survival and recurrence in patients with esophageal cancer following neoadjuvant therapy (40). As a result, clarifying the rate of lymph node metastasis at specific stations and distribution patterns after neoadjuvant treatment would provide important information for surgeons to determine the optimal surgical approach and extent of lymph node dissection, which would aid radiation oncologists in designing the dose of neoadjuvant radiotherapy. Prior to the present study, multiple studies had been published to investigate the frequency and distribution pattern of lymph node metastasis after neoadjuvant chemoradiotherapy or chemoimmunotherapy. However, the rate and distribution of lymph node metastasis in ESCC treated with nICRT remain unknown.
In this large prospective cohort, all ESCC patients were treated with standardized nCRT with or without pembrolizumab. The baseline characteristics were comparable between the two groups. Our results showed that the pCR rate of ESCC after nICRT was 44.9%, which was higher than that after nCRT (37%), but lacked statistical significance. The pCR rate was consistent with that of the CROSS trial (49%) and the NEOCRTEC5010 (43.2%) trial (6). As for major pathological response, ESCC treated with nICT (immune checkpoint inhibitors with chemotherapy) varied in pCR, ranging from 16.7% to 50%, and MPR, ranging from 41.7% to 72.2%, in a recent meta-analysis (41), which showed that nICT had no significant advantage according to the pCR and MPR rates when compared to nCRT. In the present study, the nICRT regimen significantly improved MPR rate in comparison to nCRT alone (79.5% vs. 65.4%, p = 0.024). Therefore, our results suggested that the addition of ICIs to nCRT improved the tumor regression when compared to nCRT alone. Studies on applying immunotherapy in esophageal cancer have been emerging constantly in recent years. The CheckMate 577 trial has reported the efficacy of adjuvant nivolumab following nCRT in non-pCR patients with esophageal cancer (42). According to the oral report in American Society of Clinical Oncology (ASCO) 2025, among the 532 patients who received nivolumab, the median disease-free survival was 21.8 months [95% confidence interval (CI), 16.6 to 29.7], as compared with 10.8 months (95% CI, 8.3 to 14.3) among the 262 patients who received placebo (hazard ratio for disease recurrence or death, 0.69; 96.4% CI, 0.56 to 0.86; p < 0.001). Compared to the clinical significance of postoperative immunotherapy, our regimen, adding pembrolizumab to preoperative CRT, is expected to improve the pCR rates and therefore reduce the tumor burden during surgery, which contributes to a higher R0 resection rate. Furthermore, immunotherapy has a synergistic effect with radiotherapy, and their combined application in nICRT can better improve treatment outcomes.
Among the 208 ESCC patients treated with neoadjuvant therapy followed by surgery, the overall lymph node positivity rate was 20.7%, with 27.2% in the nCRT cohort and 16.5% in the nICRT cohort. The lymph node positivity rate in the present study was significantly lower than that in patients treated with nICT as reported by Zhou H. et al. (42.8%) (43). Sun H. B. et al. (44) reported a lymph node positivity rate of 39.2% among a cohort of 398 ESCC patients treated with neoadjuvant chemoimmunotherapy and surgery. In another trial conducted by Tang H. et al., the lymph node positivity rate was 36.4% (45). There are two possible reasons for this finding: 1) our study was a retrospective cohort study based on a prospective database, and all patients were treated with the same radiotherapy dose and concurrent chemoradiotherapy according to trial protocol; however, these published studies were retrospective research, so selection bias could not be avoided. In addition, the treatment regimen significantly varied, which would be another source of heterogeneity impacting the results; 2) the previous two studies investigated lymph node metastasis in ESCC after neoadjuvant systemic therapy, and they found that the addition of radiotherapy to neoadjuvant systemic therapy could further decrease lymph node metastasis. According to the number of resected lymph nodes, a total of 1,176 and 2,230 lymph nodes had been identified in the present study, with 37 and 39 metastatic lymph nodes in the nCRT and nICRT groups, respectively (3.1% vs. 1.7%, p = 0.0069). Based on a prospective database, we found that the overall lymph node positivity rate among ESCC patients treated with nCRT with or without pembrolizumab was relatively low, and the addition of pembrolizumab had a tendency to decrease the risk of lymph node metastasis.
As for the specific station of lymph node metastasis, the common sites of lymph node metastasis after neoadjuvant treatment were station 112pulL (8.3%, 3/36 nodes positive), followed by station 104L (4.9%, 2/41 nodes positive), station 7 (4.5%, 12/266 nodes positive), and station 3a (4.3%, 10/230 nodes positive) according to 11th JCEC standard. Zhou H. et al. (43) found that the common sites of lymph node metastasis included station 107 (12.8%), station 106recR (11.7%), and station 7 (12.5%) after neoadjuvant chemoimmunotherapy. Sun H. B. et al. found that the most common metastatic sites were the right upper paratracheal (16.8%) and left gastric artery (13.1%) stations (44) among ESCC patients after neoadjuvant chemotherapy. Based on our findings, we suggest that different neoadjuvant treatment modalities may result in varying frequencies and distributions of lymph node metastasis. Then, we compared the pattern of lymph node metastasis between nCRT and nICRT and found that the metastasis rate of lymph nodes in station 2R in patients receiving nICRT was significantly lower than that of patients receiving nCRT (0.8% vs. 4.6%, p = 0.039). Based on the 11th JCEC standard, the metastasis rates of lymph nodes in station 106recR (0.8% vs. 4.6%, p = 0.042) and station 20 (0% vs. 12.5%, p = 0.030) had a significant difference between nICRT and nCRT.
The mechanism by which immunotherapy reduces lymph node metastasis in esophageal cancer is a complex, multi-layered, and interconnected process. The core of this approach lies in breaking the immune suppression of tumors and reactivating and guiding the human immune system to recognize, attack, and eliminate primary tumors and metastatic cancer cells in lymph nodes. The immune microenvironment has been shown to play a critical role in the progression of ESCC. Radiotherapy can modulate the immune microenvironment, thereby enhancing the efficacy of immunotherapy. Research has emphasized the importance of tertiary lymphoid structures (TLSs) in the therapeutic efficacy and prognosis of ESCC patients receiving nICRT (46). As for the certain nodal reduction (e.g., 106recR), the recurrent laryngeal nerve lymph node group is the core hub for the upward drainage of lymph from the cervical and upper thoracic esophagus. When tumor cells flow upward with lymph, they will be the first to reach the recurrent laryngeal nerve area (47). This unique anatomical location, extensive lymphatic drainage function, and the lymphatic reflux characteristics determine that patients have a higher risk of metastasis, so they can benefit more from immunotherapy. According to the lymph node metastasis distribution analyzed in our study, patients with preoperative radiologically suspicious lymph node metastasis in the right recurrent nerve area may benefit most from pembrolizumab addition. Patients with tumors located in the upper thoracic segments and radiologically suspicious lymph node metastasis in the upper mediastina, and those with tumors located in the middle thoracic segments and radiologically suspicious lymph node metastasis in the lower mediastina, may benefit from pembrolizumab addition as well. In summary, our study indicated that the common sites of lymph node metastasis significantly varied among ESCC patients after different neoadjuvant treatments, and nICRT could further decrease lymph node metastasis in a specific station in comparison with nCRT.
The strength of the present study was that it was a retrospective study based on a prospective cohort database, and all patients were treated with a standardized nCRT regimen with or without pembrolizumab followed by esophagectomy. All patients were treated with the same radiotherapy dose, concurrent chemotherapy, and pembrolizumab dose. However, our study has several limitations. First, this was a single-center, retrospective study, and the sample size was relatively small; therefore, selection bias could not be avoided. Second, our analysis lacked external validation. Further prospective multicenter clinical trials are recommended to confirm our findings.
In conclusion, in comparison with nCRT, nICRT followed by surgery may lead to a promising pathological response of the primary tumor and decrease the metastasis of lymph nodes. The common sites of lymph node metastasis after neoadjuvant treatment were station 112pulL, followed by station 104L, station 7, and station 3a, according to the 11th JCEC standard. The distribution of metastatic lymph nodes significantly varies according to neoadjuvant treatment strategy and the location of the primary tumor, and nICRT should be considered as a better preoperative treatment option.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethic Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent of patients was exempted due to the retrospective nature.
Author contributions
W-XQ: Conceptualization, Funding acquisition, Writing – original draft. XH: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. S-YL: Data curation, Methodology, Software, Supervision, Writing – review & editing, Resources. HL: Formal analysis, Investigation, Methodology, Writing – original draft. J-YC: Funding acquisition, Project administration, Resources, Writing – review & editing. S-GZ: Data curation, Formal analysis, Investigation, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported in part by the Beijing Science and Technology Innovation Medical Development Foundation (grant number KC2021-JX-0170-9), Clinical Research Special Project of Shanghai Municipal Health Commission Health Industry (202340226), and Shanghai Science and Technology Innovation Action Plan Medical Innovation Research Project (23Y11904700). The funding agency plays no role in the design or execution of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1700400/full#supplementary-material
Abbreviations
nCRT, neoadjuvant chemoradiotherapy; ESCC, esophageal squamous cell carcinoma; nICRT, neoadjuvant immunochemoradiotherapy; AJCC, American Joint Committee on Cancer; JCEC, Japanese Classification of Esophageal Cancer; pCR, pathological complete response; MPR, major pathological response; ICI, immune checkpoint inhibitor; TRG, tumor regression grade.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers. (2017) 3:17048. doi: 10.1038/nrdp.2017.48
3. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-Year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
4. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
5. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
6. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-Label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
7. Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2023) 21:393–422. doi: 10.6004/jnccn.2023.0019
8. Patel MA, Kratz JD, Lubner SJ, Loconte NK, and Uboha NV. Esophagogastric cancers: integrating immunotherapy therapy into current practice. J Clin Oncol. (2022) 40:2751–62. doi: 10.1200/JCO.21.02500
9. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med. (2021) 9:1623. doi: 10.21037/atm-21-5381
10. Gao L, Lu J, Zhang P, Hong ZN, and Kang M. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Oncol. (2022) 13:478–87. doi: 10.21037/jgo-22-131
11. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
12. Qiao Y, Zhao C, Li X, Zhao J, Huang Q, Ding Z, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immunol. (2022) 13:953229. doi: 10.3389/fimmu.2022.953229
13. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. (2021) 19:333. doi: 10.1186/s12957-021-02446-5
14. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: A prospective, single-arm, phase 2 trial. Front Immunol. (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
15. Sharabi AB, Lim M, DeWeese TL, and Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. (2015) 16:e498–509. doi: 10.1016/S1470-2045(15)00007-8
16. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
17. Gong J, Le TQ, Massarelli E, Hendifar AE, and Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. (2018) 6:46. doi: 10.1186/s40425-018-0361-7
18. Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res. (2022) 28:3021–31. doi: 10.1158/1078-0432.CCR-22-0413
19. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res. (2021) 27:3351–9. doi: 10.1158/1078-0432.CCR-20-4443
20. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
21. Zheng Y, Li C, Yu B, Zhao S, Li J, Chen X, et al. Preoperative pembrolizumab combined with chemoradiotherapy for esophageal squamous cell carcinoma: Trial design. JTCVS Open. (2022) 9:293–9. doi: 10.1016/j.xjon.2021.11.003
22. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. (2014) 32:385–91. doi: 10.1200/JCO.2013.51.2186
23. Ide H, Nakamura T, Hayashi K, Endo T, Kobayashi A, Eguchi R, et al. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg. (1994) 18:321–30. doi: 10.1007/BF00316810
24. Shah MA, Altorki N, Patel P, Harrison S, Bass A, and Abrams JA. Improving outcomes in patients with oesophageal cancer. Nat Rev Clin Oncol. (2023) 20:390–407. doi: 10.1038/s41571-023-00757-y
25. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, and Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. (2017) 12:36–42. doi: 10.1016/j.jtho.2016.10.016
26. Japan Esophageal S. Japanese classification of esophageal cancer. Esophagus. (2017) 14:1–36. doi: 10.1007/s10388-016-0551-7
27. Japan Esophageal S. Japanese classification of esophageal cancer. Esophagus. (2017) 14:37–65. doi: 10.1007/s10388-016-0556-2
28. Qi WX, Li S, Zhang S, Li C, Li H, Li X, et al. Characterization and dosimetric predictors for absolute lymphocyte count changes during neoadjuvant chemoradiotherapy with or without pembrolizumab for esophageal squamous cell carcinoma: an analysis of a prospective cohort. Radiat Oncol. (2025) 20:5. doi: 10.1186/s13014-024-02581-9
29. Qi WX, Li S, Li H, Chen J, and Zhao S. The addition of pembrolizumab to neoadjuvant chemoradiotherapy did not increase the risk of developing postoperative anastomotic leakage for ESCC: an analysis from a prospective cohort. BMC Cancer. (2024) 24:1029. doi: 10.1186/s12885-024-12774-w
30. Qi WX, Wang X, Li C, Li S, Li H, Xu F, et al. Pretreatment absolute lymphocyte count is an independent predictor for survival outcomes for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy and pembrolizumab: An analysis from a prospective cohort. Thorac Cancer. (2023) 14:1556–66. doi: 10.1111/1759-7714.14898
31. Cao Y, Han D, Yang S, Shi Y, Zhao S, Jin Q, et al. Effects of pre-operative enteral immunonutrition for esophageal cancer patients treated with neoadjuvant chemoradiotherapy: protocol for a multicenter randomized controlled trial (point trial, pre-operative immunonutrition therapy). BMC Cancer. (2022) 22:650. doi: 10.1186/s12885-022-09721-y
32. Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. (2005) 103:1347–55. doi: 10.1002/cncr.20916
33. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156:721–9. doi: 10.1001/jamasurg.2021.2373
34. Hirose T, Yamamoto S, and Kato K. Pembrolizumab for first-line treatment of advanced unresectable or metastatic esophageal or gastroesophageal junction cancer. Therap Adv Gastroenterol. (2023) 16:17562848221148250. doi: 10.1177/17562848221148250
35. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
36. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
37. Xu J, Li Y, Fan Q, Shu Y, Yang L, Cui T, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2). Nat Commun. (2022) 13:857. doi: 10.1038/s41467-022-28408-3
38. Abyaneh R, Ghalehtaki R, and Sanford NN. Combination of immune checkpoint inhibitors and radiotherapy in locally advanced esophagogastric junction adenocarcinoma: A review. Cancer. (2024) 130:4040–51. doi: 10.1002/cncr.35561
39. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. (2021) 161:836–843 e831. doi: 10.1016/j.jtcvs.2020.11.106
40. Visser E, van Rossum PSN, Ruurda JP, and van Hillegersberg R. Impact of lymph node yield on overall survival in patients treated with neoadjuvant chemoradiotherapy followed by esophagectomy for cancer: A population-based cohort study in the Netherlands. Ann Surg. (2017) 266:863–9. doi: 10.1097/SLA.0000000000002389
41. Li Q, Liu T, and Ding Z. Neoadjuvant immunotherapy for resectable esophageal cancer: A review. Front Immunol. (2022) 13:1051841. doi: 10.3389/fimmu.2022.1051841
42. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
43. Zhou H, Lin J, Wei W, Gao P, Wang PY, Liu SY, et al. Frequency and distribution pattern of lymph node metastasis after neoadjuvant chemoimmunotherapy for locally advanced esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. (2024) 150:476. doi: 10.1007/s00432-024-05967-0
44. Sun HB, Jiang D, Liu XB, Xing WQ, Chen PN, Feng SK, et al. Patterns and influence of lymph nodal metastases after neoadjuvant chemotherapy and surgery for thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. (2023) 30:5205–12. doi: 10.1245/s10434-023-13634-w
45. Tang H, Jiang D, Zhang S, Zeng Z, Tan L, Hou Y, et al. Residual tumor characteristics of esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. (2021) 162:1632–41. doi: 10.1016/j.jtcvs.2020.09.042
46. Saito H, Sato T, and Miyazaki M. Extramural lymphatic drainage from the thoracic esophagus based on minute cadaveric dissections: fundamentals for the sentinel node navigation surgery for the thoracic esophageal cancers. Surg Radiol Anat. (2007) 29:531–42. doi: 10.1007/s00276-007-0257-6
Keywords: esophageal squamous cell carcinoma, neoadjuvant immunochemoradiotherapy, neoadjuvant chemoradiotherapy, primary tumor residual, lymph node spread
Citation: Han X, Qi W-X, Li S-Y, Li H, Chen J-Y and Zhao S-G (2025) Residual pattern of primary tumor and lymph node in ESCC treated with nCRT with or without pembrolizumab: an analysis from a prospective cohort. Front. Immunol. 16:1700400. doi: 10.3389/fimmu.2025.1700400
Received: 06 September 2025; Accepted: 07 October 2025;
Published: 22 October 2025.
Edited by:
Elias Kouroumalis, University of Crete, GreeceCopyright © 2025 Han, Qi, Li, Li, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Xiang Qi, cWl3ZWl4aWFuZzExMTNAMTYzLmNvbQ==; Jia-Yi Chen, Y2p5MTE3NTZAcmpoLmNvbS5jbg==; Sheng-Guang Zhao, enNnMTA5MzVAcmpoLmNvbS5jbg==
†These authors have contributed equally to this work
 Xuan Han
Xuan Han Wei-Xiang Qi
Wei-Xiang Qi Shu-Yan Li1,2
Shu-Yan Li1,2 Jia-Yi Chen
Jia-Yi Chen Sheng-Guang Zhao
Sheng-Guang Zhao