- 1Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 2Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 3Consorzio Interuniversitario Biotecnologie (CIB), Trieste, Italy
The NLRP3 inflammasome is a master regulator of neuroinflammation, linking systemic perturbations to brain dysfunction and thereby influencing overall brain health. Its sensitivity to biological sex and environmental factors suggests that NLRP3 may act both as a contributor to sex-dependent disease mechanisms and a modifiable therapeutic target for pharmacological and non-pharmacological interventions. In this mini-review, we summarize emerging evidence on sex-specific differences in NLRP3 signaling that may contribute to disparities between males and females in disease incidence, symptomatology, and treatment response. Neuroinflammation-driven disorders, including atherosclerosis, neuropathic pain, substance use, and stress-related syndromes, show how sex influences NLRP3 inflammasome expression and activity with downstream effects on cognition and behavior. We also examine the modulatory influence of environmental factors, with emphasis on social behavior and environmental enrichment, as determinants of NLRP3 dynamics relevant to neurocognitive function and brain health. Overall, the findings suggest that NLRP3 acts as a central hub integrating sex and environmental influences, with broad implications for personalized interventions in brain-related disorders.
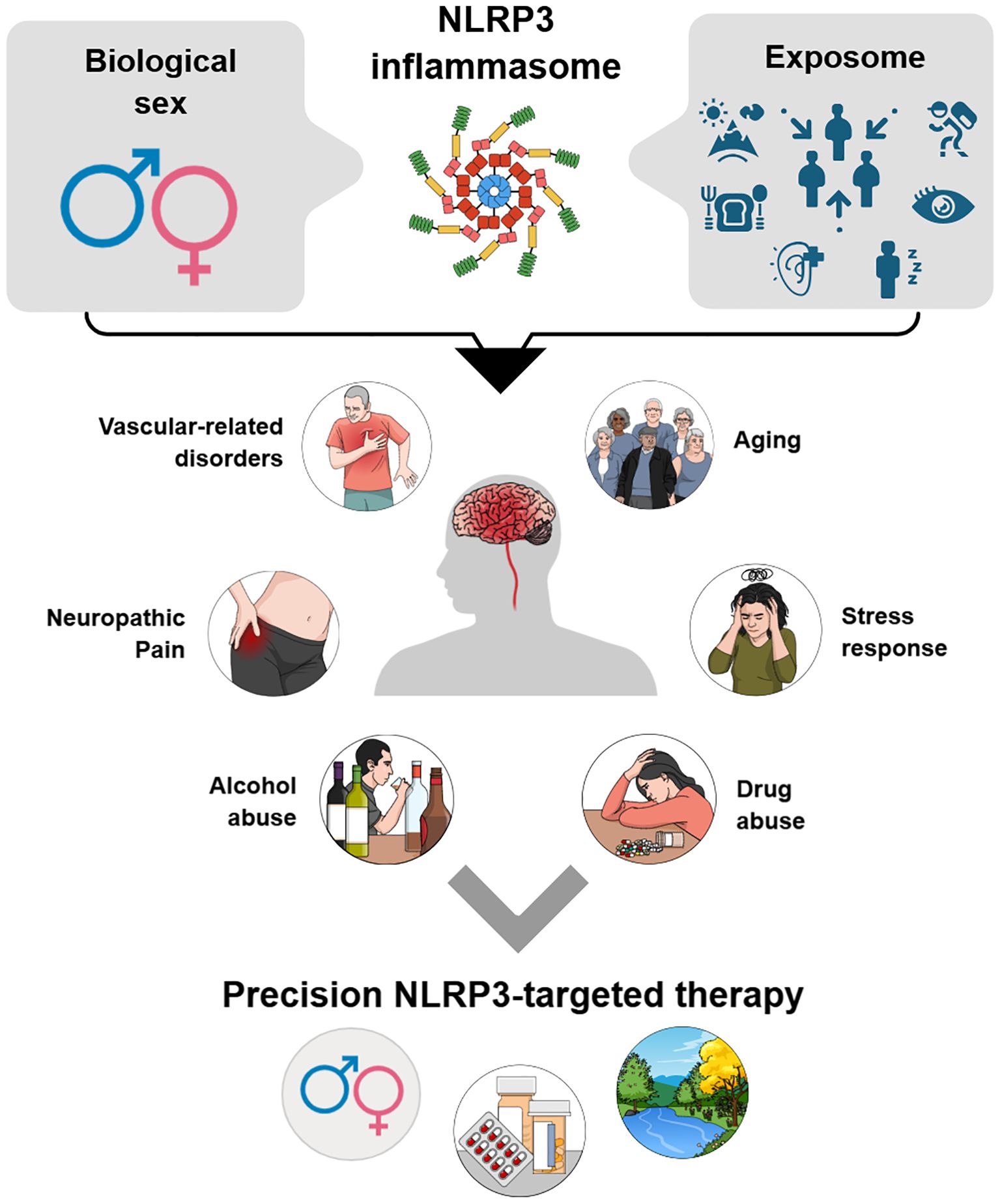
Graphical Abstract. The NLRP3 inflammasome emerges as a central hub at the intersection of biological sex and exposome influences, integrating genetic, hormonal, and lifestyle-related inputs. Its dysregulation contributes to multiple brain-related conditions, including vascular disorders, neuropathic pain, aging, stress responses, and substance abuse. Understanding these modulatory axes highlights opportunities for precision NLRP3-targeted therapies tailored to sex-specific and environmental determinants.
1 Introduction
The inflammasome family comprises cellular multiprotein complexes that detect stress and danger signals, supporting host defense and damage resolution. Key platforms include NLRP1, NLRP3, NLRC4, AIM2, and Pyrin, with NLRP3 being the best-characterized regulator of early neuroinflammatory responses (1, 2). Its activation occurs in two phases: (i) priming, defined by NF-κB-dependent transcriptional upregulation of NLRP3, pro-IL-1β, and pro-IL-18; and (ii) activation triggered by stressors such as mitochondrial dysfunction, ROS production, potassium efflux, and lysosomal rupture, leading to assembly with ASC and pro-caspase-1 (3). This cascade culminates in caspase-1 activation, GSDMD-mediated pyroptosis, and maturation of IL-1β and IL-18 (4, 5). Although protective, dysregulation fosters chronic inflammation with profound systemic consequences, including impaired brain function. NLRP3 overactivation contributes to neurodegeneration across multiple conditions, such as depression, Alzheimer’s disease (AD), ischemic injury, and metabolic-associated cognitive decline, making it a major therapeutic target (6–9).
Emerging data highlight sex-based differences in NLRP3 expression, regulation, and function, shaping disease risk, patient outcomes, and treatment efficacy, and underscoring the importance of including sex as a biological variable in neuroimmunology and inflammasome-targeted therapies (10–12). NLRP3 is also highly sensitive to environmental inputs, including sensory stimulation, physical activity, and psychosocial experiences, which modulate its activity and impact brain development, neuroplasticity and function (13–15). Thus, NLRP3 emerges as a modifiable determinant of neurobehavioral health and disease vulnerability.
Building on this, this mini-review integrates current evidence on emerging sex-related differences in NLRP3 inflammasome across conditions involving cognitive and behavioral impairments. In parallel, within the broader context of environmental influences, we examine two critical dimensions of social and environmental exposure, social isolation and environmental enrichment, and their effects on the NLRP3 pathway with downstream implications for cognition, behavior, and brain health.
2 Methods
The literature search was performed in PubMed, Web of Science, Scopus, and Google Scholar, restricted to English publications and updated to August 15, 2025. Keywords included “NLRP3 inflammasome,” “sex differences OR male OR female,” “enriched environment,” “social isolation,” “exercise OR physical activity,” “central nervous system,” “cognition”. Eligible studies were in vivo models assessing sex differences and/or environmental influences on NLRP3 activity in CNS-related conditions. Exclusion criteria were in vitro-only studies, non-English reports, narrative reviews, or works without NLRP3 measures. In total, 25 studies, mostly preclinical, were included, addressing how sex and environmental factors, conceptualized as somatosensory-rich contexts and social behaviors, modulate NLRP3 signaling in CNS pathophysiology.
3 Sex-specific modulation of the NLRP3 inflammasome in systemic disorders affecting brain health
Sex differences in immune-inflammatory responses are increasingly recognized for their impact on health and disease. Biological sex shapes immune activity at peripheral and central levels across the lifespan through interaction among hormones, chromosomal factors, and environmental influences (16–19). Males and females differ in the magnitude and quality of inflammatory responses, including immune cell activation, signaling pathways, and downstream processes (20). These variations influence disease susceptibility, progression, and therapeutic outcomes (21–23). The NLRP3 inflammasome is emerging as a pathway modulated by sex hormones, which exert context-dependent effects and contribute to vulnerability or resilience in brain disorders (23–25). Sex-related differences in NLRP3 signaling are reported both at baseline and under inflammatory challenges, varying across tissues, developmental stages, and disease models (26, 27). The following section examines CNS related conditions where NLRP3 is crucial, focusing on in vivo evidence of sex-dependent differences in its expression and activity (Table 1).
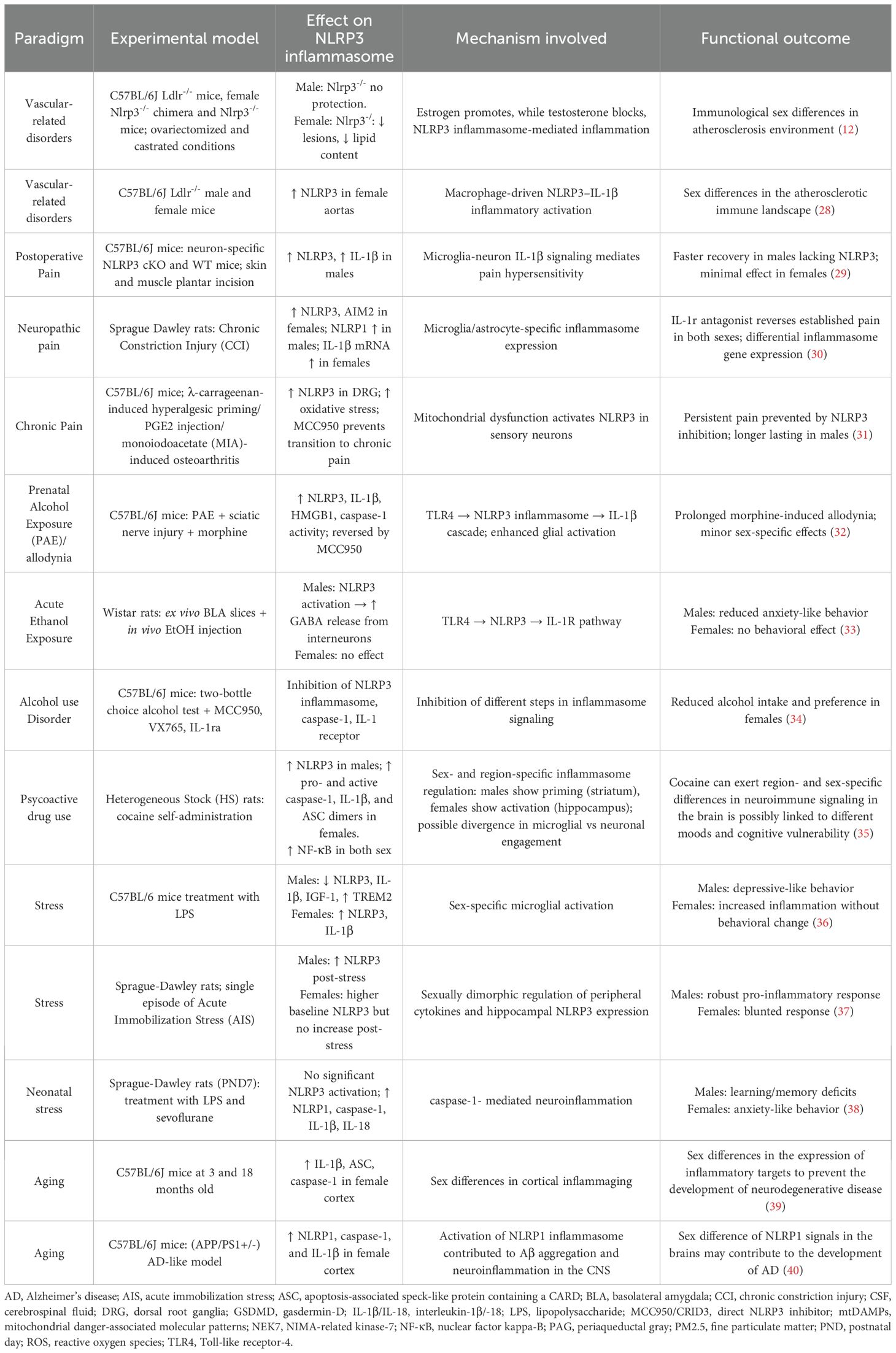
Table 1. Experimental evidence of sex differences in NLRP3 inflammasome expression/activation across systemic conditions with neurological impact.
3.1 Vascular-related disorders
Vascular dysfunction, manifesting endothelial impairment, altered cerebrovascular reactivity, or vessel damage, is a hallmark of multiple CNS affecting disorders by promoting neuroinflammation. Extensive evidence emphasizes the causal role of NLRP3 in driving endothelial dysfunction and atherogenesis (41–43). Targeting NLRP3 reduces vascular inflammation and delays CNS complications (44, 45). Differences in atherosclerosis between sexes may, in part, arise from varied activation of NLRP3 (12, 28) (Table 1). Chen et al. (2020) demonstrated that NLRP3 contributes to atherogenesis in a sex-dependent manner via estrogen-mediated regulation: their study demonstrated that ovariectomy condition in a valuable model of atherosclerosis, such as the low-density lipoprotein receptor gene knockout mice (Ldlr-/-), revealed that NLRP3 deficiency markedly reduced plaque formation in middle-aged females, lowering caspase-1 activation, IL-1β release, and immune cell infiltration. These protective effects were absent in castrated males, where testosterone depletion heightened reliance on NLRP3 activity (12).
A recent multi-modal immunophenotyping study showed that biological sex shapes immune cell composition in aged atherosclerotic plaques via NLRP3 signaling: aged Ldlr-/- females exhibited stronger pro-inflammatory macrophage responses, whereas males displayed a distinct profile marked by greater CD8+ T cell and dendritic cell involvement (28). Together, these studies indicate that estrogen amplifies NLRP3-driven vascular inflammation, while testosterone exerts a protective, anti-inflammatory role in atherosclerotic progression.
3.2 Neuropathic pain
Chronic neuropathic pain, marked by maladaptive central sensitization, involves dysfunction of the prefrontal cortex, hippocampus, and amygdala (46, 47). A shared mechanism across chronic pain syndromes (e.g. diabetic neuropathy, fibromyalgia, and spinal cord injury) is the neuroimmune activation, largely mediated by microglial NLRP3 signaling. This promotes IL-1β/IL-18 releases and sustains pain through neuroinflammatory feedback loops (48, 49). Growing recognition exists for sex-based differences in pain perception with distinct cellular and molecular inflammasome dynamics (48–51) (Table 1). In a postoperative pain model, Cowie et al. (2019) showed that males relied on both neuronal and non-neuronal NLRP3 for IL-1β-mediated hypersensitivity, whereas females depended exclusively on neuronal NLRP3, making inhibition less effective (29). Using a chronic constriction injury (CCI) model of the sciatic nerve, Green-Fulgham et al. (2024) reported sex-specific inflammasome signaling in the spinal cord: female mice showed higher IL-1β, NLRP3, and AIM2 expression, whereas males exhibited increased levels of NLRP1. Notably, IL-1 receptor antagonist treatment reversed pain behaviors in both sexes, indicating shared therapeutic pathways despite divergent molecular mechanisms (30). In an osteoarthritis model, Ribeiro et al. (2023) found that neuronal NLRP3 involvement in dorsal root ganglia, driven by mitochondrial dysfunction, mediated the transition to chronic pain. Pharmacological inhibition of NLRP3 with the selective inhibitor MCC950 was effective in both sexes, but had longer-lasting effects in males, suggesting sex-dependent regulation (31). Additionally, prenatal alcohol exposure also exacerbated adult pain via NLRP3 hyperactivation, especially in male mice, accompanied by spinal glial reactivity and upregulation of HMGB1, a key amplifier of inflammasome activation. MCC950 treatment reversed these changes, indicating long-term sex-specific neuroimmune programming. Finally, spared nerve injury-induced pain in female mice caused stronger mechanical hypersensitivity and greater NLRP3 activation in the midbrain periaqueductal gray compared with males, further supporting sex-dimorphic inflammasome activity in central pain circuits (32).
3.3 Substance use disorders
Chronic substance use induces persistent neurobiological changes impairing behavior, motor function, emotion, and cognition (52). Individuals with substance use disorders face greater risk from comorbidities, drug interactions, and polypharmacy, which exacerbate neuroinflammation (53). The NLRP3 inflammasome is a pivotal mediator of addiction, activated by alcohol and other psychoactive agents in both brain and periphery (54, 55). Indeed, its inhibition has been demonstrated to reduce drug-seeking, withdrawal anxiety, relapses, and neurobehavioral symptoms, underscoring therapeutic potential (34, 56).
3.3.1 Alcohol consumption
Alcohol abuse compromises brain immunity through NLRP3 inflammasome overactivation, driving caspase-1 activation, IL-1β release, pyroptosis, and impaired autophagy/mitophagy and mitochondrial function, which further sustain pathway activity (57–61). These mechanisms, prominent in the prefrontal cortex, hippocampus, amygdala, cerebellum, and cerebral cortex, underlie behavioral and cognitive deficits (62–65). Although microglial NLRP3 activation is central, neurons, astrocytes, and impaired microglia-neuron communication also contribute (60, 65). Notably, pharmacological NLRP3 inhibition reduces inflammation and improves behavioral outcomes in alcohol-related brain injury (66, 67).
Although early studies focused on male models, recent findings highlight sex-dependent differences in NLRP3 activation and alcohol responses (Table 1). Munshi et al. (2023) showed that acute alcohol exposure in adolescent and young adult rats activated NLRP3 in the basolateral amygdala only in males, inducing GABAergic inhibition and anxiety-like behaviors, while females remained unaffected (33). Similarly, Lowe et al. (2020) reported that inflammasome inhibition via MCC950, VX765 (caspase-1 inhibitor), or anakinra (IL-1 receptor antagonist) reduced alcohol intake in females, whereas in males only MCC950 and anakinra were effective, suggesting sex-specific differences in downstream inflammasome signaling (34). Together, these findings indicate that alcohol-related modulation of NLRP3 signaling is strongly sex-dependent. Males appeared more vulnerable to NLRP3-mediated neuroinflammatory and behavioral alterations, whereas females displayed differential sensitivity to inflammasome inhibition, suggesting distinct downstream signaling or compensatory immune mechanisms between sexes.
3.3.2 Psychoactive drug consumption
Opioids, stimulants, and other psychoactive substances trigger NLRP3 inflammasome activation in central regions such as the dorsal raphe nucleus, hippocampus, piriform cortex, and amygdala, initiating neuroinflammation that impairs cognition, mood, and pain processing, also promoting neurodegeneration (55, 68–73). Oxidative stress, mitochondrial dysfunction, and impaired autophagy, exacerbate neural vulnerability (54). NLRP3 activation occurs in microglia, astrocytes, and neurons varying by drug and cell type. Pharmacological targeting of this pathway shows promise in reducing substance-induced neurotoxicity and behavioral alterations (69). Notably, Cheng et al. (2023) demonstrated that cocaine self-administration induced neuroinflammation through sex-specific mechanisms: in females, hippocampal NLRP3 activation occurred independently of CRF signaling, which regulates the NF-κB-NLRP3 pathway via the HPA axis; in males, by contrast, striatal NLRP3 expression was increased alongside CRF signaling (35) (Table 1).
3.4 Stress response
Stress is a physiological process by which the brain reacts to physical or psychological threats. Activation of the fight-or-flight system releases stress hormones and triggers systemic changes that, while adaptive short term, become maladaptive when chronic, particularly within the CNS, increasing vulnerability to neuropsychiatric and neurodegenerative disorders (74–76). The NLRP3 inflammasome links cellular stress to neuroinflammation and its sustained activation contributes to cognitive, emotional, and structural brain alterations (77–80). Biological sex further shapes stress responses, driving divergent inflammasome activation profiles (81, 82). Evidence indicates that NLRP3 expression and responsiveness vary with sex, as well as tissue, developmental stage, and disease context (Table 1).
In adulthood, systemic inflammatory stress differentially modulates NLRP3 across sexes. Alonaizan et al. (2025) reported that acute LPS-induced neuroinflammation engaged NLRP3 in both male and female mice, yet only males developed behavioral impairments, indicating a sex-specific vulnerability despite comparable microglial activation (36). Likewise, Sood et al. (2022) observed higher basal hippocampal NLRP3 levels in females, but stress-induced upregulation occurred only in males, suggesting heightened inflammasome sensitivity in the male brain (37). Sex differences are also evident during neurodevelopment. Useinovic et al. (2022) demonstrated that neonatal (postnatal day-PND7) exposure to LPS plus the anesthetic sevoflurane enhanced hippocampal NLRP3-mediated neuroinflammation and induced long-term behavioral changes. Males showed learning and memory deficits, whereas females exhibited increased anxiety-like behaviors, underscoring the interplay between early-life inflammation, pharmacological exposure, and sex-specific inflammasome signaling (38).
3.5 Aging
Aging is a major risk factor for neurodegenerative and neuropsychiatric disorders, largely through dysregulation of immune-inflammatory pathways in the CNS. While transient inflammation may be protective, aging is accompanied by chronic low-grade inflammation (“inflammaging”) that promotes neuronal vulnerability, glial dysfunction, and cognitive decline (83). The NLRP3 inflammasome is a key mediator, sensing age-related stressors and amplifying neuroinflammation via caspase-1 activation, IL-1β/IL-18 releases, and pyroptosis (84, 85). Evidence suggests that NLRP3 signaling increases with age, and it follows sex-specific trajectories across the lifespan, fostering shaping susceptibility and disease progression (Table 1).
Cyr et al. (2023) showed that aged female mice exhibit a markedly enhanced pro-inflammatory transcriptional profile in cortical and hippocampal regions, with higher expression of caspase-1 and IL-1β compared with males (39), although these changes may reflect a broader upregulation of inflammasome pathways beyond NLRP3 specifically. By contrast, male brains displayed relatively stable or even decreased inflammasome-related gene expression across aging. Similarly, Zhang et al. (2020) reported that in an AD-like mouse model, expression of NLRP1, caspase-1, and IL-1β was more elevated in females than in males, pointing to heightened inflammasome activation in the aging female brain (40).
4 Environmental modulation of the NLRP3 inflammasome in brain health and disease
The lifelong imprint of environmental exposures, defined as the exposome, is a major determinant of brain health and disease susceptibility (86, 87). It encompasses non-genetic factors, including diet, physical activity, sleep patterns, environmental pollutants, stress, infections, sensory stimuli, and the social milieu, all strongly shaping neuroimmune function and plasticity (88–91). Many influences converge on NLRP3 inflammasome modulation through priming (e.g., NF-κB transcription) and activation triggers (e.g., mitochondrial ROS, K+ efflux) (15, 92–95).
Diet and nutraceuticals regulate priming via the gut–microbiota–brain axis, mediated by short-chain fatty acids and microbial metabolites (96–102). Circadian disruption and sleep loss heighten inflammatory tone by enhancing microglial NLRP3 activity (103–105), while pollutants amplify oxidative stress and neuroinflammation, accelerating degeneration (106–109). Conversely, physical activity broadly suppresses NLRP3 signaling, supporting cognitive resilience (110, 111). Of note, greater lifelong exposure to positive social interactions, physical activity, sensory stimulation, and cognitive engagement, beyond standard conditions, has been identified as a powerful protective factor for brain and systemic health, thereby lowering the risk of inflammatory and neurodegenerative disorders.
Social and environmental influences, from social connection to sensory engagement, profoundly modulate brain health directly link inequalities to health disparities. Indeed, favorable and adverse conditions differentially shape NLRP3 activation and, in turn, cognitive and behavioral outcomes, with important implications for brain well-being and relevance to both neurodegenerative and psychiatric disorders (7, 112). Within this exposome-informed framework, we next summarize evidence of social isolation and environmental enrichment as key exposures that regulate NLRP3 activity in vivo, producing divergent effects on cognition and behavior (Table 2).
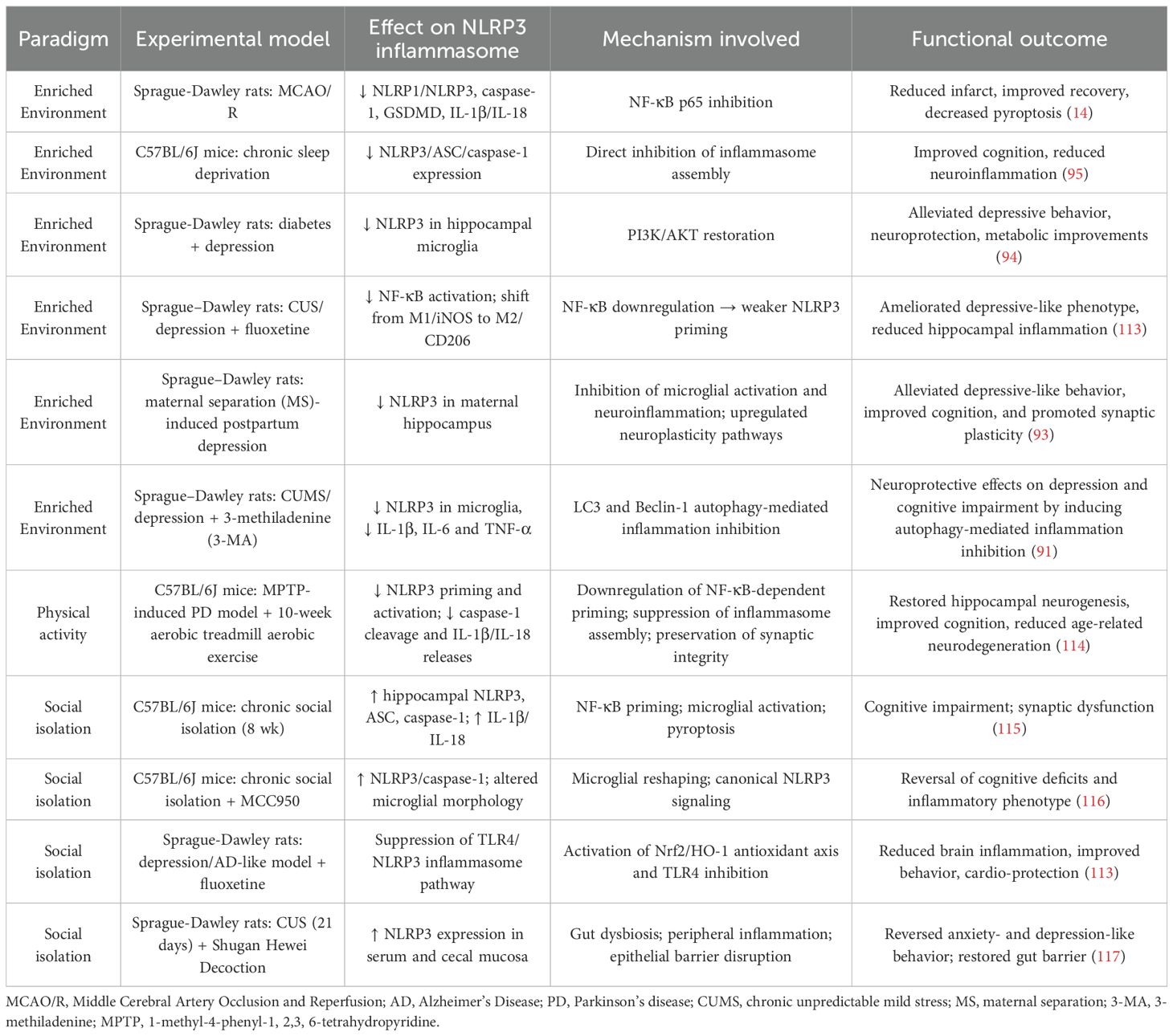
Table 2. Experimental evidence of living environment effects on NLRP3 inflammasome expression/activation across systemic conditions with neurological repercussions.
4.1 Enriched beyond an ordinary living environment
Environmental enrichment (EE), defined as enhanced sensory, cognitive, and physical stimulation beyond ordinary living conditions, promotes brain function and resilience by promoting neurogenesis, synaptic plasticity, and cognition (118). Preclinical EE paradigms provide larger housing with toys, wheels, tunnels, climbing structures, and social interaction with frequent novelty (89, 119). Compared with standard housing, EE fosters neuroplasticity, dendritic branching, and hippocampal synaptogenesis (89, 90, 120), while also influencing metabolic, endocrine, and immune processes (92, 121, 122). In contrast, impoverished settings with reduced activity and stimulation enhance microglial NLRP3 activation, exacerbating neuroinflammation and pathology (113, 115, 116). Conversely, enriched contexts dampen NLRP3-driven inflammation and improve behavioral and cognitive resilience, highlighting EE as a regulator of inflammasome signaling across conditions (89, 90, 123, 124). Environmental factors regulate NLRP3 activity, by influencing its priming and activation and integrating lifestyle inputs that shape immunometabolic and stress-response pathways (15, 94, 122). Converging preclinical evidence indicates that EE attenuates NLRP3 signaling and downstream inflammation across distinct pathophysiological contexts (Table 2).
In cerebral ischemia/reperfusion, EE improved recovery and reduced infarct size by suppressing NLRP1/NLRP3, caspase-1 activation, GSDMD-mediated pyroptosis and IL-1β/IL-18 releases via NF-κB inhibition compared to standard housing (14). In chronic sleep deprivation, EE rescued cognition and downregulated hippocampal NLRP3/ASC/caspase-1 levels, limiting inflammasome assembly (95). In diabetes with comorbid depression, EE alleviated depressive-like behavior, improved glucose regulation, reduced neuronal apoptosis, and suppressed microglial NLRP3 alongside PI3K/AKT pathway restoration, a negative regulator of inflammasome activation (94). More broadly, under stress- and depression-related paradigms, EE reprogramed microglia toward anti-inflammatory phenotypes (15) and restrained NF-κB signaling, reducing NLRP3 priming (91, 93). Beyond EE, physical activity exerts potent strong anti-inflammatory and immunomodulatory effects mediated by NLRP3 inflammasome. In middle-aged mice, Zhao et al. (2025) demonstrated that aerobic exercise restored hippocampal neurogenesis, improved cognition, and counteracted age-related neurodegeneration by reducing NLRP3 priming and activation, thereby preserving synaptic integrity and attenuating neuroinflammation (114).
4.2 Social isolation as an adverse living condition
Social connectedness supports health, whereas reduced interaction impairs cognition and behavior (125). In rodents, social isolation models psychosocial stress, driving microglial activation and NLRP3 inflammasome–mediated neuroimmune dysregulation (Table 2). Chronic social isolation increases hippocampal levels of ASC, caspase-1, and IL-1β/IL-18, driving cognitive deficits, depressive-like behavior, and impaired synaptic plasticity. Niu et al. (2020) further showed that isolated mice exhibited marked hippocampal upregulation of inflammasome proteins and cytokines without peripheral changes, underscoring a CNS-specific mechanism. Both the antibiotic minocycline and the selective inflammasome inhibitor MCC950 reversed NLRP3 activation and rescued cognition (115). Li et al. (2021) showed also that MCC950 prevented social isolation-induced depressive-like behavior, normalized microglial morphology and restored NLRP3/caspase-1 signaling in male mice, indicating that inflammasome blockade promotes behavioral resilience (116). Extending these findings, Abu-Elfotuh et al. (2022) demonstrated that the antidepressant fluoxetine counteracted isolation-exacerbated brain and cardiovascular pathology in an AD-like depression model by activating Nrf2/HO-1, an antioxidant pathway that limits ROS accumulation and thereby inhibits NLRP3 signaling (113). In contrast to CNS-focused studies, Yue et al. (2021) investigated the peripheral effects of chronic psychosocial stress using a chronic unpredictable stress (CUS) model that included social isolation as a key stressor (117). They showed their contribution to NLRP3-driven peripheral inflammation and highlighted the gut-brain axis as a parallel mediator of stress-related behavioral outcomes. Male rats exposed to 21 days of CUS developed depressive- and anxiety-like behaviors, weight loss, and gut alterations, with a significant upregulation of NLRP3 in both serum and cecal mucosa. Treatment with Shugan Hewei Decoction, a traditional multi-herbal remedy, reversed behavioral deficits, improved gut barrier function, and suppressed peripheral inflammasome activation (117).
5 Conclusion
The NLRP3 inflammasome is emerging as a central player in brain disorders and systemic conditions with neurological repercussions, underscoring its relevance within the CNS and potential as a biomarker (126). The pathway toward precision medicine will require sex-informed approaches to NLRP3 research, spanning preclinical studies and therapeutic development, rather than relying on uniform models that risk overlooking meaningful differences. While sex-dependent regulation of NLRP3 has been explored in some immune-related brain conditions, comparable investigations in mental health disorders remain limited. This gap is particularly pressing, as NLRP3 influences cognition and behavior and may contribute to sex disparities in neurocognitive and neurobehavioral disorders. Importantly, critical developmental windows such as puberty and aging act as “reset points” for inflammatory set-points in a sex-dependent manner. Fluctuating ovarian steroids (puberty, perimenopause, menopause) and androgens (puberty, aging) shape microglial phenotype and NLRP3 sensitivity, as shown in four-core genotype paradigms disentangling chromosomal and gonadal contributions (127, 128). These dynamics highlight the need for studies stratified by pubertal stage and aging stage to fully map sex-by-age interactions on NLRP3 priming and activation in CNS cells.
Concurrently, multiple environmental exposures profoundly modulate NLRP3 activity. Its heightened sensitivity to adverse inputs can drive maladaptive outcomes, including depression-like behaviors, cognitive decline, and stress vulnerability. Yet this sensitivity also offers an opportunity for lifestyle and environmental interventions, alongside pharmacological strategies, to dampen inflammation and preserve brain health, an avenue still underexplored. Moreover, biological sex, long considered static, is now recognized as dynamic in constant interplay with the exposome, with implications for sex-specific health outcomes and NLRP3-mediated mechanisms that can no longer be overlooked (129).
Given the dual influence of intrinsic (sex) and extrinsic (environment) factors on NLRP3, we suggest that a deeper understanding of their interplay, with NLRP3 as a central hub, could foster a more personalized and effective medicine, in which targeted non-pharmacological strategies complement pharmacological treatments (130). Such integration may overcome limitations of exclusive pharmacological NLRP3 inhibition, risks diverting inflammation toward alternative inflammasome pathways with harmful consequences (131–133). Addressing the complex interplay between intrinsic and extrinsic determinants of NLRP3-driven neuroimmune responses may ultimately enable more precise prevention, improved therapeutic efficacy, and better long-term neurological outcomes.
Author contributions
MC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. GR: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Funding acquisition. BB: Writing – original draft, Writing – review & editing. CB: Visualization, Writing – review & editing. SA: Visualization, Writing – review & editing. FT: Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The current work was funded by “FAR2023_Ricerca diffusa”, The current work was funded by “FAR2023_Ricerca diffusa”, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia (Principal Investigator: G.R. and JMC Blom); by PRIN-2022, Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), prot. 202277LAA7; Principal Investigator: prof. Fabio Tascedda, University of Modena and Reggio Emilia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NLRP, NOD-, LRR- and pyrin domain-containing protein; NLRC4, NOD-like receptor family CARD domain-containing protein 4; AIM2, absent in melanoma 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; ROS, reactive oxygen species; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; GSDMD, gasdermin D; CNS, central nervous system; HMGB1, high mobility group box 1; GABA, Gamma-AminoButyric Acid; Ldl, low-density lipoprotein; CRF, corticotropin-releasing factor; HPA, hypothalamic-pituitary-adrenal; LPS, lipopolysaccharide; PI3K, phosphoinositide 3-kinase; NRF2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1.
References
1. Broz P and Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. (2016) 16:407–20. doi: 10.1038/nri.2016.58
2. Paerewijck O and Lamkanfi M. The human inflammasomes. Mol Aspects Med. (2022) 88:101100. doi: 10.1016/J.MAM.2022.101100
3. Fu J and Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. (2023) 41:301–16. doi: 10.1146/annurev-immunol-081022-021207
4. Franchi L, Eigenbrod T, Muñoz-Planillo R, and Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. (2009) 10:241–7. doi: 10.1038/ni.1703
5. Pan Y, Cai W, Huang J, Cheng A, Wang M, Yin Z, et al. Pyroptosis in development, inflammation and disease. Front Immunol. (2022) 13:991044. doi: 10.3389/fimmu.2022.991044
6. Tastan B, Arioz BI, and Genc S. Targeting NLRP3 inflammasome with nrf2 inducers in central nervous system disorders. Front Immunol. (2022) 13:865772. doi: 10.3389/fimmu.2022.865772
7. Anderson FL, Biggs KE, Rankin BE, and Havrda MC. NLRP3 inflammasome in neurodegenerative disease. Trans Res. (2023) 252:21–33. doi: 10.1016/j.trsl.2022.08.006
8. Ma Q. Pharmacological inhibition of the NLRP3 inflammasome: structure, molecular activation, and inhibitor-NLRP3 interaction. Pharmacol Rev. (2023) 75:487–520. doi: 10.1124/pharmrev.122.000629
9. Xia CY, Guo YX, Lian WW, Yan Y, Ma BZ, Cheng YC, et al. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol Res. (2023) 187:106625. doi: 10.1016/j.phrs.2022.106625
10. Cignarella A, Vegeto E, Bolego C, Trabace L, Conti L, and Ortona E. Sex-oriented perspectives in immunopharmacology. Pharmacol Res. (2023) 197:106956. doi: 10.1016/J.PHRS.2023.106956
11. Zhang H, Tang Y, and Tao J. Sex-related overactivation of NLRP3 inflammasome increases lethality of the male COVID-19 patients. Front Mol Biosci. (2021) 8:671363. doi: 10.3389/fmolb.2021.671363
12. Chen S, Markman JL, Shimada K, Crother TR, Lane M, Abolhesn A, et al. Sex-specific effects of the nlrp3 inflammasome on atherogenesis in LDL receptor-deficient mice. JACC Basic Transl Sci. (2020) 5:582–98. doi: 10.1016/j.jacbts.2020.03.016
13. Martinon F, Pétrilli V, Mayor A, Tardivel A, and Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. (2006) 440:237–41. doi: 10.1038/NATURE04516;KWRD=SCIENCE
14. Liu J, Zheng J, Xu Y, Cao W, Wang J, Wang B, et al. Enriched environment attenuates pyroptosis to improve functional recovery after cerebral ischemia/reperfusion injury. Front Aging Neurosci. (2021) 13:717644. doi: 10.3389/fnagi.2021.717644
15. Gu JY, Xu YW, Feng LP, Dong J, Zhao LQ, Liu C, et al. Enriched environment mitigates depressive behavior by changing the inflammatory activation phenotype of microglia in the hippocampus of depression model rats. Brain Res Bull. (2021) 177:252–62. doi: 10.1016/j.brainresbull.2021.10.005
16. Hanamsagar R and Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. (2016) 160:127–33. doi: 10.1016/j.jsbmb.2015.09.039
17. De Felice E, Gonçalves de Andrade E, Golia MT, González Ibáñez F, Khakpour M, Di Castro MA, et al. Microglial diversity along the hippocampal longitudinal axis impacts synaptic plasticity in adult male mice under homeostatic conditions. J Neuroinflamm. (2022) 19(1):292. doi: 10.1186/S12974-022-02655-Z
18. De Felice E, Bobotis BC, Rigillo G, Khakpour M, de AEG, Benatti C, et al. Female mice exhibit similar long-term plasticity and microglial properties between the dorsal and ventral hippocampal poles. Brain Behav Immun. (2025) 124:192–204. doi: 10.1016/j.bbi.2024.11.034
19. Osborne BF, Turano A, and Schwarz JM. Sex differences in the neuroimmune system. Curr Opin Behav Sci. (2018) 23:118. doi: 10.1016/J.COBEHA.2018.05.007
20. Forsyth KS, Jiwrajka N, Lovell CD, Toothacre NE, and Anguera MC. The conneXion between sex and immune responses. Nat Rev Immunol. (2024) 24:487–502. doi: 10.1038/s41577-024-00996-9
21. Klein SL and Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/NRI.2016.90;SUBJMETA=2152,250,262,631;KWRD=ADAPTIVE+IMMUNITY,INNATE+IMMUNITY
22. Di Florio DN, Sin J, Coronado MJ, Atwal PS, and Fairweather DL. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. (2020) 31:101482. doi: 10.1016/j.redox.2020.101482
23. Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, et al. Sex-specific features of microglia from adult mice. Cell Rep. (2018) 23:3501–11. doi: 10.1016/j.celrep.2018.05.048
24. Slowik A, Lammerding L, Zendedel A, Habib P, and Beyer C. Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. J Steroid Biochem Mol Biol. (2018) 183:18–26. doi: 10.1016/j.jsbmb.2018.05.003
25. Paik S, Kim JK, Shin HJ, Park EJ, Kim IS, and Jo EK. Updated insights into the molecular networks for NLRP3 inflammasome activation. Cell Mol Immunol. (2025) 22:563–96. doi: 10.1038/s41423-025-01284-9
26. Fonseca BM, Pinto B, Costa L, Felgueira E, and Rebelo I. Increased expression of NLRP3 inflammasome components in granulosa cells and follicular fluid interleukin(IL)-1beta and IL-18 levels in fresh IVF/ICSI cycles in women with endometriosis. J Assist Reprod Genet. (2023) 40:191–9. doi: 10.1007/s10815-022-02662-2
27. Jarrot PA, Kim J, Chan W, Heger L, Schommer N, Cunin P, et al. Sex-specific NLRP3 activation in neutrophils promotes neutrophil recruitment and NETosis in the murine model of diffuse alveolar hemorrhage. Front Immunol. (2024) 15:1466234. doi: 10.3389/fimmu.2024.1466234
28. Smit V, de Mol J, Kleijn MNAB, Depuydt MAC, de Winther MPJ, Bot I, et al. Sexual dimorphism in atherosclerotic plaques of aged Ldlr–/– mice. Immun Ageing. (2024) 21:27. doi: 10.1186/s12979-024-00434-3
29. Cowie AM, Menzel AD, O’Hara C, Lawlor MW, and Stucky CL. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain. (2019) 160:1794–816. doi: 10.1097/j.pain.0000000000001555
30. Green-Fulgham SM, Ball JB, Kwilasz AJ, Harland ME, Frank MG, Dragavon JM, et al. Interleukin-1beta and inflammasome expression in spinal cord following chronic constriction injury in male and female rats. Brain Behav Immun. (2024) 115:157–68. doi: 10.1016/j.bbi.2023.10.004
31. Santos Ribeiro PS, Willemen HLDM, Versteeg S, Martin Gil C, and Eijkelkamp N. NLRP3 inflammasome activation in sensory neurons promotes chronic inflammatory and osteoarthritis pain. Immunotherapy Adv. (2023) 3(1):ltad022. doi: 10.1093/immadv/ltad022
32. Pasmay AA, Pritha AN, Carter JR, Jones A, Fernandez-Oropeza AK, Sun MS, et al. Prenatal alcohol exposure promotes nerve injury-induced pathological pain following morphine treatment via NLRP3-mediated peripheral and central proinflammatory immune actions. Brain Behav Immun. (2025) 129:736–56. doi: 10.1016/j.bbi.2025.06.041
33. Munshi S, Albrechet-Souza L, dos-Santos RC, Stelly CE, Secci ME, Gilpin NW, et al. Acute ethanol modulates synaptic inhibition in the basolateral amygdala via rapid NLRP3 inflammasome activation and regulates anxiety-like behavior in rats. J Neurosci. (2023) 43:7902–12. doi: 10.1523/JNEUROSCI.1744-22.2023
34. Lowe PP, Cho Y, Tornai D, Coban S, Catalano D, and Szabo G. Inhibition of the inflammasome signaling cascade reduces alcohol consumption in female but not male mice. Alcohol Clin Exp Res. (2020) 44:567–78. doi: 10.1111/acer.14272
35. Cheng Y, Dempsey RE, Roodsari SK, Shuboni-Mulligan DD, George O, Sanford LD, et al. Cocaine regulates NLRP3 inflammasome activity and CRF signaling in a region- and sex-dependent manner in rat brain. Biomedicines. (2023) 11(7):1800. doi: 10.3390/biomedicines11071800
36. Alonaizan R K, Alotaibi W, Alsulami A, Alkhulaifi F M, and Alomar S. Sex-differences influence depressive-like behaviour via alterations in microglial expression of GIF-1, TREM2, and IL-1β in an acute lipopolysaccharide-induced murine neuroinflammation model. Immunol Invest. (2025) 54:317–33. doi: 10.1080/08820139.2024.2440006
37. Sood A, Chaudhari PR, Tiwari P, Shah S, and Vaidya VA. Acute immobilization stress evokes sexually dimorphic peripheral and hippocampal neuroimmune responses in adult rats. Neurosci Lett. (2022) 789:136871. doi: 10.1016/j.neulet.2022.136871
38. Useinovic N, Maksimovic S, Liechty C, Cabrera OH, Quillinan N, and Jevtovic-Todorovic V. Systemic inflammation exacerbates developmental neurotoxicity induced by sevoflurane in neonatal rats. Br J Anaesth. (2022) 129:555–66. doi: 10.1016/j.bja.2022.05.008
39. Cyr B and de Rivero Vaccari JP. Sex differences in the inflammatory profile in the brain of young and aged mice. Cells. (2023) 12:1372. doi: 10.3390/CELLS12101372/S1
40. Zhang J, Pei L, Zang D, Xue Y, Wang X, Chen Y, et al. Gender differences of NLRP1 inflammasome in mouse model of alzheimer’s disease. Front Aging Neurosci. (2020) 12:512097. doi: 10.3389/FNAGI.2020.512097
41. Kastbom A, Ärlestig L, and Rantapää-Dahlqvist S. Genetic variants of the NLRP3 inflammasome are associated with stroke in patients with rheumatoid arthritis. J Rheumatol. (2015) 42:1740–5. doi: 10.3899/jrheum.141529
42. Karasawa T and Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb. (2017) 24:443–51. doi: 10.5551/jat.RV17001
43. Chen P and Li X. NLRP3 inflammasome in atherosclerosis: Mechanisms and targeted therapies. Front Pharmacol. (2024) 15:1430236. doi: 10.3389/fphar.2024.1430236
44. Lu N, Cheng W, Liu D, Liu G, Cui C, Feng C, et al. NLRP3-mediated inflammation in atherosclerosis and associated therapeutics. Front Cell Dev Biol. (2022) 10:823387. doi: 10.3389/fcell.2022.823387
45. Akif A, Nguyen TTM, Liu L, Xu X, Kulkarni A, Jiang J, et al. Targeting NLRP3 signaling with a novel sulfonylurea compound for the treatment of vascular cognitive impairment and dementia. Fluids Barriers CNS. (2025) 22:55. doi: 10.1186/s12987-025-00665-6
46. Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, et al. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. (2006) 26:12165–73. doi: 10.1523/JNEUROSCI.3576-06.2006
47. Volcheck MM, Graham SM, Fleming KC, Mohabbat AB, and Luedtke CA. Central sensitization, chronic pain, and other symptoms: Better understanding, better management. Cleve Clin J Med. (2023) 90:245–54. doi: 10.3949/ccjm.90a.22019
48. Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. (2020) 21:353–65. doi: 10.1038/s41583-020-0310-6
49. Cowie AM, Dittel BN, and Stucky CL. A novel sex-dependent target for the treatment of postoperative pain: The NLRP3 inflammasome. Front Neurol. (2019) 10:622. doi: 10.3389/fneur.2019.00622
50. Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U.S.A. (2016) 113:E3441–50. doi: 10.1073/pnas.1602070113
51. Chen C and Smith MT. The NLRP3 inflammasome: role in the pathobiology of chronic pain. Inflammopharmacology. (2023) 31:1589–603. doi: 10.1007/s10787-023-01235-8
52. Koob GF and Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. (2016) 3:760–73. doi: 10.1016/S2215-0366(16)00104-8
53. Guerzoni S, Pellesi L, Pini LA, and Caputo F. Drug-drug interactions in the treatment for alcohol use disorders: A comprehensive review. Pharmacol Res. (2018) 133:65–76. doi: 10.1016/j.phrs.2018.04.024
54. Guo ML, Roodsari SK, Cheng Y, Dempsey RE, and Hu W. Microglia NLRP3 inflammasome and neuroimmune signaling in substance use disorders. Biomolecules. (2023) 13(6):922. doi: 10.3390/biom13060922
55. Kurawa MI, Torkaman-Boutorabi A, Shafaghi L, Hassanzadeh G, Zahmatkesh M, Vousooghi N, et al. The effects of subchronic methamphetamine administration on the NLRP3 inflammasome, memory function, and hippocampal morphology. Arch Neurosci. (2024) 11(4):e145644. doi: 10.5812/ANS-145644
56. Smith C, Trageser KJ, Wu H, Herman FJ, Iqbal UH, Sebastian-Valverde M, et al. Anxiolytic effects of NLRP3 inflammasome inhibition in a model of chronic sleep deprivation. Trans Psychiatry. (2021) 11:1–15. doi: 10.1038/s41398-020-01189-3
57. Alfonso-Loeches S, Ureña-Peralta J, Morillo-Bargues MJ, Gómez-Pinedo U, and Guerri C. Ethanol-induced TLR4/NLRP3 neuroinflammatory response in microglial cells promotes leukocyte infiltration across the BBB. Neurochem Res. (2016) 41:193–209. doi: 10.1007/s11064-015-1760-5
58. Fujii C, Zorumski CF, and Izumi Y. Ethanol, neurosteroids and cellular stress responses: Impact on central nervous system toxicity, inflammation and autophagy. Neurosci Biobehav Rev. (2021) 124:168–78. doi: 10.1016/j.neubiorev.2021.01.026
59. Priyanka SH, Thushara AJ, Rauf AA, and Indira M. Alcohol induced NLRP3 inflammasome activation in the brain of rats is attenuated by ATRA supplementation. Brain Behav Immun Health. (2020) 2:100024. doi: 10.1016/j.bbih.2019.100024
60. Ahmed S, Panda SR, Kwatra M, Sahu BD, and Naidu V. Perillyl alcohol attenuates NLRP3 inflammasome activation and rescues dopaminergic neurons in experimental in vitro and in vivo models of parkinson’s disease. ACS Chem Neurosci. (2022) 13:53–68. doi: 10.1021/acschemneuro.1c00550
61. Noor S, Sun MS, Pasmay AA, Pritha AN, Ruffaner-Hanson CD, Nysus MV, et al. Prenatal alcohol exposure promotes NLRP3 inflammasome-dependent immune actions following morphine treatment and paradoxically prolongs nerve injury-induced pathological pain in female mice. Alcohol Clin Exp Res. (2023) 47:2262. doi: 10.1111/ACER.15214
62. Li J, Wang H, Liu D, Li X, He L, Pan J, et al. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav Immun. (2023) 110:60–79. doi: 10.1016/j.bbi.2023.02.001
63. Yao H, Zhang D, Yu H, Yuan H, Shen H, Lan X, et al. Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation. Mol Psychiatry. (2023) 28:919–30. doi: 10.1038/s41380-022-01841-y
64. Anton PE, Rutt LN, Kaufman ML, Busquet N, Kovacs EJ, and McCullough RL. Binge ethanol exposure in advanced age elevates neuroinflammation and early indicators of neurodegeneration and cognitive impairment in female mice. Brain Behav Immun. (2024) 116:303–16. doi: 10.1016/j.bbi.2023.12.034
65. Brezani V, Joshi RS, Ortega-Ribera M, Nagesh PT, Brezani V, Zivny A, et al. Ethanol consumption aggravates amyloid pathology and neuroinflammation in Alzheimer’s disease associated with inflammasome activation and ASC speck propagation. J Neuroinflamm. (2025) 22(1):183. doi: 10.1186/s12974-025-03501-8
66. Sun D, Li X, Xu S, Cao S, Quan Y, Cui S, et al. Dazhu Hongjingtian injection attenuated alcohol-induced depressive symptoms by inhibiting hippocampus oxidative stress and inflammation through Nrf2/HO-1/NLRP3 signaling pathway. J Ethnopharmacol. (2024) 334:118564. doi: 10.1016/j.jep.2024.118564
67. Lin X, Wang H, Zou L, Yang B, Chen W, Rong X, et al. The NRF2 activator RTA-408 ameliorates chronic alcohol exposure-induced cognitive impairment and NLRP3 inflammasome activation by modulating impaired mitophagy initiation. Free Radic Biol Med. (2024) 220:15–27. doi: 10.1016/j.freeradbiomed.2024.04.236
68. Fang Y, Sun Y, Liu Y, Liu T, Hao W, and Liao Y. Neurobiological mechanisms and related clinical treatment of addiction: a review. Psychoradiology. (2022) 2:180–9. doi: 10.1093/psyrad/kkac021
69. Carranza-Aguilar CJ, Hernández-Mendoza A, Mejias-Aponte C, Rice KC, Morales M, González-Espinosa C, et al. Morphine and fentanyl repeated administration induces different levels of NLRP3-dependent pyroptosis in the dorsal raphe nucleus of male rats via cell-specific activation of TLR4 and opioid receptors. Cell Mol Neurobiol. (2022) 42:677–94. doi: 10.1007/s10571-020-00957-5
70. Mahmoudias GR, Abbaszadeh HA, Rezaei-Tavirani M, Abdollahifar MA, Khoramgah MS, Niknazar S, et al. Nod-like receptor protein 3 and nod-like receptor protein 1 inflammasome activation in the hippocampal region of postmortem methamphetamine chronic user. Bratislava Med J. (2019) 120:769–76. doi: 10.4149/BLL_2019_129
71. Hui R, Xu J, Zhou M, Xie B, Zhou M, Zhang L, et al. Betaine improves METH-induced depressive-like behavior and cognitive impairment by alleviating neuroinflammation via NLRP3 inflammasome inhibotion. Prog Neuropsychopharmacol Biol Psychiatry. (2024) 135:111093. doi: 10.1016/j.pnpbp.2024.111093
72. Khodagholi F, Dezfouli MA, Yazdanfar N, Rashidi SK, Meymand AZ, Javadpour P, et al. Prenatal methamphetamine exposure impairs helping behaviour in male offspring: the possible role of miR-223 and NLRP3 inflammasomes in the amygdala. Int J Dev Neurosci. (2025) 85(1):e10410. doi: 10.1002/jdn.10410
73. Eidson LN and Murphy AZ. Inflammatory mediators of opioid tolerance: Implications for dependency and addiction. Peptides (NY). (2019) 115:51–8. doi: 10.1016/J.PEPTIDES.2019.01.003
74. Del Giudice M, Hinnant JB, Ellis BJ, and El-Sheikh M. Adaptive patterns of stress responsivity: A preliminary investigation. Dev Psychol. (2012) 48:775–90. doi: 10.1037/a0026519
75. Joëls M, Karst H, and Sarabdjitsingh RA. The stressed brain of humans and rodents. Acta Physiologica. (2018) 223(2):e13066. doi: 10.1111/apha.13066
76. McEwen BS, Mirsky AE, and Hatch MM. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. (2007) 87(3):873–904. doi: 10.1152/physrev.00041.2006
77. Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. (2019) 573:590–4. doi: 10.1038/s41586-019-1551-2
78. kodi T, Sankhe R, Gopinathan A, Nandakumar K, and Kishore A. New insights on NLRP3 inflammasome: mechanisms of activation, inhibition, and epigenetic regulation. J Neuroimmune Pharmacol. (2024) 19(1):7. doi: 10.1007/s11481-024-10101-5
79. Xu W, Huang Y, and Zhou R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol Immunol. (2025) 22:341–55. doi: 10.1038/s41423-025-01275-w
80. Yang L, Xing W, Shi Y, Hu M, Li B, Hu Y, et al. Stress-induced NLRP3 inflammasome activation and myelin alterations in the hippocampus of PTSD rats. Neuroscience. (2024) 555:156–66. doi: 10.1016/j.neuroscience.2024.07.028
81. Bangasser DA and Valentino RJ. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front Neuroendocrinol. (2014) 35:303–19. doi: 10.1016/j.yfrne.2014.03.008
82. Hodes GE, Bangasser D, Sotiropoulos I, Kokras N, and Dalla C. Sex differences in stress response: classical mechanisms and beyond. Curr Neuropharmacol. (2023) 22:475–94. doi: 10.2174/1570159x22666231005090134
83. Franceschi C, Garagnani P, Parini P, Giuliani C, and Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/S41574-018-0059-4
84. Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. (2012) 493:674–8. doi: 10.1038/nature11729. 7434.
85. Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, et al. The NLRP3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. (2012) 1:56–68. doi: 10.1016/j.celrep.2011.11.005
86. Gago-Ferrero P, Cousins I, Ghassabian A, Lamoree M, Schlenk D, Toms LM, et al. The exposome and human health. Environ Sci Technol. (2025) 59:991–2. doi: 10.1021/ACS.EST.4C13478
87. Hahad O, Al-Kindi S, Lelieveld J, Münzel T, and Daiber A. Supporting and implementing the beneficial parts of the exposome: The environment can be the problem, but it can also be the solution. Int J Hyg Environ Health. (2024) 255:114290. doi: 10.1016/J.IJHEH.2023.114290
88. You T, Lee T, Im GH, Jung WB, Jang MS, Lee S, et al. Differential impacts of social isolation and enriched environment on multi-sensory brain-wide functionality and network segregation. Nat Commun. (2025) 16:1–22. doi: 10.1038/s41467-025-62253-4
89. Nithianantharajah J and Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. (2006) 7:697–709. doi: 10.1038/nrn1970
90. Sale A, Berardi N, and Maffei L. Enrich the environment to empower the brain. Trends Neurosci. (2009) 32:233–9. doi: 10.1016/j.tins.2008.12.004
91. Xu L, Sun H, Qu C, Shen J, Qu C, Song H, et al. The environmental enrichment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive decline by inducing autophagy-mediated inflammation inhibition. Brain Res Bull. (2022) 187:98–110. doi: 10.1016/J.BRAINRESBULL.2022.07.001
92. Brod S, Gobbetti T, Gittens B, Ono M, Perretti M, and D’Acquisto F. The impact of environmental enrichment on the murine inflammatory immune response. JCI Insight. (2017) 2(7):e90723. doi: 10.1172/jci.insight.90723
93. Chen G, Zhang Y, Li R, Jin L, Hao K, Rong J, et al. Environmental enrichment attenuates depressive-like behavior in maternal rats by inhibiting neuroinflammation and apoptosis and promoting neuroplasticity. Neurobiol Stress. (2024) 30:100624. doi: 10.1016/J.YNSTR.2024.100624
94. Wang H, Liang Q, Wen Z, Ma W, Ji S, Zhang H, et al. Enriched environment alleviates NLRP3 inflammasome mediated neuroinflammation in diabetes complicated with depression rats. Sci Rep. (2025) 15:1–10. doi: 10.1038/S41598-025-98312-5;SUBJMETA=1414,1689,378,4081,631,704,844;KWRD=DEPRESSION,ENVIRONMENTAL+IMPACT
95. Tian R, Zhou Y, Wei T, and Yu D. Effect of enriched environment on the chronic sleep deprivation mice by NLRP3/Caspase-1 signal pathway. (2024). doi: 10.21203/rs.3.rs-5654841/v1
96. Feng Y, Wang Y, Wang P, Huang Y, and Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. (2018) 49:190–205. doi: 10.1159/000492853
97. Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. (2018) 172:162–175.e14. doi: 10.1016/J.CELL.2017.12.013
98. Zhou X, Ji S, Chen L, Liu X, Deng Y, You Y, et al. Gut microbiota dysbiosis in hyperuricaemia promotes renal injury through the activation of NLRP3 inflammasome. Microbiome. (2024) 12(1):109. doi: 10.1186/S40168-024-01826-9
99. Zhao W, Ma L, Cai C, and Gong X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol Sci. (2019) 15:1571–81. doi: 10.7150/IJBS.34211
100. Csak T, Pillai A, Ganz M, Lippai D, Petrasek J, Park JK, et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88- dependent manner in dietary steatohepatitis. Liver Int. (2014) 34:1402–13. doi: 10.1111/LIV.12537
101. Chiazza F, Couturier-Maillard A, Benetti E, Mastrocola R, Nigro D, Cutrin JC, et al. Targeting the NLRP3 inflammasome to reduce diet-induced metabolic abnormalities in mice. Mol Med. (2015) 21:1025–37. doi: 10.2119/MOLMED.2015.00104
102. Sokolova M, Yang K, Hansen SH, Louwe MC, Kummen M, Hov JER, et al. NLRP3 inflammasome deficiency attenuates metabolic disturbances involving alterations in the gut microbial profile in mice exposed to high fat diet. Sci Rep. (2020) 10:1–16. doi: 10.1038/S41598-020-76497-1;SUBJMETA=250,4019,631,692;KWRD=CARDIOLOGY,IMMUNOLOGY
103. Musiek ES and Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Sci (1979). (2016) 354:1004–8. doi: 10.1126/SCIENCE.AAH4968
104. Fan K, Yang J, Gong WY, Pan YC, Zheng P, and Yue XF. NLRP3 inflammasome activation mediates sleep deprivation-induced pyroptosis in mice. PeerJ. (2021) 7:e11609. doi: 10.7717/PEERJ.11609/SUPP-2
105. Shixing X, Xueyan H, Yuan R, Wei T, and Wei W. Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus. Transl Neurosci. (2023) 14:20220280. doi: 10.1515/tnsci-2022-0280
106. Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. (2011) 16:987–95. doi: 10.1038/MP.2011.76
107. Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. (2019) 12:eaat9900. doi: 10.1126/SCISIGNAL.AAT9900
108. Zhu J, Zhou F, Zhou Q, Xu Y, Li Y, Huang D, et al. NLRP3 activation in microglia contributes to learning and memory impairment induced by chronic lead exposure in mice. Toxicological Sci. (2023) 191:179–91. doi: 10.1093/TOXSCI/KFAC115
109. Chen L, Na R, Boldt E, and Ran Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol Aging. (2015) 36:2533–43. doi: 10.1016/j.neurobiolaging.2015.05.018
110. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, and Nimmo MA. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11:607–10. doi: 10.1038/NRI3041
111. Pedersen BK. Physical activity and muscle–brain crosstalk. Nat Rev Endocrinol. (2019) 15:383–92. doi: 10.1038/S41574-019-0174-X
112. Wo´znywo´zny-Rasała I and Ogłodek EA. NLRP3 inflammasome in stress-related neuropsychiatric disorders: mechanisms of neuron–microglia–astrocyte crosstalk, HPA axis dysregulation, and therapeutic perspective. Biomolecules. (2025) 15:1344. doi: 10.3390/BIOM15091344
113. Abu-Elfotuh K, Al-Najjar AH, Mohammed AA, Aboutaleb AS, and Badawi GA. Fluoxetine ameliorates Alzheimer’s disease progression and prevents the exacerbation of cardiovascular dysfunction of socially isolated depressed rats through activation of Nrf2/HO-1 and hindering TLR4/NLRP3 inflammasome signaling pathway. Int Immunopharmacol. (2022) 104:108488. doi: 10.1016/j.intimp.2021.108488
114. Zhao R, Tian X, Xu H, Wang Y, Lin J, and Wang B. Aerobic exercise restores hippocampal neurogenesis and cognitive function by decreasing microglia inflammasome formation through irisin/NLRP3 pathway. Aging Cell. (2025) 24:e70061. doi: 10.1111/ACEL.70061
115. Niu L, Luo SS, Xu Y, Wang Z, Luo D, Yang H, et al. The critical role of the hippocampal NLRP3 inflammasome in social isolation-induced cognitive impairment in male mice. Neurobiol Learn Mem. (2020) 175:107301. doi: 10.1016/j.nlm.2020.107301
116. Li W, Niu L, Liu Z, Xu X, Shi M, Zhang Y, et al. Inhibition of the NLRP3 inflammasome with MCC950 prevents chronic social isolation-induced depression-like behavior in male mice. Neurosci Lett. (2021) 765:136290. doi: 10.1016/j.neulet.2021.136290
117. Yue Y, Ke Y, Zheng J, Wang Z, Liu H, and Liu S. Microbiota-derived tryptophan metabolism and AMPK/mTOR pathway mediate antidepressant-like effect of Shugan Hewei Decoction. Front Pharmacol. (2024) 15:1466336. doi: 10.3389/fphar.2024.1466336
118. Liew AKY, Teo CH, and Soga T. The molecular effects of environmental enrichment on alzheimer’s disease. Mol Neurobiol. (2022) 59:7095–118. doi: 10.1007/s12035-022-03016-w
119. Simpson J and Kelly JP. The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behav Brain Res. (2011) 222:246–64. doi: 10.1016/j.bbr.2011.04.002
120. van Praag H, Kempermann G, and Gage FH. Neural consequences of enviromental enrichment. Nat Rev Neurosci. (2000) 1:191–8. doi: 10.1038/35044558;KWRD=BIOMEDICINE
121. Xiao R, Ali S, Caligiuri MA, and Cao L. Enhancing effects of environmental enrichment on the functions of natural killer cells in mice. Front Immunol. (2021) 12:695859/BIBTEX. doi: 10.3389/FIMMU.2021.695859/BIBTEX
122. de León-Guerrero SD, Salazar-León J, Meza-Sosa KF, Valle-Garcia D, Aguilar-León D, Pedraza-Alva G, et al. An enriched environment re-establishes metabolic homeostasis by reducing obesity-induced inflammation. Dis Model Mech. (2022) 15:dmm048936. doi: 10.1242/DMM.048936
123. Al Omran AJ, Shao AS, Watanabe S, Zhang Z, Zhang J, Xue C, et al. Social isolation induces neuroinflammation and microglia overactivation, while dihydromyricetin prevents and improves them. J Neuroinflamm. (2022) 19:1–12. doi: 10.1186/S12974-021-02368-9/FIGURES/6
124. Wohleb ES, Franklin T, Iwata M, and Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. (2016) 17:497–511. doi: 10.1038/NRN.2016.69
125. Xiong Y, Hong H, Liu C, and Zhang YQ. Social isolation and the brain: effects and mechanisms. Mol Psychiatry. (2023) 28:191–201. doi: 10.1038/s41380-022-01835-w
126. Ciani M, Rigillo G, Benatti C, Pani L, Blom JMC, Brunello N, et al. Time- and region-specific effect of vortioxetine on central LPS-induced transcriptional regulation of NLRP3 inflammasome. Curr Neuropharmacol. (2024) 22:1–13. doi: 10.2174/1570159X22666240705143649
127. Ocañas SR, Ansere VA, Kellogg CM, Isola JVV, Chucair-Elliott AJ, and Freeman WM. Chromosomal and gonadal factors regulate microglial sex effects in the aging brain. Brain Res Bull. (2023) 195:157. doi: 10.1016/J.BRAINRESBULL.2023.02.008
128. Wiese CB, Soliman B, and Reue K. The Four Core Genotypes mouse model: evaluating the impact of a recently discovered translocation. Biol Sex Differ. (2024) 15:1–9. doi: 10.1186/S13293-024-00665-5/FIGURES/3
129. Bucher ML, Anderson FL, Lai Y, Dicent J, Miller GW, and Zota AR. Exposomics as a tool to investigate differences in health and disease by sex and gender. Exposome. (2023) 3(1):osad003. doi: 10.1093/exposome/osad003
130. Rigillo G and Alboni S. Exploring the frontiers of neuroinflammation: new horizons in research and treatment. Curr Issues Mol Biol. (2024) 46:11665–7. doi: 10.3390/CIMB46100692
131. Ozaki E, Campbell M, and Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflammation Res. (2015) 8:15. doi: 10.2147/JIR.S51250
132. Zahid A, Li B, Kombe AJK, Jin T, and Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol. (2019) 10:2538. doi: 10.3389/FIMMU.2019.02538
Keywords: NLRP3 inflammasome, biological sex, central nervous system, social isolation, enriched environment, exposome, pharmacology
Citation: Ciani M, Rigillo G, Bertarini B, Benatti C, Alboni S and Tascedda F (2025) Neuroimmune modulatory effects of the NLRP3 inflammasome: the role of sex and living environment in brain affecting conditions. Front. Immunol. 16:1711656. doi: 10.3389/fimmu.2025.1711656
Received: 23 September 2025; Accepted: 29 October 2025;
Published: 20 November 2025.
Edited by:
Yongkui Li, Jinan University, ChinaReviewed by:
Juan Pablo de Rivero Vaccari, University of Miami, United StatesCopyright © 2025 Ciani, Rigillo, Bertarini, Benatti, Alboni and Tascedda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dr. Giovanna Rigillo, Z2lvdmFubmEucmlnaWxsb0B1bmltb3JlLml0
 Miriam Ciani
Miriam Ciani Giovanna Rigillo
Giovanna Rigillo Beatrice Bertarini2
Beatrice Bertarini2 Cristina Benatti
Cristina Benatti Silvia Alboni
Silvia Alboni Fabio Tascedda
Fabio Tascedda