A commentary on
Communication between Viruses Guides Lysis–Lysogeny Decisions
by Erez, Z., Steinberger-Levy, I., Shamir, M., Doron, S., Stokar-Avihail, A., Peleg, Y., et al. (2017). Nature 541, 488–493. doi: 10.1038/nature21049
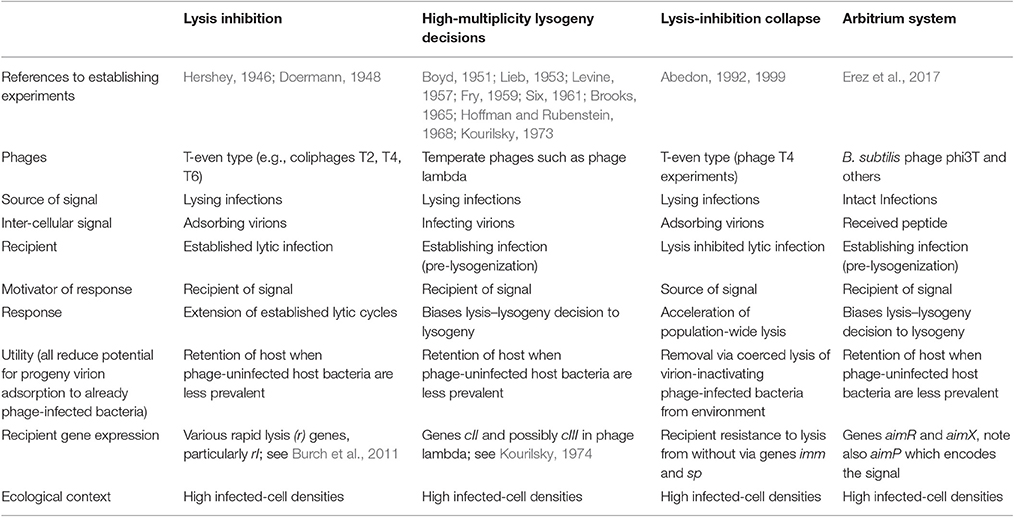
Communication between bacteria, via quorum sensing, has been a hot topic for some time (Miller and Bassler, 2001). Communication between bacteriophage- (phage-) infected bacteria has been much less studied, though predates the discovery of quorum sensing (Table 1), plus an offshoot played a prominent role in characterization of the fine structure of genes (Benzer, 1955). A conceptually related phenomenon has been observed with phage lambda involving lysis–lysogeny decisions. As I've had an interest in these systems for ~30 years, here I provide some historical as well as ecological perspective.
In a fascinating study, Erez et al. (2017) found that phage phi3T-infected Bacillus subtilis provides extracellular signals, consisting of hexapeptides, which are detected by newly phage phi3T-infected bacteria. This “arbitrium” system requires three phage genes: One produces the peptide, another serves as receptor, and the third regulates the display of lysogeny. Thus, phi3T-infected bacteria produce an extracellular signal which, if present in sufficient quantities, has the effect of increasing the likelihood of display of lysogenic cycles by newly phage-infected bacteria. If insufficient signal is present, then there is greater tendency for infections to instead display lytic cycles. Phages thus can extend their infection periods, as prophages, when potential host bacteria presumably are mostly phage infected, but exploit those bacteria lytically when neighboring bacteria are less likely to be already phage infected. Erez et al. conclude by noting that, “To our knowledge, this study is the first demonstration of actual small-molecule communication between viruses.” To my knowledge that statement is technically correct. As alluded to in the first paragraph of this commentary, however, the Erez et al. study is not the first to demonstrate communication between viruses, or more specifically between virus-infected bacteria.
The virus-to-virus communication described by Erez et al. (2017) is unidirectional, involving the release of a factor, a short peptide, which is both received by and influences the physiology of different phage-infected bacteria. That idea, however, that phage-infected bacteria can generate extracellular factors which can influence the physiology of other phage infections was, to my knowledge, first and in ways similarly presented by Doermann (1948) as a phenotype of T-even type phages; see also Hershey (1946). Here it is phage virions themselves that serve as the extracellular signal, as received in the form of secondary adsorptions (Abedon, 1994, 2015). By some as yet not fully characterized mechanism (Moussa et al., 2012), this results in an extension of the infecting phage's latent period (lytic cycle), with this extension coinciding with enhancement of the phage burst size.
The possible ecological underpinnings of the phenomenon of lysis inhibition were first pointed out and subsequently elaborated upon by myself (Abedon, 1990, 2008, 2009, 2011a, 2012). As echoed by Erez et al. (2017), “The biological logic behind this strategy is clear: when a single phage encounters a bacterial colony, there is ample prey for the progeny phages that are produced from the first cycles of infection, and hence a lytic cycle is preferred. In later stages of the infection dynamics, the number of bacterial cells is reduced to a point that progeny phages are at risk of no longer having a new host to infect.” Thus, the phages extend their infections presumably to more fully exploit increasingly rare bacterial hosts, whether using lysogenic cycles or, instead, via lysis inhibition.
A second example of communication between phage infections was also noted, by myself, within the context of lysis inhibition (Abedon, 1992). Lysis inhibited bacteria face a dilemma as a consequence of lysis inhibition (Abedon, 2008, 2009), and this stems from a display of superinfection exclusion by phage-infected bacteria (Abedon, 1994). In a population of lysis-inhibited bacteria, the first infections to lyse will expose their virion progeny to already phage-infected bacteria. Sufficiently high local densities of these phage-infected bacteria can result in rapid inactivation of those virions, i.e., as due to superinfection exclusion. A solution to this problem is to wait, via continued lysis inhibition, until the rest of the phage population has lysed before releasing phage progeny. If all local infections were to so wait, however, then the expectation would be that lysis would never occur and thereby no disseminating virions would be released to locate new hosts, hence the dilemma. One solution is for infections to lyse more or less simultaneously, which in the laboratory turns out to be just what they do. The signal that conveys this coordination between otherwise independent bacteria is supplied by other infections, again in the form of virions. The mechanism itself appears to resemble a phenomenon known as lysis from without (Abedon, 1992, 1999, 2011b).
Lysis inhibition represents a conditional increase in a phage's infection period in association with an increase in a phage's burst size. Lysogeny represents a conditional increase in a phage's infection period in association, at least potentially, with an increase in the number of phage bursts (Abedon, 2008, 2009). As Erez et al. note, the decision to enter lysogenic cycles can be influenced by secondary adsorptions, or more specifically in this case, by multiple infection of otherwise uninfected bacteria. Thus, just as with lysis inhibition, when multiple phages which are able to adsorb individual bacteria are present within an environment, then this has the effect of inducing extensions in latent periods, that is, biasing infections toward lysogenic cycles (e.g., see Weitz et al., 2008).
Erez et al. (2017) found that signals provided by predecessor infections can influence the behavior of subsequent infections, changing the behavior of the newer infections in response to the existence of high local densities of phage-infected bacteria. As noted, at least three instances have already been described of similar communication between phage infections, each also serving to mitigate issues associated with phage-infection “overcrowding.” These other mechanisms all employ whole phage virions as the signal. An important ecological question therefore is why employ peptide-based lytic cycle-suppression given use, toward similar ends, of virion-mediated communication by other phages? Perhaps to achieve redundant, sooner, or additive activity? Origination of signals also from already established lysogens is another possibility (Hynes and Moineau, 2017).
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abedon, S. T. (1990). Selection for lysis inhibition in bacteriophage. J. Theor. Biol. 146, 501–511. doi: 10.1016/S0022-5193(05)80375-3
Abedon, S. T. (1992). Lysis of lysis inhibited bacteriophage T4-infected cells. J. Bacteriol. 174, 8073–8080. doi: 10.1128/jb.174.24.8073-8080.1992
Abedon, S. T. (1994). “Lysis and the interaction between free phages and infected cells,” in The Molecular Biology of Bacteriophage T4, eds J. D. Karam, E. Kutter, K. Carlson, and B. Guttman (Washington, DC: ASM Press), 397–405.
Abedon, S. T. (1999). Bacteriophage T4 resistance to lysis-inhibition collapse. Genet. Res. 74, 1–11. doi: 10.1017/S0016672399003833
Abedon, S. T. (2008). “Phage population growth: constraints, games, adaptation,” in Bacteriophage Ecology, ed S. T. Abedon (Cambridge, UK: Cambridge University Press), 64–93. doi: 10.1017/cbo9780511541483.006
Abedon, S. T. (2009). “Bacteriophage intraspecific cooperation and defection,” in Contemporary Trends in Bacteriophage Research, ed H. T. Adams (Hauppauge, NY: Nova Science Publishers), 191–215.
Abedon, S. T. (2011a). Bacteriophages and Biofilms: Ecology, Phage Therapy, Plaques. Hauppauge, NY: Nova Science Publishers.
Abedon, S. T. (2012). Thinking about microcolonies as phage targets. Bacteriophage 2, 200–204. doi: 10.4161/bact.22444
Abedon, S. T. (2015). Bacteriophage secondary infection. Virol. Sin. 30, 3–10. doi: 10.1007/s12250-014-3547-2
Benzer, S. (1955). Fine structure of a genetic region in bacteriophage. Proc. Natl. Acad. Sci. U.S.A. 41, 344–354. doi: 10.1073/pnas.41.6.344
Boyd, J. S. (1951). Observations on the relationship of symbiotic and lytic bacteriophage. J. Pathol. Bacteriol. 63, 445–457. doi: 10.1002/path.1700630311
Brooks, K. (1965). Studies in the physiological genetics of some supporessor-sensitive mutants of bacteriophages lambda. Virology 26, 489–499. doi: 10.1016/0042-6822(65)90011-5
Burch, L. H., Zhang, L., Chao, F. G., Xu, H., and Drake, J. W. (2011). The bacteriophage T4 rapid-lysis genes and their mutational proclivities. J. Bacteriol. 193, 3537–3545. doi: 10.1128/JB.00138-11
Doermann, A. H. (1948). Lysis and lysis inhibition with Escherichia coli bacteriophage. J. Bacteriol. 55, 257–275.
Erez, Z., Steinberger-Levy, I., Shamir, M., Doron, S., Stokar-Avihail, A., Peleg, Y., et al. (2017). Communication between viruses guides lysis-lysogeny decisions. Nature 541, 488–493. doi: 10.1038/nature21049
Fry, B. A. (1959). Conditions for the infection of Escherichia coli with lambda phage and for the establishment of lysogeny. J. Gen. Microbiol. 21, 676–684. doi: 10.1099/00221287-21-3-676
Hershey, A. D. (1946). Mutation of bacteriophage with respect to type of plaque. Genetics 31, 620–640.
Hoffman, D. B. Jr., and Rubenstein, I. (1968). Physical studies of lysogeny. I. Properties of intracellular parental bacteriophage DNA from λ-infected sensitive bacteria. J. Mol. Biol. 35, 375–399. doi: 10.1016/S0022-2836(68)80001-4
Hynes, A. P., and Moineau, S. (2017). Phagebook: the social network. Mol. Cell 65, 963–964. doi: 10.1016/j.molcel.2017.02.028
Kourilsky, P. (1973). Lysogenization by bacteriophage lambda. I. Multiple infection and the lysogenic response. Mol. Gen. Genet. 122, 183–195. doi: 10.1007/BF00435190
Kourilsky, P. (1974). Lysogenization by bacteriophage lambda. II. Identification of genes involved in the multiplicity dependent processes. Biochimie 56, 1511–1516. doi: 10.1016/S0300-9084(75)80274-4
Levine, M. (1957). Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology 3, 22–41. doi: 10.1016/0042-6822(57)90021-1
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. doi: 10.1146/annurev.micro.55.1.165
Moussa, S. H., Kuznetsov, V., Tran, T. A., Sacchettini, J. C., and Young, R. (2012). Protein determinants of phage T4 lysis inhibition. Protein Sci. 21, 571–582. doi: 10.1002/pro.2042
Six, E. (1961). Inheritance of prophage P2 in superinfection experiments. Virology 14, 220–233. doi: 10.1016/0042-6822(61)90197-0
Keywords: bacteriophage ecology, extracellular signaling, lysis inhibition, multiplicity of infection, phage
Citation: Abedon ST (2017) Commentary: Communication between Viruses Guides Lysis–Lysogeny Decisions. Front. Microbiol. 8:983. doi: 10.3389/fmicb.2017.00983
Received: 24 February 2017; Accepted: 16 May 2017;
Published: 31 May 2017.
Edited by:
William Michael McShan, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Scott Van Nguyen, Agricultural Research Service (USDA), United StatesCopyright © 2017 Abedon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen T. Abedon, YWJlZG9uLjFAb3N1LmVkdQ==
 Stephen T. Abedon
Stephen T. Abedon