- 1Department of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou, China
- 2Department of Hepatobiliary Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 3Department of Gastrointestinal Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 4Government Agency Clinic, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 5Digestive Endoscopy Center, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
Gastric and colorectal cancers present significant therapeutic challenges, particularly in the elderly population, who often have comorbidities and diminished tolerance to standard treatments. This report describes an 85-year-old male with concurrent stage III gastric adenocarcinoma and stage IIIb microsatellite stable colorectal cancer, who declined both surgery and chemotherapy. Subsequently, the patient was treated with an innovative regimen consisting of endoscopic intratumoral injections of Oncolytic adenovirus H101 in combination with the PD-1 inhibitor tislelizumab. Following this combined therapeutic approach, the patient demonstrated notable tumor shrinkage and downstaging, accompanied by a reduction in serum tumor markers, including CEA and CA19-9. Additionally, there was an observed increase in CD8+ and CD4+ T-cell counts, indicating systemic immune activation. The treatment was well-tolerated, with the only reported adverse event being mild fever. The patient achieved nearly 4 months of progression-free survival and a substantial improvement in quality of life. This case highlights the potential of combining oncolytic virotherapy with PD-1 inhibition as a promising and novel personalized strategy for treating elderly patients with advanced gastrointestinal cancers who are unsuitable candidates for conventional therapies.
Introduction
With the global aging population steadily increasing, the demand for effective diagnosis and treatment of elderly cancer patients is growing exponentially. The median age of patients diagnosed with colorectal cancer is 66 years, with over 70% of gastric cancer patients aged 60 years or older (1, 2). This demographic shift highlights the importance of adapting cancer treatment protocols to better suit the needs of elderly individuals. However, the aging process is often accompanied by a decline in physiological functions, an increase in comorbidities, and diminished drug resistance, making treatment plans for elderly patients inherently more complex. One of the central challenges in this context is how to effectively balance the therapeutic outcomes with maintaining the quality of life (QoL) for these patients (3, 4).
Currently, the treatment modalities for gastric cancer include surgery, chemotherapy, targeted therapy, immunotherapy, and radiotherapy. However, these traditional approaches often fail to fully meet the needs of elderly patients (5, 6). Many elderly individuals are unable to tolerate the rigors of surgery or chemotherapy due to frailty, poor performance status, or multiple underlying health conditions. Furthermore, these treatments can negatively impact their quality of life, with some patients opting to forgo treatment altogether due to the perceived risks and potential adverse effects. A significant concern when using immune checkpoint inhibitors (ICIs) in elderly patients is the heightened risk of treatment-related adverse events (TRAEs) and the subsequent likelihood of treatment discontinuation (7, 8). These risks are further compounded by the vulnerability of the immune system in aging individuals, which can lead to increased side effects and complications. Therefore, there is a pressing need for more personalized treatment strategies that can reduce the burden of adverse events while still offering effective therapeutic outcomes (9).
Oncolytic virus therapy, a novel and emerging immunotherapy, offers a promising alternative to conventional treatments. This therapeutic approach involves viruses that selectively infect and destroy tumor cells while simultaneously stimulating the immune system to produce an anti-tumor response. Research has demonstrated the efficacy of oncolytic virus therapy in various cancer types. When used in combination with PD-1 inhibitors, this therapy can further enhance immune responses, restore T cell function, and counteract immune suppression within the tumor microenvironment. These combined effects can provide a novel treatment option for elderly cancer patients, who may not tolerate conventional therapies as well (10–12). Oncolytic adenovirus H101 (−20°C, Shanghai Sunway Biotech, Shanghai, China), a recombinant type 5 human adenovirus with a E1B55KD deletion and partial E3 region which can selectively replicate in tumor cells.
This article investigates the application of oncolytic virus endoscopic injection combined with PD-1 inhibitors in the treatment of an elderly patient with gastrointestinal tumors. The patient, who was ineligible for surgery and chemotherapy due to advanced age and cardiovascular disease, experienced significant tumor shrinkage and downstaging through a tailored treatment regimen. This case provides valuable insights into the potential benefits of integrating oncolytic virus therapy with immunotherapy for elderly cancer patients, offering a promising approach for improving treatment outcomes and quality of life for this vulnerable population.
Case presentation
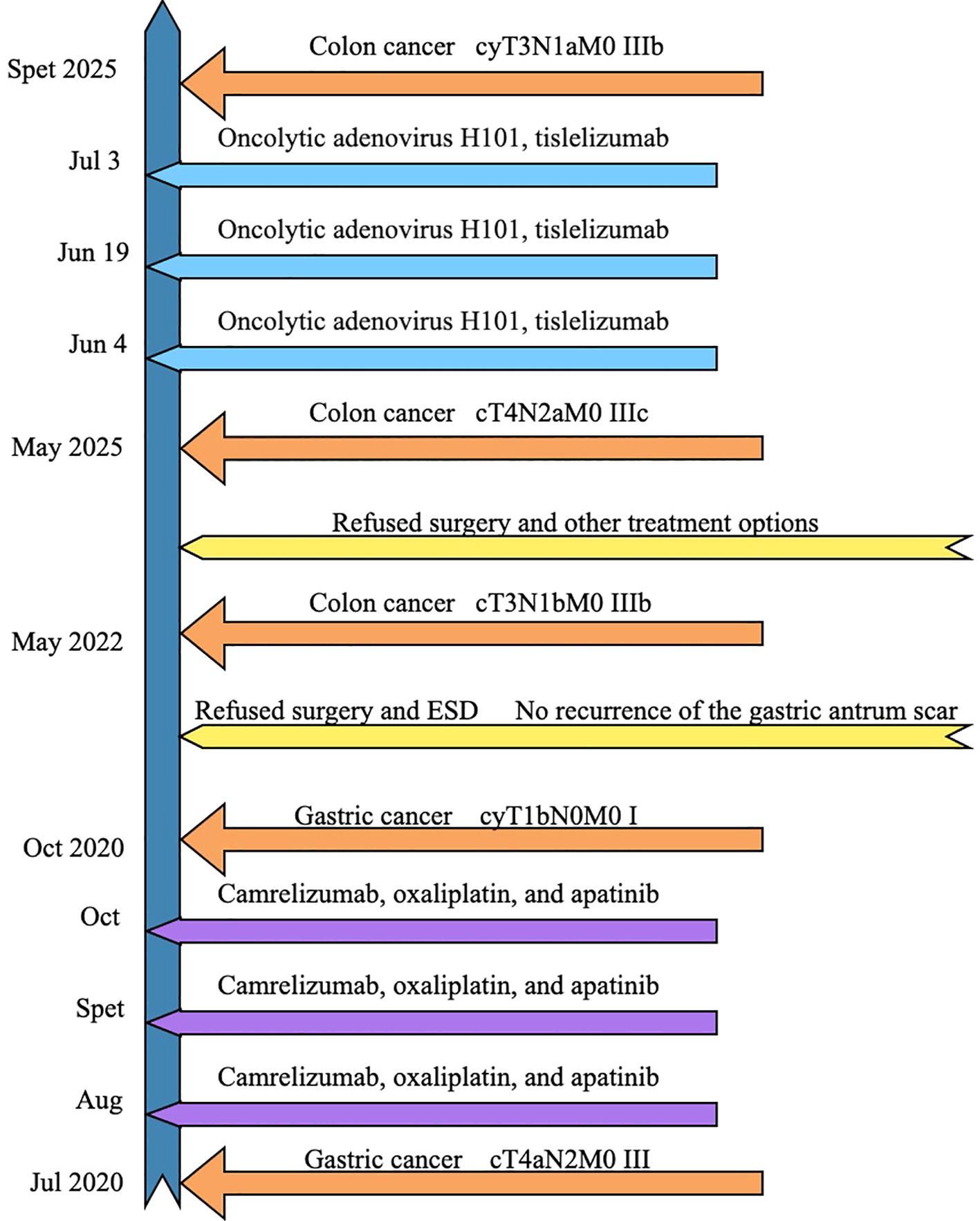
A male patient, aged 85, presented in July 2020 with abdominal pain and bloating after meals. Gastroscopy revealed a large ulcer in the gastric antrum (6×5 cm) (Figure 1a), and pathological biopsy confirmed adenocarcinoma. CT scans showed a tumor in the gastric antrum with multiple enlarged lymph nodes in the lesser curvature and surrounding areas. According to the AJCC staging criteria, the clinical stage was cT4aN2M0, stage III (Figure 2a). Following a multidisciplinary team (MDT) discussion, a neoadjuvant treatment plan was formulated, involving camrelizumab (IV 200 mg, every 3 weeks), oxaliplatin (IV 130 mg/m², every 3 weeks), and apatinib (oral 250 mg daily for 14 days, every 3 weeks), with the goal of performing surgery after 3 cycles of treatment.
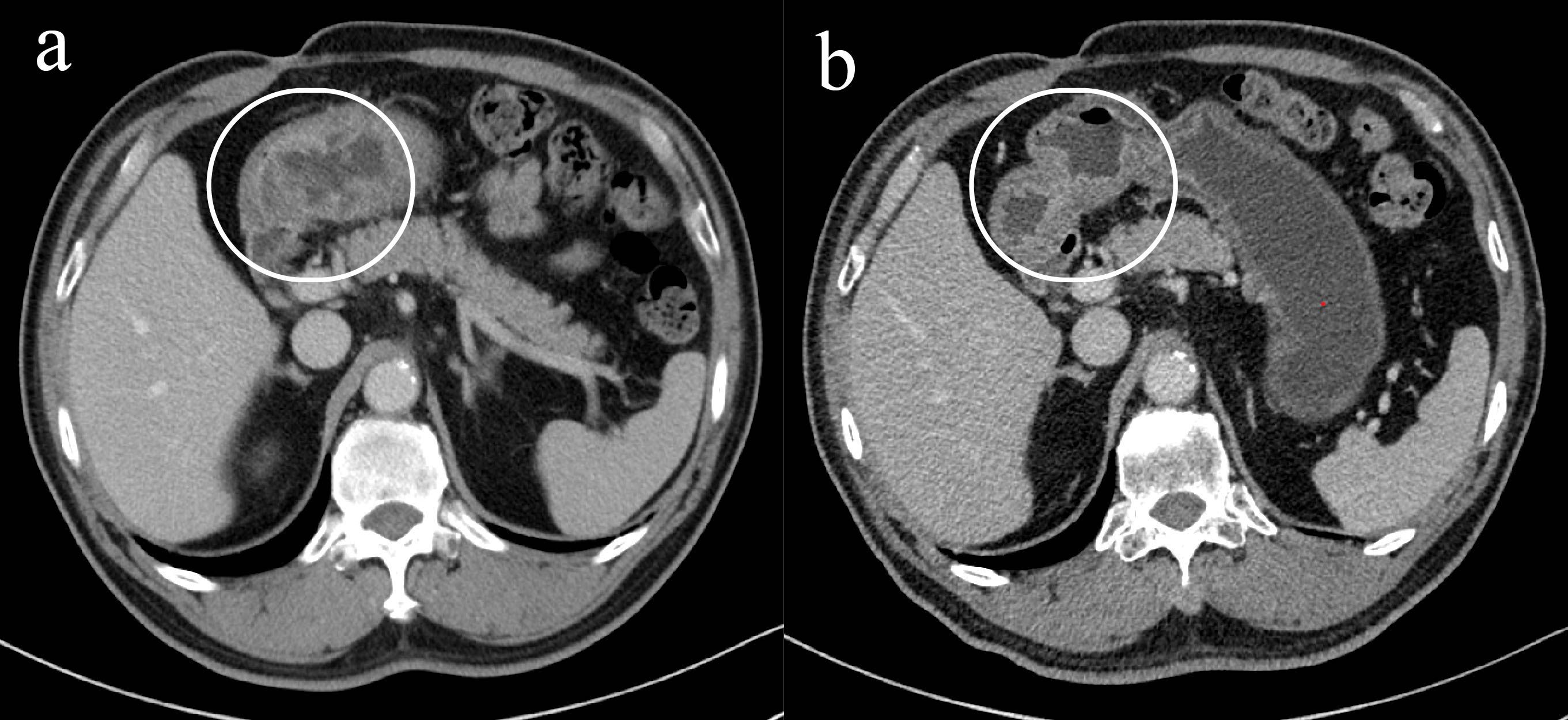
Figure 1. Changes of gastric lesions under gastroscopy, (a) On July 30, 2020, (b) On October 22, 2020.
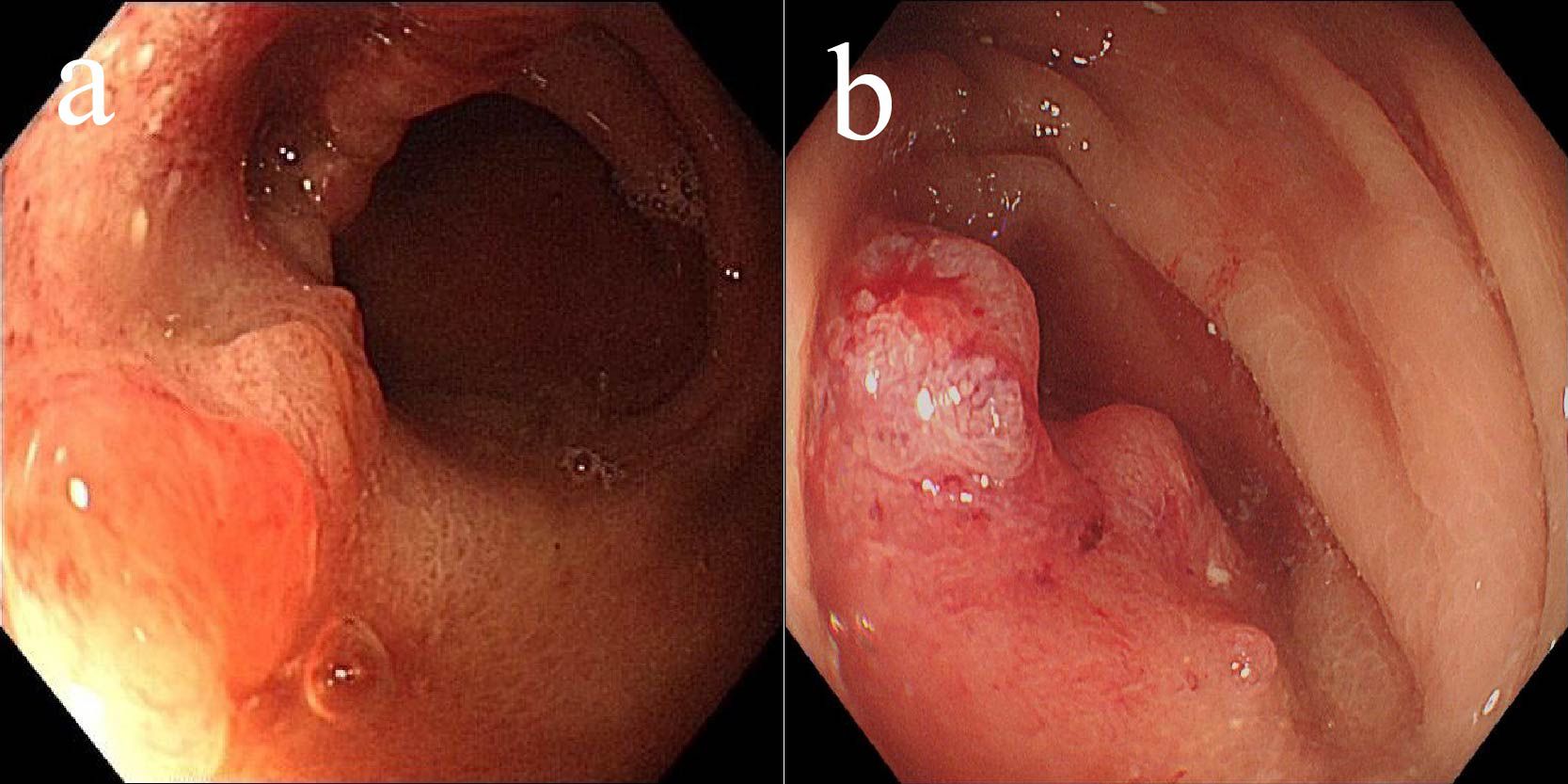
Figure 2. Imaging changes of gastric lesions after treatment, (a) On July 26, 2020 (slice thickness: 5mm, arterial phase), (b) On October 20, 2020 (slice thickness: 5mm, portal venous phase).
After the second cycle, follow-up gastroscopy revealed a significant reduction in the gastric antrum ulcer. By the third cycle, the ulcer had formed scar tissue (Figure 1b). Follow-up CT scans showed significant tumor shrinkage and downstaging, with the clinical stage revised to ycT1bN0M0, stage I, indicating a partial response (Figure 2b).
Due to a history of coronary heart disease and the implantation of six coronary stents, the patient considered surgery and endoscopic submucosal dissection (ESD) too risky and refused them. Additionally, the patient declined oral chemotherapy, opting only for regular follow-up. By the end of 2020 and throughout 2022, follow-up gastroscopy showed no significant recurrence of the gastric antrum scar (Figures 3a–d). Blood samples were routinely collected for circulating tumor DNA (ctDNA) testing, all of which returned negative results.
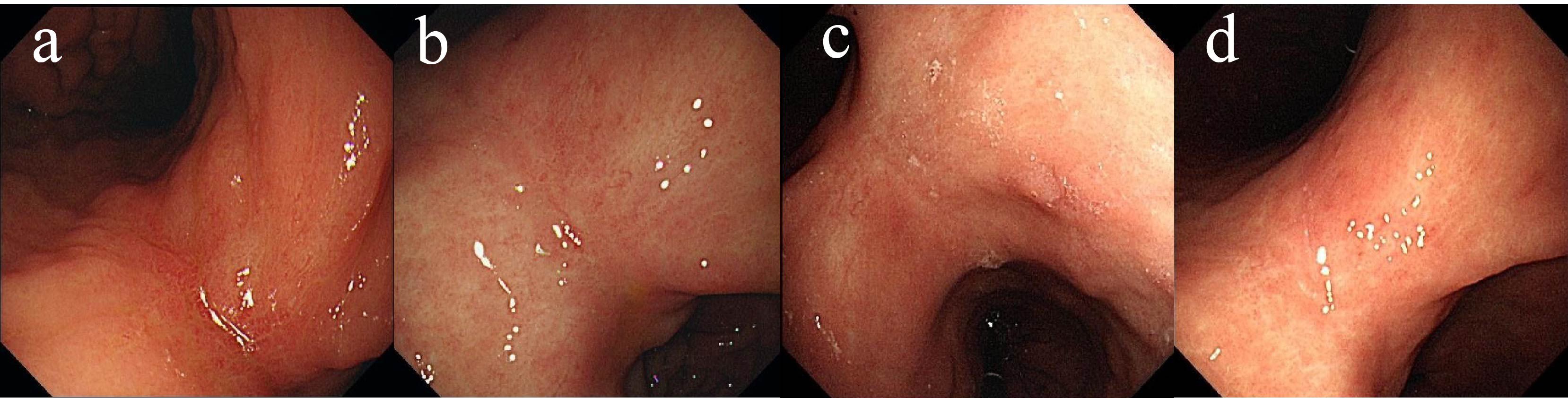
Figure 3. Changes of gastric lesions under gastroscopy, (a) On December 25, 2020, (b) On April 14, 2021, (c) On December 09, 2021, (d) On May 02, 2022.
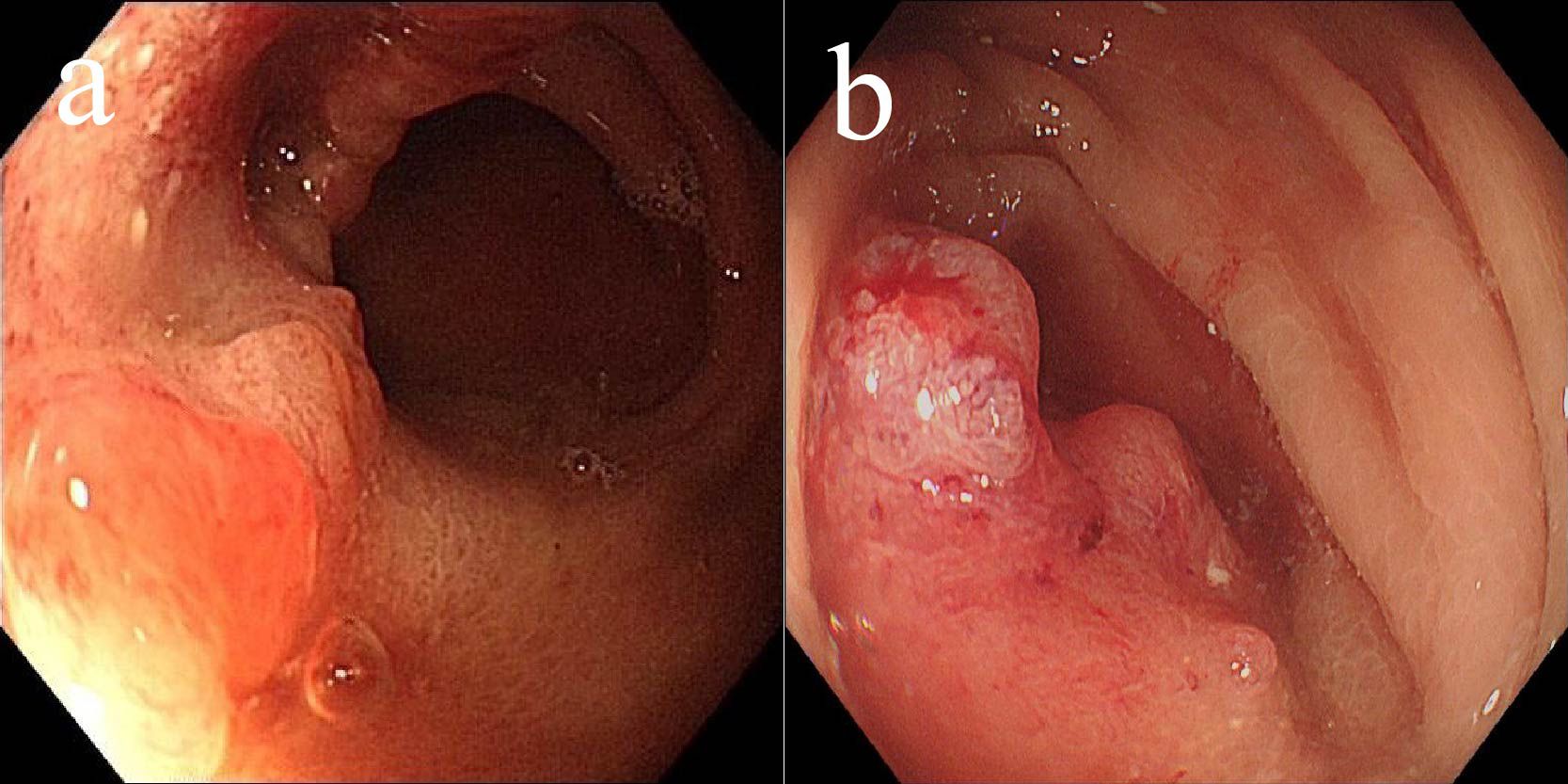
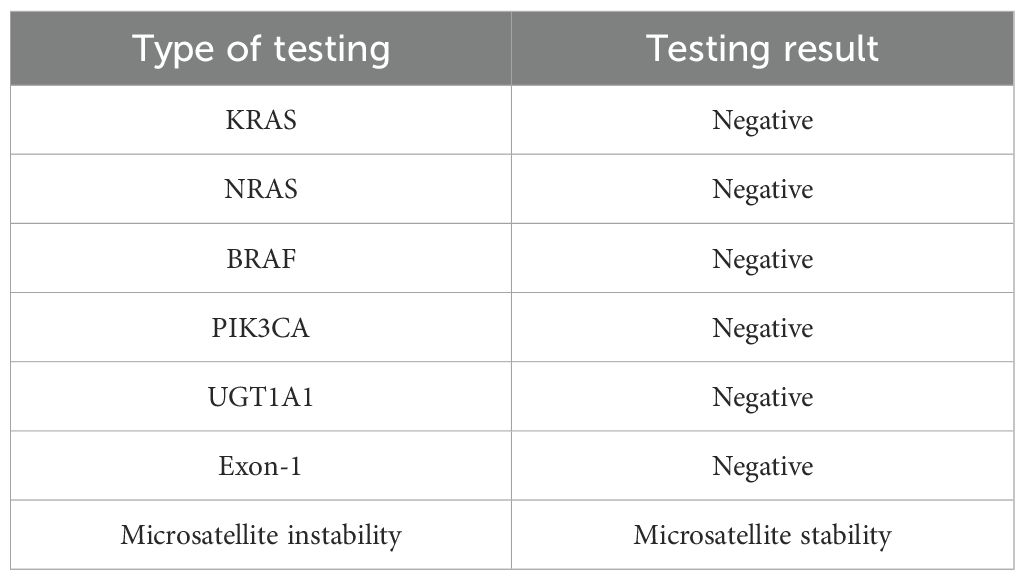
In May 2022, the patient presented with difficulty in defecation and abdominal bloating. Colonoscopy revealed an ulcerative neoplasm at the junction of the sigmoid and descending colon (Figure 4a), with pathological biopsy confirming adenocarcinoma. According to the AJCC staging criteria, the clinical stage was cT3N1bM0, stage IIIb. Genetic testing revealed wild-type KRAS, NRAS, BRAF, PIK3CA, and no mutations in the UGT1A1 promoter or Exon-1. Microsatellite stability was confirmed (Table 1), categorizing the tumor as a “cold tumor.” The patient requested non-surgical treatment and refrained from receiving any further treatment over the subsequent two years.
In May 2025, due to stenosis of the intestinal lumen, the patient underwent another colonoscopy, which revealed tumor progression (Figure 4b). Based on the AJCC staging criteria and a full abdominal CT scan (Figure 5a), the clinical stage of colorectal cancer was cT4N2aM0, stage IIIc. The department recommended surgical resection, but the patient again refused due to personal reasons. After reviewing relevant domestic and international research, as well as clinical trial results, and following MDT discussion, a decision was made to proceed with oncolytic virus endoscopic injection combined with immunotherapy. This patient received three intratumoral injections of 1.5 mL Oncolytic adenovirus H101 diluted with 4.5ml normal saline (15.0 × 1011 viral particles, 1.05 × 1011 PFU, each) on June 4, June 19, and July 3, 2025. Each injection was administered at a dose of 0.3ml on the tumor surface and within the tumor margin area. Each time, a flexible colonoscope (GIF-Q260, 9.2 mm; Olympus, Tokyo, Japan) was inserted at the junction of the sigmoid colon and descending colon, and injection was performed using an endoscopic injection needle (ATE-ZSZ-23×1800×23×5, Jiangsu, China). Immune therapy with tislelizumab (IV 200 mg) was administered the day after each injection.
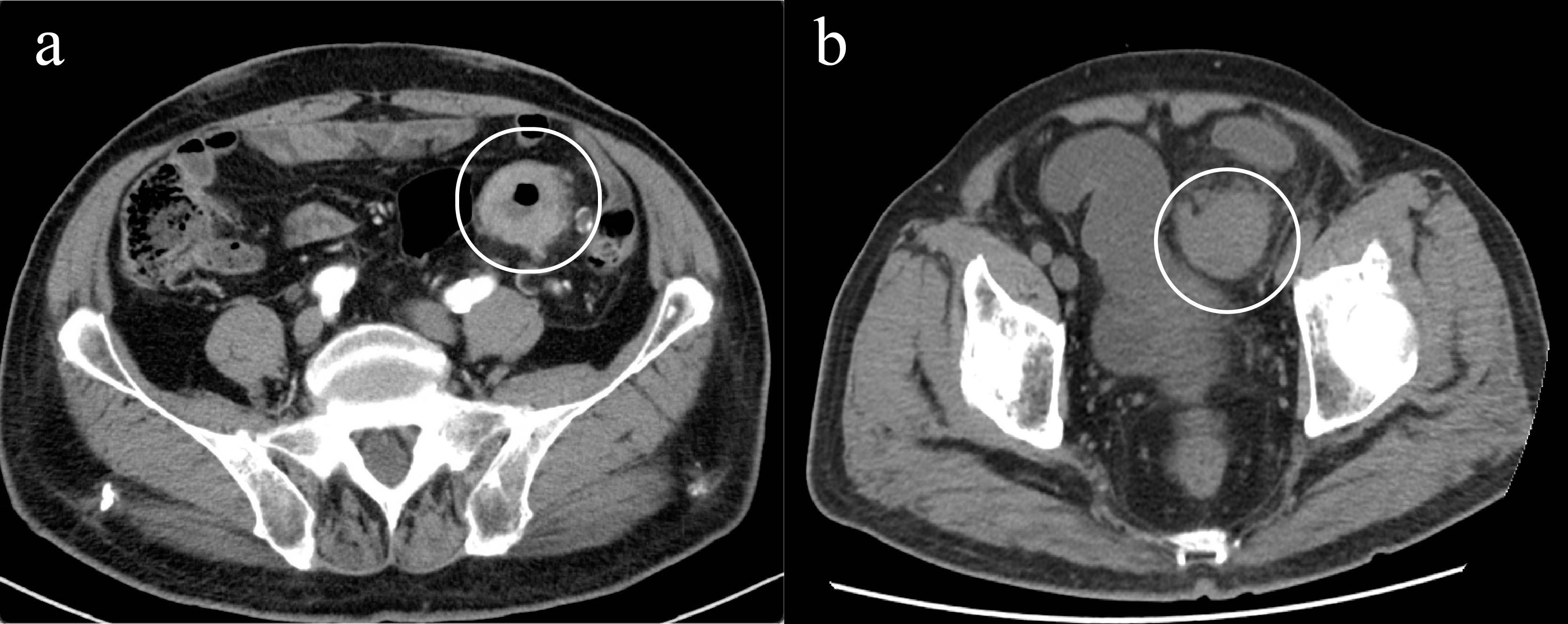
Figure 5. Imaging changes of colonic lesions, (a) On May 15, 2025 (slice thickness: 5mm, arterial phase), (b) On September 23, 2025 (slice thickness: 5mm, portal venous phase).
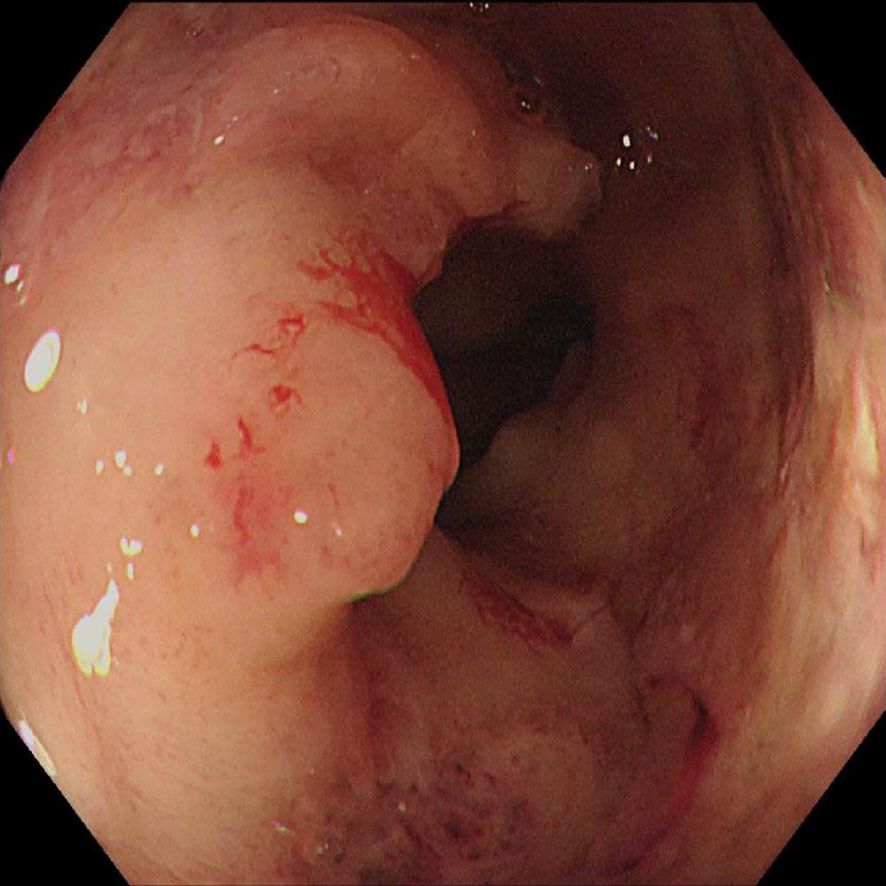
The first endoscopy revealed the tumor located 50 cm from the anus, with the intratumoral injection completed (Figure 6a). The second endoscopy showed significant tumor shrinkage, and the endoscope could barely pass through the narrowed area (Figure 6b). After the third treatment, the endoscope passed smoothly, and the tumor had further shrunk (Figure 6c). A repeat colonoscopy, performed in September 2025, revealed no enlargement of the lesion or active bleeding (Figure 7). Based on the AJCC staging criteria and a full abdominal CT scan (Figure 5b), the clinical stage of colorectal cancer was revised to cT3N1aM0, stage IIIb.
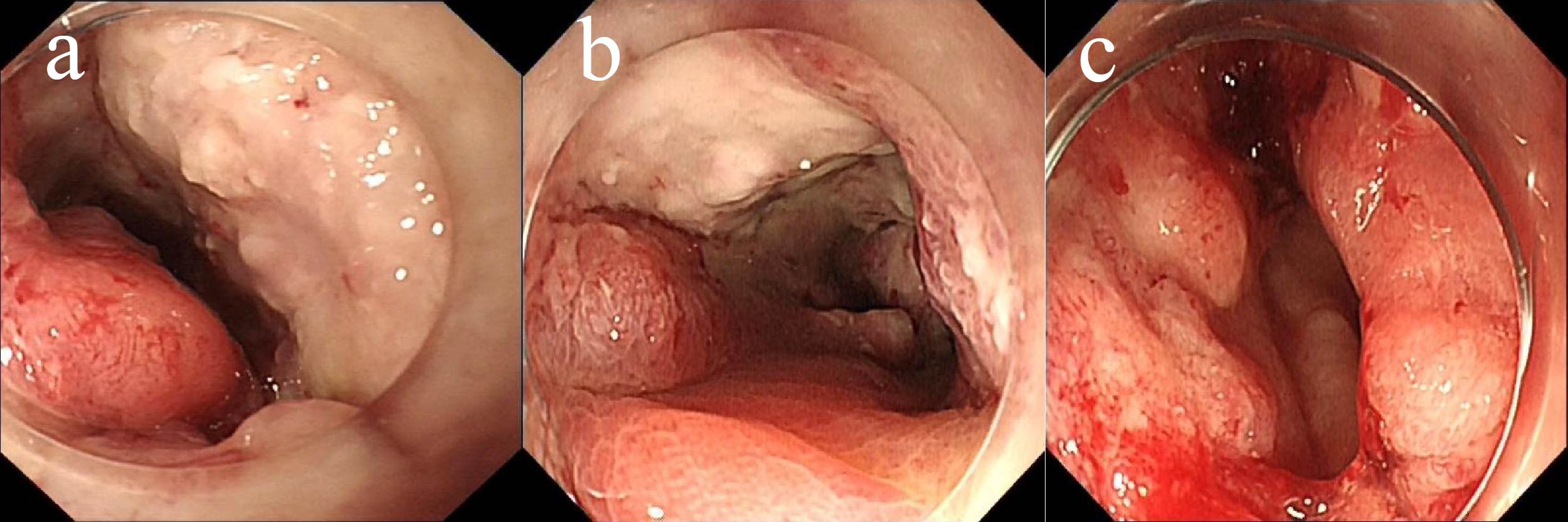
Figure 6. Endoscopic injection of colonic tumors, (a) On June 5, 2025, (b) On June 19, 2025, (c) On July 3, 2025.
Currently, the patient is eating normally and has been living without progression for nearly four months (Figure 8).
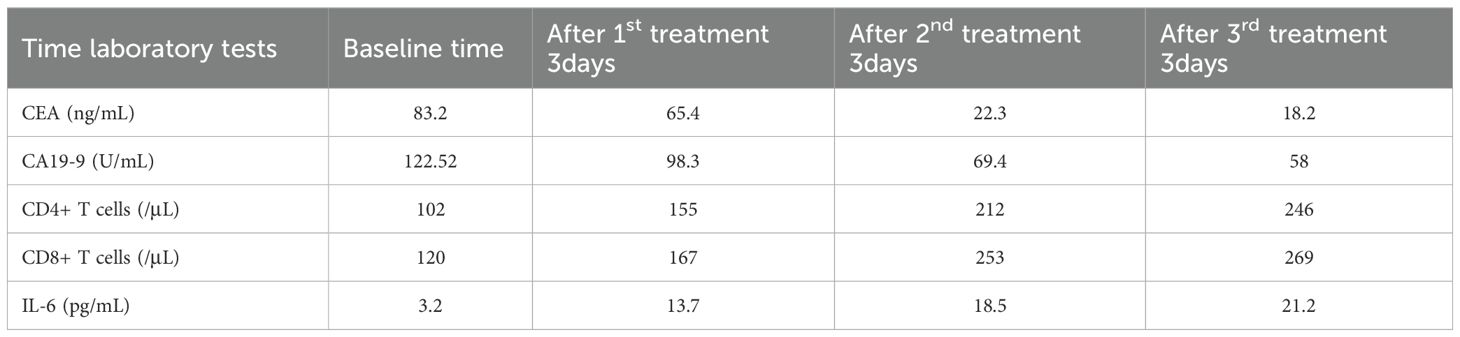
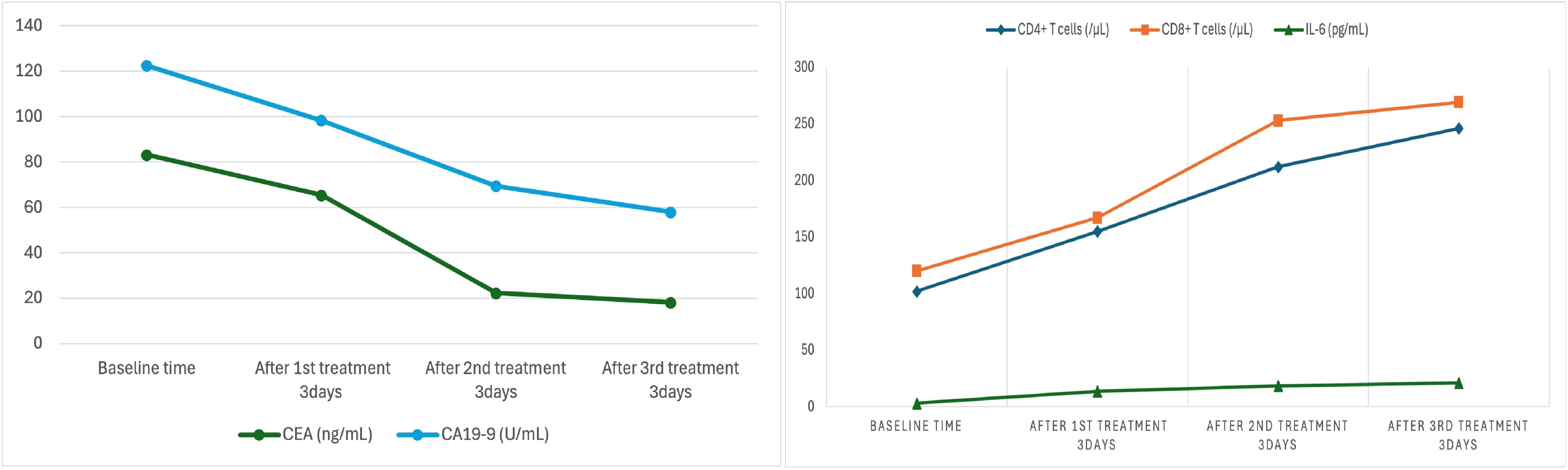
During treatment, the patient’s serum tumor markers significantly decreased: CEA decreased from 83.20 ng/mL to 18.2 ng/mL, and CA19–9 decreased from 122.62 U/mL to 58 U/mL. Immunological monitoring showed a significant increase in CD8+ T cells, from 102/μL to 246/μL, CD4++ T cells increased from 120/μL to 269/μL and interleukin-6 increased from 3.2 pg/mL to 21.2 pg/mL (Table 2) (Figure 9).
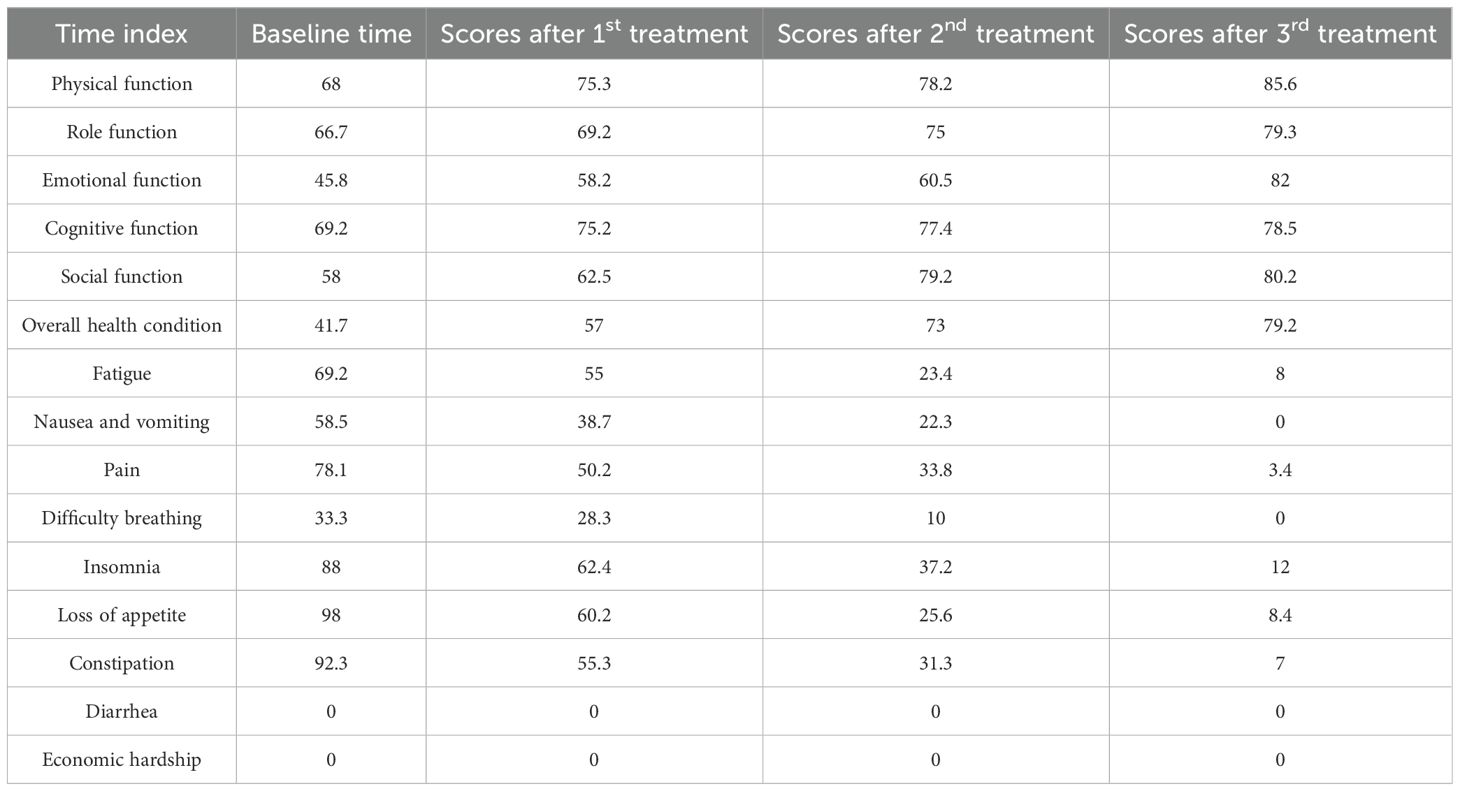
Regarding adverse reactions, no thrombocytopenia was observed during the three treatments. No fever occurred after the first treatment; however, the second treatment resulted in a rise in temperature (38.5°C), which subsided with symptomatic treatment. The third treatment caused a fever of 39°C accompanied by chills, which returned to normal after medication. Additionally, quality of life was evaluated during all three treatments using the EORTC QLQ-C30 scale (Table 3). Results showed improvements in six functional domains: physical, role, emotional, cognitive, social functions, and overall health, compared to pre-treatment levels. Fatigue, nausea and vomiting, pain, difficulty breathing, insomnia, loss of appetite, constipation, and diarrhea showed a decreasing or stable trend.
In conclusion, this case demonstrates that oncolytic virus endoscopic injection combined with immunotherapy can achieve significant therapeutic effects in an elderly patient with heterogeneous gastrointestinal malignant tumors. The treatment was associated with minimal adverse reactions and controllable safety, presenting a promising option for patients unable or unwilling to undergo surgery.
Discussion
This case report presents an 85-year-old male patient diagnosed with gastric antrum adenocarcinoma and heterogeneous colorectal cancer. Following the diagnosis of advanced colorectal cancer, the patient declined surgery and traditional chemotherapy due to advanced age and comorbidities. In response, the treatment team implemented an innovative, personalized approach: endoscopic injection of Oncolytic adenovirus H101 combined with intravenous injection of tislelizumab. This combination therapy significantly reduced tumor burden, improved the patient’s quality of life, and was associated with manageable adverse effects.
Oncolytic viruses selectively infect and destroy tumor cells while simultaneously activating immune responses that enhance anti-tumor effects. Genetically modified oncolytic viruses replicate selectively within tumor cells, releasing tumor-associated antigens (TAAs) and danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). These signals trigger T-cell-mediated immune responses (7). In this case, following treatment, there was a notable increase in the patient’s CD8+ and CD4+ T cell counts, indicating successful immune activation.
Gastrointestinal tumors typically present with an immune-suppressive tumor microenvironment (TME). However, oncolytic viruses can facilitate the polarization of tumor-associated macrophages and enhance CD8+ T cell infiltration, thus reversing the “cold” tumor microenvironment into a more immune-reactive “hot” state (13). Specifically, in colorectal cancer research, oncolytic viruses have shown promise in transforming immune-cold tumors into immune-hot tumors, thereby enhancing the efficacy of immunotherapies (14).
In recent years, the combination of oncolytic viruses and immune checkpoint inhibitors (ICIs) has garnered significant research attention. Oncolytic viruses promote T-cell infiltration into tumor tissues through the induction of tumor cell death and TME remodeling. PD-1 inhibitors can relieve T-cell functional suppression, further boosting immune responses. This “activation + suppression relief” model has demonstrated promising results in clinical studies, such as those involving liver metastatic colorectal cancer, where oncolytic viruses combined with localized chemotherapy successfully induced anti-tumor immune responses (15).
The NCT04755543 study indicated that the combination treatment exhibited good safety, with mild fever and injection site pain being the primary adverse reactions. No severe adverse events were observed. In terms of efficacy, the objective response rate was 35.9%, and partial responders experienced remission lasting up to 313 days, far surpassing the outcomes of traditional treatments (16).
Based on the viral replication dynamics, immune response activation time, and clinical feasibility of H101, a dosing interval of Day 0, Day 15, and Day 30 was chosen (25, 26). The initial injection on Day 0 initiates viral replication and triggers the early immune response, laying the foundation for subsequent immune activation. The second injection on Day 15 coincides with the peak of the immune response, further enhancing T cell activation and memory response. The third injection on Day 30 aims to maintain sustained immune pressure and prevent tumor immune escape. This regimen design references the clinical protocol and safety data from Zhang et al. on oncolytic virus therapy for malignant ascites, aiming to balance viral clearance with immune stimulation, while minimizing cumulative toxicity and ensuring adequate immune response development (24).
For elderly patients with comorbidities, treatment safety is of paramount importance. In the context of colorectal cancer treatment, research has shown that oncolytic virus M1 exerts strong oncolytic effects without inducing serious systemic toxicity (17). One study by Zhang demonstrated that oHSV2 treatment in a mouse colorectal cancer model did not cause weight loss, and no necrosis or ulcers were observed at the injection sites (18). This research provides a foundation for the clinical application of oncolytic viruses. Similarly, Emma’s study found no grade 3 or higher treatment-related adverse events in patients with liver metastatic colorectal cancer who received hepatic artery infusion of oncolytic virus TG6002 combined with oral 5-fluorocytosine (19). In this case, fever was quickly alleviated with symptomatic treatment, confirming the manageable nature of such reactions.
During the course of combination therapy with an oncolytic virus and a PD-1 inhibitor, the patient developed a transient febrile episode. Serial immunological monitoring revealed a marked post-treatment increase in peripheral CD4+, CD8+ T-cell counts and IL-6, showing a clear temporal correlation with the onset of fever. Approximately three days after the second treatment cycle, both CD4+, CD8+ T-cell and IL-6 counts peaked, coinciding precisely with the development of fever. This time-dependent relationship suggests that the febrile response was most likely driven by treatment-induced immune activation rather than by infectious causes.
Consistent with this observation, previous clinical studies involving oncolytic virus–based immunotherapy, such as talimogene laherparepvec (T-VEC), have identified fever as one of the most common adverse events, occurring in nearly 47% of treated patients (22). The underlying pathophysiology is thought to involve immune system hyperactivation and subsequent cytokine release syndrome (CRS). Activation of immune effector cells leads to the release of proinflammatory cytokines—including (IL-6), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ)—which collectively mediate systemic inflammatory responses characterized by fever, chills, and hypotension (23).
In the present case, the fever occurred early during combination therapy and was temporally associated with a rapid rise in peripheral T-lymphocyte counts. The absence of clinical or microbiological evidence of infection further supports an immune-mediated etiology. This pattern indicates robust activation of the antitumor immune response, suggesting that the oncolytic virus and PD-1 inhibitor may have exerted synergistic effects in stimulating host immunity. Nevertheless, excessive immune activation carries a potential risk of systemic inflammatory complications. Clinicians should therefore maintain close surveillance for immune-related adverse events (irAEs), particularly cytokine-mediated inflammatory responses, and initiate appropriate supportive or immunomodulatory measures when necessary to ensure treatment safety.
Taken together, the transient fever observed in this patient during oncolytic virus and PD-1 inhibitor combination therapy most likely represents an immune activation–related inflammatory response rather than an infection. This phenomenon reflects effective immune engagement and antitumor activation induced by the combined regimen. However, it also underscores the need for vigilant monitoring, early differentiation of immune-mediated versus infectious causes, and timely clinical intervention to balance therapeutic efficacy with immune-related toxicity management.
Safety has also been enhanced by altering the administration route. Local delivery via intratumoral or endoscopic injection significantly reduces the risk of systemic exposure. Several clinical trials in Japan, including those with HF10 and OBP-301 endoscopic injections, have confirmed the safety of this approach, exemplifying the benefits of local precision delivery (20, 21). This case highlights the successful application of the local delivery strategy, effectively ensuring patient safety.
It is important to acknowledge the limitations of this case report. This report is intended primarily as a means of sharing clinical experience and facilitating academic exchange. It serves to illustrate the potential of oncolytic virus endoscopic injection combined with PD-1 inhibitors as a novel and feasible therapeutic option for elderly patients who are ineligible for conventional treatments. The promising outcomes observed in this case warrant further validation through more rigorous research.
Moving forward, we plan to initiate broader, multi-center clinical investigations, including both single-arm and randomized controlled trials, to systematically evaluate the efficacy and safety of H101 in combination with ICIs across various cancer types.
This case provides a novel treatment strategy for elderly patients with gastrointestinal tumors. The combination of oncolytic viruses and immune checkpoint inhibitors offers a new perspective on the personalized treatment of elderly cancer patients, establishing a foundation for future research and clinical practice in this field.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Affiliated Hospital of Guizhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
SL: Writing – original draft, Writing – review & editing. XD: Writing – review & editing. HZ: Writing – review & editing. LD: Writing – review & editing. MZ: Writing – review & editing. ZW: Writing – review & editing. LX: Supervision, Resources, Visualization, Writing – review and editing. QW: Investigation, Writing – review & editing. XJ: Supervision, Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (82360116), Science and Technology Foundation Project of Guizhou Provincial Health Commission (gzwkj2023-033), Doctoral Research Start-up Fund Project of the Affiliated Hospital of Guizhou Medical University (gyfybsky-2021-21), and National Natural Science Foundation Cultivation Project of the Affiliated Hospital of Guizhou Medical University (gyfynsfc-2021-53).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Wagle NS, Cercek A, Smith RA, and Jemal A. Colorectal cancer statistics. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
2. Kubo T, Adachi Y, Mita H, Adachi Y, Iwata N, Yoshida Y, et al. Prognosis of elderly patients with gastric cancer treated with the best supportive care. J Gastrointest Cancer. (2024) 55:178–81. doi: 10.1007/s12029-023-00987-4
3. Dos Santos Hughes SF and Malone ML. What matters most?” for older patients who have cancer. J Am Geriatr Soc. (2023) 71:8–10. doi: 10.1111/jgs.18096
4. Huynh L and Moore J. Palliative and end-of-life care for the older adult with cancer. Curr Opin Sup Palliat Care. (2021) 15:23–8. doi: 10.1097/SPC.0000000000000541
5. Smyth EC, Nilsson M, Grabsch HI, Van Grieken NC, and Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
6. Dotan E, Walter LC, Browner IS, Clifton K, Cohen HJ, Extermann M, et al. NCCN Guidelines® Insights: Older adult oncology, version 1.2021: Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. (2021) 19:pp.1006–1019. doi: 10.6004/jnccn.2021.0043
7. Ksienski D, Wai ES, Croteau NS, Wai ES, Croteau NS, Freeman AT, Chan A, Fiorino L, et al. Association of age with differences in immune related adverse events and survival of patients with advanced nonsmall cell lung cancer receiving pembrolizumab or nivolumab. J Geriatr Oncol. (2020) 11:pp.807–813. doi: 10.1016/j.jgo.2020.01.006
8. Lee SM and Shin JS. Colorectal cancer in octogenarian and nonagenarian patients: Clinicopathological features and survivals. Ann Coloproctol. (2020) 36:323–9. doi: 10.3393/ac.2020.01.19.2
9. Joharatnam-Hogan N, Shiu KK, and Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev. (2020) 85:101980. doi: 10.1016/j.ctrv.2020.101980
10. Ma R, Li Z, Chiocca EA, Caligiuri MA, and Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. (2023) 9:122–39. doi: 10.1016/j.trecan.2022.10.003
11. Gupta A, Chavan SR, Gadepalli R, and Pareek P. Oncolytic viruses in head and neck cancers: Clinical applications and therapeutic potential. Front Microbiol. (2025) 16:1641267. doi: 10.3389/fmicb.2025.1641267
12. Narayan VM, Boorjian SA, Alemozaffar M, Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, et al. Efficacy of intravesical nadofaragene firadenovec for patients with Bacillus Calmette-Guérin–unresponsive nonmuscle-invasive bladder cancer: 5-year follow-up from a phase 3 trial. J Urol. (2024) 212:pp.74–86. doi: 10.1097/JU.0000000000004020
13. Wang J, Du L, and Chen X. Oncolytic virus: A catalyst for the treatment of gastric cancer. Front Oncol. (2022) 12:1017692. doi: 10.3389/fonc.2022.1017692
14. Pérez-Domínguez F, Quezada-Monrás C, Cárcamo L, Muñoz JP, and Carrillo-Beltrán D. Oncolytic viruses as a novel therapeutic approach for colorectal cancer: Mechanisms, current advances, and future directions. Cancers (Basel). (2025) 17:1854. doi: 10.3390/cancers17111854
15. Nakamura N, Sato-Dahlman M, Travis E, Jacobsen K, and Yamamoto M. CDX2 promoter-controlled oncolytic adenovirus suppresses tumor growth and liver metastasis of colorectal cancer. Cancer Sci. (2025) 116:1897–907. doi: 10.1111/cas.70063
16. Zhang H, Ren Y, Wang F, Ren Y, Wang F, Tu X, Tong Z, Liu L, et al. The long-term effectiveness and mechanism of oncolytic virotherapy combined with anti-PD-L1 antibody in colorectal cancer patient. Cancer Gene Ther. (2024) 31:pp.1412–1426. doi: 10.1038/s41417-024-00807-2
17. Guo L, Hu C, Liu Y, Hu C, Liu Y, Chen X, Song D, Shen R, et al. Directed natural evolution generates a next-generation oncolytic virus with a high potency and safety profile. Nat Commun. (2023) 14:p.3410. doi: 10.1038/s41467-023-39156-3
18. Zhang W, Wang F, Hu X, Liang J, Liu B, and Guan Q. & Liu, S., Inhibition of colorectal cancer liver metastasis in BALB/c mice following intratumoral injection of oncolytic herpes simplex virus type 2 for the induction of specific antitumor immunity. Oncol Lett. (2019) 17:815–22. doi: 10.3892/ol.2018.9720
19. West EJ, Sadoun A, Bendjama K, Erbs P, Smolenschi C, Cassier PA, et al. A phase I clinical trial of intrahepatic artery delivery of TG6002 in combination with oral 5-fluorocytosine in patients with liver-dominant metastatic colorectal cancer. Clin Cancer Res. (2025) 31:1243–56. doi: 10.1158/1078-0432.CCR-24-2498
20. Hirooka Y, Kasuya H, Ishikawa T, Kawashima H, Ohno E, Villalobos IB, et al. A phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer. (2018) 18:596. doi: 10.1186/s12885-018-4453-z
21. Shirakawa Y, Tazawa H, Tanabe S, Kanaya N, Noma K, Koujima T, et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur J Cancer. (2021) 153:pp.98–108. doi: 10.1016/j.ejca.2021.04.043
22. Barker CA, D'Angelo SP, Wasilewski G, Steckler AM, Lian M, Zhang Z, et al. A phase II randomized trial of talimogene laherparepvec oncolytic immunotherapy with or without radiotherapy for patients with cutaneous metastases from solid tumors. Radiother Oncol. (2024) 200:110478. doi: 10.1016/j.radonc.2024.110478
23. Fajgenbaum DC and June CH. Cytokine storm. New Engl J Med. (2020) 383:2255–73. doi: 10.1056/nejmra2026131
24. Zhang Y, Qian L, Chen K, Gu S, Meng Z, Wang J, et al. Oncolytic adenovirus in treating Malignant ascites: A phase II trial and longitudinal single-cell study. Mol Ther. (2024) 32:2000–20. doi: 10.1016/j.ymthe.2024.04.029
25. Lyu S, Li JY, Zhang R, Cui YX, and Jiang XJ. ‘Preliminary study on the efficacy of endoscopic injection of recombinant human type 5 adenovirus combined with chemotherapy for intermediate and advanced esophageal cancer and gastric cancer’. J Gen Surg Electr Ed. (2019) 7:15–21.
Keywords: gastric cancer, colorectal cancer, oncolytic virus, PD-1 inhibitors, elderly patients
Citation: Luo S, Ding X, Zhang H, Dai L, Zhang M, Wang Z, Xu L, Wang Q and Jin X (2025) Case report: Local and systemic combination therapy: endoscopic injection of an oncolytic virus with PD-1 inhibitor for an elderly patient with advanced gastrointestinal cancer. Front. Immunol. 16:1719438. doi: 10.3389/fimmu.2025.1719438
Received: 06 October 2025; Accepted: 30 October 2025;
Published: 21 November 2025.
Edited by:
Zebo Jiang, Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine, ChinaReviewed by:
Tan-Huy Chu, Tam Anh Research Institute, VietnamJie Zhang, Shanghai Jiao Tong University, China
Copyright © 2025 Luo, Ding, Zhang, Dai, Zhang, Wang, Xu, Wang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangren Jin, amlueGlhbmdyZW45MkAxNjMuY29t
†These authors share first authorship
 Siqi Luo
Siqi Luo Xue Ding1†
Xue Ding1† Xiangren Jin
Xiangren Jin