- 1Epigenomics and Biomarker of Solid Tumors Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 2Sahlgrenska Center for Cancer Research, Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Metastasis, the primary cause of cancer-related mortality, is sustained by complex interactions between tumor cells and host-derived factors. Extracellular vesicles, membrane-bound particles that mediate intercellular communication, have emerged as critical regulators of this process. Among them, platelet-derived extracellular vesicles represent the most abundant EV population in circulation and extend the multifaceted influence of platelets in cancer progression. Platelets actively contribute to metastasis by shielding circulating tumor cells from immune surveillance, promoting vascular remodelling, facilitating extravasation, and releasing soluble factors that shape the premetastatic niche. Platelet-derived extracellular vesicles further potentiate these processes by delivering a heterogeneous cargo of proteins, nucleic acids, and lipids to endothelial, stromal, and immune cells, thereby promoting angiogenesis, extracellular matrix remodelling, immune suppression, and organ-specific metastatic colonization. This review summarizes current evidence on the cooperative roles of platelets and platelet-derived extracellular vesicles in metastatic dissemination, with particular emphasis on their contribution to lung premetastatic niche formation and their emerging translational potential in oncology.
1 Introduction
Metastasis is the process by which cancer cells spread from their original location to other parts of the body, forming new tumors (1), and it represents one of the major contributors to cancer-related deaths (2). Furthermore, morbidity and mortality of metastasis are associated with the rise of paraneoplastic syndromes and complications due to treatments (2). Metastasis is a highly complex process involving numerous biological components. Identifying its key contributors is essential for the development of metastasis-targeted therapies and for improving the management of advanced disease (3).
Blood represents a complex fluid comprising different cellular (red blood cells, white blood cells, and platelets) and soluble components, many of which are actively involved in the metastatic process (4, 5). Platelets are the most abundant cell population in blood, and their primary activities are related to the coagulation cascade and hemostasis maintenance (6). Among other factors, such as cytokines and growth factors, extracellular vesicles (EVs) are highly present in the blood circulation. EVs are membrane-limited particles released by almost all cells in our body (7). Blood EVs originate primarily from blood cells and endothelial cells, although vesicles released from distant organs can also be detected in circulation (8). Platelets and platelet-derived extracellular vesicles (PEVs) are two major contributors to tumor progression and metastasis establishment, as they are involved in nearly all steps of the metastatic cascade (9). The complex bidirectional interaction between cancer cells and platelets has important clinical implications in metastasis management. This interaction can lead to platelet activation with consequent rise of thrombotic complications that represent a major cause of cancer-related deaths (10, 11). Moreover, cancer cells exploit platelets to enhance their chances of establishing metastases in distant organs and to develop resistance to cytotoxic chemotherapeutic drugs (12–14). This review summarizes the key mechanisms by which platelets contribute to metastatic establishment and how PEVs influence the formation of the premetastatic niche (PMN), with particular emphasis on PMN formation in the lungs.
2 Metastatic cascade in solid tumors
The first event in the metastatic cascade is the escape of metastatic cells from the primary tumor, followed by their invasion of nearby lymphatic vessels or the bloodstream. In this phase, metastatic cells acquire a more plastic phenotype by undergoing the epithelial-to-mesenchymal transition (EMT) and acquiring different genomic alterations (15, 16). As a consequence of these events, metastatic cells detach from surrounding cells and extracellular matrix (ECM) and migrate towards the circulation.
Intravasation is a critical bottleneck in the metastatic process. Evidence from mouse models demonstrates that, although large numbers of tumor cells are shed from solid tumors, fewer than 0.1% successfully form metastases at distant sites (17, 18). This low rate of success could be explained by the fact that once in the bloodstream, tumor cells have to face different threats, including as anoikis due to cellular detachment, shear stress, and the presence of immune cells, making the circulation a hostile environment for their survival. In the circulation, circulating tumor cells (CTCs) have been detected as both single cells and as clusters of cells, and their presence in the circulation is generally associated with a worse prognosis for patients (19, 20). Furthermore, CTCs could be circulating in association with other cells, such as neutrophils (20) and platelets, which increases their survival probability and, at the same time, avoids their recognition and consequent elimination mediated by natural killer (NK) cells and other cytotoxic immune cells (21).
As a consequence, CTCs can extravasate into distant organs and initiate metastatic colonization (22). Generally, each primary tumor shows a specific organ tropism for the metastatic seeding. This behavior forms the basis of Paget’s classic ‘seed and soil’ theory of metastasis (23). It is known that the metastatic behavior of tumor cells is affected by cell intrinsic properties but also by a plethora of different environmental cues, such as chemokines, cytokines, and EVs released by both primary tumor cells and the cell components of the host microenvironment. All these factors contribute to the formation of the so called PMN, which favors colonization and organ-specific metastatic dissemination (24).
Once in secondary organs, disseminated tumor cells (DTCs) face new challenges that undermine the establishment of metastasis. At this level, DTCs can be eliminated by patrolling immune cells (25) or nutrients’ deprivation, hypoxia (26, 27), and elevated oxidative stress (28, 29). To overcome these difficulties, DTCs can enter into dormancy, which allows their persistence in secondary organs for months or even decades, thus avoiding elimination by immune cells and chemotherapeutics (30–32). Several factors then mediate the following re-awakening of cancer cells. Integrin signaling and interactions with the ECM have been implicated in the exit from dormancy, enabling metastatic outgrowth. Integrin-β1 signaling is a well-known regulator of this process: its inhibition induces cell cycle arrest. It sustains dormancy in various cancer models (33), whereas its upregulation facilitates re-entry into the cell cycle (34). Cancer cell proliferation at metastatic sites is likewise induced by the interaction between collagen and noncanonical discoidin domain receptor 1 (DDR1) (35). Furthermore, in a model of dormant breast cancer and lung adenocarcinoma, the depletion of the WNT ligand DKK1 promotes the re-entrance into the cell cycle, highlighting an important role of WNT pathway activation for the metastatic progression (36).
2.1 Pre-metastatic niche
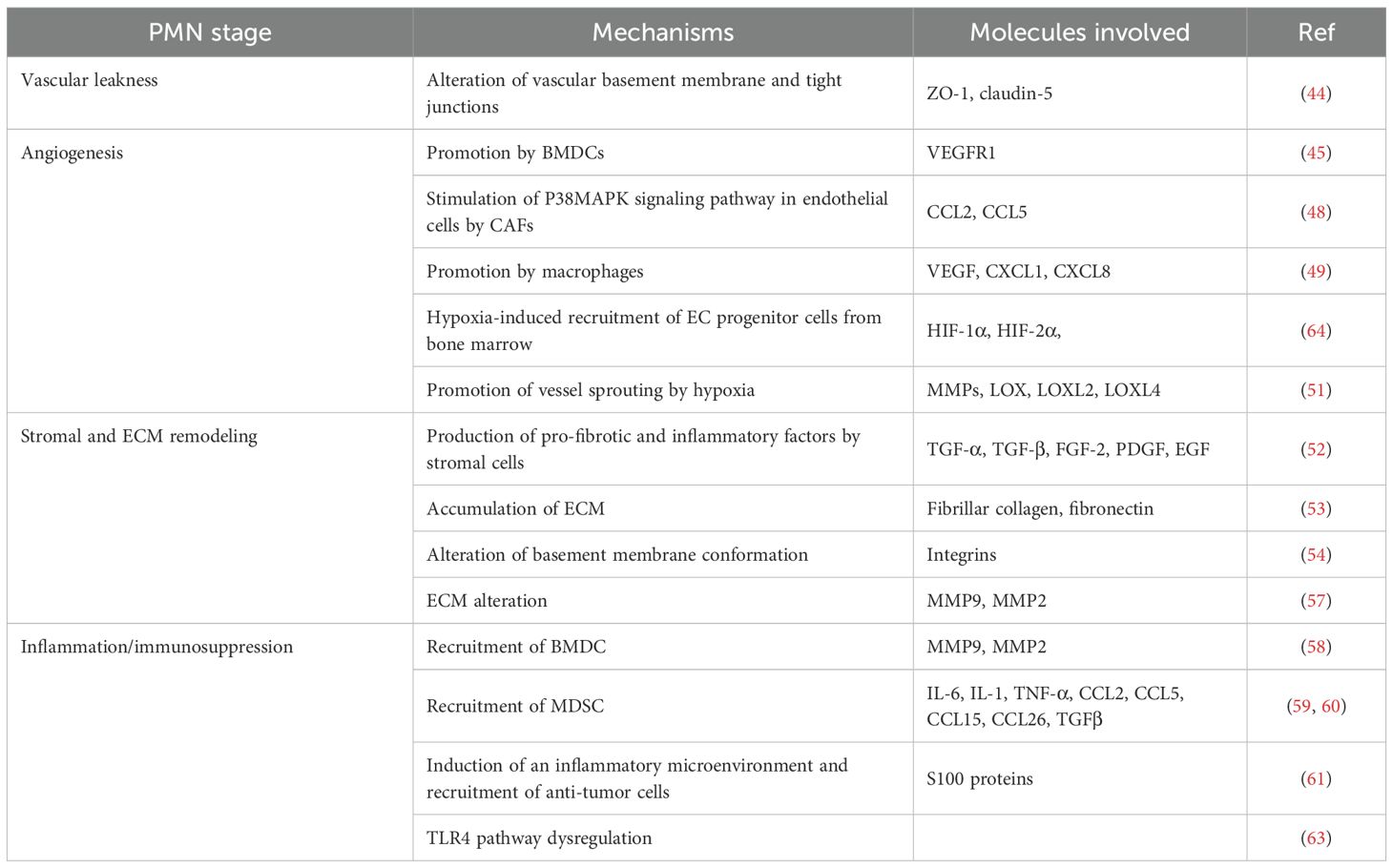
The mechanisms that establish a favorable and protective microenvironment in secondary organs, facilitating tumor cell colonization and growth before the arrival of CTCs, are referred to as PMN formation (24, 37). The concept of PMN as currently known was first introduced by Lyden and colleagues, who outperformed and ameliorated both Paget’s “seed and soil” theory and Ewing’s assumption (38, 39). Indeed, growing evidence highlighted that organotropic metastases and the development of the PMN are a combination of multiple factors and an intricate interplay among tumor-secreted molecules and microenvironment alterations (40). The PMN is characterized by some main features, including vascular permeability and angiogenesis, lymphangiogenesis, inflammation, immunosuppression, stromal and ECM remodeling, and metabolic reprogramming (41). Each step is finely regulated by an intricate interplay of cells and soluble factors that ensures the establishment of a suitable soil for the attachment and growth of primary tumor cells (42). Angiogenesis and disruption of the endothelial wall are early events in PMN formation, and as the ultimate goal, they enable tumor cell extravasation, thereby facilitating metastasis (43). Mechanistically, vascular leakage is driven by molecules that disrupt the vascular basement membrane and alter tight junctions, such as zonula occludens-1 (ZO-1), occludin and claudin-5, in endothelial cells (44). A plethora of factors released by tumor cells or tumor microenvironment (TME) cell populations are involved in the process of new vessel formation. Kaplan et al. demonstrated that VEGFR1+ bone marrow-derived cells (BMDCs) are essential for creating a suitable environment for secondary tumor attachment in response to primary tumor signals, by promoting angiogenesis in PMN (45). Other cell populations contribute to the neo-angiogenesis process, such as cancer-associated fibroblasts (CAFs) (46, 47). Indeed, they have been shown to release the lncRNA SNHG5, which upregulates CCL2 and CCL5, thereby activating the p38 MAPK signaling pathway in endothelial cells within the PMN. This activation promotes angiogenesis, enhances vascular permeability, and supports the establishment of the premetastatic microenvironment (48). Moreover, macrophages, in addition to their capacity to secrete VEGF, also release CXCL1 and CXCL8, which further promote angiogenesis (49). Hypoxia and its key transcriptional mediators, hypoxia-inducible factors 1α (HIF-1α) and hypoxia-inducible factors 2α (HIF-2α), are critical regulators of blood vessel formation. These factors promote the recruitment of endothelial progenitor cells from the bone marrow and their differentiation into endothelial cells through the regulation of VEGF expression. In addition, HIF-1α and HIF-2α facilitate angiogenic remodeling by inducing the expression of matrix metalloproteinases (MMPs) and lysyl oxidase family enzymes (LOX, LOXL2, and LOXL4), which contribute to the sprouting of pre-existing vessels and the remodeling of collagen fibers within the extracellular matrix (50, 51).
The ECM has been widely recognized as a key factor in the formation of distant pre-metastatic and metastatic niches, with its alteration and remodeling playing a crucial role in shaping the tumor microenvironment and determining the fate of tumor cells (52). The ECM is composed by a complex structure that includes various proteins, namely collagens, elastins, fibronectins, glycoproteins, laminins and ECM-associated proteins (53). By considering the alteration of ECM in relation to its abundance, tumour cells are shown to orchestrate the recruitment of stromal cells that produce various pro-fibrotic growth factors and inflammatory factors such as TGF-α, TGF-β, fibroblast growth factor (FGF)-2, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) (54). In a pro-tumorigenic context, structural modifications of the ECM play a key role. Recent studies have shown that fibrillar collagen accumulation directly promotes tumor development and progression (51), while fibronectin deposition creates a supportive niche for the adhesion of BMDCs, which are critical for PMN formation (55). Moreover, ECM modification can be mediated by the mechanical force applied by the integrins that modulate the basement membrane conformation, thus facilitating cancer cell invasion (56). Critical enzymes involved in ECM remodeling are MMPs. Namely, MMP9 is over-expressed by endothelial cells and MAC1+/VEGFR1+ myeloid cells in PMN, and its expression has not only been associated with tumor cell invasion but also with the recruitment of BMDC to the niche. Moreover, MMP9, together with MMP2 mediates tumor cells’ invasion through degradation of collagen IV (57, 58).
Besides the ECM context, inflammation and immunosuppression are two critical points, strictly correlated to each other in the PMN formation (41). It has been demonstrated that tumor cells release inflammatory cytokines (IL-6, IL-1, TNF-α), chemokines (CCL2, CCL5, CCL15, CCL26), and growth factors (TGFβ), which ultimately recruit BMDCs such as myeloid-derived suppressor cells (MDSCs) and create a favorable niche for tumor development (59, 60). For example, S100 proteins have been linked to inflammation and the recruitment of hematopoietic progenitor cells and immune suppressor cells, such as regulatory T cells (Tregs), and tumor-associated macrophages, which help tumors evade immune detection (61). Similarly, macrophages resident in premetastatic sites were shown to contribute to the establishment of an immunosuppressive microenvironment with inflammatory characteristics (62). Furthermore, deregulated TLR4 signaling in tumor cells can lead to an inflammatory response, which potentiates tumor cells’ resistance towards cell death, proliferation, invasion, and metastasis (63). All the processes involved in the PMN cascade are summarized in Table 1.
2.2 Lung metastasis formation
The lung represents a preferential organ for metastasis formation for many types of tumors due to its high vascularization, ECM composition and organization (65). In primary lung cancer, intrapulmonary metastases, either contralateral or ipsilateral, are observed in approximately 15–30% of cases (66). Moreover, in breast cancer, the lung is a frequent site of metastasis, with an incidence of about 40% in triple-negative and 20% in non-triple-negative subtypes (67). Lung metastases are also frequent in several other malignancies, occurring in approximately 46% of prostate cancers, 40–50% of renal cell carcinomas, 17–40% of sarcomas, and about 6% of hepatocellular carcinomas (66). Hematogenous dissemination to the lungs represents the most common metastatic route for many cancer types, given the continuous inflow of blood from the heart (66). Through the bloodstream, CTCs, tumor-derived extracellular vesicles (TEVs), and other soluble factors can reach the lungs, where they modulate the local microenvironment and promote PMN formation and subsequent metastatic colonization (68). Reactivation of disseminated tumor cells is promoted by remodeling of the ECM, transitioning from a disorganized to a highly aligned configuration rich in type III collagen fibers. This structural change triggers cancer cell proliferation through activation of the DDR1–STAT1 signaling axis (69). In addition to their role in ECM remodeling, lung-resident fibroblasts are essential for establishing an immunosuppressive microenvironment that facilitates metastatic growth (70). In particular, by producing prostaglandin E2, lung fibroblasts impair dendritic cell (DC) function and promote the expansion of suppressive monocytes (70). The spatial distribution of immune cells also contributes to the development of lung metastases (66). In breast cancer metastasizing to the lungs, metastatic regions have been shown to be enriched in macrophages, macrophage-regulatory cells, and monocytes producing type I interferons (IFNs) (71). In contrast, anti-tumor immune populations, such as T cells and NK cells, are primarily detected in lung regions devoid of metastases. This spatial organization supports metastatic outgrowth, with tumor-promoting immune cells concentrated within the metastatic core and anti-tumor immune cells excluded to the periphery. The recruitment and functional hijacking of immune cells further contribute to neo-angiogenesis within the metastatic niche (71). Tenascin C, produced by cancer cells, stimulates lung-associated macrophages via TLR4 to produce nitric oxide and TNF, thereby inducing an inflammatory response in endothelial cells and supporting the formation of a metastatic vascular niche (72). In an inflammatory context, endothelial cells are also responsible for the proliferation of cancer cells through the production of TGF-β1 and periostin (73). Furthermore, in breast and prostate cancer lung metastasis models, inflammation has been shown to trigger neutrophils to release extracellular traps, leading to laminin degradation and the generation of signals that awaken dormant cancer cells (74).
3 Extracellular vesicles and cancer
EVs are defined as membrane-limited particles released by all kinds of cells and cannot replicate on their own (75). Initially identified as cell waste, these particles have recently gained increasing attention for their fundamental role in intercellular communication, both in physiological and pathological conditions (76). “Extracellular vesicles” is an umbrella term that encompasses different particle subtypes that differ based on their biogenesis, size, and composition. In this review, we will use the term EVs consistently, irrespective of the terminology adopted by the cited authors.
The biological functions of EVs largely stem from their capacity to influence recipient cells by delivering bioactive molecules such as proteins, lipids, and nucleic acids either as cargo or displayed on their surface (77). EVs are released in different biofluids, allowing them to reach distant organs. Interactions with target cells occurring through specific receptors or adhesion molecules directly modulate the cell’s behaviour and function (78, 79). However, the effector functions of EVs are often exerted after internalization into cells (80). Once internalized, the EV cargo is released and can modulate multiple signaling pathways within the target cell (77).
In recent years, growing evidence has highlighted the role of EVs in nearly all stages of tumor development and progression (81). TEVs modulate the behaviour of stromal and immune cells within the microenvironment, reprogramming them to support cancer growth. They also play a pivotal role in PMN formation, underscoring their capacity to influence distant organs (82). EVs could contribute to the local and systemic cancer progression by enhancing the proliferation and survival of tumor cells as they can carry a wide range of bioactive molecules, including proteins, DNA, RNA, lipids, and metabolites (83). EV cargo includes oncogenic proteins (e.g., EGFR, c-Myc) and regulatory RNAs that enhance cancer cells’ proliferation, cell invasiveness, and metastatic potential by inducing EMT, promoting ECM degradation, and conditioning distant tissues through the establishment of PMN (84, 85). Moreover, EVs play a prominent role in angiogenesis, facilitating tumour vascularisation through the delivery of pro-angiogenic factors as VEGF, FGF, and angiogenesis-related miRNAs (86). EVs also facilitate immune evasion by impairing the function of cytotoxic immune cells and reprogramming immune responses to promote tumor tolerance (83) (71, 87).
Beyond their autocrine effects, TEVs are especially effective at corrupting cells within the tumor microenvironment (TME) to promote cancer progression. For example, lung cancer–derived EVs boost angiogenesis and vascular permeability by delivering miR-23a to endothelial cells, which causes HIF-1α accumulation and the breakdown of the tight junction protein ZO-1 (88). Fibroblasts are another common target of TEVs, often reprogrammed into CAFs. In both prostate and bladder cancer, TEV-associated TGF-β1 induces stromal fibroblasts to acquire a myofibroblast-like phenotype by activating the SMAD signaling pathway, thereby promoting tumor growth and angiogenesis in vivo (89, 90).
Another crucial aspect of TEVs is their immunomodulatory properties (91). TEVs can promote immune evasion by shutting down NK cell and cytotoxic T cell functions, as well as activating pro-tumoral immune cells, such as MDSCs (92), TAMs (93, 94), and Tregs (95).
TEVs also exhibit tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs) on their surface. They have immunogenic properties exerted on antigen-presenting cells (DCs) and can trigger an anti-tumor immune response (91). Collectively, TEVs have multifaceted roles in cancer biology, orchestrating interaction between tumor and distant organs, reshaping the TME, and modulating the immune system.
4 Platelets
In the 19th century, Giulio Bizzozero identified a third morphological cell population within the blood, separate from white and red blood cells: platelets (96). He described platelets as anucleate, disc-shaped structures, round or oval, about three times smaller than erythrocytes. Today, we know that platelets are approximately 2-4 μm in size and result from the fragmentation of megakaryocytes (MKs) (97). MKs, large multinucleated cells found in the bone marrow, spleen, and lungs, shed platelets into the bloodstream, where they have a lifespan of approximately 5–7 days (98, 99). Platelets inherit granules, mitochondria, coding and non-coding RNAs from MKs, as well as translational machinery for post-transcriptional gene regulation (100). They are primarily involved in haemostasis and coagulation processes, thanks to the release of several factors (101, 102). Platelets contain several critical structures, including α-granules, dense granules, and lysosomal granules, all of which are essential for optimal function. These proteins are released upon activation and are derived from the continuous endocytosis process of MKs and platelets (103).
4.1 Platelets’ physiological functions
Platelets play vital roles in maintaining vascular integrity and tissue homeostasis. Their classical function is the clot formation (101). Platelet-dependent coagulation can be activated through two mechanisms, which imply the activation of two distinct pathways: the extrinsic and the intrinsic pathways. The extrinsic pathway begins with endothelial injury, which triggers the release of tissue factor (factor III) into the blood, leading to its processing and activation of the typical cascade (104, 105). The intrinsic pathway begins, instead, when factor XII, also known as the Hageman factor, is activated by exposure to collagen, kallikrein, and high-molecular-weight kininogen (HMWK) (106). In this case, the cascade proceeds through the activation of factors XI and IX before merging into the common coagulation pathway shared by both routes (106, 107). The final steps of the coagulation cascade aim to produce thrombin and fibrin, creating a solid structure that prevents further bleeding (107).
Platelets preserve the vascular integrity by releasing sphingosine-1-phosphate (S1P), a bioactive lipid that protects the endothelial barrier and prevents leakages (108). They are also recognised for their involvement in other processes, including inflammation, where they recruit leukocytes to the damage site, angiogenesis, and tissue regeneration (109, 110). All these mechanisms are regulated by the release of growth factors, cytokines, and EVs by platelets that modulate the revascularisation and healing of connective tissue damage (111).
4.2 Platelets in cancer
The primary role of platelets is to maintain blood homeostasis, but they can also participate in pathological processes. Several studies show an association between platelets and the onset and progression of cancer (Figure 1). For example, platelets are responsible for the tumour’s immune evasion, tumour cell adhesion and arrest on the endothelial wall, as well as their extravasation and survival (112). Different mechanisms, dependent on the environment, are used by platelets to support tumour growth (95).
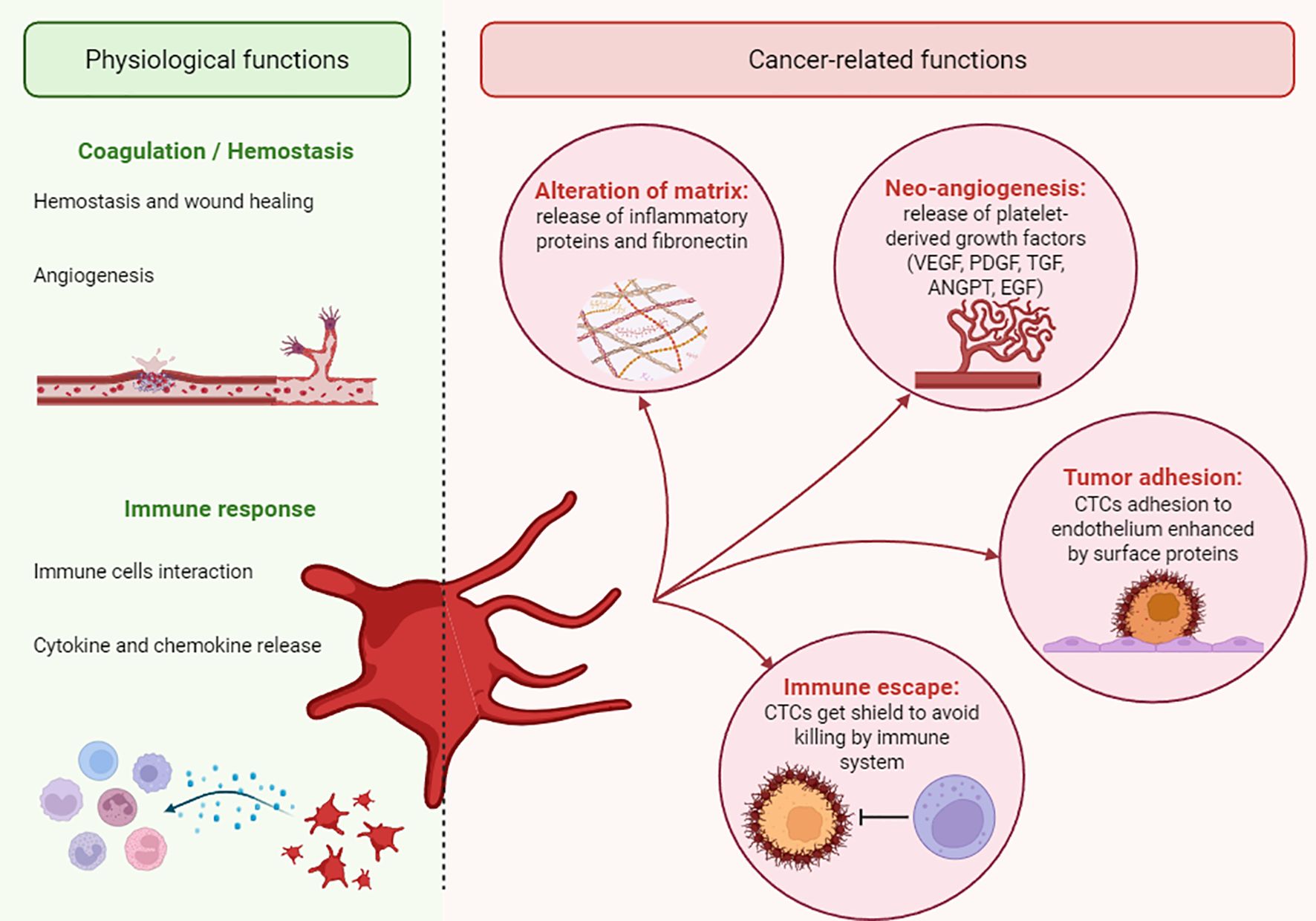
Figure 1. Physio-pathological roles of platelets. A schematic illustration of the dual role of platelets. The pathological roles of platelets in cancer are indicated as “cancer-related functions”.
In the bloodstream, platelets help CTCs released by melanoma, breast, and lung cancer to survive in the circulation by preventing immune cell binding and killing (113). Specifically, fibrinogen and tissue factor, along with NKG2D downregulation, help platelets form a shield over CTCs to prevent tumor cell recognition by NK cells (114). Additionally, the presence of selectins and integrins on the surface of platelets is crucial for the arrest and adhesion of CTCs on the endothelium (115).
Within the TME, platelets release proangiogenic factors, including interleukins, VEGF, CXCL12, and TGFβ, which promote neovascularisation, EMT, and metastatic seeding (116, 117). One example is platelets’ ability to induce the biosynthesis and esterification of 12S-hydroxyeicosatetraenoic acid (12S-HETE) in colon cancer cells, which, in turn, modulates the expression of EMT marker genes and promotes metastasis (118). Although these findings are important for the development of new therapeutic strategies, free 12S-HETE levels were not significantly different between colorectal polyps, cancer mucosa, and normal colorectal mucosa in humans. Furthermore, as the cited study was conducted exclusively in cell lines, it would be valuable to validate these results in animal models and human samples (118). Other mechanisms, such as coagulation-associated pathways and fibrinogen production, are upregulated in lung adenocarcinoma compared to squamous cell carcinoma, suggesting that platelets play a significant role in advanced and metastatic stages (119).
4.3 Platelets’ activities during tumor growth
Beyond releasing bioactive molecules, platelets also actively uptake factors from the bloodstream, thereby changing their cargo composition and undergoing a process of “education” (95). These factors may originate from the bone marrow, stromal cells, or cancer cells, influencing platelet functions and promoting a pro-tumorigenic phenotype (120). Platelets that undergo this process, known as tumor-educated platelets (TEPs), acquire a unique RNA cargo capable of distinguishing between cancer patients and those with non-malignant or inflammatory conditions (121). The RNA in TEPs undergoes extensive splicing, resulting in an enrichment of mRNAs related to vesicle transport and cytoskeletal functions, as well as miRNAs that regulate gene expression and RNA silencing in recipient cells. These findings are based on a detailed analysis of platelet RNA from healthy donors and patients with localized or metastatic cancer (121). The authors showed that sequencing could accurately identify the diagnosis and location of the primary tumor, offering hope for the future of platelet-based liquid biopsies (121). Although these results are promising, the study emphasizes that inflammatory diseases and other factors, such as cardiovascular events and non-cancerous conditions, may also influence the platelet mRNA profile. A key point in the study is the selection of the healthy control group, which consists of younger individuals than those in the cancer group. Since the RNA content of platelets varies with age and gender (122), some of the observed differences may be attributed mainly to the age gap between the two groups.
Additionally, in vitro studies in which platelets were co-cultured with cancer cells or exposed to conditioned media from cancer cell cultures demonstrated enhanced platelet activation and alterations in platelets RNA signatures (123). These interactions also promoted cancer cell survival, migration, and invasion by activating the TGFβ/Smad/PAI-1 and PI3K/AKT signalling pathways (124, 125). These findings are based on indirect interactions between platelets and CTCs, suggesting that the factors responsible for platelet education are present in the conditioned medium. In circulation, platelets could promote PMN formation and metastasis by directly interacting with CTCs (126). Recent evidence further reveals that platelets can physically cover CTCs in the bloodstream, shielding them from the immune system (127, 128).
In vivo, platelets can extravasate from the bloodstream and infiltrate the tumor stroma, where they are identified as tumor-infiltrating platelets (TIPs) through histochemical detection of CD42b expression. TIPs accumulation is elevated within tumor tissue and correlates with cancer progression and stage across different tumor types (129). The presence of TIPs could thus be used to integrate the RNM staging system as well as predict the prognosis and post-surgical survival in several cancer types, including pancreatic ductal adenocarcinoma and colorectal cancer (130, 131).
Elucidating the role of TEPs and their oncogenic cargo has significant implications for cancer diagnosis and therapy. TEPs represent promising biomarkers of cancer progression and potential targets for strategies aimed at blocking their pro-tumorigenic activities (132). Notably, key mRNA markers such as MAX, MTURN, UQCRH, and HLA-B are significantly upregulated in TEPs and have been associated with chemotherapy responses, underscoring their value as non-invasive biomarkers for cancer detection (133). Additionally, TEPs and TIPs can modulate the TME and its vascular supply by releasing factors and EVs enriched in regulatory miRNAs (132, 134).
4.3.1 PEV cargo in tumor progression
EVs carry a complex cargo that mirrors the state of their cells of origin (75). Among them, PEVs are the most abundant population in the bloodstream, generated through the continuous release of vesicles that occur during normal platelet physiology (135). PEVs inherit both cytosolic and membrane components from platelets and are characterized by specific membrane markers, including CD41, CD42a, CD42b, and CD62P (136, 137). Their concentration is closely linked to the platelet activation state, which can be altered under pathological conditions (137). In cancer, platelets and PEVs act as potent immunomodulators, exerting both suppressive and stimulatory effects. They shield CTCs, transfer PD-L1 and TGF-β, and release prostaglandin E2, collectively inhibiting CD4+ and CD8+ T cell activity and promoting an immunosuppressive TME (138, 139). Moreover, PEVs acquire diverse tumor-derived biomolecules, including proteins (such as cytokines and enzymes), nucleic acids (coding and non-coding RNAs), second messengers, and even mitochondrial components (140, 141). This heterogeneous cargo underpins the diverse biological activities of PEVs across different target cell types. Importantly, EVs can cross the bloodstream and tissue barriers, enabling PEVs to deliver their cargo to distant cells and organs (142). Through this mechanism, PEVs mediate communication between the tumor and the microenvironment, thereby fostering growth, metastasis, and overall cancer progression.
4.3.2 MicroRNAs
PEVs play a crucial role in mediating intercellular communication by transferring microRNAs between cells. MiRNAs are small nucleic acids, ranging from 19 to 25 nucleotides in length, that play an essential role in regulating RNA expression. Their presence in EVs significantly affects various types of cancers and the pathophysiology of the immune system (142). These vesicles can be internalized by recipient cells, reshaping their molecular profiles and functions (143). Specific miRNAs carried within PEVs promote cancer aggressiveness by driving invasion, migration, and angiogenesis (143). For example, it has been shown that miR-939 found in PEVs is delivered to ovarian cancer cells and is linked to increased aggressiveness (144). However, in the cited study, healthy donor platelets stimulated with thrombin were used as controls. This condition does not accurately mirror the physiological state of cancer patients and thus limits clinical relevance. Moreover, PEVs transporting miR-223 are internalized by endothelial cells, where they downregulate the tumor suppressors FBXW7 and EFNA1 (145). This study suggests that PEVs can be internalized by diverse cell types beyond tumor cells. Specific miRNAs released by PEVs, including miR-126, let-7a, and miR-320b, are implicated in the regulation of angiogenesis. Notably, miR-126 promotes angiogenesis and modulates CXCL12 and VCAM-1 expression in endothelial cells, thereby facilitating transendothelial migration and contributing to vascular inflammation (146, 147). However, as the vesicles analysed were generated through in vitro platelet lysis, it remains unclear whether they accurately reflect EVs naturally secreted by circulating platelets, limiting insight into their proper pathophysiological role. Given the central role of miR-126 in multiple angiogenic pathways, further studies are necessary to elucidate these mechanisms and establish their biological significance. PEV-mediated delivery of let-7a to endothelial cells provides an angiogenic stimulus that supports the growth of solid tumors (148). However, in this study, EVs were derived from healthy donor platelets activated with thrombin, a condition that does not fully reflect the pathological state of cancer patients.
In contrast, a different study successfully isolated plasma-derived EVs from lung cancer patients. It demonstrated the transfer of miR-320 to human umbilical vein endothelial cells (HUVECs), resulting in altered endothelial phenotype (149). More recently, studies have specifically examined miR-320b within PEVs, revealing its role in downregulating ICAM-1 expression in HUVECs (150, 151).
These studies underscore the interplay between TEPs, PEVs, and endothelial cells, which is central to the platelet-mediated regulation of tumor angiogenesis and the TME. Overall, the dynamic activity of EV-associated miRNAs shapes the tumor milieu while also modulating gene expression across diverse cell types.
4.3.3 Additional PEV cargo molecules
It is known that cancer condition alters the composition of proteins in TEP-derived EVs (95). Comparative studies have revealed considerable differences in protein expression between EVs isolated from platelets of colorectal cancer patients and healthy donors. Specifically, the authors identified 119 proteins downregulated and 89 proteins upregulated in EVs from cancer patients compared with those from healthy individuals (152). In the mentioned study, EVs were isolated from the platelets of cancer patients, thereby reflecting the pathological state of the disease. However, in this study, platelets were artificially activated with thrombin to boost EV production, a condition that may alter their composition. Functional assays demonstrated that TEP-derived EVs upregulated key EMT markers in cancer cells, including TWIST and VIM (152). In addition, both colorectal and prostate cancer cells were shown to internalize PEVs, leading to increased expression of MMPs such as MMP-2 and MMP-9, thereby enhancing their ability to remodel the distant microenvironment (153, 154). PEVs also transfer platelet-derived integrins, including CD41, to the surface of tumor cells, which strengthens the adhesion of lung, prostate, and colorectal cancer cells to the endothelium and facilitates their migration and systemic dissemination (155).
Taken together, these findings illustrate the complex interplay between TEP-derived EVs and tumor cells, underscoring their pivotal role in cancer progression and metastasis. Deciphering these mechanisms holds promise for the development of novel targeted therapies and anti-metastatic strategies.
4.4 Platelets’ activities during metastatization
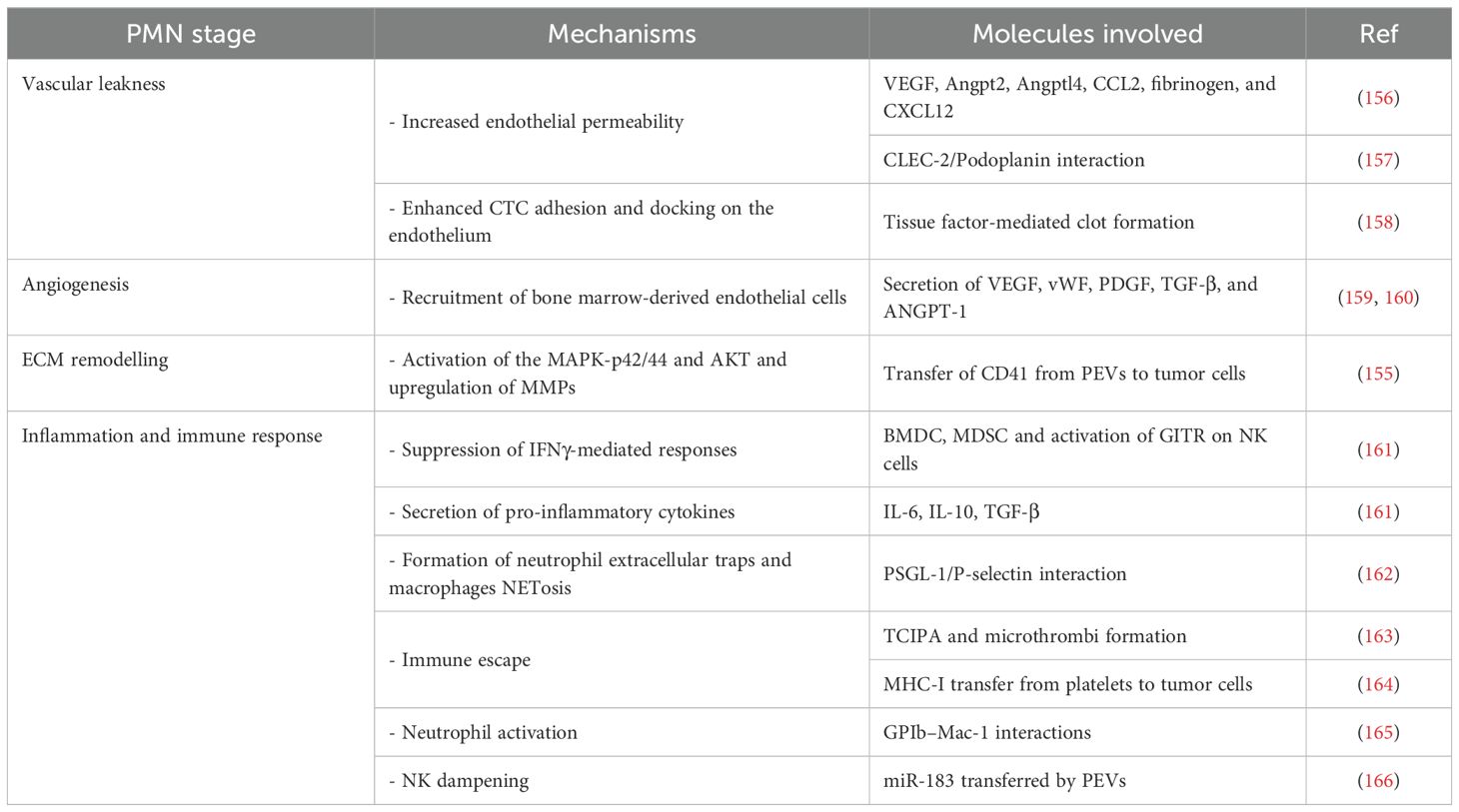
During cancer metastasis, TEPs play a pivotal role in PMN formation as illustrated in Table 2 (167). One key mechanism is tumor cell–induced platelet aggregation (TCIPA), in which platelets encapsulate CTCs, shielding them from immune surveillance and promoting metastatic spread via microthrombus formation (163). TCIPA also fosters lung metastasis in malignant melanoma models through the release of chemokines (CCL2, CXCL12, IL-1α, IL-1β) and recruitment of TAMs, driving their polarization toward an M2 phenotype (168).
At the vascular level, platelets increase endothelial permeability and vascular leakiness, facilitating CTCs extravasation through the secretion of junction-regulating molecules, including VEGF, Angpt2, Angptl4, CCL2, fibrinogen, and CXCL12 (156). They also enhance CTCs adhesion and docking on the endothelium via tissue factor-mediated clot formation, which recruits macrophages expressing CD11b, CD68, F4/80, and CX3CR1 (158). Platelet activation additionally drives granulocyte recruitment (CD11b+MMP9+Ly6G+) through the release of CXCL5 and CXCL7, which bind granulocyte CXCR2 (169, 170). Importantly, granulocyte depletion alone is insufficient to block the formation of metastatic foci, underlining the indispensable contribution of platelets in this early step (169).
Another central pathway involves platelet C-type lectin-like receptor 2 (CLEC-2), which binds podoplanin on tumor cells. This interaction is crucial for vascular permeability changes and cancer cell dissemination: podoplanin-positive lung tumor cells fail to metastasize in Clec2-deficient mice, and CLEC-2 depletion abrogates pro-metastatic thrombus formation in vivo (157, 171).
Platelets also contribute to angiogenesis, another key feature of PMN establishment (127). Upon tumor-induced activation, they serve as major transporters of VEGF (159), which triggers the release of von Willebrand factor (vWF) and subsequent secretion of PDGF, TGF-β, and angiopoietin-1 (ANGPT-1). These mediators recruit bone marrow–derived endothelial progenitor cells that drive neovascularization (160).
Beyond angiogenesis, platelets remodel the TME and modulate the immune response. They recruit BMDCs and MDSCs, which suppress IFNγ-mediated responses through Glucocorticoid-induced Tumor Necrosis Factor Receptor Ligand (GITRL) and secrete pro-inflammatory cytokines (IL-6, IL-10, and TGF-β) that enhance inflammation and metastasis (161). Platelets also activate neutrophils to produce neutrophil extracellular traps (NETs) and subsequently undergo NETosis through TLR4 signaling in a P-selectin–dependent manner, thereby promoting platelet aggregation and endothelial activation (172). Activated platelet P-selectin further mediates the recruitment of immune cells (TAMs, monocytes, neutrophils) through binding to PSGL-1 (162). Interestingly, platelets protect tumor cells from immunosurveillance through direct or indirect inhibition of immune cell engagement (173). In breast cancer cell lines, the interaction between platelets and cancer stem cells induced platelet release of TGF-β, which inhibited NK cell activity (174). Additionally, platelet-derived MHC-I can be transferred to tumor cells, shielding them from NK cell–mediated killing and thereby promoting tumor growth (164).
Collectively, platelets are central orchestrators of premetastatic and metastatic niche formation, regulating angiogenesis, immune evasion, vascular remodeling, and ECM dynamics. While many pathways have been elucidated, further studies are required to fully define their mechanistic contributions and therapeutic potential in metastasis.
4.4.1 Platelet-derived EVs and their cargo in cancer metastasis
Several studies have attributed a critical role to PEVs in regulating various tumor hallmarks, including proliferation, resistance to cell death, invasion, metabolic reprogramming, immunity, and angiogenesis (Table 3) (180) (Figure 2). Recent studies have demonstrated that the transfer of CD41 from PEVs to tumor cells stimulates the activation of the MAPK-p42/44 and AKT signalling pathways, as well as the upregulation of MMPs necessary for ECM remodelling and invasion (155). PEVs have been shown to enhance tumor cell invasion by stimulating MMP-2 synthesis and secretion, as well as by promoting the transcription of MMP-9, VEGF, IL-8, and HGF mRNAs, factors closely associated with lung cancer metastasis (181). Furthermore, PEVs can transfer miR-223 to different target cells, supporting the metastatic progression (182). In particular, miR-223 promotes invasion and influences the expression of tumour suppressors, such as EPB41L3, in lung cancer cells, thus enhancing their metastatic capability (165). Critical immunoregulatory functions within the TME are achieved by activating neutrophils via GPIb–Mac-1 interactions and by promoting macrophage polarization toward an M2 phenotype (183). Furthermore, PEVs also exhibited the capacity to transfer miR-183 to NK cells, dampening their ability to kill cancer cells (184). Moreover, the transfer of CXCR4 by PEVs, a chemokine receptor that activates key signalling pathways, enhances tumor cell migration and supports the survival of CTCs in circulation (166).
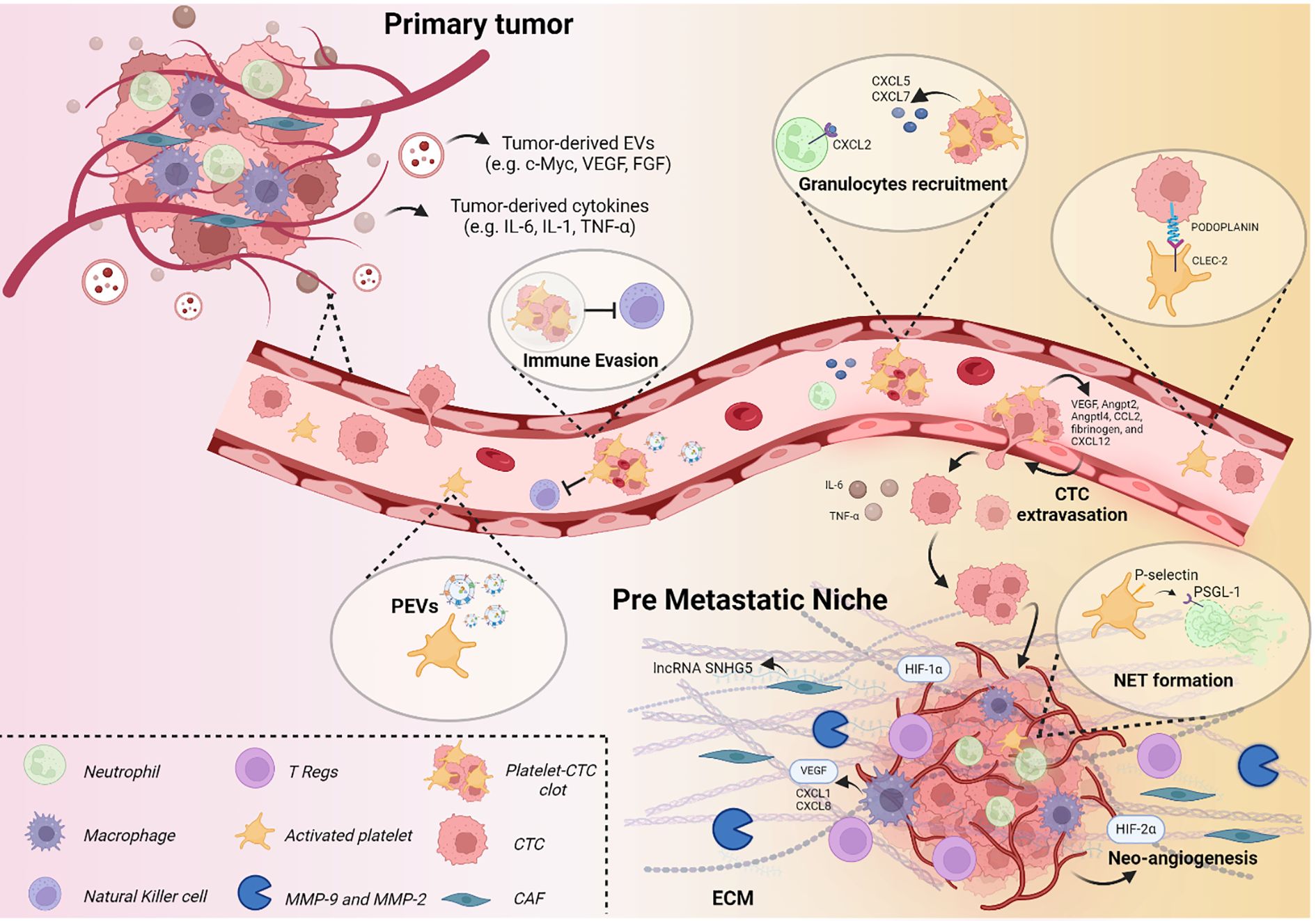
Figure 2. The role of platelets in PMN formation. Schematic representation of soluble factors and cellular composition contributing to PMN establishment, with particular focus on platelet involvement.
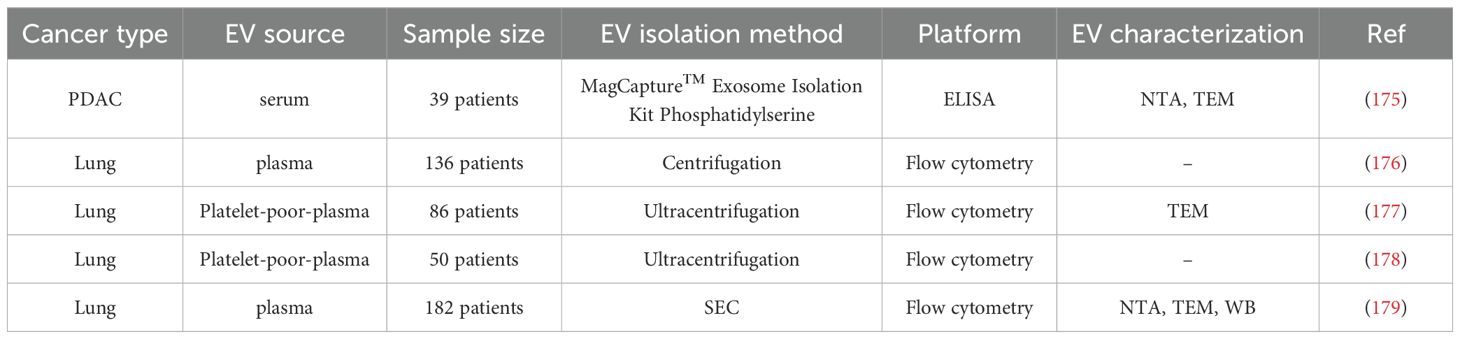
Besides the release of their cargo, the number of PEVs themselves has been shown to play a crucial role in determining the pro-tumorigenic effect (142). PEV concentrations in the blood of lung cancer patients are elevated compared with those of healthy controls. In a cohort of 136 NSCLC patients, PEV levels measured after three months of chemotherapy or targeted therapy were significantly higher in those with disease progression than in patients with controlled disease (176). In addition, Odaka et al. reported that serum levels of PEVs (CD41+-EVs and CD61+-EVs) were significantly higher in patients with pancreatic ductal adenocarcinoma (PDAC) than in healthy controls, supporting their potential as predictive biomarkers for PDAC (175). In vitro and in vivo studies by Zhao and colleagues demonstrated that the PKCα agonist PMA increased PKCα levels and enhanced PEV production, thereby promoting lung metastasis in nude mice. Conversely, treatment with the PKCα inhibitor GÖ6976 produced the opposite effect, highlighting PKCα as a key regulator of PEV release (185).
Collectively, these findings point out the pivotal role of platelets and PEVs in metastasis. Deeper investigation into their functions could inform the development of therapies aimed at disrupting platelet–tumor cell interactions. Moreover, given their small size and capacity to mediate intercellular communication, PEVs hold great promise as novel biomarkers and therapeutic targets in oncology.
5 Conclusions and future directions
Platelets and their EVs play a pivotal role in cancer progression and metastasis by modulating the TME, shaping immune responses, and promoting PMN formation.
Despite the growing body of literature highlighting the importance of platelets and their EVs in cancer progression, the lack of standardized procedures for EV isolation, purification, and characterization remains a major limitation that hampers cross-study comparability.
First, the heterogeneity of platelet activation stimuli, including thrombin, collagen, lipopolysaccharide, and calcium ionophore, affects not only the number and size of PEVs but also, more importantly, their molecular cargo. This variability complicates the interpretation of findings related to cancer metastasis. Moreover, the absence of universal reference materials and standardized protocols further undermines the accuracy and reliability of quantitative analyses.
A major shortcoming of many published studies is the lack of comprehensive in vivo validation, including dose–response assessments, biodistribution analyses, and half-life determinations of PEVs, which are essential to confirm their translational relevance. Indeed, most of the studies discussed in this review were conducted using EVs derived from cell lines or in vitro models, without clear in vivo confirmation of their functional importance in cancer progression.
To fully harness the potential of platelets and their EVs in biomedical research and therapeutic applications, these methodological and translational challenges must be addressed.
5.1 Future directions
To date, no clinical trials are underway to investigate P-EVs in cancer as diagnostic biomarkers or to explore new therapeutic strategies aimed at blocking PEV pro-tumorigenic effects (186). However, several ongoing clinical trials involving platelets focus on anti-aggregating drugs designed to prevent platelet activation, but their results remain inconclusive. For instance, randomized studies assessing aspirin for colorectal adenoma prevention (187–190) demonstrated some preventive effects, although the outcomes were not universally consistent. Likewise, some primary prevention studies have reported inconsistent results; for example, extended follow-up analyses showed that aspirin use was associated with a modest reduction in colorectal cancer incidence and mortality after twenty years (191). Because platelet activation is closely linked to EV release, it is reasonable to hypothesize that anti-aggregating drugs might also inhibit EV secretion into circulation. Further experimental validation of this mechanism could reveal new therapeutic applications for these agents as neoadjuvant treatments in oncology.
As discussed in this review, platelets hold significant potential as biomarkers for the detection and monitoring of various cancer types. However, several technical and methodological challenges, including isolation procedures, stability, storage, and detection, must be addressed before the use of platelets can be fully integrated into clinical practice. Future studies should aim to modulate platelet–tumor interactions to determine whether this approach can meaningfully influence cancer progression and serve as an effective therapeutic strategy, either alone or in combination with standard treatments. Moreover, well-designed clinical trials are essential to establish the safety, efficacy, and translational value of platelet-based strategies in oncology.
Author contributions
CL: Writing – original draft, Writing – review & editing. NF: Writing – original draft, Writing – review & editing. OF: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. RC: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by 5xmille funds for healthcare research (Italian Ministry of Health)”. RC was supported by Vetenskapsrådet Etableringsbidrag (Starting Grant from the Swedish Research Council) (Grant # 2023-02239), Assar Gabrielsson’s Foundation (Grant # FB23-01), the Serena Ehrenström foundation, the Ann-Lisa och Bror Björnssons Foundation, Wilhelm och Martina Lundgrens Vetenskapsfond (Grant # 2023-SA-4142), and Magnus Bergvalls Stiftelse (Grant # 2024-1009). This work was funded by Italian Ministry of Health - “Ricerca Corrente” funds.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. RC has developed multiple EV-associated patents for putative clinical utilization. RC owns equity in Exocure Bioscience Inc.
The authors RC and OF declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We declare that the text was created by the authors and subsequently reviewed using AI tools like Grammarly for linguistic guidance.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gerstberger S, Jiang Q, and Ganesh K. Metastasis. Cell. (2023) 186:1564–79. doi: 10.1016/j.cell.2023.03.003
2. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. (2006) 12:895–904. doi: 10.1038/nm1469
4. Gonzalez H, Hagerling C, and Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. (2018) 32:1267–84. doi: 10.1101/gad.314617.118
5. Finnigan D, Hajjaj OI, and Othman M. Red blood cell changes due to cancer and cancer treatments: a narrative review. Curr Opin Hematol. (2025) 32:93–103. doi: 10.1097/MOH.0000000000000859
6. Nash GF, Turner LF, Scully MF, and Kakkar AK. Platelets and cancer. Lancet Oncol. (2002) 3:425–30. doi: 10.1016/S1470-2045(02)00789-1
7. Nieuwland R and Siljander PR. A beginner’s guide to study extracellular vesicles in human blood plasma and serum. J Extracell Vesicles. (2024) 13:e12400. doi: 10.1002/jev2.12400
8. Alberro A, Iparraguirre L, Fernandes A, and Otaegui D. Extracellular vesicles in blood: sources, effects, and applications. Int J Mol Sci. (2021) 22(15):8163. doi: 10.3390/ijms22158163
9. Li S, Lu Z, Wu S, Chu T, Li B, Qi F, et al. The dynamic role of platelets in cancer progression and their therapeutic implications. Nat Rev Cancer. (2024) 24:72–87. doi: 10.1038/s41568-023-00639-6
10. Tuzovic M, Herrmann J, Iliescu C, Marmagkiolis K, Ziaeian B, and Yang EH. Arterial thrombosis in patients with cancer. Curr Treat Options Cardiovasc Med. (2018) 20:40. doi: 10.1007/s11936-018-0635-x
11. Sorensen HT, Pedersen L, van Es N, Buller HR, and Horvath-Puho E. Impact of venous thromboembolism on the mortality in patients with cancer: a population-based cohort study. Lancet Reg Health Eur. (2023) 34:100739. doi: 10.1016/j.lanepe.2023.100739
12. Casagrande N, Borghese C, Agostini F, Durante C, Mazzucato M, Colombatti A, et al. In ovarian cancer multicellular spheroids, platelet releasate promotes growth, expansion of ALDH+ and CD133+ Cancer stem cells, and protection against the cytotoxic effects of cisplatin, carboplatin and paclitaxel. Int J Mol Sci. (2021) 22(6):3019. doi: 10.3390/ijms22063019
13. Cacic D, Reikvam H, Nordgård O, Meyer P, and Hervig T. Platelet microparticles protect acute myelogenous leukemia cells against daunorubicin-induced apoptosis . Cancers. (2021) 13(8):1870. doi: 10.3390/cancers13081870
14. Bottsford-Miller J, Choi HJ, Dalton HJ, Stone RL, Cho MS, Haemmerle M, et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer . Clin Cancer Res. (2015) 21(3):602–10. doi: 10.1158/1078-0432.CCR-14-0870
15. Yeung KT and Yang J. Epithelial-mesenchymal transition in tumor metastasis . Mol Oncol. (2017) 11(1):28–39. doi: 10.1002/1878-0261.12017
16. Dongre A and Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer . Nat Rev Mol Cell Biol. (2019) 20(2):69–84. doi: 10.1038/s41580-018-0080-4
17. Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. (1998) 153:865–73. doi: 10.1016/S0002-9440(10)65628-3
18. Hamza B, Miller AB, Meier L, Stockslager M, Ng SR, King EM, et al. Measuring kinetics and metastatic propensity of CTCs by blood exchange between mice. Nat Commun. (2021) 12:5680. doi: 10.1038/s41467-021-25917-5
19. Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. (2019) 573:439–44. doi: 10.1038/s41586-019-1526-3
20. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566:553–7. doi: 10.1038/s41586-019-0915-y
21. Quail DF and Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. (2013) 19:1423–37. doi: 10.1038/nm.3394
22. de Visser KE and Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. (2023) 41:374–403. doi: 10.1016/j.ccell.2023.02.016
23. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8:98–101.
24. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. (2017) 17:302–17. doi: 10.1038/nrc.2017.6
25. Goddard ET, Bozic I, Riddell SR, and Ghajar CM. Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol. (2018) 20:1240–9. doi: 10.1038/s41556-018-0214-0
26. Endo H, Okami J, Okuyama H, Nishizawa Y, Imamura F, and Inoue M. The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene. (2017) 36:2824–34. doi: 10.1038/onc.2016.431
27. Fluegen G, Avivar-Valderas A, Wang Y, Padgen MR, Williams JK, Nobre AR, et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. (2017) 19:120–32. doi: 10.1038/ncb3465
28. Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. (2015) 7:308re8. doi: 10.1126/scitranslmed.aad3740
29. Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. (2015) 527:186–91. doi: 10.1038/nature15726
30. Elkholi IE, Lalonde A, Park M, and Cote JF. Breast cancer metastatic dormancy and relapse: an enigma of microenvironment(s). Cancer Res. (2022) 82:4497–510. doi: 10.1158/0008-5472.CAN-22-1902
31. Owen KL, Gearing LJ, Zanker DJ, Brockwell NK, Khoo WH, Roden DL, et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. (2020) 21:e50162. doi: 10.15252/embr.202050162
32. Friberg S and Nystrom A. Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis. (2015) 8:43–9. doi: 10.4137/CGM.S31244
33. Barkan D and Chambers AF. beta1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res. (2011) 17:7219–23. doi: 10.1158/1078-0432.CCR-11-0642
34. Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. (2008) 68:6241–50. doi: 10.1158/0008-5472.CAN-07-6849
35. Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. (2016) 166:47–62. doi: 10.1016/j.cell.2016.06.009
36. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. (2016) 165:45–60. doi: 10.1016/j.cell.2016.02.025
37. Li Y, Li M, Su K, Zong S, Zhang H, and Xiong L. Pre-metastatic niche: from revealing the molecular and cellular mechanisms to the clinical applications in breast cancer metastasis. Theranostics. (2023) 13:2301–18. doi: 10.7150/thno.82700
38. Akhtar M, Haider A, Rashid S, and Al-Nabet A. Paget’s “Seed and soil” Theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol. (2019) 26:69–74. doi: 10.1097/PAP.0000000000000219
39. Agrawal A, Javanmardi Y, Watson SA, Serwinski B, Djordjevic B, Li W, et al. Mechanical signatures in cancer metastasis. NPJ Biol Phys Mech. (2025) 2:3. doi: 10.1038/s44341-024-00007-x
40. Xie H, Sun Q, Chu X, Zhu S, and Xie F. Review of pre-metastatic niches in lung metastasis: From cells to molecules, from mechanism to clinics. Biochim Biophys Acta Rev Cancer. (2024) 1879:189081. doi: 10.1016/j.bbcan.2024.189081
41. Wang Y, Jia J, Wang F, Fang Y, Yang Y, Zhou Q, et al. Pre-metastatic niche: formation, characteristics and therapeutic implication. Signal Transduct Target Ther. (2024) 9:236. doi: 10.1038/s41392-024-01937-7
42. Liu Y and Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
43. Li Y, Zheng Y, Tan X, Du Y, Wei Y, and Liu S. Extracellular vesicle-mediated pre-metastatic niche formation via altering host microenvironments. Front Immunol. (2024) 15:1367373. doi: 10.3389/fimmu.2024.1367373
44. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. (2018) 9:5395. doi: 10.1038/s41467-018-07810-w
45. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. (2005) 438:820–7. doi: 10.1038/nature04186
46. Zeng H, Hou Y, Zhou X, Lang L, Luo H, Sun Y, et al. Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics. (2022) 12:7351–70. doi: 10.7150/thno.74753
47. Dong G, Chen P, Xu Y, Liu T, and Yin R. Cancer-associated fibroblasts: Key criminals of tumor pre-metastatic niche. Cancer Lett. (2023) 566:216234. doi: 10.1016/j.canlet.2023.216234
48. Dong Q, Liu X, Cheng K, Sheng J, Kong J, and Liu T. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front Cell Dev Biol. (2021) 9:733627. doi: 10.3389/fcell.2021.733627
49. Wang D, Sun H, Wei J, Cen B, and DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. (2017) 77:3655–65. doi: 10.1158/0008-5472.CAN-16-3199
50. Muz B, de la Puente P, Azab F, and Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). (2015) 3:83–92. doi: 10.2147/HP.S93413
51. Schito L and Rey-Keim S. Hypoxia signaling and metastatic progression. Semin Cancer Biol. (2023) 97:42–9. doi: 10.1016/j.semcancer.2023.11.001
52. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, and Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. (2020) 11:5120. doi: 10.1038/s41467-020-18794-x
53. Patras L, Paul D, and Matei IR. Weaving the nest: extracellular matrix roles in pre-metastatic niche formation. Front Oncol. (2023) 13:1163786. doi: 10.3389/fonc.2023.1163786
54. Heneberg P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit Rev Oncol Hematol. (2016) 97:303–11. doi: 10.1016/j.critrevonc.2015.09.008
55. Fang M, Yuan J, Peng C, and Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. (2014) 35:2871–82. doi: 10.1007/s13277-013-1511-7
56. Sun Z, Guo SS, and Fassler R. Integrin-mediated mechanotransduction. J Cell Biol. (2016) 215:445–56. doi: 10.1083/jcb.201609037
57. Shay G, Lynch CC, and Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. (2015) 44-46:200–6. doi: 10.1016/j.matbio.2015.01.019
58. Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. (2023) 22:48. doi: 10.1186/s12943-023-01744-8
59. Li R, Mukherjee MB, and Lin J. Coordinated regulation of myeloid-derived suppressor cells by cytokines and chemokines. Cancers (Basel). (2022) 14(5):1236. doi: 10.3390/cancers14051236
60. Li BH, Garstka MA, and Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. (2020) 117:201–15. doi: 10.1016/j.molimm.2019.11.014
61. Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. (2012) 18:883–91. doi: 10.1038/nm.2753
62. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. (2021) 33:2040–58.e10. doi: 10.1016/j.cmet.2021.09.002
63. Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. (2016) 16:35–50. doi: 10.1038/nri.2015.8
64. de la Puente P, Muz B, Azab F, and Azab AK. Cell trafficking of endothelial progenitor cells in tumor progression. Clin Cancer Res. (2013) 19:3360–8. doi: 10.1158/1078-0432.CCR-13-0462
65. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis . Nat Rev Cancer. (2019) 19(1):9–31. doi: 10.1038/s41568-018-0081-9
66. Zullo L, Filippiadis D, Hendriks LEL, Portik D, Spicer JD, Wistuba II, et al. Lung metastases. Nat Rev Dis Primers. (2025) 11:60. doi: 10.1038/s41572-025-00642-1
67. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. (2010) 28:3271–7. doi: 10.1200/JCO.2009.25.9820
68. Kurma K and Alix-Panabieres C. Mechanobiology and survival strategies of circulating tumor cells: a process towards the invasive and metastatic phenotype. Front Cell Dev Biol. (2023) 11:1188499. doi: 10.3389/fcell.2023.1188499
69. Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. (2022) 3:90–107. doi: 10.1038/s43018-021-00291-9
70. Gong Z, Li Q, Shi J, Wei J, Li P, Chang CH, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. (2022) 55:1483–1500.e9. doi: 10.1016/j.immuni.2022.07.001
71. Yofe I, Shami T, Cohen N, Landsberger T, Sheban F, Stoler-Barak L, et al. Spatial and temporal mapping of breast cancer lung metastases identify TREM2 macrophages as regulators of the metastatic boundary. Cancer Discov. (2023) 13:2610–31. doi: 10.1158/2159-8290.CD-23-0299
72. Hongu T, Pein M, Insua-Rodriguez J, Gutjahr E, Mattavelli G, Meier J, et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat Cancer. (2022) 3:486–504. doi: 10.1038/s43018-022-00353-6
73. Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. (2013) 15:807–17. doi: 10.1038/ncb2767
74. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. (2018) 361(6409):eaao4227. doi: 10.1126/science.aao4227
75. Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12404
76. van Niel G, D’Angelo G, and Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
77. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, and Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. (2022) 23:369–82. doi: 10.1038/s41580-022-00460-3
78. Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. (2017) 36:3012–28. doi: 10.15252/embj.201696003
79. Rausch L, Flaskamp L, Ashokkumar A, Trefzer A, Ried C, Buchholz VR, et al. Phosphatidylserine-positive extracellular vesicles boost effector CD8(+) T cell responses during viral infection. Proc Natl Acad Sci U.S.A. (2023) 120:e2210047120. doi: 10.1073/pnas.2210047120
80. Mulcahy LA, Pink RC, and Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. (2014) 3. doi: 10.3402/jev.v3.24641
81. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. (2016) 126:1208–15. doi: 10.1172/JCI81135
82. Zhang C, Qin C, Dewanjee S, Bhattacharya H, Chakraborty P, Jha NK, et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Mol Cancer. (2024) 23:18. doi: 10.1186/s12943-024-01932-0
83. Han T, Hao Q, Chao T, Sun Q, Chen Y, Gao B, et al. Extracellular vesicles in cancer: golden goose or Trojan horse. J Mol Cell Biol. (2024) 16(5):mjae025. doi: 10.1093/jmcb/mjae025
84. Zhang X, Liu D, Gao Y, Lin C, An Q, Feng Y, et al. The biology and function of extracellular vesicles in cancer development. Front Cell Dev Biol. (2021) 9:777441. doi: 10.3389/fcell.2021.777441
85. Lopez K, Lai SWT, Lopez Gonzalez EJ, Davila RG, and Shuck SC. Extracellular vesicles: A dive into their role in the tumor microenvironment and cancer progression. Front Cell Dev Biol. (2023) 11:1154576. doi: 10.3389/fcell.2023.1154576
86. Jurj A, Paul D, and Calin GA. Extracellular Vesicles in cancer: from isolation and characterization to metastasis, drug resistance, and clinical applications. BMC Cancer. (2025) 25:1154. doi: 10.1186/s12885-025-14375-7
87. Bao Q, Huang Q, Chen Y, Wang Q, Sang R, Wang L, et al. Tumor-derived extracellular vesicles regulate cancer progression in the tumor microenvironment. Front Mol Biosci. (2021) 8:796385. doi: 10.3389/fmolb.2021.796385
88. Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. (2017) 36:4929–42. doi: 10.1038/onc.2017.105
89. Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. (2015) 34:290–302. doi: 10.1038/onc.2013.560
90. Ringuette Goulet C, Bernard G, Tremblay S, Chabaud S, Bolduc S, and Pouliot F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFbeta Signaling. Mol Cancer Res. (2018) 16:1196–204. doi: 10.1158/1541-7786.MCR-17-0784
91. Marar C, Starich B, and Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. (2021) 22:560–70. doi: 10.1038/s41590-021-00899-0
92. Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. (2009) 124:2621–33. doi: 10.1002/ijc.24249
93. Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, et al. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J Immunol. (2017) 199:1505–15. doi: 10.4049/jimmunol.1700167
94. Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. (2022) 41:296. doi: 10.1186/s13046-022-02499-8
95. Roweth HG and Battinelli EM. Lessons to learn from tumor-educated platelets. Blood. (2021) 137:3174–80. doi: 10.1182/blood.2019003976
96. Ribatti D and Crivellato E. Giulio Bizzozero and the discovery of platelets. Leuk Res. (2007) 31:1339–41. doi: 10.1016/j.leukres.2007.02.008
97. Garraud O and Cognasse F. Are platelets cells? And if yes, are they immune cells? Front Immunol. (2015) 6:70. doi: 10.3389/fimmu.2015.00070
98. Palacios-Acedo AL, Mege D, Crescence L, Dignat-George F, Dubois C, and Panicot-Dubois L. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol. (2019) 10:1805. doi: 10.3389/fimmu.2019.01805
99. Livada AC, Pariser DN, and Morrell CN. Megakaryocytes in the lung: History and future perspectives. Res Pract Thromb Haemost. (2023) 7:100053. doi: 10.1016/j.rpth.2023.100053
100. Rowley JW and Weyrich AS. Coordinate expression of transcripts and proteins in platelets. Blood. (2013) 121:5255–6. doi: 10.1182/blood-2013-03-487991
101. Scridon A. Platelets and their role in hemostasis and thrombosis-from physiology to pathophysiology and therapeutic implications. Int J Mol Sci. (2022) 23(21):12772. doi: 10.3390/ijms232112772
102. Contursi A, Grande R, Dovizio M, Bruno A, Fullone R, and Patrignani P. Platelets in cancer development and diagnosis. Biochem Soc Trans. (2018) 46:1517–27. doi: 10.1042/BST20180159
103. Yadav S and Storrie B. The cellular basis of platelet secretion: Emerging structure/function relationships. Platelets. (2017) 28:108–18. doi: 10.1080/09537104.2016.1257786
104. Hill CN, Hernandez-Caceres MP, Asencio C, Torres B, Solis B, and Owen GI. Deciphering the role of the coagulation cascade and autophagy in cancer-related thrombosis and metastasis. Front Oncol. (2020) 10:605314. doi: 10.3389/fonc.2020.605314
105. Mackman N, Tilley RE, and Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. (2007) 27:1687–93. doi: 10.1161/ATVBAHA.107.141911
106. Grover SP and Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. (2019) 39:331–8. doi: 10.1161/ATVBAHA.118.312130
107. Wang Y, Luan J, Luo K, Fan J, and Zhu T. Model reduction of coagulation cascade based on genetic algorithm. Int J Numer Method BioMed Eng. (2022) 38:e3652. doi: 10.1002/cnm.3652
108. Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. (2009) 119:1871–9. doi: 10.1172/JCI38575
109. Ho-Tin-Noe B, Boulaftali Y, and Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. (2018) 131:277–88. doi: 10.1182/blood-2017-06-742676
110. Best MG, Wesseling P, and Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res. (2018) 78:3407–12. doi: 10.1158/0008-5472.CAN-18-0887
111. Etulain J. Platelets in wound healing and regenerative medicine. Platelets. (2018) 29:556–68. doi: 10.1080/09537104.2018.1430357
112. Gay LJ and Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
113. Zhou L, Zhang Z, Tian Y, Li Z, Liu Z, and Zhu S. The critical role of platelet in cancer progression and metastasis. Eur J Med Res. (2023) 28:385. doi: 10.1186/s40001-023-01342-w
114. Kopp HG, Placke T, and Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. (2009) 69:7775–83. doi: 10.1158/0008-5472.CAN-09-2123
115. Gupta GP and Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest. (2004) 114:1691–3. doi: 10.1172/JCI200423823
116. Labelle M, Begum S, and Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. (2011) 20:576–90. doi: 10.1016/j.ccr.2011.09.009
117. Bian X, Yin S, Yang S, Jiang X, Wang J, Zhang M, et al. Roles of platelets in tumor invasion and metastasis: A review. Heliyon. (2022) 8:e12072. doi: 10.1016/j.heliyon.2022.e12072
118. Contursi A, Schiavone S, Dovizio M, Hinz C, Fullone R, Tacconelli S, et al. Platelets induce free and phospholipid-esterified 12-hydroxyeicosatetraenoic acid generation in colon cancer cells by delivering 12-lipoxygenase. J Lipid Res. (2021) 62:100109. doi: 10.1016/j.jlr.2021.100109
119. Li X, Li M, Hu Z, Zhou L, Zheng M, Jiao D, et al. Tumor-infiltrating platelets promote the growth of lung adenocarcinoma. Transl Oncol. (2024) 39:101813. doi: 10.1016/j.tranon.2023.101813
120. Feng W, Madajka M, Kerr BA, Mahabeleshwar GH, Whiteheart SW, and Byzova TV. A novel role for platelet secretion in angiogenesis: mediating bone marrow-derived cell mobilization and homing. Blood. (2011) 117:3893–902. doi: 10.1182/blood-2010-08-304808
121. Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, et al. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. (2015) 28:666–76. doi: 10.1016/j.ccell.2015.09.018
122. Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. (2019) 5:5. doi: 10.1038/s41572-018-0055-y
123. Bhuniya A, Sarkar A, Guha A, Choudhury PR, Bera S, Sultana J, et al. Tumor activated platelets induce vascular mimicry in mesenchymal stem cells and aid metastasis. Cytokine. (2022) 158:155998. doi: 10.1016/j.cyto.2022.155998
124. Tong H, Li K, Zhou M, Wu R, Yang H, Peng Z, et al. Coculture of cancer cells with platelets increases their survival and metastasis by activating the TGFbeta/Smad/PAI-1 and PI3K/AKT pathways. Int J Biol Sci. (2023) 19:4259–77. doi: 10.7150/ijbs.85986
125. Best MG, Sol N, In ‘t Veld S, Vancura A, Muller M, Niemeijer AN, et al. Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell. (2017) 32:238–52.e9. doi: 10.1016/j.ccell.2017.07.004
126. Eslami SZ, Cortes-Hernandez LE, Glogovitis I, Antunes-Ferreira M, D’Ambrosi S, Kurma K, et al. In vitro cross-talk between metastasis-competent circulating tumor cells and platelets in colon cancer: a Malicious association during the harsh journey in the blood. Front Cell Dev Biol. (2023) 11:1209846. doi: 10.3389/fcell.2023.1209846
127. Gkolfinopoulos S, Jones RL, and Constantinidou A. The emerging role of platelets in the formation of the micrometastatic niche: current evidence and future perspectives. Front Oncol. (2020) 10:374. doi: 10.3389/fonc.2020.00374
128. Nieswandt B, Hafner M, Echtenacher B, and Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. (1999) 59:1295–300.
129. Lazar S, Wurtzel JGT, Chen X, Ma P, and Goldfinger LE. High-efficiency unassisted transfection of platelets with naked double-stranded miRNAs modulates signal-activated translation and platelet function. Platelets. (2021) 32:794–806. doi: 10.1080/09537104.2020.1809642
130. Zhang SR, Yao L, Wang WQ, Xu JZ, Xu HX, Jin W, et al. Tumor-infiltrating platelets predict postsurgical survival in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. (2018) 25:3984–93. doi: 10.1245/s10434-018-6727-8
131. Miao Y, Xu Z, Feng W, Zheng M, Xu Z, Gao H, et al. Platelet infiltration predicts survival in postsurgical colorectal cancer patients. Int J Cancer. (2022) 150:509–20. doi: 10.1002/ijc.33816
132. Liao K, Zhang X, Liu J, Teng F, He Y, Cheng J, et al. The role of platelets in the regulation of tumor growth and metastasis: the mechanisms and targeted therapy. MedComm (2020). (2023) 4:e350. doi: 10.1002/mco2.350
133. Ding S, Dong X, and Song X. Tumor educated platelet: the novel BioSource for cancer detection. Cancer Cell Int. (2023) 23:91. doi: 10.1186/s12935-023-02927-5
134. Calverley DC, Phang TL, Choudhury QG, Gao B, Oton AB, Weyant MJ, et al. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci. (2010) 3:227–32. doi: 10.1111/j.1752-8062.2010.00226.x
135. Ilvonen P, Pusa R, Harkonen K, Laitinen S, and Impola U. Distinct targeting and uptake of platelet and red blood cell-derived extracellular vesicles into immune cells. J Extracell Biol. (2024) 3:e130. doi: 10.1002/jex2.130
136. Lazar S and Goldfinger LE. Platelet microparticles and miRNA transfer in cancer progression: many targets, modes of action, and effects across cancer stages. Front Cardiovasc Med. (2018) 5:13. doi: 10.3389/fcvm.2018.00013
137. Zmigrodzka M, Witkowska-Pilaszewicz O, and Winnicka A. Platelets extracellular vesicles as regulators of cancer progression-an updated perspective. Int J Mol Sci. (2020) 21(15):5195. doi: 10.3390/ijms21155195
138. Asgari A, Lesyk G, Poitras E, Govindasamy N, Terry K, To R, et al. Platelets stimulate programmed death-ligand 1 expression by cancer cells: Inhibition by anti-platelet drugs. J Thromb Haemost. (2021) 19:2862–72. doi: 10.1111/jth.15478
139. Cai C, Liu Y, Lu R, Fan X, Zeng S, and Gan P. Platelets in cancer and immunotherapy: functional dynamics and therapeutic opportunities. Exp Hematol Oncol. (2025) 14:83. doi: 10.1186/s40164-025-00676-x
140. Zhang Q, Song X, and Song X. Contents in tumor-educated platelets as the novel biosource for cancer diagnostics. Front Oncol. (2023) 13:1165600. doi: 10.3389/fonc.2023.1165600
141. Gottardo A, Gristina V, Perez A, Di Giovanni E, Contino S, Barraco N, et al. Roles of Tumor-Educated Platelets (TEPs) in the biology of Non-Small Cell Lung Cancer (NSCLC): A systematic review. “Re-discovering the neglected biosources of the liquid biopsy family. J Liq Biopsy. (2024) 3:100136. doi: 10.1016/j.jlb.2024.100136
142. Chaudhary PK, Kim S, and Kim S. Shedding light on the cell biology of platelet-derived extracellular vesicles and their biomedical applications. Life (Basel). (2023) 13. doi: 10.3390/life13061403
143. Muttiah B, Ng SL, Lokanathan Y, Ng MH, and Law JX. Beyond blood clotting: the many roles of platelet-derived extracellular vesicles. Biomedicines. (2024) 12(8):1850. doi: 10.3390/biomedicines12081850
144. Tang M, Jiang L, Lin Y, Wu X, Wang K, He Q, et al. Platelet microparticle-mediated transfer of miR-939 to epithelial ovarian cancer cells promotes epithelial to mesenchymal transition. Oncotarget. (2017) 8:97464–75. doi: 10.18632/oncotarget.22136
145. Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. (2013) 122:253–61. doi: 10.1182/blood-2013-03-492801
146. Gottardo A, Tulone G, Pavan N, Fulfaro F, Gristina V, Bazan Russo TD, et al. Applications of platelet concentrates (PCs) in regenerative onco-urology: A systematic review of literature. Int J Mol Sci. (2024) 25(19):10683. doi: 10.3390/ijms251910683
147. Bordin A, Chirivi M, Pagano F, Milan M, Iuliano M, Scaccia E, et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. (2022) 55:e13312. doi: 10.1111/cpr.13312
148. Anene C, Graham AM, Boyne J, and Roberts W. Platelet microparticle delivered microRNA-Let-7a promotes the angiogenic switch. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:2633–43. doi: 10.1016/j.bbadis.2018.04.013
149. Pontis F, Roz L, Mensah M, Segale M, Moro M, Bertolini G, et al. Circulating extracellular vesicles from individuals at high-risk of lung cancer induce pro-tumorigenic conversion of stromal cells through transfer of miR-126 and miR-320. J Exp Clin Cancer Res. (2021) 40:237. doi: 10.1186/s13046-021-02040-3
150. He Y, Jiang Y, Wu F, Zhang X, Liang S, and Ye Z. Platelet microparticle-derived miR-320b inhibits hypertension with atherosclerosis development by targeting ETFA. Int Heart J. (2024) 65:329–38. doi: 10.1536/ihj.23-365
151. Xin X and Koenen RR. Assessing platelet-derived extracellular vesicles for potential as therapeutic targets in cardiovascular diseases. Expert Opin Ther Targets. (2025) 29:17–28. doi: 10.1080/14728222.2025.2454617
152. Contursi A, Fullone R, Szklanna-Koszalinska P, Marcone S, Lanuti P, Taus F, et al. Tumor-educated platelet extracellular vesicles: proteomic profiling and crosstalk with colorectal cancer cells. Cancers (Basel). (2023) 15(2):350. doi: 10.3390/cancers15020350
153. Dashevsky O, Varon D, and Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer. (2009) 124:1773–7. doi: 10.1002/ijc.24016
154. Kassassir H, Papiewska-Pajak I, Kryczka J, Boncela J, and Kowalska MA. Platelet-derived microparticles stimulate the invasiveness of colorectal cancer cells via the p38MAPK-MMP-2/MMP-9 axis. Cell Commun Signal. (2023) 21:51. doi: 10.1186/s12964-023-01066-8
155. Lou C and Cai X. The emerging roles of platelet-derived extracellular vesicles in disease. Ann Med. (2025) 57:2499029. doi: 10.1080/07853890.2025.2499029
156. Foss A, Munoz-Sagredo L, Sleeman J, and Thiele W. The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin Exp Metastasis. (2020) 37:47–67. doi: 10.1007/s10585-019-10009-y
157. Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. (2017) 15:513–25. doi: 10.1111/jth.13604
158. Gil-Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. (2012) 119:3164–75. doi: 10.1182/blood-2011-08-376426
159. Sabrkhany S, Griffioen AW, and Oude Egbrink MG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. (2011) 1815:189–96. doi: 10.1016/j.bbcan.2010.12.001
160. Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, et al. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. (2006) 98:e2–10. doi: 10.1161/01.RES.0000201285.87524.9e
161. Han X, Song X, Xiao Z, Zhu G, Gao R, Ni B, et al. Study on the mechanism of MDSC-platelets and their role in the breast cancer microenvironment. Front Cell Dev Biol. (2024) 12:1310442. doi: 10.3389/fcell.2024.1310442
162. Raskov H, Orhan A, Agerbaek MO, and Gogenur I. The impact of platelets on the metastatic potential of tumour cells. Heliyon. (2024) 10:e34361. doi: 10.1016/j.heliyon.2024.e34361
163. Asghar S, Parvaiz F, and Manzoor S. Multifaceted role of cancer educated platelets in survival of cancer cells. Thromb Res. (2019) 177:42–50. doi: 10.1016/j.thromres.2019.02.026
164. Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. (2012) 72:440–8. doi: 10.1158/0008-5472.CAN-11-1872
165. Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, et al. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. (2015) 14:58. doi: 10.1186/s12943-015-0327-z
166. Lazar S and Goldfinger LE. Platelets and extracellular vesicles and their cross talk with cancer. Blood. (2021) 137:3192–200. doi: 10.1182/blood.2019004119
167. Zmigrodzka M, Guzera M, Miskiewicz A, Jagielski D, and Winnicka A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biol. (2016) 37:14391–401. doi: 10.1007/s13277-016-5358-6
168. Chen Y, Zhou J, Liu Z, Wu T, Li S, Zhang Y, et al. Tumor cell-induced platelet aggregation accelerates hematogenous metastasis of Malignant melanoma by triggering macrophage recruitment. J Exp Clin Cancer Res. (2023) 42:277. doi: 10.1186/s13046-023-02856-1
169. Labelle M, Begum S, and Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U.S.A. (2014) 111:E3053–61. doi: 10.1073/pnas.1411082111
170. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. (2018) 11:125. doi: 10.1186/s13045-018-0669-2
171. Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. (2019) 134:1912–8. doi: 10.1182/blood.2019001388
172. Lucotti S and Muschel RJ. Platelets and metastasis: new implications of an old interplay. Front Oncol. (2020) 10:1350. doi: 10.3389/fonc.2020.01350
173. Schmied L, Hoglund P, and Meinke S. Platelet-mediated protection of cancer cells from immune surveillance - possible implications for cancer immunotherapy. Front Immunol. (2021) 12:640578. doi: 10.3389/fimmu.2021.640578
174. Zuo XX, Yang Y, Zhang Y, Zhang ZG, Wang XF, and Shi YG. Platelets promote breast cancer cell MCF-7 metastasis by direct interaction: surface integrin alpha2beta1-contacting-mediated activation of Wnt-beta-catenin pathway. Cell Commun Signal. (2019) 17:142. doi: 10.1186/s12964-019-0464-x
175. Odaka H, Hiemori K, Shimoda A, Akiyoshi K, and Tateno H. CD63-positive extracellular vesicles are potential diagnostic biomarkers of pancreatic ductal adenocarcinoma. BMC Gastroenterol. (2022) 22:153. doi: 10.1186/s12876-022-02228-7
176. Tasso R, Marconi S, Rossi G, Genova C, and Coco S. Platelets and their derived extracellular vesicles: The new generation of markers in non-small cell lung cancer management. Drug Discov Today. (2023) 28:103616. doi: 10.1016/j.drudis.2023.103616
177. Liu T, Wang J, Li T, Cui P, Hou B, Zhuang C, et al. Predicting disease progression in advanced non-small cell lung cancer with circulating neutrophil-derived and platelet-derived microparticles. BMC Cancer. (2021) 21:939. doi: 10.1186/s12885-021-08628-4
178. Liu T, Wang J, Liu Y, Wu J, Yuan Y, Wang C, et al. Prediction of the therapeutic effects of pembrolizumab and nivolumab in advanced non-small cell lung cancer by platelet-derived microparticles in circulating blood. Technol Cancer Res Treat. (2021) 20:1533033821997817. doi: 10.1177/1533033821997817
179. Genova C, Tasso R, Rosa A, Rossi G, Reverberi D, Fontana V, et al. Prognostic role of soluble and extracellular vesicle-associated PD-L1, B7-H3 and B7-H4 in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cells. (2023) 12(6):832. doi: 10.3390/cells12060832
180. Zhuang T, Wang S, Yu X, He X, Guo H, and Ou C. Current status and future perspectives of platelet-derived extracellular vesicles in cancer diagnosis and treatment. biomark Res. (2024) 12:88. doi: 10.1186/s40364-024-00639-0
181. Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. (2005) 113:752–60. doi: 10.1002/ijc.20657
182. Chai B, Guo Y, Cui X, Liu J, Suo Y, Dou Z, et al. MiR-223-3p promotes the proliferation, invasion and migration of colon cancer cells by negative regulating PRDM1. Am J Transl Res. (2019) 11:4516–23.
183. Salanova B, Choi M, Rolle S, Wellner M, Luft FC, and Kettritz R. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J Biol Chem. (2007) 282:27960–9. doi: 10.1074/jbc.M704039200
184. Sadallah S, Schmied L, Eken C, Charoudeh HN, Amicarella F, and Schifferli JA. Platelet-derived ectosomes reduce NK cell function. J Immunol. (2016) 197:1663–71. doi: 10.4049/jimmunol.1502658
185. Zhao J, Tian H, Zhao X, Lan L, Liu H, Sun Y, et al. PKCalpha induced the generation of extracellular vesicles in activated platelets to promote breast cancer metastasis. Int J Biol Sci. (2024) 20:3956–71. doi: 10.7150/ijbs.89822
186. Fan Z, Gan Y, and Hu Y. The potential utilization of platelet-derived extracellular vesicles in clinical treatment. Platelets. (2024) 35:2397592. doi: 10.1080/09537104.2024.2397592
187. Benamouzig R, Uzzan B, Deyra J, Martin A, Girard B, Little J, et al. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. (2012) 61:255–61. doi: 10.1136/gutjnl-2011-300113
188. Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. (2003) 348:891–9. doi: 10.1056/NEJMoa021735
189. Wojtukiewicz MZ, Hempel D, Sierko E, Tucker SC, and Honn KV. Antiplatelet agents for cancer treatment: a real perspective or just an echo from the past? Cancer Metastasis Rev. (2017) 36:305–29. doi: 10.1007/s10555-017-9683-z
190. Johnson J, Law SQK, Shojaee M, Hall AS, Bhuiyan S, Lim MBL, et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. (2023) 12:e12332. doi: 10.1002/jev2.12332
Keywords: lung cancer, extracellular vesicle (EV), platelet, metastasis, microRNA
Citation: Locatelli C, Ferrario N, Fortunato O, Ghidotti P and Crescitelli R (2025) Platelets and platelet-derived extracellular vesicles: their role in lung cancer dissemination and premetastatic niche formation. Front. Immunol. 16:1703974. doi: 10.3389/fimmu.2025.1703974
Received: 12 September 2025; Accepted: 11 November 2025; Revised: 08 November 2025;
Published: 26 November 2025.
Edited by:
Adit Ben-Baruch, Tel Aviv University, IsraelReviewed by:
Saptak Banerjee, Chittaranjan National Cancer Institute (CNCI), IndiaWeian Zhu, Sun Yat-sen University, China
Copyright © 2025 Locatelli, Ferrario, Fortunato, Ghidotti and Crescitelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orazio Fortunato, b3JhemlvLmZvcnR1bmF0b0Bpc3RpdHV0b3R1bW9yaS5taS5pdA==
†These authors have contributed equally to this work
‡These authors jointly supervised this work
 Camilla Locatelli1,2†
Camilla Locatelli1,2† Orazio Fortunato
Orazio Fortunato Patrizia Ghidotti
Patrizia Ghidotti Rossella Crescitelli
Rossella Crescitelli