- 1Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Spine Institute, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3School of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 4Key Laboratory of Ministry of Education of Theory and Therapy of Muscles and Bones, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 5State Key Laboratory of Discovery and Utilization of Functional Components in Traditional Chinese Medicine, Shanghai, China
Bone remodeling disorders such as osteoporosis, rheumatoid arthritis, and periodontitis highlight the clinical significance of osteoimmune communication. Osteoimmunology has emerged as a key interdisciplinary field elucidating the dynamic interplay between the immune and skeletal systems, with extracellular vesicles (EVs) recognized as nanosized mediators that transport proteins, lipids, and RNAs to regulate bone remodeling. Immunocyte-derived EVs modulate osteoblast and osteoclast activity through macrophage polarization, Treg-associated CD73/adenosine signaling, Th17/Treg balance, and B cell–bone interactions, exerting dual effects by promoting bone formation under physiological conditions while amplifying inflammation and bone resorption in osteoporosis, rheumatoid arthritis, and periodontitis. Bidirectional communication between bone marrow stromal cell–derived EVs and immune cells further highlights the complexity of EV-mediated regulation in bone microenvironments. Moreover, engineering approaches such as cargo loading, surface modification, and biomaterial integration are rapidly advancing the therapeutic application of EVs in bone diseases. Despite these advances, challenges remain in EV standardization, scalable production, and clinical translation, underscoring that immunocyte-derived EVs represent both pathogenic mediators and promising therapeutic agents, with future studies required to resolve mechanistic complexity and optimize their clinical utility. Engineered EVs enable targeted modulation of CD73–adenosine, NF-κB, HIF-1α, and PI3K/AKT axes, offering bone-targeting delivery and immune-instructive biomaterials as converging strategies. These insights highlight immunocyte-derived EVs as both biomarkers and therapeutic candidates in bone disorders, and underscore the need for standardized approaches to advance their clinical utility in osteoimmunology.
Introduction
Osteoimmunology is a rapidly evolving interdisciplinary field that investigates the complex bidirectional interactions between the skeletal and immune systems. It elucidates the fundamental molecular mechanisms governing both bone homeostasis and pathological conditions (1). This intricate crosstalk is particularly prominent in bone remodeling, where immune cells and their secreted cytokines directly modulate osteoblast and osteoclast activity. Extracellular vesicles (EVs) have recently emerged as essential nanoscale mediators in this intercellular communication network, facilitating the transfer of bioactive molecules between immune and bone cells (2, 3).
These membrane-bound nanoparticles, typically 40–160 nm in diameter, transport diverse molecular cargo—including proteins, lipids, mRNAs, and microRNAs (miRNAs)—, that critically regulate bone formation, resorption, angiogenesis, and immune responses within the bone microenvironment (2, 4).
The pathological relevance of EV-mediated communication is evident in multiple diseases. In periodontitis, Porphyromonas gingivalis–derived EVs suppress acetylcholine secretion by inhibiting Cyp4f40, which accelerates osteoclastogenesis and bone loss (5). Similarly, immunocyte-derived EVs facilitate bone metastasis by establishing pre-metastatic niches through integrin-mediated homing and microenvironmental modulation (6). Collectively, these studies indicate that EVs are central regulators maintaining skeletal balance under physiological conditions, but when dysregulated, they contribute to osteoporosis, rheumatoid arthritis, and periodontitis (7–9).
The bone microenvironment is increasingly recognized as an EV-rich niche, where vesicle-mediated immune–bone crosstalk contributes to both skeletal development and pathological bone loss. Here, we first summarize the biology and cargo features of immunocyte-derived EVs, then outline their bidirectional crosstalk with bone marrow stromal cells (BMSCs), examine disease-specific roles in periodontitis, osteoporosis, and rheumatoid arthritis, and finally discuss engineering and translational applications.
Biology and cargo of immunocyte-EVs
EVs are lipid-bilayer particles secreted by virtually all cell types. According to the Minimal Information for Studies of Extracellular Vesicles 2023 (MISEV2023) guidelines, “EVs” is the preferred generic term encompassing particles previously referred to as exosomes or microvesicles, which differ mainly by size and biogenesis. Accordingly, EVs are broadly categorized as BMSC small EVs (sEVs; 40–160 nm, typically of endosomal origin), microvesicles (100–1000 nm, derived from plasma-membrane budding), and apoptotic bodies (1–5 µm, released during apoptosis) (2, 10). Their molecular cargo—comprising proteins, lipids, and nucleic acids—reflects the cell of origin, activation state, and microenvironmental cues, thereby mediating intercellular communication under both physiological and pathological conditions (11–13). This heterogeneity accounts for the broad functional spectrum of EVs in skeletal homeostasis and pathological bone remodeling.
Dendritic-cell-derived EVs (DC-EVs) exemplify the immunomodulatory breadth of this system. Mature DC-EVs act as potent mucosal adjuvants that enhance both systemic and local immune responses (14). Beyond immunostimulation, alterations in circulating EV profiles offer clinically relevant biomarkers: in rheumatoid arthritis, CD14+ HLA-DR+ EVs reflect antigen-presenting-cell activation (15), and plasma RANKL+ EVs correlate with bone metastases and skeletal-related events (16).
Macrophage-derived EVs (Mφ-EVs) are central mediators of bone remodeling. Their actions depend on polarization: M1-EVs amplify inflammatory cascades and osteoclastogenesis, whereas M2-EVs resolve inflammation and enhance angiogenic–osteogenic coupling (13, 17). Mechanical and chemical cues—including lithium-doped calcium-silicate scaffolds, cyclic stretch, and shear stress—reshape EV cargo such as miR-145-5p, mitochondrial components, and miR-423-5p, thereby promoting osteogenic differentiation of bone-marrow stromal cells (13, 18, 19). M2-EVs also suppress osteoclastogenesis through the PKM2/HIF-1α axis (20). Erythropoietin-stimulated macrophages secrete miR-5107-5p-rich EVs that inhibit EGFR and restore osteogenesis under inflammatory stress (21). Electrical preconditioning enriches oxidative-phosphorylation proteins within macrophage EVs, further potentiating their regenerative activity (22). Collectively, M2-EVs exert pro-regenerative and anti-inflammatory effects, whereas M1- or pathogen-modified EVs intensify osteoclastic activation and bone resorption (13, 17–22). Conversely, adipose-tissue macrophages under estrogen deficiency release pro-inflammatory small EVs carrying miR-30e-5p that reinforce M1 polarization (23), whereas Porphyromonas gingivalis gingipains suppress miR-146a-5p in BMSC-EVs, activating TRAF6 and driving osteoclastogenesis (24).
Lymphocyte-derived EVs constitute another immunoregulatory axis in bone remodeling. Retinoic-acid-induced Treg EVs enriched in CD73 hydrolyze AMP to adenosine, suppressing IL-17A and RANKL expression and thereby alleviating alveolar bone loss in periodontitis (9). In rheumatoid arthritis, pro-inflammatory CD80+ macrophages sustain immune imbalance; EVs engineered to deliver methotrexate reprogram them toward anti-inflammatory phenotypes, enhance Treg differentiation, and suppress Th1 responses (25). B-cell-derived EVs add an additional layer of immune control. BMSC-EVs induce B10 regulatory-cell differentiation without excessive IL-10 production, indicating fine-tuned B-cell tolerance (26).
Professional antigen-presenting-cell EVs further contribute to the oste-immune network, and circulating EV signatures serve as accessible biomarkers. Collectively, immunocyte-derived EVs convey cell-of-origin-specific cargo with context-dependent effects, acting as bidirectional modulators of bone formation and resorption. Representative cargo and target pathways are summarized in Table 1.
BMSC-EVs: reciprocal crosstalk with immune cells
EV-mediated communication between immune cells and BMSCs constitutes a bidirectional regulatory axis within the bone microenvironment. Inbound M2 macrophage–derived EVs enhance BMSC osteogenic differentiation, whereas inflammatory EVs suppress lineage commitment and promote catabolic signaling. Outbound BMSC-EVs (CD73+/PD-L1+) attenuate excessive macrophage and T-cell activation; however, chronic TNF-α or IFN-γ stimulation reduces these immunosuppressive subsets (12). In diabetes, BMSC-EVs exhibit diminished CD73 expression, weakening their capacity to promote M2 polarization and bone formation. Conversely, CD73+ BMSC-EVs activate A2b-adenosine receptor and cAMP signaling to restore osteogenesis (27).
Reciprocal regulation by immune cells also shapes the BMSC-EV landscape. VPS33B-dependent EV secretion mediates autocrine signaling in BMSCs, and disruption of this pathway accelerates cellular senescence, bone loss, and remodeling imbalance (28). Importantly, transplantation of EVs from young BMSCs restores osteogenic potential and mitigates age-related skeletal decline. Therapeutically, engineered BMSC-EVs overexpressing CTLA4Ig or IL-1 receptor antagonist exhibit potent immunomodulatory effects in collagen-induced arthritis, increasing TGF-β1 expression and suppressing IL-6 and RANTES levels, thereby alleviating inflammation and promoting bone repair (29).
Collectively, BMSC-EVs act as editors of immune tone within bone niches, integrating inbound immune cues and releasing outbound immunoregulatory cargo. Dysregulation of this bidirectional communication contributes to impaired regeneration and disease progression, whereas targeted engineering of BMSC-EVs provides a promising avenue for osteo-immune modulation and skeletal regeneration (12, 27–29).
Pathogenic roles of immunocyte-EVs in bone diseases
With the mechanistic toolkit in place, these principles can be mapped onto specific disease contexts. Immunocyte-derived EVs act as double-edged mediators in bone diseases, functioning either as pathogenic drivers or as therapeutic agents depending on their cellular origin and cargo composition (3, 30).
Periodontitis
Oral infection and local dysbiosis reshape EV traffic at barrier tissues, tipping the balance toward osteoclastogenesis and inflammatory bone loss. Infection-associated EVs play a central role in periodontal bone loss. Porphyromonas gingivalis infection of oral keratinocytes induces a tenfold increase in EV release, enriching vesicles with TNF-α and IL-1β that exacerbate local inflammation and tissue damage (31). Gingipains further disrupt skeletal balance by downregulating miR-146a-5p in BMSC-derived EVs, thereby activating TRAF6 and promoting osteoclastogenesis (24). In estrogen-deficient conditions, adipose macrophages secrete pro-inflammatory sEVs (e.g., miR-30e-5p) that amplify M1-like polarization and worsen disease severity (23).
Therapeutic EVs demonstrate potential for regeneration. M2 macrophage-derived EVs reprogram immature neutrophils into Anxa1+ subsets, promoting inflamed bone repair (17). Likewise, erythropoietin-stimulated macrophage EVs deliver miR-5107-5p to BMSCs, suppress EGFR, and restore osteogenesis in inflammatory settings (21). Engineered CD80-targeting EVs carrying methotrexate selectively suppress pro-inflammatory macrophages, enhance Treg differentiation, and reduce osteoclastogenesis (25). Collectively, these findings underscore the dual role of EVs in periodontitis progression and therapy. Thus, in periodontitis, EVs can either accelerate alveolar bone destruction or serve as regenerative mediators depending on their cellular origin.
Osteoporosis
Systemic aging, endocrine factors, and microbiota-derived signals converge on EV programs that weaken bone mass and repair capacity. In osteoporosis, immunocyte- and microbiota-derived EVs contribute to bone fragility through multiple mechanisms. In glucocorticoid-induced disease, miR-370-3p regulates the TLR4/SLC7A11/GPX4 axis to enhance osteogenesis while inhibiting ferroptosis, thereby attenuating bone loss (32). Senescent cell accumulation accelerates skeletal decline, whereas engineered bifunctional EVs that promote both senolysis and efferocytosis show anti-aging therapeutic benefits (33). Gut microbiota-derived EVs also mediate the gut–bone axis, linking microbial dysbiosis to osteoporosis risk (34, 35).
Nutritional and plant-derived EVs provide additional protective effects. Milk-derived vesicles activate BMP2/MAPK pathways and enhance osteogenesis (36, 37). Plant EVs from Epimedium and sea buckthorn stimulate bone regeneration via PI3K/AKT and miRNA-mediated signaling, respectively (38, 39). These studies underscore that EVs from senescent cells, microbiota, and dietary sources collectively influence osteoporosis pathogenesis and therapy. Together, these studies highlight how diverse EV sources influence osteoporosis pathogenesis and may be harnessed for intervention.
These observations emphasize that diverse EV sources—from senescent cells to dietary vesicles—can both compromise and restore skeletal integrity.
Rheumatoid arthritis
Autoimmune inflammation reprograms joint and circulating EVs, coupling synovitis to cartilage and bone damage. Distinct EV signatures have been identified in rheumatoid arthritis (RA). Synovial fibroblast-derived sEVs carrying miRNA-15-29148 induce chondrocyte apoptosis via CIAPIN1 targeting, contributing to joint degeneration (40). Circulating EVs from RA patients are enriched in antigen-presenting cell markers such as CD14 and HLA-DR, reflecting immune activation within inflamed joints (15).
Therapeutically, probiotic-derived EVs favor M2 polarization and suppress pro-inflammatory cytokines, ameliorating disease activity (8, 41). Engineered EVs co-loaded with methotrexate and CD80 antibodies further suppress inflammatory macrophages and enhance Treg responses, illustrating the translational potential of immune-targeted vesicles (25).
Other orthopedic diseases: ONFH and OA/TMJ-OA
Beyond osteoporosis, rheumatoid arthritis, and periodontitis, accumulating evidence has linked bone-marrow-mesenchymal-stem-cell-derived extracellular vesicles (BMSC-EVs) to additional orthopedic disorders. In osteonecrosis of the femoral head (ONFH), BMSC-EVs enriched with miR-148a-3p suppress SMURF1, enhance osteogenic differentiation, and mitigate disease progression (42). In osteoarthritis, including temporomandibular-joint OA (TMJ-OA), sEVs facilitate cartilage reconstruction through the autotaxin–YAP signaling axis, reducing inflammation and restoring joint architecture (43). These findings extend the osteo-immune relevance of BMSC-EVs beyond inflammatory bone loss to degenerative skeletal disorders, highlighting SMURF1 and YAP as potential therapeutic targets.
Taken together, across bone diseases—including periodontitis, osteoporosis, rheumatoid arthritis, and degenerative entities such as ONFH and OA—immunocyte-derived EVs act as double-edged mediators: pathogenic EVs amplify inflammatory osteoclastogenesis, whereas regenerative or engineered EVs restore osteo-immune balance. Representative pathogenic and therapeutic roles are summarized in Table 2, and Figure 1 schematizes the cargo-specific signaling circuits through which EVs shape bone remodeling outcomes.
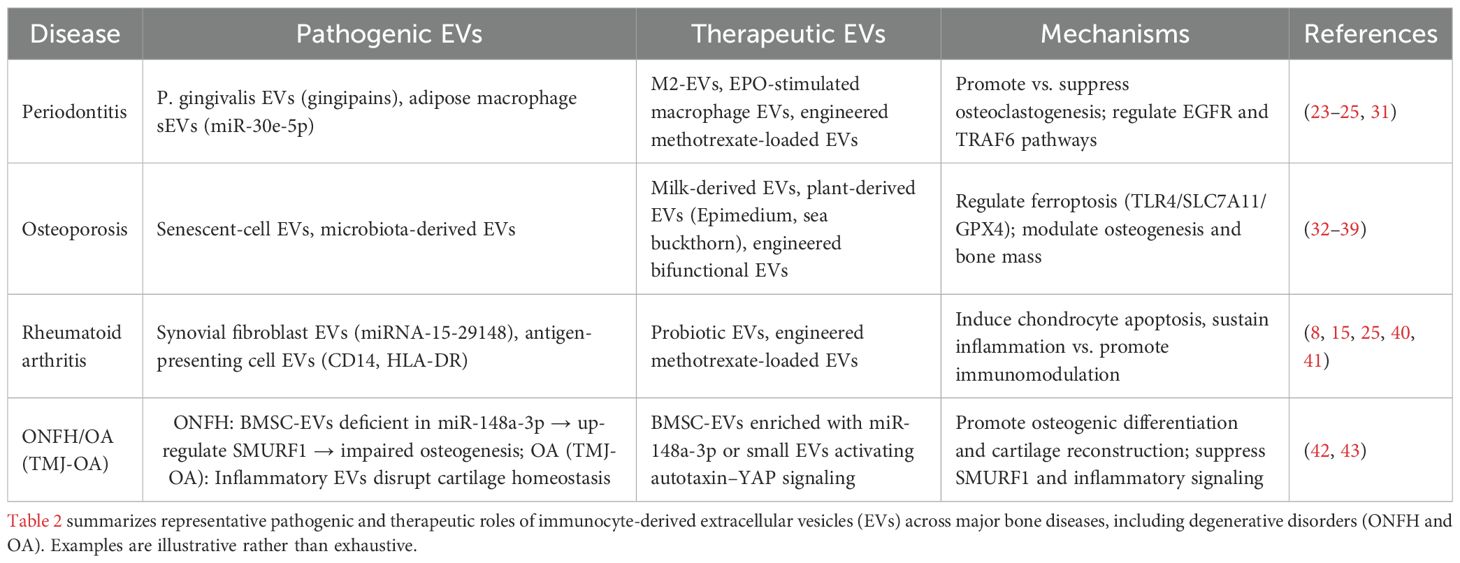
Table 2. Representative pathogenic and therapeutic roles of immunocyte-derived EVs across major bone diseases.
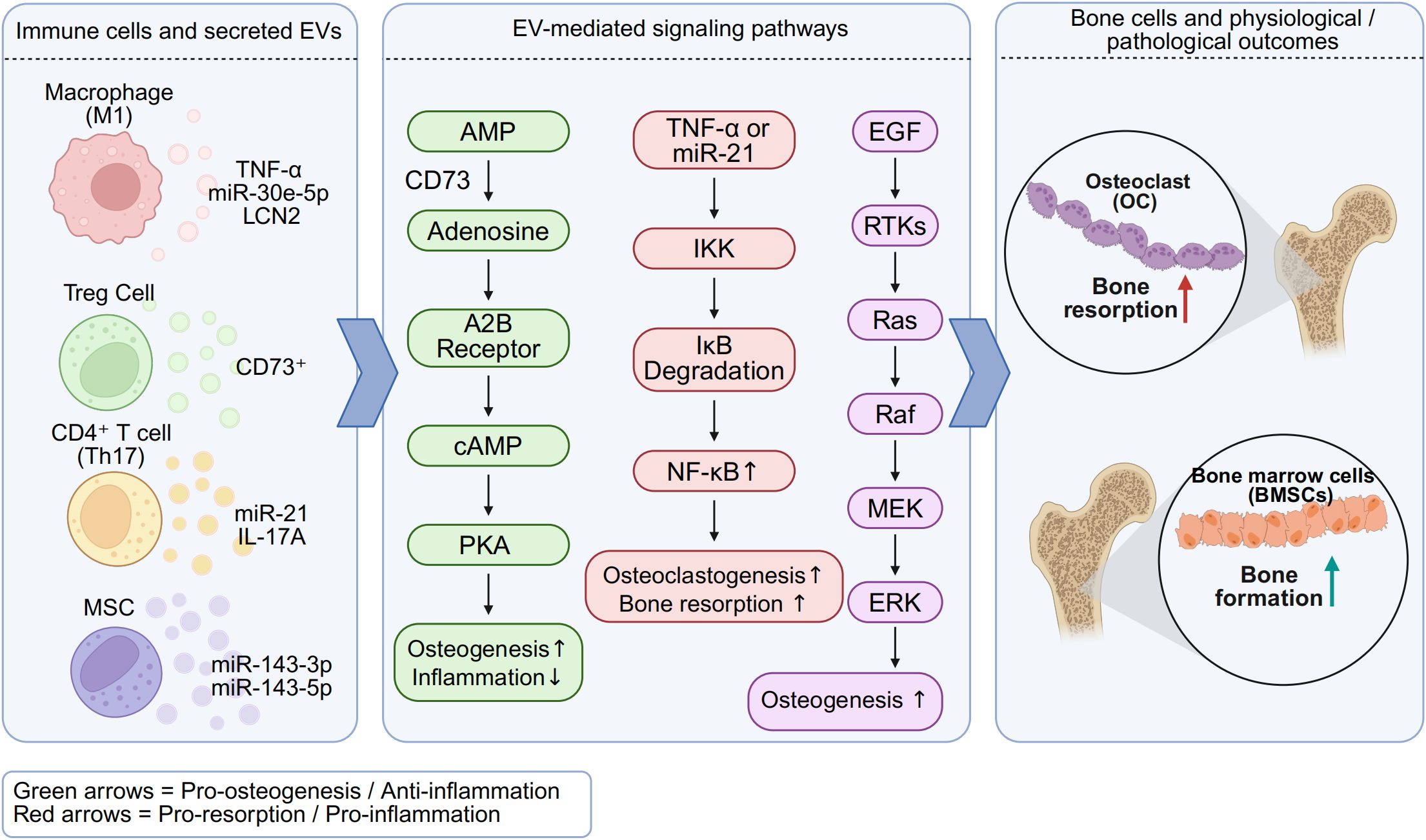
Figure 1. Osteoimmune signaling by immunocyte-derived EVs in bone remodeling. Schematic representation of cargo-specific pathways summarized in Table 2. Macrophage- and T cell-derived EVs carry distinct miRNAs and proteins that regulate osteoclastogenesis and osteogenesis via CD73–adenosine, NF-κB, and HIF-1α signaling. M1-EVs enriched in TNF-α and miR-21 promote inflammation and osteoclast activity, whereas M2-EVs and Treg-derived EVs carrying CD73 facilitate immunosuppression and osteoblast differentiation. These EV-mediated pathways shape the osteoimmune microenvironment and contribute to skeletal homeostasis or pathological bone loss. Created with BioRender.com.
Engineering and translational applications
These mechanistic and disease insights motivate engineering strategies to control EV cargo, targeting, and release. Advanced engineering approaches enhance the therapeutic utility of immunocyte-derived EVs by improving targeting specificity, sustaining release, and boosting bioactivity (44–48). These strategies mainly involve cargo loading, surface modification, and integration with biomaterials.
Hypoxia preconditioning of magnesium-activated dental pulp stem cells enriches EVs with miR-451a. These vesicles promote angiogenesis through the AKT/eNOS/NO axis (49, 50).Surface modification. Decorating EV membranes improves tissue specificity. FNDC5/irisin-enriched EVs conjugated with bone-targeting aptamers efficiently accumulated in skeletal tissues and alleviated osteoporosis (50). Antibody-modified EVs, such as CD80-coated vesicles, selectively targeted inflammatory macrophages and enhanced therapeutic outcomes in RA and periodontitis (25). Similarly, bacterial EVs engineered to express DC-STAMP directly delivered inhibitory peptides into osteoclast precursors, suppressing osteoclastogenesis in osteoporosis models (47). Surface activation strategies, such as choline phosphate modification, further improve EV binding efficiency to biomaterials (48). Surface modification enables precise tissue targeting in vivo. Such strategies enable precise tissue targeting and improve therapeutic specificity.
Biomaterial integration. Incorporation of EVs into scaffolds enhances retention and controlled release. GelMA hydrogels provide a supportive 3D matrix for EV delivery, improving bone regeneration efficiency (38, 51). Thermosensitive hydrogels constructed from polyhedral oligomeric silsesquioxane, PEG, and PPG effectively carried aspirin-treated macrophage EVs, promoting osteochondral repair (52). Electrospun membranes incorporating lipocalin-2–enriched EVs accelerated healing of large bone defects (53). Likewise, 3D-printed porous titanium scaffolds combined with stem cell EVs and anchored by zwitterionic coatings improved osteogenic repair (48). Biomaterial integration improves EV stability and prolongs functional activity. These systems stabilize EVs and provide spatiotemporal control of release in vivo.
Hybrid platforms. Integrative systems combine cargo engineering, surface targeting, and biomaterial encapsulation for synergistic benefits. For example, an Exo@Tβ4/HAMA hydrogel system, generated by grafting thymosin β4 onto modified hyaluronic acid and encapsulating BMSC-EVs (Exo@Tβ4 refers to thymosin β4-modified HAMA hydrogels loaded with EVs, promoted BMSC recruitment, neurogenesis, angiogenesis, and osteogenesis (54). Similarly, dual biomimetic scaffolds that integrated 3D-printed Ti-6Al-4V frameworks with EVs-loaded PEGDA/GelMA microspheres enabled sustained release and enhanced regenerative outcomes (55).
Collectively, engineering of EV cargo, surfaces, and biomaterial integration provides a clear trajectory toward clinical translation by improving specificity, stability, and spatiotemporal control of osteo-immune modulation.
Emerging technologies enabling EV-based targeted osteoimmune modulation
Recent advances in multi-omics and bioengineering are transforming EVs research from descriptive biology into translationally actionable platforms.
Single-EV and spatial multi-omics
Bulk analyses mask EV heterogeneity. Single-EV proteomics and transcriptomics now permit high-resolution profiling of immunocyte-derived EVs, identifying distinct signatures in macrophage, T-cell, and B-cell subsets. Spatial transcriptomics further delineates EV deposition within bone marrow and inflamed synovium, directly mapping EV-mediated osteoimmune interactions in situ (56).
Artificial intelligence and machine learning
High-dimensional EV cargo datasets (miRNAs, proteins, lipids) challenge interpretation. AI-based classifiers stratify rheumatoid arthritis (RA) patients by plasma EV signatures and predict flare risk, suggesting applicability to osteoporosis or periodontitis (57, 58). Such computational tools accelerate biomarker discovery and support clinical translation of EV diagnostics.
Immune checkpoint–associated EVs
Beyond canonical CD73–adenosine signaling, checkpoint molecules are increasingly detected in EV cargo. Programmed death-ligand 1 (PD-L1)–positive EVs suppress T-cell activation and modulate local bone remodeling by shifting the Th17/Treg balance, linking immunotherapy-relevant pathways to skeletal disease (59, 60). These data position EVs as vehicles and targets for checkpoint modulation in osteoimmunology.
Exercise-mimetic and bone-targeted EVs
Skeletal muscle–derived irisin (FNDC5)–enriched EVs reproduce exercise-induced osteogenesis. Conjugation with bone-targeting aptamers enhances skeletal accumulation and attenuates bone loss in preclinical osteoporosis models (61, 62). Such exercise-mimetic EV platforms represent precision therapeutics for patients unable to exercise.
Standardization and regulatory alignment
Translation requires adherence to the Minimal Information for Studies of Extracellular Vesicles (MISEV2023) guidelines, emphasizing multimethod characterization, functional validation, and in vivo uptake (63, 64). In parallel, scalable good manufacturing practice (GMP)–compliant EV production and transparent reporting standards and harmonization with MISEV2023 are essential for reproducibility and regulatory approval (65). To facilitate clinical translation, Table 3 summarizes key quality dimensions of EV research, aligning minimal experimental standards (MISEV2023) with preferred criteria for translational applications.
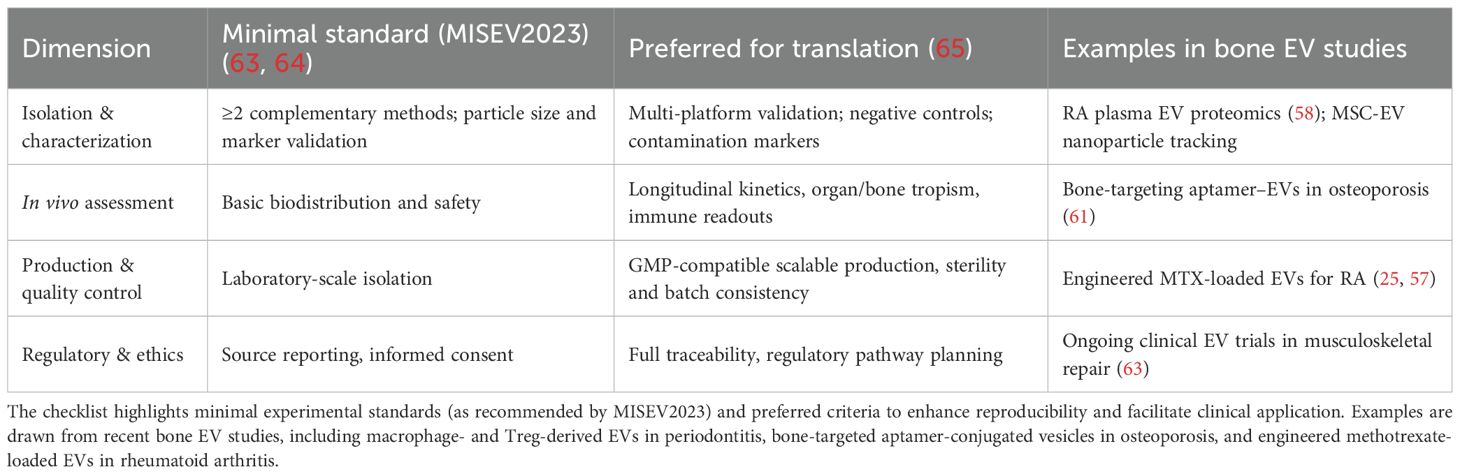
Table 3. Translational readiness checklist for EV-based osteoimmune therapeutics (aligned with MISEV2023).
Conclusion and perspectives
Research on immunocyte-derived EVs has revealed their dual roles in bone remodeling, functioning as both pathogenic mediators and therapeutic agents. Despite rapid progress, several barriers still hinder clinical translation.
Current limitations
Standardization of EV isolation and characterization remains a major challenge, with no universally accepted protocols and frequent heterogeneity across preparations (2, 66, 67). This undermines reproducibility and complicates cross-study comparisons. Scaling up production for clinical-grade applications is also technically demanding, as large-scale biomanufacturing must guarantee batch-to-batch consistency and stringent quality control (10, 68). These barriers highlight the urgent need for harmonized workflows across laboratories.
Although research on extracellular vesicles has expanded rapidly, several methodological and translational challenges remain unresolved. Heterogeneity in isolation procedures, quantification methods, and characterization markers continues to hinder reproducibility and cross-study comparison. Differences in parental cell sources, disease models, and culture conditions further complicate data interpretation. In most preclinical settings, evidence is derived from small-animal models with limited follow-up, and the long-term biodistribution, pharmacokinetics, and safety profiles of engineered EVs remain insufficiently defined. Moreover, the absence of unified potency assays and large-scale manufacturing standards restricts clinical translation. Future studies adhering to MISEV2023 recommendations, incorporating rigorous quality control, and integrating multicenter validation will be essential to establish reliable and clinically applicable EV-based osteoimmune therapies.
Emerging opportunities
Advances in single-EV analysis, imaging, and multi-omics now provide unprecedented resolution of vesicle heterogeneity and disease-specific signatures (16). Circulating immunocyte-derived EVs show promise as accessible biomarkers for diagnosis and prognosis in osteoporosis, rheumatoid arthritis (RA), and periodontitis (3, 28). Concurrently, engineering approaches—including cargo loading, surface modification, and biomaterial integration—are enhancing the specificity and efficacy of EV-based therapeutics (45, 47).
Future directions
Emerging frontiers such as single-EV omics and artificial-intelligence–driven biomarker discovery are expected to further refine mechanistic insight and diagnostic precision in osteoimmunology. Translation will require: (1) identification of key cargo molecules (miRNAs, proteins, lipids) that distinguish beneficial from pathogenic EVs; (2) implementation of standardized preparation, storage, and functional validation guidelines to ensure reproducibility (63, 69); and (3) combinatorial strategies integrating EV therapy with biomaterials, pharmacological agents, or cell-based interventions for synergistic efficacy, while addressing safety and regulatory concerns (70–73).
Taken together, immunocyte-derived EVs represent pivotal regulators of osteoimmunology, capable of driving both tissue repair and pathology. Advances in engineering and biomaterial integration provide strong momentum toward clinical application. Emerging platforms—including single-EV omics, artificial intelligence–driven biomarker discovery, and checkpoint- or exercise-mimetic vesicles—further expand the therapeutic landscape. With sustained collaboration among immunologists, bioengineers, and clinicians, these innovations delineate a realistic roadmap from mechanistic discovery to clinical translation.
Author contributions
LY: Investigation, Writing – original draft, Funding acquisition, Visualization, Software, Resources, Formal Analysis, Validation, Conceptualization, Supervision, Project administration, Methodology, Writing – review & editing, Data curation. NL: Writing – review & editing, Methodology, Supervision, Project administration. HX: Writing – review & editing, Investigation, Conceptualization. JC: Validation, Visualization, Writing – review & editing, Formal Analysis. QL: Conceptualization, Validation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 82474534); Shanghai’s Dongfang Yingcai Program Leading Project; Key projects of the Shanghai Municipal Science and Technology Commission (Grant No. 23Y31920100, 25Y12800800); the 2022 Emerging Interdisciplinary Research Special Project of the Shanghai Municipal Health Commission (Grant No. 2022JC005); the National Natural Science Foundation of China (Grant No. 82305281); the Science and Technology Innovation Action Plan in Shanghai (Grant No. 23YF1447900); and the Shanghai Education Commission Morning Light Program” project (Grant No. 23CGA53).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AKT/eNOS/NO axis, Protein kinase B/endothelial nitric oxide synthase/nitric oxide pathway; BMSCs, Bone marrow stromal cells; BMSC-EVs, Bone marrow stromal cell-derived extracellular vesicles; DC, Dendritic cell; DC-EVs, Dendritic cell-derived extracellular vesicles; EVs, Extracellular vesicles; Exo, Exosomes; Exo@Tβ4, Thymosin β4-modified HAMA hydrogels loaded with exosomes; HAMA, Hyaluronic acid methacrylate; MSC, Mesenchymal stem cell; OA, Osteoarthritis; OPG, Osteoprotegerin; RA, Rheumatoid arthritis; RANKL, Receptor activator of nuclear factor κB ligand; sEVs, Small extracellular vesicles; Tβ4, Thymosin β4; Th17, T helper 17 cell; TRAF6, TNF receptor-associated factor 6; Treg, Regulatory T cell.
References
1. Kaji H. Bone-muscle interactions. Osteoporosis Sarcopenia. (2025) 11:32–9. doi: 10.1016/j.afos.2025.04.001
2. Biswas S, Gangadaran P, Dhara C, Ghosh S, Phadikar SD, Chakraborty A, et al. Extracellular vesicles in osteogenesis: A comprehensive review of mechanisms and therapeutic potential for bone regeneration. Curr Issues Mol Biol. (2025) 47:675. doi: 10.3390/cimb47080675
3. Yue G, Dai X, Shi H, Shen J, Guo H, Liang R, et al. Harnessing extracellular vesicles as an emerging diagnostic and therapeutic strategy for osteoporosis. Biomaterials Science. (2025) 13(19):5260–77. doi: 10.1039/d5bm00537j
4. Meng F, Yang C, Li N, and Wang H. Progress in understanding the role and mechanism of miRNAs in osteoporosis. Front Endocrinol. (2025) 16:1544944. doi: 10.3389/fendo.2025.1544944
5. Chen X, Zou J, Su Z, Liang P, Ye Y, Ye Z, et al. Acetylcholine Suppression by P. gingivalis Extracellular Vesicles Drives Osteoclastogenesis and Bone Loss via the Cyp4f40. FASEB J. (2025) 39. doi: 10.1096/fj.202500112rr
6. Dabaliz A, Mahmoud H, AlMutawa R, and Mohammad KS. Sending the signal to bone: how tumor-derived EVs orchestrate pre-metastatic niche formation and skeletal colonization. Biomedicines. (2025) 13:1640. doi: 10.3390/biomedicines13071640
7. Gatti M, Beretti F, Malenchini M, Bertucci E, Ceneri E, Follo MY, et al. Mesenchymal stromal cell-derived extracellular vesicles as a therapeutic treatment for osteosarcopenia: crosstalk among neurons, muscle, and bone. Int J Mol Sci. (2025) 26:7875. doi: 10.3390/ijms26167875
8. Dell’Atti F, Abreu H, Malfa P, Raineri D, Cappellano G, and Chiocchetti A. Probiotic-derived extracellular vesicles: the next breakthrough in postbiotics for rheumatoid arthritis. Front Immunol. (2025) 16:1620185. doi: 10.3389/fimmu.2025.1620185
9. Rojas C, García M, González-Osuna L, Campos-Mora M, de León EP, Sierra-Cristancho A, et al. Induced treg-derived extracellular vesicles suppress CD4+ T-cell-mediated inflammation and ameliorate bone loss during periodontitis partly through CD73/adenosine-dependent immunomodulatory mechanisms. J Extracellular Vesicles. (2025) 14. doi: 10.1002/jev2.70118
10. Sgaglione J, Neufeld EV, Swami P, and Grande DA. Clinical status of exosomes: A review. HSS Journal®: Musculoskeletal J Hosp Special Surgery. (2025). doi: 10.1177/15563316251362179
11. Zagrodnik JL and Moore CS. From cellular waste to biomarkers; insights into past, present, and future methods to detect immune cell-derived extracellular vesicles using flow cytometry. J Pharmacol Toxicological Methods. (2025) 135:108376. doi: 10.1016/j.vascn.2025.108376
12. Castro-Manrreza M, Romano LE, López-García L, Medina-Contreras O, and Montesinos J. Persistent stimulation of human mesenchymal stem/stromal cells with TNF-α and IFN-γ Affects the release of large extracellular vesicles with immunoregulatory phenotype. Stem Cells Dev. (2025) 34:291–303. doi: 10.1089/scd.2025.0064
13. Kuo TY, Lin TL, Lin YH, Chen CY, Cho DY, Chen YW, et al. Lithium-doped calcium silicate scaffolds-activated M2-polarized macrophage-derived miR-145-5p-riched extracellular vesicles to enhance osteoimmunomodulation for accelerating bone regeneration. J Nanobiotechnology. (2025) 23. doi: 10.1186/s12951-025-03679-2
14. Dong C, Wei L, Zhu W, Kim JK, Wang Y, Omotara P, et al. Mature dendritic cell-derived extracellular vesicles are potent mucosal adjuvants for influenza hemagglutinin vaccines. ACS Nano. (2025) 19:25526–42. doi: 10.1021/acsnano.5c08831
15. Brenis Gómez CM, Plasencia-Rodríguez C, Novella-Navarro M, Martínez-Feito A, Balsa A, Calvo-Aranda E, et al. Exosomal protein biomarkers in arthritis: deciphering the inflammatory profiles of RA and OA. Biomedicines. (2025) 13:1283. doi: 10.3390/biomedicines13061283
16. Brouns AJWM, Robbesom-van den Berge IJ, Ernst SM, Steendam CMJ, Woud WW, Wu L, et al. Connecting the dots: (RANKL+) extracellular vesicle count in blood plasma in relation to bone metastases, skeletal related events and osimertinib treatment in patients with EGFR mutated non-small cell lung cancer. Trans Lung Cancer Res. (2025) 14:761–74. doi: 10.21037/tlcr-24-1007
17. Yao Y, Yin Y, Shuai F, Lam W, Zhou T, Xie Y, et al. M2 macrophage-derived extracellular vesicles reprogram immature neutrophils into anxa1hi neutrophils to enhance inflamed bone regeneration. Advanced Sci. (2025) 12. doi: 10.1002/advs.202416159
18. Li Y, Yan Z, Dai Y, Cai H, Chen Y, Chen Y, et al. Mechanical force promotes mitochondrial transfer from macrophages to BMSCs to enhance bone formation. Cell Proliferation. (2025). doi: 10.1111/cpr.70121
19. Liu W, Wang X, Zhang X, Yang Y, and Bai S. Shear stress-mediated downregulation of miR-423-5p in M2 macrophage exosomes promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Int Immunopharmacology. (2025) 164:115298. doi: 10.1016/j.intimp.2025.115298
20. Zhang Y, Liang Y, and Zhou Y. M2 polarization of RAW264.7-derived exosomes inhibits osteoclast differentiation and inflammation via PKM2/HIF-1α Axis. Immunol Investigations. (2025) 54(7):1195–209. doi: 10.1080/08820139.2025.2525896
21. Liu S, Wang Z, Li Y, Pan Z, Huang L, Cui J, et al. Erythropoietin-stimulated macrophage-derived extracellular vesicles in chitosan hydrogel rescue BMSCs fate by targeting EGFR to alleviate inflammatory bone loss in periodontitis. Advanced Sci. (2025) 12. doi: 10.1002/advs.202500554
22. Chen J, Chen J, Chen J, Lu R, Liu Z, Zhang Y, et al. Pretreated exosomes by electrical stimulation accelerate bone regeneration. Bioactive Materials. (2025) 51:383–98. doi: 10.1016/j.bioactmat.2025.04.019
23. Li D, Yang T, Li Y, Lyu X, Hu C, Yan J, et al. Adipose tissue macrophages as initiators of exacerbated periodontitis in estrogen-deficient environments via the amplifier extracellular vesicles. Advanced Science. (2025). doi: 10.1002/advs.202506121
24. Dong J, Liao Y, Sun M, Chen H, Zhou K, Zhang H, et al. Gingipains disrupt bone homeostasis via dual regulation of osteogenesis and osteoclastogenesis through exosomal miR-146a-5p/TRAF6 signaling. Front Cell Infection Microbiol. (2025) 15:1614126. doi: 10.3389/fcimb.2025.1614126
25. Yang J, Zhang H, Wang W, Yin Q, He X, Tao D, et al. CD80 antibody and MTX co-engineered extracellular vesicles targets CD80+ Macrophages to suppress inflammation and alleviate chronic inflammatory diseases. Int J Nanomedicine. (2025) 20:6379–98. doi: 10.2147/ijn.s517357
26. Wang J, Gu Y, Wang J, Zhang N, Han X, and Bai Y. Exosomes derived from BMMSCs promote B10 cell differentiation but not IL-10 production. Cell Biochem Funct. (2025) 43. doi: 10.1002/cbf.70083
27. Dou Y, Yu S, Cao S, Gao K, Lv M, He Y, et al. BMSC-derived exosomal CD73 mediated macrophage polarization promotes osteoblastic differentiation in diabetes. Exp Cell Res. (2025) 450:114653. doi: 10.1016/j.yexcr.2025.114653
28. Wang H, Tan Q, Duan Y, Wu M, Zuo B, and Li J. VPS33B-dependent exosomes modulate cellular senescence of mesenchymal stem cells via an autocrine signaling pathway. Exp Gerontology. (2025) 207:112786. doi: 10.1016/j.exger.2025.112786
29. Choi EW, Lim IR, Park JH, Song J, Choi B, and Kim S. Therapeutic effects of CTLA4Ig-overexpressing mesenchymal stem cell-derived extracellular vesicles in a mouse model of rheumatoid arthritis. Stem Cell Res Ther. (2025) 16. doi: 10.1186/s13287-025-04524-x
30. Zhang X, Gao H, and Lin L. The extracellular vesicle-based treatment: a developing strategy for periodontal diseases. Front Immunol. (2025) 16:1480292. doi: 10.3389/fimmu.2025.1480292
31. Gegout PY, Mary B, Stutz C, Hyenne V, Ginesin O, Zigdon-Giladi H, et al. Porphyromonas gingivalis infection of oral keratinocytes drives the release of pro-inflammatory extracellular vesicles. Sci Rep. (2025) 16(1):374. doi: 10.1038/s41598-025-10078-y
32. Zuo R, Cao B, Kong L, Wang F, Li S, Shan H, et al. MiR-370-3p regulate TLR4/SLC7A11/GPX4 to alleviate the progression of glucocorticoids-induced osteonecrosis of the femoral head by promoting osteogenesis and suppressing ferroptosis. J Orthopaedic Translation. (2025) 51:337–58. doi: 10.1016/j.jot.2024.10.014
33. Qu M, Liu Y, Yang G, and Xing H. Engineered bifunctional extracellular vesicles simultaneously promoting senolysis and efferocytosis for anti-aging therapy in diabetic mice. Biochem Biophys Res Commun. (2025) 778:152400. doi: 10.1016/j.bbrc.2025.152400
34. Liu H, Li R, Yang H, Situ B, Wang G, Xu K, et al. Extracellular vesicles in gut-bone axis: novel insights and therapeutic opportunities for osteoporosis. Small Sci. (2024) 5(4):2400474. doi: 10.1002/smsc.202400474
35. Chen X, Li H, Wang G, Wang Z, Lv Y, Xie H, et al. Exploring the role of intestinal pathogenic bacteria in metronidazole-induced bone loss: focus on Klebsiella variicola. Gut Pathog. (2025) 17(1):42. doi: 10.1186/s13099-025-00713-4
36. Tao SC and Guo SC. Extracellular vesicles in bone: “dogrobbers” in the “eternal battle field. Cell Communication Signaling. (2019) 17. doi: 10.1186/s12964-019-0319-5
37. Silva FRF, Heredia JE, Arntz OJ, Barrioni BR, Teixeira MM, Silva TA, et al. Bovine milk extracellular vesicles as a preventive treatment for bone dysfunction and metabolic alterations in obese mice fed a high-refined carbohydrate diet. Mol Nutr Food Res. (2025). doi: 10.1002/mnfr.70139
38. Hu W, Xie X, and Xu J. Epimedium-derived exosome-loaded gelMA hydrogel enhances MC3T3-E1 osteogenesis via PI3K/akt pathway. Cells. (2025) 14:1214. doi: 10.3390/cells14151214
39. Zhao M, Chen X, Wang W, Li M, Zhang H, Zou X, et al. Sea buckthorn-derived extracellular vesicles foster bone regeneration through aau-miR168-mediated pathways. Stem Cell Res Ther. (2025) 16. doi: 10.1186/s13287-025-04373-8
40. Zhang Z, Liu L, Ti H, Chen M, Chen Y, Du D, et al. Synovial fibroblast derived small extracellular vesicles miRNA15-29148 promotes articular chondrocyte apoptosis in rheumatoid arthritis. Bone Res. (2025) 13. doi: 10.1038/s41413-025-00430-3
41. Rossi F, Santonicola S, Giaccone V, Truant A, and Colavita G. Dairy propionibacteria: probiotic properties and their molecular bases. Biomolecules. (2025) 15:886. doi: 10.3390/biom15060886
42. Huang S, Li Y, Wu P, Xiao Y, Duan N, Quan J, et al. microRNA-148a-3p in extracellular vesicles derived from bone-marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of the femoral head. J Cell Mol Med. (2020) 24:11512–23. doi: 10.1111/jcmm.15766
43. Wang Y, Zhao M, Li W, Yang Y, Zhang Z, Ma R, et al. BMSC-derived small extracellular vesicles induce cartilage reconstruction of temporomandibular-joint osteoarthritis via autotaxin–YAP signaling axis. Front Cell Dev Biol. (2021) 9:656153. doi: 10.3389/fcell.2021.656153
44. Ehab S, Gaser OA, Oyouni AAA, Kameli N, Alzahrani F, and Abdal Dayem A. Engineered extracellular vesicles in arthritic diseases: therapeutic applications & Challenges. WIREs Nanomedicine Nanobiotechnology. (2025) 17. doi: 10.1002/wnan.70031
45. Chen O, Zhou Y, Xu Z, Liu X, Zhang D, and Bai M. Engineered biomembrane-camouflaged nanoparticles: Promising strategies to treat inflammatory skeletal diseases. J Controlled Release. (2025) 385:114025. doi: 10.1016/j.jconrel.2025.114025
46. Mao MZ, Zheng MH, Guo B, Ling YL, Lin X, Li FX, et al. FNDC5/irisin-enriched sEVs conjugated with bone-targeting aptamer alleviate osteoporosis: a potential alternative to exercise. J Nanobiotechnology. (2025) 23. doi: 10.1186/s12951-025-03587-5
47. Kong X, Liu H, Chen S, Liu Z, Chen Q, Li X, et al. Bioengineered bacterial extracellular vesicles for targeted delivery of an osteoclastogenesis-inhibitory peptide to alleviate osteoporosis. J Controlled Release. (2025) 382:113751. doi: 10.1016/j.jconrel.2025.113751
48. Cui X, Li J, Wang Y, Sun T, Weng J, Li Z, et al. Choline phosphate surface-activated 3d-printed porous titanium scaffold combined with stem cell exosomes for enhancing bone defects repair. ACS Appl materials interfaces. (2025) 17:27788–805. doi: 10.1021/acsami.5c00953
49. Bai Y, Zhao J, Abtahi M, and Liu X. Therapeutic potential of miR-10a overexpressing mesenchymal stem cell–derived extracellular vesicles in modulating inflammation in collagen-induced arthritis. In Vitro Cell Dev Biol - Animal. (2025). doi: 10.1007/s11626-025-01098-z
50. Gao Y, Li X, Ding Y, Wang Y, Du J, Chen Y, et al. MiR-451a-enriched small extracellular vesicles derived from mg2+-activated DPSCs induce vascularized bone regeneration through the AKT/eNOS/NO axis. ACS Appl Materials Interfaces. (2025) 17:31345–56. doi: 10.1021/acsami.5c02551
51. Li S, Sheng Y, You Y, Wang Y, Zhang Y, Tao J, et al. Study on VEGFA mRNA delivery via GelMA hydrogel-encapsulated extracellular vesicles for enhanced bone regeneration. Materials Today Bio. (2025) 34:102144. doi: 10.1016/j.mtbio.2025.102144
52. Peng XZ, Xie CY, Zhuo JJ, Wang Y, Sun ZH, Liu J, et al. Extracellular vesicles derived from aspirin-treated macrophages promote osteochondral tissue regeneration. J Controlled Release. (2025) 385:114052. doi: 10.1016/j.jconrel.2025.114052
53. Lv N, Li H, Hong L, Qian Z, Yuan F, Sun H, et al. An electrospun membrane incorporating lipocalin 2-enhanced exosomes effectively facilitated the repair of critical bone defects. Int J Biol Macromolecules. (2025) 322:146674. doi: 10.1016/j.ijbiomac.2025.146674
54. Xi Y, Zhang Z, Zhao Z, Qiu B, Wang W, Xu G, et al. Injectable thymosin β4-modified hyaluronic acid hydrogel with exosomes for stem cell homing and neuronic–angiogenic–osteogenic coupled cranial repair. ACS Nano. (2025) 19:22710–24. doi: 10.1021/acsnano.4c10386
55. Luo L, Zheng W, Li J, Chen T, Xue W, Lin T, et al. 3D-printed titanium trabecular scaffolds with sustained release of hypoxia-induced exosomes for dual-mimetic bone regeneration. Advanced Sci. (2025) 12. doi: 10.1002/advs.202500599
56. Rydland A, Heinicke F, Nyman TA, Trøseid A-M, Flåm ST, Stensland M, et al. Plasma extracellular vesicle proteomics distinguishes treatment-naïve rheumatoid arthritis from controls. Sci Rep. (2025) 15:10211. doi: 10.1038/s41598-025-10211-5
57. Jouybari MT, Mojtahedi F, Babaahmadi M, Faeed M, Eslaminejad MB, Taghiyar L, et al. Extracellular vesicles as diagnostic and therapeutic tools in rheumatoid arthritis: a systematic review. Front Immunol. (2024) 15:118765. doi: 10.3389/fimmu.2024.118765
58. Arntz OJ, Thurlings RM, Blaney Davidson EN, Jansen PWTC, Vermeulen M, Koenders MI, et al. Profiling of plasma extracellular vesicles identifies proteins that strongly associate with patient's global assessment of disease activity in rheumatoid arthritis. Front Med (Lausanne). (2024) 10:1247778. doi: 10.3389/fmed.2023.1247778
59. Ma F, Liu X, Zhang Y, Tao Y, Zhao L, Abusalamah H, et al. Tumor extracellular vesicle-derived PD-L1 promotes T cell senescence through lipid metabolism reprogramming. Sci Transl Med. (2025) 17(785):eadm7269. doi: 10.1126/scitranslmed.adm7269
60. Wang Q, Sun J, Jiang H, and Yu M. Emerging roles of extracellular vesicles in oral and maxillofacial areas. Int J Oral Sci. (2025) 17(1):11. doi: 10.1038/s41368-024-00341-9
61. Zhao R, Chen Y, Wang D, Zhang C, Song H, Ni G, et al. Role of irisin in bone diseases. Front Endocrinol (Lausanne). (2023) 14:1212892. doi: 10.3389/fendo.2023.1212892
62. Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. (2019) 11(43):20884–92. doi: 10.1039/c9nr02791b
63. Welsh JA, Goberdhan DC, O'Driscoll L, Théry C, and Witwer KW. MISEV2023: An updated guide to EV research and applications. J Extracell Vesicles. (2024) 13:e12416. doi: 10.1002/jev2.12416
64. Adamo G, Picciotto S, Gargano P, Paterna A, Raccosta S, Rao E, et al. DetectEV: A functional enzymatic assay to assess integrity and bioactivity of extracellular vesicles. Journal of Extracellular Vesicles. (2025) 14(1):e70030. doi: 10.1002/jev2.70030
65. Qu M, Liu Y, Yang G, and Xing H. Engineered bifunctional extracellular vesicles promoting senolysis and efferocytosis for anti-aging therapy. Biochem Biophys Res Commun. (2025) 778:152400. doi: 10.1016/j.bbrc.2025.152400
66. Cheng K and Kalluri R. Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracellular Vesicle. (2023) 2:100029. doi: 10.1016/j.vesic.2023.100029
67. Clarke EJ, Chabronova A, and Peffers MJ. Extracellular vesicles in cartilage homeostasis, osteoarthritis, and biomarker discovery. Connective Tissue Res. (2025) 66(5):428–34. doi: 10.1080/03008207.2025.2524064
68. Ahmad P, Estrin N, Farshidfar N, Zhang Y, and Miron RJ. Isolation methods of exosomes derived from dental stem cells. Int J Oral Science. (2025) 17. doi: 10.1038/s41368-025-00370-y
69. Xiang K, Hao M, Zhang Z, Zhang K, Sun H, and Zhang L. Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration. Materials Today Bio. (2025) 32:101881. doi: 10.1016/j.mtbio.2025.101881
70. Chatzopoulos MK, Fragoulis GE, Samiotaki M, Tektonidou MG, Sfikakis PP, and Vetsika EK. Small extracellular vesicle cargo as biomarkers in autoimmune rheumatic diseases: a systematic review. Rheumatol Int. (2025) 45. doi: 10.1007/s00296-025-05953-w
71. Gao YF, Zhao N, and Hu CH. Harnessing mesenchymal stem/stromal cells-based therapies for rheumatoid arthritis: mechanisms, clinical applications, and microenvironmental interactions. Stem Cell Res Ther. (2025) 16. doi: 10.1186/s13287-025-04495-z
72. Zhou G, Zhou Q, Li R, Sheng S, Gao Q, Zhou D, et al. Synthetically engineered bacterial extracellular vesicles and IL-4-encapsulated hydrogels sequentially promote osteoporotic fracture repair. ACS Nano. (2025) 19:16064–83. doi: 10.1021/acsnano.5c03106
Keywords: extracellular vesicles, osteoimmunology, bone remodeling, macrophages, rheumatoid arthritis, osteoporosis, periodontitis, biomaterial-integrated EVs
Citation: Yang L, Li N, Xu H, Chen J and Liang Q (2025) Immunocyte-derived extracellular vesicles in osteoimmunology: mechanisms, disease contexts, and translational prospects. Front. Immunol. 16:1709048. doi: 10.3389/fimmu.2025.1709048
Received: 23 September 2025; Accepted: 10 November 2025; Revised: 11 October 2025;
Published: 24 November 2025.
Edited by:
Changjun Li, Central South University, ChinaReviewed by:
Maoxiao Ma, Luoyang Orthopedic Traumatological Hospital, ChinaCopyright © 2025 Yang, Li, Xu, Chen and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianqian Liang, bGlhbmdxaWFucWlhbkBzaHV0Y20uZWR1LmNu
 Lixuan Yang
Lixuan Yang Ning Li1,2,4,5
Ning Li1,2,4,5 Qianqian Liang
Qianqian Liang