- 1Department of Pediatric Nephrology, Radboud University Medical Centre, Amalia Children’s Hospital, Nijmegen, Netherlands
- 2Department of Human Genetics, Radboud University Medical Centre, Nijmegen, Netherlands
- 3Department of Pediatric Nephrology, Emma Children’s Hospital, Amsterdam University Medical Centre, Amsterdam Institute of Infection and Immunity, Amsterdam, Netherlands
- 4Department of Pediatrics/Pediatric Nephrology, University Hospital Leuven, Leuven, Belgium
- 5Department of Development and Regeneration, University Hospital Leuven, Leuven, Belgium
A Commentary on
Complement genetic variants and FH desialylation in S. pneumoniae-haemolytic uraemic syndrome
By Gómez Delgado I, Corvillo F, Nozal P, Arjona E, Madrid Á, Melgosa M, Bravo J, Szilágyi Á, Csuka D, Veszeli N, Prohászka Z and Sánchez-Corral P (2021) 12:641656. doi: 10.3389/fimmu.2021.641656
Introduction
Hemolytic uremic syndrome caused by an invasive Streptococcus pneumoniae infection (SP-HUS) is a very rare but severe disease of which the pathogenesis is still poorly elucidated. In this recent article, Gómez Delgado and colleagues (1) presented interesting data on the desialylation of members of the Factor H (FH) protein family, which includes FH, Factor H-like 1 (FHL-1), and Complement Factor H Related proteins 1-5 (CFHR1-5), in SP-HUS patients. This desialylation is likely the result of neuraminidases released by S. pneumoniae in the circulation of these patients. Furthermore, the authors investigated whether this desialylation of FH affected its key complement-regulatory functions in the complement alternative pathway, to further understand the role of bacterial neuraminidases in the pathogenesis of SP-HUS. As patient samples were limited, they used a biological substitute, which was in vitro desialylated FH generated by incubating commercially obtained serum-purified FH with neuraminidase. The authors demonstrated that in vitro desialylated FH showed a decreased ability to regulate complement activity on sheep erythrocyte surfaces, while FH’s ability to bind C3b, degrade C3b into iC3b, and its decay accelerating activity remained unchanged. Therefore, the authors hypothesized FH desialylation may have a role in SP-HUS pathogenesis. While we acknowledge the significant contributions this study offers in elucidating the pathogenesis of SP-HUS, we have identified certain technical concerns regarding the execution of the experiments and the interpretation of the data on the desialylation of FH and its functional consequences.
Discussion
Recently, we assessed the glycosylation status of SP-HUS patient FH using a high-resolution LC-MS/MS-based N-glycoproteomics approach. While we also detected loss of sialylation of FH in our SP-HUS patients, we additionally identified loss of galactose and N-acetylglucosamine residues on the N-glycan structure of FH, which could not have been detected with the lectin blotting method used by Gómez Delgado et al. Because the glycosylation alterations were beyond desialylation and since we had sufficient patient material available, we purified FH from SP-HUS patient sera using immunoprecipitation and evaluated its functional activity (2). We were not able to detect any differences in the functionality of FH derived from SP-HUS patients compared to control FH, as measured by FH’s ability to degrade C3b into iC3b and to regulate complement on the sheep erythrocyte surface. Importantly, we also observed no functional consequence for in vitro desialylated FH derived from treating normal human serum with neuraminidase and applying the same FH purification using immunoprecipitation. The resulting in vitro desialylated FH preparation specifically lacked sialic acid residues, without detectable loss of galactose and N-acetylglucosamine. We concluded that FH derived from in vitro desialylation or SP-HUS patient material, even though having severely affected N-glycans, retains its canonical complement-inhibitory functions. Therefore, we proposed that alterations of FH’s N-glycans, including the loss of galactose and N-acetylglucosamine, are unlikely to directly contribute to complement dysregulation during SP-HUS. Our data, along with other published reports (3–5) consistently demonstrate that the impairment of FH’s N-glycosylation (to varying degrees) does not affect its canonical complement inhibitory activity.
In contrast, Gómez Delgado and colleagues (1) did report a functional consequence of FH desialylation, as they showed that in vitro desialylated FH showed increased lysis of sheep erythrocytes, indicating limited complement-regulatory function. By replicating the method for creating in vitro desialylated FH (dFH) reported by Gómez Delgado et al. (1), we indeed noticed increased hemolysis of the erythrocytes with this dFH sample when comparted to FH not treated with neuraminidase (FH) (Figure 1) (2). However, we suspected that residual neuraminidase activity in the sample was responsible for this, as the neuraminidases are not removed after the in vitro desialylation reaction but are inhibited by a high pH. When we determined the neuraminidase activity in the supernatants of the hemolytic assay using fluorometry, we indeed found residual neuraminidase activity in the dFH samples compared to FH (Figure 1) (2).
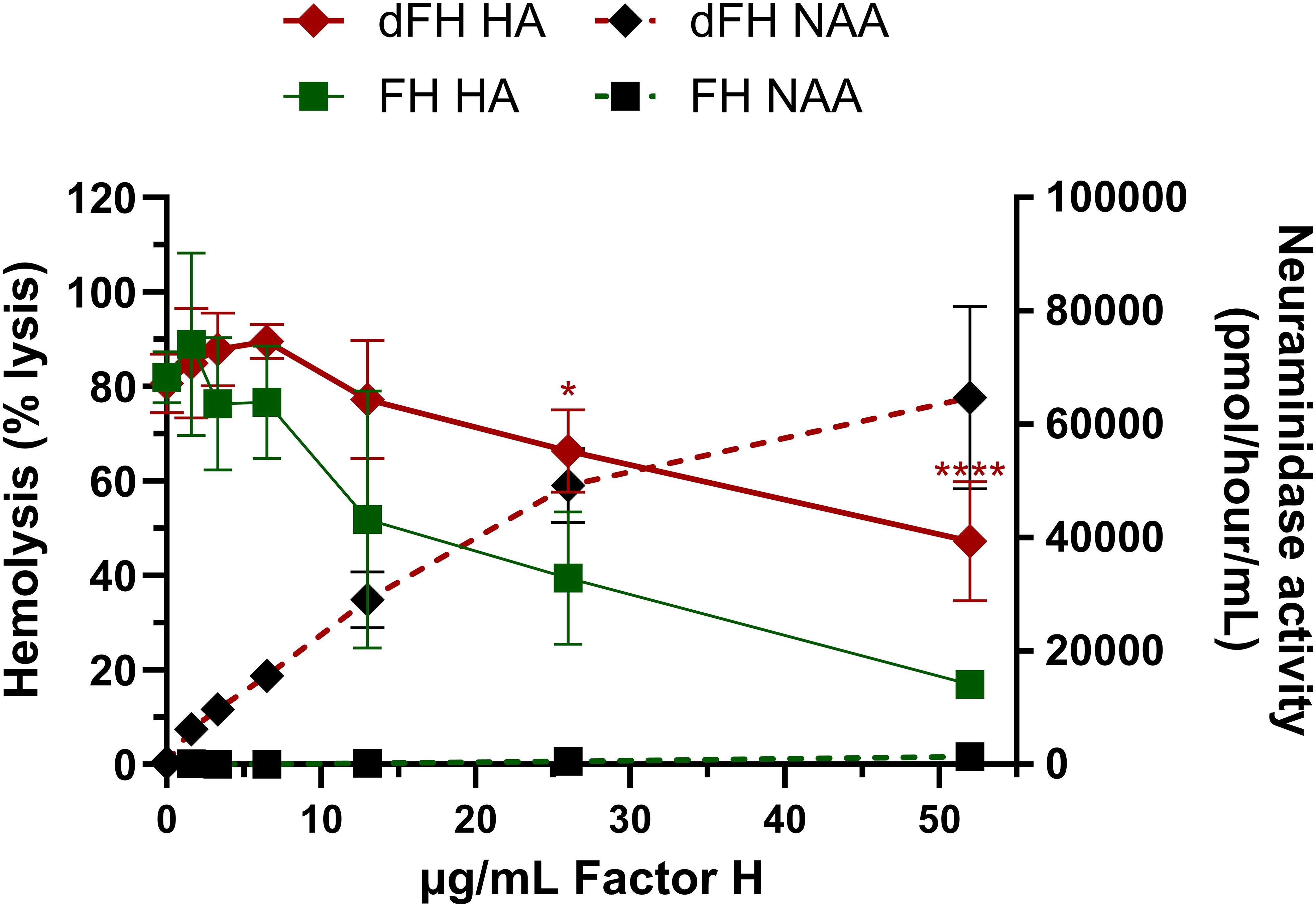
Figure 1. Evaluation of in vitro desialylated FH created according to the Gómez Delgado protocol. In vitro desialylated FH (dFH) was prepared using the Gómez Delgado protocol (1). In short, commercially available FH (CompTech) was treated with neuraminidase from C. welchii for 4 hours at 37°C. Following incubation, the reaction was halted by adjusting the pH to 10. In parallel, FH not treated with neuraminidase (FH) was included as a control. FH functionality was assessed using a sheep erythrocyte hemolytic assay (HA) as described previously (2) to evaluate FH’s capability to protect sheep erythrocytes from complement-mediated lysis. FH and dFH were added in increasing concentrations (0-52 µg/mL). The hemolysis is expressed as % the of the full lysis of erythrocytes in water. Compared to FH (green squares), dFH shows increased hemolysis (red diamonds) suggesting impaired FH complement-regulatory function. Following the HA, the neuraminidase activity assay (NAA) was performed in the supernatants of the HA by fluorometry as described previously (2). dFH shows increased neuraminidase activity in a dose-dependent manner (black diamonds). No neuraminidase activity was detected in the FH sample (black squares). HA data represents the mean ± SD of three independent preparations of dFH/FH measured in two separate experiments. Statistical significance comparing dFH and FH was determined for the HA using multiple unpaired T tests followed by Holm-Sidák’s multiple comparison test; * p ≤ 0.05; and **** p ≤ 0.0001. NAA results are shown as the mean ± SD of three independent experiments. The figure was adapted from previously published data (2), Figures 6A, B.
It is well-established that C. welchii neuraminidases have an optimal enzyme activity at pH 5 and are inhibited at pH levels above 9 (6, 7), but the reversibility of this inhibition seems not to have been addressed by the authors. Our data indicates that lowering the pH, as is the case when the desialylated FH sample (pH 10) is diluted in the hemolytic assay buffer (pH 7), restores the neuraminidase activity (at least in part), thus making the neuraminidase inhibition reversible.
In their manuscript, the authors did not present experimental evidence confirming that the enzyme activity was halted following the in vitro desialylation reaction and during subsequent functional analyses. The omission of neuraminidases in hemolytic assays is critical, as incomplete inhibition could lead to desialylation of the erythrocyte surfaces, thereby increasing their susceptibility to complement-mediated lysis (8). As a result, the observed increase in erythrocyte lysis may not reflect altered FH functionality due to desialylation of FH itself, but rather reduced recruitment of FH to the erythrocyte surface due to erythrocyte surface desialylation. While the desialylation of cell surfaces may indeed have a role in the disease pathogenesis of SP-HUS, this was outside of the scope of both of our studies and requires a different study design.
In summary, while Gómez Delgado et al. provide observations linking FH desialylation to complement dysregulation and SP-HUS pathogenesis, the lack of controls to exclude residual neuraminidase activity undermines their functional claim. The observed increased hemolysis is likely explained by erythrocyte surface desialylation driven by residual neuraminidase activity, rather than by decreased functionality of the desialylated FH itself. Without conclusive evidence that residual enzyme activity was eliminated, the authors’ interpretation overstates the role of FH desialylation in complement dysregulation in SP-HUS, and their functional data should therefore be viewed with caution.
Author contributions
LB: Writing – original draft. NK: Writing – review & editing. MM: Writing – review & editing. LH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gomez Delgado I, Corvillo F, Nozal P, Arjona E, Madrid A, Melgosa M, et al. Complement genetic variants and FH desialylation in S. pneumoniae-haemolytic uraemic syndrome. Front Immunol. (2021) 12:641656. doi: 10.3389/fimmu.2021.641656
2. Baas LM, Wijnsma KL, Zijlstra F, van de Kar NCAJ, ter Steeg L, Bouts AHM, et al. Glycoproteomics analysis of complement factor H and its complement-regulatory function during Streptococcus pneumoniae-associated hemolytic uremic syndrome. Front Immunol. (2025) 16. doi: 10.3389/fimmu.2025.1645196
3. Tschongov T, Konwar S, Busch A, Sievert C, Hartmann A, Noris M, et al. Moss-produced human complement factor H with modified glycans has an extended half-life and improved biological activity. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1383123
4. Schmidt CQ, Slingsby FC, Richards A, and Barlow PN. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expression Purification. (2011) 76:254–63. doi: 10.1016/j.pep.2010.12.002
5. Michelfelder S, Parsons J, Bohlender LL, Hoernstein SNW, Niederkrüger H, Busch A, et al. Moss-produced, glycosylation-optimized human factor H for therapeutic application in complement disorders. J Am Soc Nephrol. (2017) 28:1462–74. doi: 10.1681/ASN.2015070745
6. Li J and McClane Bruce A. The sialidases of clostridium perfringens type D strain CN3718 differ in their properties and sensitivities to inhibitors. Appl Environ Microbiol. (2014) 80:1701–9. doi: 10.1128/AEM.03440-13
7. Kurnia R, Tarigan S, Nugroho C, Silaen O, Natalia L, Ibrahim F, et al. Potency of bacterial sialidase Clostridium perfringens as antiviral of Newcastle disease infections using embryonated chicken egg in ovo model. Veterinary World. (2022) 15:1896–905. doi: 10.14202/vetworld.
Keywords: factor H, Neuraminidase, functional assays, SP-HUS, hemolytic uremic syndrome
Citation: Baas LM, van de Kar NCAJ, Michels MAHM and van den Heuvel LP (2025) Commentary: Complement genetic variants and FH desialylation in S. pneumoniae-haemolytic uraemic syndrome. Front. Immunol. 16:1707529. doi: 10.3389/fimmu.2025.1707529
Received: 17 September 2025; Accepted: 04 November 2025; Revised: 29 October 2025;
Published: 20 November 2025.
Edited by:
Roberta Bulla, University of Trieste, ItalyReviewed by:
Andrea Balduit, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyCopyright © 2025 Baas, van de Kar, Michels and van den Heuvel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lambertus P. van den Heuvel, QmVydC52YW5kZW5oZXV2ZWxAcmFkYm91ZHVtYy5ubA==
†These authors have contributed equally to this work
 Laura M. Baas
Laura M. Baas Nicole C. A. J. van de Kar
Nicole C. A. J. van de Kar Marloes A. H. M. Michels
Marloes A. H. M. Michels Lambertus P. van den Heuvel
Lambertus P. van den Heuvel