- 1Department of Nursing, Institute of International Medical Science and Technology, Shanghai Sanda University, Shanghai, China
- 2Naval Medical University, Shanghai, China
- 3Anhui Medical University, Hefei, China
As a key signaling molecule network in the tumor immune microenvironment, cytokines mediate intercellular communication through mechanisms such as autocrine and paracrine, exhibiting a significant “double-edged sword” effect during tumor initiation and progression. The dynamic regulation of this dual effect is influenced by the dependence on concentration, the variability within the tumor immune microenvironment, and the stages of tumor progression, ultimately representing the prolonged co-evolutionary result between tumors and the immune system. Cytokines, as a vital element of the immune microenvironment within tumors, influence cancer promotion by creating intricate networks. Therefore, disrupting this balance to alter the tumor growth environment is of great significance for achieving tumor suppression. In terms of clinical translation, the combined strategy of cytokine therapy and immune checkpoint blockade therapy has significantly improved treatment efficacy by synergistically enhancing immune activation and relieving immune suppression. Meanwhile, approaches such as monoclonal antibodies and bispecific molecules targeting pro-tumor cytokines have provided new insights for overcoming therapeutic resistance. In-depth clarification of the molecular mechanisms underlying the dual effects of cytokines, and breaking through the limitations of single targets from a network perspective, will provide a new paradigm for cancer immunotherapy from basic mechanisms to clinical applications. This will promote the upgrading of targeting strategies towards “dynamic regulation and synergistic intervention,” ultimately improving the prognosis of cancer patients.
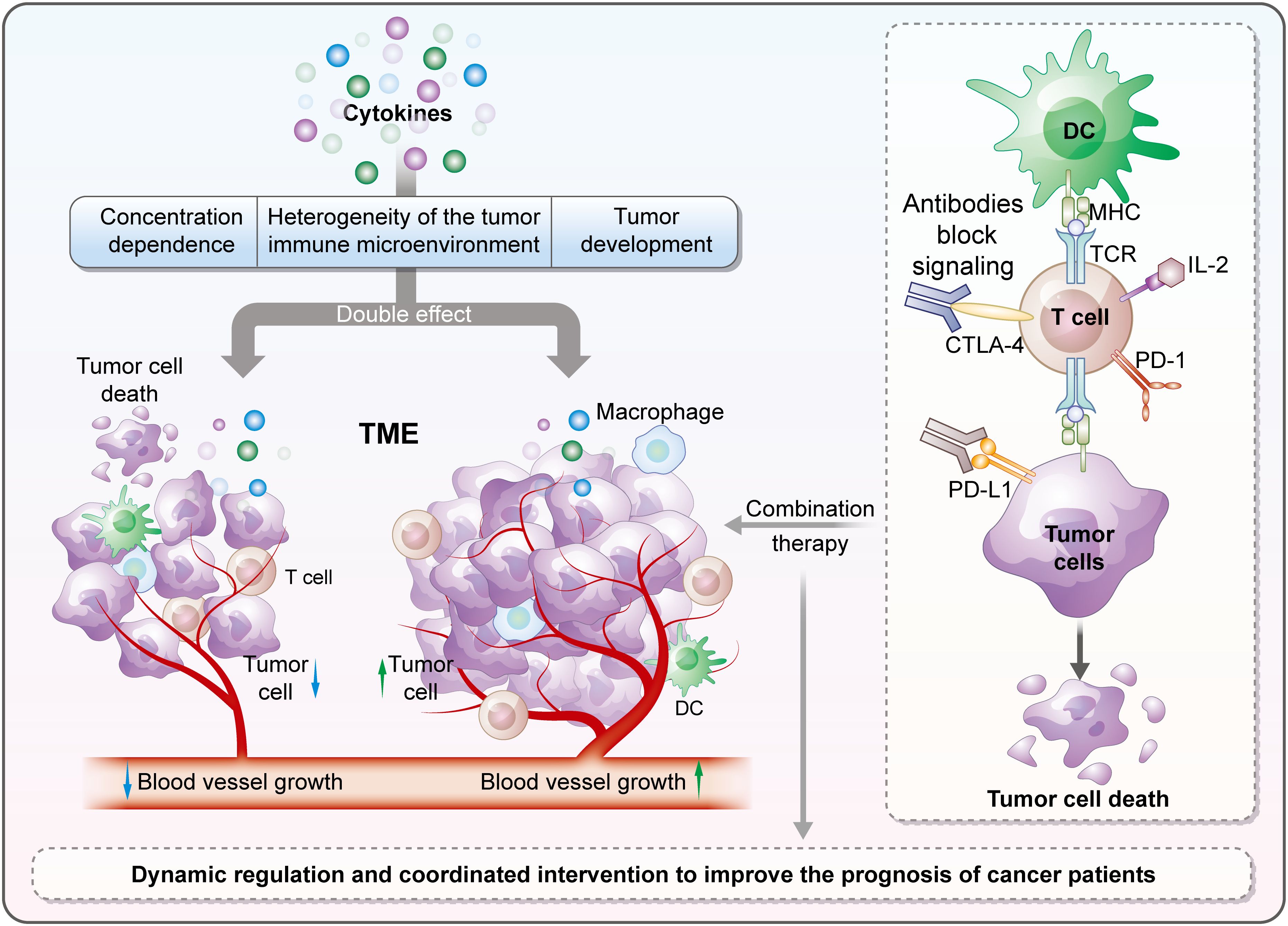
Graphical Abstract. Cytokines in the tumor immune microenvironment have a “double-edged sword” effect, regulated by multiple factors. Their combination with immune checkpoint blockade boosts efficacy; targeting pro-tumor cytokines aids overcoming resistance, promising better cancer immunotherapy and patient prognosis.
1 Introduction
Cytokines are protein or polypeptide substances with small sizes released by immune, tumor and stromal cells. These proteins will bind with receptors on the surface of target cells. This will release signals that will regulate biological processes in cells like growth, differentiation, and immune response. Cytokines can promote cell communication through autocrine, paracrine or endocrine mechanisms (1). Based on structure, origin, and function, cytokines can be classified into interleukins (IL), interferons (IFN), tumor necrosis factors (TNF), colony-stimulating factors (CSF), chemokines, and the transforming growth factor-β (TGF-β) superfamily, etc (2). Through these categories do not exist alone, the work of the immune system and tumors is highly interactive. Their interactions may be synergistic or antagonistic. The immune system performs homeostasis to avoid disease. Tumors, on the other hand, can initiate and progress to cause the disease. The efficiency of the immune system in fighting tumors relies greatly on the balance of their functions (3).
The tumor microenvironment (TME) is a complex ecosystem consisting of tumor cells, immune cells, stromal cells, and a cocktail of soluble factors (4). Cytokines, as key signaling molecules, profoundly impact tumor initiation, progression, and outcomes by modulating the function of immune cells, biological behavior of tumor cells, and angiogenesis (5). There is growing evidence that cytokines with anti-tumor function can also participate in facilitating tumor progression, indicating a functional duality (5). Certain cytokines can impact the fate of tumors through completely different pathways depending on the TMEs or developmental stages. The “double-edged sword”effect not only reveals the complex interaction with TME but also presents a new perspective for the development of precision immunotherapy. Recent advances in immunotherapy strategies have had a significant impact on the development of precision immunotherapy. The continuous emergence of innovative technologies such as CAR-T cell therapy and immune checkpoint inhibitors in recent years has rekindled people ‘s attention to the role of cytokines in tumor immunity (6, 7). This article, will first examine the dual mechanisms of cytokines and their therapeutic translational potential in enhancing tumor immunity.
2 Tumor-suppressive effects of cytokines
Cytokines like IFN-α, IFN-γ, IL-2, IL-12, IL-15 and granulocyte-macrophage colony stimulating factor (GM-CSF) have anticancer effects in tumor immunity (8). They do so by activating immune effector cells, improving antigen presentation and also acting directly on tumor cells. An important cytokine in the elimination of tumors is IFN-γ. According to research studies, Interferon gamma inhibits tumor cell growth and promotes tumor cell apoptosis. It is also known to up-regulate the expression of class I MHC on tumor cells. These mechanisms enable T cells to effectively identify and respond to tumor-associated antigens. IFN-γ can promote the activation of M1-polarized macrophages and natural killer (NK) cells, while enhancing their respective phagocytic functions and tumor-killing capabilities. Furthermore, IFN-γ can also facilitate the maturation of dendritic cells (DCs), thereby strengthening their antigen-presenting function and supporting the activation of cytotoxic T lymphocytes (CTLs) (5). IL-2 plays a critical role in driving the clonal expansion of CD4+ and CD8+ T cell populations. It not only improves the cytotoxic potential of CTLs against malignant cells but also induces the activation of NK cells and lymphokine-activated killer (LAK) cells, ultimately enhancing the ability of these cells to eliminate tumor cells (9). In contrast, IL-12 directs the differentiation of naïve T cells toward a Th1 phenotype, promotes the secretion of IFN-γ from Th1 and NK cells, and strengthens the tumor-lytic activity of CTLs and NK cells. At the same time, it limits the supply of nutrients to the tumor by suppressing angiogenesis (10). During the initial stages of tumor development, TGF-β has tumor-suppressive roles, acting by reducing cell proliferation and triggering apoptosis (11). IL-36 exerts its anti-tumor effects primarily by coordinating and activating the host’s immune system (12). Although it cannot directly stimulate effector CD8+ T cells, it exhibits a strong synergistic effect with cytokines such as IL-2 and IL-12. Together, they activate T cells and induce the latter to produce large amounts of IFN-γ (13). In vivo studies have confirmed that this immune activation can significantly inhibit the growth of tumors in models such as melanoma and fibrosarcoma. The underlying mechanism involves the induction of IL-36 receptor expression and the direct inhibition of tumor cell proliferation (14).
3 Tumor-promoting effects of cytokines
In contrast, during tumor progression, certain cytokines can assist tumors in evading immune attacks by promoting tumor proliferation, inducing an immunosuppressive microenvironment, and enhancing metastatic capacity. Examples include epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), TGF-β, TNF-α, IL-1β, IL-6, CSF-1, chemokine (CC motif) ligand 5 (CCL5), and chemokine (CXC motif) ligand 8 (CXCL8) (15). Pro-tumor cytokines are actively involved in various phases of cancer progression, which include tumor growth, metastasis, remodeling of the extracellular matrix, evasion from the immune system, and resistance to therapies. The specific mechanisms by which cytokines promote cancer progression are provided in Supplementary Figure 1.
Cytokines have the ability to trigger malignant characteristics in cancer cells, including increased proliferation, migration, and angiogenesis. IL-6, produced by tumor cells as well as by macrophages and fibroblasts within the TME, facilitates the growth of tumor cells and prevents apoptosis through the activation of the Janus kinase (JAK) - signal transducer and activator of transcription (STAT) 3 signaling pathway. Moreover, IL-6 can promote the process of epithelial-mesenchymal transition (EMT) in cancer cells, enhancing their ability to invade surrounding tissues. In addition, IL-6 supports hepatic glycogen breakdown to supply energy for tumor cells and encourages the growth of vascular endothelial cells, which plays a critical role in tumor angiogenesis (16). IL-9 can directly act on hematological malignancies expressing IL-9R, including Hodgkin’s lymphoma, anaplastic large cell lymphoma, and chronic lymphocytic leukemia (17). It promotes disease progression by enhancing the survival and proliferation of tumor cells while inhibiting their apoptosis (18). Meanwhile, IL-9 also contributes to tumor development through indirect mechanisms. For example, in solid tumors such as non-small cell lung cancer and breast cancer, IL-9 can induce the immunosuppressive effects of Treg cells and mast cells in the TME, promote angiogenesis, or upregulate the expression of PD-1 molecules on cytotoxic T lymphocytes to enhance tumor immune escape (18, 19). In lung cancer metastasis models, IL-9 can further facilitate the formation of metastatic foci through Arg1 and IL-6 secreted by IL-9R+ stromal macrophages (20). During the progressive stage of tumors, the role of TGF-β “reverses”: on one hand, it enhances the migratory and invasive abilities of tumor cells by inducing EMT; Conversely, it creates a microenvironment that suppresses the immune response by reducing the function of effector T cells and encouraging the development of immunosuppressive cells, including Treg cells (11). Tumor cells can secrete CXCL12, which recruits these immunosuppressive cells into the TME by binding to C-X-C chemokine receptor type 4 (CXCR4) on the surface of immune cells. Meanwhile, CXCL12 can induce tumor cells to express CXCR4, promoting their metastasis to tissues with high CXCL12 expression, such as bone marrow and lymph nodes (21).
During the formation of tumor immune microenvironment (TIME), cytokines show significant tumor-promoting effects. Cytokines shape the microenvironment conducive to tumor development through a variety of mechanisms, such as driving the recruitment and phenotypic differentiation of immunosuppressive cells, inhibiting the function of anti-tumor T cells, promoting the secretion of pro-tumor cytokines, and activating related signaling pathways, thus providing support for tumor growth, progression and metastasis (22). IL-10 can inhibit the antigen-presenting function of dendritic cells (DCs) and macrophages by reducing the expression of major histocompatibility complex class II (MHC-II) molecules and co-stimulatory molecules; it suppresses type 1 helper T cell (Th1)-type immune responses by decreasing the secretion of pro-inflammatory cytokines such as IFN-γ and TNF-α; at the same time, it promotes the differentiation and longevity of Treg cells, reducing the effectiveness of effector T cells in eliminating tumor cells (23). TGF-β has the capability to diminish the cytotoxic activity of CD8+ T cells by lowering the levels of granzyme and perforin. Additionally, it encourages Treg cells to produce IL-10, which further amplifies the immunosuppressive impact (24). Furthermore, TGF-β can stimulate fibroblasts within the TME to release collagen, thereby creating a thick stromal barrier that obstructs the entry of immune cells into tumor tissues (25). TNF-α induces the overexpression of programmed death ligand 1 (PD-L1) in a variety of tumors, creates an immunosuppressive TME, weakens the inhibitory effect of immune checkpoints, and induces tumor cells to develop resistance to targeted therapy (18). This suggests that in the course of treatment, targeted intervention of these pro-tumor cytokines and their related pathways can affect the composition of tumor immunosuppressive microenvironment homeostasis and enhance anti-tumor immune response.
4 Factors influencing the dual effects
Beneath the dual effects of cytokines lies a dynamic balance of multiple regulatory factors. Table 1 presents the roles of cytokines that exert dual effects in tumors, as well as the factors dependent on these roles. The concentration effect is one of the most prominent regulatory mechanisms: the unique and opposing roles of TNF-α in cancer depend on the cytokine concentration (29); IL-2 can enhance T cell function at appropriate levels but may induce T cell exhaustion when excessive (9). Specifically, low concentrations of TNF-α activate the NF-κB signaling pathway, inducing EMT in tumor cells, enhancing their invasiveness, and sustaining tumor-associated macrophages (30). In contrast, high-dose recombinant human TNF-α can induce hemorrhagic necrosis in syngeneic transplanted tumors in mouse models and human tumor xenografts (31). The core mechanism underlying this effect is the disruption of the tumor vascular bed, resulting in ischemic necrosis of tumor tissue (29, 31).
The diversity within the TME is also significant; factors like hypoxia and acidosis in the microenvironment can change the characteristics of immune cells, leading to a transformation of M1 macrophages into M2, macrophages, which subsequently modifies the cytokines they produce from pro-inflammatory to anti-inflammatory types (48). Additionally, differences in tumor development stages affect cytokine functions. For instance, TGF-β exerts a dual effect characterized by “tumor suppression in the early stage and tumor promotion in the late stage” (26, 27). In early-stage tumors, TGF-β upregulates p21 through Smad3 to inhibit the cell cycle (11). In the late stage, however, it activates Snail via Smad2/3 to induce EMT (25). The distinct effects of TGF-β on tumors at different stages depend on corresponding molecular switches, which are precisely the outcome of long-term interactions between tumors and the immune system (27).
Cytokines exhibit varying performances across different cancers. Multiple studies have shown that GM-CSF can exert either anti-tumor or pro-tumor effects (33). In colorectal cancer, GM-CSF activates the STAT 1 pathway, which recruits CD8+ T cells to the TME. These CD8+ T cells then exert cytotoxic effects against tumor cells, contributing to the anti-tumor response (34). In contrast, in bladder cancer, GM-CSF binds to CSF2Rα, activating the STAT 3 pathway. This activation promotes the expression of the anti-apoptotic protein Bcl-2 in tumor cells, enhancing their resistance to apoptosis and thereby facilitating tumor progression (35). Even more intriguing is the observed phenomenon whereby this identical cytokine can mediate opposing biological effects within the same cancer subtype (49). Acquiring a thorough and contextualized understanding of cytokine roles—whether they exert anti-tumorigenic or pro-tumorigenic functions—in a cancer-type-specific manner, alongside elucidating the molecular mechanisms that govern these dual roles, is imperative for their rational and effective application in therapeutic strategies.
5 Application of cytokines in targeted therapy
With the continuous advancement of cancer immunotherapy, this field has successfully achieved a revolutionary transition from theoretical establishment to clinical translation. Figure 1 systematically summarizes the key milestones in the development of cancer immunotherapy since 1891. While cytokines function locally, their systemic use is challenged by significant toxicity and limited effectiveness. So far, only a small number of cytokines have received approval for use in treating cancer patients (50). Currently, the U.S. Food and Drug Administration (FDA) has approved IFN-α and IL-2 for the treatment of various cancers (51–54). For IFN-α, in the adjuvant treatment of melanoma, it has been shown to increase the overall survival from 2.8 years to 3.8 years. Additionally, the proportion of patients with persistent disease-free survival has been improved by 42% (52). Notwithstanding the drawbacks associated with these therapies, including significant side effects and the requirement for high dosages, pertinent clinical research has shown the effectiveness of cytokines in enhancing patient outcomes (2).
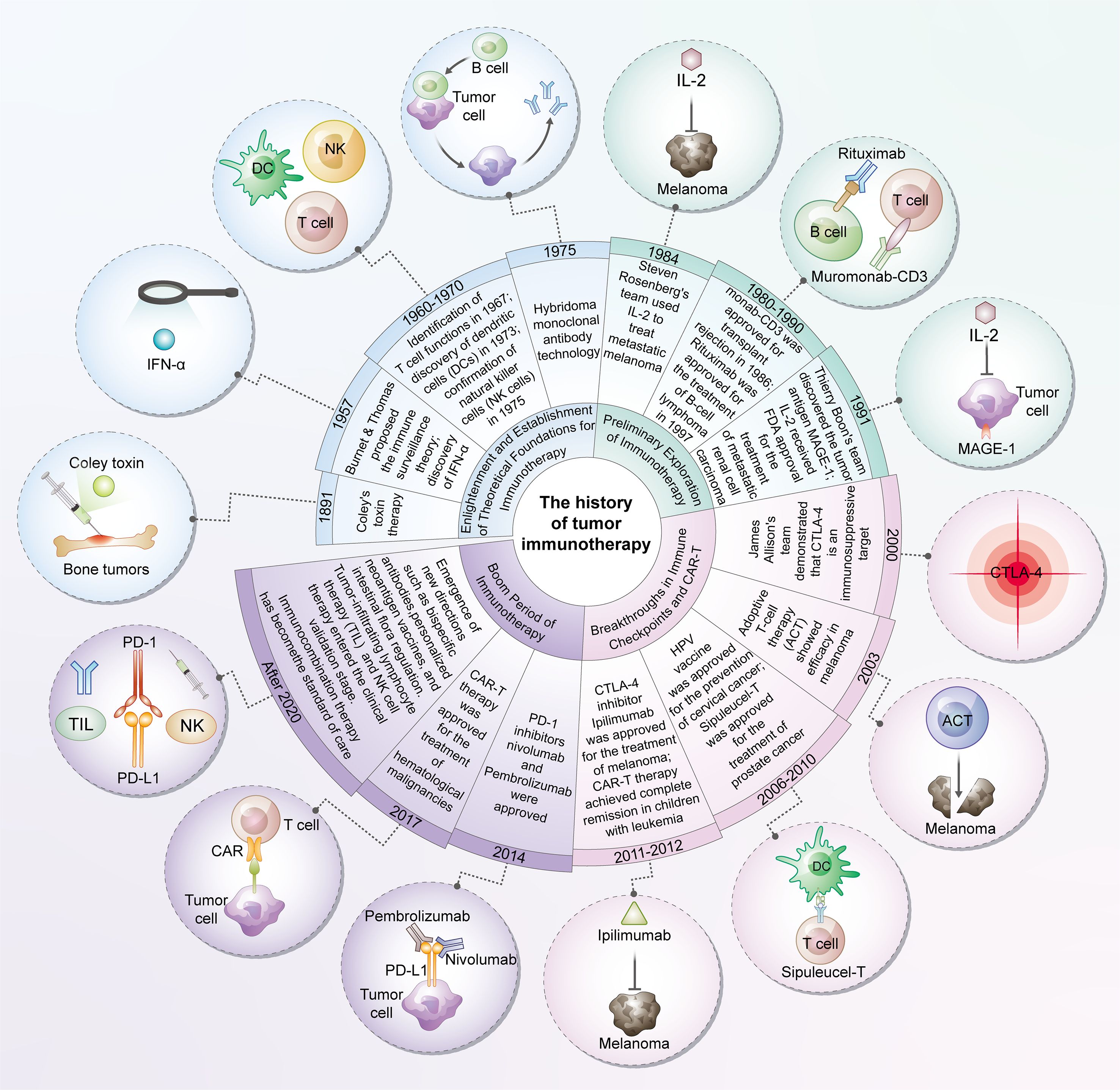
Figure 1. The history of tumor immunotherapy. The development of tumor immunotherapy represents a transformative history spanning from theoretical inception to clinical innovation. Originating initially from the establishment of fundamental theories such as immune surveillance, this field has gradually undergone exploratory phases including cytokine therapy and monoclonal antibody-based approaches, ultimately achieving major breakthroughs in immune checkpoint inhibitors and chimeric antigen receptor T-cell (CAR-T) therapy—breakthroughs that have revolutionized the treatment paradigm for multiple cancer types. In recent years, immunotherapy has entered a new era characterized by diversification and combined applications. Emerging directions such as personalized vaccines, bispecific antibodies, and microbial modulation continue to emerge, while immunotherapeutic combination strategies have become standard protocols in tumor treatment. Overall, tumor immunotherapy not only exemplifies the successful translation of basic scientific research into clinical practice but also demonstrates a distinct trend from the exploration of single mechanisms toward multi-targeted, personalized integrated therapy.
In clinical settings, alternative immunotherapies, especially immune checkpoint blockade (ICB) therapy, have predominantly supplanted these cytokines, owing to their enhanced effectiveness and improved safety profile (55, 56). Immune checkpoints constitute a group of molecules within the immune system that normally serve to modulate the strength and duration of immune responses, thereby preventing the excessive activation of immune cells that could harm healthy tissues. The typical representatives of immune checkpoint molecules include cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed death receptor 1 (PD-1) and PD-L1 (57). Within the TME, cancer cells often use these immune checkpoint molecules: by binding to the corresponding receptors on the surface of immune cells, they transmit inhibitory signals, resulting in T cells and other immune cells unable to effectively identify and attack cancer cells, thus helping tumors to evade immune surveillance and clearance (58). Immune checkpoint blockade therapy uses drugs such as specific antibodies to block the interaction between immune checkpoint molecules and their ligands, reversing this immunosuppressive state. For example, antibodies that target PD-1/PD-L1 obstruct the interaction between PD-1 and PD-L1, whereas antibodies against CTLA-4 impede the activity of CTLA-4, thereby enabling T cells to restore their functions and improve their capacity to identify and destroy cancer cells, leading to tumor management and eradication (59). At present, ICB therapy has demonstrated notable effectiveness across several cancers, including melanoma, non-small cell lung carcinoma, and renal cell carcinoma, positioning it as a key strategy in cancer immunotherapy (6). However, this therapy also has certain limitations: some patients may experience immune-related adverse reactions, not all patients can benefit from it, and there are issues such as intrinsic tumor resistance. Therefore, its clinical application is still under continuous exploration and optimization (60, 61).
The prospect of integrating cytokines with various immunotherapeutic approaches, in conjunction with progress in drug delivery systems and protein engineering, has rekindled enthusiasm for the use of cytokines in the treatment of cancer (62). In order to overcome the drug resistance mechanism of ICB monotherapy, one of the key directions of current clinical research is to combine cytokine therapy with ICB. The evaluation of the combined application of ICB with cytokines such as IFN-α and IL-12 in a number of trials has shown that such combined treatment regimens can synergistically enhance anti-tumor immunity (63). In general, cytokines represent promising yet complex targets within the TIME. Cytokines have the ability to enhance the infiltration of immune cells and facilitate the partial activation of lymphocytes; therefore, therapies that utilize cytokines might aid in overcoming both primary and acquired resistance to ICB, thereby maximizing clinical advantages across a diverse array of patients (64). Clinical data have demonstrated that the combination of IFN-α-1b and PD-1 monoclonal antibody exhibits favorable anti-tumor activity and acceptable toxicity in Chinese patients with metastatic melanoma, including those with cutaneous, acral, and mucosal subtypes (65). A latest study revealed that for microsatellite-stable colorectal cancer liver metastases—a typical type of “cold tumor”—the combined use of LIGHT cytokine and anti-CTLA-4 antibody can synergistically remodel the TME (66). Specifically, LIGHT activates T cells, while the anti-CTLA-4 antibody reverses the exhaustion state of activated T cells and eliminates inhibitory immune cells, thereby achieving effective control of tumor growth (66).
In addition, the advancement of monoclonal antibodies and receptor inhibitors aimed at pro-tumor cytokines like VEGF, IL-6, and TGF-β represents a significant achievement within cancer therapy. These cytokines, which support various dimensions of cancer progression, facilitate processes such as tumor expansion, metastasis, remodeling of the extracellular matrix, immune system avoidance, and resilience against treatment (15). By neutralizing these cancer-facilitating cytokines or inhibiting their receptors, the efficacy of cancer immunotherapy could potentially be improved. Presently, multiple approaches to inhibit these cytokines have been established, which include neutralizing antibodies, bispecific antibodies, small-molecule inhibitors, cytokine traps, small interfering RNA (siRNA), and peptides (2). Certain cytokine antagonists, including anti-TGF-β and anti-VEGF antibodies, have demonstrated considerable promise in boosting the effectiveness of different immunotherapy modalities, including ICB, while addressing treatment resistance (67).
Cytokine-centered therapies for cancer encounter two main obstacles: significant toxicity and less than ideal efficacy. The negative effects linked to cytokine treatment, including capillary leak syndrome seen with high doses of IL-2, highlight the need for approaches that can minimize toxicity without compromising effectiveness (68). Additionally, tumor heterogeneity and the complexity of the TME result in variable patient responses to these treatments, highlighting the importance of identifying biomarkers to predict treatment responses and guide therapeutic choices (63). For cytokine-based cancer therapies to be successfully translated into clinical practice, improvements are required in the following areas: optimizing pharmacokinetic and pharmacodynamic properties; refining local delivery strategies; gaining a deeper understanding of environment-dependent interactions within the TME; and optimizing combination therapies (69). Moreover, since cytokines typically act synergistically with other cytokines and chemokines to form regulatory loops, targeting multiple cytokines simultaneously may yield more effective anti-tumor effects than single-agent cytokine-targeted drugs (42). It is important to highlight that a majority of cytokines serve multiple functions and have unique effects at various phases of tumor progression. Consequently, the knowledge of the precise role that cytokines play in each patient is vital for the improvement of therapies targeting these molecules.
6 Discussion
The interplay between tumor cells and the immune system over a long period of time is basically responsible for the dual role of cytokines in tumor immunity. This can not only illustrate the complexity of tumor immune regulation, but also provide a critical breakthrough for breaking the therapeutic bottleneck. Scale inhibition of tumor development related with certain cytokines convinces investigators in a study to optimize their use in tumor treatments. Conventional therapies utilizing cytokines have not produced great results due to inevitable side effects. The next-generation targeting strategy is now shifting towards “precision regulation”: using monoclonal antibodies to block the functionality of protumor factors. Alongside this method, a genetic engineering approach will also be employed to modify cytokines and enhance their anti- tumor specificity and reduce the immunosuppressive effect. Cytokine-targeted treatment combined with immune checkpoint inhibitors can relieve immune suppression as well as enhance immune activation. As they possess complementary mechanisms of action, they can form a synergistic effect.
However, the implementation of these strategies still faces numerous challenges: How to precisely target cytokines in the TME without disrupting systemic immune homeostasis? How to develop personalized cytokine regulation protocols based on individual patient differences? To address these issues, we need to further decipher the dynamic change rules of cytokine networks and develop more precise intervention methods. In future research, only by deeply understanding the dual roles of cytokines and their dynamic variation patterns can we achieve precise regulation of tumor immune responses. Cytokine-targeted therapy, based on theoretical mechanisms and continuously integrated with other treatment modalities, is expected to expand its therapeutic potential, improve its clinical application, and ultimately enhance the prognosis of cancer patients.
Although combination therapy can partially overcome the limitations of single-target therapy, the dynamic adaptability of the cytokine network in the TME can still lead to treatment resistance in patients. Therefore, the treatment strategy needs to shift from “target intervention” to “network regulation”. The “Network equilibrium Hypothesis” holds that the cytokine network in the TME maintains a stable pro-tumor state through complex interactions and feedback loops. Breaking this balanced state rather than merely targeting individual cytokines can disrupt the tumor’s adaptability to the microenvironment, restore the anti-tumor immune response, and ultimately achieve effective tumor suppression. This shift from focusing on individual targets to regulating the entire network reflects a deeper understanding of the complexity of the TME and provides a new direction for developing more effective cancer immunotherapy strategies.
Author contributions
XY: Conceptualization, Formal Analysis, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. WZ: Methodology, Validation, Writing – original draft, Writing – review & editing, Conceptualization. GW: Conceptualization, Validation, Writing – original draft, Writing – review & editing. YL: Formal Analysis, Validation, Writing – original draft, Writing – review & editing. BD: Supervision, Writing – review & editing. LY: Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (81970640, 82370735).
Acknowledgments
The Supplementary Figure 1 was created using Figdraw (ID: ORARR3b939).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1701405/full#supplementary-material
Supplementary Figure 1 | Mechanisms of cytokines promoting cancer progression. Cytokines can promote the proliferation and survival of tumor cells; facilitate epithelial-mesenchymal transition (EMT) and stemness maintenance of tumor cells, thereby fostering a more invasive phenotype; drive angiogenesis; participate in the activation of fibroblasts into cancer-associated fibroblasts (CAFs) and the remodeling of the extracellular matrix (ECM), which in turn promote tumor immune evasion and therapeutic resistance; and pro-inflammatory cytokines induce dysregulated inflammation to support tumor initiation and progression. Additionally, cytokines act on immune cells within the tumor microenvironment to form an immunosuppressive tumor microenvironment, helping tumors evade immune surveillance and further proliferate.
Glossary
CAR-T: Chimeric Antigen Receptor T-cell
CAF: Cancer-associated fibroblast
CCL5: Chemokine (CC motif) ligand 5
CSF: Colony-stimulating factor
CTLA-4: Cytotoxic T-lymphocyte-associated antigen 4
CTL: Cytotoxic T lymphocyte
CXCL8: Chemokine (CXC motif) ligand 8
CXCL12: Chemokine (CXC motif) ligand 12
CXCR4: C-X-C chemokine receptor type 4
CXCR7: C-X-C chemokine receptor type 7
DC: Dendritic cell
ECM: Extracellular matrix
EMT: Epithelial-mesenchymal transition
EGF: Epidermal growth factor
FDA: U.S. Food and Drug Administration
GM-CSF: Granulocyte-macrophage colony-stimulating factor
ICB: Immune checkpoint blockade
IFN: Interferon
IL: Interleukin
JAK: Janus kinase
LAK: Lymphokine-activated killer
MAPK: Mitogen-activated protein kinase
MHC: Major histocompatibility complex
M-CSF: Macrophage Colony-Stimulating Factor
NF-κB: Nuclear factor-κB
NK: Natural killer
PD-1: Programmed death-1
PD-L1: Programmed death-ligand 1
PI3K/Akt: Phosphatidylinositol 3-kinase/protein kinase B
SDF-1: Stromal cell-derived factor-1
siRNA: Small interfering RNA
STAT: Signal transducer and activator of transcription
TAM: Tumor-associated macrophage
TGF-β: Transforming growth factor-β
Th1: Type 1 helper T cell
TIME: Tumor immune microenvironment
TME: Tumor microenvironment
TNF: Tumor necrosis factor
TRAIL: Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand
Treg: Regulatory T
VEGF: Vascular endothelial growth factor.
References
1. Zhang JM and An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. (2007) 45:27–37. doi: 10.1097/AIA.0b013e318034194e
2. Propper DJ and Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. (2022) 19:237–53. doi: 10.1038/s41571-021-00588-9
3. Niu T and Zhou F. Inflammation and tumor microenvironment. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2023) 48:1899–913. doi: 10.11817/j.issn.1672-7347.2023.230231
4. Anderson NM and Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r5. doi: 10.1016/j.cub.2020.06.081
5. Kureshi CT and Dougan SK. Cytokines in cancer. Cancer Cell. (2025) 43:15–35. doi: 10.1016/j.ccell.2024.11.011
6. Wei SC, Duffy CR, and Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. (2018) 8:1069–86. doi: 10.1158/2159-8290.Cd-18-0367
7. Bhagwat AS, Torres L, Shestova O, Shestov M, Mellors PW, Fisher HR, et al. Cytokine-mediated CAR T therapy resistance in AML. Nat Med. (2024) 30:3697–708. doi: 10.1038/s41591-024-03271-5
8. Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10:1–23. doi: 10.1101/cshperspect.a028472
9. Leonard WJ, Lin JX, and O'Shea JJ. The γ(c) family of cytokines: basic biology to therapeutic ramifications. Immunity. (2019) 50:832–50. doi: 10.1016/j.immuni.2019.03.028
10. Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. (1994) 153:1697–706. doi: 10.4049/jimmunol.153.4.1697
11. Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q, et al. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J Hematol Oncol. (2022) 15:135. doi: 10.1186/s13045-022-01349-6
12. Finucane M, Brint E, and Houston A. The complex roles of IL-36 and IL-38 in cancer: friends or foes? Oncogene. (2025) 44:851–61. doi: 10.1038/s41388-025-03293-4
13. Tsurutani N, Mittal P, St Rose MC, Ngoi SM, Svedova J, Menoret A, et al. Costimulation endows immunotherapeutic CD8 T cells with IL-36 responsiveness during aerobic glycolysis. J Immunol. (2016) 196:124–34. doi: 10.4049/jimmunol.1501217
14. Neurath MF. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. (2020) 55:70–9. doi: 10.1016/j.cytogfr.2020.06.006
15. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, and Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. (2021) 21:481–99. doi: 10.1038/s41568-021-00363-z
16. Kumari N, Dwarakanath BS, Das A, and Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. (2016) 37:11553–72. doi: 10.1007/s13277-016-5098-7
17. Chen N and Wang X. Role of IL-9 and STATs in hematological Malignancies (Review). Oncol Lett. (2014) 7:602–10. doi: 10.3892/ol.2013.1761
18. Bick F, Blanchetot C, Lambrecht BN, and Schuijs MJ. A reappraisal of IL-9 in inflammation and cancer. Mucosal Immunol. (2025) 18:1–15. doi: 10.1016/j.mucimm.2024.10.003
19. Heim L, Yang Z, Tausche P, Hohenberger K, Chiriac MT, Koelle J, et al. IL-9 producing tumor-infiltrating lymphocytes and treg subsets drive immune escape of tumor cells in non-small cell lung cancer. Front Immunol. (2022) 13:859738. doi: 10.3389/fimmu.2022.859738
20. Fu Y, Pajulas A, Wang J, Zhou B, Cannon A, Cheung CCL, et al. Mouse pulmonary interstitial macrophages mediate the pro-tumorigenic effects of IL-9. Nat Commun. (2022) 13:3811. doi: 10.1038/s41467-022-31596-7
21. Yang Y, Li J, Lei W, Wang H, Ni Y, Liu Y, et al. CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. Int J Biol Sci. (2023) 19:3341–59. doi: 10.7150/ijbs.82317
22. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
23. Saraiva M, Vieira P, and O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. (2020) 217:1–19. doi: 10.1084/jem.20190418
24. Wang C, Zhu C, Deng X, and Zhang W. Dual effects and balanced regulation of cytokines in sepsis. Trends Immunol. (2025) doi. doi: 10.1016/j.it.2025.10.002
25. Bierie B and Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. (2006) 17:29–40. doi: 10.1016/j.cytogfr.2005.09.006
26. Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. (2003) 112:1116–24. doi: 10.1172/jci18899
27. Reiss M. TGF-beta and cancer. Microbes Infect. (1999) 1:1327–47. doi: 10.1016/s1286-4579(99)00251-8
28. Seoane J and Gomis RR. TGF-β Family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. (2017) 9. doi: 10.1101/cshperspect.a022277
29. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. (2009) 9:361–71. doi: 10.1038/nrc2628
30. Lv N, Liu F, Cheng L, Liu F, and Kuang J. The Expression of Transcription Factors is Different in Papillary Thyroid Cancer Cells during TNF - α induced EMT. J Cancer. (2021) 12:2777–86. doi: 10.7150/jca.53349
31. Mukaida N, Sasakki S, and Popivanova BK. Tumor necrosis factor (TNF) and chemokines in colitis-associated cancer. Cancers (Basel). (2011) 3:2811–26. doi: 10.3390/cancers3032811
32. Manohar SM. At the crossroads of TNFα Signaling and cancer. Curr Mol Pharmacol. (2024) 17:e060923220758. doi: 10.2174/1874467217666230908111754
33. Kumar A, Taghi Khani A, Sanchez Ortiz A, and Swaminathan S. GM-CSF: A double-edged sword in cancer immunotherapy. Front Immunol. (2022) 13:901277. doi: 10.3389/fimmu.2022.901277
34. Nebiker CA, Han J, Eppenberger-Castori S, Iezzi G, Hirt C, Amicarella F, et al. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin Cancer Res. (2014) 20:3094–106. doi: 10.1158/1078-0432.Ccr-13-2774
35. Perez FA, Fligner CL, and Yu EY. Rapid clinical deterioration and leukemoid reaction after treatment of urothelial carcinoma of the bladder: possible effect of granulocyte colony-stimulating factor. J Clin Oncol. (2009) 27:e215–7. doi: 10.1200/jco.2009.22.4931
36. Urdinguio RG, Fernandez AF, Moncada-Pazos A, Huidobro C, Rodriguez RM, Ferrero C, et al. Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res. (2013) 73:395–405. doi: 10.1158/0008-5472.Can-12-0806
37. Alspach E, Lussier DM, and Schreiber RD. Interferon γ and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb Perspect Biol. (2019) 11. doi: 10.1101/cshperspect.a028480
38. Wawrzyniak P and Hartman ML. Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors. Mol Cancer. (2025) 24:89. doi: 10.1186/s12943-025-02294-x
39. Castro F, Cardoso AP, Gonçalves RM, Serre K, and Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. (2018) 9:847. doi: 10.3389/fimmu.2018.00847
40. Im SJ, Lee K, and Ha SJ. Harnessing IL-2 for immunotherapy against cancer and chronic infection: a historical perspective and emerging trends. Exp Mol Med. (2024) 56:1908. doi: 10.1038/s12276-024-01301-3
41. Rokade S, Damani AM, Oft M, and Emmerich J. IL-2 based cancer immunotherapies: an evolving paradigm. Front Immunol. (2024) 15:1433989. doi: 10.3389/fimmu.2024.1433989
42. Soler MF, Abaurrea A, Azcoaga P, Araujo AM, and Caffarel MM. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-007530
43. Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. (2021) 33:127–48. doi: 10.1093/intimm/dxaa078
44. Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo MJ, and Selvan SR. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res. (2011) 51:170–82. doi: 10.1007/s12026-011-8262-6
45. Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. (2023) 14:1161067. doi: 10.3389/fimmu.2023.1161067
46. Dennis KL, Blatner NR, Gounari F, and Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. (2013) 25:637–45. doi: 10.1097/cco.0000000000000006
47. Dong C, Tan D, Sun H, Li Z, Zhang L, Zheng Y, et al. Interleukin-12 delivery strategies and advances in tumor immunotherapy. Curr Issues Mol Biol. (2024) 46:11548–79. doi: 10.3390/cimb46100686
48. Zou Z, Lin H, Li M, and Lin B. Tumor-associated macrophage polarization in the inflammatory tumor microenvironment. Front Oncol. (2023) 13:1103149. doi: 10.3389/fonc.2023.1103149
49. Mercogliano MF, Bruni S, Mauro F, Elizalde PV, and Schillaci R. Harnessing tumor necrosis factor alpha to achieve effective cancer immunotherapy. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13030564
50. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. (2004) 4:11–22. doi: 10.1038/nrc1252
51. Groopman JE, Gottlieb MS, Goodman J, Mitsuyasu RT, Conant MA, Prince H, et al. Recombinant alpha-2 interferon therapy for Kaposi's sarcoma associated with the acquired immunodeficiency syndrome. Ann Intern Med. (1984) 100:671–6. doi: 10.7326/0003-4819-100-5-671
52. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, and Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. (1996) 14:7–17. doi: 10.1200/jco.1996.14.1.7
53. Golomb HM, Jacobs A, Fefer A, Ozer H, Thompson J, Portlock C, et al. Alpha-2 interferon therapy of hairy-cell leukemia: a multicenter study of 64 patients. J Clin Oncol. (1986) 4:900–5. doi: 10.1200/jco.1986.4.6.900
54. Solal-Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d'Etude des Lymphomes de l'Adulte. N Engl J Med. (1993) 329:1608–14. doi: 10.1056/nejm199311253292203
55. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. (2018) 24:1649–54. doi: 10.1038/s41591-018-0197-1
56. Konen JM, Wu H, and Gibbons DL. Immune checkpoint blockade resistance in lung cancer: emerging mechanisms and therapeutic opportunities. Trends Pharmacol Sci. (2024) 45:520–36. doi: 10.1016/j.tips.2024.04.006
57. Darvin P, Toor SM, Sasidharan Nair V, and Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
58. Zhang Y and Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv Exp Med Biol. (2020) 1248:201–26. doi: 10.1007/978-981-15-3266-5_9
59. Lynch C, Pitroda SP, and Weichselbaum RR. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. (2024) 25:e352–e62. doi: 10.1016/s1470-2045(24)00075-5
60. Fujiwara Y, Horita N, Adib E, Zhou S, Nassar AH, Asad ZUA, et al. Treatment-related adverse events, including fatal toxicities, in patients with solid tumours receiving neoadjuvant and adjuvant immune checkpoint blockade: a systematic review and meta-analysis of randomised controlled trials. Lancet Oncol. (2024) 25:62–75. doi: 10.1016/s1470-2045(23)00524-7
61. Kalbasi A and Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. (2020) 20:25–39. doi: 10.1038/s41577-019-0218-4
62. Atallah-Yunes SA and Robertson MJ. Cytokine based immunotherapy for cancer and lymphoma: biology, challenges and future perspectives. Front Immunol. (2022) 13:872010. doi: 10.3389/fimmu.2022.872010
63. Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct Target Ther. (2024) 9:176. doi: 10.1038/s41392-024-01868-3
64. Tang T, Huang X, Zhang G, Hong Z, Bai X, and Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. (2021) 6:72. doi: 10.1038/s41392-020-00449-4
65. Zhu G, Shi Q, Zhao B, Liu Y, Feng T, Li C, et al. Efficacy and safety of interferon-alpha 1b combined with PD-1 monoclonal antibody in patients with unresectable stage IV melanoma: a retrospective study. J Cancer Res Clin Oncol. (2023) 149:6263–9. doi: 10.1007/s00432-023-04596-3
66. Keenan BP, Qiao G, Kunda N, Kone L, Guldberg SM, Todeschini L, et al. Combination LIGHT overexpression and checkpoint blockade disrupts the tumor immune environment impacting colorectal liver metastases. Sci Adv. (2025) 11:eadv9161. doi: 10.1126/sciadv.adv9161
67. Li T, Wang X, Niu M, Wang M, Zhou J, Wu K, et al. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front Immunol. (2023) 14:1196970. doi: 10.3389/fimmu.2023.1196970
68. Dhupkar P and Gordon N. Interleukin-2: old and new approaches to enhance immune-therapeutic efficacy. Adv Exp Med Biol. (2017) 995:33–51. doi: 10.1007/978-3-319-53156-4_2
Keywords: cytokines, cancer, tumor immune microenvironment, immune checkpoint blockade, cancer therapy
Citation: Yin X, Zhang W, Wang G, Liu Y, Dai B and Yue L (2025) The “double-edged sword” effect of cytokines in cancer: coexisting opportunities and challenges. Front. Immunol. 16:1701405. doi: 10.3389/fimmu.2025.1701405
Received: 08 September 2025; Accepted: 03 November 2025;
Published: 19 November 2025.
Edited by:
Jun Wang, Dalhousie University, CanadaReviewed by:
Laura Patrussi, University of Siena, ItalyCopyright © 2025 Yin, Zhang, Wang, Liu, Dai and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Yue, bHB5dWVAc2FuZGF1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xuan Yin
Xuan Yin Wangzheqi Zhang
Wangzheqi Zhang Guanhua Wang3†
Guanhua Wang3† Bing Dai
Bing Dai