- 1Women’s Health Services, Middlemore Hospital, Counties Manukau Health New Zealand, Auckland, New Zealand
- 2Department of Women and Children’s, King’s College London, London, United Kingdom
- 3Department of Clinical Science, Intervention and Technology Karolinska Institutet, Stockholm, Sweden
- 4Faculty of Medical and Health Science, University of Auckland, Auckland, New Zealand
- 5Reproduction and Perinatal Centre, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Introduction: Pregnancy complications from maternal placental syndrome (MPS) are associated with the accelerated development of cardiovascular events (CVE) in the parous cohort with systemic lupus erythematosus (SLE). Preterm birth may result from MPS, which is more common in SLE. This study aimed to determine if preterm birth stratified by gestational age increases the risk of CVE in SLE.
Methods: Utilizing Swedish population databases between 1973 and 2011, those who had been pregnant with SLE were identified and stratified into three groups: term (≥37 + 0 weeks), late preterm 34 + 0–36 + 6 weeks, and early preterm <34 + 0weeks births. The primary outcome was CVE or death from CVE. The risk of CVE was calculated and adjusted for SLE-related morbidity and cardiovascular risk factors.
Results: Over the 38-year interval, there were 3,963 subjects, and the prevalence of preterm birth was 20.9%. The prevalence of CVE was 10.4% (n = 411), being highest in those who had given birth <34 + 0 weeks. After multivariable adjustment, the risk of CVE was 1.8 [adjusted hazards ratio (HR) 95% CI: 1.3–2.5] in those who birthed <34 + 0 weeks compared with others who had birthed at term. They also developed CVE earlier than those who birthed at later gestational ages.
Conclusions: Early preterm birth <34 + 0 weeks conferred a two-fold increased hazard for accelerated development of CVE. Reassuringly, those who delivered at a later gestation did not exhibit a similar risk of premature CVE. Therefore, birth <34 + 0 weeks, regardless of an underlying cause, may be a useful screening question to identify parous persons with SLE who are at greater risk of early CVE.
Introduction
Cardiovascular events (CVE) are more common in young females with systemic lupus erythematosus (SLE), despite having fewer traditional cardiovascular risk factors (1–4). While the etiology is likely multifactorial and linked with antiphospholipid syndrome, studies in the general population have demonstrated associations between adverse pregnancy outcomes especially pre-eclampsia and other complications of placental insufficiency are associated with accelerated cardiovascular events (5, 6). Since those with SLE are also more prone to pregnancy complications, it is likely that preterm birth as a result of these complications could be a marker for CVE.
Our previous work has demonstrated that females with SLE are at greater risk of pregnancy complications; maternal placental syndrome (MPS) is associated with a two-fold increased risk of primary cardiovascular death and CVE (7, 8). MPS is defined as either hypertensive disorders of pregnancy, small for gestational age (SGA), placental abruption, or stillbirth; these features of MPS are also indications for iatrogenic preterm birth to reduce either maternal or fetal morbidity and mortality. Small studies have shown that those with SLE may also have higher rates of spontaneous preterm birth especially if steroids were used at conception (9–11).
Preterm birth is broadly defined as birth before 37 weeks' gestation; many subcategories of preterm birth related to different gestational ages exist in the literature (12). The implications of preterm birth are immense and are closely related to the gestational age at birth; the earlier the gestation at birth, the greater the risk of complications, particularly for the vulnerable premature neonate. Preterm births can occur for a variety of reasons and are not limited to MPS alone. There are different classifications of preterm birth; in the general population, half are spontaneous or idiopathic. Iatrogenic causes and preterm rupture of membranes (PROM) account equally for the remaining 50% (13). In women with SLE, birth may be expedited for maternal and fetal wellbeing to avoid worsening fetal growth restriction (leading to SGA infants), other features of MPS, or active maternal disease.
We aimed to determine the association between different gestational ages of preterm birth and the risk of CVE in a parous cohort with SLE to facilitate future CVE risk stratification.
Methods
Swedish population registries and study design
This was a population-based retrospective cohort study of females with SLE in Sweden between 01/01/1973 and 31/12/2010. Females with SLE were identified using the Swedish National Patient Register (NPR). The personal identity number (PIN) linked NPR data to the Swedish Medical Birth Registry (MBR) and Cause of Death Register. Information on hospital admissions and diagnoses, pregnancies and resulting complications, and cause of death was obtained (14).
Diagnoses were coded using the Swedish International Classification of Disease (ICD) system based on the World Health Organization ICD classification (15). The recording of diagnostic codes is well-validated (15). The Medical Birth Register (MBR) founded in 1973 includes data on 97.0%–99.5% of all pregnancies and births beyond 22 weeks in Sweden (16). The Cause of Death Register contains information on all deaths of Swedish nationals, irrespective of whether the death occurred in Sweden or elsewhere (http://www.socialstyrelsen.se/statistics).
Study cohort and outcomes of interest
Persons with SLE who had a birth and gestational age at birth recorded in the MBR were included. For the survival analysis, those who had a CVE prior to pregnancy were excluded. The outcome of interest was any CVE, including acute coronary syndrome, stroke and peripheral vascular disease, or death from these causes. To explore the severity of preterm birth on the future risk of CVE, length of gestation (or gestational age at birth) was stratified into three categories: (i) <34 + 0 weeks (<239 days) gestation; (ii) 34 + 0–36 + 6 weeks (239–258 days); and (iii) term ≥37 + 0 weeks (≥259 days).
MPS was defined as hypertensive disorders of pregnancy, small for gestational age (SGA), placental abruption, or stillbirth. SGA coded independently in the MBR is defined as a birthweight of more than two standard deviations below the mean weight for gestational age, according to the Swedish sex-specific fetal growth curve (17). The other features of MPS were identified through ICD codes.
Statistics
Continuous variables were expressed in medians and interquartile ranges (IQR) and compared using Mann–Whitney U or the t-test as appropriate. The χ2 test was used for univariate comparisons of dichotomous data. The risk of CVE was calculated using Cox proportional hazard models and expressed as a hazard ratio (HR). The multivariable analyses included adjustments for age, cardiovascular risk factors (hypertension, renal disease, and diabetes), and SLE-related morbidity (number of inpatient admissions, duration of SLE, cancer, and infections). The cumulative probabilities of survival and time from pregnancy to CVE were estimated using Kaplan–Meier curves and compared using a log-rank test. All p-values were two-sided and the threshold for statistical significance was set at p < 0.05. Analyses were performed with Stata IC version 13.1 (StataCorp LP, College Station, TX, USA).
Results
Over the 38-year study interval, there were 3,977 females with SLE who had 7,410 pregnancies recorded in the Medical Birth Register, and 3,963 (99.6%) had their length of gestation recorded. At the conclusion of the period of observation, the median age of the cohort who were still alive was 50 (IQR 41–59) years; 324 (8.2%) had died with a median age at death of 52 (IQR 44–59) years. Cardiovascular deaths accounted for 32.4% of all deaths and the median age of CV-related deaths was 54 (IQR 47–60) years. The prevalence of CVE was 10.4% (n = 411), with the median age of first CVE at 51 (IQR 42–57) years. The most common CVE was coronary artery disease accounting for 45.0% of events, with age at first event of 53 (IQR 46–59) years. Stroke was the second most common (38.0%) but occurred at a much younger age [47 (IQR 38–53) years].
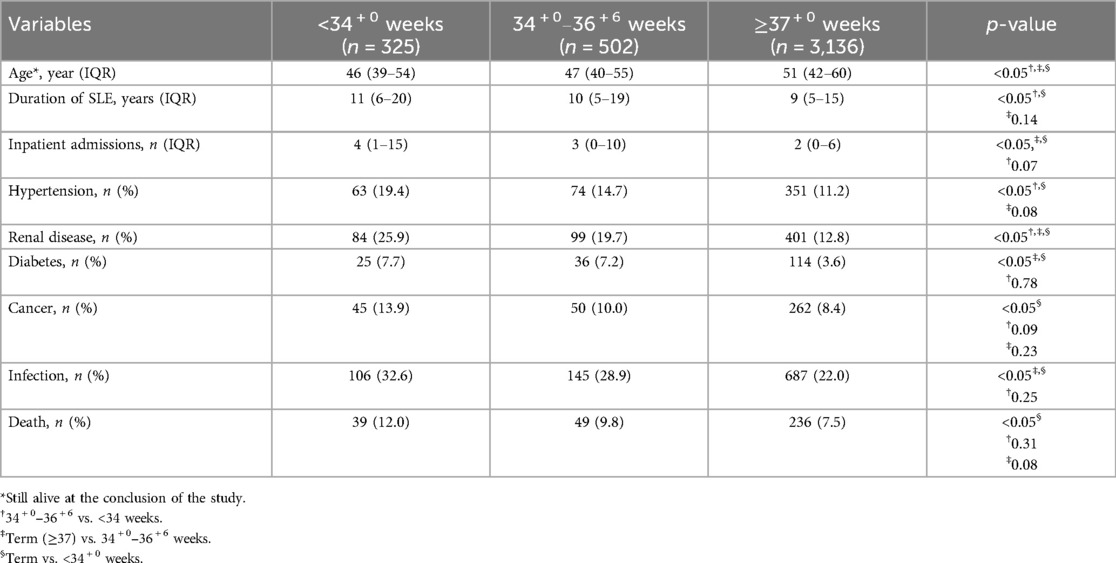
Three hundred and twenty-five (8.2%) had a history of any birth <34 + 0 weeks, 12.7% had birthed between 34 + 0 and 36 + 6 weeks, and approximately 80% had only term births of ≥37 + 0 weeks. Of those who gave birth <34 + 0 weeks, 63% had MPS complicating their pregnancies. Despite being younger than the other two groups, those who birthed <34 weeks had a longer duration of SLE and increased prevalence of all cardiovascular risk factors and SLE-related morbidity (except for inpatient admissions) compared with those who had term births (p < 0.05). Significant differences remained despite a younger age, more renal disease, inpatient admissions, diabetes, and infection between those who gave birth between 34 + 0 weeks and 36 + 6 weeks and those who birthed at term (Table 1).
Despite ages being similar across all groups, MPS and all its components were more common in the groups who birthed prior to 37 weeks (p < 0.05) (Table 2).
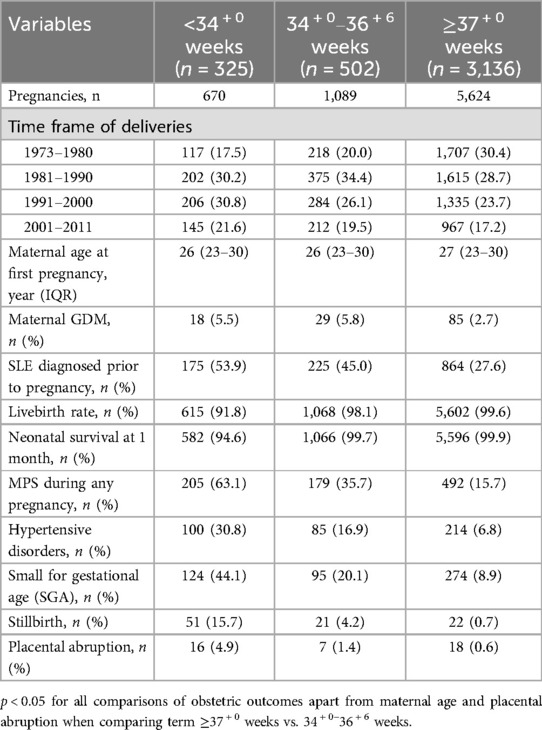
Table 2. Obstetric outcomes and subsequent cardiovascular events in parous persons with SLE according to length of gestation.
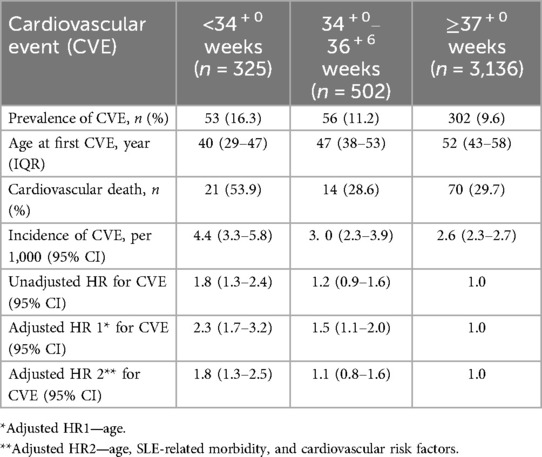
The prevalence of CVE was significantly higher in those who had a previous birth <34 + 0 weeks' gestation, even when compared with those who gave birth between 34 + 0 and 36 + 6 weeks' gestation (p < 0.05). CVE occurred much earlier in any parous person who birthed prior to 37 weeks' gestation. More than half of those who birthed <34 + 0 weeks' gestation died from cardiovascular causes (Table 3).
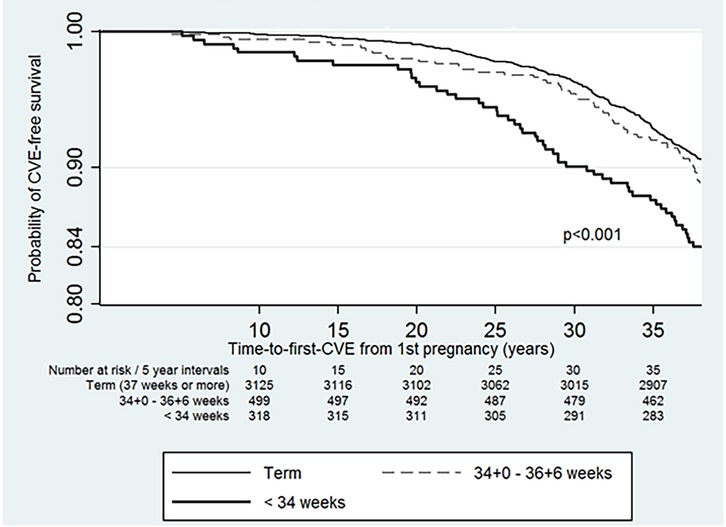
The subjects contributed 146,754.3 years of person-time to the study. The incidence of CVE in the parous cohort was 27.5 per 10,000 person-years (95% CI: 25.0–30.4). The incidence was higher at 43.7 per 10,000 person-years (95% CI: 33.92–57.5) in those who had <34 + 0-week births, and the incidence was 30.2 per 10,000 person-years (95% CI: 2.3–3.9) in those who gave birth between 34 and 36 weeks (Table 3). The risk of developing CVE was 1.8 (95% CI: 1.3–2.4) in those who birthed <34 + 0 weeks' gestation. The risk of CVE remained elevated in those who had preterm births <34 + 0 weeks even after adjustment for known CVE risk factors and SLE-related morbidity (Table 3). CVE developed earliest in those with <34 + 0-week births, compared with those who had term births (p < 0.001) (Figure 1).
Discussion
In this Swedish population of parous persons with SLE, 20.9% had one or more preterm birth <37 + 0 weeks' gestation. When stratified according to the length of gestation, those with any birth <34 + 0 weeks had the highest prevalence and incidence of CVE. This study has identified those with SLE who had the greatest need for early cardiovascular screening; this could also provide some reassurance to those who have had a preterm birth at later gestational ages.
Individuals are likely to recollect a preterm birth because of its implications and the need for special care for the neonate and long-term morbidity. Therefore, it serves as an excellent screening question (and is arguably more reliable than a history of pre-eclampsia/hypertensive disorders) for clinicians aiming to identify parous persons with SLE who are at particular risk of CVE.
Globally, preterm births are increasing, due to a variety of reasons (18). Births prior to 37 weeks' gestation in those with SLE may arise as a result of maternal disease and comorbidities, especially MPS-related complications, active SLE during pregnancy, and high doses of corticosteroids which are associated with preterm premature rupture of membranes (19). It is likely that those with severe or active SLE in pregnancy experienced earlier preterm births. Preterm birth unrelated to SLE and its complications can occur spontaneously, but this remains rare, particularly in early preterm births of <34 + 0 weeks. Only 3.5% of births occurred before 34 weeks in a low-risk American cohort, while the corresponding figure is only 1.8% for Sweden (20). Spontaneous preterm births in those with SLE are usually the result of preterm rupture of membranes which in turn are associated with steroid use (10, 21). Therefore, it is likely that these spontaneous preterm births are associated with SLE disease activity if not MPS (9, 11). It is not unusual for active SLE in pregnancy to lead to MPS and other adverse obstetric and neonatal outcomes (22). Since there are significant health implications for a neonate delivered prior to 34 weeks, any decision on medically indicated preterm birth would have been taken only after other options to prolong the pregnancy have been exhausted.
This study population is considered at high risk of MPS (22.1% in the Swedish cohort) (7); two-thirds of births prior to 34 + 0 weeks' gestation was associated with MPS-type complications, possibly related to active SLE in pregnancy. Although we were unable to discern the reasons for preterm birth—as SLE activity was not recorded in the MBR—these findings indicate that, regardless of the underlying cause for preterm birth, any birth prior to 34 weeks was associated with an increased risk of accelerated CVE.
It is probable that those with active SLE in pregnancy were more likely to develop MPS and therefore had to be birthed earlier. This is reflected in the greater SLE-related morbidity seen in those who birthed <37 + 0 weeks' gestation.
Registry-based studies are limited to the variables recorded; the present study specifically lacks information about flare or active SLE during pregnancy, the presence of lupus nephritis or other end-organ damage from SLE, steroid and hydroxychloroquine use, and antiphospholipid syndrome, all of which can affect SLE disease activity in pregnancy. The extent of disease damage due to SLE was not available, but we used surrogate markers such as hospital admissions, infections, and development of malignancies and adjusted for these factors in our multivariate analysis. There are multiple shared risk factors between cancer and cardiovascular disease, e.g., obesity, smoking, and social determinants of health, demonstrating how these two conditions are fundamentally interconnected (23). Furthermore, malignancy in SLE is associated with a higher risk of disease damage and immunosuppression (23, 24). Information on other traditional cardiovascular risk factors such as dyslipidemia, family history of premature coronary artery disease, smoking, and obesity were not available; we adjusted for those that were present including hypertension, presence of diabetes, and renal disease. However, as previously summarized in our publications, large international cohorts of SLE patients have not consistently demonstrated that these are useful markers for predicting CVE in patients with SLE, particularly young females (1, 4).
On the other hand, our data from Swedish population registries were drawn from one of the most comprehensive long-term population-based studies, followed up longitudinally over a 38-year interval. Sweden's population registries are a world-renowned example of meticulous record keeping. These data are particularly robust as the length of each gestation is recorded in the MBR and is not subject to recall bias. In addition, Sweden has the highest recorded prevalence of SLE in the Nordic countries where thorough records exist, although this may partly be due to ascertainment bias because of very reliable national records (25).
In conclusion, preterm birth <34 weeks’ gestation could be a useful screening question for clinicians identifying parous individuals with SLE at risk of CVE, as they have a two-fold increased likelihood of premature CVE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Regional Board of Ethics in Stockholm PROTOKOLL 2012/3:2. The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. CN-P: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. MW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. LM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. DP: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. MS was supported by the Rose Hellaby Medical Trust Scholarship and the Asia Pacific League Against Rheumatism Grant.
Acknowledgments
We thank Alexandra Gillette for the linkage of Swedish population registries, Paul Seed for his guidance in statistical analysis, and Headington & Co. for the formatting of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soh MC, Nelson-Piercy C, Westgren M, McCowan L, Pasupathy D. Do adverse pregnancy outcomes contribute to accelerated cardiovascular events seen in young women with systemic lupus erythematosus? Lupus. (2017) 26:1351–67. doi: 10.1177/0961203317719146
2. Urowitz MB, Gladman D, Ibañez D, Bae SC, Sanchez-Guerrero J, Gordon C, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res. (2010) 62:881–7. doi: 10.1002/acr.20122
3. Farina N, Webster J, Luo W, Garelick D, Pinto SM, Isenberg D, et al. Factors associated with cardiovascular events in systemic lupus erythematosus in a monocentric cohort with up to 40 years of follow-up. Semin Arthritis Rheum. (2023) 61:152226. doi: 10.1016/j.semarthrit.2023.152226
4. Richter P, Cardoneanu A, Rezus C, Burlui AM, Rezus E. Non-traditional pro-inflammatory and pro-atherosclerotic risk factors related to systemic lupus erythematosus. Int J Mol Sci. (2022) 23:12604. doi: 10.3390/ijms232012604
5. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health. Circ Cardiovasc Qual Outcomes. (2017) 10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497
6. Hallum S, Basit S, Kamper-Jørgensen M, Sehested TSG, Boyd HA. Risk and trajectory of premature ischaemic cardiovascular disease in women with a history of pre-eclampsia: a nationwide register-based study. Eur J Prev Cardiol. (2023) 30:506–16. doi: 10.1093/eurjpc/zwad003
7. Soh MC, Nelson-Piercy C, Dib F, Westgren M, McCowan L, Pasupathy D. Brief report: association between pregnancy outcomes and death from cardiovascular causes in parous women with systemic lupus erythematosus: a study using Swedish population registries. Arthritis Rheumatol. (2015) 67:2376–82. doi: 10.1002/art.39218
8. Soh MC, Dib F, Nelson-Piercy C, Westgren M, McCowan L, Pasupathy D. Maternal-placental syndrome and future risk of accelerated cardiovascular events in parous Swedish women with systemic lupus erythematosus-a population-based retrospective cohort study with time-to-event analysis. Rheumatology. (2016) 55:1235–42. doi: 10.1093/rheumatology/kew043
9. Clark CA, Spitzer KA, Nadler JN, Laskin CA. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol. (2003) 30:2127–32.14528505
10. Chakravarty EF, Colón I, Langen ES, Nix DA, El-Sayed YY, Genovese MC, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. (2005) 192:1897–904. doi: 10.1016/j.ajog.2005.02.063
11. Abheiden CNH, Blomjous BS, Slaager C, Landman AJEMC, Ket JCF, Salmon JE, et al. Systemic lupus erythematosus is associated with an increased frequency of spontaneous preterm births: systematic review and meta-analysis. Am J Obstet Gynecol. (2024) 231:408–416.e21. doi: 10.1016/j.ajog.2024.03.010
12. World Health Organization (WHO). Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization (2012). Available online at: https://iris.who.int/handle/10665/44864 (accessed May 20, 2025).
13. Moutquin J. Classification and heterogeneity of preterm birth. BJOG. (2003) 110:30–3. doi: 10.1046/j.1471-0528.2003.00021.x
14. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. (2009) 24:659–67. doi: 10.1007/s10654-009-9350-y
15. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. (2011) 11:450. doi: 10.1186/1471-2458-11-450
16. Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. (1990) 18:143–8. doi: 10.1177/140349489001800209
17. Maršál K, Persson P-H, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. (1996) 85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x
18. Cheong JL, Doyle LW. Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health. (2012) 48:784–8. doi: 10.1111/j.1440-1754.2012.02536.x
19. Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. (1992) 166:1318–23. doi: 10.1016/0002-9378(92)91596-3
20. Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. (2012) 17:120–5. doi: 10.1016/j.siny.2012.01.007
21. Shimada H, Wakiya R, Kanenishi K, Miyatake N, Nakashima S, Mansour MMF, et al. Preterm birth is strongly affected by the glucocorticoid dose during pregnancy in women complicated by systemic lupus erythematosus. Arthritis Res Ther. (2022) 24:10. doi: 10.1186/s13075-021-02699-1
22. Clowse MEB. Lupus activity in pregnancy. Rheum Dis Clin N Am. (2007) 33:237–52. doi: 10.1016/j.rdc.2007.01.002
23. Wilcox NS, Amit U, Reibel JB, Berlin E, Howell K, Ky B. Cardiovascular disease and cancer: shared risk factors and mechanisms. Nat Rev Cardiol. (2024) 21:617–31. doi: 10.1038/s41569-024-01017-x
24. Hardenbergh D, Molina E, Naik R, Geetha D, Chaturvedi S, Timlin H. Factors mediating cancer risk in systemic lupus erythematosus. Lupus. (2022) 31:1285–95. doi: 10.1177/09612033221122163
Keywords: systemic lupus erythematosus, pregnancy complication, cardiovascular risk (CV risk), risk stratification, preterm birth, population-based, SLE pregnancy, cardiovascular risk prediction
Citation: Soh MC, Nelson-Piercy C, Westgren M, McCowan L and Pasupathy D (2025) Differences in gestational age at preterm birth can predict future cardiovascular events in systemic lupus erythematosus (SLE). Front. Lupus 3:1473368. doi: 10.3389/flupu.2025.1473368
Received: 30 July 2024; Accepted: 5 June 2025;
Published: 27 June 2025.
Edited by:
Marjon Alina De Boer, VU Medical Center, NetherlandsReviewed by:
Gloria Riitano, Sapienza University of Rome, ItalyAbir Mokbel, Cairo University, Egypt
Madhuri Radhakrishna, AIG Hospitals, India
Copyright: © 2025 Soh, Nelson-Piercy, Westgren, McCowan and Pasupathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: May Ching Soh, bWF5LnNvaEBtaWRkbGVtb3JlLmNvLm56
 May Ching Soh
May Ching Soh Catherine Nelson-Piercy
Catherine Nelson-Piercy Magnus Westgren3
Magnus Westgren3 Lesley McCowan
Lesley McCowan Dharmintra Pasupathy
Dharmintra Pasupathy