- 1Departments of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 2Arthritis & Clinical Immunology, Oklahoma Medical Research Foundation, Oklahoma City, OK, United States
- 3Oklahoma City Department of Veterans Affairs Health Care System, Oklahoma City, OK, United States
- 4Research Services, Oklahoma City Department of Veterans Affairs Health Care System, Oklahoma City, OK, United States
- 5Departments of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 6Departments of Psychology, Oklahoma City Department of Veterans Affairs Health Care System, Oklahoma City, OK, United States
- 7Departments of Neurology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 8Departments of Neurology, Oklahoma City Department of Veterans Affairs Health Care System, Oklahoma City, OK, United States
Background: Post traumatic stress disorder (PTSD) is associated with traumatic brain injury (TBI) and autoimmune disease, including systemic lupus erythematosus (SLE). We sought to determine whether the association was related to only PTSD or the combination of PTSD and TBI.
Methods: We studied rheumatic disease autoantibodies in a cohort of 40 patients, 20 of whom had TBI without PTSD and 20 had both PTSD and TBI. None had diagnosed rheumatic autoimmune disease. We also examined a cohort of 229 TBI patients, of whom 60% had PTSD, for diagnosis of rheumatic autoimmune disease.
Results: Among the 20 subjects with PTSD and TBI, 8 had autoantibodies [1 each with anti-Ro (or SSA), anti-RNP and anti-RNP plus anti-dsDNA, the remainder with a positive ANA]. Only 1 of 20 subjects with TBI alone had autoantibodies (p = 0.0088 by Fisher's Exact test). In the cohort of 229 subjects, there were 92 with TBI but no PTSD, of whom 4 (4.3%) had a diagnosed rheumatic autoimmune disease. Of the 137 with TBI and PTSD, 17 (13.3%) had an autoimmune rheumatic disease (p = 0.02 by Fisher's exact test).
Conclusion: We found more autoantibodies in the sera of patients with TBI and PTSD than in TBI alone. In addition, we found a 3-fold increased prevalence of autoimmune rheumatic disease in patients with TBI and PTSD compared to those with TBI alone. TBI is strongly associated with PTSD but we conclude that TBI does not contribute to the increased risk of autoimmune disease in PTSD.
Introduction
Post-traumatic stress disorder (PTSD) is manifested by nightmares, intrusive memories, hypervigilance and emotional withdrawal (1). In the past, involvement in an extraordinary traumatic event, one “outside the normal range of human experience”, according to the Diagnostic and Statistical Manual (DSM) III), was considered necessary but this is no longer the case as it is now recognized that more routine human experiences can lead to PTSD.
The pathophysiology of PTSD is incompletely understood. There are distinct patterns of neurocircuitry in the frontal-limbic system but these findings are among those who have PTSD. Thus, cause and effect is not established (2, 3). There is also genetic susceptibility to PTSD (4, 5) as well as epigenetic changes (6, 7). A portion of the genetics of PTSD lies in genes involved in neuroinflammation, including the pituitary-adrenal axis (4). Several abnormalities of the pituitary-adrenal axis and the immune system have been found in PTSD including increased negative feedback resulting in low basal serum cortisol, blunted response to ACTH, and abnormal cortisol circadian rhythm (8, 9).
There are many conditions associated with PTSD, including autoimmune disease and traumatic brain injury with up to two-thirds of combat veterans with PTSD also having TBI (10, 11). Autoimmune diseases are several-fold more common among US military veterans with PTSD than among those without PTSD. Autoimmune thyroid disease, systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis and multiple sclerosis have been found in excess in PTSD cohorts. One study of SLE confirmed the diagnosis of this disease with review of medical records (12), while another study of all patients seeking care within the Department of Veterans Affairs found similar results for SLE and the other diseases mentioned by use of diagnostic codes (13). Of interest in regard to autoimmune disease, both Th1 and Th17 cells as well as microRNA 125a, which regulates interferon, are increased in the peripheral blood of those with PTSD (14). In addition, increased expression of interferon-regulated genes prior to combat exposure is a risk factor for development of PTSD (15). Many of these same interferon-regulated genes are also over-expressed in the peripheral blood cells of patients with autoimmune disease (16). Recent reviews have highlighted these autoimmune associations of PTSD and the potential pathophysiological mechanisms that might underlie such connections, as well as called for more research into these observations (9, 17, 18).
Given that those with PTSD are likely to have TBI, we undertook this study to determine whether the association of PTSD and autoimmune disease was related to the presence of TBI.
Methods
Subjects
We studied serum for autoantibodies from a cohort recruited from the Oklahoma City VA Medical Center TBI Clinic, for which one of us (CP) serves as attending physician. Forty consecutive subjects attending the TBI clinic, who agreed to participate, with and without PTSD were studied. All subjects served in Afghanistan and saw combat duty within the last 10 years prior to recruitment to the study. All 40 had a history of a combat-related closed head injury and 20 of these also had a diagnosis of PTSD associated with their combat deployment. Review of medical records confirmed that the diagnostic criteria for PTSD were met in each subject. None had a diagnosed autoimmune rheumatic illness. The average age was 37.4 years with a range of 27–54. There were 39 men and 1 woman. There were 28 non-Hispanic White, 9 Black, 2 Hispanics White and 1 Pacific Islander American in the cohort. We administered Beck Depression index-II (BDI) and the Montreal Cognitive Assessment (MOCA) at a routine clinic visit.
We screened a larger group from this same clinic for a recorded diagnosis of a rheumatic, autoimmune illness as well as other medical conditions from the electronic medical record. These subjects have been previously enrolled in a study of vascular disease in TBI, which is conducted by one of us (CP), and constitute a random sample of approximately 10% from the TBI Clinic at the Oklahoma City VAMC. The medical records of each individual with such a diagnosis were reviewed to determine whether research classification or diagnostic criteria were met for autoimmune rheumatic diseases. For this study we examined the VA medical records of each subject, including both local Computerized Patient Record System (CPRS) data and Joint Legacy View (JVL) data from the nationwide VA medical system.
Antibodies
We determined serum autoantibodies using the BioPlex2200 (Bio-Rad, Hercules, California, USA). This simultaneous bead assay determines autoantibodies to the following autoantigens: dsDNA, chromatin, ribosomal P, Ro/SSA, La/SSB, centromere B, Smith (Sm), SmRNP, RNP, Scl70 (topoisomerase I), and Jo-1 (histidyl-transfer-RNA synthetase). We determined antinuclear antibodies (ANA) by indirect immunofluorescence using Hep-2 cells (BioRad, Hercules, California, USA).
Statistics
We compared categorical variables using the Chi square analysis or Fisher's Exact test, as appropriate, with calculation of the odds ratio and 95% confidence intervals. A p value <0.05 was considered significant.
Ethics
This study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board (IRB), which is the IRB of record for the Oklahoma City VAMC, as well as the Oklahoma City VA Research & Development Committee.
Results
Autoantibodies in PTSD/TBI and TBI alone
We studied sera from the 40 subjects with diagnosed autoimmune disease using the BioPlex2200 assay. Three of the PTSD/TBI patients had rheumatic disease related autoantibodies. One had anti-Ro in high titer, one had high titer anti-RNP, and a third patient had high titer anti-RNP and intermediate titer anti-dsDNA. This result (3 of 20 vs. 0 of 20) does not quite reach statistical significance but does show a trend (p = 0.11 by Fisher's exact test) when comparing TBI alone vs. PTSD/TBI. We also tested for anti-nuclear antibody (ANA) by indirect immunofluorescence using a screening serum dilution of 1:40. There were an additional 6 positive sera for ANA, 5 among the PTSD/TBI group and 1 among the 20 TBI only group. Thus, in total among the 20 subjects with PTSD and TBI, 8 had autoantibodies [1 each with anti-Ro (or SSA), anti-RNP and anti-RNP plus anti-dsDNA, the remainder with a positive ANA], while among those with TBI alone only one had autoantibodies (8 of 20 vs. 1 of 20, p = 0.0088 by Fisher's Exact test). Thus, autoantibodies were found in excess among those with both PTSD and TBI compared to those with only TBI.
Clinical rheumatic autoimmune disease
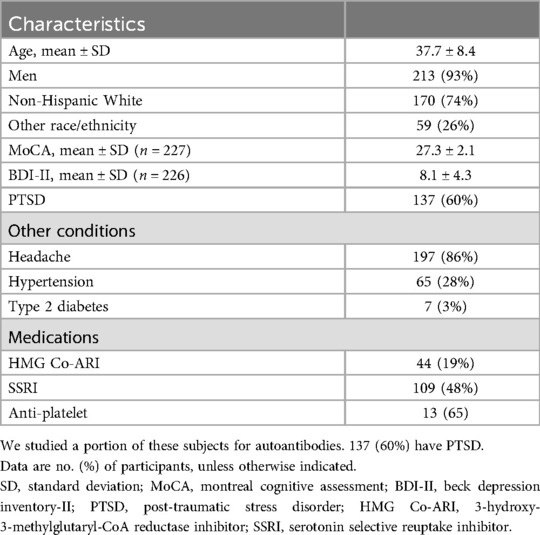
Among the 229 TBI subjects, 223 (93%) were men. The average age was 37.7 ± 8.4% and 74% were non-Hispanic White Americans. 137 (60%) had PTSD. There was no statistical difference in age, race/ethnicity, or sex distribution between those with and without PTSD. Headache was common (Table 1). Other medical conditions and the most common medications taken are given in Table 1.
We evaluated the co-morbid conditions among this 229-member cohort of patients with TBI. The subjects were relatively healthy with only 3% having type 2 diabetes mellitus, and a little more than 25% had hypertension. About 10% had moderate depression and 5% had severe by Beck Depression Index (BDI). Examining the Montreal Cognitive Assessment (MoCA), we found no subject had dementia with 40 of 229 having mild cognitive impairment (Table 1). There was no association of ANA or autoimmune rheumatic disease with the commonly taken medications, cognitive impairment or the mentioned diseases.
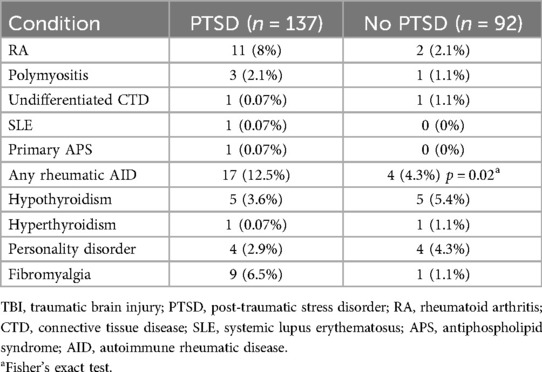
Twenty-one of 229 (14%) had a diagnosed autoimmune disease and 17 of these 33 (81%) had PTSD/TBI. Rheumatoid arthritis was over-represented among those with PTSD by 4-fold compared to the population prevalence of this disease (Table 2). The difference in myositis was also of interest but the numbers were low. Thus, when autoimmune rheumatic diseases were considered together, among those with TBI but no PTSD only 4 of 92 (4.3%) had an autoimmune disease, compared to 17 of 137 (12.41%) of those with both TBI and PTSD (p = 0.02 by Fisher's exact test, odds ratio 3.4, 95% CI: 1.1–10.5).
Discussion
The epidemiology of PTSD has been well studied, including among those who have served in the US Armed Services. Ten years after exposure to combat in Vietnam, 28% of veterans had continuing PTSD; and in long term follow up of almost 40 years, 11% of Vietnam veterans were still experiencing PTSD (19). Similar data are available from veterans of the Iraq and Afghanistan conflicts with up to 13% of combat-exposed personnel having PTSD (20, 21) with risk of PTSD directly related to degree of combat exposure (21). Among the individuals in this latest group, the prevalence of PTSD among veterans with mild traumatic brain injury is particularly high, reaching 32%–66% (10). The original definition of PTSD included exposure to trauma “outside the range of normal human experience” (quoting DSM III). Subsequently, data indicated that a significant portion of individuals suffer trauma as civilians and that PTSD is not uncommon in the general population, although the majority of persons suffering severe trauma do not develop PTSD. Lifetime risk of PTSD was found to be about 1% in one international study (22). Women are at higher risk of PTSD than men (23).
We found a 3-fold increased prevalence of rheumatic autoimmune disease among subjects with TBI and PTSD compared to those with TBI alone. This increased risk is similar to what has been reported in studies of PTSD (12, 13). In addition, among a well characterized group of subjects with TBI, of whom 60% had PTSD, we found autoantibodies more commonly among those with both PTSD and TBI, compared to those with only TBI. Thus, we conclude that TBI does not make a contribution to the risk of autoimmunity or autoimmune disease in PTSD.
Immune system abnormalities have been found among PTSD patients. This includes low basal cortisol with abnormal circadian rhythm (24) as well as elevated levels of catecholamines (25), at least in part due to an increased negative feedback loop of the hypothalamic-pituitary-adrenal axis [for comprehensive review see (8)]. Decreased glucocorticoid signaling is a pre-existing state in those who go on to develop PTSD (26, 27). In PTSD animal models, there are sex differences in the decrement of glucocorticoid signaling (28). Evidence of immune activation is found in PTSD with elevated pro-inflammatory cytokines in the cerebral spinal fluid and the peripheral blood (29–32). In a prospective study of peripheral blood cell gene expression in 188 US Marines, Breen and colleagues found over-expression of innate immunity and interferon signaling genes in those destined to develop PTSD. The findings were confirmed in an independent cohort of 96 US Marines (15). That is, development of PTSD is associated with abnormal regulation of immune genes with an interferon signature present, similar to that found in autoimmune diseases such as systemic lupus erythematosus, Sjögren's disease, and systemic sclerosis (33–35), while interferon-regulated genes are also abnormally but distinctly expressed compared to those with multiple sclerosis (36). Genome-wide association studies of PTSD show genetic association with several genes whose protein products are involved in the immune system. In addition, there are HLA genetic associations to PTSD (37–40). Thus, subjects at risk of PTSD harbor immune abnormalities that may pre-dispose to autoimmune disease.
As we have found and as previously reported, autoimmune disease is found among those with PTSD (12, 13). A study of the immune system of PTSD subjects informs these clinical studies associating autoimmune disease with PTSD as well as the studies of PTSD immune function cited above. Zhou and colleagues (14) studied peripheral blood mononuclear cells in PTSD and matched control subjects. Both Th1 and Th17T helper cells were increased in number and percentage. In addition, there were increased levels of not only IL-17 but also interferon-gamma. MicroRNA-125a was down regulated, consistent with its role in regulating interferon (14). T regulatory cells are also abnormal in PTSD patients (41). The innate immune system (42, 43) as well as Th17T cells and IL-17 (44) are critical to the pathogenesis of autoimmune disease. Thus, these changes in lymphocytes and serum cytokines are congruent with the increased risk of autoimmune disease that has been reported.
On the other hand, stress may predispose to autoimmune disease. For example, adverse childhood events are associated with autoimmune disease in adulthood (45). A recent study of 2 large nationwide databases showed self-reported adverse childhood events were associated with a prevalence ratio of 1.24 for Sjögren's disease, 1.14 for rheumatoid arthritis, and 1.13 for systemic lupus erythematosus (46). Stress in early life is associated with altered hypothalamic-pituitary-adrenal signaling (47), However, PTSD is associated with a blunted cortisol response to ACTH (9), while early childhood stress has been reported to increase signaling through this axis (48). Long-term studies showed that early life stress was associated with markers of chronic inflammation in adulthood (49, 50). Early life trauma induces epigenetic modification, which might influence risk of immune disease in adulthood (51, 52). Animal data also support the link of early life stress to future autoimmune disease (53, 54). Thus, psychological and emotional stress, especially in early life may give increased risk of autoimmune disease.
Some of the TBI and PTSD patients had autoantibodies in their sera without clinical disease. In fact, autoantibodies appear in the sera long before clinical autoimmune disease. Using the US Armed Forces Sera Repository, we previously showed a crescendo of autoantibody appearance in the serum of future SLE patients that peaked just at diagnosis. ANA, anti-Ro and anti-La were present many years, even decades, in advance of disease while anti-RNP, anti-Sm and anti-dsDNA made their appearance closer to onset of clinical disease (55). For diseases more common than SLE, such as rheumatoid arthritis or Sjögren's disease, investigators have utilized stored serum from blood donors to study autoantibodies prior to disease onset (56, 57). Studies of family members show similar results (58), as do population-based studies (59). In fact, for every autoimmune disease thus far studied, autoantibodies precede disease (60, 61). Thus, long term follow-up of TBI/PTSD patients with autoantibodies may demonstrate new onset autoimmune disease.
The present work is limited in that we studied only rheumatic autoimmune disease, while organ specific autoimmune disease (multiple sclerosis and autoimmune thyroid disease) has been associated with PTSD. In addition, we used coded diagnoses, but did review medical records to determine whether patients met the research classification criteria for the respective diseases. A study using coded diagnoses that associated autoimmune disease with PTSD (13) was confirmed by a second study in which diagnosis was confirmed by medical record review, at least for SLE (12), which might be the most troublesome disease in regards to miscoding/misdiagnosis. Fibromyalgia was in excess among those with PTSD, but this excess was found among patients with a diagnosed rheumatic disease seen by a rheumatologist. Not only are those with RA, SLE, APS, PM more likely to have fibromyalgia, we suspect rheumatologists are more likely to diagnose the condition compared to other practitioners.
In summary, TBI and PTSD are strongly associated among combat veterans. With a background of TBI, we find autoimmune rheumatic disease is associated with PTSD not TBI. These data provide further evidence that autoimmune disease and PSTD are linked, perhaps by common predisposing mechanisms. However, the cause-and-effect direction cannot be ascertained by the presently available data. That is, PTSD may induce immune changes that increase risk of autoimmune disease, or both PTSD and autoimmune disease may have underlying immune abnormalities in common.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Oklahoma Health Sciences Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. SL: Data curation, Investigation, Writing – review & editing. BK: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. TG: Data curation, Investigation, Methodology, Writing – review & editing. KS: Data curation, Investigation, Writing – review & editing. CP: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing. VL: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. VA Merit Review CX001877; NIH grants: AR053483, AI082714, GM104938.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. (2015) 1:15057. doi: 10.1038/nrdp.2015.57
2. Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. (2012) 36(9):2130–42. doi: 10.1016/j.neubiorev.2012.06.003
3. Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. (2012) 74(9):904–11. doi: 10.1097/PSY.0b013e318273bf33
4. Carvalho CM, Coimbra BM, Ota VK, Mello MF, Belangero SI. Single-nucleotide polymorphisms in genes related to the hypothalamic-pituitary-adrenal axis as risk factors for posttraumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. (2017) 174(7):671–82. doi: 10.1002/ajmg.b.32564
5. Zass LJ, Hart SA, Seedat S, Hemmings SM, Malan-Muller S. Neuroinflammatory genes associated with post-traumatic stress disorder: implications for comorbidity. Psychiatr Genet. (2017) 27(1):1–16. doi: 10.1097/YPG.0000000000000143
6. Zannas AS, Provencal N, Binder EB. Epigenetics of posttraumatic stress disorder: current evidence, challenges, and future directions. Biol Psychiatry. (2015) 78(5):327–35. doi: 10.1016/j.biopsych.2015.04.003
7. Ratanatharathorn A, Boks MP, Maihofer AX, Aiello AE, Amstadter AB, Ashley-Koch AE, et al. Epigenome-wide association of PTSD from heterogeneous cohorts with a common multi-site analysis pipeline. Am J Med Genet B Neuropsychiatr Genet. (2017) 174(6):619–30. doi: 10.1002/ajmg.b.32568
8. Yehuda R. Post-traumatic stress disorder. N Engl J Med. (2002) 346(2):108–14. doi: 10.1056/NEJMra012941
9. Lawrence S, Scofield RH. Post traumatic stress disorder associated hypothalamic-pituitary-adrenal axis dysregulation and physical illness. Brain Behav Immun Health. (2024) 41:100849. doi: 10.1016/j.bbih.2024.100849
10. Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. (2014) 71(2):149–57. doi: 10.1001/jamapsychiatry.2013.3080
11. Afari N, Harder LH, Madra NJ, Heppner PS, Moeller-Bertram T, King C, et al. PTSD, combat injury, and headache in veterans returning from Iraq/Afghanistan. Headache. (2009) 49(9):1267–76. doi: 10.1111/j.1526-4610.2009.01517.x
12. Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang SC, Koenen KC, et al. Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis Rheumatol. (2017) 69(11):2162–9. doi: 10.1002/art.40222
13. O'Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. (2015) 77(4):365–74. doi: 10.1016/j.biopsych.2014.06.015
14. Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. (2014) 9(4):e94075. doi: 10.1371/journal.pone.0094075
15. Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. (2015) 20(12):1538–45. doi: 10.1038/mp.2015.9
16. Nocturne G, Mariette X. Interferon signature in systemic autoimmune diseases: what does it mean? RMD Open. (2022) 8(2):e002687. doi: 10.1136/rmdopen-2022-002687
17. Goldschen L, Ellrodt J, Amonoo HL, Feldman CH, Case SM, Koenen KC, et al. The link between post-traumatic stress disorder and systemic lupus erythematosus. Brain Behav Immun. (2023) 108:292–301. doi: 10.1016/j.bbi.2022.12.012
18. Ploesser M, Silverman S, Diaz JDL, Zincke MT, Taylor MB. The link between traumatic stress and autoimmune rheumatic diseases: a systematic scoping review. Semin Arthritis Rheum. (2024) 69:152558. doi: 10.1016/j.semarthrit.2024.152558
19. Marmar CR, Schlenger W, Henn-Haase C, Qian M, Purchia E, Li M, et al. Course of posttraumatic stress disorder 40 years after the Vietnam war: findings from the national Vietnam veterans longitudinal study. JAMA Psychiatry. (2015) 72(9):875–81. doi: 10.1001/jamapsychiatry.2015.0803
20. Kok BC, Herrell RK, Thomas JL, Hoge CW. Posttraumatic stress disorder associated with combat service in Iraq or Afghanistan: reconciling prevalence differences between studies. J Nerv Ment Dis. (2012) 200(5):444–50. doi: 10.1097/NMD.0b013e3182532312
21. Hines LA, Sundin J, Rona RJ, Wessely S, Fear NT. Posttraumatic stress disorder post Iraq and Afghanistan: prevalence among military subgroups. Can J Psychiatry. (2014) 59(9):468–79. doi: 10.1177/070674371405900903
22. Karam EG, Friedman MJ, Hill ED, Kessler RC, McLaughlin KA, Petukhova M, et al. Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depression Anxiety. (2014) 31(2):130–42. doi: 10.1002/da.22169
23. Lehavot K, O'Hara R, Washington DL, Yano EM, Simpson TL. Posttraumatic stress disorder symptom severity and socioeconomic factors associated with veterans health administration use among women veterans. Womens Health Issues. (2015) 25(5):535–41. doi: 10.1016/j.whi.2015.05.003
24. Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. (1996) 40(2):79–88. doi: 10.1016/0006-3223(95)00451-3
25. Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci Biobehav Rev. (2013) 37(5):860–95. doi: 10.1016/j.neubiorev.2013.03.024
26. Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. (2013) 42(3):503–13. doi: 10.1016/j.ecl.2013.05.004
27. Galatzer-Levy IR, Steenkamp MM, Brown AD, Qian M, Inslicht S, Henn-Haase C, et al. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. J Psychiatr Res. (2014) 56:36–42. doi: 10.1016/j.jpsychires.2014.04.020
28. Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc Natl Acad Sci U S A. (2014) 111(37):13529–34. doi: 10.1073/pnas.1401660111
29. Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. (2012) 62(2):663–73. doi: 10.1016/j.neuropharm.2011.02.027
30. Daskalakis NP, Cohen H, Nievergelt CM, Baker DG, Buxbaum JD, Russo SJ, et al. New translational perspectives for blood-based biomarkers of PTSD: from glucocorticoid to immune mediators of stress susceptibility. Exp Neurol. (2016) 284(Pt B):133–40. doi: 10.1016/j.expneurol.2016.07.024
31. Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. (2001) 9(4):209–17. doi: 10.1159/000049028
32. Lerman I, Davis BA, Bertram TM, Proudfoot J, Hauger RL, Coe CL, et al. Posttraumatic stress disorder influences the nociceptive and intrathecal cytokine response to a painful stimulus in combat veterans. Psychoneuroendocrinology. (2016) 73:99–108. doi: 10.1016/j.psyneuen.2016.07.202
33. Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. (2010) 62(2):589–98. doi: 10.1002/art.27224
34. Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. (2003) 100(5):2610–5. doi: 10.1073/pnas.0337679100
35. Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, et al. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. (2009) 10(4):285–96. doi: 10.1038/gene.2009.20
36. Nickles D, Chen HP, Li MM, Khankhanian P, Madireddy L, Caillier SJ, et al. Blood RNA profiling in a large cohort of multiple sclerosis patients and healthy controls. Hum Mol Genet. (2013) 22(20):4194–205. doi: 10.1093/hmg/ddt267
37. Garrett ME, Qin XJ, Mehta D, Dennis MF, Marx CE, Grant GA, et al. Gene expression analysis in three posttraumatic stress disorder cohorts implicates inflammation and innate immunity pathways and uncovers shared genetic risk with major depressive disorder. Front Neurosci. (2021) 15:678548. doi: 10.3389/fnins.2021.678548
38. James LM, Georgopoulos AP. Immunogenetics of posttraumatic stress disorder (PTSD) in women veterans. Brain Behav Immun Health. (2022) 26:100567. doi: 10.1016/j.bbih.2022.100567
39. Katrinli S, Lori A, Kilaru V, Carter S, Powers A, Gillespie CF, et al. Association of HLA locus alleles with posttraumatic stress disorder. Brain Behav Immun. (2019) 81:655–8. doi: 10.1016/j.bbi.2019.07.016
40. Katrinli S, Zheng Y, Gautam A, Hammamieh R, Yang R, Venkateswaran S, et al. PTSD Is associated with increased DNA methylation across regions of HLA-DPB1 and SPATC1l. Brain Behav Immun. (2021) 91:429–36. doi: 10.1016/j.bbi.2020.10.023
41. Jergović M, Bendelja K, Vidović A, Savić A, Vojvoda V, Aberle N, et al. Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin Immunol. (2014) 10(1):43. doi: 10.1186/1710-1492-10-43
42. Pisetsky DS. The role of innate immunity in the induction of autoimmunity. Autoimmun Rev. (2008) 8(1):69–72. doi: 10.1016/j.autrev.2008.07.028
43. Theofilopoulos AN. TLRs and IFNs: critical pieces of the autoimmunity puzzle. J Clin Invest. (2012) 122(10):3464–6. doi: 10.1172/JCI63835
44. Amatya N, Garg AV, Gaffen SL. IL-17 signaling: the yin and the yang. Trends Immunol. (2017) 38(5):310–22. doi: 10.1016/j.it.2017.01.006
45. Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. (2009) 71(2):243–50. doi: 10.1097/PSY.0b013e3181907888
46. Köhler-Forsberg O, Ge F, Aspelund T, Wang Y, Fang F, Tomasson G, et al. Adverse childhood experiences, mental distress, and autoimmune disease in adult women: findings from two large cohort studies. Psychol Med. (2025) 55:e36. doi: 10.1017/S0033291724003544
47. Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. (2005) 28(9):456–63. doi: 10.1016/j.tins.2005.07.006
48. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. (2000) 284(5):592–7. doi: 10.1001/jama.284.5.592
49. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. (2007) 104(4):1319–24. doi: 10.1073/pnas.0610362104
50. Pitts C, Millar SR, Perry IJ, Phillips CM. Relationships between childhood adversity and inflammatory biomarkers in adulthood: a cross-sectional analysis of a middle-to older-aged population. SSM Popul Health. (2024) 25:101608. doi: 10.1016/j.ssmph.2024.101608
51. Neves I, Dinis-Oliveira RJ, Magalhães T. Epigenomic mediation after adverse childhood experiences: a systematic review and meta-analysis. Forensic Sci Res. (2021) 6(2):103–14. doi: 10.1080/20961790.2019.1641954
52. Soares S, Rocha V, Kelly-Irving M, Stringhini S, Fraga S. Adverse childhood events and health biomarkers: a systematic review. Front Public Health. (2021) 9:649825. doi: 10.3389/fpubh.2021.649825
53. Faraji J, Bettenson D, Yong VW, Metz GAS. Early life stress aggravates disease pathogenesis in mice with experimental autoimmune encephalomyelitis: support for a two-hit hypothesis of multiple sclerosis etiology. J Neuroimmunol. (2023) 385:578240. doi: 10.1016/j.jneuroim.2023.578240
54. Faraji J, Soltanpour N, Lotfi H, Moeeini R, Moharreri AR, Roudaki S, et al. Lack of social support raises stress vulnerability in rats with a history of ancestral stress. Sci Rep. (2017) 7(1):5277. doi: 10.1038/s41598-017-05440-8
55. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349(16):1526–33. doi: 10.1056/NEJMoa021933
56. Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary sjogren syndrome. JAMA. (2013) 310(17):1854–5. doi: 10.1001/jama.2013.278448
57. Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. (2004) 50(2):380–6. doi: 10.1002/art.20018
58. Maijer KI, Gerlag DM, Tak PP. Prevalence of anti-citrullinated protein antibodies and IgM rheumatoid factor in first-degree relatives of Dutch rheumatoid arthritis patients. Arthritis Rheumatol. (2015) 67(12):3324–6. doi: 10.1002/art.39412
59. van Zanten A, Arends S, Roozendaal C, Limburg PC, Maas F, Trouw LA, et al. Presence of anticitrullinated protein antibodies in a large population-based cohort from The Netherlands. Ann Rheum Dis. (2017) 76(7):1184–90. doi: 10.1136/annrheumdis-2016-209991
60. Rose NR. Prediction and prevention of autoimmune disease in the 21st century: a review and preview. Am J Epidemiol. (2016) 183(5):403–6. doi: 10.1093/aje/kwv292
Keywords: PTSD, systemic lupus erythematosus, autoimmune disease, autoantibodies, rheumatoid arthritis
Citation: Scofield RH, Lawrence S, Kurien BT, Gross T, Sorocco K, Prodan C and Lewis VM (2025) Rheumatic autoimmune disease in relation to post-traumatic stress disease and traumatic brain injury. Front. Lupus 3:1553510. doi: 10.3389/flupu.2025.1553510
Received: 30 December 2024; Accepted: 28 March 2025;
Published: 24 April 2025.
Edited by:
Ryusuke Yoshimi, Yokohama City University, JapanReviewed by:
Antonia Szanto, University of Debrecen, HungaryRahul Kakalij, University of Nebraska Medical Center, United States
Copyright: © 2025 Scofield, Lawrence, Kurien, Gross, Sorocco, Prodan and Lewis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Hal Scofield, aGFsLXNjb2ZpZWxkQG9tcmYub3Voc2MuZWR1
 R. Hal Scofield
R. Hal Scofield Stephanie Lawrence2,5
Stephanie Lawrence2,5 Biji T. Kurien
Biji T. Kurien Valerie M. Lewis
Valerie M. Lewis